
SNA-120 Phase 2b Trial Top-Line Results December 3, 2018 March 2017 Exhibit 99.2
Forward Looking Statements This presentation contains forward-looking statements. All statements other than descriptions of historical facts contained in this presentation, including statements regarding future operational and financial results and positions, business strategy, prospective products, potential market, commercial opportunity and market share, availability and potential sources of funding, clinical trial results, product approvals and regulatory pathways, research and development costs, timing (including but not limited to clinical development and regulatory timelines), strategies for completion and likelihood of success for our business activities, and plans for future operations, are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Such risks and uncertainties include, among others, those inherent in the preclinical and clinical development process and the regulatory approval process; the risks and uncertainties in commercialization and gaining market acceptance; the risks associated with protecting and defending our patents or other proprietary rights; the risk that our proprietary rights may be insufficient to protect our product candidates; the risk that we will be unable to obtain necessary capital when needed on acceptable terms or at all; competition from other products or procedures; our reliance on third-parties to conduct our clinical and non-clinical trials; and our reliance on single-source third-party suppliers to manufacture clinical, non-clinical and any future commercial supplies of our product candidates. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission and our future current and periodic reports. Except as required by applicable law, we assume no obligation to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
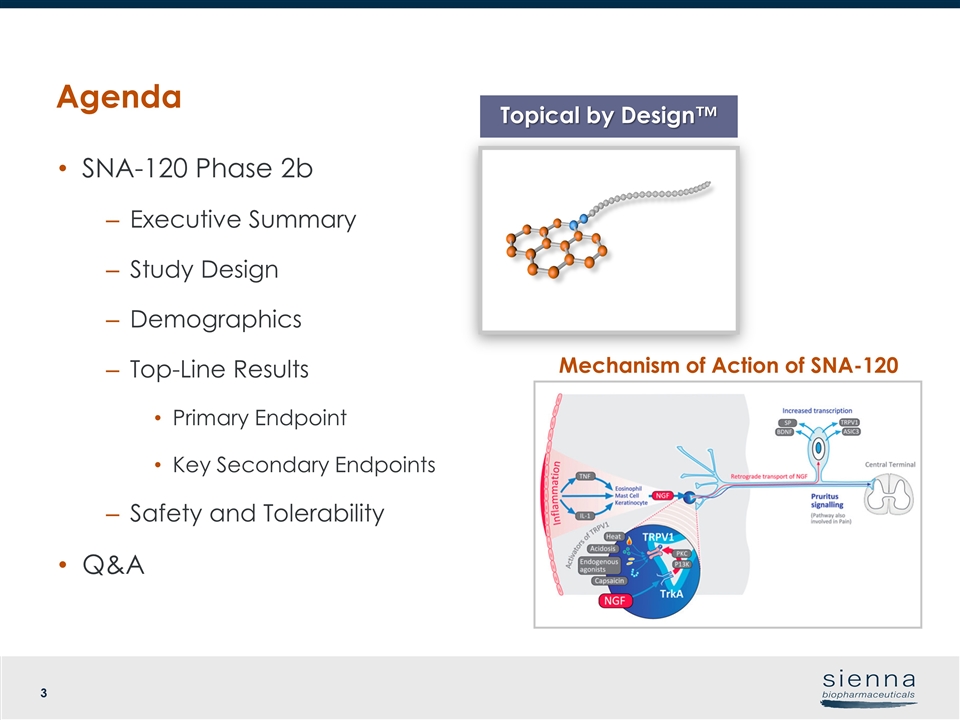
Agenda SNA-120 Phase 2b Executive Summary Study Design Demographics Top-Line Results Primary Endpoint Key Secondary Endpoints Safety and Tolerability Q&A Topical by Design™ Mechanism of Action of SNA-120
These data were not entirely as we would have expected We began this study with data suggesting we had a pruritus drug with a positive impact on psoriasis We now have data indicating we have a potential psoriasis drug with a positive impact on pruritus We had a substantial 58% reduction on itch in the primary endpoint, but it was not statistically different from Vehicle However, we had a substantial and statistically significant response on the two key psoriasis regulatory endpoints, PASI 751 and the composite IGA2, that was much stronger than we had expected The lower dose (0.05%) performed consistently better than the higher dose (0.5%) SNA-120 Phase 2b Trial Executive Summary PASI 75 = reduction of ≥ 75% in Psoriasis Area Severity Index score from baseline at Week 12 Composite IGA = proportion of subjects categorized as ‘clear’ or ‘almost clear’ on the Investigator Global Assessment and a minimum two-grade improvement from baseline at Week 12

We now have data in 500+ subjects treated with SNA-120, demonstrating that it is well tolerated and has an acceptable safety profile, and further validating our Topical by Design™ platform We intend to have an End-of-Phase 2 meeting with the FDA and then begin Phase 3 trials for psoriasis in 2H19 We believe the commercial opportunity is now significantly larger SNA-120 Phase 2b Trial Executive Summary (cont’d)
Multicenter, randomized, double-blind, Vehicle-controlled study Enrolled 208 subjects over the age of 18 with mild-to-moderate psoriasis and at least moderate itch Treated each subject twice daily for 12 weeks with either SNA-120 (0.05% or 0.5% ointment) or Vehicle Primary endpoint: Change in previous week’s mean daily scores in pruritus recorded on the I-NRS1 from baseline to Week 8 Key secondary regulatory endpoints: Proportion of subjects categorized as ‘clear’ or ‘almost clear’ on the IGA and a minimum two-grade improvement from baseline at Week 12 PASI 75: response defined as a reduction of ≥ 75% from baseline at Week 12 SNA-120 Phase 2b Study Design I-NRS = Itch Numeric Rating Scale

Refined population in this study: Included only itchy subjects with at least moderate itch (> 5 on the I-NRS) Excluded severe psoriasis subjects Longer treatment duration: 12 weeks (instead of 8 weeks) Only two SNA-120 doses: High (0.5%) and low (0.05%) concentrations from previous study I-NRS daily diary as primary itch measure (instead of itch VAS1) Important Changes from Previous Phase 2b Study VAS = Visual Analog Scale
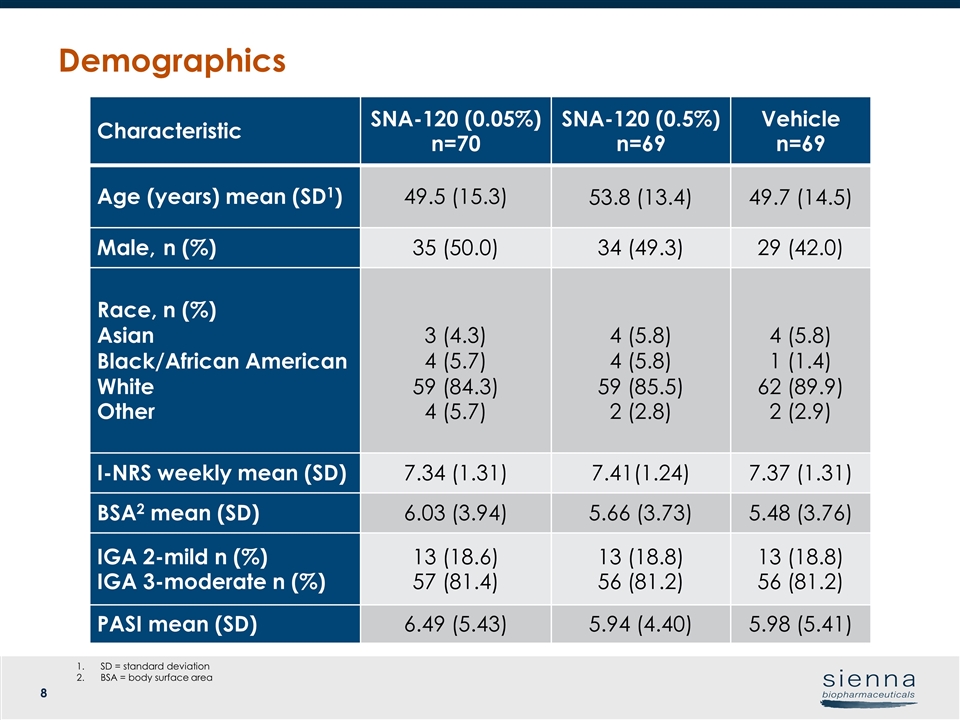
Characteristic SNA-120 (0.05%) n=70 SNA-120 (0.5%) n=69 Vehicle n=69 Age (years) mean (SD1) 49.5 (15.3) 53.8 (13.4) 49.7 (14.5) Male, n (%) 35 (50.0) 34 (49.3) 29 (42.0) Race, n (%) Asian Black/African American White Other 3 (4.3) 4 (5.7) 59 (84.3) 4 (5.7) 4 (5.8) 4 (5.8) 59 (85.5) 2 (2.8) 4 (5.8) 1 (1.4) 62 (89.9) 2 (2.9) I-NRS weekly mean (SD) 7.34 (1.31) 7.41(1.24) 7.37 (1.31) BSA2 mean (SD) 6.03 (3.94) 5.66 (3.73) 5.48 (3.76) IGA 2-mild n (%) IGA 3-moderate n (%) 13 (18.6) 57 (81.4) 13 (18.8) 56 (81.2) 13 (18.8) 56 (81.2) PASI mean (SD) 6.49 (5.43) 5.94 (4.40) 5.98 (5.41) Demographics SD = standard deviation BSA = body surface area
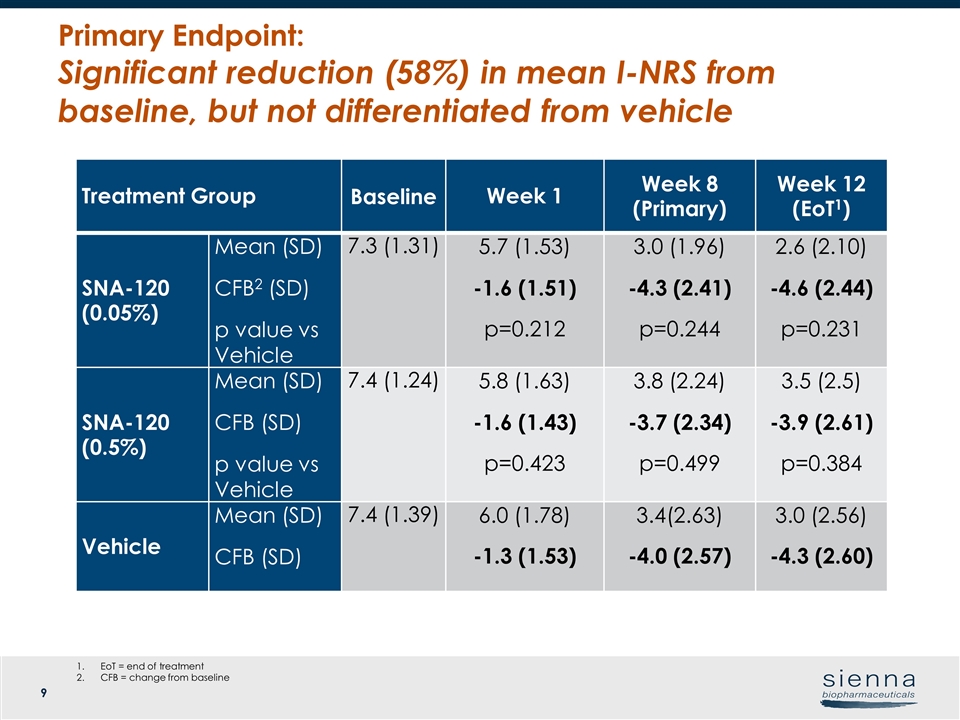
Treatment Group Baseline Week 1 Week 8 (Primary) Week 12 (EoT1) SNA-120 (0.05%) Mean (SD) CFB2 (SD) p value vs Vehicle 7.3 (1.31) 5.7 (1.53) -1.6 (1.51) p=0.212 3.0 (1.96) -4.3 (2.41) p=0.244 2.6 (2.10) -4.6 (2.44) p=0.231 SNA-120 (0.5%) Mean (SD) CFB (SD) p value vs Vehicle 7.4 (1.24) 5.8 (1.63) -1.6 (1.43) p=0.423 3.8 (2.24) -3.7 (2.34) p=0.499 3.5 (2.5) -3.9 (2.61) p=0.384 Vehicle Mean (SD) CFB (SD) 7.4 (1.39) 6.0 (1.78) -1.3 (1.53) 3.4(2.63) -4.0 (2.57) 3.0 (2.56) -4.3 (2.60) Primary Endpoint: Significant reduction (58%) in mean I-NRS from baseline, but not differentiated from vehicle EoT = end of treatment CFB = change from baseline
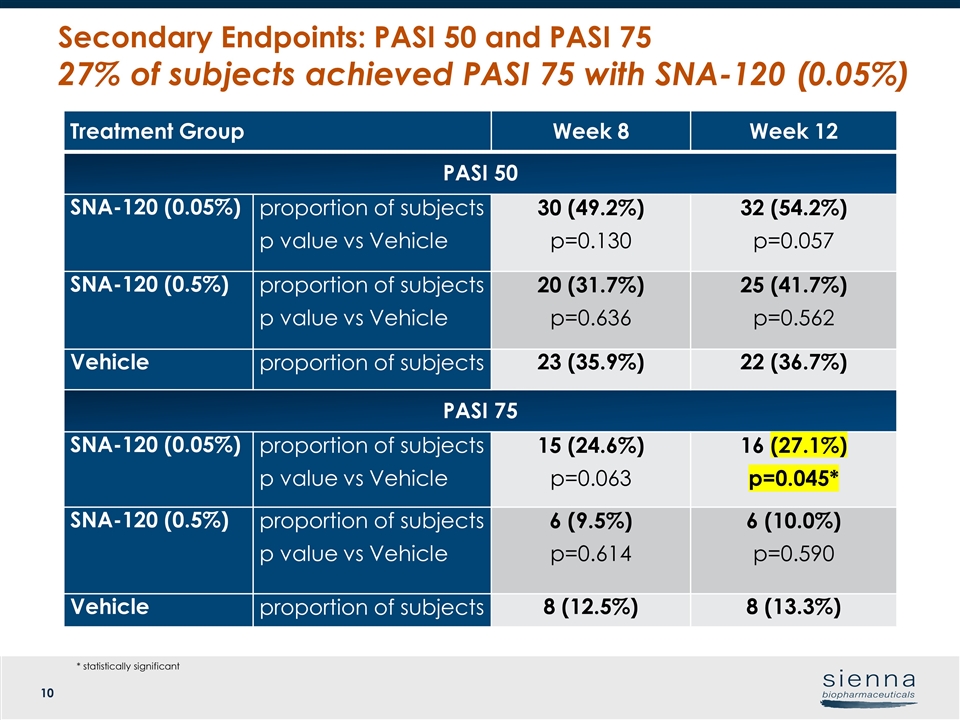
Treatment Group Week 8 Week 12 PASI 50 SNA-120 (0.05%) proportion of subjects p value vs Vehicle 30 (49.2%) p=0.130 32 (54.2%) p=0.057 SNA-120 (0.5%) proportion of subjects p value vs Vehicle 20 (31.7%) p=0.636 25 (41.7%) p=0.562 Vehicle proportion of subjects 23 (35.9%) 22 (36.7%) PASI 75 SNA-120 (0.05%) proportion of subjects p value vs Vehicle 15 (24.6%) p=0.063 16 (27.1%) p=0.045* SNA-120 (0.5%) proportion of subjects p value vs Vehicle 6 (9.5%) p=0.614 6 (10.0%) p=0.590 Vehicle proportion of subjects 8 (12.5%) 8 (13.3%) Secondary Endpoints: PASI 50 and PASI 75 27% of subjects achieved PASI 75 with SNA-120 (0.05%) * statistically significant
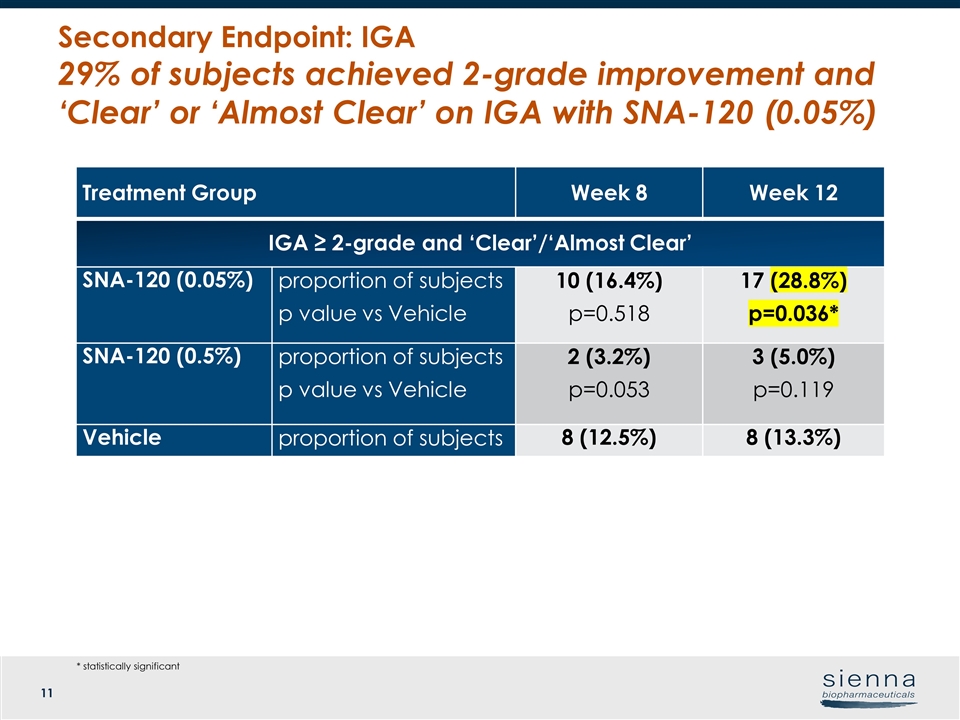
Treatment Group Week 8 Week 12 IGA ≥ 2-grade and ‘Clear’/‘Almost Clear’ SNA-120 (0.05%) proportion of subjects p value vs Vehicle 10 (16.4%) p=0.518 17 (28.8%) p=0.036* SNA-120 (0.5%) proportion of subjects p value vs Vehicle 2 (3.2%) p=0.053 3 (5.0%) p=0.119 Vehicle proportion of subjects 8 (12.5%) 8 (13.3%) Secondary Endpoint: IGA 29% of subjects achieved 2-grade improvement and ‘Clear’ or ‘Almost Clear’ on IGA with SNA-120 (0.05%) * statistically significant
Treatment-related AEs were observed in only 2 subjects and included dermatitis (0.5% group) and pain and pruritus (Vehicle group) Most common AEs (≥ 2 subjects) in any group were nasopharyngitis, nausea, diarrhea, cellulitis and urinary tract infection Majority of TEAEs2 were mild to moderate There were 6 serious AEs in 3 subjects, but none were considered drug related SNA-120 was well-tolerated with no serious treatment-related AEs1 AEs = adverse events TEAEs = treatment-emergent adverse events We now have data in 500+ subjects treated with SNA-120, demonstrating that it is well tolerated and has an acceptable safety profile, and further validating our Topical by Design™ platform
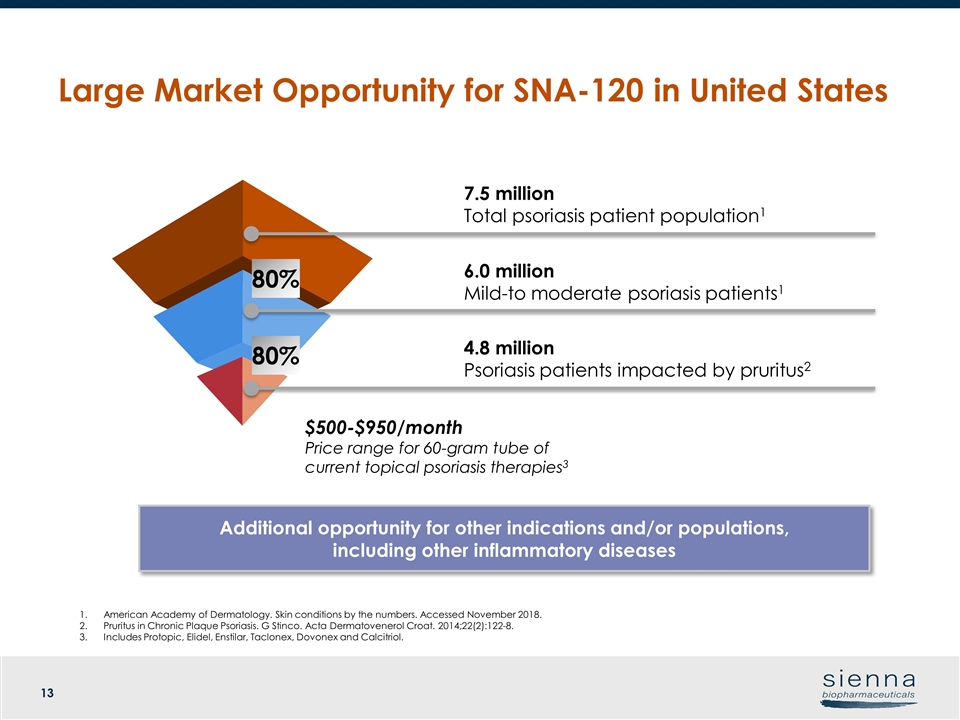
7.5 million Total psoriasis patient population1 6.0 million Mild-to moderate psoriasis patients1 $500-$950/month Price range for 60-gram tube of current topical psoriasis therapies3 American Academy of Dermatology. Skin conditions by the numbers. Accessed November 2018. Pruritus in Chronic Plaque Psoriasis. G Stinco. Acta Dermatovenerol Croat. 2014;22(2):122-8. Includes Protopic, Elidel, Enstilar, Taclonex, Dovonex and Calcitriol. Large Market Opportunity for SNA-120 in United States Additional opportunity for other indications and/or populations, including other inflammatory diseases 4.8 million Psoriasis patients impacted by pruritus2 80% 80%
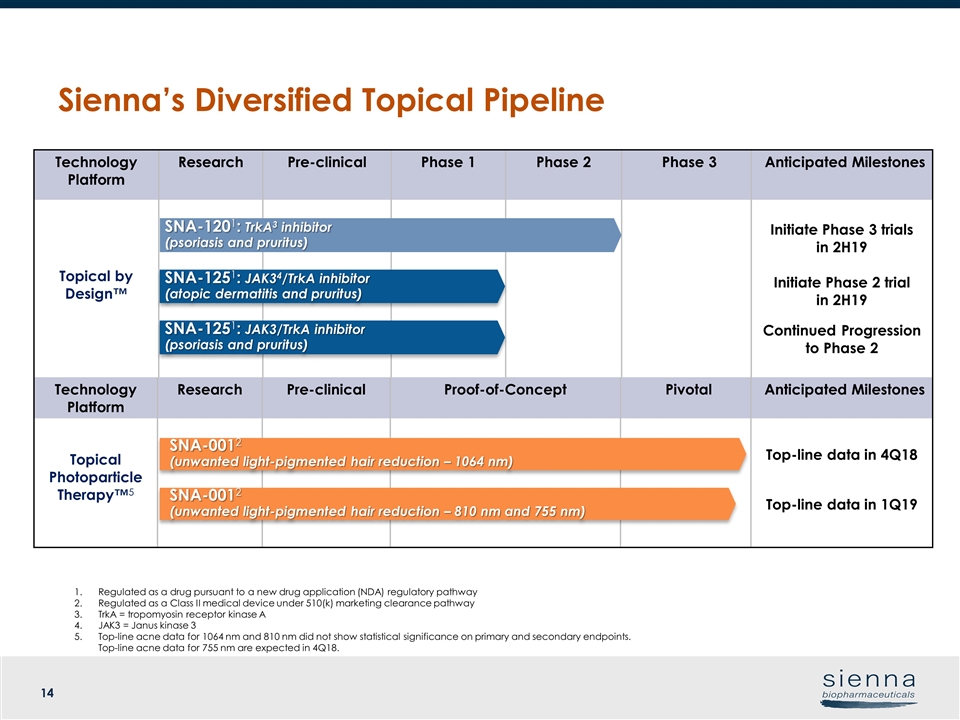
Regulated as a drug pursuant to a new drug application (NDA) regulatory pathway Regulated as a Class II medical device under 510(k) marketing clearance pathway TrkA = tropomyosin receptor kinase A JAK3 = Janus kinase 3 Top-line acne data for 1064 nm and 810 nm did not show statistical significance on primary and secondary endpoints. Top-line acne data for 755 nm are expected in 4Q18. Technology Platform Research Pre-clinical Phase 1 Phase 2 Phase 3 Anticipated Milestones Topical by Design™ Initiate Phase 3 trials in 2H19 Initiate Phase 2 trial in 2H19 Continued Progression to Phase 2 Technology Platform Research Pre-clinical Proof-of-Concept Pivotal Anticipated Milestones Topical Photoparticle Therapy™5 Top-line data in 4Q18 Top-line data in 1Q19 SNA-1201: TrkA3 inhibitor (psoriasis and pruritus) SNA-1251: JAK3/TrkA inhibitor (psoriasis and pruritus) SNA-0012 (unwanted light-pigmented hair reduction – 1064 nm) SNA-1251: JAK34/TrkA inhibitor (atopic dermatitis and pruritus) SNA-0012 (unwanted light-pigmented hair reduction – 810 nm and 755 nm) Sienna’s Diversified Topical Pipeline













