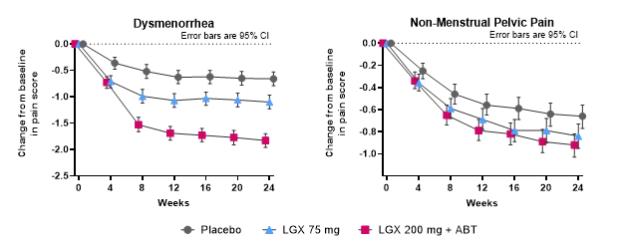
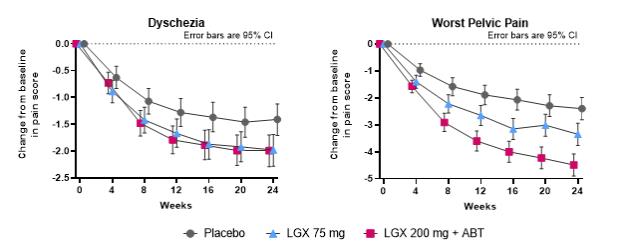
Endometriosis is a disease in which the endometrium (tissue lining the inside of the uterus) is found outside the uterus, where it induces a chronic inflammatory reaction that may result in scar tissue. It is primarily found on the pelvic peritoneum, on the ovaries, in the rectovaginal septum, on the bladder and bowel. The most common symptom of endometriosis is pelvic pain, which often correlates to the menstrual cycle. Patients may also experience painful ovulation, pain during or after sexual intercourse (dyspareunia), dyschezia (difficult or painful defecation), heavy bleeding, fatigue, and infertility. Endometriosis pain can be so severe and debilitating that it affects day-to-day activities and has a negative impact on general, physical, mental, and social well-being. Endometriosis treatments aim first to alleviate pain, then to remove or decrease the size and number of endometrial lesions, and possibly improve fertility.
About Linzagolix
Linzagolix is a novel, once daily, oral GnRH receptor antagonist with a potentially best-in-class profile.1,2,3 Linzagolix has completed clinical trial development for the treatment of uterine fibroids and is currently in late-stage clinical development for the treatment of pain associated with endometriosis. ObsEva licensed linzagolix from Kissei in late 2015 and retains worldwide commercial rights, excluding Asia, for the product. Linzagolix is not currently approved anywhere in the world.
About ObsEva
ObsEva is a biopharmaceutical company developing and commercializing novel therapies to improve women’s reproductive health and pregnancy. Through strategic in-licensing and disciplined drug development, ObsEva has established a late-stage clinical pipeline with development programs focused on new therapies for the treatment of uterine fibroids, endometriosis, and preterm labor. ObsEva is listed on the Nasdaq Global Select Market and is traded under the ticker symbol “OBSV” and on the SIX Swiss Exchange where it is traded under the ticker symbol “OBSN”. For more information, please visit www.ObsEva.com
About Kissei Pharmaceutical Co., Ltd.
Kissei is a Japanese pharmaceutical company based on the management philosophy “contributing to society through high-quality, innovative pharmaceutical products” and “serving society through our employees.” As a strong R&D-oriented corporation, it concentrates on providing innovative pharmaceuticals to patients worldwide in the focus fields of urology, nephrology/dialysis, gynecology and rare/intractable diseases.
Cautionary Note Regarding Forward-Looking Statements
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “anticipate”, “believe”, “continue”, “could”, “estimate”, “expect”, “intend”, “may”, “might”, “ongoing”, “objective”, “plan”, “potential”, “predict”, “should”, “will”, “would”, or the negative of these and similar expressions, and are based on ObsEva’s current beliefs and expectations. These forward-looking statements include expectations regarding the clinical development of ObsEva’s product candidates, including the timing, advancement of, and potential therapeutic benefits of such product candidates, including linzagolix, the anticipated milestones and pipeline updates, the timing of expected results from the EDELWEISS clinical trial, the potential for such product candidates to be commercially competitive, the success of the Company’s partnerships with third parties, expectations regarding regulatory and development milestones and ObsEva’s ability to obtain and maintain regulatory approvals for its product candidates. These statements involve risks and uncertainties