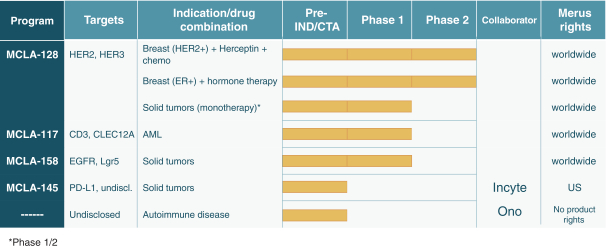
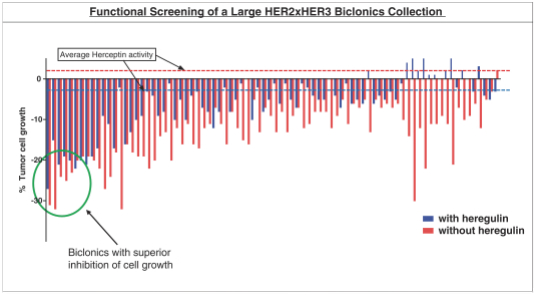
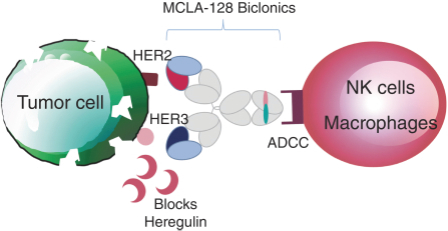
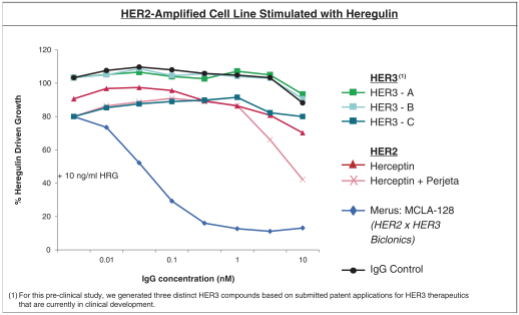
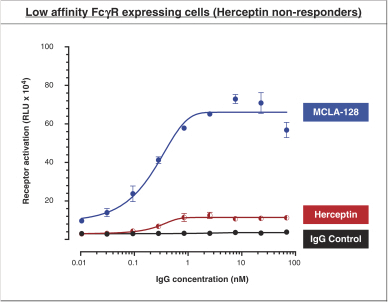
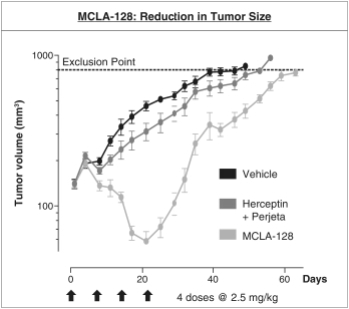
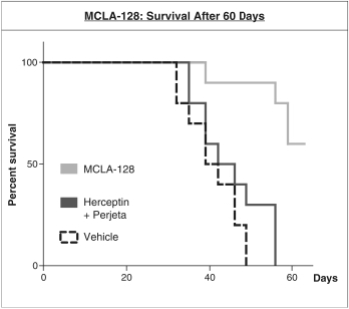
As of April 24, 2018, our patent portfolio related to our bispecific antibody candidateMCLA-128 consists of one PCT application, filed on February 27, 2015 which entered national phases in the United States, Europe and 17 other foreign countries with an expected expiry not earlier than February 27, 2035. Claims are directed toMCLA-128 composition of matter and methods of usingMCLA-128 to treat subjects having or at risk of having aErbB-2 and/or ErbB3 positive tumor. In addition, four priority patent application filings covering further methods of usingMCLA-128, including in combination therapies, to treat patients, three of which have been filed on March 31, 2017 and one filed on May 17, 2017.
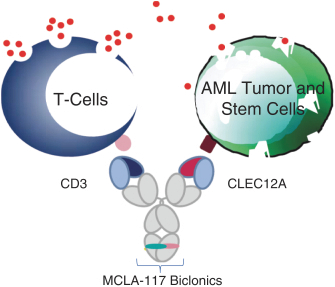
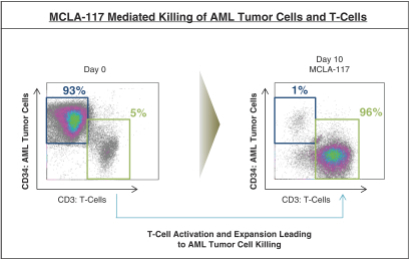
As of April 24, 2018, our patent portfolio related to our bispecific antibody candidateMCLA-117 consists of a first PCT application, filed on September 27, 2013, which entered national phases in the United States, Europe and 13 foreign countries with an expected expiry not earlier than September 27, 2033. There is currently, one pending US application, 14 pending foreign applications, and three issued patents in several foreign jurisdictions. In addition, we filed a second PCT application related toMCLA-117 on July 10, 2016, which has entered national phases in the United States, Europe and 13 foreign countries with an expected expiry not earlier than July 10, 2036. There is currently, one issued U.S. patent and one pending U.S. application, 2 pending EP applications, and 13 pending foreign applications. Claims are directed to theMCLA-117 composition of matter and methods of usingMCLA-117 in the treatment or prevention of MDS, chronic myelogenous leukemia, or CML, or AML.
As of April 24, 2018, our patent portfolio related to our bispecific antibody candidateMCLA-158 consists of one PCT filed on October 21, 2016, which entered or will enter national phases in the United States, Europe and 14 other foreign countries with an expiry no earlier than October 21, 2036. Claims are directed to theMCLA-158 composition of matter and methods of usingMCLA-158 in the treatment or prevention of various solid tumors.
As of April 24, 2018, our patent portfolio related to our MeMo® mouse consists of three issued U.S. patents, eight pending U.S. applications, 12 issued foreign patents including one issued European patent that has been validated in many countries, and 11 pending foreign applications, all with an expected expiry not earlier than June 29, 2029. Claims are directed to a common light chain mouse and methods of producing antibodies by exposing the mouse to an antigen. For a discussion concerning opposition proceedings against this patent family see Part I, Item 8. “Legal Proceedings” of our Annual Report on Form20-F for the year ended December 31, 2016 and in Note 16 to our Consolidated Financial Statements included in this Annual Report, respectively.
As of April 24, 2018, our patent portfolio related to our Spleen to ScreenTM technology consists of two issued U.S. patents, one pending U.S. application, one issued European Patent, and one issued foreign patent, with five foreign pending applications, all with an expected expiry not earlier than September 16, 2035. For a discussion concerning opposition proceedings against this patent family see Part I, Item 8 included in this Annual Report.
As of April 24, 2018, our patent portfolio related to recombinant production of mixtures of antibodies, and includes claims directed to host cells generating multi-specific antibodies consists of 5 issued U.S. patents, and 4 pending U.S. applications, 2 issued European patents, 14 issued foreign patents, and four 5 pending foreign applications, all with an expected expiry not earlier than July 18, 2022. For a discussion concerning opposition proceedings against this patent family see Note 16 to our Consolidated Financial Statements included in this Annual Report.
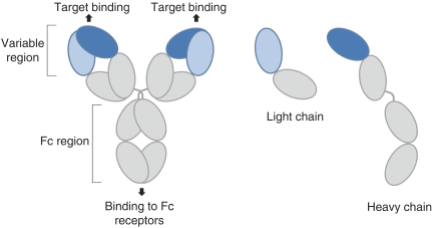
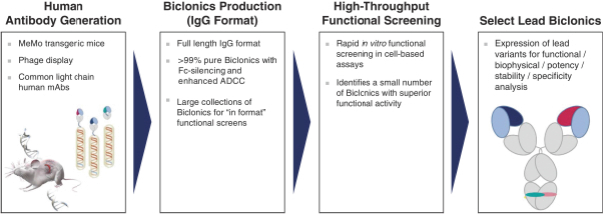
We plan to continue to expand our intellectual property estate by filing patent applications directed to dosage forms, methods of treatment and additional compositions created or identified from our Biclonics® technology platform, improvements to our Biclonics® technology platform and ongoing development of our antibody candidates. Specifically, we seek patent protection in the United States and internationally for novel compositions of matter directed to aspects of the molecules, basic structures and processes for manufacturing these molecules and the use of these molecules in a variety of therapies.
Our patent portfolio is intended to cover, but is not limited to, the composition of matter of our bispecific antibody candidates, their methods of use, the Biclonics® technology platform used to generate them, related
74