Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Filing exhibits
AVXS similar filings
- 21 Dec 16 Other Events
- 10 Nov 16 Results of Operations and Financial Condition
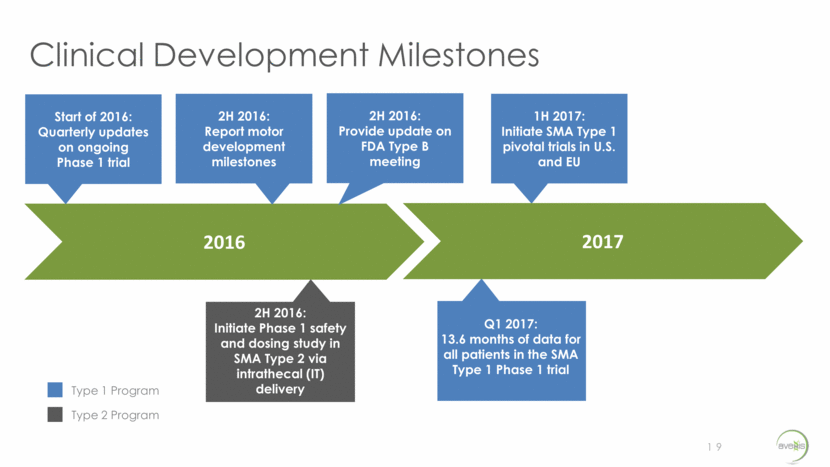
- 1 Nov 16 AveXis Announces Single-Arm Design for U.S. Pivotal Study of AVXS-101 in SMA Type 1 Patients
- 11 Oct 16 Regulation FD Disclosure
- 7 Sep 16 Other Events
- 11 Aug 16 Results of Operations and Financial Condition
- 20 Jul 16 Other Events
Filing view
External links