
Developing breakthrough therapies for highly resistant cancers May 2019 Corporate Presentation (Nasdaq: MBRX) 1

Disclaimer All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. "Risk Factors" in our most recently filed Form 10-K filed with the Securities and Exchange Commission ("SEC") and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements. More detailed information about Moleculin is set forth in our filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. 2

Moleculin Biotech, Inc. is a clinical stage pharmaceutical company focused on developing breakthrough treatments for highly resistant cancers. These highly resistant cancers include: • deadly glioblastoma brain tumors • acute myeloid leukemia • pancreatic cancer • cutaneous T-cell lymphoma (a deadly form of skin cancer) Our diverse pipeline of technologies was built around the recognition that treatment of resistant tumors tend to have a common set of traits: • Multidrug resistance • Immune evasion • Upregulation of oncogenic transcription factors • Increase in dependence on glycolysis Each of these elements is addressed by the unique and innovative mechanisms introduced by one or more of our three core technologies. 3
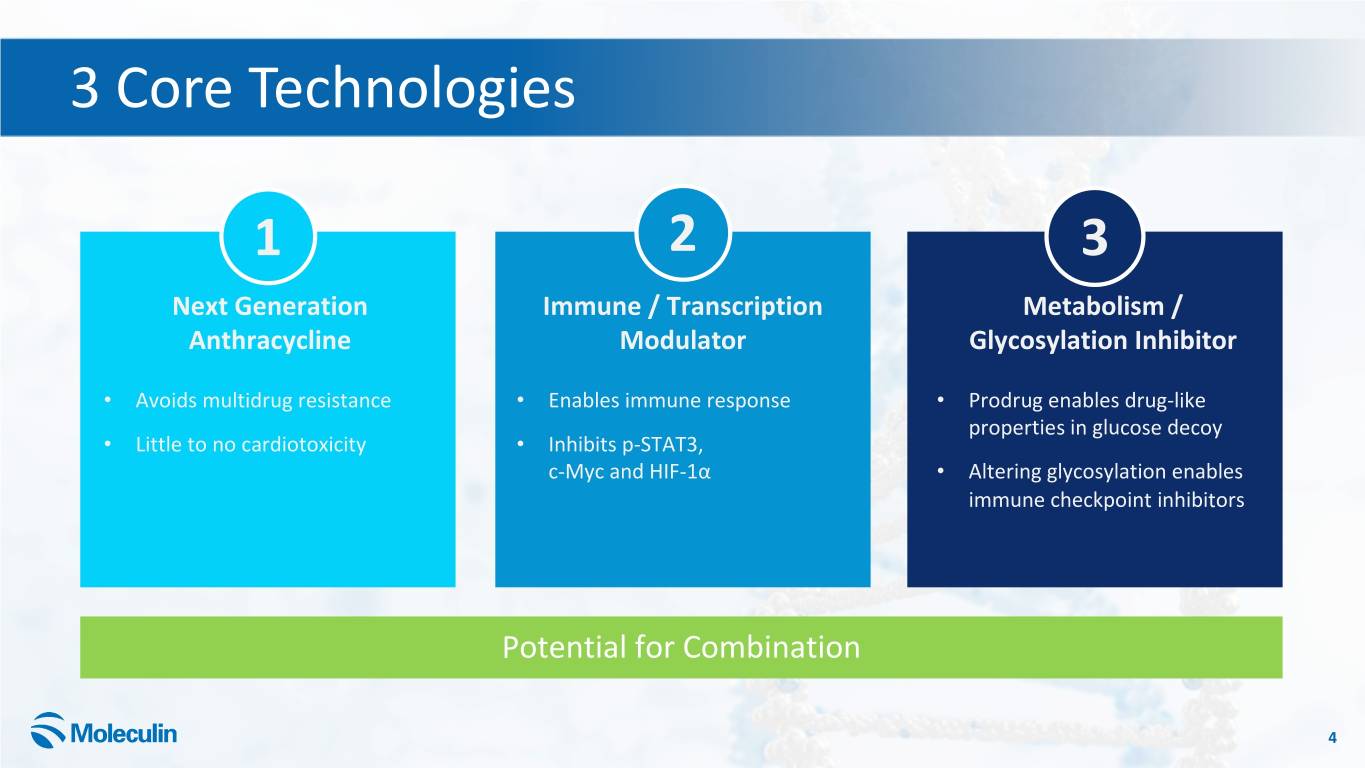
3 Core Technologies 1 2 3 Next Generation Immune / Transcription Metabolism / Anthracycline Modulator Glycosylation Inhibitor • Avoids multidrug resistance • Enables immune response • Prodrug enables drug-like properties in glucose decoy • Little to no cardiotoxicity • Inhibits p-STAT3, c-Myc and HIF-1α • Altering glycosylation enables immune checkpoint inhibitors Potential for Combination 4
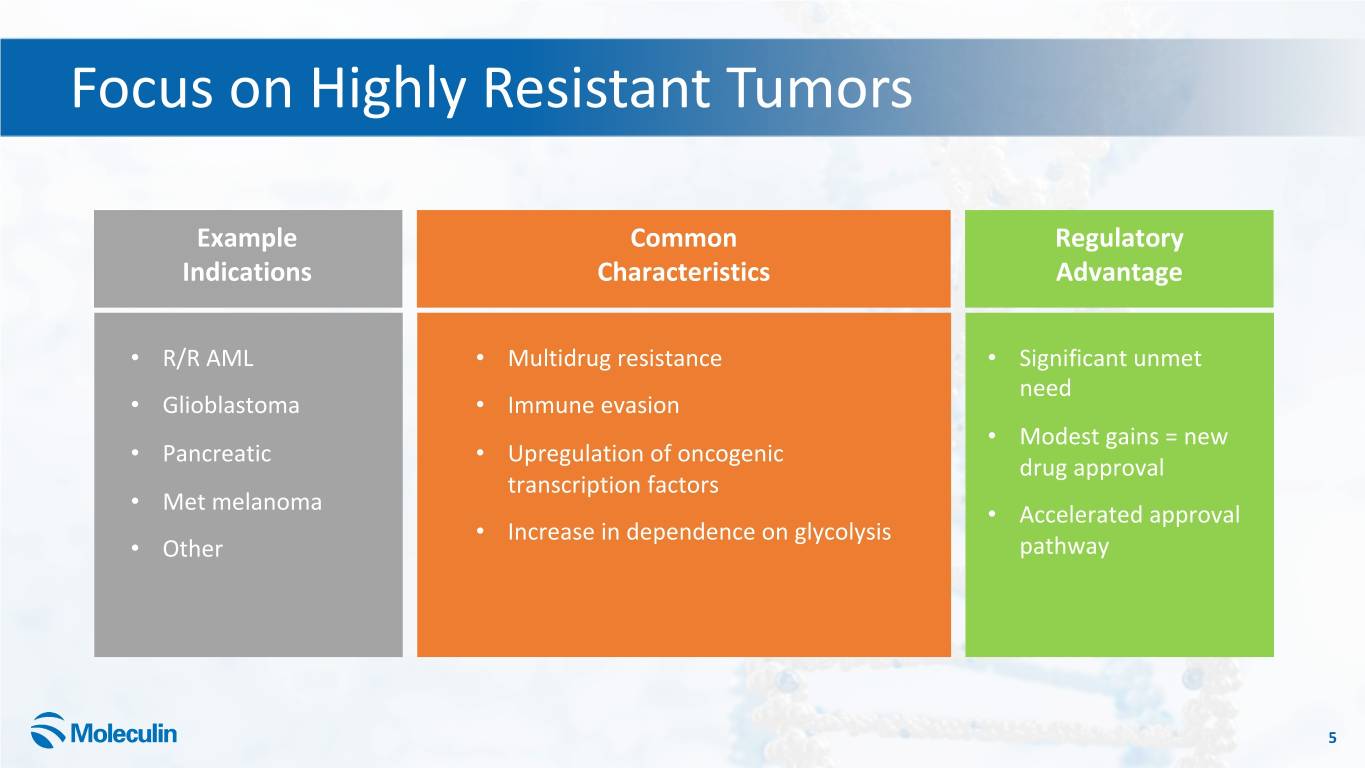
Focus on Highly Resistant Tumors Example Common Regulatory Indications Characteristics Advantage • R/R AML • Multidrug resistance • Significant unmet need • Glioblastoma • Immune evasion • Modest gains = new • Pancreatic • Upregulation of oncogenic drug approval transcription factors • Met melanoma • Accelerated approval • Increase in dependence on glycolysis • Other pathway 5

Partnerships & Collaborations Our world-leading partnerships & collaborations significantly expand our research capabilities and increase our knowledgebase 6
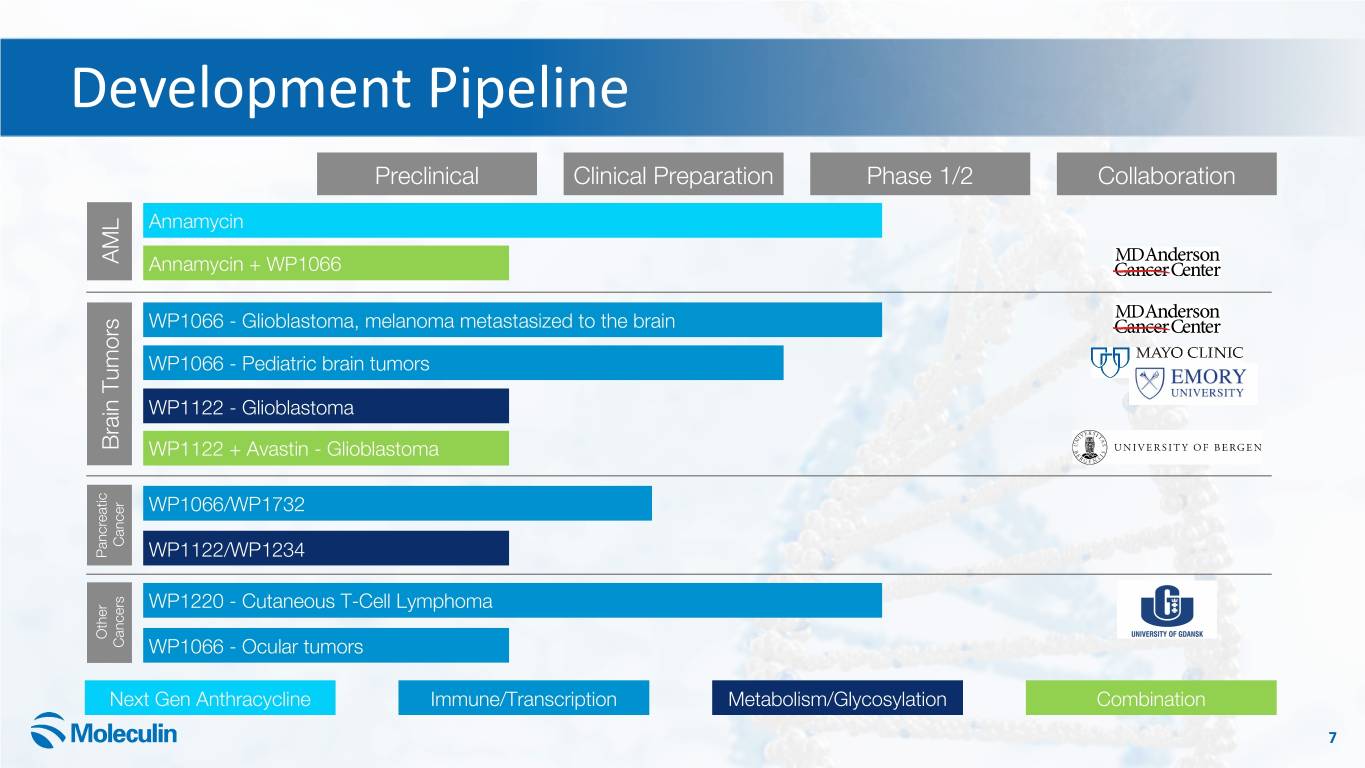
Development Pipeline Preclinical Clinical Preparation Phase 1/2 Collaboration Annamycin AML Annamycin + WP1066 WP1066 - Glioblastoma, melanoma metastasized to the brain WP1066 - Pediatric brain tumors WP1122 - Glioblastoma Brain Tumors WP1122 + Avastin - Glioblastoma WP1066/WP1732 Cancer Pancreatic WP1122/WP1234 WP1220 - Cutaneous T-Cell Lymphoma Other Cancers WP1066 - Ocular tumors Next Gen Anthracycline Immune/Transcription Metabolism/Glycosylation Combination 7

Key Developments Moleculin has three drug candidates in four clinical trials. Next Generation Anthracycline - Annamycin Immune/Transcription Modulators - WP1066 Portfolio • Positive interim safety and efficacy data from Annamycin trial in • Emory University to conduct pediatric brain tumor trial. Poland. • Phase 1 clinical trial of WP1066 in children with recurrent or refractory • After a single starting dose of 120 mg/m2 in the first cohort, 2 of 3 patients malignant brain tumors. treated responded sufficiently to qualify for a potentially curative bone • Conducted at the Aflac Cancer & Blood Disorders Center at Children's marrow transplant. Healthcare of Atlanta. • No patients in the U.S. or in the European trials, to date, have shown any signs of cardiotoxicity. • First two patients enrolled in our European clinical trial of WP1220 for the topical treatment of cutaneous T-Cell Lymphoma (CTCL). • FDA grants Fast Track Designation of Annamycin for the treatment of relapsed or refractory acute myeloid (AML). • FDA granted Orphan Drug Designation for our drug candidate WP1066 for the treatment of glioblastoma, the most aggressive form • Annamycin active against metastases to the lungs in pre-clinical of brain tumor. testing. • Significantly improved survival in an aggressive form for triple negative • WP1066, an Immune/Transduction Modulator, appears to counteract breast cancer metastasized to the lungs in animal models. resistance to checkpoint blockade therapy (specifically, immune checkpoint target PD-L1) in our own sponsored research. • U.S. clinical trial second cohort dose level of 120 mg/m2. 8
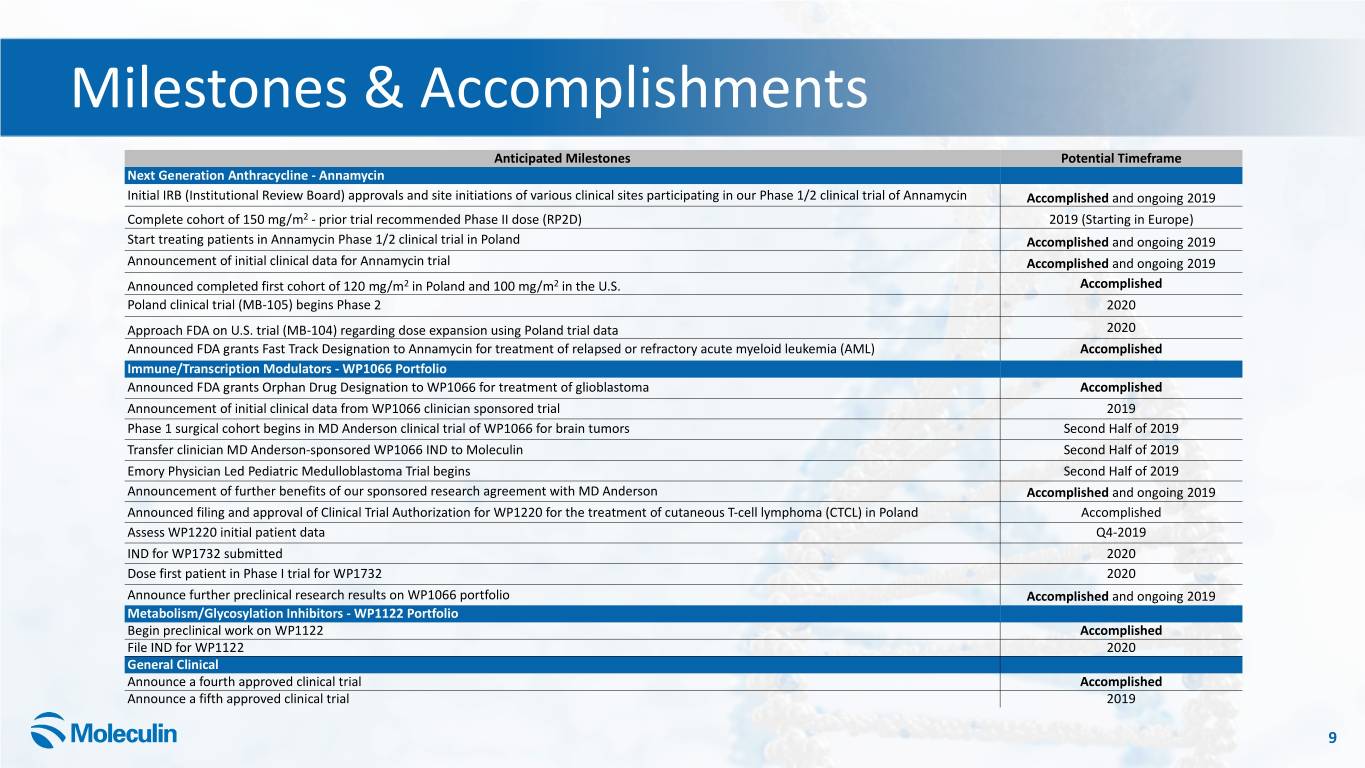
Milestones & Accomplishments Anticipated Milestones Potential Timeframe Next Generation Anthracycline - Annamycin Initial IRB (Institutional Review Board) approvals and site initiations of various clinical sites participating in our Phase 1/2 clinical trial of Annamycin Accomplished and ongoing 2019 Complete cohort of 150 mg/m2 - prior trial recommended Phase II dose (RP2D) 2019 (Starting in Europe) Start treating patients in Annamycin Phase 1/2 clinical trial in Poland Accomplished and ongoing 2019 Announcement of initial clinical data for Annamycin trial Accomplished and ongoing 2019 Announced completed first cohort of 120 mg/m2 in Poland and 100 mg/m2 in the U.S. Accomplished Poland clinical trial (MB-105) begins Phase 2 2020 Approach FDA on U.S. trial (MB-104) regarding dose expansion using Poland trial data 2020 Announced FDA grants Fast Track Designation to Annamycin for treatment of relapsed or refractory acute myeloid leukemia (AML) Accomplished Immune/Transcription Modulators - WP1066 Portfolio Announced FDA grants Orphan Drug Designation to WP1066 for treatment of glioblastoma Accomplished Announcement of initial clinical data from WP1066 clinician sponsored trial 2019 Phase 1 surgical cohort begins in MD Anderson clinical trial of WP1066 for brain tumors Second Half of 2019 Transfer clinician MD Anderson-sponsored WP1066 IND to Moleculin Second Half of 2019 Emory Physician Led Pediatric Medulloblastoma Trial begins Second Half of 2019 Announcement of further benefits of our sponsored research agreement with MD Anderson Accomplished and ongoing 2019 Announced filing and approval of Clinical Trial Authorization for WP1220 for the treatment of cutaneous T-cell lymphoma (CTCL) in Poland Accomplished Assess WP1220 initial patient data Q4-2019 IND for WP1732 submitted 2020 Dose first patient in Phase I trial for WP1732 2020 Announce further preclinical research results on WP1066 portfolio Accomplished and ongoing 2019 Metabolism/Glycosylation Inhibitors - WP1122 Portfolio Begin preclinical work on WP1122 Accomplished File IND for WP1122 2020 General Clinical Announce a fourth approved clinical trial Accomplished Announce a fifth approved clinical trial 2019 9

Technology Review 10

Annamycin (Next Generation Anthracycline) Critical Advantages Leading AML induction therapy drugs are cardiotoxic and lose efficacy due to Annamycin Process over Leading Drug multidrug resistance Annamycin has little to no cardiotoxicity, avoids multidrug resistance, has been shown to be more potent in AML cell lines and has shown activity in patients who failed standard of care Potential to Annamycin has shown the potential to significantly improve health in a Phase Significantly Improve I/II acute myeloid leukemia (AML) trial Health Orphan Drug status as single agent for relapsed or refractory AML Positioned for Annamycin appears to be well suited for an accelerated approval pathway in the Accelerated Approval US, and in Europe Absence of any approved second-line drug for most AML patients represents a significant unmet need Potentially shorter time scale for saving lives than with typical cancer drugs 11
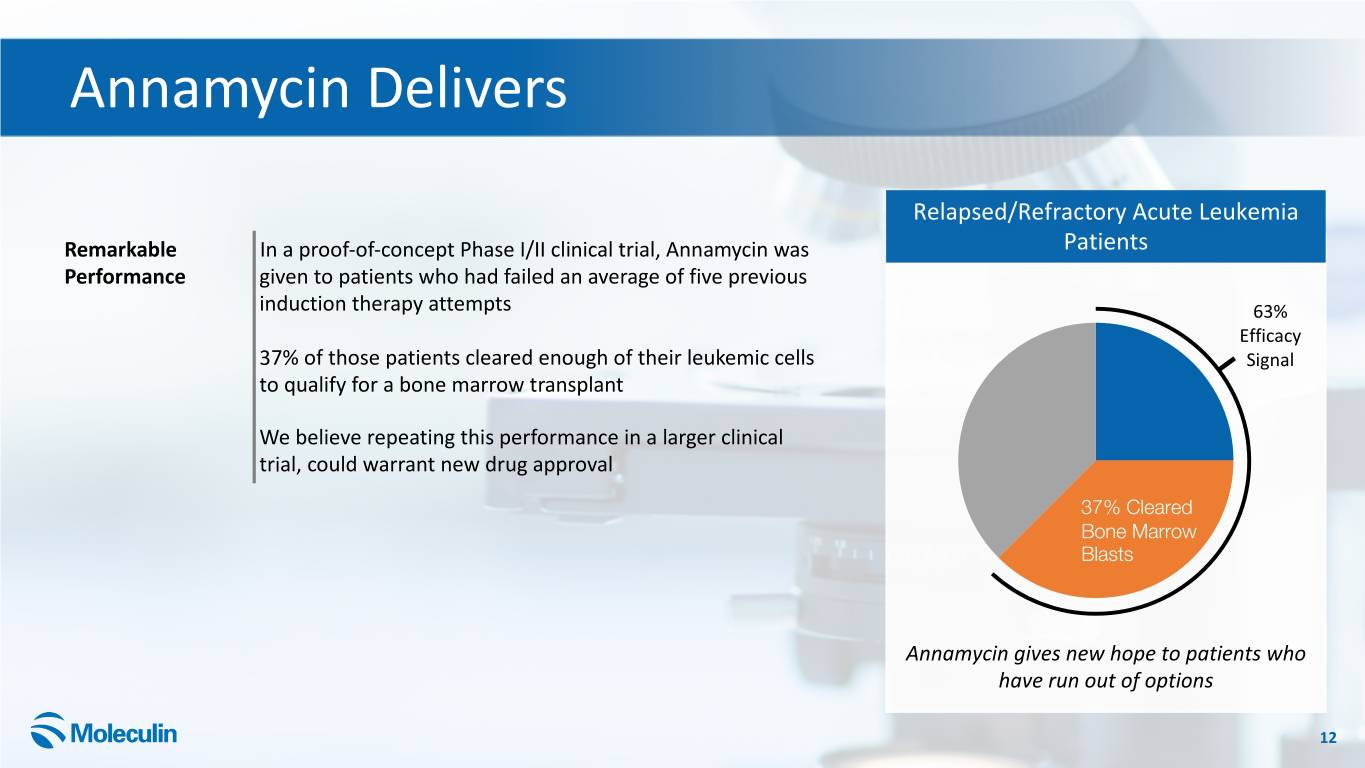
Annamycin Delivers Relapsed/Refractory Acute Leukemia Remarkable In a proof-of-concept Phase I/II clinical trial, Annamycin was Patients Performance given to patients who had failed an average of five previous induction therapy attempts 63% Efficacy 37% of those patients cleared enough of their leukemic cells Signal to qualify for a bone marrow transplant We believe repeating this performance in a larger clinical trial, could warrant new drug approval 37% Cleared Bone Marrow Blasts Annamycin gives new hope to patients who have run out of options 12
Annamycin Recap & Status • Annamycin is a “Next Generation” anthracycline designed to be non-cardiotoxic and avoid multidrug resistance • Prior developer failed financially and lost rights to license • Although impressive activity was shown in prior acute leukemia trials, developer did not properly close out or establish an appropriate RP2D • Moleculin is repeating Phase 1/2 both in US and EU • US Trial (MB-104) has begun treating patients; EU trial is about to begin • Repeat of prior results should afford Annamycin an accelerated approval pathway as a 2nd line induction therapy for R/R AML • Other indications include sarcomas, lewis lung carcinoma and squamous cell carcinoma, among others 13
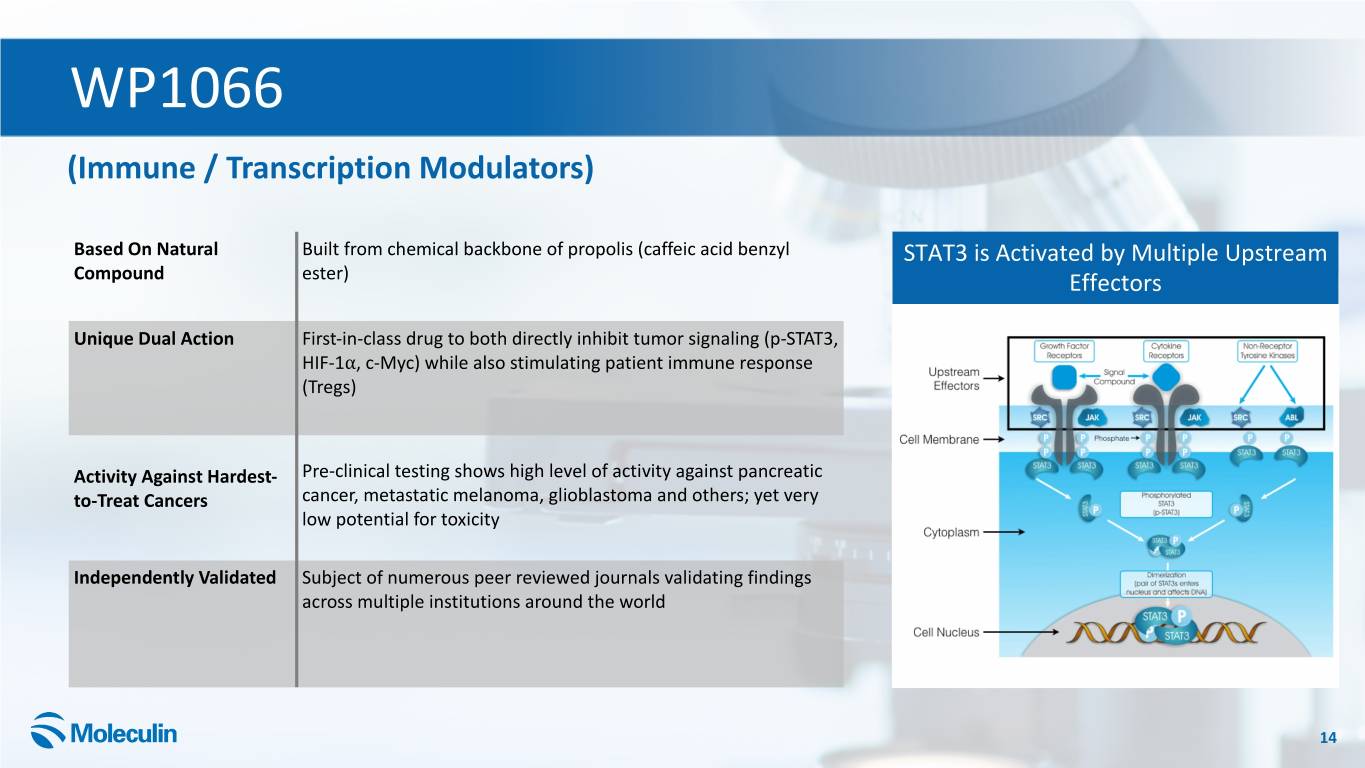
WP1066 (Immune / Transcription Modulators) Based On Natural Built from chemical backbone of propolis (caffeic acid benzyl STAT3 is Activated by Multiple Upstream Compound ester) Effectors Unique Dual Action First-in-class drug to both directly inhibit tumor signaling (p-STAT3, HIF-1⍺, c-Myc) while also stimulating patient immune response (Tregs) Activity Against Hardest- Pre-clinical testing shows high level of activity against pancreatic to-Treat Cancers cancer, metastatic melanoma, glioblastoma and others; yet very low potential for toxicity Independently Validated Subject of numerous peer reviewed journals validating findings across multiple institutions around the world 14

STAT3 is a Master Regulator of Tumor Progression Triggering oncogenic transcription activity: • Survival Activated STAT3 • Proliferation Resulting in tumor (p-STAT3) binds to • Inflammation growth and DNA • Invasion metastasis • Metastasis • Angiogenesis • Immunosuppression 15

WP1066 Affects Tumors Directly & Indirectly WP1066 WP1066 modulates transcription factors resulting in direct tumor cytotoxicity while also Immune Transcription stimulating a natural Modulation Modulation immune response by reducing Regulatory T-cells (Tregs) Tumor 16

Transcriptional Control of Cancer Immunosuppressive Oncogenic Axis HIF-1α STAT3 c-Myc Implications for: • GBM • Pancreatic cancer • Other cancers 17

WP1066 Recap & Status • WP1066 is a small molecule that inhibits p-STAT3 by accelerating proteasomal degradation (not by blocking phosphorylation) without respect to upstream signaling • WP1066 also inhibits HIF1-α, c-Myc and Tregs • Currently in Phase 1 trial at MD Anderson for GBM and melanoma metastasized to the brain (WP1066 crosses BBB); in 3rd cohort of dose ranging; planned surgical expansion • Oral administration (due to lack of solubility) is demonstrating bioavailability in patients • Recently began IND-enabling work on WP1732, a fully water soluble analog of WP1066 that does not cross BBB, and shows impressive accumulation in pancreas 18

WP1122 (Metabolism/Glycosylation Inhibitor) Addicted to Sugar A brain tumor requires as much as 18 to 37 times as much glucose to Tumors are hyper-consumers survive as a healthy brain cell of glucose and starve to death without it Starving a Tumor to This eventually led to the theory that, if we feed tumor cells a glucose Death decoy (one that can’t convert into energy), we can kill the tumor This works well in a laboratory setting, but the problem is making these decoys drugable Breakthrough design WP1122 is a prodrug of 2-deoxyglucose (2-DG) that increases half-life, enables transmission across the blood brain barrier and improves other drug-like properties Tumor cell Potential to Change WP1122 (at suboptimal doses) performs as well or better than Standard temozolomide in live human brain tumors; even better performance of Care by combining the two drugs was shown in trials Normal cell 19
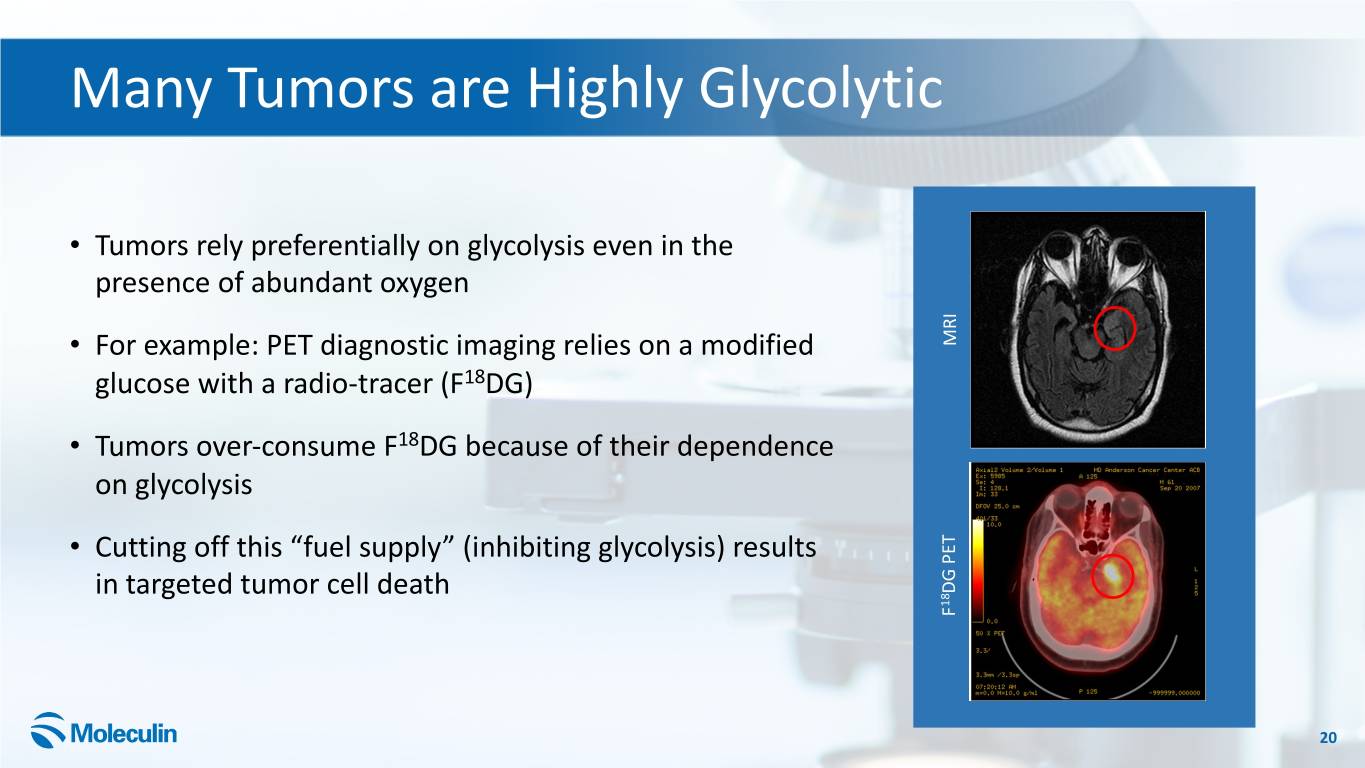
Many Tumors are Highly Glycolytic • Tumors rely preferentially on glycolysis even in the presence of abundant oxygen • For example: PET diagnostic imaging relies on a modified MRI glucose with a radio-tracer (F18DG) • Tumors over-consume F18DG because of their dependence on glycolysis • Cutting off this “fuel supply” (inhibiting glycolysis) results in targeted tumor cell death PET DG 18 F 20
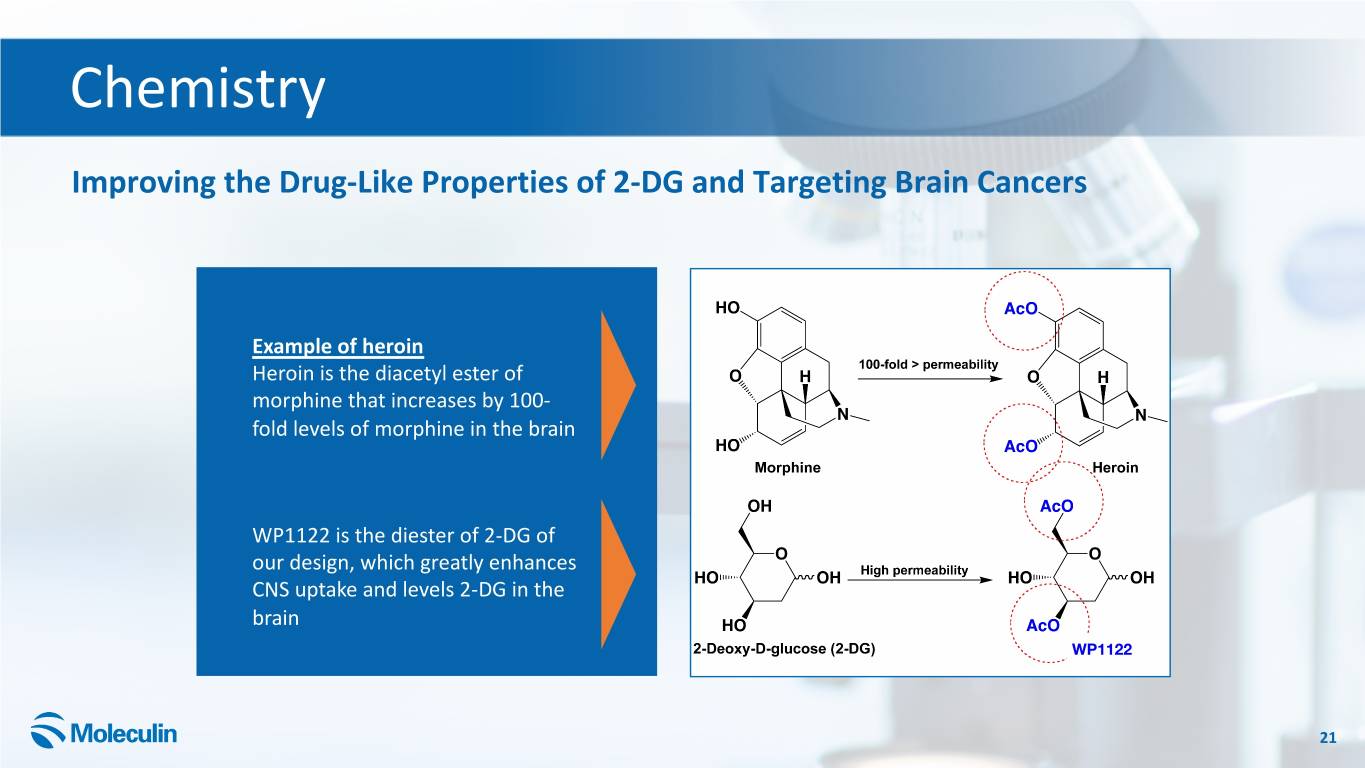
Chemistry Improving the Drug-Like Properties of 2-DG and Targeting Brain Cancers Example of heroin Heroin is the diacetyl ester of morphine that increases by 100- fold levels of morphine in the brain WP1122 is the diester of 2-DG of our design, which greatly enhances CNS uptake and levels 2-DG in the brain WP1122 21

Pharmacology (PK/PD) 1400. • 2-DG CNS distribution and retention was 2-DG WP1122 measured after oral administration of equimolar amounts of 2-DG and its 1050. diacetate WP1122 • CNS distribution and retention of 2-DG is dramatically higher when generated from 700. WP1122 • No observed systemic toxicity 350. Data presented at annual meeting of: 0. Mean CNS Peak CNS Tlast AUC Concentration Concentration (minutes) (μg/gm x hr) (μg/gm) (μg/gm) 22

WP1122 is Effective In Vivo against Gliomas July 2017 WP1122 used alone has at least the same or greater activity than temozolomide (Temodar®), a current standard of care in patients diagnosed with glioblastoma Control Temodar WP1122 10 • Human brain tumors 8 injected into mice • WP1122 performed as 6 well or better than 4 temazolomide # of Animals # of • Not shown is that a 2 Orthotopic combination of both 0 Glioblastoma performed even better 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Model in Mice Days 23
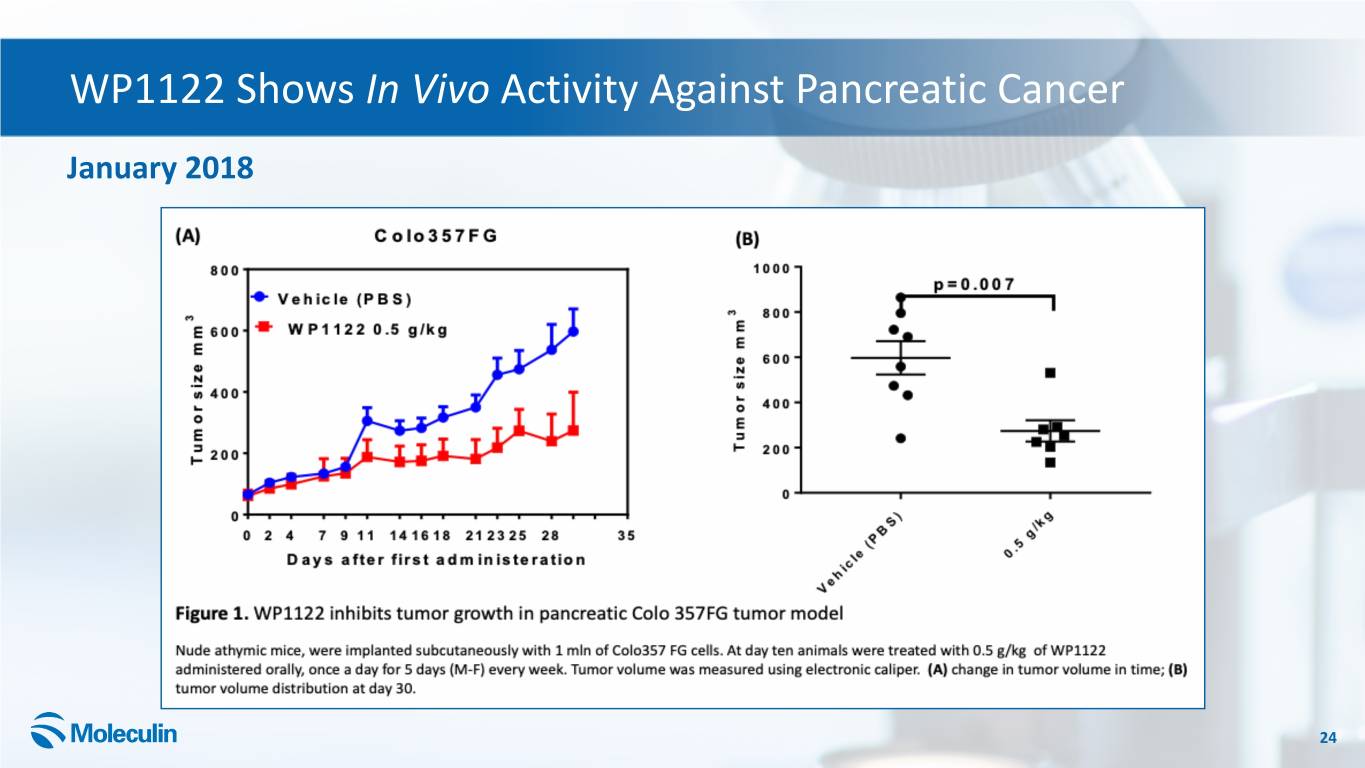
WP1122 Shows In Vivo Activity Against Pancreatic Cancer January 2018 24
Inhibitors of Sugar Metabolism in Cancer and Immunotherapeutic Implications Glycolysis Glycosylation 2-DG 2-Deoxyglucose or 2-Deoxymannose? 25
WP1122 Recap & Status • WP1122 is a prodrug of 2-DG that increases circulation time and ability to cross BBB and provides other drug-like properties • Utilizing Warburg principle, WP1122 converts to 2-DG in highly glycolytic tumor cells causing autophagy due to energy starvation • Evaluating oral versus IV administration and about to begin IND-enabling work • Recent published literature suggests that 2-DG may also enable deglycosylation of PD-L1 (see abstract in following slide) • WP1234 is an analog of WP1122 with improved organ distribution to the pancreas 26

American Journal of Cancer Research 27

Financial Review

Financial Statement Summary For the Quarter Ended For the Year Ended In thousands, except for share and per share data and shares outstanding 03/31/19 12/31/18 (Unaudited) (Unaudited) Statement of Operations Data Revenue - - Research and development $2,932 $9,728 General and administrative and depreciation 1,639 5,297 Loss from operations (4,571) (15,025) Net loss (4,041) (11,876) Net loss per common share – basic and diluted $ (0.14) $ (0.46) Basic and diluted weighted average shares outstanding 29,064,913 25,904,170 Balance Sheet Data Cash and cash equivalents $ 8,782 $ 7,134 Prepaid expenses and other 831 840 Total current assets 9,613 7,974 Total assets $21,298 $19,585 Total current liabilities 6,436 3,878 Total liabilities 6,616 5,313 Total stockholders’ equity 14,682 14,272 Total liabilities and stockholders’ equity $21,298 $19,585 The above financial information is summarized from the Company’s most recent 10-K AND 10-Q filed with the SEC (www.sec.gov) 29

Highly Experienced Leadership

Executive Management Team Selected Prior Experience Walter Klemp, Chairman, President & CEO Don Picker, PhD Chief Science Officer Jonathan P. Foster, CPA, CGMA EVP & CFO Robert Shepard, MD, FACP Chief Medical Officer Sandra Silberman, MD & PhD Chief Medical Officer – New Products 31

Science Advisory Board Waldemar Elihu Estey, Priebe, PhD MD John Paul Waymack, Marty MD, SCD Tallman, MD Jorge Cortes, James Abbruzzese, MD MD 32

Board of Directors Robert E. George - Chair of Audit and Nominating & Governance Committees Mr. George joined our board of directors upon our IPO. He was a partner with the international accounting firm of PricewaterhouseCoopers (PWC) for 27 years until 2010. Mr. George currently serves as Chairman of the Audit Committee for The University of Texas Health Science Center at Houston and, since June 2011, has been a member of The University of Texas at Austin, McCombs Graduate School of Business accounting faculty. Michael D. Cannon - Chair of Compensation Committee Between 1997 and 2004, Mr. Cannon was the Chief Science Officer, EVP and a Director of SICOR, Inc. until its acquisition by Teva Pharmaceutical Industries, Inc. SICOR focused on generic finished dosage injectable pharmaceuticals, active pharmaceutical ingredients and generic biopharmaceuticals. From July 2005 to December 2009, Mr. Cannon was a member of the scientific advisory board of Trevi Health Ventures LP. Mr. Cannon currently serves on the board of directors of other privately held biotech companies. John M. Climaco, JD – Lead Independent Director Most recently the Executive Vice President of Perma-Fix Medical S.A, a Polish subsidiary of the Perma-Fix Environmental Services, Inc. (NASDAQ: PESI) where he has served as a director since 2013. From 2003 to 2012, Mr. Climaco served as President and Chief Executive Officer, as well as a member of the Board of Directors of Axial Biotech, Inc., which he cofounded in 2003. Mr. Climaco has served as a member of the Board of Directors for Digirad Corporation (NASDAQ: DRAD), PDI, Inc. (NASDAQ: PDII) and InfuSystem Holdings, Inc. (NASDAQ: INFU). From 2001 to 2007, he practiced law for the firm of Fabian and Clendenin. Mr. Climaco holds a J.D. from the University of California Hasting College of the Law. 33

Partnerships & Collaborations The University of Texas MD Anderson Cancer Center Moleculin actively sponsors ongoing research at The University of Texas MD Anderson Cancer Center. MD Anderson is the largest cancer research center in the world and one of the country’s largest recipients of NIH medical research grants. Dr. Waldemar Priebe, a Professor of Medicinal Chemistry in the Department of Experimental Therapeutics, Division of Cancer Medicine at the University of Texas MD Anderson Cancer Center, discovered the molecules that form the basis for our lead drug candidates. MD Anderson has granted Moleculin royalty-bearing, worldwide, exclusive licenses for the patent and technology rights to all of Moleculin’s drug technologies and continues to collaborate with the Company to translate research discoveries into clinical therapies. Emory University Moleculin entered into an agreement with Emory University to enable expanded cancer research on Moleculin’s WP1066 molecule for the possible treatment of medulloblastoma, a pediatric malignant primary brain tumor. Physician-scientists at Emory University and Children’s Healthcare of Atlanta have requested support to continue research aimed at the development of a novel treatment of medulloblastoma using WP1066 and Moleculin has agreed to supply them with a pure form of WP1066 for preclinical testing for the potential treatment of medulloblastoma. Emory studies so far have indicated that medulloblastoma may be particularly vulnerable to the ability of WP1066 to block the activated form of STAT3, a key signaling protein believed to contribute to the growth and survival of many tumors, including medulloblastoma. Mayo Clinic Research Endeavor Moleculin entered into an agreement with a physician at the Mayo Clinic to enable additional research on Moleculin’s WP1066 molecule for the possible treatment of a rare form of pediatric brain tumor. Mayo Clinic physician-scientists have requested and Moleculin has agreed to supply them with WP1066 for preclinical testing for the potential treatment of pediatric Diffuse Intrinsic Pontine Gliomas (DIPG), a rare and very aggressive form of brain tumor. Mayo Clinic studies have suggested that DIPG may be particularly sensitive to the inhibition of the activated form of a cell-signaling protein called STAT3, a primary target of WP1066, and their preliminary studies have demonstrated significant anti-tumor activity of WP1066 in DIPG in vitro and in vivo tumor models. The University of Iowa Moleculin entered into an agreement with The University of Iowa Pharmaceuticals for the development of a formulation for WP1732, a new molecule for cancer treatment. Based on preclinical testing, WP1732 is believed to be a breakthrough discovery and represents a major expansion of its STAT3 inhibition capability by providing a highly soluble alternative that is ideally suited for IV administration. This agreement marks the beginning of creating a preclinical package to submit to the FDA in order to request Investigational New Drug status. 34

Partnerships & Collaborations Medical University of Gdansk The Medical University of Gdańsk (MUG) is the largest medical university in northern Poland, located in one of the most beautiful cities in Europe with an old town and beautiful sandy beaches. The MUG educates nearly 6,000 undergraduate and postgraduate students at four Faculties: Faculty of Health Sciences, Faculty of Medicine, Faculty of Pharmacy and the Intercollegiate Faculty of Biotechnology. The MUG offers Premedical Course, Medicine Doctor Programme, Nursing Programme, Nutrition and Dietetics Programme and the Ph.D. Programmes, which are taught fully in English. University of Bergen Moleculin entered into an agreement to collaborate with the University of Bergen to expand research on inhibition of brain metastasis by Moleculin’s pre-clinical drug WP1066 and its unique ability to increase immune system response to cancer and suppression of tumor cell proliferation and survival. Additionally, Moleculin entered into a second collaborative agreement with the University of Bergen to test WP1122 in combination with the drug Avastin(R) (bevacizumab) made by Roche Pharma. Roche Pharma is not a party to the collaborative agreement. DERMIN Sp. z o. o. Dermin Sp. z o. o. (Dermin) is a Polish drug development company that has sublicensed our technologies for use in limited territories, including Poland. We are collaborating with Dermin to conduct a clinical trial in Poland to study WP1220 for the topical treatment of early stage cutaneous T- cell lymphoma or CTCL patients, as well as to coordinate clinical trials of Annamycin in AML patients between clinical sites in Poland and other countries. We are also collaborating with Dermin to prepare WP1122 for clinical development for the possible treatment of glioblastoma. 35

3 6 Key Take-aways Robust pipeline 3 distinctly different technologies, all with blockbuster potential World-leading collaboration MD Anderson Cancer Center/Mayo Clinic/Emory Breakthrough disruptive technologies Annamycin for AML: designed to be non-cardiotoxic, avoids MDR1 WP1066: STAT3 inhibitor that also stimulates immune response WP1122: metabolic inhibitor with improved BBB transmission and animal model activity against pancreatic cancer Highly experienced leadership Veteran pharma/biotech, life science micro-cap managers Proprietary positioning Orphan drug and/or patents, exclusive licenses (applied and received)

Developing breakthrough therapies for highly resistant cancers Walter Klemp, CEO / Jonathan Foster, CFO info@moleculin.com (713) 300-5160 (Nasdaq: MBRX) 3 7