
Exhibit 99.2 INVESTOR DAY SEPTEMBER 24, 2019Exhibit 99.2 INVESTOR DAY SEPTEMBER 24, 2019


OPENING REMARKS ARIE BELLDEGRUN, MD, FACS Chairman, UroGen PharmaOPENING REMARKS ARIE BELLDEGRUN, MD, FACS Chairman, UroGen Pharma

UROGEN: BUILDING A GROWTH COMPANY LIZ BARRETT Chief Executive Officer, UroGen PharmaUROGEN: BUILDING A GROWTH COMPANY LIZ BARRETT Chief Executive Officer, UroGen Pharma

FORWARD LOOKING STATEMENTS This investor presentation contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including, without limitation: the potential of UGN-101 for LG UTUC; the timing for completion of the rolling NDA for UGN-101; the potential approval of UGN-101 and the timing thereof; the expectation that UGN-101, if approved, will be the first drug approved for the non-surgical treatment of LG UTUC; the timing for completion of pre-commercial activities and infrastructure build-out in anticipation of a potential commercial launch of UGN-101; the expected readiness of UroGen for a potential commercial launch of UGN-101 in 1H 2020 and the strength and timing of the potential commercial launch of UGN-101; plans for distribution and product packaging for UGN-101; plans for the retention of field-based personnel in support of the launch of UGN-101; the potential of UroGen’s proprietary RTGel™ technology platform to improve therapeutic profiles of existing drugs; the opportunity and potential of UGN-102 for LG NMIBC; plans to commence a pivotal trial for UGN-102 in LG NMIBC in 2020; UGN-102’s potential to replace current standard of care in LG NMIBC; plans to initiate a Phase 1 study with UGN-201; UroGen’s anticipated status relating to Q3’ 2019 financial guidance; plans to build a sustainable growth company; projections of revenue opportunities in markets of interest; plans to develop a global footprint; plans with Janssen to conduct an early stage feasibility evaluation in an area of mutual interest; and the anticipated completion of a Phase 2 trial of RTGel with Botox. These statements are subject to a number of risks, uncertainties and assumptions, including, but not limited to: the timing and success of clinical trials, including the OLYMPUS Phase 3 trial and the OPTIMA II Phase 2b trial and potential safety and other complications thereof; the ability to obtain regulatory approval within the timeframe expected, or at all; the ability to maintain regulatory approval; complications associated with achieving commercial readiness for the launch of a new product; the labeling and packaging for any approved product; the scope, progress and expansion of developing and commercializing UroGen’s product candidates; the size and growth of the market(s) therefor and the rate and degree of market acceptance thereof vis-à-vis alternative therapies; and UroGen’s ability to attract or retain key management, members of the board of directors and personnel. In light of these risks and uncertainties, and other risks and uncertainties that are described in the Risk Factors section of UroGen’s Form 10-Q filed with the SEC on August 9, 2019, and other filings that UroGen makes with the SEC from time to time (which are available at http://www.sec.gov), the events and circumstances discussed in such forward-looking statements may not occur, and UroGen’s actual results could differ materially and adversely from those anticipated or implied thereby. Any forward-looking statements speak only as of the date of this presentation and are based on information available to UroGen as of the date of this presentation.FORWARD LOOKING STATEMENTS This investor presentation contains forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including, without limitation: the potential of UGN-101 for LG UTUC; the timing for completion of the rolling NDA for UGN-101; the potential approval of UGN-101 and the timing thereof; the expectation that UGN-101, if approved, will be the first drug approved for the non-surgical treatment of LG UTUC; the timing for completion of pre-commercial activities and infrastructure build-out in anticipation of a potential commercial launch of UGN-101; the expected readiness of UroGen for a potential commercial launch of UGN-101 in 1H 2020 and the strength and timing of the potential commercial launch of UGN-101; plans for distribution and product packaging for UGN-101; plans for the retention of field-based personnel in support of the launch of UGN-101; the potential of UroGen’s proprietary RTGel™ technology platform to improve therapeutic profiles of existing drugs; the opportunity and potential of UGN-102 for LG NMIBC; plans to commence a pivotal trial for UGN-102 in LG NMIBC in 2020; UGN-102’s potential to replace current standard of care in LG NMIBC; plans to initiate a Phase 1 study with UGN-201; UroGen’s anticipated status relating to Q3’ 2019 financial guidance; plans to build a sustainable growth company; projections of revenue opportunities in markets of interest; plans to develop a global footprint; plans with Janssen to conduct an early stage feasibility evaluation in an area of mutual interest; and the anticipated completion of a Phase 2 trial of RTGel with Botox. These statements are subject to a number of risks, uncertainties and assumptions, including, but not limited to: the timing and success of clinical trials, including the OLYMPUS Phase 3 trial and the OPTIMA II Phase 2b trial and potential safety and other complications thereof; the ability to obtain regulatory approval within the timeframe expected, or at all; the ability to maintain regulatory approval; complications associated with achieving commercial readiness for the launch of a new product; the labeling and packaging for any approved product; the scope, progress and expansion of developing and commercializing UroGen’s product candidates; the size and growth of the market(s) therefor and the rate and degree of market acceptance thereof vis-à-vis alternative therapies; and UroGen’s ability to attract or retain key management, members of the board of directors and personnel. In light of these risks and uncertainties, and other risks and uncertainties that are described in the Risk Factors section of UroGen’s Form 10-Q filed with the SEC on August 9, 2019, and other filings that UroGen makes with the SEC from time to time (which are available at http://www.sec.gov), the events and circumstances discussed in such forward-looking statements may not occur, and UroGen’s actual results could differ materially and adversely from those anticipated or implied thereby. Any forward-looking statements speak only as of the date of this presentation and are based on information available to UroGen as of the date of this presentation.

AGENDA • Opening Remarks • Management Q & A Arie Belldegrun, MD, FACS, Chairman Liz Barrett, Mark Schoenberg, Peter Pfreundschuh, Jeff Bova + • UroGen: Building a Growth Company Extended Team Liz Barrett, CEO • Closing Remarks • Clinical Update Liz Barrett, CEO Mark Schoenberg, MD, CMO • Innovation in Practice: KOL Panel and Q&A Mark Schoenberg (Moderator), MD, CMO Karim Chamie, MD, MSHS Jennifer Linehan, MD Phil Pierorazio, MD Sandip Prasad, MD Dan Saltzstein, MDAGENDA • Opening Remarks • Management Q & A Arie Belldegrun, MD, FACS, Chairman Liz Barrett, Mark Schoenberg, Peter Pfreundschuh, Jeff Bova + • UroGen: Building a Growth Company Extended Team Liz Barrett, CEO • Closing Remarks • Clinical Update Liz Barrett, CEO Mark Schoenberg, MD, CMO • Innovation in Practice: KOL Panel and Q&A Mark Schoenberg (Moderator), MD, CMO Karim Chamie, MD, MSHS Jennifer Linehan, MD Phil Pierorazio, MD Sandip Prasad, MD Dan Saltzstein, MD

OUR TEAM Liz Barrett Peter Pfreundschuh Mark Schoenberg, MD Stephen Mullennix Jeff Bova Woody Bryan, PhD Chief Executive Officer Chief Financial Officer Chief Medical Officer Chief Operating Officer SVP, Commercial SVP, Business Development Leadership Marina Konorty, PhD Jim Ottinger, RPh Elyse Seltzer, MD John O’Reilly Dalit Strauss-Ayali SVP, R&D & Head of Israel Operations SVP, Regulatory Affairs SVP, Clinical Development VP, Associate General Counsel VP, Non-clinical Research, Science, Discovery Board of Directors Arie Belldegrun, MD, FACS Cynthia Butitta Fred E. Cohen, MD Kate Falberg Stuart Holden, MD Ran Nussbaum Shawn C. TomaselloOUR TEAM Liz Barrett Peter Pfreundschuh Mark Schoenberg, MD Stephen Mullennix Jeff Bova Woody Bryan, PhD Chief Executive Officer Chief Financial Officer Chief Medical Officer Chief Operating Officer SVP, Commercial SVP, Business Development Leadership Marina Konorty, PhD Jim Ottinger, RPh Elyse Seltzer, MD John O’Reilly Dalit Strauss-Ayali SVP, R&D & Head of Israel Operations SVP, Regulatory Affairs SVP, Clinical Development VP, Associate General Counsel VP, Non-clinical Research, Science, Discovery Board of Directors Arie Belldegrun, MD, FACS Cynthia Butitta Fred E. Cohen, MD Kate Falberg Stuart Holden, MD Ran Nussbaum Shawn C. Tomasello

UROGEN: BUILDING A GROWTH COMPANY TM • RTGel Technology • Near-Term Catalysts • UGN-101 • The Road to Anticipated Launch • UGN-102 • UGN-201 • Collaborations/Partnerships • Long-Term Strategy • Leading in Uro-Oncology & BeyondUROGEN: BUILDING A GROWTH COMPANY TM • RTGel Technology • Near-Term Catalysts • UGN-101 • The Road to Anticipated Launch • UGN-102 • UGN-201 • Collaborations/Partnerships • Long-Term Strategy • Leading in Uro-Oncology & Beyond

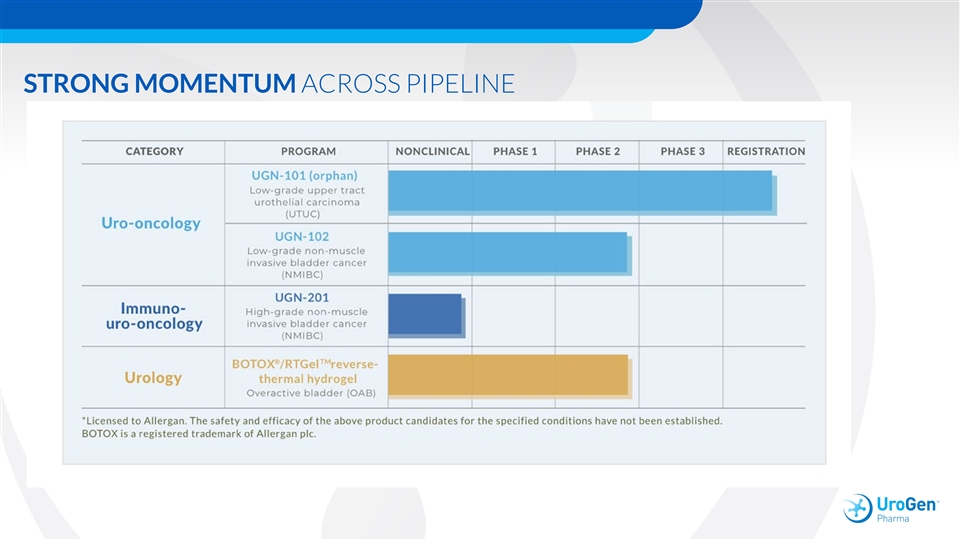
STRONG MOMENTUM ACROSS PIPELINESTRONG MOMENTUM ACROSS PIPELINE
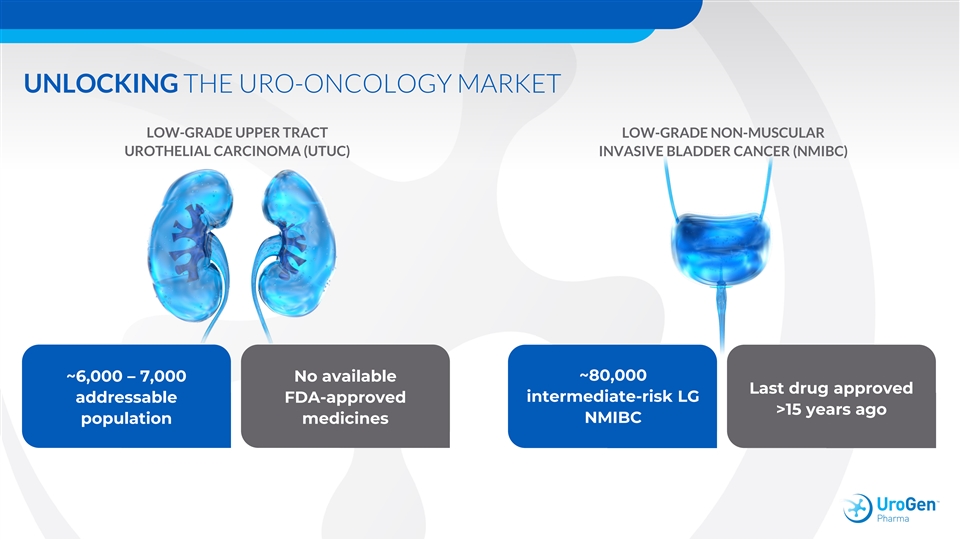
UNLOCKING THE URO-ONCOLOGY MARKET LOW-GRADE UPPER TRACT LOW-GRADE NON-MUSCULAR UROTHELIAL CARCINOMA (UTUC) INVASIVE BLADDER CANCER (NMIBC) ~80,000 ~6,000 – 7,000 No available Last drug approved intermediate-risk LG addressable FDA-approved >15 years ago NMIBC population medicinesUNLOCKING THE URO-ONCOLOGY MARKET LOW-GRADE UPPER TRACT LOW-GRADE NON-MUSCULAR UROTHELIAL CARCINOMA (UTUC) INVASIVE BLADDER CANCER (NMIBC) ~80,000 ~6,000 – 7,000 No available Last drug approved intermediate-risk LG addressable FDA-approved >15 years ago NMIBC population medicines

KEY ACCOMPLISHMENTS IN 2019 • Completed phase III OLYMPUS trial for UGN-101 in LG UTUC; obtained breakthrough therapy designation and advanced rolling submission with FDA • Fully enrolled phase 2b study of UGN-102 in intermediate-risk low-grade NMIBC and conducted interim analysis • Enhanced the pipeline with UGN-201 preclinical work and announcement of the early-stage feasibility agreement with Janssen • Organizational and commercial readiness for anticipated launch of UGN-101 • Developed long-term vision and strategy for sustainable growth • Strong cash position and delivery of guidance TM Established RTGel as the first innovation UroGen expects to bring to market KEY ACCOMPLISHMENTS IN 2019 • Completed phase III OLYMPUS trial for UGN-101 in LG UTUC; obtained breakthrough therapy designation and advanced rolling submission with FDA • Fully enrolled phase 2b study of UGN-102 in intermediate-risk low-grade NMIBC and conducted interim analysis • Enhanced the pipeline with UGN-201 preclinical work and announcement of the early-stage feasibility agreement with Janssen • Organizational and commercial readiness for anticipated launch of UGN-101 • Developed long-term vision and strategy for sustainable growth • Strong cash position and delivery of guidance TM Established RTGel as the first innovation UroGen expects to bring to market

UROGEN: BUILDING A GROWTH COMPANY OUR PIPELINE: UGN-101UROGEN: BUILDING A GROWTH COMPANY OUR PIPELINE: UGN-101

UROTHELIAL CARCINOMA COMMON, COSTLY CANCER WITH SIGNIFICANT QOL IMPACT LOW-GRADE UPPER TRACT UROTHELIAL CARCINOMA (UTUC) Urothelial carcinoma (UC) Cancer that happens in the lining of the kidneys or the ureters is the 9th most common LOW-GRADE UTUC 1 cancer globally • Kidney-sparing treatments are achievable and may decrease overtreatment and loss of renal units • 70%-80% of LG UTUC patients receive UC is the most costly cancer nephroureterectomies in the US health care system 1 on a per-patient basis Yeung et al. (2014) Pharmacoeconomics UROTHELIAL CARCINOMA COMMON, COSTLY CANCER WITH SIGNIFICANT QOL IMPACT LOW-GRADE UPPER TRACT UROTHELIAL CARCINOMA (UTUC) Urothelial carcinoma (UC) Cancer that happens in the lining of the kidneys or the ureters is the 9th most common LOW-GRADE UTUC 1 cancer globally • Kidney-sparing treatments are achievable and may decrease overtreatment and loss of renal units • 70%-80% of LG UTUC patients receive UC is the most costly cancer nephroureterectomies in the US health care system 1 on a per-patient basis Yeung et al. (2014) Pharmacoeconomics
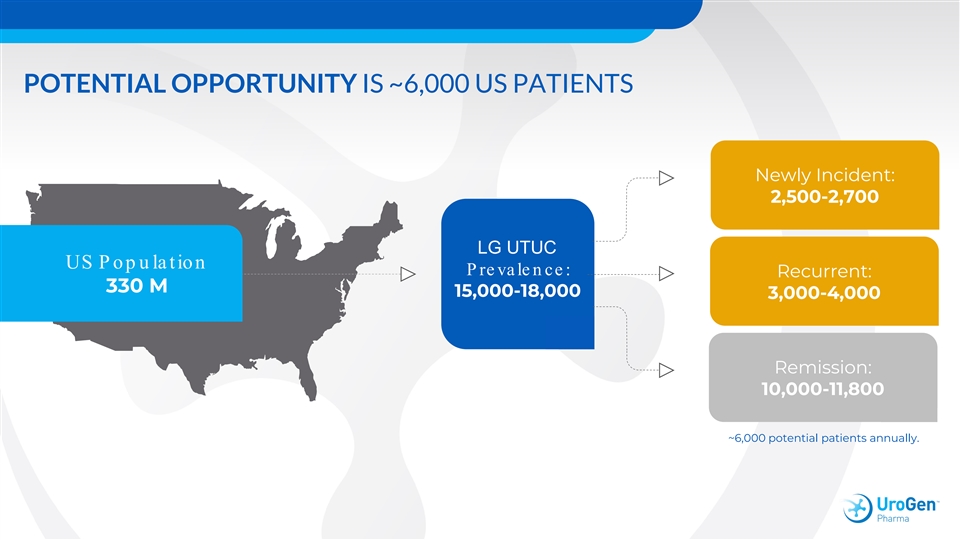
POTENTIAL OPPORTUNITY IS ~6,000 US PATIENTS Newly Incident: 2,500-2,700 LG UTUC US P op u la tion P reva len ce: Recurrent: 330 M 15,000-18,000 3,000-4,000 Remission: 10,000-11,800 ~6,000 potential patients annually.POTENTIAL OPPORTUNITY IS ~6,000 US PATIENTS Newly Incident: 2,500-2,700 LG UTUC US P op u la tion P reva len ce: Recurrent: 330 M 15,000-18,000 3,000-4,000 Remission: 10,000-11,800 ~6,000 potential patients annually.
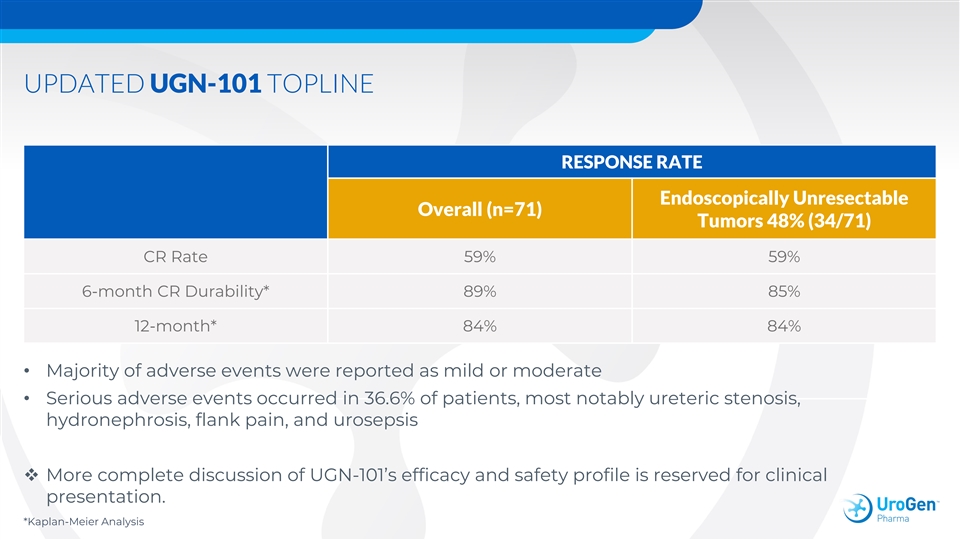
UPDATED UGN-101 TOPLINE RESPONSE RATE Endoscopically Unresectable Overall (n=71) Tumors 48% (34/71) CR Rate 59% 59% 6-month CR Durability* 89% 85% 12-month* 84% 84% • Majority of adverse events were reported as mild or moderate • Serious adverse events occurred in 36.6% of patients, most notably ureteric stenosis, hydronephrosis, flank pain, and urosepsis v More complete discussion of UGN-101’s efficacy and safety profile is reserved for clinical presentation. *Kaplan-Meier AnalysisUPDATED UGN-101 TOPLINE RESPONSE RATE Endoscopically Unresectable Overall (n=71) Tumors 48% (34/71) CR Rate 59% 59% 6-month CR Durability* 89% 85% 12-month* 84% 84% • Majority of adverse events were reported as mild or moderate • Serious adverse events occurred in 36.6% of patients, most notably ureteric stenosis, hydronephrosis, flank pain, and urosepsis v More complete discussion of UGN-101’s efficacy and safety profile is reserved for clinical presentation. *Kaplan-Meier Analysis
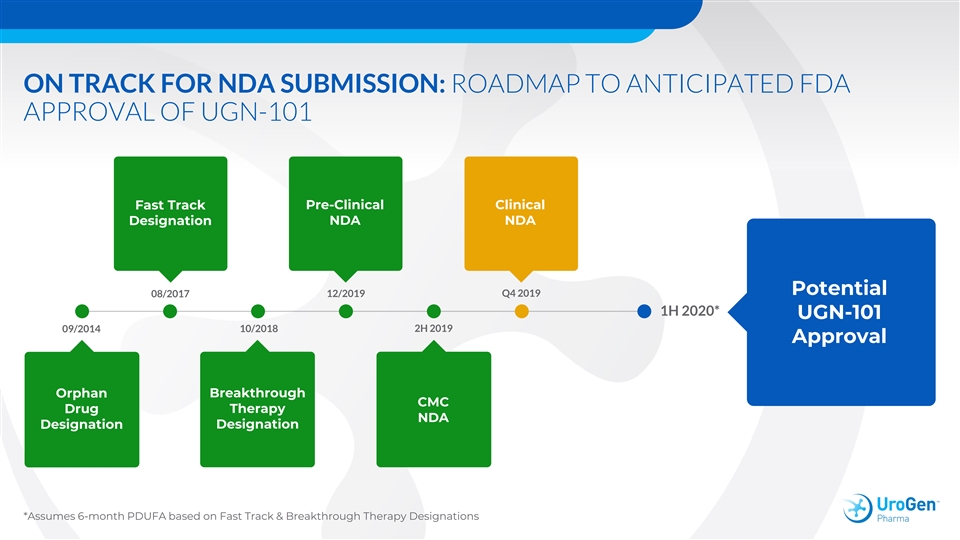
ON TRACK FOR NDA SUBMISSION: ROADMAP TO ANTICIPATED FDA APPROVAL OF UGN-101 Pre-Clinical Clinical Fast Track Designation NDA NDA Potential 08/2017 12/2019 Q4 2019 1H 2020* UGN-101 09/2014 10/2018 2H 2019 Approval Breakthrough Orphan CMC Drug Therapy NDA Designation Designation *Assumes 6-month PDUFA based on Fast Track & Breakthrough Therapy DesignationsON TRACK FOR NDA SUBMISSION: ROADMAP TO ANTICIPATED FDA APPROVAL OF UGN-101 Pre-Clinical Clinical Fast Track Designation NDA NDA Potential 08/2017 12/2019 Q4 2019 1H 2020* UGN-101 09/2014 10/2018 2H 2019 Approval Breakthrough Orphan CMC Drug Therapy NDA Designation Designation *Assumes 6-month PDUFA based on Fast Track & Breakthrough Therapy Designations

UROGEN: BUILDING A GROWTH COMPANY THE ROAD TO ANTICIPATED LAUNCH: UGN-101UROGEN: BUILDING A GROWTH COMPANY THE ROAD TO ANTICIPATED LAUNCH: UGN-101

ORGANIZATIONAL READINESS: UROGEN IS ON TRACK TO BE LAUNCH READY FOR UGN-101 BY JANUARY 2020 PREPARE THE MARKET ü Field Medical Team hired and active ü Field National Account Directors calling on payers ü Increased awareness in urology community LAUNCH PREPARE THE BRAND READINESS ü Distribution strategy set and brand name selected ü Reimbursement support HUB established PREPARE THE COMPANY ü Full commercial leadership team hired ü Field leadership team hired and recruiting sales forceORGANIZATIONAL READINESS: UROGEN IS ON TRACK TO BE LAUNCH READY FOR UGN-101 BY JANUARY 2020 PREPARE THE MARKET ü Field Medical Team hired and active ü Field National Account Directors calling on payers ü Increased awareness in urology community LAUNCH PREPARE THE BRAND READINESS ü Distribution strategy set and brand name selected ü Reimbursement support HUB established PREPARE THE COMPANY ü Full commercial leadership team hired ü Field leadership team hired and recruiting sales force
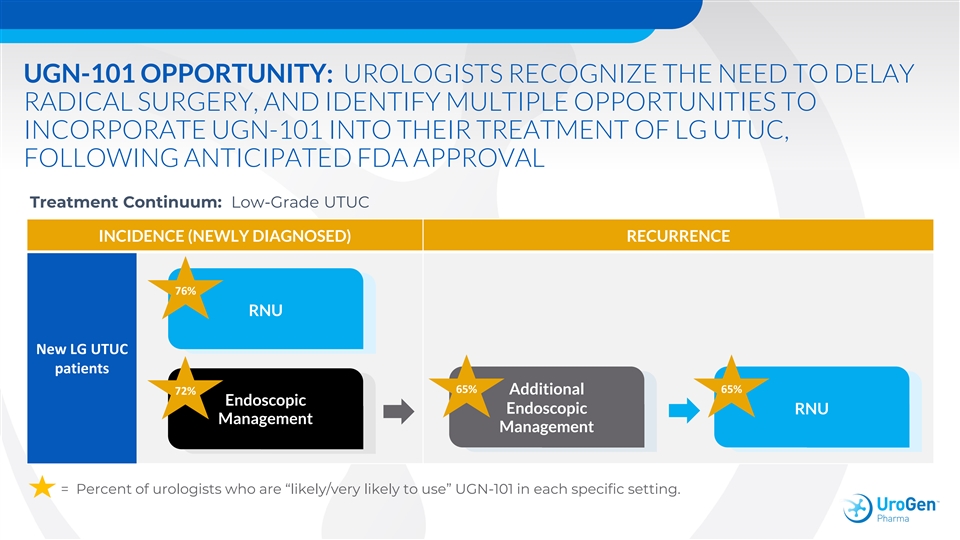
UGN-101 OPPORTUNITY: UROLOGISTS RECOGNIZE THE NEED TO DELAY RADICAL SURGERY, AND IDENTIFY MULTIPLE OPPORTUNITIES TO INCORPORATE UGN-101 INTO THEIR TREATMENT OF LG UTUC, FOLLOWING ANTICIPATED FDA APPROVAL Treatment Continuum: Low-Grade UTUC INCIDENCE (NEWLY DIAGNOSED) RECURRENCE 76% RNU New LG UTUC patients 65% 65% 72% Additional Endoscopic Endoscopic RNU Management Management = Percent of urologists who are “likely/very likely to use” UGN-101 in each specific setting.UGN-101 OPPORTUNITY: UROLOGISTS RECOGNIZE THE NEED TO DELAY RADICAL SURGERY, AND IDENTIFY MULTIPLE OPPORTUNITIES TO INCORPORATE UGN-101 INTO THEIR TREATMENT OF LG UTUC, FOLLOWING ANTICIPATED FDA APPROVAL Treatment Continuum: Low-Grade UTUC INCIDENCE (NEWLY DIAGNOSED) RECURRENCE 76% RNU New LG UTUC patients 65% 65% 72% Additional Endoscopic Endoscopic RNU Management Management = Percent of urologists who are “likely/very likely to use” UGN-101 in each specific setting.

CREATIVE SOLUTIONS TO REMOVE BARRIERS • Hire experienced field teams with expertise in uro-oncology, rare disease UGN-101 • Strong marketing awareness efforts Awareness • Real-time patient alerts where possible • Early Payer engagement -- National Account Directors in field 2019 Ensure Reimbursement • Dedicated team of Field Reimbursement Managers + independent HUB services Confidence to support appropriate coding, benefits verifications, issue resolution • National Pharmacy partner will provide pre-mixed formulation to urology clinics Product • All-in-one convenience kit will be provided to hospital pharmacies who prefer to Distribution self-mixCREATIVE SOLUTIONS TO REMOVE BARRIERS • Hire experienced field teams with expertise in uro-oncology, rare disease UGN-101 • Strong marketing awareness efforts Awareness • Real-time patient alerts where possible • Early Payer engagement -- National Account Directors in field 2019 Ensure Reimbursement • Dedicated team of Field Reimbursement Managers + independent HUB services Confidence to support appropriate coding, benefits verifications, issue resolution • National Pharmacy partner will provide pre-mixed formulation to urology clinics Product • All-in-one convenience kit will be provided to hospital pharmacies who prefer to Distribution self-mix

ROLES TO SUPPORT ACCOUNT-BASED APPROACH 33% OF ACCOUNTS HAVE 90% OF THE PATIENT POTENTIAL Regional Business 7 RBM: Regional Business Manager Manager (RBM) Responsible for all commercial activity 48 TBM: Territory Business Manager Customer lead and demand generation Field Certified Nurse Reimbursement 7 FRM: Field Reimbursement Manager Educator (CNE) Manager (FRM) Account experts on billing and coding 7 CNE: Certified Nurse Educator Provide technical training and support for mixing and product instillation Territory Business Manager (TBM)ROLES TO SUPPORT ACCOUNT-BASED APPROACH 33% OF ACCOUNTS HAVE 90% OF THE PATIENT POTENTIAL Regional Business 7 RBM: Regional Business Manager Manager (RBM) Responsible for all commercial activity 48 TBM: Territory Business Manager Customer lead and demand generation Field Certified Nurse Reimbursement 7 FRM: Field Reimbursement Manager Educator (CNE) Manager (FRM) Account experts on billing and coding 7 CNE: Certified Nurse Educator Provide technical training and support for mixing and product instillation Territory Business Manager (TBM)
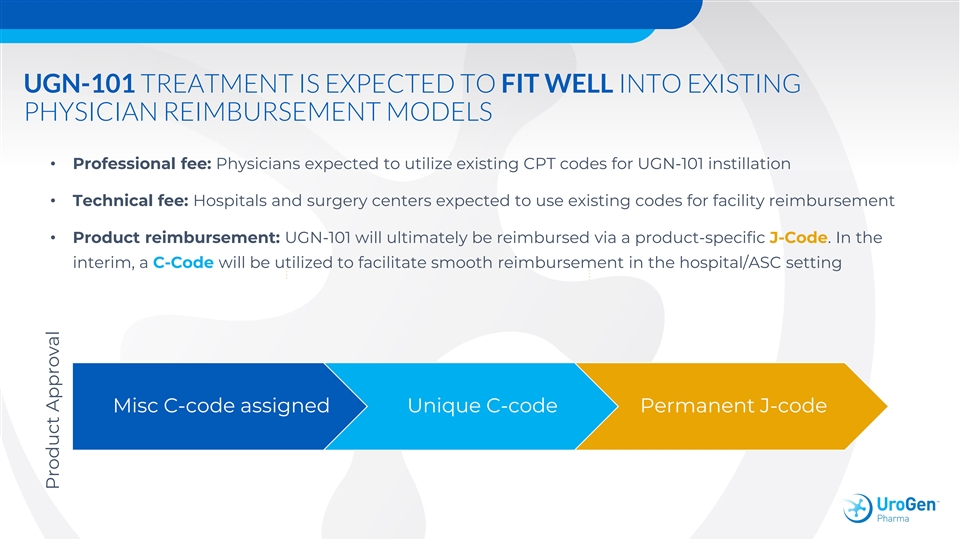
UGN-101 TREATMENT IS EXPECTED TO FIT WELL INTO EXISTING PHYSICIAN REIMBURSEMENT MODELS • Professional fee: Physicians expected to utilize existing CPT codes for UGN-101 instillation • Technical fee: Hospitals and surgery centers expected to use existing codes for facility reimbursement • Product reimbursement: UGN-101 will ultimately be reimbursed via a product-specific J-Code. In the interim, a C-Code will be utilized to facilitate smooth reimbursement in the hospital/ASC setting Misc C-code assigned Unique C-code Permanent J-code Product ApprovalUGN-101 TREATMENT IS EXPECTED TO FIT WELL INTO EXISTING PHYSICIAN REIMBURSEMENT MODELS • Professional fee: Physicians expected to utilize existing CPT codes for UGN-101 instillation • Technical fee: Hospitals and surgery centers expected to use existing codes for facility reimbursement • Product reimbursement: UGN-101 will ultimately be reimbursed via a product-specific J-Code. In the interim, a C-Code will be utilized to facilitate smooth reimbursement in the hospital/ASC setting Misc C-code assigned Unique C-code Permanent J-code Product Approval
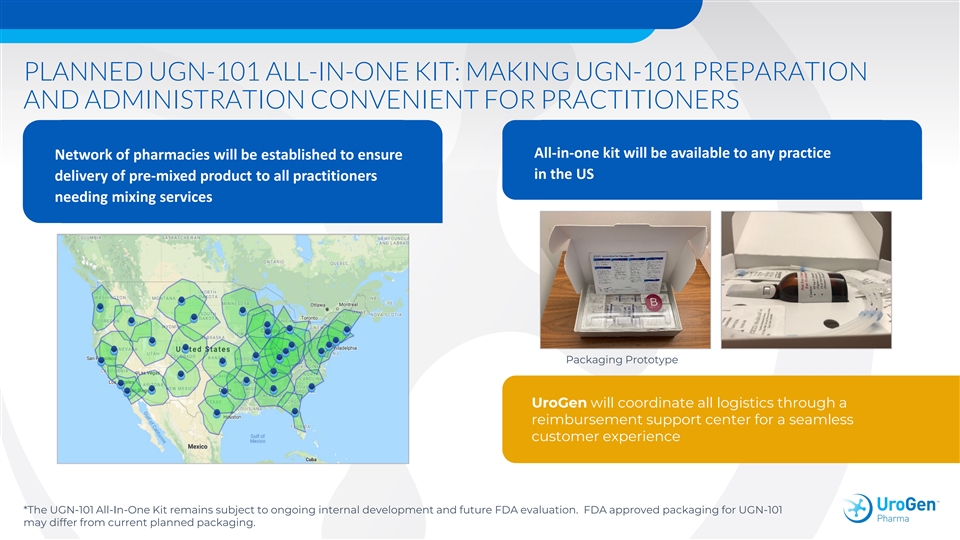
PLANNED UGN-101 ALL-IN-ONE KIT: MAKING UGN-101 PREPARATION AND ADMINISTRATION CONVENIENT FOR PRACTITIONERS All-in-one kit will be available to any practice Network of pharmacies will be established to ensure in the US delivery of pre-mixed product to all practitioners needing mixing services Packaging Prototype UroGen will coordinate all logistics through a reimbursement support center for a seamless customer experience *The UGN-101 All-In-One Kit remains subject to ongoing internal development and future FDA evaluation. FDA approved packaging for UGN-101 may differ from current planned packaging.PLANNED UGN-101 ALL-IN-ONE KIT: MAKING UGN-101 PREPARATION AND ADMINISTRATION CONVENIENT FOR PRACTITIONERS All-in-one kit will be available to any practice Network of pharmacies will be established to ensure in the US delivery of pre-mixed product to all practitioners needing mixing services Packaging Prototype UroGen will coordinate all logistics through a reimbursement support center for a seamless customer experience *The UGN-101 All-In-One Kit remains subject to ongoing internal development and future FDA evaluation. FDA approved packaging for UGN-101 may differ from current planned packaging.

EXPERIENCED TEAM IS PRIMED FOR LAUNCH UPON APPROVAL ü Hired an internal team with a track record ü Launched a successful disease education of success in oncology campaign ü Hired a veteran sales force leadership team ü Tested and validated a launch campaign with deep uro-oncology relationships with urologists ü Consulted with our customers, and we are ü Developed partnerships with seasoned ready to deliver on their needs vendors • HCPs • Pharmacists ü Aligned and prepared for • Payers launch readiness January 2020EXPERIENCED TEAM IS PRIMED FOR LAUNCH UPON APPROVAL ü Hired an internal team with a track record ü Launched a successful disease education of success in oncology campaign ü Hired a veteran sales force leadership team ü Tested and validated a launch campaign with deep uro-oncology relationships with urologists ü Consulted with our customers, and we are ü Developed partnerships with seasoned ready to deliver on their needs vendors • HCPs • Pharmacists ü Aligned and prepared for • Payers launch readiness January 2020

UROGEN: BUILDING A GROWTH COMPANY OUR PIPELINE: UGN-102UROGEN: BUILDING A GROWTH COMPANY OUR PIPELINE: UGN-102

UGN-102 TOPLINE UGN-102 enrollment complete ahead Safety: Most AEs mild to moderate in of schedule severity, related to local tolerability, no related SAEs - CR 63%* *Interim CR based on half of patients v More complete discussion of UGN-102’s efficacy and safety profile is reserved for clinical presentation.UGN-102 TOPLINE UGN-102 enrollment complete ahead Safety: Most AEs mild to moderate in of schedule severity, related to local tolerability, no related SAEs - CR 63%* *Interim CR based on half of patients v More complete discussion of UGN-102’s efficacy and safety profile is reserved for clinical presentation.
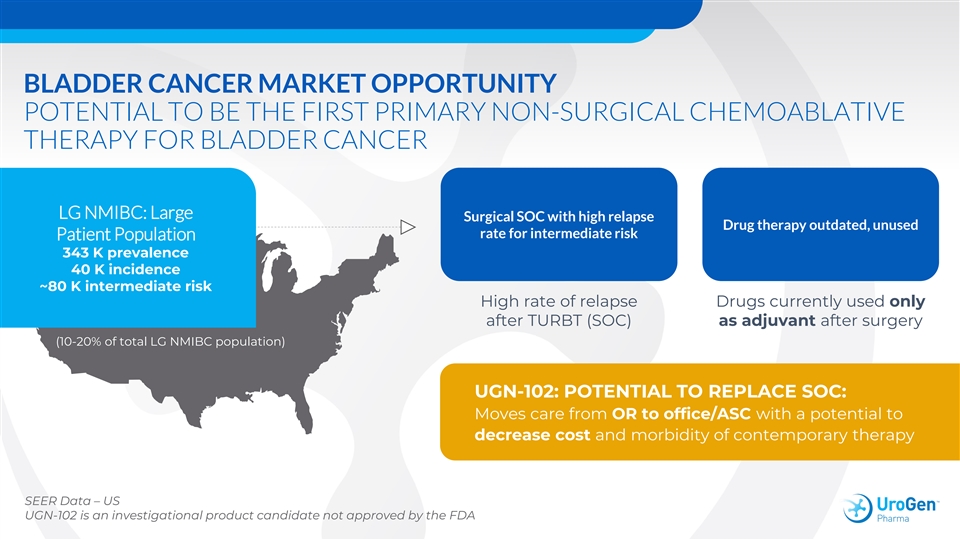
BLADDER CANCER MARKET OPPORTUNITY POTENTIAL TO BE THE FIRST PRIMARY NON-SURGICAL CHEMOABLATIVE THERAPY FOR BLADDER CANCER LG NMIBC: Large Surgical SOC with high relapse Drug therapy outdated, unused rate for intermediate risk Patient Population 343 K prevalence 40 K incidence ~80 K intermediate risk High rate of relapse Drugs currently used only after TURBT (SOC) as adjuvant after surgery (10-20% of total LG NMIBC population) UGN-102: POTENTIAL TO REPLACE SOC: Moves care from OR to office/ASC with a potential to decrease cost and morbidity of contemporary therapy SEER Data – US UGN-102 is an investigational product candidate not approved by the FDABLADDER CANCER MARKET OPPORTUNITY POTENTIAL TO BE THE FIRST PRIMARY NON-SURGICAL CHEMOABLATIVE THERAPY FOR BLADDER CANCER LG NMIBC: Large Surgical SOC with high relapse Drug therapy outdated, unused rate for intermediate risk Patient Population 343 K prevalence 40 K incidence ~80 K intermediate risk High rate of relapse Drugs currently used only after TURBT (SOC) as adjuvant after surgery (10-20% of total LG NMIBC population) UGN-102: POTENTIAL TO REPLACE SOC: Moves care from OR to office/ASC with a potential to decrease cost and morbidity of contemporary therapy SEER Data – US UGN-102 is an investigational product candidate not approved by the FDA

UROGEN: BUILDING A GROWTH COMPANY OUR PIPELINE: UGN-201UROGEN: BUILDING A GROWTH COMPANY OUR PIPELINE: UGN-201

UGN-201 PROVIDES US WITH MULTIPLE SHOTS ON GOAL • UGN-201 is a TLR 7/8 agonist that is believed to stimulate innate and adaptive antitumor immunity. It likely works in conjunction with other potent immunoregulatory molecules • Preclinical experiments as monotherapy and in combination with checkpoint inhibitors provide signals of efficacy • Plan is to optimize combinations and move into human studies as soon as is feasibleUGN-201 PROVIDES US WITH MULTIPLE SHOTS ON GOAL • UGN-201 is a TLR 7/8 agonist that is believed to stimulate innate and adaptive antitumor immunity. It likely works in conjunction with other potent immunoregulatory molecules • Preclinical experiments as monotherapy and in combination with checkpoint inhibitors provide signals of efficacy • Plan is to optimize combinations and move into human studies as soon as is feasible

UROGEN: BUILDING A GROWTH COMPANY COLLABORATIONS & PARTNERSHIPSUROGEN: BUILDING A GROWTH COMPANY COLLABORATIONS & PARTNERSHIPS

ADVANCING LOCAL DELIVERY THROUGH STRATEGIC COLLABORATIONS Strategic collaborations in urology and oncology TM TM ALLERGAN JANSSEN Overactive bladder (phase 2) Early-stage feasibility evaluation RTGel has the potential to provide meaningful improvement over the current standard of care across urologic cancers and beyond *The above trademarks are the property of their respective owners.ADVANCING LOCAL DELIVERY THROUGH STRATEGIC COLLABORATIONS Strategic collaborations in urology and oncology TM TM ALLERGAN JANSSEN Overactive bladder (phase 2) Early-stage feasibility evaluation RTGel has the potential to provide meaningful improvement over the current standard of care across urologic cancers and beyond *The above trademarks are the property of their respective owners.
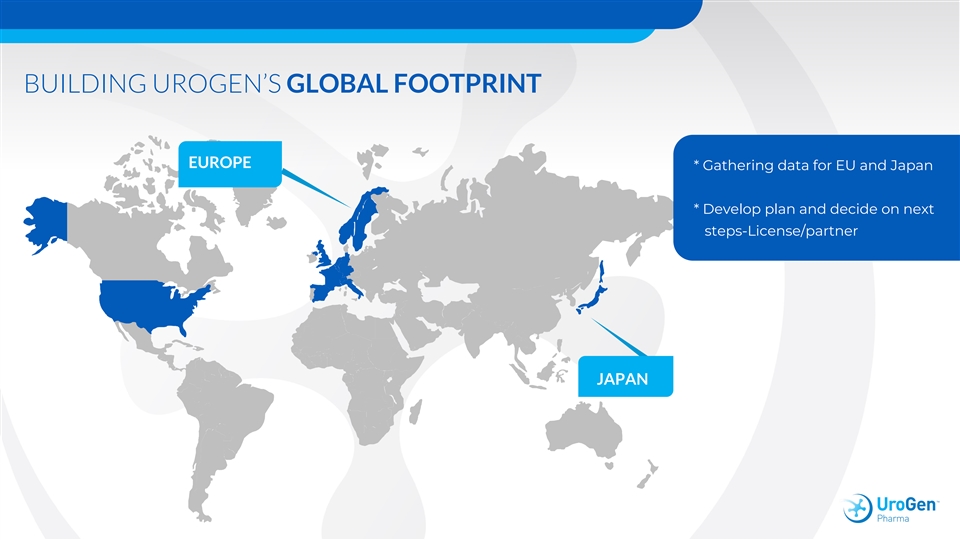
BUILDING UROGEN’S GLOBAL FOOTPRINT EUROPE * Gathering data for EU and Japan * Develop plan and decide on next steps-License/partner JAPANBUILDING UROGEN’S GLOBAL FOOTPRINT EUROPE * Gathering data for EU and Japan * Develop plan and decide on next steps-License/partner JAPAN

UROGEN: BUILDING A GROWTH COMPANY LEADING IN URO-ONCOLOGY & BEYONDUROGEN: BUILDING A GROWTH COMPANY LEADING IN URO-ONCOLOGY & BEYOND
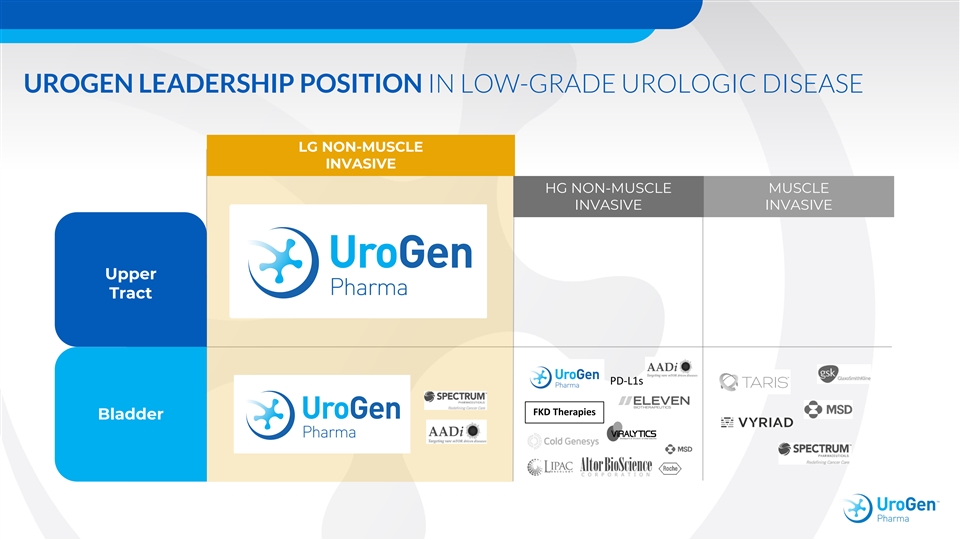
UROGEN LEADERSHIP POSITION IN LOW-GRADE UROLOGIC DISEASE LG NON-MUSCLE INVASIVE HG NON-MUSCLE MUSCLE INVASIVE INVASIVE Upper Tract PD-L1s FKD Therapies BladderUROGEN LEADERSHIP POSITION IN LOW-GRADE UROLOGIC DISEASE LG NON-MUSCLE INVASIVE HG NON-MUSCLE MUSCLE INVASIVE INVASIVE Upper Tract PD-L1s FKD Therapies Bladder

UGN-101 & UGN-102 ADVANCE SOC AND PROVIDE A STRONG FOUNDATION TO BUILD A SUSTAINABLE COMPANY DRIVING INNOVATION IN URO-ONCOLOGY WITH RTGEL LG UPPER TRACT LG NON-MUSCLE INVASIVE UROTHELIAL CANCER BLADDER CANCER UGN-101 (LG) Intermediate-Risk LG- Phase 3 NMIBC Phase 2B Annual US addressable Annual US addressable market: ~6 K market: ~80 K ~$1 BILLION POTENTIAL PEAK REVENUE OPPORTUNITYUGN-101 & UGN-102 ADVANCE SOC AND PROVIDE A STRONG FOUNDATION TO BUILD A SUSTAINABLE COMPANY DRIVING INNOVATION IN URO-ONCOLOGY WITH RTGEL LG UPPER TRACT LG NON-MUSCLE INVASIVE UROTHELIAL CANCER BLADDER CANCER UGN-101 (LG) Intermediate-Risk LG- Phase 3 NMIBC Phase 2B Annual US addressable Annual US addressable market: ~6 K market: ~80 K ~$1 BILLION POTENTIAL PEAK REVENUE OPPORTUNITY

WE BUILD NOVEL SOLUTIONS TO TREAT SPECIALTY CANCERS AND UROLOGIC DISEASES BECAUSE PATIENTS DESERVE BETTER ADDRESS CHALLENGING DISEASE NIMBLE, SOLUTION- MAXIMIZE BENEFIT OF PATIENT CENTRICITY WITH TRANSFORMATIVE LOCAL DELIVERY ORIENTED ORGANIZATION THERAPIES Ensure patients who can benefit from our medicines have Through our nimble Leverage RTGel capabilities and access to them. approach, UroGen is designed to expertise where unique solutions develop and commercialize are needed to overcome Addressing high unmet need Provide a holistic approach with medicines faster and anatomical and biological barriers diseases in Urology & gyn/GI tools that help patients manage more efficiently while creating cancers their disease and live their best a dynamic environment Opportunistically gain access to lives possible for employees additional delivery platforms Must advance SOCWE BUILD NOVEL SOLUTIONS TO TREAT SPECIALTY CANCERS AND UROLOGIC DISEASES BECAUSE PATIENTS DESERVE BETTER ADDRESS CHALLENGING DISEASE NIMBLE, SOLUTION- MAXIMIZE BENEFIT OF PATIENT CENTRICITY WITH TRANSFORMATIVE LOCAL DELIVERY ORIENTED ORGANIZATION THERAPIES Ensure patients who can benefit from our medicines have Through our nimble Leverage RTGel capabilities and access to them. approach, UroGen is designed to expertise where unique solutions develop and commercialize are needed to overcome Addressing high unmet need Provide a holistic approach with medicines faster and anatomical and biological barriers diseases in Urology & gyn/GI tools that help patients manage more efficiently while creating cancers their disease and live their best a dynamic environment Opportunistically gain access to lives possible for employees additional delivery platforms Must advance SOC


CLINICAL UPDATE: UGN-101, UGN-102, UGN-201 MARK SCHOENBERG, MD Chief Medical Officer, UroGen PharmaCLINICAL UPDATE: UGN-101, UGN-102, UGN-201 MARK SCHOENBERG, MD Chief Medical Officer, UroGen Pharma

LASER RESECTION OF LG UTUC Courtesy of Dr. Scott Hubosky, Dept. of Urology, Jefferson University, Philadelphia, PALASER RESECTION OF LG UTUC Courtesy of Dr. Scott Hubosky, Dept. of Urology, Jefferson University, Philadelphia, PA

DEFINING LOW-GRADE VERSUS HIGH-GRADE DISEASE LOW GRADE HIGH GRADE • Chronic relapse • Progression – Metastasis & death • Current treatment – Repetitive surgery • Current treatment: – TURBT – Risks – BCG – Incidence: 42 K – Clinical trials – Prevalence: ~500 K – RCP/TMT • Incidence: ~18 K • Prevalence: ~200 K BCG is not used in low-grade diseaseDEFINING LOW-GRADE VERSUS HIGH-GRADE DISEASE LOW GRADE HIGH GRADE • Chronic relapse • Progression – Metastasis & death • Current treatment – Repetitive surgery • Current treatment: – TURBT – Risks – BCG – Incidence: 42 K – Clinical trials – Prevalence: ~500 K – RCP/TMT • Incidence: ~18 K • Prevalence: ~200 K BCG is not used in low-grade disease
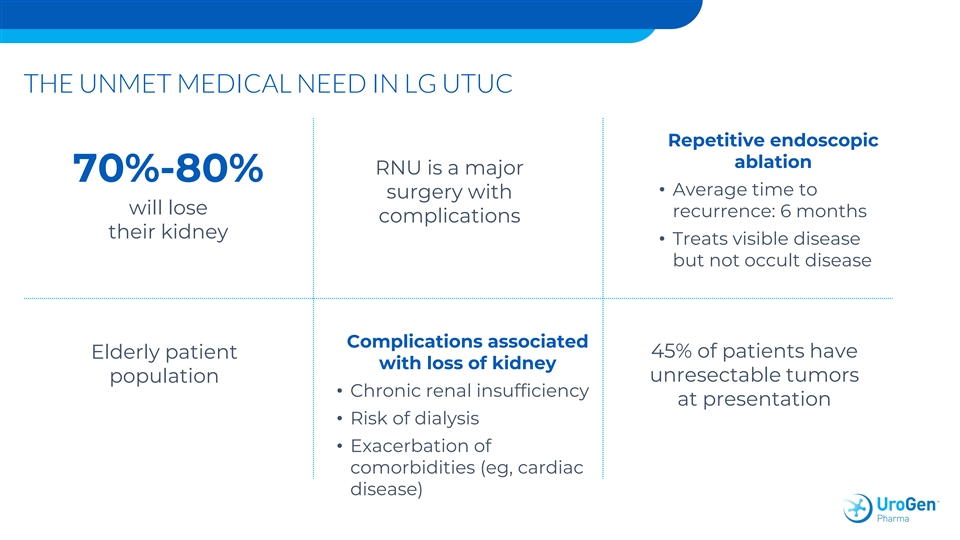
THE UNMET MEDICAL NEED IN LG UTUC Repetitive endoscopic ablation RNU is a major 70%-80% • Average time to surgery with will lose recurrence: 6 months complications their kidney • Treats visible disease but not occult disease Complications associated 45% of patients have Elderly patient with loss of kidney unresectable tumors population • Chronic renal insufficiency at presentation • Risk of dialysis • Exacerbation of comorbidities (eg, cardiac disease)THE UNMET MEDICAL NEED IN LG UTUC Repetitive endoscopic ablation RNU is a major 70%-80% • Average time to surgery with will lose recurrence: 6 months complications their kidney • Treats visible disease but not occult disease Complications associated 45% of patients have Elderly patient with loss of kidney unresectable tumors population • Chronic renal insufficiency at presentation • Risk of dialysis • Exacerbation of comorbidities (eg, cardiac disease)
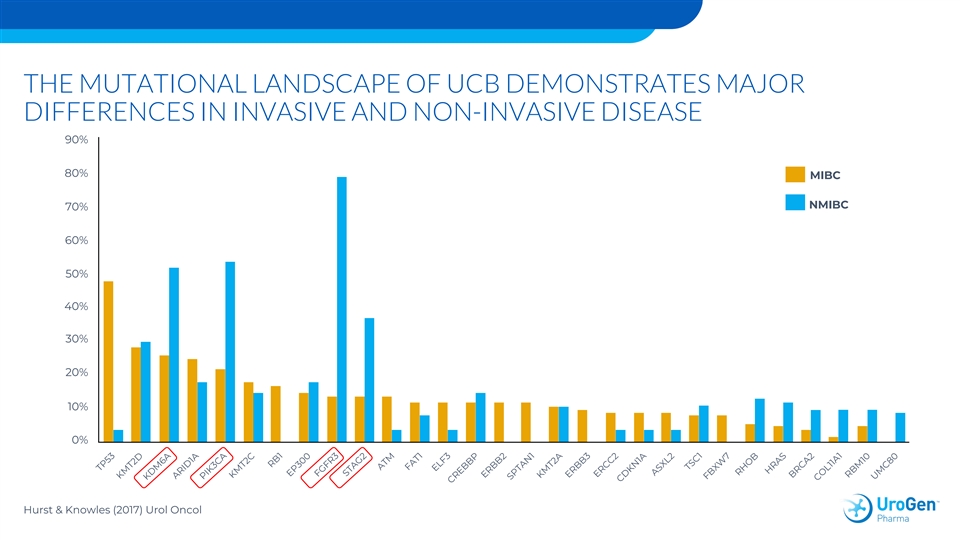
THE MUTATIONAL LANDSCAPE OF UCB DEMONSTRATES MAJOR DIFFERENCES IN INVASIVE AND NON-INVASIVE DISEASE 90% 80% MIBC NMIBC 70% 60% 50% 40% 30% 20% 10% 0% Hurst & Knowles (2017) Urol OncolTHE MUTATIONAL LANDSCAPE OF UCB DEMONSTRATES MAJOR DIFFERENCES IN INVASIVE AND NON-INVASIVE DISEASE 90% 80% MIBC NMIBC 70% 60% 50% 40% 30% 20% 10% 0% Hurst & Knowles (2017) Urol Oncol
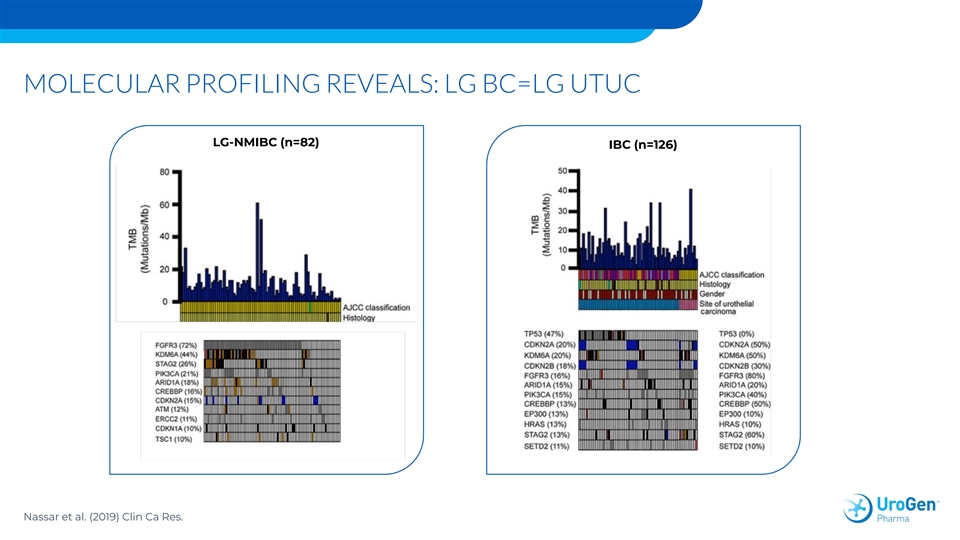
MOLECULAR PROFILING REVEALS: LG BC=LG UTUC LG-NMIBC (n=82) IBC (n=126) Nassar et al. (2019) Clin Ca Res.MOLECULAR PROFILING REVEALS: LG BC=LG UTUC LG-NMIBC (n=82) IBC (n=126) Nassar et al. (2019) Clin Ca Res.

MOLECULAR ANALYSIS REVEALS “NORMAL TISSUE” HARBORS CANCER MUTATIONS Thomsen et al. (2017) Nature/Scientific ReportsMOLECULAR ANALYSIS REVEALS “NORMAL TISSUE” HARBORS CANCER MUTATIONS Thomsen et al. (2017) Nature/Scientific Reports

OLYMPUS TRIAL Enrollment Criteria Rationale • New & recurrent LG UTUC • LG UTUC like LG NMIBC – Solitary or multifocal • Anatomic complexity • Renal pelvis • Limitation of current tools • 5 mm-15 mm • RNU ~70%-80% • No HG, CIS • Cytology negative for HG • Partial resection permitted Goal: Decrease renal loss and avoid repetitive surgeryOLYMPUS TRIAL Enrollment Criteria Rationale • New & recurrent LG UTUC • LG UTUC like LG NMIBC – Solitary or multifocal • Anatomic complexity • Renal pelvis • Limitation of current tools • 5 mm-15 mm • RNU ~70%-80% • No HG, CIS • Cytology negative for HG • Partial resection permitted Goal: Decrease renal loss and avoid repetitive surgery
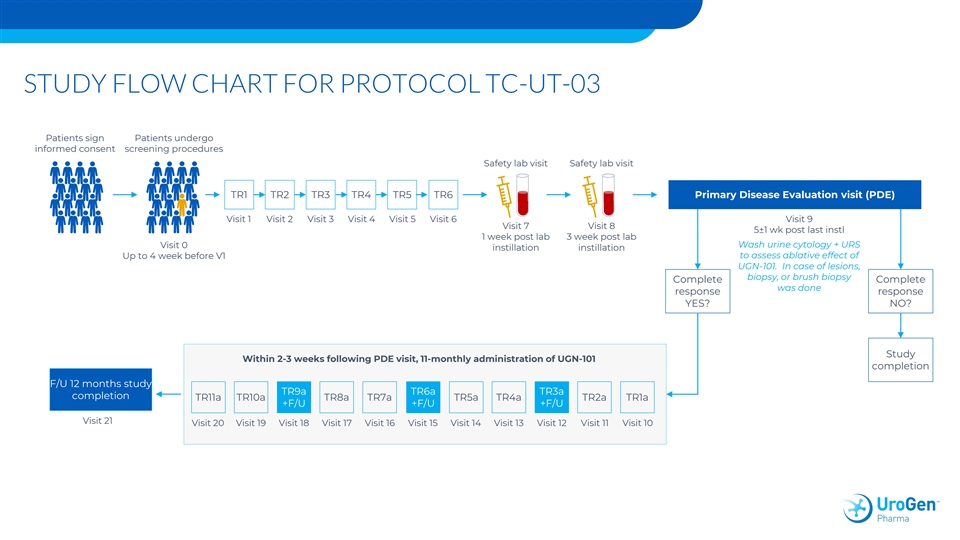
STUDY FLOW CHART FOR PROTOCOL TC-UT-03 Patients sign Patients undergo informed consent screening procedures Safety lab visit Safety lab visit TR1 TR2 TR3 TR4 TR5 TR6 Primary Disease Evaluation visit (PDE) Visit 1 Visit 2 Visit 3 Visit 4 Visit 5 Visit 6 Visit 9 Visit 7 Visit 8 5±1 wk post last instl 1 week post lab 3 week post lab Visit 0 Wash urine cytology + URS instillation instillation to assess ablative effect of Up to 4 week before V1 UGN-101. In case of lesions, biopsy, or brush biopsy Complete Complete was done response response YES? NO? Study Within 2-3 weeks following PDE visit, 11-monthly administration of UGN-101 completion F/U 12 months study TR9a TR6a TR3a completion TR11a TR10a TR8a TR7a TR5a TR4a TR2a TR1a +F/U +F/U +F/U Visit 21 Visit 20 Visit 19 Visit 18 Visit 17 Visit 16 Visit 15 Visit 14 Visit 13 Visit 12 Visit 11 Visit 10STUDY FLOW CHART FOR PROTOCOL TC-UT-03 Patients sign Patients undergo informed consent screening procedures Safety lab visit Safety lab visit TR1 TR2 TR3 TR4 TR5 TR6 Primary Disease Evaluation visit (PDE) Visit 1 Visit 2 Visit 3 Visit 4 Visit 5 Visit 6 Visit 9 Visit 7 Visit 8 5±1 wk post last instl 1 week post lab 3 week post lab Visit 0 Wash urine cytology + URS instillation instillation to assess ablative effect of Up to 4 week before V1 UGN-101. In case of lesions, biopsy, or brush biopsy Complete Complete was done response response YES? NO? Study Within 2-3 weeks following PDE visit, 11-monthly administration of UGN-101 completion F/U 12 months study TR9a TR6a TR3a completion TR11a TR10a TR8a TR7a TR5a TR4a TR2a TR1a +F/U +F/U +F/U Visit 21 Visit 20 Visit 19 Visit 18 Visit 17 Visit 16 Visit 15 Visit 14 Visit 13 Visit 12 Visit 11 Visit 10
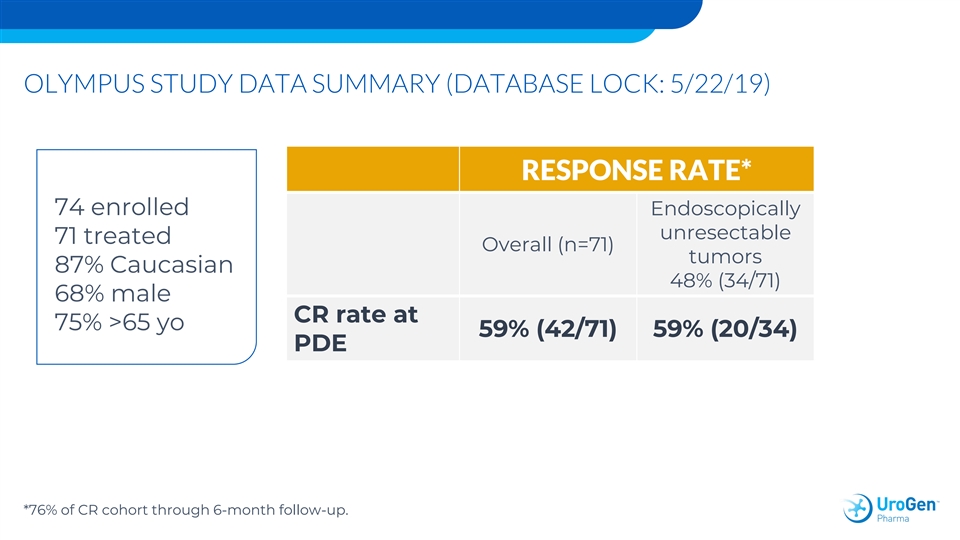
OLYMPUS STUDY DATA SUMMARY (DATABASE LOCK: 5/22/19) RESPONSE RATE* 74 enrolled Endoscopically unresectable 71 treated Overall (n=71) tumors 87% Caucasian 48% (34/71) 68% male CR rate at 75% >65 yo 59% (42/71) 59% (20/34) PDE *76% of CR cohort through 6-month follow-up.OLYMPUS STUDY DATA SUMMARY (DATABASE LOCK: 5/22/19) RESPONSE RATE* 74 enrolled Endoscopically unresectable 71 treated Overall (n=71) tumors 87% Caucasian 48% (34/71) 68% male CR rate at 75% >65 yo 59% (42/71) 59% (20/34) PDE *76% of CR cohort through 6-month follow-up.
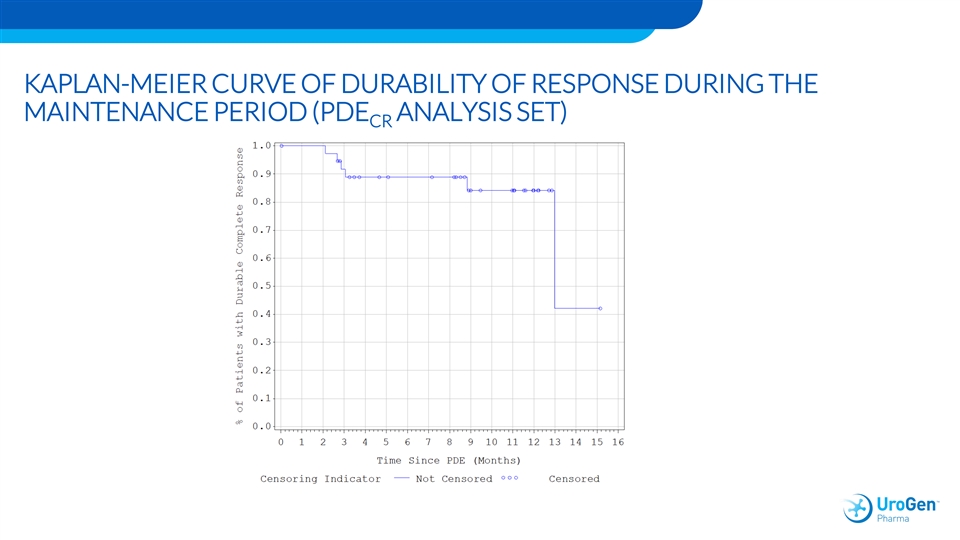
KAPLAN-MEIER CURVE OF DURABILITY OF RESPONSE DURING THE MAINTENANCE PERIOD (PDE ANALYSIS SET) CRKAPLAN-MEIER CURVE OF DURABILITY OF RESPONSE DURING THE MAINTENANCE PERIOD (PDE ANALYSIS SET) CR
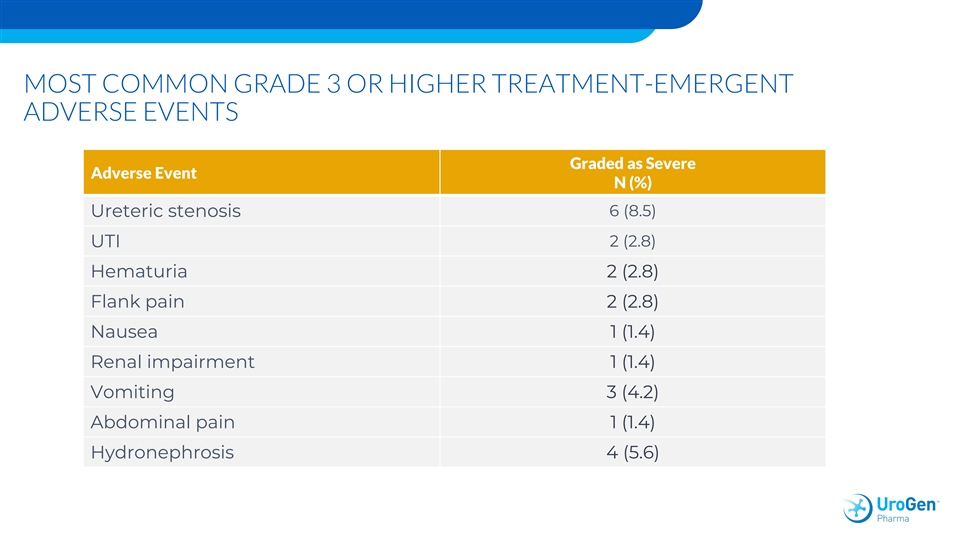
MOST COMMON GRADE 3 OR HIGHER TREATMENT-EMERGENT ADVERSE EVENTS Graded as Severe Adverse Event N (%) 6 (8.5) Ureteric stenosis UTI 2 (2.8) Hematuria 2 (2.8) Flank pain 2 (2.8) Nausea 1 (1.4) Renal impairment 1 (1.4) Vomiting 3 (4.2) Abdominal pain 1 (1.4) Hydronephrosis 4 (5.6)MOST COMMON GRADE 3 OR HIGHER TREATMENT-EMERGENT ADVERSE EVENTS Graded as Severe Adverse Event N (%) 6 (8.5) Ureteric stenosis UTI 2 (2.8) Hematuria 2 (2.8) Flank pain 2 (2.8) Nausea 1 (1.4) Renal impairment 1 (1.4) Vomiting 3 (4.2) Abdominal pain 1 (1.4) Hydronephrosis 4 (5.6)

RENAL URINARY TOXICITY 48 patients 11 (23%) no surgical intervention 24 (50%) transient stent 11 (23%) long-term stent 2 (4%) RNURENAL URINARY TOXICITY 48 patients 11 (23%) no surgical intervention 24 (50%) transient stent 11 (23%) long-term stent 2 (4%) RNU
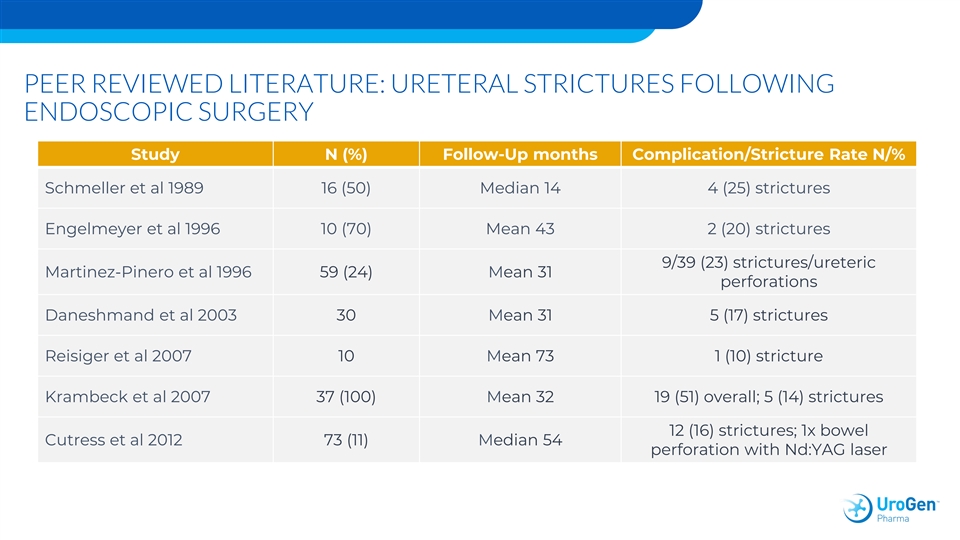
PEER REVIEWED LITERATURE: URETERAL STRICTURES FOLLOWING ENDOSCOPIC SURGERY Study N (%) Follow-Up months Complication/Stricture Rate N/% Schmeller et al 1989 16 (50) Median 14 4 (25) strictures Engelmeyer et al 1996 10 (70) Mean 43 2 (20) strictures 9/39 (23) strictures/ureteric Martinez-Pinero et al 1996 59 (24) Mean 31 perforations Daneshmand et al 2003 30 Mean 31 5 (17) strictures Reisiger et al 2007 10 Mean 73 1 (10) stricture Krambeck et al 2007 37 (100) Mean 32 19 (51) overall; 5 (14) strictures 12 (16) strictures; 1x bowel Cutress et al 2012 73 (11) Median 54 perforation with Nd:YAG laserPEER REVIEWED LITERATURE: URETERAL STRICTURES FOLLOWING ENDOSCOPIC SURGERY Study N (%) Follow-Up months Complication/Stricture Rate N/% Schmeller et al 1989 16 (50) Median 14 4 (25) strictures Engelmeyer et al 1996 10 (70) Mean 43 2 (20) strictures 9/39 (23) strictures/ureteric Martinez-Pinero et al 1996 59 (24) Mean 31 perforations Daneshmand et al 2003 30 Mean 31 5 (17) strictures Reisiger et al 2007 10 Mean 73 1 (10) stricture Krambeck et al 2007 37 (100) Mean 32 19 (51) overall; 5 (14) strictures 12 (16) strictures; 1x bowel Cutress et al 2012 73 (11) Median 54 perforation with Nd:YAG laser

OLYMPUS SUMMARY • 71 patients treated • 59% CR –Efficacy consistent in endoscopically unresectable pts • Durability stable at 6 and 12 months (84% per KM analysis) • AE profile –Consistent with upper tract manipulation for cancer –Stricture consistent with published literature • Follow-up ongoingOLYMPUS SUMMARY • 71 patients treated • 59% CR –Efficacy consistent in endoscopically unresectable pts • Durability stable at 6 and 12 months (84% per KM analysis) • AE profile –Consistent with upper tract manipulation for cancer –Stricture consistent with published literature • Follow-up ongoing

UGN-102 PROGRAM FOR INTERMEDIATE-RISK LG UBC • Similarities between LG NMIBC and LG UTUC –Histology –Recurrence pattern –Clinical behavior –High risk of recurrence –Low risk of progression • New molecular data • Sensitivity to locally administered drugs UGN-102 PROGRAM FOR INTERMEDIATE-RISK LG UBC • Similarities between LG NMIBC and LG UTUC –Histology –Recurrence pattern –Clinical behavior –High risk of recurrence –Low risk of progression • New molecular data • Sensitivity to locally administered drugs
CYSTOSCOPIC APPEARANCE OF TURBT Courtesy of Alex Sankin, MD, Montefiore Medical Center & The Albert Einstein College of Medicine, Bronx, NYCYSTOSCOPIC APPEARANCE OF TURBT Courtesy of Alex Sankin, MD, Montefiore Medical Center & The Albert Einstein College of Medicine, Bronx, NY
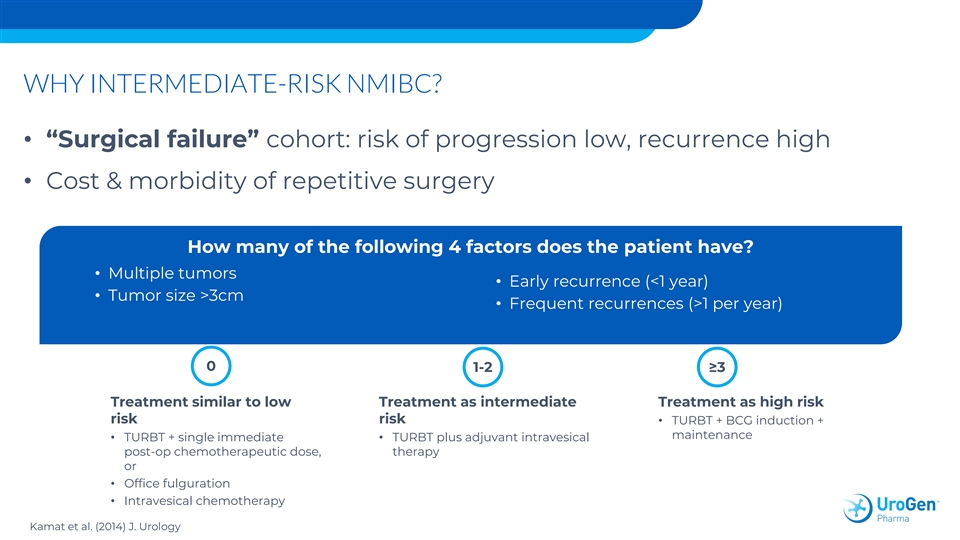
WHY INTERMEDIATE-RISK NMIBC? • “Surgical failure” cohort: risk of progression low, recurrence high • Cost & morbidity of repetitive surgery How many of the following 4 factors does the patient have? • Multiple tumors • Early recurrence (<1 year) • Tumor size >3cm • Frequent recurrences (>1 per year) 0 1-2≥3 Treatment similar to low Treatment as intermediate Treatment as high risk risk risk • TURBT + BCG induction + maintenance • TURBT + single immediate • TURBT plus adjuvant intravesical post-op chemotherapeutic dose, therapy or • Office fulguration • Intravesical chemotherapy Kamat et al. (2014) J. Urology WHY INTERMEDIATE-RISK NMIBC? • “Surgical failure” cohort: risk of progression low, recurrence high • Cost & morbidity of repetitive surgery How many of the following 4 factors does the patient have? • Multiple tumors • Early recurrence (<1 year) • Tumor size >3cm • Frequent recurrences (>1 per year) 0 1-2≥3 Treatment similar to low Treatment as intermediate Treatment as high risk risk risk • TURBT + BCG induction + maintenance • TURBT + single immediate • TURBT plus adjuvant intravesical post-op chemotherapeutic dose, therapy or • Office fulguration • Intravesical chemotherapy Kamat et al. (2014) J. Urology

OPTIMA II DESIGN • Patients with IR NMIBC (1-2/3: multifocal, lesion >3 cm, recurrence within 12 months) • UGN-102 q wk X 6 • Primary endpoint: CR at 3-month visit –CR pts followed quarterly x 9 months • Secondary endpoint: 12-month durability & safety OPTIMA II DESIGN • Patients with IR NMIBC (1-2/3: multifocal, lesion >3 cm, recurrence within 12 months) • UGN-102 q wk X 6 • Primary endpoint: CR at 3-month visit –CR pts followed quarterly x 9 months • Secondary endpoint: 12-month durability & safety
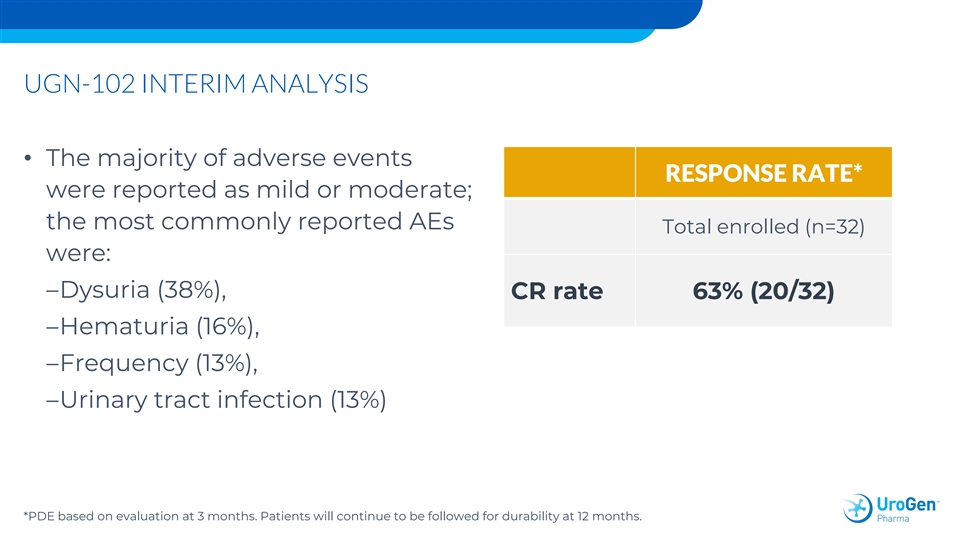
UGN-102 INTERIM ANALYSIS • The majority of adverse events RESPONSE RATE* were reported as mild or moderate; the most commonly reported AEs Total enrolled (n=32) were: –Dysuria (38%), CR rate 63% (20/32) –Hematuria (16%), –Frequency (13%), –Urinary tract infection (13%) *PDE based on evaluation at 3 months. Patients will continue to be followed for durability at 12 months.UGN-102 INTERIM ANALYSIS • The majority of adverse events RESPONSE RATE* were reported as mild or moderate; the most commonly reported AEs Total enrolled (n=32) were: –Dysuria (38%), CR rate 63% (20/32) –Hematuria (16%), –Frequency (13%), –Urinary tract infection (13%) *PDE based on evaluation at 3 months. Patients will continue to be followed for durability at 12 months.

NEXT STEPS FOR UGN-102 • Continued follow-up of patients in the trial • Discuss with FDA • Scenario planning for phase 3 pivotal study –Streamline & expedite registrational path –Randomized H2H vs TURBT likely • We are prepared to execute • Pivotal study planned for 2020NEXT STEPS FOR UGN-102 • Continued follow-up of patients in the trial • Discuss with FDA • Scenario planning for phase 3 pivotal study –Streamline & expedite registrational path –Randomized H2H vs TURBT likely • We are prepared to execute • Pivotal study planned for 2020

UGN-201 UC is an “immune” 201: TLR 7/8 agonist Hypothesis: responsive tumor • Activates “innate” Combinatorial immune response immunotherapy is • “Early” experience with feasible and clinically BCG (local) • May potentiate “adaptive” meaningful antitumor response • CPI trials for MIBC (systemic) • Phase 1b: “signal” when applied locally to CISUGN-201 UC is an “immune” 201: TLR 7/8 agonist Hypothesis: responsive tumor • Activates “innate” Combinatorial immune response immunotherapy is • “Early” experience with feasible and clinically BCG (local) • May potentiate “adaptive” meaningful antitumor response • CPI trials for MIBC (systemic) • Phase 1b: “signal” when applied locally to CIS
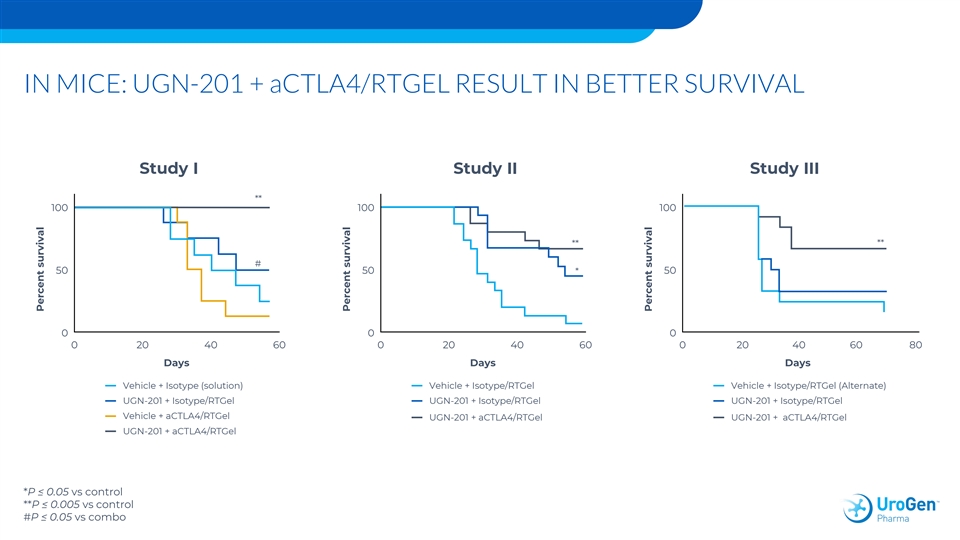
IN MICE: UGN-201 + aCTLA4/RTGEL RESULT IN BETTER SURVIVAL Study I Study II Study III ** 100 100 100 ** ** # 50 50 * 50 0 0 0 0 20 40 60 0 20 40 60 0 20 40 60 80 Days Days Days Vehicle + Isotype (solution) Vehicle + Isotype/RTGel Vehicle + Isotype/RTGel (Alternate) UGN-201 + Isotype/RTGel UGN-201 + Isotype/RTGel UGN-201 + Isotype/RTGel Vehicle + aCTLA4/RTGel UGN-201 + aCTLA4/RTGel UGN-201 + aCTLA4/RTGel UGN-201 + aCTLA4/RTGel *P ≤ 0.05 vs control **P ≤ 0.005 vs control #P ≤ 0.05 vs combo Percent survival Percent survival Percent survivalIN MICE: UGN-201 + aCTLA4/RTGEL RESULT IN BETTER SURVIVAL Study I Study II Study III ** 100 100 100 ** ** # 50 50 * 50 0 0 0 0 20 40 60 0 20 40 60 0 20 40 60 80 Days Days Days Vehicle + Isotype (solution) Vehicle + Isotype/RTGel Vehicle + Isotype/RTGel (Alternate) UGN-201 + Isotype/RTGel UGN-201 + Isotype/RTGel UGN-201 + Isotype/RTGel Vehicle + aCTLA4/RTGel UGN-201 + aCTLA4/RTGel UGN-201 + aCTLA4/RTGel UGN-201 + aCTLA4/RTGel *P ≤ 0.05 vs control **P ≤ 0.005 vs control #P ≤ 0.05 vs combo Percent survival Percent survival Percent survival

SUMMARY OF MAJOR FINDINGS FROM MURINE STUDIES • Intravesical UGN-201+aCTLA4/RTGel: –Smaller tumors and better survival rate –Decreases T regulatory cells –Increases the ratio of CD8+/T regulatory cells • Data support continued progression toward human trials • May represent a novel approach to HG NMIBC SUMMARY OF MAJOR FINDINGS FROM MURINE STUDIES • Intravesical UGN-201+aCTLA4/RTGel: –Smaller tumors and better survival rate –Decreases T regulatory cells –Increases the ratio of CD8+/T regulatory cells • Data support continued progression toward human trials • May represent a novel approach to HG NMIBC

SUMMARY UGN-101: First primary chemoablative therapy for urothelial cancer • Robust efficacy & durability in LG UTUC – CR: 59% – 12-month durability 84% (KM) including “unresectable” 59% pts 63% UGN-102 in IR NMIBC: • Interim data encouraging – CR: 63% – AE profile: mostly mild to moderate, local & transient UGN-201 Immunotherapy: • Local aCTLA4+UGN-201 increased survival UBC (Murine) SUMMARY UGN-101: First primary chemoablative therapy for urothelial cancer • Robust efficacy & durability in LG UTUC – CR: 59% – 12-month durability 84% (KM) including “unresectable” 59% pts 63% UGN-102 in IR NMIBC: • Interim data encouraging – CR: 63% – AE profile: mostly mild to moderate, local & transient UGN-201 Immunotherapy: • Local aCTLA4+UGN-201 increased survival UBC (Murine)

CREDIT WHERE CREDIT IS DUE • Elyse Seltzer, MD • Roman Bromblin • Jim Ottinger, RPh • Diana Licis • Marina Konorty, PhD • Eric Smith • Dalit Strauss-Ayali, PhD DVM • Madlen Malinowski • Ifat Klein, PhD • Tami Gerassi • Yael Agmon • Swati Chiktara • Nimrod Gabai • Ruby Salmo • Elinor Schreiber • Sunil Raju, MD • Dima Zolotaryov • Robert Kirshoff • Baruch Narotzki • Pallavi RajputCREDIT WHERE CREDIT IS DUE • Elyse Seltzer, MD • Roman Bromblin • Jim Ottinger, RPh • Diana Licis • Marina Konorty, PhD • Eric Smith • Dalit Strauss-Ayali, PhD DVM • Madlen Malinowski • Ifat Klein, PhD • Tami Gerassi • Yael Agmon • Swati Chiktara • Nimrod Gabai • Ruby Salmo • Elinor Schreiber • Sunil Raju, MD • Dima Zolotaryov • Robert Kirshoff • Baruch Narotzki • Pallavi Rajput

INNOVATION IN PRACTICE KOL Panel and Q&AINNOVATION IN PRACTICE KOL Panel and Q&A

MANAGEMENT Q&AMANAGEMENT Q&A

UROGEN: BUILDING A GROWTH COMPANY CLOSING REMARKSUROGEN: BUILDING A GROWTH COMPANY CLOSING REMARKS

IN SUMMARY UroGen is committed to leading in challenging areas of high unmet need UroGen is poised for near-term catalysts behind positive data in UGN-101 and UGN-102 UroGen will focus on urology and Gyn/GI oncology UroGen plans to leverage our proprietary expertise in RTGel but grow beyond local delivery UroGen is in a strong financial position that will take us through launch and advance the pipeline UroGen is designed to build a sustainable growth company while maintaining the culture of creativity and problem solving that got us hereIN SUMMARY UroGen is committed to leading in challenging areas of high unmet need UroGen is poised for near-term catalysts behind positive data in UGN-101 and UGN-102 UroGen will focus on urology and Gyn/GI oncology UroGen plans to leverage our proprietary expertise in RTGel but grow beyond local delivery UroGen is in a strong financial position that will take us through launch and advance the pipeline UroGen is designed to build a sustainable growth company while maintaining the culture of creativity and problem solving that got us here