Acerta Pharma Collaboration
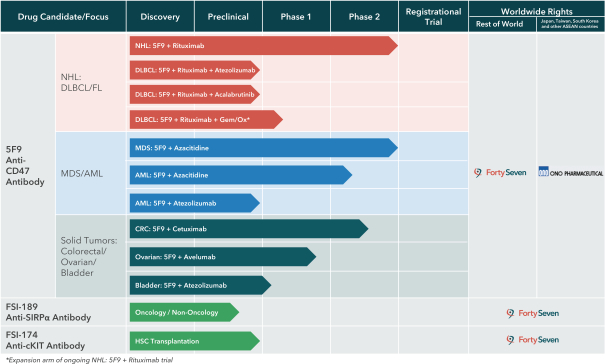
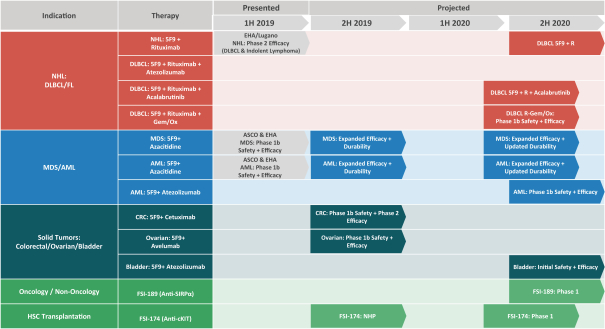
In May 2019, we entered into a collaboration with Acerta Pharma, AstraZeneca’s hematology research and development center of excellence, to evaluate 5F9 in combination with rituximab plus AstraZeneca/Acerta Pharma’s Bruton’s tyrosine kinase, or BTK, inhibitor, acalabrutinib (CALQUENCE), in patients with DLBCL. This approach aims to optimize the treatment of DLBCL patients by inhibiting BTK. InB-cells, BTK signaling results in the activation of pathways associated withB-cell proliferation, trafficking, chemotaxis and adhesion, and BTK inhibition is a proven strategy for inducing the regression ofB-cell lymphomas.
Genentech Collaboration
In April 2019, we extended our existing collaboration with Genentech, a member of the Roche Group, to include a third clinical trial evaluating 5F9 in combination with rituximab plus Genentech’santi-PD-L1 antibody, atezolizumab (TECENTRIQ), in patients with DLBCL. This approach aims to optimize the treatment of DLBCL patients whose tumors are associated with high levels of macrophages expressingPD-L1.
In June 2019, a clinical trial collaboration with Genentech, to explore 5F9 plus atezolizumab for the treatment of bladder cancer, dosed the first patient in a Phase 1 clinical trial.
Ono Pharmaceutical License and Collaboration Agreement
On July 10, 2019, we entered into an exclusive license and collaboration agreement with Ono Pharmaceutical Co., Ltd., or Ono. Under the agreement, we granted Ono an exclusive license to develop, manufacture and commercialize 5F9, our monoclonal antibody against CD47, as well as other anti-CD47 antibodies controlled by us in Japan, South Korea, Taiwan and the ASEAN countries, or the Ono Territory. We retain all rights to 5F9 and other licensed antibodies outside of the Ono Territory.
Under the agreement, the parties will collaborate on the development, manufacturing and commercialization of 5F9 and other licensed antibodies. Each party will be responsible for conducting development and commercialization of licensed antibodies in its respective territory at its own cost. Further, each party will have the right to participate, at its own cost, in global clinical studies of 5F9 and other licensed antibodies conducted by the other party. We will initially be responsible for supplying 5F9 and other licensed antibodies to Ono for development and commercialization within the Ono Territory at Ono’s cost. Ono has the right to elect that such manufacturing activities be transferred to Ono. During the term of the agreement, neither party may manufacture or commercialize any competing products in the Ono Territory.
We will receive aone-time upfront payment of approximately $15.8 million from Ono and will be eligible to receive up to an additional approximately $104 million at current exchange rates if specified future development and commercial milestones are achieved by Ono. We are also eligible to receive tiered percentage royalties spanning from themid-teens to thelow-twenties on future net sales of 5F9 and other licensed antibodies in the Ono Territory, subject to certain offsets. Ono’s obligation to pay royalties expires, on aproduct-by-product andcountry-by-country basis, on the later of (1) the expiration of the first regulatory exclusivity for such product in such country, (2) the expiration of the last to expire patent controlled by us that covers the composition of matter of a licensed antibody in such product in such country, or (3) the tenth anniversary of the first commercial sale of such product in such country.
The agreement will remain in effect until the expiration of all of Ono’s royalty obligations, after which Ono’s license shall be fullypaid-up. Ono may terminate the agreement on acountry-by-country basis for convenience upon 90 days’ prior written notice to us prior to the first commercial sale of the first licensed product in the Ono Territory, or 180 days’ prior written notice after such first sale. Either party may also