Exhibit 99.1
SUMMARY
This summary highlights selected information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. The summary may not contain all the information that you should consider before investing in our common stock. You should read this entire prospectus supplement and the accompanying prospectus carefully, including “Risk Factors” contained in this prospectus supplement and the documents incorporated by reference herein, before making an investment decision.
Overview
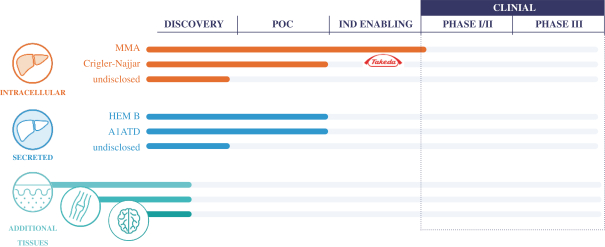
We are a company dedicated to extending the reach of genetic medicine with pioneering targeted delivery platforms. Our proprietary genome editing technology platform, GeneRide, enables the site-specific integration of a therapeutic transgene without nucleases or exogenous promoters by harnessing the native process of homologous recombination. We are developing LB-001, a wholly owned genome editing program leveraging GeneRide for the treatment of methylmalonic acidemia, or MMA. In addition, we have a research collaboration with Takeda Pharmaceutical Company Limited, or Takeda, to develop LB-301, an investigational therapy leveraging GeneRide for the treatment of the rare pediatric disease Crigler-Najjar syndrome, or CN.
We are also developing a Next Generation Capsid platform for use in gene editing and gene therapy. At the American Society of Gene & Cell Therapy, or ASGCT, conference in May 2020, data was presented showing that the capsids delivered highly efficient functional transduction of human hepatocytes in a humanized mouse model. The data also showed the capsids exhibited improved manufacturability with low levels of pre-existing neutralizing antibodies in human samples. Based on this data, we believe the top-tier capsid candidates from this effort demonstrated the potential to achieve significant improvements over benchmark adeno-associated viruses, or AAVs, that are currently in clinical development. We are developing these highly potent vectors for use in our internal development candidates and potentially for business development collaborations. We plan to announce data generated from translational animal models using these capsids in early 2021.
Based on our GeneRide technology, we are developing our lead product candidate, LB-001, to treat MMA. In August 2020, we announced the clearance of an investigational new drug application, or IND, to support the initiation of a Phase 1/2 clinical trial in pediatric patients with MMA. The SUNRISE trial is a multi-center, open-label, Phase 1/2 clinical trial designed to assess the safety and tolerability of a single intravenous infusion of LB-001 in pediatric patients with MMA characterized by methylmalonyl-CoA mutase gene (MMUT) mutations. Six leading centers in the United States are expected to participate in the SUNRISE trial.
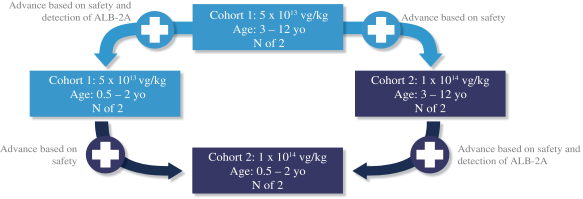
The SUNRISE Phase 1/2 clinical trial is expected to enroll eight pediatric patients with ages ranging from 6 months to 12 years, initially starting with 3 to 12 year old patients and then adding patients aged 6 months to 2 years. The SUNRISE trial will evaluate two dose cohorts of LB-001 (cohort 1 = 5 x 1013 vg/kg and cohort 2 = 1 x 1014 vg/kg). After initially starting with the lower dose in the 3 to 12 year old patient group (cohort 1, older age group, n=2), age de-escalation (cohort 1, younger age group, n=2) and dose escalation (cohort 2, older age group, n=2) are planned to occur in parallel. The decision to escalate the dose will be determined based solely on safety, whereas the decision to age de-escalate will be based on both safety and the detection of the pharmacodynamic biomarker, albumin-2A. Afterwards, based on a review of safety and/or the detection of albumin-2A, as applicable, from these two patient groups, the trial will progress to dosing additional patients in the younger age group at the higher dose (cohort 2, younger age group, n=2). The SUNRISE trial includes a six-week staggering interval between the dosing of each patient. Patients will participate in a pre-dosing observational period and will be administered a prophylactic steroid regimen. The following diagram illustrates the age de-escalation and dose escalation plan in the SUNRISE trial.