
Merger Announcement July 21, 2022 Exhibit 99.2

Forward Looking Statements This presentation contains forward-looking statements which include, but are not limited to, statements regarding expected timing, completion, effects and potential benefits of the proposed merger; the expected cash, cash equivalents and marketable securities of the combined company at closing; the expected ownership percentages in the combined company; the expected name, ticker symbol, management team and board of directors of the combined company; the design and potential benefits of neffy; ARS’s plans to submit an NDA to the FDA and MAA to the EMA for neffy, the timing thereof and optimism regarding the support therefor; the timing of the commercial launch of neffy, if approved, and the ability of the merger to provide sufficient capital for such launch; ARS’s commercialization strategy; the potential market opportunity for neffy, the projected growth thereof and neffy’s ability to capture and grow that market; and any statements of assumptions underlying any of the foregoing. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Silverback’s expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks, and changes in circumstances, including but not limited to risks and uncertainties related to: the ability of the parties to consummate the merger in a timely manner or at all; the satisfaction (or waiver) of closing conditions to the consummation of the merger, including with respect to the approval of Silverback’s stockholders; potential delays in consummating the merger; the ability of the combined company to timely and successfully achieve the anticipated benefits of the merger; the impact of health epidemics, including the COVID-19 pandemic, on the parties’ respective businesses and the actions the parties may take in response thereto; the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the merger agreement; the effect of the announcement or pendency of the merger on Silverback’s or ARS’s business relationships, operating results and business generally; costs related to the merger; the outcome of any legal proceedings that may be instituted against Silverback, ARS or any of their respective directors or officers related to the merger agreement or the transactions contemplated thereby; the ability to obtain and maintain regulatory approval for neffy; results from clinical trials may not be indicative of results that may be observed in the future; potential safety and other complications from neffy; the labelling for neffy, if approved; the scope, progress and expansion of developing and commercializing neffy; the size and growth of the market therefor and the rate and degree of market acceptance thereof vis-à-vis intramuscular injectable products; the combined company’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” and elsewhere in Silverback’s most recent filings with the SEC, including its Quarterly Report on Form 10-Q for the quarter ended March 31, 2022 and any subsequent reports on Form 10-K, Form 10-Q or Form 8-K filed with the SEC from time to time and available at www.sec.gov. These documents can be accessed on Silverback’s web page at https://ir.silverbacktx.com/ by clicking on the link “Financials & Filings.” The forward-looking statements included in this presentation are made only as of the date hereof. Silverback Therapeutics assumes no obligation and does not intend to update these forward-looking statements, except as required by law. CONFIDENTIAL

Additional Information on the Transaction Additional Information and Where to Find It In connection with the transaction, Silverback Therapeutics intends to file with the SEC preliminary and definitive proxy statements relating to the transaction and other relevant documents. The definitive proxy statement will be mailed to Silverback Therapeutics’ stockholders as of a record date to be established for voting on the transaction and any other matters to be voted on at the special meeting. BEFORE MAKING ANY VOTING DECISION, INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PRELIMINARY AND DEFINITIVE PROXY STATEMENTS, ANY AMENDMENTS OR SUPPLEMENTS THERETO AND ANY OTHER DOCUMENTS TO BE FILED WITH THE SEC IN CONNECTION WITH THE TRANSACTION OR INCORPORATED BY REFERENCE IN THE PROXY STATEMENTS WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT SILVERBACK THERAPEUTICS, ARS PHARMA AND THE TRANSACTION. Investors and security holders may obtain free copies of these documents (when they are available) on the SEC’s web site at www.sec.gov, on Silverback Therapeutics’ website at https://ir.silverbacktx.com/ or by contacting Silverback Therapeutics’ Investor Relations via email at IR@silverbacktx.com or by telephone at (206)736-7946. Participants in the Solicitation Silverback Therapeutics and its directors and certain of its executive officers may be deemed participants in the solicitation of proxies from the stockholders of Silverback Therapeutics in connection with the transaction and any other matters to be voted on at the special meeting. Information regarding the names, affiliations and interests of such directors and executive officers will be included in the preliminary and definitive proxy statements (when available). Additional information regarding such directors and executive officers is included in Silverback Therapeutics’ definitive proxy statement on Schedule 14A for the 2022 Annual Meeting of the Stockholders, which was filed with the SEC on April 28, 2022. Information regarding the persons who may, under SEC rules, be deemed participants in the solicitation of proxies of Silverback Therapeutics’ stockholders in connection with the transaction and any other matters to be voted upon at the special meeting will be set forth in the preliminary and definitive proxy statements (when available) for the transactions. These documents are available free of charge as described in the preceding paragraph. Non-Solicitation This presentation will not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor will there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. CONFIDENTIAL

Transformative Transaction for Silverback Therapeutics, Inc. In March this year, Silverback announced a strategic reprioritization, and over the last three months the company explored strategic alternatives anticipated to provide value to Silverback stockholders Merger with ARS provides Silverback stockholders with the opportunity to participate in the potential growth of the combined company: neffyTM offers a clear value proposition for a convenient, no needle, no injection replacement for epinephrine autoinjectors (e.g., Epipen) Near-term commercial product with a sizable potential market and opportunity for long-term value creation Strong management team that has a proven track record of developing and commercializing intranasal medicines Silverback will be focused on closing the proposed transaction with ARS and exploring opportunities to divest the SBT8230 program for chronic HBV and other preclinical assets
ARS – Silverback Merger Transaction Summary ARS Pharmaceuticals and Silverback Therapeutics agreed to merge on July 21, 2022 in an all-stock transaction and expected to trade on Nasdaq under new symbol SPRY Transaction expected to close in Q4 2022 Strong financial position with ~$265M in total cash balance expected at closing Silverback equity holders will own ~37% of the combined company and pre-merger ARS equity holders will own ~63%, subject to certain adjustments, including Silverback’s net cash at closing Post-merger Board of Directors of ten members: seven from ARS and three from Silverback Transaction has been approved by the Board of Directors of both companies and is subject to approval of the stockholders of both companies

neffy™ is an investigational new drug currently in clinical trials for the emergency treatment of allergic reactions (type I) including anaphylaxis. neffy™ is not approved by the US. Food and Drug Administration (FDA), European Medicines Agency or other health authorities. The efficacy and safety of neffy™ has not been established. In addition, the tradename, neffy™, is tentatively approved by FDA but has not received final approval from the FDA or any other regulatory body.

THE FIRST NO-NEEDLE, NO-INJECTION SOLUTION for Type I Allergic Reactions JULY 2022

Potential to Transform the Treatment of Type I Allergic Reactions neffy: first “no needle, no injection” solution for Type I allergic reactions to address an unmet market need Registration program demonstrates comparable PK and PD, without risk of needle-related safety concerns, fear and hesitation Significant opportunity to disrupt current epinephrine injectables market Planned Q3 2022 NDA submission and potential 2023 approval Potential multi-billion-dollar market driven by HCP and consumer preference and adoption NCE-like IP exclusivity potential until at least 2038
Proven leadership team with track record developing and commercializing intranasal and consumer-driven medicines Richard Lowenthal, M.S. Chief Executive Officer, Co-Founder Led FDA approvals for multiple nasal spray products 25+ years of experience Robert Bell, Ph.D. Chief Scientific Officer, Co-Founder 30+ years of senior R&D leadership experience including Barr and Somerset Sarina Tanimoto, M.D. Chief Medical Officer, Co-Founder Led FDA approvals for multiple nasal spray products 20+ years of experience Eric Karas Chief Commercial Officer Led Narcan® commercial ops at Emergent/Adapt, and Auxilium specialty 25+ years of experience Harris Kaplan EVP, Commercial Strategy 40+ years of commercial strategy across more than 125 product launches Dan Relovsky SVP, Sales & Marketing 30+ years of marketing, sales and operational experience across specialty and consumer markets Brian Dorsey EVP, Operations & Project Mgmt 25+ years of R&D experience as including multiple head of R&D roles including Pernix, Apricus and Somaxon Kathy Scott Chief Financial Officer 30+ years of finance experience including multiple CFO roles including Neurana, Recros and Oncternal Justin Chakma Chief Business Officer 10+ years of M&A, licensing, financing and strategy experience including Celgene, Receptos and Auspex

Top-tier board of directors, investors and partnerships Pratik Shah, Ph.D. Chairman of Board of Directors Executive Chairman at Design, Former Chairman of Synthorx (acq. $2.5B), Former CEO at Auspex (acq. $3.5B) Peter Kolchinsky, Ph.D. Managing Partner and Founder at RA Capital Rajeev Dadoo, Ph.D. Managing Partner at SR One Richard Lowenthal, M.S. Chief Executive Officer, Co-Founder Led FDA approvals for multiple nasal spray products 25+ years of experience Brent Saunders Chairman at The Beauty Health Co., Former CEO of Allergan (acq. $63B), Actavis, Forest Labs, and Bausch + Lomb (acq. $8.7B) Michael Kelly Former President, US Operations at Adapt (acq. $735M), CEO at Covis (acq. $1.2B), founder at Azur Jonathan Leff Partner at Deerfield Management Chairman of Deerfield Institute Philip Schneider Former CFO at IDEC, former Board member at Arena (acq. $6.7B), Auspex (acq. $3.5B), GenProbe (acq. $3.7B) Undisclosed large U.S.-based healthcare-focused fund European commercialization partner Undisclosed Japanese and Chinese commercialization partners
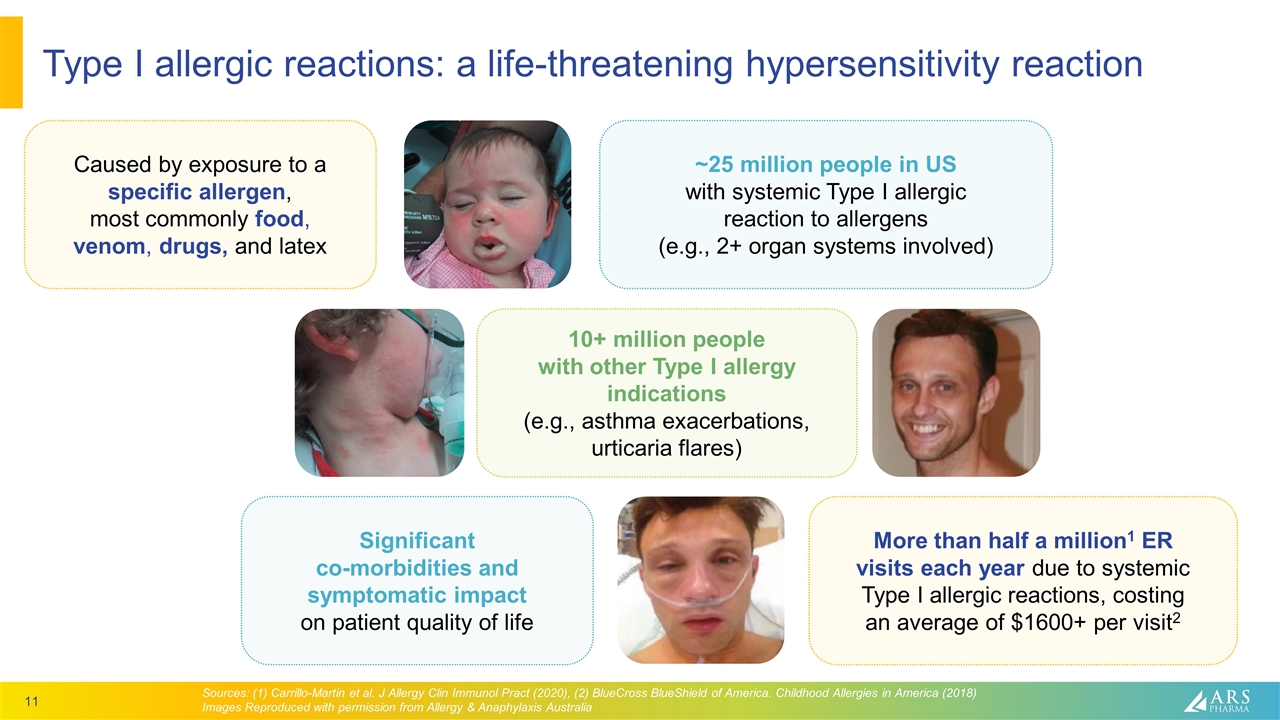
Type I allergic reactions: a life-threatening hypersensitivity reaction Sources: (1) Carrillo-Martin et al. J Allergy Clin Immunol Pract (2020), (2) BlueCross BlueShield of America. Childhood Allergies in America (2018) Images Reproduced with permission from Allergy & Anaphylaxis Australia Caused by exposure to a specific allergen, most commonly food, venom, drugs, and latex ~25 million people in US with systemic Type I allergic reaction to allergens (e.g., 2+ organ systems involved) 10+ million people with other Type I allergy indications (e.g., asthma exacerbations, urticaria flares) Significant co-morbidities and symptomatic impact on patient quality of life More than half a million1 ER visits each year due to systemic Type I allergic reactions, costing an average of $1600+ per visit2
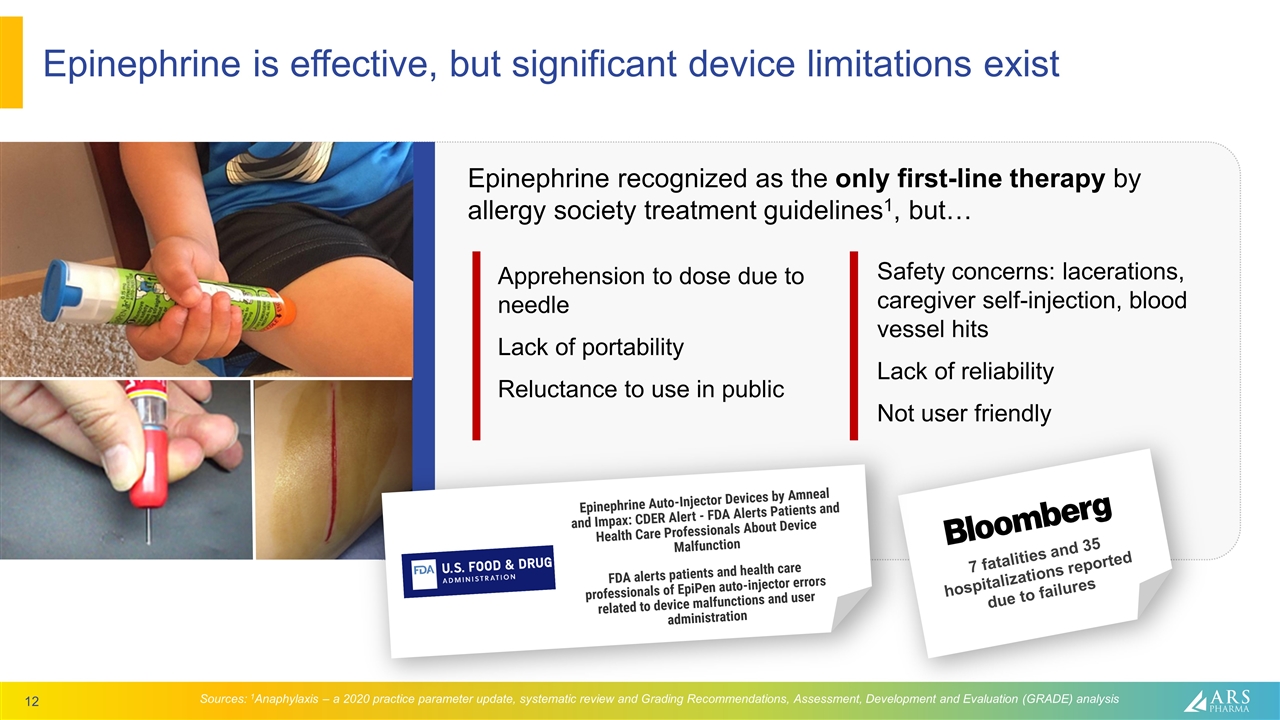
Epinephrine recognized as the only first-line therapy by allergy society treatment guidelines1, but… Epinephrine is effective, but significant device limitations exist Sources: 1Anaphylaxis – a 2020 practice parameter update, systematic review and Grading Recommendations, Assessment, Development and Evaluation (GRADE) analysis Apprehension to dose due to needle Lack of portability Reluctance to use in public Safety concerns: lacerations, caregiver self-injection, blood vessel hits Lack of reliability Not user friendly 7 fatalities and 35 hospitalizations reported due to failures
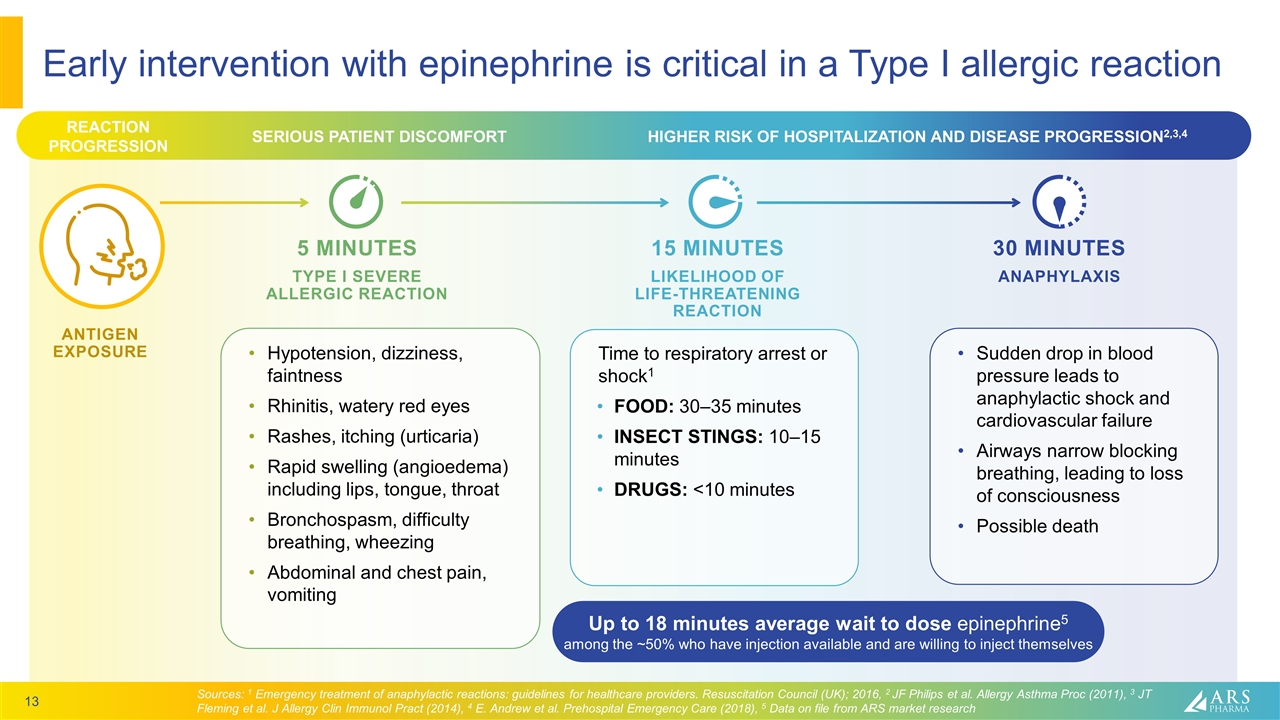
Early intervention with epinephrine is critical in a Type I allergic reaction 5 MINUTES TYPE I SEVERE ALLERGIC REACTION ANTIGEN EXPOSURE Sources: 1 Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. Resuscitation Council (UK); 2016, 2 JF Philips et al. Allergy Asthma Proc (2011), 3 JT Fleming et al. J Allergy Clin Immunol Pract (2014), 4 E. Andrew et al. Prehospital Emergency Care (2018), 5 Data on file from ARS market research SERIOUS PATIENT DISCOMFORT HIGHER RISK OF HOSPITALIZATION AND DISEASE PROGRESSION2,3,4 30 MINUTES ANAPHYLAXIS 15 MINUTES LIKELIHOOD OF LIFE-THREATENING REACTION Sudden drop in blood pressure leads to anaphylactic shock and cardiovascular failure Airways narrow blocking breathing, leading to loss of consciousness Possible death Time to respiratory arrest or shock1 FOOD: 30–35 minutes INSECT STINGS: 10–15 minutes DRUGS: <10 minutes Hypotension, dizziness, faintness Rhinitis, watery red eyes Rashes, itching (urticaria) Rapid swelling (angioedema) including lips, tongue, throat Bronchospasm, difficulty breathing, wheezing Abdominal and chest pain, vomiting Up to 18 minutes average wait to dose epinephrine5 among the ~50% who have injection available and are willing to inject themselves REACTION PROGRESSION
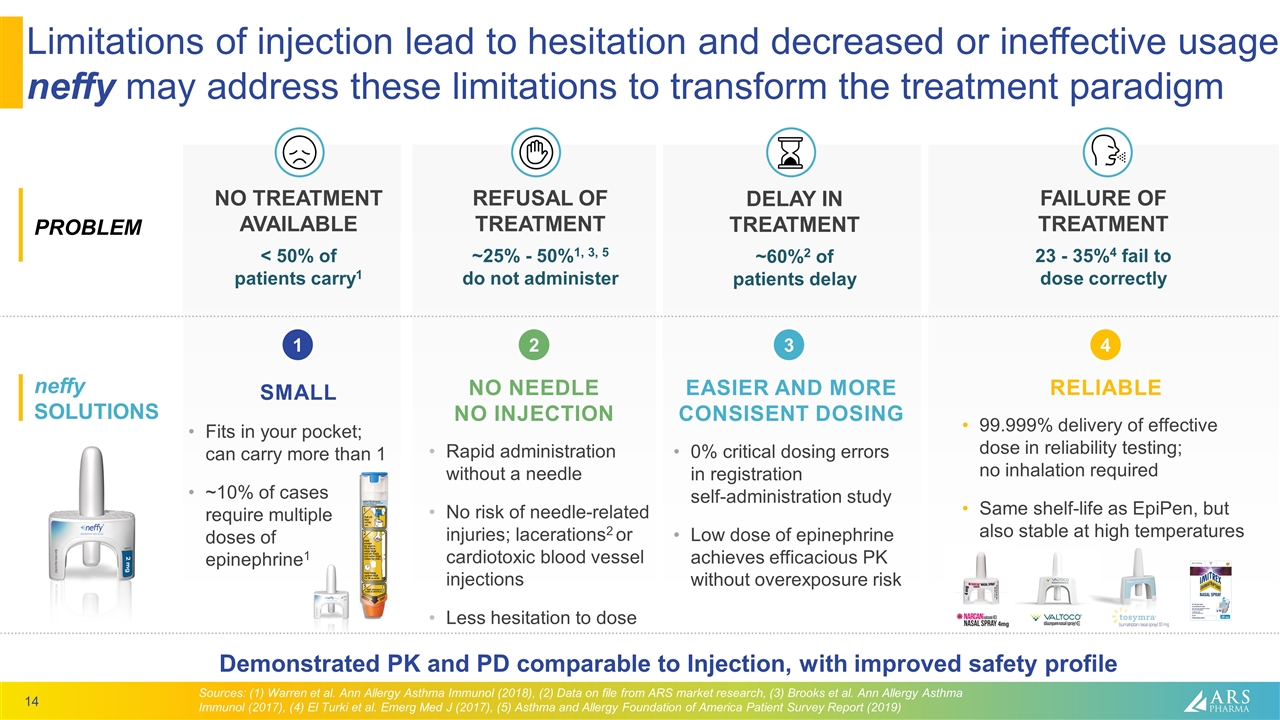
FAILURE OF TREATMENT 23 - 35%4 fail to dose correctly Limitations of injection lead to hesitation and decreased or ineffective usage neffy may address these limitations to transform the treatment paradigm Rapid administration without a needle No risk of needle-related injuries; lacerations2 or cardiotoxic blood vessel injections Less hesitation to dose NO NEEDLE NO INJECTION SMALL Fits in your pocket; can carry more than 1 ~10% of cases require multiple doses of epinephrine1 EASIER AND MORE CONSISENT DOSING 0% critical dosing errors in registration self-administration study Low dose of epinephrine achieves efficacious PK without overexposure risk RELIABLE 99.999% delivery of effective dose in reliability testing; no inhalation required Same shelf-life as EpiPen, but also stable at high temperatures DELAY IN TREATMENT ~60%2 of patients delay NO TREATMENT AVAILABLE < 50% of patients carry1 REFUSAL OF TREATMENT ~25% - 50%1, 3, 5 do not administer 3 4 2 1 neffy SOLUTIONS PROBLEM Sources: (1) Warren et al. Ann Allergy Asthma Immunol (2018), (2) Data on file from ARS market research, (3) Brooks et al. Ann Allergy Asthma Immunol (2017), (4) El Turki et al. Emerg Med J (2017), (5) Asthma and Allergy Foundation of America Patient Survey Report (2019) Demonstrated PK and PD comparable to Injection, with improved safety profile 3 4 2 1
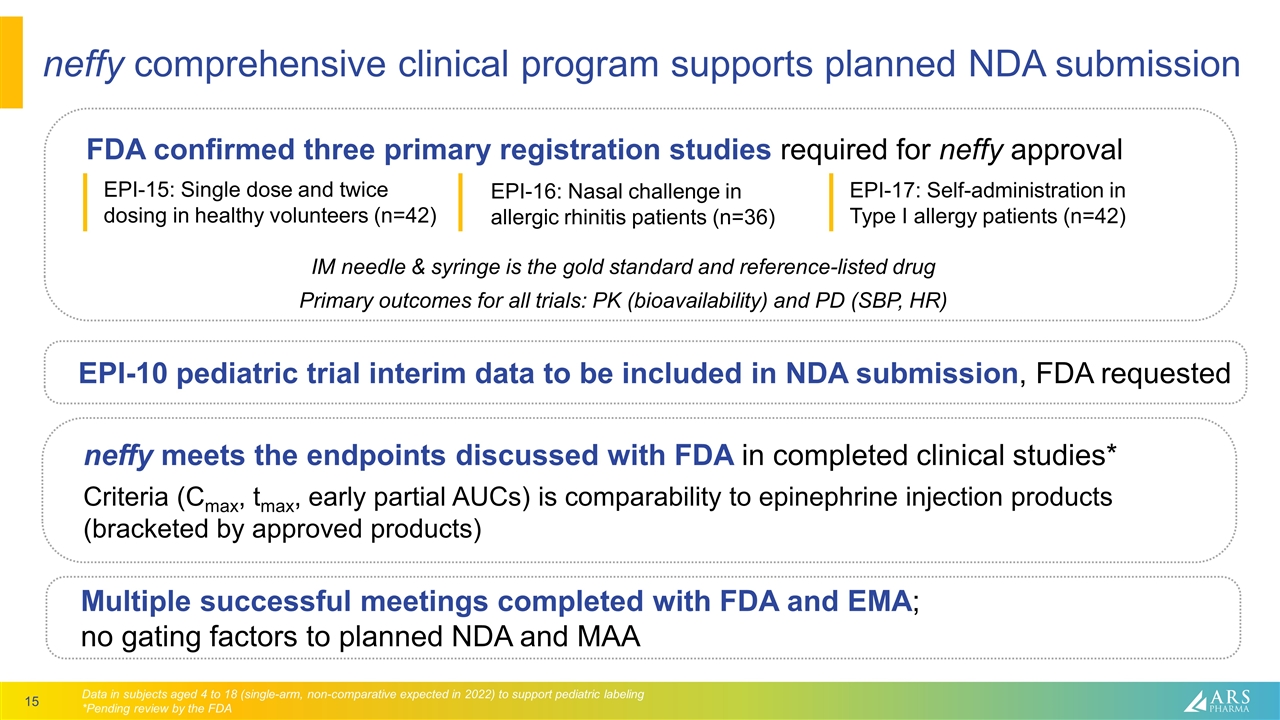
neffy comprehensive clinical program supports planned NDA submission Multiple successful meetings completed with FDA and EMA; no gating factors to planned NDA and MAA FDA confirmed three primary registration studies required for neffy approval EPI-17: Self-administration in Type I allergy patients (n=42) EPI-15: Single dose and twice dosing in healthy volunteers (n=42) EPI-16: Nasal challenge in allergic rhinitis patients (n=36) Data in subjects aged 4 to 18 (single-arm, non-comparative expected in 2022) to support pediatric labeling *Pending review by the FDA neffy meets the endpoints discussed with FDA in completed clinical studies* Criteria (Cmax, tmax, early partial AUCs) is comparability to epinephrine injection products (bracketed by approved products) IM needle & syringe is the gold standard and reference-listed drug Primary outcomes for all trials: PK (bioavailability) and PD (SBP, HR) EPI-10 pediatric trial interim data to be included in NDA submission, FDA requested
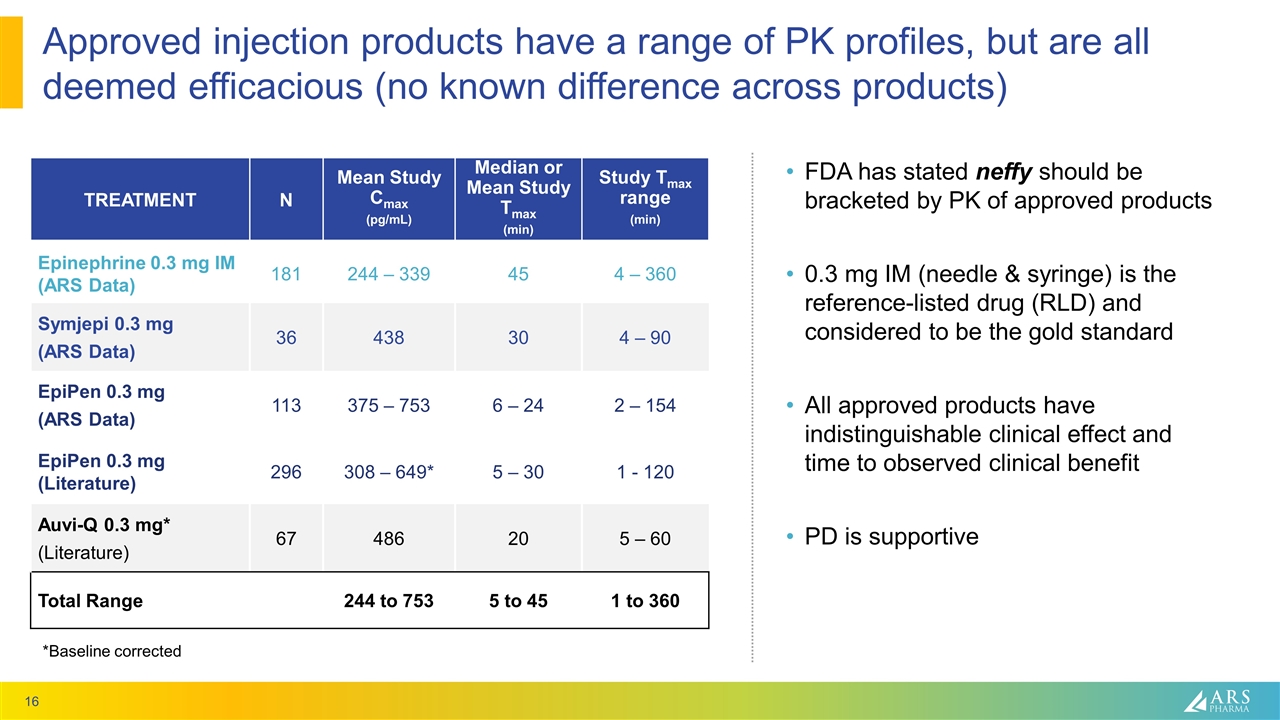
Approved injection products have a range of PK profiles, but are all deemed efficacious (no known difference across products) TREATMENT N Mean Study Cmax (pg/mL) Median or Mean Study Tmax (min) Study Tmax range (min) Epinephrine 0.3 mg IM (ARS Data) 181 244 – 339 45 4 – 360 Symjepi 0.3 mg (ARS Data) 36 438 30 4 – 90 EpiPen 0.3 mg (ARS Data) 113 375 – 753 6 – 24 2 – 154 EpiPen 0.3 mg (Literature) 296 308 – 649* 5 – 30 1 - 120 Auvi-Q 0.3 mg* (Literature) 67 486 20 5 – 60 Total Range 244 to 753 5 to 45 1 to 360 FDA has stated neffy should be bracketed by PK of approved products 0.3 mg IM (needle & syringe) is the reference-listed drug (RLD) and considered to be the gold standard All approved products have indistinguishable clinical effect and time to observed clinical benefit PD is supportive *Baseline corrected
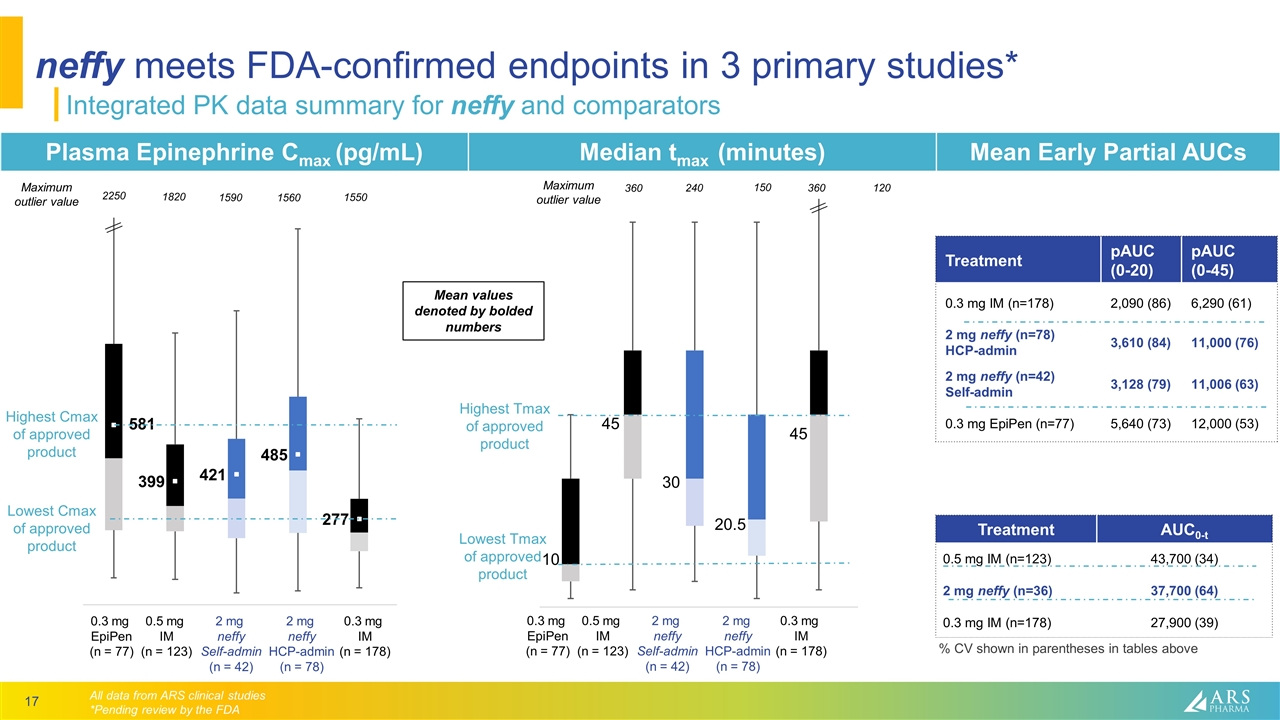
neffy meets FDA-confirmed endpoints in 3 primary studies* Integrated PK data summary for neffy and comparators Plasma Epinephrine Cmax (pg/mL) Median tmax (minutes) Mean Early Partial AUCs All data from ARS clinical studies *Pending review by the FDA 2250 0.3 mg IM (n = 178) 2 mg neffy HCP-admin (n = 78) 0.3 mg EpiPen (n = 77) 0.5 mg IM (n = 123) 2 mg neffy Self-admin (n = 42) 1820 1590 1560 1550 1000 10 45 45 20.5 30 Treatment pAUC (0-20) pAUC (0-45) 0.3 mg IM (n=178) 2,090 (86) 6,290 (61) 2 mg neffy (n=78) HCP-admin 3,610 (84) 11,000 (76) 2 mg neffy (n=42) Self-admin 3,128 (79) 11,006 (63) 0.3 mg EpiPen (n=77) 5,640 (73) 12,000 (53) 360 120 360 240 150 Treatment AUC0-t 0.5 mg IM (n=123) 2 mg neffy (n=36) 0.3 mg IM (n=178) 43,700 (34) 37,700 (64) 27,900 (39) 0.3 mg IM (n = 178) 2 mg neffy HCP-admin (n = 78) 0.3 mg EpiPen (n = 77) 0.5 mg IM (n = 123) 2 mg neffy Self-admin (n = 42) Maximum outlier value Lowest Cmax of approved product Highest Cmax of approved product Lowest Tmax of approved product Highest Tmax of approved product Maximum outlier value Mean values denoted by bolded numbers % CV shown in parentheses in tables above
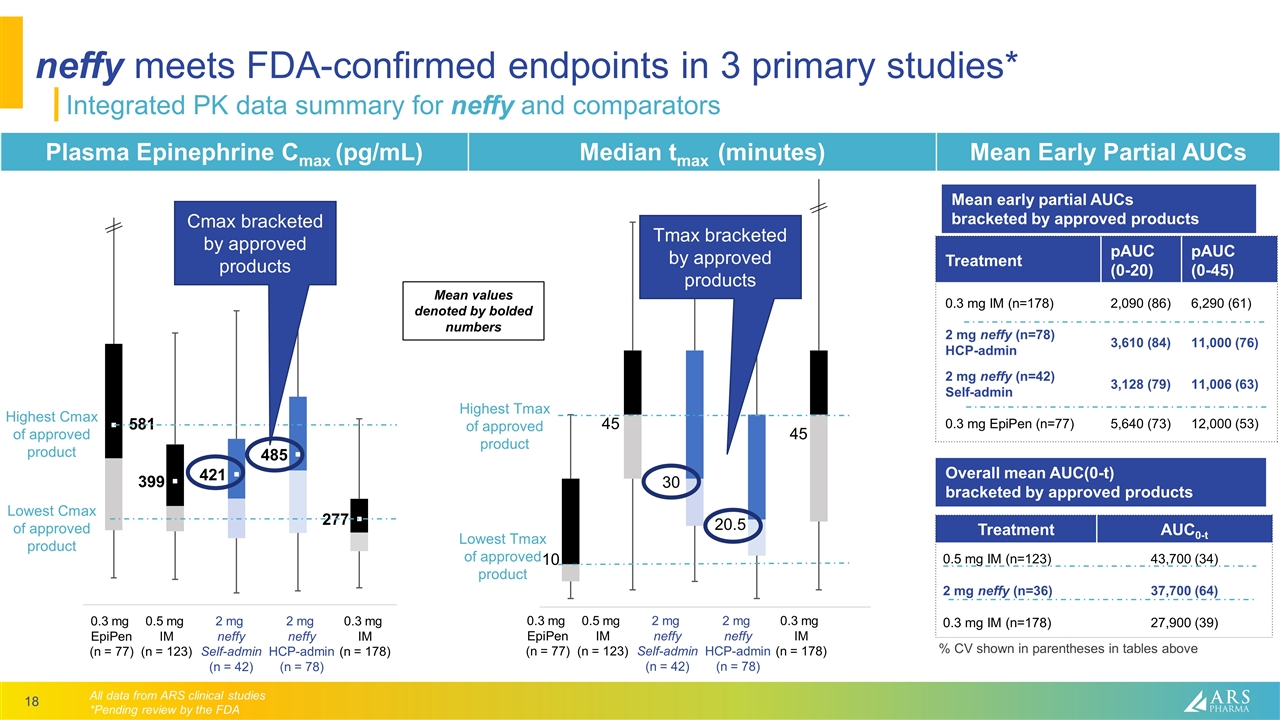
neffy meets FDA-confirmed endpoints in 3 primary studies* Integrated PK data summary for neffy and comparators Plasma Epinephrine Cmax (pg/mL) Median tmax (minutes) Mean Early Partial AUCs 0.3 mg IM (n = 178) 2 mg neffy HCP-admin (n = 78) 0.3 mg EpiPen (n = 77) 0.5 mg IM (n = 123) 2 mg neffy Self-admin (n = 42) 10 45 45 20.5 30 Treatment pAUC (0-20) pAUC (0-45) 0.3 mg IM (n=178) 2,090 (86) 6,290 (61) 2 mg neffy (n=78) HCP-admin 3,610 (84) 11,000 (76) 2 mg neffy (n=42) Self-admin 3,128 (79) 11,006 (63) 0.3 mg EpiPen (n=77) 5,640 (73) 12,000 (53) Treatment AUC0-t 0.5 mg IM (n=123) 2 mg neffy (n=36) 0.3 mg IM (n=178) 43,700 (34) 37,700 (64) 27,900 (39) Mean early partial AUCs bracketed by approved products Overall mean AUC(0-t) bracketed by approved products 0.3 mg IM (n = 178) 2 mg neffy HCP-admin (n = 78) 0.3 mg EpiPen (n = 77) 0.5 mg IM (n = 123) 2 mg neffy Self-admin (n = 42) Lowest Cmax of approved product Highest Cmax of approved product Lowest Tmax of approved product Highest Tmax of approved product Cmax bracketed by approved products Tmax bracketed by approved products Mean values denoted by bolded numbers All data from ARS clinical studies *Pending review by the FDA % CV shown in parentheses in tables above
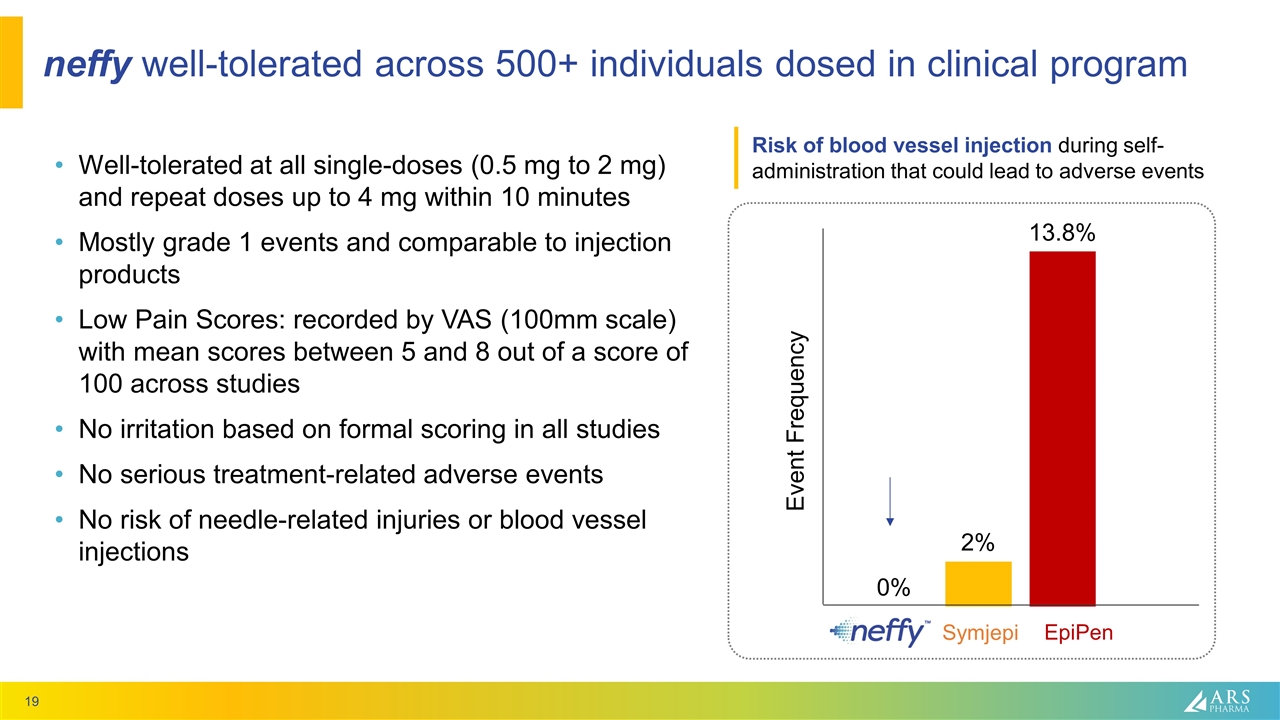
neffy well-tolerated across 500+ individuals dosed in clinical program Well-tolerated at all single-doses (0.5 mg to 2 mg) and repeat doses up to 4 mg within 10 minutes Mostly grade 1 events and comparable to injection products Low Pain Scores: recorded by VAS (100mm scale) with mean scores between 5 and 8 out of a score of 100 across studies No irritation based on formal scoring in all studies No serious treatment-related adverse events No risk of needle-related injuries or blood vessel injections Event Frequency EpiPen Symjepi Risk of blood vessel injection during self-administration that could lead to adverse events
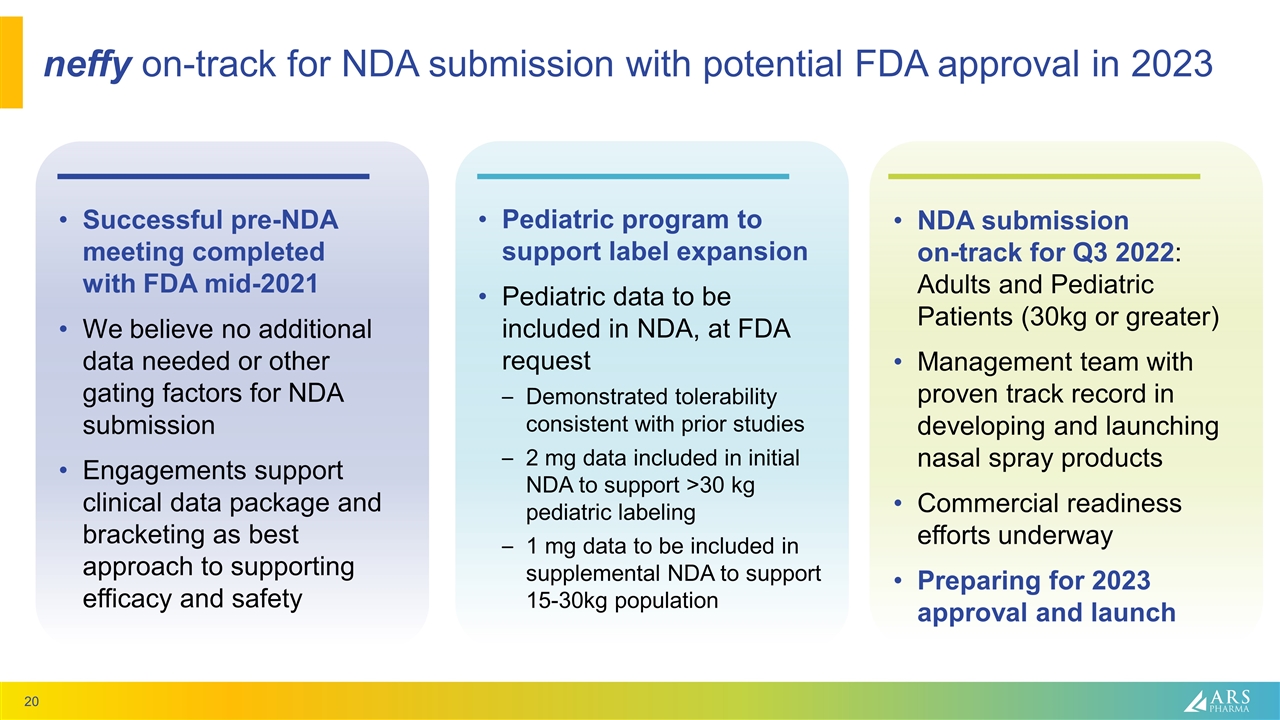
neffy on-track for NDA submission with potential FDA approval in 2023 Successful pre-NDA meeting completed with FDA mid-2021 We believe no additional data needed or other gating factors for NDA submission Engagements support clinical data package and bracketing as best approach to supporting efficacy and safety Pediatric program to support label expansion Pediatric data to be included in NDA, at FDA request Demonstrated tolerability consistent with prior studies 2 mg data included in initial NDA to support >30 kg pediatric labeling 1 mg data to be included in supplemental NDA to support 15-30kg population NDA submission on-track for Q3 2022: Adults and Pediatric Patients (30kg or greater) Management team with proven track record in developing and launching nasal spray products Commercial readiness efforts underway Preparing for 2023 approval and launch
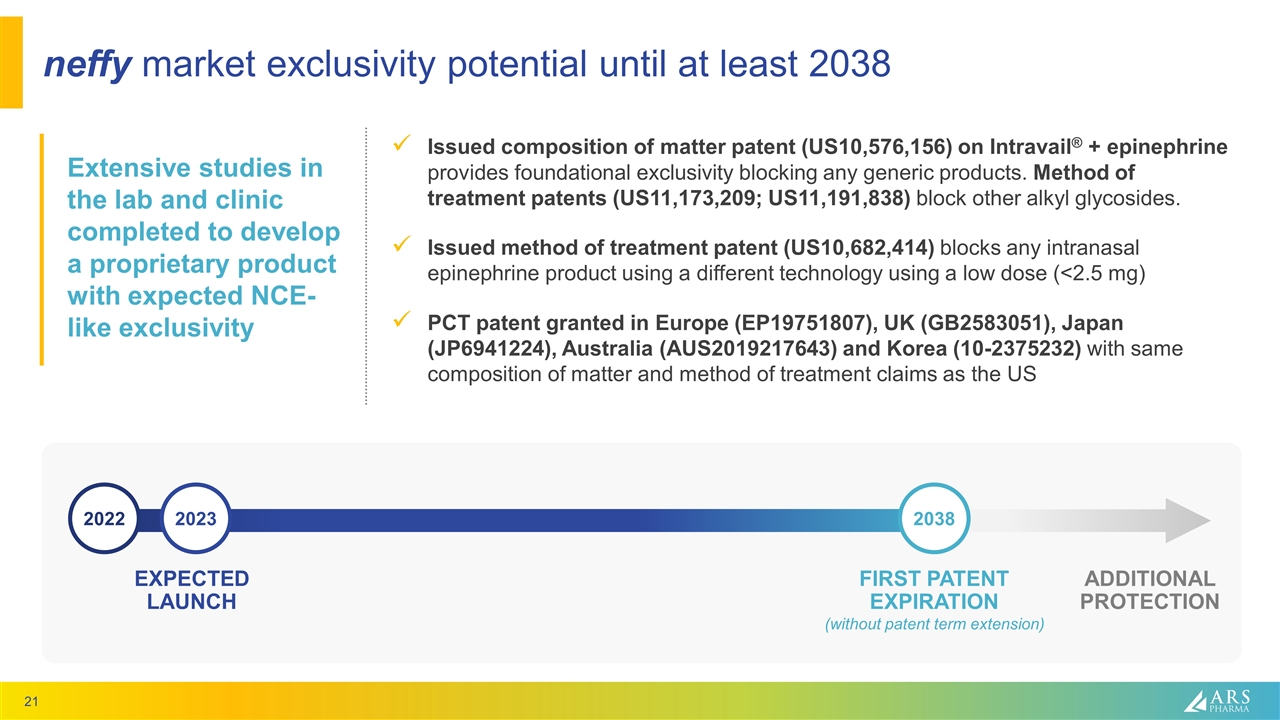
neffy market exclusivity potential until at least 2038 Issued composition of matter patent (US10,576,156) on Intravail® + epinephrine provides foundational exclusivity blocking any generic products. Method of treatment patents (US11,173,209; US11,191,838) block other alkyl glycosides. Issued method of treatment patent (US10,682,414) blocks any intranasal epinephrine product using a different technology using a low dose (<2.5 mg) PCT patent granted in Europe (EP19751807), UK (GB2583051), Japan (JP6941224), Australia (AUS2019217643) and Korea (10-2375232) with same composition of matter and method of treatment claims as the US Extensive studies in the lab and clinic completed to develop a proprietary product with expected NCE-like exclusivity EXPECTED LAUNCH FIRST PATENT EXPIRATION (without patent term extension) ADDITIONAL PROTECTION 2022 2023 2038
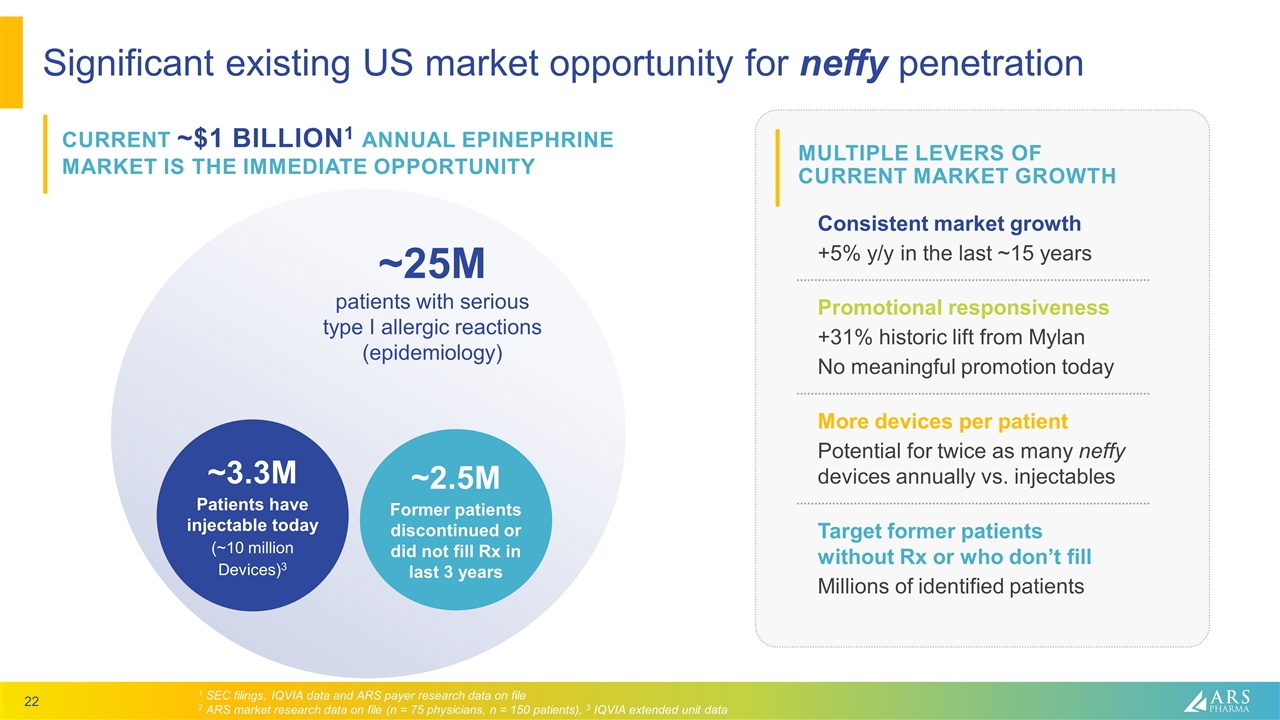
MULTIPLE LEVERS OF CURRENT MARKET GROWTH Significant existing US market opportunity for neffy penetration CURRENT ~$1 BILLION1 ANNUAL EPINEPHRINE MARKET IS THE IMMEDIATE OPPORTUNITY 1 SEC filings, IQVIA data and ARS payer research data on file 2 ARS market research data on file (n = 75 physicians, n = 150 patients), 3 IQVIA extended unit data ~3.3M Patients have injectable today (~10 million Devices)3 ~25M patients with serious type I allergic reactions (epidemiology) Consistent market growth +5% y/y in the last ~15 years Promotional responsiveness +31% historic lift from Mylan No meaningful promotion today More devices per patient Potential for twice as many neffy devices annually vs. injectables Target former patients without Rx or who don’t fill Millions of identified patients ~2.5M Former patients discontinued or did not fill Rx in last 3 years
neffy has the potential to transform the treatment of Type I allergies
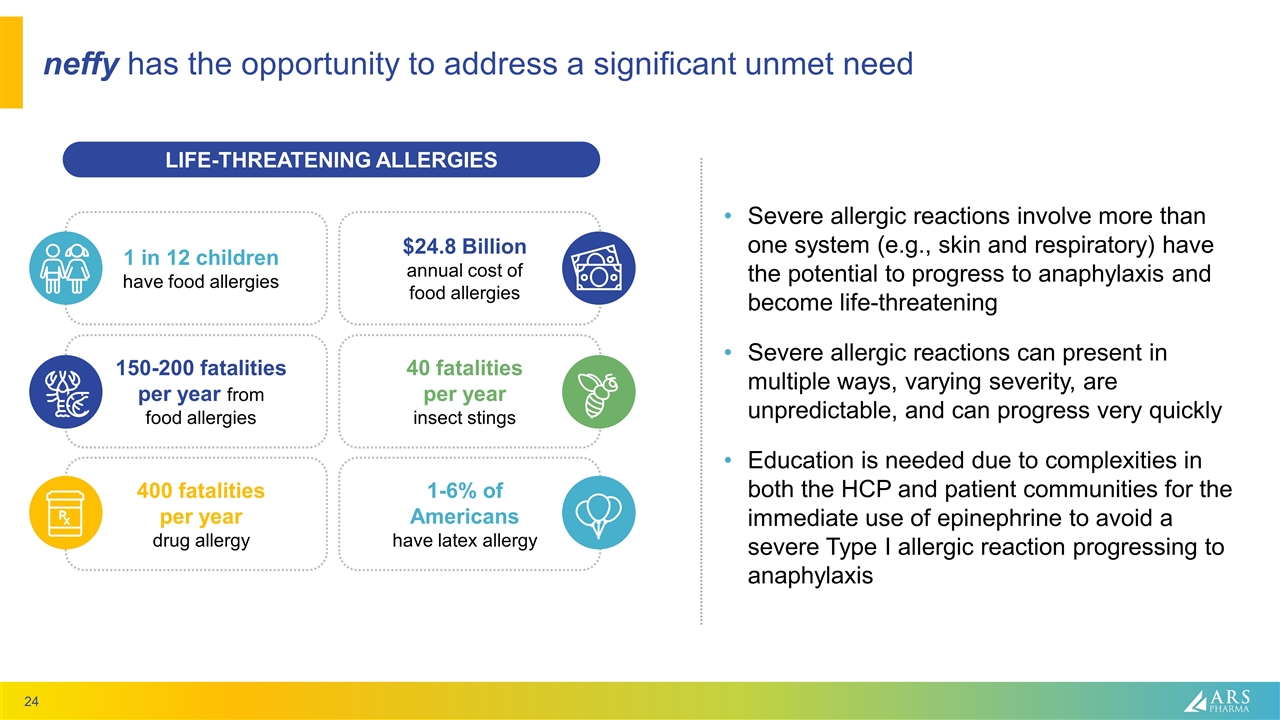
neffy has the opportunity to address a significant unmet need AllergyAsthmaNetwork.org LIFE-THREATENING ALLERGIES 1 in 12 children have food allergies $24.8 Billion annual cost of food allergies 150-200 fatalities per year from food allergies 40 fatalities per year insect stings 400 fatalities per year drug allergy 1-6% of Americans have latex allergy Severe allergic reactions involve more than one system (e.g., skin and respiratory) have the potential to progress to anaphylaxis and become life-threatening Severe allergic reactions can present in multiple ways, varying severity, are unpredictable, and can progress very quickly Education is needed due to complexities in both the HCP and patient communities for the immediate use of epinephrine to avoid a severe Type I allergic reaction progressing to anaphylaxis
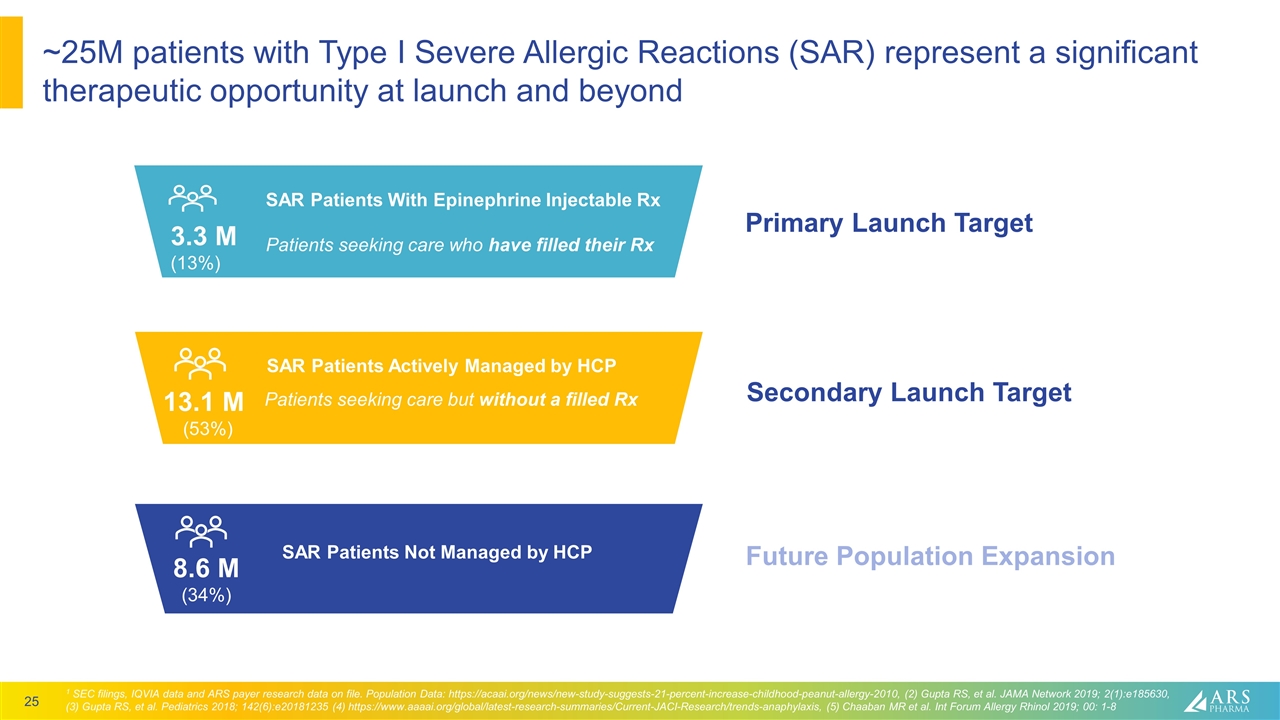
~25M patients with Type I Severe Allergic Reactions (SAR) represent a significant therapeutic opportunity at launch and beyond SAR Patients Not Managed by HCP 8.6 M (34%) Secondary Launch Target Future Population Expansion Patients seeking care but without a filled Rx 13.1 M (53%) SAR Patients Actively Managed by HCP Primary Launch Target 3.3 M (13%) SAR Patients With Epinephrine Injectable Rx Patients seeking care who have filled their Rx 1 SEC filings, IQVIA data and ARS payer research data on file. Population Data: https://acaai.org/news/new-study-suggests-21-percent-increase-childhood-peanut-allergy-2010, (2) Gupta RS, et al. JAMA Network 2019; 2(1):e185630, (3) Gupta RS, et al. Pediatrics 2018; 142(6):e20181235 (4) https://www.aaaai.org/global/latest-research-summaries/Current-JACI-Research/trends-anaphylaxis, (5) Chaaban MR et al. Int Forum Allergy Rhinol 2019; 00: 1-8
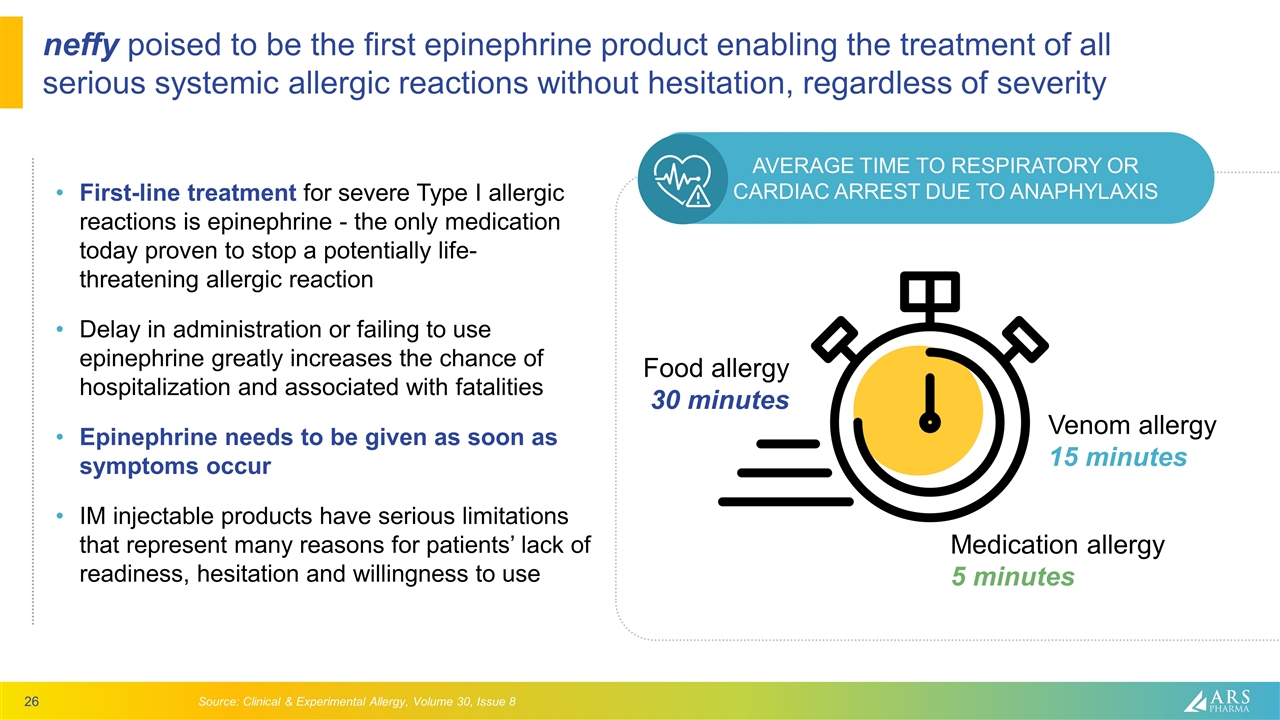
neffy poised to be the first epinephrine product enabling the treatment of all serious systemic allergic reactions without hesitation, regardless of severity First-line treatment for severe Type I allergic reactions is epinephrine - the only medication today proven to stop a potentially life-threatening allergic reaction Delay in administration or failing to use epinephrine greatly increases the chance of hospitalization and associated with fatalities Epinephrine needs to be given as soon as symptoms occur IM injectable products have serious limitations that represent many reasons for patients’ lack of readiness, hesitation and willingness to use Source: Clinical & Experimental Allergy, Volume 30, Issue 8 Food allergy 30 minutes Venom allergy 15 minutes Medication allergy 5 minutes AVERAGE TIME TO RESPIRATORY OR CARDIAC ARREST DUE TO ANAPHYLAXIS

Physicians supportive of adopting neffy into practice WOULD PRESCRIBE NEFFY IF THEIR PATIENTS ASKED FOR IT 100% 10 = MAJOR ADVANCE / 1 = NOT AN ADVANCE AT ALL 8.5 out of 10 rating viewed as a major advance in therapy n = 75 Physicians No difference in uptake of neffy by physician specialty
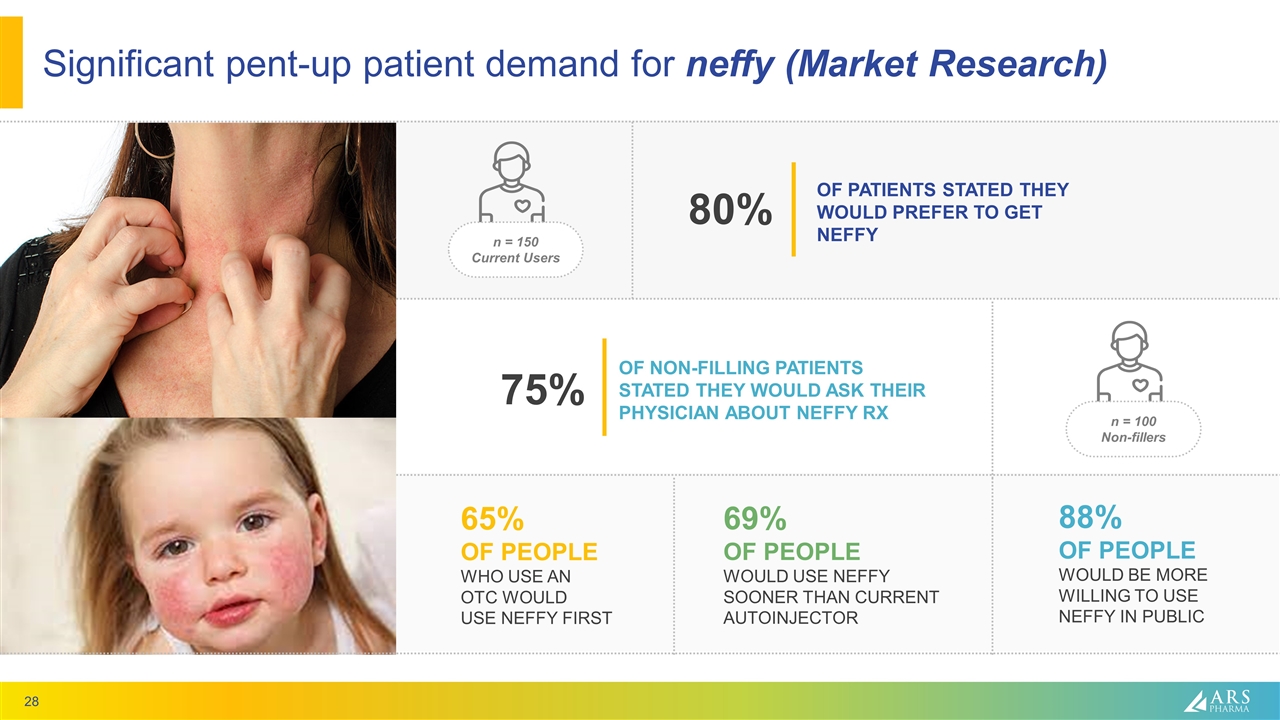
Significant pent-up patient demand for neffy (Market Research) 65% OF PEOPLE WHO USE AN OTC WOULD USE NEFFY FIRST 69% OF PEOPLE WOULD USE NEFFY SOONER THAN CURRENT AUTOINJECTOR 88% OF PEOPLE WOULD BE MORE WILLING TO USE NEFFY IN PUBLIC OF NON-FILLING PATIENTS STATED THEY WOULD ASK THEIR PHYSICIAN ABOUT NEFFY RX 75% n = 150 Current Users OF PATIENTS STATED THEY WOULD PREFER TO GET NEFFY 80% n = 100 Non-fillers
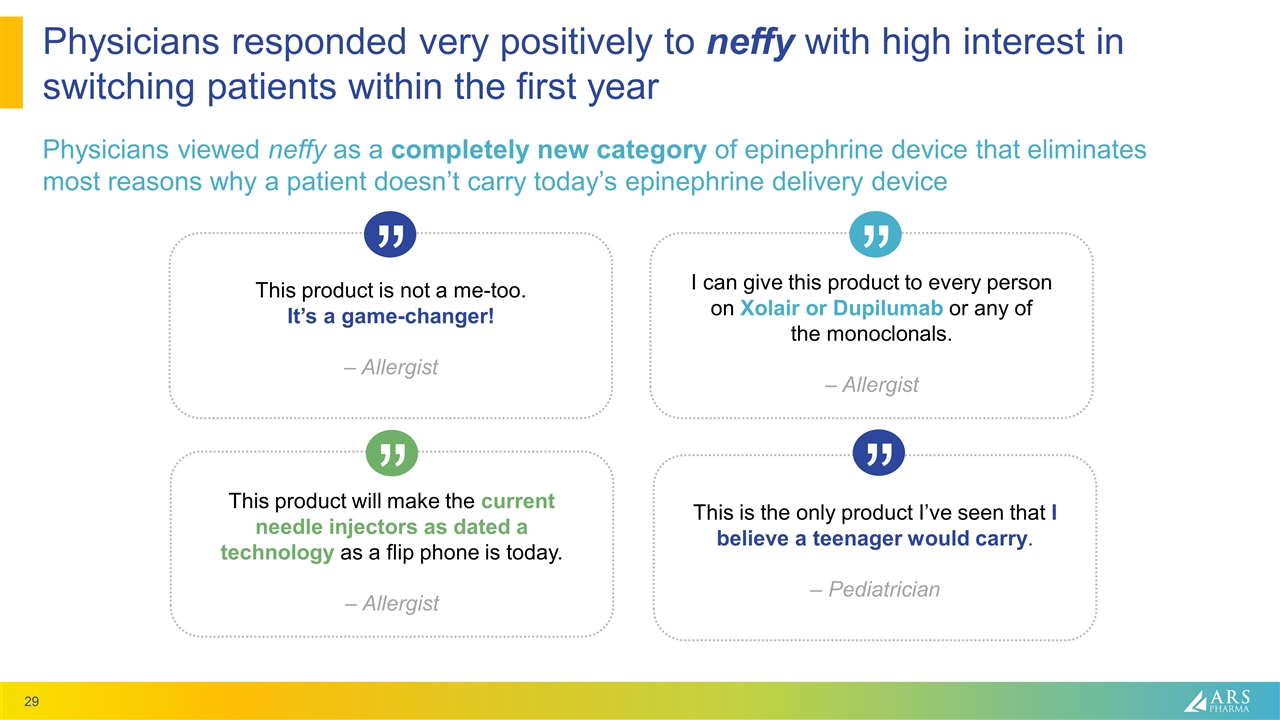
Physicians responded very positively to neffy with high interest in switching patients within the first year Physicians viewed neffy as a completely new category of epinephrine device that eliminates most reasons why a patient doesn’t carry today’s epinephrine delivery device This product is not a me-too. It’s a game-changer! – Allergist “ This product will make the current needle injectors as dated a technology as a flip phone is today. – Allergist “ I can give this product to every person on Xolair or Dupilumab or any of the monoclonals. – Allergist “ This is the only product I’ve seen that I believe a teenager would carry. – Pediatrician “
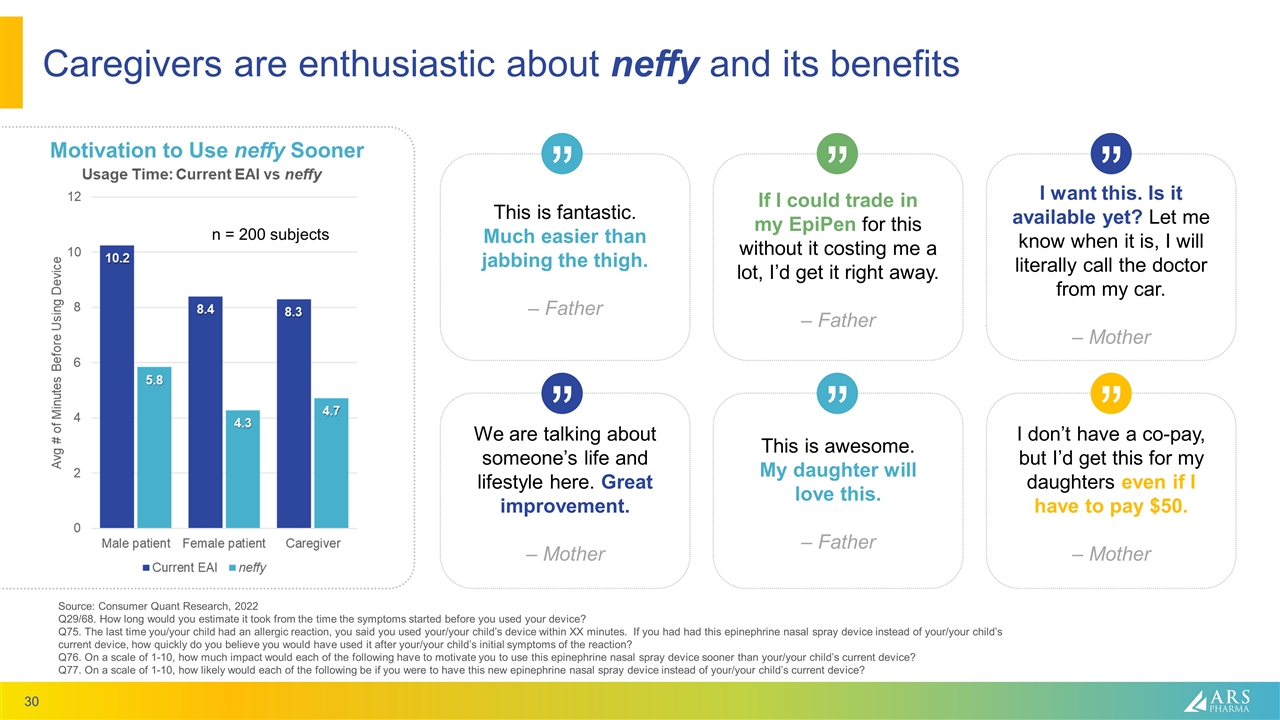
Caregivers are enthusiastic about neffy and its benefits Source: Consumer Quant Research, 2022 Q29/68. How long would you estimate it took from the time the symptoms started before you used your device? Q75. The last time you/your child had an allergic reaction, you said you used your/your child’s device within XX minutes. If you had had this epinephrine nasal spray device instead of your/your child’s current device, how quickly do you believe you would have used it after your/your child’s initial symptoms of the reaction? Q76. On a scale of 1-10, how much impact would each of the following have to motivate you to use this epinephrine nasal spray device sooner than your/your child’s current device? Q77. On a scale of 1-10, how likely would each of the following be if you were to have this new epinephrine nasal spray device instead of your/your child’s current device? Motivation to Use neffy Sooner n = 200 subjects This is fantastic. Much easier than jabbing the thigh. – Father This is awesome. My daughter will love this. – Father We are talking about someone’s life and lifestyle here. Great improvement. – Mother I don’t have a co-pay, but I’d get this for my daughters even if I have to pay $50. – Mother I want this. Is it available yet? Let me know when it is, I will literally call the doctor from my car. – Mother If I could trade in my EpiPen for this without it costing me a lot, I’d get it right away. – Father “ “ “ “ “ “
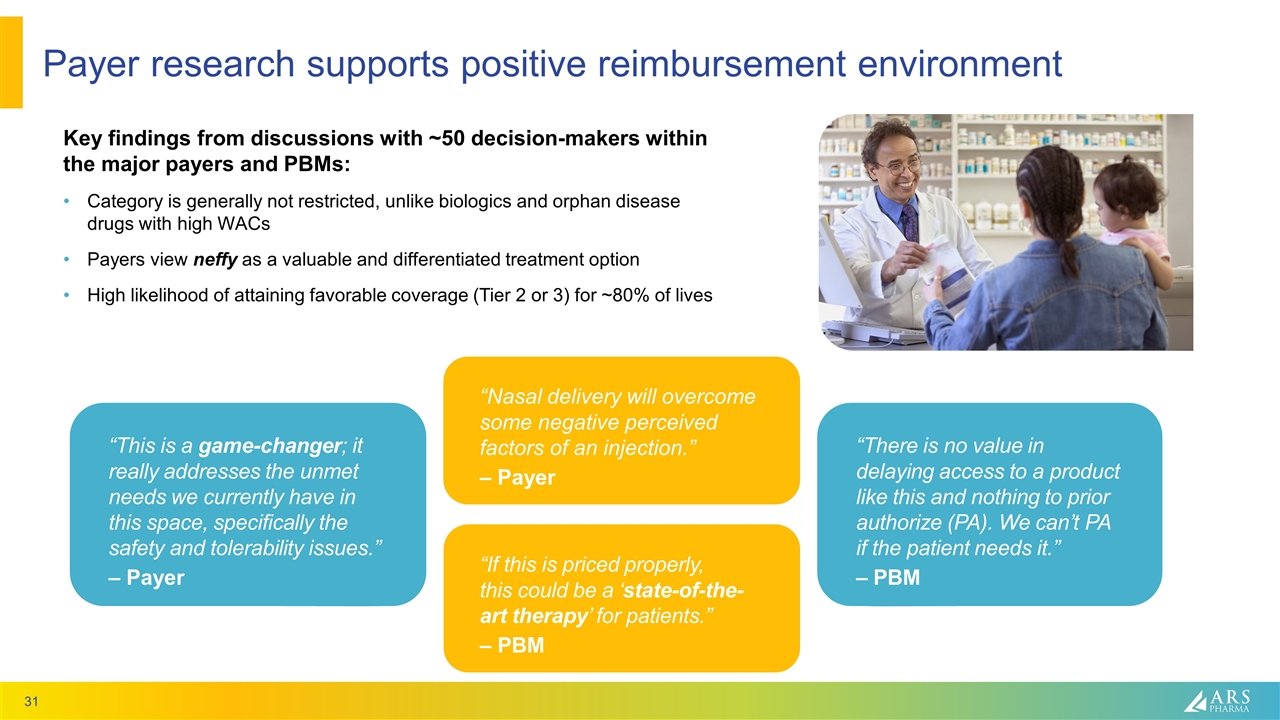
Payer research supports positive reimbursement environment Key findings from discussions with ~50 decision-makers within the major payers and PBMs: Category is generally not restricted, unlike biologics and orphan disease drugs with high WACs Payers view neffy as a valuable and differentiated treatment option High likelihood of attaining favorable coverage (Tier 2 or 3) for ~80% of lives “This is a game-changer; it really addresses the unmet needs we currently have in this space, specifically the safety and tolerability issues.” – Payer “If this is priced properly, this could be a ‘state-of-the-art therapy’ for patients.” – PBM “Nasal delivery will overcome some negative perceived factors of an injection.” – Payer “There is no value in delaying access to a product like this and nothing to prior authorize (PA). We can’t PA if the patient needs it.” – PBM
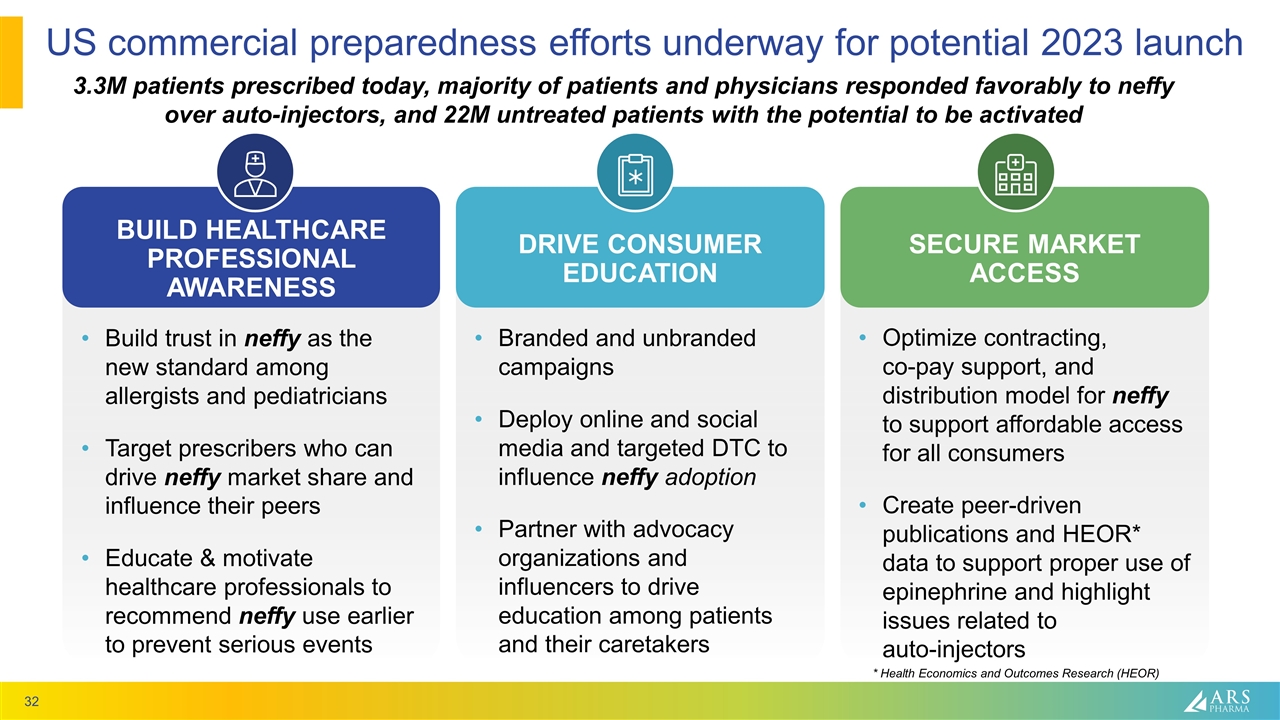
Optimize contracting, co-pay support, and distribution model for neffy to support affordable access for all consumers Create peer-driven publications and HEOR* data to support proper use of epinephrine and highlight issues related to auto-injectors Build trust in neffy as the new standard among allergists and pediatricians Target prescribers who can drive neffy market share and influence their peers Educate & motivate healthcare professionals to recommend neffy use earlier to prevent serious events Branded and unbranded campaigns Deploy online and social media and targeted DTC to influence neffy adoption Partner with advocacy organizations and influencers to drive education among patients and their caretakers US commercial preparedness efforts underway for potential 2023 launch BUILD HEALTHCARE PROFESSIONAL AWARENESS DRIVE CONSUMER EDUCATION SECURE MARKET ACCESS 3.3M patients prescribed today, majority of patients and physicians responded favorably to neffy over auto-injectors, and 22M untreated patients with the potential to be activated * Health Economics and Outcomes Research (HEOR)
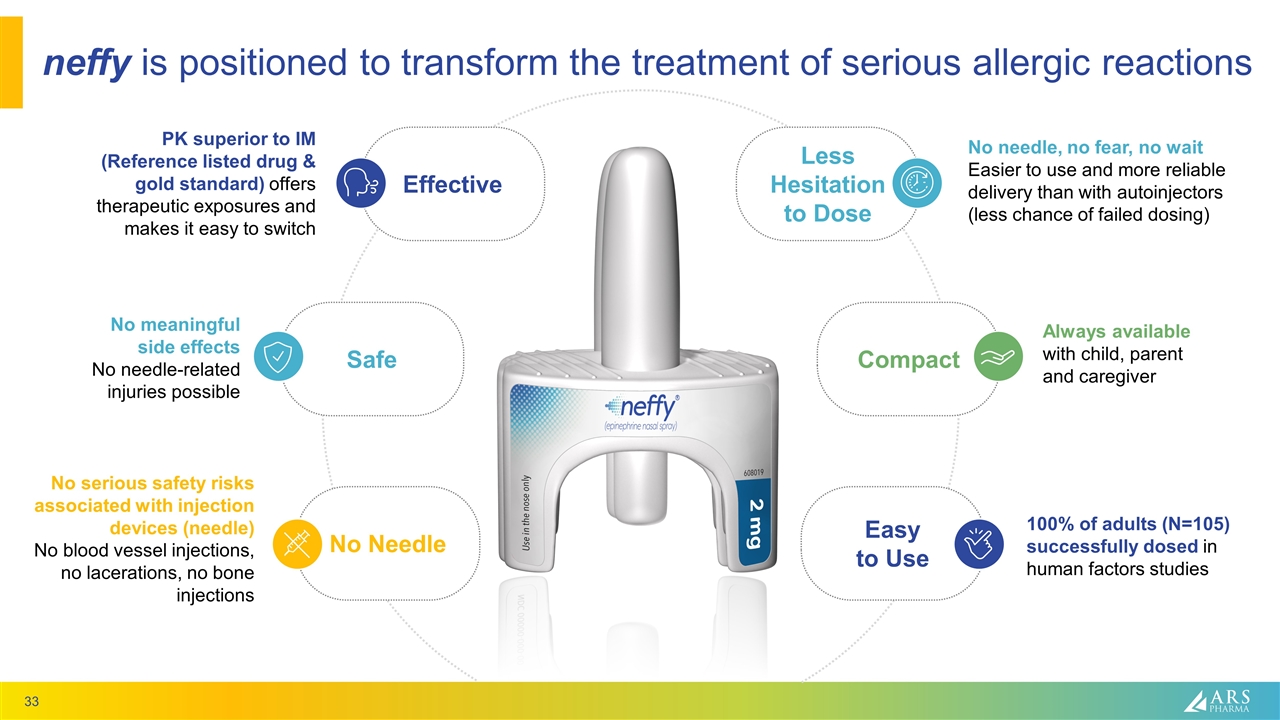
neffy is positioned to transform the treatment of serious allergic reactions Less Hesitation to Dose Effective Compact Safe Easy to Use No Needle PK superior to IM (Reference listed drug & gold standard) offers therapeutic exposures and makes it easy to switch No meaningful side effects No needle-related injuries possible No serious safety risks associated with injection devices (needle) No blood vessel injections, no lacerations, no bone injections No needle, no fear, no wait Easier to use and more reliable delivery than with autoinjectors (less chance of failed dosing) Always available with child, parent and caregiver 100% of adults (N=105) successfully dosed in human factors studies
































