
Corporate Presentation May 2021 Exhibit 99.2

Forward-looking Statements The following presentation contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the therapeutic potential of C4 Therapeutics, Inc.’s technology and products. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, as well as the fact that the product candidates that we are developing or may develop may not demonstrate success in clinical trials. Prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. C4 Therapeutics, Inc. undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. Intellectual Property C4 Therapeutics, Inc. owns various registered and unregistered trademarks in the U.S. and internationally, including, without limitation, C4 THERAPEUTICS, our housemark logo, the name of our TORPEDO platform, and the names of our BIDAC and MONODAC degrader products. All trademarks or trade names referred to in this presentation that we do not own are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but those references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. Forward-looking Statements and Intellectual Property 2
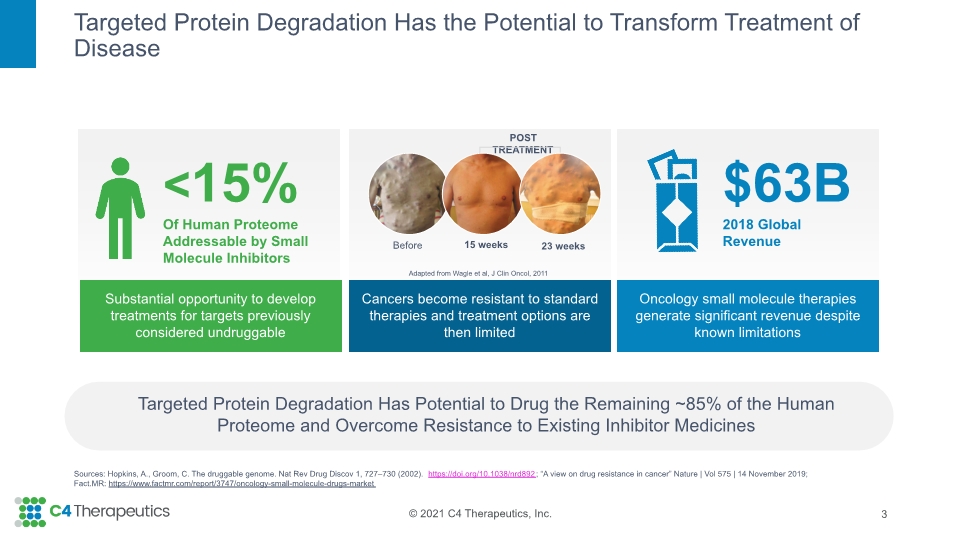
z Oncology small molecule therapies generate significant revenue despite known limitations z Targeted Protein Degradation Has the Potential to Transform Treatment of Disease 3 Targeted Protein Degradation Has Potential to Drug the Remaining ~85% of the Human Proteome and Overcome Resistance to Existing Inhibitor Medicines Substantial opportunity to develop treatments for targets previously considered undruggable <15% Of Human Proteome Addressable by Small Molecule Inhibitors $63B 2018 Global Revenue Sources: Hopkins, A., Groom, C. The druggable genome. Nat Rev Drug Discov 1, 727–730 (2002). https://doi.org/10.1038/nrd892; “A view on drug resistance in cancer” Nature | Vol 575 | 14 November 2019; Fact.MR: https://www.factmr.com/report/3747/oncology-small-molecule-drugs-market z Cancers become resistant to standard therapies and treatment options are then limited Adapted from Wagle et al, J Clin Oncol, 2011 Before 15 weeks 23 weeks POST TREATMENT
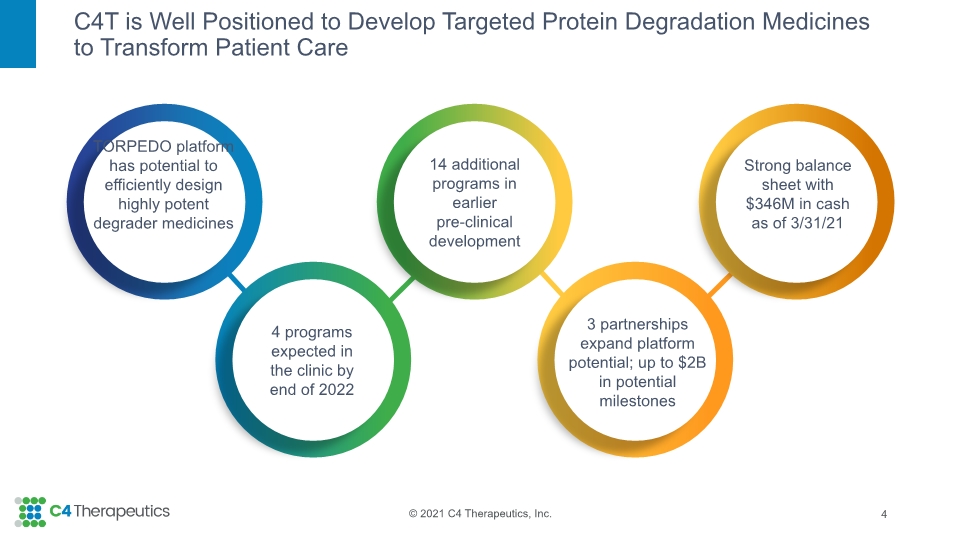
C4T is Well Positioned to Develop Targeted Protein Degradation Medicines to Transform Patient Care 4 Strong balance sheet with $346M in cash as of 3/31/21 TORPEDO platform has potential to efficiently design highly potent degrader medicines 4 programs expected in the clinic by end of 2022 14 additional programs in earlier pre-clinical development 3 partnerships expand platform potential; up to $2B in potential milestones

The Human Body Has A Natural Process to Destroy Unwanted Proteins 5 1 2 3 4
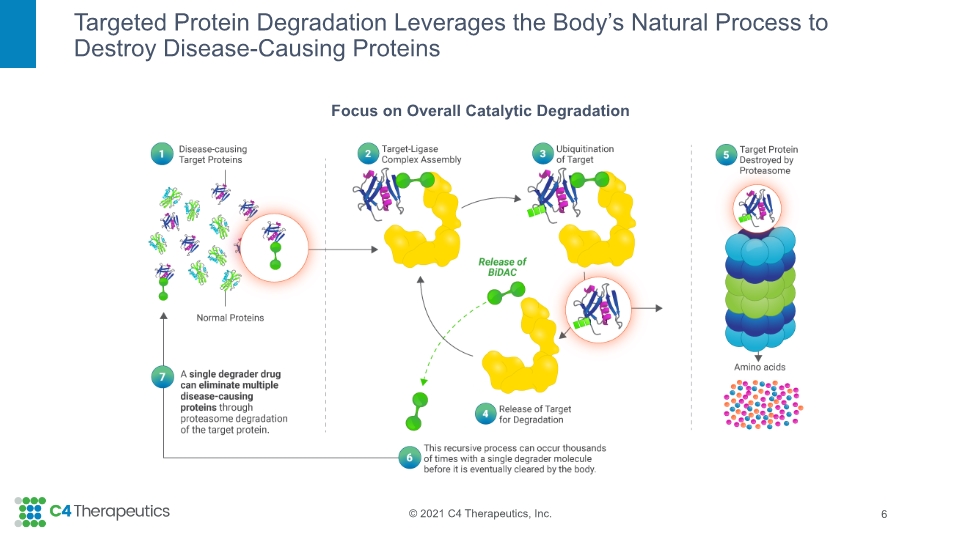
Focus on Overall Catalytic Degradation Targeted Protein Degradation Leverages the Body’s Natural Process to Destroy Disease-Causing Proteins 6
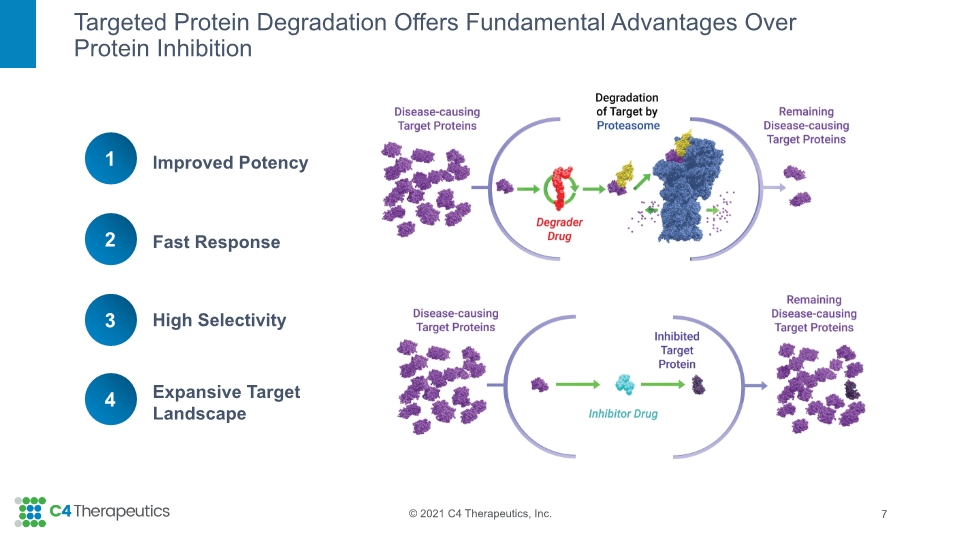
Targeted Protein Degradation Offers Fundamental Advantages Over Protein Inhibition 7 Improved Potency Fast Response High Selectivity Expansive Target Landscape 1 2 3 4

TORPEDO (Target ORiented ProtEin Degrader Optimizer) Platform
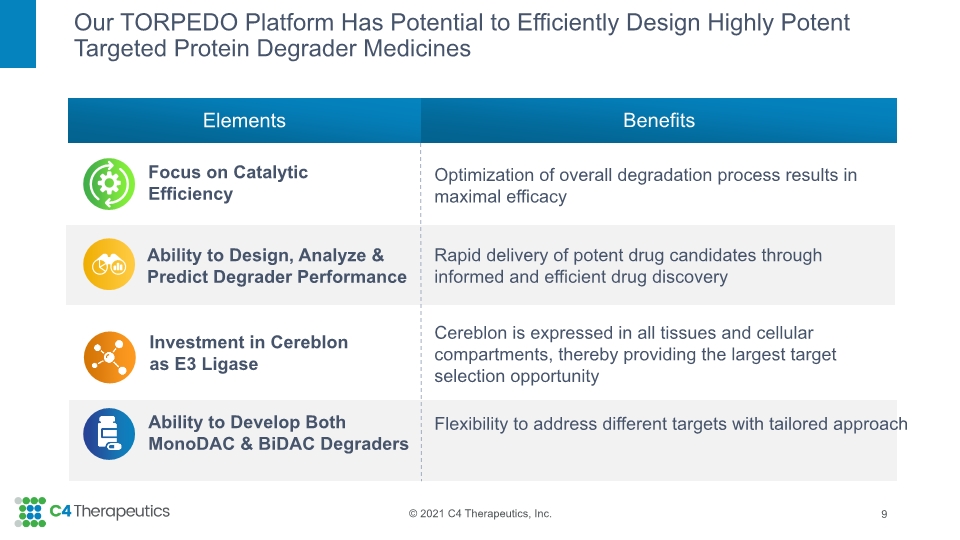
Our TORPEDO Platform Has Potential to Efficiently Design Highly Potent Targeted Protein Degrader Medicines Ability to Develop Both MonoDAC & BiDAC Degraders Focus on Catalytic Efficiency Ability to Design, Analyze & Predict Degrader Performance Investment in Cereblon as E3 Ligase Flexibility to address different targets with tailored approach Optimization of overall degradation process results in maximal efficacy Rapid delivery of potent drug candidates through informed and efficient drug discovery Cereblon is expressed in all tissues and cellular compartments, thereby providing the largest target selection opportunity Benefits Elements 9
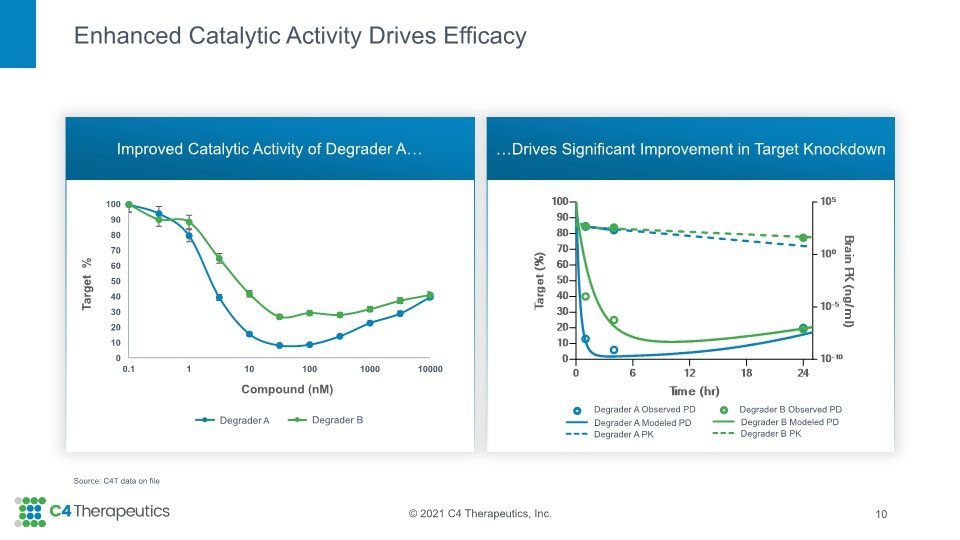
Enhanced Catalytic Activity Drives Efficacy Improved Catalytic Activity of Degrader A… …Drives Significant Improvement in Target Knockdown 10 Source: C4T data on file
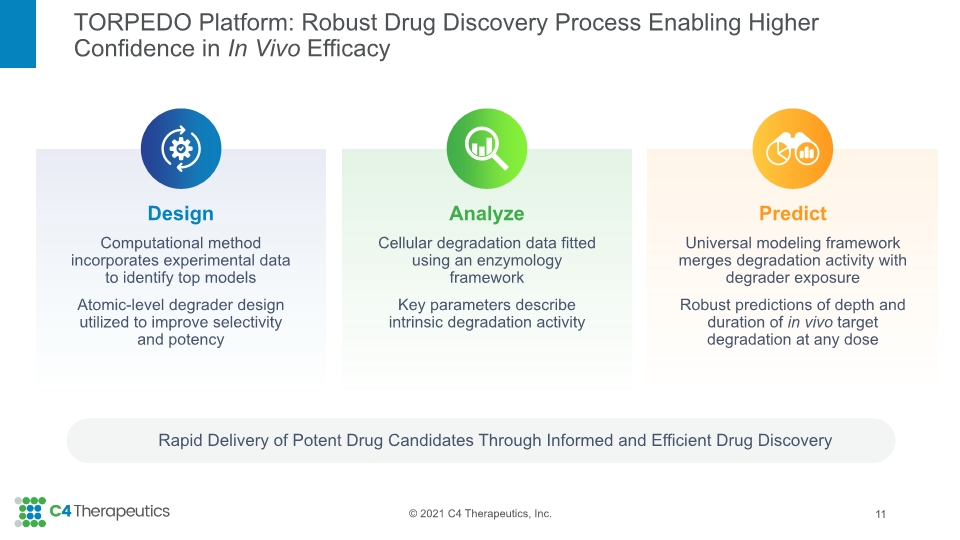
TORPEDO Platform: Robust Drug Discovery Process Enabling Higher Confidence in In Vivo Efficacy Rapid Delivery of Potent Drug Candidates Through Informed and Efficient Drug Discovery 11 Analyze Cellular degradation data fitted using an enzymology framework Key parameters describe intrinsic degradation activity Predict Universal modeling framework merges degradation activity with degrader exposure Robust predictions of depth and duration of in vivo target degradation at any dose Design Computational method incorporates experimental data to identify top models Atomic-level degrader design utilized to improve selectivity and potency
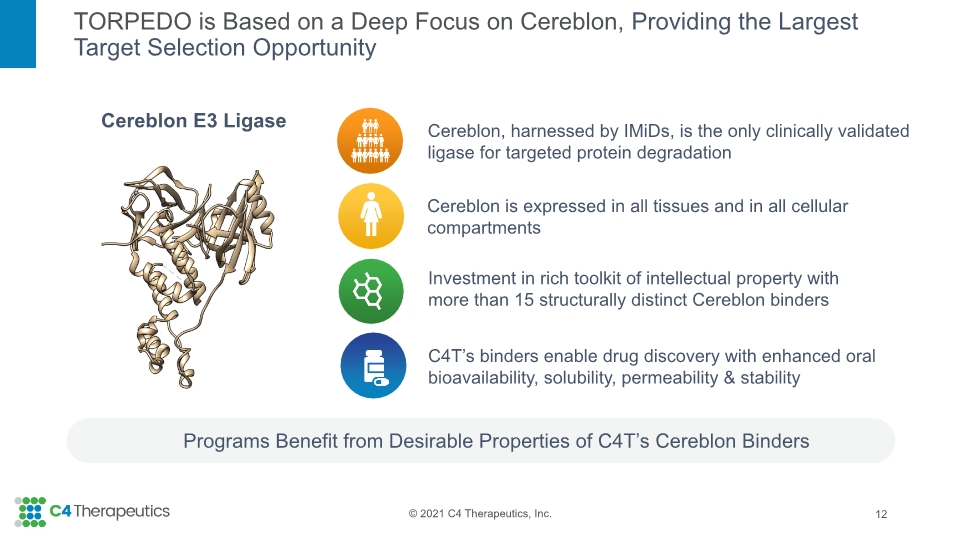
TORPEDO is Based on a Deep Focus on Cereblon, Providing the Largest Target Selection Opportunity Programs Benefit from Desirable Properties of C4T’s Cereblon Binders 12 Cereblon E3 Ligase Investment in rich toolkit of intellectual property with more than 15 structurally distinct Cereblon binders Cereblon is expressed in all tissues and in all cellular compartments Cereblon, harnessed by IMiDs, is the only clinically validated ligase for targeted protein degradation C4T’s binders enable drug discovery with enhanced oral bioavailability, solubility, permeability & stability
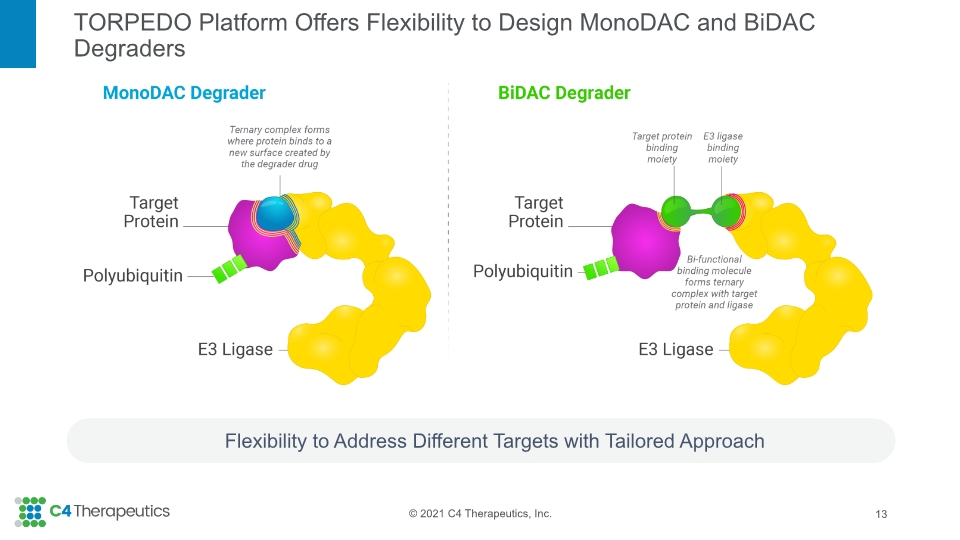
TORPEDO Platform Offers Flexibility to Design MonoDAC and BiDAC Degraders Flexibility to Address Different Targets with Tailored Approach 13
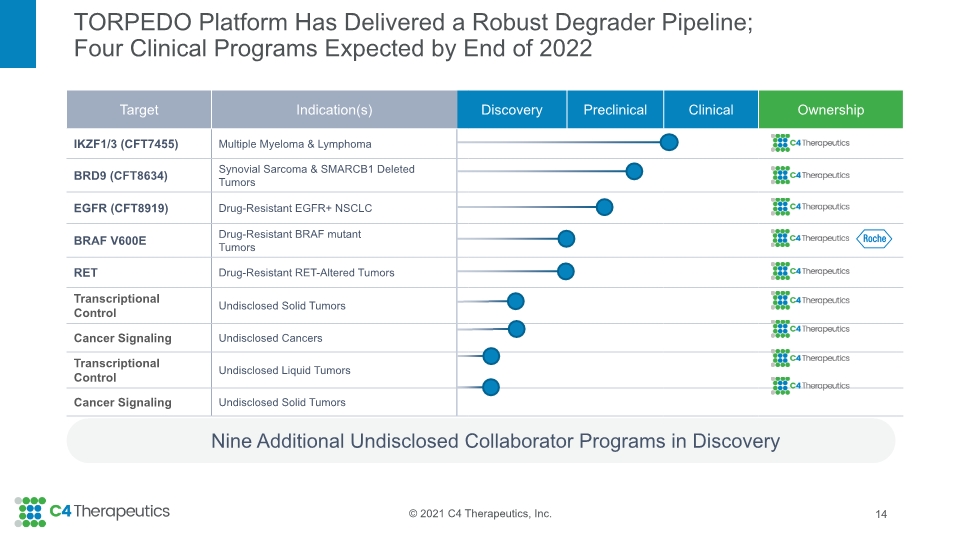
TORPEDO Platform Has Delivered a Robust Degrader Pipeline; Four Clinical Programs Expected by End of 2022 Nine Additional Undisclosed Collaborator Programs in Discovery 14
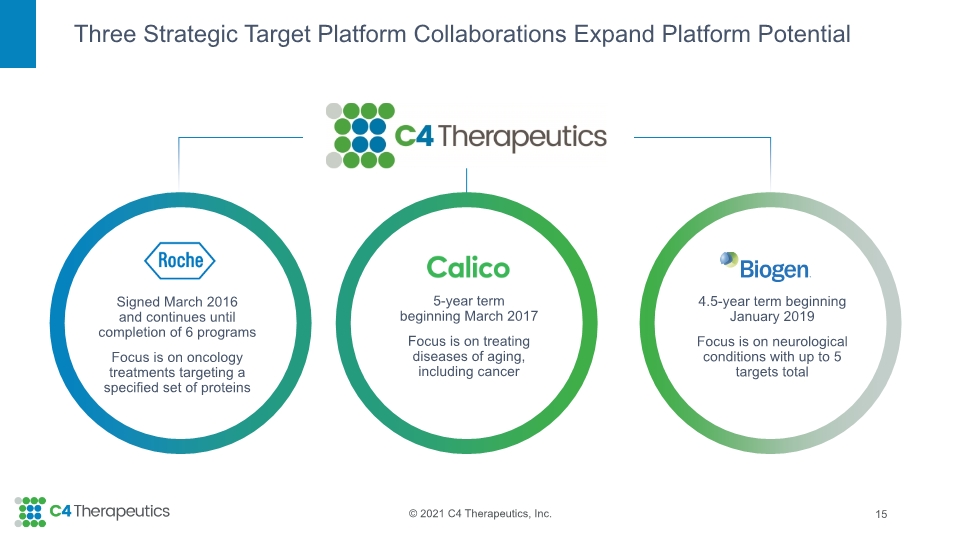
Three Strategic Target Platform Collaborations Expand Platform Potential 15 4.5-year term beginning January 2019 Focus is on neurological conditions with up to 5 targets total 5-year term beginning March 2017 Focus is on treating diseases of aging, including cancer Signed March 2016 and continues until completion of 6 programs Focus is on oncology treatments targeting a specified set of proteins

IKZF1/3 CFT7455
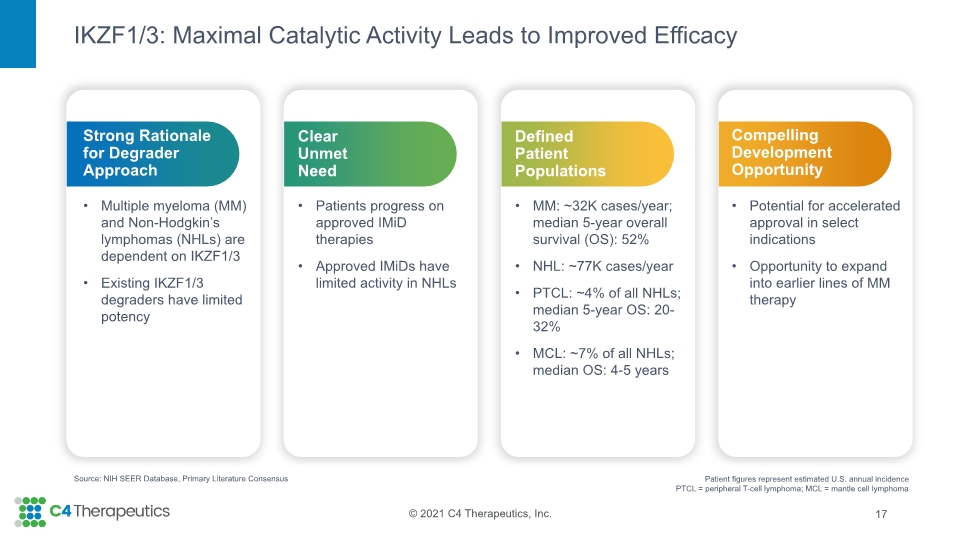
IKZF1/3: Maximal Catalytic Activity Leads to Improved Efficacy 17 Source: NIH SEER Database, Primary Literature Consensus Patient figures represent estimated U.S. annual incidence PTCL = peripheral T-cell lymphoma; MCL = mantle cell lymphoma Compelling Development Opportunity Potential for accelerated approval in select indications Opportunity to expand into earlier lines of MM therapy Defined Patient Populations MM: ~32K cases/year; median 5-year overall survival (OS): 52% NHL: ~77K cases/year PTCL: ~4% of all NHLs; median 5-year OS: 20-32% MCL: ~7% of all NHLs; median OS: 4-5 years Clear Unmet Need Patients progress on approved IMiD therapies Approved IMiDs have limited activity in NHLs Strong Rationale for Degrader Approach Multiple myeloma (MM) and Non-Hodgkin’s lymphomas (NHLs) are dependent on IKZF1/3 Existing IKZF1/3 degraders have limited potency
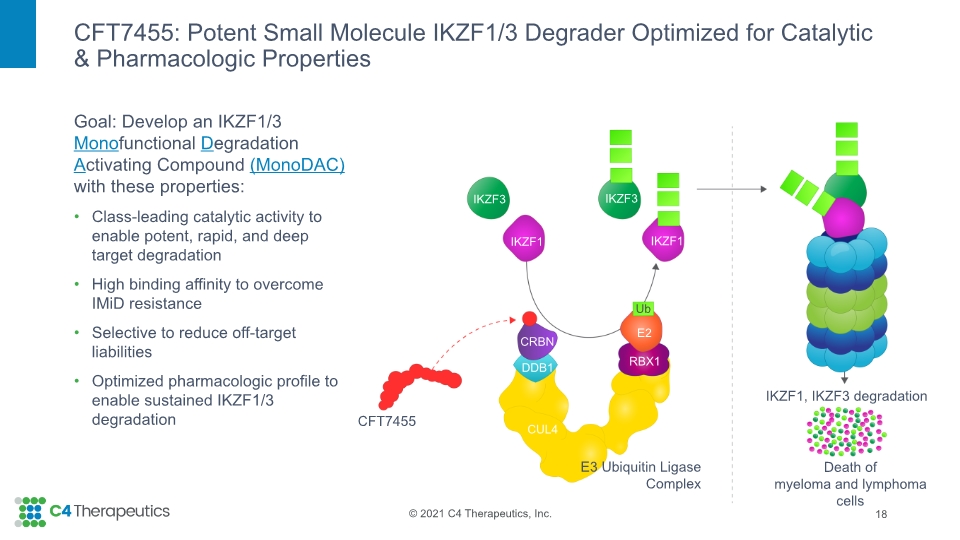
CFT7455: Potent Small Molecule IKZF1/3 Degrader Optimized for Catalytic & Pharmacologic Properties 18 CFT7455 DDB1 E3 Ubiquitin Ligase Complex CRBN RBX1 E2 IKZF1, IKZF3 degradation Death of myeloma and lymphoma cells IKZF1 IKZF1 IKZF3 IKZF3 Ub CUL4 Goal: Develop an IKZF1/3 Monofunctional Degradation Activating Compound (MonoDAC) with these properties: Class-leading catalytic activity to enable potent, rapid, and deep target degradation High binding affinity to overcome IMiD resistance Selective to reduce off-target liabilities Optimized pharmacologic profile to enable sustained IKZF1/3 degradation
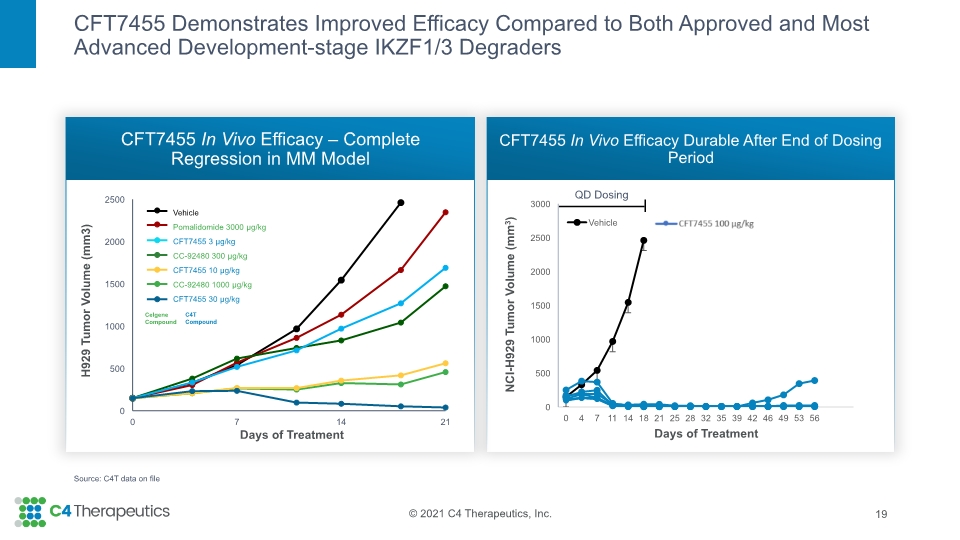
CFT7455 Demonstrates Improved Efficacy Compared to Both Approved and Most Advanced Development-stage IKZF1/3 Degraders CFT7455 In Vivo Efficacy – Complete Regression in MM Model CFT7455 In Vivo Efficacy Durable After End of Dosing Period 19 Source: C4T data on file QD Dosing NCI-H929 Tumor Volume (mm3)
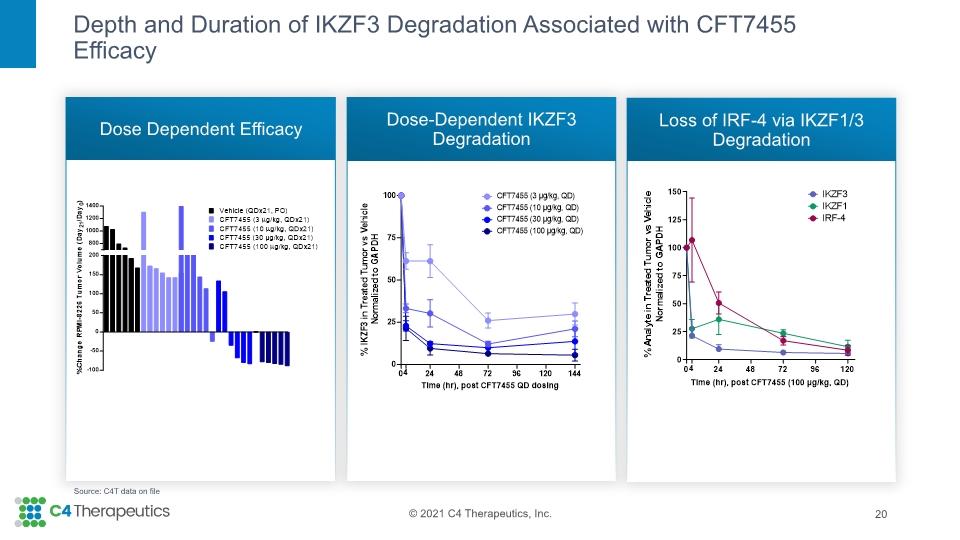
Depth and Duration of IKZF3 Degradation Associated with CFT7455 Efficacy Dose Dependent Efficacy Dose-Dependent IKZF3 Degradation Loss of IRF-4 via IKZF1/3 Degradation 20 Source: C4T data on file
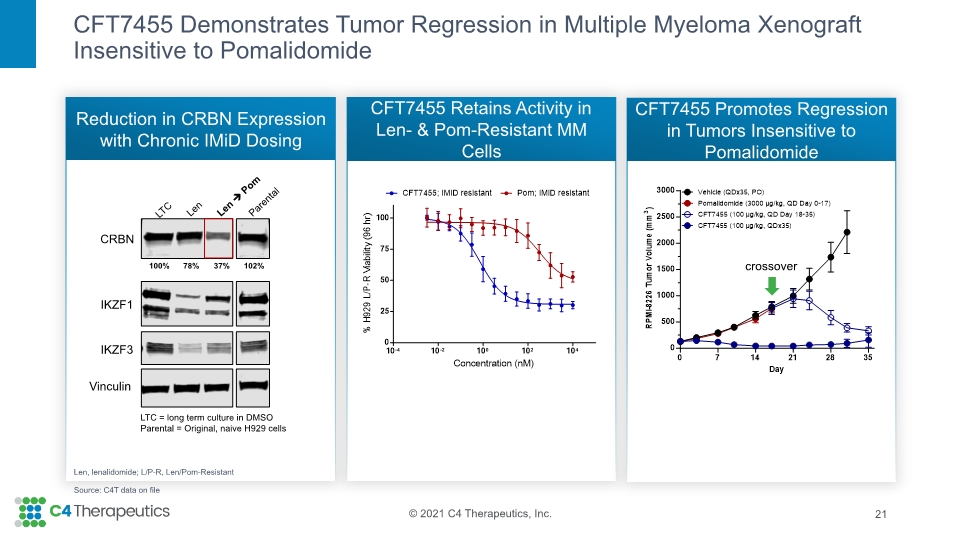
CFT7455 Demonstrates Tumor Regression in Multiple Myeloma Xenograft Insensitive to Pomalidomide Reduction in CRBN Expression with Chronic IMiD Dosing CFT7455 Retains Activity in Len- & Pom-Resistant MM Cells CFT7455 Promotes Regression in Tumors Insensitive to Pomalidomide 21 crossover Len, lenalidomide; L/P-R, Len/Pom-Resistant Source: C4T data on file
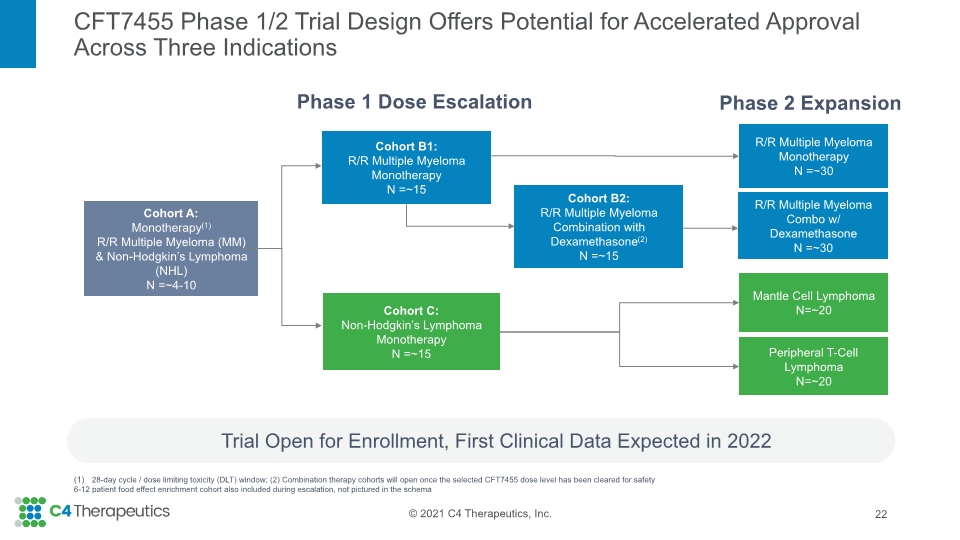
CFT7455 Phase 1/2 Trial Design Offers Potential for Accelerated Approval Across Three Indications Trial Open for Enrollment, First Clinical Data Expected in 2022 22 Phase 1 Dose Escalation R/R Multiple Myeloma Monotherapy N =~30 Cohort B1: R/R Multiple Myeloma Monotherapy N =~15 Cohort C: Non-Hodgkin’s Lymphoma Monotherapy N =~15 Mantle Cell Lymphoma N=~20 Peripheral T-Cell Lymphoma N=~20 Cohort A: Monotherapy(1) R/R Multiple Myeloma (MM) & Non-Hodgkin’s Lymphoma (NHL) N =~4-10 Phase 2 Expansion Cohort B2: R/R Multiple Myeloma Combination with Dexamethasone(2) N =~15 R/R Multiple Myeloma Combo w/ Dexamethasone N =~30 28-day cycle / dose limiting toxicity (DLT) window; (2) Combination therapy cohorts will open once the selected CFT7455 dose level has been cleared for safety 6-12 patient food effect enrichment cohort also included during escalation, not pictured in the schema

BRD9 CFT8634
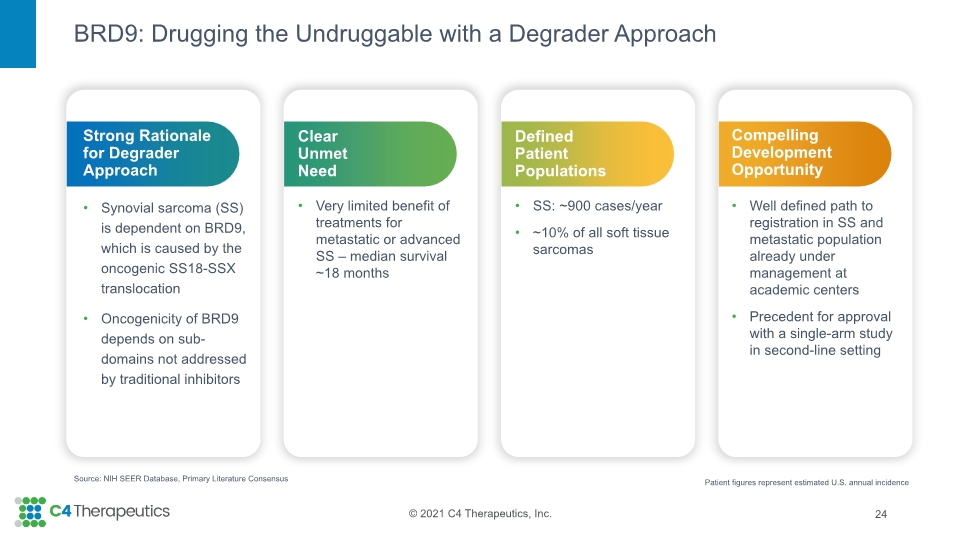
BRD9: Drugging the Undruggable with a Degrader Approach 24 Source: NIH SEER Database, Primary Literature Consensus Compelling Development Opportunity Well defined path to registration in SS and metastatic population already under management at academic centers Precedent for approval with a single-arm study in second-line setting Defined Patient Populations SS: ~900 cases/year ~10% of all soft tissue sarcomas Clear Unmet Need Very limited benefit of treatments for metastatic or advanced SS – median survival ~18 months Strong Rationale for Degrader Approach Synovial sarcoma (SS) is dependent on BRD9, which is caused by the oncogenic SS18-SSX translocation Oncogenicity of BRD9 depends on sub-domains not addressed by traditional inhibitors Patient figures represent estimated U.S. annual incidence
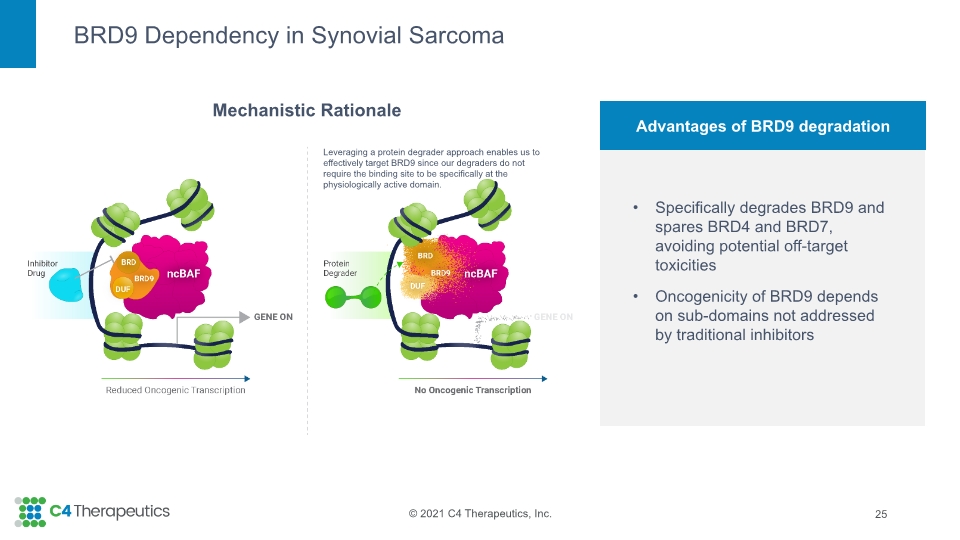
BRD9 Dependency in Synovial Sarcoma 25 Specifically degrades BRD9 and spares BRD4 and BRD7, avoiding potential off-target toxicities Oncogenicity of BRD9 depends on sub-domains not addressed by traditional inhibitors Advantages of BRD9 degradation Mechanistic Rationale Leveraging a protein degrader approach enables us to effectively target BRD9 since our degraders do not require the binding site to be specifically at the physiologically active domain.
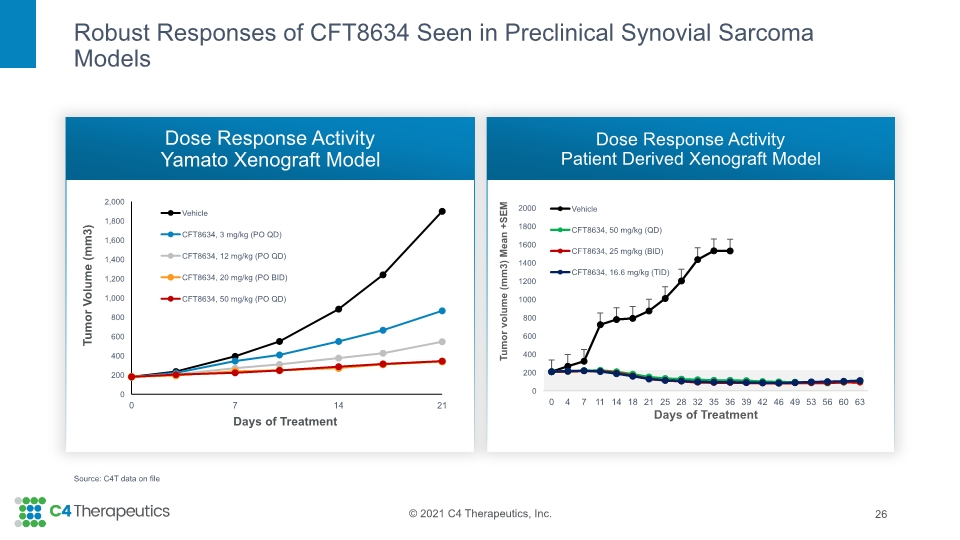
Robust Responses of CFT8634 Seen in Preclinical Synovial Sarcoma Models Dose Response Activity Yamato Xenograft Model Dose Response Activity Patient Derived Xenograft Model 26 Source: C4T data on file
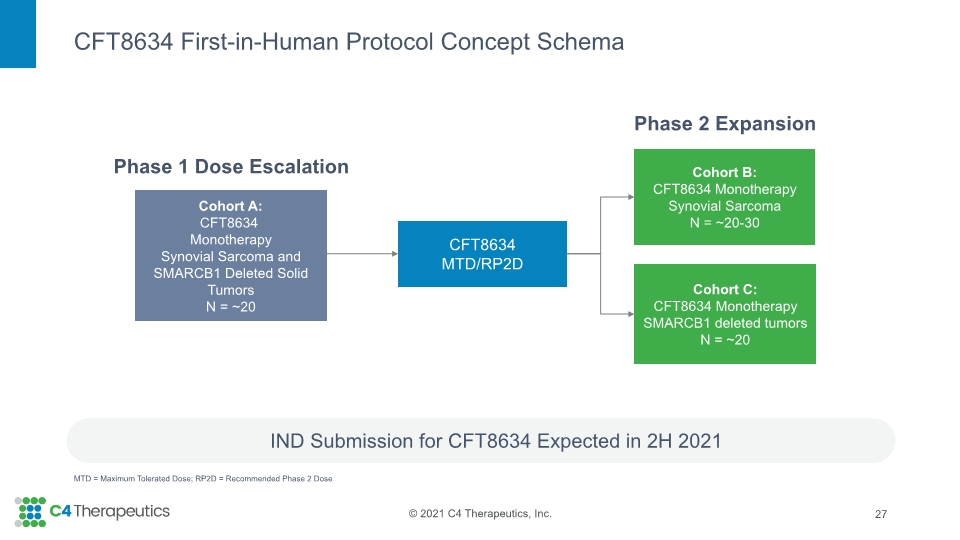
CFT8634 First-in-Human Protocol Concept Schema IND Submission for CFT8634 Expected in 2H 2021 27 MTD = Maximum Tolerated Dose; RP2D = Recommended Phase 2 Dose

EGFR CFT8919
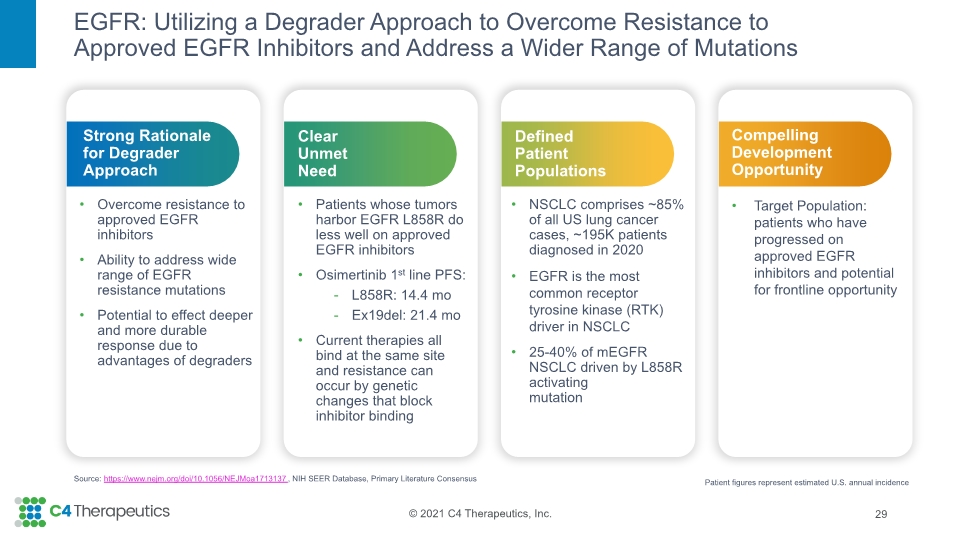
EGFR: Utilizing a Degrader Approach to Overcome Resistance to Approved EGFR Inhibitors and Address a Wider Range of Mutations 29 Source: https://www.nejm.org/doi/10.1056/NEJMoa1713137, NIH SEER Database, Primary Literature Consensus Compelling Development Opportunity Target Population: patients who have progressed on approved EGFR inhibitors and potential for frontline opportunity Defined Patient Populations NSCLC comprises ~85% of all US lung cancer cases, ~195K patients diagnosed in 2020 EGFR is the most common receptor tyrosine kinase (RTK) driver in NSCLC 25-40% of mEGFR NSCLC driven by L858R activating mutation Clear Unmet Need Patients whose tumors harbor EGFR L858R do less well on approved EGFR inhibitors Osimertinib 1st line PFS: L858R: 14.4 mo Ex19del: 21.4 mo Current therapies all bind at the same site and resistance can occur by genetic changes that block inhibitor binding Strong Rationale for Degrader Approach Overcome resistance to approved EGFR inhibitors Ability to address wide range of EGFR resistance mutations Potential to effect deeper and more durable response due to advantages of degraders Patient figures represent estimated U.S. annual incidence
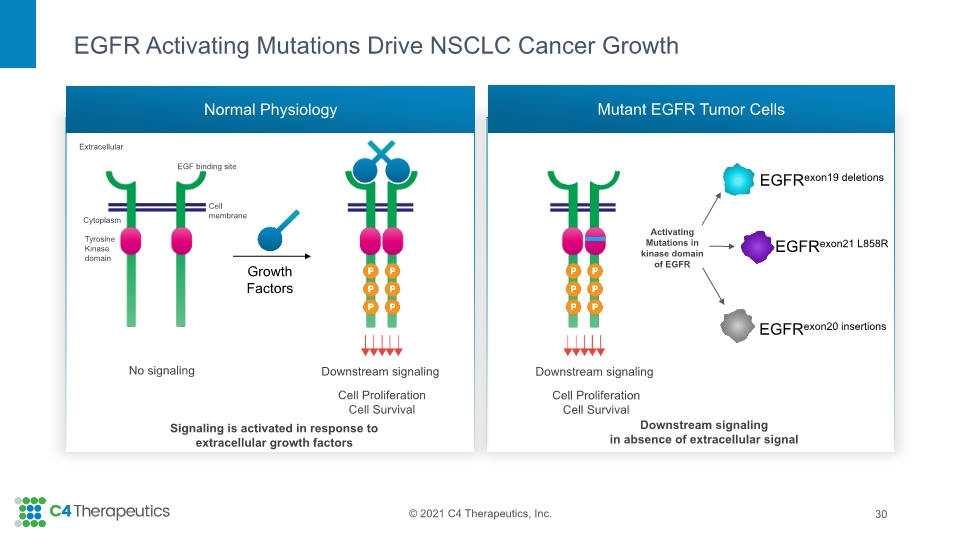
EGFR Activating Mutations Drive NSCLC Cancer Growth 30 Normal Physiology Mutant EGFR Tumor Cells No signaling Downstream signaling Downstream signaling in absence of extracellular signal Cell Proliferation Cell Survival Growth Factors Signaling is activated in response to extracellular growth factors Activating Mutations in kinase domain of EGFR Extracellular EGF binding site Cell membrane Cytoplasm Tyrosine Kinase domain Downstream signaling Cell Proliferation Cell Survival EGFRexon19 deletions EGFRexon21 L858R EGFRexon20 insertions
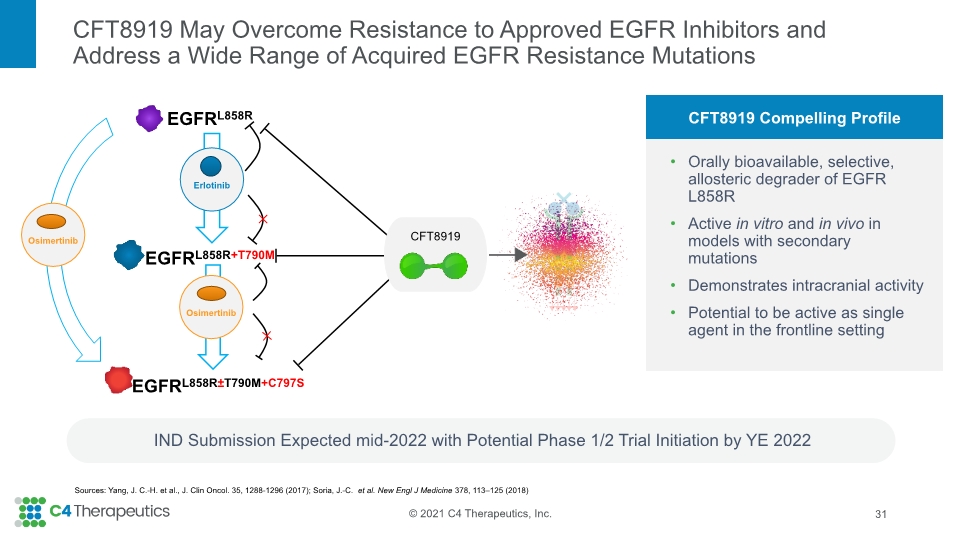
CFT8919 May Overcome Resistance to Approved EGFR Inhibitors and Address a Wide Range of Acquired EGFR Resistance Mutations 31 IND Submission Expected mid-2022 with Potential Phase 1/2 Trial Initiation by YE 2022 CFT8919 Compelling Profile Orally bioavailable, selective, allosteric degrader of EGFR L858R Active in vitro and in vivo in models with secondary mutations Demonstrates intracranial activity Potential to be active as single agent in the frontline setting Sources: Yang, J. C.-H. et al., J. Clin Oncol. 35, 1288-1296 (2017); Soria, J.-C. et al. New Engl J Medicine 378, 113–125 (2018)

BRAF
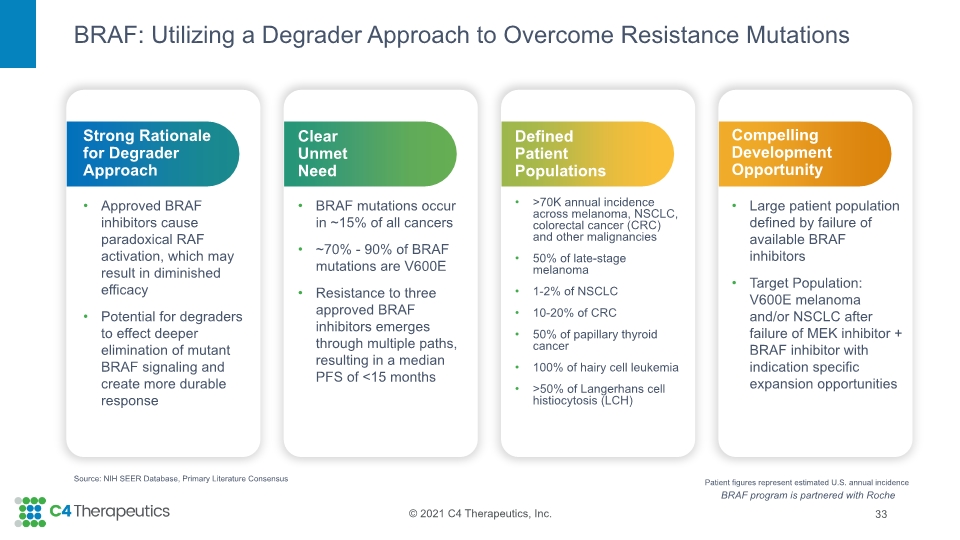
BRAF: Utilizing a Degrader Approach to Overcome Resistance Mutations 33 Source: NIH SEER Database, Primary Literature Consensus Compelling Development Opportunity Large patient population defined by failure of available BRAF inhibitors Target Population: V600E melanoma and/or NSCLC after failure of MEK inhibitor + BRAF inhibitor with indication specific expansion opportunities Defined Patient Populations >70K annual incidence across melanoma, NSCLC, colorectal cancer (CRC) and other malignancies 50% of late-stage melanoma 1-2% of NSCLC 10-20% of CRC 50% of papillary thyroid cancer 100% of hairy cell leukemia >50% of Langerhans cell histiocytosis (LCH) Clear Unmet Need BRAF mutations occur in ~15% of all cancers ~70% - 90% of BRAF mutations are V600E Resistance to three approved BRAF inhibitors emerges through multiple paths, resulting in a median PFS of <15 months Strong Rationale for Degrader Approach Approved BRAF inhibitors cause paradoxical RAF activation, which may result in diminished efficacy Potential for degraders to effect deeper elimination of mutant BRAF signaling and create more durable response Patient figures represent estimated U.S. annual incidence BRAF program is partnered with Roche
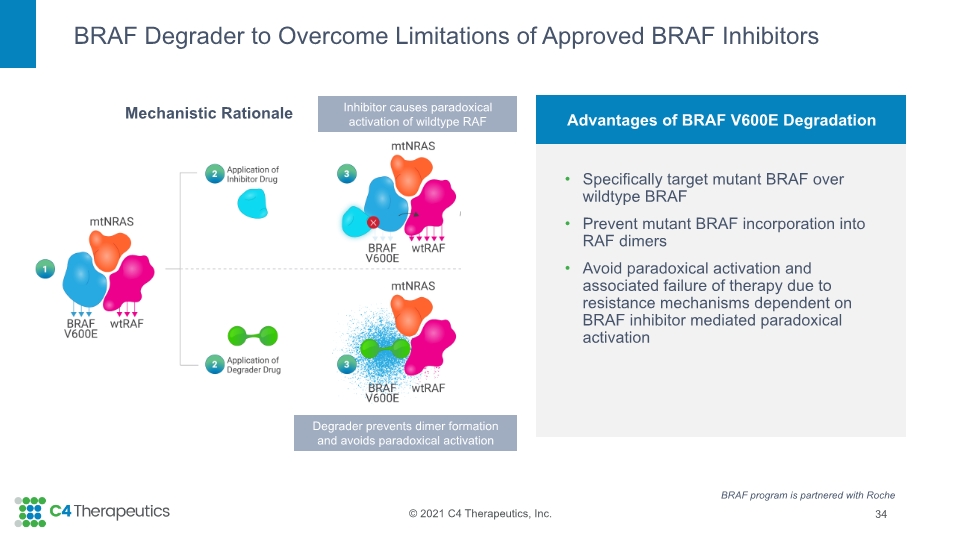
Advantages of BRAF V600E Degradation Specifically target mutant BRAF over wildtype BRAF Prevent mutant BRAF incorporation into RAF dimers Avoid paradoxical activation and associated failure of therapy due to resistance mechanisms dependent on BRAF inhibitor mediated paradoxical activation BRAF Degrader to Overcome Limitations of Approved BRAF Inhibitors 34 Mechanistic Rationale Inhibitor causes paradoxical activation of wildtype RAF Degrader prevents dimer formation and avoids paradoxical activation BRAF program is partnered with Roche
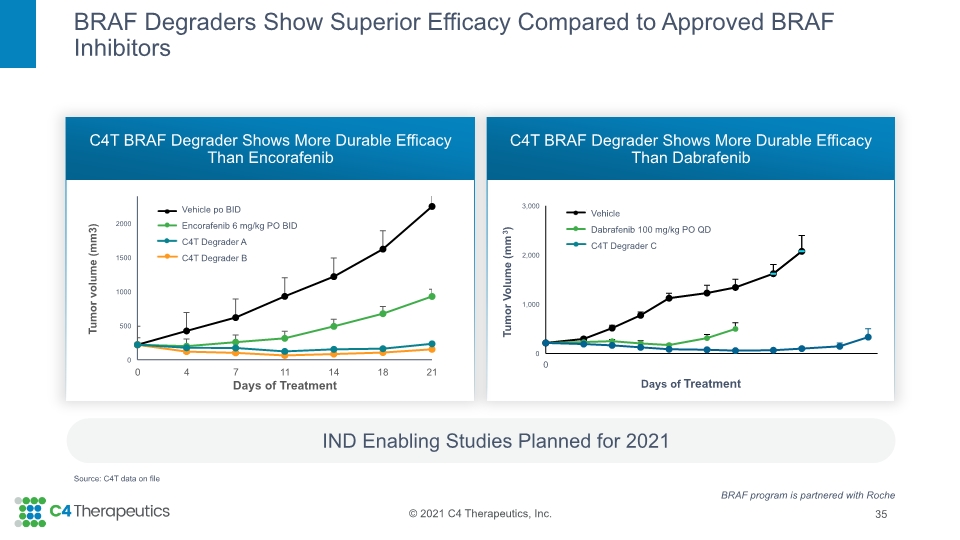
BRAF Degraders Show Superior Efficacy Compared to Approved BRAF Inhibitors C4T BRAF Degrader Shows More Durable Efficacy Than Encorafenib C4T BRAF Degrader Shows More Durable Efficacy Than Dabrafenib 35 IND Enabling Studies Planned for 2021 Tumor Volume (mm3) Days of Treatment BRAF program is partnered with Roche Source: C4T data on file Vehicle po BID Encorafenib 6 mg/kg PO BID C4T Degrader A C4T Degrader B Vehicle Dabrafenib 100 mg/kg PO QD C4T Degrader C

RET
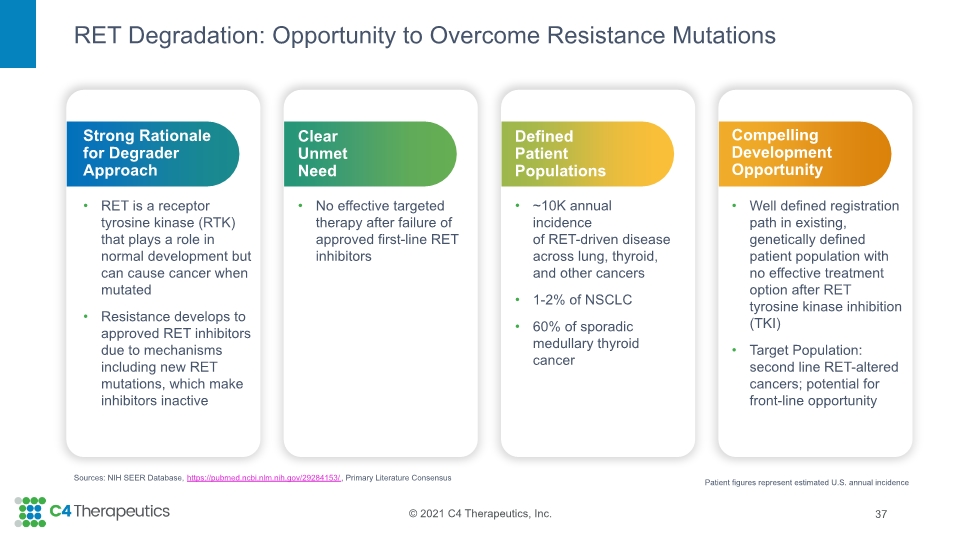
RET Degradation: Opportunity to Overcome Resistance Mutations 37 Sources: NIH SEER Database, https://pubmed.ncbi.nlm.nih.gov/29284153/, Primary Literature Consensus Compelling Development Opportunity Well defined registration path in existing, genetically defined patient population with no effective treatment option after RET tyrosine kinase inhibition (TKI) Target Population: second line RET-altered cancers; potential for front-line opportunity Defined Patient Populations ~10K annual incidence of RET-driven disease across lung, thyroid, and other cancers 1-2% of NSCLC 60% of sporadic medullary thyroid cancer Clear Unmet Need No effective targeted therapy after failure of approved first-line RET inhibitors Strong Rationale for Degrader Approach RET is a receptor tyrosine kinase (RTK) that plays a role in normal development but can cause cancer when mutated Resistance develops to approved RET inhibitors due to mechanisms including new RET mutations, which make inhibitors inactive Patient figures represent estimated U.S. annual incidence
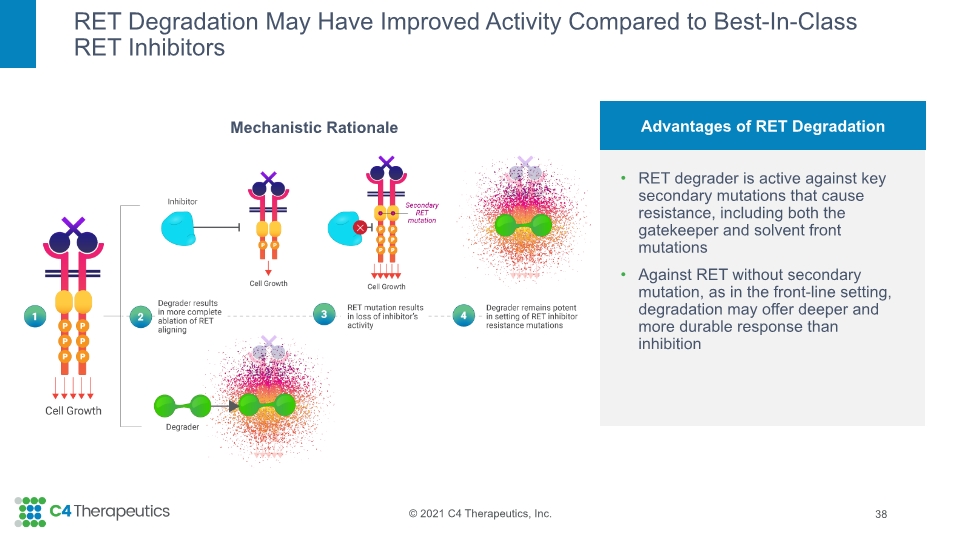
Mechanistic Rationale RET Degradation May Have Improved Activity Compared to Best-In-Class RET Inhibitors 38 Advantages of RET Degradation RET degrader is active against key secondary mutations that cause resistance, including both the gatekeeper and solvent front mutations Against RET without secondary mutation, as in the front-line setting, degradation may offer deeper and more durable response than inhibition
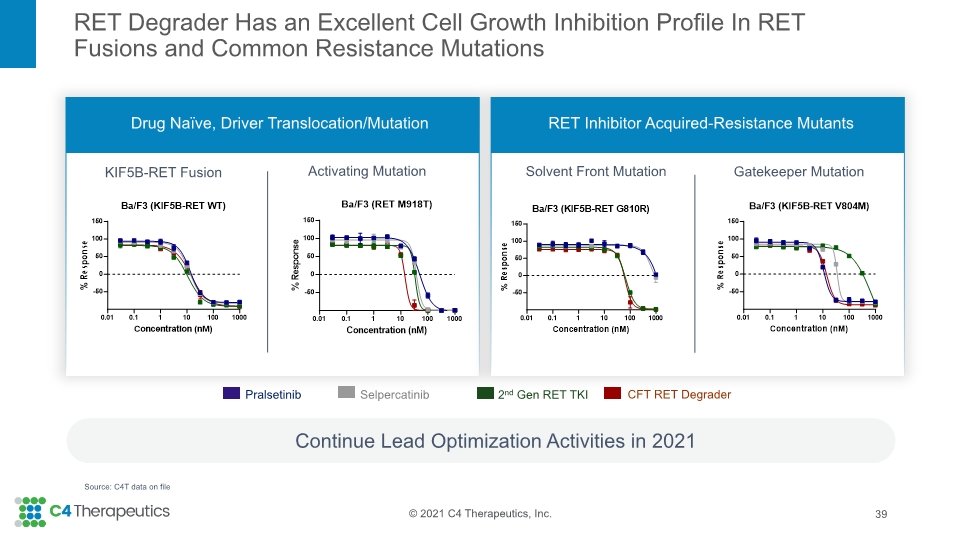
RET Degrader Has an Excellent Cell Growth Inhibition Profile In RET Fusions and Common Resistance Mutations Continue Lead Optimization Activities in 2021 39 KIF5B-RET Fusion Solvent Front Mutation Activating Mutation Drug Naïve, Driver Translocation/Mutation RET Inhibitor Acquired-Resistance Mutants Gatekeeper Mutation Source: C4T data on file
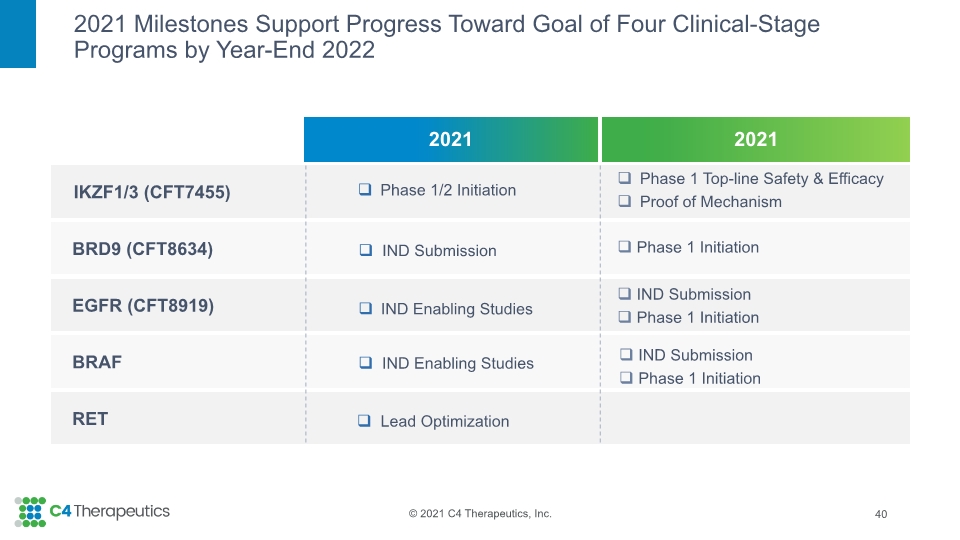
2021 Milestones Support Progress Toward Goal of Four Clinical-Stage Programs by Year-End 2022 40 Phase 1/2 Initiation IND Submission Lead Optimization IND Enabling Studies IND Enabling Studies Phase 1 Top-line Safety & Efficacy Proof of Mechanism Phase 1 Initiation IND Submission Phase 1 Initiation IND Submission Phase 1 Initiation
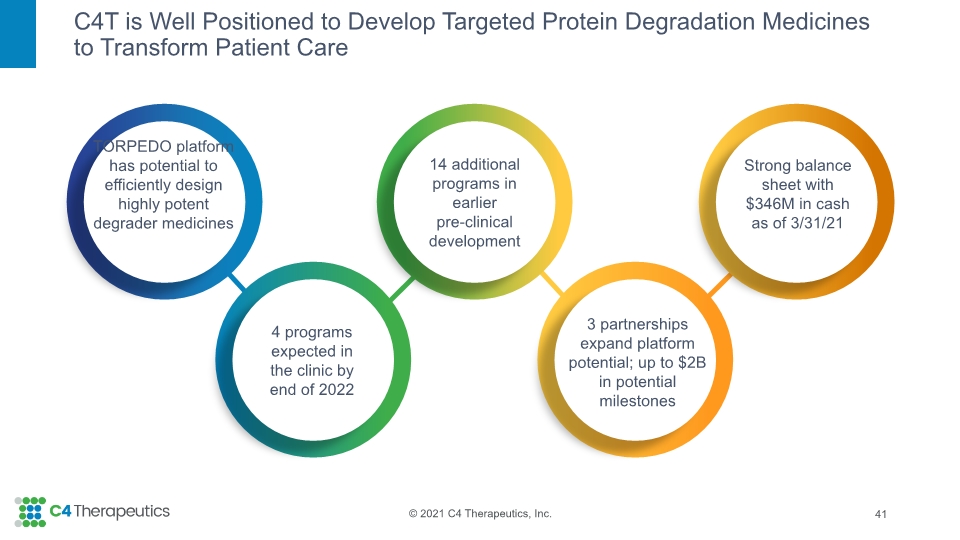
C4T is Well Positioned to Develop Targeted Protein Degradation Medicines to Transform Patient Care 41 Strong balance sheet with $346M in cash as of 3/31/21 TORPEDO platform has potential to efficiently design highly potent degrader medicines 4 programs expected in the clinic by end of 2022 14 additional programs in earlier pre-clinical development 3 partnerships expand platform potential; up to $2B in potential milestones

Thank You









































