Sienna biopharmaceuticals TM Company Overview June 2018 Mar c h 2 0 1 7 Exhibit 99.1
|
 |
2 Forward Looking Statements This presentation contains forward-looking statements. All statements other than descriptions of historical facts contained in this presentation, including statements regarding future operational and financial results and positions, business strategy, prospective products, potential market, commercial opportunity and market share, availability and potential sources of funding, clinical trial results, product approvals and regulatory pathways, research and development costs, timing (including but not limited to clinical development and regulatory timelines), strategies for completion and likelihood of success for our business activities, and plans for future operations, are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Such risks and uncertainties include, among others, those inherent in the preclinical and clinical development process and the regulatory approval process; the risks and uncertainties in commercialization and gaining market acceptance; the risks associated with protecting and defending our patents or other proprietary rights; the risk that our proprietary rights may be insufficient to protect our product candidates; the risk that we will be unable to obtain necessary capital when needed on acceptable terms or at all; competition from other products or procedures; our reliance on third-parties to conduct our clinical andnon-clinical trials; and our reliance on singlesource third-party suppliers to manufacture clinical,non-clinical and any future commercial supplies of our product candidates. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our most recent Annual Report on Form10-K filed with the Securities and Exchange Commission and our future current and periodic reports. Except as required by applicable law, we assume no obligation to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
|
 |
3 The Sienna Opportunity Addressing the Innovation Gap in Medical Dermatology Accomplished, Credible Management Team • Recent advances in dermatology have mostly benefited the more severe patient populations • Opportunity to leverage biotechnology and well-understood pathways to develop novel, targeted topical products • Unmet need fornon-steroidal topical therapies suitable for chronic use • Exploring additional opportunities in inflammation and immunology to treat unmet medical needs while minimizing systemic exposure Track record with top brands and integral involvement with multiple FDA approvals Previous leadership roles
|
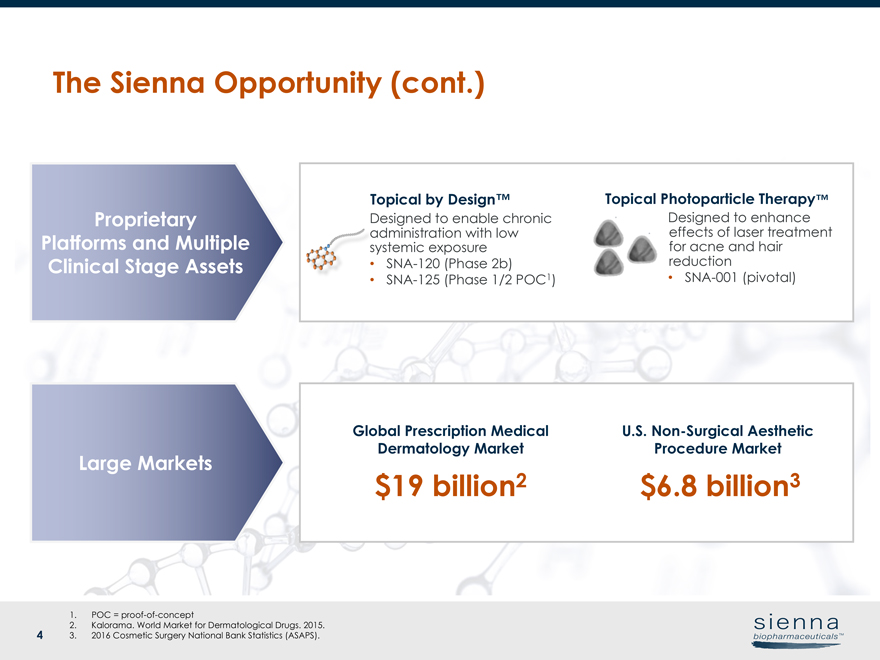 |
4 The Sienna Opportunity (cont.) Proprietary Platforms and Multiple Clinical Stage Assets Large Markets Global Prescription Medical Dermatology Market U.S.Non-Surgical Aesthetic Procedure Market Topical by Design™ Topical Photoparticle Therapy™ Designed to enable chronic administration with low systemic exposure •SNA-120 (Phase 2b) •SNA-125 (Phase 1/2 POC1) Designed to enhance effects of laser treatment for acne and hair reduction •SNA-001 (pivotal) $19 billion2 $6.8 billion3 1. POC =proof-of-concept 2. Kalorama. World Market for Dermatological Drugs. 2015. 3. 2016 Cosmetic Surgery National Bank Statistics (ASAPS).
|
 |
5 Accomplished Team in Medical Dermatology and Aesthetics Frederick C. Beddingfield, III, MD, PhD President & CEO John Smither Chief Financial Officer Senior management with extensive experience Caro Van Hove Chief Commercial Officer Paul F. Lizzul MD, PhD Chief Medical Officer Diane Stroehmann, MS, RAC Chief of Staff and Head of Regulatory Affairs & Quality Ryan Irvine, PhD Head of Medical Affairs Majed Kheir, MBA Vice President, Operations Tim Andrews, Esq. General Counsel Todd Harris, PhD Head of Corporate Development Silvio Traversa BSc, PhD Chief Scientific Officer
|
 |
6 Experienced Board and Advisors Established leaders in the industry Keith Leonard, MS, MBA Chairman, Sienna Biopharmaceuticals Former CEO, Kythera Robert Nelsen, MBACo-Founder, Managing Director, ARCH Venture Partners Kristina Burow, MBA Managing Director, ARCH Venture Partners Bob More, MBA Chairman, One Revolution Todd Harris, PhD Head of Corporate Development, Sienna Biopharmaceuticals Frederick C. Beddingfield III MD, PhDCo-Founder, President and CEO, Sienna Biopharmaceuticals Dennis Fenton, PhD Former EVP, Operations and Compliance, Amgen Dr. David E.I. Pyott Special Advisor to the CEO and Board Former CEO, Allergan Erle Mast, BScCo-Founder and former CFO and Executive Vice President, Clovis Oncology, Inc.
|
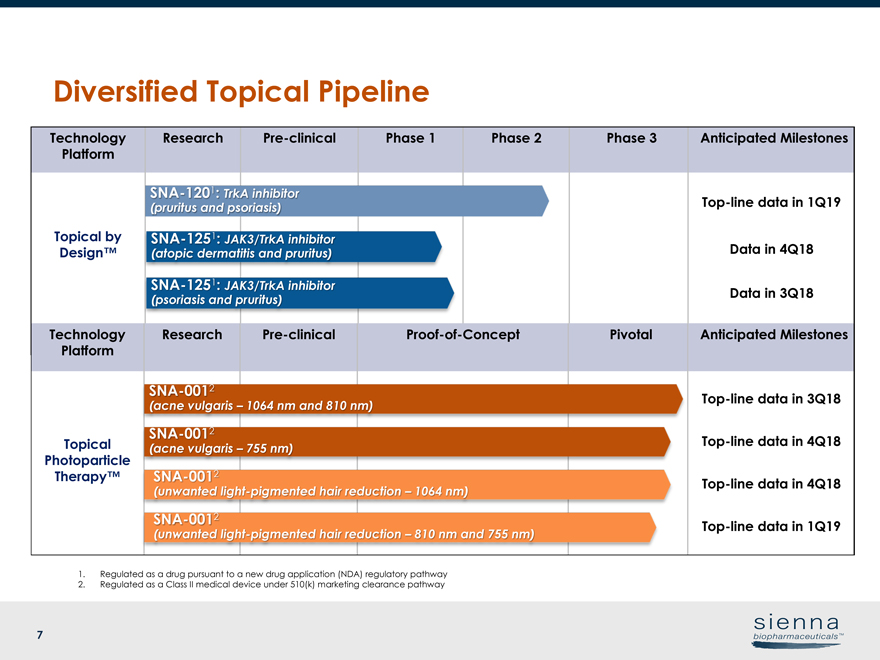 |
7 1. Regulated as a drug pursuant to a new drug application (NDA) regulatory pathway 2. Regulated as a Class II medical device under 510(k) marketing clearance pathway Technology Platform ResearchPre-clinical Phase 1 Phase 2 Phase 3 Anticipated Milestones Topical by Design™Top-line data in 1Q19 Data in 4Q18 Data in 3Q18 Technology Platform ResearchPre-clinicalProof-of-Concept Pivotal Anticipated Milestones Topical Photoparticle Therapy™Top-line data in 3Q18Top-line data in 4Q18Top-line data in 4Q18Top-line data in 1Q19SNA-1201: TrkA inhibitor (pruritus and psoriasis)SNA-1251: JAK3/TrkA inhibitor (psoriasis and pruritus)SNA-0012 (acne vulgaris – 755 nm)SNA-0012 (unwanted light-pigmented hair reduction – 1064 nm)SNA-1251: JAK3/TrkA inhibitor (atopic dermatitis and pruritus)SNA-0012 (acne vulgaris – 1064 nm and 810 nm)SNA-0012 (unwanted light-pigmented hair reduction – 810 nm and 755 nm) Diversified Topical Pipeline
|
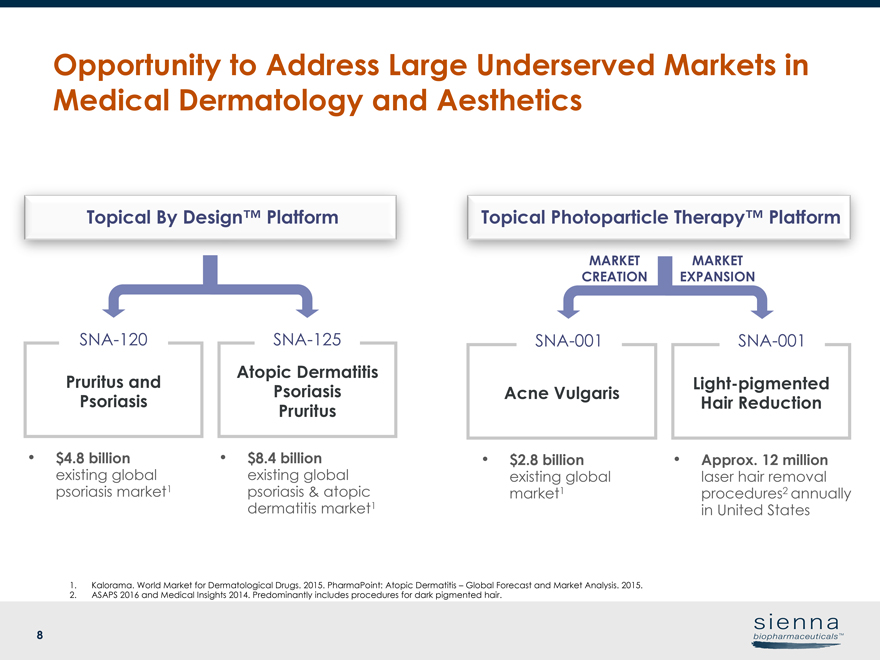 |
8 Opportunity to Address Large Underserved Markets in Medical Dermatology and Aesthetics Atopic Dermatitis Psoriasis PruritusSNA-125 Pruritus and PsoriasisSNA-120 • $8.4 billion existing global psoriasis & atopic dermatitis market1 • $4.8 billion existing global psoriasis market1 1. Kalorama. World Market for Dermatological Drugs. 2015. PharmaPoint: Atopic Dermatitis – Global Forecast and Market Analysis. 2015. 2. ASAPS 2016 and Medical Insights 2014. Predominantly includes procedures for dark pigmented hair. Topical By Design™ Platform Topical Photoparticle Therapy™ Platform Acne Vulgaris Light-pigmented Hair Reduction MARKET EXPANSION MARKET CREATION • Approx. 12 million laser hair removal procedures2 annually in United States • $2.8 billion existing global market1SNA-001SNA-001
9 Topical by Design™ Platform(SNA-120 &SNA-125)
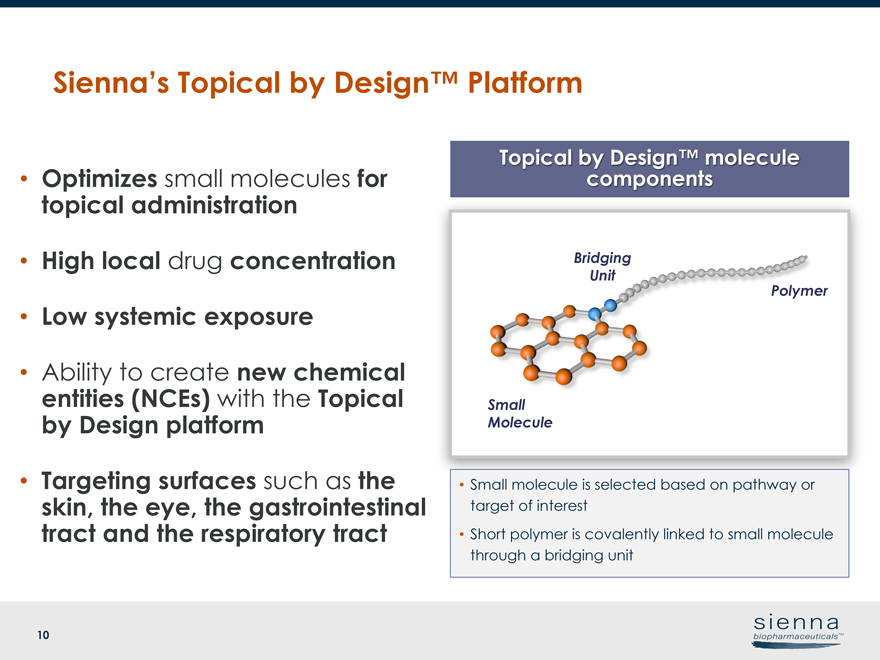
10 Topical by Design™ molecule • Optimizes small molecules for components topical administration • High local drug concentration • Low systemic exposure • Ability to create new chemical entities (NCEs) with the Topical by Design platform • Targeting surfaces such as the skin, the eye, the gastrointestinal tract and the respiratory tract Sienna’s Topical by Design™ Platform Polymer Small Molecule Bridging Unit • Small molecule is selected based on pathway or target of interest • Short polymer is covalently linked to small molecule through a bridging unit
|
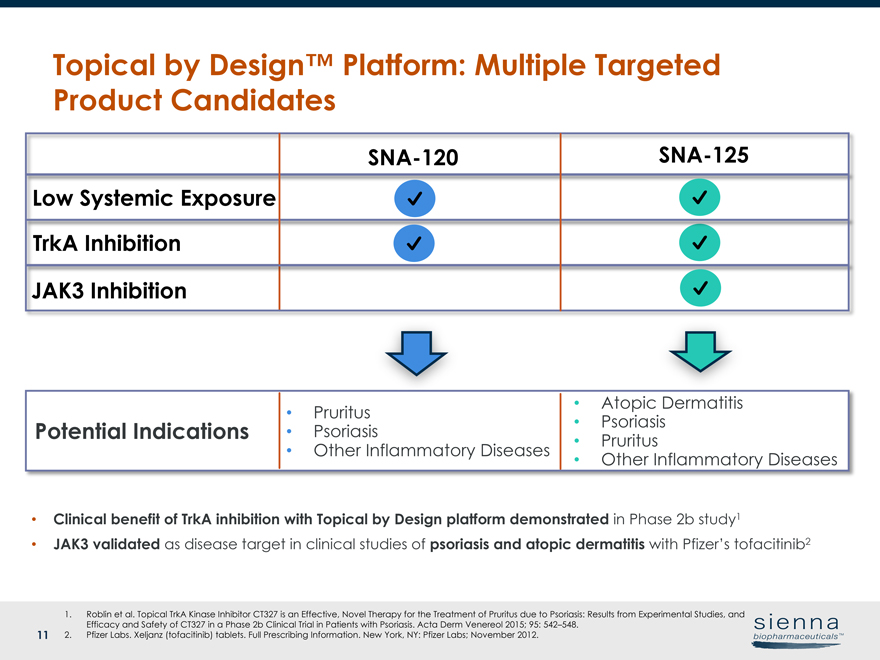 |
11 Topical by Design™ Platform: Multiple Targeted Product Candidates Low Systemic Exposure TrkA Inhibition PPPotential Indications 1. Roblin et al. Topical TrkA Kinase Inhibitor CT327 is an Effective, Novel Therapy for the Treatment of Pruritus due to Psoriasis: Results from Experimental Studies, and Efficacy and Safety of CT327 in a Phase 2b Clinical Trial in Patients with Psoriasis. Acta Derm Venereol 2015; 95: 542–548. 2. Pfizer Labs. Xeljanz (tofacitinib) tablets. Full Prescribing Information. New York, NY: Pfizer Labs; November 2012. • Clinical benefit of TrkA inhibition with Topical by Design platform demonstrated in Phase 2b study1 • JAK3 validated as disease target in clinical studies of psoriasis and atopic dermatitis with Pfizer’s tofacitinib2SNA-120SNA-125 JAK3 Inhibition • Pruritus • Psoriasis • Other Inflammatory Diseases • Atopic Dermatitis • Psoriasis • Pruritus • Other Inflammatory Diseases
|
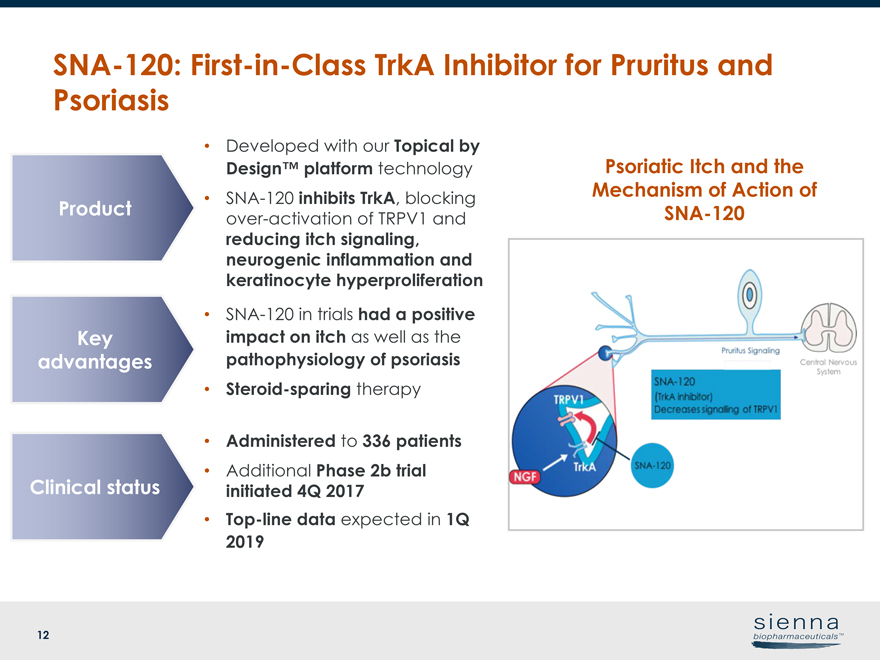 |
12 • Developed with our Topical by Design™ platform technology •SNA-120 inhibits TrkA, blocking over-activation of TRPV1 and reducing itch signaling, neurogenic inflammation and keratinocyte hyperproliferationSNA-120:First-in-Class TrkA Inhibitor for Pruritus and Psoriasis Psoriatic Itch and the Mechanism of Action of ProductSNA-120 Key advantages Clinical status •SNA-120 in trials had a positive impact on itch as well as the pathophysiology of psoriasis • Steroid-sparing therapy • Administered to 336 patients • Additional Phase 2b trial initiated 4Q 2017 •Top-line data expected in 1Q 2019
|
 |
13 • The vast majority of psoriasis patients report they suffer from itch1 • Itch intensity similar to atopic dermatitis2 • Itch significantly impacts sleep and quality of life3 • Reduced itching is a primary benefit sought by psoriatic patients3 • 80 to 90% of psoriasis patients havemild-to-moderate disease for which biologic treatments may not be ideal4 • Biologic and other systemic treatments do not resolve the itch in all patients3 No Specific, Chronic Topical Treatment is Currently Available for Pruritus Associated with Psoriasis 1. Bilac C. The relationship between symptoms and patient characteristics among psoriasis patients. Indian J. Dermatol. Venereol. Leprol. 2009;75:551. 2. Yosipovitch, G. Retrospective Analysis of Data from an Itch Center: Integrating Validated Tools in the Electronic Health Record. J Am Acad Dermatol. October 2016. 3. FDA Public Meeting. The Voice of the Patient: Psoriasis. March 17, 2016. 4. Global Data. PharmaPoint: Psoriasis Global Drug Forecast & Market Analysis to 2024. April 2016.
|
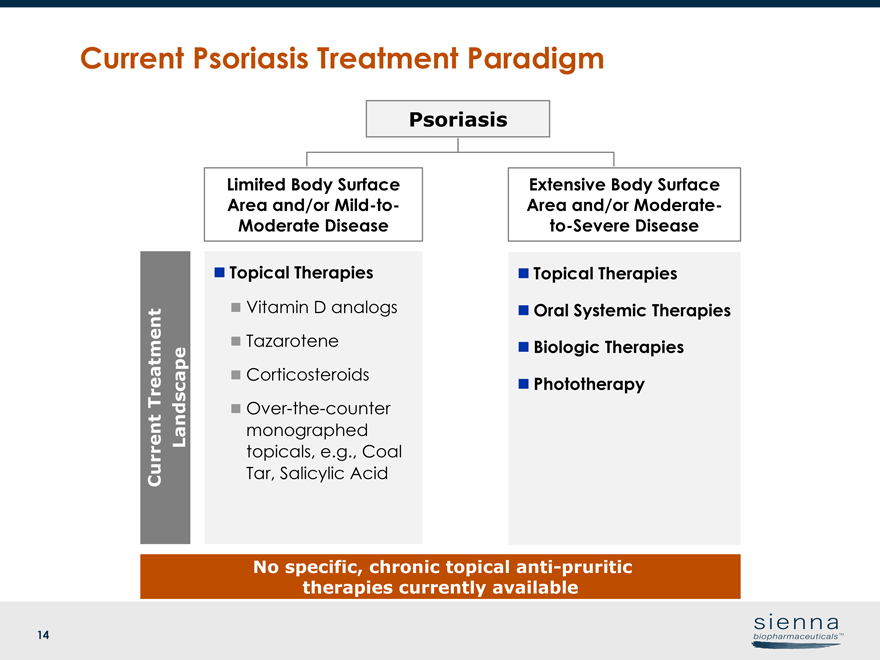 |
14 Current Psoriasis Treatment Paradigm Psoriasis n Topical Therapies n Vitamin D analogs n Tazarotene n Corticosteroids nOver-the-counter monographed topicals, e.g., Coal Tar, Salicylic Acid Limited Body Surface Area and/orMild-to- Moderate Disease Extensive Body Surface Area and/or Moderateto- Severe Disease n Topical Therapies n Oral Systemic Therapies n Biologic Therapies n Phototherapy Current Treatment Landscape No specific, chronic topical anti-pruritic therapies currently available
|
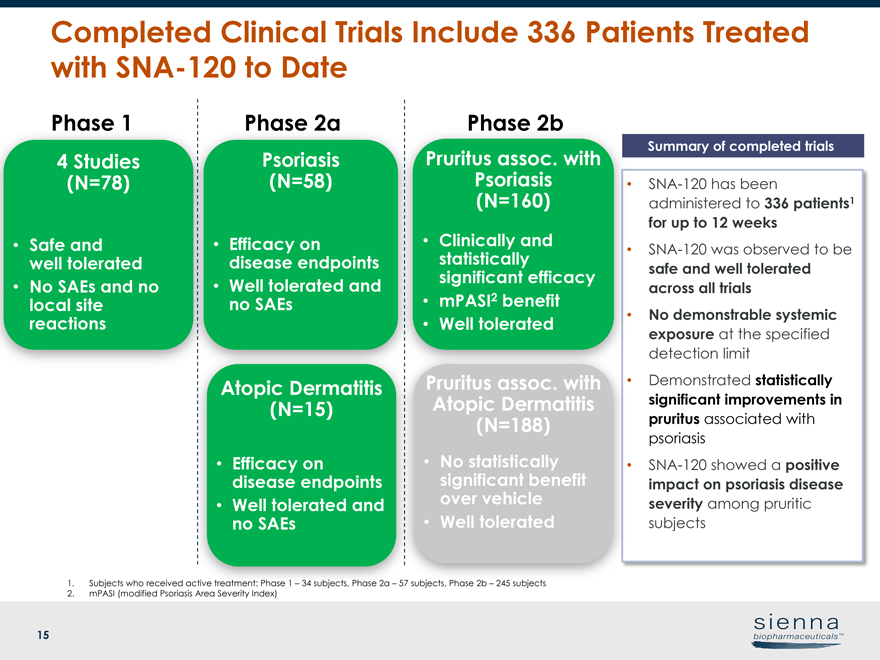 |
15 Completed Clinical Trials Include 336 Patients Treated withSNA-120 to Date •SNA-120 has been administered to 336 patients1 for up to 12 weeks •SNA-120 was observed to be safe and well tolerated across all trials • No demonstrable systemic exposure at the specified detection limit • Demonstrated statistically significant improvements in pruritus associated with psoriasis •SNA-120 showed a positive impact on psoriasis disease severity among pruritic subjects Summary of completed trials Phase 1 Phase 2a Phase 2b 4 Studies (N=78) • Safe and well tolerated • No SAEs and no local site reactions Psoriasis (N=58) • Efficacy on disease endpoints • Well tolerated and no SAEs Atopic Dermatitis (N=15) • Efficacy on disease endpoints • Well tolerated and no SAEs Pruritus assoc. with Psoriasis (N=160) • Clinically and statistically significant efficacy • mPASI2 benefit • Well tolerated Pruritus assoc. with Atopic Dermatitis (N=188) • No statistically significant benefit over vehicle • Well tolerated 1. Subjects who received active treatment: Phase 1 – 34 subjects, Phase 2a – 57 subjects, Phase 2b – 245 subjects 2. mPASI (modified Psoriasis Area Severity Index)
|
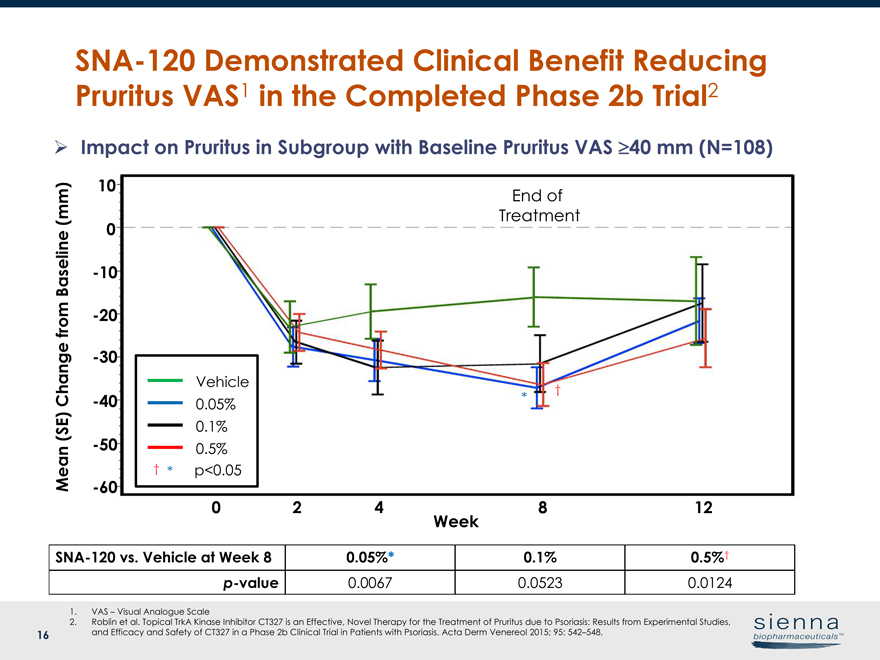 |
16SNA-120 Demonstrated Clinical Benefit Reducing Pruritus VAS1 in the Completed Phase 2b Trial2SNA-120 vs. Vehicle at Week 8 0.05%* 0.1% 0.5%†p-value 0.0067 0.0523 0.0124 Week 0 2 4 8 12 Mean (SE) Change from Baseline (mm) 0 10-10-20-30-40-50-60 Vehicle 0.05% 0.5% 0.1% * End of Treatment Ø Impact on Pruritus in Subgroup with Baseline Pruritus VAS ³40 mm (N=108) 1. VAS – Visual Analogue Scale 2. Roblin et al. Topical TrkA Kinase Inhibitor CT327 is an Effective, Novel Therapy for the Treatment of Pruritus due to Psoriasis: Results from Experimental Studies, and Efficacy and Safety of CT327 in a Phase 2b Clinical Trial in Patients with Psoriasis. Acta Derm Venereol 2015; 95: 542–548. † † * p<0.05
|
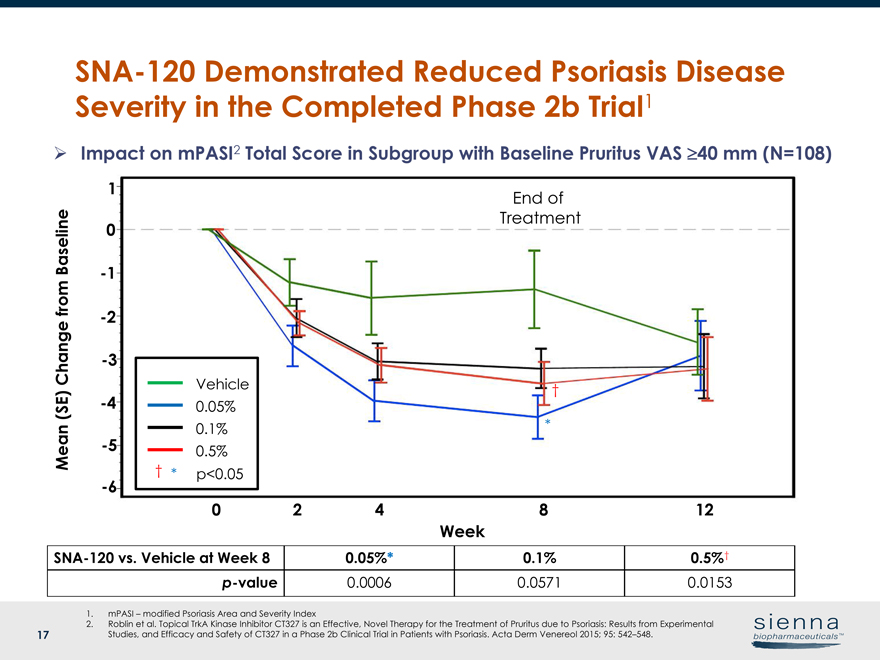 |
17SNA-120 Demonstrated Reduced Psoriasis Disease Severity in the Completed Phase 2b Trial1SNA-120 vs. Vehicle at Week 8 0.05%* 0.1% 0.5%†p-value 0.0006 0.0571 0.0153 Week 0 2 4 8 12 Mean (SE) Change from Baseline 0 1-1-2-3-4-5-6 Vehicle 0.05% 0.5% 0.1% * End of Treatment Ø Impact on mPASI2 Total Score in Subgroup with Baseline Pruritus VAS ³40 mm (N=108) 1. mPASI – modified Psoriasis Area and Severity Index 2. Roblin et al. Topical TrkA Kinase Inhibitor CT327 is an Effective, Novel Therapy for the Treatment of Pruritus due to Psoriasis: Results from Experimental Studies, and Efficacy and Safety of CT327 in a Phase 2b Clinical Trial in Patients with Psoriasis. Acta Derm Venereol 2015; 95: 542–548. † † * p<0.05
|
 |
18SNA-120 Exhibited a Favorable Safety Profile in Completed Clinical Studies1 •SNA-120 was not detected in blood samples down to a detection limit of 2.5 ng/mL – Consistent with local delivery and low systemic exposure • Low incidence of AEs, which were characterized as mostly mild or moderate • TEAEs2 occurred in33-55% of subjects in theSNA-120 group and 53% of subjects in the vehicle group • The most frequently reported AEs were pruritus, headache, nasopharyngitis and diarrhea • No drug-related application site AEs were observed • No drug-related serious AEs were observed 1. Data on file. Sienna Biopharmaceuticals. 2. TEAEs – Treatment Emergent Adverse Events
|
 |
19 Phase 2b Study forSNA-120 (ongoing) • Evaluate a refined target population • Demonstrate efficacy using the validated11-point itch Numeric Rating Scale Primary Objectives Patients Trial Design Endpoints Timing • Further evaluate impact on pruritus and psoriasis • At least moderate itch andmild-to-moderate psoriasis • Approximately 190 subjects • Multicenter, randomized, double-blind, placebo-controlled • Two doses: 0.05%, 0.5% • Topically twice daily for 12 weeks • Initiated 4Q 2017 •Top-line data expected in 1Q 2019 • Regulated as a drug pursuant to a new drug application (NDA) regulatory pathway Regulatory Pathway
|
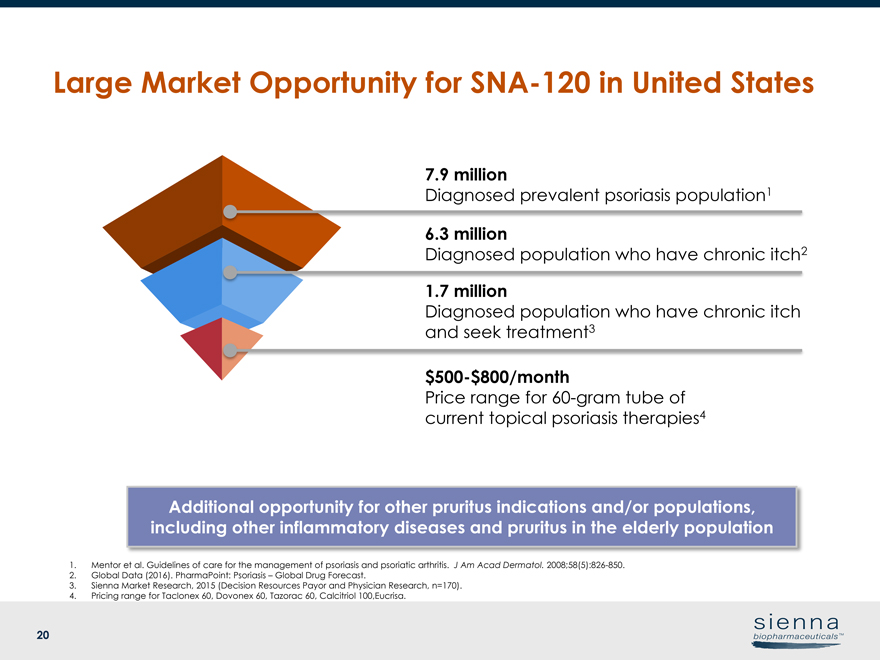 |
20 7.9 million Diagnosed prevalent psoriasis population1 6.3 million Diagnosed population who have chronic itch2 1.7 million Diagnosed population who have chronic itch and seek treatment3$500-$800/month Price range for60-gram tube of current topical psoriasis therapies4 1. Mentor et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. J Am Acad Dermatol.2008;58(5):826-850. 2. Global Data (2016). PharmaPoint: Psoriasis – Global Drug Forecast. 3. Sienna Market Research, 2015 (Decision Resources Payor and Physician Research, n=170). 4. Pricing range for Taclonex 60, Dovonex 60, Tazorac 60, Calcitriol 100,Eucrisa. Large Market Opportunity forSNA-120 in United States Additional opportunity for other pruritus indications and/or populations, including other inflammatory diseases and pruritus in the elderly population
|
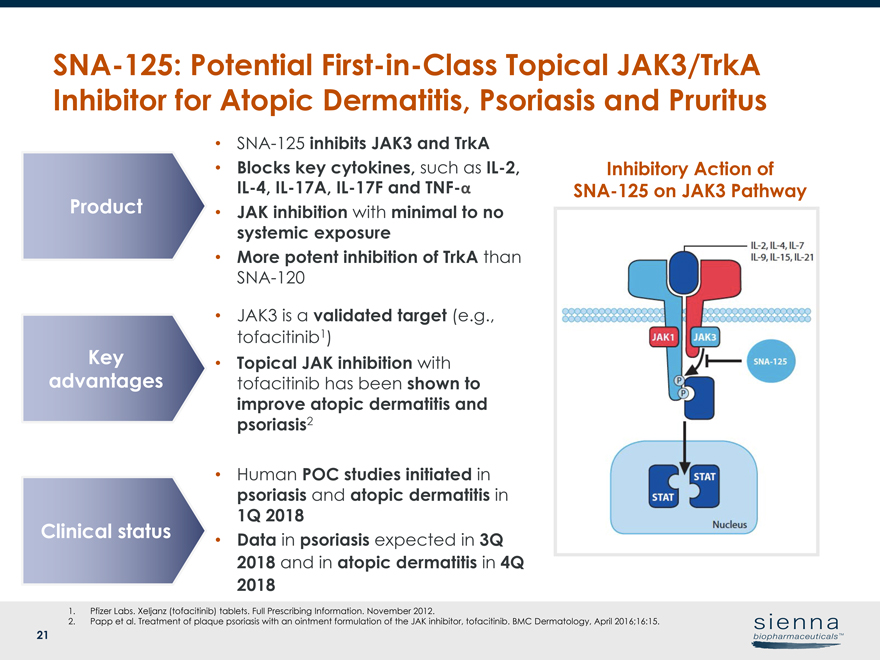 |
21SNA-125: PotentialFirst-in-Class Topical JAK3/TrkA Inhibitor for Atopic Dermatitis, Psoriasis and Pruritus 1. Pfizer Labs. Xeljanz (tofacitinib) tablets. Full Prescribing Information. November 2012. 2. Papp et al. Treatment of plaque psoriasis with an ointment formulation of the JAK inhibitor, tofacitinib. BMC Dermatology, April 2016;16:15. Inhibitory Action ofSNA-125 on JAK3 Pathway •SNA-125 inhibits JAK3 and TrkA • Blocks key cytokines, such asIL-2,IL-4,IL-17A,IL-17F andTNF-α • JAK inhibition with minimal to no systemic exposure • More potent inhibition of TrkA thanSNA-120 Product Key advantages Clinical status • JAK3 is a validated target (e.g., tofacitinib1) • Topical JAK inhibition with tofacitinib has been shown to improve atopic dermatitis and psoriasis2 • Human POC studies initiated in psoriasis and atopic dermatitis in 1Q 2018 • Data in psoriasis expected in 3Q 2018 and in atopic dermatitis in 4Q 2018
|
 |
22 • One of the most common skin diseases:2-3% of adults, up to 25% of children1 • In 2016, the prevalence of Atopic Dermatitis was estimated at 16 million in the United States2 • 75% of Atopic Dermatitis patients havemild-to-moderate disease2 • Large unmet need across the Atopic Dermatitis population – Few safe and effectivenon-steroidal options suitable for chronic use – New biologics will address only the more severe populations (dupilumab3) • Pediatric population has a need for steroid alternative – Safety concerns with steroids are very high in this population Atopic Dermatitis: Large Unmet Need for Safe TopicalNon-steroid Drug 1. Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014Feb;70(2):338-51. 2. Global Data. Pharmapoint: Atopic Dermatitis. November 2015. 3. Sanofi and Regeneron Pharmaceuticals. DUPIXENT (dupilumab) injection 300mg. Full Prescribing information. March 2017.
|
 |
23 • We are selectively pursuing the development of our current product candidatesSNA-120 andSNA-125 in additional indications • We have developed additional NCEs from our Topical by Design platform that have shown favorable data in early preclinical studies • We believe the Topical by Design technology may also address other therapeutic needs in which localized drug exposure is desirable, and are conducting preclinical work in ophthalmological, gastrointestinal and pulmonary conditions Topical by Design™ Early-Stage Pipeline
24 Topical Photoparticle TherapyTM Platform(SNA-001)
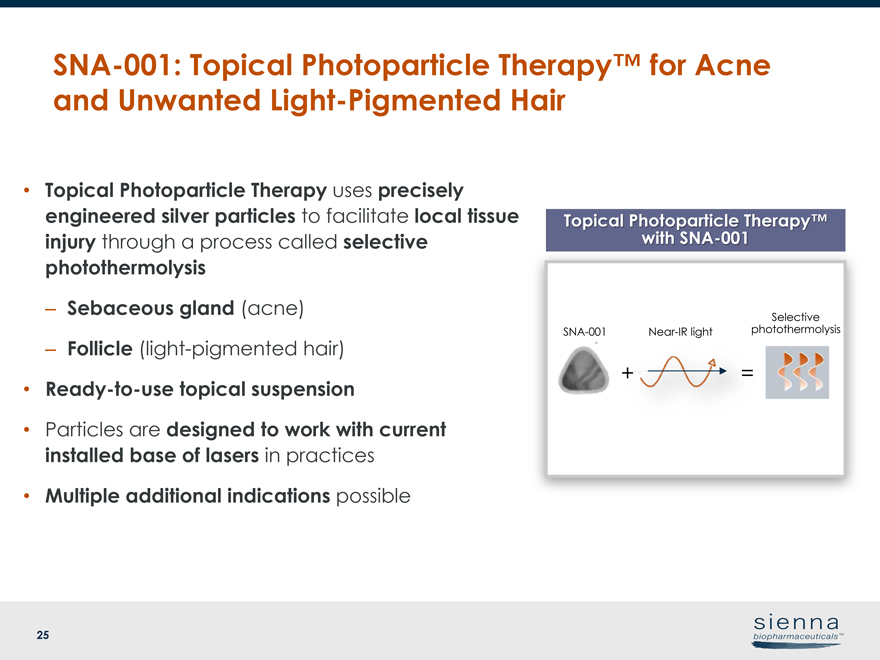
25 Topical Photoparticle Therapy™ withSNA-001 • Topical Photoparticle Therapy uses precisely engineered silver particles to facilitate local tissue injury through a process called selective photothermolysis – Sebaceous gland (acne) – Follicle (light-pigmented hair) •Ready-to-use topical suspension • Particles are designed to work with current installed base of lasers in practices • Multiple additional indications possibleSNA-001: Topical Photoparticle Therapy™ for Acne and Unwanted Light-Pigmented HairSNA-001 +Near-IR light = Selective photothermolysis
26SNA-001: A Potentially Disruptive Procedural Solution for AcneFirst-in-class, topical 1 treatment of acne 2 Favorable safety profile 3 Procedural solution with durable results PRODUCT VISION 4 Sparing the use of systemic medications (isotretinoin and antibiotics)
|
 |
27 • Current topical treatment options are limited by effectiveness, patient compliance and side effects1 • 50% do not achieve clearance in first 3 months of treatment2 •Non-facial acne is often treated with systemic therapies due to greater severity and inconvenience of applying topical therapies1 • Systemic therapies are limited due to patient, parent and physician concern about unwanted side effects • Accutane® (isotretinoin), which also targets the sebaceous gland, is highly effective but is associated with local and systemic side effects and is teratogenic3SNA-001: Opportunity to Address Current Acne Treatment Limitations 1. Global Data. Acne Vulgaris: Opportunity Analysis and Forecast to 2018. 2.SNA-001 Physician Demand Research. October 2016. 3. Roche Laboratories Inc. Accutane (isotretinoin) capsules. Full Prescribing information.
|
 |
28 Current Acne Treatment Paradigm Acne n Topical Therapies n Topical Therapies n Systemic Therapies Current Treatment Landscape Facial Acne —Mild-to-Moderate Disease Back Acne Facial Acne —Moderate-to-Severe Disease Procedural solutions have the potential to solve issues of patient compliance and provide safer alternatives to systemic therapy. n Topical Therapies n Systemic Therapies Topical therapies include: retinoids, benzoyl peroxide, antibiotics, combo therapy Systemic therapies include: antibiotics, hormonal treatments, and isotretinoin
|
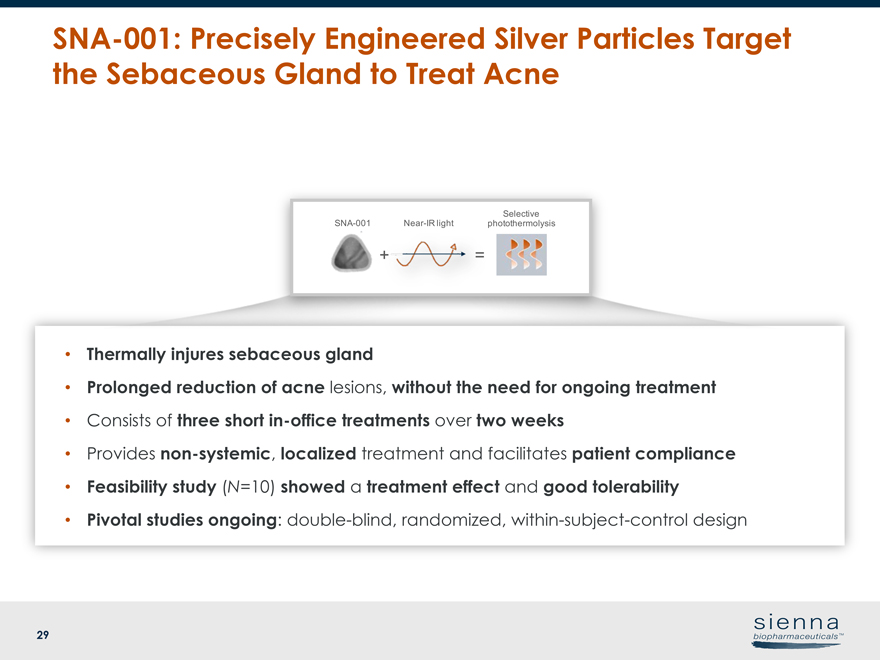 |
29SNA-001: Precisely Engineered Silver Particles Target the Sebaceous Gland to Treat Acne • Thermally injures sebaceous gland • Prolonged reduction of acne lesions, without the need for ongoing treatment • Consists of three shortin-office treatments over two weeks • Providesnon-systemic, localized treatment and facilitates patient compliance • Feasibility study (N=10) showed a treatment effect and good tolerability • Pivotal studies ongoing: double-blind, randomized, within-subject-control designSNA-001 +Near-IR light = Selective photothermolysis
|
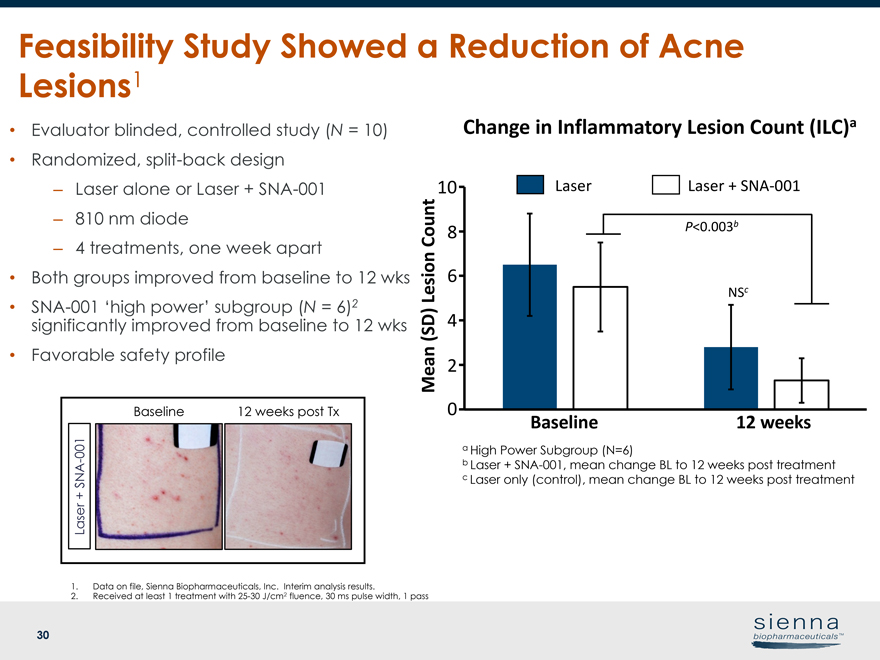 |
30 Feasibility Study Showed a Reduction of Acne Lesions1 • Evaluator blinded, controlled study (N = 10) • Randomized, split-back design – Laser alone or Laser +SNA-001 – 810 nm diode – 4 treatments, one week apart • Both groups improved from baseline to 12 wks •SNA-001 ‘high power’ subgroup (N = 6)2 significantly improved from baseline to 12 wks • Favorable safety profile Baseline 12 weeks post Tx Laser +SNA-001 1. Data on file, Sienna Biopharmaceuticals, Inc. Interim analysis results. 2. Received at least 1 treatment with25-30 J/cm2 fluence, 30 ms pulse width, 1 pass Change in Inflammatory Lesion Count (ILC)a Baseline 12 weeks P<0.003b NSc Mean (SD) Lesion Count a High Power Subgroup (N=6) b Laser +SNA-001, mean change BL to 12 weeks post treatment c Laser only (control), mean change BL to 12 weeks post treatment Laser Laser +SNA-001 6 0 8 10 4 2
|
 |
31 • Evaluate safety and efficacy ofSNA-001 followed by laser1 for the treatment of acne lesions in facial areas of subjects Primary Objectives Patients Trial Design Endpoints Timing1 • Percentage change in inflammatory lesion count from baseline through week 12 post final treatment • Patients with moderate or severe facial acne • Multicenter, randomized, double-blind, split-face study • Three treatments over two weeks • Ongoing •Top-line data expected in 3Q 2018 for 1064 nm and 810 nm and in 4Q 2018 for 755 nm 1. Three separate clinical trials are being conducted, using nearly identical protocols but different laser wavelengths corresponding to three commonly used commercial lasers, 755 nm, 810 nm and 1064 nm Acne Pivotal Studies forSNA-001 (ongoing) • Regulated as a Class II medical device under 510(k) marketing clearance pathway Regulatory Path
|
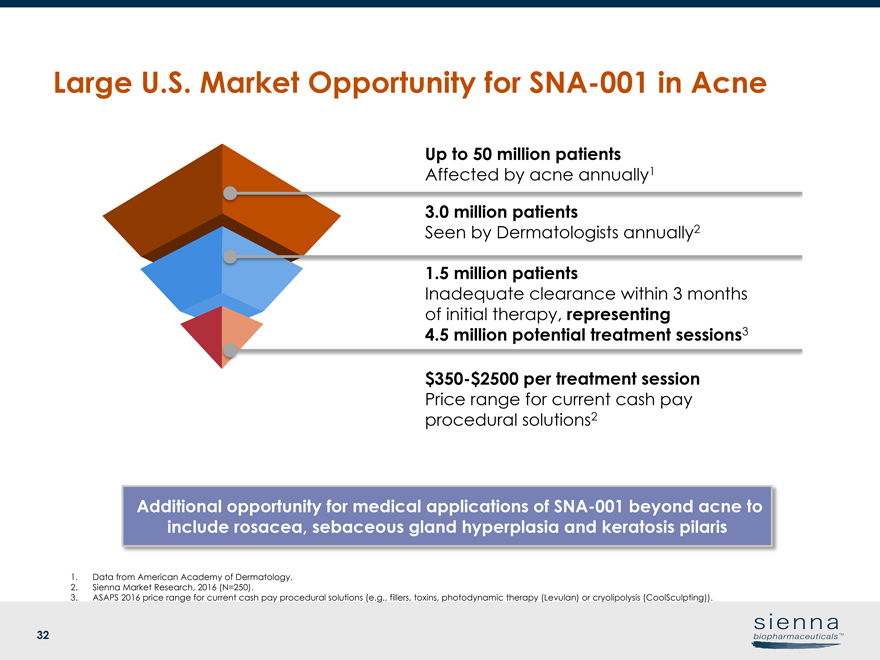 |
32 3.0 million patients Seen by Dermatologists annually2 1.5 million patients Inadequate clearance within 3 months of initial therapy, representing 4.5 million potential treatment sessions3$350-$2500 per treatment session Price range for current cash pay procedural solutions2 1. Data from American Academy of Dermatology. 2. Sienna Market Research, 2016 (N=250). 3. ASAPS 2016 price range for current cash pay procedural solutions (e.g., fillers, toxins, photodynamic therapy (Levulan) or cryolipolysis (CoolSculpting)). Large U.S. Market Opportunity forSNA-001 in Acne Additional opportunity for medical applications ofSNA-001 beyond acne to include rosacea, sebaceous gland hyperplasia and keratosis pilaris Up to 50 million patients Affected by acne annually1way
|
 |
33SNA-001: An Opportunity to Expand the Market for Laser Hair Removal First effective hair removal solution for light-pigmented hair 1 2 Safe, similar to conventional laser hair removal treatment 3 Works with existing installed base of lasers and all skin and hair types PRODUCT VISION 4 Aligned with physicians’ interest in new procedural cash pay solutions
|
 |
34 • Laser hair removal is the highest volume aesthetic procedure performed globally1 • Nevertheless, laser hair removal is largely ineffective for patients with light pigmented hair (i.e., white, gray, blond, light brown and light red)2 • As a result, patients are often turned away by healthcare practitioners if their hair color is too lightly pigmented • Some patients who are treated anyway see insufficient benefit due to light hair or a mixture of dark and light hair types and are dissatisfied SNA 001 Designed to Overcome Current Limitations Available for Light Pigmented Hair Removal 1. ASAPS Stats 2016 / Medical Insights – Laser Hair Removal Market Analysis. December 2014. 2. American Society for Laser Medicine & Surgery. Laser Hair Removal. http://www.aslms.org/for the public/treatments using lasers and energy baseddevices/ laser hair removal
|
 |
35SNA-001: Precisely Engineered Silver Particles Target the Hair Follicles to Reduce Light-Pigmented Hair • Thermally injures hair follicle for permanent reduction of unwanted, light-pigmented hair • Number and timing of treatment sessions are same as current laser hair removal treatment – Sixin-office treatments spaced6-8 weeks apart • Light-pigmented hair lacks adequate pigment to be efficiently targeted by traditional lasers • Feasibility study (N=10) showed treatment effect and good tolerability • Pivotal study ongoing: double-blind, randomized, within-subject-controlledSNA-001 +Near-IR light = Selective photothermolysis
|
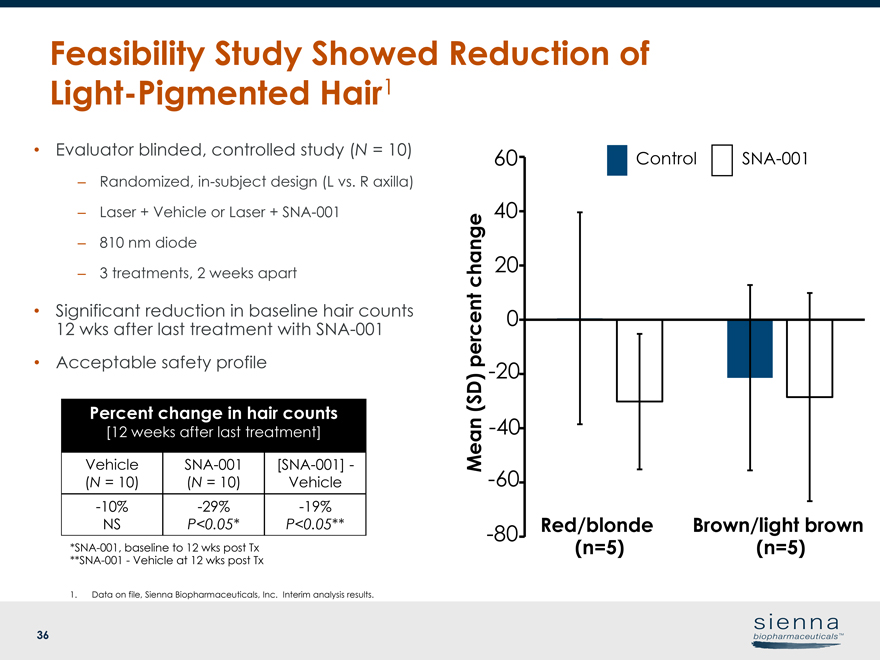 |
36 Feasibility Study Showed Reduction of Light-Pigmented Hair1 1. Data on file, Sienna Biopharmaceuticals, Inc. Interim analysis results.*SNA-001, baseline to 12 wks post Tx**SNA-001—Vehicle at 12 wks post Tx • Evaluator blinded, controlled study (N = 10) – Randomized,in-subject design (L vs. R axilla) – Laser + Vehicle or Laser +SNA-001 – 810 nm diode – 3 treatments, 2 weeks apart Percent change in hair counts [12 weeks after last treatment] Vehicle (N = 10)SNA-001 (N = 10)[SNA-001]— Vehicle-10% NS-29% P<0.05*-19% P<0.05** • Significant reduction in baseline hair counts 12 wks after last treatment withSNA-001 • Acceptable safety profile Mean (SD) percent change ControlSNA-001 Brown/light brown (n=5) Red/blonde-80 (n=5)-60-40-20 0 20 40 60
|
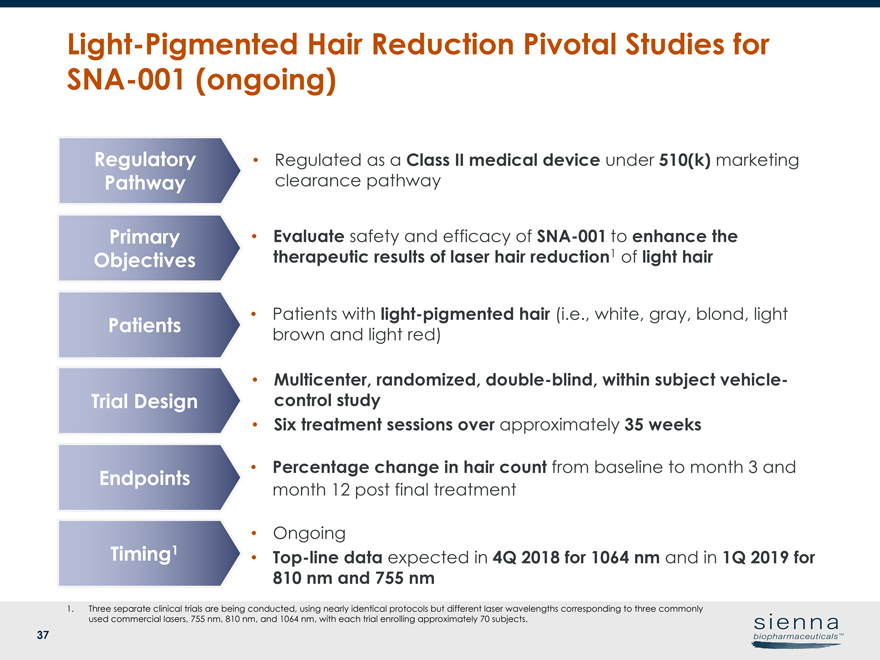 |
37 Light-Pigmented Hair Reduction Pivotal Studies forSNA-001 (ongoing) • Evaluate safety and efficacy ofSNA-001 to enhance the therapeutic results of laser hair reduction1 of light hair Primary Objectives Patients Trial Design Endpoints Timing1 • Percentage change in hair count from baseline to month 3 and month 12 post final treatment • Patients with light-pigmented hair (i.e., white, gray, blond, light brown and light red) • Multicenter, randomized, double-blind, within subject vehiclecontrol study • Six treatment sessions over approximately 35 weeks • Ongoing •Top-line data expected in 4Q 2018 for 1064 nm and in 1Q 2019 for 810 nm and 755 nm 1. Three separate clinical trials are being conducted, using nearly identical protocols but different laser wavelengths corresponding to three commonly used commercial lasers, 755 nm, 810 nm, and 1064 nm, with each trial enrolling approximately 70 subjects. • Regulated as a Class II medical device under 510(k) marketing clearance pathway Regulatory Pathway
|
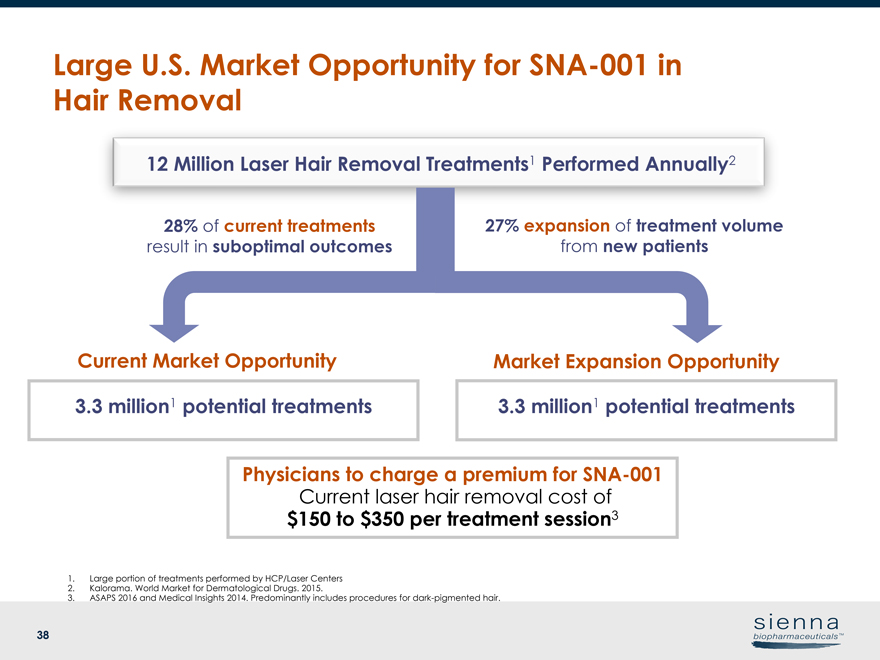 |
38 Large U.S. Market Opportunity forSNA-001 in Hair Removal 3.3 million1 potential treatments 27% expansion of treatment volume from new patients 3.3 million1 potential treatments 28% of current treatments result in suboptimal outcomes 1. Large portion of treatments performed by HCP/Laser Centers 2. Kalorama. World Market for Dermatological Drugs. 2015. 3. ASAPS 2016 and Medical Insights 2014. Predominantly includes procedures for dark-pigmented hair. 12 Million Laser Hair Removal Treatments1 Performed Annually2 Current Market Opportunity Market Expansion Opportunity Physicians to charge a premium forSNA-001 Current laser hair removal cost of $150 to $350 per treatment session3
|
 |
39 • Sienna has worldwide commercial rights toSNA-001,SNA-120 andSNA-125, with U.S. patent protection to at least 2030 • Plan to build specialized medical, sales and marketing infrastructure for U.S. • Evaluate partnerships to maximize the potential value in certain channels and geographies • Balance a diversified, multi-asset portfolio and commercial investment for long-term value creation Maximizing the Global Commercial Potential of Our Product Candidates
|
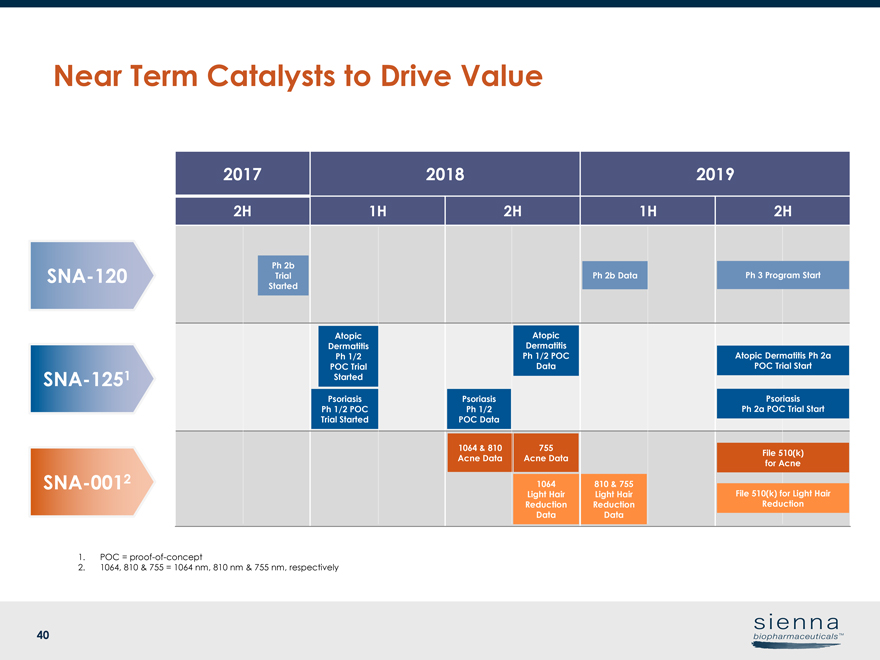 |
40 2017 2018 2019 2H 1H 2H 1H 2H 1064 & 810 Acne Data 1064 Light Hair Reduction Data Atopic Dermatitis Ph 1/2 POC Data Ph 2b Trial StartedSNA-120SNA-1251SNA-0012 Psoriasis Ph 1/2 POC Data Ph 2b Data Psoriasis Ph 1/2 POC Trial Started Atopic Dermatitis Ph 1/2 POC Trial Started 755 Acne Data 810 & 755 Light Hair Reduction Data 1. POC =proof-of-concept 2. 1064, 810 & 755 = 1064 nm, 810 nm & 755 nm, respectively Ph 3 Program Start Atopic Dermatitis Ph 2a POC Trial Start Psoriasis Ph 2a POC Trial Start File 510(k) for Acne File 510(k) for Light Hair Reduction Near Term Catalysts to Drive Value
|
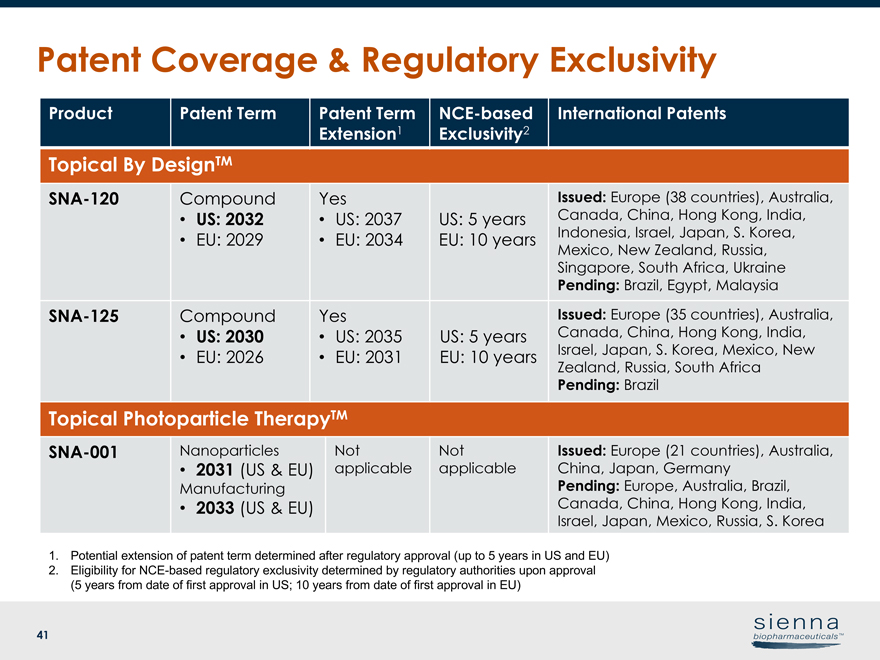 |
41 Patent Coverage & Regulatory Exclusivity Product Patent Term Patent Term Extension1NCE-based Exclusivity2 International Patents Topical By DesignTMSNA-120 Compound • US: 2032 • EU: 2029 Yes • US: 2037 • EU: 2034 US: 5 years EU: 10 years Issued: Europe (38 countries), Australia, Canada, China, Hong Kong, India, Indonesia, Israel, Japan, S. Korea, Mexico, New Zealand, Russia, Singapore, South Africa, Ukraine Pending: Brazil, Egypt, MalaysiaSNA-125 Compound • US: 2030 • EU: 2026 Yes • US: 2035 • EU: 2031 US: 5 years EU: 10 years Issued: Europe (35 countries), Australia, Canada, China, Hong Kong, India, Israel, Japan, S. Korea, Mexico, New Zealand, Russia, South Africa Pending: Brazil Topical Photoparticle TherapyTMSNA-001 Nanoparticles • 2031 (US & EU) Manufacturing • 2033 (US & EU) Not applicable Not applicable Issued: Europe (21 countries), Australia, China, Japan, Germany Pending: Europe, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan, Mexico, Russia, S. Korea 1. Potential extension of patent term determined after regulatory approval (up to 5 years in US and EU) 2. Eligibility forNCE-based regulatory exclusivity determined by regulatory authorities upon approval (5 years from date of first approval in US; 10 years from date of first approval in EU)
|
 |
42 The Sienna Opportunity Addressing the Innovation Gap in Medical Dermatology Proprietary Platforms and Multiple Clinical-Stage Assets Large Markets Accomplished Management Team