AboutAVR-RD-01
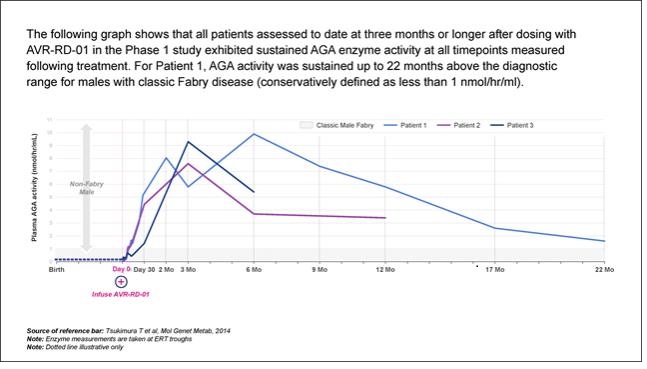
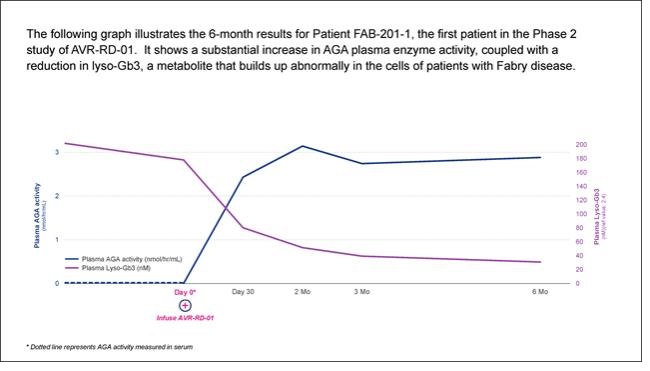
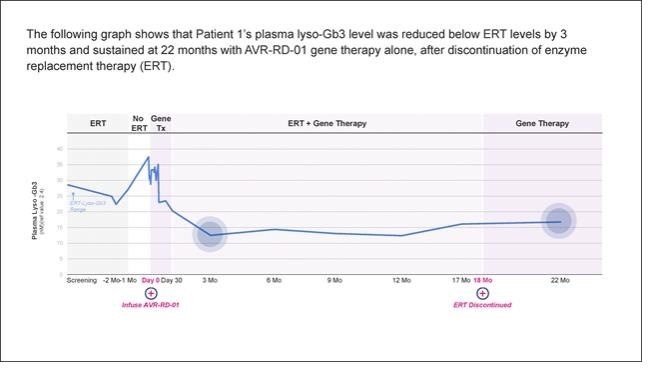
AVR-RD-01 is anex vivo lentiviral gene therapy being investigated as a single-dose therapy with the potential to provide durable and life-long potential therapeutic benefit for patients with Fabry disease.AVR-RD-01 is designed to employ astate-of-the-art lentiviral vector system that is an efficient gene transfer technology for the permanent integration of functional copies of the gene into the patient’s own stem cells. In patients with Fabry disease, hematopoietic stem cells are collected from the patient, and then transduced with lentiviral vector carrying a functional version of the GLA gene that encodes active α-galactosidase A (AGA) – the enzyme that is deficient in Fabry disease – to createAVR-RD-01 gene therapy.AVR-RD-01 is then infused back into the patient with the goal of restoring normal GLA gene expression such that functional AGA enzyme is sufficiently produced by the patient’s own body.
About AVROBIO, Inc.
AVROBIO, Inc., is a Phase 2 clinical-stage gene therapy company developing gene therapies to potentially cure rare diseases with a single dose. AVROBIO’s lentiviral-based gene therapies employ hematopoietic stem cells that are collected from the patient and then modified with a lentiviral vector to insert functional copies of the gene that is defective in the target disease. AVROBIO is focused on the development of its gene therapy,AVR-RD-01, in Fabry disease, as well as additional gene therapy programs in other lysosomal storage disorders including Gaucher disease, cystinosis and Pompe disease. The Company’s plato™ platform is a vector system and automated, closed, cell manufacturing solution designed to support worldwide commercialization. AVROBIO is headquartered in Cambridge, MA and has offices in Toronto, ON. For additional information, visit www.avrobio.com.
Forward-Looking Statements
This press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words or similar expressions that are intended to identify forward-looking statements. These forward-looking statements include, without limitation, statements regarding our business strategy, prospective products and goals, the therapeutic potential of our product candidates, the design, enrollment and timing of ongoing or planned clinical trials, clinical trial results, product approvals and regulatory pathways, potential regulatory approvals and the timing thereof, anticipated benefits of our gene therapy platform, timing and likelihood of success, plans and objectives of management for future operations, future results of anticipated products, and the market opportunity for our product candidates. Any such statements in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Results in preclinical or early stage clinical trials may not be indicative of results from later stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements, or the scientific data presented.
7