Exhibit 99.2
Corporate Update
DCC-2618
Ongoing Phase 1 Trial Update
We are studyingDCC-2618 in an ongoing Phase 1 trial in patients with advanced malignancies. As of January 18, 2018, we had enrolled 169 total patients with 113 patients receiving 150 mg ofDCC-2618 once daily, or QD. Sixty-eight patients were enrolled in the dose escalation portion and 101 patients were enrolled in the dose expansion portion of the Phase 1 trial. Of these 169 total patients, 142 were gastrointestinal stromal tumor, or GIST, patients, 100 of whom received 150 mg ofDCC-2618 QD. We are enrolling patients with select advanced malignancies, including fourth- and fourth-line plus GIST, second- and third-line GIST, advanced systemic mastocytosis, or ASM, and gliomas, including glioblastoma multiforme, or GBM, in expansion cohorts of this Phase 1 trial.
2018 American Society of Clinical Oncology Annual Meeting and Additional Interim Results
In June 2018, at the 2018 American Society of Clinical Oncology Annual Meeting, or ASCO 2018, we presented an update to the interim results from our ongoing Phase 1 trial ofDCC-2618 in 150 GIST patients shown to harbor a broad range of KIT and PDGFRa mutations who received at least one dose at or above 100 mg ofDCC-2618 daily, on or before February 26, 2018, with an efficacycut-off date of April 18, 2018.
In the data presented at ASCO 2018 and as part of our additional interim update, we observed that 83% (115 of 138) of evaluable patients, defined as those patients with at least a baseline and one follow-up tumor assessment as of the efficacycut-off date, had a best response of stable disease or partial response (defined as tumor size reduction of 30% or more), or PR, by Response Evaluation Criteria in Solid Tumors, or RECIST. In addition, we observed a disease control rate, or DCR, defined as the proportion of patients with either stable disease or a PR at a point in time, of 70% at three months in 145 evaluable patients, which excluded five patients that were on study at the efficacycut-off date, but had not received a first tumor assessment. Disease control includes stable disease, PRs and complete responses, or CRs, measured by computerized tomography, or CT scan, or magnetic resonance imaging, or MRI scan, and assessed locally by RECIST. We also observed an objective response rate, or ORR, which is the proportion of patients with either CRs or PRs by RECIST of 15% in 145 patients. In 54 second- and third-line GIST patients, we also observed an ORR of 24%, a best response of 83% and a DCR of 80% at 3 months.
Interim results, including those presented at ASCO 2018, are summarized in the below table.
| | | | | | | | | | | | | | | | | | | | |
Line of Therapy | | Total
Patients(1) | | | Active(1) | | | Best(2) Response
(CR/PR/SD) | | | DCR at 3
Months(2) | | | ORR(2) | |
2nd Line | | | 25 | | | | 68 | % | | | 84 | % | | | 79 | % | | | 24 | % |
3rd Line | | | 29 | | | | 76 | % | | | 83 | % | | | 82 | % | | | 24 | % |
³4th Line | | | 96 | | | | 53 | % | | | 77 | %(3) | | | 64 | %(3) | | | 9 | %(3) |
| Total | | | 150 | | | | 60 | % | | | 79 | %(3) | | | 70 | %(3) | | | 15 | %(3) |
| (1) | Reflects total number of GIST patients in the Phase 1 trial that received at least 100 mg ofDCC-2618 daily and that began cycle one day one, or C1D1, on or before February 26, 2018. |
| (2) | Patients with C1D1 on or before February 2, 2018, or enrolled later with an available tumor assessment, based on the April 18, 2018 efficacycut-off date. |
| (3) | Excludes five patients with C1D1 after February 2, 2018 and no assessment. |
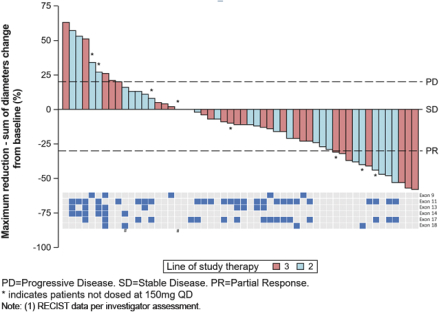
RECIST Responses inKIT- or PDGFRa-driven GIST Patients in Phase 1 Trial
At ASCO 2018, for the second- and third-line GIST patients in our Phase 1 trial ofDCC-2618 who received both a baseline and post-treatment CT scan by the efficacycut-off date (n = 54), we presented the greatest reduction or smallest increase in tumor size from baseline as measured by CT or MRI scan, or best response, for solid malignancies per RECIST as shown in the following figure.
Best Response per RECIST(1)
In Second- and Third-Line KIT & PDGFRa GIST Patients Receiving
³ 100 mgDCC-2618 Daily (n = 54)
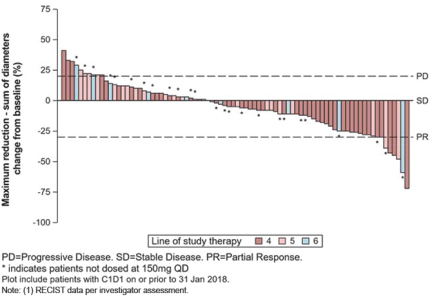
In addition to the data presented at ASCO 2018, for the fourth- and fourth-line plus GIST patients in our Phase 1 trial ofDCC-2618 who received both a baseline and post-treatment CT scan by the efficacycut-off date (n = 82), we also evaluated the best response for solid malignancies per RECIST as shown in the following figure.
Best Response per RECIST(1)
In Fourth- and Fourth-Line Plus KIT & PDGFRa GIST Patients Receiving
³ 100 mgDCC-2618 Daily (n = 82)
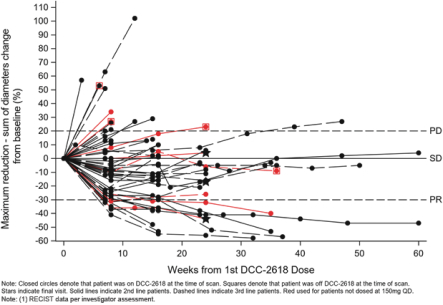
Disease Control Rate inKIT- and PDGFRa-driven GIST Patients
As presented at ASCO 2018, for the second- and third-line GIST patients in our Phase 1 trial ofDCC-2618, we observed a DCR at three months of 79% and 82%, respectively. The chart below shows durability of response in 54 second- and third-line GIST patients receivingDCC-2618 at doses of at least 100 mg daily, where each cycle has a duration of 4 weeks.
Duration of Disease Control(1)
In Second- and Third-Line KIT & PDGFRa GIST Patients Receiving
³ 100 mgDCC-2618 Daily (n = 54)
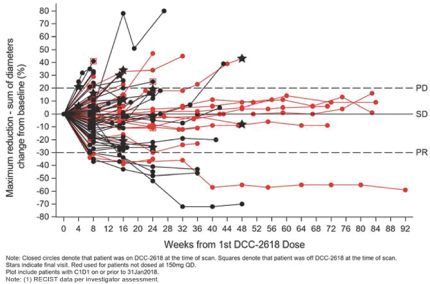
We also observed a DCR at three months of 64% in fourth- and fourth-line plus GIST patients in our Phase 1 trial ofDCC-2618. In addition to the data presented at ASCO 2018, we evaluated the durability of response in 89 fourth- and fourth-line plus GIST patients receivingDCC-2618 at doses of at least 100 mg daily, where each cycle has a duration of 4 weeks as shown in the chart below.
Duration of Disease Control(1)
In Fourth- and Fourth-Line Plus KIT & PDGFRa GIST Patients Receiving
³ 100 mgDCC-2618 Daily (n = 89)
Additional Interim Observations
In addition to the data presented at ASCO 2018, we also observed clinical activity in patients who were previously treated with the investigational drug avapritinib(BLU-285). Of the 150 patients in our ongoing Phase 1 trial ofDCC-2618, there were 10 evaluable patients withKIT-driven GIST who previously received avapritinib and who were enrolled and being treated withDCC-2618 as of January 31, 2018. Six out of 10, or 60%, of these patients achieved stable disease as best response as of the efficacycut-off date. One additional patient achieved stable disease following intra-patient dose escalation to 150 mg ofDCC-2618 twice daily from 150 mg QD. As of the efficacycut-off date, five out of 10, or 50%, of these patients remained on study and three out of 10, or 30%, of these patients had receivedDCC-2618 for at least six months. As of the efficacycut-off date, of the three patients who had receivedDCC-2618 for at least six months, two achieved continued stable disease and remained on study. The third patient with progressive disease was dose escalated and reported asoff-study as of the efficacycut-off date. The chart below summarizes the treatment duration of the 10 evaluable patients who previously received avapritinib and the responses observed.
Treatment Duration of Evaluable Patients Who Previously Received Avapritinib
(n = 10)
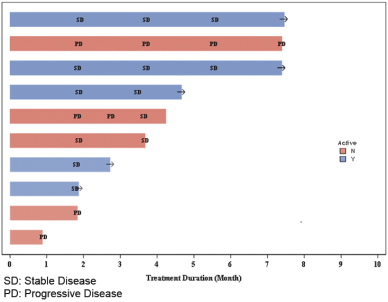
The evaluable patients in our ongoing Phase 1 clinical trial ofDCC-2618 who were previously treated with avapritinib are limited in number. We are not able to draw any conclusions about why such patients were no longer enrolled in clinical trials for avapritinib. As a result, any clinical activity observed in these patients may not be representative of future results for these patients or indicative of results for other patients who were previously treated with avapritinib. References to such patients are not intended to be comparisons between avapritinib andDCC-2618.
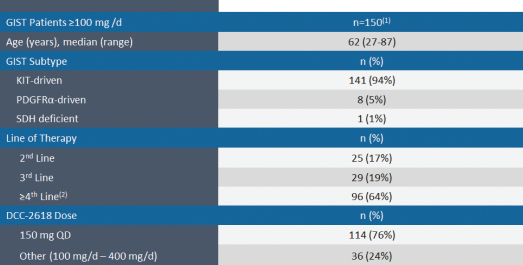
The chart below summarizes the demographic profile of the patients for whom data was presented at ASCO 2018 and the other additional interim data described above.
Demographic Profile of GIST Patients in Phase 1 Trial
AACR Annual Meeting 2018
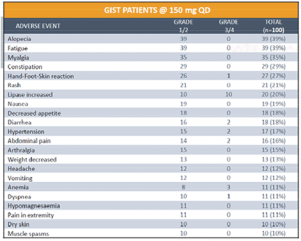
In April 2018, at the AACR Annual Meeting 2018, or AACR 2018, we reported updated safety on 100 patients with GIST who were treated at our recommended Phase 2 dose of 150 mg ofDCC-2618 QD. The data showed that in the ongoing Phase 1 trialDCC-2618 continues to be generally well-tolerated at the dose of 150 mg QD as summarized in the table below.
Treatment Emergent Adverse Events
At AACR 2018, we also reported that as of January 1, 2018, 137 GIST patients were enrolled in the Phase 1 trial ofDCC-2618 at doses of at least 100 mg ofDCC-2618 daily. As of March 19, 2018, 81 of the 137 patients remained on study, with 46, 21, 10 and seven of the patients on study for at least six, nine, 12 and 15 months, respectively.
Ongoing and Planned Phase 3 Trials forDCC-2618 in GIST
In January 2018, we initiated a pivotal Phase 3 trial, or INVICTUS, comparing treatment withDCC-2618 to placebo in 120 fourth-line plus GIST patients, 80 of whom will be treated with 150 mg ofDCC-2618 QD and 40 of whom will receive placebo. We expect to initiate a second pivotal Phase 3 trial comparing treatment with DCC-2618 to sunitinib in up to 350 second-line GIST patients in 2018. We expect half of the patients enrolled in this trial will be treated with 150 mg ofDCC-2618 QD and the other will be treated with 50 mg of sunitinib QD, with median PFS as the primary endpoint.
Preclinical Update
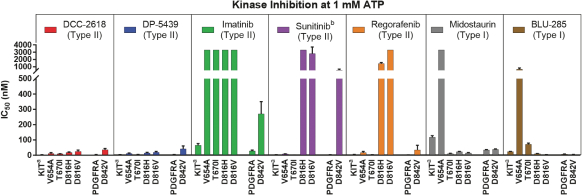
In April 2018, we presented preclinical data at AACR 2018 that describes the breadth of inhibition achieved withDCC-2618 and its active metabolite,DP-5439, across both primary and secondary KIT mutations and primary PDGFRa mutations compared to thein vitro profiles of theFDA-approved kinase inhibitors, imatinib, sunitinib, regorafenib, midostaurin and the investigational drug, avapritinib(BLU-285). Potency is measured by the concentration ofDCC-2618 orDP-5439 required to inhibit kinase activity by 50%, or the inhibitory concentration 50%, or IC50. The lower the bar in the following graphs, the greater the potency. Compared to the approved and investigational compounds tested,DCC-2618 and its active metabolite,DP-5439, exhibited the broadest profile of inhibition across primary and secondary drug-resistant KIT mutations, and primary mutations in PDGFRa.
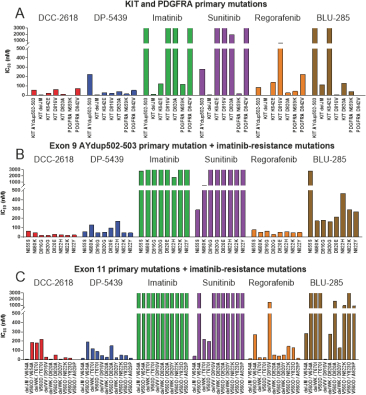
In enzyme assays at relevant cellular levels of adenosine triphosphate, or ATP,DCC-2618 broadly inhibited primary and drug-resistant KIT mutations and primary PDGFRa mutations.DCC-2618 also broadly inhibited KIT and PDGFRa mutations in a panel of GIST, mastocytosis, leukemia, lung cancer, and transfected cell assays.
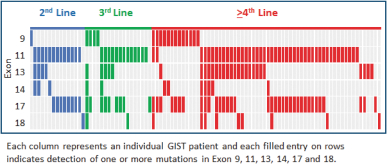
One of the exploratory objectives of our Phase 1 trial ofDCC-2618 was to understand the KIT and PDGFRa mutation status at baseline identified in plasma ctDNA of GIST patients and their association with study drug response. At ASCO 2018, we presented data on the mutational status of 131 GIST patients, of which 95 hadKIT-driven mutations in ctDNA, by exon, as summarized in the following figure.
KIT Mutations in ctDNA (n = 95)
In 131 GIST Patients by Line of Therapy
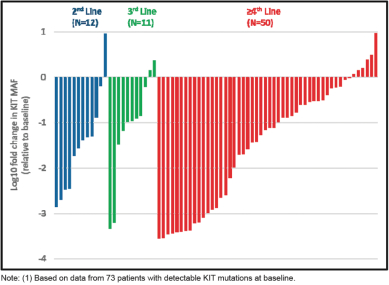
The following figure, which was also included in our ASCO 2018 presentation, shows the maximum change in ctDNA mutant allele frequency, or MAF, by exon in 73 patients with circulating KIT mutations observed at baseline and having data from at least one sample following treatment withDCC-2618. We observed in this group ofKIT-positive GIST patients that treatment withDCC-2618 resulted in reductions of least 50% in the
frequency of circulating ctDNA mutant KIT alleles in 78% (57 of 73) of patients, with 48% (35 of 73) of patients becomingKIT-negative on treatment.
Cumulative Reductions in Circulating MAF of KIT Exons 9, 11,
13, 14, 17 and 18 by Lines of Therapy (n = 73)(1)
(Note log scale:-1 =10-fold reduction,-2 =100-fold reduction)
Development ofDCC-2618 in Gliomas, including GBM
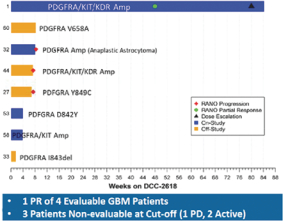
In November 2017, we presented data at the 22nd Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology, or SNO 2018, from eight patients diagnosed with malignant gliomas treated withDCC-2618. Of the five evaluable patients, four had GBM. Among these four GBM patients, we observed one PR, as defined by RANO, and three patients with progressive disease. In addition, of the three patients who werenon-evaluable, two patients remained on study and one had progressive disease.DCC-2618 produced an encouraging partial response in a GBM patient with triple amplification of PDGFRa, KIT and KDR (4q12 amplicon). There was an observed tumor reduction from baseline of 94% on Cycle 23 Day 1 per RANO. However, other patients with similar amplifications or PDGFRa alterations did not derive similar benefit from treatment withDCC-2618. This patient population is very heterogeneous, and patients often exhibit multiple genetic alterations in addition to PDGFRa alterations. We believe that this single exceptional responder warrants
further testing ofDCC-2618 in patients withKIT- and PDGFRa-driven gliomas, and there is an open cohort in the ongoing expansion phase of the Phase 1 study.
Activity Observed in GBM
Rebastinib—TIE2 Inhibitor
Rebastinib is in clinical development for the treatment of multiple solid tumors and in combination with chemotherapy in an investigator-sponsored Phase 1b trial. The investigators presented preliminary clinical data at AACR 2018 from their ongoing Phase 1b trial with rebastinib. Based on data combining rebastinib with anti-PD1 antibodies or anti-tubulin chemotherapy in preclinical studies, we are evaluating opportunities for further development of these drug candidates in combination with other immuno-oncology therapies or chemotherapy. We expect to initiate a company-sponsored Phase 1b trial in rebastinib with chemotherapy in 2018.
Market Opportunity for our Drug Candidates
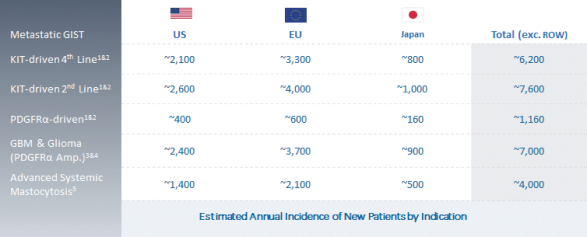
We are continuing to focus on our strategy to expand the market opportunity forDCC-2618 by pursuing development in second-line GIST, gliomas, including GBM, ASM and other solid tumors driven by KIT or PDGFRa. The chart below summarizes our estimates of the annual incidence of these diseases.
Estimated Market Opportunity
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This document contains forward-looking statements, which reflect our current views with respect to, among other things, our operations and financial performance. All statements other than statements of historical facts contained in this document, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plan, objectives of management and expected market growth are forward-looking statements. You can identify these forward-looking statements by the use of words such as “outlook,” “believes,” “expects,” “potential,” “continues,” “may,” “will,” “should,” “seeks,” “approximately,” “predicts,” “intends,” “plans,” “estimates,” “anticipates” or the negative version of these words or other comparable words. Such forward-looking statements are subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements. We believe these factors include but are not limited to those described under “Risk Factors” and include, among other things:
| | • | | the success, cost and timing of our product development activities and clinical trials, including the timing of our second planned Phase 3 trial forDCC-2618 in GIST; |
| | • | | our ability to obtain and maintain regulatory approval forDCC-2618 or any of our other current or future drug candidates, and any related restrictions, limitations, and/or warnings in the label of an approved drug candidate; |
| | • | | our expectations regarding the size of target patient populations for our drug candidates, if approved for commercial use, and any additional drug candidates we may develop; |
| | • | | our ability to obtain funding for our operations; |
| | • | | our ability to manufacture sufficient quantities ofDCC-2618 to support our planned clinical trials and, if approved, commercialization; |
| | • | | the commercialization of our drug candidates, if approved; |
| | • | | our plans to research, develop and commercialize our drug candidates; |
| | • | | our ability to attract collaborators with development, regulatory and commercialization expertise; |
| | • | | our expectations regarding our ability to obtain, maintain, enforce and defend our intellectual property protection for our drug candidates; |
| | • | | future agreements with third parties in connection with the commercialization ofDCC-2618, or any of our other current or future drug candidates; |
| | • | | the size and growth potential of the markets for our drug candidates, and our ability to serve those markets; |
| | • | | the rate and degree of market acceptance of our drug candidates, as well as the reimbursement coverage for our drug candidates; |
| | • | | regulatory and legal developments in the United States and foreign countries; |
| | • | | the performance of our third-party suppliers and manufacturers; |
| | • | | the success of competing therapies that are or may become available; |
| | • | | our ability to attract and retain key scientific or management personnel; |
| | • | | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
| | • | | our expectations regarding the period during which we qualify as an emerging growth company under the JOBS Act; and |
| | • | | our use of the proceeds from our initial public offering. |
These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere in our filings with the SEC. The forward-looking statements contained in this document are made as of the date of this document, and we undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise.












