Exhibit 99.1
AGM PRESENTATION 21 NOVEMBER, 2022 Steven Lydeamore - CEO NASDAQ: IMRN ASX: IMC 1

Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections . Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “guidance” and similar expressions are intended to identify forward - looking statements . Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain risks and uncertainties, some of which are beyond Immuron’s control, including those risks or uncertainties inherent in the process of both developing and commercializing technology . As a result, actual results could materially differ from those expressed or forecasted in the forward - looking statements . The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made . Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, circumstances or unanticipated events occurring after the date of this presentation except as required by law or by any appropriate regulatory authority . 2 SAFE HARBOR STATEMENT

EXECUTIVE SUMMARY Immuron Ltd (ASX:IMC) (NASDAQ:IMRN) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases Company Overview • Two commercially available oral immunotherapeutic products – Travelan® and Protectyn® • Three pipeline assets in four clinical programs • Market capitalisation of $18.7 million as of 17 November 2022 with cash and cash equivalates balance of $22.1 million as of 30 June 2022 • Refreshed corporate structure including three key hires - Steven Lydeamore as CEO, Flavio Palumbo as Chief Commercial Officer and Joanne Casey as R&D Manager • Flagship product Travelan® once again in demand as global travel rebounded post lockdowns; global sales increased 431% in FY22 • Addressable market continues to grow as Immuron expands its distribution capability in FY23 • Platform Technology: capable of producing highly specific orally active immunoglobulins to any enteric pathogen • Pursuing organic growth and M&A to expand commercial product sales within existing and new geographies and to increase product offering • Signed subscription and option agreement with leading gut health biotech, Ateria Health who recently launched ground - breaking Juvia Ρ for irritable bowel syndrome (IBS) • Strong balance sheet supporting refreshed organic growth strategy and new M&A strategy. Strategic investment in Ateria Health is the first milestone in this journey Business Update 3

COMPANY HIGHLIGHTS 4 Our Flagship Commercial Assets – Once Again Generating Revenue o Global sales increased by 425% in FY22 to AU $765k, compared to AU $146k in FY21 o Global sales to end October increased by 343% in the FY22 to AU$500k, compared to AU $113k LY Collaborations with U.S. DoD – Remain Strong o Awarded $6.2M to Clinically Evaluate a Military Strength Dosing Regimen for Travelan o Contact executed with US based CRO (Pharmaron) to conduct clinical trial o IND Submission – December 2022 o Study Initiation – Q2 2023 o Top Line Results – Q4 2023

COMPANY HIGHLIGHTS 5 US Naval Medical Research Center – Campylobacter & ETEC PROJECT o New Therapeutic in Clinical Development for Treatment of moderate to severe Campylobacteriosis and Infectious diarrhea caused by ETEC pathogens o IND Clinical Hold – July 2022 o Contact executed with CRO to conduct general toxicology study o AEC Approval – November 2022 o Study Initiation – December 2022 o Final Study Report – Q1 2023 US Uniformed Services University Travelers’ Diarrhea Clinical Field Trial o USU’s Infectious Diseases Clinical Research Program (IDCRP), the UK Ministry of Defence and the New York City Travel Clinic are jointly planning to conduct the randomized clinical trial to evaluate the efficacy of Travelan® and Florastor® for Travelers’ Diarrhea o Investigational medical products shipped to the clinical trial sites – October 2022 o Study Initiation – Q1 2023 o Duration – 18 Months to recruit 1302 study participants (434 per arm)
COMPANY HIGHLIGHTS 6 IMM - 529 Clinical Development for Treatment of C. difficile Infections o Opportunity Assessment Completed o C. difficile infection (CDI) affects just over ~400,000 people in the US annually o Sizable number of patients who experience at least one recurrence (~20 - 25%), o Many patients experience multiple recurrences, creating persistent unmet need for novel therapies to address recurrences o Infectious disease experts reacted favorably to the IMM - 529 MOA, and its ability to target three elements of the rCDI infection – the spores, vegetative cells, and Toxin B. o Non - antibiotic treatments (such as IMM - 529) are appealing to experts o A detailed business plan which will include a budget, key tasks, associated milestones, timelines and costs of getting IMM - 529 to market is currently being developed

M&A Strategy • By pursuing growth through M&A of a fragmented market, IMC believes that it will be able to increase market geographies, sales channels and penetration driving revenue growth and ultimately shareholder value • Our M&A Key Criteria focusses on: Expand existing customer base Cost & Earnings Synergies Strength of IP and Management Distribution network and sales & marketing by each product BUSINESS POSITIONED FOR ORGANIC GROWTH AND NEW M&A STRATEGY 1 • Expand market verticals & product offering 2 • 3 • 4 • 5 • Australia USA Canada Retail Pharmacy B2B E - commerce Established Organic Growth Strategy • Focus on commercialised products and near - term development extensions, including: • 1 Travelan®: • Sales expansion across target geographies • Growth in distribution network and sales & marketing initiatives • Product development (new formulations including once daily dosing) e.g. FDA approval • 2 Protectyn®: • Sales expansion across target geographies • Growth in distribution network and sales & marketing initiatives • Product development and broader applications 7
THANK YOU

9 ADDRESSABLE MARKET & INDUSTRY OVERVIEW Billion Dollar Market Travelers diarrhea treatment market is large and growing at a CAGR of ~7% Industry tailwinds Travel picking up significantly following COVID lockdowns Frequent Symptom 30% - 70% of travelers experience traveler’s diarrhea*** * IQVIA Consumer Health Category QuickView MAT Q1 2019 ** IMC Company Report - Travelan Market Analysis 2019 *** Centers for Disease Control and Prevention Yellow Book ~$15b+ Immuron’s products are a subset of the global digestive health market, which a multi - billion - dollar market* Ateria strategic investment establishes Immuron’s position in the large and growing IBS market which is complementary to Travelan®; both products focused on gut health ~7% CAGR Travelers diarrhea treatment market is large and growing at a CAGR of ~7% over 2019 - 2022* Travelan® has large market potential given that acute diarrhea affects millions of travelers each year $83m Based on US annual travel numbers and a penetration rate of 15%, the market potential for Travelan® is estimated at $83m** $50m Based on EU travel numbers and a penetration rate of 15%, the market potential for Travelan® is estimated at $50m** $1.7b Clostridioides difficile infections (CDIs) projected to grow to ~ $1.7 billion by 2026, according to GlobalData
10 Lumanity* Opportunity Assessment for IMM - 124E • Immuron’s development of IMM - 124E (hyperimmune bovine colostrum) as a prescription medication has the potential to address this unmet need • Primary care physicians (PCP)s impressed with clinical efficacy endpoint targets demonstrating > 80 % protection against the development of diarrhea . • If base case efficacy targets are reached, IMM - 124E would mostly be used by travelers going to the highest risk areas (e.g., rural Central America/Asia/Africa). • Based on the estimated market size and pricing, the base case yearly revenue in USA for IMM - 124 E is projected at US $ 102 M . • Reaching higher efficacy goals could broaden use. IMMURON’S CLINICAL PROGRAMS – OPPORTUNITY ASSESSMENT Compound or brand name Indication Phase I Phase II Phase III Market IMM - 124E - Travelan® Traveler’s Diarrhea ETEC challenge IMM - 529 Clostridioides difficile Infection & Recurrence Lumanity Opportunity Assessment for IMM - 529 • Infectious disease experts reacted favorably to the IMM - 529 MOA, and its unique ability to target three elements of the rCDI infection – the spores, vegetative cells, and Toxin B • Non - microbiome approaches (such as IMM - 529) are still appealing to experts, who noted that clinical trial efficacy (reduction in relapse rate) and cost/access will be the key drivers of clinical use in recurrent patients, not mechanism of action • Based on the estimated market size, anticipated payer restrictions, pricing, and competition, base case yearly revenue in USA for IMM - 529 is projected at $ 93 M . • Positioning IMM - 529 at the point of second relapse and/or efficacy targets could lead to higher uptake . • Lumanity, a leading lifescience consulting company: https://lumanity.com/company/our - story /
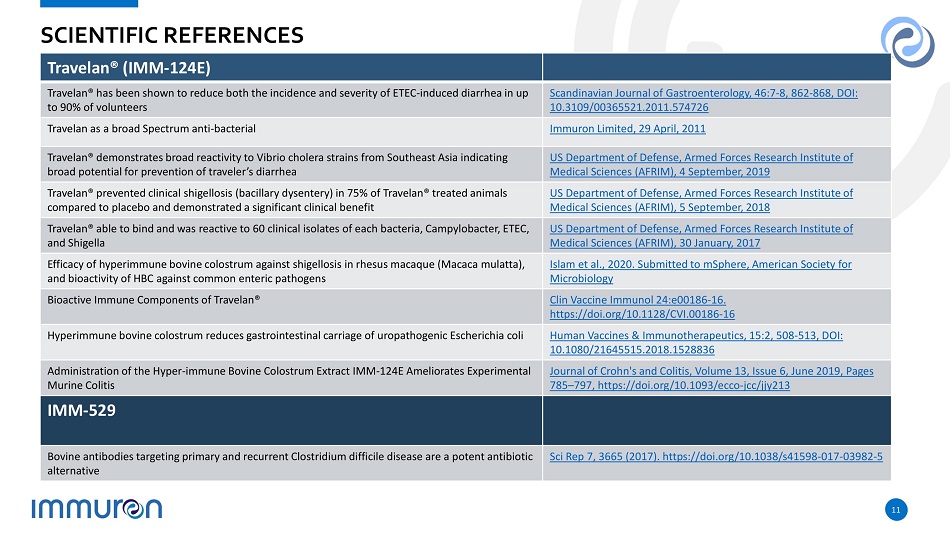
11 SCIENTIFIC REFERENCES Travelan® (IMM - 124E) Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan as a broad Spectrum anti - bacterial Immuron Limited, 29 April, 2011 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Efficacy of hyperimmune bovine colostrum against shigellosis in rhesus macaque (Macaca mulatta), and bioactivity of HBC against common enteric pathogens Islam et al., 2020. Submitted to mSphere, American Society for Microbiology Bioactive Immune Components of Travelan® Clin Vaccine Immunol 24:e00186 - 16. https://doi.org/10.1128/CVI.00186 - 16 Hyperimmune bovine colostrum reduces gastrointestinal carriage of uropathogenic Escherichia coli Human Vaccines & Immunotherapeutics, 15:2, 508 - 513, DOI: 10.1080/21645515.2018.1528836 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis Journal of Crohn's and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 IMM - 529 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5










