
PROLONG A phase 2a, double-blind, parallel group, randomised, placebo controlled, proof of concept study to assess the efficacy, safety and pharmacokinetics of EBOPIPRANT (OBE022) added-on to atosiban, after oral administration in pregnant women with spontaneous preterm labour. Main study results November 2020 Exhibit 99.2

Disclaimer Matters discussed in this presentation may constitute forward-looking statements. The forward-looking statements contained in this presentation reflect our views as of the date of this presentation about future events and are subject to risks, uncertainties, assumptions, and changes in circumstances that may cause our actual results, performance, or achievements to differ significantly from those expressed or implied in any forward-looking statement. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future events, results, performance, or achievements. Some of the key factors that could cause actual results to differ from our expectations include our plans to develop and potentially commercialize our product candidates; our planned clinical trials and preclinical studies for our product candidates; the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; the extent of clinical trials potentially required for our product candidates; the clinical utility and market acceptance of our product candidates; our commercialization, marketing and manufacturing capabilities and strategy; our intellectual property position; and our ability to identify and in-license additional product candidates. For further information regarding these risks, uncertainties and other factors that could cause our actual results to differ from our expectations, you should read he risk factors set forth in our Annual Report on Form 20-F for the year ended December 31, 2019 filed with the SEC on March 5, 2020 and the risk factors disclosed in the Report on Form 6-K filed with the SEC on November 5, 2020, and our other filings we make with the Securities and Exchange Commission from time to time. We expressly disclaim any obligation to update or revise the information herein, including the forward-looking statements, except as required by law. Please also note that this presentation does not constitute an offer to sell or a solicitation of an offer to buy any securities. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.

Preterm birth is to be born before week 37 of pregnancy Life altering and costly U.S. economic burden $26B /yr >1 In 10 babies are born preterm preterm related deaths in 2015 WW 1million LEADING cause of death in children under age 5 Babies surviving early birth face greater likelihood of lifelong disabilities Preterm birth, a costly burden per baby $16.9B+ U.S. infant medical costs $195K+ average cost per U.S. survivor infant born 24-26 weeks $50K average U.S. cost for a preterm infant WHO ‘Born Too Soon: The Global Action Report on Preterm Birth’ (2012); Kissin et al. NEJM, 2014 Behrman et al., National Academies Press, 2007

Professor Ben Mol, MD, PhD Professor of Obstetrics and Gynecology, Monash University, Australia “We desperately need new medical treatments for preterm labor to reduce the incidence of preterm birth, which accounts for about 10% of all births.” “A delay of delivery with 48 hours is extremely important as this allows transfer of women to a center with neonatal intensive care facilities, and it allows corticosteroids administered to the mother to have maximal effectiveness for the baby” “The development of ebopiprant is exciting and the results of PROLONG are promising” Melbourne, November 2020
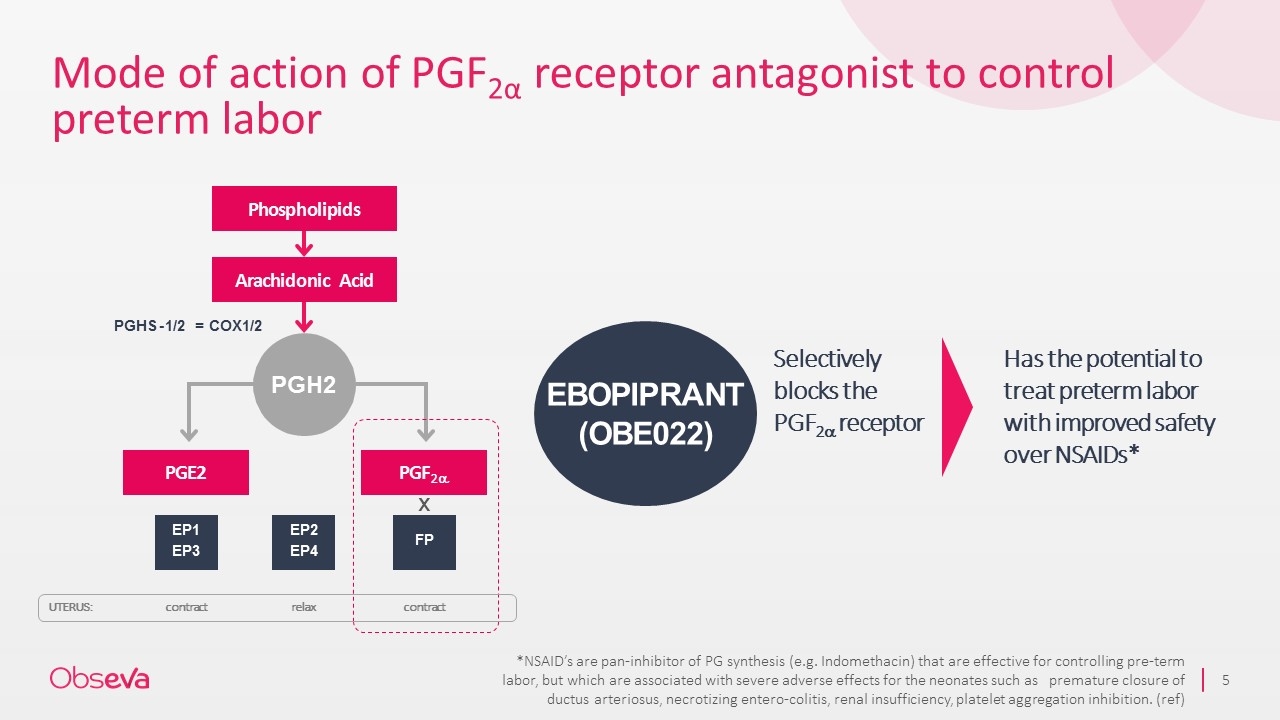
Mode of action of PGF2α receptor antagonist to control preterm labor PGE2 PGF2a PGHS - 1/2 = COX1/2 EP1 EP3 EP2 EP4 FP PGH2 contract relax contract Selectively blocks the PGF2a receptor Has the potential to treat preterm labor with improved safety over NSAIDs* EBOPIPRANT (OBE022) UTERUS: Phospholipids Arachidonic Acid x *NSAID’s are pan-inhibitor of PG synthesis (e.g. Indomethacin) that are effective for controlling pre-term labor, but which are associated with severe adverse effects for the neonates such as premature closure of ductus arteriosus, necrotizing entero-colitis, renal insufficiency, platelet aggregation inhibition. (ref)
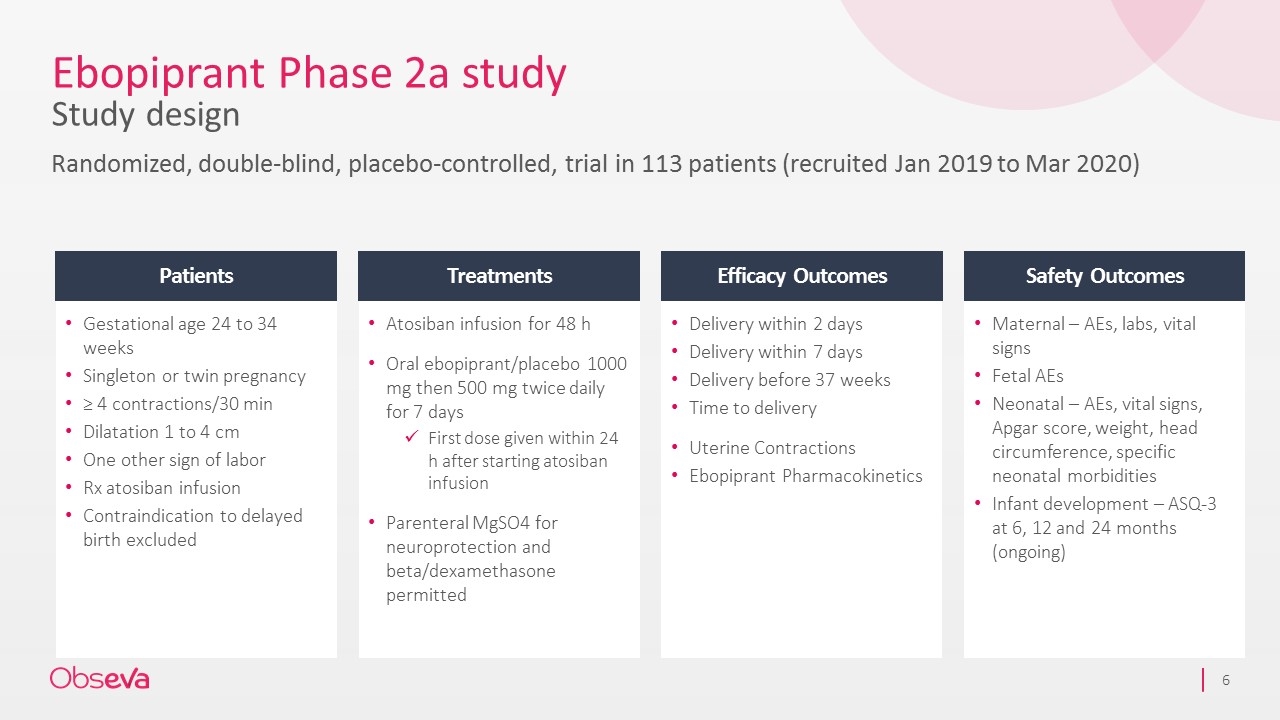
Ebopiprant Phase 2a study Study design Randomized, double-blind, placebo-controlled, trial in 113 patients (recruited Jan 2019 to Mar 2020) Gestational age 24 to 34 weeks Singleton or twin pregnancy ≥ 4 contractions/30 min Dilatation 1 to 4 cm One other sign of labor Rx atosiban infusion Contraindication to delayed birth excluded Atosiban infusion for 48 h Oral ebopiprant/placebo 1000 mg then 500 mg twice daily for 7 days First dose given within 24 h after starting atosiban infusion Parenteral MgSO4 for neuroprotection and beta/dexamethasone permitted Delivery within 2 days Delivery within 7 days Delivery before 37 weeks Time to delivery Uterine Contractions Ebopiprant Pharmacokinetics Maternal – AEs, labs, vital signs Fetal AEs Neonatal – AEs, vital signs, Apgar score, weight, head circumference, specific neonatal morbidities Infant development – ASQ-3 at 6, 12 and 24 months (ongoing) Patients Treatments Efficacy Outcomes Safety Outcomes
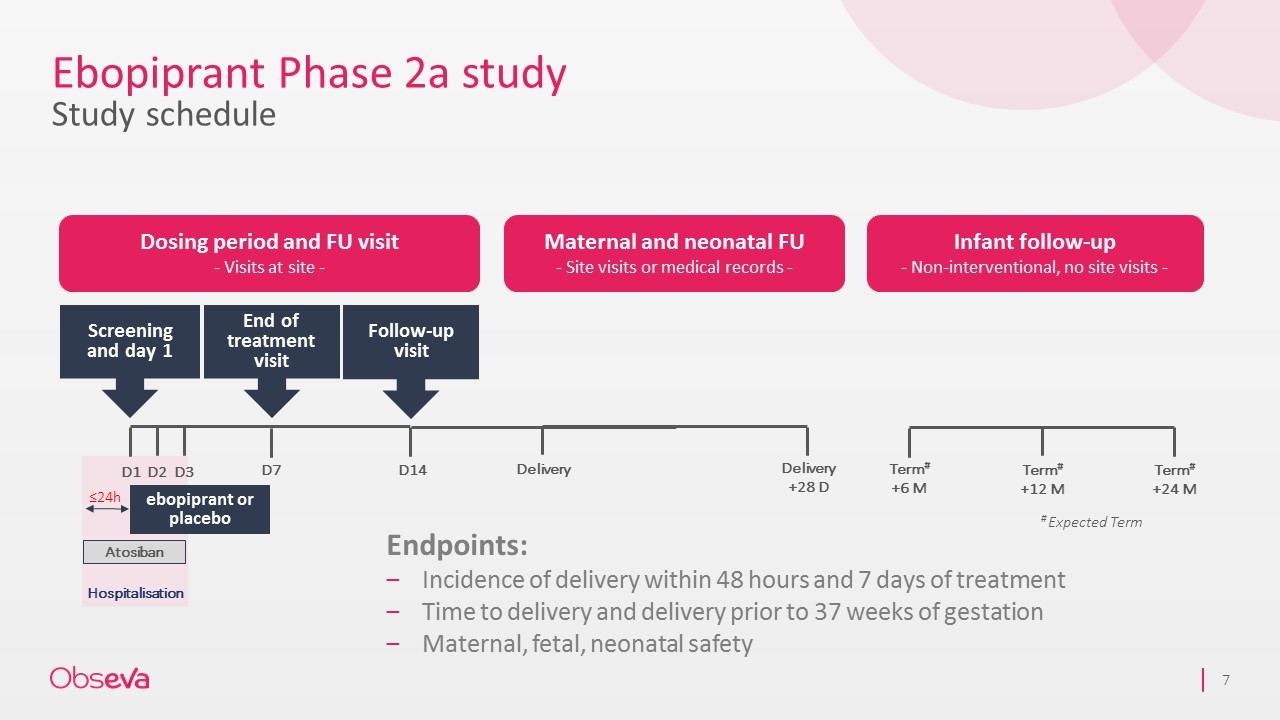
Ebopiprant Phase 2a study Study schedule Hospitalisation Dosing period and FU visit - Visits at site - Maternal and neonatal FU - Site visits or medical records - Infant follow-up - Non-interventional, no site visits - Term# +6 M Term# +12 M Term# +24 M ebopiprant or placebo Screening and day 1 Follow-up visit D1 D2 D3 D14 End of treatment visit Atosiban D7 # Expected Term ≤24h Delivery Delivery +28 D Endpoints: Incidence of delivery within 48 hours and 7 days of treatment Time to delivery and delivery prior to 37 weeks of gestation Maternal, fetal, neonatal safety
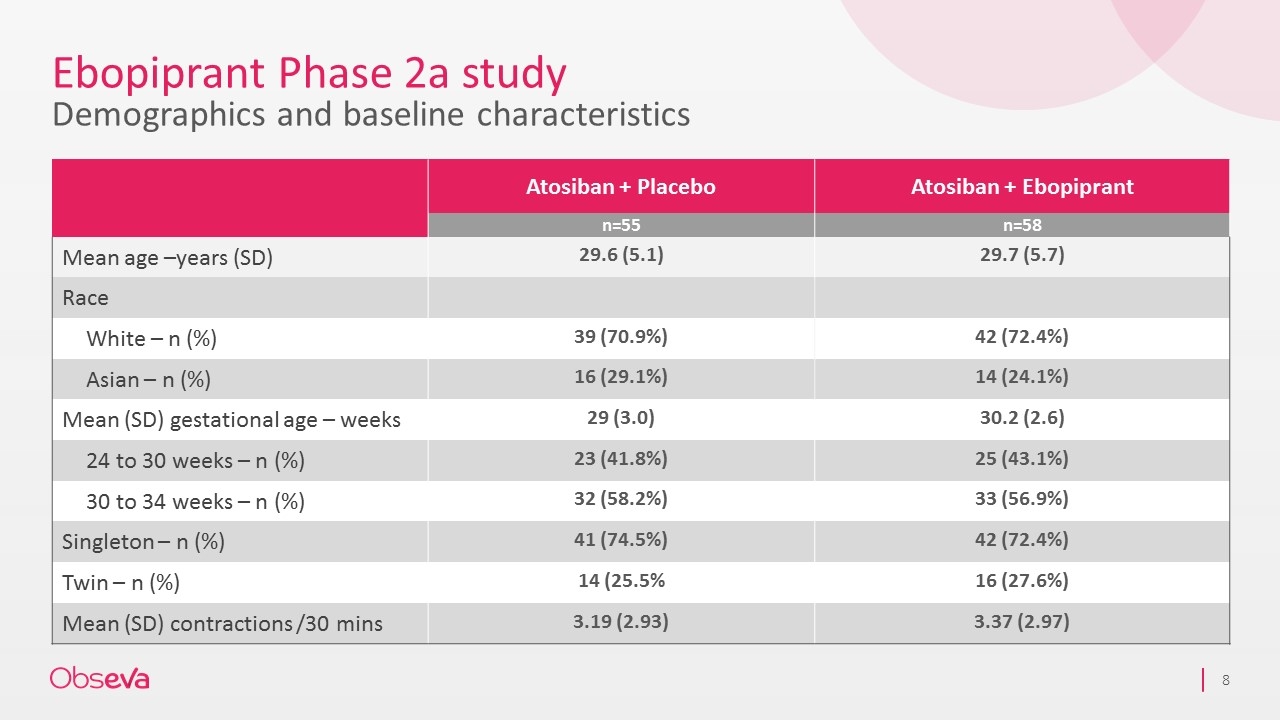
Ebopiprant Phase 2a study Demographics and baseline characteristics Atosiban + Placebo Atosiban + Ebopiprant n=55 n=58 Mean age –years (SD) 29.6 (5.1) 29.7 (5.7) Race White – n (%) 39 (70.9%) 42 (72.4%) Asian – n (%) 16 (29.1%) 14 (24.1%) Mean (SD) gestational age – weeks 29 (3.0) 30.2 (2.6) 24 to 30 weeks – n (%) 23 (41.8%) 25 (43.1%) 30 to 34 weeks – n (%) 32 (58.2%) 33 (56.9%) Singleton – n (%) 41 (74.5%) 42 (72.4%) Twin – n (%) 14 (25.5% 16 (27.6%) Mean (SD) contractions /30 mins 3.19 (2.93) 3.37 (2.97)
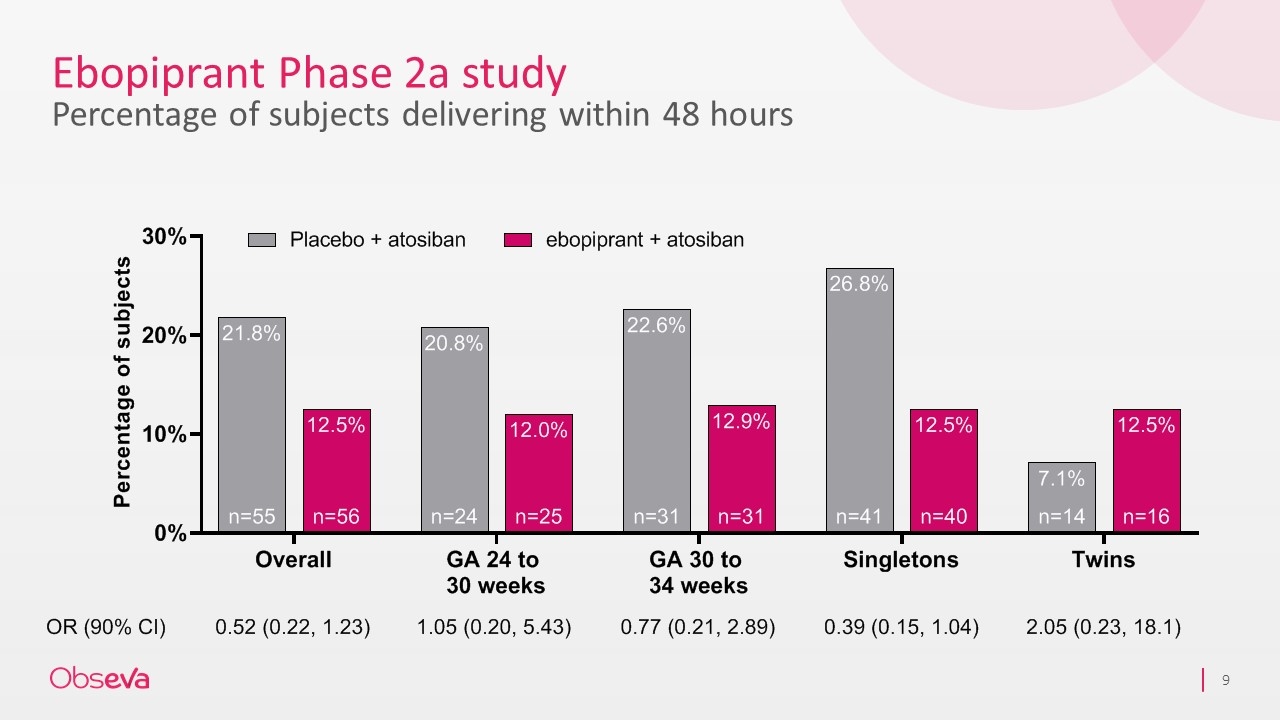
Ebopiprant Phase 2a study Percentage of subjects delivering within 48 hours
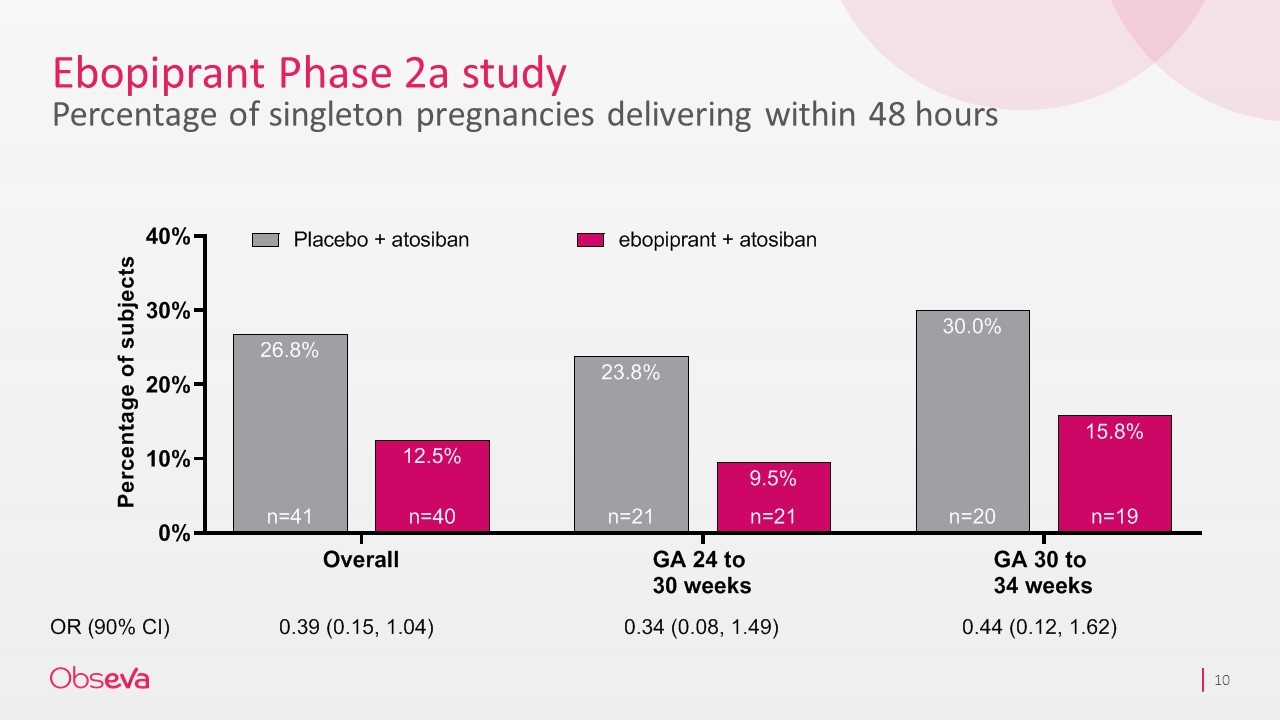
Ebopiprant Phase 2a study Percentage of singleton pregnancies delivering within 48 hours
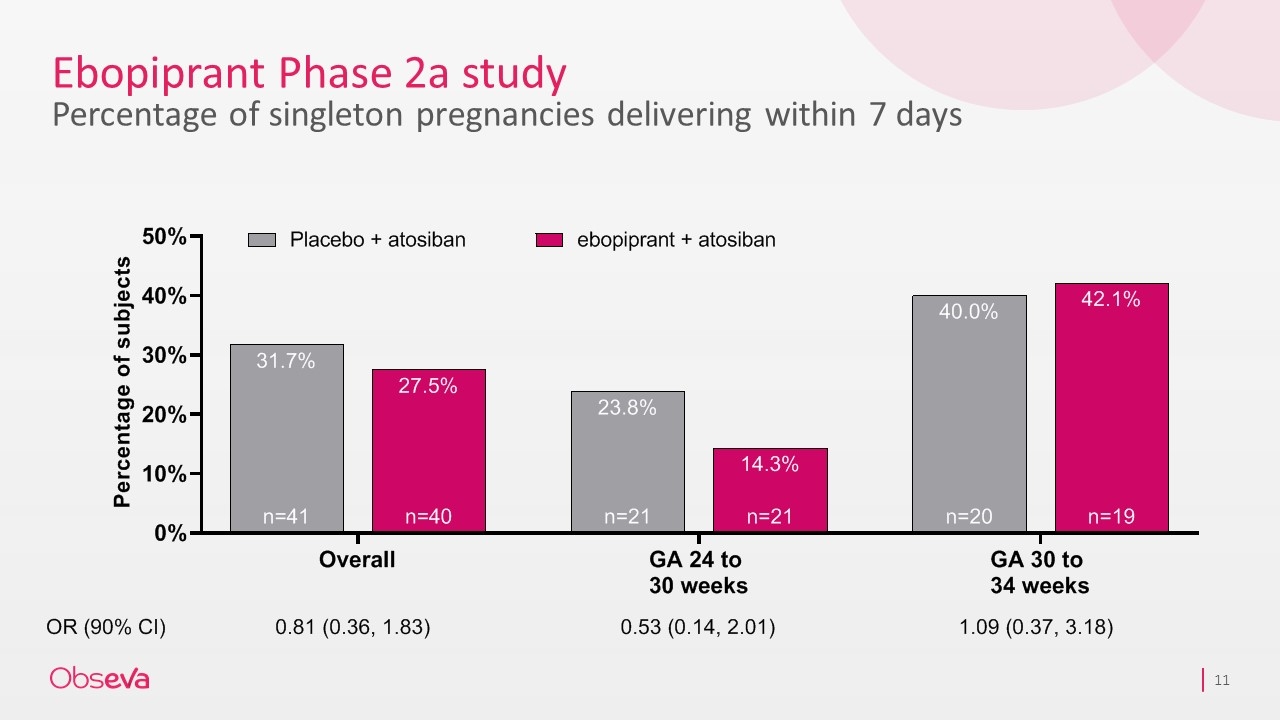
Ebopiprant Phase 2a study Percentage of singleton pregnancies delivering within 7 days
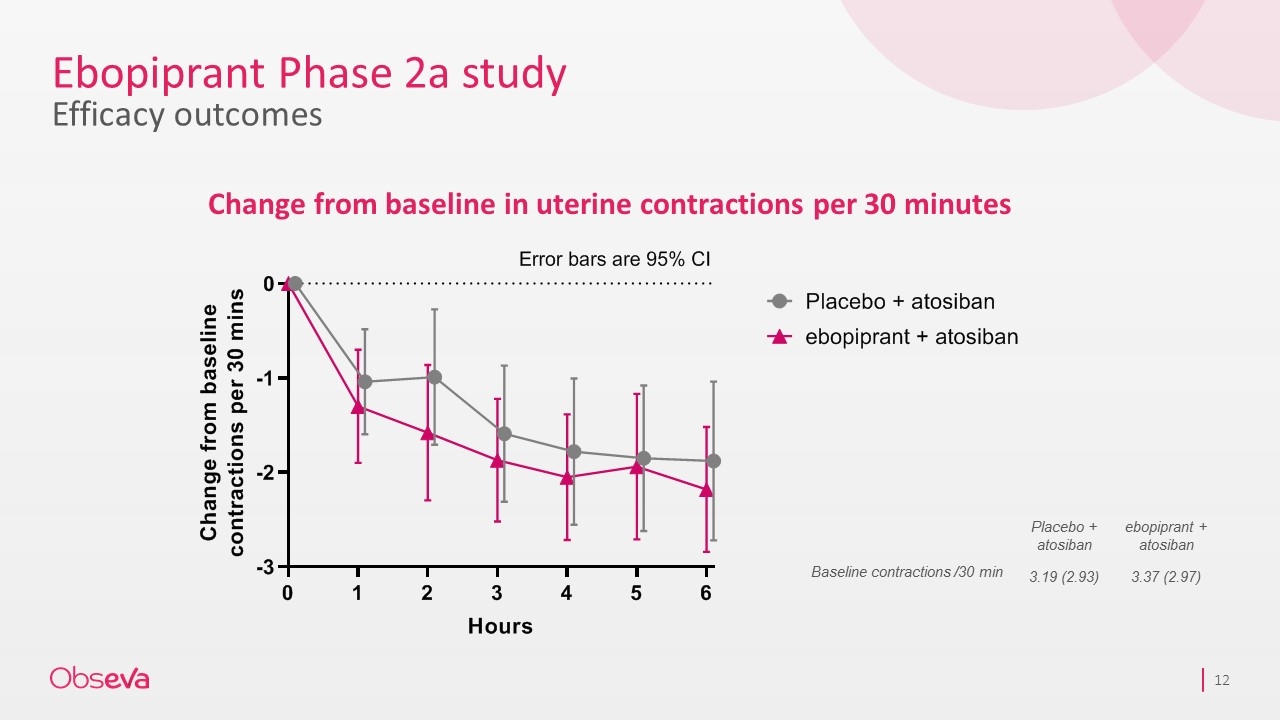
Ebopiprant Phase 2a study Efficacy outcomes Change from baseline in uterine contractions per 30 minutes Placebo + atosiban ebopiprant + atosiban Baseline contractions /30 min 3.19 (2.93) 3.37 (2.97)
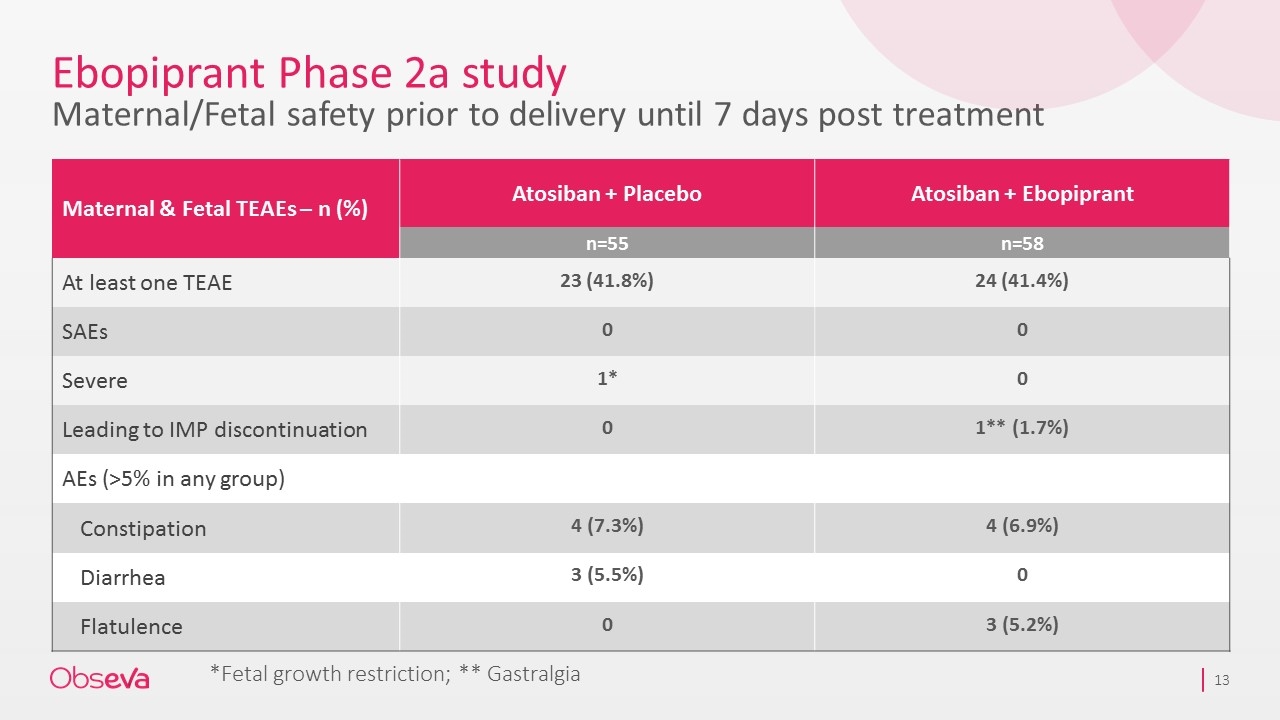
Ebopiprant Phase 2a study Maternal/Fetal safety prior to delivery until 7 days post treatment Maternal & Fetal TEAEs – n (%) Atosiban + Placebo Atosiban + Ebopiprant n=55 n=58 At least one TEAE 23 (41.8%) 24 (41.4%) SAEs 0 0 Severe 1* 0 Leading to IMP discontinuation 0 1** (1.7%) AEs (>5% in any group) Constipation 4 (7.3%) 4 (6.9%) Diarrhea 3 (5.5%) 0 Flatulence 0 3 (5.2%) *Fetal growth restriction; ** Gastralgia
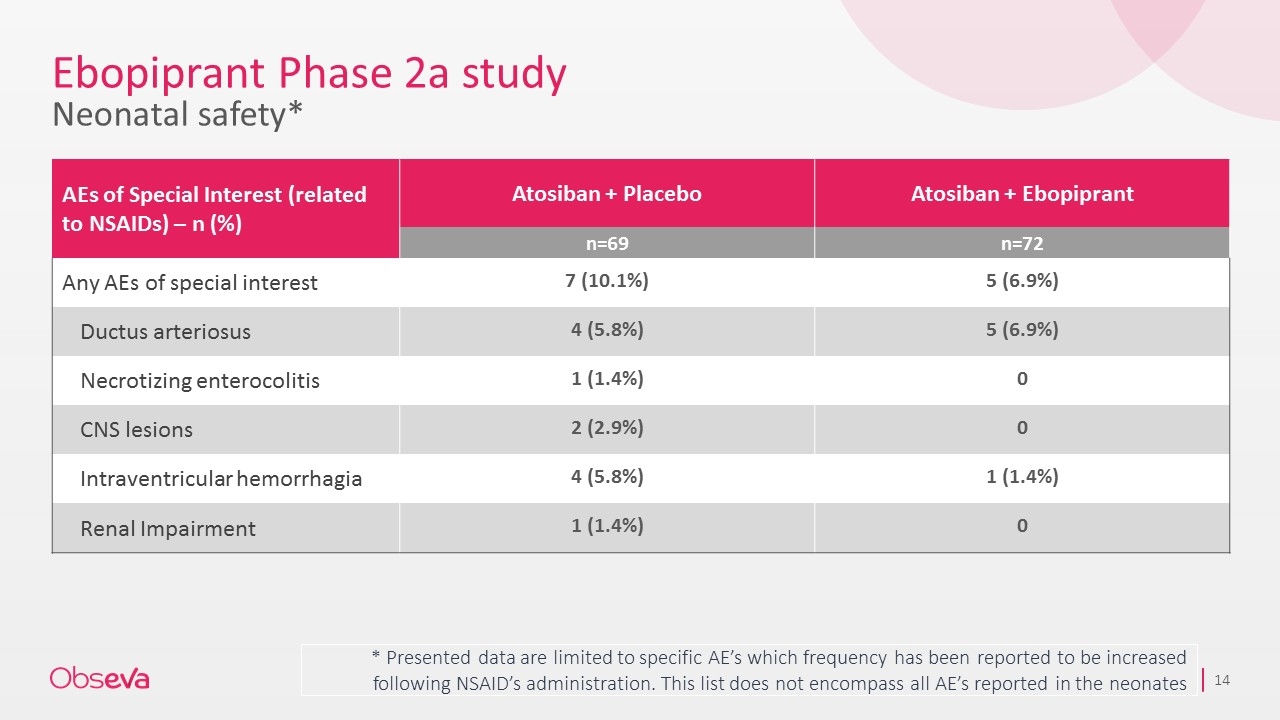
Ebopiprant Phase 2a study Neonatal safety* * Presented data are limited to specific AE’s which frequency has been reported to be increased following NSAID’s administration. This list does not encompass all AE’s reported in the neonates AEs of Special Interest (related to NSAIDs) – n (%) Atosiban + Placebo Atosiban + Ebopiprant n=69 n=72 Any AEs of special interest 7 (10.1%) 5 (6.9%) Ductus arteriosus 4 (5.8%) 5 (6.9%) Necrotizing enterocolitis 1 (1.4%) 0 CNS lesions 2 (2.9%) 0 Intraventricular hemorrhagia 4 (5.8%) 1 (1.4%) Renal Impairment 1 (1.4%) 0
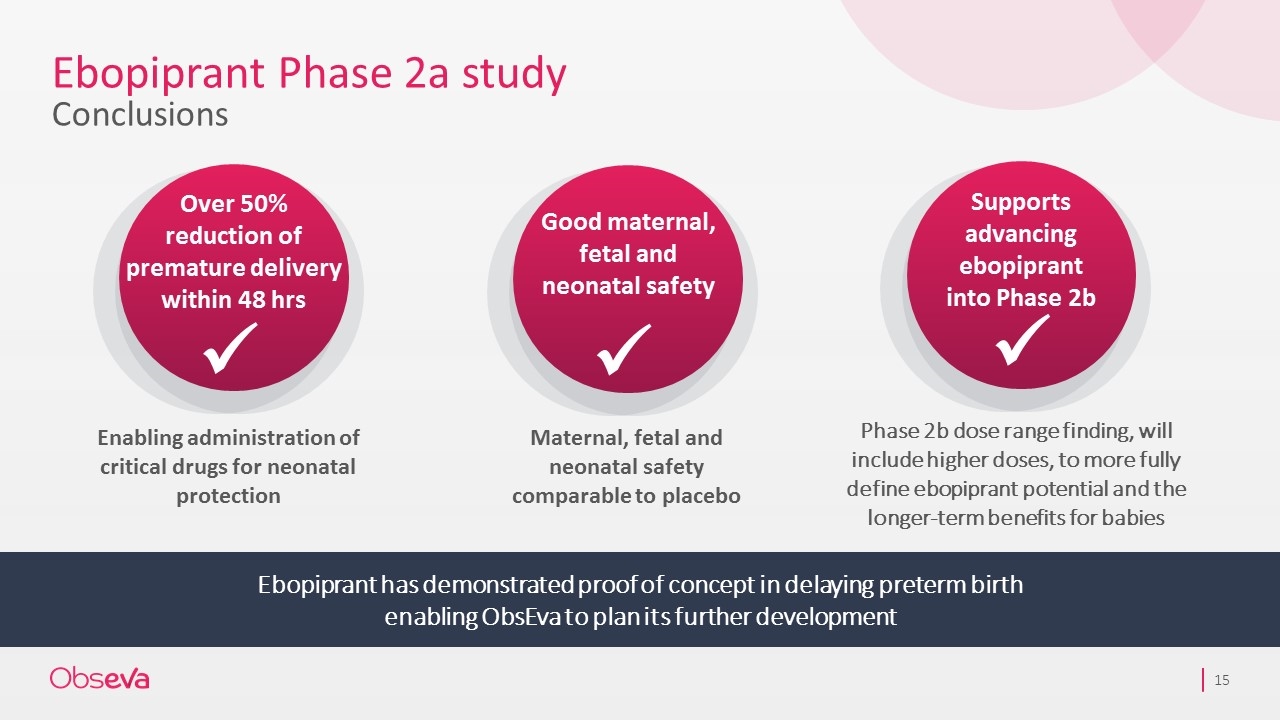
Ebopiprant Phase 2a study Conclusions Ebopiprant has demonstrated proof of concept in delaying preterm birth enabling ObsEva to plan its further development Enabling administration of critical drugs for neonatal protection Maternal, fetal and neonatal safety comparable to placebo Over 50% reduction of premature delivery within 48 hrs ü Supports advancing ebopiprant into Phase 2b ü Phase 2b dose range finding, will include higher doses, to more fully define ebopiprant potential and the longer-term benefits for babies Good maternal, fetal and neonatal safety ü














