
PRT3789 Phase 1 Interim Clinical Data Update from 2024 ESMO Congress 13 September 2024 Investor Presentation Exhibit 99.2

Forward Looking Statements This presentation contains “forward-looking” statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, anticipated discovery, preclinical and clinical development activities for Prelude’s product candidates, including product candidates that are the subjects of the Company’s collaborations and partnerships, the potential safety, efficacy, benefits and addressable market for Prelude’s product candidates, the expected timeline for clinical trial results for Prelude’s product candidates including its SMARCA2 degrader molecules. Any statements contained herein or provided orally that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by such terminology as ‘‘believe,’’ ‘‘may,’’ ‘‘will,’’ ‘‘potentially,’’ ‘‘estimate,’’ ‘‘continue,’’ ‘‘anticipate,’’ ‘‘intend,’’ ‘‘could,’’ ‘‘would,’’ ‘‘project,’’ ‘‘plan,’’ ‘‘expect’’ and similar expressions that convey uncertainty of future events or outcomes, although not all forward-looking statements contain these words. Statements, including forward-looking statements, speak only to the date they are provided (unless an earlier date is indicated). This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. These forward-looking statements are based on the beliefs of our management as well as assumptions made by and information currently available to us. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. If such assumptions do not fully materialize or prove incorrect, the events or circumstances referred to in the forward-looking statements may not occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Additional risks and uncertainties that could affect our business are included under the caption “Risk Factors” in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended December 31, 2023 and our Quarterly Reports on Form 10-Q.

Kris Vaddi, Ph.D., CEO�Prelude Therapeutics Incorporated Opening Remarks

We are on a mission to extend the promise of precision �medicine to every cancer patient in need Select the best modality to precisely target oncogenic mechanisms Strive for first- or best-in-class and anchor to patient unmet need Draw on decades of experience and proven leadership to drive innovation
Developing an Industry Leading Portfolio of SMARCA-Targeted Precision Medicines SMARCA - DACs + Lead SMARCA2 Degrader (PRT3789) Oral SMARCA2 Degrader (PRT7732) Precision ADCs with SMARCA2/4 Degrader Payload

Dr. Jane Huang, President & Chief Medical Officer�Prelude Therapeutics Incorporated A Phase 1 Trial of PRT3789, a First-in-Class SMARCA2 Degrader in Patients with Advanced Solid Tumors With a SMARCA4 Mutation� Guo, R. et. al., ESMO Congress, 13 Sept 2024 PRT3789 Overview and �Phase 1 Interim Data

Dr. Timothy Yap, University of Texas�MD Anderson Cancer Center Investigator on PRT3789-01: A Phase 1 Trial of PRT3789, a First-in-Class SMARCA2 Degrader in Patients with Advanced Solid Tumors With a SMARCA4 Mutation Expert Perspective on SMARCA4-mutated Cancer
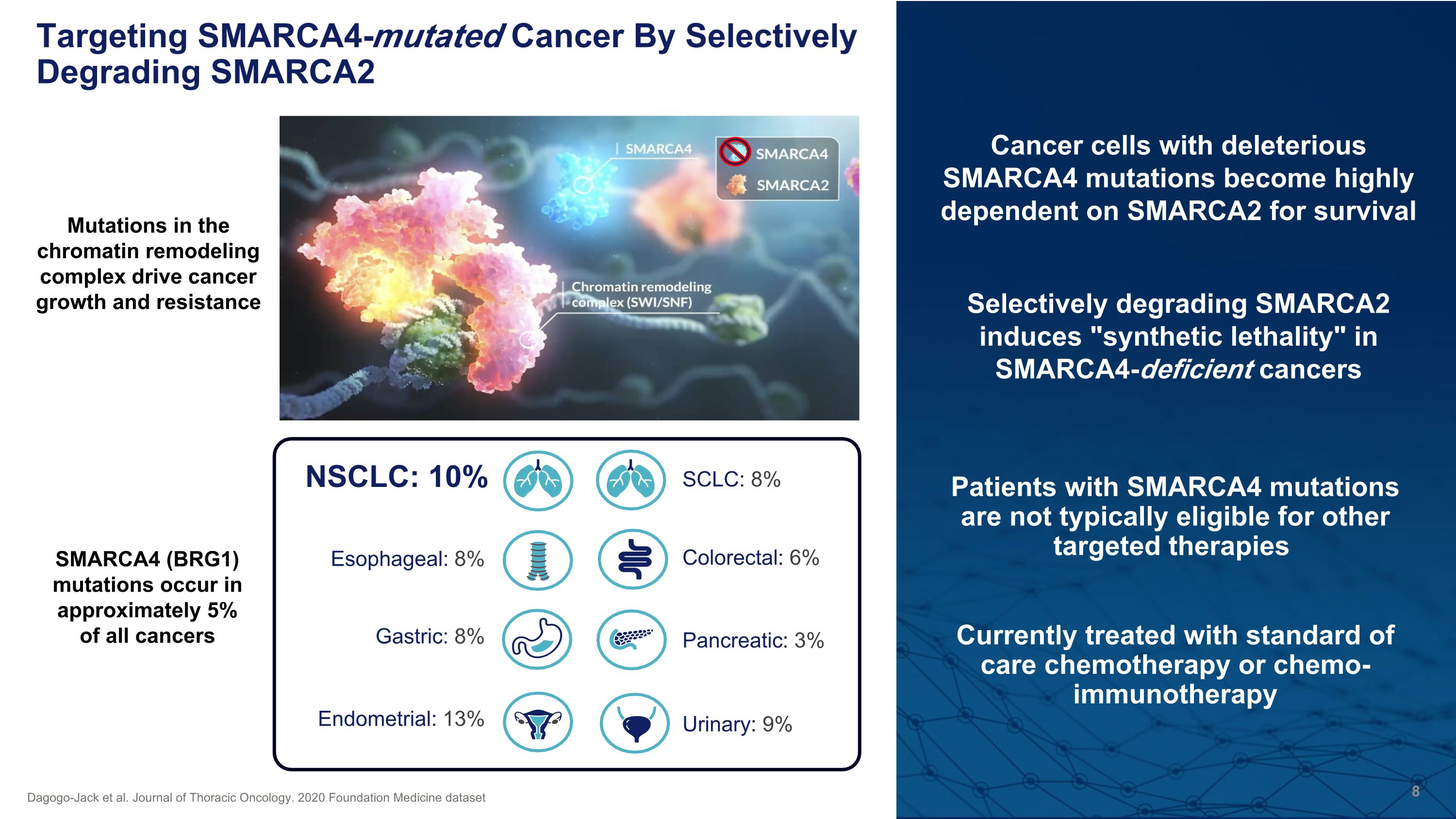
Patients with SMARCA4 mutations are not typically eligible for other targeted therapies Currently treated with standard of care chemotherapy or chemo-immunotherapy Targeting SMARCA4-mutated Cancer By Selectively Degrading SMARCA2 Pancreatic: 3% NSCLC: 10% Esophageal: 8% Gastric: 8% Endometrial: 13% SCLC: 8% Urinary: 9% Colorectal: 6% Dagogo-Jack et al. Journal of Thoracic Oncology. 2020 Foundation Medicine dataset SMARCA4 (BRG1) mutations occur in approximately 5% of all cancers Mutations in the chromatin remodeling complex drive cancer growth and resistance Cancer cells with deleterious SMARCA4 mutations become highly dependent on SMARCA2 for survival Selectively degrading SMARCA2 induces "synthetic lethality" in SMARCA4-deficient cancers
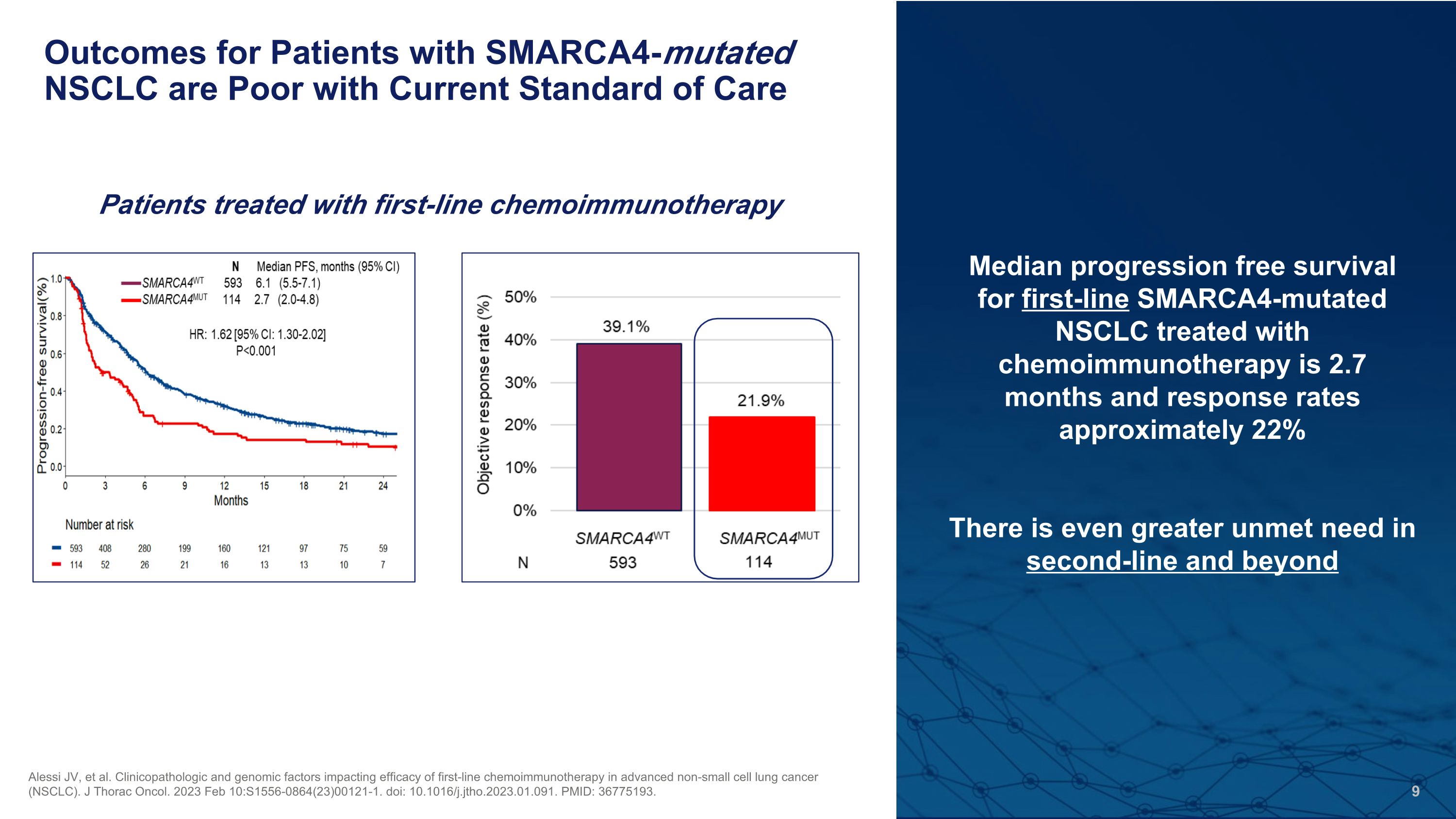
Outcomes for Patients with SMARCA4-mutated NSCLC are Poor with Current Standard of Care Alessi JV, et al. Clinicopathologic and genomic factors impacting efficacy of first-line chemoimmunotherapy in advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2023 Feb 10:S1556-0864(23)00121-1. doi: 10.1016/j.jtho.2023.01.091. PMID: 36775193. Median progression free survival for first-line SMARCA4-mutated NSCLC treated with chemoimmunotherapy is 2.7 months and response rates approximately 22% There is even greater unmet need in�second-line and beyond Patients treated with first-line chemoimmunotherapy
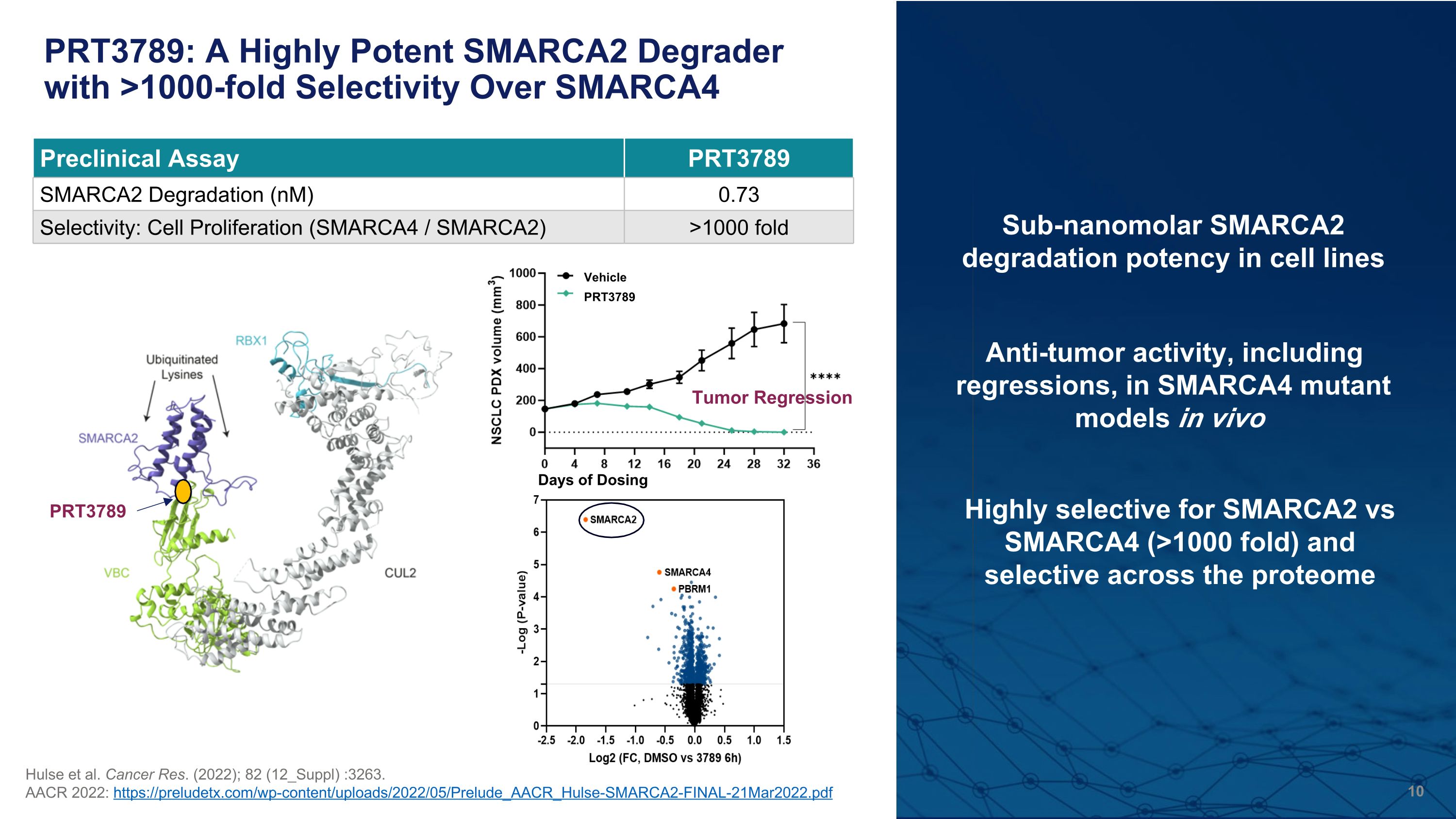
PRT3789: A Highly Potent SMARCA2 Degrader with >1000-fold Selectivity Over SMARCA4 Hulse et al. Cancer Res. (2022); 82 (12_Suppl) :3263. AACR 2022: https://preludetx.com/wp-content/uploads/2022/05/Prelude_AACR_Hulse-SMARCA2-FINAL-21Mar2022.pdf Highly selective for SMARCA2 vs SMARCA4 (>1000 fold) and�selective across the proteome Preclinical Assay PRT3789 SMARCA2 Degradation (nM) 0.73 Selectivity: Cell Proliferation (SMARCA4 / SMARCA2) >1000 fold Sub-nanomolar SMARCA2 degradation potency in cell lines Anti-tumor activity, including regressions, in SMARCA4 mutant models in vivo PRT3789 Tumor Regression PRT3789 Vehicle Days of Dosing
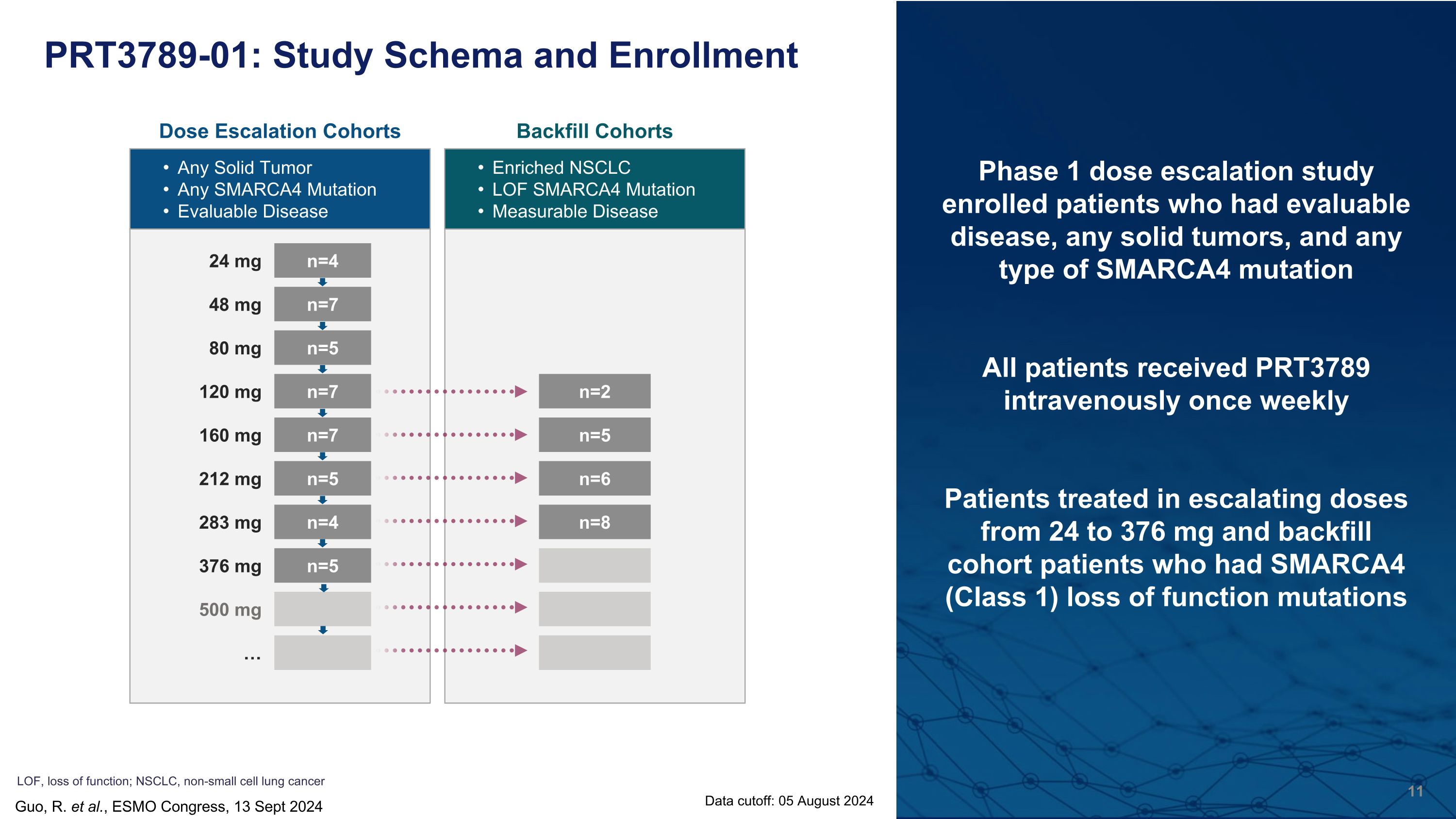
LOF, loss of function; NSCLC, non-small cell lung cancer PRT3789-01: Study Schema and Enrollment Dose Escalation Cohorts Any Solid Tumor Any SMARCA4 Mutation Evaluable Disease Backfill Cohorts Enriched NSCLC LOF SMARCA4 Mutation Measurable Disease n=2 n=5 n=6 n=8 24 mg n=4 48 mg n=7 80 mg n=5 120 mg n=7 160 mg n=7 212 mg n=5 283 mg n=4 376 mg n=5 500 mg … Phase 1 dose escalation study enrolled patients who had evaluable disease, any solid tumors, and any type of SMARCA4 mutation All patients received PRT3789 intravenously once weekly Patients treated in escalating doses from 24 to 376 mg and backfill cohort patients who had SMARCA4 (Class 1) loss of function mutations Guo, R. et al., ESMO Congress, 13 Sept 2024 Data cutoff: 05 August 2024
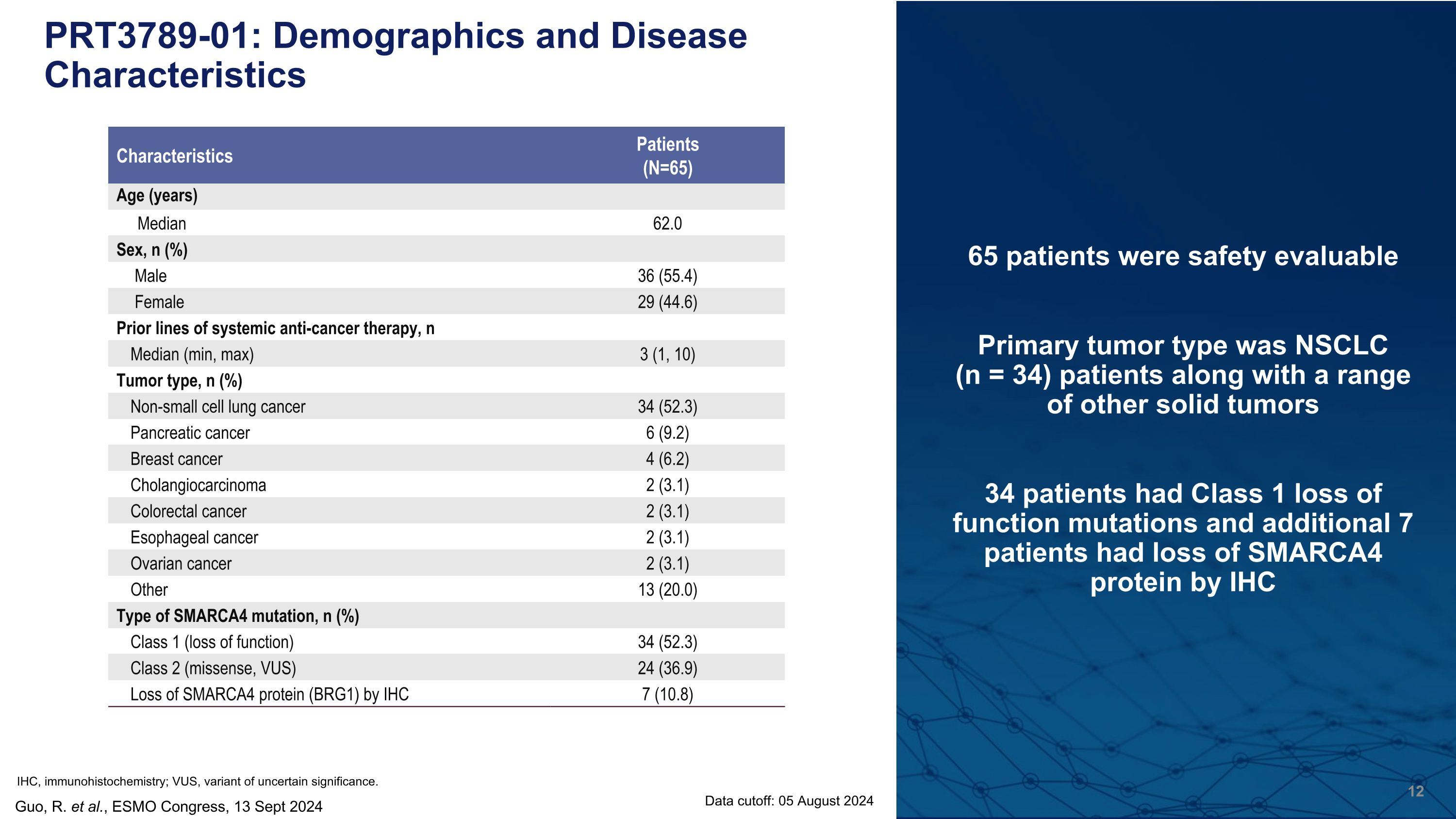
65 patients were safety evaluable Primary tumor type was NSCLC �(n = 34) patients along with a range of other solid tumors 34 patients had Class 1 loss of function mutations and additional 7 patients had loss of SMARCA4 protein by IHC PRT3789-01: Demographics and Disease Characteristics Guo, R. et al., ESMO Congress, 13 Sept 2024 Characteristics Patients (N=65) Age (years) Median 62.0 Sex, n (%) Male 36 (55.4) Female 29 (44.6) Prior lines of systemic anti-cancer therapy, n Median (min, max) 3 (1, 10) Tumor type, n (%) Non-small cell lung cancer 34 (52.3) Pancreatic cancer 6 (9.2) Breast cancer 4 (6.2) Cholangiocarcinoma 2 (3.1) Colorectal cancer 2 (3.1) Esophageal cancer 2 (3.1) Ovarian cancer 2 (3.1) Other 13 (20.0) Type of SMARCA4 mutation, n (%) Class 1 (loss of function) 34 (52.3) Class 2 (missense, VUS) 24 (36.9) Loss of SMARCA4 protein (BRG1) by IHC 7 (10.8) Data cutoff: 05 August 2024 IHC, immunohistochemistry; VUS, variant of uncertain significance.
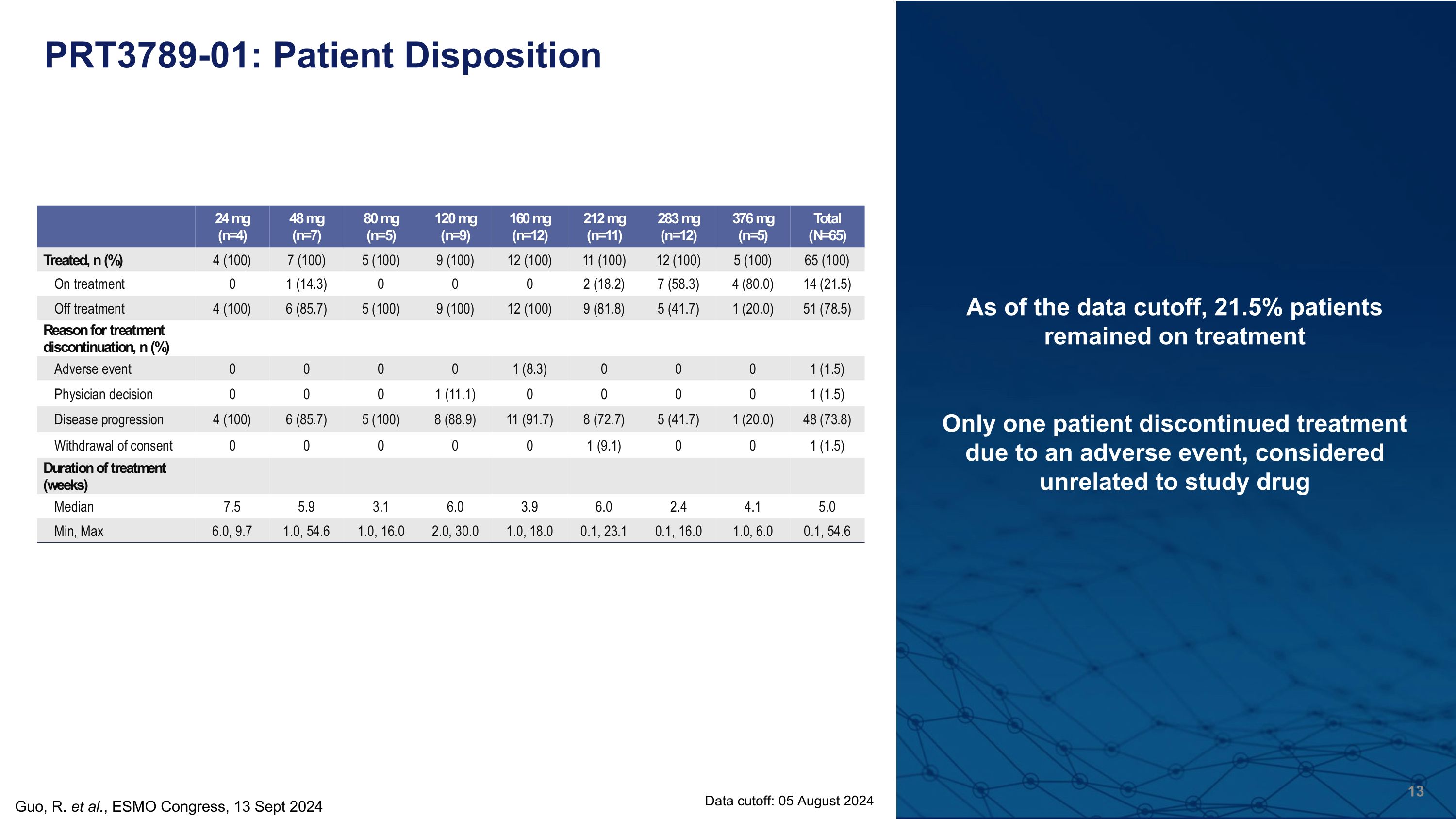
PRT3789-01: Patient Disposition As of the data cutoff, 21.5% patients remained on treatment Only one patient discontinued treatment due to an adverse event, considered unrelated to study drug Data cutoff: 05 August 2024 Guo, R. et al., ESMO Congress, 13 Sept 2024
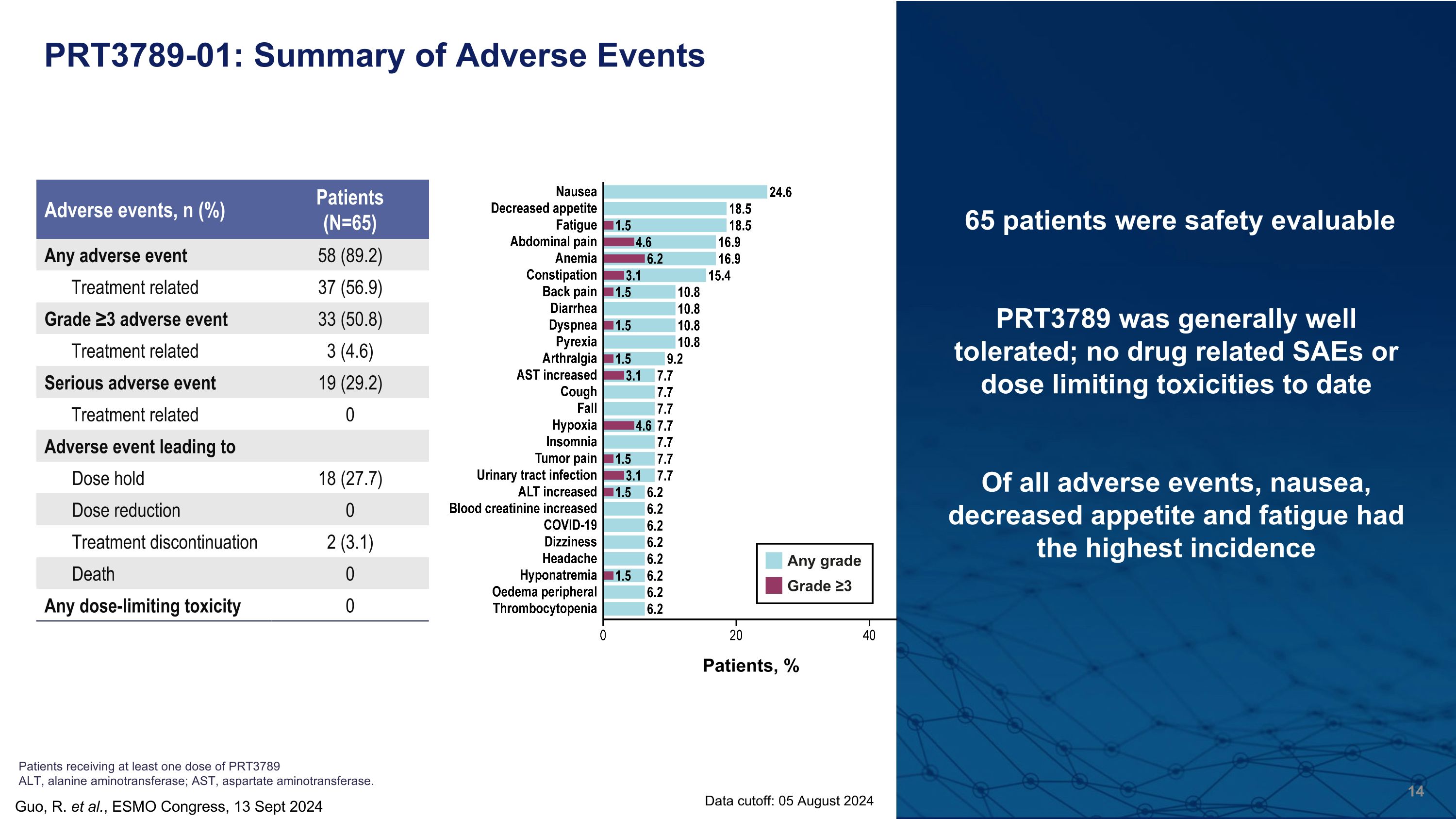
PRT3789-01: Summary of Adverse Events 65 patients were safety evaluable PRT3789 was generally well tolerated; no drug related SAEs or dose limiting toxicities to date Of all adverse events, nausea, decreased appetite and fatigue had the highest incidence Guo, R. et al., ESMO Congress, 13 Sept 2024 Data cutoff: 05 August 2024 Patients receiving at least one dose of PRT3789 ALT, alanine aminotransferase; AST, aspartate aminotransferase. Adverse events, n (%) Patients (N=65) Any adverse event 58 (89.2) Treatment related 37 (56.9) Grade ≥3 adverse event 33 (50.8) Treatment related 3 (4.6) Serious adverse event 19 (29.2) Treatment related 0 Adverse event leading to Dose hold 18 (27.7) Dose reduction 0 Treatment discontinuation 2 (3.1) Death 0 Any dose-limiting toxicity 0 Patients, %
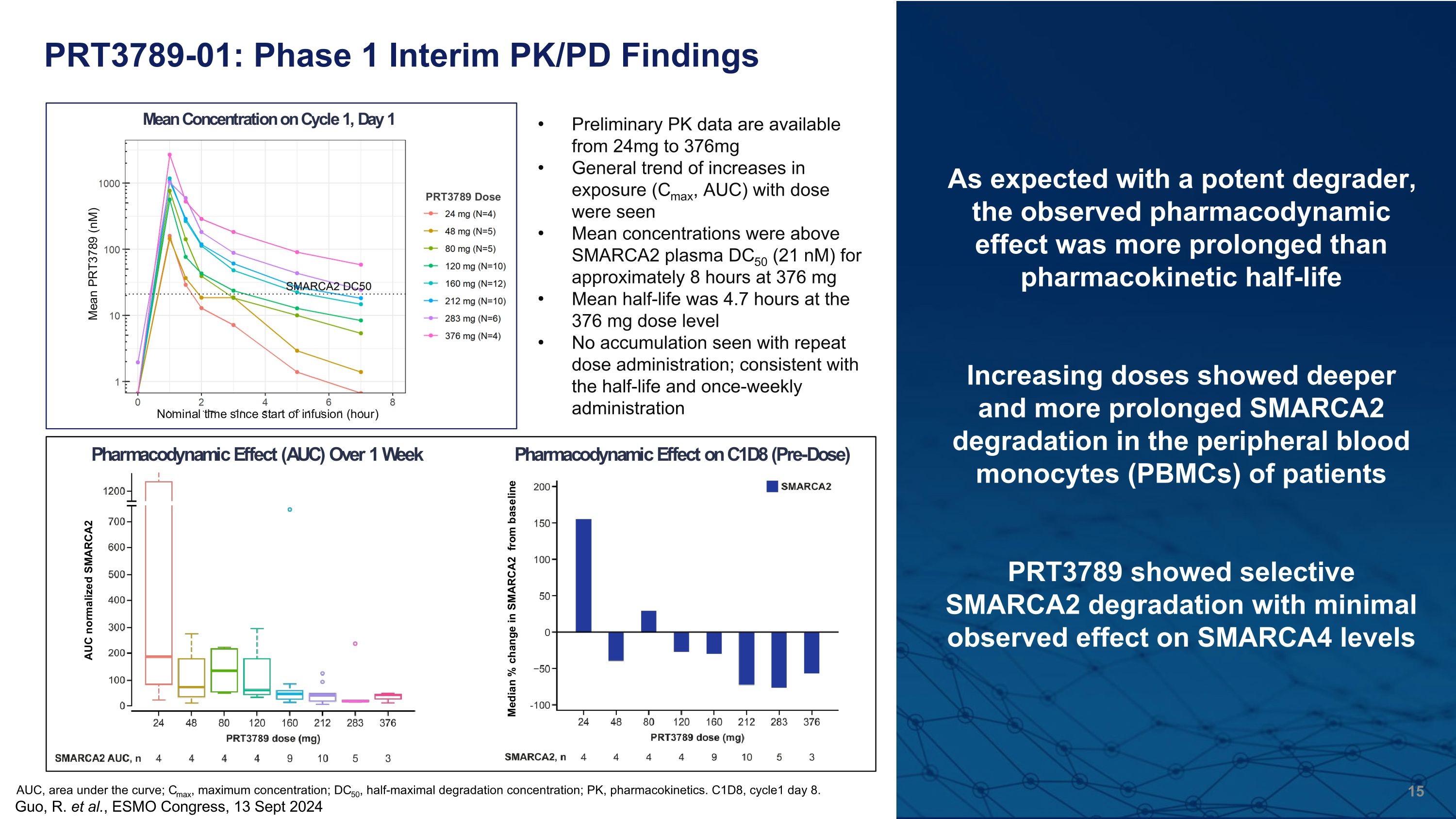
PRT3789-01: Phase 1 Interim PK/PD Findings As expected with a potent degrader, the observed pharmacodynamic effect was more prolonged than pharmacokinetic half-life Increasing doses showed deeper and more prolonged SMARCA2 degradation in the peripheral blood monocytes (PBMCs) of patients PRT3789 showed selective SMARCA2 degradation with minimal observed effect on SMARCA4 levels Preliminary PK data are available from 24mg to 376mg General trend of increases in exposure (Cmax, AUC) with dose were seen Mean concentrations were above SMARCA2 plasma DC50 (21 nM) for approximately 8 hours at 376 mg Mean half-life was 4.7 hours at the 376 mg dose level No accumulation seen with repeat dose administration; consistent with the half-life and once-weekly administration Guo, R. et al., ESMO Congress, 13 Sept 2024 AUC, area under the curve; Cmax, maximum concentration; DC50, half-maximal degradation concentration; PK, pharmacokinetics. C1D8, cycle1 day 8.
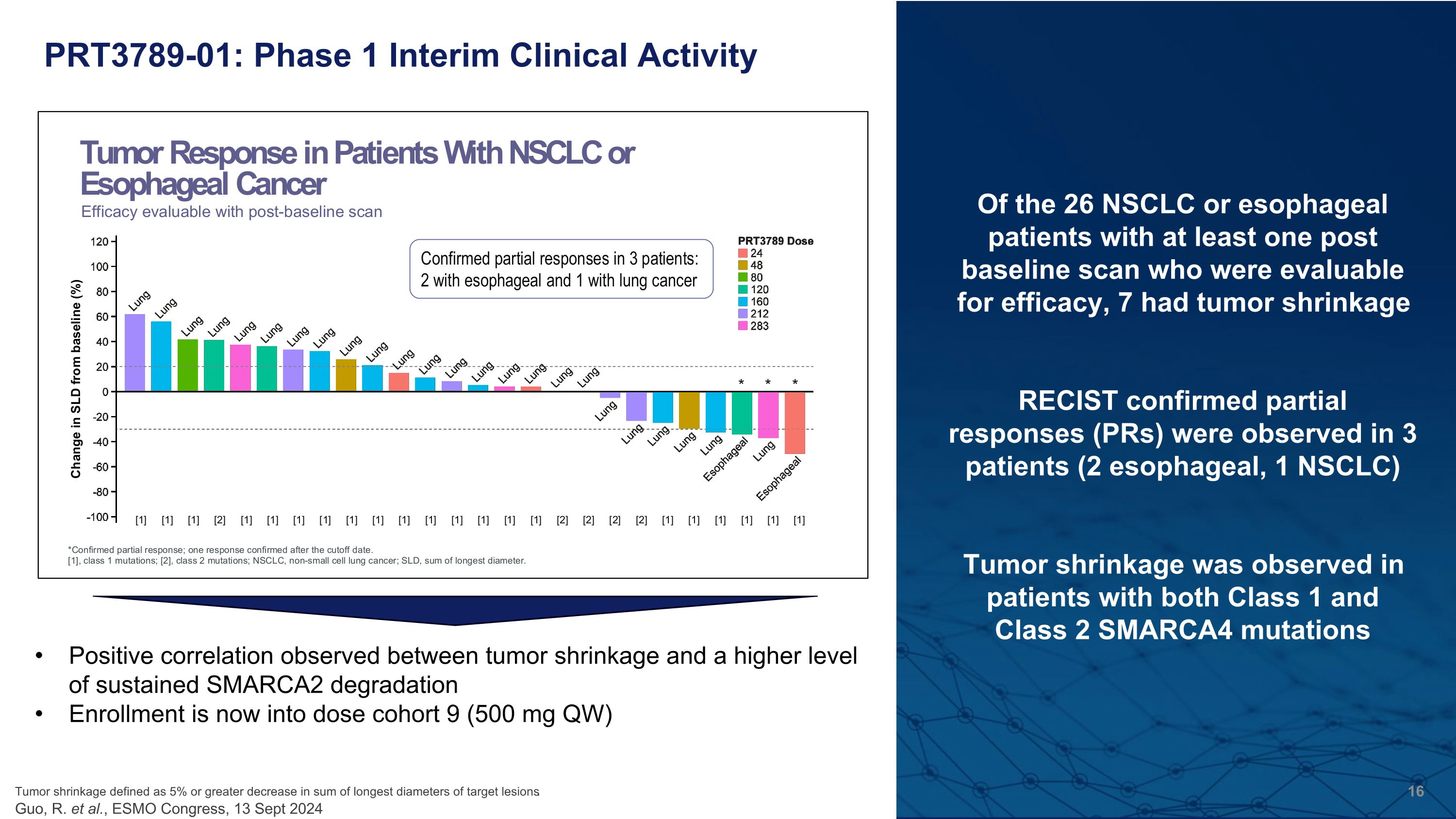
PRT3789-01: Phase 1 Interim Clinical Activity Positive correlation observed between tumor shrinkage and a higher level of sustained SMARCA2 degradation Enrollment is now into dose cohort 9 (500 mg QW) Of the 26 NSCLC or esophageal patients with at least one post baseline scan who were evaluable for efficacy, 7 had tumor shrinkage RECIST confirmed partial �responses (PRs) were observed in 3 patients (2 esophageal, 1 NSCLC) Tumor shrinkage was observed in patients with both Class 1 and �Class 2 SMARCA4 mutations Tumor shrinkage defined as 5% or greater decrease in sum of longest diameters of target lesions.�Guo, R. et al., ESMO Congress, 13 Sept 2024
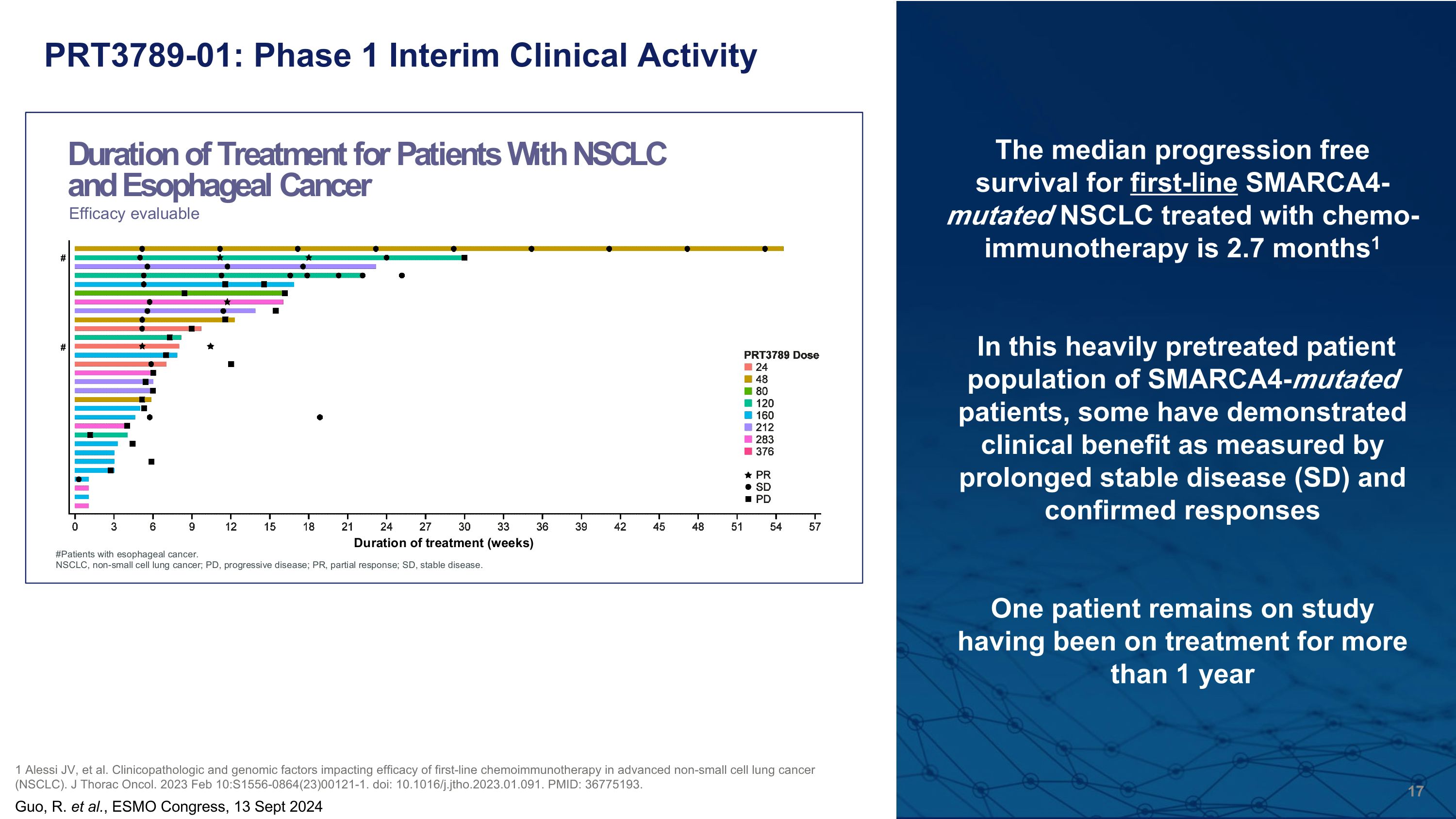
PRT3789-01: Phase 1 Interim Clinical Activity Guo, R. et al., ESMO Congress, 13 Sept 2024 The median progression free survival for first-line SMARCA4-mutated NSCLC treated with chemo-immunotherapy is 2.7 months1 In this heavily pretreated patient population of SMARCA4-mutated patients, some have demonstrated clinical benefit as measured by prolonged stable disease (SD) and confirmed responses One patient remains on study having been on treatment for more than 1 year 1 Alessi JV, et al. Clinicopathologic and genomic factors impacting efficacy of first-line chemoimmunotherapy in advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2023 Feb 10:S1556-0864(23)00121-1. doi: 10.1016/j.jtho.2023.01.091. PMID: 36775193.
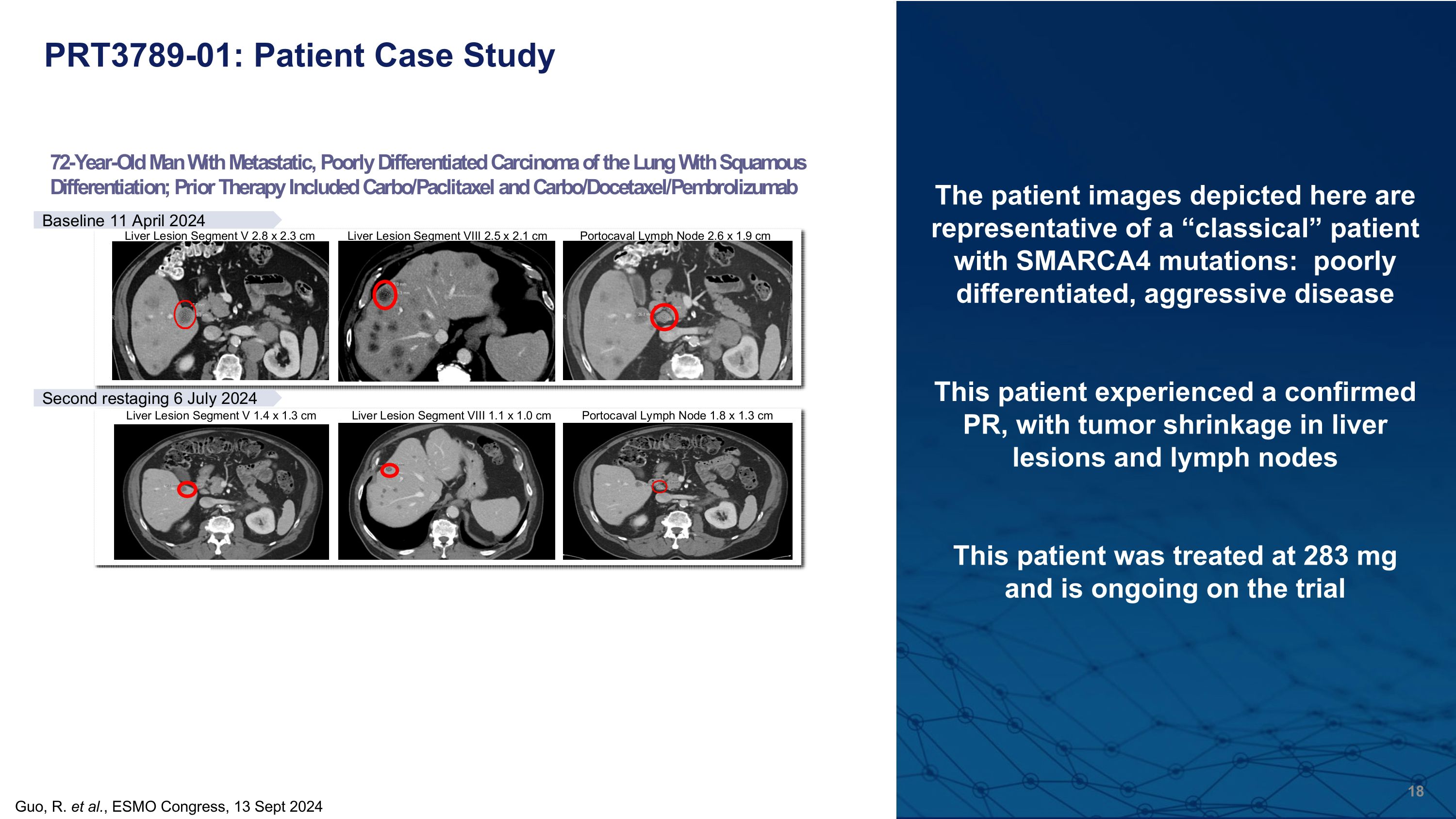
PRT3789-01: Patient Case Study Guo, R. et al., ESMO Congress, 13 Sept 2024 The patient images depicted here are representative of a “classical” patient with SMARCA4 mutations: poorly differentiated, aggressive disease This patient experienced a confirmed PR, with tumor shrinkage in liver lesions and lymph nodes This patient was treated at 283 mg and is ongoing on the trial
PRT3789-01: Key Takeaways Guo, R. et al., ESMO Congress, 13 Sept 2024 These data represent initial proof of concept that selective SMARCA2 degradation �can yield anti-tumor activity in certain SMARCA4-mutated cancers
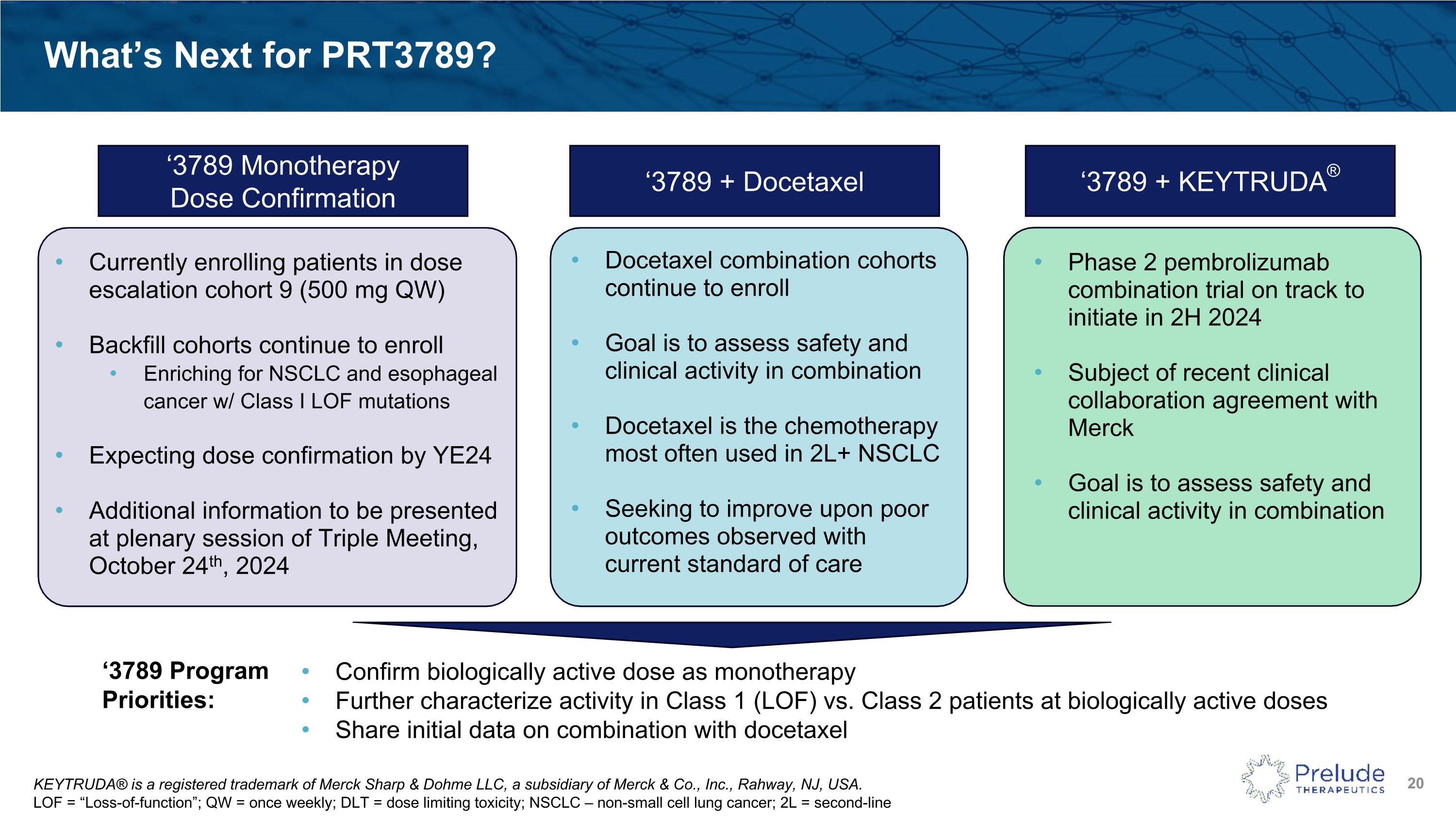
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. �LOF = “Loss-of-function”; QW = once weekly; DLT = dose limiting toxicity; NSCLC – non-small cell lung cancer; 2L = second-line What’s Next for PRT3789? ‘3789 Monotherapy �Dose Confirmation ‘3789 + KEYTRUDA® ‘3789 Program Priorities: Confirm biologically active dose as monotherapy Further characterize activity in Class 1 (LOF) vs. Class 2 patients at biologically active doses Share initial data on combination with docetaxel Currently enrolling patients in dose escalation cohort 9 (500 mg QW) Backfill cohorts continue to enroll Enriching for NSCLC and esophageal cancer w/ Class I LOF mutations Expecting dose confirmation by YE24 Additional information to be presented at plenary session of Triple Meeting, October 24th, 2024 ‘3789 + Docetaxel Phase 2 pembrolizumab combination trial on track to initiate in 2H 2024 Subject of recent clinical collaboration agreement with Merck Goal is to assess safety and clinical activity in combination Docetaxel combination cohorts continue to enroll Goal is to assess safety and clinical activity in combination Docetaxel is the chemotherapy most often used in 2L+ NSCLC Seeking to improve upon poor outcomes observed with current standard of care
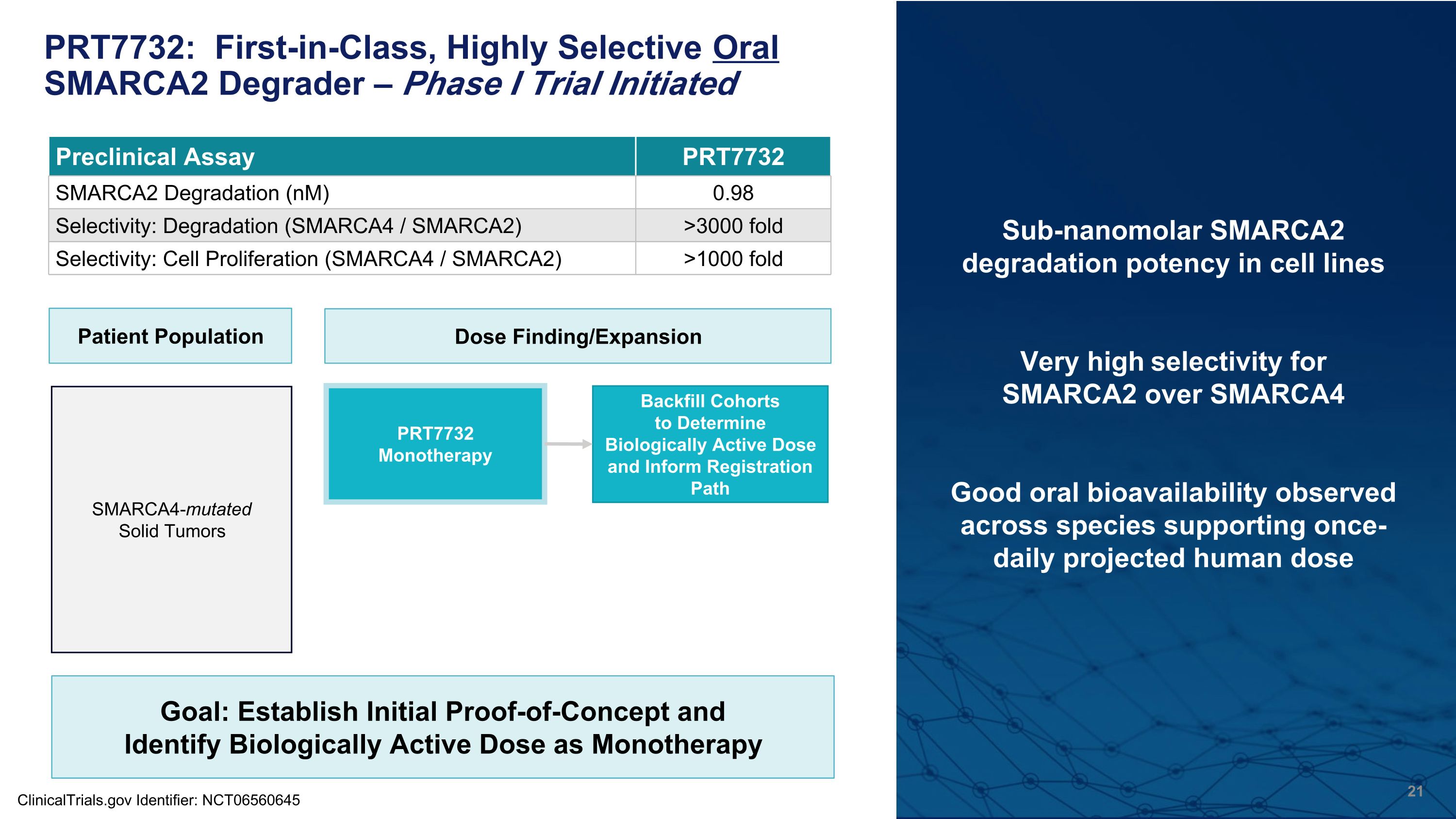
PRT7732: First-in-Class, Highly Selective Oral SMARCA2 Degrader – Phase I Trial Initiated Sub-nanomolar SMARCA2 degradation potency in cell lines Very high selectivity for �SMARCA2 over SMARCA4 Good oral bioavailability observed �across species supporting once-daily projected human dose Preclinical Assay PRT7732 SMARCA2 Degradation (nM) 0.98 Selectivity: Degradation (SMARCA4 / SMARCA2) >3000 fold Selectivity: Cell Proliferation (SMARCA4 / SMARCA2) >1000 fold ClinicalTrials.gov Identifier: NCT06560645 Patient Population Dose Finding/Expansion PRT7732 Monotherapy SMARCA4-mutated �Solid Tumors Backfill Cohorts�to Determine Biologically Active Dose and Inform Registration Path Goal: Establish Initial Proof-of-Concept and Identify Biologically Active Dose as Monotherapy

Kris Vaddi, Ph.D., CEO�Prelude Therapeutics Incorporated Closing Remarks

Open for Questions Kris Vaddi, PhD Chief Executive Officer Jane Huang M.D. President and Chief Medical Officer Sean Brusky, MBA Chief Business Officer Peggy Scherle, PhD Chief Scientific Officer Bryant Lim, J.D. Chief Legal Officer, Corporate Secretary and Interim CFO
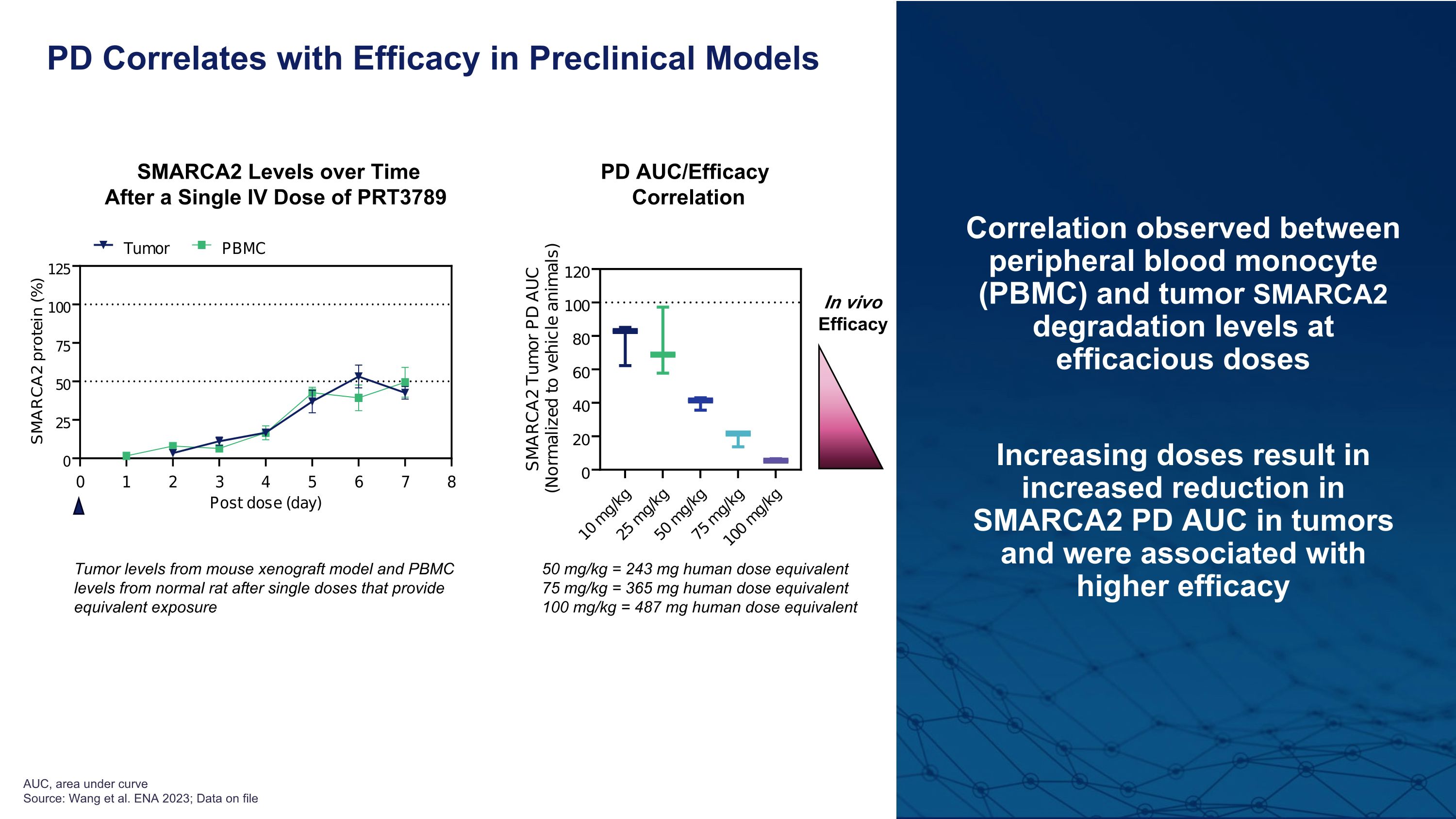
Correlation observed between peripheral blood monocyte (PBMC) and tumor SMARCA2 degradation levels at efficacious doses Increasing doses result in increased reduction in SMARCA2 PD AUC in tumors and were associated with higher efficacy PD Correlates with Efficacy in Preclinical Models In vivo Efficacy Tumor levels from mouse xenograft model and PBMC levels from normal rat after single doses that provide equivalent exposure AUC, area under curve�Source: Wang et al. ENA 2023; Data on file SMARCA2 Levels over Time After a Single IV Dose of PRT3789 PD AUC/Efficacy Correlation 50 mg/kg = 243 mg human dose equivalent 75 mg/kg = 365 mg human dose equivalent 100 mg/kg = 487 mg human dose equivalent
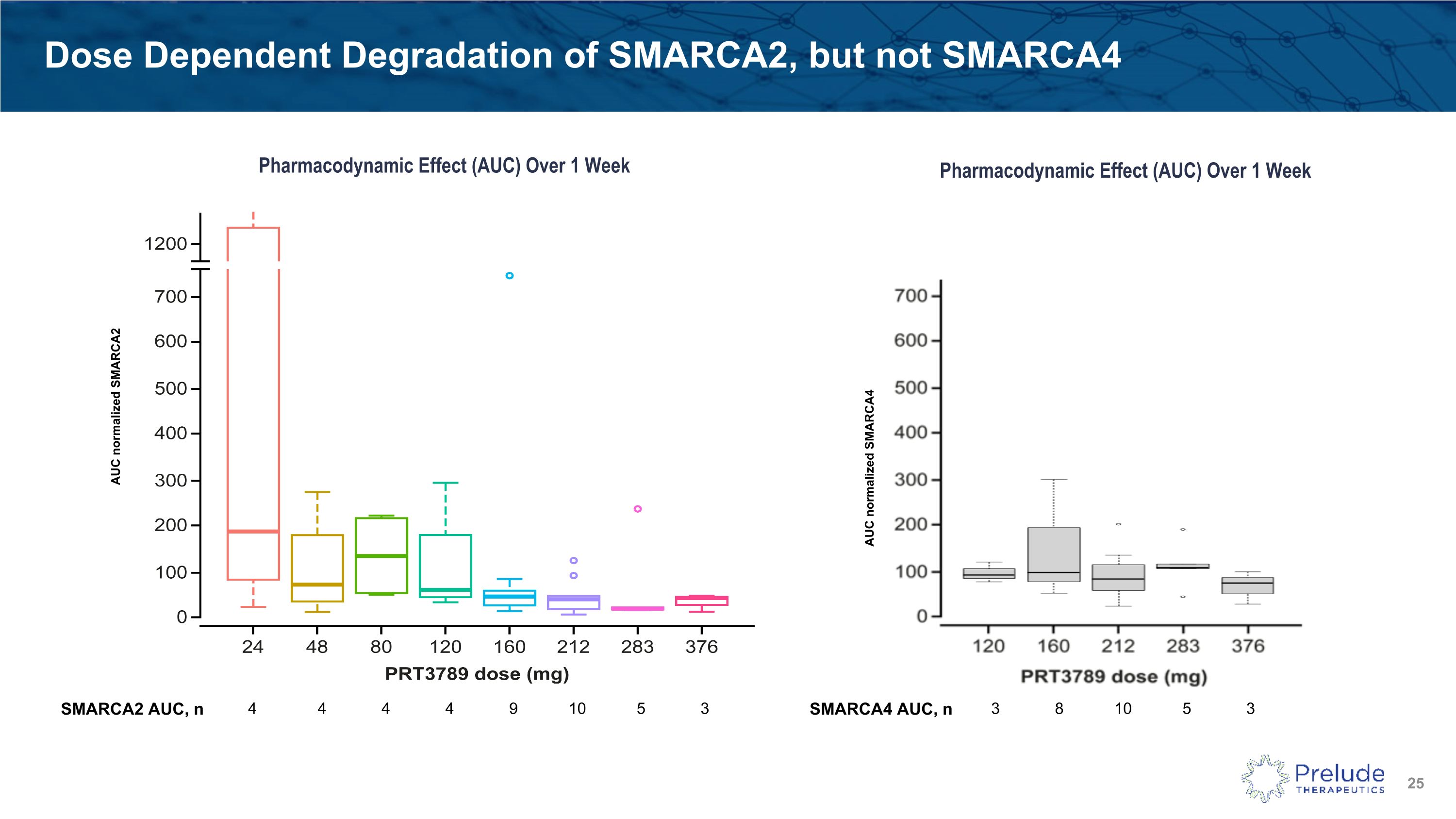
Dose Dependent Degradation of SMARCA2, but not SMARCA4 Pharmacodynamic Effect (AUC) Over 1 Week AUC normalized SMARCA2 AUC normalized SMARCA4 Pharmacodynamic Effect (AUC) Over 1 Week SMARCA2 AUC, n 4 4 4 4 9 10 5 3 SMARCA4 AUC, n 3 8 10 5 3
























