Exhibit 99.1

1 1 AGM PRESENTATION 21 NOVEMBER , 2023 NASDAQ: IMRN ASX: IMC Steven Lydeamore - CEO

2 2 Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections. Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “guidance” and similar expressions are intended to identify forward - looking statements. Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain risks and uncertainties, some of which are beyond Immuron’s control, including those risks or uncertainties inherent in the process of both developing and commercializing technology. As a result, actual results could materially differ from those expressed or forecasted in the forward - looking statements. The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made. Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, circumstances or unanticipated events occurring after the date of this presentation except as required by law or by any appropriate regulatory authority. SAFE HARBOR STATEMENT FY2024 results in this presentation are subject to audit review.

3 3 EXECUTIVE SUMMARY Immuron Ltd (ASX:IMC) (NASDAQ:IMRN) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases Company Overview • Platform Technology: capable of producing highly specific orally active immunoglobulins to any enteric pathogen • Two commercially available oral immunotherapeutic products – Travelan® and Protectyn® • Three pipeline assets in four clinical programs • Market capitalisation of $ 16.86 million as of 17 November 2023 with cash and cash equivalates balance of $17.2 million as of 30 June 2023 • G lobal sales increased 136% in FY23 to $1.80 million; Travelan® sales of $1.74 million • Record quarterly Travelan® sales of $1.55 million in Q1, FY2024 • Travelan® is now the #2 SKU and fastest growing in the Antidiarrheal category across all pharmacy in Australia 1 • Australian sales YTD FY24 have exceeded full year FY23 sales • Pursuing growth strategies to expand commercial product sales within existing and new geographies and to increase product off eri ng • Launched on Amazon in USA in July 2023 • Status of investment in leading gut health biotech, Ateria Health • Launched ground - breaking Juvia Ρ for irritable bowel syndrome (IBS) in UK (August 2022), Australia (July 2023) • September 2023: annualised revenue run rate >A$1.2 million with 40%+ growth trajectory since April 2023 • Achieved milestones in all clinical programs (Travelan® IND, Travelan® USU study, CampETEC and IMM - 529) ; near - term milestones anticipated Business Update 1. IQVIA Australia Pharmacy Scan – Antidiarrheal segment, value sales 13 weeks to 21 October 2023
4 4 COMPANY HIGHLIGHTS Strong Sales Growth o Global sales increased by 136% in the 2023 fiscal year to A$1.80 million compared to A$0.77 million in FY22 o Record quarterly global sales in Q1, FY24 of $1.57 million o Australia Net Sales o FY23: A$1.16 million o Q1, FY24 : A$1.35 million o 229% higher than pre - pandemic period Q1, FY20 o 12 months to July 2023 short term resident returns 77% of those in 2019 1 o Travelan® is now the #2 SKU and fastest growing in the Antidiarrheal category across all pharmacy in Australia 2 o Australian sales YTD FY24 have exceeded full year FY23 sales o USA Net Sales o FY23: A$0.64 million o Q1, FY24: A$0.21 million o 9% lower than pre - pandemic period Q1, FY20 o Total departures June 2023 99.4% of June 2019 3 1. https://www.abs.gov.au/statistics/industry/tourism - and - transport/overseas - arrivals - and - departures - australia/latest - release 2. IQVIA Australia Pharmacy Scan – Antidiarrheal segment, value sales 13 weeks to 21 October 2023 3. https://www.trade.gov/sites/default/files/2023 - 09/US - Outbound - to - World - Regions.xlsx
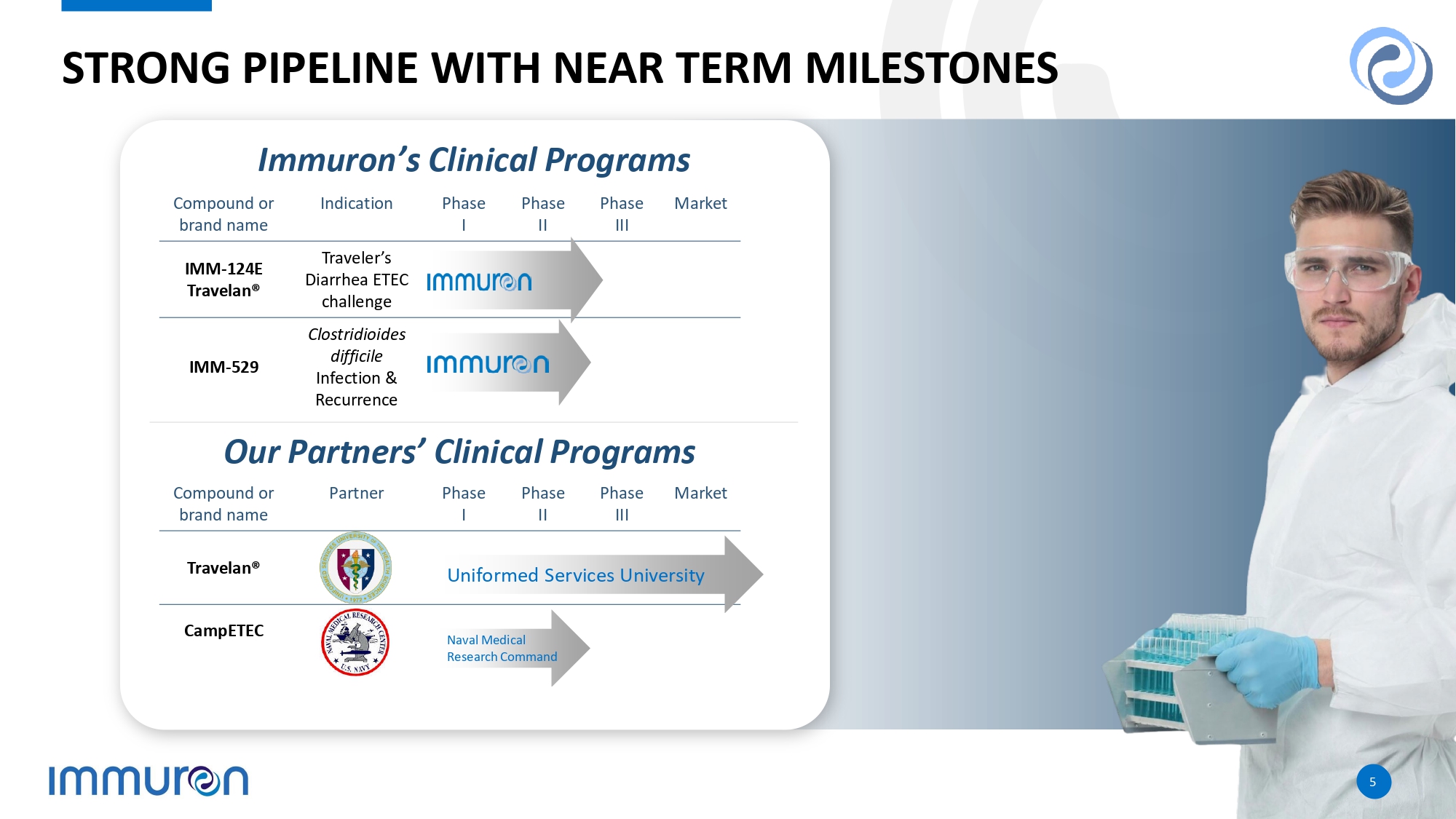
5 5 STRONG PIPELINE WITH NEAR TERM MILESTONES 5 5 Market Phase III Phase II Phase I Partner Compound or brand name Travelan® CampETEC Market Phase III Phase II Phase I Indication Compound or brand name Traveler’s Diarrhea ETEC challenge IMM - 124E Travelan® Clostridioides difficile Infection & Recurrence IMM - 529 Uniformed Services University Naval Medical Research Command Immuron’s Clinical Programs Our Partners’ Clinical Programs

6 6 COMPANY HIGHLIGHTS Collaborations with U.S. DoD – Remain Strong o Travelan® IND Approval – December 2022 o Study Initiation – May 2023 o First cohort recruitment completed – July 2023 o Presentations at Military Health System Research Symposium – August 2023 o Second cohort recruitment completed – October 2023 o Completion of In - patient phase – October 2023 o Anticipated Top Line Results and Clinical Study Report – 1H 2024 US Uniformed Services University Traveller’s Diarrhoea Clinical Field Trial o USU’s Infectious Diseases Clinical Research Program (IDCRP), the UK Ministry of Defence and the New York City Travel Clinic are jointly planning to conduct the randomized clinical trial to evaluate the efficacy of Travelan® against placebo for Traveller’s Diarrhoea o Florastor ® removed from active study arms – February 2023 o Now 868 study participants (434 per arm) o 50% recruitment milestone – October 2023 o Anticipated completion of recruitment – March 2024 o Anticipated completion of In - patient phase – June 2024

7 7 COMPANY HIGHLIGHTS US Naval Medical Research Command – Campylobacter & ETEC PROJECT o New Therapeutic in Clinical Development for Treatment of moderate to severe Campylobacteriosis and Infectious diarrhea caused by ETEC pathogens o Toxicology study completed – December 2022 o FDA removed IND Clinical Hold – May 2023 o Institutional Review Board Approval – October 2023 o Anticipated Initiation of Campylobacter Controlled Human Infection Model (CHIM) clinical trial – 1H2024 o Anticipated completion of In - patient phase – 1H2024
8 8 COMPANY HIGHLIGHTS IMM - 529 Clinical Development for Treatment of C. difficile Infections o Opportunity Assessment Completed o C. difficile infection (CDI) affects just over ~400,000 people in the US annually o S izable number of patients who experience at least one recurrence (~20 - 25%), o M any patients experience multiple recurrences, creating persistent unmet need for novel therapies to address recurrences o Infectious disease experts reacted favorably to the IMM - 529 MOA, and its ability to target three elements of the rCDI infection – the spores, vegetative cells, and Toxin B. o Non - antibiotic treatments (such as IMM - 529) are appealing to experts o 600mg solid dose active formulation development – February 2023 o Anticipated cGMP manufacture – December 2023 o Anticipated FDA pre - IND submission – 1H 2024

9 9 EMAIL: STEVE@IMMURON.COM PHONE: AUSTRALIA: +613 8892 4854 STEVEN LYDEAMORE CHIEF EXECUTIVE OFFICER IMMURON LIMITED CONTACT INFORMATION:
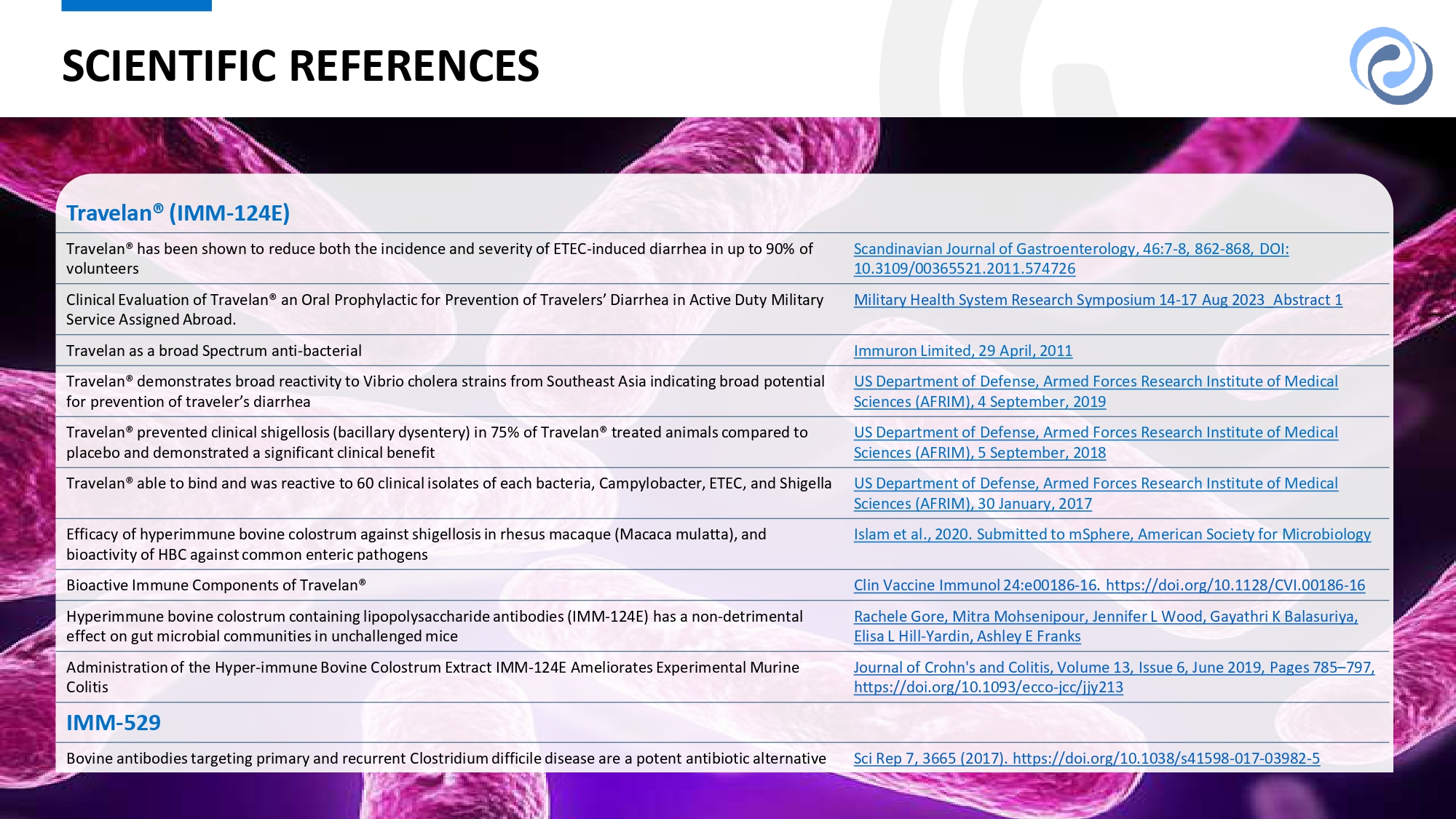
10 10 SCIENTIFIC REFERENCES Travelan® (IMM - 124E) Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers Military Health System Research Symposium 14 - 17 Aug 2023_Abstract 1 Clinical Evaluation of Travelan® an Oral Prophylactic for Prevention of Travelers’ Diarrhea in Active Duty Military Service Assigned Abroad. Immuron Limited, 29 April, 2011 Travelan as a broad Spectrum anti - bacterial US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella Islam et al., 2020. Submitted to mSphere, American Society for Microbiology Efficacy of hyperimmune bovine colostrum against shigellosis in rhesus macaque (Macaca mulatta), and bioactivity of HBC against common enteric pathogens Clin Vaccine Immunol 24:e00186 - 16. https://doi.org/10.1128/CVI.00186 - 16 Bioactive Immune Components of Travelan® Rachele Gore, Mitra Mohsenipour, Jennifer L Wood, Gayathri K Balasuriya, Elisa L Hill - Yardin, Ashley E Franks Hyperimmune bovine colostrum containing lipopolysaccharide antibodies (IMM - 124E) has a non - detrimental effect on gut microbial communities in unchallenged mice Journal of Crohn's and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis IMM - 529 Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative









