
Company Overview January 2019 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements. All statements other than descriptions of historical facts contained in this presentation, including statements regarding future operational and financial results and positions, business strategy, prospective products, potential market, commercial opportunity and market share, availability and potential sources of funding, clinical trial results, product approvals and regulatory pathways, research and development costs, timing (including but not limited to clinical development and regulatory timelines), strategies for completion and likelihood of success for our business activities, and plans for future operations, are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Such risks and uncertainties include, among others, those inherent in the preclinical and clinical development process and the regulatory approval process; the risks and uncertainties in commercialization and gaining market acceptance; the risks associated with protecting and defending our patents or other proprietary rights; the risk that our proprietary rights may be insufficient to protect our product candidates; the risk that we will be unable to obtain necessary capital when needed on acceptable terms or at all; competition from other products or procedures; our reliance on third-parties to conduct our clinical and non-clinical trials; and our reliance on single-source third-party suppliers to manufacture clinical, non-clinical and any future commercial supplies of our product candidates. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission and any subsequent current and periodic reports. Except as required by applicable law, we assume no obligation to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
The Sienna® Opportunity Addressing the Innovation Gap in Medical Dermatology Accomplished, Credible Management Team Recent advances in dermatology have mostly benefited the more severe patient populations Opportunity to leverage biotechnology and well-understood pathways to develop novel, targeted topical products Unmet need for topical non-steroidal therapies suitable for chronic use Additional opportunities in inflammation and immunology to treat unmet medical needs while minimizing systemic exposure Track record with top brands and integral involvement with multiple FDA approvals Previous leadership roles
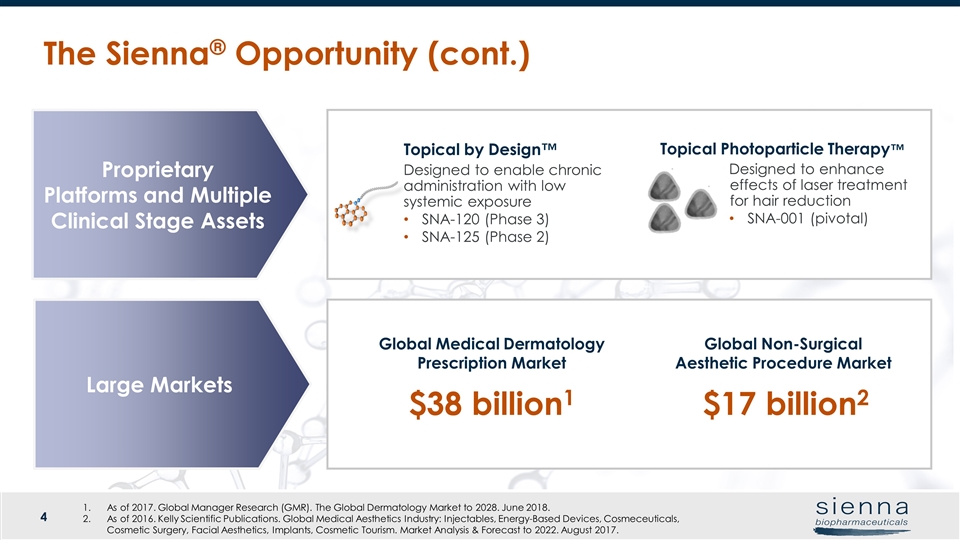
The Sienna® Opportunity (cont.) Proprietary Platforms and Multiple Clinical Stage Assets Large Markets Global Medical Dermatology Prescription Market Global Non-Surgical Aesthetic Procedure Market Topical by Design™ Topical Photoparticle Therapy™ Designed to enable chronic administration with low systemic exposure SNA-120 (Phase 3) SNA-125 (Phase 2) Designed to enhance effects of laser treatment for hair reduction SNA-001 (pivotal) $38 billion1 $17 billion2 As of 2017. Global Manager Research (GMR). The Global Dermatology Market to 2028. June 2018. As of 2016. Kelly Scientific Publications. Global Medical Aesthetics Industry: Injectables, Energy-Based Devices, Cosmeceuticals, Cosmetic Surgery, Facial Aesthetics, Implants, Cosmetic Tourism. Market Analysis & Forecast to 2022. August 2017.

Regulated as a drug pursuant to a new drug application (NDA) regulatory pathway. Regulated as a Class II medical device under 510(k) marketing clearance pathway. TrkA = tropomyosin receptor kinase A JAK3 = Janus kinase 3 Top-line acne data for 1064 nm and 810 nm did not show statistical significance on primary and secondary endpoints. Top-line acne data for 755 nm are expected in late January or early February 2019. Technology Platform Research Pre-clinical Phase 1 Phase 2 Phase 3 Anticipated Milestones Topical by Design™ Initiate Phase 3 trial in 2H19 Initiate Phase 2 trial Continued Progression to Phase 2 Technology Platform Research Pre-clinical Proof-of-Concept Pivotal Anticipated Milestones Topical Photoparticle Therapy™5 Top-line data in late January or early February 2019 SNA-1201: TrkA3 inhibitor (psoriasis and pruritus) SNA-1251: JAK3/TrkA inhibitor (psoriasis and pruritus) SNA-0012 (unwanted light-pigmented hair reduction – 1064 nm, 810 nm and 755 nm) SNA-1251: JAK34/TrkA inhibitor (atopic dermatitis and pruritus) Sienna’s Diversified Topical Pipeline
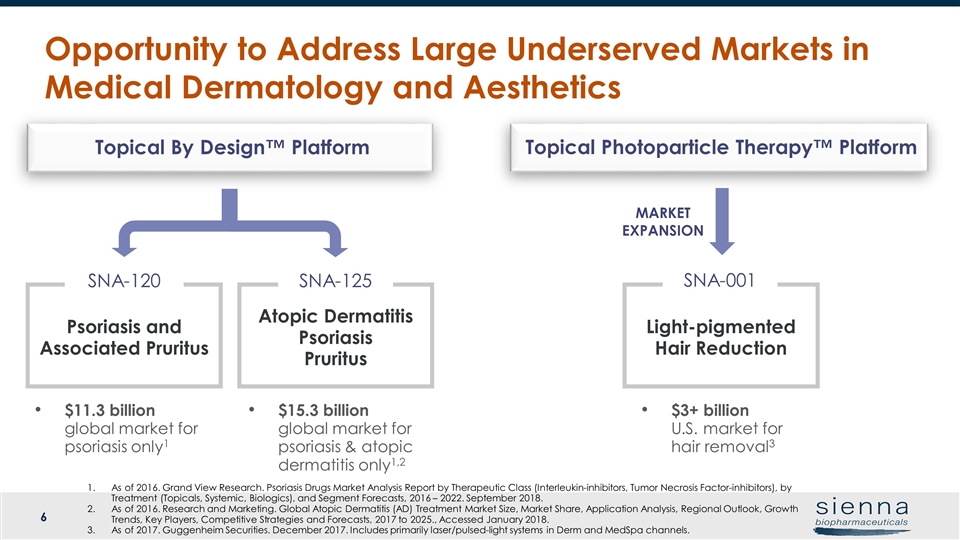
Opportunity to Address Large Underserved Markets in Medical Dermatology and Aesthetics Atopic Dermatitis Psoriasis Pruritus SNA-125 Psoriasis and Associated Pruritus SNA-120 Topical By Design™ Platform Topical Photoparticle Therapy™ Platform Light-pigmented Hair Reduction MARKET EXPANSION $3+ billion U.S. market for hair removal3 SNA-001 $15.3 billion global market for psoriasis & atopic dermatitis only1,2 $11.3 billion global market for psoriasis only1 As of 2016. Grand View Research. Psoriasis Drugs Market Analysis Report by Therapeutic Class (Interleukin-inhibitors, Tumor Necrosis Factor-inhibitors), by Treatment (Topicals, Systemic, Biologics), and Segment Forecasts, 2016 – 2022. September 2018. As of 2016. Research and Marketing. Global Atopic Dermatitis (AD) Treatment Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies and Forecasts, 2017 to 2025., Accessed January 2018. As of 2017. Guggenheim Securities. December 2017. Includes primarily laser/pulsed-light systems in Derm and MedSpa channels.

Topical by Design™ Platform (SNA-120 & SNA-125)
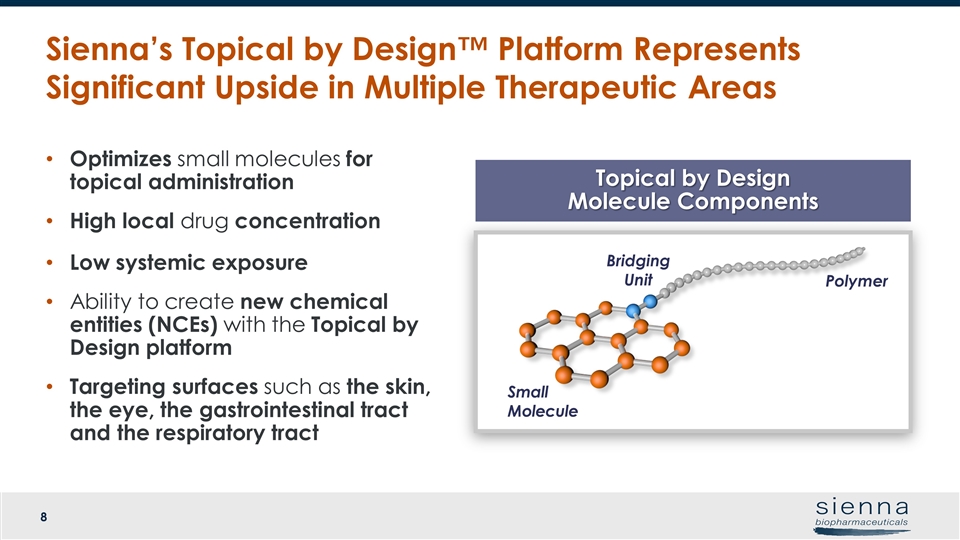
Topical by Design Molecule Components Optimizes small molecules for topical administration High local drug concentration Low systemic exposure Ability to create new chemical entities (NCEs) with the Topical by Design platform Targeting surfaces such as the skin, the eye, the gastrointestinal tract and the respiratory tract Sienna’s Topical by Design™ Platform Represents Significant Upside in Multiple Therapeutic Areas Polymer Small Molecule Bridging Unit
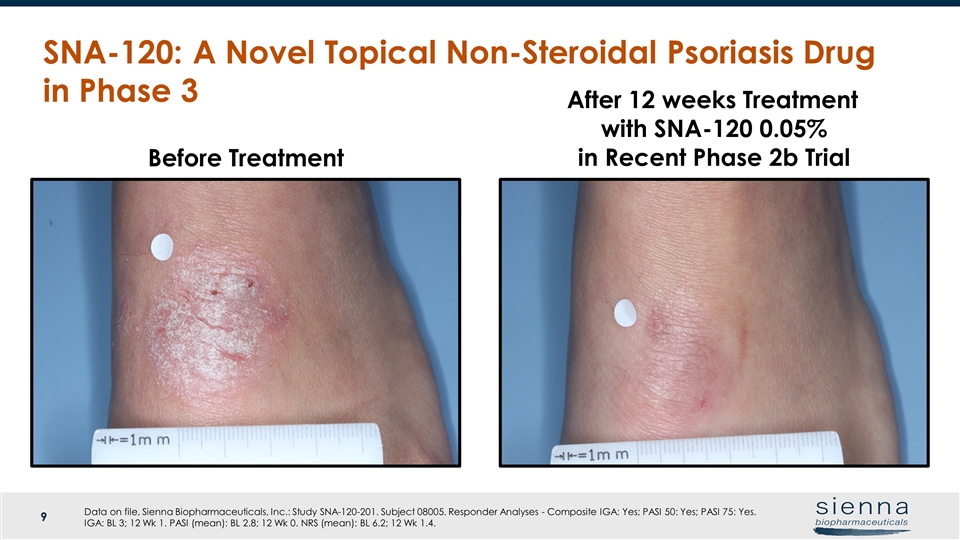
SNA-120: A Novel Topical Non-Steroidal Psoriasis Drug in Phase 3 Before Treatment After 12 weeks Treatment with SNA-120 0.05% in Recent Phase 2b Trial Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201. Subject 08005. Responder Analyses - Composite IGA: Yes; PASI 50: Yes; PASI 75: Yes. IGA: BL 3; 12 Wk 1. PASI (mean): BL 2.8; 12 Wk 0. NRS (mean): BL 6.2; 12 Wk 1.4.
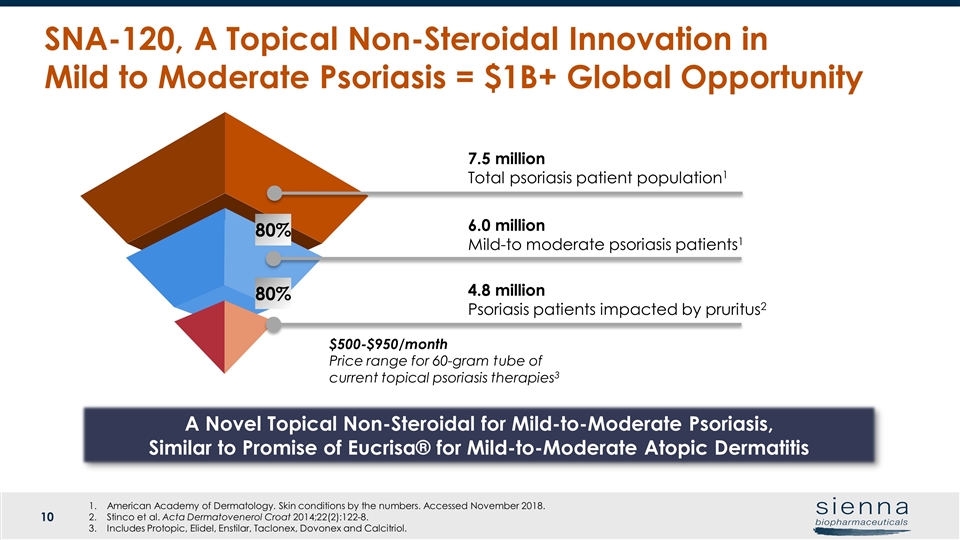
7.5 million Total psoriasis patient population1 6.0 million Mild-to moderate psoriasis patients1 $500-$950/month Price range for 60-gram tube of current topical psoriasis therapies3 American Academy of Dermatology. Skin conditions by the numbers. Accessed November 2018. Stinco et al. Acta Dermatovenerol Croat 2014;22(2):122-8. Includes Protopic, Elidel, Enstilar, Taclonex, Dovonex and Calcitriol. SNA-120, A Topical Non-Steroidal Innovation in Mild to Moderate Psoriasis = $1B+ Global Opportunity A Novel Topical Non-Steroidal for Mild-to-Moderate Psoriasis, Similar to Promise of Eucrisa® for Mild-to-Moderate Atopic Dermatitis 4.8 million Psoriasis patients impacted by pruritus2 80% 80%
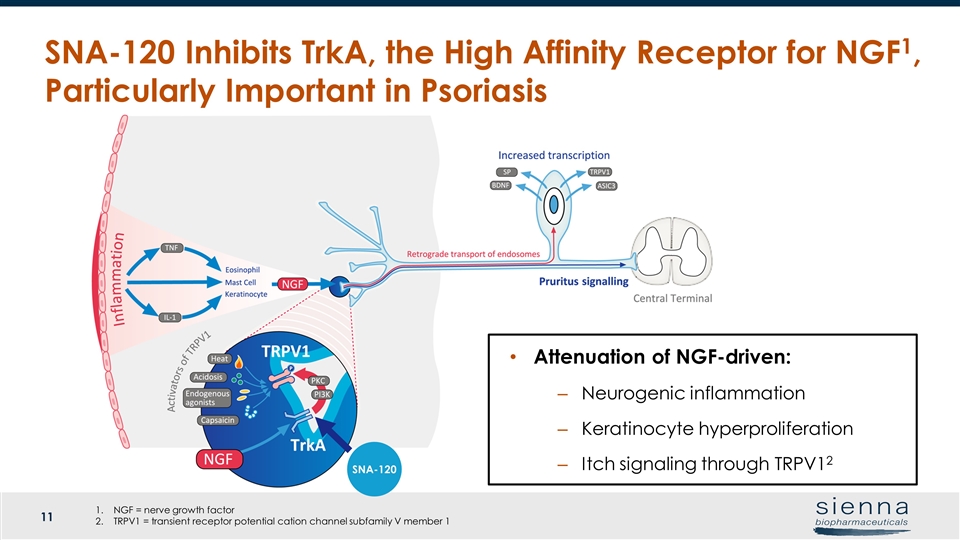
SNA-120 Inhibits TrkA, the High Affinity Receptor for NGF1, Particularly Important in Psoriasis SNA-120 Attenuation of NGF-driven: Neurogenic inflammation Keratinocyte hyperproliferation Itch signaling through TRPV12 NGF = nerve growth factor TRPV1 = transient receptor potential cation channel subfamily V member 1
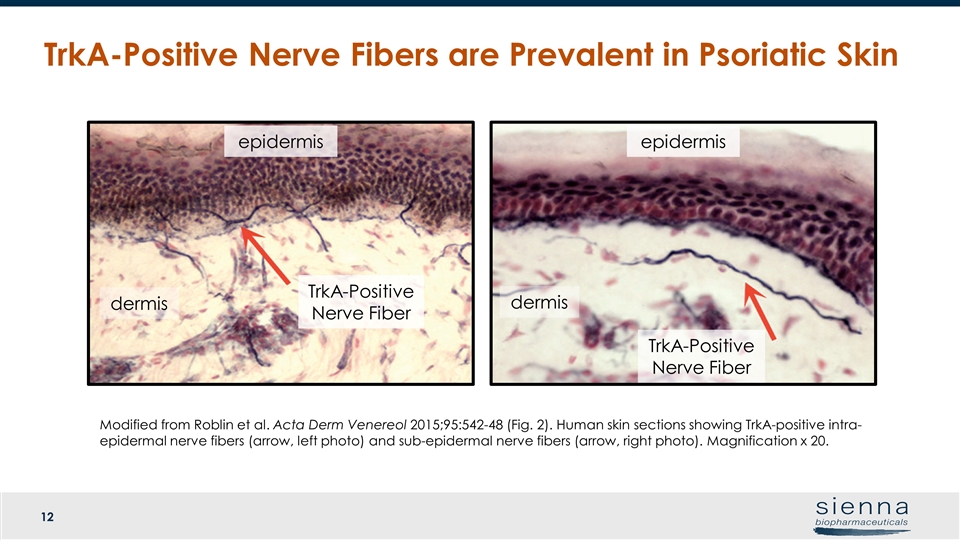
TrkA-Positive Nerve Fibers are Prevalent in Psoriatic Skin epidermis dermis TrkA-Positive Nerve Fiber TrkA-Positive Nerve Fiber epidermis dermis Modified from Roblin et al. Acta Derm Venereol 2015;95:542-48 (Fig. 2). Human skin sections showing TrkA-positive intra-epidermal nerve fibers (arrow, left photo) and sub-epidermal nerve fibers (arrow, right photo). Magnification x 20.
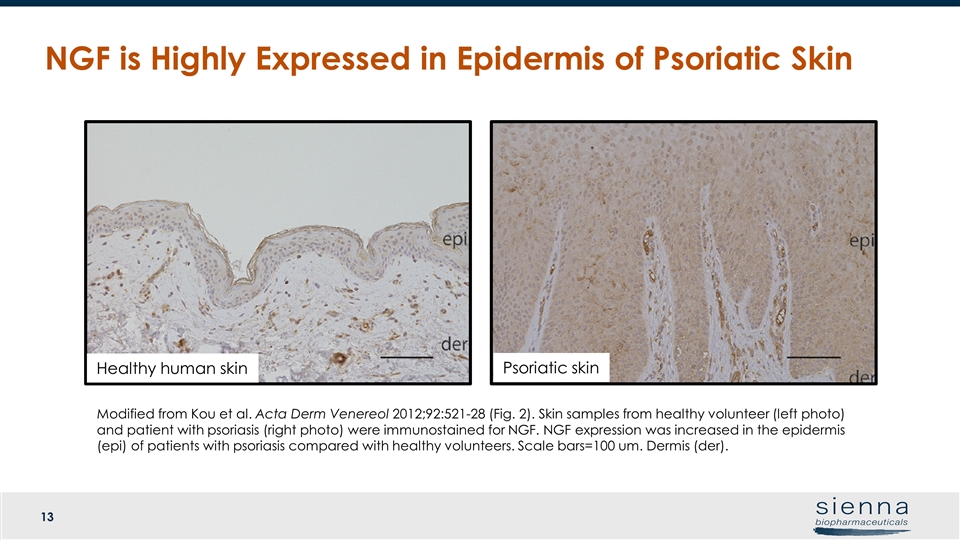
NGF is Highly Expressed in Epidermis of Psoriatic Skin Healthy human skin Psoriatic skin Modified from Kou et al. Acta Derm Venereol 2012;92:521-28 (Fig. 2). Skin samples from healthy volunteer (left photo) and patient with psoriasis (right photo) were immunostained for NGF. NGF expression was increased in the epidermis (epi) of patients with psoriasis compared with healthy volunteers. Scale bars=100 um. Dermis (der).
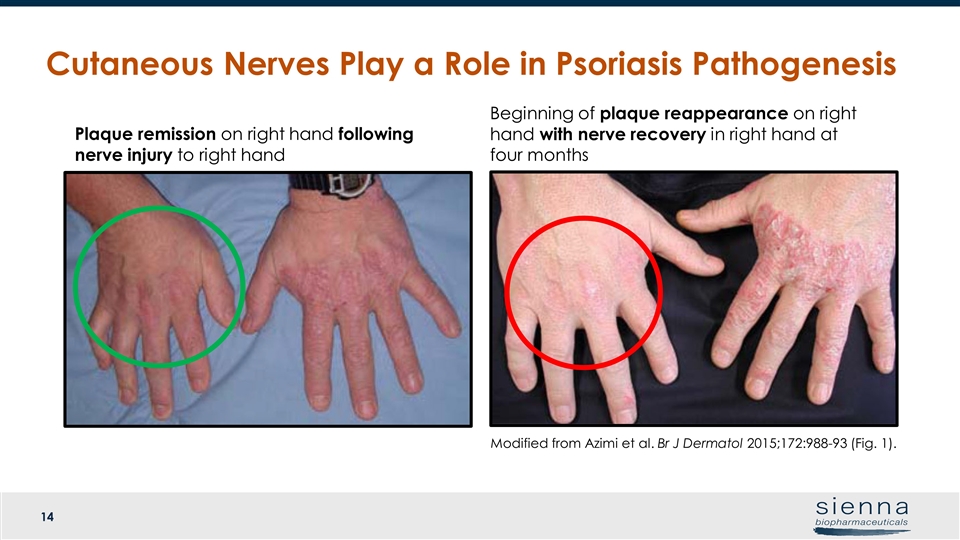
Cutaneous Nerves Play a Role in Psoriasis Pathogenesis Plaque remission on right hand following nerve injury to right hand Beginning of plaque reappearance on right hand with nerve recovery in right hand at four months Modified from Azimi et al. Br J Dermatol 2015;172:988-93 (Fig. 1).
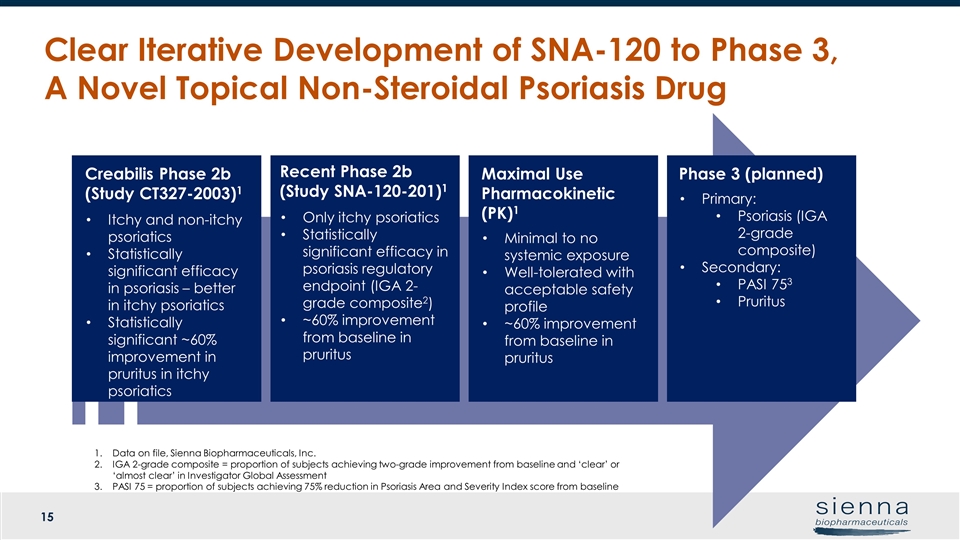
Clear Iterative Development of SNA-120 to Phase 3, A Novel Topical Non-Steroidal Psoriasis Drug Recent Phase 2b (Study SNA-120-201)1 Only itchy psoriatics Statistically significant efficacy in psoriasis regulatory endpoint (IGA 2-grade composite2) ~60% improvement from baseline in pruritus Maximal Use Pharmacokinetic (PK)1 Minimal to no systemic exposure Well-tolerated with acceptable safety profile ~60% improvement from baseline in pruritus Phase 3 (planned) Primary: Psoriasis (IGA 2-grade composite) Secondary: PASI 753 Pruritus Creabilis Phase 2b (Study CT327-2003)1 Itchy and non-itchy psoriatics Statistically significant efficacy in psoriasis – better in itchy psoriatics Statistically significant ~60% improvement in pruritus in itchy psoriatics Data on file, Sienna Biopharmaceuticals, Inc. IGA 2-grade composite = proportion of subjects achieving two-grade improvement from baseline and ‘clear’ or ‘almost clear’ in Investigator Global Assessment PASI 75 = proportion of subjects achieving 75% reduction in Psoriasis Area and Severity Index score from baseline
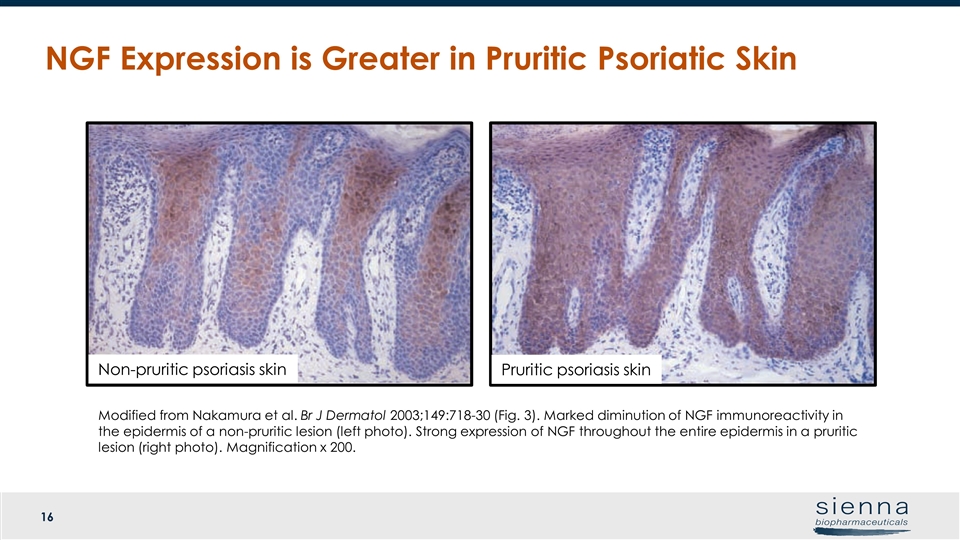
NGF Expression is Greater in Pruritic Psoriatic Skin Pruritic psoriasis skin Non-pruritic psoriasis skin Modified from Nakamura et al. Br J Dermatol 2003;149:718-30 (Fig. 3). Marked diminution of NGF immunoreactivity in the epidermis of a non-pruritic lesion (left photo). Strong expression of NGF throughout the entire epidermis in a pruritic lesion (right photo). Magnification x 200.
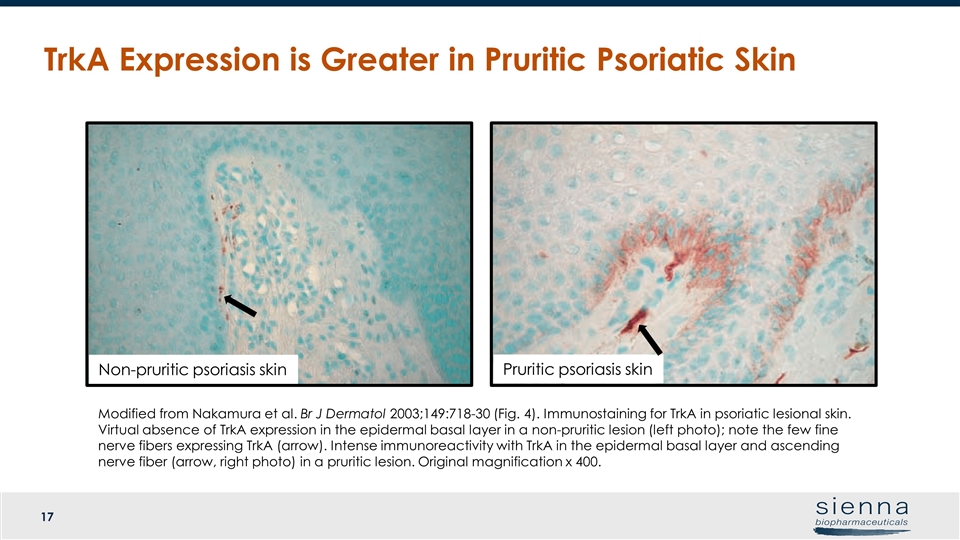
TrkA Expression is Greater in Pruritic Psoriatic Skin Modified from Nakamura et al. Br J Dermatol 2003;149:718-30 (Fig. 4). Immunostaining for TrkA in psoriatic lesional skin. Virtual absence of TrkA expression in the epidermal basal layer in a non-pruritic lesion (left photo); note the few fine nerve fibers expressing TrkA (arrow). Intense immunoreactivity with TrkA in the epidermal basal layer and ascending nerve fiber (arrow, right photo) in a pruritic lesion. Original magnification x 400. Pruritic psoriasis skin Non-pruritic psoriasis skin
SNA-120 improved both psoriasis and pruritus Greater impact in itchy patients on both psoriasis and pruritus Pruritus may be a clinical biomarker for NGF-mediated psoriasis, which SNA-120 affects as a TrkA inhibitor Treatment effect appeared to increase with time Low dose appeared similar to or more effective than high dose Very well-tolerated, with no detectable drug in blood Key Learnings from CT327-2003 Study Data on file, Sienna Biopharmaceuticals, Inc.
Confirm positive effect on both psoriasis and pruritus in larger and longer (12 week) Phase 2b study Pruritus as primary endpoint based on CT327-2003 data FDA prefers I-NRS1 (versus I-VAS2) as primary measure for pruritus Needed to test I-NRS measure before Phase 3 Demonstrate maximum impact on psoriasis Included only itchy patients Excluded patients with severe psoriasis Extended treatment period to 12 weeks Confirm low dose as optimal for both psoriasis and pruritus Rationale Behind SNA-120-201 Study Design I-NRS = itch numeric rating scale I-VAS = itch visual analog scale

Multicenter, randomized, double-blind, placebo-controlled study N = 208 ³18 years of age Mild-to-moderate psoriasis At least moderate pruritus Twice daily treatment for 12 weeks with either SNA-120 (0.05% or 0.5%) or Vehicle SNA-120-201 Study Design
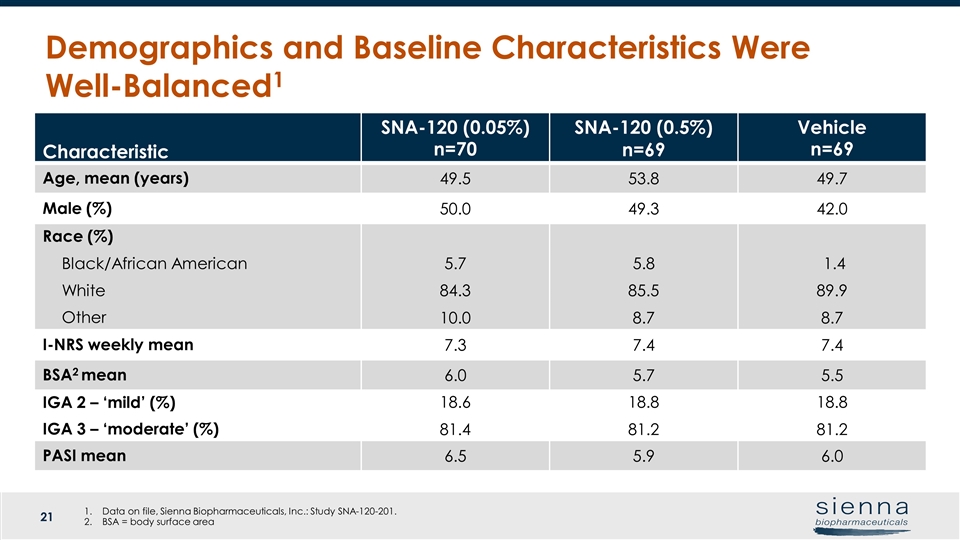
Characteristic SNA-120 (0.05%) n=70 SNA-120 (0.5%) n=69 Vehicle n=69 Age, mean (years) 49.5 53.8 49.7 Male (%) 50.0 49.3 42.0 Race (%) Black/African American White Other 5.7 84.3 10.0 5.8 85.5 8.7 1.4 89.9 8.7 I-NRS weekly mean 7.3 7.4 7.4 BSA2 mean 6.0 5.7 5.5 IGA 2 – ‘mild’ (%) IGA 3 – ‘moderate’ (%) 18.6 81.4 18.8 81.2 18.8 81.2 PASI mean 6.5 5.9 6.0 Demographics and Baseline Characteristics Were Well-Balanced1 Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201. BSA = body surface area
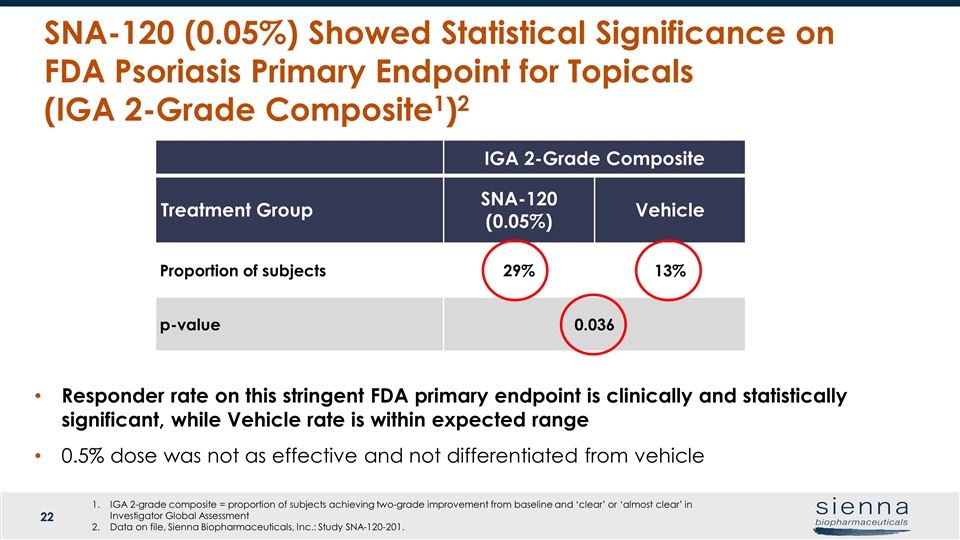
SNA-120 (0.05%) Showed Statistical Significance on FDA Psoriasis Primary Endpoint for Topicals (IGA 2-Grade Composite1)2 Treatment Group Week 8 Week 12 PASI 50 SNA-120 (0.05%) Proportion of subjects P value vs vehicle 30 (49.2%) p=0.130 32 (54.2%) p=0.057 SNA-120 (0.5%) Proportion of subjects P value vs vehicle 20 (31.7%) p=0.636 25 (41.7%) p=0.562 Vehicle Proportion of subjects 23 (35.9%) 22 (36.7%) PASI 75 SNA-120 (0.05%) Proportion of subjects P value vs vehicle 15 (24.6%) p=0.063 16 (27.1%) p=0.045* SNA-120 (0.5%) Proportion of subjects P value vs vehicle 6 (9.5%) p=0.614 6 (10.0%) p=0.590 Vehicle Proportion of subjects 8 (12.5%) 8 (13.3%) IGA 2-Grade Composite Treatment Group SNA-120 (0.05%) Vehicle Proportion of subjects 29% 13% p-value 0.036 Responder rate on this stringent FDA primary endpoint is clinically and statistically significant, while Vehicle rate is within expected range 0.5% dose was not as effective and not differentiated from vehicle IGA 2-grade composite = proportion of subjects achieving two-grade improvement from baseline and ‘clear’ or ‘almost clear’ in Investigator Global Assessment Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201.
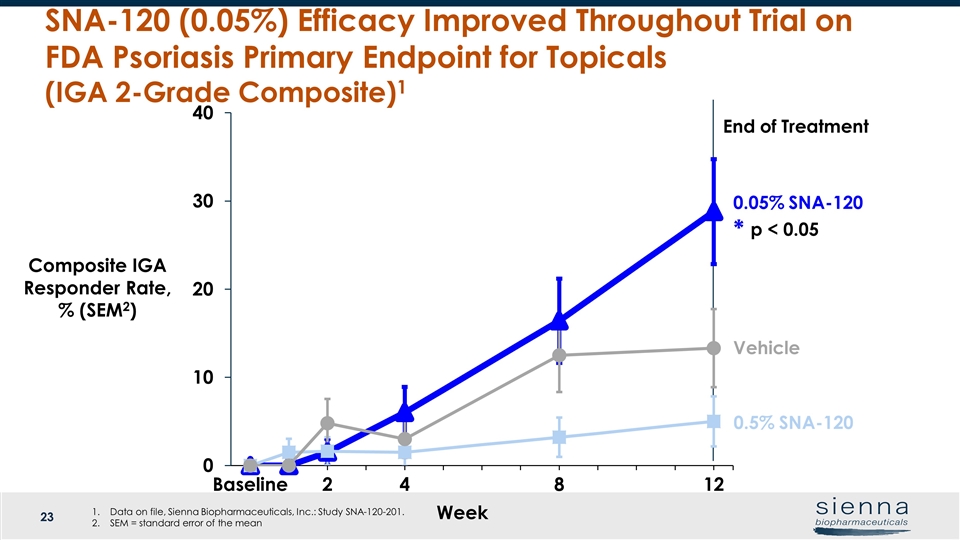
Week 0.05% SNA-120 Vehicle SNA-120 (0.05%) Efficacy Improved Throughout Trial on FDA Psoriasis Primary Endpoint for Topicals (IGA 2-Grade Composite)1 Composite IGA Responder Rate, % (SEM2) End of Treatment p < 0.05 * 0.5% SNA-120 Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201. SEM = standard error of the mean
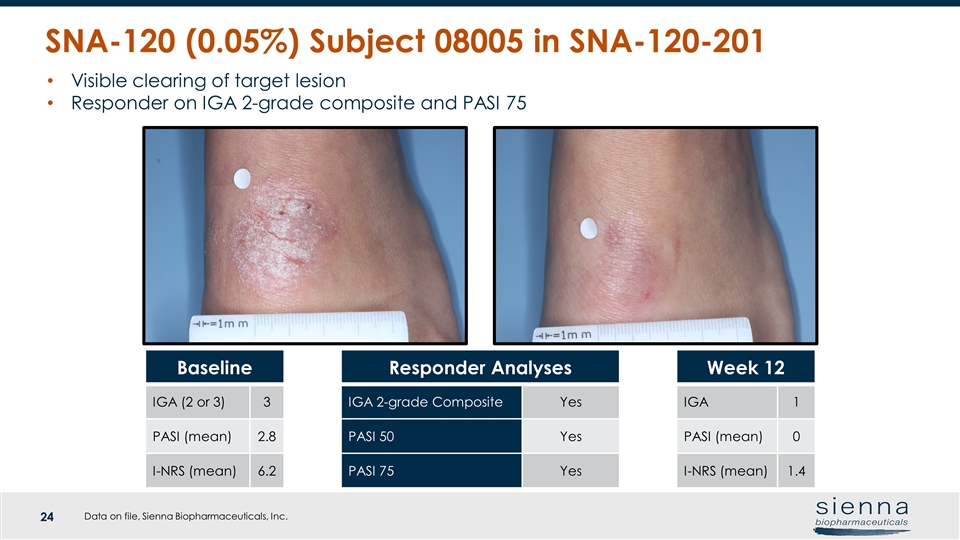
SNA-120 (0.05%) Subject 08005 in SNA-120-201 Visible clearing of target lesion Responder on IGA 2-grade composite and PASI 75 Baseline IGA (2 or 3) 3 PASI (mean) 2.8 I-NRS (mean) 6.2 Responder Analyses IGA 2-grade Composite Yes PASI 50 Yes PASI 75 Yes Week 12 IGA 1 PASI (mean) 0 I-NRS (mean) 1.4 Data on file, Sienna Biopharmaceuticals, Inc.
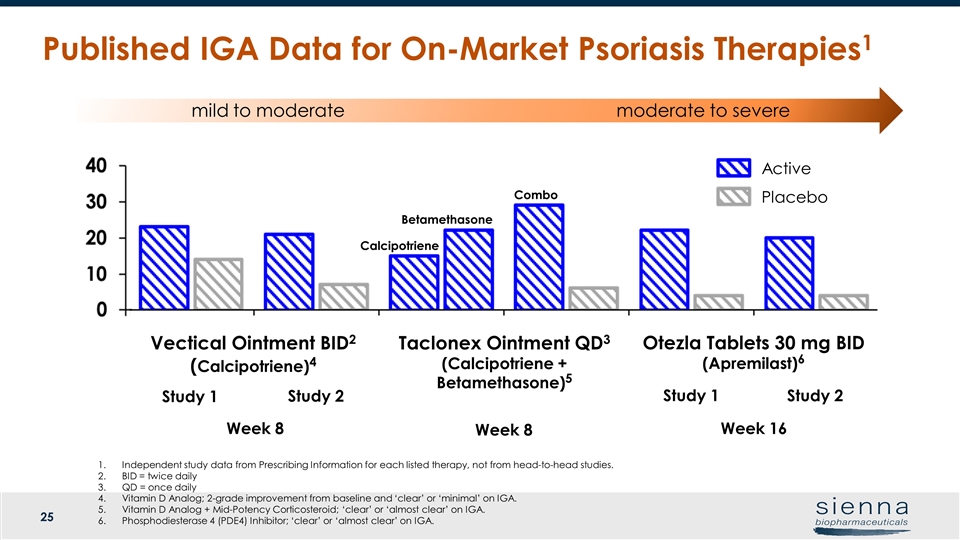
mild to moderate moderate to severe Drug Active Vehicle N % N % Vanos (fluocinonide) Cream 0.1%2 107 18% QD 54 7% QD 107 31% BID 54 5% BID Otezla (apremilast) Tablets 30 mg3 562 22% 282 4% 272 20% 137 4% Taclonex (calcipotriene / betamethasone) Ointment4 Calc Arm 480 17% 157 8% Vectical ointment (calcipotriene ) Ointment5 23.4% 14.4% 20.5% 6.6% Published IGA Data for On-Market Psoriasis Therapies1 Active Placebo Taclonex Ointment QD3 (Calcipotriene + Betamethasone)5 Otezla Tablets 30 mg BID (Apremilast)6 Study 2 Vectical Ointment BID2 (Calcipotriene)4 Study 2 Study 1 Study 1 Week 16 Week 8 Week 8 Betamethasone Combo Calcipotriene Independent study data from Prescribing Information for each listed therapy, not from head-to-head studies. BID = twice daily QD = once daily Vitamin D Analog; 2-grade improvement from baseline and ‘clear’ or ‘minimal’ on IGA. Vitamin D Analog + Mid-Potency Corticosteroid; ‘clear’ or ‘almost clear’ on IGA. Phosphodiesterase 4 (PDE4) Inhibitor; ‘clear’ or ‘almost clear’ on IGA.
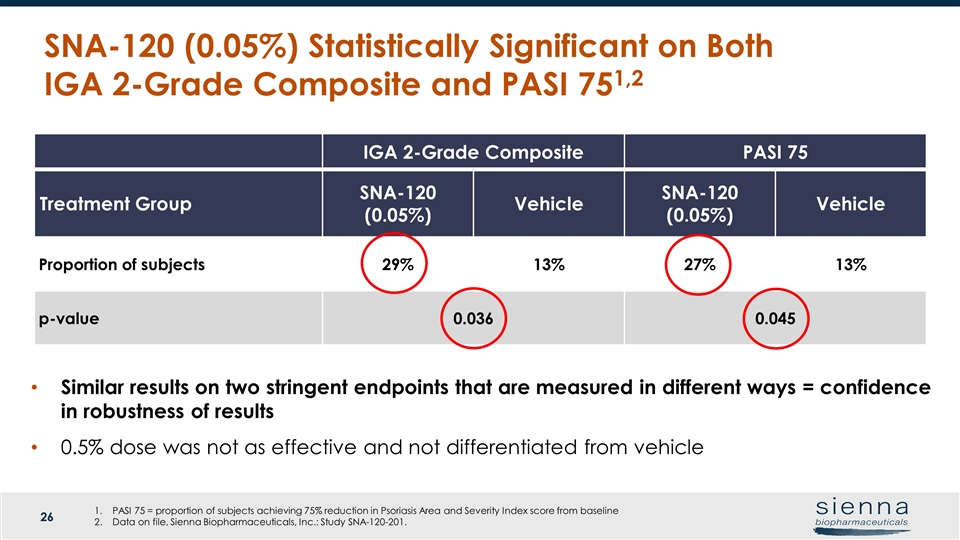
SNA-120 (0.05%) Statistically Significant on Both IGA 2-Grade Composite and PASI 751,2 Treatment Group Week 8 Week 12 PASI 50 SNA-120 (0.05%) Proportion of subjects P value vs vehicle 30 (49.2%) p=0.130 32 (54.2%) p=0.057 SNA-120 (0.5%) Proportion of subjects P value vs vehicle 20 (31.7%) p=0.636 25 (41.7%) p=0.562 Vehicle Proportion of subjects 23 (35.9%) 22 (36.7%) PASI 75 SNA-120 (0.05%) Proportion of subjects P value vs vehicle 15 (24.6%) p=0.063 16 (27.1%) p=0.045* SNA-120 (0.5%) Proportion of subjects P value vs vehicle 6 (9.5%) p=0.614 6 (10.0%) p=0.590 Vehicle Proportion of subjects 8 (12.5%) 8 (13.3%) IGA 2-Grade Composite PASI 75 Treatment Group SNA-120 (0.05%) Vehicle SNA-120 (0.05%) Vehicle Proportion of subjects 29% 13% 27% 13% p-value 0.036 0.045 Similar results on two stringent endpoints that are measured in different ways = confidence in robustness of results 0.5% dose was not as effective and not differentiated from vehicle PASI 75 = proportion of subjects achieving 75% reduction in Psoriasis Area and Severity Index score from baseline Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201.
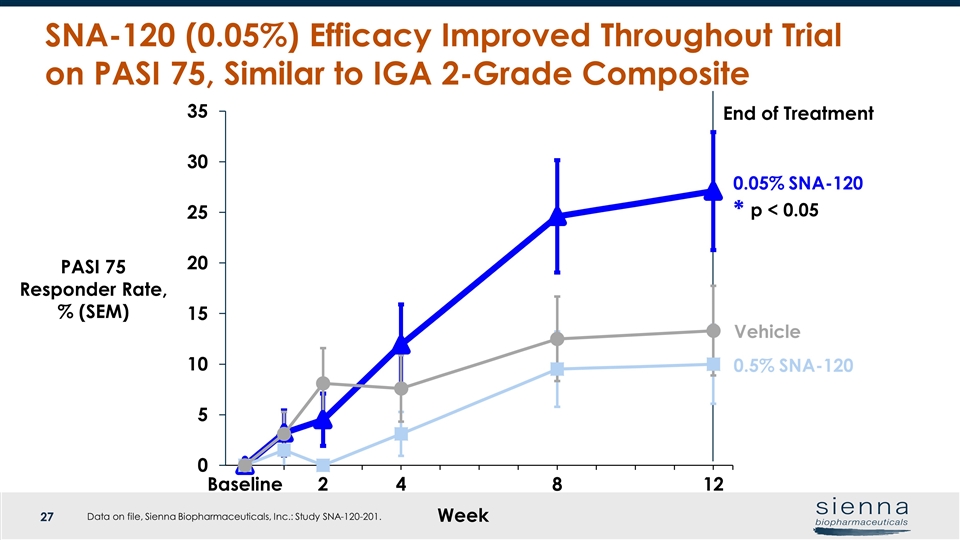
Week Vehicle PASI 75 Responder Rate, % (SEM) End of Treatment SNA-120 (0.05%) Efficacy Improved Throughout Trial on PASI 75, Similar to IGA 2-Grade Composite 0.05% SNA-120 p < 0.05 * 0.5% SNA-120 Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201.
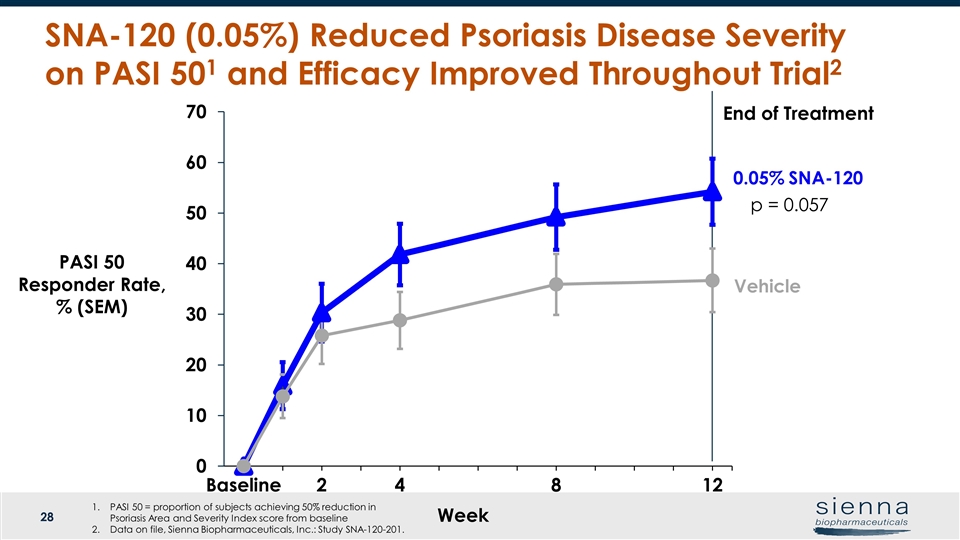
Week Vehicle PASI 50 Responder Rate, % (SEM) End of Treatment SNA-120 (0.05%) Reduced Psoriasis Disease Severity on PASI 501 and Efficacy Improved Throughout Trial2 0.05% SNA-120 p = 0.057 PASI 50 = proportion of subjects achieving 50% reduction in Psoriasis Area and Severity Index score from baseline Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201.
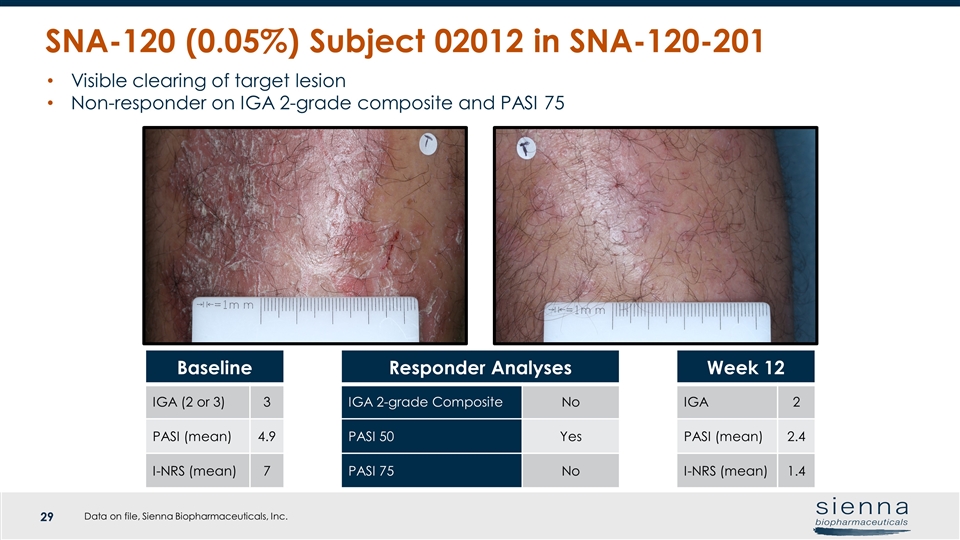
SNA-120 (0.05%) Subject 02012 in SNA-120-201 Visible clearing of target lesion Non-responder on IGA 2-grade composite and PASI 75 Baseline IGA (2 or 3) 3 PASI (mean) 4.9 I-NRS (mean) 7 Responder Analyses IGA 2-grade Composite No PASI 50 Yes PASI 75 No Week 12 IGA 2 PASI (mean) 2.4 I-NRS (mean) 1.4 Data on file, Sienna Biopharmaceuticals, Inc.
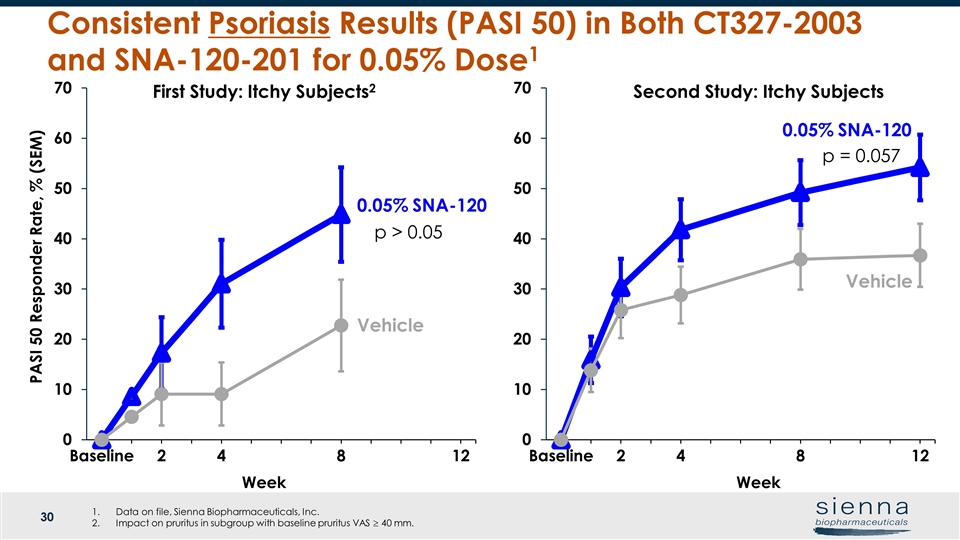
Week Vehicle Week 0.05% SNA-120 Vehicle Consistent Psoriasis Results (PASI 50) in Both CT327-2003 and SNA-120-201 for 0.05% Dose1 PASI 50 Responder Rate, % (SEM) Second Study: Itchy Subjects First Study: Itchy Subjects2 0.05% SNA-120 p > 0.05 Data on file, Sienna Biopharmaceuticals, Inc. Impact on pruritus in subgroup with baseline pruritus VAS ³ 40 mm.
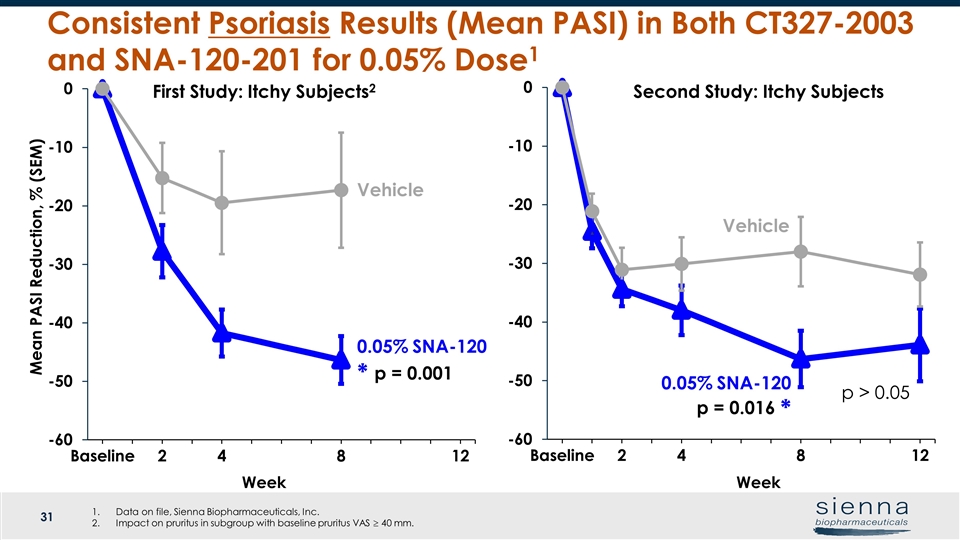
Week Vehicle Week Vehicle Consistent Psoriasis Results (Mean PASI) in Both CT327-2003 and SNA-120-201 for 0.05% Dose1 Mean PASI Reduction, % (SEM) Second Study: Itchy Subjects First Study: Itchy Subjects2 0.05% SNA-120 p = 0.001 * 0.05% SNA-120 p = 0.016 * Data on file, Sienna Biopharmaceuticals, Inc. Impact on pruritus in subgroup with baseline pruritus VAS ³ 40 mm. p > 0.05
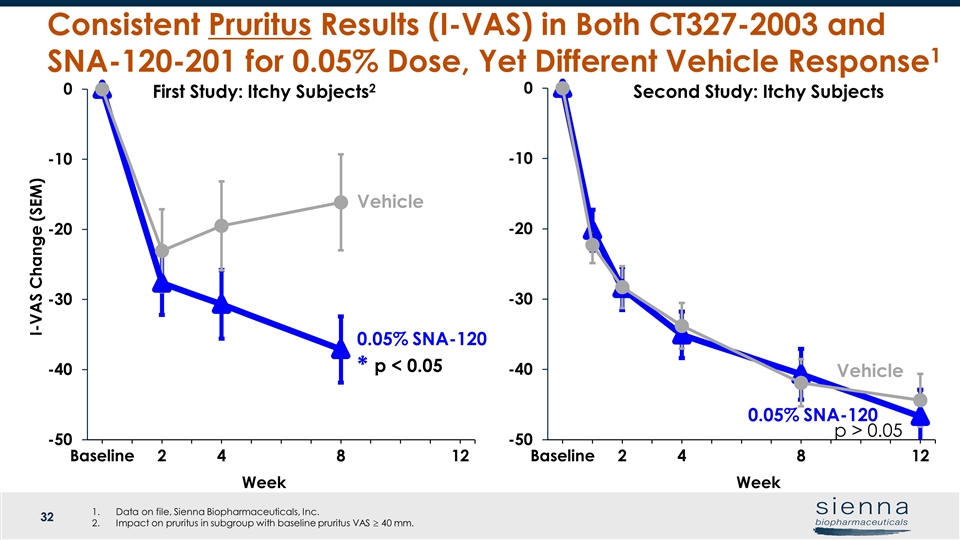
Week Vehicle Week Vehicle Consistent Pruritus Results (I-VAS) in Both CT327-2003 and SNA-120-201 for 0.05% Dose, Yet Different Vehicle Response1 I-VAS Change (SEM) Second Study: Itchy Subjects First Study: Itchy Subjects2 0.05% SNA-120 p < 0.05 * 0.05% SNA-120 p > 0.05 Data on file, Sienna Biopharmaceuticals, Inc. Impact on pruritus in subgroup with baseline pruritus VAS ³ 40 mm.
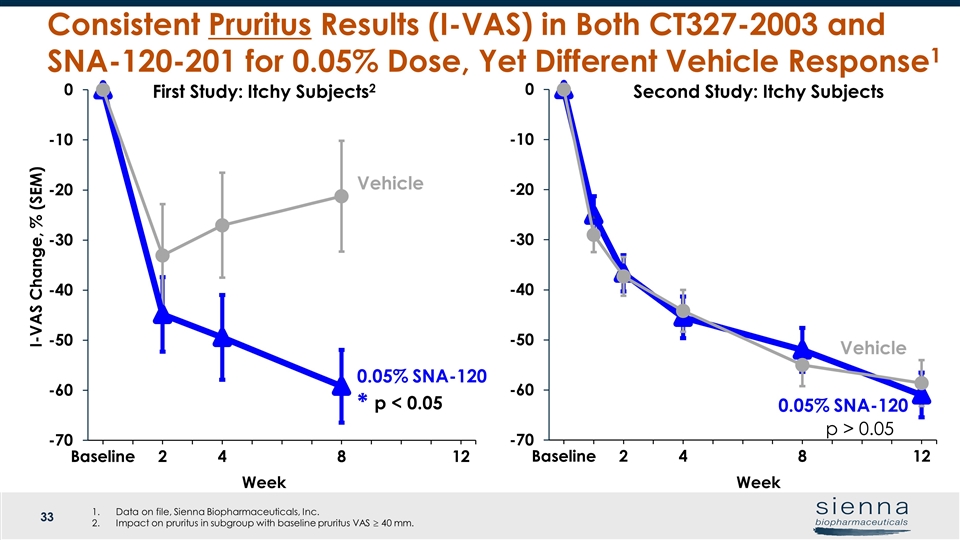
Week Vehicle Week Vehicle Consistent Pruritus Results (I-VAS) in Both CT327-2003 and SNA-120-201 for 0.05% Dose, Yet Different Vehicle Response1 I-VAS Change, % (SEM) Second Study: Itchy Subjects First Study: Itchy Subjects2 0.05% SNA-120 p < 0.05 * 0.05% SNA-120 p > 0.05 Data on file, Sienna Biopharmaceuticals, Inc. Impact on pruritus in subgroup with baseline pruritus VAS ³ 40 mm.
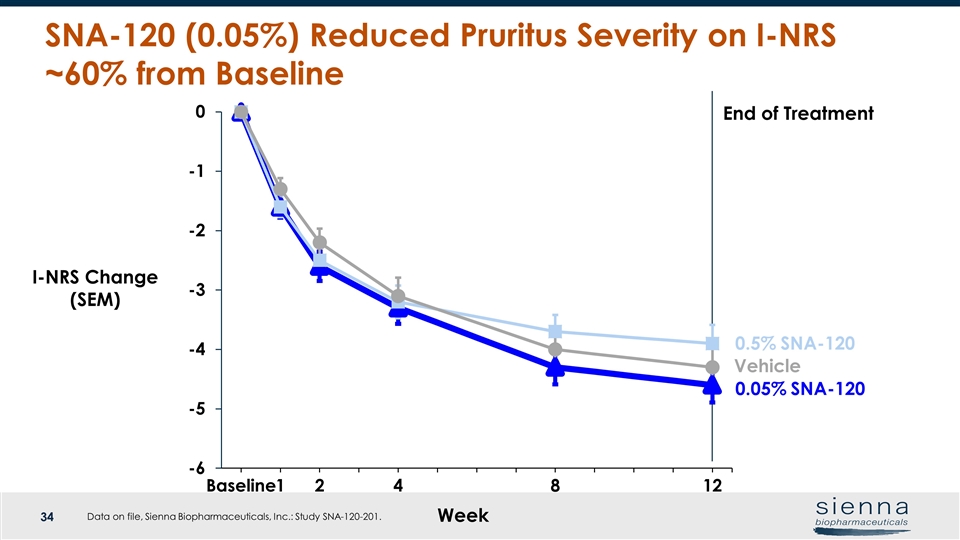
Week Vehicle I-NRS Change (SEM) End of Treatment SNA-120 (0.05%) Reduced Pruritus Severity on I-NRS ~60% from Baseline 0.05% SNA-120 0.5% SNA-120 Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201.
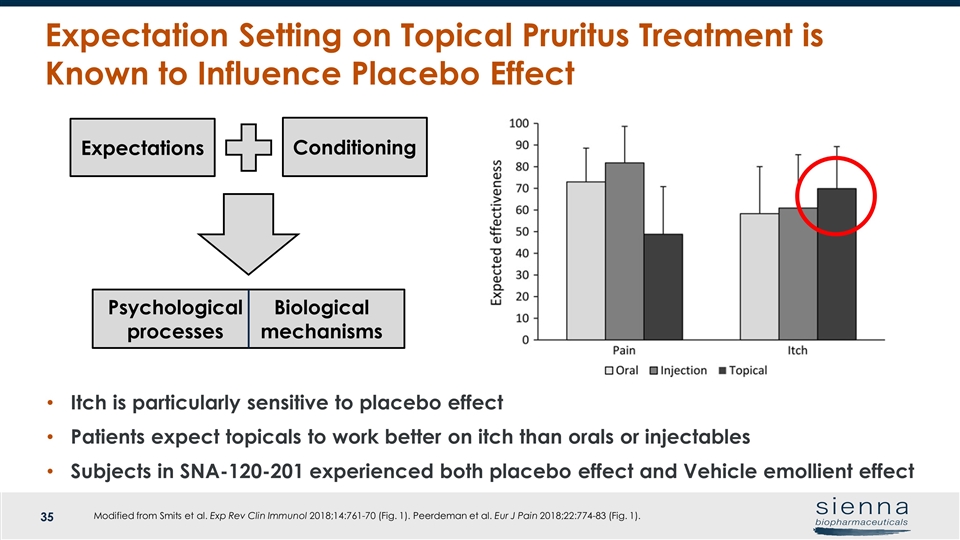
Modified from Smits et al. Exp Rev Clin Immunol 2018;14:761-70 (Fig. 1). Peerdeman et al. Eur J Pain 2018;22:774-83 (Fig. 1). Expectation Setting on Topical Pruritus Treatment is Known to Influence Placebo Effect Expectations Conditioning Psychological processes Biological mechanisms Itch is particularly sensitive to placebo effect Patients expect topicals to work better on itch than orals or injectables Subjects in SNA-120-201 experienced both placebo effect and Vehicle emollient effect
Clinically and statistically significant psoriasis results in SNA-120-2011 Even better than CT327-20031, as expected with refined patient population On stringent FDA psoriasis primary endpoint for topicals (IGA 2-Grade Composite) Also on stringent PASI 75 = confidence in robustness of results Pruritus improvement clinically meaningful, similar to CT327-2003 Yet high Vehicle response (placebo effect + emollient effect) Makes pruritus challenging as primary endpoint in Phase 3 Adjusting Phase 3 trial design to limit expectation setting around pruritus and resulting placebo effect2 Pruritus will be an important secondary endpoint in Phase 3 trials, but not the basis for approval SNA-120 (0.05%) Improved Psoriasis and Pruritus, Important Attributes for Success Data on file, Sienna Biopharmaceuticals, Inc. Smits et al. Exp Rev Clin Immunol 2018;14:761-70. Peerdeman et al. Eur J Pain 2018;22:774-83.
Treatment-related AEs were observed in only 2 subjects and included dermatitis (0.5% group) and pain and pruritus (Vehicle group) Most common AEs (≥ 2 subjects) in any group were nasopharyngitis, nausea, diarrhea, cellulitis and urinary tract infection Majority of treatment-emergent AEs were mild to moderate There were 6 serious AEs in 3 subjects, but none were considered drug related SNA-120 Was Well-Tolerated with No Serious Treatment-Related Adverse Events (AEs)1 SNA-120 has been administered to 500+ subjects for up to 12 weeks, observed to be safe and well-tolerated across all trials, with minimal to no demonstrable systemic exposure2, further validating Topical by Design™ platform Data on file, Sienna Biopharmaceuticals, Inc.: Study SNA-120-201. Excludes second Phase 2b trial, as data not yet available.
Increasing dose results in broader, less targeted kinase inhibition Consistent better performance of low vs. high dose in both CT327-2003 and SNA-120-201 Similar effect observed in SNA-125 preclinical and Phase 1 studies Kinase inhibitors can have bell-shaped dose-response curves e.g., tofacitinib1, upadacitinib2, ASN0023 Better Efficacy with Low Dose (0.05%) Was Expected Beattie et al. J Inflammation 2017;14:28. Salgado et al. Int J Clin Rheumatol 2013;8(3):315-26. Mukherjee et al. Br J Clin Pharmacol 2018;84(6):1136-45. Genovese et al. Arthritis Rheumatol 2016;68:2857–66. Kremer et al. Arthritis Rheumatol 2016;68:2867–77. Guttman-Yassky et al, EADV 2018.
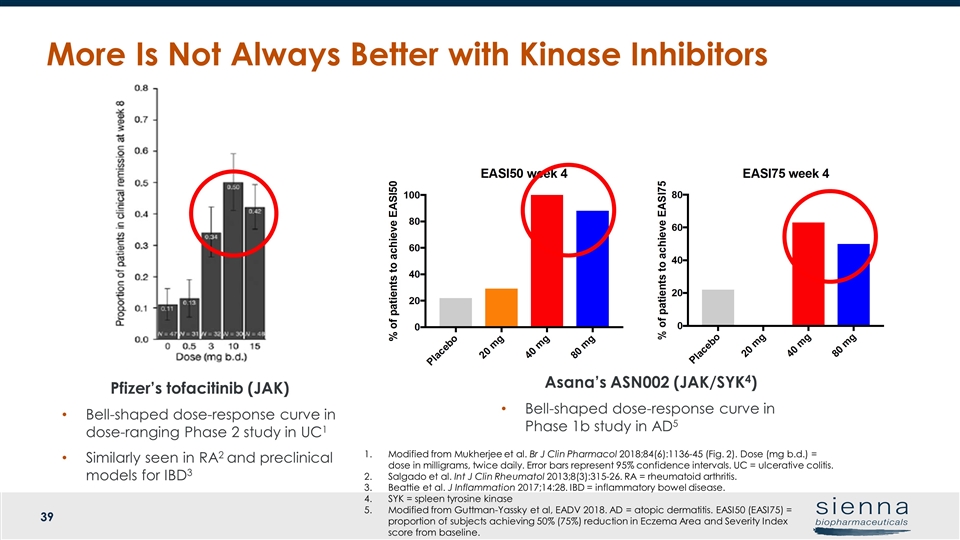
More Is Not Always Better with Kinase Inhibitors Pfizer’s tofacitinib (JAK) Bell-shaped dose-response curve in dose-ranging Phase 2 study in UC1 Similarly seen in RA2 and preclinical models for IBD3 Asana’s ASN002 (JAK/SYK4) Bell-shaped dose-response curve in Phase 1b study in AD5 Modified from Mukherjee et al. Br J Clin Pharmacol 2018;84(6):1136-45 (Fig. 2). Dose (mg b.d.) = dose in milligrams, twice daily. Error bars represent 95% confidence intervals. UC = ulcerative colitis. Salgado et al. Int J Clin Rheumatol 2013;8(3):315-26. RA = rheumatoid arthritis. Beattie et al. J Inflammation 2017;14:28. IBD = inflammatory bowel disease. SYK = spleen tyrosine kinase Modified from Guttman-Yassky et al, EADV 2018. AD = atopic dermatitis. EASI50 (EASI75) = proportion of subjects achieving 50% (75%) reduction in Eczema Area and Severity Index score from baseline.
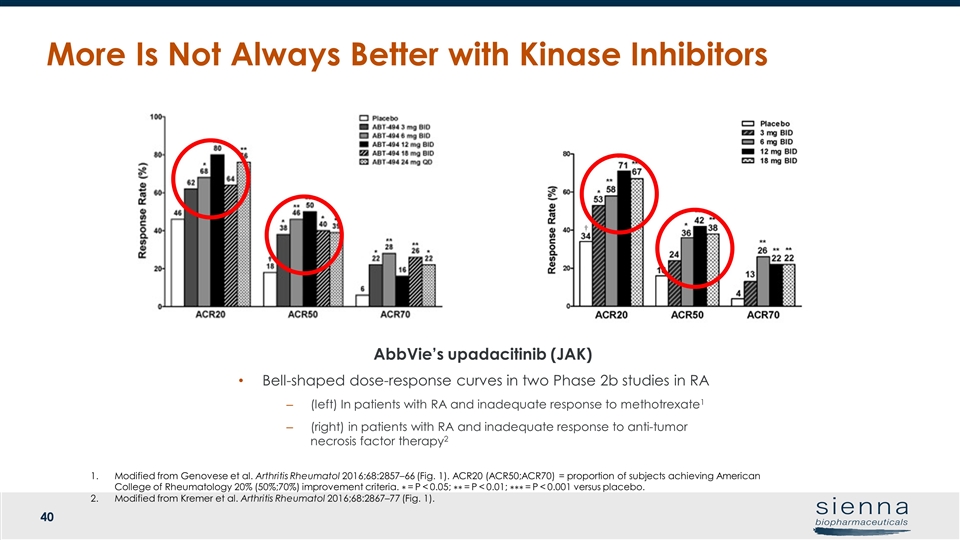
More Is Not Always Better with Kinase Inhibitors AbbVie’s upadacitinib (JAK) Bell-shaped dose-response curves in two Phase 2b studies in RA (left) In patients with RA and inadequate response to methotrexate1 (right) in patients with RA and inadequate response to anti-tumor necrosis factor therapy2 Modified from Genovese et al. Arthritis Rheumatol 2016;68:2857–66 (Fig. 1). ACR20 (ACR50;ACR70) = proportion of subjects achieving American College of Rheumatology 20% (50%;70%) improvement criteria. ∗ = P < 0.05; ∗∗ = P < 0.01; ∗∗∗ = P < 0.001 versus placebo. Modified from Kremer et al. Arthritis Rheumatol 2016;68:2867–77 (Fig. 1).
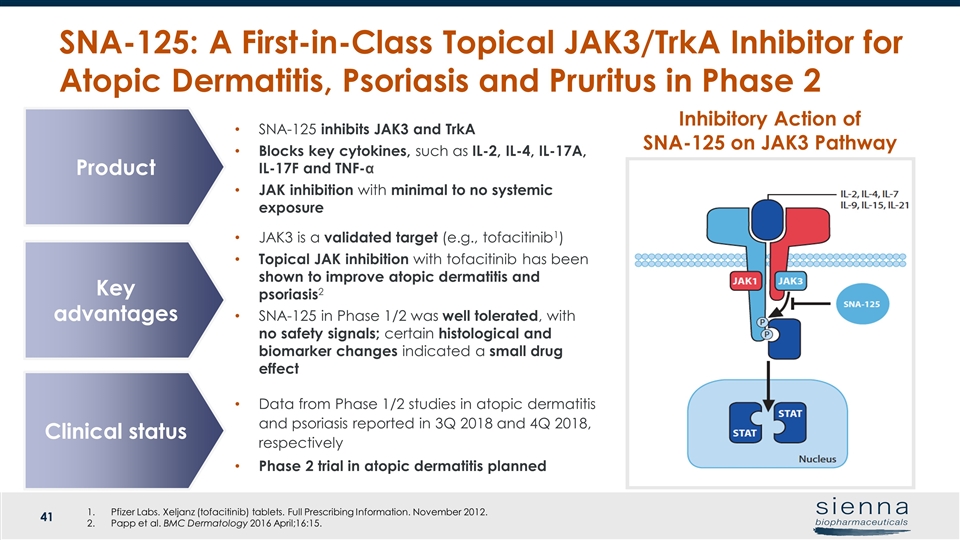
SNA-125: A First-in-Class Topical JAK3/TrkA Inhibitor for Atopic Dermatitis, Psoriasis and Pruritus in Phase 2 Pfizer Labs. Xeljanz (tofacitinib) tablets. Full Prescribing Information. November 2012. Papp et al. BMC Dermatology 2016 April;16:15. Inhibitory Action of SNA-125 on JAK3 Pathway SNA-125 inhibits JAK3 and TrkA Blocks key cytokines, such as IL-2, IL-4, IL-17A, IL-17F and TNF-α JAK inhibition with minimal to no systemic exposure Product Key advantages Clinical status JAK3 is a validated target (e.g., tofacitinib1) Topical JAK inhibition with tofacitinib has been shown to improve atopic dermatitis and psoriasis2 SNA-125 in Phase 1/2 was well tolerated, with no safety signals; certain histological and biomarker changes indicated a small drug effect Data from Phase 1/2 studies in atopic dermatitis and psoriasis reported in 3Q 2018 and 4Q 2018, respectively Phase 2 trial in atopic dermatitis planned

One of the most common skin diseases: 2-3% of adults, up to 25% of children1 In 2017, the prevalence of Atopic Dermatitis in the United States was estimated at 28 million2 85% of Atopic Dermatitis patients in the United States have mild-to-moderate disease3 Large unmet need across the Atopic Dermatitis population Few safe and effective non-steroidal options suitable for chronic use New biologics will address only the more severe populations (dupilumab4) Pediatric population has a need for steroid alternative Safety concerns with steroids are very high in this population Atopic Dermatitis: Large Unmet Need for Safe Topical Non-steroidal Drug Eichenfield et al. J Am Acad Dermatol 2014 Feb;70(2):338-51. National Eczema Association. https://nationaleczema.org/research/eczema-facts/. Accessed November 2018. Datamonitor Healthcare Atopic Dermatitis Survey. August 2015. Sanofi and Regeneron Pharmaceuticals. DUPIXENT (dupilumab) injection 300mg. Full Prescribing information. March 2017.
Opportunity for selective development of our current product candidates SNA-120 and SNA-125 in additional indications We have developed additional NCEs from our Topical by Design platform which have shown favorable data in early nonclinical studies We believe the Topical by Design technology may also address other therapeutic needs in which localized drug exposure is desirable, such as ophthalmological, gastrointestinal and pulmonary conditions Sienna’s Topical by DesignTM Platform Represents Significant Upside in Multiple Therapeutic Areas

Topical Photoparticle TherapyTM Platform (SNA-001)
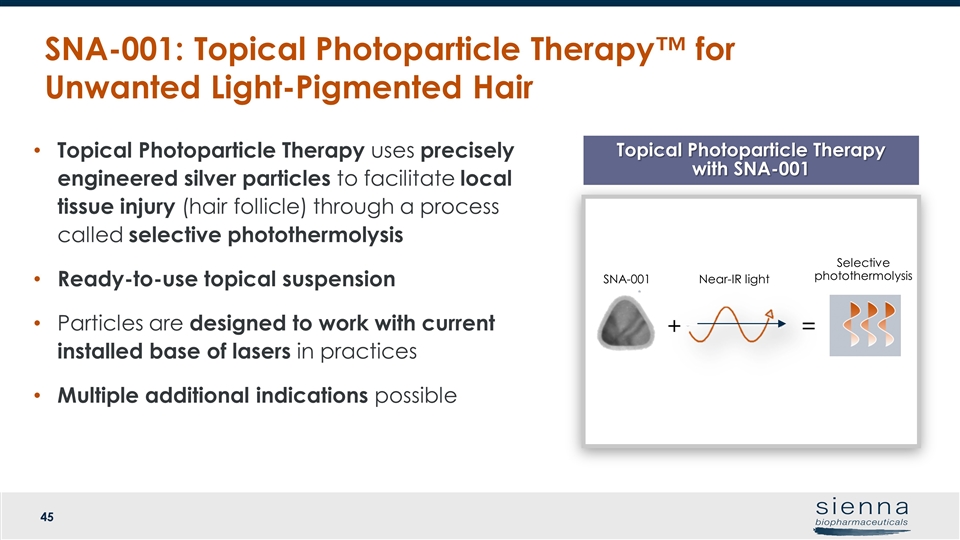
Topical Photoparticle Therapy uses precisely engineered silver particles to facilitate local tissue injury (hair follicle) through a process called selective photothermolysis Ready-to-use topical suspension Particles are designed to work with current installed base of lasers in practices Multiple additional indications possible SNA-001: Topical Photoparticle Therapy™ for Unwanted Light-Pigmented Hair Topical Photoparticle Therapy with SNA-001 SNA-001 + Near-IR light = Selective photothermolysis

SNA-001: Opportunity to Expand the Market for Laser Hair Removal First effective hair removal solution for light-pigmented hair 1 2 Safe, similar to conventional laser hair removal treatment 3 Works with existing installed base of lasers and all skin and hair types PRODUCT VISION 4 Aligned with physicians’ interest in new procedural cash pay solutions
Laser hair removal is the highest volume aesthetic procedure performed globally1 Nevertheless, laser hair removal is largely ineffective for patients with light-pigmented hair (i.e., white, gray, blond, light brown and light red)2 As a result, patients are often turned away by healthcare practitioners if their hair color is too lightly pigmented Some patients who are treated anyway see insufficient benefit due to light hair or a mixture of dark and light hair types and are dissatisfied SNA-001: Designed to Overcome Current Limitations with Light-Pigmented Hair Removal ASAPS Stats 2016 / Medical Insights – Laser Hair Removal Market Analysis. December 2014. American Society for Laser Medicine & Surgery. Laser Hair Removal. http://www.aslms.org/for-the-public/treatments-using-lasers-and-energy-based-devices/laser-hair-removal/. Accessed July 2017.
SNA-001: Precisely Engineered Silver Particles Target Hair Follicles to Reduce Light-Pigmented Hair Thermally injures hair follicle for permanent reduction of unwanted, light-pigmented hair Number and timing of treatment sessions are same as current laser hair removal treatment Six in-office treatments spaced 6-8 weeks apart Light-pigmented hair lacks adequate pigment to be efficiently targeted by traditional lasers Feasibility study (N=10) showed treatment effect and good tolerability Pivotal trial ongoing: double-blind, randomized, within-subject-controlled
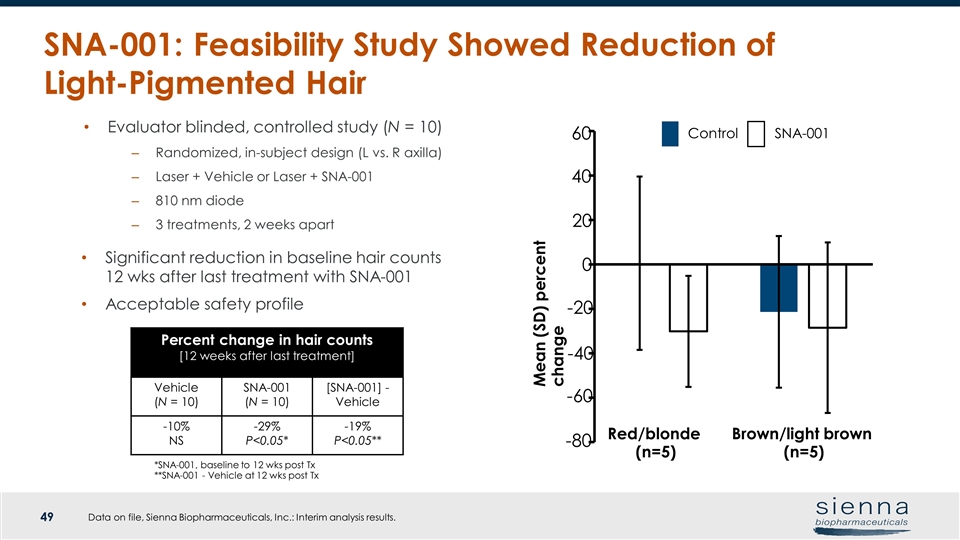
SNA-001: Feasibility Study Showed Reduction of Light-Pigmented Hair Data on file, Sienna Biopharmaceuticals, Inc.: Interim analysis results. *SNA-001, baseline to 12 wks post Tx **SNA-001 - Vehicle at 12 wks post Tx Evaluator blinded, controlled study (N = 10) Randomized, in-subject design (L vs. R axilla) Laser + Vehicle or Laser + SNA-001 810 nm diode 3 treatments, 2 weeks apart Percent change in hair counts [12 weeks after last treatment] Vehicle (N = 10) SNA-001 (N = 10) [SNA-001] - Vehicle -10% NS -29% P<0.05* -19% P<0.05** Significant reduction in baseline hair counts 12 wks after last treatment with SNA-001 Acceptable safety profile Mean (SD) percent change Control SNA-001 Brown/light brown (n=5) Red/blonde (n=5) -80 -60 -40 -20 0 20 40 60
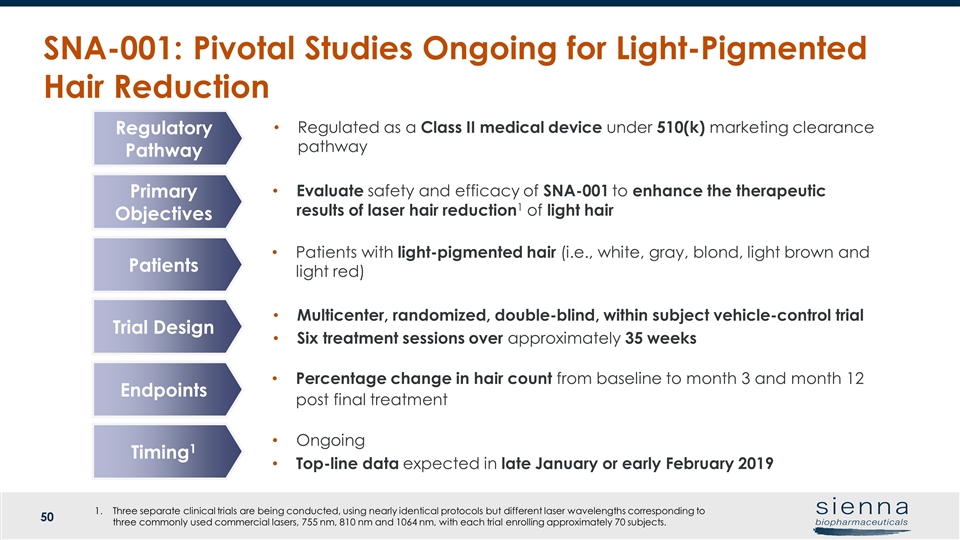
SNA-001: Pivotal Studies Ongoing for Light-Pigmented Hair Reduction Evaluate safety and efficacy of SNA-001 to enhance the therapeutic results of laser hair reduction1 of light hair Primary Objectives Patients Trial Design Endpoints Timing1 Percentage change in hair count from baseline to month 3 and month 12 post final treatment Patients with light-pigmented hair (i.e., white, gray, blond, light brown and light red) Multicenter, randomized, double-blind, within subject vehicle-control trial Six treatment sessions over approximately 35 weeks Ongoing Top-line data expected in late January or early February 2019 Three separate clinical trials are being conducted, using nearly identical protocols but different laser wavelengths corresponding to three commonly used commercial lasers, 755 nm, 810 nm and 1064 nm, with each trial enrolling approximately 70 subjects. Regulated as a Class II medical device under 510(k) marketing clearance pathway Regulatory Pathway
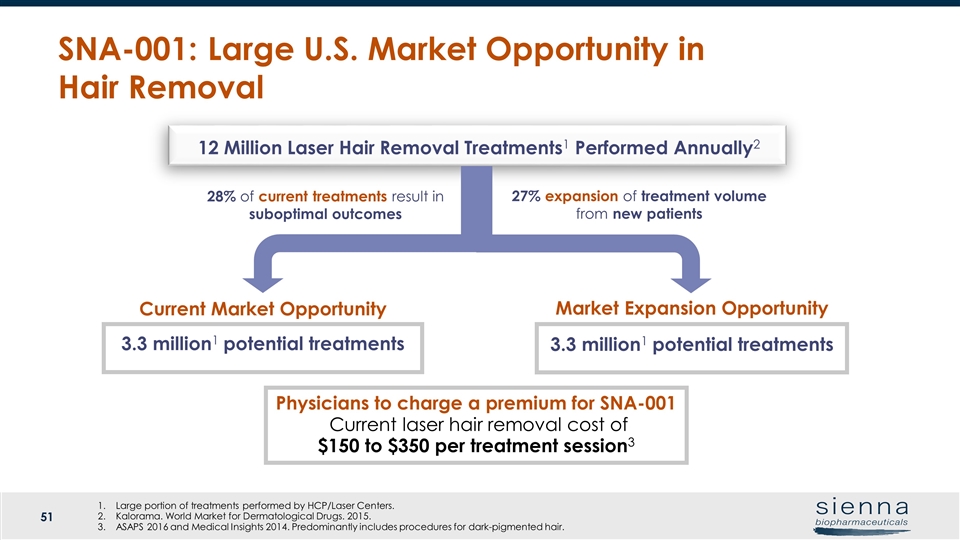
SNA-001: Large U.S. Market Opportunity in Hair Removal 3.3 million1 potential treatments 27% expansion of treatment volume from new patients 3.3 million1 potential treatments 28% of current treatments result in suboptimal outcomes Large portion of treatments performed by HCP/Laser Centers. Kalorama. World Market for Dermatological Drugs. 2015. ASAPS 2016 and Medical Insights 2014. Predominantly includes procedures for dark-pigmented hair. 12 Million Laser Hair Removal Treatments1 Performed Annually2 Current Market Opportunity Market Expansion Opportunity Physicians to charge a premium for SNA-001 Current laser hair removal cost of $150 to $350 per treatment session3
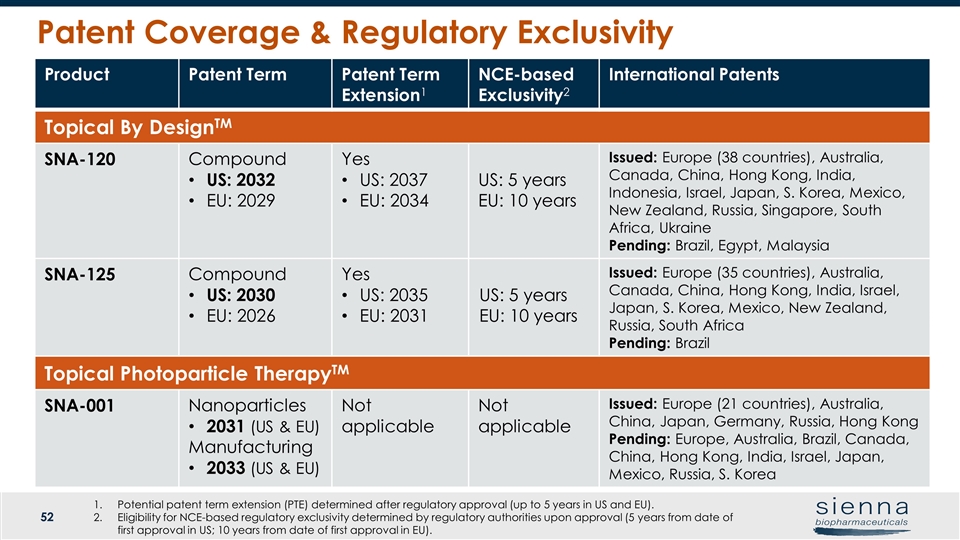
Patent Coverage & Regulatory Exclusivity Product Patent Term Patent Term Extension1 NCE-based Exclusivity2 International Patents Topical By DesignTM SNA-120 Compound US: 2032 EU: 2029 Yes US: 2037 EU: 2034 US: 5 years EU: 10 years Issued: Europe (38 countries), Australia, Canada, China, Hong Kong, India, Indonesia, Israel, Japan, S. Korea, Mexico, New Zealand, Russia, Singapore, South Africa, Ukraine Pending: Brazil, Egypt, Malaysia SNA-125 Compound US: 2030 EU: 2026 Yes US: 2035 EU: 2031 US: 5 years EU: 10 years Issued: Europe (35 countries), Australia, Canada, China, Hong Kong, India, Israel, Japan, S. Korea, Mexico, New Zealand, Russia, South Africa Pending: Brazil Topical Photoparticle TherapyTM SNA-001 Nanoparticles 2031 (US & EU) Manufacturing 2033 (US & EU) Not applicable Not applicable Issued: Europe (21 countries), Australia, China, Japan, Germany, Russia, Hong Kong Pending: Europe, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan, Mexico, Russia, S. Korea Potential patent term extension (PTE) determined after regulatory approval (up to 5 years in US and EU). Eligibility for NCE-based regulatory exclusivity determined by regulatory authorities upon approval (5 years from date of first approval in US; 10 years from date of first approval in EU).

Company Overview January 2019




















































