concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical study sites and patient registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs. Small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. The key competitive factors affecting the success of all of our product candidates, if approved, are likely to be their efficacy, safety, convenience, price, the level of branded and generic competition, and the availability of reimbursement from government and other third-party payors.
NMDAr-targeted therapies
A number of pharmaceutical, biotechnology, and specialty pharmaceutical companies are developing therapies targeting NMDArs. Most of the therapies being developed are broad antagonists and tend to have multiple target actions while our compounds are truly modulatory and have not demonstrated any off-target activity in preclinical screening. We are aware of other companies developing or commercializing NMDAr-targeted therapies, including but not limited to, Acadia Pharmaceuticals Inc., Adamas Pharmaceuticals Inc., Allergan plc (now a subsidiary of AbbVie), Avanir Pharmaceuticals, Inc., Axsome Therapeutics, Inc., Biohaven Pharmaceutical Holding Co. Ltd., Cadent Therapeutics, Inc., Cerecor Inc., Eli Lilly and Company, Genentech Inc., Intra-Cellular Therapies, Inc., Janssen Pharmaceuticals, Inc., NeuroRx, Inc., Newron Pharmaceuticals S.p.A., Otonomy, Inc., Relmada Therapeutics, Inc., Sage Therapeutics, Inc., UCB S.A., Gate Neurosciences, Inc., and Vistagen Therapeutics, Inc.
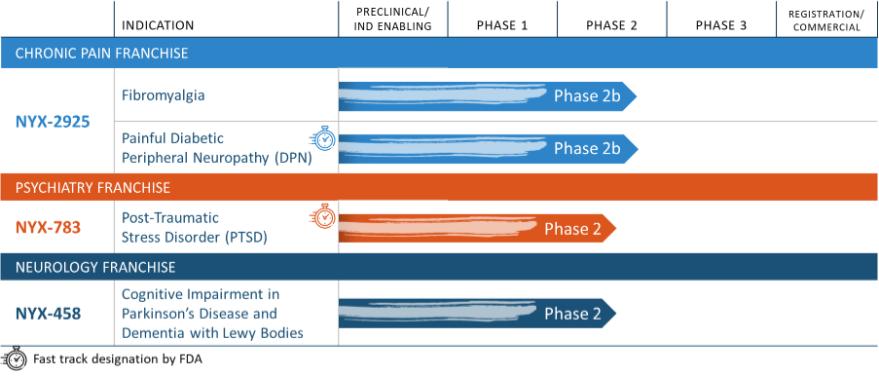
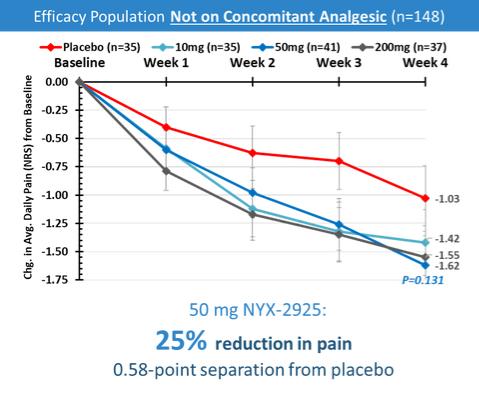
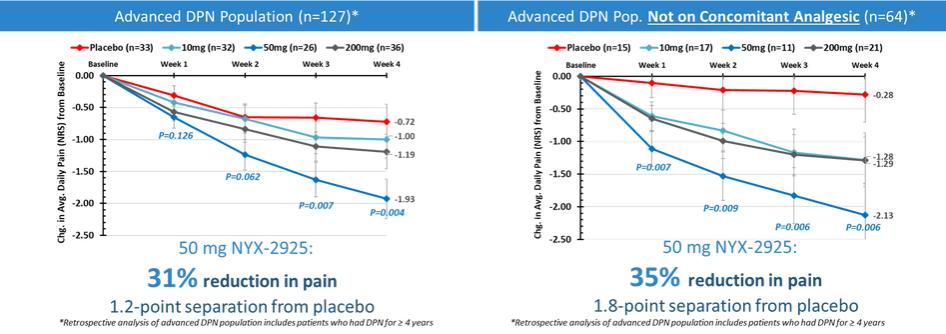
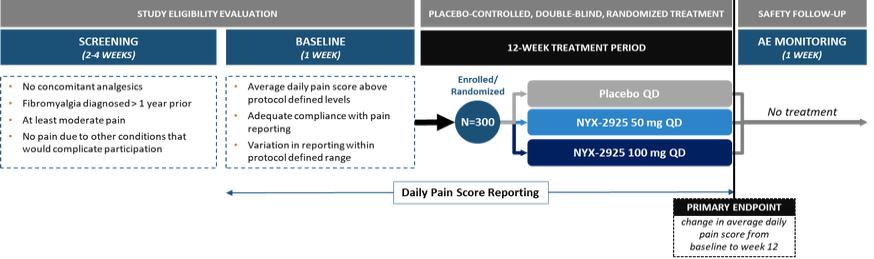
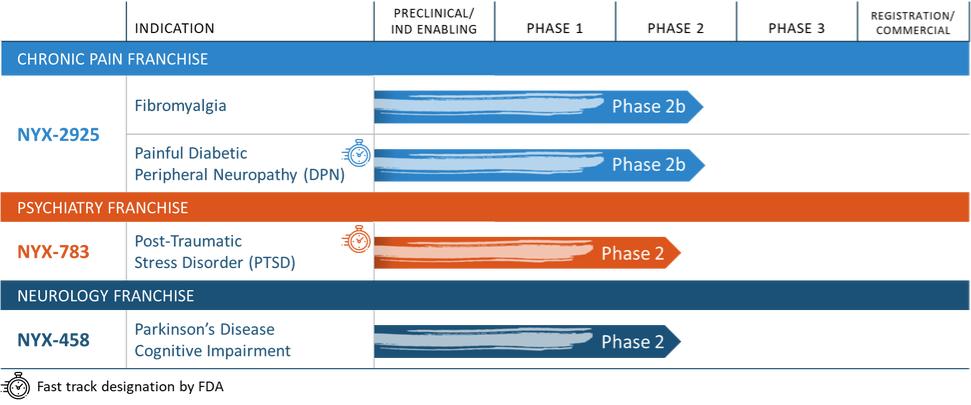
NYX-2925—neuropathic/chronic pain
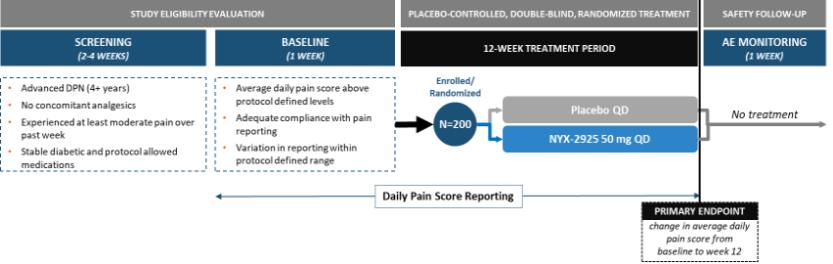
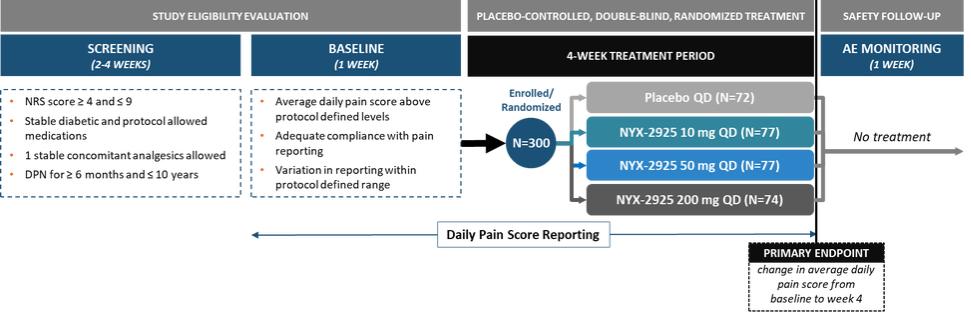
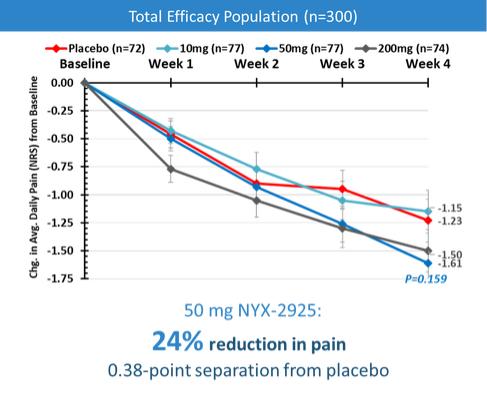
We expect that, if approved, NYX-2925 will compete with the currently approved therapies for painful DPN and fibromyalgia, including pregabalin, duloxetine, and tapentadol HCl. We are aware of a number of therapies that are approved to treat other types of neuropathic pain. We are also aware that various therapies are used off-label to treat neuropathic pain. In addition to the marketed therapies, we are aware of companies currently developing therapies for neuropathic pain, including Arbor Pharmaceuticals LLC, AstraZeneca Plc., Biogen Inc., Cara Therapeutics, Inc., Daiichi Sankyo Company, Eli Lilly and Company, GW Pharmaceuticals plc., Immune Pharmaceuticals Inc., NeuroBo Pharmaceuticals, Novaremed AG, Novartis AG, Vertex Pharmaceuticals Incorporated, and ViroMed Inc.
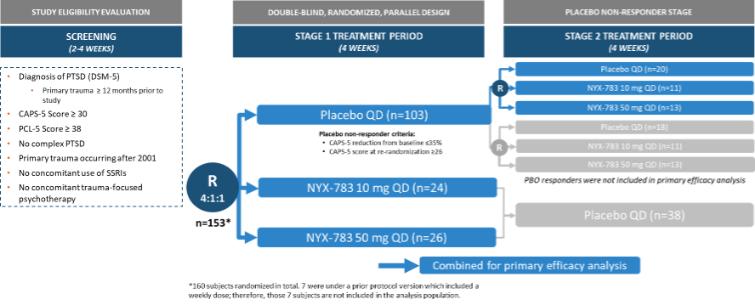
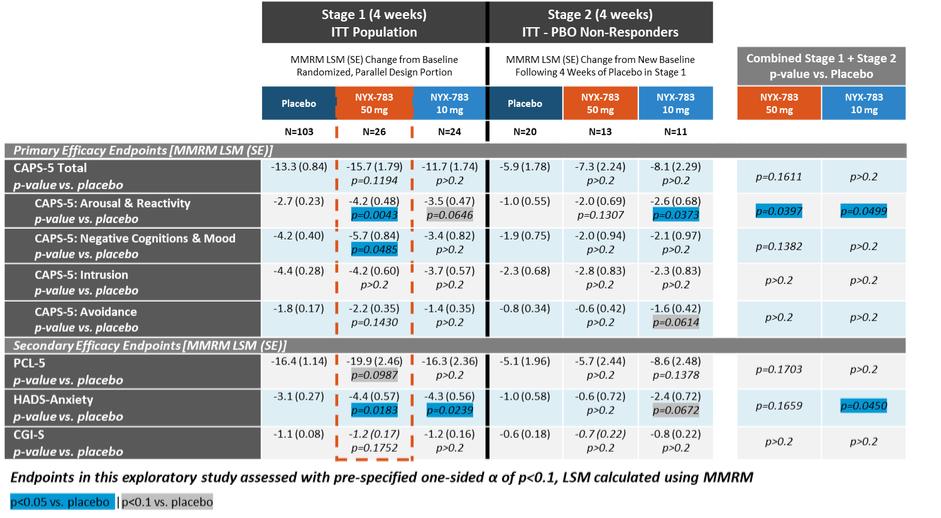
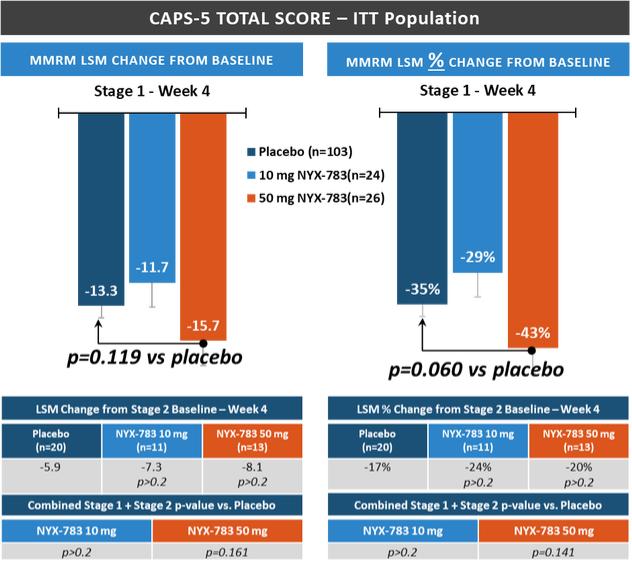
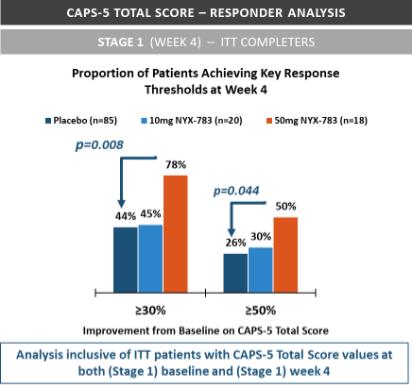
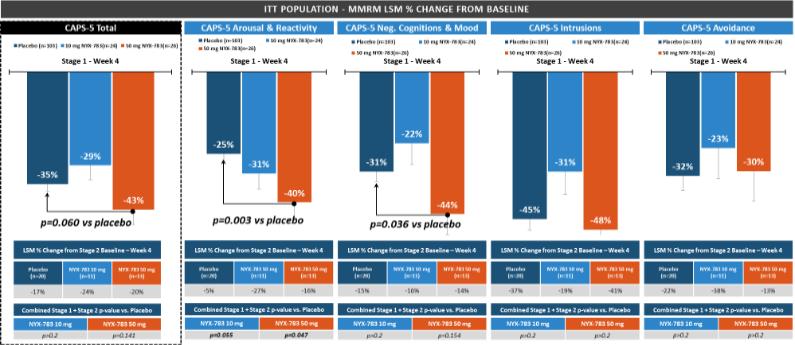
NYX-783—post-traumatic stress disorder
We expect that, if approved, NYX-783 will compete with currently approved therapies for PTSD, including paroxetine and sertraline. We are also aware of other companies developing therapies for PTSD, including but not limited to, Azevan Pharmaceuticals Inc., Bionomics Ltd., Otsuka Pharmaceutical Co., Ltd., SpringWorks Therapeutics, and Tonix Pharmaceuticals Holding Corp.
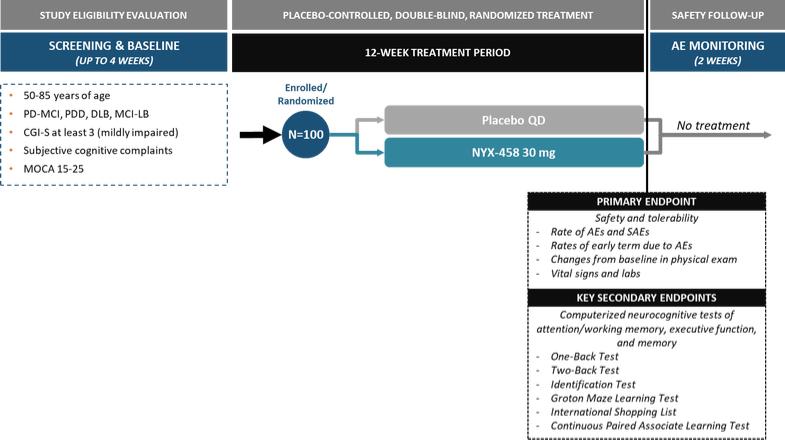
NYX-458—Parkinson’s disease cognitive impairment and dementia with Lewy bodies
We expect that, if approved, NYX-458 will compete with currently approved therapies for PDD, the only one of which in the United States is rivastigmine. We are also aware of other companies developing therapies for Parkinson’s disease cognitive impairment, including but not limited to, Anavex Life Sciences, Annovis Bio Inc, Eli Lilly and Company, Sage Therapeutics Inc, Takeda Pharmaceutical Co Ltd, and Eisai Pharmaceuticals Inc.