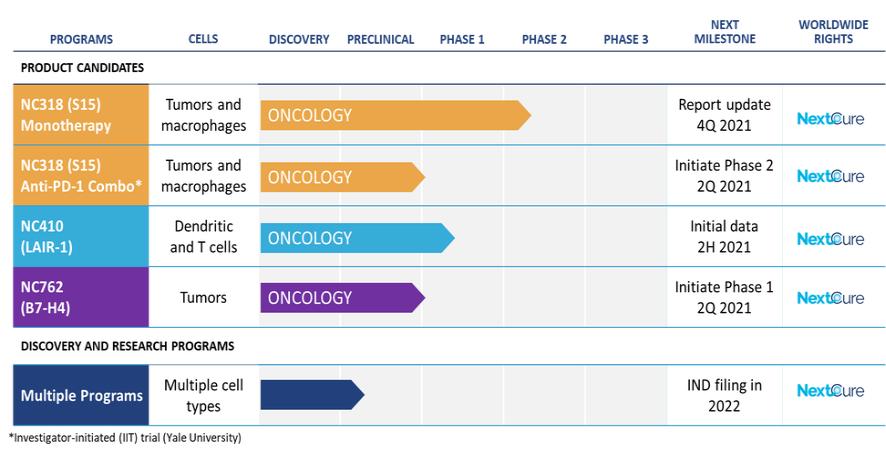
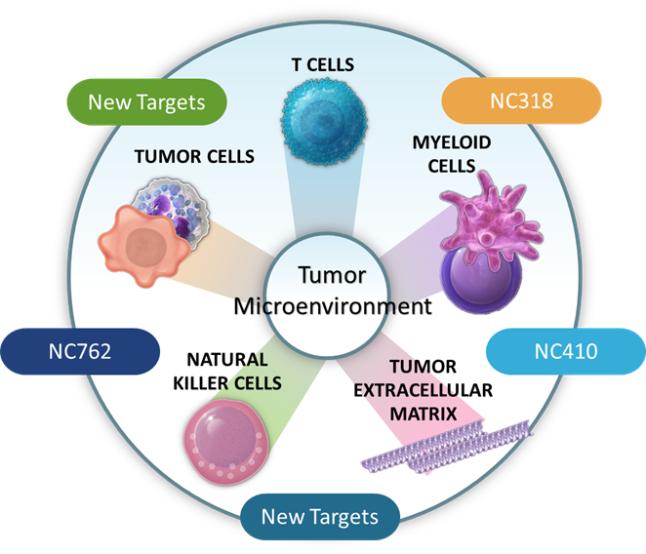
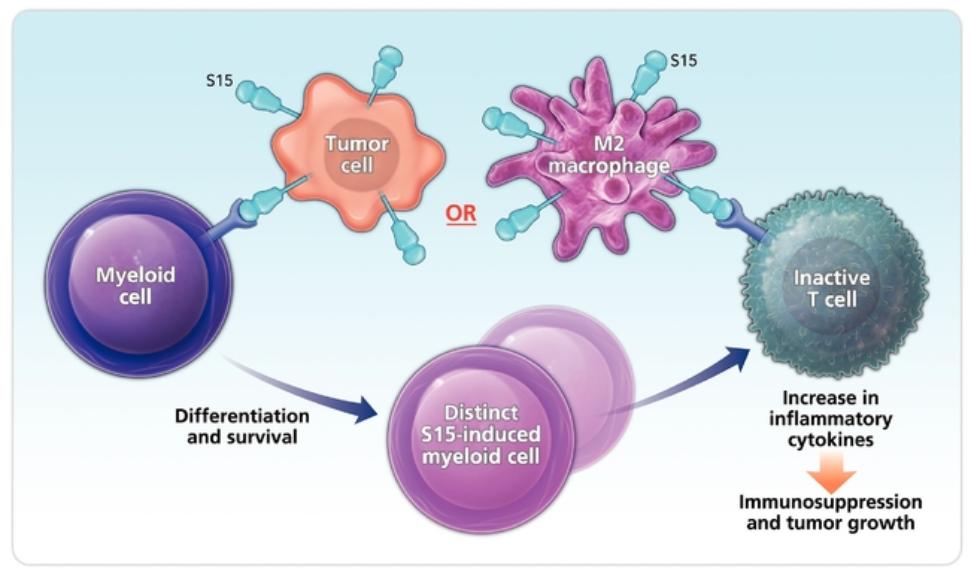
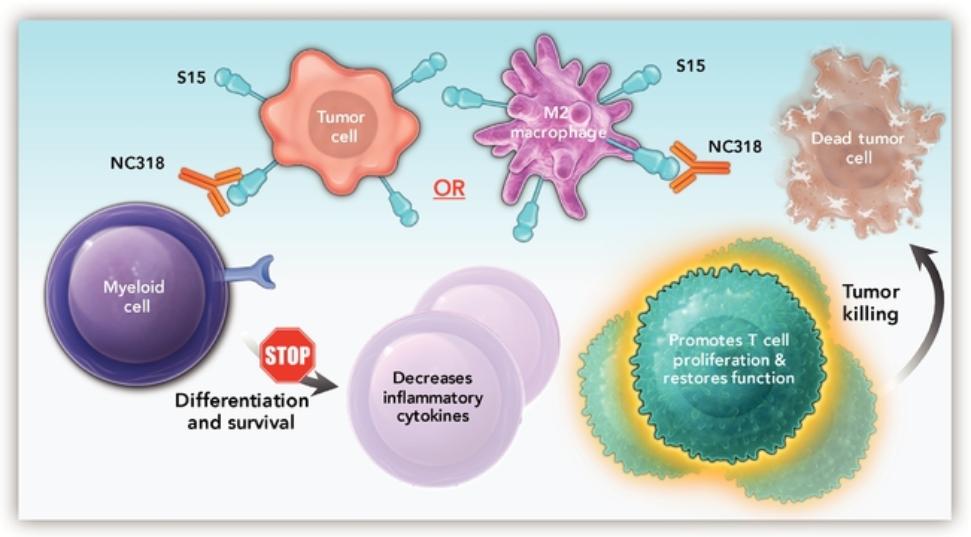
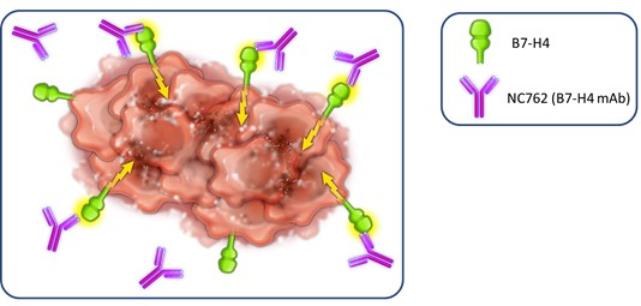
in 2037 absent any patent term adjustments or extensions and for NC762, if issued, are expected to expire beginning in 2039 absent any patent term adjustments or extensions.
In addition, as described above, under the Yale Agreement, we have an exclusive, royalty-bearing, sublicensable worldwide license from Yale for an intellectual property portfolio, including among other things patent applications relating to methods of use for S15 that covers the use of NC318 and one allowed patent relating to FIND-IO. Any patents from these patent applications, if issued, are expected to expire no earlier than 2036 absent any patent term adjustments or extensions.
For all patent applications, we determine strategy for claim scope on a case-by-case basis, taking into account advice of counsel and our business model and needs. We file patents containing claims for protection of all useful applications of our proprietary technologies and any products, as well as all new applications and/or uses we discover for existing technologies and products, based on our assessment of their strategic value. We continuously reassess the number and type of patent applications, as well as the pending and issued patent claims to ensure that maximum coverage and value are obtained for our processes and compositions, given existing patent office rules and regulations. Further, claims may be modified during patent prosecution to meet our intellectual property and business needs.
We also rely upon trade secrets, know-how and continuing technological innovation to develop and maintain our competitive position, including with respect to our FIND-IO platform. We seek to protect our proprietary technology and processes, in part, by confidentiality and invention assignment agreements with our employees, consultants, scientific advisors and other contractors. In addition, in the ordinary course of our business, we enter into agreements with other third parties for non-exclusive rights to intellectual property directed to other technologies that are ancillary to our business, including laboratory information management software and research and development tools. In addition, we have filed for trademark registration with the U.S. Patent and Trademark Office, or the USPTO, for “NextCure,” our logo and our FIND-IO platform.
Government Regulation
Government Regulation and Product Approval
The FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, recordkeeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of biological products. Along with third-party contractors, we will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our product candidates. The processes for obtaining regulatory approvals in the United States and in foreign jurisdictions, along with subsequent compliance with applicable laws and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
Government policies may change and additional government regulations may be enacted that could prevent or delay further development or regulatory approval of any product candidates, product or manufacturing changes, additional disease indications or label changes. We cannot predict the likelihood, nature or extent of government regulation that might arise from future legislative or administrative action.
Review and Approval for Licensing Biologics in the United States
In the United States, the FDA regulates our current product candidates as biological products, or biologics, under the Federal Food, Drug, and Cosmetic Act, or FDCA, the Public Health Service Act and associated implementing regulations. Biologics, like other drugs, are used for the treatment, prevention or cure of disease in humans. In contrast to small molecular weight drugs, which have a well-defined structure and can be thoroughly characterized, biologics are generally derived from living material (human, animal, or microorganism) are complex in structure, and thus are usually not fully characterized. Biologics include immunomedicines for cancer and other diseases.
Biologics are also subject to other federal, state and local statutes and regulations. The failure to comply with applicable statutory and regulatory requirements at any time during the product development process, approval process or after approval may subject a sponsor or applicant to administrative or judicial enforcement actions. These actions could