
Yselty® for Uterine Fibroids Clinical Results PRIMROSE 1 up to Week 52 PRIMROSE 2 up to Week 76 10 DEC 2020 Exhibit 99.2

Disclaimer Matters discussed in this presentation may constitute forward-looking statements. The forward-looking statements contained in this presentation reflect our views as of the date of this presentation about future events and are subject to risks, uncertainties, assumptions, and changes in circumstances that may cause our actual results, performance, or achievements to differ significantly from those expressed or implied in any forward-looking statement. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future events, results, performance, or achievements. Some of the key factors that could cause actual results to differ from our expectations include our plans to develop and potentially commercialize our product candidates; our planned clinical trials and preclinical studies for our product candidates; the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; the extent of clinical trials potentially required for our product candidates; the clinical utility and market acceptance of our product candidates; our commercialization, marketing and manufacturing capabilities and strategy; our intellectual property position; and our ability to identify and in-license additional product candidates. For further information regarding these risks, uncertainties and other factors that could cause our actual results to differ from our expectations, you should read he risk factors set forth in our Annual Report on Form 20-F for the year ended December 31, 2019 filed with the SEC on March 5, 2020 and the risk factors disclosed in the Report on Form 6-K filed with the SEC on November 5, 2020, and our other filings we make with the Securities and Exchange Commission from time to time. We expressly disclaim any obligation to update or revise the information herein, including the forward-looking statements, except as required by law. Please also note that this presentation does not constitute an offer to sell or a solicitation of an offer to buy any securities. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.

Uterine fibroids are ruining lives … No two women are the same. But millions share a common problem: suffering the daily consequences of uterine fibroids … Yselty®, designed to treat more women Yselty® 200 mg once daily without ABT Yselty® 100 mg once daily without ABT Yselty® 200 mg once daily with concomitant ABT For short-term use (up to 6 months) when rapid reduction in fibroid and uterine volume is desired For long-term use for women with a contraindication to or who prefer to avoid ABT For long-term use for women for whom ABT is appropriate
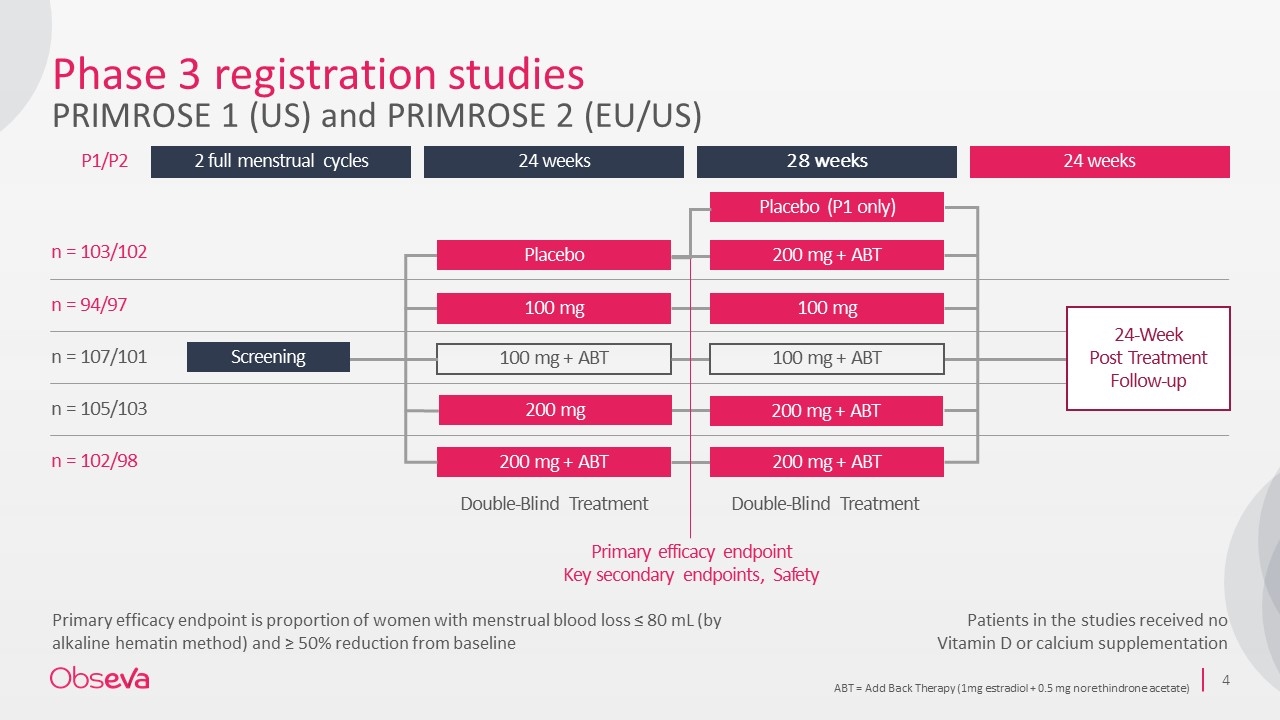
ABT = Add Back Therapy (1mg estradiol + 0.5 mg norethindrone acetate) Primary efficacy endpoint is proportion of women with menstrual blood loss ≤ 80 mL (by alkaline hematin method) and ≥ 50% reduction from baseline 2 full menstrual cycles 24 weeks 28 weeks 24 weeks n = 103/102 n = 94/97 n = 107/101 n = 105/103 n = 102/98 100 mg + ABT 200 mg + ABT 24-Week Post Treatment Follow-up Double-Blind Treatment Double-Blind Treatment Placebo 200 mg + ABT 100 mg 100 mg 100 mg + ABT 200 mg + ABT Primary efficacy endpoint Key secondary endpoints, Safety P1/P2 Screening Phase 3 registration studies PRIMROSE 1 (US) and PRIMROSE 2 (EU/US) Patients in the studies received no Vitamin D or calcium supplementation Placebo (P1 only) 200 mg 200 mg + ABT
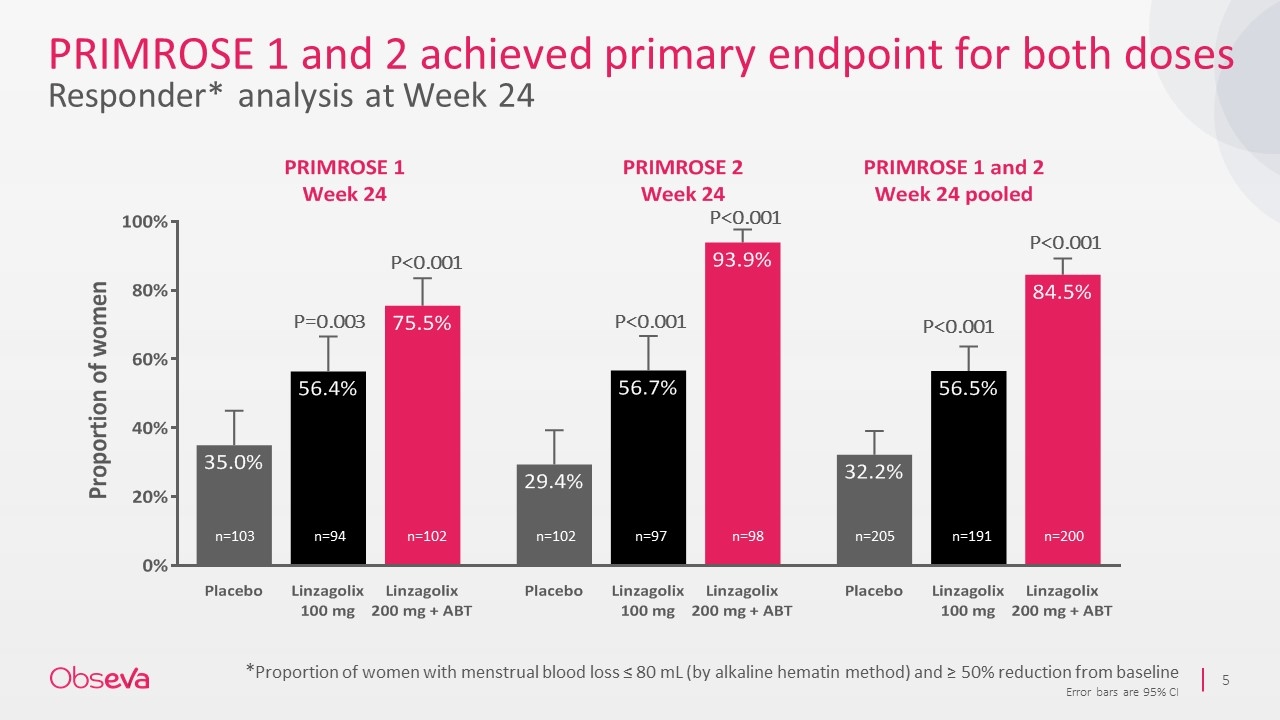
PRIMROSE 1 and 2 achieved primary endpoint for both doses Responder* analysis at Week 24 *Proportion of women with menstrual blood loss ≤ 80 mL (by alkaline hematin method) and ≥ 50% reduction from baseline Error bars are 95% CI P=0.003 P<0.001 P<0.001 P<0.001 n=103 n=94 n=102 n=102 n=97 n=98 n=205 n=191 n=200 P<0.001 P<0.001
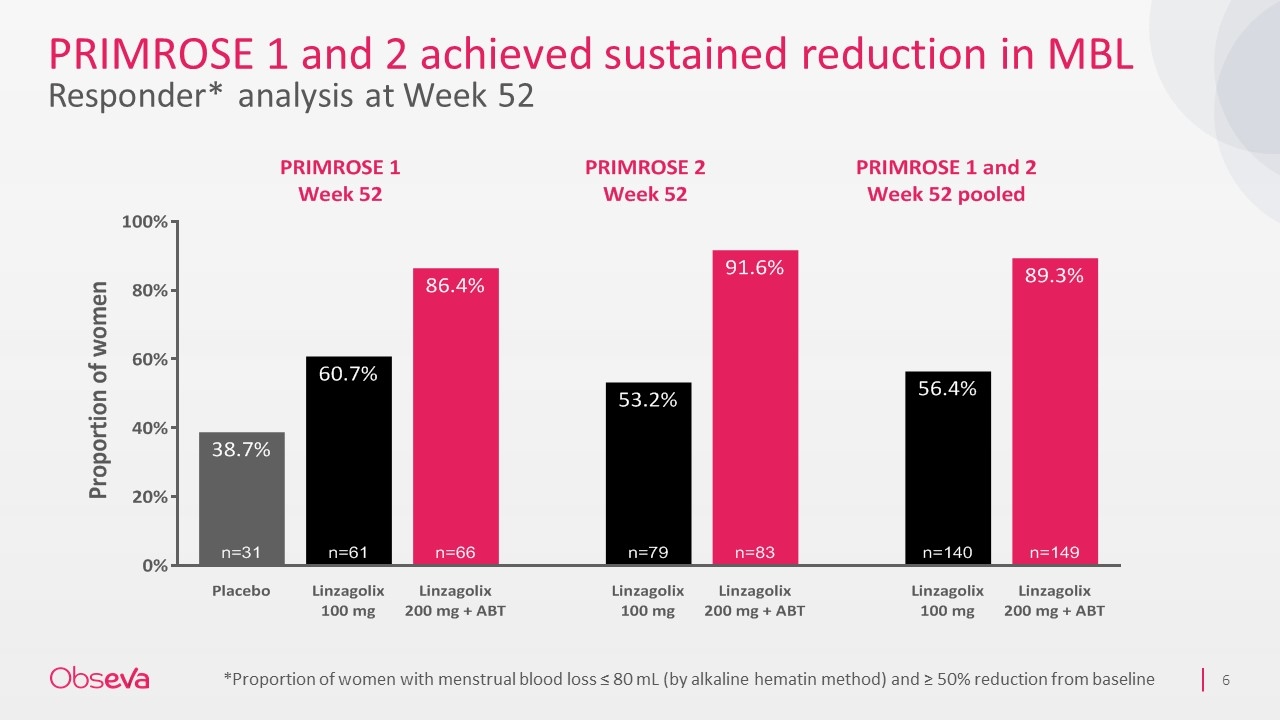
PRIMROSE 1 and 2 achieved sustained reduction in MBL Responder* analysis at Week 52 *Proportion of women with menstrual blood loss ≤ 80 mL (by alkaline hematin method) and ≥ 50% reduction from baseline
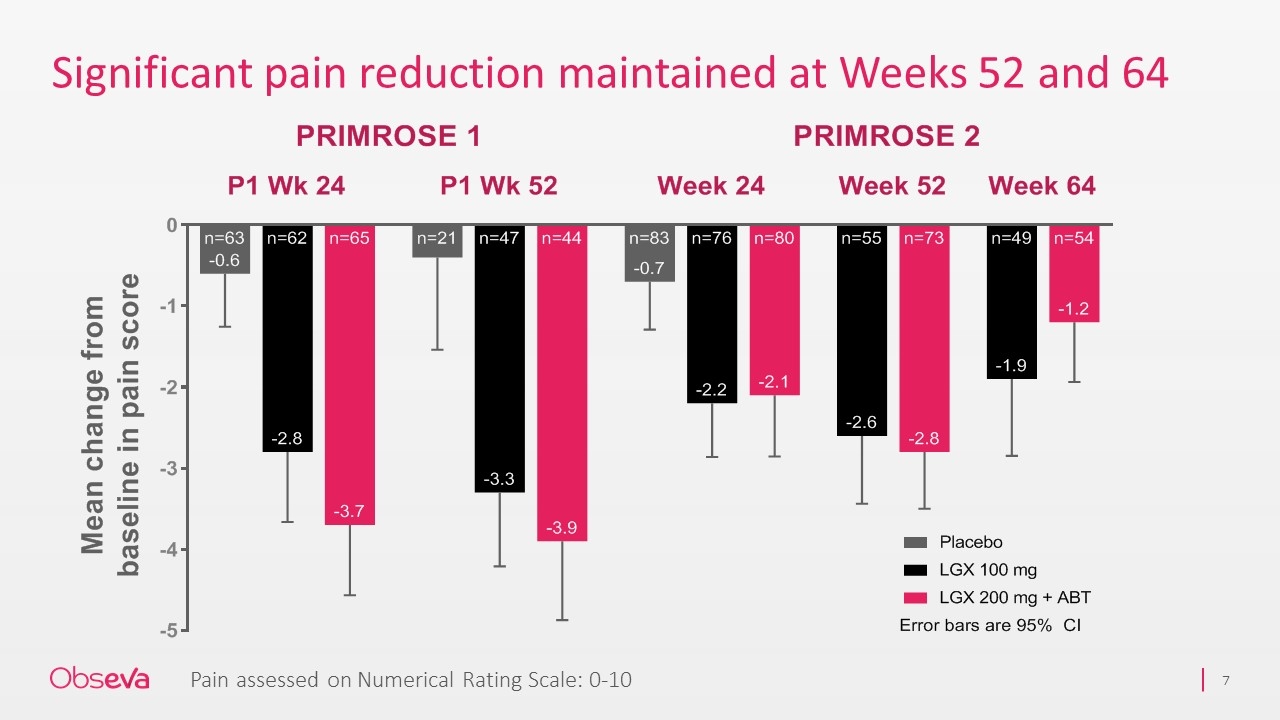
Significant pain reduction maintained at Weeks 52 and 64 Pain assessed on Numerical Rating Scale: 0-10
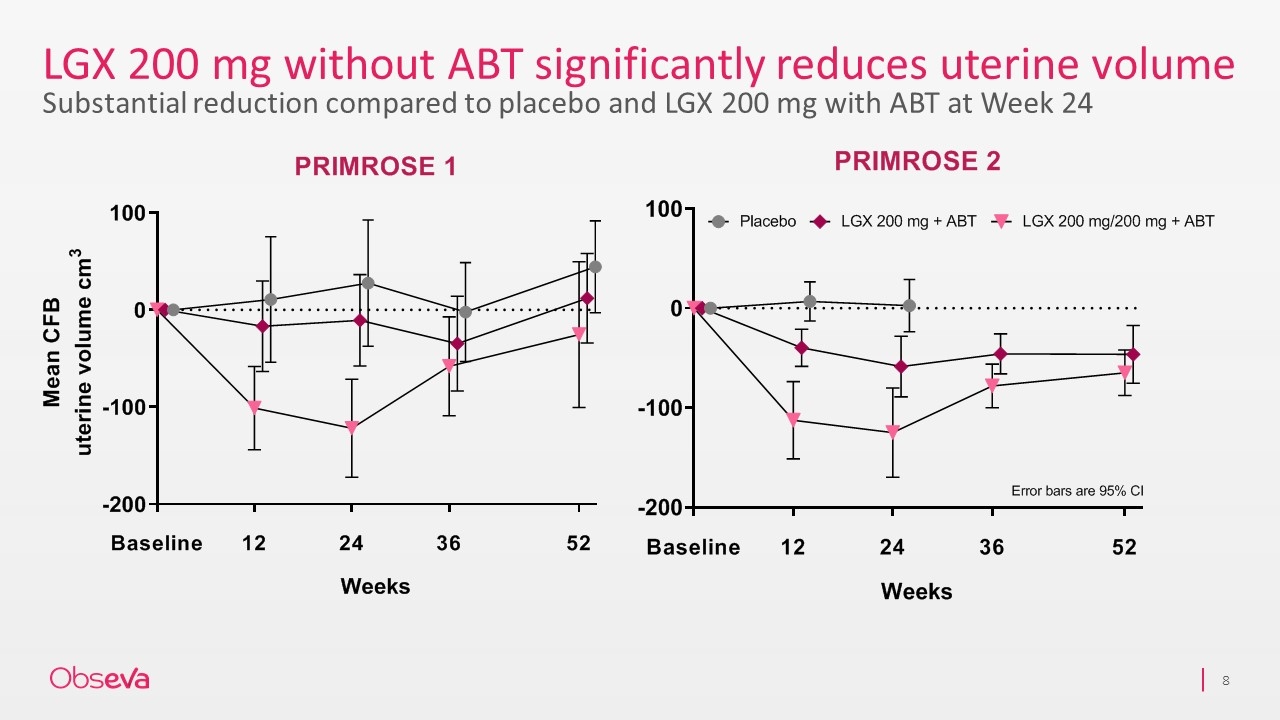
LGX 200 mg without ABT significantly reduces uterine volume Substantial reduction compared to placebo and LGX 200 mg with ABT at Week 24
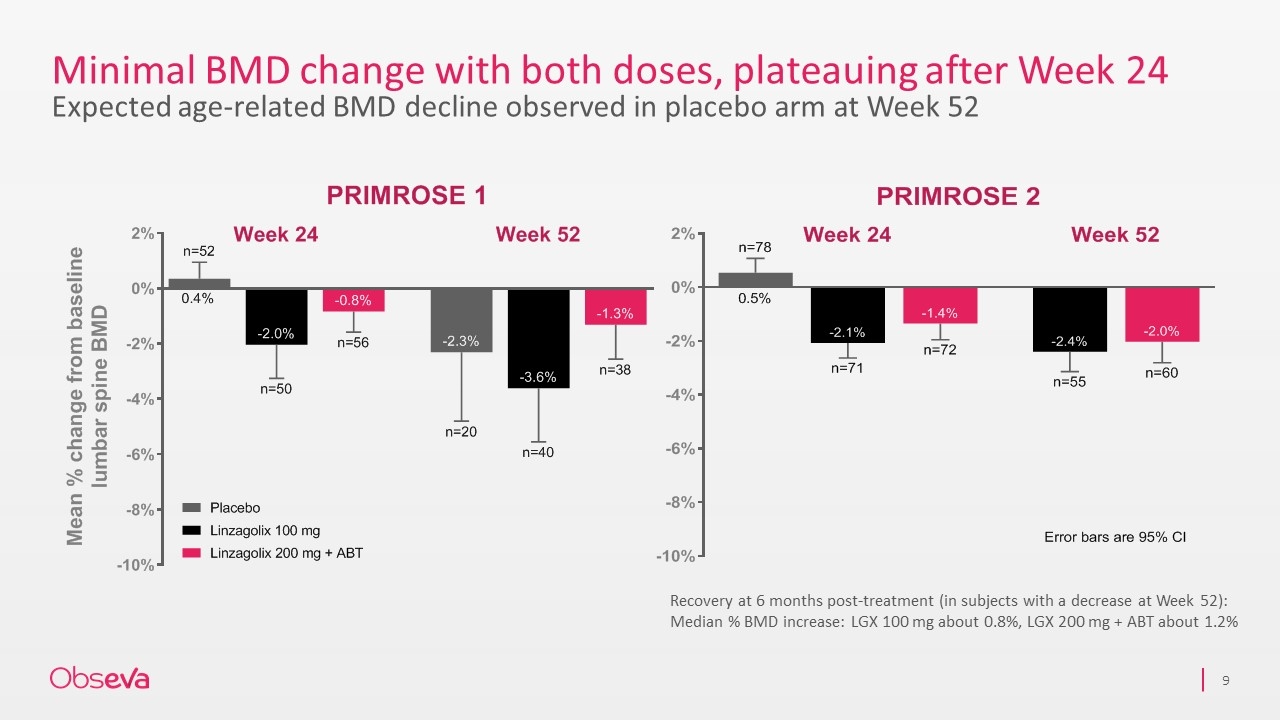
Minimal BMD change with both doses, plateauing after Week 24 Expected age-related BMD decline observed in placebo arm at Week 52 Recovery at 6 months post-treatment (in subjects with a decrease at Week 52): Median % BMD increase: LGX 100 mg about 0.8%, LGX 200 mg + ABT about 1.2%
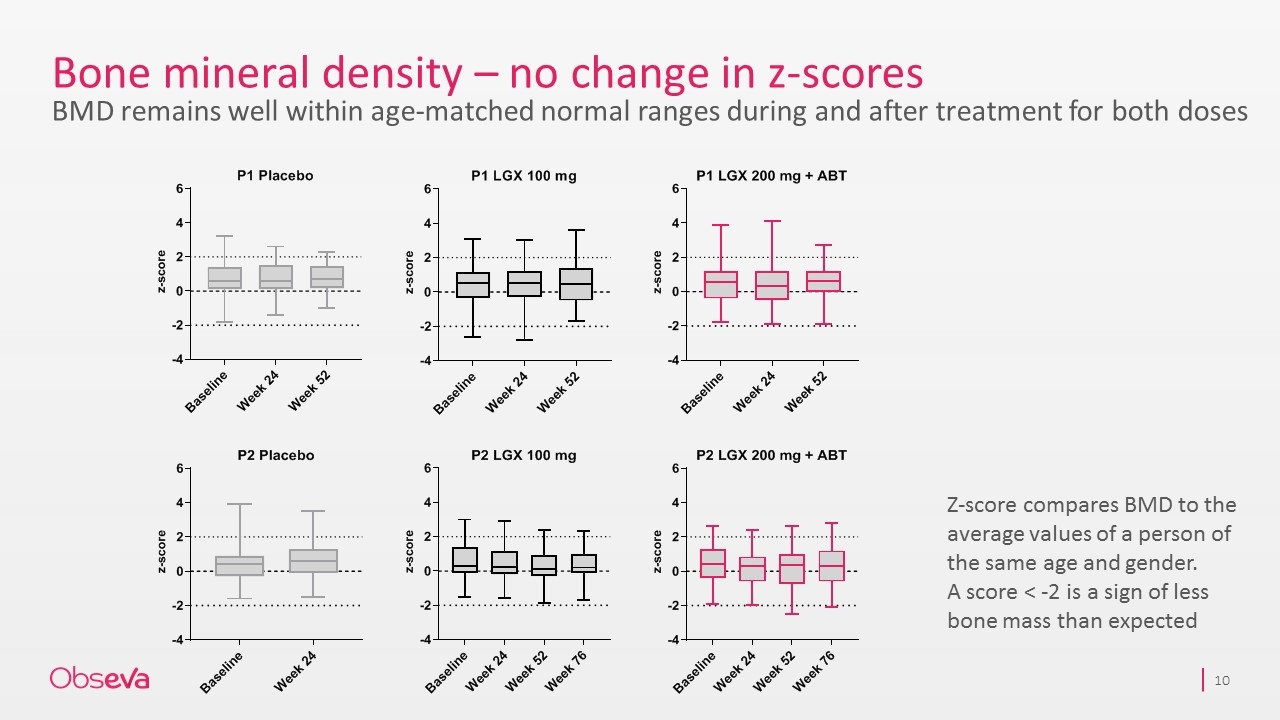
Bone mineral density – no change in z-scores BMD remains well within age-matched normal ranges during and after treatment for both doses Z-score compares BMD to the average values of a person of the same age and gender. A score < -2 is a sign of less bone mass than expected
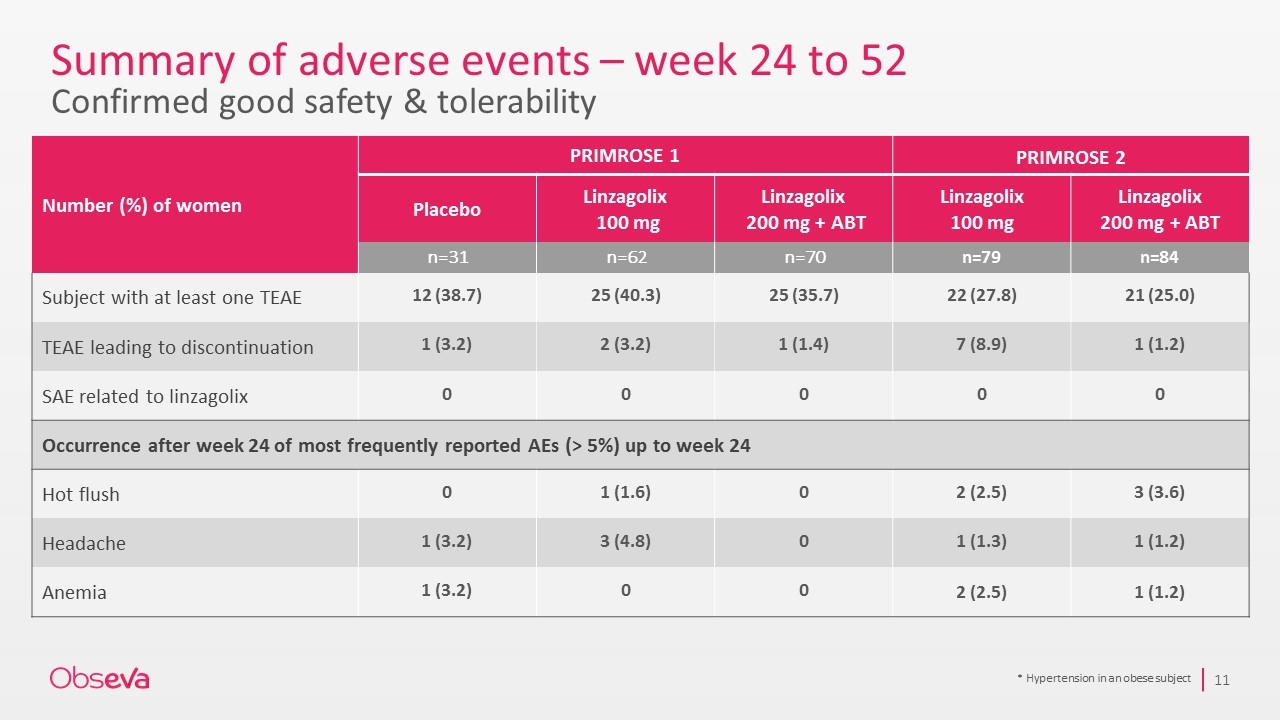
Summary of adverse events – week 24 to 52 Confirmed good safety & tolerability * Hypertension in an obese subject Number (%) of women PRIMROSE 1 PRIMROSE 2 Placebo Linzagolix 100 mg Linzagolix 200 mg + ABT Linzagolix 100 mg Linzagolix 200 mg + ABT n=31 n=62 n=70 n=79 n=84 Subject with at least one TEAE 12 (38.7) 25 (40.3) 25 (35.7) 22 (27.8) 21 (25.0) TEAE leading to discontinuation 1 (3.2) 2 (3.2) 1 (1.4) 7 (8.9) 1 (1.2) SAE related to linzagolix 0 0 0 0 0 Occurrence after week 24 of most frequently reported AEs (> 5%) up to week 24 Hot flush 0 1 (1.6) 0 2 (2.5) 3 (3.6) Headache 1 (3.2) 3 (4.8) 0 1 (1.3) 1 (1.2) Anemia 1 (3.2) 0 0 2 (2.5) 1 (1.2)
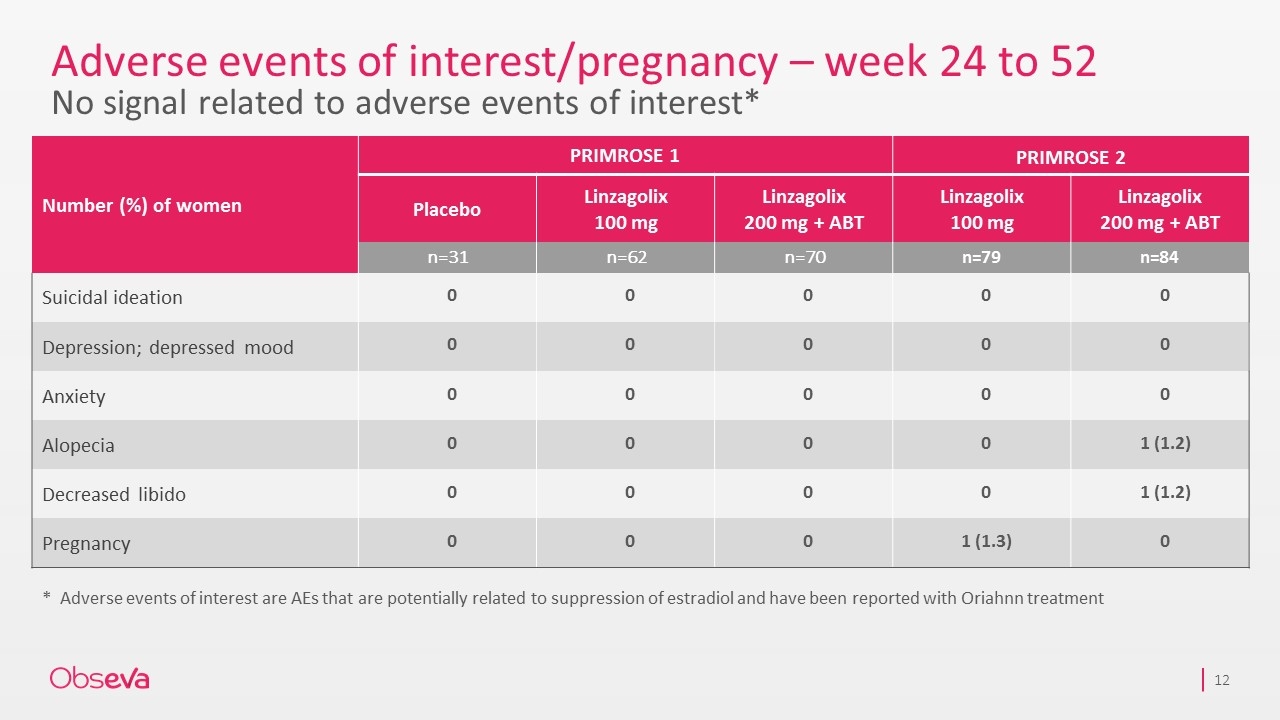
Adverse events of interest/pregnancy – week 24 to 52 No signal related to adverse events of interest* Number (%) of women PRIMROSE 1 PRIMROSE 2 Placebo Linzagolix 100 mg Linzagolix 200 mg + ABT Linzagolix 100 mg Linzagolix 200 mg + ABT n=31 n=62 n=70 n=79 n=84 Suicidal ideation 0 0 0 0 0 Depression; depressed mood 0 0 0 0 0 Anxiety 0 0 0 0 0 Alopecia 0 0 0 0 1 (1.2) Decreased libido 0 0 0 0 1 (1.2) Pregnancy 0 0 0 1 (1.3) 0 *Adverse events of interest are AEs that are potentially related to suppression of estradiol and have been reported with Oriahnn treatment
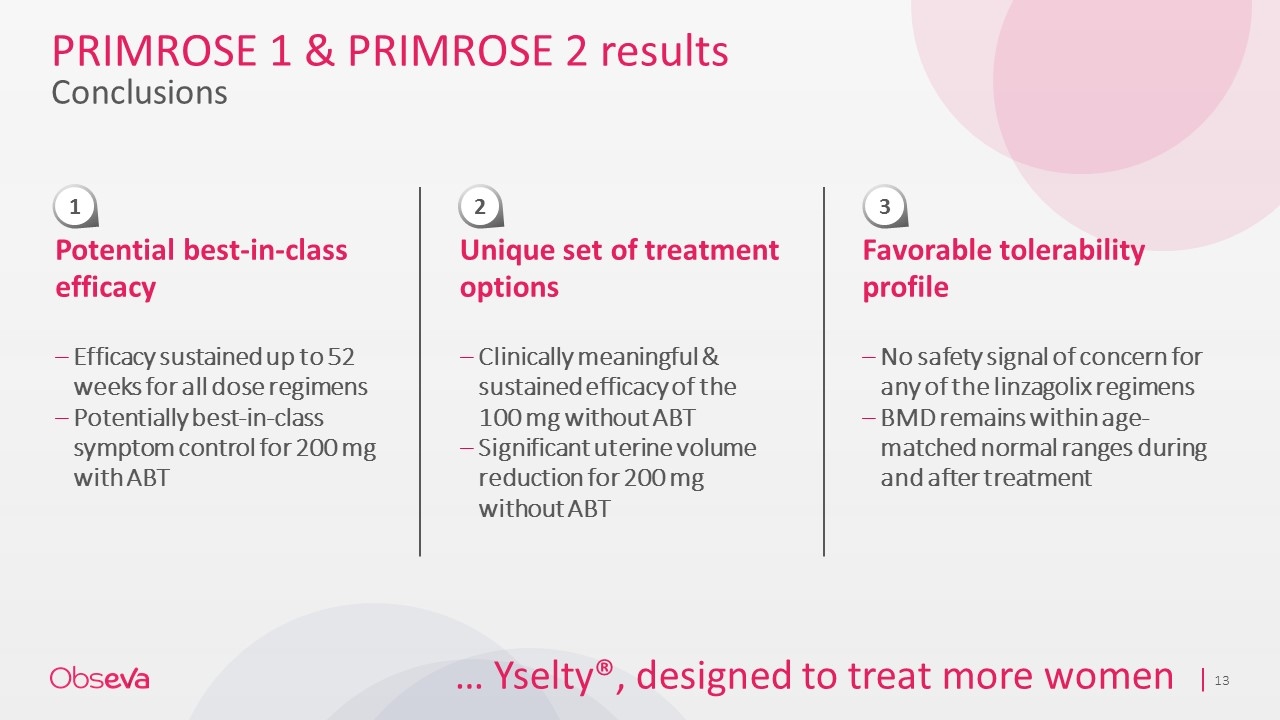
PRIMROSE 1 & PRIMROSE 2 results Conclusions Potential best-in-class efficacy Efficacy sustained up to 52 weeks for all dose regimens Potentially best-in-class symptom control for 200 mg with ABT Unique set of treatment options Clinically meaningful & sustained efficacy of the 100 mg without ABT Significant uterine volume reduction for 200 mg without ABT Favorable tolerability profile No safety signal of concern for any of the linzagolix regimens BMD remains within age-matched normal ranges during and after treatment 1 2 3 … Yselty®, designed to treat more women

Thank you













