Exhibit 99.1
FORGING COMMERCIAL & CLINICAL PATHWAYS TARGETING INFECTIOUS DISEASES WITH ORAL IMMUNOTHERAPIES – OCTOBER, 2020 JERRY KANELLOS, Ph.D. CEO NASDAQ: IMRN ASX: IMC 1
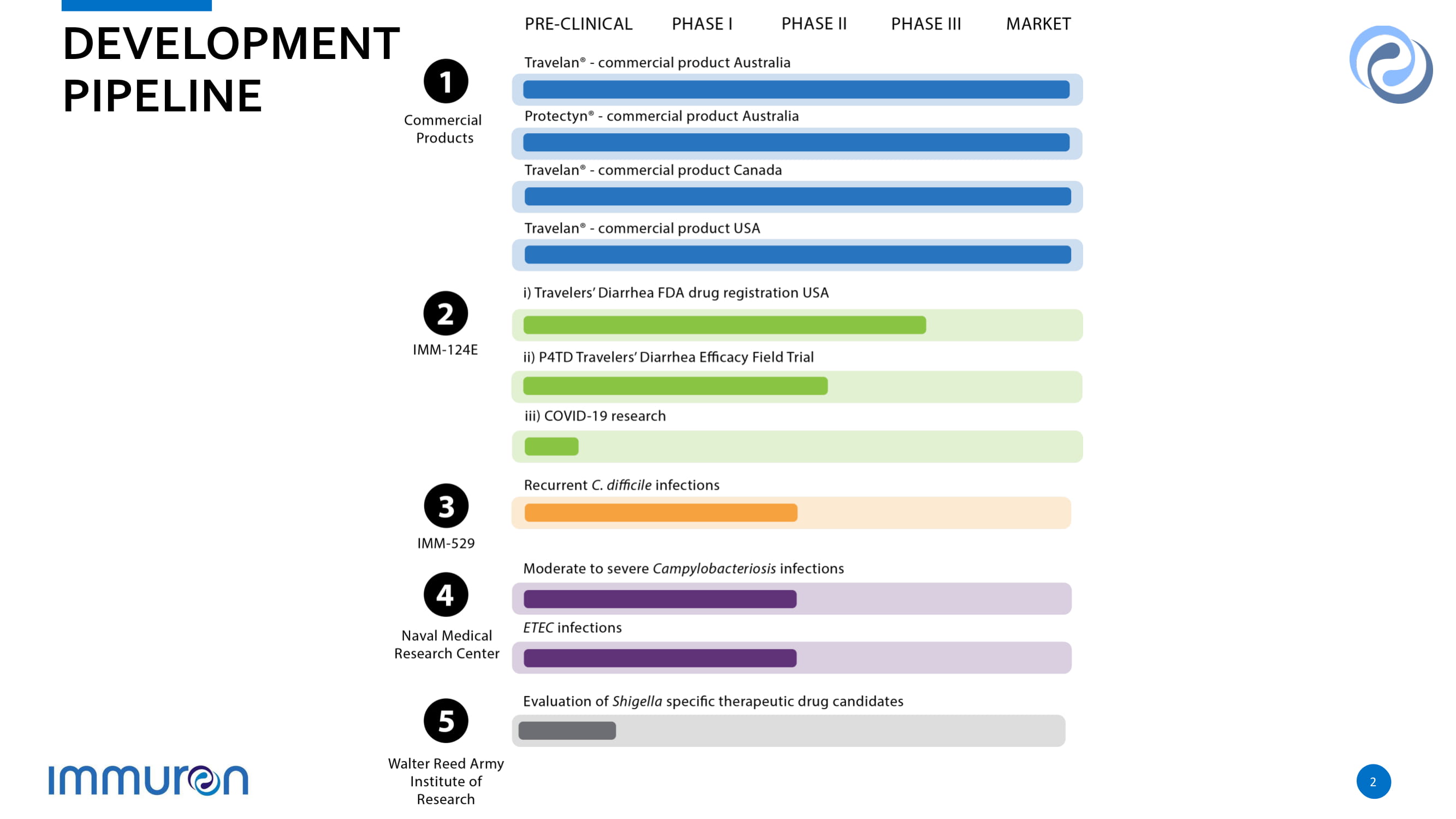
DEVE L OPMENT PIPELINE 2

TRAVELAN® COMMERCIAL PROFILE: A UD $ - 3 $500,000 $1,000,000 $1,500,000 $2,000,000 $2,500,000 $3,000,000 FY17A FY18A FY19A FY20A $1.38M $2.01M $2.6M $2.7M Global Immuron Sales (Gross) - AUD

Plan to register Travelan® as a drug in the USA with the FDA to reduce the risk of Travelers’ Diarrhea (TD) in travelers to endemic areas: IMM - 124E DRUG DEVELOPMENT PLAN Execute Prevention Trial and File Registration Package 4 File IND with Plan for Double - Blind, Placebo Controlled Trial for Prevention of Travelers’ Diarrhea Hold Pre - IND Meeting to Discuss Merits of Application to Prevent TD Assimilate Data to Support IND Submission to FDA to Prevent TD x x

US SALES FORECAST FOR TRAVELAN®: IF APPROVED AS DRUG Market potential figure derived from: 2014 figures of US citizens traveling to high risk destinations for TD (44.3 million) 1 and obtaining pretravel advice (22.2 million) 2 . Sources of pre - travel advice include primary care provider, travel medicine specialist, company doctors, pharmacist, and travel agencies 2 . Our forecast utilizes a very conservative estimate for % of US citizens purchasing Travelan® after seeking pre - travel advice. 1. U.S. Department of Commerce, International Trade Administration, National Travel and Tourism Office. U.S. Citizen Traffic to Overseas Regions, Canada & Mexico 2014. Monthly Statistics, U.S.Outbound Travel by World Regions. 2014. Available at: http://travel.trade.gov/view/m - 2014 - O - 001/index.html. Accessed June 26, 2015. 2. Mathyas Wang , MD , Thomas D. Szucs , MD, MBA, MPH, LLM , and Robert Steffen , MD. Economic Aspects of Travelers ’ Diarrhea. Journal of Travel Medicine, Volume 15, Issue 2, 2008, 110 – 118 MARKET POTENTIAL FOR TRAVELAN® SALES: USD >$100 MILLION 5

A RANDOMIZED, DOUBLE - BLIND, PLACEBO - CONTROLLED TRIAL EVALUATING THE EFFICACY OF NON - ANTIBIOTIC OTC PRODUCTS IN TRAVELERS’ DIARRHEA (TD) PREVENTION (P4TD) 6 CURRENT STATUS – PLAN TO C 0 MMENCE ENROLMENT JUNE 2021 Primary Objective : To evaluate the clinical efficacy of Travelan®, Florastor® and Bimuno® vs . placebo for maintenance of Gastrointestinal Health (GH) focusing on a 10 day window of prophylaxis during travel . STUDY DESIGN This is a randomized ( 1 : 1 : 1 : 1 allocation), double - blind, placebo controlled multicenter clinical trial comparing three dietary supplements, Travelan®, Florastor® and Bimuno®, individually against placebo to determine efficacy for maintenance of GH . A total of 1320 subjects ( 330 /arm) will be enrolled from the following populations : active duty US and UK military personnel, US DoD beneficiaries and US civilians deploying or traveling to intermediate or high GH disruption risk destinations .
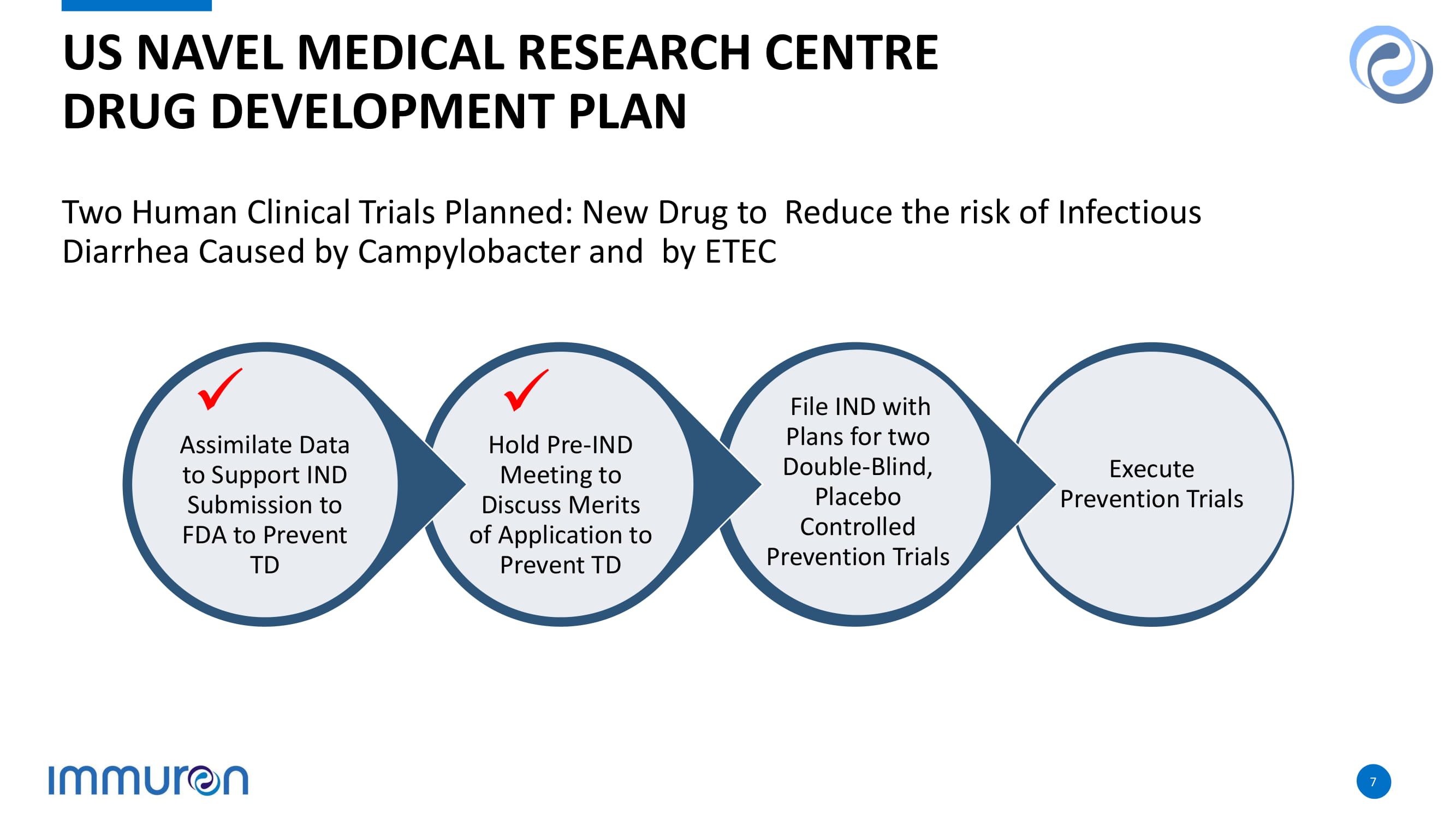
Two Human Clinical Trials Planned: New Drug to Reduce the risk of Infectious Diarrhea Caused by Campylobacter and by ETEC US NAVEL MEDICAL RESEARCH CENTRE DRUG DEVELOPMENT PLAN Execute Prevention Trials 7 File IND with Plans for two Double - Blind, Placebo Controlled Prevention Trials Hold Pre - IND Meeting to Discuss Merits of Application to Prevent TD Assimilate Data to Support IND Submission to FDA to Prevent TD x x

Develop clinical protocol for FDA approval as drug to prevent recurrent Clostridiodes difficile Infection: IMM - 529 DRUG DEVELOPMENT PLAN Execute recurrence prevention trial 8 File IND with plan for placebo controlled trial for prevention of disease recurrence Hold pre - IND meeting to discuss clinical protocol and strategy Develop clinical registration strategy for FDA submission

Immuron Reports Neutralizing activity Against SARS - CoV - 2 9 Key Points Immuron’s Hyper - immune Bovine Colostrum used to manufacture Travelan® and Protectyn® demonstrates antiviral activity against the COVID - 19 virus in laboratory studies Immuron’s technology platform offers a potential new oral therapeutic approach to target SARS - CoV - 2 in the GI Tract Melbourne, Australia, July 21, 2020: Immuron Limited (ASX: IMC; NASDAQ: IMRN), an Australian biopharmaceutical company focused on developing and commercialising oral immunotherapeutics for the prevention and treatment of gut mediated pathogens, today is pleased to announce that the hype - Immune bovine colostrum used to manufacture the company’s flag ship commercially available and over - the - counter gastrointestinal and digestive health immune supplements Travelan® and Protectyn® has demonstrated neutralizing activity against the severe acute respiratory syndrome coronavirus - 2 (SARS - CoV - 2), the virus that causes COVID - 19.

IMM - 124E SARS - COV - 2 RESEARCH & DEVELOPMENT PROPOSAL 10 CURRENT STATUS RESEARCH & DEVELOPMENT Reached out to local, national, and international potential research collaborators to advance this work and assist in the further characterization of the neutralization activity of SARS - CoV - 2 observed with IMM - 124E • Research Services Agreements » To identify the inhibitory substance/s in IMM - 124E • Preclinical Development » Access application form for a contract research project – submitted » The project aims to assess the effect of IMM - 124E in ex - vivo and animal models infected with SARS - CoV - 2

IMM - 124E SARS - COV - 2 RESEARCH & DEVELOPMENT PROPOSAL 11 CURRENT STATUS CLINICAL PROPOSALS • Consultancy agreement executed with Professor Teena Chopra, Professor of Medicine Wayne State University School of Medicine, Detroit » Professor Chopra is building a registry of the patients presenting with gastrointestinal events to better understand this cohort and the unique medical challenges they present • Clinical protocol development » Reviewing several proposals to assess the efficacy of IMM - 124E to treat patients with COVID - 19










