As filed with the Securities and Exchange Commission on April 12, 2016
Registration No. 333-209379
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 2 TO FORM F-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
NUTRITIONAL HIGH INTERNATIONAL INC.
(Exact name of registrant as specified in its charter)
Not Applicable
(Translation of Registrant's name into English)
| Canada | | 5122 | | N/A |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
77 King Street West, Suite 2905 P. O. Box 121
Toronto Ontario M5K 1H1
(416) 840-3798
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
David Posner
Chief Executive Officer
77 King Street West, Suite 2905 P. O. Box 121
Toronto Ontario M5K 1H1
(647) 958-6727
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
| Gregg E. Jaclin, Esq. |
| Szaferman Lakind Blumstein & Blader, P.C. |
| 101 Grovers Mill Road, Suite 200 |
| Lawrenceville, New Jersey |
| Phone: (609) 275-0400 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earliest effective registration statement for the same offering. ☐
CALCULATION OF REGISTRATION FEE
| Title of Securities Being Registered | | Amount Being Registered (1) | | | Proposed Maximum
Aggregate Offering Price | | | Amount of
Registration Fee (2) | |
| | | | | | | | | | |
| Common Stock ($0.0001 par value) | | | 29,178,000 | | | $ | 945,367.20 | | | $ | 95.20 | |
| | (1) | Represents the number of shares being registered for resale by the selling stockholder pursuant to an equity purchase agreement ("Purchase Agreement") that we entered into with Kodiak Capital Group, LLC ("Kodiak Capital"), The purchase price of the shares that may be sold to Kodiak Capital under the Purchase Agreement will be equal to a 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account, but at a price that is equal to no less than CDN $0.05. |
| | (2) | Calculated pursuant to Rule 457(c) based on the closing price of our Common Stock as reported on the OTC on January 29, 2016 which was $0.0324 per share. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We have filed a registration statement with the Securities and Exchange Commission relating to this preliminary prospectus. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is declared effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED: APRIL 12, 2016 |
Up to 29,178,000 Shares of Common Stock
NUTRITIONAL HIGH INTERNATIONAL INC.
________________________________________
This prospectus relates to the resale from time to time, of up to 29,178,000 shares of the common stock of Nutritional High International Inc. (hereafter, "we" "us" "our", "SPLIF" or the "Company") by Kodiak Capital Group, LLC ("Kodiak Capital"), a Delaware limited liability company. We are not selling any shares of common stock in this offering. We, therefore, will not receive any proceeds from the sale of the shares by the selling stockholders. We will, however, receive proceeds from the sale of securities pursuant to our exercise of the put right under the Purchase Agreement. Any participating broker-dealers and, if the selling stockholder is an affiliate of any such broker-dealers, may be deemed to be "underwriters" within the meaning of the Securities Act of 1933, as amended (the "Securities Act"), and any commissions or discounts given to any such broker-dealer or affiliates of a broker-dealer may be regarded as underwriting commissions or discounts under the Securities Act. The selling stockholder has informed us that it is not a broker-dealer, is not an affiliate of a broker dealer, and does not have any agreement or understanding, directly or indirectly, with any person to distribute our common stock.
The shares being registered herein are comprised of 29,178,000 common shares in the capital of the Company ("Common Stock" or "Common Shares") that are issuable pursuant to the Purchase Agreement that we entered into with Kodiak Capital on December 23, 2015. The purchase price of the shares that may be sold to Kodiak Capital under the Purchase Agreement will be equal to the greater of (i) 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account, or (ii) CDN $0.05. Kodiak Capital is an "underwriter" within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended, or the Securities Act. The total amount of shares of common stock which may be sold pursuant to this Prospectus would constitute approximately 17.1% of our issued and outstanding common stock as of April 12, 2016, assuming that the selling stockholder will sell all of the shares offered for sale.
The selling stockholder may offer all or part of the shares for resale from time to time through public or private transactions, at either prevailing market prices or at privately negotiated prices. Kodiak Capital is paying all of the registration expenses incurred in connection with the registration of the shares except for accounting fees and expenses and we will not pay any of the selling commissions, brokerage fees and related expenses.
The Company's Common Shares are currently listed on the OTCQB. Our common stock is quoted on the OTCQB under the symbol "SPLIF". The Shares registered hereunder are being offered for sale by the Selling Security Holder at prices established on the OTCQB during the term of this offering. On April 11, 2016, the closing price as reported on the OTCQB was $0.0247 per share. The Company's Common Shares are also listed on the Canadian Securities Exchange (the "CSE") under the trading symbol EAT". On April 11, 2016, the closing price as reported on the CSE was CDN $0.035 per share.
The Company qualifies as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act, which became law in April 2012 and will be subject to reduced public company reporting requirements.
An investment in our common stock is subject to many risks and an investment in our shares will also involve a high degree of risk. The shares issuable from the Purchase Agreement will dilute the ownership interest and voting power of existing stockholders. See "Risk Factors" on page 11 to read about factors you should consider before purchasing shares of our common stock.
_______________________________________
NEITHER THE SECURITIES AND EXCHANGE COMMISSION ("SEC") NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES, OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is April 12, 2016
TABLE OF CONTENTS
PROSPECTUS SUMMARY | 5 |
SUMMARY CONSOLIDATED FINANCIAL DATA | 10 |
RISK FACTORS | 11 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS | 18 |
USE OF PROCEEDS | 19 |
DETERMINATION OF OFFERING PRICE | 19 |
DILUTION | 20 |
SELLING STOCKHOLDERS | 21 |
PLAN OF DISTRIBUTION | 22 |
MARKET PRICE OF COMMON STOCK AND OTHER STOCKHOLDER MATTERS | 24 |
EXCHANGE RATE INFORMATION | 26 |
SELECTED CONSOLIDATED FINANCIAL DATA | 27 |
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 28 |
OUR CORPORATE HISTORY AND BACKGROUND | 36 |
DESCRIPTION OF BUSINESS | 38 |
MANAGEMENT | 55 |
EXECUTIVE COMPENSATION | 60 |
PRINCIPAL STOCKHOLDERS | 66 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS | 67 |
DESCRIPTION OF SECURITIES | 68 |
TAXATION | 71 |
ENFORCEABILITY OF CIVIL LIABILITIES | 77 |
EXPENSES RELATED TO THIS OFFERING | 78 |
LEGAL MATTERS | 78 |
EXPERTS | 78 |
WHERE YOU CAN FIND MORE INFORMATION | 78 |
FINANCIAL STATEMENTS | 79 |
PART II INFORMATION NOT REQUIRED IN THE PROSPECTUS | 141 |
SIGNATURES | 145 |
EXHIBIT INDEX | 146 |
You should rely only on the information contained in or incorporated by reference in this prospectus and any applicable prospectus supplement. We have not authorized anyone to provide you with different or additional information. If anyone provides you with different or inconsistent information, you should not rely on it. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of securities described in this prospectus. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus or any prospectus supplement, as well as information we have previously filed with the Securities and Exchange Commission ("SEC") and incorporated by reference herein, is accurate as of the date on the front of those documents only. Our business, financial condition, results of operations and prospects may have changed since those dates.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that you should consider before investing in the common stock. You should carefully read the entire prospectus, including "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the consolidated financial statements, before making an investment decision. Certain statements in this summary are forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from future results contemplated in the forward-looking statements. See "Cautionary Note Regarding Forward-Looking Statements." All references to "we," "us," "our," "SPLIF" and the "Company" mean Nutritional High International Inc.
All dollar amounts in this prospectus are expressed in Canadian dollars unless otherwise indicated. The Company's accounts are maintained in Canadian dollars and the Company's financial statements are prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board. All reference to "U.S. dollars", "USD", or to "US$" are to United States dollars.
Overview
The Company mainly conducts its business in the medical marijuana, retail marijuana and hemp infused products sectors in the United States, where such activity is permitted and regulated by applicable laws, through entities that hold a valid license, which we refer to as licensed operators, to produce and distribute such products in accordance with applicable regulations. The Company also pursues similar opportunities in Canada; however, it does not expect to invest significant resources in Canada due to regulatory uncertainty.
Marijuana, for the purposes of this prospectus, is referred to herein as all parts of the plant of the genus cannabis, the seeds thereof, the resin extracted from any part of the plant, and every compound, manufacture, salt, derivative, mixture, or preparation of the plant, its seeds, or its resin, but does not include industrial hemp, nor does it include fiber produced from stalks, oil, or cake made from the seeds of the plant, sterilized seed of the plant which is incapable of germination, or the weight of any other ingredient combined with marijuana to prepare topical or oral administrations, food, drink, or other product.
Traditionally, the female marijuana or cannabis plant was consumed by smoking. The flower of the plant is dried and smoked either through a pipe, a cigarette or a filtration device/apparatus such as a water pipe or a vaporizer.
Leafs, nodes and stems are not typically utilized for consuming cannabis through smoking, but are used in production of other products such as oil extracts, referring to hereinafter as marijuana concentrate, which are produced by extracting cannabinoids from marijuana by a method including, but not limited to use of solvent, water, ice, dry ice or propylene glycol, glycerin, butter, olive oil or other typical cooking fats. The marijuana concentrate is then used to manufacture a products, referring to hereinafter as marijuana-infused products, for medical or recreational adult use in jurisdictions where permitted by the applicable regulatory authorities that are intended for use or consumption other than by smoking.
Regulations differ significantly amongst the U.S. states. Some U.S. states only cultivation, processing and distribution of marijuana for medical purposes only, which we refer as medical marijuana, and may also include marijuana-infused products, which we refer as medical marijuana-infused products. Some U.S. states may also permit cultivation, processing and distribution of marijuana for recreational purposes, which we refer as retail marijuana or recreational marijuana, and may also include marijuana-infused products, which we refer as retail marijuana-infused products.
Most U.S. states that have legalized medical marijuana or retail marijuana impose a range of requirements on the business operators, including obtaining a license from state governmental authorities. Some states (such as Colorado) require licensed operators (or their shareholders) to be residents of that U.S. State, which we refer as residency requirement. Other states (such as Illinois, Nevada, Maryland and Arizona) do not impose a residency requirement. The State of Oregon permits out-state ownership, however, such ownership is subject to a number of restrictions.
For the marijuana related products, the Company's strategy in the states with residency requirements is focused on providing products or services to the industry rather than directly owning production or retail operations. The Company is currently actively pursuing the opportunities in the State of Colorado and Illinois. The State of Colorado permits the sale, possession, use, production, distribution and personal cultivation of marijuana, however, that the Colorado Supreme Court has held that even workers who use marijuana for medical reasons may be discharged for violation of federal law. On the other hand, the State of Illinois currently allows only the medical use of marijuana under very controlled circumstances.
In addition, pursuant to federal regulations, hemp-infused products (also refer to as hemp stalk oil-infused products) are excluded from the definition of "marihuana", as long as the product does not contain active levels of tetrahydrocannabinol ("THC"), the psychoactive compound of the cannabis plant. It is generally accepted that products with less than 0.3% THC do not contain active levels of THC. Changes in related state and federal regulation could have a material positive or negative impact on the Company's operations.
The Company has two distinct objectives as a part of the separate business units:
| | Ø | Marijuana-Infused Products Segment. The Company is focused on developing, acquiring and designing marijuana-infused products and marijuana concentrate products and brands for use by licensed operators entering into royalty or raw materials and packaging agreements with the Company in jurisdictions where permitted; and |
| | Ø | Hemp-Infused Products Segment. The Company is focused on developing, acquiring, manufacturing and distributing products infused with non-psychoactive constituent of the industrial hemp plant (less than 0.3% THC or otherwise as permitted by applicable law), which may be distributed in all 50 states. The Company is pursuing a retail distribution strategy through networks of retail dispensaries where THC products are sold, head shops, vitamin stores and independent grocers via direct sales and distributors and is considering online sales, and multi-level marketing. |
The two segments we described above are operating segments only and that for financial reporting purposes we combine them and report our results as one segment. The Company will establish operations only in U.S. states which have implemented adequate licensing frameworks and are in compliance with the Cole Memo as hereinafter described below.
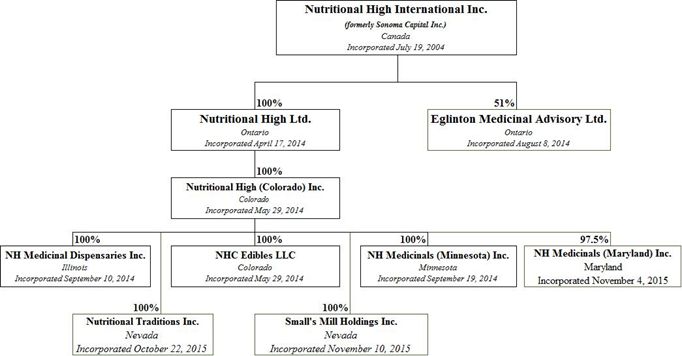
We are a holding company and we conduct our business through our operating subsidiaries as follows:
NHC Edibles LLC ("NHC") and Nutritional High (Colorado) Inc. ("NHCI") carry out the Company's operations in the Marijuana–Infused Products Segment. NHC's activities in the State of Colorado includes but not limited to acquiring and leasing real estate to licensed operators and entering into raw materials and packaging agreements and financing agreements with licensed operators who are focused in the retail market and subject to residency requirements, that do not require it to obtain a licence.
NH Medicinal Dispensaries Inc ("NHMDI") and Small's Mill Holdings Inc. ("SMHI") carry out the Company's operations in the Marijuana Sector in the State of Illinois. NHMDI has submitted an application for a dispensary license with the State of Illinois and has received an authorization to register a medical marijuana dispensary. SMHI has taken possession of the Lawrenceville Property under the Lawrenceville PSA.
Nutritional Traditions Inc. carries out the Company's operations in the Hemp-Infused Product Segment. See "Our Corporate History and Background – Our Subsidiaries"
Marijuana-Infused Products Segment
In its Marijuana-Infused Products Segment, the Company is focused on developing, acquiring and designing marijuana-infused products and marijuana concentrate products and brands for use by licensed operators who enter into raw materials and packaging agreements or royalty agreements with the Company in respect to the Company's brands, recipes and know-how ("NH Licensed Operators") or sell packaging to licensed operators with the Company's branding, as permitted by the applicable regulation. The Marijuana-Infused Products Segment is solely focused on the U.S. states where marijuana-infused products are permitted by law and regulation. We have no current agreements with licensed operators other than the agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute our oils and edibles in Illinois. The agreement provides for a right to negotiate an agreement to negotiate the framework in good faith, but provides for no financial terms in this regard. As such, the Company considers the agreement not material to its operations at this time. We currently have no commercial products.
The Company's business model in its Marijuana-Infused Products Segment differs depending on the residency requirements of the applicable jurisdiction. Most U.S. states that have legalized marijuana for medical or recreational use require the businesses or individuals to hold a valid license under applicable regulation in the respective U.S. state issued by the applicable state authorities. In some U.S. states, for a licensed operator to be eligible to be granted a license, the owners of the licensed operator must be residents of such U.S. state. As such, listed companies or other widely held enterprises are ineligible to obtain a license in those U.S. states where a licensed operator must be a state resident. In such U.S. states, the Company will work with a licensed operator to provide them with financing, licensing of its products, recipes and brands, know how, consulting services and may purchase the facilities where such license operators operate or intend to operate. In U.S. states where there are no residency requirements and where the Company may acquire licensing on its own, the Company may apply to become a license operator. The Company will not operate in jurisdictions which have not legalized marijuana, and does not intend on operating in jurisdictions which have legalized marijuana but have not developed and imposed a licensing regime for licensed operators.
In certain jurisdictions, and more specifically in the states with the residency requirements, the Company may conduct business in the value chain segments, which do not require a license from the requisite regulatory authorities. Such ancillary value chain segments do not directly handle, process, manufacture, cultivate or distribute cannabis products, and may include: unsecured lending, providing real estate to licensed operators, equipment leasing and providing non-cannabis raw materials (such as packaging, food ingredients, etc.) The focus of this strategy is to provide the services to licensed operators that are focused on extraction and processing of cannabis to enable them to attain a stronger competitive advantage in the market, while proving an acceptable economic return to the Company.
In certain circumstances the Company may pursue other value chain segments, such as operating a dispensary, in the states where the regulatory environment is unsuitable to earn an economic rate of return in the extraction/processing segment, given the Company's assessment. This aim of this strategy is to secure a foothold in such markets, through obtaining a license to operate a business that is not directly related to extraction/processing and then partner with another licensed operator who is able to operate in the extraction/processing space. The Company has employed this type of strategy in the State of Illinois, where it is working to obtain a dispensary license, but has also partnered with another licensed operator to be in a position to sell its products in Illinois.
Hemp-Infused Products Segment
In its Hemp-Infused Products Segment, the Company is focused on developing, acquiring, manufacturing and distributing products infused with non-psychoactive constituent of the industrial hemp plant (less than 0.3% THC or as otherwise permitted by applicable law), which may be distributed in all 50 U.S. states. The Company is pursuing a retail distribution strategy through networks of retail dispensaries where THC products are sold, head shops, vitamin stores and independent grocers via direct sales and distributors and is considering online sales, and multi-level marketing. Having access to the Company's expertise in manufacturing products from cannabis, these hemp-infused products are expected to have a competitive edge in the market. The products are being sold under the "Nutritional Traditions" brand name and initially available to California retail customers. Distribution will be expanded outside of California beginning in 2016.
U.S. Federal Law and Cole Memo Compliance
The use, possession, sale, cultivation and transportation of cannabis remains illegal under U.S. federal law. The concepts of "medical marijuana" and "retail marijuana" do not exist under U.S. federal law. The Federal Controlled Substances Act classifies "marihuana" as a Schedule I drug. Under U.S. federal law, a Schedule I drug or substance has a high potential for abuse, no accepted medical use in the United States, and a lack of safety for the use of the drug under medical supervision. As such, marijuana-related practices or activities, including without limitation, the manufacture, importation, possession, use or distribution of marijuana are illegal under U.S. federal law. Strict compliance with state laws with respect to marijuana will neither absolve the Company of liability under U.S. federal law, nor will it provide a defense to any federal proceeding which may be brought against the Company.
In a memorandum dated August 29, 2013, addressed to "All United States Attorneys" from James M. Cole, Deputy Attorney General (the "Cole Memo"), the U.S. Department of Justice acknowledged that certain U.S. states had enacted laws relating to the use of marijuana and outlined the U.S. federal government's enforcement priorities with respect to marijuana notwithstanding the fact that certain U.S. states have legalized or decriminalized the use, sale and manufacture of marijuana:
| | · | Preventing the distribution of marijuana to minors; |
| | · | Preventing revenue from the sale of marijuana from going to criminal enterprises, gangs, and cartels; |
| | · | Preventing the diversion of marijuana from U.S. states where it is legal under state law in some form to other U.S. states; |
| | · | Preventing U.S. State-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity; |
| | · | Preventing violence and the use of firearms in the cultivation and distribution of marijuana; |
| | · | Preventing drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use; |
| | · | Preventing the growing of marijuana on public lands and the attendant public safety and environmental dangers posed by marijuana production on public lands; and |
| | · | Preventing marijuana possession or use on U.S. federal property. |
There is no guarantee that the current presidential administration will not change its stated policy regarding the low-priority enforcement of U.S. federal laws that conflict with state laws. Additionally, any new U.S. federal government administration that follows could change this policy and decide to enforce the U.S. federal laws vigorously.
The Company's operations are compliant with the Cole Memo.
Recent Developments
Illinois Joint-Venture, Oils and Edibles Relationship and Conditional Approval for Registration of the Dispensary
The Company has significant developments in Illinois, which include:
| · | Entered into joint-venture agreement ("JV Agreement") with ILDISP LLC, which has ties to the Illinois medical marijuana industry, to build and assist in operating the Company's planned dispensary in Lawrenceville, Illinois; |
| · | Been granted conditional approval ("Conditional Approval") by the Illinois Department of Financial and Professional Regulation ("IDFPR") to establish the Lawrenceville Dispensary; |
| · | Commenced renovations at the Lawrenceville Dispensary property; and |
| · | Entered into an agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute Nutritional High's oils and edibles in Illinois. |
Under the terms of the JV Agreement ILDISP LLC will fund up to USD $300,000 of the expenses and working capital required to complete and launch the Lawrenceville Dispensary. In addition, subject to regulatory approval (as discussed below), ILDISP LLC will provide the Company with a guarantee for half the Seller Take-Back Mortgage. If ILDISP LLC makes its full contribution, in exchange for its contribution, ILDISP LLC shall receive a 50% interest in NHMD, the Company's wholly owned subsidiary which holds the Conditional Approval for the Lawrenceville Dispensary, and a 50% interest in SMHI, the Company's wholly owned subsidiary which holds the Company's interest in the Dispensary real estate property located in Lawrenceville, IL. The joint venture might require a need for additional capital infusions in excess of $300,000, which would require the Company to make additional contributions, failing to do which may result in reduction of the Company's interest in NHMDI and SMHI. In addition, ILDISP's failure to contribute may create an greater need for the Company to contribute additional capital, which may not be available to the Company on favorable terms or at all.
ILDISP LLC has already made initial advances to fund the Lawrenceville Dispensary renovations and property mortgage payments. The joint venture is subject to the approval of the IDFPR. Furthermore, it is contemplated that ILDISP LLC will contribute to the management of the Lawrenceville Dispensary and its relationships in the surrounding community will help accelerate the growth and development of the Dispensary. Upon securing IDFRP approval, it is expected that a shareholders agreement, in a form and substance acceptable to the Company and ILDISP LLC, will be entered into. While the form of shareholders agreement will not be finalized until IDFRP approval has been secured, the shareholders agreement will contain customary provisions such as governance of the rights and responsibilities of the shareholders, respective share ownership and dilution mechanism if additional capital is required, corporate governance protection and various other checks and balances as between the shareholders. The Board of Directors of both NHMD and SMHI shall be composed of an equal number of directors appointed by both ILDISP and Nutritional High, once the requisite approvals are obtained.
In addition, NHMD has been advised by the IDFPR that it has been awarded Conditional Approval to register the Lawrenceville Dispensary under the Compassionate Use of Medical Cannabis Pilot Program Act (Illinois) ("CUMCPPA"). The Conditional Approval sets out the requirements that NHMD must fulfill prior to IDFPR approving the registration of the dispensary, which includes completing the renovations and passing the final inspection to the satisfaction of IDFPR. Upon meeting IDFRP's conditions, it is expected that NHMD will be granted a final license to operate the Lawrenceville Dispensary.
The Company also has entered into an agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute Nutritional High's oils and edibles in Illinois. The cultivation and extraction facility is licensed with Illinois Department of Agriculture and was amongst the first of the companies to commence commercial cultivation and processing of cannabis products under the CUMCPPA. The Company will develop its business framework with the extraction facility over the next 18 months and will provide updates as this business initiative develops. The agreement provides for a right to negotiate an agreement to negotiate the framework in good faith, but provides for no financial terms in this regard. As such, the Company considers the agreement not material to its operations at this time.
Lawrenceville Dispensary Application and Lawrenceville Property Acquisition
The Company has taken possession of the real estate property in Lawrenceville, Illinois ("Lawrenceville Property") on November 25, 2015, under a purchase and sale agreement between NHMDI and the vendor of the Lawrenceville Property ("Lawrenceville PSA"). The final acquisition price for the Lawrenceville property was USD $350,000. The Company has also negotiated a seller take-back mortgage ("Seller Take-Back Mortgage"), which will have a 15 year amortization period, bearing an interest at the rate of 6% and be due in two years from the date of issuance as a balloon payment. Upon payment of the Seller Take-Back Mortgage, title to the Lawrenceville Property will automatically transfer SMHI (as hereinafter defined).
Private Placement
On December 2, 2015, the Company completed a non-brokered private placement of 4,200,000 units at $0.05 per unit for gross proceeds of $210,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
Hemp Product Launch
The Company has rolled out of the first three products in its Hemp-Infused Products Segment. Initial products are being made available to consumers in California include capsules, push caps for water bottles, and a push cap formulation for post-exercise recovery called "Rapid Recovery". In addition, the Company intends to commenced sales of these products in Colorado in 2016, targeting sales to marijuana dispensaries in Colorado, further developing sales channels for the Company's marijuana oil and edible brands.
Equity Purchase Agreement with Kodiak Capital
On December 23, 2015, we entered into the Purchase Agreement with Kodiak Capital. Pursuant to the terms of the Purchase Agreement, Kodiak Capital committed to purchase up to $1,000,000 of our common stock over a period until the earlier of (i) the date on which Kodiak Capital shall have purchased put shares pursuant to the Purchase Agreement for an aggregate purchase price of $1,000,000, or (ii) December 31, 2016. The purchase price of the shares that may be sold to Kodiak Capital under the Purchase Agreement will be equal to the greater of (i) 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account, or (ii) CDN $0.05.
We do not have the right to commence any sales to Kodiak Capital under the Purchase Agreement until the SEC has declared effective the registration statement of which this prospectus forms a part. Thereafter, we may, from time to time and at our sole discretion, direct Kodiak Capital to purchase shares of our common stock, but we would be unable to sell shares to them if such purchase would result in their respective beneficial ownership equaling more than 9.99% of the outstanding common stock. Except as described in this prospectus, there are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any sales of our common stock to Kodiak Capital. We may at any time in our sole discretion terminate the Purchase Agreement without fee, penalty or cost upon one business day notice. Kodiak Capital is not permitted to engage in any short shales of our common stock during the period covered by the Purchase Agreement.
In connection with the Purchase Agreement, we also entered into the Registration Rights Agreement, pursuant to which we are obligated to file a registration statement with the SEC by January 30, 2016. Kodiak Capital agreed to extend the filing deadline to February 10, 2016.
At an assumed purchase price of CDN $0.05 (equal to the greater of (i) 75% of the closing price of our common stock of CDN $0.035 on April 11, 2016, or (ii) CDN $0.05), we will be able to receive $1,000,000 in gross proceeds, assuming the sale of the entire 29,178,000 shares being registered hereunder pursuant to the Purchase Agreement, assuming the USD:CDN exchange rate of $1.4598. However, we may be required to register more shares in order to receive the full amount of the investment amount if the USD:CDN exchange rate increases at the drawdown.
Risk Factors
We face certain risks, challenges and uncertainties that may materially affect our business, financial condition, results of operations and prospects. The primary ones include:
| • | Funding from our Purchase Agreement with Kodiak Capital may be limited or be insufficient to fund our operations or to implement our strategy; |
| | |
| • | The Company has limited operating history and encounters risks and uncertainties which might significantly harm the Company's business if those where not addressed properly. In addition, there is substantial doubt upon the Company's ability to continue as a going concern. At January 31, 2016 the Company had working capital (deficiency) of $(327,988) (July 31, 2015 - $116,439), had not yet achieved profitable operations, has accumulated losses of $3,393,225 (July 31, 2015 - $2,740,442) and expects to incur further losses in the development of its business; |
| | |
| • | Our business is dependent on laws pertaining to the marijuana industry; |
| | |
| • | Marijuana-related practices or activities are illegal under U.S. federal laws. Strict enforcement of federal law regarding marijuana would likely result in our inability to proceed with our business plan; |
| | |
| • | We face an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury; |
| | |
| • | The Company may need to raise significant additional funds in order to support its growth, however, the Company cannot be sure that this additional financing, if needed, will be available on acceptable terms, or at all; |
| | |
| • | The success of the Company is dependent on the performance of its senior management. The loss of the services of these persons would have a material adverse effect on the Company's business and its prospects; |
| | |
| • | Exchange rate fluctuations may adversely affect the Company's financial position and results; |
| | |
| • | You may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. federal courts may be limited because we are incorporated under Canadian law, a substantial portion of our assets are in Canada and most of our directors and executive officers reside outside the United States; |
| | |
| • | If the selling shareholder sells a large number of shares all at once or in blocks, the market price of our shares would most likely decline; |
| | |
| • | The sale of our common stock to Kodiak Capital may cause dilution, and the sale of the shares of common stock acquired by Kodiak Capital, or the perception that such sales may occur, could cause the price of our common stock to fall; |
| | |
| • | Kodiak Capital will pay less than the then-prevailing market price for our common stock; |
| | |
| • | The Company's put right may convert into a greater number of shares than we have assumed in this prospectus; |
| | |
| • | The market price of our common stock may fluctuate significantly; |
| | |
| • | We are an "emerging growth company" and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors; |
| | |
| • | Our common stock is subject to the "penny stock" rules of the SEC and the trading market in our securities is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment in our stock; and |
| | |
| • | Because we do not intend to pay any cash dividends on our common stock, our stockholders will not be able to receive a return on their shares unless they sell them. |
You should consider the risks discussed in the "Risk Factors" section and elsewhere in this prospectus before investing in our common stock.
Emerging Growth Company
We are an "emerging growth company" as defined in Section 2(a)(19) of the Securities Act of 1933, as amended (the "Securities Act"), as modified by the Jumpstart Our Business Startups Act of 2012 (the "JOBS Act"). As such, we are eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not "emerging growth companies" including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (the "Sarbanes-Oxley Act"), reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We intend to take advantage of all of these exemptions.
In addition, Section 107 of the JOBS Act also provides that an "emerging growth company" can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards, and delay compliance with new or revised accounting standards until those standards are applicable to private companies. We have elected to take advantage of the benefits of this extended transition period.
We could be an emerging growth company until the last day of the first fiscal year following the fifth anniversary of our first common equity offering, although circumstances could cause us to lose that status earlier if our annual revenues exceed $1.0 billion, if we issue more than $1.0 billion in non-convertible debt in any three-year period or if we become a "large accelerated filer" as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the "Exchange Act").
Our principal executive offices are located at 77 King Street West, Suite 2905 P. O. Box 121, Toronto Ontario M5K 1H1 and our telephone number is (416) 840-3798. Our website address is www.nutritionalhigh.com. The information contained therein or connected thereto shall not be deemed to be incorporated into this preliminary prospectus or the registration statement of which it forms a part. The information on our website is not part of this preliminary prospectus.
The Offering
| Common stock offered for resale: | | 29,178,000 shares of common stock |
| | | |
| Common Stock outstanding after this offering: | | 171,120,514 shares of common stock(1) |
| | | |
| Use of proceeds: | | We will not receive any proceeds from the sale of shares by the selling stockholder. However, we will receive proceeds from the sale of shares to Kodiak Capital pursuant to the Purchase Agreement. We intend to use the net proceeds received from any such sales of shares to Kodiak Capital under the Purchase Agreement for general corporate and working capital purposes and acquisitions or assets, businesses or operations or for other purposes that the Board of Directors, in its good faith deem to be in the best interest of the Company. |
| | | |
| Risk factors: | | There are significant risks involved in investing in the Company. For a discussion of risk factors you should consider before buying our common stock, see "Risk Factors" beginning on page 11. |
| | | |
| Ticker symbol: | | OTCQB: "SPLIF" CSE: "EAT" |
| | | |
| Underwriter: | | Kodiak Capital Group, LLC is considered an underwriter of the Company. An underwriter must make public disclosure similar to disclosure made by an issuer in the event of purchases and sales of securities. |
| | (1) | The number of shares of our common stock outstanding after this offering is based on 141,942,514 shares of common stock outstanding as of April 12, 2016 and excludes shares of common stock issuable upon exercise of warrants and conversion of convertible notes prior to this offering. |
SUMMARY CONSOLIDATED FINANCIAL DATA
The summary financial information set forth below has been derived from the financial statements of the Company for periods presented and should be read in conjunction with the financial statements and the notes thereto included elsewhere in this prospectus and in the information set forth in the section titled "Management's Discussion and Analysis of Financial Condition and Results of Operations."
| | | Six months
ended | | | For the Year Ended | | From Incorporation (April 17, 2014) to | |
| | | January 31,
2016 (Unaudited) | | | July 31, 2015 | | July 31, 2014 | |
| Statements of Net Loss and Comprehensive Loss: | | | | | | | | |
| Total revenue | | $ | Nil | | | Nil | | Nil | |
| Total expenses | | | (832,188 | ) | | | (2,179,122 | ) | | | (681,155 | ) |
| Other income (expenses) | | | 179,558 | | | | 110,762 | | Nil | |
| Net loss and comprehensive loss | | | (661,552 | ) | | | (2,083,645 | ) | | | (681,155 | ) |
| Basic and diluted loss per common share | | | (0.005 | ) | | | (0.2 | ) | | | (0.01 | ) |
| Current Assets | | | 233,802 | | | | 388,713 | | | | 673,525 | |
| Total Assets | | | 2,608,669 | | | | 1,972,588 | | | | 695,477 | |
| Total Liabilities | | | 1,199,035 | | | | 1,142,249 | | | | 220,150 | |
| Shareholders' Equity | | $ | 1,409,634 | | | | 830,339 | | | | 475,327 | |
RISK FACTORS
You should carefully consider the risks described below together with all of the other information included in this Prospectus before making an investment decision with regard to our securities. The statements contained in or incorporated into this Prospectus that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Business
Funding from our Purchase Agreement with Kodiak Capital may be limited or be insufficient to fund our operations or to implement our strategy.
Under our Purchase Agreement with Kodiak Capital, upon effectiveness of the registration statement of which this prospectus is a part, and subject to other conditions, we may direct Kodiak Capital to purchase up to $1,000,000 of our shares of common stock until the earlier of (i) the date on which Kodiak Capital shall have purchased put shares pursuant to the Purchase Agreement for an aggregate purchase price of $1,000,000, or (ii) December 31, 2016. The purchase price of the shares that may be sold to Kodiak Capital under the Purchase Agreement will be equal to the greater of (i) 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account, or (ii) CDN $0.05.
There can be no assurance that we will be able to receive all or any of the purchase price from Kodiak Capital because the Purchase Agreement contains certain limitations, restrictions, requirements, conditions and other provisions that could limit our ability to cause Kodiak Capital to buy common stock from us. For instance, there is a floor price of CDN $0.05 pursuant to the Purchase Agreement. If the Company delivers a put notice and the purchase price is below CDN $0.05, Kodiak Capital may elect to purchase all, or any portion of the put shares for CDN $0.05. There is no assurance that Kodiak Capital is willing to pay CDN $0.05 for the put shares and as such we may not be able to receive all or any of the purchase price. Also, the Company is prohibited from issuing a drawdown notice if the amount requested in such drawdown notice would cause the Company to sell or Kodiak Capital to purchase an aggregate number of shares of the Company's common stock which would result in beneficial ownership by Kodiak Capital of more than 9.99% of the Company's common stock (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder).
The extent to which we rely on Kodiak Capital as a source of funding will depend on a number of factors, including the amount of working capital needed, the prevailing market price of our common stock and the extent to which we are able to secure working capital from other sources. If obtaining sufficient funding from Kodiak Capital were to prove unavailable or prohibitively dilutive, we would need to secure another source of funding. Even if we sell all $1,000,000 of common stock under the Purchase Agreement with Kodiak Capital, we will still need additional capital to fully implement our current business, operating plans and development plans.
The Company has limited operating history and encounters risks and uncertainties which might significantly harm the Company's business if those where not addressed properly. In addition, there is substantial doubt upon the Company's ability to continue as a going concern.
The Company has a very limited history of operations, is in the early stage of development and must be considered a start-up. As such, the Company is subject to many risks common to such enterprises, including under-capitalization, cash shortages, limitations with respect to personnel, financial and other resources and lack of revenues. There is no assurance that the Company will be successful in achieving a return on shareholders' investment and the likelihood of success must be considered in light of its early stage of operations. The Company has no history of earnings. Because the Company has a limited operating history in emerging area of business, you should consider and evaluate its operating prospects in light of the risks and uncertainties frequently encountered by early-stage companies in rapidly evolving markets. These risks may include:
| | · | risks that it may not have sufficient capital to achieve its growth strategy; |
| | · | risks that it may not develop its product and service offerings in a manner that enables it to be profitable and meet its customers' requirements; |
| | · | risks that its growth strategy may not be successful; |
| | · | risks that fluctuations in its operating results will be significant relative to its revenues; and |
| | · | risks relating to an evolving regulatory regime. |
The Company's future growth will depend substantially on its ability to address these and the other risks described in this section. If it does not successfully address these risks, its business may be significantly harmed.
In addition, at January 31, 2016 the Company had working capital (deficiency) of $(327,988) (July 31, 2015 - $116,439), had not yet achieved profitable operations, has accumulated losses of $3,393,225 (July 31, 2015 - $2,740,442) and expects to incur further losses in the development of its business, all of which describes the material uncertainties that cast significant doubt upon the Company's ability to continue as a going concern. The Company will require additional financing in order to conduct its planned business operations, meet its ongoing levels of corporate overhead and discharge its liabilities and commitments as they come due. There can be no assurance that we will have adequate capital resources to fund planned operations or that any additional funds will be available to us when needed or at all, or, if available, will be available on favorable terms or in amounts required by us. If we are unable to obtain adequate capital resources to fund operations, we may be required to delay, scale back or eliminate some or all of our operations, which may have a material adverse effect on our business, results of operations and ability to operate as a going concern.
The Company relies on securing agreements with NH Licensed Operators in the targeted jurisdictions that have been able to obtain a License with the appropriate regulatory authorities. Failure of a NH Licensed Operators to comply with the requirements of their License or any failure to maintain their License would have a material adverse impact on the business, financial condition and operating results of the Company.
The regulatory framework in most U.S. states restricts the Company from obtaining a License to grow, store, process and sell marijuana products. As such, the Company relies on securing agreements with NH Licensed Operators in the targeted jurisdictions that have been able to obtain a License with the appropriate regulatory authorities. Failure of a NH Licensed Operators to comply with the requirements of their License or any failure to maintain their License would have a material adverse impact on the business, financial condition and operating results of the Company. Should the regulatory authorities not grant a License or grant a License on different terms unfavorable to the NH Licensed Operators, and should the Company be unable to secure alternative NH Licensed Operators, the business, financial condition and results of the operation of the Company would be materially adversely affected.
If the federal government changes its approach to the enforcement of laws relating to marijuana, the Company would need to seek to replace those tenants with non-marijuana tenants, who would likely pay lower rents. It is likely that the Company would realize an economic loss on its capital acquisitions and improvements made to its capital assets specific to the marijuana industry, and the Company would likely lose all or substantially all of its investments in the markets affected by such regulatory changes.
The Company will advance significant funds to Palo Verde in a form of unsecured loans, which the Company may not be able to collect if Palo Verde fails to achieve commercial production
Palo Verde is a development stage entity with limited capacity to raise funds. There is no assurance that any or all of the amounts loaned will be recovered by the Company. If Palo Verde is unable to commence commercial production in a profitable fashion or secure an alternative source of funds, the full amount of the loan might be written off.
Our business is dependent on laws pertaining to the marijuana industry.
Our business operation is dependent on state laws pertaining to the marijuana industry. Continued development of the marijuana industry is dependent upon continued legislative authorization of marijuana at the state level. Any number of factors could slow or halt progress in this area. Further, progress, while encouraging, is not assured. While there may be ample public support for legislative action, numerous factors impact the legislative process. Any one of these factors could slow or halt use of marijuana, which would negatively impact our proposed business.
As of December 20, 2015, 23 states, the District of Columbia and Guam allow their residents to use medical marijuana. Voters in the states of Colorado and Washington approved and implemented regulations to legalize cannabis for adult use. The state laws are in conflict with the Federal Controlled Substances Act, which makes marijuana use and possession illegal on a national level. The Obama administration has made numerous statements indicating that it is not an efficient use of resources to direct federal law enforcement agencies to prosecute those lawfully abiding by state-designated laws allowing the use and distribution of medical marijuana. However, there is no guarantee that the administration will not change its stated policy regarding the low-priority enforcement of federal laws. Additionally, any new administration that follows could change this policy and decide to stringently enforce the federal laws. Any such change in the federal government's enforcement of current federal laws could cause significant financial damage to the Company and its stockholders, including the potential exposure to criminal liability.
Laws and regulations affecting our industry are constantly changing.
The constant evolution of laws and regulations affecting the marijuana industry could detrimentally affect our operations. Local, state and federal medical marijuana laws and regulations are broad in scope and subject to changing interpretations. These changes may require us to incur substantial costs associated with legal and compliance fees and ultimately require us to alter our business plan. Furthermore, violations of these laws, or alleged violations, could disrupt our business and result in a material adverse effect on our operations. In addition, we cannot predict the nature of any future laws, regulations, interpretations or applications, and it is possible that regulations may be enacted in the future that will be directly applicable to our business.
Marijuana-related practices or activities are illegal under U.S. federal laws. Strict enforcement of federal law regarding marijuana would likely result in our inability to proceed with our business plan.
The concepts of "medical marijuana" and "retail marijuana" do not exist under U.S. federal law. The Federal Controlled Substances Act classifies "marihuana" as a Schedule I drug. Under U.S. federal law, a Schedule I drug or substance has a high potential for abuse, no accepted medical use in the United States, and a lack of safety for the use of the drug under medical supervision. As such, marijuana-related practices or activities, including without limitation, the manufacture, importation, possession, use or distribution of marijuana are illegal under U.S. federal law. Strict compliance with state laws with respect to marijuana will neither absolve the Company of liability under U.S. federal law, nor will it provide a defense to any federal proceeding which may be brought against the Company.
There are regulation that may hinder the Company's ability to establish and maintain bank accounts due to the marijuana business.
The U.S. federal prohibitions on the sale of marijuana may result in NH Licensed Operators, the Company and its subsidiaries being restricted from accessing the U.S. banking system and they may be unable to deposit funds in federally insured and licensed banking institutions. Banking restrictions could be imposed due to the Company and banking institutions could refuse to accept payments from NH Licensed Operators. At times NH Licensed Operators, and in some cases the Company or its subsidiaries, may not have deposit services and are at risk that any bank accounts they have could be closed at any time. Such risks increase costs to the Company and to the NH Licensed Operators. Additionally, similar risks are associated with large amounts of cash at these businesses. These businesses require heavy security with respect to holding and transport of cash, whether or not they have bank accounts.
In the event financial service providers do not accept accounts or transactions related to the marijuana industry, it is possible that the Company or NH Licensed Operators may seek alternative payment solutions, including but not limited to crypto currencies such as Bitcoin. There are risks inherent in crypto currencies, most notably its volatility and security issues. If the industry was to move towards alternative payment solutions and accept payments in crypto currency the Company would have to adopt policies and protocols to manage its volatility and exchange rate risk exposures. The Company's inability to manage such risks may adversely affect the Company's operations and financial performance.
We face an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury.
As a licensing company (in the case of the Company) and a manufacturer and distributor of products (in the case of the licensed operators) designed to be ingested by humans, the licensed operators and the Company face an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of marijuana-infused products based on the Company's recipes and brands involve the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of the Company's and the licensed operator's products alone or in combination with other medications or substances could occur.
We may incur unexpected expenses relating to the recall and any legal proceedings that might arise in connection with the recall.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If any of the products developed by the Company and sold by NH Licensed Operators are recalled due to an alleged product defect or for any other reason, the Company could be required to incur the unexpected expense relating to the recall and any legal proceedings that might arise in connection with the recall. In addition, a product recall may require significant management attention and could harm the image of the brand and Company.
Our insurance does not cover all potential liabilities and such uninsurable risks may cause a material adverse effect on the financial condition of the Company.
The medical and retail marijuana business is subject to several risks that could result in damage to or destruction of properties or facilities or cause personal injury or death, environmental damage, delays in production and monetary losses and possible legal liability. It is not always possible to fully insure against such risks, and the Company may decide not to take out insurance against such risks as a result of high premiums or other reasons. Should such liabilities arise, they could reduce or eliminate any future profitability and result in increasing costs and a decline in the value of the securities of the Company. While the Company and its subsidiaries (as the case may be) carries insurance covering its real estate properties, it is possible that it may not obtain insurance to cover all potential liabilities that may arise as a result any of the above noted risks may cause a material adverse effect on the financial condition of the Company.
The Company may need to raise significant additional funds in order to support its growth, however, the Company cannot be sure that this additional financing, if needed, will be available on acceptable terms, or at all.
The Company may need to raise significant additional funds in order to support its growth, develop new or enhanced services and products, respond to competitive pressures, acquire or invest in complementary or competitive businesses or technologies, or take advantage of unanticipated opportunities. If its financial resources are insufficient, it will require additional financing in order to meet its plans for expansion. The Company cannot be sure that this additional financing, if needed, will be available on acceptable terms, or at all.
Furthermore, any debt financing, if available, may involve restrictive covenants, which may limit its operating flexibility with respect to business matters. If additional funds are raised through the issuance of equity securities, the percentage ownership of existing shareholders will be reduced, such shareholders may experience additional dilution in net book value, and such equity securities may have rights, preferences or privileges senior to those of its existing shareholders.
Access to public and private capital and financing continues to be negatively impacted by many factors as a result of the global financial crisis and global recession. Such factors may impact the Company's ability to obtain debt and equity financing in the future on favorable terms or obtain any financing at all. Additionally, global economic conditions may cause a long term decrease in asset values. If such global volatility, market turmoil and the global recession continue, the Company's operations and financial condition could be adversely impacted.
The Company is subject to risks generally associated with ownership of real estate.
The Company is subject to risks generally associated with ownership of real estate, including: (a) changes in general economic or local conditions; (b) changes in supply of, or demand for, similar or competing properties in the area; (c) bankruptcies, financial difficulties or defaults by tenants or other parties (including licensed operators and NH Licensed Operators); (d) increases in operating costs, such as taxes and insurance; (e) the inability to achieve full stabilized occupancy at rental rates adequate to produce targeted returns; (f) periods of high interest rates and tight money supply; (g) excess supply of rental properties in the market area; (h) liability for uninsured losses resulting from natural disasters or other perils; (i) liability for environmental hazards; and (j) changes in tax, real estate, environmental, zoning or other laws or regulations. There is no assurance that the Company's investments will yield an economic profit.
Weakness in regional and national economies could materially and adversely impact the licensed operators and NH Licensed Operators leasing the real estate properties that the Company's may acquire in the future. If the licensed operators or NH Licensed Operators suffer a business disruption or the Company's ability to collect the rents from those parties may be limited, and the recourse available to the Company can be limited. As such, this may hinder the Company's ability to service its financial obligations, and in some cases may lead to complete loss of the Company's assets if its lenders were to foreclose.
The Company may not be able to deduct certain costs from its revenue for U.S. federal taxation purposes.
U.S. federal prohibitions on the sale of marijuana may result in the Company not being able to deduct certain costs from its revenue for U.S. federal taxation purposes if the U.S. Internal Revenue Service determines that revenue sources of the Company are generated from activities which are not permitted under U.S. federal law.
We may face threats from illegal drug dealers and cartels which could negatively impact the Company, its employees and its business operations.
Currently, there are many drug dealers and cartels that cultivate, buy, sell and trade marijuana in the United States, Canada and worldwide. Many of these dealers and cartels are violent and dangerous, well financed and well organized. It is possible that these dealers and cartels could feel threatened by legalized marijuana businesses such as those with whom the Company does business and could take action against or threaten the Company, its principals, employees and/or agents and this could negatively impact the Company and its business.
The success of the Company is dependent on the performance of its senior management. The loss of the services of these persons would have a material adverse effect on the Company's business and its prospects.
The success of the Company is currently dependent on the performance of its senior management. The loss of the services of these persons would have a material adverse effect on the Company's business and prospects in the short term. There is no assurance the Company can maintain the services of its officers or other qualified personnel required to operate its business. Failure to do so could have a material adverse effect on the Company and its prospects.
The Company is in early development stage and facing many factors that may prevent realization of growth targets.
The Company is currently in the early development stage. There is a risk that the additional resources will be needed and milestones will not be achieved on time, on budget, or at all, as they can be adversely affected by a variety of factors, including some that are discussed elsewhere in these risk factors and the following as it relates to the Company and its licensed operators or NH Licensed Operators, as the case may be:
| | · | delays in obtaining, or conditions imposed by, regulatory approvals; |
| | · | facility design errors; |
| | · | environmental pollution; |
| | · | non-performance by third party contractors; |
| | · | increases in materials or labour costs; |
| | · | construction performance falling below expected levels of output or efficiency; |
| | · | breakdown, aging or failure of equipment or processes; |
| | · | contractor or operator errors; |
| | · | labour disputes, disruptions or declines in productivity; |
| | · | inability to attract sufficient numbers of qualified workers; |
| | · | disruption in the supply of energy and utilities; and |
| | · | major incidents and/or catastrophic events such as fires, explosions, earthquakes or storms. |
The Company faces increasing competition in the medicinal and recreational marijuana industry.
The marijuana industry is highly competitive. The Company will compete with numerous other businesses in the medicinal and recreational industry, many of which possess greater financial and marketing resources and other resources than the Company. The marijuana business is often affected by changes in consumer tastes and discretionary spending patterns, national and regional economic conditions, demographic trends, consumer confidence in the economy, traffic patterns, local competitive factors, cost and availability of raw material and labour, and governmental regulations. Any change in these factors could materially and adversely affect the Company's operations.
The Company expects to face additional competition from new entrants. If the number of legal users of marijuana in its target jurisdiction increases, the demand for products will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products.
The products provided by the Company to NH Licensed Operators may become subject to regulation governing food and related products.
Should the Federal government legalize marijuana for medical or recreational use nation-wide, it is possible that the U.S. Food and Drug Administration ("FDA") would seek to regulate the products under the Food, Drug and Cosmetics Act of 1938. The FDA may issue rules and regulations including certified good manufacturing practices related to the growth, cultivation, harvesting and processing of medical marijuana and marijuana-infused products. Clinical trials may be needed to verify efficacy and safety of the medical marijuana. It is also possible that the FDA would require that facilities where medical marijuana is cultivated be registered with the applicable government agencies and comply with certain federal regulations. In the event any of these regulations are imposed, The Company cannot foresee the impact on its operations and economics. If the Company or the NH Licensed Operators are unable to comply with the regulations and or registration as prescribed by the FDA or another federal agency, the Company or the NH Licensed Operators may be unable to continue to operate in its current form or at all.
The Company's operations are subject to environmental and safety laws and regulations concerning. Violation of such laws and regulations would have a material adverse effect to the Company's business.
The Company's operations are subject to environmental and safety laws and regulations concerning, among other things, emissions and discharges to water, air and land, the handling and disposal of hazardous and non-hazardous materials and wastes, and employee health and safety. The Company will incur ongoing costs and obligations related to compliance with environmental and employee health and safety matters. Failure to comply with environmental and safety laws and regulations may result in additional costs for corrective measures, penalties or in restrictions on our manufacturing operations. In addition, changes in environmental, employee health and safety or other laws, more vigorous enforcement thereof or other unanticipated events could require extensive changes to the Company's operations or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Company.
The Company is relying largely on its own market research to forecast sales.
The Company must rely largely on its own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the marijuana industry in the United States and Canada. A failure in the demand for its products to materialize as a result of competition, technological change, market acceptance or other factors could have a material adverse effect on the business, results of operations and financial condition of the Company.
We are a holding company with no material assets other than the stock of our subsidiaries and intellectual property.
As a holding company with no material assets other than the stock of the Company's operating subsidiaries and intellectual property, nearly all of the Company's funds generated from operations are generated by the Company's operating subsidiaries. The Company's subsidiaries are subject to requirements of various regulatory bodies, both domestically and internationally. Accordingly, if the Company's operating subsidiaries are unable, due to regulatory restrictions or otherwise, to pay the Company's dividends and make other payments to the Company when needed, the Company may be unable to satisfy the Company's obligations when they arise.
The Company is subject to growth related risks including the ability of the Company's management to growth effectively.
The Company may be subject to growth-related risks including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. The inability of the Company to deal with this growth may have a material adverse effect on the Company's business, financial condition, results of operations and prospects.
The Company does not anticipate paying any dividends in the foreseeable future.
The Company has no earnings or dividend record, and does not anticipate paying any dividends on the Common Shares in the foreseeable future. Dividends paid by the Company would be subject to tax and, potentially, withholdings.
Exchange rate fluctuations may adversely affect the Company's financial position and results.
Exchange rate fluctuations may adversely affect the Company's financial position and results. It is anticipated that a significant portion of the Company's business will be conducted in the United States using U.S. dollars. The Company's financial results are reported in Canadian Dollars and costs are incurred primarily in U.S. dollars in its Marijuana–Infused Products and Hemp-Infused Products segments. The depreciation of the Canadian Dollar against the U.S. Dollar could increase the actual capital and operating costs of the Company's U.S. operations and materially adversely affect the results presented in the Company's financial statements.
You may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. federal courts may be limited because we are incorporated under Canadian law, a substantial portion of our assets are in Canada and most of our directors and all of our executive officers reside outside the United States.
The Company is organized under the laws of the Canada Business Corporations Act (Canada) and our executive offices are located outside of the United States in Toronto, Ontario. A majority of our directors and officers, as well as our auditor, reside outside the United States. In addition, a substantial portion of their assets and the Company's assets are located outside of the United States. As a result, you may have difficulty serving legal process within the United States upon us or any of these persons. You may also have difficulty enforcing, both in and outside of the United States, judgments you may obtain in U.S. courts against us or these persons in any action, including actions based upon the civil liability provisions of U.S. Federal or state securities laws. Furthermore, there is substantial doubt as to the enforceability in Canada against us or against any of our directors, officers and the expert named in this prospectus who are not residents of the United States, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities based solely upon the civil liability provisions of the U.S. federal securities laws. In addition, shareholders in Canada companies may not have standing to initiate a shareholder derivative action in U.S. federal courts.
As a result, our public shareholders may have more difficulty in protecting their interests through actions against us, our management, our directors or our major shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
Non-compliance with federal, provincial or state laws and regulations, or the expansion of current, or the enactment of new laws or regulations, could adversely affect the Company's Business.
The activities of the Company are subject to regulation by governmental authorities. Achievement of the Company's Business objectives are contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all regulatory approvals, where necessary, for the sale of its products. The Company cannot predict the time required to secure all appropriate regulatory approvals for its products, or the extent of testing and documentation that may be required by governmental authorities. Any delays in obtaining, or failure to obtain regulatory approvals would significantly delay the development of markets and products and could have a material adverse effect on the Business, results of operations and financial condition of the Company.
While oil derived from industrial hemp stalk that has naturally occurring THC content equal to or less than 0.3% is excluded from the definition of marijuana under the CSA, there is no certainty that this exclusion could not be altered by court or governmental action or re-interpretation. There is no certainty that the FDA will not regulate the use of hemp oil as a drug and prohibit use as a dietary ingredient. There is no certainty that hemp oil will be considered a grandfathered dietary ingredient under the Dietary Supplement Health and Education Act of 1994 ("DSHEA"), or would otherwise be permitted for use under the DSHEA.
The Company relies on the supply of hemp stalk oil extracts, which is imported into the United States from other countries. Currently, the definition of "marijuana" in the CSA does not include the plant's 'mature stalks", which are used to create hemp (which only contains trace amounts of THC and has no psychoactive effect). Hemp stalk oil is not scheduled under the CSA and therefore, is also not under the enforcement authority of the United States Drug Enforcement Administration ("DEA"). Currently, the DEA does not take jurisdiction over hemp stalk oil products, but controls hemp cultivation, and companies that wish to cultivate hemp in the United States must apply for a permit with the DEA. If in future DEA takes jurisdiction to regulate hemp stalk oil products, the Company may become subject to additional licensing requirements, which may require additional capital. There is no assurance that the Company will be able to obtain any such licenses, or be eligible to apply for such licenses, which would adversely affect the Company's business.
We share control in joint venture projects, which limits our ability to manage third-party risks associated with these projects.
Joint venturers often have shared control over the operation of our joint venture assets, such as the joint-venture arrangement with ILDISP, LLC, and do not control all the decisions of the joint ventures. Therefore, joint venture investments may involve risks such as the possibility that a co-venturer in an investment might become bankrupt, be unable to meet its capital contribution obligations, have economic or business interests or goals that are inconsistent with our business interests or goals, or take actions that are contrary to our instructions or to applicable laws and regulations. In addition, we may be unable to take action without the approval of our joint venture partners, or our joint venture partners could take actions binding on the joint venture without our consent. Consequently, actions by a co-venturer or other third-party could expose us to claims for damages, financial penalties and reputational harm, any of which could have an adverse effect on our business and operations. In addition, we may agree to guarantee indebtedness incurred by a joint venture or co-venturer or provide standard indemnifications to lenders for loss liability or damage occurring as a result of our actions or actions of the joint venture or other co-venturers. Such a guarantee or indemnity may be on a joint and several basis with a co-venturer, in which case we may be liable in the event such co-venturer defaults on its guarantee obligation. The non-performance of such obligations may cause losses to us in excess of the capital we initially may have invested or committed under such obligations.
Preparing our financial statements requires us to have access to information regarding the results of operations, financial position and cash flows of our joint ventures. Any deficiencies in our joint ventures' internal controls over financial reporting may affect our ability to report our financial results accurately or prevent or detect fraud. Such deficiencies also could result in restatements of, or other adjustments to, our previously reported or announced operating results, which could diminish investor confidence and reduce the market price for our shares. Additionally, if our joint ventures are unable to provide this information for any meaningful period or fail to meet expected deadlines, we may be unable to satisfy our financial reporting obligations or timely file our periodic reports.
Although our joint ventures may generate positive cash flow, in some cases they may be unable to distribute that cash to the joint venture partners. Additionally, in some cases our joint venture partners control distributions and may choose to leave capital in the joint venture rather than distribute it. Because our ability to generate liquidity from our joint ventures depends in part on their ability to distribute capital to us, our failure to receive distributions from our joint venture partners could reduce our return on these investments.
Upon securing regulatory approval and ILDISP LLC completing the earn-in by contributing USD $300,000 to the dispensary in Illinois, they will earn 50% interest in each of NHMDI and SMHI. The joint venture might require a need for additional capital infusions in excess of $300,000, which might create an obligation on the Company to make additional contributions, failing to do which may result in reduction of the Company's interest in NHMDI and SMHI. In addition, ILDISP's failure to contribute may create a greater need for the Company to contribute additional capital, which may not be available to the Company on favorable terms or at all.
Joint Venture with IlDISP LLC is subject to regulatory approval.
Joint Venture with IlDISP LLC is subject to regulatory approval by agencies, which include, but not limited to IDFPR. In the case that the Company or ILDISP, as the case may be, fail to obtain requisite regulatory approvals, the Company will have an obligation to pay back all funds advanced by ILDISP to the Company up to that point within 90 days.
Risks Related to our Common Stock
If the selling shareholder sells a large number of shares all at once or in blocks, the market price of our shares would most likely decline.
The selling shareholder is offering up to 29,178,000 shares of our common stock through this prospectus. Should the selling stockholder decide to sell our shares at a price below the current market price at which they are quoted, such sales will cause that market price to decline. Moreover, we believe that the offer or sale of a large number of shares at any price may cause the market price to fall. A steep decline in the price of our common stock would adversely affect our ability to raise additional equity capital, and even if we were successful in raising such capital, the terms of such raise may be substantially dilutive to current shareholders.
The sale of our common stock to Kodiak Capital may cause dilution, and the sale of the shares of common stock acquired by Kodiak Capital, or the perception that such sales may occur, could cause the price of our common stock to fall.
On December 23, 2015, we entered into the Purchase Agreement with Kodiak Capital. Pursuant to the Purchase Agreement, Kodiak Capital has committed to purchase up to $1 million of our common stock. The shares that may be sold pursuant to the Purchase Agreement in the future may be sold by us to Kodiak Capital at our discretion from time to time, commencing after the SEC has declared effective the registration statement that includes this prospectus. The per share purchase price for the shares that we may sell to the Kodiak Capital under the Purchase Agreement will fluctuate based on the price of our common stock, and will be equal to a 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account. Depending on market liquidity at the time, sales of such shares may cause the trading price of our common stock to fall.
Kodiak Capital will pay less than the then-prevailing market price for our common stock.
The common stock to be issued to the Kodiak Capital pursuant to the Purchase Agreement will be purchased at 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account. Kodiak Capital has a financial incentive to sell our common stock immediately upon receiving the shares to realize the profit equal to the difference between the discounted price and the market price. If Kodiak Capital sells the shares, the price of our common stock could decrease. If our stock price decreases, Kodiak Capital may have a further incentive to sell the shares of our common stock that they hold. These sales may have a further impact on our stock price.
The Company's put right may convert into a greater number of shares than we have assumed in this prospectus.
We are registering up to 29,178,000 shares of our common stock for resale by the selling stockholder in this prospectus, but the actual number of shares we may end up issuing to the selling stockholder may be substantially higher. The purchase price represents a price that is the greater of (i) 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account, or (ii) CDN $0.05. For purposes of this prospectus, we have assumed a purchase price of CDN $0.05 at the USD:CDN exchange rate as at $1.4589. However, we may be required to issue a much larger number of shares of our common stock if the USD:CDN exchange rate increases. Our existing shareholders could suffer substantial dilution and we cannot guarantee that investors would not lose their entire investment.
The market price of our common stock may fluctuate significantly.
The market price and marketability of shares of our common stock may be affected significantly by numerous factors, including some over which we have no control and which may not be directly related to us. These factors include the following:
| | • | The lack of trading volume in our shares; |
| | • | Price and volume fluctuations in the stock market from time to time, which often are unrelated to our operating performance; |
| | • | Variations in our operating results; |
| | • | Any shortfall in revenue or any increase in losses from expected levels; |
| | • | Announcements of new initiatives, joint ventures, or commercial arrangements; and |
| | • | General economic trends and other external factors. |
| | • | If the trading price of our common stock falls significantly following completion of this offering, this may cause some of our shareholders to sell our shares, which would further adversely affect the trading market for, and liquidity of, our common stock. If we seek to raise capital through future equity financings, this volatility may adversely affect our ability to raise such equity capital. |
We are an "emerging growth company" and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an "emerging growth company" as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to take advantage of the benefits of this extended transition period.
Our common stock is subject to the "penny stock" rules of the SEC and the trading market in our securities is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment in our stock.
Under U.S. federal securities legislation, our common stock will constitute "penny stock". A penny stock is any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a potential investor's account for transactions in penny stocks, and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. In order to approve an investor's account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person, and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prepared by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination. Brokers may be less willing to execute transactions in securities subject to the "penny stock" rules. This may make it more difficult for investors to dispose of our common stock and cause a decline in the market value of our stock. Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Because we do not intend to pay any cash dividends on our common stock, our stockholders will not be able to receive a return on their shares unless they sell them.
We have never paid a dividend and we intend to retain any future earnings to finance the development and expansion of our business. Consequently, we do not anticipate paying any cash dividends on our common stock in the foreseeable future. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell them. We cannot assure you that stockholders will be able to sell shares when desired.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward looking statements that involve risks and uncertainties, principally in the sections entitled "Description of Business," "Risk Factors," and "Management's Discussion and Analysis of Financial Condition and Results of Operations." All statements other than statements of historical fact contained in this prospectus, including statements regarding future events, our future financial performance, business strategy and plans and objectives of management for future operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including "anticipates," "believes," "can," "continue," "could," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "should," or "will" or the negative of these terms or other comparable terminology. Although we do not make forward looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks outlined under "Risk Factors" or elsewhere in this prospectus, which may cause our or our industry's actual results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time and it is not possible for us to predict all risk factors, nor can we address the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual results to differ materially from those contained in any forward-looking statements. All forward-looking statements included in this document are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements.
You should not place undue reliance on any forward-looking statement, each of which applies only as of the date of this prospectus. Before you invest in our securities, you should be aware that the occurrence of the events described in the section entitled "Risk Factors" and elsewhere in this prospectus could negatively affect our business, operating results, financial condition and stock price. Except as required by law, we undertake no obligation to update or revise publicly any of the forward-looking statements after the date hereof to conform our statements to actual results or changed expectations.
USE OF PROCEEDS
We will not receive any proceeds from the sale of shares by the selling stockholder. However, we will receive proceeds from the sale of shares to Kodiak Capital pursuant to the Purchase Agreement. We intend to use the net proceeds received from any such sales of shares to Kodiak Capital under the Purchase Agreement for general corporate and working capital purposes and acquisitions or assets, businesses or operations or for other purposes that the Board of Directors, in its good faith deem to be in the best interest of the Company.
DETERMINATION OF OFFERING PRICE
The selling stockholder will determine at what price it may sell the offered shares, and such sales may be made at prevailing market prices or at privately negotiated prices.
DILUTION
The following information is based upon the Company's balance sheet for the three and six months ended January 31, 2016.
Dilution as used herein represents the difference between the offering price per share of shares offered hereby and the net tangible book value per share of the Company's common stock after completion of the offering. Dilution in the offering is primarily due to the losses previously recognized by the Company.
The net tangible book value of the Company at January 31, 2016 was $1,409,634 or $0.0099 per share. Net tangible book value represents the amount of total tangible assets less total liabilities. Assuming that all of the shares offered hereby were purchased by investors (a fact of which there can be no assurance) as of April 12, 2016, 141,942,514 shares of common stock outstanding prior to completion of the offering, which would constitute all of the issued and outstanding equity capital of the Company, would have a net tangible book value $1,409,634 or approximately $0.0099 per share.
At an assumed purchase price of CDN $0.05 (equal to the greater of (i) 75% of the closing price of our common stock of CDN $0.035 on April 11, 2016, or (ii) CDN $0.05), we will be able to receive USD $1,000,000 in gross proceeds, assuming the sale of the entire 29,178,000 shares being registered hereunder pursuant to the Purchase Agreement, assuming the USD:CDN exchange rate of $1.4598. However, we may be required to register more shares in order to receive the full amount of the investment amount if the USD:CDN exchange rate increases at the drawdown.
SELLING STOCKHOLDERS
We are registering for resale shares of our common stock that are issued and outstanding held by the selling stockholder identified below. We are registering the shares to permit the selling stockholder to resell the shares when and as it deems appropriate in the manner described in the "Plan of Distribution". As of the date of this Prospectus, there are 141,942,514 shares of common stock issued and outstanding.
The selling stockholder has never served as our officer or director or any of its predecessors or affiliates within the last three years, nor has the selling stockholder had a material relationship with us. The selling stockholder is neither a broker-dealer nor an affiliate of a broker-dealer. The selling stockholder did not have any agreement or understanding, directly or indirectly, to distribute any of the shares being registered at the time of purchase.
The selling stockholder may offer for sale all or part of the shares from time to time. The table below assumes that the selling stockholder will sell all of the shares offered for sale. The selling stockholder is under no obligation, however, to sell any shares pursuant to this Prospectus.
| Selling stockholders | | Shares Beneficially Owned Before this Offering(1) | | | Percentage of Outstanding Shares Beneficially Owned Before this Offering | | Shares to be Sold in this Offering | | | Number Of Shares Beneficially Owned After this Offering | | | Percentage of Outstanding Shares Beneficially Owned After this Offering | |
Kodiak Capital Group, LLC (2) | | | 0 | | | | — | | | 29,178,000 | | | | 0 | | | | — | |
| | (1) | Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, securities that are currently convertible or exercisable into shares of our common stock, or convertible or exercisable into shares of our common stock within 60 days of the date hereof are deemed outstanding. Such shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person. |
| | (2) | A Delaware limited liability company owned and controlled by Ryan C. Hodson. Mr. Hodson exercises voting and investment control with respect to the shares held by Kodiak Capital Group, LLC. Kodiak Capital Group, LLC is not a broker-dealer or affiliate of a broker dealer. |
PLAN OF DISTRIBUTION
On December 23, 2015, we entered into the Purchase Agreement with Kodiak Capital. Pursuant to the terms of the Purchase Agreement, Kodiak Capital committed to purchase up to $1,000,000 of our common stock over a period until the earlier of (i) the date on which Kodiak Capital shall have purchased put shares pursuant to the Purchase Agreement for an aggregate purchase price of USD $1,000,000, or (ii) December 31, 2016. The purchase price of the shares that may be sold to Kodiak Capital under the Purchase Agreement will be equal to the greater of (i) 25% discount to the closing bid price for the Company's common stock as reported by Bloomberg Finance, L.P., of the 5th trading day immediately following the date in which the Put Shares (as defined in the Purchase Agreement) have been deposited into the Kodiak Capital's brokerage account, or (ii) CDN $0.05.
We do not have the right to commence any sales to Kodiak Capital under the Purchase Agreement until the SEC has declared effective the registration statement of which this prospectus forms a part. Thereafter, we may, from time to time and at our sole discretion, direct Kodiak Capital to purchase shares of our common stock, but we would be unable to sell shares to them if such purchase would result in their respective beneficial ownership equaling more than 9.99% of the outstanding common stock. Except as described in this prospectus, there are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any sales of our common stock to Kodiak Capital. We may at any time in our sole discretion terminate the Purchase Agreement without fee, penalty or cost upon one business day notice.
In connection with the Purchase Agreement, we also entered into the Registration Rights Agreement, pursuant to which we are obligated to file a registration statement with the SEC by January 30, 2016. Kodiak Capital agreed to extend the filing deadline to February 10, 2016.
At an assumed purchase price of CDN $0.05 (equal to the greater of (i) 75% of the closing price of our common stock of CDN $0.035 on April 11, 2016, or (ii) CDN $0.05), we will be able to receive $1,000,000 in gross proceeds, assuming the sale of the entire 29,178,000 shares being registered hereunder pursuant to the Purchase Agreement, assuming the USD:CDN exchange rate of $1.4598. However, we may be required to register more shares in order to receive the full amount of the investment amount if the USD:CDN exchange rate increases at the drawdown.
The selling stockholder may, from time to time, sell any or all of its shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. The selling stockholder may use any one or more of the following methods when selling shares:
| | · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| | · | block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction |
| | · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| | · | an exchange distribution in accordance with the rules of the applicable exchange; |
| | · | privately negotiated transactions; |
| | · | broker-dealers may agree with the selling stockholder to sell a specified number of such shares at a stipulated price per share; |
| | · | through the writing of options on the shares; |
| | · | a combination of any such methods of sale; and |
| | · | any other method permitted pursuant to applicable law. |
The selling stockholder may also sell the shares directly to market makers acting as principals and/or broker-dealers acting as agents for themselves or their customers. Such broker-dealers may receive compensation in the form of discounts, concessions or commissions from the selling stockholder and/or the purchasers of shares for whom such broker-dealers may act as agents or to whom they sell as principal or both, which compensation as to a particular broker-dealer might be in excess of customary commissions. Market makers and block purchasers purchasing the shares will do so for their own account and at their own risk. It is possible that a selling stockholder will attempt to sell shares of common stock in block transactions to market makers or other purchasers at a price per share which may be below the then market price. The selling stockholder cannot assure that all or any of the shares offered in this Prospectus will be issued to, or sold by, the selling stockholder. The selling stockholder and any brokers, dealers or agents, upon effecting the sale of any of the shares offered in this Prospectus, are "underwriters" as that term is defined under the Securities Act, or the Exchange Act, or the rules and regulations under such acts. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act.
Discounts, concessions, commissions and similar selling expenses, if any, attributable to the sale of shares will be borne by the selling stockholder. The selling stockholder may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the shares if liabilities are imposed on that person under the Securities Act.
The selling stockholder shall acquire the securities offered hereby in the ordinary course of business and has advised us that it has not entered into any agreements, understandings or arrangements with any underwriters or broker-dealers regarding the sale of its shares of common stock, nor is there an underwriter or coordinating broker acting in connection with a proposed sale of shares of common stock by any selling stockholder. If we are notified by any selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of shares of common stock, if required, we will file a supplement to this Prospectus.
If the selling stockholder uses this Prospectus for any sale of the shares of common stock, it will be subject to the prospectus delivery requirements of the Securities Act.
Regulation M
During such time as we may be engaged in a distribution of any of the shares we are registering by this registration statement, we are required to comply with Regulation M of the Securities Exchange Act of 1934. In general, Regulation M precludes any selling security holder, any affiliated purchasers and any broker-dealer or other person who participates in a distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase, any security which is the subject of the distribution until the entire distribution is complete. Regulation M defines a "distribution" as an offering of securities that is distinguished from ordinary trading activities by the magnitude of the offering and the presence of special selling efforts and selling methods. Regulation M also defines a "distribution participant" as an underwriter, prospective underwriter, broker, dealer, or other person who has agreed to participate or who is participating in a distribution.
Regulation M prohibits, with certain exceptions, participants in a distribution from bidding for or purchasing, for an account in which the participant has a beneficial interest, any of the securities that are the subject of the distribution. Regulation M also governs bids and purchases made in order to stabilize the price of a security in connection with a distribution of the security. We have informed the selling stockholder that the anti-manipulation provisions of Regulation M may apply to the sales of their shares offered by this prospectus, and we have also advised the selling stockholder of the requirements for delivery of this prospectus in connection with any sales of the common stock offered by this prospectus.
MARKET PRICE OF COMMON STOCK AND OTHER STOCKHOLDER MATTERS
Trading Information
CSE
The principal market on which the Common Shares of the Company are traded is CSE under the symbol "EAT".
Trading the Company's common stock on the CSE began on March 23, 2015. Shown below are the ranges of high and low prices of common stock for the two most recent fiscal years as reported on www.quotemedia.com. From March 23, 2015 to August 24, 2015 the Company's Common Shares were trading on the CSE under the symbol "NHL".
| | 2015 | |
| | High | | Low | |
| Third Quarter (February 1, 2015 – April 30, 2015) (from March 23, 2015) | | $ | 0.155 | | | $ | 0.07 | |
| Fourth Quarter (May 1, 2015 – July 31, 2015) | | $ | 0.09 | | | $ | 0.035 | |
| | 2016 | |
| | High | | Low | |
| First Quarter (August 1, 2015 – October 31, 2015) | | $ | 0.075 | | | $ | 0.03 | |
| Second Quarter (November 1, 2015 – January 31, 2016 ) | | $ | 0.065 | | | $ | 0.035 | |
| Third Quarter (February 1, 2016 – April 30, 2016) (until April 11, 2016) | | $ | 0.045 | | | $ | 0.03 | |
OTCQB
In the United States, the Common Shares of the Company are quoted on the OTCQB under the symbol "SPLIF". Trading of the Company's common stock on the OTCQB began on April 23, 2015.
Shown below are the high and low trading prices of the common stock as reported by the OTCQB for the two most recent fiscal years as reported on by the OTC Markets Group Inc. These bid prices represent prices quoted by broker-dealers on the OTCBB quotation service. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commissions, and may not represent actual transactions.
| | 2015 | |
| | High | | Low | |
| Third Quarter (February 1, 2015 – April 30, 2015) | | $ | 0.084 | | | $ | 0.0765 | |
| Fourth Quarter (May 1, 2015 – July 31, 2015) | | $ | 0.0986 | | | $ | 0.0296 | |
| | 2016 | |
| | High | | Low | |
| First Quarter (August 1, 2015 – October 31, 2015) | | $ | 0.086 | | | $ | 0.017 | |
| Second Quarter (November 1, 2015 – January 31, 2016 ) | | $ | 0.0587 | | | $ | 0.03 | |
| Third Quarter (February 1, 2016 – April 30, 2016) (until April 11, 2016) | | $ | 0.038 | | | $ | 0.0224 | |
Holders
As of April 12, 2016, there were approximately 161 holders of record of the Common Shares. A total of 25 registered holders are from the United States, who hold 18,667,222 common shares, which constitutes approximately 13.2% of our issued and outstanding common stock as of April 12, 2016, prior to giving effect to this offering.
However, the Company estimates that there are significantly more beneficial holders of Common Shares as many beneficial holders hold their Common Shares through brokerage clearing houses, depositories or others in unregistered form.
Dividends
The Company currently does not anticipate paying any cash dividends in the foreseeable future on the Common Shares. Although the Company intends to retain its earnings, if any, to finance the its business growth, the Company's board of directors have the discretion to declare and pay dividends in the future. Payment of dividends in the future will depend upon the Company's earnings, capital requirements, and other factors, which our board of directors may deem relevant.
Penny Stock
Our common stock has recently traded at less than $5.00 per share and is therefore subject to the Securities and Exchange Commission's ("SEC") penny stock rules.
Penny stocks generally are equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges or quoted on the NASDAQ system). Penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer's account. The broker-dealer must also make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction. These requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security that becomes subject to the penny stock rules. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our securities, which could severely limit their market price and liquidity of the Company securities. These requirements may restrict the ability of broker-dealers to sell our common stock and may affect the ability of our stockholders to resell our common stock.
Securities Authorized for Issuance under Equity Compensation Plans
The following table sets out, as of the end of the Corporation's fiscal year ended July 31, 2015, all required information with respect to compensation plans under which equity securities of the Corporation are authorized for issuance:
| Plan Category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights | | | Weighted-average
exercise price of
outstanding
options, warrants
and rights | | | Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a)) | |
| Equity compensation plans approved by securityholders | | | 10,550,000 | | | $ | 0.10 | | | | 965,826 | |
| Equity compensation plans not approved by security holders | | Nil | | | Nil | | | Nil | |
| Total | | | 10,550,000 | | | $ | 0.10 | | | | 965,826 | |
EXCHANGE RATE INFORMATION
All dollar amounts in this prospectus are expressed in Canadian dollars unless otherwise indicated. The Company's accounts are maintained in Canadian dollars and the Company's financial statements are prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board. All reference to "U.S. dollars", "USD", or to "US$" are to United States dollars.
The following table sets forth the rate of exchange for the Canadian dollar, expressed in United States dollars in effect at the end of the periods indicated, the average of exchange rates in effect during such periods, and the high and low exchange rates during such periods based on the noon rate of exchange as reported by the Bank of Canada for conversion of Canadian dollars into United States dollars.
| | | Canada Dollar per U.S. Dollar Noon Buying Rate | |
| | | Average(1) | | High | | Low | | Period-End | |
Year ended July 31, | | | | | | | | | | | | | |
| 2011 | | | 0.9941 | | | 1.0642 | | | 0.9449 | | | 0.9538 | |
| 2012 | | | 1.0084 | | | 1.0604 | | | 0.9580 | | | 1.0014 | |
| 2013 | | | 1.0070 | | | 1.0576 | | | 0.9710 | | | 1.0287 | |
| 2014 | | | 1.0733 | | | 1.1251 | | | 1.0237 | | | 1.089 | |
| 2015 | | | 1.1924 | | | 1.3060 | | | 1.0857 | | | 1.3047 | |
Most recent six months | | | | | | | | | | | | | |
| October 2015 | | | 1.3073 | | | 1.3242 | | | 1.2904 | | | 1.3083 | |
| November 2015 | | | 1.3280 | | | 1.3360 | | | 1.3095 | | | 1.3333 | |
| December 2015 | | | 1.3705 | | | 1.3990 | | | 1.3360 | | | 1.384 | |
| January 2016 | | | 1.4223 | | | 1.4585 | | | 1.3969 | | | 1.408 | |
| February 2016 | | | 1.3796 | | | 1.404 | | | 1.3523 | | | 1.3523 | |
| March 2016 | | | 1.3226 | | | 1.3468 | | | 1.2962 | | | 1.2971 | |
| April 2016 (2) | | | 1.3081 | | | 1.3170 | | | 1.2995 | | | 1.2995 | |
| (1) | Annual and Monthly averages are calculated using the average of the daily rates during the relevant period. |
SELECTED CONSOLIDATED FINANCIAL DATA
The selected consolidated financial data set forth below as of and for the last two completed financial year ended July 31, 2015, for the period from April 17, 2014 to July 31, 2014 and unaudited interim financial information for the six months ended January 31, 2016. This table contains financial information derived from financial statements that have been prepared in accordance with the International Financial Reporting Standards ("IFRS"). You should read the following selected consolidated financial data in conjunction with, and it is qualified in its entirety by reference to our consolidated financial statements and the related notes appearing elsewhere in this prospectus and other information provided in this prospectus, including "Management's Discussion and Analysis of Financial Condition and Results of Operations." The historical results set forth below are not necessarily indicative of the results to be expected in future periods.
| | | Six Months
Ended | | | For the Year Ended | | | From Incorporation (April 17, 2014) to | |
| | | January 31, 2016 (Unaudited) | | | July 31, 2015 | | | July 31, 2014 | |
| Statements of Net Loss and Comprehensive Loss: | | | | | | | | | |
| Total revenue | | $ | Nil | | | Nil | | | Nil | |
| Total expenses | | | (832,188 | ) | | | (2,179,122 | ) | | | (681,155 | ) |
| Other income (expenses) | | | 179,558 | | | | 110,762 | | | Nil | |
| Net loss and comprehensive loss | | | (661,522 | ) | | | (2,083,645 | ) | | | (681,155 | ) |
| Basic and diluted loss per common share | | | (0.005 | ) | | | (0.02 | ) | | | (0.01 | ) |
| Current Assets | | | 233,802 | | | | 388,713 | | | | 673,525 | |
| Total Assets | | | 2,608,669 | | | | 1,972,588 | | | | 695,477 | |
| Total Liabilities | | | 1,199,035 | | | | 1,142,249 | | | | 220,150 | |
| Shareholders' Equity | | $ | 1,409,634 | | | | 830,339 | | | | 475,327 | |
(Expressed in Canadian Dollars)
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis of the results of operations and financial condition for the six months ended January 31, 2016 and 2015 and the years ended July 31, 2015 and 2014 should be read in conjunction with our financial statements and the notes to those financial statements that are contained in this prospectus.
Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors, including those set forth under the "Risk Factors," "Cautionary Note Regarding Forward-Looking Statements" and "Description of Business" sections in this prospectus. We use words such as "anticipate," "estimate," "plan," "project," "continuing," "ongoing," "expect," "believe," "intend," "may," "will," "should," "could," and similar expressions to identify forward-looking statements.
Overview
The Company mainly conducts its business in the medical marijuana, retail marijuana and hemp infused products sectors in the United States, where such activity is permitted and regulated by applicable laws, through entities that hold a valid license, which we refer to as licensed operators, to produce and distribute such products in accordance with applicable regulations. The Company also pursues similar opportunities in Canada; however, it does not expect to invest significant resources in Canada due to regulatory uncertainty.
Marijuana, for the purposes of this prospectus, is referred to herein as all parts of the plant of the genus cannabis, the seeds thereof, the resin extracted from any part of the plant, and every compound, manufacture, salt, derivative, mixture, or preparation of the plant, its seeds, or its resin, but does not include industrial hemp, nor does it include fiber produced from stalks, oil, or cake made from the seeds of the plant, sterilized seed of the plant which is incapable of germination, or the weight of any other ingredient combined with marijuana to prepare topical or oral administrations, food, drink, or other product.
Traditionally, the female marijuana or cannabis plant was consumed by smoking. The flower of the plant is dried and smoked either through a pipe, a cigarette or a filtration device/apparatus such as a water pipe or a vaporizer.
Leafs, nodes and stems are not typically utilized for consuming cannabis through smoking, but are used in production of other products such as oil extracts, referring to hereinafter as marijuana concentrate, which are produced by extracting cannabinoids from marijuana by a method including, but not limited to use of solvent, water, ice, dry ice or propylene glycol, glycerin, butter, olive oil or other typical cooking fats. The marijuana concentrate is then used to manufacture a products, referring to hereinafter as marijuana-infused products, for medical or recreational adult use in jurisdictions where permitted by the applicable regulatory authorities that are intended for use or consumption other than by smoking.
Regulations differ significantly amongst the U.S. states. Some U.S. states only cultivation, processing and distribution of marijuana for medical purposes only, which we refer as medical marijuana, and may also include marijuana-infused products, which we refer as medical marijuana-infused products. Some U.S. states may also permit cultivation, processing and distribution of marijuana for recreational purposes, which we refer as retail marijuana or recreational marijuana, and may also include marijuana-infused products, which we refer as retail marijuana-infused products.
Most U.S. states that have legalized medical marijuana or retail marijuana impose a range of requirements on the business operators, including obtaining a license from state governmental authorities. Some states (such as Colorado) require licensed operators (or their shareholders) to be residents of that U.S. State, which we refer as residency requirement. Other states (such as Illinois, Nevada, Maryland and Arizona) do not impose a residency requirement. The State of Oregon permits out-state ownership, however, such ownership is subject to a number of restrictions.
For the marijuana related products, the Company's strategy in the states with residency requirements is focused on providing products or services to the industry rather than directly owning production or retail operations. The Company is currently actively pursuing the opportunities in the State of Colorado and Illinois. The State of Colorado permits the sale, possession, use, production, distribution and personal cultivation of marijuana, however, that the Colorado Supreme Court has held that even workers who use marijuana for medical reasons may be discharged for violation of federal law. On the other hand, the State of Illinois currently allows only the medical use of marijuana under very controlled circumstances.
In addition, pursuant to federal regulations, hemp-infused products (also refer to as hemp stalk oil-infused products) are excluded from the definition of "marihuana", as long as the product does not contain active levels of THC, the psychoactive compound of the cannabis plant. It is generally accepted that products with less than 0.3% THC do not contain active levels of THC. Changes in related state and federal regulation could have a material positive or negative impact on the Company's operations.
The Company has two distinct objectives as a part of the separate business units:
| | Ø | Marijuana-Infused Products Segment. The Company is focused on developing, acquiring and designing marijuana-infused products and marijuana concentrate products and brands for sale by the Company where it has secured the required licensing, or for use by licensed operators entering into royalty or raw materials and packaging agreements with the Company in jurisdictions where permitted; and |
| | Ø | Hemp-Infused Products Segment. The Company is focused on developing, acquiring, manufacturing and distributing products infused with non-psychoactive constituent of the industrial hemp plant (less than 0.3% THC or otherwise as permitted by applicable law), which may be distributed in all 50 states. The Company is pursuing a retail distribution strategy through networks of retail dispensaries where THC products are sold, head shops, vitamin stores and independent grocers via direct sales and distributors and is considering online sales, and multi-level marketing. |
The two segments we described above are operating segments only and that for financial reporting purposes we combine them and report our results as one segment. The Company will establish operations only in U.S. states which have implemented adequate licensing frameworks and are in compliance with the Cole Memo as hereinafter described below.
We are a holding company and we conduct our business through our operating subsidiaries as follows:
NHC Edibles LLC ("NHC") and Nutritional High (Colorado) Inc. ("NHCI") carry out the Company's operations in the Marijuana–Infused Products Segment. NHC's activities in the State of Colorado includes but not limited to acquiring and leasing real estate to licensed operators and entering into raw materials and packaging agreements and financing agreements with licensed operators who are focused in the retail market and subject to residency requirements, that do not require it to obtain a licence.
NH Medicinal Dispensaries Inc ("NHMDI") and Small's Mill Holdings Inc. ("SMHI") carry out the Company's operations in the Marijuana Sector in the State of Illinois. NHMDI has submitted an application for a dispensary license with the State of Illinois and has received an authorization to register a medical marijuana dispensary. SMHI has taken possession of the Lawrenceville Property under the Lawrenceville PSA.
Nutritional Traditions Inc. carries out the Company's operations in the Hemp-Infused Product Segment. See "Our Corporate History and Background – Our Subsidiaries"
Marijuana-Infused Products Segment
In its Marijuana-Infused Products Segment, the Company is focused on developing, acquiring and designing marijuana-infused products and marijuana concentrate products and brands for sale by the Company where it has secured the required licensing, or for use by NH Licensed Operators who enter into raw materials and packaging agreements or royalty agreements with the Company in respect to the Company's brands, recipes and know-how or sell packaging to licensed operators with the Company's branding, as permitted by the applicable regulation. The Marijuana-Infused Products Segment is solely focused on the U.S. states where marijuana-infused products are permitted by law and regulation. We have no current agreements with licensed operators other than the agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute our oils and edibles in Illinois. The agreement provides for a right to negotiate an agreement to negotiate the framework in good faith, but provides for no financial terms in this regard. As such, the Company considers the agreement not material to its operations at this time. We currently have no commercial products.
The Company's business model in its Marijuana-Infused Products Segment differs depending on the residency requirements of the applicable jurisdiction. Most U.S. states that have legalized marijuana for medical or recreational use require the businesses or individuals to hold a valid license under applicable regulation in the respective U.S. state issued by the applicable state authorities. In some U.S. states, for a licensed operator to be eligible to be granted a license, the owners of the licensed operator must be residents of such U.S. State. As such, listed companies or other widely held enterprises are ineligible to obtain a license in those U.S. states where a licensed operator must be a state resident. In such U.S. states, the Company will work with a licensed operator to provide them with financing, licensing of its products, recipes and brands, know how, consulting services and may purchase the facilities where such license operators operate or intend to operate. In U.S. states where there are no residency requirements and where the Company may acquire licensing on its own, the Company may apply to become a license operator. The Company will not operate in jurisdictions which have not legalized marijuana, and does not intend on operating in jurisdictions which have legalized marijuana but have not developed and imposed a licensing regime for licensed operators.
In certain jurisdictions, and more specifically in the states with the residency requirements, the Company may conduct business in the value chain segments, which do not require a license from the requisite regulatory authorities. Such ancillary value chain segments do not directly handle, process, manufacture, cultivate or distribute cannabis products, and may include: unsecured lending, providing real estate to licensed operators, equipment leasing and providing non-cannabis raw materials (such as packaging, food ingredients, etc.) The focus of this strategy is to provide the services to licensed operators that are focused on extraction and processing of cannabis to enable them to attain a stronger competitive advantage in the market, while proving an acceptable economic return to the Company.
In certain circumstances the Company may pursue other value chain segments, such as operating a dispensary, in the states where the regulatory environment is unsuitable to earn an economic rate of return in the extraction/processing segment, given the Company's assessment. This aim of this strategy is to secure a foothold in such markets, through obtaining a license to operate a business that is not directly related to extraction/processing and then partner with another licensed operator who is able to operate in the extraction/processing space. The Company has employed this type of strategy in the State of Illinois, where it is working to obtain a dispensary license, but has also partnered with another licensed operator to be in a position to sell its products in Illinois.
Hemp-Infused Products Segment
In its Hemp-Infused Products Segment, the Company is focused on developing, acquiring, manufacturing and distributing products infused with non-psychoactive constituent of the industrial hemp plant (less than 0.3% THC or as otherwise permitted by applicable law), which may be distributed in all 50 U.S. States. The Company is pursuing a retail distribution strategy through networks of retail dispensaries where THC products are sold, head shops, vitamin stores and independent grocers via direct sales and distributors and is considering online sales, and multi-level marketing. Having access to the Company's expertise in manufacturing products from cannabis, these hemp-infused products are expected to have a competitive edge in the market. The products are being sold under the "Nutritional Traditions" brand name and initially available to California retail customers. Distribution will be expanded outside of California beginning in 2016.
U.S. Federal Law and Cole Memo Compliance
The use, possession, sale, cultivation and transportation of cannabis remains illegal under U.S. federal law. The concepts of "medical marijuana" and "retail marijuana" do not exist under U.S. federal law. The Federal Controlled Substances Act classifies "marihuana" as a Schedule I drug. Under U.S. federal law, a Schedule I drug or substance has a high potential for abuse, no accepted medical use in the United States, and a lack of safety for the use of the drug under medical supervision. As such, marijuana-related practices or activities, including without limitation, the manufacture, importation, possession, use or distribution of marijuana are illegal under U.S. federal law. Strict compliance with state laws with respect to marijuana will neither absolve the Company of liability under U.S. federal law, nor will it provide a defense to any federal proceeding which may be brought against the Company.
| | · | Preventing the distribution of marijuana to minors; |
| | · | Preventing revenue from the sale of marijuana from going to criminal enterprises, gangs, and cartels; |
| | · | Preventing the diversion of marijuana from U.S. states where it is legal under state law in some form to other U.S. states; |
| | · | Preventing U.S. State-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity; |
| | · | Preventing violence and the use of firearms in the cultivation and distribution of marijuana; |
| | · | Preventing drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use; |
| | · | Preventing the growing of marijuana on public lands and the attendant public safety and environmental dangers posed by marijuana production on public lands; and |
| | · | Preventing marijuana possession or use on U.S. federal property. |
There is no guarantee that the current presidential administration will not change its stated policy regarding the low-priority enforcement of U.S. federal laws that conflict with state laws. Additionally, any new U.S. federal government administration that follows could change this policy and decide to enforce the U.S. federal laws vigorously.
The Company's operations are compliant with the Cole Memo.
Recent Developments
Illinois Joint-Venture, Oils and Edibles Relationship and Conditional Approval for Registration of the Dispensary
The Company has significant developments in Illinois, which include:
| · | Entered into joint-venture agreement ("JV Agreement") with ILDISP, LLC with ties to the medical marijuana industry to build and operate the Company's planned dispensary in Illinois; |
| · | Been granted conditional approval ("Conditional Approval") by the Illinois Department of Financial and Professional Regulation ("IDFPR") to establish the Lawrenceville Dispensary; |
| · | Commenced renovations at the Lawrenceville Dispensary property; and |
| · | Entered into an agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute Nutritional High's oils and edibles in Illinois. |
Under the terms of the JV Agreement ILDISP, LLC will fund up to USD $300,000 of the expenses and working capital required to complete and launch the Lawrenceville Dispensary. In addition, ILDISP, LLC will provide the Company with a guarantee for half the Seller Take-Back Mortgage. In exchange for its contribution, ILDISP, LLC shall receive a 50% interest in NHMD, the Company's wholly owned subsidiary which holds the Conditional Approval for the Lawrenceville Dispensary, and a 50% interest in SMHI, the Company's wholly owned subsidiary which holds the Company's interest in the Dispensary real estate property located in Lawrenceville, IL.
ILDISP LLC has already made initial advances to fund the Lawrenceville Dispensary renovations and property mortgage payments. The joint venture is subject to the approval of the IDFPR and the entry into a shareholders agreement. Furthermore, it is contemplated that ILDISP LLC will contribute to the management of the Lawrenceville Dispensary and its relationships in the surrounding community will help accelerate the growth and development of the Dispensary. The shareholder agreement has not been entered into as of the date hereof and is expected to be finalized in due course. The parties have agreed on a form of shareholders agreement to be entered into upon receipt of IDFRP approval, which shall contain customary provisions such as governance of the rights and responsibilities of the shareholders, respective share ownership and dilution mechanism if additional capital is required. The Board of Directors of both NHMD and SMHI shall be composed of directors appointed by both ILDISP and Nutritional High, once the requisite approvals are obtained. Both parties will appoint a general manager for the dispensary, once operational, who will report to both parties and may be removed by common consent. The shareholder agreement remains subject to IDFPR's approval and there is no guarantee that the final version will be entered on the terms favorable to the Company or at all.
In addition, NHMD has been advised by the IDFPR that it has been awarded Conditional Approval to register the Lawrenceville Dispensary under the
Compassionate Use of Medical Cannabis Pilot Program Act (Illinois) ("CUMCPPA"). The Conditional Approval sets out the requirements that NHMD must fulfill prior to IDFPR approving the registration of the dispensary, which includes completing the renovations and passing the final inspection to the satisfaction of IDFPR. Upon meeting IDFRP's conditions, it is expected that NHMD will be granted a final license to operate the Lawrenceville Dispensary.
The Company also has entered into an agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute Nutritional High's oils and edibles in Illinois. The cultivation and extraction facility is licensed with Illinois Department of Agriculture and was amongst the first of the companies to commence commercial cultivation and processing of cannabis products under the CUMCPPA. The Company will develop its business framework with the extraction facility over the next 18 months and will provide updates as this business initiative develops. The agreement provides for a right to negotiate an agreement to negotiate the framework in good faith, but provides for no financial terms in this regard. As such, the Company considers the agreement not material to its operations at this time.
Lawrenceville Dispensary Application and Lawrenceville Property Acquisition
The Company has taken possession of the real estate property in Lawrenceville, Illinois on November 25, 2015, the Lawrenceville PSA. The final acquisition price for the Lawrenceville property was USD $350,000. The Company has also negotiated a Seller Take-Back Mortgage, which will have a 15 year amortization period, bearing an interest at the rate of 6% and be due in two years from the date of issuance as a balloon payment. Upon Payment of the Seller Take-Back Mortgage, title to the Lawrenceville Property will automatically transfer SMHI (as hereinafter defined).
Private Placement
On December 2, 2015, the Company completed a non-brokered private placement of 4,200,000 units at $0.05 per unit for gross proceeds of $210,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
Hemp Product Launch
The Company has rolled out of the first three products in its Hemp-Infused Products Segment. Initial products are being made available to consumers in California include capsules, push caps for water bottles, and a push cap formulation for post-exercise recovery called "Rapid Recovery". In addition, the Company intends to commenced sales of these products in Colorado in 2016, targeting sales to marijuana dispensaries in Colorado, further developing sales channels for the Company's marijuana oil and edible brands.
Plan of Operation
In the twelve month period starting upon the effective date of this Prospectus, the Company intends to develop our business in the following areas:
Marijuana-Infused Products Segment:
| | · | Advance additional funds to Palo Verde LLC ("Palo Verde") a private Company incorporated under the laws of the State of Colorado, which received a RMIP License (as defined hereinafter) and a RMC License (as defined hereinafter) on October 1, 2014. Palo Verde shall use the funds to complete Phase I of the construction at the Pueblo Location, to enable Palo Verde to produce marijuana concentrates using the Company's brands. The Company and Palo Verde have been working with architects and contractors to finalize and cost of the construction plans, as well as secure the requisite local permits necessary to for construction. The estimated cost to complete the Phase I, including Palo Verde's working capital is estimated at USD $550,000; |
| | · | Advance additional funds to Palo Verde to complete Phase II of the construction at the Pueblo Location, to enable Palo Verde to produce edible marijuana-infused products using the Company's brands. While it is expected that the Company will be able to use the revenue and fees from Palo Verde, after Palo Verde commences production of marijuana concentrates, gross capital needs are estimated at approximately at USD $450,000; |
| | · | Finalize packaging and raw materials agreement with Palo Verde prior to Palo Verde commencing commercial production, which will allow the Company to supply Palo Verde with non-marijuana materials in order to earn revenue; |
| | · | Source and install the equipment for manufacturing of marijuana concentrates to be installed at Pueblo Location. The Company estimates that the equipment for Phase I will cost approximately USD $300,000, and the equipment for Phase II will cost approximately USD $100,000; |
| | · | Complete the build-out of the Dispensary in Lawrenceville, IL, which is estimated at USD $300,000, and obtain the Dispensary License, subject to obtaining requisite regulatory approvals. |
Hemp-Infused Products Segment:
| | · | Formulation of additional products and product improvements are estimated at USD $20,000; |
| | · | The Company also expects that approximately USD $50,000 of capital will be required to fund the inventory purchase. Initially, the Company expects to use the proceeds from sales to fund the purchases of additional inventory on roll-over basis. |
Below is the summary of the expenditures outlined above ("Planned Expenditures"):
| Planned Expenditure | | Budgeted Amount
($USD) | |
| Loans to Palo Verde to complete Phase I build-out of Pueblo Location | | | |
| Construction | | $ | 425,000 | |
| Security System | | $ | 35,000 | |
| Palo Verde Working Capital and Final Permitting Costs | | $ | 90,000 | |
| Loans to Palo Verde to complete Phase II build-out of Pueblo Location | | $ | | |
| Construction | | $ | 225,000 | |
| Security System | | $ | 20,000 | |
| Palo Verde Working Capital and Final Permitting Costs | | $ | 205,000 | |
| Purchase of equipment to complete Phase I | | $ | 300,000 | |
| Purchase of equipment to complete Phase II | | $ | 100,000 | |
| Complete the build-out of the Dispensary in Lawrenceville, IL | | $ | 300,000 | |
| Complete formulation of hemp-infused products | | $ | 20,000 | |
| Purchase of hemp-infused products inventory | | $ | 50,000 | |
| Corporate General & Administrative | | $ | 560,000 | |
Interest Payments(1) | | $ | 20,000 | |
| Total: | | $ | 2,350,000 | |
Note:
| | (1) | Based on the Senior Convertible Debentures and Subordinate Convertible Debentures outstanding as of the date hereof of CDN $250,000 |
The currently available financial resources are not sufficient to fund the Company's limited levels of operations for any period of time. The Planned Expenditures represents the expenditures that the Company wishes to accomplish in the 12 months subsequent to completing this offering; however, many expenditures are subject to the Company obtaining requisite financing. The Company's priority is to provide additional loans to Palo Verde, which the Company intends to fund with the net proceeds of this offering. The Company intends to utilize the following potential sources of capital to fund the balance of the Planned Expenditures:
| | Ø | Seek secured debt financing against the Company real estate assets; |
| | Ø | Seek alternative capital funding sources, including equipment lease financing; |
| | Ø | Seek joint-venture partners to fund all or part of the Planned Expenditures in exchange for direct interest in the Company's assets or projects; |
| | Ø | Reduce or suspend certain consulting fees, which are not integral to accomplishing the main priority of providing additional loans to Palo Verde and completing Phase I of the build-out; |
| | Ø | Convert outstanding accounts payable into Common Shares; |
| | Ø | Delaying additional loans to Palo Verde and purchase of the equipment for completion of Phase II to reduce the external cash requirement; |
| | Ø | It shall be noted that the budget does not account for potential cash inflows that may result from Palo Verde, upon Palo Verde achieving commercial production. |
The Company is a development stage entity and has not generated any significant revenue to date. Long term financing beyond the maximum aggregate amount of this offering will be required to fully implement our business plan. The exact amount of funding will depend on funding required for full implementation of the Company's business plan. After a twelve-month period the Company may need additional financing, which may not be available on the terms favorable to the Company or at all. There is no guarantee that the Company will be able to commence operations as planned in the timely fashion and cannot guarantee that once we start operations we will stay in business after doing so.
Palo Verde is a development stage entity with limited capacity to raise funds outside of the Company, The Company has no reason to believe that the funds will not collectible and expects Palo Verde to be in a position to being paying off the loan, once it achieves commercial production. Advancing additional funds is necessary for Palo Verde to achieve commercial production. If Palo Verde is unable to commence commercial production in a profitable fashion or secure an alternative source of funds, the full amount of the loan might be written off.
No portion of the proceeds of this offering will be allocated to potential operations in jurisdictions other than Colorado and Illinois due to the fact that there is no assurance that the Company will be eligible to obtain such licensing or whether any of the regulatory applications associated with such operations will be approved. If the Company receives one or more of the necessary approvals and decides to proceed with such business(es) it may seek additional debt or equity financing which may not be available on terms acceptable to the Company, or at all. See "Risk Factors – The Company may not be able to accurately predict its future capital needs and may not be able to secure additional financing."
The Company had negative cash flows from operations for the most recently completed financial year as the Company began operations in April 2014. See "Risk Factors - The Company has a very limited operating history in an emerging area of business and had negative cash flows from operations in its most recently completed financial year." The Company intends to spend the available funds for the principal purposes as indicated above. Notwithstanding the foregoing, there may also be circumstances where, for sound business reasons, a reallocation of funds may be necessary for the Company to achieve its objectives. The Company may require additional funds in order to fulfill all of the Company's expenditure requirements to meet its objectives. See "Risk Factors". There is no assurance that additional funding required by the Company will be available if required. However, it is anticipated that the available funds will be sufficient to satisfy the Company's objectives over the 12 months following completion of this offering. A major portion of the Company's planned capital expenditures is in US dollars and changes in the CDN:USD exchange rate could adversely affected the Company's financial position.
The investment policy for unallocated funds will be determined by the directors of the Company. Until such time as unallocated funds are required for use as working capital, the Company plans to invest such funds in securities of, or those guaranteed by, the Government of Canada, any province or territory thereof or the Government of the United States of America, in certificates of deposit or in interest bearing accounts of Canadian chartered banks and/or trust companies, or a combination thereof.
Results of Operations
As at January 31, 2016, the Company had assets of $2,608,669, liabilities of $1,199,035 and shareholders' equity of $1,409,634. During the six months ended January 31, 2016, the Company incurred a net loss and comprehensive loss of $661,522.
At January 31, 2016, the Company had working capital deficiency of $327,988 and cash of $18,233.
Summarized selected financial information with respect to Nutritional High is as follows:
| | | Six months ended January 31, 2016 | | | Year ended July 31, 2015 | | | Period ended July 31, 2014 | |
| Total expenses | | $ | (832,188 | ) | | | (2,179,122 | ) | | | (681,155 | ) |
| Other income | | | 179,558 | | | | 110,762 | | | | - | |
| Net loss | | | (652,630 | ) | | | (2,068,360 | ) | | | (681,155 | ) |
| Foreign exchange translation | | | 8,892 | | | | (15,285 | ) | | | - | |
| Net loss and comprehensive loss | | | (661,522 | ) | | | (2,083,645 | ) | | | (681,155 | ) |
| Loss per share | | | (0.005 | ) | | | (0.02 | ) | | | (0.01 | ) |
| Total assets | | | 2,608,669 | | | | 1,972,588 | | | | 695,477 | |
| Total liabilities | | | 1,199,035 | | | | 1,142,249 | | | | 220,150 | |
| Shareholders' Equity | | $ | 1,409,634 | | | | 830,339 | | | | 475,327 | |
Three months period ended January 31, 2016 and 2015
The Company incurred a net loss of $291,228 or $0.002 per Common Share for the three month period ended January 31, 2016 compared with a net loss of $339,845 or $0.005 per Common Share for the same period ended January 31, 2015.
For the three month period ended January 31, 2016, management and consulting fees increased by $248,088 to $352,358 from $104,270 in the same period in 2015, and consisted of services provided by FMI Capital Advisory Inc., for strategic advisory services, Branson Corporate Services Ltd. for financial accounting services, including CFO services, and the President and CEO of the Company.
Six months period ended January 31, 2016 and 2015
The Company incurred a net loss of $652,630 or $0.005 per Common Share for the six month period ended January 31, 2016 compared with a net loss of $722,572 or $0.010 per Common Share for the same period ended January 31, 2015.
For the six month period ended January 31, 2016, management and consulting fees increased by $290,804 to $549,024 from $258,220 in the same period in 2015, and consisted of services provided by FMI Capital Advisory Inc., for strategic advisory services, Branson Corporate Services Ltd. for financial accounting services, including CFO services, and the President and CEO of the Company.
For the six month period ended January 31, 2016, professional fees, consisting of legal and audit fees, decreased by $295,415 to $76,625 from $372,040 for the comparable period in the prior year. The prior year included fees incurred for the going public transaction.
The Company earned $259,543 in rental income on the Pueblo location for the six month period ended January 31, 2016, and $32,519 for the comparable period in the prior year. In the prior year, there was one less lease, and two leases commenced on January 1, 2015, & therefore, included only one month of rent. As well, in the prior year, the average US exchange rate was 1.1357 vs 1.3448 in the current period.
Years ended July 31, 2015 and 2014
The Company incurred a net loss of $2,083,645 or $0.02 per common share for the year ended July 31, 2015, compared with a loss of $681,155 or $0.01 per share for the same period ended July 31, 2014.
Management and consulting fees totaled $753,432 for the year end July 31, 2015 and $176,244 during the period ended July 31, 2014, and primarily consisted of services provided by FMI Capital Advisory Inc., for strategic leadership, Branson Corporate Services Ltd. for financial accounting, including CFO services and the President and CEO of the Company. Prior year consisted only of three months, compared to twelve months in the current year.
Professional fees, consisting of legal and audit fees, totaled $468,809 for the year ended July 31, 2015 and $88,231 during the period ended July 31, 2014. Prior year consisted only of three months, compared to twelve months in the current year.
The Company incurred $294,389 in office and general expenses for the year ended July 31, 2015 and $32,857 during the period ended July 31, 2014, and consisted primarily of travel and entertainment, marketing, printing and other miscellaneous costs. Prior year consisted only of three months, compared to twelve months in the current year.
The Company incurred share based payments of $286,000 for the year ended July 31, 2015 and $28,000 for the period ended July 31, 2014. Share based payments are recorded based on the valuation of options using the Black-Scholes model.
The Company incurred listing expense for the period ended July 31, 2014 of $355,823 in connection with the reverse takeover transaction, with fair value of share consideration paid of $287,840 in excess of net liabilities acquired of $67,983.
The Company earned $202,270 in rental income on the Pueblo location for the year ended July 31, 2015, and $nil for the comparable period in the prior year. A provision of $100,000 was taken against the rent receivable in the year.
Liquidity and Capital Resources
Liquidity risk is the risk that the Company will not have sufficient cash resources to meet its financial obligations as they come due. The Company's liquidity and operating results may be adversely affected if the Company's access to the capital market is hindered, whether as a result of a downturn in stock market conditions generally or related to matters specific to the Company. The Company generates cash flow primarily from its financing activities. The Company's approach to managing liquidity risk is to ensure that it will have sufficient liquidity to meet liabilities when due. As at January 31, 2016, the Company had current assets of $233,802, current liabilities of $561,790 and working capital deficiency of $327,988.
Going Concern
At January 31, 2016 the Company had working capital (deficiency) of $(327,988) (July 31, 2015 - $116,439), had not yet achieved profitable operations, has accumulated losses of $3,393,225 (July 31, 2015 - $2,740,442) and expects to incur further losses in the development of its business, all of which describes the material uncertainties that cast significant doubt upon the Company's ability to continue as a going concern. The Company will require additional financing in order to conduct its planned business operations, meet its ongoing levels of corporate overhead and discharge its liabilities and commitments (Note 19) as they come due. There is no assurance that the Company will successfully raise this financing.
Critical Accounting Policies and Estimates
Critical Accounting Estimates
The preparation of these unaudited condensed interim consolidated financial statements requires management to make judgments and estimates and form assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. On an ongoing basis, management evaluates its judgments and estimates in relation to assets, liabilities, revenue and expenses. Management uses historical experience and various other factors it believes to be reasonable under the given circumstances as the basis for its judgments and estimates. Actual outcomes may differ from these estimates under different assumptions and conditions. The most significant estimates relate to recoverability of loan receivable and rent receivable (Note 5), valuation of deferred income tax amounts, and valuation of warrants and shares issued during private placements and measurement of derivative liability.
The most significant judgments relate to recognition of deferred tax assets and liabilities, assessment of functional currency and determination of derivative liability of convertible debt.
Share based payments
Share based payment transactions
Employees (including directors and senior executives) of the Company receive a portion of their remuneration in the form of share-based payments, whereby employees render services as consideration for equity instruments ("equity-settled transactions"). In situations where equity instruments are issued and some or all of the goods or services received by the entity as consideration cannot be specifically identified, they are measured at fair value of the share-based payment.
Equity settled transactions
The costs of equity-settled transactions with employees are measured by reference to the fair value of the equity instruments at the date on which they are granted.
The costs of equity-settled transactions are recognized, together with a corresponding increase in equity, over the period in which the performance and/or service conditions are fulfilled, ending on the date on which the relevant employees become fully entitled to the award ("the vesting date"). The cumulative expense recognized for equity-settled transactions at each reporting date until the vesting date reflects the Company's best estimate of the number of equity instruments that will ultimately vest. The profit or loss charge or credit for a period represents the movement in cumulative expense recognized as at the beginning and end of that period and the corresponding amount is represented in share option reserve.
No expense is recognized for awards that do not ultimately vest.
Loss per share
Basic loss per share is computed by dividing the net loss available to common shareholders by the weighted average number of Common Shares outstanding during the period. The computation of diluted loss per share assumes conversion, exercise or contingent issuance of securities only when such conversion, exercise or issuance would have a dilutive effect on loss per share. When there is a loss, no potential shares are included in the computation as they are anti-dilutive.
Financial assets
All financial assets are initially recorded at fair value and designated upon inception into one of the following four categories: held to maturity, available for sale, loans and receivables, or at fair value through profit or loss ("FVTPL").
Financial assets classified as FVTPL are measured at fair value with realized gains and losses recognized through earnings. The Company's cash and cash equivalents are classified as FVTPL.
Financial assets classified as loans and receivables and held to maturity are measured at amortized cost. The Company classified loans and other receivables as loans and receivables.
Financial assets classified as available for sale are measured at fair value with unrealized gains and losses recognized in other comprehensive income (loss) except for losses in value that are considered other than temporary. At January 31, 2016, the Company has not classified any financial assets as available for sale or held to maturity.
Transaction costs associated with FVTPL financial assets are expensed as incurred, while transaction costs associated with all other financial assets are included in the initial carrying amount of the asset.
Financial liabilities
All financial liabilities are initially recorded at fair value and designated upon inception as FVTPL or other financial liabilities. Financial liabilities classified as other financial liabilities are initially recognized at fair value less directly attributable transaction costs. After initial recognition, other financial liabilities are subsequently measured at amortized cost using the effective interest method. The effective interest method is a method of calculating the amortized cost of a financial liability and of allocating interest expense over the relevant period. The effective interest rate is the rate that exactly discounts estimated future cash payments through the expected life of the financial liability, or, where appropriate, a shorter period. The Company's accounts payable and accrued liabilities are classified as other financial liabilities.
Financial liabilities classified as FVTPL include financial liabilities held for trading and financial liabilities designated upon initial recognition as FVTPL. Derivatives, including separated embedded derivatives are also classified as held for trading unless they are designated as effective hedging instruments. Fair value changes on financial liabilities classified as FVTPL are recognized through the statement of comprehensive income. At January 31, 2016, the Company had not classified any financial liabilities as FVTPL.
Cash
Cash in the statement of financial position is comprised of cash in bank and held in Company's lawyers trust account.
Related party transactions
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Parties are also considered to be related if they are subject to common control or common significant influence, related parties may be individuals or corporate entities. A transaction is considered to be a related party transaction when there is a transfer of resources or obligations between related parties.
Credit Risk
Credit risk is the risk of loss associated with counterparty's inability to fulfill its payment obligations. The Company's credit risk is primarily attributable to cash, loan and other receivables. The Company has no significant concentration of credit risk arising from operations. The majority of the Company's cash is held in a trust account with the Company's lawyer. Remaining cash is held with a reputable Canadian chartered bank which is closely monitored by management. Management believes that the credit risk concentration with respect to financial instruments included in cash, loans and other receivables is minimal.
Liquidity Risk
Liquidity risk is the risk that the Company will not have sufficient cash resources to meet its financial obligations as they come due. The Company's liquidity and operating results may be adversely affected if the Company's access to the capital market is hindered, whether as a result of a downturn in stock market conditions generally or related to matters specific to the Company. The Company generates cash flow primarily from its financing activities. The Company has accumulated losses of $3,101,997 and expects to incur further losses in the development of its business. As at January 31, 2016, the Company had a cash balance of $103,135 and current liabilities of $370,138.
Recent Accounting Pronouncements
The IASB and IFRIC has issued the following new and revised Standards and Interpretations which are not yet effective for the relevant reporting periods and which the Company has not early adopted. However, the Company is currently assessing what impact the application of these standards or amendments will have on the consolidated financial statements of the Company.
Pronouncements effective for annual periods beginning on or after January 1, 2016
IAS 1 Presentation of Financial Statements Amendments are designed to further encourage companies to apply professional judgment in determining what information to disclose in their financial statements.
IAS 16 Property, Plant and Equipment and IAS 38 Intangible Assets Amendments clarify that the use of revenue-based methods to calculate the depreciation of an asset are not appropriate because revenue generated by an activity that includes the use of an asset generally reflects factors other than the consumption of the economic benefits embodied in the asset.
Pronouncements effective for annual periods beginning on or after January 1, 2018
In July 2014, the IASB issued the final version of IFRS 9, Financial Instruments. The new standard will replace IAS 39, Financial instruments: recognition and measurement. The final amendments made in the new version include guidance for the classification and measurement of financial assets and a third measurement category for financial assets, fair value through other comprehensive income. The standard also contains a new expected loss impairment model for debt instruments measured at amortized cost or fair value through other comprehensive income, lease receivables, contract assets and certain written loan commitments and financial guarantee contracts. The standard is effective for annual periods beginning on or after January 1, 2018 and must be applied retrospectively with some exceptions. Early adoption is permitted. Restatement of prior periods in relation to the classification and measurement, including impairment, is not required. The Company has not yet assessed the impact of this new standard.
Off Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a material effect on our financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources, other than as disclosed in the financial statements and notes thereto.
OUR CORPORATE HISTORY AND BACKGROUND
Name and Incorporation
The Company was incorporated under the Canada Business Corporations Act ("CBCA") on July 19, 2004 as "Sonoma Capital Inc." The Company filed a final prospectus on January 31, 2007 in Quebec, and is therefore a reporting issuer in Quebec. On June 27, 2014, the Company completed the acquisition of Nutritional High Ltd. and changed its name to "Nutritional High International Inc."
On June 27, 2014, the Company filed articles of amendment to allow for an increase in the size of the Board between meetings of shareholders.
The Acquisition
On June 27, 2014, the Company completed the acquisition of Nutritional High Ltd. ("NHL"), whereby it acquired all the issued and outstanding shares and warrants of NHL (the "Acquisition") and changed its name to "Nutritional High International Inc." In connection with the Acquisition, the Company issued an aggregate of 60,400,011 Common Shares and 13,500,006 Series I Warrants, on a one-for-one basis, in exchange for the NHL securities held by the vendors, which represented 83.99% of the total issued and outstanding shares of the Company at the closing of the Acquisition. In addition, 150,000 Series II Warrants were issued in connection with the Acquisition. See "Description of Securities – Warrants" for description of the Series I Warrants and Series II Warrants.
Prior to the completion of the Acquisition, the Company had no active business operations and was seeking business opportunities including assets or businesses with good growth potential to merge with or acquire. After completing the Acquisition, the Company has continued NHL's efforts to develop its business in the marijuana business sector.
Our Subsidiaries
Through the Acquisition, NHL became a wholly-owned subsidiary of the Company. Our current corporate structure is as follows:
NHL is a holding company and also holds certain rights to purchase interest in the two companies that have applied for licenses under means Marihuana for Medical Purposes Regulations under the Controlled Drugs and Substances Act (Canada), which are not material to the Company's operations. See "The Business – Our Products, Services and Operations – Other Jurisdiction - Canada."
NHC Edibles LLC ("NHC") and Nutritional High (Colorado) Inc. ("NHCI") carry out the Company's operations in the Marijuana–Infused Products Segment. NHC's activities in the State of Colorado includes but not limited to acquiring and leasing real estate to licensed operators and entering into raw materials and packaging agreements and financing agreements with licensed operators who are focused in the retail market and subject to residency requirements, that do not require it to obtain a licence. See "The Business – Our Products, Services and Operations – State of Colorado."
NH Medicinal Dispensaries Inc ("NHMDI") and Small's Mill Holdings Inc. ("SMHI") carry out the Company's operations in the Marijuana Sector in the State of Illinois. NHMDI has submitted an application for a dispensary license with the State of Illinois and has received an authorization to register a medical marijuana dispensary. SMHI has taken possession of the Lawrenceville Property under the Lawrenceville PSA. See "Description of Business – Our Products, Services and Operations – State of Illinois"
Eglinton Medicinal Advisory Inc. ("EMAL") is dormant.
NH Medicinals (Minnesota) Inc. ("NHMMI") has signed acquisition agreements to acquire real estate properties in various U.S. states, which are conditional on securing requisite licenses.
NH Medicinals (Maryland) Inc. ("NHMMDI") is contemplating various strategies to conduct business in the State of Maryland.
Nutritional Traditions Inc. ("NTI") carries out the Company's operations in the Hemp-Infused Product Segment.
Financing
On March 13, 2015, the Company completed its initial public offering of units, which raised gross proceeds of $1,645,000 through Jacob Securities Inc. Each unit was comprised of one Common Share and one-half Common Share purchase warrant. Each whole warrant entitles the holder there of to purchase a Common Share at a price of $0.07 per share until March 13, 2017. The Company's Common Shares commenced trading on the Canadian Securities Exchange at the opening of market on March 23, 2015 under the trading symbol "NHL". On August 24, 2015, the Company changed its trading symbol on the CSE to "EAT".
On December 2, 2015, the Company completed a non-brokered private placement of 4,200,000 units at $0.05 per unit for gross proceeds of $210,000. Each unit consisted of one Common Share and one half Common Share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
DESCRIPTION OF BUSINESS
Overview
The Company is focused on developing, manufacturing and distributing products and nationally-recognized brands in the hemp and marijuana-infused products industry, including edibles and oil extracts for medical and adult recreational use. The Company works exclusively through licensed operators in jurisdictions where such activity is permitted and regulated by requisite state laws in the United States. The Company also works to acquire licenses and operating businesses related to marijuana-infused products elsewhere in the United States. The Company's vision is to establish a leading foothold in several distinct parts of the value chain of the North American recreational and medical marijuana industry and replicate its model in other jurisdictions where permitted by law or regulation.
The Company is well positioned to take advantage of growth in the marijuana industry in various jurisdictions with its multi-faceted strategy and experienced management team. The Company is aware that the legal marijuana industry is in its infancy and is rapidly evolving which presents risks in addition to opportunities. The Company has retained legal counsel in each jurisdiction in which it operates. There is, however, no certainty that the Company will not be adversely affected by changes in government regulation and other factors in the future. The Company aims to mitigate these risks by closely monitoring regulatory changes with the assistance of legal counsel and by maintaining the highest standards possible with respect to legal, accounting and security controls, as well as proactively taking a leadership role in working with regulatory bodies and other stakeholders to build the necessary institutional infrastructure typically available to other types of businesses. In the United States, the Company will establish operations only in the states which have implemented adequate licensing frameworks and are in compliance with the Cole Memo as hereinafter described below.
The Company's objective is to take advantage of the changing regulation governing the marijuana industry in the United States and Canada. The Company's business is focused on two main segments:
| | Ø | Marijuana-Infused Products Segment. The Company is focused on developing, acquiring and designing marijuana-infused products and marijuana concentrate products and brands for use by licensed operators entering into raw materials and packaging agreements with the Company in jurisdictions where permitted; and |
| | Ø | Hemp-Infused Products Segment. The Company is focused on developing, acquiring, manufacturing and distributing products infused with non-psychoactive constituent of the industrial hemp plant (less than 0.3% THC), which may be distributed in all 50 states. The Company is pursuing several distribution strategies, which include retail dispensaries where THC products are sold, smoke shops, vitamin stores and independent grocers via direct sales and distributors. |
The two segments we described above are operating segments only and that for financial reporting purposes we combine them and report our results as one segment.
To date, other than accrued rent and interest, the Company has not yet earned any revenues from its Marijuana-Infused Products Segment. In its Hemp-Infused Products Segment, the Company has launched its product, which is available for sale, but has not yet earned any significant revenues from its hemp-infused products. As such, the Company shall be considered to be a development stage entity.
Our Industry
Marijuana-Infused Products Segment
Marijuana, for the purposes of this prospectus, is referred to herein as all parts of the plant of the genus cannabis, the seeds thereof, the resin extracted from any part of the plant, and every compound, manufacture, salt, derivative, mixture, or preparation of the plant, its seeds, or its resin, but does not include industrial hemp, nor does it include fiber produced from stalks, oil, or cake made from the seeds of the plant, sterilized seed of the plant which is incapable of germination, or the weight of any other ingredient combined with marijuana to prepare topical or oral administrations, food, drink, or other product.
Traditionally, the female marijuana or cannabis plant was consumed by smoking. The flower of the plant is dried and smoked either through a pipe, a cigarette or a filtration device/apparatus such as a water pipe or a vaporizer.
Leafs, nodes and stems are not typically utilized for consuming cannabis through smoking, but are used in production of other products such as oil extracts, referring to hereinafter as marijuana concentrate, which are produced by extracting cannabinoids from marijuana by a method including, but not limited to use of solvent, water, ice, dry ice or propylene glycol, glycerin, butter, olive oil or other typical cooking fats. The marijuana concentrate is then used to manufacture a products, referring to hereinafter as marijuana-infused products, for medical or recreational adult use in jurisdictions where permitted by the applicable regulatory authorities that are intended for use or consumption other than by smoking, including:
| | · | Edible Products. Marijuana Concentrate is used to infuse edible products such as confectionaries (chocolate bars, candies, cakes, etc), teas, drops and tinctures. |
| | · | Drinks. Teas, drink additives, sweeteners and branded drinks. |
| | · | Topical Products. Creams, massage oils, lotions, bath soaks, lip balms, etc. |
| | · | Dietary Supplements. Protein shakes, oil capsules, etc. |
| | · | Smokable Products. Vaporizer pens akin to e-cigarettes, which use cartridges infused with cannabis oil. |
Regulations differ significantly amongst the U.S. states. Some U.S. states only cultivation, processing and distribution of marijuana for medical purposes only, which we refer as medical marijuana, and may also include marijuana-infused products, which we refer as medical marijuana-infused products. Some U.S. states may also permit cultivation, processing and distribution of marijuana for recreational purposes, which we refer as retail marijuana or recreational marijuana, and may also include marijuana-infused products, which we refer as retail marijuana-infused products.
Most U.S. states that have legalized medical marijuana or retail marijuana impose a range of requirements on the business operators, including obtaining a license from state governmental authorities. Some states (such as Colorado) require licensed operators (or their shareholders) to be residents of that U.S. State, which we refer as residency requirement. Other states (such as Illinois, Nevada, Maryland and Arizona) do not impose a residency requirement. The State of Oregon permits out-state ownership, however, such ownership is subject to a number of restrictions.
For the marijuana related products, the Company's strategy in the states with residency requirements is focused on providing products or services to the industry rather than directly owning production or retail operations. The Company is currently actively pursuing the opportunities in the State of Colorado and Illinois.
In its Marijuana-Infused Products Segment, the Company may conduct business in the value chain segments which do not directly relate to marijuana-infused products, due to differing regulatory and business framework in the U.S. states where the Company operates, including auxiliary services (such as real estate, secured and unsecured lending, raw material supplier, etc.) or retail (such as a dispensary business).
Hemp-Infused Products Segment
In its Hemp-Infused Products Segment, the Company is focused on developing, acquiring, manufacturing and distributing products infused with non-psychoactive constituent of the industrial hemp plant (less than 0.3% THC or as otherwise permitted by applicable law), which may be distributed in all 50 U.S. States. The Company is pursuing a retail distribution strategy through networks of retail dispensaries where THC products are sold, head shops, vitamin stores and independent grocers via direct sales and distributors and is considering online sales, and multi-level marketing. Having access to the Company's expertise in manufacturing products from cannabis, these hemp-infused chocolate bars, hard candies, chews, gummies are expected to have a competitive edge in the market. The products are being sold under the "Nutritional Traditions" brand name and initially available to California retail customers. Distribution will be expanded outside of California beginning in 2016.
Our Products, Services and Operations
Marijuana-Infused Products Segment
In the State of Colorado, the Company plans to focus primarily on developing retail product lines and once the recreational products have been developed, they will be modified for the purpose of marketing to the medical markets. In the U.S. states other than Colorado, it is expected that the Company will focus primarily on developing and licensing the manufacture of marijuana-infused products for the medicinal and recreational markets, where permitted by state law.
Our products in the Marijuana-Infused Products Segment include marijuana concentrate products and edible marijuana-infused products. The products are in the development stage. The Company is developing the products using the recipes that it has developed or licensed to date. The Company has acquired 30 recipes for edible marijuana-infused products from Melissa Parks, the Company's previous VP Product Development. The recipes included Chocolate Chew, Colorado Peach Pound Cake, High Altitude Hard Candy and Caramel Cashew Popcorn with Chocolate Drizzle. Ms. Melissa Parks was issued 200,000 common shares at a price of $0.005 per common share on May 12, 2014 for assigning this intellectual property to the Company. The contract assigning the intellectual property to the company is provided as an exhibit.
We are also looking to acquire additional product lines/brands from other companies if such is deemed synergistic with the Company's current operations. Our products include:
Marijuana Concentrates Products:
Ø Solid Marijuana Concentrates. The products include extracts varying in strain types and potencies. This also includes a line of solid cannabinoid extracts, which do not contain THC.
Ø Liquid Marijuana Concentrates. The products include oils extracted from a variety of strains to be sold primarily to other licensed operators for infusion into edible marijuana-infused products.
Ø Cartridges. The cartridges that can be inserted into a vaporizer or "vape pen" for the purpose of consuming the product through "vaping". The Company will work with different dispensaries in the U.S. states that legalized marijuana and vaporizer manufacturers to create Marijuana Concentrates that work most effectively with their product brands.
Ø Tinctures. Flavoured liquids that can be added into to dips, food, hot drinks, water or cold drinks, or be used for sublingual consumption. The liquid form can come in a squeeze bottle allowing consumers to add the tincture to different types of food.
Edible Marijuana-Infused Products:
Ø Hard Candy. The hard candy line is small hard candy wrapped in the Company's packaging. This also includes a line of individual hard molded candies with a lower THC content.
Ø Chocolates. Variation of dark and/or milk chocolate bars, mint chocolate, peanut butter and chocolate and chocolate where almonds, hazelnuts, fruit or cookies could be added to the chocolate.
Ø Sugar/Sweetener packets. The sweetener line includes a powder that can be added to hot drinks (such as tea and coffee) added to cereal or used as sugar is presently used in foods.
Ø Chews. Chocolate chews and fruit chews with a variety of fruit flavours.
Ø Drink Additives. The line has a variety of flavours providing a variety of taste and selection to the customers.
Ø Other Products. Including protein shakes, boxed baking items and flour in jars with recipes for edible products.
The Company believes branding will be important and is focusing on developing brands that it believes will resonate with consumers. The Company intends to focus on the Jimi Hendrix products line under an agreement with Purple Haze Properties LLC ("PHP") for the marijuana related products.
On June 5, 2015, the Company entered into a license agreement with PHP granting the Company exclusive right to manufacture and distribute marijuana and hemp oil-infused products under "Jimi Hendrix" brand including gummy bears, hard candies and health and energy drinkable products, and non-exclusive rights to manufacture and distribute certain apparel and accessories, in the United States and Canada. The term of the license agreement is for five years, commencing October 1, 2015, with a renewal option for an additional five years. Under the terms of the agreement, the Company issued to PHP 4,025,765 Common Shares, valued for a total of USD $250,000, and agreed to pay annual fees of USD $200,000 per year and royalties equal to 15% of the sales of the "Jimi Hendrix" products received by the Company. The Company has satisfied its 2016 obligation to PHP on March 18, 2016 by issuing 5,000,000 Common Shares at a price of USD $0.05 per share to as payment for license fees and advanced royalties.
A variety of products under the "Edibles Experience" banner are in development including the "Purple Haze" line of THC-based products and the "Stone Free" line of hemp-based, non-psychoactive health products.
The Company is in its development stage of product packaging. The Company will generally only enter into raw materials and packaging agreements or royalty agreements with NH Licensed Operators that have the ability to produce the marijuana-infused products in a commercial kitchen, using semi-automated processes developed by the Company, as permitted by applicable regulation. The Company's staff has significant experience in operating commercial kitchens and the Company will assist NH Licensed Operators in establishing or upgrading manufacturing facilities and can provide appropriate support to NH Licensed Operators. The Company expects to acquire certain processing and confectionary manufacturing equipment, which the Company anticipates leasing to NH licensed operators. Management estimates that the processor is capable of processing up to 400 pounds of retail marijuana per month into marijuana concentrate, and the confectionary depositor/printer has the capacity to produce up to 350 pounds of confectionary products per hour (provided that each products weighs half an ounce). Management expects this capacity to be well beyond the initial production needs of an NH Licensed Operators.
The Company will protect its intellectual property with provisions in the applicable contracts and through the enforcement of copyright law and may commence trademark infringement actions in an event of a breach of its intellectual property rights.
State of Colorado
The Company has achieved a number of milestones in the development of its business. In Colorado, due to residency requirements, the Company's operations in the Marijuana-Infused Products Segment are dependent on its agreements with NH Licensed Operators. In this regard, the Company has acquired the industrial property located near West Pueblo, Colorado ("Pueblo Location") on November 17, 2014. The Pueblo Location is comprised of three main buildings, several smaller storage buildings, an old boiler building and an oversized two-car garage on approximately three acres. NHC paid an aggregate purchase price of US$885,000. Lease rates for similar properties are at a premium given the short supply of locations which meet the zoning and licensing requirements imposed on the industry.
The Company financed the purchase price of the Pueblo Location through the issuance of two secured convertible debentures in an aggregate principal amount of $600,000, the details of which are set out below. The remainder of the purchase price was funded by the Company through working capital. As of the date hereof, $250,000 of secured convertible debentures secured by the Pueblo Location were outstanding.
In connection with the purchase of the Pueblo Location, the Company issued to an arm's length party a senior secured convertible debenture (the "Senior Convertible Debenture") in the principal amount of $450,000. The Senior Convertible Debenture matures on November 17, 2016 (the "Maturity Date") and carries an interest rate of 12% per annum. The Senior Convertible Debenture is secured by a first ranking general security interest over all assets of the Company. The Senior Convertible Debenture is convertible into Common Shares at the option of lender at any time prior to the Maturity Date at a price of $0.06 per Common Share. As of the date of this prospectus, the Senior Convertible Debenture in the principal amount of $350,000 plus all accrued but unpaid interest have been converted into 5,833,333 Common Shares of the Company, and $100,000 of the Senior Convertible Debenture was outstanding.
The Company also issued a subordinated convertible Debenture ("Subordinated Convertible Debenture") in the principal amount of $150,000 to a group of lenders comprised of Adam Szweras, Statis Rizas and David Posner, all of whom are directors of the Company, in connection with the financing of the acquisition of the Pueblo Location. The Subordinated Convertible Debenture matures on the Maturity Date and carries an interest rate of 12% per annum. The Subordinated Convertible Debenture is secured by a general security interest over all assets of the Company, subordinate to the Senior Convertible Debenture. The Subordinate Convertible Debenture is convertible into Common Shares at the option of lender at any time prior to the Maturity Date at a price of $0.06 per Common Share. . On February 29, 2016 and on March 2, 2016, each of David Posner, CEO and Director, and Adam Szweras, Director and Corporate Secretary, respectively, advanced CAD $15,000 (a total of $30,000), which was added to the Subordinated Convertible Debenture. As of the date of this prospectus the total amount of Subordinated Convertible Debentures is CAD $180,000.
In the next 12 months, the Company expects to (i) advance additional funds to Palo Verde under the revolving loan agreement (though it is not obligated to do so upon provision of notice to Palo Verde to that effect), (ii) have Palo Verde commence production of the Company's edible Marijuana–Infused Products using the Company's recipes and other know-how, for distribution under the Company's brands, and (iii) assist Palo Verde in its efforts to produce and sell Marijuana Concentrate using the Company's know-how and brands. See "MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS – Plan of Operation".
Palo Verde intends to enter into a raw materials and packaging agreement with the Company and commence production of marijuana-infused products and marijuana concentrates using the Company's brands and recipes in the first quarter of 2015. Palo Verde also intends to commence cultivation of retail marijuana pursuant to its RMC License at the Pueblo Location upon finalizing the architectural drawings and sourcing appropriate equipment to commence operations. Palo Verde is in the process of renewing its RMIP License, which approval is expected to be granted in the normal course. On at January 21, 2016 Palo Verde has advised the Company that the application renewals were accepted by the MED and that the issuance of renewals is expected to be issued in calendar Q1 of 2016. Palo Verde has also advised the Company that the application for a marijuana establishment business license has been determined to be complete by the local Pueblo County authorities, subject to the public hearing which has taken place in calendar Q3 of 2015. The hearing has been completed and there were no objections and the Pueblo County has granted Palo Verde the business license for manufacturing of marijuana-infused products.
To date, the Company has entered into agreements with Palo Verde contemplating the following:
| | Ø | "The Lease Agreement (MIP)", which carries an annual rent of US$15 per square foot, subject to a 5% annual increase, and having a term of two years with an option to renew for an additional four years. The Lease Agreement (MIP) covers an area of 11,000 square feet. The rent commenced on January 1, 2015 subject to a six month deferral period. The deferred rent will accrue interest at a rate of 12% per annum and will be paid over a period of three months commencing on the expiry of the deferral period. Under the terms of the lease agreement, Palo Verde shall not sublet the leased property or any part thereof, nor assign the leases or any interest therein, without the prior written consent of NHC. |
| | Ø | "The Lease Agreement (Cultivation)" and its amendment, which carries an annual rent of US$15 per square foot, subject to a 5% annual increase, and having a term of two years with an option to renew for an additional four years. The Lease Agreement (Cultivation) covers an aggregate area of 15,000 square feet, comprised of two buildings. The vendor of the Pueblo Location vacated the premises at the end of August 2015, at which time, Palo Verde began to occupy the building pursuant to the Lease Agreement (Cultivation) and commence paying rent in accordance with such agreement. The rent payable by Palo Verde on the 5,000 square feet currently occupied by it commenced on January 1, 2015 subject to a nine month deferral period. The rent payable on the 10,000 square feet to be occupied by Palo Verde beginning on September 1, 2015 commenced on September 1, 2015 subject to a nine month deferral period. The deferred rent will accrue interest at a rate of 12% per annum and will be paid over a period of three months commencing on the expiry of the deferral period. Under the terms of the lease agreement, Palo Verde shall not sublet the leased property or any part thereof, nor assign the leases or any interest therein, without the prior written consent of NHC. |
| | Ø | A revolving loan agreement providing a US$150,000 unsecured debt facility to Palo Verde to draw funds for general day-to-day operating purposes, obtaining raw materials, hiring of staff and other ancillary costs related to starting and maintaining production. The revolving loan agreement provides that the Company is not under an obligation to advance funds to Palo Verde upon provision of notice to that effect. The loan commenced on July 23, 2014 and is effective for a period of 12 month at a rate of 12% per annum. The interest compounds on a monthly basis. Principal and accrued interest are payable at maturity of the facility. Palo Verde may extend the maturity date for up to five successive one-year terms for a total of five years, but no later than July 22, 2020. Each extension is subject to 2% origination fee. |
On October 1, 2014, Palo Verde advised the Company that it has received two marijuana licenses from the MED: RMIP License and RMC License.
On December 16, 2015 NHC and Palo Verde have amended the Lease Agreement (Cultivation) to provide for the following: (i) the rent payable under the Lease Agreement (Cultivation) and Lease Agreement (MIP) has been deferred ("Deferred Rent") until September 1, 2015, which shall be paid no later than September 1, 2016, subject to 12% interest per annum.
On August 31, 2015 NHC and Palo Verde have amended the Lease Agreement (Cultivation) to provide for the following: (i) the Deferred Rent payable under the Lease Agreement (Cultivation) and Lease Agreement (MIP) has been deferred until June 30, 2016; (ii) Deferred Rent shall accrue interest in the amount of twelve percent (12%) annually and the aggregate of the Deferred Rent and accrued interest shall be paid no later than September 30, 2017. (iii) Subject to applicable renewals, the Lease Agreement (Cultivation) and Lease Agreement (MIP) shall terminate on December 31, 2017, being three (3) years after the Commencement Date ("Termination Date"); and (iv) A security, cleaning, and damage deposit in the amount of one (1) month's rent shall be payable on June 30, 2016 ("Security Deposit"); and (v) Tenant's pro rata share of costs for purposes of the Lease Agreement (Cultivation) and Lease Agreement (MIP) shall be one hundred percent (100%).
The Company is also finalizing a raw materials and packaging agreement to provide its intellectual property including recipes, branding, packaging and other know-how to Palo Verde. The Company anticipates entering into such agreement with the Palo Verde in the first quarter of 2016. Such agreement is conditional on approval of the MED. The Company expects Palo Verde to commence production of marijuana-infused products in 2016. Palo Verde has received requisite approvals in respect to its Licenses from the MED and is in the process of obtaining final approvals from the local government to commence operations. The Company is working with Palo Verde to finalize a packaging agreement.
The development at the Pueblo Property is ongoing and the Palo Verde continues to work with architects, contractors, engineers, local authorities and service providers to bring the product manufacturing online. Definitive engineering has been completed and is in the process of being implemented. Palo Verde aims to complete construction in 2016, contingent on the date of the final inspection by the local authorities. In addition, the Company has taken a number of steps to assist the Palo Verde in developing its business ahead of completing the construction. In this regard, among other duties, the Company has been undertaking the following:
| | Ø | Working with Palo Verde to coordinate the preparation of the Pueblo build out plans |
| | Ø | Working to establish the medical cannabis dispensary in Lawrenceville, Illinois |
| | Ø | Working with consultants to develop manufacturing processes |
| | Ø | Developing marketing plans and product ideas |
| | Ø | Negotiations regarding branding and licensing opportunities |
| | Ø | Recruitment of personnel |
Palo Verde is not a related party to the Company or any of its officers or directors and does not hold any ownership interest in the Company. The officer of Palo Verde has provided ad-hoc consulting services to the Company from time to time, and the Board has approved a grant of 325,000 Company Options to the officer.
State of Illinois
On February 2, 2015, the Company was advised by the Illinois Department of Financial and Professional Regulation (the "Division" or "IDFPR") that it has been awarded authorization to register a medical marijuana dispensary under the Compassionate Use of Medical Cannabis Pilot Program Act (Illinois). This authorization permits the Company to submit a registration package to the Division, and upon their satisfaction, the Company will receive conditional approval for a dispensary license.
The Company has taken possession of the Lawrenceville Property, under the Lawrenceville PSA. The final acquisition price for the Lawrenceville Property was USD $350,000. The Company has also negotiated a Seller Take-Back Mortgage, which will have a 15 year amortization period, bearing an interest at the rate of 6% and be due in two years from the date of issuance as a balloon payment. Upon Payment of the Seller Take-Back Mortgage, title to the Lawrenceville Property will automatically transfer SMHI.
NHMDI is the only entity authorized to apply for a dispensary license in District 12. NHMDI has submitted all necessary paperwork to the Division to register a medical cannabis dispensary in Lawrenceville, IL. The Company is waiting for the approval of the registration package submission, which it believes to be substantially complete. Upon receiving final requisite approvals the Company expects to commence the build-out, and begin operations in early 2016. As NHMD indicated in its registration package, it is expected that the dispensary will operate under the name of "Small's Mill Medicinal Center". Based on its correspondence with IDFPR, the Company is confident of securing final approvals shortly.
In February 2016 the Company has significant developments in Illinois, which include:
| Ø | Entered into JV Agreement with ILDISP LLC, which has significant ties to the medical marijuana industry to build and operate the Company's planned dispensary in Lawrenceville, Illinois; |
| Ø | Been granted Conditional Approval by the IDFPR to establish the Lawrenceville Dispensary; |
| Ø | Commenced renovations at the Lawrenceville Dispensary property; and |
| Ø | Entered into an agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute Nutritional High's oils and edibles in Illinois. |
Under the terms of the JV Agreement ILDISP LLC will fund up to USD $300,000 of the expenses and working capital required to complete and launch the Lawrenceville Dispensary. In addition, ILDISP LLC will provide the Company with a guarantee for half the Seller Take-Back Mortgage. In exchange for its contribution, ILDISP LLC shall receive a 50% interest in NHMD, the Company's wholly owned subsidiary which holds the Conditional Approval for the Lawrenceville Dispensary, and a 50% interest in SMHI, the Company's wholly owned subsidiary which holds the Company's interest in the Dispensary real estate property located in Lawrenceville, IL. The joint venture might require a need for additional capital infusions in excess of $300,000, which might create an obligation on the Company to make additional contributions, failing to do which may result in reduction of the Company's interest in NHMDI and SMHI. In addition, ILDISP's failure to contribute may create an greater need for the Company to contribute additional capital, which may not be available to the Company on favorable terms or at all.
ILDISP LLC has already made initial advances to fund the Lawrenceville Dispensary renovations and property mortgage payments. The JV is subject to the approval of the IDFPR and the entry into a shareholders agreement. Furthermore, it is contemplated that ILDISP LLC will contribute to the management of the Lawrenceville Dispensary and its relationships in the surrounding community will help accelerate the growth and development of the Dispensary. The shareholder agreement has not been entered into as of the date hereof and is expected to be finalized in due course. It is expected that he shareholder agreement will contain customary provisions such as governance of the rights and responsibilities of the shareholders, respective share ownership and dilution mechanism if additional capital is required. It is expected that each of NHII and ILDISP will appoint two directors to the board of the NHMD and SMHI and each will own 50% interest in each of NHMD and SMHI, upon ILDISP completing its earn-in commitment by ILDISP. The shareholder agreement shall also provide for an arbitration mechanism if either party fails to fund the additional capital requirements and a buyout provision if either party's ownership interest falls below a certain threshold. Both parties will appoint a general manager for the dispensary, once operational, who will report to both parties and may be removed by common consent. The shareholder agreement remains subject to IDFPR's approval and there is no guarantee that the final version will be entered on the terms favorable to the Company or at all.
In addition, NHMD has been advised by the IDFPR that it has been awarded Conditional Approval to register the Lawrenceville Dispensary under the CUMCPPA. The Conditional Approval sets out the requirements that NHMD must fulfill prior to IDFPR approving the registration of the dispensary, which includes completing the renovations and passing the final inspection to the satisfaction of IDFPR. Upon meeting IDFRP's conditions, it is expected that NHMD will be granted a final license to operate the Lawrenceville Dispensary.
The Company also has entered into an agreement with an Illinois cannabis cultivation and extraction facility to develop a framework under which the extraction facility will manufacture and distribute Nutritional High's oils and edibles in Illinois. The cultivation and extraction facility is licensed with Illinois Department of Agriculture and was amongst the first of the companies to commence commercial cultivation and processing of cannabis products under the CUMCPPA. The Company will develop its business framework with the extraction facility over the next 18 months and will provide updates as this business initiative develops. The agreement provides for a right to negotiate an agreement to negotiate the framework in good faith, but provides for no financial terms in this regard. As such, the Company considers the agreement not material to its operations at this time.
Other Jurisdictions - Canada
The Company has also entered into option agreements with two separate companies pursuant to which the Company has the option to purchase all the capital stock of these two companies that have applied for licenses under means Marihuana for Medical Purposes Regulations under the Controlled Drugs and Substances Act (Canada) ("MMPR"). The options are currently of secondary priority to the Company's Marijuana-Infused Products Segment, as the Company does not anticipate that the licenses will be granted in the next twelve 12 months, due to regulatory uncertainty and a backlog of applications for licences of medical marijuana in Canada.
The Company has entered into a consulting agreement with Canopy Growth Corporation ("Canopy"), a company formed from the combination of Tweed Marijuana Inc. and Bedrocan Cannabis Inc., to assist Canopy in developing their cannabis extracts product offerings in Canada. The Company will be involved in various capacities including identifying equipment and developing manufacturing processes related to marijuana concentrates and marijuana-infused products. The Company does not expect to expend significant financial resources in the Canopy project over the next twelve months.
Hemp-Infused Products Segment
The Company's first three products in its Hemp-Infused Products Segment include gelatine capsules, push caps for water bottles and a formulation for post exercise recovery called Rapid Recovery. The products are being sold under the "Nutritional Traditions" brand name and initially available to retail stores in Northern California. Distribution will be expanded outside of California beginning in 2016. All active hemp products will be manufactured using extract from only the industrial hemp plant, containing less than 0.3% THC (trace elements only) and therefore may be distributed in all 50 states. The Active Hemp capsules are available in both 5 mg and 25 mg doses, which include non-psychoactive ingredients that naturally occur in the industrial hemp plant. All hemp used in the products is tested for its quality and purity.
In the secondary phase of product development the Company intends to pursue development of the following product lines:
| | Ø | Chocolates. Variation of dark and/or milk chocolate bars, chocolate cookies, mint chocolate, peanut butter and chocolate bars with almonds, hazelnuts or fruit |
| | Ø | Gummies. Flavored chewy candies wrapped in the Company's packaging |
| | Ø | Hard Candies. Small hard candies wrapped in the Company's packaging. This also includes a line of individual hard molded candies with a hemp content |
The Company has completed the formulation and development of the initial product line under the name "Nutritional Traditions", and is in the process of developing additional products for its Hemp-Infused Products segment. The products are currently available to retail stores in Northern California. The Company intends to expand the distribution outside of California in 2016.
Regulations
Marijuana-Infused Products Segment
The Company's Marijuana-Infused Products Segment is currently focused exclusively in the U.S. states that have legalized marijuana for medical or recreational uses and require manufacturers of marijuana products to hold a License issued by the applicable state authorities. The Company's business will be affected by both state and federal regulation governing the production and sale of marijuana in general, and edible marijuana-infused products, in particular.
Changes in both state and federal law and the enforcement of those laws could have a material positive or negative impact on the Company's operations. The Company is also looking at other states for prospective business opportunities should changes in regulations occur that are favourable to the Company's business. For further discussion regarding the risk factors relating to the Company's business. See "Risk Factors."
License and Residency Requirements
Regulations differ significantly amongst the U.S. states. Some U.S. states only permit the cultivation, processing and distribution of medical marijuana and marijuana-infused products. Some U.S. states may also permit the cultivation, processing and distribution of marijuana for recreational purposes and retail marijuana-infused products.
Most U.S. states that have legalized medical marijuana or retail marijuana impose a range of requirements on the entities wishing to become licensed operators including obtaining a license from state governmental authorities. Some states (such as the State of Colorado) impose a residency requirement, requiring licensed operators (or their shareholders) to be residents of that U.S. State. Other states (such as Illinois Nevada, Maryland and Arizona) do not impose a residency requirement. The State of Oregon permits out-state ownership, however, such ownership is subject to a number of restrictions. The Company's strategy in the states with residency requirements is focused on providing services to the industry rather than directly owning production or retail operations. The Company currently is currently actively pursuing the opportunities in the following jurisdictions:
| State | Recreational Use | Medical Use |
| Colorado* | X | X |
| Illinois | | X |
* indicates that Licensed Operators are subject to Residency Requirements
The Company is evaluating potential opportunities in the following jurisdictions, but does not currently have active operations.
| State | Recreational Use | Medical Use |
| Washington* | X | X |
| Nevada | | X |
| Oregon* | X | X |
| Maryland | | X |
| Canada | | X |
* indicates that Licensed Operators are subject to Residency Requirements
State of Colorado Regulatory Regime
On November 6, 2012, Colorado Amendment 64 was passed to amend Colorado's constitution, subsequently enacted as Article 18, section 16 of the state constitution, addressing "personal use and regulation of marijuana" for adults 21 and over, as well as commercial cultivation, manufacture, and sale, effectively regulating cannabis in a manner similar to alcohol. Pursuant to the Retail Code adopted in 2013, by the State of Colorado, licensed operators are subject to residency requirements. Marijuana Enforcement Division, Colorado Department of Revenue, a state government agency of the State of Colorado ("MED") is tasked with licensing and regulating marijuana industries in the State of Colorado.
Under the regulatory regime in Colorado, a company or individual may apply for the two types of Licenses – a license to conduct business in the retail marijuana sector ("Retail License") and a license to conduct business in the medical marijuana sector ("Medical License"). Medical License has three classes:
| | Ø | "MMC Licence" – also known as a "medical dispensary" License. A MMC License allows for the operation of a medical marijuana store from which medical marijuana registry patients may purchase medical marijuana and medical marijuana-infused products. Medical marijuana cannot lawfully be grown and medical marijuana-infused products may not be produced with this license or within this facility's premises. There are several types of MMC Licenses, depending on the number of registered patients that are serviced. |
| | Ø | "MMOPC License" – facility which grows, harvests and processes medical marijuana for sale to licensed operators holding a valid MMC License or for sale to Licensed Producers holding a valid MMIP License for use in medical marijuana-infused products. Holders of MMOPC License may only cultivate medical marijuana and must be associated with at least one licensed operator holding a valid MMIP License or MMC License. |
| | Ø | "MMIP License" – facility which produces medical marijuana-infused products. These facilities are only able to wholesale their products to MMC License holders. |
Retail License has four classes:
| | Ø | "RMS Licence" – means an entity licensed to purchase retail marijuana from RMC License holders and retail marijuana–infused products from RMIP License holders and to sell retail marijuana and retail marijuana-infused products to qualified consumers. Retail marijuana may not be cultivated or processed under this License. |
| | Ø | "RMIP License" – means an entity licensed to purchase retail marijuana; manufacture, prepare, and package retail marijuana-infused product; and sell retail marijuana and retail marijuana-infused product to other RMIP License holders and to RMS License holders, but not to consumers. Retail marijuana may not be cultivated or sold to retail customers under this License - all sales must be made wholesale to entities holding a RMS License. If a holder of this License class chooses to own and operate a cultivation facility it may not sell any of the retail marijuana that it cultivates except for the retail marijuana that is contained in its products. |
| | Ø | "RMC License" – used exclusively for the cultivation of retail marijuana plants and the harvesting of retail marijuana. If not associated with a holder of a RMIP License this licensee may sell retail marijuana to holders of a RMC License, RMS License or RMIP License. Medical marijuana cannot be lawfully cultivated with this license or within this facility's premises. |
| | Ø | "RMT License" – facility that performs testing and research on retail marijuana for other licensed operators. |
State of Illinois Regulatory Regime
On August 1, 2013, Illinois enacted the means Compassionate Use of Medical Cannabis Pilot Program Act (Illinois) ("CUMCPPA") legalizing medical marijuana in Illinois with the legislation taking effect on January 1, 2014. CUMCPPA establishes a patient registry program, protects registered qualifying patients and registered designated caregivers from "arrest, prosecution, or denial of any right or privilege," and allows for the registration of cultivation centers and dispensing organizations.
According to CUMCPPA, qualifying patients can begin applying for registration commencing September 1, 2014. Patients must submit written certification from a doctor that they need medical marijuana, proof of Illinois residency, proof of identity and age and a photo when they apply.
The CUMCPPA is regulated by three departments of the State of Illinois:
| | · | Department of Agriculture (Illinois) ("DOA"), a department of the Illinois state government that regulates various facets of the agriculture industries of Illinois, including regulating the application process for cultivation of marijuana and regulating the application process for facilities operated by an organization or businesses that are registered by the DOA to perform necessary activities to provide Dispensaries with usable medical marijuana ("Cultivation Centres"); |
| | · | Department of Public Health (Illinois) ("DPH"), a department of the Illinois state government that prevents and controls disease and injury, regulates medical practitioners, promotes sanitation and medical marijuana patient registration process under CUMCPPA; |
| | · | Department of Financial and Professional Regulation (Illinois) ("DFPR"), a department of the Illinois state government that regulates banks, other financial institutions, real estate agents, insurance firms, and insurance agents, oversees the professional qualifications of members of self-regulating professions and regulating the application process for businesses ("Dispensaries") registered in the State of Illinois to acquire medical cannabis from a registered Cultivation Center for the purpose of dispensing cannabis, paraphernalia, or related supplies and educational materials to registered qualifying patients ("Dispensary Licenses"); |
These departments are required to enact rules to implement the CUMCPPA, which were finalized and ready for implementation on July 15, 2014. There are currently no residency requirements for companies wishing to apply for Cultivation or Dispensary Licenses.
The CUMCPPA states that a medical cannabis dispensary may not be located within 1,000 feet of the property line of a pre-existing public or private preschool or elementary or secondary school or daycare center, daycare home, group daycare home, or part day child care facility. A medical cannabis dispensing organization may not be located in a house, apartment, condominium or an area zoned for residential use.
Section 85(a) of CUMCPPA provides that the DOA may register up to 22 medical marijuana cultivation centers. Section 115(a) of CUMCPPA provides that the DFPR may issue up to 60 licenses for Dispensaries.
The DOA was accepting applications for Cultivation Centres and Dispensaries between September 8, 2014 and September 22, 2014. The application for a Dispensary Licence by NHMDI required a submission of a comprehensive package, which included submissions on the following primary subjects:
| | Ø | Suitability assessment of the proposed dispensary – explanation of why the proposed location is suitable for public access, the size and layout promote safe dispensing of medical cannabis, product handling, and storage; |
| | Ø | Business and operations plan – information about principal officers, a staffing plan, business management practices, operating plan, and a general description of products, varieties and services related to medical cannabis intended to be offered and reasoning for those choices; |
| | Ø | Security plan – facility security plan, surveillance system description, product security, shipping/transportation security measures; |
| | Ø | Recordkeeping and inventory plan – recordkeeping plan, inventory control plan, patient education and support plan; |
| | Ø | Financial disclosures – disclose all relevant business transactions and financial information connected with the application; and |
| | Ø | Optional Information – labour and employment practices, research plan, community benefits plan, substance abuse prevention plan, local community/neighbourhood report and environmental plan. |
On October 2, 2014 the State of Illinois released an update indicating that the DOA received 159 submissions for Cultivation Centre licenses and the DFPR received 214 submissions for Dispensary Licenses. The update indicates that Lawrence County (the county where the Lawrenceville property is located) belongs to District 12, where four cultivation centre submissions were received and one Dispensary License submission (that of NHMDI).
On February 2, 2015, Jason Barclay, the general counsel to Illinois Governor Bruce Rauner, released a statement disclosing that 18 Cultivation Centers were awarded permits and 52 Dispensary applicants were awarded clearances from the DFPR, authorizing the organizations that submitted an application for a Dispensary License to submit a Registration Package ("Authorizations"). (Rauner announces who can grow and sell medical marijuana in Illinois. http://chicago.suntimes.com/politics/7/71/339015/rauner-announces-can-grow-sell-medical-marijuana-illinois) An Authorization permits an applicant to submit the package which provides additional detail on construction, start-up and operation of the Dispensary ("Registration Package"). Upon the DFPR's satisfaction of such Registration Package, the DFPR will issue a conditional approval, after which time the build-out can commence. Granting of the Dispensary License is then subject to final inspection by the DFPR in order to confirm that the build-out has been completed as specified in the Registration Package
U.S. Federal Law
While marijuana and marijuana-infused products are legal under the laws of several U.S. states (with vastly differing restrictions), at the present time, the concept of "medical marijuana" and "retail marijuana" do not exist under U.S. federal law. The United States Federal Controlled Substances Act classifies "marijuana" as a Schedule I drug. Under U.S. federal law, a Schedule I drug or substance has a high potential for abuse, no accepted medical use in the United States, and a lack of safety for the use of the drug under medical supervision.
The United States Supreme Court has ruled in a number of cases that the federal government does not violate the federal constitution by regulating and criminalizing cannabis, even for medical purposes. Therefore, federal law criminalizing the use of marijuana pre-empts state laws that legalizes its use for medicinal purposes.
In a memorandum dated August 29, 2013 addressed to "All United States Attorneys" from James M. Cole, Deputy Attorney General ("Cole Memo"), the U.S. Department of Justice ("DOJ") acknowledged that certain U.S. states had enacted laws relating to the use of marijuana and outlined the U.S. federal government's enforcement priorities with respect to marijuana notwithstanding the fact that certain U.S. states have legalized or decriminalized the use, sale and manufacture of marijuana:
| | · | Preventing the distribution of marijuana to minors; |
| | · | Preventing revenue from the sale of marijuana from going to criminal enterprises, gangs, and cartels; |
| | · | Preventing the diversion of marijuana from U.S. states where it is legal under state law in some form to other U.S. states; |
| | · | Preventing U.S. state-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity; |
| | · | Preventing violence and the use of firearms in the cultivation and distribution of marijuana; |
| | · | Preventing drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use; |
| | · | Preventing the growing of marijuana on public lands and the attendant public safety and environmental dangers posed by marijuana production on public lands; and |
| | · | Preventing marijuana possession or use on U.S. federal property. |
There is no guarantee that the current presidential administration will not change its stated policy regarding the low-priority enforcement of U.S. federal laws that conflict with state laws. Additionally, any new U.S. federal government administration that follows could change this policy and decide to enforce the U.S. federal laws vigorously. Any such change in the U.S. federal government's enforcement of current U.S. federal laws could cause adverse financial impact to the Company's business. See "Risk Factors".
In February 2014, FinCEN issued guidelines allowing banks to legally provide financial services to licensed operators that hold a valid license ("FinCEN Memo"). The rules re-iterated the guidance provided by the Cole Memo, stating that banks can do business with licensed operators and "may not" be prosecuted. The guidelines provide that "it is possible [for the banks] to provide financial services" to licensed operators and while remaining in compliance with federal anti-money laundering laws. The guidance falls short of the explicit legal authorization that banking industry officials anticipated and the outcome of the banks relying on this guidance in transacting with licensed operators is currently unclear. See "Risk Factors".
On December 16, 2014, President Obama signed the Cromnibus Bill, approving spending for certain federal agencies through September 30, 2015. Section 583 of the Cromnibus Bill prohibits the United States government from using federal funds to prevent states with medical marijuana laws from implementing state laws that authorize the use, distribution, possession, or cultivation of medical marijuana. In June 2015, The United States House of Representatives voted to reauthorize the amendment ("Rohrabacher-Farr Amendment") to the Commerce, Science, and Justice ("CJS") Appropriations bill for fiscal year 2015. The Rohrabacher-Farr Amendment has re-authorized the ban of DOJ from expending funds to prevent the implementation of state-level medical cannabis programs, removing funding for federal medical cannabis raids, arrests and prosecutions in states where medical cannabis is legal. The Rohrabacher-Farr Amendment has also expanded the number of states and now covers 39 states and protects access to medical cannabis programs for 275 million citizens in those states. Nevertheless, there can be no certainty that future U.S. federal funding bills will include similar provisions. See "Risk Factors".
To date, other federally-regulated agencies have taken a stance that using their services to conduct business in the marijuana sector remains illegal. On November 27, 2015 the United States Postal Service ("USPS") has issued a memo which specifies that it is illegal to "place an ad in any publication with the purpose of seeking or offering illegally to receive, buy, or distribute a Schedule I controlled substance" and that USPS cooperates with all other agencies in preserving the laws of United States. While, newspapers in some U.S. states where medical or retail marijuana are permitted by the state regulations, have been accepting payment on advertisements for legal marijuana-related businesses without legal repercussions, there is no assurance that DOJ will not enforce federal laws in similar contexts. See "Risk Factors".
Hemp-Infused Products Segment
The Company's primary focus in the Hemp-Infused Products Segment is in the development and sale of supplement products in the United States. The Company's business will be affected by both state and federal regulation governing the production and sale of hemp stalk oil-infused products in general. At present time, pursuant to federal regulations, hemp stalk oil-infused products are excluded from the definition of "marihuana", as long as the product does not contain active levels of tetrahydrocannabinol ("THC"), the psychoactive compound of the cannabis plant. It is generally accepted that products with less than 0.3% THC do not contain active levels of THC. Changes in related state and federal regulation could have a material positive or negative impact on the Company's operations. See "Risk Factors".
While oil derived from industrial hemp stalk that has naturally occurring THC content equal to or less than 0.3% is excluded from the definition of marijuana under the United States Controlled Substances Act ("CSA"), there is no certainty that this exclusion could not be altered by court or governmental action or re-interpretation. See "Risk Factors".
Business Model
Marijuana-Infused Products Segment
The Company's business model in its Marijuana-Infused Products Segment differs depending on the residency requirements of the applicable jurisdiction. Most U.S. states that have legalized marijuana for medical or recreational use require licensed operators to hold a license issued by the applicable state authorities. In some states, for a licensed operator to be eligible to be granted a license, the owners of the licensed operator must be residents of such U.S. State. As such, listed companies or other widely held enterprises are ineligible to obtain a license in those states where a licensed operator must be a U.S. state resident. In the U.S. states where the applicable regulations permit minority ownership in the licensed operators for the entities which are note residents of that U.S. State, the Company may acquire an interest in the licensed operators.
The Company will not operate in jurisdictions which have not legalized marijuana, and does not intend on operating in jurisdictions which have legalized marijuana but have not developed and imposed a licensing regime for licensed operators, in a manner compliant with the Cole Memo.
In the U.S. states without residency requirements, the Company may choose to apply for a license or acquire entities with a license and produce products itself, or work with other licensed operators using the same model as it has developed for U.S. states with a residency requirement. The licensed operators include growers of marijuana, marijuana-infused product manufacturers and retail dispensaries. Ancillary service providers may include medical and educational centres and marijuana paraphernalia shops.
In certain jurisdictions, and more specifically in the states with the residency requirements, the Company may conduct business in the value chain segments, which do not require a license from the requisite regulatory authorities. Such ancillary value chain segments do not directly handle, process, manufacture, cultivate or distribute cannabis products, and may include: unsecured lending, providing real estate to licensed operators, equipment leasing and providing non-cannabis raw materials (such as packaging, food ingredients, etc.) The focus of this strategy is to provide the services to licensed operators that are focused on extraction and processing of cannabis to enable them to attain a stronger competitive advantage in the market, while proving an acceptable economic return to the Company.
In certain circumstances the Company may pursue other value chain segments, such as operating a dispensary, in the states where the regulatory environment is unsuitable to earn an economic rate of return in the extraction/processing segment, given the Company's assessment. This aim of this strategy is to secure a foothold in such markets, through obtaining a license to operate a business that is not directly related to extraction/processing and then partner with another licensed operator who is able to operate in the extraction/processing space. The Company has employed this type of strategy in the State of Illinois, where it is working to obtain a dispensary license, but has also partnered with another licensed operator to be in a position to sell its products in Illinois.
U.S. states with Residency Requirements
In U.S. states with residency requirements, the Company will work with companies or other entities that have a valid License issued by the applicable U.S. state authorities to provide an array of services as a part of its "franchise-like" model, or will work with eligible persons applying for such Licence. The Company has developed a business model where it is undertaking a combination of the following functions with the expectation of realizing the following respective revenue streams from such activities.
In most cases the Company seeks to enter into raw materials and packaging agreements with NH Licensed Operators, whereby the Company shall supply non-marijuana raw materials and packaging for marijuana-infused products.
| Activities | | Expected Revenue Streams |
| Acquire and develop recipes, know-how and other intellectual property for the preparation of marijuana-infused products and marijuana concentrates, for use by NH Licensed Operators entering into royalty agreements with the Company, where permitted by the applicable regulation. | | Royalty fees and fees from selling packaging and non-marijuana raw materials |
| Develop recognizable brands for marijuana-infused products and marijuana concentrates for use by NH Licensed Operators entering into raw materials and packaging agreements with the Company. | | Fees from selling packaging and non-marijuana raw materials |
| Provide consulting services with respect to extraction processes, techniques, training and know-how relating to Marijuana Concentrates. | | Consulting fees
|
| Acquire real estate for lease to licensed operators. | | Leasing fees, Rent |
| Provide financing and equipment leasing to licensed operators and prospective licensed operators. | | Interest income
Loan fees (renewal, origination, etc.)
Leasing Fees |
| Provide financial and strategic support to licensed operators in securing supply of marijuana, where permitted by the applicable regulation. | | Miscellaneous consulting fees |
While the Company's core strength is development of edible marijuana-infused products and it is developing expertise in marijuana concentrate extraction techniques, it approaches each jurisdiction with a tailored strategy in order to comply with the regulatory framework, while emphasizing its core competencies in the edible marijuana-infused products market. As such, the Company may focus on different parts of the industry value chain, or focus on acquiring assets in the industries, not directly related to marijuana-infused products or marijuana concentrate in order to ensure such compliance (e.g., acquisition of real estate, retail, raw material supplier, equipment leasing, unsecured lending and consulting).
The Company has engaged legal counsel in Colorado to conduct appropriate corporate due diligence to ensure compliance of licensed operators with whom the Company conducts business. Compliance with the residency requirement in Colorado are strictly monitored by the MED. The Company intends to verify that licensed operators with whom it does business provide a copy of their state and local licenses. Any agreements entered into with the licensed operator will be subject to the licensed operator maintaining its licenses in good standing with the appropriate regulatory authority and comply with applicable laws.
U.S. states without Residency Requirements
The Company is also considering seeking licensing to manufacture and distribute edible marijuana-infused products and marijuana concentrates in certain U.S. states with or without residency requirements that the Company is able to comply with. The Company is also considering licensing its marijuana-infused products for sale in certain U.S. states for manufacturing and distribution in other U.S. states, where edible marijuana-infused products are permitted by the applicable U.S. state regulations. Due to U.S. federal regulations, the Company will evaluate each U.S. state in which the Company chooses to operate as a separate market and with a distinct business plan. Given market fragmentation due to the various U.S. state regulatory regimes, the Company expects that its products will be manufactured in micro-factories for distribution only in the U.S. state where the micro-factory is situated.
Hemp-Infused Products Segment
Initially, all products in the Hemp-Infused Products Segment will be produced by a contract manufacturer similar to other small to medium-sized companies in the space. The manufacturer is responsible for sourcing all raw materials and undertaking the manufacturing process. The final products are then shipped to the Company's customers.
The Company will evaluate installation of its own facility for manufacturing of hemp-infused products at various points during its development to deem if it may be commercially accretive. Such project may require raising additional funds to finance the capital expenditures associated with securing the facility, equipment and hiring staff to operate the line.
The Company has develops its own branding with help of third party consultants, but may consider entering into arrangements with the third parties to license their intellectual property. The Company manufactures its own labelling and packaging with help of external consultants.
Marketing
The Company's marketing efforts are subject to strict state and federal regulations, which the Company (or an NH Licensed Operator, as the case may be) must adhere to, especially considering the federal status of marijuana. In the states which have legalized medical marijuana or retail marijuana product promotion and advertisement has been mostly limited to word of mouth, online and social media as the companies are not permitted to use traditional forms of print advertisement, such as newspapers. As the interstate commerce is not permitted, there are substantial product price differentials, which are determined by the supply and demand dynamics in every market it operates in. Product distribution is largely dependent on the regulatory framework of each individual state, and in many cases the distribution channels are strictly specified by the state regulation. For these reasons, the Company's develops a distinct marketing strategy for each of the states it operates, in the Marijuana-Infused Products Segment. The marketing strategy for the Hemp-Infused Products segment was developed by taking a nation-wide approach, as the products may be distributed in all 50 states.
Marijuana-Infused Products Segment
State of Colorado
The Department of Revenue of Colorado commissioned a study which estimated 2014 annual demand for marijuana for recreational use at 130.3 metric tons (total resident and visitor demand), with approximately 77 tons expected to be sold through retail marijuana stores. This indicates that approximately 53.3 tons will be provided to the market from outside of the regulatory framework – indicating a significant supply-demand imbalance, which creates an opportunity for new market entrants to fill the gap. (Source: Market Size and Demand For Marijuana in Colorado, prepared by The Marijuana Policy Group for the Colorado Department of Revenue)
The statistics in the study are focused on the retail marijuana market, but currently provide no data specific to the retail marijuana-infused products. Although a portion of the market sales estimate includes edible marijuana-infused products, the author of the report assumes all sales represented dry weight of retail marijuana.
Based on Colorado Department of Revenue report Colorado Marijuana Tax Data, total retail sales at medical marijuana stores were US$383 million and US$362 million for the 2015 fiscal year (July 2014 to June 2015). The Legislative Council Staff of the Colorado General Assembly, reports in its research paper on economic and revenue forecast that the expected sales tax revenues for fiscal years 2015-2016, 2016-2017 and 2017-2018 from medical and recreational marijuana, are estimated at US$25.7 million, US$27.3 million and 28.4, respectively. (Source: Focus Colorado: Economic And Revenue Forecast, December 21, 2015) Dividing the expected tax revenue by the sales tax rate of 2.9%, these figures extrapolate to US$828 million and US$869 million, respectively. The same document projects the tax revenue for recreational marijuana for fiscal years 2015-2016 and 2016-2017, at US$14.3 million and US$15.8 million, respectively. These figures extrapolate to US$493 million and US$545 million gross revenues, for the respective tax years. Though the data does not state the percentage of sales allocated to marijuana-infused products versus retail marijuana, these statistics evidence the size of the potential market in the state of Colorado. The Company considers the estimates of the Legislative Counsel Staff of the Colorado General Assembly conservative based on monthly reports.
As of January 4, 2016, MED has reported the following statistics regarding licensed operators:
The Company is taking a staged approach to rolling out the products in Colorado – initially focused on marijuana concentrates in Phase I followed by edible marijuana-infused products in Phase II. The products will be distributed solely through dispensaries which hold a valid RMS License. It is intended that the Company's products sold by NH Licensed Operators will be priced competitively, with a view of introducing a premium product suite under the Jimi Hendrix brand line. The NH Licensed Operators will initially focus on distributing to dispensaries in densely populated areas and group which own more than one RMS License.
State of Illinois
According to the regulations, the patients that qualify for medical marijuana under CUMCPPA must obtain a prescription from a doctor and also submit an application to DPH to become qualified patient ("Qualified Patient") and be able to purchase medical marijuana from Dispensaries. According to the press release from DPH, as at January 6, 2016, approximately 5,200 individuals have submitted a complete application to DPH and approximately 4,000 have received approvals and are Qualified Patients.
According to DPH regulations, Qualified Patients must select a medical cannabis dispensary to purchase medical cannabis, and must fill-out a form if they want to change the dispensary, which are then processed and confirmed by the IDPH only. Given that NHMDI's is located in a rural area, with high other dispensaries being located considerably far from Lawrenceville, IL the Company expects that the patient base will be relatively "sticky" whereby there will be little incentive to switch do other dispensaries due to geographic dispersion. As such, the Company feels that NHMD is relatively well positioned in terms of competition. NHMD will work with doctors and clinics in the area with a view of facilitate the patient on boarding process and also develop a referral system to increase its patient base, once operational.
Hemp-Infused Products Segment
The products will be distributed through two primary retail channels ("Retail Establishments"):
| | 1. | Cannabis-related retail stores: medical marijuana dispensaries, vape lounges and headshops. Even though the hemp products do not contain marijuana, and licenses are therefore not required to sell the Company's hemp products, management believes dispensary clients are likely to be strong adopters; |
| | 2. | Food Supplement retail stores (eg. vitamin stores, supplement stores, etc.) |
The initial focus will be the State of California, where the Company intends to work with retail distributors who specialize in distributing the hemp products and have relationships with the retail stores. The market was initially segmented by the number of establishments by zip code, focusing on areas with highest concentration of Retail Establishments. Top 10 cities are listed below:
| City | Headshops | Dispensaries | Vape lounges | Total Retail Establishments |
| Los Angeles | 59 | 250 | 6 | 315 |
| San Diego | 24 | 52 | 5 | 81 |
| San Jose | 15 | 54 | 3 | 72 |
| Santa Ana | 10 | 50 | 0 | 60 |
| San Francisco | 22 | 26 | 2 | 50 |
| Van Nuys | 3 | 44 | 0 | 47 |
| North Hollywood | 11 | 34 | 0 | 45 |
| Sacramento | 12 | 24 | 1 | 37 |
| Riverside | 15 | 16 | 1 | 32 |
| San Bernardino | 1 | 25 | 0 | 26 |
| Other | 453 | 598 | 71 | 1122 |
| Total: | 625 | 1173 | 89 | 1887 |
Sources: www.headshopfinder.com; Leafly, http://www.thevapormap.com/; NH management research
In some cases, the Company will distribute the products through third party distributors, which have capabilities to sell to head shops, cannabis accessory stores, vitamin stores and independent grocers. The Company has been in discussions with several distributors in California who expressed an interest in carrying the Company's products.
Competition
State of Colorado
In the Marijuana-Infused Products Segment, the Company is initially focused on the State of Colorado, where marijuana-infused products are currently legal for medicinal and recreational use and is also pursuing opportunities in other U.S. states.
Since marijuana has only recently been legalized in certain jurisdictions, the Company's management believes the industry is still in infancy stages, and business, industrial and regulatory frameworks are not fully developed. Lack of traditional sources of financing, absence of an efficient supply chain network and streamlined marketing channels, and strict regulatory requirements (including the Residency Requirement) create market inefficiencies, which create a business opportunity for the Company.
In the United States, there are several public companies offering financing, incubation and strategic services to licensed operators in different U.S. states; however, few offer a similar focus on edible marijuana-infused products. Below is a summary of some of the competitors identified by the Company.
| Brand | Product Types |
| Dixie Elixirs | Beverages, chocolate treats, drops, topical creams, scripts, marijuana concentrates |
| Incredibles | Cookies, creams, chocolate bars, protein bars, marijuana concentrates |
| EdiPure | Gummies, chews, crackers, drops |
| Blue Kudu | Chocolate |
| O,pen Vape | Cartridges for vaporizers that pre-filled with marijuana concentrates |
| Top Shelf Extracts | Marijuana concentrates |
| Mahatma Concentrates | Marijuana concentrates |
| The Green Solutions | Marijuana concentrates and vaporizer cartridges |
While the Company is not directly involved in production and manufacturing of the products outlined above as the entity is required to hold a License, the Company considers the products these companies manufacture to be competing with the Company's products by virtue of the relationship with Palo Verde. As the products that Palo Verde intends to manufacture using the Company's recipes and packaging need to appeal to the customers in the Colorado market, these brands are directly competitive to those of the Company.
State of Illinois
In February 2, 2015, the State of Illinois has announced that 52 companies have been awarded the Authorizations under CUMCPPA. While most applicants have only applied for a Dispensary License in one Illinois State Police district ("District"), there were several companies which have been awarded Authorizations in several Districts. NHMDI is the only company to receive the Authorization in District 12, which covers 10 counties with a population of approximately 200,000. According to the Company's research there are no other dispensaries in over a 100 miles radius demonstrating that the NHMD is well positioned in the market in terms of competition. Furthermore, to the Company's knowledge, very few of the Dispensary Applicants who were awarded Authorizations have received a License as of the date hereof, further supporting the Company's competitive position in the State of Illinois. Below is a summary of the Companies who were awarded Authorizations, the number of Authorizations received and their respective Districts (source: Klein, Thorpe and Jenkins Ltd. http://www.ktjlaw.com/457AE3/files/2015/04/MedicalMarijuanaLicensesAwarded.pdf):
| Dispensary applicant name to receive Authorizations | | Number of Authorizations | | | Districts where Authorization is received | |
| Pharmacann, LLC | | | 4 | | | | 17, 26, 31, 34 | |
| WCCC, LLC | | | 4 | | | | 37, 38, 39, 40 | |
| Nu Med Rx, LLC | | | 3 | | | | 8, 10, 45 | |
| TGS Illinois, LLC | | | 2 | | | | 6, 11 | |
| Greenhouse Group, LLC | | | 2 | | | | 25, 33 | |
| 3C Compassionate Care Center, LLC | | | 2 | | | | 29, 34 | |
| New Age Care, LLC | | | 1 | | | | 32 | |
| Evergreen Dispensary | | | 1 | | | | 14 | |
| 420 Capital Management, LLC | | | 1 | | | | 46 | |
| Floramedex, LLC | | | 1 | | | | 35 | |
| Nature's Treatment of Illinois, Inc | | | 1 | | | | 7 | |
| Future Transactions Holdings, LLC | | | 1 | | | | 36 | |
| EarthMed, LLC | | | 1 | | | | 24 | |
| Compass Ventures, Inc | | | 1 | | | | 18 | |
| Professional Pharmacy Management, LLC | | | 1 | | | | 48 | |
| The Dispensary, LLC | | | 1 | | | | 1 | |
| MedMar, Inc | | | 1 | | | | 44 | |
| Trinity MMJ, LLC | | | 1 | | | | 8 | |
| GTI Clinic Illinois Holdings, LLC | | | 1 | | | | 27 | |
| Midwest Compassion Center, Inc | | | 1 | | | | 29 | |
| Harborside Illinois Grown Medicine, Inc | | | 1 | | | | 42 | |
| NCC, LLC | | | 1 | | | | 30 | |
| Healthway Services of West Illinois, LLC | | | 1 | | | | 26 | |
| NH Medicinal Dispensaries, Inc. | | | 1 | | | | 12 | |
| Herbal Remedies Dispensaries, LLC | | | 1 | | | | 20 | |
| PDI III, LLC | | | 1 | | | | 27 | |
| Kirkwood Pharmacy Group of Anna, LLC | | | 1 | | | | 22 | |
| Phoenix Farms of Illinois, LLC | | | 1 | | | | 10 | |
| Kirkwood Pharmacy Group of Harrisburg, LLC | | | 1 | | | | 19 | |
| Terra Herbal Health, LLC | | | 1 | | | | 13 | |
| Mapleglen Care Center, LLC | | | 1 | | | | 16 | |
| The Cannabis Group | | | 1 | | | | 44 | |
| Maribis of Chicago, LLC | | | 1 | | | | 43 | |
| Alternative Treatment, Ltd. | | | 1 | | | | 28 | |
| Union Group of Illinois, LLC | | | 1 | | | | 41 | |
| Chicago Alternative Health Center, LLC | | | 1 | | | | 43 | |
| Maribis of Springfield, LLC | | | 1 | | | | 9 | |
| MedMar Rockford, LLC | | | 1 | | | | 16 | |
Other Jurisdictions
The Company's growth plans include expansion to other U.S. states if and when legislation permits on a state-by-state basis as the Company determines that a suitable business opportunity exists. By developing product and brand designs that can be licensed to local manufacturers together with other support services, the Company believes it will be well-positioned to enter new U.S. states quickly, efficiently and to be among the first to capitalize on opportunities in the new markets in an accretive manner. To this end, the Company is currently reviewing the regulations of numerous U.S. states and has started a process of identifying suitable local partners in a number of other U.S. states. The Company is considering submitting License applications in other U.S. states which do not impose Residency Requirements.
Hemp-Infused Products Segment
Outlined below is a list of products which the Company considers to be competitive to the ones the Company is developing in its Hemp-Infused Products Segment.
Edible Products
| Product Name | Product Type | Location |
| Casa Luna | Chocolate | California |
| Iris | Chocolate | California |
| CanChew | Gum | New Jersey |
| Pharma CBD Honey | Honey | California |
| Pharma CBD Peanut Butter | Peanut Butter | California |
| IrieCBD Chocolate | Chocolate | California |
| IrieCBD Honey Sticks | Honey Sticks | California |
Gelatin Capsules
| Product Name | Product Type | Location |
| Dixie Botanicals: Cannabidiol Pills | Capsules | California |
| PlusCBD Oil Capsules | Capsules | California |
| Cibdex CBD Capsules | Capsules | California |
| Pharma CBD Capsules | Capsules | California |
Beverages
| Product Name | Product Type | Location |
| Canna Energy | Hemp Energy Soda | Colorado |
| Rocky Mountain High | Hemp Energy Soda/Iced Tea | Texas |
| Iris Whisper Water | Drink | California |
Intellectual Property
Currently, the Company's intellectual property includes recipes, branding, packaging and other know-how related to the marijuana and hemp infused products, all of which have not been registered.
The Company will protect its intellectual property with provisions in the applicable contracts and through the enforcement of copyright law and may commence trademark infringement actions in an event of a breach of its intellectual property rights.
Employees
As of April 12, 2016, the Company had no full-time employees and has utilized contract labor on as needed basis. None of the contractors are covered by collective bargaining agreements, and the Company considers its labor relations with to be in good standing.
The Company's management also has identified marijuana growers, marijuana concentrate extractors and suppliers in North America, which it can call on to fill the need for various expertise as such needs arise.
DESCRIPTION OF PROPERTIES
The Company owns the Pueblo Location, which is located at 78 N Silicon Dr #80, Pueblo West, CO 81007-1462, Pueblo County. The Pueblo Location is comprised of 3 main buildings covering an area of 26,525 square feet, while to total lot area is 131,250 square feet. The Pueblo Location is secured by the Senior Convertible Debenture and Subordinate Convertible Debenture. See "DESCRIPTION OF BUSINESS – Our Industry – State of Colorado".
The Company owns the Lawrenceville Property, which is located at 2415 State Street, Lawrenceville, IL 62439. The Lawrenceville Property contains three buildings, with the main building comprising an area of 8,271 square feet. The Company secured a Seller Take-Back Mortgage with the Vendor in the amount of USD $250,000. See "DESCRIPTION OF BUSINESS – Our Industry – State of Illinois".
The Company's executive head office is located at 77 King Street West, Suite 2905 P. O. Box 121, Toronto Ontario M5K 1H1.
MANAGEMENT
The following table sets forth the names and ages of all of our directors, executive officers and key employees.
| Name | Age | | Position | Director / Officer Since |
| David Posner | 42 | | President, Chief Executive Officer and Director | July 7, 2014 |
| Al Quong | 49 | | Chief Financial Officer | September 1, 2014 |
| Gary Margolin | 68 | | Chief Operating Officer | September 5, 2015 |
| Adam Szweras | 44 | | Secretary and Director | July 7, 2014 |
| Anne Marie Youhana | 34 | | VP Product Development and Quality Control | August 1, 2015 |
| Statis Rizas | 56 | | Chairman of the Board | July 7, 2014 |
David Caplan (1)(2) | 51 | | Director | July 7, 2014 |
Brian Presement (1)(2)(3) | 46 | | Director | October 10, 2013 |
Billy Morrison (2)(3) | 38 | | Director | June 11, 2015 |
| | (1) | Member of the Audit Committee of the Board. |
| | (2) | Member of the Compensation and Nomination Committee of the Board. |
| | (3) | Member of the Corporate Governance Committee of the Board. |
Business Experience
The following summarizes the occupation and business experience during the past five (5) years for our officers, directors and key employees as of the date of this prospectus:
David Posner, President, Chief Executive Officer and Director
David Posner was appointed Chief Executive Officer of the Corporation in April 2014. Between 2012 and April 2014, Mr. Posner served as an Acquisitions Manager for Stonegate Properties Inc. where he managed real estate properties and brokered deals in Canada and Oklahoma. He was a Managing Director of Sales and Acquisitions for Maria Chiquita Development Company from 2005 to 2012. From 2004 to 2007 he was a partner in a private investment group involved in the acquisition, re-zoning and re-positioning for sale of land holdings in Costa Rica and Panama. Mr. Posner holds a Bachelor of Arts degree from York University.
Al Quong, Chief Financial Officer
Al Quong has been the Chief Financial Officer for Branson Corporate Services since September 1, 2014 and has over 25 years of finance experience in various capacities and industries. Mr. Quong provides overall financial leadership for Branson and also serves as Chief Financial Officer for a number of Branson clients. Prior to joining Branson, Mr. Quong was Chief Financial Officer for Integris Real Estate Counsellors and the Fovere Group of Companies, from April 2011, and of Unilock Ltd. From April 2005 to April 2011. Renewable energy has been Mr. Quong's major initiative over the past three years, managing Fovere's private equity investment to become the largest owner of microFIT sites in Ontario. Mr. Quong served as a CFO of Norrock Realty Finance Corporation from May 2011 to October 2011. He is a Chartered Accountant, and Certified Public Accountant (Illinois), and holds a Bachelor of Commerce degree in Accounting from the University of Saskatchewan, and a Graduate Diploma in Forensic & Investigative Accounting from the University of Toronto, Mississauga.
Gary Margolin, Chief Operating Officer
On September 5, 2015, the Company appointed Gary Margolin as Chief Operating Officer. As COO, Mr. Margolin is responsible for the Company's transition to the operational phase of its strategic plan, including the development and launch of the Company's hemp oil infused line of products, and bringing completion of construction and commencement of operations to the Company's Pueblo, Colorado and Lawrenceville, Illinois facilities.
Mr. Margolin previously served as a key advisor to the CEO and a member of the Chief Operating Committee of Cablevision Systems Corporation, driving revenue growth from $1.4 billion to $4 billion and market capitalization from $1 billion to over $14 billion. Before joining Cablevision, Mr. Margolin was an attorney focusing on corporate and employment law, including corporate finance (venture capital, private placement, and public finance), M&A, labor relations (union and non-union) and governmental compliance. His clients included small private companies, start-up companies, large NYSE-listed companies, venture capital firms and an agency of the United States Government. Since leaving Cablevision, Mr. Margolin has consulted to executives at companies ranging in size from startup to large NYSE-listed companies and has developed a similarly strong track record of driving growth.
Adam Szweras, Secretary and Director
Mr. Szweras has practiced corporate and securities law since 1996 and is a partner in the Securities Law Group of Fogler, Rubinoff LLP. In January 2006, Mr. Szweras founded Foundation Markets Inc. and FMI Capital Advisory Inc., a Toronto brokerage firm and merchant bank respectively, where he continues on in the role of Chairman. In Mr. Szweras' law and banking practices, he focuses on working with emerging growth companies to develop and execute on their business plans, make acquisitions and to finance and list. Mr. Szweras works with companies in the marijuana and hemp sectors, mineral exploration, oil and gas, technology and the alternative energy sector. Mr. Szweras is a board member or Corporate Secretary of several publicly listed companies where he plays a role as a key advisor and stakeholder. He has written numerous articles and papers, and is a co-author of "Doing Business In Vietnam," and past editor of the Canadian Bar Association International Law newsletter and journal.
Anne Marie Youhana, VP Product Development and Quality Control
Anne Marie Youhana was appointed VP of Product Development and Quality Control on August 1, 2015. Anne Marie was introduced to the Company by Billy Morrison, director of the Company with whom she co-managed The Union Collective in California, which was one of the most successful cannabis collectives in the United States. She was responsible for introducing a variety of edible recipes (including cupcakes, cookies, loaves, cakes, candies, superfood granola, and chews) and developed proprietary recipes for cannabis butter and cookies using various infusion and preparation techniques. Anne Marie was also instrumental in developing the facilities for Capstone Analytical where she procured the lab location, ordered and set up all the lab equipment, assisted with the creation of testing standards, as well as developing home testing kits.
Statis Rizas, Chairman of the Board
Statis Rizas served as the President of Spindrift Telecom Inc. since 1992. Since 1991, he has been a successful entrepreneur and has been involved with numerous business activities, most of which have dealt with telephony. He has been Director of Capricorn Business Acquisitions Inc. since May 2008. Mr. Rizas holds a Bachelor of Arts degree from Dartmouth College and an MBA from the University of Chicago.
David Caplan, Director
David Caplan is a former Ontario politician. Mr. Caplan served as a Minister of Infrastructure during the 2003 session and as a Minister of Health and Long-Term Care during the 2007 session. He was a member of the Legislative Assembly of Ontario, and was a cabinet minister in the government of Liberal Premier Dalton McGuinty. Since retiring from the Ontario legislature on October 6, 2011 he served as a President and CEO of David Caplan Solutions Inc. a private consulting firm. Mr. Caplan served as a member on board of directors of Yee Hong Foundation since 2012, Baycrest Foundation since 2013, Epilepsy Toronto since 2010, Ontario Liberal Fund from 2007 to 2011 and as a Chair of Fundraising for Ontario Cabinet of Ministers from 2007 to 2009. He has also served as a Vice-Chairman of Global Public Affairs, privately-owned, fully-integrated public affairs firm since 2012. Mr. Caplan was born in Toronto, Ontario, and was educated at the University of Western Ontario. He worked as a commercial real estate agent with the firm of Ernest Goodman Ltd. from 1985 to 1989, and was Vice-President of Taurus Metal Trading Ltd. (a recycling company) between 1989 and 1992. Caplan was elected as a trustee to the North York Board of Education in 1991 and served in this capacity for six years, becoming the Board's Vice-Chair in 1993. He also served on the Metro Toronto Board of Education from 1994 to 1997, becoming its Vice-Chair shortly before his departure for higher office.
Brian Presement, Director
Brian Presement has been the President and CEO of Unite Communications Corporation ("UNiTE") since its inception in 2001. Under his leadership, UNiTE has grown from a regional telecom provider offering a narrow set of services to a full scale telecom provider offering services to companies of all sizes all across Canada. Mr. Presement has over 20 years of telecommunications experience. Prior to UNiTE, Mr. Presement served as Vice President Business Development of VOXX Corporation, where he was responsible for the sales and marketing of Voxx's Telecommunications Services. Mr. Presement served as a director of Aurelio Resource Corp. from Feb 2012 to Aug 2013 and a director of Sagittarius Capital Corp. since Jan 2013. From 2004 to 2007 Mr. Presement served as a General Manager of a Spam Filtering Service Called Mailgate Corp. Mr. Presement holds an Honours Bachelor of Arts Degree from York University with a double major in Mass Communications and Political Science.
Billy Morrison, Director
Mr. Morrison started his career in the cannabis sector by co-founding The Union Collective in California, which quickly became a successful medical cannabis collective in the county. He also founded Capstone Analytical LLC, which was one of the first thin layer chromatography cannabis testing facilities in the Bay Area, where he developed proprietary software for testing cannabinoid potency. In 2011, Mr. Morrison was appointed as a Chief Technology Officer of Temez Extracts/Pure Vape, where he devised closed loop extraction methods that analyzed analytical grade N-Butane and further leveraged sub-critical, refined CO2 extraction. At Pure Vape he also spearheaded a partnership with Dragon Vape to become the second largest producer of refined cannabis extracts in prefilled e-cigarettes in California. Mr. Morrison's most recent appointment is a Chief Technology Officer of Peloton Pharmaceuticals, a Canadian MMPR applicant, where he was responsible for designing, developing and deploying nearly autonomous grow system focused on producing pharmaceutical grade cannabis. Mr. Morrison holds an MBA in International Business from the University of the Incarnate Word in San Antonio, Texas and a Bachelor degree in Business Administration from the University of Texas, also in San Antonio.
Advisory Board
The Company has also formed an advisory board (the "Advisory Board") to provide expertise and advice to the senior management team regarding operational matters relating to the execution of the Company's business plan. The advisory board is comprised of Frank Galati and Debra Zwiefelhofer. The Board expects to review the structure and composition of the Advisory Board with the intention of adding additional members in the near future and as the Company grows and its business objectives and milestones evolve.
Mr. Galati has been involved in the food service and health supplement industries as a consultant and senior executive for over 20 years. He is currently Managing Partner of the Resource Practice at Bedford Consulting Group Inc., a position he has held since January 2008. Between June 2006 and January 2008, he was the President and Chief Financial Officer at Wellnx Life Sciences Inc., a consumer packaged goods company devoted to the discovery, development and marketing of weight-loss & health care supplements. From May 1997 to June 2006, Mr. Galati was President and Chief Executive Officer at Destination Products International Ltd., a company involved in providing retail branding and food development solutions to the U.S. grocery and mass merchandising sector. Mr. Galati holds an MBA from the University of Windsor, a Bachelor of Arts, Economics from the University of Toronto and a CMA designation form the Society of Management Accountants.
Ms. Zwiefelholer is a registered and licensed dietician and nutritionist with over 30 years of experience in clinical nutrition care, food service management and medical marketing. As a consultant for various clients, she markets nutrition related products to healthcare professionals with a specialty in weight management, dysphagia, and geriatrics. Ms. Zwiefelhofer previously spent 17 years with Nestlé HealthCare Nutrition (formerly Novartis Nutrition) where she held a number of marketing positions in a managerial capacity, including Interim Director of Marketing where she utilized her clinical experience and knowledge of industry in building the foundation for a successful new company by directing activities of four marketing managers, collectively responsible for over 30% of total sales volume. Ms. Zwiefelhofer belongs to numerous associations relating to nutrition and was responsible for several industry publications.
Family Relationships
Anne Marie Youhana is Billy Morrison's sister-in-law. There are no other family relationships among any of our directors or executive officers.
Term of Office
Each director of our company is to serve for a term of one year ending on the date of the subsequent annual meeting of stockholders following the annual meeting at which such director was elected. Notwithstanding the foregoing, each director is to serve until his successor is elected and qualified or until his death, resignation or removal. Our board of directors is to elect our officers and each officer is to serve until his successor is elected and qualified or until his or her death, resignation or removal.
Corporate Governance
Board of Directors
The Board currently consists of six directors. The Board has concluded that Brian Presement, Billy Morrison and David Caplan are "independent" for purposes of Board membership, as defined in National Instrument 58-101 Disclosure of Corporate Governance Practices. By virtue of their management positions or their status as promoter of the Corporation, each of David Posner, Statis Rizas and Adam K. Szweras are not considered to be "independent".
A member of the Board is considered to be independent if the member has no direct or indirect material relationship with the issuer. A material relationship means a relationship which could, in the view of the reporting issuer's Board, reasonably interfere with the exercise of a member's independent judgment.
Orientation and Continuing Education
The Board is comprised of individuals with either prior experience as a director or publicly listed issuer or a private entity or with significant business experience as a senior business manager. While the Corporation currently has no formal orientation and education program for new Board members, sufficient information (such as annual reports, prospectuses, proxy solicitation materials, budgets and operations reports) is provided to new Board members to ensure that each new director is familiar with the business of the Corporation and the functions of the Board. In addition, new directors are encouraged to meet with senior management.
Ethical Business Conduct
Ethical business conduct and behaviour is of great importance to the Board and management of the Corporation. The Corporate Governance Committee and the Board have discussed the adoption of a written code of conduct but as yet have not adopted a written code. The Corporation does expect that each of the directors, officers and employees conduct themselves ethically and within the confines of professional behaviour, including the avoidance of conflicts of interest, protection and proper use of Corporation information, compliance with laws, rules and regulations and reporting of illegal or unethical behaviour.
Any director or officer of the Corporation shall disclose in writing or request to have it entered into the minutes of Board's meeting or any of the committees of the directors the nature and extent of any interest in a material contract or a material transaction, whether made or proposed, as soon as the director or officer becomes aware of such a contract or transaction. In such a case, the director shall abstain from voting on any resolution to approve such a contract or transaction.
Audit Committee
The Audit Committee of the Corporation is responsible for the Corporation's financial reporting process and the quality of its financial reporting. The Audit Committee is charged with the mandate of providing independent review and oversight of the Company's financial reporting process, the system of internal control and management of financial risks, and the audit process, including the selection, oversight and compensation of the Company's external auditors. In performing its duties, the Audit Committee maintains effective working relationships with the Board, management, and the external auditors and monitors the independence of those auditors.
Composition of the Audit Committee
The Board members of the Corporation's Audit Committee are:
| Name | Independent(1) | Financially Literate(2) |
| Brian Presement | Yes | Yes |
| David Caplan | Yes | Yes |
Notes:
| | (1) | A member of the Audit Committee is independent if the member has no direct or indirect material relationship with the Corporation, which could, in the view of the Board, reasonably interfere with the exercise of a member's independent judgment. |
| | (2) | An individual is financially literate if he has the ability to read and understand a set of financial statements that present a breadth of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by the Corporation's financial statements. |
Audit Committee Oversight
Since the commencement of the Corporation's most recently completed financial year, there has not been a recommendation of the Audit Committee to nominate or compensate an external auditor with was not adopted by the Board.
Pre-Approval Policies and Procedures
In the event that the Corporation wishes to retain the services of the Corporation's external auditors for any non-audit services, prior approval of the Audit Committee must be obtained.
Nomination of Directors
The Board is entrusted with reviewing on a periodic basis the composition of the Board and, when appropriate, with maintaining a list of potential candidates for Board membership and interviewing potential candidates for Board membership.
Compensation
At present, no compensation other than the grant of options is paid to the Corporation's directors, in such capacity. For a description of the process by which the Board determines compensation for the Corporation's officers and directors, see "Compensation Discussion and Analysis" under Executive Compensation below.
Other Board Committees
Other than the Audit Committee, the Corporation's Board has a Compensation and Nominating Committee and a Corporate Governance Committee.
The Compensation and Nominating Committee's responsibility is to formulate and make recommendations to the directors of the Corporation in respect of compensation issues relating to directors and officers of the Corporation. The Compensation and Nominating Committee is comprised of Brian Presement (Chair), David Caplan and Billy Morrison, all of whom are independent. See "Executive Compensation – Compensation Governance".
The Corporate Governance Committee's responsibility is to assist the Board in fulfilling its oversight responsibilities in the following principal areas: (i) developing a set of corporate governance rules; (ii) reviewing and recommending the compensation of the Corporation's directors; (iii) facilitating the evaluation of the Board and committees of the Board. The Corporate Governance Committee is comprised of Billy Morrison and Brian Presement.
Assessments
The Board does not formally review the contribution and effectiveness of the Board, its members or committees. The Board believes that its size facilitates an informal review process through discussion and evaluation between the Chairman of the Board, the CEO and the Chair of the Corporate Governance Committee.
Involvement in Certain Legal Proceedings
None of our directors or executive officers have been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, or has been a party to any judicial or administrative proceeding during the past ten years that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in "Certain Relationships and Related Transactions", none of our directors, director nominees or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
This section sets out the objectives of the Corporation's executive compensation arrangements, the Corporation's executive compensation philosophy and the application of this philosophy to the Corporation's executive compensation arrangements. It also provides an analysis of the compensation design, and the decisions that the Board made in fiscal 2014 with respect to the Named Executive Officers. When determining the compensation arrangements for the Named Executive Officers, the Compensation and Nominating Committee considers the objectives of: (i) retaining an executive critical to the success of the Corporation and the enhancement of shareholder value; (ii) providing fair and competitive compensation; (iii) balancing the interests of management and Corporation's shareholders; and (iv) rewarding performance, both on an individual basis and with respect to the business in general. See the "Compensation Governance" below for more discussion on the Compensation and Nominating Committee.
Benchmarking
The Compensation and Nominating Committee considers a variety of factors when designing and establishing, reviewing and making recommendations for executive compensation arrangements for all executive officers of the Corporation. The Board typically does not position executive pay to reflect a single percentile within the industry for each executive. Rather, in determining the compensation level for each executive, the Compensation and Nominating Committee looks at factors such as the relative complexity of the executive's role within the organization, the executive's performance and potential for future advancement, the compensation paid by the other companies in medicinal and recreational marijuana industry, and pay equity considerations.
Elements of Compensation
The compensation paid to Named Executive Officers in any year consists of two primary components:
| | (a) | base salary; and |
| | (b) | long-term incentives in the form of stock options granted under the Option Plan. |
The key features of these two primary components of compensation are discussed below:
Base salary recognizes the value of an individual to the Corporation based on his or her role, skill, performance, contributions, leadership and potential. It is critical in attracting and retaining executive talent in the markets in which Corporation competes for talent. Base salaries for the Named Executive Officers are reviewed annually. Any change in base salary of a Named Executive Officer is generally determined by an assessment of such executive's performance, a consideration of competitive compensation levels in companies similar to the Corporation (in particular, companies in the marijuana industry) and a review of the performance of the Corporation as a whole and the role such executive officer played in such corporate performance.
The Corporation provides long-term incentives to Named Executive Officers in the form of stock options as part of its overall executive compensation strategy. The Compensation and Nominating Committee believes that stock option grants serve the Corporation's executive compensation philosophy in several ways: firstly, it helps attract, retain, and motivate talent; secondly, it aligns the interests of the Named Executive Officers with those of the shareholders by linking a specific portion of the officer's total pay opportunity to the share price; and finally, it provides long-term accountability for Named Executive Officers.
Risks Associated with Compensation Policies and Practices
The oversight and administration of the Corporation's executive compensation program requires the Compensation and Nominating Committee to consider risks associated with the Corporation's compensation policies and practices. Potential risks associated with compensation policies and compensation awards are considered at annual reviews and also throughout the year whenever it is deemed necessary by the Compensation and Nominating Committee.
The Corporation's executive compensation policies and practices are intended to align management incentives with the long-term interests of the Corporation and its shareholders. In each case, the Corporation seeks an appropriate balance of risk and reward. Practices that are designed to avoid inappropriate or excessive risks include (i) financial controls that provide limits and authorities in areas such as capital and operating expenditures to mitigate risk taking that could affect compensation, (ii) balancing base salary and variable compensation elements and (iii) spreading compensation across short and long-term programs.
Compensation Governance
The Compensation and Nominating Committee intends to conduct a yearly review of directors' compensation having regard to various reports on current trends in directors' compensation and compensation data for directors of reporting issuers of comparative size to the Corporation. Except for director's fees paid to the Chairman of the Board, director compensation is currently limited to the grant of stock options pursuant to the Option Plan. It is anticipated that the Chief Executive Officer will review the compensation of officers of the Corporation for the prior year and in comparison to industry standards via information disclosed publicly and obtained through copies of surveys. The Board expects that the Chief Executive Officer will make recommendations on compensation to the Compensation and Nominating Committee. The Compensation and Nominating Committee will review and make suggestions with respect to compensation proposals, and then makes a recommendation to the Board.
The Compensation and Nominating Committee is currently comprised of Brian Presement (Chair), David Caplan and Billy Morrison, all of whom are independent.
The Compensation and Nominating Committee's responsibility is to formulate and make recommendations to the directors of the Corporation in respect of compensation issues relating to directors and officers of the Corporation. Without limiting the generality of the foregoing, the Compensation and Nominating Committee has the following duties:
| | (a) | to review the compensation philosophy and remuneration policy for officers of the Corporation and to recommend to the directors of the Corporation changes to improve the Corporation's ability to recruit, retain and motivate officers; |
| | (b) | to review and recommend to the Board the retainer and fees, if any, to be paid to directors of the Corporation; |
| | (c) | to review and approve corporate goals and objectives relevant to the compensation of the CEO, evaluate the CEO's performance in light of those corporate goals and objectives, and determine (or make recommendations to the directors of the Corporation with respect to) the CEO's compensation level based on such evaluation; |
| | (d) | to recommend to the directors of the Corporation with respect to non-CEO officer and director compensation including reviewing management's recommendations for proposed stock options and other incentive-compensation plans and equity-based plans, if any, for non-CEO officer and director compensation and make recommendations in respect thereof to the directors of the Corporation; |
| | (e) | to administer the stock option plan approved by the directors of the Corporation in accordance with its terms including the recommendation to the directors of the Corporation of the grant of stock options in accordance with the terms thereof; and |
| | (f) | to determine and recommend for the approval of the directors of the Corporation bonuses to be paid to officers and employees of the Corporation and to establish targets or criteria for the payment of such bonuses, if appropriate. Pursuant to the mandate and terms of reference of the Compensation and Nominating Committee, meetings of the Committee are to take place at least once per year and at such other times as the Chair of the Compensation and Nominating Committee may determine. |
Summary Compensation Table
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to the named executive officers during the years ended July 31, 2015 and 2014.
Name and
Principal
Position | | Year | | Salary
($) | | | | | | Bonus
($) | | | Stock
Awards
($) | | | Option
Awards
($) | | | Non-Equity
Incentive
Plan
Compensation
($) | | | Nonqualified
Deferred
Compensation
Earnings
($) | | | All
Other
Compensation
($) | | | | | | Total
($) | |
| David Posner | | 2015 | | $ | 96,000 | | | | (1 | ) | | | - | | | | - | | | | 27,960 | | | | - | | | | - | | | | - | | | | | | $ | 123,960 | |
| President and Chief Executive Officer | | 2014 | | $ | 24,000 | | | | | | | | - | | | | - | | | | - | | | | - | | | | - | | | $ | 35,000 | | | | (2 | ) | | $ | 62,960 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Al Quong | | 2015 | | $ | 20,400 | | | | (3 | ) | | | - | | | | - | | | | 9,600 | | | | - | | | | - | | | | - | | | | | | | $ | 30,000 | |
| Chief Financial Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Melisa Parks | | 2015 | | $ | 22,000 | | | | | | | | - | | | | - | | | | 3,960 | | | | - | | | | - | | | | - | | | | | | | $ | 25,960 | |
| VP Product Development | | 2014 | | $ | 6,000 | | | | | | | | - | | | | - | | | | | | | | - | | | | - | | | | - | | | | | | | $ | 6,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Adam Szweras | | 2015 | | $ | - | | | | (4 | ) | | | - | | | | - | | | | 27,960 | | | | - | | | | - | | | | - | | | | | | | $ | 27,960, | |
| Secretary | | 2014 | | $ | - | | | | | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | $ | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Anne Marie Youhana | | 2015 | | $ | 2,500 | | | | | | | | - | | | | - | | | | 12,000 | | | | - | | | | - | | | | - | | | | | | | $ | 12,000 | |
| VP Product Development and Quality Control | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Marco Guidi | | 2015 | | $ | 1,000 | | | | (5 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | $ | 1,000 | |
| Chief Financial Officer | | 2014 | | $ | 1,000 | | | | | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | $ | 1,000 | |
| (1 | ) | As at July 31, 2015, $11,040 is included in accounts payable and accrued liabilities. | |
| (2 | ) | Mr. Posner was paid an initial advisory fee of $35,000 on May 1, 2014 pursuant to the Posner Agreement. | |
| (3 | ) | Mr. Quong is paid by Branson pursuant to the Branson Agreement. See "Executive Compensation – Branson Agreement." It is estimated that during fiscal 2015 Mr. Quong received approximately $1,700 per month from Branson for the services rendered in the CFO capacity of the Company. | |
| (4 | ) | Mr. Szweras, a director and corporate secretary of the Company; is an indirect 33.3% owner of FMI Capital Advisory Inc. ("FMICAI"), which receives fees from the Company in consideration for the corporate finance advisory services and executive management of Adam Szweras. Please see FMICAI Agreement "Certain Relationships and Related Transactions". Mr. Szweras does not receive regular fees from FMI. | |
| (5 | ) | Mr. Guidi resigned September 1, 2014. He was paid by Branson pursuant to the Branson Agreement. See "Executive Compensation – Branson Agreement." It is estimated that fiscal 2015 Mr, Guidi received approximately $1,000 per month from Branson for the services rendered in the CFO capacity of the Company. | |
Employment Agreements
There are currently no written contracts or agreements that provide for payment to a named executive officer at, following or in connection with any termination (whether voluntary, involuntary or constructive), resignation, retirement, a change in control of the Corporation or a change in a named executive officer's responsibilities.
NHL, an operating subsidiary of the Corporation has entered into the following consulting agreements with the following executive officers of the Corporation.
Posner Agreement
On May 1, 2014, NHL entered into consulting agreement with David Posner, President (the "Posner Agreement"). Pursuant to the Posner Agreement, David Posner has agreed to perform the services of the Chief Executive Officer of NHL and its affiliates (including the Corporation). Mr. Posner was paid an initial advisory fee of $35,000 and a base fee of $8,000 per month, subject to annual review by the Board.
Branson Agreement
On September 1, 2014, Al Quong was appointed the Chief Financial Officer of the Corporation, designated consultant to provide the services of Chief Financial Officer to the Corporation. On May 1, 2014, the Corporation entered into an agreement with Branson Corporate Services Inc. ("Branson") to provide a Chief Financial Officer, controllership and bookkeeping services, administrative services and general and back office services for a monthly fee of $3,000. Al Quong is employed by Branson and is compensated by Branson. On October 27, 2014, the Corporation amended the Branson agreement, by including a provision that the Corporation will pay Branson an additional fee of $30,000 plus applicable taxes upon completion of the initial public offering. On March 23, 2015 the Corporation entered into an amending agreement to pay Branson a monthly fee of $6,000 to provide a Chief Financial Officer, Corporate Secretarial services, controllership and bookkeeping services, administrative services, general and back office services.
Stock Option Plan and Stock Options
The Board adopted the rolling stock option plan (the "Option Plan") on July 7, 2014. A "rolling" stock option plan is one under which options may be granted equal in number to up to 10% of the issued capital of the Corporation at the time of the grant of the stock option. "Rolling" stock option plans are required to be approved by the shareholders at each annual general meeting on a yearly basis. The purpose of the Option Plan is to provide an incentive to the Corporation's directors, senior officers, employees and consultants to continue their involvement with the Corporation and to increase their efforts on the Corporation's behalf.
The Option Plan is administered by the Board or, if applicable, by the Compensation Committee (the "Compensation Committee").
As of the date of this prospectus, the Company has 141,942,514 Common Shares issued and outstanding. This means that a total of 14,194,251 Options are currently available to be granted pursuant to the Option Plan. As of the date hereof, 11,300,000 Options had been granted pursuant to the Option Plan and 2,894,251 Options were still available to be granted.
Option Plan Terms
The Option Plan authorizes the Board to grant stock options to the officers, directors, employees and consultants of the Corporation on the following terms:
| 1. | The number of shares subject to each option is determined by the Board provided that the Option Plan, together with all other previously established or proposed share compensation arrangements may not, during any 12 month period, result in: |
| | (a) | the number of shares reserved for issuance pursuant to stock options granted to any one person exceeding 5% of the issued shares of the Corporation; |
| | (b) | the issuance, within a one year period, to insiders of the Corporation of a number of shares exceeding 10%, or to one insider of a number exceeding 5%, or to a consultant of a number exceeding 2%; or to employees or consultants (as defined by the Exchange) who provides Investor Relations services of a number exceeding 2% (in the aggregate for all such employees or consultants) of the issued shares of the Corporation. |
| 2. | The aggregate number of shares which may be issued pursuant to options granted under the Option Plan may not exceed 10% of the issued and outstanding shares of the Corporation as at the date of the grant. |
| 3. | The exercise price of an option may not be set at less than the discounted market price (as provided in the Exchange regulations) for the trading day immediately preceding the date of grant of the option. |
| 4. | The options may be exercisable for a period of up to five years. |
| 5. | The options are non-assignable, except in certain circumstances. The options can only be exercised by the optionee as long as the optionee remains an eligible optionee pursuant to the Option Plan or within a period of not more than 90 days (30 days for providers of investor relations services) after ceasing to be an eligible optionee or, if the optionee dies, within one year from the date of the optionee's death. |
| 6. | On the occurrence of a takeover bid, issuer bid or going private transaction, the Board will have the right to accelerate the date on which any option becomes exercisable. |
Outstanding Option-Based Awards for Named Executive Officers
The table below reflects all option-based awards for each Named Executive Officer outstanding as at July 31, 2015. The Corporation does not have any other equity incentive plans other than its Option Plan. As of the date hereof there are no share based award plans for any of the directors or the named executive officer's of the Corporation,
| OPTION-BASED AWARDS OUTSTANDING AS AT END OF FISCAL YEAR |
| Named Executive Officer | | Number of Securities Underlying Unexercised Options | | | Option Exercise Price
($/Security) | | Option Expiration Date | Value of Unexercised In-the-Money Options(1)
($) |
David Posner President and Chief Executive Officer(3) | | | | | | $ | | | July 7, 2019 March 18, 2020 | |
Al Quong Chief Financial Officer | | | 300,000 | | | $ | 0.10 | | March 18, 2020 | Nil |
Adam Szweras Corporate Secretary | | | | | | $ | | | July 7, 2019 March 18, 2020 | |
Anne Marie Youhana VP Product Development and Quality Control | | | 250,000 | | | $ | 0.10 | | July 22,2020 | Nil |
| (1) | This column contains the aggregate value of in-the-money unexercised options as at July 31, 2015, calculated based on the difference between the market price of the Common Shares underlying the options, being $0.055. |
Incentive Award Plans
The following table provides information concerning the incentive award plans of the Corporation with respect to each Named Executive Officer during the fiscal year ended July 31, 2015. The only incentive award plan of the Corporation during such fiscal years was the Stock Option Plan.
| Named Executive Officer | Option-Based Awards – Value Vested During Year Ended July 31, 2015 | Non-Equity Incentive Plan – Value Vested During Year Ended July 31, 2015 |
| David Posner | Nil | Nil |
| Al Quong | Nil | Nil |
| Adam Szweras | Nil | Nil |
| Anne Marie Youhana | Nil | Nil |
Director Compensation for Fiscal 2015
The Compensation and Nominating Committee makes recommendations to the Board as to the appropriate level of remuneration for the directors and officers of the Corporation. The Board as a whole makes the final determination in respect of compensation matters. Remuneration is assessed and determined by taking into account such factors as the size of the Corporation and the level of compensation earned by directors and officers of companies of comparable size and industry.
Other than with respect to director fees paid to the Chairman of the Board, the only arrangements the Corporation has, standard or otherwise, pursuant to which directors are compensated by the Corporation for their services in their capacity as directors, or for committee participation, involvement in special assignments or for services as consultants or experts for the financial year ended July 31, 2015, are through the issuance of stock options. The number of options to be granted from time to time is determined by the Board in its discretion.
The table below summarizes the compensation earned by our non-employee directors for the fiscal year ended July 31, 2015.
| Name | | Fees
earned
or paid
in cash
($) | | | Stock
Awards
($) | | | | | | Option
Awards
($) | | | Non-equity
incentive plan
compensation
($) | | | Change in
pension value
and
nonqualified
deferred
compensation
earnings
($) | | | All Other
Compensation
($) | | | Total
($) | |
| Statis Rizas | | | - | | | | - | | | | | | $ | 8,012 | | | | - | | | | - | | | | - | | | $ | 8,012 | |
Michael Pesner(4) | | | - | | | | - | | | | | | $ | 4,000 | | | | - | | | | - | | | | - | | | $ | 4,000 | |
| David Caplan | | | - | | | | - | | | | | | $ | - | | | | - | | | | - | | | | - | | | $ | - | |
| Brian Presement | | | - | | | | - | | | | | | $ | - | | | | - | | | | - | | | | - | | | $ | - | |
| Billy Morrison | | | - | | | $ | 22,500 | | | | (1 | ) | | $ | 31,000 | | | | - | | | | - | | | | - | | | $ | 53,500 | |
Michael Dacks(2) | | | - | | | | - | | | | | | | $ | - | | | | - | | | | - | | | | - | | | $ | - | |
| | (1) | During the year ended July 31, 2015, the Company issued 300,000 Common Shares to Billy Morrison as compensation for services valued at $22,500. |
| | (2) | Resigned on June 11, 2015. |
| | (3) | The relevant disclosure for Mr. Szweras and Mr. Posner is provided in the Summary Compensation Table for NEO's above. |
| | (4) | Resigned on January 27, 2016. |
Director Outstanding Option-Based Awards
The table below reflects all option-based awards for each director of the Corporation outstanding as at July 31, 2015 (including option-based awards granted to a director before each such fiscal year). The Corporation does not have any equity incentive plan other than the Option Plan.
| DIRECTOR OPTION–BASED AWARDS OUTSTANDING AS AT JULY 31, 2015 |
|
| Name of Director | | Number of Securities Underlying Unexercised Options | | | Option Exercise Price ($/Security) | | Option Expiration Date | Value of Unexercised In-the-Money Options(1) |
| Statis Rizas | | | 400,000 | | | | | | $ | 0.10 | | July 7, 2019 | Nil |
| | | | 250,000 | | | | | | $ | 0.10 | | March 18, 2020 | Nil |
| Adam K. Szweras | | | | | | | | | | $ $ | | | July 7, 2019 March 18, 2020 | |
Michael Pesner(3) | | | 400,000 | | | | | | | $ | 0.10 | | October 14, 2019 | Nil |
| David Posner | | | | | | | | | | $ $ | | | July 7, 2019 March 18, 2020 | |
| David Caplan | | | 400,000 | | | | | | | $ | 0.10 | | July 7, 2019 | Nil |
| Brian Presement | | | 400,000 | | | | | | | $ | 0.10 | | July 7, 2019 | Nil |
| Billy Morrison | | | 400,000 | | | | | | | $ | 0.10 | | June 10, 2020 | Nil |
Michael Dacks(2) | | | 400,000 | | | | | | | $ | 0.10 | | July 7, 2019 | Nil |
| | (1) | This column contains the aggregate value of in-the-money unexercised options as at July 31, 2015, calculated based on the difference between the market price of the Common Shares underlying the options, being $0.055. |
| | (2) | Resigned on June 11, 2015. |
| | (3) | Resigned on January 27, 2016. |
Director Incentive Award Plans
The following table provides information concerning the incentive award plans of the Corporation with respect to each director during the fiscal year ended July 31, 2015. The only incentive award plan of the Corporation during such fiscal year was its Stock Option Plan.
INCENTIVE AWARD PLANS –
VALUE VESTED OR EARNED DURING THE FISCAL YEAR ENDED JULY 31, 2015 |
|
| Name of Director | | Option-Based Awards – Value Vested During Fiscal Year Ended July 31, 2015(1) | | Non-Equity Incentive Plan Compensation – Value Vested During Fiscal Year Ended July 31, 2015 |
Statis Rizas(3) | | $ | 8,012 | | Nil |
Adam K. Szweras(3) | | $ | 24,035 | | Nil |
Michael Pesner(1)(5) | | $ | 5,000 | | Nil |
| David Caplan | | $ | - | | Nil |
David Posner(3) | | $ | 24,035 | | Nil |
| Brian Presement | | $ | - | | Nil |
Billy Morrison(2) | | $ | 31,000 | | Nil |
Michael Dacks(4) | | $ | - | | Nil |
| | (1) | Option-based awards are valued at the share price on the date of the option grant. The fair value of each option granted is estimated at the time of grant using the Black-Scholes option-pricing model with weighted average assumptions for grants as follows: a 5 year expected term, 100% volatility, risk-free interest rate of 1.56% per annum, a dividend rate of 0% and weighted average grant-date fair value of stock options of $0.0125. |
| | (2) | Option-based awards are valued at the share price on the date of the option grant. The fair value of each option granted is estimated at the time of grant using the Black-Scholes option-pricing model with weighted average assumptions for grants as follows: a 5 year expected term, 209% volatility, risk-free interest rate of 0.95% per annum, a dividend rate of 0% and weighted average grant-date fair value of stock options of $0.0775. |
| | (3) | Option-based awards are valued at the share price on the date of the option grant. The fair value of each option granted is estimated at the time of grant using the Black-Scholes option-pricing model with weighted average assumptions for grants as follows: a 5 year expected term, 100% volatility, risk-free interest rate of 0.75% per annum, a dividend rate of 0% and weighted average grant-date fair value of stock options of $0.032. |
| | (4) | Resigned on June 11, 2015. |
| | (5) | Resigned on January 27, 2016. |
Pension Benefits
We do not have any defined benefit pension plans or any other plans providing for retirement payments or benefits.
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information regarding the beneficial ownership of our common stock as of April, 2016 by (a) each stockholder who is known to us to own beneficially 5% or more of our outstanding common stock; (b) all directors; (c) our executive officers, and (d) all executive officers and directors as a group. Except as otherwise indicated, all persons listed below have (i) sole voting power and investment power with respect to their shares of common stock, except to the extent that authority is shared by spouses under applicable law, and (ii) record and beneficial ownership with respect to their shares of common stock.
For purposes of this table, a person or group of persons is deemed to have "beneficial ownership" of any shares of common stock that such person has the right to acquire within 60 days of April, 2016. For purposes of computing the percentage of outstanding shares of our common stock held by each person or group of persons named above, any shares of common stock that such person or persons has the right to acquire within 60 days of April, 2016 is deemed to be outstanding, but is not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares of common stock listed as beneficially owned does not constitute an admission of beneficial ownership. Unless otherwise identified, the address of our directors and officers is c/o Nutritional High International Inc., 77 King Street West, Suite 2905 P. O. Box 121, Toronto Ontario M5K 1H1.
Name and address of beneficial owner | | Shares owned before
Offering(1) | |
| | | Number | | | Percent | |
| Directors and Executive Officers: | | | | | | |
| David Posner | | | 6,000,000 | | | | 4.39 | % |
| Al Quong | | | 0 | | | | - | |
| Gary Margolin | | | 0 | | | | - | |
| Adam Szweras | | | 56,000 | | | | * | |
| Anne Marie Youhana | | | 161,111 | | | | * | |
Statis Rizas (2) | | | 15,300,000 | | | | 11.19 | % |
| David Caplan | | | 0 | | | | - | |
| Brian Presement | | | 0 | | | | - | |
| Billy Morrison | | | 300,000 | | | | * | |
| All current directors and officers as a group (9 people) | | | 26,250,111 | | | | 19.19 | % |
| | | | | | | | | |
| Promoter: | | | | | | | | |
FMI Capital Advisory Inc. (3) | | | 4,433,000 | | | | 3.24 | % |
| | | | | | | | | |
| Other 5% Shareholders: | | | | | | | | |
| None. | | | | | | | | |
| | * (1) | Less than 1% Based on 141,942,514 shares outstanding as of April 12, 2016. |
| | (2) | The shares were held indirectly through Halki Holdings Inc., which is controlled by Statis Rizas. |
| | (3) | FMI Capital Advisory Inc. is a promoter of the Company and is 33.3% indirectly owned by The Goomie Trust, a family trust, of which the children of Mr. Adam Szweras are the beneficiaries. |
As of April 12, 2016, there were approximately 161 holders of record of the Common Shares. A total of 25 registered holders are from the United States, who hold 18,667,222 common shares, which constitutes approximately 13.2% of our issued and outstanding common stock as of April 12, 2016, prior to giving effect to this offering.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
NHL and FMICAI entered into an advisory and consulting agreement on May 1, 2014, FMICAI is a subsidiary of Foundation Financial Holdings Corp. ("FFHC"). FFHC is an entity in which an officer is a director of the Company. In consideration for services, NHL agreed to pay an initial advisory fee of $35,000 and a monthly fee of $8,000 commencing on May 1, 2014. An amendment to the agreement was entered into on October 27, 2014, to include a success fee of $70,000, payable upon successful completion of the IPO, half of which was paid in Units and half of the success fee in cash. A further amendment to the agreement was entered into on September 11, 2015 to increase the monthly fee to $16,000 effective January 1, 2015, for additional services to be rendered including ongoing executive management services, identifying qualifying real estate, assistance with licensing applications, implementing the business plan, seeking out state licensed providers, and assist in build-out of Pueblo facility. For the six month period ended January 31, 2016, NHL was charged $96,000 by FMICAI. At January 31, 2016, $164,930 is included in accounts payable and accrued liabilities in relation to FMICAI.
NHL and Branson entered into a management services agreement on May 1, 2014. The management services agreement includes the provision of services of the Company's Chief Financial Officer. Branson is an entity in which FFHC is a 49.0% shareholder. In consideration for services the Company agreed to pay $3,000 per month. An amendment to the agreement was entered into on October 1, 2014 to increase the fee to $5,000 per month. A further amendment to the agreement was entered into on October 27, 2014, to include a success fee of $30,000, which was paid upon the successful completion of the IPO. A further amendment to the agreement was entered into on March 1, 2015 to increase the fee to $6,000 per month. For the six month period ended January 31, 2016, the Company paid $36,000 for management services provided by Branson. As at January 31, 2016, $36,934 is included in accounts payable and accrued liabilities in relation to Branson.
For the six month period ended January 31, 2016, Fogler, Rubinoff LLP ("Fogler") a law firm in which an officer and director of the Company is also a partner, provided $16,089 of legal services, which are included in professional fees. As at January 31, 2016, $34,348 due to Fogler is included in accounts payable and accrued liabilities.
For the year ended July 31, 2015, the Company incurred a finder's fee of $4,000 and issued 160,000 finder's warrants to Foundation Markets Inc., a company with a related director. This was in connection with the October 8, 2014 closing of the 4,000,000 unit private placement.
On May 1, 2014, NHL entered into a general consulting agreement with Statis Rizas, a significant, but not controlling, shareholder. Mr. Rizas provided $15,000 of consulting services during the three month period ended October 31, 2014. The agreement was terminated on October 31, 2014, and Mr. Rizas was paid a termination fee of $10,000. As at July 31, 2015, $2,000 is included in accounts payable and accrued liabilities.
On October 31, 2014, the Company also issued 800,000 Common Shares and 800,000 warrants to Halki Holdings Inc., a company controlled by Mr. Rizas, valued at $40,000 and $2,691 respectively, in exchange for cancellation of debt in such amount owing to Mr. Rizas.
On January 27, 2015, Statis Rizas also advanced the Company a short-term, non-interest bearing loan for $15,000. This loan was repaid on March 17, 2015.
Total key management compensation paid to the Chief Executive Officer, the Chief Operating Officer, the VP Product Development, and a Director amounted to $137,713 (2015 - $86,629) for the six month period ended January 31, 2016. These expenses have been measured at their exchange amount, being the amounts negotiated and agreed to by the parties to the transactions. As at January 31, 2016, $337,704 (July 31, 2015 - $85,463) is included in accounts payable and accrued liabilities.
The Company has also issued a Subordinated Convertible Debenture in the principal amount of $150,000 to a group of lenders comprised of Adam Szweras, Statis Rizas and David Posner, all of whom are directors of the Company. During the year ended July 31, 2015, interest of $12,674 has been accrued on the Subordinated Debentures. On February 29, 2016 and on March 2, 2016, each of David Posner, CEO and Director, and 1306413 Ontario Limited, respectively, advanced CAD $15,000 (a total of $30,000), which was added to the Subordinated Convertible Debenture. As of the date of this prospectus the total amount of Subordinated Convertible Debentures is CAD $180,000.
The Company does not have a written policy with respect to related party transactions. However, all such transactions are reviewed and approved by the Board of Directors prior to finalization.
DESCRIPTION OF SECURITIES
Authorized Capital Stock
We are authorized to issue an unlimited number of common shares without par value. As of April 12, 2016, 141,942,514 shares of our common stock were issued and outstanding.
Common Stock
All outstanding shares of common stock are of the same class and have equal rights and attributes. The holders of common stock are entitled to one vote per share on all matters submitted to a vote of shareholders of the Company. All shareholders are entitled to share equally in dividends, if any, as may be declared from time to time by the board of directors out of funds legally available. In the event of liquidation, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities. The shareholders do not have cumulative or preemptive rights.
Warrants
As of April 12, 2016, the Company has outstanding warrants to purchase 27,926,638 shares of common stock. Each of the Company's warrants outstanding entitles the holder to purchase one share of the Company's common stock for each warrant share held. The exercise price of the warrants are subject to adjustment upon certain events, such as stock splits, combinations, dividends, distributions, reclassifications and mergers or other corporate change. A summary of the Company's warrants outstanding and exercisable is as follows:
| · | 2,160,000 Series I Warrants - at $0.05 expiring April 8, 2016; |
In connection with the private placement on October 8, 2014, the Company issued warrants to purchase an aggregate of 2,000,000 Common Shares. Such warrants have an exercise price of $0.05 per shares and a term of 18 months.
On April 7, 2016, 160,000 compensation options exercised for $4,000 for 160,000 common shares and 80,000 share purchase warrants, exercisable at a price of $0.05 per share until April 8, 2016.
On March 22, 2016, 160,000 compensation options exercised for $4,000 for 160,000 common shares and 80,000 share purchase warrants, exercisable at a price of $0.05 per share until April 8, 2016.
| · | 150,000 Series II Warrants - at $0.07 expiring June 26, 2016; |
On June 27, 2014, the Company issued warrants to purchase 150,000 Common Shares in connection with the acquisition of NHL. Such warrants have an exercise price of $0.07 per shares and a term of 24 months.
| · | 3,566,638 Series III Warrants - at $0.07 expiring October 26, 2016; |
On October 8, 2014, the Company closed a subscription for 4,000,000 units at $0.025 per unit for gross proceeds of $100,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.05 per share until the earlier of 36 months from the date of issuance or 18 months following the date of a business combination between the Company and a public company pursuant to a reverse take-over, merger, amalgamation, take-over bid, insider bid, reorganization, joint venture, sale or exchange of assets or similar transaction or IPO. On October 31, 2014, 3,566,638 warrants were exercised at $0.05 per warrant for gross proceeds of $178,332. An additional $17,467 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share purchase warrants have been amended to include an early exercise provision of an additional warrant exercisable into one common share at a price of $0.10 per share until 24 months from the date of issuance. As a result of the amendment, an additional 3,566,638 warrants were issued. The terms of any unexercised Company warrants outstanding at October 31, 2014 remain unchanged.
| · | 2,100,000 Series IV Warrants - at $0.07 expiring June 2, 2018; |
On December 2, 2015, the Company completed a non-brokered private placement of 4,200,000 units at $0.05 per unit for gross proceeds of $210,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
| · | 15,794,000 Unit Warrants - at $0.07 expiring March 13, 2017; |
On March 13, 2015, the Company completed its initial public offering of units, which raised gross proceeds of $1,645,000 through its agent Jacob Securities Inc. Each unit was comprised of one Common Share and one-half of one Common Share purchase warrant. Each whole warrant entitles the holder there of to purchase a Common Share at a price of $0.07 per share until March 13, 2017. An aggregate of 32,900,000 units at $0.05 per unit were issued for gross proceeds of $1,645,000 in the offering. 1,306,000 warrants have been exercised and 15,794,000 warrants are still outstanding.
| · | 6,000 Warrants - at $0.05 expiring March 16, 2017; |
On April 12, 2016, 12,000 compensation options exercised for $600 for 12,000 common shares and 6,000 share purchase warrants, exercisable at a price of $0.05 per share until March 16, 2017.
| · | 3,750,000 Warrants - at $0.06 expiring December 21, 2017. |
On December 21, 2015, the Company has issued 3,750,000 warrants in lieu of amending the terms of the Senior Convertible Debenture, with each warrant exercisable into one common share at a price of $0.06 per share for a period of 18 months from the date of issuance.
| · | 400,000 Warrants - at $0.07 expiring June 2, 2018; |
On January 31, 2016, the Company completed a non-brokered private placement of 800,000 units at $0.05 per unit for gross proceeds of $40,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
In connection with the October 8, 2014 offering, the Company paid a finder's fee of $8,000 and issued an aggregate of 320,000 finder's warrants, exercisable into one common share at a price of $0.025 per share until 18 months from the closing date.
Options
| · | Options to acquire up to 9,700,000 Common Shares at $0.10 for five years from issuance; |
During the year ended July 31, 2015, the Company granted 8,400,000 stock options to certain officers, directors, consultants and advisory board members to purchase common shares of the Company at the exercise price of $0.10 exercisable until 60 months from the date of issuance.
Vesting periods on the options granted during the year ended July 31, 2015 are as follows: 7,050,000 stock options vested immediately upon issuance, 300,000 stock options issued on March 18, 2015, 50% of which vested immediately and the remaining 50% vest monthly over 6 months, 2,000,000 stock options issued on March 18, 2015, which vest in stages over a minimum of 12 months with no more than ¼ of options vesting any three month period, and the remaining vest quarterly over next 24 months.
| · | Options to acquire up to 1,600,000 Common Shares at $0.075 for five years from issuance; |
During the period ended October 31, 2015, the Company granted 1,100,000 stock options to an officer to purchase common shares of the Company at the exercise price of $0.075 exercisable until 60 months from the date of issuance, for a stock option expense of $4,000. A stock option expense of $15,000 was recorded in relation to the vesting of the options issued through July 31, 2015.
| · | 2,408,800 Compensation Options. |
On March 13, 2015, the Company issued 2,404,800 compensation options under a warrant indenture valued at $63,000 based on the services provided. Each option is exercisable at $0.05 per unit, and comprised of one common share and one half of one share purchase warrant, exercisable into one additional common share at a price of $0.07 per share until 24 months from the date of issuance.
On March 30, 2015, 12,000 compensation options under the warrant indenture were exercised for $600 for 12,000 common shares and 6,000 share purchase warrants, exercisable at a price of $0.07 per share until March 16, 2017.
In connection with the private placement on December 2, 2015, the Company completed has issued 16,000 compensation options. Each compensation option is exercisable at $0.05 per unit, and us comprised of one common share and one half of one share purchase warrant, exercisable into one additional common share at a price of $0.07 per share until 18 months from the date of issuance.
Convertible Notes
As of April 12, 2016, we had $280,000 aggregate principal amount of outstanding convertible notes, which at the current time are convertible into 4,666,667 shares of our common stock, with conversion prices and maturity dates as listed below. The conversion price of the convertible notes are subject to adjustment upon certain events, such as stock splits, combinations, dividends, distributions, reclassifications and mergers or other corporate change.
Senior Convertible Debenture
In connection with the purchase of the Pueblo Location, the Company issued to an arm's length party a senior secured convertible debenture (the "Senior Convertible Debenture") in the principal amount of $450,000. The Senior Convertible Debenture matures on November 17, 2016 (the "Maturity Date") and carries an interest rate of 12% per annum. The Senior Convertible Debenture is secured by a first ranking general security interest over all assets of the Company. The Senior Convertible Debenture is convertible into Common Shares at the option of lender at any time prior to the Maturity Date at a price of $0.06 per Common Share. As of the date of this prospectus, the Senior Convertible Debenture in the principal amount of $350,000 plus all accrued but unpaid interest have been converted into 5,833,333 Common Shares of the Company, and $100,000 of the Senior Convertible Debenture was outstanding.
Subordinate Convertible Debenture
The Company also issued a subordinated convertible Debenture ("Subordinated Convertible Debenture") in the principal amount of $180,000 to a group of lenders comprised of Adam Szweras, Statis Rizas and David Posner, all of whom are directors of the Company, in connection with the financing of the acquisition of the Pueblo Location. The Subordinated Convertible Debenture matures on the Maturity Date and carries an interest rate of 12% per annum. The Subordinated Convertible Debenture is secured by a general security interest over all assets of the Company, subordinate to the Senior Convertible Debenture. The Subordinate Convertible Debenture is convertible into Common Shares at the option of lender at any time prior to the Maturity Date at a price of $0.06 per Common Share. The $180,000 outstanding principal amount is convertible into 3,000,000 Common Shares of the Company.
Dividends
We currently do not anticipate paying any cash dividends in the foreseeable future on our common stock. Although we intend to retain our earnings, if any, to finance the exploration and growth of our business, our board of directors will have the discretion to declare and pay dividends in the future. Payment of dividends in the future will depend upon our earnings, capital requirements, and other factors, which our board of directors may deem relevant.
Transfer Agent and Registrar
The Company's transfer agent is CST Trust Company. Their address is 320 Bay St, Toronto, ON M5H 4A6, Canada and their telephone number is (416) 682-3800.
DESCRIPTION OF ARTICLES AND BY-LAWS
Remuneration of Directors
Our By-Laws provide that the board of directors may fix the remuneration of our directors. Any remuneration paid to a director may be in addition to salary paid to the director in his or her capacity as an officer or employee of our company. Our directors are also entitled to be reimbursed for travelling and other expenses they properly incur in attending meetings of the board or any committee of the board.
Number of Directors
Our Articles provide that the board shall consist of at least one member and a maximum of ten members.
Shareholder Rights
The holders of our common shares are entitled to notice of, to attend and to one vote per share held at any meeting of our shareholders.
The holders of our common shares are, at the discretion of the board of directors and subject to applicable legal restrictions, entitled to receive any dividends declared by the board of directors.
In the event of any liquidation, dissolution or winding-up of our company, the holders of our common shares are entitled to share in the remaining property of our company, subject to the rights of the holders of our preferred shares.
Changes to Shareholder Rights
In accordance with the Canadian Business Corporation Act ("CBCA"), the rights of our common shareholders may be changed by amending our Articles or, to the extent applicable, our By-Laws. An amendment to our Articles or By-Laws, as applicable, would require the approval of the holders of our common shares in accordance with the requirements of the CBCA.
Shareholder Meetings
Subject to the provisions of the CBCA, our annual meeting of shareholders will be held at the day, time and place as the board, the chairman of our board, the managing director or the president may from time to time determine. The CBCA requires that we hold an annual meeting of our shareholders each year, no later than 15 months after the date of our prior annual meeting.
Our board of directors, our chairman of the board, our managing director or our president may call a special meeting of shareholders at any time.
Under the CBCA, notice of a meeting of shareholders must be provided at least 21 days, but not more than 50 days, prior to the date on which the meeting is to be held. The CBCA permits a record date to be set for determining shareholders entitled to receive notice of or to vote at a shareholder meeting. If no record date is set, the record date for the determination of shareholders entitled to receive notice of a meeting of shareholders shall be (i) at the close of business on the last day preceding the day on which the notice is sent, or (ii) if no notice is sent, the day on which the meeting is held. Subject to the CBCA, a meeting of shareholders may be held without notice at any time and place if (a) all the shareholders entitled to vote are present in person or duly represented or if those absent waive notice or otherwise consent and (b) the auditor and directors are present or waive notice or otherwise consent to the meeting being held, subject to certain limitations.
Our By-Laws provide that only the following persons are entitled to be present at a shareholder meeting:
| | i. | those entitled to vote at the meeting; |
| | ii. | our directors and auditor; |
| | iii. | others who, although not entitled to vote, are entitled or required under the CBCA or the Articles or By-Laws to be present at the meeting; and |
| | iv. | any other person on the invitation of the chairman of the meeting or with the consent of the meeting. |
Votes at shareholder meetings may be given either personally or by proxy. Subject to the CBCA, every question at a shareholder meeting will be decided by a show of hands, unless a ballot is required or demanded by our chairman, a shareholder or a proxyholder entitled to vote at the meeting.
If our directors or our shareholders call a shareholder meeting, the directors or the shareholders, as the case may be, may determine that the meeting shall be held, in accordance with the CBCA, entirely by electronic means, telephone or other communication facility that permits all participants to communicate adequately with each other during the meeting.
A shareholder or shareholders present in person or represented by proxy, and together representing 5% of the outstanding shares of our company constitutes a quorum.
The chairman of the meeting may, with the consent of the meeting and subject to such conditions as the meeting may decide, adjourn the meeting. If a shareholder meeting is adjourned for less than 30 days, notice of the adjourned meeting is not necessary, other than by announcement at the time of adjournment.
A resolution signed by all of the shareholders entitled to vote on that resolution at a shareholder meeting is as valid as if it had been passed at a meeting of shareholders.
TAXATION
Canadian Income Tax Consequences
We consider that the following summary fairly describes the principal Canadian federal income tax consequences applicable to a holder of our common shares who at all material times deals at arm's length with our company, who holds all common shares as capital property, who is resident in the United States, who is not a resident of Canada and who does not use or hold, and is not deemed to use or hold, his common shares of our company in connection with carrying on a business in Canada (a "non-resident holder"). It is assumed that the common shares will at all material times be listed on a stock exchange that is prescribed for purposes of the Income Tax Act (Canada) (the "ITA") and regulations thereunder. Investors should be aware that the Canadian federal income tax consequences applicable to holders of our common shares will change if, for any reason, we cease to be listed on a prescribed stock exchange. Accordingly, holders and prospective holders of our common shares should consult with their own tax advisors with respect to the income tax consequences of them purchasing, owing and disposing of our common shares should we cease to be listed on a prescribed stock exchange.
This summary is based upon the current provisions of the ITA, the regulations there under, the Canada-United States Tax Convention as amended by the Protocols thereto (the "Treaty") as at the date of the registration statement and the currently publicly announced administrative and assessing policies of the Canada Revenue Agency (the "CRA"). This summary does not take into account Canadian provincial income tax consequences. This description is not exhaustive of all possible Canadian federal income tax consequences and does not take into account or anticipate any changes in law, whether by legislative, governmental or judicial action. This summary does, however, take into account all specific proposals to amend the ITA and regulations there under, publicly announced by the Government of Canada to the date hereof.
This summary does not address potential tax effects relevant to our company or those tax considerations that depend upon circumstances specific to each investor. Accordingly, holders and prospective holders of our common shares should consult with their own tax advisors with respect to the income tax consequences to them of purchasing, owning and disposing of common shares in our company.
Dividends
The ITA provides that dividends and other distributions deemed to be dividends paid or deemed to be paid by a Canadian resident corporation (such as our company) to a non-resident of Canada shall be subject to a non-resident withholding tax equal to 25% of the gross amount of the dividend of deemed dividend. Provisions in the ITA relating to dividend and deemed dividend payments to and gains realized by non-residents of Canada, who are residents of the United States, are subject to the Treaty. The Treaty may reduce the withholding tax rate on dividends as discussed below.
Article X of the Treaty as amended by the US-Canada Protocol ratified on November 9, 1995 provides a 5% withholding tax on gross dividends or deemed dividends paid to a United States corporation which beneficially owns at least 10% of the voting stock of the company paying the dividend. In cases where dividends or deemed dividends are paid to a United States resident (other than a corporation) or a United States corporation which beneficially owns less than 10% of the voting stock of a company, a withholding tax of 15% is imposed on the gross amount of the dividend or deemed dividend paid. We would be required to withhold any such tax from the dividend and remit the tax directly to CRA for the account of the investor.
The reduction in withholding tax from 25%, pursuant to the Treaty, will not be available:
| | (a) | if the shares in respect of which the dividends are paid formed part of the business property or were otherwise effectively connected with a permanent establishment or fixed base that the holder has or had in Canada within the 12 months preceding the disposition, or |
| | (b) | the holder is a U.S. LLC which is not subject to tax in the U.S. |
The Treaty generally exempts from Canadian income tax dividends paid to a religious, scientific, literary, educational, or charitable organization or to an organization exclusively administering a pension, retirement or employee benefit fund or plan, if the organization is resident in the U.S. and is exempt from income tax under the laws of the U.S.
Capital Gains
A non-resident holder is not subject to tax under the ITA in respect of a capital gain realized upon the disposition of one of our shares unless the share represents "taxable Canadian property" to the holder thereof. Our common shares will be considered taxable Canadian property to a non-resident holder only if:
| | (a) | the non-resident holder; |
| | (b) | persons with whom the non-resident holder did not deal at arm's length - or |
| | (c) | the non-resident holder and persons with whom he did not deal at arm's length, |
owned not less than 25% of the issued shares of any class or series of our company at any time during the five year period preceding the disposition. In the case of a non-resident holder to whom shares of our company represent taxable Canadian property and who is resident in the United States, no Canadian taxes will generally be payable on a capital gain realized on such shares by reason of the Treaty unless:
| | (a) | the value of such shares is derived principally from real property (including resource property) situated in Canada, |
| | (b) | the holder was resident in Canada for 120 months during any period of 20 consecutive years preceding, and at any time during the 10 years immediately preceding, the disposition and the shares were owned by him when he ceased to be a resident of Canada, |
| | (c) | they formed part of the business property or were otherwise effectively connected with a permanent establishment or fixed base that the holder has or had in Canada within the 12 months preceding the disposition, or |
| | (d) | the holder is a U.S. LLC which is not subject to tax in the U.S. |
If subject to Canadian tax on such a disposition, the taxpayer's capital gain (or capital loss) from a disposition is the amount by which the taxpayer's proceeds of disposition exceed (or are exceeded by) the aggregate of the taxpayer's adjusted cost base of the shares and reasonable expenses of disposition. For Canadian income tax purposes, the "taxable capital gain" is equal to one-half of the capital gain.
U.S. Federal Income Tax Consequences
The following is a discussion of the material United States Federal income tax consequences, under current law, applicable to a U.S. Holder (as defined below) of our common shares who holds such shares as capital assets. This discussion does not address all potentially relevant Federal income tax matters and it does not address consequences peculiar to persons subject to special provisions of Federal income tax law, such as those described below as excluded from the definition of a U.S. Holder. In addition, this discussion does not cover any state, local, or foreign tax consequences. (See "Canadian Federal Income Tax Consequences" above.)
The following discussion is based on the Internal Revenue Code of 1986, as amended (the "Code"), Treasury Regulations, published Internal Revenue Service ("IRS") rulings, published administrative positions of the IRS and court decisions that are currently applicable, any or all of which could be materially and adversely changed, possibly on a retroactive basis, at any time. In addition, this discussion does not consider the potential effects, both adverse and beneficial, of any recently proposed legislation, which, if enacted, could be applied, possibly on a retroactive basis, at any time.
The discussion below does not address potential tax effects relevant to our company or those tax considerations that depend upon circumstances specific to each investor. In addition, this discussion does not address the tax consequences that may be relevant to particular investors subject to special treatment under certain U.S. Federal income tax laws, such as, dealers in securities, tax-exempt entities, banks, insurance companies, and non-U.S. Holders. Purchasers of the common stock should therefore satisfy themselves as to the overall tax consequences of their ownership of the common stock, including the State, local and foreign tax consequences thereof (which are not reviewed herein), and should consult their own tax advisors with respect to their particular circumstances.
U.S. Holders
As used herein, a "U.S. Holder" includes a beneficial holder of common shares of our company who is a citizen or resident of the United States, a corporation or partnership created or organized in or under the laws of the United States or of any political subdivision thereof, any trust if a US court is able to exercise primary supervision over the administration of the trust and one or more US persons have the authority to control all substantial decisions of the trust, any entity created or organized in the United States which is taxable as a corporation for U.S. tax purposes and any other person or entity whose ownership of common shares of our company is effectively connected with the conduct of a trade or business in the United States. A U.S. Holder does not include persons subject to special provisions of Federal income tax law, such as tax-exempt organizations, qualified retirement plans, financial institutions, insurance companies, real estate investment trusts, regulated investment companies, broker-dealers, non-resident alien individuals or foreign corporations whose ownership of our common shares is not effectively connected with the conduct of a trade or business in the United States and shareholders who acquired their shares through the exercise of employee stock options or otherwise as compensation.
Dividend Distribution on Shares of our Company
U.S. Holders receiving dividend distributions (including constructive dividends) with respect to the common shares of our company are required to include in gross income for United States Federal income tax purposes the gross amount of such distributions to the extent that we have current or accumulated earnings and profits, without reduction for any Canadian income tax withheld from such distributions. Such Canadian tax withheld may be deducted or may be credited against actual tax payable, subject to certain limitations and other complex rules, against the U.S. Holder's United States Federal taxable income. See "Foreign Tax Credit" below. To the extent that distributions exceed our current or accumulated earnings and profits, they will be treated first as a return of capital to the extent of the shareholder's basis in the common shares of our company and thereafter as gain from the sale or exchange of the common shares of our company. Preferential tax rates for net long term capital gains may be applicable to a U.S. Holder which is an individual, estate or trust.
In general, dividends paid on our common shares will not be eligible for the dividends received deduction provided to corporations receiving dividends from certain United States corporations.
Foreign Tax Credit
A U.S. Holder who pays (or who has had withheld from distributions) Canadian income tax with respect to the ownership of our common shares may be entitled, at the election of the U.S. Holder, to either a deduction or a tax credit for such foreign tax paid or withheld. This election is made on a year-by-year basis and generally applies to all foreign income taxes paid by (or withheld from) the U.S. Holder during that year. There are significant and complex limitations which apply to the credit, among which is the general limitation that the credit cannot exceed the proportionate share of the U.S. Holder's United States income tax liability that the U.S. Holder's foreign source income bears to his or its world-wide taxable income. In determining the application of this limitation, the various items of income and deduction must be classified into foreign and domestic sources. Complex rules govern income such as "passive income", "high withholding tax interest", "financial services income", "shipping income" and certain other classifications of income. A U.S. Holder who is treated as a domestic U.S. corporation owning 10% or more of our voting stock is also entitled to a deemed paid foreign tax credit in certain circumstances for the underlying foreign tax of our company related to dividends received or Subpart F income received from us. (See the discussion below of Controlled Foreign Corporations). The availability of the foreign tax credit and the application of the limitations on the foreign tax credit are fact specific and holders and prospective holders of our common shares should consult their own tax advisors regarding their individual circumstances.
Disposition of Common Shares
If a "U.S. Holder" is holding shares as a capital asset, a gain or loss realized on a sale of our common shares will generally be a capital gain or loss, and will be long-term if the shareholder has a holding period of more than one year. However, gains realized upon sale of our common shares may, under certain circumstances, be treated as ordinary income, if we were determined to be a "collapsible corporation" within the meaning of Code Section 341 based on the facts in existence on the date of the sale (See below for definition of "collapsible corporation"). The amount of gain or loss recognized by a selling U.S. Holder will be measured by the difference between (i) the amount realized on the sale and (ii) his tax basis in our common shares. Capital losses are deductible only to the extent of capital gains. However, in the case of taxpayers other than corporations (U.S.) $3,000 ($1,500 for married individuals filing separately) of capital losses are deductible against ordinary income annually. In the case of individuals and other non-corporate taxpayers, capital losses that are not currently deductible may be carried forward to other years. In the case of corporations, capital losses that are not currently deductible are carried back to each of the three years preceding the loss year and forward to each of the five years succeeding the loss year.
A "collapsible corporation" is a corporation that is formed or availed principally to manufacture, construct, produce, or purchase prescribed types or property that the corporation holds for less than three years and that generally would produce ordinary income on its disposition, with a view to the stockholders selling or exchanging their stock and thus realizing gain before the corporation realizes two thirds of the taxable income to be derived from prescribed property. Prescribed property includes: stock in trade and inventory; property held primarily for sale to customers in the ordinary course of business; unrealized receivables or fees, consisting of rights to payment for noncapital assets delivered or to be delivered, or services rendered or to be rendered to the extent not previously included in income, but excluding receivables from selling property that is not prescribed; and property gain on the sale of which is subject to the capital gain/ordinary loss rule. Generally, a shareholder who owns directly or indirectly 5 percent or less of the outstanding stock of the corporation may treat gain on the sale of his shares as capital gain.
Other Considerations for U.S. Holders
In the following circumstances, the above sections of this discussion may not describe the United States Federal income tax consequences resulting from the holding and disposition of common shares of the Registrant. Our management is of the opinion that there is little, if not, any likelihood that we will be deemed a "Foreign Personal Holding Company", a "Foreign Investment Company" or a "Controlled Foreign Corporation" (each as defined below) under current and anticipated conditions.
Foreign Personal Holding Company
If at any time during a taxable year more than 50% of the total combined voting power or the total value of our outstanding shares is owned, actually or constructively, by five or fewer individuals who are citizens or residents of the United States and 60% or more of our gross income for such year was derived from certain passive sources (e.g., from dividends received from its subsidiaries), we would be treated as a "foreign personal holding company." In that event, U.S. Holders that hold common shares in our capital would be required to include in income for such year their allocable portion of our passive income which would have been treated as a dividend had that passive income actually been distributed.
Foreign Investment Company
If 50% or more of the combined voting power or total value of our outstanding shares are held, actually or constructively, by citizens or residents of the United States, United States domestic partnerships or corporations, or estates or trusts other than foreign estates or trusts (as defined by the Code Section 7701(a)(31)), and we are found to be engaged primarily in the business of investing, reinvesting, or trading in securities, commodities, or any interest therein, it is possible that we might be treated as a "foreign investment company" as defined in Section 1246 of the Code, causing all or part of any gain realized by a U.S. Holder selling or exchanging our common shares to be treated as ordinary income rather than capital gains.
Passive Foreign Investment Company
A U.S. Holder who holds stock in a foreign corporation during any year in which such corporation qualifies as a passive foreign investment company ("PFIC") is subject to U.S. federal income taxation of that foreign corporation under one of two alternative tax methods at the election of each such U.S. Holder.
Section 1297 of the Code defines a PFIC as a corporation that is not formed in the United States and, for any taxable year, either (i) 75% or more of its gross income is "passive income," which includes interest, dividends and certain rents and royalties or (ii) the average percentage, by value (or, if the company is a controlled foreign corporation or makes an election, adjusted tax basis), of its assets that produce or are held for the production of "passive income" is 50% or more. For taxable years of U.S. persons beginning after December 31, 1997, and for tax years of foreign corporations ending with or within such tax years, the Taxpayer Relief Act of 1997 provides that publicly traded corporations must apply this test on a fair market value basis only. We believe that we currently do not qualify as a PFIC because our passive income producing assets are less than 50% of our total assets.
As a PFIC, each U.S. Holder must determine under which of the alternative tax methods it wishes to be taxed. Under one method, a U.S. Holder who elects in a timely manner to treat the Registrant as a Qualified Electing Fund ("QEF"), as defined in the Code, (an "Electing U.S. Holder") will be subject, under Section 1293 of the Code, to current federal income tax for any taxable year in which we qualify as a PFIC on his pro-rata share of our (i) "net capital gain" (the excess of net long-term capital gain over net short-term capital loss), which will be taxed as long-term capital gain to the Electing U.S. Holder and (ii) "ordinary earnings" (the excess of earnings and profits over net capital gain), which will be taxed as ordinary income to the Electing U.S. Holder, in each case, for the U.S. Holder's taxable year in which (or with which) our taxable year ends, regardless of whether such amounts are actually distributed. Such an election, once made shall apply to all subsequent years unless revoked with the consent of the IRS.
A QEF election also allows the Electing U.S. Holder to (i) generally treat any gain realized on the disposition of his common shares (or deemed to be realized on the pledge of his common shares) as capital gain; (ii) treat his share of our net capital gain, if any, as long-term capital gain instead of ordinary income, and (iii) either avoid interest charges resulting from PFIC status altogether (see discussion of interest charge below), or make an annual election, subject to certain limitations, to defer payment of current taxes on his share of our annual realized net capital gain and ordinary earnings subject, however, to an interest charge. If the Electing U.S. Holder is an individual, such an interest charge would be not deductible.
The procedure a U.S. Holder must comply with in making a timely QEF election will depend on whether the year of the election is the first year in the U.S. Holder's holding period in which we are a PFIC. If the U.S. Holder makes a QEF election in such first year, (sometimes referred to as a "Pedigreed QEF Election"), then the U.S. Holder may make the QEF election by simply filing the appropriate documents at the time the U.S. Holder files its tax return for such first year. If, however, we qualified as a PFIC in a prior year, then the U.S. Holder may make an "Unpedigreed QEF Election" by recognizing as an "excess distribution" (i) under the rules of Section 1291 (discussed below), any gain that he would otherwise recognize if the U.S. Holder sold his stock on the qualification date (Deemed Sale Election) or (ii) if we are a controlled foreign corporation ("CFC"), the Holder's pro rata share of the corporation's earnings and profits (Deemed Dividend Election) (But see "Elimination of Overlap Between Subpart F Rules and PFIC Provisions"). The effect of either the deemed sale election or the deemed dividend election is to pay all prior deferred tax, to pay interest on the tax deferral and to be treated thereafter as a Pedigreed QEF as discussed in the prior paragraph. With respect to a situation in which a Pedigreed QEF election is made, if we no longer qualify as a PFIC in a subsequent year, normal Code rules and not the PFIC rules will apply.
If a U.S. Holder has not made a QEF Election at any time (a "Non-electing U.S. Holder"), then special taxation rules under Section 1291 of the Code will apply to (i) gains realized on the disposition (or deemed to be realized by reason of a pledge) of his common shares and (ii) certain "excess distributions", as specially defined, by our company. An "excess distribution" is any current-year distribution in respect of PFIC stock that represents a ratable portion of the total distributions in respect of the stock during the year that exceed 125 percent of the average amount of distributions in respect of the stock during the three preceding years.
A Non-electing U.S. Holder generally would be required to pro-rate all gains realized on the disposition of his common shares and all excess distributions over the entire holding period for the common shares. All gains or excess distributions allocated to prior years of the U.S. Holder (other than years prior to our first taxable year during such U.S. Holder's holding period and beginning after January, 1987 for which it was a PFIC) would be taxed at the highest tax rate for each such prior year applicable to ordinary income. The Non-electing U.S. Holder also would be liable for interest on the deferred tax liability for each such prior year calculated as if such liability had been due with respect to each such prior year. A Non-electing U.S. Holder that is an individual is not allowed a deduction for interest on the deferred tax liability. The portions of gains and distributions that are not characterized as "excess distributions" are subject to tax in the current year under the normal tax rules of the Internal Revenue Code.
If we are a PFIC for any taxable year during which a Non-electing U.S. Holder holds common shares, then we will continue to be treated as a PFIC with respect to such common Shares, even if it is no longer by definition a PFIC. A Non-electing U.S. Holder may terminate this deemed PFIC status by electing to recognize gain (which will be taxed under the rules discussed above for Non-Electing U.S. Holders) as if such common shares had been sold on the last day of the last taxable year for which it was a PFIC.
Under Section 1291(f) of the Code, the Department of the Treasury has issued proposed regulations that would treat as taxable certain transfers of PFIC stock by Non-electing U.S. Holders that are generally not otherwise taxed, such as gifts, exchanges pursuant to corporate reorganizations, and transfers at death. If a U.S. Holder makes a QEF Election that is not a Pedigreed Election (i.e., it is made after the first year during which we are a PFIC and the U.S. Holder holds our shares) (a "Unpedigreed Election"), the QEF rules apply prospectively but do not apply to years prior to the year in which the QEF first becomes effective. U.S. Holders should consult their tax advisors regarding the specific consequences of making a Non-Pedigreed QEF Election.
Certain special, generally adverse, rules will apply with respect to the common shares while we are a PFIC whether or not it is treated as a QEF. For example under Section 1297(b)(6) of the Code (as in effect prior to the Taxpayer Relief Act of 1997), a U.S. Holder who uses PFIC stock as security for a loan (including a margin loan) will, except as may be provided in regulations, be treated as having made a taxable disposition of such stock.
The foregoing discussion is based on currently effective provisions of the Code, existing and proposed regulations thereunder, and current administrative rulings and court decisions, all of which are subject to change. Any such change could affect the validity of this discussion. In addition, the implementation of certain aspects of the PFIC rules requires the issuance of regulations which in many instances have not been promulgated and which may have retroactive effect. There can be no assurance that any of these proposals will be enacted or promulgated, and if so, the form they will take or the effect that they may have on this discussion. Accordingly, and due to the complexity of the PFIC rules, U.S. Holders of the Registrant are strongly urged to consult their own tax advisors concerning the impact of these rules on their investment in our company. For a discussion of the impact of the Taxpayer Relief Act of 1997 on a U.S. Holder of a PFIC, see "Mark-to-Market Election For PFIC Stock Under the Taxpayer Relief Act of 1997" and "Elimination of Overlap Between Subpart F Rules and PFIC Provisions" below.
Mark-to-Market Election for PFIC Stock under the Taxpayer Relief Act of 1997
The Taxpayer Relief Act of 1997 provides that a U.S. Holder of a PFIC may make a mark-to-market election with respect to the stock of the PFIC if such stock is marketable as defined below. This provision is designed to provide a current inclusion provision for persons that are Non-Electing Holders. Under the election, any excess of the fair market value of the PFIC stock at the close of the tax year over the Holder's adjusted basis in the stock is included in the Holder's income. The Holder may deduct any excess of the adjusted basis of the PFIC stock over its fair market value at the close of the tax year. However, deductions are limited to the net mark-to-market gains on the stock that the Holder included in income in prior tax years, or so called "unreversed inclusions." For purposes of the election, PFIC stock is marketable if it is regularly traded on (1) a national securities exchange that is registered with the SEC, (2) the national market system established under Section II A of the Exchange Act, or (3) an exchange or market that the IRS determines has rules sufficient to ensure that the market price represents legitimate and sound fair market value.
A Holder's adjusted basis of PFIC stock is increased by the income recognized under the mark-to-market election and decreased by the deductions allowed under the election. If a U.S. Holder owns PFIC stock indirectly through a foreign entity, the basis adjustments apply to the basis of the PFIC stock in the hands of the foreign entity for the purpose of applying the PFIC rules to the tax treatment of the U.S. owner.
Similar basis adjustments are made to the basis of the property through which the U.S. persons hold the PFIC stock.
Income recognized under the mark-to-market election and gain on the sale of PFIC stock with respect to which an election is made is treated as ordinary income. Deductions allowed under the election and loss on the sale of PFIC with respect to which an election is made, to the extent that the amount of loss does not exceed the net mark-to-market gains previously included, are treated as ordinary losses. The U.S. or foreign source of any income or losses is determined as if the amount were a gain or loss from the sale of stock in the PFIC.
If PFIC stock is owned by a CFC (discussed below), the CFC is treated as a U.S. person that may make the mark-to-market election. Amounts includible in the CFC's income under the election are treated as foreign personal holding company income, and deductions are allocable to foreign personal holding company income.
The above provisions apply to tax years of U.S. persons beginning after December 31, 1997, and to tax years of foreign corporations ending with or within such tax years of U.S. persons.
The rules of Code Section 1291 applicable to nonqualified funds as discussed above generally do not apply to a U.S. Holder for tax years for which a mark-to-market election is in effect. If Code Section 1291 is applied and a mark-to-market election was in effect for any prior tax year, the U.S. Holder's holding period for the PFIC stock is treated as beginning immediately after the last tax year of the election. However, if a taxpayer makes a mark-to-market election for PFIC stock that is a nonqualified fund after the beginning of a taxpayer's holding period for such stock, a co-ordination rule applies to ensure that the taxpayer does not avoid the interest charge with respect to amounts attributable to periods before the election.
Controlled Foreign Corporation Status
If more than 50% of the voting power of all classes of stock or the total value of the stock of our company is owned, directly or indirectly, by U.S. Holders, each of whom own after applying rules of attribution 10% or more of the total combined voting power of all classes of stock of our company, we would be treated as a "controlled foreign corporation" or "CFC" under Subpart F of the Code. This classification would bring into effect many complex results including the required inclusion by such 10% U.S. Holders in income of their pro rata shares of "Subpart F income" (as defined by the Code) of our company and our earnings invested in "U.S. property" (as defined by Section 956 of the Code). In addition, under Section 1248 of the Code if we are considered a CFC at any time during the five year period ending with the sale or exchange of its stock, gain from the sale or exchange of common shares of our company by such a 10% U.S. Holder of our common stock at any time during the five year period ending with the sale or exchange is treated as ordinary dividend income to the extent of our earnings and profits attributable to the stock sold or exchanged. Because of the complexity of Subpart F, and because we may never be a CFC, a more detailed review of these rules is beyond the scope of this discussion.
Elimination of Overlap between Subpart F Rules and PFIC Provisions
Under the Taxpayer Relief Act of 1997, a PFIC that is also a CFC will not be treated as a PFIC with respect to certain 10% U.S. Holders. For the exception to apply, (i) the corporation must be a CFC within the meaning of section 957(a) of the Code and (ii) the U.S. Holder must be subject to the current inclusion rules of Subpart F with respect to such corporation (i.e., the U.S. Holder is a "United States Shareholder," see "Controlled Foreign Corporation," above). The exception only applies to that portion of a U.S. Holder's holding period beginning after December 31, 1997. For that portion of a United States Holder before January 1, 1998, the ordinary PFIC and QEF rules continue to apply.
As a result of this new provision, if we were ever to become a CFC, U.S. Holders who are currently taxed on their pro rata shares of Subpart F income of a PFIC which is also a CFC will not be subject to the PFIC provisions with respect to the same stock if they have previously made a Pedigreed QEF Election. The PFIC provisions will however continue to apply to U.S Holders for any periods in which Subpart F does not apply (for example he is no longer a 10% Holder or we are no longer a CFC) and to U.S. Holders that did not make a Pedigreed QEF Election unless the U.S. Holder elects to recognize gain on the PFIC shares held in our company as if those shares had been sold.
ALL PROSPECTIVE INVESTORS ARE ADVISED TO CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE SPECIFIC TAX CONSEQUENCES OF PURCHASING THE COMMON SHARES OF OUR COMPANY.
ENFORCEABILITY OF CIVIL LIABILITIES
The Company is organized under the laws of the Canada Business Corporations Act (Canada) and our executive offices are located outside of the United States in Toronto, Ontario. A majority of our directors and officers, as well as our auditor, reside outside the United States. In addition, a substantial portion of their assets and the Company's assets are located outside of the United States. As a result, you may have difficulty serving legal process within the United States upon us or any of these persons. You may also have difficulty enforcing, both in and outside of the United States, judgments you may obtain in U.S. courts against us or these persons in any action, including actions based upon the civil liability provisions of U.S. Federal or state securities laws. Furthermore, there is substantial doubt as to the enforceability in Canada against us or against any of our directors, officers and the expert named in this prospectus who are not residents of the United States, in original actions or in actions for enforcement of judgments of U.S. courts, of liabilities based solely upon the civil liability provisions of the U.S. federal securities laws.
EXPENSES RELATED TO THIS OFFERING
All amounts are estimates other than the SEC's registration fee. Kodiak Capital is paying all of the registration expenses incurred in connection with the registration of the shares except for accounting fees and expenses. No portion of these expenses will be borne by the selling stockholders. The selling stockholders, however, will pay any other expenses incurred in selling their common stock, including any brokerage commissions or costs of sale.
| Securities and Exchange Commission Registration Fee | | $ | 95.20 | |
| Blue Sky Fees and Expenses | | $ | 404.80 | |
| Legal Fees and Expenses | | $ | 15,000 | |
| Accounting Fees and Expenses | | $ | 5,000 | |
| Miscellaneous | | $ | 2,500 | |
| | | | | |
| Total | | $ | 23,000 | |
LEGAL MATTERS
Certain legal matters concerning this offering will be passed upon for us by Szaferman, Lakind, Blumstein & Blader, PC, Lawrenceville, New Jersey. Certain legal matters with respect to matters of Canada law including the validity of the common shares offered by this prospectus will be passed upon for us by Fogler, Rubinoff LLP, Toronto, Canada.
EXPERTS
Our audited consolidated financial statements appearing in this prospectus and registration statement have been audited by Collins Barrow Toronto LLP, an independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein and in the registration statement, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form F-1 under the Securities Act with the SEC with respect to the shares of our common stock offered through this prospectus. This prospectus is filed as a part of that registration statement but does not contain all of the information contained in the registration statement and exhibits. Statements made in the registration statement are summaries of the material terms of the referenced contracts, agreements or documents of our Company. You may inspect the registration statement, exhibits and schedules filed with the SEC at the SEC's principal office in Washington, D.C. Copies of all or any part of the registration statement may be obtained from the Public Reference Section of the SEC, at 100 F Street, NE, Washington, D.C. 20549, on official business days during the hours of 10:00 a.m. to 3:00 p.m. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference rooms. The SEC also maintains a web site at http://www.sec.gov that contains reports, proxy statements and information regarding registrants that file electronically with the SEC. Our registration statement and the referenced exhibits can also be found on this site.
FINANCIAL STATEMENTS
Unaudited Condensed Interim Consolidated Financial Statements for the three and six months ended January 31, 2016 and 2015
| | · | MANAGEMENT'S RESPONSIBILITY FOR FINANCIAL REPORTING |
| | · | Unaudited Condensed Interim Consolidated Statements of Financial Position |
| | · | Unaudited Condensed Interim Consolidated Statements of Loss and Comprehensive Loss |
| | · | Unaudited Condensed Interim Consolidated Statements of Changes in Shareholders' Equity |
| | · | Unaudited Condensed Interim Consolidated Statement of Cash Flows |
| | · | Notes to the unaudited condensed interim consolidated Financial Statements |
Audited financial statements for the years ended July 31, 2015 and 2014
| | · | MANAGEMENT'S RESPONSIBILITY FOR FINANCIAL REPORTING |
| | · | INDEPENDENT AUDITORS' REPORT to the Shareholders of Nutritional High International Inc. |
| | · | Unaudited Condensed Interim Consolidated Statements of Financial Position |
| | · | Unaudited Condensed Interim Consolidated Statements of Loss and Comprehensive Loss |
| | · | Unaudited Condensed Interim Consolidated Statements of Changes in Shareholders' Equity |
| | · | Unaudited Condensed Interim Consolidated Statement of Cash Flows |
| | · | Notes to the unaudited condensed interim consolidated Financial Statements |
Nutritional High International Inc.
Unaudited Condensed Interim Consolidated Financial Statements
For the three and six months ended January 31, 2016 and 2015
MANAGEMENT'S RESPONSIBILITY FOR FINANCIAL REPORTING
The accompanying unaudited condensed interim consolidated financial statements of Nutritional High International Inc., are the responsibility of the management and Board of Directors of the Company.
The unaudited condensed interim consolidated financial statements have been prepared by management, on behalf of the Board of Directors, in accordance with the accounting policies disclosed in the notes to the consolidated financial statements. Where necessary, management has made informed judgments and estimates in accounting for transactions which were not complete at the consolidated statement of financial position date. In the opinion of management, the consolidated financial statements have been prepared within acceptable limits of materiality and are in accordance with International Financial Reporting Standards using accounting policies consistent with International Financial Reporting Standards appropriate in the circumstances.
Management has established systems of internal control over the financial reporting process, which are designed to provide reasonable assurance that relevant and reliable financial information is produced.
The Board of Directors is responsible for reviewing and approving the unaudited condensed interim consolidated financial statements together with other financial information of the Company and for ensuring that management fulfills its financial reporting responsibilities. An Audit Committee assists the Board of Directors in fulfilling this responsibility. The Audit Committee meets with management to review the financial reporting process and the unaudited condensed interim consolidated financial statements together with other financial information of the Company. The Audit Committee reports its findings to the Board of Directors for its consideration in approving the unaudited condensed interim consolidated financial statements together with other financial information of the Company for issuance to the shareholders.
Management recognizes its responsibility for conducting the Company's affairs in compliance with established financial standards, and applicable laws and regulations, and for maintaining proper standards of conduct for its activities.
"David Posner" , Director and CEO "Al Quong" , CFO
David Posner Al Quong
Nutritional High International Inc.
Unaudited Condensed Interim Consolidated Statements of Financial Position
(Expressed in Canadian Dollars)
The accompanying notes are an integral part of these unaudited condensed interim consolidated financial statements
| | | |
| | | January 31, 2016 | | | |
| Assets | | | | | |
| Current | | | | | |
| Cash | | $ | 18,233 | | | $ | 19,567 |
HST receivable (Note 4) | | | 66,420 | | | | 121,572 |
| Other receivable | | | - | | | | 9,459 |
Prepaids (Note 19) | | | 122,549 | | | | 238,115 |
Inventory (Note 5) | | | 26,600 | | | | - |
| | | | 233,802 | | | | 388,713 |
| Non-current assets | | | | | | | |
Amounts due from Palo Verde LLC (Note 6) | | | 430,444 | | | | 197,842 |
Prepaid license (Note 19) | | | 233,128 | | | | 249,780 |
Investment property (Note 7) | | | 1,697,289 | | | | 1,129,582 |
Property deposits (Note 8) | | | 14,006 | | | | 6,671 |
| | | $ | 2,608,669 | | | $ | 1,972,588 |
| | | | | | | | |
| Liabilities | | | | | | | |
| Current | | | | | | | |
Accounts payable and accrued liabilities (Notes 9 and 11) | | $ | 548,074 | | | $ | 272,274 |
Promissory note payable (Note 10) | | | 13,716 | | | | - |
| | | | 561,790 | | | | 272,274 |
| Non-current liabilities | | | | | | | |
Convertible debentures (Note 16) | | | 211,935 | | | | 539,910 |
Derivative liability (Note 16) | | | 91,291 | | | | 330,065 |
Promissory note payable, net of current portion (Note 10) | | | 334,019 | | | | - |
| | | | 1,199,035 | | | | 1,142,249 |
| Shareholders' Equity | | | | | | | |
Share Capital (Note 12) | | | 3,677,086 | | | | 2,719,740 |
| Shares to be issued | | | 11,250 | | | | - |
Reserve for share based payments (Note 13) | | | 348,000 | | | | 314,000 |
Reserve for warrants (Note 12 and 14) | | | 804,620 | | | | 566,399 |
| Reserve for foreign currency translation | | | (24,177 | ) | | | (15,285 |
Non-controlling interest (Note 15) | | | (8,920 | ) | | | (9,073 |
Loss on acquisition of non-controlling interest (Note 15) | | | (5,000 | ) | | | (5,000 |
| Accumulated deficit | | | (3,393,225 | ) | | | (2,740,442 |
| | | | 1,409,634 | | | | 830,339 |
| | | $ | 2,608,669 | | | $ | 1,972,588 |
Nature of Operations and Going concern (Note 1)
Licensing and Royalty Agreement (Note 19)
Subsequent Events (Note 21)
Approved on behalf of the Board:
"Adam Szweras" Director "David Posner" Director
The accompanying notes are an integral part of these unaudited condensed interim consolidated financial statements
Nutritional High International Inc.
Unaudited Condensed Interim Consolidated Statements of Loss and Comprehensive Loss
(Expressed in Canadian Dollars)
| | | | | | | | |
| | | | | | | | |
| | | Three months ended January 31, 2016 | | | Three months ended January 31, 2015 | | | Six months ended January 31, 2016 | | | Six months ended January 31, 2015 | |
| Administrative expenses | | | | | | | | | | | | |
Management and consulting fees (Note 11) | | | 352,358 | | | | 104,270 | | | | 549,024 | | | | 258,220 | |
Professional fees (Note 11) | | | 51,908 | | | | 193,967 | | | | 76,625 | | | | 372,040 | |
| Office and general | | | 118,936 | | | | 95,005 | | | | 192,008 | | | | 145,718 | |
| Foreign exchange gain | | | (119,202 | ) | | | (26,744) | | | | (120,222) | | | | (29,658) | |
Share based payments (Note 13) | | | 15,000 | | | | - | | | | 34,000 | | | | 4,000 | |
| Other expenses | | | | | | | | | | | | | | | | |
Change in fair value of derivative liability (Note 16) | | | (81,902 | ) | | | - | | | | 11,288 | | | | - | |
Finance costs (Note 16) | | | 19,784 | | | | - | | | | 52,204 | | | | - | |
Amortization (Note 7 and 19) | | | 23,014 | | | | 7,150 | | | | 37,261 | | | | 7,150 | |
| Total expenses | | | (379,896 | ) | | | (373,648 | ) | | | (832,188 | ) | | | (757,470 | ) |
| Other income (Expense) | | | | | | | | | | | | | | | | |
Rental income (Note 6) | | | 133,856 | | | | 32,519 | | | | 259,543 | | | | 32,519 | |
| Interest | | | 19,812 | | | | 1,284 | | | | 30,015 | | | | 2,379 | |
Allowance for amounts receivable (Note 6) | | | (65,000 | ) | | | - | | | | (110,000 | ) | | | - | |
| Net loss | | | (291,228 | ) | | | (339,845 | ) | | | (652,630 | ) | | | (722,572 | ) |
| Other Comprehensive loss | | | | | | | | | | | | | | | | |
| Item that may be reclassified subsequently to net income/loss | | | | | | | | | | | | | | | | |
| Exchange differences on translating foreign operations | | | (8,946 | ) | | | (27,806 | ) | | | (8,892 | ) | | | (30,085 | ) |
| Net loss and comprehensive loss | | $ | (300,174 | ) | | $ | (367,651 | ) | | $ | (661,522 | ) | | $ | (752,657 | ) |
| | | | | | | | | | | | | | | | | |
Net loss attributable to non-controlling interest (Note 15) | | $ | - | | | $ | (3,695 | ) | | $ | 153 | | | $ | (4,754 | ) |
| Net loss attributable to parent company | | | (291,228 | ) | | | (336,150 | ) | | | (652,783 | ) | | | (717,818 | ) |
| | | $ | (291,228 | ) | | $ | (339,845 | ) | | $ | (652,630 | ) | | $ | (722,572 | ) |
Net loss and comprehensive loss attributable to non-controlling interest (Note 15) | | $ | - | | | $ | (3,695 | ) | | $ | 153 | | | $ | (4,760 | ) |
| Net loss and comprehensive loss attributable to parent company | | | (300,174 | ) | | | (363,956 | ) | | | (661,675 | ) | | | (747,897 | ) |
| | | $ | (300,174 | ) | | $ | (367,651 | ) | | $ | (661,522 | ) | | $ | (752,657 | ) |
| Weighted average number of shares outstanding | | | | | | | | | | | | | | | | |
| - basic and diluted (Note 3.2) | | | 131,276,498 | | | | 79,480,270 | | | | 125,846,162 | | | | 76,196,951 | |
Loss per share | | | | | | | | | | | | | | | | |
| - basic and diluted (Note 3.2) | | $ | (0.002 | ) | | $ | (0.004 | ) | | $ | (0.005 | ) | | $ | (0.009 | ) |
| | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these unaudited condensed interim consolidated financial statements
Nutritional High International Inc.
Unaudited Condensed Interim Consolidated Statements of Changes in Shareholders' Equity
(Expressed in Canadian Dollars)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Share Capital | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Number of shares | | | Amount | | | Loss on acquisition of non-controlling interest | | | Reserve for Share based payments | | | Reserve for Warrants | | | Reserve for Foreign Exchange | | | Shares to be issued | | | Accumulated Deficit | | | Attributable to owners of Parent | | | Non-controlling interest | | | Total | |
| Balance at July 31, 2014 | | | 71,913,631 | | | $ | 1,063,482 | | | $ | - | | | $ | 28,000 | | | $ | 65,000 | | | $ | - | | | $ | - | | | $ | (681,155 | ) | | $ | 475,327 | | | | | | $ | - | | | $ | 475,327 | |
| Private placements (Note 12) | | | 4,000,000 | | | | 100,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 100,000 | | | | | | | - | | | | 100,000 | |
| Finders warrants issued in connection with private placements (Note 12) | | | - | | | | (24,000 | ) | | | - | | | | - | | | | 24,000 | | | | - | | | | - | | | | - | | | | - | | | | | | | - | | | | - | |
| Shares issued on exercise of warrants | | | 3,566,638 | | | | 195,799 | | | | - | | | | - | | | | (17,467 | ) | | | - | | | | - | | | | - | | | | 178,332 | | | | | | | - | | | | 178,332 | |
| Share based payments | | | - | | | | - | | | | - | | | | 4,000 | | | | - | | | | - | | | | - | | | | - | | | | 4,000 | | | | | | | - | | | | 4,000 | |
| Share issue costs | | | - | | | | (10,219 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (10,219 | ) | | | | | | - | | | | (10,219 | ) |
| Exchange gain on translating foreign operations | | | - | | | | - | | | | - | | | | - | | | | - | | | | (30,079 | ) | | | - | | | | - | | | | (30,079 | ) | | | | | | (6 | ) | | | (30,085 | ) |
| Net loss and comprehensive loss for the period | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (717,818 | ) | | | (717,818 | ) | | | | | | (4,754 | ) | | | (722,572 | ) |
| Balance at January 31, 2015 | | | 79,480,269 | | | $ | 1,325,062 | | | $ | - | | | $ | 32,000 | | | $ | 71,533 | | | $ | (30,079 | ) | | $ | - | | | $ | (1,398,973 | ) | | $ | (457 | ) | | | | | $ | (4,760 | ) | | $ | (5,217 | ) |
| Initial public offering (Note 12) | | | 32,900,000 | | | | 1,282,000 | | | | - | | | | - | | | | 363,000 | | | | - | | | | - | | | | - | | | | 1,645,000 | | | | | | | - | | | | 1,645,000 | |
| Compensation warrants issued in connection with initial public offering (Note 12) | | | - | | | | (63,000 | ) | | | - | | | | - | | | | 63,000 | | | | - | | | | - | | | | - | | | | - | | | | | | | - | | | | - | |
| Warrants issued in connection to early and regular exercise of warrants (Note 14) | | | - | | | | (71,000 | ) | | | - | | | | - | | | | 71,000 | | | | - | | | | - | | | | - | | | | - | | | | | | | - | | | | - | |
| Shares issued on exercise of warrants (Note 12) | | | 1,478,000 | | | | 133,153 | | | | - | | | | - | | | | (37,134 | ) | | | - | | | | - | | | | - | | | | 96,019 | | | | | | | - | | | | 96,019 | |
| Issued for non-cash consideration: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issued for success fee (Note 11 and 12) | | | 700,000 | | | | 16,000 | | | | - | | | | - | | | | 19,000 | | | | - | | | | - | | | | - | | | | 35,000 | | | | | | | - | | | | 35,000 | |
| Issued for extension fee (Note 12 and 16) | | | 600,000 | | | | 14,000 | | | | - | | | | - | | | | 16,000 | | | | - | | | | - | | | | - | | | | 30,000 | | | | | | | - | | | | 30,000 | |
| Issued for services (Note 12) | | | 811,111 | | | | 57,500 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 57,500 | | | | | | | - | | | | 57,500 | |
| Issed for license and royalty fees (Note 12 and 19) | | | 3,333,334 | | | | 250,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 250,000 | | | | | | | - | | | | 250,000 | |
| Issued for acquisition of non-controlling interest (Note 12 and 15) | | | 66,667 | | | | 5,000 | | | | (5,000 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | - | | | | - | |
| Share based payments | | | - | | | | - | | | | - | | | | 282,000 | | | | - | | | | - | | | | - | | | | - | | | | 282,000 | | | | | | | - | | | | 282,000 | |
| Share issue costs | | | - | | | | (228,975 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (228,975 | ) | | | | | | - | | | | (228,975 | ) |
| Exchange gain on translating foreign operation | | | - | | | | - | | | | - | | | | - | | | | - | | | | 14,794 | | | | - | | | | - | | | | 14,794 | | | | | | | - | | | | 14,794 | |
| Net loss and comprehensive loss for the period | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (1,341,469 | ) | | | (1,341,469 | ) | | | | | | (4,313 | ) | | | (1,345,782 | ) |
| Balance at July 31, 2015 | | | 119,369,381 | | | $ | 2,719,740 | | | $ | (5,000 | ) | | $ | 314,000 | | | $ | 566,399 | | | $ | (15,285 | ) | | | - | | | $ | (2,740,442 | ) | | $ | 839,412 | | | | | | $ | (9,073 | ) | | $ | 830,339 | |
| Private placements (Note 12) | | | 5,000,000 | | | | 137,000 | | | | | | | | | | | | 113,000 | | | | | | | | | | | | | | | | 250,000 | | | | | | | | | | | 250,000 | |
| Shares issued on exercise of warrants (Note 12) | | | 2,608,000 | | | | 143,179 | | | | - | | | | - | | | | (12,779 | ) | | | - | | | | - | | | | - | | | | 130,400 | | | | | | | - | | | | 130,400 | |
| Warrants issued pursuant to debenture agreement (Note 14) | | | | | | | (138,000 | ) | | | | | | | | | | | 138,000 | | | | | | | | | | | | | | | | - | | | | | | | | | | | - | |
| Shares to be issued | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 11,250 | | | | - | | | | 11,250 | | | | | | | - | | | | 11,250 | |
| Issued for non-cash consideration: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issued for services (Note 12) | | | 456,668 | | | | 22,833 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 22,833 | | | | | | | - | | | | 22,833 | |
| Issued for license and royalty fees (Note 12 and 19) | | | 692,431 | | | | 51,932 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 51,932 | | | | | | | - | | | | 51,932 | |
| Issued for conversion of debentures (Note 12 and 16) | | | 5,833,334 | | | | 600,062 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 600,062 | | | | | | | - | | | | 600,062 | |
| Issued for debt settlements (Note 12) | | | 2,822,700 | | | | 141,135 | | | | | | | | | | | | | | | | | | | | | | | | | | | | 141,135 | | | | | | | | | | | 141,135 | |
| Share based payments | | | | | | | - | | | | - | | | | 34,000 | | | | - | | | | - | | | | - | | | | - | | | | 34,000 | | | | | | | - | | | | 34,000 | |
| Share issue costs | | | | | | | (795 | ) | | | | | | | | | | | | | | | | | | | | | | | | | | | (795 | ) | | | | | | | | | | (795 | ) |
| Exchange gain on translating foreign operation | | | - | | | | - | | | | - | | | | - | | | | - | | | | (8,892 | ) | | | - | | | | - | | | | (8,892 | ) | | | | | | - | | | | (8,892 | ) |
| Net loss for the period | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (652,783 | ) | | | (652,783 | ) | | | | | | 153 | | | | (652,630 | ) |
| Balance at January 31, 2016 | | | 136,782,514 | | | $ | 3,677,086 | | | $ | (5,000 | ) | | $ | 348,000 | | | $ | 804,620 | | | $ | (24,177 | ) | | $ | 11,250 | | | $ | (3,393,225 | ) | | $ | 1,418,554 | | | | | | $ | (8,920 | ) | | $ | 1,409,634 | |
The accompanying notes are an integral part of these unaudited condensed interim consolidated financial statements
Nutritional High International Inc.
Unaudited Condensed Interim Consolidated Statement of Cash Flows
(Expressed in Canadian Dollars)
| For the six months ended January 31 | | | 2016 | | | 2015 | |
| OPERATING ACTIVITIES | | | | | | | |
| Net Loss | | | $ | (652,630 | ) | | $ | (722,572 | ) |
| Item not affecting cash: | | | | | | | | | |
| Amortization | | | | 37,261 | | | | 7,150 | |
| Interest accretion | | | | 22,025 | | | | 1,954 | |
| Shares issued for services | | | | 22,833 | | | | - | |
Share based payments (Note 13) | | | | 34,000 | | | | 4,000 | |
Allowances for amounts receivable (Note 6) | | | | 110,000 | | | | - | |
| Change in the fair value of derivative liability | | | | 11,288 | | | | - | |
| Net change in non-cash working capital: | | | | | | | | | |
| HST receivables and other receivables | | | | 64,611 | | | | (33,329 | ) |
| Prepaids | | | | 115,564 | | | | (19,533 | ) |
| Inventory | | | | (26,600 | ) | | | - | |
Amounts due from Palo Verde LLC (Note 6) | | | | (303,193 | ) | | | (2,151 | ) |
| Accounts payable and accrued liabilities | | | | 467,869 | | | | 419,661 | |
| Cash Flow From (Used) in Operating Activities | | | | (96,972 | ) | | | (344,820 | ) |
| INVESTING ACTIVITIES | | | | | | | | | |
Amounts due from Palo Verde LLC (Note 6) | | | | (37,009 | ) | | | - | |
| Purchase of investment property | | | | (509,202 | ) | | | (1,003,719 | ) |
Property deposits (Note 8) | | | | (6,724 | ) | | | 16,398 | |
| Cash Flow Used in Investing Activities | | | | (552,935 | ) | | | (987,321 | ) |
| FINANCING ACTIVITIES | | | | | | | | | |
Issuance of share capital, net of share issue costs (Note 12) | | | | 380,605 | | | | 168,113 | |
Promissory note payable (Note 10) | | | | 347,735 | | | | - | |
| Loan payable | | | | - | | | | 15,000 | |
Convertible debentures issued, net of issue costs (Note 16) | | | | - | | | | 591,000 | |
| Shares to be issued | | | | 11,250 | | | | - | |
| Cash Flow From Financing Activities | | | | 739,590 | | | | 774,113 | |
| Net increase (decrease) in cash | | | $ | 89,683 | | | $ | (558,028 | ) |
| Effects of exchange rate changes on cash | | | | (91,017 | ) | | | (36,524 | ) |
| Cash at beginning of period | | | | 19,567 | | | | 617,066 | |
| Cash at end of period | | | $ | 18,233 | | | $ | 22,514 | |
| 1. | Nature of Operations and Going Concern |
The accompanying notes are an integral part of these unaudited condensed interim consolidated financial statements
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
| 1. | Nature of Operations and Going Concern |
Nutritional High International Inc. ("Nutritional High" or "the Company" or "NHII"), formerly Sonoma Capital Inc. is the parent company of Nutritional High Ltd., Nutritional High (Colorado) Inc., NHC Edibles LLC, NH Medicinal Dispensaries Inc., NH Medicinal (Minnesota) Inc., Eglinton Medicinal Advisory Ltd., NH Medicinals (Maryland) Inc., Small's Mill Holdings Inc., and Nutritional Traditions Inc. The Company's objective is to take advantage of the changing regulation governing the marijuana industry in the United States and Canada. The address of the Company's registered office is 77 King Street West, Suite 2905, Toronto, Ontario M5K 1H1. The Company is listed on the Canadian Securities Exchange (CSE) under symbol "EAT". The Company is also listed on the OTCQB Marketplace under US symbol: SPLIF.
The Company was incorporated under the name "Sonoma Capital Inc." on July 19, 2004 under the Canada Business Corporations Act.
The condensed interim consolidated financial statements were approved by the Board of Directors on March 31, 2016.
The Company is in the process of developing brands, trademark applications, and packaging for a confectionery line. The Company is also developing a licensing/franchising system to work with licensed marijuana edibles manufacturers and in this regard, is negotiating with parties who are licensed or seeking a manufacturing license.
The Company has earned rental income but not yet realized any revenue from its operations and will not be able to do so until a license/franchise arrangement is negotiated with licensed parties. As such, there is uncertainty with respect to the Company's ability to continue as a going concern, dependent upon such events as financing, entering into agreements with licensed parties, commencement of sales and market demand conditions. There is no assurance that any prospective project in the medical marijuana industry will be successfully initiated or completed. As is common with development stage companies, the Company is dependent upon obtaining necessary financing from time to time to finance its on-going and planned activities and to cover administrative costs.
At January 31, 2016 the Company had working capital (deficiency) of $(327,988) (July 31, 2015 - $116,439), had not yet achieved profitable operations, has accumulated losses of $3,393,225 (July 31, 2015 - $2,740,442) and expects to incur further losses in the development of its business, all of which describes the material uncertainties that cast significant doubt upon the Company's ability to continue as a going concern. The Company will require additional financing in order to conduct its planned business operations, meet its ongoing levels of corporate overhead and discharge its liabilities and commitments (Note 19) as they come due. There is no assurance that the Company will successfully raise this financing.
2.1 Statement of compliance
The condensed consolidated interim financial statements have been prepared in conformity with IAS 34 Interim Financial Reporting on the basis of International Financial Reporting Standards and do not include all the information required for full annual consolidated financial statements in accordance with IFRS and should be read in conjunction with the audited consolidated financials for the year ended July 31, 2015.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
| 2. | Basis of Presentation (continued) |
2.2 Basis of presentation
The unaudited condensed interim consolidated financial statements have been prepared on the historical cost basis except for certain financial instruments, which are measured at fair value, as explained in the accounting policies set out in Note 3.
2.3 Basis of consolidation
The unaudited condensed interim consolidated financial statements include the accounts of Nutritional High International Inc. and its wholly–owned subsidiaries Nutritional High Ltd., Nutritional High (Colorado), Inc., NHC Edibles, LLC, NH Medicinal (Minnesota) Inc., NH Medicinals (Maryland) Inc., Small's Mill Holdings Inc., and Nutritional Traditions Inc. with jurisdiction in US, and 51% owned subsidiary Eglinton Medicinal Advisory Ltd.
The subsidiaries are entities controlled by the Company. Control exists when the Company has power over an investee, when the Company is exposed, or has rights, to variable returns from the investee and when the Company has the ability to affect those returns through its power over the investee. The financial statements of subsidiaries are included in the consolidated financial statements from the date that control commences until the date control ceases.
Non-controlling interest is shown as a component of equity on the statement of financial position and the share of the loss attributable to non-controlling interest is shown as a component of loss for the year in the statement of loss and comprehensive loss.
The functional currency of the Company, Nutritional High Ltd. and Eglinton Medical Advisory Ltd. is the Canadian dollar, which is the presentation currency of the unaudited condensed interim consolidated financial statements. The functional currency of US subsidiaries (Nutritional High (Colorado), Inc, NHC Edibles, LLC, NH Medicinal (Minnesota), Inc., NH Medicinals (Maryland) Inc., Small's Mill Holdings Inc., and Nutritional Traditions Inc.) is the US dollar.
Intercompany balances and transactions, and unrealized gains arising from intercompany transactions are eliminated in preparing the unaudited condensed interim consolidated financial statements.
2.4 New and revised standards and interpretations to be adopted in the future
At the date of authorization of these unaudited condensed interim consolidated financial statements, the IASB and IFRIC has issued the following new and revised Standards and Interpretations which are not yet effective for the relevant reporting periods and which the Company has not early adopted. However, the Company is currently assessing what impact the application of these standards or amendments will have on the consolidated financial statements of the Company.
| · | Pronouncements effective for annual periods beginning on or after January 1, 2016 |
IAS 1 Presentation of Financial Statements Amendments are designed to further encourage companies to apply professional judgment in determining what information to disclose in their financial statements.
IAS 16 Property, Plant and Equipment and IAS 38 Intangible Assets Amendments clarify that the use of revenue-based methods to calculate the depreciation of an asset are not appropriate because revenue generated by an activity that includes the use of an asset generally reflects factors other than the consumption of the economic benefits embodied in the asset.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
| 2. | Basis of Presentation (continued) |
2.4 New and revised standards and interpretations to be adopted in the future (continued)
| · | Pronouncements effective for annual periods beginning on or after January 1, 2018 |
In July 2014, the IASB issued the final version of IFRS 9, Financial Instruments. The new standard will replace IAS 39, Financial instruments: recognition and measurement. The final amendments made in the new version include guidance for the classification and measurement of financial assets and a third measurement category for financial assets, fair value through other comprehensive income. The standard also contains a new expected loss impairment model for debt instruments measured at amortized cost or fair value through other comprehensive income, lease receivables, contract assets and certain written loan commitments and financial guarantee contracts. The standard is effective for annual periods beginning on or after January 1, 2018 and must be applied retrospectively with some exceptions. Early adoption is permitted. Restatement of prior periods in relation to the classification and measurement, including impairment, is not required. The Company has not yet assessed the impact of this new standard.
IFRS 16 Leases was issued in January 2016 and replaces IAS 17 Leases. Under IAS 17, lessees were required to make a distinction between a finance lease and an operating lease. If the lease was classified as a finance lease, a lease liability was included on the statement of financial position. IFRS 16 now requires lessees to recognize a right of use asset and lease liability reflecting future lease payments for virtually all lease contracts. The right of use asset is treated similarly to other non-financial assets and depreciated accordingly. The lease liability accrues interest. The IASB has included an optional exemption for certain short term leases and leases of low value assets; however, this exemption can only be applied by lessees. Under IFRS 16, a contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. Control is conveyed where the customer has both the right to direct the identified asset's use and obtain substantially all the economic benefits from that use. IFRS 16 is effective for annual periods beginning on or after January 1, 2019 with early adoption permitted if IFRS 15, Revenue from Contracts with Customers, is also applied. The Company is currently evaluating the impact of the standard on the Company's financial statements.
| 3. | Summary of Significant Accounting Policies |
3.1 Share based payments
Share based payment transactions
Employees (including directors and senior executives) of the Company receive a portion of their remuneration in the form of share based payment transactions, whereby they render services as consideration for equity instruments ("equity settled transactions").
In situations where equity instruments are issued to non-employees and some or all of the goods or services received by the entity as consideration cannot be specifically measured, they are measured at fair value of the share based payment. The fair value of the share based payments is recognized together with a corresponding increase in equity over a period that services are provided or goods are received.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
Equity settled transactions
The costs of equity settled transactions with employees are measured by reference to the fair value of the equity instrument at the date on which they are granted.
The costs of equity settled transactions are recognized, together with a corresponding increase in equity, over the period in which the performance and/or service conditions are fulfilled, ending on the date on which the relevant employees become fully entitled to the award ("the vesting date"). The cumulative cost is recognized for equity settled transactions at each reporting date until the vesting date reflects the Company's best estimate of the number of equity instruments that will ultimately vest. The profit or loss charge or credit for a period represents the movement in cumulative expense recognized as at the beginning and end of that period and the corresponding amount is represented in share based payment reserve.
No expense is recognized for awards that do not ultimately vest.
Where the terms of an equity settled award are modified, the minimum expense recognized is the expense as if the terms had not been modified. An additional expense is recognized for any modification which increases the total fair value of the share based payment arrangement, or is otherwise beneficial to the employee as measured at the date of modification. The dilutive effect of outstanding options is reflected as additional dilution in the computation of loss per share.
3.2 Loss per share
Basic loss per share is calculated using the weighted number of shares outstanding. Diluted loss per share is calculated using the weighted average number of common and potential common shares outstanding during the period. In order to determine diluted loss per share, it is assumed that any proceeds from the exercise of dilutive stock options and warrants would be used to repurchase common shares at the average market price during the period, with the incremental number of shares being included in the denominator of the diluted loss per share calculation. The diluted loss per share calculation excludes any potential conversion of options and warrants that would increase earnings per share or decrease loss per share. Total shares issuable from warrants were excluded from the computation of diluted loss per share because they were anti-dilutive for the period ended January 31, 2016.
3.3 Taxation
Current income tax
Current income tax assets and liabilities for the current and prior periods are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted by the date of the statement of financial position.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
Deferred income tax
Deferred income tax is provided using the liability method on temporary differences at the date of the statement of financial position between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes.
Deferred income tax liabilities are recognized for all taxable temporary differences, except:
| · | where the deferred income tax liability arises from the initial recognition of goodwill or of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss; and |
| · | in respect of taxable temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, where the timing of the reversal of the temporary differences can be controlled and it is probable that the temporary differences will not reverse in the foreseeable future. |
Deferred income tax assets are recognized for all deductible temporary differences, carry forward of unused tax credits and unused tax losses, to the extent that it is probable that taxable profit will be available against which the deductible temporary differences and the carry forward of unused tax credits and unused tax losses can be utilized except:
| · | where the deferred income tax asset relating to the deductible temporary difference arises from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss; and |
| · | in respect of deductible temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, deferred income tax assets are recognized only to the extent that it is probable that the temporary differences will reverse in the foreseeable future and taxable profit will be available against which the temporary differences can be utilized. |
The carrying amount of deferred income tax assets is reviewed at each date of the statement of financial position and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized. Unrecognized deferred income tax assets are reassessed at each date of the statement of financial position and are recognized to the extent that it has become probable that future taxable profit will allow the deferred tax asset to be recovered.
Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the date of the statement of financial position.
Deferred income tax relating to items recognized directly in equity is recognized in equity and not in the statement of comprehensive loss.
3.4 Financial assets
All financial assets are initially recorded at fair value and designated upon inception into one of the following four categories: held to maturity, available for sale, loans and receivables or at fair value through profit or loss ("FVTPL").
Financial assets classified as FVTPL are measured at fair value with unrealized gains and losses recognized through earnings. The Company's cash is classified as FVTPL.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
3.4 Financial assets (continued)
Financial assets classified as loans and receivables and held to maturity are measured at amortized cost using the effective interest rate method. The Company's amounts due from Palo Verde LLC and other receivables except for HST receivable are classified as loans and receivables.
Financial assets classified as available for sale are measured at fair value with unrealized gains and losses recognized in other comprehensive loss except for losses in value that are considered other than temporary. At January 31, 2016, the Company has not classified any financial assets as available for sale.
Transactions costs associated with FVTPL financial assets are expensed as incurred, while transaction costs associated with all other financial assets are included in the initial carrying amount of the asset.
3.5 Financial liabilities
All financial liabilities are initially recorded at fair value and designated upon inception as FVTPL or other financial liabilities.
Financial liabilities classified as other financial liabilities are initially recognized at fair value less directly attributable transaction costs. After initial recognition, other financial liabilities are subsequently measured at amortized cost using the effective interest method. The effective interest method is a method of calculating the amortized cost of a financial liability and of allocating interest expense over the relevant period. The effective interest rate is the rate that exactly discounts estimated future cash payments through the expected life of the financial liability, or, where appropriate, a shorter period. The Company's accounts payable and accrued liabilities, and convertible debentures are classified as other financial liabilities.
Financial liabilities classified at FVTPL include financial liabilities held for trading and financial liabilities designated upon initial recognition at FVTPL. Derivatives, including separated embedded derivatives are classified as held for trading unless they are designated as effective hedging instruments. Fair value changes on financial liabilities classified at FVTPL are recognized through the statement of comprehensive loss. At January 31, 2016, the Company has classified the conversion feature of the convertible debentures as financial liabilities at FVTPL.
3.6 Impairment of financial assets
The Company assesses at each date of the statement of financial position whether a financial asset is impaired. Management has concluded that additional impairment for amounts due from Palo Verde LLC are recognized as at January 31, 2016 (Note 6).
Assets carried at amortized cost
If there is objective evidence that an impairment loss on assets carried at amortized cost has been incurred, the amount of the loss is measured as the difference between the asset's carrying amount and the present value of estimated future cash flows discounted at the financial asset's original effective interest rate. The carrying amount of the asset is then reduced by the amount of the impairment. The amount of the loss is recognized in profit or loss.
If, in a subsequent period, the amount of the impairment loss decreases and the decrease can be related objectively to an event occurring after the impairment was recognized, the previously recognized impairment loss is reversed to the extent that the carrying value of the asset does not exceed what the amortized cost would have been had the impairment not been recognized. Any subsequent reversal of an impairment loss is recognized in profit or loss.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
3. Summary of Significant Accounting Policies (continued)
Available-for-sale
If an available for sale asset is impaired, an amount comprising the difference between its cost and its current fair value, less any impairment loss previously recognized in profit or loss, is transferred from equity to profit or loss. Reversals in respect of equity instruments classified as available for sale are not recognized in profit or loss.
3.7 Provisions
Provisions are recognized when the Company has a present obligation (legal or constructive) that has arisen as a result of a past event and it is probable that a future outflow of resources will be required to settle the obligation, provided that a reliable estimate can be made of the amount of the obligation.
Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax rate that reflects current market assessments of the time value of money and the risk specific to the obligation. The increase in the provision due to passage of time is recognized as interest expense.
3.8 Related party transactions
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Parties are also considered to be related if they are subject to common control or common significant influence, related parties may be individuals or corporate entities. A transaction is considered to be a related party transaction when there is a transfer of resources or obligations between related parties.
3.9 Leases
Leases are classified as finance leases whenever the terms of the lease transfer substantially all the risks and rewards of ownership to the lessee. All other leases are classified as operating leases.
The Company as lessor
Rental income from operating leases is recognized on a straight-line basis over the term of the relevant lease. Initial direct costs incurred in negotiating and arranging an operating lease are added to the carrying amount of the leased asset and recognized on a straight-line basis over the lease term.
3.10 Inventory
Inventory is valued at the lower of cost or net realizable value. Cost is calculated based on a first-in, first-out basis. Cost includes the acquisition cost in USD converted at the date of purchase and costs directly attributable to bringing the asset to the location and condition necessary for distribution to customers. Net realizable value is the estimated selling price, in the ordinary course of business, less appropriate selling and distribution expenses.
When inventory is sold, the carrying amount of the inventory is recognized as an expense in cost of goods sold in the period in which the related revenue is recognized.
3.11 Intangibles
Intangibles arise on the purchase of a license, which is accounted for using the cost model whereby capitalized costs are amortized on a straight-line basis over their estimated useful lives, as these
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
3. Summary of Significant Accounting Policies (continued)
3.11 Intangibles (continued)
assets are considered finite. Useful lives are reviewed annually and intangibles are subject to impairment testing.
3.12 Investment property
Investment property earns rental income and is not for sale in the ordinary course of business, is not used in the production or supply of goods or services or for administrative purposes. Investment property is carried at historical cost less any accumulated depreciation and impairment losses.
Amortization is computed using the declining balance methods based on the estimated useful life of the assets. Useful life is reviewed at the end of each reporting period. Amortization is provided at rates as follows:
Building 4% Declining balance
3.13 Impairment of non-financial assets
At each date of the statement of financial position, the Company reviews the carrying amounts of its tangible and intangible assets to determine whether there is an indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss (if any). Where it is not possible to estimate the recoverable amount of an individual asset, the Company estimates the recoverable amount of the cash generating unit to which the assets belong.
Recoverable amount is the higher of fair value less costs of disposal and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
If the recoverable amount of an asset (or cash generating unit) is estimated to be less than its carrying amount, the carrying amount of the asset (or cash generating unit) is reduced to its recoverable amount. An impairment loss is recognized immediately in the statement of comprehensive loss.
For assets excluding goodwill, an assessment is made at each reporting date as to whether there is any indication that previously recognised impairment losses may no longer exist or may have decreased. If such indication exists, the Company estimates the asset's or cash-generating unit's recoverable amount. A previously recognised impairment loss is reversed only if there has been a change in the assumptions used to determine the asset's recoverable amount since the last impairment loss was recognised. The reversal is limited so that the carrying amount of the asset does not exceed its recoverable amount, nor exceed the carrying amount that would have been determined, net of depreciation, had no impairment loss been recognised for the asset in prior years. Such reversal is recognised in profit or loss and the carrying amount of the asset (cash-generating unit) is increased to the revised estimate of its recoverable amount.
3.14 Share issuance costs
Costs incurred in connection with the issuance of share capital are netted against the proceeds received net of tax. Costs related to the issuance of share capital and incurred prior to issuance are recorded as deferred share issuance costs and subsequently netted against proceeds when they are received.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
3. Summary of Significant Accounting Policies (continued)
3.15 Share capital
In situations where the Company issues units, the value of warrants is bifurcated and is included as the separate reserve of the Company's equity.
3.16 Convertible debentures
The proceeds received on issue of the Company's convertible debentures have been recorded as a liability on the consolidated statement of financial position. The convertible debentures contain an embedded derivative. The Company has designated the derivative as a financial liability at fair value through profit or loss. The Company revalues the convertible debenture derivative liability using recent arm's length market transactions and option pricing models at each reporting period.
3.17 Significant accounting judgments and estimates
The preparation of these unaudited condensed interim consolidated financial statements requires management to make judgments and estimates and form assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. On an ongoing basis, management evaluates its judgments and estimates in relation to assets, liabilities, revenue and expenses. Management uses historical experience and various other factors it believes to be reasonable under the given circumstances as the basis for its judgments and estimates. Actual outcomes may differ from these estimates under different assumptions and conditions. The most significant estimates relate to recoverability of loan receivable and rent receivable (Note 5), valuation of deferred income tax amounts, and valuation of warrants and shares issued during private placements and measurement of derivative liability.
The most significant judgments relate to recognition of deferred tax assets and liabilities, assessment of functional currency and determination of derivative liability of convertible debt.
3.18 Foreign currency translation
Monetary assets and liabilities denominated in currencies other than Canadian dollars are translated into Canadian dollars at the rate of exchange in effect at the statement of financial position date. Non-monetary assets and liabilities are translated at the historical rates. Revenues and expenses are translated at the transaction exchange rate. Foreign currency gains and losses resulting from translation are reflected in net comprehensive loss for the period.
The assets and liabilities of entities with a functional currency that differs from the presentation currency are translated to the presentation currency as follows:
| · | Assets and liabilities are translated at the closing rate at the financial period end; |
| · | Income and expenses are translated at average exchange rates (unless the average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case, income and expenses are translated at the rate on the dates of the transactions); |
| · | Equity transactions are translated using the exchange rate at the date of the transaction; and |
| · | All resulting exchange differences are recognized as a separate component of equity as reserve for foreign exchange. |
When a foreign operation is disposed of, the relevant amount in the reserve for foreign exchange in other comprehensive income is transferred to profit or loss as part of the profit or loss on disposal.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
3. Summary of Significant Accounting Policies (continued)
3.18 Foreign currency translation (continued)
On the partial disposal of a subsidiary that includes a foreign operation, the relevant proportion of such cumulative amount is reattributed to non-controlling interest. In any other partial disposal of a foreign operation, the relevant proportion is reclassified to profit or loss.
Foreign exchange gains or losses arising from a monetary item receivable from or payable to a foreign operation, the settlement of which is neither planned nor likely to occur in the foreseeable future, and which in substance, is considered to form part of the net investment in the foreign operation, are recognized in the reserve for foreign exchange.
4. HST receivable
HST consists of harmonized sales tax ("HST") due from the Canadian government.
5. Inventory
Inventory consists of gel caps, pushcaps and labels. All inventories are valued at the lower of cost and net realizable value.
6. Amounts due from Palo Verde LLC
The Company has lent Palo Verde LLC ("Palo") monies pursuant to a credit agreement and has leased property to Palo pursuant to two lease agreements, as disclosed below. The Company intends on entering into a branding arrangement with Palo in respect of its licensing arrangement (note 19).
| | | | | | | |
| | | January 31, 2016 | | | July 31, 2015 | |
Rental income receivable(i) | | $ | 451,694 | | | $ | 183,000 | |
Loan receivable(ii) | | | 151,433 | | | | 105,424 | |
| Interest receivable | | | 37,317 | | | | 9,418 | |
| | | | 640,444 | | | | 297,842 | |
Impairment on amounts receivable(iii) | | | (210,000 | ) | | | (100,000 | ) |
| | | $ | 430,444 | | | $ | 197,842 | |
| (i) | Rental income receivable is on the investment property in Colorado. Rent is deferred for the first 6, 9 and 9 months of the three respective leases, two leases commencing January 1, 2015 and the third on September 1, 2015 and accrues interest at 12% per annum to be paid no later than June 30, 2017 and September 30, 2017. |
| (ii) | Revolving line of credit of USD $150,000 to Palo Verde LLC, of which $151,433 (USD $108,120) (July 31, 2015 – USD $80,600) and accrued interest of $37,317 (USD $26,644) (July 31, 2015 – USD $5,717) was receivable as at January 31, 2016. Advances through a promissory note are unsecured; bear interest at 12% per annum which are due on September 30, 2016. Accrued interest is payable in three monthly installments on September 30, 2016, October 31, 2016 and November 30, 2016. Palo Verde may extend the maturity date for up to an additional four successive one-year terms for a total of five years, but no later than July 22, 2020 for a fee equal to 1% of the outstanding revolving credit loan. |
Nutritional High International Inc .
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
6. Amounts due from Palo Verde LLC
| (iii) | An allowance was recorded in July 31, 2015 on account of the uncertainties surrounding recoverability of the loan and rents receivable in respect of timing and unexpected financing delays. An additional allowance of $110,000 was recorded in the six month period ended January 31, 2016. |
7. Investment property
Cost | | | |
| Balance at July 31, 2014 | $ - | $ - | $ - |
| Additions | 141,200 | 858,496 | 999,696 |
| Effect of movement in exchange rates | 22,300 | 135,584 | 157,884 |
| Balance at July 31, 2015 | 163,500 | 994,080 | 1,157,580 |
| Additions | 62,479 | 446,723 | 509,202 |
| Effect of movement in exchange rates | 11,575 | 70,376 | 81,951 |
| Balance at January 31, 2016 | 237,554 | 1,511,179 | 1,748,733 |
Accumulated Amortization | | | |
| Balance at July 31, 2014 | - | - | - |
| Amortization for the year | - | 25,523 | 25,523 |
| Effect of movement in exchange rates | - | 2,475 | 2,475 |
| Balance at July 31, 2015 | - | 27,998 | 27,998 |
| Amortization for the period | - | 20,609 | 20,609 |
| Effect of movement in exchange rates | - | 2,837 | 2,837 |
| Balance at January 31, 2016 | - | 51,444 | 51,444 |
Carrying Amounts | | | |
| Balance at July 31, 2014 | - | - | - |
| Balance at July 31, 2015 | 163,500 | 966,082 | 1,129,582 |
| Balance at January 31, 2016 | $ 237,554 | $ 1,459,735 | $ 1,697,289 |
The fair value of the investment property as at January 31, 2016 and July 31, 2015 approximates the carrying value as the investment properties were acquired during the year ended July 31, 2015 and period ended January 31, 2016. The direct operating expenses arising from investment property that generated rental income were USD $85,622 for the period ended January 31, 2016.
8. Property Deposits
The Company through its owned subsidiary NH Medicinal (Minnesota) Inc. made a refundable deposit of $7,003 (USD $5,000) on the purchase of the commercial property located in Oregon, USA, with a remaining commitment of USD $394,000 upon closing. The settlement date is to be within 30 days after the Company receives a processors license.
The Company through its subsidiary NH Medicinals (Minnesota) Inc. made a refundable deposit of $7,003 (USD $5,000) on the purchase of the commercial property located in Maryland, USA. The acquisition price for the property is USD $350,000. The settlement date is to be within 45 days after the Company receives Stage 1 license approval, but in no event, later than 365 days after the execution date of this contract.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
9. Accounts Payable and Accrued Liabilities
Accounts payable and accrued liabilities of the Company are principally comprised of amounts outstanding for trade purchases relating to regular business activities and amounts payable for financing activities. The usual credit period taken for purchases is between 30 to 90 days.
The following is an aged analysis of accounts payable and accrued liabilities:
| | | January 31, | | | July 31, | |
| | | 2016 | | | 2015 | |
| | | | | | | |
| Less than 1 month | | $ | 154,400 | | | $ | 97,370 | |
| Over 1 month | | | 393,674 | | | | 174,904 | |
Total accounts payable and accrued liabilities | | $ | 548,074 | | | $ | 272,274 | |
10. Promissory Note Payable
On November 4, 2015, the vendor of the real estate property in Illinois where the Company's dispensary will be located provided a buyer take-back mortgage in the amount of USD $250,000. The mortgage has a 15 year amortization period, bearing an interest at the rate of 6%, payable USD $2,110 monthly including interest and be due in two years from the date of issuance as a balloon payment.
| | | January 31, 2016 | | | July 31, 2015 | |
| Opening balance | | $ | - | | | $ | - | |
| Advance | | | 350,150 | | | | - | |
| Repayments | | | (2,415 | ) | | | - | |
| | | | 347,735 | | | | - | |
| Current | | | 13,716 | | | | - | |
| Long-term | | $ | 334,019 | | | $ | - | |
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
11. Related Parties and Key Management
Key management includes the Company's directors, officers and any employees with authority and responsibility for planning, directing and controlling the activities of an entity, directly or indirectly.
For the six month period ended January 31, 2016, the Company incurred professional fees of $36,000 (2015 - $26,000) from Branson Corporate Services, a company in which a company with a related director has a 49% interest.
For the six month period ended January 31, 2016, the Company incurred consulting fees of $96,000 (2015 - $48,000) from FMI Capital Advisory Inc. (formerly Foundation Opportunities Inc.), a company with a related director.
For the six month period ended January 31, 2016, the Company incurred professional fees of $16,089 (2015 - $276,771) from Fogler Rubinoff, LLP, a law firm in which a director of the company is a partner.
Total key management compensation paid to the Chief Executive Officer, the Chief Operating Officer, the VP Product Development, and a Director amounted to $137,713 (2015 - $86,629) for the six month period ended January 31, 2016.
These expenses have been measured at their exchange amount, being the amounts negotiated and agreed to by the parties to the transactions. As at January 31, 2016, $337,704 (July 31, 2015 - $85,463) is included in accounts payable and accrued liabilities.
The Company is authorized to issue an unlimited number of common shares without par value.
On October 8, 2014, the Company closed a subscription for 4,000,000 units at $0.025 per unit for gross proceeds of $100,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.05 per share until the earlier of 36 months from the date of issuance or 18 months following the date of a business combination between the Company and a public company pursuant to a reverse take-over, merger, amalgamation, take-over bid, insider bid, reorganization, joint venture, sale or exchange of assets or similar transaction or IPO. In connection with the subscription, the Company paid a finder's fee of
$8,000 and issued an aggregate of 320,000 finder's warrants (Note 11), exercisable into one common share at a price of $0.025 per share until 18 months from the closing date.
On October 31, 2014, 3,566,638 warrants were exercised at $0.05 per warrant for gross proceeds of $178,332. An additional $17,467 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share purchase warrants have been amended to include an early exercise provision of an additional warrant exercisable into one common share at a price of $0.10 per share until 24 months from the date of issuance. As a result of the amendment, an
additional 3,566,638 warrants were issued, as described in Note 14. The terms of any unexercised Company warrants outstanding at October 31, 2014 remain unchanged.
On March 13, 2015, 32,900,000 units at $0.05 per unit were issued for gross proceeds of $1,645,000 pursuant to the IPO. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance.
On March 13, 2015, the Company issued 2,404,800 compensation options under a warrant indenture valued at $63,000 based on the services provided. Each option is exercisable at $0.05 per unit, and comprised of one common share and one half of one share purchase warrant, exercisable into one additional common share at a price of $0.07 per share until 24 months from the date of issuance.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
12. Share Capital (continued)
On March 18, 2015, the Company paid an extension fee of $30,000 by issuing an aggregate of 600,000 units and one half of one share purchase warrant, exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance (Note 16).
On March 18, 2015, the Company paid a going public success fee of $35,000 by issuing an aggregate of 700,000 units and one half of one share purchase warrant, exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance to FMI Capital Advisory (formerly Foundation Opportunities Inc.), a company with a related director.
On March 26, 2015, 400,000 warrants were exercised for gross proceeds of $28,000. An additional $8,827 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share price on the date of exercise was $0.11.
On March 27, 2015, 400,000 warrants were exercised for gross proceeds of $28,000. An additional $8,827 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share price on the date of exercise was $0.11.
On March 30, 2015, 12,000 compensation options under the warrant indenture were exercised for $600 for 12,000 common shares and 6,000 share purchase warrants, exercisable at a price of $0.07 per share until March 16, 2017. An additional $314 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On April 7, 2015, 160,000 warrants were exercised for $4,000 for 160,000 common shares and 80,000 share purchase warrants, exercisable at a price of $0.05 per share until April 8, 2016. An additional $2,000 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share price on the date of exercise was $0.095.
On April 8, 2015, 6,000 warrants were exercised for gross proceeds of $420. An additional $132 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share price on the date of exercise was $0.095.
On April 20, 2015, 500,000 warrants were exercised for gross proceeds of $35,000. An additional $11,034 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share price on the date of exercise was $0.10.
On June 5, 2015, the Company issued 3,333,334 shares valued at $250,000 to satisfy the initial licensing and royalty commitment as described in Note 19.
On June 6, 2015, the Company issued 66,667 shares valued at $5,000 in exchange for 2% interest in NH Medicinal Dispensaries Inc. as described in Note 15.
On June 7, 2015, the Company issued 700,000 shares valued at $52,500 as compensation for services where the fair value of shares was determined based on the value of services received.
On July 31, 2015, the Company issued 111,111 shares valued at $5,000 as compensation for services where the fair value of shares was determined based on the value of services received.
Cash costs in connection with the transactions amounted to $239,194.
On August 20, 2015, the Company issued 692,431 shares valued at $51,932 as the final instalment on its initial licensing obligations, as described in Note 19.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
12. Share Capital (continued)
On September 7, 2015, the Company issued 406,668 shares valued at $20,333 as compensation for services where the fair value of shares was determined based on the value of services received.
On October 5, 2015, 8,000 warrants were exercised for gross proceeds of $400. An additional $39 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share price on the date of exercise was $0.05.
On October 23, 2015, a holder of the convertible debentures (Note 16) converted $180,000 in convertible debentures into 3,000,000 common shares of the Company at a share price of $0.06 per share.
On October 26, 2015, the Company issued 50,000 shares valued at $2,500 as compensation for services where the fair value of shares was determined based on the value of services received.
On November 5, 2015, a holder of the convertible debentures (Note 16) converted $100,000 in convertible debentures into 1,666,667 common shares of the Company at a share price of $0.06 per share.
On November 5, 2015, 1,600,000 warrants were exercised for gross proceeds of $80,000. The share price on the date of exercise was $0.05.
On November 12, 2015, a holder of the convertible debentures (Note 16) converted $70,000 in convertible debentures into 1,166,667 common shares of the Company at a share price of $0.06 per share.
On November 17, 2015, 1,000,000 warrants were exercised for gross proceeds of $50,000. The share price on the date of exercise was $0.05.
On December 2, 2015, the Company completed a non-brokered private placement of 4,200,000 units at $0.05 per unit for gross proceeds of $210,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
On January 28, 2016, the Company has issued 2,822,700 shares to settle $141,135.00 of debt, where the fair value of shares was determined based on share price at the date of issuance.
On January 31, 2016, the Company completed a non-brokered private placement of 800,000 units at $0.05 per unit for gross proceeds of $40,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
13. Reserve for Share Based Payments
The Company established a stock option plan to provide additional incentive to its officers, directors, employees and consultants in their effort on behalf of the Company in the conduct of its affairs. Options vest immediately, unless otherwise stated, and expire on the fifth anniversary from the date of issue unless otherwise specified. The maximum number of common shares reserved for issuance for options that may be granted under the Plan is 10% of the total issued and outstanding Common shares, which was 13,678,251 at January 31, 2016.
The following table reflects the continuity of options for the period ended January 31, 2016:
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
13. Reserve for Share Based Payments (continued)
| | | Number of Options | | | Amount | |
| Balance – July 31, 2014 | | | 2,800,000 | | | $ | 28,000 | |
| Granted | | | 8,400,000 | | | | 286,000 | |
| Expired | | | (200,000 | ) | | | - | |
| Balance – July 31, 2015 | | | 11,000,000 | | | | 314,000 | |
| Granted | | | 1,600,000 | | | | 34,000 | |
| Expired | | | (1,300,000 | ) | | | - | |
| Balance – January 31, 2016 | | | 11,300,000 | | | $ | 348,000 | |
During the period ended January 31, 2016, the Company granted 1,600,000 stock options to an officer to purchase common shares of the Company at the exercise price of $0.075 exercisable until
60 months from the date of issuance, for a stock option expense of $17,000. These stock options are vesting quarterly in equal amounts over 3 years. A stock option expense of $17,000 was recorded in relation to the vesting of the options issued through July 31.
During the year ended July 31, 2015, the Company granted 8,400,000 stock options to certain officers, directors, consultants and advisory board members to purchase common shares of the Company at the exercise price of $0.10 exercisable until 60 months from the date of issuance.
Vesting periods on the options granted during the year ended July 31, 2015 are as follows: 7,050,000 stock options vested immediately upon issuance, 300,000 stock options issued on March 18, 2015, 50% of which vested immediately and the remaining 50% vest monthly over 6 months, 2,000,000 stock options issued on March 18, 2015, which vest in stages over a minimum of 12 months with no more than ¼ of options vesting any three month period, and the remaining vest quarterly over next 24 months.
The estimated fair value of share based compensation during the period ended January 31, 2016 was determined using the Black-Scholes option pricing model with the following assumptions:
| | | | | | | |
| | | | | | | | |
| | | September 16, 2015 | | | December 21, 2015 | | Total |
| Number of options | | | 1,100,000 | | | | 500,000 | | |
| Share price | | $ | 0.030 | | | $ | 0.045 | | |
| Risk-free interest rate | | | 0.81 | % | | | 0.49 | % | |
| Expected life of options | | 5 years | | | 5 years | | |
| Expected volatility | | | 202 | % | | | 201 | % | |
| Expected dividend yield | | | 0 | % | | | 0 | % | |
| Fair value | | $ | 32,000 | | | $ | 22,000 | | $54,000 |
The estimated fair value of share based compensation during the year ended July 31, 2015 was determined using the Black-Scholes option pricing model with the following assumptions:
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | October 10, 2014 | | | March 18, 2015 | | | April 1, 2015 | | | April 6, 2015 | | | June 10, 2015 | | | July 22, 2015 | | Total |
| Number of options | | | 400,000 | | | | 6,950,000 | | | | 250,000 | | | | 150,000 | | | | 400,000 | | | | 250,000 | | |
| Share price | | $ | 0.025 | | | $ | 0.050 | | | $ | 0.095 | | | $ | 0.110 | | | $ | 0.080 | | | $ | 0.050 | | |
| Risk-free interest rate | | | 1.56 | % | | | 0.75 | % | | | 0.98 | % | | | 0.98 | % | | | 0.95 | % | | | 0.83 | % | |
| Expected life of options | | 5 years | | | 5 years | | | 5 years | | | 5 years | | | 5 years | | | 5 years | | |
| Expected volatility | | | 100 | % | | | 100 | % | | | 209 | % | | | 209 | % | | | 209 | % | | | 209 | % | |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | |
| Fair value | | $ | 5,000 | | | $ | 199,000 | | | $ | 23,000 | | | $ | 16,000 | | | $ | 31,000 | | | $ | 12,000 | | $286,000 |
Option pricing models require the input of highly subjective assumptions including the expected price volatility. Changes in the subjective input assumptions can materially affect the fair value estimated,
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
13. Reserve for Share Based Payments (continued)
and therefore, the existing models do not necessarily provide a reliable measure of the fair value of the Company's stock options. Expected volatility is based on comparable companies.
The weighted average remaining contractual life for outstanding options is as follows:
| Exercise Price | | | Number of Options | | | Weighted Average Exercise Price | | | Weighted Average Remaining Life (years) | | | Number of Options - exercisable | |
| | | | | | | | | | | | | | |
| $ | 0.075 | | | | 1,600,000 | | | $ | 0.075 | | | | 4.69 | | | | 91,667 | |
| $ | 0.10 | | | | 9,700,000 | | | $ | 0.10 | | | | 3.98 | | | | 9,300,000 | |
| $ | 0.010 - $0.075 | | | | 11,300,000 | | | $ | 0.096 | | | | 4.08 | | | | 9,391,667 | |
14. Reserve for Warrants
The following table reflects the continuity of warrants for the period ended January 31, 2016:
| | | Number of Warrants | | | Amount | |
| Balance – July 31, 2014 | | | 13,650,006 | | | $ | 65,000 | |
| Warrants pursuant to private placement and option agreement (Note 12) | | | 5,966,638 | | | | 88,000 | |
| Warrants pursuant to IPO (Note 12) | | | 16,450,000 | | | | 363,000 | |
| Compensation warrants (Note 12) | | | 2,410,800 | | | | 64,000 | |
| Warrants issued for success and extension fees (Note 12) | | | 650,000 | | | | 35,000 | |
| Warrants exercised (Note 12) | | | (5,044,638 | ) | | | (48,601 | ) |
| Balance – July 31, 2015 | | | 34,082,806 | | | | 566,399 | |
| Warrants pursuant to private placement (Note 12) | | | 2,516,000 | | | | 113,000 | |
| Warrants pursuant to debenture agreement (Note 16) | | | 3,750,000 | | | | 138,000 | |
| Warrants exercised (Note 12) | | | (2,608,000 | ) | | | (12,779 | ) |
| Balance – January 31, 2016 | | | 37,740,806 | | | $ | 804,620 | |
During the period ended January 31, 2016, the Company issued warrants to purchase common shares, valued at $251,000 using the Black-Scholes option pricing model using the following assumptions:
| | | December 02, 2015 | | | December 02, 2015 | | | December 21, 2015 | | | January 31, 2016 | | Total |
| Number of warrants | | | 2,100,000 | | | | 16,000 | | | | 3,750,000 | | | | 400,000 | | |
| Share price | | $ | 0.055 | | | $ | 0.055 | | | $ | 0.045 | | | $ | 0.040 | | |
| Risk-free interest rate | | | 0.61 | % | | | 0.61 | % | | | 0.49 | % | | | 0.43 | % | |
| Expected life of warrants | | 2.5 years | | | 2.5 years | | | 2 years | | | 1.5 years | | |
| Expected volatility | | | 201 | % | | | 201 | % | | | 201 | % | | | 199 | % | |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | |
| Valued at | | $ | 101,000 | | | $ | 1,000 | | | $ | 138,000 | | | $ | 11,000 | | $251,000 |
During the year ended July 31, 2015, the Company issued warrants to purchase common shares, valued at $550,000 using the Black-Scholes option pricing model using the following assumptions:
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
14. Reserve for Warrants (continued)
| | | October 8, 2014 | | | October 31, 2014 | | | March 13, 2015 | | | March 18, 2015 | | | March 30, 2015 | | | April 7, 2015 | | Total |
| Number of warrants | | | 2,320,000 | | | | 3,566,638 | | | | 18,854,800 | | | | 650,000 | | | | 6,000 | | | | 80,000 | | |
| Share price | | $ | 0.025 | | | $ | 0.100 | | | $ | 0.050 | | | $ | 0.130 | | | $ | 0.110 | | | $ | 0.100 | | |
| Risk-free interest rate | | | 1.05 | % | | | 1.04 | % | | | 0.49 | % | | | 0.49 | % | | | 0.49 | % | | | 0.67 | % | |
| Expected life of warrants | | 1.5 years | | | 2 years | | | 2 years | | | 2 years | | | 2 years | | | 2 years | | |
| Expected volatility | | | 100 | % | | | 100 | % | | | 100 | % | | | 209 | % | | | 209 | % | | | 209 | % | |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | |
| Valued at | | $ | 18,000 | | | $ | 64,000 | | | $ | 426,000 | | | $ | 35,000 | | | $ | 1,000 | | | $ | 6,000 | | $550,000 |
Warrants to purchase common shares carry exercise prices and terms to maturity at January 31, 2016 as follows:
| | | | | | | | |
| Date of Issue | | No. of warrants | | | Exercise Price ($) | | Expiry Date |
| | | | | | | | |
| October 8, 2014 | | | 2,000,000 | | | | 0.050 | | April 7, 2016 |
| October 8, 2014 | | | 160,000 | | | | 0.025 | | April 7, 2016 |
| June 27, 2014 | | | 150,000 | | | | 0.100 | | June 26, 2016 |
| October 31, 2014 | | | 3,566,638 | | | | 0.100 | | October 31, 2016 |
| May 16, 2014 | | | 4,936,789 | | | | 0.050 | | March 27, 2016 |
| May 30, 2014 | | | 1,082,399 | | | | 0.050 | | March 27, 2016 |
| June 20, 2014 | | | 1,306,180 | | | | 0.050 | | March 27, 2016 |
| March 13, 2015 | | | 15,800,000 | | | | 0.070 | | March 12, 2017 |
| March 13, 2015 | | | 2,392,800 | | | | 0.050 | | March 12, 2017 |
| April 7, 2015 | | | 80,000 | | | | 0.050 | | April 6, 2017 |
| December 2, 2015 | | | 2,100,000 | | | | 0.070 | | June 2, 2018 |
| December 2, 2015 | | | 16,000 | | | | 0.050 | | June 2, 2018 |
| December 21, 2015 | | | 3,750,000 | | | | 0.060 | | December 21, 2017 |
| January 31, 2016 | | | 400,000 | | | | 0.070 | | July 31, 2017 |
| | | | 37,740,806 | | | | | | |
On December 21, 2015, the company has amended the exercise period of its share purchase warrants issued on June 27, 2014 from eighteen months to twenty one months. 5,563,348 Series I Warrants with expiry date of December 27, 2015 will now expire on expire on March 27, 2016. The Warrants exercise price of $0.05 per Common Share remains unchanged.
15. Non-controlling Interest
The Company's 51% interest in Eglinton Medicinal Advisory Ltd. is consolidated into the Company's consolidated financial statements. The 49% interest attributable to a minority shareholder is then presented as "non-controlling interest" within shareholders' equity on the consolidated statement of financial position. Net loss and comprehensive loss is allocated between the Company's 51% ownership and non-controlling 49% ownership interest. The Company recorded $153 of the subsidiary's net loss and comprehensive loss related to the non-controlling interest during the six month period ended January 31, 2016.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
16. Convertible Debentures
On November 17, 2014, the Company closed its non-brokered private placement of secured convertible debentures for total gross proceeds of $600,000 as follows:
| a. | Senior convertible debenture of $450,000, bearing interest at 12%, maturing in 24 months from |
16. Convertible Debentures (continued)
date of issue, and secured by a first ranking general security interest over all assets of the Company. The senior convertible debenture is convertible into common shares of the Company at any time prior to the maturity date at a price equal to a 20% premium to the price at which the Company completes its going public transaction or $0.06 per Company share ("Conversion Price"). If the Company fails to complete the going public transaction on or before January 31, 2015, the Conversion Price will be reduced to $0.05 per Company share. If the Company completes the going public transaction on or before January 31, 2015, but less than $1,000,000
is raised, the Conversion Price will be equal to the price at which the Company completes the
going public transaction ("Conversion Price Adjustment") and the Company will issue to the holder 450,000 Company shares immediately prior to closing the going public transition. On January 19, 2015, an amendment was made to the agreement to extend the going public date from January 31, 2015 to March 16, 2015, in consideration for $30,000, convertible into units at the offering price. On October 23, 2015, $180,000 of the debentures were converted into 3,000,000 common shares (Note 12). On November 5, 2015, an additional $100,000 of the debentures was converted into 1,666,667 common shares (Note 12). On November 12, 2015, an additional $70,000 of the debentures was converted into 1,166,667 common shares (Note 12). The amount of the debenture remaining is $100,000.
| b. | Subordinate convertible debenture of $150,000, bearing interest at 12%, maturing in 24 months from date of issue, and secured by a general security interest over all assets of the Company, subordinate to the senior convertible debenture. The group of lenders is comprised of directors of the Company. The subordinate convertible debenture carries the same Conversion Prices and Conversion Price Adjustment as the senior convertible debenture described above. On January 19, 2015, an amendment was made to the agreement to extend the going public date from January 31, 2015 to March 16, 2015. |
The debentures are classified as a liability at amortized cost for the host component and its embedded derivative was classified at fair value through profit and loss as the conversion feature of debentures failed equity classification. The fair value of the derivative was calculated using the Black-Scholes model with the following assumptions: (November 17, 2014 and July 31, 2015: share price: $.02 and $.045; risk free interest rate: 1%; expected life: 2 and 1.3 years; expected volatility 100% and 209%; dividend yield 0%). The assumptions used in the Black-Scholes model are based on management's estimate of the rates that are reflective for the Company. Changes in these estimates could result in significant changes in the fair value of the derivative. The discount is being accreted over the term of the debenture utilizing the effective interest rate method at a 5.22% discount rate.
A fair value adjustment loss on the convertible debentures for the period ended January 31, 2016, of $11,288 (July 31, 2015: $277,753) has been reflected in the consolidated statement of comprehensive loss as a change in the fair value of the derivative liability. The fair value of the derivative liability at the inception was $52,312. The interest and accretion expense in the amount of $25,677 and $22,025 are recorded as a finance cost.
On December 21, 2015, the Company has entered into an amending agreement with the holder of Senior Convertible Debenture ("Holder") to provide for the prepayment. The Company has agreed to issue 3,750,000 Common Share purchase warrants ("Warrants") to the holder of the Secured Convertible Debenture immediately, and pay a fee in the amount of $27,000 if the Company elects to proceed with pre-payment. Each Warrant entitles the Holder to purchase one Common Share at an exercise price of $0.06 per Common Share for a period of two years from the date of the Warrant.
The debt issue costs in the amount of $30,000 were recorded against the debentures liability and amortized using the effective interest method. As at January 31, 2016, $13,909 is recorded as accrued interest in accounts payable and accrued liabilities.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
17. Management of Capital
The Company manages its capital structure and makes adjustments to it, based on the funds available to the Company, in order to support the development of its planned business activities. The Board of Directors does not establish quantitative return on capital criteria for management, but rather relies on the expertise of the Company's management to sustain future development of the business. In order to carry out the planned business activities and pay for administrative costs, the Company will spend its existing working capital and raise additional funds as needed. Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable. There were no changes in the Company's approach to capital management during the period ended January 31, 2016. The Company is not subject to externally imposed capital requirements.
The Company considers its capital to be shareholders' equity, which is comprised of share capital, reserve for warrants, share based payments and deficit, which as at January 31, 2016 totaled $1,447,731.
The Company's objective when managing capital is to obtain adequate levels of funding to support its business activities, to obtain corporate and administrative functions necessary to support organizational functioning and obtain sufficient funding to further the development of its business. The Company raises capital, as necessary, to meet its needs and take advantage of perceived opportunities and, therefore, does not have a numeric target for its capital structure. Funds are primarily secured through equity capital raised by way of private placements, initial public offering and issuance of convertible debentures. There can be no assurance that the Company will be able to continue raising equity capital in this manner.
18. Financial Instruments
Fair Value of Financial Instruments
The fair value hierarchy that reflects the significance of inputs used in making fair value measurements as follows:
Level 1 quoted prices in active markets for identical assets or liabilities;
| Level 2 | inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. from derived prices); and |
Level 3 inputs for the asset or liability that are not based upon observable market data.
Assets are classified in their entirety based on the lowest level of input that is significant to the fair value measurement.
The Company designated its cash as fair value through profit and loss, which is measured at fair value and is classified as Level 1. The Company designated its derivative liability from convertible debentures as fair value through profit and loss which is measured at fair value and classified as level 2.
The carrying value of the Company's other receivable, amounts due from Palo Verde LLC and accounts payable and accrued liabilities and convertible debentures (except for derivative liability which is recorded at fair value) approximate their fair value due to the relatively short periods to maturity of these instruments.
Fair value estimates are made at a specific point in time, based on relevant market information and information about financial instruments. These estimates are subject to and involve uncertainties and matters of significant judgment, therefore cannot be determined with precision. Changes in assumptions could significantly affect the estimates.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
18. Financial Instruments
Fair Value of Financial Instruments (continued)
A summary of the Company's risk exposures as it relates to financial instruments are reflected below:
Credit risk
Credit risk is the risk of loss associated with counterparty's inability to fulfill its payment obligations. The Company's credit risk is primarily attributable to cash and amounts due from Palo Verde LLC and rent receivable (Note 6). The Company has no significant concentration of credit risk arising from operations. Cash is held with a reputable Canadian chartered bank which is closely monitored by management. The Board of Directors meets on a quarterly basis to review and assess the risk profile of the loan. Management believes that the credit risk concentration with respect to financial instruments included in cash and other receivable is not material for the Company.
Liquidity risk
Liquidity risk is the risk that the Company will not have sufficient cash resources to meet its financial obligations as they come due. The Company's liquidity and operating results may be adversely affected if the Company's access to the capital market is hindered, whether as a result of a downturn in stock market conditions generally or related to matters specific to the Company. The Company generates cash flow primarily from its financing activities. The Company's approach to managing liquidity risk is to ensure that it will have sufficient liquidity to meet liabilities when due. As at January 31, 2016, the Company had current assets of $233,802 (July 31, 2015 - $388,713) and current liabilities of $561,790 (July 31, 2015 - $272,274). All of the Company's financial liabilities and receivables, excluding a loan receivable (Note 6), have contractual maturities of less than 90 days and are subject to normal trade terms. As at January 31, 2016, working capital (deficiency) of the Company is $(327,988) (July 31, 2015 - $116,439).
Foreign currency exchange risk
The Company conducts a portion of its purchases in US dollars which results in the foreign currency exchange risk. The Company does not consider its exposure to foreign currency exchange risk to be material.
An increase (decrease) of 10% in the currency exchange rate of the Canadian dollar versus US dollar would have impacted net loss by $2,491 ($2,491) as a result of the Company's exposure to currency exchange rate fluctuations.
Interest rate risk
Interest rate risk is the potential for financial loss arising from changes in interest rates. Financial instruments that potentially subject the Company to interest rate risk include financial liabilities with fixed interest rates.
The Company manages interest rate risk by monitoring market conditions and the impact of interest rate fluctuations on its debt.
Net earnings are sensitive to the impact of a change in interest rates on the average balance of interest bearing financial liabilities during the year.
An increase (decrease) of 25 basis points would have impacted net loss by $2,595 ($2,595) as a result of the Company's exposure to interest rate fluctuations.
Nutritional High International Inc.
Notes to the unaudited condensed interim consolidated Financial Statements
For the three and six month period ended January 31, 2016
(expressed in Canadian Dollars)
19. Licensing and Royalty Agreement
On June 5, 2015, the Company entered into the agreement with Purple Haze Properties LLC for the exclusive right to manufacture and distribute marijuana and hemp oil-infused products, and non-exclusive rights to manufacture and distribute certain apparel and accessories in the United States and Canada.
Under the terms of the agreement, the Company is to issue 3,333,334 common shares (Note 12) valued at USD$250,000 to pay for the annual exclusivity fee (USD $200,000) for the first out of five years and royalties USD $50,000 which was due on signing the agreement. The agreement provides for annual exclusivity fees and royalties of no less than USD $1,000,000 over five years with an additional renewal option for an additional five years. The agreement term commenced on October 1, 2015, with minimums payable in cash or shares at the Company's option.
20. Segment Information
The Company has one reportable segment which is Marijuana-Infused Products. The segment reflects the basis on which management measures performance and makes decisions regarding the allocation of resources.
21. Subsequent events
On January 6, 2016, the Company entered into an equity purchase agreement ("EPA") with Kodiak Capital Group LLC ("Kodiak"), pursuant to which Kodiak has agreed to purchase up to USD $1 million of Nutritional High's common shares. A Form F-1 Registration Statement was filed with the United States Securities Commission ("SEC") as a condition of the $1 million financing.
On February 5, 2016, the Company was granted conditional approval by the Illinois Department of Financial and Professional Regulation to establish the Lawrenceville, Illinois Dispensary.
On February 11, 2016, the Company entered into a joint venture agreement with an Illinois investor group to build and operate the planned Lawrenceville dispensary. Under the terms of the joint venture agreement, the investor group will fund up to USD $300,000 for expenses and working capital, and provide a guarantee for half of the USD $250,000 Lawrenceville mortgage, in exchange for a 50% interest in NH Medicinals Dispensaries Inc., the wholly owned subsidiary which holds the conditional approval to operate the dispensary, and a 50% interest in Small's Mill Holdings Inc., the wholly owned subsidiary which holds the Company's interest in the real estate property located in Lawrenceville, Illinois. The joint venture agreement is subject to a final agreement that would be subject to all requisite regulatory approvals from the relevant government authorities.
On March 18, 2016, the Company amended the terms of its agreement with Purple Haze Properties LLC to provide for early payment of the 2016 exclusivity fee and advance royalty in exchange for 5,000,000 Common shares of the Company at an effective price of USD $0.05 per share rather than the market price as originally agreed.
Nutritional High International Inc.
Consolidated Financial Statements
For the years ended July 31, 2015 and 2014
MANAGEMENT'S RESPONSIBILITY FOR FINANCIAL REPORTING
The accompanying consolidated financial statements of Nutritional High International Inc., are the responsibility of the management and Board of Directors of the Company.
The consolidated financial statements have been prepared by management, on behalf of the Board of Directors, in accordance with the accounting policies disclosed in the notes to the consolidated financial statements. Where necessary, management has made informed judgments and estimates in accounting for transactions which were not complete at the consolidated statement of financial position date. In the opinion of management, the consolidated financial statements have been prepared within acceptable limits of materiality and are in accordance with International Financial Reporting Standards using accounting policies consistent with International Financial Reporting Standards appropriate in the circumstances.
Management has established systems of internal control over the financial reporting process, which are designed to provide reasonable assurance that relevant and reliable financial information is produced.
The Board of Directors is responsible for reviewing and approving the consolidated financial statements together with other financial information of the Company and for ensuring that management fulfills its financial reporting responsibilities.
Management recognizes its responsibility for conducting the Company's affairs in compliance with established financial standards, and applicable laws and regulations, and for maintaining proper standards of conduct for its activities.
"David Posner" , Director and CEO "Al Quong" , CFO
David Posner Al Quong
| Collins Barrow Toronto LLP Collins Barrow Place 11 King Street West Suite 700, Box 27 Toronto, Ontario M5H 4C7 Canada T. 416.480.0160 F. 416.480.2646 www.collinsbarrow.com |
INDEPENDENT AUDITORS' REPORT
To the Directors of Nutritional High International Inc.
We have audited the accompanying consolidated financial statements of Nutritional High International Inc. and its subsidiaries, which comprise the consolidated statements of financial position as at July 31, 2015 and July 31, 2014, and the consolidated statements of loss and comprehensive loss, changes in shareholders' equity and cash flows for the year ended July 31, 2015 and for the period from incorporation, April 17, 2014 to July 31, 2014 and a summary of significant accounting policies and other explanatory information.
Management's Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board, and for such internal control as management determines is necessary to enable the preparation of the consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditors' Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor's judgement, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity's preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity's internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Nutritional High International Inc. and its subsidiaries, as at July 31, 2015 and July 31, 2014, and its financial performance and its cash flows for the year ended July 31, 2015 and for the period from incorporation, April 17, 2014 to July 31, 2014 in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Emphasis of Matter
Without qualifying our opinion, we draw attention to Note 1 in the consolidated financial statements which describes the material uncertainties that cast significant doubt about the Company's ability to continue as a going concern.
This office is independently owned and operated by Collins Barrow Toronto LLP

The Collins Barrow trademarks are used under License.
Chartered Professional Accountants
Licensed Public Accountants
Toronto, Canada
March 11, 2016
| | | | | | | |
| As at July 31, | | 2015 | | | 2014 | |
| Assets | | | | | | |
| Current | | | | | | |
| Cash | | $ | 19,567 | | | $ | 617,066 | |
HST receivable (Note 4) | | | 121,572 | | | | 23,747 | |
| Other receivable | | | 9,459 | | | | - | |
Prepaids (Note 19) | | | 238,115 | | | | - | |
| | | | 388,713 | | | | 673,525 | |
| Non-current assets | | | | | | | | |
Amounts due from Palo Verde LLC (Note 5) | | | 197,842 | | | | 32,712 | |
Prepaid license (Note 19) | | | 249,780 | | | | - | |
Investment property (Note 6) | | | 1,129,582 | | | | - | |
Property deposit (Note 7) | | | 6,671 | | | | 21,952 | |
| | | $ | 1,972,588 | | | $ | 695,477 | |
| | | | | | | | | |
| Liabilities | | | | | | | | |
| Current | | | | | | | | |
Accounts payable and accrued liabilities (Notes 8 and 9) | | $ | 272,276 | | | $ | 120,150 | |
| Unissued share capital (Note 10) | | | - | | | | 100,000 | |
| | | | 272,276 | | | | 220,150 | |
| Non-current liabilities | | | | | | | | |
Convertible debentures (Note 15) | | | 539,910 | | | | - | |
Derivative liability (Note 15) | | | 330,065 | | | | - | |
| | | | 1,142,249 | | | | 220,150 | |
| Shareholders' Equity | | | | | | | | |
Share Capital (Note 10) | | | 2,719,740 | | | | 1,063,482 | |
Reserve for share based payments (Note 11) | | | 314,000 | | | | 28,000 | |
Reserve for warrants (Note 10 and 12) | | | 566,399 | | | | 65,000 | |
| Reserve for foreign currency translation | | | (15,285 | ) | | | - | |
Non-controlling interest (Note 14) | | | (9,073 | ) | | | - | |
Loss on acquisition of non-controlling interest (Note 14) | | | (5,000 | ) | | | - | |
| Accumulated deficit | | | (2,740,442 | ) | | | (681,155 | ) |
| | | | 830,339 | | | | 475,327 | |
| | | $ | 1,972,588 | | | $ | 695,477 | |
Nature of Operations and Going concern (Note 1)
Licensing and Royalty Agreement (Note 19)
Subsequent Events (Note 21)
Approved on behalf of the Board:
"Adam Szweras" Director "David Posner" Director
The accompanying notes are an integral part of these consolidated financial statements
Nutritional High International Inc. Consolidated Statements of Loss and Comprehensive Loss (Expressed in Canadian Dollars) |
| | | For the Year Ended July 31, 2015 | | | For the Period from Incorporation April 17, 2014 to July 31, 2014 | |
| | | | | | | |
| Administrative expenses | | | | | | |
Management and consulting fees (Note 9) | | $ | 753,432 | | | $ | 176,244 | |
Professional fees (Note 9) | | | 468,809 | | | | 88,231 | |
| Office and general | | | 294,389 | | | | 32,857 | |
Share based payments (Note 11) | | | 286,000 | | | | 28,000 | |
| Other Expenses | | | | | | | | |
Change in fair value of derivative liability (Note 15) | | | 277,753 | | | | - | |
Listing expense (Note 13) | | | - | | | | 355,823 | |
Finance costs (Note 15) | | | 73,216 | | | | - | |
Amortization (Note 6) | | | 25,523 | | | | - | |
| Total expenses | | | (2,179,122 | ) | | | (681,155 | ) |
| Other income (expense) | | | | | | | | |
| Interest | | | 8,492 | | | | - | |
Rental income (Note 5) | | | 202,270 | | | | - | |
Allowance for amounts receivable (Note 5) | | | (100,000 | ) | | | - | |
| Net loss | | | (2,068,360 | ) | | | (681,155 | ) |
| Other Comprehensive loss | | | | | | | | |
| Items that may be reclassified subsequently to net income/loss | | | | | | | | |
| Exchange differences on translating foreign operations | | | (15,285 | ) | | | - | |
| Net loss and comprehensive loss | | $ | (2,083,645 | ) | | $ | (681,155 | ) |
| | | | | | | | | |
Net loss attributable to non-controlling interest (Note 14) | | $ | (9,073 | ) | | $ | - | |
| Net loss attributable to parent company | | | (2,059,287 | ) | | | (681,155 | ) |
| | | $ | (2,068,360 | ) | | $ | (681,155 | ) |
| | | | | | | | | |
Net loss and comprehensive loss attributable to non-controlling interest (Note 14) | | $ | (9,073 | ) | | $ | - | |
| Net loss and comprehensive loss attributable to parent company | | | (2,074,572 | ) | | | (681,155 | ) |
| | | $ | (2,083,645 | ) | | $ | (681,155 | ) |
| | | | | | | | | |
| Weighted average number of shares outstanding | | | | | | | | |
- Basic and diluted (Note 3.2) | | | 91,731,676 | | | | 55,591,495 | |
| Loss per share | | | | | | | | |
- Basic and diluted (Note 3.2) | | $ | (0.2 | ) | | $ | (0.01 | ) |
| | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements
Nutritional High International Inc.
Consolidated Statements of Changes in Shareholders' Equity
(Expressed in Canadian Dollars)
| | | Share Capital | | | | | | | | | | | | | | | | | | | |
Note | | Number of Shares | | | Amount | | | Loss on acquisition of non-controlling interest | | | Reserve for Share based payments | | | Reserve for Warrants | | | Reserve for Foreign Exchange | | | Accumulated Deficit | | | Attributable to owners of parent | | | Non-controlling interest | | | Total | |
| Founders shares issued (Note 10) | | | 33,400,000 | | | $ | 167,000 | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | 167,000 | | | $ | - | | | $ | 167,000 | |
| Private placements (Note 10) | | | 27,000,011 | | | | 675,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 675,000 | | | | - | | | | 675,000 | |
| Warrants issued (Note 12) | | | - | | | | (65,000 | ) | | | - | | | | - | | | | 65,000 | | | | - | | | | - | | | | - | | | | - | | | | | |
| Issuance of shares to former Sonoma Capital Inc. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | - | | | | | |
| Shareholders (Note 13) | | | 11,513,620 | | | | 287,840 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 287,840 | | | | - | | | | 287,840 | |
| Share based payments (Note 11) | | | - | | | | | | | | - | | | | 28,000 | | | | - | | | | - | | | | - | | | | 28,000 | | | | - | | | | 28,000 | |
| Share issue costs (Note 10) | | | - | | | | (1,358 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | (1,358 | ) | | | - | | | | (1,358 | ) |
| Net loss and comprehensive loss for the year | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (681,155 | ) | | | (681,155 | ) | | | - | | | | (681,155 | ) |
| Balance at July 31, 2014 | | | 71,913,631 | | | | 1,063,482 | | | | - | | | | 28,000 | | | | 65,000 | | | | - | | | | (681,155 | ) | | | 475,327 | | | | - | | | | 475,325 | |
| Private placements (Note 10) | | | 4,000,000 | | | | 86,000 | | | | - | | | | - | | | | 14,000 | | | | - | | | | - | | | | 100,000 | | | | - | | | | 100,000 | |
| Finders warrants issued in connection with private placement (Note 10) | | | | | | | (4,000 | ) | | | - | | | | - | | | | 4,000 | | | | - | | | | - | | | | | | | | - | | | | | |
| Initial public offering (Note 10) | | | 32,900,000 | | | | 1,282,000 | | | | - | | | | - | | | | 363,000 | | | | - | | | | - | | | | 1,645,000 | | | | - | | | | 1,645,000 | |
| Compensation warrants issued in connection with initial public offering (Note 10) | | | | | | | (63,000 | ) | | | - | | | | - | | | | 63,000 | | | | - | | | | - | | | | 63,000 | | | | - | | | | 63,000 | |
| Warrants issued in connection to early and regular exercise of warrants (Note 12) | | | - | | | | (71,000 | ) | | | - | | | | - | | | | 71,000 | | | | - | | | | - | | | | | | | | - | | | | | |
| Shares issued on exercise of warrants (Note 10) | | | 5,044,638 | | | | 322,952 | | | | - | | | | - | | | | (48,601 | ) | | | - | | | | - | | | | 274,351 | | | | - | | | | 274,351 | |
| Issued for non-cash consideration: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | - | | | | | |
| Issued for success fee (Note 9 and 10) | | | 700,000 | | | | 16,000 | | | | - | | | | - | | | | 19,000 | | | | - | | | | - | | | | 35,000 | | | | - | | | | 35,000 | |
| Issued for extension fee (Note 10 and 15) | | | 600,000 | | | | 14,000 | | | | - | | | | - | | | | 16,000 | | | | - | | | | - | | | | 30,000 | | | | - | | | | 30,000 | |
| Issued for services (Note 10) | | | 811,111 | | | | 57,500 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 57,500 | | | | - | | | | 57,500 | |
| Issued for license and royalty fees (Note 10 and 19) | | | 3,333,334 | | | | 250,000 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 250,000 | | | | - | | | | 250,000 | |
| Issued for acquisition of non-controlling interest (Note 10 and 14) | | | 66,667 | | | | 5,000 | | | | (5,000 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | | - | |
| Share based payments (Note 11) | | | - | | | | - | | | | - | | | | 286,000 | | | | - | | | | - | | | | - | | | | 286,000 | | | | - | | | | 286,000 | |
| Share issue costs (Note 10) | | | - | | | | (239,194 | ) | | | - | | | | - | | | | - | | | | - | | | | - | | | | (239,194 | ) | | | - | | | | (239,194 | ) |
| Exchange loss on translating foreign operations | | | - | | | | - | | | | - | | | | - | | | | - | | | | (15,285 | ) | | | - | | | | (15,285 | ) | | | - | | | | (15,285 | ) |
| Net loss and comprehensive loss for the year | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,059,287 | ) | | | (2,059,287 | ) | | | (9,073 | ) | | | (2,068,360 | ) |
| Balance at July 31, 2015 | | | 119,369,381 | | | $ | 2,719,740 | | | $ | (5,000 | ) | | $ | 314,000 | | | $ | 566,399 | | | $ | (15,285 | ) | | $ | (2,740,442 | ) | | $ | 839,412 | | | $ | (9,073 | ) | | $ | 830,339 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements
Nutritional High International Inc.
Consolidated Statement of Cash Flows
(Expressed in Canadian Dollars)
| | | For the Year Ended July 31, 2015 | | | For the Period from Incorporation April 17, 2014 to July 31, 2014 | |
| OPERATING ACTIVITIES | | | | | | |
| Net Loss | | $ | (2,068,360 | ) | | $ | (681,155 | ) |
| Item not affecting cash: | | | | | | | | |
| Amortization | | | 25,523 | | | | - | |
| Interest accretion | | | 22,222 | | | | - | |
| Shares issued for services | | | 122,500 | | | | - | |
Listing expense (Note 13) | | | - | | | | 355,823 | |
| Interest paid | | | (46,157 | ) | | | - | |
Share based payments (Note 11) | | | 286,000 | | | | 28,000 | |
| Allowance for amounts receivable (Note 5) | | | 100,000 | | | | - | |
| Prepaids | | | (175,668 | ) | | | - | |
| Change in the fair value of derivative liability | | | 277,753 | | | | - | |
| Net change in non-cash working capital: | | | | | | | | |
| HST receivables and other receivables | | | (107,284 | ) | | | - | |
| Amounts due from Palo Verde LLC (Note 5) | | | (191,674 | ) | | | (23,747 | ) |
| Accounts payable and accrued liabilities | | | 159,577 | | | | (69,112 | ) |
| Cash Flow Used in Operating Activities | | | (1,595,567 | ) | | | (390,191 | ) |
| INVESTING ACTIVITIES | | | | | | | | |
Amounts due from Palo Verde LLC (Note 5) | | | (60,335 | ) | | | (32,712 | ) |
Property deposit (Note 7) | | | (5,962 | ) | | | (21,952 | ) |
Purchase of investment property (Note 6) | | | (977,744 | ) | | | - | |
| Cash acquired in reverse takeover transaction | | | - | | | | 121,279 | |
| Cash Flow From Investing Activities | | | (1,044,041 | ) | | | 66,615 | |
| FINANCING ACTIVITIES | | | | | | | | |
Issuance of share capital, net of share issue costs (Note 10) | | | 1,680,157 | | | | 940,642 | |
Convertible debentures issued, net of issue costs (Note 15) | | | 543,000 | | | | - | |
| Cash Flow From Financing Activities | | | 2,223,157 | | | | 940,642 | |
| Net increase (decrease) in cash | | $ | (416,451 | ) | | $ | 617,066 | |
| Effects of exchange rate changes on cash | | | (181,047 | ) | | | - | |
| Cash at beginning of year | | | 617,066 | | | | - | |
| Cash at end of year | | $ | 19,567 | | | $ | 617,066 | |
The accompanying notes are an integral part of these consolidated financial statements
| 1. | Nature of Operations and Going Concern |
Nutritional High International Inc. ("Nutritional High" or "the Company" or "NHII"), formerly Sonoma Capital Inc. is the parent company of Nutritional High Ltd., Nutritional High (Colorado) Inc., NHC Edibles LLC, NH Medicinal Dispensaries Inc., NH Medicinal (Minnesota) Inc. and Eglinton Medicinal Advisory Ltd. The Company's objective is to take advantage of the changing regulation governing the marijuana industry in the United States and Canada. The address of the Company's registered office is 77 King Street West, Suite 2905, Toronto, Ontario M5K 1H1. The Company is listed on the Canadian Securities Exchange (CSE) under symbol "EAT". The Company is also listed on the OTCQB Marketplace under US symbol: SPLIF.
The Company was incorporated under the name "Sonoma Capital Inc." on July 19, 2004 under the Canada Business Corporations Act (Note 13).
The consolidated financial statements were approved by the Board of Directors on March 10, 2016.
The Company is in the process of developing brands, trademark applications, and packaging for a confectionery line. The Company is also developing a licensing/franchising system to work with licensed marijuana edibles manufacturers and in this regard, is negotiating with parties who are licensed or seeking a manufacturing license.
The Company has earned rental income but not yet realized any revenue from its operations and will not be able to do so until a license/franchise arrangement is negotiated with licensed parties. As such, there is uncertainty with respect to the Company's ability to continue as a going concern, dependent upon such events as financing, entering into agreements with licensed parties, commencement of sales and market demand conditions. There is no assurance that any prospective project in the medical marijuana industry will be successfully initiated or completed. As is common with development stage companies, the Company is dependent upon obtaining necessary financing from time to time to finance its on-going and planned activities and to cover administrative costs.
At July 31, 2015 the Company had working capital of $116,437 (2014 - $453,375), had not yet achieved profitable operations, has accumulated losses of $2,740,442 (2014 - $681,155) and expects to incur further losses in the development of its business, all of which describes the material uncertainties that cast significant doubt upon the Company's ability to continue as a going concern. The Company will require additional financing in order to conduct its planned business operations, meet its ongoing levels of corporate overhead and discharge its liabilities and commitments (Note 19) as they come due. There is no assurance that the Company will successfully raise this financing.
2.1 Statement of compliance
The Company's consolidated financial statements have been prepared in accordance with and using accounting policies in full compliance with the International Financial Reporting Standards ("IFRS") and International Accounting Standards ("IAS") issued by the International Accounting Standards Board ("IASB") and IFRS Interpretations Committee, effective for the Company's reporting for the year ended July 31, 2015.
2.2 Basis of presentation
The consolidated financial statements have been prepared on the historical cost basis except for certain financial instruments, which are measured at fair value, as explained in the accounting policies set out in Note 3.
The accompanying notes are an integral part of these consolidated financial statements
| 2. | Basis of Presentation (continued) |
2.3 Basis of consolidation
The consolidated financial statements include the accounts of Nutritional High International Inc. and its wholly–owned subsidiaries Nutritional High Ltd., Nutritional High (Colorado), Inc., NHC Edibles, LLC, and NH Medicinal (Minnesota) Inc. with jurisdiction in US, and 51% owned subsidiary Eglinton Medicinal Advisory Ltd.
The subsidiaries are entities controlled by the Company. Control exists when the Company has power over an investee, when the Company is exposed, or has rights, to variable returns from the investee and when the Company has the ability to affect those returns through its power over the investee. The financial statements of subsidiaries are included in the consolidated financial statements from the date that control commences until the date control ceases.
Non-controlling interest is shown as a component of equity on the statement of financial position and the share of the loss attributable to non-controlling interest is shown as a component of loss for the year in the statement of loss and comprehensive loss.
The functional currency of the Company, Nutritional High Ltd. and Eglinton Medical Advisory Ltd. is the Canadian dollar, which is the presentation currency of the consolidated financial statements. The functional currency of US subsidiaries (Nutritional High (Colorado), Inc, NHC Edibles, LLC and NH Medicinal (Minnesota)) is the US dollar.
Intercompany balances and transactions, and unrealized gains arising from intercompany transactions are eliminated in preparing the consolidated financial statements.
2.4 New and revised standards and interpretations to be adopted in the future
At the date of authorization of these consolidated financial statements, the IASB and IFRIC has issued the following new and revised Standards and Interpretations which are not yet effective for the relevant reporting periods and which the Company has not early adopted. However, the Company is currently assessing what impact the application of these standards or amendments will have on the consolidated financial statements of the Company.
-
| | · | Pronouncements effective for annual periods beginning on or after January 1, 2016 |
-
- IAS 1 Presentation of Financial Statements Amendments are designed to further encourage companies to apply professional judgement in determining what information to disclose in their financial statements.
-
- IAS 16 Property, Plant and Equipment and IAS 38 Intangible Assets Amendments clarify that the use of revenue-based methods to calculate the depreciation of an asset are not appropriate because revenue generated by an activity that includes the use of an asset generally reflects factors other than the consumption of the economic benefits embodied in the asset.
-
-
The accompanying notes are an integral part of these consolidated financial statements
| 2. | Basis of Presentation (continued) |
2.4 New and revised standards and interpretations to be adopted in the future (continued)
-
| | · | Pronouncements effective for annual periods beginning on or after January 1, 2018 |
-
In July 2014, the IASB issued the final version of IFRS 9, Financial Instruments. The new standard will replace IAS 39, Financial instruments: recognition and measurement. The final amendments made in the new version include guidance for the classification and measurement of financial assets and a third measurement category for financial assets, fair value through other comprehensive income. The standard also contains a new expected loss impairment model for debt instruments measured at amortized cost or fair value through other comprehensive income, lease receivables, contract assets and certain written loan commitments and financial guarantee contracts. The standard is effective for annual periods beginning on or after January 1, 2018 and must be applied retrospectively with some exceptions. Early adoption is permitted. Restatement of prior periods in relation to the classification and measurement, including impairment, is not required. The Company has not yet assessed the impact of this new standard.
-
| 3. | Summary of Significant Accounting Policies |
3.1 Share based payments
Share based payment transactions
Employees (including directors and senior executives) of the Company receive a portion of their remuneration in the form of share based payment transactions, whereby they render services as consideration for equity instruments ("equity settled transactions").
In situations where equity instruments are issued to non-employees and some or all of the goods or services received by the entity as consideration cannot be specifically measured, they are measured at fair value of the share based payment. The fair value of the share based payments is recognized together with a corresponding increase in equity over a period that services are provided or goods are received.
Equity settled transactions
The costs of equity settled transactions with employees are measured by reference to the fair value of the equity instrument at the date on which they are granted.
The costs of equity settled transactions are recognized, together with a corresponding increase in equity, over the period in which the performance and/or service conditions are fulfilled, ending on the date on which the relevant employees become fully entitled to the award ("the vesting date"). The cumulative cost is recognized for equity settled transactions at each reporting date until the vesting date reflects the Company's best estimate of the number of equity instruments that will ultimately vest. The profit or loss charge or credit for a period represents the movement in cumulative expense recognized as at the beginning and end of that period and the corresponding amount is represented in share based payment reserve.
No expense is recognized for awards that do not ultimately vest.
Where the terms of an equity settled award are modified, the minimum expense recognized is the expense as if the terms had not been modified. An additional expense is recognized for any modification which increases the total fair value of the share based payment arrangement, or is otherwise beneficial to the employee as measured at the date of modification. The dilutive effect of outstanding options is reflected as additional dilution in the computation of loss per share.
The accompanying notes are an integral part of these consolidated financial statements
| 3. | Summary of Significant Accounting Policies (continued) |
3.2 Loss per share
Basic loss per share is calculated using the weighted number of shares outstanding. Diluted loss per share is calculated using the weighted average number of common and potential common shares outstanding during the period. In order to determine diluted loss per share, it is assumed that any proceeds from the exercise of dilutive stock options and warrants would be used to repurchase common shares at the average market price during the period, with the incremental number of shares being included in the denominator of the diluted loss per share calculation. The diluted loss per share calculation excludes any potential conversion of options and warrants that would increase earnings per share or decrease loss per share. Total shares issuable from warrants were excluded from the computation of diluted loss per share because they were anti-dilutive for the year ended July 31, 2015 and for the period from incorporation April 17, 2014 to July 31, 2014.
3.3 Taxation
Current income tax
Current income tax assets and liabilities for the current and prior periods are measured at the amount expected to be recovered from or paid to the taxation authorities. The tax rates and tax laws used to compute the amount are those that are enacted or substantively enacted by the date of the statement of financial position.
Deferred income tax
Deferred income tax is provided using the liability method on temporary differences at the date of the statement of financial position between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes.
Deferred income tax liabilities are recognized for all taxable temporary differences, except:
| | · | where the deferred income tax liability arises from the initial recognition of goodwill or of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss; and |
-
| | · | in respect of taxable temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, where the timing of the reversal of the temporary differences can be controlled and it is probable that the temporary differences will not reverse in the foreseeable future. |
Deferred income tax assets are recognized for all deductible temporary differences, carry forward of unused tax credits and unused tax losses, to the extent that it is probable that taxable profit will be available against which the deductible temporary differences and the carry forward of unused tax credits and unused tax losses can be utilized except:
| | · | where the deferred income tax asset relating to the deductible temporary difference arises from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither the accounting profit nor taxable profit or loss; and |
-
-
-
-
The accompanying notes are an integral part of these consolidated financial statements
| 3. | Summary of Significant Accounting Policies (continued) |
3.3 Taxation (continued)
Deferred income tax (continued)
-
| | · | in respect of deductible temporary differences associated with investments in subsidiaries, associates and interests in joint ventures, deferred income tax assets are recognized only to the extent that it is probable that the temporary differences will reverse in the foreseeable future and taxable profit will be available against which the temporary differences can be utilized. |
The carrying amount of deferred income tax assets is reviewed at each date of the statement of financial position and reduced to the extent that it is no longer probable that sufficient taxable profit will be available to allow all or part of the deferred income tax asset to be utilized. Unrecognized deferred income tax assets are reassessed at each date of the statement of financial position and are recognized to the extent that it has become probable that future taxable profit will allow the deferred tax asset to be recovered.
Deferred income tax assets and liabilities are measured at the tax rates that are expected to apply to the year when the asset is realized or the liability is settled, based on tax rates (and tax laws) that have been enacted or substantively enacted at the date of the statement of financial position.
Deferred income tax relating to items recognized directly in equity is recognized in equity and not in the statement of comprehensive loss.
3.4 Financial assets
All financial assets are initially recorded at fair value and designated upon inception into one of the following four categories: held to maturity, available for sale, loans and receivables or at fair value through profit or loss ("FVTPL").
Financial assets classified as FVTPL are measured at fair value with unrealized gains and losses recognized through earnings. The Company's cash is classified as FVTPL.
Financial assets classified as loans and receivables and held to maturity are measured at amortized cost using the effective interest rate method. The Company's amounts due from Palo Verde LLC and other receivables except for HST receivable are classified as loans and receivables.
Financial assets classified as available for sale are measured at fair value with unrealized gains and losses recognized in other comprehensive loss except for losses in value that are considered other than temporary. At July 31, 2015, the Company has not classified any financial assets as available for sale.
Transactions costs associated with FVTPL financial assets are expensed as incurred, while transaction costs associated with all other financial assets are included in the initial carrying amount of the asset.
The accompanying notes are an integral part of these consolidated financial statements
| 3. | Summary of Significant Accounting Policies (continued) |
3.5 Financial liabilities
All financial liabilities are initially recorded at fair value and designated upon inception as FVTPL or other financial liabilities.
Financial liabilities classified as other financial liabilities are initially recognized at fair value less directly attributable transaction costs. After initial recognition, other financial liabilities are subsequently measured at amortized cost using the effective interest method. The effective interest method is a method of calculating the amortized cost of a financial liability and of allocating interest expense over the relevant period. The effective interest rate is the rate that exactly discounts estimated future cash payments through the expected life of the financial liability, or, where appropriate, a shorter period. The Company's accounts payable and accrued liabilities, and convertible debentures are classified as other financial liabilities.
Financial liabilities classified at FVTPL include financial liabilities held for trading and financial liabilities designated upon initial recognition at FVTPL. Derivatives, including separated embedded derivatives are classified as held for trading unless they are designated as effective hedging instruments. Fair value changes on financial liabilities classified at FVTPL are recognized through the statement of comprehensive loss. At July 31, 2015, the Company has classified the conversion feature of the convertible debentures as financial liabilities at FVTPL.
3.6 Impairment of financial assets
The Company assesses at each date of the statement of financial position whether a financial asset is impaired. Management has concluded that there is impairment for amounts due from Palo Verde LLC as at July 31, 2015 (Note 5).
Assets carried at amortized cost
If there is objective evidence that an impairment loss on assets carried at amortized cost has been incurred, the amount of the loss is measured as the difference between the asset's carrying amount and the present value of estimated future cash flows discounted at the financial asset's original effective interest rate. The carrying amount of the asset is then reduced by the amount of the impairment. The amount of the loss is recognized in profit or loss.
If, in a subsequent period, the amount of the impairment loss decreases and the decrease can be related objectively to an event occurring after the impairment was recognized, the previously recognized impairment loss is reversed to the extent that the carrying value of the asset does not exceed what the amortized cost would have been had the impairment not been recognized. Any subsequent reversal of an impairment loss is recognized in profit or loss.
Available-for-sale
If an available for sale asset is impaired, an amount comprising the difference between its cost and its current fair value, less any impairment loss previously recognized in profit or loss, is transferred from equity to profit or loss. Reversals in respect of equity instruments classified as available for sale are not recognized in profit or loss.
The accompanying notes are an integral part of these consolidated financial statements
| 3. | Summary of Significant Accounting Policies (continued) |
3.7 Provisions
Provisions are recognized when the Company has a present obligation (legal or constructive) that has arisen as a result of a past event and it is probable that a future outflow of resources will be required to settle the obligation, provided that a reliable estimate can be made of the amount of the obligation.
Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax rate that reflects current market assessments of the time value of money and the risk specific to the obligation. The increase in the provision due to passage of time is recognized as interest expense.
3.8 Related party transactions
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Parties are also considered to be related if they are subject to common control or common significant influence, related parties may be individuals or corporate entities. A transaction is considered to be a related party transaction when there is a transfer of resources or obligations between related parties.
3.9 Leases
Leases are classified as finance leases whenever the terms of the lease transfer substantially all the risks and rewards of ownership to the lessee. All other leases are classified as operating leases.
The Company as lessor
Rental income from operating leases is recognized on a straight-line basis over the term of the relevant lease. Initial direct costs incurred in negotiating and arranging an operating lease are added to the carrying amount of the leased asset and recognized on a straight-line basis over the lease term.
3.10 Intangibles
Intangibles arise on the purchase of a license, which is accounted for using the cost model whereby capitalized costs are amortized on a straight-line basis over their estimated useful lives, as these assets are considered finite. Useful lives are reviewed annually and intangibles are subject to impairment testing. As at July 31, 2015, the license term (Note 19) has not commenced.
3.11 Investment property
Investment property earns rental income and is not for sale in the ordinary course of business, is not used in the production or supply of goods or services or for administrative purposes. Investment property is carried at historical cost less any accumulated depreciation and impairment losses. Amortization is computed using the declining balance methods based on the estimated useful life of the assets. Useful life is reviewed at the end of each reporting period. Amortization is provided at rates as follows:
Building 4% Declining balance
The accompanying notes are an integral part of these consolidated financial statements
| 3. | Summary of Significant Accounting Policies (continued) |
3.12 Impairment of non-financial assets
At each date of the statement of financial position, the Company reviews the carrying amounts of its tangible and intangible assets to determine whether there is an indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss (if any). Where it is not possible to estimate the recoverable amount of an individual asset, the Company estimates the recoverable amount of the cash generating unit to which the assets belong.
Recoverable amount is the higher of fair value less costs of disposal and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset.
If the recoverable amount of an asset (or cash generating unit) is estimated to be less than its carrying amount, the carrying amount of the asset (or cash generating unit) is reduced to its recoverable amount. An impairment loss is recognized immediately in the statement of comprehensive loss.
For assets excluding goodwill, an assessment is made at each reporting date as to whether there is any indication that previously recognised impairment losses may no longer exist or may have decreased. If such indication exists, the Company estimates the asset's or cash-generating unit's recoverable amount. A previously recognised impairment loss is reversed only if there has been a change in the assumptions used to determine the asset's recoverable amount since the last impairment loss was recognised. The reversal is limited so that the carrying amount of the asset does not exceed its recoverable amount, nor exceed the carrying amount that would have been determined, net of depreciation, had no impairment loss been recognised for the asset in prior years. Such reversal is recognised in profit or loss and the carrying amount of the asset (cash-generating unit) is increased to the revised estimate of its recoverable amount.
3.13 Share issuance costs
Costs incurred in connection with the issuance of share capital are netted against the proceeds received net of tax. Costs related to the issuance of share capital and incurred prior to issuance are recorded as deferred share issuance costs and subsequently netted against proceeds when they are received.
3.14 Share capital
In situations where the Company issues units, the value of warrants is bifurcated and is included as the separate reserve of the Company's equity.
3.15 Convertible debentures
The proceeds received on issue of the Company's convertible debentures have been recorded as a liability on the consolidated statement of financial position. The convertible debentures contain an embedded derivative. The Company has designated the derivative as a financial liability at fair value through profit or loss. The Company revalues the convertible debenture derivative liability using recent arm's length market transactions and option pricing models at each reporting period.
The accompanying notes are an integral part of these consolidated financial statements
| 3. | Summary of Significant Accounting Policies (continued) |
3.16 Significant accounting judgments and estimates
The preparation of these consolidated financial statements requires management to make judgments and estimates and form assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. On an ongoing basis, management evaluates its judgments and estimates in relation to assets, liabilities, revenue and expenses. Management uses historical experience and various other factors it believes to be reasonable under the given circumstances as the basis for its judgments and estimates. Actual outcomes may differ from these estimates under different assumptions and conditions. The most significant estimates relate to recoverability of loan receivable and rent receivable (note 5), valuation of deferred income tax amounts, and valuation of warrants and shares issued during private placements and measurement of derivative liability.
The most significant judgments relate to recognition of deferred tax assets and liabilities, assessment of functional currency and determination of derivative liability of convertible debt.
3.17 Foreign currency translation
Monetary assets and liabilities denominated in currencies other than Canadian dollars are translated into Canadian dollars at the rate of exchange in effect at the statement of financial position date. Non-monetary assets and liabilities are translated at the historical rates. Revenues and expenses are translated at the transaction exchange rate. Foreign currency gains and losses resulting from translation are reflected in net comprehensive loss for the period.
The assets and liabilities of entities with a functional currency that differs from the presentation currency are translated to the presentation currency as follows:
| | · | Assets and liabilities are translated at the closing rate at the financial period end; |
| | · | Income and expenses are translated at average exchange rates (unless the average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case, income and expenses are translated at the rate on the dates of the transactions); |
| | · | Equity transactions are translated using the exchange rate at the date of the transaction; and |
| | · | All resulting exchange differences are recognized as a separate component of equity as reserve for foreign exchange. |
When a foreign operation is disposed of, the relevant amount in the reserve for foreign exchange in other comprehensive income is transferred to profit or loss as part of the profit or loss on disposal.
On the partial disposal of a subsidiary that includes a foreign operation, the relevant proportion of such cumulative amount is reattributed to non-controlling interest. In any other partial disposal of a foreign operation, the relevant proportion is reclassified to profit or loss.
Foreign exchange gains or losses arising from a monetary item receivable from or payable to a foreign operation, the settlement of which is neither planned nor likely to occur in the foreseeable future, and which in substance, is considered to form part of the net investment in the foreign operation, are recognized in the reserve for foreign exchange.
HST consists of harmonized sales tax ("HST") due from the Canadian government.
The accompanying notes are an integral part of these consolidated financial statements
| 5. | Amounts due from Palo Verde LLC |
The Company has lent Palo Verde LLC ("Palo") monies pursuant to a credit agreement and has leased property to Palo pursuant to two lease agreements, as disclosed below. The Company intends on entering into a branding arrangement with Palo in respect of its licensing arrangement (note 19).
| | | 2015 | | | 2014 | |
Rental income receivable(i) | | $ | 183,000 | | | $ | - | |
Loan receivable(ii) | | | 105,424 | | | | 32,712 | |
| Interest receivable | | | 9,418 | | | | - | |
| | | | 297,842 | | | | 32,712 | |
Impairment on amounts receivable(iii) | | | (100,000 | ) | | | - | |
| | | $ | 197,842 | | | $ | 32,712 | |
| | (i) | Rental income receivable is on the investment property in Colorado. Rent is deferred for the first 6 and 9 months of the two respective leases, commencing January 1, 2015 and accrues interest at 12% per annum. Subsequent to the year ended July 31, 2015, two lease agreements were amended whereas the deferred rent and accrued interest of 12% are to be paid no later than June 30, 2017 and September 30, 2017. |
| | (ii) | Revolving line of credit of USD $150,000 to Palo Verde LLC, of which $105,425 (USD $80,600) (2014 – USD $30,000) and accrued interest of $7,478 (USD $5,717) (2014 – USD $nil) was receivable as at July 31, 2015. Advances through a promissory note are unsecured; bear interest at 12% per annum which were due on July 22, 2015. On July 23, 2015, the agreement was amended to extend maturity date of principal and interest to September 30, 2016. Accrued interest is payable in three monthly installments on September 30, 2016, October 31, 2016 and November 30, 2016. Palo Verde may extend the maturity date for up to an additional four successive one-year terms for a total of five years, but no later than July 22, 2020 for a fee equal to 1% of the outstanding revolving credit loan. |
| | (iii) | An allowance was recorded in 2015 on account of the uncertainties surrounding recoverability of the loan and rents receivable in respect of timing and unexpected financing delays. |
The accompanying notes are an integral part of these consolidated financial statements
| | | Land | | | Building | | | Total | |
Cost | | | | | | | | | |
| Balance at July 31, 2014 | | $ | - | | | $ | - | | | $ | - | |
| Additions | | | 141,200 | | | | 858,496 | | | | 999,696 | |
| Effect of movement in exchange rates | | | 22,300 | | | | 135,584 | | | | 157,884 | |
| Balance at July 31, 2015 | | | 163,500 | | | | 994,080 | | | | 1,157,580 | |
Accumulated Amortization | | | | | | | | | |
| Balance at July 31, 2014 | | | - | | | | - | | | | - | |
| Amortization for the year | | | - | | | | 25,523 | | | | 25,523 | |
| Effect of movement in exchange rates | | | - | | | | 2,475 | | | | 2,475 | |
| Balance at July 31, 2015 | | | - | | | | 27,998 | | | | 27,998 | |
Carrying Amounts | | | | | | | | | |
| Balance at July 31, 2014 | | | - | | | | - | | | | - | |
| Balance at July 31, 2015 | | | 163,500 | | | | 966,082 | | | | 1,129,582 | |
The Company through its wholly owned subsidiary NHC Edibles, LLC purchased the commercial investment property, land and building located in Colorado, USA, through making a refundable deposit of USD $20,000 with a remaining commitment of USD $865,000 upon closing. The Company completed its acquisition of the commercial property on November 17, 2014, and the deposit was applied against the purchase price. The fair value of the investment property as at July 31, 2015 approximates the carrying value as the investment property was acquired during the year ended July 31, 2015 and management used the independent real estate appraiser to establish the acquisition price of the investment property. The direct operating expenses arising from investment property that generated rental income were USD $110,370 for the year ended July 31, 2015.
The Company through its owned subsidiary NH Medicinal Dispensaries Inc. made a refundable deposit of $6,671 (USD $5,000) on the purchase of the commercial property located in Illinois, USA, with a remaining commitment of USD $345,000 upon closing (Note 21).
| 8. | Accounts Payable and Accrued Liabilities |
Accounts payable and accrued liabilities of the Company are principally comprised of amounts outstanding for trade purchases relating to regular business activities and amounts payable for financing activities. The usual credit period taken for purchases is between 30 to 90 days.
The following is an aged analysis of accounts payable and accrued liabilities:
| | | | | | | |
| | | 2015 | | | 2014 | |
| | | $ | | | | $ | | |
| | | | | | | | | |
| Less than 1 month | | | 97,370 | | | | 62,321 | |
| Over 1 month | | | 174,904 | | | | 57,829 | |
Total accounts payable and accrued liabilities | | | 272,274 | | | | 120,150 | |
The accompanying notes are an integral part of these consolidated financial statements
| 9. | Related Parties and Key Management |
Key management includes the Company's directors, officers and any employees with authority and responsibility for planning, directing and controlling the activities of an entity, directly or indirectly.
For the year ended July 31, 2015, the Company incurred professional fees of $98,000 (2014 - $9,000) from Branson Corporate Services, a company in which a company with a related director has a 49% interest. The agreement includes a success fee of $30,000, paid upon successful completion of the public offering.
For the year ended July 31, 2015, the Company incurred consulting fees of $227,000 (2014 - $59,000) from FMI Capital Advisory Inc. (formerly Foundation Opportunities Inc.), a company with a related director. The agreement includes a success fee of $70,000, paid upon successful completion of the initial public offering, $35,000 paid in cash and $35,000 in units (Note 10).
For the year ended July 31, 2015, the Company incurred professional fees of $354,007 (2014 - $59,781) from Fogler Rubinoff, LLP, a law firm in which a director of the company is a partner.
For the year ended July 31, 2015, the Company incurred a finder's fee of $4,000 and issued 160,000 finder's warrants to Foundation Markets Inc., a company with a related director. This was in connection with the closing of the 4,000,000 unit subscription as described in Note 10.
The Company issued a Subordinate Convertible Debenture in the principal amount of $150,000 to a group of lenders comprised of directors of the company.
Total key management compensation paid was $147,829 and compensation paid in shares was $25,000 to the Chief Executive Officer, the VP Product Development, a Director, and a Chairman amounted to $172,829 (2014 - $80,491) for the year ended July 31, 2015.
These expenses have been measured at their exchange amount, being the amounts negotiated and agreed to by the parties to the transactions. As at July 31, 2015, $85,463 (2014 - $106,003) is included in accounts payable and accrued liabilities.
The Company is authorized to issue an unlimited number of common shares without par value.
On April 17, 2014, the Company issued 33,000,000 shares, valued at $0.005 per share for gross proceeds of $165,000. On May 12, 2014, the Company issued 400,000 shares, valued at $0.005 per share for gross proceeds of $2,000.
On May 16, 2014, the Company completed the first tranche of its non-brokered private placement of 22,106,853 units at $0.025 per unit for gross proceeds of $552,671. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.05 per share until the earlier of 36 months from the date of issuance or 18 months following the date of a qualifying transaction between the Company and a public company pursuant to a reverse take‑over, merger, amalgamation, take‑over bid, insider bid, reorganization, joint venture, sale or exchange of assets or similar transaction or IPO.
The accompanying notes are an integral part of these consolidated financial statements
| 10. | Share Capital (continued) |
On May 30, 2014, the Company completed the second tranche of its non-brokered private placement of 2,180,798 units at $0.025 per unit for gross proceeds of $54,520. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.05 per share until the earlier of 36 months from the date of issuance or 18 months following the date of a qualifying transaction between the Company and a public company pursuant to a reverse take‑over, merger, amalgamation, take‑over bid, insider bid, reorganization, joint venture, sale or exchange of assets or similar transaction or IPO.
On June 20, 2014, the Company completed the third tranche of its non-brokered private placement of 2,712,360 units at $0.025 per unit for gross proceeds of $67,809. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one
common share at a price of $0.05 per share until the earlier of 36 months from the date of issuance or 18 months following the date of a qualifying transaction between the Company and a public company pursuant to a reverse take‑over, merger, amalgamation, take‑over bid, insider bid, reorganization, joint venture, sale or exchange of assets or similar transaction or IPO.
For the year ended July 31, 2014, the Company issued 11,440,798 shares valued at $76,020 as compensation for services where the fair value of shares was determined based on the value of services received.
Cash costs in connection with the transactions above amounted to $1,358.
On October 8, 2014, the Company closed a subscription for 4,000,000 units at $0.025 per unit for gross proceeds of $100,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.05 per share until the earlier of 36 months from the date of issuance or 18 months following the date of a business combination between the Company and a public company pursuant to a reverse take-over, merger, amalgamation, take-over bid, insider bid, reorganization, joint venture, sale or exchange of assets or similar transaction or IPO. In connection with the subscription, the Company paid a finder's fee of $8,000 and issued an aggregate of 320,000 finder's warrants (Note 9), exercisable into one common share at a price of $0.025 per share until 18 months from the closing date.
On October 31, 2014, 3,566,638 warrants were exercised at $0.05 per warrant for gross proceeds of $178,332. An additional $17,467 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share purchase warrants have been amended to include an early exercise provision of an additional warrant exercisable into one common share at a price of $0.10 per share until 24 months from the date of issuance. As a result of the amendment, an additional 3,566,638 warrants were issued, as described in Note 12. The terms of any unexercised Company warrants outstanding at October 31, 2014 remain unchanged.
On March 13, 2015, 32,900,000 units at $0.05 per unit were issued for gross proceeds of $1,645,000 pursuant to the IPO. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance.
On March 13, 2015, the Company issued 2,404,800 compensation options under a warrant indenture valued at $63,000 based on the services provided. Each option is exercisable at $0.05 per unit, and comprised of one common share and one half of one share purchase warrant, exercisable into one additional common share at a price of $0.07 per share until 24 months from the date of issuance.
The accompanying notes are an integral part of these consolidated financial statements
| 10. | Share Capital (continued) |
On March 18, 2015, the Company paid an extension fee of $30,000 by issuing an aggregate of 600,000 units and one half of one share purchase warrant, exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance (Note 15).
On March 18, 2015, the Company paid a going public success fee of $35,000 by issuing an aggregate of 700,000 units and one half of one share purchase warrant, exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance to FMI Capital Advisory (formerly Foundation Opportunities Inc.), a company with a related director.
On March 26, 2015, 400,000 warrants were exercised for gross proceeds of $28,000. An additional $8,827 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On March 27, 2015, 400,000 warrants were exercised for gross proceeds of $28,000. An additional $8,827 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On March 30, 2015, 12,000 compensation options under the warrant indenture were exercised for $600 for 12,000 common shares and 6,000 share purchase warrants, exercisable at a price of $0.07 per share until March 16, 2017. An additional $314 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On April 7, 2015, 160,000 warrants were exercised for $4,000 for 160,000 common shares and 80,000 share purchase warrants, exercisable at a price of $0.05 per share until April 8, 2016. An additional $2,000 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On April 8, 2015, 6,000 warrants were exercised for gross proceeds of $420. An additional $132 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On April 20, 2015, 500,000 warrants were exercised for gross proceeds of $35,000. An additional $11,034 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On June 5, 2015, the Company issued 3,333,334 shares valued at $250,000 to satisfy the initial licensing and royalty commitment as described in Note 19.
On June 6, 2015, the Company issued 66,667 shares valued at $5,000 in exchange for 2% interest in NH Medicinal Dispensaries Inc. as described in Note 14.
On June 7, 2015, the Company issued 700,000 shares valued at $52,500 as compensation for services where the fair value of shares was determined based on the value of services received.
On July 31, 2015, the Company issued 111,111 shares valued at $5,000 as compensation for services where the fair value of shares was determined based on the value of services received.
Cash costs in connection with the transactions amounted to $239,194.
The accompanying notes are an integral part of these consolidated financial statements
| 11. | Reserve for Share Based Payments |
The Company established a stock option plan to provide additional incentive to its officers, directors, employees and consultants in their effort on behalf of the Company in the conduct of its affairs. Options vest immediately, unless otherwise stated, and expire on the fifth anniversary from the date of issue unless otherwise specified. The maximum number of common shares reserved for issuance for options that may be granted under the Plan is 10% of the total issued and outstanding Common shares, which was 11,936,938 at July 31, 2015.
The following table reflects the continuity of options for the year ended July 31, 2015:
| | | Number of Options | | | Amount $ | |
| Balance – July 31, 2013 | | | - | | | | - | |
Granted (i) | | | 2,800,000 | | | | 28,000 | |
| Balance – July 31, 2014 | | | 2,800,000 | | | | 28,000 | |
| Granted | | | 8,400,000 | | | | 286,000 | |
| Expired | | | (200,000 | ) | | | - | |
| Balance – July 31, 2015 | | | 11,000,000 | | | | 314,000 | |
| | (i) | The options granted on July 7, 2014 and have a fair value of $28,000, which was estimated using the Black-Scholes option pricing model and the following assumptions: |
| Risk-free interest rate | | | 1.14 | % | Expected volatility (Based upon comparable public companies) | | | 100 | % |
| Dividend yield | | Nil | | Expected life-options | | 60 months | |
| | | | | | Share price | | $ | 0.025 | |
During the year, the Company granted 8,400,000 stock options to certain officers, directors, consultants and advisory board members to purchase common shares of the Company at the exercise price of $0.10 exercisable until 60 months from the date of issuance.
Vesting periods on the options granted in 2015 are as follows: 7,050,000 stock options vested immediately upon issuance, 300,000 stock options issued on March 18, 2015, 50% of which vested immediately and the remaining 50% vest monthly over 6 months, 2,000,000 stock options issued on March 18, 2015, which vest in stages over a minimum of 12 months with no more than ¼ of options vesting any three month period, and the remaining vest quarterly over next 24 months.
The accompanying notes are an integral part of these consolidated financial statements
The estimated fair value of share based compensation during the year ended July 31, 2015 was determined using the Black-Scholes option pricing model with the following assumptions:
Options | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | October 10, 2014 | | | March 18, 2015 | | | April 1, 2015 | | | April 6, 2015 | | | June 10, 2015 | | | July 22, 2015 | | Total |
| Number of options | | | 400,000 | | | | 6,950,000 | | | | 250,000 | | | | 150,000 | | | | 400,000 | | | | 250,000 | | |
| Share price | | $ | 0.025 | | | $ | 0.050 | | | $ | 0.095 | | | $ | 0.110 | | | $ | 0.080 | | | $ | 0.050 | | |
| Risk-free interest rate | | | 1.56 | % | | | 0.75 | % | | | 0.98 | % | | | 0.98 | % | | | 0.95 | % | | | 0.83 | % | |
| Expected life of options | | 5 years | | | 5 years | | | 5 years | | | 5 years | | | 5 years | | | 5 years | | |
| Expected volatility | | | 100 | % | | | 100 | % | | | 209 | % | | | 209 | % | | | 209 | % | | | 209 | % | |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | |
| Fair value | | $ | 5,000 | | | $ | 199,000 | | | $ | 23,000 | | | $ | 16,000 | | | $ | 31,000 | | | $ | 12,000 | | $286,000 |
Option pricing models require the input of highly subjective assumptions including the expected price volatility. Changes in the subjective input assumptions can materially affect the fair value estimated, and therefore, the existing models do not necessarily provide a reliable measure of the fair value of the Company's stock options. Expected volatility is based on comparable companies.
The weighted average remaining contractual life for outstanding options is as follows:
Exercise Price | | Number of Options | | Weighted Average Exercise Price | | Weighted Average Remaining Life (years) | | Number of Options - exercisable | |
| | | | | | | | | |
| | $ | 0.10 | | | | 11,000,000 | | | $ | 0.10 | | | | 4.46 | | | | 9,350,000 | |
The following table reflects the continuity of warrants for the year ended July 31, 2015:
| | | Number of Warrants | | | Amount $ | |
| Balance - from incorporation | | | - | | | | - | |
Warrants pursuant to private placement and option agreement (ii) | | | 13,650,006 | | | | 65,000 | |
| Balance – July 31, 2014 | | | 13,650,006 | | | | 65,000 | |
| Warrants pursuant to private placement and option agreement (Note 10) | | | 5,966,638 | | | | 88,000 | |
| Warrants pursuant to IPO (Note 10) | | | 16,450,000 | | | | 363,000 | |
| Compensation warrants (Note 10) | | | 2,410,800 | | | | 64,000 | |
| Warrants issued for success and extension fees (Note 10) | | | 650,000 | | | | 35,000 | |
| Warrants exercised (Note 10) | | | (5,044,638 | ) | | | (48,601 | ) |
| Balance – July 31, 2015 | | | 34,082,806 | | | | 566,399 | |
The accompanying notes are an integral part of these consolidated financial statements
| 12. | Reserve for Warrants (continued) |
| | (ii) | The warrants issued pursuant to the private placement and option agreement which have a fair value of $65,000, were estimated using the Black-Scholes option pricing model and the following assumptions. |
| Risk-free interest rate | 1.04-1.12% | | Expected volatility (Based upon comparable public companies) | 100% |
| Dividend yield | nil | | Expected life-options | 18 months |
During the year ended July 31, 2015, the Company issued warrants to purchase common shares, valued at $591,000 using the Black-Scholes option pricing model using the following assumptions:
Warrants | | | | | | | | | | | | | |
| | October 8, 2014 | | October 31, 2014 | | March 13, 2015 | | March 18, 2015 | | March 30, 2015 | | April 7, 2015 | | Total |
| Number of options | | | 2,320,000 | | | | 3,566,638 | | | | 18,854,800 | | | | 650,000 | | | | 6,000 | | | | 80,000 | | |
| Share price | | $ | 0.025 | | | $ | 0.100 | | | $ | 0.050 | | | $ | 0.130 | | | $ | 0.110 | | | $ | 0.100 | | |
| Risk-free interest rate | | | 1.05 | % | | | 1.04 | % | | | 0.49 | % | | | 0.49 | % | | | 0.49 | % | | | 0.67 | % | |
| Expected life of warrants | 1.5 years | | 2 years | | 2 years | | 2 years | | 2 years | | 2 years | | |
| Expected volatility | | | 100 | % | | | 100 | % | | | 100 | % | | | 209 | % | | | 209 | % | | | 209 | % | |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | |
| Valued at | | $ | 18,000 | | | $ | 64,000 | | | $ | 426,000 | | | $ | 35,000 | | | $ | 1,000 | | | $ | 6,000 | | $550,000 |
Warrants to purchase common shares carry exercise prices and terms to maturity at July 31, 2015 as follows:
| Date of Issue | | No. of warrants | | | Exercise Price ($) | | Expiry Date |
| | | | | | | | |
| October 8, 2014 | | | 2,000,000 | | | | 0.050 | | April 7, 2016 |
| October 8, 2014 | | | 160,000 | | | | 0.025 | | April 7, 2016 |
| June 27, 2014 | | | 150,000 | | | | 0.100 | | June 26, 2016 |
| October 31, 2014 | | | 3,566,638 | | | | 0.100 | | October 31, 2016 |
| May 16, 2014 | | | 7,536,789 | | | | 0.050 | | December 27, 2015 |
| May 30, 2014 | | | 1,090,399 | | | | 0.050 | | December 27, 2015 |
| June 20, 2014 | | | 1,306,180 | | | | 0.050 | | December 27, 2015 |
| March 13, 2015 | | | 15,800,000 | | | | 0.070 | | March 12, 2017 |
| March 13, 2015 | | | 2,392,800 | | | | 0.050 | | March 12, 2017 |
| April 7, 2015 | | | 80,000 | | | | 0.050 | | April 6, 2017 |
| | | | 34,082,806 | | | | | | |
| 13. | Reverse Takeover Transaction |
On June 27, 2014, Sonoma Capital Inc. ("Sonoma") closed its reverse takeover transaction ("the Transaction") with Nutritional High whereby it acquired all of the issued and outstanding securities of Nutritional High from the Vendors and changed its name to Nutritional High International Inc. Pursuant to the transaction, the Vendors exchanged an aggregate of 60,400,011 Nutritional shares and 13,650,006 warrants, being all of the issued and outstanding Nutritional shares and warrants held by the Vendors, for the same number of shares and warrants of Sonoma.
The accompanying notes are an integral part of these consolidated financial statements
| 13. | Reverse Takeover Transaction (continued) |
Although the Amalgamation will result in a legal combination of Nutritional High and Sonoma to form the Resulting Issuer, because Sonoma does not meet the criteria for a business per IFRS 3, from an accounting perspective, the Amalgamation is considered to be a takeover transaction. The Amalgamation is not considered to be a business combination but a capital transaction whereby Nutritional High was considered to issue additional shares in return for the net liabilities of Sonoma.
For financial reporting purposes, this is considered a continuation of Nutritional High, the legal subsidiary, which was incorporated on April 17, 2014.
The transaction is a reverse acquisition of Sonoma and has been accounted for under IFRS 2, Share-based Payments. Accordingly, the transaction has been accounted for at the fair value of the equity instruments granted by the shareholders of Nutritional High to the shareholders of Sonoma. The difference between the fair value of the consideration paid of $287,840 (based upon the fair value of Nutritional High shares just prior to the reverse acquisition) and the Sonoma net liabilities acquired of $67,983, in the amount of $355,823, has been recognized as a listing expense in the statement of comprehensive loss for the period ended July 31, 2014.
The results of operations of Sonoma are included in the consolidated financial statements of Nutritional High from the date of the reverse acquisition, June 27, 2014.
The following represents management's estimate of the fair value of the net liabilities acquired at June 27, 2014 as a result of the reverse acquisition.
| Fair value of share consideration paid (11,513,620 shares at $0.025 per share) | | | $ | 287,840 | |
| | | | | |
| | | | | |
| Cash | $ | 121,279 | | | | |
| Accounts payables and accrued liabilities | | (189,262 | ) | | |
| Net liabilities acquired | | (67,983 | ) | | | (67,983 | ) |
| Listing expense | | | | 355,823 | |
| 14. | Non-controlling Interest |
On June 6, 2015, the Company acquired the minority shareholder's 2% interest in NH Medicinal Dispensaries Inc., in exchange for 66,667 shares of NHII, valued at $0.075 per share. The loss on acquisition in the amount of $5,000 is presented as "loss on acquisition of non-controlling interest" within shareholders' equity.
The Company's 51% interest in Eglinton Medicinal Advisory Ltd. is consolidated into the Company's consolidated financial statements. The 49% interest attributable to a minority shareholder is then presented as "non-controlling interest" within shareholders' equity on the consolidated statement of financial position. Net loss and comprehensive loss is allocated between the Company's 51% ownership and non-controlling 49% ownership interest. The Company recorded $9,073 (2014 - $nil) of the subsidiary's net loss and comprehensive loss related to the non-controlling interest during the year ended July 31, 2015.
The accompanying notes are an integral part of these consolidated financial statements
| 15. | Convertible Debentures |
On November 17, 2014, the Company closed its non-brokered private placement of secured convertible debentures for total gross proceeds of $600,000 as follows:
-
| | a. | Senior convertible debenture of $450,000, bearing interest at 12%, maturing in 24 months from date of issue, and secured by a first ranking general security interest over all assets of the Company. The senior convertible debenture is convertible into common shares of the Company at any time prior to the maturity date at a price equal to a 20% premium to the price at which the Company completes its going public transaction or $0.06 per Company share ("Conversion Price"). If the Company fails to complete the going public transaction on or before January 31, 2015, the Conversion Price will be reduced to $0.05 per Company share. If the Company completes the going public transaction on or before January 31, 2015, but less than $1,000,000 is raised, the Conversion Price will be equal to the price at which the Company completes the going public transaction ("Conversion Price Adjustment") and the Company will issue to the holder 450,000 Company shares immediately prior to closing the going public transition. On January 19, 2015, an amendment was made to the agreement to extend the going public date from January 31, 2015 to March 16, 2015, in consideration for $30,000, convertible into units at the offering price. |
| | b. | Subordinate convertible debenture of $150,000, bearing interest at 12%, maturing in 24 months from date of issue, and secured by a general security interest over all assets of the Company, subordinate to the senior convertible debenture. The group of lenders is comprised of directors of the Company. The subordinate convertible debenture carries the same Conversion Prices and Conversion Price Adjustment as the senior convertible debenture described above. On January 19, 2015, an amendment was made to the agreement to extend the going public date from January 31, 2015 to March 16, 2015. |
The debentures are classified as a liability at amortized cost for the host component and its embedded derivative was classified at fair value through profit and loss as the conversion feature of debentures failed equity classification. The fair value of the derivative was calculated using the Black-Scholes model with the following assumptions: (November 17, 2014 and July 31, 2015: share price: $.02 and $.045; risk free interest rate: 1%; expected life: 2 and 1.3 years; expected volatility 100% and 209%; dividend yield 0%). The assumptions used in the Black-Scholes model are based on management's estimate of the rates that are reflective for the Company. Changes in these estimates could result in significant changes in the fair value of the derivative. The discount is being accreted over the term of the debenture utilizing the effective interest rate method at a 5.22% discount rate.
A fair value adjustment loss on the convertible debentures for the year ended July 31, 2015, of $277,753 (2014: $Nil) has been reflected in the consolidated statement of comprehensive loss as a change in the fair value of the derivative liability. The fair value of the derivative liability at the inception was $52,312. The interest and accretion expense in the amount of $50,992 and $22,224 are recorded as a finance cost.
The debt issue costs in the amount of $30,000 were recorded against the debentures liability and amortized using the effective interest method. As at July 31, 2015, $4,835 is recorded as accrued interest in accounts payable and accrued liabilities.
The accompanying notes are an integral part of these consolidated financial statements
The Company manages its capital structure and makes adjustments to it, based on the funds available to the Company, in order to support the development of its planned business activities. The Board of Directors does not establish quantitative return on capital criteria for management, but rather relies on the expertise of the Company's management to sustain future development of the business. In order to carry out the planned business activities and pay for administrative costs, the Company will spend its existing working capital and raise additional funds as needed. Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable. There were no changes in the Company's approach to capital management during the year ended July 31, 2015. The Company is not subject to externally imposed capital requirements.
The Company considers its capital to be shareholders' equity, which is comprised of share capital, reserve for warrants, share based payments and deficit, which as at July 31, 2015 totaled $859,697.
The Company's objective when managing capital is to obtain adequate levels of funding to support its business activities, to obtain corporate and administrative functions necessary to support organizational functioning and obtain sufficient funding to further the development of its business. The Company raises capital, as necessary, to meet its needs and take advantage of perceived opportunities and, therefore, does not have a numeric target for its capital structure. Funds are primarily secured through equity capital raised by way of private placements, initial public offering and issuance of convertible debentures. There can be no assurance that the Company will be able to continue raising equity capital in this manner.
Fair Value of Financial Instruments
The fair value hierarchy that reflects the significance of inputs used in making fair value measurements as follows:
| | Level 1 | quoted prices in active markets for identical assets or liabilities; |
| | Level 2 | inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. from derived prices); and |
| | Level 3 | inputs for the asset or liability that are not based upon observable market data. |
Assets are classified in their entirety based on the lowest level of input that is significant to the fair value measurement.
The Company designated its cash as fair value through profit and loss, which is measured at fair value and is classified as Level 1. The Company designated its derivative liability from convertible debentures as fair value through profit and loss which is measured at fair value and classified as level 2.
The carrying value of the Company's other receivable, amounts due from Palo Verde LLC and accounts payable and accrued liabilities and convertible debentures (except for derivative liability which is recorded at fair value) approximate their fair value due to the relatively short periods to maturity of these instruments.
The accompanying notes are an integral part of these consolidated financial statements
Fair Value of Financial Instruments (continued)
Fair value estimates are made at a specific point in time, based on relevant market information and information about financial instruments. These estimates are subject to and involve uncertainties and matters of significant judgment, therefore cannot be determined with precision. Changes in assumptions could significantly affect the estimates.
A summary of the Company's risk exposures as it relates to financial instruments are reflected below:
Credit risk
Credit risk is the risk of loss associated with counterparty's inability to fulfill its payment obligations. The Company's credit risk is primarily attributable to cash and amounts due from Palo Verde LLC and rent receivable (Note 5). The Company has no significant concentration of credit risk arising from operations. Cash is held with a reputable Canadian chartered bank which is closely monitored by management. The Board of Directors meets on a quarterly basis to review and assess the risk profile of the loan. Management believes that the credit risk concentration with respect to financial instruments included in cash, amounts due from Palo Verde LLC and other receivable is not material for the Company.
Liquidity risk
Liquidity risk is the risk that the Company will not have sufficient cash resources to meet its financial obligations as they come due. The Company's liquidity and operating results may be adversely affected if the Company's access to the capital market is hindered, whether as a result of a downturn in stock market conditions generally or related to matters specific to the Company. The Company generates cash flow primarily from its financing activities. The Company's approach to managing liquidity risk is to ensure that it will have sufficient liquidity to meet liabilities when due. As at July 31, 2015, the Company had current assets of $388,713 (2014 - $673,525) and current liabilities of $272,274 (2014 - $220,150). All of the Company's financial liabilities and receivables, excluding a loan receivable (Note 5), have contractual maturities of less than 90 days and are subject to normal trade terms. As at July 31, 2015, working capital of the Company is $116,437 (2014 - $453,375).
Foreign currency exchange risk
The Company conducts a portion of its purchases in US dollars which results in the foreign currency exchange risk. The Company does not consider its exposure to foreign currency exchange risk to be material.
An increase (decrease) of 10% in the currency exchange rate of the Canadian dollar versus US dollar would have impacted net loss by $16,923 ($16,923) as a result of the Company's exposure to currency exchange rate fluctuations.
Interest rate risk
Interest rate risk is the potential for financial loss arising from changes in interest rates. Financial instruments that potentially subject the Company to interest rate risk include financial liabilities with fixed interest rates.
The Company manages interest rate risk by monitoring market conditions and the impact of interest rate fluctuations on its debt.
The accompanying notes are an integral part of these consolidated financial statements
| 17. | Financial Instruments (continued) |
Interest rate risk (continued)
Net earnings are sensitive to the impact of a change in interest rates on the average balance of interest bearing financial liabilities during the year.
An increase (decrease) of 25 basis points would have impacted net loss by $2,442 ($2,442) as a result of the Company's exposure to interest rate fluctuations.
Provision for income taxes
No deferred tax asset has been recognized because of the uncertainty as to the utilization of the losses for income tax purposes. The Company has accumulated losses for Canadian income tax purposes expiring as follows:
The Company has share issue costs of $192,126 available for deduction against future Canadian taxable income over the next four years.
| | | 2015 | | | 2014 | |
| | | | | | | |
| Loss before income taxes | | $ | (2,068,360 | ) | | $ | (681,155 | ) |
| Tax rate | | | 26.5 | % | | | 26.5 | % |
| Calculated income tax recovery | | | (548,115 | ) | | | (180,506 | ) |
| Non-deductible expense and other | | | 6,851 | | | | 8,061 | |
| Non-deductible listing expense | | | 5,889 | | | | 94,293 | |
| Change in deferred taxes not recognized | | | 535,375 | | | | 78,152 | |
| Income tax expense | | $ | - | | | $ | - | |
The tax effects of temporary differences that give rise to future income tax assets and liabilities are as follows:
| | | 2015 | | | 2014 | |
| Deferred income tax assets | | | | | | |
| Non-capital loss carry forwards | | $ | 620,812 | | | $ | 77,864 | |
| Issue costs | | | (14,409 | ) | | | 288 | |
| Investment property | | | 6,764 | | | | - | |
| | | | (613,167 | ) | | | 78,152 | |
| Less: Deferred taxes not recognized | | | (613,167 | ) | | | (78,152 | ) |
| | | $ | - | | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements
| 19. | Licensing and Royalty Agreement |
On June 5, 2015, the Company entered into the agreement with Purple Haze Properties LLC for the exclusive right to manufacture and distribute marijuana and hemp oil-infused products, and non-exclusive rights to manufacture and distribute certain apparel and accessories in the United States and Canada.
Under the terms of the agreement, the Company is to issue 3,333,334 common shares (Note 10) valued at USD$250,000 to pay for the annual exclusivity fee (USD $200,000) for the first out of five years and royalties USD $50,000 which was due on signing the agreement. The agreement provides for annual exclusivity fees and royalties of no less than USD $1,000,000 over five years with an additional renewal option for an additional five years. The agreement term commences the earlier of the first product sale or October 1, 2015, with minimums payable in cash or shares at the Company's option.
As at July 31, 2015, the Company recorded $249,780 as a prepaid license and $62,445 as a prepaid expense for the advance on royalties and accrued $65,170 as the remaining liability for the first year of exclusivity and royalty obligation under the terms of the agreement.
The Company has one reportable segment which is Marijuana-Infused Products. The segment reflects the basis on which management measures performance and makes decisions regarding the allocation of resources.
On August 20, 2015, the Company issued 692,431 shares valued at $51,932 to Purple Haze Properties LLC as the final instalment on its initial licensing obligations, as described in Note 19.
On October 23, 2015, a holder of the convertible debentures (Note 15) converted $180,000 in convertible debentures into 3,000,000 common shares of the Company at a share price of $0.06 per share.
On October 26, 2015, the Company through its owned subsidiary NH Medicinals Inc. made a refundable deposit of $6,581 (USD $5,000) on the purchase of the commercial property located in Somerset County, Maryland, USA. The acquisition price for the property is USD $350,000. The settlement date is to be within 45 days after the Company receives Stage 1 license approval, but in no event, later than 365 days after the execution date of this contract.
On November 5, 2015, a holder of the convertible debentures (Note 15) converted $100,000 in convertible debentures into 1,666,667 common shares of the Company at a share price of $0.06 per share.
On November 5, 2015, 1,600,000 warrants were exercised for gross proceeds of $80,000.
The accompanying notes are an integral part of these consolidated financial statements
| 21. | Subsequent events (continued) |
On November 11, 2015, a holder of the convertible debentures (Note 15) converted $70,000 in convertible debentures into 1,166,667 common shares of the Company at a share price of $0.06 per share.
During the year ended July 31, 2015, the Company through its owned subsidiary NH Medicinal Dispensaries Inc. made a refundable deposit of $6,671 (USD $5,000) on the purchase of the commercial property located in Lawrenceville, Illinois, USA. On November 4, 2015, the vendor of the real estate property in Illinois where the Company's dispensary will be located agreed to provide a buyer take-back mortgage in the amount of USD $250,000. The mortgage will have a 15 year amortization period, bearing an interest at the rate of 6% and be due in two years from the date of issuance as a balloon payment. The acquisition price for the commercial property was USD $350,000 and the Company closed the acquisition on November 25, 2015.
On November 17, 2015, 1,000,000 warrants were exercised for gross proceeds of $50,000.
The accompanying notes are an integral part of these consolidated financial statements
Until [_________], 2016 (___ days after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Nutritional High International Inc.
29,178,000 Shares of Common Stock
____________________________
PROSPECTUS
____________________________
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 6. Indemnification of Directors and Officers
Sections 136 of the Business Corporations Act (Ontario) ("OBCA") and 124 of the Canada Business Corporations Act ("CBCA") allow for the indemnification of directors and officers in relatively narrow circumstances. Both Acts permit a corporation to indemnify a director or officer of the corporation, either in their former or present capacity, against:
"…all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of that association with the corporation or other entity."
However, the corporation may only do this if the individual:
"acted honestly and in good faith with a view to the best interests of the corporation…and, in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the individual had reasonable grounds for believing that the individual's conduct was lawful."
In the case of a derivative action where the individual has met the above criteria, the individual can only be indemnified "with the approval of a court". In all cases, if an individual met the above requirement and additionally "was not judged by the court or other competent authority to have committed any fault or omitted to do anything that the individual ought to have done," then the corporation must indemnify the individual.
The court has shown a willingness to follow these sections strictly, stating that "according to s. 136(1), indemnification is permitted if the directors acted honestly, in good faith and in the reasonable belief that their conduct was lawful. If [the directors] failed to meet these requirements, [a separate] order is superfluous because [the company] is prohibited from indemnifying them under s. 136(1). If they did act honestly, in good faith and in the reasonable belief that their conduct was lawful, [a separate] order contradicts the legislative scheme of the Ontario Business Corporations Act".
Our directors and officers are currently covered by a directors' and officers' liability insurance policy. As of the date of this prospectus, no claims for directors' and officers' liability insurance have been filed under this policy and we are not aware of any pending or threatened litigation or proceeding involving any of our office holders, including our directors, in which indemnification is sought.
Item 7. Recent Sales of Unregistered Securities
During the past two years, we have issued and sold the securities described below without registering the securities under the Securities Act. None of these transactions involved any underwriters' underwriting discounts or commissions, or any public offering. We believe that each of the following issuances was exempt from registration under the Securities Act in reliance on Regulation S under the Securities Act regarding sales by an issuer in offshore transactions, Regulation D under the Securities Act, Rule 701 under the Securities Act or pursuant to Section 4(a)(2) of the Securities Act regarding transactions not involving a public offering.
On April 17, 2014, the Company issued 33,000,000 common shares, valued at $0.005 per share for gross proceeds of $165,000. On May 12, 2014, the Company issued 400,000 Common Shares, valued at $0.005 per share for gross proceeds of $2,000.
On June 27, 2014, in connection with the acquisition of NHL, the Company issued an aggregate of 60,400,011 Common Shares and 13,500,006 Series I Warrants, on a one-for-one basis, in exchange for the NHL securities held by the vendors. In addition, 150,000 Series II Warrants were issued in connection with the Acquisition.
For the year ended July 31, 2014 the Company issued 11,440,798 Common Shares valued at $76,020 as compensation for services where the fair value of shares was determined based on the value of services received.
On October 8, 2014, the Company completed a private placement offering of 4,000,000 units at $0.025 per unit for gross proceeds of $100,000. Each unit consisted of one Common Share and one-half of one Company Warrant, with each whole Company Warrant exercisable into one Common Share at a price of $0.05 per share for a period of 18 months from closing.
On October 31, 2014, 3,566,638 warrants were exercised at $0.05 per warrant for gross proceeds of $178,332. An additional $17,467 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants. The share purchase warrants have been amended to include an early exercise provision of an additional warrant exercisable into one common share at a price of $0.10 per share until 24 months from the date of issuance. As a result of the amendment, an additional 3,566,638 warrants were issued. The terms of any unexercised Company warrants outstanding at October 31, 2014 remain unchanged.
On March 13, 2015, 32,900,000 units at $0.05 per unit were issued for gross proceeds of $1,645,000 pursuant to the IPO. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance.
On March 13, 2015, the Company issued 2,404,800 compensation options under a warrant indenture valued at $63,000 based on the services provided. Each option is exercisable at $0.05 per unit, and comprised of one common share and one half of one share purchase warrant, exercisable into one additional common share at a price of $0.07 per share until 24 months from the date of issuance.
On March 18, 2015, the Company paid an extension fee of $30,000 by issuing an aggregate of 600,000 units and one half of one share purchase warrant, exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance.
On March 18, 2015, the Company paid a going public success fee of $35,000 by issuing an aggregate of 700,000 units and one half of one share purchase warrant, exercisable into one common share at a price of $0.07 per share until 24 months from the date of issuance.
On March 26, 2015, 400,000 warrants were exercised for gross proceeds of $28,000. An additional $8,827 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On March 27, 2015, 400,000 warrants were exercised for gross proceeds of $28,000. An additional $8,827 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On March 30, 2015, 12,000 compensation options under the warrant indenture were exercised for $600 for 12,000 common shares and 6,000 share purchase warrants, exercisable at a price of $0.07 per share until March 16, 2017. An additional $314 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On April 7, 2015, 160,000 warrants were exercised for $4,000 for 160,000 common shares and 80,000 share purchase warrants, exercisable at a price of $0.05 per share until April 8, 2016. An additional $2,000 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On April 8, 2015, 6,000 warrants were exercised for gross proceeds of $420. An additional $132 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On April 20, 2015, 500,000 warrants were exercised for gross proceeds of $35,000. An additional $11,034 credited to share capital represents a transfer of the reserve for warrants in respect of the exercised warrants.
On June 5, 2015, the Company issued 3,333,334 shares valued at $250,000 to satisfy the initial licensing and royalty commitment as described in Note 19.
On June 6, 2015, the Company issued 66,667 shares valued at $5,000 in exchange for 2% interest in NH Medicinal Dispensaries Inc. as described in Note 14.
On June 7, 2015, the Company issued 700,000 shares valued at $52,500 as compensation for services where the fair value of shares was determined based on the value of services received.
On July 31, 2015, the Company issued 111,111 shares valued at $5,000 as compensation for services where the fair value of shares was determined based on the value of services received.
On August 20, 2015, the Company issued 692,431 shares valued at $51,932 as the final instalment on its initial licensing obligations.
On September 7, 2015, the Company issued 406,668 shares valued at $20,333 as compensation for services where the fair value of shares was determined based on the value of services received.
On October 5, 2015, 8,000 warrants were exercised for gross proceeds of $400.
On October 23, 2015, a holder of the convertible debentures converted $180,000 in convertible debentures into 3,000,000 common shares of the Company at a share price of $0.06 per share.
On October 26, 2015, the Company issued 50,000 shares valued at $2,500 as compensation for services where the fair value of shares was determined based on the value of services received.
On November 5, 2015, 1,600,000 warrants were exercised for gross proceeds of $80,000.
On November 5, 2015, a holder of the convertible debentures converted $100,000 in convertible debentures into 1,666,667 common shares of the Company at a share price of $0.06 per share.
On November 11, 2015, a holder of the convertible debentures converted $70,000 in convertible debentures into 1,166,667 common shares of the Company at a share price of $0.06 per share.
On November 17, 2015, 1,000,000 warrants were exercised for gross proceeds of $50,000.
On December 2, 2015, the Company completed a non-brokered private placement of 4,200,000 units at $0.05 per unit for gross proceeds of $210,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
On January 28, 2015, the Company issued 2,822,700 Common Shares valued at $$141,135 as compensation for services where the fair value of shares was determined based on the value of services received.
On January 31, 2016, the Company completed a non-brokered private placement of 800,000 units at $0.05 per unit for gross proceeds of $40,000. Each unit consisted of one common share and one half of one share purchase warrant, with each warrant exercisable into one common share at a price of $0.07 per share for a period of 18 months from the date of issuance.
On February 29, 2016 and on March 2, 2016, each of David Posner, CEO and Director, and 1306413 Ontario Limited, respectively, advanced CAD $15,000 (a total of $30,000), which was added to the Subordinated Convertible Debenture. As of the date of this prospectus the total amount of Subordinated Convertible Debentures is CAD $180,000.
On March 18, 2016, the Company issued 5,000,000 Common Shares at a price of USD $0.05 per share to Purple Haze Properties LLC per the license agreement addendum with Purple Haze Properties LLC.
Item 8. Exhibits and Financial Statement Schedules
(a) Exhibits
See the Exhibit Index for a list of exhibits filed as part of this registration statement on Form F-1, which Exhibit Index is incorporated herein by reference.
The agreements included as exhibits to this registration statement contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties were made solely for the benefit of the other parties to the applicable agreement and (i) were not intended to be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; (ii) may have been qualified in such agreement by disclosures that were made to the other party in connection with the negotiation of the applicable agreement; (iii) may apply contract standards of "materiality" that are different from "materiality" under the applicable securities laws; and (iv) were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement.
We acknowledge that, notwithstanding the inclusion of the foregoing cautionary statements, we are responsible for considering whether additional specific disclosures of material information regarding material contractual provisions are required to make the statements in this registration statement not misleading.
(b) Financial Statement Schedules
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the Consolidated Financial Statements or the Notes thereto.
Item 9. Undertakings
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement.
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) To file a post-effective amendment to the registration statement to include any financial statements required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that the Registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (4) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by Section 10(a)(3) of the Act or Rule 3-19 of Regulation S-X if such financial statements and information are contained in periodic reports filed with or furnished to the Commission by the Registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the Form F-3.
(5) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(6) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereonto duly authorized in Toronto, Canada, on April 12, 2016.
| | | |
| | Nutritional High International Inc. | |
| | | | |
| Date: April 12, 2016 | By: | /s/ David Posner | |
| | | David Posner | |
| | | Chief Executive Officer and Director
(Principal Executive Officer) | |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | | Title | | Date | |
| | | | | | |
/s/ David Posner | |
President, Chief Executive Officer (Principal Executive Officer) and Director
| | April 12, 2016 | |
| David Posner | | | | | |
| | | | | | |
/s/ Al Quong | | Chief Financial Officer (Principal Financial and Accounting Officer) | | April 12, 2016 | |
| Al Quong | | | | | |
| | | | | | |
/s/ Gary Margolin | | Chief Operating Officer | | April 12, 2016 | |
| Gary Margolin | | | | | |
| | | | | | |
/s/ Adam Szweras | | Director and Corporate Secretary | | April 12, 2016 | |
| Adam Szweras | | | | | |
| | | | | | |
/s/ Anne Marie Youhana | | VP Product Development and Quality Control | | April 12, 2016 | |
| Anne Marie Youhana | | | | | |
/s/ Statis Rizas | | Chairman of the Board | | April 12, 2016 |
| Statis Rizas | | | | |
/s/ David Caplan | | Independent Director | | April 12, 2016 | |
| David Caplan | | | | | |
| | | | | | |
/s/ Brian Presement | | Independent Director | | April 12, 2016 | |
| Brian Presement | | | | | |
| | | | | | |
/s/ Billy Morrison | | Independent Director | | April 12, 2016 | |
| Billy Morrison | | | | | |
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the requirements of the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of Nutritional High International Inc. has signed this registration statement or amendment thereto in the City of San Jose, State of California, on April 12, 2016.
| | |
| | | |
| | By: | /s/ Billy Morrison |
| | Name: | Billy Morrison, Director |
EXHIBIT INDEX
| Exhibit Number | Exhibit Description | Form | File Number | Date of First Filing | Exhibit Number | Filed Herewith |
| 2.1 | Securities Exchange Agreement | F-1 | 333-209379 | 2/4/2016 | 2.1 | |
| 3.1 | Certificate of Incorporation | F-1 | 333-209379 | 2/4/2016 | 3.1 | |
| 3.1 (a) | Certificate of Amendment, dated June 27, 2014, to change the name of the Company | F-1 | 333-209379 | 2/4/2016 | 3.1 (a) | |
| 3.1 (b) | Certificate of Amendment, dated June 27, 2014 | F-1 | 333-209379 | 2/4/2016 | 3.1 (b) | |
| 3.2 | Bylaws | F-1 | 333-209379 | 2/4/2016 | 3.2 | |
| 4.1 | Form of Series I Warrant | F-1 | 333-209379 | 2/4/2016 | 4.1 | |
| 4.2 | Form of Series II Warrant | F-1 | 333-209379 | 2/4/2016 | 4.2 | |
| 4.3 | Form of Series III Warrant | F-1 | 333-209379 | 2/4/2016 | 4.3 | |
| 4.4 | Form of Unit Warrant | F-1 | 333-209379 | 2/4/2016 | 4.4 | |
| 4.5 | Form of Series IV Warrant | F-1 | 333-209379 | 2/4/2016 | 4.5 | |
| 4.6 | Form of Finder's Warrant | F-1 | 333-209379 | 2/4/2016 | 4.6 | |
| 4.7 | Form of Senior Convertible Note | F-1 | 333-209379 | 2/4/2016 | 4.7 | |
| 4.8 | Form of Subordinated Convertible Note | F-1 | 333-209379 | 2/4/2016 | 4.8 | |
| 4.8 (a) | Subordinated Convertible Note issued to Adam Szweras, Statis Rizas and David Posner, dated November 18, 2014 | F-1/A | 333-209379 | 3/11/2016 | 4.8 (a) | |
| 4.8 (b) | Second Amendment To The Amended and Restated Secured Convertible Debenture made as of the 18th day of January, 2015 | F-1/A | 333-209379 | 3/11/2016 | 4.8 (b) | |
| 4.9 | Promissory Note dated November 20, 2015 | F-1 | 333-209379 | 2/4/2016 | 4.9 | |
| Opinion of Fogler, Rubinoff LLP | | | | | X |
| 10.1 (a) | Lease Agreement (MIP) dated July 23, 2014 and subsequently amended on August 31, 2015 and December 17, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.1 (a) | |
| 10.1 (b) | Lease Agreement (MIP) Amendment dated August 31, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.1 (b) | |
| 10.1 (c) | Lease Agreement (MIP) Amendment and Lease Agreement (Cultivation) Amendment dated December 17, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.1 (c) | |
| 10.2 (a) | Lease Agreement (Cultivation) dated July 23, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.2 (a) | |
| 10.2 (b) | Lease Agreement (Cultivation) Amendment dated December 16, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.2 (b) | |
| 10.2 (c) | Lease Agreement (Cultivation) Amendment dated August 31, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.2 (c) | |
| 10.3 (a) | Lawrenceville PSA dated August 24, 2014, and amended on September 3, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.3 (a) | |
| 10.3 (b) | Lawrenceville PSA Amendment dated September 3, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.3 (b) | |
| 10.4 | Installment Contract for Sale dated August 24, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.4 | |
| 10.5 | Guaranty of Payment dated November 18, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.5 | |
| 10.6 | Warranty Deed dated November 25, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.6 | |
| 10.7 (a) | Revolving Loan Agreement dated July 23, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.7 (a) | |
| 10.7 (b) | Revolving Loan Agreement Amendment dated July 23, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.7 (b) | |
| 10.8 | Escrow Agreement dated March 13, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.8 | |
| 10.9 | Consulting Agreement between Nutritional High Ltd. and David Posner dated May 1, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.9 | |
| 10.10 | Management Services Agreement between Nutritional High Ltd. and Branson Corporate Services Inc. dated May 1, 2014 and subsequently amended on October 1, 2014, October 26, 2014 and October 27, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.10 | |
| 10.11 | Stock Option Plan dated July 7, 2014 | F-1 | 333-209379 | 2/4/2016 | 10.11 | |
| 10.12 | Audit Committee Charter | F-1 | 333-209379 | 2/4/2016 | 10.12 | |
| 10.13 | Equity Purchase Agreement with Kodiak Capital, dated December 23, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.13 | |
| 10.14 | Registration Rights Agreement with Kodiak Capital, dated December 23, 2015 | F-1 | 333-209379 | 2/4/2016 | 10.14 | |
| 10.15 | Amendment to Registration Rights Agreement with Kodiak Capital, dated March 4, 2016 | F-1/A | 333-209379 | 3/11/2016 | 10.15 | |
| 10.16 (a) | Advisory and Consulting Agreement with FMI Capital Advisory Inc., dated May 1, 2014 | F-1/A | 333-209379 | 3/11/2016 | 10.16 (a) | |
| 10.16 (b) | Advisory and Consulting Agreement Amendment with FMI Capital Advisory Inc., dated October 27, 2014 | F-1/A | 333-209379 | 3/11/2016 | 10.16 (b) | |
| 10.16 (c) | Advisory and Consulting Agreement Amendment with FMI Capital Advisory Inc., dated September 11, 2015 | F-1/A | 333-209379 | 3/11/2016 | 10.16 (c) | |
| 10.17 | Assignment of Intellectual Property Agreement netween Nutritional High Ltd. and Melissa Parks dated June 6, 2015 | F-1/A | 333-209379 | 3/11/2016 | 10.17 | |
| 10.18 | Restated Letter Agreement between ILDISP, LLC, NHCI, NHII, NHMD and SMHI dated April 4, 2016 | | | | | X |
| 10.19 | Cannabis Products License Agreement between Purple Haze Properties LLC and Nutritional High International Inc. dated June 5, 2015 | | | | | X |
| 10.20 | Caabis Products License Agreement Addendum between Purple Haze Properties LLC and Nutritional High International Inc. dated March 7, 2016 | | | | | X |
| Consent of Collins Barrow Toronto LLP | | | | | X |
| 23.2 | Consent of Fogler, Rubinoff LLP (included as part of Exhibit 5.1 herein) | | | | | X |
147