Topline Phase 2 Results from Atumelnant in �Congenital Adrenal Hyperplasia (CAH) January 10, 2025 Exhibit 99.2

Safe Harbor Statement This presentation contains forward-looking statements. Crinetics Pharmaceuticals, Inc. (“Crinetics,” the “company,” “we,” “us,” or “our”) cautions you that statements contained in this presentation regarding matters that are not historical facts are forward-looking statements. These statements are based on the company’s current beliefs and expectations. Such forward-looking statements include, but are not limited to, statements regarding: the plans and timelines for the clinical development of atumelnant, including the therapeutic potential and clinical benefits or safety profile in patients with CAH or Cushing’s Disease and the expected plans and timing for ongoing clinical studies and related initiatives. In some cases, you can identify forward-looking statements by terms such as “may,” “believe,” “anticipate,” “could,” “should,” “estimate,” “expect,” “intend,” “plan,” “project,” “will,” “contemplate,” “predict,” “continue,” “forecast,” “aspire,” ”lead to,” “designed to,” “goal,” “potential,” “target” or the negative of or other similar terms. These statements speak only as of the date of this presentation, involve known and unknown risks, uncertainties, assumptions, and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, without limitation: topline and initial data that we report may change following completion or a more comprehensive review of the data related to the clinical studies and such data may not accurately reflect the complete results of a clinical study, and the FDA and other regulatory authorities may not agree with our interpretation of such results; the possibility of unfavorable new clinical data and further analyses of existing clinical data; potential delays in the commencement, enrollment and completion of clinical studies and the reporting of data therefrom; the FDA or other regulatory agencies may suggest changes to our planned clinical studies; international conflicts may disrupt our business and that of the third parties on which we depend, including delaying or otherwise disrupting our clinical studies and preclinical studies, manufacturing and supply chain, or impairing employee productivity; our dependence on third parties in connection with product manufacturing, research and preclinical and clinical testing; regulatory developments or price restrictions in the United States and foreign countries; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval and/or commercialization; clinical studies and preclinical studies may not proceed at the time or in the manner expected, or at all; the timing and outcome of research, development and regulatory review is uncertain, and our drug candidates may not advance in development or be approved for marketing; our ability to obtain and maintain intellectual property protection for our product candidates; we may use our capital resources sooner than we expect; and other risks described under the heading “Risk Factors” in documents we file from time to time with the Securities and Exchange Commission (“SEC”). Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and, except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
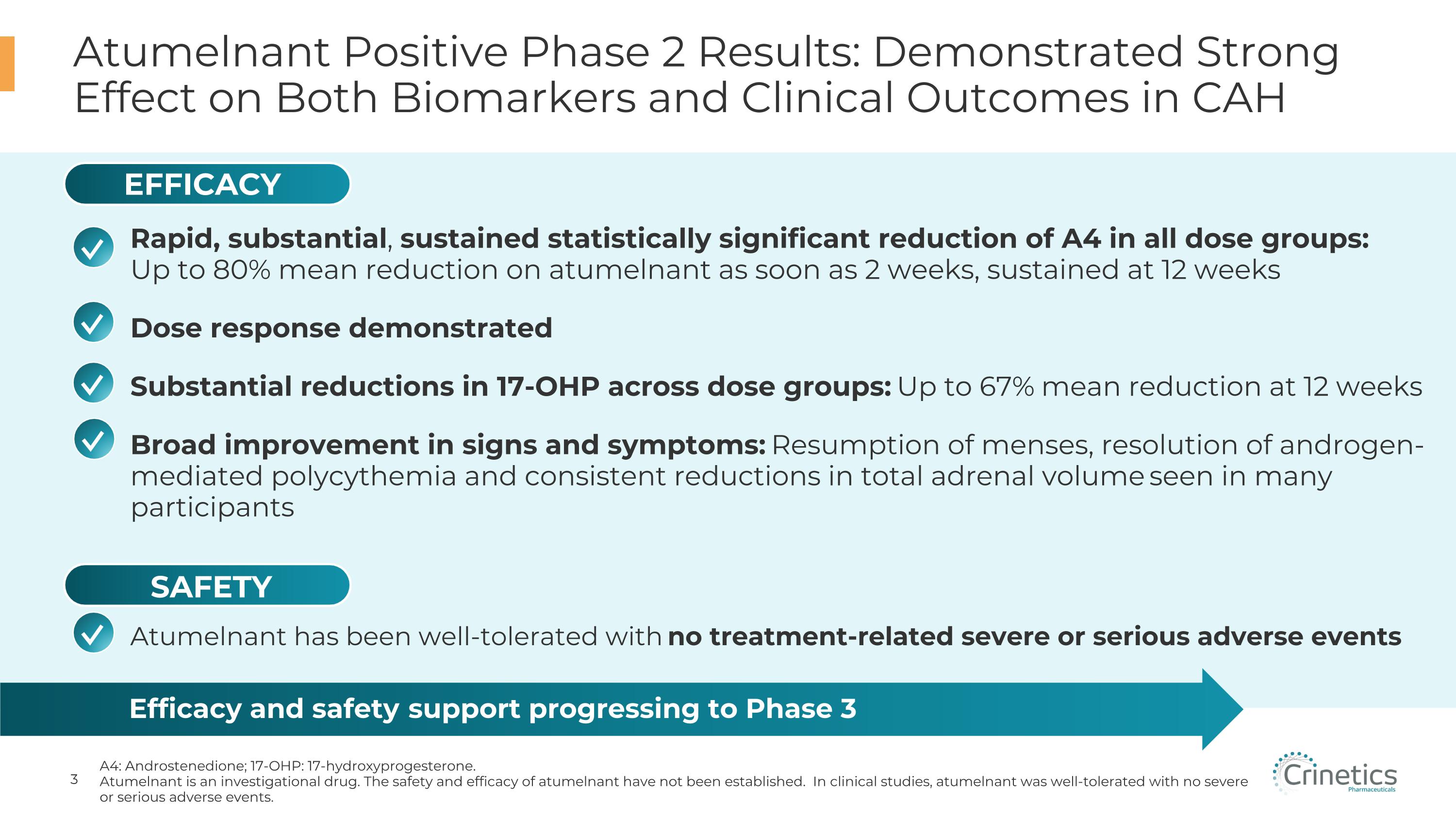
Atumelnant Positive Phase 2 Results: Demonstrated Strong Effect on Both Biomarkers and Clinical Outcomes in CAH A4: Androstenedione; 17-OHP: 17-hydroxyprogesterone. Atumelnant is an investigational drug. The safety and efficacy of atumelnant have not been established. In clinical studies, atumelnant was well-tolerated with no severe or serious adverse events. Efficacy and safety support progressing to Phase 3 Atumelnant has been well-tolerated with no treatment-related severe or serious adverse events ✓ ✓ ✓ EFFICACY SAFETY ✓ Rapid, substantial, sustained statistically significant reduction of A4 in all dose groups: Up to 80% mean reduction on atumelnant as soon as 2 weeks, sustained at 12 weeks Dose response demonstrated Substantial reductions in 17-OHP across dose groups: Up to 67% mean reduction at 12 weeks Broad improvement in signs and symptoms: Resumption of menses, resolution of androgen-mediated polycythemia and consistent reductions in total adrenal volume seen in many participants ✓
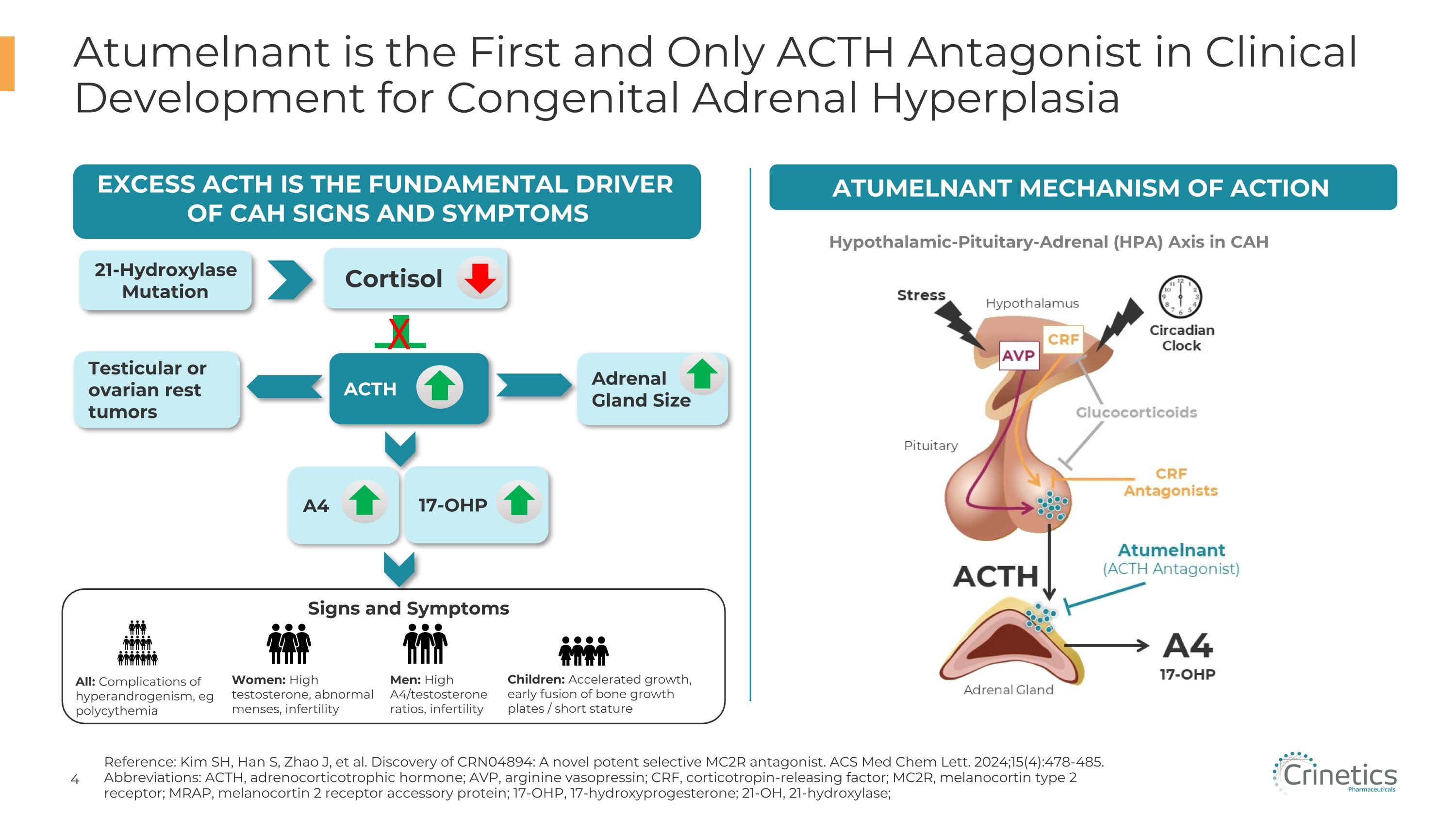
Atumelnant is the First and Only ACTH Antagonist in Clinical Development for Congenital Adrenal Hyperplasia Reference: Kim SH, Han S, Zhao J, et al. Discovery of CRN04894: A novel potent selective MC2R antagonist. ACS Med Chem Lett. 2024;15(4):478-485. Abbreviations: ACTH, adrenocorticotrophic hormone; AVP, arginine vasopressin; CRF, corticotropin-releasing factor; MC2R, melanocortin type 2 receptor; MRAP, melanocortin 2 receptor accessory protein; 17-OHP, 17-hydroxyprogesterone; 21-OH, 21-hydroxylase; Atumelnant Mechanism of Action Hypothalamic-Pituitary-Adrenal (HPA) Axis in CAH Excess ACTH IS THE FUNDAMENTAL DRIVER OF CAH Signs and symptoms 21-Hydroxylase Mutation Adrenal Gland Size A4 Cortisol ACTH Signs and Symptoms All: Complications of hyperandrogenism, eg polycythemia Women: High testosterone, abnormal menses, infertility Men: High A4/testosterone ratios, infertility Children: Accelerated growth, early fusion of bone growth plates / short stature 17-OHP Testicular or ovarian rest tumors X

CAH Affects ~17,000 Addressable Adult and Pediatric Patients in the US Treatment Goals in Adults with CAH Reduction of A4 and other androgens to address hyperandrogenism, which can manifest as excessive facial hair, acne and polycythemia Restore normal menstrual cycles and fertility in women Shrink testicular adrenal rest tumors, alleviate pain and restore fertility in men Eliminate excessive exposure to glucocorticoids to minimize steroid therapy related adverse effects including weight gain, cardiovascular issues, diabetes, and osteoporosis Abram Living with �CAH “There's so many different facets of a person's life that it can affect. It can demoralize people. It takes a toll.” – Spouse of a CAH Patient CAH Has a Range of Clinical Implications
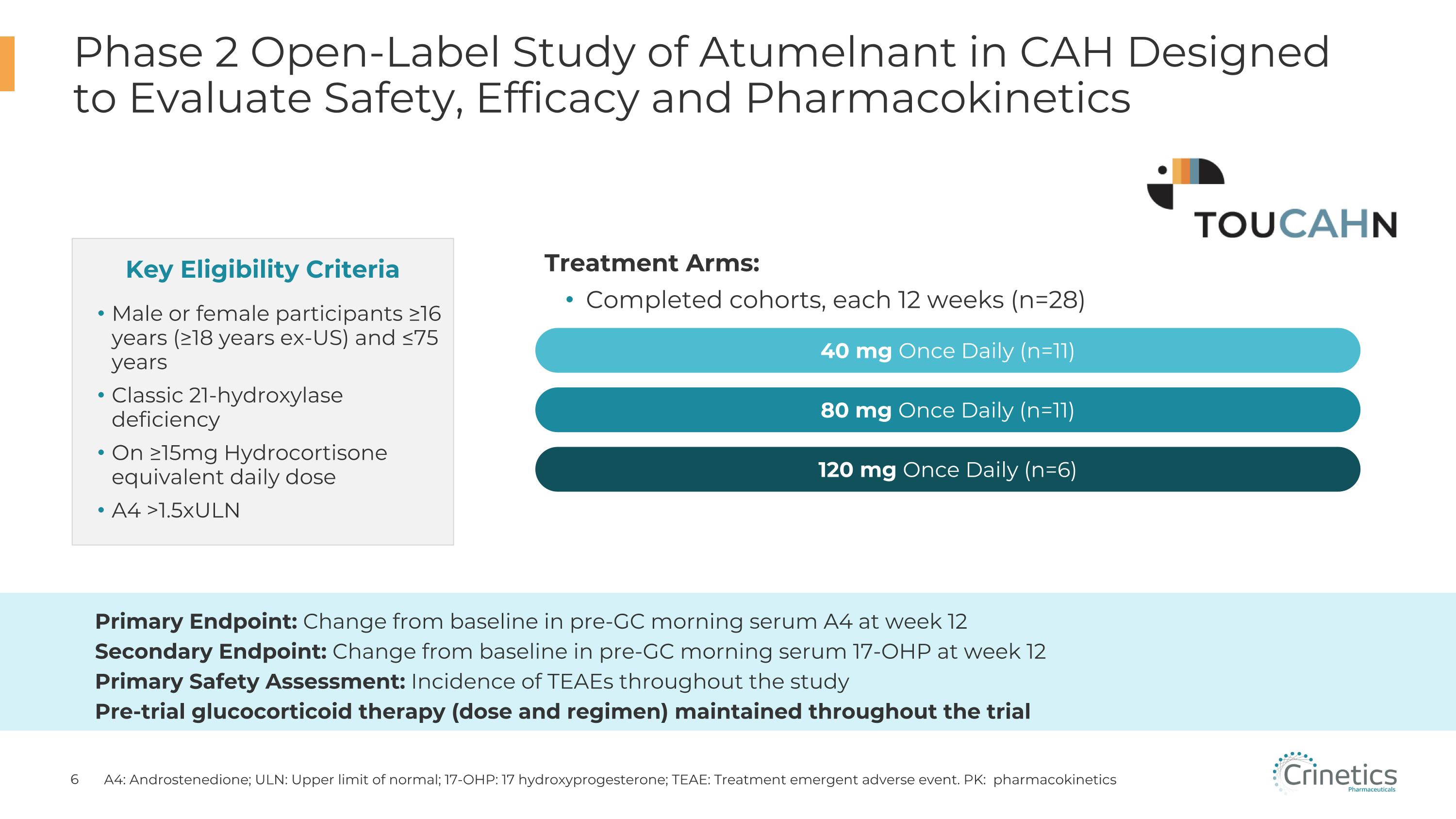
Male or female participants ≥16 years (≥18 years ex-US) and ≤75 years Classic 21-hydroxylase deficiency On ≥15mg Hydrocortisone equivalent daily dose A4 >1.5xULN Phase 2 Open-Label Study of Atumelnant in CAH Designed to Evaluate Safety, Efficacy and Pharmacokinetics A4: Androstenedione; ULN: Upper limit of normal; 17-OHP: 17 hydroxyprogesterone; TEAE: Treatment emergent adverse event. PK: pharmacokinetics Key Eligibility Criteria Treatment Arms: Completed cohorts, each 12 weeks (n=28) Primary Endpoint: Change from baseline in pre-GC morning serum A4 at week 12 Secondary Endpoint: Change from baseline in pre-GC morning serum 17-OHP at week 12 Primary Safety Assessment: Incidence of TEAEs throughout the study Pre-trial glucocorticoid therapy (dose and regimen) maintained throughout the trial 80 mg Once Daily (n=11) 40 mg Once Daily (n=11) 120 mg Once Daily (n=6)
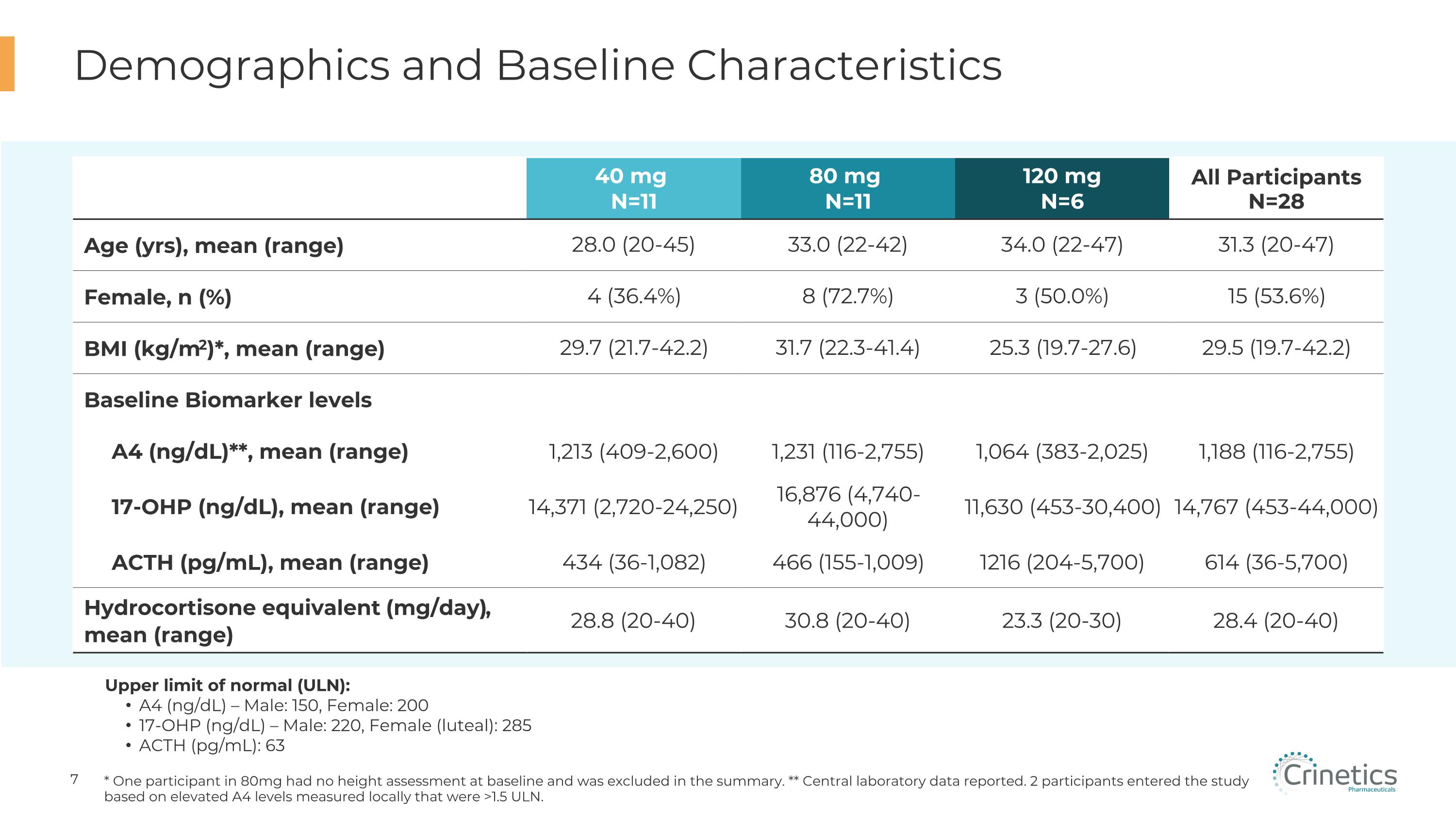
Demographics and Baseline Characteristics * One participant in 80mg had no height assessment at baseline and was excluded in the summary. ** Central laboratory data reported. 2 participants entered the study based on elevated A4 levels measured locally that were >1.5 ULN. Upper limit of normal (ULN): A4 (ng/dL) – Male: 150, Female: 200 17-OHP (ng/dL) – Male: 220, Female (luteal): 285 ACTH (pg/mL): 63 40 mg N=11 80 mg N=11 120 mg N=6 All Participants N=28 Age (yrs), mean (range) 28.0 (20-45) 33.0 (22-42) 34.0 (22-47) 31.3 (20-47) Female, n (%) 4 (36.4%) 8 (72.7%) 3 (50.0%) 15 (53.6%) BMI (kg/m2)*, mean (range) 29.7 (21.7-42.2) 31.7 (22.3-41.4) 25.3 (19.7-27.6) 29.5 (19.7-42.2) Baseline Biomarker levels A4 (ng/dL)**, mean (range) 1,213 (409-2,600) 1,231 (116-2,755) 1,064 (383-2,025) 1,188 (116-2,755) 17-OHP (ng/dL), mean (range) 14,371 (2,720-24,250) 16,876 (4,740-44,000) 11,630 (453-30,400) 14,767 (453-44,000) ACTH (pg/mL), mean (range) 434 (36-1,082) 466 (155-1,009) 1216 (204-5,700) 614 (36-5,700) Hydrocortisone equivalent (mg/day), �mean (range) 28.8 (20-40) 30.8 (20-40) 23.3 (20-30) 28.4 (20-40)
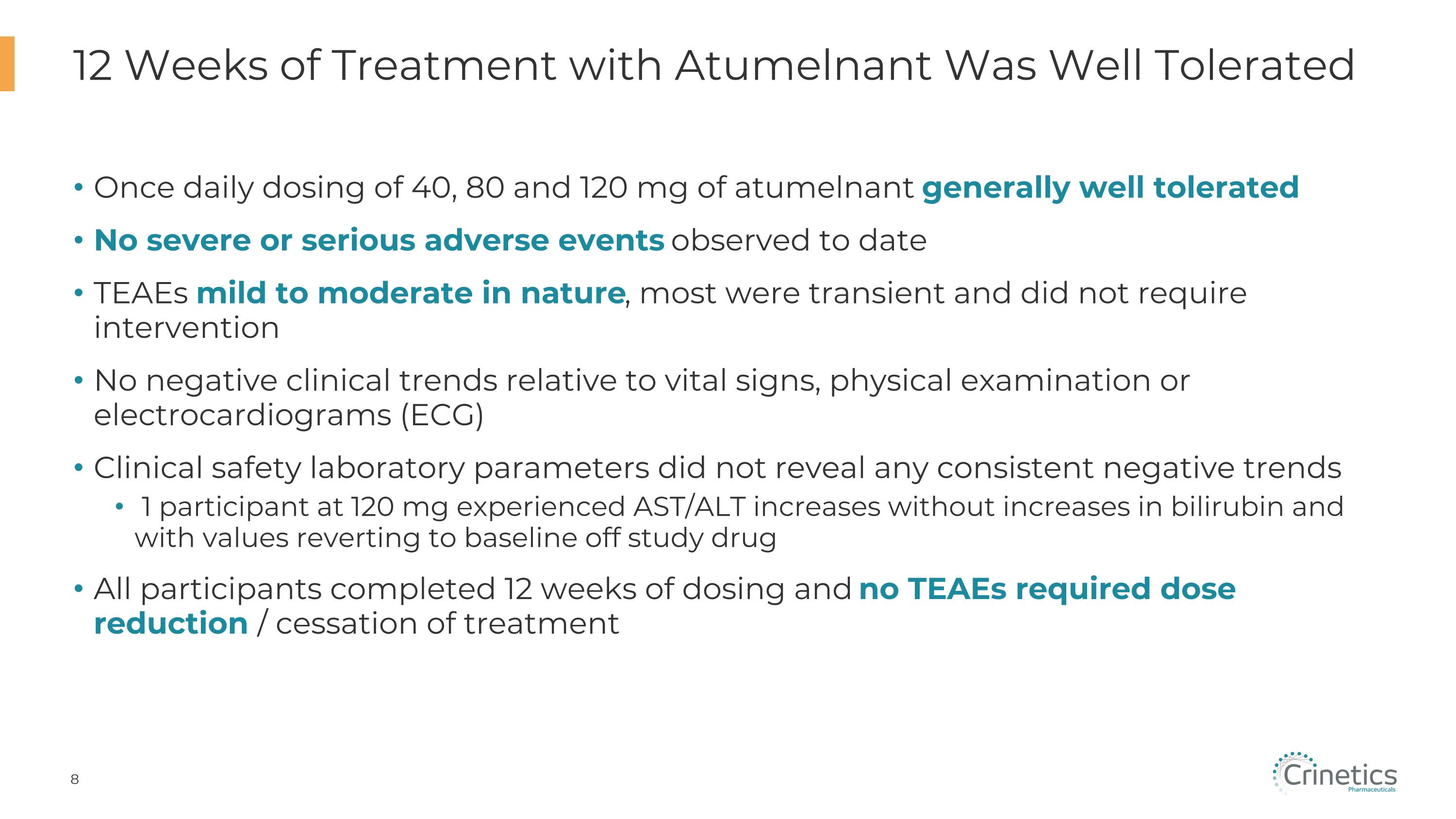
Once daily dosing of 40, 80 and 120 mg of atumelnant generally well tolerated No severe or serious adverse events observed to date TEAEs mild to moderate in nature, most were transient and did not require intervention No negative clinical trends relative to vital signs, physical examination or electrocardiograms (ECG) Clinical safety laboratory parameters did not reveal any consistent negative trends 1 participant at 120 mg experienced AST/ALT increases without increases in bilirubin and with values reverting to baseline off study drug All participants completed 12 weeks of dosing and no TEAEs required dose reduction / cessation of treatment 12 Weeks of Treatment with Atumelnant Was Well Tolerated
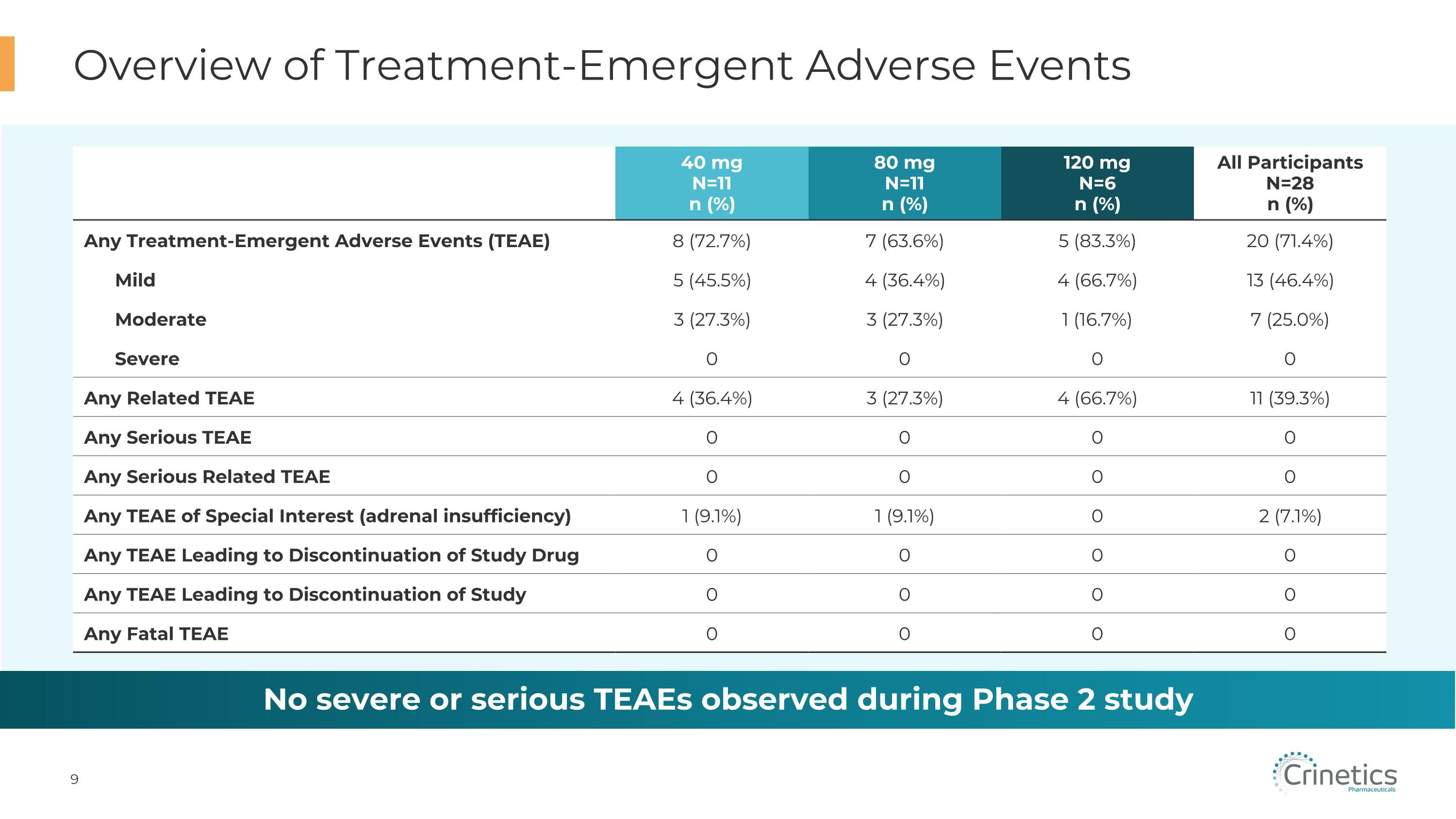
40 mg N=11 n (%) 80 mg N=11 n (%) 120 mg N=6 n (%) All Participants�N=28 n (%) Any Treatment-Emergent Adverse Events (TEAE) 8 (72.7%) 7 (63.6%) 5 (83.3%) 20 (71.4%) Mild 5 (45.5%) 4 (36.4%) 4 (66.7%) 13 (46.4%) Moderate 3 (27.3%) 3 (27.3%) 1 (16.7%) 7 (25.0%) Severe 0 0 0 0 Any Related TEAE 4 (36.4%) 3 (27.3%) 4 (66.7%) 11 (39.3%) Any Serious TEAE 0 0 0 0 Any Serious Related TEAE 0 0 0 0 Any TEAE of Special Interest (adrenal insufficiency) 1 (9.1%) 1 (9.1%) 0 2 (7.1%) Any TEAE Leading to Discontinuation of Study Drug 0 0 0 0 Any TEAE Leading to Discontinuation of Study 0 0 0 0 Any Fatal TEAE 0 0 0 0 Overview of Treatment-Emergent Adverse Events No severe or serious TEAEs observed during Phase 2 study
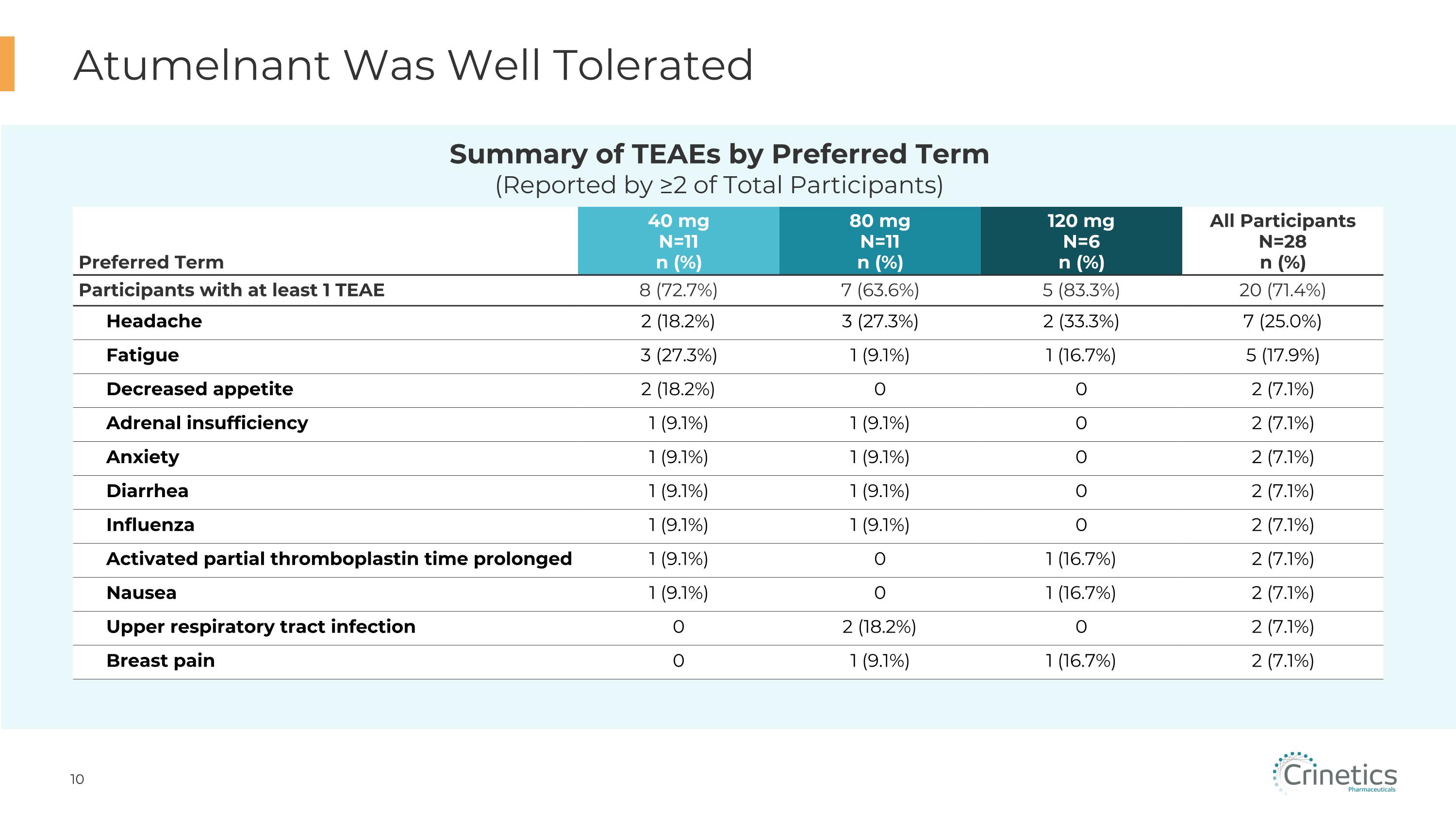
Atumelnant Was Well Tolerated Preferred Term 40 mg N=11 n (%) 80 mg N=11 n (%) 120 mg N=6 n (%) All Participants�N=28 n (%) Participants with at least 1 TEAE 8 (72.7%) 7 (63.6%) 5 (83.3%) 20 (71.4%) Headache 2 (18.2%) 3 (27.3%) 2 (33.3%) 7 (25.0%) Fatigue 3 (27.3%) 1 (9.1%) 1 (16.7%) 5 (17.9%) Decreased appetite 2 (18.2%) 0 0 2 (7.1%) Adrenal insufficiency 1 (9.1%) 1 (9.1%) 0 2 (7.1%) Anxiety 1 (9.1%) 1 (9.1%) 0 2 (7.1%) Diarrhea 1 (9.1%) 1 (9.1%) 0 2 (7.1%) Influenza 1 (9.1%) 1 (9.1%) 0 2 (7.1%) Activated partial thromboplastin time prolonged 1 (9.1%) 0 1 (16.7%) 2 (7.1%) Nausea 1 (9.1%) 0 1 (16.7%) 2 (7.1%) Upper respiratory tract infection 0 2 (18.2%) 0 2 (7.1%) Breast pain 0 1 (9.1%) 1 (16.7%) 2 (7.1%) Summary of TEAEs by Preferred Term�(Reported by ≥2 of Total Participants)
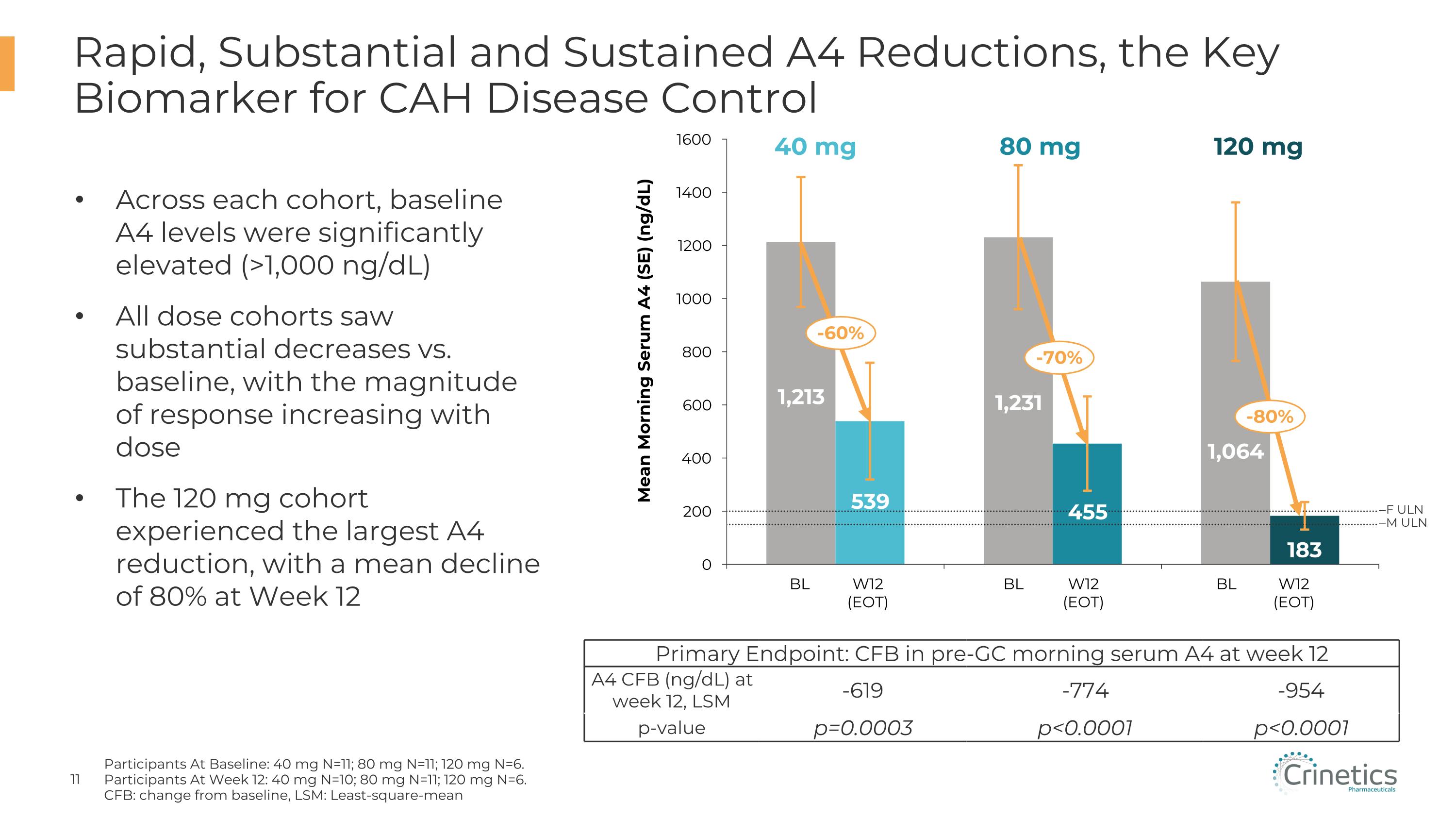
Rapid, Substantial and Sustained A4 Reductions, the Key Biomarker for CAH Disease Control Participants At Baseline: 40 mg N=11; 80 mg N=11; 120 mg N=6. Participants At Week 12: 40 mg N=10; 80 mg N=11; 120 mg N=6. CFB: change from baseline, LSM: Least-square-mean Across each cohort, baseline A4 levels were significantly elevated (>1,000 ng/dL) All dose cohorts saw substantial decreases vs. baseline, with the magnitude of response increasing with dose The 120 mg cohort experienced the largest A4 reduction, with a mean decline of 80% at Week 12 Mean Morning Serum A4 (SE) (ng/dL) BL W12�(EOT) BL W12�(EOT) BL W12�(EOT) F ULN M ULN -60% -70% -80% Primary Endpoint: CFB in pre-GC morning serum A4 at week 12 A4 CFB (ng/dL) at week 12, LSM -619 -774 -954 p-value p=0.0003 p<0.0001 p<0.0001 40 mg 80 mg 120 mg
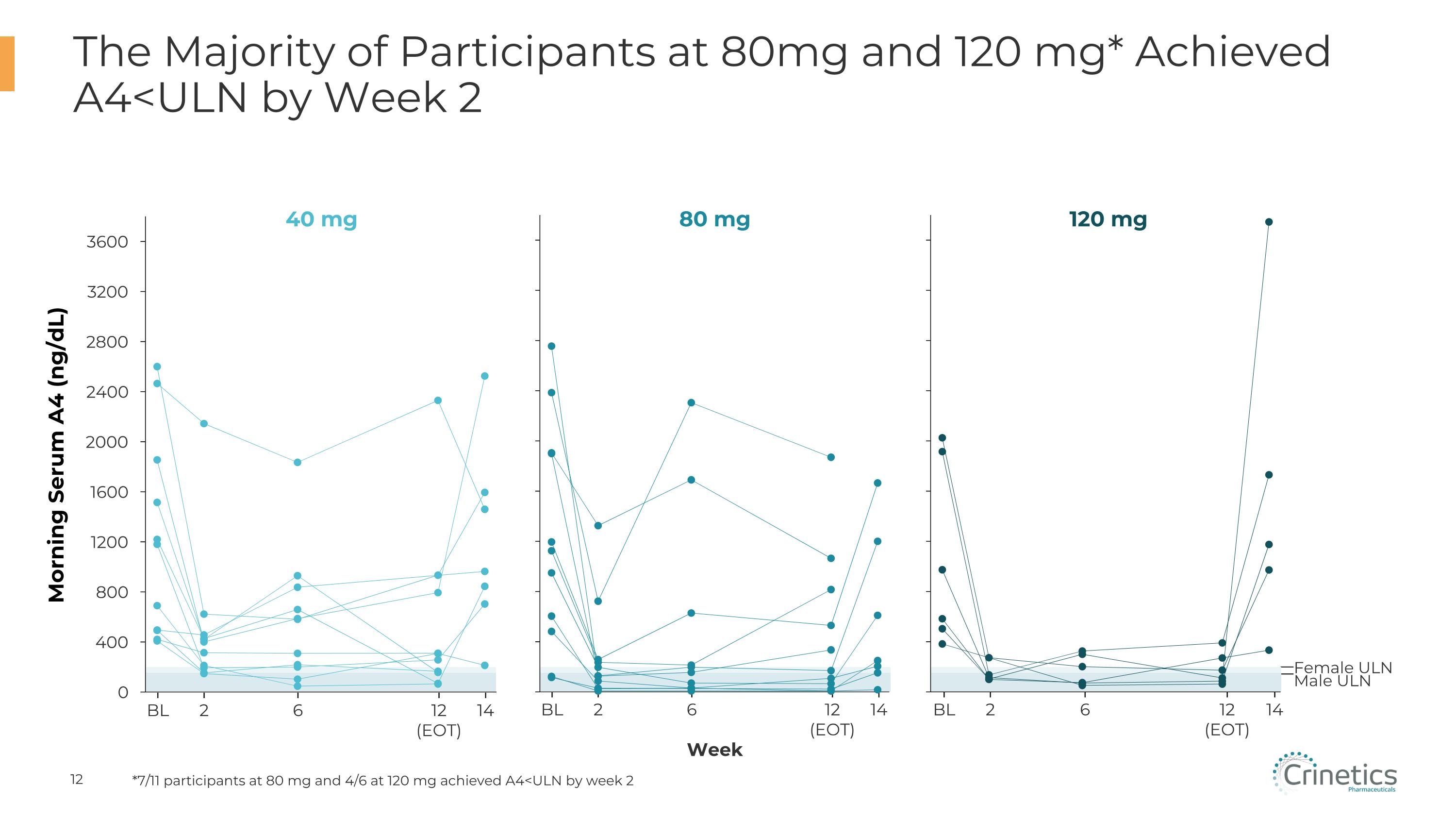
Female ULN Male ULN 2 BL 6 12�(EOT) 14 The Majority of Participants at 80mg and 120 mg* Achieved A4<ULN by Week 2 40 mg Morning Serum A4 (ng/dL) 80 mg Week 120 mg 2 BL 6 12�(EOT) 14 2 BL 6 12�(EOT) 14 *7/11 participants at 80 mg and 4/6 at 120 mg achieved A4<ULN by week 2
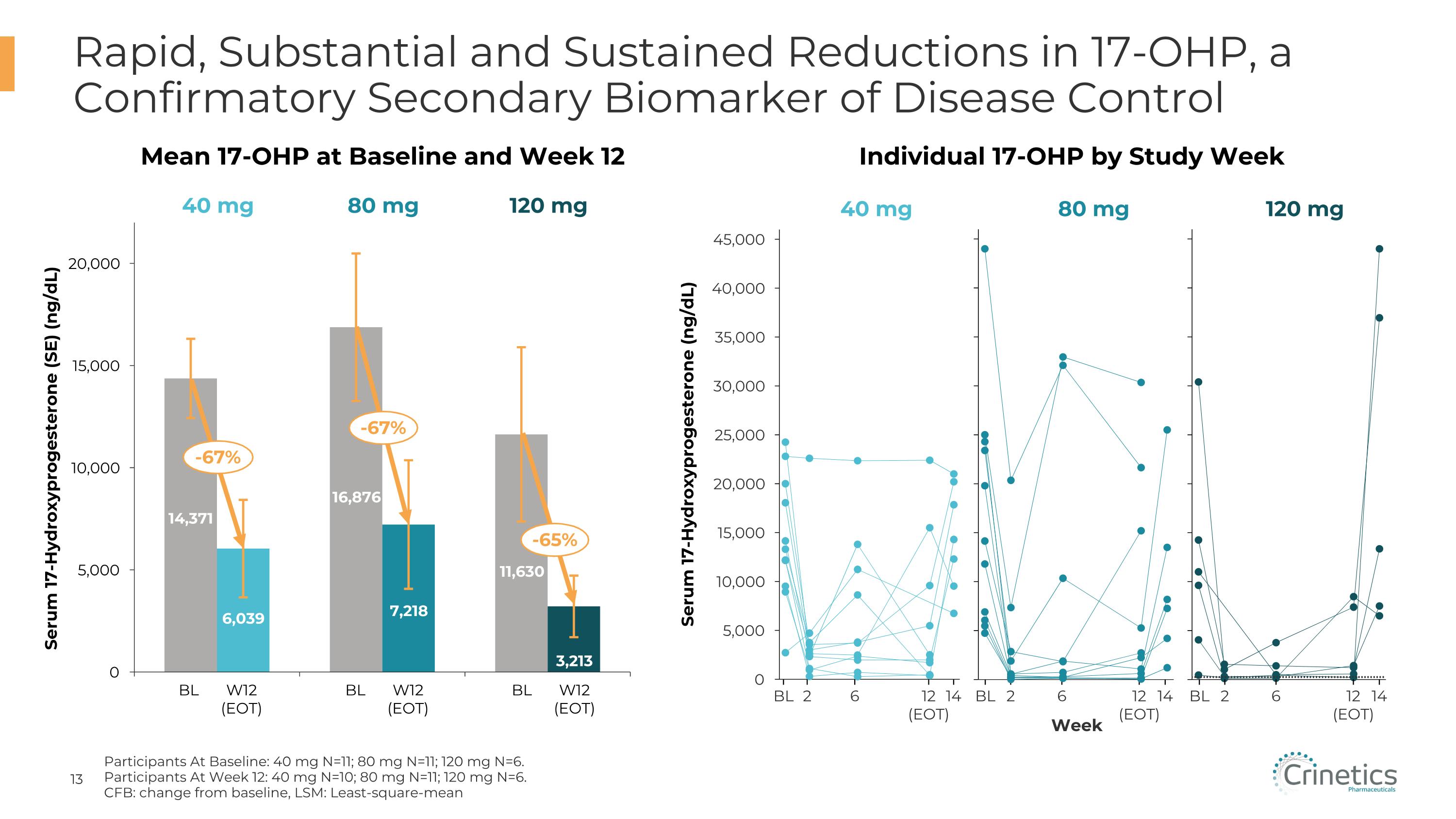
Rapid, Substantial and Sustained Reductions in 17-OHP, a Confirmatory Secondary Biomarker of Disease Control Serum 17-Hydroxyprogesterone (SE) (ng/dL) BL W12�(EOT) BL W12�(EOT) BL W12�(EOT) 2 BL 6 12�(EOT) 14 Serum 17-Hydroxyprogesterone (ng/dL) Week 2 BL 6 12�(EOT) 14 2 BL 6 12�(EOT) 14 40 mg 80 mg 120 mg 40 mg 80 mg 120 mg Mean 17-OHP at Baseline and Week 12 Individual 17-OHP by Study Week -67% -67% -65% Participants At Baseline: 40 mg N=11; 80 mg N=11; 120 mg N=6. Participants At Week 12: 40 mg N=10; 80 mg N=11; 120 mg N=6. CFB: change from baseline, LSM: Least-square-mean
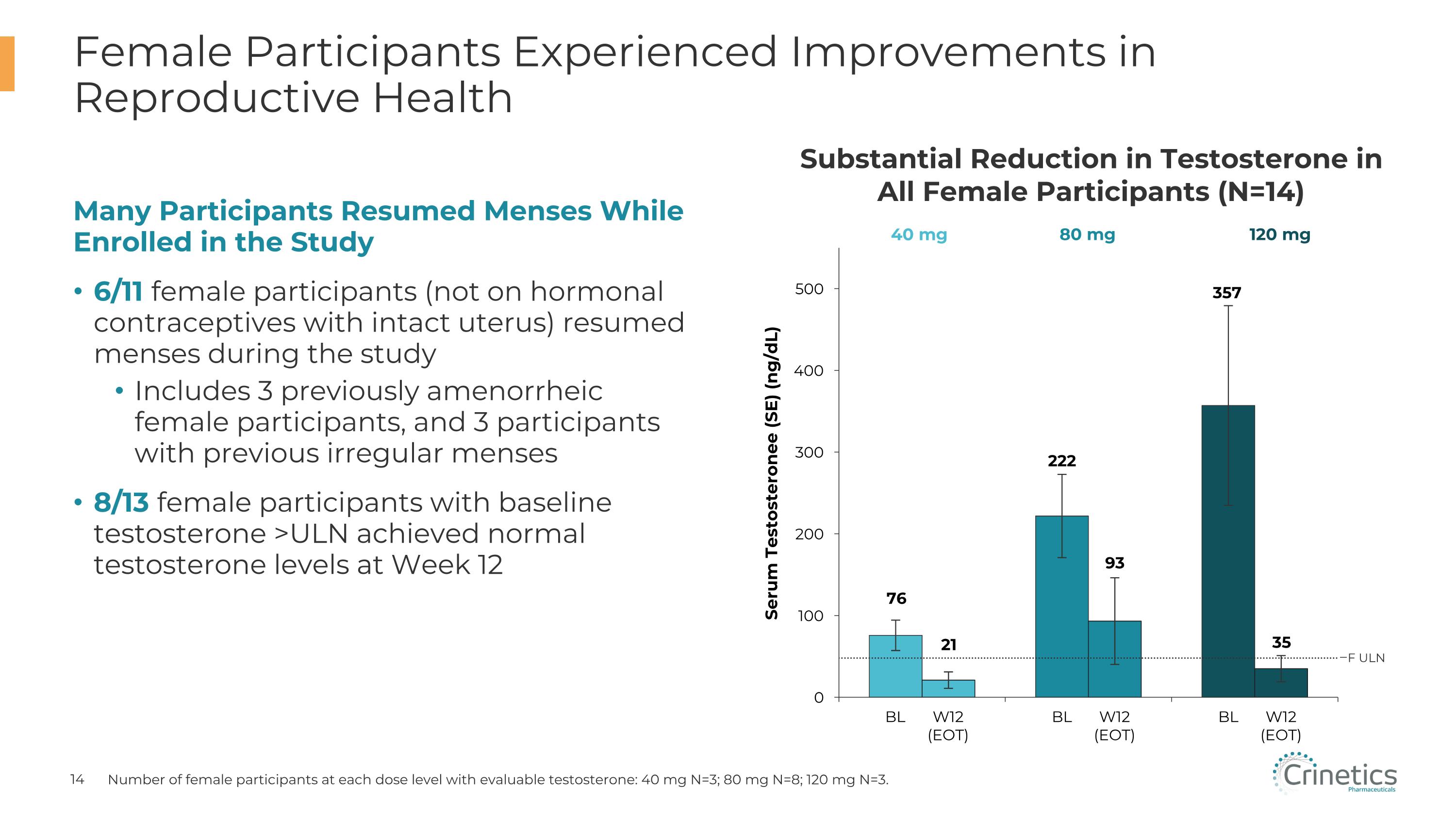
Many Participants Resumed Menses While Enrolled in the Study 6/11 female participants (not on hormonal contraceptives with intact uterus) resumed menses during the study Includes 3 previously amenorrheic female participants, and 3 participants with previous irregular menses 8/13 female participants with baseline testosterone >ULN achieved normal testosterone levels at Week 12 Female Participants Experienced Improvements in Reproductive Health Number of female participants at each dose level with evaluable testosterone: 40 mg N=3; 80 mg N=8; 120 mg N=3. Substantial Reduction in Testosterone in All Female Participants (N=14) Serum Testosteronee (SE) (ng/dL) 40 mg 80 mg 120 mg BL W12�(EOT) BL W12�(EOT) BL W12�(EOT) F ULN
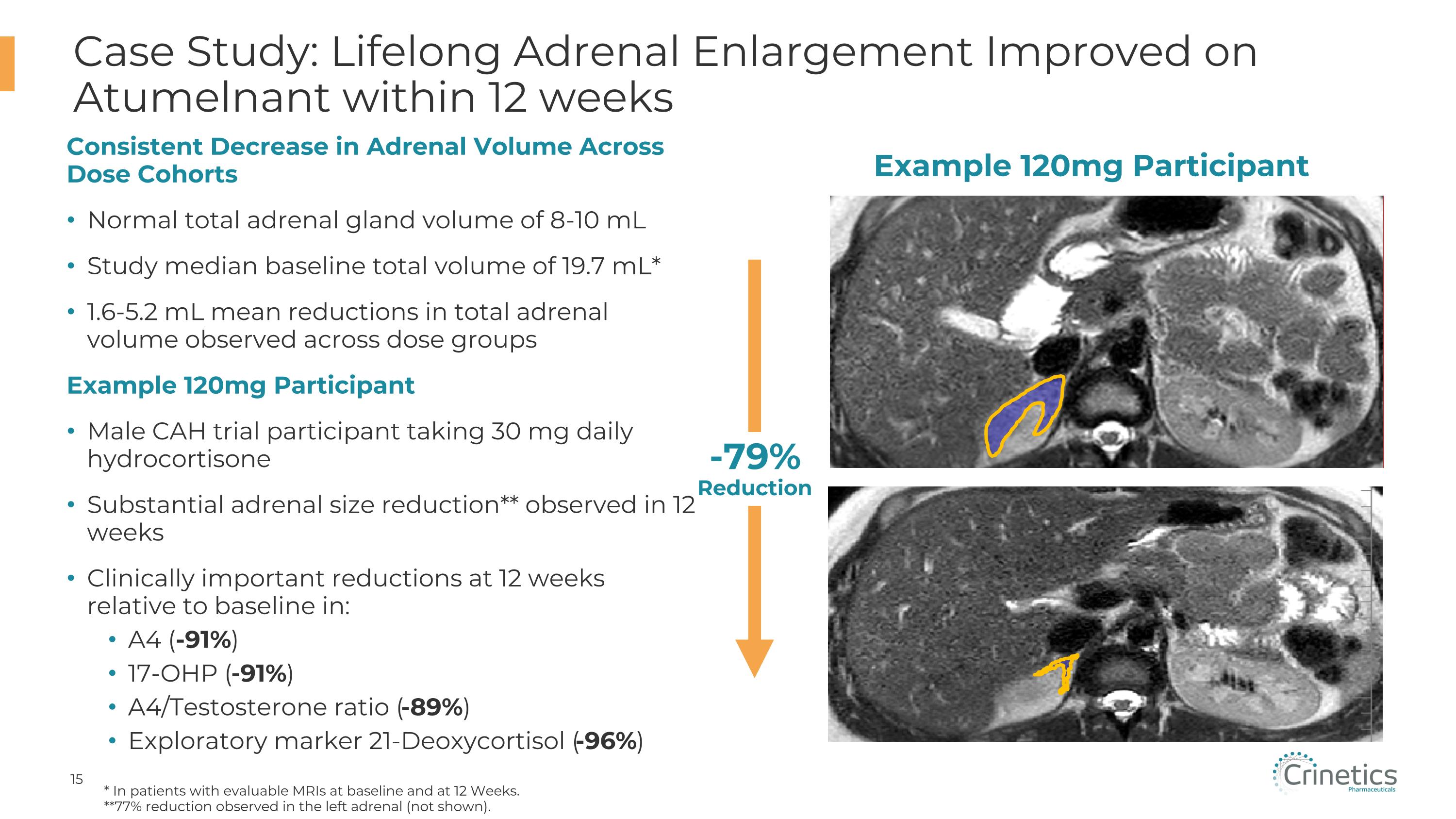
Case Study: Lifelong Adrenal Enlargement Improved on Atumelnant within 12 weeks * In patients with evaluable MRIs at baseline and at 12 Weeks. **77% reduction observed in the left adrenal (not shown). CONFIDENTIAL Example 120mg Participant -79%�Reduction Consistent Decrease in Adrenal Volume Across Dose Cohorts Normal total adrenal gland volume of 8-10 mL Study median baseline total volume of 19.7 mL* 1.6-5.2 mL mean reductions in total adrenal volume observed across dose groups Example 120mg Participant Male CAH trial participant taking 30 mg daily hydrocortisone Substantial adrenal size reduction** observed in 12 weeks Clinically important reductions at 12 weeks relative to baseline in: A4 (-91%) 17-OHP (-91%) A4/Testosterone ratio (-89%) Exploratory marker 21-Deoxycortisol (-96%)
Significant Clinical Improvements Achieved with Atumelnant Treatment CAH Manifestations Achieved following 12 weeks of treatment with atumelnant Overproduction of androgens, �and androgen precursors Normalization of A4 in many participants and substantial reduction in 17-OHP levels (across dose groups) Females: Elevated testosterone levels Absent/irregular menses Testosterone substantially reduced/normalized in the majority of participants; 6/11 participants resumed menses Males: Elevated A4/testosterone ratio Clinically relevant reductions in many participants Adrenal gland hyperplasia Consistent reductions in adrenal volume Androgen mediated polycythemia�(linked to increased cardiovascular risks) Resolution in 5/6 participants with polycythemia Hirsutism and acne Improvements reported, longer treatment likely needed for full effects ✓ ✓ ✓ ✓ ✓ ✓
Summary of Results and Next Steps Summary: Successful Phase 2 Results Rapid and sustained treatment effect, including in participants with high baseline A4 Clinical activity observed across all doses (once daily 40, 80 and 120 mg) Dose response demonstrated 80 mg and 120 mg as the likely therapeutic doses of choice Evidence of meaningful improvement in multiple clinical signs and symptoms No serious adverse events and no severe related adverse events All participants completed 12 weeks of treatment Next Steps Initiate Phase 3 pivotal trial in adult CAH population in 1H25 Start Phase 2/3 pivotal trial in pediatric CAH population
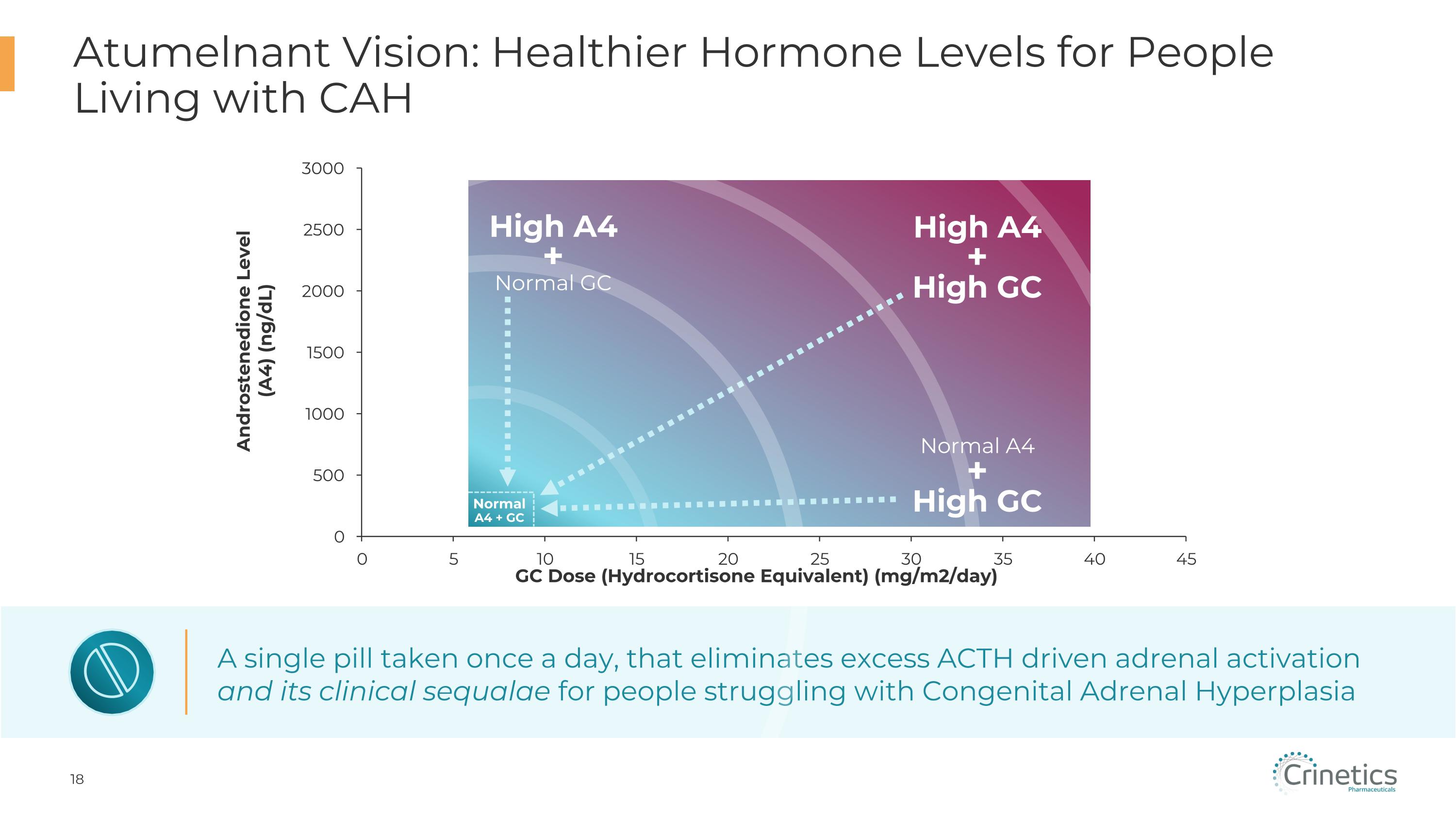
A single pill taken once a day, that eliminates excess ACTH driven adrenal activation and its clinical sequalae for people struggling with Congenital Adrenal Hyperplasia Normal�A4 + GC High A4 + Normal GC High A4 + High GC Normal A4 + High GC Atumelnant Vision: Healthier Hormone Levels for People Living with CAH Androstenedione Level (A4) (ng/dL)

Q&A Scott Struthers, Ph.D. Founder and Chief Executive Officer Dana Pizzuti, M.D. Chief Medical & Development Officer Alan Krasner, M.D. Chief Endocrinologist Marc Wilson Chief Financial Officer
Abbreviations CAH Congenital adrenal hyperplasia CRF Corticotropin releasing factor EOT End of treatment GC Glucocorticoid TEAE Treatment emergent adverse event TART Testicular adrenal rests tumors ULN Upper limit of normal 17-OHP 17-hydroxyprogesterone A4 Androstenedione ACTH Adrenocorticotrophic hormone ALT Alanine Transaminase AST Aspartate Transaminase AVP Arginine Vasopressin BL Baseline BMI Body Mass Index



















