
Corporate Presentation March 2025 Update Exhibit 99.2

Forward Looking Statements This presentation contains “forward-looking” statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, anticipated discovery, preclinical and clinical development activities for Prelude’s product candidates, the potential safety, efficacy, benefits and addressable market for Prelude’s product candidates, the expected timeline for clinical trial results for Prelude’s product candidates including its SMARCA2 degrader molecules. Any statements contained herein or provided orally that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by such terminology as ‘‘believe,’’ ‘‘may,’’ ‘‘will,’’ ‘‘potentially,’’ ‘‘estimate,’’ ‘‘continue,’’ ‘‘anticipate,’’ ‘‘intend,’’ ‘‘could,’’ ‘‘would,’’ ‘‘project,’’ ‘‘plan,’’ ‘‘expect’’ and similar expressions that convey uncertainty of future events or outcomes, although not all forward-looking statements contain these words. Statements, including forward-looking statements, speak only to the date they are provided (unless an earlier date is indicated). This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. These forward-looking statements are based on the beliefs of our management as well as assumptions made by and information currently available to us. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. If such assumptions do not fully materialize or prove incorrect, the events or circumstances referred to in the forward-looking statements may not occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Additional risks and uncertainties that could affect our business are included under the caption “Risk Factors” in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended December 31, 2023.

We are on a mission to extend the promise of precision �medicine to every cancer patient in need Select the best modality to precisely target oncogenic mechanisms Strive for first-in-class science focused on areas of highest patient unmet need Draw on decades of experience and proven leadership to drive innovation

Kris Vaddi, PhD Chief Executive Officer Jane Huang M.D. President and Chief Medical Officer Andrew Combs, PhD Chief Chemistry Officer Sean Brusky, MBA Chief Business Officer Peggy Scherle, PhD Chief Scientific Officer Bryant Lim, J.D. Chief Financial Officer,�Chief Legal Officer, and Corporate Secretary Experienced Leadership Team With Proven Track Records in Precision Oncology
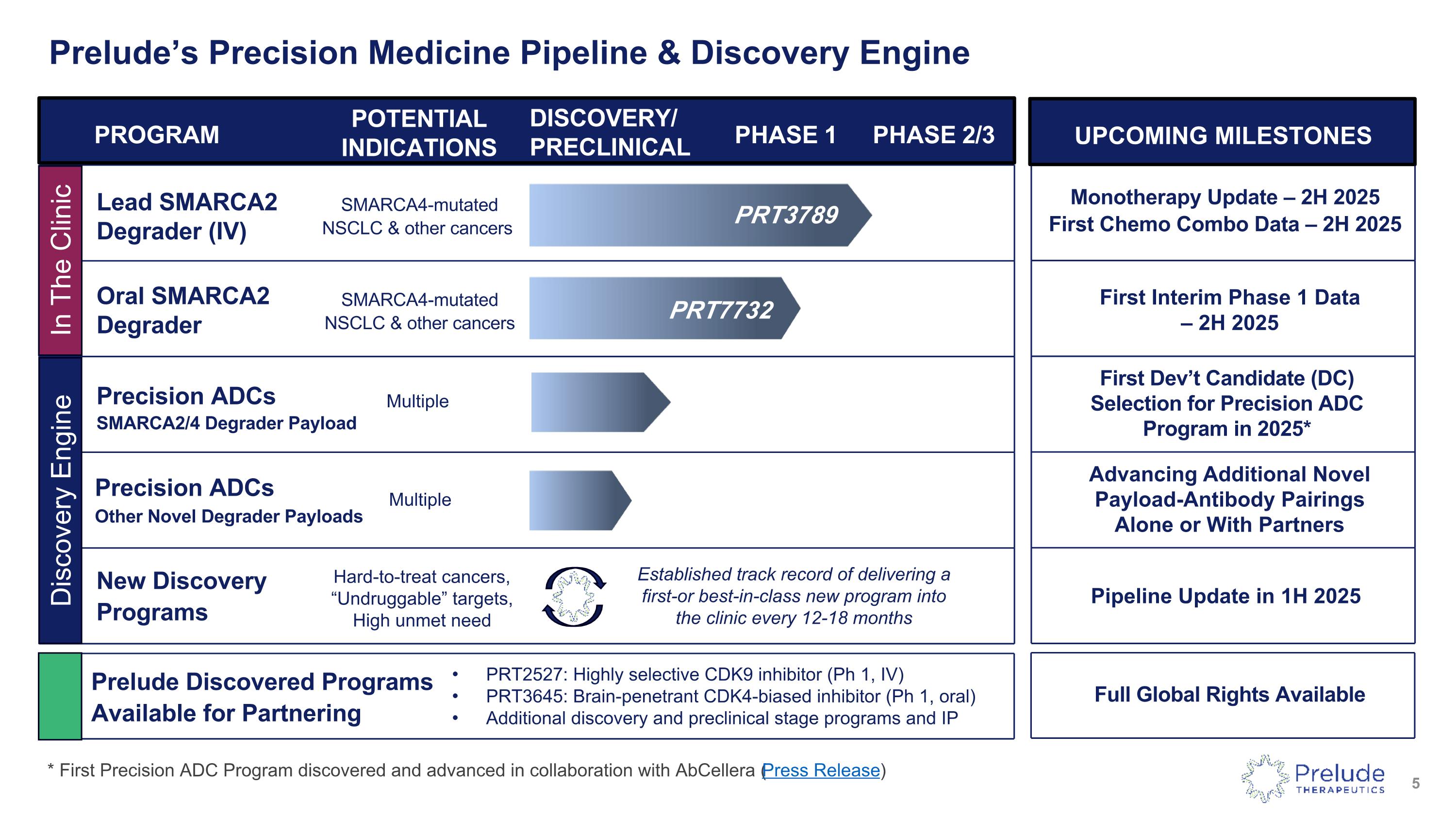
Prelude’s Precision Medicine Pipeline & Discovery Engine PROGRAM POTENTIAL INDICATIONS DISCOVERY/�PRECLINICAL PHASE 1 PHASE 2/3 Lead SMARCA2�Degrader (IV) SMARCA4-mutated NSCLC & other cancers Oral SMARCA2�Degrader SMARCA4-mutated NSCLC & other cancers Precision ADCs�SMARCA2/4 Degrader Payload Monotherapy Update – 2H 2025 First Chemo Combo Data – 2H 2025 UPCOMING MILESTONES First Dev’t Candidate (DC) Selection for Precision ADC Program in 2025* First Interim Phase 1 Data �– 2H 2025 PRT3789 Multiple * First Precision ADC Program discovered and advanced in collaboration with AbCellera (Press Release) PRT7732 Full Global Rights Available Precision ADCs Other Novel Degrader Payloads Multiple Advancing Additional Novel Payload-Antibody Pairings Alone or With Partners Prelude Discovered Programs Available for Partnering PRT2527: Highly selective CDK9 inhibitor (Ph 1, IV) PRT3645: Brain-penetrant CDK4-biased inhibitor (Ph 1, oral) Additional discovery and preclinical stage programs and IP New Discovery Programs Pipeline Update in 1H 2025 Hard-to-treat cancers, “Undruggable” targets, High unmet need Discovery Engine Established track record of delivering a first-or best-in-class new program into the clinic every 12-18 months In The Clinic
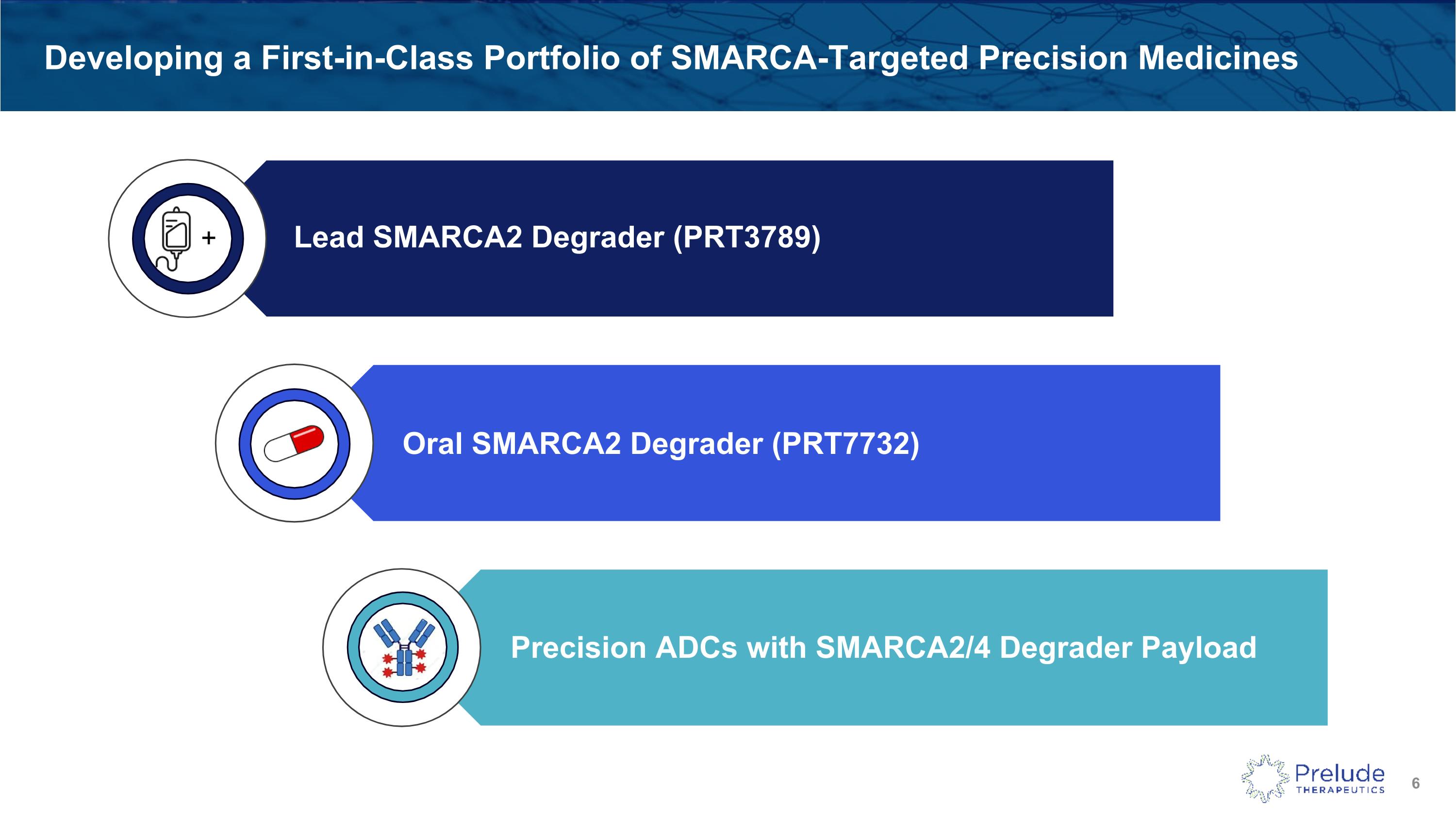
Developing a First-in-Class Portfolio of SMARCA-Targeted Precision Medicines SMARCA - DACs + Lead SMARCA2 Degrader (PRT3789) Oral SMARCA2 Degrader (PRT7732) Precision ADCs with SMARCA2/4 Degrader Payload
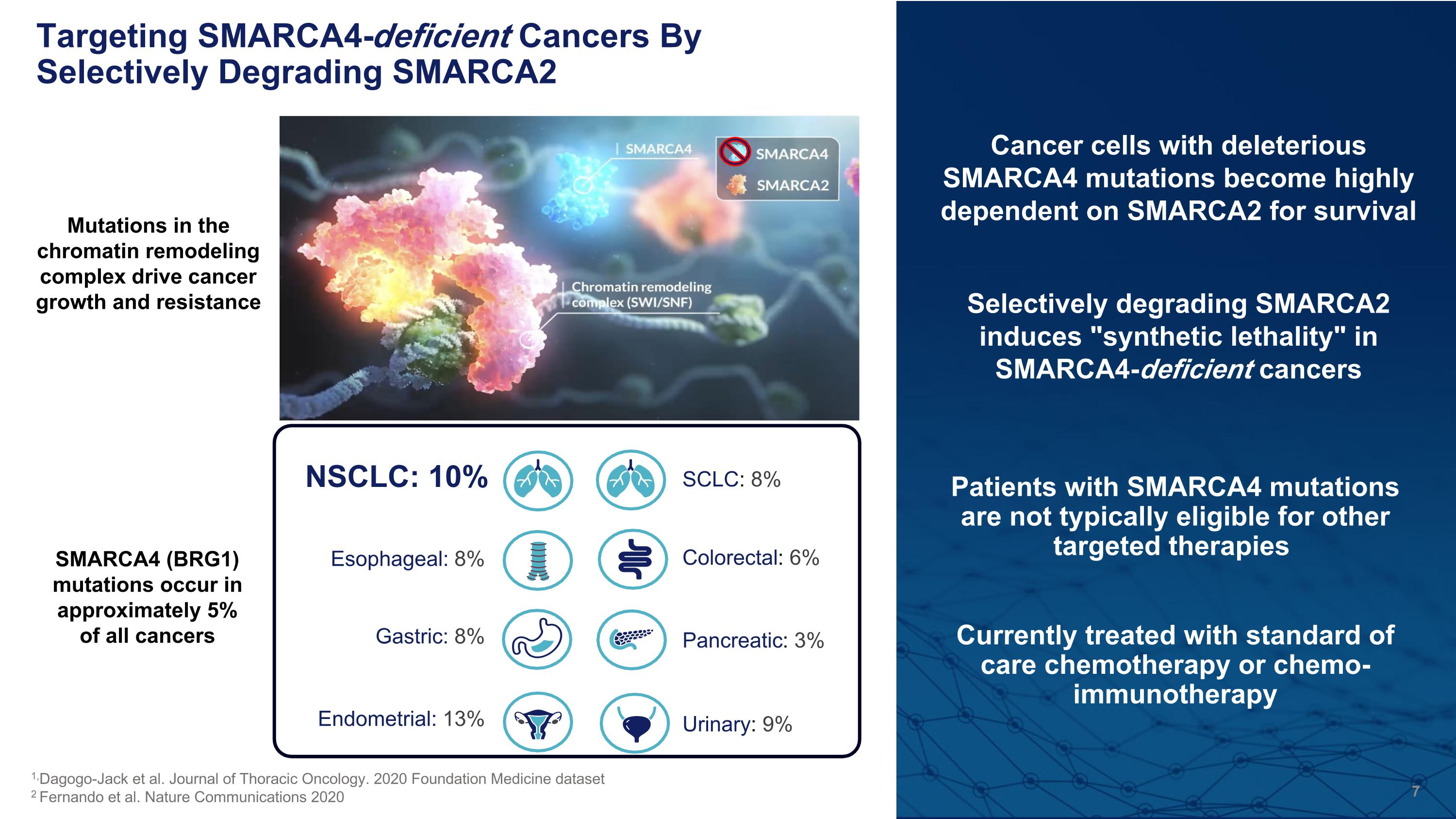
Patients with SMARCA4 mutations are not typically eligible for other targeted therapies Currently treated with standard of care chemotherapy or chemo-immunotherapy Targeting SMARCA4-deficient Cancers By Selectively Degrading SMARCA2 Pancreatic: 3% NSCLC: 10% Esophageal: 8% Gastric: 8% Endometrial: 13% SCLC: 8% Urinary: 9% Colorectal: 6% 1,Dagogo-Jack et al. Journal of Thoracic Oncology. 2020 Foundation Medicine dataset 2 Fernando et al. Nature Communications 2020 SMARCA4 (BRG1) mutations occur in approximately 5% of all cancers Mutations in the chromatin remodeling complex drive cancer growth and resistance Cancer cells with deleterious SMARCA4 mutations become highly dependent on SMARCA2 for survival Selectively degrading SMARCA2 induces "synthetic lethality" in SMARCA4-deficient cancers
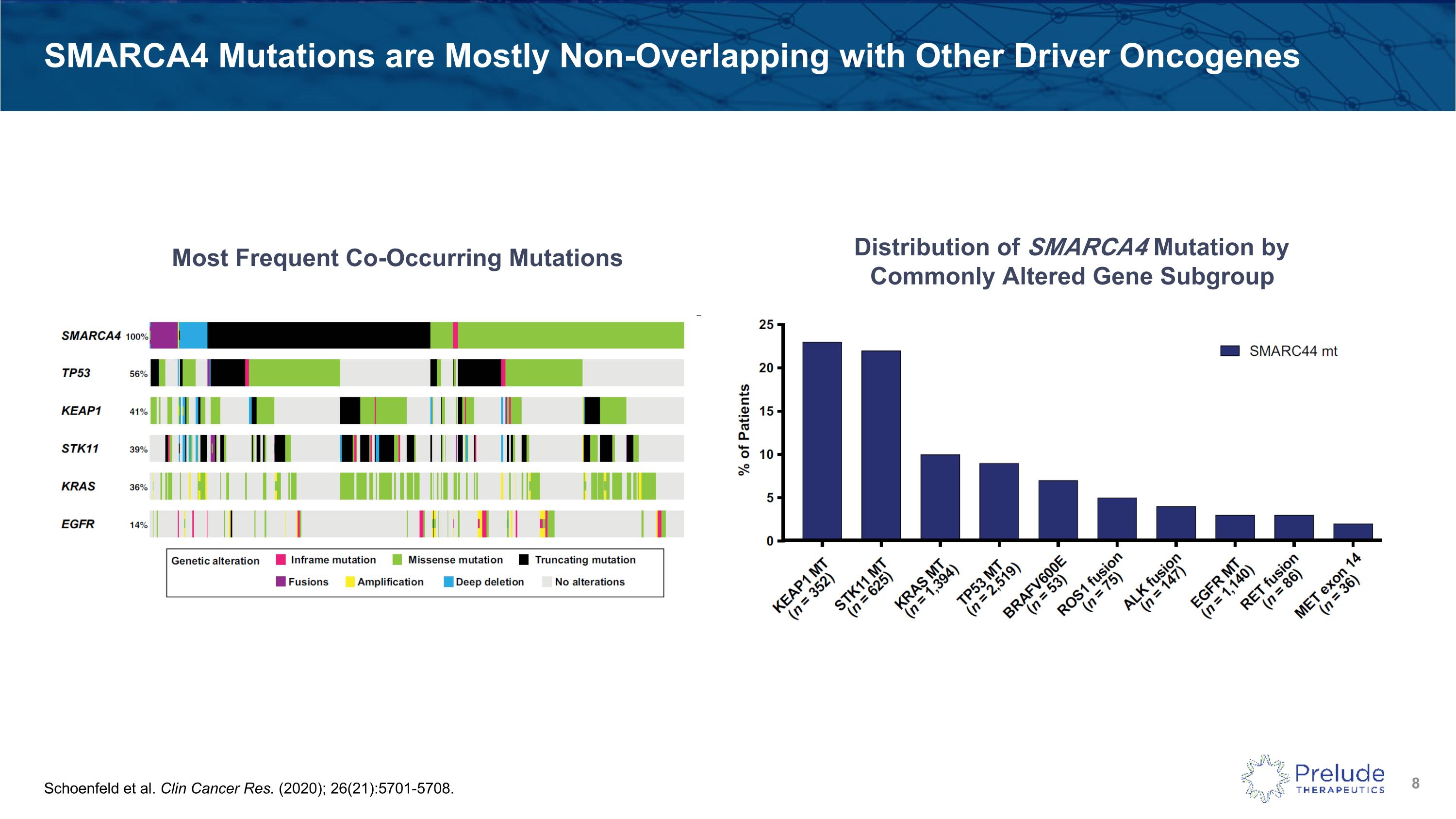
SMARCA4 Mutations are Mostly Non-Overlapping with Other Driver Oncogenes Schoenfeld et al. Clin Cancer Res. (2020); 26(21):5701-5708. Most Frequent Co-Occurring Mutations Distribution of SMARCA4 Mutation by Commonly Altered Gene Subgroup
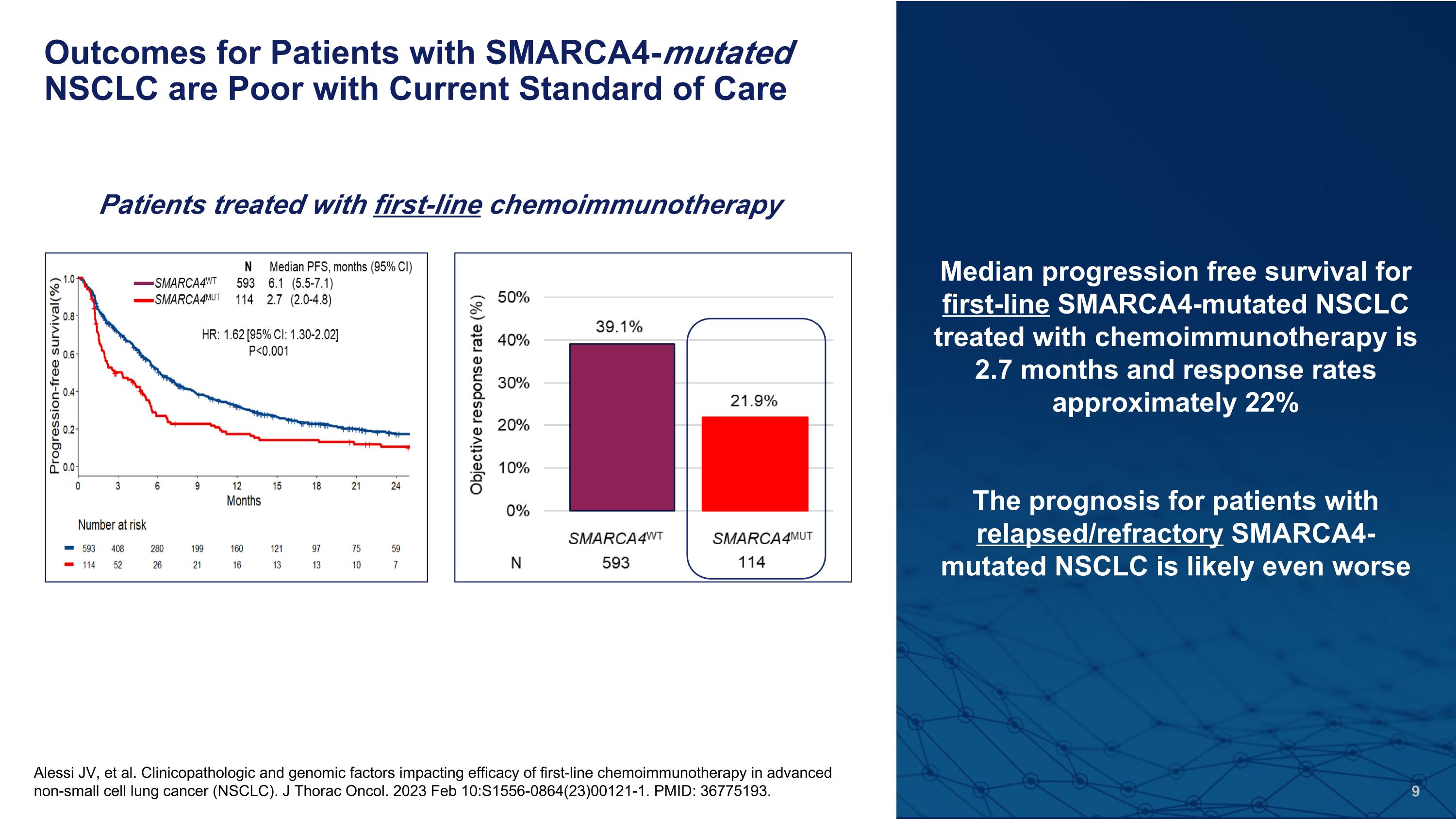
Outcomes for Patients with SMARCA4-mutated NSCLC are Poor with Current Standard of Care Alessi JV, et al. Clinicopathologic and genomic factors impacting efficacy of first-line chemoimmunotherapy in advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2023 Feb 10:S1556-0864(23)00121-1. PMID: 36775193. Median progression free survival for first-line SMARCA4-mutated NSCLC treated with chemoimmunotherapy is 2.7 months and response rates approximately 22% The prognosis for patients with relapsed/refractory SMARCA4-mutated NSCLC is likely even worse Patients treated with first-line chemoimmunotherapy
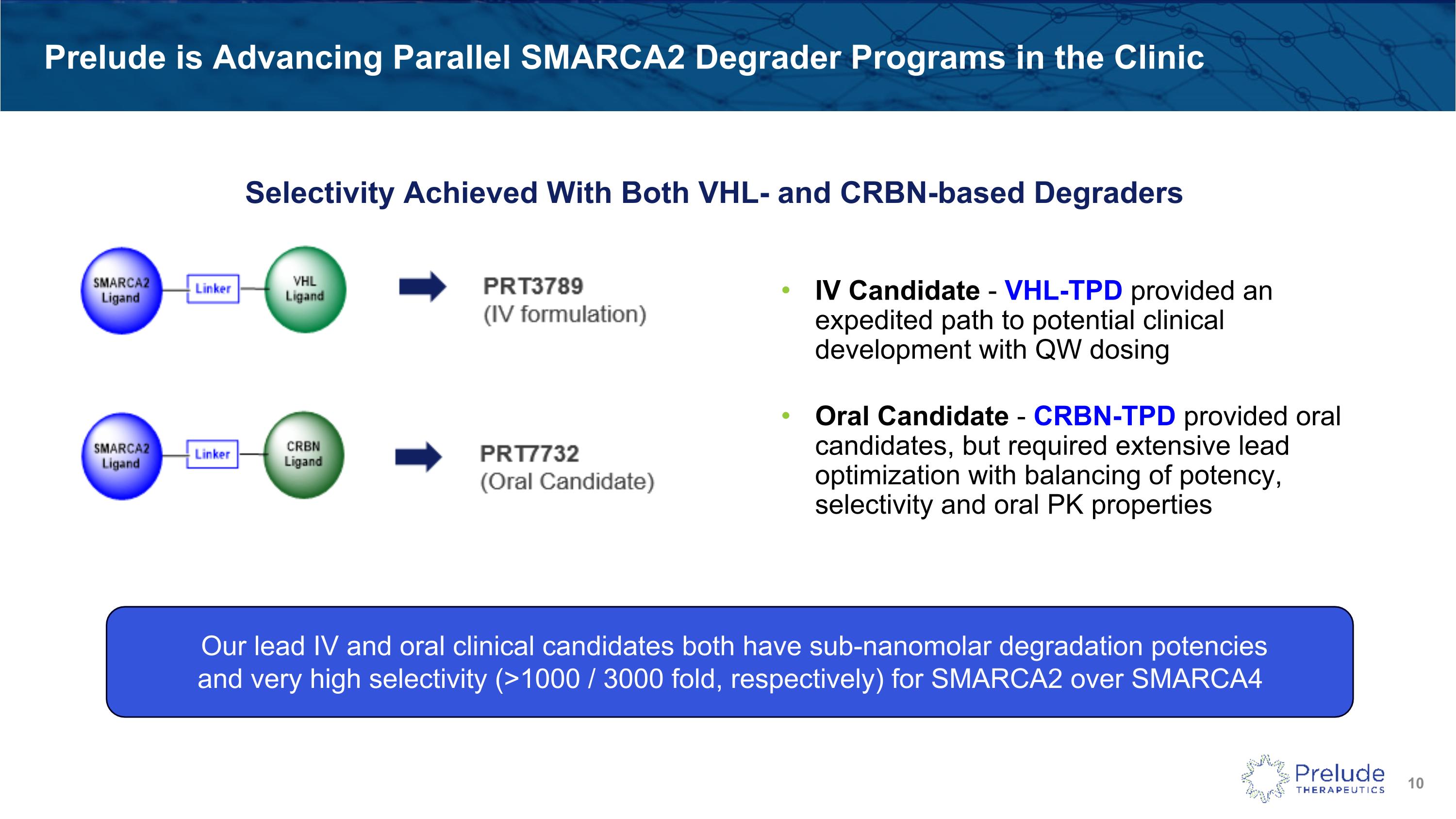
Prelude is Advancing Parallel SMARCA2 Degrader Programs in the Clinic Selectivity Achieved With Both VHL- and CRBN-based Degraders IV Candidate - VHL-TPD provided an expedited path to potential clinical development with QW dosing� Oral Candidate - CRBN-TPD provided oral candidates, but required extensive lead optimization with balancing of potency, selectivity and oral PK properties Our lead IV and oral clinical candidates both have sub-nanomolar degradation potencies �and very high selectivity (>1000 / 3000 fold, respectively) for SMARCA2 over SMARCA4
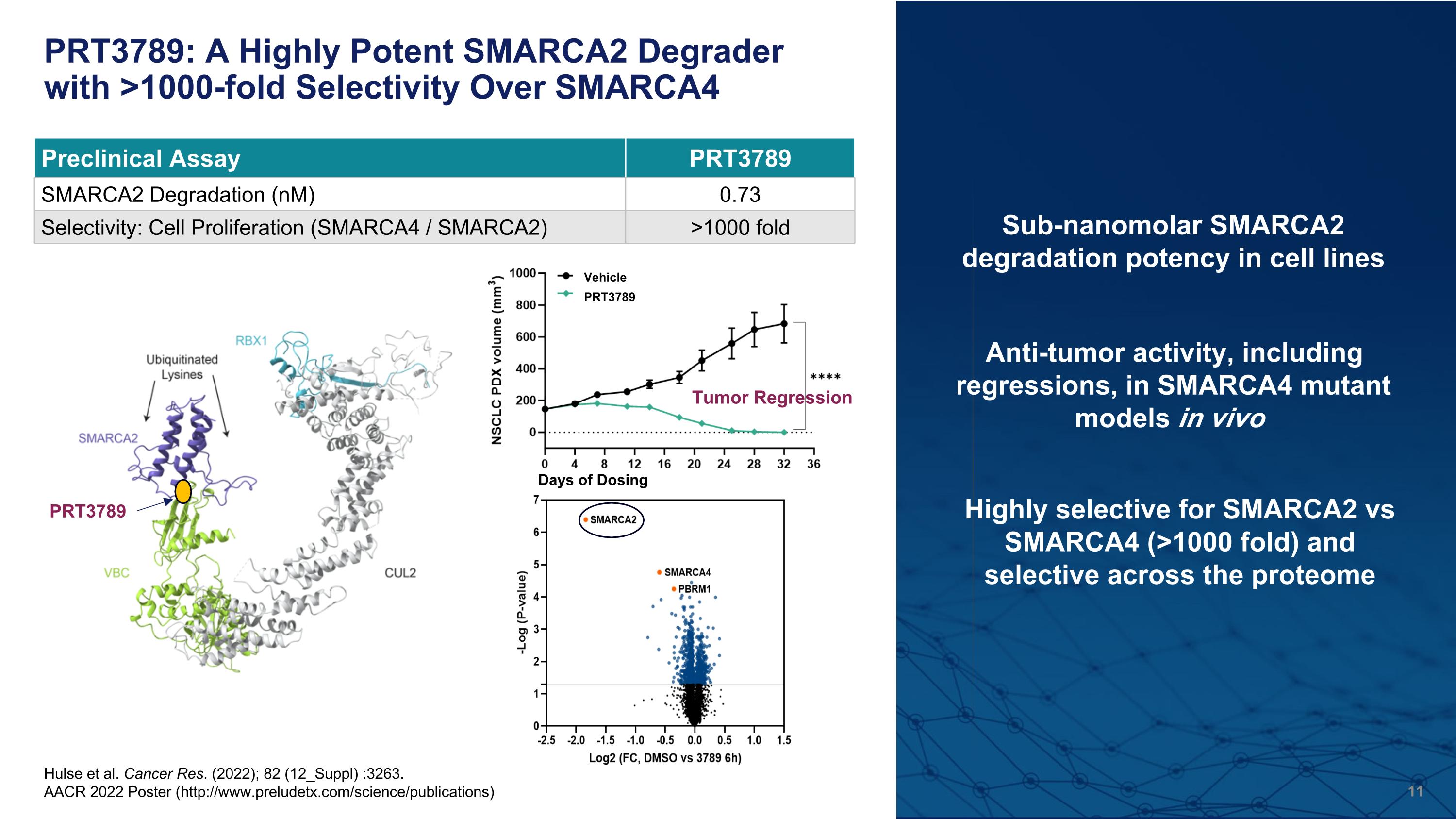
PRT3789: A Highly Potent SMARCA2 Degrader with >1000-fold Selectivity Over SMARCA4 Hulse et al. Cancer Res. (2022); 82 (12_Suppl) :3263. AACR 2022 Poster (http://www.preludetx.com/science/publications) Highly selective for SMARCA2 vs SMARCA4 (>1000 fold) and�selective across the proteome Preclinical Assay PRT3789 SMARCA2 Degradation (nM) 0.73 Selectivity: Cell Proliferation (SMARCA4 / SMARCA2) >1000 fold Sub-nanomolar SMARCA2 degradation potency in cell lines Anti-tumor activity, including regressions, in SMARCA4 mutant models in vivo PRT3789 Tumor Regression PRT3789 Vehicle Days of Dosing
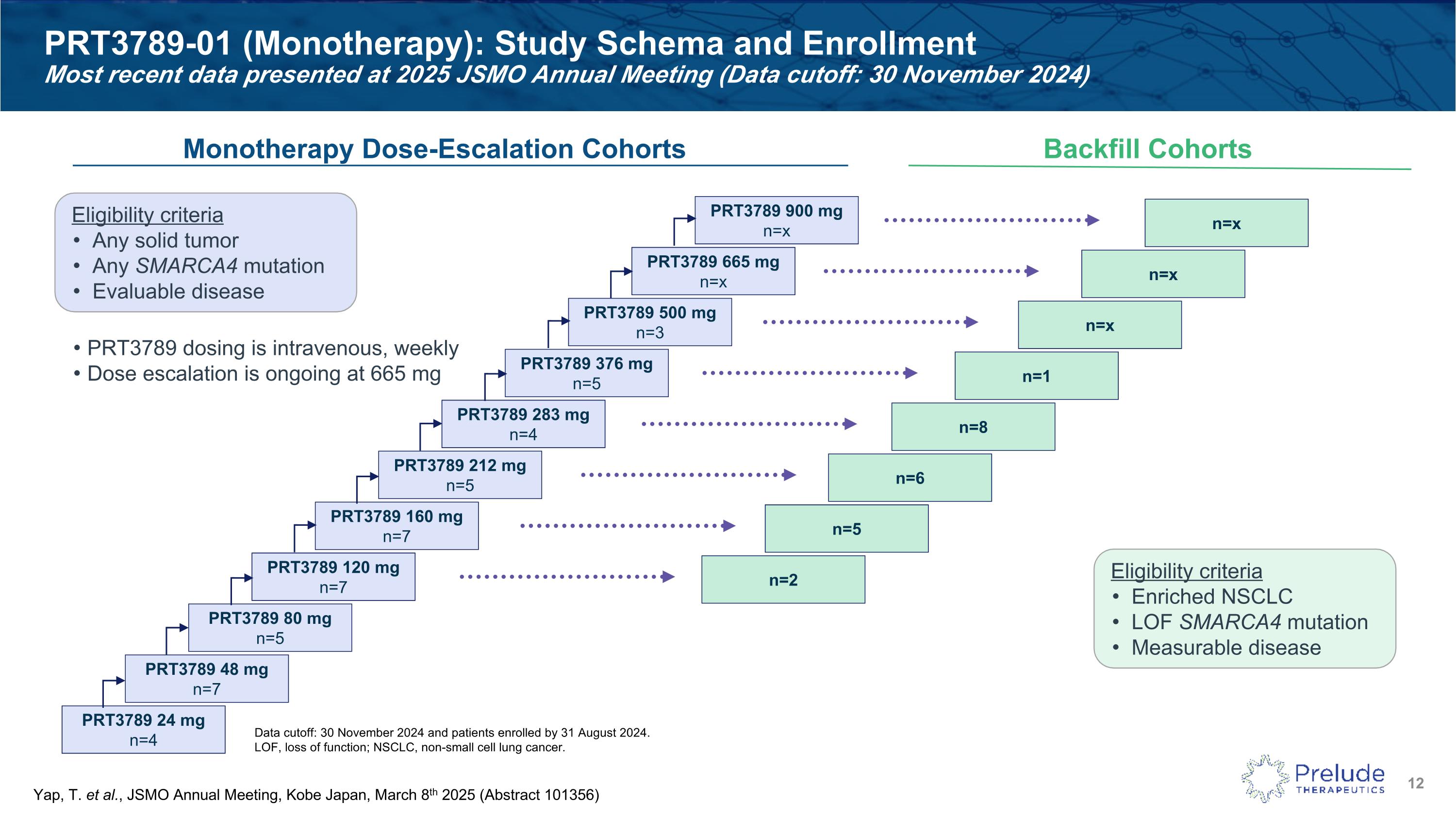
Data cutoff: 30 November 2024 and patients enrolled by 31 August 2024. LOF, loss of function; NSCLC, non-small cell lung cancer. PRT3789-01 (Monotherapy): Study Schema and Enrollment�Most recent data presented at 2025 JSMO Annual Meeting (Data cutoff: 30 November 2024) Monotherapy Dose-Escalation Cohorts Backfill Cohorts Eligibility criteria Any solid tumor Any SMARCA4 mutation Evaluable disease PRT3789 dosing is intravenous, weekly Dose escalation is ongoing at 665 mg PRT3789 24 mg n=4 Eligibility criteria Enriched NSCLC LOF SMARCA4 mutation Measurable disease PRT3789 48 mg n=7 PRT3789 80 mg n=5 PRT3789 120 mg n=7 PRT3789 160 mg n=7 PRT3789 212 mg n=5 PRT3789 283 mg n=4 PRT3789 376 mg n=5 PRT3789 500 mg n=3 PRT3789 665 mg n=x PRT3789 900 mg n=x n=2 n=5 n=6 n=8 n=1 n=x n=x n=x Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
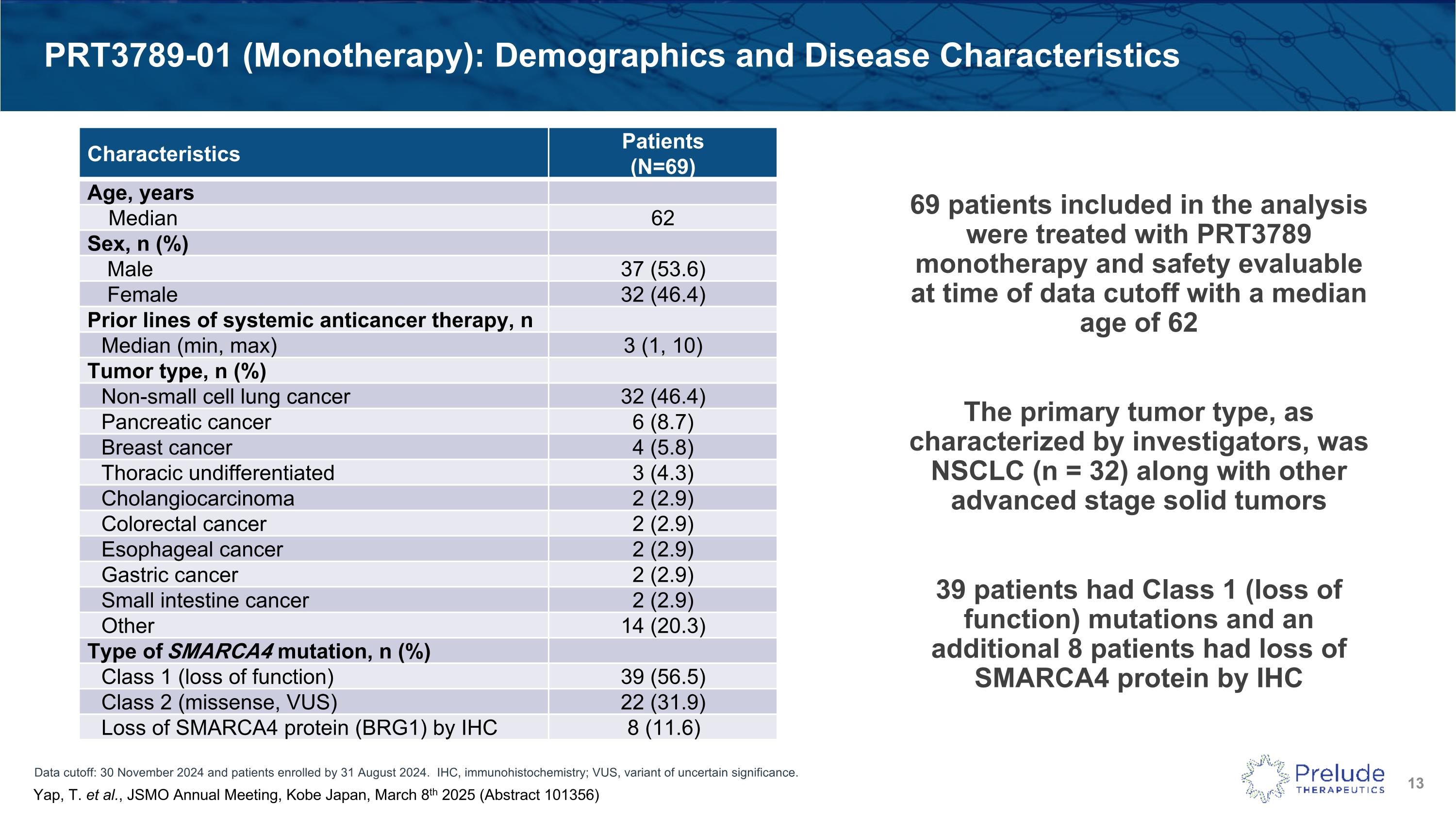
Data cutoff: 30 November 2024 and patients enrolled by 31 August 2024. IHC, immunohistochemistry; VUS, variant of uncertain significance. PRT3789-01 (Monotherapy): Demographics and Disease Characteristics Characteristics Patients (N=69) Age, years Median 62 Sex, n (%) Male 37 (53.6) Female 32 (46.4) Prior lines of systemic anticancer therapy, n Median (min, max) 3 (1, 10) Tumor type, n (%) Non-small cell lung cancer 32 (46.4) Pancreatic cancer 6 (8.7) Breast cancer 4 (5.8) Thoracic undifferentiated 3 (4.3) Cholangiocarcinoma 2 (2.9) Colorectal cancer 2 (2.9) Esophageal cancer 2 (2.9) Gastric cancer 2 (2.9) Small intestine cancer 2 (2.9) Other 14 (20.3) Type of SMARCA4 mutation, n (%) Class 1 (loss of function) 39 (56.5) Class 2 (missense, VUS) 22 (31.9) Loss of SMARCA4 protein (BRG1) by IHC 8 (11.6) Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356) 69 patients included in the analysis were treated with PRT3789 monotherapy and safety evaluable at time of data cutoff with a median age of 62 The primary tumor type, as characterized by investigators, was NSCLC (n = 32) along with other advanced stage solid tumors 39 patients had Class 1 (loss of function) mutations and an additional 8 patients had loss of SMARCA4 protein by IHC
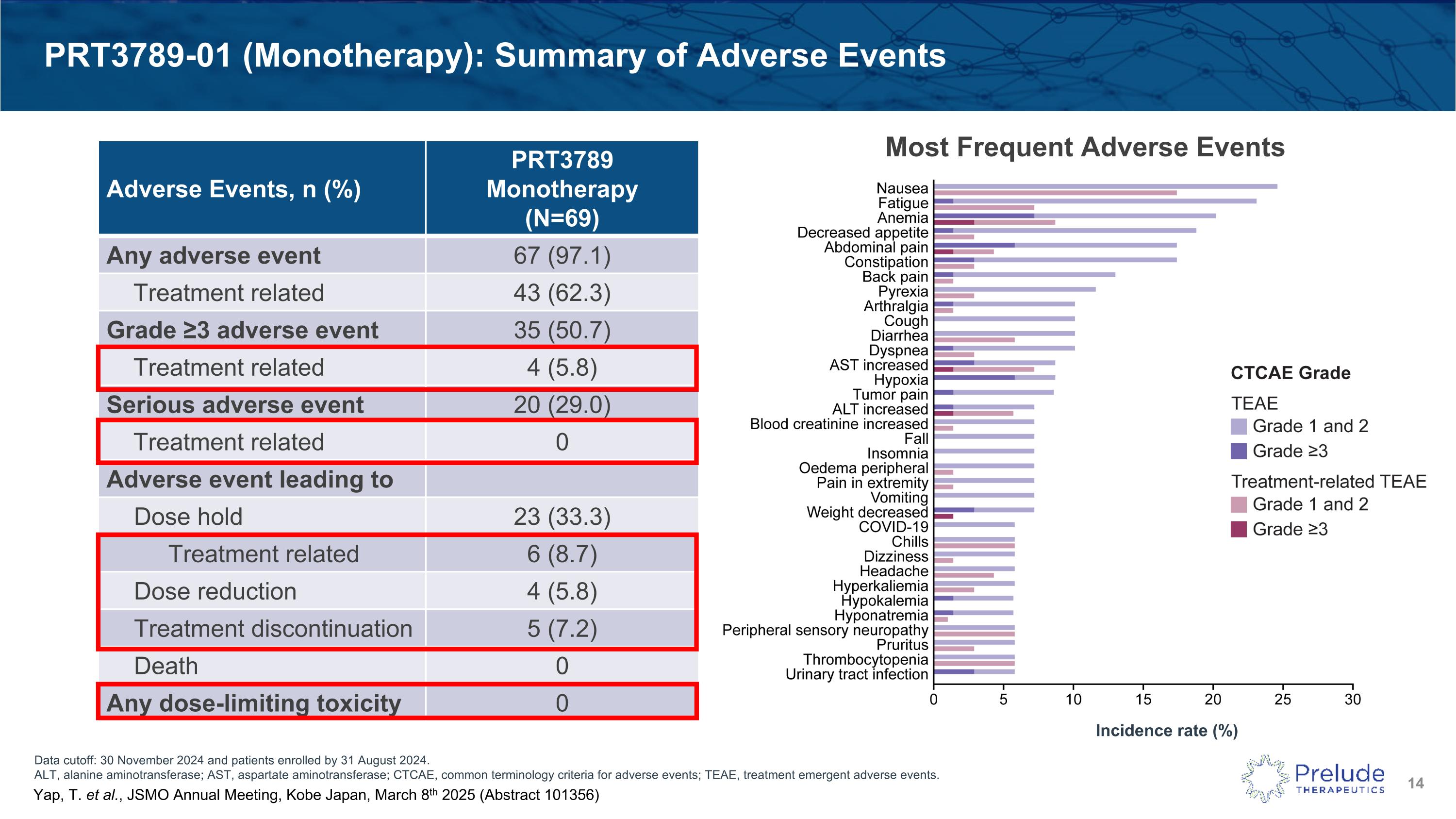
Data cutoff: 30 November 2024 and patients enrolled by 31 August 2024. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, common terminology criteria for adverse events; TEAE, treatment emergent adverse events. PRT3789-01 (Monotherapy): Summary of Adverse Events Adverse Events, n (%) PRT3789 Monotherapy (N=69) Any adverse event 67 (97.1) Treatment related 43 (62.3) Grade ≥3 adverse event 35 (50.7) Treatment related 4 (5.8) Serious adverse event 20 (29.0) Treatment related 0 Adverse event leading to Dose hold 23 (33.3) Treatment related 6 (8.7) Dose reduction 4 (5.8) Treatment discontinuation 5 (7.2) Death 0 Any dose-limiting toxicity 0 Most Frequent Adverse Events Incidence rate (%) Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
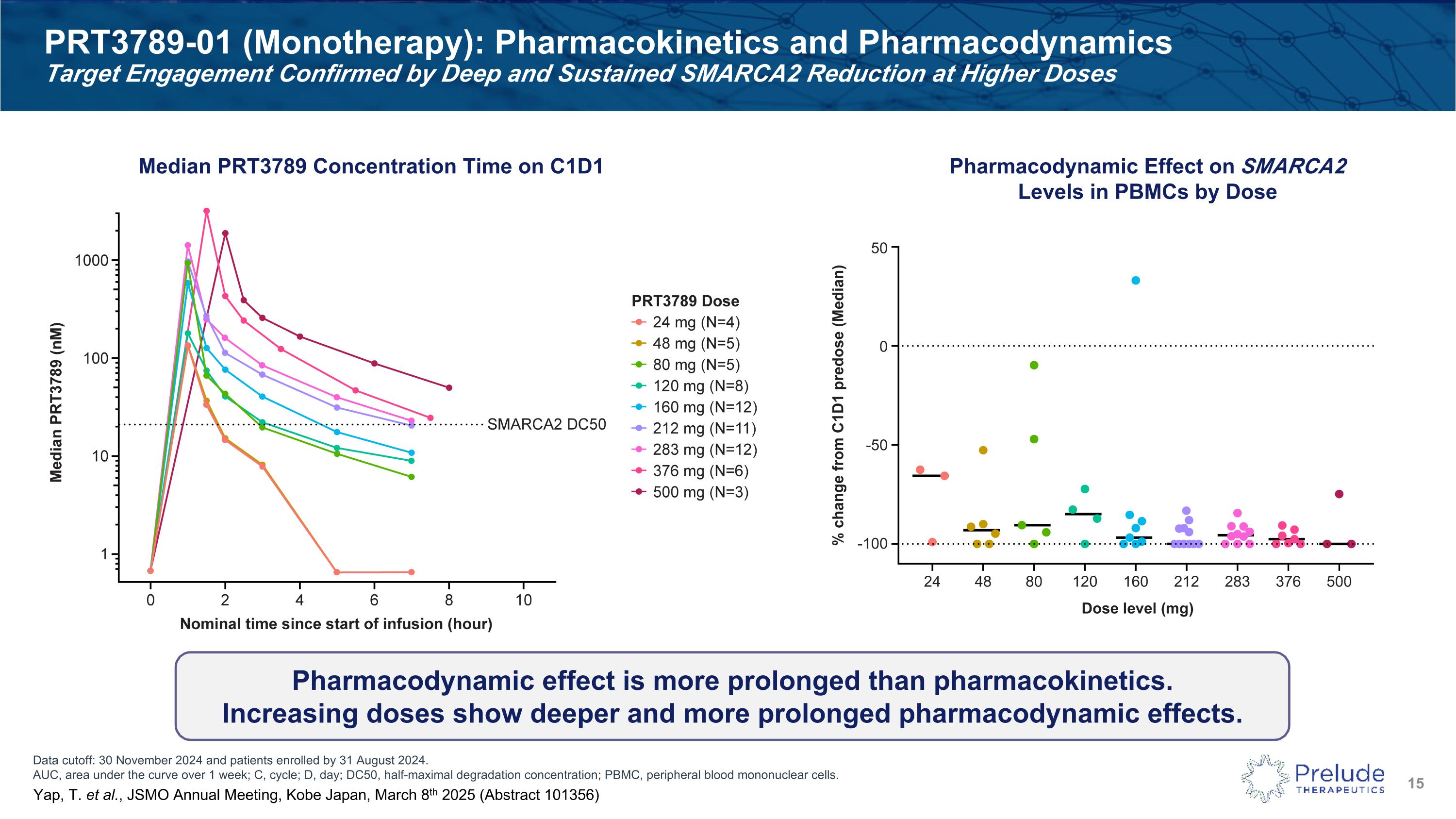
Data cutoff: 30 November 2024 and patients enrolled by 31 August 2024. AUC, area under the curve over 1 week; C, cycle; D, day; DC50, half-maximal degradation concentration; PBMC, peripheral blood mononuclear cells. PRT3789-01 (Monotherapy): Pharmacokinetics and Pharmacodynamics �Target Engagement Confirmed by Deep and Sustained SMARCA2 Reduction at Higher Doses Pharmacodynamic effect is more prolonged than pharmacokinetics. Increasing doses show deeper and more prolonged pharmacodynamic effects. Median PRT3789 Concentration Time on C1D1 Pharmacodynamic Effect on SMARCA2 Levels in PBMCs by Dose Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
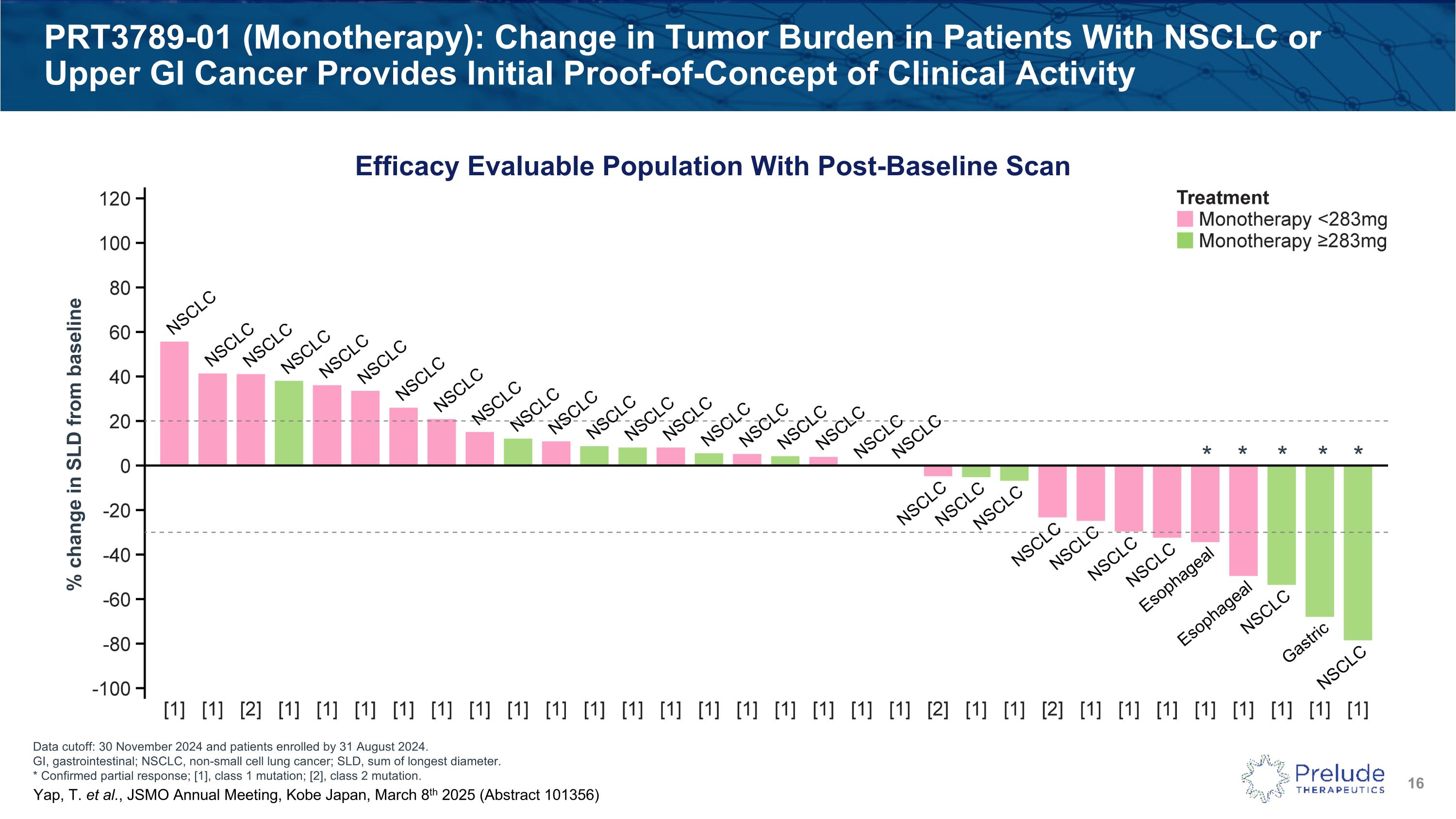
Data cutoff: 30 November 2024 and patients enrolled by 31 August 2024. GI, gastrointestinal; NSCLC, non-small cell lung cancer; SLD, sum of longest diameter. * Confirmed partial response; [1], class 1 mutation; [2], class 2 mutation. PRT3789-01 (Monotherapy): Change in Tumor Burden in Patients With NSCLC or Upper GI Cancer Provides Initial Proof-of-Concept of Clinical Activity Efficacy Evaluable Population With Post-Baseline Scan % change in SLD from baseline * * * * * Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
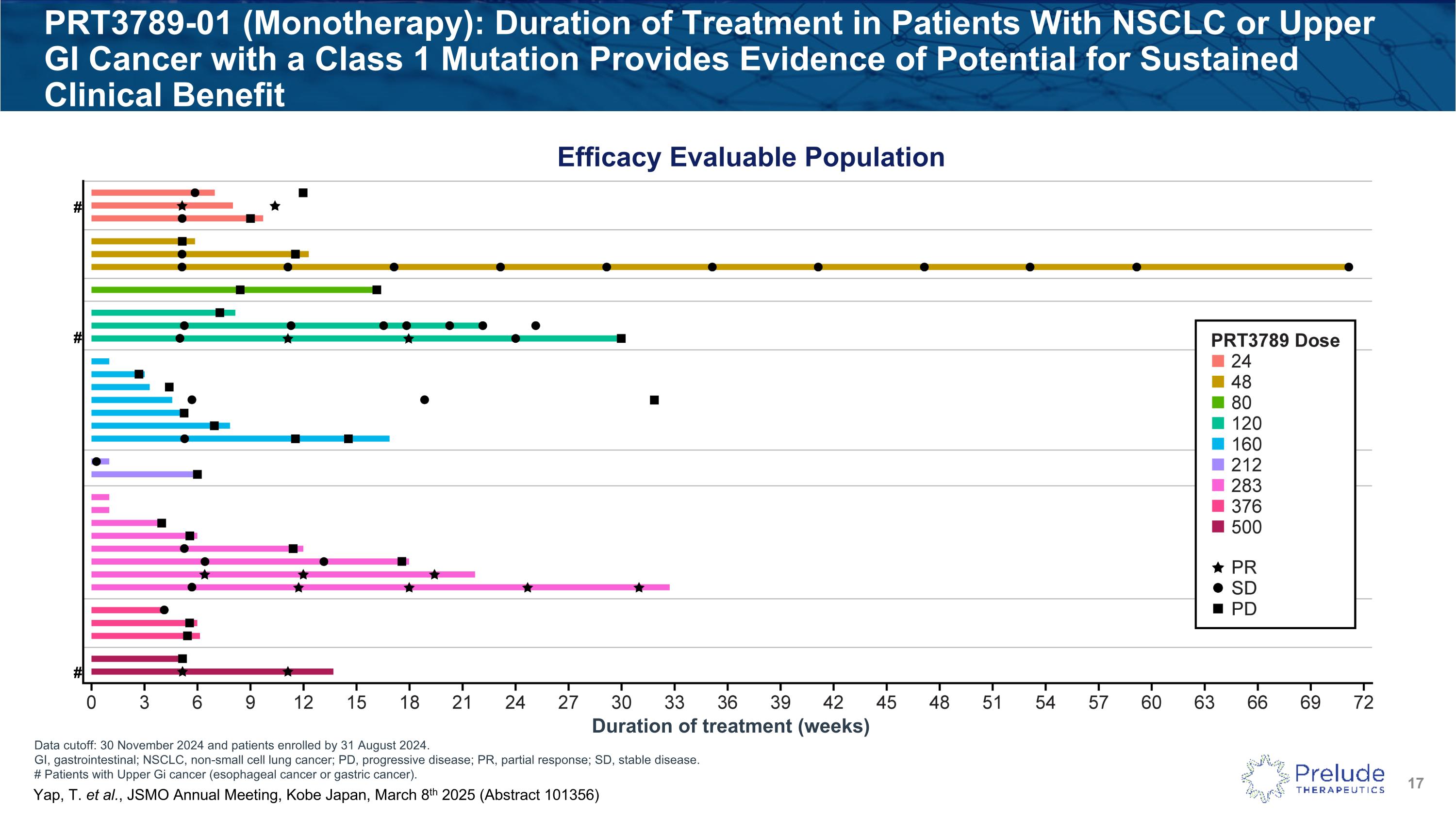
Data cutoff: 30 November 2024 and patients enrolled by 31 August 2024. GI, gastrointestinal; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease. # Patients with Upper Gi cancer (esophageal cancer or gastric cancer). PRT3789-01 (Monotherapy): Duration of Treatment in Patients With NSCLC or Upper GI Cancer with a Class 1 Mutation Provides Evidence of Potential for Sustained Clinical Benefit Efficacy Evaluable Population # # # Duration of treatment (weeks) Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
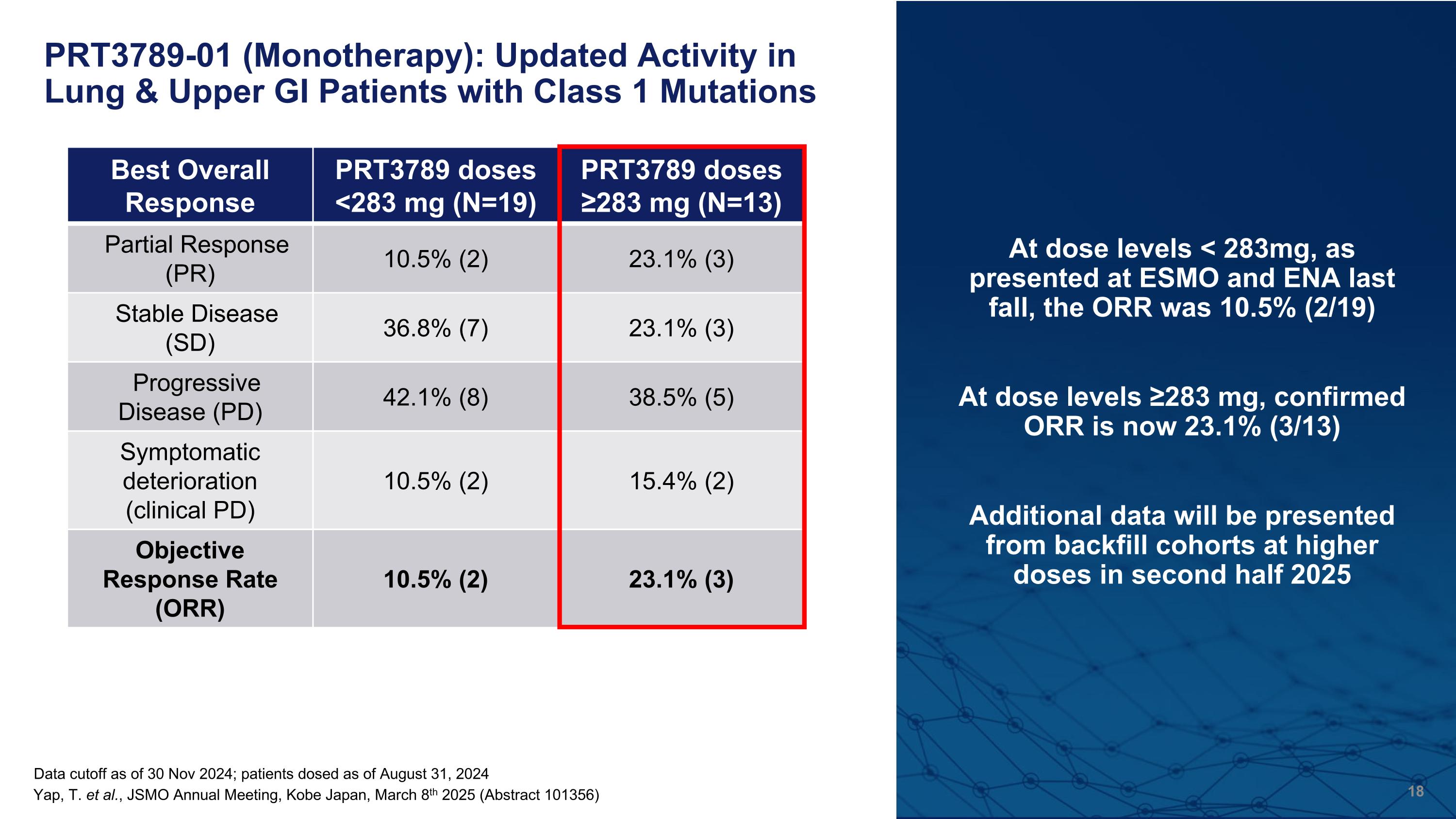
Data cutoff as of 30 Nov 2024; patients dosed as of August 31, 2024 PRT3789-01 (Monotherapy): Updated Activity in Lung & Upper GI Patients with Class 1 Mutations Best Overall Response PRT3789 doses <283 mg (N=19) PRT3789 doses ≥283 mg (N=13) Partial Response (PR) 10.5% (2) 23.1% (3) Stable Disease (SD) 36.8% (7) 23.1% (3) Progressive Disease (PD) 42.1% (8) 38.5% (5) Symptomatic deterioration (clinical PD) 10.5% (2) 15.4% (2) Objective Response Rate (ORR) 10.5% (2) 23.1% (3) At dose levels < 283mg, as presented at ESMO and ENA last fall, the ORR was 10.5% (2/19) At dose levels ≥283 mg, confirmed ORR is now 23.1% (3/13) Additional data will be presented from backfill cohorts at higher doses in second half 2025 Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
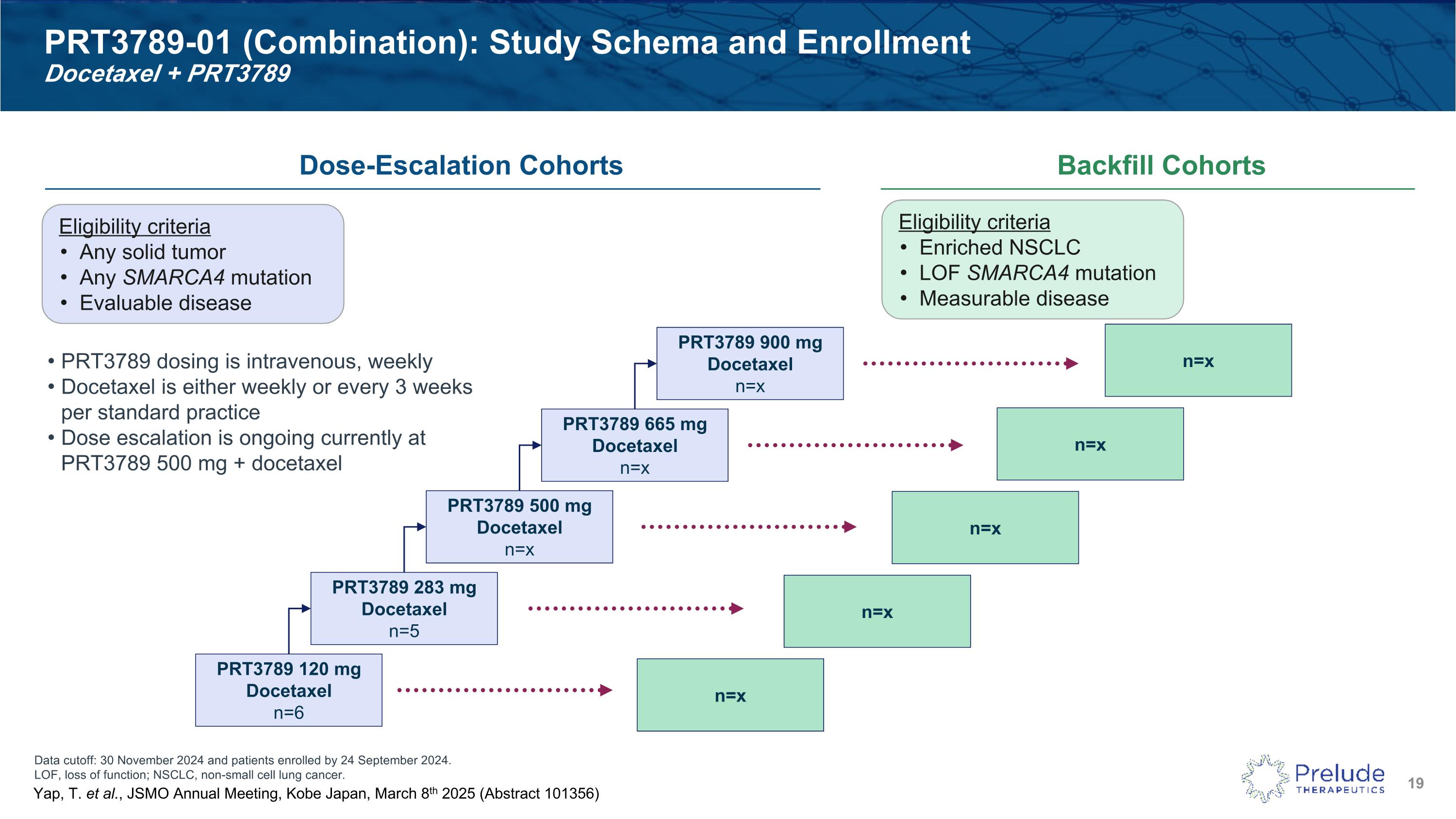
Data cutoff: 30 November 2024 and patients enrolled by 24 September 2024. LOF, loss of function; NSCLC, non-small cell lung cancer. PRT3789-01 (Combination): Study Schema and Enrollment�Docetaxel + PRT3789 Dose-Escalation Cohorts Backfill Cohorts Eligibility criteria Any solid tumor Any SMARCA4 mutation Evaluable disease PRT3789 dosing is intravenous, weekly Docetaxel is either weekly or every 3 weeks per standard practice Dose escalation is ongoing currently at PRT3789 500 mg + docetaxel PRT3789 120 mg Docetaxel n=6 PRT3789 283 mg Docetaxel n=5 PRT3789 500 mg Docetaxel n=x PRT3789 665 mg Docetaxel n=x PRT3789 900 mg Docetaxel n=x Eligibility criteria Enriched NSCLC LOF SMARCA4 mutation Measurable disease n=x n=x n=x n=x n=x Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
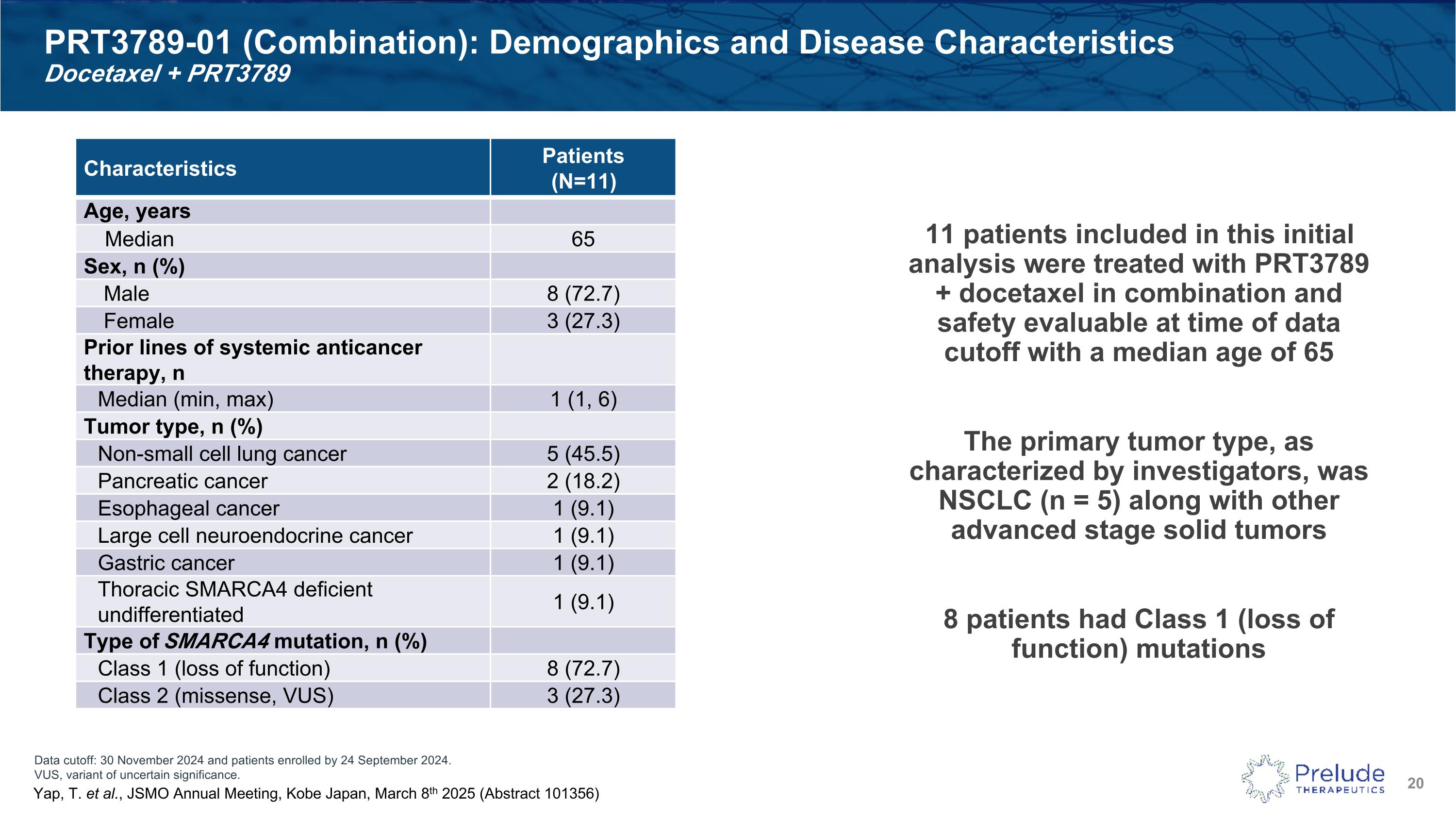
Data cutoff: 30 November 2024 and patients enrolled by 24 September 2024. VUS, variant of uncertain significance. PRT3789-01 (Combination): Demographics and Disease Characteristics�Docetaxel + PRT3789 Characteristics Patients (N=11) Age, years Median 65 Sex, n (%) Male 8 (72.7) Female 3 (27.3) Prior lines of systemic anticancer therapy, n Median (min, max) 1 (1, 6) Tumor type, n (%) Non-small cell lung cancer 5 (45.5) Pancreatic cancer 2 (18.2) Esophageal cancer 1 (9.1) Large cell neuroendocrine cancer 1 (9.1) Gastric cancer 1 (9.1) Thoracic SMARCA4 deficient undifferentiated 1 (9.1) Type of SMARCA4 mutation, n (%) Class 1 (loss of function) 8 (72.7) Class 2 (missense, VUS) 3 (27.3) Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356) 11 patients included in this initial analysis were treated with PRT3789 + docetaxel in combination and safety evaluable at time of data cutoff with a median age of 65 The primary tumor type, as characterized by investigators, was NSCLC (n = 5) along with other advanced stage solid tumors 8 patients had Class 1 (loss of function) mutations
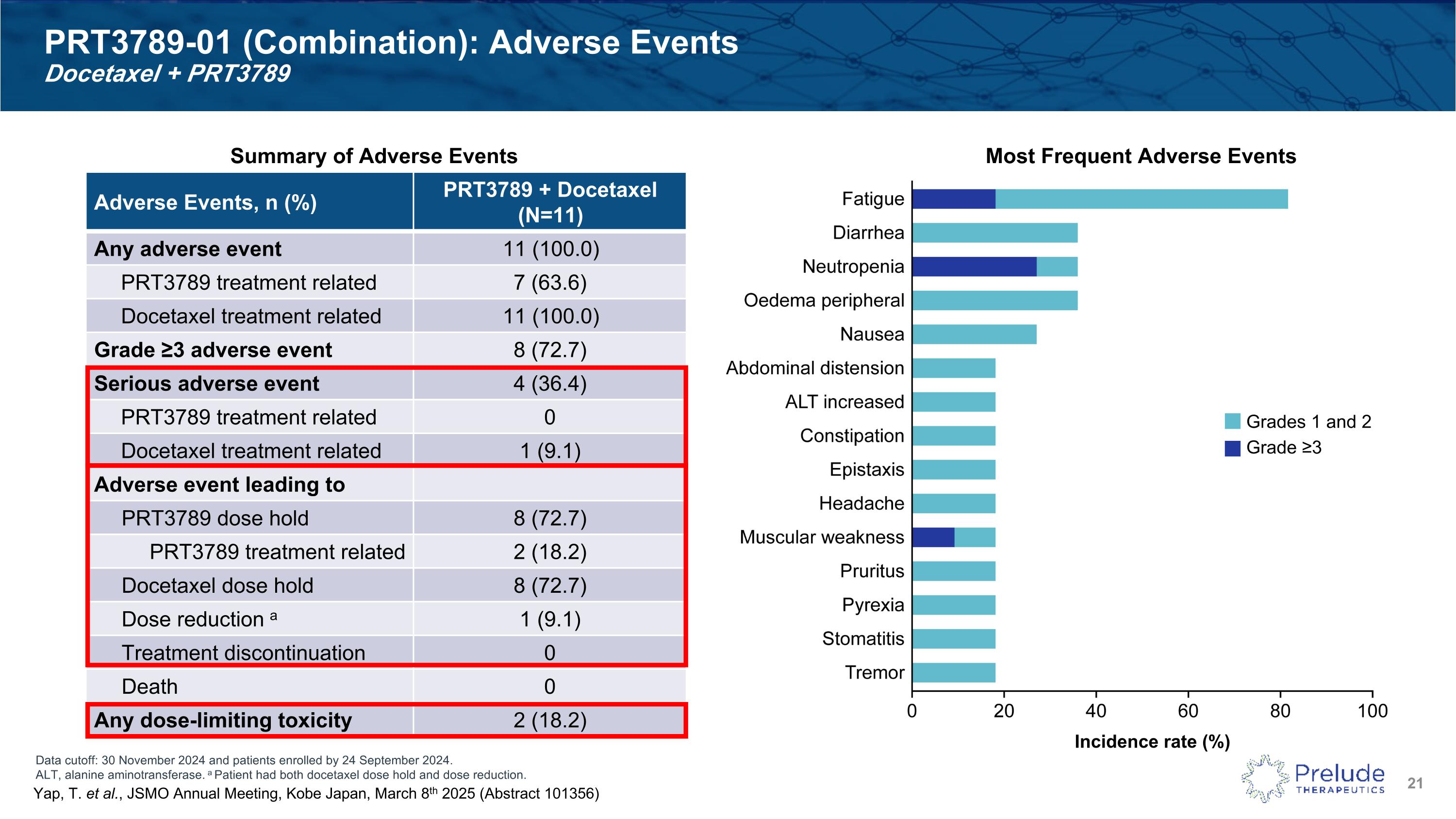
Data cutoff: 30 November 2024 and patients enrolled by 24 September 2024. ALT, alanine aminotransferase. a Patient had both docetaxel dose hold and dose reduction. PRT3789-01 (Combination): Adverse Events�Docetaxel + PRT3789 Adverse Events, n (%) PRT3789 + Docetaxel (N=11) Any adverse event 11 (100.0) PRT3789 treatment related 7 (63.6) Docetaxel treatment related 11 (100.0) Grade ≥3 adverse event 8 (72.7) Serious adverse event 4 (36.4) PRT3789 treatment related 0 Docetaxel treatment related 1 (9.1) Adverse event leading to PRT3789 dose hold 8 (72.7) PRT3789 treatment related 2 (18.2) Docetaxel dose hold 8 (72.7) Dose reduction a 1 (9.1) Treatment discontinuation 0 Death 0 Any dose-limiting toxicity 2 (18.2) Summary of Adverse Events Most Frequent Adverse Events Incidence rate (%) Grades 1 and 2 Grade ≥3 Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
Prelude’s First-in-Class Oral SMARCA2 Degrader Has Several Advantages + Lead SMARCA2 Degrader (PRT3789) Oral SMARCA2 Degrader (PRT7732) Distinct new chemical entity with PK supporting once-daily dosing Potential to expand patient access and provide optionality Phase I underway and enrollment proceeding rapidly Precision ADCs with SMARCA2/4 Dual Degrader Payload
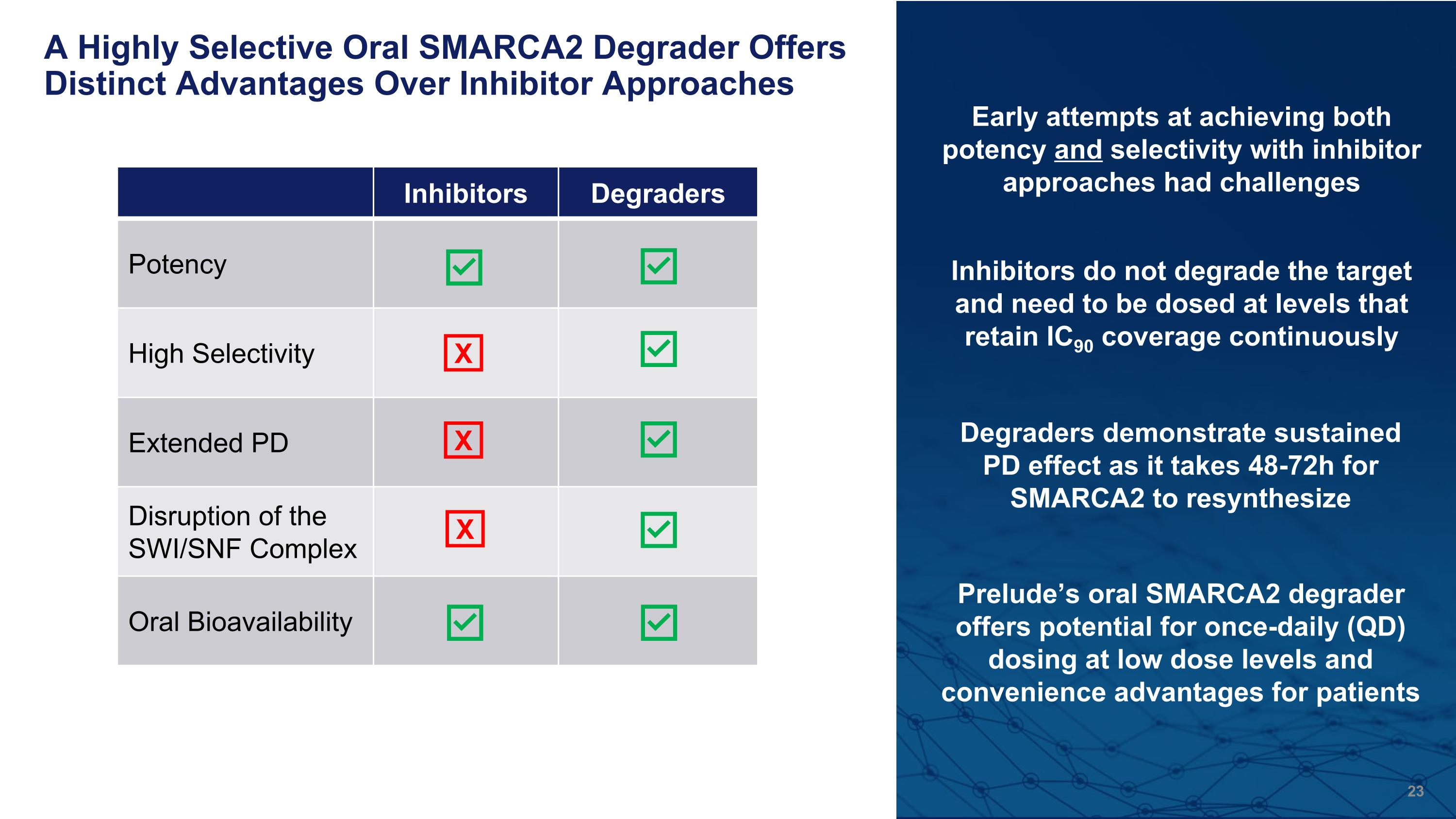
A Highly Selective Oral SMARCA2 Degrader Offers Distinct Advantages Over Inhibitor Approaches Inhibitors Degraders Potency High Selectivity Extended PD Disruption of the SWI/SNF Complex Oral Bioavailability X X Early attempts at achieving both potency and selectivity with inhibitor approaches had challenges Inhibitors do not degrade the target and need to be dosed at levels that retain IC90 coverage continuously Degraders demonstrate sustained PD effect as it takes 48-72h for SMARCA2 to resynthesize Prelude’s oral SMARCA2 degrader offers potential for once-daily (QD) dosing at low dose levels and convenience advantages for patients X
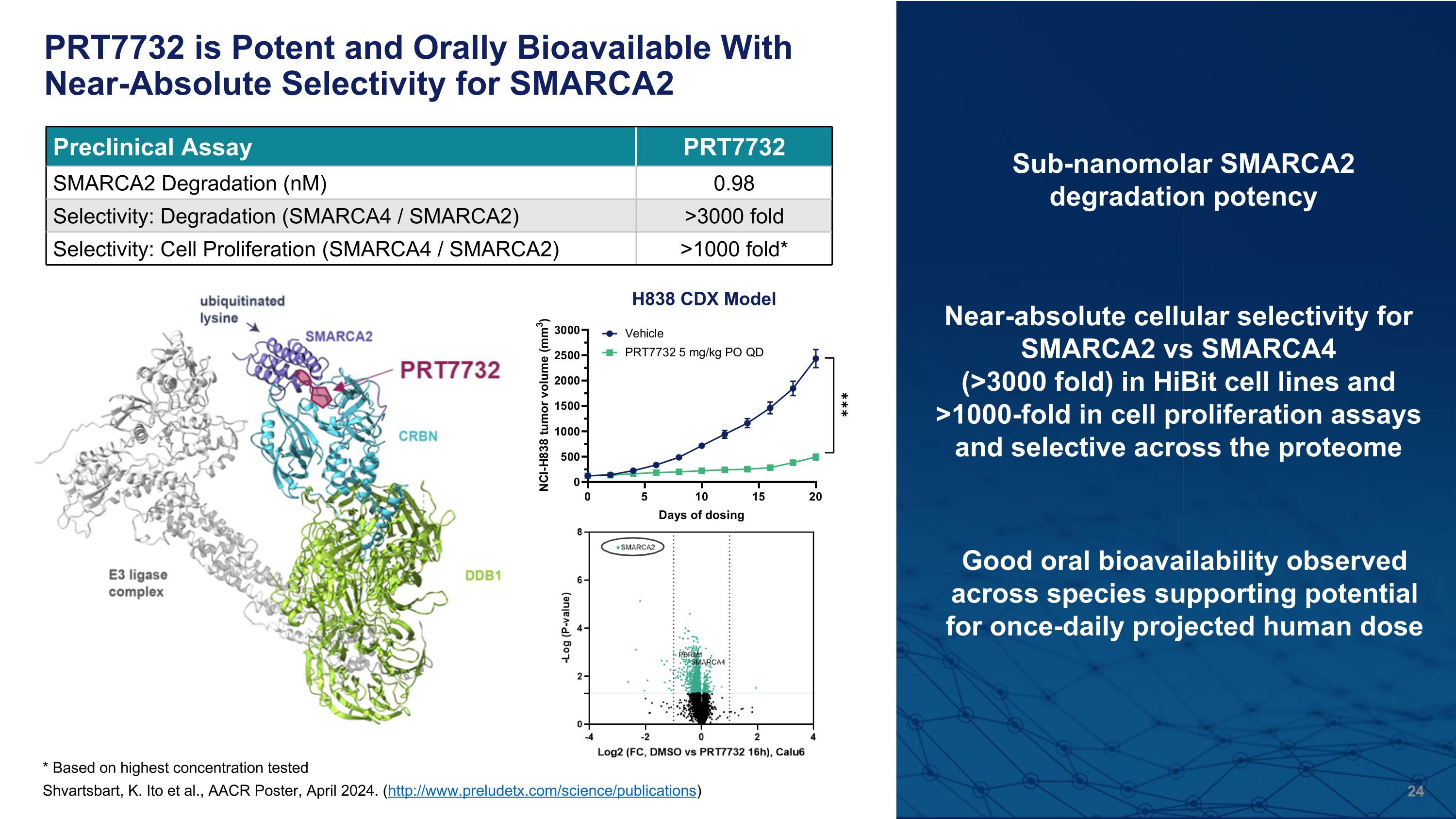
PRT7732 is Potent and Orally Bioavailable With Near-Absolute Selectivity for SMARCA2 Near-absolute cellular selectivity for SMARCA2 vs SMARCA4�(>3000 fold) in HiBit cell lines and >1000-fold in cell proliferation assays and selective across the proteome Sub-nanomolar SMARCA2 degradation potency * Based on highest concentration tested Good oral bioavailability observed across species supporting potential for once-daily projected human dose Preclinical Assay PRT7732 SMARCA2 Degradation (nM) 0.98 Selectivity: Degradation (SMARCA4 / SMARCA2) >3000 fold Selectivity: Cell Proliferation (SMARCA4 / SMARCA2) >1000 fold* Shvartsbart, K. Ito et al., AACR Poster, April 2024. (http://www.preludetx.com/science/publications) H838 CDX Model
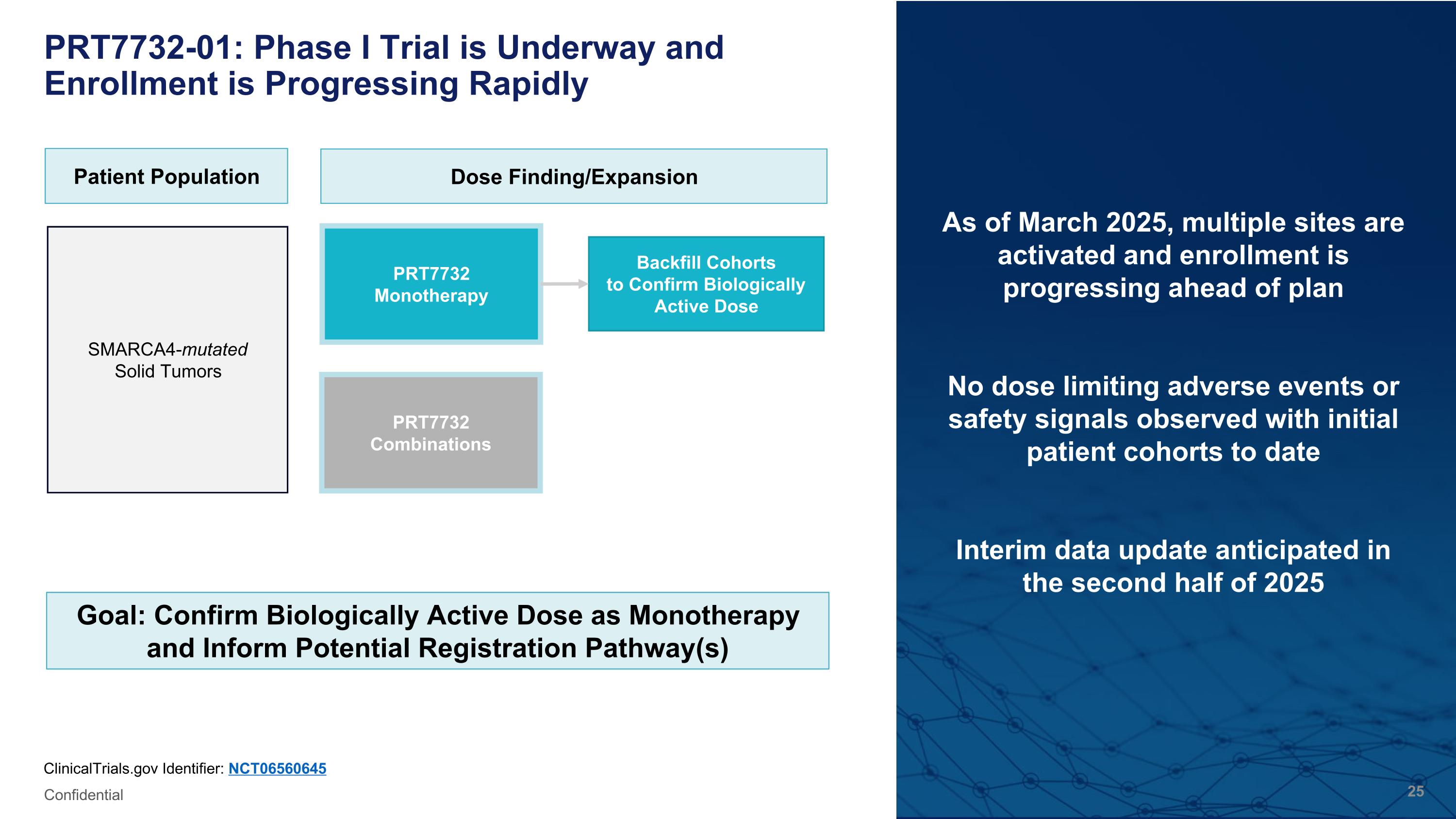
PRT7732-01: Phase I Trial is Underway and Enrollment is Progressing Rapidly As of March 2025, multiple sites are activated and enrollment is progressing ahead of plan No dose limiting adverse events or safety signals observed with initial patient cohorts to date Interim data update anticipated in the second half of 2025 ClinicalTrials.gov Identifier: NCT06560645 Patient Population Dose Finding/Expansion PRT7732 Monotherapy SMARCA4-mutated �Solid Tumors Backfill Cohorts�to Confirm Biologically Active Dose Goal: Confirm Biologically Active Dose as Monotherapy and Inform Potential Registration Pathway(s) PRT7732 Combinations Confidential
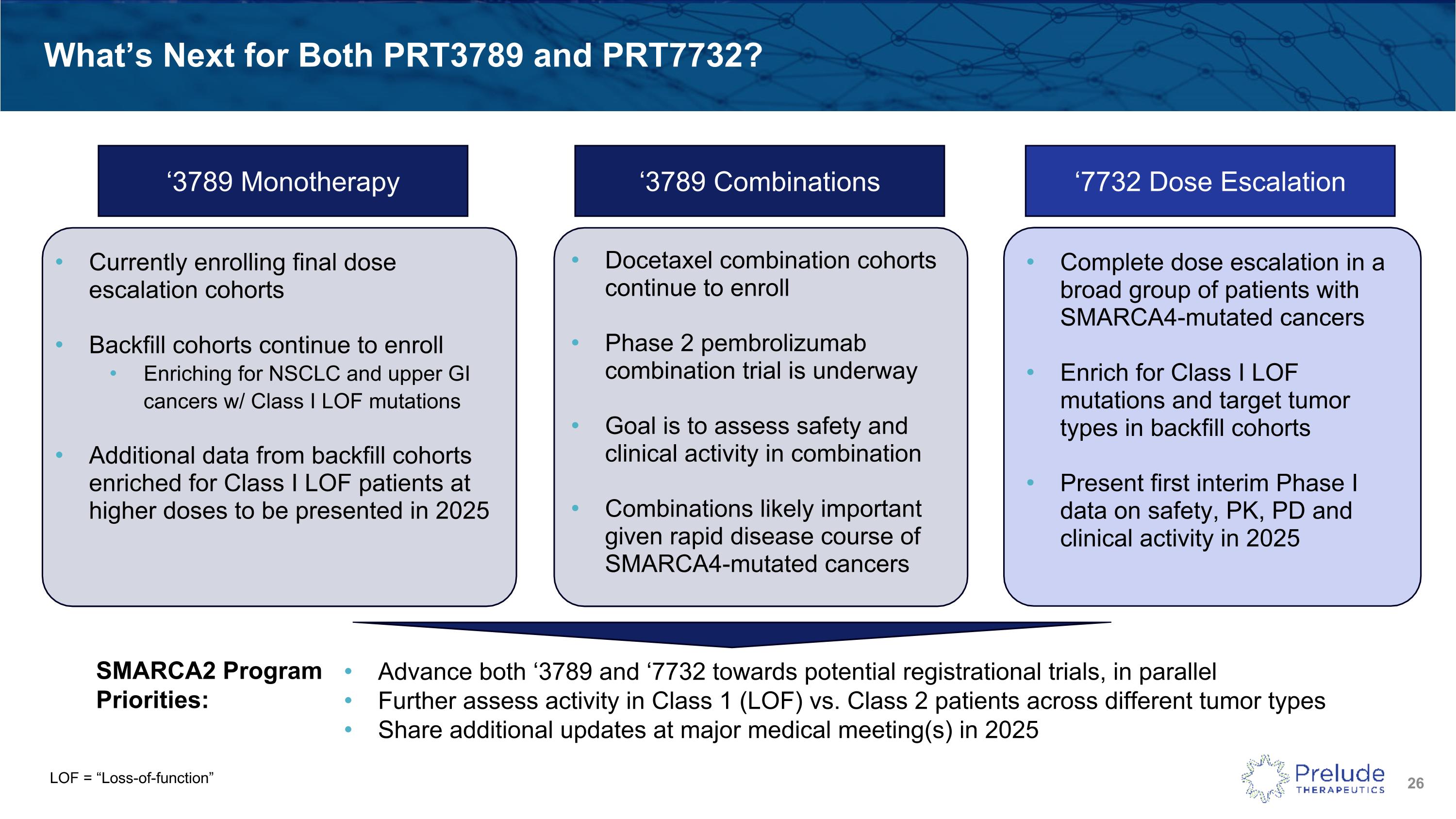
LOF = “Loss-of-function” What’s Next for Both PRT3789 and PRT7732? ‘3789 Monotherapy ‘7732 Dose Escalation SMARCA2 Program Priorities: Advance both ‘3789 and ‘7732 towards potential registrational trials, in parallel Further assess activity in Class 1 (LOF) vs. Class 2 patients across different tumor types Share additional updates at major medical meeting(s) in 2025 Currently enrolling final dose escalation cohorts Backfill cohorts continue to enroll Enriching for NSCLC and upper GI cancers w/ Class I LOF mutations Additional data from backfill cohorts enriched for Class I LOF patients at higher doses to be presented in 2025 ‘3789 Combinations Docetaxel combination cohorts continue to enroll Phase 2 pembrolizumab combination trial is underway Goal is to assess safety and clinical activity in combination Combinations likely important given rapid disease course of SMARCA4-mutated cancers Complete dose escalation in a broad group of patients with SMARCA4-mutated cancers Enrich for Class I LOF mutations and target tumor types in backfill cohorts Present first interim Phase I data on safety, PK, PD and clinical activity in 2025
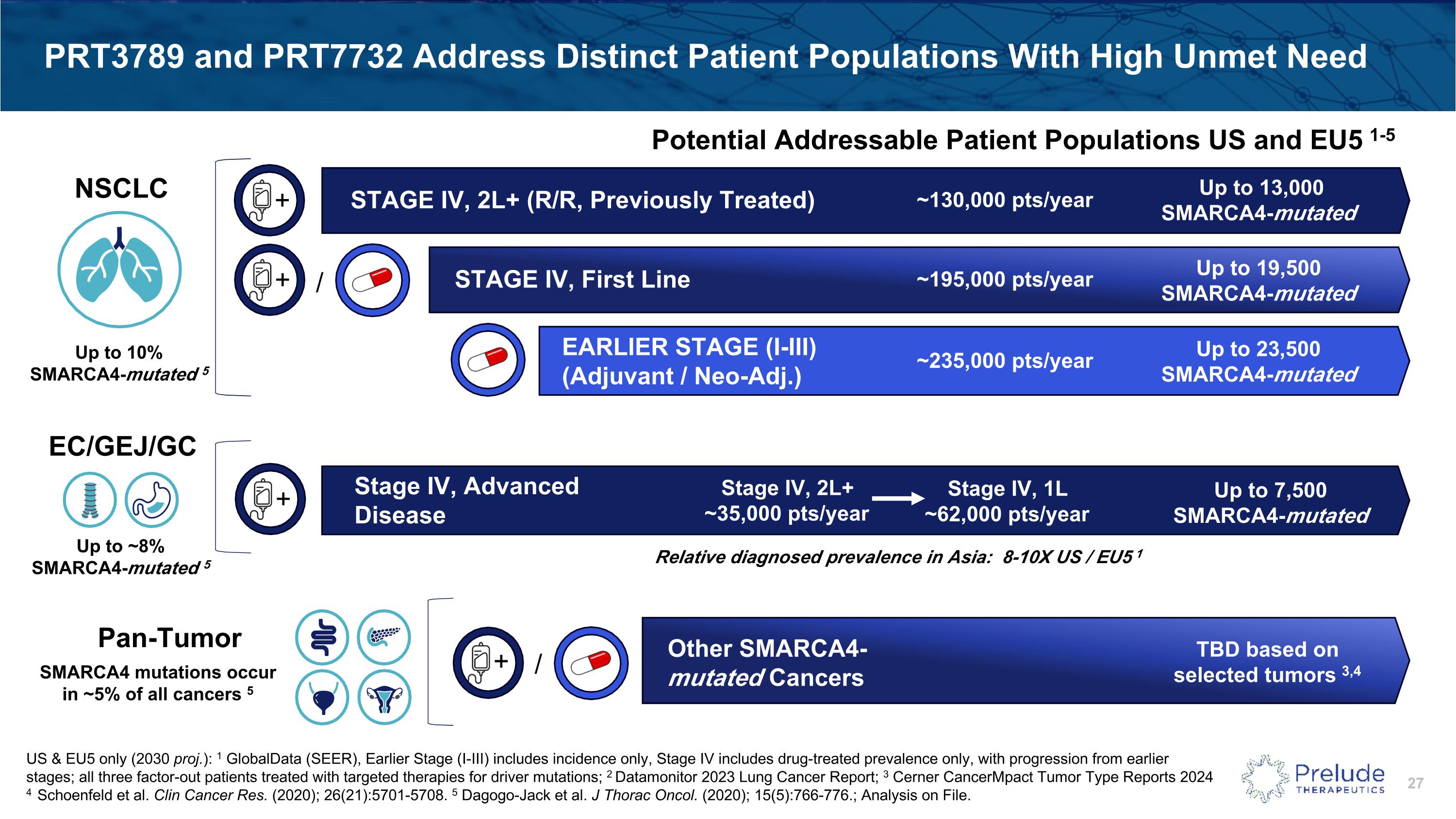
PRT3789 and PRT7732 Address Distinct Patient Populations With High Unmet Need ~195,000 pts/year Up to 19,500 �SMARCA4-mutated STAGE IV, First Line ~130,000 pts/year Up to 13,000 �SMARCA4-mutated STAGE IV, 2L+ (R/R, Previously Treated) + US & EU5 only (2030 proj.): 1 GlobalData (SEER), Earlier Stage (I-III) includes incidence only, Stage IV includes drug-treated prevalence only, with progression from earlier stages; all three factor-out patients treated with targeted therapies for driver mutations; 2 Datamonitor 2023 Lung Cancer Report; 3 Cerner CancerMpact Tumor Type Reports 2024 4 Schoenfeld et al. Clin Cancer Res. (2020); 26(21):5701-5708. 5 Dagogo-Jack et al. J Thorac Oncol. (2020); 15(5):766-776.; Analysis on File. ~235,000 pts/year Up to 23,500 �SMARCA4-mutated EARLIER STAGE (I-III)�(Adjuvant / Neo-Adj.) + / Potential Addressable Patient Populations US and EU5 1-5 + / Other SMARCA4-mutated Cancers TBD based on selected tumors 3,4 NSCLC SMARCA4 mutations occur in ~5% of all cancers 5 Up to 10% �SMARCA4-mutated 5 Stage IV, Advanced Disease + Up to ~8% �SMARCA4-mutated 5 EC/GEJ/GC Stage IV, 2L+�~35,000 pts/year Up to 7,500 �SMARCA4-mutated Stage IV, 1L�~62,000 pts/year Relative diagnosed prevalence in Asia: 8-10X US / EU5 1 Pan-Tumor
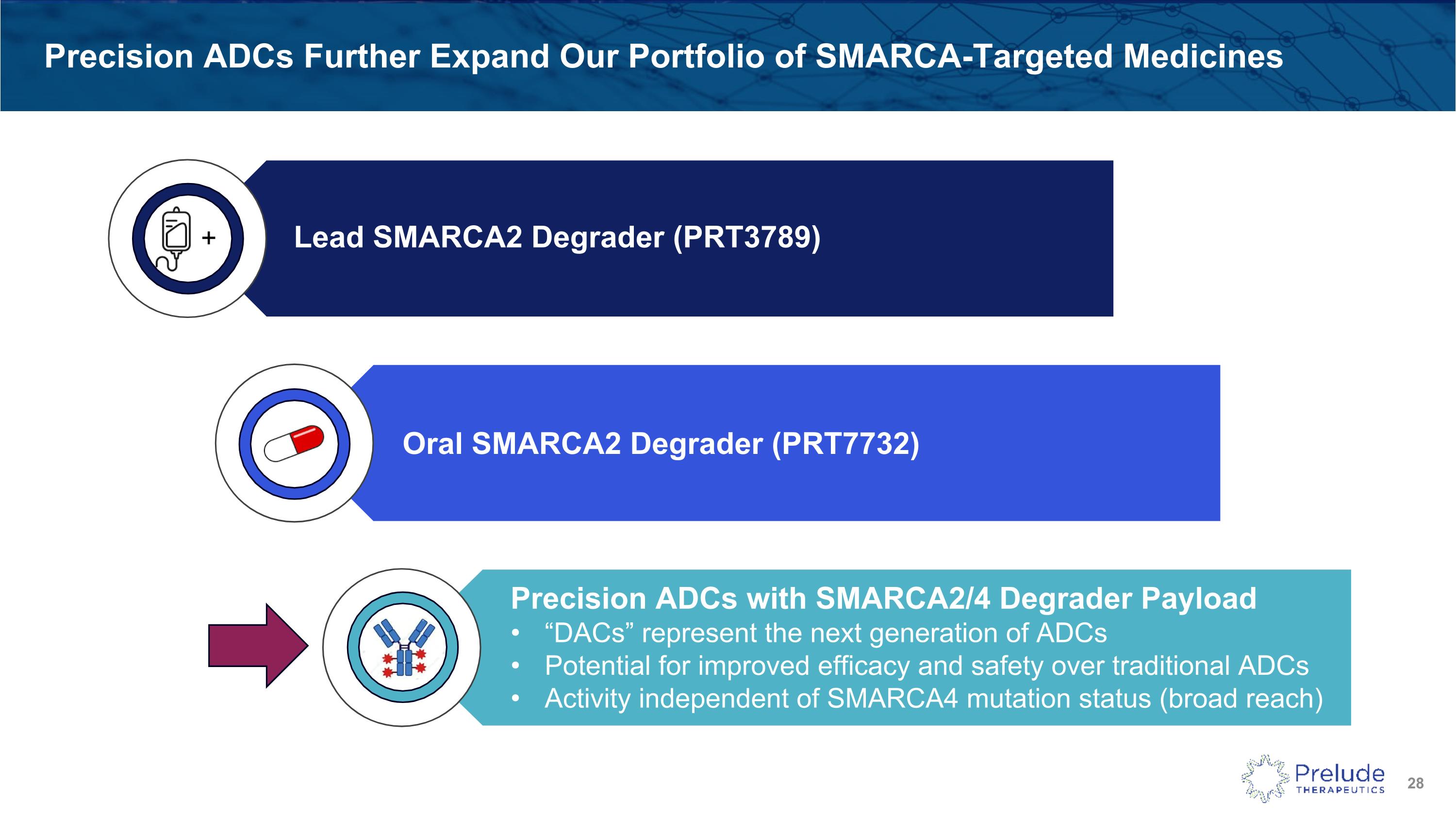
Precision ADCs Further Expand Our Portfolio of SMARCA-Targeted Medicines Precision ADCs with SMARCA2/4 Degrader Payload “DACs” represent the next generation of ADCs Potential for improved efficacy and safety over traditional ADCs Activity independent of SMARCA4 mutation status (broad reach) SMARCA - DACs + Lead SMARCA2 Degrader (PRT3789) Oral SMARCA2 Degrader (PRT7732)
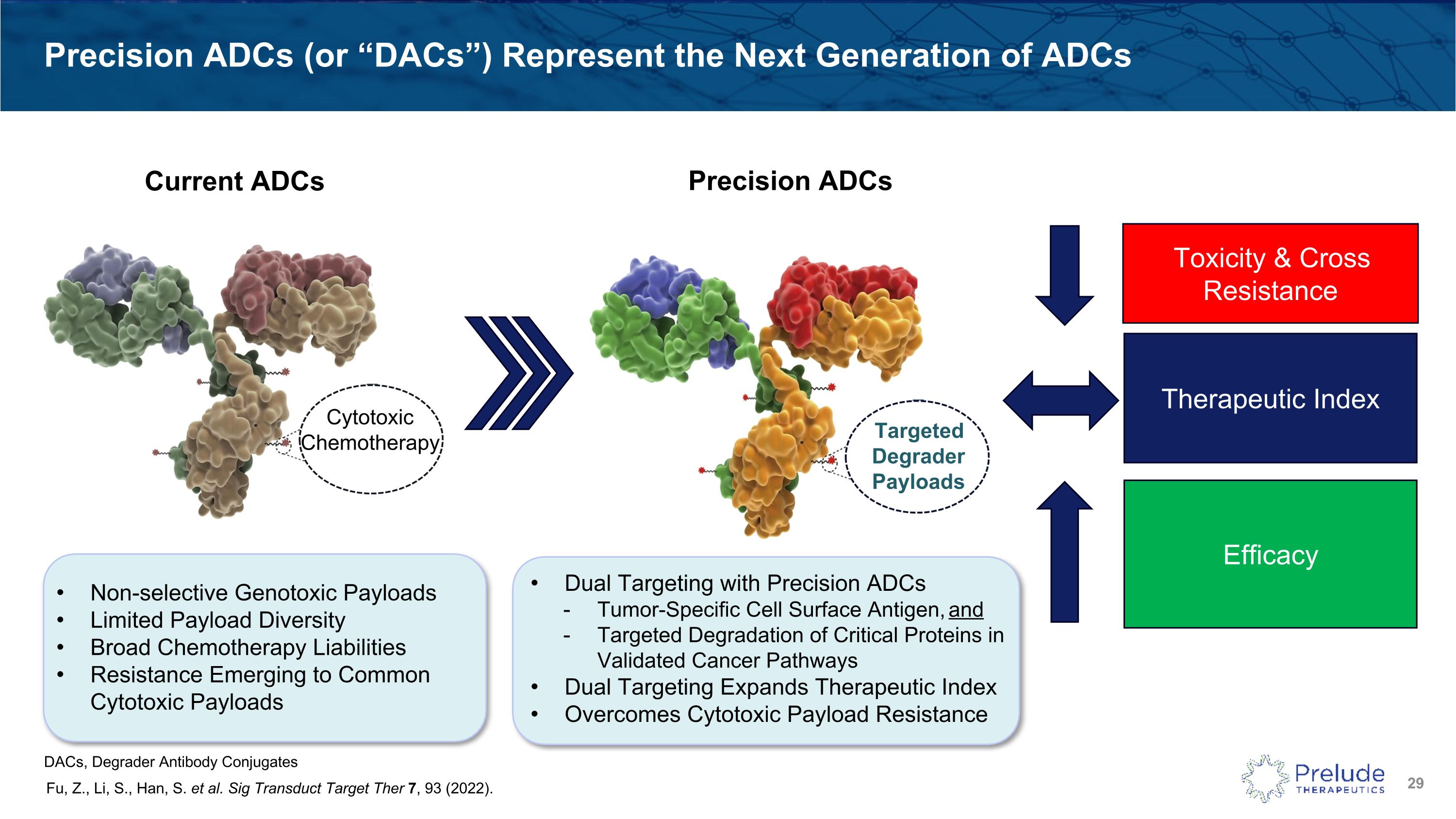
Precision ADCs (or “DACs”) Represent the Next Generation of ADCs Fu, Z., Li, S., Han, S. et al. Sig Transduct Target Ther 7, 93 (2022). Toxicity & Cross Resistance Efficacy Therapeutic Index Non-selective Genotoxic Payloads Limited Payload Diversity Broad Chemotherapy Liabilities Resistance Emerging to Common Cytotoxic Payloads Dual Targeting with Precision ADCs Tumor-Specific Cell Surface Antigen, and Targeted Degradation of Critical Proteins in Validated Cancer Pathways Dual Targeting Expands Therapeutic Index Overcomes Cytotoxic Payload Resistance Targeted�Degrader Payloads Current ADCs Precision ADCs Cytotoxic Chemotherapy DACs, Degrader Antibody Conjugates
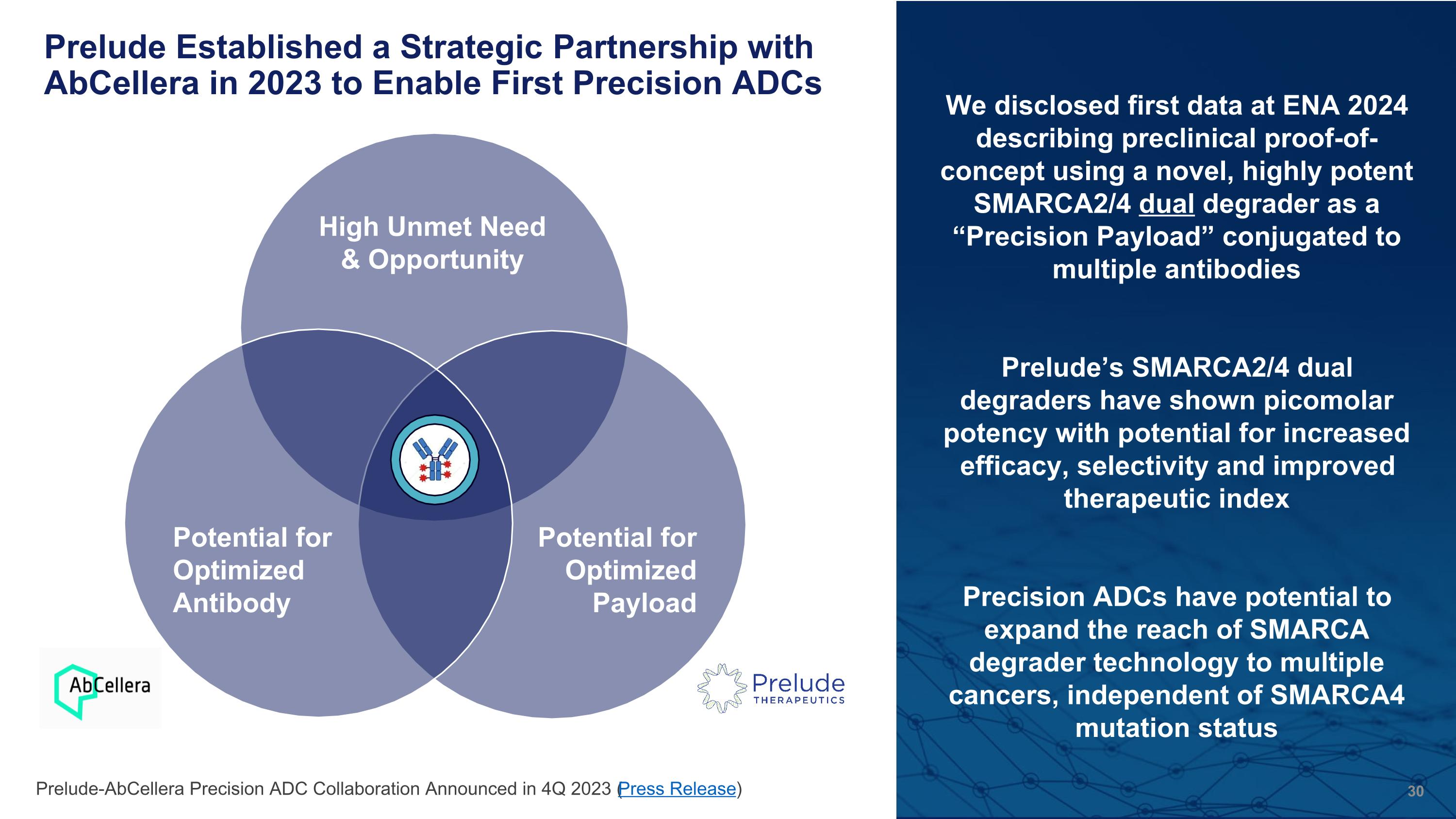
Prelude Established a Strategic Partnership with AbCellera in 2023 to Enable First Precision ADCs High Unmet Need & Opportunity Potential for Optimized Payload Potential for Optimized Antibody We disclosed first data at ENA 2024 describing preclinical proof-of-concept using a novel, highly potent SMARCA2/4 dual degrader as a “Precision Payload” conjugated to multiple antibodies Prelude’s SMARCA2/4 dual degraders have shown picomolar potency with potential for increased efficacy, selectivity and improved therapeutic index Precision ADCs have potential to expand the reach of SMARCA degrader technology to multiple cancers, independent of SMARCA4 mutation status Prelude-AbCellera Precision ADC Collaboration Announced in 4Q 2023 (Press Release)
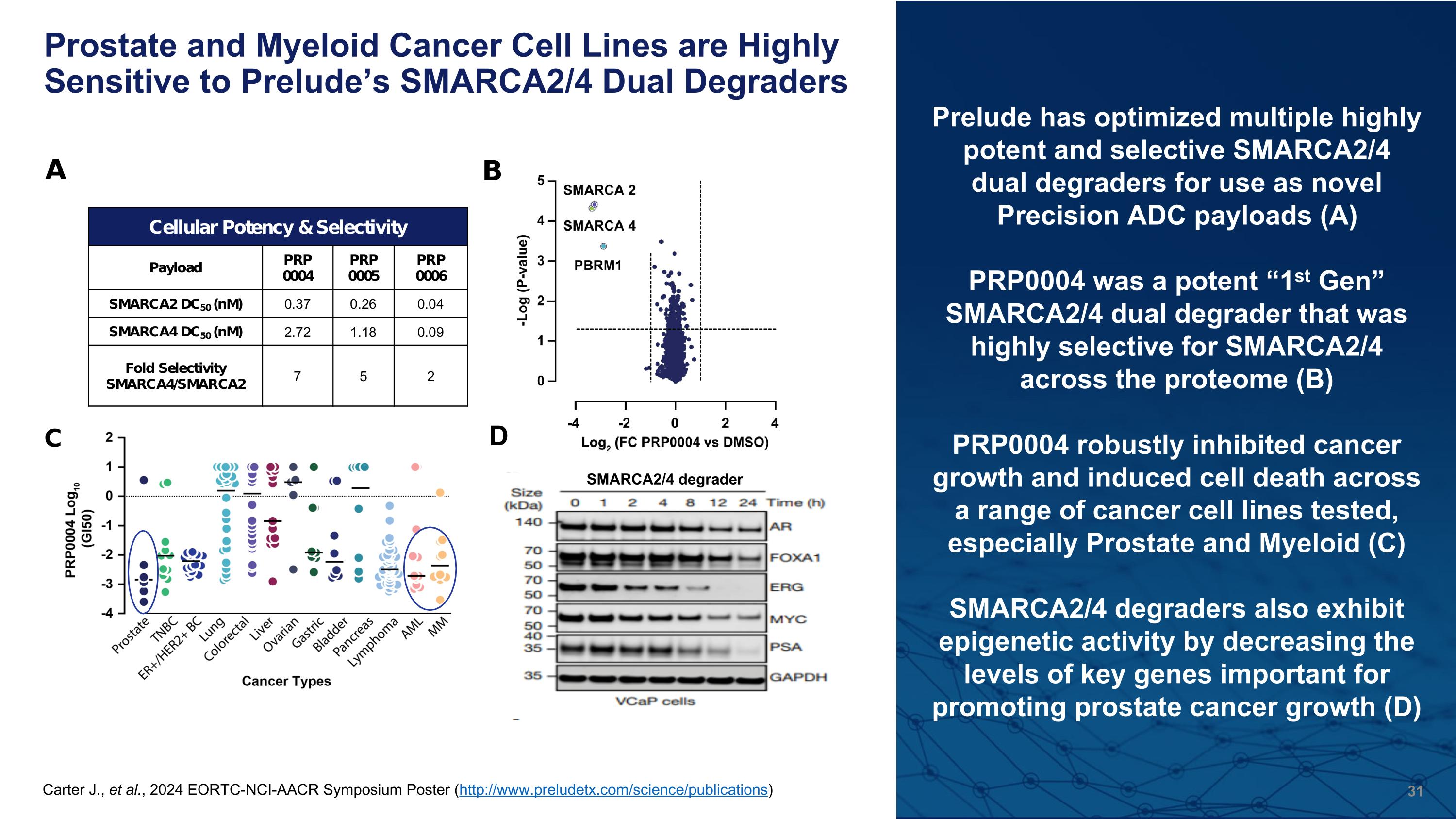
Prostate and Myeloid Cancer Cell Lines are Highly Sensitive to Prelude’s SMARCA2/4 Dual Degraders Carter J., et al., 2024 EORTC-NCI-AACR Symposium Poster (http://www.preludetx.com/science/publications) Prelude has optimized multiple highly potent and selective SMARCA2/4 dual degraders for use as novel�Precision ADC payloads (A) PRP0004 was a potent “1st Gen” SMARCA2/4 dual degrader that was highly selective for SMARCA2/4 across the proteome (B) PRP0004 robustly inhibited cancer growth and induced cell death across a range of cancer cell lines tested, especially Prostate and Myeloid (C) SMARCA2/4 degraders also exhibit epigenetic activity by decreasing the levels of key genes important for promoting prostate cancer growth (D) D SMARCA2/4 degrader
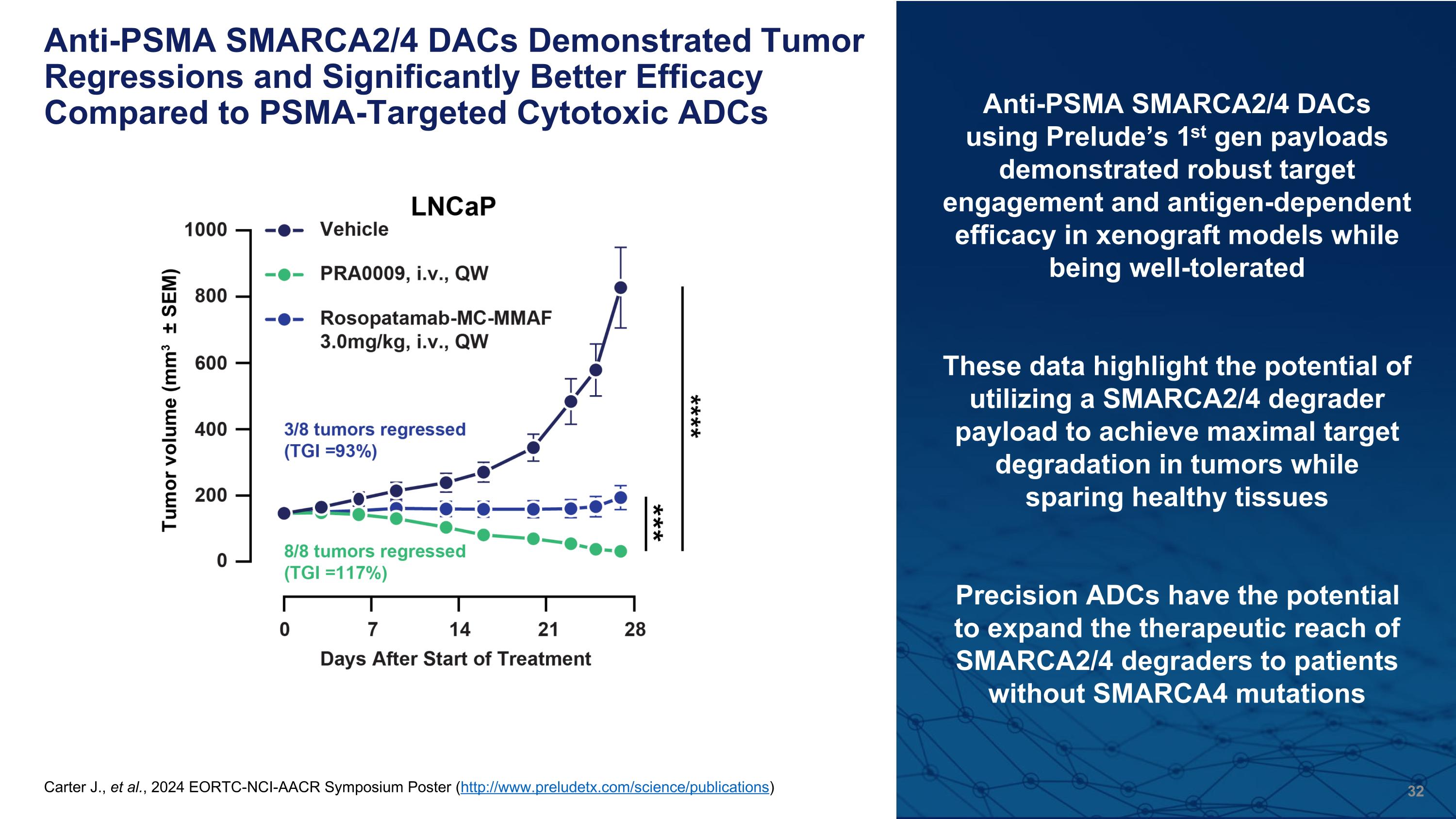
Anti-PSMA SMARCA2/4 DACs Demonstrated Tumor Regressions and Significantly Better Efficacy Compared to PSMA-Targeted Cytotoxic ADCs Anti-PSMA SMARCA2/4 DACs using Prelude’s 1st gen payloads demonstrated robust target engagement and antigen-dependent efficacy in xenograft models while being well-tolerated These data highlight the potential of utilizing a SMARCA2/4 degrader payload to achieve maximal target degradation in tumors while sparing healthy tissues Precision ADCs have the potential to expand the therapeutic reach of SMARCA2/4 degraders to patients without SMARCA4 mutations (A) SMARCA2/4 protein expression was analyzed in DAC PRA0002 and payload PRP0004-treated LNCaP tumors at the indicated time points following a single dose. Graphs are quantitation of western blots. (B) Weekly i.v. administration of PRA0002 was well-tolerated and demonstrated significant tumor growth inhibition (89%) of PSMA+ LNCaP tumors. (C) Weekly i.v. administration of PRA0002 did not induce significant tumor growth inhibition in PSMA- PC3 tumors, in comparison to PRP0004 which was efficacious, but caused mouse body weight loss and death (D) Weekly i.v. administration of PRA0009 demonstrated tumor regression and significantly better efficacy compared to a PSMA cytotoxic ADC (Rosopatamab-MC-MMAF, DAR2) in LNCaP tumors. Carter J., et al., 2024 EORTC-NCI-AACR Symposium Poster (http://www.preludetx.com/science/publications)
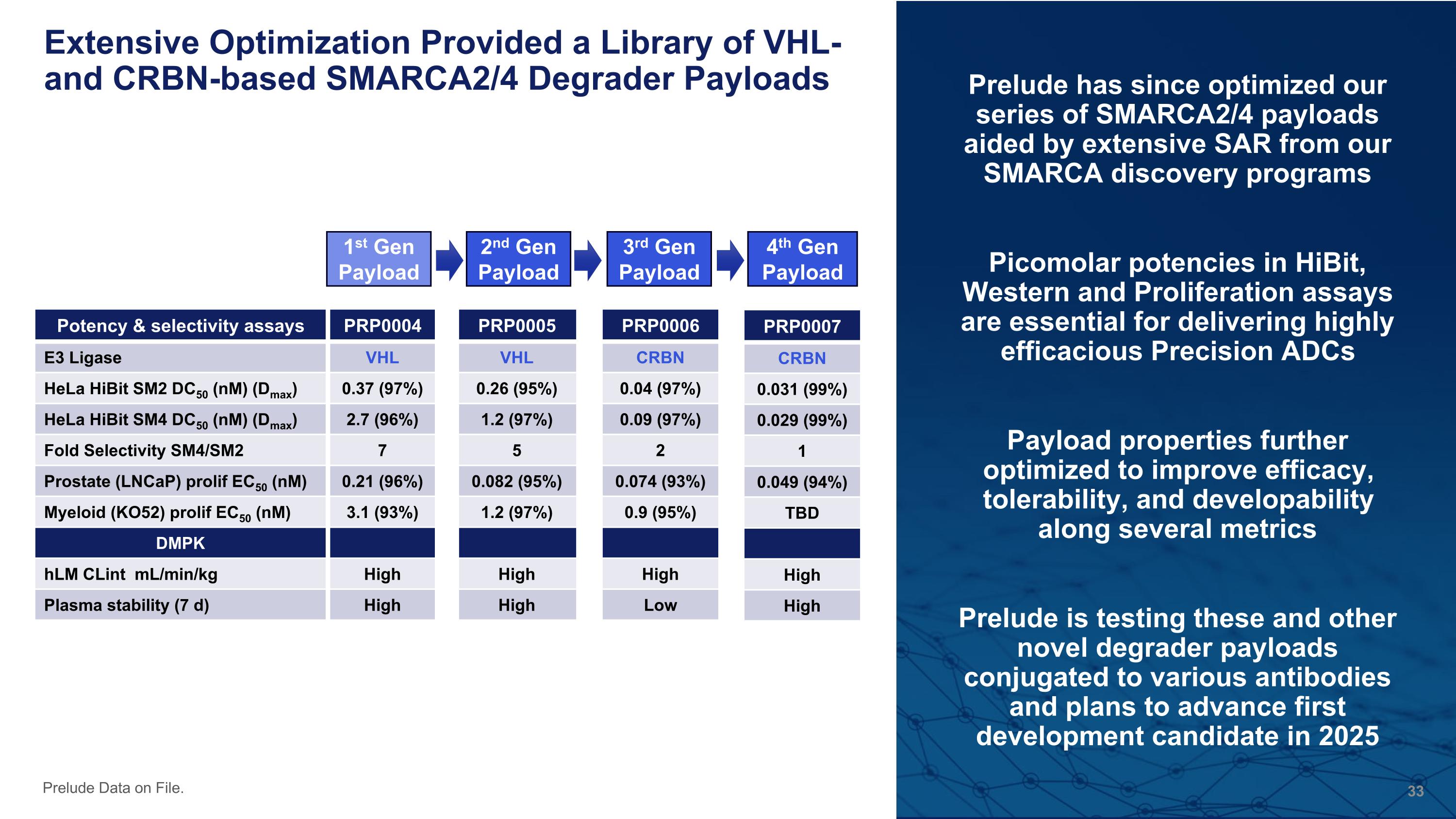
Extensive Optimization Provided a Library of VHL- and CRBN-based SMARCA2/4 Degrader Payloads Prelude has since optimized our series of SMARCA2/4 payloads aided by extensive SAR from our SMARCA discovery programs Picomolar potencies in HiBit, Western and Proliferation assays are essential for delivering highly efficacious Precision ADCs Payload properties further optimized to improve efficacy, tolerability, and developability along several metrics Prelude is testing these and other novel degrader payloads conjugated to various antibodies and plans to advance first development candidate in 2025 Prelude Data on File. Potency & selectivity assays PRP0004 E3 Ligase VHL HeLa HiBit SM2 DC50 (nM) (Dmax) 0.37 (97%) HeLa HiBit SM4 DC50 (nM) (Dmax) 2.7 (96%) Fold Selectivity SM4/SM2 7 Prostate (LNCaP) prolif EC50 (nM) 0.21 (96%) Myeloid (KO52) prolif EC50 (nM) 3.1 (93%) DMPK hLM CLint mL/min/kg High Plasma stability (7 d) High PRP0006 CRBN 0.04 (97%) 0.09 (97%) 2 0.074 (93%) 0.9 (95%) High Low PRP0005 VHL 0.26 (95%) 1.2 (97%) 5 0.082 (95%) 1.2 (97%) High High 1st Gen Payload PRP0007 CRBN 0.031 (99%) 0.029 (99%) 1 0.049 (94%) TBD High High 2nd Gen Payload 3rd Gen Payload 4th Gen Payload
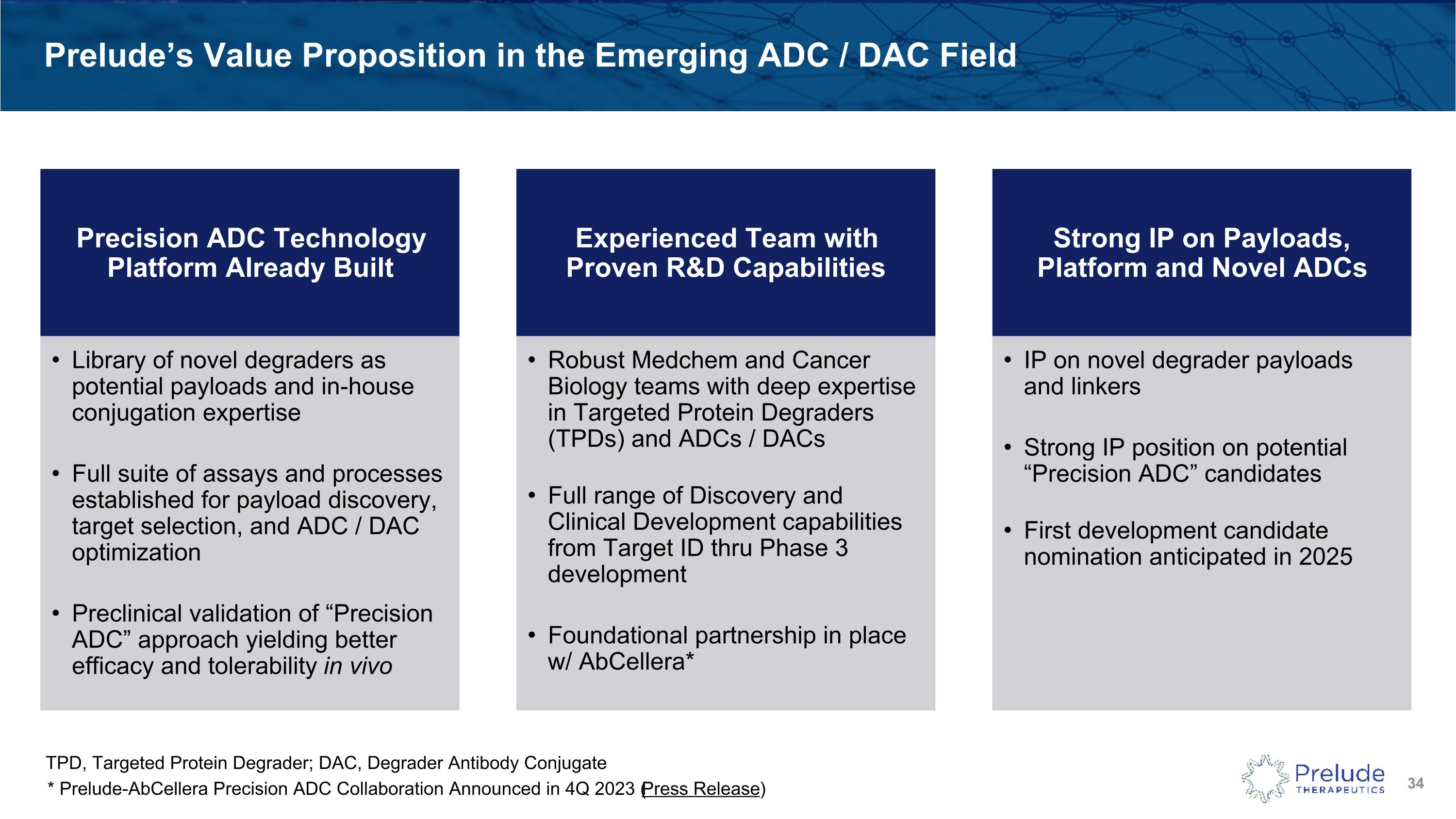
Prelude’s Value Proposition in the Emerging ADC / DAC Field Precision ADC Technology Platform Already Built Library of novel degraders as potential payloads and in-house conjugation expertise Full suite of assays and processes established for payload discovery, target selection, and ADC / DAC optimization Preclinical validation of “Precision ADC” approach yielding better efficacy and tolerability in vivo Experienced Team with Proven R&D Capabilities Robust Medchem and Cancer Biology teams with deep expertise in Targeted Protein Degraders (TPDs) and ADCs / DACs Full range of Discovery and Clinical Development capabilities from Target ID thru Phase 3 development Foundational partnership in place w/ AbCellera* Strong IP on Payloads, Platform and Novel ADCs IP on novel degrader payloads and linkers Strong IP position on potential “Precision ADC” candidates First development candidate nomination anticipated in 2025 TPD, Targeted Protein Degrader; DAC, Degrader Antibody Conjugate * Prelude-AbCellera Precision ADC Collaboration Announced in 4Q 2023 (Press Release)

In 2025 Our Focus is Continued Execution Across Strategic Priorities PRT2527 CDK9 PROGRAM Report Phase 1 monotherapy clinical results Report Phase 1 docetaxel combination interim data PRT3789 Lead IV SMARCA2 Degrader Selective CDK Inhibitor PRT2527 Report first Phase 1 interim data (Safety, PK/PD, Clinical Activity) PRT7732 Oral SMARCA2�Degrader Discovery�Engine Precision ADCs & Other Advance next first-in-class, novel small molecule candidate(s) Advance first Precision ADC candidate in partnership w/ AbCellera EXPECTED DELIVERABLE MILESTONE BY 2H 2025 2H 2025 2H 2025 2025 2025 Cash, cash equivalents and marketable securities of $133.6 Million as of 12/31/2024

Thank You Contact Us: Robert Doody SVP, Investor Relations rdoody@preludetx.com



































