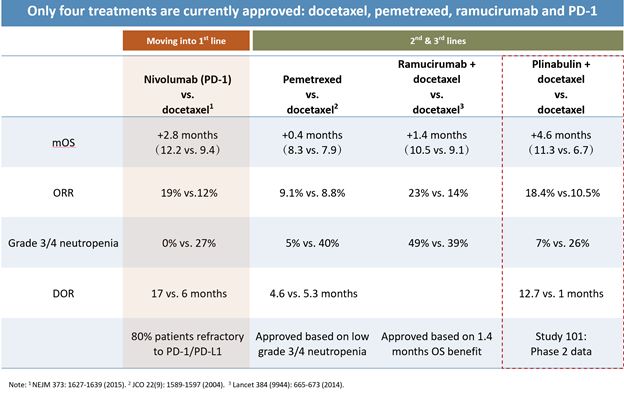
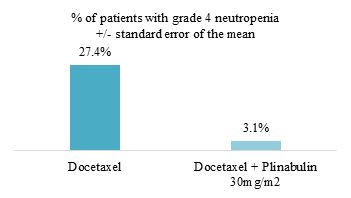
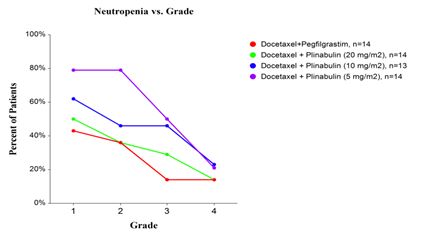
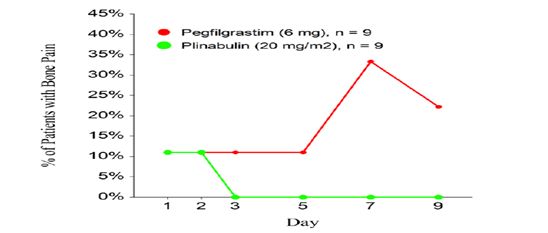
Other programs
In addition to exploring Plinabulin’s therapeutic potential in combination with immuno-oncology agents, we have a pipeline of preclinical immuno-oncology product candidates and have utilized our research collaborators to advance these programs.
BPI-002 and BPI-003 programs
Our BPI-002 program is based on an oral small molecule agent, similar to a CTLA-4 antibody, which induces T-cell activation and preclinical studies have shown that it is synergistic to checkpoint inhibitors in immune competent colon cancer animal model. BPI-002 is currently in preclinical studies and we plan to initiate a clinical study in 2020. Our IKK program, BPI-003, is based on a novel small molecule inhibitor of IKK, a protein kinase. IKK is involved in survival of some tumor cells as well as in the production of a number of cytokines and growth factors that serve as survival factors for various tumors. Our IKK inhibitor has shown promising activity in multiple animal models of pancreatic cancer.
BPI-004 program
Our BPI-004 program is focused on a small molecule that induces the production of neo-antigens by tumor cells, allowing tumors containing no immune cells to be infiltrated by the immune system. A large proportion of human cancers do not produce antigens that are recognized by the immune system. As a result, these tumors do not respond to treatments that work through interaction with the patient’s immune response. For example, these tumors will not respond to treatment with PD-1 inhibitors. A treatment that induces the tumor cells to produce antigens has the potential to make these cancers responsive to PD-1 inhibitors.
Ubiquitination drug development platform
We are also investigating an alternative approach to cancer treatment in which disease-causing proteins are marked for early degradation. This approach uses a protein called a ubiquitin E3 ligase to target and promote the destruction of disease-causing proteins, such as oncogenes. To trigger degradation, the target protein is labeled with poly-ubiquitin by a specific ubiquitin ligase enzyme. Poly-ubiquitin acts as an indicating tag to cellular proteasome machinery that the target protein should be destroyed. One approach to tagging the target protein is using a “molecular glue” to bind the ubiquitin ligase to the target protein.
We are collaborating with Dr. Ning Zheng, a Howard Hughes Medical Institute Investigator, and his group at the University of Washington on a unique “molecular glue” used to selectively tag certain oncogene proteins with E3 ligase, one of the ubiquitin ligase enzymes. Dr. Huang, co-founder of BeyondSpring, and Dr. Zheng were the first to discover the crystal structure of the only two classes of E3 ligases. This work forms the structural basis for the selection of the small molecules to be studied as a potential “molecular glue.” The first target protein is expected to be oncogene KRAS. KRAS is frequently mutated in pancreas, colon, lung and uterus cancers. This novel platform technology has the potential to significantly reduce the amount of oncogene protein in the cell and such disease-causing protein is not targeted by current therapeutic approaches.
Plinabulin in other indications
Tumors with RAS mutations
We have identified that tumors that have mutations in an oncogene called RAS are particularly sensitive to Plinabulin. An oncogene is a gene that is a changed or mutated form of a gene involved in normal cell growth, which has the potential to cause cancer. A particular type of oncogene is the mutation of the RAS gene (HRAS, KRAS and NRAS), which is frequently found in human tumors. We believe that based on data from preclinical studies, Plinabulin will work together with standard-of-care agents in tumors with RAS mutations, including NSCLC and colorectal cancer. Mutations in KRAS are found in a large proportion of tumors including 16% of NSCLC, 36% of colon adenocarcinomas, and 69% of pancreatic ductal adenocarcinomas.
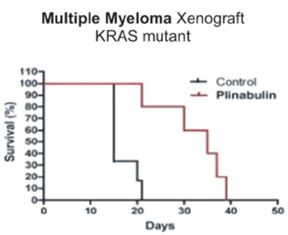
In a preclinical study, Plinabulin led to increased survival in a mouse multiple myeloma model containing a mutant KRAS gene. The figure below shows the survival of mice containing a mutant KRAS gene when treated with Plinabulin compared to those who were not treated with Plinabulin. Mice receiving Plinabulin at a dose level of 7.5 mg/kg twice weekly for three weeks had median survival of 35 days compared to 15 days in the control group (p=0.0041).
While specific KRAS mutations are not believed to be a major cause of glioblastoma, systems analyses have estimated that signaling through the KRAS pathway is altered in 88% of glioblastoma tumors. Plinabulin is able to cross the blood-brain barrier and led to a significant survival advantage in a KRAS-driven mouse model of glioblastoma.
While we continue to be primarily focused on the use of Plinabulin in advanced NSCLC, in CIN and in combination with immuno-oncology agents, if the necessary resources and financing are available, we may decide to further investigate the effect of Plinabulin in RAS mutant tumors.
Principal Investigators and Scientific Advisors
Our clinical trials are led by world renowned leaders in the clinical community, which we believe demonstrates their confidence in of our clinical trials.
NSCLC
Dr. David Ettinger, Chairman of the NCCN guidelines for NSCLC in the U.S. has guided the study design and is assisting with Study 103. Dr. Ettinger is Alex Grass Professor of Oncology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
Dr. Yan Sun, our lead clinical investigator for NSCLC in China is Chairman of the NCCN guidelines for NSCLC in China and the Director of National GCP Center for Anticancer Agents Cancer Hospital in Beijing, a hospital that treats 320,000 patients a year. In 1997, Dr. Sun also co-founded the Steering Committee of the Chinese Society of Clinical Oncology and served as its Chairman and President from 1997 to 2013. Dr. Sun was the lead clinical investigator for the Phase 3 trials of other lung cancer drugs that received approval from the NMPA, including icotinib.
CIN
Dr. Douglas Blayney of Stanford University, a board member of the NCCN and contributor to the NCCN guidelines for neutropenia management, is our principal investigator for both Study 105 and Study 106. Dr. Blayney is the former president of ASCO and a former member of the FDA’s Oncologic Drugs Advisory Committee.
Dr. Jeffrey Crawford is DSMB Chairman for Study 105 and Study 106. He is Chairman of NCCN Guidelines for Neutropenia Management in U.S. and the lead investigator of the U.S. multicenter, randomized trial of Filgrastim (G-CSF, Neupogen), leading to FDA approval. Dr. Crawford is Professor of Medicine at Duke University.
Dr. Yuankai Shi, Chairman of the NCCN guidelines for neutropenia management in China, is our principal investigator for the Chinese portion of both studies. Dr. Shi is Director of Oncology Department at Cancer Hospital Chinese Academy of Medical Sciences.
Ubiquitination platform
Dr. Avram Hershko is our SAB member of ubiquitination platform. He brought in nearly 50 years of research leadership in ubiquitination pathway and is the winner of 2004 Nobel Prize in Chemistry for discovery of ubiquitin-mediated protein degradation. Dr. Hershko is Distinguished Professor at Rappaport Faculty of Medicine at Technion in Haifa.
Intellectual Property
The proprietary nature of, and protection for, our product candidates and their methods of use are an important part of our strategy to develop and commercialize novel medicines, as described in more detail below. We have obtained U.S. patents and filed patent applications in the U.S. and other countries relating to certain of our product candidates, and are pursuing additional patent protection for them and for other of our product candidates and technologies.
Our success will depend significantly on our ability to obtain and maintain patent and other proprietary protection for our product candidates and other commercially important products, technologies, inventions and know-how, as well as on our ability to defend and enforce our patents including any patent that we have or may issue from our patent applications, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and proprietary rights of other parties.
As of March 31, 2019, we owned or co-owned 76 patents, in 36 jurisdictions, including 20 issued U.S. patents. We also owned seven pending U.S. non-provisional patent applications as well as corresponding patent applications pending in other jurisdictions and seven pending U.S. provisional patent applications. In addition, we owned five pending international patent applications related to Plinabulin and Plinabulin analogs filed under the PCT, which we plan to file nationally in the U.S. and in other jurisdictions directed to reduction of CIN, the therapeutic use of tubulin binding compounds, dosage regimens, the treatment of thrombocytopenia, and use in combination with G-CSF therapy.
Our patent portfolio as of March 29, 2019 included sixteen issued U.S. patents directed to Plinabulin and Plinabulin analogs, their synthesis and their use in the treatment of various disorders. In particular, we owned nine issued U.S. patents directed to the Plinabulin composition of matter, methods of synthesizing Plinabulin, polymorphic forms of Plinabulin, and methods of treating various disorders with Plinabulin including various cancers such as lung cancer, NSCLC, breast cancer, skin cancer, prostate cancer, myeloma, RAS mutant tumors, and brain tumors, and fungal infections, and methods of using Plinabulin for inhibiting cell proliferation, promotion of microtubule depolymerization, and inducement of vascular collapse in a tumor. These U.S. patents were scheduled to expire between 2021 and 2036, excluding any potential patent term restorations. The patent portfolio also contained counterpart patents granted in 35 foreign jurisdictions including Japan, South Korea, China, Europe and other countries.
The term of individual patents may vary based on the countries in which they are obtained. In most countries in which we file including the U.S., the term of an issued patent is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. In the U.S., the term of a patent may be lengthened in some cases by a patent term adjustment, which extends the term of a patent to account for administrative delays by the USPTO, in excess of a patent applicant’s own delays during the prosecution process, or may be shortened if a patent is terminally disclaimed over a commonly owned patent having an earlier expiration date. In addition, in certain instances, the term of one patent for a given drug product can be restored (extended) to recapture a portion of the term effectively lost as a result of the FDA regulatory review period. However, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. We plan to seek such an extension of one of our U.S. patents directed to Plinabulin or its use when appropriate.
In certain foreign jurisdictions similar extensions as compensation for regulatory delays are also available. The actual protection afforded by a patent varies on a claim by claim and country by country basis and depends upon many factors, including the type of patent, the scope of its coverage, the availability of any patent term extensions or adjustments, the availability of legal remedies in a particular country and the validity and enforceability of the patent. In particular, up to a five-year extension may be available in the EU and Japan. We plan to seek such extensions as appropriate.
Furthermore, the patent positions of biotechnology and pharmaceutical products and processes like those we intend to develop and commercialize are generally uncertain and involve complex legal and factual questions. No consistent policy regarding the breadth of claims allowed in such patents has emerged to date in the U.S. The scope of patent protection outside the U.S. is even more uncertain. Changes in the patent laws or in interpretations of patent laws in the U.S. and other countries have diminished, and may further diminish, our ability to protect our inventions and enforce our intellectual property rights and, more generally, could affect the value of intellectual property.
Additionally, while we have already secured a number of issued patents directed to our product candidates, we cannot predict the breadth of claims that may issue from our pending patent applications or may have or may be issued from patents and patent applications owned by others. Substantial scientific and commercial research has been conducted for many years in the areas in which we have focused our development efforts, which has resulted in other parties having a number of issued patents and pending patent applications relating to such areas. Patent applications in the U.S. and elsewhere are generally published only after 18 months from the priority date, and the publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made. Therefore, patents and patent applications relating to drugs similar to our current product candidates and any future drugs, discoveries or technologies we might develop may have already been issued or filed, which could prohibit us from commercializing our product candidates.
The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights. Our ability to maintain and solidify our proprietary position for our product candidates and technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any of the pending patent applications that we currently own, may file or license from others will result in the issuance of any patents. The issued patents that we own or may receive in the future, may be challenged, invalidated or circumvented, and the rights granted under any issued patents may not provide us with proprietary protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may be able to independently develop and commercialize similar drugs or duplicate our technology, business model or strategy without infringing our patents. Because of the extensive time required for clinical development and regulatory review of a drug we may develop, it is possible that, before any of our product candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of any such patent.
We may rely, in some limited circumstances, on trade secrets and unpatented know-how to protect aspects of our technology. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with consultants, scientific advisors and contractors and invention assignment agreements with our employees. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Our commercial success will also depend in part on not infringing the proprietary rights of other parties. The existence of any patent by others with claims covering or related to aspects of our product candidates would require us to alter our development of commercial strategies, redesign our product candidates or processes, obtain licenses or cease certain activities. Such licenses may not be available on reasonable commercial terms or at all, which could require us to cease development or commercialization of our product candidates. In addition, our breach of any license agreements or failure to obtain a license to proprietary rights that we may require to develop or commercialize our product candidates would have a material adverse impact on us. If others have prepared and filed patent applications in the U.S. that also claim technology to which we have filed patent applications or otherwise wish to challenge our patents, we may have to participate in interferences, post-grant reviews, inter partes reviews, derivation or other proceedings in the USPTO and other patent offices to determine issues such as priority of claimed invention or validity of such patent applications as well as our own patent applications and issued patents.
For more information on these and other risks related to intellectual property, see “Item 3. Key Information—D. Risk Factors—Risks Related to Our Intellectual Property.”
Competition
Our industry is highly competitive and subject to rapid and significant change. While we believe that our development and commercialization experience, scientific knowledge and industry relationships provide us with competitive advantages, we face competition from pharmaceutical and biotechnology companies, including specialty pharmaceutical companies, and generic drug companies, academic institutions, government agencies and research institutions.
There are a number of large pharmaceutical and biotechnology companies that currently market and sell drugs or are pursuing the development of drugs for the treatment of cancer for which we are developing our product candidates. For example, both Genetech Inc. and Eli Lilly and Company currently market and sell drugs, Tarceva and Cyramza. Cyramza is approved to treat NSCLC once the disease has progressed after platinum-containing chemotherapy. Moreover, a number of additional drugs are currently in ongoing Phase 3 clinical trials as second and third line treatments of NSCLC, and may become competitors if and when they receive regulatory approval.
Neutropenia can be prevented or treated by G-CSF, a protein that promotes the survival, proliferation and differentiation of neutrophils. Recombinant G-CSF therapies, such as filgrastim (Neupogen), a short-acting drug, and pegfilgrastim (Neulasta), a long-acting drug, are commonly used to prevent and treat CIN. The major manufacturer of these competing therapies is Amgen.
While we are investigating an alternative approach to cancer treatment by using molecular glue technology to tag oncogene proteins with ubiquitin ligase and destroy such proteins, there are a number of companies who are also working on using such technology to target and destroy oncogene proteins. See “—Plinabulin, Our Lead Drug Candidate—Other programs.”
Many of our competitors have longer operating histories, better name recognition, stronger management capabilities, better supplier relationships, a larger technical staff and sales force and greater financial, technical or marketing resources than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Our commercial opportunity could be reduced or eliminated if our competitors develop or market products or other novel therapies that are more effective, safer or less costly than our current product candidates, or any future product candidates we may develop, or obtain regulatory approval for their products more rapidly than we may obtain approval for our current product candidates or any such future product candidates. Our success will be based in part on our ability to identify, develop and manage a portfolio of product candidates that are safer and more effective than competing products.
Government Regulation
Government authorities in the U.S. at the federal, state and local level and in other countries extensively regulate, among other things, the research and clinical development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing, pricing, export and import of drug products, such as those we are developing. Generally, before a new drug can be marketed, considerable data demonstrating its quality, safety and efficacy must be obtained, organized to address the requirements of and in the format specific to each regulatory authority, submitted for review and approved by the regulatory authority. This process is very lengthy and expensive, and success is uncertain.
Drugs are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable regulatory requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the regulatory authority’s refusal to approve pending applications, withdrawal of an approval, clinical holds, untitled or warning letters, voluntary product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution, injunctions, disbarment, fines, refusals of government contracts, restitution, disgorgement, or civil or criminal penalties. Any such administrative or judicial enforcement action could have a material adverse effect on us.
U.S. Regulation
U.S. Government Regulation and Product Approval
Government authorities in the U.S. at the federal, state and local level extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing, export and import of drug products such as those we are developing. In the U.S., the FDA regulates drugs under the FDCA and its implementing regulations and biologics under the FDCA and the Public Health Service Act and its implementing regulations.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and policies are often revised or reinterpreted by the agency in ways that may significantly affect our business and our product candidates or any future product candidates we may develop. It is impossible to predict whether further legislative or FDA regulation or policy changes will be enacted or implemented and what the impact of such changes, if any, may be.
U.S. Drug Development Process
The process of obtaining regulatory approvals and maintaining compliance with appropriate federal, state and local statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process, or after approval, may subject an applicant to administrative or judicial sanctions or lead to voluntary product recalls. Administrative or judicial sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, untitled or warning letters, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. The process required by the FDA before a drug may be marketed in the U.S. generally involves the following:
| • | completion of nonclinical laboratory tests, preclinical studies and formulation studies according to Good Laboratory Practices, or GLP, regulations; |
| • | submission to the FDA of an IND, which must become effective before human clinical trials may begin; |
| • | approval by an independent IRB, at each clinical site before each trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials according to GCP, to establish the safety and efficacy of the proposed product for its intended use; |
| • | preparation and submission to the FDA of an NDA, for a drug; |
| • | satisfactory completion of an FDA advisory committee review, if applicable; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with cGMP; and |
| • | payment of user fees and FDA review and approval of the NDA. |
The testing and approval process requires substantial time, effort and financial resources and we cannot be certain that any approvals for our product candidates, or any future product candidates we may develop, will be granted on a timely basis, if at all.
Once a drug product candidate is identified for development, it enters the nonclinical testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity, formulation and stability, as well as preclinical studies. An Investigational New Drug, or IND, sponsor must submit the results of the nonclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, to the FDA as part of the IND prior to commencing any testing in humans. An IND sponsor must also include a protocol detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated if the initial clinical trial lends itself to an efficacy evaluation. Some nonclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions related to a proposed clinical trial and places the trial on a clinical hold within that 30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during clinical trials due to safety concerns or noncompliance, and may be imposed on all products within a certain class of products. The FDA also can impose partial clinical holds, for example, prohibiting the initiation of clinical trials of a certain duration or for a certain dose.
We are conducting our current clinical trials under two INDs. Our investigators in connection with investigator-led clinical trials are being conducted under separate INDs.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCP regulations. These regulations include the requirement that all research subjects provide informed consent in writing before their participation in any clinical trial. Further, an IRB representing each institution participating in a clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. An IRB is responsible for protecting the rights of clinical trial subjects and considers, among other things, whether the risks to individuals participating in the clinical trial are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the information regarding the clinical trial and the consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed. Each new clinical protocol and any amendments to the protocol must be submitted for FDA review, and to the IRBs for approval.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| • | Phase 1. The product is initially introduced into a small number of healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of some products for severe or life-threatening diseases, especially when the product is suspected or known to be unavoidably toxic, the initial human testing may be conducted in patients. |
| • | Phase 2. The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage and schedule. |
| • | Phase 3. The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate data to evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product. |
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and safety reports must be submitted to the FDA and clinical investigators within 15 calendar days for serious and unexpected suspected adverse events, any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator’s brochure, or any findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the drug candidate. Additionally, a sponsor must notify FDA of any unexpected fatal or life-threatening suspected adverse reaction no later than 7 calendar days after the sponsor’s receipt of the information. Phase 1, Phase 2 and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product has been associated with unexpected serious harm to subjects.
Concurrent with clinical trials, companies usually complete additional preclinical studies and must also develop additional information about the chemistry and physical characteristics of the product and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product drug and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product drug does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
The results of product development, nonclinical studies and clinical trials, together with other detailed information regarding the manufacturing process, analytical tests conducted on the product, proposed labeling and other relevant information, are submitted to the FDA as part of an NDA requesting approval to market the new drug. Under the Prescription Drug User Fee Act, as amended, applicants are required to pay fees to the FDA for reviewing an NDA. These user fees, as well as the annual fees required for commercial manufacturing establishments and for approved products, can be substantial. The NDA review fee alone can currently exceed $2.4 million, and is likely to increase over time. The user fee requirement is subject to certain limited deferrals, waivers and reductions.
The FDA reviews all NDAs submitted within 60 days of submission to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be re-submitted with the additional information. The re-submitted application also is subject to review before the FDA accepts it for filing.
Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA’s established goal is to review 90% of NDA applications given “Priority” status – where there is evidence that the proposed product would be a significant improvement in the safety or effectiveness in the treatment, diagnosis, or prevention of a serious condition – within 6 months, and 90% of applications given “Standard” status within 10 months, whereupon a review decision is to be made. The FDA, however, may not approve a drug within these established goals, and its review goals are subject to change from time to time. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use. The FDA also evaluates whether the product’s manufacturing is cGMP-compliant to assure the product’s identity, strength, quality and purity. Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is or will be manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. The FDA also may refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. An advisory committee is a panel of experts, including clinicians and other scientific experts, who provide advice and recommendations when requested by the FDA. The FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations when making decisions.
The approval process is lengthy and difficult and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical data or other data and information. Even if such data and information are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive, and the FDA may interpret data differently than we interpret the same data. The FDA will issue a complete response letter if the agency decides not to approve the NDA in its present form. The complete response letter usually describes all of the specific deficiencies that the FDA identified in the NDA that must be satisfactorily addressed before it can be approved. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application or request an opportunity for a hearing.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require post-approval studies, including Phase 4 clinical trials, to further assess a product’s safety and effectiveness after NDA approval and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized. The FDA also may conclude that an NDA may only be approved with a REMS designed to mitigate risks through, for example, a medication guide, physician communication plan, or other elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools.
Post-Approval Requirements
Any products for which we receive FDA approval would be subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements. The FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market. Products may be promoted only for the approved indications and in accordance with the provisions of the approved label. Further, manufacturers must continue to comply with cGMP requirements, which are extensive and require considerable time, resources and ongoing investment to ensure compliance. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented and other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
Manufacturers and other entities involved in the manufacturing and distribution of approved products are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. The cGMP requirements apply to all stages of the manufacturing process, including the production, processing, sterilization, packaging, labeling, storage and shipment of the product. Manufacturers must establish validated systems to ensure that products meet specifications and regulatory requirements, and test each product batch or lot prior to its release. We rely, and expect to continue to rely, on third parties for the production of clinical quantities of our product candidates and any future product candidates we may develop. Future FDA and state inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution or may require substantial resources to correct.
The FDA may withdraw a product approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product’s marketing or even complete withdrawal of the product from the market. Further, the failure to maintain compliance with regulatory requirements may result in administrative or judicial actions, such as fines, untitled or warning letters, holds on clinical trials, product seizures, product detention or refusal to permit the import or export of products, refusal to approve pending applications or supplements, restrictions on marketing or manufacturing, injunctions or consent decrees, or civil or criminal penalties, or may lead to voluntary product recalls.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of the use of our product candidates, or any future product candidates we may develop, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application, except that this review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, if available, we intend to apply for restorations of patent term for some of our currently owned patents beyond their current expiration dates, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant NDA; however, any such extension may not be granted to us.
Market exclusivity provisions under the FDCA can also delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the U.S. to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the nonclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA-regulated products, including drugs are required to register and disclose certain clinical trial information, which is publicly available at www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any products for which we may obtain regulatory approval. In the U.S., sales of any products for which we may receive regulatory approval for commercial sale will depend in part on the availability of coverage and reimbursement from third-party payors. Third-party payors include government authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the reimbursement rate that the payor will pay for the product. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the FDA-approved products for a particular indication. Moreover, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost- effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of any products, in addition to the costs required to obtain regulatory approvals. Our product candidates, or any future product candidates we may develop, may not be considered medically necessary or cost-effective. If third-party payors do not consider a product to be cost-effective compared to other available therapies, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit.
The U.S. government, including the Trump administration, and state legislatures have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement, requirements for substitution of generic products for branded prescription drugs, and increased transparency around drug pricing practices. For example, the Affordable Care Act contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. There also has been increased public and governmental scrutiny of the cost of drugs and drug pricing strategies, including by the U.S. Senate and federal and state prosecutors. In May 2018, President Trump released the Blueprint which, along with related drug pricing measures proposed since the Blueprint, could cause significant operational and reimbursement changes for the pharmaceutical industry. Adoption of government controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals including our product candidates, if any achieve approval.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, the emphasis on cost containment measures in the U.S. has increased and we expect will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates also may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
If we obtain regulatory approval of our products, we may be subject to various federal and state laws targeting fraud and abuse in the healthcare industry. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order, or recommendation of, an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| • | federal civil and criminal false claims laws, false statement laws, and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, or making a false statement or record material to payment of a false claim or avoiding, decreasing, or concealing an obligation to pay money to the federal government; |
| • | HIPAA, which imposes federal criminal and civil liability for executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
| • | the federal transparency laws, including the federal Physician Payments Sunshine Act, which is part of the Affordable Care Act, that requires applicable manufacturers of covered drugs to disclose payments and other transfers of value provided to physicians and teaching hospitals and physician ownership and investment interests; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and are not preempted by HIPAA, thus complicating compliance efforts. |
The Affordable Care Act broadened the reach of the fraud and abuse laws by, among other things, amending the intent requirement of the federal Anti-Kickback Statute and the applicable criminal healthcare fraud statutes contained within 42 U.S.C. § 1320a-7b. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act provides that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act or the civil monetary penalties statute. Many states have adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
Although we would not submit claims directly to payors, manufacturers can be held liable under the federal False Claims Act and other healthcare laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our future activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state, and third-party reimbursement for our products, and the sale and marketing of our products, will be subject to scrutiny under the False Claims Act. Penalties for a False Claims Act violation include three times the actual damages sustained by the government, plus mandatory civil penalties, and the potential for exclusion from participation in federal healthcare programs. The applicable civil penalties are subject to an annual increase based on inflation; effective January 29, 2018, the penalties are between $11,181 and $22,363 for each separate false claim. In addition, although the federal False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes. Further, private individuals have the ability to bring actions under the federal False Claims Act and certain states have enacted laws modeled after the federal False Claims Act.
Patient Protection and the Affordable Care Act
The Affordable Care Act, enacted in March 2010, includes measures that have or will significantly change the way health care is financed in the U.S. by both governmental and private insurers. Among the provisions of the Affordable Care Act of greatest importance to the pharmaceutical industry are the following:
| • | The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The Affordable Care Act increased pharmaceutical manufacturers’ rebate liability on most branded prescription drugs from 15.1% of the average manufacturer price to 23.1% of the average manufacturer price, added a new rebate calculation for line extensions of solid oral dosage forms of branded products, and modified the statutory definition of average manufacturer price. The Affordable Care Act also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization and expanding the population potentially eligible for Medicaid drug benefits. |
| • | In order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The Affordable Care Act expanded the types of entities eligible to receive discounted 340B pricing. |
| • | The Affordable Care Act imposed a requirement on manufacturers of branded drugs to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., the “donut hole”). |
| • | The Affordable Care Act imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs, apportioned among these entities according to their market share in certain government healthcare programs, although this fee does not apply to sales of certain products approved exclusively for orphan indications. |
In addition to these provisions, the Affordable Care Act established a number of bodies whose work may have a future impact on the market for certain pharmaceutical products. These include the Patient-Centered Outcomes Research Institute, established to oversee, identify priorities in, and conduct comparative clinical effectiveness research and the Center for Medicare and Medicaid Innovation within the Centers for Medicare and Medicaid Services, to test innovative payment and service delivery models to lower Medicare and Medicaid spending.
The Affordable Care Act has been subject to challenges and numerous ongoing efforts to repeal or amend the Act in whole or in part. Since the November 2016 U.S. election, President Trump and the U.S. Congress have made numerous efforts to repeal or amend the Affordable Care Act in whole or in part. For example, the Tax Cuts and Jobs Act, which President Trump signed into law in December 2017, repealed the Affordable Care Act’s individual health insurance mandate, which is considered a key component of the Affordable Care Act. Thus, the full impact of the Affordable Care Act, or any law replacing elements of it, on our business remains unclear. These and other laws may result in additional reductions in healthcare funding, which could have a material adverse effect on customers for our product candidates, if we gain approval for any of them. Although we cannot predict the full effect on our business of the implementation of existing legislation or the enactment of additional legislation pursuant to healthcare and other legislative reform, we believe that legislation or regulations that would reduce reimbursement for, or restrict coverage of, our products could adversely affect how much or under what circumstances healthcare providers will prescribe or administer our product candidates if we gain approval for any of them.
Chinese Regulation
In China, we operate in an increasingly complex legal and regulatory environment. We are subject to a variety of Chinese laws, rules and regulations affecting many aspects of our business. This section summarizes the principal Chinese laws, rules and regulations relevant to our business and operations.
General Regulations on China Food and Drug Administration
In China, the NMPA monitors and supervises the administration of pharmaceutical products, as well as medical devices and equipment. The NMPA’s primary responsibility includes evaluating, registering and approving new drugs, generic drugs, imported drugs and traditional Chinese medicines; approving and issuing permits for the manufacture, export and import of pharmaceutical products and medical appliances; approving the establishment of enterprises for pharmaceutical manufacture and distribution; formulating administrative rules and policies concerning the supervision and administration of food, cosmetics and pharmaceuticals; and handling significant accidents involving these products. The local provincial drug administrative authorities are responsible for supervision and administration of drugs within their respective administrative regions.
The Drug Administration Law of the People’s Republic of China, or the PRC Drug Administration Law, promulgated by the Standing Committee of the National People’s Congress in 1984, as amended in 2001, 2013 and 2015, respectively, and the Implementing Measures of the PRC Drug Administration Law promulgated by the State Council in 2002, as amended in 2016, set forth the legal framework for the administration of pharmaceutical products, including the research, development and manufacturing of drugs.
The PRC Drug Administration Law was revised in December 2001, December 2013 and again in April 2015. The purpose of the revisions was to strengthen the supervision and administration of pharmaceutical products and to ensure the quality and safety of those products for human use. The revised PRC Drug Administration Law applies to entities and individuals engaged in the development, production, trade, application, supervision and administration of pharmaceutical products. It regulates and prescribes a framework for the administration of pharmaceutical preparations of medical institutions and for the development, research, manufacturing, distribution, packaging, pricing and advertisement of pharmaceutical products. A draft amendment on the PRC Drug Administration Law was published by the National People’s Congress on November 1, 2018, which has yet to take effect. The draft amendment incorporates the Drug Marketing Authorization Holder System and change the responsible drug administrative authorities from provincial level to county level. Revised Implementing Measures of the PRC Drug Administration Law promulgated by the State Council took effect in September 2002, as amended in 2016, providing detailed implementing regulations for the revised PRC Drug Administration Law.
Under these regulations, we need to follow related regulations for nonclinical research, clinical trials and production of new drugs.
Good Laboratories Practice Certification for Nonclinical Research
To improve the quality of animal research, the CFDA promulgated the Administrative Measures for Good Laboratories Practice of Preclinical Laboratory in 2003, which was amended in July, 2017, and began to conduct the certification program of the GLP. In April 2007, the CFDA issued the Circular on Measures for Certification of Good Laboratory Practice, or CFDA Circular 214, providing that the NMPA is responsible for certification of nonclinical research institutions. Under CFDA Circular 214, the NMPA decides whether an institution is qualified for undertaking pharmaceutical nonclinical research upon the evaluation of the institution’s organizational administration, its research personnel, its equipment and facilities and its operation and management of nonclinical pharmaceutical projects. If all requirements are met, a GLP Certification will be issued by the NMPA and the result will be published on the NMPA’s website.
Currently for all our ongoing projects, we cooperated with NMPA certified GLP laboratories operated by Joinn Laboratories to conduct the studies following GLP based on NMPA requirements.
Approval for Clinical Trials and Production of New Drugs
According to the Provisions for Drug Registration promulgated by the CFDA in 2007, Drug Administration Law promulgated and amended by the Standing Committee of the National People’s Congress in 2015, Circular on Regulations for Special Approval on New Drug Registration issued by the CFDA in 2009, and Circular on Information Publish Platform for Pharmaceutical Clinical Trials issued by the CFDA in 2013, we must comply with the following procedures and obtain several approvals for clinical trials and production of new drugs.
Clinical Trial Application
Upon completion of its nonclinical research, a research institution must apply for approval of a Clinical Trial Application before conducting clinical trials.
Special Examination and Approval for Domestic Category 1 Pharmaceutical Products
Domestic Category 1 New Drugs Are Eligible for Special Examination and Approval
According to Provisions for Drug Registration promulgated by the CFDA in 2007, drug registration applications are divided into three different types, namely Domestic New Drug Application, Domestic Generic Drug Application, and Imported Drug Application. Drugs fall into one of three categories, namely chemical medicine, biological product or traditional Chinese or natural medicine. A Category 1 drug is a new drug that has never been marketed in any country and will be manufactured in China. Our product candidates qualify as a domestic Category 1 new drug.
According to Provisions on the Administration of Special Examination and Approval of Registration of New Drugs, or the Special Examination and Approval Provisions promulgated by the CFDA in January 2009, the NMPA conducts special examination and approval for new drugs registration application when:
| 1. | the effective constituent of drug extracted from plants, animals, minerals, etc. as well as the preparations thereof have never been marketed in China, and the material medicines and the preparations thereof are newly discovered; |
| 2. | the chemical raw material medicines as well as the preparations thereof and the biological product have not been approved for marketing home and abroad; |
| 3. | the new drugs are for treating AIDS, malignant tumors and rare diseases, etc., and have obvious advantages in clinic treatment; or |
| 4. | the new drugs are for treating diseases with no effective methods of treatment. |
The Special Examination and Approval Provisions provide that the applicant may file for special examination and approval at the stage of Clinical Trial Application if the drug candidate falls within item (1) or (2). The provisions provide that for product candidates that fall within items (3) or (4), the application for special examination and approval must be made when filing for production.
We believe that our current product candidates fall within items (2) and (3) above. Therefore, we may file an application for special examination and approval at the Clinical Trial Application stage, which may enable us to pursue a more expedited path to approval in China and bring therapies to patients more quickly.
The Advantages of Category 1 New Drugs over Category 5 Drugs
Prior to the enactment of Reform Plan for Registration Category of Chemical Medicine, Category 3 drugs are drugs which have already been marketed abroad by multinational companies, but are not yet approved in China, and Category 3 drugs are reclassified as Category 5 according to the Reform Plan for Registration Category of Chemical Medicine issued by CFDA in March 2016. Compared with the application for Category 5 drugs, the application for Category 1 domestic new drugs has a more straight-forward registration pathway. According to Provisions for Drug Registration, where a special examination and approval treatment is granted, the application for clinical trial and manufacturing will be handled with priority and with enhanced communication with the Center for Drug Evaluation of the NMPA, or the CDE, which will establish a working mechanism for communicating with the applicants. If it becomes necessary to revise the clinical trial scheme or make other major alterations during the clinical trial, the applicant may file an application for communication. When an application for communication is approved, the CDE will arrange the communication with the applicant within one month.
In comparison, according to Provisions for Drug Registration, the registration pathway for Category 5 drugs is complicated and evolving. Category 5 drug applications may only be submitted after a company obtains an NDA approval and receives the CPP granted by a major regulatory authority, such as the FDA or the EMA. Multinational companies may need to apply for conducting multi-regional clinical trials, which means that companies do not have the flexibility to design the clinical trials to fit the Chinese patients and standard-of-care. Category 5 product candidates may not qualify to benefit from fast track review with priority at the Clinical Trial Application stage. Moreover, a requirement to further conduct local clinical trials can potentially delay market access by several years from its international NDA approval. Further, according to Opinions on Reforming the Review and Approval Process for Pharmaceutical Products and Medical Devices issued by the State Council in August 2015, which is a guideline for future legislation and NMPA examination, the drugs which have already been marketed abroad may no longer be categorized as new drugs under Chinese law in the future, and therefore may not be able to enjoy any preferential treatment for new drugs. In order to implement this guideline, in March 2016, the CFDA issued the Reform Plan for Registration Category of Chemical Medicine, which changed the registration category of chemical medicine stipulated in Provisions for Drug Registration. According to the Interpretation of Reform Plan for Registration Category of Chemical Medicine issued by CFDA, a new drug refers to a drug that has never been marketed in China or abroad.
Our product candidates are all new therapeutic agents and we expect that all of our current product candidates fall under the Category 1 application process. Although the regulatory framework normally requires approval of separate Clinical Trial Applications prior to initiating each phase of clinical development, in December 2015, the CFDA approved our Clinical Trial Application including all phases of clinical trials for Plinabulin as a direct anticancer agent in NSCLC when combined with docetaxel and for the treatment of CIN.
Changes to the Review and Approval Process
In August 2015, the State Council issued Opinions on Reforming the Review and Approval Process for Pharmaceutical Products and Medical Devices, providing several potential policy changes that could benefit the pharmaceutical industry:
| • | A plan to accelerate innovative drug approval with a special review and approval process, with a focus on areas of high unmet medical needs, including drugs for HIV, cancer, serious infectious diseases, orphan diseases and drugs on national priority lists; |
| • | A plan to adopt a policy which would allow companies to act as the marketing authorization holder and to hire contract manufacturing organizations to produce drug products; |
| • | A plan to improve the review and approval of clinical trials, and to allow companies to conduct clinical trials in China at the same time as they are doing so in other countries and encourage local clinical trial organizations to participate in international multi-center clinical trials. |
In November 2015, the Standing Committee of the National People’s Congress issued the Decision on Authorizing the State Council to Conduct the Pilot Program of the System of the Holders of Drug Marketing Licenses in Certain Areas and the Relevant Issues, which authorized the State Council to conduct the pilot program of the system of the holders of drug marketing licenses in Beijing, Tianjin, Hebei, Shanghai, Jiangsu, Zhejiang, Fujian, Shandong, Guangdong and Sichuan, and authorized the State Council to conduct reforms of registration category for drugs.
In November 2015, the CFDA released the Circular Concerning Several Policies on Drug Registration Review and Approval, which further clarified the following policies potentially simplifying and accelerating the approval process of clinical trials:
| • | A one-time umbrella approval procedure allowing approval of all phases of a new drug’s clinical trials at once, rather than the current phase-by-phase approval procedure, will be adopted for new drugs’ clinical trial applications; |
| • | A fast track drug registration or clinical trial approval pathway will be available for the following applications: (1) registration of innovative new drugs treating HIV, cancer, serious infectious diseases and orphan diseases; (2) registration of pediatric drugs; (3) registration of geriatric drugs that treat China-prevalent diseases; (4) registration of drugs sponsored by national science and technology grants; (5) registration of innovative drugs using advanced technology, using innovative treatment methods, or having distinctive clinical benefits; (6) registration of foreign innovative drugs to be manufactured locally in China; (7) concurrent applications for new drug clinical trials which are already approved in the U.S. or European Union, or concurrent drug registration applications for drugs which have applied for marketing authorization and passed onsite inspections in the U.S. or European Union and are manufactured using the same production line in China; and (8) clinical trial applications for drugs with urgent clinical need and patent expiry within three years, and marketing authorization applications for drugs with urgent clinical need and patent expiry within one year. |
In December 2017, the CFDA released the Opinions on Encouraging the Prioritized Evaluation and Approval for Drug Innovations, which further stipulated the scope of priority review and approval. The following drugs will be entitled to priority review and approval:
| • | Applications for drugs with obvious clinical benefits if any of the following circumstances applies: (1) registration applications for innovative drugs that are not marketed in China and abroad; (2) registration applications for innovative drugs to be manufactured locally in China; (3) registration applications for drugs using advanced formulation technology, using innovative treatment methods, or having distinctive clinical benefits; (4) clinical trial applications for patented drugs with patent to be expired in three years and manufacturing applications for drugs with patent to be expired in one year; (5) concurrent applications for new drug clinical trials which are already approved in the U.S. or European Union, or concurrent drug registration applications for drugs which have applied for marketing authorization and passed onsite inspections in the U.S. or European Union and are manufactured using the same production line in China; (6) registration applications for traditional Chinese drugs (including ethnic drugs) with clear clinical directions in the prevention and treatment of severe diseases; (7) registration applications for new drugs sponsored by national science and technology grants and of which clinical trials were conducted by a national clinical medical research center and approved by the administration department of such center. |
| • | Applications for drugs with obvious clinical benefits in the prevention and treatment of following diseases: (1) HIV; (2) pulmonary tuberculosis; (3) viral hepatitis; (4) orphan diseases; (5) malignant tumor; (6) pediatric drugs; (7) senile diseases. |
In May 2016, the General Office of the State Council issued Circular 41, which signals that the Drug Marketing Authorization Holder System is finally put into implementation. Circular 41 allows institutions of drugs research and development and research specialist staffs in Beijing, Tianjin, Hebei, Shanghai, Jiangsu, Zhejiang, Fujian, Shandong, Guangdong and Sichuan, to act as the applicant of drugs registration and to submit applications for drug clinical trials and drugs marketing. For the drugs newly registered after the effective date of Circular 41, applicants are allowed to submit applications for becoming a drugs marketing authorization holder at the same time as they submit applications for drug clinical trials or drugs marketing. In August 2016, the CFDA issued Circular on Conducting Works Regarding the Pilot Program for the Drug Marketing Authorization Holder System, which further detail the application procedures stipulated in Circular 41. In August 2017, the CFDA issued the Circular on the Matters Relating to Promotion of the Pilot Program for the Drug Marketing Authorization Holder System. This notice is issued, among other things, to advance implementation of a system pilot program for holders of drug marketing authorization, to delineate the rights and obligations of such holders, to enhance the quality of drug manufacturing process and to improve the responsibility system over drug manufacturing and marketing supply chains. In October 2018, the NMPA issued the Decisions on Extending the Period of the Pilot Program for the Drug Marketing Authorization Holder System in Several Regions (Draft), which extends the expiration date of the pilot program from November 4, 2018 to the implementation date of the revised Drug Administration Law.
Non-Inferiority Standard
In China, a drug may receive regulatory approval without showing superiority in its primary endpoint. Rather, a drug may be approved for use if it shows non-inferiority in its primary endpoint and superiority in one of its secondary endpoints.
Accelerated or Conditional Approval
In October 2017, the Central Committee of the Communist Party of China and General Office of the State Council issued the Opinions on Deepening the Reform of the Evaluation and Approval System and Inspiring Innovation of Drugs and Medical Devices. This opinion provides that, among other things:
| • | the review and approval process should be accelerated for drugs or medical devices that are urgently in need for clinical practice; |
| • | for drugs or medical devices that are (i) for treatment of severe and life threatening diseases that cannot be cured in an effective manner, or (ii) urgently in need to improve public health, if early and mid-term indicators in clinical trials for the aforementioned drugs or medical devices show efficacy and potential clinical value, the marketing of these drugs and medical devices may be approved conditionally, and companies who desire to market such drugs or medical devices shall develop risk control plans for conducting researches according to applicable requirements; |
| • | extension of the patent term for certain new drugs may be granted, given that clinical trials and the review and approval process may cause delay in bringing new drugs to the market; and |
| • | clinical trial data obtained from foreign countries may be used to register drugs and medical devices in China if such data meet applicable requirements for the registration of drugs and medical devices in China. |
Four Phases of Clinical Trials
A clinical trial consists of Phases 1, 2, 3 and 4. Phase 1 refers to the initial clinical pharmacology and safety evaluation studies in humans. Phase 2 refers to the preliminary evaluation of a drug candidate’s therapeutic effectiveness and safety for particular indication(s) in patients, which provides evidence and support for the design of Phase 3 clinical trial and settles the administrative dose regimen. Phase 3 refers to clinical trials undertaken to confirm the therapeutic effectiveness of a drug. Phase 3 is used to further verify the drug’s therapeutic effectiveness and safety on patients with target indication(s), to evaluate overall benefit-risk relationships of the drug, and ultimately to provide sufficient evidence for the review of drug registration application. Phase 4 refers to a new drug’s post-marketing study to assess therapeutic effectiveness and adverse reactions when the drug is widely used, to evaluate overall benefit-risk relationships of the drug when used among general population or specific groups, and to adjust the administration dose, etc.
Drug Clinical Practice Certification
To improve the quality of clinical trial, the CFDA promulgated the Administration of Quality of Drug Clinical Practice in August 2003. In February 2004, the CFDA issued the Circular on Measures for Certification of Drug Clinical Practice (trial), providing that the NMPA is responsible for certification of clinical trial institutions, and that the National Health and Family Planning Commission of the PRC, formerly known as the Ministry of Health is responsible for relevant things in respect of certification of clinical trial institutions within its duties. Under the Circular on Measures for Certification of Drug Clinical Practice (trial), the NMPA and the National Health and Family Planning Commission of the PRC decide whether an institution is qualified for undertaking pharmaceutical clinical trial upon the evaluation of the institution’s organizational administration, its research personnel, its equipment and facilities, its management system and its standard operational rules. If all requirements are met, a GCP Certification will be issued by the NMPA and the result will be published on the NMPA’s website.
New Drug Application
When Phase 1, 2 and 3 of the clinical trials have been completed, the applicant must apply to the NMPA for approval of an NDA. The NMPA then determines whether to approve the application according to the comprehensive evaluation opinion provided by the CDE of the NMPA. We have obtained approval of our Clinical Trial Application for Plinabulin as a direct anticancer agent in NSCLC when combined with docetaxel in China, and we initiated clinical trials in June 2016. We must obtain approval of an NDA before our drugs can be manufactured and sold in the Chinese market.
Good Manufacturing Practice
All facilities and techniques used in the manufacture of products for clinical use or for sale in China must be operated in conformity with cGMP guidelines as established by the NMPA. Failure to comply with applicable requirements could result in the termination of manufacturing and significant fines. The NMPA issued the Good Manufacturing Practice for Drugs Used in Clinical Trial (Exposure Draft for Public Comment) on July 2018, which provides the requirements on quality management, personnel, facilities and equipment, packaging and certain other issues relating to drugs used in clinical trials. Such exposure draft has yet to take effect.
Animal Test Permits
According to Regulations for the Administration of Affairs Concerning Experimental Animals promulgated by the State Science and Technology Commission in November 1988 and amended in January 2011, July 2013 and March 2017 and Administrative Measures on the Certificate for Animal Experimentation promulgated by the State Science and Technology Commission and other regulatory authorities in December 2001, performing experimentation on animals requires a Certificate for Use of Laboratory Animals. Applicants must satisfy the following conditions:
| • | Laboratory animals must be qualified and sourced from institutions that have Certificates for Production of Laboratory Animals; |
| • | The environment and facilities for the animals’ living and propagating must meet state requirements; |
| • | The animals’ feed and water must meet state requirements; |
| • | The animals’ feeding and experimentation must be conducted by professionals, specialized and skilled workers, or other trained personnel; |
| • | The management systems must be effective and efficient; and |
| • | The applicable entity must follow other requirements as stipulated by Chinese laws and regulations. |
International Multi-Center Clinical Trials Regulations
On January 30, 2015, the CFDA promulgated Notice on Issuing the International Multi-Center Clinical Trial Guidelines (Trial), or the Multi-Center Clinical Trial Guidelines, which took effect as of March 1, 2015, aiming to provide guidance for the regulation of application, implementation and administration of international multi-center clinical trials in China. Pursuant to the Multi-Center Clinical Trial Guidelines, international multi-center clinical trial applicants may simultaneously perform clinical trials in different centers using the same clinical trial protocol. Where the applicant plans to make use of the data derived from the international multi-center clinical trials for application to NMPA for approval of an NDA, such international multi-center clinical trials shall satisfy, in addition to the requirements set forth in Drug Administration Law and its implementation regulations, Provisions for Drug Registration and relevant laws and regulations, the following requirements:
| • | The applicant shall first conduct an overall evaluation on the global clinical trial data and further make trend analysis of the Asian and Chinese clinical trial data. In the analysis of Chinese clinical trial data, the applicant shall consider the representativeness of the research subjects i.e. the participating patients; |
| • | The applicant shall analyze whether the amount of Chinese research subjects is sufficient to assess and adjudicate the safety and effectiveness of the drug under clinical trial, and satisfy the statistical and relevant legal requirements; and |
| • | The onshore and offshore international multi-centers clinical trial research centers shall be subject to on-site inspections of competent PRC governmental agencies. |
International multi-center clinical trials shall follow international prevailing GCP principles and ethics requirements. Applications shall ensure the truthfulness, reliability and trustworthiness of clinical trials results; the researchers shall have the qualification and capability to perform relevant clinical trials; ethics committee shall continuously review the trials and protect the subjects’ interests, benefits and safety. Before the performance of the international multi-center clinical trial applicants shall obtain clinical trial approvals or complete filings pursuant to requirements under the local regulations where clinical trials are conducted, and register and disclose the information of all major researcher, clinical trial organizations on the NMPA drug clinical trial information platform.
When using international multi-center clinical trial data to support NDAs in China, applicants shall submit the completed global clinical trial report, statistical analysis report and database, along with relevant supporting data in accordance with ICH-CTD (International Conference on Harmonization-Common Technical Document) content and format requirements; subgroup research results summary and comparative analysis shall also be conducted concurrently.
Leveraging the clinical trial data derived from international multi-center clinical trials, we may avoid unnecessary repetitive clinical trials and thus further accelerate the NDA process in China.
PRC Enterprise Income Tax Law and Its Implementation
The EIT Law and its implementation rules permit certain High and New Technologies Enterprises, or HNTEs, to enjoy a preferential enterprise income tax rate subject to these HNTEs meeting certain qualification criteria. One of our Chinese subsidiaries enjoys such preferential tax treatment.
On March 23, 2016, the Ministry of Finance and the SAT issued the Circular on Comprehensively Promoting the Pilot Program of the Collection of Value-added Tax in Lieu of Business Tax. Effective from May 1, 2016, the PRC tax authorities collect VAT in lieu of business tax in all regions and industries. VAT is applicable at a rate of 6% in lieu of business taxes for certain services and 17% for the sale of goods and provision of tangible property lease services. VAT payable on goods sold or taxable services provided by a general VAT taxpayer for a taxable period is the net balance of the output VAT for the period after crediting the input VAT for the period.
Regulations Relating to Intellectual Property Rights
Patent
General
Pursuant to the Patent Law of the PRC, most recently amended in December 2008, and its implementation rules, most recently amended in January 2010, patents in China fall into three categories, namely invention patent, utility model and design patent. Invention patent refers to a new technical solution proposed in respect of a product, method or its improvement; utility model refers to a new technical solution that is practicable for application and proposed in respect of the shape, structure or a combination of both of a product; and design patent refers to the new design of a certain product in shape, pattern or a combination of both and in color, shape and pattern combinations aesthetically suitable for industrial application. Under the Patent Law of the PRC, the term of patent protection starts from the date the patent was filed. Patents relating to utility-models and designs are effective for ten years from the initial date the patent application was filed, and patents relating to invention are effective for twenty years from the initial date the patent application was filed. The Patent Law of the PRC adopts the principle of “first to file,” which means where more than one person files a patent application for the same invention, a patent will be granted to the person who first filed the application.
Existing patents can become invalid or unenforceable due to a number of factors, including lack of novelty, and/or lack of inventive step in technology, and deficiencies in patent application. In China, a patent must have novelty, inventive step and practical applicability. Under the Patent Law of the PRC, novelty means that before a patent application is filed, no identical invention or utility model has been publicly disclosed in any publication in China or abroad or has been publicly used or made known to the public by any other means, whether in or outside of China, nor has any other person filed with the patent authority an application that describes an identical invention or utility model and is recorded in patent application documents or patent documents published after the filing date. Inventive step means, compared with existing technology, an invention has prominent substantial features and represents notable progress, and a utility model has substantial features and represents any progress; practical applicability means an invention or utility model can be manufactured or used and may produce positive results. Patents in China are filed with the National Intellectual Property Administration of the PRC, or CNIPA. Normally, the CNIPA publishes an application for an invention patent 18 months after the application is filed, which may be shortened upon request by the applicant. The applicant must apply to the CNIPA for a substantive examination within three years from the date the application is filed.
Article 20 of the Patent Law of the PRC provides that, for an invention or utility model completed in China, any applicant (not just Chinese companies and individuals), before filing a patent application outside of China, must first submit it to the CNIPA for a confidential examination. Failure to comply with this requirement will result in the denial of any Chinese patent for the subject invention. This added requirement of confidential examination by the CNIPA has raised concerns by foreign companies who conduct research and development activities in China or outsource research and development activities to service providers in China. Currently we have three invention patents published by CNIPA and one invention patent under the application process.
Patent Enforcement
Unauthorized use of patents without consent from owners of patents, forgery of the patents belonging to other persons, or engagement in other infringement acts against patent rights, will subject the infringers to tortious liabilities. Serious offences of forgery of the patents belonging to other persons may be subject to criminal penalties.
When a dispute arises as a result of infringement of the patent owner’s patent right, Chinese law requires that the parties first attempt to settle the dispute through consultation between them. However, if the dispute cannot be settled through consultation, the patent owner, or an interested party who believes the patent is being infringed, may either file a civil legal suit or file an administrative complaint with the relevant patent administration authority. A Chinese court may issue a preliminary injunction upon the patent owner’s or an interested party’s request before instituting any legal proceedings or during the proceedings. Damages for infringement are calculated as the loss suffered by the patent holder arising from the infringement, and if the loss suffered by the patent holder arising from the infringement is uncertain, the damages for infringement shall be calculated as the benefit gained by the infringer from the infringement. If it is difficult to ascertain damages in this manner, damages may be determined by using a reasonable multiple of the license fee under a contractual license. As in other jurisdictions, with one notable exception, the patent owner in China has the burden of proving that the patent is being infringed. However, if the owner of an invention patent for manufacturing process of a new product alleges infringement of its patent, the alleged infringer has the burden of proving that it has not infringed. To our knowledge, there are no disputes as to our infringement of any third party’s patent.
Medical Patent Compulsory License
According to the Patent Law of the PRC, for the purpose of public health, the CNIPA may grant a compulsory license for manufacturing patented drugs and exporting them to countries or regions covered under relevant international treaties to which the People’s Republic of China has acceded.
Exemptions for Unlicensed Manufacture, Use, Sell or Import of Patented Drugs
The Patent Law of the PRC provides five exceptions for unlicensed manufacture, use, sell or import of patented drugs. None of following circumstances are deemed an infringement of the patent rights, and any person may manufacture, use, sell or import patented drugs without authorization granted by patent owner as follows:
| • | Any person who uses, promises to sell, sells or imports any patented product or product directly obtained in accordance with the patented methods after such product is sold by the patent owner or by its licensed entity or individual; |
| • | Any person who has manufactured an identical product, has used an identical method or has made necessary preparations for manufacture or use prior to the date of patent application continues to manufacture such product or use such method only within the original scope; |
| • | Any foreign transportation facility that temporarily passes through the territory, territorial waters or territorial airspace of China uses the relevant patents in its devices and installations for its own needs in accordance with any agreement concluded between China and that country to which the foreign transportation facility belongs, or any international treaty to which both countries are party, or on the basis of the principle of reciprocity; |
| • | Any person who uses the relevant patents solely for the purposes of scientific research and experimentation; or |
| • | Any person who manufactures, uses or imports patented drugs or patented medical equipment for the purpose of providing information required for administrative approval, or manufactures, uses or imports patented drugs or patented medical equipment for the abovementioned person. |
However, even if patented drugs are utilized on the ground of exemptions for unlicensed manufacture, use, sell or import of patented drugs prescribed in Patent Law of the PRC, such patented drugs cannot be manufactured, used, sold or imported for any commercial purposes without authorization granted by the patent owner.
Trademarks
Trademarks are protected by the Trademark Law of the People’s Republic of China, or the PRC Trademark Law, adopted on August 23, 1982 and subsequently amended on February 22, 1993, October 27, 2001 and August 30, 2013, respectively, as well as the Implementation Regulation of the PRC Trademark Law adopted by the State Council on August 3, 2002 and amended on April 29, 2014. The Trademark Office of the CNIPA handles trademark registrations and grants a term of ten years to registered trademarks and another ten years if requested upon expiry of the first or any renewed ten-year term. The PRC Trademark Law has adopted a “first-to-file” principle with respect to trademark registration.
Trade Secrets
According to the Law Against Unfair Competition of the People’s Republic of China, or the Anti-Unfair Competition Law, of China promulgated in September 1993 and amended in November 2017, the term “trade secrets” refers to technical information and business information that is unknown to the public, that has utility and may create business interest or profit for its legal owners or holders, and that is maintained as a secret by its legal owners or holders.
Under this law, business persons are prohibited from employing the following methods to infringe trade secrets: (1) obtaining the trade secrets from the legal owners or holders by any unfair methods such as stealing, solicitation or coercion; (2) disclosing, using or permitting others to use the trade secrets obtained illegally under item (1) above; or (3) disclosing, using or permitting others to use the trade secrets, in violation of any contractual agreements or any requirements of the legal owners or holders to keep such trade secrets in confidence. If a third party knows or should have known of the above-mentioned illegal conduct but nevertheless obtains, uses or discloses trade secrets of others, the third party may be deemed to have committed a misappropriation of the others’ trade secrets. The parties whose trade secrets are being misappropriated may petition for administrative corrections, and regulatory authorities may stop any illegal activities and fine infringing parties in the amount of RMB100,000 to RMB500,000, and where the infringement is material, the fine shall range from RMB500,000 to RMB3,000,000. Alternatively, persons whose trade secrets are being misappropriated may file lawsuits in a Chinese court for loss and damages caused by the misappropriation.
The measures to protect trade secrets include oral or written agreements or other reasonable measures to require the employees of, or persons in business contact with, legal owners or holders to keep trade secrets confidential. Once the legal owners or holders have asked others to keep trade secrets confidential and have adopted reasonable protection measures, the requested persons bear the responsibility for keeping the trade secrets confidential.
Regulations Relating to Foreign Exchange and Dividend Distribution
Foreign Exchange Regulation
The Foreign Exchange Administration Regulations, most recently amended in August 2008, are the principal regulations governing foreign currency exchange in China. Under Chinese foreign exchange regulations, payments of current account items, such as trade and service-related foreign exchange transactions, may be made in foreign currencies without prior approval from SAFE, by complying with certain procedural requirements. In contrast, approval from or registration with appropriate government authorities is required when RMB is to be converted into a foreign currency and remitted out of China to pay capital expenses such as the repayment of foreign currency-denominated loans.
In November 2012, SAFE promulgated the Circular of Further Improving and Adjusting Foreign Exchange Administration Policies on Foreign Direct Investment, which substantially amends and simplifies the current foreign exchange procedure. Pursuant to this circular, the opening of various special purpose foreign exchange accounts, such as pre-establishment expenses accounts, foreign exchange capital accounts and guarantee accounts, the reinvestment of RMB proceeds by foreign investors in China, and remittance of foreign exchange profits and dividends by a Foreign Investment Enterprise, or FIE, to its foreign shareholders no longer require the approval or verification of SAFE, and multiple capital accounts for the same entity may be opened in different provinces, which was not previously possible. In addition, SAFE promulgated the Circular on Printing and Distributing the Provisions on Foreign Exchange Administration over Domestic Direct Investment by Foreign Investors and the Supporting Documents in May 2013, which specifies that the administration by SAFE or its local branches over direct investment by foreign investors in China will be conducted by way of registration, and banks must process foreign exchange business relating to the direct investment in China based on the registration information provided by SAFE and its branches.
Under the Circular of the SAFE on Further Improving and Adjusting the Policies for Foreign Exchange Administration under Capital Accounts promulgated by SAFE on January 10, 2014 and effective from February 10, 2014, administration over the outflow of the profits by domestic institutions has been further simplified. In principle, a bank is no longer required to examine transaction documents when handling the outflow of profits of no more than the equivalent of $50,000 by a domestic institution. When handling the outflow of profits exceeding the equivalent of $50,000, the bank, in principle, is no longer required to examine the financial audit report and capital verification report of the domestic institution, provided that it must examine, according to the principle of transaction authenticity, the profit distribution resolution of the board of directors (or the profit distribution resolution of the partners) relating to this profit outflow and the original copy of its tax record-filing form. After each profit outflow, the bank must affix its seal to and endorsements on the original copy of the relevant tax record-filing form to indicate the actual amount of the profit outflow and the date of the outflow.
On March 30, 2015, SAFE promulgated the Circular on Reforming the Management Approach regarding the Settlement of Foreign Exchange Capital of Foreign-invested Enterprises, or SAFE Circular 19, which became effective on June 1, 2015. According to SAFE Circular 19, the foreign exchange capital of foreign-invested enterprises may be settled on a discretionary basis, meaning that the foreign exchange capital in the capital account of an FIE for which the rights and interests of monetary contribution has been confirmed by the local foreign exchange bureau (or the book-entry registration of monetary contribution by the banks) can be settled at the banks based on the actual operational needs of the FIE. The proportion of such discretionary settlement is temporarily determined as 100%. The RMB converted from the foreign exchange capital will be kept in a designated account, and if an FIE needs to make further payment from such account, it still must provide supporting documents and go through the review process with the banks.
Furthermore, SAFE Circular 19 stipulates that the use of capital by FIEs must adhere to the principles of authenticity and self-use within the business scope of enterprises. The capital of an FIE and capital in RMB obtained by the FIE from foreign exchange settlement must not be used for the following purposes:
| • | directly or indirectly used for the payment beyond the business scope of the enterprises or the payment prohibited by relevant laws and regulations; |
| • | directly or indirectly used for investment in securities, unless otherwise provided by relevant laws and regulations; |
| • | directly or indirectly used for granting the entrusted loans in RMB, unless permitted by the scope of business, repaying the inter-enterprise borrowing (including advances by the third party), or repaying the bank loans in RMB that have been sub-lent to the third party; and/or |
| • | paying the expenses related to the purchase of real estate that is not for self-use, except for the foreign-invested real estate enterprises. |
On June 9, 2016, SAFE promulgated the Circular on Reforming and Regulation of Administrative Policy on Settlement of Foreign Exchange of Capital Account, or SAFE Circular 16, which became effective on June 9, 2016. According to SAFE Circular 16, the foreign exchange capital of FIEs, foreign debt and funds raised through offshore listing may be settled on a discretionary basis, and can be settled at the banks. The proportion of such discretionary settlement is temporarily determined as 100%. The RMB converted from relevant foreign exchange will be kept in a designated account, and if a domestic enterprise needs to make further payment from such account, it still must provide supporting documents and go through the review process with the banks.
Furthermore, SAFE Circular 16 reiterates that the use of capital by domestic enterprises must adhere to the principles of authenticity and self-use within the business scope of enterprises. The foreign exchange income of capital account and RMB obtained by domestic enterprise from foreign exchange settlement must not be used for the following purposes:
| • | directly or indirectly used for the payment beyond the business scope of the enterprises or the payment prohibited by relevant laws and regulations; |
| • | directly or indirectly used for investment in securities and investment in wealth management products except for principal-guaranteed bank wealth management products, unless otherwise provided by relevant laws and regulations; |
| • | directly or indirectly used for extending the entrusted loans to non-affiliate enterprises, unless permitted by the scope of business; and/or |
| • | used for construction or purchase of real estate that is not for self-use, except for the foreign-invested real estate enterprises. |
On January 26, 2017, SAFE issued the Notice on Improving the Examination of Authenticity and Compliance to Further Promote Foreign Exchange Administration, or the SAFE Circular 3, which stipulates several capital control measures with respect to the outbound remittance of profit from domestic entities to offshore entities, including (i) under the principle of genuine transaction, banks shall check board resolutions regarding profit distribution, the original version of tax filing records and audited financial statements; and (ii) domestic entities shall hold income to account for previous years’ losses before remitting the profits. Moreover, pursuant to SAFE Circular 3, domestic entities shall make detailed explanations of the sources of capital and utilization arrangements, and provide board resolutions, contracts and other proof when completing the registration procedures in connection with an outbound remittance.
Our Chinese subsidiaries’ distributions to the offshore parent and carrying out cross-border foreign exchange activities shall comply with the various SAFE registration requirements described above.
Share Option Rules
Under the Administration Measures on Individual Foreign Exchange Control issued by the People’s Bank of China on December 25, 2006, all foreign exchange matters involved in employee share ownership plans and share option plans in which Chinese citizens participate require approval from SAFE or its authorized branch. In addition, under the Notices on Issues concerning the Foreign Exchange Administration for Domestic Individuals Participating in Share Incentive Plans of Overseas Publicly-Listed Companies, or Share Option Rules, issued by the SAFE on February 15, 2012, Chinese residents who are granted shares or share options by companies listed on overseas stock exchanges under share incentive plans are required to (1) register with the SAFE or its local branches; (2) retain a qualified Chinese agent, which may be a Chinese subsidiary of the overseas listed company or another qualified institution selected by the Chinese subsidiary, to conduct the SAFE registration and other procedures with respect to the share incentive plans on behalf of the participants; and (3) retain an overseas institution to handle matters in connection with their exercise of share options, purchase and sale of shares or interests and funds transfers.
SAFE Regulations on Offshore Special Purpose Companies Held by Chinese Residents or Citizens
SAFE promulgated SAFE Circular 37 on July 4, 2014, which replaced the former circular commonly known as “SAFE Circular 75” promulgated by SAFE on October 21, 2005. SAFE Circular 37 regulates foreign exchange matters in relation to the use of special purpose vehicles, or SPVs, by Chinese residents to seek overseas investment and financing and conduct round trip investment in China. Under SAFE Circular 37, an SPV refers to an offshore entity established or controlled, directly or indirectly, by Chinese residents or entities for the purpose of overseas investment and financing, with Chinese residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or interests, while “round trip investment” refers to the direct investment in China by Chinese residents through SPVs, namely, establishing FIEs to obtain the ownership, control rights and management rights. Pursuant to SAFE Circular 37, before making contribution into an SPV, Chinese residents are required to complete foreign exchange registration with SAFE or its local branch. SAFE Circular 37 further requires amendment to the registration in the event of any significant changes with respect to the special purpose vehicle, such as increase or decrease of capital contributed by PRC individuals, share transfer or exchange, merger, division or other material event. Failure to comply with the registration procedures set forth in SAFE Circular 37, or making misrepresentation on or failure to disclose controllers of an FIE that is established through round-trip investment, may result in restrictions on the foreign exchange activities of the relevant FIE, including payment of dividends and other distributions, such as proceeds from any reduction in capital, share transfer or liquidation, to its offshore parent or affiliate, and the capital inflow from the offshore parent, and may also subject relevant Chinese residents to penalties under PRC foreign exchange administration regulations.
Pursuant to SAFE Circular 37, PRC residents who participate in share incentive plans in overseas non-publicly-listed companies may submit applications to SAFE or its local branches for the foreign exchange registration with respect to offshore SPV. For more information on compliance with SAFE Circular 37, please see “Item 3. Key Information—D. Risk Factors—Risks Related to Our Doing Business in China—Chinese regulations relating to investments in offshore companies by Chinese residents may subject our future Chinese resident beneficial owners or our Chinese subsidiaries to liability or penalties, limit our ability to inject capital into our Chinese subsidiaries or limit our Chinese subsidiaries’ ability to increase their registered capital or distribute profits.”
We have completed the foreign exchange registration of PRC resident shareholders of Dr. Lan Huang, Mr. Linqing Jia and Mr. Dong Liang.
Regulation of Dividend Distribution
The principal laws, rules and regulations governing dividend distribution by FIEs in China are the Company Law of the PRC, most recently amended in December 2013, the Wholly Foreign-owned Enterprise Law, most recently amended in October 2016, and its implementation regulations, most recently amended in February 2014, and the Sino-Foreign Equity Joint Venture Law, most recently amended in October 2016, and its implementation regulations, most recently amended in February 2014. Under these laws, rules and regulations, FIEs may pay dividends only out of their accumulated profit, if any, as determined in accordance with Chinese accounting standards and regulations. Both Chinese domestic companies and wholly-foreign owned Chinese enterprises are required to allocate at least 10% of their respective accumulated after-tax profits each year, if any, to fund certain capital reserve funds until the aggregate amount of these reserve funds have reached 50% of the registered capital of the enterprises. A Chinese company is not permitted to distribute any profits until any losses from prior fiscal years have been offset. Profits retained from prior fiscal years may be distributed together with distributable profits from the current fiscal year.
Labor Laws and Social Insurance
Pursuant to the PRC Labor Law promulgated in July 1994 and amended in August 2009, and the PRC Labor Contract Law promulgated in June 2007 and amended in December 2012, employers must execute written labor contracts with full-time employees. All employers must comply with local minimum wage standards. Violations of the PRC Labor Contract Law and the PRC Labor Law may result in the imposition of fines and other administrative and criminal liability in the case of serious violations.
In addition, according to the PRC Social Insurance Law promulgated in October 2010 and Administrative Regulations on the Housing Provident Fund promulgated in April 1999 and amended in March 2002, employers like our Chinese subsidiaries in China must provide employees with welfare schemes covering pension insurance, unemployment insurance, maternity insurance, work-related injury insurance, medical insurance, and housing provident fund.
Foreign Investment Law
On March 15, 2019, the National People’s Congress approved the Foreign Investment Law, which will take effect on January 1, 2020 and replace the trio of existing laws regulating foreign investment in China, namely, the Sino-foreign Equity Joint Venture Enterprise Law, the Sino-foreign Cooperative Joint Venture Enterprise Law and the Wholly Foreign-invested Enterprise Law, together with their implementation rules and ancillary regulations. According to the Foreign Investment Law, “foreign investment” refers to investment activities directly or indirectly conducted by one or more natural persons, business entities, or other organizations of a foreign country in China.
According to the Foreign Investment Law, the State Council will publish a catalogue for special administrative measures, or the “negative list”, which provides the scope of “restricted” or “prohibited” industries that have certain restriction on foreign investment such as market entry clearance. Foreign investment in industries not included in the “negative list” are granted national treatment. Our principal business, the development of innovative cancer therapies, is not included in the current “negative list” which became effective on July 28, 2018. The new “negative list” has yet to be published, and it is unclear whether the “negative list” effective at the time when Foreign Investment Law comes into effect will defer from the current “negative list”.
Rest of the World Regulation
For other countries outside of the U.S. and China, the requirements governing the conduct of clinical trials, drug licensing, pricing and reimbursement vary from country to country. In all cases the clinical trials must be conducted in accordance with GCP requirements and the applicable regulatory requirements and the ethical principles having their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Manufacturing and Supply
We outsource the production of the active pharmaceutical ingredient of Plinabulin to an external service provider, Johnson Mattey, and the production of the final drug formulation to Pharmaceutics International Inc. and for contingency planning purposes, we have also established relationships with other contract manufacturing organizations. We expect to continue our outsourcing relationships with contract manufacturers to meet the ongoing requirements for the development of Plinabulin. We do not have a long-term agreement with these third parties. We have framework agreements with these external service providers, under which they provide services to us on a short-term, project-by-project basis.
Currently, our contract manufacturers obtain materials for the manufacturing activities they perform for us from multiple suppliers who we believe have sufficient capacity to meet our demands. In addition, we believe that adequate alternative sources for such supplies exist. However, a risk exists that an interruption supplies would materially harm our business. We typically order materials and services on a purchase order basis and do not enter into long-term dedicated capacity or minimum supply arrangements.
We rely on BASF SE as the sole supplier of the stabilizing agent, Solutol, used in Plinabulin’s current formulation. If BASF SE becomes unable or unwilling to supply Solutol, we will not be able to replace BASF SE and we would be required to reformulate Plinabulin.
Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements governing record keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among others. The contract manufacturing organizations we plan to use to manufacture our current product candidates, or any future product candidates we may develop, will be required to operate under cGMP conditions. These cGMP conditions are regulatory requirements for the production of pharmaceuticals that will be used in humans.
Legal Proceedings
From time to time we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, if determined adversely to us, would individually or taken together have a material adverse effect on our business, results of operations, financial condition or cash flows. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
| C. | Organizational Structure |
The diagram below depicts our current organizational structure that resulted from an internal restructuring consummated in July 2015.
| D. | Property, Plants and Equipment |
In May 2015, we began to lease office space of 276 leasable, 610 rentable, square feet in New York, with a total rent of $2,938.25 per month.
From August 2015 to February 2016, we leased additional office space in New York, increasing total space to 411 leasable, 908 rentable, square feet and the rent increased to $4,384.97 per month.
From March 2016 to April 2016, we leased additional office space in New York, increasing total space to 695 leasable, 1,535 rentable, square feet and the rent increased to $7,666.82 per month.
From May 2016 to February 2017, we leased additional office space in New York, increasing total space to 976 leasable, 2,156 rentable, square feet and the rent increased to $9,972.07 per month.
From March 2017 to April 2018, we leased additional office space in New York, increasing total space to 1,121 leasable, 2,476 rentable, square feet and the rent increases to $11,252.15 per month.
After March 2018, we leased additional office space in New York, increasing total space to 2,541 leasable, 5,614 rentable, square feet at $26,382.39 per month through February 2020.
After October 2018, we leased additional office space in New York, increasing total space to 7,238 rentable square feet at $60,159.72 per month through December 2023. Payments under operating leases are expensed on a straight-line basis over the periods of the respective leases, and the terms of the leases do not contain rent escalation, contingent rent, renewal or purchase options.
We leased office space of 12,721 square feet in Dalian, China under multiple leases through August 31, 2016. In accordance with our lease agreement with the Chinese government, rent for the office facilities in Dalian, China was free during the above leasing period. We leased offices in Dalian, China at approximately $2,000 per month starting September 2016. The rent was increased to approximately $2,500 per month starting September 2018.
We also leased office space of 189 square feet in San Diego at $2,359 per month from February 2016 to January 2018. We leased additional office space, increasing total space to 297 square feet at $3,708 per month through February 2018. After January 2018, we leased additional office space, increasing total space to 386 square feet at $4,618.8 per month through January 2020 and this lease agreement was terminated in June 2018.
We leased office space in Hong Kong at $15,552.26 per month from May 27, 2018 to January 15, 2019.
| Unresolved Staff Comments |
Not applicable.
Item 5. | Operating and Financial Review and Prospects |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our audited consolidated financial statements and the related notes included elsewhere in this annual report on Form 20-F. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those discussed in the section titled “Item 3. Key Information—D. Risk Factors” and in other parts of this annual report on Form 20-F. Our consolidated financial statements have been prepared in accordance with U.S. GAAP. The functional currency of BeyondSpring Inc. is the U.S. dollar.
Overview
We are a global clinical stage biopharmaceutical company focused on the development and commercialization of innovative immuno-oncology cancer therapies. Our lead asset, Plinabulin, is being studied in late stage clinical trials as an anti-cancer agent in combination with docetaxel in advanced NSCLS and for its potential benefit in the prevention of high and intermediate risk CIN. Plinabulin is also currently being studied in two investigator-initiated trials for its therapeutic potential in combination with various immuno-oncology agents, including in combination with nivolumab for the treatment of NSCLC at UCSD, the Fred Hutchinson Center, and the University of Washington and in combination with PD-1 and CTLA-4 antibodies for the treatment of SCLC at the Rutgers University. We own global rights to Plinabulin in all countries except China. We own a 60% interest in our China subsidiary, which subsequently owns 100% of the rights to Plinabulin in China. We are also developing three small molecule immune agents, currently in preclinical stages, and a drug development platform using ubiquitin mediated protein degradation pathway.
Our strategy is to develop a pipeline of product candidates that apply Plinabulin to different indications and other product candidates with the potential to be important components of multiple-agent combination regimes for the benefit of cancer patients. To implement our strategy, we use a novel, highly scalable business model that integrates clinical resources in the U.S. and China. We believe that our strategy of dual development in the U.S. and China has provided and will continue to provide significant developmental advantages including the ability to conduct trials in China, which could result in faster enrollment, lower costs and expedited approval process, as well as access to China’s large cancer population . Our drug development capabilities are facilitated by strong interest from clinical investigators in the U.S. as well as by our understanding of the pharmaceutical industry, clinical resources and regulatory system in China. In addition, this model represents significant commercial advantages for Plinabulin, as the U.S. and China are the two largest pharmaceutical markets in the world.
In addition to the clinical development programs in NSCLC and CIN for Plinabulin, we are utilizing our research collaborations to advance Plinabulin in clinical trials to investigate its therapeutic potential as an immuno-oncology agent. We provide financial support for these various investigator-initiated clinical trials as well as the drug supply of Plinabulin. In addition to exploring Plinabulin’s therapeutic potential in combination with immuno-oncology agents, we have a pipeline of preclinical immuno-oncology product candidates and de novo drug discovery with ubiquitin-mediated degradation pathway in collaboration with the University of Washington. We have utilized our research collaborations effectively and efficiently to advance these programs.
We intend to commercialize Plinabulin, if approved, in China through our subsidiary, Wanchun Bulin because China recognized Plinabulin as a National Science and Technology Major Project for “essential new drug research and development.” With receipt of the 2017 Grant, Plinabulin has been included in the National Drug Priority Review List. We believe that, pending drug approval and successful pricing negotiations with the Chinese government, the 2017 Grant could assist with inclusion of Plinabulin in the National Insurance System, which would allow for faster access to patients and reimbursement. We plan to either partner with one or more national pharmaceutical companies or build our own commercial organization for marketing Plinabulin for advanced NSCLC and in CIN in Beijing, Shanghai and Guangzhou areas, which represent 80% of China’s geographic cancer care market. We also plan to retain a contract sales organization for selling efforts in secondary markets in China. In the U.S. and for the rest of world, we intend to work with one or multiple potential pharmaceutical partners for commercialization.
Since the inception of Wanchun Biotech, the former holding company of our U.S. subsidiary, in 2010, our operations have focused on organizing and staffing our company, business planning, raising capital, establishing our intellectual property portfolio, including protecting the rights to Plinabulin, and conducting studies in animals and clinical trials of Plinabulin. We do not have any product candidates approved for sale and have not generated any revenue from product sales. We have financed our operations through a combination of equity financings and loans from related and third parties, which have been either assumed by Wanchun Biotech as part of our internal restructuring, converted into an equity investment, or repaid by us. On March 14, 2017, we completed our IPO, in which we issued 174,286 ordinary shares at $20.00 per share for gross proceeds of approximately $3.5 million. In conjunction with our IPO, we issued 2,541,048 ordinary shares in a private placement to certain investors at $20.00 per share for gross proceeds of approximately $50.8 million. As of December 31, 2018, we had cash of $3.9 million.
Since inception we have incurred operating losses. Our net losses were $12.5 million , $96.4 million and $57.5 million for the years ended December 31, 2016, 2017 and 2018, respectively. As of December 31, 2017 and 2018, we had an accumulated deficit of $123.9 million and $178.8 million, respectively. Substantially all of our losses have resulted from funding our clinical trials, manufacturing our drug product, our research and development programs and from general and administrative costs associated with our operations. We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate that our expenses will increase significantly in connection with our ongoing activities, as we:
| • | continue preclinical studies and clinical development of our programs including in connection with the clinical development programs for Plinabulin in CIN and NSCLC; |
| • | hire additional research, development and business personnel; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | launch and commercialize Plinabulin in China; |
| • | fund the discovery and development of new product candidates; and |
| • | incur additional costs associated with operating as a public company. |
We will need substantial additional funding to support our operating activities as we advance our product candidates through clinical development, seek regulatory approval and prepare for and, if any of our product candidates are approved, proceed to commercialization. Adequate funding may not be available to us on acceptable terms, or at all.
We have no manufacturing facilities, and all our manufacturing activities are contracted out to third parties. Additionally, we currently utilize third-party CROs to carry out our clinical development and trials. We do not yet have a sales organization in China but intend to establish one beginning in 2019.
Components of Results of Operations
Revenue
To date, we have not generated any revenue from product sales and do not expect to generate any revenue from the sale of products in the foreseeable future. In the future, we may generate revenue from a combination of product sales, reimbursements, up-front payments, milestone payments and royalties in connection with future collaborations. If we fail to complete the development of our product candidates in a timely manner or fail to obtain their regulatory approval, we will not generate revenue in the future.
Expenses
Research and Development Expenses
The largest component of our total operating expenses has historically been our investment in research and development activities. Research and development expenses consist of costs associated with our research and development activities, including the purchase of the Plinabulin global rights from Nereus, conducting preclinical studies and clinical trials of Plinabulin and development of our pipeline of immuno-oncology product candidates and de novo drug discovery using our ubiquitin-mediated degradation platform. Research and development expenses also include activities related to:
| • | employee-related expenses, including salaries, benefits and travel expense for research and development personnel; |
| • | expenses incurred under agreements with CROs, contract manufacturing organizations, and consultants that conduct and support clinical trials and preclinical studies; |
| • | costs associated with preclinical studies and development activities; |
| • | costs associated with regulatory operations; |
| • | costs associated with protecting intellectual property; |
| • | share-based compensation to employees, directors and non-employee consultants; and |
| • | other expenses, which include direct and allocated expenses for rent, insurance and other supplies used in research and development activities. |
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect our research and development expenses to increase significantly over the next several years as we continue to develop our product pipeline through preclinical studies and clinical trials and prepare for our Plinabulin NDA filings in China and the U.S., including preparing commercial manufacturing batches of Plinabulin and building up inventories before approval. We expect to add additional personnel to support these activities, which would increase personnel cost, including equity-based compensation. Our current research and development activities mainly relate to:
| • | Study 105, an approximately 160 patient Phase 2/3 clinical trial for Plinabulin to prevent CIN in intermediate risk chemotherapy; |
| • | Study 106, an approximately 400 patient Phase 2/3 clinical trial for Plinabulin to prevent CIN in high risk chemotherapy (TAC); |
| • | Study 103, an approximately 554 patient Phase 3 clinical trial of Plinabulin in combination with docetaxel for second and third line treatments of advanced NSCLC; |
| • | Three Phase 1/2 investigator-initiated clinical trials of Plinabulin in combination with various immuno-oncology agents; |
| • | Preclinical studies investigating both Plinabulin’s, and our preclinical product candidates’, potential in combination with immunotherapies; and |
| • | Preclinical study investigating the ubiquitin-mediated degradation pathway. |
We expense research and development costs when we incur them. We record costs for some development activities, such as clinical trials, based on an evaluation of the progress to completion of specific tasks using data such as subject enrollment, clinical site activations or information our vendors provide to us.
There are numerous factors associated with the successful commercialization of any of our product candidates, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development. Additionally, future commercial and regulatory factors beyond our control will impact our clinical development programs and plans.
The successful development of our product candidates is highly uncertain. Due to the inherently unpredictable nature of preclinical studies and clinical development and commercialization of product candidates, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the remainder of the development of, or when, if ever, material net cash inflows may commence from, any of our other product candidates. This unpredictability is due to the numerous risks and uncertainties associated with the duration and cost of clinical trials and commercialization of product candidates, which vary significantly over the life of a project as a result of many factors, including:
| • | the number of clinical sites included in the trials; |
| • | the design of the trial and changes to the design of the trial; |
| • | establishing an appropriate safety profile; |
| • | the length of time required to enroll suitable patients; |
| • | the number of patients that ultimately participate in the trials; |
| • | the number of doses patients receive; |
| • | the duration of patient follow-up; |
| • | the results of our clinical trials; |
| • | making arrangements with third-party manufacturers; |
| • | receipt of marketing approvals from applicable regulatory authorities; |
| • | commercializing the product candidates, if and when approved, whether alone or in collaboration with others; |
| • | obtaining and maintaining patent and trade secret protection and regulatory exclusivity for our product candidates; |
| • | continued acceptable safety profiles of the products following approval; and |
| • | retention of key research and development personnel. |
A change in the outcome of any of these variables with respect to the development of any of our product candidates would significantly change the costs, timing and viability associated with the development of that product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of share-based compensation costs, personnel costs, including executive, finance and human resource functions, and information technology. Other general and administrative expenses include professional fees for legal, consulting, auditing and tax services as well as other direct expenses for rent, insurance and supplies used in general and administrative activities. We expect increases in general and administrative expenses related to pre-commercialization activities and launch activities if Plinabulin is approved. These increases will likely include increased headcount, expanded infrastructure and increased costs for insurance. We also incur legal, compliance, accounting, directors’ and officers’ insurance, and investor and public relations expenses associated with being a public company.
Other Income (Expenses)
Other income for 2018 consists primarily of interest income earned on our cash and government grants. The government grants are approximately $315,000 (RMB 2 million) from Chinese government. Such government grants for Dalian Wanchun Pharmaceutical Co., Ltd. (“Wanchun Pharma”) were received in December 2014. The government grant was transferred to Wanchun Bulin since Wanchun Pharma was liquidated in August 2015. The Company previously included such government grant under current liabilities as the amendment procedures for changing the beneficiary to Wanchun Bulin were still under review by the local government. In January 2018, the Company obtained approval from local government and became eligible for the government grant and recorded the government grant as other income in the consolidated statements of comprehensive loss during the year of 2018.
Other expenses consist primarily of foreign exchange losses in 2018.
Results of Operations
Comparison of Years Ended December 31, 2018 and 2017
The following table summarizes the results of our operations for the years ended December 31, 2018 and 2017, respectively, together with the changes in those items in dollars:
| | | | |
| | | | | | | | | | |
| | | (in thousands of U.S. Dollars (“$”)) | | | % | |
| Revenue | | | — | | | | — | | | | — | |
| Operating expenses | | | | | | | | | | | | |
| Research and development | | | (51,618 | ) | | | (88,928 | ) | | | -42 | % |
| General and administrative | | | (5,927 | ) | | | (9,053 | ) | | | -35 | % |
| Loss from operations | | | (57,545 | ) | | | (97,981 | ) | | | -41 | % |
| Other (expense) income | | | | | | | | | | | | |
| Foreign exchange (loss)gain, net | | | (455 | ) | | | 555 | | | | -182 | % |
| Interest income | | | 211 | | | | 120 | | | | 76 | % |
| Other income | | | 315 | | | | 918 | | | | -66 | % |
| Total other income | | | 71 |
| | | 1,593 | | | | -104 | % |
| Net loss before income tax | | | (57,474 | ) | | | (96,388 | ) | | | -40 | % |
| Income tax benefit | | | — | | | | — | | | | — | |
| Net loss | | | (57,474 | ) | | | (96,388 | ) | | | -40 | % |
Research and Development
Research and development expenses decreased by $37.3 million to $51.6 million for the year ended December 31, 2018 from $88.9 million for the year ended December 31, 2017. In 2017, the Company issued 2,112,963 ordinary shares to Nereus Trust as a result of our performance of the termination agreement described below, which is recorded as an expense of $42.3 million. Without this non-cash expense, research and development expenses would have increased by $5.0 million from $46.6 million in 2017 to $51.6 million in 2018. The increase would have been mainly due to an increase of $15.9 million in our clinical trial expenses for Study 103, Study 105 and Study 106 and three investigator initiated Phase 1/2 clinical trials of Plinabulin in combination with various immuno-oncology agents as well as costs related to the manufacturing of Plinabulin, offset by a decrease of $10.9 million in non-cash share based compensation.
| R&D activities(1) | | | |
| | | | | | | | | | |
| | | (in thousands of U.S. Dollars (“$”)) | | | % | |
| Study 103 | | | 10,020 | | | | 8,138 | | | | 23 | % |
| Study 105 | | | 9,837 | | | | 6,375 | | | | 54 | % |
| Study 106 | | | 5,443 | | | | 3,615 | | | | 51 | % |
| Preclinical | | | 1,553 | | | | 2,348 | | | | -34 | % |
| Other clinical trials | | | 1,634 | | | | 1,230 | | | | 33 | % |
| Employee-related expenses | | | 4,015 | | | | 2,881 | | | | 39 | % |
| Share-based compensation | | | 6,821 | | | | 17,753 | | | | -62 | % |
| Consultant and other | | | 12,295 | | | | 4,329 | | | | 184 | % |
| Share-based payment to Nereus(2) | | | - | | | | 42,259 | | | | -100 | % |
| Total research and development | | | 51,618 | | | | 88,928 | | | | -42 | % |
NM: Not meaningful
| (1) | Due to the inherently unpredictable nature of preclinical and clinical development, we do not track all of our internal research and development expenses on a program-by-program basis as they primarily relate to personnel, early research, manufacturing and development, which are deployed across multiple projects under development. These costs are therefore shown separately. |
| (2) | On February 2, 2015, we, Wanchun Biotech and Fortis Advisors LLC, in its capacity as an agent of the former stakeholders of the seller of the patent of Plinabulin transferred to Wanchun Biotech, entered into an agreement to terminate royalty payment arrangements due by us on Plinabulin sales, if any, to the patent sellers. The termination agreement was effective upon the consummation of our IPO and required us to issue a number of ordinary shares representing 10% of our fully-diluted equity capitalization immediately prior to the offering to an entity designated by the seller in lieu of the royalty payment. In satisfaction of that obligation, we issued 2,112,963 ordinary shares to Nereus Trust, upon consummation of our IPO on March 14, 2017, and the royalty payment arrangements were terminated. |
General and Administrative Expense
General and administrative expenses decreased by $3.2 million to $5.9 million for the year ended December 31, 2018 from $9.1 million for the year ended December 31, 2017. The decrease was primarily attributable to a decrease in non-cash share compensation expenses of $5.2 million, offset by an increase of $2.0 million due to an increase in headcount and professional fees, including an increase in personnel and increased legal expenses.
Other Income
Other income decreased by $1.5 million to $0.1 million for the year ended December 31, 2018 from $1.6 million for the year ended December 31, 2017. This decrease was primarily attributable to a $0.6 million decrease of government grants and a decrease of $1.0 million from foreign exchange gain to foreign exchange loss..
Comparison of Years Ended December 31, 2017 and 2016
The following table summarizes the results of our operations for the years ended December 31, 2017 and 2016, respectively, together with the changes in those items:
| | | | |
| | | | | | | | | | |
| | | (in thousands of U.S. Dollars (“$”)) | | | % | |
| Revenue | | | — | | | | — | | | | — | |
| Operating expenses | | | | | | | | | | | | |
| Research and development | | | (88,928 | ) | | | (10,437 | ) | | | 752 | % |
| General and administrative | | | (9,053 | ) | | | (1,931 | ) | | | 369 | % |
| Loss from operations | | | (97,981 | ) | | | (12,368 | ) | | | 692 | % |
| Other income (expense) | | | | | | | | | | | | |
| Foreign exchange gain (loss), net | | | 555 | | | | (195 | ) | | | -385 | % |
| Interest income | | | 120 | | | | 18 | | | | 567 | % |
| Other income | | | 918 | | | | — | | | | 100 | % |
| Total other income (expense) | | | 1,593 | | | | (177 | ) | | | -1000 | % |
| Net loss before income tax | | | (96,388 | ) | | | (12,545 | ) | | | 668 | % |
| Income tax benefit | | | — | | | | — | | | | — | |
| Net loss | | | (96,388 | ) | | | (12,545 | ) | | | 668 | % |
Research and Development
Research and development expenses increased by $78.5 million to $88.9 million for the year ended December 31, 2017 from $10.4 million for the year ended December 31, 2016. Approximately $60.1 million of the increase was due to non-cash expense increases, of which approximately $17.8 million was due to an increase in non-cash share compensation expense; and $42.3 million was due to the issuance of 2,112,963 ordinary shares to Nereus Trust as a result of our performance of the termination agreement. The remaining $18.4 million of the increase was primarily attributable to the continuation of our clinical trials, Study 103, Study 105 and Study 106 and two investigator initiated Phase 1/2 clinical trials of Plinabulin in combination with various immuno-oncology agents and costs related to the manufacturing of Plinabulin.
General and Administrative Expense
General and administrative expenses increased by $7.2 million to $9.1 million for the year ended December 31, 2017 from $1.9 million for the year ended December 31, 2016. The increase was primarily attributable to an increase in non-cash share compensation expenses of $4.9 million and the additional costs of operating as a public company, including an increase in personnel and increased legal expenses.
Other (Expense) Income
Other income increased by $1.8 million to $1.6 million for the year ended December 31, 2017 from $0.2 million for the year ended December 31, 2016. This increase was primarily attributable to a total of approximately $0.9 million cash grants we received, approximately $450,000 (RMB 3 million) from Financial Service Development Council of Dalian and approximately $470,000 (RMB 3.1 million) from Financial and Monetary Bureau of Jinpu New Area of Dalian, and an increase in foreign exchange gains of $0.8 million.
Critical Accounting Policies and Significant Judgments and Estimates
Research and Development Costs
We account for research and development costs in accordance with Accounting Standards Codification, or ASC 730, Research and Development. Research and development costs are primarily comprised of costs incurred in performing research and development activities, including related personnel and consultant’s salaries, benefits and related costs, raw materials and supplies to develop product candidates, patent-related costs incurred in connection with filing patent applications and external costs of outside vendors engaged to conduct clinical development activities and trials. We expense research and development costs as they are incurred.
The costs incurred relate to nonrefundable advance payments for goods or services that will be used in future research and development activities are deferred and capitalized. The capitalized amounts are expensed as research and development costs when the related goods are delivered or the services are performed, or when we do not expect we will need the goods to be delivered or the services to be rendered.
Research Contract Costs and Accruals
We have entered into various research and development contracts with research institutions and other companies in China, the U.S., Europe and Australia. Related payments are recorded as research and development expenses as incurred. We record accruals for estimated ongoing research costs. When evaluating the adequacy of the accrued liabilities, we analyze progress of the studies, including the phase or completion of events, invoices received and contracted costs. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period. Actual results could differ from our estimates. Our historical accrual estimates have not been materially different from the actual costs.
Foreign Currency Translation and Transactions
We currently use U.S. dollar as our functional currency for all our entities, except for entities in China, which adopt RMB as the functional currency, and BeyondSpring Pharmaceuticals Australia PTY Ltd., which adopts the Australian dollar as the functional currency. The determination of the respective functional currency is based on the criteria of ASC 830, Foreign Currency Matters. We use U.S. dollars as our reporting currency. For subsidiaries whose functional currencies are not the U.S. dollar, we use the average exchange rate for the year and the exchange rate at the balance sheet date to translate the operating results and financial position to U.S. dollar, the reporting currency, respectively. Translation differences are recorded in accumulated other comprehensive income or loss, a component of shareholders’ equity.
We measure transactions denominated in currencies other than the functional currency by translating into the functional currency at the exchange rates prevailing on the transaction dates. Foreign currency denominated financial assets and liabilities are re-measured at the exchange rates prevailing at the balance sheet date. We include exchange gains and losses in the consolidated statements of comprehensive loss.
Income Taxes
We use the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of assets and liabilities and are measured using enacted tax rates that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
In accordance with Accounting Standards Update (“ASU”) No. 2015-17, Income Taxes (Topic 740) all deferred income tax assets and liabilities are classified as non-current on the consolidated balance sheets. We evaluate our uncertain tax positions using the provisions of ASC 740, Income Taxes, which prescribes a recognition threshold that a tax position is required to meet before being recognized in the financial statements. We recognize in the financial statements the benefit of a tax position which is “more likely than not” to be sustained under examination based solely on the technical merits of the position assuming a review by tax authorities having all relevant information. Tax positions that meet the recognition threshold are measured using a cumulative probability approach, at the largest amount of tax benefit that has a greater than fifty percent likelihood of being realized upon settlement. It is our policy to recognize interest and penalties related to unrecognized tax benefits, if any, as a component of income tax expense.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-2, Leases (Topic 842) (“ASU 2016-02”), which requires lessees to recognize assets and liabilities related to lease arrangements longer than 12 months on the balance sheet. This standard also requires additional disclosures by lessees and contains targeted changes to accounting by lessors. The updated guidance is effective for interim and annual periods beginning after December 15, 2018, and early adoption is permitted. The Company will adopt the new standard effective January 1, 2019 using the modified retrospective method and will not restate comparative periods. The Company will elect the package of practical expedients permitted under the transition guidance within the new standard, which permits the Company not to reassess under the new standard its prior conclusions about lease identification, lease classification and initial direct costs. The Company currently believes the most significant change will be related to the recognition of right-of-use assets and lease liabilities on the Company’s balance sheet for certain in-scope operating leases. The Company does not expect any material impact on net assets and the consolidated statement of comprehensive income as a result of adopting the new standard.
In June 2018, the FASB issued ASU 2018-7, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-7”). This update expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. This update also specifies that Topic 718 applies to all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. This update is effective in fiscal years, including interim periods, beginning after December 15, 2018. Early adoption is permitted, but no earlier than an entity’s adoption date of Topic 606. The Company is currently evaluating the impact on its financial statements of adopting this guidance.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework- Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”). The update eliminates, modifies, and adds certain disclosure requirements for fair value measurements. This update is effective in fiscal years, including interim periods, beginning after December 15, 2019, and early adoption is permitted. The added disclosure requirements and the modified disclosure on the narrative description of measurement uncertainty should be applied prospectively for only the most recent interim or annual period presented. All other changes to disclosure requirements in this update should be applied retrospectively to all periods presented upon their effective date. The Company is currently evaluating the impact on its financial statements of adopting this guidance.
JOBS Act
Under Section 107(b) of the JOBS Act, an “emerging growth company” can delay the adoption of new or revised accounting standards until such time as those standards would apply to private companies. We have irrevocably elected not to avail ourselves of this exemption and, as a result, we will adopt new or revised accounting standards at the same time as other public companies that are not emerging growth companies. There are other exemptions and reduced reporting requirements provided by the JOBS Act that we are currently evaluating. For example, as an emerging growth company, we are exempt from Sections 14A(a) and (b) of the Exchange Act which would otherwise require us to (1) submit certain executive compensation matters to shareholder advisory votes, such as “say-on-pay,” “say-on-frequency” and “golden parachutes”; and (2) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of our chief executive officer’s compensation to our median employee compensation. We intend to rely on an exemption from the rule requiring us to provide an auditor’s attestation report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and the rule requiring us to comply with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will continue to remain an “emerging growth company” until the earliest of the following: (1) the last day of the fiscal year following the fifth anniversary of March 14, 2017, (2) the last day of the fiscal year in which our total annual gross revenue is equal to or more than $1.07 billion, (3) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years, or (4) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
We comply with the reporting requirements under the Exchange Act as a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| • | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; |
| • | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; |
| • | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events; and |
| • | Regulation FD, which regulates selective disclosures of material information by issuers. |
| B | Liquidity and Capital Resources |
Since inception, we have incurred net losses and negative cash flows from our operations. Substantially all of our negative cash flows have resulted from funding our research and development programs and general and administrative costs associated with our operations. We incurred consolidated net losses of $56.9 million, $96.4 million and $12.5 million for the years ended December 31, 2018, 2017 and 2016, respectively. As of December 31, 2018 and 2017, we had an accumulated deficit of $178.1 million and $123.9 million, respectively. Our primary use of cash is to fund research and development costs and for general and administrative costs. Our operating activities used $40.0 million, $28.8 million and $13.7 million of cash during the years ended December 2018, 2017 and 2016, respectively. We have financed our operations principally through proceeds from public offering and private placements of ordinary shares and loans from related and third parties, including our IPO and the concurrent private placement consummated in March 2017, in which we received net proceeds of $47.2 million, after deducting underwriting discounts and commissions and the offering expenses. Additionally, in May 2018, we offered 739,095 ordinary shares to certain institutional investors for potential gross proceeds of $20 million before deducting expenses, of which we have received $14.0 million as of the date of this annual report on Form 20-F. We do not expect to receive the remaining $6.0 million in the near future.
Our liquidity is affected by financing activities, our clinical trials, and research and development and general and administrative expenses. In order to operate as a going concern in the foreseeable future, we will need, among other things, additional capital resources. We are pursuing various financing alternatives to fund our operations so we can continue as a going concern in the medium to long term, including equity and debt financings, potential licensing and partnership arrangements, and sales of products after obtaining regulatory approvals. There can be no assurance that capital will be available as necessary to meet our working capital requirements or, if the capital is available, that it will be on terms acceptable us. The issuances of additional equity securities by us may result in dilution in the equity interests of our current shareholders. Obtaining commercial loans, assuming those loans will be available, will increase our liabilities and future cash commitments and may include financial covenants and restrictions. If we are unable to obtain financing in the amounts and on terms deemed acceptable, our business and future success will be materially and adversely affected. These factors raise substantial doubt regarding our ability to continue as a going concern.
Cash Flows
The following table provides information regarding our cash flows for the years ended December 31, 2018, 2017 and 2016:
| | | | |
| | | | | | | | | | |
| | | (in thousands of U.S. Dollars (“$”)) | |
| Net cash used in operating activities | | | (39,955 | ) | | | (28,796 | ) | | | (13,698 | ) |
| Net cash provided by/(used in) investing activities | | | 2,867 | | | | (3,150 | ) | | | (64 | ) |
| Net cash provided by financing activities | | | 13,245 | | | | 47,722 | | | | 14,713 | |
| Net effect of foreign exchange rate changes | | | 251 | | | | 18 | | | | (85 | ) |
| Net increase in cash and cash equivalents | | | (23,592 | ) | | | 15,794 | | | | 866 | |
Net Cash Used in Operating Activities
The cash used in operating activities for the years ended December 31, 2018, 2017 and 2016 resulted primarily from our net losses of $56.9 million, $96.4 million and $12.5 million, respectively, adjusted for non-cash charges and changes in components of working capital. During 2018 these non-cash charges mainly consist of the $6.6 million of non-cash share-based compensation. Net cash used in operating activities was $40.0 million for the year ended December 31, 2018, compared to $28.8 million for the year ended December 31, 2017. The $11.2 million increase was primarily due to an increase in research and development expenses as we expanded our clinical trial activities related to Plinabulin and an increase in general and administrative expenses as we expanded our business. Net cash used in operating activities was $28.8 million for the year ended December 31, 2017, compared to $13.7 million for the year ended December 31, 2016. The increase of $15.1 million in cash used in operating activities was primarily due to a $13.5 million increase in research and development and a $2.8 million increase in general and administrative expenses.
The primary use of our cash in the periods presented was to fund the development of our research and development, regulatory and other clinical trial costs, and related supporting administration. Our advances to suppliers and other current assets, accounts payable and accrued expense balances in all periods presented were affected by the timing of vendor invoicing and payments.
Net Cash Provided by/(Used in) Investing Activities
Net cash provided by investing activities for the year ended December 31, 2018 was $2.9 million, net cash used in investing activities for the year ended December 31, 2017 was $3.2 million, and net cash provided by investing activities for the year ended December 31, 2016 was $0.06 million. During 2018, net cash was primarily provided by the maturity of a short-term investment. During 2017, net cash was primarily used in purchasing a one-year short-term investment. During 2016, net cash is primarily used in acquisitions of furniture and fixtures for our offices in Dalian, China.
Net Cash Provided by Financing Activities
Net cash provided by financing activities for the year ended December 31, 2018 decreased by $34.5 million to $13.2 million from $47.7 million for the year ended December 31, 2017. The decrease was primarily due to our IPO and concurrent private placement in March 2017, in which we received net proceeds of $47.2 million, while in our offering of ordinary shares to certain institutional investors in May 2018 we only received gross proceeds of $14 million. Net cash provided by financing activities for the year ended December 31, 2017 was $47.7 million compared to $14.7 million for the year ended December 31, 2016. Net cash provided by financing activities during 2017 was primarily due to our IPO and concurrent private placement in March 2017, in which we received net proceeds of $47.2 million.
Operating Capital Requirements
We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize any of our current product candidates. We anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of, and seek regulatory approvals for, our current product candidates and incur pre-commercialization expenses that are expected to occur prior to regulatory approval. In addition, subject to obtaining regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing and manufacturing. Accordingly, we anticipate that we will need substantial additional funding in connection with our future operations.
Our liquidity is affected by financing activities, our clinical trials, research and development and general and administrative expenses. In order to operate as a going concern in the foreseeable future, we will need, among other things, additional capital resources. There can be no assurance that capital will be available as necessary to meet our working capital requirements or, if the capital is available, that it will be on terms acceptable to us. The issuances of additional equity securities by us may result in dilution in the equity interests of its current shareholders. Obtaining commercial loans, assuming those loans will be available, will increase our liabilities and future cash commitments and may include financial covenants and restrictions. If we are unable to obtain financing in the amounts and on terms deemed acceptable, our business and future success will be materially and adversely affected. These factors raise substantial doubt regarding our ability to continue as a going concern. Thus, we need to raise additional capital in order to continue our business activities. We have based our estimates on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures necessary to complete the development and commercialization of our product candidates.
Our future capital requirements will depend on many factors, including:
| • | the costs, timing and outcome of regulatory reviews and approvals; |
| • | the ability of our product candidates to progress through clinical development successfully; |
| • | the initiation, progress, timings, costs and results of studies in animals and clinical trials for our other programs and potential product candidates; |
| • | the number and characteristics of the product candidates we pursue; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | the extent to which we acquire or in-license other products and technologies; |
| • | our ability to establish and maintain arrangements partnership with other pharmaceutical companies for the development, licensing and commercialization of our assets; and |
| • | our ability to maintain and establish collaboration arrangements on favorable terms, if at all. |
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity and debt financing, potential licensing and partnership arrangements, and sale of products after obtaining regulatory approvals. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our shareholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our shareholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends and may require the issuance of warrants, which could potentially dilute the ownership interest of our shareholders. If we raise additional funds through collaborations, strategic alliances, marketing or distribution arrangements or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or research programs or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market products or product candidates that we would otherwise prefer to develop and market ourselves.
| C. | Research and Development, Patents and Licenses, etc. |
Research and Development
Our research and development expenses primarily are comprised of costs incurred in performing research and development activities, including related personnel and consultant’s salaries, benefits and related costs, raw materials and supplies to develop product candidates, patent-related costs incurred in connection with filing patent applications and external costs of outside vendors engaged to conduct clinical development activities and trials. See “—A. Operating Results—Components of Results of Operations—Expenses—Research and Development Expenses.”
Intellectual Property
As of March 31, 2019, we owned or co-owned 76 patents, in 36 jurisdictions, including 20 issued U.S. patents. We also owned seven pending U.S. non-provisional patent applications as well as corresponding patent applications pending in other jurisdictions and seven pending U.S. provisional patent applications. In addition, we owned five pending international patent applications related to Plinabulin and Plinabulin analogs filed under the PCT, which we plan to file nationally in the U.S. and in other jurisdictions directed to reduction of CIN, the therapeutic use of tubulin binding compounds, dosage regimens, the treatment of thrombocytopenia, and use in combination with G-CSF therapy. See “Item 4. Information on the Company—B. Business Overview—Intellectual Property.”
We are a clinical stage company and cannot predict with any degree of accuracy the outcome of our research and development efforts. As such, we cannot predict with any degree of accuracy any significant trends, uncertainties or events that are reasonably likely to have a material effect on our net loss, liquidity or capital resources, or cause financial information to not be indicative of future operating results or financial condition. However, to the extent possible, certain trends, uncertainties, demands, commitments and events are described in this “Item 5. Operating and Financial Review and Prospects.”
| E. | Off-Balance Sheet Arrangements |
We did not have in fiscal years 2016, 2017 and 2018, and we do not currently have, any off-balance sheet arrangements, as defined under SEC rules, such as relationships with unconsolidated entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on our consolidated balance sheets.
| F. | Contractual Obligations and Commitments |
Lease commitments
Our principal commitments consist of obligations under our operating leases for equipment and office space. The following table summarizes our significant contractual obligations as of payment due date by period at December 31, 2018:
| | | | | | | |
| | | | | | | | | | | | | | | | |
| | | (in thousands) | |
| Operating lease commitments | | $ | 3,958 | | | | 792 | | | | 1,584 | | | | 1,582 | | | | — | |
We lease all of our facilities and believe our current facilities are sufficient to meet our needs. Our principal executive offices are located in New York, and we also have offices in Dalian, China. In May 2015, we began to lease offices in New York at $2,938.25 per month. From August 2015 to February 2016, we leased additional office space in New York, and the rent increased to $4,384.97 per month. From March 2016 to February 2017, we leased additional office space in New York, and the rent increased to $9,972.07 per month. From March 2017 to April 2018, we leased additional office space in New York, and the rent increased to $11,252.15 per month. After that we leased additional office space in New York, and the rent increased to $26,382.39 per month through February 2020. After October 2018, we leased additional office space in New York, and the rent increased to $63,372.72 per month until February 2020. Starting March 2020, the rent will increase to $64,571.39 until December 2023, with the annual license fee of $3,619.00 being increased on each anniversary of the lease agreement commencement date. Payments under operating leases are expensed on a straight-line basis over the periods of the respective leases, and the terms of the leases do not contain rent escalation, contingent rent, renewal or purchase options.
We leased offices in Dalian, China under multiple operating leases through August 31, 2016. In accordance with our lease agreement with the Chinese government, rent for the office facilities in Dalian, China is free during above leasing period. We lease offices in Dalian, China at approximately $2,000 per month starting September 2016, and the rent increased to approximately $2,500 per month starting September 2018.
We also leased office space in San Diego at $2,359 per month from February 2016 to January 2017. After that we leased additional office space and the rent increased to $3,708 per month through February 2018, After January 2018, we leased addition office space for $4618.8 per month through January 2020 and this lease agreement was terminated in June 2018.
We leased office space in Hong Kong at $15,552.26 per month from May 27, 2018 to January 15, 2019.
Loan agreements
In January 2019, we entered into a three-month RMB 20.0 million loan agreement with He’en Weiye (Shenzhen) Assets Management Center L.P. at an annual interest rate of 15%. The loan is secured by Mr. Linqing Jia and repayable upon maturity. As of the date of this annual report on Form 20-F, we have drawn down RMB 10.0 million pursuant to this agreement with the remaining RMB 10.0 million expected to be drawn down in the second quarter of 2019. In April 2019, the maturity of this loan was extended to April 20, 2020.
In March 2019, we entered into a three-year RMB 10.0 million loan agreement with China Construction Bank at an annual interest rate of 5.7%. The loan is secured by Shenzhen Sangel Capital Management Limited Company and Mulong Liu, and repayable upon maturity.
In April 2019, we entered into a one-year $1.0 million loan agreement with Shenzhen Sangel Zhichuang Investment Co., Ltd. at an annual interest rate of 15%. The loan is secured by Mr. Linqing Jia and repayable upon maturity.
Other contractual obligations
We enter into agreements in the normal course of business with CROs and institutions to license intellectual property. We have not included these future payments in the table of contractual obligations above since the contracts are cancelable at any time by us with prior written notice.
This annual report on Form 20-F contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act and as defined in the Private Securities Litigation Reform Act of 1995. See section titled “Forward-Looking Statements.”
Item 6. | Directors, Senior Management and Employees |
| A. | Directors and Senior Management |
Below is a list of the names and ages of our co-founder, directors and executive officers (including officers of BeyondSpring Pharmaceuticals, Inc., or BeyondSpring U.S.) as of March 31, 2019, and a brief account of the business experience of each of them. The business address for our directors and officers and the officers of BeyondSpring U.S. is c/o BeyondSpring Inc., 28 Liberty Street, 39th Floor, New York, NY 10005.
| | | | |
| Executive Officers | | | | |
| Lan Huang, Ph.D. | | 48 | | Co-Founder, Chairman and Chief Executive Officer |
| Edward Dongheng Liu | | 37 | | Chief Financial Officer |
| Ramon W. Mohanlal, M.D., Ph.D. | | 60 | | Chief Medical Officer and Executive Vice President of Research and Development |
| G. Kenneth Lloyd, Ph.D. | | 74 | | Chief Scientific Officer |
| Gordon L. Schooley, Ph.D. | | 72 | | Chief Regulatory Officer |
| Richard Daly, M.B.A. | | 58 | | Chief Operating Officer |
| James R. Tonra, Ph.D. | | 53 | | Senior Vice President of Preclinical Development |
| Non-Employee Directors | | | | |
| Patrick Fabbio, M.B.A. | | 51 | | Director |
| Matthew Kirkby, M.A. | | 50 | | Director |
| Quanqi Song, Ph.D. | | 54 | | Director |
| Yanbin Xie, M.D. | | 61 | | Director |
| Christine Ying Zhao, M.B.A. | | 46 | | Director |
Executive Officers
Lan Huang, Ph.D. is our Co-founder, Chairman and Chief Executive Officer and has been a member of the board of directors since November 2014. Dr. Huang brings over ten years of entrepreneurial experience in the Chinese and U.S. biotechnology industries. In 2010, Dr. Huang founded Wanchun Biotech, the former holding company of our U.S. subsidiary. In 2007, Dr. Huang co-founded Wuxi MTLH Biotechnology Co. Ltd, where she served as CEO in 2010 and continues to hold a directorship. The rights related to the development and marketing of the peptide drug in China, which drug Dr. Huang designed while at Wuxi MTLH Biotechnology Co. Ltd, were sold to Shanghai Pharmaceutical Group in 2010. Additionally, in 2008, Dr. Huang co-founded Paramax International Inc., a CRO that conducts clinical trials for global biopharmaceutical and medical device companies. Paramax International Inc. was acquired by ReSearch Pharmaceutical Services, Inc. in 2009. Dr. Huang was trained at Memorial Sloan Kettering Cancer Center from 1998 to 2002, where her research in cancer signaling pathways involving P53 degradation was published in Science. Her translational research in cancer signaling pathways involving RAS was published in two Nature papers. She has invented and holds patents for a number of biotech products for oncology and dermatology indications. Dr. Huang received her B.A., Magna Cum Laude and Phi Beta Kappa, from Lawrence University, where she served as a trustee from 2012 to 2015. She received her Ph.D. in chemistry from the University of California at Berkeley, where she won the international-level Women’s Opportunity Award given by Soroptimist International. She also studied at Fudan University in Shanghai, China.
Edward Dongheng Liu has served as our Chief Financial Officer since March 26, 2018. Mr. Liu brings decade-long experience in senior banking roles, supporting corporate financings and strategic transactions for clients in the Asia-Pacific region. Through his experiences in equity investments and investment banking, he developed an expertise in financial analysis with a particular focus in healthcare. From 2016 to 2018, Mr. Liu was a Partner and Executive Director at Epiphron Capital, a cross-border, healthcare-focused investment fund, also an early investor of BeyondSpring. From 2013 to 2016, he served as Vice President and subsequently as Senior Vice President of the Investment Banking and Capital Markets team at Jefferies Hong Kong Limited in Hong Kong. Prior to that, he held various roles with increasing responsibilities at J.P. Morgan Securities (Asia Pacific) Limited, where he focused on investment banking in the Asia-Pacific region. Mr. Liu received his bachelor’s degree in economics and mathematics from Yale University. He also completed a year of biomedical engineering coursework at Tsinghua University before attending Yale.
Ramon W. Mohanlal, M.D., Ph.D. has served as our Chief Medical Officer since October 2015, and he also serves as Executive Vice President of Research and Development. Most recently, from July 2015 to October 2015, Dr. Mohanlal served as a consultant for AstraZeneca on its immuno-oncology programs to help support and manage several Phase 1/2 and 2/3 studies. Prior to that, from January 2012 to July 2015, Dr. Mohanlal served as the Clinical Head of Established Products Oncology for Novartis AG, a global healthcare company. From 2009 to 2012, Dr. Mohanlal was a consultant for Syntium Inc., a biopharmaceutical company, where he was responsible for partnering, deal-making and creating business plans around drug development assets. From 2007 to 2009, Dr. Mohanlal served as CEO and Chief Medical Officer for BioPremiere, Inc., a biopharmaceutical company focused on developing biologics for serious diseases, where he was responsible for fundraising and partnering activities. From 2005 to 2007, Dr. Mohanlal managed drug development and diagnostic development as Chief Medical Officer of Interleukin Genetics, Inc., a personalized health company that develops genetic tests for use in the personalized health market. Dr. Mohanlal received his M.B.A. from the American Intercontinental University in Illinois and earned both his M.D. and Ph.D. in experimental CV pharmacology from the University of Leiden, The Netherlands.
G. Kenneth Lloyd, Ph.D. has served as our Chief Scientific Officer since June 1, 2015. Dr. Lloyd also served as the Chief Scientific Officer of Wanchun Biotech, the former holding company of our U.S. subsidiary, from 2013 to 2014. From 2012 to 2015 Dr. Lloyd served as a scientific consultant for Triphase Accelerator Corporation, a company focused on clinically enabling and out-licensing oncology assets. From 2000 to 2012, Dr. Lloyd served as the Chief Scientific Officer of Nereus, where he oversaw the company’s research and development and drug discovery programs including the company’s development of Plinabulin. In addition to serving as a director of GKOL Inc., a consulting firm he co-founded, Dr. Lloyd also holds a directorship at Verne Mendel Medical Corporation, a company focused on developing pharmaceutical product candidates to treat mitochondrial degenerative diseases. He is widely published in journals that include Science, Nature and the New England Journal of Medicine. Dr. Lloyd received his undergraduate education and M.S. in biochemistry at McGill University and earned his Ph.D. in pharmacology and toxicology from the University of Toronto. He completed a post-doctoral fellowship at F. Hoffmann-La Roche AG, a global healthcare company in Basel, Switzerland.
Gordon L. Schooley, Ph.D. has served as our Chief Regulatory Officer since September 2016. Dr. Schooley also served as our Senior Vice President of Regulatory Affairs from 2013 to September 2016. Dr. Schooley served as President of Advanced Analytics and Informatics LLC from June 2008 to June 2016. From 2008 to 2009 Dr. Schooley served as a director of Progen Pharmaceuticals and from 2005 to 2009 he served as Regulatory and Biostatistical Consultant. Dr. Schooley served as Chief Science Officer and Senior Vice President of Clinical and Regulatory Affairs at both SkyePharma PLC and Pacira Pharmaceuticals, Inc. from January 1999 to June 2008, and Vice President of Clinical and Regulatory Affairs at Alliance Pharmaceuticals Inc. from January 1989 to January 1999. Dr. Schooley received his undergraduate training and M.S. at Brigham Young University, and his Ph.D. in biostatistics and medical care organization and administration at the University of Michigan School of Public Health.
Richard Daly, M.B.A. has served as our Chief Operating Officer since August 2018. Mr. Daly has more than 25 years of experience heading business and commercial operations for leading pharmaceutical and biotech companies. Most recently, from February 2016 to July 2018, Mr. Daly served as CEO, President and Chairman of Neuralstem, Inc., a clinical stage biotech company focused in central nervous system disorders. From October 2014 to September 2016, Mr. Daly was the lead partner at RavineRock Partners, a commercial consulting practice focused on biotech and pharmaceuticals. Before that, Mr. Daly served as the President of AstraZeneca Diabetes U.S. from February 2014 to September 2014, and from August 2013 to January 2014, Mr. Daly served as the President of BMS-AstraZeneca Diabetes Alliance U.S. Prior to this position, Mr. Daly was a Founder and a Partner at SagePath Partners, a commercial service company serving the biotech and pharmaceutical industries, from October 2011 to July 2013. Prior to the foregoing positions, from 1998 to 2011, Mr. Daly served in several capacities at Takeda North America, where he was instrumental in building Takeda North America from 14 people to more than 3,000 employees and $5 billion in sales in less than seven years. During his 13-year tenure, he served as Executive Vice President, U.S., from 2008 to 2011, where he was responsible for business development for the Americas and for expanding the company’s commercial footprint across North and South America and into new therapeutic areas including oncology Mr. Daly currently sits on the board of directors of Catalyst Pharmaceuticals and Opiant Pharmaceuticals. Mr. Daly earned an M.B.A. from Northwestern University’s Kellogg School of Management and holds a B.S. in microbiology from University of Notre Dame.
James R. Tonra, Ph.D. has served as our Senior Vice President of Preclinical Development since April 2018. Dr. Tonra has worked for over 20 years in biotechnology, leading and utilizing in-house, contracted and sponsored research efforts to generate definitive data packages that enable the prioritization of research projects and guide clinical development at Regeneron Pharmaceuticals, Millennium Pharmaceuticals, ImClone Systems/Eli Lilly, and Kadmon Holdings. Dr. Tonra has collaborated with and lead multidisciplinary teams to develop biologic and small molecule drug candidates for disease indications including inflammation, oncology, diabetes, and CNS disorders. He has authored over 40 peer-reviewed publications and is an inventor on numerous use-patents. At ImClone Systems, prior to the successful acquisition by Eli Lilly, Dr. Tonra’s efforts significantly contributed to the IND filing and clinical strategy development for 8 novel drugs, 3 of which are now approved therapies for cancer: Cyramza, Portrazza and Lartruvo. Dr. Tonra received his Ph.D. in Physiology and Biophysics from SUNY at Stony Brook and B.S. in Physics, Summa Cum Laude, from SUNY at Stony Brook.
Non-Employee Directors
Patrick Fabbio, M.B.A. has served on our board since January 2, 2018. Mr. Fabbio is currently the Chief Financial Officer of Progenics Pharmaceuticals, Inc. a public biotech company. Prior to joining Progenics in November 2015, he was Chief Financial Officer of electroCore LLC, a privately-held bioelectric medicine healthcare company, and Vice President, Finance for NPS Pharmaceuticals, Inc., a publicly traded, global rare disease company that was acquired by Shire. Mr. Fabbio has more than 20 years of financial leadership experience in both public and private life science and pharmaceutical companies in various roles, including: Vice President, Finance of Catalent Pharma Solutions Inc.; Chief Financial Officer of Ikano Therapeutics Inc.; senior corporate finance, commercial and transactional roles at Sanofi; and Corporate Controller for Biomatrix Inc., a publicly traded biotechnology company that was acquired by Genzyme. He received his B.B.A. in accounting at Pace University and M.B.A. from the Stern School of Business at New York University.
Matthew Kirkby, M.A. has served on our board since October 13, 2016. Mr. Kirkby brings over 20 years of banking experience to our board. He has held senior management positions in London, Hong Kong and Singapore. From 2015 to 2016 Mr. Kirkby served as Head of Corporate Banking Asia Pacific for HSBC in Hong Kong. From 2012 to 2015 Mr. Kirkby was the CEO North Asia and Co-Head of Investment Banking for CIMB in Hong Kong. He served as Managing Director, Global Head of ECM and Corporate Finance from 2008 to 2010 and Managing Director, Head of Global Banking Asia Pacific from 2010 to 2012 at the Royal Bank of Scotland. Between 1999 and 2007 Mr. Kirkby held various positions at ABN AMRO. He is currently a director or adviser to a number of privately held companies. He received his M.A. in jurisprudence at Pembroke College, University of Oxford in the United Kingdom.
Quanqi Song, Ph.D. has served on our board since May 1, 2016. Since 2013, Dr. Song has served as director of China Coal International Leasing Co., Ltd., a company providing international leasing, consultant and management services, where he continues to hold a directorship. Additionally, since 2007, Dr. Song has served as an investment manager and executive director of Shenjin Investment Limited. Dr. Song received his undergraduate degree in geography, followed by a postgraduate degree in natural resources, from Henan University in Kaifeng, China. Dr. Song earned his Ph.D. in finance and banking from Renmin University of China in Beijing.
Yanbin Xie, M.D. has served on our board since May 1, 2016. Dr. Xie has had a long career in drug development research. From 1997 to January 1, 2018, Dr. Xie was the General Manager of ICON Clinical Research (Beijing No. 2) Co., Limited, formerly BeijingWits Medical Consulting Ltd., a provider of outsourced development services to the biotechnology, pharmaceutical and medical device industries. At ICON, Dr. Xie was responsible for coordinating strategic goals and development, training management teams and overseeing regulatory compliance. Dr. Xie founded BeijingWits, the first Chinese joint venture CRO, in 1997 with the goal of bringing international standards for clinical trials to China. Dr. Xie continues to hold a directorship at Beijing Wits Science & Technology Co. Ltd., which he has held since 1995. Since 2011, Dr. Xie has also served as a director of Med-Sonics Corp., a company focused on the development of ultrasonic devices. Dr. Xie received his M.D. from Shanxi Medical University, in Taiyuan, China, specializing in clinical medicine.
Christine Ying Zhao, M.B.A. has served on our board since October 13, 2016. Since 2016, Ms. Zhao has been a Managing Partner of Yuanming Capital, a VC/PE fund focused on cross-border investment and acquisition opportunities between the U.S. and China, particularly in the healthcare industry. Previously Ms. Zhao served as the Group Chief Financial Officer of BEST Logistics Technology (China) Co., Ltd. Prior to this, Ms. Zhao served as a Managing Director at Bank of America Merrill Lynch and as an Executive Director at JPMorgan, where she held senior positions (including regional CFO and COO) in global corporate and investment banking units. Ms. Zhao has worked for a number of corporations in various roles including strategy and corporate venture investing at American Express in New York, London and Singapore, investment banking at Goldman Sachs in Hong Kong and corporate development at FedEx Corporation in the U.S. She brings to the board unique management experience as she has managed teams across four continents. Ms. Zhao currently serves as a board member of the Chinese Finance Association, a non-profit organization with over 6,000 members worldwide. A Chartered Financial Analyst, or CFA, Ms. Zhao holds an M.B.A. from Harvard Business School and a B.S. in Economics with distinction from Fudan University in Shanghai, China.
Changes in Directors and Officers in 2018
Resignation of Richard Brand
Richard A. Brand resigned as our Chief Financial Officer effective as of February 20, 2018. Mr. Brand’s departure was not the result of any disagreement with us or our Board of Directors on any matter relating to our operations, policies or practices. In connection with Mr. Brand’s departure, we entered into a separation agreement with Richard Brand, as described below. Our former Controller, Amy Yang, acted as the Company’s interim Chief Financial Officer until Edward Dongheng Liu was appointed as Chief Financial Officer on March 26, 2018.
In connection with his resignation, we entered into a Severance Agreement and General Release with Mr. Brand, dated February 20, 2018, pursuant to which we agreed to pay Mr. Brand one month base salary and to pay health insurance premiums for Mr. Brand and his dependents through May 31, 2018, in exchange for execution by Mr. Brand of a general release of claims against us. Mr. Brand will continue to be bound by the restrictive covenants contained in his employment agreement and his mutual confidentiality and non-disclosure agreement. Mr. Brand’s unvested restricted share awards were forfeited as of the date of his resignation.
Resignation of Nanxing He and Mulong Liu
On January 2, 2018, each of Dr. Nanxing He and Mr. Mulong Liu resigned from the board.
Compensation of Executive Officers and Directors in 2018
With respect to the year ended December 31, 2018, the aggregate cash compensation, including benefits in kind, accrued or paid by us and our subsidiaries to our directors and executive officers (including our former CFO Richard Brand (resigned February 20, 2018) and former directors Nanxing He and Mulong Liu (each resigned January 2, 2018).) was $2 million, and the aggregate equity compensation by us and our subsidiaries to our directors and executive officers was $6 million (including stock options, as described in “—2017 Omnibus Incentive Plan” below). This amount does not include business travel, relocation, professional and business association dues and expenses reimbursed to such persons, and other benefits commonly reimbursed or paid by companies in our industry. For the year ended December 31, 2018, we did not separately set aside any amounts for pensions, retirement or other benefits for our directors and executive officers.
Director Agreements
We have entered into a director agreement, or the Director Agreement, with each of our non-employee directors. Under the terms of each Director Agreement, the annual compensation payable to our non-employee directors consists of:
| • | an annual cash retainer fee equal to $30,000 (pro-rated for any partial year of service), plus an additional cash retainer fee equal to $3,750 (pro-rated for any partial year of service) for any director serving as chairman of either the Compensation Committee, the Nominating and Corporate Governance Committee, or the Audit Committee, and |
| • | an annual grant of restricted shares with a grant date value of $30,000 (pro-rated for any partial year of service), plus an additional grant of restricted shares with a grant date value of $3,750 (pro-rated for any partial year of service, but not pro-rated in respect of fiscal year 2017) for any director serving as chairman of either the Compensation Committee, the Nominating and Corporate Governance Committee, or the Audit Committee, in each case paid on a fiscal year basis in arrears. |
Such restricted shares are issued under the BeyondSpring Inc. 2017 Omnibus Incentive Plan, or the 2017 Omnibus Incentive Plan and vest in equal installments on the first three anniversaries of the grant date, subject to the director’s continued service as our director through the applicable vesting dates. Any grant of restricted shares made to a non-employee director will be subject to the terms and conditions of the 2017 Omnibus Incentive Plan and the applicable restricted share award agreement memorializing such grant.
On December 20, 2018, we made the following grants of restricted shares to our non-employee directors in respect of their annual share grant entitlements under their Director Agreements (subject to vesting as described above): Mr. Fabbio, 1,704 shares; Mr. Kirkby, 1,704 shares; Dr. Song, 1,515 shares; Dr. Xie, 1,515 shares; and Ms. Zhao, 1,704 shares.
As of March 25, 2019, our non-employee directors held the following number of restricted shares: Mr. Fabbio, 1,704 shares (of which none were vested); Mr. Kirkby, 2,550 shares (of which 282 were vested); Dr. Song, 2,250 shares (of which 245 were vested); Dr. Xie, 2,250 shares (of which 245 were vested); and Ms. Zhao, 2,550 shares (of which 282 were vested). No former directors hold any shares under the 2017 Omnibus Incentive Plan.
For additional information regarding equity-based grants under our 2017 Omnibus Incentive Plan, see “—2017 Omnibus Incentive Plan.”
Employment Agreements
Lan Huang, Ph.D.
Dr. Lan Huang, our Co-founder, Chairman and Chief Executive Officer, is party to an amended and restated employment agreement with BeyondSpring U.S. dated as of November 10, 2016. Dr. Huang’s employment agreement provides for an annual base salary of $300,000, which is subject to review and adjustment in accordance with company policy. Dr. Huang’s base salary for fiscal year 2018 was $300,000. Dr. Huang is eligible to participate in any bonus program, on a basis consistent with that applicable to other employees at her level, in accordance with company policy. Dr. Huang is also eligible to receive payment for the cost of her medical insurance. Dr. Huang is required to use her best efforts to remain in our employment for a minimum of three years, but her employment is at will, and can be terminated by us at any time or by Dr. Huang upon four weeks’ notice. Dr. Huang’s employment agreement contains a two year non-solicit of employees, a confidentiality provision and an assignment of intellectual property provision.
Edward Dongheng Liu
Mr. Edward Dongheng Liu, our Chief Financial Officer, is party to an employment agreement with BeyondSpring U.S., dated as of March 26, 2018. Mr. Liu’s employment agreement provides for an annual base salary of $300,000, which is subject to review and adjustment in accordance with company policy. Mr. Liu is eligible for an annual merit bonus of two-month salary based on certain pre-specified milestones. Mr. Liu is also eligible to receive payment for the cost of his medical insurance up to a maximum of $500 per month. Mr. Liu is required to use his best efforts to remain in our employment for a minimum of four years, but his employment is at will, and can be terminated by us at any time or by Mr. Liu upon four weeks’ notice. Mr. Liu’s employment agreement contains a two year non-solicit of employees, a confidentiality provision, and an assignment of intellectual property provision.
Ramon Mohanlal, M.D., Ph.D.
Dr. Ramon Mohanlal, our Chief Medical Officer, is party to an amended and restated employment agreement with BeyondSpring U.S., dated as of November 10, 2016. Dr. Mohanlal’s employment agreement provides for an annual base salary of $300,000, which is subject to review and adjustment in accordance with company policy. Dr. Mohanlal’s base salary for fiscal year 2018 was $300,000. Dr. Mohanlal is eligible for an annual merit bonus of 30% of his annual salary based on certain pre-specified milestones. Dr. Mohanlal is also eligible to receive payment for the cost of his medical insurance up to a maximum of $500 per month. Dr. Mohanlal is required to use his best efforts to remain in our employment for a minimum of five years, but his employment is at will, and can be terminated by us at any time and by Dr. Mohanlal upon four weeks’ notice. Dr. Mohanlal’s employment agreement contains a two year non-solicit of employees, a confidentiality provision and an assignment of intellectual property provision.
Gordon L. Schooley, Ph.D.
Dr. Gordon Schooley, our Chief Regulatory Officer, is party to an amended and restated employment agreement with BeyondSpring U.S., dated as of November 10, 2016. Dr. Schooley’s employment agreement provides for an annual base salary of $250,000, which is subject to review and adjustment in accordance with company policy. Dr. Schooley’s base salary for fiscal year 2018 was $250,000. Dr. Schooley is eligible for an annual merit bonus of 20% of his annual salary based on certain pre-specified milestones. Dr. Schooley is also eligible to receive payment for the cost of medical insurance for him and his wife. Dr. Schooley is required to use his best efforts to remain in our employment for a minimum of three years, but his employment is at will, and can be terminated by us at any time and by Dr. Schooley upon four weeks’ notice. Dr. Schooley’s employment agreement contains a two year non-solicit of employees, a confidentiality provision and an assignment of intellectual property provision.
Richard J. Daly
Mr. Richard Daly, our Chief Operating Officer, is party to an employment with BeyondSpring U.S. dated as of June 8, 2018. Mr. Daly’s employment agreement provides for an annual base salary of $300,000, which is subject to review and adjustment in accordance with company policy. Mr. Daly is eligible for an annual merit bonus of 30% of his annual salary based on certain pre-specified milestones. In addition, Mr. Daly is eligible for certain performance-based incentive bonuses, based on attainment of various milestones relating to business development and global sales. If earned, any such amounts shall be paid in either cash or fully vested ordinary shares, at Mr. Daly’s election.
Mr. Daly is required to use commercially reasonable efforts to remain in our employment for a minimum of five years, but his employment is at will, and can be terminated by us at any time or by Mr. Daly upon 4 weeks’ notice. If we terminate Mr. Daly’s employment other than for Cause (as defined in his employment agreement) or due to death or disability, or if Mr. Daly terminates his employment for Good Reason (as defined in his employment agreement), then Mr. Daly is entitled (subject to his execution of a release of claims) to: (i) 9 months of salary continuation; (ii) a pro-rated portion of any bonus earned for the year in which his termination occurs, based on actual performance results, paid at the same time as other senior executives; and (iii) a reduced portion, determined based on length of service and length of period between termination and achievement, of certain of his performance-based incentive bonuses, if any, subsequently achieved. Mr. Daly’s employment agreement contains a two year non-solicit of employees, a confidentiality provision and an assignment of intellectual property provision.
James Tonra, Ph.D.
Dr. James Tonra, our Senior Vice President, Preclinical Development, is party to an employment with BeyondSpring U.S. dated as of March 16, 2018. Mr. Tonra’s employment agreement provides for an annual base salary of $280,000, which is subject to review and adjustment in accordance with company policy. Dr. Tonra is eligible for an annual merit bonus of 30% of his annual salary based on certain pre-specified milestones. Dr. Tonra is also eligible to receive payment for the cost of his medical insurance up to a maximum of $500 per month. Dr. Tonra is required to use best efforts to remain in our employment for a minimum of four years, but his employment is at-will and can be terminated by us at any time or by Dr. Tonra upon four weeks’ notice. If we terminate Dr. Tonra’s employment without Cause (as defined in his employment agreement), Dr. Tonra is entitled to three months of severance. Dr. Tonra’s employment agreement contains a two year non-solicit of employees, a confidentiality provision and an assignment of intellectual property provision.
Consulting Arrangements
G. Kenneth Lloyd, Ph.D.
Dr. G. Kenneth Lloyd, our Chief Scientific Officer, has provided consulting services to BeyondSpring U.S., and prior to the formation of BeyondSpring U.S., to our subsidiary Wanchun Pharma, since December 16, 2012. Based on the consulting service contracts entered into with Dr. Lloyd, the consulting service fees were $207,500 for his services provided during the year ended December 31, 2018. As part of his consulting agreement, Dr. Lloyd is also reimbursed for the cost of health insurance for him and his wife as well as certain consulting expenses.
2017 Omnibus Incentive Plan
In connection with our initial public offering, we adopted the 2017 Omnibus Incentive Plan to provide additional incentives to selected directors, officers, employees and consultants, and to enable our company to obtain and retain the services of these individuals. The 2017 Omnibus Incentive Plan enables us to grant restricted shares, options or other awards to our directors, employees and consultants. Up to 2,137,037 ordinary shares were authorized pursuant to awards under the 2017 Omnibus Incentive Plan. Awards will be made pursuant to award agreements and may be subject to vesting and other restrictions as determined by the board of directors.
Restricted Share Awards
We have granted restricted share awards under the 2017 Omnibus Incentive Plan to certain of our employees and consultants, including certain of our executive officers. Under the terms of the restricted share award agreements, if an executive officer is terminated without “cause” within 12 months of a “change in control” (each as defined in the 2017 Omnibus Incentive Plan), then any unvested time-based restricted shares will become fully vested on the termination date. If the executive officer’s employment or engagement terminates due to death or disability, the next tranche of time-based restricted shares that would have vested had the executive officer remained in service with us through the applicable vesting date will become fully vested on the termination date, and any remaining unvested time-based restricted shares as of the termination date will be forfeited. Upon any other termination of employment or engagement, all unvested time-based restricted shares as of the termination date will be forfeited. Upon any termination of service for any reason, all unvested performance-based restricted shares as of the termination date will be forfeited.
We did not grant any restricted shares to our executive officers in 2018. In February 2018, Mr. Richard Brand forfeited 75,000 restricted shares following his resignation from our company. In May 2018, Dr. Schooley forfeited 20,000 restricted shares following his failure to pay consideration due on a call by us under our memorandum and articles of association.
On April 25, 2019, we granted Mr. Liu 100,000 restricted shares, which will vest as follows: (i) 50,000 shares will vest in four equal installments on each of May 1, 2019, March 26, 2020, 2021 and 2022; and (ii) 50,000 shares will vest on the attainment of various milestones related to capital markets activities.
As of March 25, 2019, our executive officers held the following number of restricted shares: Dr. Huang, 0 shares; Mr. Liu, 0 shares; Dr. Mohanlal, 227,220 shares (82,200 of which were vested); Dr. Lloyd, 27,100 shares (all of which were vested); Dr. Schooley, 7,000 shares (all of which were vested); Mr. Daly, 0 shares; and Dr. Tonra, 0 shares.
Option Awards
We have also granted non-qualified stock options to purchase our ordinary shares under the 2017 Omnibus Incentive Plan, or “options,” to certain of our executive officers. The options are subject to a combination of time-based vesting and performance-based vesting (as described further below), subject to the executive officer’s continued service with us through the applicable vesting date. The options, if not earlier exercised or forfeited, expire on the tenth anniversary of the date of grant.
Under the terms of the 2017 Omnibus Incentive Plan and option agreements, (i) if the options are assumed or substituted for in the change in control, if the executive officer is terminated without cause within 12 months of such change in control, then any unvested options will become vested and will remain exercisable for the 90-day period following the termination date, and (ii) if the options are not assumed or substituted for in the change of control, then any unvested options will become vested upon such change of control and otherwise be treated as determined by the plan administrator.
If the executive officer’s employment or engagement terminates due to death or disability, the next tranche of time-based options that would have vested had the executive officer remained employed or engaged through the applicable vesting date will become fully vested on the termination date (and will remain exercisable for one year following such termination), and any remaining unvested time-based options will be forfeited. On a termination by us for cause, all vested and unvested options are forfeited. On a termination for any other reason, vested options remain exercisable for three months following such termination date. Upon any termination of employment, any unvested performance-based options as of the termination date will be forfeited.
In 2018, we granted the following options to our executive officers:
| • | Mr. Daly: 100,000 options, granted on August 2, 2018, at an exercise price of $24.41 per share, which will vest as follows: (i) 10,000 options will vest on the first anniversary of the grant date; (ii) 40,000 options will vest in sixteen equal installments on each September 30, December 31, March 31, and June 30 thereafter (with the last installment vesting on June 30, 2023); and (iii) 50,000 options will vest on the attainment of various component milestones related to the equity raise and the launch of commercial sales of any product in China and the U.S. In addition, with respect to 40,000 of the performance-based options, Mr. Daly will be entitled to post-termination vesting (either in full or pro-rated) upon achievement of certain milestones, with such options remaining exercisable for 3 months thereafter, and then forfeited if not exercised. |
| • | Dr. Tonra: 30,000 options, granted on March 16, 2018, at an exercise price of $27.30 per share, which will vest as follows: (i) 3,500 options will vest on May 1, 2019; (ii) 12,000 options will vest in twelve equal installments on each August 1, November 1, February 1 and May 1 thereafter (with the last installment vesting on May 1, 2022); and (iii) 14,500 options will vest on the attainment of various milestones in the research, development, testing and realization of Plinabulin, BPI-002 and BPI- 004. |
As of March 25, 2019, our executive officers held the following options:
| • | Dr. Mohanlal: 170,000 options, granted on December 20, 2017 at an exercise price of $29.00 per share, all of which were vested. |
| • | Dr. Lloyd: 72,900 options, granted on December 20, 2017 at an exercise price of $29.00 per share, all of which were vested. |
| • | Dr. Schooley: 93,000 options, granted on December 20, 2017 at an exercise price of $29.00 per share, all of which were vested. |
| • | Mr. Daly: 100,000 options, as described above. |
| • | Dr. Tonra: 30,000 options, as described above. |
Stock Bonus Awards
On April 11, 2017, we granted a stock bonus award opportunity to Dr. Mohanlal under the 2017 Omnibus Incentive Plan. Dr. Mohanlal will be eligible to receive up to a maximum of 300,000 ordinary shares payable in installments upon our recognition of specified amounts of cumulative net income in connection with the attainment of various component milestones in the research, development, testing and realization of the drug BPI-002, subject to his continued employment through the attainment of each applicable milestone. Dr. Mohanlal will also be eligible to receive up to a maximum of 300,000 additional ordinary shares payable in installments upon our recognition of specified amounts of cumulative net income in connection with the attainment of various component milestones in the research, development, testing and realization of the drug BPI-004, subject to his continued employment through the attainment of each applicable milestone.
Under the terms of the applicable stock bonus award agreements, if Dr. Mohanlal’s employment terminates for any reason (other than in connection with a change in control, as described below), any bonus payment for which the applicable milestone has not been attained as of the termination date will be forfeited, provided, that if we terminate Dr. Mohanlal’s employment without cause and within six months following such termination any milestone is attained, then he will be eligible to receive the number of ordinary shares that he would have received upon the attainment of such milestone had he still been employed on such date. If we terminate Dr. Mohanlal’s employment without cause within 12 months of a change in control (as defined in the 2017 Omnibus Incentive Plan) following which the stock bonus award agreement is assumed by the successor entity, then the next bonus tranche that would have become payable upon attainment of the next milestone will become fully vested on the termination date. If the stock bonus award agreement is not assumed by the successor entity in the change in control, then the next bonus tranche that would have become payable upon attainment of the next milestone will become fully vested on the date of the change in control.
Board of Directors
Our board of directors currently consists of six members, all of whom were elected pursuant to our current articles of association. Our nominating and governance committee and board of directors consider a broad range of factors relating to the qualifications and background of nominees, which may include diversity and is not limited to race, gender or national origin. We have no formal policy regarding board diversity. Our nominating and governance committee’s and board of directors’ priority in selecting board members is identification of persons who will further the interests of our shareholders through his or her established record of professional accomplishment, the ability to contribute positively to the collaborative culture among board members, knowledge of our business, understanding of the competitive landscape and professional and personal experiences and expertise relevant to our growth strategy.
There is no Cayman Islands law requirement that a director must hold office for a certain term and stand for re-election unless the resolutions appointing the director impose a term on the appointment. Our amended and restated articles of association provide that our directors shall hold office until the expiration of his or her term and until his or her successor shall have been elected and qualified.
A director may be elected by ordinary resolution either to fill a casual vacancy on the board of directors or as an addition to the existing board of directors. The directors by the affirmative vote of a simple majority of the remaining directors present and voting at a board meeting shall have the power from time to time and at any time to appoint any person as a director to fill a casual vacancy on the board of directors or as an addition to the existing board of directors, subject to our compliance with director nomination procedures required under applicable corporate governance rules of the NASDAQ Capital Market, as long as our company’s securities are traded on the NASDAQ Capital Market. A director may be removed from office by ordinary resolution at any time before the expiration of his or her term. The Director Agreement does not provide for any benefits upon termination of service to our directors.
Director Independence
Our board of directors has determined that Patrick Fabbio, Matthew Kirkby, Quanqi Song, Yanbin Xie and Christine Ying Zhao are independent, as determined in accordance with the rules of the NASDAQ Capital Market. In making such independence determination, our board of directors considered the relationships that each such non-employee director has with us and all other facts and circumstances that the board of directors deemed relevant in determining their independence, including the beneficial ownership of our share capital by each non-employee director and the transactions involving them described in “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions.” The composition and functioning of our board of directors and each of our committees comply with all applicable requirements of the NASDAQ Capital Market and the rules and regulations of the SEC. There are no family relationships among any of our directors or executive officers.
Board Committees
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee, each of which operates pursuant to a separate charter adopted by our board of directors. The composition and functioning of all of our committees comply with all applicable requirements of the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the NASDAQ Capital Market and SEC rules and regulations.
Audit Committee
Patrick Fabbio, Matthew Kirkby and Quanqi Song currently serve on the audit committee, which is chaired by Patrick Fabbio. Our board of directors has determined that each member of the audit committee is “independent” for audit committee purposes as that term is defined in the rules of the SEC and the applicable rules of the NASDAQ Capital Market. The audit committee’s responsibilities include:
| • | selecting and appointing our independent registered public accounting firm, and approving the audit and permitted non-audit services to be provided by our independent registered public accounting firm; |
| • | evaluating the performance and independence of our independent registered public accounting firm; |
| • | monitoring the integrity of our financial statements and our compliance with legal and regulatory requirements as they relate to our financial statements or accounting matters; |
| • | reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures; |
| • | establishing procedures for the receipt, retention and treatment of accounting-related complaints and concerns; |
| • | reviewing and discussing with the independent registered public accounting firm the results of our year-end audit, and recommending to our board of directors, based upon such review and discussions, whether our financial statements shall be included in our annual report on Form 20-F; |
| • | reviewing all related party transactions for potential conflict of interest situations and approving all such transactions; and |
| • | reviewing the type and presentation of information to be included in our earnings press releases, as well as financial information and earnings guidance provided by us to analysts and rating agencies. |
Compensation Committee
Matthew Kirkby, Yanbin Xie and Christine Ying Zhao currently serve on the compensation committee, which is chaired by Matthew Kirkby. Our board of directors has determined that each member of the compensation committee is “independent” as that term is defined in the applicable rules of the NASDAQ Capital Market. The compensation committee’s responsibilities include:
| • | reviewing the goals and objectives of our executive compensation plans, as well as our executive compensation plans in light of such goals and objectives; |
| • | evaluating the performance of our executive officers in light of the goals and objectives of our executive compensation plans and recommending to our board of directors with respect to the compensation of our executive officers; |
| • | reviewing the goals and objectives of our general compensation plans and other employee benefit plans, as well as our general compensation plans and other employee benefit plans in light of such goals and objectives; |
| • | retaining and approving the compensation of any compensation advisors; |
| • | reviewing all equity-compensation plans to be submitted for shareholder approval under the NASDAQ listing rules, and reviewing and approving all equity-compensation plans that are exempt from such shareholder approval requirement; |
| • | evaluating the appropriate level of compensation for board and board committee service by non-employee directors; and |
| • | reviewing and approving description of executive compensation included in our annual report on Form 20-F. |
Nominating and Corporate Governance Committee
Matthew Kirkby, Yanbin Xie and Christine Ying Zhao currently serve on the nominating and corporate governance committee, which is chaired by Christine Ying Zhao. Our board of directors has determined that each member of the nominating and corporate governance committee is “independent” as that term is defined in the applicable rules of the NASDAQ Capital Market. The nominating and corporate governance committee’s responsibilities include:
| • | assisting our board of directors in identifying prospective director nominees and recommending nominees for election by the shareholders or appointment by our board of directors; |
| • | advising the board of directors periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to our board of directors on all matters of corporate governance and on any corrective action to be taken; |
| • | overseeing the evaluation of our board of directors; and |
| • | recommending members for each board committee of our board of directors. |
Our board of directors may establish other committees from time to time.
As of December 31, 2018, we had 52 full-time employees. Of these, 38 were engaged in full-time research and development and laboratory operations and 14 were engaged in full-time general and administrative functions. As of December 31, 2018, 18 of our employees were located in China and 34 were located in the U.S. We have also engaged and may continue to engage independent contractors who are not full-time employees, to assist us with our operations. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We have never experienced any employment related work stoppages, and we consider our relations with our employees to be good. The following table sets out our total number of employees by function for the last three years.
| | | | | | | | | | |
| Research and Development and Laboratory Operations | | | 12 | | | | 22 | | | | 38 | |
| General and Administrative Functions | | | 12 | | | | 10 | | | | 14 | |
For information regarding the share ownership of our directors and executive officers, see “Item 7. Major Shareholders and Related Party Transactions—A. Major Shareholders.” For information regarding equity-based grants to our directors, executive officers and other employees, see “Item 6. Directors, Senior Management and Employees—B. Compensation—Director Agreements” and “Item 6. Directors, Senior Management and Employees—B. Compensation—2017 Omnibus Incentive Plan.”
| Major Shareholders and Related Party Transactions |
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of March 25, 2019 by:
| • | each person or group of affiliated persons known by us to own beneficially 5% or more of our outstanding ordinary shares; |
| • | each of our directors and executive officers individually; and |
| • | all of our executive officers and directors as a group. |
The beneficial ownership of our ordinary shares is determined in accordance with the rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power, and includes the ordinary shares issuable pursuant to stock options that are exercisable within 60 days of March 25, 2019. Ordinary shares issuable pursuant to stock options are deemed outstanding for computing the percentage of the person holding such options but are not outstanding for computing the percentage of any other person. As of March 25, 2019, there were 349,400 ordinary shares issuable pursuant to stock options exercisable within 60 days thereof.
The calculation of percentage of ordinary shares beneficially owned in the table below is based on 23,184,612 ordinary shares outstanding as of March 25, 2019. Except where otherwise indicated, we believe, based on information furnished to us by such owners, that the beneficial owners of the ordinary shares listed below have sole investment and voting power with respect to such shares.
Unless otherwise noted below, each shareholder’s address is c/o BeyondSpring Inc., 28 Liberty Street, 39th Floor, New York, NY 10005.
Name of Beneficial Owner | | Number of Ordinary Shares Beneficially Owned | | | | |
| 5% Shareholders | | | | | | |
| Ever Regal Group Limited(1) | | | 3,660,000 | | | | 15.79 | |
| Fairy Eagle Investment Limited(1) | | | 4,620,000 | | | | 19.93 | |
| Rosy Time Holdings Limited(1) | | | 2,190,000 | | | | 9.45 | |
| NPBSIPO Liquidating Trust(2) | | | 1,932,963 | | | | 8.34 | |
| Entities affiliated with Sangel Venture Capital(3) | | | 2,105,494 | | | | 9.08 | |
| Executive Officers and Directors | | | | | | | | |
| Lan Huang(1) | | | 11,207,037 | | | | 48.34 | |
| Edward Dongheng Liu(4) | | | 55,555 | | | | * | |
| Ramon W. Mohanlal(5) | | | 397,220 | | | | 1.68 | |
| G. Kenneth Lloyd(6) | | | 100,000 | | | | * | |
| Gordon L. Schooley(7) | | | 100,000 | | | | * | |
| Richard Daly(8) | | | 10,000 | | | | * | |
| James R. Tonra(9) | | | 3,500 | | | | * | |
| Patrick Fabbio(10) | | | 1,704 | | | | * | |
| Matthew Kirkby(11) | | | 2,550 | | | | * | |
| Quanqi Song(12) | | | 687,788 | | | | 2.97 | |
| Yanbin Xie(13) | | | 2,250 | | | | * | |
| Christine Ying Zhao(14) | | | 2,550 | | | | * | |
| All Directors and Executive Officers as a group (12 people) | | | 12,570,154 | | | | 53.41 | |
| * | Amounts represent less than 1% of outstanding ordinary shares. |
| (1) | Dr. Lan Huang, our Co-founder, Chairman and Chief Executive Officer, is the sole owner of Ever Regal Group Limited. Mr. Linqing Jia, Dr. Huang’s spouse, is the sole owner of Fairy Eagle Investments Limited and Rosy Time Holdings Limited. Dr. Huang and Mr. Jia collectively also own approximately 90% of the equity interest in Wanchun Biotech. Dr. Huang and Mr. Jia may be deemed to have shared voting and dispositive power over the shares held by each of Ever Regal Group Limited, Fairy Eagle Investments Limited, Rosy Time Holdings Limited and Wanchun Biotech, and the 137,037 restricted shares, all of which have been vested, held of record by Mr. Jia. The amount also includes 600,000 ordinary shares held by Lan Huang 2018 Grantor Retained Annuity Trust, over which Dr. Huang as the trustee has the sole voting and dispositive power. |
| (2) | Consisting of 1,932,963 ordinary shares owned by NPBSIPO Liquidating Trust. BSIPO Trustee MGR LLC is the Administrator Trustee of NPBSIPO Liquidating Trust. Mr. Kobi Sethna has sole discretion to direct the Administrator Trustee with respect to voting and investment power over the shares owned by NPBSIPO Liquidating Trust. |
| (3) | Consisting of 800,000 ordinary shares owned by Beijing Sangel Fang Sheng Venture Capital (Limited Partnership), 300,000 ordinary shares owned by Shenzhen Sangel Jing Rui Venture Capital (Limited Partnership), 200,000 ordinary shares owned by Shenzhen Sangel Sino-Euro Venture Capital (Limited Partnership), 444,444 ordinary shares owned by Beijing Huarong Sangel Venture Capital (Limited Partnership) (previously named Beijing Sangel Venture Capital (Limited Partnership)) (“Beijing Huarong Sangel”) and 361,050 ordinary shares owned by Sangel Star Biomedical Fund LP. Shenzhen Sangel Capital Management Limited Company (“Sangel Venture Capital”) is the sole general partner of Beijing Sangel Fang Sheng Venture Capital (Limited Partnership), Shenzhen Sangel Jing Rui Venture Capital (Limited Partnership) and Shenzhen Sangel Sino-Euro Venture Capital (Limited Partnership). Sangel Star Biomedical Fund LP is an affiliate of Sangel Venture Capital. The managing members of Sangel Venture Capital are Mr. Mulong Liu (who is a founding partner of Sangel Venture Capital and one of our former directors), Dr. Feng Fang, Dr. Nanxing He (who is one of our former directors), Dr. Jinglong Liu, Mr. Xiaoming Yang and Dr. Huali Zhang. The address of Sangel Venture Capital is 8th Floor, Design House, Donghua Garden, Nanshan District, Shenzhen, China. Sangel Venture Capital and Huarong Tianze Investments Limited (“Huarong Tianze”) are the general partners of Beijing Huarong Sangel Venture Capital (Limited Partnership). The managing members of Huarong Tianze are Mr. Xiaoming Ran and Mr. Xiaodong Wu. The address of Huarong Tianze is 2nd Floor, Building A, No. 8 Financial Street, Xicheng District, Beijing, China. |
| (4) | Consisting of 55,555 ordinary shares held of record by Mr. Edward Dongheng Liu. |
| (5) | Consisting of (i) 227,220 restricted shares, 82,200 of which have been vested, held of record by Dr. Ramon W. Mohanlal, granted under the 2017 Omnibus Incentive Plan and (ii) vested options to purchase 170,000 ordinary shares, granted under the 2017 Omnibus Incentive Plan. |
| (6) | Consisting of (i) 27,100 restricted shares, all of which have been vested, held of record by Dr. G. Kenneth Lloyd, granted under the 2017 Omnibus Incentive Plan and (ii) vested options to purchase 72,900 ordinary shares. |
| (7) | Consisting of (i) 7,000 restricted shares, all of which have been vested, held of record by Dr. Gordon Schooley granted under the 2017 Omnibus Incentive Plan, and (ii) vested options to purchase 93,000 ordinary shares. |
| (8) | Consisting of options to purchase 10,000 ordinary shares granted under the 2017 Omnibus Incentive Plan. |
| (9) | Consisting of options to purchase 3,500 ordinary shares granted under the 2017 Omnibus Incentive Plan, which will vest on May 1, 2019. |
| (10) | Consisting of 1,704 restricted shares, none of which have been vested, held of record by Mr. Patrick Fabbio granted under the 2017 Omnibus Incentive Plan. |
| (11) | Consisting of 2,550 restricted shares, 282 of which have been vested, held of record by Mr. Matthew Kirkby granted under the 2017 Omnibus Incentive Plan. |
| (12) | Consisting of 687,788 ordinary shares owned by Hover Dragon Investment Limited, an entity wholly owned by Winning View Investments Limited and 2,250 restricted shares, 245 of which have been vested, held by record by Dr. Quanqi Song. Dr. Quanqi Song, a member of our board of directors, is the sole shareholder of Winning View Investments Limited. |
| (13) | Consisting of 2,250 restricted shares, 245 of which have been vested, held of record by Dr. Yanbin Xie granted under the 2017 Omnibus Incentive Plan. |
| (14) | Consisting of 2,550 restricted shares, 282 of which have been vested, held of record by Ms. Christine Ying Zhao granted under the 2017 Omnibus Incentive Plan. |
We have one class of ordinary shares, and each holder of our ordinary shares is entitled to one vote per share. None of our shareholders has different voting rights from other shareholders.
As of March 25, 2019, approximately 7,130,665 of our outstanding ordinary shares were held by 15 record holders in the U.S.
We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
| B. | Related Party Transactions |
Since January 1, 2018, there has not been, nor is there currently proposed, any material transaction or series of similar material transactions to which we were or are a party to and in which any of the members of our board of directors or senior management, holders of more than 10% of ordinary shares, or any member of the immediate family of any of the foregoing persons, had or will have a direct or indirect material interest, other than the transactions we describe below.
On December 27, 2018, we entered into a loan agreement with Dr. Mohanlal, pursuant to which we made a $481,034.44 loan to Dr. Mohanlal at an annual interest rate of 0%. The loan was made in connection with the tax withholding payment due by Dr. Mohanlal on his vested restricted shares. As of the date of this annual report on Form 20-F, the loan has been fully repaid.
On March 19, 2019, we entered into loan agreements with each of Dr. Huang, Dr. Schooley and Ms. Yue Jia, our International Finance Manager, pursuant to which each of Dr. Huang, Dr. Schooley and Ms. Jia made loans to us which, in the aggregate, totaled $350,000.00, each at an annual interest rate of 0%. The loans were made to provide cash for our operating activities. As of the date of this annual report on Form 20-F, the total principal amount remains outstanding, which shall be repaid in full by December 31, 2019 or earlier at our discretion.
Employment Agreements
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment Agreements.”
Consulting Arrangements
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Consulting Arrangements.”
Director Agreement
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Director Agreements.”
2017 Omnibus Incentive Plan
See “Item 6. Directors, Senior Management and Employees—B. Compensation—2017 Omnibus Incentive Plan.”
Indemnification Agreements
Cayman Islands law does not limit the extent to which a company’s memorandum and articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our amended and restated memorandum and articles of association require us to indemnify every director, alternate director, secretary, assistant secretary, or other officer for the time being and from time to time of our company (but not including our auditors) and the personal representatives of the same against all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by such indemnified person, other than by reason of such indemnified person’s own dishonesty, willful default or fraud, in or about the conduct of our company’s business or affairs (including as a result of any mistake of judgment) or in the execution or discharge of his duties, powers, authorities or discretions, including without prejudice to the generality of the foregoing, any costs, expenses, losses or liabilities incurred by such indemnified person in defending (whether successfully or otherwise) any civil proceedings concerning us or our affairs in any court whether in the Cayman Islands or elsewhere. This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation.
In addition, we have entered into indemnification agreements with each of our directors and executive officers that provide such persons with additional indemnification beyond that provided in our amended and restated memorandum and articles of association.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us under the foregoing provisions, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
| C. | Interests of Experts and Counsel |
Not applicable.
| A. | Consolidated Financial Statements and Other Financial Information |
See “Item 18. Financial Statements.”
Legal Proceedings
See “Item 4. Information on the Company—B. Business Overview—Legal Proceedings.”
Dividend Policy
We have never declared or paid cash dividends to our shareholders, and we do not intend to pay cash dividends in the foreseeable future. We intend to reinvest any earnings in developing and expanding our business. Any future determination relating to our dividend policy will be at the discretion of our board of directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, our strategic goals and plans to expand our business, applicable law and other factors that our board of directors may deem relevant.
See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Ordinary Shares—Because we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of the ordinary shares for return on your investment” and “Item 10. Additional Information—B. Memorandum and Articles of Association—Dividends.”
We are a holding company incorporated in the Cayman Islands. We will rely to some extent on dividends from our U.S., Australia and PRC subsidiaries for payment of any dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to make such dividend payments to us. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Doing Business in China—In the future, we may rely to some extent on dividends and other distributions on equity from our principal operating subsidiaries to fund offshore cash and financing requirements” and “Item 4. Information on the Company—B. Business Overview—Government Regulation—Chinese Regulation—Regulations of Dividend Distribution.”
Except as disclosed elsewhere in this annual report on Form 20-F, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report on Form 20-F.
| A. | Offer and Listing Details |
Our ordinary shares have been listed on the NASDAQ Capital Market since March 9, 2017 under the symbol “BYSI.”
Not applicable.
Our ordinary shares have been listed on the NASDAQ Capital Market since March 9, 2017 under the symbol “BYSI.”
Not applicable.
Not applicable.
Not applicable.
Not applicable.
| B. | Memorandum and Articles of Association |
We are a Cayman Islands exempted company with limited liability and our affairs are governed by our memorandum and articles of association, as amended and restated from time to time, and the Companies Law (2018 Revision) of the Cayman Islands, which is referred to as the Companies Law below, and the common law of the Cayman Islands.
The following are summaries of material provisions of our current amended and restated memorandum and articles of association that became effective immediately prior to the completion of our initial public offering in March 2017, insofar as they relate to the material terms of our ordinary shares.
Objects of Our Company
Under our amended and restated memorandum and articles of association, the objects of our company are unrestricted and we have the full power and authority to carry out any object not prohibited by the law of the Cayman Islands.
Board of Directors
See “Item 6. Directors, Senior Management and Employees—C. Board Practices.”
Ordinary Shares
Our ordinary shares are issued in registered form and are issued when registered in our register of members. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their shares.
Dividends
The holders of our ordinary shares are entitled to such dividends as may be declared by our board of directors. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our directors. Under Cayman Islands law, dividends may be declared and paid only out of funds legally available therefor, namely out of either profit or our share premium account, provided that in no circumstances may a dividend be paid if this would result in our company being unable to pay its debts as they fall due in the ordinary course of business.
Voting Rights
Voting at any shareholders’ meeting is by show of hands unless a poll is demanded. A poll may be demanded by the chairman of such meeting or any one or more shareholders who together hold not less than 10% of the voting share capital of our company present in person or by proxy.
A quorum required for a meeting of shareholders consists of one or more shareholders present and holding not less than a majority of all voting share capital of our company in issue. Shareholders may be present in person or by proxy or, if the shareholder is a legal entity, by its duly authorized representative. Shareholders’ meetings may be convened by our board of directors on its own initiative or upon a request to the directors by shareholders holding at the date of deposit of the requisition not less than ten percent of our voting share capital in issue. Advance notice of at least seven calendar days is required for the convening of our annual general shareholders’ meeting and any other general shareholders’ meeting.
An ordinary resolution to be passed at a meeting by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the ordinary shares cast at a meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes attaching to the ordinary shares cast at a meeting. Both ordinary resolutions and special resolutions may also be passed by a unanimous written resolution signed by all the shareholders of our company, as permitted by the Companies Law and our amended and restated memorandum and articles of association. A special resolution will be required for important matters such as a change of name or making changes to our amended and restated memorandum and articles of association. Holders of the ordinary shares may, among other things, divide or combine their shares by ordinary resolution.
Transfer of Ordinary Shares
Subject to the restrictions set out below, any of our shareholders may transfer all or any of his or her ordinary shares by an instrument of transfer in the usual or common form or any other form approved by our board of directors.
Our board of directors may, in its absolute discretion, decline to register any transfer of any ordinary share which is not fully paid up or on which we have a lien. Our board of directors may also decline to register any transfer of any ordinary share unless:
| • | the instrument of transfer is lodged with us, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; |
| • | the instrument of transfer is in respect of only one class of shares; |
| • | the instrument of transfer is properly stamped, if required; |
| • | in the case of a transfer to joint holders, the number of joint holders to whom the ordinary share is to be transferred does not exceed four; and |
| • | a fee of such maximum sum as the Nasdaq Capital Market may determine to be payable or such lesser sum as our directors may from time to time require is paid to us in respect thereof. |
If our directors refuse to register a transfer they shall, within two months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal.
The registration of transfers may, after compliance with any notice required of the Nasdaq Capital Market, be suspended and the register closed at such times and for such periods as our board of directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register closed for more than 30 days in any year as our board may determine.
Liquidation
On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of shares), assets available for distribution among the holders of ordinary shares shall be distributed among the holders of our ordinary shares on a pro rata basis. If our assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our shareholders proportionately.
Calls on Shares and Forfeiture of Shares
Our board of directors may from time to time make calls upon shareholders for any amounts unpaid on their shares in a notice served to such shareholders at least 14 calendar days prior to the specified time or times of payment. The shares that have been called upon and remain unpaid are subject to forfeiture.
Redemption, Repurchase and Surrender of Ordinary Shares
We may issue shares on terms that such shares are subject to redemption, at our option or at the option of the holders thereof, on such terms and in such manner as may be determined, before the issue of such shares, by our board of directors. Our company may also repurchase any of our shares (including any redeemable shares) provided that the manner and terms of such purchase have been approved by our board of directors or by ordinary resolution of our shareholders, or are otherwise authorized by our amended and restated memorandum and articles of association. Under the Companies Law, the redemption or repurchase of any share may be paid out of our company’s profits or out of the proceeds of a fresh issue of shares made for the purpose of such redemption or repurchase, or out of capital (including share premium account and capital redemption reserve) if the company can, immediately following such payment, pay its debts as they fall due in the ordinary course of business. In addition, under the Companies Law no such share may be redeemed or repurchased (a) unless it is fully paid up, (b) if such redemption or repurchase would result in there being no shares outstanding, or (c) if the company has commenced liquidation. In addition, our company may accept the surrender of any fully paid share for no consideration.
Variations of Rights of Shares
The rights attached to any class or series of shares (unless otherwise provided by the terms of issue of the shares of that class or series) may, subject to our amended and restated memorandum and articles of association, be varied with the consent in writing of the holders of not less than two-thirds of the issued shares of that class or series or with the sanction of a special resolution passed at a general meeting of the holders of the shares of that class or series. The rights conferred upon the holders of the shares of any class issued shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such existing class of shares.
Issuance of Additional Shares
Our amended and restated memorandum and articles of association authorize our board of directors to issue additional ordinary shares from time to time as our board of directors shall determine, to the extent of available authorized but unissued shares.
Our amended and restated memorandum and articles of association also authorize our board of directors to establish from time to time one or more series of preferred shares and to determine, with respect to any series of preferred shares, the terms and rights of that series, including:
| • | the designation of the series; |
| • | the number of shares of the series; |
| • | the dividend rights, dividend rates, conversion rights, voting rights; and |
| • | the rights and terms of redemption and liquidation preferences. |
Our board of directors may issue preferred shares without action by our shareholders to the extent authorized but unissued. Issuance of these shares may dilute the voting power of holders of ordinary shares.
Inspection of Books and Records
Holders of our ordinary shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we will provide our shareholders with annual audited financial statements. See “—H. Documents on Display.”
Anti-Takeover Provisions
Some provisions of our amended and restated memorandum and articles of association may discourage, delay or prevent a change of control of our company or management that shareholders may consider favorable, including provisions that:
| • | authorize our board of directors to issue preferred shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preferred shares without any further vote or action by our shareholders; and |
| • | limit the ability of shareholders to requisition and convene general meetings of shareholders. |
However, under Cayman Islands law, our directors may only exercise the rights and powers granted to them under our memorandum and articles of association for a proper purpose and for what they believe in good faith to be in the best interests of our company.
General Meetings of Shareholders and Shareholder Proposals
Our shareholders’ general meetings may be held in such place within or outside the Cayman Islands as our board of directors considers appropriate.
As a Cayman Islands exempted company, we are not obliged by the Companies Law to call shareholders’ annual general meetings. Our amended and restated memorandum and articles of association provide that we may (but are not obliged to) hold a general meeting in each year as our annual general meeting.
Shareholders’ annual general meetings and any other general meetings of our shareholders may be convened by a majority of our board of directors. Our board of directors shall give not less than seven calendar days’ written notice of a shareholders’ meeting to those persons whose names appear as members in our register of members on the date the notice is given (or on any other date determined by our directors to be the record date for such meeting) and who are entitled to vote at the meeting.
Cayman Islands law provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. However, these rights may be provided in a company’s articles of association. Our amended and restated memorandum and articles of association allow our shareholders holding shares representing in aggregate not less than ten percent of our voting share capital in issue, to requisition an extraordinary general meeting of our shareholders, in which case our directors are obliged to call such meeting and to put the resolutions so requisitioned to a vote at such meeting; however, our amended and restated memorandum and articles of association do not provide our shareholders with any right to put any proposals before annual general meetings or extraordinary general meetings not called by such shareholders.
Exempted Company
We are an exempted company with limited liability under the Companies Law. The Companies Law distinguishes between ordinary resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary company except that an exempted company:
| • | does not have to file an annual return of its shareholders with the Registrar of Companies; |
| • | is not required to open its register of members for inspection; |
| • | does not have to hold an annual general meeting; |
| • | may issue negotiable or bearer shares or shares with no par value; |
| • | may obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the first instance); |
| • | may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands; |
| • | may register as a limited duration company; and |
| • | may register as a segregated portfolio company. |
“Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on the shares of the company (except in exceptional circumstances, such as involving fraud, the establishment of an agency relationship or an illegal or improper purpose or other circumstances in which a court may be prepared to pierce or lift the corporate veil).
Register of Members
Under Cayman Islands law, we must keep a register of members and there should be entered therein:
| • | the names and addresses of the members, a statement of the shares held by each member, and of the amount paid or agreed to be considered as paid, on the shares of each member; |
| • | the date on which the name of any person was entered on the register as a member; and |
| • | the date on which any person ceased to be a member. |
Under Cayman Islands law, the register of members of our company is prima facie evidence of the matters set out therein (i.e. the register of members will raise a presumption of fact on the matters referred to above unless rebutted) and a member registered in the register of members should be deemed as a matter of Cayman Islands law to have legal title to the shares as set against its name in the register of members. Once our register of members has been updated, the shareholders recorded in the register of members should be deemed to have legal title to the shares set against their name.
If the name of any person is incorrectly entered in, or omitted from, our register of members, or if there is any default or unnecessary delay in entering on the register the fact of any person having ceased to be a member of our company, the person or member aggrieved (or any member of our company or our company itself) may apply to the Cayman Islands Grand Court for an order that the register be rectified, and the Court may either refuse such application or it may, if satisfied of the justice of the case, make an order for the rectification of the register.
In connection with our initial public offering and the concurrent private placement, we entered into several share subscription agreements with certain investors on February 27, 2017, pursuant to which such investors purchased an aggregate of approximately $50.8 million of ordinary shares from us at $20.00 per share in the concurrent private placement in reliance on an exemption from registration contained in either Regulation S or Regulation D under the Securities Act. On March 8, 2017, we entered into an underwriting agreement with Rodman & Renshaw, a unit of H.C. Wainwright & Co., LLC, or Rodman, pursuant to which Rodman served as underwriter for our initial public offering and received a commission of 7% of the total offering price of our ordinary shares in our initial public offering. On March 8, 2017, we also entered into a private placement agent agreement with Rodman, pursuant to which Rodman served as placement agent for the concurrent private placement and received a placement agent fee equal to 7% of the total purchase price of our ordinary shares sold in the concurrent private placement.
On May 29, 2018, we entered into a series of securities purchase agreements with the investors described therein pursuant to which we issued and sold an aggregate of 739,095 shares of our ordinary shares at a purchase price of $27.06 per share. The gross proceeds from the offering were $20.0 million before deducting expenses. The ordinary shares were offered pursuant to a registration statement on Form F-3 (File No. 333-224437), which was filed with the Commission on April 25, 2018 and was declared effective on May 3, 2018.
See “Item 4. Information on the Company—B. Business Overview—Chinese Regulation—Regulations Relating to Foreign Exchange and Dividend Distribution.”
The following discussion is a summary of the Cayman Islands, Chinese and U.S. federal income tax considerations relevant to the ownership and disposition of the ordinary shares. The discussion is not intended to be, nor should it be construed as, legal or tax advice to any particular holder. The discussion is based on laws and relevant interpretations thereof as of the date of this annual report on Form 20-F, all of which are subject to change or different interpretations, possibly with retroactive effect. The discussion does not address U.S. state or local tax laws, or tax laws of jurisdictions other than the Cayman Islands, China and the United States. You should consult your tax advisors with respect to the consequences of the ownership and disposition of the ordinary shares. To the extent that the discussion relates to matters of Cayman Islands tax law, it represents the opinion of Maples and Calder (Hong Kong) LLP, our special Cayman Islands counsel; to the extent the discussion relates to PRC tax law, it is the opinion of Han Kun Law Offices, our special PRC counsel.
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation, and there is no taxation in the nature of inheritance tax or estate duty or withholding tax applicable to us or to any holder of the ordinary shares. There are no other taxes likely to be material to us levied by the Government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or after execution brought within, the jurisdiction of the Cayman Islands. No stamp duty is payable in the Cayman Islands on the issue of shares by, or any transfers of shares of, Cayman Islands companies (except those which hold interests in land in the Cayman Islands). The Cayman Islands is not party to any double tax treaties that are applicable to any payments made to or by our company. There are no exchange control regulations or currency restrictions in the Cayman Islands.
Payments of dividends and capital in respect of the ordinary shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of the ordinary shares, as the case may be, nor will gains derived from the disposal of the ordinary shares be subject to Cayman Islands income or corporation tax.
People’s Republic of China Taxation
Under the EIT Law, an enterprise established outside of China with a “de facto management body” within China is considered a “resident enterprise,” which means that it is treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. Although the implementation rules of the EIT Law define “de facto management body” as a managing body that exercises substantive and overall management and control over the production and business, personnel, accounting books and assets of an enterprise, the only official guidance for this definition currently available is set forth in Circular 82, issued by the State Administration of Taxation, which provides guidance on the determination of the tax residence status of a Chinese-controlled offshore incorporated enterprise, defined as an enterprise that is incorporated under the laws of a foreign country or territory and that has a Chinese enterprise or enterprise group as its primary controlling shareholder. Although BeyondSpring Inc. does not have a Chinese enterprise or enterprise group as our primary controlling shareholder and is therefore not a Chinese-controlled offshore incorporated enterprise within the meaning of Circular 82, in the absence of guidance specifically applicable to us, we have applied the guidance set forth in Circular 82 to evaluate the tax residence status of BeyondSpring Inc. and its subsidiaries organized outside China.
According to Circular 82, a Chinese-controlled offshore incorporated enterprise will be regarded as a PRC tax resident by virtue of having a “de facto management body” in China and will be subject to Chinese enterprise income tax on its worldwide income only if all of the following criteria are met:
| • | the primary location of the enterprise’s senior executives of the day-to-day operational management and senior management departments performing their duties is in China; |
| • | decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in China; |
| • | the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder meeting minutes are located or maintained in China; and |
| • | 50% or more of voting board members or senior executives habitually reside in China. |
Currently, some of the members of our management team are located in China. However, we do not believe that we meet all of the conditions outlined in the immediately preceding paragraph. BeyondSpring Inc. and its offshore subsidiaries are incorporated outside China. As a holding company, our key assets and records, including the resolutions and meeting minutes of our board of directors and the resolutions and meeting minutes of our shareholders, are located and maintained outside China. Moreover, we are not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a Chinese “resident enterprise” by the Chinese tax authorities. Accordingly, we believe that BeyondSpring Inc. and its offshore subsidiaries should not be treated as a “resident enterprise” for Chinese tax purposes if the criteria for “de facto management body” as set forth in Circular 82 were deemed applicable to us. However, as the tax residency status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body” as applicable to our offshore entities, we will continue to monitor our tax status.
The implementation rules of the EIT Law provide that, (1) if the enterprise that distributes dividends is domiciled in China or (2) if gains are realized from transferring equity interests of enterprises domiciled in China, then such dividends or capital gains are treated as China-sourced income. It is not clear how “domicile” may be interpreted under the EIT Law, and it may be interpreted as the jurisdiction where the enterprise is a tax resident. Therefore, if we are considered as a Chinese tax resident enterprise for Chinese tax purposes, any dividends we pay to our overseas shareholders as well as gains realized by such shareholders from the transfer of our shares may be regarded as China-sourced income. If we are considered a “non-resident enterprise” by the PRC tax authorities, the dividends paid to us by our PRC subsidiaries will be subject to a 10% withholding tax. The EIT Law also imposes a withholding income tax of 10% on dividends distributed by an foreign invested enterprise to its immediate holding company outside of China, if such immediate holding company is considered as a non-resident enterprise without any establishment or place within China or if the received dividends have no connection with the establishment or place of such immediate holding company within China, unless such immediate holding company’s jurisdiction of incorporation has a tax treaty with China that provides for a different withholding arrangement. The Cayman Islands, where we are incorporated does not have such tax treaty with China. Under the arrangement between the PRC and the Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion with Respect to Taxes on Income and Capital, the dividend withholding tax rate may be reduced to 5%, if a Hong Kong resident enterprise that receives a dividend is considered a non-PRC tax resident enterprise and holds at least 25% of the equity interests in the PRC enterprise distributing the dividends, subject to approval of the PRC local tax authority. However, if the Hong Kong resident enterprise is not considered to be the beneficial owner of such dividends under applicable PRC tax regulations, such dividends may remain subject to withholding tax at a rate of 10%. In February, 2018, the SAT promulgated the Announcement on Issues Relating to “Beneficial Owner” in Tax Treaties, which provides the criteria of determination of “Beneficial Owner”. For determination of “Beneficial Owner”, actual conditions of the specific case shall be taken into account to conduct a comprehensive analysis. Accordingly, BeyondSpring HK may be able to enjoy the 5% withholding tax rate for the dividends it receives from its PRC subsidiaries if it satisfies the relevant conditions under tax rules and regulations and obtains the approvals as required.
U.S. Federal Income Tax Considerations
The following discussion is a summary of U.S. federal income tax considerations generally applicable to the ownership and disposition of our ordinary shares. Except where noted, this summary deals only with U.S. Holders (as defined below) that hold our ordinary shares as capital assets for U.S. federal income tax purposes. This summary does not address all U.S. federal income tax considerations that may be relevant to a particular U.S. Holder (as defined below) and does not represent a detailed description of all of the U.S. federal income tax considerations applicable to shareholders that may be subject to special treatment under U.S. federal income tax laws, including:
| • | banks, financial institutions or insurance companies; |
| • | real estate investment trusts, regulated investment companies or grantor trusts; |
| • | brokers or dealers in securities, commodities or currencies; |
| • | traders in securities who have elected the mark-to-market method of accounting for their securities; |
| • | tax-exempt entities or organizations, including individual retirement accounts or other tax-deferred accounts; |
| • | former citizens or long-term residents of the United States; |
| • | persons that received our shares as compensation for the performance of service, including shareholders who acquired shares pursuant to the exercise of an employee stock option; |
| • | persons that will hold our shares as part of a “hedging,” “integrated” or “conversion” transaction or other risk reduction strategy or as a position in a “straddle” for U.S. federal income tax purposes; |
| • | entities classified as partnerships for U.S. federal income tax purposes or other pass-through entities, or holders that will hold our shares through such an entity; |
| • | entities classified as partnerships for U.S. federal income tax purposes or other pass-through entities, or holders that will hold our shares through such an entity; |
| • | persons whose “functional currency” is not the U.S. dollar; or |
| • | holders that own or have owned directly, indirectly or constructively 10.0% or more of the voting power or value of our shares. |
The discussion below is based upon the provisions of the Code, current, proposed and temporary U.S. Treasury regulations, judicial and administrative interpretations thereof and the income tax treaty between the U.S. and China, or the Treaty, in each case as in effect and available on the date hereof. Such authorities may be replaced, revoked or modified, perhaps retroactively, and may be subject to differing interpretations which could result in U.S. federal income tax consequences different from those discussed below.
This summary does not address all aspects of U.S. federal income tax, does not deal with all tax considerations that may be relevant to shareholders in light of their personal circumstances and does not address the Medicare tax imposed on certain net investment income or any state, local, foreign, gift, estate or alternative minimum tax considerations. Holders should consult their tax advisors concerning the U.S. federal, state, local and foreign tax consequences of owning and disposing of our ordinary shares in their particular circumstances. As used herein, the term “U.S. Holder” means a beneficial owner of an ordinary share that is, for U.S. federal income tax purposes:
| • | an individual citizen or resident of the United States; |
| • | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons has or have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
The U.S. federal income tax treatment of a partner in an entity or arrangement treated as a partnership for U.S. federal income tax purposes that is the beneficial owner of our ordinary shares will generally depend on the status of the partner and the activities of the partnership. A partner in such a partnership should consult its tax advisor regarding the tax consequences of the ownership and disposition of our ordinary shares.
Tax Residence of BeyondSpring Inc. for U.S. Federal Income Tax Purposes
Under current U.S. federal income tax law, a corporation is generally considered a tax resident in the jurisdiction of its organization or incorporation. Thus, as a corporation organized under the laws of the Cayman Islands, we should generally be classified as a non-U.S. corporation (and therefore a non-U.S. tax resident) for U.S. federal income tax purposes. In certain circumstances, however, under section 7874 of the Code, or section 7874, a corporation organized outside the United States. will be treated as a U.S. corporation (and, therefore, a U.S. tax resident) unless one or more exceptions apply.
Section 7874 is generally implicated when a non-U.S. corporation acquires all of the stock of a U.S. corporation. If, immediately after such an acquisition, former shareholders of the U.S. corporation are considered to hold, for purposes of section 7874, 80% or more (by vote and value) of the stock of the acquiring non-U.S. corporation and certain other circumstances exist, the acquiring non-U.S. corporation will be treated as a U.S. corporation for U.S. federal income tax purposes. In such event, the acquiring non-U.S. corporation will be subject to U.S. corporate income tax on its worldwide income and the income of its non-U.S. subsidiaries will be subject to U.S. tax when repatriated (with a deduction available for the foreign-source portion of such income) or when deemed recognized under the U.S. federal income tax rules for controlled foreign subsidiaries. Additionally, any deferred foreign income of its non-U.S. subsidiaries that has not previously been subject to U.S. taxation, determined as of November 2, 2017 or December 31, 2017 (whichever amount is greater), will be subject to a “transition tax” imposed under the Tax Cuts and Jobs Act. Moreover, the gross amount of any dividends paid to a non-U.S. shareholder will be subject to U.S. withholding tax at a rate of 30% unless the non-U.S. shareholder is eligible for an exemption or reduced withholding rate under an applicable income tax treaty.
The determination of the percentage of stock of the acquiring non-U.S. corporation treated as held by former shareholders of the U.S. corporation for purposes of section 7874, or the section 7874 ownership percentage, is subject to various adjustments and exceptions, including an “internal group restructuring exception” and a “foreign-parented group exception,” both of which, when they apply, generally operate to reduce the section 7874 ownership percentage (and the likelihood that the acquiring non-U.S. corporation will be treated as a U.S. corporation for U.S. federal income tax purposes). The internal group restructuring exception, when applicable, effectively permits the acquisition of a U.S. corporation by certain of its non-U.S. affiliates without triggering the adverse effects of section 7874. The foreign-parented group exception, when applicable, ensures that certain post-acquisition transfers of the non-U.S. acquiring corporation do not defeat the otherwise appropriate application of the internal group restructuring exception to the acquisition of a U.S. corporation. Section 7874 also contains an anti-abuse rule pursuant to which the transfer of property, including stock, may be disregarded if the transfer is part of a plan a principal purpose of which is to avoid the purposes of section 7874.
In July of 2015, we completed our internal restructuring. See Note 1 to our consolidated financial statements included elsewhere in this annual report on Form 20-F for additional information regarding the internal restructuring. As part of the internal restructuring, Wanchun Biotech contributed all of the stock of BeyondSpring U.S., a U.S. corporation, to BVI Biotech, a non-U.S. entity, in exchange for all of the outstanding interests in BVI Biotech, or the BeyondSpring U.S. transfer. Shortly thereafter, Wanchun Biotech transferred all of the interests in BVI Biotech to us in exchange for 300,000 of our ordinary shares, or the BVI Biotech transfer.
Based on the rules in effect under section 7874 at the time of the internal restructuring, we believe that the BVI Biotech transfer does not constitute the indirect acquisition of substantially all of the properties held directly or indirectly by a U.S. corporation and that, therefore, the BVI Biotech transfer, in and of itself, does not implicate section 7874. Moreover, we believe that the BVI Biotech transfer satisfies the foreign-parented group exception and, therefore, does not prevent the application of the internal group restructuring exception to the BeyondSpring U.S. transfer. As a result, we believe that, under the law in effect in July of 2015, the section 7874 ownership percentage with respect to the BeyondSpring U.S. transfer was less than 80% and that neither the BeyondSpring U.S. transfer nor the BVI Biotech transfer triggered the application of section 7874. Accordingly, we expect that we will not be treated as a U.S. corporation for U.S. federal income tax purposes.
Notwithstanding, the determination of the section 7874 percentage and the application of the various exceptions are complex and subject to factual and legal uncertainties. Moreover, changes to section 7874 or the U.S. Treasury regulations promulgated thereunder (or other relevant provisions of U.S. federal income tax law), which could be given prospective or retroactive effect, could adversely affect the section 7874 analysis with respect to our status as a non-U.S. corporation for U.S. federal income tax purposes. As a result, there can be no assurance that the IRS will agree with the position that we should not be treated as a U.S. corporation for U.S. federal income tax purposes. If the IRS were to prevail with an assertion that the exceptions described above do not apply with respect to the internal restructuring or that the internal restructuring runs afoul of the section 7874 anti-abuse rules or other substance-over-form or similar principles, we could be treated as a U.S. corporation for U.S. federal income tax purposes.
The remainder of this discussion assumes that we will not be treated as a U.S. corporation for U.S. federal income tax purposes.
Taxation of Dividends
Subject to the discussion under “—Passive Foreign Investment Company” below, the gross amount of distributions on our ordinary shares (including any amounts withheld in respect of Chinese withholding taxes) will generally be taxable as dividends to the extent paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles, and will be includable in your gross income as ordinary income on the day actually or constructively received by you. Such dividends will not be eligible for the dividends received deduction generally allowed to U.S. corporations under the Code. The following discussion assumes that any dividends will be paid in U.S. dollars.
If you are a non-corporate U.S. Holder, dividends received by you from a qualified foreign corporation may be eligible for reduced rates of taxation. A foreign corporation is treated as a qualified foreign corporation with respect to dividends received from that corporation on ordinary shares that are “readily tradable” on an “established securities market” in the United States. Our ordinary shares are listed on the NASDAQ Capital Market, however, our ordinary shares may not be considered readily tradable on an established securities market in the current year or subsequent years. If you do not meet a minimum holding period requirement during which you are not protected from the risk of loss or you elect to treat dividend income as “investment income” pursuant to section 163(d)(4) of the Code, you will not be eligible for the reduced rates of taxation. In addition, the rate reduction will not apply to dividends if the recipient of a dividend is obligated to make related payments with respect to positions in substantially similar or related property, even if the minimum holding period has been met. The rate reduction also will not apply if we are a PFIC in the taxable year in which such dividends are paid or in the preceding taxable year. In light of the discussion in “—Passive Foreign Investment Company” below, if you are a non-corporate U.S. Holder, you should assume that dividends generally will not constitute “qualified dividend income” eligible for reduced rates of taxation.
In the event that we are deemed to be a Chinese resident enterprise under the EIT Law, you may be subject to Chinese withholding taxes on distributions paid to you with respect to the ordinary shares. See “—People’s Republic of China Taxation.” In that case, subject to certain conditions and limitations, Chinese withholding taxes on dividends will generally be treated as foreign taxes eligible for credit against your U.S. federal income tax liability. For purposes of calculating the foreign tax credit, dividends paid on our ordinary shares will be treated as foreign-source income and will generally constitute passive category income. However, in certain circumstances, if you have held our ordinary shares for less than a specified minimum period during which you are not protected from risk of loss, or are obligated to make payments related to the dividends, you will not be allowed a foreign tax credit for any Chinese withholding taxes imposed on dividends paid on our ordinary shares. If you are eligible for Treaty benefits, any Chinese taxes on dividends will not be creditable against your U.S. federal income tax liability to the extent withheld at a rate exceeding the applicable Treaty rate. The rules governing the foreign tax credit are complex. You should consult your tax advisor regarding the availability of the foreign tax credit in your particular circumstances. In lieu of claiming a credit, you may elect to deduct such Chinese taxes in computing your taxable income, subject to applicable limitations. An election to deduct foreign taxes instead of claiming foreign tax credits must apply to all foreign taxes paid or accrued in the taxable year.
To the extent that the amount of any distribution on the ordinary shares exceeds our current and accumulated earnings and profits for a taxable year, as determined under U.S. federal income tax principles, the distribution will first be treated as a tax-free return of capital, causing a reduction in your adjusted tax basis in the ordinary shares, and the balance in excess of adjusted tax basis will be taxed as capital gain recognized on a sale or exchange, as described below under “—Sale, Exchange or Other Taxable Disposition of Ordinary Shares.” However, we may not calculate earnings and profits in accordance with U.S. federal income tax principles. Therefore, a distribution to you may be treated as a dividend (as discussed above).
Sale, Exchange or Other Taxable Disposition of Ordinary Shares
You will recognize gain or loss on the sale, exchange or other taxable disposition of our ordinary shares equal to the difference between the amount realized on such sale, exchange or other taxable disposition and your adjusted tax basis in our ordinary shares. The adjusted tax basis in an ordinary share generally will be equal to the cost of such ordinary share. Subject to the discussion under “—Passive Foreign Investment Company” below, such gain or loss will generally be capital gain or loss, which will be long-term capital gain or loss if your holding period for the shares exceeds one year at the time of disposition. Long-term capital gains are generally eligible for a preferential rate of taxation for certain non-corporate U.S. Holders. The deductibility of capital losses for U.S. federal income tax purposes is subject to limitations under the Code.
Any gain or loss recognized by you will generally be treated as U.S. source gain or loss. However, if we were to be treated as a Chinese resident enterprise for EIT Law purposes and Chinese tax were imposed on any gain, and if you are eligible for the benefits of the Treaty, you may elect to treat such gain as Chinese source gain under the Treaty. If you are not eligible for the benefits of the Treaty or you fail to make the election to treat any gain as Chinese source, then you may not be able to use the foreign tax credit arising from any Chinese tax imposed on the disposition of the ordinary shares unless such credit can be applied (subject to applicable limitations) against tax due on other income derived from foreign sources. You are also urged to consult your tax advisor regarding the tax consequences in case any Chinese tax is imposed on gain on a disposition of the ordinary shares, including the availability of the foreign tax credit and the election to treat any gain as Chinese source, under your particular circumstances.
Passive Foreign Investment Company
If a non-U.S. company is classified as a PFIC in any taxable year, a U.S. Holder of such PFIC’s shares will be subject to special rules generally intended to reduce or eliminate any benefits from the deferral of U.S. federal income tax that such U.S. Holder could derive from investing in a non-U.S. company that does not distribute all of its earnings on a current basis.
Under the PFIC rules, any excess distributions received or gain realized with respect to a PFIC’s stock are allocated ratably over a U.S. Holder’s holding period in such stock. Amounts allocated to the U.S. Holder’s current taxable year and any taxable year prior to PFIC classification are taxed as ordinary income, while amounts allocated to other taxable years are taxed at the highest rate of tax (plus any interest deemed deferred) in effect for such U.S. Holder in such years.
A non-U.S. company will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which, after applying certain look-through rules with respect to the income and assets of subsidiaries, either:
| • | at least 75% of its gross income is “passive income”; or |
| • | at least 50% of the average quarterly value of its total gross assets is attributable to assets that produce “passive income” or are held for the production of passive income. |
Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions and the excess of gains over losses from the disposition of assets which produce passive income. If a non-U.S. company owns at least 25% by value of the stock of another company, the non-U.S. company is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other company and as receiving directly its proportionate share of the other company’s income. For publicly traded companies, the PFIC asset test described above is applied using the fair market value of the non-U.S. company’s assets.
Based on current business plans and financial expectations, it is likely that we will be a PFIC for 2018, the current taxable year and in future taxable years. However, because PFIC status is based on our income, assets and activities for the entire taxable year, it is not possible to determine whether we will be characterized as a PFIC for the current taxable year or other years until after the close of the taxable year. Moreover, we must determine our PFIC status annually based on tests which are factual in nature, and our status in future years will depend on our income, assets and activities in each of those years, and, as a result, cannot be predicted with certainty as of the date hereof.
If we are classified as a PFIC in any year with respect to which you own our ordinary shares, we will continue to be treated as a PFIC with respect to you in all succeeding years during which you own our ordinary shares, regardless of whether we continue to meet the tests described above, unless you make certain elections (as described below) with respect to our ordinary shares that may mitigate some of the adverse tax consequences resulting from PFIC treatment. If you own our ordinary shares during any year in which we are classified as a PFIC, you will generally be required to file an IRS Form 8621 with respect to the company with your federal income tax return for that year.
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election with respect to such stock in order to avoid taxation under the rules described above. If a U.S. Holder were to make a valid mark-to-market election for the ordinary shares of a PFIC, such U.S. Holder would include in income, for each year that such company is treated as a PFIC, an amount equal to the excess, if any, of the fair market value of the PFIC’s ordinary shares held by such U.S. Holder as of the close of the year over such U.S. Holder’s adjusted basis in such ordinary shares. Amounts included in your income under a mark-to-market election, as well as any gain on the actual sale or other disposition of ordinary shares, will be treated as ordinary income. The mark-to-market election is available only for “marketable stock,” which is stock that is traded in other than de minimis quantities on at least 15 days during each calendar quarter, or regularly traded, on a qualified exchange or other market, as defined in applicable U.S. Treasury regulations. Our ordinary shares are listed on the NASDAQ Capital Market, which is a qualified exchange or other market for these purposes. If our ordinary shares continue to be listed on the NASDAQ Capital Market and are regularly traded, and you are a holder of our ordinary shares, we expect that the mark-to-market election would be available to you were we to be or become a PFIC. However, because, as a technical matter, a mark-to-market election cannot be made for any lower-tier PFICs of a PFIC, you would technically continue to be subject to the PFIC rules with respect to any indirect interest in any investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes.
Alternatively, a U.S. Holder of shares in a PFIC may avoid taxation under the rules described above by making a “qualified electing fund” election, to include in income its share of the PFIC’s income on a current basis. However, a U.S. Holder can only make a qualified electing fund election with respect to ordinary shares in a PFIC if such company agrees to furnish such U.S. Holder with certain tax information annually. We currently do not intend to prepare or provide such information. Therefore you should assume that you will not receive such information from us and would therefore be unable to make a qualified electing fund election with respect to any of our ordinary shares were we to be or become a PFIC.
You should consult your tax advisor regarding whether we are a PFIC as well as the potential U.S. federal income tax consequences of holding and disposing of our ordinary shares if we are or become classified as a PFIC, including the possibility of making a mark-to-market election or a qualifying electing fund election.
U.S. Holders are urged to consult their tax advisors regarding the consequences of owning and disposing our ordinary shares in light of their particular circumstances.
| F. | Dividends and Paying Agents |
Not applicable.
Not applicable.
We have previously filed with the SEC a registration statement on Form F-1 (File No. 333-214610), as amended, with respect to our ordinary shares. As allowed by the SEC, in Item 19 of this annual report on Form 20-F, we incorporate by reference certain information we previously filed with the SEC. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to be part of this annual report on Form 20-F.
We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Copies of reports and other information, when so filed, may be inspected without charge and may be obtained at prescribed rates at the SEC’s Public Reference Room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. The public may obtain information regarding the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website at www.sec.gov that contains reports and other information regarding registrants that file electronically with the SEC. Our annual report on Form 20-F and other information submitted by us to the SEC may be accessed through this website.
As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we are required to file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and to submit to the SEC, on Form 6-K, unaudited quarterly financial information for the first three quarters of each fiscal year.
We maintain a corporate website at www.beyondspringpharma.com. In accordance with NASDAQ Stock Market Rule 5250(d), we will post this annual report on Form 20-F on our website. Information contained on our website is not incorporated by reference into this annual report on Form 20-F. In addition, we will provide hardcopies of our annual report on Form 20-F free of charge to shareholders upon request.
Not applicable.
| Qualitative and Quantitative Disclosures About Market Risk |
Interest and Credit Risk
We are not exposed to typical interest rate risk, which is the impact of interest rates on the cost of servicing and repaying debt. Our exposure to interest rate risk arises through movements in regard to interest income we earn on our deposits and, historically, on the imputed interest on an interest-free loan from one of our co-founders. Such loan was retired as part of our internal restructuring in 2015. We had cash short-term investments in the amount of $3.9 million and $30.6 million at December 31, 2018 and December 31, 2017, respectively. Our cash is held at financial institutions that we believe to be of high credit quality. We have not used derivative financial instruments in our investment portfolio. Interest-earning instruments carry a degree of interest rate risk. We have not been exposed nor do we anticipate being exposed to material risks due to changes in market interest rates. However, our future interest income may fall short of expectations due to changes in market interest rates.
Foreign Currency Exchange Rate Risk
We are exposed to foreign exchange risk arising from various currency exposures. Our functional currency is primarily U.S. dollars, but a portion of our operating transactions and assets and liabilities are in other currencies, such as RMB and AUD. We do not believe that we currently have any significant direct foreign exchange risk and have not used any derivative financial instruments to hedge exposure to such risk.
RMB is not freely convertible into foreign currencies for capital account transactions. The value of RMB against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions and China’s foreign exchange prices. To the extent that we need to convert U.S. dollars into RMB for our operations, appreciation of the RMB against the U.S. dollar would have an adverse effect on the RMB amount we receive from the conversion. Conversely, if we decide to convert RMB into U.S. dollars for the purpose of making payments for dividends on our ordinary shares or for other business purposes, appreciation of the U.S. dollar against the RMB would have a negative effect on the U.S. dollar amounts available to us.
| Description of Securities Other than Equity Securities |
Not applicable.
Not applicable.
Not applicable.
| D. | American Depositary Shares |
Not applicable.
PART II
| Defaults, Dividend Arrearages and Delinquencies |
None.
| Material Modifications to the Rights of Security Holders and Use of Proceeds |
In connection with our initial public offering, we have adopted an amended and restated memorandum and articles of association, which became effective and replaced our pre-offering memorandum and articles of association in its entirety in March 2017. For a description of the rights of holders of our ordinary shares, see “Item 10. Additional Information—B. Memorandum and Articles of Association.”
The following “Use of Proceeds” information relates to the registration statement on Form F-1, as amended (File No. 333-214610) in relation to our initial public offering, which was declared effective by the SEC on March 8, 2017. In March 2017, we completed our initial public offering and the concurrent private placement, in which we issued and sold 174,286 ordinary shares in the initial public offering and 2,541,048 ordinary shares in the current private placement. We received an aggregate amount of net proceeds of approximately $47.2 million, after deducting underwriting discounts and commissions and the offering expenses.
For the period from March 8, 2017, the date that the registration statement on Form F-1 was declared effective by the SEC, to December 31, 2018, we used the net proceeds from our initial public offering and the concurrent private placement as follows:
| • | Approximately $13,700,000 to conduct a Phase 2/3 clinical trial of Plinabulin in combination with docetaxel for the reduction of docetaxel chemotherapy-induced severe neutropenia and a Phase 2/3 clinical trial of Plinabulin in combination with other chemotherapeutic agents for the prevention of non-docetaxel chemotherapy-induced severe neutropenia; |
| • | Approximately $1,500,000 to fund our collaboration with the Fred Hutchinson Center and the University of Washington; |
| • | Approximately $7,500,000 to pay salaries and consultant fees and to cover research and development costs, which includes consulting fees paid to Dr. Lloyd as well as salaries paid to our executive officers; |
| • | Approximately $17,000,000 to pay certain expenses in connection with a Phase 3 clinical trial of Plinabulin as an anticancer agent in combination with docetaxel in advanced NSCLC; |
| • | Approximately $13,300,000 to pay salaries and consulting fees and to cover general and administrative costs; and |
| (a) | Disclosure Controls and Procedures |
Our management has evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, and has concluded that our disclosure controls and procedures were effective as of December 31, 2018.
| (b) | Management’s Annual Report on Internal Control Over Financial Reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with the U.S. GAAP and includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of our company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with GAAP, and that receipts and expenditures of our company are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of the unauthorized acquisition, use or disposition of our company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As required by Section 404 of the Sarbanes-Oxley Act and related rules as promulgated by the SEC, our management including our Chief Executive Officer and Chief Financial Officer assessed the effectiveness of internal control over financial reporting as of December 31, 2018 using the criteria set forth in the report “Internal Control—Integrated Framework (2013)” published by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2018.
Our independent registered public accounting firm, Ernst & Young Hua Ming LLP, was not required to perform an evaluation of our internal control over financial reporting as of December 31, 2018.
| (c) | Attestation Report of the Registered Public Accounting Firm |
See statement in Section (b) above. As an “emerging growth company,” as defined in the JOBS Act, we may take advantage of certain temporary exemptions from various reporting requirements, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act (and the SEC rules and regulations thereunder). When these exemptions cease to apply, we expect to incur additional expenses and devote increased management effort toward ensuring compliance with them.
| (d) | Changes in Internal Control Over Financial Reporting |
There were no changes in our internal control over financial reporting during the period covered by this annual report on Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 16A. | Audit Committee Financial Expert |
Patrick Fabbio is an audit committee financial expert. Our board of directors is of the opinion that retaining a financial expert is not necessary at this time since our company is at its start-up stage.
In connection with our initial public offering, we have adopted a written code of ethics that applies to all of our directors, executive officers and employees. The code of ethics is available in the investors section of our website (www.beyondspringpharma.com).
| Item 16C. | Principal Accountant Fees and Services |
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by Ernst & Young Hua Ming LLP, our principal external auditors, for the periods indicated.
| | | | |
| | | | | | | |
| Audit Fees(1) | | $ | 426 | | | $ | 468
| |
| Audit-Related Fees(2) | | | — | | | | — | |
| Tax Fees(3) | | | — | | | | — | |
| All Other Fees(4) | | | — | | | | — | |
| Total | | $ | 426 | | | $ | 468
| |
| (1) | “Audit Fees” represents the aggregate fees for the interim reviews and annual audit of our financial statements for 2018 as well as other assurance service. |
| (2) | “Audit-Related Fees” represents the aggregate fees billed for each of the fiscal years listed for the assurance and related services rendered by our principal auditors that are reasonably related to the performance of the audit or review of our financial statements and not reported under “Audit Fees.” |
| (3) | “Tax Fees” represents the aggregate fees billed for each of the fiscal years listed for the professional tax services rendered by our principal auditors. |
| (4) | “All Other Fees” represents the aggregate fees for services rendered by our principal auditors other than services reported under “Audit Fees,” “Audit-related Fees” and “Tax Fees.” |
Audit Committee Pre-Approval Policies and Procedures
Our Audit Committee has adopted a policy pursuant to which we will not engage our auditors to perform any non-audit services unless the audit committee pre-approves the service.
| Item 16D. | Exemptions from the Listing Standards for Audit Committees |
Not applicable.
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
Not applicable.
| Item 16F. | Change in Registrant’s Certifying Accountant |
Not applicable.
| Item 16G. | Corporate Governance |
As a Cayman Islands company listed on the Nasdaq Capital Market, we are subject to the Nasdaq corporate governance listing standards. However, the Nasdaq rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in the Cayman Islands, which is our home country, may differ significantly from the Nasdaq corporate governance listing standards. Maples and Calder (Hong Kong) LLP, our Cayman Islands counsel, has provided a letter to the Nasdaq Stock Market certifying that under Cayman Islands law, we are not required to hold annual shareholders meetings every year. We follow home country practice with respect to annual meetings and did not hold an annual meeting of shareholders in 2018. We will, however, hold annual shareholders meetings in the future if there are matters that require shareholders’ approval.
Other than the annual meeting practice described above, there are no significant differences between our corporate governance practices and those followed by U.S. domestic companies under Nasdaq Stock Market Rules.
However, if we choose to follow other home country practice in the future, our shareholders may be afforded less protection than they otherwise would under the Nasdaq corporate governance listing standards applicable to U.S. domestic issuers. See “Item 3.D. Key Information—Risk Factors—Risks Related to Our Ordinary Shares—As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq Capital Market corporate governance listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.”
| Item 16H. | Mine Safety Disclosure |
Not applicable.
PART III
| Item 17. | Financial Statements |
We have elected to provide financial statements pursuant to Item 18.
| Item 18. | Financial Statements |
The consolidated financial statements of BeyondSpring Inc. are included at the end of this annual report on Form 20-F.
| Amended and Restated Memorandum and Articles of Association of BeyondSpring Inc. |
| Specimen Certificate for Ordinary Shares of BeyondSpring Inc. |
| Consulting Agreement, dated as of June 18, 2013, between Wanchun Pharma and GKOL Inc. |
| First Amendment to the Consulting Agreement, dated as of March 30, 2014, among Wanchun Pharma, BeyondSpring U.S. and GKOL Inc. |
| Second Amendment to the Consulting Agreement, dated as of June 1, 2015, between BeyondSpring U.S. and GKOL Inc. |
| Termination Agreement, dated as of February 2, 2015, among BeyondSpring Inc., Wanchun Biotech and Nereus |
| Amended and Restated Employment Agreement, dated as of November 10, 2016, between BeyondSpring U.S. and Richard Brand |
| Amended and Restated Employment Agreement, dated as of November 10, 2016, between BeyondSpring U.S. and Lan Huang |
| Amended and Restated Employment Agreement, dated as of November 10, 2016, between BeyondSpring U.S. and Ramon Mohanlal |
| Amended and Restated Employment Agreement, dated as of November 10, 2016, between BeyondSpring U.S. and Gordon L. Schooley |
| Form of Director and Executive Officer Indemnification Agreement |
| BeyondSpring Inc. 2017 Omnibus Incentive Plan and related form agreements |
| Form of Director Agreement |
| Form of Share Subscription Agreement between BeyondSpring Inc. and Regulation S investors |
| Share Subscription Agreement, dated as of February 27, 2017, between BeyondSpring Inc. and Sangel Star Biomedical Fund LP |
| Letter Agreement with respect to BPI-002 Milestone Stock Bonus Award, dated as of April 11, 2017, between BeyondSpring Inc. and Ramon Mohanlal |
| Letter Agreement with respect to BPI-004 Milestone Stock Bonus Award, dated as of April 11, 2017, between BeyondSpring Inc. and Ramon Mohanlal |
| Severance Agreement and General Release, dated as of February 20, 2018, between BeyondSpring U.S. and Richard Brand |
| Employment Agreement, dated as of March 26, 2018, between BeyondSpring U.S. and Edward Dongheng Liu |
| Securities Purchase Agreement dated as of May 29, 2018, by and between BeyondSpring Inc. and China Southern Dragon Dynamic Fund |
| Securities Purchase Agreement dated as of May 29, 2018, by and between BeyondSpring Inc. and Everbright Sun Hung Kai Co., Ltd. |
| Securities Purchase Agreement dated as of May 29, 2018, by and between BeyondSpring Inc. and New China Asset Management (Hong Kong) Limited |
| Securities Purchase Agreement dated as of May 29, 2018, by and between BeyondSpring Inc. and Tianyi HongKong Development Ltd. |
| Employment Agreement, dated as of June 8, 2018, between BeyondSpring Pharmaceuticals, Inc. and Richard Daly |
| Employment Agreement, dated as of March 16, 2018, between BeyondSpring Pharmaceuticals, Inc. and James Tonra |
| List of Subsidiaries of BeyondSpring Inc. |
| Certification by Principal Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| Certification by Principal Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| Certification by Principal Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| Certification by Principal Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| Consent of Ernst & Young Hua Ming LLP |
| Consent of Maples and Calder (Hong Kong) LLP |
| Consent of Han Kun Law Offices |
| 101.INS | XBRL Instance Document |
| 101.SCH | XBRL Taxonomy Extension Schema Document |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
| * | Previously filed with the Registration Statement on Form F-1 (File No. 333-214610), as amended, initially filed on November 15, 2016, and incorporated herein by reference. |
| ** | Furnished with this annual report on Form 20-F. |
| *** | Incorporated by reference to the 2016 annual report on Form 20-F of BeyondSpring Inc. filed with the SEC on April 28, 2017. |
| **** | Previously filed with Form 6-K of BeyondSpring Inc., initially filed with the SEC on May 30, 2018, and incorporated herein by reference. |
| ***** | Incorporated by reference to the 2017 annual report on Form 20-F of BeyondSpring Inc. filed with the SEC on April 3, 2018. |
In reviewing the agreements included as exhibits to this annual report on Form 20-F, please remember they are included to provide you with information regarding their terms and are not intended to provide any other factual or disclosure information about us or the other parties to the agreements.
The agreements may contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties have been made solely for the benefit of the other parties to the applicable agreement and:
| • | should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; |
| • | have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement; |
| • | may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and |
| • | were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments. |
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time.
SIGNATURES
BeyondSpring Inc. hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on Form 20-F on its behalf.
| | BeyondSpring Inc. |
| | |
| | By: | /s/ Lan Huang |
| | Name: |
| Lan Huang |
| | Title: |
| Chief Executive Officer |
| Date: April 30, 2019 | | |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | Page
|
| Audited Consolidated Financial Statements of BeyondSpring Inc. | |
| Report of Independent Registered Public Accounting Firm | F-2
|
| Consolidated balance sheets as of December 31, 2018 and 2017 | F-3
|
| Consolidated statements of comprehensive loss for the years ended December 31, 2018, 2017 and 2016 | F-4
|
| Consolidated statements of shareholders’ equity (deficit) for the years ended December 31, 2018, 2017 and 2016 | F-5 |
| Consolidated statements of cash flows for the years ended December 31, 2018, 2017 and 2016 | F-6 |
| Notes to consolidated financial statements | F-7
|
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of BeyondSpring Inc.:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of BeyondSpring Inc. (the “Company”) as of December 31, 2018 and 2017, the related consolidated statements of comprehensive loss, shareholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2018, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018, in conformity with U.S. generally accepted accounting principles.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations, has a working capital deficiency, and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to fraud or error, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young Hua Ming LLP
We have served as the Company’s auditor since 2015.
Beijing, People’s Republic of China
April 30, 2019
BEYONDSPRING INC.
CONSOLIDATED BALANCE SHEETS
(Amounts in thousands of U.S. Dollars (“$”), except for number of shares and per share data)
| | |
| | | As of December 31 | |
| | | Note | | | 2017 | | | 2018 | |
| | |
| | | $
| | | | $ | |
| | | | | |
| | | |
| | |
| Assets | | | | | | | | | | | |
| | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | |
| Cash | | | | | | 27,481 | | | | 3,889 | |
| Short term investment | | | | | | 3,074 | | | | - | |
| Advances to suppliers | | | | | | 1,525 | | | | 1,209 | |
| Due from related parties | | 4 | | | | - | | | | 481 | |
| Prepaid expenses and other current assets | | | | | | 264 | | | | 292 | |
| Total current assets | | | | | | 32,344 | | | | 5,871 | |
| | |
| | |
| | | | | |
| Noncurrent assets: | | | | | | | | | | | |
| Property and equipment, net | | 3 | | | | 123 | | | | 282 | |
| Other noncurrent assets | | | | | | 361 | | | | 910 | |
| Total noncurrent assets | | | | | | 484 | | | | 1,192 | |
| | | | | | | | | | | | |
| Total assets | | | | | | 32,828 | | | | 7,063 | |
| | | | | | | | | | | | |
| Liabilities and equity | | | | | | | | | | | |
| | | | | | | | | | | | |
| Current liabilities: | | | | | | | | | | | |
| Accounts payable | | | | | | 3,379 | | | | 9,586 | |
| Government grants | | | | | | 307 | | | | - | |
| Accrued expenses | | | | | | 807 | | | | 5,495 | |
| Other current liabilities | | | | | | 299 | | | | 1,364 | |
| Total current liabilities | | | | | | 4,792 | | | | 16,445 | |
| | | | | | | | | | | | |
| Total liabilities | | | | | | 4,792 | | | | 16,445 | |
| | | | | | | | | | | | |
| Commitments and contingencies | | 10 | | | | | | | | | |
| | | | | | | | | | | | |
| Equity (deficit): | | | | | | | | | | | |
| Ordinary shares ($0.0001par value; 500,000,000 shares authorized; 22,530,702 shares and 23,184,612 shares issued and outstanding as of December 31, 2017 and 2018, respectively) | | | | | | 2 | | | | 2 | |
| Additional paid-in capital | | | | | | 151,147 | | | | 170,950 | |
| Accumulated deficit | | | | | | (123,891 | ) | | | (178,760 | ) |
| Accumulated other comprehensive loss (gain) | | | | | | (182 | ) | | | 42 | |
| | | | | | | | | | | | |
| Total BeyondSpring Inc. shareholders’ equity (deficit) | | | | | | 27,076 | | | | (7,766 | ) |
| Noncontrolling interests | | | | | | 960 | | | | (1,616 | ) |
| Total equity (deficit) | | | | | | 28,036 | | | | (9,382 | ) |
| | | | | | | | | | | | |
| Total liabilities and equity (deficit) | | | | | | 32,828 | | | | 7,063 | |
The accompanying notes are an integral part of these consolidated financial statements.
BEYONDSPRING INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(Amounts in thousands of U.S. Dollars (“$”), except for number of shares and per share data)
| | | | | | Year ended December 31, | |
| | | Note | | | 2016 | | | 2017 | | | 2018 | |
| | | | | | $ | | | $ | | | $ | |
| | | | | | | | | | | | | | | | |
| Revenue | | | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | |
| Research and development, including patent cost of $42,259 for the year ended December 31, 2017 | | | | | | (10,437 | ) | | | (88,928 | ) | | | (51,618 | ) |
| General and administrative | | | | | | (1,931 | ) | | | (9,053 | ) | | | (5,927 | ) |
| | | | | | | | | | | | | | | | |
| Loss from operations | | | | | | (12,368 | ) | | | (97,981 | ) | | | (57,545 | ) |
| Foreign exchange (loss) gain, net | | | | | | (195 | ) | | | 555 | | | | (455 | ) |
| Interest income | | | | | | 18 | | | | 120 | | | | 211 | |
| Other income | | | | | | - | | | | 918 | | | | 315 | |
| | | | | | | | | | | | | | | | |
| Net loss before income tax | | | | | | (12,545 | ) | | | (96,388 | ) | | | (57,474 | ) |
| Income tax benefit | | 5 | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | |
| Net loss | | | | | | (12,545 | ) | | | (96,388 | ) | | | (57,474 | ) |
| Less: Net loss attributable to noncontrolling interests | | | | | | (535 | ) | | | (4,625 | ) | | | (2,605 | ) |
| Net loss attributable to BeyondSpring Inc. | | | | | | (12,010 | ) | | | (91,763 | ) | | | (54,869 | ) |
| | | | | | | | | | | | | | | | |
| Net loss per share | | | | | | | | | | | | | | | |
| Basic and diluted | | 6 | | | | (0.75 | ) | | | (4.40 | ) | | | (2.42 | ) |
| | | | | | | | | | | | | | | | |
| Weighted average shares outstanding | | | | | | | | | | | | | | | |
| Basic and diluted | | 6 | | | | 16,086,419 | | | | 20,866,084 | | | | 22,665,265 | |
| | | | | | | | | | | | | | | | |
| Other comprehensive loss | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment (loss) gain | | | | | | (64 | ) | | | (1 | ) | | | 251 | |
| Total comprehensive loss | | | | | | (12,609 | ) | | | (96,389 | ) | | | (57,223 | ) |
| Less: Comprehensive loss attributable to noncontrolling interests | | | | | | (561 | ) | | | (4,535 | ) | | | (2,578 | ) |
| Comprehensive loss attributable to BeyondSpring Inc. | | | | | | (12,048 | ) | | | (91,854 | ) | | | (54,645 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
BEYONDSPRING INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY (DEFICIT)
(Amounts in thousands of U.S. Dollars (“$”), except for number of shares and per share data)
| | BeyondSpring Inc.’s shareholders | | | | | | | |
| | |
| | | | | | | | | | | | Subtotal | | | | | | | |
| Ordinary share |
| Shares | | | Amount |
| | | | | |
| $ | | |
| $ | | |
| $ | | |
| $ | | |
| $ | | |
| $ | | |
| $ | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances at January 1, 2016 | | | 15,750,000 | | | | 2 | | | | 29,119 | | | | (20,118 | ) | | | (53 | ) | | | 8,950 | | | | 708 | | | | 9,658 | |
| Issuance of ordinary shares (Note 1) | | | 1,129,628 | | | | - | | | | 15,250 | | | | - | | | | - | | | | 15,250 | | | | - | | | | 15,250 | |
| Foreign currency translation adjustment loss | | | - | | | | - | | | | - | | | | - | | | | (38 | ) | | | (38 | ) | | | (26 | ) | | | (64 | ) |
| Net loss | | | - | | | | - | | | | - | | | | (12,010 | ) | | | - | | | | (12,010 | ) | | | (535 | ) | | | (12,545 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances at December 31, 2016 | | | 16,879,628 | | | | 2 | | | | 44,369 | | | | (32,128 | ) | | | (91 | ) | | | 12,152 | | | | 147 | | | | 12,299 | |
| Issuance of ordinary shares (Note 1) | | | 4,828,297 | | | | - | | | | 89,443 | | | | - | | | | - | | | | 89,443 | | | | - | | | | 89,443 | |
| Effect of capital contribution to a subsidiary | | | - | | | | - | | | | (1,480 | ) | | | - | | | | - | | | | (1,480 | ) | | | 1,480 | | | | - | |
| Share-based compensation (Note 7) | | | 822,777 | | | | - | | | | 18,815 | | | | - | | | | - | | | | 18,815 | | | | 3,868 | | | | 22,683 | |
Foreign currency translation adjustment (loss) gain | | | - | | | | - | | | | - | | | | - | | | | (91 | ) | | | (91 | ) | | | 90 | | | | (1 | ) |
| Net loss | | | - | | | | - | | | | - | | | | (91,763 | ) | | | - | | | | (91,763 | ) | | | (4,625 | ) | | | (96,388 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances at December 31, 2017 | | | 22,530,702 | | | | 2 | | | | 151,147 | | | | (123,891 | ) | | | (182 | ) | | | 27,076 | | | | 960 | | | | 28,036 | |
| Issuance of ordinary shares (Note 1) | | | 739,095 | | | | - | | | | 13,245 | | | | - | | | | - | | | | 13,245 | | | | - | | | | 13,245 | |
| Share-based compensation (Note 7) | | | (85,185 | ) | | | - | | | | 6,558 | | | | - | | | | - | | | | 6,558 | | | | 2 | | | | 6,560 | |
Foreign currency translation adjustment gain | | | - | | | | - | | | | - | | | | - | | | | 224 | | | | 224 | | | | 27 | | | | 251 | |
| Net loss | | | - | | | | - | | | | - | | | | (54,869 | ) | | | - | | | | (54,869 | ) | | | (2,605 | ) | | | (57,474 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances at December 31, 2018 | | | 23,184,612 | | | | 2 | | | | 170,950 | | | | (178,760 | ) | | | 42 | | | | (7,766 | ) | | | (1,616 | ) | | | (9,382 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
BEYONDSPRING INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands of U.S. Dollars (“$”), except for number of shares and per share data)
| |
| | | | |
| | Note | | | 2016 | | | 2017 | | | 2018 | |
| | | | | $ | | | $ | | | $ | |
| Cash flows from operating activities: | | | | | | | | | | | | | | | |
| Net loss | |
| | | | (12,545 | ) | | | (96,388 | ) | | | (57,474 | ) |
| Adjustments to reconcile net loss to cash used in operating activities: | | | | | | | | | | | | | | | |
| Depreciation expenses | | 3 | | | | 18 | | | | 32 | | | | 48 | |
| Share-based compensation | | | 7 | | | | - | | | | 22,683 | | | | 6,560 | |
Research and development expense settled by share issuance | | | 10 | | | | - | | | | 42,259 | | | | - | |
| Other income from government grant | | | | | | | - | | | | - | | | | (307 | ) |
| | | | | | | | | | | | | | | | | |
| Changes in assets and liabilities: | | | | | | | | | | | | | | | | |
| Advances to suppliers | | | | | | | (710 | ) | | | (726 | ) | | | 316 | |
| Due from related parties | | | | | | | - | | | | - | | | | (481 | ) |
| Prepaid expenses and other current assets | | | | | | | (334 | ) | | | 96 | | | | (28 | ) |
| Other noncurrent assets | | | | | | | (121 | ) | | | (240 | ) | | | (549 | ) |
| Accounts payable | | | | | | | 144 | | | | 2,935 | | | | 6,207 | |
| Due to related parties | | | | | | | (222 | ) | | | (210 | ) | | | - | |
| Accrued expenses | | | | | | | (102 | ) | | | 699 | | | | 4,688 | |
| Other current liabilities | | | | | | | 174 | | | | 64 | | | | 1,065 | |
| Net cash used in operating activities | | | | | | | (13,698 | ) | | | (28,796 | ) | | | (39,955 | ) |
| | | | | | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| Acquisitions of property and equipment | | | | | | | (64 | ) | | | (76 | ) | | | (207 | ) |
| Purchase of short-term investments | | | | | | | - | | | | (3,074 | ) | | | - | |
| Proceeds from maturity of short-term investments | | | | | | | - | | | | - | | | | 3,074 | |
| Net cash (used in) provided by investing activities | | | | | | | (64 | ) | | | (3,150 | ) | | | 2,867 | |
| | | | | | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| Proceeds from issuance of ordinary shares | | | 1 | | | | 15,250 | | | | 50,505 | | | | 14,000 | |
| Payments of shares offering costs | | | | | | | (537 | ) | | | (2,783 | ) | | | (755 | ) |
| Net cash provided by financing activities | | | | | | | 14,713 | | | | 47,722 | | | | 13,245 | |
| | | | | | | | | | | | | | | | | |
| Effect of foreign exchange rate changes | | | | | | | (85 | ) | | | 18 | | | | 251 | |
| | | | | | | | | | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | | | | | 866 | | | | 15,794 | | | | (23,592 | ) |
| Cash at beginning of year | | | | | | | 10,821 | | | | 11,687 | | | | 27,481 | |
| Cash at end of year | | | | | | | 11,687 | | | | 27,481 | | | | 3,889 | |
| | | | | | | | | | | | | | | | | |
| Non-cash activities: | | | | | | | | | | | | | | | | |
| Initial public offering costs accrued in accrued expenses | | | | | | | 1,324 | | | | - | | | | - | |
Research and development expense settled by share issuance | | | 10 | | | | - | | | | 42,259 | | | | - | |
The accompanying notes are an integral part of these consolidated financial statements.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
BeyondSpring Inc. (the “Company”) was incorporated in the Cayman Islands on November 21, 2014. The Company and its subsidiaries (collectively, the “Group”) are principally engaged in clinical stage biopharmaceutical activities focused on the development of innovative cancer therapies. The Company is under the control of Mr. Linqing Jia and Dr. Lan Huang as a couple (collectively, the “Founders”) since its incorporation.
In September 2016, the Company issued 1,129,628 ordinary shares of the Company to several third-party investors for an aggregate cash consideration of $15,250 or $13.5 per share.
On March 14, 2017, the Company completed its initial public offering (“IPO”) on the NASDAQ Capital Market. 174,286 ordinary shares were sold at $20.00 per share (the “IPO Price”). In conjunction with the IPO, 2,541,048 ordinary shares were sold in a private placement to certain investors at the same IPO Price. Net proceeds from the IPO and private placement after deducting underwriting discount and offering expenses were $47,184. The underwriting discount and offering expenses including those recorded as deferred IPO costs were recorded as a reduction of the proceeds received from the IPO in the shareholders’ equity. Immediately prior to the IPO, the Company issued 2,112,963 ordinary shares to NPBSIPO Liquidating Trust, or Nereus Trust in connection with termination of royalty payment arrangement (Note 10).
In May 2018, the Company entered into various agreements with certain third-party investors to issue 739,095 ordinary shares of the Company with a par value $0.0001 per share for an aggregate cash consideration of $20,000 or $27.06 per ordinary share. To date, the Company received gross proceeds of $14,000 (net proceeds of $13,245) from the issuance.
On May 21, 2018, Beijing Wanchun Pharmaceutical Technology Ltd. was incorporated in the People’s Republic of China (“PRC”) as a wholly owned subsidiary of Dalian Wanchunbulin Pharmaceuticals Ltd.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 1. | Nature of the business (continued) |
As at December 31, 2018, the subsidiaries of the Company are as follows:
| Name of company | Place of incorporation | Date of incorporation | Percentage of ownership by the Company | Principal activities |
| | | | | |
BeyondSpring Pharmaceuticals Inc. (“BeyondSpring US”) | Delaware,
United States of America (“U.S.”) | June 18, 2013 | 100% | Clinical trial activities |
| | | | | |
| BeyondSpring Ltd. | The British Virgin Islands (“BVI”) | December 3, 2014 | 100% | Holding company |
| | | | |
| BeyondSpring (HK) Limited (“BeyondSpring HK”) | Hong Kong | January 13, 2015 | 100% | Holding company |
| | | | | |
Wanchun Biotechnology Limited (“BVI Biotech”) | BVI | April 1, 2015 | 100% | Holding company |
| | | | | |
Wanchun Biotechnology (Shenzhen) Ltd. (“Wanchun Shenzhen”) | The People’s Republic of China (“PRC”) | April 23, 2015 | 100% | Holding company |
| | | | | |
Dalian Wanchunbulin Pharmaceuticals Ltd. (“Wanchunbulin”) | PRC | May 6, 2015 | 60% | Clinical trial activities |
| | | | | |
BeyondSpring Pharmaceuticals Australia PTY Ltd. (“BeyondSpring Australia”) | Australia | March 3, 2016 | 100% | Clinical trial activities |
| | | | | |
| Beijing Wanchun Pharmaceutical Technology Ltd. (“Beijing Wanchun”) | PRC | May 21, 2018 | 60% | Clinical trial activities |
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies |
Basis of presentation
The consolidated financial statements of the Company have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”).
Going concern
According to Accounting Standards Codification (“ASC”) 205-40, Presentation of Financial Statements - Going Concern (“ASC 205-40”), management must evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued. This evaluation initially does not take into consideration the potential mitigating effect of management’s plans that have not been fully implemented as of the date the financial statements are issued. When substantial doubt exists under this methodology, management evaluates whether the mitigating effect of its plans sufficiently alleviates substantial doubt about the Company’s ability to continue as a going concern. The mitigating effect of management’s plans, however, is only considered if both (1) it is probable that the plans will be effectively implemented within one year after the date that the financial statements are issued, and (2) it is probable that the plans, when implemented, will mitigate the relevant conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued.
The Company has incurred operating losses and negative cash flows from operations since inception. To date, the Company has no product revenue and management expects operating losses to continue for the foreseeable future, and has primarily funded these losses through equity financings. The Company incurred a net loss of $57,474 during 2018 and has an accumulated deficit of $178,760 as of December 31, 2018. Net cash used in operations was approximately $39,955 for the year of 2018. As of December 31, 2018, the Company had $3,889 of cash on hand, with current liabilities amounting to $16,445. Losses are anticipated in the ongoing development of the Company’s business and therefore can be no assurance that the Company will be able to achieve profitability.
The continuing operations of the Company is depending upon the Company’s ability to obtain necessary financing to fund its working capital requirement. These financial statements have been prepared in accordance with U.S. GAAP, on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. These financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts, or amounts and classification of liabilities that might result from this uncertainty.
In order to enable the Company to operate as a going concern in the foreseeable future, the Company will need, among other things, additional capital resources. There can be no assurance that capital will be available as necessary to meet the Company’s working capital requirements or, if the capital is available, that it will be on terms acceptable to the Company. The issuances of additional equity securities by the Company may result in dilution in the equity interests of its current shareholders. Obtaining commercial loans, assuming those loans will be available, will increase the Company’s liabilities and future cash commitments. If the Company is unable to obtain financing in the amounts and on terms deemed acceptable, the business and future success will be adversely affected including suspension of business operations. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Basics of consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries. All intercompany transactions and balances between the Company and its subsidiaries are eliminated upon consolidation.
Use of estimates
The preparation of the consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the period. Areas where management uses subjective judgment include, but are not limited to, share-based compensation, clinical trial accrual, valuation allowance for deferred tax assets, and estimating of useful life for property and equipment. Management bases the estimates on historical experience, known trends and various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results could differ from these estimates.
Research and development (“R&D”) costs
The Company accounts for R&D costs in accordance with ASC 730, Research and Development. R&D costs primarily are comprised of costs incurred in performing research and development activities, including related personnel and consultant’s salaries, benefits and related costs, raw materials and supplies to develop product candidates, patent-related costs incurred in connection with filing patent applications and external costs of outside vendors engaged to conduct clinical development activities and trials. The Company expenses R&D costs as they are incurred.
The costs incurred relate to nonrefundable advance payments for goods or services that will be used in future research and development activities are deferred and capitalized. The capitalized amounts are expensed as R&D costs when the related goods are delivered or the services are performed, or when the Company does not expect it will need the goods to be delivered or the services to be rendered.
Research contract costs and accruals
The Company has entered into various research and development contracts with research institutions and other companies primarily in the PRC, the United States, and Australia. Related payments are recorded as research and development expenses as incurred. The Company records accruals for estimated ongoing research costs. When evaluating the adequacy of the accrued liabilities, the Company analyzes progress of the studies, including the phase or completion of events, invoices received and contracted costs. Significant judgments and estimates are made in determining the accrued balances at the end of any reporting period. Actual results could differ from the Company’s estimates. The Company’s historical accrual estimates have not been materially different from the actual costs.
Foreign currency translation and transactions
Functional currency
The Company currently uses U.S. dollar as the functional currency for all its entities, except for entities in the PRC, which adopt RMB as the functional currency, and BeyondSpring Australia, which adopts the Australian dollar as the functional currency. The determination of the respective functional currency is based on the criteria of ASC 830, Foreign Currency Matters. The Company uses the U.S. dollar as its reporting currency.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Foreign currency translation and transactions (continued)
Foreign currency translation
For subsidiaries whose functional currencies are not the U.S. dollar, the Company uses the average exchange rate for the year and the exchange rate at the balance sheet date, to translate the operating results and financial position to U.S. dollar, the reporting currency, respectively. Translation differences are recorded in accumulated other comprehensive income/(loss), a component of shareholders’ equity (deficit). Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing on the transaction dates. Foreign currency denominated financial assets and liabilities are remeasured at the exchange rates prevailing at the balance sheet date. Exchange gains and losses are included in the consolidated statements of comprehensive loss.
Cash
Cash consist of cash on hand and bank deposits. As of December 31, 2017 and 2018, the Company had no cash equivalents.
Short-term investments
All liquid investments with an original maturity greater than three months but less than one year are considered to be short-term investments. As of December 31, 2017, the short-term investments are one-year time deposits amounting to $3,074 (RMB20,000) placed with China Merchants Bank.
Advances to suppliers
Advances to suppliers consist of cash to contractors and vendors for services and materials that have not been provided or received. Advances to suppliers are reviewed periodically to determine whether their carrying values have become impaired. The Company considers the assets to be impaired if it is doubtful that the services and materials will be or can be provided by the suppliers. As of December 31, 2017 and 2018, there were no allowances provided.
Leases
Leases are classified at the inception date as either a capital lease or an operating lease. The Company assesses a lease to be a capital lease if any of the following conditions exist: (a) ownership is transferred to the lessee by the end of the lease term, (b) there is a bargain purchase option, (c) the lease term is at least 75% of the property’s estimated remaining economic life or (d) the present value of the minimum lease payments at the beginning of the lease term is 90% or more of the fair value of the leased property to the lessor at the inception date. A capital lease is accounted for as if there was an acquisition of an asset and an incurrence of an obligation at the inception of the lease. The Company had no capital leases for the years presented.
All other leases are accounted for as operating leases wherein rental payments are expensed on a straight-line basis over the periods of their respective lease terms. The Company leases office space under operating lease agreements. The lease term begins on the date of initial possession of the lease property for purposes of recognizing lease expense on straight-line basis over the term of the lease.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Government grants
Government grants relating to assets are recognized in the consolidated balance sheets upon receipt and amortized as other income over the weighted average useful life of the related assets. Government grants relating to income that involves no conditions or continuing performance obligations of the Company are recognized as other income upon receipt.
Government grants for Dalian Wanchun Pharmaceutical Co., Ltd. (“Wanchun Pharma”) amounting to $316 (RMB2,000) were received in December 2014. The government grant was transferred to Wanchunbulin since Wanchun Pharma was liquidated in August 2015. The Company previously included such government grant under current liabilities as the amendment procedures for changing the beneficiary to Wanchunbulin was still under review of the local government. In January 2018, the Company obtained approval from local government and became eligible for the government grant and recorded the government grant as other income in the consolidated statements of comprehensive loss during the year of 2018.
The Company received government grants of $914 which was recorded as other income in the consolidated statements of comprehensive loss for the year ended December 31, 2017.
Property and equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the respective assets as follows:
Category | Estimated useful life |
| | |
| Office equipment | 5 years |
| Laboratory equipment | 2-5 years |
| Motor vehicles | 10 years |
| Leasehold improvements | Lower of lease term or economic life |
Repair and maintenance costs are charged to expense as incurred, whereas the cost of renewals and betterment that extends the useful lives of plant and equipment are capitalized as additions to the related assets. Retirements, sales and disposals of assets are recorded by removing the cost and accumulated depreciation from the assets and accumulated depreciation accounts with any resulting gain or loss reflected in the consolidated statements of comprehensive loss.
Impairment of long-lived assets
Long-lived assets are reviewed for impairment in accordance with authoritative guidance for impairment or disposal of long-lived assets. Long-lived assets are reviewed for events or changes in circumstances, which indicate that their carrying value may not be recoverable. Long-lived assets are reported at the lower of carrying amount or fair value less cost to sell. For the years ended December 31, 2018, 2017 and 2016, the Company did not record any impairment losses on its long-lived assets.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Fair value measurements
Financial instruments of the Company primarily include cash, short-term investments, amounts due from related parties and accounts payable. As of December 31, 2017 and 2018, the carrying values of these financial instruments approximated their fair value due to their short term nature.
The Company applies ASC 820, Fair Value Measurements and Disclosures (“ASC 820”), in measuring fair value. ASC 820 defines fair value, establishes a framework for measuring fair value and requires disclosures to be provided on fair value measurement.
ASC 820 establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
| • | Level 1—Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| • | Level 2 —Other inputs that are directly or indirectly observable in the marketplace. |
| • | Level 3—Unobservable inputs which are supported by little or no market activity. |
ASC 820 describes three main approaches to measuring the fair value of assets and liabilities: (1) market approach; (2) income approach and (3) cost approach. The market approach uses prices and other relevant information generated from market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value amount. The measurement is based on the value indicated by current market expectations about those future amounts. The cost approach is based on the amount that would currently be required to replace an asset.
Segment information
The Company’s chief operating decision maker, the Chief Executive Officer, reviews the consolidated results when making decisions about allocating resources and assessing performance of the Company as a whole and hence in accordance with ASC 280, Segment Reporting, the Company has only one reportable segment. The Company does not distinguish between markets or segments for the purpose of internal reporting.
The Company had no revenue for all years presented. The following table summarizes property and equipment of the Group by geographical location:
| | | December 31, | |
| | | 2017 | | | 2018 | |
| | | | | | | |
| PRC | | | 90 | | | | 73 | |
| U.S. | | | 30 | | | | 208 | |
| Australia | | | 3 | | | | 1 | |
| | | | | | | | | |
| Total | | | 123 | | | | 282 | |
Comprehensive income (loss)
Comprehensive loss is defined as the changes in equity of the Company during a period from transactions and other events and circumstances excluding transactions resulting from investments by owners and distributions to owners. For each of the periods presented, the Company’s comprehensive loss includes net loss and foreign currency translation adjustments.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Income taxes
The Company uses the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of assets and liabilities and are measured using enacted tax rates that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
In accordance with Accounting Standards Update (“ASU”) No. 2015-17, Income Taxes (Topic 740), all deferred income tax assets and liabilities are classified as non-current on the consolidated balance sheets.
The Company evaluates its uncertain tax positions using the provisions of ASC 740, Income Taxes, which prescribes a recognition threshold that a tax position is required to meet before being recognized in the financial statements. The Company recognizes in the financial statements the benefit of a tax position which is “more likely than not” to be sustained under examination based solely on the technical merits of the position assuming a review by tax authorities having all relevant information. Tax positions that meet the recognition threshold are measured using a cumulative probability approach, at the largest amount of tax benefit that has a greater than fifty percent likelihood of being realized upon settlement. It is the Company’s policy to recognize interest and penalties related to unrecognized tax benefits, if any, as a component of income tax expense.
Share-based compensation
Awards granted to employees
The Company applies ASC 718, Compensation—Stock Compensation (“ASC 718”), to account for its employee share-based payments. In accordance with ASC 718, the Company determines whether an award should be classified and accounted for as a liability award or equity award. All the Company’s grants of share-based awards to employees were classified as equity awards and are recognized in the financial statements based on their grant date fair values. Specifically, the grant date fair value of share options is calculated using an option pricing model, and the grant date fair value of restricted shares is based on the quoted market price of the Company’s ordinary shares. The Company has elected to recognize compensation expense on a straight-line basis over the requisite service period for each separately vesting portion of the award as if the award was, in-substance, multiple awards for all employee equity awards granted with graded vesting based on service condition. The Company uses the accelerated method for all awards granted with graded vesting based on performance conditions. The Company elected to account for forfeitures in the period they occur as a reduction to expense.
Awards granted to non-employees
The Company has accounted for equity instruments issued to non-employees in accordance with the provisions of ASC 718 and ASC 505, Equity. All transactions in which goods or services are received in exchange for equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date of the fair value of the equity instrument issued is the date on which the counterparty’s performance is completed as there is no associated performance commitment. The expense is recognized in the same manner as if the Company had paid cash for the services provided by the non-employees in accordance with ASC 505-50, Equity-based Payments to Non-Employees. The Company estimated the fair value of share options granted to non-employees using the same method as employees.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Share-based compensation (continued)
Modification of awards
A change in the terms or conditions of the awards is accounted for as a modification of the award. Incremental compensation cost is measured as the excess, if any, of the fair value of the modified award over the fair value of the original award immediately before its terms are modified, measured based on the fair value of the awards and other pertinent factors at the modification date. For vested awards, the Company recognizes incremental compensation cost in the period the modification occurs. For unvested awards, the Company recognizes over the remaining requisite service period, the sum of the incremental compensation cost and the remaining unrecognized compensation cost for the original award on the modification date. If the fair value of the modified award is lower than the fair value of the original award immediately before modification, the minimum compensation cost the Company recognizes is the cost of the original award. There were no modifications to the awards during the year ended December 31, 2018 and 2017.
Loss per share
Loss per share is calculated in accordance with ASC 260, Earnings per Share. Basic loss per ordinary share is computed by dividing net loss attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period.
Diluted loss per share is calculated by dividing net loss attributable to ordinary shareholders as adjusted for the effect of dilutive ordinary equivalent shares, if any, by the weighted average number of ordinary and dilutive ordinary equivalent shares outstanding during the period. Ordinary equivalent shares consist of the ordinary shares issuable upon the conversion of the share options and the vesting of restricted shares, using the treasury stock method. Ordinary share equivalents are excluded from the computation of diluted loss per share if their effects would be anti-dilutive. Basic and diluted loss per ordinary share is presented in the Company’s consolidated statements of comprehensive loss.
Concentration of credit risk
Concentration of credit risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash. The Company’s cash is held at financial institutions that management believes to be of high credit quality. The Company has not experienced any losses on cash to date. The Company does not believe that it is subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Business, customer, political, social and economic risks
The Company participates in a dynamic high technology industry and believes that changes in any of the following areas could have a material adverse effect on the Company’s future financial position, results of operations or cash flows: changes in the overall demand for services and products; competitive pressures due to new entrants; advances and new trends in new technologies and industry standards; changes in clinical research organizations; changes in certain strategic relationships or customer relationships; regulatory considerations; copyright regulations; and risks associated with the Company’s ability to attract employees necessary to support its growth. The Company’s operations could be also adversely affected by significant political, economic and social uncertainties in the PRC.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Concentration of credit risk (continued)
Business risk
The Company relies on third parties to support clinical development activities, trials and manufacturing process of product candidates. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, the Company may not be able to obtain regulatory approval for the Company´s drug candidates and the Company’s business could be substantially impacted. The Company’s main activities are located in U.S. and PRC.
Currency convertibility risk
The Company incurs portions of expenses in currencies other than the U.S. dollars, in particular, the RMB. On January 1, 1994, the PRC government abolished the dual rate system and introduced a single rate of exchange as quoted daily by the People’s Bank of China (the “PBOC”). However, the unification of the exchange rates does not imply that the RMB may be readily convertible into United States dollars or other foreign currencies. All foreign exchange transactions continue to take place either through the PBOC or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the PBOC. Approvals of foreign currency payments by the PBOC or other institutions require submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts.
Additionally, the value of the RMB is subject to changes in central government policies and international economic and political developments affecting supply and demand in the PRC foreign exchange trading system market.
Foreign currency exchange rate risk
From July 21, 2005, the RMB is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. The depreciation of RMB against the U.S. dollar was approximately 5.7% for the year ended December 31, 2018, the appreciation of RMB against the U.S. dollar was approximately 6.5% for the year ended December 31, 2017 and the depreciation of RMB against the U.S. dollar was approximately 6.3% for the year ended December 31, 2016. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the RMB and the U.S. dollar in the future.
To the extent that the Company needs to convert U.S. dollar into RMB for capital expenditures and working capital and other business purposes, appreciation of RMB against U.S. dollar would have an adverse effect on the RMB amount the Company would receive from the conversion. Conversely, if the Company decides to convert RMB into U.S. dollar for the purpose of making payments for dividends on ordinary shares, strategic acquisitions or investments or other business purposes, appreciation of U.S. dollar against RMB would have a negative effect on the U.S. dollar amount available to the Company. In addition, a significant depreciation of the RMB against the U.S. dollar may significantly reduce the U.S. dollar equivalent of the Company’s earnings or losses.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 2. | Summary of significant accounting policies (continued) |
Recent accounting pronouncements
In February 2016, the FASB issued ASU No. 2016-2, Leases (Topic 842) (“ASU 2016-02”), which requires lessees to recognize assets and liabilities related to lease arrangements longer than 12 months on the balance sheet. This standard also requires additional disclosures by lessees and contains targeted changes to accounting by lessors. The updated guidance is effective for interim and annual periods beginning after December 15, 2018, and early adoption is permitted. The Company will adopt the new standard effective January 1, 2019 using the modified retrospective method and will not restate comparative periods. The Company will elect the package of practical expedients permitted under the transition guidance within the new standard, which permits the Company not to reassess under the new standard its prior conclusions about lease identification, lease classification and initial direct costs. The Company currently believes the most significant change will be related to the recognition of right-of-use assets and lease liabilities on the Company’s balance sheet for certain in-scope operating leases. The Company does not expect any material impact on net assets and the consolidated statement of comprehensive income as a result of adopting the new standard.
In June 2018, the FASB issued ASU 2018-7, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-7”). This update expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. This update also specifies that Topic 718 applies to all share-based payment transactions in which a grantor acquires goods or services to be used or consumed in a grantor’s own operations by issuing share-based payment awards. This update is effective in fiscal years, including interim periods, beginning after December 15, 2018. Early adoption is permitted, but no earlier than an entity’s adoption date of Topic 606. The Company is currently evaluating the impact on its financial statements of adopting this guidance.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework- Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”). The update eliminates, modifies, and adds certain disclosure requirements for fair value measurements. This update is effective in fiscal years, including interim periods, beginning after December 15, 2019, and early adoption is permitted. The added disclosure requirements and the modified disclosure on the narrative description of measurement uncertainty should be applied prospectively for only the most recent interim or annual period presented. All other changes to disclosure requirements in this update should be applied retrospectively to all periods presented upon their effective date. The Company is currently evaluating the impact on its financial statements of adopting this guidance.
| 3. | Property and equipment, net |
Property and equipment consisted of the following:
| | | December 31, | |
| | | 2017 | | | 2018 | |
| | | | | | | |
| Office equipment | | | 39 | | | | 143 | |
| Laboratory equipment | | | 104 | | | | 111 | |
| Motor vehicles | | | 23 | | | | 23 | |
| Leasehold improvements | | | 13 | | | | 109 | |
| | | | | | | | | |
| | | | 179 | | | | 386 | |
| Less: accumulated depreciation | | | (56 | ) | | | (104 | ) |
| | | | | | | | | |
| Property and equipment, net | | | 123 | | | | 282 | |
Depreciation expenses for the years ended December 31, 2016, 2017 and 2018 were $18, $32 and $48, respectively.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 4. | Related party transactions |
In addition to transactions disclosed elsewhere in the consolidated financial statements, the related party transactions for the years presented were as follows:
Dr. Lan Huang
Dr. Lan Huang provided consulting services to BeyondSpring US at a fee of $75, nil and nil for the years ended December 31, 2016, 2017 and 2018 respectively.
Wanchun Biotech as a noncontrolling shareholder
On November 28, 2016 and January 13, 2017, Wanchunbulin entered into purchase contracts with Wanchun Biotech to purchase drugs from Wanchun Biotech for clinical research purpose. During the years ended December 31, 2016, 2017, and 2018, Wanchun Biotech purchased drugs amounting to $110 (RMB754), $547 (RMB3,770) and nil from third party vendors and sold to Wanchunbulin without any margin, respectively.
Dr. Ramon Mohanlal, the Chief Medical Officer
In December 2018, the Company provided an interest-free loan amounting to $481 to Dr. Ramon Mohanlal which was fully repaid in April 2019.
Cayman Islands
The Company is incorporated in the Cayman Islands, and is not subject to income tax under the current laws of the Cayman Islands.
British Virgin Islands
BeyondSpring Ltd. and BVI Biotech are incorporated in the British Virgin Islands, and are not subject to income tax under the current laws of the British Virgin Islands.
U.S.
BeyondSpring US is incorporated in Delaware, the U.S. It is subject to statutory U.S. Federal corporate income tax at a rate of 21% for the year ended December 31, 2018, and 35% for the years ended December 31, 2017 and 2016. BeyondSpring US had no taxable income for all years presented and therefore, no provisions for income taxes were recorded.
In December 2017, the Tax Cuts and Jobs Act (the “2017 Tax Act”) was enacted. The 2017 Tax Act includes a number of changes to existing U.S. tax laws that impact the Company, most notably a reduction of the U.S. corporate income tax rate from 35% to 21% for tax years beginning after December 31, 2017.
Australia
BeyondSpring Australia incorporated in Australia is subject to corporate income tax at a rate of 30%. BeyondSpring Australia has no taxable income for all years presented and therefore, no provision for income taxes is required.
Hong Kong
BeyondSpring HK is in incorporated in Hong Kong. Companies registered in Hong Kong are subject to Hong Kong Profits Tax on the taxable income as reported in their respective statutory financial statements adjusted in accordance with relevant Hong Kong tax laws. The applicable tax rate is 16.5% in Hong Kong. BeyondSpring HK has no taxable income for all years presented and therefore, no provision for income taxes is required.
PRC
Wanchun Shenzhen, Wanchunbulin and Beijing Wanchun are subject to the statutory tax rate of 25% in accordance with the PRC Enterprise Income Tax Law (“EIT Law”), which was effective since January 1, 2008.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 5. | Income taxes (continued) |
The components of losses before income taxes are as follows:
| | | Year Ended December 31, | |
| | | 2016 | | | 2017 | | | 2018 | |
| | | | | | | | | | |
| Cayman Islands | | | 894 | | | | 2,045 | | | | 3,305 | |
| U.S. | | | 9,840 | | | | 80,008 | | | | 23,347 | |
| PRC | | | 1,338 | | | | 11,341 | | | | 6,742 | |
| BVI | | | - | | | | 2,498 | | | | 22,979 | |
| Australia | | | 473 | | | | 496 | | | | 1,101 | |
| | | | | | | | | | | | | |
| Net loss before income taxes | | | 12,545 | | | | 96,388 | | | | 57,474 | |
There were no provisions for current and deferred income taxes because the Company and all of its subsidiaries were loss making and were at cumulative losses for the years presented.
A reconciliation of the differences between income tax benefits and the amounts computed by applying the U.S. Federal corporate income tax rate of 35% for the years of 2016 and 2017, and 21% for the year of 2018 are as follows:
| | | Year Ended December 31, | |
| | | 2016 | | | 2017 | | | 2018 | |
| | | | | | | | | | |
| Net loss before income taxes | | | 12,545 | | | | 96,388 | | | | 57,474 | |
| | | | | | | | | | | | | |
| Expected income tax benefit | | | 4,391 | | | | 33,736 | | | | 12,069 | |
| Tax rate differential | | | 581 | | | | 5,796 | | | | (2,121 | ) |
| Non-deductible expenses | | | (152 | ) | | | (25,299 | ) | | | (880 | ) |
| Deemed disposal gain* | | | - | | | | (10,506 | ) | | | - | |
| Impact of U.S. statutory tax rate change | | | - | | | | (2,943 | ) | | | - | |
| Non-taxable income | | | - | | | | 227 | | | | 75 | |
| Others | | | (63 | ) | | | 74 | | | | (143 | ) |
| Change in valuation allowance | | | (4,757 | ) | | | (1,085 | ) | | | (9,000 | ) |
| | | | | | | | | | | | | |
| Total income tax benefit | | | - | | | | - | | | | - | |
*Arose from intragroup transfer of certain intellectual property rights.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 5. | Income taxes (continued) |
Net deferred tax assets as of December 31, 2017 and 2018 consisted of the following:
| | | December 31, | |
| | | 2017 | | | 2018 | |
| | | | | | | |
| Deferred tax assets: | | | | | | |
| Net operating loss carryforward | | | 7,244 | | | | 13,403 | |
| Intangible asset | | | 329 | | | | 191 | |
| Deferral of tax deduction of R&D expense | | | 1,239 | | | | 2,891 | |
| Share based compensation | | | 1,427 | | | | 2,754 | |
| Less: valuation allowance | | | (10,239 | ) | | | (19,239 | ) |
| | | | | | | | | |
| Net deferred tax assets | | | - | | | | - | |
Valuation allowances have been provided on the deferred tax assets where, based on all available evidence, it was considered more likely than not that some portion or all of the recorded deferred tax assets will not be realized in future periods. The Company recorded a full valuation allowance against deferred tax assets.
As of December 31, 2018, the Company had U.S. and PRC tax loss carryforwards of approximately $38,938 and $688, respectively. For losses occurred in the U.S. in years after December 31, 2017, the Tax Cuts and Jobs Act included a limitation of the deduction for net operating losses to 80% of current year taxable income and a provision where such losses can be carried forward indefinitely. Loss carryforwards in 2017 and prior years are not limited in their current usage, and can be carried forward for 20 years after the year they were generated. Whereas the PRC unused tax losses can be carryforward for 5 years and $688 will fully expire by 2023 if not utilized.
As of December 31, 2017 and 2018, the Company determined that it had no material unrecognized tax benefit and accordingly no material related interest and penalty. Management does not expect that the amount of unrecognized tax benefit will increase significantly within the next 12 months.
The Company’s subsidiaries in the U.S., Australian and PRC filed income tax returns in the U.S., Australia and PRC, respectively. An entity in the U.S. is subject to U.S. federal and state income tax examination by tax authorities for tax years beginning in 2014. An entity in Australia’s tax returns are open to examination by Australian Taxation Office for tax years beginning in 2016. For entities in the PRC the tax returns for tax years after 2015 are open to examination by the PRC taxing authorities.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
Basic and diluted net loss per share attributable to ordinary shareholders was calculated as follows:
| | | Year Ended December 31, | |
| | | 2016 | | | 2017 | | | 2018 | |
| | | | | | | | | | |
| Numerator: | | | | | | | | | |
Net loss attributable to BeyondSpring Inc.—basic and diluted | | $ | (12,010 | ) | | $ | (91,763 | ) | | $ | (54,869 | ) |
| | | | | | | | | | | | | |
| Denominator: | | | | | | | | | | | | |
| Weighted average number of ordinary shares outstanding—basic and diluted | | | 16,086,419 | | | | 20,866,084 | | | | 22,665,265 | |
| | | | | | | | | | | | | |
| Net loss per share—basic and diluted | | $ | (0.75 | ) | | $ | (4.40 | ) | | $ | (2.42 | ) |
The effects of all share options and unvested restricted shares were excluded from the calculation of diluted loss per share as their effect would have been anti-dilutive during the years ended December 31, 2016, 2017 and 2018.
| 7. | Share based compensation |
General
On February 24, 2017, in connection with the IPO, the Company’s board of directors and shareholders approved an equity compensation plan, the 2017 Omnibus Incentive Plan (the “2017 Plan”), which became effective on March 9, 2017, to provide an additional incentive to selected officers, employees, non-employee directors, independent contractors and consultants of the Company (the “Participants”) under certain conditions. Under the 2017 Omnibus Incentive Plan, the maximum number of the Company’s ordinary shares reserved for issuance is 2,137,037 shares.
During 2017, the Company granted a total of 1,141,477 restricted shares to employee and non-employee under the 2017 Plan, among which 318,700 restricted shares was forfeited during 2017. During 2018, the Company granted a total of 9,815 restricted shares to employee and non-employee under the 2017 Plan. The restricted shares were subject to various service condition schedule and certain performance conditions with respect to the research and development progress.
During 2017, the Company granted up to 600,000 common shares to the Chief Medical Officer of the Company under the 2017 Plan. The common shares were subject to certain performance conditions with respect to the research and development progress, and none of the common shares were vested as of December 31, 2018.
During 2017, the Company granted a total of 343,000 nonqualified share options, with an exercise price of US$29.00 per ordinary share. During 2018, the Company granted a total of 130,000 nonqualified share options, with a weighted average exercise price of US$25.08 per ordinary share. The share options have a contractual term of 10 years based on certain service or performance conditions.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 7. | Share based compensation (continued) |
Restricted shares
The following table summarizes the Company’s employee restricted share activities under the 2017 Plan:
| | | Numbers of shares | | | Weighted average grant date fair value | |
|
| | | | | | $
| |
| | | | | | | | |
| Outstanding at December 31, 2016 | | | - | | | | - | |
| Granted | | | 1,141,477 | | | | 20.76 | |
| Vested | | | (434,615 | ) | | | 22.19 | |
| Forfeited | | | (318,700 | ) | | | 19.80 | |
| Outstanding at December 31, 2017 | | | 388,162 | | | | 19.93 | |
| | | | | | | | | |
| Granted | | | 9,815 | | | | 19.50 | |
| Vested | | | (147,727 | ) | | | 19.88 | |
| Forfeited | | | (95,000 | ) | | | 19.96 | |
| Outstanding at December 31, 2018 | | | 155,250 | | | | 19.94 | |
| Expected to vest at December 31, 2018 | | | 125,250 | | | | 19.97 | |
As of December 31, 2018, the unrecognized compensation cost related to unvested restricted shares expected to vest was $1,635. This unrecognized compensation will be recognized over an estimated weighted-average amortization period of 3.4 years.
Share options
The following table summarizes the Company’s share option activities under the 2017 Plan:
| | | | | | | | | | | | | | | | |
| | | | | | $ | | | $
| | | Years | | | $ | |
| | | | | | | | | | | | | | | | | | | |
| Outstanding at December 31, 2016 | | | - | | | | - | | | | | | | | | | | | |
| Granted | | | 343,000 | | | | 29.00 | | | | 19.91 | | | | | | | | |
| Outstanding at December 31, 2017 | | | 343,000 | | | | 29.00 | | | | | | | | 9.98 | | | | 27 | |
| | | | | | | | | | | | | | | | | | | | | |
| Granted | | | 130,000 | | | | 25.08 | | | | 14.49 | | | | | | | | | |
| Forfeited | | | 7,100 | | | | 29.00 | | | | 19.91 | | | | | | | | | |
| Outstanding at December 31, 2018 | | | 465,900 | | | | 27.91 | | | | | | | | 9.12 | | | | - | |
| Exercisable as of December 31, 2018 | | | 335,900 | | | | 29.00 | | | | | | | | 8.98 | | | | - | |
Vested and expected to vest at December 31, 2018 | | | 441,400 | | | | 28.00 | | | | | | | | 9.11 | | | | - | |
As of December 31, 2018, the unrecognized compensation cost related to 441,400 unvested share options expected to vest was $1,066. This unrecognized compensation will be recognized over an estimated weighted-average amortization period of 1.65 years.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 7. | Share based compensation (continued) |
Fair value of options
The Black-Scholes-Merton formula was applied in determining the estimated fair value of the options granted. The model requires the input of highly subjective assumptions including the estimated expected share price volatility and the expected terms of awards. These estimates involve inherent risk and uncertainties and the application of management’s judgment. The Company historically has limited available historical data to demonstrate consistent early exercise behavior. To determine the expected term of the awards, the Company applied a simplified method considering factors including the timing of achieving various performance conditions and their respective probabilities as well as the contractual life of the options. The risk-free interest rates for the periods within the expected term of the option are based on the U.S. Treasury rate. The Company historically has been a private company and lack company-specific historical and implied volatility information, therefore, the Company estimates the expected volatility based on the historical volatility of a group of similar companies, which are publicly-traded. The Company’s management was ultimately responsible for the determination of the estimated fair value of its share options.
The following table presents the assumptions used to estimate the fair values of the share options granted for the years ended December 31, 2017 and 2018:
| | | December 31, | |
| | | 2017 | | | 2018 | |
| | | | | | | |
| Fair value of ordinary share | | | 34.00 | | | 24.87~26.09 | |
| Risk-free interest rate | | | 2.28% |
| | 2.73%~2.89% | |
| Expected term | | 5.5 years | | | 5.9~6.2 years | |
| Expected volatility | | | 60% |
| | | 60% |
|
| Expected dividend yield | | | 0% |
| | | 0% |
|
| Contractual life | | 10 years | | | 10 years | |
The following table summarizes total share-based compensation expense recognized for the year ended December 31, 2017 and 2018:
| | | December 31, | |
| | | 2017 | | | 2018 | |
| | | | | | | |
| Research and development | | | 17,753 | | | | 6,821 | |
| General and administrative | | | 4,930 | | | | (261) |
|
| | | | | | | | | |
| Total | | | 22,683 | | | | 6,560 | |
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 8. | Employee defined contribution plan |
Full time employees of the Company in the PRC participate in a government mandated defined contribution plan, pursuant to which certain pension benefits, medical care, employee housing fund and other welfare benefits are provided to employees. Chinese labor regulations require that the Company’s PRC subsidiaries make contributions to the government for these benefits based on certain percentages of the employees’ salaries. The Company has no legal obligation for the benefits beyond the contributions made. The total amounts for such employee benefits, which were expensed as incurred, were $22, $38 and $54 for the years ended December 31, 2016, 2017 and 2018, respectively.
The Company’s ability to pay dividends may depend on the Company receiving distributions of funds from its PRC subsidiaries. Relevant PRC statutory laws and regulations permit payments of dividends by the Company’s PRC subsidiaries only out of its retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. The results of operations reflected in the consolidated financial statements prepared in accordance with U.S. GAAP differ from those reflected in the statutory financial statements of the Company’s PRC subsidiaries.
In accordance with the PRC Regulations on Enterprises with Foreign Investment and their articles of association, a foreign invested enterprise established in the PRC is required to provide certain statutory reserves, namely general reserve fund, the enterprise expansion fund and staff welfare and bonus fund which are appropriated from net profit as reported in the enterprise’s PRC statutory accounts. A foreign invested enterprise is required to allocate at least 10% of its annual after-tax profit to the general reserve until such reserve has reached 50% of its respective registered capital based on the enterprise’s PRC statutory accounts. Appropriations to the enterprise expansion fund and staff welfare and bonus fund are at the discretion of the board of directors for all foreign invested enterprises. The aforementioned reserves can only be used for specific purposes and are not distributable as cash dividends. Wanchun Shenzhen was established as a foreign invested enterprise and therefore is subject to the above mandated restrictions on distributable profits.
Additionally, in accordance with the Company Law of the PRC, a domestic enterprise is required to provide statutory common reserve of at least 10% of its annual after-tax profit until such reserve has reached 50% of its respective registered capital based on the enterprise’s PRC statutory accounts. A domestic enterprise is also required to provide discretionary surplus reserve, at the discretion of the board of directors, from the profits determined in accordance with the enterprise’s PRC statutory accounts. The aforementioned reserves can only be used for specific purposes and are not distributable as dividends. Wanchunbulin was established as domestic invested enterprises and therefore is subject to the above mandated restrictions on distributable profits.
Foreign exchange and other regulations in the PRC further restrict the Company’s PRC subsidiaries from transferring funds to the Company in the form of loans, advances or cash dividends. As of December 31, 2017 and 2018, restricted net assets of the Company’s PRC subsidiaries were $2,399 and $nil, respectively.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 10. | Commitments and contingencies |
Operating lease commitments
The Company has several operating leases, primarily for offices. Payments under operating leases are expensed on a straight-line basis over the periods of their respective leases, and the terms of the leases do not contain rent escalation, contingent rent, renewal, or purchase options.
Rental expenses incurred under the operating leases for the years ended December 31, 2016, 2017 and 2018 amounted to $136, $200 and $548 respectively.
The following table summarizes the future minimum lease payments under the operating lease as of December 31, 2018:
| | |
| $ | |
| | | | | |
| Year ending December 31, 2019 | | | 792 | |
| Year ending December 31, 2020 | | | 798 | |
| Year ending December 31, 2021 | | | 786 | |
| Year ending December 31, 2022 | | | 789 | |
| Year ending December 31, 2023 | | | 793 | |
| | | | | |
| Total | | | 3,958 | |
Royalty payment
As part of the consideration to the seller for acquiring the worldwide patent of Plinabulin excluding the PRC and Hong Kong, Wanchun Biotech was required to pay royalties on a quarterly basis equal to 20% of gross proceeds from the sales of the product, commencing on the first commercial sale of such product for ten years.
On February 2, 2015, the Company, Wanchun Biotech and Fortis Advisors LLC, in its capacity as an agent of the former stakeholders of the seller of the patent of Plinabulin transferred to Wanchun Biotech, entered into an agreement to terminate such royalty payment arrangements. The termination agreement would be effective upon the consummation of the Company’s initial public offering (“IPO”) in the United States. If the IPO was consummated within three years following the agreement date, the Company was required to issue and allot such number of ordinary shares representing 10% of the Company’s fully-diluted equity capitalization immediately prior to the IPO to a single corporate entity designated by the seller in lieu of the royalty payment. In connection with the Company IPO on the NASDAQ Capital Market completed in March 2017, the Company issued 2,112,963 ordinary shares to Nereus Trust, an entity designated by the seller, and the royalty payment arrangements were terminated. The cost of such patent acquired and expensed as research and development expense was $42,259, which is determined based on the fair value of such issued ordinary shares of $20 per share.
On January 21, 2019, the Company entered into a $2,986 (RMB20,000) loan arrangement with a third party, with an annual interest rate of 15%, and is guaranteed by the Founder, Mr. Linqing Jia. The original maturity date of the loan is April 20, 2019, and the Company has received $1,493 (RMB10,000) by the date of these financial statements. The repayment period was extended to October 20, 2020 pursuant to a supplementary agreement for those amount received.
On March 28, 2019, the Company borrowed a three-year term loan of $1,493 (RMB10,000) from China Construction Bank, with an annual interest rate of 5.7%, guaranteed by its shareholder, Shenzhen Sangel Capital Management Limited Company and Mulong Liu.
BEYONDSPRING INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
FOR THE YEARS ENDED DECEMBER 31, 2018, 2017 AND 2016
(Amounts in thousands of U.S. Dollars (“$”) and Renminbi (“RMB”),
except for number of shares and per share data)
| 11. | Subsequent events (continued) |
On April 26, 2019, the Company entered into a $1,000 loan agreement with Shenzhen Sangel Zhichuang Investment Co., Ltd., with an annual interest rate of 15%, and is guaranteed by the Founder, Mr. Linqing Jia. The Company has fully drawn down the loan of $1,000 in April 2019.