
Protein degraded. Disease targeted. Lives transformed. January 2025

Forward-looking Statements and Intellectual Property 2 FORWARD-LOOKING STATEMENTS The following presentation contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the therapeutic potential of C4 Therapeutics, Inc.’s technology and products. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, as well as the fact that the product candidates that we are developing or may develop may not demonstrate success in clinical trials. Prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The forward-looking statements included in this presentation are subject to a variety of risks and uncertainties, including those set forth in our most recent and future filings with the Securities and Exchange Commission. Our actual results could vary significantly from those anticipated in this presentation, and our financial condition and results of operations could be materially adversely affected. C4 Therapeutics, Inc. undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. This presentation also contains estimates, projections and other information concerning the markets for C4 Therapeutics, Inc.’s product candidates, including data regarding the estimated size of those markets, and the incidence and prevalence of certain medical conditions and patient use of medicines. Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject to uncertainties and actual events, and circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, the Company obtained this industry, business, market and other data from reports, research surveys, clinical trials studies and similar data prepared by market research firms and other third parties, from industry, medical and general publications, from other publicly available information, and from government data and similar sources. INTELLECTUAL PROPERTY C4 Therapeutics, Inc. owns various registered and unregistered trademarks and service marks in the U.S. and internationally, including, without limitation, C4 THERAPEUTICS, our housemark logo, the name of our TORPEDO platform, and the names of our BIDAC and MONODAC degrader products. All trademarks, service marks, or trade names referred to in this presentation that we do not own are the property of their respective owners. Solely for convenience, the trademarks, service marks, and trade names in this presentation are referred to without the symbols ®, SM and , but those references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights to. © 2025 C4 Therapeutics, Inc.
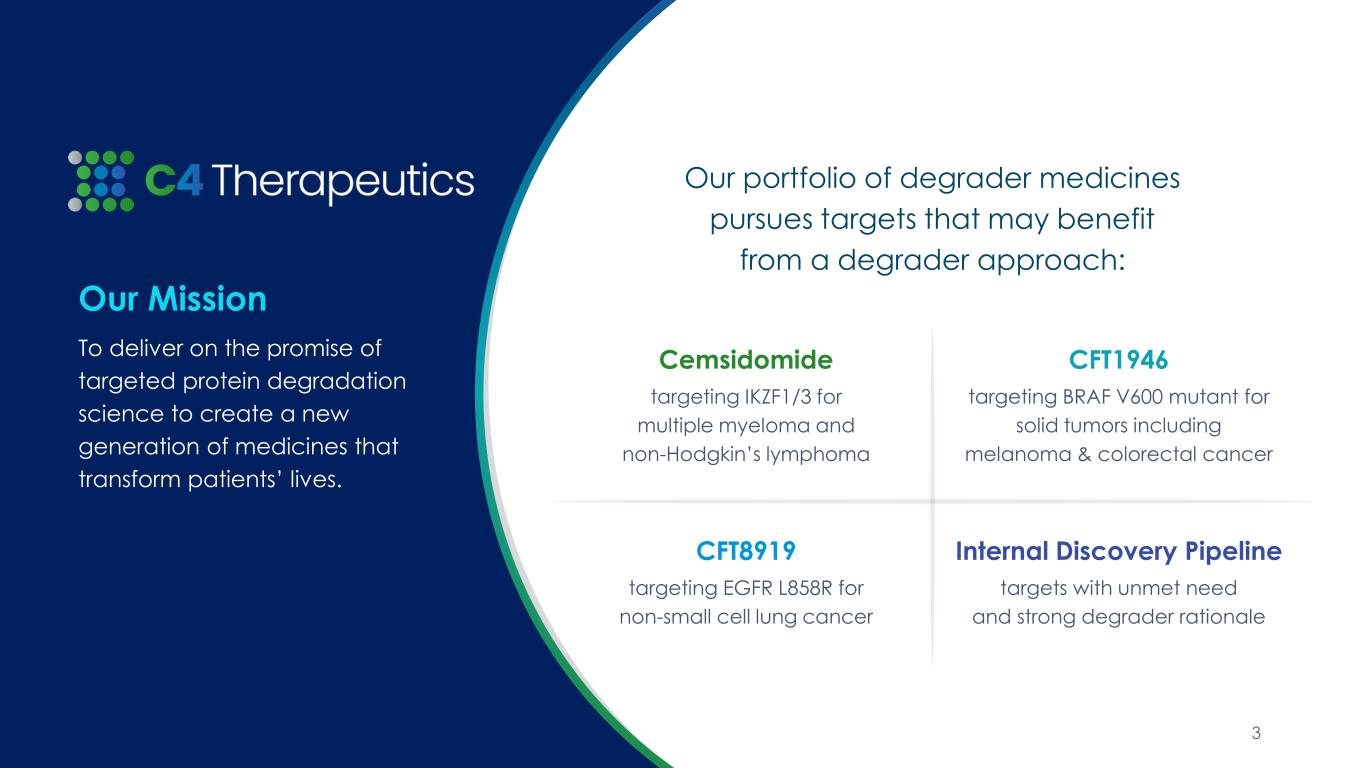
Our Mission To deliver on the promise of targeted protein degradation science to create a new generation of medicines that transform patients’ lives. Our portfolio of degrader medicines pursues targets that may benefit from a degrader approach: Cemsidomide targeting IKZF1/3 for multiple myeloma and non-Hodgkin’s lymphoma CFT8919 targeting EGFR L858R for non-small cell lung cancer CFT1946 targeting BRAF V600 mutant for solid tumors including melanoma & colorectal cancer Internal Discovery Pipeline targets with unmet need and strong degrader rationale 3
C4T Has Been at the Forefront of TPD Science and Is On the Path to Becoming a Fully Integrated Biotechnology Company © 2025 C4 Therapeutics, Inc. Built TORPEDO platform to design highly catalytic, orally bioavailable degraders Established collaborations with leading global pharmaceutical companies, building expertise across a range of diseases and target classes Assembled strong catalog of intellectual property Progressed four development candidates into clinical trials, including three trials underway today Delivered two development candidates to a collaborator for non-oncology targets Achieved blood-brain barrier penetration in several development candidates Advancing clinical programs to approval for patients with high unmet needs Leveraging TORPEDO platform to develop a sustainable pipeline against targets of high unmet need with strong degrader rationale in large commercial markets Expanding application of targeted protein degradation through high-value collaborations 4 Leading the Way in Designing Orally Bioavailable Degraders 2015 – 2020 Delivering on the Promise of Targeted Protein Degradation 2025 and beyond Demonstrating Proof of Concept 2020 – 2025

C4T Is Advancing a Portfolio of Rationally Designed Degraders Against Targets of High Unmet Need in Oncology TORPEDO platform has produced highly catalytic, brain penetrant, oral degraders across a broad range of target classes Degrader Rationale CFT8919 Targeting EGFR L858R Receptor Tyrosine Kinase CFT1946 Targeting BRAF V600X Scaffolding Kinase Cemsidomide Targeting IKZF1/3 Transcription Factor Degradation has the potential to avoid resistance mechanisms and enable a longer duration of response Clinical Progress ✓ Clinical trial initiated in Greater China1 Approved IKZF1/3 degraders were not rationally designed and provide less than optimal potency and selectivity 1License and collaboration agreement with Betta Pharmaceuticals for development and commercialization in Greater China 2 NCI SEER, consulting engagements with Health Advances and Clearview. 32024 Evaluate Ltd. US + EU4 + UK population 4 EvaluatePharma, consulting engagements with Health Advances and Clearview. Complete metabolic response (CMR); dexamethasone (dex); multiple myeloma (MM); Overall response rate (ORR); peripheral T-cell lymphoma (PTCL); Germany, Italy, France, and Spain (EU4); Standard of care (SOC) Data to date supports best-in-class profile: ✓ Differentiated safety profile ✓ Competitive ORR in combination with dex at 75 µg in MM ✓ Immune activity demonstrated as monotherapy ✓ Encouraging ORR and CMR rate in PTCLL Potential Patient Population Commercial Rights Data to date demonstrates: ✓ Proof of mechanism established ✓ Early signs of anti-tumor activity in Phase 1 dose escalation Across U.S., EU4 and UK: • MM: ~65,0002 • PTCL: ~16,0002 Across U.S., EU4 and UK: • Melanoma: ~66,0003 • Colorectal cancer: ~33,0003 Across U.S., EU4, UK and China: • EGFR L858R Mutated NSCLC: ~219,0004 © 2025 C4 Therapeutics, Inc. 5 Degradation facilitates targeting an allosteric L858R- specific binding site distinct from current inhibitors leading to increased selectivity over wild-type, activity against inhibitor resistance mutations & an opportunity for use in conjunction with current SOC

Multiple 2025 Data Based Inflection Points to Unlock Value Across Entire Portfolio © 2025 C4 Therapeutics, Inc. 6 Cemsidomide IKZF1/3 2025: Enable initiation of the next phase of clinical development for MM and PTCL. These new studies are expected to initiate in early 2026 2H 2025: Complete Phase 1 dose escalation trial in MM and NHL and present data 2H 2025: Open expansion cohort(s) in PTCL in the ongoing Phase 1/2 trial 1H 2025: Complete monotherapy Phase 1 dose escalation trial in BRAF V600 mutant solid tumors 2H 2025: Generate data from Phase 1 cohorts evaluating CFT1946 as a monotherapy in melanoma, in combination with trametinib in melanoma, and in combination with cetuximab in CRC to define and enable next phase of development 2H 2025: Present Phase 1 data: monotherapy in BRAF V600 mutant solid tumors; monotherapy expansion cohorts in melanoma; combination with cetuximab in colorectal cancer CFT8919 EGFR L858R Year-end 2025: Utilize data from Phase 1 dose escalation trial in Greater China to inform ex-China clinical development 2025: Present and publish preclinical work from internal pipeline and TORPEDO platform 2025: Advance internal and collaboration programs to key discovery milestonesDiscovery Multiple myeloma (MM); peripheral T-cell lymphoma (PTCL), a subtype of NHL CFT1946 BRAF V600 Mutant

PROGRAM TARGET INDICATIONS DISCOVERY PRECLINICAL EARLY DEVELOPMENT LATE DEVELOPMENT RIGHTS Cemsidomide IKZF1/3 Multiple Myeloma & Non-Hodgkin’s Lymphoma CFT1946 BRAF V600 Mutant V600 Mutant Cancers CFT89191 EGFR L858R Non-Small Cell Lung Cancer Discovery Stage Programs Various Cancers 1License and collaboration agreement with Betta Pharmaceuticals for development and commercialization in Greater China © 2025 C4 Therapeutics, Inc. 7 MM NHL CRC Melanoma Other BRAF V600 Mutant Cancers Advancing a Portfolio of Degrader Medicines Targeting Areas of High Unmet Need
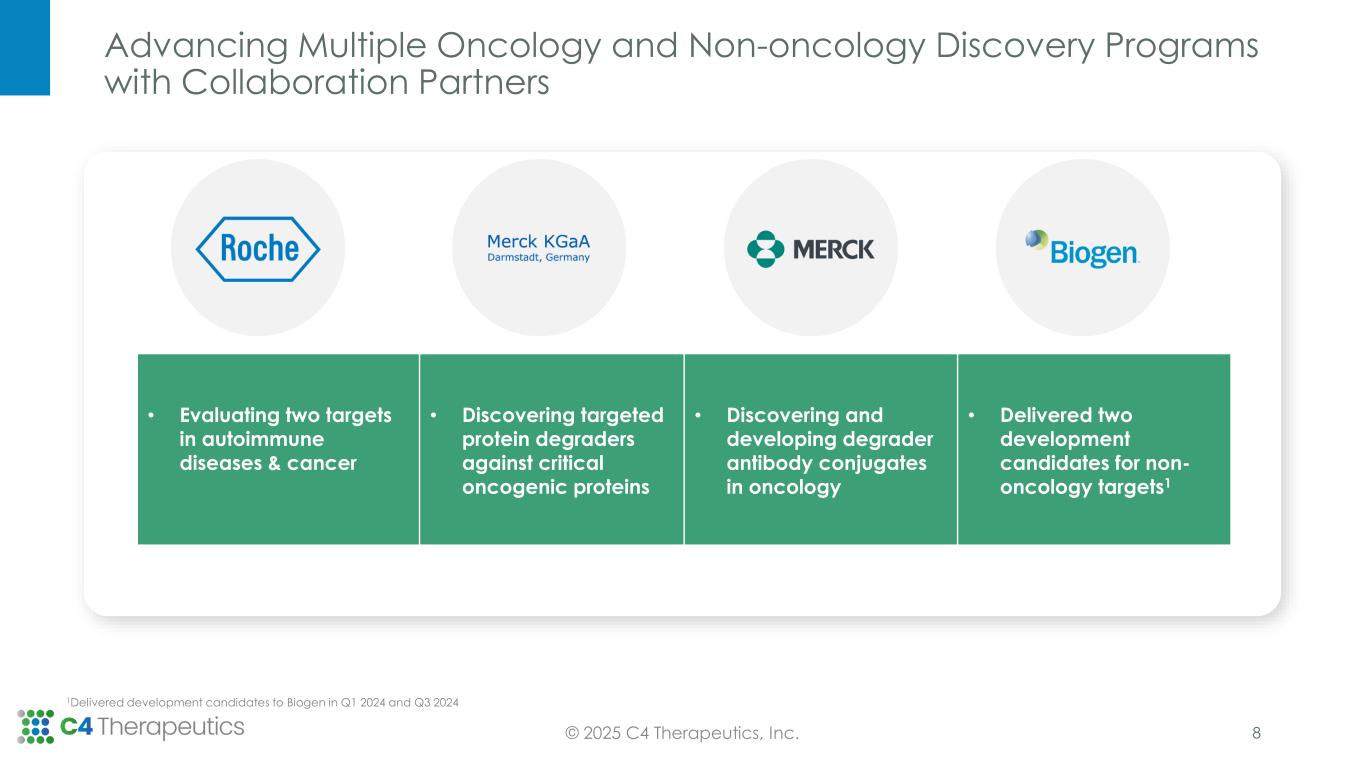
Advancing Multiple Oncology and Non-oncology Discovery Programs with Collaboration Partners © 2025 C4 Therapeutics, Inc. • Evaluating two targets in autoimmune diseases & cancer • Discovering targeted protein degraders against critical oncogenic proteins • Discovering and developing degrader antibody conjugates in oncology • Delivered two development candidates for non- oncology targets1 1Delivered development candidates to Biogen in Q1 2024 and Q3 2024 8

Cemsidomide IKZF1/3 Degrader Multiple Myeloma & Non-Hodgkin’s Lymphoma

IKZF1/3 Are Key Promoters of Myeloma and Lymphoma Cell Survival and Will Remain Important Therapeutic Targets for These Indications © 2025 C4 Therapeutics, Inc. Multiple myeloma (MM); non-Hodgkin’s lymphoma (NHL) 10 Hematopoietic Stem Cell Common Myeloid Progenitor Cell Common Lymphoid Progenitor Cell Neutrophil Platelets T-Cell B-Cell Plasma Cell Oncogenic Mutations/Aberrations T-Cell Lymphoma B-Cell Lymphoma Multiple Myeloma IKZF1/3 IRF4 ↑ IKZF1/3 and IRF4 Key Roles of IKZF1/3: • Multiple myeloma and lymphoma cells rely on IKZF1/3 and IRF4 for survival • Degrading IKZF1/3 leads to down regulation of IRF4, promoting myeloma and lymphoma cell death and on-target neutropenia • IKFZ1/3 degradation combined with MM immune-based regimens have potential to enhance activity through T-cell activation and cancer cell death by downregulation of IRF4 Degrader Advantages of Cemsidomide: Cemsidomide is more potent than approved and development stage IKZF1/3 degraders Increased selectivity for IKZF1/3 resulting in reduced off-target toxicity
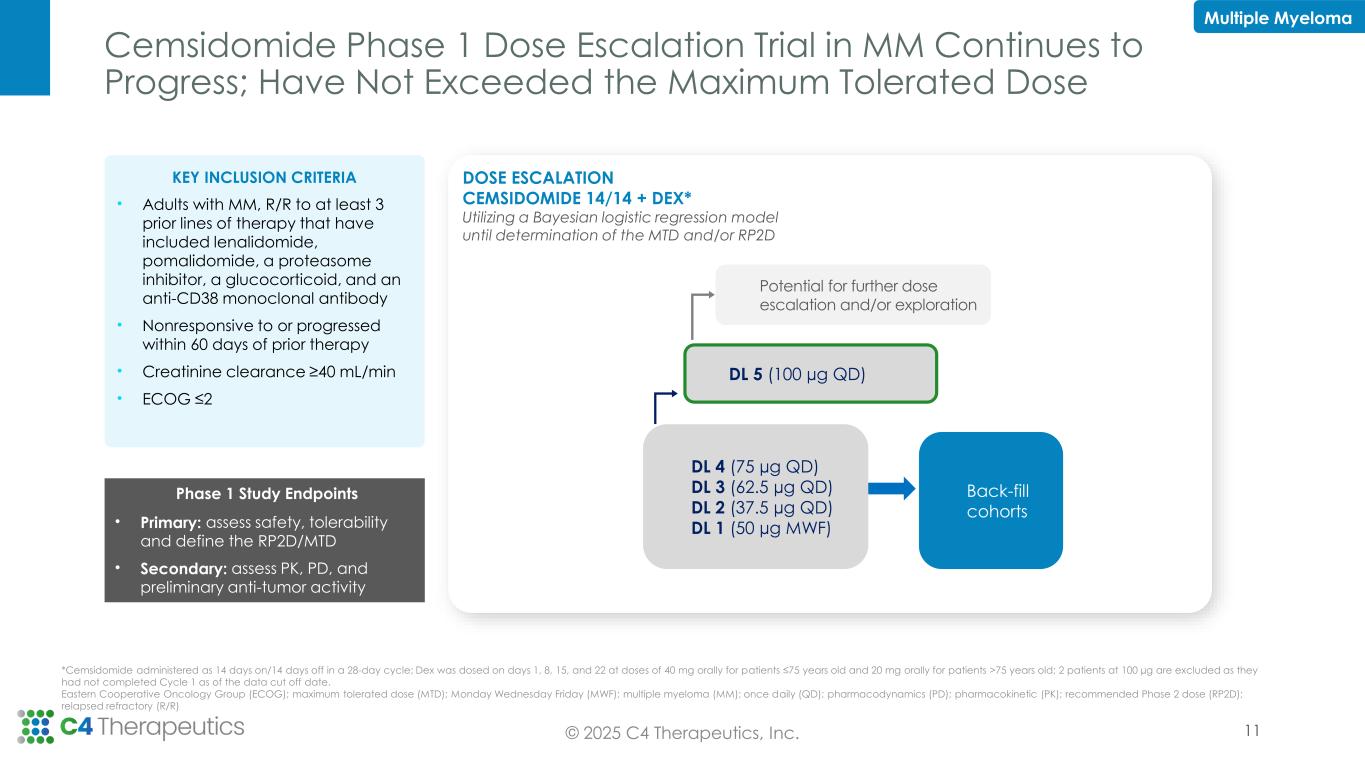
Cemsidomide Phase 1 Dose Escalation Trial in MM Continues to Progress; Have Not Exceeded the Maximum Tolerated Dose DL 4 (75 µg QD) DL 3 (62.5 µg QD) DL 2 (37.5 µg QD) DL 1 (50 µg MWF) Potential for further dose escalation and/or exploration DL 5 (100 µg QD) Back-fill cohorts DOSE ESCALATION CEMSIDOMIDE 14/14 + DEX* Utilizing a Bayesian logistic regression model until determination of the MTD and/or RP2D Multiple Myeloma © 2025 C4 Therapeutics, Inc. KEY INCLUSION CRITERIA • Adults with MM, R/R to at least 3 prior lines of therapy that have included lenalidomide, pomalidomide, a proteasome inhibitor, a glucocorticoid, and an anti-CD38 monoclonal antibody • Nonresponsive to or progressed within 60 days of prior therapy • Creatinine clearance ≥40 mL/min • ECOG ≤2 Phase 1 Study Endpoints • Primary: assess safety, tolerability and define the RP2D/MTD • Secondary: assess PK, PD, and preliminary anti-tumor activity 11 *Cemsidomide administered as 14 days on/14 days off in a 28-day cycle; Dex was dosed on days 1, 8, 15, and 22 at doses of 40 mg orally for patients ≤75 years old and 20 mg orally for patients >75 years old; 2 patients at 100 µg are excluded as they had not completed Cycle 1 as of the data cut off date. Eastern Cooperative Oncology Group (ECOG); maximum tolerated dose (MTD); Monday Wednesday Friday (MWF); multiple myeloma (MM); once daily (QD); pharmacodynamics (PD); pharmacokinetic (PK); recommended Phase 2 dose (RP2D); relapsed refractory (R/R)

With a Potentially Best-in-Class Profile, Cemsidomide Is Positioned to Be an IKZF1/3 Degrader of Choice Across Various Combinations Post-Transplant Maintenance1 Anti-CD38 Combos Proteasome Inhibitor Combos CAR-Ts (+/- Maintenance Therapy) Other MOAs2 1Approximately 30% of multiple myeloma patients undergo a hematopoietic stem cell transplant and receive post-transplant maintenance therapy. 2 Other MOAs approved in MM include dexamethasone combos, anti-SLAMF7 mAbs and XPO1 inhibitors. Potential future treatment options include FcRH5 bispecific T-cell engagers, BCL-2 inhibitors, and others. Sources: EvaluatePharma (accessed 1/8/25), NCCN guidelines, consulting engagements with Health Advances and Clearview. B-cell maturation antigen (BCMA); G protein-coupled receptor, class C, group 5, member D (GPRC5D); monoclonal antibodies (mAbs); mechanism of action (MOA); Germany, Italy, France, and Spain (EU4) BCMA/GPRC5D T-cell Engagers and ADC Combos © 2025 C4 Therapeutics, Inc. CEMSIDOMIDE OPPORTUNITY • IKZF1/3 degraders are currently used across lines of therapy, from post-transplant maintenance to 5L+ doublets, and will remain backbone therapies in various combination approaches • Cemsidomide has the potential to become the IKZF1/3 degrader of choice in numerous regimens across lines of therapy given its potent anti-myeloma activity, differentiated safety profile, and immunomodulatory effects EVOLVING MULTIPLE MYELOMA TREATMENT LANDSCAPE Multiple Myeloma 12 2L ~56,000 1L ~65,000 3L ~49,000 4L ~42,000 5L+ ≥23,000 U.S. + EU4 + UK Addressable Patients (2024) Treatment Line

TEMRA TEM 0 20 40 60 80 100 % positive cells (C1D21 vs C1D1) % p o s it iv e c e ll s C1D1 C1D21 © 2025 C4 Therapeutics, Inc. Clinical Evidence of Immune T-cell Activation With Cemsidomide Monotherapy ** **** TEMRA: Terminally differentiated T-cells TEM: Effector memory T-cells Peripheral blood mononuclear cells (PBMCs); daily dosing (QD); Monday, Wednesday, Friday dosing (MWF); multiple myeloma (MM) Source: C4T data on file as of 11/28/2023 (https://ir.c4therapeutics.com/static-files/ec59b02e-3074-484d-ad88-e81831bf37ed) • 19 patient samples (PBMCs) analyzed by flow cytometry • Aggregate data of 25 µg, 50 µg, and 75 µg Supports potential of cemsidomide as a maintenance therapy option and in combination with other MM agents to improve efficacy: ✓ Cemsidomide induces CD8+ T- cell activation by increasing effector memory T-cell subset ✓ T-cell activation is observed at well-tolerated monotherapy clinical doses ✓ Clinical data consistent with the preclinical in vitro data reported for cemsidomide 13 Multiple Myeloma

Cemsidomide Is Well-Tolerated With Manageable Neutropenia and No Treatment Emergent Adverse Events Lead to Dose Reductions Common (>20% All Grades) TEAEs and Events of Interest, n (%) All Grades (N=47) Grade 3 (N=47) Grade 4 (N= 47) Grade 5 (N=47) Neutropenia 22 (47) 6 (13) 12 (26) 0 Infections Pneumonia Upper respiratory tract infection Septic shock 18 (38) 5 (11) 7 (15) 1 (2) 7 (15) 5 (11) 1 (2) 0 0 0 0 0 1(2) 0 0 1(2) Anemia 17 (36) 10 (21) 0 0 Fatigue 14 (30) 0 0 0 Thrombocytopenia 10 (21) 3 (6) 2 (4) 0 Diarrhea 10 (21) 0 0 0 Lymphopenia 9 (19) 6 (13) 0 0 Febrile neutropenia 3 (6) 3 (6) 0 0 2 patients experienced Grade 5 AEs (septic shock and subdural hematoma), both deemed unrelated to cemsidomide • 1 DLT (Grade 4 neutropenia lasting >7 days at the 62.5 µg dose level) • No TEAEs lead to dose reductions • TEAEs leading to dose interruption: 32% (15/47) • TEAEs leading to discontinuation1: 4% (2/47) 1Primary reason of discontinuation of patient at 37.5 µg was due to withdrawal of consent; primary reason of discontinuation of patient at 75 µg was due to death unrelated to cemsidomide. Adverse events (AEs); dose limiting toxicity (DLT); treatment emergent adverse events (TEAEs) Source: ASH 2024; C4T data as of 10/11/2024 (https://ir.c4therapeutics.com/static-files/32ae4fdb-d4d9-4a17-a77d-83289c66e91f) Multiple Myeloma 14 © 2025 C4 Therapeutics, Inc.

75 µg Cemsidomide Dose Level Resulted in Compelling Anti-Myeloma Activity With a 36% ORR and 45% CBR 15 33% (2) 8% (1) 18% (2) 12% (5) 50% (3) 58% (7) 46% (6) 36% (4) 48% (20) 17% (2) 23% (3) 9% (1) 14% (6) 17% (1) 8% (1) 15% (2) 36% (4) 19% (8) 8% (1) 8% (1) 5% (2)8% (1) 2% (1) 0% 25% 50% 75% 100% 50 µg MWF (N=6) 37.5 µg QD (N=12) 62.5 µg QD (N=13) 75 µg QD (N=11) TOTAL (N=42) B e st R e sp o n se % ( N ) sCR VGPR PR MR SD PD CBR 17% CBR 42% CBR 46% ORR 17% ORR 25% ORR 23% ORR 36% ORR 26% CBR 40% Best Response: Multiple Myeloma – Cemsidomide + Dex* a CBR 45% © 2025 C4 Therapeutics, Inc *Investigator assessed response a1 patient in the 37.5 µg cohort achieved a PR based on light chains, no follow up M protein available; 1 patient in the 62.5 µg cohort had an unconfirmed PR as of the data cutoff date; 1 patient in the 75 µg cohort had an unconfirmed PR as of the data cutoff date. Minimal response (MR); Monday Wednesday Friday (MWF); once daily (QD); partial response (PR); progressive disease (PD); stable disease (SD); stringent complete response (sCR); very good partial response (VGPR) Overall Response Rate (≥ PR) (ORR); Clinical Benefit Rate (≥ MR) (CBR) Source: ASH 2024; C4T data as of 10/11/2024 (https://ir.c4therapeutics.com/static-files/32ae4fdb-d4d9-4a17-a77d-83289c66e91f) Multiple Myeloma
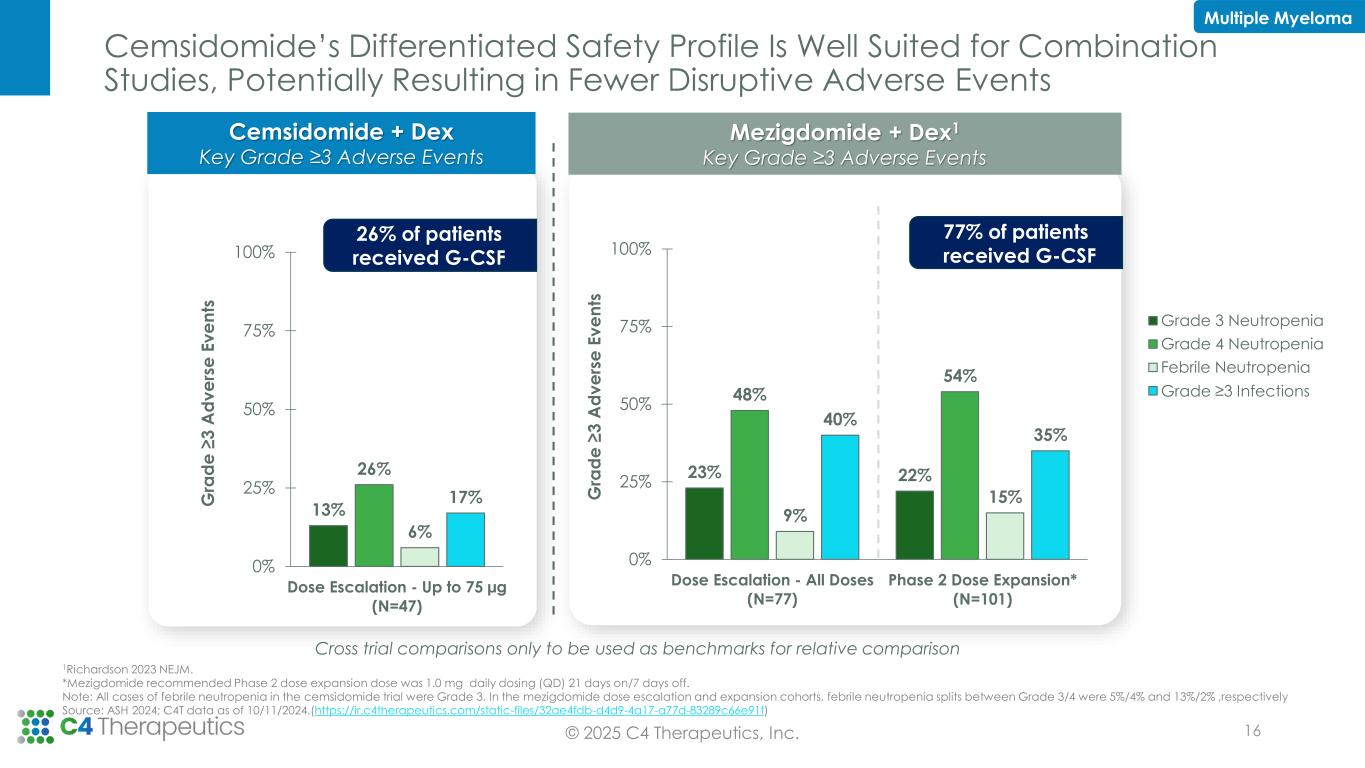
Cemsidomide’s Differentiated Safety Profile Is Well Suited for Combination Studies, Potentially Resulting in Fewer Disruptive Adverse Events © 2025 C4 Therapeutics, Inc. 16 Cemsidomide + Dex Key Grade ≥3 Adverse Events 23% 22% 48% 54% 9% 15% 40% 35% 0% 25% 50% 75% 100% Dose Escalation - All Doses (N=77) Phase 2 Dose Expansion* (N=101) G ra d e ≥ 3 A d v e rs e E v e n ts 13% 26% 6% 17% 0% 25% 50% 75% 100% Dose Escalation - Up to 75 µg (N=47) G ra d e ≥ 3 A d v e rs e E v e n ts Cross trial comparisons only to be used as benchmarks for relative comparison 1Richardson 2023 NEJM. *Mezigdomide recommended Phase 2 dose expansion dose was 1.0 mg daily dosing (QD) 21 days on/7 days off. Note: All cases of febrile neutropenia in the cemsidomide trial were Grade 3. In the mezigdomide dose escalation and expansion cohorts, febrile neutropenia splits between Grade 3/4 were 5%/4% and 13%/2% ,respectively Source: ASH 2024; C4T data as of 10/11/2024.(https://ir.c4therapeutics.com/static-files/32ae4fdb-d4d9-4a17-a77d-83289c66e91f) Mezigdomide + Dex1 Key Grade ≥3 Adverse Events Grade 3 Neutropenia Grade 4 Neutropenia Febrile Neutropenia Grade ≥3 Infections 26% of patients received G-CSF 77% of patients received G-CSF Multiple Myeloma

Cemsidomide Has the Potential to Become a Treatment Option Across Lines of Therapy and Address a Growing Patient Population 2L ~56,000 1L ~65,000 3L ~49,000 4L ~42,000 5L+ ≥23,000 U.S. + EU4 + UK Addressable Patients (2024)1 Treatment Line Phase 1/2 (Escalation/Expansion) Cemsidomide/Dex + Anti-CD38 and Cemsidomide + BCMA Bispecific Phase 2 (Single Arm) Cemsidomide + Dex (Post anti-BCMA) Randomized Phase 3 Cemsidomide/Dex + Anti-CD38 (2-4 prior lines; post anti-BCMA) Randomized Phase 3 Cemsidomide + BCMA Bispecific (1-3 prior lines) Studies Currently Being Enabled Studies for Registrational Intent Potential Accelerated Approval INITIAL CEMSIDOMIDE DEVELOPMENT PATH IN MM 1 EvaluatePharma (accessed 1/8/25), consulting engagements with Health Advances and Clearview. EU4 = Germany, Italy, France, and Spain. B-cell maturation antigen (BCMA); dexamethasone (Dex); T-Cell engager (TCE); multiple myeloma (MM) Multiple Myeloma 17© 2025 C4 Therapeutics, Inc. Development Rationale Potentially enhances response durability and treatment duration of BCMA bispecific by preventing T-cell exhaustion Provides post anti-BCMA patients a potentially highly efficacious combo where there are limited proven options Potential to provide highly refractory patients a treatment option that is tolerable and efficacious where there are limited options

U.S. + EU4 + UK Annual Incidence (2024)1 ~54,000 ~33,000 ~12,000 ~8,000 ~16,000 Lenalidomide FDA Approved2 Lenalidomide in NCCN Guidelines DLBCL Diffuse Large B-Cell Lymphoma FL Follicular Lymphoma MZL Marginal Zone Lymphoma MCL Mantle Cell Lymphoma PTCL Subtypes Peripheral T-Cell Lymphoma B-Cell Lymphomas T-Cell Lymphomas Cemsidomide Opportunity • Lenalidomide is approved across NHL subtypes • Cemsidomide has the potential to be developed as a monotherapy in the R/R setting and in combination with front-line standard of care regimens 1 EvaluatePharma (accessed 1/8/25), American Cancer Society, Leukemia & Lymphoma Society. EU4 = Germany, Italy, France, and Spain. 2 FL, MZL, and MCL FDA approved in the Revlimid (lenalidomide) label and DLBCL approved in the Monjuvi (tafasitamab) label. U.S. Food and Drug Administration (FDA); National Comprehensive Cancer Network (NCCN); non-Hodgkin’s lymphoma (NHL); relapsed refractory (R/R) Cemsidomide Has the Potential to be Developed Across NHL Subtypes and Lines of Treatment 18© 2025 C4 Therapeutics, Inc. NHL

Cemsidomide Phase 1 Dose Escalation Trial in NHL Continues to Progress 19 DOSE ESCALATION CEMSIDOMIDE 14/14 Utilizing a Bayesian logistic regression model until determination of the MTD and/or RP2D DL 4 (62.5 µg QD) DL 3 (37.5 µg QD) DL 2 (50 µg QD) DL 1 (25 µg MWF) Potential to resume enrollment at 100 µg pending outcome at 75 µg cohortDL 5 (100 µg QD) 2 DLTs 75 µg QD Back-fill cohort at 37.5 µg QD NHL © 2025 C4 Therapeutics, Inc. KEY INCLUSION CRITERIA • Adults with NHL, R/R to prior therapy • PTCL patients must have received at least 1 prior alkylator-based chemotherapy • ALCL patients must have also received a CD-30 mAb • Nonresponsive to or progressed within 60 days of prior therapy • Creatinine clearance ≥40 mL/min • ECOG ≤2 Phase 1 Study Endpoints • Primary: assess safety, tolerability and define the RP2D/MTD • Secondary: assess PK, PD, and preliminary anti-tumor activity Anaplastic large cell lymphoma (ALCL); dose escalation meeting (DEM); dose limiting toxicities (DLT); Eastern Cooperative Oncology Group (ECOG); monoclonal antibody (mAb); maximum tolerated dose (MTD); Monday Wednesday Friday (MWF); non-Hodgkin’s lymphoma (NHL);once daily (QD); pharmacodynamic (PD); pharmacokinetic (PK); peripheral T-cell lymphoma (PTCL); recommended Phase 2 dose (RP2D); relapsed refractory (R/R)

Cemsidomide Was Well-tolerated With Manageable Incidents of On- target Neutropenia Common (>20% All Grades) TEAEs and Events of Interest*, n (%) All Grade (N=23) Grade 3 (N=23) Grade 4 (N=23) Infections Upper respiratory tract infection Sepsis Bacteremia Pneumonia 15 (65) 4 (17) 1(4) 1(4) 2 (9) 4 (17) 0 0 0 2 (9) 2 (9) 0 1 (4) 1 (4) 0 Neutropenia 11 (48) 4 (17) 7 (30) Fatigue 11 (48) 1 (4) 0 Cough 7 (30) 0 0 Anemia 6 (26) 4 (17) 0 Peripheral edema 5 (22) 0 0 Febrile neutropenia* 4 (17) 4 (17) 0 Thrombocytopenia* 4 (17) 1 (4) 2 (9) Maculopapular rash* 3 (13) 2 (9) 0 One patient experienced a Grade 5 AE (hip fracture resulting in transfer to hospice) • 2 DLTs occurred at 100 µg QD (Grade 4 thrombocytopenia and Grade 3 febrile neutropenia) • TEAEs leading to discontinuation: 9% (2/23) • 39% (9/23) of patients received G-CSF • 3 of 9 patients received G-CSF in Cycle 1 © 2025 C4 Therapeutics, Inc. *Events of Interest Adverse event (AE); dose limiting toxicities (DLTs); granulocyte colony-stimulating factor (G-CSF); once daily (QD); treatment emergent adverse events (TEAEs) Source: ASH 2024; C4T data as of 10/11/2024.(https://ir.c4therapeutics.com/static-files/32ae4fdb-d4d9-4a17-a77d-83289c66e91f) 20 NHL

Compelling and Deep Responses Achieved Across PTCL Subtypes 19% (1) 25% (1) 20% (1) 20% (1) 25% (3) 75% (3) 50% (1) 0% 25% 50% 75% 100% All PTCL (N=16) AITL (N=4) ALCL (N=2) ATLL (N=5) PTCL-NOS (N=5) B e st R e sp o n se % ( N ) CMR PMR PET-CT–based Assessment of PMR or Better by PTCL Subtype* (N=16) ORR 20% ORR 20% ORR 100% ORR 50% © 2025 C4 Therapeutics, Inc. ORR 44% *Investigator assessed response; 2 patients were evaluated based on CT scan and were PD but not evaluable based on PET-CT, both patients are included as PMD for PET-CT based assessment; 2 additional subjects that came off study prior to follow up scans were not considered efficacy evaluable. Angioimmunoblastic T-cell lymphoma (AITL); anaplastic large cell lymphoma (ALCL); adult T-cell lymphoma (ATLL); complete metabolic response (CMR); overall response rate (ORR); partial metabolic response (PMR); peripheral T- cell lymphoma (PTCL); peripheral T-cell lymphoma not-otherwise specified (PTCL-NOS) Source: ASH 2024; C4T data as of 10/11/2024.(https://ir.c4therapeutics.com/static-files/32ae4fdb-d4d9-4a17-a77d-83289c66e91f) • Cemsidomide monotherapy produced responses in all four PTCL subtypes • All AITL patients (4/4) experienced a metabolic response 21 NHL

Cemsidomide NHL Data Supports Further Development in PTCL, Which Provides the Fastest Path to Market Phase 2 (Single Arm) Cemsidomide Monotherapy (2L+ R/R PTCL) Randomized Phase 3 Cemsidomide + SOC2 (treatment naïve) Study Currently Being Enabled Study for Registrational Intent Potential Accelerated Approval INITIAL CEMSIDOMIDE DEVELOPMENT PATH IN PTCL Development Rationale Potentially enhance response durability and decrease chemotherapy use, thus providing a more tolerable and durable option Potentially provides R/R patients a treatment option that is tolerable and efficacious where there are limited options 1 EvaluatePharma, ACS, consulting engagements with Health Advances and Clearview. 2 Standard of care (SOC) for 1L patients with CD30+ disease is brentuximab vedotin +/- chemotherapy and for CD30- patients it is the CHOP chemotherapy regimen (cyclophosphamide, doxorubicin, vincristine, prednisone) Germany, Italy, France, and Spain (EU4); peripheral t-cell lymphoma (PTCL); relapsed refractory (R/R); standard of care (SOC) 1L 2L+ ~16,000 ≤12,000 U.S. + EU4 + UK Addressable Patients (2024)1 Treatment Line 22© 2025 C4 Therapeutics, Inc. NHL

2 Cemsidomide Has a Strategic Path to Become a Potential Backbone Therapy for MM and NHL Across Various Lines of Treatment Dex combination in late-line MM In combination with MM agents in 2/3L Establish as backbone treatment across MM (1L+) • Highest unmet patient need opportunities • Fastest path to label • Combination strategies with other MM agents to expand to 2L+ MM • Combine with standard of care in front-line NHLs • Maximize broad applicability • Drive revenue growth Monotherapy in 2L+ PTCL Expand to 1L PTCL in combination with SOC Establish as backbone treatment across NHL Multiple myeloma (MM); non-Hodgkin’s lymphoma (NHL); peripheral T-cell lymphoma (PTCL); standard of care (SOC) 1 3 © 2025 C4 Therapeutics, Inc. 23

CFT1946 BRAF Mutant V600 Degrader Colorectal Cancer, Melanoma & Other BRAF Mutant Solid Tumors

CFT1946 Is an Oral, Potent Degrader of BRAF V600 Mutants With Potential to Improve Outcomes for Patients 1Kreger B et al. Abstract 1658, AACR 2024 Adverse event (AE);); colorectal cancer (CRC); central nervous system (CNS); mitogen- activated protein kinase (MAPK);progression free survival (PFS); BRAF inhibitor (BRAFi). Degrader Benefit in BRAF V600 Mutant Monomer and Dimer-dependent Diseases Causes of Dimer-Driven Resistance Melanoma: Acquired NRAS mutation, BRAF V600 mutant amplification, BRAF V600 mutant splice variant CRC: EGFR-mediated pathway reactivation Current Approved BRAF Inhibitors Have Limitations: • Resistance mechanisms lead to limited duration of response • Toxicities associated with inhibition of wild- type BRAF limit tolerability Potential Degrader Advantages of CFT1946: Enables deep elimination of mutant BRAF signaling to avoid resistance mechanisms and enable a longer duration of response Spares wild-type BRAF1, likely avoiding AEs associated with inhibition of wild-type BRAF Ability to cross the blood-brain barrier and highlight the potential for drug delivery to CNS tumors, with Kpu,u values ranging from 0.34 to 0.88 25© 2025 C4 Therapeutics, Inc.

CFT1946 Phase 1/2 Trial Continues to Progress Across BRAF V600 Mutant Driven Solid Tumors MONOTHERAPY DOSE ESCALATION 160 mg BID PK, PD, ANTI-TUMOR ACTIVITY EVALUATION 1 640 mg BID 1Evaluating additional patients for pharmacodynamic assessment via pre- and post-drug exposure biopsies Colorectal cancer (CRC); dose Level (DL); twice daily (BID; pharmacokinetic (PK); pharmacodynamic (PD) 320 mg BID Phase 1B: CFT1946 in combination with cetuximab in CRC (160 mg BID, 320 mg BID, 640 mg BID) Exploratory Expansions: CFT1946 monotherapy in melanoma (320 mg and 640 mg BID) Phase 1B: CFT1946 in combination with trametinib for melanoma (320 mg BID) Potential for further dose escalation and/or exploration DL 5 (640 mg BID) DL 4 (320 mg BID) DL 3 (160 mg BID) DL 2 (80 mg BID) DL 1 (20 mg BID) 26© 2025 C4 Therapeutics, Inc.

Initial CFT1946 Pharmacokinetic and Pharmacodynamic Data Support Proof of Mechanism 27© 2025 C4 Therapeutics, Inc. Mean plasma concentration shown for n > 2 Exhibited dose-dependent bioavailability measured by IHC in paired different dose levels α -B R A F -V 6 0 0 E 100um BRAF V600E degradation determined by H-score of paired biopsies from different tumor types Immunohistochemistry (IHC); Twice Daily (BID, Cycle 1, Day 15 (C1D15); Pharmacokinetic (PK) Source: ESMO Congress 2024; C4T data on file as of 7/19/2024 (https://ir.c4therapeutics.com/static-files/b648e3ae-c2db-4f5e-9d47-324b0c8a2b2b) IHC on paired biopsy of a melanoma patient dosed at 320 mg BRAF V600E degradation in paired biopsies at different dose levels (n=1, 80 mg; n=3,160 mg; n=3, 320 mg) Screening C1D15 0 100 200 300 B R A F V 6 0 0 E a c ti v ty (H -s c o re ) CFT1946 C1D15 Pharmacokinetics H-Score Calculation: • IHC staining is measured using BRAF VE1 (BRAF V600E) clone from Roche Laboratories • H-scores (combining both proportion of cells and intensity of cell staining) are used as a surrogate for quantitative BRAF V600E levels 0 4 8 12 0 500 1000 1500 2000 2500 Time (h) P la s m a P K ( n g /m L ) 20 mg BID (N=3) 80 mg BID (N=5) 160 mg BID (N=9) 320 mg BID (N=9) 640 mg BID (N=4) Mean plasma concentration shown for n > 2 Screening C1D15

Preferred Term Grade 1 n (%) Grade 2 n (%) Grade 3 n (%) Grade 4 n (%) Grade 5 n (%) Total (n=36) n (%) Patients with any TEAEs^ 3 (8) 14 (39) 11 (31) 2 (6) 1 (3)# 31 (86) Anemia 1 (3) 4 (11) 2 (6) 0 0 7 (19) Abdominal pain 4 (11) 1 (3) 2 (6) 0 0 7 (19) Peripheral edema 5 (14) 1 (3) 0 0 0 6 (17) Pyrexia 4 (11) 2 (6) 0 0 0 6 (17) Fatigue 1 (3) 4 (11) 0 0 0 5 (14) Lipase increased 3 (8) 2 (6) 0 0 0 5 (14) Back pain 1 (3) 2 (6) 1 (3) 0 0 4 (11) Hypophosphatemia 1 (3) 3 (8) 0 0 0 4 (11) Constipation 1(3) 2 (6) 0 0 0 4 (11)* Source: ESMO Congress 2024; C4T data as of 7/19/2024 (https://ir.c4therapeutics.com/static-files/b648e3ae-c2db-4f5e-9d47-324b0c8a2b2b) Well-Tolerated Monotherapy Safety Profile, Consistent With BRAF V600 Mutant Selectivity Design of CFT1946 28 Summary of TEAEs ≥ 10% of 36 patients treated with CFT1946 • No DLTs • Majority of TEAEs observed were mild to moderate • No treatment-related SAEs • No Grade > 3 treatment-related cutaneous adverse events • No new primary malignancies Serious adverse events (SAEs); Dose limiting toxicities (DLTs); Treatment-emergent adverse events (TEAEs) ^A patient is only counted once with the highest severity and preferred term #Patient had a fatal cerebrovascular accident not related to CFT1946 CTCAE v5.0 grading criteria; *Grade missing for 1 patient with TEAE © 2025 C4 Therapeutics, Inc.

PR PD PRSD SD SDV 60 0K V 60 0K V 60 0R PR PD PRSD SD SD # 29 Source: ESMO Congress 2024; C4T data on file as of 7/19/2024 (https://ir.c4therapeutics.com/static-files/b648e3ae-c2db-4f5e-9d47-324b0c8a2b2b) Early Signs of CFT1946 Anti-tumor Activity: 59% of Patients Demonstrated Target Lesion Tumor Reductions * 1 2 3 4 5 *Other tumor types include cholangiocarcinoma, non-small cell lung cancer, pancreatic carcinoma, and small intestine cancer; BRAF V600 mutation is V600E unless otherwise specified; #This patient did not receive prior BRAF inhibitor therapy, all other patients received prior BRAF inhibitor therapy. Dotted lines represent partial response (-30%, blue line) and progressive disease (20%, gray line) per RECIST v1.1. 1 Patient on 160 mg BID had 56.2% reduction on target lesion, progression on non-target lesion and a new lesion, hence assessed as PD for overall response; 2 Patient on 640 mg BID had PR confirmed after data cut off, and as of ESMO Congress (9/13/2024); 3 Patient on 160 mg BID had PD following first PR (- 43.9%), hence assessed as SD for overall response; 4 Patient on 20 mg BID had unconfirmed PR, hence assessed as SD for overall response; 5 Patient on 160 mg BID had -29% reduction on target lesion, hence assessed as SD (As of 7/19/2024) © 2025 C4 Therapeutics, Inc.
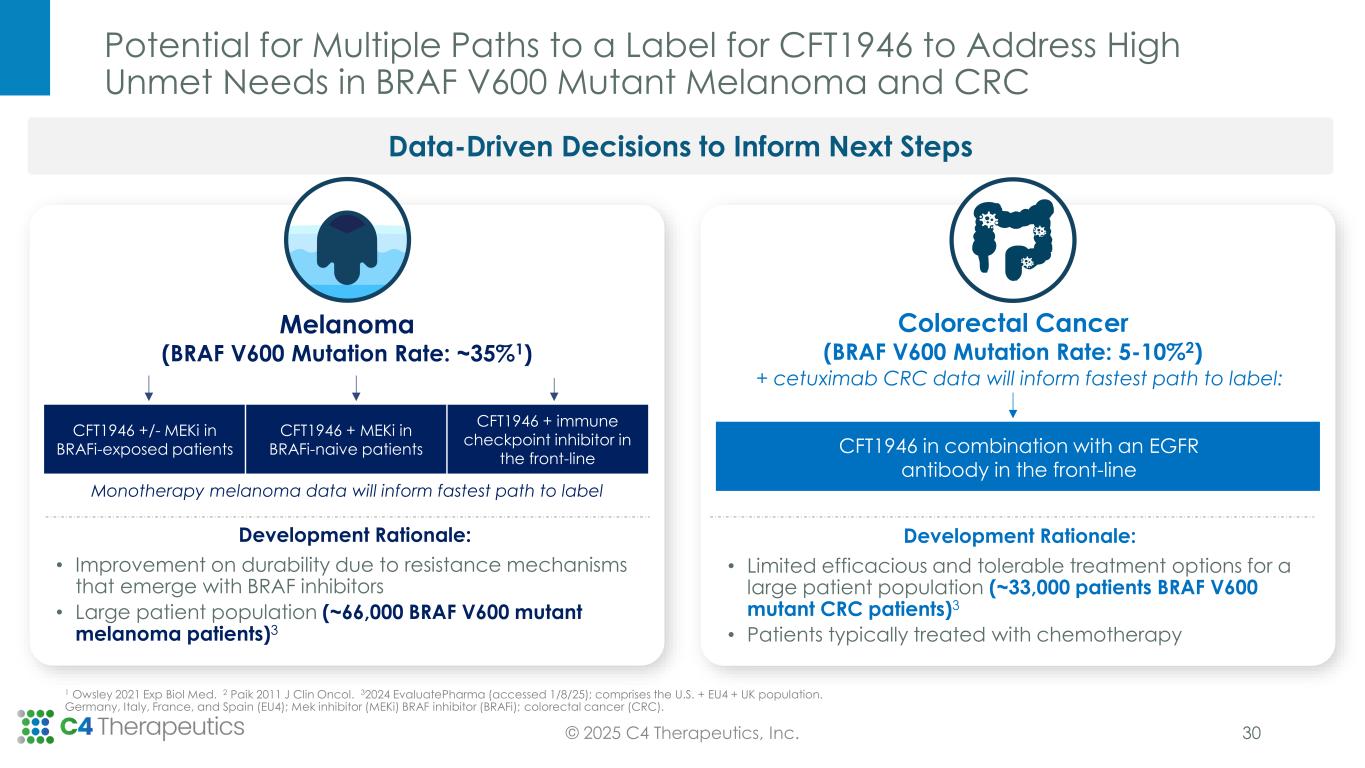
Potential for Multiple Paths to a Label for CFT1946 to Address High Unmet Needs in BRAF V600 Mutant Melanoma and CRC Data-Driven Decisions to Inform Next Steps + cetuximab CRC data will inform fastest path to label: Melanoma (BRAF V600 Mutation Rate: ~35%1) Development Rationale: • Limited efficacious and tolerable treatment options for a large patient population (~33,000 patients BRAF V600 mutant CRC patients)3 • Patients typically treated with chemotherapy Monotherapy melanoma data will inform fastest path to label CFT1946 +/- MEKi in BRAFi-exposed patients CFT1946 + MEKi in BRAFi-naive patients CFT1946 + immune checkpoint inhibitor in the front-line CFT1946 in combination with an EGFR antibody in the front-line Colorectal Cancer (BRAF V600 Mutation Rate: 5-10%2) Development Rationale: • Improvement on durability due to resistance mechanisms that emerge with BRAF inhibitors • Large patient population (~66,000 BRAF V600 mutant melanoma patients)3 30© 2025 C4 Therapeutics, Inc. 1 Owsley 2021 Exp Biol Med. 2 Paik 2011 J Clin Oncol. 32024 EvaluatePharma (accessed 1/8/25); comprises the U.S. + EU4 + UK population. Germany, Italy, France, and Spain (EU4); Mek inhibitor (MEKi) BRAF inhibitor (BRAFi); colorectal cancer (CRC).

CFT8919 EGFR L858R Degrader Non-Small Cell Lung Cancer
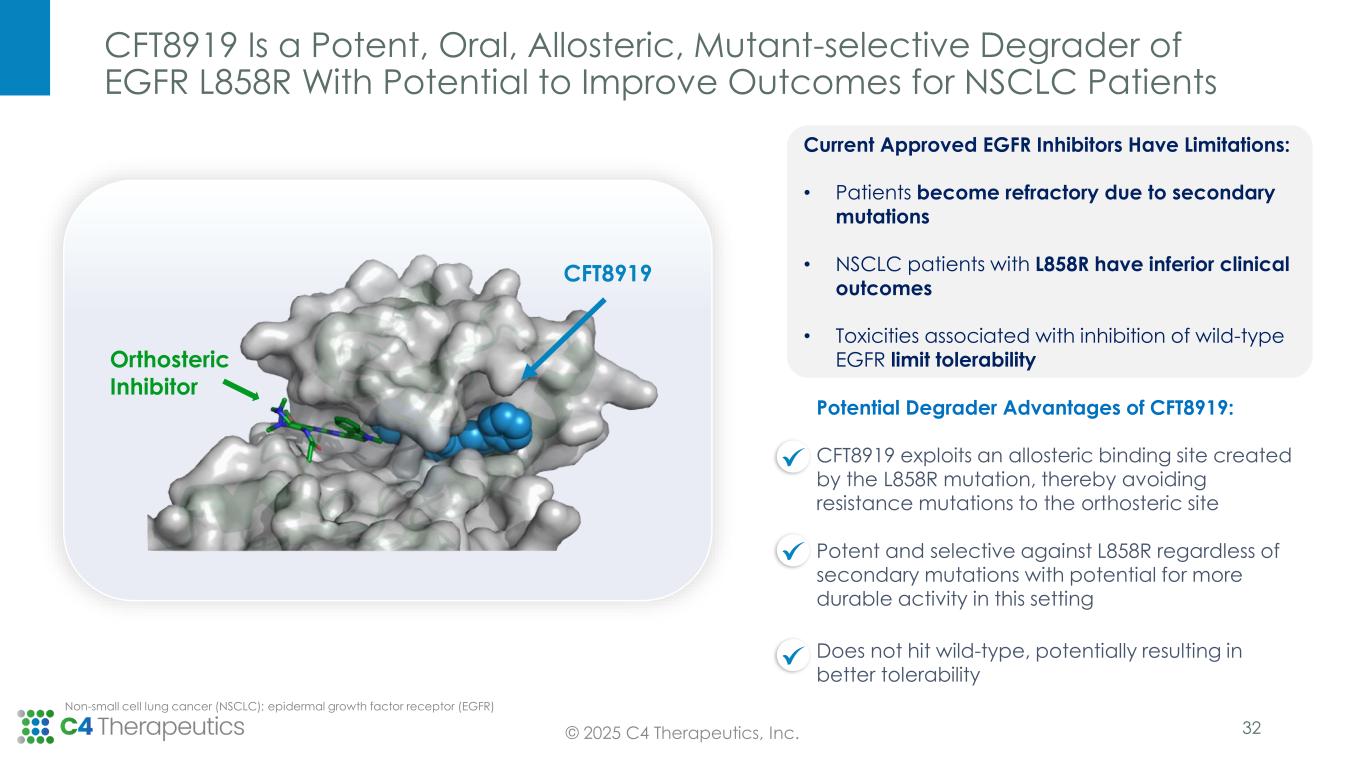
CFT8919 Is a Potent, Oral, Allosteric, Mutant-selective Degrader of EGFR L858R With Potential to Improve Outcomes for NSCLC Patients CFT8919 Orthosteric Inhibitor Potential Degrader Advantages of CFT8919: CFT8919 exploits an allosteric binding site created by the L858R mutation, thereby avoiding resistance mutations to the orthosteric site Potent and selective against L858R regardless of secondary mutations with potential for more durable activity in this setting Does not hit wild-type, potentially resulting in better tolerability Non-small cell lung cancer (NSCLC); epidermal growth factor receptor (EGFR) © 2025 C4 Therapeutics, Inc. Current Approved EGFR Inhibitors Have Limitations: • Patients become refractory due to secondary mutations • NSCLC patients with L858R have inferior clinical outcomes • Toxicities associated with inhibition of wild-type EGFR limit tolerability 32

CFT8919 Binds to Allosteric Site, Avoiding Impact of L858R Non-classical Co-mutations in the Orthosteric Binding Pocket Sources: 1. From Black Diamond’s analyses of 94,939 sequencing reports from treatment naive NSCLC (Guardant Health) presented at AACR 2024 (https://blackdiamondtherapeutics.com/assets/files/AACR_2024__BDTX-1535_FINAL_Presentation_20240405.pdf) 2. Gitenbeek, et al. 2023 Progression free survival (PFS) © 2025 C4 Therapeutics, Inc. CFT8919 binds to the allosteric site, potentially avoiding the impact of non-classical co- mutations with L858R, where inhibitors demonstrate lower PFS in this patient population than those with EXON 19 deletion 1 2 Overall survival by type of mutation in patients with Stage IV EGFR mutated NSCLC and brain metastasis who received first-line treatment with osimertinib EGFR-L858R Tumors More Frequently Co-express Non-classical EGFR Mutations Before Exposure to EGFR TKI1 Patients with L858R Do Less Well on Osimertinib Therapy vs Ex19del 33

CFT8919 Is Selective for EGFR L858R and Active in a Setting of Osimertinib Resistance in Preclinical Models © 2025 C4 Therapeutics, Inc. Source: C4T data on file; Presented at Keystone Symposium 2021 (https://c4therapeutics.com/wp-content/uploads/Preclinical-Evaluation-of-CFT8919-as-a-Mutant-Selective-Degrader- of-EGFR-with-L858R-Activating-Mutations-for-the-Treatment-of-Non-Small-Cell-Lung-Can.pdf) Investigational new drug application (IND) Specific for EGFR Exon 21 Mutants EGFR-L858R EGFR-L861Q Active in Setting of EGFR C797S 0 5 10 15 0 500 1000 1500 2000 Days of Treatment B a F 3 E G F R L 8 5 8 R /T 7 9 0 M /C 7 9 7 S T u m o r v o lu m e ( m m 3 ) Vehicle PO BID Osimertinib 25 mpk PO QD CFT8919 10 mpk PO BID CFT8919 25 mpk PO BID CFT8919 50 mpk PO BID 34

CFT8919 Demonstrates Activity in Brain Metastasis Model Source: C4T data on file; presented at TPD Summit 2021 (https://c4therapeutics.com/wp-content/uploads/C4_CFT8919_TPD_Summit_Presentation.pdf) By mouth (PO); twice daily (BID) © 2025 C4 Therapeutics, Inc. Mean Plasma & Tumor Concentration In vivo Efficacy In vivo Body Weight Change 4 6 8 10 12 10 100 1000 10000 Time (hr) C F T 8 9 1 9 c o n c e n tr a ti o n in p la s m a ( n g /m l) a n d t u m o r (n g /g ) Plasma Tumor in brain Plasma clearance t1/2 = 3.1 hrs 0 5 10 15 0 200 400 600 Study Day B L I (p h o to n s /s × 1 0 6 ) Vehicle CFT8919 (50mg/kg PO BID) 0 5 10 15 -20 -10 0 10 20 Study Day B o d y W e ig h t C h a n g e ( % ) * *Body weight loss due to tumor burden 50 mg/kg single dose PO 35

CFT8919 Has the Potential to Address Multiple Opportunities with High Unmet Needs CFT8919’s Fastest Path to Market Is in 2L+ With Potential to Expand Into Front-Line Development Rationale: • Fast path to market • Lack of therapies after patients relapse with secondary mutation (i.e., C797S) 2L+ Development Rationale: • Large patient opportunity • Potential to increase responses and durability in L858R patients Front-line Dose escalation in Greater China is advancing; C4T to utilize data to inform ex-China clinical development 2024 Annual Incidence of EGFR L858R Mutated NSCLC1: • U.S.: ~17,000 • China: ~189,000 • EU4 + UK: ~13,000 1 EvaluatePharma (accessed on 1/10/25), consulting engagements with Health Advances and Clearview. Germany, Italy, France, and Spain (EU4) 36© 2025 C4 Therapeutics, Inc.
Multiple 2025 Inflection Points to Unlock Value Across Entire Portfolio © 2025 C4 Therapeutics, Inc. C4T is on a path to become a fully integrated biotechnology company focused on orally bioavailable degraders KEY CATALYSTSVALUE DRIVERS Cemsidomide IKZF1/3 Further development in multiple myeloma and non- Hodgkin’s lymphoma positions cemsidomide to potentially be best-in-class IKZF1/3 degrader CFT1946 BRAF V600 Mutant Phase 1 data updates to further validate initial anti- tumor activity and safety profile in melanoma and colorectal cancer CFT8919 EGFR L858R Phase 1 data from Greater China clinical trial to inform U.S. and rest-of-world development plans Develop orally bioavailable degraders in oncology and non-oncology targets for internal research and collaborations TORPEDO Platform 37




































