Exhibit 99.1

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Pioneering Precision Medicine for Neurodegeneration NASDAQ: ACIU | Annual General Meeting, June 23, 2023 www.acimmune.com Version: 19.06.2023

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Disclaimer This presentation contains statements that constitute “forward - looking statements” within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Forward - looking statements are statements other than historical fact and may include statements that address future operating, financial or business performance or AC Immune’s strategies or expectations . In some cases, you can identify these statements by forward - looking words such as “may,” “might,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “potential,” “outlook” or “continue,” and other comparable terminology . Forward - looking statements are based on management’s current expectations and beliefs and involve significant risks and uncertainties that could cause actual results, developments and business decisions to differ materially from those contemplated by these statements . These risks and uncertainties include those described under the captions “Item 3 . Key Information – Risk Factors” and “Item 5 . Operating and Financial Review and Prospects” in AC Immune’s Annual Report on Form 20 - F and other filings with the Securities and Exchange Commission . These include : the impact of Covid - 19 on our business, suppliers, patients and employees and any other impact of Covid - 19 . Forward - looking statements speak only as of the date they are made, and AC Immune does not undertake any obligation to update them in light of new information, future developments or otherwise, except as may be required under applicable law . All forward - looking statements are qualified in their entirety by this cautionary statement . This presentation is strictly confidential, is being distributed to a limited range of invited persons solely for their own information, may not be distributed to the press or any other person, and may not be reproduced or published, in whole or in part, in any form . SupraAntigen ® is a registered trademark of AC Immune SA in the following territories : AU, CH, EU, GB, JP, RU, SG and USA . Morphomer ® is a registered trademark of AC Immune SA in CH, CN, GB, JP, KR, NO and RU . 2

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission 3 Agenda 1. AC Immune’s approach to neurodegenerative diseases 2. Business strategy and pipeline update 3. Clinical - stage vaccine programs 4. Achievements and key milestones 2022/23 5. Financial figures 6. Summary and Strategic outlook
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission AC Immune pioneering new ways to treat neurodegenerative diseases Combining Precision Medicine and early, targeted treatment 4 Broad, diverse pipeline – 16 programs 1 Phase 3 program and 5 in Phase 2 Key differentiation: Precision Medicine Integrates therapeutics and diagnostics Multiple global partnerships >CHF 3 billion in potential milestones Clinically validated technology platforms Best - in - class small molecules and biologics Strong Balance sheet Funded into Q3 2024 (1) As of March 31, 2023; excluding treasury shares; (2) As of March 31, 2023 ■ Based in Lausanne, Switzerland ■ ~150 employees ■ Listed September 2016 (NASDAQ: ACIU) ■ 83.6 million shares outstanding 1 ■ Cash of CHF 105.4 million 2 (~USD 115 million)
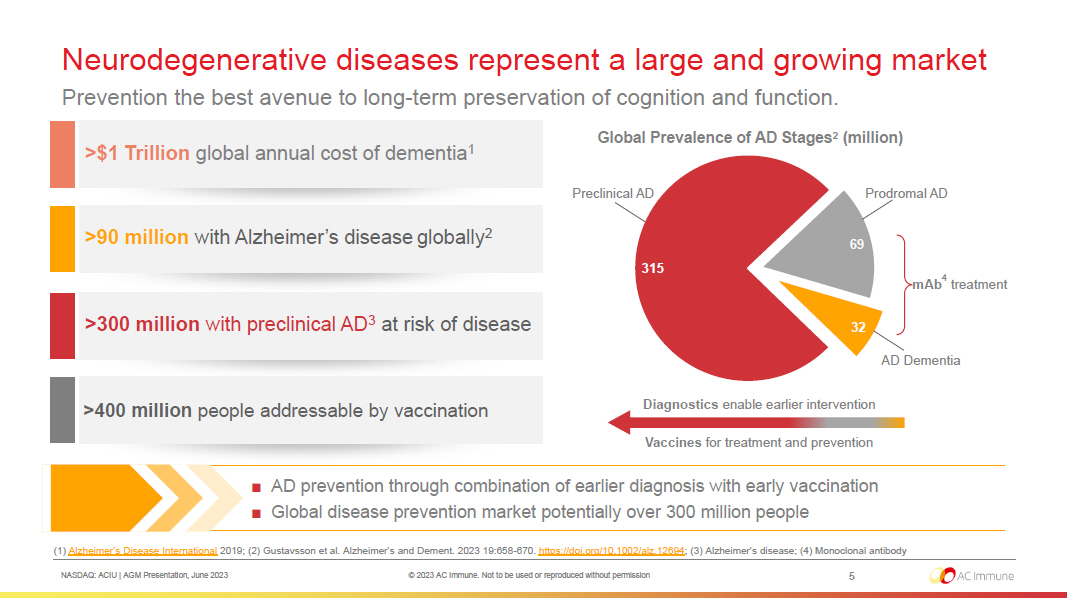
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Neurodegenerative diseases represent a large and growing market Prevention the best avenue to long - term preservation of cognition and function. 5 >$1 Trillion global annual cost of dementia 1 >90 million with Alzheimer’s disease globally 2 >300 million with preclinical AD 3 at risk of disease (1) Alzheimer’s Disease International 2019 ; (2) Gustavsson et al. Alzheimer’s and Dement. 2023 19:658 - 670. https://doi.org/10.1002/alz.12694 ; (3) Alzheimer’s disease; (4) Monoclonal antibody ■ AD prevention through combination of earlier diagnosis with early vaccination ■ Global disease prevention market potentially over 300 million people >400 million people addressable by vaccination mAb 4 treatment Global Prevalence of AD Stages 2 (million) 315 69 32 Vaccines for treatment and prevention Diagnostics enable earlier intervention Preclinical AD Prodromal AD AD Dementia
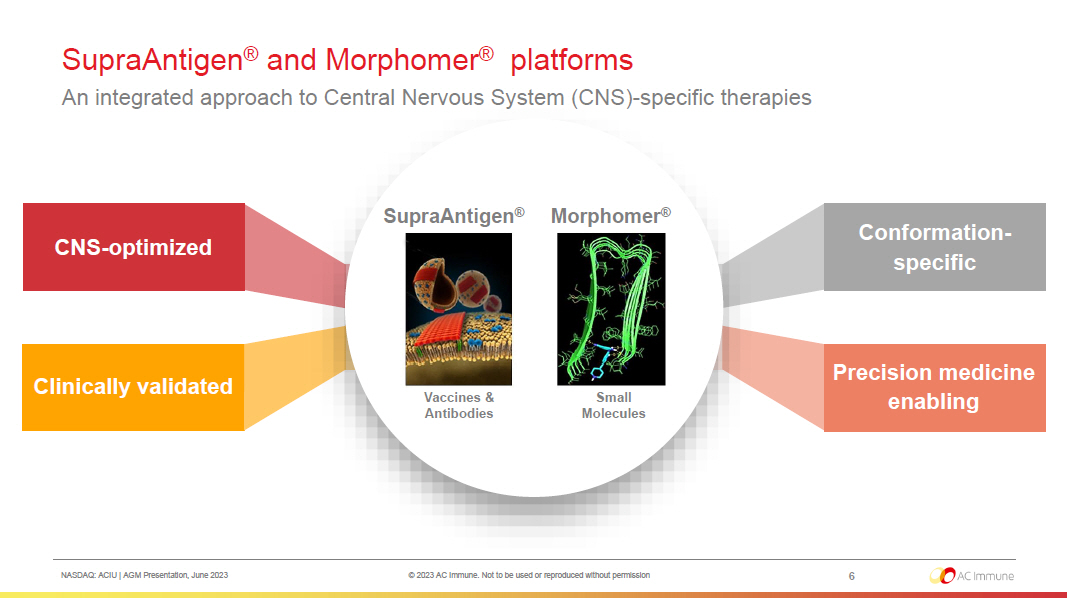
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission SupraAntigen ® and Morphomer ® platforms An integrated approach to Central Nervous System (CNS) - specific therapies 6 Clinically validated CNS - optimized Precision medicine enabling Conformation - specific SupraAntigen ® Morphomer ® Vaccines & Antibodies Small Molecules

2. Business strategy and pipeline update
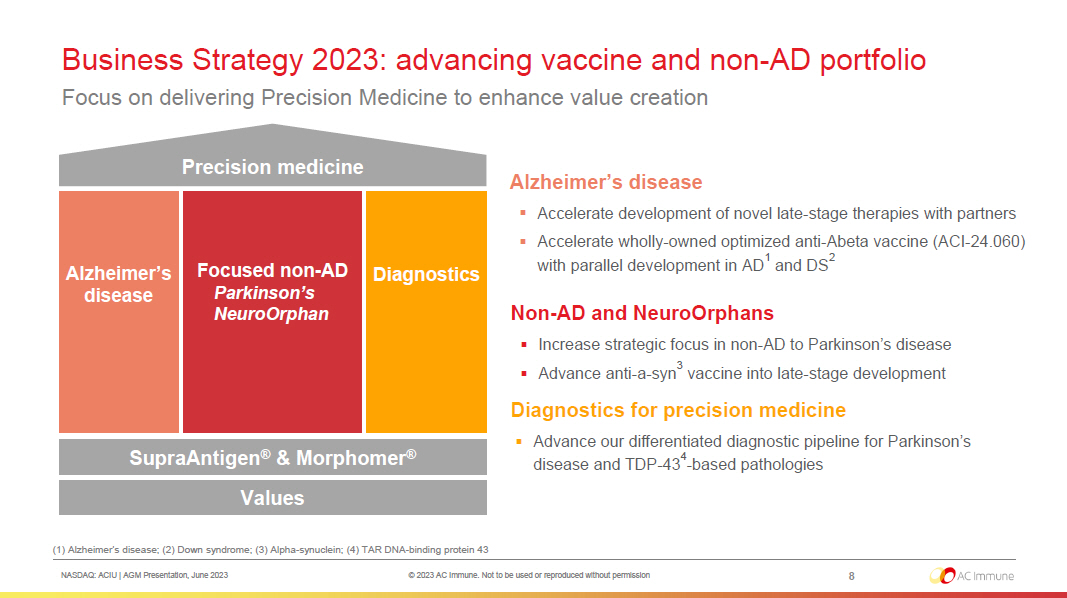
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Business Strategy 2023: advancing vaccine and non - AD portfolio Focus on delivering Precision Medicine to enhance value creation Precision medicine Alzheimer’s disease Focused non - AD Parkinson’s NeuroOrphan Diagnostics SupraAntigen ® & Morphomer ® Values (1) Alzheimer’s disease; (2) Down syndrome; (3) Alpha - synuclein; (4) TAR DNA - binding protein 43 Alzheimer’s disease ▪ Accelerate development of novel late - stage therapies with partners ▪ Accelerate wholly - owned optimized anti - Abeta vaccine (ACI - 24.060) with parallel development in AD 1 and DS 2 Non - AD and NeuroOrphans ▪ Increase strategic focus in non - AD to Parkinson’s disease ▪ Advance anti - a - syn 3 vaccine into late - stage development Diagnostics for precision medicine ▪ Advance our differentiated diagnostic pipeline for Parkinson’s disease and TDP - 43 4 - based pathologies 8
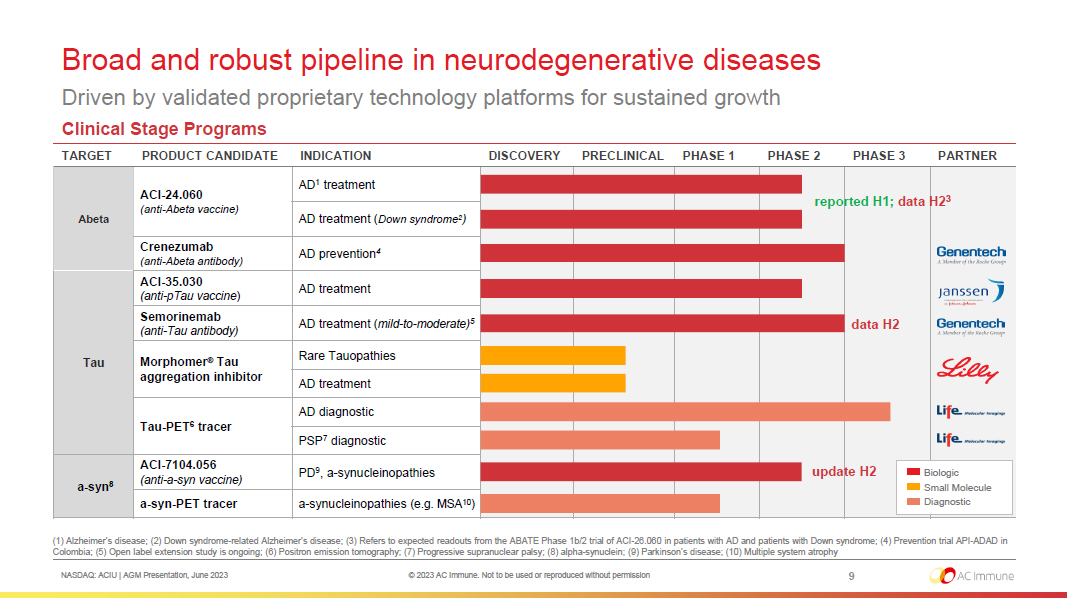
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Broad and robust pipeline in neurodegenerative diseases Driven by validated proprietary technology platforms for sustained growth 9 (1) Alzheimer’s disease; (2) Down syndrome - related Alzheimer’s disease ; (3) Refers to expected readouts from the ABATE Phase 1b/2 trial of ACI - 26.060 in patients with AD and patients with Down syndro me ; (4) Prevention trial API - ADAD in Colombia ; (5) Open label extension study is ongoing; (6) Positron emission tomography; (7) Progressive supranuclear palsy ; (8) alpha - synuclein; (9) Parkinson’s disease; (10) Multiple system atrophy Clinical Stage Programs TARGET PRODUCT CANDIDATE INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PARTNER Abeta ACI - 24.060 (anti - Abeta vaccine) AD 1 treatment AD treatment ( Down syndrome 2 ) Crenezumab (anti - Abeta antibody) AD prevention 4 Tau ACI - 35.030 (anti - pTau vaccine ) AD treatment Semorinemab (anti - Tau antibody) AD treatment ( mild - to - moderate) 5 Morphomer ® Tau aggregation inhibitor Rare Tauopathies AD treatment Tau - PET 6 tracer AD diagnostic PSP 7 diagnostic a - syn 8 ACI - 7104.056 (anti - a - syn vaccine) PD 9 , a - synucleinopathies a - syn - PET tracer a - synucleinopathies (e.g. MSA 10 ) Biologic Small Molecule Diagnostic data H2 reported H1; data H2 3 update H2
3. Clinical - stage vaccine programs
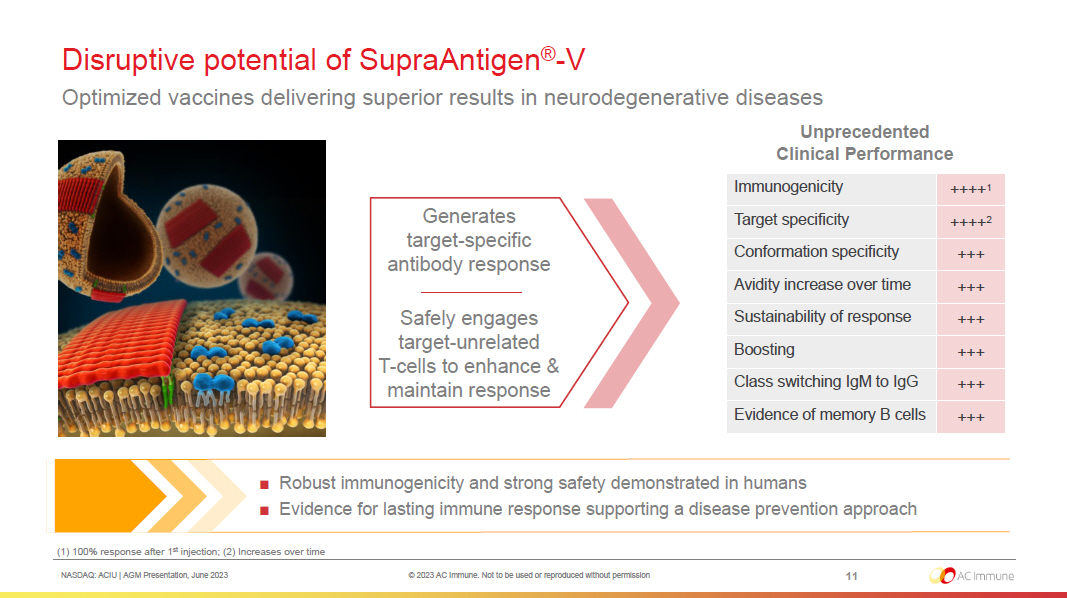
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Disruptive potential of SupraAntigen ® - V Optimized vaccines delivering superior results in neurodegenerative diseases 11 (1) 100% response after 1 st injection; (2) Increases over time ■ Robust immunogenicity and strong safety demonstrated in humans ■ Evidence for lasting immune response supporting a disease prevention approach Immunogenicity ++++ 1 Target specificity ++++ 2 Conformation specificity +++ Avidity increase over time +++ Sustainability of response +++ Boosting +++ Class switching IgM to IgG +++ Evidence of memory B cells +++ Unprecedented Clinical Performance Generates target - specific antibody response Safely engages target - unrelated T - cells to enhance & maintain response
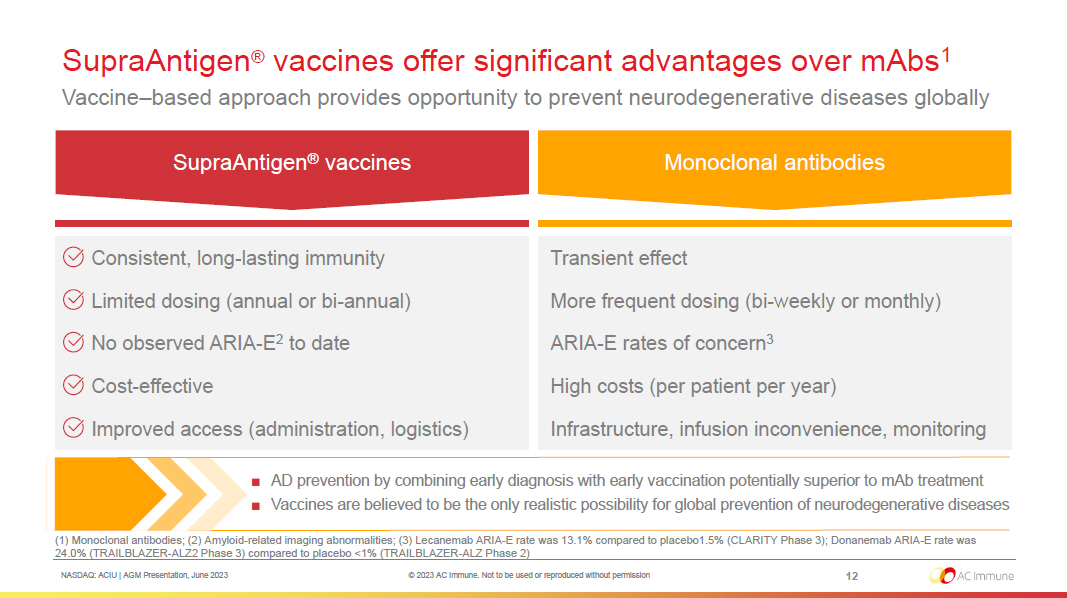
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission SupraAntigen ® vaccines offer significant advantages over mAbs 1 Vaccine – based approach provides opportunity to prevent neurodegenerative diseases globally SupraAntigen ® vaccines Monoclonal antibodies (1) Monoclonal antibodies; (2) Amyloid - related imaging abnormalities; (3) Lecanemab ARIA - E rate was 13.1% compared to placebo1.5 % (CLARITY Phase 3); Donanemab ARIA - E rate was 24.0% (TRAILBLAZER - ALZ2 Phase 3) compared to placebo <1% (TRAILBLAZER - ALZ Phase 2) Consistent, long - lasting immunity Limited dosing (annual or bi - annual) No observed ARIA - E 2 to date Cost - effective Improved access (administration, logistics) Transient effect More frequent dosing (bi - weekly or monthly) ARIA - E rates of concern 3 High costs (per patient per year) Infrastructure, infusion inconvenience, monitoring ■ AD prevention by combining early diagnosis with early vaccination potentially superior to mAb treatment ■ Vaccines are believed to be the only realistic possibility for global prevention of neurodegenerative diseases 12
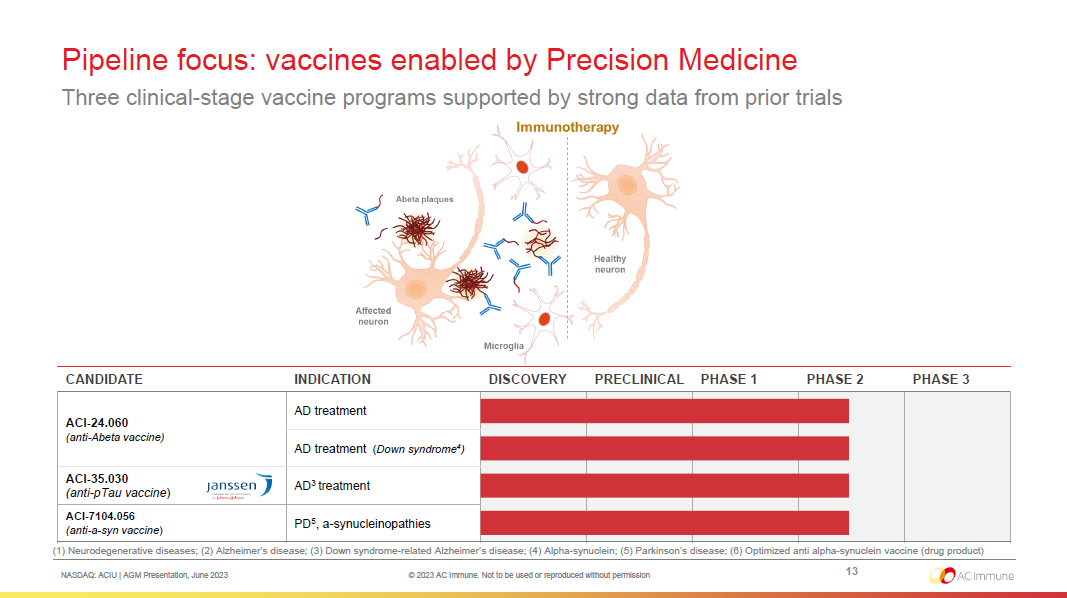
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission (1) Neurodegenerative diseases ; (2) Alzheimer’s disease; (3) Down syndrome - related Alzheimer’s disease; (4) Alpha - synuclein; (5) Parkinson’s disease; (6) Optimized anti alpha - synuclein vaccine (drug product) CANDIDATE INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 ACI - 24.060 (anti - Abeta vaccine) AD treatment AD treatment ( Down syndrome 4 ) ACI - 35.030 (anti - pTau vaccine ) AD 3 treatment ACI - 7104.056 (anti - a - syn vaccine ) PD 5 , a - synucleinopathies 13 Pipeline focus: vaccines enabled by Precision Medicine Three clinical - stage vaccine programs supported by strong data from prior trials
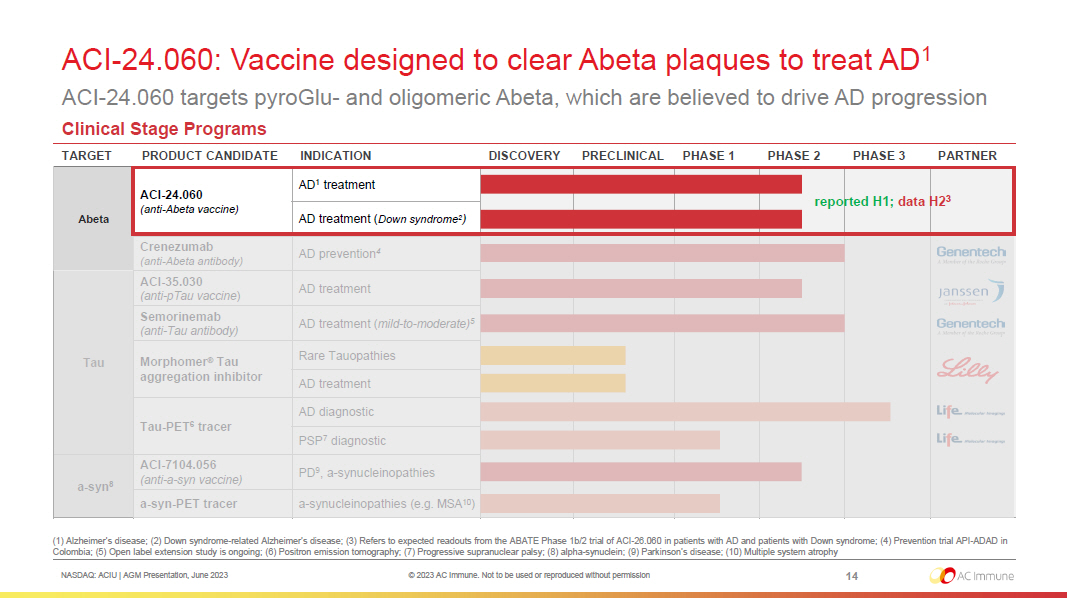
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission ACI - 24.060: Vaccine designed to clear Abeta plaques to treat AD 1 ACI - 24.060 targets pyroGlu - and oligomeric Abeta, which are believed to drive AD progression 14 (1) Alzheimer’s disease; (2) Down syndrome - related Alzheimer’s disease ; (3) Refers to expected readouts from the ABATE Phase 1b/2 trial of ACI - 26.060 in patients with AD and patients with Down syndro me ; (4) Prevention trial API - ADAD in Colombia ; (5) Open label extension study is ongoing; (6) Positron emission tomography; (7) Progressive supranuclear palsy ; (8) alpha - synuclein; (9) Parkinson’s disease; (10) Multiple system atrophy Clinical Stage Programs TARGET PRODUCT CANDIDATE INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PARTNER Abeta ACI - 24.060 (anti - Abeta vaccine) AD 1 treatment AD treatment ( Down syndrome 2 ) Crenezumab (anti - Abeta antibody) AD prevention 4 Tau ACI - 35.030 (anti - pTau vaccine ) AD treatment Semorinemab (anti - Tau antibody) AD treatment ( mild - to - moderate) 5 Morphomer ® Tau aggregation inhibitor Rare Tauopathies AD treatment Tau - PET 6 tracer AD diagnostic PSP 7 diagnostic a - syn 8 ACI - 7104.056 (anti - a - syn vaccine) PD 9 , a - synucleinopathies a - syn - PET tracer a - synucleinopathies (e.g. MSA 10 ) reported H1; data H2 3
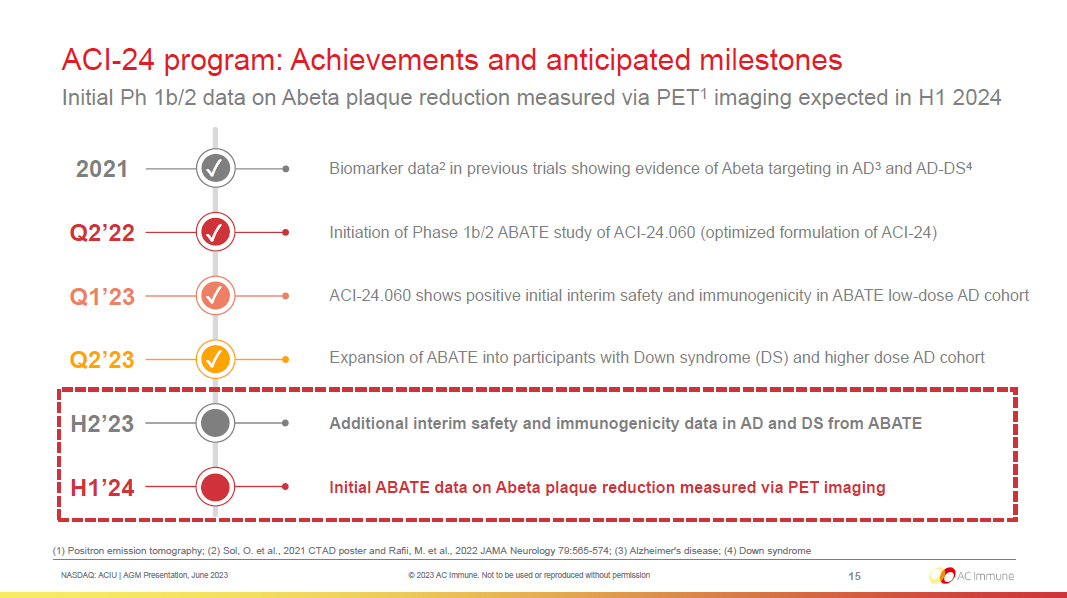
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission ACI - 24 program: Achievements and anticipated milestones Initial Ph 1b/2 data on Abeta plaque reduction measured via PET 1 imaging expected in H1 2024 15 H1’24 Additional interim safety and immunogenicity data in AD and DS from ABATE H2’23 2021 Biomarker data 2 in previous trials showing evidence of Abeta targeting in AD 3 and AD - DS 4 Q1’23 Q2’23 Q2’22 Expansion of ABATE into participants with Down syndrome (DS) and higher dose AD cohort Initiation of Phase 1b/2 ABATE study of ACI - 24.060 (optimized formulation of ACI - 24) ACI - 24.060 shows positive initial interim safety and immunogenicity in ABATE low - dose AD cohort Initial ABATE data on Abeta plaque reduction measured via PET imaging ✓ ✓ ✓ ✓ (1) Positron emission tomography; (2) Sol, O. et al., 2021 CTAD poster and Rafii, M. et al., 2022 JAMA Neurology 79:565 - 574; (3) Alzheimer's disease; (4) Down syndrome
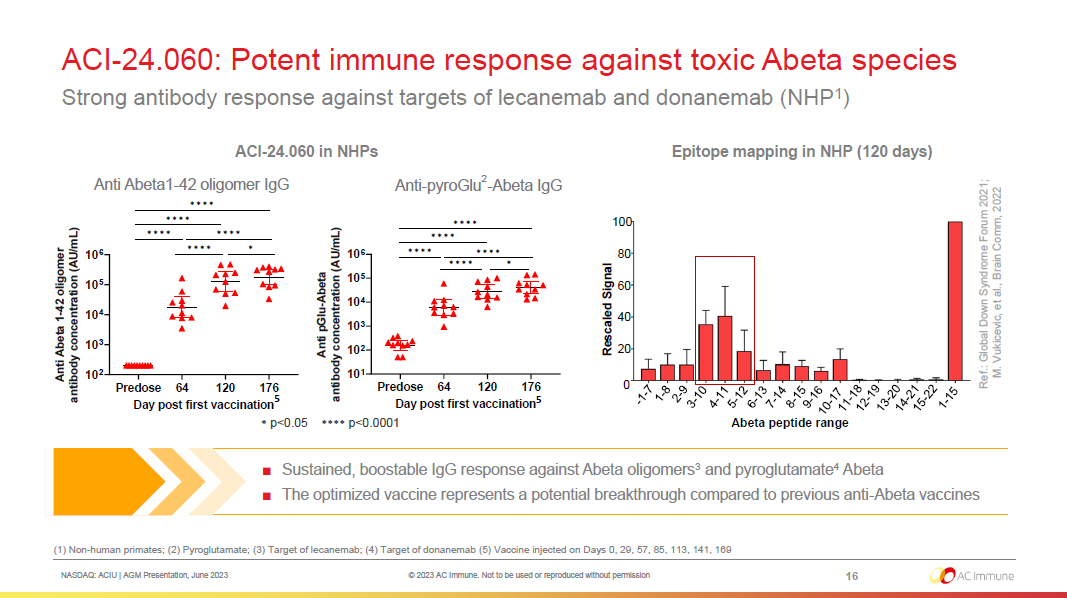
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission ACI - 24.060: Potent immune response against toxic Abeta species Strong antibody response against targets of lecanemab and donanemab (NHP 1 ) (1) Non - human primates; (2) Pyroglutamate; (3) Target of lecanemab; (4) Target of donanemab (5) Vaccine injected on Days 0, 29, 57, 85, 113, 141, 169 ■ Sustained, boostable IgG response against Abeta oligomers 3 and pyroglutamate 4 Abeta ■ The optimized vaccine represents a potential breakthrough compared to previous anti - Abeta vaccines Epitope mapping in NHP (120 days) Rescaled Signal 20 40 60 80 100 Abeta peptide range 0 Ref.: Global Down Syndrome Forum 2021; M. Vukicevic, et al., Brain Comm, 2022 16 Anti - pyroGlu 2 - Abeta IgG Anti Abeta1 - 42 oligomer IgG ACI - 24.060 in NHPs * p<0.05 **** p<0.0001 5 5
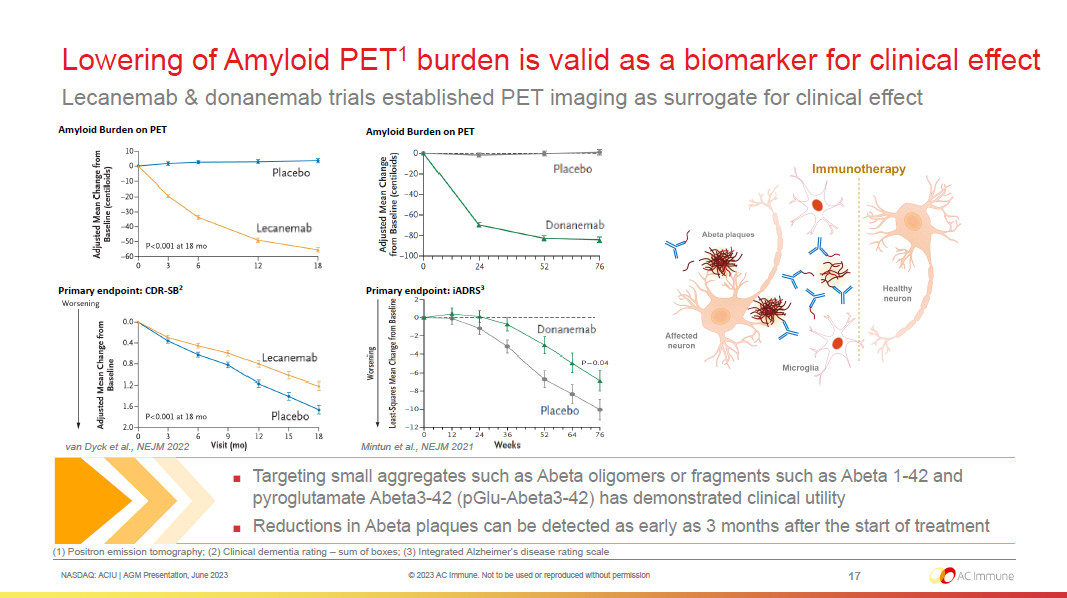
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Lowering of Amyloid PET 1 burden is valid as a biomarker for clinical effect Lecanemab & donanemab trials established PET imaging as surrogate for clinical effect 17 ■ Targeting small aggregates such as Abeta oligomers or fragments such as Abeta 1 - 42 and pyroglutamate Abeta3 - 42 (pGlu - Abeta3 - 42) has demonstrated clinical utility ■ Reductions in Abeta plaques can be detected as early as 3 months after the start of treatment Amyloid Burden on PET Primary endpoint: CDR - SB 2 Amyloid Burden on PET Primary endpoint: iADRS 3 Mintun et al., NEJM 2021 (1) Positron emission tomography; (2) Clinical dementia rating – sum of boxes; (3) Integrated Alzheimer’s disease rating scale van Dyck et al., NEJM 2022
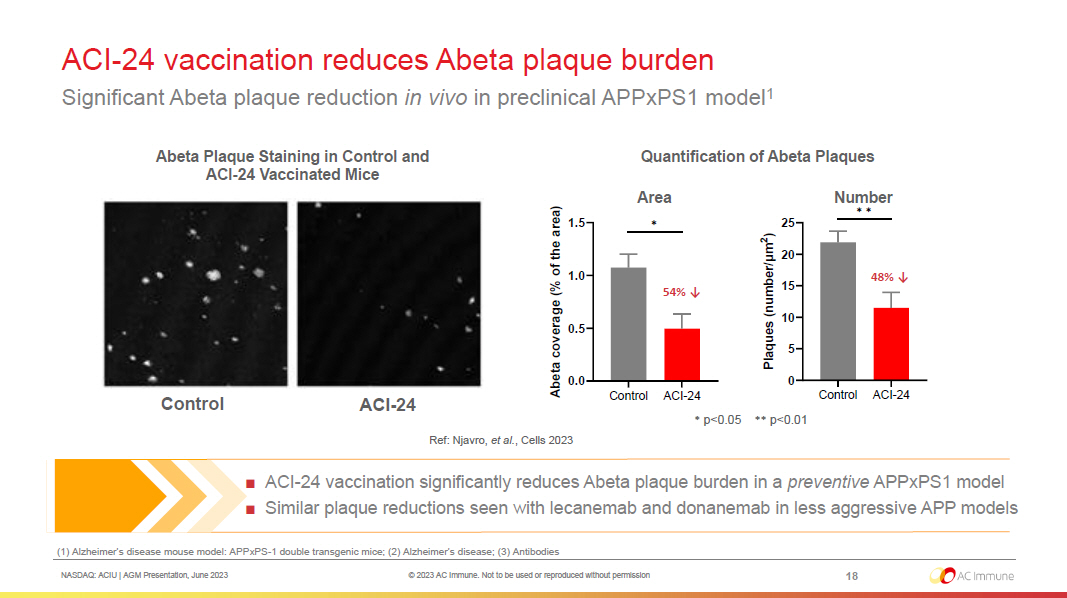
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission ACI - 24 vaccination reduces Abeta plaque burden Significant Abeta plaque reduction in vivo in preclinical APPxPS1 model 1 18 (1) Alzheimer’s disease mouse model: APPxPS - 1 double transgenic mice; (2) Alzheimer’s disease; (3) Antibodies ■ ACI - 24 vaccination significantly reduces Abeta plaque burden in a preventive APPxPS1 model ■ Similar plaque reductions seen with lecanemab and donanemab in less aggressive APP models * p<0.05 ** p<0.01 Ref: Njavro, et al. , Cells 2023 Number Area 54% ↓ 48% ↓ Abeta Plaque Staining in Control and ACI - 24 Vaccinated Mice ACI - 24 Control Quantification of Abeta Plaques
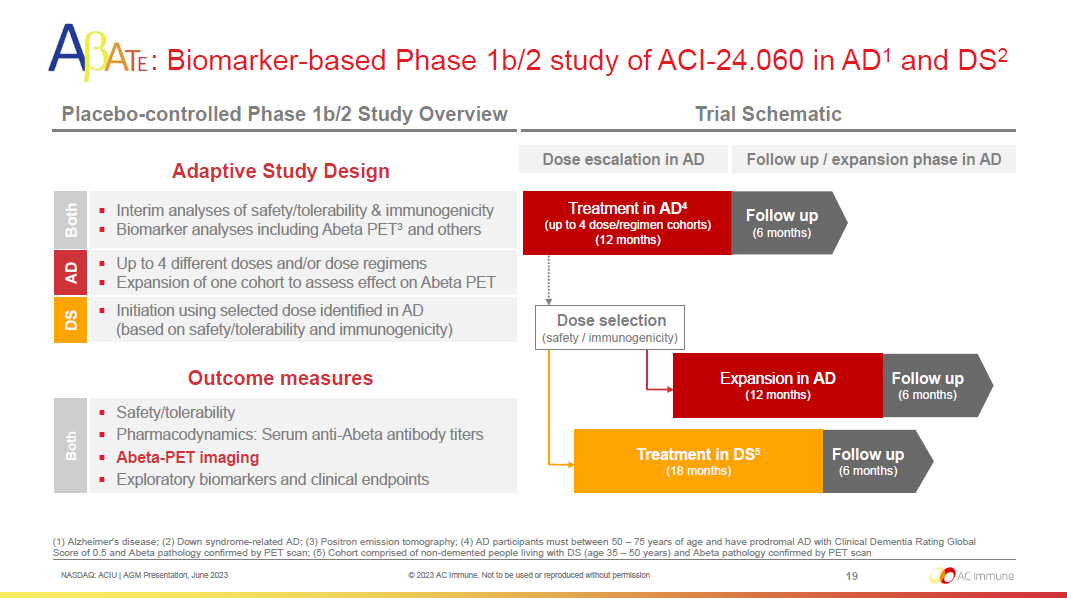
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Treatment in AD 4 (up to 4 dose/regimen cohorts) (12 months) 19 : Biomarker - based Phase 1b/2 study of ACI - 24.060 in AD 1 and DS 2 Placebo - controlled Phase 1b/2 Study Overview Trial Schematic Adaptive Study Design Follow up (6 months) Outcome measures ▪ Safety/tolerability ▪ Pharmacodynamics: Serum anti - Abeta antibody titers ▪ Abeta - PET imaging ▪ Exploratory biomarkers and clinical endpoints Both Both AD DS ▪ Interim analyses of safety/tolerability & immunogenicity ▪ Biomarker analyses including Abeta PET 3 and others ▪ Initiation using selected dose identified in AD (based on safety/tolerability and immunogenicity) ▪ Up to 4 different doses and/or dose regimens ▪ Expansion of one cohort to assess effect on Abeta PET Dose selection (safety / immunogenicity) Dose escalation in AD Follow up / expansion phase in AD Follow up (6 months) Expansion in AD (12 months) Follow up (6 months) Treatment in DS 5 (18 months) (1) Alzheimer's disease; (2) Down syndrome - related AD; (3) Positron emission tomography; (4) AD participants must between 50 – 7 5 years of age and have prodromal AD with Clinical Dementia Rating Global Score of 0.5 and Abeta pathology confirmed by PET scan; (5) Cohort comprised of non - demented people living with DS (age 35 – 50 years) and Abeta pathology confirmed by PET scan
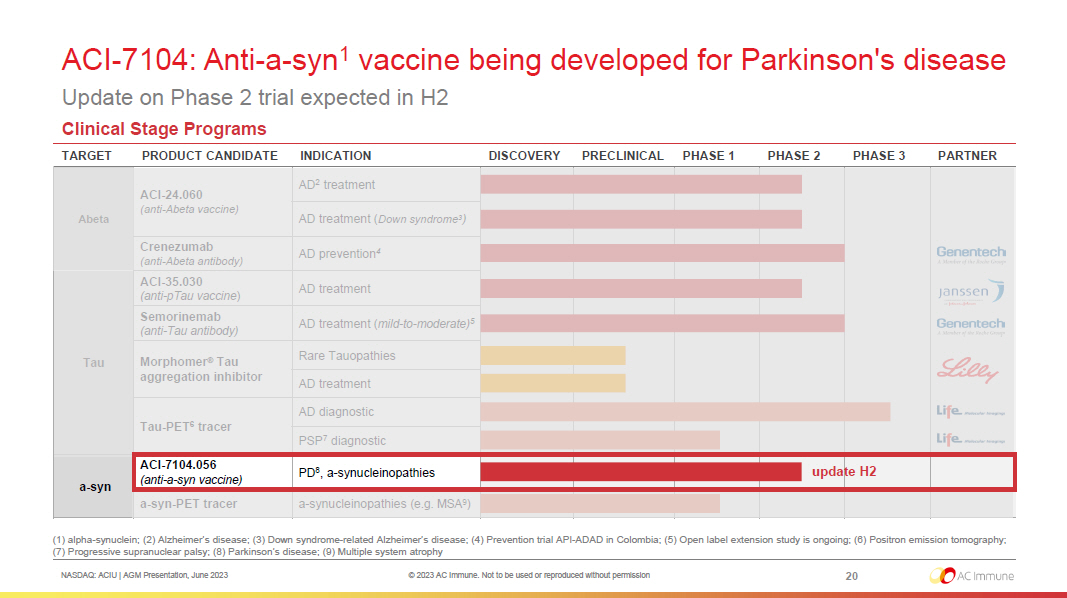
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission ACI - 7104: Anti - a - syn 1 vaccine being developed for Parkinson's disease Update on Phase 2 trial expected in H2 20 (1) alpha - synuclein; (2) Alzheimer’s disease; (3) Down syndrome - related Alzheimer’s disease ; (4) Prevention trial API - ADAD in Colombia ; (5) Open label extension study is ongoing; (6) Positron emission tomography; (7) Progressive supranuclear palsy ; (8) Parkinson’s disease; (9) Multiple system atrophy Clinical Stage Programs TARGET PRODUCT CANDIDATE INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PARTNER Abeta ACI - 24.060 (anti - Abeta vaccine) AD 2 treatment AD treatment ( Down syndrome 3 ) Crenezumab (anti - Abeta antibody) AD prevention 4 Tau ACI - 35.030 (anti - pTau vaccine ) AD treatment Semorinemab (anti - Tau antibody) AD treatment ( mild - to - moderate) 5 Morphomer ® Tau aggregation inhibitor Rare Tauopathies AD treatment Tau - PET 6 tracer AD diagnostic PSP 7 diagnostic a - syn ACI - 7104.056 (anti - a - syn vaccine) PD 8 , a - synucleinopathies a - syn - PET tracer a - synucleinopathies (e.g. MSA 9 ) update H2
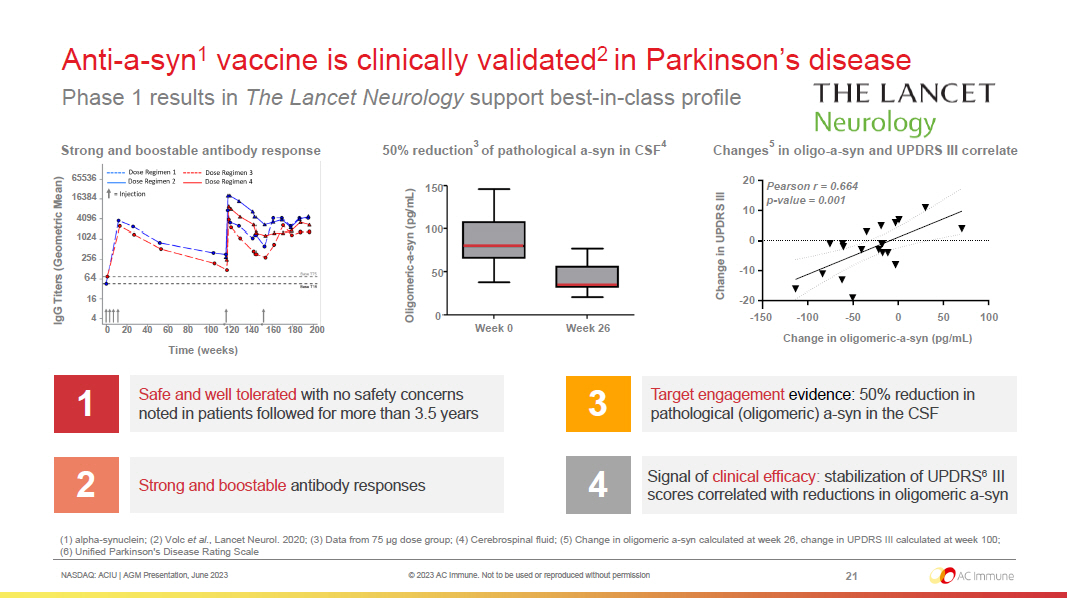
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Anti - a - syn 1 vaccine is clinically validated 2 in Parkinson’s disease Phase 1 results in The Lancet Neurology support best - in - class profile 21 (1) alpha - synuclein; (2) Volc et al. , Lancet Neurol. 2020; (3) Data from 75 µg dose group; (4) Cerebrospinal fluid; (5) Change in oligomeric a - syn calculated at week 26, change in UPDRS III calculated at week 100; (6) Unified Parkinson's Disease Rating Scale Safe and well tolerated with no safety concerns noted in patients followed for more than 3.5 years 1 Strong and boostable antibody responses 2 Target engagement evidence: 50% reduction in pathological (oligomeric) a - syn in the CSF 3 Signal of clinical efficacy : stabilization of UPDRS 6 III scores correlated with reductions in oligomeric a - syn 4 50% reduction 3 of pathological a - syn in CSF 4 Oligomeric - a - syn (pg/mL) Week 0 Week 26 0 50 100 150 Strong and boostable antibody response IgG Titers (Geometric Mean) Time (weeks) 0 20 4 0 60 8 0 100 120 140 160 180 200 4 16 64 256 1024 4096 16384 65536 Change in oligomeric - a - syn (pg/mL) Change in UPDRS III - 20 - 10 0 10 20 - 150 - 100 - 50 0 50 100 Pearson r = 0.664 p - value = 0.001 Changes 5 in oligo - a - syn and UPDRS III correlate
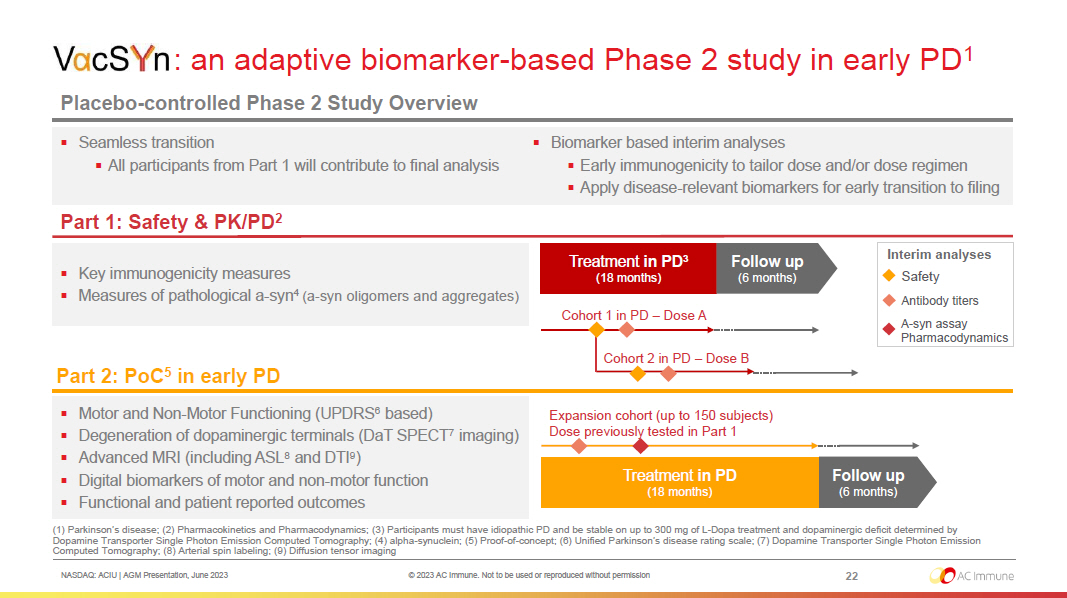
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission 22 : : an adaptive biomarker - based Phase 2 study in early PD 1 Placebo - controlled Phase 2 Study Overview ▪ Key immunogenicity measures ▪ Measures of pathological a - syn 4 (a - syn oligomers and aggregates) (1) Parkinson’s disease; (2) Pharmacokinetics and Pharmacodynamics; (3) Participants must have idiopathic PD and be stable on up to 300 mg of L - Dopa treatment and dopaminergic deficit determined by Dopamine Transporter Single Photon Emission Computed Tomography; (4) alpha - synuclein; (5) Proof - of - concept; (6) Unified Parkinso n’s disease rating scale; (7) Dopamine Transporter Single Photon Emission Computed Tomography; (8) Arterial spin labeling; (9) Diffusion tensor imaging Treatment in PD 3 (18 months) Cohort 1 in PD – Dose A Cohort 2 in PD – Dose B Safety Antibody titers A - syn assay Pharmacodynamics Interim analyses Follow up (6 months) Part 2: PoC 5 in early PD ▪ Motor and Non - Motor Functioning (UPDRS 6 based) ▪ Degeneration of dopaminergic terminals (DaT SPECT 7 imaging) ▪ Advanced MRI (including ASL 8 and DTI 9 ) ▪ Digital biomarkers of motor and non - motor function ▪ Functional and patient reported outcomes Expansion cohort (up to 150 subjects) Dose previously tested in Part 1 Treatment in PD (18 months) Follow up (6 months) ▪ Seamless transition ▪ All participants from Part 1 will contribute to final analysis ▪ Biomarker based interim analyses ▪ Early immunogenicity to tailor dose and/or dose regimen ▪ Apply disease - relevant biomarkers for early transition to filing Part 1: Safety & PK/PD 2
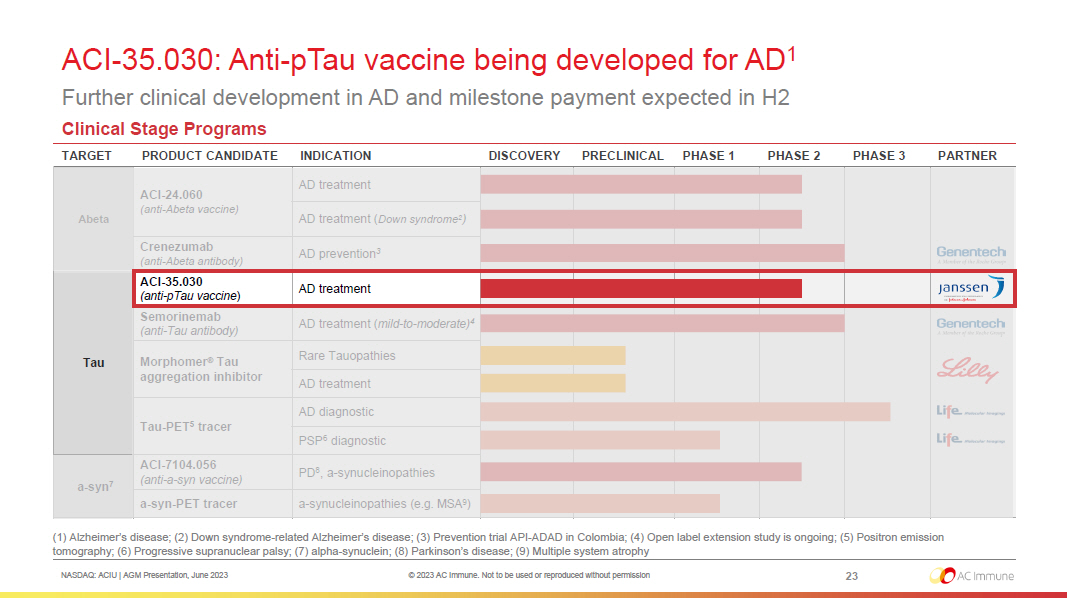
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission ACI - 35.030: Anti - pTau vaccine being developed for AD 1 Further clinical development in AD and milestone payment expected in H2 23 (1) Alzheimer’s disease; (2) Down syndrome - related Alzheimer’s disease ; (3) Prevention trial API - ADAD in Colombia; (4) Open label extension study is ongoing ; (5) Positron emission tomography; (6) Progressive supranuclear palsy ; (7) alpha - synuclein; (8) Parkinson’s disease; (9) Multiple system atrophy Clinical Stage Programs TARGET PRODUCT CANDIDATE INDICATION DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PARTNER Abeta ACI - 24.060 (anti - Abeta vaccine) AD treatment AD treatment ( Down syndrome 2 ) Crenezumab (anti - Abeta antibody) AD prevention 3 Tau ACI - 35.030 (anti - pTau vaccine ) AD treatment Semorinemab (anti - Tau antibody) AD treatment ( mild - to - moderate) 4 Morphomer ® Tau aggregation inhibitor Rare Tauopathies AD treatment Tau - PET 5 tracer AD diagnostic PSP 6 diagnostic a - syn 7 ACI - 7104.056 (anti - a - syn vaccine) PD 8 , a - synucleinopathies a - syn - PET tracer a - synucleinopathies (e.g. MSA 9 )
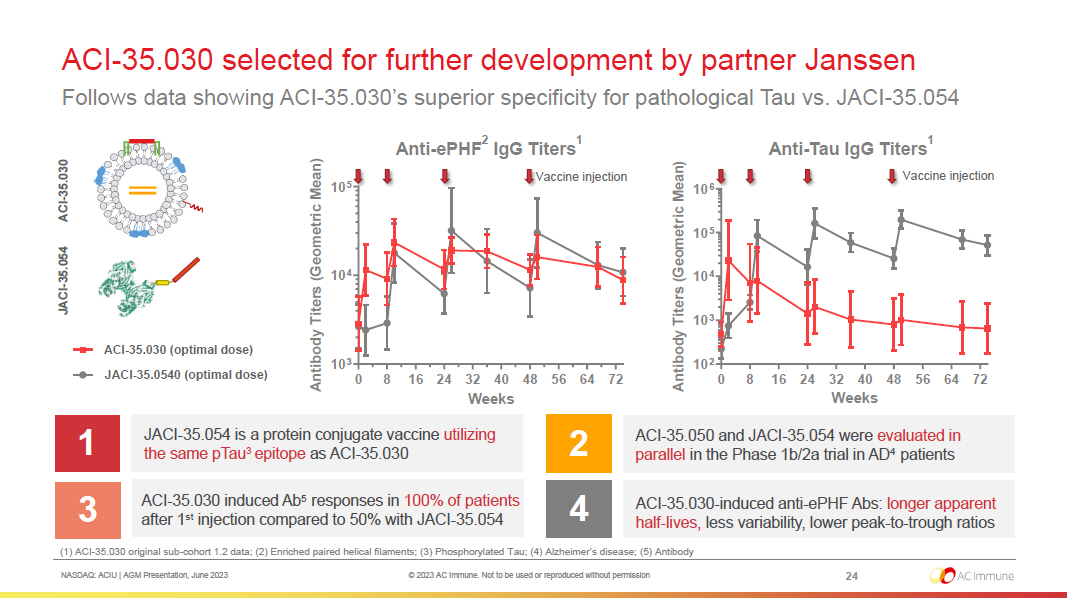
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission 0 8 16 24 32 40 48 56 64 72 10 3 10 4 10 5 Weeks A n t i b o d y T i t e r s ( G e o m e t r i c M e a n ) low dose JACI-35.054 mid dose ACI-35.030 0 8 16 24 32 40 48 56 64 72 10 2 10 3 10 4 10 5 10 6 Weeks A n t i b o d y T i t e r s ( G e o m e t r i c M e a n ) low dose JACI-35.054 mid dose ACI-35.030 ACI - 35.030 selected for further development by partner Janssen Follows data showing ACI - 35.030’s superior specificity for pathological Tau vs. JACI - 35.054 1 JACI - 35.054 is a protein conjugate vaccine utilizing the same pTau 3 epitope as ACI - 35.030 3 ACI - 35.030 induced Ab 5 responses in 100% of patients after 1 st injection compared to 50% with JACI - 35.054 2 4 Anti - ePHF 2 IgG Titers 1 ACI - 35.030 - induced anti - ePHF Abs: longer apparent half - lives, less variability, lower peak - to - trough ratios ACI - 35.050 and JACI - 35.054 were evaluated in parallel in the Phase 1b/2a trial in AD 4 patients ACI - 35.030 JACI - 35.054 (1) ACI - 35.030 original sub - cohort 1.2 data; (2) Enriched paired helical filaments; (3) Phosphorylated Tau; (4) Alzheimer’s disease; (5) Antibody Anti - Tau IgG Titers 1 24 Vaccine injection Vaccine injection ACI - 35.030 (optimal dose) JACI - 35.0540 (optimal dose)
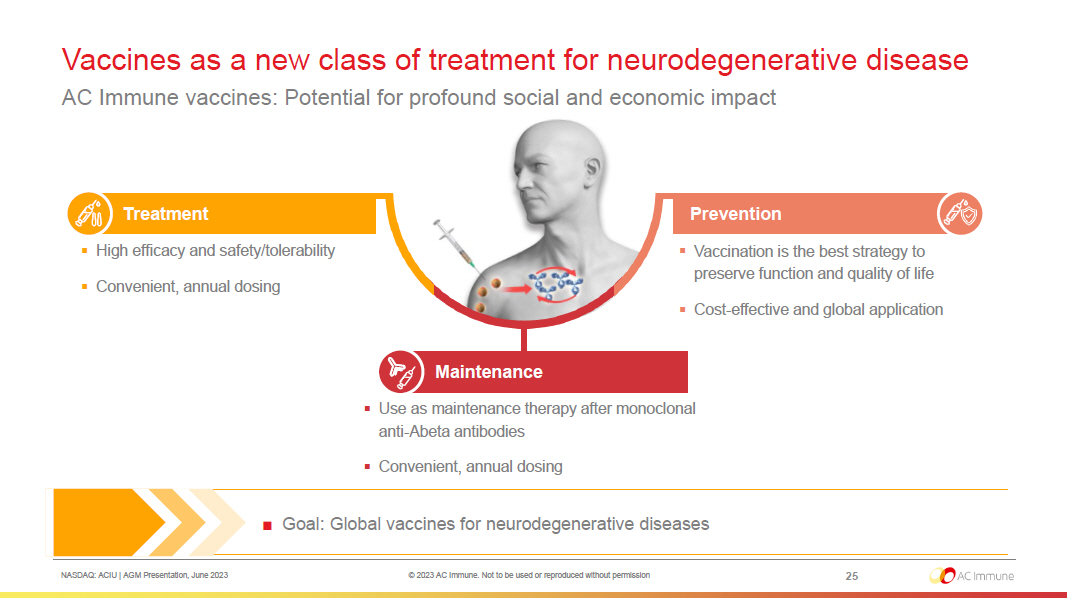
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission 25 Vaccines as a new class of treatment for neurodegenerative disease AC Immune vaccines: Potential for profound social and economic impact ■ Goal: Global vaccines for neurodegenerative diseases Treatment Maintenance Prevention ▪ Use as maintenance therapy after monoclonal anti - Abeta antibodies ▪ Convenient, annual dosing ▪ Vaccination is the best strategy to preserve function and quality of life ▪ Cost - effective and global application ▪ High efficacy and safety/tolerability ▪ Convenient, annual dosing

4. Achievements in 2022 and key milestones for 2023
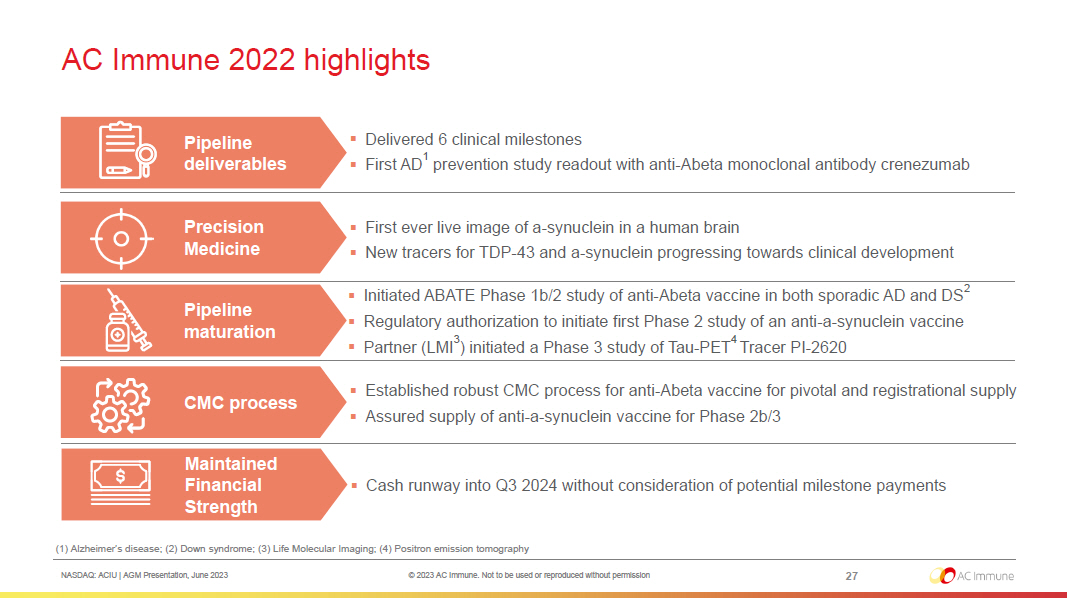
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission AC Immune 2022 highlights 27 Precision Medicine ▪ First ever live image of a - synuclein in a human brain ▪ New tracers for TDP - 43 and a - synuclein progressing towards clinical development Pipeline maturation ▪ Initiated ABATE Phase 1b/2 study of anti - Abeta vaccine in both sporadic AD and DS 2 ▪ Regulatory authorization to initiate first Phase 2 study of an anti - a - synuclein vaccine ▪ Partner (LMI 3 ) initiated a Phase 3 study of Tau - PET 4 Tracer PI - 2620 CMC process ▪ Established robust CMC process for anti - Abeta vaccine for pivotal and registrational supply ▪ Assured supply of anti - a - synuclein vaccine for Phase 2b/3 Maintained Financial Strength ▪ Cash runway into Q3 2024 without consideration of potential milestone payments (1) Alzheimer’s disease; (2) Down syndrome; (3) Life Molecular Imaging; (4) Positron emission tomography Pipeline deliverables ▪ Delivered 6 clinical milestones ▪ First AD 1 prevention study readout with anti - Abeta monoclonal antibody crenezumab

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Vaccines H1 H2 ACI - 24.060 Abeta Initiation of Down syndrome cohort of Phase 1b/2 ABATE study IND submission to enable expansion of ABATE study to U.S. Two interim analyses in AD 1 – safety, immunogenicity Interim analysis in Down syndrome – safety, immunogenicity ACI - 35.030 Tau Further development with initiation of next trial in AD followed by milestone payment ACI - 7104.056 a - syn 2 Phase 2 VACSYN study in PD update Monoclonal antibodies Semorinemab Tau Phase 2 Lauriet Trial Open Label Extension results Monoclonal antibody TDP - 43 3 Candidate into preclinical development (tox) Diagnostics a - syn - PET 4 tracer a - syn Next clinical candidate declaration for PD 5 TDP - 43 - PET tracer TDP - 43 Clinical candidate declaration Key milestones for value creation in 2023 Multiple clinical readouts for wholly - owned vaccines (1) Alzheimer’s disease; ( 2) Alpha - synuclein; (3) TAR DNA - binding protein 43 ; (4) Positron emission tomography; (5) Parkinson’s disease 28 Clinical readouts Other development events Achieved

5. Financial figures
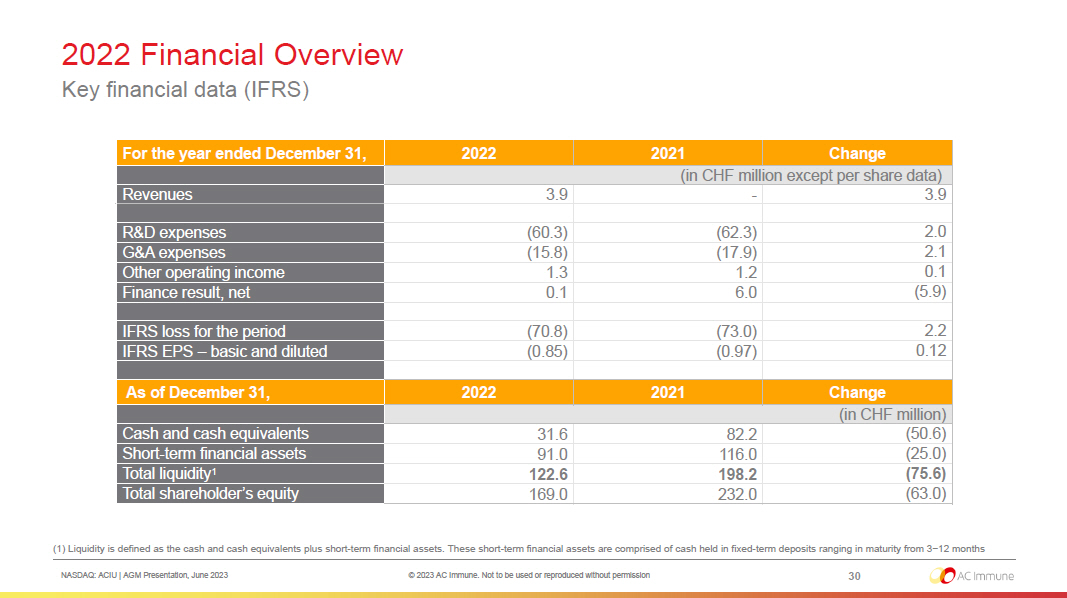
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission 2022 Financial Overview 30 Key financial data (IFRS) For the year ended December 31, 2022 2021 Change (in CHF million except per share data) Revenues 3.9 - 3.9 R&D expenses (60.3) (62.3) 2.0 G&A expenses (15.8) (17.9) 2.1 Other operating income 1.3 1.2 0.1 Finance result, net 0.1 6.0 (5.9) IFRS loss for the period (70.8) (73.0) 2.2 IFRS EPS – basic and diluted (0.85) (0.97) 0.12 As of December 31, 2022 2021 Change (in CHF million) Cash and cash equivalents 31.6 82.2 (50.6) Short - term financial assets 91.0 116.0 (25.0) Total liquidity 1 122.6 198.2 (75.6) Total shareholder’s equity 169.0 232.0 (63.0) (1) Liquidity is defined as the cash and cash equivalents plus short - term financial assets. These short - term financial assets are comprised of ca sh held in fixed - term deposits ranging in maturity from 3−12 months

6. Summary and Strategic Outlook
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Today’s strengths predict future success Precision Medicine for mono - and combination therapy 32 (1) Neurodegenerative diseases Treating the right proteinopathy - in the right patient - at the right time Unique Precision Medicine approach in NDD 1 Broad and diverse product pipeline 2 clinically validated platforms
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission 33 Shifting the treatment paradigm for neurodegenerative disease towards precision medicine and disease prevention AC Immune: Pioneering science and precision medicine

Agenda items and proposals of the Board of Directors

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 1 2022 IFRS Consolidated Financial Statements, 2022 Statutory Financial Statements and 2022 Compensation Report 35 1.1 Approval of 2022 IFRS Consolidated Financial Statements and 2022 Statutory Financial Statements ■ The Board of Directors proposes to approve the 2022 IFRS Consolidated Financial Statements and the 2022 Statutory Financial Statements and to take note of the Reports of the Auditors.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 1 2022 IFRS Consolidated Financial Statements, 2022 Statutory Financial Statements and 2022 Compensation Report 36 1.2 Advisory vote on the 2022 Compensation Report ■ The Board of Directors proposes that the 2022 Compensation Report be endorsed (non - binding advisory vote).
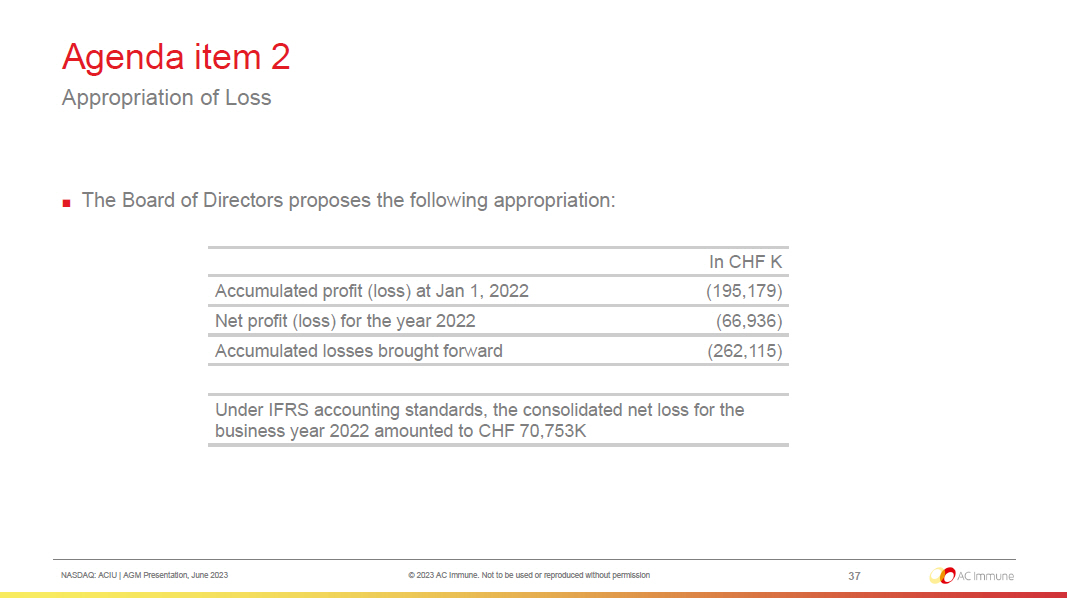
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 2 Appropriation of Loss 37 ■ The Board of Directors proposes the following appropriation: In CHF K Accumulated profit (loss) at Jan 1, 2022 (195,179) Net profit (loss) for the year 2022 (66,936) Accumulated losses brought forward (262,115) Under IFRS accounting standards, the consolidated net loss for the business year 2022 amounted to CHF 70,753K

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 3 Discharge of the Board of Directors and of the Executive Committee 38 ■ The Board of Directors proposes that all members of the Board of Directors and of the Executive Committee be discharged from their liabilities for their activities in the financial year 2022.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 4 Compensation for the Members of the Board of Directors and the Executive Committee 39 4.1 Binding vote on Maximum Aggregate Compensation for Members of the Board of Directors from 1 July 2023 to 30 June 2024 ■ The Board of Directors proposes the approval of the total maximum amount of compensation for the Board of Directors of CHF 862K (cash - based and equity or equity linked instruments at grant value, excluding employer social security contributions) covering the period from 1 July 2023 to 30 June 2024.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 4 Compensation for the Members of the Board of Directors and the Executive Committee 40 4.2 Binding vote on Maximum Aggregate Compensation for Members of the Executive Committee for the calendar year 2024 ■ The Board of Directors proposes the approval of the total maximum compensation for the Executive Committee with maximum value of CHF 7,581K (cash - based compensation, variable compensation including equity and equity linked instruments at grant value, excluding employer social security and pension contributions) from 1 January 2024 to 31 December 2024.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 5 Election and re - elections 41 5.1 Re - election of Members of the Board of Directors ■ The Board of Directors proposes that each of the following persons be re - elected as directors for a term of office until the end of the Annual General Meeting 2024: ■ Douglas Williams as Member of the Board of Directors and Chair And as Members of the Board of Directors: ■ Monika Bütler ■ Carl June ■ Werner Lanthaler ■ Andrea Pfeifer ■ Monica Shaw ■ Roy Twyman

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 5 Election and re - elections 42 5.2 Election and re - elections of Members of the Compensation, Nomination and Corporate Governance Committee ■ The Board of Directors proposes that: ■ Monika Bütler (election) ■ Roy Twyman (re - election) ■ Douglas Williams (re - election) be elected or re - elected as Members of the Compensation, Nomination and Corporate Governance Committee for a term of office until the end of the Annual General Meeting 2024.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 5 Election and re - elections 43 5.3 Re - election of the Statutory Auditors ■ The Board of Directors proposes that PricewaterhouseCoopers SA, in Pully, Switzerland, be re - elected as Statutory Auditors for a term of office of one year.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 5 Election and re - elections 44 5.4 Re - election of the Independent Proxy ■ The Board of Directors proposes that Reymond & Associés, Lausanne, which will be represented by any of their attorneys for this purpose, be re - elected as Independent Proxy for a term of office until the end of the Annual General Meeting 2024.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 6 Changes in the Articles of Association 45 ■ The Board of Directors is proposing to the Shareholders to accept certain changes to current Article 3b of the Company’s Articles of Association on the Conditional Capital for bonds and similar debt instruments. This would enable the use of this Conditional Capital for the issuance of standalone warrants and similar instruments. Accordingly, the Board of Directors proposes to repeal Article 3b (Conditional Share Capital Increase for Bonds and Similar Debt Instruments) of the Company's Articles of Association, and to adopt a new Article 3b as follows: Article 3b: Conditional capital for financing and other purposes Bonds and Similar Debt Instruments Proposed revisions are highlighted, additions in red text and deletions in barred text.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 6 (continued) Changes in the Articles of Association 46 1 st Paragraph: “The share capital of the Company shall be increased by a maximum amount of CHF 100 , 000 through the issue of a maximum of 5 , 000 , 000 registered shares, payable in full, each with a nominal value of CHF 0 . 02 through the optional exercise or mandatory exercise of conversion, exchange, and or option, or warrant or similar rights or obligations for the subscription of shares granted to shareholders or third parties on a standalone basis or in connection with bonds , notes, options, warrants or other securities or contractual obligations of similar instruments issued or to be issued by the Company or by any subsidiaries of the Company, including convertible debt instruments , as may be amended or novated from time to time . ” Proposed revisions are highlighted, additions in red text and deletions in barred text.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 6 (continued) Changes in the Articles of Association 47 2 nd Paragraph: “Shareholders' subscription rights are excluded . Shareholders' advance subscription rights with regard to the new bonds , warrants or similar instruments may be restricted or excluded by decision of the Board of Directors in order to finance or re - finance the acquisition of companies, parts of companies or holdings, or new investments planned by the Company, or in order to issue convertible bonds and warrants on the international capital markets or through private placement . If advance subscription rights are excluded, then ( 1 ) the instruments are to be placed at market conditions, ( 2 ) the exercise period is not to exceed ten years from the date of issue for warrants and twenty years for conversion rights and ( 3 ) the conversion or exercise price for the new shares is to be set at least in line with the market conditions prevailing at the date on which the instruments are issued . The respective holders of conversion and/or option or warrant rights are entitled to subscribe the new shares . ” Proposed revisions are highlighted, additions in red text and deletions in barred text.

NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission Agenda item 6 (continued) Changes in the Articles of Association 48 3 rd Paragraph: “The exercise of conversion or option rights, as well as the waiver of such rights, may be exercised by written declaration or by electronic means . ” 4 th Paragraph : “The acquisition of registered shares through the exercise of conversion rights or warrants and any transfers of registered shares shall be subject to the restrictions specified in Article 4 of the Articles of Association . ” Proposed revisions are highlighted, additions in red text and deletions in barred text.
NASDAQ: ACIU | AGM Presentation, June 2023 © 2023 AC Immune. Not to be used or reproduced without permission 49 We thank y ou for your attendance and your continued support.
















































