As filed with the Securities and Exchange Commission on April 27, 2017.
Registration No. 333-215204
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
To
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
IMMURON LIMITED
(Exact name of registrant as specified in its charter)
| Australia | | 2834 | | Not Applicable |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
Suite 1, 1233 High Street, Armadale, Victoria, Australia 3143
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Delaney Corporate Services Ltd.
99 Washington Avenue, Suite 805A
Albany, New York 12210
Tel: (518) 465-9242
Fax: (518) 465-7883
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Gregory Sichenzia, Esq. Darrin Ocasio, Esq. David B. Manno, Esq. Sichenzia Ross Ference Kesner LLP 61 Broadway New York, New York 10006
Tel: (212) 930-9700
Fax: (212) 930-9725 | | | Mitchell S. Nussbaum, Esq. Norwood P. Beveridge, Jr. Esq. Lili Taheri, Esq.
Loeb & Loeb LLP
345 Park Avenue
New York, NY 10154
Telephone: (212) 407-4000
Facsimile: (212) 407-4990 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earliest effective registration statement for the same offering.¨
CALCULATION OF REGISTRATION FEE
Title of Each Class of
Securities to be Registered(1)(2) | | Proposed
Maximum
Aggregate
Offering Price(2)(3) | | | Amount of
Registration Fee | |
| Ordinary shares, no par value, represented by American Depositary Shares(1) | | $ | 17,250,000 | | | $ | 1,999.27 | * |
| Warrants to purchase American Depository Shares | | $ | — | | | $ | — | |
| Ordinary shares underlying the American Depository Shares issuable upon exercise of Warrants | | $ | 10,781,250 | | | $ | 1,249.55 | |
| Representative’s Warrant | | | — | | | $ | — | |
| Ordinary Shares underlying Representative’s Warrants, no par value(4) | | $ | 1,078,125 | | | $ | 124.96 | |
| Total | | $ | 29,109,375 | | | $ | 3,373.78 | |
| (1) | American depositary shares, or ADSs, issuable upon deposit of the ordinary shares registered hereby have been registered under a separate registration statement on Form F-6 (File No. 333-148037). Each ADS represents 40 ordinary shares. |
| (2) | Includes additional ordinary shares that are issuable upon the exercise of the underwriters’ option to purchase additional shares to cover over-allotments, if any. |
| (3) | Estimated solely for the purpose of determining the amount of registration fee in accordance with Rule 457(o) under the Securities Act of 1933. |
| (4) | We have agreed to issue warrants exercisable within five years after the effective date of this registration statement representing 5% of the securities issued in the offering (the “Representative’s Warrants”) to Joseph Gunnar & Co., LLC andRodman & Renshaw. The Representative’s Warrants are exercisable at an effective per ordinary share price equal to 125% of the ADS public offering price. Resales of the Representative’s Warrants on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, are registered hereby. Resales of ordinary shares issuable upon exercise of the Representative’s Warrants are also being similarly registered on a delayed or continuous basis hereby. See “Underwriting.” In accordance with Rule 457(g) under the Securities Act, because the ordinary shares of the Registrant underlying the Representative’s Warrants are registered hereby, no separate registration fee is required with respect to the warrants registered hereby. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED APRIL 27, 2017 |
American Depositary Shares
Representing [*] Ordinary Shares
Warrants to Purchase [*]American Depositary Shares

Immuron Limited
We are offering American Depositary Shares (each, an “ADS” and, collectively the “ADSs”) and warrants to purchase ADSs (each a “Warrant”) and, collectively the “Warrants”) at an offering price of $ per Warrant. Each ADS will represent forty (40) of our ordinary shares. Each Warrant will have a per ADS exercise price of $ , will be exercisable immediately and will expire five years from the date of issuance. We expect that the initial public offering price will be between $ and $ per ADS.
Prior to this offering, the ADSs have not been listed on any stock exchange. We have applied for a listing of the ADSs and Warrants on The NASDAQ Capital Market under the symbol “IMRN” and “IMRNW”, respectively. No assurance can be given that our application will be approved.
Our ordinary shares are listed on the Australian Securities Exchange under the symbol “IMC.” On April 24, 2017, the closing price of our ordinary shares on the Australian Securities Exchange was AUD$0.51 per ordinary share, equivalent to $15.42 per ADS based on an exchange rate of AUD$1.00 to $0.7559 (as published by the Reserve Bank of Australia as of April 24, 2017).
We are an “emerging growth company,” as that term is used in the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and, as such, we have elected to comply with certain reduced public company reporting requirements for future filings.
Investing in our ordinary shares in the form of ADSs involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | | Per ADS | | | Per Warrant | | | Total | |
| Public offering price | | $ | | | | $ | | | | $ | | |
| Underwriting discounts and commissions(1) | | $ | | | | $ | | | | $ | | |
| Proceeds, before expenses, to us | | $ | | | | $ | | | | $ | | |
| (1) | Does not include a non-accountable expense allowance equal to 1% of the gross proceeds of this offering payable to Joseph Gunnar & Co. and Rodman & Renshaw, the representative of the underwriters. See ‘‘Underwriting’’ for a description of compensation payable to the underwriters. |
We have granted a 45-day option to the representative of the underwriters to purchase up to ADSs and/or Warrants solely to cover over-allotments, if any.
The underwriters expect to deliver our ADSs and Warrants to the purchasers on or about , 2017.
| Joseph Gunnar & Co. | Rodman & Renshaw, |
| | a unit of H.C. Wainwright & Co. |
WallachBeth Capital, LLC
Prospectus dated , 2017.
TABLE OF CONTENTS
You may rely only on the information contained in this prospectus. Neither we nor any of the underwriters have authorized anyone to provide information different from that contained in this prospectus. When you make a decision about whether to invest in the ADSs and Warrants, you should not rely upon any information other than the information in this prospectus. Neither the delivery of this prospectus nor the sale of ADSs and Warrants means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or solicitation of an offer to buy the ADSs and Warrants in any circumstances under which the offer of solicitation is unlawful.
We have not taken any action to permit a public offering of the ADSs and Warrants outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of the ADSs and Warrants and the distribution of this prospectus outside of the United States.
CONVENTIONS THAT APPLY TO THIS PROSPECTUS
Unless otherwise indicated or the context implies otherwise:
| • | “we,” “us,” “our” or “Immuron” refers to Immuron Limited, an Australian corporation; |
| • | “shares” or “ordinary shares” refers to our ordinary shares; |
| • | “ADSs” refers to American Depositary Shares, each of which represents 40 ordinary shares; |
| • | “ADRs” refers to American Depositary Receipts, which evidence the ADSs; and |
| • | Warrants” refers to warrants to purchase ADSs. |
Our reporting and functional currency is the Australian dollar. Solely for the convenience of the reader, this prospectus contains translations of some Australian dollar amounts into U.S. dollars at specified rates. Except as otherwise stated in this prospectus, all translations from Australian dollars to U.S. dollars are based on the rate published by the Reserve Bank of Australia on the date indicated. See “Exchange Rate Information.” No representation is made that the Australian dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars at such rate.
Unless otherwise noted, all industry and market data in this prospectus, including information provided by independent industry analysts, is presented in U.S. dollars. Unless otherwise noted, all other financial and other data related to Immuron Limited in this prospectus is presented in Australian dollars. All references to “$” in this prospectus (other than in our audited consolidated financial statements) refer to U.S. dollars. All references to “AUD$” or “AUD” in this prospectus mean Australian dollars.
Our fiscal year end is June 30. References to a particular “fiscal year” are to our fiscal year ended June 30 of that calendar year.
Unless otherwise indicated, the consolidated financial statements and related notes included in this prospectus have been prepared in accordance with International Accounting Standards (IAS) and also comply with International Financial Reporting Standards, or IFRS, and interpretations issued by the International Accounting Standards Board, or IASB, which differ in certain significant respects from Generally Accepted Accounting Principles in the United States, or GAAP.
INDUSTRY AND MARKET DATA
This prospectus includes information with respect to market and industry conditions and market share from third-party sources or based upon estimates using such sources when available. We believe that such information and estimates are reasonable and reliable. We also believe the information extracted from publications of third-party sources has been accurately reproduced.
TRADEMARKS AND TRADENAMES
We have rights to trademarks and tradenames (both registered and unregistered) used in this prospectus which are important to our business.
These trademarks are as follows:
| - | Immuron (registration in process) |
| - | Travelan (registration in U.S., Australia and China) |
| - | Protectyn (registration in Australia and Europe) |
Solely for convenience, trademarks and trade names referred to in this prospectus appear without the “®” or “™” symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. Each trademark, trade name or service mark of any other company appearing in this prospectus is the property of its respective holder.
PROSPECTUS SUMMARY
This summary provides a brief overview of information contained elsewhere in this prospectus and is qualified in its entirety by the more detailed information and the financial statements and notes thereto included elsewhere in this prospectus. This summary does not contain all of the information that you should consider before investing in the ADSs and Warrants. You should read the entire prospectus carefully before making an investment decision, including the information presented under the headings “Risk Factors,” “Cautionary Note Regarding Forward-Looking Statements” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the historical consolidated financial statements and the related notes to those financial statements included elsewhere in this prospectus.
Overview
We are a clinical-stage biopharmaceutical company with a proprietary technology platform focused on the development and commercialization of a novel class of immunomodulator polyclonal antibodies that we believe can address significant unmet medical needs. Immunomodulator polyclonol antibodies are blood proteins that are produced by the immune system to attack foreign substances such as bacteria and can be specifically utilized to increase and/or decrease the activity of the immune system. Our oral polyclonal antibodies offer targeted delivery within the gastrointestinal (GI) track but do not cross into the bloodstream. We believe that our two lead immunomodulator product candidates, IMM-124E and IMM-529, have the potential to transform the existing treatment paradigms for NASH (Non Alcoholic Steatohepatitis) and for Clostridium difficile (C. difficile), respectively. We also market an OTC product, Travelan, has been designated by the Therapeutic Goods Administration (“TGA”) in Australia and Health Canada as being preventative to Traveler’s Diarrhea . Travelan is also based on the same technology. Generally Regarded as Safe (“GRAS”) is a Food and Drug Administration (“FDA”) designation which designates a chemical or substance that is added to food as “safe” by experts exempting such chemical or substance from Federal Food, Drug and Cosmetic Act (“FFDCA”) food additive tolerance requirements. We previously submitted a GRAS notification to the FDA as part of our investigational new drug (“IND”) application with respect to IMM-124E which designation, to date, has not challenged by the FDA. The safety profile of our compounds, which has a GRAS status, enables us to commercialize our platform-derived products through a range of regulatory pathways, including prescription (Rx), medical foods, over-the-counter (OTC) medicines and dietary supplements. As of December 31, 2016 and June 30, 2016, our accumulated deficit was AUD$46,175,581 and AUD$42,821,357, respectively
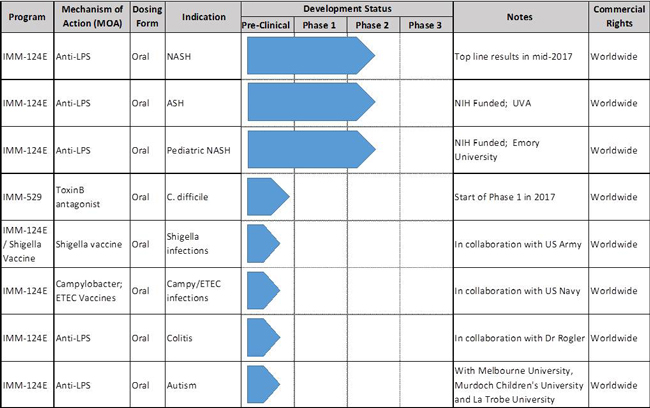
Lipopolysaccharide (“LPS”) is the major component of the outer membrane of Gram-negative bacteria, and IMM-124E is a first-in-class oral anti-LPS polyclonal antibody and a powerful regulator agent of regulatory T-cells. IMM-124E is currently in Phase 2 clinical trial in the United States, Australia and Israel for the treatment of nonalcoholic steatohepatitis (NASH). We expect that top line results will be available in mid-2017. IMM-124E is also the investigational drug of two National Institute of Health (“NIH”) sponsored Phase 2 clinical trials in alcoholic steatohepatitis (ASH) and Pediatric NASH. NIH is an agency of the United States Department of Health and is one of the world’s foremost medical research centers.
IMM-529 targets the C. difficile bacterium and contains polyclonal antibodies to the Toxin B, the spores and the vegetative cells. We successfully completed our pre-clinical program in January 2016 and are currently preparing our clinical supplies to support our Phase 1/2 clinical trials, which will enroll as many as 60 patients. We plan to initiate our Phase 1/2 clinical trial in Israel in the second quarter of 2017. As the clinical trial will be conducted outside of the United States, specifically, in Israel, the Company is not required to apply for an IND.
Because our assets target different biological pathways and act on diseases in completely novel ways, we believe that both IMM-124E and IMM-529 have the potential to be the backbone therapies in their respective fields.
Our Major Markets
Our two lead assets target two prevalent diseases with major unmet need: NASH and C.difficile.
NASH, which is now an epidemic of global proportion, is driven by obesity and a “western lifestyle”. NASH is a severe disease of the liver caused by chronic inflammation and a buildup of fat in the liver, and is one of the most severe manifestations of NAFLD (nonalcoholic fatty-liver disease). The presentation of NASH resembles alcoholic liver disease but occurs in people who drink little or no alcohol. Current estimates place NASH prevalence at approximately 24 million people in the United States, or approximately 7% of the population, with similar prevalence in other major developed markets. There are currently no approved therapies for the treatment of NASH, making this disease one of the largest unmet medical needs in the world today, and a key therapeutic area (TA) targeted by large pharmas. A major analyst report by Deutche Bank in 2014, forecasted a $35B-$40B market by 2025.
Clostridiumdifficile, or C.difficile, is a gram-positive, toxin-producing, spore-forming bacterium that generally causes severe and persistent diarrhea in infected individuals, but can also lead to more severe outcomes, including in the most serious cases, death. C.difficileinfection (CDI) is most often associated with the prior use of antibiotics. The U.S. Centers for Disease Control has identified CDI as one of the top three most urgent antibiotic-resistant bacterial threats in the United States. It is the most common cause of hospital acquired infection in the United States and has overtaken methicillin-resistant Staphylococcus aureus in prevalence with an estimated 450,000 acute infections per year, and nearly 100,000 cases of first recurrences. CDI is responsible for the death of approximately 29,000 Americans each year.
The versatility of our platform allows us to target other immune mediated diseases such as Inflammatory Bowel Disease (IBD) and infectious agents such as shigella and campylobacter. Both therapeutic areas ecompass millions of potential patients and present significant unmet medical needs.
Our Platform
Immuron’s platform technology is based on producing antigen targeted, hyper-immune bovine colostrum powder (BCP) suitable for pharmaceutical use. Polyclonal antibodies are collected from the first milking of a cow after calving. Prior to calving, cows are immunized with proprietary vaccines to ensure maximum immunogenicity and after calving, the first milk is harvested and purified. This proprietary process ensures that the colostrum contains a high concentration of antibodies and high concentrations of Immunoglobulin G1. The technology is safe (classified as GRAS by the FDA), low cost, and can be applied to a variety of diseases.
The underlying nature of Immuron’s platform technology enables the development of medicines across a large range of diseases, including infectious diseases and immune mediated disorders. The platform can be used to influence the cell-mediated immune system through regulatory T cell populations, or it can directly block viruses or bacteria at mucosal surfaces (such as the GI tract) and neutralize the toxins they produce. Additionally, the dairy origins of Immuron’s antibodies enables us to commercialize our platform through most regulatory pathways, including prescription (Rx), medical foods, over-the-counter medicines, and dietary supplements. The GRAS status of our technology platform allow us to advance our preclinical programs into clinical trials faster relative to other companies due to the platform’s proven safety profile.
Our Pipeline
IMM-124E is currently in an ongoing Phase 2 clinical study for the treatment of NASH. Over 100 patients have been recruited to date out of a target of 120. We estimate that top line results will be available in mid-2017. The mechanism of action for IMM-124E is unique and acts on multiple pathways to significantly reduce inflammation in the liver by (1) targeting the gut innate immune system to upregulate regulatory T cell (Treg) populations and (2) by neutralizing intestinal bacterial-LPS, thereby decreasing the translocation of these toxins into the liver, and thus reducing pro-inflammatory burden in the gut and in the liver.
In addition to NASH,IMM-124E is also intwo NIH-sponsored Phase 2 clinical trials for the treatment ofASH, in collaboration with Dr. Arun Sanyal at the University of Virginia, and inPediatric NASH, in collaboration with Dr. Miriam Vos at Emory University. We are unaware of another company in the field of fatty-liver diseases with such a comprehensive set of Phase 2 trials.
IMM-529has successfully completed its pre-clinical program inCDIand we estimate that we will begin Phase 1/2 trials in Israel in the second quarter of 2017. IMM-529 which was developed in collaboration with world leading C.difficileresearcher Dr. Dena Lyras and her team at Monash University, targets the virulent Toxin B, the spores and the vegetative cells. It is a three pronged approach that is unique and which has yielded exceptional results in the pre-clinical studies including (1) Prevention of primary disease, (2) Treatment of primary disease and (3) Suppression of recurrence. To our knowledge, it is to date the only investigational drug that has showed therapeutic benefits in all three phases of the disease.
In addition to these programs, we also have two research collaborations with the U.S. Department of Defense including with theU.S. Navy and with theU.S. Army, for the study ofshigella, campylobacter and Enterotoxigenic Escherichia coli (“ETEC”) vaccines. ETEC is a type of E-coli and is one of the leading bacterial causes of diarrhea in the developing world, as well as the most common cause of travelers’ diarrhea.
We also started a pre-clinical program inIBD, in collaboration with renowned IBD KOL, Professor Gerhard Rogler, MD, PhD. and the university of Zurich, Switzerland.
We believe that the breath/depth of our assets and the support we are receiving from the NIH and the DoD, makes us truly a unique and attractive player in the TAs that we target.
Our Marketed Assets
Travelan – A Unique OTC: Travelan is the only product currently on the market designated by TGA and Health Canada for the prevention of Traveler’s Diarrhea (TD). Travelan uses hyperimmune BCP from cows vaccinated against various strains of ETEC to protect against TD and to reduce the risk of TD, along with the symptoms of minor gastrointestinal disorders. Two independent, double-blinded, placebo-controlled clinical trials in Europe in 90 healthy volunteers showed protection of up to 90% against infection with the type of E. coli that causes TD, along with indicating a significant reduction in abdominal cramps and stomach pain. There were no reported side effects in the clinical trials. Importantly, because Travelan is not an antibiotic, it does not have the side-effect profile of antibiotics and also does not contribute to the worldwide concerns about bacterial drug resistance. Sales in fiscal year 2016, were AUD$1.0M.
Travelan is now marketed in four countries: Australia, U.S.A, China and Canada (with our partner Paladin Labs, a branch of Endo Pharmaceuticals that sells products for the Canadian Market), and we plan to launch the products in additional countries. Effective February 23, 2017, the Company terminated its marketing and distribution agreement with Paladin for failure to purchase the minimum quantities of product pursuant to the agreement;provided, however, that Paladin shall be entitled to continue selling existing inventory in the Territory (as defined in the agreement) for a period of 6 months from the date of termination. The Company may, but is not required to, repurchase any inventory from Paladin. After termination of the agreement with Paladin, the Company will continue to sell Travelan in Canada.
Protectyn – For Gut Dysbiosis.During fiscal year 2016, we launched Protectyn in Australia, which is an OTC targeting LPS bacteria in the gut to prevent gut dysbiosis, improve bacterial clearance, reduce chronic inflammation and improve immune function. This product has been formulated to help maintain healthy digestive function and help support the liver. The product was launched in late 2015 and is currently in its market ramp up phase.
Our Strategy
Our goal is to become one of the leading biopharmaceutical company developing and commercializing therapeutics to address increased unmet medical needs in inflammation-mediated diseases and anti-infectious diseases. The critical components of our strategy include:
| · | Rapidly advance our two lead oral polyclonal antibodies, IMM-124E and IMM-529: |
| - | IMM-124E/NASH:Continue progressing our IMM-124E Phase 2 for the treatment of NASH with a target for top line read-out of mid-2017; and |
| - | IMM-529/CDI:Finalize development of clinical supplies and Phase 1/2 protocol with a target Phase 1/2 start in second quarter of 2017. |
| · | Leverage our technology platform and our collaborations to expand our differentiated polyclonal-based product pipeline across multiple indications including ASH, Pediatric NASH and various novel and potentially game-changing anti-infective programs with the DoD (U.S. Army and U.S. Navy). |
| · | Partner our fatty-liver programsatthe right time and with the right commercial / development partner(s) for NASH, ASH and pediatric NASH. |
| · | Continue investing in and growing Travelan Worldwide including in the U.S., Australia, Canada and China, and in new markets. |
| · | Continue investing in mechanism of action studies that expands our understanding of our unique MOA across our targeted diseases and conditions, and potentially identify new opportunities for investment. |
| · | Protect and leverage our intellectual property portfolio and patents. We believe that our intellectual property protection strategy, grounded in securing composition of matter patents on the biologics we develop, as well as broader patents to protect our technology platform, has best positioned us to gain broad and strong protection of our assets. We have 13 issued patents and 23 pending patent applications worldwide. We have been issued patents in the U.S., Australia, Canada, India, Japan and New Zealand. |
Our Management Team
Our management and development team has extensive experience in designing and developing therapeutics, ensuring a stable manufacturing supply chain and bringing products to the market (either in partnerships/BD or organically), gained across both large pharmas (e.g., Pfizer) and leading and emerging biotechnology companies (e.g., CSL).
Our advisory Board includes some of the foremost experts in the their field including Dr. Arun Sanyal in NASH and Professor Dena Lyras in C.difficile.
Dual Listing - Australian Securities Exchange and The NASDAQ Capital Market
Our ordinary shares are currently listed on the Australian Securities Exchange, or ASX under the symbol “IMC”, and we have applied for a listing of the ADSs and Warrants on The NASDAQ Capital Market under the symbol “IMRN” and “IMRNW”, respectively. The investor in our convertible notes issued in February 2016 has the right to require that we delist from NASDAQ at any time when our primary listing is not on the ASX.
Risk Factors
You should carefully consider the risks described under the “Risk Factors” section beginning on page 10. Some of these risks are:
| · | Risk associated with our profitability including, but not limited to: |
| o | We have incurred operating losses since we began operations and may not be profitable in the future. |
| · | Risk associated with clinical trials, the development of our products and acceptance in the market of our products including, but not limited to: |
| o | Clinical trials are expensive and time consuming, and their outcome is uncertain; |
| o | We may experience delays in our clinical trials that could adversely affect our business and operations; |
| o | We rely on third parties to conduct our preclinical studies and clinical trials and if such third parties do not meet our deadlines or otherwise conduct the studies as required, we may be delayed in progressing, or ultimately may not be able to progress, product candidates to clinical trials, our clinical development programs could be delayed or unsuccessful, and we may not be able to commercialize or obtain regulatory approval for our product candidates when expected, or at all; |
| o | We may not be able to secure and maintain research institutions to conduct our future trials; |
| o | We have limited large scale manufacturing experience with our product candidates. Delays in manufacturing sufficient quantities of such materials to the required standards for pre-clinical and clinical trials may negatively impact our business and operations; |
| o | We may not be able to complete the development of IMM-124E or develop other pharmaceutical products; |
| o | We may need to prioritize the development of our most promising candidates at the expense of the development of other products; |
| o | Physicians, patients, third-party payors or others in the medical community may not be receptive to our product candidates, and we may not generate any future revenue from the sale or licensing of our product candidates; and |
| o | If healthcare insurers and other organizations do not pay for our products, or impose limits on reimbursement, our future business may suffer. |
| · | Risks associated with intellectual property including, but not limited to: |
| o | We may not be successful in obtaining or maintaining other necessary rights necessary to the development of our pipeline through acquisitions and in-licenses; |
| · | Risks associated with competition and manufacturing including, but not limited to: |
| o | We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours; and |
| o | We depend upon a sole manufacturer of our lead compound and on a sole manufacturer to encapsulate the compound and could incur significant costs and delays if we are unable to promptly find a replacement for either of them. |
| · | Risks associated with government regulation including, but not limited to: |
| o | If we do not obtain the necessary governmental approvals, we will be unable to commercialize our pharmaceutical products; |
| o | We will not be able to commercialize any current or future product candidates if we fail to adequately demonstrate their safety, efficacy and superiority over existing therapies; |
| o | Even if we obtain regulatory approval for a product candidate, our products may remain subject to regulatory scrutiny; and |
| o | Healthcare reform measures and other statutory or regulatory changes could adversely affect our business. |
| · | Risk associated with the ADSs, Warrants and this Offering including, but not limited to: |
| o | If we are classified as a “passive foreign investment company,” our U.S. shareholders could suffer adverse tax consequences as a result; |
| o | The market price and trading volume of the ADSs and Warrants may be volatile and may be affected by economic conditions beyond our control; |
| o | Investors purchasing the ADSs will suffer immediate and substantial dilution; |
| o | Currency fluctuations may adversely affect the price of our ordinary shares, ADSs and Warrants; and |
| o | The dual listing of our ordinary shares and the ADSs following this offering may adversely affect the liquidity and value of the ADSs, and the investor in our convertible notes issued in February 2016 has the right to require that we delist from NASDAQ at any time when our primary listing is not on the ASX. |
These and other risks described in this prospectus could materially and adversely impact our business, financial condition, operating results and cash flow, which could cause the trading price of our ADSs and Warrants to decline and could result in a loss of your investment.
Corporate Information
Immuron Limited was incorporated under the laws of Australia in 1994 and has been listed on the Australian Securities Exchange, or ASX, since April 30, 1999.
Our headquarters are located at Suite 1, 1233 High Street, Armadale, Victoria, Australia 3143. Our telephone number is +61 (0)3 9824 5254. Our website address is www.immuron.com. Information on our website and the websites linked to it do not constitute part of this prospectus or the registration statement to which this prospectus forms a part. Our agent for service of process in the United States is Delaney Corporate Services Ltd., 99 Washington Avenue, Suite 805A, Albany, New York 12210
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, with less than $1 billion innon-convertible debt securities issued in the past three years, and that is pursuing a first registered equity offering in the United States, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may avail itself of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. For example, we have elected to rely on an exemption from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, relating to internal control over financial reporting, and we will not provide such an attestation from our auditors for as long as we qualify as an emerging growth company.
We will remain an emerging growth company until the earliest of:
| • | the end of the fiscal year in which the fifth anniversary of the completion of this offering occurs; |
| • | the end of the first fiscal year in which the market value of our ordinary shares held by non-affiliates exceeds US $700 million as of the end of the second quarter of such fiscal year; |
| • | the end of the first fiscal year in which we have total annual gross revenues of at least US $1 billion; and |
| • | the date on which we have issued more than US $1 billion in non-convertible debt securities in any rolling three-year period. |
Once we cease to be an emerging growth company, we will not be entitled to the exemptions provided for by the JOBS Act.
Implications of Being a Foreign Private Issuer
We are also considered a “foreign private issuer” pursuant to Rule 405 under the Securities Act of 1933, as amended, or the Securities Act. In our capacity as a foreign private issuer, we are exempt from certain rules under the Securities Exchange Act of 1934, as amended, or the Exchange Act, that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our ordinary shares. Moreover, we are not required to file periodic reports and financial statements with the U.S. Securities and Exchange Commission, or SEC, as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. In addition, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (1) the majority of our executive officers or directors are U.S. citizens or residents; (2) more than 50% of our assets are located in the United States; or (3) our business is administered principally in the United States. We are required to determine our status as a foreign private issuer on an annual basis at the end of our second fiscal quarter.
THE OFFERING
| Securities offered by us | ADSs (or ADSs if the underwriters exercise their option to purchase additional ADSs in full). Warrants to purchase ADSs.Each Warrant will have a per ADS exercise price of $ will be exercisable immediately and will expire five years from the date of issuance. To better understand the terms of the Warrants, you should carefully read the section in this prospectus entitled “Description of the Warrants.” |
| | |
| ADSs to be outstanding immediately after this offering | ADSs (or ADSs if the underwriters exercise their option to purchase additional ADSs in full). |
| | |
| Ordinary shares to be outstanding immediately after this offering, including shares underlying ADSs | ordinary shares |
| | |
Underwriters’ option to purchase additional ADSs and/or Warrants | We have granted the underwriters a 45-day option to purchase up to an additional ADSs and/or Warrants to cover overallotments, if any. |
| | |
| The ADSs | Each ADS represents 40 ordinary shares. |
| | |
| | The depositary (as identified below) will be the holder of the ordinary shares underlying the ADSs and you will have the rights of an ADS holder as provided in the deposit agreement among us, the depositary and holders and beneficial owners of ADSs from time to time. |
| | |
| | You may surrender your ADSs to the depositary to withdraw the ordinary shares underlying your ADSs. The depositary will charge you a fee for such an exchange. |
| | |
| | We may amend or terminate the deposit agreement for any reason without your consent. Any amendment that imposes or increases fees or charges or which materially prejudices any substantial existing right you have as an ADS holder will not become effective as to outstanding ADSs until 30 days after notice of the amendment is given to ADS holders. If an amendment becomes effective, you will be bound by the deposit agreement as amended if you continue to hold your ADSs. |
| | |
| | To better understand the terms of the ADSs, you should carefully read the section in this prospectus entitled “Description of American Depositary Shares.” We also encourage you to read the deposit agreement, which is an exhibit to the registration statement to which this prospectus forms a part. |
| | |
| Depositary | The Bank of New York Mellon. |
| | |
| Offering Price | On April 24, 2017, the last reported sale price of our ordinary shares on the ASX was AUD$0.51 per ordinary share, equivalent to approximately $15.42 per ADS. See “Underwriting” for a discussion of factors considered in determining the price of public ADSs. |
| | |
| Shareholder approval of offering | Under Australian law, certain steps necessary for the consummation of this offering require the approval of our shareholders voting at a general meeting of shareholders. We expect to receive all such required approvals from our shareholders prior to the completion of this offering. |
| Use of proceeds | We estimate that the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, will be $ million, assuming the ADSs are offered at $ per ADS, which is the midpoint of the estimated price range set forth on the cover page of this prospectus and $ per Warrant. We intend to use the net proceeds from this offering to advance our products and platform technologies and for working capital and for general corporate purposes. See “Use of Proceeds” for a description of the intended use of proceeds from this offering. |
| | |
| Risk factors | You should carefully read and consider the information in this prospectus under the heading “Risk Factors” beginning on page 10 and other information included in this prospectus before deciding to invest in the ADSs. |
| | |
| NASDAQ Capital Market | We have applied for the listing of the ADSs and Warrants on NASDAQ under the symbol “IMRN” and “IMRNW”, respectively |
| | |
| Lock-up Agreements | We and our directors and executive officers have agreed with the underwriters, subject to certain exceptions, not to sell or transfer any of the ordinary shares, the ADSs or securities convertible into or exchangeable or exercisable for ordinary shares or ADSs for a period of (i) twelve months after the date of this prospectus in the case of our directors and officers and (ii)180 days after the date of this prospectus in the case of the Company without the prior written consent of the representative of the underwriters. See “Underwriting.” |
The number of ordinary shares shown above that will be outstanding immediately following the completion of this offering:
| • | is based on ordinary shares outstanding as of , 2017; and |
| • | excludes an aggregate of ordinary shares issuable upon the exercise of options outstanding at 2017, at a weighted average exercise price of AUD$ , of which options to purchase ordinary shares were vested at a weighted average exercise price of AUD$ . |
Except as otherwise indicated herein, all information in this prospectus assumes no exercise by the underwriters of their option to purchase up to additional ADSs.
SUMMARY HISTORICAL CONSOLIDATED FINANCIAL DATA
The following tables set forth summary historical consolidated financial data for the periods indicated.
The consolidated statement of profit or loss and other comprehensive income data for the years ended June 30, 2016, 2015 and 2014 and consolidated statement of financial position data as of June 30, 2016 and 2015 are derived from the audited consolidated financial statements included in this prospectus. The consolidated statement of profit or loss and other comprehensive income data for the six months ended December 31, 2016 and 2015 and consolidated statement of financial position data as of the six months ended December 31, 2016 are derived from the unaudited consolidated financial statements included in this prospectus.
Our consolidated financial statements have been prepared in Australian dollars and in accordance with International Accounting Standards and IFRS, as issued by the IASB.
You should read the summary historical consolidated financial data in conjunction with our consolidated financial statements and related notes beginning on page F-1 of this prospectus, and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. Our historical results do not necessarily indicate our expected results for any future periods. Financial results for the year ended June 30, 2016 and the six months ended December 31, 2016 are not necessarily indicative of the results that may be expected for the year ending June 30, 2017.
| | | For the year ended June 30, | | | For the six months ended
December 31, | |
| | | 2016 AUD$ | | | 2015 AUD$ | | | 2014 AUD$ | | | 2016 AUD$ | | | 2015 AUD$ | |
| Consolidated Statement of Profit or Loss and Other Comprehensive Income Data: | | | | | | | | | | | | | | | |
| Revenue: | | | | | | | | | | | | | | | | | | | | |
| Operating Revenue | | $ | 1,001,077 | | | $ | 1,002,380 | | | $ | 981,051 | | | $ | 703,099 | | | $ | 497,220 | |
| Total Operating Revenue | | | 1,001,077 | | | | 1,002,380 | | | | 981,051 | | | | 703,099 | | | | 497,220 | |
| Cost of Goods Sold | | | (301,435 | ) | | | (316,128 | ) | | | (277,928 | ) | | | (223,394 | ) | | | (153,640 | ) |
| Gross Profit | | | 699,642 | | | | 686,252 | | | | 703,123 | | | | 479,705 | | | | 343,580 | |
| Sales and Marketing Costs | | | (133,781 | ) | | | (76,794 | ) | | | (79,796 | ) | | | (93,520 | ) | | | (52,659 | ) |
| Freight Costs | | | (134,967 | ) | | | (116,379 | ) | | | (114,278 | ) | | | (62,590 | ) | | | (61,299 | ) |
| Total Gross Profit less Direct Selling Costs | | | 430,894 | | | | 493,079 | | | | 509,049 | | | | 323,595 | | | | 229,622 | |
| | | | | | | | | | | | | | | | | | | | | |
| Other Income | | | 1,539,015 | | | | 1,591,021 | | | | 804,477 | | | | 816,932 | | | | 763,563 | |
| | | | | | | | | | | | | | | | | | | | | |
| Expenses: | | | | | | | | | | | | | | | | | | | | |
| Amortisation | | | - | | | | - | | | | (680,587 | ) | | | - | | | | - | |
| Consulting, Employee and Director | | | (2,840,037 | ) | | | (728,140 | ) | | | (555,487 | ) | | | (907,390 | ) | | | (1,113,214 | ) |
| Corporate Administration | | | (1,320,570 | ) | | | (557,422 | ) | | | (492,465 | ) | | | (790,103 | ) | | | (708,625 | ) |
| Depreciation | | | (3,892 | ) | | | (3,719 | ) | | | (3,989 | ) | | | (1,975 | ) | | | (1,944 | ) |
| Finance Costs | | | (341,600 | ) | | | - | | | | (463,685 | ) | | | (13,183 | ) | | | - | |
| Impairment of Inventory | | | (4,176 | ) | | | (35,340 | ) | | | (50,204 | ) | | | (135,170 | ) | | | (169 | ) |
| Marketing and Promotion | | | (487,591 | ) | | | (304,687 | ) | | | (235,176 | ) | | | (471,735 | ) | | | (201,724 | ) |
| Research and Development | | | (3,623,961 | ) | | | (3,018,294 | ) | | | (1,289,675 | ) | | | (2,117,867 | ) | | | (1,839,990 | ) |
| Travel and Entertainment | | | (416,849 | ) | | | (128,318 | ) | | | (37,327 | ) | | | (112,453 | ) | | | (195,605 | ) |
| Loss before income tax | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) | | | (3,409,349 | ) | | | (3,068,086 | ) |
| Income tax expense | | | - | | | | - | | | | - | | | | - | | | | - | |
| Loss for the period | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) | | | (3,409,349 | ) | | | (3,068,086 | ) |
| Other Comprehensive Income / (Losses) | | | 8,846 | | | | (12,581 | ) | | | - | | | | (41,425 | ) | | | (4,781 | ) |
| Total Comprehensive Loss for the Period | | | (7,059,921 | ) | | | (2,704,401 | ) | | | (2,495,069 | ) | | | (3,450,774 | ) | | | (3,072,867 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Loss per share, basic and diluted (cent per share) | | $ | 9.248 | | | $ | 3.592 | | | $ | 5.947 | | | $ | 3.300 | | | $ | 4.065 | |
| Weighted-average number of shares outstanding, basic and diluted | | | 76,435,993 | | | | 74,935,902 | | | | 41,955,199 | | | | 103,328,491 | | | | 75,470,006 | |
| | | As of June 30, | | | As of
December 31, | |
| | | 2016 AUD$ | | | 2015 AUD$ | | | 2016 AUD$ | |
| | | | | | | | | | |
| Consolidated Statement of Financial Position Data: | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 2,290,639 | | | $ | 3,116,074 | | | $ | 3,103,683 | |
| Total current assets | | | 8,809,421 | | | | 5,998,898 | | | | 5,837,834 | |
| Total assets | | | 8,827,484 | | | | 6,018,412 | | | | 5,855,801 | |
| Total current liabilities | | | 3,886,921 | | | | 1,207,810 | | | | 2,302,621 | |
| Total liabilities | | | 3,886,921 | | | | 1,207,810 | | | | 2,302,621 | |
| Total equity | | | 4,940,563 | | | | 4,810,602 | | | | 3,553,180 | |
RISK FACTORS
An investment in the ADSs involves significant risks. You should carefully consider the risks described below and the other information in this prospectus, including our consolidated financial statements and related notes included elsewhere in this prospectus, before you decide to invest in the ADSs. If any of the following risks actually occurs, our business, prospects, financial condition and results of operations could be materially and adversely affected, the trading price of the ADSs could decline and you could lose all or part of your investment.
Risks Related to Our Financial Condition
We have incurred operating losses since we began operations and may not be profitable in the future. We will need to secure additional financing and may not be successful in obtaining sufficient funding.
We have incurred losses in every period since we began operations in 1994 and reported net losses of AUD$7,068,767, AUD$2,691,820 and AUD$2,495,069 during the fiscal years ended June 30, 2016, 2015 and 2014, respectively. For the six-month periods ended December 31, 2016 and 2015, the reported net losses were AUD$3,409,349 and AUD$3,068,086, respectively. As of December 31, 2016 and June 30, 2016, our accumulated deficit was AUD$46,175,581 and AUD$42,821,357, respectively. We expect to continue to incur additional operating losses over at least the next several years as we expand our research and development activities in fatty-liver diseases and new trials for our product candidate IMM-529 for C.difficile, and potential other assets/indications. We may never be able to achieve or maintain profitability.
Our actual cash requirements may vary materially from those now planned and will depend upon numerous factors, including:
| · | the continued progress of our research and development programs; |
| · | the timing, scope, results and costs of pre-clinical studies and clinical trials; |
| · | the cost, timing and outcome of regulatory submissions and approvals; |
| · | determinations as to the commercial potential of our product candidates; |
| · | spending on our marketed assets; |
| · | our ability to successfully expand our contract manufacturing services; |
| · | our ability to establish and maintain collaborative arrangements; and |
| · | the status and timing of competitive developments. |
As of December 31, 2016 and June 30, 2016, our cash and cash equivalents were AUD$3,103,683 and AUD$2,290,639. Developing prescription products is expensive and we will need to secure additional financing in order to continue to meet our longer term business objectives, including advancement of our research and development programs. We may also require additional funds to pursue regulatory clearances, defend our intellectual property rights, establish commercial scale manufacturing facilities, develop marketing and sales capabilities and fund operating expenses. We intend to seek such additional funding through public or private financings and/or through licensing of our assets or strategic alliances or other arrangements with corporate partners. The global economic climate could adversely impact our ability to obtain such funding, license our assets or enter into alliances or other arrangements with corporate partners. Any shortfall in funding could result in our having to curtail or cease our operations, including our research and development activities, which would be expected to adversely affect our business, financial condition and results of operations.
We have never generated any revenue from prescription product sales and may never be profitable.
Our ability to generate significant revenue from prescription products and achieve profitability depends on our ability to, alone or with strategic collaboration partners, successfully complete the development of and obtain the regulatory approvals for our prescription product candidates, to manufacture sufficient supply of our product candidates, to establish a sales and marketing organization or suitable third-party alternative for the marketing of any approved products and to successfully commercialize any approved products on commercially reasonable terms. All of these activities will require us to raise sufficient funds to finance business activities. Currently, we do not expect any milestone payments from our collaborative partners to be significant in the foreseeable future however we are actively pursuing potential partner collaboration. In addition, we do not anticipate generating revenue from commercializing product candidates for the foreseeable future, if ever.
Our ability to generate future revenues from commercializing Company owned IP assets depends heavily on our success in:
| • | establishing proof of concept in preclinical studies and clinical trials for our product candidates; |
| • | successfully completing clinical trials of our product candidates; |
| • | obtaining regulatory and marketing approvals for product candidates for which we complete clinical trials; |
| • | maintaining, protecting and expanding our intellectual property portfolio, and avoiding infringing on intellectual property of third parties; |
| • | establishing and maintaining successful licenses, collaborations and alliances with third parties; |
| | • | developing a sustainable, scalable, reproducible and transferable manufacturing process for our product candidates; |
| • | establishing and maintaining supply and manufacturing relationships with third parties that can provide products and services adequate, in amount and quality, to support clinical development and commercialization of our product candidates, if approved; |
| • | launching and commercializing any product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales, marketing and distribution infrastructure; |
| • | obtaining market acceptance of any product candidates that receive regulatory approval as viable treatment options; |
| • | obtaining favorable coverage and reimbursement rates for our products from third-party payors; |
| • | addressing any competing technological and market developments; |
| • | identifying and validating new product candidates; and |
| • | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter. |
The process of developing product candidates for fatty-liver and anti-infective conditions contains a number of inherent risks and uncertainties, including clinical and regulatory risks.
Even if one or more of our product candidates is approved for commercial sale, we may incur significant costs associated with commercializing any approved product candidate. As one example, our expenses could increase beyond expectations if we are required by the Food and Drug Administration, or FDA, or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that we currently anticipate. Even if we are able to generate revenues from the sale of any approved products, we may not become profitable and may need to obtain additional funding to continue operations, which could have an adverse effect on our business, financial condition, results of operations and prospects.
We are a development stage company of pharmaceutical products and our success is uncertain.
We are a development stage company of our pharmaceutical products which are designed to treat a range of anti-inflammatory and anti-infectives. Other than Travelan product, we have not sufficiently advanced the development of any of our products, including our current lead product candidate, IMM-124E, to market or generate revenues from their commercial application. Our current or any future product candidates, if successfully developed, may not generate sufficient or sustainable revenues to enable us to be profitable.
We receive Australian government research and development income tax concession refunds. If our research and development expenditures are not deemed to be eligible for the refund, we may encounter difficulties in the funding of future research and development projects, which could harm our operating results.
We have historically received, and expect to continue to receive, refunds from the Australian Federal Government’s Research and Development Tax Incentive program, under which the government provides a cash refund for the 45% of eligible research and development expenditures by small Australian entities, which are defined as Australian entities with less than AUD$20 million in revenue, having a tax loss.
The Research and Development Tax Incentive refund is made by the Australian federal government for eligible research and development purposes based on the filing of an annual application. We received Research and Development Tax Concession Incentive refunds in the fiscal years ended June 30, 2014, June 30, 2015 and June 30, 2016 of AUD$713,632, AUD$1,478,581 and AUD$1,512,840 respectively. This refund is available for our research and development activities in Australia, as well as activities in the United States to the extent such U.S.-based expenses relate to our activities in Australia, do not exceed half the expenses for the relevant activities and are approved by the Australian government. To the extent our research and development expenditures are deemed to be “ineligible,” then our refunds would decrease. In addition, the Australian government may in the future modify the requirements of, reduce the amounts of the refunds available under, or discontinue the Research and Development Tax Incentive program. Any such change in the Research and Development Tax Incentive program would have a negative effect on our future cash flows.
Risks Related To Our Business
We are faced with uncertainties related to our research.
Our research programs are based on scientific hypotheses and experimental approaches that may not lead to desired results. In addition, the timeframe for obtaining proof of principle and other results may be considerably longer than originally anticipated, or may not be possible given time, resource, financial, strategic and collaborator scientific constraints. Success in one stage of testing is not necessarily an indication that the particular program will succeed in later stages of testing and development. It is not possible to predict whether any of the drugs designed for these programs will prove to be safe, effective, and suitable for human use. Each drug will require additional research and development, scale-up, formulation and extensive clinical testing in humans. Unsatisfactory results obtained from a particular study relating to a program may cause us to abandon our commitment to that program or to the lead compound or product candidate being tested. The discovery of toxicities, lack of sufficient efficacy, unacceptable pharmacology, inability to increase scale of manufacture, market attractiveness, regulatory hurdles, competition, as well as other factors, may make our targets, lead therapies or product candidates unattractive for further development or unsuitable for human use, and we may abandon our commitment to that program, target, lead therapy or product candidate. Any delay in obtaining or failure to obtain required approvals could materially and adversely affect our ability to generate revenue from the particular product candidate, which likely would result in significant harm to our financial position and adversely impact the price of the ADSs. Furthermore, any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product. These limitations may limit the size of the market for the product.
Clinical trials are expensive and time consuming, and their outcome is uncertain.
In order to obtain approvals to market a new drug product, we or our potential partners must demonstrate proof of safety and efficacy in humans. To meet these requirements, we or our potential partners will have to conduct extensive preclinical testing and “adequate and well-controlled” clinical trials. Conducting clinical trials is a lengthy, time-consuming and expensive process. The length of time may vary substantially according to the type, complexity, novelty and intended use of the product candidate, and often can be several years or more per trial. Even if we obtain positive results from preclinical or initial clinical trials, we may not achieve the same success in future trials. Clinical trials may not demonstrate statistically sufficient safety and effectiveness to obtain the requisite regulatory approvals for product candidates employing our technology. The failure of clinical trials to demonstrate safety and efficacy for a particular desired indication could harm development of that product candidate for other indications as well as other product candidates.
We expect to commence new clinical trials from time to time in the course of our business as our product development work continues. Any change in, or termination of, our clinical trials could materially harm our business, financial condition and results of operations.
We rely on third parties to conduct our preclinical studies and clinical trials. If these third parties do not meet our deadlines or otherwise conduct the studies as required, we may be delayed in progressing, or ultimately may not be able to progress, product candidates to clinical trials, our clinical development programs could be delayed or unsuccessful, and we may not be able to commercialize or obtain regulatory approval for our product candidates when expected, or at all.
We do not have the ability to conduct all aspects of our preclinical testing or clinical trials ourselves. We are dependent on third parties to conduct the clinical trials for IMM-124E and IMM-529, and preclinical studies for our other product candidates, and therefore the timing of the initiation and completion of these trials and studies is reliant on third parties and may occur at times substantially different from our estimates or expectations.
If we cannot contract with acceptable third parties on commercially reasonable terms, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for the conduct of preclinical studies or clinical trials or meet expected deadlines, our clinical development programs could be delayed or discontinued.
We may experience delays in our clinical trials that could adversely affect our business and operations.
We do not know whether planned clinical trials will begin on time or whether we will complete any of our clinical trials on schedule or at all or within budget. Our ability to commence and complete clinical trials may be delayed by many factors, including:
| · | government or regulatory delays, including delays in obtaining approvals from applicable hospital ethics committees and internal review boards; |
| · | slower than expected patient enrollment; |
| · | our inability to manufacture sufficient quantities of our new proprietary compound or our other product candidates or matching controls; |
| · | unforeseen safety issues; or |
| · | lack of efficacy or unacceptable toxicity during the clinical trials or non-clinical studies. |
Patient enrollment is a function of, among other things, the nature of the clinical trial protocol, the existence of competing protocols, the size and longevity of the target patient population, and the availability of patients who comply with the eligibility criteria for the clinical trial. Delays in planned patient enrollment may result in increased costs, delays or termination of the clinical trials. Moreover, we rely on third parties such as clinical research organizations to assist us in clinical trial management functions including; clinical trial database management, statistical analyses, site management and monitoring. Any failure by these third parties to perform under their agreements with us may cause the trials to be delayed or result in a failure to complete the trials.
If we experience delays in testing or approvals or if we need to perform more, larger or more complex clinical trials than planned, our product development costs may increase. Significant delays could adversely affect the commercial prospects of our product candidates and our business, financial condition and results of operations.
We may not be successful in obtaining or maintaining other necessary rights necessary to the development of our pipeline through acquisitions and in-licenses.
Our product candidates may require specific formulations to work effectively and efficiently and rights to such formulations may be held by others. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify on terms that we find acceptable, or at all. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we sometimes collaborate with U.S. and foreign academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
We rely on research institutions to conduct our clinical trials and we may not be able to secure and maintain research institutions to conduct our future trials.
We rely on research institutions to conduct our clinical trials. Our reliance upon research institutions, including public and private hospitals and clinics, provides us with less control over the timing and cost of clinical trials, clinical study management personnel and the ability to recruit subjects. If we are unable to reach agreements with suitable research institutions on acceptable terms, or if any resulting agreement is terminated, we may be unable to secure, maintain or quickly replace the research institution with another qualified institution on acceptable terms.
We grant licenses to our collaborators to use our hyper-immune colostrum technology exclusively for the development of product candidates for certain conditions.
We may out-license to our collaborators the right to use our hyper-immune colostrum technology for the development of product candidates for certain conditions, so long as our collaborators comply with certain requirements. That means that once our technology is licensed to a collaborator for a specified condition, we are generally prohibited from developing product candidates for that condition and from licensing the to any third party for that condition. The limitations imposed by these exclusive licenses could prevent us from expanding our business and increasing our development of product candidates with new collaborators, both of which could adversely affect our business and results of operations.
We may not be able to complete the development of IMM-124E; IMM-529 or develop other pharmaceutical products.
We may not be able to progress with the development of our current or any future pharmaceutical product candidates to a stage that will attract a suitable collaborative partner for the development of any current or future pharmaceutical product candidates. The projects initially specified in connection with any such collaboration and any associated funding may change or be discontinued as a result of changing interests of either the collaborator or us, and any such change may change the budget for the projects under the collaboration. Additionally, our research may not lead to the discovery of additional product candidates, and any of our current and future product candidates may not be successfully developed, prove to be safe and efficacious in clinical trials, meet applicable regulatory standards and receive regulatory approval, be capable of being produced in commercial quantities at reasonable costs, or be successfully or profitably marketed, either by us or a collaborative partner. The products we develop may not be able to penetrate the potential market for a particular therapy or indication or gain market acceptance among health care providers, patients and third-party payers. We cannot predict if or when the development of IMM-124E, IMM-529 or any future pharmaceutical product will be completed or commercialized, whether funded by us, as part of a collaboration or through a grant.
We may need to prioritize the development of our most promising candidates at the expense of the development of other products.
We may need to prioritize the allocation of development resources and/or funds towards what we believe to be our most promising product or products. The nature of the drug development process is such that there is a constant availability of new information and data which could positively or adversely affect a product in development. We cannot predict how such new information and data may impact in the future the prioritization of the development of our current or future product candidates or that any of our products, regardless of its development stage or the investment of time and funds in its development, will continue to be funded or developed.
Our research and development efforts will be seriously jeopardized if we are unable to retain key personnel and cultivate key academic and scientific collaborations.
Our future success depends to a large extent on the continued services of our senior management and key scientific personnel, including Thomas Liquard, our Chief Executive Officer, Dr. Jerry Kanellos (PhD), Chief Operating and Scientific Officer and Dr. Dan Peres (MD), Medical Director. The loss of their services could negatively affect our business. Competition among biotechnology and pharmaceutical companies for qualified employees is intense, including competition from larger companies with greater resources, and we may not be able to continue to attract and retain qualified management, technical and scientific personnel critical to our success. Our success is highly dependent on our ability to develop and maintain important relationships with leading academic institutions and scientists who conduct research at our request or assist us in formulating our research and development strategies. These academic and scientific collaborators are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, these collaborators may have arrangements with other companies to assist such companies in developing technologies that may prove competitive to ours.
If we are unable to successfully keep pace with technological change or with the advances of our competitors, our technology and products may become obsolete or non-competitive.
The biotechnology and pharmaceutical industries are subject to rapid and significant technological change. Our competitors are numerous and include major pharmaceutical companies, biotechnology firms, universities and other research institutions. These competitors may develop technologies and products that are more effective than any that we are developing, or which would render our technology and products obsolete or non-competitive. Many of these competitors have greater financial and technical resources and manufacturing and marketing capabilities than we do. In addition, many of our competitors have much more experience than we do in pre-clinical testing and human clinical trials of new or improved drugs, as well as in obtaining regulatory approvals.
We know that competitors are developing or manufacturing various technologies or products for the treatment of diseases that we have targeted for product development. Some of these competitive products use therapeutic approaches that compete directly with our product candidates. Our ability to further develop our products may be adversely affected if any of our competitors were to succeed in obtaining regulatory approval for their competitive products sooner than us.
Physicians, patients, third-party payors or others in the medical community may not be receptive to our product candidates, and we may not generate any future revenue from the sale or licensing of our product candidates.
Even if we obtain approval for a product candidate, we may not generate or sustain revenue from sales of the product if the product cannot be sold at a competitive cost or if it fails to achieve market acceptance by physicians, patients, third-party payors or others in the medical community. These market participants may be hesitant to adopt a novel treatment based on hyper-immune colostrum technology, and we may not be able to convince the medical community and third-party payors to accept and use, or to provide favorable reimbursement for, any product candidates developed by us or our existing or future collaborators. Market acceptance of our product candidates will depend on, among other factors:
| · | the establishment and demonstration of the safety and clinical efficacy of our product candidates and their potential advantages over existing therapeutics and technologies; |
| · | our ability to offer our products for sale at competitive prices; |
| · | the relative convenience and ease of administration of our product candidates; |
| · | the prevalence and severity of any adverse side effects associated with our product candidates; |
| · | the receipt, timing and terms of regulatory approvals and the countries in which approvals are obtained; |
| · | limitations or warnings contained in any labeling approved by the FDA or comparable foreign regulatory authorities; |
| · | conditions upon the approval imposed by FDA or comparable foreign regulatory authorities, including, but not limited to, a REMS; |
| · | the willingness of patients to try new treatments and of physicians to prescribe these treatments; |
| · | the availability of government and other third-party payor coverage and adequate reimbursement; and |
| · | availability of alternative effective treatments for the disease indications our product candidates are intended to treat and the relative risks, benefits and costs of those treatments. |
Additional risks apply in relation to any disease indications we pursue which are classified as rare diseases and allow for orphan drug designation by regulatory agencies in major commercial markets, such as the United States or European Union. If pricing is not approved or accepted in the market at an appropriate level for any approved product for which we pursue and receive an orphan drug designation, such product may not generate enough revenue to offset costs of development, manufacturing, marketing and commercialization despite any benefits received from the orphan drug designation, such as market exclusivity, for a period of time. Orphan exclusivity could temporarily delay or block approval of one of our products if a competitor obtains orphan drug designation for its product first. However, even if we obtain orphan exclusivity for one of our products upon approval, our exclusivity may not block the subsequent approval of a competitive product that is shown to be clinically superior to our product.
Market size is also a variable in disease indications not classified as rare. Our estimates regarding potential market size for any indication may be materially different from what we discover to exist at the time we commence commercialization, if any, for a product, which could result in significant changes in our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours. If these companies develop technologies or product candidates more rapidly than we do or their technologies, including delivery technologies, are more effective, our ability to develop and successfully commercialize product candidates may be compromised.
The development and commercialization of pharmaceutical products is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. Our competitors have developed, are developing or could develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community, patients and third-party payors, and any new treatments that enter the market.
We believe that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which we are developing, and may in the future try to develop, product candidates. We are aware of multiple companies that are working in the field of fatty-liver diseases and C.difficile therapeutics, including Intercept, Gilead, Genfit, Tobira, Galmed are all developing therapeutics for fatty-liver diseases and Seres, Synthetic Biotechnology and Assembly Biotechnology for C.difficile.
We have limited large scale manufacturing experience with our product candidates. Delays in manufacturing sufficient quantities of such materials to the required standards for pre-clinical and clinical trials may negatively impact our business and operations.
While we have extensive experience in producing therapeutic colostrum, we may not be able to manufacture sufficient quantities of our product candidates in a cost-effective or timely manner. Manufacturing includes the production, formulation and stability testing of an active pharmaceutical ingredient and its formulation into pharmaceutical products, such as capsules or tablets. Any delays in production would delay our pre-clinical and human clinical trials, which could adversely affect our business, financial condition and operations.
We may be required to enter into contracting arrangements with third parties to manufacture our product candidates for large-scale, pre-clinical and/or clinical trials. We may not be able to make the transition from laboratory-scale to development-scale or from development-scale to commercial production. We may need to develop additional manufacturing resources, enter into collaborative arrangements with other parties who have established manufacturing capabilities, or have third parties manufacture our products on a contract basis. We may not have access on acceptable terms to the necessary and substantial financing that would be required to scale-up production and develop effective commercial manufacturing processes and technologies. We may not be able to enter into collaborative or contracting arrangements on acceptable terms with parties that will meet our requirements for quality, quantity and timeliness.
We expect that we will be required to design and develop new synthetic pathways for most, if not all, of the product candidates that we currently intend to develop or may develop in the future. We cannot predict the success of such efforts, the purity of the products that may be obtained or the nature of the impurities that may result from such efforts. If we are not able to obtain an acceptable purity for any product candidate or an acceptable product specification, pre-clinical and clinical trials would be delayed, which could adversely affect the priority of the development of our product candidates, our business, financial condition and results of operations. We also cannot guarantee that the active pharmaceutical ingredient will be suitable for high throughput encapsulation to produce drug products. This may adversely impact the cost of goods or feasibility of market scale manufacture.
Our product candidates and the process for administering our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any potential marketing approval.
Treatment with our product candidates may produce undesirable side effects or adverse reactions or events. If any such adverse events occur, our clinical trials could be suspended or discontinued and the FDA or comparable foreign regulatory authorities could order us to cease further development or deny approval of our product candidates for any or all targeted indications. The product-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial. If we elect or are required to delay, suspend or discontinue any clinical trial of any of our product candidates, the commercial prospects of such product candidates will be harmed and our ability to generate product revenues from any of these product candidates will be delayed or eliminated. Any of these occurrences may harm our business, financial condition and prospects significantly.
Currently we depend upon a sole manufacturer of our lead compound and on a sole manufacturer to encapsulate the compound and could incur significant costs and delays if we are unable to promptly find a replacement for either of them.
At this time, we are relying on a single manufacturer to develop Good Manufacturing Practice, processes for our lead compounds. Our lead compound, IMM-124E, is manufactured by Synlait based in New Zealand. This manufacturer enables efficient large scale manufacture of colostrum to provide drug substance for the current and prospective trials in fatty-liver and c.difficile patients. We also rely on a sole manufacturer Catalent Australia, to encapsulate all of our marketed and investigational drug products. We are actively seeking an additional and back up manufacturer but may be unsuccessful in our efforts, or may incur material additional costs and substantial delays.
The failure to establish sales, marketing and distribution capability would materially impair our ability to successfully market and sell our pharmaceutical products.
We currently havelimitedexperience in marketing, sales or distribution of pharmaceutical products. If we develop any commercially marketable pharmaceutical products and decide to perform our own sales and marketing activities, we will require additional management, will need to hire sales and marketing personnel and will require additional capital. Qualified personnel may not be available in adequate numbers or at a reasonable cost. Further, our sales staff may not achieve success in their marketing efforts. Alternatively, we may be required to enter into marketing arrangements with other parties who have established appropriate marketing, sales and distribution capabilities. We may not be able to enter into marketing arrangements with any marketing partner, or if such arrangements are established, our marketing partners may not be able to commercialize our products successfully. Other companies offering similar or substitute products may have well-established and well-funded marketing and sales operations in place that will allow them to market their products more successfully. Failure to establish sufficient marketing capabilities would materially impair our ability to successfully market and sell our pharmaceutical products.
If healthcare insurers and other organizations do not pay for our products, or impose limits on reimbursement, our future business may suffer.
The drugs we hope to develop may be rejected by the marketplace due to many factors, including cost. The continuing efforts of governments, insurance companies, health maintenance organizations and other payors of healthcare costs to contain or reduce healthcare costs may affect our future revenues and profitability and those of our potential customers, suppliers and collaborative partners, as well as the availability of capital. In Australia and certain foreign markets, the pricing or profitability of prescription pharmaceuticals is already subject to government control. We expect initiatives for similar government control at both the state and federal level to continue in the United States and elsewhere. The adoption of any such legislative or regulatory proposals could adversely affect our business and prospects.
Our ability to commercially exploit our products successfully will depend in part on the extent to which reimbursement for the cost of our products and related treatment will be available from government health administration authorities, private health coverage insurers and other organizations. Third-party payors, such as government and private health insurers, are increasingly challenging the price of medical products and services. Uncertainty exists as to the reimbursement status of newly approved health care products and in foreign markets, including the United States. If third-party coverage is not available to patients for any of the products we develop, alone or with collaborators, the market acceptance of these products may be reduced, which may adversely affect our future revenues and profitability. In addition, cost containment legislation and reductions in government insurance programs may result in lower prices for our products and could materially adversely affect our ability to operate profitably.
We may be exposed to product liability claims, which could harm our business.
The testing, marketing and sale of human health care products also entails an inherent risk of product liability. We may incur substantial liabilities or be required to limit development or commercialization of our products if we cannot successfully defend ourselves against product liability claims. We have historically obtained no fault compensation insurance for our clinical trials and intend to obtain similar coverage for future clinical trials. Such coverage may not be available in the future on acceptable terms, or at all. This may result in our inability to pursue further clinical trials or to obtain adequate protection in the event of a successful claim. We may not be able to obtain product liability insurance in the event of the commercialization of a product or such insurance may not be available on commercially reasonable terms. Even if we have adequate insurance coverage, product liability claims or recalls could result in negative publicity or force us to devote significant time, attention and financial resources to those matters.
Breaches of network or information technology security, natural disasters or terrorist attacks could have an adverse effect on our business.
Cyber-attacks or other breaches of network or information technology (IT) security, natural disasters, terrorist acts or acts of war may cause equipment failures or disrupt our research and development operations. In particular, both unsuccessful and successful cyber-attacks on companies have increased in frequency, scope and potential harm in recent years. Such an event may result in our inability, or the inability of our partners, to operate the research and development facilities, which even if the event is for a limited period of time, may result in significant expenses and/or significant damage to our experiments and trials. In addition, a failure to protect employee confidential data against breaches of network or IT security could result in damage to our reputation. Any of these occurrences could adversely affect our results of operations and financial condition.
To date, we have not had any such occurrence of cyber-attacksto our networks and IT infrastructure through cyber-attack, malware, computer viruses and other means of unauthorized access or other cyber incidents, individually or in the aggregate, however, should this occur in the future, it may result in a material impact to our operations or financial condition.
We expect to expand our drug development, regulatory and business development capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and consultants and the scope of our operations, particularly in the areas of drug development, regulatory affairs and business development. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations, and have a materially adverse effect on our business.
Preliminary positive results from the clinical trial of our leading product candidate, IMM-124E, are not necessarily predictive of the final results of the trial, and positive results from preclinical studies of our product candidates are not necessarily predictive of the results of our planned clinical trials of our product candidates.
In 2012, 10 biopsy-proven NASH patients were dosed for 30 days with IMM-124E in a Phase 1 study aimed to assess the safety of IMM-124E in NASH patients. The preliminary results from this trial are not necessarily predictive of the final results of the trial. The biological effect observed in this trial has been observed in only those 10 patients, is not statistically significant and might not be observed in any other patients treated with IMM-124E.
In addition, positive results in preclinical proof-of-concept and animal studies of our product candidates may not result in positive results in clinical trials in humans. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical trials after achieving positive results in preclinical development or early stage clinical trials, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in clinical trials, including adverse events. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain FDA or other regulatory authority approval. If we fail to produce positive results in our clinical trials of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be negatively impacted.
Our future prospects may also be dependent on our or our collaborators’ ability to successfully develop a pipeline of additional product candidates, and we and our collaborators may not be successful in efforts to use our platform technologies to identify or discover additional product candidates.
The success of our business depends primarily upon our ability to identify, develop and commercialize products based on our platform technology. We only have two product candidates currently in clinical development (IMM-124E) or ready to start clinical development trials phase (IMM-529).
Our other product candidates derived from our platform technology may not successfully complete IND-enabling studies, and our research programs may fail to identify other potential product candidates for clinical development for a number of reasons. Our and our collaborators’ research methodology may be unsuccessful in identifying potential product candidates, our potential product candidates may not demonstrate the necessary preclinical outcomes to progress to clinical studies, or our product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
If any of these events occur, we may be forced to discontinue our development efforts for a program or programs. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
We may not be able to obtain orphan drug exclusivity for some of our product candidates.
Of our current product candidates, the only one designed for treatment of an indication that would likely qualify for rare disease status is IMM-529 for the treatment of recurrent C.difficile. Regulatory authorities in some jurisdictions, including the United States and the European Union, may designate drugs or biological products for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a product intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States. The FDA may also designate a product as an orphan drug if it is intended to treat a disease or condition of more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Under the European Union orphan drug legislation, a rare disease or condition means a disease or condition which affects not more than five in ten thousand persons in the European Union at the time of the orphan drug designation application.
Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for that time period. During the marketing exclusivity period, in the European Union, the European Medicines Agency, or the EMA, is precluded from approving a similar drug with an identical therapeutic indication. The applicable period is seven years in the United States and ten years in the European Union. The European Union exclusivity period can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
Even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition, and the same drug could be approved for a different condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug, made by a competitor, for the same condition if the FDA concludes that the competitive product is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In the European Union, the EMA can approve a competitive product if the orphan drug no longer meets the criteria for orphan designation (including sufficient profitability), if the competitive product is safer, more effective or otherwise clinically superior, or if the orphan drug cannot be supplied in sufficient quantities.
We have not entered into agreements with any third-party manufacturers to support commercialization of our pharmaceutical product candidates. Additionally, no manufacturers have experience producing our product candidates at commercial levels, and any manufacturer that we work with may not achieve the necessary regulatory approvals or produce our product candidates at the quality, quantities, locations and timing needed to support commercialization.
We have not yet secured manufacturing capabilities for commercial quantities of our product candidates or established facilities in the desired locations to support commercialization of our product candidates. We intend to rely on third-party manufacturers for commercialization, and currently we have only entered into agreements with such manufacturers to support our clinical trials for IMM-124E. We may be unable to negotiate agreements with third-party manufacturers to support our commercialization activities on commercially reasonable terms.
We may encounter technical or scientific issues related to manufacturing or development that we may be unable to resolve in a timely manner or with available funds. Currently, we do not have the capacity to manufacture our product candidates on a commercial scale. In addition, our product candidates are novel, and no manufacturer currently has experience producing our product candidates on a large scale. If we are unable to engage manufacturing partners to produce our product candidates on a larger scale on reasonable terms, our commercialization efforts will be harmed.
Even if we timely develop a manufacturing process and successfully transfer it to the third-party manufacturers of our product candidates, if such third-party manufacturers are unable to produce the necessary quantities of our product candidates, or do so in compliance with cGMP or with pertinent foreign regulatory requirements, and within our planned time frame and cost parameters, the development and sales of our product candidates, if approved, may be impaired.
Our auditors have identified certain matters involving our internal controls over our financial reporting that are material weaknesses under standards established by the PCAOB.
Material weaknesses were identified in some aspects of our internal control over financial reporting for the fiscal years ended June 30, 2014, 2015, and 2016 and for the six months ended December 31, 2016 and 2015. Given these material weaknesses, management concluded that we did not maintain effective internal control over financial reporting. Once identified, we commenced the evaluation of the material weaknesses noted in our internal control over financial reporting specifically surrounding the assessment of certain significant transactions and properly performing certain reviews and monitoring controls in the preparation of the financial statements in accordance with IFRS, as promulgated by IASB. This resulted in the restatements disclosed in the financial statements. In order to address the Company’s internal control over financial reporting material weaknesses the Company has improved the training of its finance team with respect to the applicable financial reporting requirements during fiscal 2017. The cost of implementing the improved internal control process was negligible because the additional training was incorporated into the existing training programs at no additional cost.
The elements of our remediation plan can only be proven over time and we can offer no assurance that these initiatives will ultimately have the intended effects.
Any failure to maintain such internal controls could adversely impact our ability to report our financial results on a timely and accurate basis, which could result in our inability to satisfy our reporting obligations or result in material misstatements in our financial statements. If our financial statements are not accurate, investors may not have a complete understanding of our operations or may lose confidence in our reported financial information, which could result in a material adverse effect on our business or have a negative effect on the trading price of our common stock.
Risks Related to Government Regulation
If we do not obtain the necessary governmental approvals, we will be unable to commercialize our pharmaceutical products.
Our ongoing research and development activities are, and the production and marketing of our pharmaceutical product candidates derived from such activities will be, subject to regulation by numerous international regulatory authorities. Prior to marketing, any therapeutic product developed must undergo rigorous pre-clinical testing and clinical trials and, to the extent that any of our pharmaceutical products under development are marketed abroad, by the relevant international regulatory authorities. For example, in Australia, principally the Therapeutics Goods Administration, or TGA;the FDA, in the United States; the Medicines and Healthcare products Regulatory Agency, or MHRA, in the United Kingdom; the Medical Products Agency, or MPA, in Sweden; and the European Medicines Agency, or EMA. These processes can take many years and require the expenditure of substantial resources. Governmental authorities may not grant regulatory approval due to matters arising from pre-clinical animal toxicology, safety pharmacology, drug formulation and purity, clinical side effects or patient risk profiles, or medical contraindications. Failure or delay in obtaining regulatory approvals would adversely affect the development and commercialization of our pharmaceutical product candidates. We may not be able to obtain the clearances and approvals necessary for clinical testing or for manufacturing and marketing our pharmaceutical product candidates.
We will not be able to commercialize any current or future product candidates if we fail to adequately demonstrate their safety, efficacy and superiority over existing therapies.
Before obtaining regulatory approvals for the commercial sale of any of our pharmaceutical products, we must demonstrate through pre-clinical testing and clinical studies that our product candidates are safe and effective for use in humans for each target indication. Results from early clinical trials may not be predictive of results obtained in large-scale, later-stage clinical testing. Even though a potential drug product shows promising results in clinical trials, regulatory authorities may not grant the necessary approvals without sufficient safety and efficacy data.
We may not be able to undertake further clinical trials of our current and future product candidates as therapies for fatty-liver disease, C.difficileor other indications or to demonstrate the safety and efficacy or superiority of any of these product candidates over existing therapies or other therapies under development, or enter into any collaborative arrangement to commercialize our current or future product candidates on terms acceptable to us, or at all. Clinical trial results that show insufficient safety and efficacy could adversely affect our business, financial condition and results of operations.
Even if we obtain regulatory approval for a product candidate, our products may remain subject to regulatory scrutiny.
Even if we obtain regulatory approval in a jurisdiction, the regulatory authority may still impose significant restrictions on the indicated uses or marketing of our product candidates, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. For example, the holder of an approved biologics license application (BLA) is obligated to monitor and report to the FDA adverse events and any failure of a product to meet the specifications in the BLA. The holder of an approved BLA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable foreign, federal and state laws.
If we fail to comply with applicable regulatory requirements following approval of any of our product candidates, a regulatory agency may:
| · | issue a warning letter asserting that we are in violation of the law; |
| · | seek an injunction or impose civil or criminal penalties or monetary fines; |
| · | suspend or withdraw regulatory approval; |
| · | suspend any ongoing clinical trials; |
| · | refuse to permit government reimbursement of our product by government-sponsored third-party payors; |
| · | refuse to approve a pending BLA or supplements to a BLA submitted by us for other indications or new product candidates; |
| · | refuse to allow us to enter into or continue supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenues.
Healthcare reform measures and other statutory or regulatory changes could adversely affect our business.
In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could impact our business. For example, the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the “ACA”), enacted in March 2010, substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. With regard to pharmaceutical products, among other things, the ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs and make changes to the coverage requirements under the Medicare D program.
If we obtain FDA approval for any of our product candidates and begin commercializing those products in the United States, our operations may be directly or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations.
The pharmaceutical and biotechnology industries are subject to extensive regulation, and from time to time legislative bodies and governmental agencies consider changes to such regulations that could have significant impact on industry participants. For example, in light of certain highly-publicized safety issues regarding certain drugs that had received marketing approval, the U.S. Congress has considered various proposals regarding drug safety, including some which would require additional safety studies and monitoring and could make drug development more costly. Additional legislation or regulation, if any, relating to safety or other aspects of drug development may be enacted in the future, which could have an adverse effect on our business.
Our product candidates are based on our hyper-immune colostrum technology. Currently, no prescription product candidates utilizing our technology have been approved for commercial sale and our approach to the development of our technology may not result in safe, effective or marketable products.
We have concentrated our product research and development efforts on our hyper-immune colostrum technology, and our future success depends on successful clinical development of this technology. We plan to develop a pipeline of product candidates using our technology and deliver therapeutics for a number of chronic and life-threatening conditions, including fatty-liver diseases and C.difficile.
The scientific research that forms the basis of our efforts to develop product candidates is based on the pre-clinical and clinical data in conditions such as Traveler’s Diarrhea, NASH and C.difficile, and the identification, optimization and delivery ofhyper-immunecolostrum-based product candidates is relatively new. The scientific evidence to support the feasibility of successfully developing therapeutic treatments based on our is preliminary and limited. There can be no assurance that any development and technical problems we experience in the future will not cause significant delays or unanticipated costs, or that such development problems can be solved. We may be unable to reach agreement on favorable terms, or at all, with providers of vectors needed to optimize delivery of our product candidates to target disease cells and we may also experience unanticipated problems or delays in expanding our manufacturing capacity or transferring our manufacturing process to commercial partners, any of which may prevent us from completing our clinical trials or commercializing our products on a timely or profitable basis, if at all.
Only a few product candidates based on our technology have been tested in either animals or humans. We may discover that the applications of IMM-124E and IMM-529 do not possess properties required for a therapeutic benefit, such as the ability to sufficiently suppress the immune system for the period of time required to be approved as a NASH therapeutic. In addition, application of hyper-immune-based products in humans may result in safety problems. We currently have only limited long-term data, and no conclusive evidence, to suggest that we can effectively produce effective therapeutic treatments using our hyper-immunecolostrum technology.
We are early in our product development efforts and have only two product candidates in early-stage (Phase 1 ready) and mid-stage (Phase 2) clinical trials. All of our other current product candidates are still in preclinical development. We have no late-stage clinical trials (post-proof of concept) and may not be able to obtain regulatory approvals for the commercialization of some or all of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of biologics is subject to extensive regulation by the FDA and other regulatory authorities, and these regulations differ from country to country. We do not have any products on the market and are early in our development efforts. We have only one product candidate in clinical trials and all of our other product candidates are in preclinical development. All of our current and future product candidates are subject to the risks of failure typical for development of biologics. The development and approval process is expensive and can take many years to complete, and its outcome is inherently uncertain. In addition, the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results.
We have not submitted an application, or received marketing approval, for any of our product candidates and will not submit any applications for marketing approval for several years. We have limited experience in conducting and managing clinical trials necessary to obtain regulatory approvals for prescription product candidates. To receive approval, we must, among other things, demonstrate with evidence from clinical trials that the product candidate is both safe and effective for each indication for which approval is sought, and failure can occur in any stage of development. Satisfaction of the approval requirements typically takes several years and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product. We cannot predict if or when we might receive regulatory approvals for any of our product candidates currently under development.
The FDA and foreign regulatory authorities also have substantial discretion in the pharmaceutical product approval process. The numbers, types and sizes of preclinical studies and clinical trials that will be required for regulatory approval varies depending on the product candidate, the disease or condition that the product candidate is designed to address and the regulations applicable to any particular product candidate. Approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions, and there may be varying interpretations of data obtained from preclinical studies or clinical trials, any of which may cause delays or limitations in the approval or the decision not to approve an application.Regulatory agencies can delay, limit or deny approval of a product candidate for many reasons, including:
| · | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| · | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| · | the results of clinical trials may not meet the level of statistical or clinical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| · | the patients recruited for a particular clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval; |
| · | the results of clinical trials may not confirm the positive results from earlier preclinical studies or clinical trials; |
| · | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| · | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| · | the data collected from clinical trials of our product candidates may not be sufficient to the satisfaction of FDA or comparable foreign regulatory authorities to support the submission of a biologics license application, or BLA, or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere; |
| · | the FDA or comparable foreign regulatory authorities may only agree to approve a product candidate under conditions that are so restrictive that the product is not commercially viable; |
| · | regulatory agencies might not approve or might require changes to our manufacturing processes or facilities; or |
| · | regulatory agencies may change their approval policies or adopt new regulations in a manner rendering our clinical data insufficient for approval. |
Any delay in obtaining or failure to obtain required approvals could materially and adversely affect our ability to generate revenue from the particular product candidate, which likely would result in significant harm to our financial position and adversely impact the price of the ADSs and Warrants. Furthermore, any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product. These limitations may limit the size of the market for the product.
We are not permitted to market our product candidates in the United States or in other countries until we receive approval of a BLA from the FDA or marketing approval from applicable regulatory authorities outside the United States. Obtaining approval of a BLA can be a lengthy, expensive and uncertain process. If we fail to obtain FDA approval to market our product candidates, we will be unable to sell our product candidates in the United States, which will significantly impair our ability to generate any revenues. In addition, failure to comply with FDA and non-U.S. regulatory requirements may, either before or after product approval, if any, subject us to administrative or judicially imposed sanctions, including:
| · | restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials; |
| · | restrictions on the products, manufacturers or manufacturing process; |
| · | civil and criminal penalties; |
| · | suspension or withdrawal of regulatory approvals; |
| · | product seizures, detentions or import bans; |
| · | voluntary or mandatory product recalls and publicity requirements; |
| · | total or partial suspension of production; |
| · | imposition of restrictions on operations, including costly new manufacturing requirements; and |
| · | refusal to approve pending BLAs or supplements to approved BLAs. |
Even if we do receive regulatory approval to market a product candidate, any such approval may be subject to limitations on the indicated uses for which we may market the product. It is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain the appropriate regulatory approvals necessary for us or our collaborators to commence product sales. Any delay in obtaining, or an inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability.
We could be adversely affected by violations of the U.S. Foreign Corrupt Practices Act.
Our business operations may be subject to anti-corruption laws and regulations, including restrictions imposed by the U.S. Foreign Corrupt Practices Actthe FCPA.The FCPA and similar anti-corruption laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. We cannot provide assurance that our internal controls and procedures will always protect us from criminal acts committed by our employees or third parties with whom we work. If we are found to be liable for violations of the FCPA or similar anti-corruption laws in international jurisdictions, either due to our own acts or out of inadvertence, or due to the acts or inadvertence of others, we could suffer from criminal or civil penalties which could have a material and adverse effect on our results of operations, financial condition and cash flows.
Risks Related to Intellectual Property
Our success depends upon our ability to protect our intellectual property and our proprietary technology, to operate without infringing the proprietary rights of third parties and to obtain marketing exclusivity for our products and technologies.
Any future success will depend in large part on whether we can:
| · | obtain and maintain patents to protect our own products and technologies; |
| · | obtain orphan designation for our products and technologies; |
| · | obtain licenses to the patented technologies of third parties; |
| · | operate without infringing on the proprietary rights of third parties; and |
| · | protect our trade secrets, know-how and other confidential information. |
Patent matters in biotechnology are highly uncertain and involve complex legal and factual questions. Accordingly, the availability and breadth of claims allowed in biotechnology and pharmaceutical patents cannot be predicted. Any of the pending or future patent applications filed by us or on our behalf may not be approved, we may not develop additional proprietary products or processes that are patentable, or we may not be able to license any other patentable products or processes.
Our products may be eligible for orphan designation for particular therapeutic indications that are of relatively low prevalence and for which there is no effective treatment. Orphan drug designation affords market exclusivity post marketing authorization for a product for a specified therapeutic utility. The period of orphan protection is dependent on jurisdiction, for example, seven years in the United States and ten years in Europe. The opportunity to gain orphan drug designation depends on a variety of requirements specific to each marketing jurisdiction and can include; a showing of improved benefit relative to marketed products, that the mechanism of action of the product would provide plausible benefit and the nature of the unmet medical need within a therapeutic indication. It is uncertain if our products will be able to obtain orphan drug designation for the appropriate indications and in the jurisdictions sought.
Our commercial success will also depend, in part, on our ability to avoid infringement of patents issued to others. If a court determines that we were infringing any third party patents, we could be required to pay damages, alter our products or processes, obtain licenses or cease certain activities. Licenses required under patents held by third parties may not be made available on terms acceptable to us or at all. To the extent that we are unable to obtain such licenses, we could be foreclosed from the development, export, manufacture or commercialization of the product requiring such license or encounter delays in product introductions while we attempt to design around such patents, and any of these circumstances could adversely affect our business, financial condition and results of operations.
We may have to resort to litigation to enforce any patents issued or licensed to us or to determine the scope and validity of third party proprietary rights. We may have to defend the validity of our patents in order to protect or enforce our rights against a third party. Third parties may in the future assert against us infringement claims or claims that we have infringed a patent, copyright, trademark or other proprietary right belonging to them. Any infringement claim, even if not meritorious, could result in the expenditure of significant financial and managerial resources and could negatively affect our profitability. While defending our patents, the scope of the claim may be reduced in breadth and inventorship of the claimed subject matter, and proprietary interests in the claimed subject matter may be altered or reduced. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Any such litigation orproceedings, regardlessof outcome, could be expensive and time consuming, and adverse determinations in any such litigation or proceedings could prevent us from developing, manufacturing or commercializing our products and could adversely affect our business, financial condition and results of operations.
The patents for our product candidates have varying expiration dates and, if these patents expire, we may be subject to increased competition and we may not be able to recover our development costs or market any of our approved products profitably. In some of the larger potential market territories, such as the United States and Europe, patent term extension or restoration may be available to compensate for time taken during aspects of the product’s development and regulatory review or by procedural delays before the relevant patent office. However, such an extension may not be granted, or if granted, the applicable time period or the scope of patent protection afforded during any extension period may not be sufficient. In addition, even though some regulatory authorities may provide some other exclusivity for a product under their own laws and regulations, we may not be able to qualify the product or obtain the exclusive time period. If we are unable to obtain patent term extension/restoration or some other exclusivity, we could be subject to increased competition and our opportunity to establish or maintain product revenue could be substantially reduced or eliminated. Furthermore, we may not have sufficient time to recover our development costs prior to the expiration of our U.S. and non-U.S. patents.
We may face difficulties in certain jurisdictions in protecting our intellectual property rights, which may diminish the value of our intellectual property rights in those jurisdictions.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the United States and the European Union, and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we or our collaboration partners encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired and our business, financial condition and results of operations may be adversely affected.
Intellectual property rights do not address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| · | Others may be able to make products that are similar to ours but that are not covered by the claims of the patents that we own. |
| · | Others may independently develop similar or alternative technologies or otherwise circumvent any of our technologies without infringing our intellectual property rights. |
| · | We or any of our collaboration partners might not have been the first to conceive and reduce to practice the inventions covered by the patents or patent applications that we own, license or will own or license. |
| · | We or any of our collaboration partners might not have been the first to file patent applications covering certain of the patents or patent applications that we or they own or have obtained a license, or will own or will have obtained a license. |
| · | It is possible that our pending patent applications will not lead to issued patents. |
| · | Issued patents that we own may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges. |
| · | Our competitors might conduct research and development activities in countries where we do not have patent rights, or in countries where research and development safe harbor laws exist, and then use the information learned from such activities to develop competitive products for sale in our major commercial markets. |
| · | The patents of third parties or pending or future applications of third parties, if issued, may have an adverse effect on our business. |
| · | Compulsory licensing provisions of certain governments to patented technologies that are deemed necessary for the government to access. |
Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products or product candidates.
As is the case with other pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents involves both technological complexity and legal complexity and is costly, time-consuming and inherently uncertain. In addition, the America Invents Act was recently enacted in the United States, resulting in significant changes to the U.S. patent system. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office,or USPTO, thelaws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. Similarly, the complexity and uncertainty of European patent laws has also increased in recent years. In addition, the European patent system is relatively stringent with regard to the type of amendments that are allowed during prosecution. These changes could limit our ability to obtain new patents in the future that may be important for our business.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and protect other proprietary information.
We consider proprietary trade secrets and/or confidential know-how and unpatented know-how to be important to our business. We may rely on trade secrets and/or confidential know-how to protect our technology, especially where patent protection is believed by us to be of limited value. However, trade secrets and/or confidential know-how can be difficult to maintain as confidential.
To protect this type of information against disclosure or appropriation by competitors, our policy is to require our employees, consultants, contractors and advisors to enter into confidentiality agreements with us. However, current or former employees, consultants, contractors and advisers may unintentionally or willfully disclose our confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Enforcing a claim that a third-party obtained illegally and is using trade secrets and/or confidential know-how is expensive, time consuming and unpredictable. The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction.
Failure to obtain or maintain trade secrets and/or confidential know-how trade protection could adversely affect our competitive position. Moreover, our competitors may independently develop substantially equivalent proprietary information and may even apply for patent protection in respect of the same. If successful in obtaining such patent protection, our competitors could limit our use of our trade secrets and/or confidential know-how.
Risks Related to the ADSs, Warrants and this Offering
The market price and trading volume of the ADSs and Warrants may be volatile and may be affected by economic conditions beyond our control.
The market price of the ADSs and Warrants may be highly volatile and subject to wide fluctuations. In addition, the trading volume of the ADSs and Warrants may fluctuate and cause significant price variations to occur. If the market price of the ADSs or Warrants declines significantly, you may be unable to resell your ADSs or Warrants at or above the purchase price, if at all. We cannot assure you that the market price of the ADSs and Warrants will not fluctuate or significantly decline in the future.
Some specific factors that could negatively affect the price of the ADSs and Warrants or result in fluctuations in their price and trading volume include:
| · | actual or expected fluctuations in our operating results; |
| · | changes in market valuations of similar companies; |
| · | changes in our key personnel; |
| · | changes in financial estimates or recommendations by securities analysts; |
| · | trading prices of our ordinary shares on the ASX; |
| · | changes in trading volume of ADSs or WArrants on The NASDAQ Capital Market, or NASDAQ, and of our ordinary shares on the ASX; |
| · | sales of the ADSs or ordinary shares by us, our executive officers or our shareholders in the future; and |
| · | conditions in the financial markets or changes in general economic conditions. |
An active trading market for the ADSs and Warrants may not develop or be liquid enough for you to sell your ADSs and Warrants quickly or at market price.
Prior to this offering, there has not been any public market in the United States for the ADSs or Warrants. If an active public market in the United States for the ADSs and Warrants does not develop after this offering, the market price and liquidity of the ADSs and Warrants may be adversely affected. While we have applied for the listing of the ADSs and Warrants on NASDAQ, a liquid public market in the United States for the ADSs and Warrants may not develop or be sustained after this offering. The public offering price for the ADSs and Warrants will be determined by negotiation among us and the underwriters, and the price at which the ADSs and Warrants are traded after this offering may decline below the initial public offering price, which means you may experience a decrease in the value of your ADSs and Warrants regardless of our operating performance or prospects. In the past, following periods of volatility in the market price of a company’s securities, shareholders often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of senior management and, if adversely determined, could have cause us significant financial harm.
Following this offering, the market value of the Warrants is uncertain and there can be no assurance that the market value of the Warrants will equal or exceed their public offering price.
The Warrants offered in this offering do not confer any rights of ordinary share ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of the ADS at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the Warrants may exercise their right to acquire ADS and pay an exercise price of _____ this offering, prior to five years from the date of issuance, after which date any unexercised Warrants will expire and have no further value. Moreover, following this offering, the market value of the Warrants is uncertain and there can be no assurance that the market value of the Warrants will equal or exceed their public offering price. There can be no assurance that the market price of the ADS will ever equal or exceed the exercise price of the Warrants, and consequently, whether it will ever be profitable for holders of the Warrants to exercise the Warrants.
Investors purchasing the ADSs will suffer immediate and substantial dilution.
The public offering price for the ADSs will be substantially higher than the net tangible book value per ADS of the underlying ordinary shares immediately after this offering. If you purchase ADSs in this offering, you will incur substantial and immediate dilution in the net tangible book value of your investment. Net tangible book value per ADS represents the amount of total tangible assets less total liabilities, divided by the number of ordinary shares then outstanding, multiplied by forty, the number of ordinary shares underlying each ADS. To the extent that options or any convertible securities that are currently outstanding are exercised or converted, there will be further dilution to your investment. We may also issue additional ordinary shares, ADSs, performance rights, options and other securities in the future that may result in further dilution of your ADSs. See “Dilution” for a calculation of the extent to which your investment will be diluted.
The dual listing of our ordinary shares and the ADSs following this offering may adversely affect the liquidity and value of the ADSs.
Following this offering and after the ADSs are listed on NASDAQ, our ordinary shares will continue to be listed on the ASX. We cannot predict the effect of this dual listing on the value of our ordinary shares and ADSs. However, the dual listing of our ordinary shares and ADSs may dilute the liquidity of these securities in one or both markets and may impair the development of an active trading market for the ADSs in the United States. The trading price of the ADSs could also be adversely affected by trading in our ordinary shares on the ASX. The investor in our convertible notes issued in February 2016 has the right to require that we delist from NASDAQ at any time when our primary listing is not on the ASX.
Future sales of our ordinary shares or ADSs, or the perception that such sales may occur, could depress the trading price of our ordinary shares and ADSs.
After the completion of this offering, we expect to have ADSs outstanding and ordinary shares outstanding, including the shares underlying the ADSs we are selling in this offering, which may be resold in the public market immediately after this offering. We and all of our directors and executive officers have signed lock-up agreements for a period of (i) twelve months after the date of this prospectus in the case of our directors and officers and (ii)180 days after the date of this prospectus in the case of the Company without the prior written consent of the representative of the underwriters subject to specified exceptions. See “Underwriting.”
The underwriters may, in their sole discretion and without notice, release all or any portion of the ordinary shares or ADSs subject to lock-up agreements. As restrictions on resale end, the market price of our ADSs and ordinary shares could drop significantly if the holders of these ADSs or ordinary shares sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of our ordinary shares, ADSs or other securities.
As a foreign private issuer, we are permitted and we expect to follow certain home country corporate governance practices in lieu of certain NASDAQ requirements applicable to domestic issuers. This may afford less protection to holders of the ADSs and Warrants.
As a foreign private issuer whose ADSs and Warrants will be listed on NASDAQ, we will be permitted to follow certain home country corporate governance practices in lieu of certain NASDAQ requirements. For example, we may follow home country practice with regard to the composition of the board of directors and quorum requirements applicable to shareholder meetings. A foreign private issuer must disclose in its annual reports filed with the SEC the requirements with which it does not comply followed by a description of its applicable home country practice. The Australian home country practices described above may afford less protection to holders of the ADSs and Warrants than that provided under NASDAQ rules. See “Description of Share Capital—Exemptions from Certain NASDAQ Corporate Governance Rules.”
As a foreign private issuer, we are permitted to file less information with the SEC than a company incorporated in the United States. Accordingly, there may be less publicly available information concerning us than there is for companies incorporated in the United States.
As a foreign private issuer, we are exempt from certain rules under the Exchange Act, that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as a company that files as a U.S. company whose securities are registered under the Exchange Act, nor are we required to comply with the SEC’s Regulation FD, which restricts the selective disclosure of material non-public information. Accordingly, there may be less information publicly available concerning us than there is for a company that files as a domestic issuer.
We are an emerging growth company as defined in the JOBS Act and the reduced disclosure requirements applicable to emerging growth companies may make the ADSs less attractive to investors and, as a result, adversely affect the price of the ADSs and result in a less active trading market for the ADSs and Warrants.
We are an emerging growth company as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. For example, we have elected to rely on an exemption from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act relating to internal control over financial reporting, and we will not provide such an attestation from our auditors for so long as we qualify as an emerging growth company.
We may avail ourselves of these disclosure exemptions until we are no longer an emerging growth company. We cannot predict whether investors will find the ADSs less attractive because of our reliance on some or all of these exemptions. If investors find the ADSs less attractive, it may cause the trading price of the ADSs and Warrants to decline and there may be a less active trading market for the ADSs.
We will cease to be an emerging growth company upon the earliest of:
| · | the end of the fiscal year in which the fifth anniversary of completion of this offering occurs; |
| · | the end of the first fiscal year in which the market value of our ordinary shares held by non-affiliates exceeds $700 million as of the end of the second quarter of such fiscal year; |
| · | the end of the first fiscal year in which we have total annual gross revenues of at least $1 billion; and |
| · | the date on which we have issued more than $1 billion in non-convertible debt securities in any rolling three-year period. |
If we fail to establish and maintain proper internal financial reporting controls, our ability to produce accurateconsolidatedfinancial statements or comply with applicable regulations could be impaired.
Section 404(a) of the Sarbanes-Oxley Act requires that, beginning with our second annual report after the completion of this offering, our management assess and report annually on the effectiveness of our internal controls over financial reporting and identify any material weaknesses in our internal controls over financial reporting. Although Section 404(b) of the Sarbanes-Oxley Act requires our independent registered public accounting firm to issue an annual report that addresses the effectiveness of our internal controls over financial reporting, we have opted to rely on the exemptions provided in the JOBS Act, and consequently will not be required to comply with SEC rules that implement Section 404(b) of the Sarbanes-Oxley Act until such time as we are no longer an emerging growth company.
Our first Section 404(a) assessment will take place beginning with our second annual report after the completion of this offering. The presence of material weaknesses could result in financial statement errors which, in turn, could lead to errors in our financial reports and/or delays in our financial reporting, which could require us to restate our operating results. We might not identify one or more material weaknesses in our internal controls in connection with evaluating our compliance with Section 404(a) of the Sarbanes-Oxley Act. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal controls over financial reporting, we will need to expend significant resources and provide significant management oversight. Implementing any appropriate changes to our internal controls may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective in maintaining the adequacy of our internal control.
If we are unable to conclude that we have effective internal controls over financial reporting, investors may lose confidence in our operating results, the price of the ADSs and Warrants could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, the ADSs and Warrants may not be able to remain listed on NASDAQ.
ADS and Warrant holders may be subject to additional risks related to holding ADSs and Warrants rather than ordinary shares.
ADS and Warrant holders do not hold ordinary shares directly and, as such, are subject to, among others, the following additional risks:
| · | As an ADS or Warrant holder, we will not treat you as one of our shareholders and you will not be able to exercise shareholder rights, except through the ADR depositary as permitted by the deposit agreement. |
| · | distributions on the ordinary shares represented by your ADSs will be paid to the ADR depositary, and before the ADR depositary makes a distribution to you on behalf of your ADSs, any withholding taxes that must be paid will be deducted. Additionally, if the exchange rate fluctuates during a time when the ADR depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution. |
| · | We and the ADR depositary may amend or terminate the deposit agreement without the ADS holders’ consent in a manner that could prejudice ADS holders. |
You must act through the ADR depositary to exercise your voting rights and, as a result, you may be unable to exercise your voting rights on a timely basis.
As a holder of ADSs (and not the ordinary shares underlying your ADSs), we will not treat you as one of our shareholders, and you will not be able to exercise shareholder rights. The ADR depositary will be the holder of the ordinary shares underlying your ADSs, and ADS holders will be able to exercise voting rights with respect to the ordinary shares represented by the ADSs only in accordance with the deposit agreement relating to the ADSs. There are practical limitations on the ability of ADS holders to exercise their voting rights due to the additional procedural steps involved in communicating with these holders. For example, holders of our ordinary shares will receive notice of shareholders’ meetings by mail and will be able to exercise their voting rights by either attending the shareholders meeting in person or voting by proxy. ADS holders, by comparison, will not receive notice directly from us. Instead, in accordance with the deposit agreement, we will provide notice to the ADR depositary of any such shareholders meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date. If we so instruct, the ADR depositary will mail to holders of ADSs the notice of the meeting and a statement as to the manner in which voting instructions may be given by holders as soon as practicable after receiving notice from us of any such meeting. To exercise their voting rights, ADS holders must then instruct the ADR depositary as to voting the ordinary shares represented by their ADSs. Due to these procedural steps involving the ADR depositary, the process for exercising voting rights may take longer for ADS holders than for holders of ordinary shares. The ordinary shares represented by ADSs for which the ADR depositary fails to receive timely voting instructions will not be voted.
If we are classified as a “passive foreign investment company,” then our U.S. shareholders could suffer adverse tax consequences as a result.
Generally, if, for any taxable year, at least 75% of our gross income is passive income (including our pro rata share of the gross income of our 25% or more owned corporate subsidiaries) or at least 50% of the average quarterly value of our gross assets (including our pro rata share of the gross assets of our 25% or more owned corporate subsidiaries) is attributable to assets that produce passive income or are held for the production of passive income, including cash, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest, and gains from the sale or exchange of investment property and rents and royalties other than rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. If we are characterized as a PFIC, a U.S. holder of our ordinary shares or ADSs may suffer adverse tax consequences, including having gains recognized on the sale of our ordinary shares or ADSs treated as ordinary income, rather than capital gain, the loss of the preferential rate applicable to dividends received on our ordinary shares or ADSs by individuals who are U.S. holders, and having interest charges added to their tax on distributions from us and on gains from the sale of our ordinary shares or ADSs. See “Taxation—U.S. Federal Income Tax Considerations—Passive Foreign Investment Company.”
Since cash is viewed by U.S. tax authorities as a passive asset, our status as a PFIC may also depend, in part, on how quickly we utilize the cash proceeds from this offering in our business. Since PFIC status depends on the composition of our income and the composition and value of our assets, which may be determined in large part by reference to the market value of our ordinary shares or ADSs, which may be volatile, there can be no assurance that we will not be a PFIC for any taxable year. While we expect that we will not be a PFIC for our taxable year ending June 30, 2017, since the PFIC tests are applied only at the end of a taxable year no assurance of our PFIC status can be provided for such taxable year or future taxable years. Prospective U.S. investors should discuss the issue of our possible status as a PFIC with their tax advisors.
Currency fluctuations may adversely affect the price of our ordinary shares, ADSs and Warrants.
Our ordinary shares are quoted in Australian dollars on the ASX and the ADSs and Warrants will be quoted in U.S. dollars on NASDAQ. Movements in the Australian dollar/U.S. dollar exchange rate may adversely affect the U.S. dollar price of the ADSs and Warrants. In the past year the Australian dollar has generally weakened against the U.S. dollar. However, this trend may not continue and may be reversed. If the Australian dollar weakens against the U.S. dollar, the U.S. dollar price of the ADSs and Warrants could decline, even if the price of our ordinary shares in Australian dollars increases or remains unchanged.
We have never declared or paid dividends on our ordinary shares and we do not anticipate paying dividends in the foreseeable future.
We have never declared or paid cash dividends on our ordinary shares. For the foreseeable future, we currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to compliance with applicable laws and covenants under current or future credit facilities, which may restrict or limit our ability to pay dividends, and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. We do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. As a result, a return on your investment will only occur if our ADS or Warrant price appreciates.
You may not receive distributions on our ordinary shares represented by the ADSs or any value for such distribution if it is illegal or impractical to make them available to holders of ADSs.
While we do not anticipate paying any dividends on our ordinary shares in the foreseeable future, if such a dividend is declared, the depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of our ordinary shares your ADSs represent. However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to take any other action to permit the distribution of the ADSs, ordinary shares, rights or anything else to holders of the ADSs. This means that you may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical to make them available to you. These restrictions may have a material adverse effect on the value of your ADSs.
Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares, ADSs or Warrants.
We are incorporated in Australia and are subject to the takeover laws of Australia. Among other things, we are subject to the Australian Corporations Act 2001, or the Corporations Act. Subject to a range of exceptions, the Corporations Act prohibits the acquisition of a direct or indirect interest in our issued voting shares if the acquisition of that interest will lead to a person’s voting power in us increasing to more than 20%, or increasing from a starting point that is above 20% and below 90%. Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares. This may have the ancillary effect of entrenching our board of directors and may deprive or limit our shareholders’, ADS holders’ or Warrant holders’ opportunity to sell their ordinary shares, ADSs or Warrants and may further restrict the ability of our shareholders and ADS and Warrant holders to obtain a premium from such transactions. See “Description of Share Capital—Change of Control.”
Our Constitution and Australian laws and regulations applicable to us may adversely affect our ability to take actions that could be beneficial to our shareholders.
As an Australian company, we are subject to different corporate requirements than a corporation organized under the laws of the states of the United States. Our Constitution, as well as the Australian Corporations Act, set forth various rights and obligations that are unique to us as an Australian company. These requirements may operate differently than those of many U.S. companies. You should carefully review the summary of these matters set forth under the section entitled, “Description of Share Capital” as well as our Constitution, which is included as an exhibit to this registration statement to which this prospectus forms a part, prior to investing in the ADSs.
You will have limited ability to bring an action against us or against our directors and officers, or to enforce a judgment against us or them, because we are incorporated in Australia and certain of our directors and officers reside outside the United States.
We are incorporated in Australia, certain of our directors and officers reside outside the United States and substantially all of the assets of those persons are located outside the United States. As a result, it may be impracticable or at least more expensive for you to bring an action against us or against these individuals in Australia in the event that you believe that your rights have been infringed under the applicable securities laws or otherwise.
You may not be able to participate in rights offerings and may experience dilution of your holdings as a result.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we may not, and under the Deposit Agreement for the ADSs, the depositary will not, offer those rights to ADS holders unless both the rights and the underlying securities to be distributed to ADS holders are registered under the Securities Act, or the distribution of them to ADS holders is exempted from registration under the Securities Act with respect to all holders of ADSs. We are under no obligation to file a registration statement with respect to any such rights or underlying securities or to endeavor to cause such a registration statement to be declared effective. In addition, we may not be able to rely on an exemption from registration under the Securities Act to distribute such rights and securities. Accordingly, holders of the ADSs may be unable to participate in our rights offerings and may experience dilution in their holdings as a result.
You may be subject to limitations on transfer of the ADSs.
The ADSs are only transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deem it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the Deposit Agreement, or for any other reason.
Australian companies may not be able to initiate shareholder derivative actions, thereby depriving shareholders of the ability to protect their interests.
Australian companies may not have standing to initiate a shareholder derivative action in a federal court of the United States. The circumstances in which any such action may be brought, and the procedures and defenses that may be available in respect to any such action, may result in the rights of shareholders of an Australian company being more limited than those of shareholders of a company organized in the United States. Accordingly, shareholders may have fewer alternatives available to them if they believe that corporate wrongdoing has occurred. Australian courts are also unlikely to recognize or enforce against us judgments of courts in the United States based on certain liability provisions of U.S. securities law and to impose liabilities against us, in original actions brought in Australia, based on certain liability provisions of U.S. securities laws that are penal in nature. There is no statutory recognition in Australia of judgments obtained in the United States, although the courts of Australia may recognize and enforce the non-penal judgment of a foreign court of competent jurisdiction without retrial on the merits, upon being satisfied about all the relevant circumstances in which that judgment was obtained.
Anti-takeover provisions in our Constitution and our right to issue preference shares could make a third-party acquisition of us difficult.
Some provisions of our Constitution may discourage, delay or prevent a change in control of our company or management that shareholders may consider favorable, including provisions that only require one-third of our board of directors to be elected annually and authorize our board of directors to issue an unlimited number of shares of capital stock and preference shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preference shares by amending the Constitution.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. All statements, other than statements of historical fact included in this prospectus, regarding our strategy, future operations, financial position, projected costs, prospects, plans and objectives of management are forward looking statements. When used in this prospectus, the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential,” “project,” or the negative of these terms, and similar expressions are intended to identify forward looking statements, although not all forward looking statements contain such identifying words. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a combination of facts and important factors currently known by us and our expectations of the future, about which we cannot be certain.
Forward-looking statements may include statements about:
| · | our plans to develop and potentially commercialize our product candidates; |
| · | the timing of the initiation and completion of preclinical studies and clinical trials; |
| · | the timing of patient enrollment and dosing in clinical trials; |
| · | the timing of the availability of data from clinical trials; |
| · | the timing of expected regulatory filings; |
| · | expectations about the plans of licensees of our technology; |
| · | potential future out-licenses and collaborations; |
| · | our expectations regarding expenses, ongoing losses, future revenue, capital needs and needs for additional financing; |
| · | our use of proceeds from this offering; |
| · | the length of time over which we expect our cash and cash equivalents and the proceeds from this offering to be sufficient; and |
| · | our intellectual property position and the duration of our patent portfolio. |
All forward-looking statements speak only as of the date of this prospectus. You should not place undue reliance on these forward-looking statements. Although we believe that our plans, objectives, expectations and intentions reflected in or suggested by the forward-looking statements we make in this prospectus are reasonable, we can give no assurance that these plans, objectives, expectations or intentions will be achieved. We disclose important factors that could cause our actual results to differ materially from our expectations under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this prospectus.
The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events.
USE OF PROCEEDS
We estimate that the net proceeds from the sale of shares of our ordinary shares in the form of ADSs in this offering will be approximately $ million, or $ million if the underwriters exercise in full their option to purchase additional ADSs, assuming an initial public offering price of $ per ADS, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We estimate that the net proceeds from the sale of the Warrants in this offering will be approximately $ million assuming an offering price of $ , after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Assuming no exercise of Warrants, a $1.00 increase or decrease in the assumed initial public offering price of $ per ADS, the midpoint of the price range set forth on the cover page of this prospectus, would increase or decrease our net proceeds from this offering by approximately $ million, assuming that the number of ADSs offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions. An increase or decrease of 1,000,000 ADSs in the number of ADSs offered by us, as set forth on the cover page of this prospectus, would increase or decrease our net proceeds from this offering by approximately $ million, assuming no change in the assumed initial public offering price per ADS and after deducting the estimated underwriting discounts and commissions.
We currently estimate that we will use the net proceeds from this offering, together with our existing cash, cash equivalents and investments, as follows:
| · | approximately $ million to advance the clinical development of IMM-124E for the treatment of fatty-liver diseases, which we expect will be sufficient to complete our Phase 2 clinical programs in NASH, ASH and Pediatric NASH; |
| · | approximately $ million to advance development of IMM-529 and complete our planned Phase 1/ 2 in the prevention of CDI recurrence in patients suffering from recurrent CDI; |
| · | approximately $ million to support other programs including our colitis pre-clinical program and our collaboration with the US Army and US Navy; and |
| · | the remainder, to fund manufacturing costs of clinical supplies and Travelan, marketing initiatives for Travelan in the United States and Australia, current and feature research and development activities, for working capital and other general corporate purposes. |
This expected use of the net proceeds from this offering represents our intentions based upon our current plans and business conditions. We may also use a portion of the net proceeds to in-license, acquire, or invest in additional businesses, technologies, products or assets, although currently we have no specific agreements, commitments or understandings in this regard. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the closing of this offering or the amounts that we will actually spend on the uses set forth above. Predicting the cost necessary to develop product candidates can be difficult and we anticipate that we will need additional funds to complete the development of IMM-124E, IMM-529 and any other product candidates we identify. The amounts and timing of our actual expenditures and the extent of clinical development may vary significantly from our expectations depending upon numerous factors, including the progress of our research and development efforts, progress of our clinical trial, our operating costs and factors described under “Risk Factors” in this prospectus. .. Accordingly, we will retain broad discretion over the allocation of the net proceeds from this offering and we reserve the right to change the allocation of the net proceeds described above.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds from this offering in investment-grade, interest-bearing instruments and U.S. government securities or certificates of deposit.
PRICE RANGE OF ORDINARY SHARES
The following table presents, for the periods indicated, the high and low market prices for our ordinary shares reported on the ASX, under the symbol IMC. All prices are in Australian dollars.
| | | High | | | Low | |
| | | AUD$ | | | AUD$ | |
| Annual: | | | | | | | | |
| Fiscal year ended June 30, | | | | | | | | |
| 2016 | | $ | 0.58 | | | $ | 0.21 | |
| 2015 | | | 0.32 | | | | 0.15 | |
| 2014 | | | 0.80 | | | | 0.12 | |
| 2013 | | | 0.88 | | | | 0.08 | |
| 2012 | | | 3.00 | | | | 0.60 | |
| | | | | | | | | |
| Quarterly: | | | | | | | | |
| Fiscal year ending June 30, 2016 | | | | | | | | |
| Fourth quarter | | | 0.40 | | | | 0.24 | |
| Third quarter | | | 0.52 | | | | 0.28 | |
| Second quarter | | | 0.57 | | | | 0.38 | |
| First quarter | | | 0.51 | | | | 0.22 | |
| Fiscal year ending June 30, 2015 | | | | | | | | |
| Fourth quarter | | | 0.30 | | | | 0.18 | |
| Third quarter | | | 0.24 | | | | 0.15 | |
| Second quarter | | | 0.32 | | | | 0.18 | |
| First quarter | | | 0.32 | | | | 0.20 | |
| Fiscal year ended June 30, 2014 | | | | | | | | |
| Fourth quarter | | | 0.28 | | | | 0.16 | |
| Third quarter | | | 0.52 | | | | 0.20 | |
| Second quarter | | | 0.80 | | | | 0.16 | |
| First quarter | | | 0.32 | | | | 0.12 | |
| | | | | | | | | |
| Most Recent Six Months: | | | | | | | | |
| March 2017 | | | 0.40 | | | | 0.27 | |
| February 2017 | | | 0.32 | | | | 0.29 | |
| January 2017 | | | 0.31 | | | | 0.27 | |
| December 2016 | | | 0.32 | | | | 0.27 | |
| November 2016 | | | 0.34 | | | | 0.29 | |
| October 2016 | | | 0.37 | | | | 0.25 | |
On April 24, 2017, the closing price of our ordinary shares as traded on the ASX was AUD$0.51 per ordinary share ($0.39 per share based on the foreign exchange rate of AUD$1.00 to $0.7559 as published by the Reserve Bank of Australia as of such date).
As of April 24, 2017, the Company had 105,641,417 ordinary shares issued and 103,641,417 ordinary shares outstanding held by a total of 1,386 shareholders and 33,177,523 options over ordinary shares outstanding. Of the total shares on issue, 256,011 of these issued ordinary shares were held in the U.S. by 6 holders on this date.
A large number of our ordinary shares are held in nominee companies so we cannot be certain of the identity of those beneficial owners.
DIVIDEND POLICY
We have not declared or paid any dividends on our ordinary shares, and we do not anticipate paying any dividends in the foreseeable future. Our board of directors presently intends to reinvest all earnings in the continued development and operation of our business.
Payment of dividends in the future, if any, will be at the discretion of our board of directors. If our board of directors elects to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial conditions, contractual restrictions and other factors that our board of directors may deem relevant.
EXCHANGE RATE INFORMATION
The Australian dollar is convertible into U.S. dollars at freely floating rates. There are no legal restrictions on the flow of Australian dollars between Australia and the United States. Any remittances of dividends or other payments by us to persons in the United States are not and will not be subject to any exchange controls.
Our consolidated financial statements are prepared and presented in Australian dollars.
The table below sets forth for the periods identified the number of U.S. dollars per Australian dollar as published by the Reserve Bank of Australia. We make no representation that any Australian dollar or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or Australian dollars, as the case may be, at any particular rate, the rates stated below, or at all.
| | | At Period
End
AUD$ | | | Average
Rate
AUD$ | | | High
AUD$ | | | Low
AUD$ | |
| Fiscal year ended June 30, | | | | | | | | | | | | | | | | |
| 2016 | | $ | 0.7426 | | | $ | 0.7265 | (1) | | $ | 0.7812 | | | $ | 0.6867 | |
| 2015 | | $ | 0.7680 | | | $ | 0.8288 | (1) | | $ | 0.9458 | | | $ | 0.7590 | |
| 2014 | | $ | 0.9420 | | | $ | 0.9148 | (1) | | $ | 0.9672 | | | $ | 0.8716 | |
| 2013 | | $ | 0.9275 | | | $ | 1.0239 | (1) | | $ | 1.0593 | | | $ | 0.9202 | |
| 2012 | | $ | 1.0191 | | | $ | 1.0362 | (1) | | $ | 1.1055 | | | $ | 0.9500 | |
| 2011 | | $ | 1.0739 | | | $ | 0.9990 | (1) | | $ | 1.0939 | | | $ | 0.8366 | |
| Month ended: | | | | | | | | | | | | | | | | |
| March, 2017 | | $ | 0.7644 | | | $ | 0.7624 | | | $ | 0.7724 | | | $ | 0.7514 | |
| February, 2017 | | $ | 0.7688 | | | $ | 0.7668 | | | $ | 0.7717 | | | $ | 0.7566 | |
| January, 2017 | | $ | 0.7567 | | | $ | 0.7453 | | | $ | 0.7576 | | | $ | 0.7234 | |
| December, 2016 | | $ | 0.7236 | | | $ | 0.7361 | | | $ | 0.7497 | | | $ | 0.7202 | |
| November, 2016 | | $ | 0.7474 | | | $ | 0.7536 | | | $ | 0.7700 | | | $ | 0.7324 | |
| October, 2016 | | $ | 0.7613 | | | $ | 0.7616 | | | $ | 0.7683 | | | $ | 0.7537 | |
| September, 2016 | | $ | 0.7630 | | | $ | 0.7595 | | | $ | 0.7698 | | | $ | 0.7469 | |
| August, 2016 | | $ | 0.7514 | | | $ | 0.7630 | | | $ | 0.7711 | | | $ | 0.7514 | |
| July, 2016 | | $ | 0.7522 | | | $ | 0.7526 | | | $ | 0.7626 | | | $ | 0.7436 | |
| June, 2016 | | $ | 0.7426 | | | $ | 0.7396 | | | $ | 0.7533 | | | $ | 0.7239 | |
| (1) | Determined by averaging the published rate on the last day of each full month during the fiscal year. |
CAPITALIZATION
The following table sets forth our cash and cash equivalents and our capitalization as of December 31, 2016 presented both in Australian dollars and U.S. dollars:
| • | on an as adjusted basis to give effect to the sale of ADSs in this offering and the receipt of the net proceeds at an assumed price of $ per ADS, which is the midpoint of the price range set forth on the cover of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, and assuming no exercise of the underwriters’ over-allotment option and no other change to the number of ADSs offered as set forth on the cover page of this prospectus. |
You should read this table together with our consolidated financial statements and the related notes, which we include elsewhere in this prospectus, and with the information under “Selected Historical Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| | | As of December 31, 2016 | |
| | | Actual | | | As adjusted(1) | |
| | | | |
| Cash and cash equivalents | | AUD$ | 3,103,683 | | | $ | 2,245,825 | |
| | | | | | | | | |
| Liabilities: | | | | | | | | |
| Borrowings | | | - | | | | - | |
| Other financial liabilities | | | 678,000 | | | | 490,601 | |
| Total current debt | | | 678,000 | | | | 490,601 | |
| | | | | | | | | |
| Equity: | | | | | | | | |
| Issued capital | | | 47,485,700 | | | | 34,360,653 | |
| Reserves | | | 2,243,061 | | | | 1,623,079 | |
| Accumulated losses | | | (46,175,581 | ) | | | (33,412,650 | ) |
| Total Equity | | AUD$ | 3,553,180 | | | $ | 2,571,082 | |
| (1) | The amounts have been translated into U.S. dollars from Australian dollars based upon the exchange rate as published by the Reserve Bank of Australia as of December 31, 2016. These translations are merely for the convenience of the reader and should not be construed as representations that the Australian dollar amounts actually represent such U.S. dollar amounts or could be converted into U.S. dollars at the rate indicated. |
The share information above:
| | • | | is based on 103,641,417 ordinary shares outstanding as of December 31, 2016; and |
| | • | | excludes an aggregate of 34,177,523 ordinary shares issuable upon the exercise of options outstanding at December 31, 2016, at a weighted average exercise price of AUD$0.543 of which options to purchase 33,177,523 ordinary shares were vested, at a weighted average exercise price of AUD$0.544. |
An increase or decrease in the initial public offering price of $1.00 per ADS would increase or decrease cash and cash equivalents, total equity and total capitalization on an as adjusted basis by AUD$ million and $ million, assuming the number of ADSs offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions. Similarly, each increase or decrease of one million in the number of ADSs offered by us would increase or decrease cash and cash equivalents, total equity and total capitalization on an as adjusted basis by AUD$ million and $ million, assuming the assumed initial public offering price remains the same and after deducting estimated underwriting discounts and commissions.
DILUTION
If you invest in the ADSs, your interest will be diluted to the extent of the difference between the initial public offering price per ADS and our net tangible book value per ADS after this offering. Dilution results from the fact that the initial public offering price per ADS is substantially in excess of the net tangible book value per ordinary shares underlying the ADSs. Our net tangible book value as at December 31, 2016 was AUD$3.55 million ($2.57 million), or AUD$ ($ ) per ordinary share, equivalent to AUD$ ($ ) per ADS. Net tangible book value per ADS represents the amount of total tangible assets, minus the amount of total liabilities, divided by the total number of ordinary shares outstanding and multiplied by 40, the number of ordinary shares underlying each ADS. Dilution is determined by subtracting net tangible book value per ADS from the assumed initial public offering price per ADS.
Without taking into account any other changes in our net tangible book value after December 31, 2016 other than to give effect to our sale of ADSs offered in this offering at the assumed initial public offering price of U.S.$ per ADS, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our adjusted net tangible book value as at December 31, 2016 would have been $ million, or $ per ADS. This represents an immediate increase in net tangible book value of $ per ADS to existing shareholders and an immediate dilution in net tangible book value of $ , (or $ ) per ADS to purchasers of ADSs in this offering. The following table presents this dilution to new investors purchasing ADSs in the offering:
| Assumed initial public offering price | | | | | | $ | | |
| | | | | | | | | |
| Net tangible book value as at December 31, 2016 | | $ | 2,571,082 | | | | | |
| | | | | | | | | |
| Increase in net tangible book value attributable to new investors | | | | | | | | |
| As-adjusted net tangible book value immediately after the offering | | | | | | | | |
| Dilution to new investors | | | | | | $ | | |
Each increase or decrease in the initial public offering price of $1.00 per ADS would increase or decrease the as-adjusted net tangible book value after this offering by $ per ADS, and the dilution to investors in the offering by $ per ADS, assuming the number of ADSs offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions. Similarly, each increase or decrease of one million in the number of ADSs offered by us would increase or decrease the as-adjusted net tangible book value after this offering by $ per ADS, and the dilution to investors in the offering by $ per ADS, assuming the assumed initial public offering price remains the same and after deducting estimated underwriting discounts and commissions.
The following table summarizes, as of December 31, 2016 on the as-adjusted basis described above, the differences between the existing shareholders as of December 31, 2016 and the new investors in this offering with respect to the number of ADSs, or equivalent number of ordinary shares, purchased from us, the total consideration paid to us and the average price per ADS, or equivalent number of ordinary shares, based on an assumed initial public offering price of $ per ADS, which is the midpoint of the price range set forth on the cover page of this prospectus, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| | | ADS or Equivalent
Ordinary Shares
Purchased(1) | | | Total
Consideration | | | Average Price
Per
ADS or
Equivalent
Ordinary
Shares(1) | |
| | | Number | | | % | | | Amount | | | % | | | | |
| Existing shareholders | | | | | | | | % | | $ | | | | | | % | | $ | | |
| New investors | | | | | | | | | | | | | | | | | | | | |
| Total | | | | | | | 100 | % | | $ | | | | | 100 | % | | | | |
| (1) | Reflects forty ordinary shares as equivalent to each ADS. |
Each increase or decrease in the initial public offering price of $1.00 per ADS would increase or decrease the total consideration paid by new investors by $ million, or $ per ADS, assuming that the number of ADSs offered by us, as set forth on the cover page of this prospectus, remains the same and before deducting estimated underwriting discounts and commissions. Each increase or decrease of one million in the number of ADSs offered by us would increase or decrease the total consideration paid by new investors by $ million, or $ per ADS, assuming the assumed initial public offering price remains the same and before deducting estimated underwriting discounts and commissions.
To the extent that we grant options or other equity awards to our employees or members of our management in the future, and those options or other equity awards are exercised or become vested or other issuance of our ordinary shares are made, there will be further dilution to new investors.
The share information above:
| | • | is based on 103,641,417 ordinary shares outstanding as of December 31, 2016; and |
| | • | excludes an aggregate of 34,177,523 ordinary shares issuable upon the exercise of options outstanding at December 31, 2016, at a weighted average exercise price of AUD$0.543 of which options to purchase 33,177,523 ordinary shares were vested, at a weighted average exercise price of AUD$0.544. |
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA
The following tables set forth selected historical consolidated financial data for the periods indicated.
The consolidated statement of profit or loss and other comprehensive income data for the fiscal years ended June 30, 2016, 2015 and 2014 are derived from the audited consolidated financial statements included in this prospectus. The consolidated statement of profit or loss and other comprehensive income data for the six months ended December 31, 2016 and 2015 are derived from the unaudited consolidated financial statements included in this prospectus. In our management’s opinion, these consolidated financial statements include all adjustments necessary for the fair presentation of our financial condition as of such dates and our results of operations for such periods.
Our consolidated financial statements have been prepared in Australian dollars and in accordance with International Accounting Standards. Our consolidated financial statements comply with IFRS, as issued by the IASB.
You should read the selected consolidated financial data in conjunction with our consolidated financial statements and related notes beginning on page F-1 of this prospectus, and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. Our historical results do not necessarily indicate our expected results for any future periods. Financial results for the year ended June 30, 2016 and six months ended December 31, 2016 are not necessarily indicative of the results that may be expected for the full fiscal year ending June 30, 2017.
| | | For the year ended June 30, | | | For the six months ended Dec 31, | |
| | | 2016
AUD$ | | | 2015
AUD$ | | | 2014
AUD$ | | | 2016
AUD$ | | | 2015
AUD$ | |
| Consolidated Statement of Profit or Loss and Other Comprehensive Income Data: | | | | | | | | | | | | | | | | | | | | |
| Revenue: | | | | | | | | | | | | | | | | | | | | |
| Operating Revenue | | $ | 1,001,077 | | | $ | 1,002,380 | | | $ | 981,051 | | | $ | 703,099 | | | $ | 497,220 | |
| Total Operating Revenue | | | 1,001,077 | | | | 1,002,380 | | | | 981,051 | | | | 703,099 | | | | 497,220 | |
| Cost of Goods Sold | | | (301,435 | ) | | | (316,128 | ) | | | (277,928 | ) | | | (223,394 | ) | | | (153,640 | ) |
| Gross Profit | | | 699,642 | | | | 686,252 | | | | 703,123 | | | | 479,705 | | | | 343,580 | |
| Sales and Marketing Costs | | | (133,781 | ) | | | (76,794 | ) | | | (79,796 | ) | | | (93,520 | ) | | | (52,659 | ) |
| Freight Costs | | | (134,967 | ) | | | (116,379 | ) | | | (114,278 | ) | | | (62,590 | ) | | | (61,299 | ) |
| Total Gross Profit less Direct Selling Costs | | | 430,894 | | | | 493,079 | | | | 509,049 | | | | 323,595 | | | | 229,622 | |
| | | | | | | | | | | | | | | | | | | | | |
| Other Income | | | 1,539,015 | | | | 1,591,021 | | | | 804,477 | | | | 816,932 | | | | 763,563 | |
| Expenses: | | | | | | | | | | | | | | | | | | | | |
| Amortization | | | - | | | | - | | | | (680,587 | ) | | | - | | | | - | |
| Consulting, Employee and Director | | | (2,840,037 | ) | | | (728,140 | ) | | | (555,487 | ) | | | (907,390 | ) | | | (1,113,214 | ) |
| Corporate Administration | | | (1,320,570 | ) | | | (557,422 | ) | | | (492,465 | ) | | | (790,103 | ) | | | (708,625 | ) |
| Depreciation | | | (3,892 | ) | | | (3,719 | ) | | | (3,989 | ) | | | (1,975 | ) | | | (1,944 | ) |
| Finance Costs | | | (341,600 | ) | | | - | | | | (463,685 | ) | | | (13,183 | ) | | | - | |
| Impairment of Inventory | | | (4,176 | ) | | | (35,340 | ) | | | (50,204 | ) | | | (135,170 | ) | | | (169 | ) |
| Marketing and Promotion | | | (487,591 | ) | | | (304,687 | ) | | | (235,176 | ) | | | (471,735 | ) | | | (201,724 | ) |
| Research and Development | | | (3,623,961 | ) | | | (3,018,294 | ) | | | (1,289,675 | ) | | | (2,117,867 | ) | | | (1,839,990 | ) |
| Travel and Entertainment | | | (416,849 | ) | | | (128,318 | ) | | | (37,327 | ) | | | (112,453 | ) | | | (195,605 | ) |
| Loss before income tax | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) | | | (3,409,349 | ) | | | (3,068,086 | ) |
| Income tax expense | | | - | | | | - | | | | - | | | | - | | | | - | |
| Loss for the period | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) | | | (3,409,349 | ) | | | (3,068,086 | ) |
| Other Comprehensive Income / (Losses) | | | 8,846 | | | | (12,581 | ) | | | - | | | | (41,425 | ) | | | (4,781 | ) |
| Total Comprehensive Loss for the Period | | | (7,059,921 | ) | | | (2,704,401 | ) | | | (2,495,069 | ) | | | (3,450,774 | ) | | | (3,072,867 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Loss per share, basic and diluted (cent per share) | | $ | 9.248 | | | $ | 3.592 | | | $ | 5.947 | | | $ | 3.300 | | | $ | 4.065 | |
| Weighted-average number of shares outstanding, basic and diluted | | | 76,435,993 | | | | 74,935,902 | | | | 41,955,199 | | | | 103,328,491 | | | | 75,470,006 | |
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the section entitled “Selected Historical Consolidated Financial Data” and our consolidated financial statements and related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including, but not limited to, those set forth under “Risk Factors” and elsewhere in this prospectus.
Our financial statements have been prepared in Australian dollars and in accordance with International Financial Reporting Standards (IFRS). Our financial statements comply with IFRS, as issued by the IASB. Our fiscal year end is June 30. References to a particular “fiscal year” are to our fiscal year ended June 30 of that year.
Overview
We are a clinical-stage publicly listed Australian biopharmaceutical company with a proprietary technology platform focused on developing a novel class of biological polyclonal antibodies. Our first-in-class oral polyclonal antibodies drugs can target specific antigens to directly block bacteria at mucosal surfaces and/or to influence the cell-mediated immune system through regulatory T-cell populations. These unique antibodies can target a large range of human diseases, including infectious diseases and immune mediated disorders.
We are advancing our lead asset, IMM-124E, in several Phase 2 clinical trials for NASH, ASH and pediatric NASH. Both the Phase 2s in ASH and Pediatric are funded by the NIH, highlighting the potentially of our technology. The success of any of these human trials would be a key step toward therapeutic use and toward ultimately commercializing the product if it achieves approval. In addition to IMM-124E, we are pursuing other programs in the pre-clinical or Phase I stage such as IMM-529, which is a C.difficile ToxinB antagonist, and a shigella vaccine, that is currently in development with the Department of Defense (DoD). Currently, we are selling over-the-counter Travelan product, the only preventative treatment targeting Traveler’s Diarrhea (TD). In the future, we also expect to earn revenues from commercializing our primary therapeutic product candidates in our targeted markets, if they receive approval.
Since we were incorporated in 1994, we have devoted the majority of our resources to development efforts relating to oral immunotherapy for human beings. We have funded our operations primarily from public offerings in Australia and private placements of ordinary shares. We have also been awarded research and development tax incentive refunds for eligible research and development expenditure from the Australian federal government, totaling nearly AUD$1.6 million for the fiscal year ended June 30, 2016. We are currently collaborating with the U.S. Department of Defense for the research of Shigella, Campylobacter and ETEC vaccines.
We have incurred losses from operations in each year since inception. Our net losses were AUD$3.4 million and AUD$3.1 million for the six-month periods ended December 31, 2016 and 2015 respectively, and AUD$7.1 million, AUD$2.7 million, and AUD$2.5 million for the fiscal years ended June 30, 2016, 2015, and 2014 respectively. The majority of our net losses resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years.
We generated AUD$0.7 million and AUD$0.5 million of revenue from the sale of our existing consumer product Travelan for the six-month periods ended December 31, 2016 and 2015 respectively, and AUD$1.0 million for the fiscal years ended June 30, 2016 and 2015, and AUD$981K for fiscal year 2014. We are not currently, but may in the future, generate revenue from licensing programs, strategic alliances, and collaboration arrangement with other pharmaceutical companies for the use of our pipeline products. Whilst we do not have any of these arrangements in place, it is likely that these possibilities will manifest as our pipeline products become more advanced following our ongoing research efforts. We do not expect to generate revenue from our current clinical trial products until we have successfully completed sufficient clinical development having also obtained the necessary regulatory approvals, which we expect will take a number of years, which is subject to significant uncertainty and may never occur.
We expect that the net proceeds from this offering, and our existing cash and cash equivalents, will be sufficient to enable us to advance the planned preclinical programs and clinical trials for certain of our key product candidates for approximately the next 24 months. See “Use of Proceeds.” In addition, we will continue to pursue licensing programs, strategic alliances, and collaboration arrangements with major pharmaceutical companies, governmental entities, and universities to assist us to fund our trial development and commercialization process.
Basis of Preparation – For the audited consolidated financial statements
The financial report is a general-purpose financial report, which has been prepared in accordance with the requirements of International Financial Reporting Standards (IFRS), required for a for-profit entity.
The financial report has been prepared on an accruals basis and is primarily based on historical costs. The financial report is presented in Australian dollars, which is the Company’s functional and presentation currency. All values are rounded to the nearest dollar unless otherwise stated.
Management is required to make judgements, estimates and assumptions about carrying values of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstance, the results of which form the basis of making the judgements. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognised in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods if the revision affects both current and future periods.
Judgements made by management in the application of IFRS that have significant effects on the financial statements and estimates with a significant risk of material adjustments in the next year are disclosed, where applicable, in the relevant notes to the financial statements.
Accounting policies are selected and applied in a manner which ensures that the resulting financial information satisfies the concepts of relevance and reliability, thereby ensuring that the substance of the underlying transactions or other events is reported.
Statement of Compliance
Our consolidated financial statements comply with the International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board.
New, revised or amending Accounting Standards and Interpretations adopted
The Company has adopted all of the new, revised or amending Accounting Standards and Interpretations issued by the International Accounting Standards Board ('IASB') that are mandatory for the current reporting period.
There were no significant new standards adopted during the reporting periods.
Management has determined that the standards that have been adopted in fiscal year 2017 have not had a material impact on the Group. Management is currently assessing the impact of the standards to be adopted in fiscal year 2018 and forward on the Group.
Critical Accounting Policies
The following is a summary of the material accounting policies adopted by the Company in the preparation of the financial report. The accounting policies have been consistently applied, unless otherwise stated.
Basis of Consolidation
The consolidated financial statements incorporate the financial statements of the Company and entities controlled by the Company (its subsidiaries) referred to as ‘the Group’ in the financial statements. Control is achieved where the consolidated entity is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the consolidated entity. They are de-consolidated from the date that control ceases.
A list of controlled entities is contained in Note 11 to the financial statements. All controlled entities have a June 30 financial year-end.
All intra-group transactions, balances, income and expenses are eliminated in full on consolidation.
Accounting policies of subsidiaries have been changed where necessary to ensure consistency with those policies applied by the parent entity. Subsidiaries are accounted for at cost in the parent entity.
The results of subsidiaries acquired or disposed of during the year are included in profit or loss from the effective date of acquisition or up to the effective date of disposal, as appropriate.
Revenue Recognition
Revenue is measured at the fair value of the consideration received or receivable. Amounts disclosed as revenue are net of returns, trade allowances, rebates and amounts collected on behalf of third parties.
The Company recognizes revenue when the amount of the revenue can be reliably measured, it is probable that the future economic benefits will flow to the entity and specific criteria have been met for each of the activities as described below. The amount of the revenue is not considered to be reliably measured until all contingencies relating to the sale have been resolved.
The following specific revenue criteria must be met before revenue is recognized:
| (i) | Sale of Goods and services | – | Significant risks and rewards of ownership of goods has passed to the buyer and an invoice for the goods or services is issued; |
| (ii) | Interest | – | Interest income is recognized using the effective interest rate method; |
| (iii) | R & D Tax Refunds | – | Income is recognized in the year the research and development expenses were incurred. |
A difference of AUD$644,149 in the Accumulated losses balance at June 30, 2013 between the consolidated financial statements appearing elsewhere in this prospectus and the original statement lodged with ASX relates to the previous recognition of fiscal year 2013 R&D refund in fiscal year 2014, which does not significantly affect the overall financial position and results presented in the original statement. For the fiscal year 2014, 2015 and 2016, the Company has reassessed and made changes to the amount of R&D Tax Refund recognized as Other Income for the period as compared to the previous statements lodged with the ASX. Effectively, these changes resulted in an increase of AUD$49,481 and AUD$756,131 in Other Income, resulting in a related decrease in the net loss, for the period on the Consolidated Statement of Profit or Loss and Other Comprehensive Income for the fiscal year 2014 and 2015. These changes also resulted in a decrease in Other Income of AUD$1,469,763, and a related increase in Net Loss, for the period on the Consolidated Statement of Profit or Loss and Other Comprehensive Income for fiscal year 2016.
These adjustments were the result of additional information being made available to the Company subsequent to the previous lodgements with ASX which, as a result, changed the timing of recognition, but not the actual amount of the R&D Refund received.
The Company has worked with experienced advisors to improve its internal process on advanced findings of the R&D activities, which includes determining and evaluating the eligibility of R&D related expenditure to support its submission of the R&D Tax Refund claim.
Intangible Assets - Research & Development
Expenditure on research activities, undertaken with the prospect of obtaining new scientific or technical knowledge and understanding, is recognized in the statement of profit or loss and other comprehensive income as an expense when it is incurred.
Expenditure on development activities, being the application of research findings or other knowledge to a plan or design for the production of new or substantially improved products or services before the start of commercial production or use, is capitalized if it is probable that the product or service is technically and commercially feasible, will generate probable economic benefits and adequate resources are available to complete development and cost can be measured reliably. Other development expenditure is recognized in the statement of profit or loss and other comprehensive income as an expense as incurred.
Interest Bearing Loans and Borrowings
Generally, loans and borrowings are initially recognized at cost, being the fair value of the consideration received net of issue costs associated with the borrowing. After initial recognition, interest bearing loans and borrowings are subsequently measured at amortized cost using the effective interest method. Amortized cost is calculated by taking into account any issue costs and any discount or premium on settlement.
The component of the convertible notes that were issued in connection with the February 2016 financing arrangement, that exhibits characteristics of a liability is recognised as a liability in the statement of financial position. On the date of issuance and each subsequent reporting period, the Company records the entire hybrid instrument as measured at fair value through profit and loss. The associated transaction costs have also been expensed as incurred and are recorded as Finance and Termination costs in the Statement of Profit or Loss and Other Comprehensive Income.
Fair Value of Convertible Notes
The convertible notes were measured and disclosed as a level 3 instrument, using a three level hierarchy, based on the lowest level of input that is significant to the entire fair value measurement, as defined below:
| · | Level 1: Quoted price (unadjusted) in active markets for identical assets or liabilities that the entity can access at the measurement date |
| · | Level 2: Inputs other than quoted prices included within Level 1 that are observable for the asset and liability, either directly or indirectly |
| · | Level 3: Unobservable inputs for the asset or liability |
No transfers between the levels of the fair value hierarchy occurred during the current year.
Inventories
Raw materials, work in progress and finished goods are stated at the lower of cost and net realizable value. Where appropriate, cost comprises direct materials, direct labor and an appropriate proportion of variable and fixed overheads expenditure, the latter being allocated on the basis of normal operating capacity. The Company classifies inventory as a current asset as all amounts are held for the purpose of trading. Costs are assigned to individual items of inventory on basis of weighted average costs. Net realizable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale.
The Company’s Inventory balance increased from AUD$1,146,267 as of June 30, 2015 to AUD$ 2,056,067 as of June 30, 2016 as the Company increased its stocks of raw colostrum to ensure there would be sufficient supply of product available to ensure no stock-outs occurred from the increase in sales volumes expected from the Company’s expansion into the new geographic markets of USA and China.
Share-based payments
Share-based compensation benefits may be provided through the issue of fully paid ordinary shares under the Immuron Employee Share and Option Plan. Options are also granted to employees and consultants in accordance with the terms of their respective employment and consultancy agreements. Any options granted are made in accordance with the terms of the Company’s Employee Share and Option Plan (ESOP).
The fair value of options granted under employment and consultancy agreements are recognized as an employee benefit expense with a corresponding increase in equity. The fair value is measured at grant date and recognized over the period during which the employees become unconditionally entitled to the options.
The fair value at grant date is determined using a Black-Scholes option pricing model that takes into account the exercise price, the term of the option, the vesting and performance criteria, the impact of dilution, the non-tradeable nature of the option, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk-free interest rate for the term of the option.
The fair value of the options granted excludes the impact of any non-market vesting conditions (for example, profitability and sales growth targets). Non-market vesting conditions are included in assumptions about the number of options that are expected to become exercisable. At each reporting date, the entity revises its estimate of the number of options that are expected to become exercisable. The employee benefit expense recognized each period takes into account the most recent estimate. The impact of the revision to original estimates, if any, is recognized in the statement of profit or loss and other comprehensive in come with a corresponding adjustment to equity.
Upon the exercise of options, the balance of the share-based payments reserve relating to those options is transferred to contributed equity.
Previously issued Financial Statements
Reclassification:
The Company has reclassified certain items in the statement of profit or loss and other comprehensive income for the years ended June 30, 2015 and 2014 to conform with the current year presentation and reclassified certain items in the statement of change in equity for the year ended June 30, 2016, as follows:
Statement of profit or loss and other comprehensive income:
| | | 2015 | | | 2014 | |
| | | Previously
Issued | | | Reclassification | | | Revised | | | Previously
Issued | | | Reclassification | | | Revised | |
| Cost of Goods Sold | | | (316,128 | ) | | | - | | | | (316,128 | ) | | | (332,686 | ) | | | 54,758 | | | | (277,928 | ) |
| Sales and Marketing Costs | | | (360,073 | ) | | | * 283,279 | | | | (76,794 | ) | | | (401,811 | ) | | | ** 322,015 | | | | (79,796 | ) |
| Freight Costs | | | (116,379 | ) | | | - | | | | (116,379 | ) | | | (38,445 | ) | | | (75,833 | ) | | | (114,278 | ) |
| Amortisation | | | - | | | | - | | | | - | | | | (680,567 | ) | | | (20 | ) | | | (680,587 | ) |
| Consulting, Employee and Director | | | (728,140 | ) | | | - | | | | (728,140 | ) | | | (555,487 | ) | | | - | | | | (555,487 | ) |
| Corporate Administration | | | (557,422 | ) | | | - | | | | (557,422 | ) | | | (367,514 | ) | | | (124,951 | ) | | | (492,465 | ) |
| Depreciation | | | (3,719 | ) | | | - | | | | (3,719 | ) | | | (4,010 | ) | | | 21 | | | | (3,989 | ) |
| Finance Costs | | | - | | | | - | | | | - | | | | (588,636 | ) | | | 124,951 | | | | (463,685 | ) |
| Impairment of Inventory | | | (35,340 | ) | | | - | | | | (35,340 | ) | | | - | | | | (50,204 | ) | | | (50,204 | ) |
| Marketing and Promotion | | | (142,735 | ) | | | (161,952 | ) | | | (304,687 | ) | | | (52,085 | ) | | | (183,091 | ) | | | (235,176 | ) |
| Research and Development | | | (3,018,294 | ) | | | - | | | | (3,018,294 | ) | | | (1,285,121 | ) | | | (4,554 | ) | | | (1,289,675 | ) |
| Travel and Entertainment | | | (128,318 | ) | | | - | | | | (128,318 | ) | | | (37,326 | ) | | | (1 | ) | | | (37,327 | ) |
| * | Amount includes AUD121,327 that has been reflected as a restatement to decrease both Operating revenue and Sales and Marketing Costs. |
| ** | Amount includes AUD63,091 that has been reflected as a restatement to decrease both Operating revenue and Sales and Marketing Costs. |
Statement of change in equity:
| | | 2016 | |
| | | Previously Issued | | | Reclassification | | | Revised | |
| Shares issued, net of costs | | | 1,658,504 | | | | (71,875 | ) | | | 1,586,629 | |
| Options exercised | | | (71,875 | ) | | | 71,875 | | | | - | |
The reclassifications had no impact on the net loss for each period.
Restatement:
| · | The Company revised all customer discounts and allowances previously recognized as Selling and Marketing Costs as reduction to Operating revenue. These revisions resulted in decreases in both Operating revenue and Selling and Marketing Costs of AUD$154,446, AUD$121,327 and AUD$63,091 for the fiscal year ended June 30, 2016, 2015 and 2014, respectively. |
| · | In the previously issued financial statements, the basic and diluted loss per share was 5.705 cents, 4.603 cents, 3.398 cents and the weighted average number of ordinary shares outstanding was 76,944,879, 74,907,491, 74,891,316 for the years ended 30 June 2016, 2015 and 2014, respectively. The revised basic and diluted loss per share and the weighted average number of ordinary shares outstanding were 9.248 cents, 3.592 cents, 5.947 cents and 76,435,993, 74,935,902, 41,955,199 for the years ended 30 June 2016, 2015 and 2014, respectively. |
| · | An adjustment was made in relation to the treasury shares which resulted in a decrease of AUD$800,000 in Non-current assets and Equity as compared to the previous statement lodged with ASX. |
| · | An adjustment of AUD$1,209,338 was made to the Total reserves balance at 30 June 2016 as compared to the previous statement lodged with ASX, as a result of a change in volatility assessment. Effectively, this resulted in an increase in Consulting, Employee and Director expense and the Loss for the period on the Statement of Profit or Loss and Other Comprehensive income. |
| · | Changes were made to the Consolidated Statement of Cash Flows for the year 2016 as compared to the previous statement lodged with ASX, details as follows: |
| | | Previously issued | | | Restatement | | | Revised | |
| Receipts from customers | | | 1,242,884 | | | | (128,288 | ) | | | 1,114,596 | |
| Payments to suppliers and employees | | | (7,639,088 | ) | | | (71,909 | ) | | | (7,710,997 | ) |
| Interest and other costs of finance paid | | | - | | | | (43,863 | ) | | | (43,863 | ) |
| Net Cash Flows Used In Operating Activities | | | (4,914,276 | ) | | | (244,060 | ) | | | (5,158,336 | ) |
| | | | | | | | | | | | | |
| Proceeds from issues of securities | | | 2,282,861 | | | | 200,000 | | | | 2,482,861 | |
| Repayment of borrowings | | | (1,121,080 | ) | | | 43,860 | | | | (1,077,220 | ) |
| Net Cash Flows Provided By Financing Activities | | | 4,091,482 | | | | 243,860 | | | | 4,335,342 | |
| Net increase/(decrease) in cash and cash equivalents | | | (825,235 | ) | | | (200 | ) | | | (825,435 | ) |
| Effects of exchange rate changes on cash and cash equivalents | | | (200 | ) | | | 200 | | | | - | |
| · | Changes were made to the Consolidated Statement of Cash Flows for the year 2014 as compared to the previous statement lodged with ASX, details as follows: |
| | | Previously issued | | | Restatement | | | Revised | |
| Proceeds from borrowings | | | - | | | | 420,000 | | | | 420,000 | |
| Repayment of borrowings | | | (1,485,001 | ) | | | (420,000 | ) | | | (1,905,001 | ) |
In addition to these restatements, the Company has made revisions to Notes 1, 3, 4, 7, 8, 9, 13, 15, 16, 19, 21, 22, 23,24
Critical Accounting Estimates and Judgments
Management evaluates estimates and judgments incorporated into the financial statements based on historical knowledge and best available current information. Estimates assume a reasonable expectation of future events are based on current trends and economic data, obtained both externally and within the group.
The value attributed to share options and remunerations shares issued is an estimate calculated using an appropriate mathematical formula based on an option pricing model. The choice of models and the resultant option value require assumptions to be made in relation to the likelihood and timing of the conversion of the options to shares and the value of volatility of the price of the underlying shares.
| (ii) | Impairment of Inventories |
The provision for impairment of inventories assessment requires a degree of estimation and judgment. The level of the provision is assessed by taking into account the recent sales experience, the ageing of inventories and in particular the shelf life of inventories that affects obsolescence.
| (iii) | Fair value measurement hierarchy |
The preparation of the financial statements requires management to make judgments, estimates and assumptions that affect the reported amounts in the financial statements. Management continually evaluates its judgments, estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgments, estimates, and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgments and estimates will seldom equal the related actual results. The judgments, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed within the relevant sections where applicable.
The fair value of convertible note classified as level 3 is determined by the use of valuation model. These include discounted cash flow analysis and the use of observable inputs that required significant adjustments based on unobservable inputs.
As at June 30, 2016, management has assessed the terms of the convertible notes and determined that in their view the fair value of the debt component is equal to the proceeds such that there is no residual amount to be allocated to an equity component. In making this determination, management is of the view that the value of the consideration received, net of costs, provided reliable evidence of the fair value of the debt component of the convertible note. Fair value has been determined by the income approach based on a discounted cash flow analysis, with the most significant inputs being the discount rate that reflects the investors credit risk.A slight increase or decrease in the discount rate used would not be material to the financial statements.
Basis of Preparation – For the unaudited consolidated financial statements
The general purpose financial report for the interim six months reporting periods ended December, 31 2016 and 2015 have been prepared in accordance with International Accounting Standard (IAS) 134 Interim Financial Reporting, under the International Financial Reporting Standards, as issued by the International Accounting Standards Board
This half year financial report does not include all notes of the type normally included in an annual financial report. Accordingly, this report is to be read in conjunction with the annual report for the years ended June 30, 2016, 2015 and 2014 included in the Form F-1 and any public announcements made by Immuron Limited and its controlled entities (the “Group”), during the interim reporting periods.
The half year financial report reflects all adjustments which are, in the opinion of management, necessary to a fair statement of the results for the interim periods presented. All adjustments are of a normal recurring nature.
Critical Accounting Policies
All accounting policies adopted are consistent with the most recent Annual Financial Report for the years ended June 30, 2016, 2015 and 2014. The consolidated entity has adopted all of the new, revised or amending Accounting Standards and Interpretations issued by the International Accounting Standards Board ('IASB') that are mandatory for the current reporting period. The adoption of these Accounting Standards and Interpretations did not have any significant impact on the financial performance or position of the consolidated entity.
Fair value measurement
Due to the nature of the Group’s operating profile, the Directors and management do not consider that the fair values of the Group’s financial assets and liabilities are materially different from their carrying amounts at December, 31 2016
Going Concern
For the six months ended December, 31 2016 the Company experienced a net cash outflow of AUD$2,256,539 (2015: AUD$3,454,247) from operating activities. Net loss for the period was AUD$3,409,349 which included AUD$2,117,867 of expenditures associated with research, development and commercialization programs predominantly surrounding the Non- Alcoholic Steatohepatitis (NASH) Clinical Trial (2015: AUD$3,068,086 and AUD$1,839,990, respectively). These factors raise substantial doubt on the Company’s ability to continue as a going concern.
Whilst the Company is projecting further net losses and a net cash outflow from operations for the remainder of the 2017 financial year, management is in the process of enhancing its internal cost control procedures to further reduce operating costs. Additionally, the Company also plans to raise additional capital from domestic or oversea market. The following historical successes achieved in the past 6 months further support the Company’s position in future capital raising and cost reduction:
| · | successfully completion of an oversubscribed AUD$6.32M Right Issue Capital Raising (before costs); |
| · | completion of patient recruitment in the Company’s (NASH) IMM-124E Phase 2 clinical trial. |
Notwithstanding the above discussion, there exists a degree of uncertainty in the Company’s ability to continue as a going concern.
Having considered all the above, the Directors have prepared the financial statements on a going concern basis. As such, the financial statements do not include any adjustments as to the recoverability and classification of recorded asset amounts or to the amounts and classification of liabilities that might be necessary should the entity not continue as a going concern.
Restatements:
The Company has restated certain items in the previously issued financial statements as at and for the six months ended December 31, 2015, as follows:
Consolidated statement of profit or loss and other comprehensive income:
| | | Previously
Issued | | | Adjustments | | | Revised | |
| Other income* | | | 1,476,906 | | | | (713,343 | ) | | | 763,563 | |
| Sales and Marketing Costs | | | (242,150 | ) | | | 189,491 | | | | (52,659 | ) |
| Marketing and Promotion | | | (12,233 | ) | | | (189,491 | ) | | | (201,724 | ) |
Consolidated statement of changes in equity:
| | | Previously
Issued | | | Adjustments | | | Revised | |
| Balance at June 30, 2015 / Accumulated losses* | | | (37,542,572 | ) | | | 1,469,762 | | | | (36,072,810 | ) |
| Lapse or exercise of share options | | | (58,615 | ) | | | 58,615 | | | | - | |
| Shares issued, net of costs | | | 480,879 | | | | (58,615 | ) | | | 422,264 | |
Consolidated statement of cash flows:
| | | Previously
Issued | | | Adjustments | | | Revised | |
| Receipts from customers | | | 613,528 | | | | (60,122 | ) | | | 553,406 | |
| Payments to suppliers and employees | | | (4,074,918 | ) | | | 60,122 | | | | (4,014,796 | ) |
* These adjustments were the result of the timing of the recognition of the R&D refund.
Restatements were also made to the Consolidated Statement of Cash Flows for the six months ended December 31, 2016 as compared to the previous statement lodged with ASX, details as follows:
| | | Previously issued | | | Adjustments | | | Revised | |
| Receipts from customers | | | 664,900 | | | | 108,576 | | | | 773,476 | |
| Payments to suppliers and employees | | | (4,423,797 | ) | | | (148,497 | ) | | | (4,572,294 | ) |
| Net Cash Flows Used In Operating Activities | | | (2,216,618 | ) | | | (39,921 | ) | | | (2,256,539 | ) |
| | | | | | | | | | | | | |
| Capital raising costs | | | (120,285 | ) | | | (29,634 | ) | | | (149,919 | ) |
| Repayment of borrowings | | | (1,271,555 | ) | | | 69,555 | | | | (1,202,000 | ) |
| Net Cash Flows Provided By Financing Activities | | | 3,031,394 | | | | 39,921 | | | | 3,071,315 | |
As described in Note 8, the Company has restated its previously issued financial statements for the six months ended December 31, 2016. In addition to these restatements, the Company has made revisions to Notes 3, 6, 7 and 9.
Results of Operations
The following discussion relates to our consolidated results of operations, financial condition and capital resources. You should read this discussion in conjunction with our consolidated financial statements and the notes thereto contained elsewhere in this prospectus.
Comparison of the six-month periods ended December 31, 2016 and 2015
Revenue and Other income
| | | For the six months ended December 31, | | | Increase/ | |
| | | 2016 | | | 2015 | | | Decrease | |
| Revenue: | | | | | | | | | | | | |
| Sale of goods | | AUD$ | 703,099 | | | AUD$ | 497,220 | | | AUD$ | 205,879 | |
| | | | | | | | | | | | | |
| Other income: | | | | | | | | | | | | |
| Australian Federal R&D Tax Concession Refund | | | 779,826 | | | | 756,420 | | | | 23,406 | |
| Interest income | | | 6,791 | | | | 7,143 | | | | (352 | ) |
| Other | | | 30,315 | | | | - | | | | 30,315 | |
| Total Revenue and Other income | | AUD$ | 1,520,031 | | | AUD$ | 1,260,783 | | | AUD$ | 259,248 | |
Revenues received from the sale of goods remain increased in the fiscal half year 2016 in comparison to the fiscal hand year of 2015. This increase in revenues can be attributed to the establishment and maturing of our U.S. sales channel as we achieved a major advancement by releasing the Company’s flagship product Travelan in the U.S. by means of sales through customers such as PassportHealth and Medique, whilst also opening a new distribution channels into the Chinese market. The Company does not have any formal agreements with PassportHealth for the sale of Travelan. There was also an increase in sales in the Canada market. Whilst our Australian product sales remained constant, we applied our resources and marketing spend to these new emerging market opportunities in the US and China, whilst instigated programs to reengage the Australian consumers. As these new markets mature over the coming 12 months, when combined with our existing market presence in Australia, we anticipate that revenues received from the sale of our Travelan product to increase.
Our R&D tax concession refund for the fiscal half year 2016 appears to be slightly higher in comparison to fiscal half year 2015 by AUD$23 thousand due to the higher R&D expenditures recognized during the fiscal half year 2016 as compared to that of fiscal half year 2015. This research and development increase was predominantly due to the increased expenditures of our major Phase 2 NASH clinical trial as the program recruitment accelerated and more patients entered the trial.
Cost of Goods Sold, Gross Profit and Direct Selling Costs
| | | For the six months ended December 31, | | | Increase/ | |
| | | 2016 AUD$ | | | 2015 AUD$ | | | Decrease AUD$ | |
| | | | | | | | | | |
| Total Operating Revenue | | $ | 703,099 | | | $ | 497,220 | | | $ | 205,879 | |
| Cost of Goods Sold | | | (223,394 | ) | | | (153,640 | ) | | | (69,754 | ) |
| Gross Profit | | | 479,705 | | | | 343,580 | | | | 136,125 | |
| Less Direct Selling Costs: | | | | | | | | | | | | |
| Sales and Marketing Costs | | | (93,520 | ) | | | (52,659 | ) | | | (40,861 | ) |
| Freight Costs | | | (62,590 | ) | | | (61,299 | ) | | | (1,291 | ) |
| Total Gross Profit less Direct Selling Costs | | | 323,595 | | | | 229,622 | | | | 93,973 | |
Immuron’s strong mature relationships with its key manufacturing partners for the Company’s flagship consumer product Travelan, has enabled the Company maintain consistent Cost of Goods Sold ratios across the two fiscal half years for 2016 and 2015 of 32% and 31% respectively. These key manufacturing partners provide Immuron with steady, reliable product for a known price which from a strategic point of view provides certainty around the manufacturing margins.
These strong manufacturing partnerships have also given rise to greater efficiencies in the manufacturing processes which not only resulted in the improvement in Gross Profit ratio but also an overall increase in Gross Profit.
Sales and Marketing Costs increased by AUD$40 thousand in fiscal half years for 2016 compared fiscal half years for 2015 due to increased marketing activities in oversea markets. Freight Costs, despite the increased number of sales, remained constant due to our more established logistical channels for shipping Travelan from Australia to the overseas countries.
Expenses
| | | For the six months ended December 31, | | | Increase/ | |
| | | 2016 AUD$ | | | 2015 AUD$ | | | Decrease AUD$ | |
| Expenses: | | | | | | | | | | | | |
| Consulting, Employee and Director | | | (907,390 | ) | | | (1,113,214 | ) | | | 205,824 | |
| Corporate Administration | | | (790,103 | ) | | | (708,625 | ) | | | (81,478 | ) |
| Depreciation | | | (1,975 | ) | | | (1,944 | ) | | | (31 | ) |
| Finance Costs | | | (13,183 | ) | | | - | | | | (13,183 | ) |
| Impairment of Inventory | | | (135,170 | ) | | | (169 | ) | | | (135,001 | ) |
| Marketing and Promotion | | | (471,735 | ) | | | (201,724 | ) | | | (270,011 | ) |
| Research and Development | | | (2,117,867 | ) | | | (1,839,990 | ) | | | (277,877 | ) |
| Travel and Entertainment | | | (112,453 | ) | | | (195,605 | ) | | | 83,152 | |
| Total expenses | | AUD$ | (4,549,876 | )) | | AUD$ | (4,061,271 | ) | | AUD$ | (488,605 | ) |
Consulting, Employee and Director. Consulting, Employee and Director expense decreased by AUD$0.21 million from the six months ended December 31, 2015 to the six months ended December 31, 2016 due primarily to the fact the company issued options to directors during the 2015 half year resulting in a higher expense. Director fees and staff salaries have remained constant.
Corporate Administration. Corporate Administration expense increased by AUD$0.08 million, or 11.50%, from the six months ended December 31, 2015 to the six months ended December 31, 2016 due to both a general increase in the size of the business in combination with additional resources being implemented to assist the Company for its growth, as well as the cost of additional specialized services required to initiate the NASDAQ listing including additional legal, accounting and audit costs which were not present during the 2015 fiscal half year.
Finance Costs. Finance Costs incurred by the Company in the six months ended December 31, 2016 of AUD$0.01 million which is directly attributable to the finance costs associated with the SBI Investment Fund Convertible Loan Facility which the Company executed in February 2016. This facility provided Immuron with the short to medium-term cash flow requirements it needed to ensure the Company’s momentum surrounding its pipeline research programs was not diminished. In comparison, there were no Finance Costs incurred in the six months ended December 31, 2015.
Impairment of Inventory. Impairment of Inventory incurred by the Company in the six months ended December 31, 2016 of AUD$0.14 million related to the writing down of Colostrum from the Company’s inventory balance as it reached expiry date.
Marketing and Promotion. Marketing and Promotion expenses increased by AUD$0.27 million from the six months ended December 31, 2015 to the six months ended December 31, 2016 as the Company increased its promotional efforts of its existing flagship consumer product Travelan. These increased costs included costs associated with the expansion of the product’s reach via a launch in both the US and China consumer markets. We hope to see the benefits of these expansions come to fruition during calendar year 2017 as these groundwork costs incurred mature into increased revenue in these large new markets. The higher Marketing and Promotional Costs incurred during the fiscal half year 2016 in comparison to the fiscal half year 2015, can also be attributed to the Company increasing both its investor relations and public relations profiles ahead of the NASDAQ listing.
Research and Development. Research and Development expense increased by AUD$0.28 million from the six months ended December 31, 2015 to the six months ended December 31, 2016, primarily due to the significant increase in the Company’s Phase 2 NASH clinical trial, together with the advancement of its other early pipeline products.
During fiscal year 2016, Immuron brought the management of its Phase 2 NASH clinical trial program in-house through the appointment of Medical Director another clinical trial support staff member, thereby alleviating the need for some of the external outsourced management whilst also providing Immuron with greater control over the program.
Whilst costs were reduced by bringing the management of the trial in-house, the ramping up of this trial from development to extensive patient recruitment and testing, began to see the majority of the costs of the trial coming through causing an expected increase in the overall research and development expenditures of the Company.
There were also some additional costs incurred through the establishment of additional clinical trial sites in a bid to lift the clinical trial recruitment rates.
Travel and Entertainment. Travel and Entertainment expense decreased by AUD$0.08 million from fiscal from the six months ended December 31, 2015 to the six months ended December 31, 2016 as the Company’s management reduced its frequency of travel due to being required to remain in their base-countries due to necessary projects which needed to be delivered locally, whilst also ensure cash conservation.
Loss for the period. As a result of the foregoing, our loss for the period after income tax benefit increased by AUD$0.34 million, or 11.12%, from AUD$3.07 million in the six months ended December 31, 2015 to AUD$3.41 million in the six months ended December 31, 2016.
Given our, and our subsidiaries’ history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we, or our subsidiaries, will generate sufficient future taxable income against which we can utilized these unused tax losses and any uncalculated potential deferred tax assets, together with any other temporary differences. Should the need arise, the Company can, and will, revisit this position.
Comparison of the fiscal years ended June 30, 2016 and 2015
Revenue and Other income
| | | For the fiscal year ended
June 30, | | | Increase/ | |
| | | 2016 | | | 2015 | | | Decrease | |
| Revenue: | | | | | | | | | | | | |
| Sale of goods | | AUD$ | 1,001,077 | | | AUD$ | 1,002,380 | | | AUD$ | (1,303 | ) |
| | | | | | | | | | | | | |
| Other income: | | | | | | | | | | | | |
| Australian Federal R&D Tax Concession Refund | | | 1,512,840 | | | | 1,478,581 | | | | 34,259 | |
| Interest income | | | 12,165 | | | | 112,440 | | | | (100,275 | ) |
| Other | | | 14,010 | | | | - | | | | 14,010 | |
| Total Revenue and Other income | | AUD$ | 2,540,092 | | | AUD$ | 2,593,401 | | | AUD$ | (53,309 | ) |
Revenues received from the sale of goods remain consistent for fiscal 2015 and 2016. Whilst there appears to be no real perceived growth in our revenues, our geographic sales mix has changed as we achieved a major advancement by releasing the Company’s flagship product Travelan in the U.S. by means of sales through customers such as PassportHealth and Medique, whilst also opening a new distribution channels into the Chinese market. The Company does not have any formal agreements with PassportHealth for the sale of Travelan. Whilst our Australian product sales remained constant, we applied our resources and marketing spend to these new emerging market opportunities in the US and China, we have instigated programs to reengage the Australian consumers. As these new markets mature over the coming 12 months, when combined with our existing market presence in Australia, we anticipate that revenues received from the sale of our Travelan product to increase.
R&D tax concession refund increased by AUD$0.03 million, or 2.32%, from AUD$1.48 million in fiscal 2015 to AUD$1.51 million in fiscal 2016 due to an increased level of eligible research and development expenditures being incurred during the fiscal 2016. This research and development increase was predominantly due to the increased expenditures of our major Phase 2 NASH clinical trial as the program recruitment accelerated and more patients entered the trial.
Interest income decreased by AUD$0.10 million or 89.18%, from AUD$0.11 million in fiscal 2015 to AUD$0.01 million in fiscal 2016 as we depleted our cash reserves through applying our financial resources to the increased areas of expenditure within the Company. The lower cash reserves therefore generated and received less interest revenue.
Cost of Goods Sold, Gross Profit and Direct Selling Costs
| | | For the fiscal year ended
June 30, | | | Increase/ | |
| | | 2016 | | | 2015 | | | Decrease | |
| | | | | | | | | | |
| Total Operating Revenue | | AUD$ | 1,001,077 | | | AUD$ | 1,002,380 | | | AUD$ | (1,303 | ) |
| Cost of Goods Sold | | | (301,435 | ) | | | (316,128 | ) | | | 14,693 | |
| Gross Profit | | AUD$ | 699,642 | | | AUD$ | 686,252 | | | AUD$ | 13,390 | |
| Less Direct Selling Costs: | | | | | | | | | | | | |
| Sales and Marketing Costs | | | (133,781 | ) | | | (76,794 | ) | | | (56,987 | ) |
| Freight Costs | | | (134,967 | ) | | | (116,379 | ) | | | (18,588 | ) |
| Total Gross Profit less Direct Selling Costs | | AUD$ | 430,894 | | | AUD$ | 493,079 | | | AUD$ | (62,185 | ) |
Immuron’s strong mature relationships with its key manufacturing partners for the Company’s flagship consumer product Travelan, has enabled the Company maintain consistent Cost of Goods Sold ratios from 32% of Operating Revenue, and then down to 30% of Operating Revenue, for the 2015 and 2016 fiscal years, respectively. These key manufacturing partners provide Immuron with steady, reliable product for a known price which from a strategic point of view provides certainty around the manufacturing margins.
These strong manufacturing partnerships have also given rise to greater efficiencies in the manufacturing processes which not only resulted in the improvement in Gross Profit ratio but also an overall increase in Gross Profit.
The Company’s push of its Travelan product into the new overseas markets of the US and China required greater Sales and Marketing support to ensure it gained traction. The expenditure to support this expansion resulted in a AUD$57 thousand increase in Sales and Marketing Costs in fiscal year 2016 in comparison to fiscal year 2015, and also a AUD$19 thousand increase in Freight Costs through the additional logistical implications of shipping Travelan from Australia to the overseas countries.
Expenses
| | | For the fiscal year ended
June 30, | | | Increase/ | |
| | | 2016
AUD$ | | | 2015
AUD$ | | | Decrease
AUD$ | |
| Expenses: | | | | | | | | | | | | |
| Amortization | | | - | | | | - | | | | | |
| Consulting, Employee and Director | | | (2,840,037 | ) | | | (728,140 | ) | | | (2,111,897 | ) |
| Corporate Administration | | | (1,320,570 | ) | | | (557,422 | ) | | | (763,148 | ) |
| Depreciation | | | (3,892 | ) | | | (3,719 | ) | | | (173 | ) |
| Finance Costs | | | (341,600 | ) | | | - | | | | (341,600 | ) |
| Impairment of Inventory | | | (4,176 | ) | | | (35,340 | ) | | | 31,164 | |
| Marketing and Promotion | | | (487,591 | ) | | | (304,687 | ) | | | (182,904 | ) |
| Research and Development | | | (3,623,961 | ) | | | (3,018,294 | ) | | | (605,667 | ) |
| Travel and Entertainment | | | (416,849 | ) | | | (128,318 | ) | | | (288,531 | ) |
| Total expenses | | AUD$ | (9,038,676 | ) | | AUD$ | (4,775,920 | ) | | AUD$ | (4,262,756 | ) |
Consulting, Employee and Director. Consulting, Employee and Director expense increased by AUD$2.11 million from fiscal 2015 to fiscal 2016 due primarily to the Company employing more permanent full-time senior management, and additional operational employees within the organization due to the Company’s expansion, which was offset by the use of less part-time consulting and advisory providers.
Following the appointment of an additional Director in May 2015, who subsequently became Executive Vice Chairman, this resulted in an increase in the overall Director’s fees paid during fiscal year 2016, in comparison to fiscal year 2015 as the Company went from three to four directors.
On top of this increase, there was a AUD$1.6 million share-based payments expense realized during fiscal year 2016 which was not present in fiscal year 2015, which pertained to the issuance of 6 million unlisted options in the company exercisable at AUD$0.50 per option expiring on November 27, 2019 to the four Directors of the Company. The unlisted options were issued to the Directors in lieu of cash payment for additional services each director has performed which were deemed to be far over and above those services usually performed by Non-Executive Directors of a company of Immuron’s positioning. The issuance of these options was designed to also encapsulate the additional services the Director’s will be required to perform over the subsequent 12 – 24 month period as the Company matures through a number of key milestone inflection points, where their guidance will be regularly required, during both the 2017 and 2018 fiscal years.
Corporate Administration. Corporate Administration expense increased by AUD$0.76 million, or 137%, from fiscal 2015 to fiscal 2016 due to the general increase in the size of the business in combination with increases in consequent expenses due to additional resources being implemented to assist the Company for its growth.
The increase was also the result of an increase in a number of back-office support costs, fees associated with the Company’s OTCQB listing, legal fees surrounding the initial U.S. NASADAQ listing and additional programs and contracts required for the organization as it employed new employees and raised further capital whilst generally expanding, and increased conference and seminar costs as the Company lifted its public profile around the world.
There was also a significant increase in the company’s foreign currency realized losses as the overseas expenditure, predominantly in $, became more expensive for our AUD$ financially denominated Company as the $ strengthened against the AUD$ throughout the fiscal year 2016, in comparison to the relative strength of the AUD$ against the $ in fiscal year 2015, where the AUD$ was either on parity of at times even above parity.
Finance Costs. Finance Costs incurred by the Company in fiscal 2016 of AUD$0.34 million which directly pertained to the establishment of the SBI Investment Fund Convertible Loan Facility in February 2016. This facility provided Immuron with the short to medium-term cash flow requirements it needed to ensure the Company’s momentum surrounding its pipeline research programs was not diminished. In comparison, there were no Finance Costs incurred in fiscal 2015.
Marketing and Promotion. Marketing and Promotion expenses increased by AUD$0.18 million from fiscal 2015 to fiscal 2016 as the Company increased its promotional efforts of its existing flagship consumer product Travelan. These increased costs included costs associated with the expansion of the product’s reach via a launch in both the US and China consumer markets. We hope to see the benefits of these expansions come to fruition during calendar year 2017 as these groundwork costs incurred mature into increased revenue in these large new markets.
Research and Development. Research and Development expense increased by AUD$0.61 million from fiscal 2015 to fiscal 2016, primarily due to the significant increase in the Company’s Phase 2 NASH clinical trial, together with the advancement of its other early pipeline products.
During fiscal year 2016, Immuron brought the management of its Phase 2 NASH clinical trial program in-house through the appointment of Medical Director another clinical trial support staff member, thereby alleviating the need for some of the external outsourced management whilst also providing Immuron with greater control over the program.
Whilst costs were reduced by bringing the management of the trial in-house, the ramping up of this trial from development to extensive patient recruitment and testing, began to see the majority of the costs of the trial coming through causing an expected increase in the overall research and development expenditures of the Company.
Travel and Entertainment. Travel and Entertainment expense increased by AUD$0.3 million from fiscal 2015 to fiscal 2016 as the Company’s activities expanded into the US through the appointment of a U.S.-based Chief Executive Officer and US Sales Director, as well as an Israeli-based Medical Director. These overseas appointments were critical for the Company to begin its expansion into the U.S. capital markets through the initial OTCQB listing, and now into the NASDAQ as part of this offering, and into the product markets for Travelan’s expansion into the US. Having an Israeli-based Medical Director also brought us within close proximity to our major-research collaboration partner Hadasit Hospital in Israel.
Loss for the period. As a result of the foregoing, our loss for the period after income tax benefit increased by AUD$4.38 million, or 163%, from AUD$2.69 million in fiscal 2015 to AUD$7.07 million in fiscal 2016.
Given our, and our subsidiaries’ history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we, or our subsidiaries, will generate sufficient future taxable income against which we can utilized these unused tax losses and any uncalculated potential deferred tax assets, together with any other temporary differences. Should the need arise, the Company can, and will, revisit this position.
Comparison of the fiscal years ended June 30, 2015 and 2014
Revenue and Other income
| | | For the fiscal year ended
June 30, | | | Increase/ | |
| | | 2015 | | | 2014 | | | Decrease | |
| Revenue: | | | | | | | | | | | | |
| Sale of goods | | AUD$ | 1,002,380 | | | AUD$ | 981,051 | | | AUD$ | 21,329 | |
| | | | | | | | | | | | | |
| Other income: | | | | | | | | | | | | |
| Australian Federal R&D Tax Concession Refund | | | 1,478,581 | | | | 713,632 | | | | 764,949 | |
| Interest income | | | 112,440 | | | | 88,345 | | | | 24,095 | |
| Other | | | - | | | | 2,500 | | | | (2,500 | ) |
| Total Revenue and Other income | | AUD$ | 2,593,401 | | | AUD$ | 1,785,528 | | | AUD$ | 807,873 | |
Sale of Goods. Revenues received from the sale of goods increased by AUD$0.02 million, from AUD$981 thousand in fiscal 2014 to AUD$1 million in fiscal 2015. Fiscal year 2013 and into 2014 were proving to be a slow sales years for the Company’s flagship product Travelan as Immuron’s licensee selling Travlean went through a period of internal restructuring. Accordingly, in June 2013 Immuron terminated the license agreement with the third party and commenced a new direct-to-wholesaler sales strategy in Australia and New Zealand which achieved immediate results in fiscal years 2014 and 2015.
Australian Federal R&D Tax Concession Refund The Company’s R&D tax concession refund more than doubled from AUD$714 thousand in fiscal year 2014, to AUD$1.48 million in fiscal 2015. This significant increase in the R&D Tax Concession refund was the direct result of the significant increase in research and development expenditures as the Company commenced its major Phase 2 NASH clinical trial program.
Interest IncomeInterest income increased marginally from AUD$88 thousand in fiscal year 2014 to AUD$0.1 million in fiscal 2015 as the company increased its cash reserves during 2015 through the completion of a capital consolidation followed by a AUD$9.66 million (before costs) Rights Issue capital raising. The higher cash reserves held by the Company, following such a significant rights issue capital raising, generated an increase in the interest revenue paid to Immuron for the cash reserves.
Cost of Goods Sold, Gross Profit and Direct Selling Costs
| | | For the fiscal year ended
June 30, | | | Increase/ | |
| | | 2015 | | | 2014 | | | Decrease | |
| | | | | | | | | | |
| Total Operating Revenue | | AUD$ | 1,002,380 | | | AUD$ | 981,051 | | | AUD$ | 21,329 | |
| Cost of Goods Sold | | | (316,128 | ) | | | (277,928 | ) | | | (38,200 | ) |
| Gross Profit | | AUD$ | 686,252 | | | AUD$ | 703,123 | | | AUD$ | (16,871 | ) |
| Less Direct Selling Costs: | | | | | | | | | | | | |
| Sales and Marketing Costs | | | (76,794 | ) | | | (79,796 | ) | | | 3,002 | |
| Freight Costs | | | (116,379 | ) | | | (114,278 | ) | | | (2,101 | ) |
| Total Gross Profit less Direct Selling Costs | | AUD$ | 493,079 | | | AUD$ | 509,049 | | | AUD$ | (15,970 | ) |
Following its shift to a direct-to-consumer wholesale sales model in early fiscal year 2014, Immruon’s Gross Profit ratio increased from just 41% in fiscal year 2013, to 72% in fiscal year 2014, and 68% in fiscal year 2015 respectively.
This improved Gross Profit margin was a combination of a shift to the direct-to-consumer wholesale sales model, and also the establishment of a relationship with a major new colostrum supplier in New Zealand who provided certainty not only to the level of colostrum powder they could manufacture for Immuron’s flagship product Travelan, but also the quality GMP processes the new supplier were able to offer.
The variances in Sales and Marketing and Freight costs between fiscal year 2014 and 2015 were negligible.
Expenses
| | | For the fiscal year ended
June 30, | | | Increase/ | |
| | | 2015
AUD$ | | | 2014
AUD$ | | | Decrease
AUD$ | |
| Expenses: | | | | | | | | | | | | |
| Amortization | | | - | | | | (680,587 | ) | | | 680,587 | |
| Consulting, Employee and Director | | | (728,140 | ) | | | (555,487 | ) | | | (172,653 | ) |
| Corporate Administration | | | (557,422 | ) | | | (492,465 | ) | | | (64,957 | ) |
| Depreciation | | | (3,719 | ) | | | (3,989 | ) | | | 270 | |
| Finance Costs | | | - | | | | (463,685 | ) | | | 463,685 | |
| Impairment of Inventory | | | (35,340 | ) | | | (50,204 | ) | | | 14,864 | |
| Marketing and Promotion | | | (304,687 | ) | | | (235,176 | ) | | | (69,511 | ) |
| Research and Development | | | (3,018,294 | ) | | | (1,289,675 | ) | | | (1,728,619 | ) |
| Travel and Entertainment | | | (128,318 | ) | | | (37,327 | ) | | | (90,991 | ) |
| Total expenses | | AUD$ | (4,775,920 | ) | | AUD$ | (3,808,595 | ) | | AUD$ | (967,325 | ) |
Amortization. In fiscal year 2014 the Company expensed the remaining amortization of the intellectual property it acquired through its platform technologies from Hadasit Hospital, Israel in 2009. At the end of fiscal year 2012, the estimated useful life of the intellectual property was reviewed, in accordance with the application of accounting standards, and it was determined that the Intellectual Property had a finite remaining useful life of two years. Accordingly, there was no amortization expense in any subsequent fiscal years after 2014 as the intellectual property asset had been fully provided for.
Consulting, Employee and Director. Consulting, Employee and Director expense increased by AUD$173 thousand from fiscal 2014 to fiscal 2015 as the Company employed some additional staff and consultants to assist in the areas of manufacturing and research and development ahead of the Phase 2 clinical trial commencement.
Corporate Administration. Corporate Administration expense increased by AUD$0.06 million from fiscal 2014 to fiscal 2015 due to the general increase in the size of the business in combination with an increase in consequent expenses due to additional resources being implemented to assist the company for its growth.
The increase was also the result of an increase in a number of back-office support costs, consultant and advisory fees associated with the planning and implementation of the Company’s clinical trials and other research and development programs. Additional expenses were also incurred through the necessary legal and compliance costs surrounding the capital restructuring and subsequent significant capital raising program.
Finance Costs. Following the company significant rights issue capital raising in February 2014 (fiscal year 2014) the Company was able to extinguish its outstanding convertible note loan liability due to Paladin which in turn removed any further finance expense from being incurred in fiscal year 2014 and no financing costs being incurred in fiscal year 2015.
Marketing and Promotion. Marketing and Promotion expenses increased by AUD$70 thousand from fiscal 2014 to fiscal 2015 as the Company marginally extended some of the marketing campaigns surrounding the Company’s flagship consumer product Travelan.
Research and Development. Research and Development expenses more than doubled between fiscal years 2014 and 2015 from AUD$1.3 million to AUD$3 million as the Company began the clinical trial program commencement for the Company’s Phase 2 NASH clinical trial, together with the advancement of its other early pipeline products. The research and development program leader, together with the other members of their team, were all contracted advisors.
Travel and Entertainment. Travel and Entertainment expense increased from AUD$37 thousand in fiscal 2014 to AUD$0.128 million in fiscal 2015 as the Company promoted the commencement of the trial overseas, and also travels to establish new overseas markets for Travelan from which Canadian, Korean, and Chinese opportunities arose.
Loss for the period. As a result of the above areas, our loss for the period after income tax benefit increased by almost AUD$0.2 million, to AUD$2.7 million. Whilst the increase has an overall negative affect on the Company, the change in direction of the Company throughout fiscal year 2015 resulted in a stronger balance sheet, with increased cash reserves, no convertible note debt, and also the completion of any ongoing amortization expenditures. This overall resulted in a much stronger, recapitalized and re-focused Company by the end of fiscal year 2015.
Given our, and our subsidiaries’ history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we, or our subsidiaries, will generate sufficient future taxable income against which we can utilized these unused tax losses and any uncalculated potential deferred tax assets, together with any other temporary differences. Should the need arise, the Company can, and will, revisit this position.
Liquidity and Capital Resources
We have incurred cumulative losses and negative cash flows from operations since the Company’s inception in 1994, and as of June 30, 2016 we had accumulated losses of AUD$42.8 million. We anticipate for the foreseeable future that we will continue to incur losses for at least the next several years. We expect that as we continue research efforts and the development of our product candidates, hire additional staff, including clinical, scientific, operational, financial and management personnel, and incur additional costs associated with being both an Australian and NASDAQ public company and, as a result, we will need additional capital to fund our operations, which we may raise through a combination of equity offerings, debt financings, other third-party funding and other collaborations, strategic alliances and licensing arrangements.
We plan to continue to fund losses from operations and capital funding needs through future debt and equity financing, as well as potential additional collaborations or strategic partnerships with other companies or through non-dilutive financings. The sale of additional equity or convertible debt could result in additional dilution to our stockholders. The incurrence of indebtedness would result in debt service obligations and could result in operating and financing covenants that would restrict our operations. We can provide no assurance that financing will be available in the amounts we need or on terms acceptable to us, if at all. I f we are not able to secure adequate additional funding we may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, and/or suspend or curtail planned programs. Any of these actions could materially harm our business
We have had nil and AUD$1.90 million of net borrowings/repayments in fiscal 2015 and fiscal 2016 respectively, and do not currently have any credit facilities in place.
As of June 30, 2016, we had cash and cash equivalents of AUD$2.29 million. Additionally, the Company also recognized a total of AUD$4,387,772 in receivables, including a AUD$1,512,840 related to R&D Tax Concession, which was received in November 2016. On this basis, even though the company has been in loss making position historically, management is satisfied that the Group is a going concern and are of the opinion that no asset is likely to be realized for an amount lower than the amount at which it is recorded in the Consolidated Statement of Financial Position at June 30, 2016.
We estimate that our net proceeds from this offering will be approximately AUD$ million after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Along with our existing cash and cash equivalents of AUD$2.3 million as of June 30, 2016, we expect that the net proceeds from this offering will be sufficient to fund our capital requirements for at least 24 months from the issuance date of the financial statements.
Cash flows
The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
| | | For the year ended
June 30, | | | For the six months ended December 31, | |
| | | 2016 | | | 2015 | | | 2014 | | | | 2016 | | | | 2015 | |
| | | | | | | | | | | | | | | | | | |
| Net cash used in operating activities | | AUD$ | (5,158,336 | ) | | AUD$ | (3,020,933 | ) | | AUD$ | (2,650,577 | ) | | AUD$ | (2,256,539 | ) | | AUD$ | (3,454,246 | ) |
| Net cash used in investing activities | | | (2,441 | ) | | | (3,168 | ) | | | (15,901 | ) | | | (1,879 | ) | | | (2,441 | ) |
| Net cash provided by (used in) financing activities | | | 4,335,342 | | | | (1,614 | ) | | | 7,361,555 | | | | 3,071,315 | | | | 1,334,764 | |
Comparison of the six months ended December 31, 2016 and 2015
Operating activities. For the six months ended December 31, 2016 and 2015, net cash used in operating activities decreased by AUD$1.20 million from AUD$3.45 million to AUD$2.26 million respectively. The use of net cash in all periods resulted from our ordinary business operations. Cash flows from operating activities for the six months ended December, 2016 and 2015 also included inflows of AUD$1.59 million and AUD$ nil, respectively in relation to refunds received through the Australian Federal Government’s Research and Development Income Tax Incentive program for eligible expenditure.
Investing activities. Net cash used in investing activities in the six months ended December, 2016 and 2015 were AUD$1,879 and AUD$2,441, which solely pertains to purchases of office equipment.
Financing activities. For the six months ended December 31, 2016, net cash provided by financing activities was AUD$3.07 million, which comprised of (i) proceeds from issue of securities and exercise of options of AUD$4.27 million, net of capital raising costs, (ii) repayments of borrowing principal of AUD$1.20 million. For the six months ended December 31, 2015, net cash provided by financing activities of AUD$1.33 million comprised of (i) proceeds from issue of securities and exercise of options of AUD$0.33 million and (ii) proceeds from borrowings of AUD$1 million.
Comparison of the fiscal years ended June 30, 2016 and 2015
Operating activities. For the twelve months ended June 30, 2016 and 2015, net cash used in operating activities increased by AUD$2.14 million from AUD$3.02 million to AUD$5.16 million respectively. The use of net cash in all periods resulted from our ordinary business operations. Cash flows from operating activities for the year ended 2016 and 2015 also included inflows of AUD$1.47 million and AUD$0.72 million, respectively in relation to refunds received through the Australian Federal Government’s Research and Development Income Tax Incentive program for eligible expenditure. As discussed earlier, the major increase of net cash outflows surrounding Operating Activities, results from the significant increase in the costs associated with the Company’s research and development programs, as well as the Company’s overall general internal expansion and shift to overseas markets.
Investing activities. Net cash used in investing activities in fiscal 2016 and 2015 was AUD$2,441 and AUD$3,168, which solely pertains to purchases of office equipment.
Financing activities. For the twelve months ended June 30, 2016, net cash provided by financing activities was AUD$4.34 million, which comprised of (i) proceeds from issue of securities and exercise of options of AUD$2.46 million, net of capital raising costs, (ii) proceeds from the issuance of convertible notes pertaining to the convertible loan funding arrangement established in February 2016, and other borrowings of AUD$2.95 million less repayments of AUD$1.08 million related to these borrowings. In the fiscal year ended June 30, 2015, net cash used in financing activities of AUD$1,614 was related to the payment of minor subsequent capital raising costs.
Comparison of the fiscal years ended June 30, 2015 and 2014
Operating activities. For the twelve months ended June 30, 2015 and 2014, net cash used in operating activities increased by AUD$370 thousand from AUD$2.65 million to AUD$3 million respectively. The use of net cash in all periods resulted from our ordinary business operations. The significant increase of AUD$0.86 million in receipts from customers from fiscal year 2014 to fiscal year 2015 was the direct result of moving Immuron's sales strategy from a license agreement for selling Travelan through a third party, to a direct to wholesaler consumer distribution model.
This significant increase in receipts from customers between fiscal years 2014 to 2015 from increased sales allowed Immuron, combined with the large capital raising the company performed in February 2014 which gave the company the confidence to increase its expenditures associated with the Company’s research and development programs as it commenced the major clinical trial program for which the funds from the February raising were applied.
Cash inflows in operating activities included inflows in fiscal year 2014 and 2015 of AUD$0.67 million and AUD$0.72 million in relation to refunds received through the Australian Federal Government’s Research and Development Income Tax Incentive program for eligible expenditure. Interest received also increased between the periods by AUD$24 thousand following receipt of funds from the February 2014 major rights issue capital raising being invested in cash term deposits.
Investing activities. The variance in the difference of cash flows used in investing activities in fiscal years 2014 and 2015 were negligible and solely pertains to purchases of office equipment.
Financing activities. There were negligible net financing cash flows in fiscal year 2015 as Immuron had performed its significant rights issue capital raising during fiscal year 2014 resulting in a total net cash inflow of AUD$7.4M. The funds raised from the capital raising were also supported from a short-term loan the Company received. This inflows were off-set by the repayment of this short-term loan, and also repayment of the convertible loan totaling AUD$1.9 million.
Operating capital requirements
In the future, we expect our revenue streams will be generated mostly through a combination of sales from our flagship consumer product Travelan as the US and China expansion plans come to fruition, and through opportunities which arise from the maturing of our pipeline portfolio products through collaboration, license, partnership or sale with major pharmaceutical or investment companies.
We have based our projections of operating capital requirements on assumptions, that may prove to be incorrect, and we may use all of our available capital resources sooner than we expect.
Our future funding requirements will depend on many factors, including, but not limited to:
| | • | the timing and costs of our planned clinical trials for our product candidates; |
| | | |
| | • | the timing and costs of our planned preclinical studies for our product candidates; |
| | | |
| | • | the number and characteristics of product candidates that we pursue; |
| | | |
| | • | the outcome, timing and costs of seeking regulatory approvals; |
| | | |
| | • | revenue received from commercial sales of any of our product candidates that may receive regulatory approval; |
| | | |
| | • | the terms and timing of any future collaborations, licensing, consulting or other arrangements that we may establish; |
| | | |
| | • | the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| | | |
| | • | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| | | |
| | • | the extent to which we need to in-license or acquire other products and technologies. |
Off-balance sheet arrangements
We did not have over the past three fiscal years, and we currently do not have, any off-balance sheet arrangements as defined in the rules and regulations of the Securities and Exchange Commission. To the extent we have any contingent assets or liabilities, these have been captured and audited within the accompanying consolidated financial statements.
Quantitative and qualitative disclosures about market risks
We are exposed to market risk related to changes in interest rates and exchange rates.
As of December 31, 2016, we had cash and cash equivalents of AUD$3.1M, primarily held in bank accounts and term deposits. Our primary exposure to market risk is interest rate sensitivity, which is affected primarily by changes in the general level of Australian interest rates. The Company is exposed to interest rate risks via the cash and cash equivalents and borrowings that it holds. Interest rate risk is the risk that a financial instruments value will fluctuate as a result of changes in market interest rates.
We are exposed to fluctuations in foreign currencies that arise from foreign currencies held in bank accounts and the translation of results from our operations outside Australia. Our foreign exchange exposure is primarily the U.S. dollar and New Zealand dollar. Foreign currency risks arising from commitments in foreign currencies are managed by holding cash in that currency. Foreign currency translation risk is not hedged.
BUSINESS
Overview
We are a clinical-stage biopharmaceutical company focused on the development and commercialization of a novel class of immunomodulator polyclonal antibodies to treat liver diseases, infectious diseases and other immune-mediated diseases, such as colitis. Our lead product candidate, IMM-124E, is a proprietary immunomodulator agent targeted at GI immune mediated diseases including fatty-liver diseases. We are developing IMM-124E for the treatment of nonalcoholic steatohepatitis, or NASH, for which we are currently in Phase 2. IMM-124E is also the investigational drug of two NIH-sponsored Phase 2 clinical trials in alcoholic steatohepatitis (ASH) and Pediatric NASH. Dr. Arun Sanyal, one of NASH’s foremost thought leaders, is the principal investigator of our NASH Phase 2 trial.
IMM-124E is a first in class oral, LPS antibody, with strong anti-inflammatory and anti-fibrotic properties, making NASH an ideal target for this compound. IMM-124E binds to the LPS receptors of gram-negative bacteria and influence the cell-mediated immune system through regulatory T cell populations, creating a downstream decrease of liver inflammation.
NASH is a severe type of nonalcoholic fatty liver disease (NAFLD). NAFLD is the most common liver disease and is associated with obesity and type-2 diabetes, and is characterized by the accumulation of fat in the liver with no other apparent causes. Approximately 10%-20% of people with NAFLD will progress to NASH. Current estimates place NASH prevalence at approximately 24 million people in the United States, or 7% of the population, with similar prevalence in other major developed markets.
There are currently no treatment approved for NASH and other compounds in development target primarily one biological pathway believed to impact NASH. However, NASH is now increasingly recognized as a multi-factorial disease, creating a unique opportunity for IMM-124E given our broad and upstream anti-inflammatory properties.
Our second lead compound, IMM-529, targets the C.difficile bacterium and contains polyclonal antibodies to the Toxin B, the spores and the vegetative cells. We recently successfully completed our pre-clinical program, and we plan to initiate a Phase 1/2 clinical trial in Israel in the second quarter of 2017. As the clinical trial will be conducted outside of the United States, specifically, in Israel, the Company is not required to apply for an IND.. IMM-529 was developed and tested extensively in pre-clinical models at Monash University, Australia in collaboration with Dr. Dena Lyras, one of the world’s foremost expert in C.difficile. Although the Company entered into an agreement with Monash University with respect to the development and pre-clinical testing of IMM-529, the agreement expired in February 2016, and the Company currently has no collaboration agreements with either Dr. Lyras or Monash University.
Clostribdiumdifficile, or C.difficile, is a gram-positive, toxin-producing, spore-forming bacterium that generally causes severe and persistent diarrhea in infected individuals, but can also lead to more severe outcomes, including in the most serious cases, death. C.difficileinfection (CDI) is most often associated with the prior use of antibiotics. The U.S. Centers for Disease Control has identified CDI as one of the top three most urgent antibiotic-resistant bacterial threats in the United States. It is the most common cause of hospital acquired infection in the United States and has overtaken methicillin-resistant Staphylococcus aureus in prevalence. CDI is responsible for the death of approximately 29,000 Americans each year.
We also market an OTC product, Travelan, in Australia, Canada (with our partner Paladin Labs, a branch of Endo Pharmaceuticals that sells products for the Canadian market) and in the U.S., for the prevention of Traveler’s Diarrhea. Travelan has been shown to be up to 90% effective in the prevention of diarrhea in several E-coli challenge placebo controlled studies. Travelan is based in the same platform and targets 13 strains of E-coli. Travelan sales for FY2016 were AUD$1.0M.
In addition to these two programs, we are also targeting other anti-infectious and anti-inflammation diseases such as shigella, campylobacter and colitis. These early programs are pursued in cooperation with some of the leading research institutions in the world including the U.S. Army, U.S. Navy and Zurich University.
Below is our clinical and pre-clinical pipeline. Of note, Immuron has successfully put together one of the most comprehensive portfolio of fatty-liver disease programs in the industry, with three Phase 2 clinical trials including NASH, ASH and Pediatric NASH.
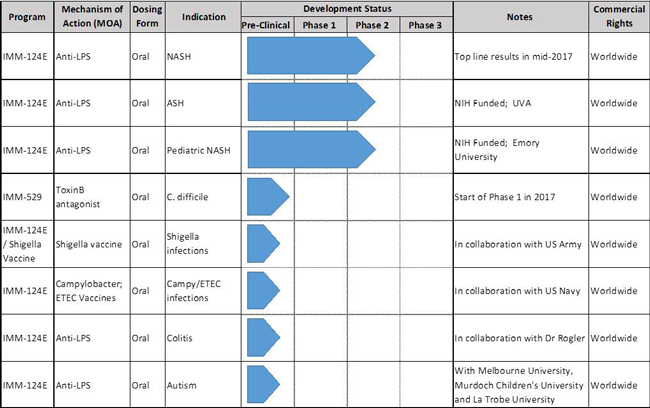
Our Strategy
Our goal is to become one of the leading biopharmaceutical company developing and commercializing therapeutics to address increased unmet medical needs in inflammation-mediated diseases and anti-infectives. The critical components of our strategy include:
| · | Rapidly advance our two lead oral polyclonal antibodies, IMM-124E and IMM-529: |
| - | IMM-124E/NASH:Continue progressing our IMM-124E Phase 2 for the treatment of NASH with a target for top line read-out of mid-2017; |
| | - | IMM-529/CDI:Finalize development of clinical supplies Phase 1/2 protocol with a target Phase 1/2 start in the second quarter of 2017; |
| · | Leverage our technology platform and our collaborations to expand our differentiated polyclonal-based product pipeline across multiple indications including ASH, Pediatric NASH and various novel and potentially game-changing anti-infective programs with the DoD (U.S. Army and U.S. Navy) |
| · | Partner our fatty-liver programsat the right time and with the right commercial / development partner(s) for NASH, ASH and pediatric NASH |
| · | Continue investing in and growing Travelan Worldwide including in the U.S., Australia, Canada and China, and in new markets |
| · | Continue investing in mechanism of action studies that expands our understanding of our unique MOA across our targeted diseases and conditions, and potentially identify new opportunities for investment |
| · | Protect and leverage our intellectual property portfolio and patents.We believe that our intellectual property protection strategy, grounded in securing composition of matter patents on the biologics we develop, as well as broader patents to protect our technology platform, has best positioned us to gain broad and strong protection of our assets. We have 13 issued patents and 23 pending patent applications worldwide. We have been issued patents in the U.S., Australia, Canada, India, Japan and New Zealand. |
Fatty-Liver Diseases Overview
NASH is a severe type of nonalcoholic fatty liver disease (NAFLD) and is characterized by the accumulation of fat in the liver with no other apparent causes. The rising prevalence of obesity-related disorders has contributed to a rapid rise in the prevalence of NASH and NAFLD. In the United States, NAFLD affects approximately 27%-34% of the population, or an estimated 86 million to 108 million people. Approximately 10%-20% of people with NAFLD will progress to NASH. Current estimates place NASH prevalence at approximately 24 million people in the United States, or approximately 7% of the population, with similar prevalence in other major developed markets. Prevalence is also rising in developing regions, likely due to the adoption of a more sedentary lifestyle and westernized diet consisting of processed food with high fat and fructose content.
NASH is a progressive disease that displays an increasing burden of liver fibrosis as the disease gets progressively worse. It is estimated that 63% of all NASH patients, or approximately 15 million people, have either no scaring of the liver (F0) or present with evidence of mild fibrosis (F1). The other 37%, or approximately 9M people, will present with either moderate (F2) or severe fibrosis (F3).
The high level of investment activity in the space, including licensing and M&A (collectively, “LM&A”), is indicative of the high level of unmet need. This is driven by a few factors including the size of the population that might need interventional agents, the increasing recognition that NASH is a severe disease that needs to be treated and the belief that because NASH is a multi-factorial disease, there will be room for multiple therapies to offer choices to physicians and patients. An often-quoted analysts report by Deutche Bank estimate that the NASH market will be $35B by 2025. This is not unreasonable given that the statin branded market peaked at nearly $30B worldwide and span multiple blockbuster drugs.
Since 2014, there have been multiple LM&A transactions and we expect this LM&A trend to continue in 2017 and beyond, especially as the increasing rates of obesity and type-2 diabetes around the world continue driving the prevalence of NASH.
| · | In 2014, Shire acquired Lumena Pharmaceuticals for $260 million. Lumena had two Phase 2 assets for NASH and cholestatic liver disease |
| · | In 2015, Boehringer Ingelheim acquired Pharmaxis Limited’s NASH asset in Phase 1 for AUD$39 million. Total potential deal value of AUD$600 million including milestones. |
| · | In 2015, Gilead acquired Phenex Pharmaceutical’s NASH asset in Phase 2 for $470 million. Undisclosed upfront fees and milestones payments. |
| · | In 2016, Allergan acquired Tobira Therapeutics for approximately $330 million and up to $1.7 billion in total payments. |
| · | In 2016, Allergan licensed Akarna Therapeutics’ pre-clinical NASH asset for approximately $50 million upfront plus other undisclosed milestones payments. |
| · | In 2016, BMS acquired the worldwide rights to Nitto Denko’s NASH asset, ND-L02-s0201, for $100 million upfront plus additional undisclosed clinical and regulatory milestone payments, royalties, sales-based milestone payments as well as option exercise payments for other indications. |
| · | In 2016, Novartis announced that it paid $50 million upfront plus undisclosed milestones and other payments for the exclusive rights to Conatus Pharmaceuticals’ Emricasan, a Phase 2 pan-caspase inhibitor for the treatment of NASH with advanced fibrosis scarring and cirrhosis. |
| · | In 2017, JNJ entered into a collaboration and option agreement with Bird Rock Bio which is evaluating a Cannabinoid receptor 1 (CB1)-targeting antibody, namacizumab, which is in Phase 1 clinical trial. JNJ will collaborate with Bird Rock Bio during the trial and has the exclusive right to acquire the Bird Rock Bio following the Phase 1 data readout. Specific terms of the transaction were not disclosed. |
Pathophysiology of NASH
NAFLD/NASH is a disease that can evolve over time as the liver is subjected to an increasing amount of injury, which deepens liver inflammation and fibrosis, and can eventually lead to end-stage liver failure and liver cancer.
Inflammation plays a key role in the pathogenesis of NASH as conditions linked to the metabolic syndrome, including obesity, are all associated with an elevated state of chronic inflammation that cause damage to organs such as the pancreas and the liver. The pathogenesis is thought to be multi-factorial, and is a multiple-hit process involving insulin resistance, oxidative stress, apoptosis, and adipokines brought on by fatty diet, obesity, sedentary lifestyle and genetic pre-disposition.
In addition to the elevated state of inflammation suffered by NASH patients which perpetuates liver injury, it has also been shown that fatty diets, sugar and obesity are linked to an overgrowth of gram-negative bacteria within the gut. These gram-bacteria produce LPS (LipoPolySaccharides) products that elicit strong innate and cell-mediated immune responses in animals and humans, both from within the gut and through circulating endotoxins, particularly via Toll-like Receptor 4 on cells. The intraluminal LPS concentration is additionally thought to increase gut permeability, also known as "leaky gut", enabling passage of endotoxins into the bloodstream and increasing the inflammatory response especially within the liver since 75% of the liver’s blood supply comes from the portal vein.
The importance of this LPS-driven inflammatory process is unfortunately often overlooked since there are no therapeutics that can effectively block gram-negative bacteria in the gut, except for broad-spectrum antibiotics which are not an option for long-term use in NASH patients.
The immune and inflammatory response to liver cell damage caused by these insults is mediated through a well-described signaling network of liver and immune cells. Kupffer cells, also known as resident liver macrophages, sense tissue injury and are the first responders to liver cell damage. Activated Kupffer cells initiate an inflammatory response to the liver injury and can activate HSCs (Hematopoietic Stem Cells) to transdifferentiate into myofibroblasts, the primary collagen-producing cell type responsible for liver fibrosis. The extent of this fibrosis can vary, and it is described in several stages. A normal liver is at a stage between F0 and F1. Stage F2 denotes light fibrosis, and F3 is severe fibrosis. Cirrhosis is defined from stage F4, when scar tissue exists throughout the liver.
Pediatric NASH is also a growing concern in many countries, and similar to NASH, Pediatric NASH is a progressive form of liver disease associated with excessive fat storage in the liver together with inflammation, which can then lead to liver fibrosis and cirrhosis. Pediatric NASH is believed to affect up to 5% - 10% of the US pediatric population. A U.S. landmark study that examined the incidence of disease in 742 autopsy children who had died of an accident, found that 17.3% of the children aged 15 to 19 years had NAFLD. There are currently no approved drug therapies for pediatric NASH.
ASH is one of the hepatitis manifestation of alcohol abuse and typically occurs in an individual with long-standing history of alcohol intake. As in NASH, inflammation plays a key part in the development and worsening of ASH. More than 90% of heavy drinkers have steatosis, 10% to 35% have ASH, and 8% to 20% have alcoholic cirrhosis. While the consumption of alcohol is certainly a driving factor, especially if intake is high, other factors can contribute to the development of ASH in these patients, including diet, age and ethnicity. It is estimated that the prevalence of alcoholism in the U.S. is 8% of the U.S. population, or more than 15 million people. It is thought that at least 20% of patients with alcoholism have ASH or 3 million people in the U.S. alone.
IMM-124E for the treatment of fatty-liver diseases
IMM-124E, which is made of LPS polyclonal antibodies, is manufactured from colostrum which is harvested from dairy cows that have been immunized against bacterial LPS of the most common strains of Enterotoxigenic Escherichia coli (ETEC). Such inoculation activates a generalized immune response in the host animal to produce antibody clones which recognize and bind with the bacterial cell-surface epitopes presented. These antibodies are present in high concentration within our raw material.
IMM-124E contains at least 40% immunoglobulins (Ig), composed mainly of IgG (mostly IgG1), some IgA with small concentrations of IgM and IgE. Our studies have shown that these antibodies bind to bacterial LPS specific sites, as per the method by which they were designed and produced. It had been additionally demonstrated that these antibodies cross react with other types of bacteria such as shigella and salmonella.
The pre-clinical evidence gathered so far supports IMM-124E’s MOA as well the Company's position that IMM-124E is a unique investigational therapeutic for fatty-liver diseases including NASH, ASH and Pediatric NASH. This has been shown given this agent's anti-fibrotic and anti-inflammatory properties.
Pre-Clinical and Clinical Studies
An IND was initially filed for the commencement of clinical trials for IMM-124E on November 11, 2011 and was sponsored by the Company. The subject of the IND was protocol number IMM-124E-2001, a Phase 2, randomized, double-blind, placebo-controlled study of IMM-124E for patients with NASH.
Oral administration of IMM-124E has been tested in a Carbon-tetrachloride (CCl4) fibrosis model and in a NASH ob/ob metabolic model. Results of this pre-clinical study demonstrate that IMM-124E has strong anti-fibrotic and anti-inflammatory effects on the liver and is associated with multiple biomarkers showing a similar response. IMM-124E had also been tested in a Phase 1 study of 10 (ten) biopsy-proven NASH and diabetes patients, conducted by investigators at Hadassah Medical Center, Jerusalem, Israel.
Powerful Anti-Fibrotic and Anti-Inflammatory Effect Shown in CCl4 Mice Models
IMM-124E was tested in a Carbon-Tetrachloride (CCl4) mouse model to determine the efficacy of orally administered IMM-124E to prevent hepatic inflammation and. 4 groups of mice (n = 6/8 per group) were utilized for the study as follow:
| - | Group A: (Positive Control) Intraperitoneal (IP) CCl4 |
| | - | Group B: IMM-124E (negative control) administered to naïve mice to assess the safety of IMM-124E in "healthy" mice |
| | - | Group C: IP CCl4 + IMM-124E: IMM-124E initiated in proximity to the CCl4 insult. |
| | - | Group D: IP CCl4 + IMM-124E (IMM-124E was administered two weeks before initiation of CCl4). It is customary to assess the effectiveness of investigational treatments to prevent the disease, especially in acute models such as the one presented. |
The IMM-124E treatment group (Group C) demonstrated the following when compared to Control Group (Group A):
| | - | Statistically significantly (p<0.05 and p<0.03) reduction and near-normal Alanine aminotransferase (“ALT”) and Aspartate aminotransferase (“AST”) respectively at days 21 and 30 post insult. ALT is an enzyme found in various body tissues, and elevated serum levels of ALT suggest liver injury. AST is an enzyme found in various body tissues, and elevated serum levels of AST, most commonly together with serum ALT, suggest liver injury. |
| | - | Statistically significant (p<0.0001) reduction in serum bilirubin levels compared to positive control group (Group A). Bilirubin is a compound used to process waste products by the liver, and elevated serum levels of Bilirubin suggest liver injury. |
| | - | Statistically significant (P<0.0009) in decreased METAVIR Score and reduced inflammation on liver histology. METAVIR Score is a standardized scoring system used to describe the level of liver fibrosis and inflammation found within a given liver biopsy. Fibrosis is scored on a range from 0 to 4 and level of inflammation is scored on a range from 0 to 3. An increased METAVIR Score describes a more advanced and active liver disease. |
| | - | Liver weight of treated mice, suggesting of level of fibrosis, was noted as significantly lower higher than Group A. Spleen weight of treated mice was significantly lower than Group A suggesting spleens were less enlarged in this group (spleen enlargement is associated with cirrhosis). |
| - | Reduction in activated Macrophages F4/80highon liver tissue FACS analysis and Immunohistochemistry (IHC) staining. |
The p-value is the probability that the results achieved would be false. It is customary, in statistical terms and in discussions with the FDA, to define p lower than 0.05 as sign of acceptable evidence, also known as "significance”.
Kuppfer Cells 4/80 Flow Cytometry and IHC
A representative macroscopic views of the livers from Group A (Positive Control) demonstrates the widespread fibrotic liver associated with this chemical insult, while the liver from the IMM-124E treated group (Group C) shows a normal liver, demonstrating the protective effect of IMM-124E even with the most aggressive of fibrosis models.
Additionally, when comparing the treated Group A to the negative control B, no statistically significant differences were noted apart from the histological fibrosis score (METAVIR) and histological inflammation. This proves the treated mice were near normal despite the insult.
Furthermore, Group D did not show differentiation from Group A in any of the labs or macroscopic parameters (spleen/liver weight) suggesting the insult did propagate an extensive necroinflammatory response. However, this group did demonstrate a statistically significant lower METAVIR score when compared to Group A.
The effect of this anti-fibrotic effect is evident both macroscopically as well as histology and on liver associated labs all pointing to the potential mechanism by which fibrosis is halted.
Ob/Ob Mice Models Show Significant and Sustained Anti-Inflammatory and Anti-Metabolic Effects
We also conducted a study using ob/ob mice, which are a well-accepted mouse model for the metabolic syndrome showing insulin resistance, dyslipidemia, liver steatosis and elevated liver enzymes.
4 groups of ob/obmice were fed for 6 weeks with either IMM-124E or immunoglobulins purified from IMM-124E (3 doses). A positive Control was also established in parallel to the treatment groups. Mice were evaluated for their liver injury (serum ALT) metabolic state and immunological / inflammatory response using Flow cytometry to determine alterations in regulatory T-cell (Treg) and cytokines. Mice were also followed for liver enzymes, glucose levels, glucose tolerance test (GTT), hepatic and serum triglycerides (TGs) levels.
| · | High dose of IMM-124E derived immunoglobulins demonstrated a statistically significant reduction in serum ALT (P<0.05) and liver/serum TG levels (P<0.05 for cf ddH20 and P<0.01 for cf NormalColostrum) over controls which did not reach statistical significance; |
| · | High dose of IMM-124E derived immunoglobulins ameliorated glucose intolerance (P<0.05 cf ddH20 and P<0.01 cf Normal Colostrum); |
| · | Glucose tolerance test (GTT) was improved within this group but did not reach statistical significance (P>0.05); |
| · | IMM-124E demonstrated a similar metabolic effect, with significant reduction in hepatic (P<0.05) and serum triglycerides levels (P<0.05) and an increase in CD3+NK1.1+ cells (P<0.05 cf ddH20 and Normal Colostrum); and |
| · | IMM-124E demonstrated a similar metabolic effect, with statistically significant reduction in hepatic and serum triglycerides levels (P<0.05) and an increase in CD4-CD25-FoxP3 cells (P>0.05) – Not statistically significant. |
Phase 1/2 - IMM-124E Demonstrated Safety and Significant Anti-Metabolic Effect in Patients with Biopsy-Proven NASH and Impaired Glucose Metabolism (i.e. type II DM or Insulin resistance)
The company conducted a Phase 1/2 clinical trial to evaluate the safety and preliminary efficacy of IMM-124E in humans. This study was an open-label, single-center, 10 patient trial, conducted in subjects with impaired glucose tolerance and biopsy-proven NASH.
All patients were treated for 30 days and were monitored for their liver enzymes, glucose metabolism markers, serum lipids and metabolic associated hormonal signals such as Adiponectin and GLP-1. Inflammation associated cytokines and regulatory immune cells were evaluated as well.
The primary endpoint of the study was safety and all patients completed the study according to protocol. No treatment related adverse events were reported by the investigators for any of the clinical or laboratory parameters during the treatment and follow-up.
We also observed multiple impact on the underlying disease relevant parameters including:
| | - | All patients exhibited a clinically meaningful reduction in the hemoglobin A1c by day 30 of treatment of at least 0.5 constituting 14.8% reduction on average. Since all 10 subjects demonstrated significant change such reduction was deemed statistically significant when comparing baseline to day 30 (p<0.03). |
| | - | 7 out of 10 subjects demonstrated at least 10% reduction in one or more enzymes (AST/ALT) between the two time points (0 and 30 days) but did not reach significance. |
| | - | Five patients demonstrated lower fasting blood glucose levels at day 30. 6.3 vs. 5.8 mmol/L for days 1 and 30 respectively, however did not demonstrate statistical significance. |
| | - | A decrease in serum cholesterol and LDL levels was noted in the 9 of the 10 patients treated with IMM-214E. The mean total serum cholesterol was 5.3 μM/dl at Baseline and 4.7 μM/dl at day 30. 8 out of 10 subjects were noted to improve their serum LDL with the mean serum LDL level reduced from 3.92 μM/dl to 3.13 μM/dl at baseline and day 30, respectively. |
| | - | An increase in adiponectin and GLP-1 was recorded in 8 and 6 subjects respectively showing a serum level increase 6181 vs. 7069 ng/mL and 6.31 vs. 6.78 × 104 pM respectively, however both did not reach statistical significance. |
| | - | Peripheral blood mononuclear cells (PBMCs) were isolated from all subjects and analyzed using flow cytometry. Levels of regulatory T-cells were measured in the isolates. CD4+CD25+, CD4+CD25+HLA-DR+, CD4+CD25+FOXP3+ and CD4+CD62L+ were numerically increased in the majority of patients for all cell types, however did not reach significance. |
The combined results of these pre-clinical studies have clearly shown that IMM-124E exerts an immunomodulatory and anti-inflammatory effects resulting in metabolic and liver related biomarkers improvements, and showed strong inhibition of fibrosis. In addition, the oral administration of IMM-124E in the Phase I clinical study was reported to be well-tolerated with no treatment-related adverse events reported. We believe that the combination of the pre-clinical and the clinical studies, as well as the supporting literature linking LPS to NASH, establishes IMM-124E as a unique and potentially paradigm-changing option for NASH patients.
Global Phase 2 Clinical Trial in NASH
In November 2014, Immuron initiated its global Phase 2, multicenter, double blind placebo control, randomized clinical study with sites in the U.S.A, Australia and Israel. The study's goal is to assess the safety and effectiveness of IMM-124E for the treatment of NASH. A total of 120 biopsy-proven NASH patients are being enrolled comparing 2 doses of IMM-124E to placebo within a 6 month treatment period. The study design had been reviewed by the FDA and finalized under the agency’s recommendation. The top line results of the study are expected by mid-2017.
The trial Principal Investigator is Dr. Arun Sanyal, one of the world’s foremost leaders in NASH. Dr. Sanyal is Professor of Medicine and Former Chairman of the Division of Gastroenterology, Hepatology and Nutrition at VCU Medical Center, Virginia, U.S.A. Dr. Sanyal is an internationally renowned expert in liver diseases. He is a former President of the AASLD (American Association for the Study of Liver Diseases) and is the current Chair of the Liver Study Section at the NIH.
To date 104 subjects had been randomized into the study, at 24 active sites. We expect to complete enrollment by the end of 2016 and to have top-line data by Mid-2017.
NIH Funded Phase 2 Clinical Trials in ASH and in Pediatric NASH
In addition to the Company's phase 2 clinical study for the treatment of NASH, 2 studies evaluating IMM-124E were chosen to be funded by the American National Institute of Health (NIH):
| - | The first is a Phase 2 clinical study for the treatment of Alcoholic SteatoHepatitis (ASH),in collaboration with Dr. Arun Sanyal at Virginia Commonwealth University (VCU). The trial is a 66 patient, randomized to placebo. The study is expected to generate safety as well as preliminary efficacy data and should be completed in 2018. |
| - | The second, is also a Phase 2 clinical study for the treatment of Pediatric NASH, in collaboration with Dr. Miriam Vos at Emory University, Atlanta. This Phase 2 trial aims to enroll 40 pediatric patients for three months treatment and aims at determining the safety and exploratory efficacy of IMM-124 in Pediatric NASH patients. |
IMM-124E – Competitive Advantage
We believe that IMM-124E has a significant competitive advantage when compared to other assets in development:
| - | Multi-Factorial / Broad Anti-Inflammatory Upstream Effect – It is now increasingly acknowledged that NASH is a multi-factorial disease, and that targeting only one or two pathways is likely to only have a marginal effect on the disease. IMM-124E offers hope for long-lasting effects because of its broad upstream anti-inflammatory effects which induces the release of regulatory T-cells and anti-inflammatory cytokines while decreasing levels of pro-inflammatory cytokines. |
| | - | Attractive Profile for Long-Term Chronic Use – Because of its tolerable safety profile, which is derived from a GRAS (Generally Regarded as Safe) platform, we believe that data will support the use of IMM-124E as a chronic / long-term treatment, providing a unique advantage over other NASH therapies as some have already shown significant side effect profile (e.g., increased cholesterol). |
| | - | Potential for Use as Backbone Agent for both Early and Severe Disease – While other more toxic agents in development are likely to be confined to severe populations, we believe that IMM-124E will be able to be used in all NASH patients, including for those with mild fibrosis (F1) / no scarring (F0), and potentially in NAFLD patients as well, to reduce their elevated inflammation state. We believe that this will be unique to IMM-124E, hence potentially putting nearly 15 million of mild NASH patients within reach of IMM-124E but out of reach of our competition. |
| | - | Potential for Use in Combination Therapy –Because of its delivery method and GRAS profile, it is likely that IMM-124E can not only be used as monotherapy, but also in combination with other NASH agents, if these are approved, and if physicians feel that this is warranted for their patients. |
C.difficile Infections (CDI)
Clostridiumdifficile, or C.difficile, is a gram-positive, toxin-producing, spore/vegetative cells-forming bacterium that can cause symptoms ranging from diarrhea to life-threatening inflammation of the colon. In the most serious cases, CDIs can lead to fulminant colitis, megacolon and even death from colon perforation and peritonitis.
Cdifficileis acquired from contact with humans or objects harboring these bacteria. It can be commonly acquired during hospitalization with up to 30% of those who have spent a prolonged period in the hospital leaving carrying these bacteria in the bowel flora, especially if antibiotics have been administered. This is because CDI is most often associated with the prior use of broad-spectrum antibiotics, which decrease the natural resistance of the body to C.difficile. Chronic CDI is estimated to occur in perhaps 15-30% of those infected. In some, reinfections can occur with the same or with a different strain. Risk factors for relapse include the number of previous episodes, the need to use antibiotics recurrently, and older age groups.
Human infection occurs through ingestion and if the bacterium survives acid and bile on its passage into the bowel, it may be eradicated by the normal bowel flora since the microbes that collectively make up the flora provide colonization resistance against pathogenic species through competition for essential nutrients and attachment sites to the gut wall. However, if the bowel flora is suppressed because of concomitant use of antibiotics, or if the bowel flora has a deficiency, C.difficile can colonize the flora and remain with the patient. In some individuals, it seems that antibiotics are not required for colonization to take place, which may be related to inadequate defense of the naturally occurring flora within the bowel.
When C.difficiletakes hold, the toxins produced by the bacterium, especially Toxin B, act by inactivation of Rho GTPases leading to cell death, and stimulation of an inflammatory cascade that exacerbates tissue damage, diarrhea, and pseudomembranous colitis. When faced with a CDI infection, the standard of care are either a course of Vancomycin or metronidazole, both of which are broad spectrum antibiotics. While these agents are very effective at treating the primary infections, they also severely impact the rest of the gut flora, creating an ideal environment for the C.difficile spores to once again take hold. This creates a vicious cycle, as more courses of antibiotic treatments worsen recurrence. Vancomycin and metronidazole are plagued by increasing rate of CDI recurrences, underscoring the need for new treatments. There is also growing concern of resistance to Vancomycin treatment.
C.difficileis a very hardy organism most likely because it shed spores and these spores are unable to be eradicated by any known antibiotics. Since C.difficile spores are able to survive for long periods of time outside of the body, and because healthcare settings are often sites of significant antibiotic use, CDI transmission rates in hospitals, long-term acute care facilities and nursing homes have been increasing. CDI is also a cause of morbidity and mortality among hospitalized cancer patients and bone marrow transplant patients, as their immune systems are suppressed by cytotoxic drugs and sometimes by antibiotics that are administered to prevent opportunistic infections.
The U.S. Centers for Disease Control has identified CDI as one of the top three most urgent antibiotic-resistant bacterial threats in the United States and is now the most common cause of hospital acquired infection in the U.S. CDI is responsible for approximately 29,000 deaths in the U.S. annually. The prevalence of CDI is estimated at more than 450,000 infections annually, with nearly 100,000 cases of first recurrences. Research suggests that the risk of recurrence is approximately 25% after the primary occurrence of CDI, 40% after a first recurrence and greater than 60% for those experiencing two or more recurrences. CDI leads to an increased length of hospitalization and an estimated $1.1 billion in health care costs annually in the U.S. The rise of community-acquired CDI is now a growing problem and led to the recognition that CDI is not simply limited to just hospitals. This increase in CDI incidence, which is now a growing problem worldwide due to the widespread and increase use of antibiotics, is the driver behind a growing market for C.difficile therapeutics, which is estimated to reach $1.5B by 2024, up from $350M today.
IMM-529 – A Potentially Revolutionary Treatment for CDI
IMM-529 is an oral biologic which does not destroy the microbiome like antibiotic treatments, allowing the microbiome to return to a healthy state, while treating the virulent CDI. The antibodies in IMM-529 have been demonstrated to be cross-reactive with a variety of human and animal C.difficile isolates and to their associated Toxin B, vegetative cells and spore components.
The antibodies in IMM-529 have also been shown to neutralize Toxin B from a historical C.difficile strain (630), and from a hypervirulent (HV) strain which caused recent worldwide outbreaks. Immunoglobulin G comprised almost 90% of total immunoglobulin present in IMM-529, with major subclass IgG1 making up over 65% of total immunoglobulins.
IMM-529 has successfully completed its pre-clinical program in CDI. IMM-529, which was developed in collaboration with world-leading C.difficile Key Opinion Leader (“KOL”) Dr. Dena Lyras and her team at Monash University, has a unique Triple-Action MOA (antibodies to Toxin B + Spores + Vegetative Cells):
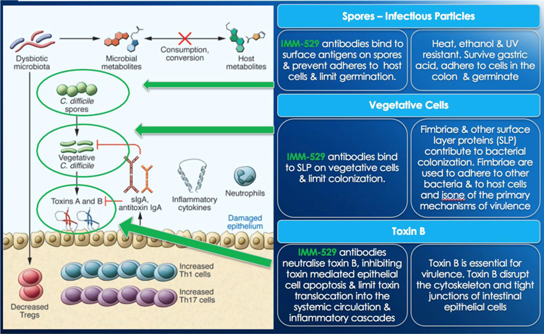
It is a three pronged approach that is unique and which has yielded exceptional results in the pre-clinical studies including (1) Prevention of primary disease, (2) Treatment of primary disease and (3) Suppression of recurrence. To our knowledge, it is to date the only investigational drug that has showed positive therapeutic benefits in all three phases of the disease. All results were highly statistically significant:
| | · | Prevention of C.difficile infection: Mice treated with IMM-529 demonstrated a significant increase in survival rates (P<0.0001) compared to untreated controls of approximately 70% (17/24) survival vs. 0% survival in the control groups. Mice that were untreated or treated with non-hyperimmune colostrum succumbed rapidly to infection with no significant difference in survival observed (P=0.3511): |
| o | Control group #1 (0/14) treated with water |
| o | Control group #2 (0/15) treated with non-hyperimmune colostrum |

| | · | Treatment: Mice treated with IMM-529 demonstrated a significant increase in survival rates (P<0.0001) compared to untreated controls of approximately 80% survival (11/14) vs. <7% survival in the control groups. Mice that were untreated or treated with non-hyperimmune colostrum showed no significant difference in survival with 0% or 6.7% survival rates (P=0.3178), respectively: |
| o | Control group #1 (0/14): Treated with water alone following vancomycin treatment |
| o | Control group #2 (1/15): Treated with non-hyperimmune colostrum following vancomycin treatment |
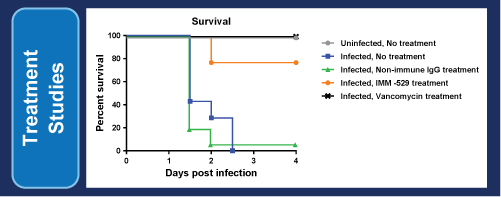
| | · | Relapse: Mice treated with IMM-529 demonstrated a significant increase in survival rates (P<0.0027) compared to mice treated with only vancomycin of approximately 90% survival in IMM-529 + vancomycin group (n=7/9); vs. 11% survival in the control group which received vancomycin alone (n=1/9) |

We have submitted these results for peer review and for publication.
We are currently in the process of manufacturing clinical supplies for our Phase 1/2 and are finalizing the Phase 1/2 protocol. We aim to start our Phase 1/2 in Israel in the second quarter of 2017. As the clinical trial will be conducted outside of the United States, specifically, in Israel, the Company is not required to apply for an IND.
IMM-529 – Competitive Advantage
We believe that IMM-529 has a unique competitive advantage:
| · | Triple Mechanism of Action – IMM-529 not onlytargets the Toxin B, but it also contains antibodies to thespores and the vegetative cells. This is unique among all assets currently in development. |
| · | Effective vs Virulent Strains – As discussed above, IMM-529 has been shown to be effective vs both the normal strains as well as the virulent strains of CDI, providing a strong Proof-of-Concept (POC) model that IMM-529 can be a front line agent in the battle vs hypervirulent and difficult to treat strains. |
| · | Effective in All phases of the Disease – IMM-529 has shown that it can be an effective agent in all phases of the disease including prevention of infection, treatment of primary disease and recurrence. This is unique among all of our competitors and indicate a much larger potential use than current development programs which primarily target recurrence. |
| · | Oral Therapy – IMM-529 is anoral therapy lessening costs/burden on the patient, hospitals and the healthcare system overall. |
| · | Not an Antibiotic – IMM-529 isnot an antibiotic, and hence is only targeted at C.difficile its Toxin B, spores and vegetative cells. It therefore does not negatively impact the rest of the flora and allows the flora to return to normal, while fighting the primary infection / recurrence. |
We are excited about the future prospects of this asset given the lack of treatment options that are available for this devastating disease. One of our major competitors, Seres Therapeutics, recently announced the interim results of a phase 2 with their SER-109 CDI therapeutic which failed to achieve the primary endpoint of reducing the risk of CDI recurrence. SER-109 (an ecology of bacterial spores enriched and purified from healthy, screened human donors) does not specifically target Toxin B, which has been proved time and again, especially through the work of Dr. Dena Lyras, to be the main driver of disease morbidity and mortality. We are confident in the long-term success of IMM-529 given its unique triple-action MOA which targets the Toxin B, the spores and the vegetative cells. We look forward to more data in the years ahead as we continue to develop this highly differentiated asset.
Other Development Programs
In addition to the IMM-124E and IMM-529 programs, we also have two research collaborations ongoing with the U.S. Department of Defense including one with theU.S. Army and a second collaboration with theU.S. Navy, for the study ofshigella, campylobacter and ETEC vaccines. We also started a pre-clinical program targetingIrritable Bowel Diseases (IBD), in collaboration with renowned IBD KOL, Dr. Gerhard Rogler and the university of Zurich, Switzerland, and a pre-clinical Autism study with several universities / hospitals in Australia.
Pre-Clinical - Colitis: The University of Zurich’s world renowned inflammatory bowel diseases researcher, Professor Gerhard Rogler, has teamed with Immuron to launch a pre-clinical development program in colitis. The three stage program will use well-known acute and chronic colitis models and will take place throughout 2016/2017.
Colitis, manifesting as a group of inflammatory bowel conditions, including Crohn’s Disease and Ulcerative Colitis, affects millions of people around the world. The global market for treatments of IBD, of which colitis is a significant component, is expected to reach an annual U.S. $10 billion by 2021.
Professor Rogler is Professor of Gastroenterology and Hepatology and Consultant Gastroenterologist at Zurich University Hospital Switzerland. He is also principal investigator of the Swiss Irritable Bowel Diseases cohort study, and the author of 200 original peer-reviewed articles.
Collaborations with U.S. Army and U.S. Navy: Our collaborations with the DoD are a powerful validation of the potential of our platform to develop novel anti-infectives. These collaborations also open the door to explore and develop potentially low risk / low cost therapeutics with some of the most advanced research facilities in the world.
U.S. Army –In June 2016, Immuron signed an agreement with the Walter Reed Army Institute of Research (WRAIR) to develop a vaccine for a form of dysentery that kills up to one million people a year. WRAIR is the largest and most diverse biomedical research laboratory in the Department of Defense. Immuron will provide the clinical supply for the study.
The project aims to develop a vaccine for shigellosis, a severe form of dysentery that affects about 165 million people a year, mostly children, and causes up to a million deaths. Symptoms of shigellosis, also known as bacillary dysentery, include severe and bloody diarrhea, fever, and stomach cramps. WRAIR aims to develop the vaccine for both civilian and military use in areas where endemic diseases such as shigellosis can compromise the health and readiness of the local community, travelers, contractors and defense personnel.
U.S. Navy– In August 2016, we signed an agreement with the U.S. Navy to test the reactivity and therapeutic effectiveness of Travelan against campylobacter and Enterotoxigenic Escherichia coli (ETEC), two common gram-negative bacterium. The next step would be to develop new vaccines. Immuron will provide the clinical supply for the study.
Campylobacter’s main reservoir is poultry, however humans can contract the disease from contaminated food. At least a dozen species of Campylobacter have been implicated in human disease, with C. jejuni and C. coli being the most common. C. jejuni is now recognized as one of the main causes of bacterial foodborne disease in many developed countries as well as developing countries were poultry is common.
Enterotoxigenic Escherichia coli (ETEC) is a type of Escherichia coli (E-coli) and one of the leading bacterial causes of diarrhea in the developing world, as well as the most common cause of travelers' diarrhea. Conservative estimates suggest that each year, about 157,000 deaths occur, mostly in children, from ETEC, but no vaccines exist, highlighting the need for new treatment modalities.
Autism:In July 2016, we announced a strategic partnership with three leading Australian research institutes focused on understanding how the genetic basis underlying Autism Spectrum Disorder (ASD) relates to changes to the gut, and how Immuron’s anti-LPS IMM-124E compound affects changes in mouse models for autism. This effort involves the University of Melbourne, La Trobe University and Murdoch Children’s Hospital.
Except for clinical supplies, this unique collaboration involves very little R&D investment from Immuron, but has the potential for tremendous upside given that are no treatments approved for autism, and very few assets in development. This could also potentially open the door for other development partnerships in Central Nervous System (CNS) conditions such as Alzheimer’s.
In summary, we believe that the breath/depth of our assets and the support we are receiving from the NIH, the DoD and other leading institutions and KOL, demonstrates the importance of our platform and makes us truly a unique and attractive player in the therapeutic areas we target.
Our Advisory Board
The company’s programs are supported by world-renowned KOLs:
· Dr. Arun Sanyal (MD) – University of Virginia. Professor of Medicine and Former Chairman of the Division of Gastroenterology, Hepatology and Nutrition, VCU Medical Center. Dr. Sanyal is an internationally renowned expert in liver diseases. He is a former President of the AASLD (American Association for the Study of Liver Diseases) and is the current Chair of the Liver Study Section at the NIH.
· Dr. Stephen Harrison (MD) – Professor of Medicine, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Physician, San Antonio Military Medical Center, Fort Sam Houston, San Antonio, Texas. Chief of Residents, Internal Medicine, Brooke U.S. Army Medical Center. Dr. Harrison is an internationally renowned expert in NASH and his group has published seminal work on many aspects in the field. Dr. Harrison is the Principal Investigator of Galectin’s GR-MD-02’s Phase 2 trial and hold's key roles in other leading clinical NASH studies.
· Dr. Manal Abdelmalek (MD) – Duke University Medical Center. Dr. Abdelmalek is Associate Professor of Medicine at Duke Medical University Medical Center, Division of Gastroenterology & Hepatology, Section of Hepatobiliary Diseases & Liver Transplantation. Dr. Abdelmalek is a leading investigator in the field of NASH.
· Dr. Gerhard Rogler (MD, PhD) – Zurich University. Dr. Rogler is the Chairman of the Scientific Advisory Board of the University of Zurich and Professor of Gastroenterology and Hepatology and Consultant Gastroenterologist at the Division of Gastroenterology & Hepatology, Department of Medicine, Zürich University Hospital, Switzerland. Prof. Rogler is a leader in the field of Colitis and has authored approximately 200 original peer-reviewed articles.
· Dr. Miriam Vos (MD) – Emory University. Dr. Vos is an associate professor of pediatrics at the Emory University School of Medicine, and an attending Hepatologist at Children’s Healthcare of Atlanta. She specializes in the treatment of gastrointestinal disease in children as well as fatty liver disease and obesity. Dr. Vos is also the author of The No-Diet Obesity Solution for Kids.
· Dr. Dena Lyras (PhD) – Monash University. Dr. Lyras is associate professor at Monash University, is one of the world’s leading expert in C.difficile. Dr. Lyras has spent her research career developing world-leading knowledge of C.difficile. She was the lead author of a seminal study published in Nature in 2009, which shed new light on the essential role specific toxins play in causing disease, a discovery that disproved prevailing opinion.
Competition
We believe that we will face competition in differing levels of intensity in all of the areas in which we are conducting research. Our competitors, which are located worldwide, are numerous and include, among others, major pharmaceutical companies such as Gilead; biotechnology firms such as Intercept with its product, obeticholic acid, Genfit, with its product Elafibrinor, Tobira with its product Cenicriviroc, Seres with its product SER-109 and Synthetic Biologics with its product ribaxamase; universities and other research institutions. These competitors may develop technologies and products that are more effective than any that we are developing, or which would render our technology and products obsolete or non-competitive. Many of these competitors have greater financial, research and screening capabilities, technical resources and manufacturing and marketing capabilities than we do. In addition, many of our competitors have much more experience than we do in pre-clinical testing and human clinical trials of new or improved drugs, as well as in obtaining FDA, EMA, TGA and other regulatory approvals.
Our Marketed Assets
Travelan – A Unique Product: Travelan is the only product currently sold for the prevention of Traveler’s Diarrhea (TD). Travelan uses hyperimmune BCP from cows vaccinated against various strains of ETEC to protect against TD and reduces the risk of TD, along with the symptoms of minor gastrointestinal disorders. Travelan prevents TD by up to 90%. Travelan is not an antibiotic and so it does not have the side-effect profile of antibiotics and does not contribute to the worldwide concerns about bacterial drug resistance. Two independent, double-blinded, placebo-controlled clinical trials in Europe in 90 healthy volunteers showed protection of up to 90% against infection with the type of E.coli that causes TD, along with indicating a significant reduction in abdominal cramps and stomach pain. There were no reported side effects in the clinical trials. Sales in FY2016, were AUD$1.0M.
Travelan is now marketed in four countries including Australia, U.S.A, China and Canada (with our partner Paladin Labs, a branch of Endo Pharmaceuticals that sells products for the Canadian market), and we planned to launched the products in additional countries. In Australia, Canada and China, Travelan is regulated as an OTC had therefore has disease claims. In the United States, Travelan is sold as a dietary supplement and as such does not require approval of a biologics license application from the FDA.
Our marketing and sales strategies vary by territory. In Australia, Travelan is sold within most pharmacies, and we utilize trade promotions strategies, as well as a contracted field force, to ensure that our products are appropriately distributed throughout our partner pharmacies. In the United States, we are heavily focused on driving demand through the travel clinics market, such as PassportHealth, and also by partnering with large distributors such as Medique. In Canada, our partner Paladin Labs, a branch of Endo Pharmaceuticals that sells products for the Canadian market will actively promote Travelan in both retail and pharmacies until approximately August 23, 2017. In China, our partner QBID, has an extensive presence on JD.com and Travelan is sold through their online stores. In all of our markets, we invest in social media marketing, trade marketing, traditional media marketing and PR to drive awareness and pull through of Travelan.
Overall, over 50 million people from developed nations travel to developing countries each year and 35%-50% of people traveling to developing countries will suffer from TD while 70% of these TD episodes will be caused by Enterotoxigenic Escherichia coli (ETEC). TD is the most common health problem faced by these travelers; given this, we believe that an expanded sales and marketing campaign for Travelan would lead to a strong increase in sales. We are in the process of finding partners for other priority markets outside of Australia, the U.S. and Canada. Our market research has shown that TD is a $600M market worldwide including therapeutics approved for the treatment of TD, off-label use of non-TD approved therapeutics such as antibiotics and OTCs. As the only preventative treatment on the market, we believe that the potential worldwide peak sales for Travelan is 20% of the worldwide TD market, or $120M per year.
Distribution and License Agreement with Paladin Labs Inc.
Effective November 28, 2011, we entered into a Distribution and License Agreement (the “Distribution Agreement”) with Paladin Labs Inc. (“Paladin”) pursuant to which we appointed Paladin as our exclusive distributor for marketing and selling Travelan in certain territories (the “Paladin Territories”). In addition to marketing, promoting, importing and selling Travelan in the Paladin Territories, Paladin has (i) the right to manufacture packs of Travelan and engage in clinical development and associated activities in connection with obtaining regulatory approvals in the Paladin Territories and (ii) grant sublicenses to its affiliates with respect to the foregoing rights. The term of the Distribution Agreement is 15 years after the first commercial sale of Travelan, which sale occurred in 2015, unless earlier terminated in accordance with the terms of the Distribution Agreement. Pursuant to the terms of the Distribution Agreement, Paladin (i) advanced the Company a secured facility in the amount of up to CAD $1,500,000, (ii) paid the Company a non-refundable fee of CAD$10,000 for the use of the Travelan trademark and (iii) paid the Company CAD$490,000 for exclusive distribution rights in the Paladin Territories. These amounts were paid in fiscal year 2012. Moreover, Paladin will pay the Company, the following amounts, as one-time milestone payments within 30 days of the end of the year in which Paladin first obtains certain aggregate Net Sales (as defined in the Distribution Agreement);provided, however,that the maximum amount of milestone payments shall not exceed CAD$115,250,000:
Milestone Payment (all expressed
in Canadian dollars) | | | Milestone the first time
that Net Sales in the
Paladin Territory in a calendar
year are equal or greater
than | |
| | | | | | | |
| $ | 100,000 | | | $ | 1,000,000 | |
| | | | | | | |
| | 150,000 | | | | 1,500,000 | |
| | | | | | | |
| | 200,000 | | | | 2,000,000 | |
| | | | | | | |
| | 250,000 | | | | 2,250,000 | |
| | | | | | | |
| | 300,000 | | | | 3,000,000 | |
| | 500,000 | | | | 5,000,000 | |
| | | | | | | |
| | 750,000 | | | | 7,500,000 | |
| | | | | | | |
| | 1,000,000 | | | | 10,000,000 | |
| | | | | | | |
| | 2,000,000 | | | | 20,000,000 | |
| | | | | | | |
| | 5,000,000 | | | | 30,000,000 | |
| | | | | | | |
| | 10,000,000 | | | | 40,000,000 | |
| | | | | | | |
| | 10,000,000 | | | | 50,000,000 | |
| | | | | | | |
| | 10,000,000 | | | | 60,000,000 | |
| | | | | | | |
| | 15,000,000 | | | | 70,000,000 | |
| | | | | | | |
| | 15,000,000 | | | | 80,000,000 | |
| | | | | | | |
| | 15,000,000 | | | | 100,000,000 | |
| | | | | | | |
| | 15,000,000 | | | | 125,000,000 | |
| | | | | | | |
| | 15,000,000 | | | | 150,000,000 | |
Paladin must order Product from the Company in multiples of a minimum of 20,000 packs. Paladin is to use commercially reasonable efforts to retain required regulatory approvals as set forth in the Distribution Agreement, and is responsible for sales and marketing expenses. To date, no milestones have been met under the Distribution Agreement. “Product” means hyper-immune bovine colostrum enriched with antibodies that are reactive to Enterotoxigenic E. Coli and all improvements thereto.
Effective February 23, 2017, the Company terminated the Distribution Agreement for Paladin’s for failure to purchase the minimum quantities of product pursuant to the agreement;provided, however, that Paladin shall be entitled to continue selling existing inventory in the Paladin Territories for a period of 6 months from the date of termination. The Company may, but is not required to, repurchase any inventory from Paladin.
Marketing and Master Distribution Agreement with UniFirst-First Aid Corporation d/b/a Medique Products
Effective June 28, 2016, the Company entered into a Marketing and Master Distribution Agreement (the “Marketing Agreement”) with UniFirst-First Aid Corporation d/b/a Medique Products (“Medique”) pursuant to which Medique was appointed as the exclusive marketing representative and exclusive distributor to certain parties in the U.S. as identified in the Marketing Agreement. The term of the Marketing Agreement is two years which will automatically be extended for additional one year periods unless earlier terminated in accordance with the terms of the Marketing Agreement. Pursuant to the Marketing Agreement, Medique will (i) assist the Company in the development of marketing, advertising and sales plans for certain of the Company’s products, (ii) implementing such plans, (iii) establishing customer relations, (iv) assisting the Company with receiving and responding to customer complaints and (v) performing such other services and providing such other assistance as may be reasonably requested by the Company. The prices that the Company will charge Medique for products will be equal to the Company’s wholesale prices for the products being charged to third parties, which prices may be increased one annually upon written notice to Medique. In addition, Medique has been granted the nontransferable right to use the Company’s trade names, trademarks and copyrights to distribute the products in the markets as contemplated by the Marketing Agreement. Medique shall have no right to manufacture any of the Company’s products.
The Marketing Agreement can be terminated sooner than the end of the tem immediately upon the giving of notice to the non-defaulting party if any of the following occur:
| (i) | Any assignment of the Marketing Agreement, or any transfer or attempted transfer by the defaulting party of the Marketing Agreement; or transfer by operation of law or otherwise of the principal assets of the defaulting party that are required to permit the defaulting party to perform its obligations under the Marketing Agreement; or change in the direct or indirect ownership of a controlling interest in the voting securities or operating management of the defaulting party however accomplished without the prior written consent of the non-defaulting party; |
| | (ii) | Any misrepresentation by the defaulting party in obtaining the Marketing Agreement or in obtaining any refund, credit, rebate, incentive, allowance, discount, reimbursement or payment from the non-defaulting party or submission by the defaulting party of any false or fraudulent application, claim or report in connection with its sales operations; |
| | (iii) | If the defaulting party is insolvent, unable to meet its debts as they mature, files a petition or answer consenting to or acquiescing in a petition against it in bankruptcy or under any chapter of the Bankruptcy Reform Act of 1978 or any similar law for the relief of debtors, federal or state, whether now existing or hereafter enacted, or suffers such a petition to be filed against it which is not vacated or stayed within 60 days, has a receiver appointed for or execution levied upon all or a material part of its business or assets, makes any arrangement with its creditors generally, goes into liquidation or dissolution, or fails for any other reason to function as a going business; |
| | (iv) | Any act by the defaulting party or any person involved in the ownership or operations of the defaulting party which violates any law and adversely affects the defaulting party’s operations or the Company’s products covered under the Marketing Agreement or any conduct or unfair business practice by the defaulting party or any person involved in the ownership or operations of the defaulting party which adversely affects the defaulting party’s operations or the goodwill and reputation of the defaulting party, the non-defaulting party or the products; and |
| | (v) | Failure by the defaulting party to fulfill any of its other obligations or agreements with respect to or arising under this Marketing Agreement and such failure continues for a period of 30 days or is repeated after written notice thereof is given by the non-defaulting party to the defaulting party. |
Protectyn – For Gut Dysbiosis. This year we launched Protectyn in Australia, an health product targeting LPS bacteria in the gut to prevent gut dysbiosis, improve bacterial clearance, reduce chronic inflammation and improve immune function. This product has been formulated to help maintain healthy digestive function and help support the liver. Protectyn is currently only sold through the Natural Healthcare Practitioners (Naturopath).
Our Technology Platform – Targeted Polyclonal Antibodies
Overview
Immuron’s hyper-immune platform technology is safe (GRAS rated), low cost, and can be applied to a variety of diseases. Immuron’s platform technology is based on producing antigen targeted, hyper-immune bovine colostrum powder (BCP) suitable for pharmaceutical use. Polyclonal antibodies are collected from the first milking of a cow after calving. Prior to calving, cows are immunized with proprietary vaccines to ensure immunogenicity and after calving, the first milk is harvested. This proprietary process ensures that the colostrum contains a high concentration of antibodies and high concentration of Immunoglobulin G.
The underlying nature of Immuron’s platform technology enables the development of medicines across a large range of diseases, including infectious diseases and immune-mediated disorders. The platform can be used to influence the cell-mediated immune system through regulatory T cell populations, or it can directly block viruses or bacteria at mucosal surfaces (such as the GI tract) and neutralize the toxins they produce. Additionally, the dairy origins of Immuron’s antibodies enables us to commercialize our platform through most regulatory pathways, including prescription (Rx), medical foods, over-the-counter medicines, and dietary supplements. The GRAS status of our technology platform will allow us to advance our preclinical programs into clinical trials in other diseases faster relative to other companies due to these characteristics.
In general, Immuron’s immunotherapy uses the inherent ability of the gastrointestinal tract’s immune system to control unwanted systemic immune responses by using specific antigens to induce the release of regulatory T-cells and anti-inflammatory cytokines while decreasing levels of pro-inflammatory cytokines. These T-cells fight systemic inflammation and restore inflammation to a normalized level. While our compound produces an anti-inflammatory effect, it does not involve general immune suppression, thus does not lead to potential serious side effects such as increased infection susceptibility.
Technology Platform – Targeted Polyclonal Antibodies
Immuron’s active ingredient is Bovine Colostrum Powder (BCP) purified from dairy cows that have been immunized with patented vaccines to produce very high levels of specific antibodies against targeted antigens.
These antibodies have powerful anti-inflammatory effect and work through two modes of action:
Immuron’s active ingredient targets a specific antigen. When bound by polyclonal antibodies, the antigens are prevented from infecting the cells of the patient.
Immuron’s active ingredient modulates the body’s own immune system by boosting the right T-cells and suppressing “unwanted” T-cells through a cell-mediated immune response that functions against the antigen. The antibodies, once orally ingested, are presented in the peyers patches to the lymph system's dendritic cells which sample the antibodies, thereby eliciting the cell mediated immune response. This response is associated with increased distribution of T regulatory lymphocytes and anti-inflammatory cytokines and decreased levels of pro-inflammatory cytokines.
Manufacturing Process
Effective June 28, 2013, we entered into a Development and Supply Agreement, as amended on June 21, 2016 (the “Development Agreement”) with Synlait Milk Ltd., a New Zealand company which specializes in the processing of infant formula and special milk powders with expertise in on-farm manipulation of milk (including colostrum), processing technologies, product development and marketing. Pursuant to the terms of the Development Agreement, we obtain our lead compound, IMM-124E, from Synlait.
Our lead compound, IMM-124E, is obtained from Synlait. Immuron’s active ingredient is manufactured under full cGMP conditions in an Australian TGA-licensed facility. Many of the active ingredients are the same as those of normal cow’s milk. However, the main differentiation between milk and Immuron’s active ingredient constituents is the presence of antibodies in the order of 35-45% by weight of dry colostrum powder. The main classes of immunoglobulins found in the active ingredient are IgG with smaller amounts of IgM and IgA. The major class of immunoglobulin found in bovine colostrum is IgG1 making up between 65% and 90% of total immunoglobulins, in contrast to milk which comprises predominantly IgA.
The Development Agreement shall expire on July 21, 2018 unless extended or earlier terminated by the parties.in accordance with the Development Agreement. Pursuant to the terms of the Development Agreement, the Company granted Synlait a non-exclusive, non-transferable license to use the Company’s intellectual property for vaccination of dairy herds with the Company’s vaccines and for collection and production of hyper-immune bovine colostrum (“HIC”) in the South Island of New Zealand. The Company shall supply Synlait with vaccines ready to use for formulation and/or component parts that are required for the production of HIC and information required by Synlait to enable it to attain regulatory registration within New Zealand for the lawful vaccination of dairy cows for the production of HIC. The Company paid Synlait NZ$260,000 in relation to its purchase of product in fiscal year 2014 for the Development (as defined in the Development Agreement); provided, however,Synlait will discount the purchase price of HIC in the first year by an amount equal to the foregoing fee. During the first year of the Development Agreement, the Company will pay Synlait as follows for HIC: (i) 10% of the expected order price upon the Company placing an order for HIC, (ii) 40% of the expected order price within two weeks of Synlait delivering written notice to the Company with respect to the commencement of processing the HIC and (ii) 50% of the expected order price within 30 days from the date the Company receives export documentation with respect to its order. After the first year, the Company will pay Synlait as follows for HIC: (i) 30% of the expected order price within two weeks of Synlait delivering written notice to the Company with respect to the commencement of processing the HIC and (ii) 70% of the expected order price within 30 days from the date the Company receives export documentation with respect to its order.
If any party is in material default or breach of any of terms of the Development Agreement, including payments due under the Development Agreement, and such breach or default s not remedied within 30 days after written notice thereof is provided by the other party, such other party may, in its sole discretion, terminate the Development Agreement in its entirety by delivering 30 days’ written notice to such effect.
In the event Synlait breaches the Development Agreement, the Company shall also be entitled to terminate the license granted to Synlait to the extent that it relates to the production of HIC on Synlait’s behalf. Additionally, each party may terminate the Development Agreement by giving written notice to the other party to such effect if such other party shall make an arrangement for the benefit of creditors, or if proceedings a party enters voluntary or involuntary liquidation or bankruptcy, or if a receiver or trustee of the property of such party shall be appointed, or if any proceedings are commenced by or against such party and in respect of such party under any provisions of any law relating to bankruptcy, liquidation or insolvency, or, in the case of Synlait, if its agreement with Bright Dairy is amended with respect to Bright Dairy’s access to Synlait’s and/or the Company’s intellectual property or with respect to its control over Synlait’s operations that use the Company’s intellectual property.
Upon termination of the Development Agreement, Synlait shall:
| (a) | immediately cease all activities permitted under the licenses and all rights granted to Synlait thereunder shall revert to the Company; |
| (b) | immediately deliver to the Company all vaccine that the Company supplied to Synlait and all the Company’s trade secrets related to the production of HIC; and |
| (c) | remain liable to the Company for all HIC ordered by the Company prior to the date of termination. |
Vaccination
The active drug substance is prepared using the first milking colostrum of dairy cows that have been immunized with a patented vaccine to produce very high levels of specific antibodies against selected surface antigens. Pregnant dairy cows at Australian commercial dairy farms are immunized through a proprietary process that is approved by an independent animal ethics committee.
Colostrum
The colostrum which is sourced from Synlait is harvested from immunized Holstein Friesian and Jersey cows. They are all Australian government registered for milk production for human consumption and at the time of harvesting are free from antibiotics. They are not given steroids at any stage of the process. Colostrum is harvested at the first milking which will be within twelve hours of calving, leaving plenty for the calf to feed on.
Risk management on the source of colostrum must focus on assurance of freedom from Bovine Spongiform Encephalopathy (BSE or commonly known as Mad Cow Disease) of the liquid raw product. A small number of countries, including Australia and New Zealand, have been judged by the World Organization for Animal Health (OIE) to have the highest BSE free rating on the basis that they have never experienced BSE at any time. The definition of this status means that both Australia and New Zealand are currently certified as BSE free countries.
Once harvested, preparation of the active ingredient complies with processes that are regulated by Dairy Safe standards in addition to the TGA, which is a Federal requirement and known globally for its stringent criteria. The raw colostrum material is centrifuged using a milk separator to remove somatic cells, cell debris, some bacteria and fat. It is then pasteurized, cooled and subjected to membrane ultra-filtration, removing much of the water, salts and lactose. The colostrum wet concentrate is then lyophilized to produce a powder, which is milled to 200 microns. The processes are typical for the dairy industry and for production of dairy foods. After freeze drying, the active ingredient is ready for further processing into the oral dosage form.
Tableting
The product excipients are all standard, FDA acceptable oral compounds that are granulated, milled and finally compressed into caplets and blister packaged (pharmaceutical grade packaging materials).
Batch Consistency
The Ab% component of Immuron’s active ingredient ranges between 36% and 40%. The parameters are stable within batches and across batches. Immuron’s product is stable according to ICH guidelines and the IgG component of Immuron’s active ingredient is stable over time. The IgG component is also stable across batches primarily as a function of harvesting colostrum from at least 100s of dairy cattle for any one batch, thereby attaining consistency by reason of averaging over a population. Furthermore, Immuron’s active ingredient is manufactured under full cGMP conditions with all associated QA and QC processes ensuring the stability of these parameters.
Regulatory
Our clinical assets are regulated as biologics by the FDA conferring it 12 years of market exclusivity in the U.S. New products in Europe have 10 years of market exclusivity.
Intellectual Property
Worldwide, we have 13 issued patents and 23 pending patent applications. We have been issued patents in the U.S., Australia, Canada, India, Japan and New Zealand. All of these patents are owned by Immuron and are not licensed. These patents enhance the market exclusivity offered by the fact that our compounds are classified as biologics by the FDA.
| Number | | Country | | Status | | Expiry |
| |
| Composition and Method for the Treatment and Prevention of Enteric Bacterial Infections |
| | | | | | | |
| 2004216920 | | Australia | | Granted | | 4 March 2024 |
| 0408085-8 | | Brazil | | Pending | | 4 March 2024 |
| 2,517,911 | | Canada | | Pending | | 4 March 2024 |
| 102698258 | | China | | Pending | | 4 March 2024 |
| 230664 B | | India | | Granted | | 4 March 2024 |
| 542088 | | New Zealand | | Granted | | 4 March 2024 |
| 9,402,902 | | USA | | Granted | | 4 March 2024 |
| 8,637,025 | | USA | | Granted | | 25 February 2028 |
| |
| Immuno-Modulating Compositions for the Treatment of Immune-Mediated Disorders |
| | | | | | | |
| 2009222965 | | Australia | | Granted | | 11 March 2029 |
| 2,718,381 | | Canada | | Pending | | 11 March 2029 |
| EP 2268669 | | Europe | | Pending | | 11 March 2029 |
| 587901 | | New Zealand | | Granted | | 11 March 2029 |
| 13/715,371 | | USA | | Pending | | 11 March 2029 |
| |
| Method and Apparatus for the Collection of Fluids |
| | | | | | | |
| 2,527,260 | | Canada | | Granted | | 10 June 2024 |
| 2004244673 | | Australia | | Granted | | 10 June 2024 |
| 544198 | | New Zealand | | Granted | | 10 June 2024 |
| |
| Anti LPS Enriched Immunoglobulin for the Treatment and/or Prophylaxis of a Pathologic Disorder |
| | | | | | | |
| 2010243205 | | Australia | | Granted | | 27 April 2030 |
| 2760096 | | Canada | | Pending | | 27 April 2030 |
| 13/265,252 | | USA | | Pending | | 27 April 2030 |
| 2424890 | | Europe | | Pending | | 27 April 2030 |
| 12103554.8 | | Hong Kong | | Published | | 27 April 2030 |
| 315924 | | Israel | | Pending | | 27 April 2030 |
| 5740390 | | Japan | | Granted | | 27 April 2030 |
| 10-2011-7027634 | | Korea | | Pending | | 27 April 2030 |
| 335793 | | Mexico | | Pending | | 27 April 2030 |
| 201171304 | | Eurasia | | Pending | | 27 April 2030 |
| |
| Anti LPS Enriched Immunoglobulin Preparation For Use In Treatment and/or Prophylaxis of a Pathologic Disorder |
| | | | | | | |
| 2011290478 | | Australia | | Granted | | 27 April 2030 |
| 2808361 | | Canada | | Pending | | 27 April 2030 |
| 2605791 | | Europe | | Granted | | 27 April 2030 |
| 13/817,414 | | USA | | Pending | | 27 April 2030 |
| 1185016 | | Hong Kong | | Published | | 27 April 2030 |
| |
| Methods and Compositions for the Treatment and/or Prophylaxis of Clostridium Difficile Associated Disease |
| | | | | | | |
| 2014253685 | | Australia | | Pending | | 17 April 2034 |
| 2,909,636 | | Canada | | Pending | | 17 April 2034 |
| 2986316 | | Europe | | Pending | | 17 April 2034 |
| 14/785,527 | | USA | | Pending | | 17 April 2034 |
| 201480034857.3 | | China | | Pending | | 17 April 2034 |
| 713233 | | NZ | | Pending | | 17 April 2034 |
Our Development Team includes Thomas Liquard (CEO), Dr. Dan Peres (MD) (CMO) and Dr. Jerry Kanellos (PhD) (CSO) and has extensive experience in the field. Bios for our development are set forth in the Management Section of this prospectus.
Government Regulation
As a pharmaceutical and biological product company that wishes to conduct clinical trials and ultimately obtain marketing approval in the United States, we are subject to extensive regulation by the FDA, and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, the Public Health Service Act, or PHS Act, and their implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. A failure to comply explicitly with any requirements during the product development, approval, or post-approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an IRB, of a suspension on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for the testing and marketing of our products in other countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in the European Union are addressed in a centralized way through the EMA, but country-specific regulation remains essential in many respects.
Government regulation may delay or prevent testing or marketing of our products and impose costly procedures upon our activities. The testing and approval process, and the subsequent compliance with appropriate statutes and regulations, requires substantial time, effort, and financial resources, and we cannot be certain that the FDA or any other regulatory agency will grant approvals for our products or any future products on a timely basis, if at all. The FDA’s or any other regulatory agency’s policies may change and additional governmental regulations may be enacted that could prevent or delay regulatory approval of our products or any future products or approval of new indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative, judicial, or administrative action, either in the United States or abroad.
Marketing Approval
In the United States, for premarket approval purposes, the FDA regulates our products as biologics under the FDC Act, the PHS Act and related regulations.
The steps required before a new biologic may be marketed in the United States generally include:
| • | nonclinical pharmacology and toxicology laboratory and animal tests according to good laboratory practices, or GLPs, and applicable requirements for the humane use of laboratory animals or other applicable regulations; |
| • | submission of an IND application which must become effective before human clinical trials may begin; |
| • | adequate and well-controlled human clinical trials according to GCPs and any additional requirements for the protection of human research subjects and their health information to establish the safety and efficacy of the investigational product for each targeted indication; |
| • | submission of a biologics license application, or BLA, to the FDA; |
| • | FDA’s pre-approval inspection of manufacturing facilities to assess compliance with cGMPs; |
| • | FDA’s audit of clinical trial sites that generated data in support of the BLA; and |
| • | FDA approval of a BLA, which must occur before a product can be marketed or sold. |
Product Development Process
Before testing any biologic in humans, the product enters the nonclinical, or preclinical, testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity, and formulation, as well as animal studies to assess the potential safety and activity of the product. The conduct of nonclinical tests must comply with federal regulations and requirements including GLPs. Because our product are Generally Regarded as Safe (GRAS), some toxicity and animal studies are not demanded by the FDA.
The product sponsor submits the results of the nonclinical testing, together with manufacturing information, analytical data, any available clinical data or literature, and a proposed clinical protocol, to the FDA in an IND, which is a request for authorization from the FDA to administer an investigational product to humans. Some nonclinical testing may continue even after the IND application is submitted. IND authorization is required before interstate shipping and administration of any new product to humans that is not the subject of an approved BLA. The IND automatically becomes effective 30 days after receipt by the FDA unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial and places the clinical trial on a clinical hold. In such case, the IND sponsor must resolve any outstanding concerns with the FDA before the clinical trial may begin. Further, an IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. If the site has an IBC, it may also have to review and approve the proposed clinical trial. Clinical trials involve the administration of the investigational product to patients under the supervision of qualified investigators following GCPs, requirements meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, investigators, and monitors. Clinical trials are conducted under protocols that detail, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, the parameters to be used in monitoring safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur, and the efficacy criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND. The informed written consent of each participating subject is required and the form and content of the informed consent must be approved by each IRB.
The clinical investigation of an investigational product is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of an investigation are as follows:
| • | Phase 1 includes the initial introduction of an investigational product into humans. Phase 1 clinical trials may be conducted in patients with the target disease or condition or on healthy volunteers. These studies are designed to evaluate the safety, metabolism, pharmacokinetics and pharmacologic actions of the investigational product in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase 1 clinical trials, sufficient information about the investigational product’s pharmacokinetics and pharmacological effects may be obtained to permit the design of Phase 2 clinical trials. |
| • | Phase 2 includes the controlled clinical trials conducted to evaluate the effectiveness of the investigational product for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the product. Phase 2 clinical trials are typically well-controlled, closely monitored, and conducted in a limited patient population, usually involving no more than several hundred participants. Phase 2a trials provide information on the impact of dose ranging on safety, biomarkers and proof of concept, while Phase 2 trials are patient dose-ranging efficacy trials. |
| • | Phase 3 clinical trials are controlled clinical trials conducted in an expanded patient population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the investigational product has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the product, and to provide an adequate basis for product approval. Phase 3 clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase 3 clinical trials to demonstrate the efficacy of the product. FDA may accept a single Phase 3 trial with other confirmatory evidence in rare instances where the trial is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible. |
Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events; any findings from other studies, tests in laboratory animals orin vitro testing that suggest a significant risk for human subjects; or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information.
The decision to terminate a clinical trial of an investigational biologic may be made by the FDA or other regulatory authority, an IRB, an IBC, or institutional ethics committee, or by a company for various reasons. The FDA may place a clinical hold and order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. If the FDA imposes a clinical hold, trials may not recommence without FDA and IRB authorization and then only under terms authorized by the FDA and IRB. In some cases, clinical trials are overseen by an independent group of qualified experts organized by the trial sponsor, or the clinical monitoring board or DSMB. This group provides authorization for whether or not a trial may move forward at designated check points. These decisions are based on the limited access to data from the ongoing trial. The suspension or termination of a clinical trial can occur during any phase of clinical trials if it is determined that the participants or patients are being exposed to an unacceptable health risk. In addition, there are requirements for the registration of ongoing clinical trials of drugs and biologics on public registries and the disclosure of certain information pertaining to the trials as well as clinical trial results after completion.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational product information is submitted to the FDA in the form of a BLA for a biologic to request marketing approval for the product in specified indications.
Biologics License Application Approval Process
In order to obtain approval to market a biologic in the United States, a BLA must be submitted to the FDA that provides data from nonclinical studies and clinical trials and manufacturing information establishing to the FDA’s satisfaction the safety, purity, and potency or efficacy of the investigational product for the proposed indication. The BLA must be accompanied by a substantial user fee payment unless a waiver or exemption applies.
The FDA will initially review the BLA for completeness before it accepts it for filing. Under the FDA’s procedures, the agency has 60 days from its receipt of a BLA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the BLA submission is accepted for filing, the FDA reviews the BLA to determine, among other things, whether the proposed product is safe, pure and potent, which includes determining whether it is effective for its intended use, and whether the product is being manufactured in accordance with cGMP, to assure and preserve the product’s identity, safety, strength, quality, potency and purity, and in accordance with biological product standards. The FDA will inspect the facilities at which the product is manufactured to ensure the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications. For a human cellular or tissue product, the FDA also will not approve the product if the manufacturer is not in compliance with the GTPs. These are FDA regulations that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues, and cellular and tissue based products, which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue based products are manufactured in a manner designed to prevent the introduction, transmission, and spread of communicable disease. Additionally, before approving a BLA, the FDA may inspect one or more clinical sites to assure compliance with GCP.
If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information, or corrective action for a manufacturing facility. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the BLA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee. The FDA also may determine a REMS is necessary to assure the safe use of the biologic, in which case the BLA sponsor must submit a proposed REMS. The REMS may include, but is not limited to, a Medication Guide, a communications plan, and other elements to assure safe use, such as restrictions on distribution, prescribing, and dispensing.
After the FDA completes its initial review of a BLA, it will either license, or approve, the product, or issue a complete response letter to communicate that it will not approve the BLA in its current form and to inform the sponsor of changes that the sponsor must make or additional clinical, nonclinical or manufacturing data that must be received before the FDA can approve the application, with no implication regarding the ultimate approvability of the application. If a complete response letter is issued, the sponsor may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
The testing and approval process for both a drug and biologic requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical trials are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
Biosimilars
We believe that any of our product candidates approved under a BLA should qualify for a 12-year period of exclusivity against biosimilar competition currently permitted by the Biologics Price Competition and Innovation Act, or BPCIA. Specifically, as part of the Patient Protection and Affordable Care Act enacted in 2010, as amended by the Health Care and Education Affordability Reconciliation Act, collectively the Affordable Care Act, the BPCIA established an abbreviated pathway for the approval of biosimilar and interchangeable biological products. The new abbreviated regulatory pathway provides legal authority for the FDA to review and approve biosimilar biologics based on their similarity to an existing brand product, referred to as a reference product, including the possible designation of a biosimilar as interchangeable with a brand product. Under the BPCIA, an application for a biosimilar product cannot be approved by the FDA until 12 years after the original brand product was approved under a BLA. There is a risk that, as proposed by President Obama, Congress could amend the BPCIA to significantly shorten this exclusivity period or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for biosimilar competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological drug products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. The BPCIA is complex and is only beginning to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning is subject to uncertainty. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes that operate to limit the scope or length of exclusivity afforded by the BPCIA could have a material adverse effect on the future commercial prospects for our biological products. In addition, the period of exclusivity provided by the BPCIA only operates against third parties seeking approval via the abbreviated pathway, but would not prevent third parties from pursuing approval via the traditional approval pathway. In addition, foreign regulatory authorities may also provide for exclusivity periods for approved biological products. For example, biological products in the European Union may be eligible for at least a ten-year period of exclusivity.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product candidate intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Orphan product designation must be requested before submitting a BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product candidate that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug or biological product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity. Orphan drug status in the European Union has similar, but not identical, benefits.
Expedited Development and Review Programs
The FDA has a fast track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for fast track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address significant unmet medical needs for the condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new biologic or drug may request the FDA to designate the biologic or drug as a fast track product at any time during the clinical development of the product. Unique to a fast track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a fast track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new biological or drug product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Biological or drug products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a biological or drug product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Lastly, under the provisions of the new Food and Drug Administration Safety and Innovation Act, enacted in 2012, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug or biological that is intended, alone or in combination with one or more other drugs or biological, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or biological may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs and biologicals designated as breakthrough therapies are also eligible for accelerated approval and receive the same benefits as drugs and biologicals with Fast Track designation. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Fast Track designation, and priority review may expedite the product approval process, but do not change the standards for approval. Accelerated approval and breakthrough therapy designation do change the standards for product approval and thus may expedite the development and/or approval process.
FDA Additional Requirements
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 clinical trials may be made a condition to be satisfied for continuing drug and biologic approval. The results of Phase 4 clinical trials can confirm the efficacy of a product candidate and can provide important safety information. In addition, the FDA has express statutory authority to require sponsors to conduct post-market studies to specifically address safety issues identified by the agency.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of an onerous REMS, restrictions on distribution, or post-marketing study requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
FDA Post-Approval Requirements
Any products manufactured or distributed by us or on our behalf pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping, reporting of adverse experiences with the biologic or drug, and submitting biological product deviation reports to notify the FDA of unanticipated changes in distributed products. Manufacturers are required to register their facilities with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements, which impose certain quality processes, manufacturing controls and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality, purity and potency characteristics that it purports to have. In November 2013, the Drug Quality and Security Act, or DQSA, became law and establishes requirements to facilitate the tracing of prescription drug and biological products through the pharmaceutical supply distribution chain. This law includes a number of new requirements that will be implemented over time and will require us to devote additional resources to satisfy these requirements, including serializing the product and using new technology and data storage to electronically trace the product from manufacturer to dispenser. If our products are not covered by the serialization and tracing requirements of the DQSA, they may be subject to state pedigree and traceability requirements. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, refuse to approve any BLA, force us to recall a product from distribution, shut down manufacturing operations or withdraw approval of the applicable BLA. Noncompliance with cGMP or other requirements can result in issuance of warning or untitled letters, civil and criminal penalties, seizures, and injunctive action.
The FDA and other federal and state agencies closely regulate the labeling, marketing and promotion of drugs and biologics. Government regulators, including the Department of Justice and the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities, recently have increased their scrutiny of the promotion and marketing of drugs and biologics. While doctors are free to prescribe any product approved by the FDA for any use, a company can only make claims relating to safety and efficacy of a product that are consistent with FDA approval, and the company is allowed to market a product only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must, among other things, be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning or untitled letters, corrective advertising, injunctions, potential civil and criminal penalties, criminal prosecution, and agreements with governmental agencies that materially restrict the manner in which a company promotes or distributes products.
Pediatric Research Equity Act
Under the Pediatric Research Equity Act, or PREA, as amended, a BLA or supplement must contain data to assess the safety and efficacy of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. Manufacturers must submit a pediatric study plan to the IND not later than 60 days after the end-of-phase 2 meeting with the FDA; if there is no such meeting, before the initiation of any phase 3 studies or a combined phase 2 and phase 3 study; or if no such study will be conducted, no later than 210 days before a marketing application or supplement is submitted. The intent of PREA is to compel sponsors whose products have pediatric applicability to study those products in pediatric populations, rather than ignoring pediatric indications for adult indications that could be more economically desirable. The FDA may grant deferrals for submission of data or full or partial waivers. By its terms, PREA does not apply to any product for an indication for which orphan designation has been granted, unless the FDA issues regulations stating otherwise. Because the FDA has not issued any such regulations, submission of a pediatric assessment is not required for an application to market a product for an orphan-designated indication.
Patent Term Restoration and Marketing Exclusivity
Depending on the timing, duration and specifics of FDA marketing approval of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent and within sixty days of approval of the biological. TheUSPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
The Biologics Price Competition and Innovation Act of 2009, which was included within the Patient Protection and Affordable Care Act, created an abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product, and grants a reference biologic twelve years of exclusivity from the time of first licensure. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structure of biological products, as well as the process by which such products are manufactured, pose significant hurdles to implementation that are still being worked out by the FDA.
Pediatric exclusivity is another type of exclusivity in the United States. Pediatric exclusivity, if granted, provides an additional six months of exclusivity to be attached to any existing exclusivity, e.g., twelve year exclusivity, or patent protection for a drug. This six month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric trial in accordance with an FDA-issued “Written Request” for such a trial.
Government Regulation Outside the United States
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In the European Union, for example, a request for a clinical trial authorization, or CTA, must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
The requirements and process governing the conduct of clinical trials, product approval or licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of a biological product under European Union regulatory systems, we must submit a marketing authorization application. The application required in the European Union is similar to a BLA in the United States, with the exception of, among other things, country-specific document requirements. The European Union also provides opportunities for market exclusivity. For example, in the European Union, upon receiving marketing authorization, a new biological generally receives eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a biosimilar application. During the additional two-year period of market exclusivity, a biosimilar marketing authorization can be submitted, and the innovator’s data may be referenced, but no biosimilar product can be marketed until the expiration of the market exclusivity. The innovator may obtain an additional one year of market exclusivity if the innovator obtains an additional authorization during the initial eight year period for one or more new indications that demonstrate significant clinical benefit over existing therapies. This data and market exclusivity regime in the European Union of a total of 10 or 11 years protects against generic competition, but does not protect against the launch of a competing product if the competitor, rather than referencing the clinical data of the originator, has conducted its own clinical trials to support its marketing authorization application.
Orphan drugs in the European Union are eligible for 10-year market exclusivity. This 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Additionally, marketing authorization may be granted to a similar product for the same indication at any time if:
| • | the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; |
| • | the applicant consents to a second orphan medicinal product application; or |
| • | the applicant cannot supply enough orphan medicinal product. |
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Pharmaceutical Coverage, Pricing and Reimbursement
Sales of our products, when and if approved for marketing, will depend, in part, on the extent to which our products will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly reducing reimbursements for medical products, biologicals, drugs and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of interchangeable products. Adoption of price controls and cost containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor not to cover our product candidates could reduce physician usage of our products once approved and have a material adverse effect on our sales, results of operations and financial condition.
The containment of healthcare costs has become a priority of federal, state and foreign governments. Third-party payors are increasingly challenging the prices charged for drug products and medical services, examining the medical necessity and reviewing the cost effectiveness of drug products and medical services, in addition to questioning safety and efficacy. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after FDA approval or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit.
In the United States and some foreign jurisdictions, there have been, and likely will continue to be, a number of legislative and regulatory changes and proposed changes regarding the healthcare system directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the ACA, was enacted. The ACA includes measures that have significantly changed, and are expected to continue to significantly change, the way healthcare is financed by both governmental and private insurers. Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2024 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Other Healthcare Laws
We also may be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and other countries in which we conduct our business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security and physician sunshine and open payment laws and regulations, many of which may become more applicable to us if our product candidates are approved and we begin commercialization. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Facilities
Our corporate headquarters are located at Suite 1, 1233 High Street, Armadale, Victoria, 3143, Australia and consist of approximately 60 square feet of office space which is provided as part of a services agreement which expires at-will upon six months written notice. We do not have a lease agreement for our corporate headquarters.
Our principle office is located at Suite 10-25 Chapman Street, Blackburn North, Victoria 3130 and consists of approximately 1,500 square feet of leased office and warehouse space under a lease agreement which expires on December 31, 2018, with an ongoing further three year option for extension. Our Company has no dedicated research and development facility as the Company’s research and development activities are provided to the company by third party suppliers whom are responsible for their own premises. We believe that our existing facilities are adequate for our current needs.
Employees
We have 8 full-time employees, many of whom hold PhD, DR, CA, MBA or other post-graduate degrees in their respective fields. Of these full-time employees, 4 are engaged in research and development activities and 4 are engaged in finance, legal, human resources, facilities and general management. Our employees are located in Australia, Israel and the United States.
Legal Proceedings
We are not currently a party to any material legal proceedings. From time to time, we may be subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Although the results of litigation and claims cannot be predicted with certainty, any such future litigation could have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
DIRECTORS and MANAGEMENT
Directors and Executive Officers
The following table sets forth information covering our current directors and executive officers.
| Name | | Age | | Position |
| Dr. Roger Aston | | 60 | | Non-Executive Chairman |
| Peter Anastasiou | | 56 | | Executive Vice Chairman |
| Daniel Pollock | | 56 | | Non-Executive Director |
| Stephen Anastasiou | | 59 | | Non-Executive Director |
| Thomas Liquard | | 45 | | Chief Executive Officer |
| Dr. Jerry Kanellos (PhD) | | 55 | | Chief Operating and Scientific Officer |
| Dr. Dan Peres (MD) | | 40 | | Head of Medical |
| Phillip Hains | | 57 | | Joint-Chief Financial Officer and Company Secretary |
| Peter Vaughan | | 33 | | Joint-Chief Financial Officer and Company Secretary |
| Professor Ravi Savarirayan | | 50 | | Non-Executive Director (Appointed April 1, 2017) |
Roger Astonhas been a member of our board of directors and the board’s non-executive Chairman since March 2012. Dr. Aston is both a scientist and a seasoned biotechnology entrepreneur, with a successful track record in both fields, and brings to the Board more than 20 years of experience in the pharmaceutical and biotech industries. Dr. Aston has been closely involved in start-up companies and major pharmaceutical companies. Aspects of his experience include FDA and EU product registration, clinical trials, global licensing agreements, fundraising through private placements, and a network of contacts within the pharmaceutical, banking and stock broking sectors.
Dr. Aston has had extensive experience on boards of many biopharmaceutical companies including Directorships/Chairmanships with Clinuvel Limited (ASX:CUV), HalcyGen Limited (ASX:HGN) and Ascent Pharma Health Limited (ASX:APH). During 2007 and 2008, Dr Aston was a member of the AusIndustry Biological Committee advising the Industry Research and Development Board. More recently, Dr Aston was Executive Chairman of Mayne Pharma Group from 2009 to 2011 and CEO of Mayne Pharma Group until 2012.
Dr. Aston has also been a director of IDT (ASX:IDT) Limited, Cynata Limited (ASX:CYP), Calzada Limited (now Polynovo Limited), Biolife Limited (now Immugene ASX:IMU, Director and Chairman of Regeneus Limited (ASX:RGS), Director and Chairman of Oncosil Medical Limited (ASX:OSL) and Director and Chairman of ResApp Limited (ASX:RAP). Roger Aston is also currently the Executive Chairman of Pharmaust Ltd (ASX:PAA).
Peter Anastasiouhas been a member of our board of directors and our Executive Vice Chairman since May 2015. Mr. Anastasiou’s involvement with Immuron commenced in May 2013 following his substantial underwriting support of the Company’s Renounceable Rights Issue, which was surpassed by his further funding support of the $9.66M capital raising in February 2014 resulting in an ownership of approx. 15% of the Company via his associated investment funds. Mr. Anastasiou is an entrepreneur and investor with extensive experience in business both in Australia and overseas. Mr. Anastasiou was the founding Chairman of the ACSI Group of Companies, which has owned and managed successful consumer companies such as SABCO, Britex Carpet care, Rug Doctor and Crystal Clear. Mr. Anastasiou also has a number of philanthropic interests including being a patron of the Identity Theatre for men, a prior board member and supporter of the Indigenous Eye Health Unit at Melbourne University, a supporter of the John Fawcett Foundation in Bali, and a founding investor and Director of Melbourne Victory Football Club. Mr. Anastasiou is the brother of Stephen Anastasiou.
Daniel Pollockhas been a member of our board of directors since October 2012. Mr. Pollock is a lawyer admitted in both Scotland and Australia and holding Practicing Certificates in both Jurisdictions. He is sole practitioner in his own legal firm based in Melbourne, Australia which operates internationally and specializes in commercial law. Mr Pollock is Chairman and Company Secretary of Amaero Pty Ltd, a company established to commercialize laser based additive manufacturing emerging from Monash University. He is also Executive Director and co-owner of Great Accommodation P/L a property management business operating in Victoria.
Stephen Anastasiouhas been a member of our board of directors since May 2013. Mr. Anastasiou has over 20 years’ experience in general management, marketing and strategic planning within the healthcare industry. His breadth of experience incorporates medical diagnostics, pharmaceuticals, hospital, dental and OTC products, with companies including the international pharmaceutical company Bristol - Myers Squibb. While working with KPMG Peat Marwick as a management consultant, Mr. Anastasiou has previously led project teams in a diverse range of market development and strategic planning projects in both the public and private sector. He is also a director and shareholder of a number of unlisted private companies, covering a variety of industry sectors that include healthcare and funds management. Mr. Anastasiou is the brother of Peter Anastasiou.
Thomas Liquard has been our Chief Executive Officer since 2015. Mr. Liquard has held various product development and leadership roles with large pharma and biotech companies. From 2013 to 2014, Mr. Liquard was Chief Operating Officer and later Chief Executive Officer of Australian Biotech Company Alchemia where he led all major development and corporate development activities. Prior to joining Alchemia from 2013 to 2014 Mr. Liquard was employed by Pfizer in New York for 7 years where he held various senior commercial positions in business and portfolio development. Mr Liquard spent three years as a key member of Pfizer's Established Products U.S. brands P&L Leadership Team where he engineered the groups $700M acquisition of Next Wave Pharmaceuticals, Inc. (NextWave). Mr. Liquard led the pre and post-acquisition integration efforts of Next Wave into the existing Pfizer business. The development products acquired with the NextWave acquisition are now approved and generate more than $250M in annual sales in the U.S. Mr. Liquard holds an MBA from Columbia Business School and a Bachelor of Science Degree from the University of Southern California.
Dr. Jerry Kanellos(PhD)has been our Chief Operating and Scientific Officer since July 2015. Dr. Jerry Kanellos has over twenty five years’ experience in the pharmaceutical and biotechnology industry, and has held leadership roles in executive management, business development, project management, intellectual property portfolio management research and development. From 2008 until 2012, Dr. Kanellos was the Chief Operating Officer of TransBio Limited where he was responsible for the strategic identification, development and maintenance of commercial partnerships globally, along with development, management and maintenance responsibility for the intellectual property portfolio, research and development and technology transfer. Prior to this, Dr. Kanellos work for five years as a consultant to the biotechnology industry and has provided development and commercialization strategies for various bodies including academic institutes, private and publicly listed companies and government departments both national and international. He has also been involved in the establishment and management of several startup biotechnology companies. During his ten years tenure in research and development at CSL Limited, a global specialty biotherapeutics company that develops and delivers innovative biotherapies, Dr. Kanellos gained considerable experience in the international drug development process, formulation development through to pharmaceutical scale up and cGMP manufacture successfully leading the Chemistry Manufacturing and Controls (CMC) programs for the approval, manufacture and launch of several products. Dr. Kanellos holds a PhD in Medicine from the University of Melbourne.
Dr. Dan Peres(MD) has served in various clinical and medical managerial roles in pharmaceutical and medical device companies such as Exalenz Bioscience, CarboFix Orthopedics Ltd, NMB Medical Applications Ltd, ByPass Makafim Ltd, IOPtima Ltd and NovoNordisk Israel. In addition, Dr. Peres has been responsible for operational, marketing and business development activities throughout his career in the life sciences industry. Dr. Peres began his career as a physician and medical director in the Israel army. Dr. Peres’ expertise lies with medical strategy, research and development, and the management of clinical studies and other laboratory processors. He has extensive knowledge of the leading International Centers for Liver Disease and established relationships with key Opinion leaders, including those currently participating in Immuron’s NASH and ASH trials. Dr. Peres has been a certified physician since 2002 when he graduated from the Sackler School of Medicine at Tel-Aviv University.
Phillip Hainshas been our joint Chief Financial Officer (CFO) and Company Secretary since April 2013. Mr Hains is a Chartered Accountant and specialist in the public company environment. He has served the needs of a number of public company boards of directors and related committees. He has over 20 years’ experience in providing accounting, administration, compliance and general management services. He holds a Masters of Business Administration from RMIT and a Public Practice Certificate from the Institute of Chartered Accountants of Australia.
Peter Vaughanhas been our joint Chief Financial Officer (CFO) and Company Secretary since April 2013. Mr Vaughan is a Chartered Accountant who has worked in the listed company environment for 13 years across a number of industries. He has served on, and provided accounting, administration, compliance and general management services to a number of private, not-for-profit, and listed public company boards of directors and related committees. Mr Vaughan is also currently studying a Senior Executive Masters of Business Administration at Melbourne University.
Professor Ravi Savarirayanwas appointed as a Non-Executive Director of the Company on April 1, 2017. He is a consultant clinical geneticist at the Victorian Clinical Genetics Services since August 1999, as well as Professor and Research Group Leader (Skeletal Biology and Disease) at the Murdoch Children’s Research Institute since September 2000. Mr. Savarirayanis a founding member of the Skeletal Dysplasia Management Consortium since January 2011 and has been the Chair of the Specialist Advisory Committee in Clinical Genetics, Royal Australasian College of Physicians since February 2009 . He was president of the International Skeletal Dysplasia Society from July 2009 to June 2011 and has been an invited member of several International Working Committees on Constitutional Diseases of Bone. Mr. Savarirayan’s primary research focus is on inherited disorders of the skeleton causing short stature, arthritis and osteoporosis. He has published over 150 peer-reviewed articles, collaborating with peers from over 30 countries, and has been on the editorial board of Human Mutation since January 2009, European Journal of Human Genetics since July 2007, American Journal of Medical Genetics since December 2011 and Journal of Medical Genetics since June 2005. Mr. Savarirayan received his MBBS from the University of Adelaide in 1990 and became a fellow of the Royal Australasian College of Physicians in December 1997. He was certified as a specialist in clinical genetics from the Human Genetics Society of Australasia in 1998 and received his Doctor of Medicine from the University of Melbourne in 2004, for his thesis “Clinical and Molecular Studies in the Osteochondrodysplasias.”
Board of Directors
Our board of directors currently consists of five members. Directors are elected at each annual general meeting of our shareholders and serve until their successors are elected or appointed, unless their office is earlier vacated. We believe that each of our directors has relevant industry experience. The membership of our board of directors is directed by the following requirements:
| • | our Constitution specifies that there must be a minimum of three directors and a maximum of 10, and our board of directors may determine the number of directors within those limits; |
| • | as set forth in our Board Charter, the membership of the board of directors should consist of a majority of independent directors who satisfy the criteria recommended by the Australian Securities Exchange (ASX) Corporate Governance Principles and Recommendations of the Australian Securities and Investments Commission (ASIC); |
| • | the Chairman of our Board should be an independent director who satisfies the criteria for independence recommended by the ASX Corporate Governance Principles and Recommendations; and |
| • | our board of directors should, collectively, have the appropriate level of personal qualities, skills, experience, and time commitment to properly fulfill its responsibilities or have ready access to such skills where they are not available. |
Our board of directors has delegated responsibility for the conduct of our businesses to the Chief Executive Officer, but remains responsible for overseeing the performance of management. Our board of directors has established delegated limits of authority, which define the matters that are delegated to management and those that require board of directors approval. Under the Corporations Act, at least two of our directors must be resident Australians. None of our directors have any service contracts with Immuron that provide for benefits upon termination of employment.
Committees
To assist our board of directors with the effective discharge of its duties, it has established a Remuneration and Nomination Committee and an Audit and Risk Committee, which committees operate under a specific charter approved by our board of directors.
Remuneration and Nomination Committee
The members of our Remuneration and Nomination Committee are Roger Aston and Daniel Pollock, each of whom our board of directors has determined meets the criteria for independence under NASDAQ Listing Rule 5605(a)(2). Dr. Aston acts as chairman of the committee. The committee’s role involves:
| · | identifying, evaluating and recommending qualified nominees to serve on our board of directors; |
| · | evaluating, adopting and administering our compensation plans and similar programs advisable for us, as well as modifying or terminating existing plans and programs; |
| · | establishing policies with respect to equity compensation arrangements; and |
| · | overseeing, reviewing and reporting on various remuneration matters to our board of directors. |
Audit and Risk Committee
The members of our Audit and Risk Committee are Daniel Pollock and Roger Aston, each of whom our board of directors has determined meets the criteria for independence of audit committee members set forth in Rule 10A-3(b)(1) under theSecurities Exchange Act of 1934, as amended, or the Exchange Act, and the applicable rules of the NASDAQ Capital Market. Each member of our audit committee meets the financial literacy requirements of the listing standards of the NASDAQ Capital Market. Daniel Pollock acts as the chairman of the audit committee. The principal duties and responsibilities of our audit committee include, among other things:
| • | overseeing and reporting on various auditing and accounting matters to our board of directors, including the selection of our independent accountants, the scope of our annual audits, fees to be paid to the independent accountants, the performance of our independent accountants and our accounting practices; |
| • | overseeing and reporting on various risk management matters to our board of directors; |
| • | considering and approving or disapproving all related-party transactions; |
| • | reviewing our annual and semi-annual financial statements and reports and discussing the statements and reports with our independent registered public accounting firm and management; |
| • | reviewing and pre-approving the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services; |
| • | evaluating the performance of our independent registered public accounting firm and deciding whether to retain their services; and |
| • | establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters. |
Code of Conduct
We have established a Corporate Governance Statement, which includes a code of conduct. Our Corporate Governance Statement, sets out the standards of behavior that apply to every aspect of our dealings and relationships, both within and outside Immuron. The following standards of behavior apply to all directors, executive officers and employees of Immuron:
| • | comply with all laws that govern us and our operations; |
| • | act honestly and with integrity and fairness in all dealings with others and each other; |
| • | avoid or manage conflicts of interest; |
| • | use our assets responsibly and in the best interests of Immuron; and |
| • | be responsible and accountable for our actions. |
The Code of Conduct is available on our website at Immuron.com.
Remuneration
Our remuneration policy ensures that directors and senior management are appropriately remunerated having regard to their relevant experience, their performance, the performance of the Company, industry norms and standards and the general pay environment as appropriate. The Remuneration Policy has been established to enable the Company to attract, motivate and retain suitably qualified directors and senior Management who will create value for shareholders.
Our Remuneration Policy is not directly based on our earnings. Our earnings have remained negative since inception due to the nature of the Company. Shareholder wealth reflects this speculative and volatile market sector. No dividends have ever been declared by us. We continue to focus on the research and development of our intellectual property portfolio with the objective of achieving key development and commercial milestones in order to add further shareholder value
The Company's performance over the previous five financial years is as follows:
| Financial Year | | Net Loss | | | Share price at year end | |
| | | AUD$ | | | AUD$ | |
| 2016 | | $ | 7,068,767 | | | $ | 0.25 | |
| 2015 | | $ | 2,691,820 | | | $ | 0.23 | |
| 2014 | | $ | 2,495,069 | | | $ | 0.20 | * |
| 2013 | | $ | 3,539,117 | | | $ | 0.16 | * |
| 2012 | | $ | 2,297,520 | | | $ | 0.72 | * |
| 2011 | | $ | 2,595,179 | | | $ | 2.52 | * |
*Share prices have been adjusted to reflect a 40:1 capital consolidation which was completed in November 2014.
Non-Executive Director Remuneration
Objective
The Remuneration Policy ensures that Non-Executive Directors are appropriately remunerated having regard to their relevant experience, individual performance, the performance of the Company, industry norms/standards and the general pay environment as appropriate.
Structure
The Company's Constitution and the ASX Listing Rules specify that the aggregate remuneration of Non-Executive Directors shall be determined from time to time by a Meeting of Shareholders. An amount (not exceeding the amount approved at the Shareholders Meeting) is determined by the Board and then divided between the Non-Executive Directors as agreed. The latest determination was at the Shareholders Meeting held on November 8, 2005 when shareholders approved the aggregate maximum sum to be paid or provided as remuneration to the Directors as a whole (other than the Managing Director and Executive Directors) for their services as $350,000 per annum. This compensation is cashed based and does not include stock based compensation.
In the year ended June 30, 2016, the Non-Executive Directors were remunerated an aggregate AUD$1,471,938 per annum, including superannuation.
The manner in which the aggregate remuneration is apportioned amongst Non-Executive Directors is reviewed periodically.
The Board is responsible for reviewing its own performance. Board, and Board committee performance, is monitored on an informal basis throughout the year with a formal review conducted during the financial year.
No retirement benefits are payable other than statutory superannuation, if applicable.
Executive Director and Executive Officer Remuneration
Objective
Our policy ensures that Executive Directors are appropriately remunerated having regard to their relevant experience, individual performance, the performance of the Company, industry norms/standards and the general pay environment as appropriate.
Structure
The Non-Executive Directors are responsible for evaluating the performance of the Chief Executive Officer (CEO) who in turn evaluates the performance of the other Senior Executives. The evaluation process is intended to assess the Company's business performance, whether long-term strategic objectives are being achieved and the achievement of individual performance objectives.
The performance of the CEO and Senior Executives are monitored on an informal basis throughout the year and a formal evaluation is performed annually.
Fixed Remuneration
Executives' fixed remuneration comprises salary and superannuation and is reviewed annually by the CEO, and in turn, the Remuneration Committee. This review takes into account the Executives' experience, performance in achieving agreed objectives and market factors as appropriate.
Variable Remuneration - Short Term Incentive Scheme
Executives may be entitled to receive a combination of short term incentive (STI) and long term incentive (LTl)’s as part of their total remuneration if they achieve certain performance indicators as set by the Board. These STI /LTI may be paid either by cash, or a combination of cash and the issue of equity in the Company, at the determination of the Board and Remuneration Committee.
The Remuneration Committee would approve the issue of bonuses following the recommendations of the CEO in the annual review of the performance of the Executives, and the Company as a whole, against agreed Key Performance Indicators (KPI’s).
Variable Remuneration - Long Term Incentive Scheme
Executives may also be provided with longer-term incentives through the Company's Employee Share and Option Plan (ESOP), that was approved by shareholders at the Annual General Meeting held on November 13, 2014. The aim of the ESOP is to allow the Executives to participate in, and benefit from, the growth of the Company as a result of their efforts and to assist in motivating and retaining those key employees over the long term. Continued service is the condition attached to the vesting of the options. The Board at its discretion determines the total number of options granted to each Executive.
Details of Remuneration for fiscal 2016
(in AUD$’s)
| | | Short term | | | Post employment | | | Equity
awards | | | | | | % of remuneration | |
| | | Salary
and
Fees | | | Cash
bonus | | | Non-
monetary
benefits | | | Superannuation | | | Termination
benefits | | | Shares/
Options | | | Total | | | consisting
of options | | | Performance
based | |
| Directors: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dr. Roger Aston | | | 62,500 | | | | - | | | | - | | | | 5,938 | | | | - | | | | 730,125 | | | | 798,563 | | | | 91 | % | | | - | % |
| Mr. Daniel Pollock | | | 45,000 | | | | - | | | | - | | | | 4,275 | | | | - | | | | 292,050 | | | | 341,325 | | | | 86 | % | | | - | % |
| Mr. Stephen Anastasiou | | | 40,000 | | | | - | | | | - | | | | - | | | | - | | | | 292,050 | | | | 332,050 | | | | 88 | % | | | - | % |
| Mr. Peter Anastasiou | | | 40,000 | | | | - | | | | - | | | | - | | | | - | | | | 292,050 | | | | 332,050 | | | | 88 | % | | | - | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Key Management Personnel: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Mr. Thomas Liquard | | | 287,485 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 287,485 | | | | - | | | | - | % |
| Dr. Jerry Kanellos | | | 149,744 | | | | - | | | | - | | | | 14,226 | | | | - | | | | - | | | | 163,970 | | | | - | | | | - | % |
| Dr. Leearne Hinch | | | 27,785 | | | | - | | | | - | | | | 1,565 | | | | - | | | | - | | | | 29,350 | | | | - | | | | - | % |
| Total | | | 652,514 | | | | - | | | | - | | | | 26,004 | | | | - | | | | 1,606,275 | | | | 2,284,793 | | | | | | | | | |
Employment Agreements with Executive Officers
The Company has contracts with all of its senior management and employees, but does not have any employment contracts with any of its directors.
Thomas Liquard
On August 24, 2016, we entered into an Executive Service Agreement (the “Liquard Agreement”) with Thomas Liquard, pursuant to which Mr. Liquard is serving as the Company’s Chief Executive Officer. The term of the Liquard Agreement is for three years unless terminated earlier in accordance with the Liquard Agreement. Pursuant to the Liquard Agreement, the Company will pay Mr. Liquard AUD$300,000 per annum. The Company also pays up to AUD$15,000 for Mr. Liquard’s health insurance. Under the Liquard Agreement, if the Short-Term Incentive Milestones (as defined below) are achieved within the required time frame, Mr. Liquard will be paid a AUD$80,000 bonus payable in ordinary shares of the Company at an issue price determined by the seven day volume weighted average price (“VWAP”) immediately prior to the issuance. If all of the Long Term Incentive Milestones (as defined below) are achieved within the required timeframe, Mr. Liquard will be issued 1,000,000 shares over ordinary shares, subject to shareholder approval.
The Short-Term Incentive Milestones are:
1) AUD$5 million of Travelan Sales worldwide, AUD$1 million of which must have been earned from China; and
2) A successful NASDAQ listing; and
3) A successful capital raise of $10 million (or AUD$ equivalent) either in conjunction to, or separate from the NASDAQ listing.
The Long-Term Incentive Milestones are:
1) A Company listed market capitalization value of $100 million;
2) AUD$10 million of Travelan Sales worldwide; and
3) The successful execution of a licensing agreement the parameters of which are to be agreed between the Board and Mr. Liquard.
If a change of control of the Company occurs within the first twelve months of the effective date of the Liquard Agreement, the Short-Term Incentive Milestones shall be automatically deemed to have been achieved. If a change of control of the Company occurs twelve months after the effective date, but prior to the first three year anniversary of the effective date, the Long-Term Incentive Milestones shall be automatically deemed to have been achieved.
At any time either the Company or Mr. Liquard can terminate the Liquard Agreement without cause on six months’ written notice. Subject to the applicable rules and laws (including the rules of the ASX Limited), the Company may elect to pay six months base salary and superannuation in lieu of notice.
The Company may also terminate the Liquard Agreement if (i) within twelve months of the effective date Mr. Liquard has not achieved two of the three Short-Term Incentive Milestones or (ii) within three years of the effective date Mr. Liquard has not achieved two of the three Long-Term Incentive Milestones.
Jerry Kanellos
On July 23, 2015, we entered into an Executive Service Agreement (the “Kanellos Agreement") with Dr. Jerry Kanellos, pursuant to which Dr. Kanellos is serving as the Company’s Chief Operating & Scientific Officer. Pursuant to the Kanellos Agreement, the Company will pay Dr. Kanellos AUD$160,000 per annum. Although the Kanellos Agreement provides that the Board will consider a short and long term share and/or share option incentive package for Dr. Kanellos after twelve months of continuous employment, subject to any applicable shareholder approval, no such term share and/or share option incentive package has been established as of the date hereof. The Company’s Board will consider a short and long term share and/or share option incentive package for Dr. Kanellos after twelve months of continuous employment, subject to any applicable shareholder approval. The Company or Dr. Kanellos may terminate the Kanellos Agreement without cause on thirty days’ written notice. Subject to applicable laws and rules, the Company may elect to pay Dr. Kanellos thirty days’ base salary in lieu of notice. The Company may also terminate the Kanellos Agreement for Cause (as defined in the Kanellos Agreement). Although the Kanellos Agreement provides that Dr. Kanellos’ remuneration will be reviewed six months from the effective date of the Kanellos Agreement and every six months thereafter, no changes have been made to Dr. . Kanellos’ remuneration as of the date hereof.
Dan Peres
On April 1, 2015, we entered into a Consultancy Agreement (the “Peres Agreement") with Dan Peres. Pursuant to the terms of the Peres Agreement, the Company will provide the Services (defined hereafter). Services shall include: oversight of the clinical trial of IMM-124E-2001 for treatment of NASH; and active involvement in all other Company activities related to pipeline products in the United States and elsewhere. Dr. Peres will introduce the Company to qualified personnel for the assistance in the delivery of the Services at the Company’s cost. Dr. Peres will require two persons in Israel which will be contracted directly by the Company and the Company shall pay a monthly sum of approximately $9,500 for such personnel. The term of the Peres Agreement commenced on April 1, 2015 and will terminate on April 30, 2017. The Company shall pay Dr. Peres $16,667 per month and Dr. Peres will be entitled to receive 1,000,000 options to purchase the Company’s ordinary shares at AUD$0.500 per ordinary share, at any time until April 1, 2017. The 1,000,000 options were subsequently vested and issued on December 9, 2016 following the successful completion of related milestone pertaining to a minimum recruitment of 100 patients into the Company’s NASH Phase 2 clinical trial. The Peres Agreement may be terminated in writing by either the Company or Dr. Peres at any time with three months’ notice.
Employee Share Option Plan
Employee Share&Option Plan (ESOP)
At the General Meeting of shareholders held on 13 November 2014 shareholders approved the rules of the ESOP and authorized directors to issue options at their discretion in accordance with the rules from time to time. Under the rules of the ESOP the Board may offer options to key management staff and consultants and in special circumstances may provide financial assistance to an entitled option holder to assist in the exercise of the ESOP options.
The aggregate number of shares that may be issued upon the exercise of the ESOP options, together with all other share purchase plans for eligible persons, shall not at any time exceed 5% of the total number of the Company's ordinary shares on issue.
During the year no options were issued under the rules of the ESOP to any Directors or Key Management Personnel:
The terms and conditions of each grant of options affecting remuneration in the current or future reporting period are as follows
| Issue Date | | Number of Options | | | Vesting Conditions | | Expiry Date | | Exercise Price
AUD$ | |
| 4 Dec 2013 | | | 1,000,000 | | | Nil | | 4 Dec 2016 | | AUD$ | 0.456 | |
| 27 Nov 2015 | | | 6,000,000 | | | (i) | | 27 Nov 2019 | | AUD$ | 0.500 | |
| 9 Dec 2016 | | | 1,000,000 | | | (ii) | | 1 Apr 2017 | | AUD$ | 0.500 | |
(i) The options with an issue date of 27 November 2015, entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$0.500. Options vest based on month of continuous services completed as per the following:
| - | 5,000,000 Options which will vest on 6th August 2016 – subject to completion of 12 months’ continuous services as a Director of the Company. |
| - | 1,000,000 Options which will vest on 6th August 2017 – subject to completion of 24 months’ continuous services as a Director of the Company. |
The assessed fair value of options granted to personnel at their grant date is allocated equally over the period from grant date to vesting date, and the amount for the 2016 financial year is included in the remuneration table as set out above. Fair values at grant date are determined using the Black-Scholes option pricing model that takes into account the exercise price, the term of the option, the impact of dilution, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk-free interest rate for the term of the option. The expected price volatility is based on the historic volatility (based on the remaining life of the options), adjusted for any expected changes to future volatility due to publically available information.
(ii) Pursuant to an agreement entered between the Company and a consultant on April 1, 2015, the Company granted 1,000,000 options, which became vested and issued on December 9, 2016, and entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$0.500. These options were vested and issued following the successful completion of related milestone pertaining to a minimum recruitment of 100 patients into the Company’s NASH Phase 2 clinical trial.
All options granted under the ESOP are deemed to be granted for no consideration.
PRINCIPAL SHAREHOLDERS
The following table and accompanying footnotes present certain information regarding the beneficial ownership of our ordinary shares based on 103,641,417 ordinary shares outstanding as of April 24, 2017 by:
| • | each person known by us to be the beneficial owner of more than 5% of our ordinary shares; |
| • | each of our directors and executive officers individually; and |
| • | all of our directors and executive officers as a group. |
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options that are exercisable within 60 days of April 24, 2017. Information with respect to beneficial ownership has been furnished to us by each director, executive officer, or 5% or more shareholder, as the case may be. Ordinary shares subject to options currently exercisable or exercisable within 60 days of April 24, 2017 are deemed to be outstanding for computing the percentage ownership of the person holding these options and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person.
Ordinary shares subject to options currently exercisable or exercisable within 60 days of April 24, 2017 are deemed to be outstanding for computing the percentage ownership of the person holding these options and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person.
Based on information known to us, as of April 24, 2017, we had 6 shareholders in the United States. These shareholders held an aggregate of 256,011 of our outstanding ordinary shares, or approximately 0.24% of our outstanding ordinary shares. A large number of our ordinary shares are held by nominee companies so we cannot be certain of the identity of those beneficial owners.
Unless otherwise indicated, to our knowledge each shareholder possesses sole voting and investment power over the ordinary shares listed subject to community property laws, where applicable. None of our shareholders have different voting rights from other shareholders. Unless otherwise indicated, the address for each of the persons listed in the table below is Immuron Limited, Suite 1, 1233 High Street, Armadale, Victoria, Australia 3143.
| | | Ordinary Shares
Beneficially
Owned Prior to
Offering | | | Ordinary Shares
Beneficially
Owned After the
Offering(1) | |
| Shareholder Name and Address | | Number | | | Percent | | | Number | | | Percent | |
| 5% Shareholders | | | | | | | | | | | | | | | | |
| Grandlodge Capital Pty Lrd. (2) Unit 10, 25-37 Chapman Street
Blackburn, North VIC 3130 | | | 13,663,364 | | | | 12.93 | % | | | | | | | | % |
Inverarey Pty Ltd and Associates Lvl 4, 349 Collins Street
Melbourne, VIC 3000 | | | 5,875,567 | | | | 5.56 | % | | | | | | | | |
| Mr Chris Retzos | | | 5,620,000 | | | | 5.32 | % | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Officers and Directors | | | | | | | | | | | | | | | | |
| Dr. Roger Aston | | | 607,116 | | | | * | % | | | | | | | | |
| Mr. Peter Anastasiou(2) | | | 13,663,364 | | | | 12.93 | % | | | | | | | | |
| Mr. Daniel Pollock | | | 300,000 | | | | * | % | | | | | | | | |
| Mr. Stephen Anastasiou | | | 4,067,857 | | | | 3.85 | % | | | | | | | | |
| Mr. Thomas Liquard | | | 134,694 | | | | * | % | | | | | | | | |
| Dr. Jerry Kanellos (PhD) | | | - | | | | - | % | | | | | | | | |
| Dr. Dan Peres (MD) | | | 79,899 | | | | * | % | | | | | | | | |
| Mr. Phillip Hains | | | 816,804 | | | | * | % | | | | | | | | |
Prof. Ravi Savarirayan | | | - | | | | - | % | | | | | | | | |
| Mr. Peter Vaughan | | | - | | | | - | % | | | | | | | | |
| Officers and directors as a group (10 persons) | | | 19,669,734 | | | | 18.62 | % | | | | | | | | |
| * | Represents beneficial ownership of less than 1% of the outstanding ordinary shares of Immuron. |
| (1) | Assumes that the underwriters will not exercise their option to purchase additional ADSs. |
| (2) | Peter Anastasiou is the Chairman of Grandlodge Capital Pty Ltd. (“Grandlodge”) and in such capacity has voting and dispositive power over the securities held by Grandlodge. |
To our knowledge, there have not been any significant changes in the ownership of our ordinary shares by major shareholders over the past three years (which is based upon substantial shareholder notices filed with the ASX).
RELATED PARTY TRANSACTIONS
Other than as disclosed below, from July 1, 2013 to December 31, 2016 we did not enter into any transactions or loans with any: (i) enterprises that directly or indirectly, through one or more intermediaries, control, are controlled by or are under common control with us; (ii) associates; (iii) individuals owning, directly or indirectly, an interest in our voting power that gives them significant influence over us, and close members of any such individual’s family; (iv) key management personnel and close members of such individuals’ families; or (v) enterprises in which a substantial interest in our voting power is owned, directly or indirectly, by any person described in (iii) or (iv) or over which such person is able to exercise significant influence.
The transactions with related parties are as follows:
| | | For the fiscal year ended
June 30, | |
| | | 2016 | | | 2015 | | | 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | |
| Short-term Loan from Grandlodge Capital Pty Ltd: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Grandlodge Capital Pty Ltd (Grandlodge) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Mr. David Plush is also an owner of Grandlodge, and its associated entities | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| On 1st December 2015 and on 6th June 2016, Immuron executed a short-term funding agreement with Grandlodge for a principle amount of AUD$1,000,000 (interest rate of 13%) and AUD$750,000 (interest rate of 15%), respectively, plus interest charges. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The short-term funding is a cash advance against the anticipated refund Immuron will receive from the Australian Taxation Office under the Research and Development Income Tax Concession Incentive for the Company's eligible R&D expenditure incurred for financial year of 2015 and 2016. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Loan from 1st December 2015 has been repaid to Grandlodge on 10th February 2016. The June 2016, loan from Grandlodge, plus applicable fees, will be repaid by the Company upon receipt of the FY2016 R&D Tax Incentive refund which was received in November 2016. Interest paid was approximately $43,000 in 2016 and loan fees paid to Grandlodge were approximately $20,000 and $15,000 in 2016, respectively. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Loans from October and December 2013 were repaid in fiscal 2014. These loan agreements were for a period of 6 months or the receipt of the R&D Tax Incentive Refund if sooner, bearing an interest rate of 18% per annum. Interest paid was approximately $15,000 in 2014. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Total paid by the Company to Grandlodge Pty Ltd during the year: | | | 1,043,863 | | | | N/A | | | | 435,495 | |
| | | | | | | | | | | | | |
| At year end the Company owed Grandlodge Pty Ltd: | | | 772,397 | | | | N/A | | | | N/A | |
| | | | | | | | | | | | | |
The Company fully repaid the loan in the amount of approximately $772,000 outstanding as of June 30, 2016 to Grandlodge. | | | | | | | | | | | | |
| | | For the fiscal year ended
June 30, | |
| | | 2016 | | | 2015 | | | 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | |
| Services rendered by Grandlodge Pty Ltd to Immuron Ltd: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Grandlodge, and its associated entities, are marketing, warehousing and distribution logistics companies. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Commencing on 1 June 2013, Grandlodge was verbally contracted on terms to provide warehousing, distribution and invoicing services for Immuron’s products for AUD$70,000 per annum. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| These fees will be payable in new fully paid ordinary shares in Immuron Limited at a set price of AUD$0.16 per share representing Immuron Limited’s share price at the commencement of the verbal agreement. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The shares to be issued to Grandlodge, or its associated entities, as compensation in lieu of cash payment for the services rendered under this verbal agreement have been subject to the approval of Immuron shareholders at Company shareholder meetings held over the past 18 months. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Grandlodge will also be reimbursed in cash for all reasonable costs and expenses incurred in accordance with their scope of works under the verbal agreement, unless both parties agree to an alternative method of payment. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The verbal agreement is cancellable by either party upon providing the other party with 30 days written notice of the termination of the agreement. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Service fees paid to Grandlodge Pty Ltd during the year through the issue of equity: | | | 87,500 | | | | 11,667 | | | | 75,833 | |
| | | | | | | | | | | | | |
| Total paid by the Company to Grandlodge Pty Ltd during the year: | | | 87,500 | | | | 11,667 | | | | 75,833 | |
| | | | | | | | | | | | | |
| At year end the Company owed Grandlodge Pty Ltd: | | | 35,000 | | | | 58,333 | | | | - | |
| | | For the fiscal year ended
June 30, | |
| | | 2016 | | | 2016 | | | 2016 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | |
| Premises Rental services received from Wattle Laboratories Pty Ltd to Immuron Ltd: | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Wattle Laboratories Pty Ltd (Wattle) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Commencing on 1 January 2016, Immuron executed a Lease Agreement with Wattle whereby Immuron will lease part of their Blackburn office facilities for Immuron's operations at a rental rate of AUD$38,940 per annum, payable in monthly installments. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The lease is for a 3 year term with an additional 3 year option period. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The lease is cancellable by either party upon 6 months written notice of termination of the agreement. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Rental fees paid to Wattle Laboratories Pty Ltd during the year through the issue of equity: | | | Nil | | | | N/A | | | | N/A | |
| | | | | | | | | | | | | |
| Total paid by the Company to Wattle Laboratories Pty Ltd during the year: | | | 19,470 | | | | N/A | | | | N/A | |
| | | | | | | | | | | | | |
| At year end the Company owed Wattle Laboratories Pty Ltd: | | | 21,417 | | | | N/A | | | | N/A | |
For the six months ended December 31, 2016:
Short-Term Loan
Grandlodge Capital Pty Ltd (Grandlodge) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou, and owns a top 20 shareholding in Immuron Limited. On 6th June 2016, Immuron executed a short-term funding agreement with Grandlodge for a principal amount of AUD$750,000, plus interest charges.
The short-term funding is a cash advance against the anticipated refund Immuron will receive from the Australian Taxation Office under the Research and Development Income Tax Concession Incentive for the Company's eligible R&D expenditure incurred for financial year of 2016.
Loan has been repaid to Grandlodge on 2nd December 2016. Interest expense for the six months ended December 31, 2016 was AUD $47,158.
Service rendered by Grandlodge Pty Ltd to Immuron Ltd
Grandlodge, and its associated entities, are marketing, warehousing and distribution logistics. Mr David Plush is also an owner of Grandlodge, and its associated entities.
Commencing on 1st June 2013, Grandlodge was verbally contracted to provide warehousing, distribution and invoicing services for Immuron’s products for AUD$70,000 per annum. These fees will be payable in new fully paid ordinary shares in Immuron Limited at a set price of AUD$0.16 per share representing Immuron Limited’s share price at the commencement of the verbal agreement.
The shares to be issued to Grandlodge, or its associated entities, as compensation in lieu of cash payment for the services rendered under this verbal agreement have been subject to the approval of Immuron shareholders at Company shareholder meetings held over the past 18 months.
Grandlodge will also be reimbursed in cash for all reasonable costs and expenses incurred in accordance with their scope of works under the verbal agreement, unless both parties agree to an alternative method of payment.
The verbal agreement is cancellable by either party upon providing the other party with 30 days written notice of the termination of the agreement.
During the six months period ended December 31, 2016, an amount of AUD$70,000 was charged to the Group’s expenses in relation to service rendered by Grandlodge. This amount remained payable as at the period end.
Premises Rental services received from Wattle Laboratories Pty Ltd to Immuron Ltd:
Wattle Laboratories Pty Ltd (Wattle) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou. Commencing on 1st January 2016, Immuron executed a Lease Agreement with Wattle whereby Immuron will lease part of their Blackburn office facilities for Immuron's operations at a rental rate of AUD$38,940 per annum, payable in monthly installments.
The lease is for a 3 year term with an additional 3 year option period.
The lease is cancellable by either party upon 6 months written notice of termination of the agreement.
During the six months period ended December 31, 2016, an amount of AUD$19,470 (excluding GST) was charged to the Group’s expenses in relation to service rendered by Wattle. This amount was paid during the period.
Other related party transactions:
On August 25th on behalf of Immuron, Grandlodge purchased US$1,500,000 at the cost of AUD$1,968,762. On the same day Immuron paid Grandlodge AUD$1,968,762 to settle this transaction. On Sept 12th Grandlodge returned the USD$1,500,000 purchase to Immuron. Grandlodge received no financial gains or benefits from this transaction.
DESCRIPTION OF SHARE CAPITAL
General
The following description of our ordinary shares is only a summary. We encourage you to read our Constitution, which is included as an exhibit to this registration statement, of which this prospectus forms a part.
We are a public company limited by shares registered under the Corporations Act by the Australian Securities and Investments Commission, or ASIC. Our corporate affairs are principally governed by our Constitution, the Corporations Act and the ASX Listing Rules. Our ordinary shares trade on the ASX, and we are applying to list the ADSs on NASDAQ.
The Australian law applicable to our Constitution is not significantly different than a U.S. company’s charter documents except we do not have a limit on our authorized share capital, the concept of par value is not recognized under Australian law and as further discussed under “—Our Constitution.”
Subject to restrictions on the issue of securities in our Constitution, the Corporations Act and the ASX Listing Rules and any other applicable law, we may at any time issue shares and grant options or warrants on any terms, with the rights and restrictions and for the consideration that our board of directors determine.
The rights and restrictions attaching to ordinary shares are derived through a combination of our Constitution, the common law applicable to Australia, the ASX Listing Rules, the Corporations Act and other applicable law. A general summary of some of the rights and restrictions attaching to our ordinary shares are summarized below. Each ordinary shareholder is entitled to receive notice of, and to be present, vote and speak at, general meetings.
Changes to Our Share Capital
As of December 31, 2016 and June 30, 2016, we had (i) 103,641,417 and 78,099,646 ordinary shares outstanding, respectively and (ii) outstanding options to purchase an aggregate of 34,177,523 and 9,937,629 ordinary shares, respectively at a weighted average exercise price of AUD$0.543 and AUD$0.529, respectively.
During the last three and a half years, the following changes have been made to our ordinary share capital:
During the Six Months ended December, 31 2016, the Company issued the following securities:
| Date | | Details | | No. | | | Issue Price AUD$ | | | Total Value AUD$ | |
| 7 July 2016 | | Right issue* | | | 18,045,512 | | | | - | | | | - | |
| 7 July 2016 | | Right issue | | | 3,275,466 | | | | 0.250 | | | | 818,867 | |
| 29 September 2016 | | Right issue to oversubscribes and private placement | | | 3,968,916 | | | | 0.250 | | | | 992,229 | |
| 9 December 2016 | | Shares under ESOP – for 6 months service (vesting monthly) | | | 251,877 | | | | 0.123 | | | | 30,855 | |
| | | | | | 25,541,771 | | | | | | | | 1,841,951 | |
*As at June 30, 2016, the Company was committed to issue 18,045,512 of ordinary shares in relation to the $4,511,378 received in capital raising. These shares were subsequently issued to respective holders on July 7, 2016. 2,418,129 of these new fully paid ordinary shares were issued to Grandlodge on the same terms and conditions as all other subscribers.
During the Full Year ended 30 June 2016, the Company issued the following securities:
Date | | Details | | No. | | | Issue Price AUD$ | | | Total Value AUD$ | |
| 18 Sep 2015 | | Exercise of IMCAI Unlisted Options | | | 218,750 | | | | 0.376 | | | | 82,250 | |
| 30 Sep 2015 | | Exercise of IMCAI Unlisted Options | | | 93,750 | | | | 0.376 | | | | 35,250 | |
| 19 Oct 2015 | | Exercise of IMCAI Unlisted Options by Grandlodge | | | 556,000 | | | | 0.376 | | | | 209,056 | |
| 13 Nov 2015 | | Exercise of IMCAI Unlisted Options | | | 41,666 | | | | 0.376 | | | | 15,667 | |
| 27 Nov 2015 | | Issue of Shares in lieu of cash payment for services as per Resolution 4 of the Annual General Meeting (AGM) held on 25 Nov 2015 | | | 546,875 | | | | 0.160 | | | | 87,500 | |
| 24 Feb 2016 | | Issue in accordance with executed funding agreement with a New York based Investment fund provider announced to the ASX on 17 Feb 2016 | | | 294,118 | | | | 0.340 | | | | 100,000 | |
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | AUD$ | | | AUD$ | |
| 24 Feb 2016 | | Issue of fully paid escrow shares as security for any repayment default of the Convertible Loan in accordance with executed funding agreement with a New York based Investment fund provider and announced to the ASX on 17 Feb 2016 | | | 2,000,000 | | | | 0.400 | | | | 800,000 | |
| 13 Apr 2016 | | Issue in accordance with executed funding agreement with a New York based Investment fund provider announced to the ASX on 17 Feb 2016 | | | 326,797 | | | | 0.306 | | | | 100,000 | |
| 18 Apr 2016 | | First repayment of Convertible Note Security in accordance with executed funding agreement with a New York based investment fund provider announced to the ASX on 17 Feb 2016 | | | 241,764 | | | | 0.312 | | | | 75,333 | |
| 16 May 2016 | | Exercise of IMCAI Unlisted Options | | | 150,000 | | | | 0.276 | | | | 41,400 | |
| 16 May 2016 | | Second repayment of Convertible Note Security in accordance with executed funding agreement with a New York based investment fund provider announced to the ASX on 17 Feb 2016 | | | 265,694 | | | | 0.284 | | | | 75,333 | |
| 31 May 2016 | | Issue of Shares in lieu of cash payment for services received | | | 400,000 | | | | 0.250 | | | | 100,000 | |
| 30 Jun 2016 | | Shares to be Issued from Capital Raising as at 30 June 2016 | | | - | | | | - | | | | 4,511,378 | |
| Total 2016 Movement | | | | 5,135,414 | | | | | | | | 6,233,167 | |
During the Full Year ended 30 June 2015, the Company issued the following securities:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | | | |
| 20 Nov 2014 | | Capital Consolidation on a 40:1 basis approved by shareholders at the Company's Annual General Meeting held on 13 Nov 2014 | | | (2,920,770,804 | ) | | | - | | | | - | |
| 21 Nov 2014 | | Issue of shares to supplier in lieu of cash payment for services rendered approved by shareholders at the Company's Annual general Meeting held on 13 Nov 2014 | | | 72,916 | | | | 0.160 | | | | 11,667 | |
| Total 2015 Movement | | | (2,920,697,888 | ) | | | | | | | 11,667 | |
During the Full Year ended 30 June 2014, the Company issued the following securities:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | $ | | | $ | |
| | | | | | | | | | | | |
| 6 Dec 2013 | | Issue of shares as per resolution 4 approved by shareholders at the Annual General Meeting of the Company held on 29 Nov 2013 | | | 8,750,000 | | | | 0.004 | | | | 35,000 | |
| 6 Dec 2013 | | Issue of shares as per resolutions 5, 6, & 8 approved by shareholders at the Annual General Meeting of the Company held on 29 Nov 2013 | | | 9,479,167 | | | | 0.006 | | | | 56,875 | |
| 3 Feb 2014 | | Exercise of IMCOA options | | | 29,075 | | | | 0.040 | | | | 1,163 | |
| 3 Mar 2014 | | Issue of shares through fully underwritten rights issue | | | 1,670,642,320 | | | | 0.005 | | | | 8,353,212 | |
| 3 Mar 2014 | | Issue of shares to Grandlodge & related owners as part of fully underwritten rights issue | | | 261,103,082 | | | | 0.005 | | | | 1,305,516 | |
| 29 May 2014 | | Issue of shares as per resolution 2 approved by shareholders at the General Meeting of the Company held on 27 May 2014 | | | 10,208,333 | | | | 0.004 | | | | 40,833 | |
| Total 2014 Movement | | | 1,960,211,977 | | | | | | | | 9,792,599 | |
Our Constitution
Our Constitution is similar in nature to the bylaws of a U.S. corporation. It does not provide for or prescribe any specific objectives or purposes of Immuron. Our Constitution is subject to the terms of the ASX Listing Rules and the Corporations Act. It may be amended or repealed and replaced by special resolution of shareholders, which is a resolution passed by at least 75% of the votes cast by shareholders entitled to vote on the resolution.
Under Australian law, a company has the legal capacity and powers of an individual both within and outside Australia. The material provisions of our Constitution are summarized below. This summary is not intended to be complete nor to constitute a definitive statement of the rights and liabilities of our shareholders. Our Constitution is filed as an exhibit to the registration statement, of which this prospectus forms a part.
Interested Directors
A director may not vote in respect of any contract or arrangement in which the director has, directly or indirectly, any material interest according to our Constitution. Such director must not be counted in a quorum, must not vote on the matter and must not be present at the meeting while the matter is being considered. However, that director may execute or otherwise act in respect of that contract or arrangement notwithstanding any material personal interest.
Unless a relevant exception applies, the Corporations Act requires our directors to provide disclosure of certain interests or conflicts of interests and prohibits directors from voting on matters in which they have a material personal interest and from being present at the meeting while the matter is being considered. In addition, the Corporations Act and the ASX Listing Rules require shareholder approval of any provision of related party benefits to our directors.
Borrowing Powers Exercisable by Directors
Pursuant to our Constitution, the management and control of our business affairs are vested in our board of directors. Our board of directors has the power to raise or borrow money, and charge any of our property or business or any uncalled capital, and may issue debentures or give any other security for any of our debts, liabilities or obligations or of any other person, in each case, in the manner and on terms it deems fit.
Retirement of Directors
Pursuant to our Constitution and the ASX Listing Rules, there must be an election of Directors at each annual general meeting. The directors, other than the managing director, who are to stand for election at each annual general meeting are: (i) any Director required to retire after a period of 3 years in office, (ii) any Director appointed by the other Directors in the year preceding the annual general meeting, (iii) any new directors, or (iv) if no person is standing for election for the aforementioned reasons then the director longest in office since last being elected. A director, other than the director who is the Chief Executive Officer, must retire from office at the conclusion of the third annual general meeting after which the director was elected. Retired directors are eligible for a re-election to the board of directors unless disqualified from acting as a director under the Corporations Act or our Constitution.
Rights and Restrictions on Classes of Shares
The rights attaching to our ordinary shares are detailed in our Constitution. Our Constitution provides that our directors may issue shares with preferred, deferred or other special rights, whether in relation to dividends, voting, return of share capital, or otherwise as our board of directors may determine. Subject to any approval which is required from our shareholders under the Corporations Act and the ASX Listing Rules (see “—Exemptions from Certain NASDAQ Corporate Governance Rules” and “—Change of Control”), any rights and restrictions attached to a class of shares, we may issue further shares on such terms and conditions as our board of directors resolve. Currently, our outstanding share capital consists of only one class of ordinary shares.
Dividend Rights
Our board of directors may from time to time determine to pay dividends to shareholders. All dividends unclaimed for one year after having been declared may be invested or otherwise made use of by our board of directors for our benefit until claimed or otherwise disposed of in accordance with our Constitution.
Voting Rights
Under our Constitution, and subject to any voting exclusions imposed under the ASX Listing Rules (which typically exclude parties from voting on resolutions in which they have an interest), the rights and restrictions attaching to a class of shares, each shareholder has one vote on a show of hands at a meeting of the shareholders unless a poll is demanded under the Constitution or the Corporations Act. On a poll vote, each shareholder shall have one vote for each fully paid share and a fractional vote for each share held by that shareholder that is not fully paid, such fraction being equivalent to the proportion of the amount that has been paid to such date on that share. Shareholders may vote in person or by proxy, attorney or representative. Under Australian law, shareholders of a public company are not permitted to approve corporate matters by written consent. Our Constitution does not provide for cumulative voting.
Note that ADS holders may not directly vote at a meeting of the shareholders but may instruct the depositary to vote the number of deposited ordinary shares their ADSs represent.
Right to Share in Our Profits
Pursuant to our Constitution, our shareholders are entitled to participate in our profits only by payment of dividends. Our board of directors may from time to time determine to pay dividends to the shareholders; however, no dividend is payable except in accordance with the thresholds set out in the Corporations Act.
Rights to Share in the Surplus in the Event of Liquidation
Our Constitution provides for the right of shareholders to participate in a surplus in the event of our liquidation, subject to the rights attaching to a class of shares.
No Redemption Provision for Ordinary Shares
There are no redemption provisions in our Constitution in relation to ordinary shares. Under our Constitution, any preference shares may be issued on the terms that they are, or may at our option be, liable to be redeemed.
Variation or Cancellation of Share Rights
Subject to the terms of issue of shares of that class, the rights attached to shares in a class of shares may only be varied or cancelled by a special resolution of Immuron together with either:
| • | a special resolution passed at a separate general meeting of members holding shares in the class; or |
| • | the written consent of members with at least 75% of the shares in the class. |
Directors May Make Calls
Our Constitution provides that subject to the terms on which the shares have been issued directors may make calls on a shareholder for amounts unpaid on shares held by that shareholder, other than monies payable at fixed times under the conditions of allotment. Shares represented by the ADSs issued in this offering will be fully paid and will not be subject to calls by directors.
General Meetings of Shareholders
General meetings of shareholders may be called by our board of directors. Except as permitted under the Corporations Act, shareholders may not convene a meeting. The Corporations Act requires the directors to call and arrange to hold a general meeting on the request of shareholders with at least 5% of the votes that may be cast at a general meeting or at least 100 shareholders who are entitled to vote at the general meeting. Notice of the proposed meeting of our shareholders is required at least 28 clear days prior to such meeting under the Corporations Act.
Foreign Ownership Regulation
There are no limitations on the rights to own securities imposed by our Constitution. However, acquisitions and proposed acquisitions of securities in Australian companies may be subject to review and approval by the Australian Federal Treasurer under the Foreign Acquisitions and Takeovers Act 1975, or the FATA, which generally applies to acquisitions or proposed acquisitions:
| • | by a foreign person (as defined in the FATA) or associated foreign persons that would result in such persons having an interest in 20% or more of the issued shares of, or control of 20% or more of the voting power in, an Australian company; and |
| • | by non-associated foreign persons that would result in such foreign person having an interest in 40% or more of the issued shares of, or control of 40% or more of the voting power in, an Australian company, where the Australian company is valued above the monetary threshold prescribed by FATA. |
However, no such review or approval under the FATA is required if the foreign acquirer is a U.S. entity and the value of the target is less than AUD$1,094 million.
The Australian Federal Treasurer may prevent a proposed acquisition in the above categories or impose conditions on such acquisition if the Treasurer is satisfied that the acquisition would be contrary to the national interest. If a foreign person acquires shares or an interest in shares in an Australian company in contravention of the FATA, the Australian Federal Treasurer may order the divestiture of such person’s shares or interest in shares in that Australian company.
Ownership Threshold
There are no provisions in our Constitution that require a shareholder to disclose ownership above a certain threshold. The Corporations Act, however, requires a shareholder to notify us and the ASX once it, together with its associates, acquires a 5% interest in our ordinary shares, at which point the shareholder will be considered to be a “substantial” shareholder. Further, once a shareholder owns a 5% interest in us, such shareholder must notify us and the ASX of any increase or decrease of 1% or more in its holding of our ordinary shares, and must also notify us and the ASX on its ceasing to be a “substantial” shareholder. Upon becoming a U.S. public company, our shareholders will also be subject to disclosure requirements under U.S. securities laws.
Issues of Shares and Change in Capital
Subject to our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, we may at any time issue shares and grant options or warrants on any terms, with preferred, deferred or other special rights and restrictions and for the consideration and other terms that the directors determine.
Subject to the requirements of our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, including relevant shareholder approvals, we may consolidate or divide our share capital into a larger or smaller number by resolution, reduce our share capital (provided that the reduction is fair and reasonable to our shareholders as a whole and does not materially prejudice our ability to pay creditors) or buy back our ordinary shares whether under an equal access buy-back or on a selective basis.
Change of Control
Takeovers of listed Australian public companies, such as Immuron are regulated by the Corporations Act, which prohibits the acquisition of a “relevant interest” in issued voting shares in a listed company if the acquisition will lead to that person’s or someone else’s voting power in Immuron increasing from 20% or below to more than 20% or increasing from a starting point that is above 20% and below 90%, subject to a range of exceptions.
Generally, a person will have a relevant interest in securities if the person:
| • | is the holder of the securities; |
| • | has power to exercise, or control the exercise of, a right to vote attached to the securities; or |
| • | has the power to dispose of, or control the exercise of a power to dispose of, the securities, including any indirect or direct power or control. |
If, at a particular time, a person has a relevant interest in issued securities and the person:
| • | has entered or enters into an agreement with another person with respect to the securities; |
| • | has given or gives another person an enforceable right, or has been or is given an enforceable right by another person, in relation to the securities (whether the right is enforceable presently or in the future and whether or not on the fulfillment of a condition); |
| • | has granted or grants an option to, or has been or is granted an option by, another person with respect to the securities; or |
| • | the other person would have a relevant interest in the securities if the agreement were performed, the right enforced or the option exercised; |
the other person is taken to already have a relevant interest in the securities.
There are a number of exceptions to the above prohibition on acquiring a relevant interest in issued voting shares above 20%. In general terms, some of the more significant exceptions include:
| • | when the acquisition results from the acceptance of an offer under a formal takeover bid; |
| • | when the acquisition is conducted on market by or on behalf of the bidder under a takeover bid, the acquisition occurs during the bid period, the bid is for all the voting shares in a bid class and the bid is unconditional or only conditioned on prescribed matters set out in the Corporations Act; |
| • | when shareholders of Immuron approve the takeover by resolution passed at general meeting; |
| • | an acquisition by a person if, throughout the six months before the acquisition, that person or any other person has had voting power in Immuron of at least 19% and, as a result of the acquisition, none of the relevant persons would have voting power in Immuron more than three percentage points higher than they had six months before the acquisition; |
| • | when the acquisition results from the issue of securities under a rights issue; |
| • | when the acquisition results from the issue of securities under dividend reinvestment schemes; |
| • | when the acquisition results from the issue of securities under underwriting arrangements; |
| • | when the acquisition results from the issue of securities through operation of law; |
| • | an acquisition that arises through the acquisition of a relevant interest in another listed company which is listed on a prescribed financial market or a financial market approved by ASIC; |
| • | an acquisition arising from an auction of forfeited shares conducted on-market; or |
| • | an acquisition arising through a compromise, arrangement, liquidation or buy-back. |
Breaches of the takeovers provisions of the Corporations Act are criminal offenses. ASIC and the Australian Takeover Panel have a wide range of powers relating to breaches of takeover provisions, including the ability to make orders canceling contracts, freezing transfers of, and rights attached to, securities, and forcing a party to dispose of securities. There are certain defenses to breaches of the takeover provisions provided in the Corporations Act.
Access to and Inspection of Documents
Inspection of our records is governed by the Corporations Act. Any member of the public has the right to inspect or obtain copies of our registers on the payment of a prescribed fee. Shareholders are not required to pay a fee for inspection of our registers or minute books of the meetings of shareholders. Other corporate records, including minutes of directors’ meetings, financial records and other documents, are not open for inspection by shareholders. Where a shareholder is acting in good faith and an inspection is deemed to be made for a proper purpose, a shareholder may apply to the court to make an order for inspection of our books.
Exemptions from Certain NASDAQ Corporate Governance Rules
The NASDAQ listing rules allow for a foreign private issuer, such as Immuron, to follow its home country practices in lieu of certain of the NASDAQ’s corporate governance standards. In connection with our NASDAQ Listing Application, we expect to rely on exemptions from certain corporate governance standards that are contrary to the laws, rules, regulations or generally accepted business practices in Australia. These exemptions being sought are described below:
| • | We expect to rely on an exemption from the independence requirements for a majority of our board of directors as prescribed by NASDAQ Listing Rules. The ASX Listing Rules do not require us to have a majority of independent directors although ASX Corporate Governance Principles and Recommendations do recommend a majority of independent directors. During fiscal 2016, we did not, have a majority of directors who were “independent” as defined in the ASX Corporate Governance Principles and Recommendations, which definition differs from NASDAQ’s definition. Accordingly, because Australian law and generally accepted business practices in Australia regarding director independence differ to the independence requirements under NASDAQ Listing Rules, we seek to claim this exemption. |
| • | We expect to rely on an exemption from the requirement that our independent directors meet regularly in executive sessions under NASDAQ Listing Rules. The ASX Listing Rules and the Corporations Act do not require the independent directors of an Australian company to have such executive sessions and, accordingly, we seek to claim this exemption. |
| • | We expect to rely on an exemption from the quorum requirements applicable to meetings of shareholders under NASDAQ Listing Rules. In compliance with Australian law, our Constitution provides that three shareholders present, in person or by proxy, attorney or a representative, shall constitute a quorum for a general meeting. NASDAQ Listing Rules require that an issuer provide for a quorum as specified in its by-laws for any meeting of the holders of ordinary shares, which quorum may not be less than 33% (1/3) of the outstanding shares of an issuer’s voting ordinary shares. Accordingly, because applicable Australian law and rules governing quorums at shareholder meetings differ from NASDAQ’s quorum requirements, we seek to claim this exemption. |
| • | We expect to rely on an exemption from the requirement prescribed by NASDAQ Listing Rules that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, private placements of securities, or the establishment or amendment of certain stock option, purchase or other compensation plans. Applicable Australian law and the ASX Listing Rules differ from NASDAQ requirements, with the ASX Listing Rules providing generally for prior shareholder approval in numerous circumstances, including (i) issuance of equity securities exceeding 15% of our issued share capital in any 12-month period (but, in determining the 15% limit, securities issued under an exception to the rule or with shareholder approval are not counted), (ii) issuance of equity securities to related parties (as defined in the ASX Listing Rules) and (iii) issuances of securities to directors or their associates under an employee incentive plan. Due to differences between Australian law and rules and the NASDAQ shareholder approval requirements, we seek to claim this exemption. |
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
American Depositary Shares
The Bank of New York Mellon, as depositary, will register and deliver American Depositary Shares, also referred to as ADSs. Each ADS will represent forty (40) shares (or a right to receive forty (40) shares) deposited with the principal Melbourne, Victoria, Australia offices of Australia and New Zealand Banking Group Ltd, Hongkong Bank of Australia and National Australia Bank Limited as custodian for the depositary. Each ADS will also represent any other securities, cash or other property which may be held by the depositary. The depositary’s office at which the ADSs will be administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at 225 Liberty Street, New York, New York 10286.
You may hold ADSs either (A) directly (i) by having an American Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs, registered in your name, or (ii) by having ADSs registered in your name in the Direct Registration System, or (B) indirectly by holding a security entitlement in ADSs through your broker or other financial institution. If you hold ADSs directly, you are a registered ADS holder, also referred to as an ADS holder. This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
The Direct Registration System, also referred to as DRS, is a system administered by The Depository Trust Company, also referred to DTC, under which the depositary may register the ownership of uncertificated ADSs, which ownership is confirmed by statements sent by the depositary to the registered holders of uncertificated ADSs.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. Australian law governs shareholder rights. The depositary will be the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs
The following is a summary of the material provisions of the deposit agreement. Because it is a summary, it does not contain all the information that may be important to you. For more complete information, you should read the entire deposit agreement and the form of ADR which summarizes certain terms of your ADSs. A copy of the deposit agreement is filed as an exhibit to the registration statement of which this prospectus forms a part. You may also obtain a copy of the deposit agreement at the SEC’s Public Reference Room which is located at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-732-0330. You may also find the registration statement and the deposit agreement on the SEC’s website at http://www.sec.gov.
Dividends and Other Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, after deducting its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent.
| · | Cash. The depositary will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest. |
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. It will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution.
| · | Shares.The depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary will only distribute whole ADSs. It will sell shares which would require it to deliver a fraction of an ADS (or ADSs representing those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed shares sufficient to pay its fees and expenses in connection with that distribution (or ADSs representing those shares). |
| · | Rights to purchase additional shares. If we offer holders of our securities any rights to subscribe for additional shares or any other rights, the depositary may make these rights available to ADS holders. If the depositary decides it is not legal and practical to make the rights available but that it is practical to sell the rights, the depositary will use reasonable efforts to sell the rights and distribute the proceeds in the same way as it does with cash. The depositary will allow rights that are not distributed or sold to lapse. In that case, you will receive no value for them. |
If the depositary makes rights available to ADS holders, it will exercise the rights and purchase the shares on your behalf. The depositary will then deposit the shares and deliver ADSs to the persons entitled to them. It will only exercise rights if you pay it the exercise price and any other charges the rights require you to pay.
U.S. securities laws may restrict transfers and cancellation of the ADSs represented by shares purchased upon exercise of rights. For example, you may not be able to trade these ADSs freely in the United States. In this case, the depositary may deliver restricted depositary shares that have the same terms as the ADSs described in this section except for changes needed to put the necessary restrictions in place.
| · | Other Distributions. The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is legal, fair and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its fees and expenses in connection with that distribution. |
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs at the depositary’s office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your request, risk and expense, the depositary will deliver the deposited securities at its office, if feasible.
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Alternatively, upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do you vote?
ADS holders may instruct the depositary how to vote the number of deposited shares their ADSs represent. Otherwise, you won’t be able to exercise your right to vote unless you withdraw the shares. However, you may not know about the meeting enough in advance to withdraw the shares.
The depositary will notify ADS holders of shareholders’ meetings and arrange to deliver our voting materials to them if we ask it to. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they much reach the depositary by a date set by the depositary.
The depositary will try, as far as practical, subject to the laws of Australia and of our articles of association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. The depositary will only vote or attempt to vote as instructed.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise your right to vote and there may be nothing you can do if your shares are not voted as you requested.
In order to give you a reasonable opportunity to instruct the Depositary as to the exercise of voting rights relating to Deposited Securities, if we request the Depositary to act, we agree to give the Depositary notice of any such meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date.
Fees and Expenses
| Persons depositing or withdrawing shares or ADS holders must pay: | | For: |
| | | |
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | | Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
| | | |
| $.05 (or less) per ADS | | Any cash distribution to ADS holders |
| | | |
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | | Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to ADS holders |
| | | |
| $.05 (or less) per ADS per calendar year | | Depositary services |
| | | |
| Registration or transfer fees | | Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
| | | |
| Expenses of the depositary | | Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) converting foreign currency to U.S. dollars |
| | | |
| Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes | | As necessary |
| | | |
| Any charges incurred by the depositary or its agents for servicing the deposited securities | | As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, subject to the depositary’s obligations under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request.
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your American Depositary Shares to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
Reclassifications, Recapitalizations and Mergers
| If we: | Then: |
| | |
· Change the nominal or par value of our shares · Reclassify, split up or consolidate any of the deposited securities · Distribute securities on the shares that are not distributed to you · Recapitalize, reorganize, merge, liquidate, sell all or substantially all of our assets, or take any similar action | The cash, shares or other securities received by the depositary will become deposited securities. Each ADS will automatically represent its equal share of the new deposited securities. The depositary may distribute new ADSs representing the new deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities. |
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will terminate the deposit agreement at our direction by mailing notice of termination to the ADS holders then outstanding at least 30 days prior to the date fixed in such notice for such termination. The depositary may also terminate the deposit agreement by mailing notice of termination to us and the ADS holders if 60 days have passed since the depositary told us it wants to resign but a successor depositary has not been appointed and accepted its appointment.
After termination, the depositary and its agents will do the following under the deposit agreement but nothing else:
| · | collect distributions on the deposited securities, |
| · | sell rights and other property, and |
| · | deliver shares and other deposited securities upon cancellation of ADSs. |
Four months after termination, the depositary may sell any remaining deposited securities by public or private sale. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement for the pro rata benefit of the ADS holders that have not surrendered their ADSs. It will not invest the money and has no liability for interest. The depositary’s only obligations will be to account for the money and other cash. After termination our only obligations will be to indemnify the depositary and to pay fees and expenses of the depositary that we agreed to pay.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
| · | are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith; |
| · | are not liable if we are or it is prevented or delayed by law or circumstances beyond our or its control from performing our or its obligations under the deposit agreement; |
| · | are not liable if we or it exercises discretion permitted under the deposit agreement; |
| · | are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential or punitive damages for any breach of the terms of the deposit agreement; |
| · | have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person; |
| · | are not liable for the acts or omissions of any securities depository, clearing agency or settlement system; and |
| · | may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person. |
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
| · | payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities; |
| · | satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and |
| · | compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying your ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying shares at any time except:
| · | when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books; (ii) the transfer of shares is blocked to permit voting at a shareholders' meeting; or (iii) we are paying a dividend on our shares; |
| · | when you owe money to pay fees, taxes and similar charges; or |
| · | when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities. |
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Pre-release of ADSs
The deposit agreement permits the depositary to deliver ADSs before deposit of the underlying shares. This is called a pre-release of the ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are canceled before the pre-release transaction has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to the depositary. The depositary may receive ADSs instead of shares to close out a pre-release. The depositary may pre-release ADSs only under the following conditions: (1) before or at the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing that it or its customer owns the shares or ADSs to be deposited; (2) the pre-release is fully collateralized with cash or other collateral that the depositary considers appropriate; and (3) the depositary must be able to close out the pre-release on not more than five business days' notice. In addition, the depositary will limit the number of ADSs that may be outstanding at any time as a result of pre-release, although the depositary may disregard the limit from time to time if it thinks it is appropriate to do so.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge thatthe DRS and Profile Modification System, or Profile, will apply to uncertificated ADSs upon acceptance thereof to DRS by DTC. DRS is the system administered by DTC under which the depositary may register the ownership of uncertificated ADSs, which ownership will be confirmed by statements sent by the depositary to the registered holders of uncertificated ADSs. Profile is a required feature of DRS that allows a DTC participant, claiming to act on behalf of a registered holder of ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery described in the paragraph above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that the depositary’s reliance on and compliance with instructions received by the depositary through the DRS/Profile System and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder communications; inspection of register of holders of ADSs
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities. The depositary will send you copies of those communications if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs.
Disclosure of Interests
We may from time to time request ADS holders to provide information as to the capacity in they own or owned ADSs and regarding the identity of any other persons then or previously interested in such ADSs and the nature of such interest. Each ADS holder agrees to provide any information of that kind that is requested by us or the depositary. To the extent that provisions of or governing the deposited securities or the rules or regulations of any governmental authority or securities exchange or automated quotation system may require the disclosure of beneficial or other ownership of deposited securities, other shares and other securities to us or other persons and may provide for blocking transfer and voting or other rights to enforce such disclosure or limit such ownership, the depositary has agreed to use its reasonable efforts to comply with our written instructions in respect of any such enforcement or limitation.
DESCRIPTION OF THE WARRANT
The following summary of certain terms of the Warrants is not complete and is subject to, and qualified in its entirety by the provisions of the ADS Warrant Agent Agreement and Form of Global Warrant to Purchase ADSs, which are filed as exhibits to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms set forth in the ADS Warrant Agent Agreement and Form of Global Warrant to Purchase ADSs. Warrants issued in connection with this offering will be administered by The Bank of New York Mellon as Warrant Agent.
• Global Certificates, Book-entry Interests. The Warrants will be represented by one or more global certificates in registered form. The global certificate will be deposited with the Warrant Agent as custodian for DTC and registered in the name of Cede & Co., as nominee of DTC. Ownership of interests in the global warrant certificate will be limited to persons that have accounts with DTC or persons that have accounts with DTC participants. Book-entry interests in the Warrants will be shown on, and transfers of such interests will be effected only through records maintained by DTC and its participants. So long as the Warrants are held in global form, DTC will be considered the sole holder of the Warrants. Beneficial owners must rely on the procedures of the participants through which they own book-entry interests to exercise their Warrants or transfer their Warrants.
• Exercisability. The Warrants are exercisable immediately upon issuance and at any time up to the date that is five years from the date of issuance. The Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice accompanied by payment in full for the number of ADSs purchased upon such exercise. The Company will pay the ADS issuance fee of US$[0.05] per ADS and any other applicable charges and taxes in connection with any such exercise.
• Maximum Percentage. A holder of a Warrant shall not have the right to exercise such Warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates and certain other persons), would beneficially own in excess of 4.99% (the “Maximum Percentage”) of the ordinary shares outstanding immediately after giving effect to such exercise. Subject to certain exceptions, “beneficial ownership” for purposes of determining the Maximum Percentage is calculated in accordance with Section 13(d) of the Exchange Act and the regulations of the SEC thereunder. Upon request by a Warrant holder, we will provide current information regarding the number of our outstanding ordinary shares.
• Exercise Price. The initial exercise price per ADS purchasable upon exercise of the Warrants is equal to US$[___]. The Warrants may not be exercised on a “cashless” or “net exercise” basis.
• Restrictive Legend Events. We will notify the Warrant Agent and each holder if we are unable to deliver ADSs via DTC transfer or otherwise (without restrictive legend), because (a) the SEC has issued a stop order with respect to the registration statement relating to the ADSs, (b) the SEC otherwise has suspended or withdrawn the effectiveness of such registration statement, either temporarily or permanently, (c) we have suspended or withdrawn the effectiveness of the registration statement, either temporarily or permanently, or (d) otherwise (each a “Restrictive Legend Event”). If a Restrictive Legend Event occurs after a Warrant holder has exercised a Warrant in accordance with its terms but prior to the delivery of the ADSs, or if we do not cause the depositary to timely deliver ADSs to a Warrant holder upon exercise of the Warrants, we will be obligated to pay a cash buy-in amount to the holder of the Warrants who did not receive ADSs upon such holder’s exercise of Warrants.
• Anti-Dilution Provisions. The exercise price per Warrant and the numbers of Warrants shall be subject to adjustment from time to time in accordance with the ASX Listing Rules upon the occurrence of certain stock dividends and distributions, stock splits, stock subdivisions and combinations, reclassifications, rights issues, or similar events affecting our ADSs or ordinary shares, or upon the occurrence of a change in ADS ratio.
• Transferability. Subject to applicable laws, the Warrants may be transferred at the option of the holders upon surrender of the Warrants to us together with the appropriate instruments of transfer.
• Warrant Agent and Exchange Listing. The Warrants will be issued in registered form under an ADS Warrant Agent Agreement between The Bank of New York Mellon, as warrant agent and us and listed on The NASDAQ Capital Market under the symbol “IMRNW”.
• Rights as a Shareholder. Except as otherwise provided in the ADS Warrant Agent Agreement or by virtue of such holder’s ownership of ADSs or ordinary shares, the holder of Warrants does not have rights or privileges of a holder of ADSs or ordinary shares, including any voting rights, until the holder exercises the Warrant.
SHARES ELIGIBLE FOR FUTURE SALE
Upon completion of this offering, there will be outstanding ordinary shares, including shares underlying the ADSs, and ADSs, representing approximately % of our outstanding ordinary shares.
Future sales of substantial amounts of our ordinary shares or ADSs in the public market in the United States or in Australia, including ordinary shares issued upon exercise of outstanding options, or the possibility of such sales, could negatively affect the market price in the United States of the ADSs and our ability to raise equity capital in the future.
All of the ADSs sold in the offering will be freely transferable in the United States by persons other than our “affiliates,” as that term is defined in Rule 144 under the Securities Act. As defined in Rule 144, an “affiliate” of an issuer is a person that directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, the issuer. ADSs purchased by one of our affiliates may not be resold, except pursuant to an effective registration statement or an exemption from registration, including Rule 144 under the Securities Act (as described below).
Lock-up Agreements
We and our executive officers and directors have generally agreed not to sell or transfer any ordinary shares, ADSs or other capital stock of Immuron or securities convertible into or exchangeable or exercisable for ordinary shares, ADSs or other capital stock of Immuron for (i) 12 months after the date of this prospectus in the case of our directors and officers and (ii) 180 days after the date of this prospectus in the case of the Company without first obtaining the written consent of Joseph Gunner & Co., LLC. Specifically, we and these other persons have agreed, with certain limited exceptions, not to directly or indirectly:
| • | offer to sell, sell, pledge, contract to sell, purchase any option to sell, grant any option for the purchase of, lend, or otherwise dispose of directly or indirectly, including the filing or participation in a filing with the SEC of a registration statement under the Securities Act to register, any of our ordinary shares or ADSs or any securities convertible into, or exercisable or exchangeable for our ordinary shares, ADSs, options or warrants or other rights to acquire ordinary shares or ADSs; or |
| • | enter into any swap or other agreement, arrangement, hedge or transaction that transfers, in whole or in part, directly or indirectly, the economic benefits or risks of ownership of any ordinary shares, ADSs or other capital stock or any securities convertible into or exercisable or exchangeable for ordinary shares, ADSs or other capital stock. |
For more detail on the lock-up agreements, see “Underwriting.”
Rule 144
In general, under Rule 144 of the Securities Act and beginning 90 days after the date of this prospectus, a person who is not deemed to have been our affiliate at any time during the three months preceding a sale and who has beneficially owned “restricted securities” within the meaning of Rule 144 for more than six months would be entitled to sell an unlimited number of shares, subject only to the availability of current public information about us. A non-affiliate who has beneficially owned “restricted securities” for at least one year from the later of the date these shares were acquired from us or from our affiliate would be entitled to freely sell those shares.
A person who is deemed to be an affiliate of ours and who has beneficially owned “restricted securities” for at least six months would be entitled to sell, within any three-month period, a number of shares that is not more than the greater of:
| • | 1.0% of the number of our ordinary shares then outstanding; or |
| • | the average weekly reported trading volume of our ordinary shares on NASDAQ during the four calendar weeks preceding the date on which a notice of the sale on Form 144 is filed with the SEC by such person. |
Sales under Rule 144 of the Securities Act by persons who are deemed to be our affiliates are also subject to manner-of-sale provisions, notice requirements and the availability of current public information about us as specified in Rule 144. In addition, in each case, these shares would remain subject to lock-up arrangements and would only become eligible for sale when the lock-up period expires.
Regulation S
Regulation S provides generally that sales made in offshore transactions are not subject to the registration or prospectus delivery requirements of the Securities Act.
Rule 701
In general, under Rule 701 of the Securities Act, each of our employees, consultants or advisors who purchases our ordinary shares from us in connection with a compensatory stock plan or other written agreement executed prior to the completion of this offering is eligible to resell such ordinary shares in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144.
TAXATION
The following is a summary of material U.S. federal and Australian income tax considerations to U.S. holders, as defined below, of the acquisition, ownership and disposition of ordinary shares and ADSs. This discussion is based on the laws in force as of the date of this registration statement, and is subject to changes in the relevant income tax law, including changes that could have retroactive effect. The following summary does not take into account or discuss the tax laws of any country or other taxing jurisdiction other than the United States and Australia. Holders are advised to consult their tax advisors concerning the overall tax consequences of the acquisition, ownership and disposition of ordinary shares and ADSs in their particular circumstances. This discussion is not intended, and should not be construed, as legal or professional tax advice.
This summary does not address the effects of U.S. federal estate and gift tax laws, the alternative minimum tax, or any state and local tax considerations within the United States, and is not a comprehensive description of all U.S. federal or Australian income tax considerations that may be relevant to a decision to acquire or dispose of ordinary shares or ADSs. Furthermore, this summary does not address U.S. federal or Australian income tax considerations relevant to holders subject to taxing jurisdictions other than, or in addition to, the United States and Australia, and does not address all possible categories of holders, some of which may be subject to special tax rules.
U.S. Federal Income Tax Considerations
The following summary describes the material U.S. federal income tax consequences to U.S. holders (as defined below) of the acquisition, ownership and disposition of our ordinary shares and ADSs as of the date hereof. Subject to the qualifications, assumptions and limitations set forth herein, this discussion of the material U.S. federal income tax consequences to U.S. holders of our ordinary shares and ADSs represents the opinion of, our U.S. counsel. Except where noted, this summary deals only with ordinary shares or ADSs acquired in the initial offering and held as capital assets within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”).
This section does not discuss the tax consequences to any particular holder, nor any tax considerations that may apply to holders subject to special tax rules, such as:
| • | individual retirement and other tax-deferred accounts; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | individuals who are former U.S. citizens or former long-term U.S. residents; |
| • | brokers or dealers in securities or currencies; |
| • | traders that elect to use a mark-to-market method of accounting; |
| • | investors in pass-through entities for U.S. federal income tax purposes; |
| | • | persons that hold ordinary shares or ADSs as a position in a straddle or as part of a hedging, constructive sale, conversion or other integrated transaction for U.S. federal income tax purposes; |
| • | persons that have a functional currency other than the U.S. dollar; |
| • | persons that own (directly, indirectly or constructively) 10% or more of our equity; or |
| • | persons that are not U.S. holders (as defined below). |
In this section, a “U.S. holder” means a beneficial owner of ordinary shares or ADSs that is, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation created or organized in or under the laws of the United States or any state thereof or the District of Columbia; |
| • | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust (i) the administration of which is subject to the primary supervision of a court in the United States and for which one or more U.S. persons have the authority to control all substantial decisions or (ii) that has an election in effect under applicable income tax regulations to be treated as a U.S. person. |
As used in this section, a “non-U.S. holder” is a beneficial owner of ordinary shares or ADSs that is not a U.S. holder. An entity or arrangement treated as a partnership or other pass-through entity for U.S. federal income tax purposes is considered a non-U.S. holder for purposes of the tax discussion in this section . If an entity or arrangement treated as a partnership for U.S. federal income tax purposes acquires, owns or disposes of ordinary shares or ADSs, the U.S. federal income tax treatment of a partner generally will depend on the status of the partner and the activities of the partnership. Partners of partnerships that acquire, own or dispose of ordinary shares or ADSs should consult their tax advisors.
The discussion below is based upon the provisions of the Code, and the U.S. Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be replaced, revoked or modified, possibly with retroactive effect, so as to result in U.S. federal income tax consequences different from those discussed below. In addition, this summary is based, in part, upon representations made by the depositary to us and assumes that the deposit agreement, and all other related agreements, will be performed in accordance with their terms.
You are urged to consult your own tax advisor with respect to the U.S. federal, as well as state, local and non-U.S., tax consequences to you of acquiring, owning and disposing of ordinary shares or ADSs in light of your particular circumstances, including the possible effects of changes in U.S. federal and other tax laws.
ADSs
If you hold ADSs, you generally will be treated, for U.S. federal income tax purposes, as the owner of the underlying ordinary shares that are represented by such ADSs. Accordingly, deposits or withdrawals of ordinary shares for ADSs will not be treated as transactions subject to U.S. federal income tax.
Distributions
Subject to the passive foreign investment company (“PFIC”) rules discussed below, U.S. holders generally will include as dividend income the U.S. dollar value of the gross amount of any distributions of cash or property (without deduction for any withholding tax), other than certain pro rata distributions of ordinary shares, with respect to ordinary shares to the extent the distributions are made from our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. A U.S. holder will include the dividend income on the day actually or constructively received by the holder, in the case of ordinary shares, or by the depositary, in the case of ADSs. To the extent, if any, that the amount of any distribution by us exceeds our current and accumulated earnings and profits, as so determined, the excess will be treated first as a tax-free return of the U.S. holder’s tax basis in the ordinary shares or ADSs and thereafter as capital gain. Notwithstanding the foregoing, we do not intend to maintain calculations of earnings and profits, as determined for U.S. federal income tax purposes. Consequently, any distributions generally will be reported as dividend income for U.S. information reporting purposes. See “Backup Withholding Tax and Information Reporting Requirements” below. Dividends paid by us will not be eligible for the dividends-received deduction generally allowed to U.S. corporate shareholders.
Subject to certain exceptions for short-term and hedged positions, the U.S. dollar amount of dividends received by an individual, trust or estate with respect to the ordinary shares or ADSs will be subject to taxation at a maximum rate of 20% if the dividends are “qualified dividends.” Dividends will be treated as qualified dividends if (a) certain holding period requirements are satisfied, (b) we are eligible for benefits under the Convention between the Government of the United States of America and the Government of Australia for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, as amended (the “Treaty”) or our ordinary shares or ADSs are readily tradable on a U.S. securities market, and (c) we were not, in the taxable year prior to the year in which the dividend was paid, and are not, in the taxable year in which the dividend is paid, a PFIC. The Treaty has been approved for the purposes of the qualified dividend rules and we have applied to list the ADSs on NASDAQ. We do not believe we were a PFIC for our taxable year ended June 30, 2016, and do not expect to be a PFIC for our taxable year ended June 30, 2017. However, our status as a PFIC in the current taxable year ending June 30, 2017 and future taxable years will depend in part upon our use of the funds from the offering, as well as our income and assets (which for this purpose depends in part on the market value of our shares) in those years. See the discussion below under “—Passive Foreign Investment Company”. Dividends included by U.S. Holders in the amount of their net investment income when calculating limitations on the deductibility of interest income are not treated as qualified dividends. You should consult your tax advisor regarding the availability of the reduced tax rate on any dividends paid with respect to our ordinary shares or ADSs.
Dividends received by an individual, trust or estate will be counted as investment income that is subject to the 3.8% surtax on net investment income. You should consult your tax advisor to determine whether, based on all your investment income, you are subject to this tax.
Includible distributions paid in Australian dollars, including any Australian withholding taxes, will be included in the gross income of a U.S. holder in a U.S. dollar amount calculated by reference to the spot exchange rate in effect on the date of actual or constructive receipt, regardless of whether the Australian dollars are converted into U.S. dollars at that time. If Australian dollars are converted into U.S. dollars on the date of actual or constructive receipt, the tax basis of the U.S. holder in those Australian dollars will be equal to their U.S. dollar value on that date and, as a result, a U.S. holder generally should not be required to recognize any foreign exchange gain or loss.
If Australian dollars so received are not converted into U.S. dollars on the date of receipt, the U.S. holder will have a basis in the Australian dollars equal to their U.S. dollar value on the date of receipt. Any gain or loss on a subsequent conversion or other disposition of the Australian dollars generally will be treated as ordinary income or loss to such U.S. holder and generally will be income or loss from sources within the United States for foreign tax credit limitation purposes.
Dividends received by a U.S. holder with respect to ordinary shares or ADSs will be treated as foreign source income, which may be relevant in calculating the holder’s foreign tax credit limitation. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For these purposes, dividends generally will be categorized as “passive” or “general” income depending on a U.S. holder’s circumstance.
Subject to certain complex limitations, a U.S. holder generally will be entitled, at its option, to claim either a credit against its U.S. federal income tax liability or a deduction in computing its U.S. federal taxable income in respect of any Australian taxes withheld. If a U.S. holder elects to claim a deduction, rather than a foreign tax credit, for Australian taxes withheld for a particular taxable year, the election will apply to all foreign taxes paid or accrued by or on behalf of the U.S. holder in the particular taxable year.
You may not be able to claim a foreign tax credit (and instead may claim a deduction) for non-U.S. taxes imposed on dividends paid on the ordinary shares or ADSs if you (i) have held the ordinary shares or ADSs for less than a specified minimum period during which you are not protected from risk of loss with respect to such shares, or (ii) are obligated to make payments related to the dividends (for example, pursuant to a short sale).
The availability of the foreign tax credit and the application of the limitations on its availability are fact specific and are subject to complex rules. You are urged to consult your own tax advisor as to the consequences of Australian withholding taxes and the availability of a foreign tax credit or deduction. See “Australian Tax Considerations—Taxation of Dividends.”
Sale, Exchange or other Disposition of Ordinary Shares or ADSs
Subject to the PFIC rules discussed below, a U.S. holder generally will, for U.S. federal income tax purposes, recognize capital gain or loss on a sale, exchange or other disposition of ordinary shares or ADSs equal to the difference between the amount realized on the disposition and the U.S. holder’s tax basis (in U.S. dollars) in the ordinary shares or ADSs. This recognized gain or loss will generally be long-term capital gain or loss if the U.S. holder has held the ordinary shares or ADSs for more than one year. Generally, for U.S. holders who are individuals (as well as certain trusts and estates), long-term capital gains are subject to U.S. federal income tax at preferential rates. For foreign tax credit limitation purposes, gain or loss recognized upon a disposition generally will be treated as from sources within the United States. However, in limited circumstances, the Treaty can re-source U.S. source income as Australian source income. The deductibility of capital losses is subject to limitations for U.S. federal income tax purposes.
You should consult your own tax advisor regarding the availability of a foreign tax credit or deduction in respect of any Australian tax imposed on a sale or other disposition of ordinary shares or ADSs. See “Australian Tax Considerations—Tax on Sales or other Dispositions of Shares.”
Exercise, Sale, and Expiration of the Warrants
A U.S. Holder’s tax basis in the Warrants will be equal to the fair market value of the Warrants on the date of acquisition (and the holder’s basis in his ordinary shares will be reduced by the amount of the investment allocated to the Warrants). If a U.S. Holder subsequently sells the Warrants, such holder will recognize capital gain or loss in an amount equal to the amount by which the consideration received in the sale exceeds, or is less than, such holder's tax basis in the Warrants. The capital gain or loss will be long-term if the U.S. holder's holding period in the Warrants is more than one year.
A U.S. Holder who subsequently exercises the Warrants will have no income, gain or loss upon such exercise. Such holder's tax basis in the ordinary shares acquired on the exercise of the Warrants will equal the sum of the exercise price paid for the ordinary shares and the U.S. holder's tax basis in the Warrants. The holder's holding period for the ordinary shares acquired in the exercise of the Warrants will begin on the date the Warrants are exercised.
If a U.S. holder allows the acquired Warrants to expire unexercised, the expiration of the Warrants will be treated as a sale or exchange of a capital asset on the expiration date and the holder will realize a capital loss equal to the holder’s basis in the Warrants. Because the term of the Warrants is more than one year, the U.S. holder's resulting capital loss will be a long-term capital loss.
Passive Foreign Investment Company
The Code provides special, generally adverse, rules regarding certain distributions received by U.S. holders with respect to, and sales, exchanges and other dispositions, including pledges, of, shares of stock of a PFIC. A foreign corporation will be a PFIC for any taxable year if at least 75% of its gross income for the taxable year is passive income or at least 50% of its gross assets during the taxable year, based on a quarterly average and generally determined by value, produce or are held for the production of passive income. Passive income for this purpose generally includes, among other things, dividends, interest, rents, royalties, gains from commodities and securities transactions and gains from the disposition of assets that produce or are held for the production of passive income. In determining whether a foreign corporation is a PFIC, a pro-rata portion of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
Based on our business results for the last fiscal year and the composition of our assets, we believe that we were not a PFIC for U.S. federal income tax purposes for the taxable year ended June 30, 2016. Similarly, based on our business projections and the anticipated composition of our assets for our current taxable year ending June 30, 2017, we expect that we will not be a PFIC for such taxable year. However, the determination of PFIC status is a factual determination that must be made annually at the close of each taxable year and therefore, there can be no certainty as to our PFIC status for a taxable year until the close of that taxable year. Our PFIC status could change depending upon, among other things, a decrease in the trading price of our ordinary shares or ADSs and how quickly we make use of the proceeds from the offering, as well as changes in the composition and relative values of our assets and the composition of our income. Moreover, the rules governing whether certain assets are active or passive are complex and in some cases their application can be uncertain. If we were a PFIC in any year during a U.S. holder’s holding period for the ordinary shares or ADSs, we generally would continue to be treated as a PFIC for each subsequent year during which the U.S. holder owned the ordinary shares or ADSs.
If we are a PFIC for any taxable year during which a U.S. holder holds ordinary shares or ADSs, any “excess distribution” that the holder receives and any gain recognized from a sale or other disposition (including a pledge) of such ordinary shares or ADSs will be subject to special tax rules, unless the holder makes a mark-to-market election or qualified electing fund election, as discussed below. Any distribution in a taxable year that is greater than 125% of the average annual distribution received by a U.S. holder during the shorter of the three preceding taxable years or such holder’s holding period for the ordinary shares or ADSs will be treated as an excess distribution. Under these special tax rules:
| • | the excess distribution or gain will be allocated ratably over the U.S. holder’s holding period for the ordinary shares or ADSs; |
| • | the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which we were a PFIC in the U.S. holder’s holding period, will be treated as ordinary income arising in the current taxable year; and |
| • | the amount allocated to each other year will be subject to income tax at the highest rate in effect for that year and applicable to the U.S. holder and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
If we are a PFIC, the tax liability for amounts allocated to years prior to the year of disposition or excess distribution cannot be offset by any net operating loss, and gains (but not losses) recognized on the transfer of the ordinary shares or ADSs cannot be treated as capital gains, even if the ordinary shares or ADSs are held as capital assets. In addition, non-corporate U.S. holders will not be eligible for reduced rates of taxation on any dividends that we pay if we are a PFIC for either the taxable year in which the dividend is paid or the preceding year. Furthermore, unless otherwise provided by the U.S. Treasury Department, each U.S. holder of a PFIC is required to file an annual report (currently Form 8621) describing the holder’s interest in the PFIC, making an election on how to report PFIC income, and providing other information about the holder’s share of the PFIC’s income.
If we are a PFIC for any taxable year during which any of our non-U.S. subsidiaries is also a PFIC, a U.S. holder of ordinary shares or ADSs during such year would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules to such subsidiary. You should consult your tax advisor regarding the tax consequences if the PFIC rules apply to any of our subsidiaries.
In certain circumstances, in lieu of being subject to the special tax rules discussed above, you may make an election to include gain on the stock of a PFIC as ordinary income under a mark-to-market method, provided that such stock is regularly traded on a qualified exchange. Under current law, the mark-to-market election may be available to U.S. holders of ADSs if the ADSs are listed on NASDAQ, which constitutes a qualified exchange, although there can be no assurance that the ADSs will be “regularly traded” for purposes of the mark-to-market election. It should also be noted that it is intended that only the ADSs and not the ordinary shares will be listed on NASDAQ. While we would expect the Australian Stock Exchange, on which the ordinary shares are listed, to be considered a qualified exchange, no assurance can be given as to whether the Australian Stock Exchange is a qualified exchange, or that the ordinary shares would be traded in sufficient frequency to be considered regularly traded for these purposes. Additionally, because a mark-to-market election cannot be made for equity interests in any lower-tier PFIC that we may own, a U.S. holder that makes a mark-to-mark election with respect to us may continue to be subject to the PFIC rules with respect to any indirect investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. If you make an effective mark-to-market election, you will include in each year that we are a PFIC as ordinary income the excess of the fair market value of your ordinary shares or ADSs at the end of your taxable year over your adjusted tax basis in the ordinary shares or ADSs. You will be entitled to deduct as an ordinary loss in each such year the excess of your adjusted tax basis in the ordinary shares or ADSs over their fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. If you make an effective mark-to-market election, any gain you recognize upon the sale or other disposition of your ordinary shares or ADSs in a year that we are a PFIC will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. Any gain or loss you recognize upon the sale or other disposition of your ordinary shares or ADSs in a year when we are not a PFIC will be a capital gain or loss. See “—Sale, Exchange or other Disposition of Ordinary Shares or ADSs” above for the treatment of capital gains and losses.
Your adjusted tax basis in the ordinary shares or ADSs will be increased by the amount of any income inclusion and decreased by the amount of any deductions under the mark-to-market rules. If you make a mark-to-market election, it will be effective for the taxable year for which the election is made and all subsequent taxable years unless the ordinary shares or ADSs are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. You are urged to consult your tax advisors about the availability of the mark-to-market election, and whether making the election would be advisable in your particular circumstances. In the case of a valid mark-to-market election, any distributions we make would generally be subject to the rules discussed above under “—Taxation of Dividends,” except the reduced rates of taxation on any dividends received from us would not apply if we are a PFIC.
Alternatively, you can sometimes avoid the PFIC rules described above by electing to treat us as a “qualified electing fund” under Section 1295 of the Code. However, this option will not be available to you because we do not intend to comply with the requirements necessary to permit you to make this election.
U.S. holders are urged to contact their own tax advisors regarding the determination of whether we are a PFIC and the tax consequences of such status.
Backup Withholding Tax and Information Reporting Requirements
Payments of dividends with respect to the ordinary shares or ADSs and proceeds from the sale, exchange or other disposition of the ordinary shares or ADSs, by a U.S. paying agent or other U.S. intermediary, or made into the United States, will be reported to the IRS and to the U.S. holder as may be required under applicable Treasury regulations. Backup withholding may apply to these payments if the U.S. holder fails to provide an accurate taxpayer identification number or certification of exempt status or fails to comply with applicable certification requirements. Certain U.S. holders (including, among others, corporations) are not subject to backup withholding and information reporting. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a U.S. holder will be refunded (or credited against such U.S. holder’s U.S. federal income tax liability, if any), provided the required information is timely furnished to the IRS. Prospective investors should consult their own tax advisors as to their qualification for exemption from backup withholding and the procedure for establishing an exemption.
Certain individual U.S. holders (and under Treasury regulations, certain entities) may be required to report to the IRS (on Form 8938) information with respect to their investment in the ordinary shares or ADSs not held through an account with a U.S. financial institution. U.S. holders who fail to report required information could become subject to substantial penalties. U.S. holders are encouraged to consult with their own tax advisors regarding foreign financial asset reporting requirements with respect to their investment in the ordinary shares or ADSs.
U.S. holders who acquire any of the ordinary shares or ADSs for cash may be required to file an IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) with the IRS and to supply certain additional information to the IRS if (i) immediately after the transfer, the U.S. holder owns directly or indirectly (or by attribution) at least 10% of our total voting power or value or (ii) the amount of cash transferred to us in exchange for the ordinary shares or ADSs when aggregated with all related transfers under applicable regulations, exceeds U.S.$100,000. Substantial penalties may be imposed on a U.S. holder that fails to comply with this reporting requirement. Each U.S. holder is urged to consult with its own tax advisor regarding this reporting obligation.
The discussion above is not intended to constitute a complete analysis of all tax considerations applicable to an investment in ordinary shares or ADSs. You should consult with your own tax advisor concerning the tax consequences to you in your particular situation.
Australian Tax Considerations
In this section, we discuss the material Australian income tax, stamp duty and goods and services tax considerations related to the acquisition, ownership and disposal by the absolute beneficial owners of the ordinary shares or ADSs. This discussion represents the opinion of, Australian counsel to Immuron.
It is based upon existing Australian tax law as of the date of this registration statement, which is subject to change, possibly retrospectively. This discussion does not address all aspects of Australian tax law which may be important to particular investors in light of their individual investment circumstances, such as shares held by investors subject to special tax rules (for example, financial institutions, insurance companies or tax exempt organizations). In addition, this summary does not discuss any foreign or state tax considerations, other than stamp duty and goods and services tax.
Prospective investors are urged to consult their tax advisors regarding the Australian and foreign income and other tax considerations of the acquisition, ownership and disposition of the shares. As used in this summary a “Non-Australian Shareholder” is a holder that is not an Australian tax resident and is not carrying on business in Australia through a permanent establishment.
Nature of ADSs for Australian Taxation Purposes
Ordinary shares represented by ADSs held by a U.S. holder will be treated for Australian taxation purposes as held under a “bare trust” for such holder. Consequently, the underlying ordinary shares will be regarded as owned by the ADS holder for Australian income tax and capital gains tax purposes. Dividends paid on the underlying ordinary shares will also be treated as dividends paid to the ADS holder, as the person beneficially entitled to those dividends. Therefore, in the following analysis we discuss the tax consequences to Non-Australian Shareholders of ordinary shares for Australian taxation purposes. We note that the holder of an ADS will be treated for Australian tax purposes as the owner of the underlying ordinary shares that are represented by such ADSs.
Taxation of Dividends
Australia operates a dividend imputation system under which dividends may be declared to be “franked” to the extent of tax paid on company profits. Fully franked dividends are not subject to dividend withholding tax. An exemption for dividend withholding tax can also apply to unfranked dividends that are declared to be conduit foreign income, or CFI, and paid to Non-Australian Shareholders. Dividend withholding tax will be imposed at 30%, unless a shareholder is a resident of a country with which Australia has a double taxation agreement and qualifies for the benefits of the treaty. Under the provisions of the current Treaty, the Australian tax withheld on unfranked dividends that are not declared to be CFI paid by us to a resident of the United States which is beneficially entitled to that dividend is limited to 15% where that resident is a qualified person for the purposes of the Treaty.
If a Non-Australian Shareholder is a company and owns a 10% or more interest, the Australian tax withheld on dividends paid by us to which a resident of the United States is beneficially entitled is limited to 5%. In limited circumstances the rate of withholding can be reduced to zero.
Tax on Sales or other Dispositions of Shares—Capital gains tax
Non-Australian Shareholders will not be subject to Australian capital gains tax on the gain made on a sale or other disposal of ordinary shares, unless they, together with associates, hold 10% or more of our issued capital, at the time of disposal or for 12 months of the last 2 years prior to disposal.
Non-Australian Shareholders who own a 10% or more interest would be subject to Australian capital gains tax if more than 50% of our direct or indirect assets, determined by reference to market value, consists of Australian land, leasehold interests or Australian mining, quarrying or prospecting rights. The Treaty is unlikely to limit Australia’s right to tax any gain in these circumstances. Net capital gains are calculated after reduction for capital losses, which may only be offset against capital gains.
Tax on Sales or other Dispositions of Shares—Shareholders Holding Shares on Revenue Account
Some Non-Australian Shareholders may hold shares on revenue rather than on capital account for example, share traders. These shareholders may have the gains made on the sale or other disposal of the shares included in their assessable income under the ordinary income taxing provisions of the income tax law, if the gains are sourced in Australia.
Non-Australian Shareholders assessable under these ordinary income provisions in respect of gains made on shares held on revenue account would be assessed for such gains at the Australian tax rates for non-Australian residents, which start at a marginal rate of 32.5%. This rate does not include the Temporary Budget Repair Levy of 2% that applies in certain circumstances. Some relief from Australian income tax may be available to Non-Australian Shareholders under the Treaty.
To the extent an amount would be included in a Non-Australian Shareholder’s assessable income under both the capital gains tax provisions and the ordinary income provisions, the capital gain amount would generally be reduced, so that the shareholder would not be subject to double tax on any part of the income gain or capital gain.
Dual Residency
If a shareholder is a resident of both Australia and the United States under those countries’ domestic taxation laws, that shareholder may be subject to tax as an Australian resident. If, however, the shareholder is determined to be a U.S. resident for the purposes of the Treaty, the Australian tax would be subject to limitation by the Treaty. Shareholders should obtain specialist taxation advice in these circumstances.
Stamp Duty
No stamp duty is payable by Australian residents or non-Australian residents on the issue and trading of shares that are quoted on the ASX or NASDAQ at all relevant times and the shares do not represent 90% or more of all of our issued shares.
Australian Death Duty
Australia does not have estate or death duties. As a general rule, no capital gains tax liability is realized upon the inheritance of a deceased person’s shares. The disposal of inherited shares by beneficiaries may, however, give rise to a capital gains tax liability if the gain falls within the scope of Australia’s jurisdiction to tax.
Goods and Services Tax
The issue or transfer of shares to a non-Australian resident investor will not incur Australian goods and services tax.
UNDERWRITING
We and the underwriters named below have entered into an underwriting agreement, dated , 2017, with respect to the ADSs being offered. Subject to certain conditions, each underwriter has severally agreed to purchase the number of ADSs and Warrants indicated in the following table. Joseph Gunner & Co., LLC is the representative of the underwriters.
| Underwriters | | Number of
ADSs | | Number of Warrants | |
| Joseph Gunner & Co., LLC | | | | | | | |
| Rodman & Rendshaw, a unit of H.C. Wainwright & Co., LLC | | | | | | | |
| WallachBeth Capital, LLC | | | | | | | |
| | | | | | | | |
| Total | | | | | | | |
All of the ADSs and Warrants to be purchased by the underwriters will be purchased from us.
The underwriting agreement provides that the obligations of the underwriters to pay for and accept delivery of the ADSs and Warrants offered by us in this prospectus are subject to various conditions and representations and warranties, including the approval of certain legal matters by their counsel and other conditions specified in the underwriting agreement. The ordinary shares in the form of ADSs are offered by the underwriters, subject to prior sale, when, as and if issued to and accepted by them. The underwriters reserve the right to withdraw, cancel or modify the offer to the public and to reject orders in whole or in part.
The underwriting agreement provides that the underwriters are obligated to take and pay for all of the ordinary shares in the form of ADSs and Warrants offered by this prospectus if any such ADSs and Warrants are taken, other than those ADSs covered by the over-allotment option described below. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement.
Over-Allotment Option
We have granted an option to the underwriters to purchase up to 15% of the total number of ordinary shares in the form of ADSs and Warrants at the initial public offering price per share and per Warrant, less the underwriting discount, set forth on the cover page of this prospectus. This option is exercisable during the 45-day period after the date of this prospectus. The underwriters may exercise this option only to cover over-allotments made in connection with this offering. If the underwriters exercise this option in whole or in part, then the underwriters will be severally committed, subject to the conditions described in the underwriting agreement, to purchase the additional ADSs in proportion to their respective commitments set forth in the prior table.
Discounts and Commissions
The representative has advised us that the underwriters propose to offer the ordinary shares in the form of ADSs and Warrants to the public at the initial public offering price per share set forth on the cover page of this prospectus. The underwriters may offer shares to securities dealers at that price less a concession of not more than $ per ADS and $ per Warrant, of which up to $ per ADS and $ per Warrant may be reallowed to other dealers. After the initial offering to the public, the public offering price and other selling terms may be changed by the representative.
The following table summarizes the underwriting discounts and commissions and proceeds, before expenses, to us assuming both no exercise and full exercise by the underwriters of their over-allotment option:
| | | | | | | | Total | |
| | | Per ADS | | | Per Warrant | | Without
Option | | | With
Option | |
| Public offering price | | $ | | | | | | $ | | | | $ | | |
| Underwriting discounts and commissions (7%) | | $ | | | | | | $ | | | | $ | | |
| Non-accountable expense allowance (1%)(1) | | $ | | | | | | $ | | | | $ | | |
| Proceeds, before expenses, to us | | $ | | | | | | $ | | | | $ | | |
| (1) | The non-accountable expense allowance of 1% is not payable with respect to the ADS and per Warrant sold upon exercise of the underwriters’ over-allotment option. |
We have paid an expense deposit of $25,000 to the representative, which will be applied against the out-of-pocket accountable expenses that will be paid by us to the underwriters in connection with this offering, and will be reimbursed to us to the extent not incurred.
In addition, we have also agreed to pay the following expenses of the underwriters relating to the offering: (a) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $15,000 in the aggregate; (b) all filing fees and communication expenses associated with the review of this offering by FINRA; (c) all fees, expenses and disbursements relating to the registration, qualification or exemption of securities offered under the securities laws of foreign jurisdictions designated by the underwriter, including the reasonable fees and expenses of the underwriter’s blue sky counsel up to $5,000; (d) $29,500 for the underwriters’ use of Ipreo’s book-building, prospectus tracking and compliance software for this offering; (e) the underwriter’s legal fees incurred in connection with this offering in an amount up to $50,000; (f) $20,000 of the representative’s actual accountable road show expenses for the offering; and (g) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and Lucite tombstones in an amount not to exceed $2,500.
We estimate the expenses of this offering payable by us, not including underwriting discounts and commissions, will be approximately $ .
.
Representative Warrants
Upon the closing of this offering, we have agreed to issue to the representative warrants, or the Representative’s Warrants, to purchase a number of ordinary shares equal to 5% of the total ordinary shares sold in the form of ADSs in this public offering. The Representative’s Warrants will be exercisable at a per share exercise price equal to 125% of the effective per ordinary share initial public offering price. The Representative’s Warrants are exercisable at any time and from time to time, in whole or in part, during the four year period commencing one year from the effective date of the registration statement related to this offering.
The Representative’s Warrants and the ordinary shares underlying the Representative’s Warrants have been deemed compensation by the Financial Industry Regulatory Authority, or FINRA, and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The representative, or permitted assignees under such rule, may not sell, transfer, assign, pledge, or hypothecate the Representative’s Warrants or the securities underlying the Representative’s Warrants, nor will the representative engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Representative’s Warrants or the underlying ordinary shares for a period of 180 days from the effective date of the registration statement. Additionally, the Representative’s Warrants may not be sold transferred, assigned, pledged or hypothecated for a 180-day period following the effective date of the registration statement except to any underwriter and selected dealer participating in the offering and their bona fide officers or partners. The Representative’s Warrants will provide for adjustment in the number and price of the Representative’s Warrants and the ordinary shares underlying such Representative’s Warrants in the event of recapitalization, merger, stock split or other structural transaction, or a future financing undertaken by us.
Right of First Refusal
Until twelve (12) months from the effective date of this registration statement, the representative shall have an irrevocable right of first refusal to act as sole investment banker, sole book-runner and/or sole placement agent, at the representative sole discretion, for each and every future public or private equity or debt offerings, including any equity linked financing, for the Company, or any successor to or any subsidiary of the Company, on terms customary to the representative. The representative shall have the sole right to determine whether or not any other broker-dealer shall have the right to participate in any such offering and the economic terms of any such participation. The representative will not have more than one opportunity to waive or terminate the right of first refusal in consideration of any payment or fee.
Lock-Up Agreements
We and each of our directors and officers have agreed for a period of (i) twelve months after the date of this prospectus in the case of our directors and officers and (ii) 180 days after the date of this prospectus in the case of the Company, without the prior written consent of the representative, not to directly or indirectly:
| · | issue (in the case of us), offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of any ordinary shares, ordinary shares in the form of ADSs or other capital stock or any securities convertible into or exercisable or exchangeable for our ordinary shares, ordinary shares in the form of ADSs or other capital stock; or |
| · | in the case of us, file or cause the filing of any registration statement under the Securities Act with respect to any ordinary shares, ordinary shares in the form of ADSs or other capital stock or any securities convertible into or exercisable or exchangeable for our ordinary shares, ordinary shares in the form of ADSs or other capital stock; or |
| · | complete any offering of debt securities of the Company, other than entering into a line of credit with a traditional bank; or |
| · | enter into any swap or other agreement, arrangement, hedge or transaction that transfers to another, in whole or in part, directly or indirectly, any of the economic consequences of ownership of our ordinary shares, ordinary shares in the form of ADSs or other capital stock or any securities convertible into or exercisable or exchangeable for our ordinary shares, ordinary shares in the form of ADSs or other capital stock, whether any transaction described in any of the foregoing bullet points is to be settled by delivery of our ordinary shares, ordinary shares in the form of ADSs or other capital stock, other securities, in cash or otherwise, or publicly announce an intention to do any of the foregoing. |
Indemnification of Underwriters
The underwriting agreement provides that we will indemnify the underwriters against certain liabilities that may be incurred in connection with this offering, including liabilities under the Securities Act, or to contribute payments that the underwriters may be required to make in respect thereof.
Stabilization
In connection with this offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our ordinary shares, including ordinary shares in the form of ADSs. Specifically, the underwriters may over-allot in connection with this offering by selling more ADSs than they are obligated to purchase under the underwriting agreement, creating a short position in our ADSs. The short position may be either a covered short position or a naked short position. In a covered short position, the number of ADSs over-allotted by the underwriter is not greater than the number of ADSs that it may purchase in the over-allotment option. In a naked short position, the number of ADSs involved is greater than the number of ADSs in the over-allotment option. To close out a short position or to stabilize the price of our ordinary shares, the underwriters may bid for, and purchase, ordinary shares, including ordinary shares in the form of ADSs, in the open market. The underwriters may also elect to reduce any short position by exercising all or part of the over-allotment option. In determining the source of ordinary shares, including ordinary shares in the form of ADSs, to close out the short position, the underwriters will consider, among other things, the price of ordinary shares available for purchase in the open market as compared to the price at which it may purchase ADSs through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying ordinary shares, including ordinary shares in the form of ADSs, in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the ADSs in the open market after pricing that could adversely affect investors who purchase in the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing our ordinary shares in the form of ADSs in this offering because the underwriter repurchases that stock in stabilizing or short covering transactions.
Finally, the underwriters may bid for, and purchase, our ordinary shares, including ordinary shares in the form of ADSs, in market making transactions.
The foregoing transaction may stabilize or maintain the market price of our ADSs at a price that is higher than the price that might otherwise exist in the absence of these activities. The underwriters are not required to engage in these activities, and may discontinue any of these activities at any time without notice. These transactions may be effected on NASDAQ or otherwise.
EXPENSES RELATING TO THIS OFFERING
Set forth below is an itemization of the estimated expenses, excluding underwriting discounts, that are expected to be incurred in connection with our offer and sale of the ADSs. Expenses for the offering will be borne by us.
| SEC registration fee | | | $3,373.78 | |
| NASDAQ listing fee | | | | |
| Financial Industry Regulatory Authority Inc. filing fee | | | $3,249.22 | |
| Printing expenses | | | | |
| Legal fees and expenses | | | | |
| Accounting fees and expenses | | | | |
| Roadshow expenses | | | | |
| Other fees and expenses | | | | |
| Total | | $ | | |
LEGAL MATTERS
The validity of the ordinary shares represented by the ADSs and the Warrants to be issued in this offering will be passed upon for us by Francis Abourizk Lightowlers, our Australian counsel. Certain matters as to U.S. federal law and New York state law will be passed upon for us by Sichenzia Ross Ference Kesner LLP, our U.S. counsel. Loeb & Loeb LLP is U.S. counsel to the underwriters.
EXPERTS
The audited consolidated financial statements as of June 30, 2016 and 2015 and for the years ended June 30, 2016, 2015 and 2014 included in this prospectus and elsewhere in the registration statement, have been so included in reliance upon the report of Marcum LLP, independent registered public accounting firm, upon the authority of said firm as experts in accounting and auditing.
ENFORCEABILITY OF CIVIL LIABILITIES
We are a public limited company incorporated under the laws of Australia. Certain of our directors are non-residents of the United States and substantially all of their assets are located outside the United States. As a result, it may not be possible for you to:
| • | effect service of process within the United States upon our non-U.S. resident directors or on us; |
| • | enforce in U.S. courts judgments obtained against our non-U.S. resident directors or us in the United States courts in any action, including actions under the civil liability provisions of U.S. securities laws; |
| • | enforce in U.S. courts judgments obtained against our non-U.S. resident directors or us in courts of jurisdictions outside the United States in any action, including actions under the civil liability provisions of U.S. securities laws; or |
| • | bring an original action in an Australian court to enforce liabilities against our non-U.S. resident directors or us based solely upon U.S. securities laws. |
You may also have difficulties enforcing in courts outside the United States judgments that are obtained in U.S. courts against any of our non-U.S. resident directors or us, including actions under the civil liability provisions of the U.S. securities laws.
With that noted, there are no treaties between Australia and the United States that would affect the recognition or enforcement of foreign judgments in Australia. We also note that investors may be able to bring an original action in an Australian court against us to enforce liabilities based in part upon U.S. federal securities laws.
The disclosure in this section is not based on the opinion of counsel.
We have appointed Delaney Corporate Services Ltd. at 99 Washington Avenue, Suite 805A, Albany, New York 12210, as our agent to receive service of process with respect to any action brought against us in the U.S. District Court for the Southern District of New York under the federal securities laws of the United States or any action brought against us in the Supreme Court of the State of New York in the County of New York under the securities laws of the State of New York.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1, including relevant exhibits and schedules, under the Securities Act with respect to the ordinary shares to be sold in this offering. This prospectus, which constitutes a part of the registration statement, summarizes material provisions of contracts and other documents that we refer to in this prospectus. Since this prospectus does not contain all of the information contained in the registration statement, you should read the registration statement and its exhibits and schedules for further information with respect to us and our ordinary shares represented by ADSs. Statements contained in this prospectus regarding the contents of any agreement, contract or other document referred to are not necessarily complete and reference is made in each instance to the copy of the contract or document filed as an exhibit to the registration statement. All information we file with the SEC is available through the SEC’s Electronic Data Gathering, Analysis and Retrieval system, which may be accessed through the SEC’s website at www.sec.gov. Information filed with the SEC may also be inspected and copied at the public reference room maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of these documents upon payment of a duplicating fee, by writing to the SEC. Please visit the SEC’s website at www.sec.gov for further information on the SEC’s public reference room.
Immediately upon completion of this offering, we will become subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Our annual reports on Form 20-F for the year ending June 30, 2017 and subsequent years will be due within four months following the fiscal year end. We are not required to disclose certain other information that is required from U.S. domestic issuers. As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act and Regulation FD (Fair Disclosure), which was adopted to ensure that select groups of investors are not privy to specific information about an issuer before other investors.
We are, however, still subject to the anti-fraud and anti-manipulation rules of the SEC, such as Rule 10b-5. Since many of the disclosure obligations required of us as a foreign private issuer are different than those required by companies filing as a domestic issuer, our shareholders, potential shareholders and the investing public in general should not expect to receive information about us in the same amount and at the same time as information is received from, or provided by, companies filing as a domestic issuer. We are liable for violations of the rules and regulations of the SEC, which do apply to us as a foreign private issuer.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of the
Board of Directors and Shareholders
of Immuron Limited
We have audited the accompanying consolidated statements of financial position of Immuron Limited (the “Company”) as of June 30, 2016 and 2015, and the related consolidated statements of profit or loss and other comprehensive income, changes in equity and cash flows for the years ended June 30, 2016, 2015 and 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Immuron Limited as of June 30, 2016 and 2015, and the consolidated results of its operations and its cash flows for the years ended June 30, 2016, 2015 and 2014 in conformity with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
As described in Notes 2, 17, 18 and 20, the consolidated financial statements as of June 30, 2016 and for the years ended June 30, 2016, 2015 and 2014 have been restated to give effect for errors in the classification of customer discounts and allowances as a reduction to revenue, measurement and recognition of share-based payments, the accounting for equity issued in connection with convertible debt and certain amounts reflected in the statements of cash flows. Further, as described in Note 6 in the consolidated financial statements, the loss per share for each year has been restated. In addition, as described in Note 1t(ii), the Company has made certain revisions to the footnotes to the consolidated financial statements.
/s/ Marcumllp
Marcumllp
Philadelphia, Pennsylvania
December 20, 2016
| Consolidated Statement of Profit or Loss and Other Comprehensive Income |
| For the year ended 30 June |
| | | | | 2016
(Restated) | | | 2015
(Restated) | | | 2014
(Restated) | |
| | | Notes | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| Revenue | | | | | | | | | | | | | | |
| Operating Revenue | | 2 | | | 1,001,077 | | | | 1,002,380 | | | | 981,051 | |
| Total Operating Revenue | | | | | 1,001,077 | | | | 1,002,380 | | | | 981,051 | |
| | | | | | | | | | | | | | | |
| Cost of Goods Sold | | | | | (301,435 | ) | | | (316,128 | ) | | | (277,928 | ) |
| Gross Profit | | | | | 699,642 | | | | 686,252 | | | | 703,123 | |
| | | | | | | | | | | | | | | |
| Direct Selling Costs | | | | | | | | | | | | | | |
| Sales and Marketing Costs | | | | | (133,781 | ) | | | (76,794 | ) | | | (79,796 | ) |
| Freight Costs | | | | | (134,967 | ) | | | (116,379 | ) | | | (114,278 | ) |
| Total Gross Profit less Direct Selling Costs | | | | | 430,894 | | | | 493,079 | | | | 509,049 | |
| | | | | | | | | | | | | | | |
| Other Income | | 2 | | | 1,539,015 | | | | 1,591,021 | | | | 804,477 | |
| | | | | | | | | | | | | | | |
| Expenses | | | | | | | | | | | | | | |
| Amortisation | | | | | - | | | | - | | | | (680,587 | ) |
| Consulting, Employee and Director | | 3 | | | (2,840,037 | ) | | | (728,140 | ) | | | (555,487 | ) |
| Corporate Administration | | 3 | | | (1,320,570 | ) | | | (557,422 | ) | | | (492,465 | ) |
| Depreciation | | | | | (3,892 | ) | | | (3,719 | ) | | | (3,989 | ) |
| Finance Costs | | | | | (341,600 | ) | | | - | | | | (463,685 | ) |
| Impairment of Inventory | | | | | (4,176 | ) | | | (35,340 | ) | | | (50,204 | ) |
| Marketing and Promotion | | | | | (487,591 | ) | | | (304,687 | ) | | | (235,176 | ) |
| Research and Development | | | | | (3,623,961 | ) | | | (3,018,294 | ) | | | (1,289,675 | ) |
| Travel and Entertainment | | | | | (416,849 | ) | | | (128,318 | ) | | | (37,327 | ) |
| Loss Before Income Tax | | | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) |
| Income Tax Expense | | 4 | | | - | | | | - | | | | - | |
| Loss for the Period | | | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) |
| Other Comprehensive Income (Loss) | | | | | 8,846 | | | | (12,581 | ) | | | - | |
| Total Comprehensive Loss for the Period | | | | | (7,059,921 | ) | | | (2,704,401 | ) | | | (2,495,069 | ) |
| | | | | | | | | | | | | | | |
| Basic/Diluted Loss per Share (cents per share) | | 6 | | | 9.248 | | | | 3.592 | | | | 5.947 | |
The accompanying notes form part of these financial statements.
| Consolidated Statement of Financial Position |
| As of 30 June |
| | | | | 2016
(Restated) | | | 2015 | |
| | | Notes | | AUD$ | | | AUD$ | |
| ASSETS | | | | | | | | | | |
| Current Assets | | | | | | | | | | |
| Cash and cash equivalents | | 7 | | | 2,290,639 | | | | 3,116,074 | |
| Trade and other receivables | | 8 | | | 4,387,772 | | | | 1,691,629 | |
| Inventories | | 9 | | | 2,056,067 | | | | 1,146,267 | |
| Other | | 10 | | | 74,943 | | | | 44,928 | |
| Total Current Assets | | | | | 8,809,421 | | | | 5,998,898 | |
| | | | | | | | | | | |
| Non-Current Assets | | | | | | | | | | |
| Property, plant and equipment | | 12 | | | 18,063 | | | | 19,514 | |
| Total Non-Current Assets | | | | | 18,063 | | | | 19,514 | |
| TOTAL ASSETS | | | | | 8,827,484 | | | | 6,018,412 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| LIABILITIES | | | | | | | | | | |
| Current liabilities | | | | | | | | | | |
| Trade and other payables | | 14 | | | 1,986,407 | | | | 1,207,810 | |
| Borrowings | | 22 | | | 772,397 | | | | - | |
| Other financial liabilities | | 15 | | | 1,128,117 | | | | - | |
| Total Current Liabilities | | | | | 3,886,921 | | | | 1,207,810 | |
| TOTAL LIABILITIES | | | | | 3,886,921 | | | | 1,207,810 | |
| NET ASSETS | | | | | 4,940,563 | | | | 4,810,602 | |
| | | | | | | | | | | |
| EQUITY | | | | | | | | | | |
| Issued capital | | 17 | | | 45,633,354 | | | | 40,335,347 | |
| Reserves | | 18 | | | 2,128,566 | | | | 548,065 | |
| Accumulated losses | | | | | (42,821,357 | ) | | | (36,072,810 | ) |
| TOTAL EQUITY | | | | | 4,940,563 | | | | 4,810,602 | |
The accompanying notes form part of these financial statements.
| Consolidated Statement of Changes in Equity |
| For the year ended 30 June |
| | | Issued capital | | | Reserves | | | Accumulated
Losses | | | Total | |
| | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Balance as at 30 June 2013 (see Note 1(d)) | | | 31,357,697 | | | | 1,208,271 | | | | (31,756,833 | ) | | | 809,135 | |
| Total comprehensive loss for the period | | | - | | | | - | | | | (2,495,069 | ) | | | (2,495,069 | ) |
| Transactions with owners in their capacity as owners | | | | | | | | | | | | | | | | |
| Shares issued, net of costs | | | 8,967,598 | | | | - | | | | - | | | | 8,967,598 | |
| Options issued | | | - | | | | 211,721 | | | | - | | | | 211,721 | |
| Employee and consultant share options | | | - | | | | 7,191 | | | | - | | | | 7,191 | |
| Lapse or exercise of share options | | | - | | | | (760,591 | ) | | | 760,591 | | | | - | |
| Balance as at 30 June 2014 | | | 40,325,295 | | | | 666,592 | | | | (33,491,311 | ) | | | 7,500,576 | |
| Loss after income tax expense for the year | | | - | | | | - | | | | (2,691,820 | ) | | | (2,691,820 | ) |
| Other comprehensive loss for the period | | | - | | | | (12,581 | ) | | | - | | | | (12,581 | ) |
| Total comprehensive loss for the period | | | - | | | | (12,581 | ) | | | (2,691,820 | ) | | | (2,704,401 | ) |
| Transactions with owners in their capacity as owners | | | | | | | | | | | | | | | | |
| Employee and consultant share options | | | - | | | | 4,375 | | | | - | | | | 4,375 | |
| Lapse or exercise of share options | | | - | | | | (110,321 | ) | | | 110,321 | | | | - | |
| Shares issued, net of costs | | | 10,052 | | | | - | | | | - | | | | 10,052 | |
| Balance as at 30 June 2015 | | | 40,335,347 | | | | 548,065 | | | | (36,072,810 | ) | | | 4,810,602 | |
| Loss after income tax expense for the year (restated) | | | - | | | | - | | | | (7,068,767 | ) | | | (7,068,767 | ) |
| Other comprehensive income for the period | | | - | | | | 8,846 | | | | - | | | | 8,846 | |
| Total comprehensive loss for the period (restated) | | | - | | | | 8,846 | | | | (7,068,767 | ) | | | (7,059,921 | ) |
| Transactions with owners in their capacity as owners | | | | | | | | | | | | | | | | |
| Options issued/expensed (restated) | | | - | | | | 1,891,875 | | | | - | | | | 1,891,875 | |
| Lapse or exercise of share options | | | - | | | | (320,220 | ) | | | 320,220 | | | | - | |
| Shares issued, net of costs | | | 1,586,629 | | | | - | | | | - | | | | 1,586,629 | |
| Share to be issued | | | 4,511,378 | | | | - | | | | - | | | | 4,511,378 | |
| Treasury shares (restated) | | | (800,000 | ) | | | - | | | | - | | | | (800,000 | ) |
| Balance as at 30 June 2016 (Restated) | | | 45,633,354 | | | | 2,128,566 | | | | (42,821,357 | ) | | | 4,940,563 | |
The accompanying notes form part of these financial statements.
| Consolidated Statement of Cash Flows |
| For the year ended 30 June |
| | | | | 2016
(Restated) | | | 2015 | | | 2014
(Restated) | |
| | | Note | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| Cash flows Related to Operating Activities | | | | | | | | | | | | | | |
| Receipts from customers | | | | | 1,114,596 | | | | 1,402,958 | | | | 541,788 | |
| Payments to suppliers and employees | | | | | (7,710,997 | ) | | | (5,286,772 | ) | | | (3,787,497 | ) |
| Interest received | | | | | 12,165 | | | | 112,440 | | | | 88,345 | |
| Interest and other costs of finance paid | | | | | (43,863 | ) | | | 27,991 | | | | (159,864 | ) |
| Other - R&D Tax Concession Refund | | | | | 1,469,763 | | | | 722,450 | | | | 666,651 | |
| Net Cash Flows Used In Operating Activities | | 20 | | | (5,158,336 | ) | | | (3,020,933 | ) | | | (2,650,577 | ) |
| | | | | | | | | | | | | | | |
| Cash Flows Related to Investing Activities | | | | | | | | | | | | | | |
| Payment for purchases of plant and equipment | | | | | (2,441 | ) | | | (3,168 | ) | | | (15,901 | ) |
| Net Cash Flows Used In Investing Activities | | | | | (2,441 | ) | | | (3,168 | ) | | | (15,901 | ) |
| | | | | | | | | | | | | | | |
| Cash Flows Related to Financing Activities | | | | | | | | | | | | | | |
| Proceeds from issues of securities | | | | | 2,482,861 | | | | - | | | | 9,665,724 | |
| Capital raising costs | | | | | (20,299 | ) | | | (1,614 | ) | | | (819,168 | ) |
| Proceeds from borrowings | | | | | 2,950,000 | | | | - | | | | 420,000 | |
| Repayment of borrowings | | | | | (1,077,220 | ) | | | - | | | | (1,905,001 | ) |
| Net Cash Flows From/(Used In) Financing Activities | | | | | 4,335,342 | | | | (1,614 | ) | | | 7,361,555 | |
| | | | | | | | | | | | | | | |
| Net increase/(decrease) in cash and cash equivalents | | | | | (825,435 | ) | | | (3,025,715 | ) | | | 4,695,077 | |
| Cash and cash equivalents at the beginning of the year | | | | | 3,116,074 | | | | 6,141,789 | | | | 1,446,712 | |
| Cash and Cash Equivalents at the End of the Year | | | | | 2,290,639 | | | | 3,116,074 | | | | 6,141,789 | |
The accompanying notes form part of these financial statements.
| Notes to the Consolidated Financial Statements |
Note 1. Summary of Significant Accounting Policies
Corporate Information
The consolidated financial report of Immuron Limited (‘the Company’, ‘Group’) for the year ended 30 June 2016, 2015 and 2014 was authorised for issue in accordance with a resolution of the Directors on December 20, 2016.
Immuron Limited is a listed public company limited by shares incorporated and domiciled in Australia whose shares are publicly traded on the Australian Securities Exchange (ASX).
The principal activity of the Company is a product development driven biopharmaceutical Company focused on the research and development of polyclonal antibodies for the treatment and prevention of major diseases.
Basis of Preparation
The financial report is a general-purpose financial report, which has been prepared in accordance with the requirements of International Financial Reporting Standards (IFRS), required for a for-profit entity.
The financial report has been prepared on an accruals basis and is based primarily on historical costs. The financial report is presented in Australian dollars, which is the Company’s functional and presentation currency. All values are rounded to the nearest dollar unless otherwise stated.
Management is required to make judgements, estimates and assumptions about carrying values of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstance, the results of which form the basis of making the judgements. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognised in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods if the revision affects both current and future periods.
Judgements made by management in the application of IFRS that have significant effects on the financial statements and estimates with a significant risk of material adjustments in the next year are disclosed, where applicable, in the relevant notes to the financial statements.
Accounting policies are selected and applied in a manner which ensures that the resulting financial information satisfies the concepts of relevance and reliability, thereby ensuring that the substance of the underlying transactions or other events is reported.
Statement of Compliance
This financial report complies with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board.
New, revised or amending Accounting Standards and Interpretations adopted
The Company has adopted all of the new, revised or amending Accounting Standards and Interpretations issued by the International Accounting Standards Board ('IASB') that are mandatory for the current reporting period.
There were no significant new standards adopted during the reporting periods.
Accounting Standards and Interpretations that have recently been issued or amended but are not yet effective and have not been adopted by the Group for the annual reporting period ending 30 June 2016 are outlined in the table below.
| Standard | | Mandatory date for annual
reporting periods
beginning on or after | | Reporting period standard
adopted by the company |
| Clarification of Acceptable Methods of Depreciation and Amortisation (Amendments to IAS 16 and IAS 38) | | 1 January 2016 | | 1 July 2016 |
| IFRS 15 Revenue from Contracts with Customers | | 1 January 2018 | | 1 July 2018 |
| Annual improvements 2012 – 2014 cycle | | 1 January 2016 | | 1 July 2016 |
| Disclosure Initiative (Amendments to IAS 1) | | 1 January 2016 | | 1 July 2016 |
| IFRS 16 - Leases | | 1 January 2019 | | 1 July 2019 |
| IFRS 2 Share-based payments - Amendments | | 1 January 2018 | | 1 July 2018 |
| IAS 12 Income tax – Amendments on recognition of deferred tax assets for unrealized losses | | 1 January 2017 | | 1 July 2017 |
| IAS 7 Statement of cash flows – Amendments on additional disclosures | | 1 January 2017 | | 1 July 2017 |
Management has determined that the standards that have been adopted in fiscal year 2017 have not had a material impact on the Group. Management is currently assessing the impact of the standards to be adopted in fiscal year 2018 and forward on the Group. The Company has adopted IFRS 9 (2014) prior to its effective date. This adoption did not have a material impact on its financial statements.
Accounting Policies
The following is a summary of the material accounting policies adopted by the Company in the preparation of the financial report. The accounting policies have been consistently applied, unless otherwise stated.
| (a) | Basis of Consolidation |
The consolidated financial statements incorporate the financial statements of the Company and entities controlled by the Company (its subsidiaries) referred to as ‘the Group’ in the financial statements. Control is achieved where the consolidated entity is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the consolidated entity. They are de-consolidated from the date that control ceases.
A list of controlled entities is contained in Note 11 to the financial statements. All controlled entities have a 30 June financial year-end.
All intra-group transactions, balances, income and expenses are eliminated in full on consolidation.
Accounting policies of subsidiaries have been changed where necessary to ensure consistency with those policies applied by the parent entity. Subsidiaries are accounted for at cost in the parent entity.
The results of subsidiaries acquired or disposed of during the year are included in profit or loss from the effective date of acquisition or up to the effective date of disposal, as appropriate.
The Company determines and presents operating segments using the ‘management approach’ where the information presented is on the same basis as the internal reports provided to the Chief Operating Decision Makers ('CODM'). The CODM are responsible for the allocation of resources to operating segments and assessing their performance and provide the strategic direction and management oversight of the day to day activities of the entity in terms of monitoring results, providing approval for research and development expenditure decisions and challenging and approving strategic planning for the business.
| (c) | Foreign Currency Translation |
Functional and Presentation Currency
Items included in the financial statements are measured using the currency of the primary economic environment in which the entity operates (“the functional currency”). The financial statements are presented in Australian dollars, which is the Company’s functional and presentation currency.
Transactions and Balances
Transactions in foreign currencies are translated into the functional currency using the rates of exchange ruling at the date of each transaction. At reporting date, amounts outstanding in foreign currencies are translated into the functional currency using the rate of exchange ruling at the end of the financial year. Refer to Note 3 for the foreign currency gains and losses recognized during the periods.
Foreign exchange gains and losses that relate to borrowings are presented in the statement of profit or loss and other comprehensive income, within finance costs. All other foreign exchange gains and losses are presented in the statement of profit or loss and other comprehensive income on a net basis within Corporate Administration Costs.
Immuron Inc., a subsidiary of the Group, has USD as its functional currency. Accordingly, this entity’s balance sheet and income statement balances have been translated to the Group’s presentation currency (which is AUD$) at the reporting date. A gain arising from this translation of AUD$8,846 (2015: loss of AUD$12,581) are recognized as Other Comprehensive Income for the year.
Revenue is measured at the fair value of the consideration received or receivable. Amounts disclosed as revenue are net of returns, trade allowances, rebates and amounts collected on behalf of third parties.
The Company recognises revenue when the amount of the revenue can be reliably measured, it is probable that the future economic benefits will flow to the entity and specific criteria have been met for each of the activities as described below. The amount of the revenue is not considered to be reliably measured until all contingencies relating to the sale have been resolved.
The following specific revenue criteria must be met before revenue is recognized:
| (iv) | Sale of Goods and services | – | Significant risks and rewards of ownership of goods has passed to the buyer and an invoice for the goods or services is issued; |
| | | | |
| (v) | Interest | – | Interest income is recognized using the effective interest rate method; |
| | | | |
| (vi) | R & D Tax Refund | – | Income is recognized in the year the research and development expenses were incurred. |
A difference of AUD$644,149 in the Accumulated losses balance at 30 June 2013 between this statement and the original statement lodged with ASX relates to the previous recognition of FY13 R&D refund in FY14, which does not significantly affect the overall financial position and results presented in the original statement. For the fiscal year 2014, 2015 and 2016, the Company has reassessed and made changes to the amount of R&D Tax Refund recognised as Other Income for the period as compared to the previous statements lodged with the ASX. Effectively, these changes resulted in an increase of AUD$49,481 and AUD$756,131 in Other income, resulting in a related decrease in the net loss, for the period on the Consolidated Statement of Profit or Loss and Other Comprehensive Income for the fiscal year 2014 and 2015. These changes also resulted in a decrease in Other income of AUD$1,469,763, and a related increase in Net Loss, for the period on the Consolidated Statement of Profit or Loss and Other Comprehensive Income for fiscal year 2016.
These adjustments were the result of additional information being made available to the Company subsequent to the previous lodgements with ASX which changed the timing of recognition, but not the actual amount of the R&D refund; and
The Company has worked with experienced advisors to improve its internal process on advanced findings of the R&D activities, which includes determining and evaluating the eligibility of R&D related expenditure to support its submission of the R&D Tax Refund claim.
Grants from the government are recognized at their fair value where there is a reasonable assurance that the grant will be received and the Company will comply with all attached conditions.
Government grants relating to costs to be incurred are deferred or accrued such that they are recognized in the statement of profit or loss and other comprehensive income over the period necessary to match them with the costs that they are intended to compensate.
The income tax expense or revenue for the period is the tax payable or tax rebate receivable on the current period’s taxable income adjusted by changes in deferred tax assets and liabilities attributable to temporary differences between the tax base of assets and liabilities and their carrying amounts in the financial statements, and to unused tax losses.
Deferred income tax is provided in full, using the liability method on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. However, the deferred income tax is not accounted for if it arises from initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects neither the accounting nor taxable profit or loss. Deferred income tax is determined using tax rates (and laws) that have been enacted or substantially enacted by the balance sheet date and are expected to apply when the related deferred income tax is realized or the deferred income tax liability is settled.
Deferred tax assets are recognized for deductible temporary differences and unused tax losses only if it is probable that future taxable amounts will be available to utilize those temporary differences and losses.
Deferred tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets and liabilities and when the deferred balances relate to the same taxation authority. Current tax assets and tax liabilities are offset where the entity has a legally enforceable right to offset and intends either to settle on a net basis, or to realize the asset and settle the liability simultaneously.
Current and deferred tax balances attributable to amounts recognized directly in equity are also recognized directly in equity.
Assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs to sell and value in use.
| (h) | Cash and Cash Equivalents |
For presentation purposes, cash and cash equivalents includes cash on hand, deposits held at call with financial institutions, other short-term, highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value, and bank overdrafts.
Trade receivables are recognized initially at fair value and subsequently measured at amortized cost using the effective interest rate method, less provision for impairment. Trade receivables are due for settlement no more than 30 days from the date of recognition.
Collectability of trade receivables is reviewed on an ongoing basis. Debts which are known to be uncollectible are written off. A provision for impairment of trade receivables is established when there is objective evidence that the Company will not be able to collect all amounts due according to the original terms of receivables.
Significant financial difficulties of the debtor, probability that the debtor will enter bankruptcy or financial reorganisation and default or delinquency in payment (more than 30 days overdue) are considered indicators that the trade receivable is impaired. The amount of the provision is the difference between the asset’s carrying amount and the present value of estimated future cash flows, discounted at the original effective interest rate. Cash flows relating to short term receivables are not discounted if the effect of discounting is immaterial. The amount of the provision is recognized in the statement of profit or loss and other comprehensive income.
Raw materials, work in progress and finished goods are stated at the lower of cost and net realisable value. Where appropriate, cost comprises direct materials, direct labor and an appropriate proportion of variable and fixed overheads expenditure, the latter being allocated on the basis of normal operating capacity. The Company classifies inventory as a current asset as all amounts are held for the purpose of trading.
Costs are assigned to individual items of inventory on basis of weighted average costs. Net realisable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale.
| (k) | Property, Plant & Equipment |
Plant and equipment are stated at historical cost less depreciation. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
Repairs and maintenance are charged to the statement of profit or loss and other comprehensive income during the financial period in which they are incurred.
Depreciation on assets is calculated using the straight line method to allocate their cost, net of their residual values, over their estimated useful lives, as follows:
| - | Plant & Equipment | (3 - 15 years) |
| | | |
| - | Computer Equipment | (2 - 4 years) |
| | | |
| - | Furniture & Fittings | (3 - 15 years) |
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, annually.
An asset’s carrying amount is written down immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount (Note 1(g)).
Gains and losses on disposals are determined by comparing proceeds with carrying amount. These are included in the statement of profit or loss and other comprehensive income.
| (i) | Research & Development |
Expenditure on research activities, undertaken with the prospect of obtaining new scientific or technical knowledge and understanding, is recognized in the statement of profit or loss and other comprehensive income as an expense when it is incurred.
Expenditure on development activities, being the application of research findings or other knowledge to a plan or design for the production of new or substantially improved products or services before the start of commercial production or use, is capitalized if it is probable that the product or service is technically and commercially feasible, will generate probable economic benefits and adequate resources are available to complete development and cost can be measured reliably. Other development expenditure is recognized in the statement of profit or loss and other comprehensive income as an expense as incurred.
| (m) | Trade and Other Payables |
These amounts represent liabilities for goods and services provided to the entity prior to the end of the financial year and which are unpaid. The amounts are unsecured and are usually paid within 30 days of recognition. Trade payables are recognized initially at fair value and subsequently measured at amortized cost using the effective interest rate method.
| (i) | Short-term obligations |
Liabilities for wages and salaries, annual leave and long service leave that are expected to be settled wholly within 12 months after the end of the period in which the employees render the related service are recognized in respect of employees’ services up to the end of the reporting period and are measured at the amounts expected to be paid when the liabilities are settled.
| (ii) | Other long-term employee benefits obligations |
The liabilities for long service leave and annual leave that are not expected to be settled wholly within 12 months after the end of the period in which the employees render the related service are recognized in the provision for employee benefits and measured as the present value of expected future payments to be made in respect of services provided by employees up to the end of the reporting period using the projected unit credit method. Consideration is given to expected future wage and salary levels, experience of employee departures and periods of service. Expected future payments are discounted using market yields at the end of the reporting period of government bonds with terms and currencies that match, as closely as possible, the estimated future cash outflows. The obligations are presented as current liabilities in the Statement of financial position if the entity does not have an unconditional right to defer settlement for at least twelve months after the reporting period, regardless of when the actual settlement is expected to occur.
| (iii) | Retirement benefit obligations |
Contributions to the defined contribution superannuation funds are recognized as an expense as they become payable. Prepaid contributions are recognized as an asset to the extent that a cash refund or a reduction in the future payments is available.
Share-based compensation benefits may be provided through the issue of fully paid ordinary shares under the Immuron Employee Share and Option Plan. Options are also granted to employees and consultants in accordance with the terms of their respective employment and consultancy agreements. Any options granted are made in accordance with the terms of the Company’s Employee Share and Option Plan (ESOP).
The fair value of options granted under employment and consultancy agreements are recognized as an employee benefit expense with a corresponding increase in equity. The fair value is measured at grant date and recognized over the period during which the employees become unconditionally entitled to the options.
| | The fair value at grant date is determined using a Black-Scholes option pricing model that takes into account the exercise price, the term of the option, the vesting and performance criteria, the impact of dilution, the non-tradeable nature of the option, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk-free interest rate for the term of the option. |
| | The fair value of the options granted excludes the impact of any non-market vesting conditions (for example, profitability and sales growth targets). Non-market vesting conditions are included in assumptions about the number of options that are expected to become exercisable. At each reporting date, the entity revises its estimate of the number of options that are expected to become exercisable. The employee benefit expense recognized each period takes into account the most recent estimate. The impact of the revision to original estimates, if any, is recognized in the statement of profit or loss and other comprehensive in come with a corresponding adjustment to equity. |
| | Upon the exercise of options, the balance of the share-based payments reserve relating to those options is transferred to contributed equity. |
Termination benefits are payable when employment is terminated before the normal retirement date, or when an employee accepts voluntary redundancy in exchange for these benefits.
The Company recognises termination benefits when it is demonstrably committed to either terminating the employment of current employees according to a detailed formal plan without possibility of withdrawal or providing termination benefits as a result of an offer made to encourage voluntary redundancy.
Benefits falling due more than 12 months after reporting date are discounted to present value.
| (o) | Interest Bearing Loans and Borrowings |
Generally, loans and borrowings are initially recognized at cost, being the fair value of the consideration received net of issue costs associated with the borrowing. After initial recognition, interest bearing loans and borrowings are subsequently measured at amortized cost using the effective interest method. Amortized cost is calculated by taking into account any issue costs and any discount or premium on settlement.
The component of the convertible notes that were issued in connection with the February 2016 financing arrangement, that exhibits characteristics of a liability is recognised as a liability in the statement of financial position. On the date of issuance and each subsequent reporting period, the Company records the entire hybrid instrument as measured at fair value through profit and loss as the embedded derivative does significantly modify the cash flows under the contract.The associated transaction costs have also been expensed as incurred and are recorded as Finance and Termination costs in the Statement of Profit or Loss and Other Comprehensive Income.
Fair Value of Convertible Notes
The convertible notes were measured and disclosed as a level 3 instrument, using a three level hierarchy, based on the lowest level of input that is significant to the entire fair value measurement, as defined below:
| · | Level 1: Quoted price (unadjusted) in active markets for identical assets or liabilities that the entity can access at the measurement date |
| · | Level 2: Inputs other than quoted prices included within Level 1 that are observable for the asset and liability, either directly or indirectly |
| · | Level 3: Unobservable inputs for the asset or liability |
No transfers between the levels of the fair value hierarchy occurred during the current year.
Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction from the proceeds.
| (i) | Basic earnings per share |
Basic earnings per share is calculated by dividing the profit or loss attributable to equity holders of the Company, excluding any costs of servicing equity other than ordinary shares, by the weighted average number of ordinary shares outstanding during the full year, adjusted for bonus elements in ordinary shares issued during the full year.
| (ii) | Diluted earnings per share |
Diluted earnings per share adjusts the figures used in the determination of basic earnings per share to take into account the after income tax effect of interest and other financing costs associated with dilutive potential ordinary shares and the weighted average number of shares assumed to have been issued for no consideration in relation to dilutive potential ordinary shares.
| (r) | Goods and Services Tax (GST) |
Revenues, expenses and assets are recognized net of the amount of associated GST, unless the GST incurred is not recoverable from the taxation authority. In this case it is recognized as part of the cost of acquisition of the asset or as part of the expense.
Receivables and payables are stated inclusive of the amount of GST recoverable or payable. The net amount of GST recoverable from, or payable to, the taxation authorities is included with other receivable or payables in the statement of financial position.
Cash flows are presented on a gross basis. The GST components of cash flow arising from investing or financing activities which are recoverable for, or payable to, the taxation authorities are presented as operating cash flow.
Leases in which a significant portion of the risk and reward of ownership are not transferred to the Company as lessee are classified as operating leases. Payments made under operating leases (net of any incentives received from the lessor) are charged to the statement of profit or loss and other comprehensive income on a straight-line basis over the period of the lease.
| (t) | Previously issued Financial Statements |
The Company has reclassified certain items in the statement of profit or loss and other comprehensive income for the years ended 30 June 2015 and 2014 to conform with the current year presentation and reclassified certain items in the statement of change in equity for the year ended 30 June 2016, as follows:
Statement of profit or loss and other comprehensive income:
| | | 2015 | | | 2014 | |
| | | Previously
Issued | | | Reclassification | | | Revised | | | Previously
Issued | | | Reclassification | | | Revised | |
| Cost of Goods Sold | | | (316,128 | ) | | | - | | | | (316,128 | ) | | | (332,686 | ) | | | 54,758 | | | | (277,928 | ) |
| Sales and Marketing Costs | | | (360,073 | ) | | | *283,279 | | | | (76,794 | ) | | | (401,811 | ) | | | **322,015 | | | | (79,796 | ) |
| Freight Costs | | | (116,379 | ) | | | - | | | | (116,379 | ) | | | (38,445 | ) | | | (75,833 | ) | | | (114,278 | ) |
| Amortisation | | | - | | | | - | | | | - | | | | (680,567 | ) | | | (20 | ) | | | (680,587 | ) |
| Consulting, Employee and Director | | | (728,140 | ) | | | - | | | | (728,140 | ) | | | (555,487 | ) | | | - | | | | (555,487 | ) |
| Corporate Administration | | | (557,422 | ) | | | - | | | | (557,422 | ) | | | (367,514 | ) | | | (124,951 | ) | | | (492,465 | ) |
| Depreciation | | | (3,719 | ) | | | - | | | | (3,719 | ) | | | (4,010 | ) | | | 21 | | | | (3,989 | ) |
| Finance Costs | | | - | | | | - | | | | - | | | | (588,636 | ) | | | 124,951 | | | | (463,685 | ) |
| Impairment of Inventory | | | (35,340 | ) | | | - | | | | (35,340 | ) | | | - | | | | (50,204 | ) | | | (50,204 | ) |
| Marketing and Promotion | | | (142,735 | ) | | | (161,952 | ) | | | (304,687 | ) | | | (52,085 | ) | | | (183,091 | ) | | | (235,176 | ) |
| Research and Development | | | (3,018,294 | ) | | | - | | | | (3,018,294 | ) | | | (1,285,121 | ) | | | (4,554 | ) | | | (1,289,675 | ) |
| Travel and Entertainment | | | (128,318 | ) | | | - | | | | (128,318 | ) | | | (37,326 | ) | | | (1 | ) | | | (37,327 | ) |
| * | Amount includes AUD121,327 that has been reflected as a restatement to decrease both Operating revenue and Sales and Marketing Costs. |
| ** | Amount includes AUD63,091 that has been reflected as a restatement to decrease both Operating revenue and Sales and Marketing Costs. |
Statement of change in equity:
| | | 2016 | |
| | | Previously Issued | | | Reclassification | | | Revised | |
| Shares issued, net of costs | | | 1,658,504 | | | | (71,875 | ) | | | 1,586,629 | |
| Options exercised | | | (71,875 | ) | | | 71,875 | | | | - | |
The reclassifications had no impact on the net loss for each period.
As described in Notes 2, 6, 17, 18 and 20, the Company has restated its previously issued 2016, 2015 and 2014 financial statements.
In addition to these restatements, the Company has made revisions to Notes 1, 3, 4, 7, 8, 9, 13, 15, 16, 19, 21, 22, 23 and 24.
Critical Accounting Estimates and Judgments
Management evaluates estimates and judgments incorporated into the financial statements based on historical knowledge and best available current information. Estimates assume a reasonable expectation of future events are based on current trends and economic data, obtained both externally and within the group.
The value attributed to share options and remunerations shares issued is an estimate calculated using an appropriate mathematical formula based on an option pricing model. The choice of models and the resultant option value require assumptions to be made in relation to the likelihood and timing of the conversion of the options to shares and the value of volatility of the price of the underlying shares. Refer to note 21 for more details.
| (ii) | Impairment of Inventories |
The provision for impairment of inventories assessment requires a degree of estimation and judgment. The level of the provision is assessed by taking into account the recent sales experience, the ageing of inventories and in particular the shelf life of inventories that affects obsolescence.
| (iii) | Fair value measurement hierarchy |
The preparation of the financial statements requires management to make judgments, estimates and assumptions that affect the reported amounts in the financial statements. Management continually evaluates its judgments, estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgments, estimates, and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgments and estimates will seldom equal the related actual results. The judgments, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed within the relevant sections where applicable.
The fair value of convertible note classified as level 3 is determined by the use of valuation model. These include discounted cash flow analysis and the use of observable inputs that required significant adjustments based on unobservable inputs.
As at 30 June 2016, management has assessed the terms of the convertible notes and determined that in their view the fair value of the debt component is equal to the proceeds such that there is no residual amount to be allocated to an equity component. In making this determination, management is of the view that the value of the consideration received, net of costs, provided reliable evidence of the fair value of the debt component of the convertible note. Fair value has been determined by the income approach based on a discounted cash flow analysis, with the most significant inputs being the discount rate that reflects the investors credit risk. A slight increase or decrease in the discount rate used would not be material to the financial statements.
Reconciliation of level 3 fair value measurements:
| | | Convertible notes/debentures | |
| | | AUD$ | |
| Balance at 30 June 2015 | | - | |
| - Issue | | | 1,200,000 | |
| - Change in fair value (*) | | | 156,000 | |
| - Repayments | | | (227,883 | ) |
| Balance at 30 June 2016 (Note 15) | | | 1,128,117 | |
(*) These amounts are recorded in the Finance Costs on the Statement of Profit or Loss and Other Comprehensive Income.
| Note 2. | Revenue and other income(Restated) |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | |
| Revenue | | | | | | | | | | | | |
| Revenue from Operating Activities | | | | | | | | | | | | |
| Sale of goods | | | 1,001,077 | | | | 1,002,380 | | | | 981,051 | |
| Total Revenue from Operating Activities | | | 1,001,077 | | | | 1,002,380 | | | | 981,051 | |
| | | | | | | | | | | | | |
| Other Income | | | | | | | | | | | | |
| Other income | | | 14,010 | | | | - | | | | 2,500 | |
| Interest income | | | 12,165 | | | | 112,440 | | | | 88,345 | |
| R&D tax concession refund | | | 1,512,840 | | | | 1,478,581 | | | | 713,632 | |
| Other Income from Non-Operating Activities | | | 1,539,015 | | | | 1,591,021 | | | | 804,477 | |
| Total Revenue and Other Income | | | 2,540,092 | | | | 2,593,401 | | | | 1,785,528 | |
The Company revised all customer allowances and discounts, previously recognised as Selling and Marketing Costs as reduction to Operating revenue. These revisions resulted in decreases in both Operating revenue and Selling and Marketing Costs of AUD$154,446, AUD$121,327 and AUD$63,091 for the fiscal year ended 30 June 2016, 2015 and 2014, respectively.
| | | | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| Expenses |
| a) | | Consulting, Employee and Director Expenses | | | | | | | | | | | | |
| | | Consulting expenses | | | 46,775 | | | | 38,955 | | | | - | |
| | | Wages and salaries expenses | | | 956,737 | | | | 543,975 | | | | 238,263 | |
| | | Superannuation and other employee related expenses | | | 32,537 | | | | 23,122 | | | | 1,351 | |
| | | Director expenses | | | 197,713 | | | | 117,713 | | | | 96,659 | |
| | | Share- based payments (restated) | | | 1,606,275 | | | | 4,375 | | | | 219,214 | |
| | | Total Consulting, Employee and Director Expenses | | | 2,840,037 | | | | 728,140 | | | | 555,487 | |
| | | | | | | | | | | | | | | |
| b) | | Corporate Administrative Costs | | | | | | | | | | | | |
| | | Audit and accounting fees | | | 62,825 | | | | 84,250 | | | | 70,708 | |
| | | Insurances | | | 100,609 | | | | 85,316 | | | | 41,852 | |
| | | Foreign exchange (gain) / losses | | | 217,904 | | | | 63,015 | | | | 62,254 | |
| | | Corporate administration costs | | | 939,232 | | | | 324,841 | | | | 326,870 | |
| | | Provisions for doubtful debts | | | - | | | | - | | | | (9,230 | ) |
| | | Total Corporate Administrative Costs | | | 1,320,570 | | | | 557,422 | | | | 492,465 | |
| Note 4. | Income Tax Benefit |
| | | | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| (a) | | The prima facie tax on loss from ordinary activities before the loss is reconciled to the income tax as follows: | | | | | | | | | | | | |
| | | Loss before income tax | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) |
| | | Income tax benefit calculated at 30% (2015, 2014:30%) | | | (2,120,630 | ) | | | (807,546 | ) | | | (748,521 | ) |
| | | | | | | | | | | | | | | |
| | | Impairment and amortization expenses | | | 1,168 | | | | 1,116 | | | | 205,373 | |
| | | Equity-based payments expenses | | | 530,842 | | | | 65,674 | | | | 49,972 | |
| | | Other expenses not deductible | | | 264,848 | | | | 47,699 | | | | 116,076 | |
| | | Non-deductible amounts associated with R&D rebates | | | 606,177 | | | | 538,913 | | | | 261,665 | |
| | | Temporary differences not recognized | | | 27,992 | | | | (44,895 | ) | | | (199,495 | ) |
| | | Deferred tax assets relating to tax losses not recognized | | | 689,603 | | | | 199,039 | | | | 314,930 | |
| | | Income tax expense | | | - | | | | - | | | | - | |
The Company has estimated total tax losses of AUD$27,955,616, representing a Deferred Tax Asset of AUD$8,386,685 (at 30%) that has not been recognized in the Financial Statements, refer to Note 1(f).
| Note 5. | Key Management Personnel Compensation |
Note 5 details the nature and amount of remuneration for each Director of Immuron Limited, and for the Key Management Personnel.
The Directors of Immuron Limited during the year ended 30 June 2016 were:
| Dr. Roger Aston | Independent Non-Executive Chairman |
| Mr. Peter Anastasiou | Executive Vice Chairman |
| Mr. Daniel Pollock | Non-Executive Director |
| Mr. Stephen Anastasiou | Non-Executive Director |
The Key Management Personnel of Immuron Limited during the year were:
| Ms. Leearne Hinch1 | Chief Executive Officer (CEO) |
| Mr. Thomas Liquard1 | Chief Executive Officer (CEO) |
| Dr. Jerry Kanellos (PhD)1 | Chief Operating & Scientific Officer (COSO) |
| 1 | Denotes a person(s) who was appointed or resigned during or after the year. |
The aggregate compensation made to Directors and Other Key Management Personnel of the Company is set out below:
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | |
| Key Management Personnel Compensation | | | | | | | | | | | | |
| Short-term employee benefits | | | 652,514 | | | | 358,908 | | | | 308,413 | |
| Post-employment benefits | | | 26,004 | | | | 14,908 | | | | 8,245 | |
| Share-based payments | | | 1,606,275 | | | | - | | | | 184,602 | |
| Total Key Management Personnel Compensation | | | 2,284,793 | | | | 373,816 | | | | 501,260 | |
| Note 6. | Loss per Share (Restated) |
| | | | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| Basic/Diluted loss per share (cents) | | 9.248 | | | 3.592 | | | 5.947 | |
| | | | | | | | | | | | | | | |
| a) | | Net loss used in the calculation of basic and diluted loss per share | | | 7,068,767 | | | | 2,691,820 | | | | 2,495,069 | |
| | | | | | | | | | | | | | | |
| b) | | Weighted average number of ordinary shares outstanding during the period used in the calculation of basic and diluted loss per share | | | 76,435,993 | * | | | 74,935,902 | | | | 41,955,199 | ** |
| * | This amount includes 182,169 of weighted average shares for ordinary shares in relation to the $4,511,378 received in capital raising that was not issued as of 30 June 2016. |
| ** | The 2014 weighted average number of ordinary shares outstanding during the period used in the calculation of basic and diluted loss per share was recalculated to account for the post-consolidation impact. |
The company is currently in a loss making position any thus the impact of any potential shares is concluded as anti-dilutive which includes the company’s stock options and convertible notes payable. Treasury shares are excluded from the calculation of weighted average number of ordinary shares.
In the previously issued financial statements, the basic and diluted loss per share was 5.705 cents, 4.603 cents, 3.398 cents and the weighted average number of ordinary shares outstanding was 76,944,879, 74,907,491, 74,891,316 for the years ended 30 June 2016, 2015 and 2014, respectively.
| Note 7. | Cash and Cash Equivalents |
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Cash at Bank: | | | | | | | | |
| Cash at bank | | | 2,290,639 | | | | 3,116,074 | |
| Total Cash and Cash Equivalents | | | 2,290,639 | | | | 3,116,074 | |
The interest rates on cash at bank at 30 June 2016 ranged from 0.95% to 0.03% (2015: from 2.55% to 0.30%)
| Note 8. | Trade and Other Receivables |
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Current | | | | | | | | |
| Trade receivables | | | 154,217 | | | | 216,207 | |
| Accrued income1 | | | 1,621,416 | | | | 1,475,422 | |
| Subscription receivables2 | | | 2,612,139 | | | | - | |
| Total Trade and Other Receivables | | | 4,387,772 | | | | 1,691,629 | |
| * | All trade receivables are non-interest bearing. |
| 1 | Primarily comprises of receivables from the Australian Tax Office in relation to R&D tax concession for the year. |
| 2 | Represents uncleared funds from the Capital Raising as at 30 June 2016. Funds received 7 July 2016 upon the issuance of shares. |
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Inventory | | | | | | | | |
| Raw materials | | | 1,259,445 | | | | 932,895 | |
| Work in Progress | | | 121,513 | | | | 64,960 | |
| Finished goods | | | 269,156 | | | | 51,863 | |
| Prepaid inventory | | | 405,953 | | | | 96,549 | |
| Total Inventory | | | 2,056,067 | | | | 1,146,267 | |
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Current | | | | | | | | |
| Prepayments | | | 74,943 | | | | 44,928 | |
| Total Other Assets | | | 74,943 | | | | 44,928 | |
| Note 11. | Controlled Entities |
| | | Country of | | Percentage of Ownership | |
| | | Incorporation | | 30 Jun 2016 | | | 30 Jun 2015 | |
| | | | | | | | | |
| Parent Entity: | | | | | | | | | | |
| Immuron Limited | | Australia | | | - | | | | - | |
| | | | | | | | | | | |
| Subsidiaries of Immuron Limited: | | | | | | | | | | |
| Immuron Inc. | | USA | | | 100 | % | | | 100 | % |
| Anadis EPS Pty Ltd1 | | Australia | | | 100 | % | | | 100 | % |
| 1 | Shares in subsidiary company – Anadis ESP Pty Ltd |
This company is a wholly owned subsidiary of Immuron Limited and was formed for the sole purpose to act as trustee for the Immuron Limited Executive Officer Share Plan Trust. All costs associated with the operations of this company are borne by Immuron Limited. Consolidated accounts have not been prepared as the net assets and trading activity of Anadis ESP Pty Ltd are not material.
| Note 12. | Plant and Equipment |
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Plant & Equipment | | | | | | | | |
| At cost | | | 304,215 | | | | 304,215 | |
| Accumulated depreciation | | | (290,705 | ) | | | (289,568 | ) |
| Total Plant & Equipment | | | 13,510 | | | | 14,647 | |
| | | | | | | | | |
| Computer Equipment | | | | | | | | |
| At cost | | | 29,627 | | | | 27,186 | |
| Accumulated depreciation | | | (25,886 | ) | | | (24,132 | ) |
| Total Computer Equipment | | | 3,741 | | | | 3,054 | |
| | | | | | | | | |
| Furniture & Fittings | | | | | | | | |
| At cost | | | 34,177 | | | | 34,177 | |
| Accumulated depreciation | | | (33,365 | ) | | | (32,364 | ) |
| Total Furniture & Fittings | | | 812 | | | | 1,813 | |
| Total Plant and Equipment | | | 18,063 | | | | 19,514 | |
| | | Plant & | | | Computer | | | Furniture & | | | | |
| | | Equipment | | | Equipment | | | Fittings | | | Total | |
| | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Carrying Amount as at 30 June 2014 | | | 15,783 | | | | - | | | | 4,282 | | | | 20,065 | |
| Additions | | | - | | | | 3,168 | | | | - | | | | 3,168 | |
| Depreciation expenses | | | (1,136 | ) | | | (114 | ) | | | (2,469 | ) | | | (3,719 | ) |
| Carrying Amount as at 30 June 2015 | | | 14,647 | | | | 3,054 | | | | 1,813 | | | | 19,514 | |
| | | | | | | | | | | | | | | | | |
| Additions | | | - | | | | 2,441 | | | | - | | | | 2,441 | |
| Depreciation expenses | | | (1,137 | ) | | | (1,754 | ) | | | (1,001 | ) | | | (3,892 | ) |
| Carrying Amount as at 30 June 2016 | | | 13,510 | | | | 3,741 | | | | 812 | | | | 18,063 | |
| Note 13. | Intangible Assets |
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Intellectual Property | | | | | | | | |
| At cost | | | 1,460,587 | | | | 1,460,587 | |
| Accumulated depreciation | | | (1,460,587 | ) | | | (1,460,587 | ) |
| Total Intellectual Property | | | - | | | | - | |
The intellectual property was acquired from Hadasit Medical Research Services and Development Limited in 2009. At the end of fiscal year 2012, the estimated useful life of the intellectual property wasreviewed, and it was determined to have a remaining finite useful life of two years and was fully amortized by the end of the 2014 fiscal year.
| Note 14. | Trade and Other Payables |
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Current | | | | | | | | |
| Trade payables | | | 1,517,255 | | | | 918,493 | |
| Accrued expenses | | | 417,090 | | | | 253,607 | |
| Other payables | | | 52,062 | | | | 35,710 | |
| Total | | | 1,986,407 | | | | 1,207,810 | |
| Note 15. | Other Financial Liabilities |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | |
| Other Financial Liabilities | | | | | | | | | | | | |
| Convertible Note | | | 1,128,117 | | | | - | | | | - | |
| Total | | | 1,128,117 | | | | - | | | | - | |
On 17 February 2016, the Company secured AUD$1,700,000 in funding with a New York-based Investment Fund. The facility is being used to fund the immediate start of the clinical phase for IMM-529 in Clostridiumdifficile.
The investment is structured in 3 tranches with a mix of equity financing and convertible securities:
| Tranche #1 | - AUD$100,000 private placement of securities plus a AUD$600,000 repayable Convertible Note with AUD$78,000 finance charge; |
| Tranche #2 | - 45 days after issuance of the tranche 1, the company can call a second Tranche as per Tranche 1 terms. |
Tranche #3 | - by mutual consent, AUD$339,000 Face Value repayable Convertible Note issued on same terms as Tranche 1 and 2. Tranche #3 has not been issued as of the issuance date of the consolidated financial statements. |
The Convertible Notes are repayable monthly over an 18 month period with each repayment to be settled at Immuron’s discretion monthly by:
| a) | the issuance of new shares at a 10% discount to a 5 Day Volume Weighted Average Price (VWAP) over the 20 trading days immediately prior to a repayment due date; or |
| b) | cash repayment plus a 2.5% premium to the repayment amount. |
Immuron repaid AUD$150,666 in shares as disclosed under Note 18, together with a cash amount of AUD$77,217 prior to 30 June 2016.
Due to the Capital Raising that was closed on 7 July 2016, Immuron has executed its commitment to shareholders and will repay all future obligations pertaining to the Convertible Note in cash, rather than via the issuance of new securities.
On 15th May 2014 Immuron Limited fully repaid the convertible debenture debt of CAD$1,500,000 to Paladin Labs Inc utilising funds raised from the renounceable pro-rata rights issue announced on 22nd January 2014. The balance as of June 30, 2013 was $1,150,319 and the payment made on 22 January 2014 was in the amount of $1,504,443. This payment resulted in a charge to earnings of approximately $335,000 and the Company incurred approximately $129,000 of interest expense during the year ended 30 June 2014.
| Note 16. | Commitments and Contingencies |
| | | | | | 30 June 2016 | |
| | | Note | | | AUD$ | |
| | | | | | | |
| Lease commitments not recognized in the financial statements: | | | | | | | | |
| - not later than 12 months | | | 1 | | | | 38,940 | |
| - between 1 and 5 years | | | | | | | 58,410 | |
| Total | | | | | | | 97,350 | |
| 1 | The property lease is a non-cancellable lease with a 3 year term, with rent payable monthly in advance. The minimum lease payments shall be increased by CPI per annum. An option exists to renew the lease at the end of the 3 year term for an additional term of 3 years. The current 3 year lease period expires in December 2018. |
The Group has recognised AUD$25,501, AUD$41,624 and AUD$35,274 of rental expenses in its Statement of Profit or Loss and Other Comprehensive Income for the year 2016, 2015 and 2014, respectively, as Corporate Administration Expense.
Pursuant to the Executive Service Agreement between Immuron and its CEO, the Company commits to pay a bonus of AUD$80,000 in ordinary shares and to issue 1,000,000 ordinary shares of the Company to the CEO if certain Short and Long Term Incentive Milestones are met, respectively.
| Note 17. | Contributed Equity |
| | | 30 June 2016 (Restated) | | | 30 June 2015 | | | 30 June 2014 | |
| | | No. | | | AUD$ | | | No. | | | AUD$ | | | No. | | | AUD$ | |
| | | | | | | | | | | | | | | | | | | |
| Fully Paid Ordinary Shares (No par value) | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at beginning of year | | | 74,964,232 | | | | 40,335,347 | | | | 2,995,662,120 | | | | 40,325,295 | | | | 1,035,450,143 | | | | 31,357,697 | |
| Capital consolidation (40:1) | | | - | | | | - | | | | (2,920,770,804 | ) | | | - | | | | - | | | | - | |
| Shares issued during the year | | | 5,135,414 | | | | 1,721,789 | | | | 72,916 | | | | 11,667 | | | | 1,960,211,977 | | | | 9,792,599 | |
| Shares to be issued (*) | | | - | | | | 4,511,378 | | | | - | | | | - | | | | - | | | | (5,833 | ) |
| Treasury shares (**) | | | - | | | | (800,000 | ) | | | - | | | | - | | | | - | | | | - | |
| Transactions costs (cash-based) | | | - | | | | (135,160 | ) | | | - | | | | (1,615 | ) | | | - | | | | (819,168 | ) |
| Total Contributed Equity | | | 80,099,646 | | | | 45,633,354 | | | | 74,964,232 | | | | 40,335,347 | | | | 2,995,662,120 | | | | 40,325,295 | |
(*) As at 30 June 2016, the Company was committed to issue 18,045,512 of ordinary shares in relation to the $4,511,378 received in capital raising (see note 24).
(**) An adjustment was made in relation to the treasury shares which resulted in a decrease of AUD$800,000 in Non-current assets and Equity as compared to the previous statement lodged with ASX.
During the Full Year ended 30 June 2016, the Company issued the following securities:
| | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | AUD$ | | | AUD$ | |
| 18 Sep 2015 | | Exercise of IMCAI Unlisted Options | | | 218,750 | | | | 0.376 | | | | 82,250 | |
| 30 Sep 2015 | | Exercise of IMCAI Unlisted Options | | | 93,750 | | | | 0.376 | | | | 35,250 | |
| 19 Oct 2015 | | Exercise of IMCAI Unlisted Options by Grandlodge | | | 556,000 | | | | 0.376 | | | | 209,056 | |
| 13 Nov 2015 | | Exercise of IMCAI Unlisted Options | | | 41,666 | | | | 0.376 | | | | 15,667 | |
| 27 Nov 2015 | | Issue of Shares in lieu of cash payment for services as per Resolution 4 of the Annual General Meeting (AGM) held on 25 Nov 2015 | | | 546,875 | | | | 0.160 | | | | 87,500 | |
| 24 Feb 2016 | | Issue in accordance with executed funding agreement with a New York based Investment fund provider announced to the ASX on 17 Feb 2016 | | | 294,118 | | | | 0.340 | | | | 100,000 | |
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | AUD$ | | | AUD$ | |
| 24 Feb 2016 | | Issue of fully paid escrow shares as security for any repayment default of the Convertible Loan in accordance with executed funding agreement with a New York based Investment fund provider and announced to the ASX on 17 Feb 2016 | | | 2,000,000 | | | | 0.400 | | | | 800,000 | |
| 13 Apr 2016 | | Issue in accordance with executed funding agreement with a New York based Investment fund provider announced to the ASX on 17 Feb 2016 | | | 326,797 | | | | 0.306 | | | | 100,000 | |
| 18 Apr 2016 | | First repayment of Convertible Note Security in accordance with executed funding agreement with a New York based investment fund provider announced to the ASX on 17 Feb 2016 | | | 241,764 | | | | 0.312 | | | | 75,333 | |
| 16 May 2016 | | Exercise of IMCAI Unlisted Options | | | 150,000 | | | | 0.276 | | | | 41,400 | |
| 16 May 2016 | | Second repayment of Convertible Note Security in accordance with executed funding agreement with a New York based investment fund provider announced to the ASX on 17 Feb 2016 | | | 265,694 | | | | 0.284 | | | | 75,333 | |
| 31 May 2016 | | Issue of Shares in lieu of cash payment for services received | | | 400,000 | | | | 0.250 | | | | 100,000 | |
| 30 Jun 2016 | | Shares to be Issued from Capital Raising as at 30 June 2016 | | | - | | | | - | | | | 4,511,378 | |
| Total 2016 Movement | | | 5,135,414 | | | | | | | | 6,233,167 | |
During the Full Year ended 30 June 2015, the Company issued the following securities:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| 20 Nov 2014 | | Capital Consolidation on a 40:1 basis approved by shareholders at the Company's Annual General Meeting held on 13 Nov 2014 | | | (2,920,770,804 | ) | | | - | | | | - | |
| 21 Nov 2014 | | Issue of shares to supplier in lieu of cash payment for services rendered approved by shareholders at the Company's Annual General Meeting held on 13 Nov 2014 | | | 72,916 | | | | 0.160 | | | | 11,667 | |
| Total 2015 Movement | | | (2,920,697,888 | ) | | | | | | | 11,667 | |
During the Full Year ended 30 June 2014, the Company issued the following securities:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | $ | | | $ | |
| | | | | | | | | | | | |
| 6 Dec 2013 | | Issue of shares as per resolution 4 approved by shareholders at the Annual General Meeting of the Company held on 29 Nov 2013 | | | 8,750,000 | | | | 0.004 | | | | 35,000 | |
| 6 Dec 2013 | | Issue of shares as per resolutions 5, 6, & 8 approved by shareholders at the Annual General Meeting of the Company held on 29 Nov 2013 | | | 9,479,167 | | | | 0.006 | | | | 56,875 | |
| 3 Feb 2014 | | Exercise of IMCOA options | | | 29,075 | | | | 0.040 | | | | 1,163 | |
| 3 Mar 2014 | | Issue of shares through fully underwritten rights issue | | | 1,670,642,320 | | | | 0.005 | | | | 8,353,212 | |
| 3 Mar 2014 | | Issue of shares to Grandlodge & related owners as part of fully underwritten rights issue | | | 261,103,082 | | | | 0.005 | | | | 1,305,516 | |
| 29 May 2014 | | Issue of shares as per resolution 2 approved by shareholders at the General Meeting of the Company held on 27 May 2014 | | | 10,208,333 | | | | 0.004 | | | | 40,833 | |
| Total 2014 Movement | | | 1,960,211,977 | | | | | | | | 9,792,599 | |
The value of all share based payments of stock is per the terms of an underlying agreement or based on the fair value of the stock on the date of the transaction.
Ordinary shares participate in dividends and the proceeds on winding up of the Company in proportion to the number of shares held. At shareholder meetings each ordinary share is entitled to one vote when a poll is called, otherwise each shareholder has one vote on a show of hands. The ordinary shares have no par value.
Nature and Purpose of the Reserve
The reserve recognises option reserves which are the expense recognised in respect of share based payments, and foreign currency translation reserve (“FCTR”) arising from translation of foreign subsidiary.
| | | 30 June 2016 (Restated) | | | 30 June 2015 | | | 30 June 2014 | |
| | | No. | | | AUD$ | | | No. | | | AUD$ | | | No. | | | AUD$ | |
| | | | | | | | | | | | | | | | | | | |
Options over Fully Paid Ordinary Shares | | | | | | | | | | | | | | | | | | | | | | | | |
| Opening balance | | | 7,188,676 | | | | 560,646 | | | | 365,542,766 | | | | 666,592 | | | | 289,860,577 | | | | 1,208,271 | |
| Capital consolidation (40:1) | | | - | | | | - | | | | (356,404,893 | ) | | | - | | | | - | | | | - | |
| Options issued during the year | | | 7,425,532 | | | | 285,600 | | | | - | | | | - | | | | 87,963,494 | | | | 211,721 | |
Granted options to be issued* | | | - | | | | - | | | | 1,000,000 | | | | - | | | | - | | | | - | |
| Options exercised during the year | | | (1,060,166 | ) | | | (71,875 | ) | | | - | | | | - | | | | (29,075 | ) | | | - | |
| Expense of vested options | | | - | | | | 1,606,275 | | | | - | | | | 4,375 | | | | - | | | | 7,191 | |
| Lapse of unexercised options | | | (3,616,413 | ) | | | (248,345 | ) | | | (2,949,197 | ) | | | (110,321 | ) | | | (12,252,230 | ) | | | (760,591 | ) |
| Closing Balance | | | 9,937,629 | | | | 2,132,301 | | | | 7,188,676 | | | | 560,646 | | | | 365,542,766 | | | | 666,592 | |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | No. | | | AUD$ | | | No. | | | AUD$ | | | No. | | | AUD$ | |
| | | | | | | | | | | | | | | | | | | |
| Foreign currency translation reserve | | | | | | | | | | | | | | | | | | | | | | | | |
| Opening balance | | | | | | | (12,581 | ) | | | | | | | - | | | | | | | | - | |
| Movement during the year | | | | | | | 8,846 | | | | | | | | (12,581 | ) | | | | | | | - | |
| Closing balance | | | | | | | (3,735 | ) | | | | | | | (12,581 | ) | | | | | | | - | |
| Total Reserves | | | 9,937,629 | | | | 2,128,566 | | | | 7,188,676 | | | | 548,065 | | | | 365,542,766 | | | | 666,592 | |
An adjustment of AUD$1,209,338 was made to the Total reserves balance at 30 June 2016 as compared to the previous statement lodged with ASX, as a result of a change in volatility assessment. Effectively, this resulted in an increase in Consulting, Employee and Director expense and the Loss for the period on the Statement of Profit or Loss and Other Comprehensive income.
* On 9 December 2016, the Company issued 1 million options exercisable at $0.50 per option expiring on April 1, 2017 to an employee under the Company's Employee Share and Options Plan (ESOP) following the successful completion of related milestone pertaining to a minimum recruitment of 100 patients into the Company’s NASH Phase 2 clinical trial
During the Full Year ended 30 June 2016, the Company issued the following options:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | | | No. | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| 27 Nov 2015 | | Issue of Unlisted Options in lieu of cash payment for additional services as per Resolution 5A - 5D of the AGM held on 25 Nov 2015 | | | 6,000,000 | | | | - | | | | 1,606,275 | |
| 18 Feb 2016 | | Issue in accordance with executed funding agreement with a New York based Investment fund provider announced to the ASX on 17 Feb 2016 | | | 1,000,000 | | | | 0.186 | | | | 185,600 | |
| 31 May 2016 | | Issue of Unlisted Options in lieu of cash payment for services received | | | 425,532 | | | | 0.235 | | | | 100,000 | |
| Total 2016 Movement | | | 7,425,532 | | | | | | | | 1,891,875 | |
During the Full Year ended 30 June 2015, the Company issued the following options:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | | | No. | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| 20 Nov 2014 | | Capital Consolidation on a 40:1 basis approved by shareholders at the Company's Annual General Meeting held on 13 Nov 2014 | | | (356,404,893 | ) | | | - | | | | - | |
| 1 April 2015 | | Granted unlisted options to be issued to employees under ESOP | | | 1,000,000 | | | | - | | | | - | |
| Total 2015 Movement | | | (355,404,893 | ) | | | | | | | - | |
During the Full Year ended 30 June 2014, the Company issued the following options:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | | | No. | | | $ | | | $ | |
| | | | | | | | | | | | |
| 4 Jul 2013 | | Issue of unlisted options to employees under ESOP | | | 31,746,031 | | | | 0.0022 | | | | 70,159 | |
| 4 Dec 2013 | | Issue of options as per resolutions 5, 6, 7, & 8 approved by shareholders at the Annual General Meeting of the Company held on 29 Nov 2013 | | | 50,000,000 | | | | 0.0025 | | | | 127,000 | |
| 3 Mar 2014 | | Issue of options in lieu of cash payment for consulting services rendered | | | 615,222 | | | | 0.0019 | | | | 1,173 | |
| 29 May 2014 | | Issue if unlisted options to employees under ESOP | | | 5,602,241 | | | | 0.0024 | | | | 13,389 | |
| Total 2014 Movement | | | 87,963,494 | | | | | | | | 211,721 | |
| Note 19. | Segments Reporting |
Primary Reporting Format - Business Segments
The entity has identified its operating segments based on the internal reports that are reviewed and used by the executive management team in assessing performance and determining the allocation of resources.
The executive management team considers the business from both a product and a geographic perspective and has identified three reportable segments.
Segments
Research and Development (R&D)
| – | Income and expenses directly attributable to the Company’s research and development projects performed in Australia and Israel. |
HyperImmune Products
| – | Income and expenses directly attributable to Travelan activities which occur in Australia, New Zealand, Canada and the United States. In 2016, the Company earned 90% and 10% of its revenues from customers located in Australia and Canada, respectively. In 2015, the Company earned 75%, 2% and 23% of its revenues from customers located in Australia, United States and Canada, respectively. In 2014, the Company earned 80%, 16% and 4% of its revenues from customers from customers located in Australia, United States and Canada, respectively. |
Corporate
| – | Other items of income and expenses not directly attributable to R&D or HyperImmune Products segment are disclosed as corporate costs. Corporate activities primarily occur within Australia. This segment includes interest expenses from financing activities and depreciation. |
| | | Research & | | | HyperImmune | | | | | | | |
| | | Development | | | Products | | | Corporate | | | Total | |
| 30 June 2016 | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Segment Revenue & Other income | | | | | | | | | | | | | | | | |
| Revenue from external customers | | | - | | | | 1,001,077 | | | | - | | | | 1,001,077 | |
| R&D tax concession refund | | | 1,512,840 | | | | - | | | | - | | | | 1,512,840 | |
| Interest income | | | - | | | | - | | | | 12,165 | | | | 12,165 | |
| Other income | | | - | | | | 10,200 | | | | 3,810 | | | | 14,010 | |
| Total Segment Revenues & Other income | | | 1,512,840 | | | | 1,011,277 | | | | 15,975 | | | | 2,540,092 | |
| | | | | | | | | | | | | | | | | |
| Segment Expenses | | | | | | | | | | | | | | | | |
| Depreciation & amortization expenses | | | - | | | | - | | | | (3,892 | ) | | | (3,892 | ) |
| Finance costs | | | - | | | | - | | | | (156,000 | ) | | | (156,000 | ) |
| Share-based payments | | | - | | | | - | | | | (2,079,375 | ) | | | (2,079,375 | ) |
| Other operating expenses | | | (3,623,961 | ) | | | (570,183 | ) | | | (3,175,448 | ) | | | (7,369,592 | ) |
| Total Segment Expenses | | | (3,623,961 | ) | | | (570,183 | ) | | | (5,414,715 | ) | | | (9,608,859 | ) |
| Income Tax Expenses | | | - | | | | - | | | | - | | | | - | |
| (Loss)/Profit for the Period | | | (2,111,121 | ) | | | 441,094 | | | | (5,398,740 | ) | | | (7,068,767 | ) |
| | | | | | | | | | | | | | | | | |
| Assets | | | | | | | | | | | | | | | | |
| Segment assets | | | 1,512,840 | | | | 2,318,860 | | | | 4,995,784 | | | | 8,827,484 | |
| Total Assets | | | 1,512,840 | | | | 2,318,860 | | | | 4,995,784 | | | | 8,827,484 | |
| | | | | | | | | | | | | | | | | |
| Liabilities | | | | | | | | | | | | | | | | |
| Segment liabilities | | | (769,434 | ) | | | (538,806 | ) | | | (2,578,681 | ) | | | (3,886,921 | ) |
| Total Liabilities | | | (769,434 | ) | | | (538,806 | ) | | | (2,578,681 | ) | | | (3,886,921 | ) |
| | | Research & | | | HyperImmune | | | | | | | |
| | | Development | | | Products | | | Corporate | | | Total | |
| 30 June 2015 | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Segment Revenue & Other income | | | | | | | | | | | | | | | | |
| Revenue from external customers | | | - | | | | 1,002,380 | | | | - | | | | 1,002,380 | |
| R&D tax concession refund | | | 1,478,581 | | | | - | | | | - | | | | 1,478,581 | |
| Interest income | | | - | | | | - | | | | 112,440 | | | | 112,440 | |
| Total Segment Revenues & Other income | | | 1,478,581 | | | | 1,002,380 | | | | 112,440 | | | | 2,593,401 | |
| | | | | | | | | | | | | | | | | |
| Segment Expenses | | | | | | | | | | | | | | | | |
| Depreciation & amortization expenses | | | - | | | | - | | | | (3,719 | ) | | | (3,719 | ) |
| Finance costs | | | - | | | | - | | | | - | | | | - | |
| Share-based payments | | | - | | | | - | | | | (16,042 | ) | | | (16,042 | ) |
| Other operating expenses | | | (3,018,294 | ) | | | (509,301 | ) | | | (1,737,865 | ) | | | (5,265,460 | ) |
| Total Segment Expenses | | | (3,018,294 | ) | | | (509,301 | ) | | | (1,757,626 | ) | | | (5,285,221 | ) |
| Income Tax Expenses | | | - | | | | - | | | | - | | | | - | |
| (Loss)/Profit for the Period | | | (1,539,713 | ) | | | 493,079 | | | | (1,645,186 | ) | | | (2,691,820 | ) |
| | | | | | | | | | | | | | | | | |
| Assets | | | | | | | | | | | | | | | | |
| Segment assets | | | 1,478,581 | | | | 1,359,315 | | | | 3,180,516 | | | | 6,018,412 | |
| Total Assets | | | 1,478,581 | | | | 1,359,315 | | | | 3,180,516 | | | | 6,018,412 | |
| | | | | | | | | | | | | | | | | |
| Liabilities | | | | | | | | | | | | | | | | |
| Segment liabilities | | | (502,178 | ) | | | (494,647 | ) | | | (210,985 | ) | | | (1,207,810 | ) |
| Total Liabilities | | | (502,178 | ) | | | (494,647 | ) | | | (210,985 | ) | | | (1,207,810 | ) |
| | | Research & | | | HyperImmune | | | | | | | |
| | | Development | | | Products | | | Corporate | | | Total | |
| 30 June 2014 | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Segment Revenue & Other income | | | | | | | | | | | | | | | | |
| Revenue from external customers | | | - | | | | 981,051 | | | | - | | | | 981,051 | |
| R&D tax concession refund | | | 713,632 | | | | - | | | | - | | | | 713,632 | |
| Interest income | | | - | | | | - | | | | 88,345 | | | | 88,345 | |
| Other income | | | - | | | | - | | | | 2,500 | | | | 2,500 | |
| Total Segment Revenues & Other income | | | 713,632 | | | | 981,051 | | | | 90,845 | | | | 1,785,528 | |
| | | | | | | | | | | | | | | | | |
| Segment Expenses | | | | | | | | | | | | | | | | |
| Depreciation & amortization expenses | | | - | | | | - | | | | (684,576 | ) | | | (684,576 | ) |
| Finance costs | | | - | | | | - | | | | (463,685 | ) | | | (463,685 | ) |
| Share-based payments | | | - | | | | - | | | | (351,619 | ) | | | (351,619 | ) |
| Other operating expenses | | | (1,289,675 | ) | | | (472,002 | ) | | | (1,019,040 | ) | | | (2,780,717 | ) |
| Total Segment Expenses | | | (1,289,675 | ) | | | (472,002 | ) | | | (2,518,920 | ) | | | (4,280,597 | ) |
| Income Tax Expenses | | | - | | | | - | | | | - | | | | - | |
| (Loss)/Profit for the Period | | | (576,043 | ) | | | 509,049 | | | | (2,428,075 | ) | | | (2,495,069 | ) |
Information on major customers:
During the years ended 30 June 2016, 2015 and 2014, the Company had the following major customers (and their respective contribution to the Group’s total revenue):
| | | 2016 | | | 2015 | | | 2014 | |
| Customer A | | | 16 | % | | | 17 | % | | | 28 | % |
| Customer B | | | 43 | % | | | 33 | % | | | 31 | % |
| Customer C | | | 22 | % | | | 26 | % | | | 34 | % |
| Customer D | | | * | | | | 28 | % | | | 11 | % |
* Less than 10% of revenue for the respective year.
No other single customers contributed 10% or more to the Group’s revenue for all periods.
| Note 20. | Cash Flow Information |
| (a) | Reconciliation of cash flow from operations with loss after income tax |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | |
| Net Loss for the Year | | | (7,068,767 | ) | | | (2,691,820 | ) | | | (2,495,069 | ) |
| | | | | | | | | | | | | |
| Non-Cash | | | | | | | | | | | | |
| Add depreciation expense | | | 3,892 | | | | 3,719 | | | | 3,989 | |
| Add amortisation expense | | | - | | | | - | | | | 680,587 | |
| Add change in fair value and interest accrued on borrowings | | | 178,401 | | | | - | | | | 334,681 | |
| Add back equity issued for non-cash consideration | | | 187,500 | | | | 11,667 | | | | 132,708 | |
| Add back share based payments expense | | | 1,891,875 | | | | 4,375 | | | | 218,912 | |
| | | | | | | | | | | | | |
| Changes in Working Capital | | | | | | | | | | | | |
| Add (increases) in current trade and other receivables | | | (84,004 | ) | | | (460,204 | ) | | | (551,835 | ) |
| Add (increases) / decreases in other current assets | | | (30,015 | ) | | | 329,130 | | | | (314,260 | ) |
| Add (increases) in inventory | | | (909,800 | ) | | | (580,310 | ) | | | (274,263 | ) |
| Add increases / (decreases) in current trade and other payables | | | 672,582 | | | | 362,510 | | | | (386,027 | ) |
| | | | (5,158,336 | ) | | | (3,020,933 | ) | | | (2,650,577 | ) |
| (b) | Non-cash financing and investing activities |
See Note 8 for details on the uncleared funds of AUD$2,612,139 from capital raising as at 30 June 2016.
An amount of AUD$114,861 of capital raising costs were recognised as expenses but remained unpaid during the period as at 30 June 2016.
See Note 21 for details regarding issues of options to employees and for details surrounding the issue of shares to suppliers.
Changes were made to the Consolidated Statement of Cash Flows for the year 2016 as compared to the previous statement lodged with ASX, details as follows:
| | | Previously issued | | | Restatement | | | Revised | |
| Receipts from customers | | | 1,242,884 | | | | (128,288 | ) | | | 1,114,596 | |
| Payments to suppliers and employees | | | (7,639,088 | ) | | | (71,909 | ) | | | (7,710,997 | ) |
| Interest and other costs of finance paid | | | - | | | | (43,863 | ) | | | (43,863 | ) |
| Net Cash Flows Used In Operating Activities | | | (4,914,276 | ) | | | (244,060 | ) | | | (5,158,336 | ) |
| | | | | | | | | | | | | |
| Proceeds from issues of securities | | | 2,282,861 | | | | 200,000 | | | | 2,482,861 | |
| Repayment of borrowings | | | (1,121,080 | ) | | | 43,860 | | | | (1,077,220 | ) |
| Net Cash Flows Provided By Financing Activities | | | 4,091,482 | | | | 243,860 | | | | 4,335,342 | |
| Net increase/(decrease) in cash and cash equivalents | | | (825,235 | ) | | | (200 | ) | | | (825,435 | ) |
| Effects of exchange rate changes on cash and cash equivalents | | | (200 | ) | | | 200 | | | | - | |
Changes were made to the Consolidated Statement of Cash Flows for the year 2014 as compared to the previous statement lodged with ASX, details as follows:
| | | Previously issued | | | Restatement | | | Revised | |
| Proceeds from borrowings | | | - | | | | 420,000 | | | | 420,000 | |
| Repayment of borrowings | | | (1,485,001 | ) | | | (420,000 | ) | | | (1,905,001 | ) |
| Note 21. | Share-based Payments |
Executives and consultants may be provided with longer-term incentives through the Company’s Employee Share and Option Plan (ESOP), to allow the executives and consultants to participate in, and benefit from, the growth of the Company as a result of their efforts and to assist in motivating and retaining these key employees over the long term.
| (a) | Options Issued under the ESOP |
The following table illustrates the number and weighted average exercise price of and movement in share options issued under the scheme during the year:
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | | | | Weighted
Avg | | | | | | Weighted
Avg | | | | | | Weighted
Avg | |
| | | Number of | | | Exercise
Price | | | Number of | | | Exercise
Price | | | Number of | | | Exercise
Price | |
| | | Options | | | AUD$ | | | Options | | | AUD$ | | | Options | | | AUD$ | |
| | | | | | | | | | | | | | | | | | | |
| Outstanding at the beginning of the year | | | 1,856,150 | | | | 0.440 | | | | 36,246,031 | | | | 0.011 | | | | 14,000,000 | | | | 0.051 | |
| Capital consolidation (40:1) | | | - | | | | - | | | | (35,339,881 | ) | | | - | | | | - | | | | - | |
| Options granted during the year | | | - | | | | - | | | | - | | | | - | | | | 31,746,031 | | | | 0.008 | |
Granted options to be issued* | | | - | | | | - | | | | 1,000,000 | | | | 0.500 | | | | - | | | | - | |
| Options exercised | | | (150,000 | ) | | | 0.276 | | | | - | | | | - | | | | - | | | | - | |
| Lapse of unexercised options | | | (643,650 | ) | | | 0.276 | | | | (50,000 | ) | | | (1.556 | ) | | | (9,500,000 | ) | | | (0.052 | ) |
| Options Outstanding at End of the Year | | | 1,062,500 | | | | 0.562 | | | | 1,856,150 | | | | 0.440 | | | | 36,246,031 | | | | 0.011 | |
| Options Exercisable at the End of the Year | | | 62,500 | | | | 1.556 | | | | 856,150 | | | | 0.369 | | | | 34,996,031 | | | | 0.011 | |
* On 9 December 2016, the Company issued 1 million options exercisable at $0.50 per option expiring on April 1, 2017 to an employee under the Company's Employee Share and Options Plan (ESOP) following the successful completion of related milestone pertaining to a minimum recruitment of 100 patients into the Company’s NASH Phase 2 clinical trial.
The options outstanding at 30 June 2016 have a weighted average remaining contractual life of 0.79 years and exercise prices ranging from $0.500 to $1.556.
| (b) | Options Issued to Directors |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | | | | Weighted
Avg | | | | | | Weighted
Avg | | | | | | Weighted
Avg | |
| | | Number of | | | Exercise
Price | | | Number of | | | Exercise
Price | | | Number of | | | Exercise
Price | |
| | | Options | | | AUD$ | | | Options | | | AUD$ | | | Options | | | AUD$ | |
| | | | | | | | | | | | | | | | | | | |
| Outstanding at the beginning of the year | | | 1,000,000 | | | | 0.456 | | | | 40,000,000 | | | | 0.011 | | | | - | | | | - | |
| Capital consolidation (40:1) | | | - | | | | - | | | | (39,000,000 | ) | | | - | | | | - | | | | - | |
| Options granted during the year | | | 6,000,000 | | | | 0.500 | | | | - | | | | - | | | | 40,000,000 | | | | 0.0114 | |
| Lapse of unexercised options | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Options Outstanding at End of the Year | | | 7,000,000 | | | | 0.494 | | | | 1,000,000 | | | | 0.456 | | | | 40,000,000 | | | | 0.0114 | |
| Options Exercisable at the End of the Year | | | 1,000,000 | | | | 0.456 | | | | 1,000,000 | | | | 0.456 | | | | 40,000,000 | | | | 0.0114 | |
The options outstanding at 30 June 2016 have a weighted average remaining contractual life of 2.94 years and exercise prices ranging from $0.456 to 0.500.
| (c) | Options Issued to third parties |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | | | | Weighted
Avg | | | | | | Weighted
Avg | | | | | | Weighted
Avg | |
| | | Number of | | | Exercise
Price | | | Number of | | | Exercise
Price | | | Number of | | | Exercise
Price | |
| | | Options | | | AUD$ | | | Options | | | AUD$ | | | Options | | | AUD$ | |
| | | | | | | | | | | | | | | | | | | |
| Outstanding at the beginning of the year | | | 4,332,526 | | | | 0.400 | | | | 289,296,735 | | | | 0.022 | | | | 275,860,577 | | | | 0.024 | |
| Capital consolidation (40:1) | | | - | | | | - | | | | (282,065,012 | ) | | | - | | | | - | | | | - | |
| Options granted during the year | | | 1,425,532 | | | | 0.549 | | | | - | | | | - | | | | 16,217,463 | | | | 0.012 | |
| Options exercised | | | (910,166 | ) | | | 0.376 | | | | - | | | | | | | | (29,075 | ) | | | 0.040 | |
| Lapse of unexercised options | | | (2,972,763 | ) | | | 0.376 | | | | (2,899,197 | ) | | | 1.556 | | | | (2,752,230 | ) | | | 0.120 | |
| Options Outstanding at End of the Year | | | 1,875,129 | | | | 0.561 | | | | 4,332,526 | | | | 0.400 | | | | 289,296,735 | | | | 0.022 | |
| Options Exercisable at the End of the Year | | | 1,875,129 | | | | 0.561 | | | | 4,332,526 | | | | 0.400 | | | | 289,296,735 | | | | 0.022 | |
The options outstanding at 30 June 2016 have a weighted average remaining contractual life of 2.85 years and exercise prices ranging from $0.300 to $1.944.
| (d) | Vesting Terms of Options |
The following summarizes information about options held by employees, Directors and third parties as at 30 June 2016:
| Issue Date | | Number of Options | | | Vesting Conditions | | Expiry Date | | Exercise Price
AUD$ | |
| 29 Jun 2012 | | | 14,493 | | | Nil | | 30 Nov 2021 | | AUD$ | 1.944 | |
| 29 Jun 2012 | | | 29,668 | | | Nil | | 17 Jan 2022 | | AUD$ | 1.876 | |
| 15 Nov 2012 | | | 62,500 | | | 25% per annum | | 1 Nov 2017 | | AUD$ | 1.556 | |
| 4 Dec 2013 | | | 1,000,000 | | | Nil | | 4 Dec 2016 | | AUD$ | 0.456 | |
| 4-Dec-13 | | | 250,000 | | | Nil | | 4 Dec 2016 | | AUD$ | 0.456 | |
| 3-Mar-14 | | | 15,380 | | | Nil | | 28 Feb 2019 | | AUD$ | 1.892 | |
| 29-May-14 | | | 140,056 | | | Nil | | 28 May 2019 | | AUD$ | 0.300 | |
| 27 Nov 2015 | | | 6,000,000 | | | See below | | 27 Nov 2019 | | AUD$ | 0.500 | |
| 18 Feb 2016 | | | 1,000,000 | | | Nil | | 24 Feb 2019 | | AUD$ | 0.570 | |
| 31 May 2016 | | | 425,532 | | | Nil | | 27 Nov 2019 | | AUD$ | 0.500 | |
| 9 Dec 2016 | | | 1,000,000 | | | Performance based | | 1 Apr 2017 | | AUD$ | 0.500 | |
November 2012 Options
The options with an issue date of 15 November 2012, entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$1.556*. There are no performance conditions attached to the options as the options vest accordingly to the following anniversary dates:
| - | 25% of the total quantum of these options issued vested immediately upon issue |
| - | 25% of the total quantum of these options issued vest on 1 July 2013 |
| - | 25% of the total quantum of these options issued vest on 1 July 2014 |
| - | 25% of the total quantum of these options issued vest on 1 July 2015 |
July 2013 Options
The options with an issue date of 4 July 2013, entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of $0.30*. There are no performance conditions attached to the options. The options were deemed to have been fully vested on their date on issue.
December 2013 Options
The options with an issue date of 4thDecember 2013, entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$0.456*. There are no performance conditions attached to the options. The options were deemed to have been fully vested on their date on issue.
November 2015 Options
The options with an issue date of 27 November 2015, entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$0.500. Options vest based on month of continuous services completed as per the following:
| - | 5,000,000 Options which will vest on 6th August 2016 – subject to completion of 12 months’ continuous services as a Director of the Company |
| - | 1,000,000 Options which will vest on 6th August 2017 – subject to completion of 24 months’ continuous services as a Director of the Company |
February 2016 Options
The options with an issue date of 18 February 2016, entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$0.570. There are no performance conditions attached to the options. The options were deemed to have been fully vested on their date on issue.
May 2016 Options
The options with an issue date of 31 May 2016, entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$0.500. There are no performance conditions attached to the options. The options were deemed to have been fully vested on their date on issue.
| * | The above value has been adjusted for 40:1 share consolidation which was completed on 20 Nov 2014. |
December 2016 Options
Pursuant to an agreement entered between the Company and a consultant on 1 April 2015, the Company granted 1,000,000 options, which became vested and issued on 9 December 2016, and entitle the holder to purchase one ordinary share in Immuron Limited at an exercise price of AUD$0.500. These options were vested and issued following the successful completion of related milestone pertaining to a minimum recruitment of 100 patients into the Company’s NASH Phase 2 clinical trial.
| (e) | Deemed Valuation of Options |
The fair value of the options granted under the Company’s Executive Share and Option Plan (ESOP) is estimated as at the grant date using Black-Scholes model taking into account the terms and conditions upon which the options were granted.
November 2012 Options
The following table lists the inputs to the model used to determine the weighted average value of the options expensed during the year:
| Vesting date | | | As per above | |
| Dividend yield | | | - | |
| Expected volatility | | | 70 | % |
| Risk-free interest rate | | | 3.25 | % |
| Expected life of option (years) | | | 5 years | |
| Option exercise price | | AUD$ | 0.04 | |
| Weighted average share price at grant date | | AUD$ | 0.017 | |
| Value per option | | AUD$ | 0.280 | * |
| * | The above value has been adjusted for 40:1 share consolidation which was completed on 20 Nov 2014. |
At 30 June 2016 the Australian Securities Exchange (ASX) market share price for Immuron was AUD$0.25.
July 2013 Options
The following table lists the inputs to the model used to determine the weighted average value of the options expensed during the year:
| Vesting date | | | N/A | |
| Dividend yield | | | - | |
| Expected volatility | | | 62 | % |
| Risk-free interest rate | | | 2.79 | % |
| Expected life of option (years) | | | 3 years | |
| Option exercise price | | $ | 0.0075 | |
| Weighted average share price at grant date | | $ | 0.0060 | |
| Value per option | | $ | 0.088 | * |
* The above value has been adjusted for 40:1 share consolidation which was completed on 20 Nov 2014.
At 30 June 2016 the Australian Securities Exchange (ASX) market share price for Immuron was AUD$0.25.
December 2013 Options
The following table lists the inputs to the model used to determine the weighted average value of the options expensed during the year:
| Vesting date | | | N/A | |
| Dividend yield | | | - | |
| Expected volatility | | | 62 | % |
| Risk-free interest rate | | | 3.03 | % |
| Expected life of option (years) | | | 3 years | |
| Option exercise price | | AUD$ | 0.0114 | |
| Weighted average share price at grant date | | AUD$ | 0.0080 | |
| Value per option | | AUD$ | 0.1016 | * |
| * | The above values have been adjusted for 40:1 share consolidation which was completed on 20 Nov 2014. |
At 30 June 2016 the Australian Securities Exchange (ASX) market share price for Immuron was AUD$0.25.
The expected life of the option is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
November 2015 Options
The following table lists the inputs to the model used to determine the weighted average value of the options expensed during the year:
| Vesting date | | | As per above | |
| Dividend yield | | | - | |
| Expected volatility | | | 100 | % |
| Risk-free interest rate | | | 2.11 | % |
| Expected life of option (years) | | | 4 years | |
| Option exercise price | | AUD$ | 0.5000 | |
| Weighted average share price at grant date | | AUD$ | 0.465 | |
| Value per option | | AUD$ | 0.3186 | |
At 30 June 2016 the Australian Securities Exchange (ASX) market share price for Immuron was AUD$0.25.
The expected life of the option is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
February 2016 Options
The following table lists the inputs to the model used to determine the weighted average value of the options expensed during the year:
| Vesting date | | | N/A | |
| Dividend yield | | | - | |
| Expected volatility | | | 97 | % |
| Risk-free interest rate | | | 1.73 | % |
| Expected life of option (years) | | | 3 years | |
| Option exercise price | | AUD$ | 0.5700 | |
| Weighted average share price at grant date | | AUD$ | 0.36 | |
| Value per option | | AUD$ | 0.1856 | |
At 30 June 2016 the Australian Securities Exchange (ASX) market share price for Immuron was AUD$0.25.
The expected life of the option is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
May 2016 Options
The following table lists the inputs to the model used to determine the weighted average value of the options expensed during the year:
| Vesting date | | | N/A | |
| Dividend yield | | | - | |
| Expected volatility | | | 84 | % |
| Risk-free interest rate | | | 2.11 | % |
| Expected life of option (years) | | | 4 years | |
| Option exercise price | | AUD$ | 0.5000 | |
| Weighted average share price at grant date | | AUD$ | 0.41 | |
| Value per option | | AUD$ | 0.235 | |
At 30 June 2016 the Australian Securities Exchange (ASX) market share price for Immuron was AUD$0.25.
The expected life of the option is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
| Note 22. | Related Party Transactions |
The transactions with related parties are as follows:
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| Short-term Loan from Grandlodge Capital Pty Ltd: | | | | | | | | | | | | |
| Grandlodge Capital Pty Ltd (Grandlodge) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Mr David Plush is also an owner of Grandlodge, and its associated entities | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| On 1st December 2015 and on 6th June 2016, Immuron executed a short-term funding agreement with Grandlodge for a principle amount of AUD$1,000,000 (interest rate of 13%) and AUD$750,000 (interest rate of 15%) respectively, plus interest charges. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The short-term funding is a cash advance against the anticipated refund Immuron will receive from the Australian Taxation Office under the Research and Development Income Tax Concession Incentive for the Company's eligible R&D expenditure incurred for financial year of 2015 and 2016. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Loan from 1st December 2015 has been repaid to Grandlodge on 10th February 2016. The June 2016, loan from Grandlodge, plus applicable fees, will be repaid by the Company upon receipt of the FY2016 R&D Tax Incentive refund which was received in November 2016. Interest paid was approximately $43,000 in 2016 and loan fees paid to Grandlodge were approximately $20,000 and $15,000 in 2016, respectively. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Loans from October and December 2013 were repaid in fiscal 2014. These loan agreements were for a period of 6 months or the receipt of the R&D Tax Incentive Refund if sooner, bearing an interest rate of 18% per annum. Interest paid was approximately $15,000 in 2014. | | | | | | | | | | | | |
| Total paid by the Company to Grandlodge Pty Ltd during the year: | | | 1,043,863 | | | | N/A | | | | 435,495 | |
| At year end the Company owed Grandlodge Pty Ltd: | | | 772,397 | | | | N/A | | | | N/A | |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| Services rendered by Grandlodge Pty Ltd to Immuron Ltd: | | | | | | | | | | | | |
| Grandlodge, and its associated entities, are marketing, warehousing and distribution logistics companies. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Commencing on 1 June 2013, Grandlodge was verbally contracted on terms to provide warehousing, distribution and invoicing services for Immuron’s products for AUD$70,000 per annum. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| These fees will be payable in new fully paid ordinary shares in Immuron Limited at a set price of AUD$0.16 per share representing Immuron Limited’s share price at the commencement of the verbal agreement. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The shares to be issued to Grandlodge, or its associated entities, as compensation in lieu of cash payment for the services rendered under this verbal agreement have been subject to the approval of Immuron shareholders at Company shareholder meetings held over the past 18 months. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Grandlodge will also be reimbursed in cash for all reasonable costs and expenses incurred in accordance with their scope of works under the verbal agreement, unless both parties agree to an alternative method of payment. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The verbal agreement is cancellable by either party upon providing the other party with 30 days written notice of the termination of the agreement. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Service fees paid to Grandlodge Pty Ltd during the year through the issue of equity: | | | 87,500 | | | | 11,667 | | | | 75,833 | |
| Total paid by the Company to Grandlodge Pty Ltd during the year: | | | 87,500 | | | | 11,667 | | | | 75,833 | |
| At year end the Company owed Grandlodge Pty Ltd: | | | 35,000 | | | | 58,333 | | | | - | |
| | | 30 June 2016 | | | 30 June 2015 | | | 30 June 2014 | |
| | | AUD$ | | | AUD$ | | | AUD$ | |
| Premises Rental services received from Wattle Laboratories Pty Ltd to Immuron Ltd: | | | | | | | | | | | | |
| Wattle Laboratories Pty Ltd (Wattle) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Commencing on 1 January 2016, Immuron executed a Lease Agreement with Wattle whereby Immuron will lease part of their Blackburn office facilities for Immuron's operations at a rental rate of AUD$38,940 per annum, payable in monthly installments. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The lease is for a 3 year term with an additional 3 year option period. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| The lease is cancellable by either party upon 6 months written notice of termination of the agreement. | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Rental fees paid to Wattle Laboratories Pty Ltd during the year through the issue of equity: | | | Nil | | | | N/A | | | | N/A | |
| Total paid by the Company to Wattle Laboratories Pty Ltd during the year: | | | 19,470 | | | | N/A | | | | N/A | |
| At year end the Company owed Wattle Laboratories Pty Ltd: | | | 21,417 | | | | N/A | | | | N/A | |
| Note 23. | Financial Risk Management Objectives and Policies |
The Company's financial instruments consist of cash and cash equivalents, trade and other receivables and trade and other payables:
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Cash and cash equivalents | | | 2,290,639 | | | | 3,116,074 | |
| Trade and other receivables | | | 4,387,772 | | | | 1,691,629 | |
| Trade and other payables | | | (1,986,407 | ) | | | (1,207,810 | ) |
| Borrowings (See Note 22) | | | (772,397 | ) | | | - | |
| Convertible notes | | | (1,128,117 | ) | | | - | |
The fair values of cash and cash equivalents, trade and other receivables and trade and other payables approximate their carrying amounts largely due to being liquid assets and payables will be settled within 12 months.
| (b) | Risk Management Policy |
The Board is responsible for overseeing the establishment and implementation of the risk management system, and reviews and assesses the effectiveness of the Company's implementation of that system on a regular basis.
The Board and Senior Management identify the general areas of risk and their impact on the activities of the Company, with Management performing a regular review of:
| Ø | the major risks that occur within the business; |
| Ø | the degree of risk involved; |
| Ø | the current approach to managing the risk; and |
| Ø | if appropriate, determine: |
| o | any inadequacies of the current approach; and |
| o | possible new approaches that more efficiently and effectively address the risk. |
Management report risks identified to the Board through the monthly Operations Report.
The Company seeks to ensure that its exposure to undue risk which is likely to impact its financial performance, continued growth and survival is minimised in a cost effective manner.
| (c) | Significant Accounting Policies |
Details of significant accounting policies and methods adopted, including the criteria for recognition, the basis for measurement and the basis on which income and expenses are recognised, in respect of each class of financial asset, financial liability and equity instrument are disclosed in Note 1 to the financial statements.
The carrying amounts of cash and cash equivalents, trade and other receivables, trade and other payables and financial liabilities represents their fair values determined in accordance with the accounting policies disclosed in Note 1. Interest income on cash and cash equivalents is disclosed in Note 2.
| (d) | Capital Risk Management |
The Company's objectives when managing capital are to safeguard the Company's ability to continue as a going concern and to maintain an optimal capital structure so as to maximise shareholder value.
In order to maintain or achieve an optimal capital structure, the Company may issue new shares or reduce its capital, subject to the provisions of the Company's constitution. The capital structure of the Company consists of equity attributed to equity holders of the Company, comprising contributed equity, reserves and accumulated losses disclosed in Notes 17 and 18.
By monitoring undiscounted cash flow forecasts and actual cash flows provided to the Board by the Company's Management the Board monitors the need to raise additional equity from the equity markets.
Financial Risk Management
The main risks the Company is exposed to through its operations are interest rate risk, foreign exchange risk, credit risk and liquidity risk.
Interest Rate Risk
The Company is exposed to interest rate risks via the cash and cash equivalents and borrowings that it holds. Interest rate risk is the risk that a financial instruments value will fluctuate as a result of changes in market interest rates. The objective of managing interest rate risk is to minimise the Company's exposure to fluctuations in interest rate that might impact its interest revenue and cash flow.
Interest rate risk is considered when placing funds on term deposits. The Company considers the reduced interest rate received by retaining cash and cash equivalents in the Company's operating account compared to placing funds into a term deposit. This consideration also takes into account the costs associated with breaking a term deposit should early access to cash and cash equivalents be required.
There has been no change to the Company's exposure to interest rate risk or the manner in which it manages and measures its risk in the year ended 30 June 2016.
Foreign Currency Risk
The Company is exposed to foreign currency risk via the trade and other receivables and trade and other payables that it holds. Foreign currency risk is the risk that the value of a financial instrument will fluctuate due to changes in foreign exchange rates. The Company aims to take a conservative position in relation to foreign currency risk hedging when budgeting for overseas expenditure however, the Company does not have a policy to hedge overseas payments or receivables as they are highly variable in amount and timing, due to the reliance on activities carried out by overseas entities and their billing cycle.
The following financial assets and liabilities are subject to foreign currency risk:
| | | 30 June 2016 | | | 30 June 2015 | |
| | | AUD$ | | | AUD$ | |
| Cash and cash equivalents (AUD/USD) | | | 40,702 | | | | 6,840 | |
| Trade and other receivable (AUD/USD) | | | 127,110 | | | | - | |
| Trade and other payables (AUD/USD) | | | 564,104 | | | | 193,443 | |
| Trade and other payables (AUD/CHF) | | | 10,069 | | | | - | |
| Trade and other payables (AUD/NZD) | | | 452,599 | | | | 394,064 | |
| Trade and other payables (AUD/ISL) | | | 46,260 | | | | 9,613 | |
Foreign currency risk is measured by regular review of cash forecasts, monitoring the dollar amount and currencies that payment are anticipated to be paid in. The Company also considers the market fluctuations in relevant currencies to determine the level of exposure. If the level of exposure is considered by Management to be too high, then Management has authority to take steps to reduce the risk.
Steps to reduce risk may include the acquisition of foreign currency ahead of the anticipated due date of an invoice, or may include negotiations with suppliers to make payment in our functional currency, or may include holding receipted foreign currency funds in a foreign currency denominated bank account to make future payments denominated in that same currency. Should Management determine that the Company consider taking out a hedge to reduce the foreign currency risk, they would need to seek Board approval.
The Company conducts some activities outside of Australia which exposes it to transactional currency movements, where the Company is required to pay in a currency other than its functional currency.
There has been no change in the manner the Company manages and measures its risk in the year ended 30 June 2016.
The Company is exposed to fluctuations in the United States and New Zealand dollars. Analysis is conducted on a currency by currency basis using sensitivity variables.
The Company has conducted a sensitivity analysis of the Company's exposure to foreign currency risk. The analysis shows that if the Company's exposure to foreign currency risk was to fluctuate as disclosed below and all other variables had remained constant, then the foreign currency sensitivity impact on the Company's loss after tax and equity would be as follows:
| | | 30 June 2016 | | | 30 June 2015 | |
| | (Higher) / Lower | | | (Higher) / Lower | |
| Trade and Other Payables | | AUD$ | | | AUD$ | |
| AUD / USD: 2016 +8.00% (2015: +8.00%) | | | 45,128 | | | | 15,475 | |
| AUD / USD: 2016 -8.00% (2015:-8.00%) | | | (45,128 | ) | | | (15,475 | ) |
| AUD / CHF: 2016 +11.00% | | | 1,108 | | | | - | |
| AUD / CHF: 2016 -11.00% | | | (1,108 | ) | | | - | |
| AUD / NZD: 2016 +11.00% (2015: +11.00%) | | | 49,786 | | | | 43,347 | |
| AUD / NZD: 2016 -11.00% (2015: -11.00%) | | | (49,786 | ) | | | (43,347 | ) |
| AUD / ISL: 2016 +11.00% (2015: +11.00%) | | | 5,089 | | | | 1,057 | |
| AUD / ISL: 2016 -11.00% (2015: -11.00%) | | | (5,089 | ) | | | (1,057 | ) |
Credit Risk
The Company is exposed to credit risk via its cash and cash equivalents and trade and other receivables. Credit risk is the risk that a counter-party will default on its contractual obligations resulting in a financial loss to the Company. To reduce risk exposure for the Company's cash and cash equivalents, it places them with high credit quality financial institutions.
The Company’s major ongoing customers are the large pharmaceutical companies for the distribution of Travelan and other Hyperimmune products, and Government bodies for the receipt of GST refunds and Research and Development Tax Concession amounts due to the Company from the Australian Tax Office.
The Company has a policy that limits the credit exposure to customers and regularly monitors its credit exposure. The Board believes that the Company does not have significant credit risk at this time in respect of its trade and other receivables. Regarding customers with over 30-day debt balance, management has maintained on-going communication with relevant counter parties in regard of repayment schedule, and concluded that there have been no changes to the initial assessment of credit risk.
The Company has analyzed its trade and other receivables below:
| | | 0 - 30 days | | | 31 - 60 days | | | 61 - 90 days | | | 90 days + | | | Total | |
| | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | | | | |
| 2016 Trade and other receivables | | | 2,822,116 | | | | 45,687 | | | | - | | | | 7,129 | | | | 2,874,932 | |
| 2016 R&D tax concession refund | | | n/a | | | | n/a | | | | n/a | | | | n/a | | | | 1,512,840 | |
| | | | | | | | | | | | | | | | | | | | | |
| 2015 Trade and other receivables | | | 99,622 | | | | 72,202 | | | | 37,325 | | | | 3,899 | | | | 213,048 | |
| 2015 R&D tax concession refund | | | n/a | | | | n/a | | | | n/a | | | | n/a | | | | 1,478,581 | |
R&D tax concession refund in each period is recovered upon finalization of the Australian Tax Office’s review of the Company’s annual R&D tax concession claim.
Liquidity Risk
The Company is exposed to liquidity risk via its trade and other payables and its recurring and projected losses.
Liquidity risk is the risk that the Company will encounter difficulty in raising funds to meet the commitments associated with its financial instruments. Responsibility for liquidity risk rests with the Board who manage liquidity risk by monitoring undiscounted cash flow forecasts and actual cash flows provided to them by the Company's Management at Board meetings to ensure that the Company continues to be able to meet its debts as and when they fall due.
Contracts are not entered into unless the Board believes that there is sufficient cash flow to fund the additional activity. The Board considers when reviewing its undiscounted cash flow forecasts whether the Company needs to raise additional funding from the equity markets.
The Company has analysed its trade and other payables below:
| | | 0 - 30 days | | | 31 - 60 days | | | 61 - 90 days | | | 90 days + | | | Total | |
| | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | | | | |
| 2016 Trade and other payables and notes payable | | | 1,008,089 | | | | 659,494 | | | | 299,239 | | | | 19,585 | | | | 1,986,407 | |
| 2016 Borrowings (see Note 22 for repayment terms) | | | n/a | | | | n/a | | | | n/a | | | | n/a | | | | 772,397 | |
| 2016 Convertible notes (note 15 for repayment terms) | | | n/a | | | | n/a | | | | n/a | | | | n/a | | | | 1,128,117 | |
| | | | | | | | | | | | | | | | | | | | | |
| 2015 Trade and other payables | | | 923,181 | | | | 96,888 | | | | 15,318 | | | | 172,423 | | | | 1,207,810 | |
As at 30 June 2016, the Company maintained a cash and cash equivalents balance of AUD$2,290,639. Additionally, the Company also recognised a total of AUD$4,387,772 in receivables, including a AUD$1,512,840 related to R&D Tax Concession, which was received in November 2016. On this basis, even though the company has been in loss making position historically, management is satisfied that the Group is a going concern and are of the opinion that no asset is likely to be realized for an amount lower than the amount at which it is recorded in the Consolidated Statement of Financial Position at 30 June 2016.
| Note 24. | Events after the Reporting Date |
7 July 2016:
| - | The Company issued 21,320,978 new fully paid ordinary shares in the Company to subscribers and shortfall participants of the Rights Issue Capital Raising. 2,418,129 of these new fully paid ordinary shares were issued to Grandlodge on the same terms and conditions as all other subscribers. The Rights Issue capital Raising raised a total of AUD$5,330,245. An amount of AUD$4,511,378 was recorded as Share to be issued as at 30 June 2016. |
| - | The Company is also committed to issue 21,320,978 free-attaching 1:1 new Unlisted Options exercisable at AUD$0.55 expiring 3 years from the date of issue to subscribers and shortfall participants of the Rights Issue. This issuance of these options is subject to shareholder approval. |
25 August 2016:
| - | On August 25th on behalf of Immuron, Grandlodge purchased US$1,500,000 at the cost of AUD$1,968,762. On the same day Immuron paid Grandlodge AUD$1,968,762 to settle this transaction. On Sept 12th Grandlodge returned the USD$1,500,000 purchase to Immuron. Grandlodge received no financial gains or benefits from this transaction. |
4 October 2016:
| - | The Company issued 3,968,816 of new fully paid ordinary shares and 3,968,816 Unlisted Options exercisable at AUD$0.55 expiring 3 years from the date of issue to Shortfall Participants of the Rights Issue as described in the Offer Booklet announced to the ASX on 31 May 2016 and to the over-subscribers of the Rights Issue, which raised a total of AUD$992,229. |
30 November 2016:
| - | The Company issued 251,877 fully paid ordinary shares to three employees under the Company's Employee Share and Options Plan (ESOP) which were issued in lieu of cash payment for future salary services rendered over the period October through March 2016. |
6 December 2016:
| - | The Company fully repaid the loan in the amount of approximately $772,000 outstanding as of June 30, 2016 to Grandlodge. |
9 December 2016:
| - | The Company issued 1million options exercisable at $0.50 per option expiring on 1 April 2017 to an employee under the Company's Employee Share and Options Plan (ESOP) |
| - | The Company issued 200,000 options exercisable at $0.50 per option expiring on 27 November 2019 to an employee under the Company's Employee Share and Options Plan (ESOP) |
Other than the events listed above, there have not been any other matters or circumstances that have arisen since the end of the financial year, which significantly affected, or may significantly affect, the operations of Immuron Limited, the results of those operations.
The registered office of the Company is:
Suite 1, 1233 High Street, Armadale, Victoria, Australia 3143.
The principal place of business of the Company is:
Suite 1, 1233 High Street, Armadale, Victoria, Australia 3143
| Consolidated Statement of Profit or Loss and Other Comprehensive Income |
| For the six months ended December 31, |
| | | | | 2016
(Unaudited) | | | 2015
(Unaudited) | |
| | | Notes | | AUD$ | | | AUD$ | |
| | | | | | | | (Restated) | |
| Revenue | | | | | | | | | | |
| Operating Revenue | | | | | 703,099 | | | | 497,220 | |
| Total Operating Revenue | | | | | 703,099 | | | | 497,220 | |
| | | | | | | | | | | |
| Cost of Goods Sold | | | | | (223,394 | ) | | | (153,640 | ) |
| Gross Profit | | | | | 479,705 | | | | 343,580 | |
| | | | | | | | | | | |
| Direct Selling Costs | | | | | | | | | | |
| Sales and Marketing Costs | | | | | (93,520 | ) | | | (52,659 | ) |
| Freight Costs | | | | | (62,590 | ) | | | (61,299 | ) |
| Total Gross Profit less Direct Selling Costs | | | | | 323,595 | | | | 229,622 | |
| | | | | | | | | | | |
| Other Income | | | | | 816,932 | | | | 763,563 | |
| | | | | | | | | | | |
| Expenses | | | | | | | | | | |
| Consulting, Employee and Director | | | | | (907,390 | ) | | | (1,113,214 | ) |
| Corporate Administration | | | | | (790,103 | ) | | | (708,625 | ) |
| Depreciation | | | | | (1,975 | ) | | | (1,944 | ) |
| Finance Costs | | | | | (13,183 | ) | | | - | |
| Impairment of Inventory | | | | | (135,170 | ) | | | (169 | ) |
| Marketing and Promotion | | | | | (471,735 | ) | | | (201,724 | ) |
| Research and Development | | | | | (2,117,867 | ) | | | (1,839,990 | ) |
| Travel and Entertainment | | | | | (112,453 | ) | | | (195,605 | ) |
| Loss Before Income Tax | | | | | (3,409,349 | ) | | | (3,068,086 | ) |
| Income Tax Expense | | | | | - | | | | - | |
| Loss for the Period | | | | | (3,409,349 | ) | | | (3,068,086 | ) |
| Other Comprehensive Loss | | | | | (41,425 | ) | | | (4,781 | ) |
| Total Comprehensive Loss for the Period | | | | | (3,450,774 | ) | | | (3,072,867 | ) |
| | | | | | | | | | | |
| Basic/Diluted Loss per Share (cents per share) | | 8 | | | 3.300 | | | | 4.065 | |
The accompanying notes form part of these financial statements.
| Consolidated Statement of Financial Position |
| |
| | | | | December 31, 2016 (Unaudited) | | | June 30, 2016 | |
| | | Notes | | AUD$ | | | AUD$ | |
| ASSETS | | | | | | | | | | |
| Current Assets | | | | | | | | | | |
| Cash and cash equivalents | | | | | 3,103,683 | | | | 2,290,639 | |
| Trade and other receivables | | | | | 900,347 | | | | 4,387,772 | |
| Inventories | | 4 | | | 1,751,871 | | | | 2,056,067 | |
| Other | | | | | 81,933 | | | | 74,943 | |
| Total Current Assets | | | | | 5,837,834 | | | | 8,809,421 | |
| | | | | | | | | | | |
| Non-Current Assets | | | | | | | | | | |
| Property, plant and equipment | | | | | 17,967 | | | | 18,063 | |
| Total Non-Current Assets | | | | | 17,967 | | | | 18,063 | |
| TOTAL ASSETS | | | | | 5,855,801 | | | | 8,827,484 | |
| | | | | | | | | | | |
| LIABILITIES | | | | | | | | | | |
| Current liabilities | | | | | | | | | | |
| Trade and other payables | | | | | 1,624,621 | | | | 1,986,407 | |
| Borrowings | | 9 | | | - | | | | 772,397 | |
| Other financial liabilities | | | | | 678,000 | | | | 1,128,117 | |
| Total Current Liabilities | | | | | 2,302,621 | | | | 3,886,921 | |
| TOTAL LIABILITIES | | | | | 2,302,621 | | | | 3,886,921 | |
| NET ASSETS | | | | | 3,553,180 | | | | 4,940,563 | |
| | | | | | | | | | | |
| EQUITY | | | | | | | | | | |
| Issued capital | | 6 | | | 47,485,700 | | | | 45,633,354 | |
| Reserves | | 7 | | | 2,243,061 | | | | 2,128,566 | |
| Accumulated losses | | | | | (46,175,581 | ) | | | (42,821,357 | ) |
| TOTAL EQUITY | | | | | 3,553,180 | | | | 4,940,563 | |
The accompanying notes form part of these financial statements.
| Consolidated Statement of Changes in Equity |
| For the six months ended December 31, 2016 and 2015 (unaudited) |
| | | Issued capital | | | Reserves | | | Accumulated
Losses | | | Total | |
| | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Balance as at June 30, 2015 (Restated) | | | 40,335,347 | | | | 548,065 | | | | (36,072,810 | ) | | | 4,810,602 | |
| Loss after income tax expense for the period (Restated) | | | - | | | | - | | | | (3,068,086 | ) | | | (3,068,086 | ) |
| Other comprehensive loss for the period | | | - | | | | (4,781 | ) | | | - | | | | (4,781 | ) |
| Total comprehensive loss for the period | | | - | | | | (4,781 | ) | | | (3,068,086 | ) | | | (3,072,867 | ) |
| Transactions with owners in their capacity as owners | | | | | | | | | | | | | | | | |
| Employee and consultant share options | | | - | | | | 537,309 | | | | - | | | | 537,309 | |
| Lapse or exercise of share options (Restated) | | | 58,615 | | | | (58,615 | ) | | | - | | | | - | |
| Shares issued, net of costs (Restated) | | | 422,264 | | | | - | | | | - | | | | 422,264 | |
| Balance as at December 31, 2015 (Restated) | | | 40,816,226 | | | | 1,021,978 | | | | (39,140,896 | ) | | | 2,697,308 | |
| | | | | | | | | | | | | | | | | |
| Balance as at June 30, 2016 | | | 45,633,354 | | | | 2,128,566 | | | | (42,821,357 | ) | | | 4,940,563 | |
| Loss after income tax expense for the period | | | - | | | | - | | | | (3,409,349 | ) | | | (3,409,349 | ) |
| Other comprehensive income for the period | | | - | | | | (41,425 | ) | | | - | | | | (41,425 | ) |
| Total comprehensive loss for the period | | | - | | | | (41,425 | ) | | | (3,409,349 | ) | | | (3,450,774 | ) |
| Transactions with owners in their capacity as owners | | | | | | | | | | | | | | | | |
| Employee and consultant share options | | | - | | | | 282,920 | | | | - | | | | 282,920 | |
| Lapse or exercise of share options | | | 71,875 | | | | (127,000 | ) | | | 55,125 | | | | - | |
| Shares issued, net of costs | | | 1,780,471 | | | | - | | | | - | | | | 1,780,471 | |
| Balance as at December 31, 2016 | | | 47,485,700 | | | | 2,243,061 | | | | (46,175,581 | ) | | | 3,553,180 | |
The accompanying notes form part of these financial statements.
| Consolidated Statement of Cash Flows |
| For the six months ended December 31, |
| | | | | 2016 (Unaudited) | | | 2015 (Unaudited) | |
| | | Note | | AUD$ | | | AUD$ | |
| | | | | (Restated) | | | (Restated) | |
| Cash flows Related to Operating Activities | | | | | | | | | | |
| Receipts from customers | | | | | 773,476 | | | | 553,406 | |
| Payments to suppliers and employees | | | | | (4,572,294 | ) | | | (4,014,796 | ) |
| Interest received | | | | | 6,791 | | | | 7,143 | |
| Interest and other costs of finance paid | | | | | (54,555 | ) | | | - | |
| Other - R&D Tax Concession Refund | | | | | 1,590,043 | | | | - | |
| Net Cash Flows Used In Operating Activities | | | | | (2,256,539 | ) | | | (3,454,247 | ) |
| | | | | | | | | | | |
| Cash Flows Related to Investing Activities | | | | | | | | | | |
| Payment for purchases of plant and equipment | | | | | (1,879 | ) | | | (2,441 | ) |
| Net Cash Flows Used In Investing Activities | | | | | (1,879 | ) | | | (2,441 | ) |
| | | | | | | | | | | |
| Cash Flows Related to Financing Activities | | | | | | | | | | |
| Proceeds from issues of securities | | | | | 4,423,234 | | | | 342,223 | |
| Capital raising costs | | | | | (149,919 | ) | | | (7,458 | ) |
| Proceeds from borrowings | | | | | - | | | | 1,000,000 | |
| Repayment of borrowings | | 9 | | | (1,202,000 | ) | | | - | |
| Net Cash Flows Provided By Financing Activities | | | | | 3,071,315 | | | | 1,334,765 | |
| | | | | | | | | | | |
| Net increase/(decrease) in cash and cash equivalents | | | | | 812,897 | | | | (2,121,923 | ) |
| Cash and cash equivalents at the beginning of the year | | | | | 2,290,639 | | | | 3,116,074 | |
| Effects of exchange rate changes on cash and cash equivalents | | | | | 147 | | | | - | |
| Cash and Cash Equivalents at the End of the Year | | | | | 3,103,683 | | | | 994,151 | |
The accompanying notes form part of these financial statements.
| Notes to the Consolidated Financial Statements (unaudited) |
Note 1. Basis of preparation
Basis of Preparation
The general purpose financial report for the interim six months reporting periods ended December, 31 2016 and 2015 have been prepared in accordance with International Accounting Standard (IAS) 134 Interim Financial Reporting, under the International Financial Reporting Standards, as issued by the International Accounting Standards Board
This half year financial report does not include all notes of the type normally included in an annual financial report. Accordingly, this report is to be read in conjunction with the annual report for the years ended June 30, 2016, 2015 and 2014 included in the Form F-1 and any public announcements made by Immuron Limited and its controlled entities (the “Group”), during the interim reporting periods.
The half year financial report reflects all adjustments which are, in the opinion of management, necessary to a fair statement of the results for the interim periods presented. All adjustments are of a normal recurring nature.
Accounting Policies
All accounting policies adopted are consistent with the most recent Annual Financial Report for the years ended June 30, 2016, 2015 and 2014. The consolidated entity has adopted all of the new, revised or amending Accounting Standards and Interpretations issued by the International Accounting Standards Board ('IASB') that are mandatory for the current reporting period. The adoption of these Accounting Standards and Interpretations did not have any significant impact on the financial performance or position of the consolidated entity.
Fair value measurement
Due to the nature of the Group’s operating profile, the Directors and management do not consider that the fair values of the Group’s financial assets and liabilities are materially different from their carrying amounts at December, 31 2016.
Going Concern
For the six months ended December, 31 2016 the Company experienced a net cash outflow of AUD$2,256,539 (2015: AUD$3,454,247) from operating activities. Net loss for the period was AUD$3,409,349 which included AUD$2,117,867 of expenditures associated with research, development and commercialisation programs predominantly surrounding the Non- Alcoholic Steatohepatitis (NASH) Clinical Trial (2015: AUD$3,068,086 and AUD$1,839,990, respectively). These factors raise substantial doubt on the Company’s ability to continue as a going concern.
Whilst the Company is projecting further net losses and a net cash outflow from operations for the remainder of the 2017 financial year, management is in the process of enhancing its internal cost control procedures to further reduce operating costs. Additionally, the Company also plans to raise additional capital from domestic or oversea market. The following historical successes achieved in the past 6 months further support the Company’s position in future capital raising and cost reduction:
| · | successfully completion of an oversubscribed AUD$6.32M Right Issue Capital Raising (before costs); |
| · | completion of patient recruitment in the Company’s (NASH) IMM-124E Phase 2 clinical trial. |
Notwithstanding the above discussion, there exists a degree of uncertainty in the Company’s ability to continue as a going concern.
Having considered all the above, the Directors have prepared the financial statements on a going concern basis. As such, the financial statements do not include any adjustments as to the recoverability and classification of recorded asset amounts or to the amounts and classification of liabilities that might be necessary should the entity not continue as a going concern.
Restatements:
The Company has restated certain items in the previously issued financial statements as at and for the six months ended December 31, 2015, as follows:
Consolidated statement of profit or loss and other comprehensive income:
| | | Previously
Issued | | | Adjustments | | | Revised | |
| Other income* | | | 1,476,906 | | | | (713,343 | ) | | | 763,563 | |
| Sales and Marketing Costs | | | (242,150 | ) | | | 189,491 | | | | (52,659 | ) |
| Marketing and Promotion | | | (12,233 | ) | | | (189,491 | ) | | | (201,724 | ) |
Consolidated statement of changes in equity:
| | | Previously
Issued | | | Adjustments | | | Revised | |
| Balance at June 30, 2015 / Accumulated losses* | | | (37,542,572 | ) | | | 1,469,762 | | | | (36,072,810 | ) |
| Lapse or exercise of share options | | | (58,615 | ) | | | 58,615 | | | | - | |
| Shares issued, net of costs | | | 480,879 | | | | (58,615 | ) | | | 422,264 | |
Consolidated statement of cash flows:
| | | Previously
Issued | | | Adjustments | | | Revised | |
| Receipts from customers | | | 613,528 | | | | (60,122 | ) | | | 553,406 | |
| Payments to suppliers and employees | | | (4,074,918 | ) | | | 60,122 | | | | (4,014,796 | ) |
* These adjustments were the result of the timing of the recognition of the R&D refund.
Restatements were also made to the Consolidated Statement of Cash Flows for the six months ended December 31, 2016 as compared to the previous statement lodged with ASX, details as follows:
| | | Previously issued | | | Adjustments | | | Revised | |
| Receipts from customers | | | 664,900 | | | | 108,576 | | | | 773,476 | |
| Payments to suppliers and employees | | | (4,423,797 | ) | | | (148,497 | ) | | | (4,572,294 | ) |
| Net Cash Flows Used In Operating Activities | | | (2,216,618 | ) | | | (39,921 | ) | | | (2,256,539 | ) |
| | | | | | | | | | | | | |
| Capital raising costs | | | (120,285 | ) | | | (29,634 | ) | | | (149,919 | ) |
| Repayment of borrowings | | | (1,271,555 | ) | | | 69,555 | | | | (1,202,000 | ) |
| Net Cash Flows Provided By Financing Activities | | | 3,031,394 | | | | 39,921 | | | | 3,071,315 | |
As described in Note 8, the Company has restated its previously issued financial statements for the six months ended December 31, 2016. In addition to these restatements, the Company has made revisions to Notes 3, 6, 7 and 9.
Note 2. Dividends
The Group has not declared any dividends in the period ended December 31, 2016. (2015: AUD$Nil)
Note 3. Segment Information
The entity has identified its operating segments based on the internal reports that are reviewed and used by the executive management team in assessing performance and determining the allocation of resources.
The executive management team considers the business from both a product and a geographic perspective and has identified three reportable segments.
Segments
Research and Development (R&D) – Income and expenses directly attributable to the company’s research and development projects performed in Australia and Israel.
HyperImmune Products – Income and expenses directly attributable to primarily Travelan activities which occur in Australia, New Zealand, Canada and United States.
Corporate – Other items of income and expenses not directly attributable to R&D or HyperImmune Products segment are disclosed as corporate costs. Corporate activities primarily occur within Australia. This segment includes interest expenses from financing activities and depreciation.
| | | Research & | | | HyperImmune | | | | | | | |
| | | Development | | | Products | | | Corporate | | | Total | |
| December 31, 2016 | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Segment Revenue & Other income | | | | | | | | | | | | | | | | |
| Revenue from external customers | | | - | | | | 703,099 | | | | - | | | | 703,099 | |
| R&D tax concession refund | | | 779,826 | | | | - | | | | - | | | | 779,826 | |
| Interest income | | | - | | | | - | | | | 6,791 | | | | 6,791 | |
| Other income | | | 25,007 | | | | 5,308 | | | | - | | | | 30,315 | |
| Total Segment Revenues & Other income | | | 804,833 | | | | 708,407 | | | | 6,791 | | | | 1,520,031 | |
| | | | | | | | | | | | | | | | | |
| Segment Expenses | | | | | | | | | | | | | | | | |
| Depreciation & amortization expenses | | | - | | | | - | | | | (1,975 | ) | | | (1,975 | ) |
| Finance costs | | | - | | | | - | | | | (13,183 | ) | | | (13,183 | ) |
| Share-based payments | | | (80,308 | ) | | | - | | | | (233,467 | ) | | | (313,775 | ) |
| Other operating expenses | | | (2,197,867 | ) | | | (514,674 | ) | | | (1,887,906 | ) | | | (4,600,447 | ) |
| Total Segment Expenses | | | (2,278,175 | ) | | | (514,674 | ) | | | (2,136,531 | ) | | | (4,929,380 | ) |
| Income Tax Expenses | | | - | | | | - | | | | - | | | | - | |
| (Loss)/Profit for the Period | | | (1,473,342 | ) | | | 193,733 | | | | (2,129,740 | ) | | | (3,409,349 | ) |
| | | | | | | | | | | | | | | | | |
| Assets | | | | | | | | | | | | | | | | |
| Segment assets | | | 702,623 | | | | 1,949,595 | | | | 3,203,583 | | | | 5,855,801 | |
| Total Assets | | | 702,623 | | | | 1,949,595 | | | | 3,203,583 | | | | 5,855,801 | |
| | | | | | | | | | | | | | | | | |
| Liabilities | | | | | | | | | | | | | | | | |
| Segment liabilities | | | (708,474 | ) | | | (131,630 | ) | | | (1,462,517 | ) | | | (2,302,621 | ) |
| Total Liabilities | | | (708,474 | ) | | | (131,630 | ) | | | (1,462,517 | ) | | | (2,302,621 | ) |
| | | Research & | | | HyperImmune | | | | | | | |
| | | Development | | | Products | | | Corporate | | | Total | |
| December 31, 2015 | | AUD$ | | | AUD$ | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | | |
| Segment Revenue & Other income | | | | | | | | | | | | | | | | |
| Revenue from external customers | | | - | | | | 497,220 | | | | - | | | | 497,220 | |
| R&D tax concession refund | | | 756,420 | | | | - | | | | - | | | | 756,420 | |
| Interest income | | | - | | | | - | | | | 7,143 | | | | 7,143 | |
| Total Segment Revenues & Other income | | | 756,420 | | | | 497,220 | | | | 7,143 | | | | 1,260,783 | |
| | | | | | | | | | | | | | | | | |
| Segment Expenses | | | | | | | | | | | | | | | | |
| Depreciation & amortization expenses | | | - | | | | - | | | | (1,944 | ) | | | (1,944 | ) |
| Finance costs | | | - | | | | - | | | | - | | | | - | |
| Share-based payments | | | - | | | | (87,500 | ) | | | (537,309 | ) | | | (624,809 | ) |
| Other operating expenses | | | (1,909,734 | ) | | | (180,098 | ) | | | (1,612,284 | ) | | | (3,702,116 | ) |
| Total Segment Expenses | | | (1,909,734 | ) | | | (267,598 | ) | | | (2,151,537 | ) | | | (4,328,869 | ) |
| Income Tax Expenses | | | - | | | | - | | | | - | | | | - | |
| (Loss)/Profit for the Period | | | (1,153,314 | ) | | | 229,622 | | | | (2,144,394 | ) | | | (3,068,086 | ) |
Information on major customers:
During the six months ended December 31, 2016 and 2015, the Group had the following major customers (and their respective contribution to the Group’s total revenue):
| | | 2016 | | | 2015 | |
| Customer A | | | 33 | % | | | 49 | % |
| Customer B | | | 19 | % | | | * | |
| Customer C | | | 16 | % | | | 24 | % |
| Customer D | | | 15 | % | | | 17 | % |
| Customer E | | | 14 | % | | | * | |
* Less than 10% of revenue for the respective year.
No other single customers contributed 10% or more to the Group’s revenue for all periods.
Note 4. Inventories
| | | December 31, 2016 | | | June 30, 2016 | |
| | | AUD$ | | | AUD$ | |
| | | | | | | |
| Inventory | | | | | | | | |
| Raw materials | | | 1,118,912 | | | | 1,259,445 | |
| Work in Progress | | | 48,285 | | | | 121,513 | |
| Finished goods | | | 289,791 | | | | 269,156 | |
| Prepaid inventory | | | 294,883 | | | | 405,953 | |
| Total Inventory | | | 1,751,871 | | | | 2,056,067 | |
As certain raw materials held by the Company at December 31, 2016 may approach their expiration date for clinical use in the future, management has alternative options to utilize these inventories as for R&D purpose, or sale as non-clinical products.
Note 5.Contingent Liabilities and Assets
There has been no change in contingent liabilities and assets since the last annual reporting date.
Note 6.Contributed Equity
| | | December 31, 2016 | | | (Restated) December 31, 2015 | |
| | | No. | | | AUD$ | | | No. | | | AUD$ | |
| | | | | | | | | | | | | |
| Fully Paid Ordinary Shares (No par value) | | | | | | | | | | | | | | | | |
| Balance at beginning of the year | | | 80,099,646 | | | | 45,633,354 | | | | 74,964,232 | | | | 40,335,347 | |
| Shares issued during the period | | | 25,541,771 | | | | 1,841,951 | | | | 1,457,041 | | | | 429,723 | |
| Transfer from reserve from exercise of options | | | - | | | | 71,875 | | | | - | | | | 58,615 | |
| Transactions costs | | | - | | | | (61,480 | ) | | | - | | | | (7,459 | ) |
| Total Contributed Equity | | | 105,641,417 | | | | 47,485,700 | | | | 76,421,273 | | | | 40,816,226 | |
During the six months ended December, 31 2016 the Company issued the following Ordinary Shares:
Date | | Details | | No. | | | Issue
Price AUD$ | | | Total
Value AUD$ | |
| 7 July 2016 | | Right issue* | | | 18,045,512 | | | | - | | | | - | |
| 7 July 2016 | | Right issue | | | 3,275,466 | | | | 0.250 | | | | 818,867 | |
| 29 September 2016 | | Right issue to oversubscribes and private placement | | | 3,968,916 | | | | 0.250 | | | | 992,229 | |
| 9 December 2016 | | Shares under ESOP – for 6 months service (vesting monthly) | | | 251,877 | | | | 0.123 | | | | 30,855 | |
| | | | | | 25,541,771 | | | | | | | | 1,841,951 | |
*As at June 30, 2016, the Company was committed to issue 18,045,512 of ordinary shares in relation to the $4,511,378 received in capital raising. These shares were subsequently issued to respective holders on July 7, 2016. 2,418,129 of these new fully paid ordinary shares were issued to Grandlodge on the same terms and conditions as all other subscribers.
Note 7.Reserves
| | | December 31, 2016 | | | December 31, 2015 | |
| | | No. | | | AUD$ | | | No. | | | AUD$ | |
| | | | | | | | | | | | | |
| Options over Fully Paid Ordinary Shares | | | | | | | | | | | | | | | | |
| Opening balance | | | 9,937,629 | | | | 2,132,301 | | | | 7,188,676 | | | | 560,646 | |
| Options issued during the period | | | 25,489,894 | | | | 28,620 | | | | 6,000,000 | | | | 537,309 | |
| Options exercised during the period | | | - | | | | - | | | | (910,166 | ) | | | (58,615 | ) |
| Expense of vested options | | | - | | | | 254,300 | | | | - | | | | - | |
| Lapse of unexercised options | | | (1,250,000 | ) | | | (127,000 | ) | | | - | | | | - | |
| Closing Balance | | | 34,177,523 | | | | 2,288,221 | | | | 12,278,510 | | | | 1,039,340 | |
| | | December 31, 2016 | | | December 31, 2015 | |
| | | No. | | | AUD$ | | | No. | | | AUD$ | |
| | | | | | | | | | | | | |
| Foreign currency translation reserve | | | | | | | | | | | | | | | | |
| Opening balance | | | | | | | (3,735 | ) | | | | | | | (12,581 | ) |
| Movement during the period | | | | | | | (41,425 | ) | | | | | | | (4,781 | ) |
| Closing balance | | | | | | | (45,160 | ) | | | | | | | (17,362 | ) |
| Total Reserves | | | 34,177,523 | | | | 2,243,061 | | | | 12,278,510 | | | | 1,021,978 | |
During the six months ended December, 31 2016 the Company issued the following Options:
Date | | Details | | No. | | | Issue Price AUD$ | | | Total Value AUD$ | |
| 7 July 2016 | | Right issue* | | | 18,045,512 | | | | - | | | | - | |
| 7 July 2016 | | Right issue | | | 3,275,466 | | | | - | | | | - | |
| 29 September 2016 | | Right issue to oversubscribes and private placement | | | 3,968,916 | | | | - | | | | - | |
| 9 December 2016 | | Unlisted options in lieu of services | | | 200,000 | | | | 0.143 | | | | 28,620 | |
| | | | | | 25,489,894 | | | | | | | | 28,620 | |
*As at June 30, 2016, the Company was committed to issue 18,045,512 of options in relation to the $4,511,378 received in capital raising. These options were subsequently issued to respective holders on July 7, 2016. 2,418,129 of these options were issued to Grandlodge on the same terms and conditions as all other subscribers.
The fair value of the options granted during the period is estimated as at the grant date using Black-Scholes model taking into account the terms and conditions upon which the options were granted.
December 2016 Options
The following table lists the inputs to the model used to determine the weighted average value of the options expensed during the year:
| Vesting date | | | N/A | |
| Dividend yield | | | - | |
| Expected volatility | | | 100 | % |
| Risk-free interest rate | | | 1.61 | % |
| Expected life of option (years) | | | 3.17 years | |
| Option exercise price | | AUD$ | 0.5000 | |
| Weighted average share price at grant date | | AUD$ | 0.275 | |
| Value per option | | AUD$ | 0.1431 | |
At December 31, 2016 the Australian Securities Exchange (ASX) market share price for Immuron was AUD$0.27.
The expected life of the option is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
Note 8.Loss per share (Restated)
| | | | | December 31, 2016 | | | December 31, 2015 |
| | | | | AUD$ | | | AUD$ |
| | | | | | | | |
| Basic/Diluted loss per share (cents) | | 3.300 | | | 4.065 |
| | | | | | | | | | |
| a) | | Net loss used in the calculation of basic and diluted loss per share | | | 3,409,349 | | | | 3,068,086 |
| | | | | | | | | | |
| b) | | Weighted average number of ordinary shares outstanding during the period used in the calculation of basic and diluted loss per share | | | 103,328,491 | | | | 75,470,006 |
In the previously issued financial statements, (i) the basic and diluted loss per share was 3.318 cents and 3.120 cents, for the six months ended December 31 2016 and 2015, respectively; (ii) the weighted average number of ordinary shares outstanding was 102,756,793 for the six months ended December 31 2016.
Note 9.Related party transactions
Short-Term Loan
Grandlodge Capital Pty Ltd (Grandlodge) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou, and owns a top 20 shareholding in Immuron Limited. On 6th June 2016, Immuron executed a short-term funding agreement with Grandlodge for a principal amount of AUD$750,000, plus interest charges.
The short-term funding is a cash advance against the anticipated refund Immuron will receive from the Australian Taxation Office under the Research and Development Income Tax Concession Incentive for the Company's eligible R&D expenditure incurred for financial year of 2016.
Loan has been repaid to Grandlodge on 2nd December 2016. Interest expense for the six months ended December 31, 2016 was AUD $47,158.
Service rendered by Grandlodge Pty Ltd to Immuron Ltd
Grandlodge, and its associated entities, are marketing, warehousing and distribution logistics. Mr David Plush is also an owner of Grandlodge, and its associated entities.
Commencing on 1st June 2013, Grandlodge was verbally contracted to provide warehousing, distribution and invoicing services for Immuron’s products for AUD$70,000 per annum. These fees will be payable in new fully paid ordinary shares in Immuron Limited at a set price of AUD$0.16 per share representing Immuron Limited’s share price at the commencement of the verbal agreement.
The shares to be issued to Grandlodge, or its associated entities, as compensation in lieu of cash payment for the services rendered under this verbal agreement have been subject to the approval of Immuron shareholders at Company shareholder meetings held over the past 18 months.
Grandlodge will also be reimbursed in cash for all reasonable costs and expenses incurred in accordance with their scope of works under the verbal agreement, unless both parties agree to an alternative method of payment.
The verbal agreement is cancellable by either party upon providing the other party with 30 days written notice of the termination of the agreement.
During the six months period ended December 31, 2016, an amount of AUD$70,000 was charged to the Group’s expenses in relation to service rendered by Grandlodge. This amount remained payable as at the period end.
Premises Rental services received from Wattle Laboratories Pty Ltd to Immuron Ltd:
Wattle Laboratories Pty Ltd (Wattle) is an entity part-owned and operated by Immuron Directors Peter and Stephen Anastasiou. Commencing on 1st January 2016, Immuron executed a Lease Agreement with Wattle whereby Immuron will lease part of their Blackburn office facilities for Immuron's operations at a rental rate of AUD$38,940 per annum, payable in monthly installments.
The lease is for a 3 year term with an additional 3 year option period.
The lease is cancellable by either party upon 6 months written notice of termination of the agreement.
During the six months period ended December 31, 2016, an amount of AUD$19,470 (excluding GST) was charged to the Group’s expenses in relation to service rendered by Wattle. This amount was paid during the period.
Other related party transactions:
On August 25th on behalf of Immuron, Grandlodge purchased US$1,500,000 at the cost of AUD$1,968,762. On the same day Immuron paid Grandlodge AUD$1,968,762 to settle this transaction. On Sept 12th Grandlodge returned the USD$1,500,000 purchase to Immuron. Grandlodge received no financial gains or benefits from this transaction.
Note 10.Fair value measurement
The fair value of convertible note classified as level 3 is determined by the use of valuation model, which includes discounted cash flow analysis and the use of observable inputs that required significant adjustments based on unobservable inputs.
As at December 31, 2016, management has assessed the terms of the convertible notes and determined that in their view the fair value of the debt component is equal to the proceeds such that there is no residual amount to be allocated to an equity component. In making this determination, management is of the view that the value of the consideration received, net of costs, provided reliable evidence of the fair value of the debt component of the convertible note. Fair value has been determined by the income approach based on a discounted cash flow analysis, with the most significant inputs being the discount rate that reflects the investors credit risk. A slight increase or decrease in the discount rate used would not be material to the financial statements. The fair value of the convertible note approximated its carrying value as at December 31, 2016.
Reconciliation of level 3 fair value measurements:
| | | Convertible notes/debentures | |
| | | AUD$ | |
| Balance at 30 June 2016 | | 1,128,117 | |
| - Finance Costs (*) | | | 1,883 | |
| - Repayments | | | (452,000 | ) |
| Balance at 31 December 2016 | | | 678,000 | |
(*) These amounts are recorded in the Finance Costs on the Statement of Profit or Loss and Other Comprehensive Income.
Note 11.Events Occurring after the Reporting Date
There have not been any matters or circumstances in the financial statements or notes thereto, that have arisen since the end of the financial half year, which significantly affected, or may significantly affect, the operations of Immuron Limited, the results of those operations or the state of affairs of Immuron Limited in future financial years.
American Depositary Shares
Representing Ordinary Shares
Warrants to Purchase up to American Depository Shares

PROSPECTUS
| Joseph Gunnar & Co. | Rodman & Renshaw, |
| | a unit of H.C. Wainwright & Co. |
WallachBeth Capital, LLC
, 2017
Until , 2017 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers
Australian law. Australian law provides that a company or a related body corporate of the company may provide for indemnification of officers and directors, except to the extent of any of the following liabilities incurred as an officer or director of the company:
| • | a liability owed to the company or a related body corporate of the company; |
| • | a liability for a pecuniary penalty order made under section 1317G or a compensation order under section 961M, 1317H, 1317HA or 1317HB of the Australian Corporations Act 2001; |
| • | a liability that is owed to someone other than the company or a related body corporate of the company and did not arise out of conduct in good faith; or |
| • | legal costs incurred in defending an action for a liability incurred as an officer or director of the company if the costs are incurred: |
| • | in defending or resisting proceedings in which the officer or director is found to have a liability for which they cannot be indemnified as set out above; |
| • | in defending or resisting criminal proceedings in which the officer or director is found guilty; |
| • | in defending or resisting proceedings brought by the Australian Securities & Investments Commission or a liquidator for a court order if the grounds for making the order are found by the court to have been established (except costs incurred in responding to actions taken by the Australian Securities & Investments Commission or a liquidator as part of an investigation before commencing proceedings for a court order); or |
| • | in connection with proceedings for relief to the officer or a director under the Corporations Act, in which the court denies the relief. |
Constitution. Our Constitution provides, except to the extent prohibited by the law and the Corporations Act, for the indemnification of every person who is or has been an officer or a director of the company against liability (other than legal costs that are unreasonable) incurred by that person as an officer or director. This includes any liability incurred by that person in their capacity as an officer or director of a subsidiary of the company where the company requested that person to accept that appointment.
Indemnification Agreements. Pursuant to Deeds of Access, Insurance and Indemnity, the form of which is filed as Exhibit 10.9 to this registration statement, we have agreed to indemnify our directors against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being such a director.
SEC Position. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Pursuant to the underwriting agreement for this offering, the form of which is filed as Exhibit 1.1 to this registration statement, the underwriters will agree to indemnify our directors and officers and persons controlling us, within the meaning of the Securities Act, against certain liabilities that might arise out of or are based upon certain information furnished to us by any such underwriter.
Item 7. Recent Sales of Unregistered Securities
During the prior three and a half years, we issued and sold to third parties the securities listed below without registering the securities under the Securities Act. None of these transactions involved any public offering. All our securities were sold through private placement either (i) outside the United States or (ii) in the United States to a limited number of investors in transactions not involving any public offering. As discussed below, we believe that each issuance of these securities was exempt from, or not subject to, registration under the Securities Act.
During the six months ended December, 31 2016 the Company issued the following securities:
Date | | Details | | No. | | | Issue Price AUD$ | | | Total Value AUD$ | |
| July 7, 2016 | | Right issue* | | | 18,045,512 | | | | - | | | | - | |
| July 7, 2016 | | Right issue | | | 3,275,466 | | | | 0.250 | | | | 818,867 | |
| September 29, 2016 | | Right issue to oversubscribes and private placement | | | 3,968,916 | | | | 0.250 | | | | 992,229 | |
| December 9, 2016 | | Shares under ESOP – for 6 months service (vesting monthly) | | | 251,877 | | | | 0.123 | | | | 30,855 | |
| | | | | | 25,541,771 | | | | | | | | 1,841,951 | |
*As at June 30, 2016, the Company was committed to issue 18,045,512 of ordinary shares in relation to the $4,511,378 received in capital raising. These shares were subsequently issued to respective holders on July 7, 2016. 2,418,129 of these new fully paid ordinary shares were issued to Grandlodge on the same terms and conditions as all other subscribers.
During the Full Year ended June 30 2016, the Company issued the following securities:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | AUD$ | | | AUD$ | |
| 18 Sep 2015 | | Exercise of IMCAI Unlisted Options | | | 218,750 | | | | 0.376 | | | | 82,250 | |
| 30 Sep 2015 | | Exercise of IMCAI Unlisted Options | | | 93,750 | | | | 0.376 | | | | 35,250 | |
| 19 Oct 2015 | | Exercise of IMCAI Unlisted Options by Grandlodge | | | 556,000 | | | | 0.376 | | | | 209,056 | |
| 13 Nov 2015 | | Exercise of IMCAI Unlisted Options | | | 41,666 | | | | 0.376 | | | | 15,667 | |
| 27 Nov 2015 | | Issue of Shares in lieu of cash payment for services as per Resolution 4 of the Annual General Meeting (AGM) held on 25 Nov 2015 | | | 546,875 | | | | 0.160 | | | | 87,500 | |
| 24 Feb 2016 | | Issue in accordance with executed funding agreement with a New York based Investment fund provider announced to the ASX on 17 Feb 2016 | | | 294,118 | | | | 0.340 | | | | 100,000 | |
| 24 Feb 2016 | | Issue of fully paid escrow shares as security for any repayment default of the Convertible Loan in accordance with executed funding agreement with a New York based Investment fund provider and announced to the ASX on 17 Feb 2016 | | | 2,000,000 | | | | 0.400 | | | | 800,000 | |
| 13 Apr 2016 | | Issue in accordance with executed funding agreement with a New York based Investment fund provider announced to the ASX on 17 Feb 2016 | | | 326,797 | | | | 0.306 | | | | 100,000 | |
| 18 Apr 2016 | | First repayment of Convertible Note Security in accordance with executed funding agreement with a New York based investment fund provider announced to the ASX on 17 Feb 2016 | | | 241,764 | | | | 0.312 | | | | 75,333 | |
| 16 May 2016 | | Exercise of IMCAI Unlisted Options | | | 150,000 | | | | 0.276 | | | | 41,400 | |
| 16 May 2016 | | Second repayment of Convertible Note Security in accordance with executed funding agreement with a New York based investment fund provider announced to the ASX on 17 Feb 2016 | | | 265,694 | | | | 0.284 | | | | 75,333 | |
| 31 May 2016 | | Issue of Shares in lieu of cash payment for services received | | | 400,000 | | | | 0.250 | | | | 100,000 | |
| 30 Jun 2016 | | Shares to be Issued from Capital Raising as at 30 June 2016 | | | - | | | | - | | | | 4,511,378 | |
Total 2016 Movement | | | 5,135,414 | | | | | | | | 6,233,167 | |
During the Full Year ended June 30, 2015, the Company issued the following securities:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | AUD$ | | | AUD$ | |
| | | | | | | | | | | | |
| 20 Nov 2014 | | Capital Consolidation on a 40:1 basis approved by shareholders at the Company's Annual General Meeting held on 13 Nov 2014 | | | (2,920,770,804 | ) | | | - | | | | - | |
| 21 Nov 2014 | | Issue of shares to supplier in lieu of cash payment for services rendered approved by shareholders at the Company's Annual General Meeting held on 13 Nov 2014 | | | 72,916 | | | | 0.160 | | | | 11,667 | |
| Total 2015 Movement | | | (2,920,697,888 | ) | | | | | | | 11,667 | |
During the Full Year ended June 30, 2014, the Company issued the following securities:
| | | | | | | | Issue Price | | | Total Value | |
| Date | | Details | | No. | | | $ | | | $ | |
| | | | | | | | | | | | |
| 6 Dec 2013 | | Issue of shares as per resolution 4 approved by shareholders at the Annual General Meeting of the Company held on 29 Nov 2013 | | | 8,750,000 | | | | 0.004 | | | | 35,000 | |
| 6 Dec 2013 | | Issue of shares as per resolutions 5, 6, & 8 approved by shareholders at the Annual General Meeting of the Company held on 29 Nov 2013 | | | 9,479,167 | | | | 0.006 | | | | 56,875 | |
| 3 Feb 2014 | | Exercise of IMCOA options | | | 29,075 | | | | 0.040 | | | | 1,163 | |
| 3 Mar 2014 | | Issue of shares through fully underwritten rights issue | | | 1,670,642,320 | | | | 0.005 | | | | 8,353,212 | |
| 3 Mar 2014 | | Issue of shares to Grandlodge & related owners as part of fully underwritten rights issue | | | 261,103,082 | | | | 0.005 | | | | 1,305,516 | |
| 29 May 2014 | | Issue of shares as per resolution 2 approved by shareholders at the General Meeting of the Company held on 27 May 2014 | | | 10,208,333 | | | | 0.004 | | | | 40,833 | |
| Total 2014 Movement | | | 1,960,211,977 | | | | | | | | 9,792,599 | |
Item 8. Exhibits and Financial Statement Schedules
| | See Exhibit Index beginning on page II-7 of this registration statement. |
| (b) | Financial Statement Schedules |
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the Consolidated Financial Statements or the Notes thereto.
Item 9. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by a registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initialbona fide offering thereof. |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Sydney, Australia on April 27, 2017.
| | IMMURON LIMITED |
| | | |
| | By: | /s/ Thomas Liquard |
| | | Name: Thomas Liquard |
| | | Title: Chief Executive Officer |
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | | Title | | Date |
| | | | | |
| * | | Non-Executive Chairman | | April 27, 2017 |
| Name: Roger Aston | | | | |
| | | | | |
| /s/ Thomas Liquard | | Chief Executive Officer and Managing Director | | April 27, 2017 |
| Name: Thomas Liquard | | (principal executive officer) | | |
| | | | | |
| * | | Joint Chief Financial Officer and Secretary | | April 27, 2017 |
| Name: Peter Vaughan | | (principal financial officer and principal
accounting officer) | | |
| | | | | |
| * | | Joint Chief Financial Officer and Secretary | | April 27, 2017 |
| Name: Phillip Hains | | | | |
| | | | | |
| * | | Director | | April 27, 2017 |
| Name: Stephen Anastasiou | | | | |
| | | | | |
| * | | Director | | April 27, 2017 |
| Name: Daniel Pollock | | | | |
| | | | | |
| * | | Executive Vice Chairman | | April 27, 2017 |
| Name: Peter Anastasiou | | | | |
| | | | | |
| | Director | | April 27, 2017 |
| Name: Ravi Savarirayan | | | | |
| * By: | /s/ Thomas Liquard | |
| | Thomas Liquard | |
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of Immuron Limited, has signed this registration statement or amendment thereto in New York, New York on April 27, 2017.
| | Authorized U.S. Representative |
| | | |
| | By: | /s/ Thomas Liquard |
| | | Thomas Liquard |
EXHIBIT INDEX
| Exhibits | | Description |
| | | |
| 1.1 | | Form of Underwriting Agreement* |
| | | |
| 3.1 | | Constitution of Immuron Limited** |
| | | |
| 4.1 | | Form of Deposit Agreement between Immuron Limited and The Bank of New York Mellon, as depositary, and Owners and Holders of the American Depositary Shares* |
| | | |
| 4.2 | | Form of American Depositary Receipt evidencing American Depositary Shares (included in Exhibit 4.1)* |
| | | |
| 4.3 | | Form of Representative’s Warrant (included in Exhibit 1.1)* |
| | | |
| 4.4 | | Form of ADS Warrant Agent Agreement* |
| | | |
| 5.1 | | Opinion of Francis Abourizk Lightowlers regarding the validity of the ordinary shares being issued* |
| | | |
| 8.1 | | Opinion regarding material U.S. tax matters* |
| | | |
| 10.1 | | Development and Supply Agreement by and between Immuron Limited and Synlait Milk Ltd. dated June 28, 2013**(1) |
| | | |
| 10.2 | | Variation of Development and Supply Agreement by and between the Company and Synlait Milk Ltd. dated June 21, 2016**(1) |
| | | |
| 10.3 | | Marketing and Master Distribution Agreement by and between the Company and UniFirst-First Aid Corporation d/b/a MEDIQUE Products dated as of June 28, 2016**(1) |
| | | |
| 10.4 | | Distribution and License Agreement by and between the Company and Paladin Labs Inc. dated November 28, 2011**(1) |
| | | |
10.5 | | Consultancy Agreement by and between Immuron Limited and Dan Peres dated April 1, 2015** |
| | | |
| 10.6 | | Commercial Lease Agreement with Wattle Laboratories Pty Ltd.** |
| | | |
| 10.7 | | Executive Share Option Plan** |
| | | |
| 10.8 | | Convertible Security and Share Purchase Agreement by and between Immuron Limited and SBI Investments dated February 16, 2016** |
| | | |
| 10.9 | | Executive Service Agreement by and between Immuron Limited and Thomas Liquard dated August 24, 2015** |
| | | |
| 10.10 | | Executive Service Agreement by and between Immuron Limited and Dr. Jerry Kanellos dated July 23, 2015** |
| | | |
| 10.11 | | Termination of Distribution Agreement letter dated February 23, 2017** |
| | | |
| 21.1 | | List of significant subsidiaries of Immuron Limited** |
| | | |
| 23.1 | | Consent of Francis Abourizk Lightowlers (see Exhibit 5.1)* |
| | | |
| 23.2 | | Consent of Marcum LLP |
| | | |
| 24.1 | | Power of Attorney** |
| * | To be filed by amendment |
(1) A redacted version of this Exhibit is filed herewith. An un-redacted version of this Exhibit has been separately filed with the Securities and Exchange Commission pursuant to an application for confidential treatment. The confidential portions of the Exhibit have been omitted and are marked by an asterisk.









