
A WORLD OF CONNECTION FOR PEOPLE WITH HEARING AND BALANCE DISORDERSTM 41st Annual Healthcare Meeting January 2023 Exhibit 99.1

This presentation contains forward looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding Decibel Therapeutics, Inc.’s (the “Company”) strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, the therapeutic potential for the Company's product candidates and preclinical programs, the expected timeline for submitting investigational new drug applications and achieving other anticipated milestones and the sufficiency of Decibel's existing cash resources, are forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the conduct of research activities and the initiation and completion of preclinical studies and clinical trials, the timing of and the Company's ability to submit and obtain approval to initiate clinical development of its product candidates, whether results from preclinical studies will be predictive of the results of later preclinical studies and clinical trials, whether Company's cash resources are sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements, uncertainties related to the impact of the COVID-19 pandemic on Company's business and operations, as well as the risks and uncertainties identified in Company's filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation. Forward-Looking Statements

Inner Ear: Optimal Characteristics to Realize Promise of Gene Therapy Maturing Pipeline: Monogenic Hearing Loss, Hair Cell Regeneration Hearing & Balance: No Approved Therapies, Rising Awareness of Opportunity Differentiated Platform: Unique Transgene Regulation Capabilities Capitalized to Achieve Milestones: Initial Human Clinical Data, Pipeline Milestones

Achievements and Anticipated Milestones Positive DB-020 IA Data Observed ototoxicity rate of 88% in placebo-treated ears Protected hearing in 87% of patients who experienced ototoxicity Generally well-tolerated DB-OTO in Phase 1/2 Orphan Drug, Rare Pediatric Disease designations from FDA FDA cleared IND to initiate Phase 1/2 clinical trial Regeneron commitment through human proof-of-concept Anticipated Milestones Clearance of DB-OTO CTAs Global initiation of DB-OTO Phase 1/2 clinical trial Milestone payments from Regeneron: DB-OTO, AAV.103 DB-020 data, reg. feedback Safety, tolerability, efficacy from initial DB-OTO Phase 1/2 cohort

Hearing Loss Profoundly Limits Connection to People and Environment Potential neurodevelopmental emergency Delays in language development Risk of impairment in executive function Highest relative risk of dementia Loss of connection often results in limited social interaction, feelings of loneliness and isolation across all ages.
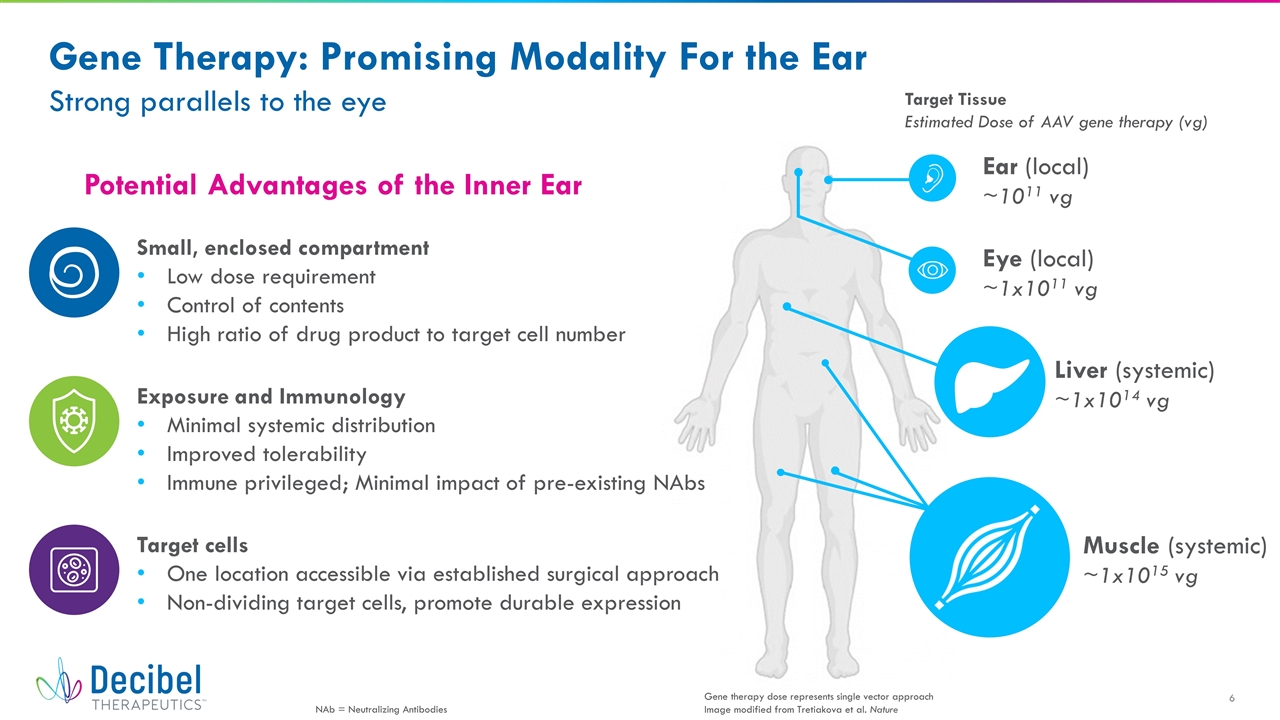
Potential Advantages of the Inner Ear Gene Therapy: Promising Modality For the Ear Strong parallels to the eye Small, enclosed compartment Low dose requirement Control of contents High ratio of drug product to target cell number Target cells One location accessible via established surgical approach Non-dividing target cells, promote durable expression Exposure and Immunology Minimal systemic distribution Improved tolerability Immune privileged; Minimal impact of pre-existing NAbs Ear (local) ~1011 vg Eye (local) ~1x1011 vg Liver (systemic) ~1x1014 vg Muscle (systemic) ~1x1015 vg Gene therapy dose represents single vector approach Image modified from Tretiakova et al. Nature Target Tissue Estimated Dose of AAV gene therapy (vg) NAb = Neutralizing Antibodies
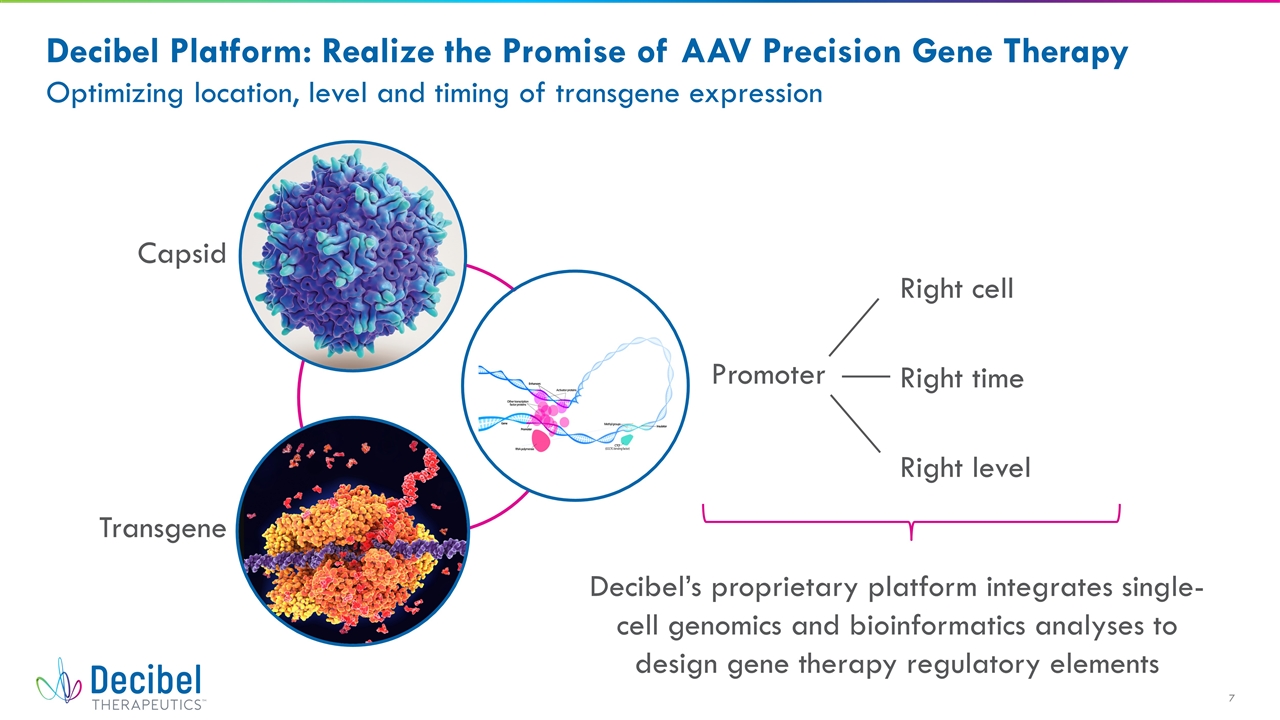
Capsid Transgene Promoter Right cell Right level Right time Decibel’s proprietary platform integrates single-cell genomics and bioinformatics analyses to design gene therapy regulatory elements Decibel Platform: Realize the Promise of AAV Precision Gene Therapy Optimizing location, level and timing of transgene expression

DBTX Pipeline: Building from Rare to Common Ear Disorders Inner Ear Gene Therapy Proof of Concept DB-OTO OTOF-related hearing loss Acquired Hearing and Balance Disorders Cochlear and Vestibular Hair Cell Regeneration Programs Larger Genetically-Defined Indications AAV.103 GJB2-related hearing loss AAV.104 STRC-related hearing loss 20,000 70,000 (STRC) 280,000 (GJB2) Millions+ Prevalent population estimates from US and EU

Pipeline: Decibel Retains 100% Worldwide Commercial Rights REGENERON Co-funds Development Decibel Commercialization Rights *Initiation of phase 1/2 clinical trial of DB-OTO in pediatric patients expected in the first half of 2023 *

Gene Therapies for Congenital, Monogenic Hearing Loss
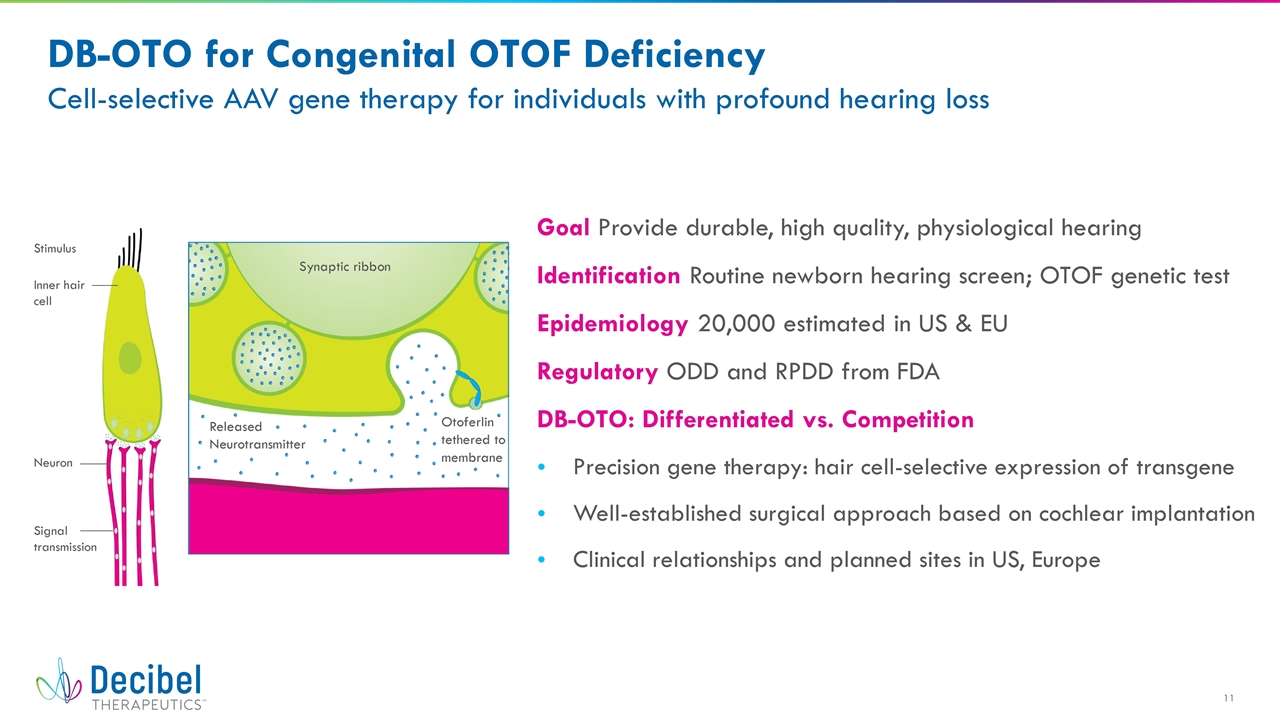
DB-OTO for Congenital OTOF Deficiency Cell-selective AAV gene therapy for individuals with profound hearing loss Goal Provide durable, high quality, physiological hearing Identification Routine newborn hearing screen; OTOF genetic test Epidemiology 20,000 estimated in US & EU Regulatory ODD and RPDD from FDA DB-OTO: Differentiated vs. Competition Precision gene therapy: hair cell-selective expression of transgene Well-established surgical approach based on cochlear implantation Clinical relationships and planned sites in US, Europe
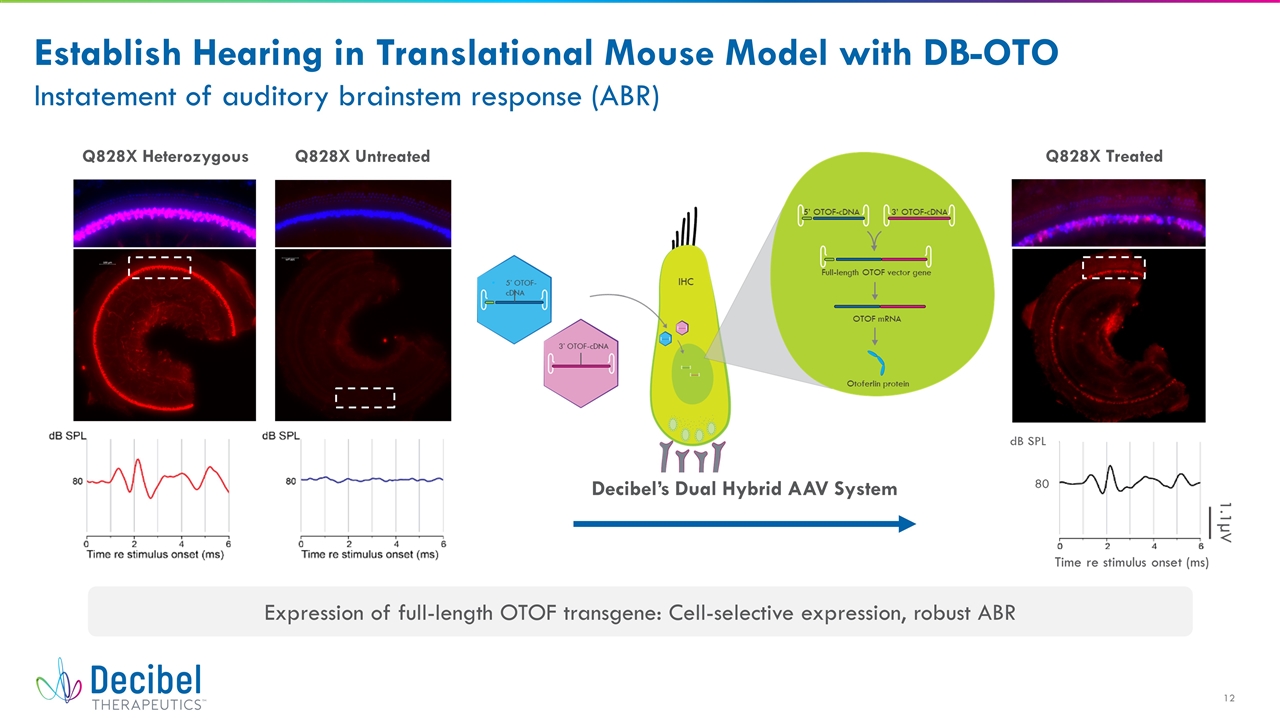
Establish Hearing in Translational Mouse Model with DB-OTO Instatement of auditory brainstem response (ABR) Decibel’s Dual Hybrid AAV System Q828X Heterozygous Q828X Untreated Q828X Treated Expression of full-length OTOF transgene: Cell-selective expression, robust ABR
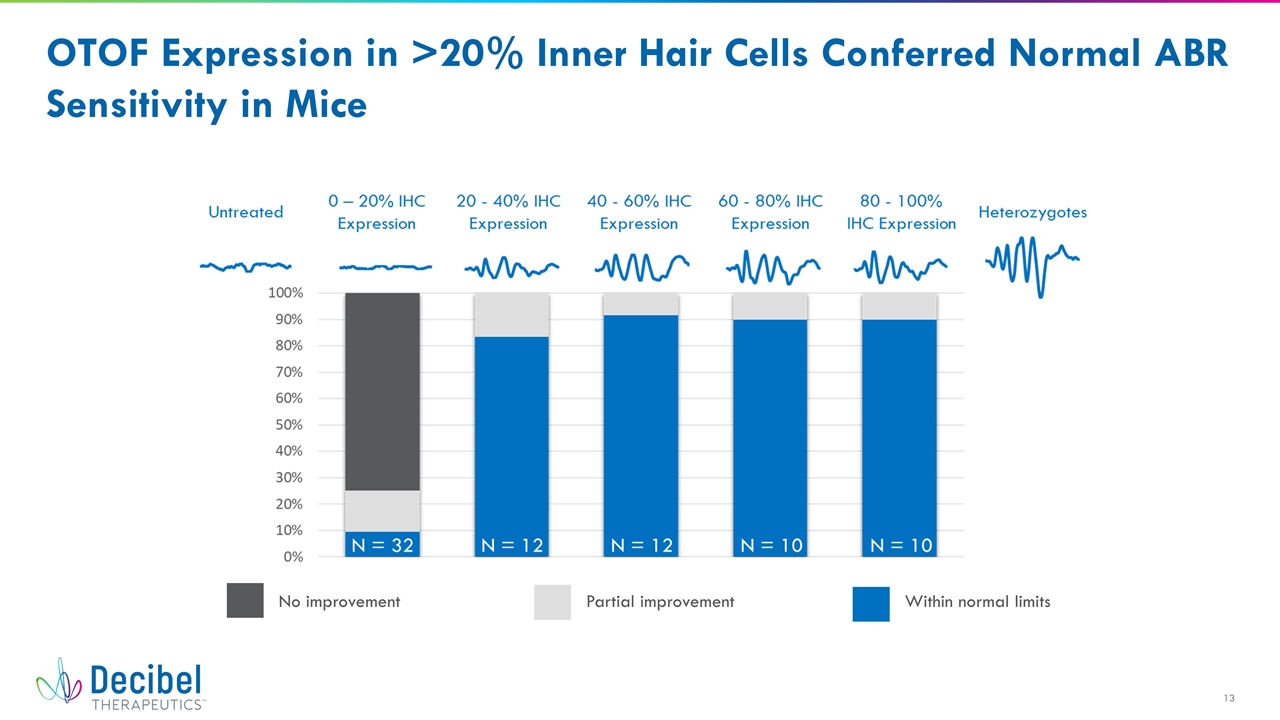
OTOF Expression in >20% Inner Hair Cells Conferred Normal ABR Sensitivity in Mice No improvement Partial improvement Within normal limits
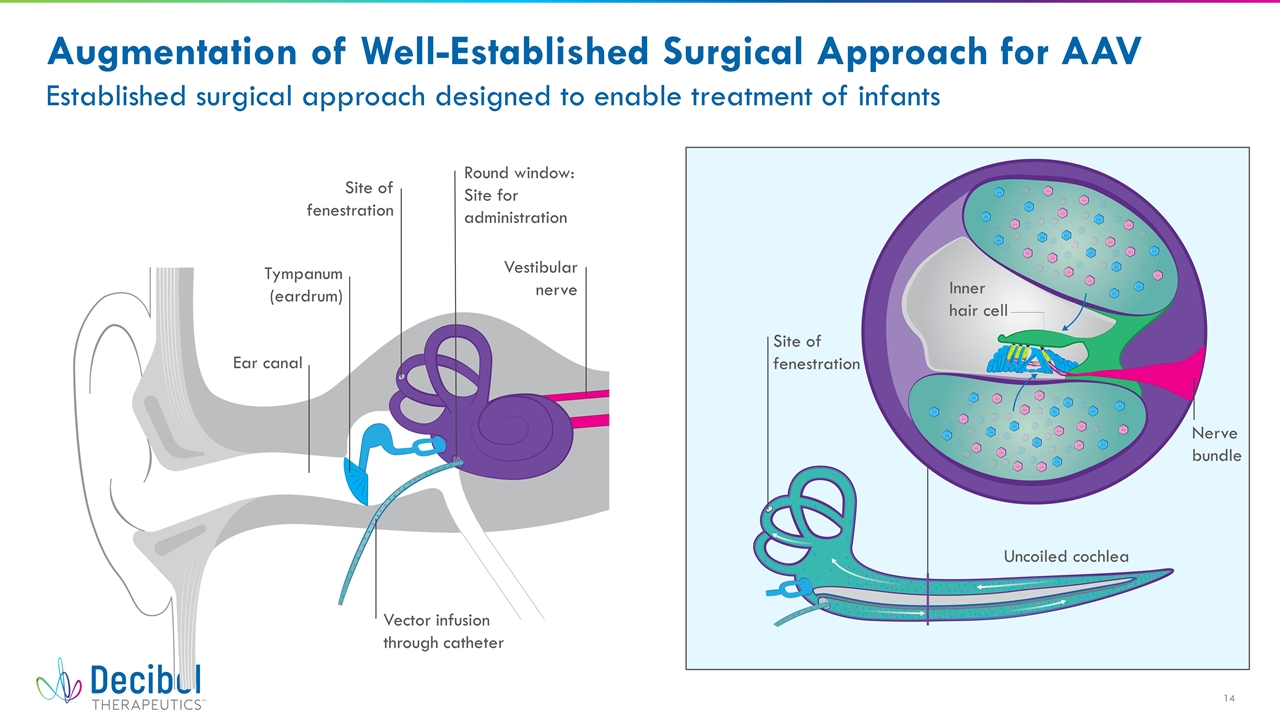
Augmentation of Well-Established Surgical Approach for AAV Established surgical approach designed to enable treatment of infants Ear canal Tympanum (eardrum) Site of fenestration Round window: Site for administration Vestibular nerve Vector infusion through catheter Site of fenestration Uncoiled cochlea Nerve bundle Inner hair cell

Today Jul 15 Oct 15 Oct 16 Nov 15 Jan 15 Apr 1 May 23 - Jul 15 Jul 1 - Oct 15 Jan 1 - Sep 30 Jul 1 - Mar 31 Apr 1 - Dec 31 Oct 1 - Mar 31 Jun 1 - Mar 31 Apr 1 - Dec 31 Jan 1 - May 15 DB-OTO Phase 1/2 Trial Phase 1/2 Clinical Trial Design Enrollment of pediatric patients, including infants Unilateral dosing, dose escalation Evaluating safety, tolerability, efficacy Efficacy endpoints: Objective, physiologic measurement of hearing function (ABR) Age-appropriate behavioral measurements of hearing Manufacturing Relationship with Catalent, leading commercial manufacturer Timeline IND US cleared by FDA; CTAs submitted to UK and Spain Commenced trial site startup activities Initiation of Phase 1/2 clinical trial anticipated in first half of 2023 Initial data from first cohort of patients anticipated in first quarter of 2024
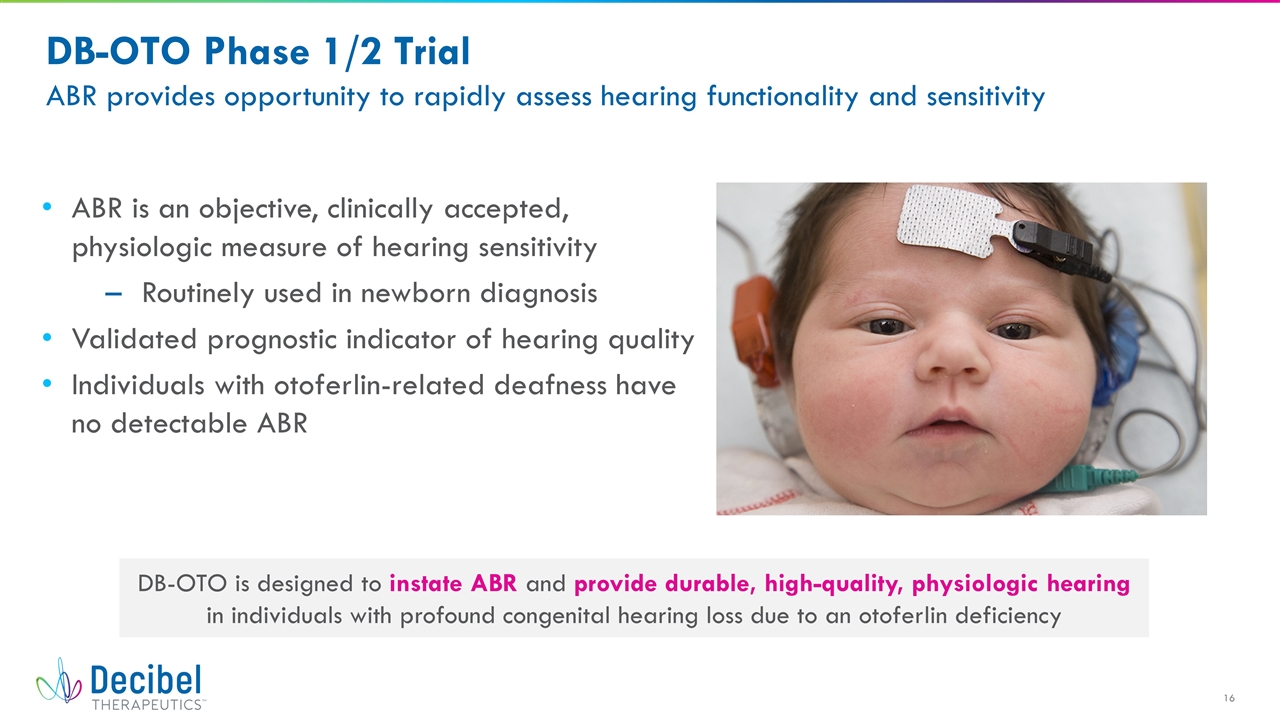
DB-OTO Phase 1/2 Trial ABR provides opportunity to rapidly assess hearing functionality and sensitivity ABR is an objective, clinically accepted, physiologic measure of hearing sensitivity Routinely used in newborn diagnosis Validated prognostic indicator of hearing quality Individuals with otoferlin-related deafness have no detectable ABR DB-OTO is designed to instate ABR and provide durable, high-quality, physiologic hearing in individuals with profound congenital hearing loss due to an otoferlin deficiency
AAV.103 Program for Congenital GJB2 Deficiency Cell-selective AAV gene therapy for individuals with GJB2-related hearing loss GJB2 Biology GJB2 encodes the connexin 26 gap junction protein Identification Routine newborn hearing screen; GJB2 genetic test Epidemiology 280,000 estimated in US & EU AAV.103: Status Proprietary cell-selective promoter to express GJB2 in non-sensory support cells Established hearing in preclinical studies in relevant rodent model Next step: initiate IND-enabling activities with product candidate
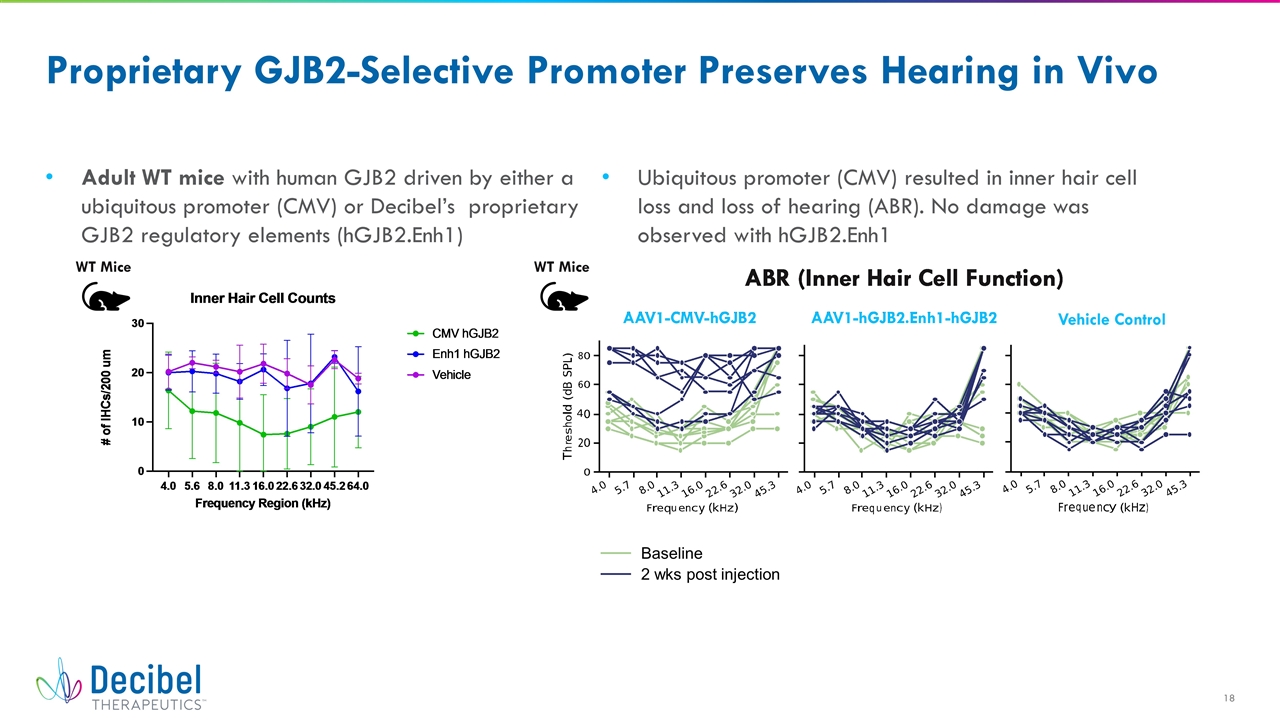
Proprietary GJB2-Selective Promoter Preserves Hearing in Vivo Adult WT mice with human GJB2 driven by either a ubiquitous promoter (CMV) or Decibel’s proprietary GJB2 regulatory elements (hGJB2.Enh1) Ubiquitous promoter (CMV) resulted in inner hair cell loss and loss of hearing (ABR). No damage was observed with hGJB2.Enh1 Baseline 2 wks post injection WT Mice WT Mice
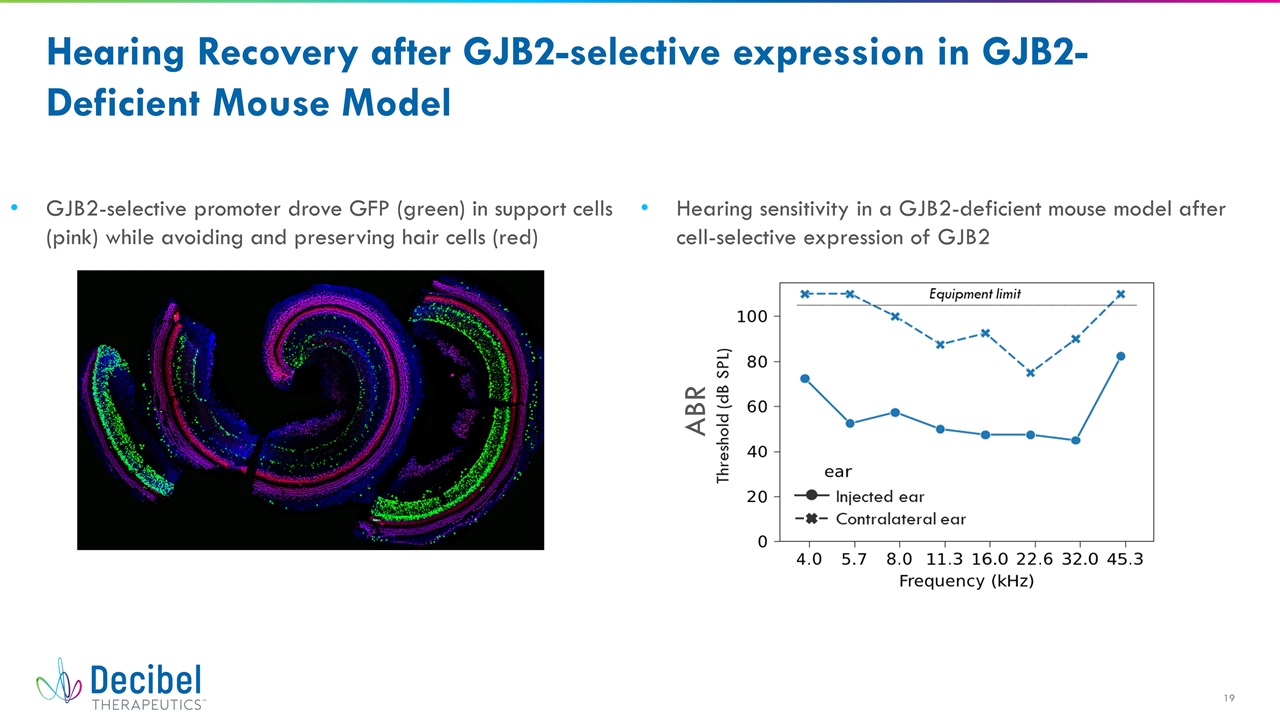
ABR Hearing Recovery after GJB2-selective expression in GJB2-Deficient Mouse Model GJB2-selective promoter drove GFP (green) in support cells (pink) while avoiding and preserving hair cells (red) Hearing sensitivity in a GJB2-deficient mouse model after cell-selective expression of GJB2
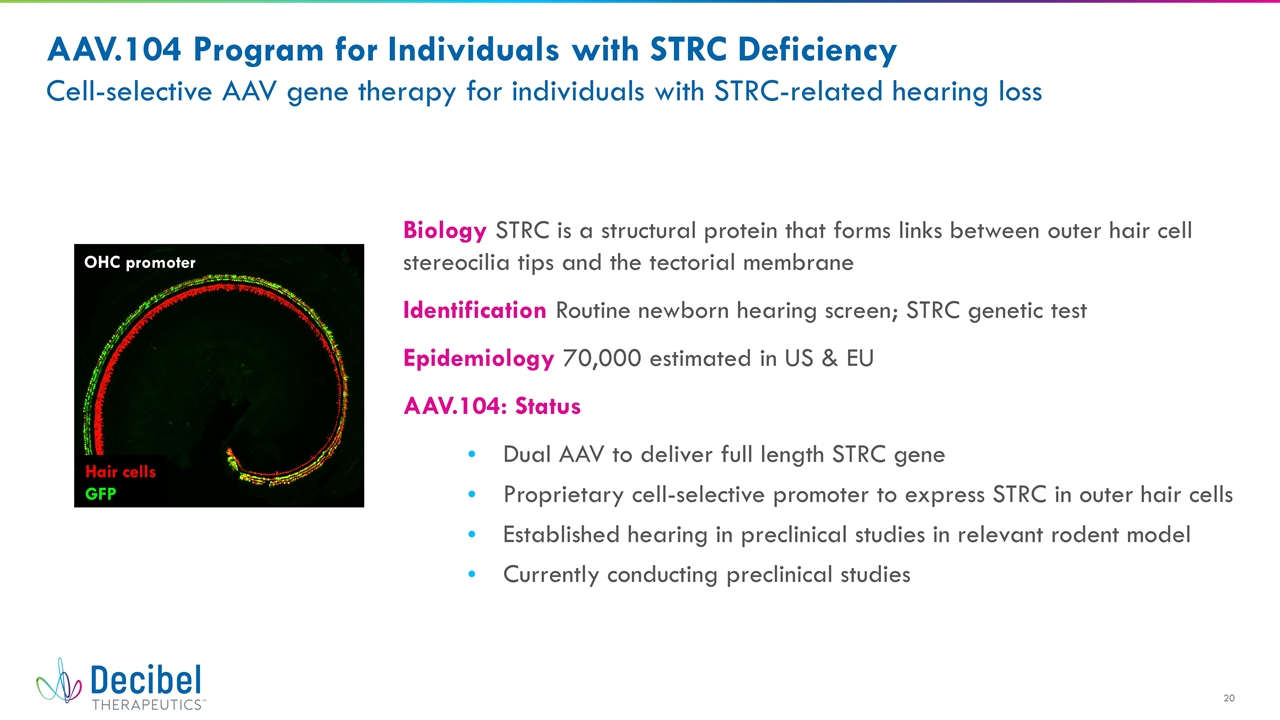
AAV.104 Program for Individuals with STRC Deficiency Cell-selective AAV gene therapy for individuals with STRC-related hearing loss Biology STRC is a structural protein that forms links between outer hair cell stereocilia tips and the tectorial membrane Identification Routine newborn hearing screen; STRC genetic test Epidemiology 70,000 estimated in US & EU AAV.104: Status Dual AAV to deliver full length STRC gene Proprietary cell-selective promoter to express STRC in outer hair cells Established hearing in preclinical studies in relevant rodent model Currently conducting preclinical studies OHC promoter Hair cells GFP

DB-020 for Chemotherapy-Induced Ototoxicity
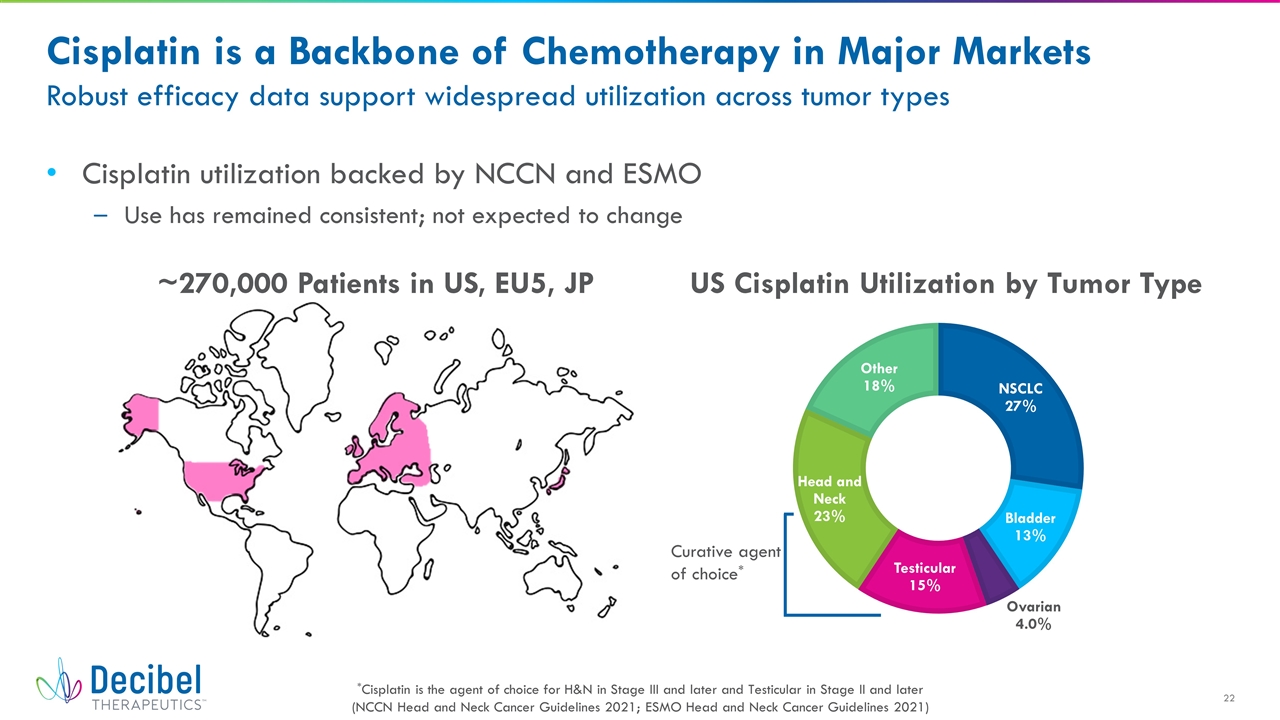
Cisplatin is a Backbone of Chemotherapy in Major Markets Robust efficacy data support widespread utilization across tumor types Cisplatin utilization backed by NCCN and ESMO Use has remained consistent; not expected to change US Cisplatin Utilization by Tumor Type ~270,000 Patients in US, EU5, JP Curative agent of choice* *Cisplatin is the agent of choice for H&N in Stage III and later and Testicular in Stage II and later (NCCN Head and Neck Cancer Guidelines 2021; ESMO Head and Neck Cancer Guidelines 2021)
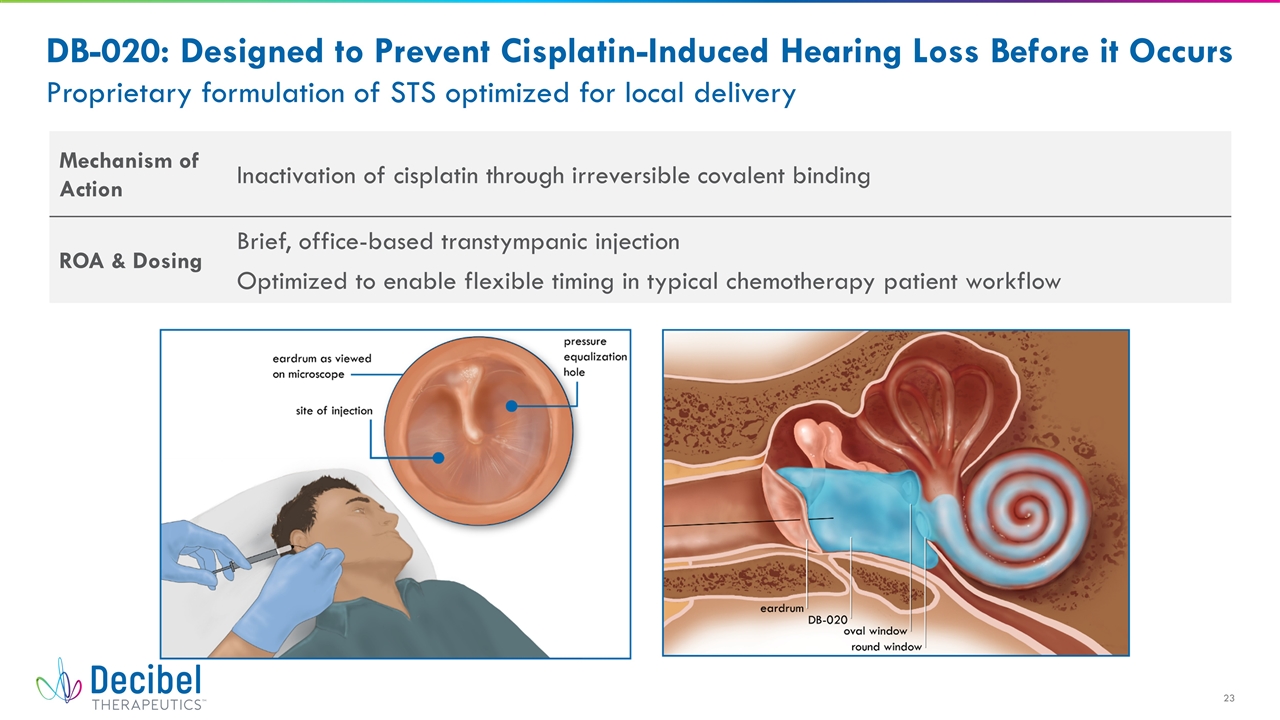
DB-020: Designed to Prevent Cisplatin-Induced Hearing Loss Before it Occurs Proprietary formulation of STS optimized for local delivery Mechanism of Action Inactivation of cisplatin through irreversible covalent binding ROA & Dosing Brief, office-based transtympanic injection Optimized to enable flexible timing in typical chemotherapy patient workflow
Summary of Interim Analysis DB-020 Proprietary formulation of sodium thiosulfate (STS) optimized for delivery to the ear Safety and Tolerability DB-020 was generally well tolerated with no significant safety issues observed Trial Design Enrolled patients randomized to receive one of two doses of DB-020 in one ear and placebo in contralateral ear Ototoxicity 88% of patients experienced ototoxicity in placebo-treated ears Interim Analysis Cohort 19 cisplatin-naïve cancer patients being treated with high dose cisplatin Efficacy 87% of patients who experienced ototoxicity in the placebo ear were partially (33%) or completely (53%) protected from ototoxicity Positive data supports continued development of DB-020 as a potential therapy to protect against hearing loss in patients receiving cisplatin chemotherapy ASHA Ototoxicity Criteria applied from 250-8000 Hz

Summary

Courtney Shanks VP, Program Management, Strategy and Operations Anna Trask, MA EVP, Chief People Officer Decibel Leadership Team Laurence Reid, PhD Chief Executive Officer John Lee Chief Development Officer Joe Burns, PhD SVP, Discovery Bridget Cleff, MBA VP, New Product Planning and Corporate Strategy Elaine Cope, M.Phil, MBA VP, Operations Jonathon Whitton, AuD, PhD Senior Clinical Advisor Elaine Kernbauer, MS VP, Development Operations Vassili Valayannopoulos, MD, PhD, MBA VP, Clinical Development

Innovative Discovery Partnership with Regeneron Integrated collaboration on research established in 2017; extended in 2021 Shared discovery teams; Decibel leadership Access to Regeneron’s world-class mouse and human genetics research platforms; gene therapy capabilities Principal focus area: monogenic hearing loss, gene therapy Target-focused collaboration; currently focused on DB-OTO, AAV.103, AAV.104 Regeneron co-funds research and development $25M upfront plus $25M in Series B equity For each collaboration product, eligible for up to $35.5M in milestones through initiation of Phase 2 Development and regulatory costs from initiation of registration trial are shared 50/50 Decibel retains worldwide development and commercial rights Regeneron eligible to receive tiered royalties

Inner Ear: Optimal Characteristics to Realize Promise of Gene Therapy Maturing Pipeline: Monogenic Hearing Loss, Hair Cell Regeneration Hearing & Balance: No Approved Therapies, Rising Awareness of Opportunity Differentiated Platform: Unique Transgene Regulation Capabilities Capitalized to Achieve Milestones: Initial Human Clinical Data, Pipeline Milestones



























