
Aptevo Therapeutics Investor Presentation November 2017 Exhibit 99.2

This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including our financial guidance, product portfolio, product sales, capabilities and any other statements containing the words “believes”, “expects”, “anticipates”, “intends”, “plans”, “forecasts”, “estimates” and similar expressions in conjunction with, among other things, discussions of financial performance or financial condition, growth strategy, product sales, manufacturing capabilities, product development, regulatory approvals or expenditures are forward-looking statements. These forward-looking statements are based on our current intentions, beliefs and expectations regarding future events. We cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this presentation, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events or circumstances. There are a number of important factors that could cause Aptevo's actual results to differ materially from those indicated by such forward-looking statements, including possible negative effects on Aptevo’s business operations, assets or financial results as a result of the separation; a deterioration in the business or prospects of Aptevo; adverse developments in Aptevo’s customer-base or markets; our ability to enter into and maintain selective collaboration and partnership arrangements; the timing of and our ability to achieve milestones in collaboration and partnership contracts; our ability and the ability of our contractors and suppliers to maintain compliance with cGMP and other regulatory obligations; the results of regulatory inspections; the rate and degree of market acceptance and clinical utility of our products; the success of our ongoing and planned development programs; the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; and our commercialization, marketing and manufacturing capabilities and strategy and changes in regulatory, social and political conditions. Additional risks and factors that may affect results are set forth in our filings with the Securities and Exchange Commission, including Aptevo’s most recent Annual Report on Form 10-K, as filed on March 31, 2017, and its subsequent reports on Form 10-Q and current reports on Form 8-K The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the SEC, when evaluating our forward-looking statements. Aptevo™, ADAPTIR and any and all Aptevo Therapeutics Inc. brand, product, service, and feature names, logos, and slogans are trademarks or registered trademarks of Aptevo Therapeutics Inc. or its subsidiaries in the United States or other countries. All rights reserved. Forward-Looking Statements Aptevo therapeutics

Aptevo: At a Glance Leading Oncology Platform Innovative ADAPTIR platform technology utilizing a novel approach in the highly attractive immuno-oncology field Leveraging Technology Targeted investments in bispecific ADAPTIR therapeutics Robust IP Estate Own and exclusively licensed patents and trade secrets which support our commercial products and pipeline Focus Oncology/Hematology Commercial Products IXINITY® Product Pipeline Clinical: 2 Preclinical: Multiple Platform Technology ADAPTIR™ Employees ~120 Headquarters Seattle, WA 2016 Product Revenue $36M 2015 Product Revenue $28M Cash Position $107M (9/30/2017) Aptevo therapeutics

Aptevo – A Compelling Investment Opportunity Solid cash position to advance R&D and commercial strategy 1 2 3 4 5 Strong leadership with a track record of execution Advancing ADAPTIR to generate novel first-in-class therapeutics Broad pipeline of wholly-owned clinical and preclinical candidates Commercial asset (IXINITY) with growth potential Aptevo therapeutics

Agenda Executing on our Strategy ADAPTIR – Developing Novel Protein Therapeutics Impressive Clinical and Preclinical Portfolio IXINITY – A Growing Commercial Opportunity Summary Aptevo therapeutics innovating

Experienced Leadership Team Senior Management Marvin White – President & CEO Former Emergent Director; Former CFO, St. Vincent’s Health; Former Exec. Director & CFO, Lilly USA Jeff Lamothe – SVP, CFO Former Emergent VP, Finance; Former CFO, Cangene Corporation Randy Maddux – SVP, Operations Former VP, Global Mfg & Supply, GSK; Former VP, Mfg Ops & Quality, Human Genome Sciences Dr. Scott Stromatt – SVP, CMO Former Emergent SVP, CMO; Former CMO, Trubion Dr. Jane Gross – SVP, CSO Former Emergent VP, Research/Non-Clinical Development; Former VP Immunology Research ZymoGenetics Inc. Mike Adelman – VP, Commercial Ops. Former Emergent VP, Commercial Operations; Former, VP Commercial Operations, Cangene Corporation Shawnte Mitchell – VP, Gen’l Counsel/HR Former Emergent VP, Associate General Counsel Board of Directors Marvin White Former Emergent Director; Former CFO, St. Vincent’s Health; Former Exec. Director & CFO, Lilly USA Fuad El-Hibri Founder, Executive Chairman, Emergent BioSolutions Daniel Abdun-Nabi President & CEO, Emergent BioSolutions Grady Grant, III Reckitt Benckiser Group (formerly Mead Johnson Nutrition); Eli Lilly & Co. Zsolt Harsanyi, Ph.D. N-Gene Research Labs; Exponential Biotherapies; Porton Int’l Barbara Lopez Kunz DIA; Battelle; Thermo Fisher Scientific; ICI/Uniqema John Niederhuber, M.D. Inova Translational Medicine Institute; NCI; Johns Hopkins Univ. Deep R&D, Manufacturing, Commercial and Financial Expertise and Experience Aptevo therapeutics
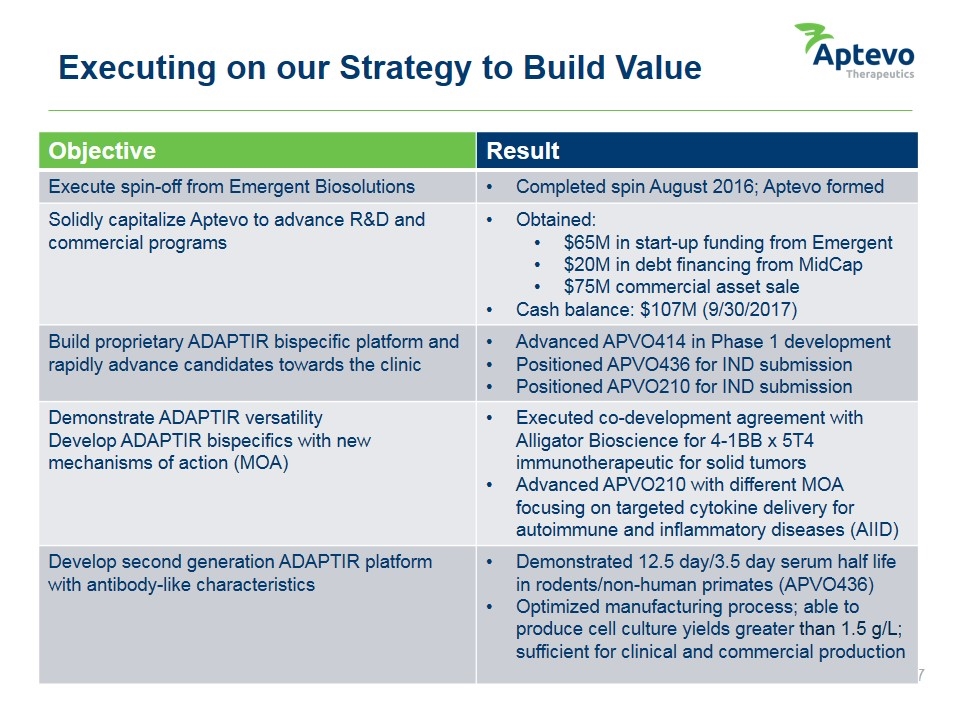
Executing on our Strategy to Build Value Objective Result Execute spin-off from Emergent Biosolutions Completed spin August 2016; Aptevo formed Solidly capitalize Aptevo to advance R&D and commercial programs Obtained: $65M in start-up funding from Emergent $20M in debt financing from MidCap $75M commercial asset sale Cash balance: $107M (9/30/2017) Build proprietary ADAPTIR bispecific platform and rapidly advance candidates towards the clinic Advanced APVO414 in Phase 1 development Positioned APVO436 for IND submission Positioned APVO210 for IND submission Demonstrate ADAPTIR versatility Develop ADAPTIR bispecifics with new mechanisms of action (MOA) Executed co-development agreement with Alligator Bioscience for 4-1BB x 5T4 immunotherapeutic for solid tumors Advanced APVO210 with different MOA focusing on targeted cytokine delivery for autoimmune and inflammatory diseases (AIID) Develop second generation ADAPTIR platform with antibody-like characteristics Demonstrated 12.5 day/3.5 day serum half life in rodents/non-human primates (APVO436) Optimized manufacturing process; able to produce cell culture yields greater than 1.5 g/L; sufficient for clinical and commercial production Aptevo therapeutics
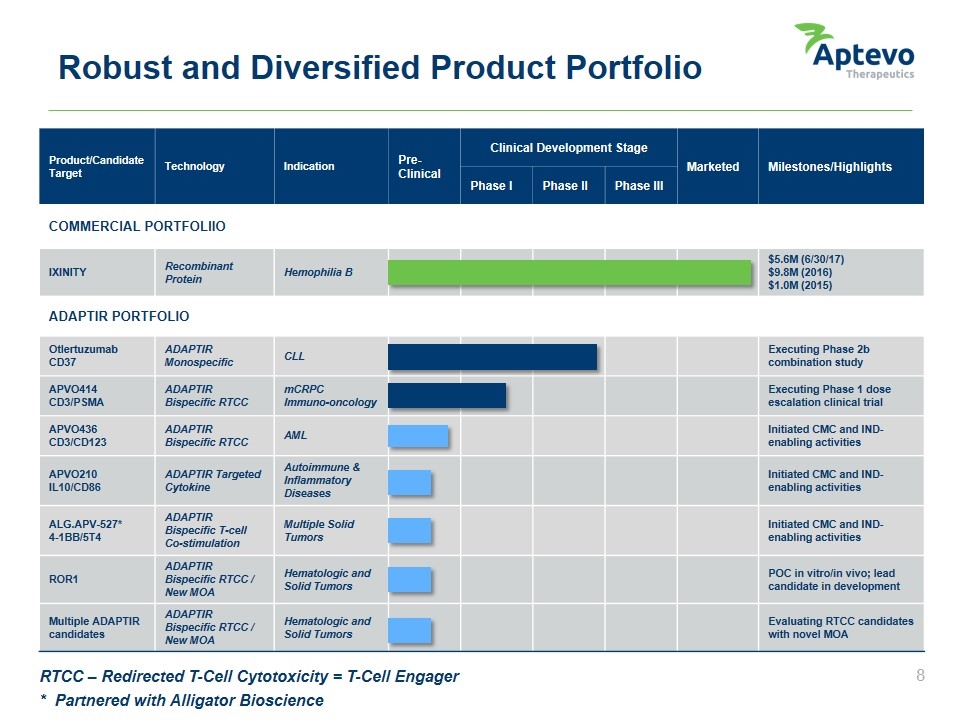
Product/Candidate Target Technology Indication Pre-Clinical Clinical Development Stage Marketed Milestones/Highlights Phase I Phase II Phase III COMMERCIAL PORTFOLIIO IXINITY Recombinant Protein Hemophilia B $5.6M (6/30/17) $9.8M (2016) $1.0M (2015) ADAPTIR PORTFOLIO Otlertuzumab CD37 ADAPTIR Monospecific CLL Executing Phase 2b combination study APVO414 CD3/PSMA ADAPTIR Bispecific RTCC mCRPC Immuno-oncology Executing Phase 1 dose escalation clinical trial APVO436 CD3/CD123 ADAPTIR Bispecific RTCC AML Initiated CMC and IND-enabling activities APVO210 IL10/CD86 ADAPTIR Targeted Cytokine Autoimmune & Inflammatory Diseases Initiated CMC and IND-enabling activities ALG.APV-527* 4-1BB/5T4 ADAPTIR Bispecific T-cell Co-stimulation Multiple Solid Tumors Initiated CMC and IND-enabling activities ROR1 ADAPTIR Bispecific RTCC / New MOA Hematologic and Solid Tumors POC in vitro/in vivo; lead candidate in development Multiple ADAPTIR candidates ADAPTIR Bispecific RTCC / New MOA Hematologic and Solid Tumors Evaluating RTCC candidates with novel MOA Robust and Diversified Product Portfolio * Partnered with Alligator Bioscience RTCC – Redirected T-Cell Cytotoxicity = T-Cell Engager Aptevo therapeutics

Agenda Executing On Our Strategy ADAPTIR – Developing Novel Protein Therapeutics Impressive Clinical and Preclinical Portfolio IXINITY – A Growing Commercial Opportunity Summary Aptevo therapeutics innovating
ADAPTIR is Aptevo’s platform technology for generating novel monospecific and bispecific antibody therapeutics for immuno-oncology and autoimmune/inflammatory diseases ADAPTIR is a robust, flexible platform that can be used to generate bispecific molecules with different mechanisms of action The ADAPTIR platform and structure provides distinct advantages over other bispecific technologies and therapeutic approaches Advancing ADAPTIR Technology to Generate Novel First-In-Class Therapeutics Aptevo therapeutics
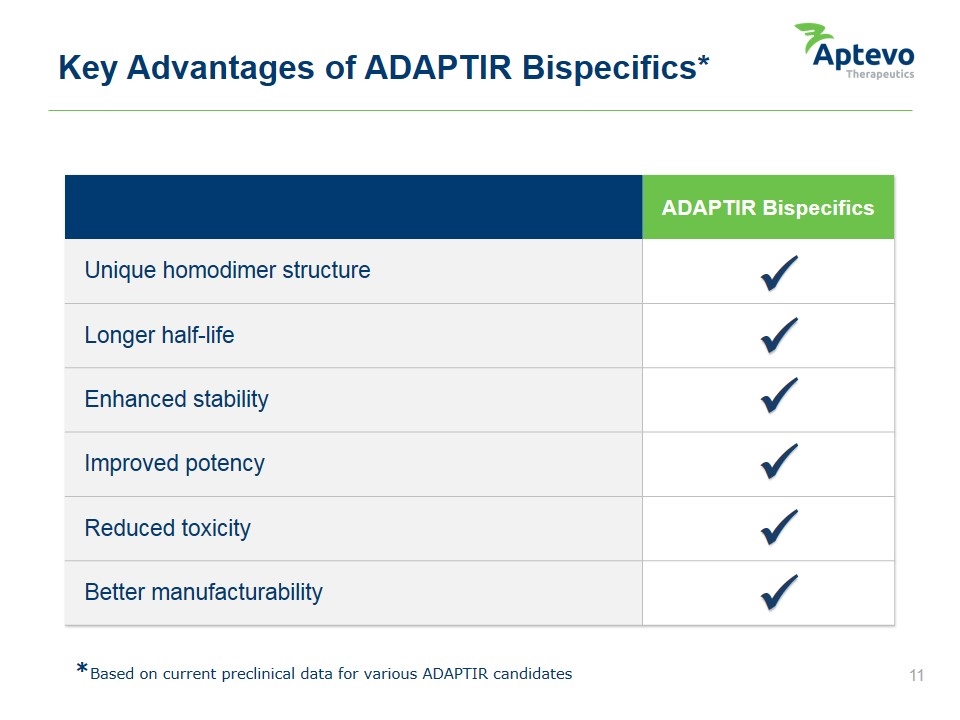
Key Advantages of ADAPTIR Bispecifics* ADAPTIR Bispecifics Unique homodimer structure Longer half-life Enhanced stability Improved potency Reduced toxicity Better manufacturability ü ü ü ü ü ü *Based on current preclinical data for various ADAPTIR candidates Aptevo therapeutics
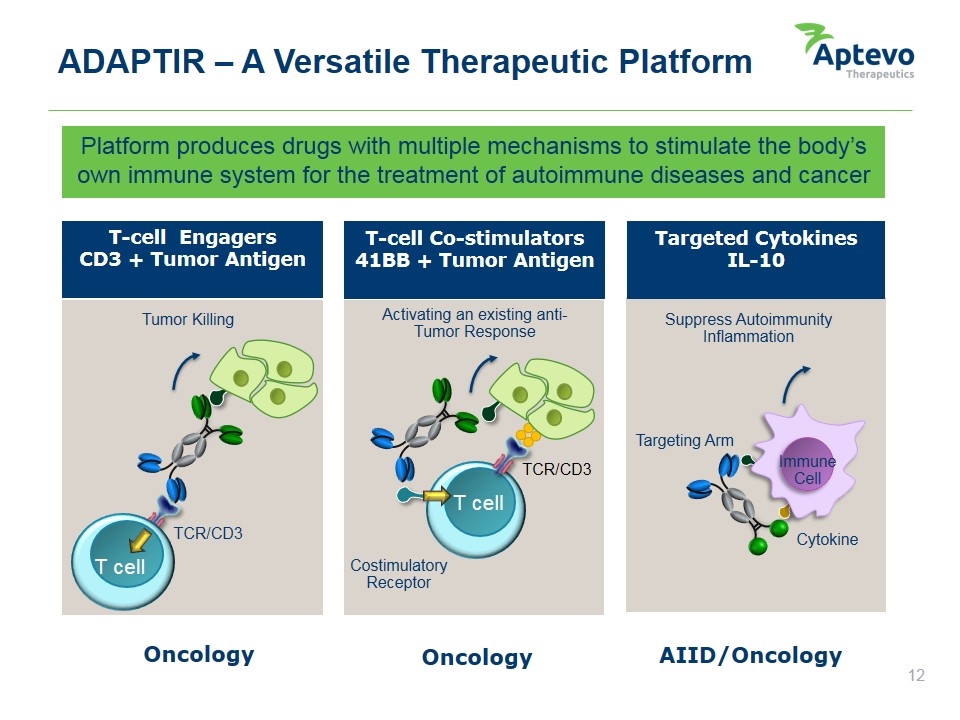
Platform produces drugs with multiple mechanisms to stimulate the body’s own immune system for the treatment of autoimmune diseases and cancer ADAPTIR – A Versatile Therapeutic Platform T-cell Engagers CD3 + Tumor Antigen T-cell Co-stimulators 41BB + Tumor Antigen Targeted Cytokines IL-10 AIID/Oncology Oncology Oncology Cytokine Targeting Arm Costimulatory Receptor T cell Activating an existing anti-Tumor Response T cell TCR/CD3 TCR/CD3 Immune Cell Suppress Autoimmunity Inflammation Tumor Killing Aptevo therapeutics
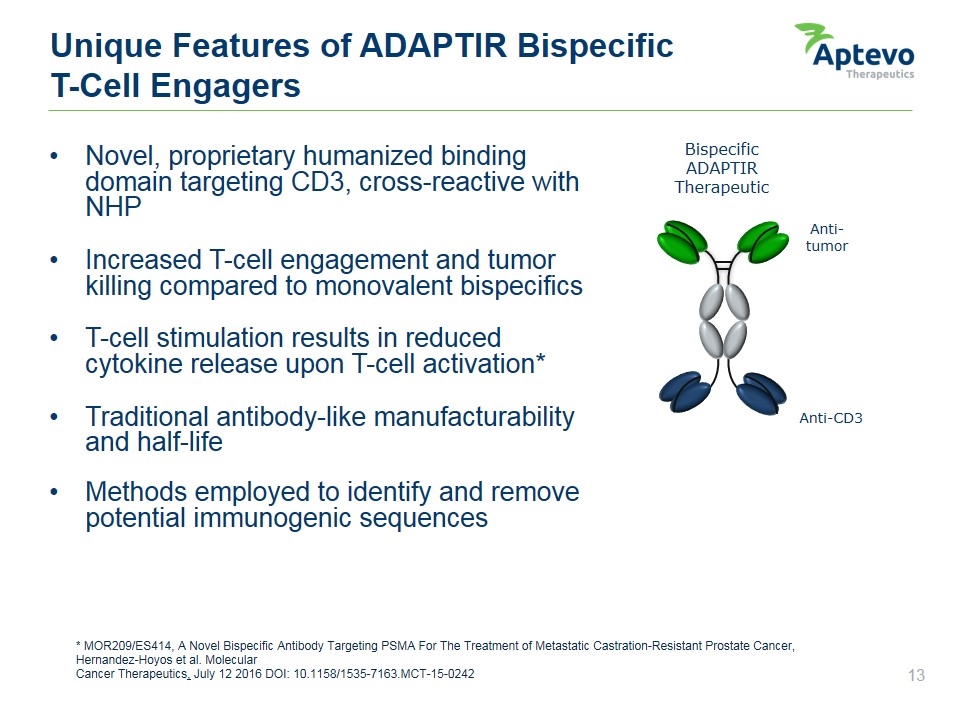
Bispecific ADAPTIR Therapeutic Anti-tumor Anti-CD3 * MOR209/ES414, A Novel Bispecific Antibody Targeting PSMA For The Treatment of Metastatic Castration-Resistant Prostate Cancer, Hernandez-Hoyos et al. Molecular Cancer Therapeutics, July 12 2016 DOI: 10.1158/1535-7163.MCT-15-0242 Unique Features of ADAPTIR Bispecific T-Cell Engagers Novel, proprietary humanized binding domain targeting CD3, cross-reactive with NHP Increased T-cell engagement and tumor killing compared to monovalent bispecifics T-cell stimulation results in reduced cytokine release upon T-cell activation* Traditional antibody-like manufacturability and half-life Methods employed to identify and remove potential immunogenic sequences Aptevo therapeutics
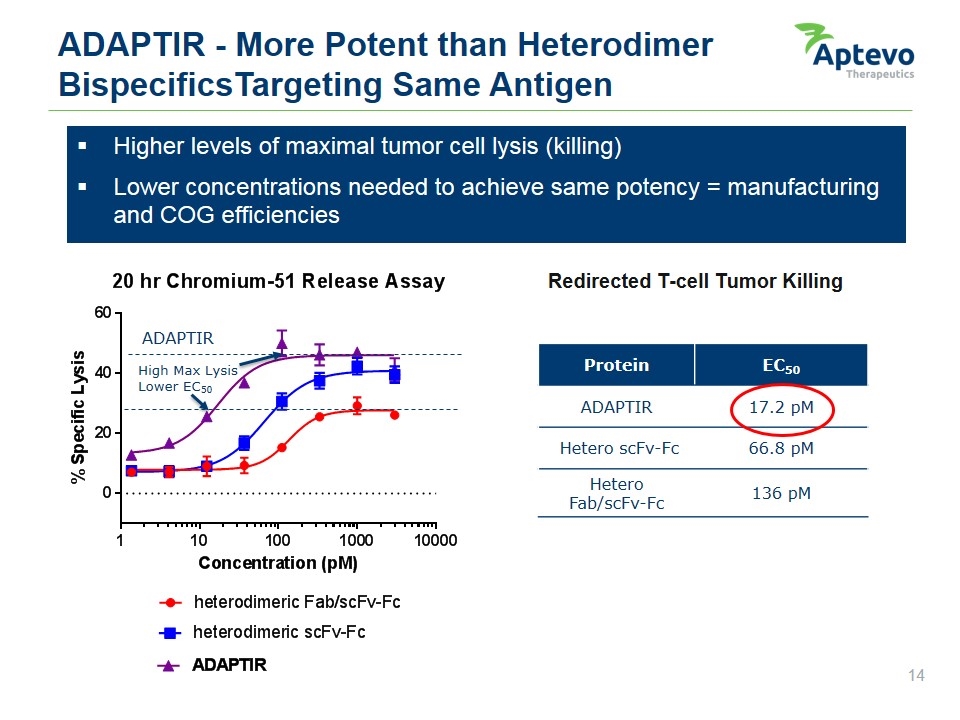
ADAPTIR - More Potent than Heterodimer BispecificsTargeting Same Antigen Higher levels of maximal tumor cell lysis (killing) Lower concentrations needed to achieve same potency = manufacturing and COG efficiencies Redirected T-cell Tumor Killing ADAPTIR High Max Lysis Lower EC50 Protein EC50 ADAPTIR 17.2 pM Hetero scFv-Fc 66.8 pM Hetero Fab/scFv-Fc 136 pM % specific lysis 20 hr chromium-51 release assay Adaptir High max lysis lower EC50 0 20 40 60 10 100 1000 10000 Concentration (pM) heterodimeric fab/scfv-fc heterodimeric scfv-fc ADAPTIR Aptevo therapeutics

Agenda Executing On Our Strategy ADAPTIR – Developing Novel Protein Therapeutics Impressive Clinical and Preclinical Portfolio IXINITY – A Growing Commercial Opportunity Summary Aptevo therapeutics innovating
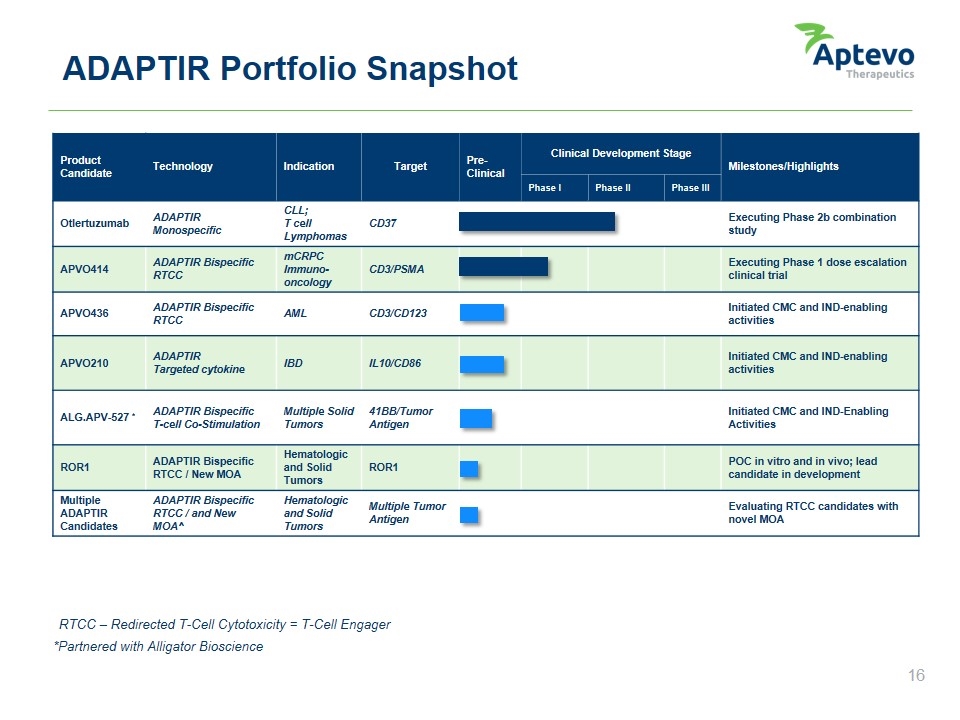
Product Candidate Technology Indication Target Pre-Clinical Clinical Development Stage Milestones/Highlights Phase I Phase II Phase III Otlertuzumab ADAPTIR Monospecific CLL; T cell Lymphomas CD37 Executing Phase 2b combination study APVO414 ADAPTIR Bispecific RTCC mCRPC Immuno-oncology CD3/PSMA Executing Phase 1 dose escalation clinical trial APVO436 ADAPTIR Bispecific RTCC AML CD3/CD123 Initiated CMC and IND-enabling activities APVO210 ADAPTIR Targeted cytokine IBD IL10/CD86 Initiated CMC and IND-enabling activities ALG.APV-527 * ADAPTIR Bispecific T-cell Co-Stimulation Multiple Solid Tumors 41BB/Tumor Antigen Initiated CMC and IND-Enabling Activities ROR1 ADAPTIR Bispecific RTCC / New MOA Hematologic and Solid Tumors ROR1 POC in vitro and in vivo; lead candidate in development Multiple ADAPTIR Candidates ADAPTIR Bispecific RTCC / and New MOA^ Hematologic and Solid Tumors Multiple Tumor Antigen Evaluating RTCC candidates with novel MOA ADAPTIR Portfolio Snapshot *Partnered with Alligator Bioscience RTCC – Redirected T-Cell Cytotoxicity = T-Cell Engager Aptevo therapeutics
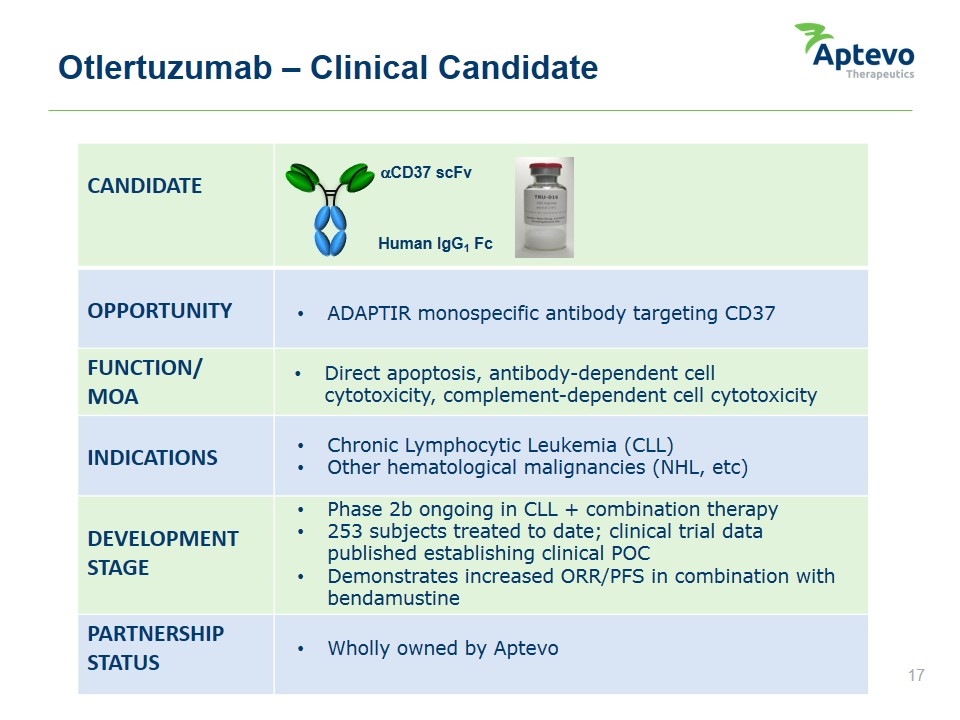
Otlertuzumab – Clinical Candidate CANDIDATE OPPORTUNITY FUNCTION/ MOA INDICATIONS DEVELOPMENT STAGE PARTNERSHIP STATUS aCD37 scFv Human IgG1 Fc ADAPTIR monospecific antibody targeting CD37 Direct apoptosis, antibody-dependent cell cytotoxicity, complement-dependent cell cytotoxicity Chronic Lymphocytic Leukemia (CLL) Other hematological malignancies (NHL, etc) Phase 2b ongoing in CLL + combination therapy 253 subjects treated to date; clinical trial data published establishing clinical POC Demonstrates increased ORR/PFS in combination with bendamustine Wholly owned by Aptevo Aptevo therapeutics
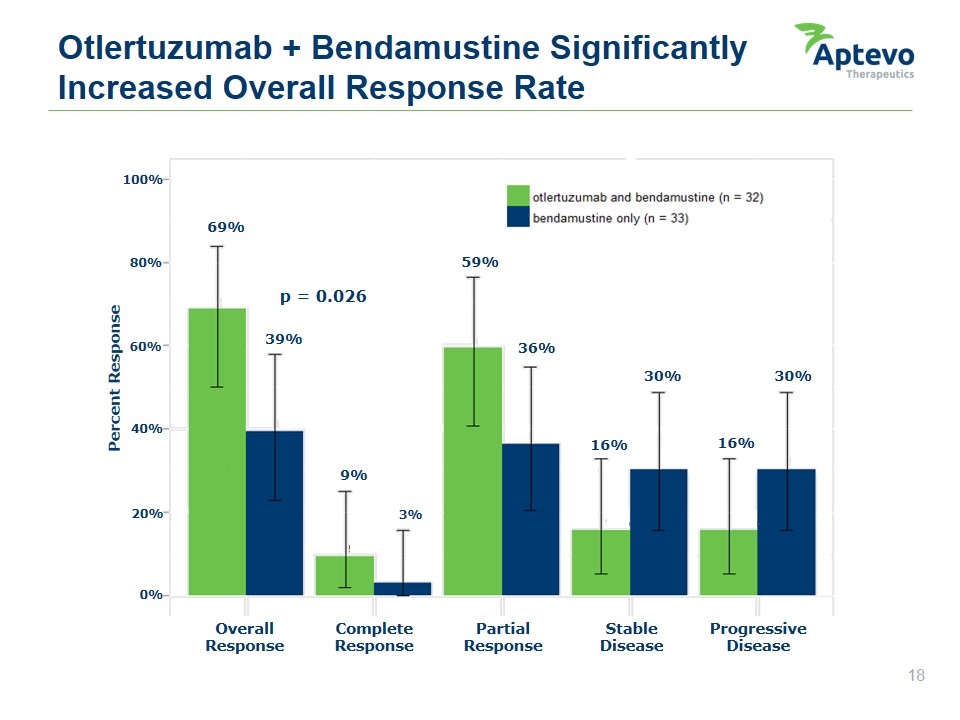
Otlertuzumab + Bendamustine Significantly Increased Overall Response Rate Overall Response Complete Response Partial Response Stable Disease Progressive Disease Percent Response 100% 80% 60% 40% 20% 0% 69% 39% 59% 9% 3% 36% 16% 30% 16% 30% p = 0.026 Aptevo therapeutics otlertuzumab and bendamustine (n = 32) bendamustine only (n = 33)
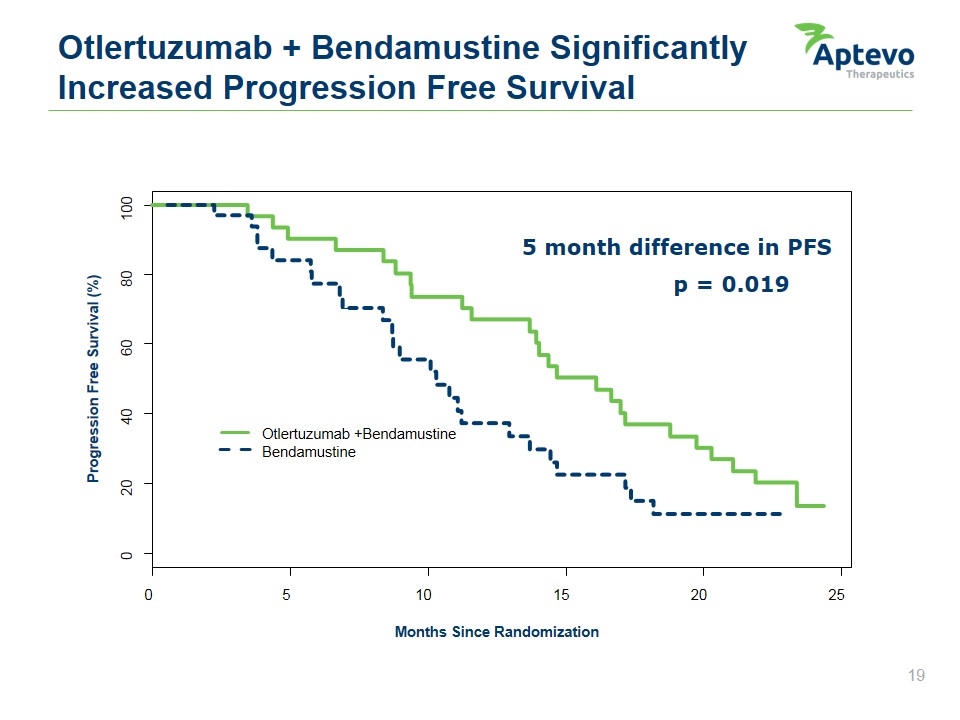
5 month difference in PFS Otlertuzumab + Bendamustine Significantly Increased Progression Free Survival p = 0.019 0 5 10 15 20 25 0 20 40 60 80 100 Months Since Randomization Progression Free Survival (%) Otlertuzumab +Bendamustine Bendamustine Aptevo therapeutics
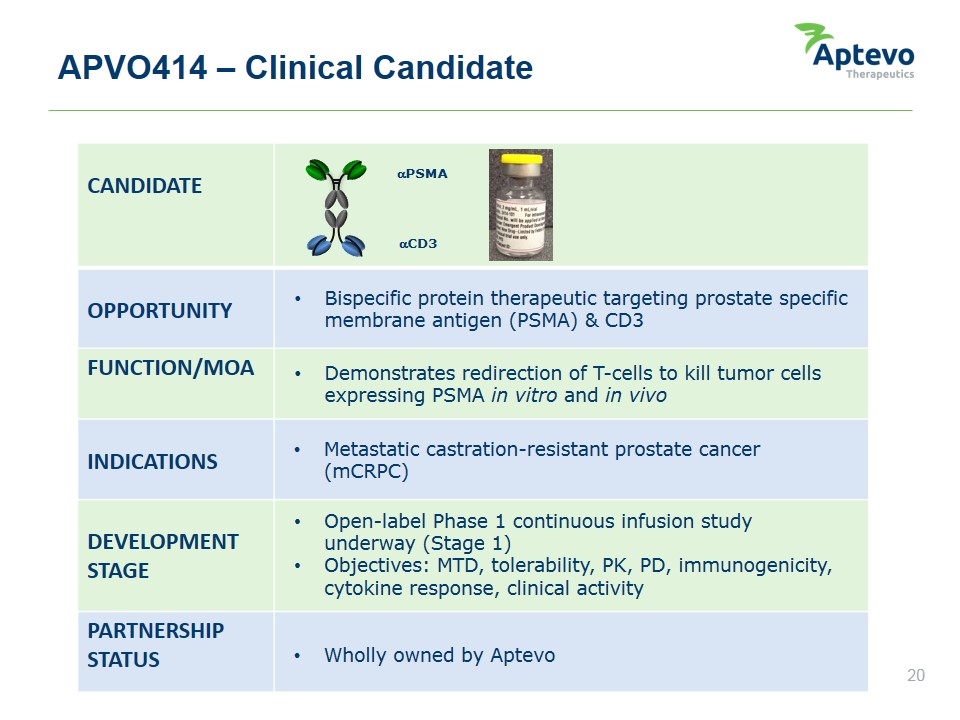
APVO414 – Clinical Candidate CANDIDATE OPPORTUNITY FUNCTION/MOA INDICATIONS DEVELOPMENT STAGE PARTNERSHIP STATUS Bispecific protein therapeutic targeting prostate specific membrane antigen (PSMA) & CD3 Demonstrates redirection of T-cells to kill tumor cells expressing PSMA in vitro and in vivo Metastatic castration-resistant prostate cancer (mCRPC) Open-label Phase 1 continuous infusion study underway (Stage 1) Objectives: MTD, tolerability, PK, PD, immunogenicity, cytokine response, clinical activity Wholly owned by Aptevo aPSMA aCD3 Aptevo therapeutics
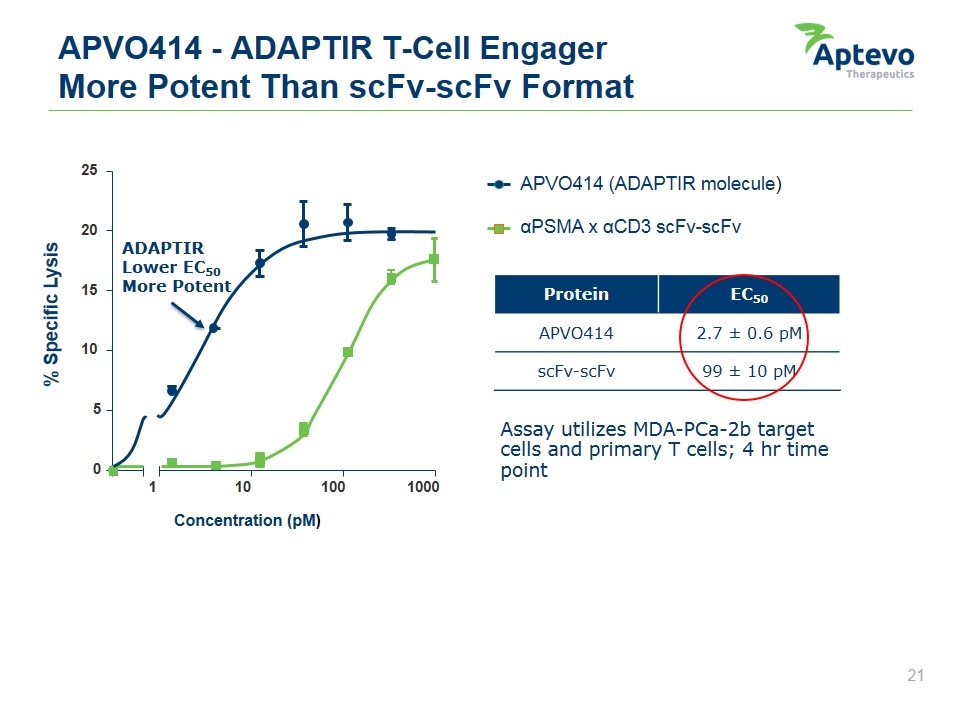
APVO414 - ADAPTIR T-Cell Engager More Potent Than scFv-scFv Format Assay utilizes MDA-PCa-2b target cells and primary T cells; 4 hr time point Protein EC50 APVO414 2.7 ± 0.6 pM scFv-scFv 99 ± 10 pM Concentration (pM) % Specific Lysis 0 5 10 15 20 25 1 10 100 1000 APVO414 (ADAPTIR molecule) αPSMA x αCD3 scFv-scFv ADAPTIR Lower EC50 More Potent Aptevo therapeutics
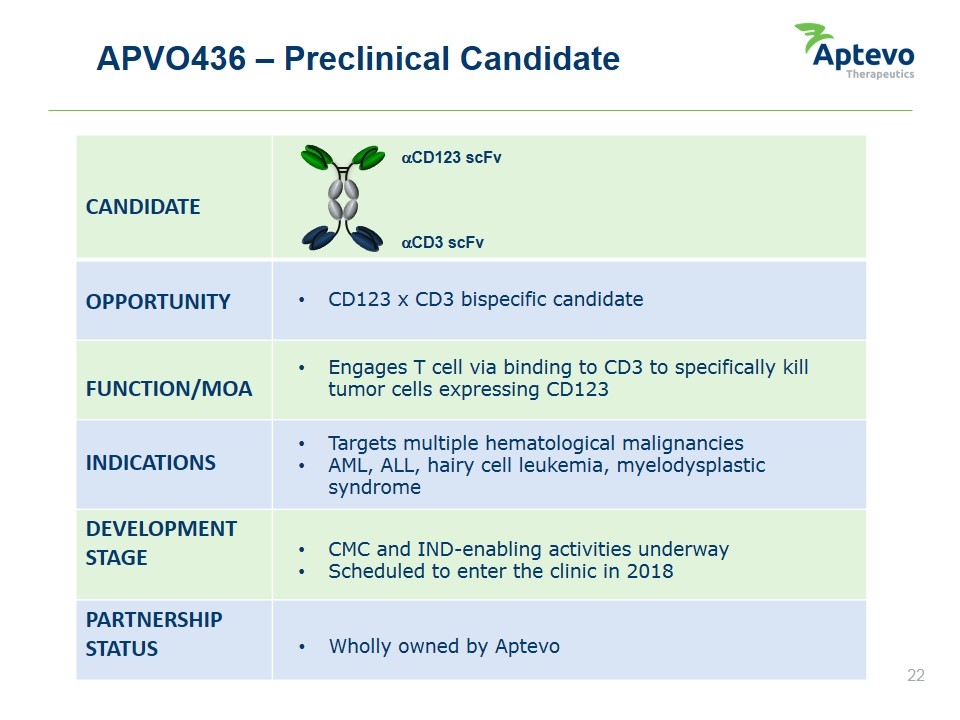
APVO436 – Preclinical Candidate CANDIDATE OPPORTUNITY FUNCTION/MOA INDICATIONS DEVELOPMENT STAGE PARTNERSHIP STATUS CD123 x CD3 bispecific candidate Engages T cell via binding to CD3 to specifically kill tumor cells expressing CD123 Targets multiple hematological malignancies AML, ALL, hairy cell leukemia, myelodysplastic syndrome CMC and IND-enabling activities underway Scheduled to enter the clinic in 2018 Wholly owned by Aptevo aCD3 scFv aCD123 scFv Aptevo therapeutics
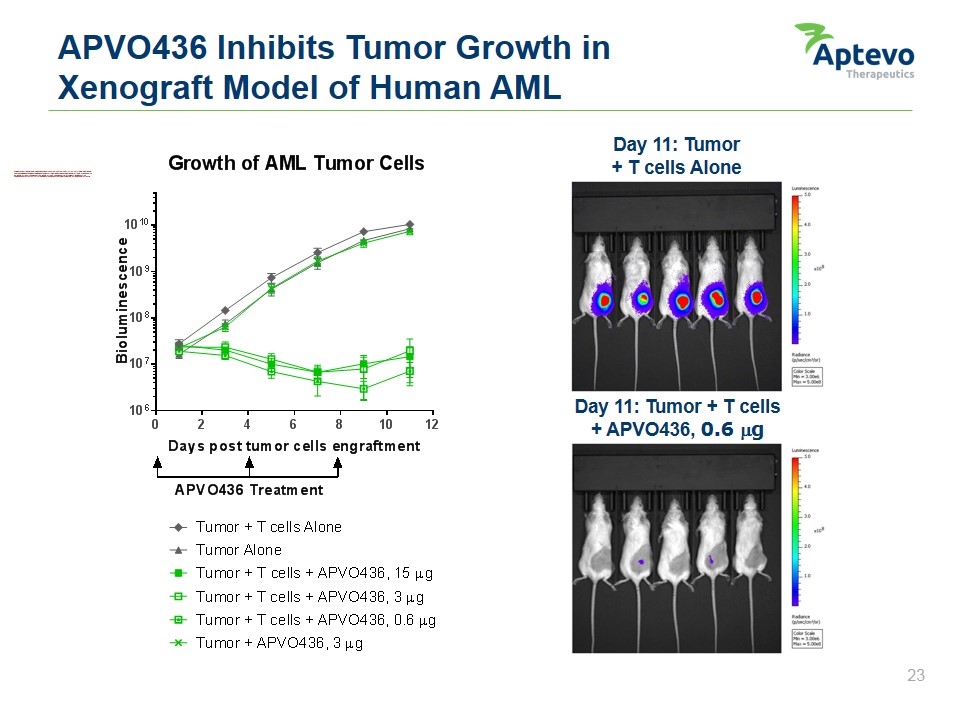
APVO436 Inhibits Tumor Growth in Xenograft Model of Human AML Day 11: Tumor + T cells Alone Day 11: Tumor + T cells + APVO436, 0.6 mg Growth of AML tumor cells bioluminescence 1010 109 108 107 106 0 2 4 6 8 10 12 days post tumor cells engraftment apvo436 treatment tumor + t cells alone tumor alone tumor + t cells + apvo436, 15 µg tumor + t cells + apvo436, 3 µg tumor + t cells + apvo436, 0.6 µg tumor + apvo436, 3 µg luminescence 5.0 4.0 3.0 2.0 1.0 Radiance (p/sec/cm2.sr) color scale min = 3.00e6 max = 5.00 e8 Aptevo therapeutics
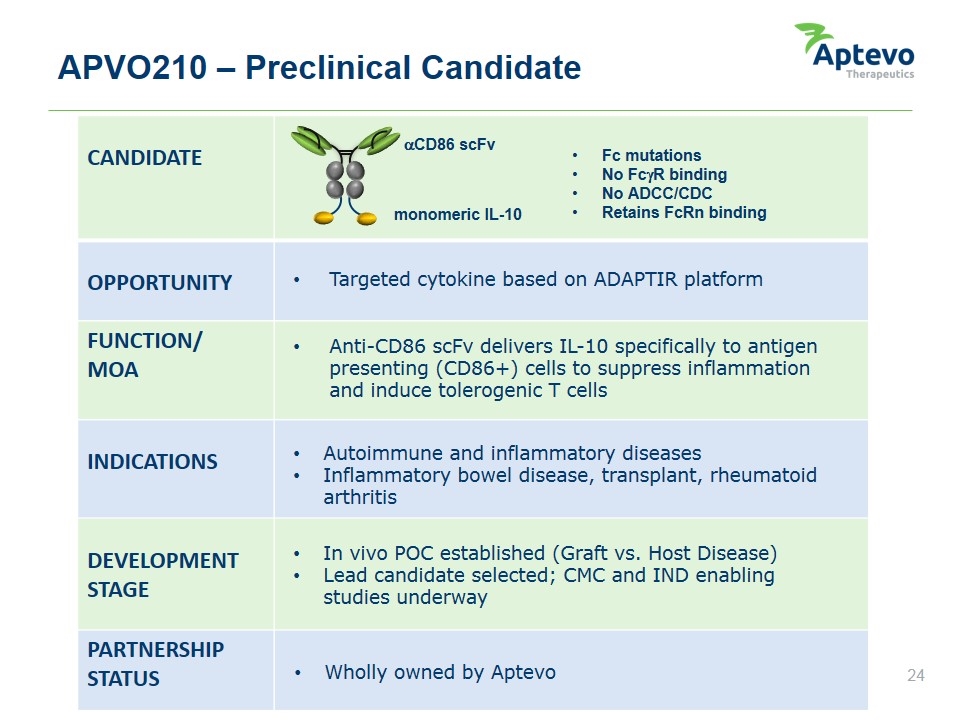
APVO210 – Preclinical Candidate CANDIDATE OPPORTUNITY FUNCTION/ MOA INDICATIONS DEVELOPMENT STAGE PARTNERSHIP STATUS Targeted cytokine based on ADAPTIR platform Anti-CD86 scFv delivers IL-10 specifically to antigen presenting (CD86+) cells to suppress inflammation and induce tolerogenic T cells Autoimmune and inflammatory diseases Inflammatory bowel disease, transplant, rheumatoid arthritis In vivo POC established (Graft vs. Host Disease) Lead candidate selected; CMC and IND enabling studies underway Wholly owned by Aptevo monomeric IL-10 Fc mutations No FcgR binding No ADCC/CDC Retains FcRn binding aCD86 scFv Aptevo therapeutics
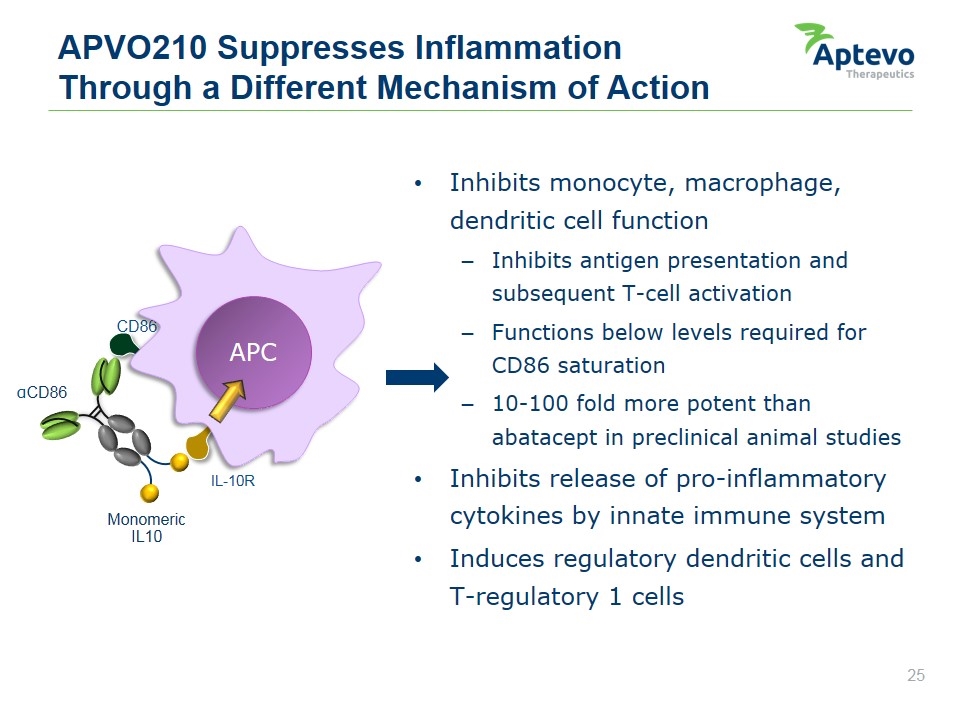
Inhibits monocyte, macrophage, dendritic cell function Inhibits antigen presentation and subsequent T-cell activation Functions below levels required for CD86 saturation 10-100 fold more potent than abatacept in preclinical animal studies Inhibits release of pro-inflammatory cytokines by innate immune system Induces regulatory dendritic cells and T-regulatory 1 cells APVO210 Suppresses Inflammation Through a Different Mechanism of Action IL-10R αCD86 Monomeric IL10 CD86 APC Aptevo therapeutics
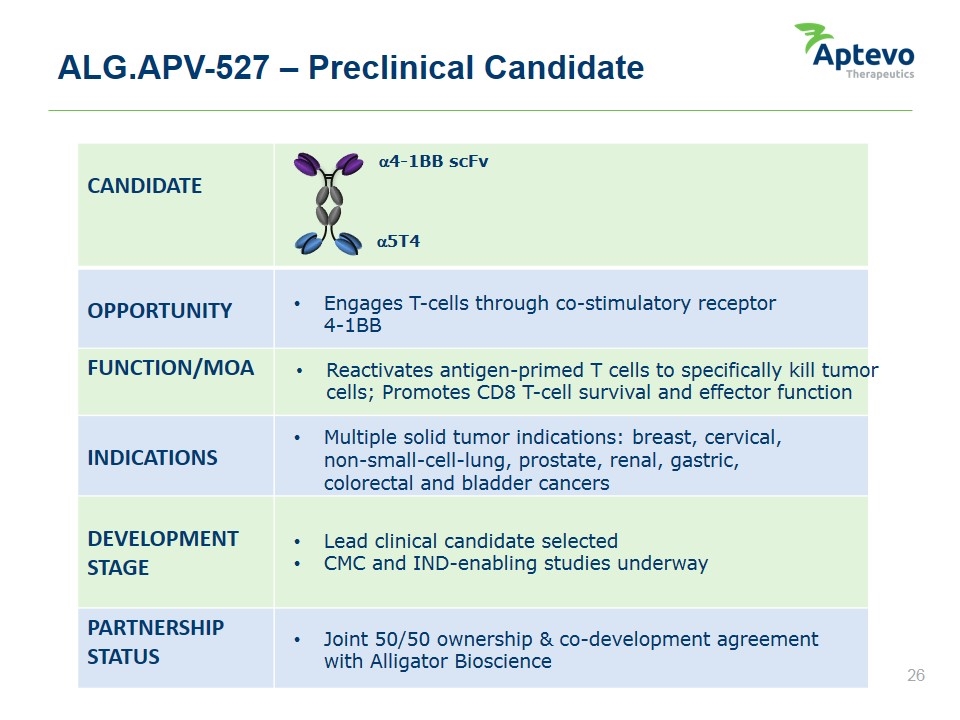
ALG.APV-527 – Preclinical Candidate CANDIDATE OPPORTUNITY FUNCTION/MOA INDICATIONS DEVELOPMENT STAGE PARTNERSHIP STATUS Engages T-cells through co-stimulatory receptor 4-1BB Reactivates antigen-primed T cells to specifically kill tumor cells; Promotes CD8 T-cell survival and effector function Multiple solid tumor indications: breast, cervical, non-small-cell-lung, prostate, renal, gastric, colorectal and bladder cancers Lead clinical candidate selected CMC and IND-enabling studies underway Joint 50/50 ownership & co-development agreement with Alligator Bioscience a4-1BB scFv a5T4 Aptevo therapeutics
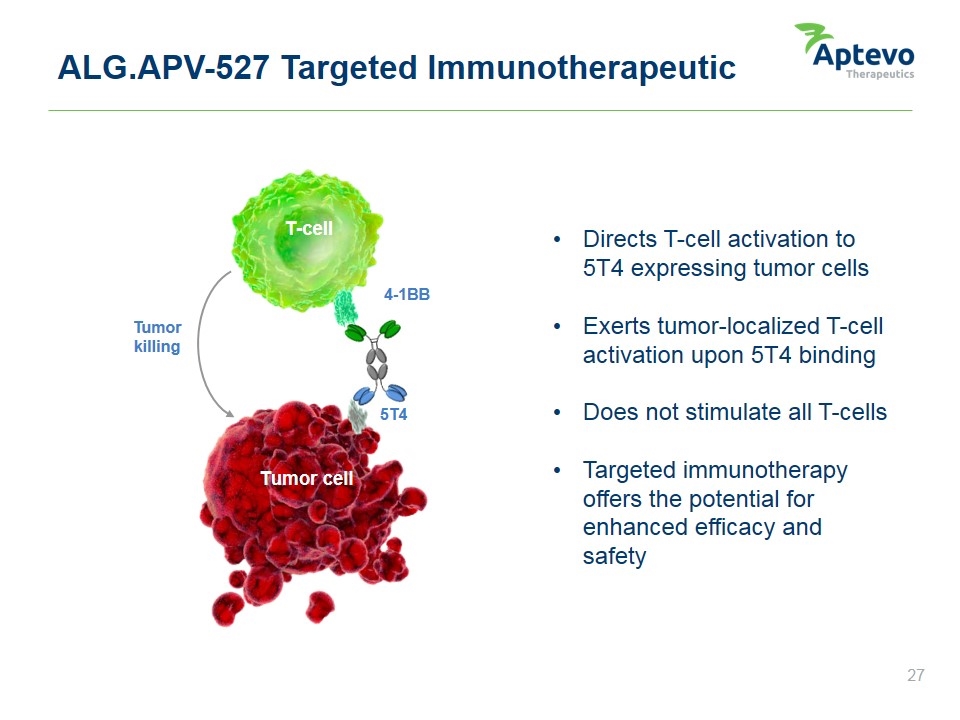
ALG.APV-527 Targeted Immunotherapeutic Directs T-cell activation to 5T4 expressing tumor cells Exerts tumor-localized T-cell activation upon 5T4 binding Does not stimulate all T-cells Targeted immunotherapy offers the potential for enhanced efficacy and safety Tumor cell 5T4 T-cell 4-1BB Tumor killing Aptevo therapeutics
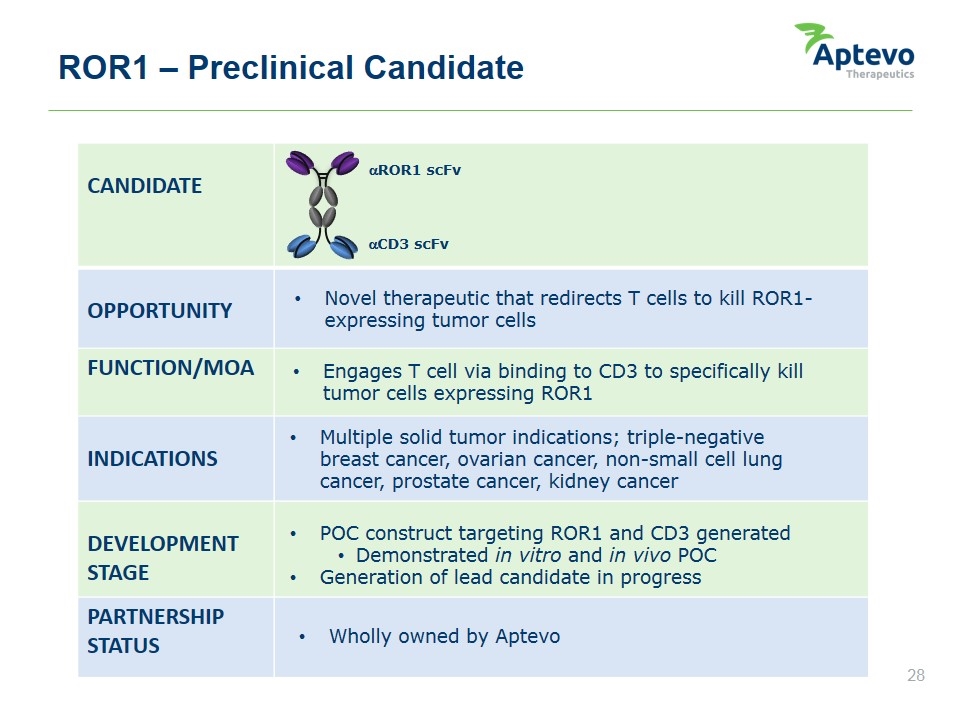
ROR1 – Preclinical Candidate CANDIDATE OPPORTUNITY FUNCTION/MOA INDICATIONS DEVELOPMENT STAGE PARTNERSHIP STATUS Novel therapeutic that redirects T cells to kill ROR1-expressing tumor cells Engages T cell via binding to CD3 to specifically kill tumor cells expressing ROR1 Multiple solid tumor indications; triple-negative breast cancer, ovarian cancer, non-small cell lung cancer, prostate cancer, kidney cancer POC construct targeting ROR1 and CD3 generated Demonstrated in vitro and in vivo POC Generation of lead candidate in progress Wholly owned by Aptevo aROR1 scFv aCD3 scFv Aptevo therapeutics
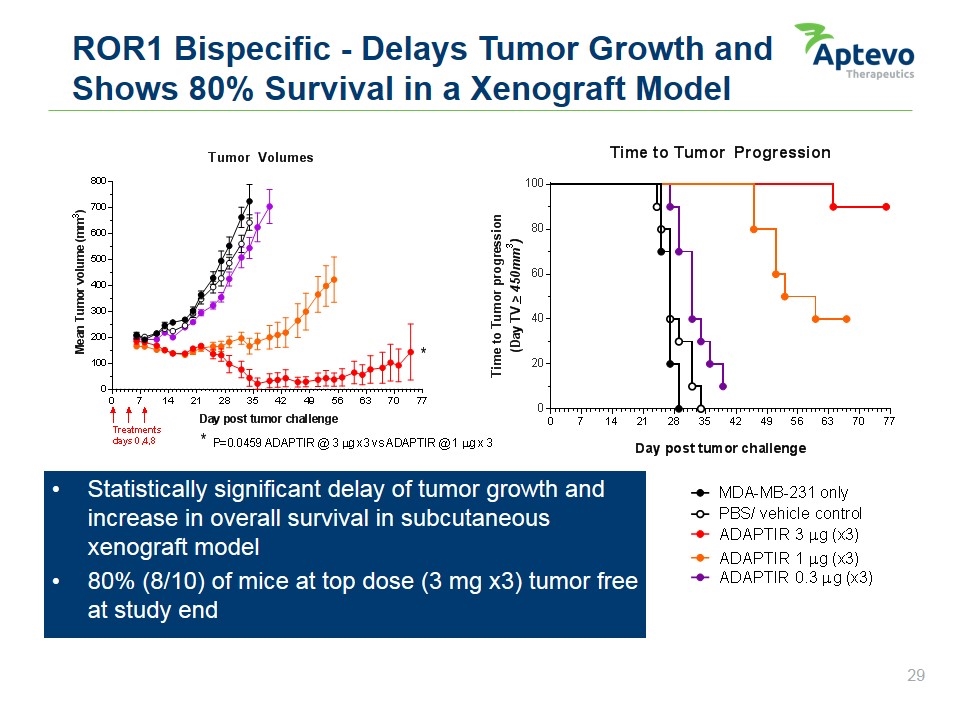
ROR1 Bispecific - Delays Tumor Growth and Shows 80% Survival in a Xenograft Model Statistically significant delay of tumor growth and increase in overall survival in subcutaneous xenograft model 80% (8/10) of mice at top dose (3 mg x3) tumor free at study end Aptevo therapeutics Mean tumor volume (mm3) 800 700 600 500 400 300 200 100 0 7 14 21 28 35 42 49 56 63 70 77 80 60 40 20 tumor volumes time to tumor progression (day tv ≥450 mm3) day post tumor challenge treatments days 0,4,8 * p=0.0459 adpatir @ 3µg x3 vs adaptire @ 1µg x 3 mda-mb-231 only pbs/vehicle control adaptir 3 µg (X3) adaptir 1 µg (x3) adaptir 0.3 µg (X3)

Agenda Executing On Our Strategy ADAPTIR – Developing Novel Protein Therapeutics Impressive Clinical and Preclinical Portfolio IXINITY – A Growing Commercial Opportunity Summary Aptevo therapeutics innovating
Intravenous blood coagulation therapy to replace factor IX in individuals with Hemophilia B U.S. launch: June 2015 Strong growth opportunity in US and ROW Worldwide rights owned by Aptevo* Opportunity to partner for U.S. and ex-U.S. markets Indication: Individuals with hemophilia B ages 12 and older IXINITY – Targeting the Hemophilia B Market with a Unique Strategy *Certain IP owned by UNC and exclusively licensed to Aptevo Aptevo therapeutics ixinity® coagulation factor IX (recombinant)
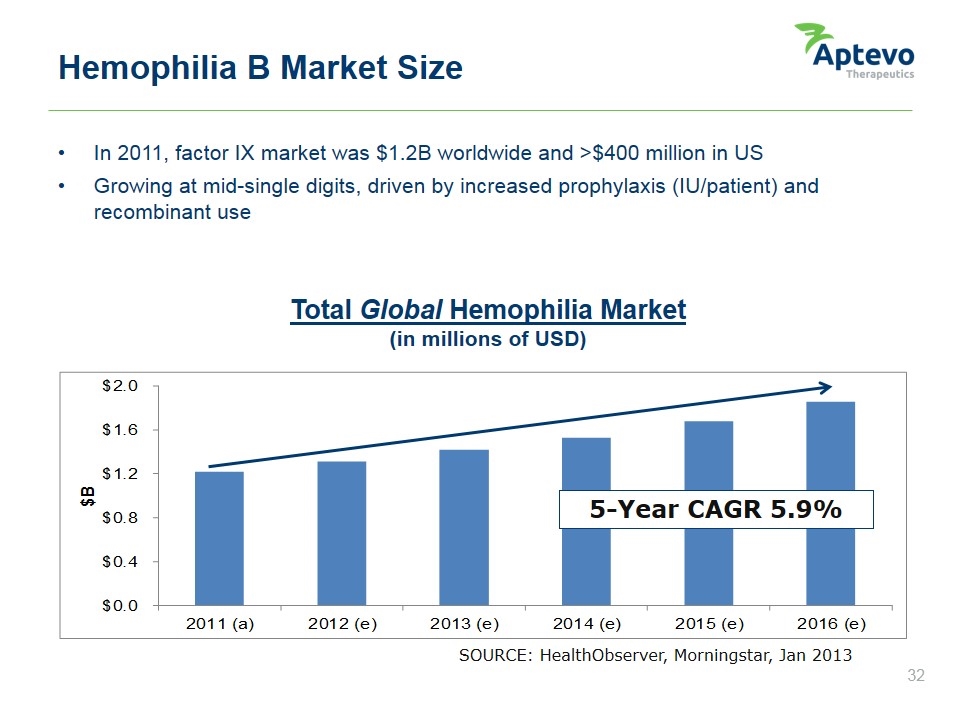
In 2011, factor IX market was $1.2B worldwide and >$400 million in US Growing at mid-single digits, driven by increased prophylaxis (IU/patient) and recombinant use Hemophilia B Market Size Total Global Hemophilia Market (in millions of USD) SOURCE: HealthObserver, Morningstar, Jan 2013 (a) actual (e) estimated 5-Year CAGR 5.9% Aptevo therapeutics $b $2.0 $1.6 $1.2 $0.8 $0.4 $0.0 2011 (a) 2012 (e) 2013 (e) 2014 (e) 2015 (e) 2016 (e) 5 year cagr 5.9%
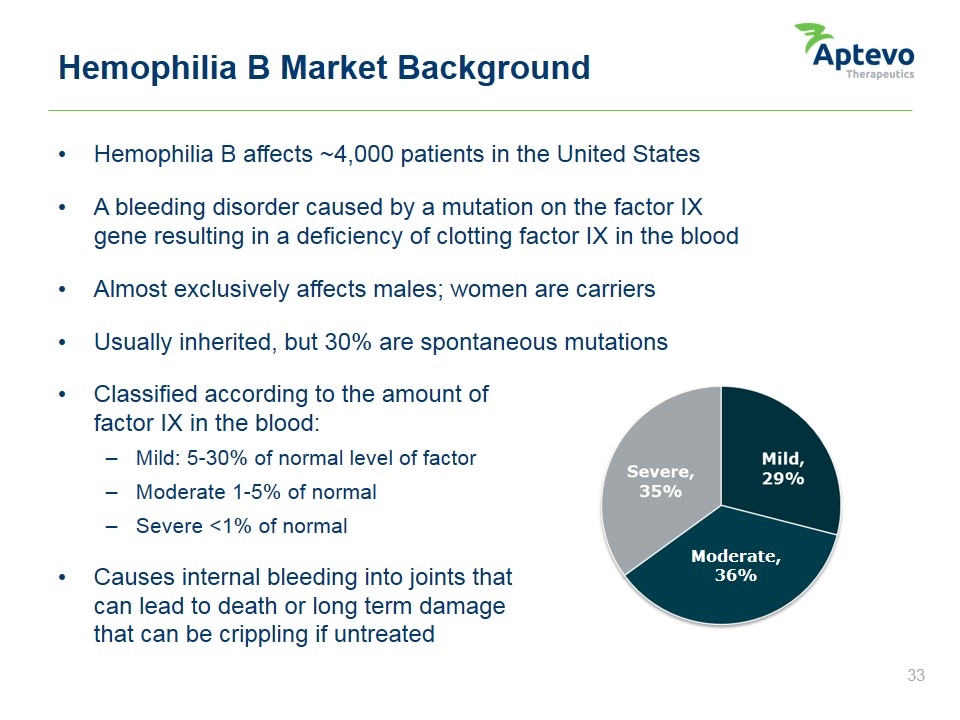
Hemophilia B affects ~4,000 patients in the United States A bleeding disorder caused by a mutation on the factor IX gene resulting in a deficiency of clotting factor IX in the blood Almost exclusively affects males; women are carriers Usually inherited, but 30% are spontaneous mutations Classified according to the amount of factor IX in the blood: Mild: 5-30% of normal level of factor Moderate 1-5% of normal Severe <1% of normal Causes internal bleeding into joints that can lead to death or long term damage that can be crippling if untreated Hemophilia B Market Background Moderate, 36% SOURCE: UDC Data, December 2011 Hemophilia B Market Overview Aptevo therapeutics Severe, 35% Mild, 29% Moderate, 36%

Agenda Executing On Our Strategy ADAPTIR – Developing Novel Protein Therapeutics Imprssive Clinical and Preclinical Pipeline IXINITY – A Growing Commercial Opportunity Summary Aptevo therapeutics innovating
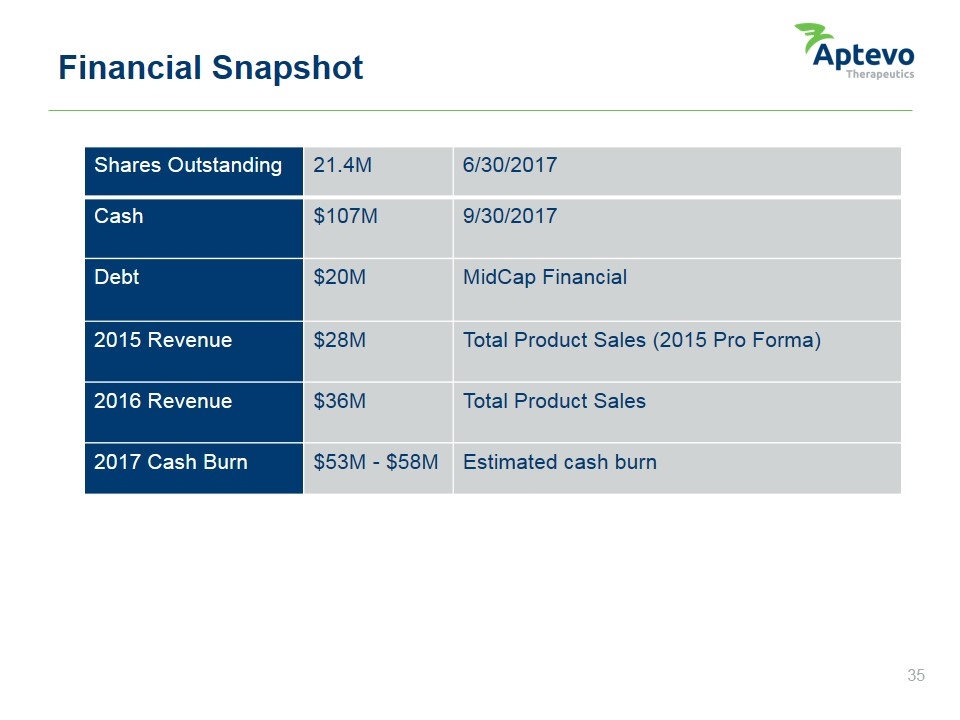
Financial Snapshot Shares Outstanding 21.4M 6/30/2017 Cash $107M 9/30/2017 Debt $20M MidCap Financial 2015 Revenue $28M Total Product Sales (2015 Pro Forma) 2016 Revenue $36M Total Product Sales 2017 Cash Burn $53M - $58M Estimated cash burn Aptevo therapeutics

Upcoming Milestones – 2017-2018 Program Announce planned IND filings for multiple ADAPTIR candidates Commence expanded Phase 2 otlertuzumab clinical program Complete enrollment of Phase 1 dosing cohorts in APVO414 clinical trial Announce APVO414 Phase 1 dose escalation preliminary clinical data Expand application of ADAPTIR-based candidates into new MOA Capture increased market share of Hemophilia B market with expanded U.S. sales of IXINITY Continue potential partnering discussions around platform / product candidate opportunities Timeframe Q4 2017 Q4 2017 Q3 2018 Q4 2018 Ongoing Ongoing Ongoing Aptevo therapeutics
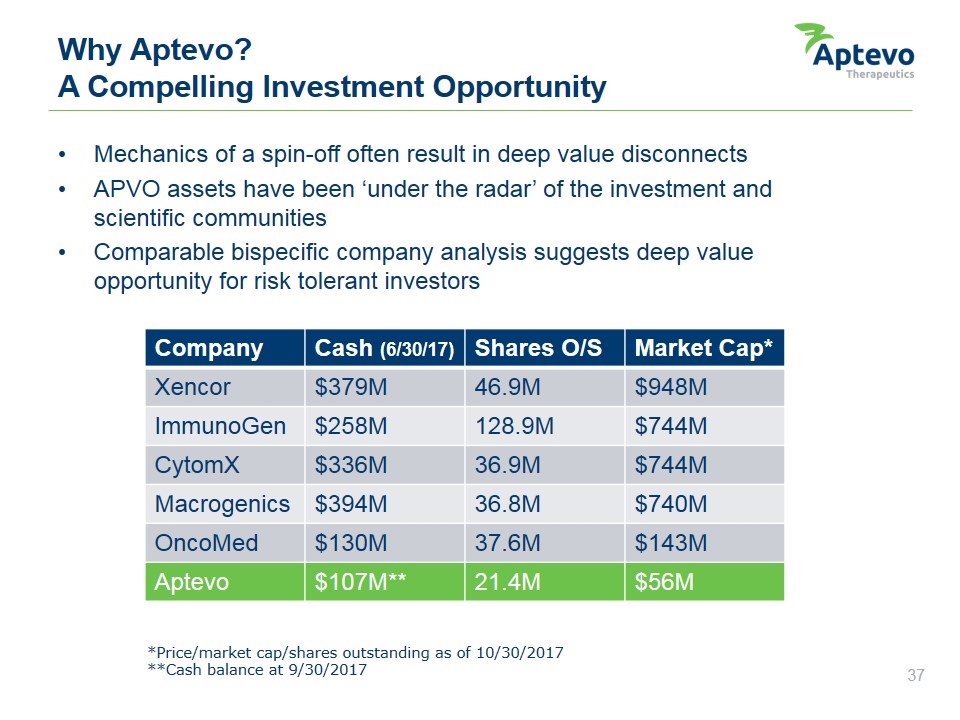
Mechanics of a spin-off often result in deep value disconnects APVO assets have been ‘under the radar’ of the investment and scientific communities Comparable bispecific company analysis suggests deep value opportunity for risk tolerant investors Why Aptevo? A Compelling Investment Opportunity Company Cash (6/30/17) Shares O/S Market Cap* Xencor $379M 46.9M $948M ImmunoGen $258M 128.9M $744M CytomX $336M 36.9M $744M Macrogenics $394M 36.8M $740M OncoMed $130M 37.6M $143M Aptevo $107M** 21.4M $56M *Price/market cap/shares outstanding as of 10/30/2017 **Cash balance at 9/30/2017 Aptevo therapeutics

Aptevo – A Compelling Investment Opportunity Solid cash position to advance R&D and commercial strategy 1 2 3 4 5 Strong leadership with a track record of execution Advancing ADAPTIR™ to generate novel first-in-class therapeutics Broad pipeline of wholly-owned clinical and preclinical candidates Commercial asset (IXINITY) with growth potential Aptevo therapeutics





































