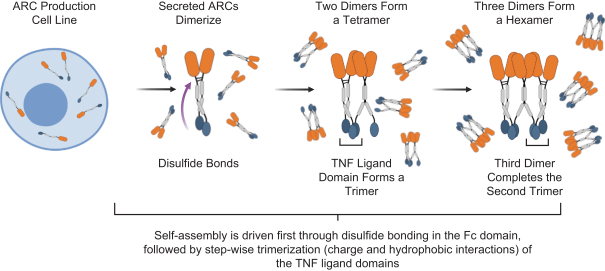
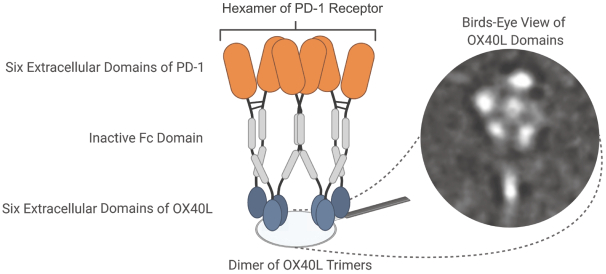
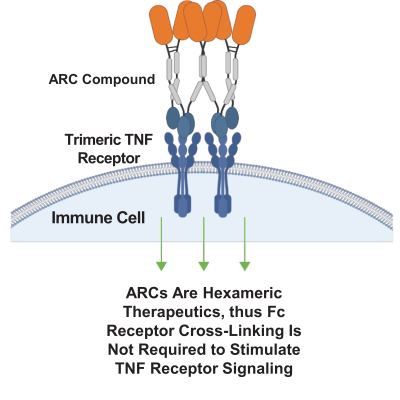
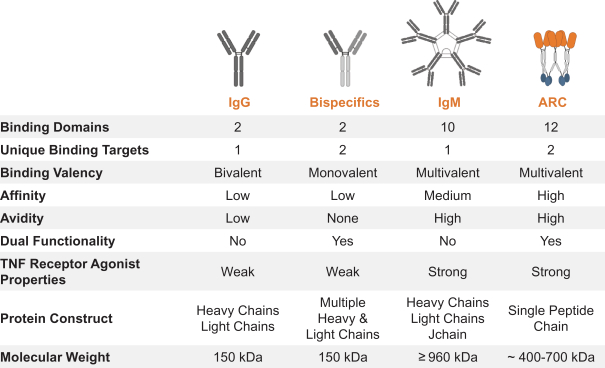
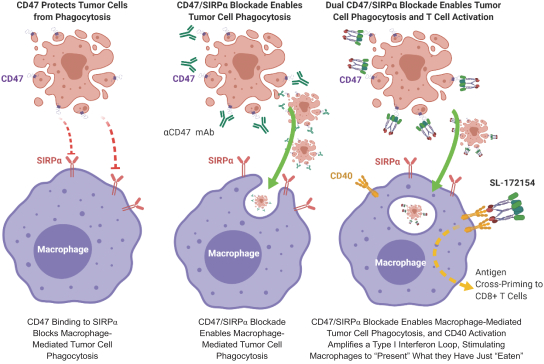
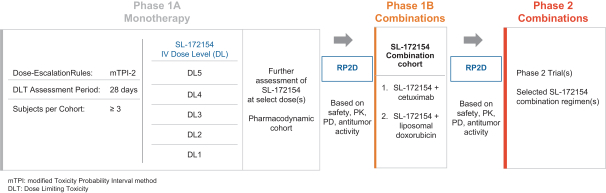
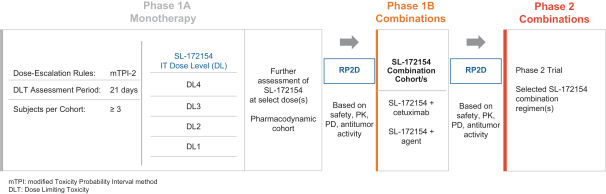
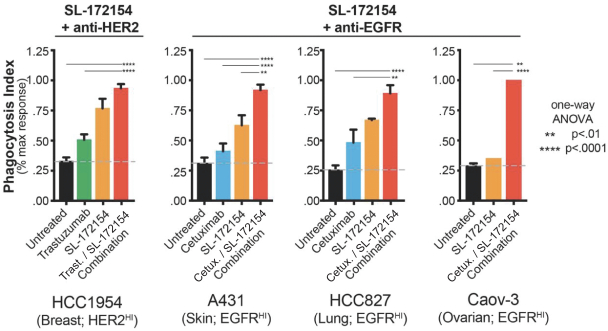
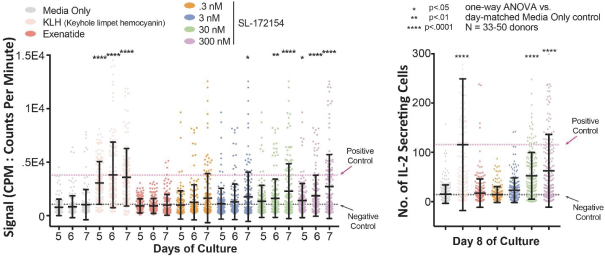
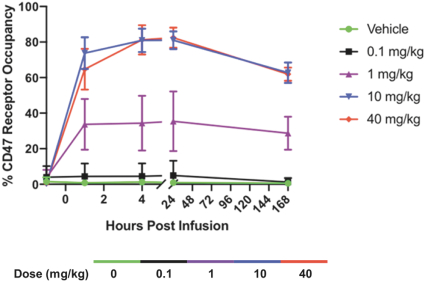
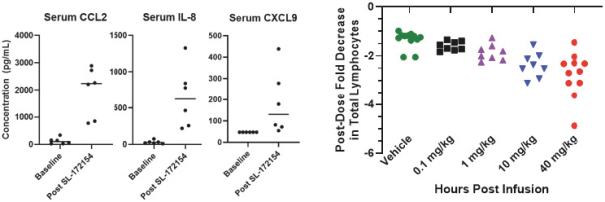
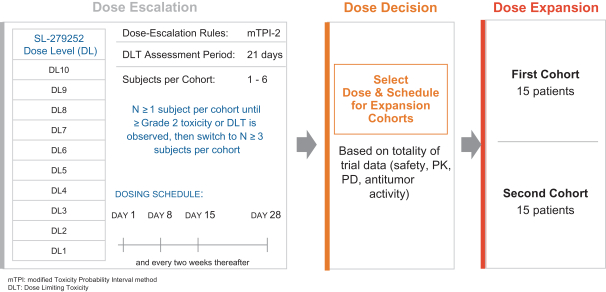
Takeda holds an exclusive option to these patent families in connection with the Collaboration Agreement discussed elsewhere herein.
SL-172154 Product Candidate
The patent portfolio for our SL-172154 product candidate is based upon our owned and in-licensed patent portfolio, which includes patents and patent applications directed generally to compositions of matter, pharmaceutical compositions, and methods of treatment. We have two granted patents in the United States, from the in-licensed patent portfolio, covering compositions of matter of a genus of molecules, and the SL-172154 product candidate specifically, pharmaceutical compositions, and methods of treating cancer. Patent applications are pending in the United States and various foreign jurisdictions and regions, including Australia, Brazil, Canada, China, Europe, Hong Kong, Indonesia, Israel, India, Japan, Korea, Mexico, Malaysia, Philippines, Russia, Saudi Arabia, Singapore, Thailand, Ukraine, and Vietnam. Patent applications in this family, if granted, are expected to expire in 2036, without taking potential patent term extensions or patent term adjustment into account.
Pending coverage, from the Company-owned patent portfolio, that relates to our SL-172154 product candidate also includes methods of treatment with various combination agents (one pending PCT application, and pending applications in the United States, Canada, China, Europe, and Japan). Patent applications in these families, if granted, are expected to expire in 2038 or 2039, without taking potential patent term extensions or patent term adjustment into account.
Preclinical Product Candidates
The Company also has taken steps to protect various preclinical product candidates. The Company owns or exclusively licenses various granted U.S. patents, and pending U.S., foreign and PCT applications covering ARC compounds that may develop into product candidates.
Six licensed U.S. granted patents and one Company-owned U.S. granted patent cover PD-1-, CSF1R-, TIM3-, SIRP1a-, and TIGIT-based ARC compounds, with OX40L, CD40L, and 4-1BBL, covering compositions of matter of a genus of compounds, and the preclinical product candidates specifically, pharmaceutical compositions, and methods of treating. Patent applications are pending in the United States and various foreign jurisdictions and regions, including Australia, Brazil, Canada, China, Europe, Hong Kong, Indonesia, Israel, India, Japan, Korea, Mexico, Malaysia, Philippines, Russia, Saudi Arabia, Singapore, Thailand, Ukraine, and Vietnam that could cover various product candidates. Patent applications in these families, if granted, are expected to expire in 2038 or 2039, without taking potential patent term extensions or patent term adjustment into account.
Trademark Protection
As of September 1, 2020, we owned an allowed trademark application for “ARC” and a pending application for “GADLEN” with the U.S. Patent and Trademark Office. We plan to register trademarks in connection with our biological products.
Licensed Intellectual Property from Heat Biologics, Inc.
In June 2016, we entered into an exclusive license agreement with Heat, pursuant to which we received an exclusive (as to the patent rights), non-transferable, sublicensable, worldwide, royalty-bearing, non-field restricted license to certain patent rights and know-how, including rights related to the ARC platform. We paid Heat an initial license fee of $50,000, and we are obligated to pay Heat fees upon receipt of certain sublicensing income, achievement of certain milestones, and royalties upon sales of commercial products. The Heat license provides us rights in the patent family that arose from PCT/US16/54598 and is the source of ten granted U.S.
133