
Exhibit 99.2 Enabling the development of November 2-3, 2023 • MADRID serum [TTR] as a biomarker for treatment of ATTR amyloidosis Julian D. Gillmore National Amyloidosis Centre, Division of Medicine, University College London on behalf of: 2 3 4 5 5 Jörg Täubel, Ed Gane, Björn Pilebro, Michael L. Maitland, Ricardo Rocha, 5 5 5 5 5 Joy Olbertz, Adia Leung, Derek Smith, Michael D. Pickard, Carri Boiselle, 5 5 6 5 7 Yuanxin Xu, Peijuan Zhu, David Gutstein, Liron Walsh, David Adams 2 Richmond Pharmacology, St. George’s University of London, London, UK 3 New Zealand Clinical Research, Auckland, New Zealand 4 Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden 5 Intellia Therapeutics, Cambridge, MA, USA 6 Regeneron Pharmaceuticals, Tarrytown, NY, USA 7 Department of Neurology, CHU Bicetre, University Paris Saclay, AP-HP, Le Ktremin-Bicetre, France

Disclosures • Adviser for Alnylam, AstraZeneca, Attralus, BridgeBio, Ionis, Pfizer, and Intellia
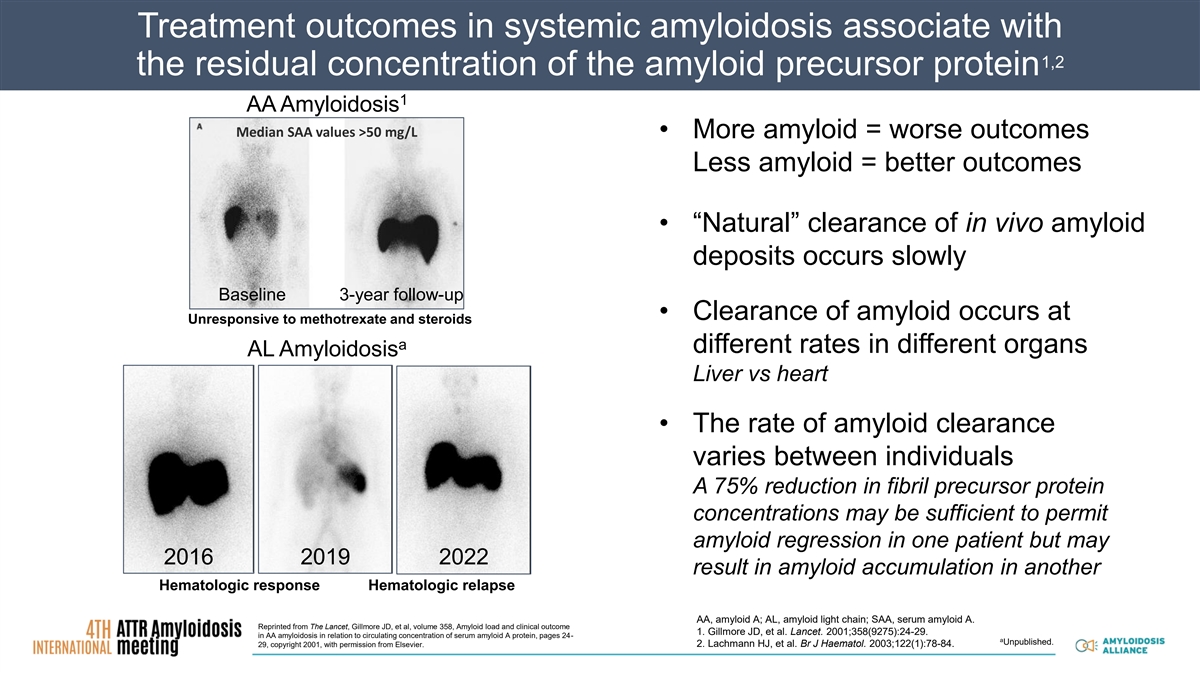
Treatment outcomes in systemic amyloidosis associate with 1,2 the residual concentration of the amyloid precursor protein 1 AA Amyloidosis Median SAA values >50 mg/L • More amyloid = worse outcomes Less amyloid = better outcomes • “Natural” clearance of in vivo amyloid deposits occurs slowly Baseline 3-year follow-up • Clearance of amyloid occurs at Unresponsive to methotrexate and steroids a different rates in different organs AL Amyloidosis Liver vs heart • The rate of amyloid clearance varies between individuals A 75% reduction in fibril precursor protein concentrations may be sufficient to permit amyloid regression in one patient but may 2016 2019 2022 result in amyloid accumulation in another Hematologic response Hematologic relapse AA, amyloid A; AL, amyloid light chain; SAA, serum amyloid A. Reprinted from The Lancet, Gillmore JD, et al, volume 358, Amyloid load and clinical outcome 1. Gillmore JD, et al. Lancet. 2001;358(9275):24-29. in AA amyloidosis in relation to circulating concentration of serum amyloid A protein, pages 24- a Unpublished. 29, copyright 2001, with permission from Elsevier. 2. Lachmann HJ, et al. Br J Haematol. 2003;122(1):78-84.
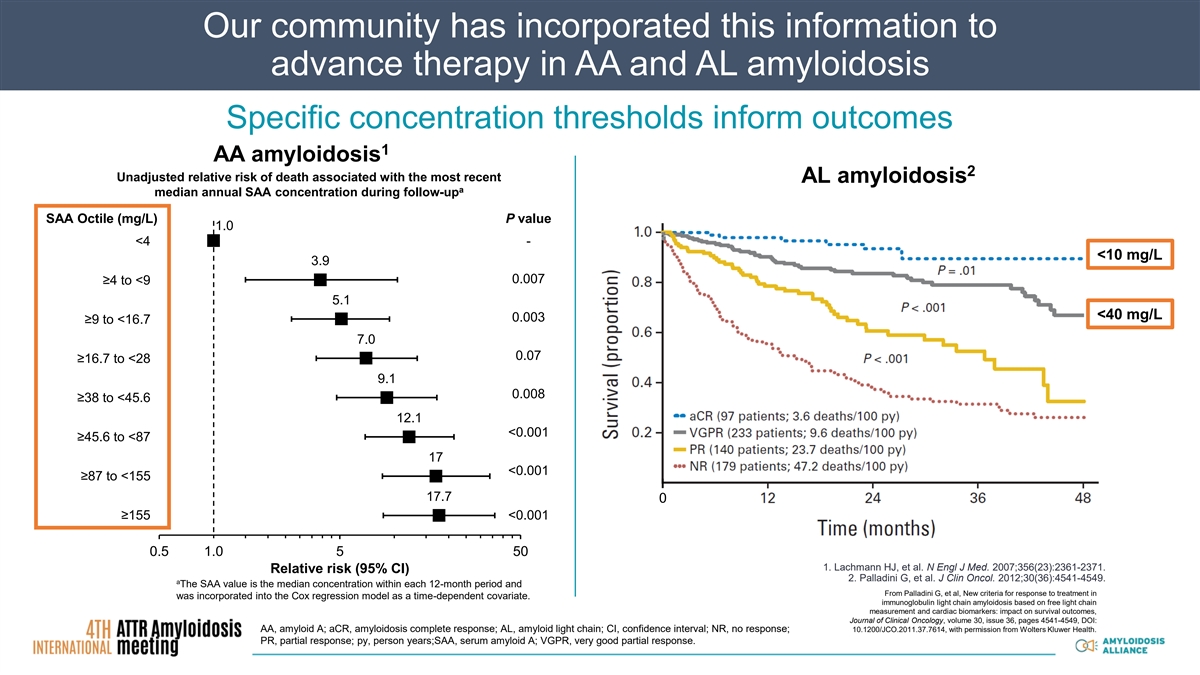
Our community has incorporated this information to advance therapy in AA and AL amyloidosis Specific concentration thresholds inform outcomes 1 AA amyloidosis 2 Unadjusted relative risk of death associated with the most recent AL amyloidosis a median annual SAA concentration during follow-up SAA Octile (mg/L) P value 1.0 <4 - <10 mg/L 3.9 0.007 ≥4 to <9 5.1 <40 mg/L 0.003 ≥9 to <16.7 7.0 0.07 ≥16.7 to <28 9.1 0.008 ≥38 to <45.6 12.1 <0.001 ≥45.6 to <87 17 <0.001 ≥87 to <155 17.7 0 ≥155 <0.001 0.5 1.0 5 50 1. Lachmann HJ, et al. N Engl J Med. 2007;356(23):2361-2371. Relative risk (95% CI) 2. Palladini G, et al. J Clin Oncol. 2012;30(36):4541-4549. a The SAA value is the median concentration within each 12-month period and From Palladini G, et al, New criteria for response to treatment in was incorporated into the Cox regression model as a time-dependent covariate. immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes, Journal of Clinical Oncology, volume 30, issue 36, pages 4541-4549, DOI: AA, amyloid A; aCR, amyloidosis complete response; AL, amyloid light chain; CI, confidence interval; NR, no response; 10.1200/JCO.2011.37.7614, with permission from Wolters Kluwer Health. PR, partial response; py, person years;SAA, serum amyloid A; VGPR, very good partial response.
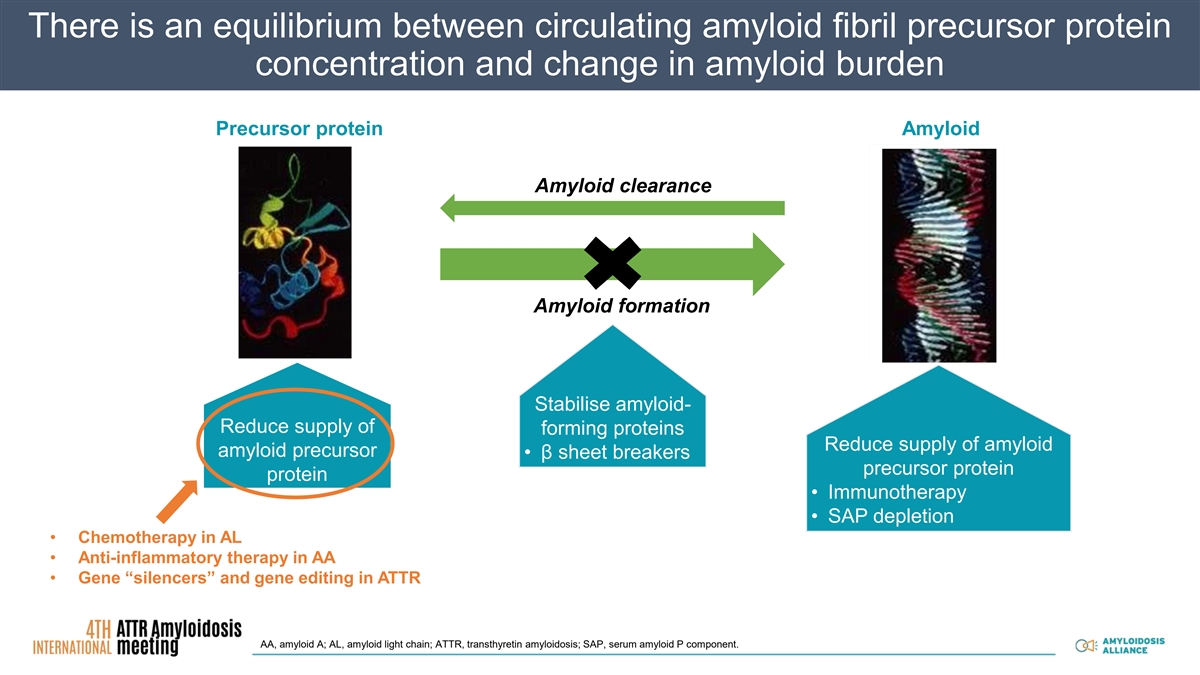
There is an equilibTherape rium bet utic str ween ategies cir in cu am lat yloing idos amyl is oid fibril precursor protein Therapeutic strategies in amyloidosis concentration and change in amyloid burden Pr Precu ecur rso sor r p pr ro ote teiin n A Amyl mylo oiid d Precursor protein Amyloid Precursor protein Amyloid Reversion to normally folded protein Reversion to normally folded protein Precursor protein Amyloid Amyloid clearance Fibril formation Fibril formation F Fiib br riill fo for rma mati tio on n Amyloid formation Stabilise amyloid- Stabilise amyloid- forming proteins forming proteins ▪β sheet breakers ▪β sheet breakers Enhance removal of Stabilise amyloid- Enhance removal of existing amyloid Reduce supply of existing amyloid forming proteins Reduce supply of Reduce supply of Reduce supply of amyloid amyloid precursor • β sheet breakers amyloid ▪ Immunotherapy amyloid ▪ Immunotherapy precursor protein protein precursor ▪ SAP depletion precursor ▪ SAP depletion • Immunotherapy protein protein • SAP depletion • Chemotherapy in AL • Anti-inflammatory therapy in AA • Gene “silencers” and gene editing in ATTR AA, amyloid A; AL, amyloid light chain; ATTR, transthyretin amyloidosis; SAP, serum amyloid P component.
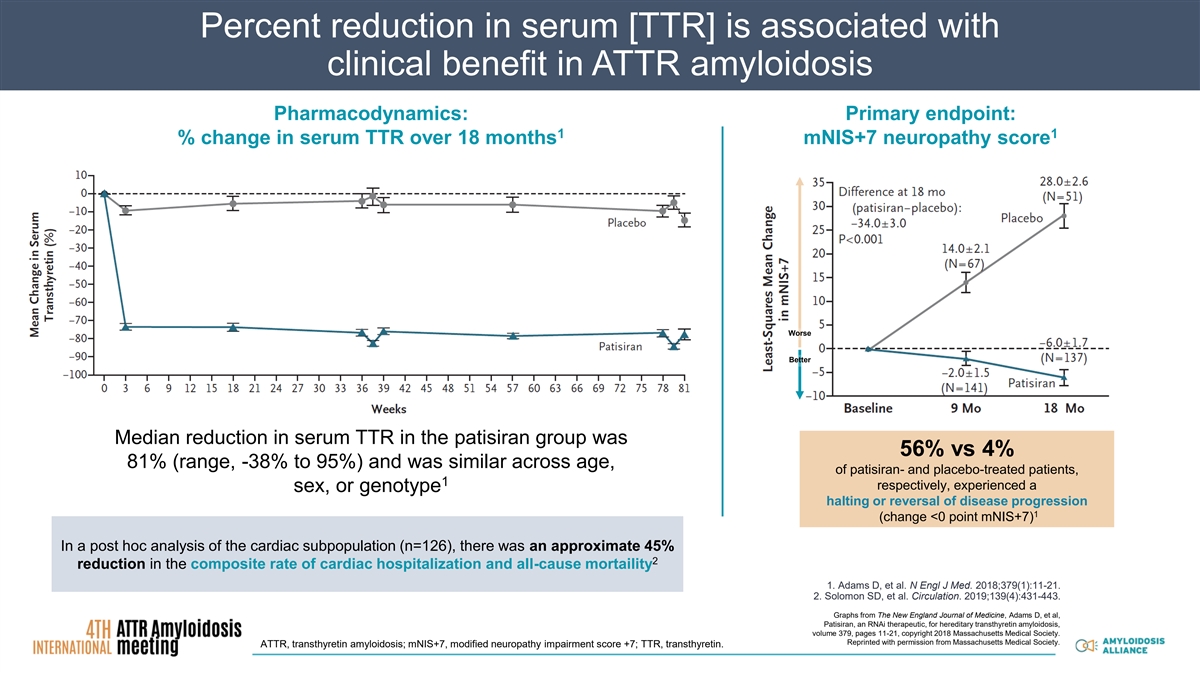
Percent reduction in serum [TTR] is associated with clinical benefit in ATTR amyloidosis Pharmacodynamics: Primary endpoint: 1 1 % change in serum TTR over 18 months mNIS+7 neuropathy score Worse Better Median reduction in serum TTR in the patisiran group was 56% vs 4% 81% (range, -38% to 95%) and was similar across age, of patisiran- and placebo-treated patients, 1 respectively, experienced a sex, or genotype halting or reversal of disease progression 1 (change <0 point mNIS+7) In a post hoc analysis of the cardiac subpopulation (n=126), there was an approximate 45% 2 reduction in the composite rate of cardiac hospitalization and all-cause mortaility 1. Adams D, et al. N Engl J Med. 2018;379(1):11-21. 2. Solomon SD, et al. Circulation. 2019;139(4):431-443. Graphs from The New England Journal of Medicine, Adams D, et al, Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis, volume 379, pages 11-21, copyright 2018 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. ATTR, transthyretin amyloidosis; mNIS+7, modified neuropathy impairment score +7; TTR, transthyretin.
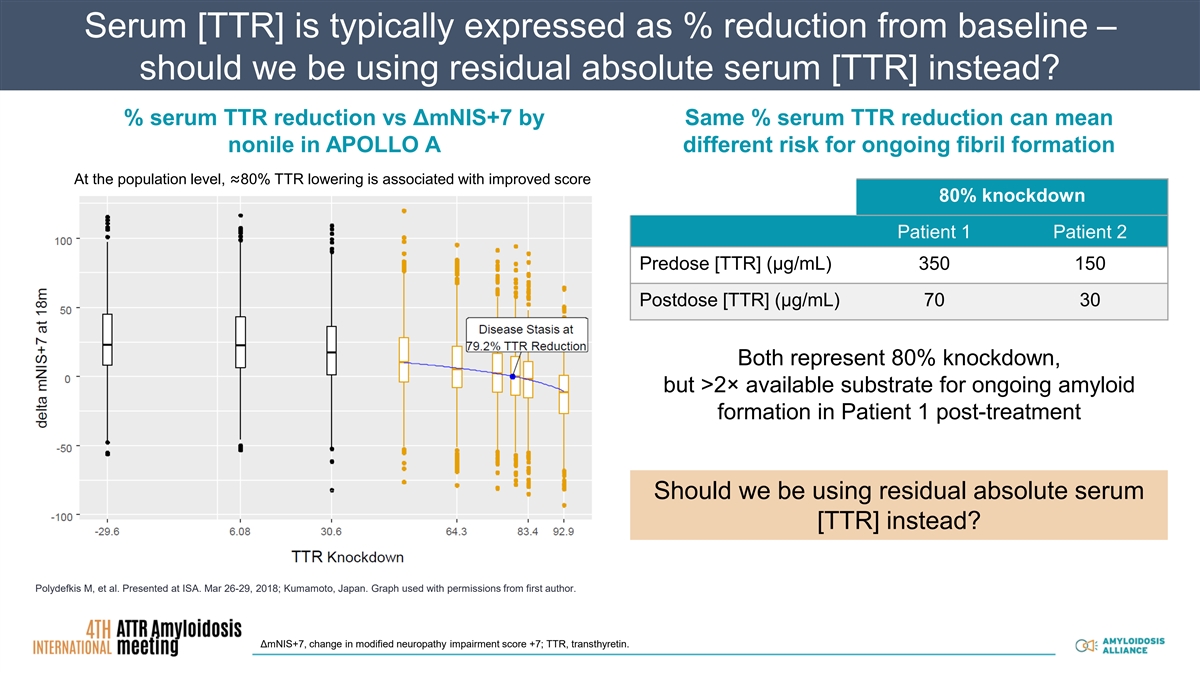
Serum [TTR] is typically expressed as % reduction from baseline – should we be using residual absolute serum [TTR] instead? % serum TTR reduction vs ΔmNIS+7 by Same % serum TTR reduction can mean nonile in APOLLO A different risk for ongoing fibril formation At the population level, ≈80% TTR lowering is associated with improved score 80% knockdown Patient 1 Patient 2 Predose [TTR] (µg/mL) 350 150 Postdose [TTR] (µg/mL) 70 30 Both represent 80% knockdown, but >2× available substrate for ongoing amyloid formation in Patient 1 post-treatment Should we be using residual absolute serum [TTR] instead? Polydefkis M, et al. Presented at ISA. Mar 26-29, 2018; Kumamoto, Japan. Graph used with permissions from first author. ΔmNIS+7, change in modified neuropathy impairment score +7; TTR, transthyretin.
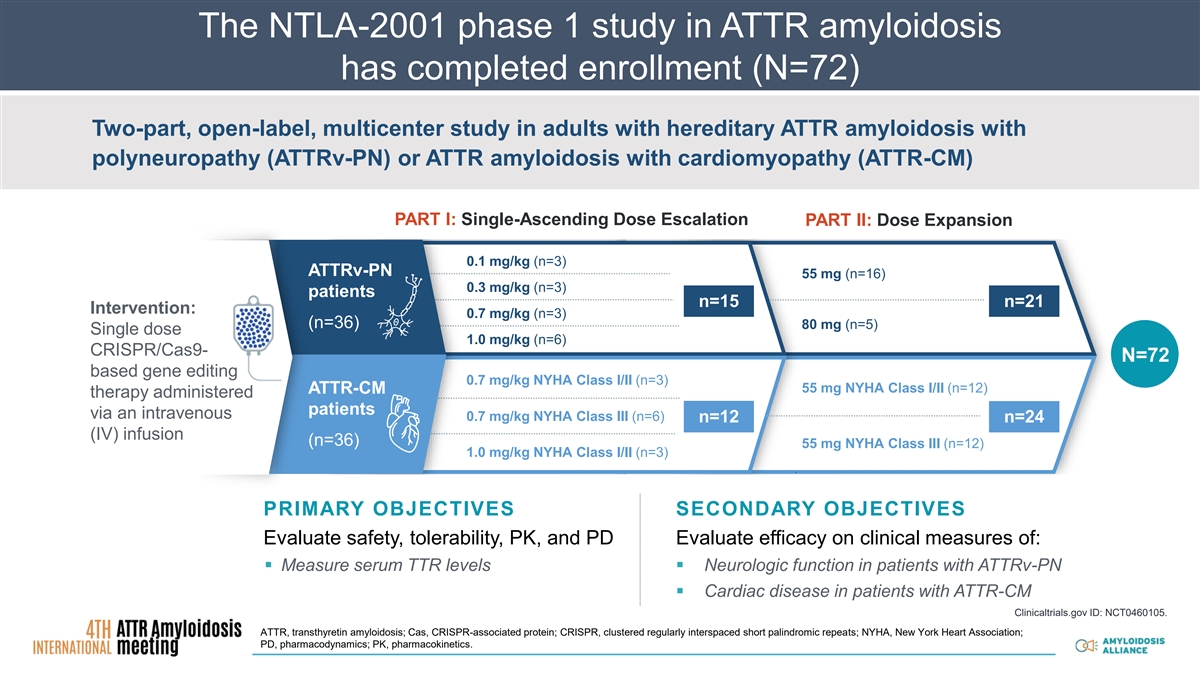
The NTLA-2001 phase 1 study in ATTR amyloidosis has completed enrollment (N=72) Two-part, open-label, multicenter study in adults with hereditary ATTR amyloidosis with polyneuropathy (ATTRv-PN) or ATTR amyloidosis with cardiomyopathy (ATTR-CM) PART I: Single-Ascending Dose Escalation PART II: Dose Expansion 0.1 mg/kg (n=3) ATTRv-PN 55 mg (n=16) 0.3 mg/kg (n=3) patients n=15 n=21 Intervention: 0.7 mg/kg (n=3) (n=36) 80 mg (n=5) Single dose 1.0 mg/kg (n=6) CRISPR/Cas9- N=72 based gene editing 0.7 mg/kg NYHA Class I/II (n=3) 55 mg NYHA Class I/II (n=12) ATTR-CM therapy administered 1.0 mg/kg patients via an intravenous 0.7 mg/kg NYHA Class III (n=6) n=12 n=24 (IV) infusion (n=36) 55 mg NYHA Class III (n=12) 1.0 mg/kg NYHA Class I/II (n=3) PRIMARY OBJECTIVES SECONDARY OBJECTIVES Evaluate safety, tolerability, PK, and PD Evaluate efficacy on clinical measures of: ▪ Measure serum TTR levels▪ Neurologic function in patients with ATTRv-PN ▪ Cardiac disease in patients with ATTR-CM Clinicaltrials.gov ID: NCT0460105. ATTR, transthyretin amyloidosis; Cas, CRISPR-associated protein; CRISPR, clustered regularly interspaced short palindromic repeats; NYHA, New York Heart Association; PD, pharmacodynamics; PK, pharmacokinetics.
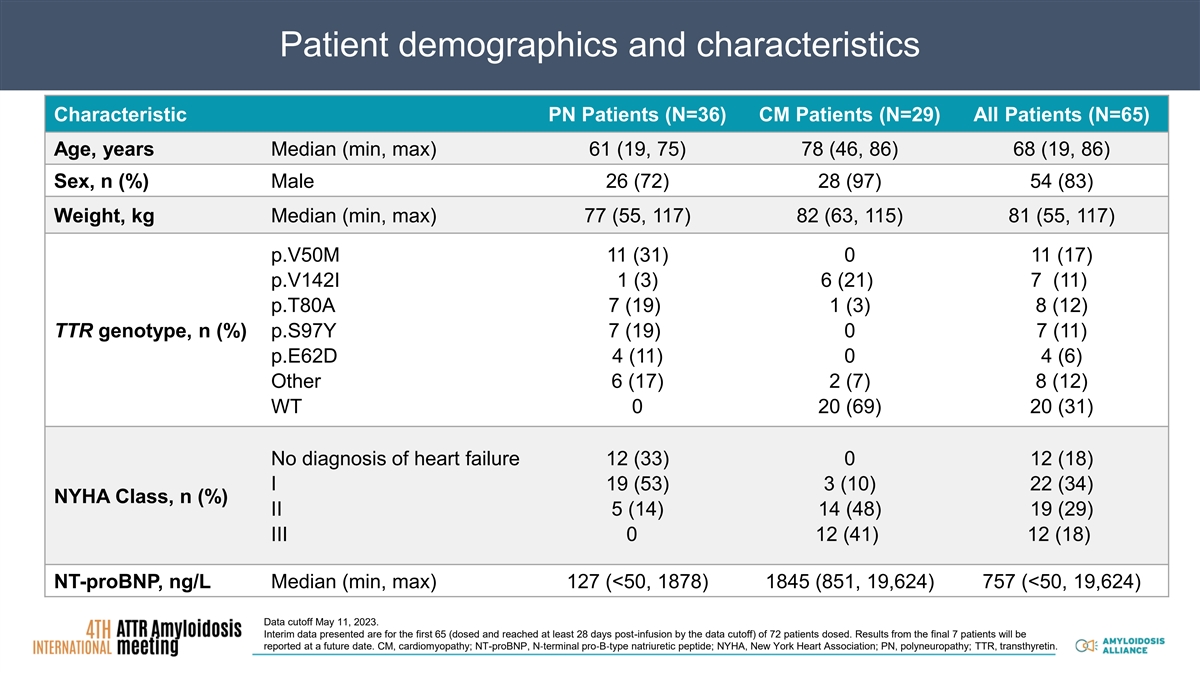
Patient demographics and characteristics Characteristic PN Patients (N=36) CM Patients (N=29) All Patients (N=65) Age, years Median (min, max) 61 (19, 75) 78 (46, 86) 68 (19, 86) Sex, n (%) Male 26 (72) 28 (97) 54 (83) Weight, kg Median (min, max) 77 (55, 117) 82 (63, 115) 81 (55, 117) p.V50M 11 (31) 0 11 (17) p.V142I 1 (3) 6 (21) 7 (11) p.T80A 7 (19) 1 (3) 8 (12) TTR genotype, n (%) p.S97Y 7 (19) 0 7 (11) p.E62D 4 (11) 0 4 (6) Other 6 (17) 2 (7) 8 (12) WT 0 20 (69) 20 (31) No diagnosis of heart failure 12 (33) 0 12 (18) I 19 (53) 3 (10) 22 (34) NYHA Class, n (%) II 5 (14) 14 (48) 19 (29) III 0 12 (41) 12 (18) NT-proBNP, ng/L Median (min, max) 127 (<50, 1878) 1845 (851, 19,624) 757 (<50, 19,624) Data cutoff May 11, 2023. Interim data presented are for the first 65 (dosed and reached at least 28 days post-infusion by the data cutoff) of 72 patients dosed. Results from the final 7 patients will be reported at a future date. CM, cardiomyopathy; NT-proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PN, polyneuropathy; TTR, transthyretin.
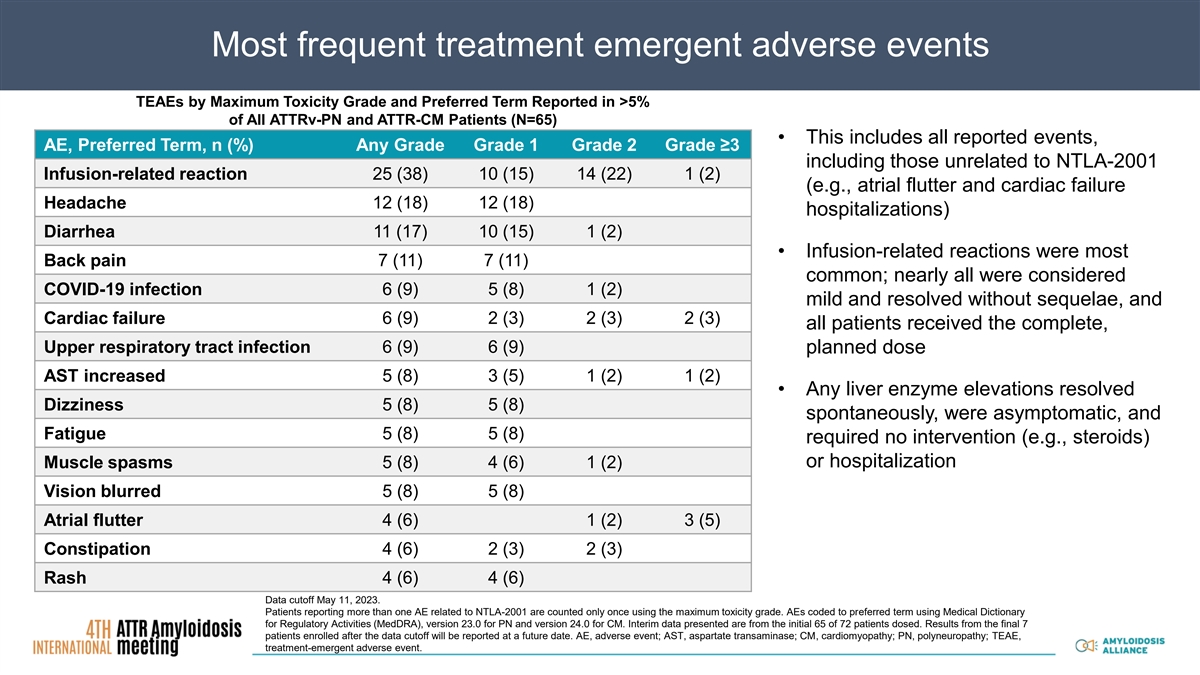
Most frequent treatment emergent adverse events TEAEs by Maximum Toxicity Grade and Preferred Term Reported in >5% of All ATTRv-PN and ATTR-CM Patients (N=65) • This includes all reported events, AE, Preferred Term, n (%) Any Grade Grade 1 Grade 2 Grade ≥3 including those unrelated to NTLA-2001 Infusion-related reaction 25 (38) 10 (15) 14 (22) 1 (2) (e.g., atrial flutter and cardiac failure Headache 12 (18) 12 (18) hospitalizations) Diarrhea 11 (17) 10 (15) 1 (2) • Infusion-related reactions were most Back pain 7 (11) 7 (11) common; nearly all were considered COVID-19 infection 6 (9) 5 (8) 1 (2) mild and resolved without sequelae, and Cardiac failure 6 (9) 2 (3) 2 (3) 2 (3) all patients received the complete, Upper respiratory tract infection 6 (9) 6 (9) planned dose 5 (8) 3 (5) 1 (2) 1 (2) AST increased • Any liver enzyme elevations resolved Dizziness 5 (8) 5 (8) spontaneously, were asymptomatic, and Fatigue 5 (8) 5 (8) required no intervention (e.g., steroids) or hospitalization Muscle spasms 5 (8) 4 (6) 1 (2) Vision blurred 5 (8) 5 (8) Atrial flutter 4 (6) 1 (2) 3 (5) Constipation 4 (6) 2 (3) 2 (3) Rash 4 (6) 4 (6) Data cutoff May 11, 2023. Patients reporting more than one AE related to NTLA-2001 are counted only once using the maximum toxicity grade. AEs coded to preferred term using Medical Dictionary for Regulatory Activities (MedDRA), version 23.0 for PN and version 24.0 for CM. Interim data presented are from the initial 65 of 72 patients dosed. Results from the final 7 patients enrolled after the data cutoff will be reported at a future date. AE, adverse event; AST, aspartate transaminase; CM, cardiomyopathy; PN, polyneuropathy; TEAE, treatment-emergent adverse event.
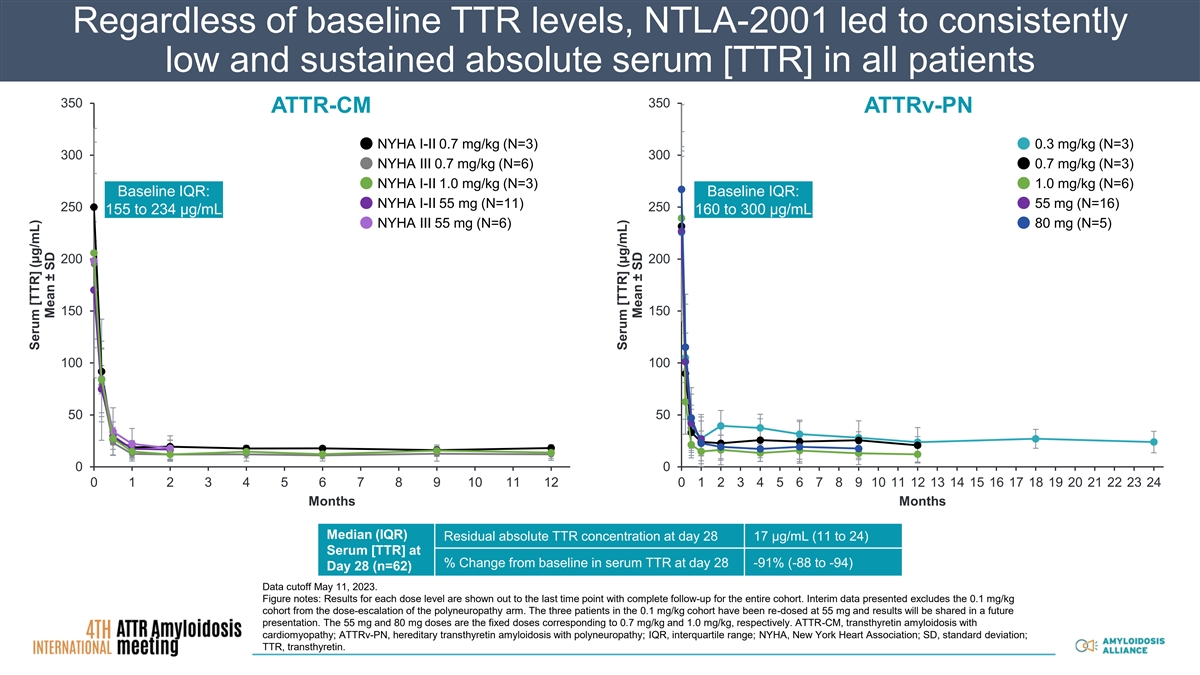
Regardless of baseline TTR levels, NTLA-2001 led to consistently low and sustained absolute serum [TTR] in all patients 350 350 ATTR-CM ATTRv-PN NYHA I-II 0.7 mg/kg (N=3) 0.3 mg/kg (N=3) 300 300 NYHA III 0.7 mg/kg (N=6) 0.7 mg/kg (N=3) NYHA I-II 1.0 mg/kg (N=3) 1.0 mg/kg (N=6) Baseline IQR: Baseline IQR: NYHA I-II 55 mg (N=11) 55 mg (N=16) 250 250 155 to 234 µg/mL 160 to 300 µg/mL NYHA III 55 mg (N=6) 80 mg (N=5) 200 200 150 150 100 100 50 50 0 0 0 1 2 3 4 5 6 7 8 9 10 11 12 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Months Months Median (IQR) Residual absolute TTR concentration at day 28 17 µg/mL (11 to 24) Serum [TTR] at % Change from baseline in serum TTR at day 28 -91% (-88 to -94) Day 28 (n=62) Data cutoff May 11, 2023. Figure notes: Results for each dose level are shown out to the last time point with complete follow-up for the entire cohort. Interim data presented excludes the 0.1 mg/kg cohort from the dose-escalation of the polyneuropathy arm. The three patients in the 0.1 mg/kg cohort have been re-dosed at 55 mg and results will be shared in a future presentation. The 55 mg and 80 mg doses are the fixed doses corresponding to 0.7 mg/kg and 1.0 mg/kg, respectively. ATTR-CM, transthyretin amyloidosis with cardiomyopathy; ATTRv-PN, hereditary transthyretin amyloidosis with polyneuropathy; IQR, interquartile range; NYHA, New York Heart Association; SD, standard deviation; TTR, transthyretin. Serum [TTR] (µg/mL) Mean ± SD Serum [TTR] (µg/mL) Mean ± SD
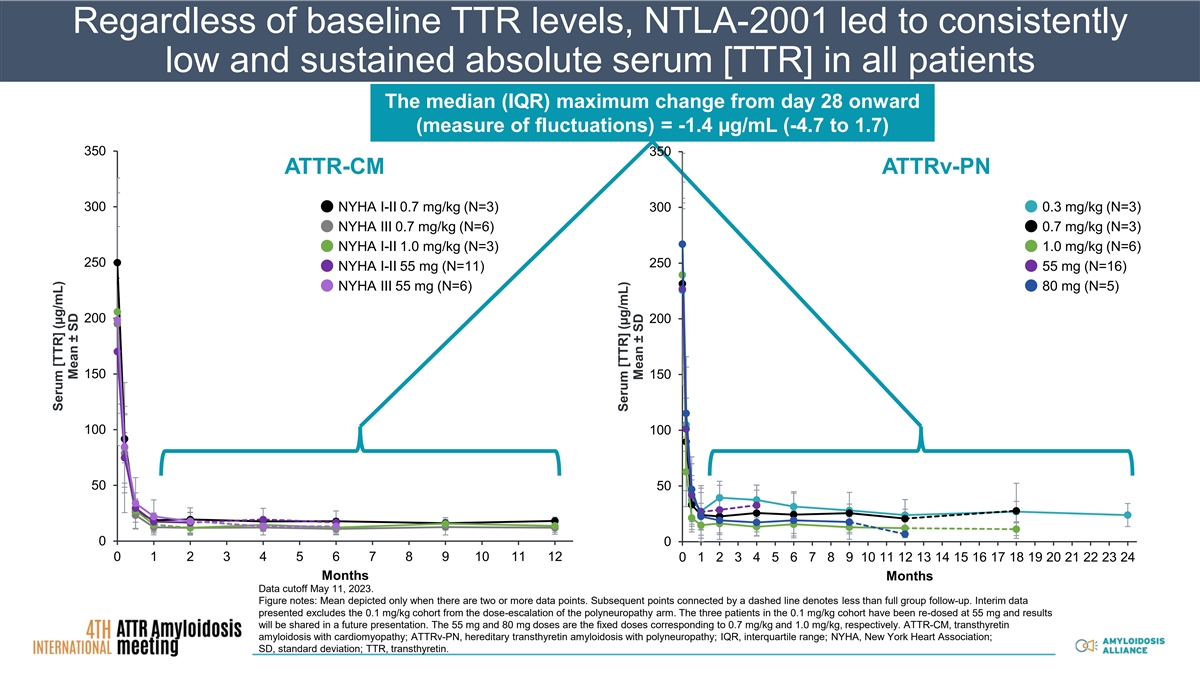
Regardless of baseline TTR levels, NTLA-2001 led to consistently low and sustained absolute serum [TTR] in all patients The median (IQR) maximum change from day 28 onward (measure of fluctuations) = -1.4 µg/mL (-4.7 to 1.7) 350 350 ATTR-CM ATTRv-PN 300 NYHA I-II 0.7 mg/kg (N=3) 300 0.3 mg/kg (N=3) NYHA III 0.7 mg/kg (N=6) 0.7 mg/kg (N=3) NYHA I-II 1.0 mg/kg (N=3) 1.0 mg/kg (N=6) 250 250 NYHA I-II 55 mg (N=11) 55 mg (N=16) NYHA III 55 mg (N=6) 80 mg (N=5) 200 200 150 150 100 100 50 50 0 0 0 1 2 3 4 5 6 7 8 9 10 11 12 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Months Months Data cutoff May 11, 2023. Figure notes: Mean depicted only when there are two or more data points. Subsequent points connected by a dashed line denotes less than full group follow-up. Interim data presented excludes the 0.1 mg/kg cohort from the dose-escalation of the polyneuropathy arm. The three patients in the 0.1 mg/kg cohort have been re-dosed at 55 mg and results will be shared in a future presentation. The 55 mg and 80 mg doses are the fixed doses corresponding to 0.7 mg/kg and 1.0 mg/kg, respectively. ATTR-CM, transthyretin amyloidosis with cardiomyopathy; ATTRv-PN, hereditary transthyretin amyloidosis with polyneuropathy; IQR, interquartile range; NYHA, New York Heart Association; SD, standard deviation; TTR, transthyretin. Serum [TTR] (µg/mL) Mean ± SD Serum [TTR] (µg/mL) Mean ± SD
Summary • In other systemic amyloidoses, the residual, absolute concentration of the amyloid precursor protein is closely associated with clinical outcomes • Interim data from 62 patients with ATTR amyloidosis treated with NTLA-2001 continue to show a favorable safety and tolerability profile, with rapid, consistent, and durable reductions of serum [TTR] to low levels in all patients • For treatments that reduce total serum [TTR], with nonfluctuating steady state measures, the residual absolute serum [TTR] could be a robust biomarker of ATTR amyloidosis therapy outcomes • With collaboration, this approach to biomarker development has facilitated progress in care and better outcomes in AA and AL amyloidosis AA, amyloid A; AL, amyloid light chain; ATTR, transthyretin amyloidosis; TTR, transthyretin.

Acknowledgments We extend our gratitude to the patients who United Kingdom participated in the NTLA-2001 Phase 1 study National Amyloidosis Centre and their family and caregiver networks Physicians Histology Staff executing the study on behalf of: Prof. Mariana Janet Gilbertson Richmond Pharmacology, London Fontana New Zealand Clinical Research, Auckland Prof. Philip Hawkins Imaging Umea University, Sweden CHU Bicetre, University of Paris-Saclay Laboratory Dorota Rowczenio Intellia Therapeutics Regeneron Pharmaceuticals Prof. Paul Simons Genetics Guglielmo Verona David Hutt Editorial support was provided by James Banigan, PhD, and Shannon Davis of Apollo Medical Communications, part of Helios Global Group, and funded by Intellia Therapeutics

Appendix
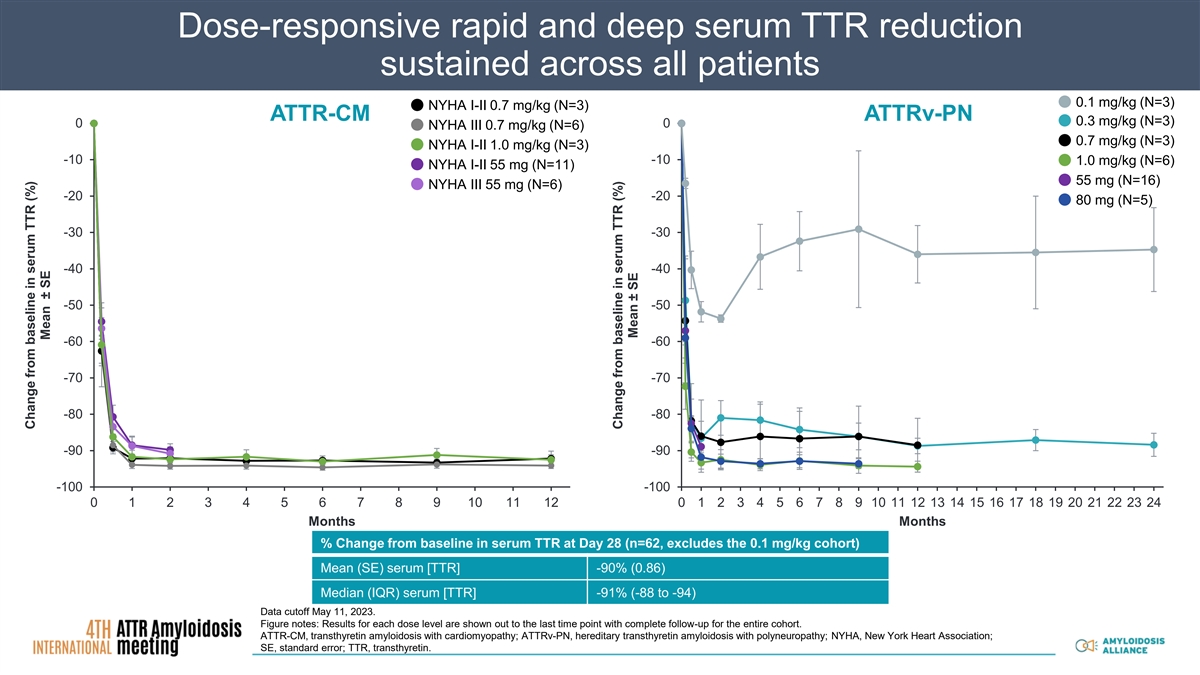
Dose-responsive rapid and deep serum TTR reduction sustained across all patients 0.1 mg/kg (N=3) NYHA I-II 0.7 mg/kg (N=3) ATTR-CM ATTRv-PN 0.3 mg/kg (N=3) 0 0 NYHA III 0.7 mg/kg (N=6) 0.7 mg/kg (N=3) NYHA I-II 1.0 mg/kg (N=3) -10 -10 1.0 mg/kg (N=6) NYHA I-II 55 mg (N=11) 55 mg (N=16) NYHA III 55 mg (N=6) -20 -20 80 mg (N=5) -30 -30 -40 -40 -50 -50 -60 -60 -70 -70 -80 -80 -90 -90 -100 -100 0 1 2 3 4 5 6 7 8 9 10 11 12 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Months Months % Change from baseline in serum TTR at Day 28 (n=62, excludes the 0.1 mg/kg cohort) Mean (SE) serum [TTR] -90% (0.86) Median (IQR) serum [TTR] -91% (-88 to -94) Data cutoff May 11, 2023. Figure notes: Results for each dose level are shown out to the last time point with complete follow-up for the entire cohort. ATTR-CM, transthyretin amyloidosis with cardiomyopathy; ATTRv-PN, hereditary transthyretin amyloidosis with polyneuropathy; NYHA, New York Heart Association; SE, standard error; TTR, transthyretin. Change from baseline in serum TTR (%) Mean ± SE Change from baseline in serum TTR (%) Mean ± SE















