Exhibit 99.1
1 1 FORGING COMMERCIAL & CLINICAL PATHWAYS TARGETING INFECTIOUS DISEASES WITH ORAL IMMUNOTHERAPIES – NOVEMBER, 2019 NASDAQ: IMRN ASX: IMC GARY S. JACOB, Ph.D. CEO

2 2 Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections . Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “guidance” and similar expressions are intended to identify forward - looking statements . Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain risks and uncertainties, some of which are beyond Immuron’s control, including those risks or uncertainties inherent in the process of both developing and commercializing technology . As a result, actual results could materially differ from those expressed or forecasted in the forward - looking statements . The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made . Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, circumstances or unanticipated events occurring after the date of this presentation except as required by law or by any appropriate regulatory authority . SAFE HARBOR STATEMENT

3 3 COMPANY HIGHLIGHTS We are a commercial and clinical - stage biopharmaceutical company focusing on infectious diseases with oral immunoglobulin - based therapies • Validated Technology Platform – with One Registered Asset, Travelan ® Generating Revenue • IMM - 124E & IMM - 529, in Clinical Development for Treatment of Gastrointestinal Disorders and C. difficile Infections • Plan for Accelerated R egulatory Path to Approval for IMM - 124E ( Travelan ®) as Drug to Prevent Travelers’ Diarrhea in USA

4 4 DEVELOPMENT PIPELINE: THREE - PRONGED PLAN DEVELOPMENT STAGE HIGHLIGHTS PRE - CLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET ANTI - INFLAMMATORY PROGRAMS Travelan ® Commercial product - Australia Commercial product - Canada Commercial product - USA IMM - 124E ( Travelan ®) PLAN TO DEVELOP AS DRUG TO PREVENT TRAVELERS’ DIARRHEA IN USA IMM - 529 TO PREVENT RECURRENCE IN C. DIFFICILE PATIENTS Dietary supplement (2015) Health Canada NPN 80046016 (2015) TGA ARTG Aust L106709 (2004) 1 2 Department of Defense Program 3 US ARMY – SHIGELLA US NAVY - CAMPLYLOBACTER
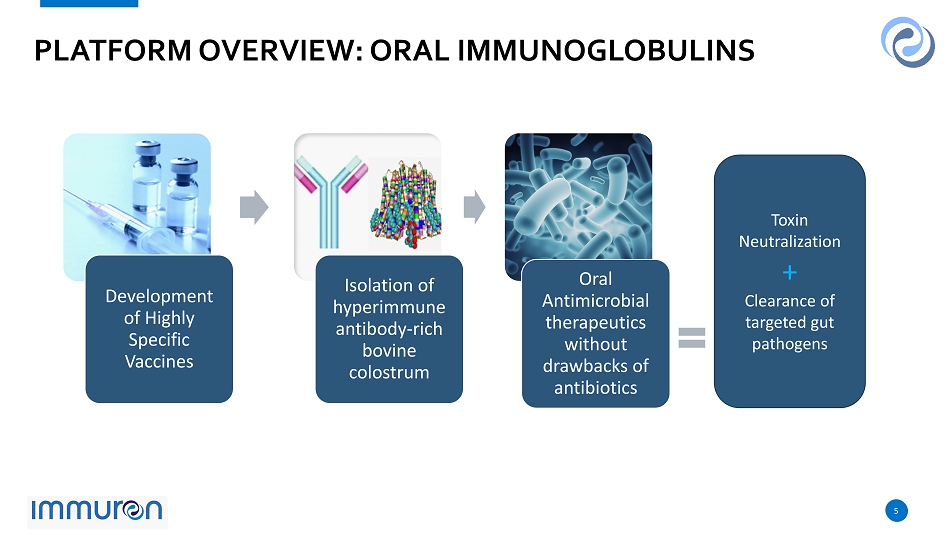
5 5 PLATFORM OVERVIEW: ORAL IMMUNOGLOBULINS Development of Highly Specific Vaccines Isolation of hyperimmune antibody - rich bovine colostrum Oral Antimicrobial therapeutics without drawbacks of antibiotics Toxin Neutralization + Clearance of targeted gut pathogens
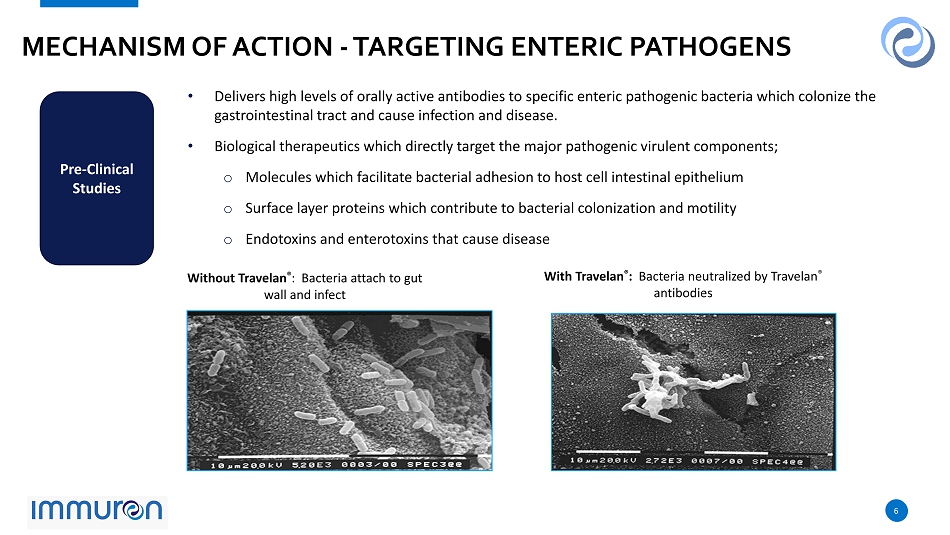
6 6 MECHANISM OF ACTION - TARGETING ENTERIC PATHOGENS 6 Pre - Clinical Studies • Delivers high levels of orally active antibodies to specific enteric pathogenic bacteria which colonize the gastrointestinal tract and cause infection and disease. • Biological therapeutics which directly target the major pathogenic virulent components; o Molecules which facilitate bacterial adhesion to host cell intestinal epithelium o Surface layer proteins which contribute to bacterial colonization and motility o Endotoxins and enterotoxins that cause disease Without Travelan ® : Bacteria attach to gut wall and infect With Travelan ® : Bacteria neutralized by Travelan ® antibodies

7 7 US DOD R&D COLLABORATION AGREEMENTS • Armed Forces Research Institute of Medical Sciences (AFRIMS) – June 2016 • Naval Medical Research Center (NMRC) – August 2016 • Walter Reed Army Institute of Research (WRAIR) – June 2016 • Travelan® binds 180 pathogenic strains of bacteria from infected personnel deployed in Bhutan, Cambodia, Nepal and Thailand Collaboration on Development: 1) Shigella - Specific Target – US Army 2) Campylobacter - specific Target – US Navy

8 8 New U.S. Department of Defense Research Collaboration with Immuron to Develop and Clinically evaluate a New Therapeutic against Campylobacter Key Highlights: AU $5.5 (USD $3.7) million funding approved by the U.S. Department of Defense to develop and clinically evaluate a new oral therapeutic targeting Campylobacter and ETEC Naval Medical Research Center will fund the manufacture and therapeutic evaluation of the new therapeutic to protect against acute infectious diarrhea Two human clinical trials to be conducted with new therapeutic under terms of grant Melbourne, Australia, October 02, 2019: Immuron Limited (ASX: IMC; NASDQ: IMRN), an Australian biopharmaceutical company focused on developing and commercializing oral immunotherapeutics for the prevention and treatment of gut mediated pathogens, is pleased to announce the funding of a new research agreement with the Naval Medical Research Center (NMRC), Silver Spring, MD, USA.
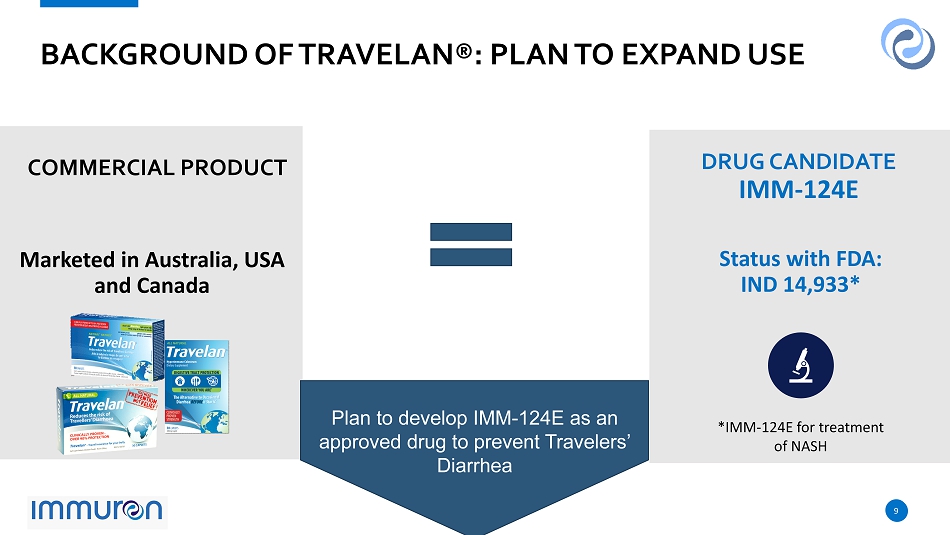
9 9 BACKGROUND OF TRAVELAN ®: PLAN TO EXPAND USE Marketed in Australia, USA and Canada Status with FDA: IND 14,933* COMMERCIAL PRODUCT DRUG CANDIDATE IMM - 124E Plan to develop IMM - 124E as an approved drug to prevent Travelers’ Diarrhea *IMM - 124E for treatment of NASH
10 10 WHAT IS TRAVELERS’ DIARRHEA? • Caused by consuming food or water infected with pathogens. Three or more unformed stools in 24 hours. • Bacterial pathogens are the predominant risk 1 . • Enterotoxigenic E. coli (ETEC) are the predominant pathogens 2,3 : 42% in Latin America 28% in Southeast Asia • Up to 70% of travelers suffer from travelers’ diarrhea 4 . 1 – Steffen, R. 2017 Epidemiology of travelers’ diarrhea. Journal of Travel Medicine 24(1) 2 – Leder , K. 2015 Advising Travellers about Management of Travelers’ Diarrhea. Australian Family Physician, vol 44 No. 1 - 2 Jan. Feb 2015 3 – Castelli et. al., Epidemiology of Travelers’ Diarrhea, J. Travel Medicine 2001; 8 (Suppl2) S26 - S30 4 – CDC Yellow Book 2018, Chapter 2 Travelers’ Diarrhea.

11 11 International Society of Travel Medicine, 2017 guidelines for treating Travelers’ Diarrhea included 1 : • Antibiotics should NOT be used routinely, except patients at high risk of complications • Rifaximin recommended when antibiotic prophylaxis is indicated • Fluoroquinolones not recommended for prophylaxi s 2 • Insufficient evidence to recommend prebiotics or probiotics 1 Riddle et al. 2017. Guidelines for the prevention and treatment of travellers’ diarrhea: a graded expert panel report. Jour nal of Travel Medicine 24(1). 2 Tribble, D. 2017 Resistant pathogens as causes of traveller’s diarrhea globally and impact(s) on treatment failure and reco mme ndations. Journal of Travel Medicine 24(1) ANTIBIOTIC RESISTANCE: OPPORTUNITY FOR TRAVELAN ® The opportunity: Travelan ®, the alternative to antibiotic treatment of TD
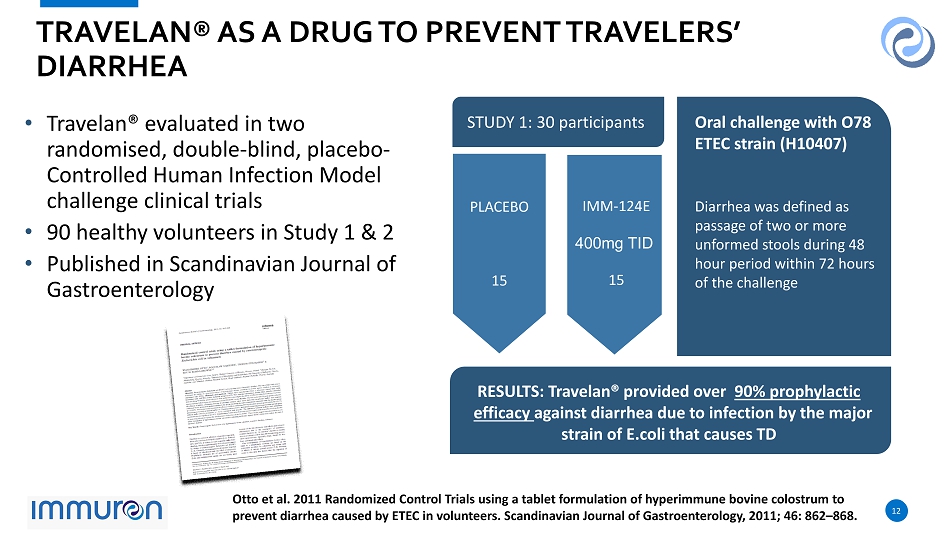
12 12 TRAVELAN ® AS A DRUG TO PREVENT TRAVELERS’ DIARRHEA Otto et al. 2011 Randomized Control Trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by ETEC in volunteers. Scandinavian Journal of Gastroenterology, 2011; 46: 862 – 868. Oral challenge with O78 ETEC strain (H10407) Diarrhea was defined as passage of two or more unformed stools during 48 hour period within 72 hours of the challenge • RESULTS: Travelan® provided over 90% prophylactic efficacy against diarrhea due to infection by the major strain of E.coli that causes TD PLACEBO 15 IMM - 124 E 400 mg TID 15 STUDY 1: 30 participants • Travelan ® evaluated in two randomised, double - blind, placebo - Controlled Human Infection Model challenge clinical trials • 90 healthy volunteers in Study 1 & 2 • Published in Scandinavian Journal of Gastroenterology
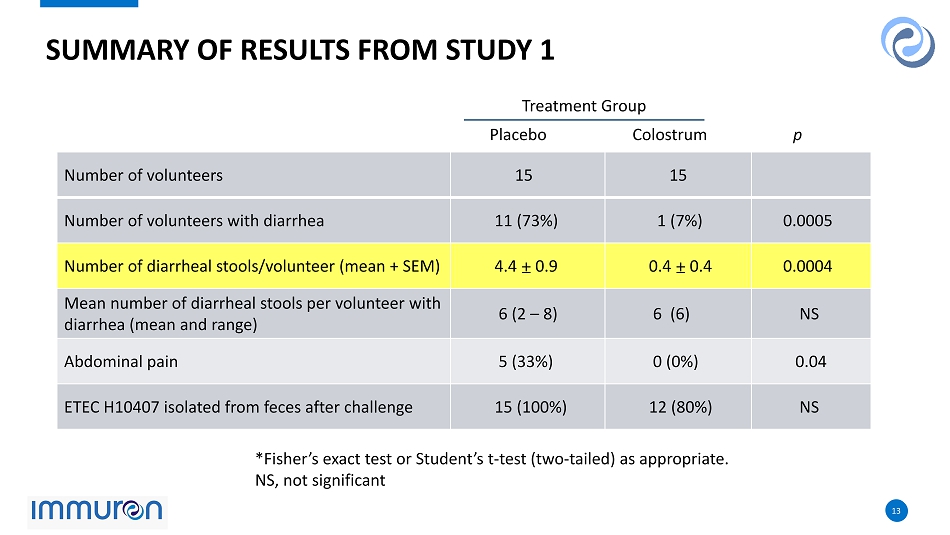
13 13 SUMMARY OF RESULTS FROM STUDY 1 Number of volunteers 15 15 Number of volunteers with diarrhea 11 (73%) 1 (7%) 0.0005 Number of diarrheal stools/volunteer (mean + SEM) 4.4 ± 0.9 0.4 ± 0.4 0.0004 Mean number of diarrheal stools per volunteer with diarrhea (mean and range) 6 (2 – 8) 6 (6) NS Abdominal pain 5 (33%) 0 (0%) 0.04 ETEC H10407 isolated from feces after challenge 15 (100%) 12 (80%) NS Treatment Group Placebo Colostrum p *Fisher’s exact test or Student’s t - test (two - tailed) as appropriate. NS, not significant

14 14 • 12 juvenile rhesus monkeys randomly assigned to Travelan ® (n=8) or placebo (high protein milk powder) (n=4) treatment groups • Travelan ® or placebo (500mg) was administered 2x daily for 6 - days, starting on day 0 • Each monkey challenged with 2.8 x 10 9 Shigella flexneri 2a intragastrically on day 3 • Travelan ® /placebo treatment stopped on day - 6. Monkeys monitored through to day 14 • Faecal samples taken 2 x daily and cultured to establish presence/absence of Shigella flexneri • Animals continually monitored for clinical signs TRAVELAN ®: ORAL CHALLENGE STUDY PREVENTION OF SHIGELLOSIS (BACILLARY DYSENTERY) IN PRIMATES* *Collaborative animal model study with AFRIMS & WRAIR
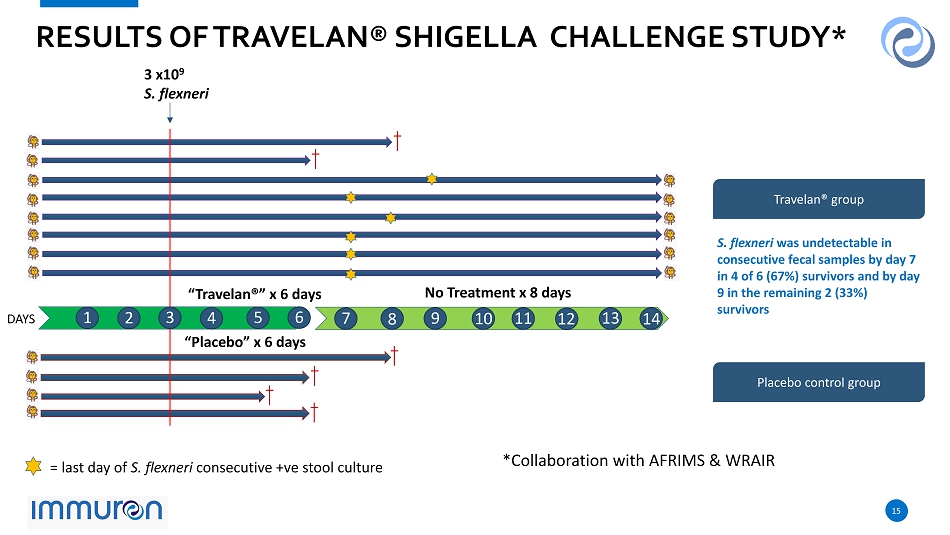
15 15 “ Travelan ® ” x 6 days “ Placebo ” x 6 days 3 x10 9 S. flexneri = last day of S. flexneri consecutive + ve stool culture No Treatment x 8 days RESULTS OF TRAVELAN ® SHIGELLA CHALLENGE STUDY* Travelan ® group Placebo control group DAYS 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 *Collaboration with AFRIMS & WRAIR S. flexneri was undetectable in consecutive fecal samples by day 7 in 4 of 6 ( 67 %) survivors and by day 9 in the remaining 2 ( 33 %) survivors
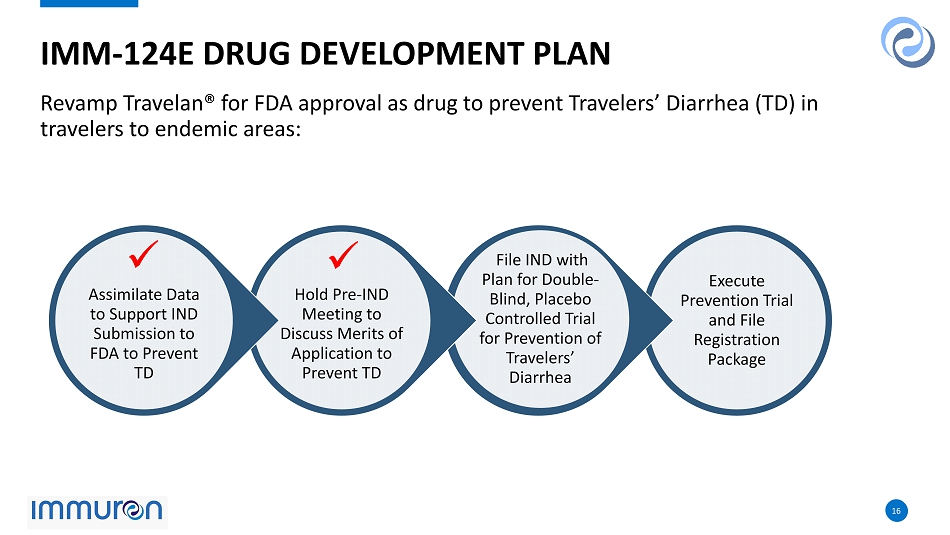
16 16 Revamp Travelan® for FDA approval as drug to prevent Travelers’ Diarrhea (TD) in travelers to endemic areas: IMM - 124E DRUG DEVELOPMENT PLAN Execute Prevention Trial and File Registration Package File IND with Plan for Double - Blind, Placebo Controlled Trial for Prevention of Travelers’ Diarrhea Hold Pre - IND Meeting to Discuss Merits of Application to Prevent TD Assimilate Data to Support IND Submission to FDA to Prevent TD x x
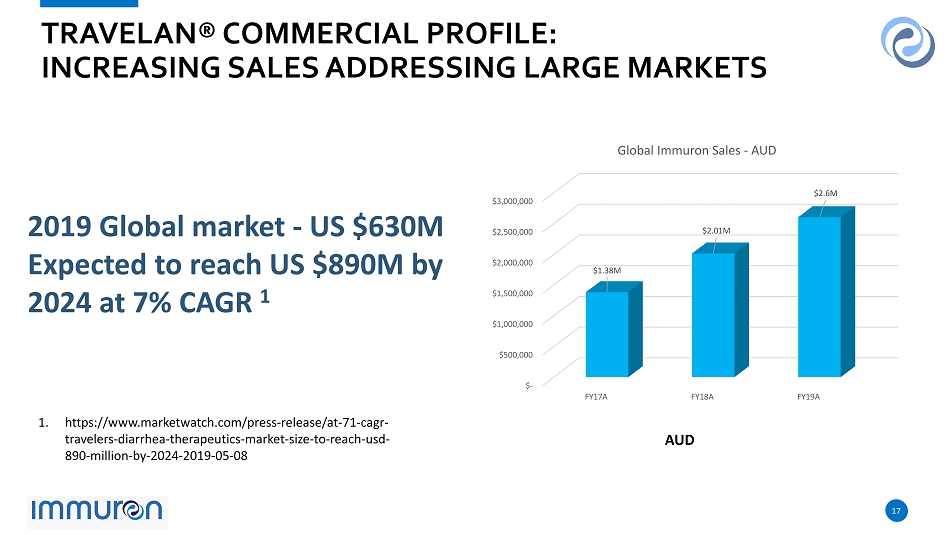
17 17 TRAVELAN® COMMERCIAL PROFILE: INCREASING SALES ADDRESSING LARGE MARKETS 2019 Global market - US $630M Expected to reach US $890M by 2024 at 7% CAGR 1 AUD 1. https://www.marketwatch.com/press - release/at - 71 - cagr - travelers - diarrhea - therapeutics - market - size - to - reach - usd - 890 - million - by - 2024 - 2019 - 05 - 08 $- $500,000 $1,000,000 $1,500,000 $2,000,000 $2,500,000 $3,000,000 FY17A FY18A FY19A $1.38M $2.01M $ 2.6 M Global Immuron Sales - AUD
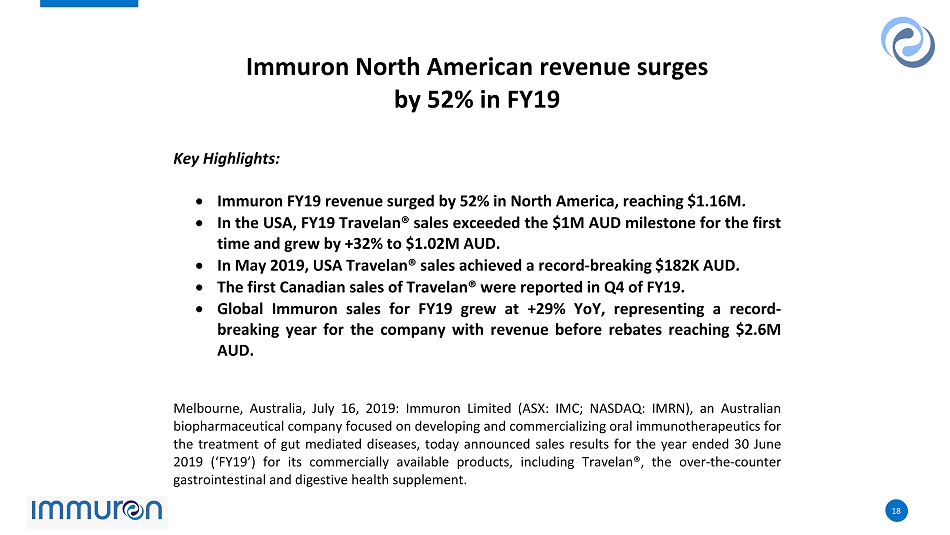
18 18 Immuron North American revenue surges by 52% in FY19 Key Highlights: Immuron FY19 revenue surged by 52% in North America, reaching $1.16M. In the USA, FY19 Travelan® sales exceeded the $1M AUD milestone for the first time and grew by +32% to $1.02M AUD. In May 2019, USA Travelan® sales achieved a record-breaking $182K AUD. The first Canadian sales of Travelan® were reported in Q4 of FY19. Global Immuron sales for FY19 grew at +29% YoY, representing a record- breaking year for the company with revenue before rebates reaching $2.6M AUD. Melbourne, Australia, July 16, 2019: Immuron Limited (ASX: IMC; NASDAQ: IMRN), an Australian biopharmaceutical company focused on developing and commercializing oral immunotherapeutics for the treatment of gut mediated diseases, today announced sales results for the year ended 30 June 2019 (‘FY19’) for its commercially available products, including Travelan®, the over-the-counter gastrointestinal and digestive health supplement.

19 19 Immuron North American Q1 Sales Up 111% YoY Key Highlights: First Quarter of FY2020 (Q1) North American sales grew by 111% YoY to $269K AUD Q1 US Travelan® sales increased by +81% YoY to $232K AUD Global Immuron sales continued an upward trend in Q1 with a +54% YoY sales increase, reaching $741K AUD In Australia, Q1 Travelan® sales increased by +34% YoY to $458K AUD Melbourne, Australia, October 14, 2019: Immuron Limited (ASX: IMC; NASDAQ: IMRN), an Australian biopharmaceutical company focused on developing and commercializing oral immunotherapeutics for the prevention and treatment of gut-mediated pathogens, is pleased to announce sales results for its commercially available, over-the-counter gastrointestinal and digestive health supplement Travelan®, for the quarter ended September 30, 2019, of fiscal year 2020 (Q1).

20 20 • Increased sales in 190 Passport Health Clinics across the USA. • Training & incentive program for travel clinic nurses. FY20 MARKETING OVERVIEW: USA • USA website launched June 2019. • Travel blogger & influencer collaborations to target travellers (28 globally). • 2 podcast campaigns to stimulate Amazon sales (80% uplift in YoY Medique sales). TRAVEL CLINICS CONSUMER MARKETING FY 19 REVIEW FY20 APPROACH • Integrated consumer awareness campaign planned for Feb 2019 (influencer, travel advertorial & earned media components). • Amazon cross - promotions with travel influencers - starting Nov 19. • Amazon paid promotions. • 4 podcast interviews to stimulate Amazon sales. • Expansion into additional 35+ Passport Health travel clinics. • Continued training programme for travel nurses. • Podcast interview with Passport Health Travel Nurse for consumer targeted advertising.

21 21 US SALES FORECAST FOR TRAVELAN ®: IF APPROVED AS DRUG TO PREVENT TD MARKET POTENTIAL FOR TRAVELAN® SALES: Market potential figure derived from: 2014 figures of US citizens traveling to high risk destinations for TD ( 44.3 million) 1 and obtaining pretravel advice ( 22.2 million) 2 . Sources of pre - travel advice include primary care provider, travel medicine specialist, company doctors, pharmacist, and travel agencies 2 . Our forecast utilizes a very conservative estimate for % of US citizens purchasing Travelan ® after seeking pre - travel advice. 1. U.S. Department of Commerce, International Trade Administration, National Travel and Tourism Office. U.S. Citizen Traffic to Overseas Regions, Canada & Mexico 2014. Monthly Statistics, U.S.Outbound Travel by World Regions. 2014. Available at: http://travel.trade.gov/view/m - 2014 - O - 001/index.html. Accessed June 26, 2015. 2. Mathyas Wang , MD , Thomas D. Szucs , MD, MBA, MPH, LLM , and Robert Steffen , MD. Economic Aspects of Travelers ’ Diarrhea. Journal of Travel Medicine, Volume 15, Issue 2, 2008, 110 – 118 USD >$100 MILLION
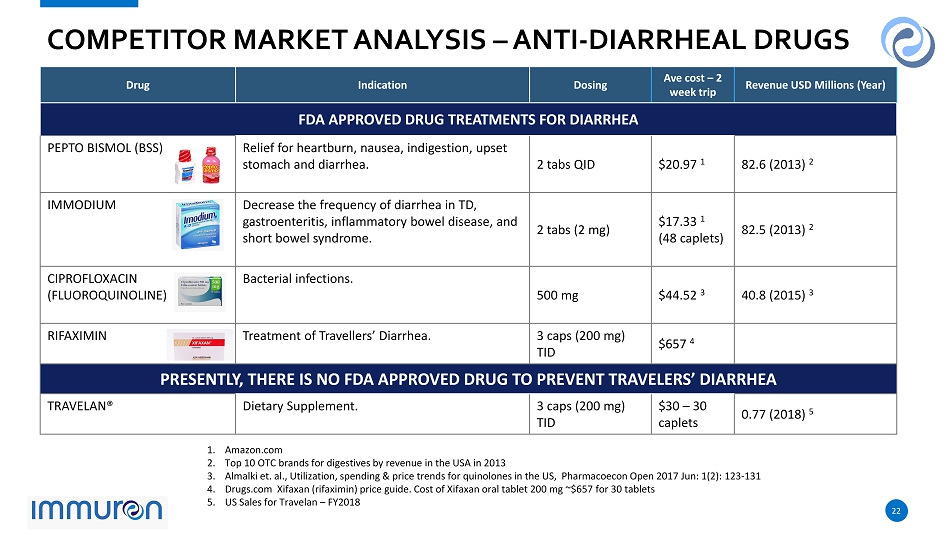
22 22 COMPETITOR MARKET ANALYSIS – ANTI - DIARRHEAL DRUGS Drug Indication Dosing Ave cost – 2 week trip Revenue USD Millions (Year) FDA APPROVED DRUG TREATMENTS FOR DIARRHEA PEPTO BISMOL (BSS) Relief for heartburn, nausea, indigestion, upset stomach and diarrhea. 2 tabs QID $20.97 1 82.6 (2013) 2 IMMODIUM Decrease the frequency of diarrhea in TD, gastroenteritis, inflammatory bowel disease, and short bowel syndrome. 2 tabs (2 mg) $17.33 1 (48 caplets) 82.5 (2013) 2 CIPROFLOXACIN (FLUOROQUINOLINE) Bacterial infections. 500 mg $44.52 3 40.8 (2015) 3 RIFAXIMIN Treatment of Travellers’ Diarrhea. 3 caps (200 mg) TID $657 4 PRESENTLY, THERE IS NO FDA APPROVED DRUG TO PREVENT TRAVELERS’ DIARRHEA TRAVELAN® Dietary Supplement. 3 caps (200 mg) TID $30 – 30 caplets 0.77 (2018) 5 1. Amazon.com 2. Top 10 OTC brands for digestives by revenue in the USA in 2013 3. Almalki et. al., Utilization, spending & price trends for quinolones in the US, Pharmacoecon Open 2017 Jun: 1 ( 2 ): 123 - 131 4. Drugs.com Xifaxan (rifaximin) price guide. Cost of Xifaxan oral tablet 200 mg ~$ 657 for 30 tablets 5. US Sales for Travelan – FY 2018
23 23 NEUTRALIZING CLOSTRIDIUM DIFFICILE , WHILE SPARING THE MICROBIOME IMM - 529
24 24 CLOSTRIDIUM DIFFICILE MARKET OPPORTUNITY 1. https//www.globaldata.com/global - clostridium - difficle - infection - market - approach - 2016 - 2026 2. Jagai , et.al., BMC Gastroenterology, 2014:14:211 Trends in gastroenteritis - associated mortality in the USA. 3. K. Desai, BMC Infect. Dis., 2016,16:303 • Therapeutic market expected to grow from USD $630 million in 2016 to over $1.7 billion by 2026 – CAGR 15% 1 • Leading cause of gastroenteritis - associated mortality in U.S. 2 • Approx. 44,500 patients 3 died in 2014 from C. difficile infections (U.S.) • Potential orphan disease (7 years market exclusivity and premium pricing) Clostridium difficile ( C. difficile ) is a bacterium that causes diarrhea and more serious intestinal conditions such as colitis

25 25 THE UNMET NEED • Current standard of care for C. difficile includes vancomycin, metronidazole & fidaxomicin • Therapies plagued by significant CDI recurrences ( * 1st relapse: 25%; 2nd: 40%; 3rd: 60%) underscoring need for new treatments • Growing resistance to vancomycin treatment • Some treatments are administered intravenously rather than via the gut where C. difficile resides * Isobel Ramsay, Nicholas Brown and David Enoch. Recent Progress for the Effective Prevention and Treatment of Recurrent Clostridium difficile Infection. Infectious Diseases: Research and Treatment Volume 11: 1 – 4 (2018). DOI: 10.1177/1178633718758023
26 26 • IMM - 529 highly differentiated – neutralizes C. difficile but does not impact microbiome • Targets not only toxin B but also spores and vegetative cells responsible for recurrence • Potential use in combination with standard of care • Targets many isolates IMM - 529 OPPORTUNITY Toxin B
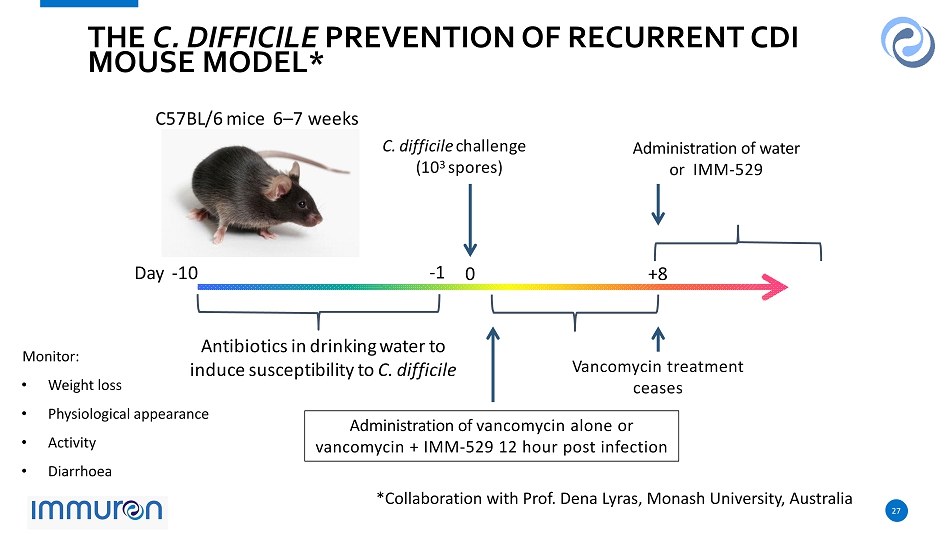
27 27 D a y - 10 - 1 0 A n t i b i o t i c s i n d ri n k i n g w a t e r t o i ndu c e s u s c e p t i b i l i t y t o C. d i ff i c il e C. d i ff i c il e c h a ll e n g e ( 10 3 s po r e s ) C 57 B L / 6 m i c e 6 – 7 w ee ks THE C. DIFFICILE PREVENTION OF RECURRENT CDI MOUSE MODEL * + 8 Administration of vancomycin alone or vancomycin + IMM - 529 12 hour post infection Administration of water or IMM - 529 V ancomycin treatment ceases Monitor: • Weight loss • Physiological appearance • Activity • Diarrhoea *Collaboration with Prof. Dena Lyras , Monash University, Australia
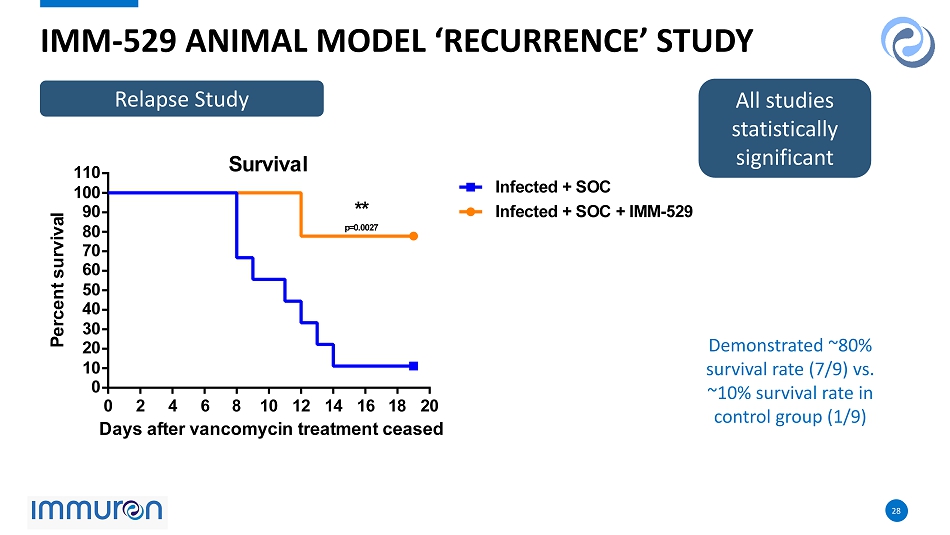
28 28 0 2 4 6 8 10 12 14 16 18 20 0 10 20 30 40 50 60 70 80 90 100 110 Survival Days after vancomycin treatment ceased P e r c e n t s u r v i v a l Infected + SOC Infected + SOC + IMM-529 ** p=0.0027 IMM - 529 ANIMAL MODEL ‘RECURRENCE’ STUDY All studies statistically significant Demonstrated ~80% survival rate (7/9) vs. ~10% survival rate in control group (1/9) Relapse Study
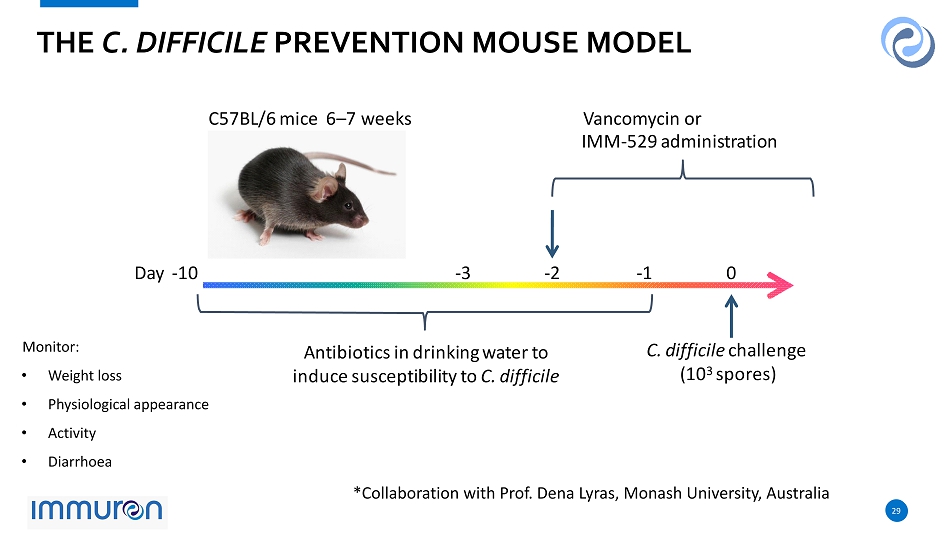
29 29 Monitor: • Weight loss • Physiological appearance • Activity • Diarrhoea D a y - 10 - 3 0 A n t i b i o t i c s i n d ri n k i n g w a t e r t o i ndu c e s u s c e p t i b i l i t y t o C. d i ff i c il e C. d i ff i c il e c h a ll e n g e ( 10 3 s po r e s ) C 57 B L / 6 m i c e 6 – 7 w ee ks Vancomycin or I M M - 52 9 a d m i n i s t r at i o n - 2 THE C. DIFFICILE PREVENTION MOUSE MODEL - 1 *Collaboration with Prof. Dena Lyras , Monash University, Australia
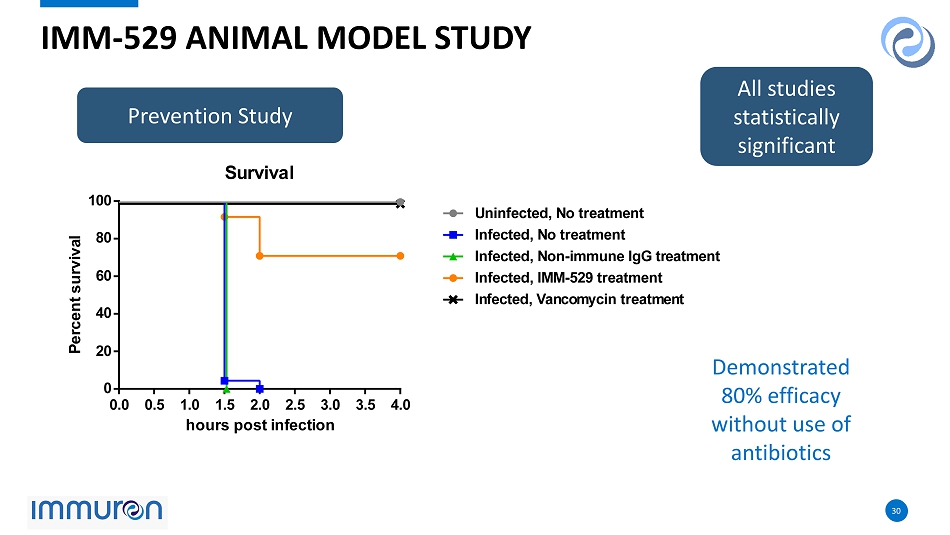
30 30 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 0 20 40 60 80 100 Survival hours post infection P e r c e n t s u r v i v a l Uninfected, No treatment Infected, No treatment Infected, Non-immune IgG treatment Infected, IMM-529 treatment Infected, Vancomycin treatment IMM - 529 ANIMAL MODEL STUDY All studies statistically significant Demonstrated 80 % efficacy without use of antibiotics Prevention Study
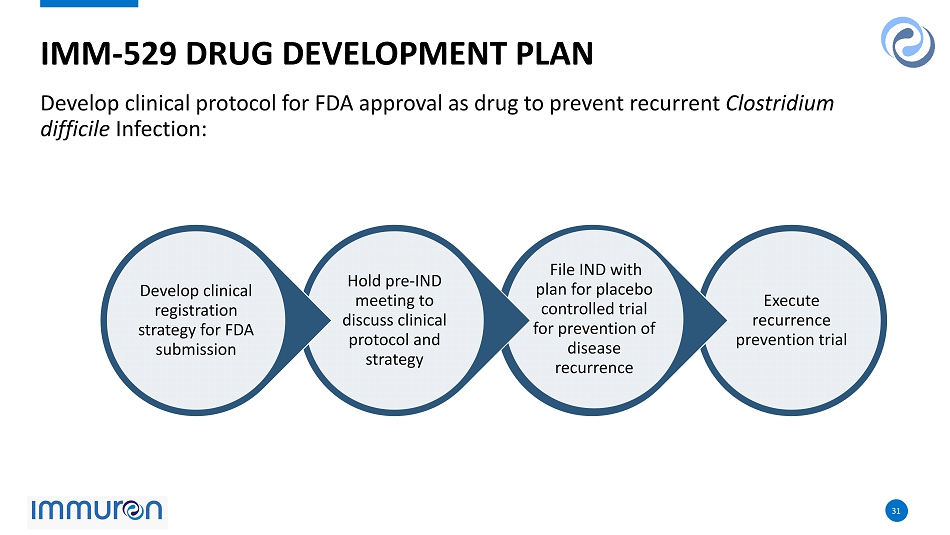
31 31 Develop clinical protocol for FDA approval as drug to prevent recurrent Clostridium difficile Infection: IMM - 529 DRUG DEVELOPMENT PLAN Execute recurrence prevention trial File IND with plan for placebo controlled trial for prevention of disease recurrence Hold pre - IND meeting to discuss clinical protocol and strategy Develop clinical registration strategy for FDA submission
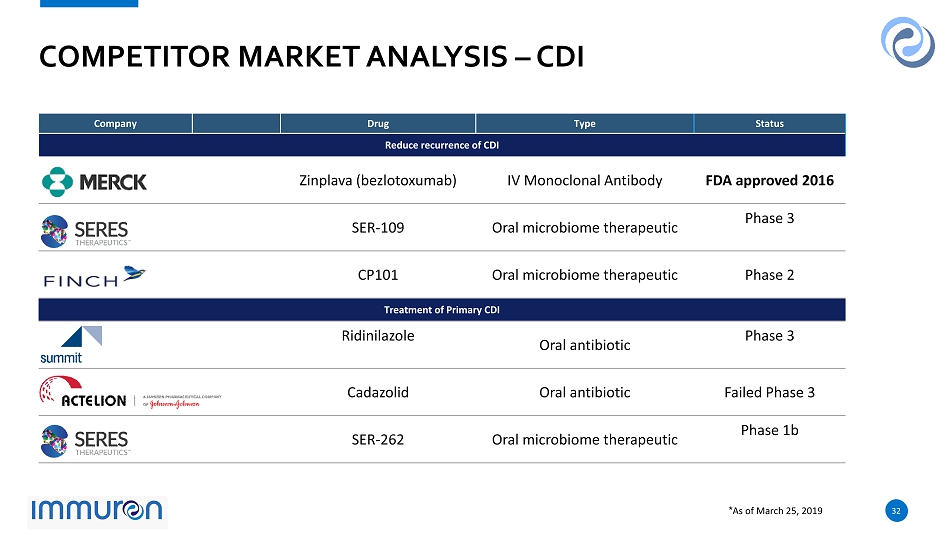
32 32 COMPETITOR MARKET ANALYSIS – CDI Company Drug Type Status Reduce recurrence of CDI Zinplava ( bezlotoxumab ) IV Monoclonal Antibody FDA approved 2016 SER - 109 Oral microbiome therapeutic Phase 3 CP101 Oral microbiome therapeutic Phase 2 Treatment of Primary CDI Ridinilazole Oral antibiotic Phase 3 Cadazolid Oral antibiotic Failed Phase 3 SER - 262 Oral microbiome therapeutic Phase 1b * As of March 25, 2019
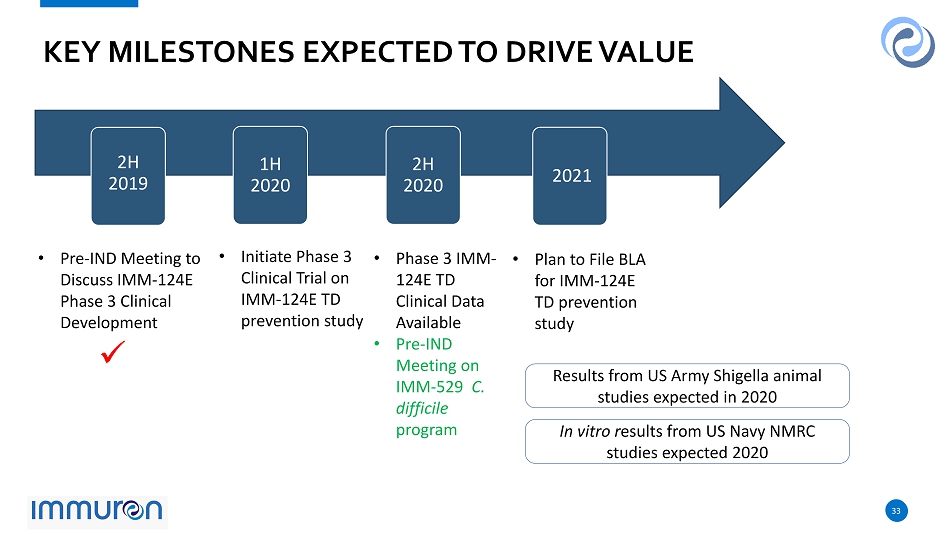
33 33 KEY MILESTONES EXPECTED TO DRIVE VALUE Results from US Army Shigella animal studies expected in 2020 2H 2019 1H 2020 2H 2020 2021 • Pre - IND Meeting to Discuss IMM - 124E Phase 3 Clinical Development • Initiate Phase 3 Clinical Trial on IMM - 124E TD prevention study • Phase 3 IMM - 124E TD Clinical Data Available • Pre - IND Meeting on IMM - 529 C. difficile program • Plan to File BLA for IMM - 124E TD prevention study In vitro r esults from US Navy NMRC studies expected 2020 x

34 34 MANAGEMENT • Chief Executive Officer of Immuron Limited since November 16, 2018. • Over 30 years of experience in pharmaceutical and biotechnology - including R&D, operations, business development and capital financing. • Co - founder and founding CEO of Synergy Pharmaceuticals. • Co - inventor of TRULANCE® ( plecanatide ), an FDA approved drug to treat chronic GI disorders. • Raised over USD $500 million of capital in the public markets to support Synergy from founding to approval of TRULANCE® in 2017. • Ph.D. in Biochemistry; University of Wisconsin - Madison and BS in Chemistry from the University of Missouri. • Former Acting CEO of Immuron Ltd. Over twenty years’ experience in pharmaceutical and biotechnology industries. • Former Chief Operating Officer of TransBio Ltd. Responsible for strategic identification, development and maintenance of global commercial partnerships, along with development, management and IP portfolio, R&D and technology transfer. • Leadership roles in business development, project management, IP portfolio management, R&D, senior management. • Consultant to academic institutes, private and publicly listed companies and government departments specializing in development and commercialization strategies. • PhD in medicine from the University of Melbourne.

35 35 BOARD OF DIRECTORS – CHAIRMAN & EXECUTIVE VICE CHAIRMAN Dr Aston has more than 20 years experience in the pharmaceutical and biotech industries. He was Chief Executive Officer of Mayne Pharma Group Limited, after leading HalcyGen’s acquisition of Mayne Limited in 2009. He has extensive experience with FDA and EU product registration, clinical trials, global licensing, private placement fundraising and prospectus preparation. Dr Aston has held numerous other board positions in the sector including with Clinuvel Limited, HalyGen Limited and Ascent Pharma Health Limited, recently acquired by Watson. Mr. Anastasiou has extensive business experience in a wide range of organisations. He has been a successful entrepreneur from and early age with his first biotech venture, Neuro Developments Australia, seeded at age 24. Mr. Anastasiou was the founder of Investment Group Grandlodge , and ACS International both of which have generated significant wealth through Investment and Management Dr Roger Aston CHAIRMAN - B . Sc . (Hons), PhD . Peter Anastasiou EXECUTIVE VICE CHAIRMAN - BBSc NON - EXECUTIVE DIRECTORS • Dr Gary Jacob • Stephen Anastasiou • Daniel Pollock • Professor Ravi Savarirayan


































