Exhibit 99.2

Formosa – Eyenovia Collaboration

• Publicly traded (6838.TWO) Taiwanese clinical stage biotechnology company – Market cap $200M – Ophthalmology and oncology (biosimilars) focus • In topical eye care, Formosa’s proprietary nanoparticle formulation technology improves the dissolution and bioavailability of drugs – Drugs used in suspensions and possibly emulsions • Ophthalmic products in development using this technology include: – Post - surgical inflammation and pain eye drop (to be filed with the US FDA in 2023) – Anti - infective eye drop with potential for meibomian gland dysfunction and dry eye disease 1 Formosa Pharmaceuticals
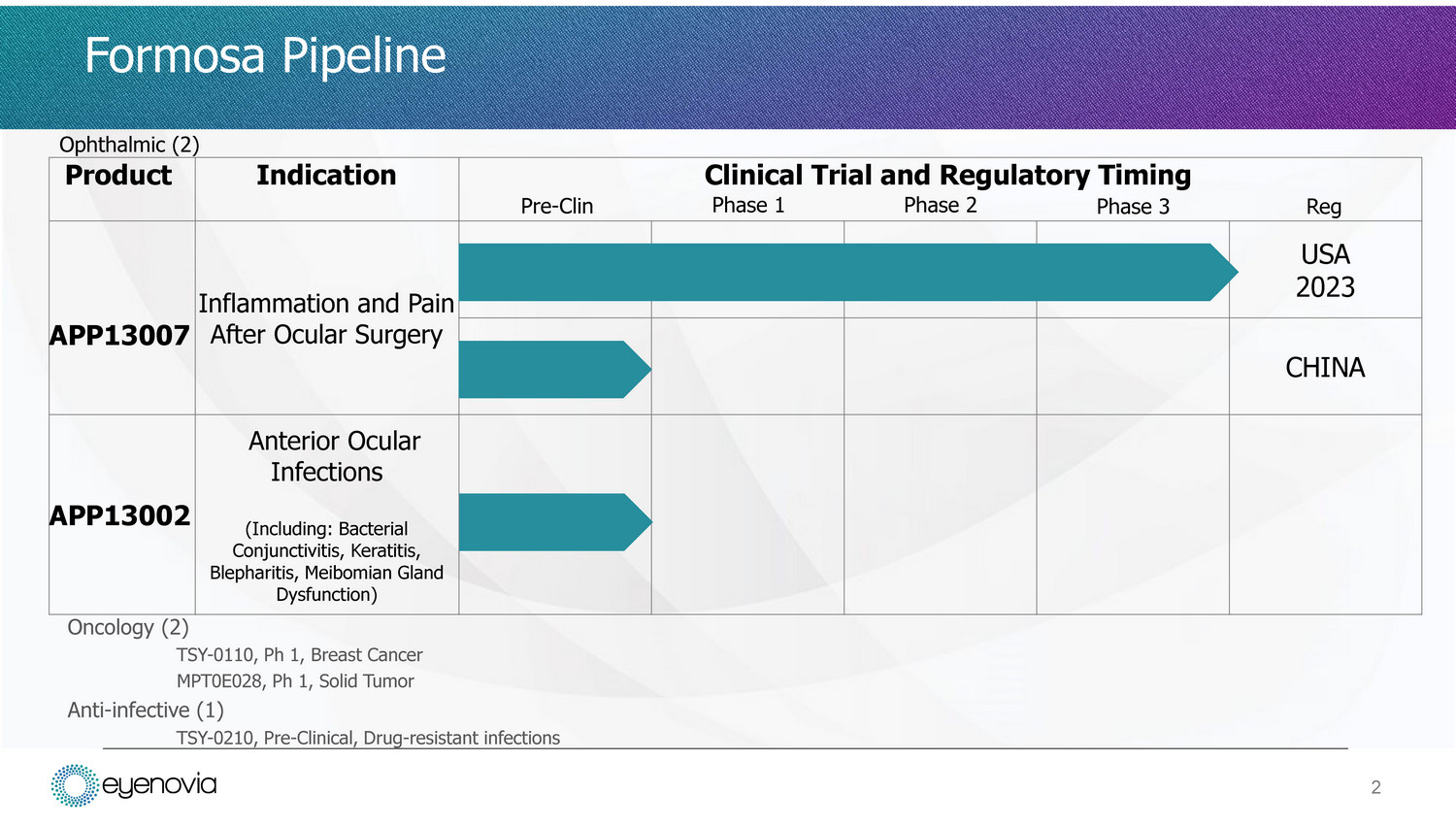
Formosa Pipeline Product Indication Pre - Clin Clinical Trial and Regulatory Timing Phase 1 Phase 2 Phase 3 Reg APP13007 Inflammation and Pain After Ocular Surgery USA 202 3 CHINA A PP 13 0 02 Anterior Ocular Infections (Including: Bacterial Conjunctivitis, Keratitis, Blepharitis, Meibomian Gland Dysfunction) Oncology (2) TSY - 0110, Ph 1, Breast Cancer MPT0E028, Ph 1, Solid Tumor Anti - infective (1) TSY - 0210, Pre - Clinical, Drug - resistant infections Ophthalmic (2) 2

• APNT Œ (Active Pharmaceutical Nanoparticle Technology) works to reduce particle size leading to improved dissolution, bioavailability, and lowers the risk of contamination – Reduces particle size by using common salts and sugars as milling media which can remain a part of the final formulation – The resulting formulation has high uniformity and purity allowing for penetration and enhanced bioavailability • Leading product candidate clobetasol propionate ophthalmic nanosuspension for post - surgical inflammation has completed Phase 3 trials and is being prepared for NDA submission – In clinical trials, shows comparable safety profile to placebo with a low incidence of IOP elevation, and potent clearance of inflammation with rapid pain relief 3 APNT Œ Technology

• Formosa’s APNT Œ technology can adjust the particle size of formulations increasing the probability of success with the Optejet ® Broadens the Optejet’s usability to existing suspension and emulsion products with viscosities that currently make them incompatible • Ophthalmic suspensions and emulsions make up over $1.5B in US sales in the dry eye, glaucoma, inflammation, anti - infective/inflammation combination categories • The APNT Œ platform may: – improve stability and lead to longer shelf life – improve dispersion properties eliminating shake before use requirements – improve bioavailability that when combined with Optejet’s targeted corneal delivery could lead to potent efficacy results 4 Synergistic Technology

• Eyenovia and Formosa Pharmaceuticals have agreed to a collaborate in the development of an APNT Œ formulation to be dispensed using the Optejet ® • In 2023, Eyenovia will conduct feasibility testing of novel APNT Œ formulations in the Optejet and request a pre - IND meeting with the FDA • Formosa will develop and optimize new APNT Œ formulations for use in the Optejet and deliver to Eyenovia for device qualification and validation • If successful, the companies will discuss an agreement for the co - development of a differentiated asset in a multi - billion dollar market 5 Collaboration Agreement





