Exhibit 99.1

DELIVERING A NEW GENERATION OF INTEGRIN MEDICINES October 2020

Forward Looking Statements This presentation contains “forward - looking” statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Morphic’s or our partners’ plans to develop and commercialize oral small - molecule integrin therapeutics and Morphic’s expectations about timing and ability to commence, enroll or complete clinical studies and to obtain regulatory approvals for MORF - 057, MORF - 720, MORF - 627 and other candidates in development, the ability of MORF - 057 to treat inflammatory bowel diseases, including ulcerative colitis or Crohn’s disease, the ability of MORF - 720 or MORF - 627 to treat idiopathic pulmonary fibrosis or liver fibrosis, the ability of our platform to discove r additional developable candidates (including against α v β 8 and α v β 1 ) or suitable indications (including in solid tumors or fibrotic diseases), the potential impact of the COVID - 19 pandemic and the sufficiency of our cash, cash equivalents and investments to fu nd our operations. Statements including words such as “believe,” “plan,” “continue,” “expect,” “will,” “develop,” “signal,” “potential,” or “ong oin g” and statements in the future tense are forward - looking statements. These forward - looking statements involve risks and uncertainties, as well as assumptions, which, if they do not fully materialize or prove incorrect, could cause our results to differ materially fr om those expressed or implied by such forward - looking statements. Forward - looking statements are subject to risks and uncertainties that may cause Morphic’s actual activities or results to diffe r significantly from those expressed in or implied by any forward - looking statement, including risks and uncertainties related to the forward - looking statements in this presentation and other risks set forth in our filings with the Securities and Exchange Commission. These forward - looking statements speak only as of the date hereof and Morphic specifically disclaims any obligation to update these forward - looking statements or reasons why actual results might differ, whether as a result of new information, future events or otherwise, except as required by law. 2

3 Morphic : "Cracking the Code" of Oral Integrin Therapies Unique MInT Platform to Interrogate the Integrin Target Class Lead Asset in IBD Targeting Validated Biology in Blockbuster Space Broad Preclinical Pipeline Addressing Multiple Disease Areas Partnerships with Marquee Pharmaceutical Companies Strong Cash Position and Seasoned Management Team
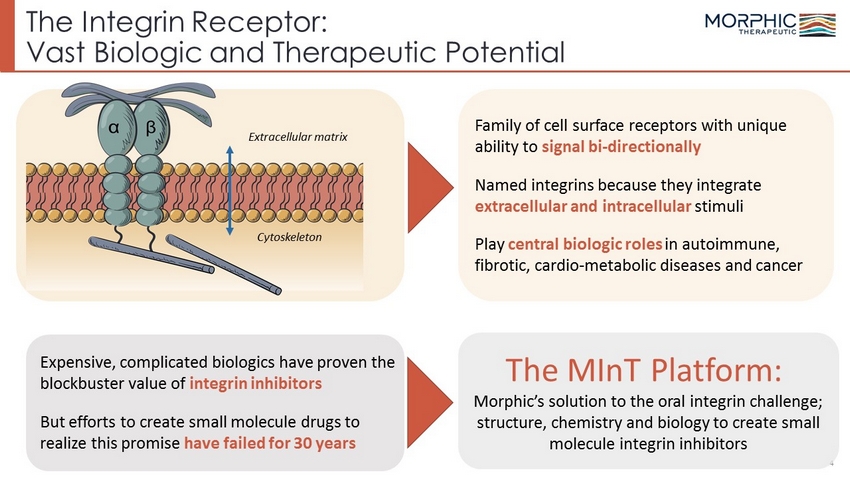
The Integrin Receptor: Vast Biologic and Therapeutic Potential 4 Extracellular matrix Cytoskeleton Expensive, complicated biologics have proven the blockbuster value of integrin inhibitors But efforts to create small molecule drugs to realize this promise have failed for 30 years Family of cell surface receptors with unique ability to signal bi - directionally Named integrins because they integrate extracellular and intracellular stimuli Play central biologic roles in autoimmune, fibrotic, cardio - metabolic diseases and cancer The MInT Platform: Morphic’s solution to the oral integrin challenge; structure, chemistry and biology to create small molecule integrin inhibitors
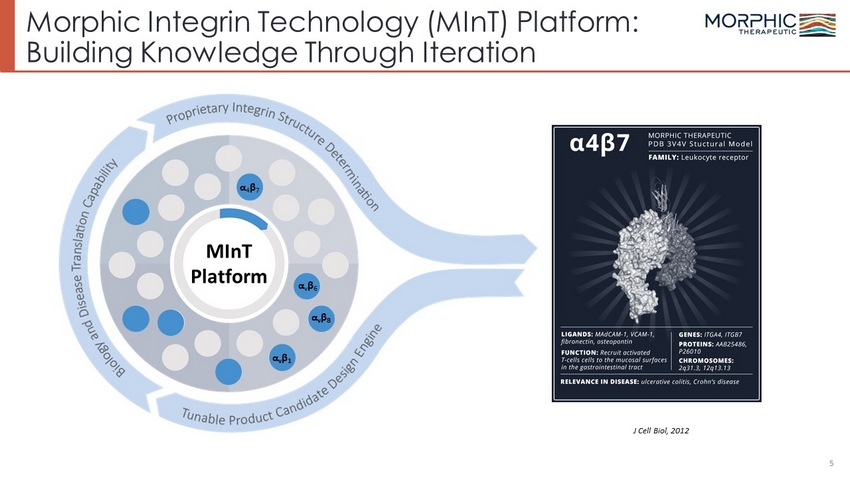
Morphic Integrin Technology (MInT) Platform: Building Knowledge Through Iteration J Cell Biol, 2012 5 α 4 β 7 α v β 6 α v β 8 α v β 1 MInT Platform
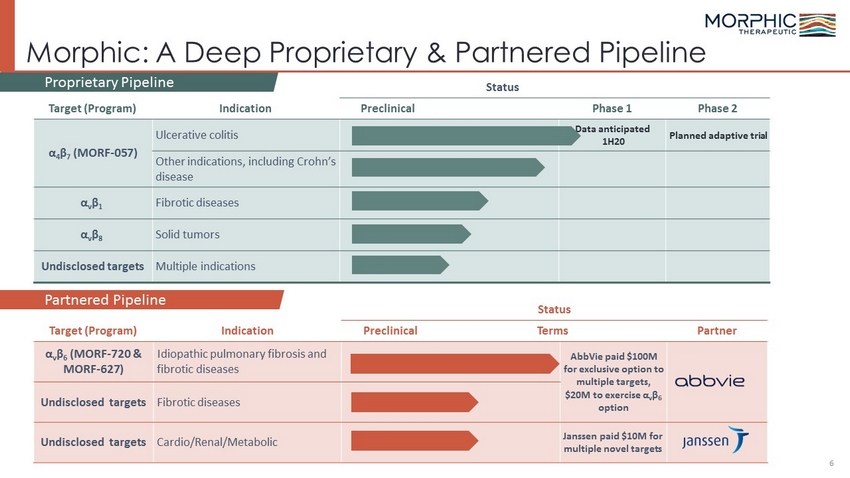
Status Target (Program) Indication Preclinical Terms Partner α v β 6 ( MORF - 720 & MORF - 627) Idiopathic pulmonary fibrosis and fibrotic diseases AbbVie paid $100M for exclusive option to multiple targets, $20M to exercise α v β 6 option Undisclosed targets Fibrotic diseases Undisclosed targets Cardio/Renal/Metabolic Janssen paid $10M for multiple novel targets Morphic: A Deep Proprietary & Partnered Pipeline Status Target (Program) Indication Preclinical Phase 1 Phase 2 α 4 β 7 ( MORF - 057) Ulcerative colitis Data anticipated 1H20 Planned adaptive trial Other indications, including Crohn’s disease α v β 1 Fibrotic diseases α v β 8 Solid tumors Undisclosed targets Multiple indications 6 Partnered Pipeline Proprietary Pipeline
MORF - 057 Targeting α 4 β 7 , a validated mechanism, for Inflammatory Bowel Disease 7 α 4 β 7

Highly Selective Oral α 4 β 7 Inhibitor for Inflammatory Bowel Disease 8 MORF - 057 MECHANISM INDICATIONS SUPPORTING DATA CLINICAL PLANS Highly selective orally available small molecule inhibitor of α 4 β 7 A safe, oral IBD treatment is needed and c ould address the need for safe and effective oral IBD therapy for early use prior to biologic step - up Inhibition of α 4 β 7 on T - lymphocytes prevents their migration to gut tissue and the resulting inflammation Well validated MOA in inflammatory bowel disease via approved monoclonal antibody, vedolizumab Inflammatory bowel disease with initial focus on ulcerative colitis (UC) Possible to expand Crohn’s Disease and / or other relevant indications High selectivity of MORF - 057 for α 4 β 7 over other integrins Saturation of α 4 β 7 integrin in non - human primate receptor occupancy studies Currently enrolling three - part Phase 1 study Receptor occupancy data may provide early proof of concept in Phase 1 Planned adaptive Phase 2 study in UC

Approved antibody Entyvio® (vedolizumab) Sub - endothelial space - gut Vascular space - blood T - cell a 4 b 7 MAdCAM - 1 Endothelial cell • α 4 β 7 + T - cells are trafficked to gut tissue via MAdCAM - 1 binding. These T - cells cause inflammation that leads to inflammatory bowel disease. • Vedolizumab, an anti - α 4 β 7 antibody, inhibits T - cell trafficking via well validated mechanism to treat UC and Crohn’s disease • Since approval, >150,000 patients have received Vedolizumab 1 • Vedolizumab generated $3.1B sales in FY19 2 α 4 β 7 Inhibition is a Proven Mechanism to Treat IBD 9 1 Takeda 2 Global Data ENTYVIO® is a registered trademark of Millennium Pharmaceuticals, Inc .
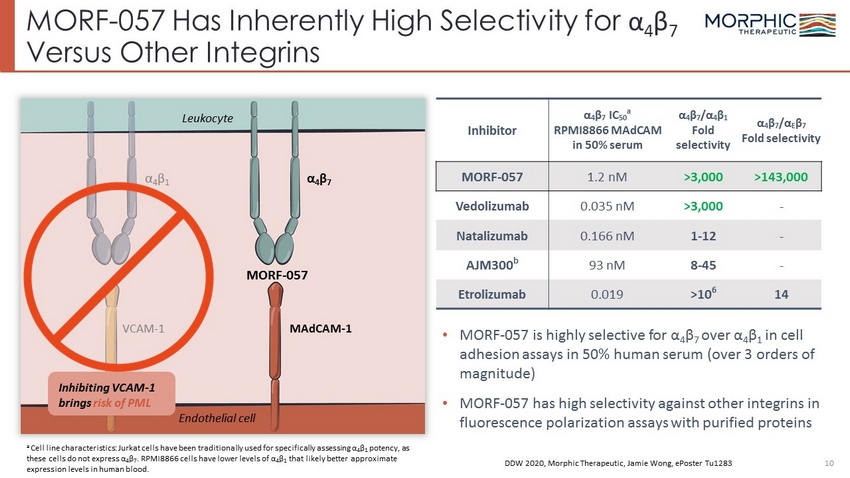
MORF - 057 Has Inherently High Selectivity for α 4 β 7 Versus Other Integrins 10 α 4 β 7 MAdCAM - 1 α 4 β 1 Endothelial cell Leukocyte Inhibiting VCAM - 1 brings risk of PML VCAM - 1 • MORF - 057 is highly selective for α 4 β 7 over α 4 β 1 in cell adhesion assays in 50% human serum (over 3 orders of magnitude) • MORF - 057 has high selectivity against other integrins in fluorescence polarization assays with purified proteins DDW 2020, Morphic Therapeutic, Jamie Wong, ePoster Tu1283 MORF - 057 Inhibitor α 4 β 7 IC 50 a RPMI8866 MAdCAM in 50% serum α 4 β 7 /α 4 β 1 Fold selectivity α 4 β 7 /α E β 7 Fold selectivity MORF - 057 1.2 nM >3,000 >143,000 Vedolizumab 0.035 nM >3,000 - Natalizumab 0.166 nM 1 - 12 - AJM300 b 93 nM 8 - 45 - Etrolizumab 0.019 >10 6 14 a Cell line characteristics: Jurkat cells have been traditionally used for specifically assessing α 4 β 1 potency, as these cells do not express α 4 β 7 . RPMI8866 cells have lower levels of α 4 β 1 that likely better approximate expression levels in human blood.

MORF - 057: Dose - dependent Anti - inflammatory Activity Comparable to Anti - α 4 β 7 mAb in Pre - clinical Studies 11 • p<0.05, ** p<0.01, ***p<0.001, and ****p<0.0001 vs. vehicle by One Way Anova followed by Dunnett’s multiple comparisons % of CFSE+ in T - cells MORF - 057, mpk mLN **** **** **** **** **** **** 0.0 0.1 0.2 0.3 Mean ± SEM T lymphocyte homing into mesenteric lymph nodes (mLN) Reduction of lymphocytes homing to mesenteric lymph nodes demonstrates engagement of same anti - inflammatory mechanism as vedolizumab *DATK32 is the mouse surrogate for vedolizumab

MORF - 057 and Related Compounds Impact T mem Biomarker in a Dose - dependent Manner 12 The biomarker response with MR - 5288 (closely related tool compound of MORF - 057) is dose responsive Means and SD of data normalized per individual at timepoint 0h (first dose administration). T test analysis was performed at each timepoint Statistical significance determined using the Holm - Sidak method, with alpha = 0.05. ** p < 0.01, *** p < 0.001 MR - 5288 Dose Response, N=8 Means and SD represented MORF - 057 inhibits α 4 β 7 + CD4 + T cell trafficking to mucosal sites in NHP ( 15mpk, 5 days; N=5) Fold change of % α 4 β 7 high cells from CD4 + T mem , t=0 ** ** ** ** *** Days 0 1 2 3 4 5 1.0 1.5 2.0 Vehicle PO MORF - 057 Fold change of % α 4 β 7 high cells from CD4 + T mem , t=0 MR - 5288 Day 6 1.0 1.2 1.4 1.6 1.8 Increase in circulating T mem demonstrates α 4 β 7 mechanistic activity: T cells are prevented from migrating through mucosa thus reducing IBD - associated inflammation
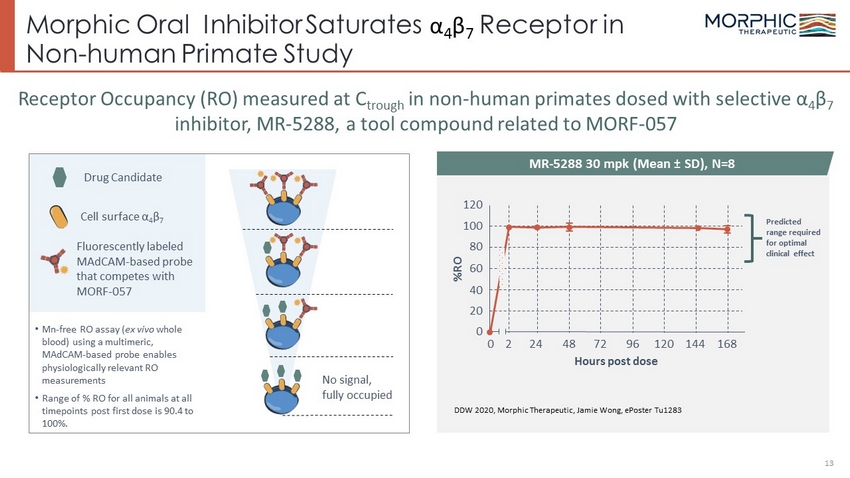
Morphic Oral Inhibitor Saturates α 4 β 7 Receptor in Non - human Primate Study 13 • Mn - free RO assay ( ex vivo whole blood) using a multimeric, MAdCAM - based probe enables physiologically relevant RO measurements • Range of % RO for all animals at all timepoints post first dose is 90.4 to 100%. Receptor Occupancy (RO) measured at C trough in non - human primates dosed with selective α 4 β 7 inhibitor, MR - 5288, a tool compound related to MORF - 057 DDW 2020, Morphic Therapeutic, Jamie Wong, ePoster Tu1283 Drug Candidate Cell surface α 4 β 7 Fluorescently labeled MAdCAM - based probe that competes with MORF - 057 No signal, fully occupied MR - 5288 30 mpk (Mean ± SD), N=8 0 2 24 48 72 96 120 144 168 Hours post dose %RO 120 100 80 40 20 0 60 Predicted range required for optimal clinical effect

MORF - 057: Phase 1 Trial with Biomarkers Provides Opportunity for Early Proof of Concept 14 1. SAD cohort: safety, tolerability, and PK/PD 2. Food Effect Cohort to determine PK of a single, projected clinically relevant dose 3. MAD cohort: safety, tolerability, and PK/PD, including receptor occupancy data Three - part Phase 1 Design: *SAD: single ascending dose **MAD: multiple ascending dose Phase 1: SAD* PHASE 1 Phase 1: Food - effect PHASE 2 Phase 1: MAD* Controlled adaptive trial in UC patients Phase 2 N ≥ 40, 5 cohorts N ≥ 12, 2 cohorts N ≥ 24, 3 cohorts 1H21
Proprietary Pipeline 15 α v β 8 Creating the next generation of proprietary integrin inhibitor candidates α v β 1 α 4 β 7 α 4 β 7

Latent TGF - β Active TGF - β α v β 1 TGF - β activation Fibrogenesis Chronic injury α v β 1 Inhibition: Potential to Treat Fibrosis Across Multiple Organ Systems α v β 1 inhibition is believed to block the activation of latent TGF - β , leading to the reduction of collagen depositions that drive fibrosis 16 • Morphic lead compounds active across multiple animal models of fibrosis • Extends and leverages Morphic development expertise in fibrosis
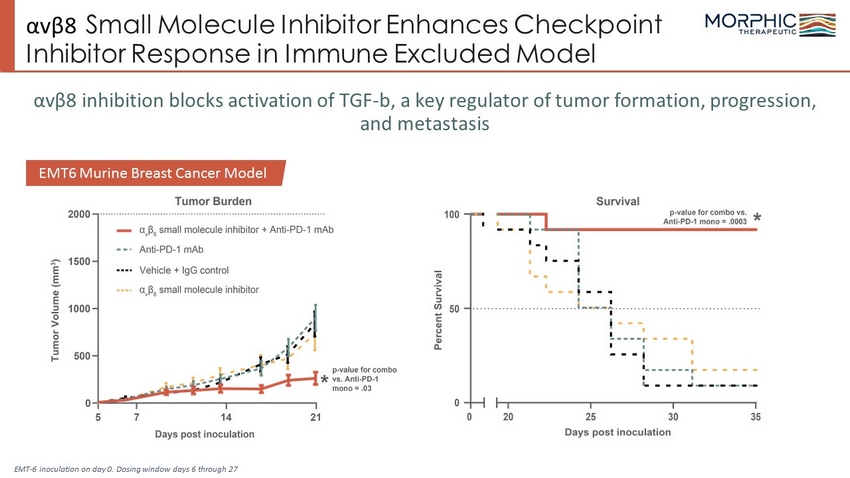
αv β 8 Small Molecule Inhibitor Enhances Checkpoint Inhibitor Response in Immune Excluded Model αv β 8 inhibition blocks activation of TGF - b, a key regulator of tumor formation, progression, and metastasis EMT6 Murine Breast Cancer Model EMT - 6 inoculation on day 0. Dosing window days 6 through 27

Partnered Programs Leveraging the MInT platform to create new oral integrin candidate for premier collaborators 18 α v β 6

Platform - validating Partnerships 19 • $100 million upfront partnership agreement to develop multiple selective orally available small molecule integrin inhibitors • Initial focus on α v β 6 inhibition to prevent TGF - β activation and collagen deposition leading to fibrosis • AbbVie exercised $20 million option for α v β 6 inhibitors, MORF - 720 and MORF - 627 • Morphic is entitled to milestones and royalties on commercialized products and retains opt - in rights for certain indications • Novel target discovery collaboration • Undisclosed targets in cardio/renal/ metabolic space • $10 million upfront payment • Milestones and royalties on commercial products

Deep Specialist Expertise Across Management, and Board of Directors PRAVEEN TIPIRNENI, MD President and Chief Executive Officer BRUCE ROGERS, PhD Chief Scientific Officer ALEXEY A. LUGOVSKOY, PhD Chief Development Officer MARC SCHEGERIN, MD Chief Financial Officer Chief Operating Officer PETER LINDE, MD Chief Medical Officer BOARD OF DIRECTORS Gustav Christensen, MBA Chairman Morphic Board of Directors, former CEO, Dyax Timothy Springer, PhD Founder, Morphic Therapeutic, Member of Morphic Scientific Advisory Board; Latham Family Professor, Professor of Biological Chemistry and Molecular Pharmacology; Professor of Medicine, Harvard Medical School Norbert Bischofberger, PhD President and Chief Executive Officer, Kronos Bio; EVP of R&D, CSO, Gilead Otello Stampacchia, PhD Founder and Managing Partner, Omega Funds Amir Nashat, PhD Managing Partner, Polaris Partners Nilesh Kumar, PhD Partner , Novo Ventures; Merck Joseph P. Slattery, CPA F ormer CFO, Transenterix, Baxano, Digene Vikas Goyal, MBA Treasurer and Senior Vice President, Pandion Therapeutics; SROne Praveen Tipirneni, MD President and CEO Morphic Therapeutic

21 Morphic : "Cracking the Code" of Oral Integrin Therapies Unique MInT Platform to Access the Integrin Target Class Lead Asset in IBD Targeting Validated Biology in Blockbuster Space Broad Preclinical Pipeline Addressing Multiple Disease Areas Partnerships with Marquee Pharmaceutical Companies Strong Cash Position and Seasoned Management Team

DELIVERING A NEW GENERATION OF INTEGRIN MEDICINES





















