
December 23, 2024 Corporate Presentation Exhibit 99.3

Disclaimer Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development plans; current and future drug candidates, including the in-license of RPT904 (JYB-1904); the license agreement related to RPT904 and potential future milestone payments and royalties; the development of RPT904, including the expected timing of clinical trials and the availability of data therefrom and regulatory interactions; our anticipated cash runway; the therapeutic potential of RPT904; the potential commercial opportunity for RPT904; the ability to obtain necessary regulatory approvals; business strategy and plans; regulatory pathways; and our ability to achieve certain milestones. Words such as "believe," "anticipate," "plan," "expect," "will," "may," "upcoming," "milestone," "potential," "target" or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the current beliefs of the Company's management with respect to future events and trends and are subject to known and unknown risks and uncertainties that may cause our actual performance or achievements to be materially different from any future performance or achievements expressed or implied by the forward-looking statements in this Presentation. Risks and uncertainties that may cause actual results to differ materially include: risks inherent in the initiation, progress and completion of clinical trials and clinical development of our product candidates; the risk that clinical trials may have unsatisfactory outcomes; risks associated with preclinical development of product candidates; regulatory authorities, including the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our drug candidates; we may decide, or regulatory authorities may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our drug candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our drug candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could delay or prevent regulatory approval or commercialization; uncertainties inherent in the conduct of clinical trials, our reliance on third parties over which we may not always have full control; our ability to enter into strategic partnerships on commercially reasonable terms; our ability to obtain additional financing; the uncertainty regarding the macroeconomic environment and other risks and uncertainties that are described in the “Risk Factors” section of our most recent Form 10-Q filed with the Securities and Exchange Commission, and any current and periodic reports filed thereafter. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that any assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of such assumptions, fully stated in the Presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Although we believe that the beliefs and assumptions reflected in the forward-looking statements are reasonable, we cannot guarantee future performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Presentation. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration FDA. No representation is made as to the safety or effectiveness of any drug candidates for any use for which such drug candidates are being studied. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
RAPT is Developing Transformative Therapies for High-Value Inflammatory Diseases RPT904 (JYB1904) is a half-life extended (HLE) anti-IgE omalizumab “bio-better” antibody licensed from Jemincare, a leading Chinese pharmaceutical company License terms: $35M upfront, ~$672M milestones, royalties of high-single to low-double digits Omalizumab (marketed as Xolair®), a first-generation anti-IgE mAb, is approved for food allergy (FA), chronic spontaneous urticaria (CSU), asthma and chronic rhinosinusitis Phase 1 data supports RPT904 potential best-in-class profile with less frequent dosing and increased patient compliance RAPT plans to initiate Phase 2b FA trial 2H 2025; topline data expected 1H 2027 Jemincare Phase 2 trials in China for asthma and CSU; topline data expected 2H 2025 and 1H 2026, respectively $150M PIPE plus current cash expected to provide runway through Phase 2b FA data * XOLAIR® is a registered trademark of Novartis AG

Commercial Opportunity RPT904
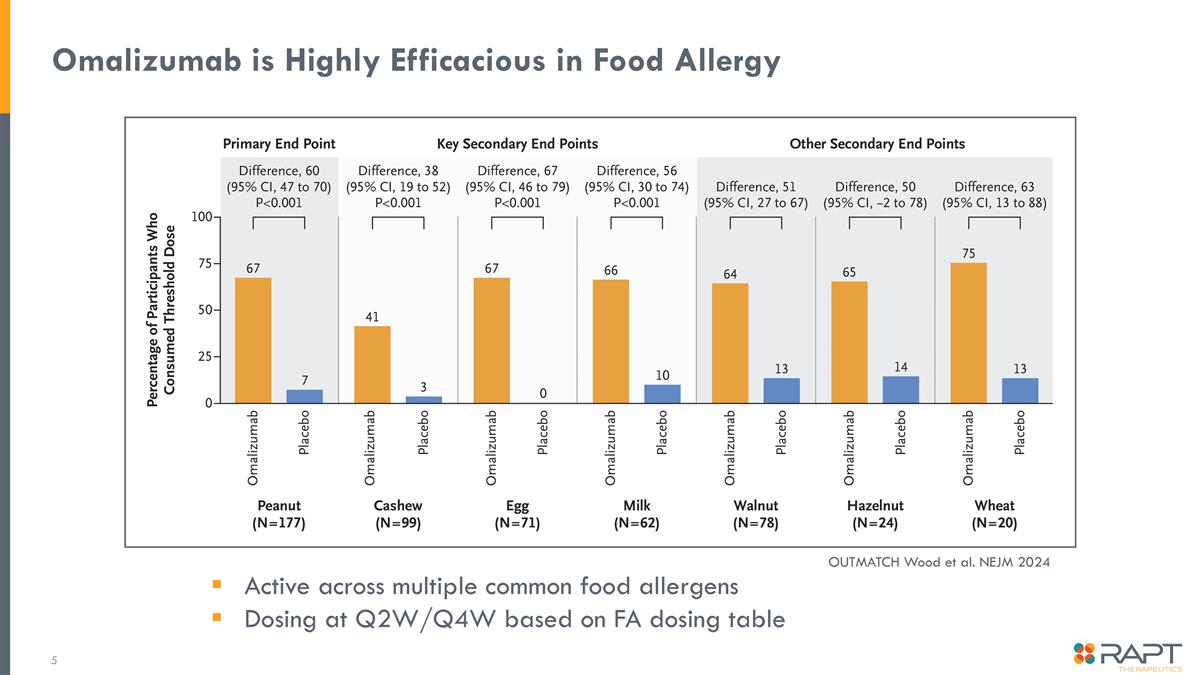
Omalizumab is Highly Efficacious in Food Allergy Active across multiple common food allergens Dosing at Q2W/Q4W based on FA dosing table OUTMATCH Wood et al. NEJM 2024
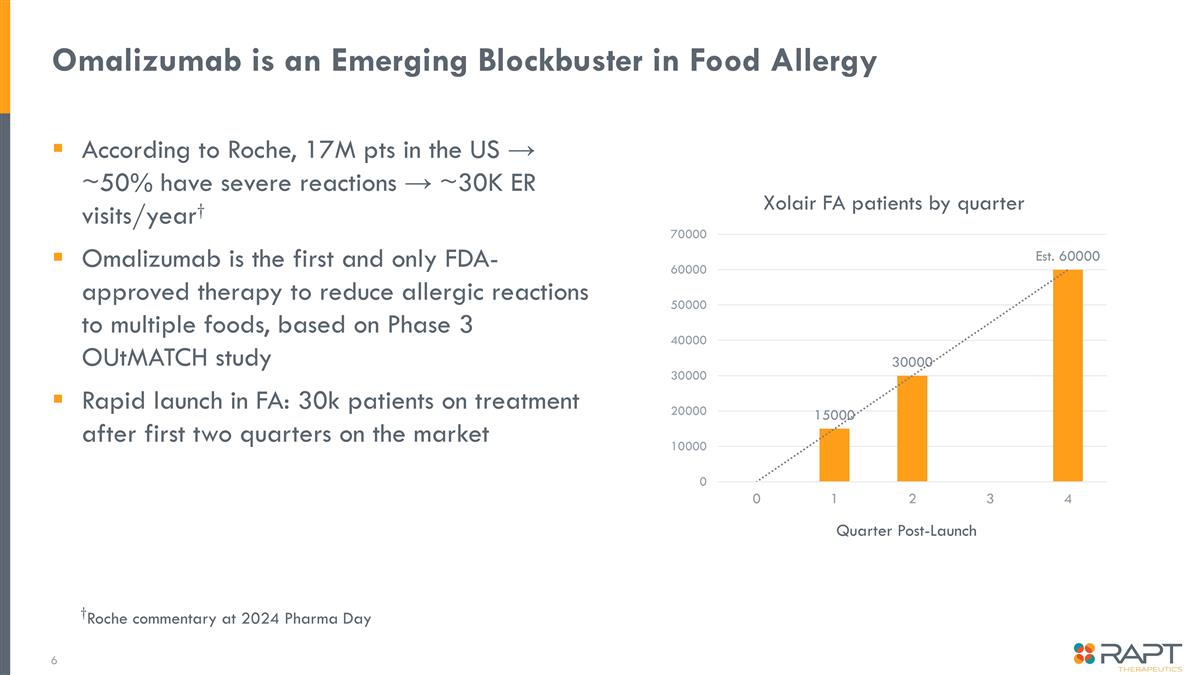
Omalizumab is an Emerging Blockbuster in Food Allergy According to Roche, 17M pts in the US → ~50% have severe reactions → ~30K ER visits/year† Omalizumab is the first and only FDA-approved therapy to reduce allergic reactions to multiple foods, based on Phase 3 OUtMATCH study Rapid launch in FA: 30k patients on treatment after first two quarters on the market †Roche commentary at 2024 Pharma Day Quarter Post-Launch

High Unmet Need and Large Commercial Opportunity in Food Allergy (FA) Treatment environment is dominated by inconvenient treatments with room to improve compliance and efficacy Food avoidance and single allergen desensitization (e.g. oral immunotherapy) Rapid uptake of omalizumab demonstrates high unmet need for a more convenient multi-allergen option Despite omalizumab’s early success, payers and prescribers would welcome a longer-acting treatment like RPT904 for increased compliance and convenience* RPT904 TPP: equivalent efficacy and safety to omalizumab with Q8W dosing Prescribers expect to use RPT904 in 16% of moderate-to-severe FA patients Payers can support at least ~30% premium over omalizumab biosimilar Estimate ~$4.5B in peak US sales for FA * Based on primary market research n=140 prescribers, Oct 2024 and n=45 payers, Nov 2024

CSU Offers an Additional Commercial Opportunity CSU affects >1M patients in the US(1) Antihistamines are first treatment step, but ~400k patients not controlled on antihistamines(2) Omalizumab is only approved biologic for CSU after failure of antihistamines RPT904 positioned to be preferred choice in front-line setting due to improved convenience and compliance over omalizumab Estimate ~$1B in peak US revenues in CSU (1) Roche, Pharma Day 2024, and Nature 2022 (2) Globaldata report, Aug 2024 and various equity research reports

A Potential Best-in-Class Anti-IgE mAb RPT904
RPT904 is a Potential Best-in-Class Omalizumab Bio-better mAb RPT904 is a half-life extended (HLE) anti-IgE monoclonal antibody with improved potency Incorporates proven HLE technology using the YTE mutation Q8W/Q12W dosing versus Q2W/Q4W with omalizumab Uses clinically validated epitope identical to omalizumab – proven MOA Potential to treat patients omalizumab cannot, e.g. restrictions due to high weight or IgE level RPT904 composition of matter patent to 2041 without PTE or formulation/device patents
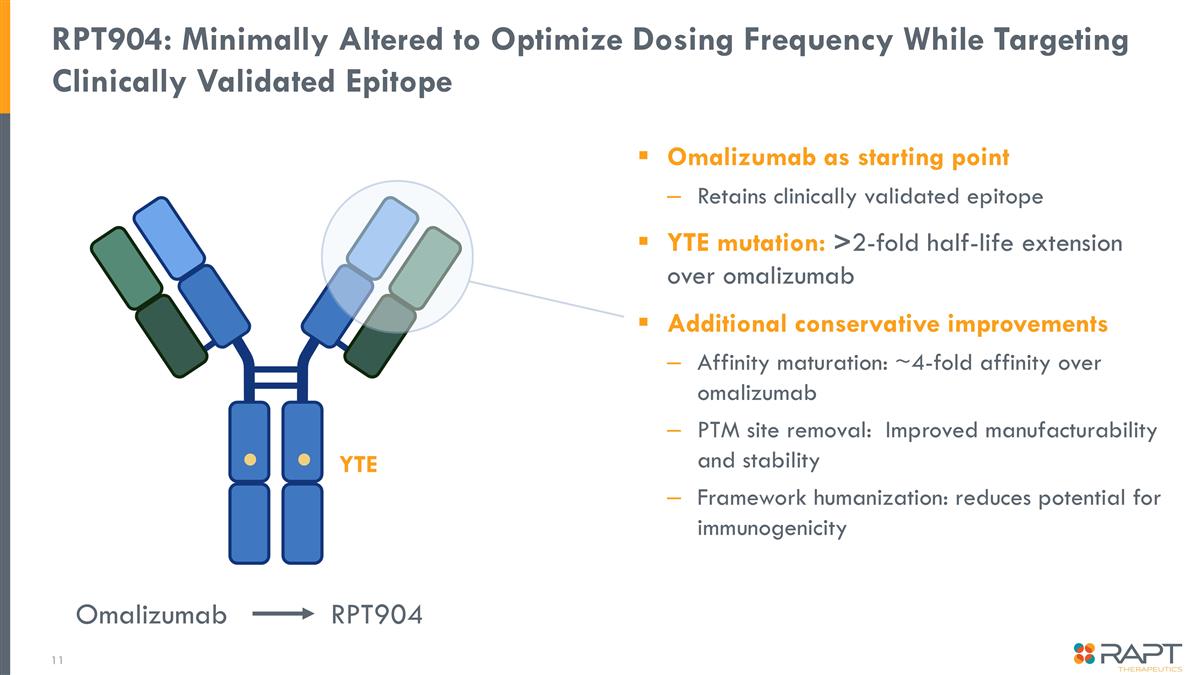
RPT904: Minimally Altered to Optimize Dosing Frequency While Targeting Clinically Validated Epitope Omalizumab as starting point Retains clinically validated epitope YTE mutation: >2-fold half-life extension over omalizumab Additional conservative improvements Affinity maturation: ~4-fold affinity over omalizumab PTM site removal: Improved manufacturability and stability Framework humanization: reduces potential for immunogenicity Omalizumab RPT904 YTE

Phase 1 Healthy Volunteer Data and Dose Estimations RPT904
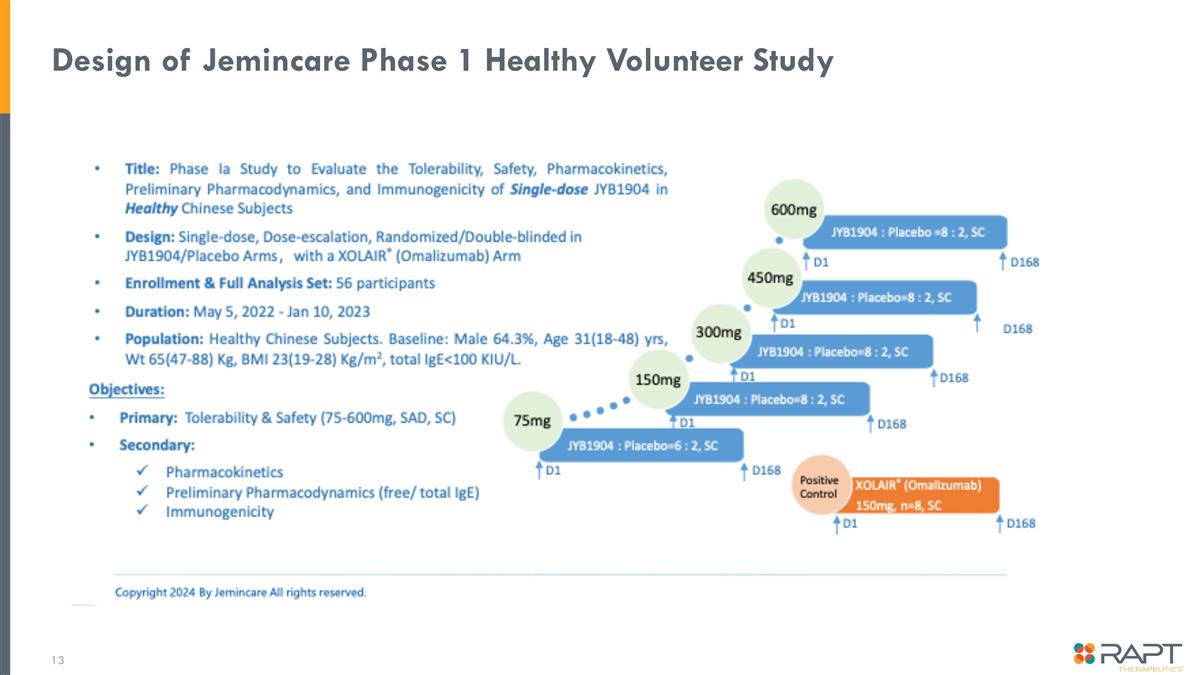
Design of Jemincare Phase 1 Healthy Volunteer Study
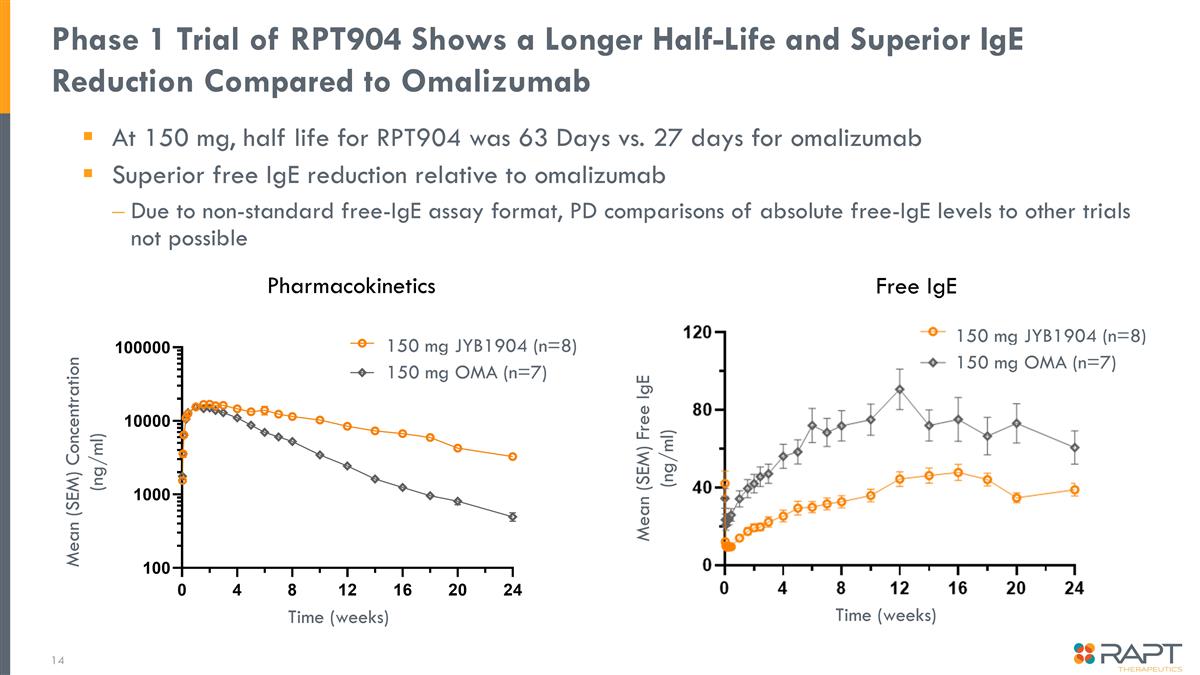
Phase 1 Trial of RPT904 Shows a Longer Half-Life and Superior IgE Reduction Compared to Omalizumab At 150 mg, half life for RPT904 was 63 Days vs. 27 days for omalizumab Superior free IgE reduction relative to omalizumab Due to non-standard free-IgE assay format, PD comparisons of absolute free-IgE levels to other trials not possible Pharmacokinetics Free IgE Time (weeks) Mean (SEM) Free IgE (ng/ml) Mean (SEM) Concentration (ng/ml) Time (weeks) 150 mg JYB1904 (n=8) 150 mg OMA (n=7) 150 mg JYB1904 (n=8) 150 mg OMA (n=7)
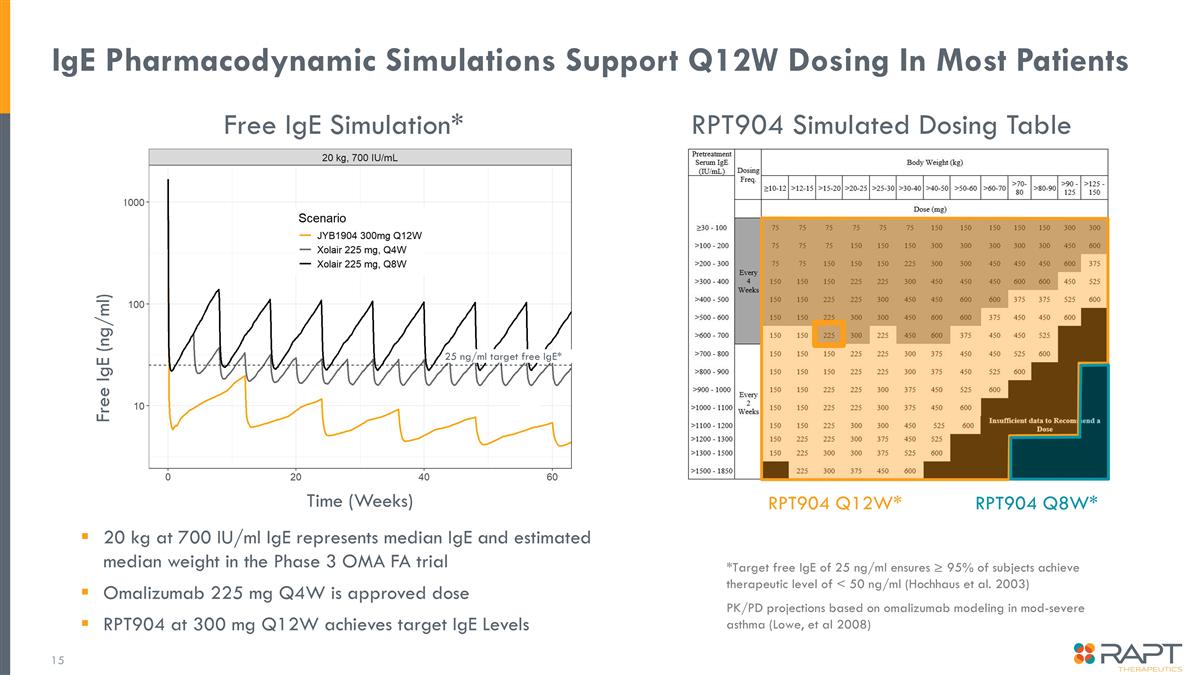
IgE Pharmacodynamic Simulations Support Q12W Dosing In Most Patients 20 kg at 700 IU/ml IgE represents median IgE and estimated median weight in the Phase 3 OMA FA trial Omalizumab 225 mg Q4W is approved dose RPT904 at 300 mg Q12W achieves target IgE Levels *Target free IgE of 25 ng/ml ensures ≥ 95% of subjects achieve therapeutic level of < 50 ng/ml (Hochhaus et al. 2003) PK/PD projections based on omalizumab modeling in mod-severe asthma (Lowe, et al 2008) 25 ng/ml target free IgE* Free IgE (ng/ml) Time (Weeks) RPT904 Simulated Dosing Table Free IgE Simulation* RPT904 Q12W* RPT904 Q8W*

Phase 2 Trial Designs RPT904
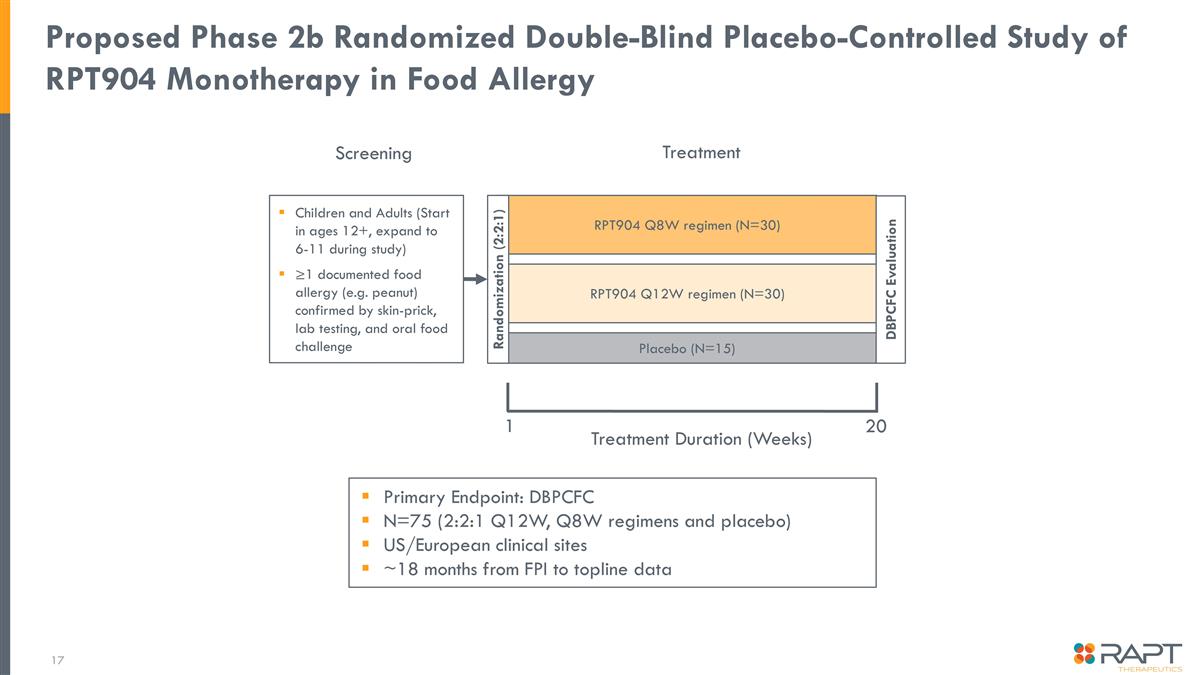
RPT904 Q12W regimen (N=30) Primary Endpoint: DBPCFC N=75 (2:2:1 Q12W, Q8W regimens and placebo) US/European clinical sites ~18 months from FPI to topline data Proposed Phase 2b Randomized Double-Blind Placebo-Controlled Study of RPT904 Monotherapy in Food Allergy Children and Adults (Start in ages 12+, expand to 6-11 during study) ≥1 documented food allergy (e.g. peanut) confirmed by skin-prick, lab testing, and oral food challenge Screening RPT904 Q8W regimen (N=30) Placebo (N=15) Randomization (2:2:1) Treatment 1 Treatment Duration (Weeks) DBPCFC Evaluation 20
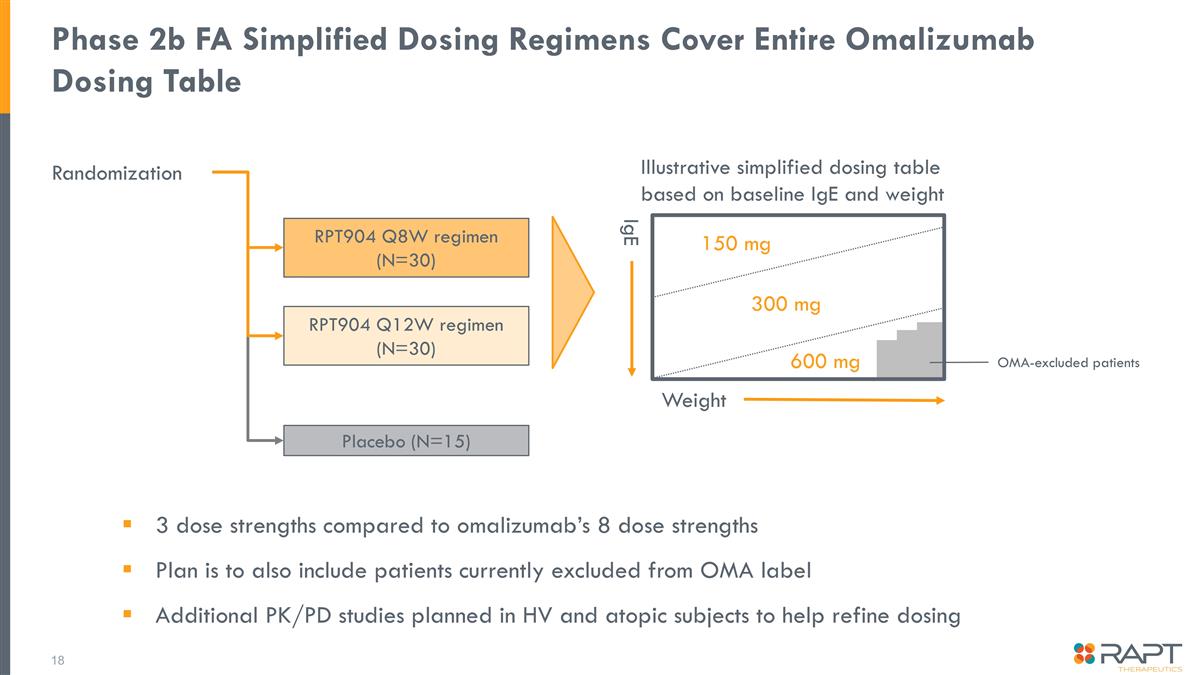
Phase 2b FA Simplified Dosing Regimens Cover Entire Omalizumab Dosing Table Randomization Illustrative simplified dosing table based on baseline IgE and weight 3 dose strengths compared to omalizumab’s 8 dose strengths Plan is to also include patients currently excluded from OMA label Additional PK/PD studies planned in HV and atopic subjects to help refine dosing RPT904 Q12W regimen (N=30) RPT904 Q8W regimen (N=30) Placebo (N=15) 150 mg 300 mg 600 mg Weight IgE OMA-excluded patients
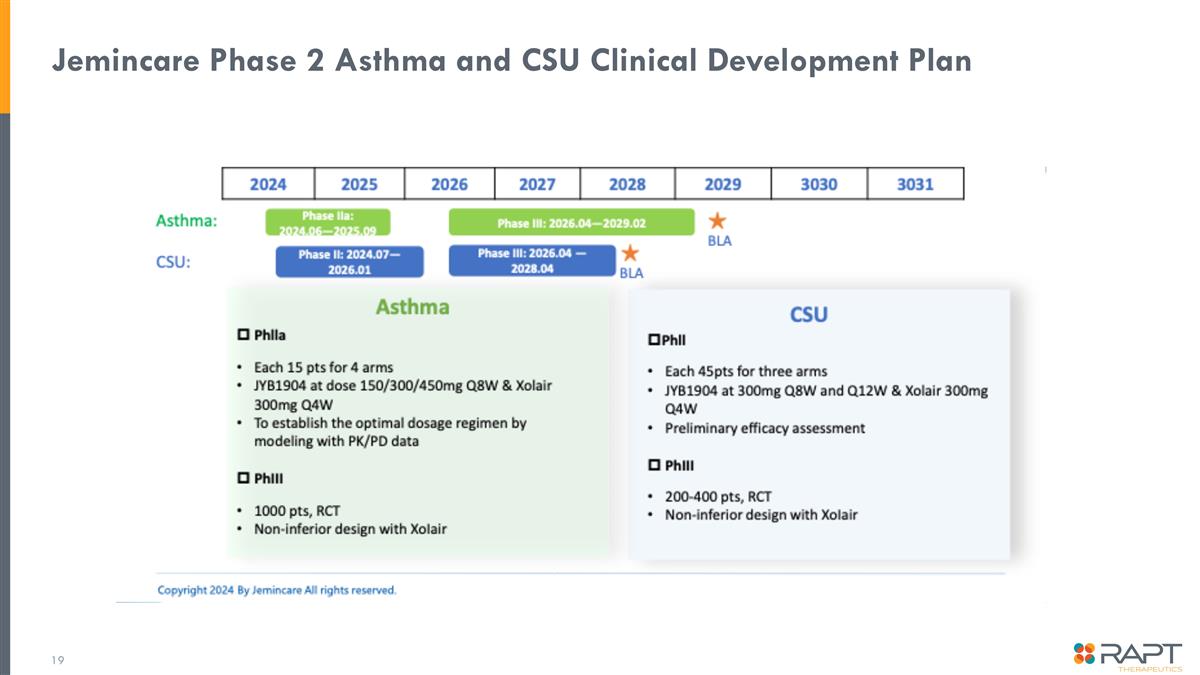
Jemincare Phase 2 Asthma and CSU Clinical Development Plan
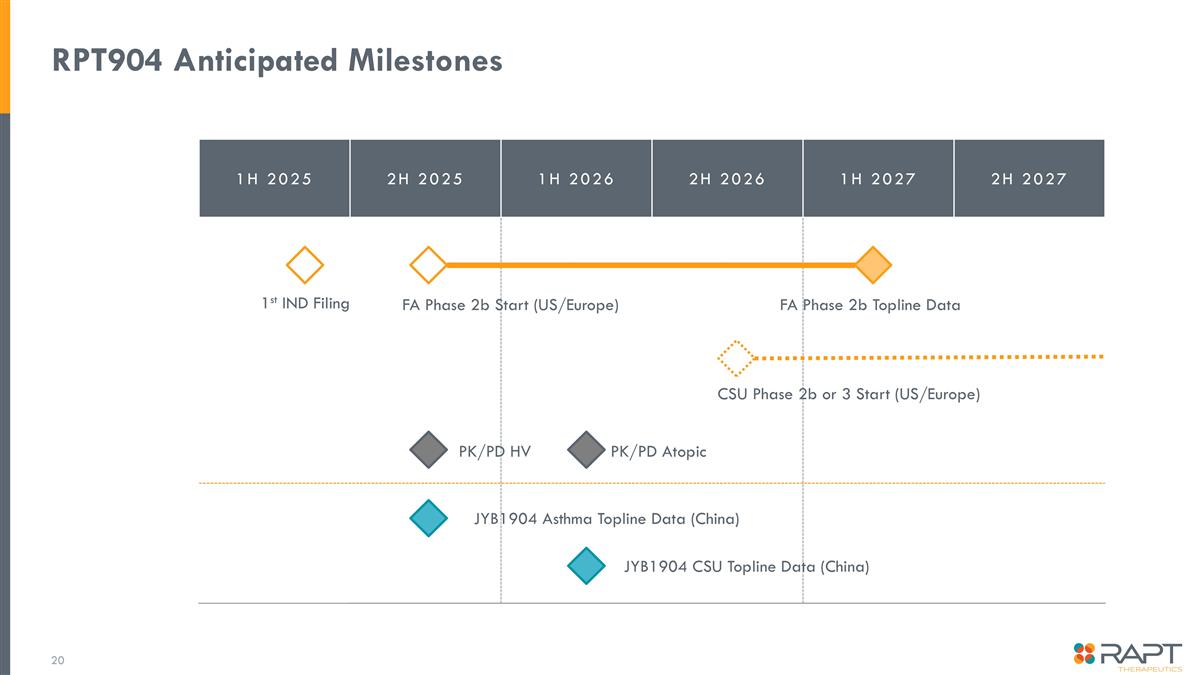
RPT904 Anticipated Milestones 1H 2025 2H 2025 1H 2026 2H 2026 1H 2027 2H 2027 FA Phase 2b Start (US/Europe) FA Phase 2b Topline Data CSU Phase 2b or 3 Start (US/Europe) JYB1904 Asthma Topline Data (China) 1st IND Filing JYB1904 CSU Topline Data (China) PK/PD HV PK/PD Atopic

Thank You




















