Changing lives with next generation peptide therapeutics Zealand Pharma Corporate Presentation January 2019

Forward-looking statements This presentation contains information pertaining to Zealand Pharma A/S (“Zealand"). Neither Zealand nor its management, directors, employees or representatives make any representation or warranty, express or implied, as to the accuracy or completeness of any of the information contained in this presentation or any other information transmitted or made available to the viewer or recipient hereof, whether communicated in written or oral form. This presentation does not constitute or form part of, and should not be construed as, an offer to sell or issue or the solicitation of an offer to buy or acquire Zealand securities, in any jurisdiction, or an inducement to enter into investment activity, nor shall there be any sale of Zealand securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. No part of this presentation, nor the fact of its distribution, should form the basis of, or be relied on in connection with, any contract or commitment or investment decision whatsoever. This presentation contains forward-looking statements that reflect management's current views with respect to Zealand's product candidates' development, clinical and regulatory timelines and anticipated results, market opportunity, potential financial performance and other statements of future events or conditions. Although Zealand believes that the expectations reflected in such forward-looking statements are reasonable, no assurance can be given that such expectations will prove to have been correct. Accordingly, results could differ materially from those set out in the forward-looking statements as a result of various factors, many of which are beyond Zealand’s control. No reliance should be made on such forward-looking statements. Zealand does not intend to update the presentation, including the forward-looking statements contained therein, following distribution, beyond what is required by applicable law or applicable stock exchange regulations if and when circumstances arise that lead to changes compared to the date when these statements were provided. 1

Zealand in brief Danish Biotech Founded in Copenhagen (HQ) in 1998 with U.S. subsidiary Leading Peptide Platform A world leading peptide platform, with two product candidates brought to market 4 Late Stage Programs Accelerating several late stage programs to launch new products into major markets in 2 to 4 years Expanding Capabilities Transforming into a fully integrated biopharma company with U.S. commercial organization Experienced team 155 employees of which 82% are in R&D Dual Nasdaq Listing Traded in Copenhagen and New York 2
2018 Achievements Glepaglutide for SBS: Phase 3 Program ongoing Dasiglucagon HypoPal® rescue pen: Positive results reported from two Phase 3 trials Dasiglucagon for congenital hyperinsulinism: Phase 3 program initiated Dasiglucagon for dual hormone pumps: New agreement with Beta Bionics to initiate Phase 2b in 2019 GLP-1/glucagon analog for obesity/ type 2 diabetes: Positive Phase 1a results reported1 Amylin analog for obesity/ type 2 diabetes : Decision to progress into Phase 1 in 2019 with new lead candidate1 GLP-1/GLP-2 dual agonist for SBS: Selected as new internal drug candidate to enter Phase 1 in 2019 USD 205m Royalty Deal: sold future royalties and related milestones from Sanofi to Royalty Pharma 3 1 Partnered with Boehringer Ingelheim

We change lives with next generation peptide therapeutics Changing Lives We work every day with patient communities and thought leaders to change the lives of patients with severe unmet medical needs Transforming Peptides We leverage our 20 years of leading R&D experience to transform peptides into next generation therapeutics Engaging Partnerships We engage with development and commercial partners to enhance innovation and expand opportunities across markets and therapeutics areas Approaching Commercialization We are building a fully integrated commercial organization with U.S. operations to market our own therapies for rare diseases 4
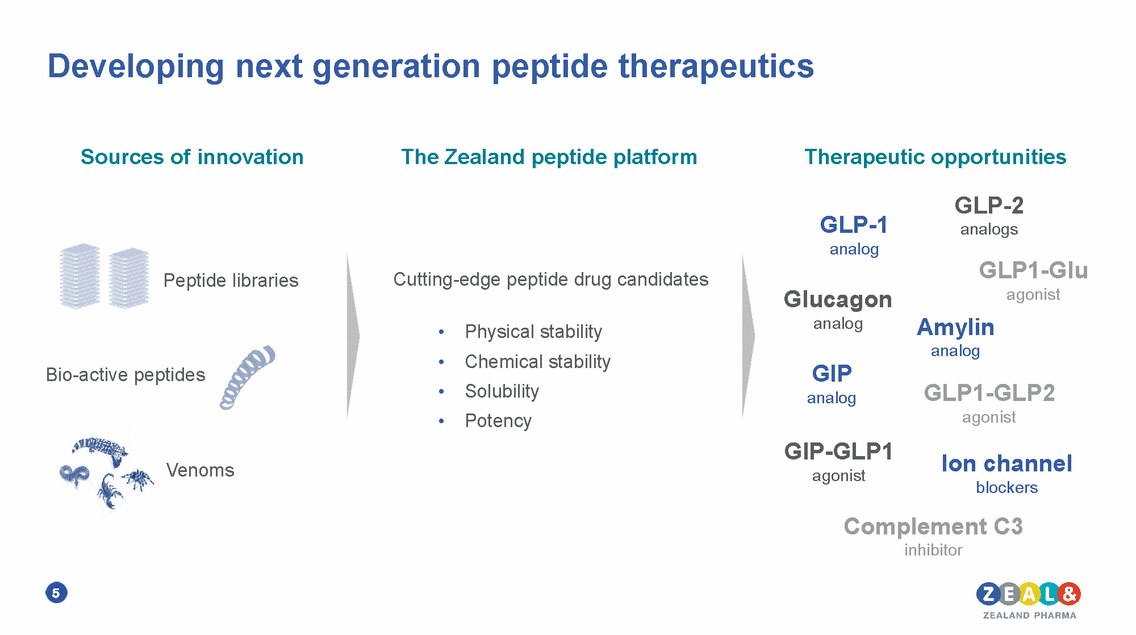
Developing next generation peptide therapeutics Sources of innovation The Zealand peptide platform Therapeutic opportunities GLP-2 analogs GLP-1 analog GLP1-Glu agonist Amylin analog Cutting-edge peptide drug candidates Peptide libraries Glucagon analog • • • • Physical stability Chemical stability Solubility Potency GIP analog Bio-active peptides GLP1-GLP2 agonist GIP-GLP1 agonist Ion channel blockers Venoms Complement C3 inhibitor 5
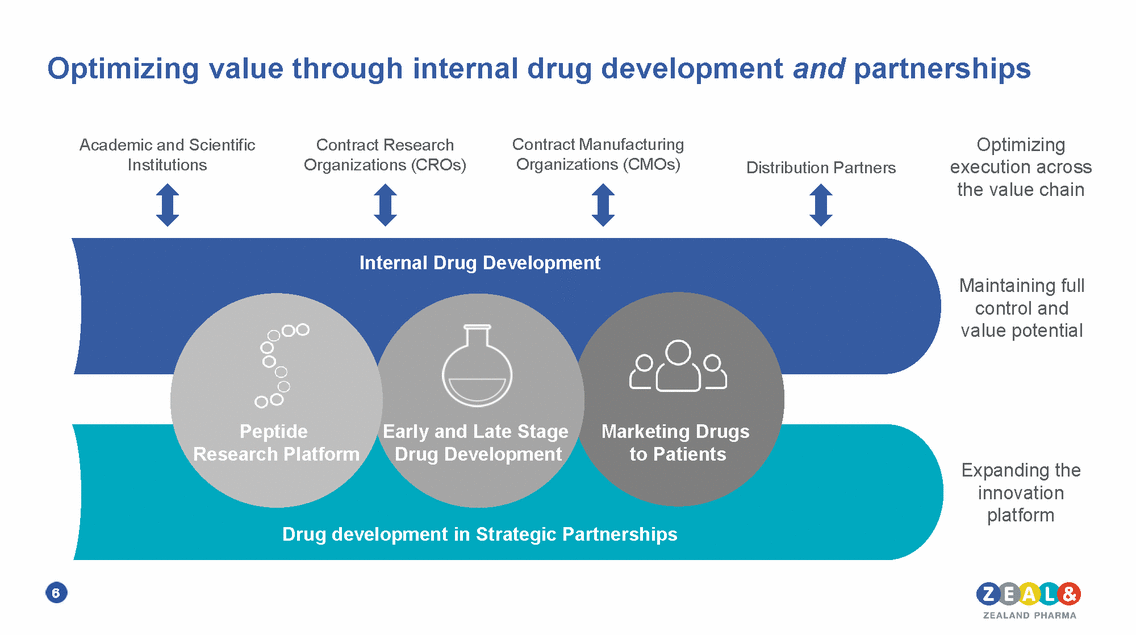
Optimizing value through internal drug development and partnerships Optimizing execution across the value chain Contract Manufacturing Organizations (CMOs) Academic and Scientific Institutions Contract Research Organizations (CROs) Distribution Partners Maintaining full control and value potential Expanding the innovation platform 6 PeptideEarly and Late StageMarketing Drugs Research PlatformDrug Developmentto Patients Drug development in Strategic Partnerships Internal Drug Development
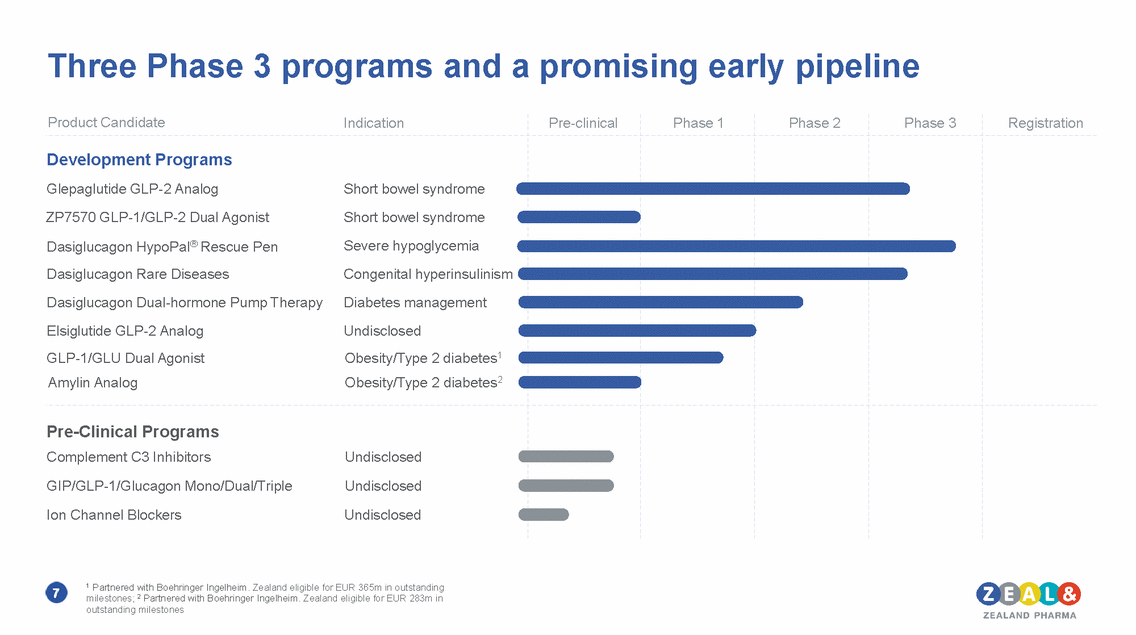
Three Phase 3 programs and a promising early pipeline 1 Partnered with Boehringer Ingelheim. Zealand eligible for EUR 365m in outstanding milestones; 2 Partnered with Boehringer Ingelheim. Zealand eligible for EUR 283m in outstanding milestones 7 Product CandidateIndication Pre-clinical Phase 1 Phase 2 Phase 3 Registration Development Programs Glepaglutide GLP-2 AnalogShort bowel syndrome ZP7570 GLP-1/GLP-2 Dual AgonistShort bowel syndrome Dasiglucagon HypoPal® Rescue PenSevere hypoglycemia Dasiglucagon Rare DiseasesCongenital hyperinsulinism Dasiglucagon Dual-hormone Pump TherapyDiabetes management Elsiglutide GLP-2 AnalogUndisclosed GLP-1/GLU Dual AgonistObesity/Type 2 diabetes1 Amylin AnalogObesity/Type 2 diabetes2 Pre-Clinical Programs Complement C3 InhibitorsUndisclosed GIP/GLP-1/Glucagon Mono/Dual/TripleUndisclosed Ion Channel BlockersUndisclosed
Marianne lives with short bowel syndrome and is dependent on daily parenteral support I \ I I It I ZEALAND PHARMA

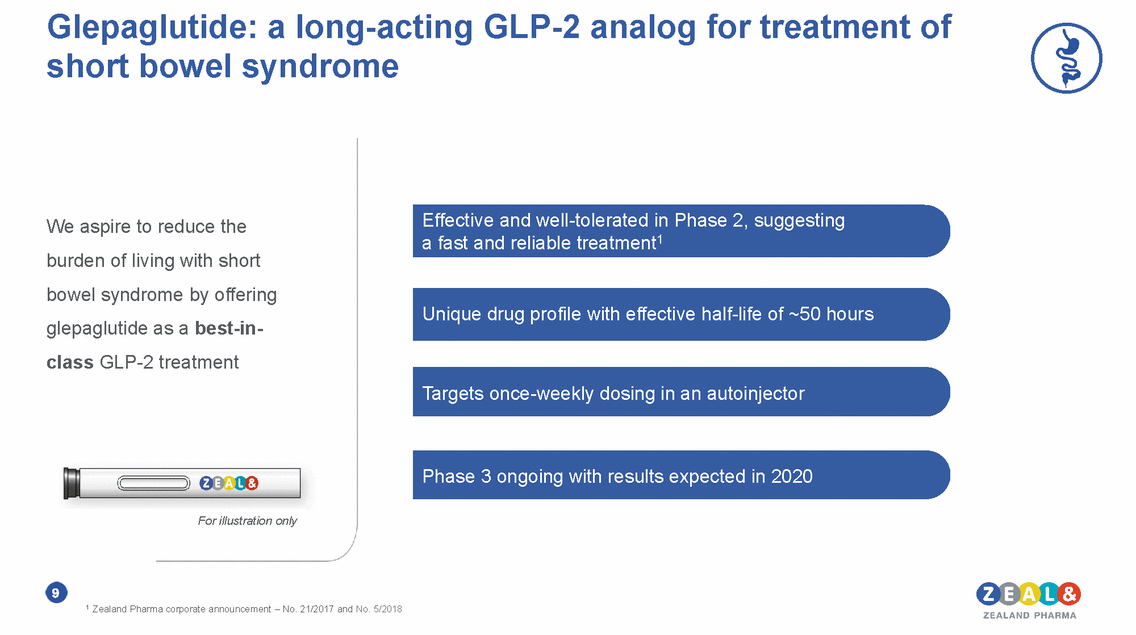
Glepaglutide: a long-acting GLP-2 analog for treatment short bowel syndrome of Effective and well-tolerated in Phase 2, suggesting a fast and reliable treatment1 We aspire to reduce the burden of living with short bowel syndrome by offering glepaglutide as a best-in-class GLP-2 treatment Unique drug profile with effective half-life of ~50 hours Targets once-weekly dosing in an autoinjector Phase 3 ongoing with results expected in 2020 For illustration only 9 1 Zealand Pharma corporate announcement – No. 21/2017 and No. 5/2018
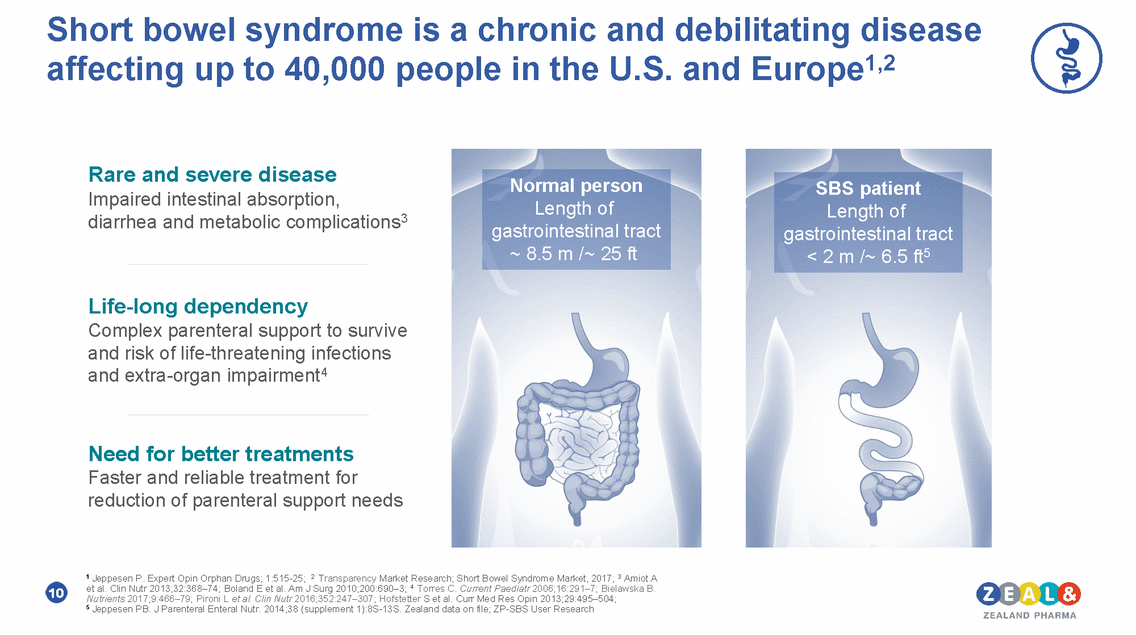
Short bowel syndrome is a chronic and debilitating disease Europe1,2 affecting up to 40,000 people in the U.S. and Rare and severe disease Impaired intestinal absorption, diarrhea and metabolic complications 3 Life-long dependency Complex parenteral support to survive and risk of life-threatening infections and extra-organ impairment4 Need for better treatments Faster and reliable treatment for reduction of parenteral support needs 1 Jeppesen P. Expert Opin Orphan Drugs; 1:515-25; 2 Transparency Market Research; Short Bowel Syndrome Market, 2017; 3 Amiot A et al. Clin Nutr 2013;32:368–74; Boland E et al. Am J Surg 2010;200:690–3; 4 Torres C. Current Paediatr 2006;16:291–7; Bielawska B. Nutrients 2017;9:466–79; Pironi L et al. Clin Nutr 2016;352:247–307; Hofstetter S et al. Curr Med Res Opin 2013;29:495–504; 5 Jeppesen PB. J Parenteral Enteral Nutr. 2014;38 (supplement 1):8S-13S. Zealand data on file; ZP-SBS User Research 10 SBS patient Length of gastrointestinal tract < 2 m /~ 6.5 ft5 Normal person Length of gastrointestinal tract ~ 8.5 m /~ 25 ft
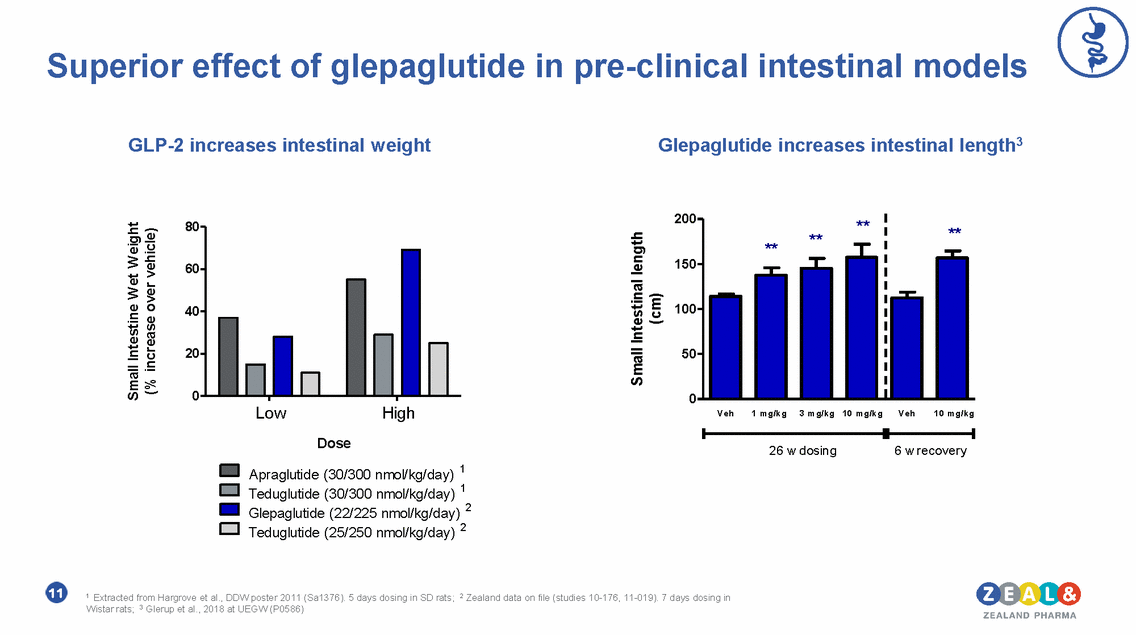
Superior effect of glepaglutide in pre-clinical intestinal models GLP-2 increases intestinal weight Glepaglutide increases intestinal length3 200 ** 80 ** ** ** 150 60 100 40 50 20 0 0 Low High Veh 1 mg/kg 3 mg/kg 10 mg/kg Veh 10 mg/kg Dose Apraglutide (30/300 nmol/kg/day) 1 Teduglutide (30/300 nmol/kg/day) 1 Glepaglutide (22/225 nmol/kg/day) 2 Teduglutide (25/250 nmol/kg/day) 2 26 w dosing 6 w recovery 26 week dosing followed by 6 week recovery in Wistar rats Data presented Glerup et al., 2018 at UEGW (P0586) 1-5 days dosing SD rats. Extracted from Hargrove et al., DDW poster 2011 (Sa1376) 2-7 day dosing in Wistar rats. Data on file at Zealand (studies 10-176, 11-019 ) 1 Extracted from Hargrove et al., DDW poster 2011 (Sa1376). 5 days dosing in SD rats; 2 Zealand data on file (studies 10-176, 11-019). 7 days dosing in Wistar rats; 3 Glerup et al., 2018 at UEGW (P0586) 11 Small Intestine W et W eight (% increase over vehicle) Small Intestinal length (cm)
Strong Phase 2 data with clinically significant increases in intestinal absorption following 3 weeks’ glepaglutide treatment Change in wet weight absorption (g/day)1 Clear dose-response on multiple endpoints1 • Significant increase in intestinal fluid and energy absorption • Significant reduction in fecal wet weight output • Significant increase in urine production • Significant increase in body weight • Safe and well-tolerated • Dose-response provided basis for Phase 3 dose selection Dose 12 1 Naimi, R., ASPEN 2018 Nutrition Science and Practice Conference (Abstract number 2829969t). Mean Baseline Wet Weight Absorption (g/day) 525538288

Pivotal Phase 3 trial evaluates once-and twice-weekly glepaglutide dosing over 24 weeks – results expected in 2020 Trial design1 • Double-blind, placebo controlled trial in 129 SBS patients evaluating safety and efficacy of twice and once weekly dosing over 24 weeks ScreSecrneeinnging RRun-inin TreTartematmenent t FoFlololloww--up Primary and key secondary endpoints • Reduction in weekly parenteral support (PS) volume > 20% reduction in PS volume Reduction in weekly days on PS • • -8 to -2 0 4 8 12 16 20 24 28 WeeWkesekfrsofmromfirfisrsttstrceraetemneinntg 13 1 www.clinicaltrials.gov 1:1:1 randomization 1:1:1 randomization GlepagGluletipdaeg1lu0timdegttwwiciceeawweekkly (43 subjects) GlepaGgluptidagelu10tidmegonocnecea weeekly (43 subjects) PlacebPola(c4e3bsoubjects)

Opportunity to take majority share of a potential > $1.5 billion market with glepaglutide Estimated number of treated SBS patients and market value potential across major markets Major growth drivers for GLP-2 treatments Increasing SBS patient prevalence5 Improving GLP-2 treatments options Improving therapy adherence Glepaglutide as potential best-in-class long acting GLP-2 analog 1 2018 patient and value estimate based on Shire PLC. Q3 2018 results presentation, based on Truven Redbook, WAC and 20-25% discount; 2 2025 forecast based on Transparency Market Research; Short Bowel Syndrome Market, 2017. Number of patients estimated by dividing with U.S. average price; 3 2025 forecast based on Zealand feasibility study 2018 and annual expected growth of 5%. Value based on existing GLP-2 WAC price; 4 No GLP-2 treatment currently approved in Japan. Patient forecast based on MHLW estimate. Price level to be defined by first GLP-2 introduction; 5 SBS prevalence doubled in one decade due to increased awareness and improved care (Brandt, 2016, Journal of Parenteral and Enteral Nutrition) 14 RoW Potential to be determined EU51,3 # PatientsValue 2018300$ ~0.1b 2025>2,000$ >0.4b Japan4 # PatientsValue 2018 0-2025>1,000TBD The US1,2 # PatientsValue 20181.000$ ~0.4b 2025>4,000$ >1.0b
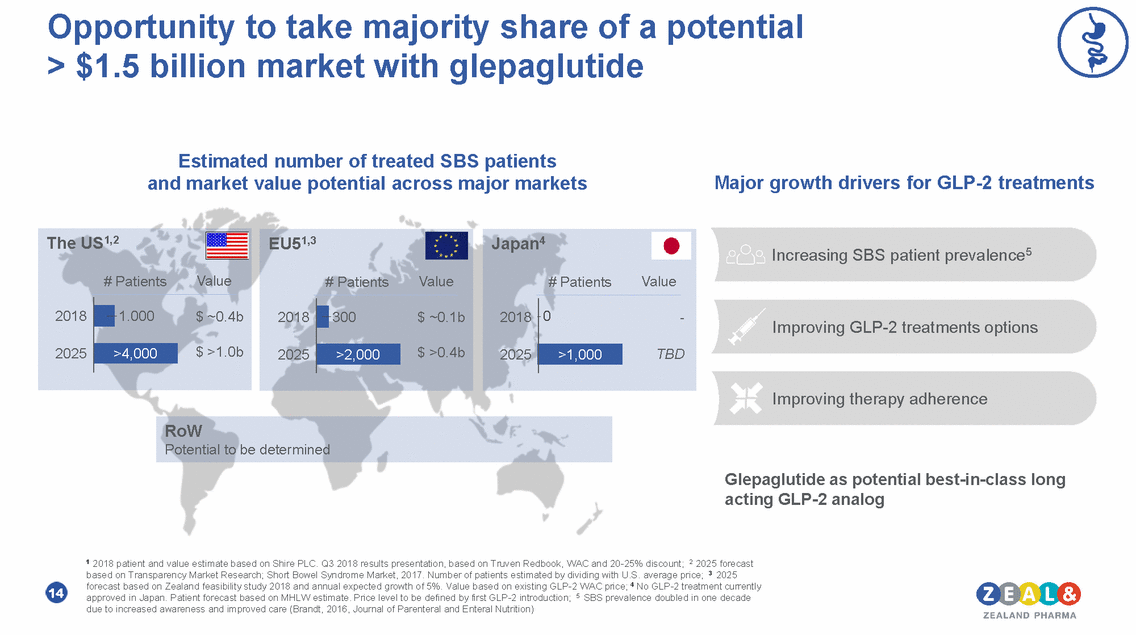
ZP7570 as next generation therapy for patients with short bowel syndrome – Phase 1 initiation in 2019 Superior pre-clinical effects on small intestine surface area2 ZP7570 • Novel long-acting GLP-1/ GLP-2 dual peptide agonist Once weekly dosing Concept proven in SBS patients1 IP protection until at least 2037 Vehicle GLP-2 agonist GLP1/GLP-2 dual agonist • • • 1000 *** 800 600 400 200 0 15 1 Hvistendahl et al, 2015, Gastroenterology 148(4):S-188-S-189, April 2015; Madsen et al, Regulatory Peptides 184 (2013) 30-39. Liraglutide tested in 8 SBS patients with 8 weeks dosing; 2 Zealand data on file Sma l Small intestine - Total surface area l inte stine - Tota l surfa ce a re a (cm 2 SEM) ***
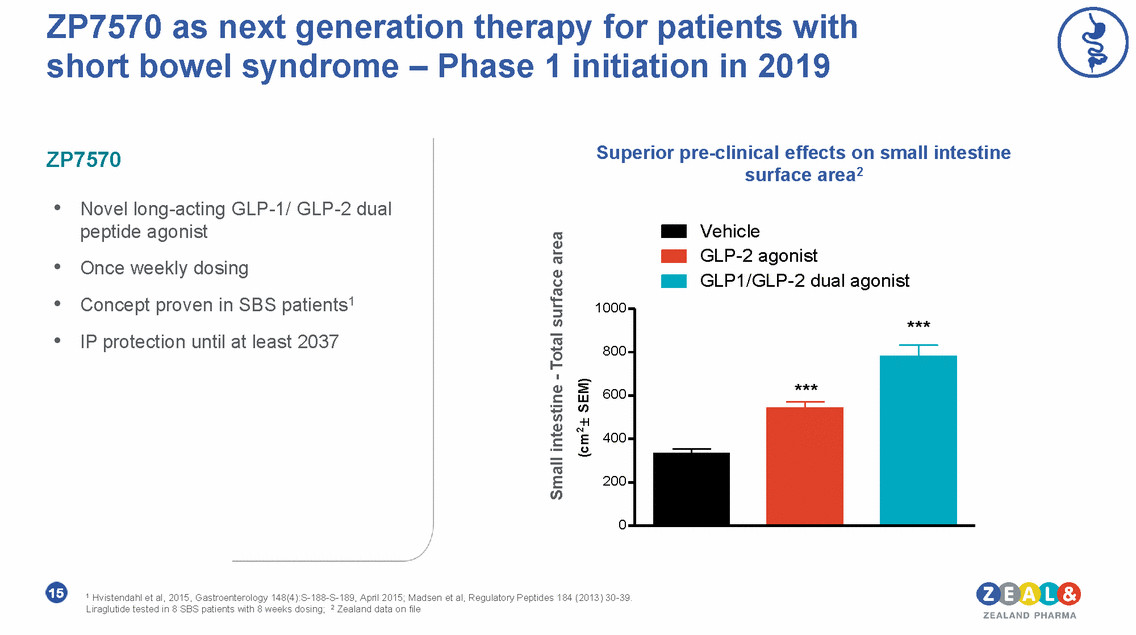
Dasiglucagon • • • • Novel stable glucagon analog Fast onset-of-action Unique stability in liquid formulation Suitable for multiple indication Diabetes care HypoPal® rescue pen for severe hypoglycemia Positive results in pivotal Phase 3 For illustration only Automated diabetes care with a dual hormone pump Phase 2b ready Rare disease Pump treatment for recurrent low blood glucose in congenital hyperinsulinism (CHI) Phase 3 initiated 16
Dasiglucagon has the potential to be the fastest-acting rescue treatment for severe hypoglycemia

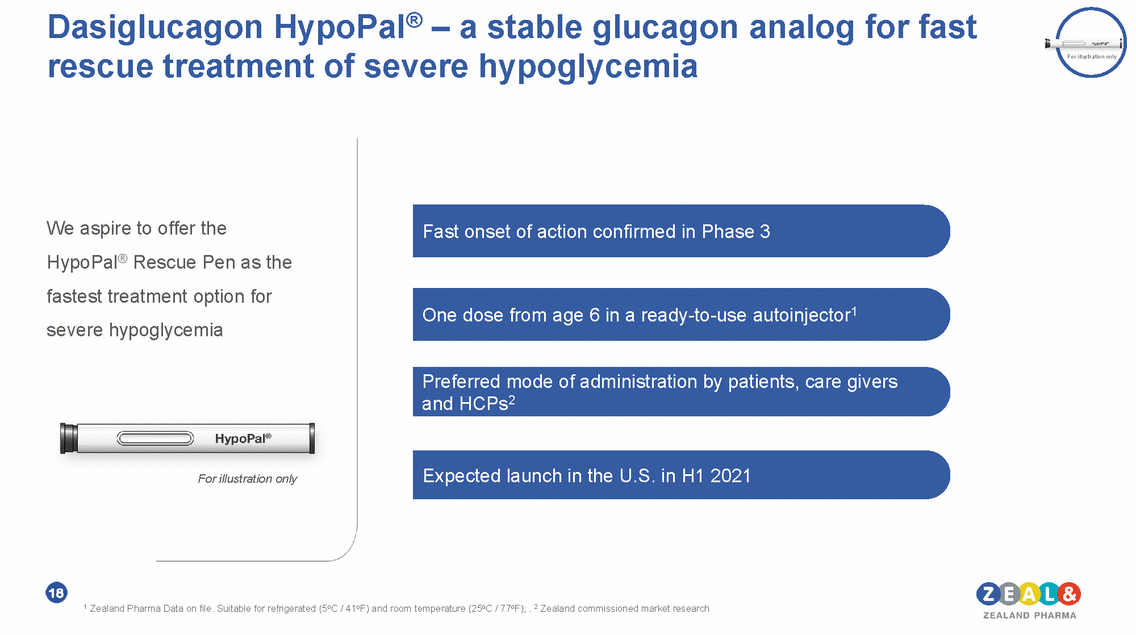
Dasiglucagon HypoPal® – a stable glucagon analog for fast rescue treatment of severe hypoglycemia For illustration only We aspire to offer the HypoPal® Rescue Pen as the fastest treatment option for severe hypoglycemia Fast onset of action confirmed in Phase 3 One dose from age 6 in a ready-to-use autoinjector1 Preferred mode of administration by patients, care givers and HCPs2 Expected launch in the U.S. in H1 2021 For illustration only 18 1 Zealand Pharma Data on file. Suitable for refrigerated (5oC / 41oF) and room temperature (25oC / 77oF); . 2 Zealand commissioned market research
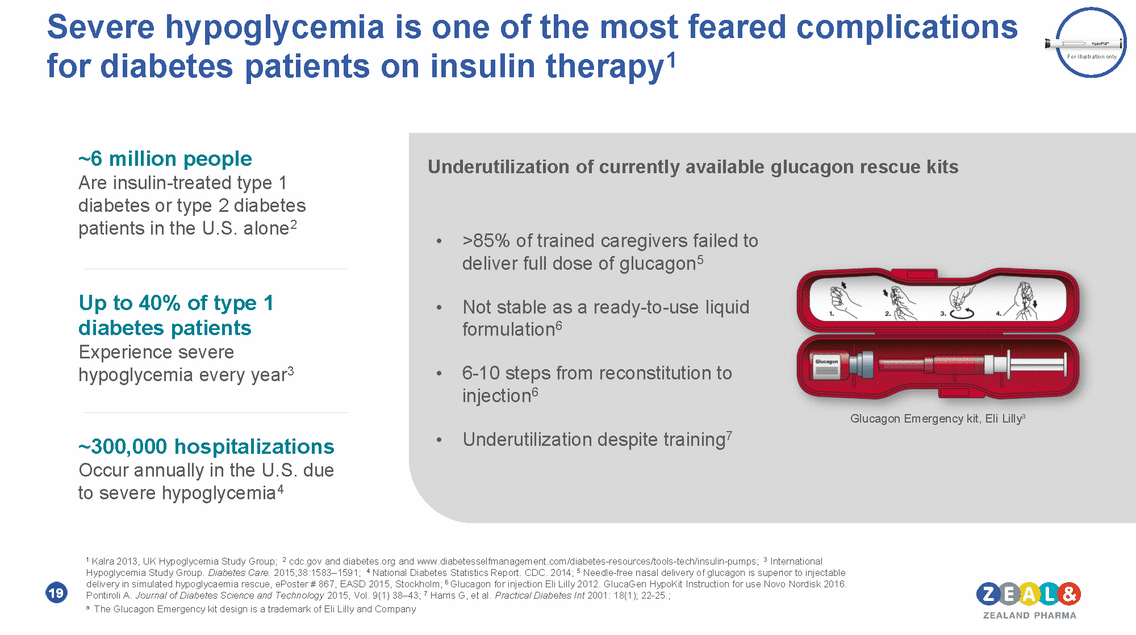
Severe hypoglycemia is one of the most feared complications insulin therapy1 for diabetes patients on For illustration only ~6 million people Are insulin-treated type 1 diabetes or type 2 diabetes patients in the U.S. alone2 Underutilization of currently available glucagon rescue kits • >85% of trained caregivers failed to deliver full dose of glucagon5 Up to 40% of type 1 diabetes patients Experience severe hypoglycemia every year3 • Not stable as a ready-to-use liquid formulation6 • 6-10 steps from reconstitution to injection6 Glucagon Emergency kit, Eli Lillya • Underutilization despite training7 ~300,000 hospitalizations Occur annually in the U.S. due to severe hypoglycemia4 1 Kalra 2013, UK Hypoglycemia Study Group; 2 cdc.gov and diabetes.org and www.diabetesselfmanagement.com/diabetes-resources/tools-tech/insulin-pumps; 3 International Hypoglycemia Study Group. Diabetes Care. 2015;38:1583–1591; 4 National Diabetes Statistics Report. CDC. 2014; 5 Needle-free nasal delivery of glucagon is superior to injectable delivery in simulated hypoglycaemia rescue, ePoster # 867, EASD 2015, Stockholm; 6 Glucagon for injection Eli Lilly 2012. GlucaGen HypoKit Instruction for use Novo Nordisk 2016. Pontiroli A. Journal of Diabetes Science and Technology 2015, Vol. 9(1) 38–43; 7 Harris G, et al. Practical Diabetes Int 2001: 18(1); 22-25.; a The Glucagon Emergency kit design is a trademark of Eli Lilly and Company 19
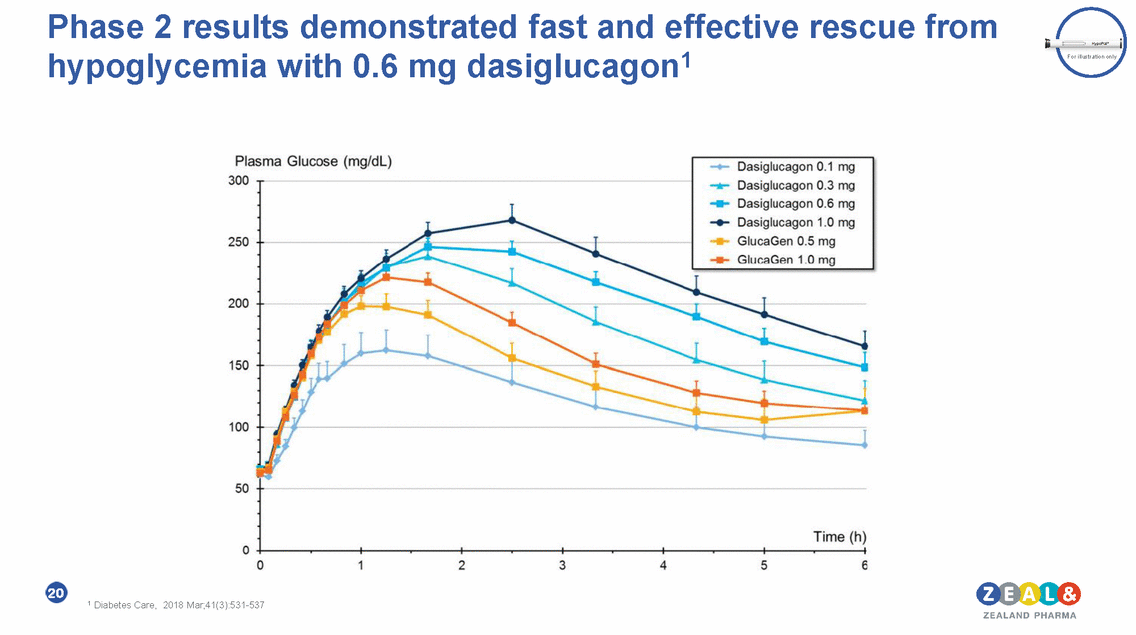
Phase 2 results demonstrated fast and effective rescue from dasiglucagon1 hypoglycemia with 0.6 mg Plasma Glucose (mg/d ) 300 250 200 150 100 50 Time (h) 0 -----+----1 --+------------- ---- ------ 5 0 2 3 4 6 1 Diabetes Care. 2018 Mar:41(3)531-537 ZEALAND PHARMA Dasiglucagon 0.1 mg .....-Dasiglucagon 0.3 mg .,... Dasiglucagon 0.6 mg ..... Dasiglucagon 1.0 mg GlucaGen 0.5 mg
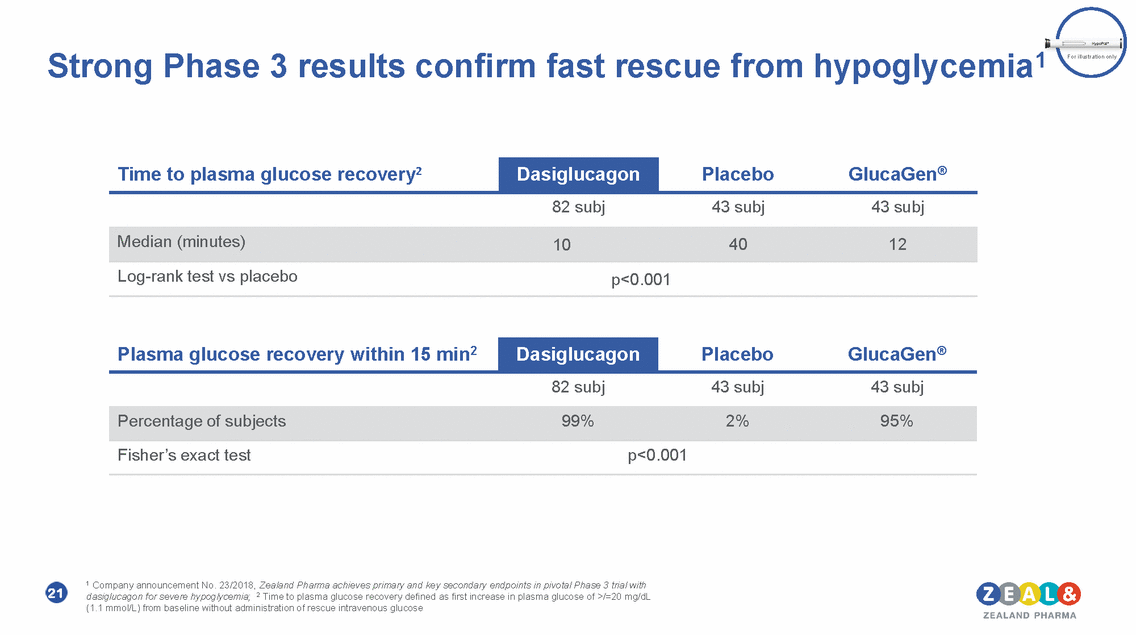
hypoglycemia1 Strong Phase 3 results confirm fast rescue from For illustration only 82 subj 43 subj 43 subj Log-rank test vs placebo p<0.001 82 subj 43 subj 43 subj Fisher’s exact test p<0.001 1 Company announcement No. 23/2018, Zealand Pharma achieves primary and key secondary endpoints in pivotal Phase 3 trial with dasiglucagon for severe hypoglycemia; 2 Time to plasma glucose recovery defined as first increase in plasma glucose of >/=20 mg/dL (1.1 mmol/L) from baseline without administration of rescue intravenous glucose 21 Percentage of subjects99%2%95% Plasma glucose recovery within 15 min2 Dasiglucagon PlaceboGlucaGen® Median (minutes)104012 Time to plasma glucose recovery2 Dasiglucagon PlaceboGlucaGen®
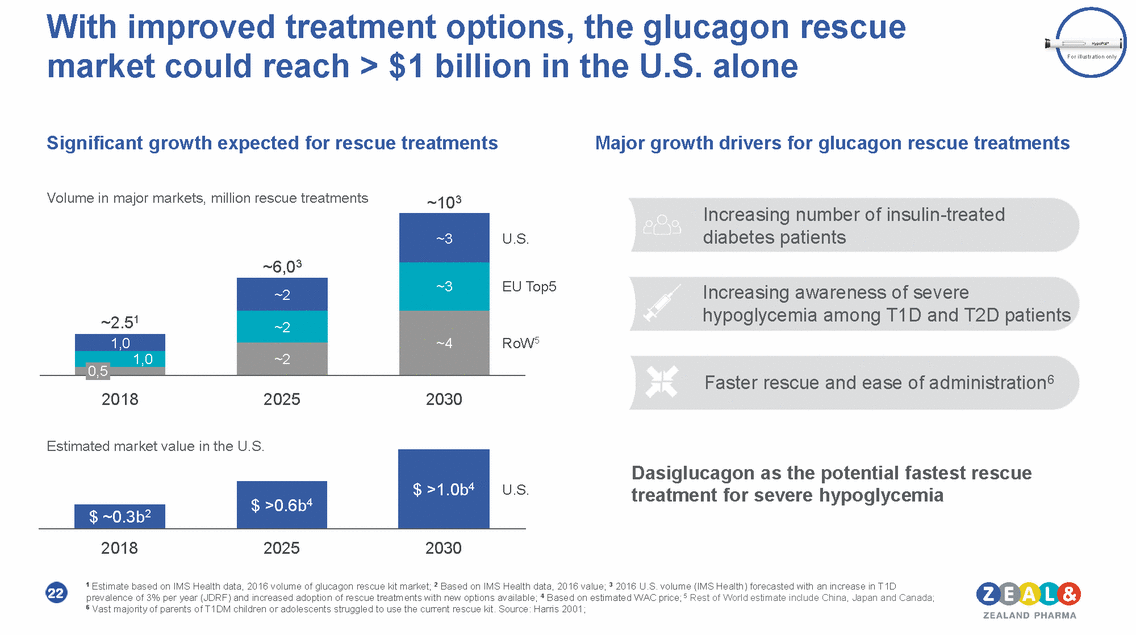
With improved treatment options, the glucagon rescue market could reach > $1 billion in the U.S. alone For illustration only Significant growth expected for rescue treatments Major growth drivers for glucagon rescue treatments Volume in major markets, million rescue treatments ~103 Increasing number of insulin-treated diabetes patients . Top5 Increasing awareness of severe hypoglycemia among T1D and T2D patients W5 Faster rescue and ease of administration6 2018 2025 2030 Estimated market value in the U.S. Dasiglucagon as the potential fastest rescue treatment for severe hypoglycemia . 2018 2025 2030 1 Estimate based on IMS Health data, 2016 volume of glucagon rescue kit market; 2 Based on IMS Health data, 2016 value; 3 2016 U.S. volume (IMS Health) forecasted with an increase in T1D prevalence of 3% per year (JDRF) and increased adoption of rescue treatments with new options available; 4 Based on estimated WAC price; 5 Rest of World estimate include China, Japan and Canada; 6 Vast majority of parents of T1DM children or adolescents struggled to use the current rescue kit. Source: Harris 2001; 22 $ >1.0b4 U.S $ >0.6b4 $ ~0.3b2 ~6,03 ~3 U.S EU Ro ~3 ~2.51 ~2 ~4 ~2 1,0 ~2 1,0 0,5
Improving the lives of insulin-dependent diabetic patients with dasiglucagon for fully automated diabetes care 23

Dasiglucagon could be the first to market glucagon analog for fully automated diabetes care 2 Glucose sensor/CGM2 We aspire to launch dasiglucagon as the first stable glucagon analog for fully automated diabetes care Pre-filled insulin and glucagon cartridges 3 Integrated insulin and glucagon pump and infusion set1 1 Dasiglucagon 4 mg/ml 24 1 Example of pump device – www.betabionics.com; 2 Example of sensor devise – www.dexcom.com Dasiglucagon Insulin
Phase 2 initiation with leading dual hormone pump developer Dasiglucagon for dual hormone pumps • Non-exclusive partnership with pump developer Beta Bionics1 • Beta Bionics received IDE approval from FDA in Q2 2018 • Phase 2 trial in the iLet™ Gen 3.2 pump device expected to complete in H1 2019 + • Aim for Phase 3 trial initiation in 2020 The iLet™ device GEN 41 Dasiglucagon 4 mg/ml 25 1 www.betabionics.com; Beta Bionics closed $ 63 million Series B financing Dec 2018
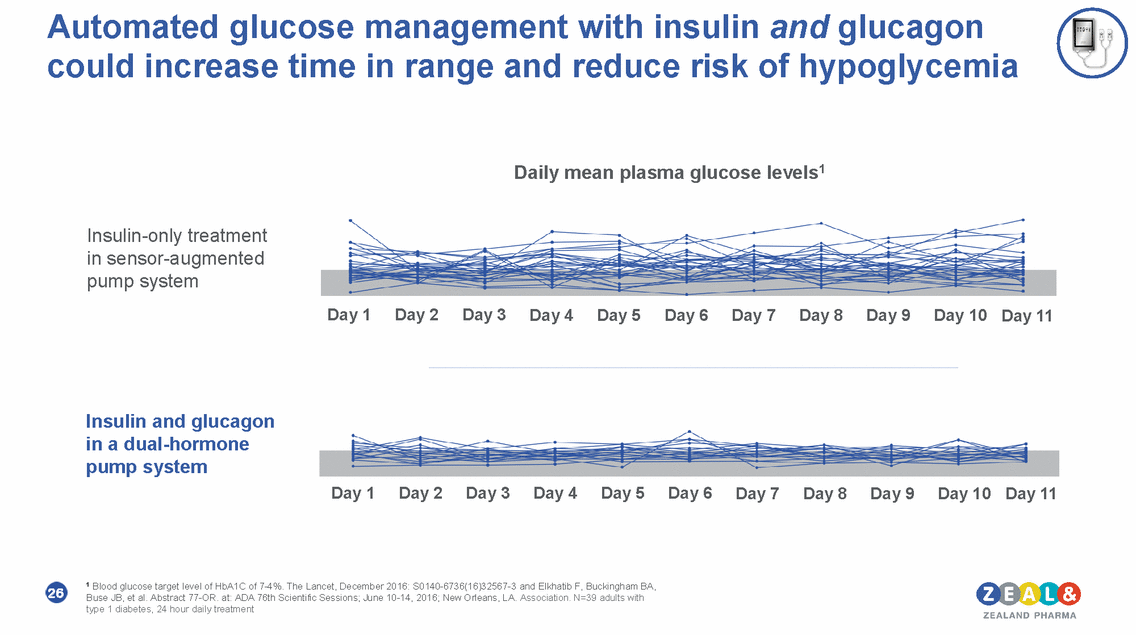
Automated glucose management with insulin and glucagon could increase time in range and reduce risk of hypoglycemia Daily mean plasma glucose levels1 Insulin-only treatment in sensor-augmented pump system Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day 8 Day 9 Day 10 Day 11 Insulin and glucagon in a dual-hormone pump system Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day 8 Day 9 Day 10 Day 11 1 Blood glucose target level of HbA1C of 7-4%. The Lancet, December 2016: S0140-6736(16)32567-3 and Elkhatib F, Buckingham BA, Buse JB, et al. Abstract 77-OR. at: ADA 76th Scientific Sessions; June 10-14, 2016; New Orleans, LA. Association. N=39 adults with type 1 diabetes, 24 hour daily treatment 26
Chronic toxicology of long-term usage studies support human testing of dasiglucagon effects to native glucagon1 Long-term safety and tolerability study of dasiglucagon1 Dasiglucagon demonstrate similar physiological • 26 and 39 week toxicity studies in rats and dogs • Chronic administration was not associated with organ toxicity • All findings were consistent with known physiological effects on glucagon 27 1 J. Castle et al, poster at ADA 2018, Long-term Safety and Tolerability Study of Dasiglucagon, a Stable-in-Solution Glucagon analog

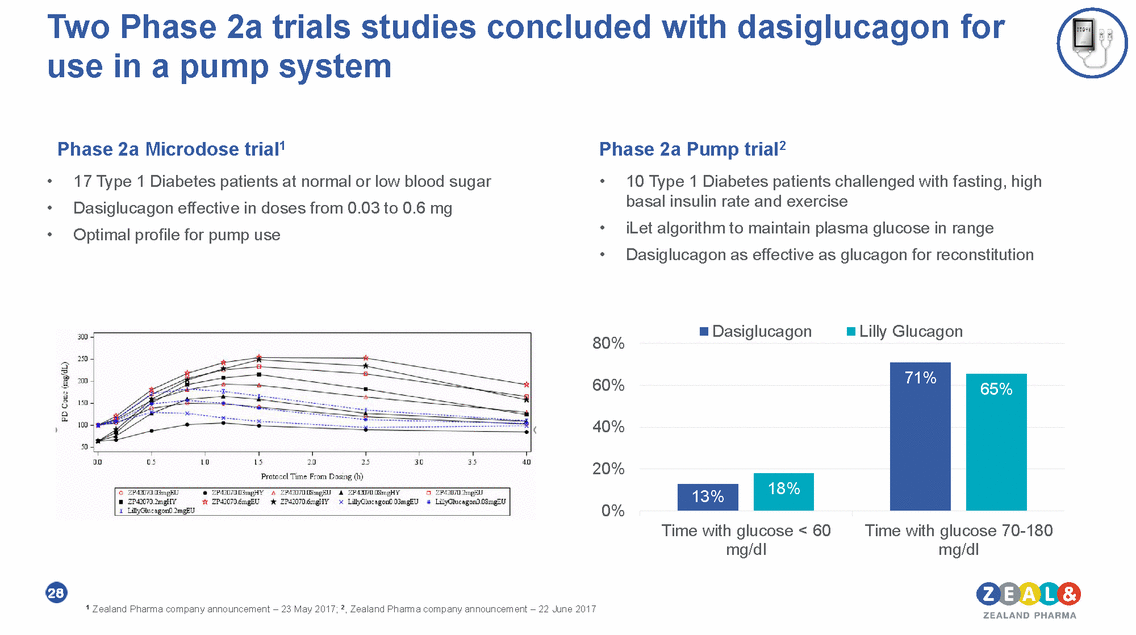
Two Phase 2a trials studies concluded with dasiglucagon for use in a pump system Phase 2a Microdose trial1 17 Type 1 Diabetes patients at normal or low blood sugar Dasiglucagon effective in doses from 0.03 to 0.6 mg Optimal profile for pump use Phase 2a Pump trial2 • • • • 10 Type 1 Diabetes patients challenged with fasting, high basal insulin rate and exercise iLet algorithm to maintain plasma glucose in range Dasiglucagon as effective as glucagon for reconstitution • • Dasiglucagon Lilly Glucagon 80% 60% 40% 20% 0% Time with glucose < 60 mg/dl Time with glucose 70-180 mg/dl 28 1 Zealand Pharma company announcement – 23 May 2017; 2, Zealand Pharma company announcement – 22 June 2017 71% 65% 18% 13%
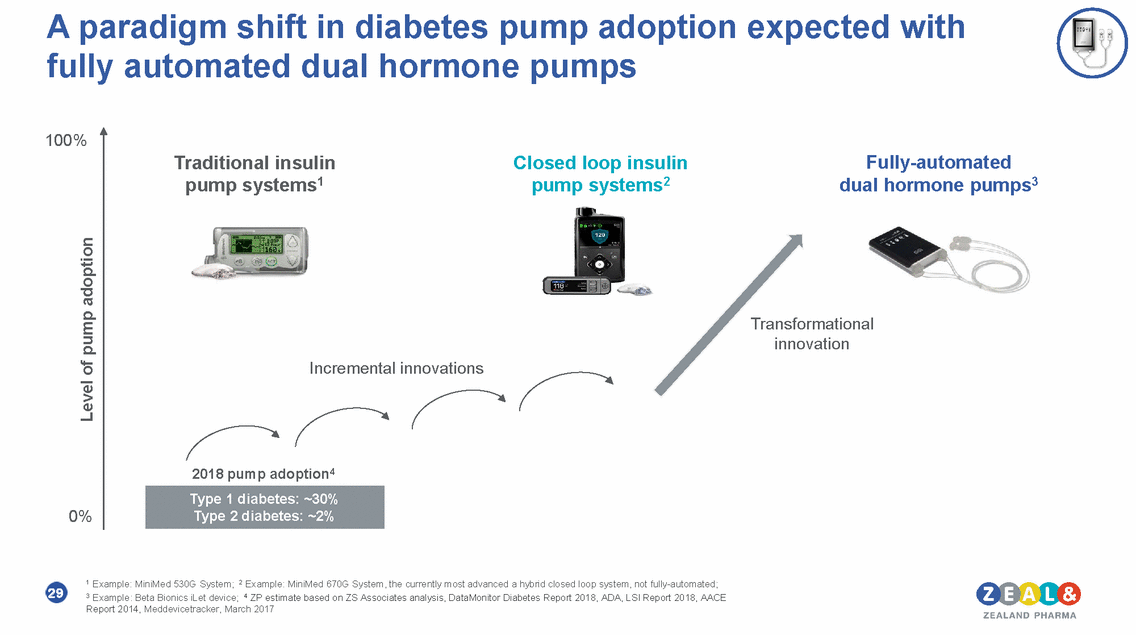
A paradigm shift in diabetes pump adoption expected with fully automated dual hormone pumps 100% Traditional insulin pump systems1 Closed loop insulin pump systems2 Fully-automated dual hormone pumps3 Transformational innovation Incremental innovations 2018 pump adoption4 0% 1 Example: MiniMed 530G System; 2 Example: MiniMed 670G System, the currently most advanced a hybrid closed loop system, not fully-automated; 3 Example: Beta Bionics iLet device; 4 ZP estimate based on ZS Associates analysis, DataMonitor Diabetes Report 2018, ADA, LSI Report 2018, AACE Report 2014, Meddevicetracker, March 2017 29 Level of pump adoption Type 1 diabetes: ~30% Type 2 diabetes: ~2%
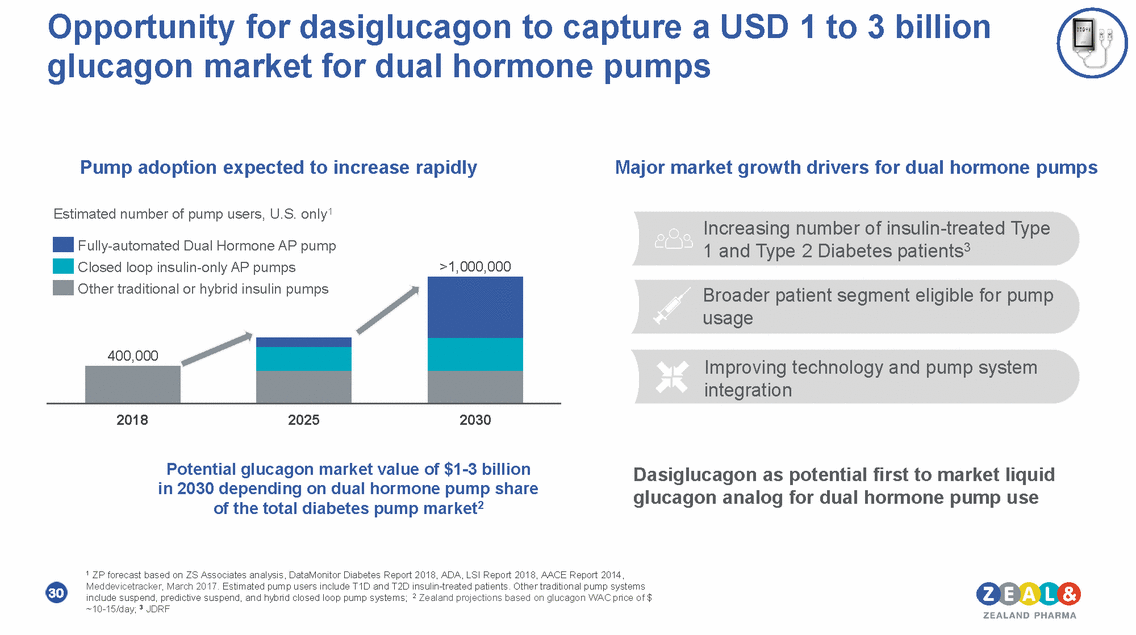
Opportunity for dasiglucagon to capture a USD 1 to 3 billion glucagon market for dual hormone pumps Pump adoption expected to increase rapidly Major market growth drivers for dual hormone pumps Estimated number of pump users, U.S. only1 Increasing number of insulin-treated Type 1 and Type 2 Diabetes patients3 Fully-automated Dual Hormone AP pump Closed loop insulin-only AP pumps >1,000,000 Broader patient segment eligible for pump usage Improving technology and pump system integration 2018 2025 2030 Potential glucagon market value of $1-3 billion in 2030 depending on dual hormone pump share of the total diabetes pump market2 Dasiglucagon as potential first to market liquid glucagon analog for dual hormone pump use 1 ZP forecast based on ZS Associates analysis, DataMonitor Diabetes Report 2018, ADA, LSI Report 2018, AACE Report 2014, Meddevicetracker, March 2017. Estimated pump users include T1D and T2D insulin-treated patients. Other traditional pump systems include suspend, predictive suspend, and hybrid closed loop pump systems; 2 Zealand projections based on glucagon WAC price of $ ~10-15/day; 3 JDRF 30 Other traditional or hybrid insulin pumps 400,000
Transforming the lives of children with congenital hyperinsulinism and their families by offering continues infusion of dasiglucagon 31

Dasiglucagon as long-term treatment for children with congenital hyperinsulinism newborns and We aspire to transform and the lives of children with CHI Providing a non-surgical treatment option Liquid formulation, stable at room and body temperature First Phase 3 trial initiated EU and U.S. orphan drug designation granted For illustration only 32
Congenital hyperinsulinism (CHI) is devastating disorder in newborns an ultra-rare and An ultra-rare disease in newborns and children ~300 newborns are diagnosed every year with genetically determined CHI in the U.S. and EU1,2 Persistent episodes of hypoglycemia Most common cause of serious episodes of hypoglycemia during childhood due to inappropriate insulin secretion2,3 Substantial burden of disease • • • • High resistance to existing medical treatment3 High risk of seizures and permanent brain injury4 Most severe cases require pancreatic surgery5 Prolonged hospitalization and intolerable burden to patients, families, caregivers, and healthcare systems6,2 1 https://www.orpha.net/consor/cgi-bin/ (not including transient cases due to perinatal stress or diabetic mother); 2 Congenital Hyperinsulinism International. Available at: http://congenitalhi.org; 3 De Leon et al. Nat Clin Pract Endocrinol Metab 2007;3:57-68, Lubchenco and Bard. Pediatrics. 1971 May;47(5):831-8, Hussain et al. Diabetes 2005 54:2946–2951; 3 Thornton PS et al., J Pediatr. 2015;167(2):238-45; ); 4 Meissner T et al., Long-term follow-up of 114 patients with congenital hyperinsulinism. Eur J Endocrinol 2003;149:43-510; 5 Yorifuji et al. Pediatrics International 2014;56:467; 6 Eljamel et al. Orphanet Journal of Rare Diseases 2018;13:123 33
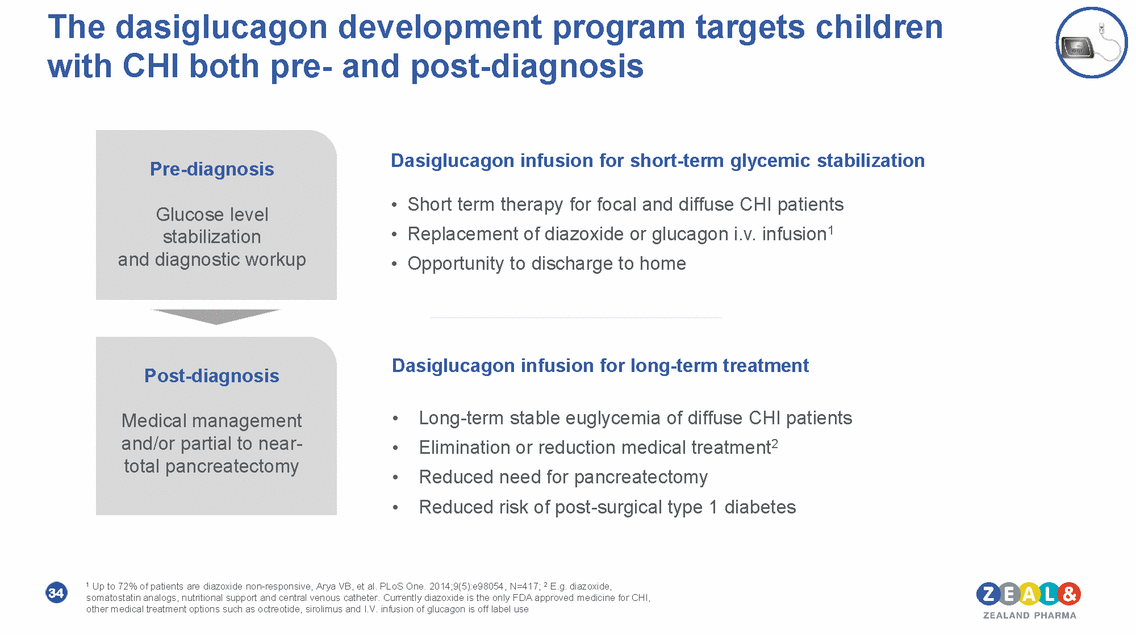
The dasiglucagon development program targets children with CHI both pre-and post-diagnosis Dasiglucagon infusion for short-term glycemic stabilization Pre-diagnosis • • • Short term therapy for focal and diffuse CHI patients Replacement of diazoxide or glucagon i.v. infusion1 Opportunity to discharge to home Glucose level stabilization and diagnostic workup Dasiglucagon infusion for long-term treatment Post-diagnosis • • • • Long-term stable euglycemia of diffuse CHI patients Elimination or reduction medical treatment2 Reduced need for pancreatectomy Reduced risk of post-surgical type 1 diabetes Medical management and/or partial to near-total pancreatectomy 1 Up to 72% of patients are diazoxide non-responsive, Arya VB, et al. PLoS One. 2014;9(5):e98054, N=417; 2 E.g. diazoxide, somatostatin analogs, nutritional support and central venous catheter. Currently diazoxide is the only FDA approved medicine for CHI, other medical treatment options such as octreotide, sirolimus and I.V. infusion of glucagon is off label use 34
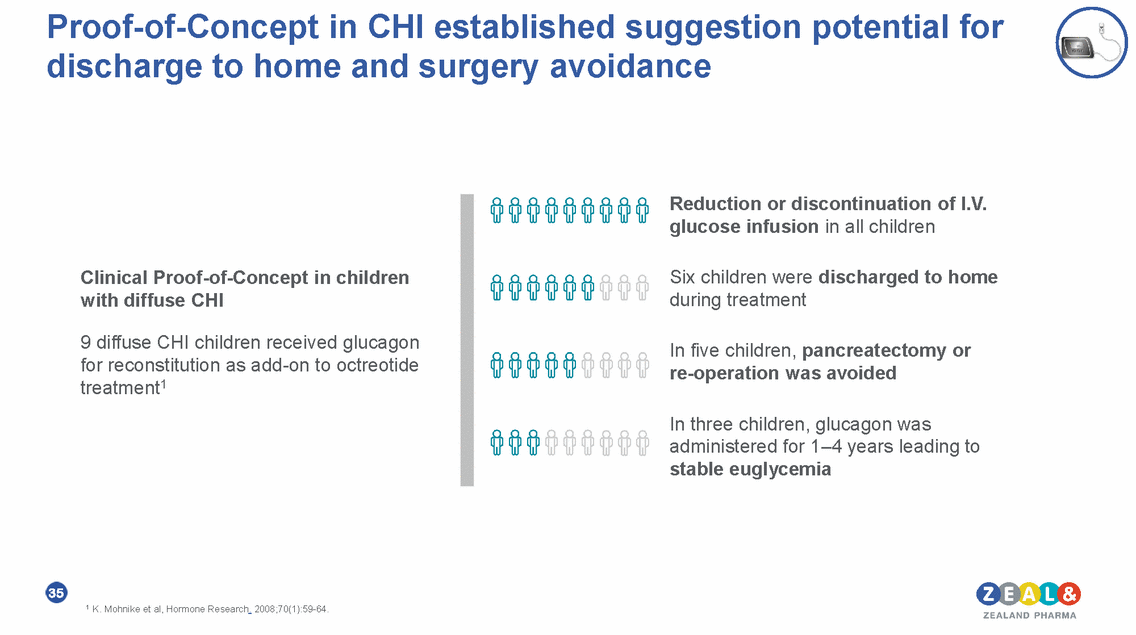
Proof-of-Concept in CHI established suggestion potential for discharge to home and surgery avoidance Reduction or discontinuation of I.V. glucose infusion in all children Six children were discharged to home during treatment Clinical Proof-of-Concept in children with diffuse CHI 9 diffuse CHI children received glucagon for reconstitution as add-on to octreotide treatment1 In five children, pancreatectomy or re-operation was avoided In three children, glucagon was administered for 1–4 years leading to stable euglycemia 35 1 K. Mohnike et al, Hormone Research. 2008;70(1):59-64.
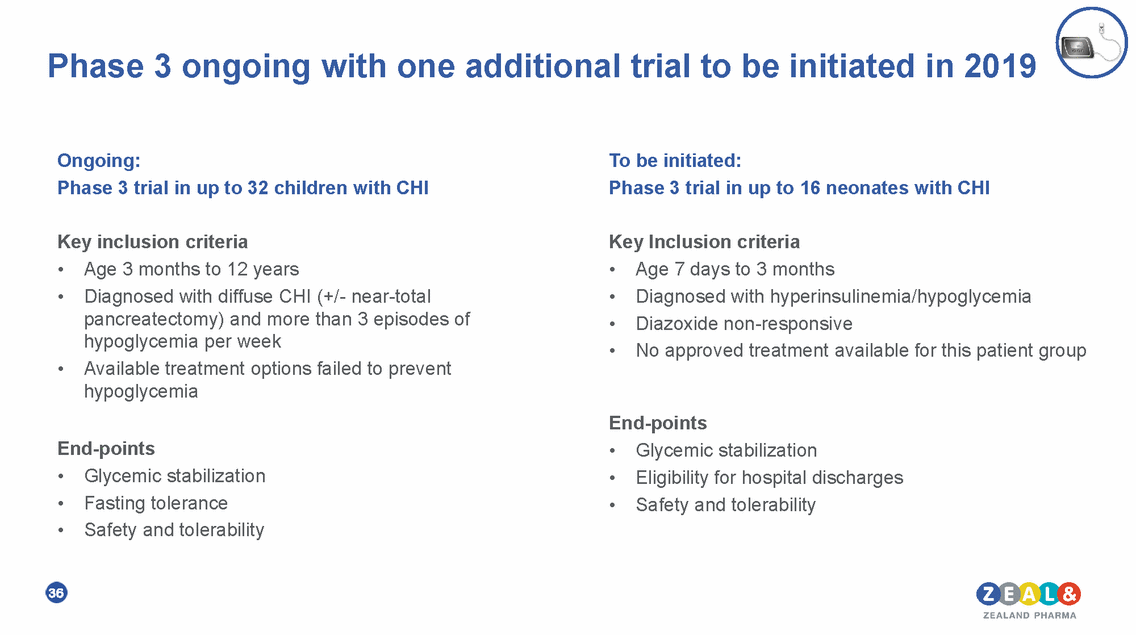
Phase 3 ongoing with one additional trial to be initiated in 2019 Ongoing: Phase 3 trial in up to 32 children with CHI To be initiated: Phase 3 trial in up to 16 neonates with CHI Key inclusion criteria Key Inclusion criteria • • Age 3 months to 12 years Diagnosed with diffuse CHI (+/-near-total pancreatectomy) and more than 3 episodes of hypoglycemia per week Available treatment options failed to prevent hypoglycemia • • • • Age 7 days to 3 months Diagnosed with hyperinsulinemia/hypoglycemia Diazoxide non-responsive No approved treatment available for this patient group • End-points End-points • • • Glycemic stabilization Eligibility for hospital discharges Safety and tolerability • • • Glycemic stabilization Fasting tolerance Safety and tolerability 36
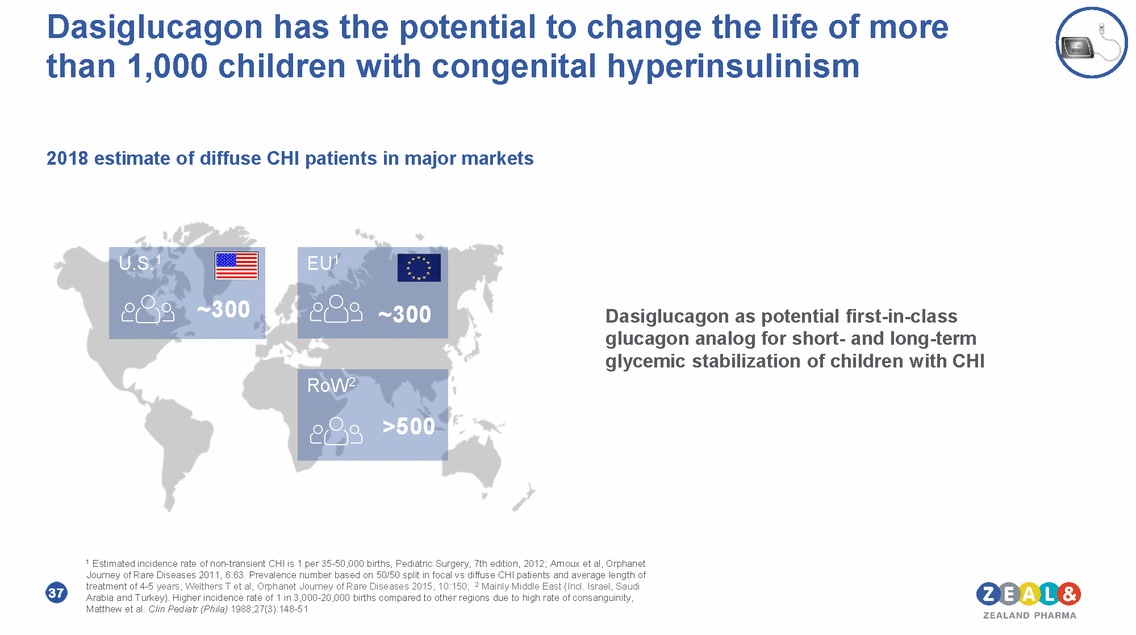
Dasiglucagon has the potential to change the life of more than 1,000 children with congenital hyperinsulinism 2018 estimate of diffuse CHI patients in major markets Dasiglucagon as potential first-in-class glucagon analog for short-and long-term glycemic stabilization of children with CHI 1 Estimated incidence rate of non-transient CHI is 1 per 35-50,000 births, Pediatric Surgery, 7th edition, 2012; Arnoux et al, Orphanet Journey of Rare Diseases 2011, 6:63. Prevalence number based on 50/50 split in focal vs diffuse CHI patients and average length of treatment of 4-5 years, Welthers T et al, Orphanet Journey of Rare Diseases 2015, 10:150; 2 Mainly Middle East (Incl. Israel, Saudi Arabia and Turkey). Higher incidence rate of 1 in 3,000-20,000 births compared to other regions due to high rate of consanguinity, Matthew et al. Clin Pediatr (Phila) 1988;27(3):148-51 37 RoW2 >500 U.S.1 ~300 EU1 ~300
Other Programs 38
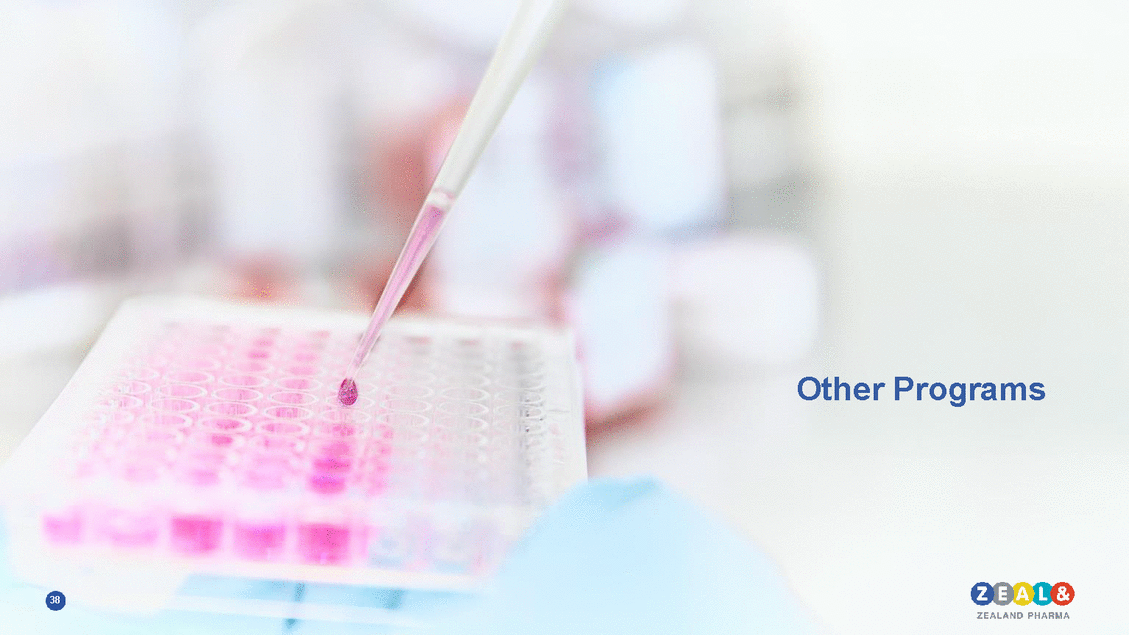
Boehringer Ingelheim partnership program: GLP-1/glucagon analog for obesity and type 2 diabetes in Phase 1 Body weight reduction with GLP-1/glucagon dual analog in a model of obesity2 GLP-1/glucagon dual analog in Phase 1b1 • • • • • • Product candidate for obesity/ T2D Potential to improve blood sugar and weight loss control Once weekly dosing Phase 1b results expected in 2019 Zealand eligible for EUR 365m in outstanding milestones Royalties on global sales Amylin analog with Phase 1 initiation in 20191 • • • • Product candidate for obesity/ T2D Once weekly dosing Zealand eligible for EUR 283m in outstanding milestones Royalties on global sales Time (days) GLP-1 ag (10 nmol/kg) ZP-GLP-1/Glu (10 nmol/kg) 39 1 Boehringer Ingelheim holds global development and commercial rights by; 2 Daugaard et al, ADA, Orlando FL, USA, 2010; 3. IDF, Global Burden of Obesity in 2005 and projections to 2030 and IDF Diabetes Atlas, Eighth Edition, 2017 Body Weight (g) ZP-GLP1/Glucagon
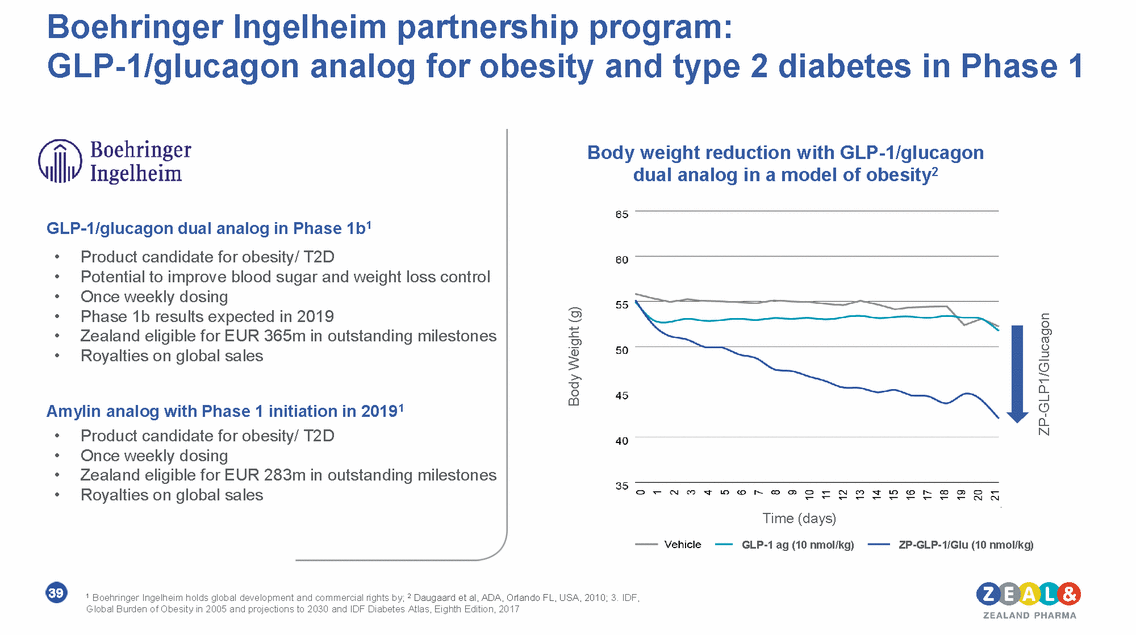
Pre-clinical progress in long-acting C3 complement inhibitor The complement cascade system2 C3 complement inhibitor • Modulation of the complement cascade is clinically validated as therapeutic approach1 • Multiple opportunities for intervention points for novel targeted therapeutics • Novel long-acting peptide inhibitor of C3 identified • Potential to start clinical development in 2020 • IP protection until at least 2038 40 1 E.g. paroxysmal nocturnal hemoglobinuria, cold agglutinin disease, myasthenia gravis and C3 glomeropathy 2 Ricklin et al, 2016, Nature Reviews Drug Discovery, 12:383-401
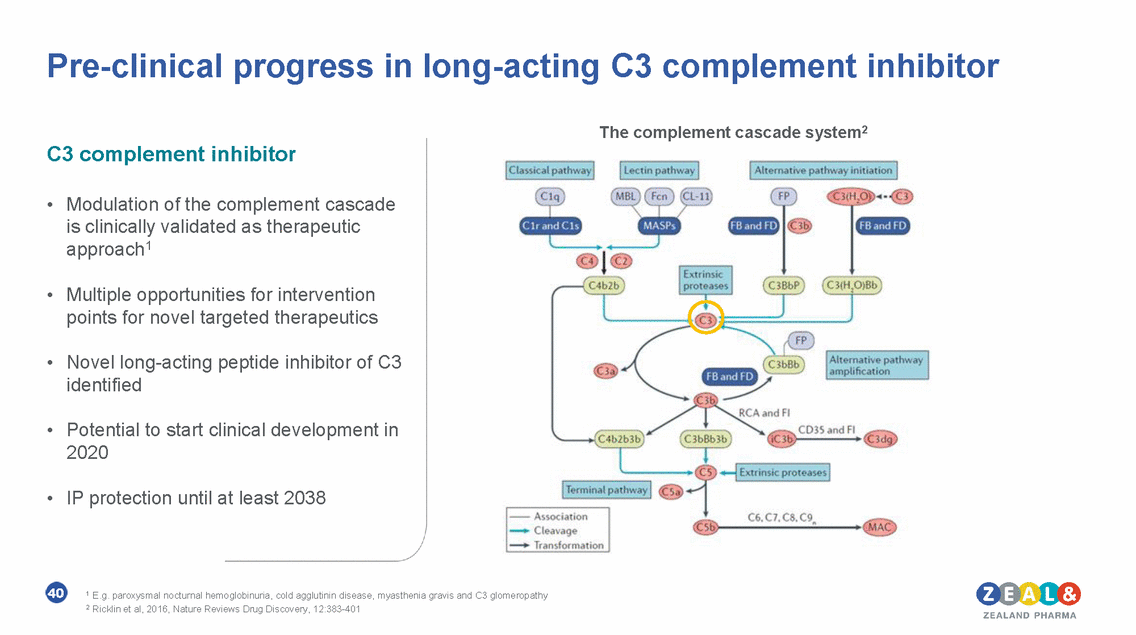
Pre-clinical GIP portfolio with compelling evidence for therapeutic development in multiple major diseases The GIP pre-clinical agonists • GIP MONO agonist with potential in T2D and obesity Vehicle 1 & 2 GLP-1 + Vehicle 2 GLP-1 + ZP GIP high dose GLP-1 + ZP GIP low dose GIP mono agonist2 • GIP/GLP-1 DUAL agonist with potential in T2D, obesity, NASH and Parkinson’s disease. significant difference vs GLP-1 + vehicle 2 • Clinically validated for weight reduction and improved glycemic control1 • Triple agonists identified for optimization GIP/GLP-1 dual agonist3 • Potential to start clinical development in 2020 41 1 www.investor.lilly.com. +ve Ph2b reported for LY3298176 in 2018; 2 www.ncbi.nlm.nih.gov/pubmed/28598027; data on file. NOTE: Liraglutide dosed once-daily. GIP agonist dosed every 3rd day. 3 Zealand Pharma
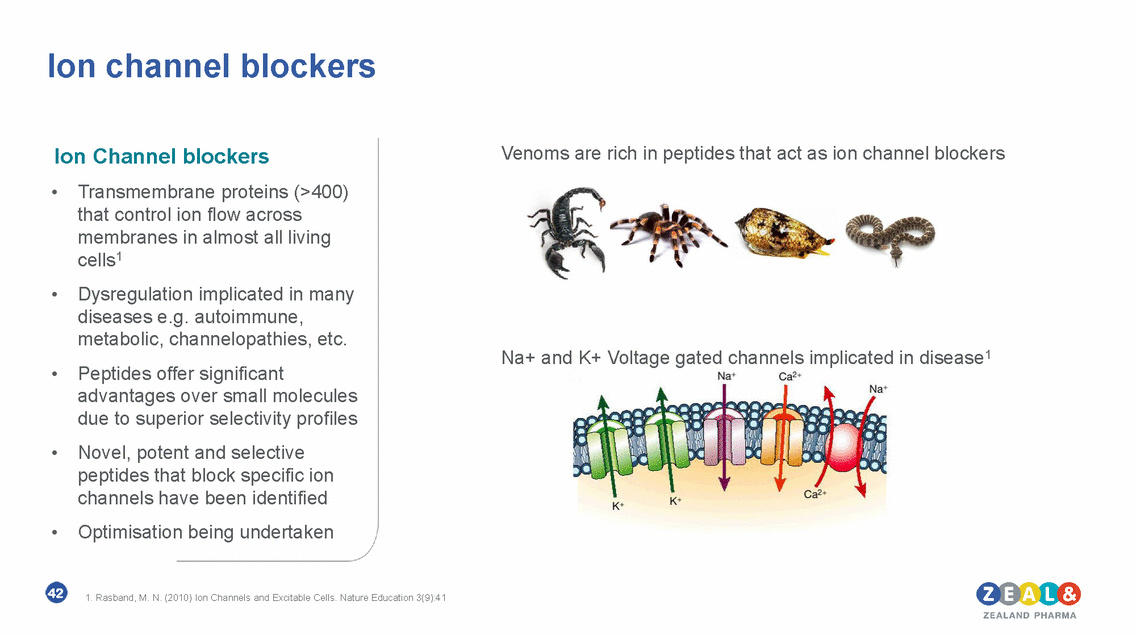
Ion channel blockers Venoms are rich in peptides that act as ion channel blockers Ion Channel blockers • Transmembrane proteins (>400) that control ion flow across membranes in almost all living cells1 Dysregulation implicated in many diseases e.g. autoimmune, metabolic, channelopathies, etc. Peptides offer significant advantages over small molecules due to superior selectivity profiles Novel, potent and selective peptides that block specific ion channels have been identified Optimisation being undertaken • Na+ and K+ Voltage gated channels implicated in disease1 • • • 42 1. Rasband, M. N. (2010) Ion Channels and Excitable Cells. Nature Education 3(9):41
Additional Company Information 43

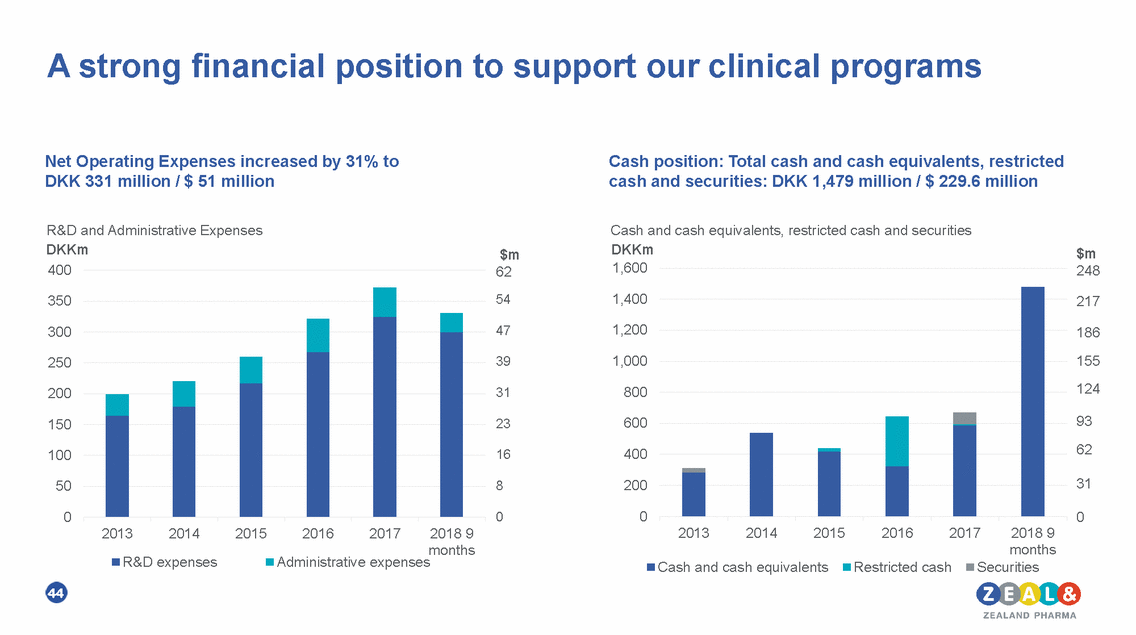
A strong financial position to support our clinical programs Net Operating Expenses increased by 31% to DKK 331 million / $ 51 million Cash position: Total cash and cash equivalents, restricted cash and securities: DKK 1,479 million / $ 229.6 million R&D and Administrative Expenses DKKm Cash and cash equivalents, restricted cash and securities DKKm $m 248 $m 62 54 1,600 400 1,400 350 217 1,200 47 300 186 155 124 1,000 39 250 800 200 31 93 62 600 150 23 16 400 100 31 200 50 8 0 0 0 0 2013 2014 2015 2016 2017 2018 9 2013 2014 2015 2016 2017 2018 9 months months Administrative expenses R&D expenses Cash and cash equivalents Restricted cash Securities 44
Our Team Corporate Management Britt Meelby Jensen President, Chief Executive Officer Mats Blom Executive Vice President, Chief Financial Officer Adam Steensberg Executive Vice President, Chief Medical & Development Officer Andrew Parker Executive Vice President, Chief Science Officer Ivan Møller Senior Vice President, Technical Development & Operations Marino Garcia Senior Vice President, Corporate & Business Development Locations Organization Highly-skilled and diverse team of 155 employees Headquarter Copenhagen, Denmark 32% Research New York, USA 50% Development and Operations 18% Other 45
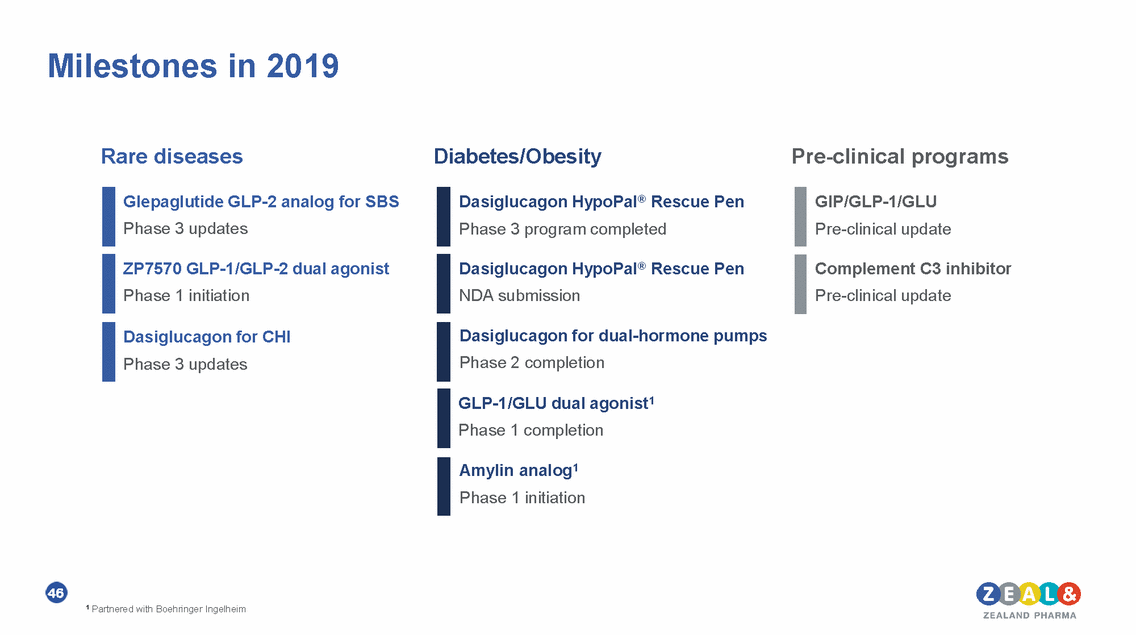
Milestones in 2019 Rare diseases Diabetes/Obesity Pre-clinical programs Glepaglutide GLP-2 analog for SBS Phase 3 updates Dasiglucagon HypoPal® Rescue Pen Phase 3 program completed GIP/GLP-1/GLU Pre-clinical update ZP7570 GLP-1/GLP-2 dual agonist Phase 1 initiation Dasiglucagon HypoPal® Rescue Pen NDA submission Complement C3 inhibitor Pre-clinical update Dasiglucagon for dual-hormone pumps Phase 2 completion Dasiglucagon for CHI Phase 3 updates GLP-1/GLU dual agonist1 Phase 1 completion Amylin analog1 Phase 1 initiation 46 1 Partnered with Boehringer Ingelheim
The Zealand Investment Thesis Four late stage development product candidates in 2019 expecting to launch into major markets in 2 to 4 years • • • • Glepaglutide for short bowel syndrome in Phase 3 Dasiglucagon HypoPal® Rescue Pen with positive phase 3 results Dasiglucagon for congenital hyperinsulinism in Phase 3 Dasiglucagon for dual hormone pumps ready for Phase 2b Validated peptide platform with promising options in early pipeline • • • • • GLP-1/glucagon analog in Phase 1b Amylin analog ready for Phase 1 GLP-1/GLP-2 dual agonist for SBS advancing to Phase 1 Novel long-acting C3 complement inhibitor GIP portfolio with compelling evidence in multiple disease areas Financial strength and a strong team with ability to deliver 47 47
