
Corporate PRESENTATION q1 2020 Exhibit 99.1

This presentation includes forward-looking statements intended to qualify for the Safe Harbor from liability established by the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “continues,” “could,” “seeks,” “estimates,” “targets,” “guidance,” “expects,” “intends,” “may,” “ongoing,” “plans,” “potential,” “predicts,” “prospects,” “projects,” “should,” “will,” “would,” or similar expressions intended to identify statements about the future and the negatives of those terms, although not all forward-looking statements contain these identifying words. These statements are based on management’s current beliefs and expectations. These statements, including, but not limited to, statements regarding clinical utility tests, regulatory action, third-party payer reimbursement, and demand for our test, are subject to substantial known and unknown risks, certainties, and other factors that could cause actual results to differ materially from those suggested or implied by these forward-looking statements. These factors include, but are not limited to, the following: regulatory action with respect to our Pigmented Lesion Assay and adhesive biopsy (together, the “Test”); the commercial launch and future sales of the Test or any other of our future products or tests; our ability to achieve favorable pricing for the Test; third-party payor reimbursement for the Test; the accuracy of our estimates regarding anticipated capital requirements and our needs for additional financing; market size and market adoption of the Test by dermatologists; the timing, cost and other aspects of the commercial launch of the Test; the timing and cost of clinical utility tests for the Test, including whether such tests will be conducted at all; our ability to develop and commercialize additional tests and products; and our ability to enter into necessary transactions for licensing, acquisitions and strategic operations, as applicable. DermTech may not actually achieve the plans, intentions or expectations disclosed in its forward-looking statements, and you should not place undue reliance on DermTech’s forward-looking statements. The risks and uncertainties that may cause actual results to differ materially from DermTech’s current expectations are more fully described in DermTech’s reports filed with the Securities and Exchange Commission (the “SEC”). You may obtain these reports for free by visiting EDGAR on the SEC website at www.sec.gov. DermTech assumes no obligation to update any forward-looking statements after the date of this presentation or to conform any forward-looking statements to actual results, and has no intention of doing so except to the extent required by applicable law. You should, therefore, not rely on the forward-looking statements in this presentation as representing DermTech’s views as of any date subsequent to the date of this presentation. Disclaimer

Leadership Team John Dobak, MD Chief Executive Officer Todd Wood Chief Commercial Officer Kevin Sun, MS, MBA Chief Financial Officer Zuxu Yao, PhD Chief Scientific Officer Burkhard Jansen, MD Chief Medical Officer Claudia Ibarra Chief Operating Officer Founder & Chairman, 10xBio (aesthetic medicine drugs) Chairman, Pantherics (anti-inflammatory drugs) MD, UCSD, Bachelors, UCLA Allergan, VP US Sales, Dermatology, Ophthalmology, Aesthetics Dexcom, VP Finance, Corporate Controller and Treasury, Interim CFO Biosite, FP&A, SEC Reporting, SOX Compliance MBA, MS, Kelley School of Business at Indiana University Senior roles at Nexogen, Advance, Celula, Nanogen Post-doctoral, UCSD PhD, Memorial University of Newfoundland Dermatologist FDA Consultant Post-doctoral, University of Minnesota; MD, University of Graz Exagen, Sr. VP Lab Operations Genoptix, Director Lab Operations Daniel Visage, MBA Sr. VP, Payer Access Progenity, VP Payer Access OPKO/BRL, VP Managed Markets LabCorp Florida Blue, Kaiser, Carecentrix
DermTech, Inc. (NASDAQ: DMTK) is a leader in precision dermatology enabled by a non-invasive skin genomics platform. DERMTECH Mission: To bring precision to the practice of dermatology through non-invasive genomic assessment of the skin. We address very large market opportunities in skin cancer and skin inflammatory disease We operate a CLIA-certified and CAP-accredited commercial laboratory in San Diego, CA. Our skin cancer product is available in all 50 U.S. states. We are commercial stage and currently offer the Pigmented Lesion Assay (PLA) for early melanoma detection We are commencing scale up around our recent Medicare coverage policy and new CPT Code (0089U) with favorable reimbursement
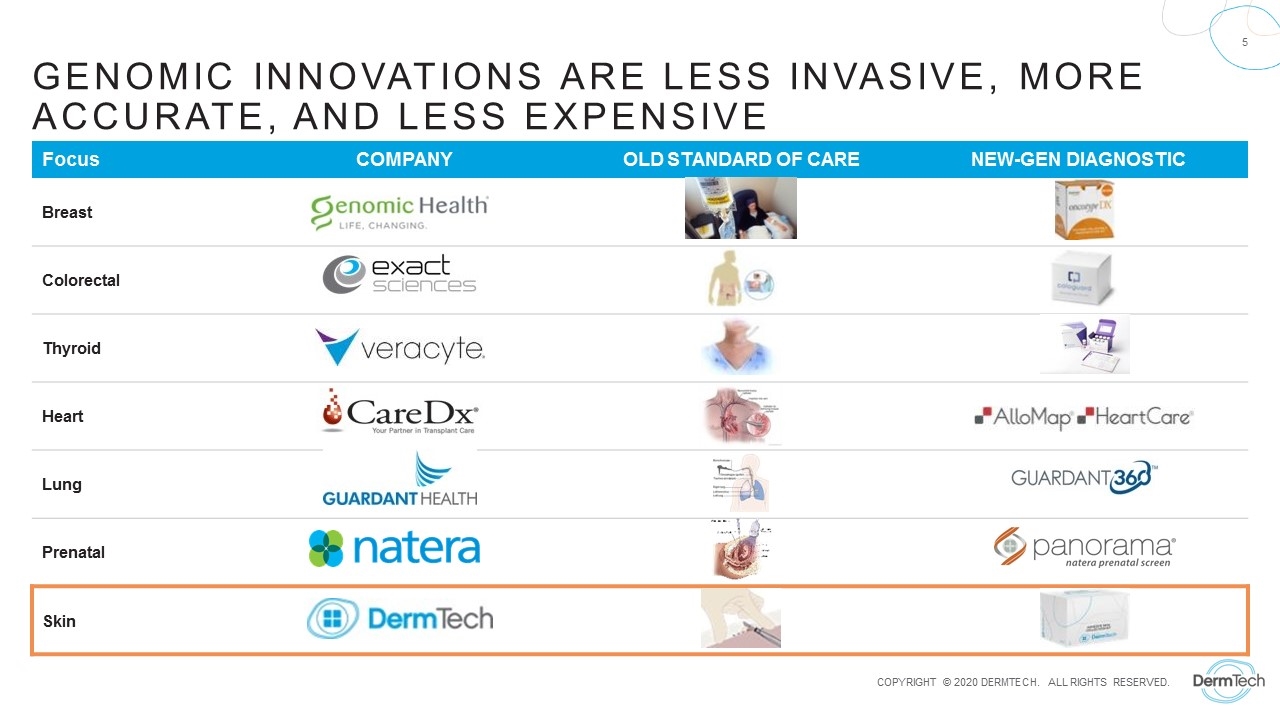
Genomic Innovations Are Less Invasive, More accurate, and less expensive Focus COMPANY OLD STANDARD OF CARE NEW-GEN DIAGNOSTIC Breast Colorectal Thyroid Heart Lung Prenatal Skin
Skin Cancer Melanoma, Basal Cell, and Squamous Cell Carcinoma More people are diagnosed with skin cancer than all other cancers combined and 1 in 5 Americans will develop skin cancer by the age of 70 A Snapshot of the U.S. Skin Cancer Market Annual cost of treatment is estimated to be $8.1 billion, $4.8 billion for non-melanoma and $3.3 billion for melanoma (2018) ~4.5 million cases of basal cell and squamous cell diagnosed per year in the US, ~11 MM diagnostic biopsies, with ~20,000 deaths ~180,000 new cases of melanoma were reported in 2018, ~4.0 MM diagnostic biopsies, with ~10,000 deaths Source: Cancer Facts & Figures 2018. American Cancer Society.
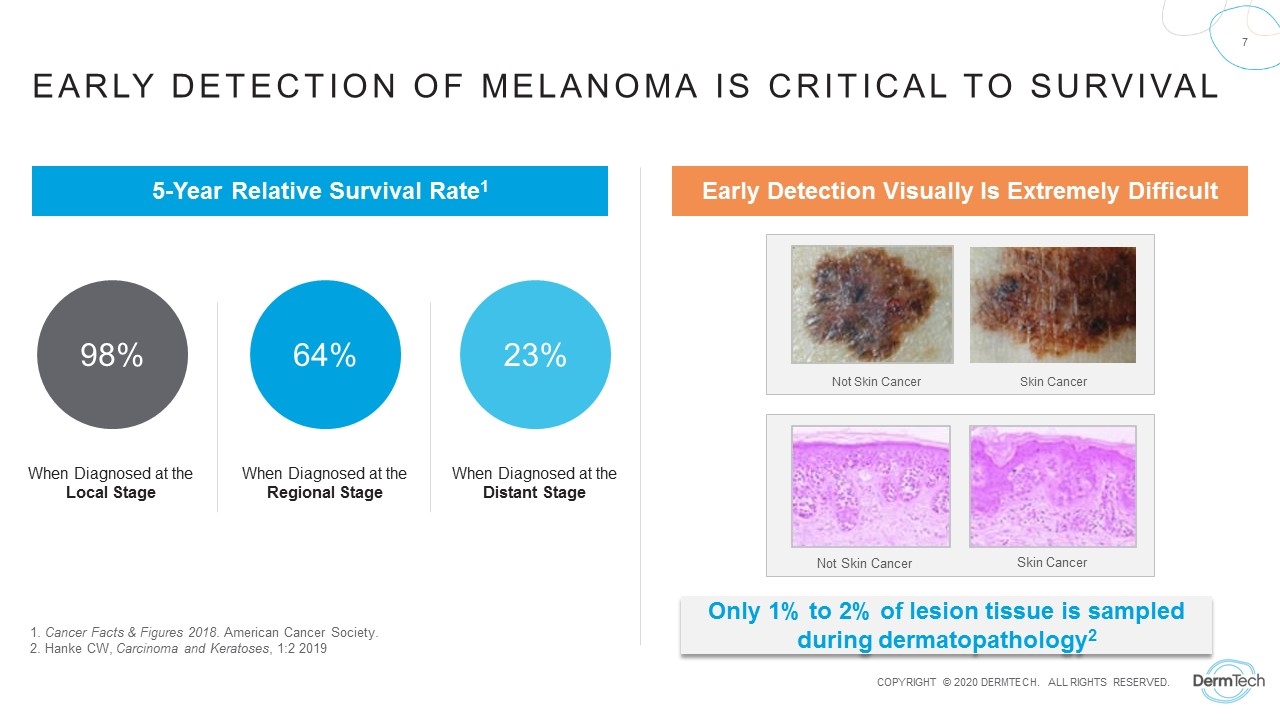
When Diagnosed at the Local Stage 98% When Diagnosed at the Regional Stage 64% When Diagnosed at the Distant Stage 23% 1. Cancer Facts & Figures 2018. American Cancer Society. 2. Hanke CW, Carcinoma and Keratoses, 1:2 2019 Not Skin Cancer Skin Cancer Not Skin Cancer Skin Cancer 5-Year Relative Survival Rate1 Early Detection Visually Is Extremely Difficult Early Detection of Melanoma Is Critical to Survival Only 1% to 2% of lesion tissue is sampled during dermatopathology2
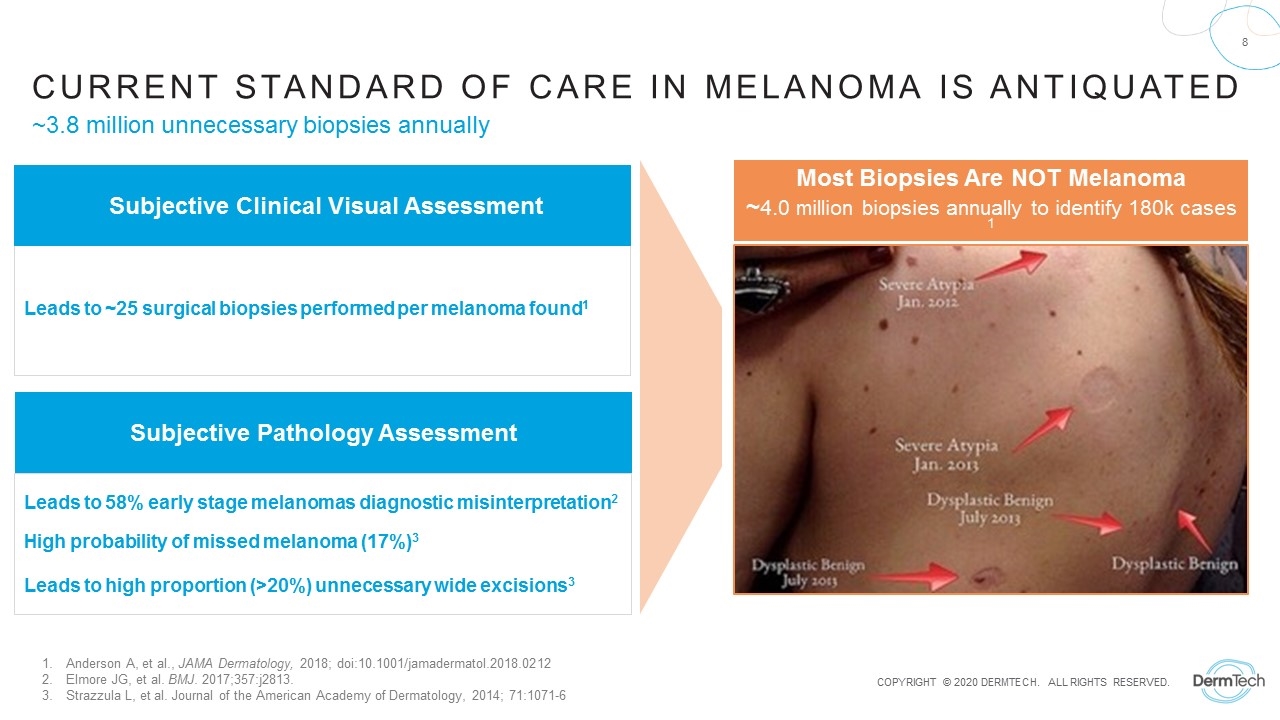
Subjective Pathology Assessment Current Standard of Care in melanoma is Antiquated Anderson A, et al., JAMA Dermatology, 2018; doi:10.1001/jamadermatol.2018.0212 Elmore JG, et al. BMJ. 2017;357:j2813. Strazzula L, et al. Journal of the American Academy of Dermatology, 2014; 71:1071-6 Most Biopsies Are NOT Melanoma ~4.0 million biopsies annually to identify 180k cases 1 Leads to ~25 surgical biopsies performed per melanoma found1 Leads to 58% early stage melanomas diagnostic misinterpretation2 High probability of missed melanoma (17%)3 Leads to high proportion (>20%) unnecessary wide excisions3 Subjective Clinical Visual Assessment ~3.8 million unnecessary biopsies annually
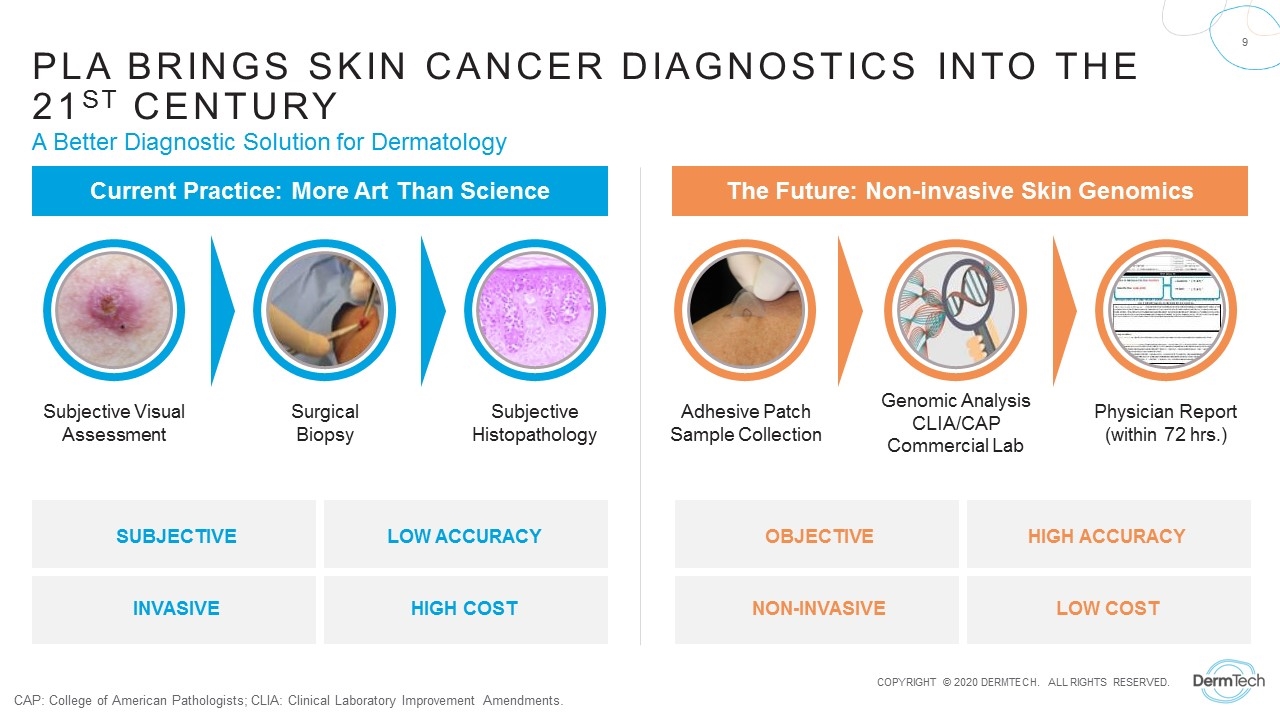
PLA Brings Skin cancer diagnostics into the 21st century A Better Diagnostic Solution for Dermatology Subjective Visual Assessment Surgical Biopsy Subjective Histopathology Adhesive Patch Sample Collection Genomic Analysis CLIA/CAP Commercial Lab Physician Report (within 72 hrs.) SUBJECTIVE LOW ACCURACY INVASIVE HIGH COST OBJECTIVE HIGH ACCURACY NON-INVASIVE LOW COST Current Practice: More Art Than Science The Future: Non-invasive Skin Genomics CAP: College of American Pathologists; CLIA: Clinical Laboratory Improvement Amendments.
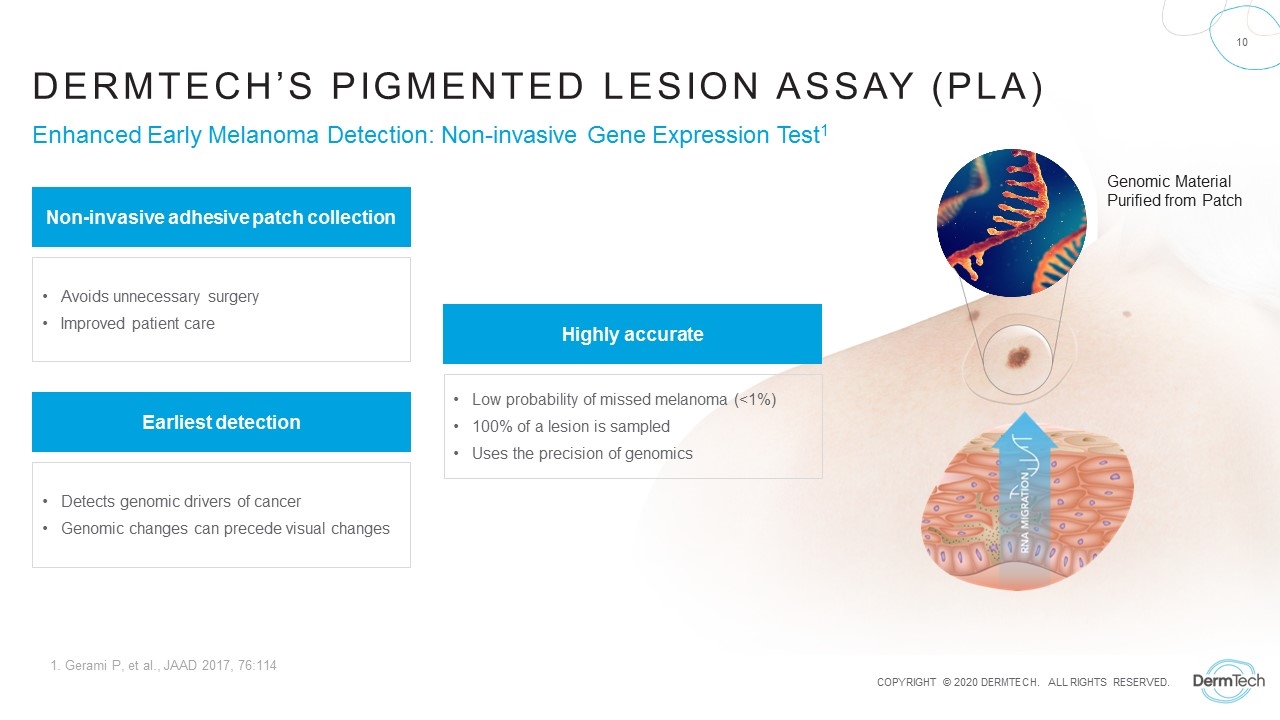
Genomic Material Purified from Patch Enhanced Early Melanoma Detection: Non-invasive Gene Expression Test1 Dermtech’s pigmented lesion assay (pla) Non-invasive adhesive patch collection Avoids unnecessary surgery Improved patient care Highly accurate Low probability of missed melanoma (<1%) 100% of a lesion is sampled Uses the precision of genomics Earliest detection Detects genomic drivers of cancer Genomic changes can precede visual changes 1. Gerami P, et al., JAAD 2017, 76:114
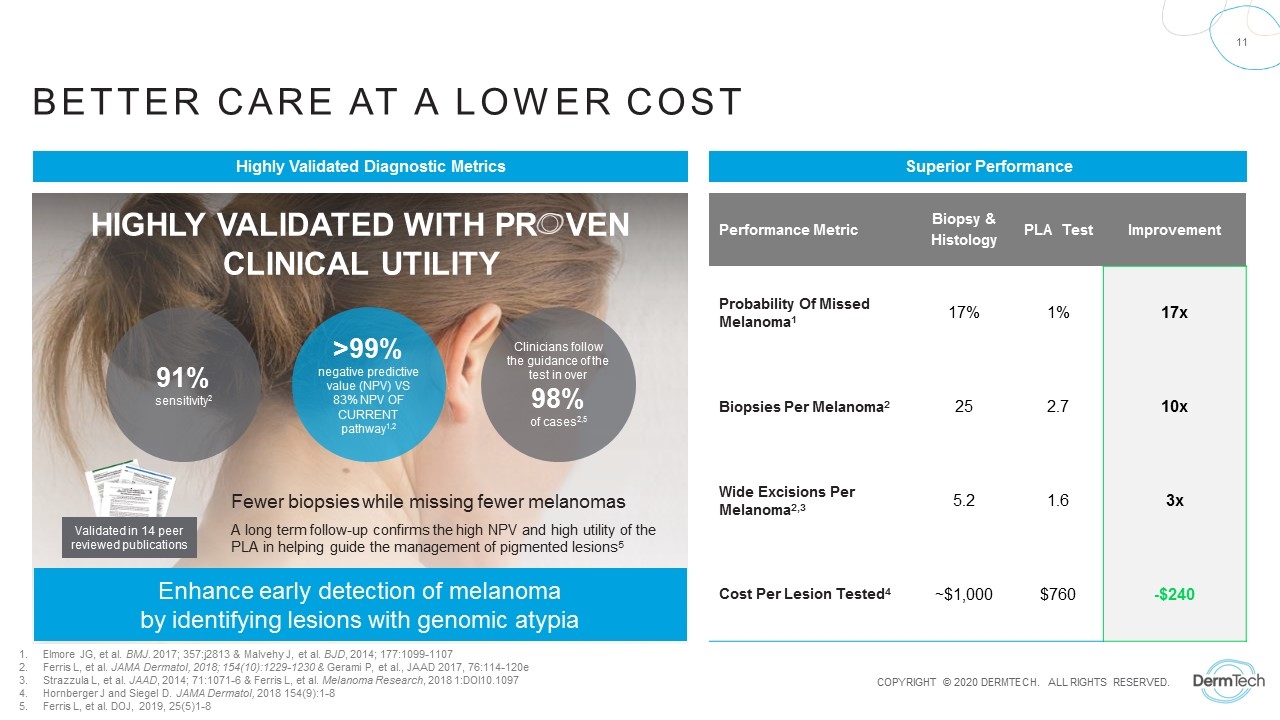
Better care at a lower cost Performance Metric Biopsy & Histology PLA Test Improvement Probability Of Missed Melanoma1 17% 1% 17x Biopsies Per Melanoma2 25 2.7 10x Wide Excisions Per Melanoma2,3 5.2 1.6 3x Cost Per Lesion Tested4 ~$1,000 $760 -$240 Fewer biopsies while missing fewer melanomas A long term follow-up confirms the high NPV and high utility of the PLA in helping guide the management of pigmented lesions5 91% sensitivity2 >99% negative predictive value (NPV) VS 83% NPV OF CURRENT pathway1,2 Highly validated with Pr ven Clinical Utility Clinicians follow the guidance of the test in over 98% of cases2,5 Enhance early detection of melanoma by identifying lesions with genomic atypia Validated in 14 peer reviewed publications Elmore JG, et al. BMJ. 2017; 357:j2813 & Malvehy J, et al. BJD, 2014; 177:1099-1107 Ferris L, et al. JAMA Dermatol, 2018; 154(10):1229-1230 & Gerami P, et al., JAAD 2017, 76:114-120e Strazzula L, et al. JAAD, 2014; 71:1071-6 & Ferris L, et al. Melanoma Research, 2018 1:DOI10.1097 Hornberger J and Siegel D. JAMA Dermatol, 2018 154(9):1-8 Ferris L, et al. DOJ, 2019, 25(5)1-8 Superior Performance Highly Validated Diagnostic Metrics
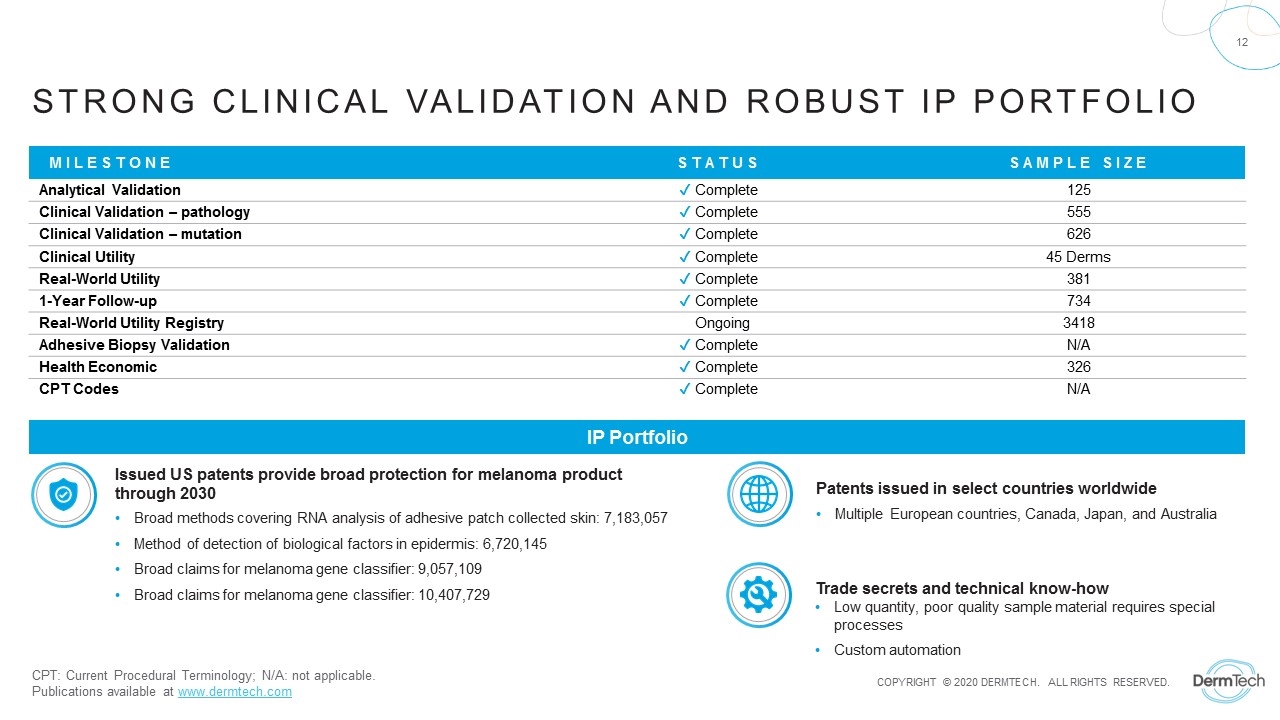
Strong clinical Validation and Robust IP Portfolio MILESTONE STATUS SAMPLE SIZE Analytical Validation ✔ Complete 125 Clinical Validation – pathology ✔ Complete 555 Clinical Validation – mutation ✔ Complete 626 Clinical Utility ✔ Complete 45 Derms Real-World Utility ✔ Complete 381 1-Year Follow-up ✔ Complete 734 Real-World Utility Registry Ongoing 3418 Adhesive Biopsy Validation ✔ Complete N/A Health Economic ✔ Complete 326 CPT Codes ✔ Complete N/A CPT: Current Procedural Terminology; N/A: not applicable. Publications available at www.dermtech.com Broad methods covering RNA analysis of adhesive patch collected skin: 7,183,057 Method of detection of biological factors in epidermis: 6,720,145 Broad claims for melanoma gene classifier: 9,057,109 Broad claims for melanoma gene classifier: 10,407,729 Issued US patents provide broad protection for melanoma product through 2030 Multiple European countries, Canada, Japan, and Australia Patents issued in select countries worldwide Low quantity, poor quality sample material requires special processes Custom automation Trade secrets and technical know-how IP Portfolio
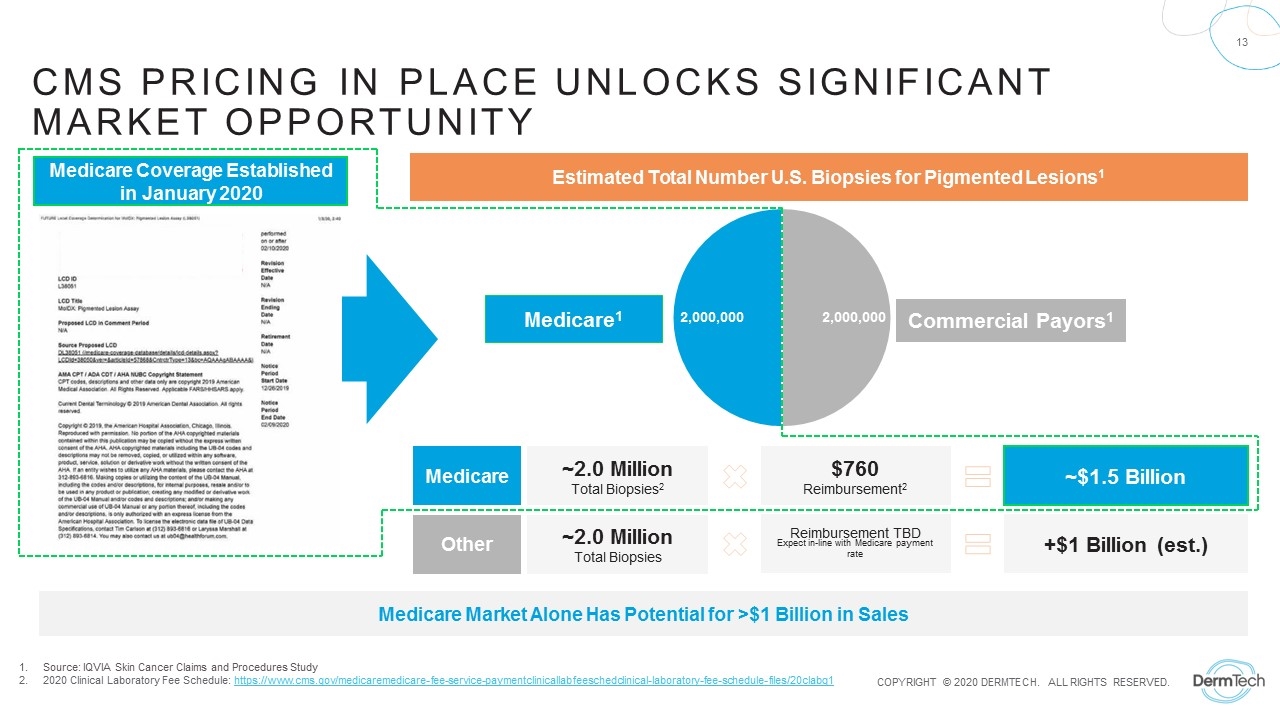
Source: IQVIA Skin Cancer Claims and Procedures Study 2020 Clinical Laboratory Fee Schedule: https://www.cms.gov/medicaremedicare-fee-service-paymentclinicallabfeeschedclinical-laboratory-fee-schedule-files/20clabq1 CMS pricing in place unlocks Significant Market Opportunity ~2.0 Million Total Biopsies2 ~$1.5 Billion $760 Reimbursement2 ~2.0 Million Total Biopsies Medicare Coverage Established in January 2020 Estimated Total Number U.S. Biopsies for Pigmented Lesions1 Reimbursement TBD Expect in-line with Medicare payment rate Medicare1 Commercial Payors1 +$1 Billion (est.) Medicare Other Medicare Market Alone Has Potential for >$1 Billion in Sales
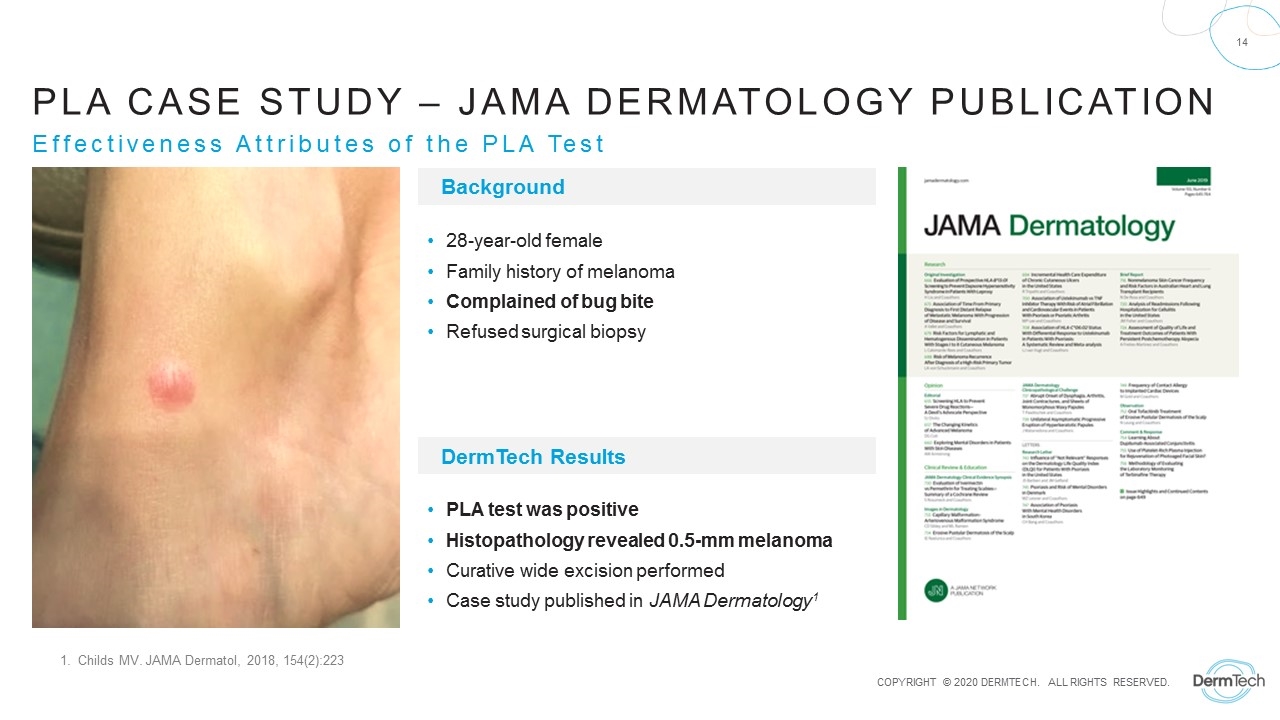
Effectiveness Attributes of the PLA Test Background DermTech Results 28-year-old female Family history of melanoma Complained of bug bite Refused surgical biopsy PLA test was positive Histopathology revealed 0.5-mm melanoma Curative wide excision performed Case study published in JAMA Dermatology1 1. Childs MV. JAMA Dermatol, 2018, 154(2):223 PLA Case Study – JAMA Dermatology Publication

COMMERCIAL ACTIVITIES

Commercial Growth Strategy 1 Establish broader payer coverage Leverage new Medicare coverage to Medicare Advantage plans and commercial payers 2 Drive adoption through optimized clinician messaging Improved and tested professional campaign Actively engage and mobilize the patient Scale our digital patient campaign 4 Aim to expand sales force to up to ~53 reps in 12-24 months Optimize market coverage and call frequency Define PLA product positioning and comparable practice economics 3 Expands testing to ambiguous lesions followed for change and enhances detection by avoiding delays 5
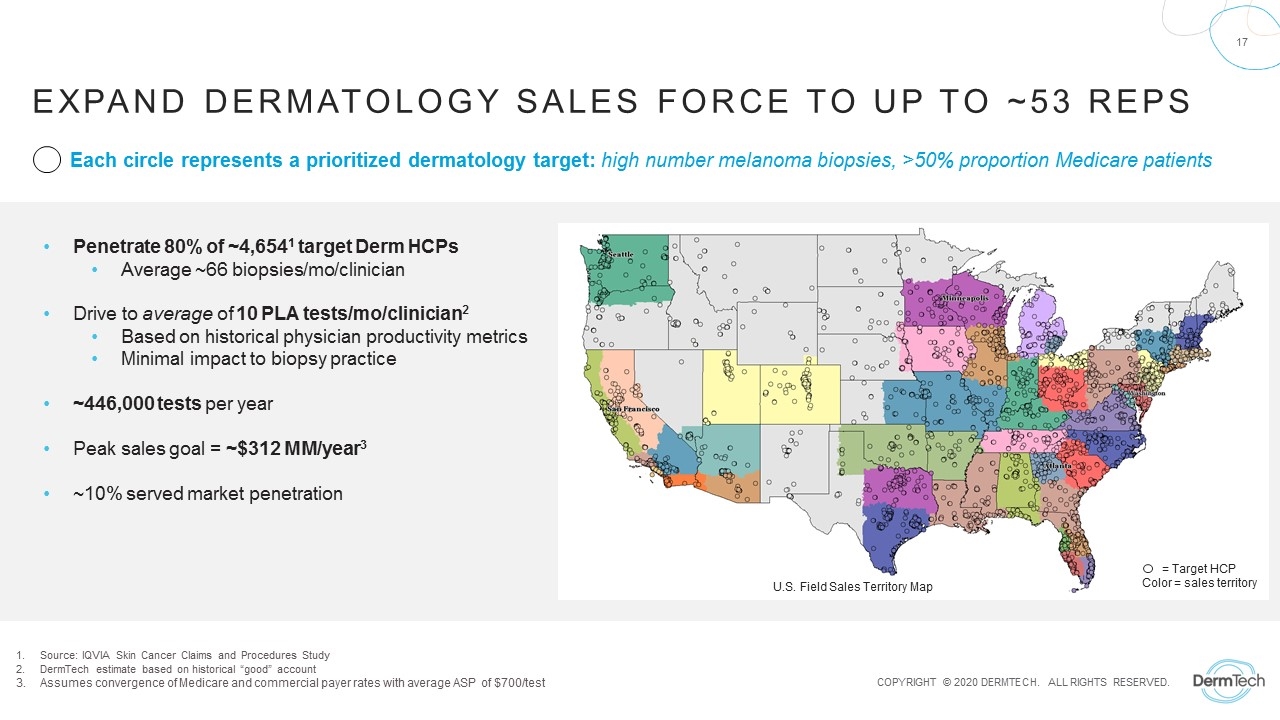
= Target HCP Color = sales territory Each circle represents a prioritized dermatology target: high number melanoma biopsies, >50% proportion Medicare patients Penetrate 80% of ~4,6541 target Derm HCPs Average ~66 biopsies/mo/clinician Drive to average of 10 PLA tests/mo/clinician2 Based on historical physician productivity metrics Minimal impact to biopsy practice ~446,000 tests per year Peak sales goal = ~$312 MM/year3 ~10% served market penetration U.S. Field Sales Territory Map Expand Dermatology sales force to up to ~53 reps Source: IQVIA Skin Cancer Claims and Procedures Study DermTech estimate based on historical “good” account Assumes convergence of Medicare and commercial payer rates with average ASP of $700/test

PLA Test Biopsy VS Samples the entire lesion Provides additional objective genomic information Measures malignant changes that cannot be seen visually Samples less than 1-2% of a potentially partial surgical biopsy Relies on subjective visual criteria Morphologic changes must be present and in the field of observation 01 02 03 Optimized CORE Clinician messaging PLA Enhances the Current Standard of Care
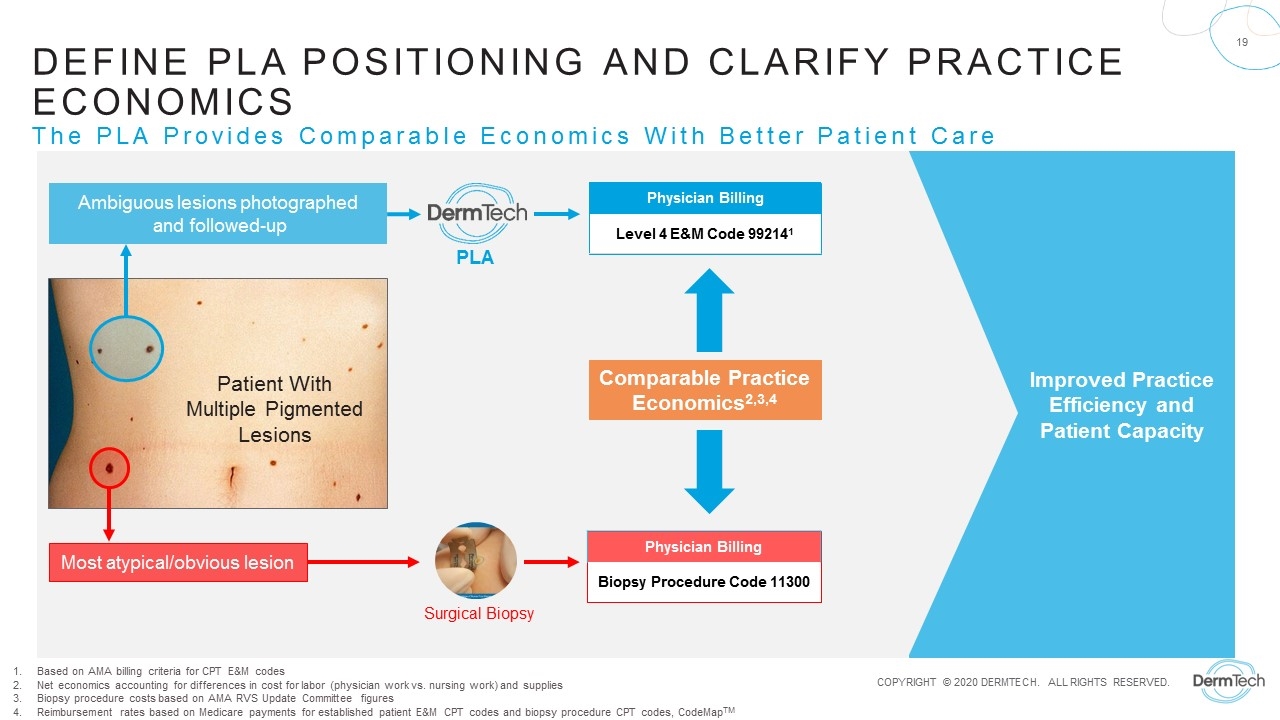
Improved Practice Efficiency and Patient Capacity Ambiguous lesions photographed and followed-up Most atypical/obvious lesion Patient With Multiple Pigmented Lesions Physician Billing Biopsy Procedure Code 11300 Physician Billing Level 4 E&M Code 992141 Surgical Biopsy PLA Define PLA Positioning and Clarify Practice Economics The PLA Provides Comparable Economics With Better Patient Care Based on AMA billing criteria for CPT E&M codes Net economics accounting for differences in cost for labor (physician work vs. nursing work) and supplies Biopsy procedure costs based on AMA RVS Update Committee figures Reimbursement rates based on Medicare payments for established patient E&M CPT codes and biopsy procedure CPT codes, CodeMapTM Comparable Practice Economics2,3,4
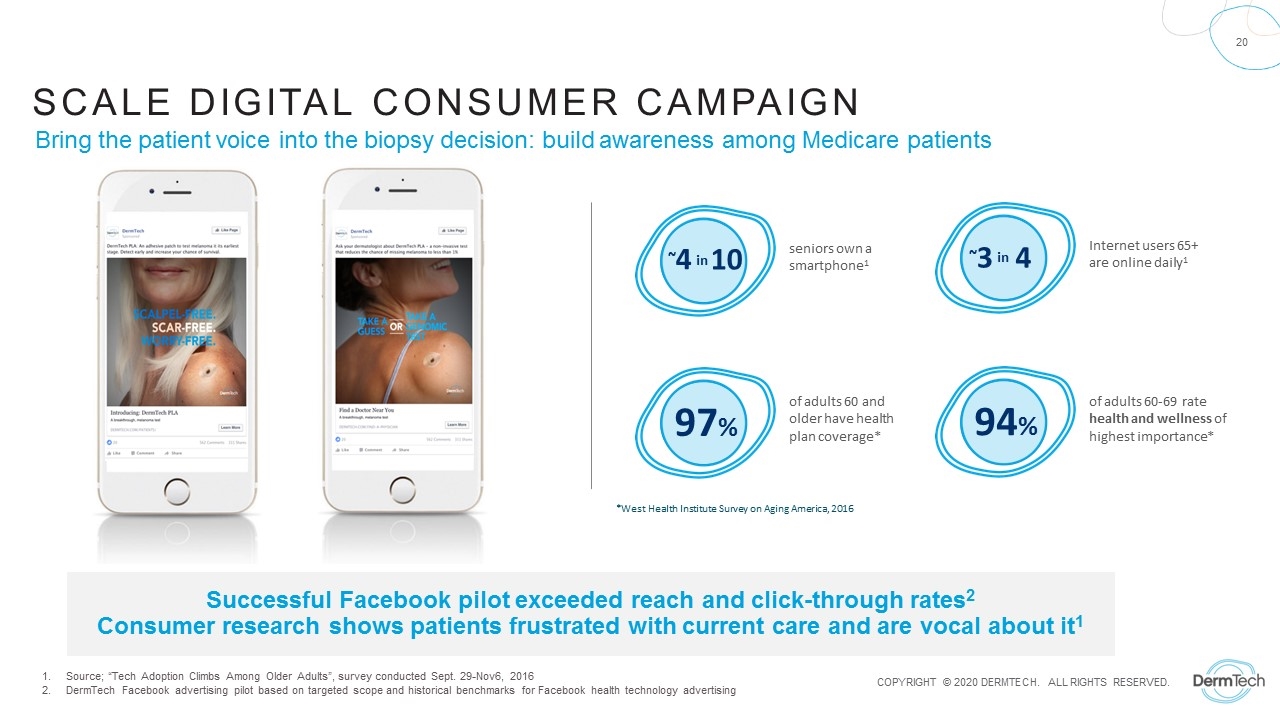
Successful Facebook pilot exceeded reach and click-through rates2 Consumer research shows patients frustrated with current care and are vocal about it1 *West Health Institute Survey on Aging America, 2016 of adults 60 and older have health plan coverage* 97% of adults 60-69 rate health and wellness of highest importance* 94% Bring the patient voice into the biopsy decision: build awareness among Medicare patients seniors own a smartphone1 ~4 in 10 Internet users 65+ are online daily1 ~3 in 4 SCALE DIGITAL CONSUMER Campaign Source; “Tech Adoption Climbs Among Older Adults”, survey conducted Sept. 29-Nov6, 2016 DermTech Facebook advertising pilot based on targeted scope and historical benchmarks for Facebook health technology advertising
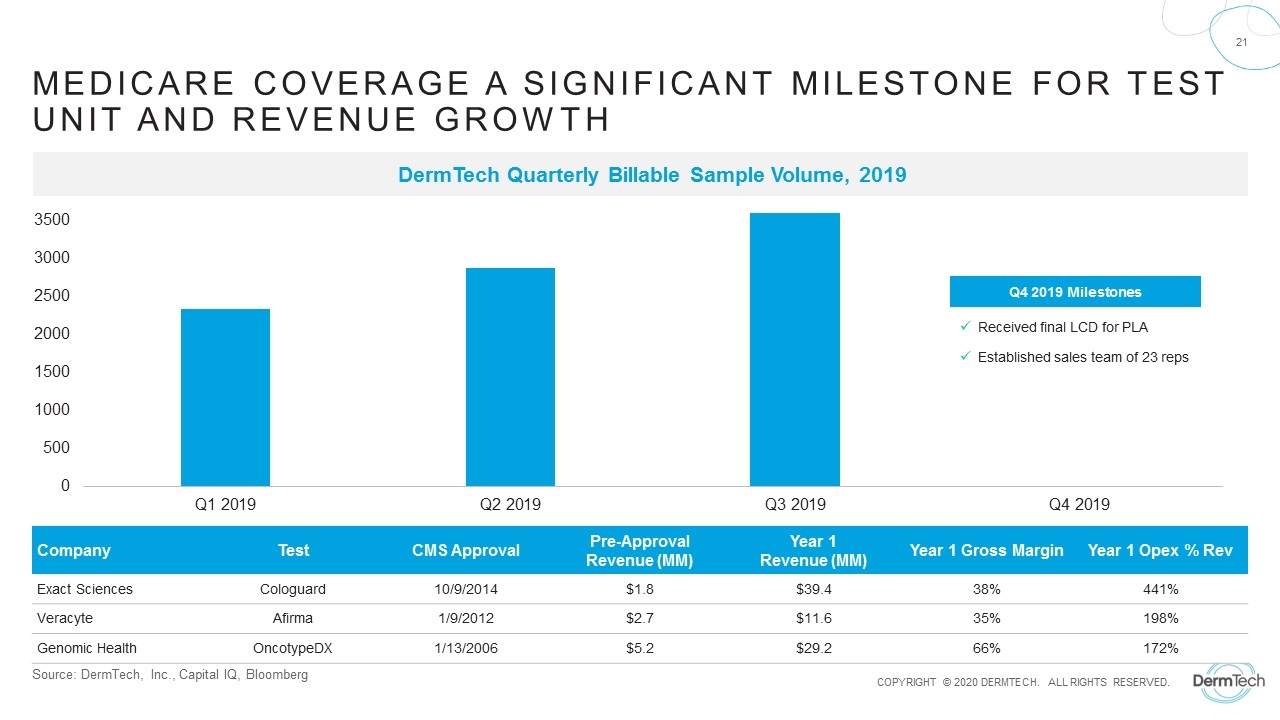
Source: DermTech, Inc., Capital IQ, Bloomberg Medicare Coverage A Significant Milestone for Test Unit and Revenue Growth DermTech Quarterly Billable Sample Volume, 2019 Company Test CMS Approval Pre-Approval Revenue (MM) Year 1 Revenue (MM) Year 1 Gross Margin Year 1 Opex % Rev Exact Sciences Cologuard 10/9/2014 $1.8 $39.4 38% 441% Veracyte Afirma 1/9/2012 $2.7 $11.6 35% 198% Genomic Health OncotypeDX 1/13/2006 $5.2 $29.2 66% 172% Q4 2019 Milestones Received final LCD for PLA Established sales team of 23 reps

LINC: Long Intergenic Non-protein Coding RNA 518; PCR: Polymerase Chain Reaction; Mut: Mutation; PRAME: Preferentially Expressed Antigen in Melanoma; R/O: Rule Out; Tx: Treatment; TERT: Telomerase Reverse Transcriptase Product and research Partnership Pipeline Product Test Purpose Assay Type Gene Targets Stage Pigmented Lesion Assay (PLA) Melanoma R/O PCR LINC, PRAME On Market PLA plus Next Gen Melanoma R/O Mut/PCR LINC, PRAME, TERT Q1 2020 Introduction Luminate Non-melanoma Skin Cancer Risk Mut Not Disclosed Development Carcinome Basal & Squamous Cell R/O PCR Not Disclosed Development ResponseAD Atopic Dermatitis Tx Response PCR Th2 Development Big Pharma Collaborations ~$9.5 million in research programs booked to date Robust pipeline of opportunities and pending contracts Independent performance development Expansion to late-stage trials (phase ll, lll)

Commercial Lab Operations CLIA-licensed laboratory in State of California and all states requiring out-of-state licensure CAP-accredited ~9000-square-foot commercial lab space Current capacity 50,000 tests per year Adding automation to increase to 100k+/year Expansion preparation to 500k/year PLA assay turnaround time is ~72 hours