may also terminate the agreement if we fail to make any changes to the clinical trial protocol regarding the use of KEYTRUDA® that are reasonably requested by Merck to address any concern raised by Merck that KEYTRUDA® is being used in the clinical trial in an unsafe manner.
Competition
The biotechnology and pharmaceutical industries, including the oncology and inflammatory disease fields, are characterized by rapidly advancing technologies, strong competition and an emphasis on intellectual property protection. We face substantial competition from many different sources, including large and specialty pharmaceutical and biotechnology companies, academic research institutions, governmental agencies and public and private research institutions. We believe that the key competitive factors affecting the success of any of our drug candidates will include patient selection strategies, efficacy (single and combination strategies), safety profile, method of administration, cost, level of promotional activity and intellectual property protection.
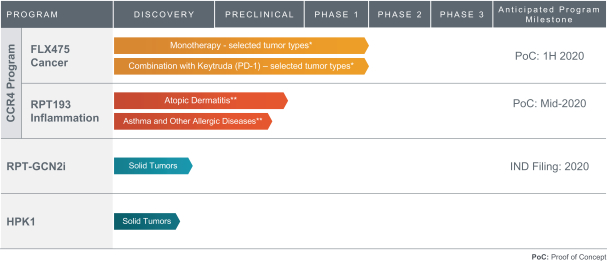
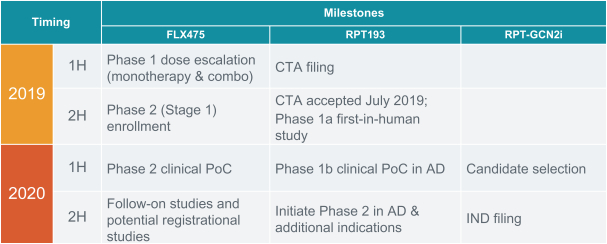
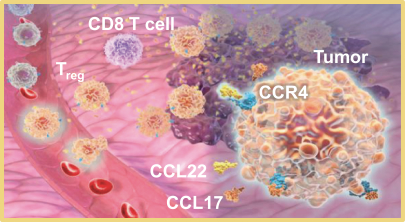
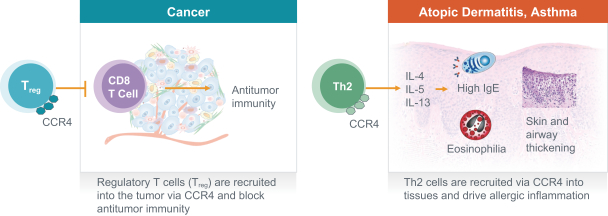
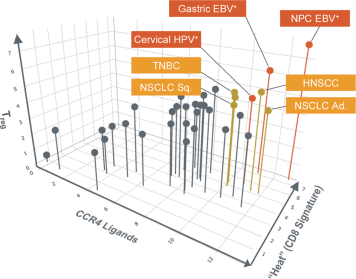
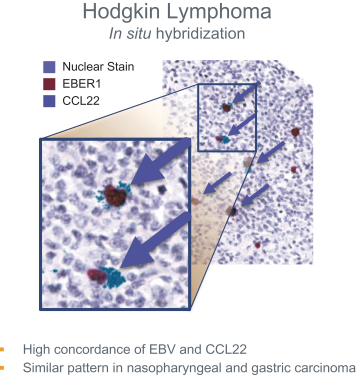
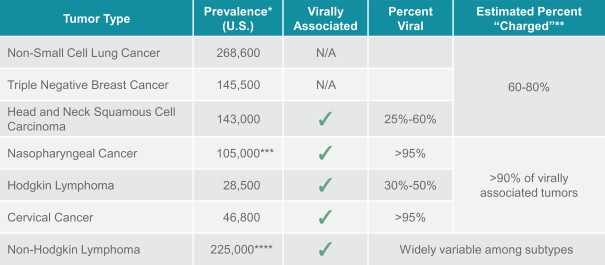
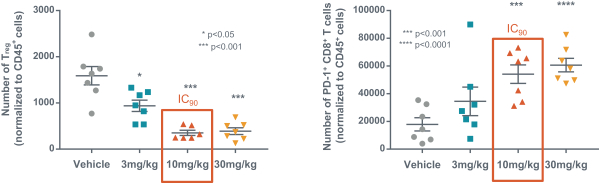
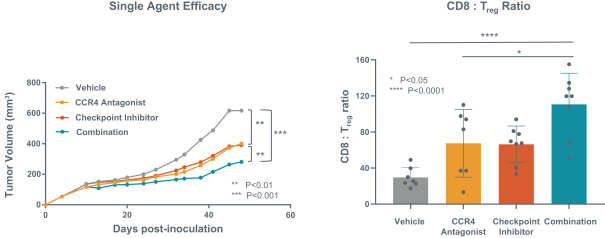
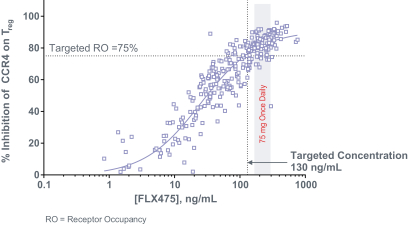
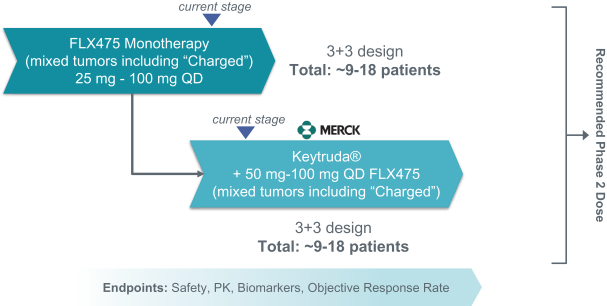
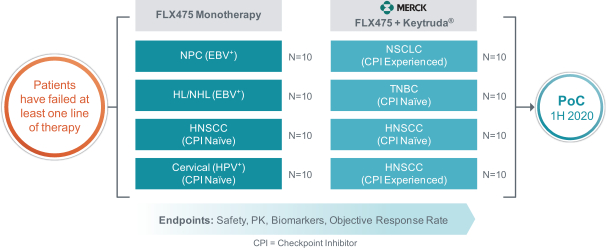
If approved, FLX475 will compete with current therapies approved for the treatment of cancer, particularly immuno-oncology. Potential immuno-oncology therapeutics are being developed or marketed by many large and specialty pharmaceutical and biotechnology companies such as Merck, Bristol-Myers Squibb, Novartis, AstraZeneca, Pfizer and Roche/Genentech. Additionally, there is one approved CCR4-targeting Treg-depleting antibody, mogamulizumab developed by Kyowa Hakko Kirin, as well as other Treg-targeting agents currently in early development by companies such as ChemoCentryx, Tusk/Roche and Agenus/Gilead.
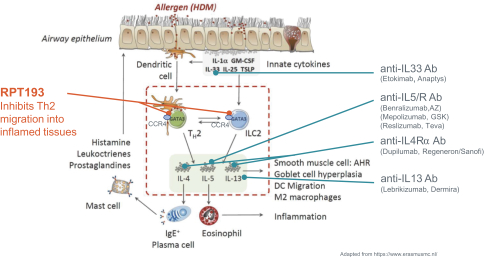
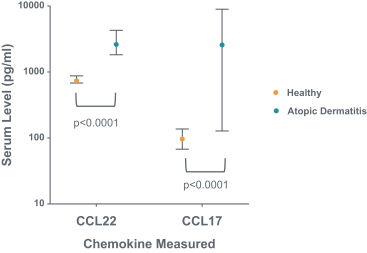
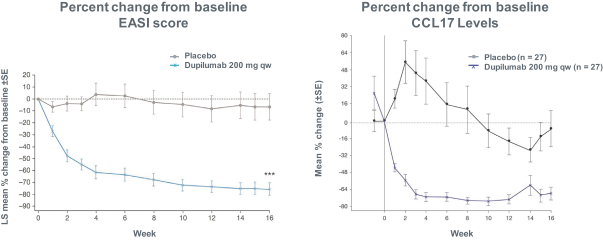
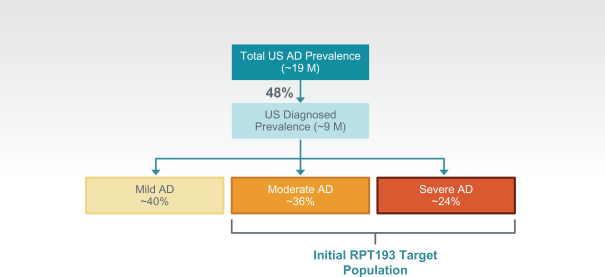
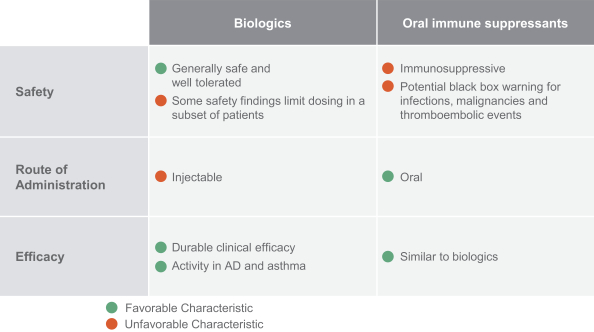
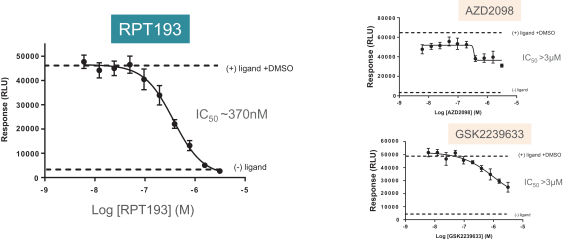
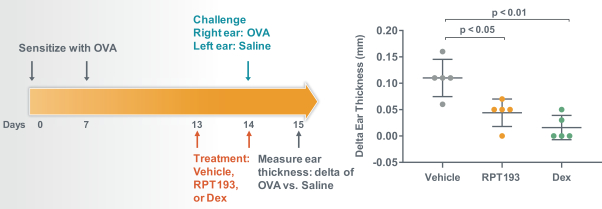
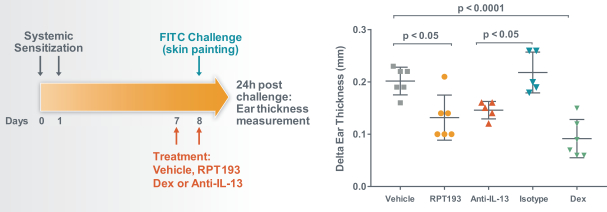
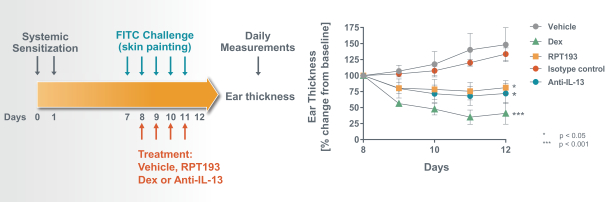
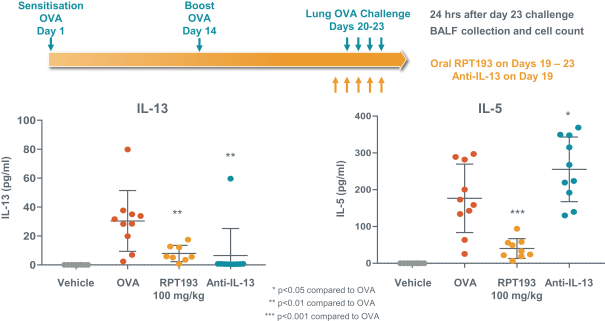
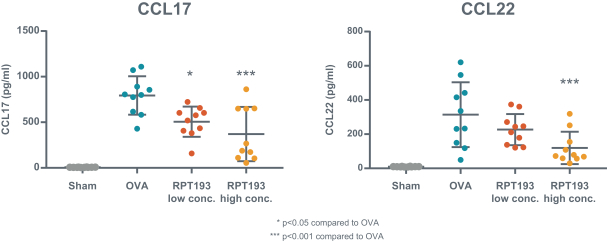
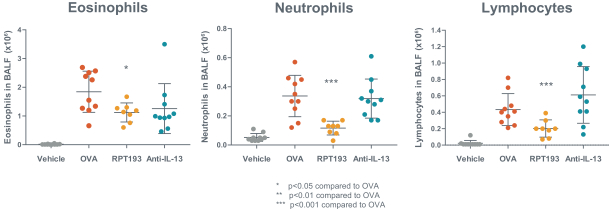
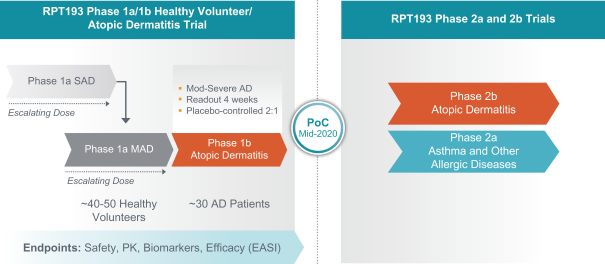
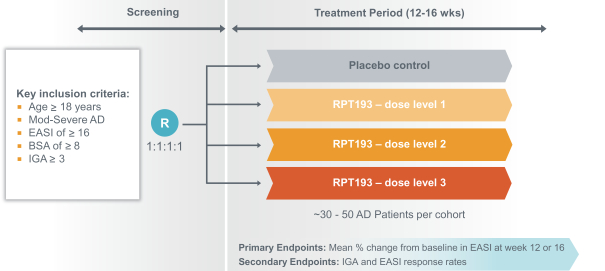
RPT193 is a CCR4 antagonist intended to treat allergic disease, including AD and other diseases along the atopic march. If approved for AD, we will face branded competition from dupilumab (marketed by Regeneron Pharmaceuticals, Inc. and Sanofi S.A. as Dupixent), a biologic recently approved. In addition, there are several companies developing treatments that may be approved for AD, including large pharmaceutical and biotechnology companies such as Pfizer, Lilly/Incyte, AbbVie, AnaptysBio, Dermira and Amgen/AstraZeneca.
There are several large and specialty pharmaceutical companies, as well as biotechnology companies with marketed or late stage assets targeting the Th2 pathway along the atopic march, which includes Amgen, AstraZeneca, Chiesi Farmaceutici, GSK, Novartis, Roche, Sanofi and Teva Pharmaceuticals.
Many of the companies against which we may compete have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trials sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Government Regulation
The FDA and other regulatory authorities at federal, state, and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring, and post-approval reporting of drug products such as those we are developing. We, along with third-party contractors, will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our drug candidates.
The process required by the FDA before drug candidates may be marketed in the United States generally involves the following:
| | • | | completion of preclinical laboratory tests and animal studies performed in accordance with the FDA’s current Good Laboratory Practices (“GLP”), regulation; |
125