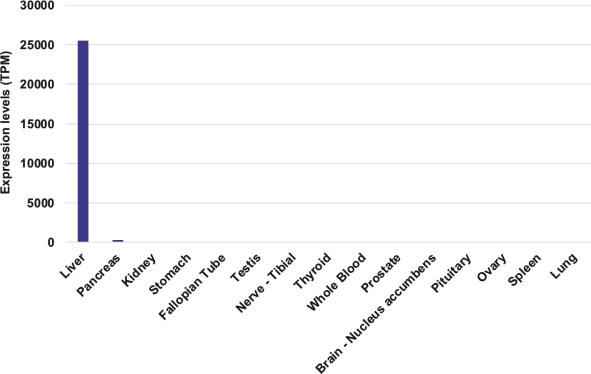
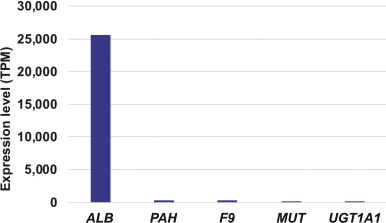
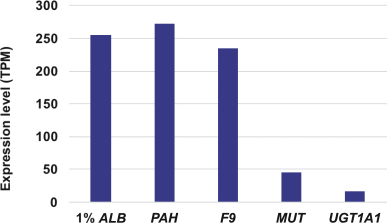
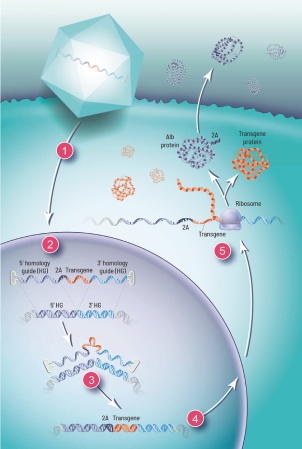
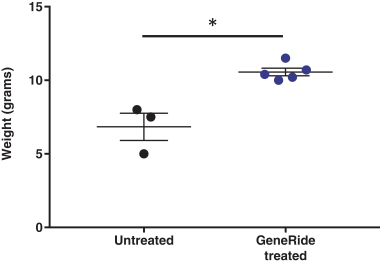
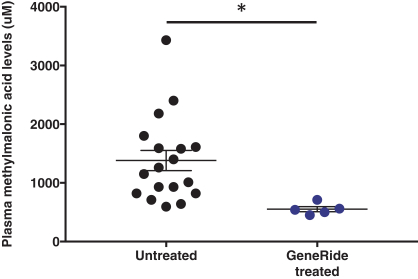
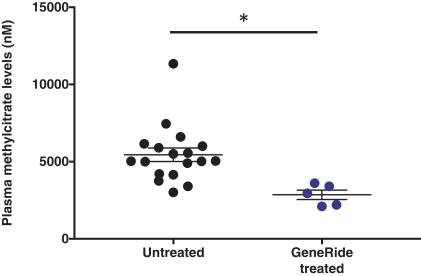
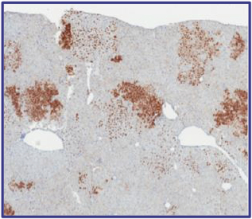
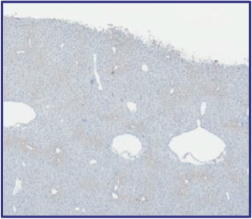
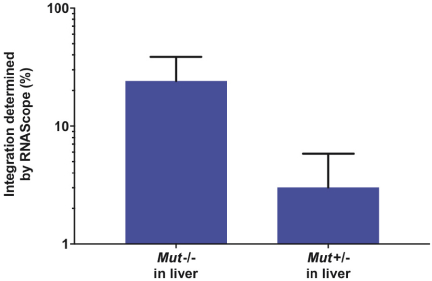
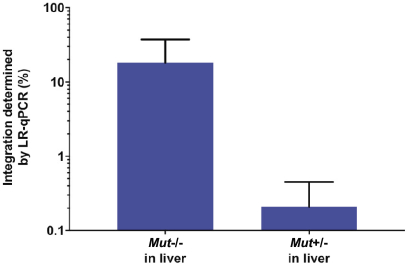
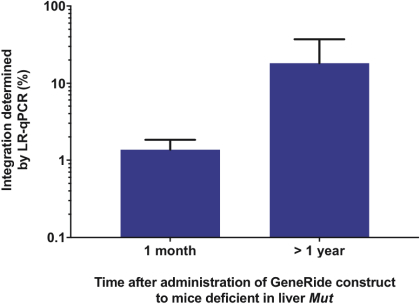
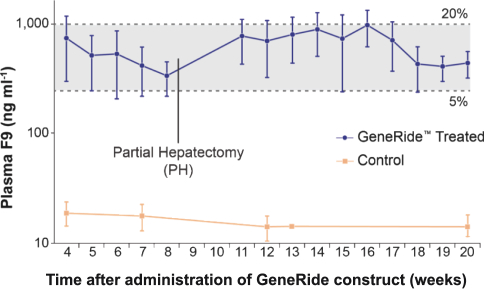
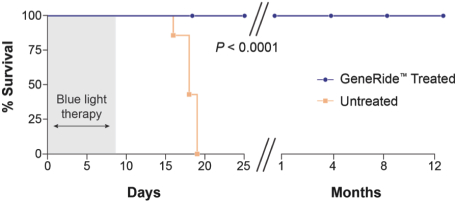
The Board of Regents of the University of Texas System License Agreement
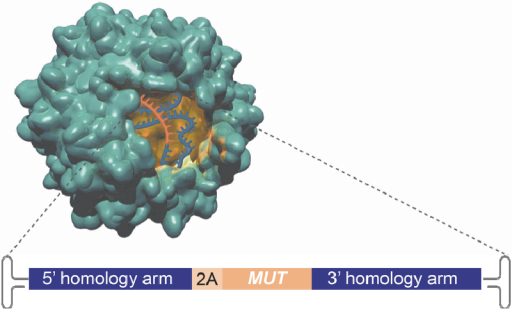
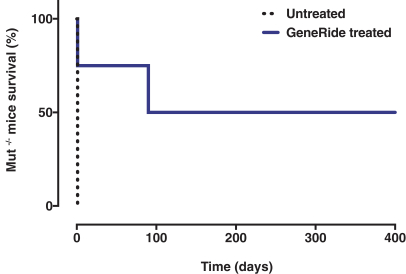
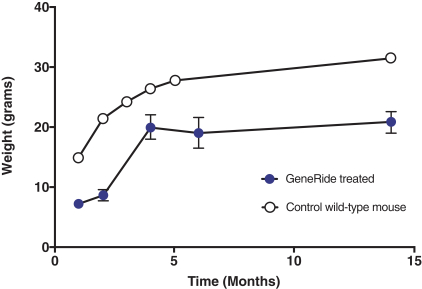
In May 2018, we entered into a license agreement with the University of Texas, pursuant to which we obtained an exclusive, worldwide license to manufacture, have manufactured, distribute, have distributed, use, offer for sale, sell, lease, loan or import products covered by certain patent rights to the GeneRide technology owned by the University of Texas (jointly with Stanford) within certain fields of use.
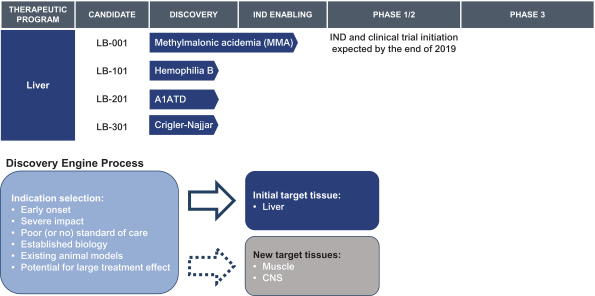
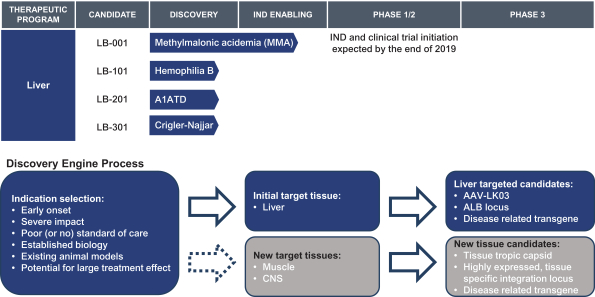
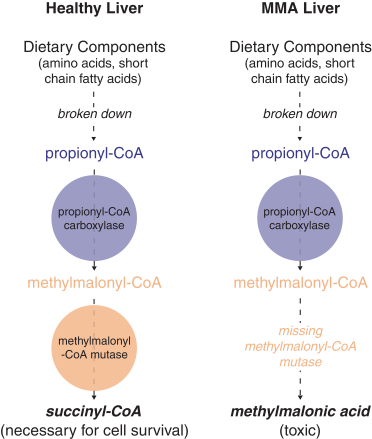
This exclusive license grant is limited to the following fields: (a) human therapeutics to treat methylmalonic acidemia, propionic acidemia, HIV, influenza, malaria, Crigler-Najjar Syndrome, Tyrosinemia Type I, Wilson’s disease, hemophilia B, Glycogen Storage Disease 1, Glycogen Storage Disease 3 and any other human disease of liver tissue that affects less than 200,000 persons in the United States as of the effective date of the agreement, and (b) the prevention, treatment or diagnosis via genome editing without a nuclease of certain additional indications with respect to certain tissues to be nominated by us, subject to the terms of the agreement.
Pursuant to the University of Texas license agreement, we also obtained a non-exclusive license to certain related know-how.
The rights licensed to us are sublicensable through multiple tiers without the consent of the University of Texas.
Under the terms of the agreement we paid a one-time, non-refundable upfront fee of $25,000. We are required to pay the University of Texas low single-digit royalties on all net sales of products as well as a portion of any sublicensing revenues. We are also obligated to pay annual maintenance fees, which are fully creditable against any royalty payments made by us, and certain development and sales milestone payments up to $3.0 million. We are also required to reimburse the University of Texas for all future patent prosecution costs on a pro rata basis with other licensees. We do not have the right to control patent prosecution with respect to the licensed patent applications, but we do have the first right to enforce any patents which may issue from these patent applications.
The term of the license agreement will continue on a country-by-country and product-by-product basis until the later of the expiration of the last-to-expire valid claim of a licensed patent that covers such product in such country and 10 years from the date of the first commercial sale of such product in such country. The University of Texas may terminate the agreement in its entirety or with respect to any applicable part of the licensed subject matter, field of use or licensed territory, or convert the exclusive license to a non-exclusive license, if (i) we fail to timely make a required payment to the University of Texas under the agreement; (ii) we are in material breach of a provision of the agreement and fail to timely cure such breach; (iii) we breach any payment obligation under the agreement three or more times in any 12-month period; (iv) we initiate, or an affiliate or sublicense initiates, a patent challenge against a licensed patent; or (v) we become bankrupt or insolvent, our board elects to liquidate our assets or dissolve the business, we cease business operations, we make an assignment to the benefit of our creditors, or our business or assets are otherwise placed in the hands of a receiver, assignee or trustee. We may terminate the agreement in its entirety or with respect to any applicable part of the licensed subject matter, field of use or licensed territory upon at least 30 days’ notice to the University of Texas.
Government Regulation
Government authorities in the United States at the federal, state and local level and in other countries and jurisdictions extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, sale, distribution, post-approval monitoring and reporting, marketing and export and import of drug products. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized into a format specific for each regulatory authority, submitted for review and approved by the regulatory authority.
U.S. Government Regulation of Biological Products
In the United States, biological products, including gene therapy products, are subject to regulation by the U.S. Food and Drug Administration, or FDA, under the Federal Food, Drug, and Cosmetic Act, or FDCA, and the Public Health Service Act, or PHSA, and their implementing regulations. Products also are subject to other federal, state and local statutes and regulations. Each clinical study protocol for a gene therapy product must be reviewed by the FDA and, in some instances, the National Institute of Health, through its Recombinant DNA Advisory Committee, or RAC. FDA approval must be obtained before marketing of biological products.
113