
Exhibit 99.1 TX45 Phase 1b PH-HFpEF Interim Data Release for Single Dose Hemodynamic Trial January 2025

2 DISCLAIMER Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as anticipates, believes, expects, intends, “plans,” “potential,” projects,” “would” and future or similar expressions are intended to identify forward-looking statements. Each of these forward-looking statements involves substantial risks and uncertainties that could cause actual results to differ significantly from those expressed or implied by such forward- looking statements. Forward-looking statements contained in this presentation include, but are not limited to, statements regarding: the design, objectives, initiation, timing, progress and results of current and future preclinical studies and clinical trials of our product candidates, including the ongoing Phase 1b and Phase 2 clinical trial for TX45, in Group 2 Pulmonary Hypertension; the expected timing of program updates and data disclosures; the timing of filing INDs and other regulatory documents; the timing and likelihood of seeking regulatory approval for our product candidates including TX45; the competitive landscape for and market potential of our product candidates; and our ability to identify and develop additional product candidates as well as pursue additional indications. These forward-looking statements reflect our current beliefs and expectations. Many factors may cause differences between current expectations and actual results, including the early stage of our development efforts; success in preclinical testing and earlier clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidates; clinical site activation rates or clinical trial enrollment rates that are lower than expected; changes in expected or existing competition; changes in the regulatory environment; the uncertainties and timing of the regulatory approval process; the impact of macroeconomic conditions, including the conflict in Ukraine and the conflict in the Middle East, heightened inflation and uncertain credit and financial markets, on our business, clinical trials and financial position; and unexpected litigation or other disputes. These and other risks are described more fully in our filings with the Securities and Exchange Commission (“SEC”), including the risks detailed in our Quarterly Report on Form 10-Q filed with the SEC on November 12, 2024, and other documents we subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except as required by law, we assume no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.

3 Tectonic Tx: GPCR-Targeted Therapies for High-Value Opportunities TECX focused on discovery & development of GPCR-target biologics with significant unmet need Clinical-Stage Biotech • Founded in 2019 by Tim Springer and Andrew Kruse, TECX went public via reverse merger (AVROBIO) in June 2024 with a concurrent private placement of approximately $131 million Tenured Team Executive team with numerous accomplishments, resulting in 20 “first” approvals Long-acting relaxin in Phase 2 trial, supported by Phase 1b interim results TX45 Lead Pipeline Asset • Initial indication targeting Group 2 Pulmonary Hypertension (PH) associated with Heart Failure with Preserved Ejection Fraction (HFpEF), or PH-HFpEF, with Phase 2 trial enriched for CpcPH • Positive Phase 1a clinical trial results support best-in-class potential, including favorable safety profile • Positive interim Phase 1b clinical trial results achieved or exceeded all hemodynamic goal targets, supporting Phase 2 trial Relaxin Potentially Relaxin physiologic and hemodynamic effects further demonstrated in prior clinical studies Ideal for PH-HFpEF • Prior clinical development of relaxin by Novartis adds to clinical rationale for TX45 targeting PH-HFpEF * PH-HFpEF Significant ~1.4M+ Group 2 PH-HFpEF patients in the U.S. with no approved therapy ; high 5-year mortality * Market Potential • Potential peak multi-billion-dollar revenue potential for Group 2 PH-HFpEF patients with EF > 40% • Astra Zeneca is pursuing a Group 2 PH relaxin program targeting both HFpEF and HFrEF patients Targeting rare bleeding disorder called Hereditary Hemorrhagic Telangiectasia (HHT) TX2100 Second Pipeline Asset • Significant market potential, no approved therapies for HHT, estimated ~75K patients in the U.S. alone (15-20% severe) • Phase 1 clinical trial initiation expected in Q4’25 / Q1’26 Potential to Broaden TX45 Phase 1b, hemodynamic topline results in PH-HFrEF subjects expected in 2H’25 TX45 to HFrEF Patients * • Positive PH-HFrEF results could potentially expand the addressable TX45 patient population by ~1.1M patients in the U.S. ~$159 million in cash as of 9/30/24, expected to provide a cash runway into mid-2027 Well-Capitalized * Estimates based on company sponsored market analysis conducted by Health Advances
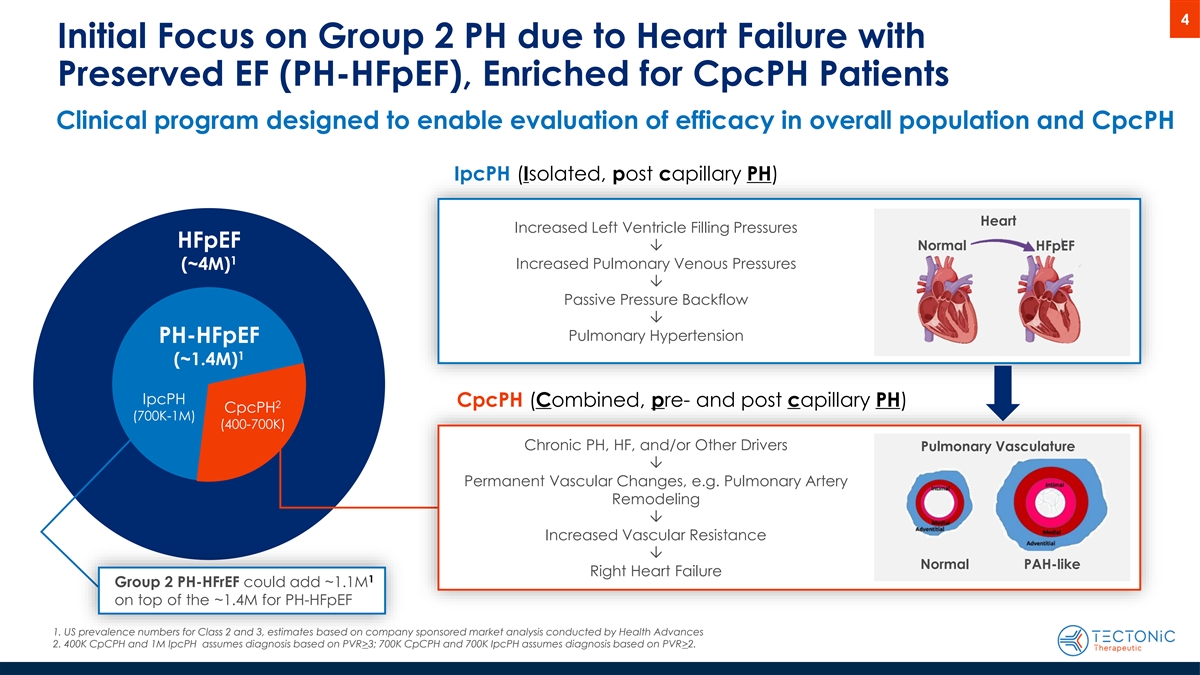
4 Initial Focus on Group 2 PH due to Heart Failure with Preserved EF (PH-HFpEF), Enriched for CpcPH Patients Clinical program designed to enable evaluation of efficacy in overall population and CpcPH IpcPH (Isolated, post capillary PH) Heart Increased Left Ventricle Filling Pressures HFpEF Normal Ô HFpEF 1 Increased Pulmonary Venous Pressures (~4M) Ô Passive Pressure Backflow Ô Pulmonary Hypertension PH-HFpEF 1 (~1.4M) IpcPH CpcPH (Combined, pre- and post capillary PH) 2 CpcPH (700K-1M) (400-700K) Chronic PH, HF, and/or Other Drivers Pulmonary Vasculature Ô Permanent Vascular Changes, e.g. Pulmonary Artery Remodeling Ô Increased Vascular Resistance Ô Normal PAH-like Right Heart Failure 1 Group 2 PH-HFrEF could add ~1.1M on top of the ~1.4M for PH-HFpEF 1. US prevalence numbers for Class 2 and 3, estimates based on company sponsored market analysis conducted by Health Advances 2. 400K CpCPH and 1M IpcPH assumes diagnosis based on PVR>3; 700K CpCPH and 700K IpcPH assumes diagnosis based on PVR>2.
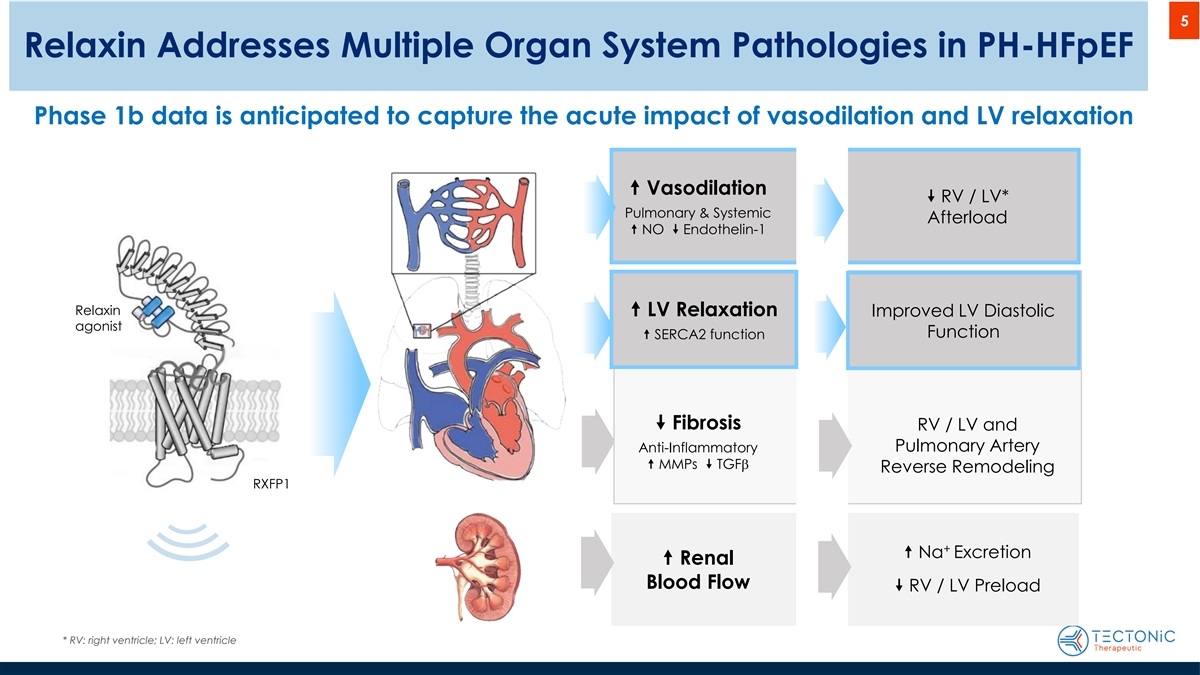
5 Relaxin Addresses Multiple Organ System Pathologies in PH-HFpEF Phase 1b data is anticipated to capture the acute impact of vasodilation and LV relaxation “ Vasodilation ” RV / LV* Pulmonary & Systemic Afterload “ NO ” Endothelin-1 Relaxin “ LV Relaxation Improved LV Diastolic agonist Function “ SERCA2 function ” Fibrosis RV / LV and Pulmonary Artery Anti-Inflammatory “ MMPs ” TGFb Reverse Remodeling RXFP1 + “ Na Excretion “ Renal Blood Flow ” RV / LV Preload * RV: right ventricle; LV: left ventricle

6 TX45 Background and Clinical Program Status • TX45 is a human relaxin-2-Fc fusion protein with a potential best in class half-life of 2-3 weeks • The Phase 1b hemodynamic clinical trial is ongoing: • Part A - Enrollment and dosing of PH-HFpEF subjects is completed (N=19) • Efficacy data is available for 16 of 19 subjects 3 • Hemodynamic data on the last 3 subjects are in line with the findings on the first 16 • Last 3 patients all have IpcPH, therefore the CpcPH cohort data is final • Safety data is available on 16 patients, 15 of whom have completed the full 43-day safety follow-up 2 • Part B - Enrollment in PH-HFrEF subjects is expected to initiate near term with data in 2H’25 1 • TX45 is currently enrolling in a Phase 2 trial for subjects with PH-HFpEF enriched for CpcPH with results expected in 2026 1 PH-HFpEF = Pulmonary Hypertension due to Heart Failure with Preserved Ejection Fraction (LVEF ≥ 40%) 2 PH-HFrEF = Pulmonary Hypertension due to Heart Failure with Reduced Ejection Fraction 3 Based on data available to date
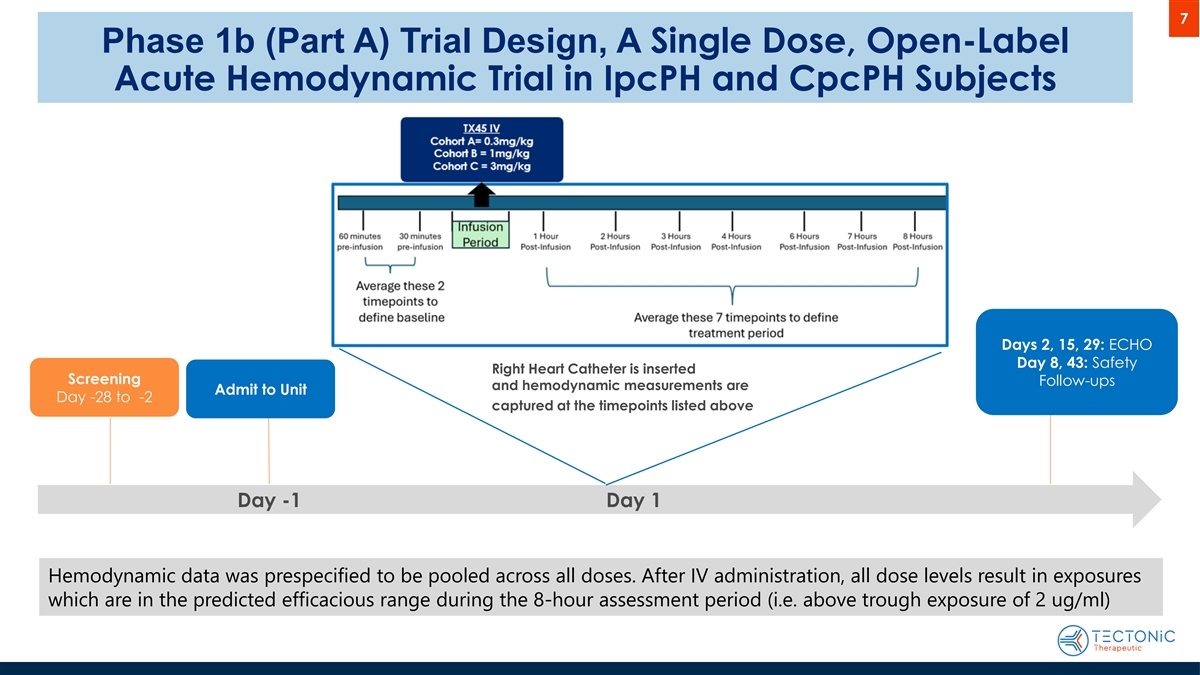
7 Phase 1b (Part A) Trial Design, A Single Dose, Open-Label Acute Hemodynamic Trial in IpcPH and CpcPH Subjects Days 2, 15, 29: ECHO Day 8, 43: Safety Right Heart Catheter is inserted Screening Follow-ups and hemodynamic measurements are Admit to Unit Day -28 to -2 captured at the timepoints listed above Day -1 Day 1 Hemodynamic data was prespecified to be pooled across all doses. After IV administration, all dose levels result in exposures which are in the predicted efficacious range during the 8-hour assessment period (i.e. above trough exposure of 2 ug/ml)
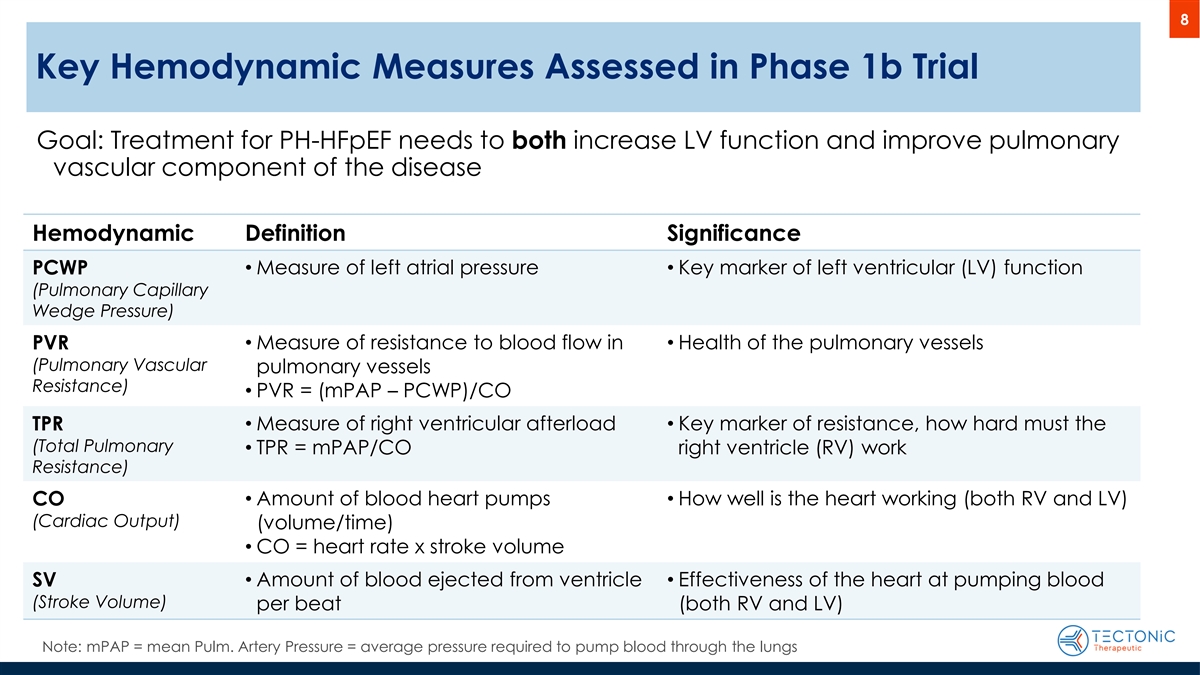
8 Key Hemodynamic Measures Assessed in Phase 1b Trial Phase 1b trial results that help predict APEX trial success Goal: Treatment for PH-HFpEF needs to both increase LV function and improve pulmonary vascular component of the disease Hemodynamic Definition Significance PCWP • Measure of left atrial pressure • Key marker of left ventricular (LV) function (Pulmonary Capillary Wedge Pressure) PVR • Measure of resistance to blood flow in • Health of the pulmonary vessels (Pulmonary Vascular pulmonary vessels Resistance) • PVR = (mPAP – PCWP)/CO • Measure of right ventricular afterload • Key marker of resistance, how hard must the TPR (Total Pulmonary • TPR = mPAP/CO right ventricle (RV) work Resistance) • Amount of blood heart pumps • How well is the heart working (both RV and LV) CO (Cardiac Output) (volume/time) • CO = heart rate x stroke volume SV • Amount of blood ejected from ventricle • Effectiveness of the heart at pumping blood (Stroke Volume) per beat (both RV and LV) Note: mPAP = mean Pulm. Artery Pressure = average pressure required to pump blood through the lungs
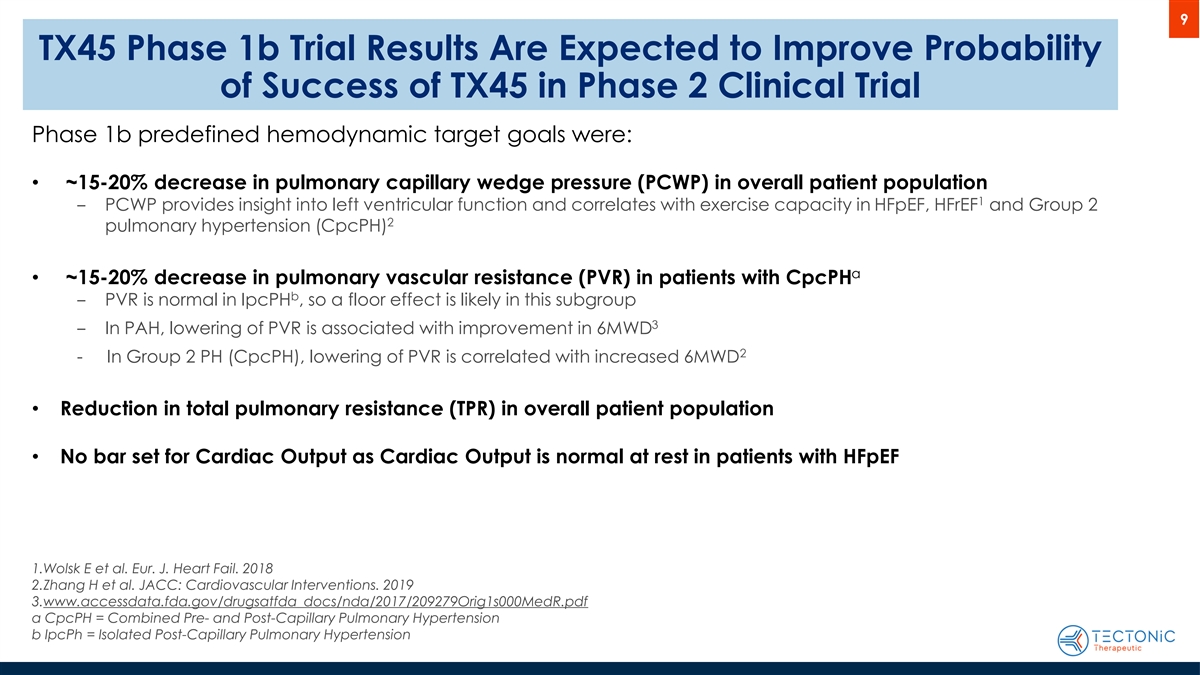
9 TX45 Phase 1b Trial Results Are Expected to Improve Probability of Success of TX45 in Phase 2 Clinical Trial Phase 1b predefined hemodynamic target goals were: • ~15-20% decrease in pulmonary capillary wedge pressure (PCWP) in overall patient population 1 ‒ PCWP provides insight into left ventricular function and correlates with exercise capacity in HFpEF, HFrEF and Group 2 2 pulmonary hypertension (CpcPH) a • ~15-20% decrease in pulmonary vascular resistance (PVR) in patients with CpcPH b ‒ PVR is normal in IpcPH , so a floor effect is likely in this subgroup 3 ‒ In PAH, lowering of PVR is associated with improvement in 6MWD 2 - In Group 2 PH (CpcPH), lowering of PVR is correlated with increased 6MWD • Reduction in total pulmonary resistance (TPR) in overall patient population • No bar set for Cardiac Output as Cardiac Output is normal at rest in patients with HFpEF 1.Wolsk E et al. Eur. J. Heart Fail. 2018 2.Zhang H et al. JACC: Cardiovascular Interventions. 2019 3.www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209279Orig1s000MedR.pdf a CpcPH = Combined Pre- and Post-Capillary Pulmonary Hypertension b IpcPh = Isolated Post-Capillary Pulmonary Hypertension
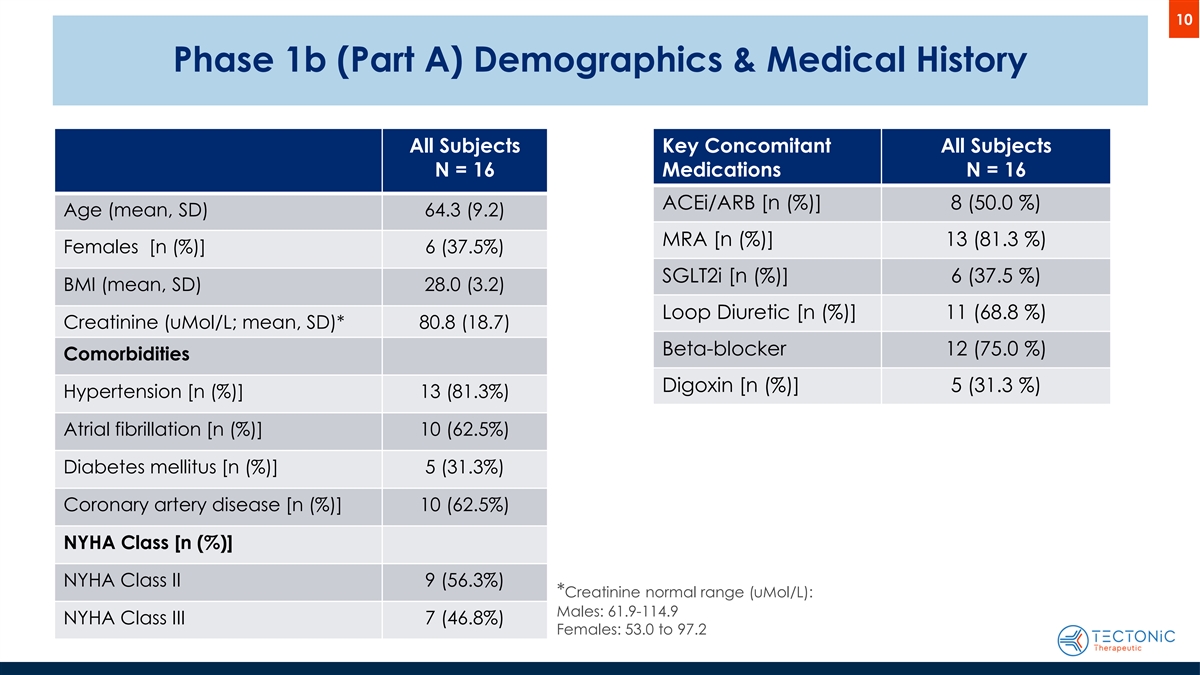
10 Phase 1b (Part A) Demographics & Medical History All Subjects Key Concomitant All Subjects N = 16 Medications N = 16 ACEi/ARB [n (%)] 8 (50.0 %) Age (mean, SD) 64.3 (9.2) MRA [n (%)] 13 (81.3 %) Females [n (%)] 6 (37.5%) SGLT2i [n (%)] 6 (37.5 %) BMI (mean, SD) 28.0 (3.2) Loop Diuretic [n (%)] 11 (68.8 %) Creatinine (uMol/L; mean, SD)* 80.8 (18.7) Beta-blocker 12 (75.0 %) Comorbidities Digoxin [n (%)] 5 (31.3 %) Hypertension [n (%)] 13 (81.3%) Atrial fibrillation [n (%)] 10 (62.5%) Diabetes mellitus [n (%)] 5 (31.3%) Coronary artery disease [n (%)] 10 (62.5%) NYHA Class [n (%)] NYHA Class II 9 (56.3%) *Creatinine normal range (uMol/L): Males: 61.9-114.9 NYHA Class III 7 (46.8%) Females: 53.0 to 97.2

11 Phase 1b (Part A) - Summary of Baseline Hemodynamics PVR < 2WU 2 WU ≤ PVR < 3WU PVR ≥3 WU Parameter Baseline Value [mean, SD] 7 4 5 Heart Rate (bpm) 68.1 (10.9) Systolic Blood Pressure (mm Hg) 128.8 (11.6) If CpcPH is defined as PVR >3: Total IpcPH = 11 Diastolic Blood Pressure (mm Hg) 78.5 (6.0) Total CpcPH = 5 Mean Pulmonary Artery Pressure (mm Hg) 26.6 (4.4) Pulmonary Capillary Wedge Pressure (mm Hg) 16.7 (3.2) If CpcPH is defined as PVR >2: Pulmonary Vascular Resistance (Woods Units) 2.45 (1.1) Total IpcPH = 7 Cardiac Output (L/min) 4.3 (1.0) Total CpcPH = 9 Stroke Volume mL 65 (18) Total Pulmonary Resistance (Woods Units) 6.5 (1.6)
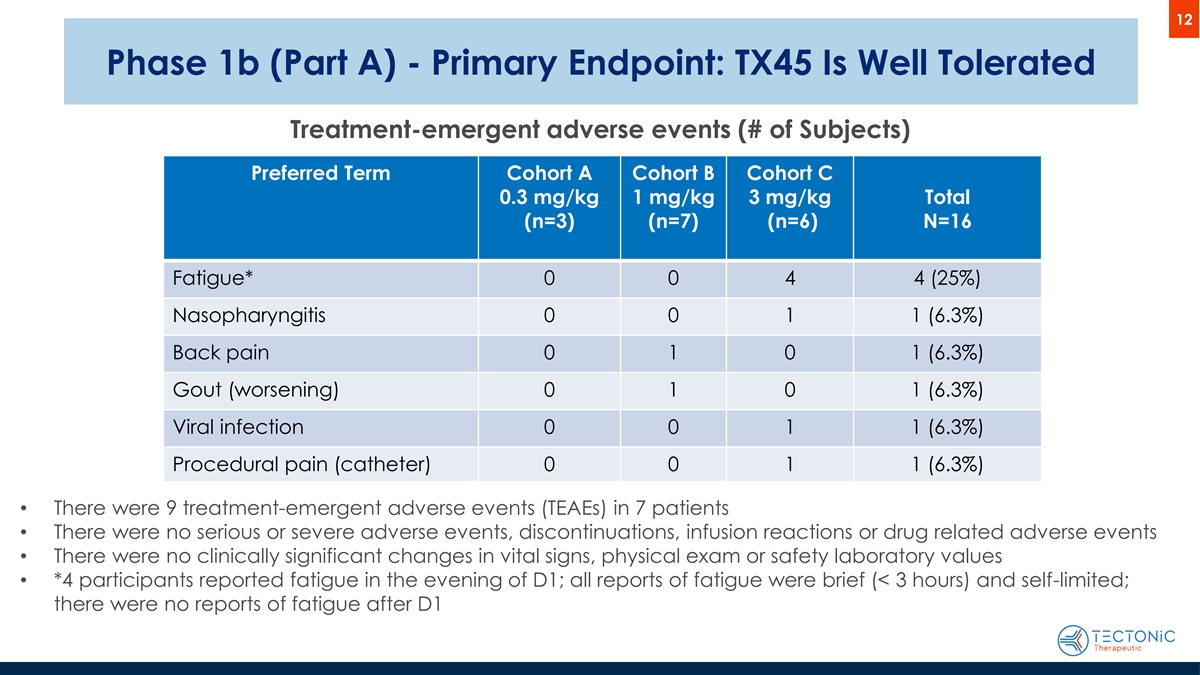
12 Phase 1b (Part A) - Primary Endpoint: TX45 Is Well Tolerated Treatment-emergent adverse events (# of Subjects) Preferred Term Cohort A Cohort B Cohort C 0.3 mg/kg 1 mg/kg 3 mg/kg Total (n=3) (n=7) (n=6) N=16 Fatigue* 0 0 4 4 (25%) Nasopharyngitis 0 0 1 1 (6.3%) Back pain 0 1 0 1 (6.3%) Gout (worsening) 0 1 0 1 (6.3%) Viral infection 0 0 1 1 (6.3%) Procedural pain (catheter) 0 0 1 1 (6.3%) • There were 9 treatment-emergent adverse events (TEAEs) in 7 patients • There were no serious or severe adverse events, discontinuations, infusion reactions or drug related adverse events • There were no clinically significant changes in vital signs, physical exam or safety laboratory values • *4 participants reported fatigue in the evening of D1; all reports of fatigue were brief (< 3 hours) and self-limited; there were no reports of fatigue after D1
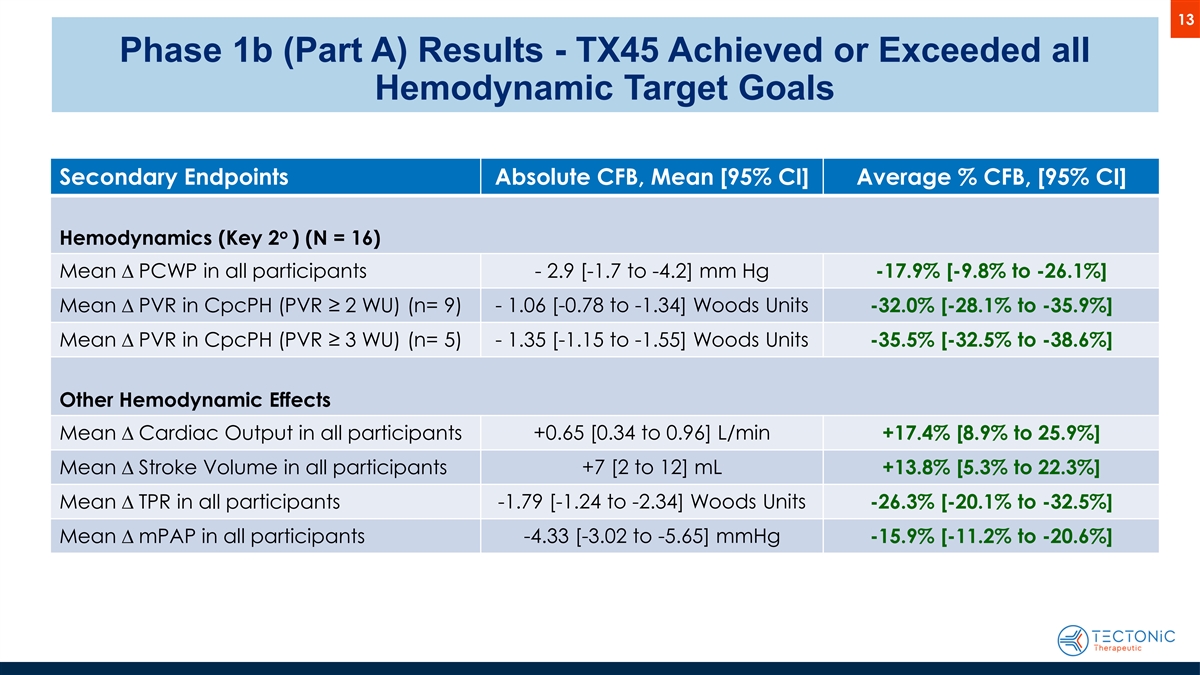
13 Phase 1b (Part A) Results - TX45 Achieved or Exceeded all Hemodynamic Target Goals Secondary Endpoints Absolute CFB, Mean [95% CI] Average % CFB, [95% CI] o Hemodynamics (Key 2 ) (N = 16) Mean D PCWP in all participants - 2.9 [-1.7 to -4.2] mm Hg -17.9% [-9.8% to -26.1%] Mean D PVR in CpcPH (PVR ≥ 2 WU) (n= 9) - 1.06 [-0.78 to -1.34] Woods Units -32.0% [-28.1% to -35.9%] Mean D PVR in CpcPH (PVR ≥ 3 WU) (n= 5) - 1.35 [-1.15 to -1.55] Woods Units -35.5% [-32.5% to -38.6%] Other Hemodynamic Effects Mean D Cardiac Output in all participants +0.65 [0.34 to 0.96] L/min +17.4% [8.9% to 25.9%] +7 [2 to 12] mL Mean D Stroke Volume in all participants +13.8% [5.3% to 22.3%] Mean D TPR in all participants -1.79 [-1.24 to -2.34] Woods Units -26.3% [-20.1% to -32.5%] -4.33 [-3.02 to -5.65] mmHg Mean D mPAP in all participants -15.9% [-11.2% to -20.6%]
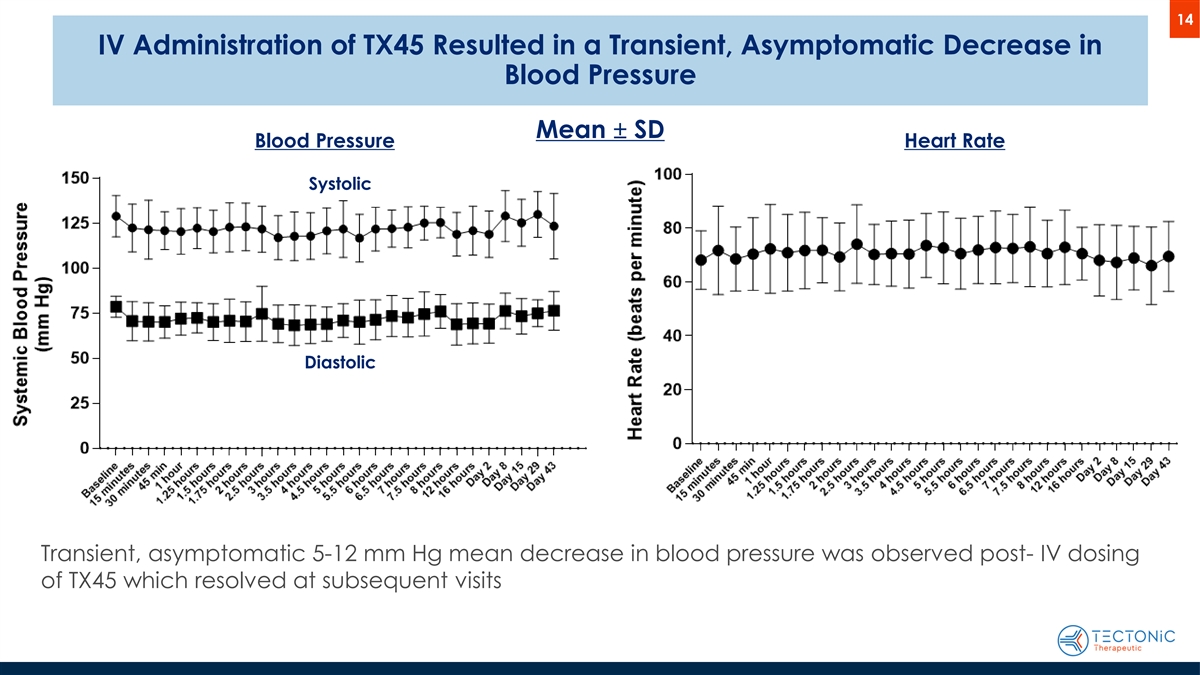
14 IV Administration of TX45 Resulted in a Transient, Asymptomatic Decrease in Blood Pressure Mean ± SD Blood Pressure Heart Rate Systolic Diastolic Transient, asymptomatic 5-12 mm Hg mean decrease in blood pressure was observed post- IV dosing of TX45 which resolved at subsequent visits
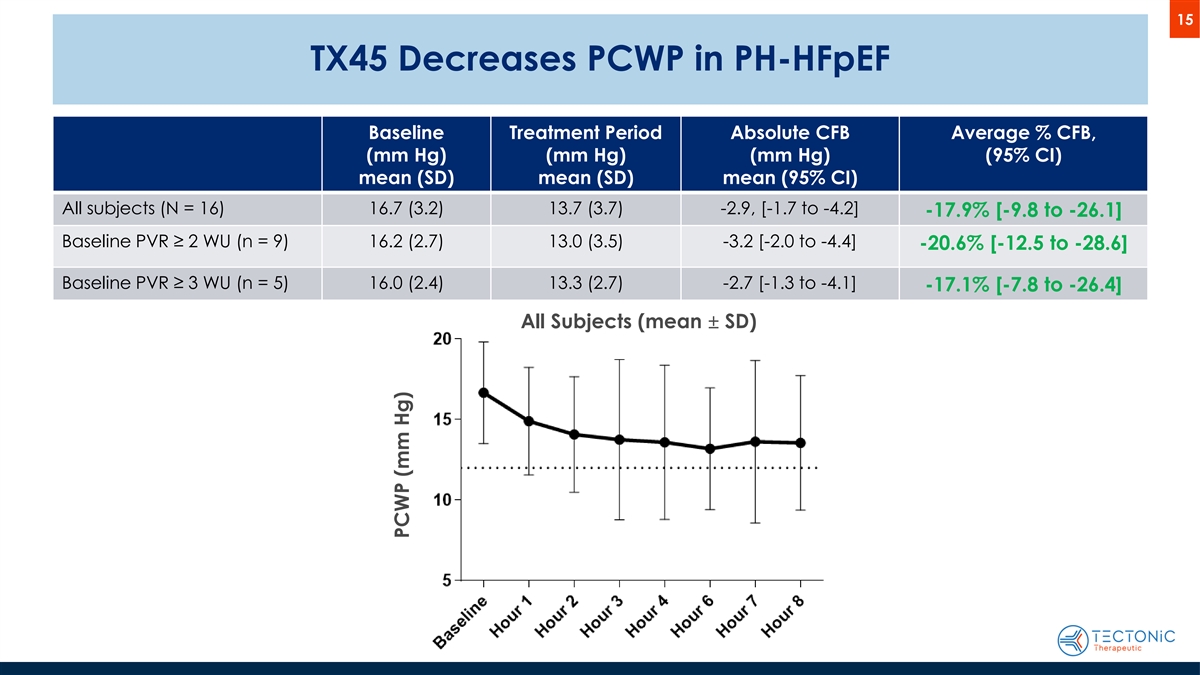
15 TX45 Decreases PCWP in PH-HFpEF Baseline Treatment Period Absolute CFB Average % CFB, (mm Hg) (mm Hg) (mm Hg) (95% CI) mean (SD) mean (SD) mean (95% CI) All subjects (N = 16) 16.7 (3.2) 13.7 (3.7) -2.9, [-1.7 to -4.2] -17.9% [-9.8 to -26.1] Baseline PVR ≥ 2 WU (n = 9) 16.2 (2.7) 13.0 (3.5) -3.2 [-2.0 to -4.4] -20.6% [-12.5 to -28.6] Baseline PVR ≥ 3 WU (n = 5) 16.0 (2.4) 13.3 (2.7) -2.7 [-1.3 to -4.1] -17.1% [-7.8 to -26.4] All Subjects (mean ± SD) PCWP (mm Hg)
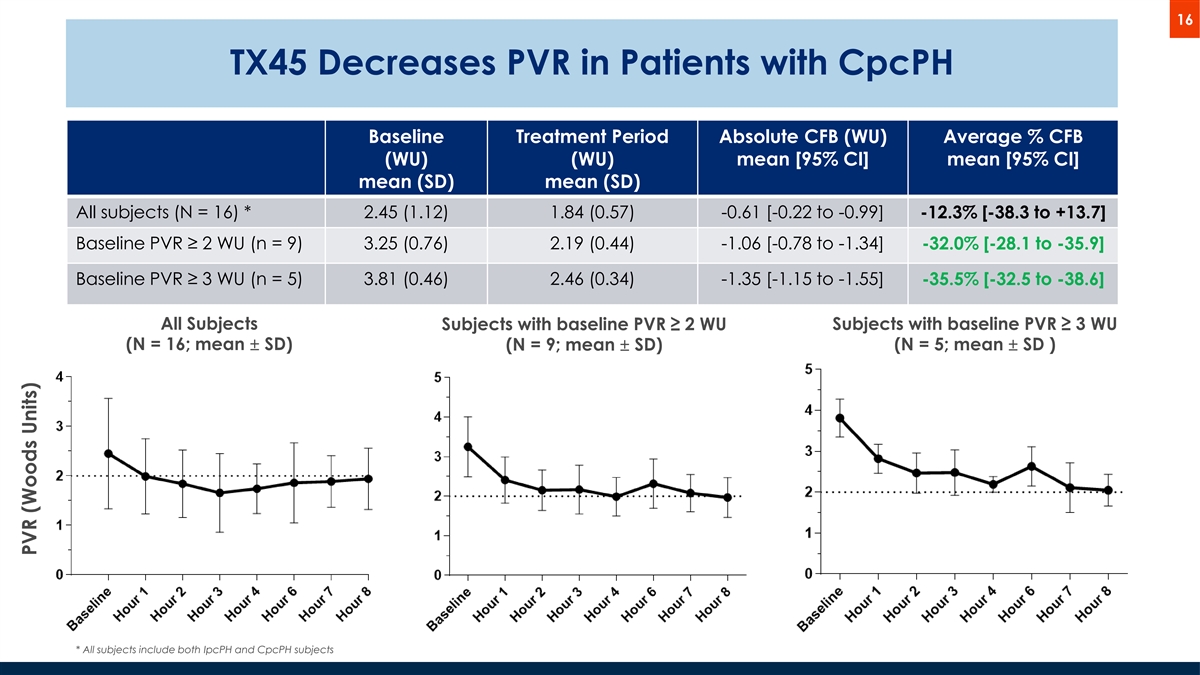
16 TX45 Decreases PVR in Patients with CpcPH Baseline Treatment Period Absolute CFB (WU) Average % CFB (WU) (WU) mean [95% CI] mean [95% CI] mean (SD) mean (SD) All subjects (N = 16) * 2.45 (1.12) 1.84 (0.57) -0.61 [-0.22 to -0.99] -12.3% [-38.3 to +13.7] Baseline PVR ≥ 2 WU (n = 9) 3.25 (0.76) 2.19 (0.44) -1.06 [-0.78 to -1.34] -32.0% [-28.1 to -35.9] Baseline PVR ≥ 3 WU (n = 5) 3.81 (0.46) 2.46 (0.34) -1.35 [-1.15 to -1.55] -35.5% [-32.5 to -38.6] All Subjects Subjects with baseline PVR ≥ 2 WU Subjects with baseline PVR ≥ 3 WU (N = 16; mean ± SD) (N = 5; mean ± SD ) (N = 9; mean ± SD) * All subjects include both IpcPH and CpcPH subjects PVR (Woods Units)

17 TX45 Improves Other Relevant Hemodynamics in PH-HFpEF TPR (WU) Cardiac Output (L/min) mPAP (mm Hg) All Subjects (N = 16) Baseline (mean, SD) 6.46 (1.65) 4.31 (1.01) 26.6 (4.4) Treatment Period (mean, SD) 4.68 (1.01) 4.96 (0.87) 22.3 (3.8) Absolute CFB (mean, 95% CI) -1.79 [-1.24 to -2.34] +0.65 [0.34 to 0.96] -4.3 (-3.0 to -5.7) Average % CFB (mean, 95% CI) -26.3% [-20.1 to -32.5] +17.4% [8.9 to 25.9] -15.9% [-11.2 to -20.6] Mean Pulmonary Artery Pressure Total Pulmonary Resistance Cardiac Output (N = 16; mean ± SD) (N = 16; mean ± SD) (N = 16; mean ± SD) TPR (Woods Units) CO (L/min) mPAP (mm Hg)
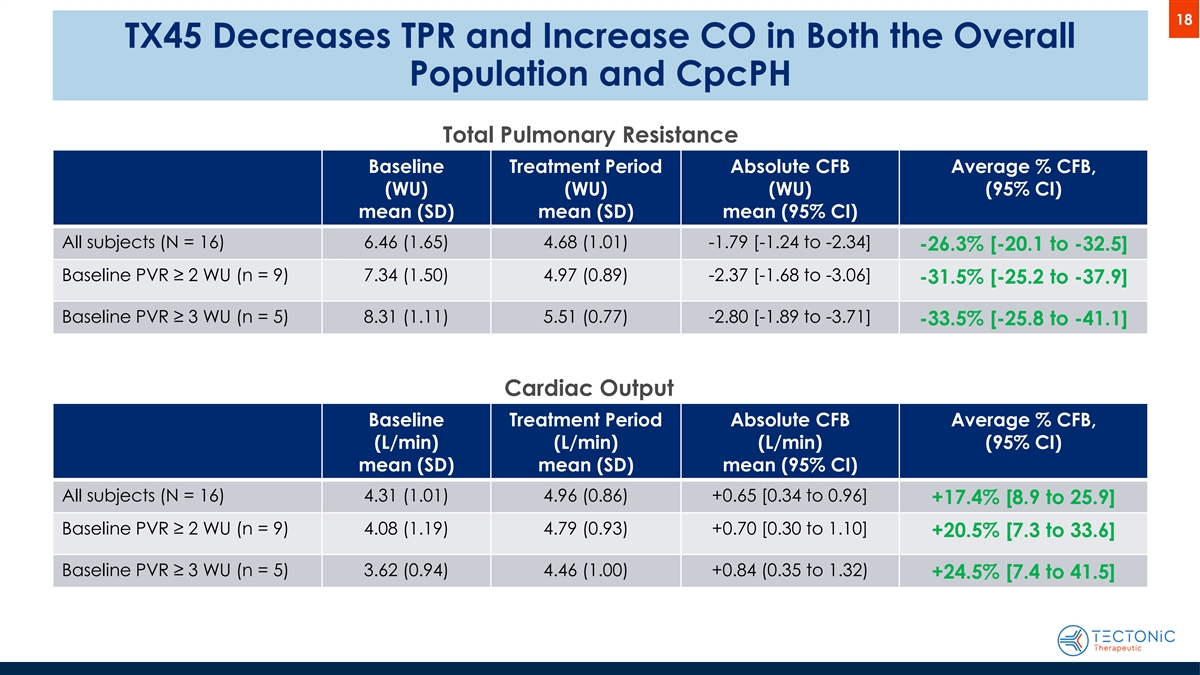
18 TX45 Decreases TPR and Increase CO in Both the Overall Population and CpcPH Total Pulmonary Resistance Baseline Treatment Period Absolute CFB Average % CFB, (WU) (WU) (WU) (95% CI) mean (SD) mean (SD) mean (95% CI) All subjects (N = 16) 6.46 (1.65) 4.68 (1.01) -1.79 [-1.24 to -2.34] -26.3% [-20.1 to -32.5] Baseline PVR ≥ 2 WU (n = 9) 7.34 (1.50) 4.97 (0.89) -2.37 [-1.68 to -3.06] -31.5% [-25.2 to -37.9] Baseline PVR ≥ 3 WU (n = 5) 8.31 (1.11) 5.51 (0.77) -2.80 [-1.89 to -3.71] -33.5% [-25.8 to -41.1] Cardiac Output Baseline Treatment Period Absolute CFB Average % CFB, (L/min) (L/min) (L/min) (95% CI) mean (SD) mean (SD) mean (95% CI) All subjects (N = 16) 4.31 (1.01) 4.96 (0.86) +0.65 [0.34 to 0.96] +17.4% [8.9 to 25.9] Baseline PVR ≥ 2 WU (n = 9) 4.08 (1.19) 4.79 (0.93) +0.70 [0.30 to 1.10] +20.5% [7.3 to 33.6] Baseline PVR ≥ 3 WU (n = 5) 3.62 (0.94) 4.46 (1.00) +0.84 (0.35 to 1.32) +24.5% [7.4 to 41.5]

19 Combined Decrease in PCWP and PVR Appears to Enhance Improvement in Exercise Capacity • Decreasing PCWP alone is expected to improve exercise capacity 1 o SGLT2 inhibitor dapagliflozin decreased resting PCWP 20% and increased 6MWT by 20m in 2 HFpEF • Decreasing both PCWP and PVR appears to further increase in exercise capacity o CpcPH patients undergoing pulmonary artery denervation surgery achieved a treatment- adjusted average decrease of 19% in PCWP and 32% in PVR, and increased 6MWT distance 3 69m [NOTE: This was a severe population of CpcPH patients (mean PVR>6 WU) and we expect impact on 6MWD will be clinically important but not as large as demonstrated in this study] 1 Borlaug B et al. Circulation 2023 2 Lewis GD et al. Circ. Heart Failure 2023 3 Zhang H et al. JACC Cardiovasc. Interv. 2019
20 TX45 Phase 1b Interim Data in PH-HFpEF Met/Exceeded our Expectations, Increasing Probability of Phase 2 Success • TX45 was well-tolerated • Transient asymptomatic decreases in BP were observed over the first 24 hours after an IV dose • TX45 observed to improve left heart function and pulmonary hemodynamics, which together should increase probability of success of Phase 2 Trial • Left Heart Function: 17.9% decrease in PCWP and 17.4% increase in Cardiac Output in overall population • Pulmonary Hemodynamics: 32.0-35.5% decrease in PVR in CpcPH and 26.3% decrease in TPR in overall pop. • TX45 has a differentiated profile compared with PAH drugs which improved pulmonary hemodynamics without an improvement in left heart function and failed in PH-HFpEF • These data support our focus on PH-HFpEF as the first indication for TX45, with enrichment in our Phase 2 trial for subjects with CpcPH where the benefit could be the greatest
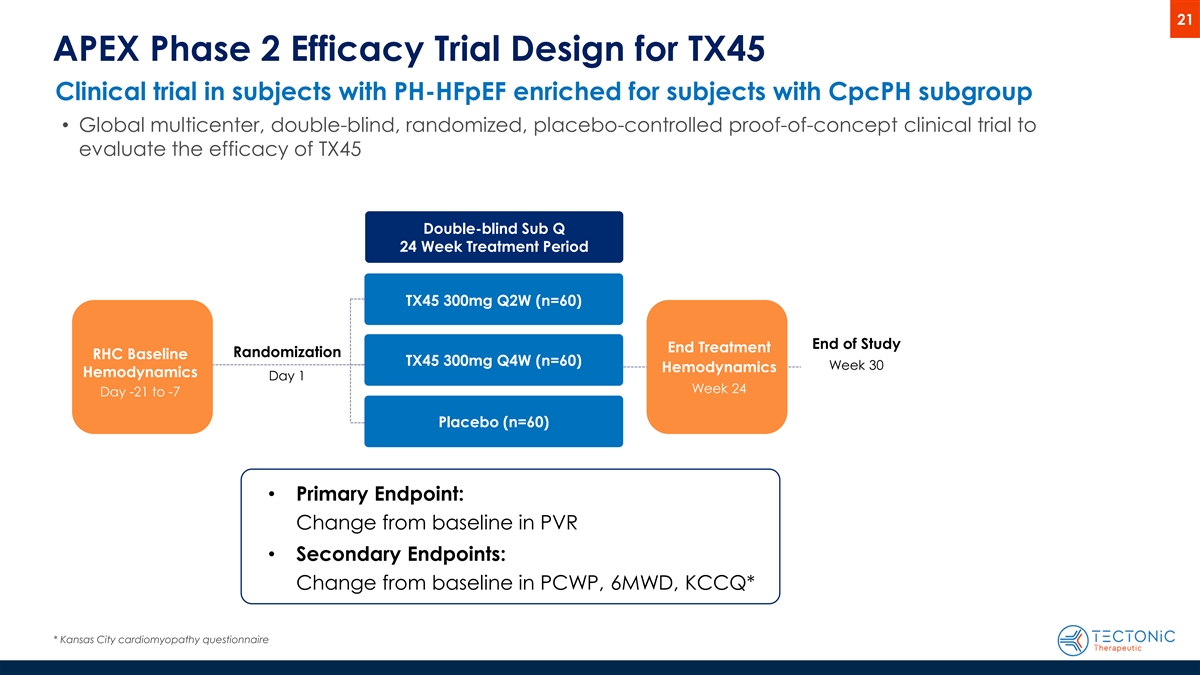
21 APEX Phase 2 Efficacy Trial Design for TX45 Clinical trial in subjects with PH-HFpEF enriched for subjects with CpcPH subgroup • Global multicenter, double-blind, randomized, placebo-controlled proof-of-concept clinical trial to evaluate the efficacy of TX45 Double-blind Sub Q 24 Week Treatment Period TX45 300mg Q2W (n=60) End of Study End Treatment Randomization RHC Baseline TX45 300mg Q4W (n=60) Week 30 Hemodynamics Hemodynamics Day 1 Week 24 Day -21 to -7 Placebo (n=60) • Primary Endpoint: Change from baseline in PVR • Secondary Endpoints: Change from baseline in PCWP, 6MWD, KCCQ* * Kansas City cardiomyopathy questionnaire
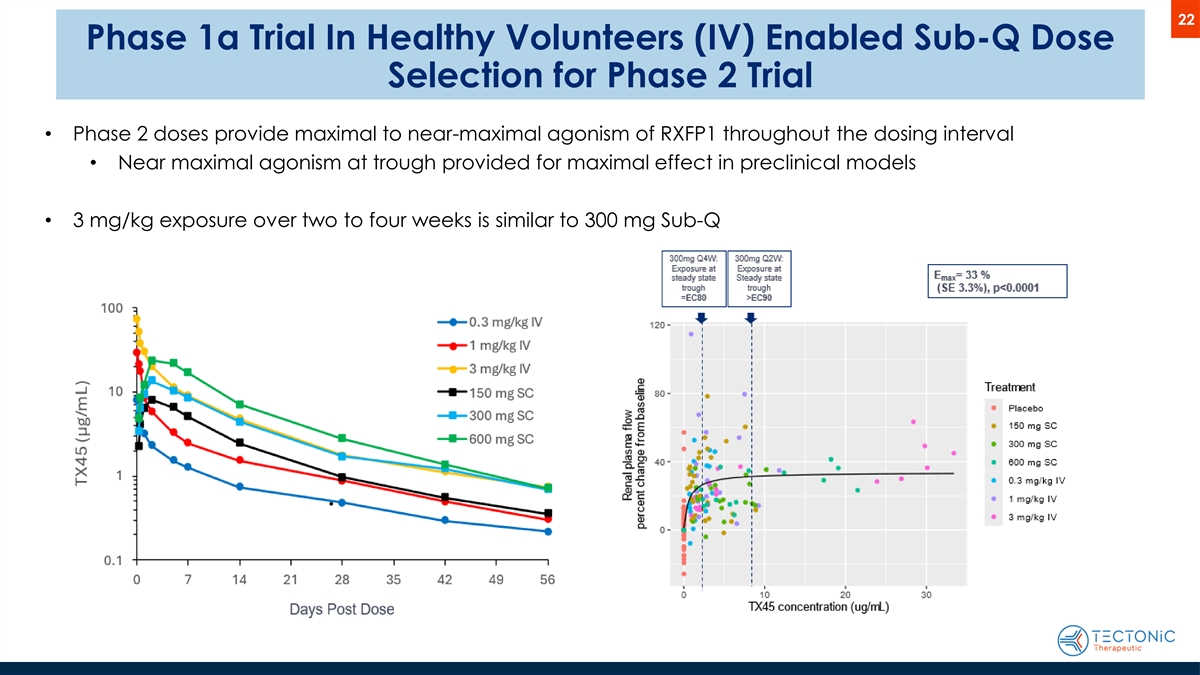
22 Phase 1a Trial In Healthy Volunteers (IV) Enabled Sub-Q Dose Selection for Phase 2 Trial • Phase 2 doses provide maximal to near-maximal agonism of RXFP1 throughout the dosing interval • Near maximal agonism at trough provided for maximal effect in preclinical models • 3 mg/kg exposure over two to four weeks is similar to 300 mg Sub-Q
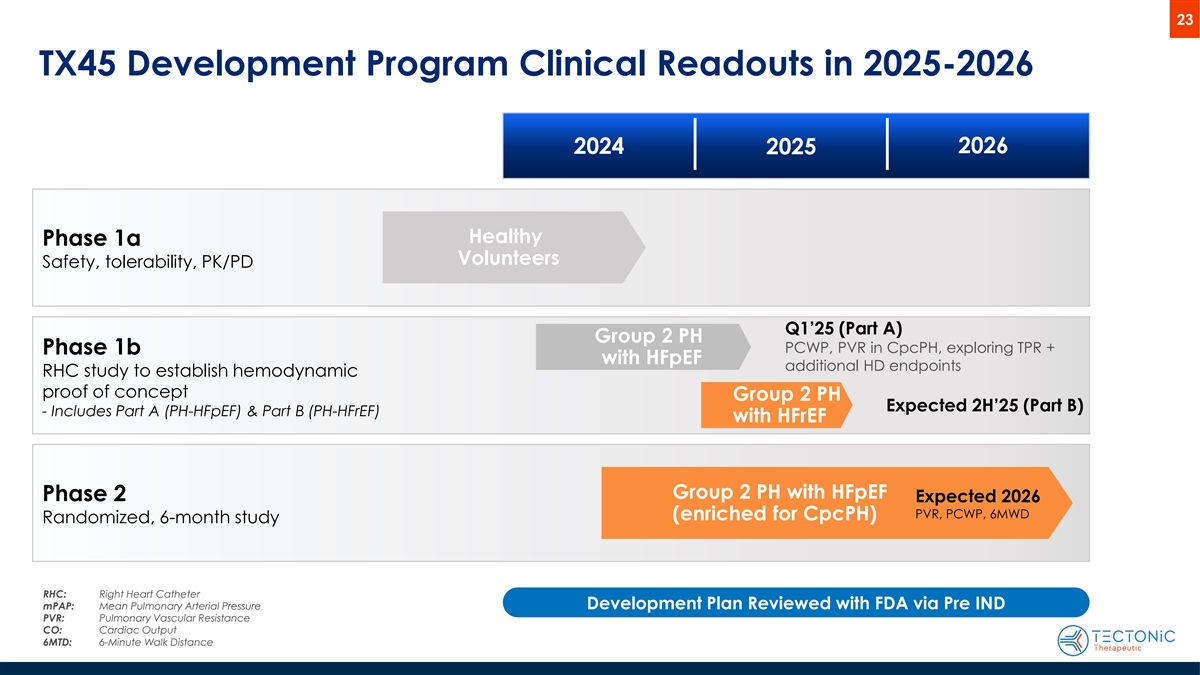
23 TX45 Development Program Clinical Readouts in 2025-2026 2024 2026 2025 Healthy Phase 1a Volunteers Safety, tolerability, PK/PD Q1’25 (Part A) Group 2 PH PCWP, PVR in CpcPH, exploring TPR + Phase 1b with HFpEF additional HD endpoints RHC study to establish hemodynamic proof of concept Group 2 PH Expected 2H’25 (Part B) - Includes Part A (PH-HFpEF) & Part B (PH-HFrEF) with HFrEF Group 2 PH with HFpEF Phase 2 Expected 2026 PVR, PCWP, 6MWD (enriched for CpcPH) Randomized, 6-month study RHC: Right Heart Catheter mPAP: Mean Pulmonary Arterial Pressure Development Plan Reviewed with FDA via Pre IND PVR: Pulmonary Vascular Resistance CO: Cardiac Output 6MTD: 6-Minute Walk Distance