treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s). In December 2019, we filed a New Drug Submission, or NDS, with Health Canada, and a market authorisation application, or AUS MAA, with the Therapeutic Goods Administration, or TGA, in Australia, for ripretinib in advanced GIST, under the FDA’s Project Orbis pilot program, or Project Orbis. Project Orbis is an initiative of the OCE and, according to the FDA, provides a framework for concurrent submission and review of oncology products among international partners. Both the NDS and the AUS MAA have received priority review. Acceptance into the RTOR and Orbis pilot programs does not guarantee or influence approvability of our NDA, NDS, and AUS MAA for ripretinib in advanced GIST, which are subject to the standard benefit-risk evaluation by the FDA, and the review standards of Health Canada and TGA, and we may not derive any benefit from inclusion in these pilot programs, including, but not limited to, a more efficient review process. These pilot programs are not formal regulatory pathways and may be changed, suspended, or halted at any time.
We are actively engaged in commercial preparations to support the potential U.S. launch of ripretinib for the treatment of patients with advanced GIST who have received prior treatment with imatinib, sunitinib, and regorafenib, if approved. We expect to file a marketing authorisation application, or MAA, with the European Medicines Agency, or EMA, in the European Union, or EU, for ripretinib in advanced GIST in the second half of 2020, and we are exploring whether to partner, or build our own, Europeango-to-market capabilities, to support a potential EU approval. In June 2019, we entered into a License Agreement, or the Zai License Agreement, with an affiliate of Zai Lab (Shanghai) Co., Ltd., or Zai, pursuant to which we granted Zai exclusive rights to develop and commercialize ripretinib, including certainfollow-on compounds, or the Licensed Products, in Mainland China, Hong Kong, Macau, and Taiwan, collectively Greater China.
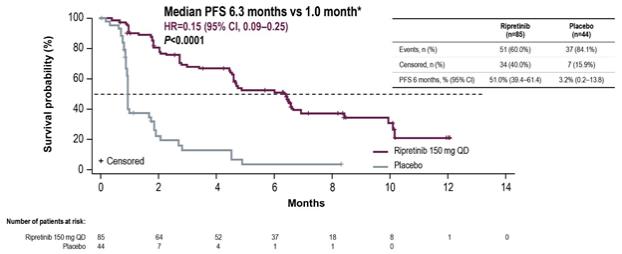
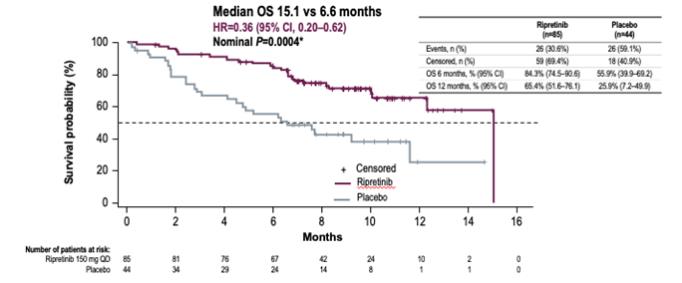
In addition, we are studying ripretinib in our global pivotal Phase 3 study, INTRIGUE, in second-line GIST patients, comparing ripretinib to sunitinb. As of January 6, 2020, we had 106 sites open for enrollment in INTRIGUE in 18 countries. We expect to complete enrollment in INTRIGUE in the second half of 2020.
We also have an ongoing Phase 1 trial studying ripretinib in patients with different stages of GIST following treatment with at least imatinib, as well as in patients with systemic mastocytosis other than indolent systemic mastocytosis, or SM, and other solid tumors driven by KIT or PDGFRα including gliomas, melanoma,non-small cell lung cancer, or NSCLC, germ cell cancer, penile cancer, and soft tissue sarcomas, as well as a cohort for GIST and other solid tumors with renal impairment. We expect to report data from one or more of these expansion cohorts in the second half of 2020.
Beyond ripretinib, we are developing two other clinical-stage drug candidates,DCC-3014 and rebastinib, which target the macrophage tumor microenvironment.
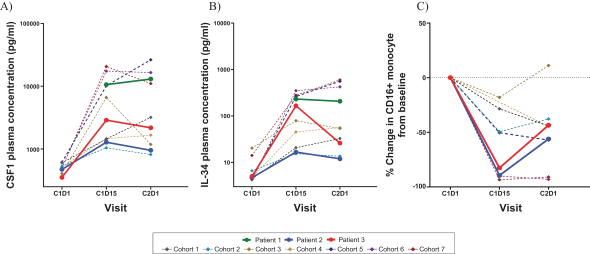
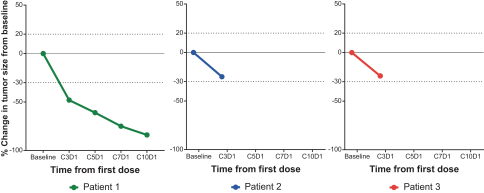
DCC-3014 is an investigational, orally administered, potent, and highly selective inhibitor of CSF1R, a kinase that controls the survival and function of certain immunosuppressive tumor associated macrophages, or TAMs. We are currently studyingDCC-3014 in a Phase 1 dose escalation study that includes patients with advanced malignancies as well as patients with a type of tenosynovial giant cell tumors, or TGCT, known as diffuse-type TGCT. The dose escalation Phase 1 study is designed to determine a Phase 2 dose for an expansion study. During 2019, we announced positive, preliminary data from the ongoing dose escalation Phase 1 study withDCC-3014 in patients with advanced malignancies and preliminary data from three initial patients diagnosed with TGCT. To explore the potential ofDCC-3014 in this target population, we intend to continue to enroll TGCT patients in the dose escalation study, and, in the second half of 2020, provide a data update on TGCT patients. Subject to favorable results from the dose escalation study, in the second half of 2020 we intend to determine a Phase 2 dose for, and initiate an expansion study withDCC-3014, including in patients with TGCT. We will also continue to evaluate the potential to studyDCC-3014 in advanced malignancies in combination with immuno-oncology, or I/O, therapies.
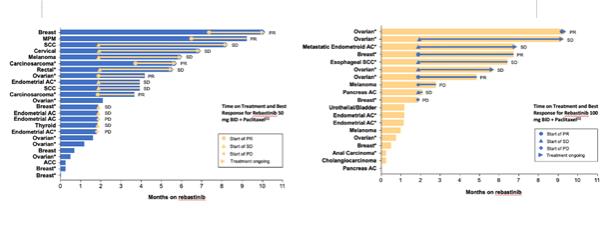
Rebastinib is an investigational, orally administered, potent, and selective inhibitor of TIE2 kinase, which plays an important role in regulating tumor angiogenesis, invasiveness, metastasis, and immunotolerance. We are currently studying rebastinib in two Phase 1b/2 studies in combination with chemotherapy. In October 2018, we initiated an open-label, multicenter, Phase 1b/2 study of rebastinib in combination with paclitaxel to assess safety, tolerability, pharmacokinetics, or PK, and efficacy in patients with advanced or metastatic solid tumors. Part 2 of this study is currently ongoing and we expect to present Phase 1b/2 data from this study in the second half of 2020.