Exhibit 99.1
Corporate Update
DCC-2618
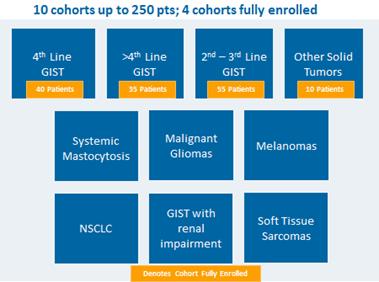
Ongoing Phase 1 Trial Update
We are studyingDCC-2618 in an ongoing Phase 1 trial in patients with advanced malignancies, including gastrointestinal stromal tumors, or GIST. Our ongoing Phase 1 trial is comprised of two parts: a dose escalation portion and a dose expansion portion, as detailed below:
Part 1: Dose Escalation

Part 2: Dose Expansion
European Society of Medical Oncology (ESMO) 2018 Congress and Additional Interim Results
On October 19, 2018, Deciphera Pharmaceuticals, Inc., or the Company, announced updated preliminary results from its ongoing Phase 1 clinical study ofDCC-2618, the Company’s broad-spectrum KIT and PDGFRα inhibitor, in patients with GIST at a presentation at the European Society of Medical Oncology 2018 Congress, or ESMO 2018.
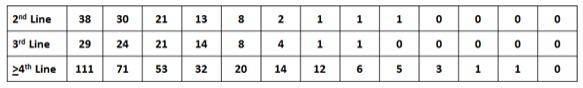
The chart below summarizes the demographic profile of the patients for whom data was presented at ESMO 2018:
Phase 1 Demographics and Baseline Characteristics of GIST Patients
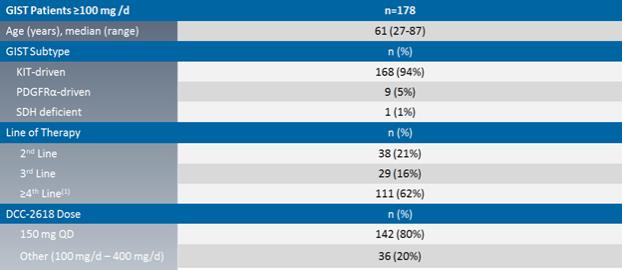
Notes: (1) Mean number of prior regimens for³4th line patients was 3.6.
At ESMO 2018, we reported preliminary results with a cutoff date of August 31, 2018 that include investigator-assessed median progression free survival, or mPFS, objective response rates by best response, or ORR, and disease control rates at 3 months, or DCR, as determined by Response Evaluation Criteria in Solid Tumors, or RECIST, version 1.1 across 178 patients receivingDCC-2618 at doses of>100mg daily.
Preliminary results, including those presented at ESMO 2018, are summarized in the below table.
Line of Therapy | GIST Patients (n) | DCR at 3 Months | ORR(1) | Median Progression(5) Free Survival | ||||||||||
2nd Line(4, 6 & 7) | 38 | 79 | % | 18 | %(2) | 42 weeks | ||||||||
3rd Line(4, 6 & 7) | 29 | 83 | % | 24 | % | 40 weeks | ||||||||
³4th Line(6 & 7) | 111 | 66 | % | 9 | %(3) | 24 weeks | ||||||||
2nd & 3rd Line(4, 6 & 7) | 67 | 81 | % | 21 | % (2 & 3) | 40 weeks | ||||||||
Notes: (1) Includes nine unconfirmed responses: 2nd line (n=1), 3rd line (n=3) and³4th line (n=5); (2) Does not reflect one PR reported after cutoff date, which would result in an ORR in 2nd line of 21% and an ORR in 2nd line and 3rd line combined of 22%; (3) Excludes five patients due to missing data at the time of data cutoff (n=2), lack of first tumor assessment (n=1), withdrawal of consent prior to first assessment (n=1) and unrelated death at C1D4 prior to first assessment (n=1); (4) 59 of 67 combined 2nd line and 3rd line patients received 150mg once daily; (5) Censored patients for mPFS were 2nd line (58%), 3rd line (52%), 4th line and 4th line plus (35%) and 2nd and 3rd line (55%); (6) As of the cutoff date, active patients by line of therapy were as follows: 2nd line were 61%; 3rd line were 59%; 4th line and 4th line plus were 44% and 2nd and 3rd line were 60%; and (7) Median Treatment Duration for 2nd line is 48 weeks, for 3rd line is NR, for 4th line and 4th line plus is 28 weeks and for 2nd and 3rd line is 52 weeks. Includes 14 patients who elected for intra-patient dose escalation.
Preliminary ORR Data by Best Response withDCC-2816 @³ 100 mg/d
At ESMO 2018, for the second- and third-line GIST patients in our Phase 1 trial ofDCC-2618 who received both a baseline and post-treatment CT scan by the efficacy cutoff date (n = 67), we presented the greatest reduction or smallest increase in tumor size from baseline as measured by CT or MRI scan, or best response, for solid malignancies per RECIST as shown in the following figure.
Best Response per RECIST(1)(2)
In Second- and Third-Line KIT & PDGFRα
Patients Receiving³ 100 mg/d (n=67)
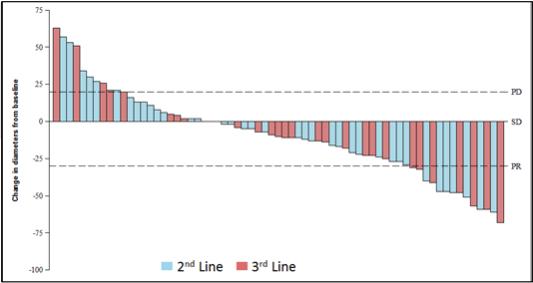
Notes: (1) Based on cutoff date of August 31, 2018; RECIST data per investigator assessment; (2) Includes unconfirmed responses in 2nd line (n=1) and 3rd line (n=3). Does not reflect one PR in 2nd line reported after cutoff date. Includes 14 patients who elected for intra-patient dose escalation.
Preliminary mPFS Data in GIST Patients withDCC-2816 @³ 100 mg/d
At ESMO 2018, for the second-, third- and fourth- and fourth-line plus GIST patients in our Phase 1 trial ofDCC-2618, we presented preliminary mPFS data for patients receiving doses of³100mg daily as shown in the following figures.
Tumor Control per RECIST(1)
KIT & PDGFRα Patients at³ 100 mg/d (n=178)
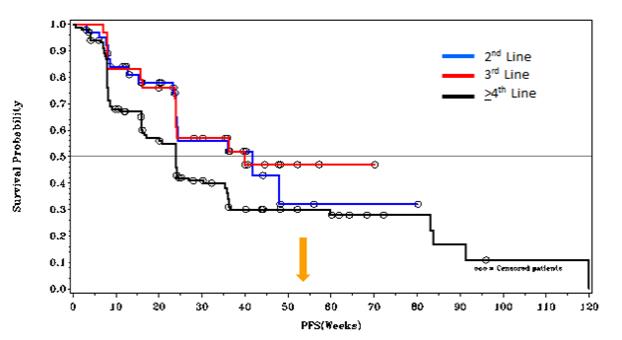
Therapy(1) | mPFS | Number Censored | ||||||||
DCC-2618 2nd Line(2) | 42 weeks | 22 (58%) | ||||||||
DCC-2618 3rd Line(2) | 40 weeks | 15 (52%) | ||||||||
DCC-2618 4th and 4th Line Plus | 24 weeks | 40 (35%) | ||||||||
DCC-2618 2nd Line and 3rd Line | 40 weeks | 37 (55%) | ||||||||
Notes: (1) Based on cutoff date of August 31, 2018; RECIST data per investigator assessment; (2) PFS at 52 weeks was 32% for 2nd Line and 47% for 3rd Line.
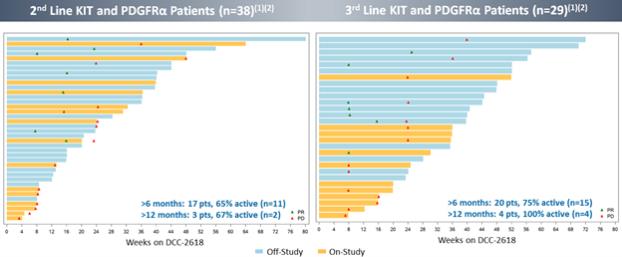
Duration of Treatment of GIST Patients in Ongoing Phase 1 Trial
The following figure shows the duration of treatment withDCC-2618, as of the cutoff date, for second- and third-line GIST patients in the Phase 1 trial who had received³ 100 mg ofDCC-2618 daily. In the second-line GIST patients, as of the cutoff date, 17 patients receivedDCC-2618 at doses of³100mg daily for more than 6 months with 65% (11 of 17) of these patients remaining on study. In third-line GIST patients, as of the cutoff date, 20 patients receivedDCC-2618 at doses of³100mg daily for more than 6 months with 75% (15 of 20) of these patients remaining on study.
Preliminary Data withDCC-2618 at³ 100 mg Daily Shows Emerging Durability
Notes: (1) Based on cutoff date of August 31, 2018; RECIST data per investigator assessment; (2) Includes unconfirmed responses in 2nd line (n=1) and 3rd line (n=3). Does not reflect one PR in 2nd line reported after cutoff date. Includes 14 patients who elected for intra-patient dose escalation.
In addition to the data presented at ESMO 2018, we also observed the following in the fourth-line and fourth-line-plus GIST patients. As of the cutoff date, of the 46 combined fourth- and fourth-line plus GIST patients that receivedDCC-2618 at doses of³100mg daily for more than 6 months, 74% (34 of 46) of these patients remained on study.
Tolerability Results in Phase 1 Trial
DCC-2618 was generally well tolerated at all doses administered and the treatment-emergent adverse events by Grades 1/2 and Grades 3/4 are summarized in the tables below.
For the 178 GIST patients treated at doses above³100mg daily,DCC-2618 was generally well tolerated. Among the treatment-emergent adverse events, or TEAEs, reported by investigators (>10%), regardless of relationship toDCC-2618, the most common were: alopecia (50%), myalgia (44%), fatigue (43%), constipation (34%), hand-foot skin reaction (32%), nausea (30%), decreased appetite (28%), weight decreased (24%), abdominal pain (23%), diarrhea (23%) and lipase increase (23%). Clinically asymptomatic lipase elevations were the most frequent Grade 3 TEAE. Among the 178 GIST patients treated at doses of>100mg daily: 14% (24 patients) experienced dose reductions due to TEAEs and 11% (19 patients) experienced treatment discontinuations due to TEAEs.
Treatment Emergent Adverse Events
in³ 10% of GIST Patients at³ 100mg Daily (n = 178)
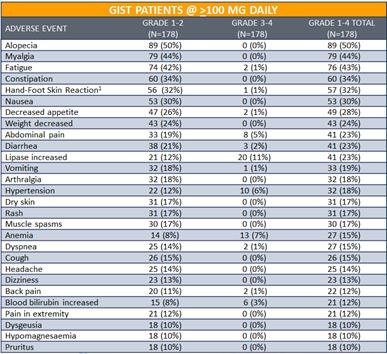
Notes: Data presented at ESMO 2018 based on cutoff as of August 31, 2018. (1) Palmar-plantar erythrodysaesthesia syndrome reported in 19 patients.
For 142 GIST patients treated at 150 mg daily,DCC-2618 was also generally well tolerated. Clinically asymptomatic lipase elevations were the most frequent Grade 3 treatment-emergent adverse event. Among the 142 GIST patients treated at 150 mg daily: 13% (18) patients experienced dose reductions due to TEAEs and 11% (15) experienced treatment discontinuations due to TEAEs.
Treatment Emergent Adverse Events
in³ 10% of GIST Patients at 150mg Daily (n = 142)
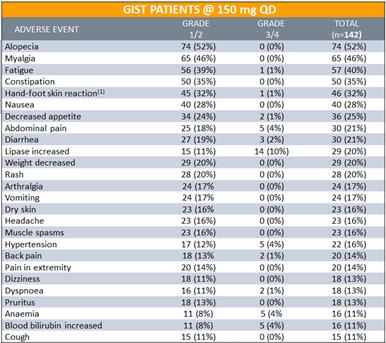
Note: Data presented at ESMO 2018 based on cutoff as of August 31, 2018. (1) Palmar-plantar erythrodysaesthesia syndrome reported in 18 patients.
PlannedPhase 3 Trial INTRIGUE
We expect to initiate our planned randomized (1:1) open label Phase 3 trial, INTRIGUE, comparing treatment withDCC-2618 at 150mg daily to sunitinib at 50mg daily in up to 358 second-line GIST patients, in the fourth quarter of 2018.
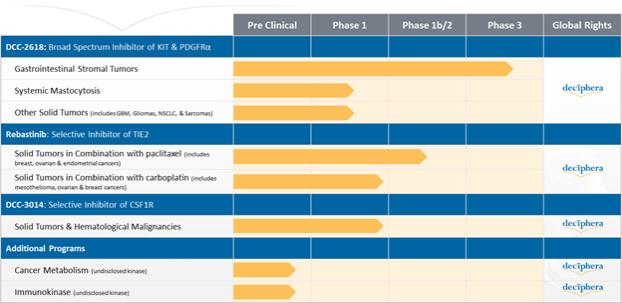
Our Drug Candidates - Pipeline
We currently retain global development and commercialization rights to our drug candidates, including the lead programs summarized in the following figure:
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This document contains forward-looking statements, which reflect our current views with respect to, among other things, our clinical programs, operations and financial performance. All statements other than statements of historical facts contained in this document, including statements regarding our strategy, future operations, future clinical programs, future financial position, future revenue, projected costs, prospects, plan, objectives of management and expected growth are forward-looking statements. You can identify these forward-looking statements by the use of words such as “outlook,” “believes,” “expects,” “potential,” “continues,” “may,” “will,” “should,” “seeks,” “approximately,” “predicts,” “intends,” “plans,” “estimates,” “anticipates” or the negative version of these words or other comparable words. Such forward-looking statements are subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements. We believe these factors include but are not limited to those described under “Risk Factors” in our Quarterly Report on Form10-Q for the quarter ended June 30, 2018 and include, among other things:
| • | the success, cost and timing of our product development activities and clinical trials, including the timing of our second planned Phase 3 trialfor DCC-2618 in GIST; |
| • | our ability to obtain and maintain regulatory approvalfor DCC-2618 or any of our other current or future drug candidates, and any related restrictions, limitations, and/or warnings in the label of an approved drug candidate; |
| • | our expectations regarding the size of target patient populations for our drug candidates, if approved for commercial use, and any additional drug candidates we may develop; |
| • | our ability to obtain funding for our operations; |
| • | our ability to manufacture sufficient quantitiesof DCC-2618 to support our planned clinical trials and, if approved, commercialization; |
| • | the commercialization of our drug candidates, if approved; |
| • | our plans to research, develop and commercialize our drug candidates, including the timing of our second planned Phase 3 trialfor DCC-2618 in GIST; |
| • | our ability to attract collaborators with development, regulatory and commercialization expertise; |
| • | our expectations regarding our ability to obtain, maintain, enforce and defend our intellectual property protection for our drug candidates; |
| • | future agreements with third parties in connection with the commercializationof DCC-2618, or any of our other current or future drug candidates; |
| • | the size and growth potential of the markets for our drug candidates, and our ability to serve those markets; |
| • | the rate and degree of market acceptance of our drug candidates, as well as the reimbursement coverage for our drug candidates; |
| • | regulatory and legal developments in the United States and foreign countries; |
| • | the performance of our third-party suppliers and manufacturers; |
| • | the success of competing therapies that are or may become available; |
| • | our ability to attract and retain key scientific or management personnel; |
| • | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
| • | our expectations regarding the period during which we qualify as an emerging growth company under the JOBS Act; and |
| • | our use of the proceeds from our initial public offering and ourfollow-on public offering. |
These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included elsewhere in our filings with the SEC. The forward-looking statements contained in this document are made as of the date of this document, and we undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise.