
Updated Data in Multiple Myeloma and First Data in Non-Hodgkin’s Lymphoma from the Ongoing Cemsidomide Phase 1/2 Trial American Hematology Annual Meeting (ASH) December 8, 2024

Forward-looking Statements and Intellectual Property 2 FORWARD-LOOKING STATEMENTS The following presentation contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the therapeutic potential of C4 Therapeutics, Inc.’s technology and products. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, as well as the fact that the product candidates that we are developing or may develop may not demonstrate success in clinical trials. Prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The forward-looking statements included in this presentation are subject to a variety of risks and uncertainties, including those set forth in our most recent and future filings with the Securities and Exchange Commission. Our actual results could vary significantly from those anticipated in this presentation, and our financial condition and results of operations could be materially adversely affected. C4 Therapeutics, Inc. undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. INTELLECTUAL PROPERTY C4 Therapeutics, Inc. owns various registered and unregistered trademarks and service marks in the U.S. and internationally, including, without limitation, C4 THERAPEUTICS, our housemark logo, the name of our TORPEDO platform, and the names of our BIDAC and MONODAC degrader products. All trademarks, service marks, or trade names referred to in this presentation that we do not own are the property of their respective owners. Solely for convenience, the trademarks, service marks, and trade names in this presentation are referred to without the symbols ®, SM and , but those references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights to. © 2024 C4 Therapeutics, Inc.

Cemsidomide Phase 1 MM & NHL Data & Next Steps Len Reyno, M.D., CMO Concluding Remarks & Q&A Session Andrew Hirsch, President and CEO Len Reyno, M.D., CMO Kendra Adams, CFO Introductions Courtney Solberg, Senior Manager of IR Opening Remarks Andrew Hirsch, President and CEO Today’s Agenda 3© 2024 C4 Therapeutics, Inc.

Opening Remarks Andrew Hirsch President and Chief Executive Officer

C4T Has Delivered a Steady Flow of Clinical Updates and Innovative Collaborations Over the Past 12 Months… Cemsidomide Collaborations Have Further Validated TORPEDO Platform CFT1946 CFT8919 Significant Progress Across Clinical Programs © 2024 C4 Therapeutics, Inc. 5 ✓ Compelling activity in both multiple myeloma and non-Hodgkin’s lymphoma ✓ Modest and manageable neutropenia ✓ Emerging data demonstrate positive exposure-response relationship ✓ Evidence of immunomodulatory effects, consistent with the class ✓ Monotherapy anti-tumor activity, including tumor reductions across various V600 mutation types ✓ Dose-dependent bioavailability ✓ Well-tolerated; no Grade ≥ 3 cutaneous adverse events commonly seen with BRAF inhibitors ✓ Preclinical data demonstrate ability to cross blood-brain barrier ✓ Clinical trial initiated in Greater China in partnership with Betta Pharmaceuticals ✓ Announced collaboration to discover targeted protein degraders against critical oncogenic proteins ✓ Established partnership to discover and develop degrader antibody conjugates ✓ Delivered two development candidates for non-oncology targets
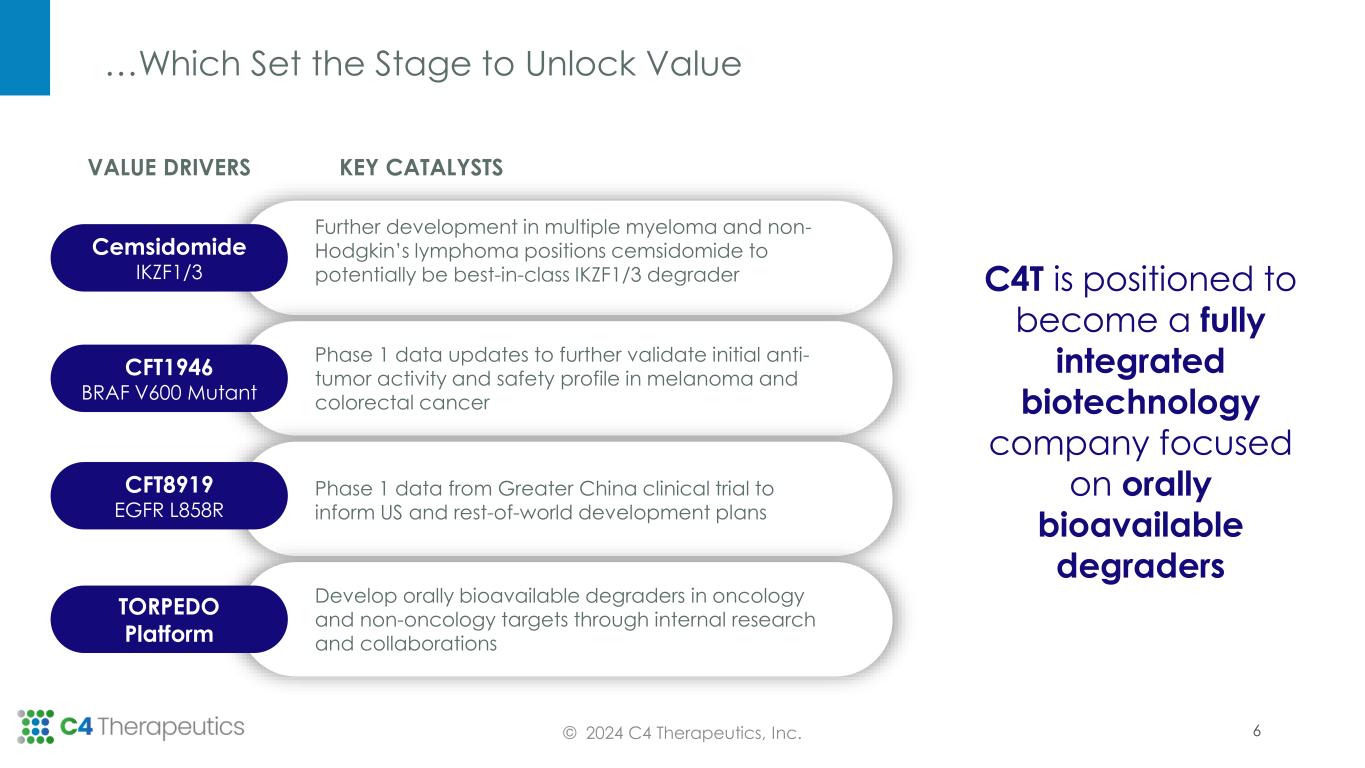
…Which Set the Stage to Unlock Value © 2024 C4 Therapeutics, Inc. C4T is positioned to become a fully integrated biotechnology company focused on orally bioavailable degraders KEY CATALYSTSVALUE DRIVERS Cemsidomide IKZF1/3 Further development in multiple myeloma and non- Hodgkin’s lymphoma positions cemsidomide to potentially be best-in-class IKZF1/3 degrader CFT1946 BRAF V600 Mutant Phase 1 data updates to further validate initial anti- tumor activity and safety profile in melanoma and colorectal cancer CFT8919 EGFR L858R Phase 1 data from Greater China clinical trial to inform US and rest-of-world development plans Develop orally bioavailable degraders in oncology and non-oncology targets through internal research and collaborations TORPEDO Platform 6

Cemsidomide First-in-Human Clinical Program Relapsed Refractory Multiple Myeloma and Non-Hodgkin's Lymphoma

IKZF1/3 Are Key Promoters of Myeloma and Lymphoma Cell Survival and Will Remain Important Therapeutic Targets in MM and NHL © 2024 C4 Therapeutics, Inc. Key Roles of IKZF1/3 Physiological Functions: • IKZF1/3 are key transcriptional regulators of hematopoietic stem cell differentiation • IKZF1/3 directly regulate the activity of IRF4, another transcription factor that regulates downstream immune cell differentiation Oncogenic Functions: • Multiple myeloma and lymphoma cells rely on IKZF1/3 and IRF4 for survival IKZF1/3 Degradation Leads to: • Downregulation of IRF4, promoting the death of myeloma and lymphoma cells • On-target neutropenia 8 Hematopoietic Stem Cell Common Myeloid Progenitor Cell Common Lymphoid Progenitor Cell Neutrophil Platelets T-Cell B-Cell Plasma Cell Oncogenic Mutations/Aberrations T-Cell Lymphoma B-Cell Lymphoma Multiple Myeloma IKZF1/3 IRF4 ↑ IKZF 1/3 and IRF4 Multiple myeloma (MM); non-Hodgkin’s lymphoma (NHL)
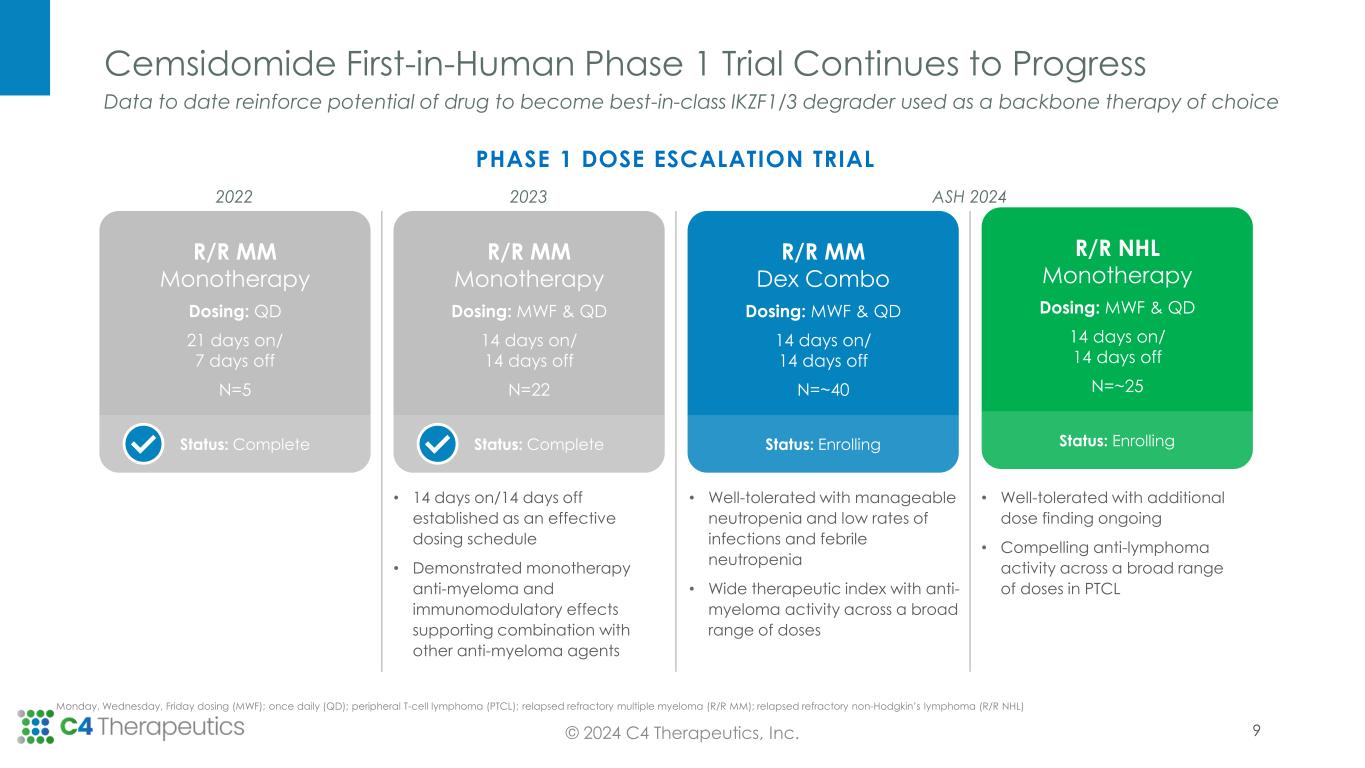
R/R NHL Monotherapy Dosing: MWF & QD 14 days on/ 14 days off N=~25 Status: Enrolling R/R MM Dex Combo Dosing: MWF & QD 14 days on/ 14 days off N=~40 Status: Enrolling R/R MM Monotherapy Dosing: MWF & QD 14 days on/ 14 days off N=22 Status: Complete Cemsidomide First-in-Human Phase 1 Trial Continues to Progress © 2024 C4 Therapeutics, Inc. Monday, Wednesday, Friday dosing (MWF); once daily (QD); peripheral T-cell lymphoma (PTCL); relapsed refractory multiple myeloma (R/R MM); relapsed refractory non-Hodgkin’s lymphoma (R/R NHL) Data to date reinforce potential of drug to become best-in-class IKZF1/3 degrader used as a backbone therapy of choice • 14 days on/14 days off established as an effective dosing schedule • Demonstrated monotherapy anti-myeloma and immunomodulatory effects supporting combination with other anti-myeloma agents • Well-tolerated with manageable neutropenia and low rates of infections and febrile neutropenia • Wide therapeutic index with anti- myeloma activity across a broad range of doses • Well-tolerated with additional dose finding ongoing • Compelling anti-lymphoma activity across a broad range of doses in PTCL 9 R/R MM Monotherapy Dosing: QD 21 days on/ 7 days off N=5 Status: Complete PHASE 1 DOSE ESCALATION TRIAL 2022 2023 ASH 2024

Multiple Myeloma Cemsidomide + Dexamethasone
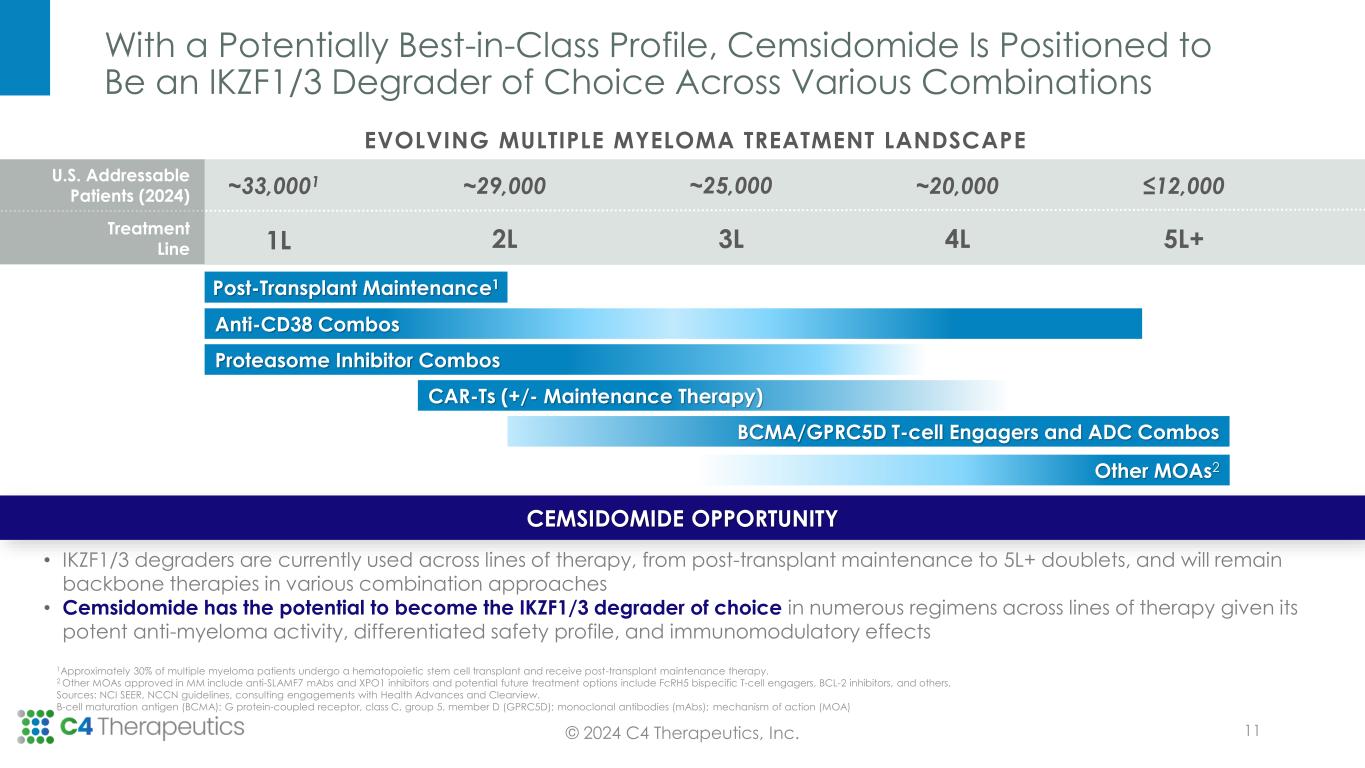
With a Potentially Best-in-Class Profile, Cemsidomide Is Positioned to Be an IKZF1/3 Degrader of Choice Across Various Combinations Post-Transplant Maintenance1 Anti-CD38 Combos Proteasome Inhibitor Combos CAR-Ts (+/- Maintenance Therapy) Other MOAs2 1Approximately 30% of multiple myeloma patients undergo a hematopoietic stem cell transplant and receive post-transplant maintenance therapy. 2 Other MOAs approved in MM include anti-SLAMF7 mAbs and XPO1 inhibitors and potential future treatment options include FcRH5 bispecific T-cell engagers, BCL-2 inhibitors, and others. Sources: NCI SEER, NCCN guidelines, consulting engagements with Health Advances and Clearview. B-cell maturation antigen (BCMA); G protein-coupled receptor, class C, group 5, member D (GPRC5D); monoclonal antibodies (mAbs); mechanism of action (MOA) BCMA/GPRC5D T-cell Engagers and ADC Combos 11© 2024 C4 Therapeutics, Inc. CEMSIDOMIDE OPPORTUNITY • IKZF1/3 degraders are currently used across lines of therapy, from post-transplant maintenance to 5L+ doublets, and will remain backbone therapies in various combination approaches • Cemsidomide has the potential to become the IKZF1/3 degrader of choice in numerous regimens across lines of therapy given its potent anti-myeloma activity, differentiated safety profile, and immunomodulatory effects 1L 5L+2L 3L 4L ~29,000 ~33,0001 ~25,000 ~20,000 ≤12,000 EVOLVING MULTIPLE MYELOMA TREATMENT LANDSCAPE U.S. Addressable Patients (2024) Treatment Line

Cemsidomide + Dexamethasone Dose Escalation Trial in MM Continues to Progress; Have Not Exceeded the Maximum Tolerated Dose 12© 2024 C4 Therapeutics, Inc. DOSE ESCALATION CEMSIDOMIDE 14/14 + DEX* Utilizing a Bayesian logistic regression model until determination of the MTD and/or RP2D 50 µg MWF Back-fill cohort; N=1 37.5 µg QD Back-fill cohort; N=8 62.5 µg QD Back-fill cohort; N=9 75 µg QD Back-fill cohort; N=10 DL 1 (50 µg MWF) N=5 DL 2 (37.5 µg QD) N=4 DL 4 (75 µg QD) N=4 DL 3 (62.5 µg QD) N=6 | 1 DLTa Potential for further dose escalation and/or exploration DL 5 (100 µg QD) Currently enrolling KEY INCLUSION CRITERIA • Adults with MM, R/R to at least 3 prior lines of therapy that have included lenalidomide, pomalidomide, a proteasome inhibitor, a glucocorticoid, and an anti- CD38 monoclonal antibody • Nonresponsive to or progressed within 60 days of prior therapy • Creatinine clearance ≥40 mL/min • ECOG ≤2 Phase 1 Study Endpoints • Primary: assess safety, tolerability and define the RP2D/MTD • Secondary: assess PK, PD, and preliminary anti-tumor activity BACK-FILL COHORT(S) Data cutoff: 10/11/24 *Cemsidomide administered as 14 days on/14 days off in a 28-day cycle; Dex was dosed on days 1, 8, 15, and 22 at doses of 40 mg orally for patients ≤75 years old and 20 mg orally for patients >75 years old; 2 patients at 100 µg are excluded as they had not completed Cycle 1 as of the data cut off date. aDLT at 62.5 µg QD was due to Grade 4 neutropenia lasting >7 days. Eastern Cooperative Oncology Group (ECOG); maximum tolerated dose (MTD); Monday Wednesday Friday (MWF); multiple myeloma (MM); once daily (QD); pharmacodynamics (PD); pharmacokinetic (PK); recommended Phase 2 dose (RP2D); relapsed refractory (R/R)

© 2024 C4 Therapeutics, Inc. Characteristics Safety Population (N=47) Age, median (range) 67 (39-82 years) Male, n (%) 25 (53) Years since initial diagnosis, median (range) 7 (2-18) ECOG performance status, n (%) 0 1 2 10 (21) 34 (72) 3 (7) Black or African American, n (%) White, n (%) Other, n (%) 9 (19) 33 (70) 5 (11) Revised ISS at screening, n (%) Stage 1 Stage 2 Stage 3 Missing 21 (45) 15 (32) 5 (11) 6 (13) Presence of EMD, n (%) 14 (30) Characteristics Safety Population (N=47) Prior therapies, median (range) 6 (3-22) Prior lenalidomide, n (%) 47 (100) Prior pomalidomide, n (%) 46 (98) Prior anti-CD38 mAb, n (%) 47 (100) Prior CAR-T therapy, n (%) 19 (40) Prior TCE therapy, n (%) 21 (45) Prior CAR-T or TCE therapy, n (%) 31 (66) Prior CAR-T and TCE therapy, n (%) 9 (19) Prior BCMA therapy, n (%) 33 (70) Triple-class exposed*, n (%) 47 (100) Penta-class exposed†, n (%) 40 (85) Baseline Characteristics Prior Therapies Data cutoff: 10/11/24 Heavily Pre-Treated Patient Population With Majority Having Received Prior CAR-T, BCMA, or T-Cell Engager Therapy *Defined as exposed to ≥1 immunomodulatory agent, ≥1 proteasome inhibitor, and 1 anti-CD38 monoclonal antibody. †Defined as exposed to ≥2 immunomodulatory agents, ≥2 proteasome inhibitors, and 1 anti-CD38 monoclonal antibody. B-cell maturation antigen (BCMA); Eastern Cooperative Oncology Group (ECOG); extramedullary disease (EMD); International Staging System (ISS); monoclonal antibody (mAb); T-cell engager (TCE) 13
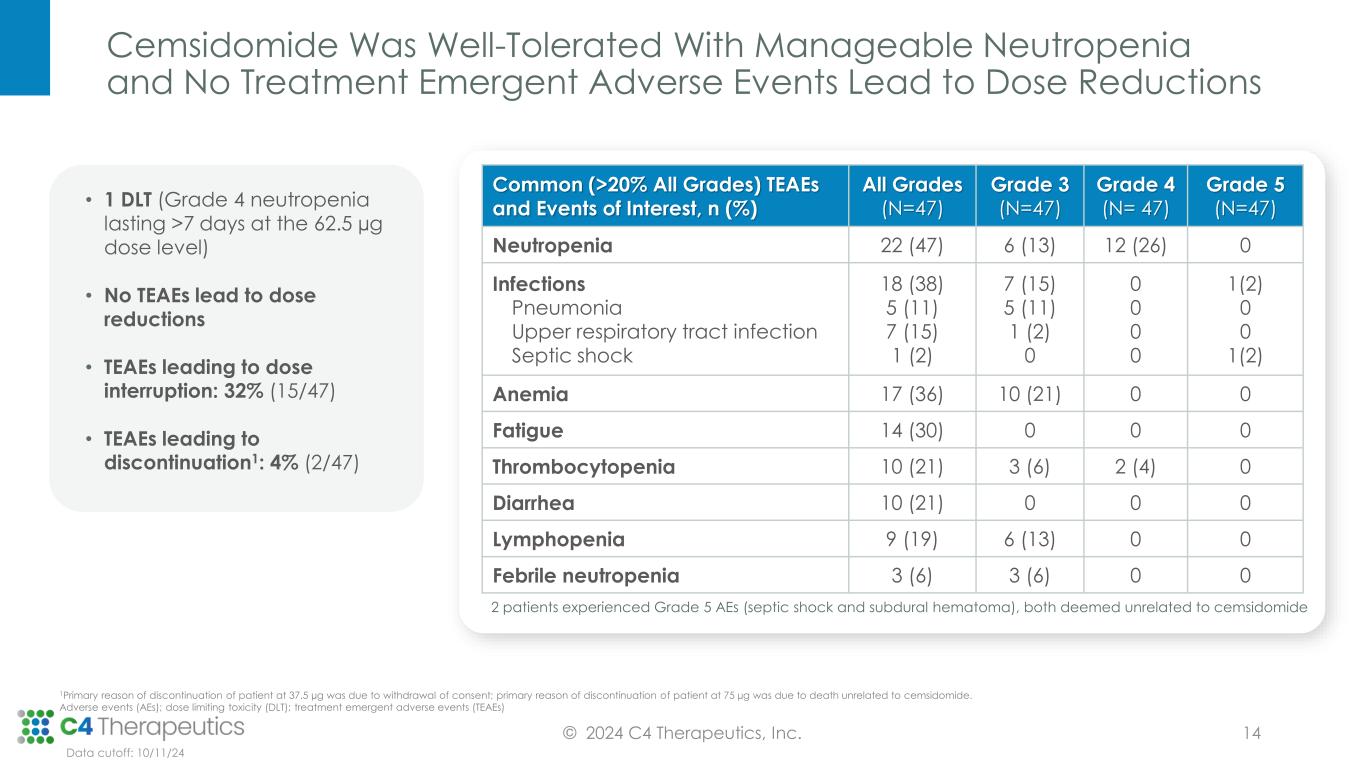
Cemsidomide Was Well-Tolerated With Manageable Neutropenia and No Treatment Emergent Adverse Events Lead to Dose Reductions © 2024 C4 Therapeutics, Inc. 14 Common (>20% All Grades) TEAEs and Events of Interest, n (%) All Grades (N=47) Grade 3 (N=47) Grade 4 (N= 47) Grade 5 (N=47) Neutropenia 22 (47) 6 (13) 12 (26) 0 Infections Pneumonia Upper respiratory tract infection Septic shock 18 (38) 5 (11) 7 (15) 1 (2) 7 (15) 5 (11) 1 (2) 0 0 0 0 0 1(2) 0 0 1(2) Anemia 17 (36) 10 (21) 0 0 Fatigue 14 (30) 0 0 0 Thrombocytopenia 10 (21) 3 (6) 2 (4) 0 Diarrhea 10 (21) 0 0 0 Lymphopenia 9 (19) 6 (13) 0 0 Febrile neutropenia 3 (6) 3 (6) 0 0 2 patients experienced Grade 5 AEs (septic shock and subdural hematoma), both deemed unrelated to cemsidomide Data cutoff: 10/11/24 • 1 DLT (Grade 4 neutropenia lasting >7 days at the 62.5 µg dose level) • No TEAEs lead to dose reductions • TEAEs leading to dose interruption: 32% (15/47) • TEAEs leading to discontinuation1: 4% (2/47) 1Primary reason of discontinuation of patient at 37.5 µg was due to withdrawal of consent; primary reason of discontinuation of patient at 75 µg was due to death unrelated to cemsidomide. Adverse events (AEs); dose limiting toxicity (DLT); treatment emergent adverse events (TEAEs)
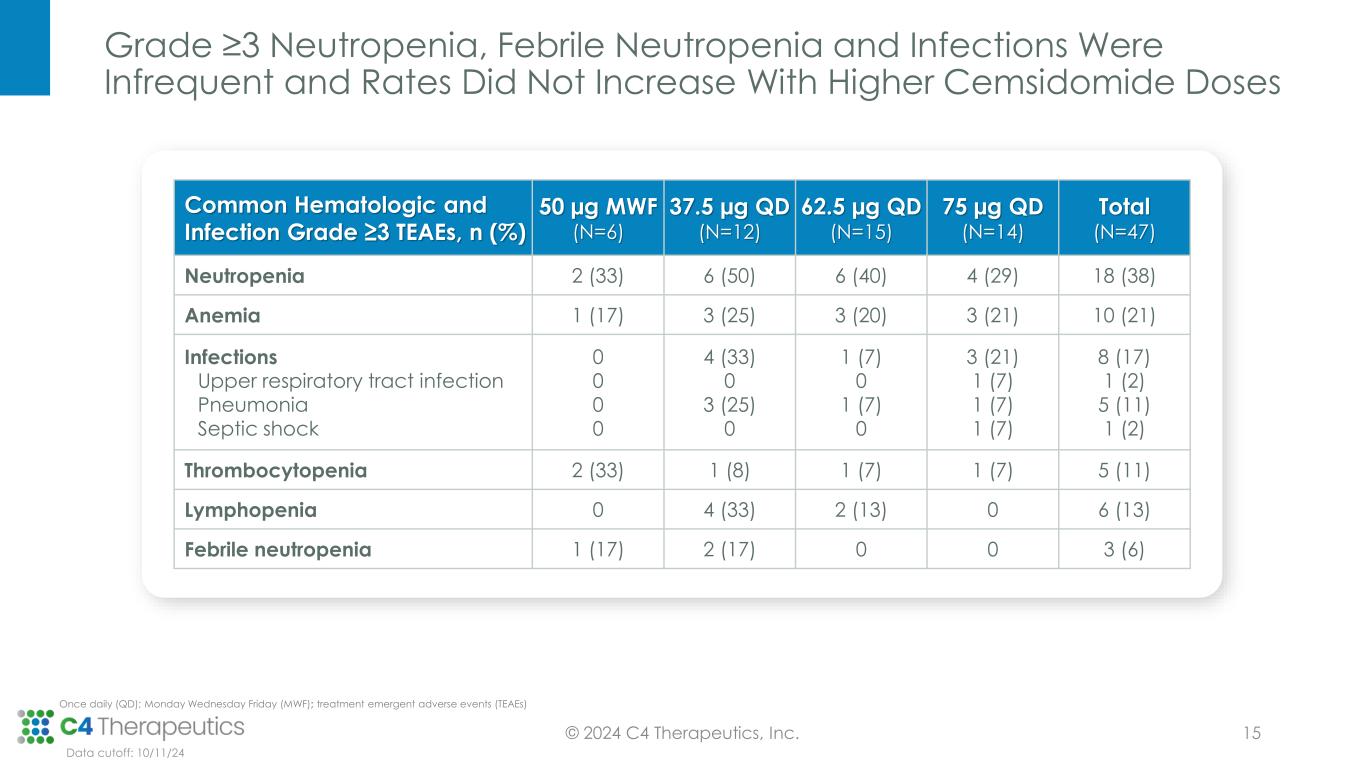
Grade ≥3 Neutropenia, Febrile Neutropenia and Infections Were Infrequent and Rates Did Not Increase With Higher Cemsidomide Doses © 2024 C4 Therapeutics, Inc. 15 Common Hematologic and Infection Grade ≥3 TEAEs, n (%) 50 µg MWF (N=6) 37.5 µg QD (N=12) 62.5 µg QD (N=15) 75 µg QD (N=14) Total (N=47) Neutropenia 2 (33) 6 (50) 6 (40) 4 (29) 18 (38) Anemia 1 (17) 3 (25) 3 (20) 3 (21) 10 (21) Infections Upper respiratory tract infection Pneumonia Septic shock 0 0 0 0 4 (33) 0 3 (25) 0 1 (7) 0 1 (7) 0 3 (21) 1 (7) 1 (7) 1 (7) 8 (17) 1 (2) 5 (11) 1 (2) Thrombocytopenia 2 (33) 1 (8) 1 (7) 1 (7) 5 (11) Lymphopenia 0 4 (33) 2 (13) 0 6 (13) Febrile neutropenia 1 (17) 2 (17) 0 0 3 (6) Data cutoff: 10/11/24 Once daily (QD); Monday Wednesday Friday (MWF); treatment emergent adverse events (TEAEs)
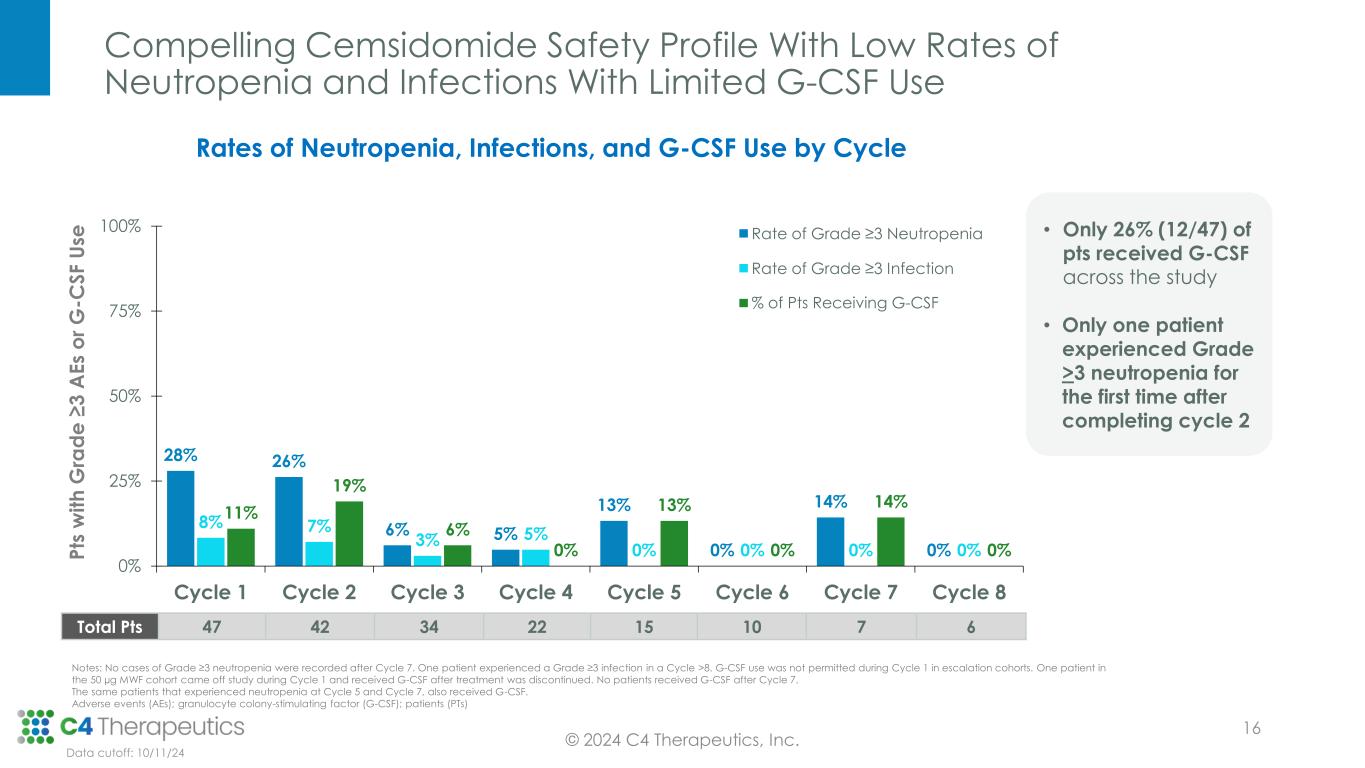
Compelling Cemsidomide Safety Profile With Low Rates of Neutropenia and Infections With Limited G-CSF Use 28% 26% 6% 5% 13% 0% 14% 0% 8% 7% 3% 5% 0% 0% 0% 0% 11% 19% 6% 0% 13% 0% 14% 0% 0% 25% 50% 75% 100% Cycle 1 Cycle 2 Cycle 3 Cycle 4 Cycle 5 Cycle 6 Cycle 7 Cycle 8 P ts w it h G ra d e ≥ 3 A E s o r G -C S F U se Rate of Grade ≥3 Neutropenia Rate of Grade ≥3 Infection % of Pts Receiving G-CSF • Only 26% (12/47) of pts received G-CSF across the study • Only one patient experienced Grade >3 neutropenia for the first time after completing cycle 2 Rates of Neutropenia, Infections, and G-CSF Use by Cycle Data cutoff: 10/11/24 © 2024 C4 Therapeutics, Inc. Total Pts 47 42 34 22 15 10 7 6 Notes: No cases of Grade ≥3 neutropenia were recorded after Cycle 7. One patient experienced a Grade ≥3 infection in a Cycle >8. G-CSF use was not permitted during Cycle 1 in escalation cohorts. One patient in the 50 µg MWF cohort came off study during Cycle 1 and received G-CSF after treatment was discontinued. No patients received G-CSF after Cycle 7. The same patients that experienced neutropenia at Cycle 5 and Cycle 7, also received G-CSF. Adverse events (AEs); granulocyte colony-stimulating factor (G-CSF); patients (PTs) 16

Across Doses, 40% (14/35) of Multiple Myeloma Patients With Elevated Light Chains Demonstrated at Least a 50% Decrease in dFLC 17© 2024 C4 Therapeutics, Inc Best Change in dFLC from Baseline (Cemsidomide + Dex) Multiple Myeloma Patients w/ Elevated Light Chain Disease (N=35)* Data cutoff: 10/11/24 0 6 12 18 24 30 36 42 48 0.00 0.05 0.10 0.15 0.20 0.25 Time (h) P la s m a P K ( n g /m L ) 62.5 μg QD (N=8) 37.5 μg QD (N=11) 50 μg MWF (N=6) 75 μg QD (N=7) • Overall geometric mean half-life estimate is approximately 2 days Dose Proportional Exposure • 69% (24/35) of patients with elevated light chain disease demonstrated a decrease in dFLC *Only included treated patients who meet both criterion (A) and (B). (A) baseline kappa free light chain value >19.4 mg/L or baseline lambda free light chain value >26.3 mg/L. (B) ratio of baseline free light chain kappa over baseline free light chain value lambda >4:1 or <1:2. Difference in involved and uninvolved free light chain (dFLC); once daily (QD); Monday Wednesday Friday (MWF); multiple myeloma (MM); pharmacokinetic (PK)

Exposure (AUC) Quartiles <Q1 (N=9) Q1-Q2 (N=8) Q2-Q3 (N=8) >Q3 (N=9) Mean AUC0-28d (ng*h/mL) 14.6 28.8 37.9 65.2 Mean Change in dFLC from Baseline +10% -12% -20% -53% Cemsidomide + Dex Dose ~17 μg QD ~35 μg QD ~45 μg QD ~78 μg QD 18© 2024 C4 Therapeutics, Inc. Data cutoff: 10/11/24 Cemsidomide 75 µg Dose Level or Greater Drives Sufficient Exposure Resulting in Meaningful Reductions in Light Chains 0 20 40 60 80 100 -100% -50% 0% 50% 100% 150% 200% PopPK-derived AUC0-28d (ngh/mL) % d F L C C h n a g e f ro m B a s e li n e 50 μg MWF (N=5) 37.5 μg QD (N=11) 62.5 μg QD (N=11) 75 μg QD (N=7) Emax Model Fit Cemsidomide + Dex PK Exposure vs. dFLC Change N=34 with abnormal baseline sFLC defined as (A) kappa FLC >19.4 mg/L or lambda FLC >26.3 mg/L and (B) kappa-to-lambda FLC ratio >4 or <0.5. Cemsidomide dose was back-calculated based on the population PK model. Area under the curve (AUC); difference in involved and uninvolved free light chain (dFLC); maximum response (Emax); Monday Wednesday Friday (MWF); once daily (QD); population pharmacokinetics (popPK); pharmacokinetic (PK)

a Cemsidomide Demonstrated Anti-Myeloma Activity Across Dose Levels 19© 2024 C4 Therapeutics, Inc. Data cutoff: 10/11/24 As of the data cutoff: • 26% ORR and 40% clinical benefit rate across all dose levels evaluated • At the two highest dose levels evaluated to date (62.5 μg and 75 μg), 62% of all patients remain on treatment1 b 50 MWF 37.5 QD 62.5 QD 75 QD a1 patient in the 37.5 µg cohort achieved a PR based on light chains, no follow up M protein available; 1 patient in the 62.5 µg cohort had an unconfirmed PR as of the data cutoff date; 1 patient in the 75 µg cohort had an unconfirmed PR as of the data cutoff date. b Patient came off study due to unrelated death. 1 Includes all 47 patients, including only safety evaluable patients. Complete response (CR); minimal response (MR); Monday Wednesday Friday (MWF); non-evaluable (NE); once daily (QD); partial response (PR); progressive disease (PD); stable disease (SD); stringent complete response (sCR); T-cell-engaging antibodies (TCE); very good partial response (VGPR); Clinical Benefit Rate (≥ MR) (CBR)
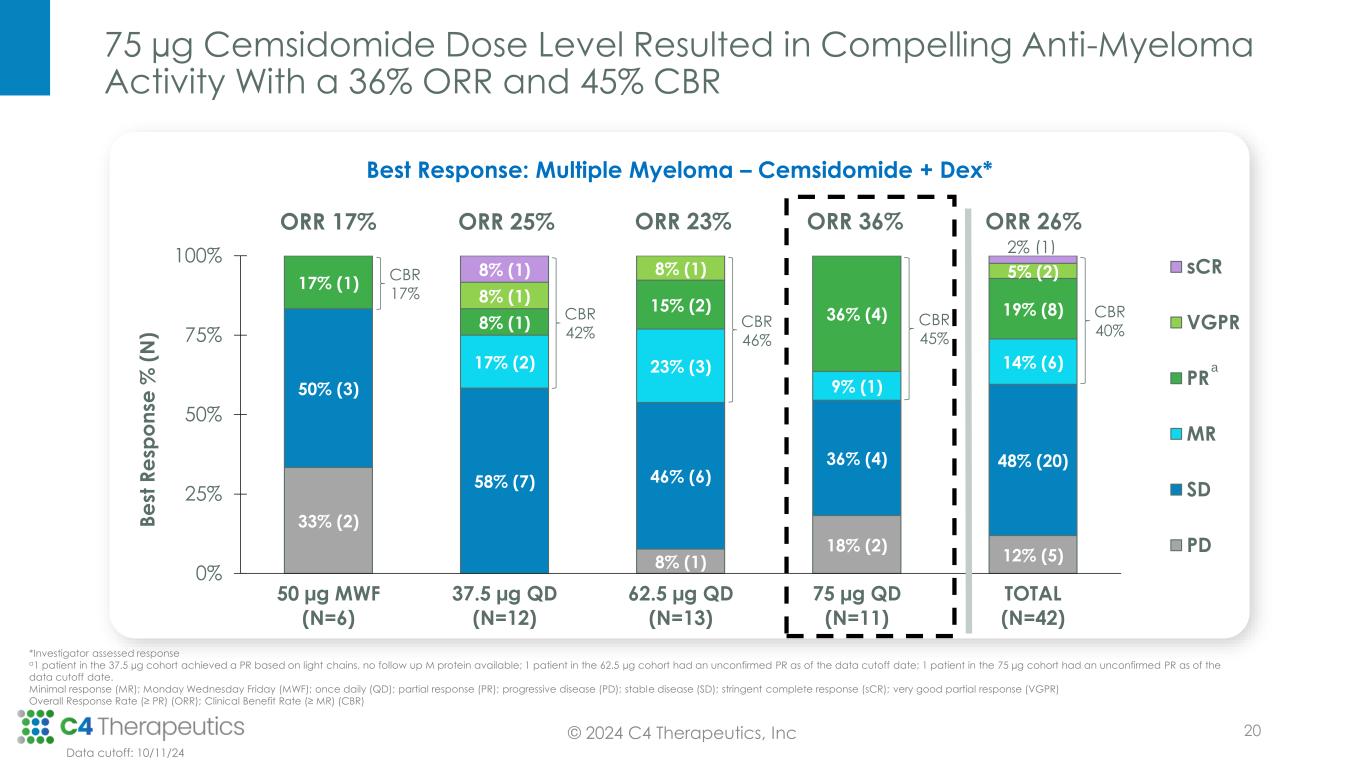
75 µg Cemsidomide Dose Level Resulted in Compelling Anti-Myeloma Activity With a 36% ORR and 45% CBR 20 33% (2) 8% (1) 18% (2) 12% (5) 50% (3) 58% (7) 46% (6) 36% (4) 48% (20) 17% (2) 23% (3) 9% (1) 14% (6) 17% (1) 8% (1) 15% (2) 36% (4) 19% (8) 8% (1) 8% (1) 5% (2)8% (1) 2% (1) 0% 25% 50% 75% 100% 50 µg MWF (N=6) 37.5 µg QD (N=12) 62.5 µg QD (N=13) 75 µg QD (N=11) TOTAL (N=42) B e st R e sp o n se % ( N ) sCR VGPR PR MR SD PD CBR 17% CBR 42% CBR 46% ORR 17% ORR 25% ORR 23% ORR 36% ORR 26% CBR 40% Best Response: Multiple Myeloma – Cemsidomide + Dex* a Data cutoff: 10/11/24 CBR 45% © 2024 C4 Therapeutics, Inc *Investigator assessed response a1 patient in the 37.5 µg cohort achieved a PR based on light chains, no follow up M protein available; 1 patient in the 62.5 µg cohort had an unconfirmed PR as of the data cutoff date; 1 patient in the 75 µg cohort had an unconfirmed PR as of the data cutoff date. Minimal response (MR); Monday Wednesday Friday (MWF); once daily (QD); partial response (PR); progressive disease (PD); stable disease (SD); stringent complete response (sCR); very good partial response (VGPR) Overall Response Rate (≥ PR) (ORR); Clinical Benefit Rate (≥ MR) (CBR)

Cemsidomide’s Differentiated Safety Profile Is Well Suited for Combination Studies, Potentially Resulting in Fewer Disruptive Adverse Events © 2024 C4 Therapeutics, Inc. 21 Cemsidomide + Dex Key Grade ≥3 Adverse Events 23% 22% 48% 54% 9% 15% 40% 35% 0% 25% 50% 75% 100% Dose Escalation - All Doses (N=77) Phase 2 Dose Expansion* (N=101) G ra d e ≥ 3 A d v e rs e E v e n ts Data cutoff: 10/11/24 13% 26% 6% 17% 0% 25% 50% 75% 100% Dose Escalation - Up to 75 µg (N=47) G ra d e ≥ 3 A d v e rs e E v e n ts Cross trial comparisons only to be used as benchmarks for relative comparison 1Richardson 2023 NEJM. *Mezigdomide recommended Phase 2 dose expansion dose was 1.0 mg daily dosing (QD) 21 days on/7 days off. Note: All cases of febrile neutropenia in the cemsidomide trial were Grade 3. In the mezigdomide dose escalation and expansion cohorts, febrile neutropenia splits between Grade 3/4 were 5%/4% and 13%/2% ,respectively. Mezigdomide + Dex1 Key Grade ≥3 Adverse Events Grade 3 Neutropenia Grade 4 Neutropenia Febrile Neutropenia Grade ≥3 Infections 26% of patients received G-CSF 77% of patients received G-CSF

Cemsidomide + dex was well-tolerated with a compelling safety profile – 38% of patients experienced Grade 3/4 neutropenia, which was manageable, and no cases resulted in cemsidomide discontinuation Cemsidomide Has the Potential to Be a Backbone Therapy of Choice Where IKZF1/3 Degradation Is Warranted Data cutoff: 10/11/24 © 2024 C4 Therapeutics, Inc. Cemsidomide is well suited for further development across treatment lines and in combination with other anti-myeloma agents Cemsidomide + dex demonstrated compelling anti-myeloma activity across a broad range of doses, highlighting a wide therapeutic range – 36% ORR at the highest dose level evaluated to date (75 µg QD) – 26% ORR across all dose levels 75 μg is a target dose for various + dex regimens with potential for higher doses to also be considered for development as dose escalation continues – For immune-based combination strategies, doses lower than 75 μg are optimal based on anti- myeloma activity and immune activation observed in the monotherapy data set1 1Monotherapy cemsidomide dataset in multiple myeloma was presented in December 2023 Once daily (QD); overall response rate (ORR) 22
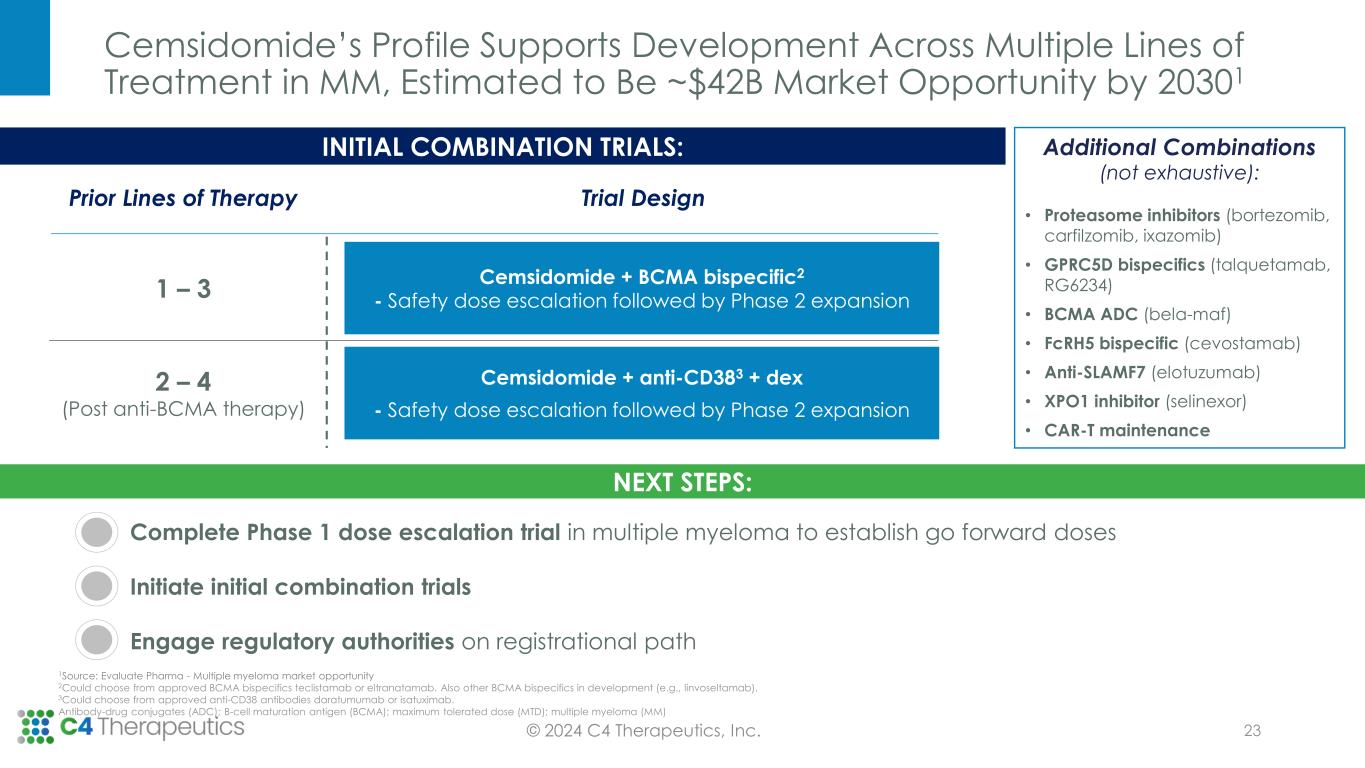
NEXT STEPS: Complete Phase 1 dose escalation trial in multiple myeloma to establish go forward doses Initiate initial combination trials Engage regulatory authorities on registrational path © 2024 C4 Therapeutics, Inc. INITIAL COMBINATION TRIALS: Trial DesignPrior Lines of Therapy Cemsidomide + BCMA bispecific2 - Safety dose escalation followed by Phase 2 expansion Cemsidomide + anti-CD383 + dex - Safety dose escalation followed by Phase 2 expansion 1 – 3 2 – 4 (Post anti-BCMA therapy) Additional Combinations (not exhaustive): • Proteasome inhibitors (bortezomib, carfilzomib, ixazomib) • GPRC5D bispecifics (talquetamab, RG6234) • BCMA ADC (bela-maf) • FcRH5 bispecific (cevostamab) • Anti-SLAMF7 (elotuzumab) • XPO1 inhibitor (selinexor) • CAR-T maintenance 1Source: Evaluate Pharma - Multiple myeloma market opportunity 2Could choose from approved BCMA bispecifics teclistamab or eltranatamab. Also other BCMA bispecifics in development (e.g., linvoseltamab). 3Could choose from approved anti-CD38 antibodies daratumumab or isatuximab. Antibody-drug conjugates (ADC); B-cell maturation antigen (BCMA); maximum tolerated dose (MTD); multiple myeloma (MM) Cemsidomide’s Profile Supports Development Across Multiple Lines of Treatment in MM, Estimated to Be ~$42B Market Opportunity by 20301 23

Non-Hodgkin’s Lymphoma (NHL) Monotherapy Cemsidomide

U.S. Annual Incidence (2023)1 ~26,000 ~15,000 ~5,000 ~4,000 ~5,000 Lenalidomide FDA Approved2 Lenalidomide in NCCN Guidelines 25 DLBCL Diffuse Large B-Cell Lymphoma FL Follicular Lymphoma MZL Marginal Zone Lymphoma MCL Mantle Cell Lymphoma PTCL Subtypes Peripheral T-Cell Lymphoma B-Cell Lymphomas T-Cell Lymphomas Cemsidomide Opportunity • IKZF1/3 degraders (e.g., lenalidomide) are widely used across NHL subtypes • Cemsidomide has the potential to be developed as a monotherapy in the R/R setting and in combination with frontline standard of care regimens © 2024 C4 Therapeutics, Inc. 1SEER, American Cancer Society, Lymphoma Research Foundation. 2FL, MZL, and MCL FDA approved in the Revlimid (lenalidomide) label and DLBCL approved in the Monjuvi (tafasitamab) label. U.S. Food and Drug Administration (FDA); National Comprehensive Cancer Network (NCCN); non-Hodgkin’s lymphoma (NHL); relapsed refractory (R/R) Cemsidomide Has the Potential to Be Developed Across NHL Subtypes and Lines of Treatment
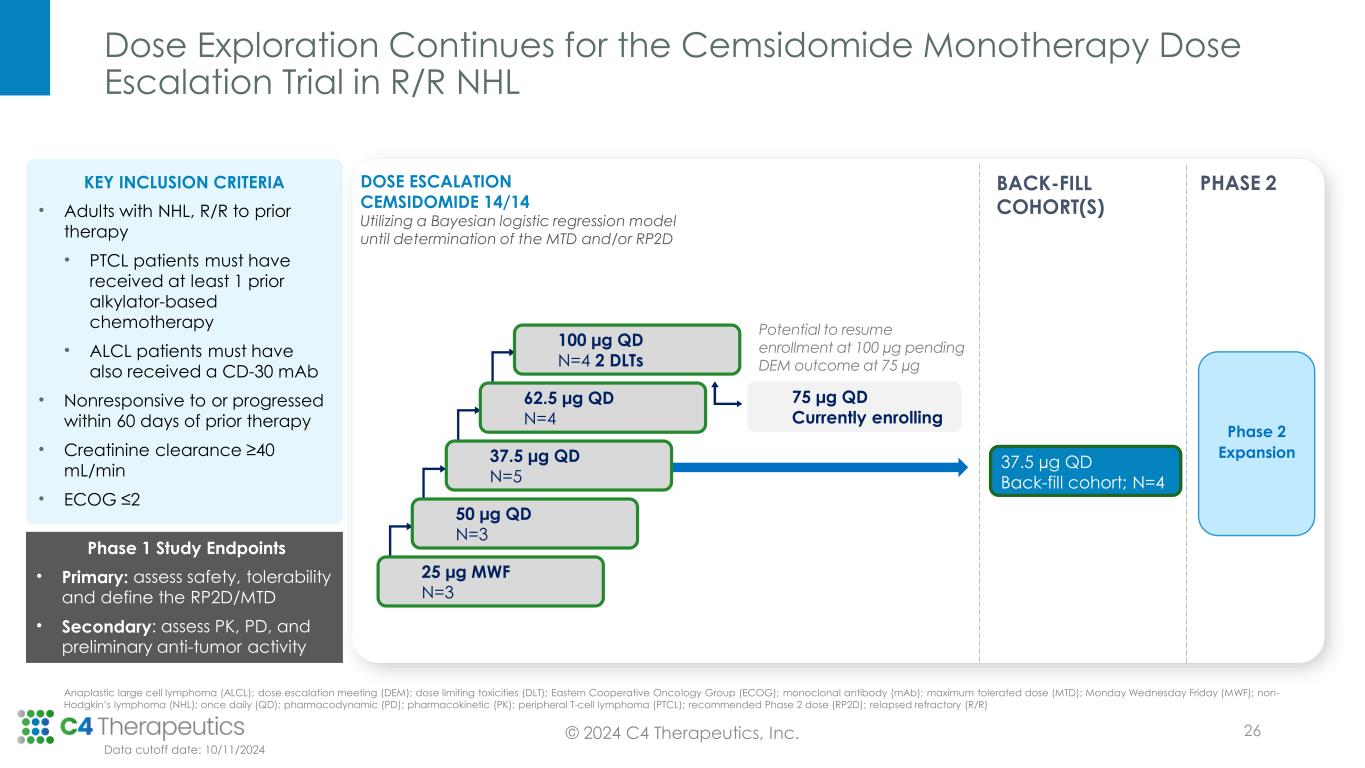
Dose Exploration Continues for the Cemsidomide Monotherapy Dose Escalation Trial in R/R NHL KEY INCLUSION CRITERIA • Adults with NHL, R/R to prior therapy • PTCL patients must have received at least 1 prior alkylator-based chemotherapy • ALCL patients must have also received a CD-30 mAb • Nonresponsive to or progressed within 60 days of prior therapy • Creatinine clearance ≥40 mL/min • ECOG ≤2 Phase 1 Study Endpoints • Primary: assess safety, tolerability and define the RP2D/MTD • Secondary: assess PK, PD, and preliminary anti-tumor activity DOSE ESCALATION CEMSIDOMIDE 14/14 Utilizing a Bayesian logistic regression model until determination of the MTD and/or RP2D 25 µg MWF N=3 50 µg QD N=3 62.5 µg QD N=4 37.5 µg QD N=5 Potential to resume enrollment at 100 µg pending DEM outcome at 75 µg 100 µg QD N=4 2 DLTs BACK-FILL COHORT(S) 75 µg QD Currently enrolling 37.5 µg QD Back-fill cohort; N=4 Phase 2 Expansion PHASE 2 © 2024 C4 Therapeutics, Inc. Data cutoff date: 10/11/2024 Anaplastic large cell lymphoma (ALCL); dose escalation meeting (DEM); dose limiting toxicities (DLT); Eastern Cooperative Oncology Group (ECOG); monoclonal antibody (mAb); maximum tolerated dose (MTD); Monday Wednesday Friday (MWF); non- Hodgkin’s lymphoma (NHL); once daily (QD); pharmacodynamic (PD); pharmacokinetic (PK); peripheral T-cell lymphoma (PTCL); recommended Phase 2 dose (RP2D); relapsed refractory (R/R) 26

Heavily Pre-Treated Population Where Majority of Patients Were Diagnosed With PTCL, Reflecting High Unmet Need 27 Data cutoff: 10/11/24 © 2024 C4 Therapeutics, Inc. Characteristics Safety Population (N=23) Age, median (range) 68 (28-85 years) Male, n (%) 14 (61) Years since initial diagnosis, median (range) 2 (0.4-21) ECOG performance status, n (%) 0 1 2 Missing 11 (48) 9 (39) 2 (9) 1 (4) Black or African American, n (%) White, n (%) Other, n (%) 6 (26) 13 (57) 4 (17) IPI at screening, n (%) 1 2 3 4 Missing 2 (9) 6 (26) 7 (30) 3 (13) 5 (22) Characteristics Safety Population (N=23) Prior therapies, median (range) 1 2 3 ≥4 3 (1-14) 2 (9) 7 (30) 3 (13) 11 (48) PTCL, n (%) PTCL-NOS AITL ALCL ATLL 17 (74) 5 (22) 4 (17) 3 (13) 5 (22) B-cell lymphoma, n (%) DLBCL MCL MZL/MALT 6 (26) 4 (17) 1 (4) 1 (4) Prior CAR-T therapy, n (%) 4 (17) Prior HCT, n (%) Autologous Allogenic 4 (17) 3 (13) 1(4) Angioimmunoblastic T-cell lymphoma (AITL); anaplastic large cell lymphoma (ALCL); adult T-cell leukemia/lymphoma (ATLL); diffuse large B-Cell lymphoma (DLBCL); Eastern cooperative oncology group (ECOG); hematopoietic cell transplantation (HCT); International Prognostic Index (IPI); mantle cell lymphoma (MCL); marginal zone lymphoma/mucosa-assisted lymphoid tissue (MZL/MALT); peripheral T-cell lymphoma (PTCL); PTCL-not otherwise specified (PTCL-NOS)
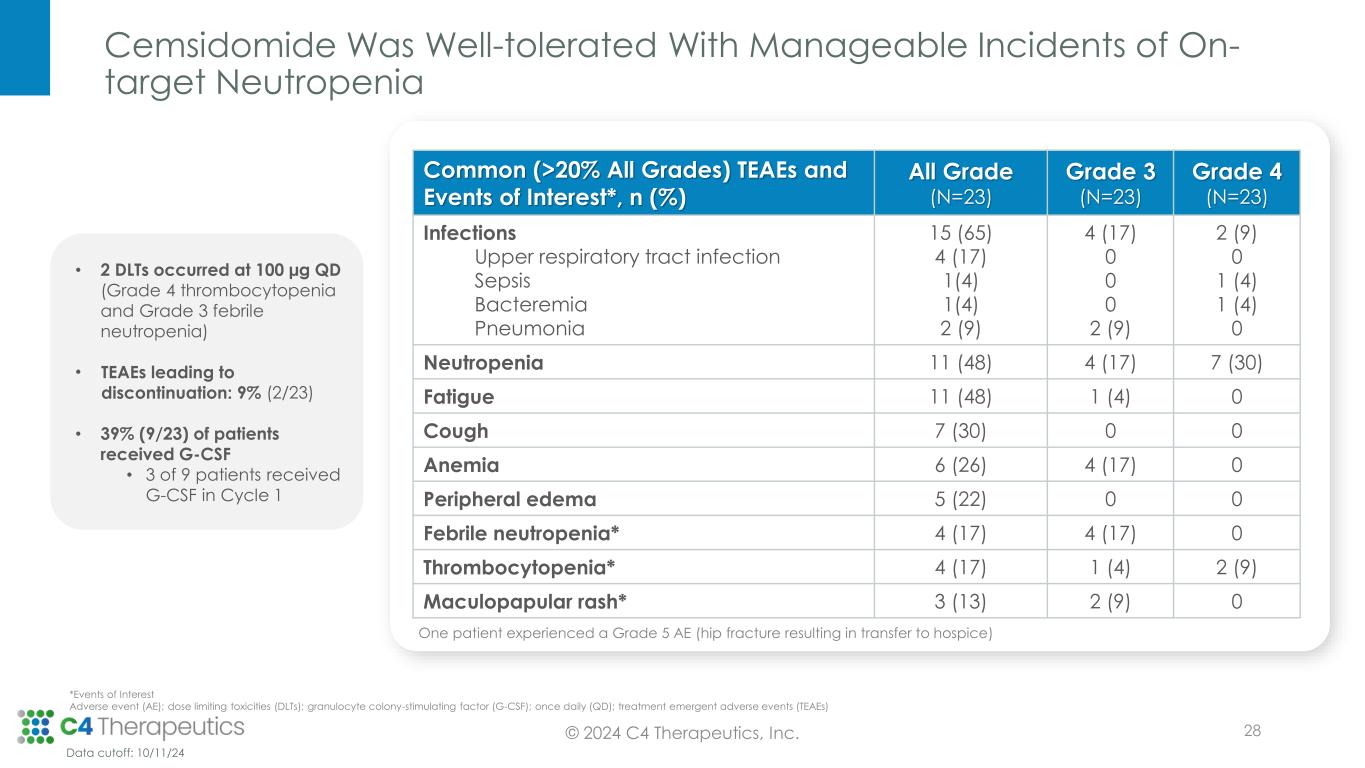
Cemsidomide Was Well-tolerated With Manageable Incidents of On- target Neutropenia Common (>20% All Grades) TEAEs and Events of Interest*, n (%) All Grade (N=23) Grade 3 (N=23) Grade 4 (N=23) Infections Upper respiratory tract infection Sepsis Bacteremia Pneumonia 15 (65) 4 (17) 1(4) 1(4) 2 (9) 4 (17) 0 0 0 2 (9) 2 (9) 0 1 (4) 1 (4) 0 Neutropenia 11 (48) 4 (17) 7 (30) Fatigue 11 (48) 1 (4) 0 Cough 7 (30) 0 0 Anemia 6 (26) 4 (17) 0 Peripheral edema 5 (22) 0 0 Febrile neutropenia* 4 (17) 4 (17) 0 Thrombocytopenia* 4 (17) 1 (4) 2 (9) Maculopapular rash* 3 (13) 2 (9) 0 One patient experienced a Grade 5 AE (hip fracture resulting in transfer to hospice) • 2 DLTs occurred at 100 µg QD (Grade 4 thrombocytopenia and Grade 3 febrile neutropenia) • TEAEs leading to discontinuation: 9% (2/23) • 39% (9/23) of patients received G-CSF • 3 of 9 patients received G-CSF in Cycle 1 © 2024 C4 Therapeutics, Inc. Data cutoff: 10/11/24 *Events of Interest Adverse event (AE); dose limiting toxicities (DLTs); granulocyte colony-stimulating factor (G-CSF); once daily (QD); treatment emergent adverse events (TEAEs) 28

Cemsidomide Monotherapy Adverse Events by Dose Level Common Grade ≥3 TEAEs, n (%) 25 µg MWF (N=3) 50 µg MWF (N=3) 37.5 µg QD (N=9) 62.5 µg QD (N=4) 100 µg QD (N=4) Total (N=23) Neutropenia 0 0 5 (56) 4 (100) 2 (50) 11 (48) Infections Pneumonia Sepsis Urinary tract infection Bacteremia Skin infection 0 0 0 0 0 0 0 0 0 0 0 0 3 (33) 1 (11) 1 (11) 1 (11) 0 0 0 0 0 0 0 0 3 (75) 1 (25) 0 0 1 (25) 1 (25) 6 (26) 2 (9) 1 (4) 1 (4) 1 (4) 1 (4) Anemia 0 0 3 (33) 0 1 (25) 4 (17) Febrile neutropenia 0 0 1 (11) 1 (25) 2 (50) 4 (17) Thrombocytopenia 0 0 1 (11) 0 2 (50) 3 (13) Maculopapular rash 0 0 1 (11) 1 (25) 0 2 (9) © 2024 C4 Therapeutics, Inc. Data cutoff: 10/11/24 Once daily (QD); Monday Wednesday Friday (MWF); Treatment Emergent Adverse Events (TEAEs) 29
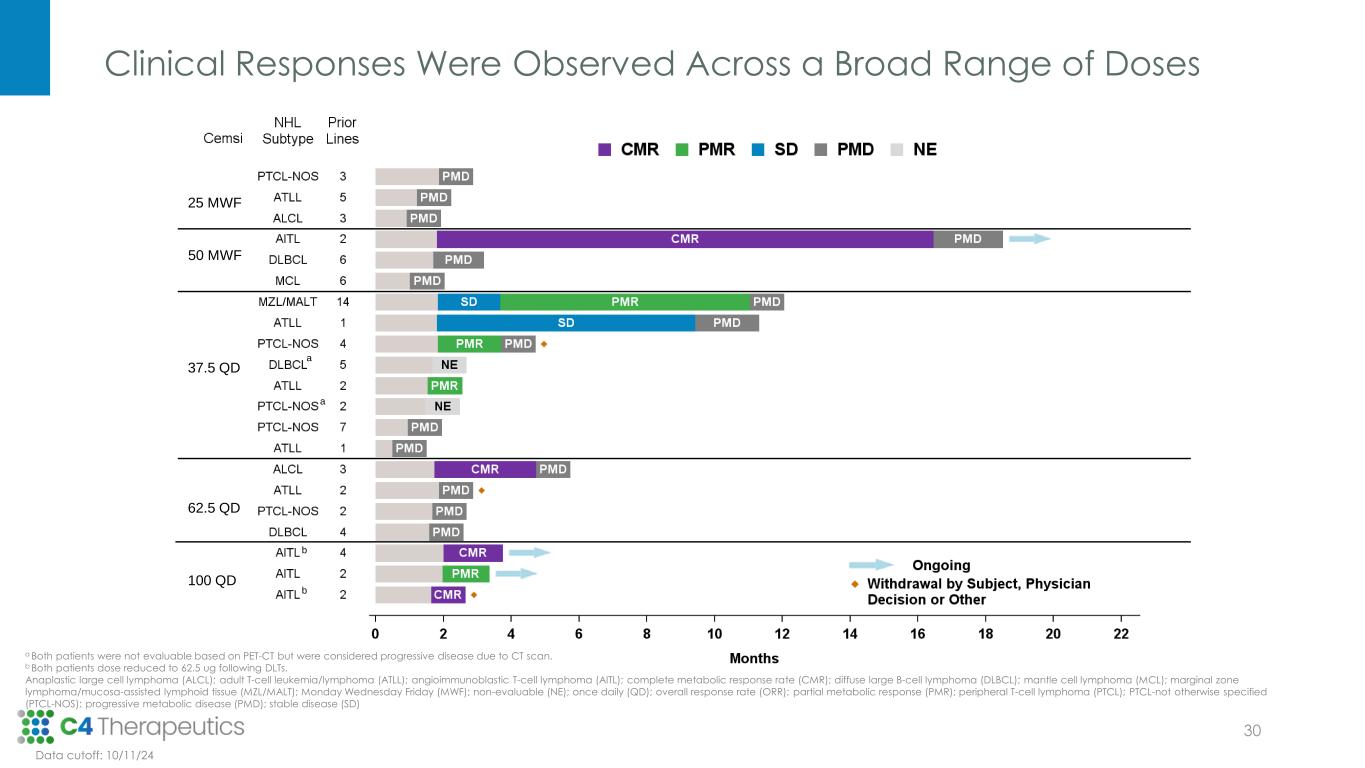
Clinical Responses Were Observed Across a Broad Range of Doses a a b b a Both patients were not evaluable based on PET-CT but were considered progressive disease due to CT scan. b Both patients dose reduced to 62.5 ug following DLTs. Anaplastic large cell lymphoma (ALCL); adult T-cell leukemia/lymphoma (ATLL); angioimmunoblastic T-cell lymphoma (AITL); complete metabolic response rate (CMR); diffuse large B-cell lymphoma (DLBCL); mantle cell lymphoma (MCL); marginal zone lymphoma/mucosa-assisted lymphoid tissue (MZL/MALT); Monday Wednesday Friday (MWF); non-evaluable (NE); once daily (QD); overall response rate (ORR); partial metabolic response (PMR); peripheral T-cell lymphoma (PTCL); PTCL-not otherwise specified (PTCL-NOS); progressive metabolic disease (PMD); stable disease (SD) Data cutoff: 10/11/24 b b a a 25 MWF 50 MWF 37.5 QD 62.5 QD 100 QD 30

Compelling and Deep Responses Achieved Across PTCL Subtypes 19% (1) 25% (1) 20% (1) 20% (1) 25% (3) 75% (3) 50% (1) 0% 25% 50% 75% 100% All PTCL (N=16) AITL (N=4) ALCL (N=2) ATLL (N=5) PTCL-NOS (N=5) B e st R e sp o n se % ( N ) CMR PMR PET-CT–based Assessment of PMR or Better by PTCL Subtype* (N=16) ORR 20% ORR 20% ORR 100% ORR 50% © 2024 C4 Therapeutics, Inc. Data cutoff: 10/11/24 ORR 44% *Investigator assessed response; 2 patients were evaluated based on CT scan and were PD but not evaluable based on PET-CT, both patients are included as PMD for PET-CT based assessment; 2 additional subjects that came off study prior to follow up scans were not considered efficacy evaluable. Angioimmunoblastic T-cell lymphoma (AITL); anaplastic large cell lymphoma (ALCL); adult T-cell lymphoma (ATLL); complete metabolic response (CMR); overall response rate (ORR); partial metabolic response (PMR); peripheral T-cell lymphoma (PTCL); peripheral T-cell lymphoma not-otherwise specified (PTCL-NOS) • Cemsidomide monotherapy produced responses in all four PTCL subtypes • All AITL patients (4/4) experienced a metabolic response 31

32© 2024 C4 Therapeutics, Inc. Data cutoff: 10/11/24 Bayesian logistic regression model (BLRM); complete metabolic response (CMR); dose limiting toxicities (DLTs); overall response rate (ORR); peripheral t-cell lymphoma (PTCL) Cemsidomide was well-tolerated with additional dose finding ongoing – 2 DLTs at 100 µg (Grade 4 thrombocytopenia) with enrollment at 75 µg currently ongoing – Per BLRM, maximum tolerated dose not yet exceeded – Grade 3/4 neutropenia cases were manageable with no cases resulting in discontinuation Profile supports cemsidomide’s development as a monotherapy in relapsed refractory settings and potentially in combination in NHL subtypes across treatment lines Cemsidomide Is Well-suited for Further Development in Earlier Lines of Treatment and in Combination Cemsidomide as a single agent demonstrated compelling anti-lymphoma activity across a broad range of doses in PTCL patients, suggesting a wide therapeutic index – 44% ORR was observed in PTCL with a 25% CMR rate

Cemsidomide Profile Supports Development Across Multiple Lines of Treatment in NHL, Estimated to Be ~$30B Market Opportunity by 20301 33© 2024 C4 Therapeutics, Inc. NEXT STEPS: Complete Phase 1 dose escalation trial in NHL and identify go forward dose Initiate expansion cohort for PTCL Engage regulatory authorities on registrational path 1Source: Evaluate Pharma – NHL Market Opportunity: Non-Hodgkin’s lymphoma (NHL); peripheral T-cell lymphoma (PTCL)
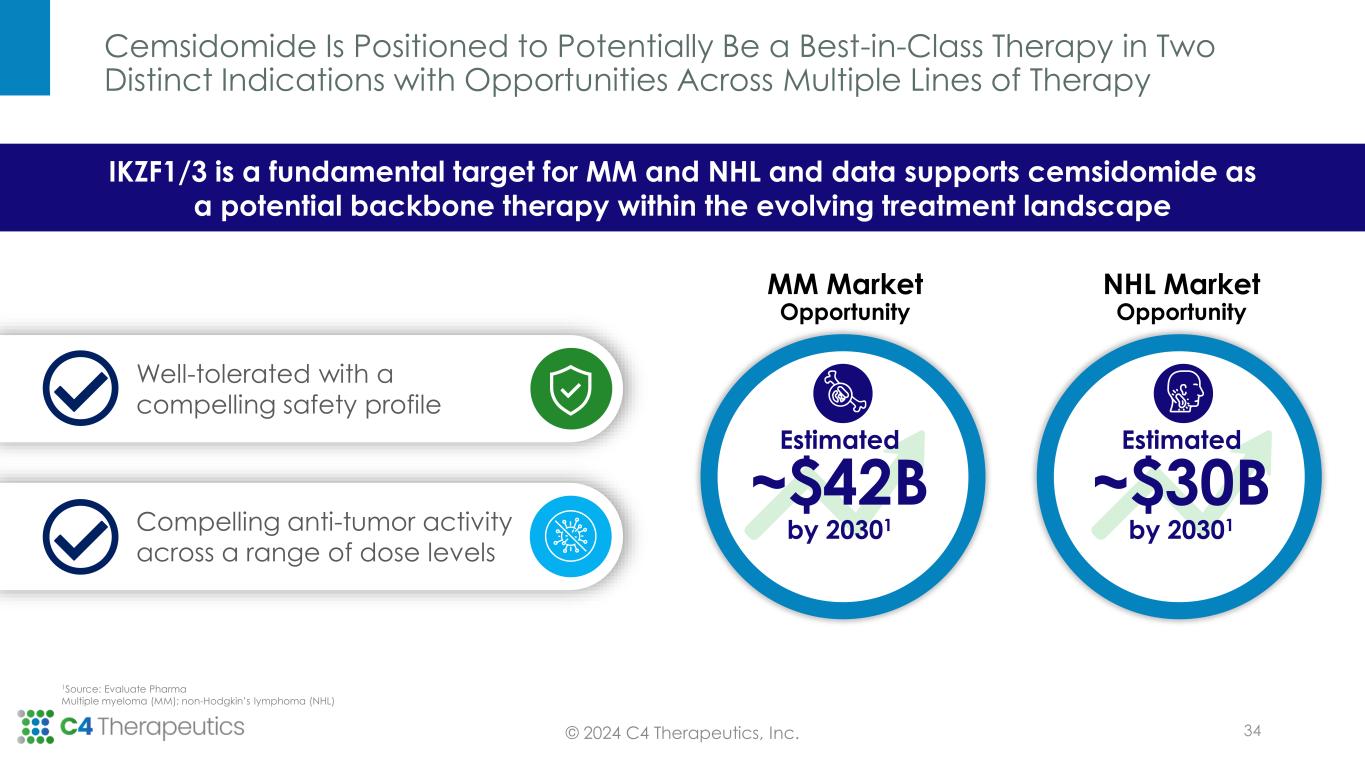
Cemsidomide Is Positioned to Potentially Be a Best-in-Class Therapy in Two Distinct Indications with Opportunities Across Multiple Lines of Therapy © 2024 C4 Therapeutics, Inc. IKZF1/3 is a fundamental target for MM and NHL and data supports cemsidomide as a potential backbone therapy within the evolving treatment landscape Compelling anti-tumor activity across a range of dose levels Well-tolerated with a compelling safety profile MM Market Opportunity Estimated ~$42B by 20301 NHL Market Opportunity Estimated ~$30B by 20301 34 1Source: Evaluate Pharma Multiple myeloma (MM); non-Hodgkin’s lymphoma (NHL)

Q&A


































