Exhibit 99.2

Making it Possible October 2019

Except for historical information, all of the statements, expectations, and assumptions contained in this presentation are forward - looking statements . Forward - looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions, including estimated market opportunities in the United States for our product candidates . These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management . These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict . Therefore, actual outcomes and results may, and are likely to, differ materially from what is expressed or forecasted in the forward - looking statements due to numerous factors discussed from time to time in documents which we file with the SEC . In addition, such statements could be affected by risks and uncertainties related to, among other things : the potential success of our reprioritized pipeline ; our ability to identify new product candidates ; the rate and degree of the market acceptance and clinical utility of our product candidates ; our estimates regarding the potential market opportunity for our product candidates ; the potential advantages of our product candidates ; risks involved in clinical trials, including, but not limited to, the initiation, timing, progress and results of such trials ; the timing and our ability to submit applications for, and obtain and maintain regulatory approvals for, our product candidates ; our ability to timely develop and implement manufacturing, commercialization, and marketing capabilities and strategies for existing product candidates ; fluctuations in our financial results ; our ability to raise money ; intellectual property risks ; changes in legal, regulatory and legislative environments in the markets in which we operate and the impact of these changes on our ability to obtain regulatory approval for our products ; and our competitive position . Any forward - looking statements speak only as of the date on which they are made, and except as may be required under applicable securities laws, we do not undertake any obligation to update any forward - looking statements . 1 Forward - Looking Statements
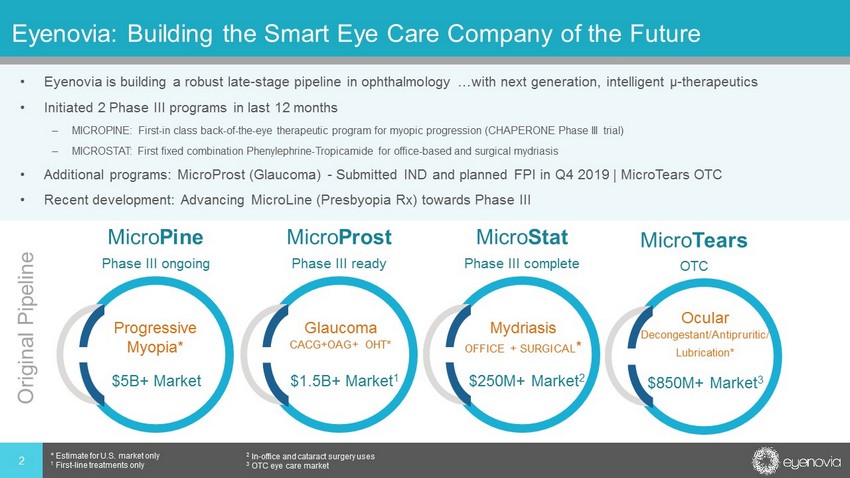
Eyenovia: Building the Smart Eye Care Company of the Future • Eyenovia is building a robust late - stage pipeline in ophthalmology …with next generation, intelligent µ - therapeutics • Initiated 2 Phase III programs in last 12 months – MICROPINE: First - in class back - of - the - eye therapeutic program for myopic progression (CHAPERONE Phase III trial) – MICROSTAT: First fixed combination Phenylephrine - Tropicamide for office - based and surgical mydriasis • Additional programs: MicroProst (Glaucoma) - Submitted IND and planned FPI in Q4 2019 | MicroTears OTC • Recent development: Advancing MicroLine (Presbyopia Rx) towards Phase III 2 * Estimate for U.S. market only 1 First - line treatments only 2 In - office and cataract surgery uses 3 OTC eye care market Micro Pine Phase III ongoing Micro Prost Phase III ready Micro Stat Phase III complete Micro Tears OTC Progressive Myopia* Glaucoma CACG+OAG+ OHT* Mydriasis OFFICE + SURGICAL * $5B+ Market $1.5B+ Market 1 $250M+ Market 2 Ocular Decongestant/Antipruritic/ Lubrication* $850M+ Market 3 Original Pipeline

Eyenovia Updated Pipeline: Prioritize Presbyopia Phase III Program • PRESBYOPIA: Prioritize MicroLine for the pharmacologic treatment of presbyopia – Initiate and complete MicroLine Phase III VISION study in 2020 • MYOPIC PROGRESSION: Continue CHAPERONE Ph III trial with complete enrollment in 2020 • MYDRIASIS: Phase III studies successful, NDA on track for 2020 filing • DEFERRED PROGRAMS: MicroProst and MicroTears 3 * Estimate for U.S. market only 1 In - office and cataract surgery uses PRESBYOPIA* MYDRIASIS OFFICE + SURGICAL * $2B+ Market $250M+ Market 1 Micro Line Ph III VISION Micro Stat Ph III MIST - 1 MIST - 2 Micro Pine Ph III CHAPERONE PROGRESSIVE MYOPIA* $5B+ Market

4 Multiple Late Stage Clinical Assets Back - of - the - eye Indications Front - of - the - eye Indications Preclinical/Formulation Phase I Phase II Phase III Anticipated 2020 Milestones Micro Pine Reduction of Pediatric Myopia Progression CHAPERONE Enrollment Completion Micro Line Improvement in near vision in patients with presbyopia VISION Initiation and Completion Micro Stat Pharmacologic Mydriasis (Pupil Dilation) NDA Filing
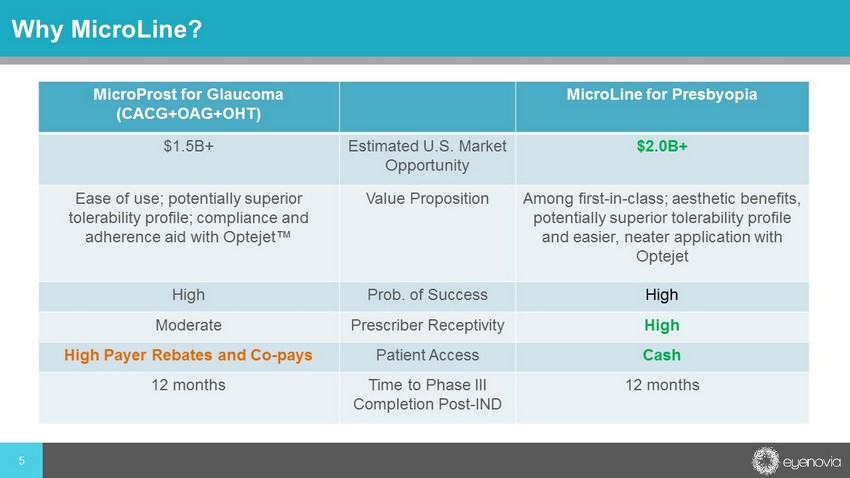
5 Why MicroLine ? Glaucoma US Market $1.5Bn MicroProst Addressable Market $150 - 200M Phase III Results H2 2020 FDA Approval est. H1 2022 Presbyopia US Market $ Bn MicroLine Addressable Market $ M Phase III Results H2 2020 FDA Approval est. H1 2022 MicroProst for Glaucoma (CACG+OAG+OHT) MicroLine for Presbyopia $1.5B+ Estimated U.S. Market Opportunity $2.0B+ Ease of use; potentially superior tolerability profile; compliance and adherence aid with Optejet ™ Value Proposition Among first - in - class; aesthetic benefits, potentially superior tolerability profile and easier, neater application with Optejet High Prob. of Success High Moderate Prescriber Receptivity High High Payer Rebates and Co - pays Patient Access Cash 12 months Time to Phase III Completion Post - IND 12 months

Etiology • Non - preventable, age - related hardening of the lens Symptoms • Tendency to hold reading material farther away to make the letters clearer • Blurred vision at normal reading distance • Eye strain , headaches after reading or doing close - up work Risk Factors • Age • Medical conditions and co - morbidities such as cardiovascular conditions, Multiple sclerosis, Type 2 diabetes can increase risk of premature presbyopia • Drugs associated with premature symptoms: antidepressants, anti - histamines, diuretics Diagnosis • Basic eye exam, with refraction assessment 6 Presbyopia: the Progressive Loss of Ability to Focus on Nearby Objects Sources: Mayo Clinic Presbyopia Overview. Wollfsohn et al. Prog Retin Eye Res. Fernandez et al. J Ophthalm . Accessed December 2018
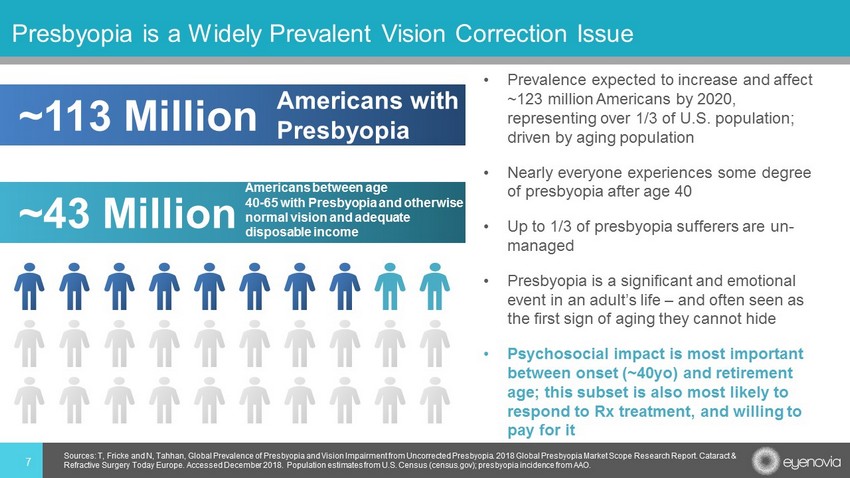
• Prevalence expected to increase and affect ~123 million Americans by 2020, representing over 1/3 of U.S. population; driven by aging population • Nearly everyone experiences some degree of presbyopia after age 40 • Up to 1/3 of presbyopia sufferers are un - managed • Presbyopia is a significant and emotional event in an adult’s life – and often seen as the first sign of aging they cannot hide • Psychosocial impact is most important between onset (~40yo) and retirement age; this subset is also most likely to respond to Rx treatment, and willing to pay for it 7 Presbyopia is a Widely Prevalent Vision Correction Issue Sources: T, Fricke and N, Tahhan , Global Prevalence of Presbyopia and Vision Impairment from Uncorrected Presbyopia. 2018 Global Presbyopia Market Scope Rese arc h Report. Cataract & Refractive Surgery Today Europe. Accessed December 2018. Population estimates from U.S. Census (census.gov); presbyopia inci den ce from AAO. ~43 Million Americans between age 40 - 65 with Presbyopia and otherwise normal vision and adequate disposable income ~113 Million Americans with Presbyopia

Indication • For the Improvement in near vision in patients with presbyopia • Provides approximately 3 - 4 hours of near vision with a single microRx spray Program Overview • 2 Phase III trials ready for initiation in 2020 Commercial • Estimated addressable population: Adults between 40 - 65 years old with otherwise normal vision and adequate disposable income • Estimated addressable U.S. market: $2B+ • Anticipated reimbursement: Cash pay Competition • Anticipated among first to market, including Allergan’s pilocarpine Phase III eyedrop program • Eyenovia is the only presbyopia product with piezo - print horizontal delivery and microdosing , designed to address potential pilocarpine side effects and improve user experience 8 MicroLine : Pharmacologic Treatment of Presbyopia Phase III ready program for the Improvement in near vision in patients with presbyopia
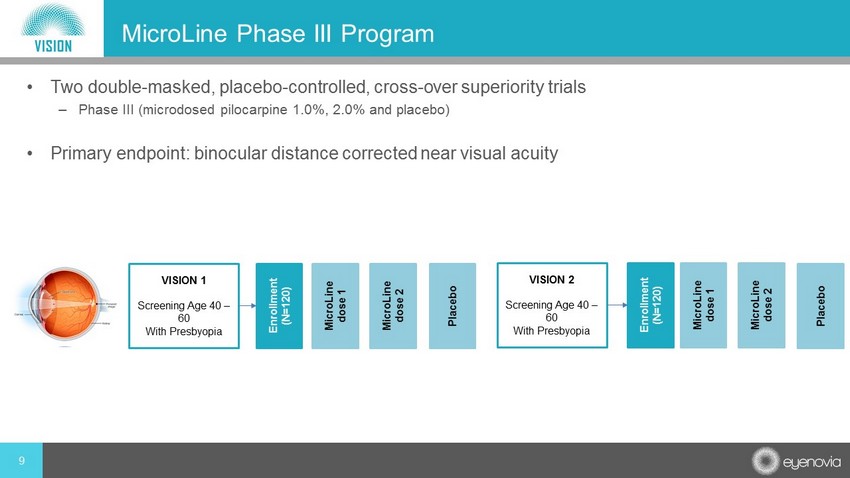
9 MicroLine Phase III Program • Two double - masked, placebo - controlled, cross - over superiority trials – Phase III (microdosed pilocarpine 1.0%, 2.0% and placebo) • Primary endpoint: binocular distance corrected near visual acuity VISION 1 Screening Age 40 – 60 With Presbyopia VISION 2 Screening Age 40 – 60 With Presbyopia Enrollment (N=120) Enrollment (N=120) MicroLine dose 1 MicroLine dose 2 Placebo MicroLine dose 1 MicroLine dose 2 Placebo
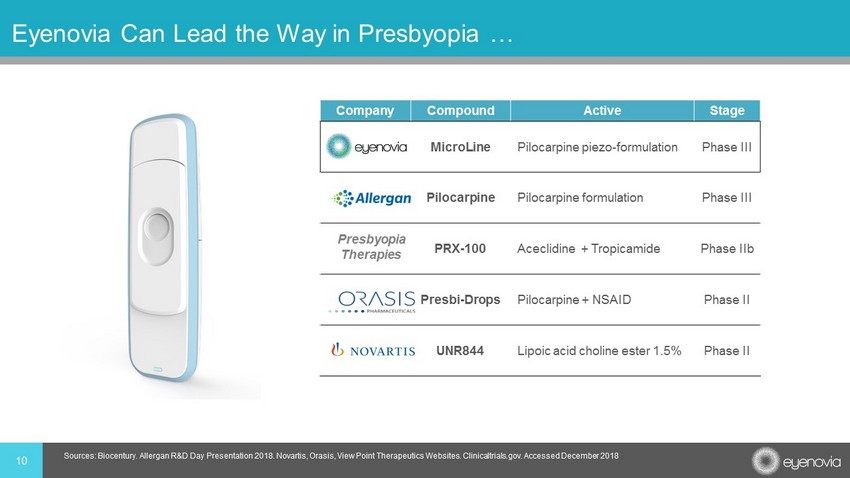
10 Eyenovia Can Lead the Way in Presbyopia … Company Compound Active Stage MicroLine Pilocarpine piezo - formulation Phase III Pilocarpine Pilocarpine formulation Phase III PRX - 100 Aceclidine + Tropicamide Phase IIb Presbi - Drops Pilocarpine + NSAID Phase II UNR844 Lipoic acid choline ester 1.5% Phase II Presbyopia Therapies Sources: Biocentury . Allergan R&D Day Presentation 2018. Novartis, Orasis , View Point Therapeutics Websites. Clinicaltrials.gov. Accessed December 2018

Making it Possible October 2019











