UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
(Mark One)
|
|
☐ |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
|
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2021
OR
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
|
|
☐ |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 001-40852
LUMIRADX LIMITED
(Exact name of Registrant as specified in its charter)
Cayman Islands
(Jurisdiction of incorporation or organization)
LumiraDx Limited
c/o Ocorian Trust (Cayman) Limited
PO Box 1350, Windward 3, Regatta Office Park
Grand Cayman KY1-1108
Cayman Islands
(Address of principal executive offices)
Dorian LeBlanc, Chief Financial Officer
LumiraDx, Inc.
221 Crescent Street, 5th Floor
Waltham, MA 02543
(888) 586-4721
Email: IR@lumiradx.com
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered, pursuant to Section 12(b) of the Act.
|
|
|
|
|
|
|
|
|
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common shares, par value $0.0000028 per share |
|
LMDX |
|
The Nasdaq Stock Market |
Warrants exercisable to purchase common shares |
|
LMDXW |
|
The Nasdaq Stock Market |
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital stock or common stock as of the close of the period covered by the annual report.
As of December 31, 2021, the issuer had 45,241,767 common shares and 207,462,080 ordinary shares outstanding.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
Non-accelerated filer |
|
☒ |
|
Emerging growth company |
|
☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards † provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
|
|
|
|
|
|
|
|
|
U.S. GAAP ☐ |
|
International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ |
|
Other ☐ |
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
TABLE OF CONTENTS
i
ii
PRESENTATION OF FINANCIAL AND OTHER INFORMATION
Unless otherwise stated or the context otherwise indicates, references to the “LumiraDx”, the “Company”, “we”, “our” or “us” refer to LumiraDx Limited, an exempted company with limited liability incorporated under the laws of the Cayman Islands, and its consolidated subsidiaries.
Trademarks, Service Marks
LumiraDx and its respective subsidiaries own or have rights to trademarks, trade names and service marks that they use in connection with the operation of their business. In addition, their names, logos and website names and addresses are their trademarks or service marks. Other trademarks, trade names and service marks appearing in this Annual Report on Form 20-F (“Annual Report”) are the property of their respective owners. Solely for convenience, in some cases, the trademarks, trade names and service marks referred to in this Annual Report are listed without the applicable ®, ™ and SM symbols, but they will assert, to the fullest extent under applicable law, their rights to these trademarks, trade names and service marks.
Financial Information
The terms “dollar,” “USD” or “$” refer to the U.S. dollar and the term “euro,” “EUR” or “€” refer to the euro, unless otherwise indicated. The exchange rate used for conversion between U.S. dollars and euros is based on the ECB euro reference exchange rate published by the European Central Bank.
Our consolidated financial statements have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”). None of the consolidated financial statements were prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”). We have made rounding adjustments to some of the figures included in this Annual Report. Accordingly, any numerical discrepancies in any table between totals and sums of the amounts listed are due to rounding.
Market and Industry Data
Certain information included in this Annual Report concerning LumiraDx’s industry, including its total addressable market (“TAM”), the volume of tests and the shift of tests from the central lab to the point-of-care (“POC”) are based on its good faith estimates and assumptions derived from management’s knowledge of the industry and other information currently available to LumiraDx. This Annual Report also includes industry and market data that LumiraDx has obtained from periodic industry publications, third-party studies and surveys and other filings of public companies in its industry. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. This industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. In addition, LumiraDx does not know all of the assumptions regarding general economic conditions or growth that were used in preparing the forecasts from the sources relied upon or cited herein. LumiraDx is responsible for all of the disclosure contained in this Annual Report, and it believes the industry and market data that it obtained from third-party sources are reliable.
The industry in which LumiraDx operates, as well as the assumptions and estimates of its future performance and the future performance of the industry in which it operates, are subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section of this Annual Report titled “Item 3. Key Information—D. Risk Factors” and elsewhere in this Annual Report, that could cause results to differ materially from those expressed in these estimates.
iii
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains forward-looking statements regarding our current expectations or forecasts of future events. All statements other than statements of historical facts contained in this Annual Report, including statements regarding our future results of operations and financial position, business strategy, the LumiraDx Platform, tests, ongoing and planned preclinical studies and clinical trials, regulatory submissions and approvals, research and development costs, timing and likelihood of success, as well as plans and objectives of management for future operations are forward-looking statements. Many of the forward-looking statements contained in this Annual Report can be identified by the use of forward-looking words such as “anticipate,” “believe,” “could,” “expect,” “should,” “plan,” “intend,” “estimate,” “will” and “potential,” among others.
Forward-looking statements appear in a number of places in this Annual Report and include, but are not limited to, statements regarding our intent, belief or current expectations. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Such statements are subject to risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward-looking statements due to various factors, including, but not limited to, those identified under “Item 3. Key Information—D. Risk Factors.” These forward-looking statements include:
•our ability to compete in the highly competitive markets in which we operate, and potential adverse effects of this competition;
•our ability to maintain revenues if our products and services do not achieve and maintain broad market acceptance, or if we are unable to keep pace with or adapt to rapidly changing technology, evolving industry standards and changing regulatory requirements;
•uncertainty, downturns and changes in the markets we serve;
•our expectations regarding the size of the POC market for the LumiraDx Platform (the “Platform”), which is an integrated system comprised of the POC diagnostic instrument (the “Instrument”) precise, low-cost microfluidic test strips, and seamless, secure digital connectivity, the size of the various addressable markets for certain tests and our ability to penetrate such markets by driving the conversion of healthcare providers’ testing needs onto the Platform;
•our commercialization strategy, including our plans to initially focus our sales efforts on large healthcare systems, government organizations and national pharmacy chains that want to deploy comprehensive POC testing across their networks;
•our belief that we will be able to drive commercialization of the Platform through the launch of our SARS-CoV-2 antigen and SARS-CoV-2 antibody tests;
•the willingness of healthcare providers to use a POC system over central lab systems and the rate of adoption of the Platform by healthcare providers and other users;
•the scalability and commercial viability of our manufacturing methods and processes, especially in light of the anticipated demand for the Platform and our minimum commitments to supply the Platform to customers;
•our ability to source suitable raw materials and components for the manufacture of the Instrument and test strips in a timely fashion;
•our ability to maintain our current relationships, or enter into new relationships, with diagnostics or research and development companies, third party manufacturers and commercial distribution collaborators;
•our ability to effectively manage our anticipated growth;
•our ability to rapidly develop and commercialize diagnostics tests that are accurate and cost-effective;
•the timing, progress and results of our diagnostics tests, including statements regarding launch plans and commercialization plans for such tests, all which may be delayed by or halted due to a number of factors, including the impact of the COVID-19 pandemic;
•the timing, scope or likelihood of regulatory submissions, filings, approvals, authorizations or clearances;
•the pricing, coverage and reimbursement of the Instrument and tests, if approved;
•our ability to repay or service our debt obligations and meet the financial covenants related to such debt obligations;
•our ability to enforce our intellectual property rights and to operate our business without infringing, misappropriating, or otherwise violating the intellectual property rights and proprietary technology of third parties; 1
iv
•developments and projections relating to our competitors and our industry;
•our ability to develop effective internal controls over financial reporting;
•our ability to attract, motivate and retain qualified employees, including members of our senior management team;
•the effects of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business or operations;
•our expectations regarding the time during which we will be an emerging growth company under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) and a foreign private issuer;
•the future trading price of common shares and impact of securities analysts’ reports on these prices;
•our ability to fully derive anticipated benefits from existing or future acquisitions, joint ventures, investments or dispositions;
•exchange rate fluctuations and volatility in global currency markets;
•potential adverse tax consequences resulting from the international scope of our operations, corporate structure and financing structure;
•U.S. tax legislation enacted in 2017, which could materially adversely affect our financial condition, results of operations and cash flows;
•increased risks resulting from our international operations;
•our ability to comply with various trade restrictions, such as sanctions and export controls, resulting from our international operations;
•government and agency demand for our products and services and our ability to comply with government contracting regulations; and
•our ability to operate in a litigious environment.
These forward-looking statements speak only as of the date of this Annual Report and are subject to a number of risks, uncertainties and assumptions described under the sections of this Annual Report titled “Item 3. Key Information—D. Risk Factors” and “Item 5. Operating and Financial Review and Prospects” and elsewhere in this Annual Report. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
v
SUMMARY OF RISK FACTORS
The following is a summary of certain, but not all, of the risks that could adversely affect our business, operations and financial results. If any of the risks actually occur, our business could be materially impaired, the trading price of our common shares and warrants could decline, and you could lose all or part of your investment.
•We are at a pivotal point in the commercialization of our Platform, and we may not succeed for a variety of reasons.
•Our short-term revenue prospects will vary with the amount of demand for COVID-19 tests, the presence of variants, which may be further adversely impacted by wide-spread implementation of authorized vaccines or other vaccines or boosters that are subsequently approved or authorized.
•We may not obtain regulatory approval, authorization, certification or clearance of additional tests on our Platform or our Amira System, and we may not be able to successfully develop and commercialize additional tests on the Platform or the Amira System, including scaling up manufacturing and sales capacity.
•Our strategy to globally launch a broad menu of tests may not be as successful as currently envisioned.
•We may not be able to generate sufficient revenue from our Platform to achieve and maintain profitability.
•We may not be able to continue as a going concern if we require and are not able to obtain waivers of covenant violations or restructure our existing debt obligations.
•Business or economic disruptions or global health concerns, such as the ongoing COVID-19 pandemic, have harmed and may continue to seriously harm our business and increase our costs and expenses.
•We rely on a limited number of suppliers for the components of our Platform and our Amira System and for other materials and may not be able to find replacements or immediately transition to alternative suppliers.
•We may experience problems in scaling our manufacturing and commercial operations, and scaling may impact performance of our products.
•Our business and reputation will suffer if our products do not perform as expected.
•We currently derive a significant portion of our revenue from a small number of tests and key customers, and loss of any of these customers could cause a material reduction in revenues. A significant portion of our revenue remains COVID-19 related, and we may not be able to scale other assays sufficiently fast.
•The loss of any member of our senior management team or an inability to attract and retain highly skilled scientists, engineers, clinicians and salespeople could adversely affect our business.
•Our business and sale of our products are subject to extensive regulatory requirements and our products may not be compliant with the new regulatory framework applicable in the European Union (“E.U.”) beginning May 26, 2022, and approvals of products under the new regulatory regime may be delayed and consequently our ability to continue to commercialize such products in the E.U. may be impacted and this could impact revenues.
•If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenue or achieve and sustain profitability.
•The dual class structure of our ordinary shares and our common shares has the effect of concentrating voting control with those holders of our share capital prior to the merger of our wholly owned subsidiary, LumiraDx Merger Sub, Inc., with and into CA Healthcare Acquisition Corp., a Delaware corporation (the “Merger”).
•If we are unable to obtain and maintain patent and other intellectual property protection for our products and technology, our ability to successfully commercialize any products we develop may be adversely affected.
•If any of our facilities were damaged or destroyed, or if we experience a significant disruption in the expansion of our operations for any reason, our ability to continue to operate our business and meet increased demand could be materially harmed.
vi
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
A. Reserved
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
We operate in a market environment that is difficult to predict and that involves significant risks, many of which are beyond our control. You should carefully consider the risks described below before you decide to purchase our securities. Additional risks and uncertainties not presently known to us or that we do not currently believe are important to an investor, if they materialize, also may adversely affect us. If any of the events, contingencies, circumstances or conditions described in the following risks actually occur, our business, financial condition or results of operations could be seriously harmed.
Risks Related to Our Business and Strategy
We are at a pivotal point in the commercialization of our Platform, and we may not succeed for a variety of reasons.
Since our inception in 2014 until December 31, 2021, we have incurred $460.6 million in research and development costs to develop our Platform, our mass screening testing system (the “Amira System”), and our Fast Lab Solutions products which support high-complexity laboratory testing (“Fast Labs Solutions”). As of the date of this Annual Report, we have eight POC diagnostic tests developed and launched for our Platform: our SARS-CoV-2 antigen test and SARS-CoV-2 antibody test, commercially available under Emergency Use Authorization (“EUA”) and CE Marks, which we introduced to the U.S. and European markets in 2019; our INR test, our SARS-CoV-2 antigen pool test, our D-Dimer test, our CRP test and our SARS-CoV-2 Ag & Flu A/B tests, all of which are CE Marked. We also received EUAs for our molecular lab reagent kits, LumiraDx SARS-CoV-2 RNA STAR and LumiraDx SARS-CoV-2 RNA STAR Complete, achieved CE mark for LumiraDx SARS-CoV-2 RNA STAR Complete and commenced sales in the U.S. and Europe. We have submitted an EUA request to the U.S. Food and Drug Administration (“FDA”) for our SARS-CoV-2 Ag & Flu A/B tests. To date we have not yet received authorization for this combo test and currently the FDA has indicated that authorization will be not be provided as further information is required, amongst other things, additional data points related to Flu A/B testing. There can therefore be no guarantee that authorization will be granted by the FDA and timing on updated submissions depends on the prevalence of Flu A/B and our ability to collect further data.
We have engaged in a large, broad-scale launch of our SARS-CoV-2 antigen test and we are relying on such test to create brand awareness and a revenue base to support our cost infrastructure as well as to create an installed base of the Instrument.
While we have launched certain tests, including our SARS-CoV-2 antigen test and our SARS-CoV-2 antibody test, we have limited commercial experience with our Platform. The launch of additional tests may be delayed, be less successful than we anticipate, or fail for any of the reasons that large commercial launches are ultimately unsuccessful. For example:
•Our tests, produced at large scale, might not perform to standards that we have experienced to date. We therefore may not obtain or maintain regulatory approval, authorization, certification or clearance for some of our
1
diagnostic tests in research and development, which may have a significant impact on the commercialization of our Platform.
•We have a number of diagnostic tests in our near-term pipeline. We may not receive relevant regulatory approval, authorization, certification or clearance for some or all of these in a timely fashion, or at all, and this may impact significantly on the commercialization of our Platform.
•Unexpected or inconsistent clinical data from existing and future clinical trials, or a regulator’s or the market’s perception of these clinical data when compared to our internal comparative data, may adversely impact our ability to obtain regulatory approval, authorization, certification or clearance for, or market acceptance of, our diagnostic tests.
•We make our Instrument, Amira System and test strips on sophisticated manufacturing systems, and these may not operate at large scale as anticipated.
•We may have difficulty sourcing raw materials and components, including micro processing or semiconductor chips or capacitors, to make our Instrument, Amira System and test strips in a timely fashion in necessary quantities, or these materials and components might not comply with our specifications, which are exacting.
•We may not be able to supply our Platform through sales channels that are effective and efficient.
•Potential users of our Platform might not accept our Platform as being better than those POC systems already available, at the prices we charge or at all.
•Governmental and third-party payors might decline to cover our products or reimburse our users for the cost of our Instrument and test strips at favorable rates or at all.
•We may not be able to scale-up and sustain operations to a level that allows our investments in technology, equipment, personnel and other resources to achieve sustainable and profitable commercial activities.
•Our management, manufacturing, sales and marketing, logistics, research and development, regulatory and other personnel might not be able to sustain the high level of operations that we anticipate and that we will require to produce our anticipated revenue and allow us to operate profitably.
•External factors, such as the ongoing COVID-19 pandemic, or political or social instability or unrest in our principal markets, such as the recent conflict between Russia and Ukraine, and its potential impact might adversely affect us in ways that we have not planned for.
Operations of the type and scope that we plan are subject to many uncertainties, and many that are undertaken are unsuccessful. We cannot be certain that we will be able to achieve our business objectives as described in this Annual Report, and if our assumptions regarding these risks and uncertainties are incorrect or change, or if we do not address these risks successfully, our results of operations could differ materially from our expectations and our business, financial condition and results of operations could be adversely affected.
Our short-term revenue prospects will vary with the amount of demand for COVID-19 tests.
Our short-term revenue prospects will continue to vary with the amount of demand for our SARS-CoV-2 antigen test, SARS-CoV-2 antigen pool test and SARS CoV-2 antibody test and the presence of various variants of the virus. Since the beginning of the COVID-19 pandemic, three vaccines for COVID-19 have received EUAs from the FDA and two of those later received marketing approval. As additional effective COVID-19 vaccines or treatments are developed, approved or authorized and rolled out to protect against and treat the virus, demand for our SARS-CoV-2 antigen test, SARS-CoV-2 antigen pool test and SARS CoV-2 antibody test may be impacted and the size of our market opportunity for such tests may be impacted. While we believe that our SARS-CoV-2 antigen, SARS-CoV-2 antigen pool test and SARS-CoV-2 antibody tests will remain in demand as new variants could appear and COVID-19 vaccines or boosters are rolled out and following such rollout to be used as a verification tool to test the efficacy of such vaccines in triggering an immune response, and to facilitate maintaining open economies, the presence of variants, their impact or the availability and efficacy of such vaccines/boosters or the mitigation of the COVID-19 pandemic earlier than expected for any other reason could negatively impact demand for our Platform and sales of our Instrument, test strips and other products. While our SARS-CoV-2 antigen test detects major global SARS-CoV-2 variants including Delta, Gamma, Epsilon, Alpha, Beta and Omicron variants, there is no guarantee that our tests will be able to accurately detect all variants of concern. In addition, competitors may produce more accurate tests or tests which receive more favorable demand, both of which may impact our revenue streams and profitability. It is not unreasonable to expect COVID-19 may become a more seasonal flu-like illness, subject to seasonality which will impact revenue cycles.
2
Our molecular lab reagent kits, LumiraDx SARS-CoV-2 RNA STAR and LumiraDx SARS-CoV-2 RNA STAR Complete will also be subject to these demand fluctuations. In addition, we have announced the plan to roll out a five-minute SARS-CoV-2 Ag Ultra test and a five-minute SARS-CoV-2 Ag Ultra Pool test. The launch of such tests may be subject to further validation and clinical trials and regulatory clearances. Launches may be delayed as clinical trials require the presence of virus for clinical testing. Completion of trials may be impacted or delayed in case of low prevalence of the virus.
We have also submitted an EUA request to the FDA for our SARS-CoV-2 Ag & Flu A/B tests. To date we have not yet received authorization for this combo test and currently the FDA has indicated that authorization will be not be provided as further information is required, amongst other things, additional data points related to Flu A/B testing. There can therefore be no guarantee that authorization will be granted by the FDA and timing on updated submissions depends on the prevalence of Flu A/B and our ability to collect further data.
New product development involves a lengthy and complex process and we may be unable to commercialize additional tests on our Platform on a timely basis, or at all.
The launch of additional tests on our Platform may be delayed or may not be successful. There can be no assurance that our Platform will accurately and rapidly identify biomarkers associated with conditions and diseases of importance to our customers, including COVID-19, for a variety of technical reasons or that our Platform will compete with market alternatives or gain market acceptance. Our diagnostic tests which are in development will take time to develop and commercialize, if we are able to commercialize them at all.
Many other POC testing systems are designed for one or few related tests, increasing the odds of creating a successful test but decreasing the odds of developing a system with broad testing abilities. Our strategy involves designing a platform that is diverse and powerful enough to produce high-quality testing abilities for a broad array of tests. While we believe this strategy will result in an industry-leading standard for POC tests, it also creates a very high hurdle for success, which we may not ultimately clear. Various tests may require improved product performance specifications over time.
Further, there can be no assurance that any new diagnostic tests we develop will have acceptable clinical performance. Before we can commercialize any new diagnostic tests, we will need to expend significant funds in order to:
•conduct substantial research and development, including validation studies and potentially clinical trials;
•further develop and scale our research and development efforts to accommodate different test strip designs or adjustments; and
•further develop and scale our infrastructure to be able to analyze increasingly large amounts of data.
Our Platform development process involves a high degree of risk, and development efforts may fail for many reasons, including:
•failure of the products to perform as expected at the research or development stage;
•lack of validation data; or
•failure to demonstrate the clinical utility of the products or pass clinical trials or obtain relevant regulatory approval, authorization, certification or clearance.
As we develop our Platform and our diagnostic tests, we will have to make significant investments in product development, marketing and selling resources. In addition, competitors may develop and commercialize competing products faster than we are able to do so.
Our Amira System may not obtain regulatory approval, authorization, certification or clearance, and we may not be able to successfully develop and commercialize our Amira System, including scaling up manufacturing and sales capacity.
We have started adjusting our high performing SARS-CoV-2 antigen test for mass screening applications with our Amira System, which is based upon our Platform and our SARS-CoV-2 antigen test. Our Amira System is still under development, which we may not be able to complete successfully. We currently have a prototype Amira System including strips, device and patient mobile device application. We are moving to design freeze of the system and clinical validation. We are simultaneously tooling up high volume manufacturing lines, for the strip and instrument, while we progress through design freeze and the verification and validation (“V&V”) phase. Even if successfully developed, our Amira System will require regulatory approval, authorization, certification or clearance prior to commercialization. In addition, we may need to seek regulatory approval, authorization, certification or clearance for specific or limited use cases based on our commercialization plans and then seek separate approval, authorization, certification or clearance over time for other settings, such as home-use settings. For example, we submitted a pre-EUA request to FDA in February 2021 and expect to complete POC clinical testing and achieve CE Mark during the first half of 2022. We started clinical testing of our Amira System in the first quarter
3
of 2022, but we may not receive positive clinical data or we may need to perform additional clinical testing to obtain regulatory approval, authorization, certification or clearance for our Amira System. Revenues related to the Amira System depend on development of mass screening opportunities and continued need for COVID-19 testing in keeping economies open and mass screening testing requirements.
We expect to continue to devote significant operational and financial resources to the development and commercialization of our Amira System to meet expected demand for mass screening applications, including at schools, airports, universities, for return-to-work screening and over time for testing in the home. Our ability to produce the planned volume of Amira COVID-19 tests will be dependent on our ability, and the ability of our contract manufacturers, to successfully and rapidly scale up manufacturing and sales capacities. These efforts may divert management’s attention and resources from other diagnostic tests, including our SARS-CoV-2 antigen test and our SARS-CoV-2 antibody test, available on our Platform. We may encounter significant difficulties in our efforts to scale, manufacture and supply our Amira System and we cannot guarantee that any of these challenges will be met in a timely manner or at all.
We may not be able to generate sufficient revenue from our Platform to achieve and maintain profitability.
We believe our commercial success is dependent upon our ability to successfully market and sell our Platform to customers, including large healthcare systems, government organizations, national pharmacy chains and community-based healthcare settings, to launch and commercialize our Instrument and diagnostic tests, including those for COVID-19, to continue to expand our current relationships and develop new relationships with diagnostic companies, and to develop and commercialize new POC diagnostic tests. We are scaling our operations assuming a rapid uptake of our Instrument and our SARS-CoV-2 antigen and SARS-CoV-2 antibody tests, but the demand for our Platform may not increase for a number of reasons, including due to the evolving nature of the COVID-19 pandemic, or unsuccessful execution of our strategy designed to meet the increased demand for COVID-19 tests, or otherwise. If we are unsuccessful in the commercialization of our SARS-CoV-2 antigen and antibody tests, then we will need significant financial resources to maintain our operations. We have experienced early revenue growth from the sale of our Platform to healthcare professionals, principally for our SARS-CoV-2 antigen tests, INR tests and from the sale of third-party distribution products and our anticoagulation management programs. We may not be able to continue revenue growth or maintain existing revenue levels.
Our existing customers and collaborators may decide to decrease or discontinue their use of our Platform due to changes in research and product development plans, changes in the occurrence of certain diseases, such as COVID-19, failures in clinical trials, financial constraints, or utilization of internal testing resources or tests performed by other parties, which are circumstances outside of our control. In addition to reducing our revenue, this may reduce our exposure to early stage research that facilitates the incorporation of newly-developed information about various tests into our Platform.
We are currently not profitable. Even if we succeed in increasing the adoption of our Platform by large healthcare systems, government organizations, national pharmacy chains and community-based healthcare settings, maintaining and creating relationships with our existing and new customers and collaborators and developing and commercializing additional POC diagnostic tests, we may not be able to generate sufficient revenue to achieve or maintain profitability.
Business or economic disruptions or global health concerns, such as the ongoing COVID-19 pandemic, have and may continue to seriously harm our business and increase our costs and expenses.
The global impact of the ongoing COVID-19 pandemic has been rapidly evolving in many countries, including the U.K. where our main research, development and manufacturing operations are located, as well as in other countries, and has led to the implementation of various responses, including government-imposed quarantines, travel restrictions, business and school closures and other public health safety measures. These responses to the COVID-19 pandemic have impacted and may continue to materially and adversely impact our business and results of operations due to, among other factors:
•a potential for delays in launches of our non-COVID-19 diagnostic tests given reduced and limited access to clinical trial sites for our other tests and social distancing and other measures that restrict ability to work on such tests;
•a delay in regulatory approval, authorization, certification or clearance by FDA, and other applicable regulators to some of our diagnostic assays in development, if such regulators focus their resources on and give priority to COVID-19 testing and treatments or to a specific form of COVID-19 testing that is different than our tests;
•a disproportionate impact on the healthcare groups and other healthcare professionals with whom we contract;
•supply shortages for materials used to manufacture our COVID-19 products, including of swabs and extraction buffers necessary for use with our SARS-CoV-2 antigen test and of chips and other components that are subject to global shortages and necessary to manufacture our Instrument and our Amira System;
4
•disruptions to our supply chains and sales and marketing efforts due to restrictions on courier delivery services and other transportation systems;
•disruptions to operations at our current and future manufacturing systems and facilities and those of our third-party vendors, collaborators, and suppliers;
•difficulty accessing the capital and credit markets on favorable terms, or at all, a severe disruption and instability in the global financial markets, and deteriorations in credit and financing conditions which could affect our access to capital necessary to fund our existing and scaled business operations or address maturing liabilities on a timely basis;
•the potential negative impact on the health or productivity of employees, especially if a significant number of them are impacted;
•a deterioration in our ability to ensure business continuity during a disruption; and
•social, economic, and labor instability in the countries in which we or the third parties with whom we engage operate, including any impact of the current conflict between Russia and Ukraine.
This pandemic, as well as intensified measures undertaken to contain the spread of COVID-19, including variants such as Omicron, could decrease healthcare industry spending; adversely affect demand for our Platform; cause one or more of our customers to file for bankruptcy protection or go out of business; cause one or more of our customers to fail to renew, terminate, or renegotiate their contracts; affect the ability of our business development team to travel worldwide to potential customers and the ability of our professional services teams to conduct in-person services and trainings; impact expected spending from new customers; negatively impact collections of accounts receivable; lead to the closure of our existing or future manufacturing facilities or any of our other production, research and/or distribution facilities; and restrict the movement of people and goods, which could negatively impact employee availability (particularly, in respect of our research and development (“R&D”) and sales and marketing teams), any of which would harm our business, results of operations, and financial condition. In addition, while we have taken remote work, group isolation and other measures to prevent an outbreak among our employees, further waves of the COVID-19 pandemic, including new variants, could further disrupt our operations as the success of the measures we have implemented is uncertain. Any changes in the regulations, travel restrictions and other public safety measures that have been or may be imposed by countries in response to the COVID-19 pandemic could impact our COVID-19 testing volumes and, in particular, as such regulations, travel restrictions and public safety measures are lifted, our COVID-19 testing volumes may decrease.
The loss of any member of our senior management team or our inability to attract and retain highly skilled scientists, engineers, software developers, technicians and salespeople could adversely affect our business.
Our success depends on the skills, experience and performance of key members of our senior management team, including Ron Zwanziger, our Chairman and Chief Executive Officer, Dave Scott, Ph.D., our Chief Technology Officer, and Jerry McAleer, Ph.D., our Chief Scientist. The individual and collective efforts of these employees will be essential as we continue to develop our Platform and additional products, and as we expand our commercial activities. The loss or incapacity of existing members of our executive management team or key scientists and engineers could adversely affect our operations, particularly if we experience difficulties hiring qualified successors. We do not have any employment agreements (other than brief at-will offer letters) or non-compete agreements with our co-founders (i.e., Ron Zwanziger, Dave Scott and Jerry McAleer), and because of their knowledge of the industry and our operations, we believe the loss of any one of their services, or any of them leaving and providing services to any of our competitors, could result in a disruption of our operations and/or put us at a competitive disadvantage, which will likely have a material adverse effect on our business.
Our R&D programs and manufacturing operations depend on our ability to attract and retain highly skilled scientists, engineers, software developers and technicians. We may not be able to attract or retain a sufficient number of qualified scientists, engineers, software developers and technicians in the future due to the competition for qualified personnel in our industry. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. We may also have difficulties locating, recruiting or retaining a sufficient number of qualified sales people to successfully scale up our sales and marketing efforts to meet expected demands. Recruiting and retention difficulties can limit our ability to support our R&D and sales and marketing programs. In addition, all of our employees in the U.S. are at-will, which means that either we or the employee may terminate their employment at any time. We also do not maintain “key person” insurance on any of our employees.
5
Our Platform may never achieve significant commercial market acceptance.
Our Platform may never gain significant acceptance in the marketplace and, therefore, may never generate substantial revenue or profits for us. Our ability to achieve commercial market acceptance for our Platform will depend on several factors, including:
•our ability to demonstrate the clinical utility and cost effectiveness of our Platform and its potential advantages over existing POC systems, or for certain tests, over central lab counterparts, to the medical community;
•our ability, and that of our collaborators, to secure and maintain FDA and other applicable regulatory clearance, authorization or approval for certain components of our Platform;
•our ability to expand our test menu and provide a broad range of tests on our Platform while maintaining consistency and precision;
•our ability to obtain relevant regulatory approval, authorization, certification or clearance for our diagnostic assays in development, particularly those in our near-term pipeline;
•the agreement by commercial third-party payors and government payors to cover and to reimburse our Instrument and test strips, the scope and extent of which will affect healthcare providers’ willingness to pay for our Instrument and test strips and likely heavily influence their decisions to recommend use of our Platform;
•the willingness of healthcare providers to use a POC system over central lab counterparts and the rate of adoption of our Platform by healthcare providers and other users; and
•the impact of our investments in our Platform innovation and commercial growth.
We believe that the successful completion of clinical trials, publication of scientific and medical results in peer-reviewed journals, and presentations at leading conferences will be important to facilitate the broad adoption of our Platform. Publication in leading medical journals is subject to a peer-review process, and peer reviewers may not consider the results of studies involving our Platform sufficiently novel or worthy of publication.
The failure of our Platform to be listed in physician guidelines or of our clinical trials to produce favorable results or to be published in peer-reviewed journals could limit the adoption of our Platform. We may not be successful in addressing these or other factors that might affect the market acceptance of our Platform and technologies. Failure to achieve widespread market acceptance of our Platform would materially harm our business, financial condition and results of operations.
A limited number of customers currently represent a substantial portion of our revenue. If we fail to retain these customers, our revenue could decline significantly.
We currently derive a substantial portion of our revenue from sales to certain key customers, including CVS Pharmacy Inc. (“CVS”) in the U.S. and the National Health Service (“NHS”) in the U.K. As a result, our revenue could fluctuate materially and could be materially and disproportionately impacted by purchasing decisions of these customers or any other significant future customers. Our agreements with CVS and NHS do not have minimum purchase requirements. Any of our significant customers may decide to purchase less than they have in the past, may alter their purchasing patterns at any time with limited notice, or may decide not to continue to use our Platform and test strips at all, any of which could cause our revenue to decline and adversely affect our financial condition and results of operations.
We rely on a limited number of suppliers or, in some cases, sole source suppliers, for the components of our Platform and our Amira System and for other materials and may not be able to find, or immediately transition to, alternative suppliers.
We rely on several sole source suppliers for certain components or accessories and materials used in our Instrument and our test strips, such as reagents. In addition, we currently rely solely on Flextronics Ltd. (“Flextronics”), as the sole manufacturer of our Instrument, with components and assemblies supplied by Flextronics and by outside vendors, and our facilities as the sole suppliers of our test strips.
In the case of any alternative supplier for our Instrument, the components of our Instrument or our test strips, there can be no assurance that replacement components or, with regards to the test strips, reagents, swabs or other accessories will be available or will meet our quality control and performance requirements for our operations or products. For example, in November 2020, there was a shortage of a component for use in our Instrument which significantly constrained the production and delivery of our Instrument to customers until we added an additional supplier. We may also have difficulty sourcing raw materials and components, including micro processing or semiconductor chips or capacitors, in a timely fashion in necessary quantities, or these materials and components might not comply with our specifications, which are exacting. An interruption in our ability to develop and produce our Instrument, Amira System or test strips could occur if we encounter delays or difficulties in securing components of our Instrument, Amira System or our test strips, including due to the
6
COVID-19 pandemic, and if we cannot then obtain an acceptable substitute. Any changes in such materials could lead to required changes in performance, regulatory approval, authorization, certification or clearance processes. If we encounter delays or difficulties in securing, reconfiguring or revalidating the equipment and reagents we require for our Platform, our business, financial condition, results of operations and reputation could be adversely affected.
Because of a long lead-time to delivery of certain components of our manufacturing system and Platform, we are required to place orders for a variety of items well in advance of scheduled production runs. We have increased our flexibility to purchase strategic components within shorter lead times by entering into scale up arrangements with the suppliers of these components. Although we attempt to match our inventory and production capabilities to estimates of marketplace demand, to the extent Instrument and test strip orders materially vary from our estimates, we may experience continued constraints in our Platform production and delivery capacity, which could adversely impact our financial condition and results of operations. Should our need for raw materials and components used in production continue to fluctuate, we could incur additional costs associated with either expediting or postponing delivery of those materials. In an effort to control costs, we have implemented a lean manufacturing system. Managing the change from discrete to continuous flow production requires time and management commitment. Lean initiatives and limitations in our supply chain capabilities may result in component shortages that delay shipments and cause fluctuations in revenue.
Further, we believe that there are a limited number of other equipment manufacturers that are currently capable of supplying and servicing the equipment necessary for the manufacturing of our Instrument, Amira System and test strips. We have spent significant time and resources developing our manufacturing processes with our existing collaborators, and the use of equipment or materials furnished by these replacement suppliers would require us to significantly alter our operations. It could take a very long time to obtain a new manufacturing system for test strips if additional capacity were needed. Transitioning to a new supplier would therefore be time consuming and expensive, may result in interruptions or delays in our operations, could affect the performance specifications of our operations or could require that we revalidate our Platform and could require us to obtain additional clearance, authorization, approval, accreditation or licensure for the changes. There can be no assurance that we will be able to secure alternative equipment, reagents, and other materials, and bring such equipment, reagents, and materials on line and revalidate them without experiencing interruptions in our workflow.
We may experience manufacturing problems or delays that could limit the growth of our revenue or increase our losses.
Our current and planned manufacturing operations are critical to our commercialization plans, and these operations may not be sufficient to withstand the demands we intend to place on them. Any disruption in the operation of any of our facilities or the facilities of our suppliers could impact our supply chain and operation of our Platform and our ability to conduct our business and generate revenue. We may encounter unforeseen situations that would result in delays or shortfalls in our production as well as delays or shortfalls caused by our outsourced manufacturing suppliers and by other third-party suppliers who manufacture components for our Platform, including delays caused by or constraints on capacity as a result of the COVID-19 pandemic. If we are unable to keep up with demand for our Platform, our revenue could be impaired, market acceptance for our Platform could be adversely affected and our customers might instead purchase our competitors’ products. Our inability to successfully manufacture the components of our Platform would have a material adverse effect on our operating results.
If our or our suppliers’ or collaborators’ present or future facilities were to be damaged, destroyed or otherwise unable to operate, whether due to fire, floods, storms, tornadoes, earthquakes, other inclement weather events or natural disasters, employee malfeasance, terrorist acts, public health crises or pandemics, power outages, or otherwise, it may render it difficult or impossible for us to increase our manufacturing and other operations sufficiently to meet increased demand, and our business could be severely disrupted. Our facilities and the equipment we use to manufacture our Platform would be costly to replace and could require substantial lead time to repair or replace.
As we continue to expand our business, we may experience problems in scaling our manufacturing and commercial operations, and if we are unable to support demand for our Platform, our Amira System and our future tests, including ensuring that we have adequate capacity to meet increased demand, or we are unable to successfully manage the evolution of our Platform or our Amira System, our business could suffer.
In connection with the commercialization of our Platform, we have added, and expect to continue to add, personnel in the areas of sales, marketing, manufacturing, regulatory, quality assurance, customer and technical service and other support functions. We also continue to scale our manufacturing, sales and marketing capabilities. As our sales volume grows, we will need to continue to increase our workflow capacity for sales, customer service, billing and general process improvements, expand our internal quality assurance program and to scale up our manufacturing systems for our Platform quickly. We will need additional sales, scientific and technical personnel to market our Platform and our Amira System and follow up on any reported quality issues. Our Amira System is focused on mass screening opportunities and over-the-counter (“OTC”) sales, and marketing channels for professional and OTC sales vary significantly and may require additional support. We will also need to secure additional facilities, purchase additional equipment, some of which can take several months or more to
7
procure, setup, and validate, and to significantly and rapidly increase our capacity to meet increased demand. There is no assurance that any of these increases in scale, expansion of personnel, equipment, software and computing capacities, or process enhancements will be successfully implemented on a timely basis, or at all, or that we will have adequate space in our facilities to accommodate such required expansion. Even if these and other measures are implemented successfully, we still expect to experience continued capacity constraints as we commercialize our products.
As additional diagnostic products are commercialized and new tests are developed, we may need to implement adjustments to our Platform and our processes and hire new personnel with different qualifications. Failure to manage this growth or transition could result in delays in the development of new test strips, higher product costs, declining product quality, deteriorating customer service, and slower responses to competitive challenges. A failure in any one of these areas could make it difficult for us to meet market expectations for our Platform and our Amira System and could damage our reputation and the prospects for our business.
If any of our facilities were damaged or destroyed, or if we experience a significant disruption in the expansion of our operations for any reason, our ability to continue to operate our business and meet increased demand could be materially harmed.
As we expand our capacity, we believe it may be necessary to both expand our existing facilities and to add one or more new facilities to meet anticipated demand. We are also in the process of scaling our manufacturing facilities and adding warehouse and office space, which are expected to continue to be rolled out in the next few years, with necessary adjustments based on market needs. Failure to complete, or timely complete, these expansion projects on time or at all, may significantly delay our workflows and operations, which may adversely affect our business, financial condition and results of operation. In addition, our financial condition may be adversely affected if we are unable to complete these expansion projects on budget and otherwise on terms and conditions acceptable to us. Finally, our financial condition will be adversely affected if demand for our Platform and our Amira System does not materialize in line with our current expectations and if, as a result, we end up building excess capacity that does not yield a reasonable return on our investment.
We have devoted and continue to devote significant resources for the scale-up and commercialization of our COVID-19 tests.
We are working toward the large-scale technical development and manufacturing scale-up in several countries and larger scale deployment of our COVID-19 tests, including our SARS-CoV-2 antigen test, SARS-CoV-2 antibody test, SARS-CoV-2 antigen pool test, our SARS-CoV-2 RNA STAR and SARS-CoV-2 RNA STAR Complete molecular lab reagent kits, as well as our five-minute SARS-CoV-2 Ag Ultra test, five-minute SARS-CoV-2 Ag Ultra Pool test and our Amira System. The number of potential tests that we are able to produce and bring to market is dependent on our ability, and the ability of our contract manufacturers, to successfully and rapidly scale up manufacturing capacity and our ability to scale up our marketing and sales capacities. To support these scale-ups, we will need to expend significant resources and capital quickly, and we therefore have diverted and expect to continue to divert resources and capital from our other non-COVID-19 diagnostic tests. Our ability to produce and successfully bring to market our COVID-19 tests will also depend on our ability to further scale up on our manufacturing, sales and marketing capacities.
We have entered into, and may continue to enter into, contractual arrangements with customers, suppliers, distributors, manufacturers or other collaborators that contain restrictions or minimum commitments which limit our ability to develop, manufacture, supply, commercialize and distribute our COVID-19 tests. If we fail to meet contractual obligations under our agreements or if we enter into agreements that restrict our ability to develop, manufacture, supply, commercialize and distribute our COVID-19 tests, we may be required to pay damages to the counterparty or contest disagreements or disputes, which could have a material and adverse effect on our financial condition and operations.
Given the rapidity of both the onset of the COVID-19 pandemic and our commercialization efforts with respect to our COVID-19 tests, as well as the complexity of the economics of a diagnostic test for a pandemic, we are still considering how to adjust our pricing strategy for these tests and cannot provide assurance as to the ultimate impact of each COVID-19 test on our financial condition and results of operations. Focus on such COVID-19 tests could have the lasting impacts of significant diversions of resources and attention away from the development of other non-COVID-19 diagnostic tests; a possible reduction in our ability to rapidly pivot research, development and commercialization back to other areas of focus; and lost time associated with addressing the demand for our COVID-19 tests. We have started diverting focus from COVID-19 tests onto other tests, such as the roll-out of recently CE marked D-Dimer and CRP tests in Europe, but this transition and our focus may depend on the onset of new variants and peaks in demand for additional COVID-19 testing, as such there may be various fluctuations in supply and demand and impact on overall sales and sales of other tests.
8
We are continuously updating and improving our Platform based on the needs of various tests, and this may impact changes, such as upgrades or new versions of our Instrument.
Our Platform is continuously evolving and will continue to do so as more tests are added to our Platform. A specific test may require specific test strip or design changes which could also impact Instrument set up. In addition, we are continuously improving our Instrument and have a pipeline of upgrades to make the Instrument more robust and further lower the costs over time. This may require regular updates to our Instrument, including software upgrades and in certain cases the need to swap out the Instrument for an updated version. Despite our rigorous testing and quality assurance processes, it is possible that our Instrument may prove to operate less reliably than we anticipated or degrade in efficacy over time. If this occurs, this may likewise necessitate updates to our design or software or replacement of Instruments, which could adversely affect our financial condition, results of operations and/or reputation. The replacement or refurbishment for further use of an Instrument may require sales and customer support and may lead to older versions of our Platform becoming obsolete which could have an adverse impact on our financial results. The need for an upgrade to an Instrument may impact the commercialization of certain diagnostic assays which require an upgraded Instrument.
Our current tests or any tests that we develop to cover additional menu or diagnostic testing may not be successfully developed or commercialized or gain the acceptance of the public or the medical community.
We plan to implement a broad range of tests on our Platform over time. Each test requires a significant amount of R&D and comes with its own technical challenges. In addition, we aim for all tests to provide lab-comparable results based on comparison against the lab standard reference for such test, where such lab reference is available. In light of the technical and complicated nature of some test strips, R&D timelines may be delayed and lab-comparable results or expected performance criteria may not be met or only be met over time as improvements are rolled out. This may affect our ability to launch or commercialize our tests and could have an adverse impact on our financial results. While we have encouraging internal data for many diagnostic tests, we have not yet performed multi-site, external clinical analyses of most of these tests or otherwise compared these results against clinical results.
While our SARS-CoV-2 antigen test detects major global SARS-CoV-2 variants including Delta, Gamma, Epsilon, Alpha, Beta and Omicron variants, there is no guarantee that our tests will be able to accurately detect all variants of concern. Sensitivity and specificity concerns with respect to COVID-19 tests generally could negatively affect demand for our Platform and therefore our business, revenues and profits. Similar concerns about our collaborators, though unrelated to us, could likewise create negative publicity, which could negatively impact demand for our Platform or harm our reputation. These concerns could be wrongly attributed to our tests and could negatively affect sales of our Instrument. Additionally, concerns about COVID-19 tests generally could adversely affect our business as the general public may associate our SARS-CoV-2 antigen, SARS-CoV-2 antigen pool test and SARS-CoV-2 antibody tests with them. In addition, the medical community is continuously learning and publishing scientific literature about COVID-19 and the success of our COVID-19 tests will depend, in part, on the ability of the tests to detect the virus (or antibodies) or variants of the virus and on acceptance of the test results by the public and medical community. If any of our tests or those of other parties developing similar products receive negative or unfavorable publicity, or the medical community publishes information criticizing the accuracy, effectiveness or utility of COVID-19 tests, whether or not ours, it could result in a decrease in demand for any product that we may develop. In addition, responses by the U.S. federal, state or foreign governments to negative public perception or ethical concerns related to COVID-19 tests may result in new legislation or regulations that could limit our ability to develop or commercialize any product, obtain or maintain regulatory approval, authorization, certification or clearance, if applicable, identify alternate regulatory pathways to market or otherwise achieve profitability. More restrictive statutory regimes, government regulations or negative public opinion would have an adverse effect on our business, financial condition, results of operations and prospects, and may delay or impair the development and commercialization of our products or demand for any products we may commercialize.
We have limited data on the performance of our Platform to date and limited experience in marketing and selling our Platform, and if we are unable to expand our direct sales and marketing force to adequately address our customers’ needs, our business may be adversely affected.
We have limited data on the performance of our Platform to date and limited experience in marketing and selling our Platform, which had its formal commercial launch in Europe in 2019 with our INR test and 2021 for our CRP and D-Dimer tests in Europe. We do not currently have, and may not be successful in developing, the capacity to market, sell, or distribute our Platform or other products we may develop effectively or in volumes high enough to support our planned growth.
We currently and will continue to sell our Platform on a region or country specific basis across our footprint in Europe, the U.S., South America, Africa and Asia using a combination of direct sales and sales through our distributors. Our future sales will depend in large part on our ability to develop and substantially expand our sales force and to significantly increase the scope of our marketing efforts. Our target market of identifying customers in healthcare systems, government organizations, national pharmacy chains and community-based healthcare settings is a large and diverse market. As a result, we believe it is
9
necessary to develop a large sales force that includes sales representatives with a variety of specific technical backgrounds. We will also need to attract and develop a significant amount of marketing personnel with industry expertise. Competition for such employees is intense. We may not be able to attract and retain personnel or be able to build an efficient and effective sales and marketing force, which could negatively impact sales and market acceptance of our products and limit our revenue growth and potential profitability.
Our expected future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, and integrate additional employees. Our future financial performance and our ability to commercialize our products and to compete effectively will depend, in part, on our ability to manage this potential future growth effectively, without compromising quality.
We also enlist distributors, and we may potentially enlist local collaborators, to assist with sales, distribution, and customer support. Locating, qualifying, and engaging a significant number of distribution collaborators with local industry experience and knowledge will be necessary to effectively market and sell our products. We may not be successful in finding, attracting, and retaining a sufficient number of distributors or other collaborators or we may not be able to enter into such arrangements on favorable terms, or at all. Our sales in low and middle income countries also depend on support from our global health partners, such as the Bill and Melinda Gates Foundation (“BMGF”) and from national governments. Developing such relationships may require significant resources, time and management attention and could adversely affect our ability to make sales.
Sales practices utilized by our distributors that are locally acceptable may not comply with sales practices standards required under the laws of the U.K., U.S. or other jurisdictions that apply to us, which could create additional compliance costs and risk and demand additional resources, time and management attention. If our sales and marketing efforts are not successful, we may not achieve significant market acceptance for our products, which would materially and adversely impact our business and anticipated financial condition and results of operations.
If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenue or achieve and sustain profitability.
The diagnostics industry, including in vitro diagnostics (“IVD”) and POC systems, is rapidly evolving, and we face competition from companies that offer products in our targeted application areas. Our principal competition comes from established diagnostic companies. Our competitors include laboratory or POC companies such as Abbott Laboratories, Becton, Dickinson and Company, Danaher Corporation, GenMark Diagnostics, Inc., Laboratory Corporation of America Holdings, Quest Diagnostics Incorporated, Quidel Corporation, Roche Diagnostics Corporation, Siemens Healthineers AG, Inc. and many others. In addition to diagnostic systems, we believe these companies may also develop their own approved or cleared diagnostic kits, which can be sold to the clients who have purchased their systems. In addition, new and existing companies could seek to develop tests that compete with ours.
For each of our eight available tests, we face competition from other commercially available tests, including:
•For our SARS-CoV-2 antigen test and SARS-CoV-2 antigen pool test: Quidel Sofia, BD Veritor Plus System, Abbott BinaxNOW COVID-19 Ag Card, general lateral flow tests and others.
•For our SARS CoV-2 antibody test: Roche Elecsys Anti-SARS-CoV-2, Accelerate Diagnostics BioCheck SARS-CoV-2 Antibody Test Kits, SD Biosensor Q COVID-19 IgM/IgG Rapid Test and others.
•For our SARS-CoV-2 Ag & Flu A/B tests: same as above antigen tests, Roche and SD Biosensor SARS-CoV-2 and Influenza A/B tests.
•For our INR test: Roche Coaguchek and others.
•For our D-Dimer test: Roche Cobas h232 and others.
•For our CRP test: Affinion (Abbott) and others.
Our tests in development are designed and validated against their respective lab standard.
Many of our current and future competitors are either publicly traded, or are divisions of publicly-traded companies, and may enjoy a number of competitive technological, financial and market access advantages over us, including:
•greater name and brand recognition;
•substantially greater financial and human resources and expertise;
•broader or superior product lines;
•larger sales forces and more established distributor networks;
10
•substantial intellectual property portfolios;
•larger and more established customer bases, relationships with healthcare professionals and third-party payors; and
•better established, larger scale, and lower cost manufacturing capabilities.
We believe that the principal competitive factors in all of our target markets include:
•cost of instruments and consumables;
•flexibility and ease of use;
•accuracy, including sensitivity and specificity, and reproducibility of results;
•reputation among customers;
•innovation in product offerings; and
•compatibility with existing processes.
Furthermore, even if we do develop new marketable products or services, our current and future competitors may develop products and services that are more commercially attractive than ours, and they may bring those products and services to market earlier or more effectively than us. If we are unable to compete successfully against current or future competitors, we may be unable to increase market acceptance for and sales of our Platform, which could prevent us from increasing or sustaining our revenues or achieving sustained profitability. Our competitors may also use their patent portfolios, developed in connection with developing their tests, to allege that our Platform infringes their patents, and we could face litigation with respect to such allegations and the validity of such patents.
The diagnostic industry is subject to rapidly changing technology which could make our Platform and other products we develop obsolete.
Our industry is characterized by rapid technological changes, frequent new product introductions and enhancements and evolving industry standards, all of which could make our Platform and the other products we are developing obsolete. Our future success will depend on our ability to anticipate and keep pace with the evolving needs of our customers on a timely and cost-effective basis and to pursue new market opportunities that develop as a result of technological and scientific advances. The attractiveness of our Platform partly depends on the ability to continue to add additional assays and tests in a timely manner. Failure to deliver such tests in the timelines suggested may affect our business plan and ability to obtain greater market penetration, or otherwise cause us to lose market share.
In recent years, there have been advances in methods used to analyze very large amounts of information. We must continuously enhance our Platform and develop new products to keep pace with evolving standards of care. If we do not update our Platform, including successfully developing new tests for our Instrument, such as multiplex test strips with the ability to detect an increased number of markers in a single sample, it could become obsolete and sales of our Platform and any new products could decline, which would have a material adverse effect on our business, financial condition, and results of operations.
Our business and reputation will suffer if our products do not perform as expected, particularly as test strip volume increases, or if we are unable to establish and comply with stringent quality standards to assure that the highest level of quality is observed in the performance of our Platform, our Amira System and our Fast Lab Solutions.
Inherent risks are involved in providing and marketing diagnostic tests and related services. Our success depends on the market’s confidence that we can provide reliable, high-quality diagnostic products and information that may be used to make critical healthcare decisions. There is no guarantee that the accuracy and reproducibility we have demonstrated to date will continue as our volume of test strips increases or as we commercialize additional tests. We believe that our customers are likely to be particularly sensitive to product defects and errors, including if our products fail to detect certain diseases with high accuracy from clinical specimens. As a result, the failure of any of our products to perform as expected could significantly impair our operating results and our reputation. We may be subject to legal claims arising from any defects or errors.
We must maintain top service standards and government-mandated and other quality controls. Past or future performance or accuracy defects, incomplete or improper process controls, or mishandling of samples or test strips due to inadequate training can lead to incorrect diagnostic results and potentially result in adverse outcomes for patients. These events could lead to voluntary or legally mandated safety alerts relating to our Platform, our Amira System, our Fast Lab Solutions or our
11
facilities and could result in the removal of our Platform, our Amira System or our Fast Lab Solutions from the market. Insufficient quality controls and any resulting negative outcomes could result in significant costs and litigation, as well as negative publicity that could reduce demand for our products and payors’ willingness to cover our products. Even if we maintain adequate controls and procedures, damaging and costly errors may occur.
If we cannot maintain our current relationships, or enter into new relationships, with diagnostics or research and development companies, or if our collaborators do not perform as expected, our product development could be delayed.
We rely on research and development collaborators to research and develop certain tests for our Platform. We have existing research and development agreements with well-established diagnostic companies that have market-leading capabilities in specific disease areas or targets, such as infectious diseases, respiratory assays, enteric diseases and others. The inability of these companies to deliver on research and development projects or our inability to use or have sufficient access to required reagents derived from such projects could have an adverse effect on our ability to launch additional tests and thus on our financial condition and results of operations.
Our success in the future depends in part on our ability to maintain these relationships and to enter into new relationships. This can be difficult due to several factors, including internal and external constraints placed on these organizations that can limit the number and type of relationships with companies such as ours that can be considered and consummated. In addition, collaboration, manufacturing and supply agreements can be complex and contain certain provisions that may be susceptible to multiple interpretations. The resolution of any interpretation disagreement that may arise could be adverse to us, for example, by increasing our royalties payable to third parties, by narrowing what we believe to be the scope of our rights to certain intellectual property, or increasing what we believe to be our financial or other obligations under these agreements, and any such outcome could have a material adverse effect on our business, financial condition, results of operations, and prospects. In addition, we expect that we will have capacity constraints on demand for our overall test portfolio, and we will need to make decisions regarding allocation of supply of such tests, which could have an adverse effect on new or existing relationships with third parties and governments.
We are currently engaged, and expect to continue to engage, in discussions with companies regarding commercial opportunities. There is no assurance that any of these discussions will result in commercial agreements, or if an agreement is reached, that the resulting engagement will be successful and that such companies will perform as expected or that clinical, sales and marketing activities conducted as part of the engagement will produce successful outcomes.
Additionally, speculation in the industry about our existing or potential engagements with life science companies may be a catalyst for adverse speculation about us, our products, and our technology, which may result in harm to our reputation and our business.
We may acquire other businesses or form joint ventures or make investments in other companies or technologies that could negatively affect our operating results, dilute our shareholders’ ownership, increase our debt or cause us to incur significant expense.
We may pursue acquisitions of businesses and assets as well as strategic alliances and joint ventures that leverage our Platform and industry experience to expand our offerings or distribution. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in the incurrence of debt, contingent liabilities or future write-offs of intangible assets or goodwill, any of which could have a material adverse effect on our financial condition, results of operations, and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that we would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could have a material negative effect on our results of operations and financial condition. We may not realize the anticipated benefits of any acquisition, technology license, strategic alliance, or joint venture.
To finance any acquisitions or joint ventures, we may choose to issue our common shares as consideration, which would dilute the ownership of our shareholders. Additional funds may not be available on terms that are favorable to us, or at all. If the price of our common shares is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our common shares as consideration.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks.
In addition to the various direct sales units that have already been established in the U.S., most Western European countries, Japan, India, South Africa, Colombia and Brazil, we are planning to both continue to grow direct sales operations as well as extend distribution agreements for our Instrument and test strips in various countries. In addition, in Africa, we plan to continue to collaborate with non-governmental organizations, such as BMGF, to build programs that utilize our Platform to improve patient outcomes across multiple countries on the African continent. We plan to maintain sales representatives and
12
distributor relationships, to conduct healthcare provider and patient association outreach activities, to extend research and development capabilities and to expand payor relationships internationally. Doing business internationally involves a number of risks, including:
•multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, economic sanctions, export and import restrictions, employment laws, regulatory requirements, and other governmental approvals, permits, and licenses;
•potential competition from existing or future local and regional product offerings;
•difficulties in complying with a multitude of product regulations in various jurisdictions, including evolving regulatory pathways in response to the ongoing COVID-19 pandemic;
•failure by us or our distributors to obtain regulatory approvals, authorizations or clearance for the use of our products in various countries;
•additional potentially relevant third-party patent rights;
•complexities and difficulties in obtaining protection and enforcing our intellectual property;
•difficulties in staffing and managing foreign operations;
•complexities associated with managing multiple payor reimbursement regimes, government payors, or patient self-pay systems;
•our dependence on cooperation and donor funding of local aid sources and private foundations, particularly in developing regions such as Africa, as well as cooperation from national healthcare programs and governments;
•logistics and regulations associated with shipping samples, including infrastructure conditions and transportation delays;
•limits in our ability to penetrate international markets if we are not able to conduct our tests locally;
•financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products, and exposure to foreign currency exchange rate fluctuations;
•the risk that regional or local distributors may not commit the necessary resources to market and sell our products to the level of our expectations or may choose to favor marketing the products of our regional or local competitors;
•natural disasters, political and economic instability, including wars, terrorism, and political and civil unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions; and
•regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors’ activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act of 1977, as amended (“FCPA”), or its books and records or anti-bribery provisions, or similar anti-bribery or anti-corruption laws or regulations in other jurisdictions, such as the United Kingdom’s Bribery Act 2010.
Any of these factors could significantly harm our future international expansion and operations and, consequently, our business, financial condition and results of operations.
Our commercial success in Africa will be dependent on continued donor funding of healthcare initiatives in Africa from a wide variety of sources such as the African Medical Supplies Platform (“AMSP”), Partnership for Supply Chain Management (“PSCM”), The Global Fund to Fight AIDS, Tuberculosis and Malaria, the World Health Organization, the United Nations Children Fund, Médecins Sans Frontières and private foundations such as BMGF, the Clinton Health Access Initiative and the Rockefeller Foundation. Our ability to work collaboratively with these funders and with national healthcare programs will be important to our success in utilizing our Platform to help transform primary care delivery in Africa and improve patient outcomes and delays in such efforts may impact the roll out of these programs, as a lot of parties are involved and we do not control operations of such complex entities.
If we were sued for product liability or professional liability we could face substantial liabilities that exceed our resources.
The marketing, sale and use of our products could lead to the filing of product liability claims were someone to allege that our Platform, our Amira System or other products identified inaccurate or incomplete information or otherwise failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of, or inappropriate reliance upon,
13
the information we provide in the ordinary course of our business activities. A product liability or professional liability claim could result in substantial damages and be costly and time-consuming for us to defend.
We maintain product and professional liability insurance, but this insurance may not fully protect us from the financial impact of defending against, settling, or paying damages in respect of product liability or professional liability claims and such policies will be subject to limitations and exclusions. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could damage our reputation, cause current customers to terminate existing agreements, or cause potential customers to seek other suppliers, any of which could adversely impact our business, financial condition and results of operations.
We are subject to, and may in the future become subject to, claims and litigation that could result in significant expenses and could ultimately result in unfavorable outcomes for us.
From time to time, we may be involved in litigation and other proceedings, including matters related to product liability claims, commercial disputes and intellectual property claims, as well as regulatory, employment, and other claims related to our business. Litigation related to our Company, our business, and our operations or financial performance may also involve customers, competitors, suppliers, patients, shareholders, governmental authorities or other third parties, including potential whistleblower claims and other employee-related claims. Our Amira System may be marketed OTC and could thus bring consumer liability claims with it. Litigation can be lengthy, expensive and disruptive to our operations, and results cannot be predicted with certainty. An adverse decision could result in significant settlement amounts, monetary damages, fines or injunctive relief that could affect our financial condition or results of operations. Even if lawsuits do not result in an unfavorable outcome, the costs of defending or prosecuting such lawsuits may be material to our business and our operations. Moreover, these lawsuits may divert management’s attention from the operation of our business, which could adversely affect our business and results of operations.
Our employees, principal investigators, consultants, and collaborators may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants, and collaborators. Misconduct by these parties could include intentional failures to comply with the regulations of FDA and other applicable regulators, comply with healthcare fraud and abuse laws and regulations in the U.K., U.S. and abroad, report financial information or data inaccurately, or fail to disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation. We currently have a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and our code of conduct and anti-bribery policies and the other precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations, particularly as we seek to rapidly expand our business on a global scale. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, which could have a significant impact on our business. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations.
We depend on our information technology systems, and any failure of these systems could harm our business.
We depend on information technology and telecommunications systems for significant elements of our operations, including all connectivity solutions associated with our Platform, our research and development data and quality management system, our knowledge and inventory management system, our factory controls, our customer provisioning and analytics reporting and our patient care database management. We have installed, and expect to expand, a number of enterprise software systems that affect a broad range of business processes and functional areas, including for example, systems handling human resources, financial controls and reporting, contract management, regulatory compliance, and other infrastructure operations. In addition to the aforementioned business systems, we intend to extend the capabilities of both our preventative and detective security controls by augmenting the monitoring and alerting functions, the network design, and the automatic countermeasure operations of our technical systems. These information technology and telecommunications systems support a variety of functions, including operations, test validation, sample processing, quality control, customer service support, research and development activities, scientific and medical curation, and general administrative activities. In addition, our
14
third-party billing and collections provider depends upon technology and telecommunications systems provided by outside vendors.
Information technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses, and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our information technology and telecommunications systems, failures or significant downtime of our information technology or telecommunications systems or those used by our third-party service providers could prevent our Platform from functioning properly and conducting analyses or prevent us from preparing and providing reports, conducting research and development activities, and managing the administrative aspects of our business. Any disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could have an adverse effect on our business.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business or prevent us from accessing critical information and expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we may collect and store sensitive data, including legally protected health information, personal information, intellectual property and proprietary business information owned or controlled by ourselves or our customers, payors, and collaborators. We manage and maintain our applications and data utilizing a combination of on-site systems, managed data center systems, and cloud-based data center systems. We may communicate sensitive patient data to customers through our Platform. These applications and data encompass a wide variety of business-critical information and regulated information including research and development information, commercial information, and business and financial information. We face risks relative to protecting this critical information, including: loss of access risk; inappropriate disclosure risk; inappropriate modification risk; and the risk of our being unable to adequately monitor our controls over these risks.
The secure processing, storage, maintenance, and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure, and that of our third-party service providers, may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance, or other disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost, or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the federal Health Insurance Portability and Accountability Act of 1996, as amended (“HIPAA”), and its implementing regulations, and regulatory penalties. Although we have implemented commercially reasonable security measures and a formal, dedicated enterprise security program to prevent unauthorized access to patient data, our Platform gives broad access to physicians, where the physicians control any other access to our Platform, and there is no guarantee we can continue to protect our online portal and mobile application from breach. Further, as we develop products and features that may be used or accessed outside of the traditional healthcare setting, there will be additional challenges to protecting the security of information and systems. Unauthorized access, loss or dissemination could also disrupt our operations, including our Platform’s ability to conduct analyses and provide test results and our ability to provide customer assistance services, conduct research and development activities, collect, process, and prepare company financial information, provide information about our products and other patient and healthcare provider education and outreach efforts through our website or otherwise, or to manage the administrative aspects of our business, and may damage our reputation, any of which could adversely affect our business.
The U.S. Department of Health and Human Services (“HHS”) Office of Civil Rights may impose significant penalties on a covered entity or a business associate for a failure to comply with a requirement of HIPAA. Penalties will vary significantly depending on a variety of factors such as the date of the violation or whether the failure to comply was known or should have been known, or whether failure to comply was due to willful neglect. Additionally, a person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty and imprisonment. The U.S. Department of Justice is responsible for criminal prosecutions under HIPAA. HIPAA also authorizes state attorneys general to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of protected health information. Furthermore, in the event of a breach as defined by HIPAA, we may be required to comply with specific reporting requirements under the HIPAA regulations, which may include notification to the general public, depending on the scale of the breach.
15
In addition, the interpretation and application of consumer, health-related, and data protection laws in the U.S., Europe and elsewhere are often uncertain, contradictory, and in flux. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices. If so, this could result in government-imposed fines or orders requiring that we change our practices, which could adversely affect our business or reputation. In addition, these privacy regulations may differ from country to country, and may vary based on whether testing is performed in the U.S. or in the local country. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices and compliance procedures in a manner adverse to our business.
Global economic or business instability may have a negative impact on our business.
Continuing concerns over the economic impact of the COVID-19 pandemic, health care reform legislation, geopolitical issues, such as the conflict between Russia and Ukraine, the availability and cost of credit, and government stimulus programs in the U.S. and other countries have contributed to volatility for the global economy. The current military conflict between Russia and Ukraine could disrupt or otherwise adversely impact the global economy and related sanctions, export controls or other actions that may be initiated by nations, including the U.K., the E.U., the U.S. and Russia could adversely affect our business and/or our supply and distribution chains, manufacturers, suppliers or customers. If the economic climate does not improve, our business, including our access to patient samples and the addressable market for diagnostic tests that we may successfully develop, as well as the financial condition of our suppliers and our commercial third-party payors, could be adversely affected, resulting in a negative impact on our business, financial condition, and results of operations. Additionally, the instability has resulted in diminished liquidity and credit availability in the market, which could impair our ability to access capital if required or adversely affect our operations. In the event of further economic slowdown, investment in research and development may also experience a further corresponding slowdown.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
Our borrowing arrangements contain restrictions that limit our flexibility in operating our business.
In March 2021, LumiraDx Investment Limited, one of our subsidiaries, entered into a senior secured term loan, as amended from time to time (the “2021 Senior Secured Loan”), with BioPharma Credit Investments V (Master) LP and BPCR Limited Partnership, as lenders and BioPharma Credit PLC (collectively, “Pharmakon”), as collateral agent. We have borrowed $300 million under the 2021 Senior Secured Loan, part of which was used to prepay the senior secured term loan originally dated as of October 6, 2020, as amended on October 16, 2020 and as further amended on January 15, 2021, between LumiraDx Group Limited (“LumiraDx Group”), one of our subsidiaries, and Silicon Valley Bank, as lender and Jefferies Finance LLC (“Jefferies”), as lender and administrative and collateral agent pursuant to which Jefferies originally made available to LumiraDx Group a $100 million senior secured term loan facility and, pursuant to an incremental term loan notice dated as of January 15, 2021, Silicon Valley Bank had provided an incremental term loan facility of an additional $40 million. The 2021 Senior Secured Loan is subject to an interest rate of 8.0% per annum payable in quarterly cash installments. The 2021 Senior Secured Loan matures on March 29, 2024. The 2021 Senior Secured Loan has been guaranteed and secured by LumiraDx and certain of our subsidiaries. The 2021 Senior Secured Loan contains various covenants that limit our ability to engage in specified types of transactions without the prior consent of Pharmakon, including:
•making certain restricted payments, including paying dividends on, or repurchasing or making distributions with respect to, our shares subject to certain exceptions;
•selling, transferring, leasing or disposing of certain assets;
•encumbering or permitting liens on certain assets;
•incurring certain indebtedness; and
•entering into certain transactions with affiliates.
16
The 2021 Senior Secured Loan also includes certain financial covenants which require:
•a minimum liquidity level to be maintained which is tested on a monthly basis; and
•a minimum net sales threshold to be met on a trailing twelve-month net sales basis.
It cannot be guaranteed that minimum liquidity or minimum net sales thresholds would be met in any given period (considering fluctuation in demand of our products also). This may lead to a breach of our debt arrangements or a requirement to renegotiate certain portions.
Upon the occurrence of a change in control, the 2021 Senior Secured Loan also requires mandatory prepayment of amounts outstanding thereunder. Such change in control may involve one of (i) the persons who are the direct or indirect shareholders of LumiraDx as at March 23, 2021, ceasing to beneficially own, directly or indirectly, 30% of the then-outstanding share capital of LumiraDx, (ii) a sale of all or substantially all of the consolidated assets of LumiraDx Investment Limited and its subsidiaries, (iii) LumiraDx ceasing to own, directly or indirectly, 100% of the equity interests in LumiraDx Investment Limited or (iv) a merger or consolidation of one of LumiraDx, LumiraDx Group or LumiraDx Investment Limited, as applicable, in which such entity is not the surviving entity.
A breach of any of the covenants under the 2021 Senior Secured Loan could result in a default. Upon the occurrence of an event of default under the 2021 Senior Secured Loan, Pharmakon could elect to declare all amounts outstanding to be immediately due and payable and terminate all commitments to extend further credit. Upon the occurrence of insolvency and insolvency proceedings events of default in respect of our U.S. subsidiaries all amounts outstanding will automatically be immediately due and payable. If we are unable to repay those amounts, Pharmakon could proceed against the collateral granted to secure such indebtedness.
We have also borrowed $18 million from BMGF pursuant to a note, which is structurally subordinated to the 2021 Senior Secured Loan. In the event of certain triggering events under such note, BMGF may exercise its rights under our other agreements with BMGF to require us to perform certain technology transfers to a third party to allow for the use of the related technology and to manufacture the relevant products under a license granted by us to BMGF. If we were required by BMGF to make a technology transfer, it could have a significant adverse effect on us and our business, as we would be transferring significant intellectual property for no consideration.
Our outstanding convertible Notes, as described below, limit our ability to incur additional debt and may restrict our ability to expand debt financing.
In addition, we may seek additional debt or restructure or refinance our existing indebtedness. We may not be able obtain additional debt or restructure or refinance our existing indebtedness on commercially reasonable terms or at all and, even if successful, those alternative actions may cause us to enter into borrowing arrangements with additional restrictions.
Risks Related to Government Regulation
If commercial third-party payors or government payors fail to provide coverage or adequate reimbursement for our Platform or future products we develop, if any, our revenue and prospects for profitability would be harmed.
In both domestic and foreign markets, the commercial success of our Platform and any future products we may develop will depend on the extent to which we obtain and maintain coverage and adequate reimbursement from governments or third-party payors. These third-party payors include government healthcare programs (such as Medicare and Medicaid in the U.S. or national or regional health services or payors in other jurisdictions), managed care organizations, health maintenance organizations, private health insurers, and other organizations. Physicians may not use our Platform or diagnostic tests unless commercial third-party payors and government payors pay for all, or a substantial portion, of the list price, and certain commercial third-party payors may not agree to reimburse our Platform if the Centers for Medicare & Medicaid Services (“CMS”), or pricing and reimbursement authorities in other jurisdictions do not issue a positive coverage decision.
In the U.S., CMS decides whether and to what extent a product will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. Therefore, we believe that obtaining and maintaining a favorable reimbursement rate from CMS for our Platform will be a necessary element in achieving material commercial success. Healthcare providers and patients may not order our Platform unless third-party payors cover and pay for all, or a substantial portion, of the list price, and certain commercial third-party payors may not agree to reimburse our Platform if CMS does not provide adequate coverage and reimbursement. Further, while due to the COVID-19 pandemic, millions of individuals have lost or will be losing employer-based insurance coverage, which may adversely affect our ability to commercialize our products, as part of the Families First Coronavirus Response Act, the Paycheck Protection Program and Health Care Enhancement Act, the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”), and the Coronavirus Response and Relief Supplemental Appropriations Act, HHS has previously provided claims reimbursement to health care providers generally at Medicare rates for testing uninsured individuals for COVID-19 on or after February 4, 2020. This
17
program stopped accepting claims for reimbursement in March 2022 and it is unclear whether the program will resume at a future date. It is also unclear whether providers would use such avenue for reimbursement for our products if it was available.
If CMS denies reimbursement of our Platform, withdraws its coverage policies after reimbursement is obtained, reviews and adjusts the rate of reimbursement, or stops paying for our Platform altogether, our revenue and results of operations would be adversely effected. Additionally, we could experience negative consequences, including:
•we could be forced to rely on private insurance coverage, which would greatly decrease our intended market opportunity for our Platform;
•a negative coverage determination could adversely affect our ability to enter into partnerships with leading healthcare systems; and
•we may need to conduct additional clinical validation, utility and other studies as part of an appeal of a negative Medicare coverage decision, and even if we expended the substantial time and resources to conduct such studies, they may not be successful and they may not result in a positive Medicare coverage determination.
Coverage and reimbursement of diagnostic tests by third-party payors may depend on a number of factors, including a payor’s determination that our Platform or other products are:
•not experimental or investigational and are otherwise authorized for marketing in the jurisdiction;
•appropriate for the specific patient;
•supported by peer-reviewed publications;
•included in clinical practice guidelines; and
•supported by clinical utility studies.
In the U.S., no uniform policy for coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for our products can differ significantly from payor to payor. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the reimbursement rate that the payor will pay for the product. One payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product. Moreover, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. If coverage and adequate reimbursement is not maintained or made available, or is available only to limited levels, we may not be able to successfully commercialize our Platform. We cannot be sure that coverage and reimbursement will be maintained or made available for, or accurately estimate the potential revenue from, our Platform or assure that coverage and reimbursement will be available for any product that we have or may develop. If we cannot maintain or obtain coverage and adequate reimbursement from third party payors for our Platform or any future products, demand for such products may decline or may not grow as we expect, which could limit our ability to generate revenue and have a material adverse effect on our financial condition, results of operations and cash flow.
In both domestic and foreign jurisdictions, third-party payors, including government payors, are increasingly attempting to contain healthcare costs by demanding price discounts or rebates and limiting both coverage on which diagnostic products they will pay for and the amounts that they will pay for new diagnostic products. Because of the cost-containment trends, third-party payors that currently provide reimbursement for, or in the future cover, our Platform may reduce, suspend, revoke, or discontinue reimbursement or coverage at any time.
As a result, there is significant uncertainty surrounding whether the use of products that incorporate new technology, such as our Platform, will be eligible for coverage by third-party payors or, if eligible for coverage, what the reimbursement rates will be for those products. The fact that a diagnostic product has been covered and reimbursed in the past, for any particular indication or in any particular jurisdiction, does not guarantee that such a diagnostic product will remain covered or reimbursed or that similar or additional diagnostic products will be covered or reimbursed in the future.
In addition, we may develop new assays that may require obtaining a Current Procedure Terminology (“CPT”) procedure code. CMS prices the new clinical diagnostic laboratory test codes using a “crosswalking” or “gapfilling” process. “Crosswalking” occurs when a new test or substantially revised test is determined to be similar to an existing test, multiple existing test codes, or a portion of an existing test code, which can then be utilized to determine reimbursement. “Gapfilling” is a process by which CMS will refer the codes to the Medicare Administrative Contractors (“MACs”) to allow them to
18
determine an appropriate price, since there is no comparable, existing code. After a year of reimbursement at the local MAC rates, CMS calculates a national limitation amount based on the median of rates for the test code across all MACs. In addition, CMS may not provide coverage for certain of the new codes for Multi-analyte Assays with Algorithmic Analyses (“MAAAs”) due to concerns that clinical efficacy and usefulness have not been widely established and documented. CMS has left the approval of new codes for MAAAs under the purview of the MACs. Our reimbursement could be adversely affected by CMS’ action in this area, including by a negative national coverage determination. If it limits coverage or reduces reimbursement for the new test codes or does not pay for our new MAAA codes, then our revenue will be adversely affected. There can be no guarantees that Medicare and other payors will establish positive or adequate coverage policies or reimbursement rates. We cannot predict whether future health care initiatives will be implemented at the federal or state level, or how any future legislation or regulation may affect us. The expansion of government’s role in the U.S. health care industry, and changes to the reimbursement amounts paid by Medicare and other payors for our current tests and our planned future tests, may reduce our profits, if any, and have a materially adverse effect on our business, financial condition, results of operations and cash flows.
In some foreign countries, the proposed pricing for a product must be approved before it may be lawfully marketed. The requirements governing pricing vary widely from country to country. For example, in the E.U. while most Member States apply some sort of pricing measures or controls, pricing and reimbursement of IVDs is not harmonized at a European level. Member States in the E.U. have exclusive competence to determine pricing and reimbursement of IVDs within their jurisdiction. In addition, many jurisdictions reimburse IVDs as part of the costs associated with certain treatments or procedures. In those cases, the pricing and reimbursement of our tests will be determined by the costs allocated to testing as part of the procedure and whether the relevant health service will select and procure our products. Therefore, the price we obtain for our products will vary depending on the different statutory health schemes within each Member State. There can be no assurance that any country that has price controls or reimbursement limitations for diagnostic products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the E.U. do not follow price structures of the U.S. and generally prices tend to be significantly lower.
The U.S. and foreign governments continue to propose and enact or promulgate legislation, regulations, guidance and other policies designed to reduce the cost of healthcare. For example, in some foreign markets, the government controls the pricing of many healthcare products. We expect that there will continue to be federal and state proposals to implement governmental controls or impose healthcare requirements. In addition, the Medicare program and increasing emphasis on managed care in the U.S. will continue to put pressure on product pricing. Cost control initiatives could decrease the price that we would receive for any products in the future, which would limit our revenue and profitability.
Payors from whom we may receive reimbursement are able to withdraw or decrease the amount of reimbursement provided for our products at any time in the future.
Our commercial success also depends on our ability to maintain coverage and adequate reimbursement from those payors that decide to cover and reimburse our Platform. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor. Payors could withdraw coverage and stop providing reimbursement for our products in the future or may reimburse our products only on a case-by-case basis.
Further, even if we obtain written agreements regarding coverage and reimbursement with certain payors, these agreements are not guarantees of indefinite coverage in an adequate amount. For example, these agreements are typically terminable without cause by either party and are typically renewable annually, and the applicable payor could opt against renewal upon expiration. In addition, the terms of certain of our written arrangements may require pre-approval from the payor or other controls and procedures prior to use by a healthcare provider. To the extent these requirements are not followed, our Platform may fail to receive some or all of the reimbursement payments to which it is otherwise entitled. These payors must also conclude that claims for our Platform satisfy the applicable contractual criteria. In addition, our written agreements regarding reimbursement with payors may not guarantee the receipt of reimbursement payments at what we believe to be the applicable reimbursement rate for such claims. If payors withdraw coverage for our products or reduce the reimbursement amounts for our products, our ability to generate revenue could be limited, which may have a material adverse effect on our financial condition, results of operations and cash flow.
Our business and the sale of our products are subject to extensive regulatory requirements, including compliance with labeling, manufacturing and reporting controls. If we fail or are unable to timely obtain the necessary authorizations, approvals or clearances for new products, our ability to generate revenue could be materially harmed.
Our products are classified as IVDs in the U.K. and the E.U. and as medical devices in the U.S. and are subject to extensive regulation in the U.K., E.U. and the U.S. by the FDA and other federal, state and local authorities and by similar regulatory authorities in other jurisdictions. Our products should be used in line with applicable Instructions for Use (“IFUs”) and product authorizations. Customers may choose to use our products “off label”. However, as a manufacturer, our obligation is
19
to limit our marketing and promotion to “on label” uses only. Government regulation of medical devices is meant to assure their safety and effectiveness, and includes regulation of, among other things:
•design, development and manufacturing;
•testing and labeling, including directions for use, processes, controls, quality assurance and packaging;
•storage, distribution, installation and servicing;
•preclinical studies and clinical trials;
•establishment registration and listing;
•product safety and effectiveness;
•marketing, sales and distribution;
•premarket approval, de novo classification, 510(k) clearance and EUA;
•recordkeeping procedures;
•advertising and promotion;
•complaint handling, corrections and removals, and recalls;
•post-market surveillance, including reporting of deaths or serious injuries, and malfunctions that, if they were to recur, would be likely to cause or contribute to a death or serious injury; and
•product import and export.
In the U.S., before we can market a new medical device, or a new use of, or claim for, an existing product, we must first receive either 510(k) clearance, de novo classification, Premarket Approval (“PMA”) or EUA from FDA, unless an exemption applies.
The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or longer, from the time the application is submitted to FDA until an approval is obtained. The process of obtaining 510(k) clearances or PMA approvals to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals on a timely basis, if at all.
An EUA may be granted for unapproved medical products, including IVDs, which authorizes the products to be marketed in the context of an actual or potential emergency that has been designated by the government. The COVID-19 pandemic has been designated such a national emergency. EUAs authorize the use of specific products based on criteria established by statute, including that the product at issue may be effective in diagnosing, treating, or preventing serious or life-threatening diseases when there are no adequate, approved, and available alternatives. An EUA is subject to additional conditions and restrictions and is product-specific. An EUA terminates when the emergency determination underlying the EUA terminates.
We cannot assure you that we will be able to obtain any 510(k) clearance, de novo classification, PMA approval or any additional EUAs. FDA can delay, limit or deny 510(k) clearance, de novo classification, PMA approval or EUA of a device for many reasons, including:
•we may not be able to demonstrate to FDA’s satisfaction that our products are safe and effective for their intended uses;
•the data from our preclinical studies and clinical trials may be insufficient to support clearance, classification, approval or authorization, where required; and
•the manufacturing process or facilities we use may not meet applicable requirements.
FDA may refuse our requests for 510(k) clearance, de novo classification, premarket approval or EUA of new products, new intended uses or modifications to existing products. Additionally, even if obtained, 510(k) clearances, de novo classifications, premarket approvals or EUAs could be withdrawn or revoked at any time for a number of reasons, including the failure of our Platform or other products to perform as expected. In particular, other companies have had their FDA approvals, or authorizations, including EUAs, revoked due to sensitivity and specificity concerns, and we cannot predict the circumstances under which the FDA would revoke an EUA for a COVID-19 test, including ours, as an understanding of the virus and the efficacy of tests and treatments is continuously evolving.
If we receive approval, authorization, certification, classification or clearance for our tests, we will be subject to ongoing FDA obligations and continued regulatory oversight and review, such as compliance with the Quality System Regulation,
20
inspections by the FDA, continued adverse event and malfunction reporting, corrections and removals reporting, registration and listing, and promotional restrictions, and we may also be subject to additional FDA post-marketing obligations. If we are not able to maintain regulatory compliance, we may not be permitted to market our tests and/or may be subject to fines, injunctions, and civil penalties; recall or seizure of products; operating restrictions; and criminal prosecution. In addition, we may be subject to similar regulatory compliance actions of foreign jurisdictions.
We may recall, replace, or make corrections to our Instrument, test strips or other products which could negatively impact manufacturing, supply and customer relationships, and may result in adverse regulatory action, including revision or revocation of an EUA. For example, beginning in early January 2021, based on reports of suspected false positive results, we initiated recalls of some test strips for our SARS-CoV-2 antigen test. As per applicable regulations, we notified and are in contact with FDA, Medicines and Healthcare Products Regulatory Agency (“MHRA”), U.K. regulatory authority and the national competent regulatory authorities of the affected E.U. countries regarding these actions. To mitigate further potential interference effects or false positives, we also added error checking measures in the Instrument, manufacturing process controls and quality control testing and release criteria, as well as a mandatory software update rolled out in February 2021 and a subsequent voluntary software update rolled out in March 2021. We cannot guarantee that no issues shall arise with regards to batches in the field where customers do not implement proposed software updates or batches manufactured prior to changes being implemented. We continue to monitor and investigate any complaints. The impact of the existence of various SARS-CoV-2 variants, change in seasons or mucus composition mix further impact our current SARS-CoV-2 antigen test.
We will need to submit numerous applications for approval, authorization, certification, classification or clearance for each test as it becomes available, which could put significant pressure on R&D and regulatory staff, resulting in delays. From time to time, legislation is drafted and introduced in the U.K., other European jurisdictions or the U.S. that could significantly change the statutory provisions governing any regulatory approval, authorization, certification, classification or clearance that we receive in such jurisdictions. In addition, in the U.S., FDA may change its authorization, clearance, classification and approval policies, adopt additional regulations or revise existing regulations, or take other actions that may prevent or delay, approval, authorization, certification, classification or clearance of our products under development or impact our ability to modify any marketed products on a timely basis.
Changes in the way FDA and other comparable regulatory authorities regulate or notified bodies assess products developed, manufactured, validated and marketed by commercial manufacturers like us could result in delay or additional expense in offering our products and products that we may develop in the future.
In the U.S., we have marketed our SARS-CoV-2 antigen test and our SARS-CoV-2 antibody test, pursuant to the “Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency” issued by FDA on February 29, 2020 and most recently revised on November 15, 2021. This policy originally allowed for the limited development and distribution of diagnostic test kits and antibody tests to detect viral particles and identify antibodies of the SARS-CoV-2 virus by commercial manufacturers prior to or without an EUA, subject to certain notification requirements. As of November 2021, the FDA ended this notification policy and generally expects the submission and issuance of an EUA prior to the distribution of such tests. We have obtained EUAs from FDA for our SARS-CoV-2 antigen test and our SARS-CoV-2 antibody test, and such tests are authorized for use at the POC under the EUA granted for each such test. Our tests should be used in line with approved IFUs and within their approved authorizations. A wave of regulatory applications in the U.S., combined with COVID-19 operational challenges, including potential staff shortages at regulatory agencies and elsewhere, could result in delays in approvals, authorizations or clearances for our SARS-CoV-2 tests or otherwise. FDA or other comparable regulatory agencies may prioritize certain applications or submissions based on the testing methodologies or other factors. In addition, FDA has issued and may issue further guidance (such as the draft Transition Plan discussed below) or change regulatory requirements at any time, which may delay our marketing and sales efforts and/or necessitate costly measures to maintain regulatory compliance with respect to these and any future products, which would have a detrimental effect on our business.
Our LumiraDx SARS-CoV-2 antigen test, LumiraDx SARS-CoV-2 antibody test, LumiraDx SARS-CoV-2 RNA STAR, and the LumiraDx SARS-CoV-2 RNA STAR Complete have not been cleared or approved by FDA. The LumiraDx SARS-CoV-2 antigen test has been authorized by FDA under an EUA only for the qualitative detection of SARS-CoV-2 nucleocapsid protein. The LumiraDx SARS-CoV-2 antibody test has been authorized by FDA under an EUA only for the qualitative detection of total antibodies to SARS-CoV-2. LumiraDx SARS-CoV-2 RNA STAR and LumiraDx SARS-CoV-2 RNA STAR Complete have been authorized by FDA under an EUA only for the qualitative detection of nucleic acid from SARS-CoV-2. They have not been authorized for use to detect any other viruses or pathogens. The tests are authorized in the U.S. for the duration of the declaration that circumstances exist justifying the authorization of emergency use of IVD tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. We have also submitted a request for an EUA for our SARS-CoV-2 Ag & Flu A/B tests, but we have not yet received authorization for this combo test and currently the FDA has indicated that authorization will be not be provided as further information is required, amongst other things, additional
21
data points related to Flu A/B testing. There can therefore be no guarantee that authorization will be granted by the FDA and timing on updated submissions depends on the prevalence of Flu A/B and our ability to collect further data.
For our IVD devices for other indications, we may not market these devices for the POC until we have received the requisite regulatory approvals, clearances, classifications or certifications for each product. Our product development program may be curtailed, redirected, eliminated or delayed at any time for many reasons, including if FDA, other regulators or notified bodies change how these devices are regulated or assessed, and we cannot predict whether we will successfully develop and commercialize these devices. FDA or a comparable regulatory authority may require more information, including additional clinical data, to support approval, clearance, classification or certification, which may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program. Any of the foregoing scenarios could materially harm the commercial prospects of our products.
Healthcare policy changes, including legislation reforming the U.S. health care system, may have a material adverse effect on our financial condition, results of operations and cash flows.
In the U.S. and in some foreign jurisdictions, there have been, and likely will continue to be, a number of legislative initiatives and regulatory changes regarding the healthcare system directed at broadening the availability of healthcare, improving the quality of healthcare, and containing or lowering the cost of healthcare. For example, in March 2010, the Patient Protection and Affordable Care Act (“ACA”) was enacted, which made a number of substantial changes in the way health care is financed by both governmental and private insurers. Among other things, the ACA required each certain medical device manufacturer to pay an excise tax (“Medical Device Excise Tax”) equal to 2.3% of the price for which such manufacturer sells its medical devices that are listed with FDA. However, this tax was permanently eliminated as part of the 2020 federal spending package, effective January 1, 2020.
Some of the provisions of the ACA have yet to be fully implemented, while certain provisions have been subject to judicial and congressional challenges. Congress previously considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, the Tax Cuts and Jobs Act of 2017 (the “Tax Act”), includes a provision that decreased the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year, commonly referred to as the “individual mandate,” to $0, effective January 1, 2019. On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, or the Texas District Court Judge, ruled that the individual mandate is a critical and inseverable feature of the ACA, and therefore, because it was repealed as part of the Tax Cuts and Jobs Act of 2017, the remaining provisions of the ACA are invalid as well. On December 18, 2019, the Fifth Circuit U.S. Court of Appeals held that the individual mandate is unconstitutional, and remanded the case to the lower court to reconsider its earlier invalidation of the full ACA. Following an appeal made by certain defendants, on June 17, 2021, the U.S. Supreme Court dismissed the plaintiffs’ challenge to the ACA without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an Executive Order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The Executive Order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how other healthcare reform measures of the Biden administration or other efforts, if any, to challenge repeal or replace the ACA, will impact our business.
While the current U.S. presidential administration has signaled its intent to pursue policies strengthening the ACA, the prior U.S. presidential administration sought to modify, repeal, or otherwise invalidate all, or certain provisions of, the ACA. From January 2017 to January 2021, former President Trump signed several Executive Orders and other directives designed to delay the implementation of certain provisions of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. One Executive Order directed federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Another Executive Order terminated the cost-sharing subsidies that reimburse insurers under the ACA. Several state Attorneys General filed suit to stop the administration from terminating the subsidies, but their request for a restraining order was denied by a federal judge in California on October 25, 2017. Further, on June 14, 2018, the U.S. Court of Appeals for the Federal Circuit ruled that the federal government was not required to pay the more than $12 billion in ACA risk corridor payments to third-party payors who argued that such payments were owed to them. This decision was appealed to the U.S. Supreme Court, which on April 27, 2020, reversed the U.S. Court of Appeals for the Federal Circuit’s decision and remanded the case to the U.S. Court of Federal Claims, concluding the government has an obligation to pay these risk corridor payments under the relevant formula. The effects of this gap in reimbursement on third-party payors, the viability of the ACA marketplace, providers, and potentially our business, are not yet known.
22
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. The Protecting Access to Medicare Act of 2014 (“PAMA”) was signed to law on April 1, 2014, and, among other things, significantly altered the payment methodology under the Clinical Laboratory Fee Schedule (“CLFS”). The CFLS applies to a wide variety of laboratories, including national chains, physician offices, and hospital laboratories. Regulations finalized in 2016 stipulated that for the reporting period beginning in 2017 and every three years thereafter (or annually in the case of advanced diagnostic laboratory tests), applicable clinical laboratories must report laboratory test payment data for each Medicare-covered clinical diagnostic laboratory test that it furnishes during the specified time period. The reported data must include the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payor (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). Additionally, effective January 1, 2018, the Medicare payment rate for a test on the CLFS is equal to the weighted median of private payor rates determined for the test, based on the data of applicable laboratories that are collected during a specified data collection period and reported to CMS during a specified data reporting period. The payment amount for a test cannot drop more than 10 percent as compared to the previous year’s payment amount for the first three years after implementation of the new payment system, and not more than 15 percent per year for the subsequent three years. Under the Laboratory Access to Beneficiaries (“LAB Act”), Congress delayed reporting for applicable clinical laboratory tests that are not advanced diagnostic laboratory tests by one year. Applicable clinical laboratory test data that was supposed to be reported between January 1, 2020 to March 31, 2020, was delayed until January 1, 2021 to March 31, 2021. The CARES Act further delayed the reporting period for another year, until January 1, 2022 to March 31, 2022. The CARES Act also delayed the 15 percent payment reduction cap under PAMA by one year. For 2020, the rates for clinical laboratory tests that are not advanced diagnostic laboratory tests or new clinical laboratory tests may not be reduced by more than 10% of the rates for 2019. There is no payment reduction for 2021, and there will be a 15% reduction cap for each of 2022, 2023, and 2024. Also, under PAMA, CMS is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by FDA. For an existing test that is cleared or approved by FDA and for which Medicare payment is made as of April 1, 2014, CMS is required to assign a unique billing code if one has not already been assigned by the agency. In addition to assigning the code, CMS is required to publicly report payment for the tests. We cannot determine at this time the full impact of PAMA on our business, financial condition and results of operations.
Additionally, the Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee did not achieve its targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers and suppliers of up to 2% per fiscal year. These reductions went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030 unless additional action is taken by Congress. Pursuant to the Coronavirus Aid, Relief, and Economic Security Act, also known as the CARES Act, as well as subsequent legislation, these reductions have been suspended from May 1, 2020 through March 31, 2022 due to the COVID-19 pandemic. Following the suspension, a 1% payment reduction will occur beginning April 1, 2022 through June 30, 2022, and the 2% payment reduction will resume on July 1, 2022. The full impact of the sequester law on our business is uncertain. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. In addition, the Middle-Class Tax Relief and Job Creation Act of 2012 mandated an additional change in Medicare reimbursement for clinical laboratory tests.
Additionally, the previous administration announced several executive orders since July 24, 2020 relating to implementing several of the administration’s healthcare proposals and in response the COVID-19 pandemic. For example, on August 6, 2020, the Trump administration issued an executive order that directed the FDA to identify a list of essential medicines, medical countermeasures and critical inputs that are medically necessary to have available at all times in an amount adequate to serve patient needs and in the appropriate dosage forms. In response, on October 30, 2020, the FDA published a list of 227 drug and biological product essential medicines and medical countermeasures, and a list of 96 device medical countermeasures. It is unclear what impact this order and list will have on our business.
Additionally, recent regulatory changes regarding health information may impact our products such as the Connect Manager, EHR Connect, the Connect Hub and the Engage app. On March 9, 2020, the HHS, Office of the National Coordinator for Health Information Technology (“ONC”) and CMS promulgated final rules aimed at supporting seamless and secure access, exchange, and use of electronic health information (“EHI”) by increasing innovation and competition by giving patients and their healthcare providers secure access to health information and new tools, allowing for more choice in care and treatment. The final rules are intended to clarify and operationalize provisions of the 21st Century Cures Act (the “Cures Act”), regarding interoperability and “information blocking,” and create significant new requirements for health care industry participants. Information blocking is defined as activity that is likely to interfere with, prevent, or materially discourage access, exchange, or use of EHI, where a health information technology developer, health information network or health
23
information exchange knows or should know that such practice is likely to interfere with access to, exchange or use of EHI. The new rules create significant new requirements for health care industry participants, and require certain electronic health record technology to incorporate standardized application programming interfaces (“APIs”), to allow individuals to securely and easily access structured EHI using smartphone applications. The ONC will also implement provisions of the Cures Act requiring that patients can electronically access all of their EHI (structured and/or unstructured) at no cost. Finally, to further support access and exchange of EHI, the final ONC rule implements the information blocking provisions of the Cures Act and identified eight “reasonable and necessary activities” as exceptions to information blocking activities, as long as specific conditions are met. In light of the COVID-19 public health emergency, ONC stated that it intends to exercise enforcement discretion for three months at the end of certain ONC Health IT Certification Program compliance dates associated with the Cures Act final ONC rule. Pursuant to the final rule, health IT developers were initially to be subject to requirements such as prohibitions on participating in any action that constitutes information blocking, providing certification to the Secretary of HHS that they will not take actions that constitute information blocking, and other requirements regarding information blocking six months from May 1, 2020, when the final rule was published in the Federal Register. However, on October 29, 2020, HHS released an Interim Final Rule, effective December 4, 2020, pushing compliance with such requirements to April 5, 2021. Certified API Developers must now comply with new administrative requirements and must provide all certified API technology by December 31, 2022.
These rules seek to implement significant reforms regarding the access, use and exchange of patient data. These rules may benefit us in that they make it more difficult for EHR vendors to engage in data blocking activity, promote common standards for data exchange, and provide for easier patient access to their EHI. However, these rules may also make it easier for other similar companies to enter the market, creating increased competition and reducing our market share. It is unclear at this time what the costs of compliance with the final rules will be, and what additional risks there may be to our business.
We expect that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, greater use of new technology assessment review boards for determination of cost and comparative effectiveness, lower reimbursement, and new payment methodologies. This could lower the price that we receive for any approved product. Any denial in coverage or reduction in reimbursement from Medicare or other government-funded programs may result in a similar denial or reduction in payments from private payors, which may prevent us from being able to generate sufficient revenue, attain profitability or commercialize our product candidates, if approved. Congress has proposed on several occasions to impose a 20% coinsurance on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. Because of the relatively low reimbursement for many clinical laboratory tests, in the event that Congress were to ever enact such legislation, the cost of billing and collecting for these tests would often exceed the amount actually received from the patient and effectively increase our costs of billing and collecting.
The regulatory pathway for our COVID-19 tests and healthcare professionals’ understanding of the novel coronavirus is continually evolving and may result in unexpected or unforeseen challenges.
We have obtained EUAs from FDA for our SARS-CoV-2 antigen test and our SARS-CoV-2 antibody test. We also received EUAs for our molecular lab reagent kits, SARS-CoV-2 RNA STAR and SARS-CoV-2 RNA STAR Complete. Our SARS-CoV-2 antigen pool test is not FDA authorized, cleared or approved and can be used for surveillance purposes only in the U.S. Additionally, in the E.U./European Economic Area we affixed a CE Mark (following self-certification against the relevant E.U. Directive) to our SARS-CoV-2 antigen test, SARS-CoV-2 antibody test, SARS-CoV-2 antigen pool test, SARS-CoV-2 Ag & Flu A/B tests and our SARS-CoV-2 RNA STAR Complete molecular lab reagent kit and we may submit such tests for regulatory approval, authorization, certification or clearance in other jurisdictions. Following the U.K.’s departure from the E.U., our E.U. CE Mark will continue to be recognized in Great Britain (“G.B.”) until June 30, 2023 and then a U.K. Conformity Assessed Mark (“UKCA mark”) will be required (whereas, in Northern Ireland a CE Mark or CE UKNI Mark will be required). In addition, in the U.K. any COVID-related tests are further subject to coronavirus test device approval (“CTDA”). To date, we have only obtained CTDA approval for our SARS-CoV-2 antigen test and our SARS-CoV-2 RNA STAR Complete molecular lab reagent kit. The volume of tests being developed for COVID-19 and the speed at which parties are acting to create and test many diagnostic tests for COVID-19 is unusual and evolving or changing plans or priorities within regulatory authorities, including changes based on new knowledge of COVID-19 and how the disease affects the human body, may significantly affect the regulatory timeline for our SARS-CoV-2 antigen test, SARS-CoV-2 antigen pool test and SARS-CoV-2 antibody test. The circumstances surrounding the pandemic may adversely impact the regulatory approval timeline for our Platform and its components both in relation to the COVID-19 tests, and our other tests generally if regulatory authorities prioritize tests for COVID-19 over other diseases. Results from clinical testing, the identification of new variants, may raise new questions and require us to proceed with additional reviews or clinical trials, including revising proposed endpoints or adding new clinical trial sites or cohorts of subjects. Additionally, our understanding of COVID-19, its infectiveness and other effects on the human body, the ability of individuals to develop antibodies against the virus and the effectiveness of any immune response in preventing future infections are constantly evolving, with new research suggesting
24
sometimes surprising results being published on a frequent basis. New discoveries, new variants or changed understanding of how the virus affects the human body, particularly of its infectivity, impact of various variants and individuals’ immune response to it, could render existing tests, including ours, technologically or commercially obsolete or inferior to new methods that we may or may not be able to develop on a timely basis without significant resources and funding.
Even though we have obtained EUAs for our SARS-CoV-2 antigen test, our SARS-CoV-2 antibody test and our molecular lab reagent kits, SARS-CoV-2 RNA STAR and SARS-CoV-2 RNA STAR Complete, and even if we obtain EUAs for additional tests in the future, an EUA terminates when the emergency determination underlying the EUA terminates. Moreover, FDA may revoke an EUA at any time if it determines that the legal criteria for issuing the EUA are no longer met, including if the product may not be effective or the product’s potential benefits for such use do not outweigh its known and potential risks, and we therefore cannot predict how long, if ever, any EUA applicable to our Platform would remain in place. Any revocation or termination of an EUA applicable to our Platform could adversely impact our business in a variety of ways, including if we and our manufacturing collaborators have invested significantly in the supply chain to produce our SARS-CoV-2 tests.
In December 2021, the FDA issued the draft Transition Plan for Medical Devices Issued Emergency Use Authorizations (EUAs) During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency (the “Transition Plan”) for public comment which, among other things, proposes a 180-day transition period for manufacturers to submit permanent marketing applications (e.g., 510(k) clearance, de novo classification or PMA) prior to the date that an EUA termination becomes effective. After the 180 days, a manufacturer may continue to market its device while the application is pending, provided that the FDA has accepted the application for substantive review prior to the end of the 180-day period. Manufacturers will be expected to comply with all regulatory requirements at the end of the 180-day period, even if their marketing applications are still pending. The final Transition Plan ultimately published by the FDA may deviate, potentially significantly, from the draft Transition Plan and it is therefore impossible to know exactly how the final Transition Plan will impact our business and regulatory compliance requirements.
In addition, since the regulatory path to authorization of any COVID-19 test is evolving in various jurisdictions and other third parties are simultaneously focused on bringing their COVID-19 tests to market, there may be a widely used product in circulation in a specific country prior to our receipt of regulatory approval, authorization, certification or clearance or before we can CE Mark our Instrument in such country, which would limit our ability to market and gain traction on sale of our Platform. Unexpected issues, including any that we have not yet observed, could lead to significant reputational damage for us and our Platform going forward and other issues, including delays in our other programs, the need for re-design of our clinical trials and the need for significant additional financial resources.
If we fail to comply with the complex federal, state, local and foreign laws and regulations that apply to our business, we could suffer severe consequences that could materially and adversely affect our operating results and financial condition.
We are or expect to become subject to broadly applicable healthcare laws, including fraud and abuse, transparency, and privacy and security laws, which are regulated and enforced by both the federal government and the states in which we conduct our business. These health care laws and regulations include, for example:
•the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual, or the purchase, lease, order, arrangement, or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. The ACA amended the intent element of the federal Anti-Kickback Statute to clarify that a person or entity can be found guilty of violating the statute without actual knowledge of the statute or specific intent to violate it. The term remuneration has been interpreted broadly to include anything of value. Further, courts have found that if “one purpose” of the remuneration is to induce referrals, the federal Anti-Kickback Statute is violated. Violations are subject to significant civil and criminal fines and penalties for each violation, imprisonment, and exclusion from government healthcare programs. In addition, a claim submitted for payment to any federal healthcare program that includes items or services that were made as a result of a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act (“FCA”). There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, though the exceptions and safe harbors are drawn narrowly and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor;
•the federal civil and criminal false claims laws, including the FCA, and civil monetary penalty laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false, fictitious or fraudulent claims for payment to, or approval by Medicare, Medicaid, or other federal healthcare
25
programs; knowingly making, using, or causing to be made or used, a false record or statement material to a false, fictitious or fraudulent claim or an obligation to pay or transmit money or property to the federal government; or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. The FCA also permits a private individual acting as a “whistleblower” to bring qui tam actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery or settlement. When an entity is determined to have violated the FCA, the government may impose civil fines and penalties for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs;
•HIPAA, which created additional federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private), and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false fictitious or fraudulent statement or entry in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating HIPAA fraud provisions without actual knowledge of the statute or specific intent to violate it;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (the “HITECH Act”), and their respective implementing regulations, which impose, among other things, certain requirements relating to the privacy, security and transmission of individually identifiable health information on certain covered healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their respective “business associates,” or third parties that create, receive, maintain, transmit or obtain protected health information in connection with providing a service on behalf of a covered entity. The HITECH Act also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, there may be additional federal, state and non-U.S. laws which govern the privacy and security of health and other personal information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts;
•the federal Physician Payments Sunshine Act, created under the ACA, as amended by the Health Care and Education Reconciliation Act of 2010, and its implementing regulations, which require manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to CMS information related to direct or indirect payments and other transfers of value made to U.S.-licensed physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by the physicians and their immediate family members. Effective January 1, 2022, these reporting obligations now extend to include transfers of value made in the previous year to certain non-physician providers, including physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives;
•federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and
•analogous U.S. state, local and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers and may be broader in scope than their federal equivalents; state and foreign laws that require medical device companies to comply with the medical device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources, state and foreign laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or product pricing; state and local laws that require the registration of medical device sales representatives; state laws that prohibit other specified practices, such as (i) billing physicians for testing that they order or waiving coinsurance, copayments, deductibles, and other amounts owed by patients, and (ii) billing a state Medicaid program at a price
26
that is higher than what is charged to one or more other payors; and state and foreign laws governing the privacy and security of health information, some of which may be more stringent than those in the U.S. (such as the E.U., which adopted the General Data Protection Regulation) in certain circumstances, and may differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert the Company’s attention from the business.
It is possible that governmental and enforcement authorities will conclude that our business practices, including our arrangements with physicians and other healthcare providers, some of whom may receive stock options as compensation for services provided, may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of the laws described above or any other government regulations that apply to us, we may be subject to significant sanctions, including civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, reputational harm, exclusion from participation in federal and state funded healthcare programs, contractual damages and the curtailment or restricting of our operations, as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws. Further, if any of the physicians or other healthcare providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to similar penalties. Any action for violation of these laws, even if successfully defended, could incur significant legal expenses and divert management’s attention from the operation of the business. In addition, the marketing authorization and commercialization of any product we develop outside the U.S. will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws. All of these could harm our ability to operate our business and our financial results.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. The shifting compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance or reporting requirements increases the possibility that we may run afoul of one or more of the requirements.
Sales of our products in other jurisdictions, including the E.U./European Economic Area and the U.K., will also be subject to equivalent or comparable laws and failure to comply with these laws could have serious financial, as well as reputational, consequences for the Company. Key laws and regulations that apply to our business in the E.U. and U.K. include, amongst others:
•the General Data Protection Regulation (Regulation (EU) 2016/679), which sets out the data protection laws across the E.U. and is particularly important for the collection, storage and use of patient data;
•the U.K. General Data Protection Regulation, read alongside the Data Protection Act 2018 (the “U.K. DPA”) set out data protection laws for the U.K.; and
•relevant anti-bribery and corruption laws enacted by the Member States of the E.U./European Economic Area (the applicable regime in the U.K. is the Bribery Act 2010).
Additionally, our failure to comply could lead to civil and/or criminal penalties in individual Member States.
When we seek to commercially distribute our POC IVD devices in the U.S., if our devices are not considered CLIA waived or if we are delayed in or unable to obtain a CLIA waiver for such devices, our business may be harmed.
In the U.S., our IVD devices are subject to compliance with the Clinical Laboratory Improvements Act of 1988 (the “CLIA”) and its implementing regulations. CLIA establishes quality standards for all laboratory testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test is performed. A laboratory is broadly defined to include any facility that performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the impairment of, or assessment of health. Under CLIA, FDA categorizes IVD tests by their degree of complexity: (1) waived; (2) moderately complex; and (3) highly complex. When a test is categorized as waived, it may be performed by laboratories that have a Certificate of Waiver.
Tests that are waived by the CLIA regulations are automatically categorized as waived following 510(k) clearance or PMA approval. Otherwise, following clearance or approval, tests may be categorized either as moderate or high complexity according to the CLIA categorization criteria. A manufacturer of a test categorized as moderate complexity may request categorization of the test as waived through a CLIA Waiver by Application (“CW”) submission to FDA. In a CW submission, the manufacturer provides evidence to FDA that a test meets the CLIA statutory criteria for waiver. Specifically, waived tests are simple laboratory examinations and procedures that have an insignificant risk of an erroneous result,
27
including those that (A) employ methodologies that are so simple and accurate as to render the likelihood of erroneous results by the user negligible, or (B) FDA has determined pose no unreasonable risk of harm to the patient if performed incorrectly. Further, when FDA authorizes tests for use at the POC under an EUA, such tests are deemed to be CLIA waived tests. As such, such tests can be performed in a patient care setting that is qualified to have the test performed there as a result of operating under a CLIA Certificate of Waiver for the duration of the emergency declaration. A CLIA waiver is critical to the marketability of a product into the POC diagnostics market. With regard to future products for which we may seek a CLIA waiver from FDA, any failure or material delay to obtain such waiver could harm our business and could harm the marketability of our products to the POC diagnostics market.
We are subject to stringent and changing privacy laws, information security laws, regulations, policies and contractual obligations related to data privacy and security. Our actual or perceived failure to comply with such obligations could harm our reputation, subject us to significant fines and liability, or otherwise adversely affect our business.
We collect, store, process and transmit sensitive data, including legally protected health information, personal information, intellectual property and proprietary business information. As we seek to expand our business, we are, and will increasingly become, subject to numerous state, federal and foreign laws, regulations and standards, as well as contractual obligations, relating to the collection, use, retention, security, disclosure, transfer and other processing of sensitive and personal information in the jurisdictions in which we operate. In many cases, these laws, regulations and standards apply not only to third-party transactions, but also to transfers of information between or among us, our subsidiaries and other parties with which we have commercial relationships and our subsidiaries’ own data collection and processing practices. These laws, regulations and standards may be interpreted and applied differently over time and from jurisdiction to jurisdiction, and it is possible that they will be interpreted and applied in ways that will materially and adversely affect our business, financial condition and results of operations. The regulatory framework for data privacy, data security and data transfers worldwide is rapidly evolving, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business, and as a result, interpretation and implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future. Failure to comply with any of these laws and regulations could result in enforcement actions against us, including fines, imprisonment of company officials and public censure, claims for damages by affected individuals, damage to our reputation and loss of goodwill, any of which could have a material adverse effect on our business.
There are numerous E.U., U.K. and U.S. federal and state laws and regulations related to the privacy and security of health information. These laws and regulations include HIPAA, as amended by the HITECH Act, and their respective implementing regulations, which establish a set of national privacy and security standards for the protection of protected health information by health plans, healthcare clearinghouses and certain healthcare providers, referred to as covered entities, and the business associates with whom such covered entities contract for services. HIPAA requires covered entities and business associates to develop and maintain policies and procedures with respect to protected health information that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect such information and ensure the confidentiality, integrity and availability of electronic protected health information. The HHS may impose penalties for a failure to comply with a requirement of HIPAA. Penalties will vary significantly depending on factors such as the date of the violation, whether the failure to comply was known or should have been known, or whether the failure was due to willful neglect. These penalties include significant civil monetary penalties, criminal penalties and, in certain instances, imprisonment. HIPAA also authorizes state attorneys general to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of protected health information. Furthermore, in the event of a breach as defined by HIPAA, we may be required to comply with specific reporting requirements under HIPAA regulations. In the event of a significant breach, the reporting requirements could include notification to the general public. Enforcement activity can result in reputational harm, and responses to such enforcement activity can consume significant internal resources. Additionally, if we are unable to properly protect the privacy and security of protected health information we create, receive, maintain, or transmit on behalf of our covered entity customers, we could be found to have breached our contracts as well as HIPAA and other applicable data privacy and security laws. Determining whether protected health information has been handled in compliance with applicable privacy standards and our contractual obligations can be complex and we cannot be sure how these regulations will be interpreted, enforced or applied to our operations.
In addition, many states in which we operate have laws that protect the privacy and security of sensitive and personal information. Certain state laws may be more stringent or broader in scope, or offer greater individual rights, with respect to sensitive and personal information than federal, international or other state laws, and such laws may differ from each other, which may complicate compliance efforts. Where state laws are more protective than HIPAA, we must comply with the state laws we are subject to, in addition to HIPAA. In certain cases, it may be necessary to modify our planned operations and procedures to comply with these more stringent state laws. For example, the California Consumer Privacy Act of 2018 (the
28
“CCPA”), which increases privacy rights for California residents and imposes stringent data privacy and security obligations on companies that process their personal information, came into effect on January 1, 2020. Among other things, the CCPA requires covered companies to provide disclosures to California consumers and provide such consumers with data protection and privacy rights, including the ability to opt-out of certain sales of personal information. However, certain personal information, such as information that is subject to HIPAA or clinical trial regulations, is exempt from the CCPA. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that result in the loss of personal information. This private right of action may increase the likelihood of, and risks associated with, data breach litigation. While any information we maintain in our role as a business associate may be exempt from the CCPA, other records and information we maintain on our customers may be subject to the CCPA. Additionally, the California Privacy Rights Act (the “CPRA”), was passed in California in November 2020. While it also would likely exempt personal information that we handle as a business associate, the CPRA will impose additional data protection obligations on companies doing business in California, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions will go into effect on January 1, 2023, and additional compliance investment and potential business process changes may be required. Further, new health information standards, whether implemented pursuant to HIPAA, the HITECH Act, congressional action or otherwise, could have a significant effect on the manner in which we handle health-related information, and the cost of complying with these standards could be significant. If we do not comply with existing or new laws and regulations related to patient health information, we could be subject to criminal or civil sanctions. New legislation and state constitutional amendments proposed or enacted in several U.S. states impose, or have the potential to impose, additional obligations on companies that collect, store, use, retain, disclose, transfer and otherwise process confidential, sensitive and personal information, and will continue to shape the data privacy environment nationally. State laws are changing rapidly and there is discussion in Congress of a new federal data protection and privacy law to which we could become subject if it is enacted. All of these evolving compliance and operational requirements impose significant costs that are likely to increase over time, may require us to modify our data processing practices and policies, divert resources from other initiatives and projects, and could restrict the way products and services involving data are offered, all of which may have a material and adverse impact on our business, financial condition and results of operations.
Laws, regulations and standards in many foreign jurisdictions apply broadly to the collection, use, retention, security, disclosure, transfer and other processing of personal information, which impose significant compliance obligations. For example, the processing of personal data, including clinical trial data, is governed by the provisions of the General Data Protection Regulation (Regulation (EU) 2016/679) (the “GDPR”) in the E.U. The U.K. GDPR read alongside the U.K. DPA are the applicable laws in the U.K. Following the U.K.’s withdrawal from the E.U. on January 31, 2020, pursuant to the transitional arrangements agreed between the U.K. and E.U., the GDPR continued to have effect in U.K. law until December 31, 2020. Following December 31, 2020, the GDPR does not have direct effect in the U.K. However, the U.K.’s E.U. (Withdrawal) Act 2018 incorporated the GDPR (as it existed on December 31, 2020 but subject to certain U.K. specific amendments) into U.K. law (referred to as the U.K. GDPR). The U.K. GDPR (as amended) and U.K. DPA set out the U.K.’s data protection regime, which is independent from but equivalent to the E.U.’s regime. The requirements for processing personal data under the U.K. GDPR and U.K. DPA largely align with those under the GDPR. The GDPR, the U.K. GDPR and U.K. DPA impose stringent data privacy and security requirements on both processors and controllers of personal data, including health data and other personal data collected during clinical trials. In particular, the GDPR imposes requirements relating to ensuring there is a lawful basis for processing personal data, extends the rights of individuals to whom the personal data relates, materially expands the definition of what is expressly noted to constitute personal data, requires additional disclosures about how personal data is to be used, imposes limitations on retention of personal data, imposes strict rules on the transfer of personal data out of the European Economic Area and/or U.K. to third countries (see below), creates mandatory data breach notification requirements in certain circumstances, and establishes onerous new obligations on service providers who process personal data simply on behalf of others in connection with their E.U. or U.K. establishment.
In July 2020, the Court of Justice of the European Union invalidated the Privacy Shield agreement facilitating the transfer of personal data from the E.U. to the U.S. Negotiations are ongoing between the E.U. and the U.S. on a new transfer solution but timescales are uncertain. Adequate safeguards must be implemented to enable the transfer of personal data outside of the E.U. or the U.K., in particular to the U.S., in compliance with E.U. and U.K. data protection laws. On June 4, 2021, the European Commission (“EC”) issued new forms of standard contractual clauses for data transfers from controllers or processors in the E.U. (or otherwise subject to the GDPR), to controllers or processors established outside the E.U. (and not subject to the GDPR). The new standard contractual clauses replace the standard contractual clauses that were adopted previously under the Data Protection Directive. The U.K. is not subject to the EC’s new standard contractual clauses but has published its own transfer mechanism, the International Data Transfer Agreement (“IDTA”), which enables transfers from the U.K. We will be required to implement these new safeguards when conducting restricted data transfers under the GDPR and the UK GDPR and doing so will require significant effort and cost. In addition, additional measures may be required even when relying on standard contractual clauses or the IDTA, where the laws of the importer’s country do not offer an
29
adequate level of protection, such as the U.S. Currently, we rely on standard contractual clauses to facilitate international data transfers although we are hoping to introduce an approved set of Binding Corporate Rules. On June 28, 2021, the European Commission announced a decision of “adequacy” concluding that the U.K. ensures an equivalent level of data protection to the GDPR, which provides some relief regarding the legality of continued personal data flows from the E.U., to the U.K. Some uncertainty remains, however, as this adequacy determination must be renewed after four years and may be modified or revoked in the interim. The U.K. Government has confirmed that transfers from the U.K. to the E.U. may currently continue to flow freely. Changes with respect to any of these matters may lead to additional costs and increase our overall risk exposure.
The GDPR and the U.K. GDPR and U.K. DPA authorize competent authorities to impose penalties and fines for certain violations of up to 4% of an undertaking’s total global annual revenue for the preceding financial year or €20 million (or £17.5 million under the U.K. DPA), whichever is greater. In addition to administrative fines, a wide variety of other potential enforcement powers are available to competent authorities in respect of potential and suspected violations of the GDPR and the U.K. GDPR and U.K. DPA, including extensive audit and inspection rights, and powers to order temporary or permanent bans on all or some processing of personal data carried out by noncompliant actors. European data protection authorities, and the U.K. Information Commissioner’s Office, may interpret the GDPR and the U.K. GDPR and U.K. DPA, and national laws differently and impose additional requirements, which contributes to the complexity of processing personal data in or from, or between, the European Economic Area and/or U.K. Given the breadth and depth of changes in data protection obligations, complying with its requirements has caused us to expend significant resources and such expenditures are likely to continue into the near future as we respond to new interpretations, additional guidance, and potential enforcement actions and patterns. While we have taken steps to comply with the GDPR and the U.K. GDPR and U.K. DPA, and implementing legislation in the U.K. and applicable Member States, including by seeking to establish appropriate lawful bases for the various processing activities we carry out as a controller, reviewing our security procedures, and entering into data processing agreements with relevant customers and business partners, we cannot assure you that our efforts to achieve and remain in compliance have been, and/or will continue to be, fully successful.
We make public statements about our use and disclosure of personal information through our privacy policy, information provided on our internet platform and press statements. Although we endeavor to comply with our public statements and documentation, we may at times fail to do so or be alleged to have failed to do so. Moreover, despite our efforts, we may not be successful in achieving compliance if our employees, consultants, or vendors fail to comply with our published policies, certifications and documentation. The publication of our privacy policy and other statements that provide promises and assurances about data privacy and security can subject us to potential government or legal action if they are found to be deceptive, unfair or misrepresentative of our actual practices. Any failure, real or perceived, by us to comply with our posted privacy policies or with any legal or regulatory requirements, standards, certifications or orders or other privacy or consumer protection-related laws and regulations applicable to us could cause our customers to reduce their use of our products and services and could materially and adversely affect our business, financial condition and results of operations. In many jurisdictions, enforcement actions and consequences for non-compliance can be significant and are rising. In addition, from time to time, concerns may be expressed about whether our products, services or processes compromise the privacy of customers and others. Concerns about our practices with regard to the collection, use, retention, security, disclosure, transfer and other processing of personal information or other privacy-related matters, even if unfounded, could damage our reputation and materially and adversely affect our business, financial condition and results of operations.
Many statutory requirements, both in the U.S. and abroad, include obligations for companies to notify individuals of security breaches involving certain personal information, which could result from breaches experienced by us or our third-party service providers. For example, laws in all 50 U.S. states and the District of Columbia require businesses to provide notice to consumers whose sensitive personal information has been disclosed as a result of a data breach. These laws are not consistent, and compliance in the event of a widespread data breach is difficult and may be costly. Moreover, states have been frequently amending existing laws, requiring attention to changing regulatory requirements. We also may be contractually required to notify customers or other counterparties of a security breach. Although we may have contractual protections with our third-party service providers, contractors and consultants, any actual or perceived security breach could harm our reputation and brand, expose us to potential liability or require us to expend significant resources on data security and in responding to any such actual or perceived breach. Any contractual protections we may have from our third-party service providers, contractors or consultants may not be sufficient to adequately protect us from any such liabilities and losses, and we may be unable to enforce any such contractual protections. As many businesses, we have suffered an increased number of phishing and hacking attempts and have increased protection and training to avoid these, there can however be no guarantee some of these may be successful. In 2020, we suffered one incident of unlawful access to our financing invoices accounts in Colombia with payment being made to an unauthorized party, we were able to submit a complaint and recover such funds. In addition, one of our external logistics and fulfillment partners suffered a cyber-attack which impacted their operations and had a short-term impact on our ability to make deliveries. Our business continuity plans operated well in this
30
instance but a more sustained prolonged incident with one of our key providers could impact our operations and disrupt supply.
In addition to the possibility of fines, lawsuits, regulatory investigations, public censure, other claims and penalties, and significant costs for remediation and damage to our reputation, we could be materially and adversely affected if legislation or regulations are expanded in a manner that requires changes in our data processing practices and policies or if governing jurisdictions interpret or implement their legislation or regulations in ways that negatively impact our business. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices and compliance procedures in a manner adverse to our business. Any inability to adequately address data privacy or security-related concerns, even if unfounded, or to comply with applicable laws, regulations, standards and other obligations relating to data privacy and security, could result in additional cost and liability to us, harm our reputation and brand, damage our relationships with customers and have a material and adverse impact on our business.
If the validity of an informed consent from a patient enrolled in a clinical trial was challenged, we could be forced to stop using some of our resources, which would hinder our product development efforts.
We have implemented measures to ensure that all clinical data and genetic and other biological samples that we receive from our collaborators have been collected from subjects who have provided appropriate informed consent for purposes which extend to our product development activities. We seek to ensure these data and samples are provided to us on a subject de-identified or pseudonymized manner. We also have measures in place to ensure that the subjects from whom the data and samples are collected do not retain or have conferred on them any proprietary or commercial rights to the data or any discoveries derived from them. Our clinical research organization (“CRO”) collaborators conduct clinical trials in a number of different countries, and, to a large extent, we rely upon them to comply with the subject’s informed consent and with local law and international regulation. The collection of data and samples in many different countries results in complex legal questions regarding the adequacy of informed consent and the status of genetic material under a large number of different legal systems. The subject’s informed consent obtained in any particular country could be challenged and/or withdrawn in the future, and those informed consents could prove invalid, unlawful, or otherwise inadequate for our purposes. Any findings against us, or our collaborators, could deny us access to or force us to stop using some of our clinical samples, which would hinder our product development efforts. We could become involved in legal challenges, which could consume our management and financial resources.
The sales of our products in the E.U. and, for the time being, the U.K., are regulated through a process that either requires self-certification or certification by a European Notified Body in order to affix a CE Mark. Such processes are uncertain, particularly in light of changes to the regulatory framework. There may be a risk of delay in placing our products on the market and, once on the market, a risk of review and challenges to certain certified statuses.
Currently, until May 25, 2022, the majority of our products (including our Instrument for use with the INR test by users other than for self-testing, the INR test and control, INRstar, SARS-CoV-2 antigen test, SARS-CoV-2 antigen pool test, SARS CoV-2 antibody test, SARS-CoV-2 Ag & Flu A/B tests, SARS-CoV-2 RNA Star Complete, our CRP test and D-Dimer test) are regulated through a self-declaration process, whereby we declare that the product meets the essential requirements of the European Directive on In-Vitro Diagnostic Devices (98/79/EC). We also have a number of products that we expect to come to market in the European Economic Area that we will self-declare compliant against this directive. After the launch of any products, we may be subject to challenges by European Regulatory Authorities if there are issues that arise that question the safety and performance of these products. Such challenges may arise from a routine audit by a regulatory authority, due to device vigilance reports submitted by us, Field Safety Corrective Actions being initiated by us or the regulatory authority, or complaints made by competitors, whether those complaints are founded or not.
We also have a number of products (including our Instrument for use by patients for self-testing and certain test strips that would fall within Annex II of the European Directive on In-Vitro Diagnostic Devices (98/79/EC)), which will likely enter the market prior to May 26, 2022 and that cannot be regulated through a self-declaration process under the European Directive on In-Vitro Diagnostic Devices (98/79/EC). Such products will require their compliance with this directive reviewed and certified by a European Notified Body. We have engaged with the European Notified Bodies (GMED, BSI, TÜV SÜD, TÜV Rheinland, DEKRA GmbH and DEKRA B.V.) and have started detailed discussions with some of them. However, there is a risk of delay in getting these products to market if the Notified Body has capacity constraints and/or if the Notified Body has any issues with our technical documentation.
For devices placed on the market prior to May 26, 2022, the ability to continue to sell products using the self-declaration process of the European Directive on In-Vitro Diagnostic Devices (98/79/EC) is unaffected by the European Regulation on In-Vitro Diagnostic Devices (Regulation (EU) 2017/746) and we will be able to continue to sell existing CE Mark products through a transitional period, depending on a risk-based product classification scheme. Products deemed lower risk (B) may stay on the market until May 26, 2027, products with medium risk (C) may stay on the market until May 26, 2026 and products with higher risk (D) may stay on the market until May 26, 2025.
31
It should be appreciated that there is a severe shortage of capacity of the European Notified Bodies to assess all IVD devices that will require Notified Body certification under the Regulation, and that it is widely recognized that not all applications for assessment by Notified Bodies will be approved before the deadline of May 26, 2022. While we have taken a proactive approach to mitigate this risk, including approaching all the IVDR accredited Notified Bodies (GMED, BSI, TÜV SÜD, TÜV Rheinland, DEKRA GmbH and DEKRA B.V.) and restructuring our quality management systems and technical documentation to align with the IVDR requirements, there can be no assurance that our ability to market IVD devices in the E.U. in the future will not be interrupted and this could, in turn, have a negative impact on our business and operating results.
We take our responsibilities as a manufacturer of medical devices seriously and where possible take all voluntary measures to have independent third parties assess our designs and processes. This includes certification to the international standard for quality management, ISO 13485:2016 by LRQA, an accredited management systems certification body, testing of our Instrument to the international standard for electrical safety, IEC 61010-1:2015 / IEC 61010-2-101:2015 by CSA International an independent and accredited safety certification body, and for the international standard for electromagnetic compatibility, IEC 61326-2-6:2012 by ETS Ltd, an independent and accredited EMC test laboratory.
We also offer a number of products that we do not believe come within the scope of the European Directive on In-Vitro Diagnostic Devices (98/79/EC) or the European Regulation on In-Vitro Diagnostic Devices (Regulation (EU) 2017/746) nor come within the scope of the European Directive on Medical Devices (93/42/EEC) or the European Regulation on Medical Devices (Regulation (EU) 2017/745). There is a risk we may be subject to challenges by European Regulatory Authorities regarding the classification of these products, particularly if there was a question about safety or performance stemming from a user or a complaint from a competitor.
LumiraDx UK Limited is the legal manufacturer and regulatory owner of our products and is based in the U.K. The U.K.’s departure from the E.U. (“Brexit”), and the future relationship of the U.K. with the E.U., remains uncertain and there may be delays and barriers in obtaining access to the European Economic Area.
Following the U.K.’s prior departure from the E.U., the U.K. continued to follow the same regulations as the E.U. until the end of 2020 (the “Transition Period”). Now that the Transition Period has ended, there will be some regulatory divergence in the U.K. from the E.U. and the new UKCA mark will replace the E.U. CE Mark in G.B. (CE Marks or CE UKNI Marks will be required in Northern Ireland). E.U. CE Marks will continue to be recognized in G.B. for medical devices until June 30, 2023, however all medical devices and IVDs must be registered with the MHRA, in order to be placed on the G.B. market (subject to certain grace periods depending on the risk class of the medical device/IVD). The E.U. legal framework remains applicable in Northern Ireland (indeed any products placed on the market in the NI must be compliant with E.U. law). From July 1, 2023 a UKCA mark will be required in order to place a device on the G.B. market, however manufacturers can use the UKCA mark on a voluntary basis prior to July 1, 2023 if they wish to do so. The nature of any new regulation in the U.K. is uncertain, and as such, we may experience delays in obtaining future access to the U.K. and other European markets. The U.K.’s prior departure from the E.U. has also impacted customs regulations and impacted timing and easy of shipments into the E.U. from the U.K.
Under the European Directive on In-Vitro Diagnostic Devices (98/79/EC) and then under the In-Vitro Diagnostic Regulation (2017/746), legal manufacturers located outside of the E.U./European Economic Area are required to appoint an authorized representative that is domiciled in a Member State within the E.U./European Economic Area. Given the uncertainty at the end of the Transition Period, we have established our own dedicated authorized representative in the E.U. After considering a number of factors, including location, language capabilities, communication efficiencies and transparency considerations, we appointed LumiraDx AB, a LumiraDx affiliate domiciled in Sweden, as an authorized representative. Our regulatory experts are actively engaged through relevant industry bodies, such as the British In-Vitro Diagnostics Association (“BIVDA”), to proactively communicate with the U.K. government on any new proposed regulatory regime applicable in the U.K.
We intend to export our products to numerous countries outside of the European Economic Area. Many other countries require certificates of free sales (“CFS”), and/or certificates of foreign government (“CFG”) as a condition of allowing the importation of medical devices from a relevant country of origin. One of the typical prerequisites to the issuance of CFS and CFG certificates is the requirement that the products being certified are legally marketed in their country of origin. Now that the Transition Period has ended and the U.K. has its own independent regulatory regime, we may face delays due to the new U.K. regulatory regime, which may in turn cause us to experience delays in obtaining requisite certificates and regulatory clearance in other countries. Additionally, as a result of Brexit, we, as a U.K. based manufacturer, will no longer be able to utilize a number of Mutual Recognition Agreements and Technical Cooperation Programs that the E.U. has agreed with other countries (subject to any agreement reached to the contrary), and therefore we may suffer delays in obtaining requisite regulatory clearances in other countries. The occurrence of any of the foregoing could have a material adverse effect on our financial condition and results of operations.
32
We could be adversely affected by violations of the FCPA and other worldwide anti-bribery laws, export and import controls, sanctions, embargoes, and anti-money laundering laws and regulations.
Various of our activities may be subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. Our reliance on independent distributors to sell our Platform internationally demands a high degree of vigilance in maintaining our policy against participation in corrupt activity, because these distributors could be deemed to be our agents, and we could be held responsible for their actions. Other U.S. companies in the medical device and pharmaceutical field have faced criminal penalties under the FCPA for allowing their agents to deviate from appropriate practices in doing business with these individuals. We are also subject to similar anti-bribery laws in the other jurisdictions in which we operate, including the U.K.’s Bribery Act 2010, which also prohibits commercial bribery and makes it a crime for companies to fail to prevent bribery. These laws are complex and far-reaching in nature, and, as a result, we cannot assure you that we would not be required in the future to alter one or more of our practices to be in compliance with these laws or any changes in these laws or the interpretation thereof. Any violations of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction, involve significant costs and expenses, including legal fees, and could result in a material adverse effect on our business, prospects, financial condition, or results of operations or our reputation. We could also suffer severe penalties, including substantial criminal and civil penalties, imprisonment, disgorgement, reputational harm and other remedial measures. We ship a significant number of our Platforms into Africa as part of our collaboration with BMGF. Various countries, including countries in Africa, have export control and embargo restrictions which need to be managed and monitored.
Our activities in the U.S. subject us to various laws relating to foreign investment and the export of certain technologies, and our failure to comply with these laws or adequately monitor the compliance of our suppliers and others we do business with could subject us to substantial fines, penalties and even injunctions, the imposition of which on us could have a material adverse effect on the success of our business.
Because we have a U.S. subsidiary and substantial operations in the U.S., we are subject to U.S. laws and regulations that regulate foreign investments in U.S. businesses and access by foreign persons to technology developed and produced in the U.S. These laws include Section 721 of the Defense Production Act of 1950, as amended by the Foreign Investment Risk Review Modernization Act of 2018, and the regulations at 31 C.F.R. Parts 800, 801, and 802, as amended, administered by the Committee on Foreign Investment in the U.S.; and the Export Control Reform Act of 2018, which is being implemented in part through Commerce Department rulemakings to impose new export control restrictions on “emerging and foundational technologies” yet to be fully identified. Application of these laws, including as they are implemented through regulations being developed, may negatively impact our business in various ways, including by restricting our access to capital and markets; limiting the collaborations we may pursue; regulating the export, reexport, and transfer (in-country) of our products, services, and technology from the U.S. and abroad; increasing our costs and the time necessary to obtain required authorizations and to ensure compliance; and threatening monetary fines and other penalties if we do not.
Intellectual Property Risks Related to Our Business
If we are unable to obtain and maintain patent and other intellectual property protection for products we develop and for our technology, or if the scope of intellectual property protection obtained is not sufficient, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize any products we may develop may be adversely affected.
Our success depends in large part on our ability to obtain and maintain patent and other intellectual property protection in the U.S. and other countries for our Platform in its current or an updated form and other products. Patent law as applied to inventions in the fields in which we operate is complex and uncertain, so we cannot make any assurances that we will be able to obtain or maintain patent or other intellectual property rights, or that the patent and other intellectual property rights we may obtain will be valuable, provide an effective barrier to competitors or otherwise provide competitive advantages. If we are unable to obtain or maintain patent or other intellectual property protection with respect to our proprietary products, our business, financial condition, results of operations, and prospects could be materially harmed.
Changes in the patent laws or in the interpretation thereof in the U.S. and other countries may diminish our ability to protect our inventions and to obtain, maintain, and enforce our intellectual property rights; more generally, such changes could affect the value of our intellectual property, including by limiting the potential scope of patent coverage that we can obtain. We
33
cannot predict whether any particular patent applications we are currently pursuing will be granted as a patent or whether the claims of any particular patents, if obtained, will provide sufficient exclusivity over our competitors.
The patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patent applications or patents at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patent-eligible aspects of our research and development output in time to obtain patent protection. Although we enter into non-disclosure and confidentiality agreements with employees, consultants, and other parties who have access to confidential aspects of our research and development output, including aspects that may be patent-eligible, any of these parties may breach the agreements and disclose such output before we are able to file a patent application directed to the disclosed subject matter, thereby jeopardizing our ability to seek patent protection for that subject matter. In addition, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after the priority date, or in some cases not at all prior to issuance. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions.
The patent position of companies in our industry generally is unsettled, involves complex legal and factual questions, and has been the subject of much litigation in recent years. Whether our pending and future patent applications will be granted and the scope, validity, enforceability, and commercial value of any patents we have obtained are highly uncertain. Our pending and future patent applications may not result in patents that protect any new products or our Platform in its current or an updated form. Our pending and future patent applications may not effectively prevent others from commercializing competitive products.
Moreover, the claim scope being pursued in a patent application may need to be significantly reduced or otherwise altered in order to achieve grant of a patent, and the scope of a patent can be reinterpreted after issuance. Even if a patent application is issued as a patent, the granted claims may not provide us with any meaningful protection, prevent others from competing with us, or otherwise provide us with any competitive advantage. Any patents that we hold may be challenged, narrowed, circumvented, or invalidated by third parties. Consequently, we do not know whether any of our products will be protectable or remain protected by valid and enforceable patents. Our competitors and other third parties may be able to circumvent our patents by developing similar or alternative products and solutions in a non-infringing manner. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Litigation or other proceedings or third-party claims of intellectual property infringement could require us to spend significant time and money and could prevent us from selling our products or impact our stock price.
Third parties may assert that we are using their patented or other proprietary technology without their authorization. As we continue to commercialize our Platform in its current or an updated form, launch new products, and enter new markets, we expect that, as part of business strategies designed to impede our successful commercialization and entry into new markets or otherwise, competitors will claim that our products or services infringe their intellectual property rights. Third parties, including, for example, one or more of our competitors listed in the section of this Annual Report titled “Item 4.B. Business Overview—Competition” beginning on page 79, may have obtained, and may in the future obtain, patents under which such third parties may claim that the use of our technologies constitutes patent infringement. For example, we are aware of third-party patents in the U.S. and Europe that expired in 2021 that contain claims that may be relevant to our SARS-CoV-2 RNA STAR and SARS-CoV-2 RNA STAR Complete molecular test kits, which are commercially available in the U.S. (pursuant to an EUA) and in Europe. If a patent infringement action based on one or more of these patents were to be brought against us, we might have to argue that our kits or the manufacture or use thereof do not infringe any valid claim of the asserted patent(s); and there would be no assurance that a court would find in our favor on issues of infringement or validity of such patents. Furthermore, because a patent application generally is unavailable to the public until 18 months from the priority date (and, at least in the U.S., can optionally be kept secret until the patent is granted), we have no way of knowing, at any given time, whether others have filed new patent applications directed to technologies that we or our collaborators will use.
Intellectual property litigation is costly, and even if we prevail, the substantial cost of such litigation could affect our business and financial condition. Intellectual property litigation may also be lengthy and time-consuming and may divert the attention of our management and technical personnel in defending ourselves against any of these claims. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a material adverse impact on our cash position, reputation and stock price. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize, and sell products. In the event of a successful claim against us of infringement or misappropriation, we may be required to pay substantial damages to and obtain one or more licenses from third parties, or we may be prohibited from selling certain products, all of which could have a material adverse impact on our cash position and business and financial condition. Moreover, any licenses that we are compelled to obtain may be nonexclusive, and therefore our competitors may have access to the same technology licensed to us. If we fail to obtain a
34
required license or are unable to design around a patent, we may be unable to sell some of our products, which could have a material adverse effect on our business and financial condition.
In addition, we may be unable to obtain any required licenses at a reasonable cost, if at all. We could therefore incur substantial costs relating to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. Moreover, we could encounter delays in product introductions while we attempt to develop alternative methods or products. Defense of any lawsuit or failure to obtain any required licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of any of our products would materially affect our ability to grow and maintain profitability and would have a material adverse impact on our business.
Developments in patent law could have a negative impact on our business.
Changes in either the patent laws or interpretation of patent laws could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of granted patents. From time to time, the U.S. Supreme Court (“Supreme Court”), other federal courts, the U.S. Congress, the U.S. Patent and Trademark Office (“USPTO”), or courts or patent offices or authorities in other jurisdictions may change the standards of patentability or patent eligibility, and any such changes could have a negative impact on our business. Generally, jurisdictions outside the U.S. have a “first to file” patent system. In the U.S., prior to March 2013, the “first to invent” a claimed invention was entitled to the patent (assuming that all other requirements were met). Following the passage of the Leahy-Smith America Invents Act (the “America Invents Act”), the U.S. transitioned to a “first inventor to file” system, under which the first inventor to file a patent application on an invention is entitled to the patent (assuming that all other requirements are met) even if another party was the first to invent the claimed invention. The America Invents Act also included a number of significant changes that affect the way patent applications are prosecuted and that also may affect patent litigation. These include the introduction of derivation proceedings; expansion of the permitted content of third-party submissions to the USPTO during patent prosecution; and additional procedures to challenge the validity of a patent after issuance, including post-grant review and inter partes review. The America Invents Act and its continued implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in our industry are particularly uncertain. Recent Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. For example, diagnostic method claims and “gene patents” were considered in two landmark Supreme Court cases, Mayo Collaborative v. Prometheus Laboratories (“Prometheus”), and Association for Molecular Pathology v. Myriad Genetics (“Myriad”). In Prometheus, a case involving patent claims to a medical testing method directed to optimizing the amount of drug administered to a specific patient, patentee’s claims were deemed not to incorporate inventive content, above and beyond merely describing underlying natural correlations, sufficient to render the claimed processes patent-eligible. In Myriad, a case brought by multiple plaintiffs challenging the validity of patent claims relating to the breast cancer susceptibility genes BRCA1 and BRCA2, the court held that isolated genomic DNA that exists in nature, such as the DNA constituting the BRCA1 and BRCA2 genes, is not patent-eligible subject matter, but that cDNA, which is an artificial construct created from RNA transcripts of genes, may be patent eligible. The Federal Circuit has begun to apply the holdings in Prometheus and Myriad. For example, in 2015, the Federal Circuit in Ariosa v. Sequenom (“Ariosa”) applying Prometheus, found claims to a prenatal diagnostic method that relied on a natural product to be patent ineligible. A number of appeals to the Federal Circuit in subsequent cases, such as Athena v. Mayo (“Athena”) have been decided in a similar way.
We cannot fully predict what impact the Supreme Court’s decisions in Prometheus and Myriad and other decisions, such as the Federal Circuit’s decisions in Ariosa and Athena, may have on our ability or the ability of companies or other entities to obtain or enforce patents relating to diagnostic and therapeutic methods, DNA, genes, or genomic-related discoveries in the future. Despite the precedent set forth in these decisions, the contours of when claims reciting laws of nature, natural phenomena, or abstract ideas may meet the patent eligibility requirements are not clear and may take years to develop via application at the USPTO and interpretation in the courts. There are many patents claiming nucleic acids and diagnostic methods based on natural correlations that were issued before the recent Supreme Court decisions discussed above, and although many of these patents may be invalid under the standards set forth in the Supreme Court’s recent decisions, until successfully challenged, these patents are presumed valid and enforceable, and the patentees could allege that we infringe, or request that we obtain a license to, one or more of these patents. Whether the patents were issued prior to or after these Supreme Court decisions, we might have to defend ourselves against claims of infringement, or we might have to obtain licenses, if available. In any of the foregoing or in other situations involving third-party intellectual property rights, if we are unsuccessful in defending against claims of patent infringement, we could be forced to pay damages or be subjected to an injunction that would prevent us from using the patented subject matter in question if we are unable to obtain a license on reasonable terms or at all. Such outcomes could materially affect our ability to offer our products and services and could have a material adverse impact on our business. Even if we are able to obtain a license or to successfully defend against claims of patent infringement, the cost and distraction associated with the defense or settlement of these claims could have a material
35
adverse impact on our business. Any of the foregoing could materially harm our business, prospects, financial condition and results of operations.
We may be unable to protect or enforce our intellectual property effectively, which could harm our competitive position.
Obtaining and maintaining a strong patent position is important to our business. No patent application is guaranteed to mature into a patent, and we cannot predict the total pendency of any application that does become a patent. Moreover, the granted patent rights may not be sufficiently broad to prevent others from marketing products similar to ours or from designing around our patents. Patent law relating to the scope and validity of claims in the technology fields in which we operate is complex and uncertain, so we cannot be certain that we will be able to obtain or maintain patent rights, or that the patent rights we may obtain will be valuable, provide an effective barrier to competitors, or otherwise provide competitive advantages. Others may have filed, and in the future may file, patent applications directed to subject matter similar or even identical to ours. To determine the priority of inventions or demonstrate that we did not derive our invention from another, we may have to participate in interference or derivation proceedings that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. We cannot be certain that our patent applications will prevail over those filed by others. Also, our intellectual property rights may be subject to other challenges by third parties. Patents we obtain have been and in the future could be challenged in litigation or in administrative proceedings such as ex parte reexamination, inter partes review, or post grant review in the U.S. or opposition proceedings in Europe or other jurisdictions and may be found to be invalid or unenforceable.
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, and other governmental fees for patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the costs associated with complying with numerous procedural provisions during the patent application process. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in irrevocable abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the respective jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or other proceedings against those who have infringed our patent rights, and we may or may not choose to monitor for infringing activity, taking into consideration the expense and time commitment associated with such enforcement and monitoring. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations.
We depend on trademarks to establish a market identity for our Company and our Platform. To maintain the value of our trademarks, we may have to file lawsuits against third parties to prevent them from using trademarks confusingly similar to or dilutive of our registered or unregistered trademarks. We also may not obtain registrations for our pending or future trademark applications and might have to defend our registered trademarks and pending applications from challenges by third parties. Enforcing or defending our registered and unregistered trademarks might result in significant litigation costs and, if we are unsuccessful, might result in damages, including the inability to continue using certain trademarks.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, we also rely upon trade secret protection, as well as non-disclosure agreements and invention assignment agreements with our employees, consultants and third-parties, to protect our confidential and proprietary information. For example, significant elements of our Platform, including, for example, the manufacture of our test strips, are protected by trade secrets and know-how that are not publicly disclosed. In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and any recourse we may have against such misconduct may not result in a remedy that protects our interests fully. Enforcing a claim that a party has illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, information that is a trade secret may be independently developed by others, which would prevent legal recourse for us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information were to be independently developed by a competitor, our competitive position could be harmed.
36
Our use of “open source” software could adversely affect our ability to offer our services and subject us to possible litigation.
We may use open source software in connection with our products and services. Companies that incorporate open source software into their products have, from time to time, faced claims challenging the use of open source software and/or compliance with open source licensing terms. As a result, we could be subject to suits by parties claiming ownership of what we believe to be open source software or claiming noncompliance with open source licensing terms. Some open source software licenses require users who distribute software containing open source software to publicly disclose all or part of the source code to such software and/or make available any derivative works of the open source code, which could include valuable proprietary code of the user, on unfavorable terms or at no cost. While we monitor the use of open source software and try to ensure that none is used in a manner that would require us to disclose our proprietary source code or that would otherwise breach the terms of an open source agreement, such use could inadvertently occur, in part because open source licensing terms are often ambiguous. Any requirement to disclose our proprietary source code or pay damages for breach of contract could have a material adverse effect on our business, financial condition and results of operations and could help our competitors develop products and services that are similar to or better than ours.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some countries do not protect intellectual property rights to the same extent as do the laws of the U.K. or of the U.S. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain such countries. The legal systems of some countries, particularly low and middle income countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to medical diagnostics. This could make it difficult for us to prevent or stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against parties such as government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Because patent and other intellectual property laws differ in each country, our intellectual property rights may not receive the same degree of protection in foreign countries as they would in the U.K. or the U.S. Accordingly, we may choose not to seek patent protection in certain countries, and if so, we will not have the benefit of patent protection in such countries. Moreover, we may not be able to predict all of the countries where patent protection ultimately will be desirable, for commercialization or marketing purposes or otherwise. If we fail to timely file a patent application for an invention in any country, we may be precluded from doing so at a later date, and we therefore would be unable to obtain patent protection for that invention in that country.
Additionally, the laws pertaining to patent ownership and assignment may differ from country to country. If we fail to obtain proper assignments for any inventions developed by us and/or our employees, or for any invention that we otherwise acquire rights to, we may lose rights to patent protection for those inventions, which may cause our competitive position to suffer.
Proceedings to enforce our patent rights in jurisdictions worldwide could result in substantial costs and divert our efforts and attention from other aspects of our business. Our efforts to protect our intellectual property rights in any particular jurisdiction may be inadequate. In addition, changes in the law and legal decisions by courts in jurisdictions worldwide may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
Many of our employees, including members of our senior management, were previously employed at other diagnostic companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
In addition, while we typically require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in obtaining such an agreement from each party who in fact develops intellectual property during the course of employment, consultancy, or contractual arrangement, respectively, which may result in claims by or against us relating to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may
37
lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest.
Our pending and future trademark applications in the U.K., the U.S. and other jurisdictions may not be allowed or may be opposed. Once filed and registered, our trademarks or trade names may be challenged, infringed, circumvented or declared generic. Our use of our trademarks or trade names may be determined to infringe the trademarks or trade names of others. To enforce our trademark rights and prevent infringement, we may be required to file trademark claims against third parties or initiate trademark opposition proceedings. This can be expensive and time-consuming, particularly for a company of our size. We may not ultimately be able to protect our trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we fail to comply with our obligations under our licenses with third parties, we could lose license rights that are important to our business.
We are a party to license agreements pursuant to which we in-license certain patents and other intellectual property. Each of our existing licenses imposes various obligations on us. If we fail to comply with these obligations, our licensors may have the right to terminate the license, in which event we would not be able to use the licensed intellectual property.
We may have limited control over the maintenance and prosecution of these in-licensed rights, activities or any other intellectual property that may be related to our in-licensed intellectual property. For example, we cannot be certain that such activities by these licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. We have limited control over the manner in which our licensors initiate an infringement proceeding against a third-party infringer of the intellectual property rights, or defend certain of the intellectual property that is licensed to us. It is possible that the licensors’ infringement proceeding or defense activities may be less vigorous than had we conducted them ourselves.
Risks Related to Our Financial Condition and Capital Requirements
We are an early, commercial-stage company and have a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
We are an early, commercial-stage company and have a limited operating history. We began operations in 2014 under the original parent company of the group, LumiraDx Group (incorporated in England and Wales) and the current parent company was incorporated in the Cayman Islands in 2016. Our limited operating history, particularly in light of our business model based upon sales of diagnostic tests enabled by our Platform, may make it difficult to evaluate our current business and predict our future performance. Any assessment of our profitability or prediction about our future success or viability is subject to significant uncertainty. We have encountered and will continue to encounter risks and difficulties frequently experienced by early, commercial-stage companies in rapidly evolving industries. If we do not address these risks successfully, our business will suffer.
We have a history of net losses. We may incur net losses in the future and we may never achieve sustained profitability.
We have historically incurred substantial net losses, including a net loss of $100.8 million during the year ended December 31, 2021. From our inception in 2014 through to December 31, 2021, we had an accumulated deficit of $676.2 million. Our losses may continue as a result of ongoing research and development expenses and increased sales and marketing costs, as well as other factors. These losses have had, and may continue to have, an adverse effect on our working capital, total assets, and shareholders’ equity. Because of the numerous risks and uncertainties associated with our research, development, and commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations, and cash flows.
38
We may require additional capital to fund our existing operations, develop our Platform and Amira System, commercialize new products and expand our operations as currently planned.
If our available cash balances and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements including because of lower demand for our products or as a result of lower than currently expected rates of reimbursement from commercial third-party payors and government payors or other risks described in this Annual Report, we may seek to sell common or preferred equity or convertible debt securities, enter into an additional credit facility or another form of third-party funding, or seek other debt financing.
We may consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities, or for other reasons, including to:
•increase our sales and marketing efforts to drive market adoption of our Platform and address competitive developments;
•seek approvals, authorizations or clearances from regulatory authorities for our existing and new products;
•fund development and marketing efforts of any future products;
•rapidly expand our manufacturing, sales and marketing efforts, including for our SARS-CoV-2 tests and Amira System;
•expand our technologies to cover additional tests;
•acquire, license or invest in technologies;
•acquire or invest in complementary businesses or assets; and
•finance capital expenditures and general and administrative expenses.
Our present and future funding requirements will depend on many factors, including:
•our ability to achieve revenue growth;
•the cost of rapidly expanding our operations and offerings, including our manufacturing, sales and marketing efforts;
•our rate of progress in, and cost of the sales and marketing activities associated with, establishing adoption of and reimbursement for our Platform;
•our rate of progress in, and cost of research and development activities associated with, products in research and early development;
•the effect of competing technological and market developments;
•costs related to rapid international expansion;
•our rate of progress in establishing reimbursement arrangements with domestic and international commercial third-party payors and government payors; and
•the potential cost of and delays in product development as a result of any regulatory oversight applicable to our products.
The various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, dilution to our shareholders could result. Any equity securities issued also could provide for rights, preferences, or privileges senior to those of holders of our common shares. If we raise funds by issuing debt securities, those debt securities may have rights, preferences, and privileges senior to those of holders of our common shares. Our Notes, for example, provide various restrictions on raising or adding additional debt, which may restrict our ability to expand operations. The terms of debt securities issued or borrowings pursuant to a credit agreement could also impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our Platform technologies or products or grant licenses on terms that are not favorable to us.
The global financial markets have experienced a period of disruption and instability as a result of the COVID-19 pandemic and the conflict between Russia and Ukraine, generally increasing the difficulty of accessing the capital and credit markets and resulting in intervention from national governments around the world. Accordingly, additional equity or debt financing might not be available on reasonable terms, if at all.
39
If we cannot secure additional funding when needed, we may have to delay, reduce the scope of, or eliminate one or more research and development programs, international expansion and commercial launch plans or sales and marketing initiatives. In addition, we may have to work with a third party on one or more of our development programs, which could lower the economic value of those programs to us.
We will continue to incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
We expect to continue to incur significant legal, accounting, reporting, and other expenses that we did not incur as a private company. As a public company, we also incur costs which we have not incurred previously, including, but not limited to, costs and expenses for increased directors and officers insurance, investor relations, and various other costs of a public company. We anticipate that we will continue to incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act, as well as rules implemented by the SEC and the Nasdaq Global Market (“Nasdaq”). We expect these rules and regulations to increase our legal and financial compliance costs and make some management and corporate governance activities more time-consuming and costly, particularly after we are no longer an “emerging growth company.” These rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. This could have an adverse impact on our ability to retain, recruit and bring on qualified board members. We expect that the additional costs we incur as a public company, including costs associated with corporate governance requirements, will be considerable relative to our costs as a private company.
The additional demands associated with being a public company may disrupt regular operations of our business by diverting the attention of some of our senior management team away from revenue producing activities to management and administrative oversight, adversely affecting our ability to attract and complete business opportunities and increasing the difficulty in both retaining professionals and managing and growing our businesses. Any of these effects could harm our business, financial condition and results of operations.
Furthermore, after the date we are no longer an emerging growth company, our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting. Even if our management concludes that our internal controls over financial reporting are effective, our independent registered public accounting firm may still decline to attest to our management’s assessment or may issue a report that is qualified if it is not satisfied with our controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us. In addition, in connection with the implementation of the necessary procedures and practices related to internal control over financial reporting, we may identify deficiencies that we may not be able to remediate in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements of Section 404. Failure to comply with Section 404 could subject us to regulatory scrutiny and sanctions, impair our ability to raise capital, cause investors to lose confidence in the accuracy and completeness of our financial reports and negatively affect our share price or cause it to be more volatile.
The ability of our U.S. subsidiaries to use net operating loss carryforwards and other tax attributes to offset future taxable income may be subject to certain limitations.
As of December 31, 2021, our U.S. subsidiaries had $41.1 million in gross net operating losses. In general, under Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), a corporation that undergoes an “ownership change” is subject to annual limitations on its ability to use its pre-change net operating loss carryforwards or other tax attributes (“NOLs”), to offset future taxable income or reduce taxes. We have not determined whether past changes in the ownership of our equity have resulted in an ownership change under Section 382 of the Code with respect to our U.S. subsidiaries. In addition, future changes in the ownership of our equity, some of which may be outside of our control, could result in ownership changes under Section 382 of the Code with respect to our U.S. subsidiaries. Furthermore, our ability to use NOLs of companies that we may acquire in the future may be subject to limitations. For these reasons, we may not be able to use a material portion of the NOLs, even if we attain profitability.
Our results of operations could be materially adversely affected by fluctuations in foreign currency exchange rates.
Although a significant portion of our revenues is currently derived in U.S. dollars, we also have significant revenues currently being denominated in other currencies. In addition, we have raised funds in U.S. dollars but a large part of our costs is in pound sterling. Unfavorable fluctuations in foreign currency exchange rates could have a material adverse effect on our results of operations.
Because our consolidated financial statements are presented in U.S. dollars, we must translate revenues, expenses and income, as well as assets and liabilities, into U.S. dollars at exchange rates in effect during or at the end of each reporting period. Therefore, changes in the value of the U.S. dollar against other currencies will affect our net revenues, operating income and the value of balance-sheet items originally denominated in other currencies. These changes cause our growth in
40
consolidated earnings stated in U.S. dollars to be higher or lower than our growth in local currency when compared against other periods.
As we continue to leverage our global delivery model, more of our expenses will be incurred in currencies other than those in which we bill for the related services. An increase in the value of certain currencies against the U.S. dollar or U.K. pound sterling could increase costs for delivery of services at off-shore sites by increasing labor and other costs that are denominated in local currency. There can be no assurance that our contractual provisions will offset their impact, or that any future currency hedging activities, which are designed to partially offset this impact, will be successful. In addition, our future currency hedging activities could themselves be subject to risk. These could include risks related to counterparty performance under future hedging contracts and risks related to currency fluctuations. We also face risks that extreme economic conditions, political instability or hostilities or disasters of the type described below could impact our underlying exposures, perhaps eliminating them. Such an event could lead to losses being recognized on any future currency hedges then in place, not offset by anticipated changes in the underlying hedge exposure.
Risks Related to Ownership of Our Securities and Tax Considerations
An active trading market for our common shares may not be sustained, which would adversely affect the liquidity and price of our common shares.
The market price and trading volume of our common shares may fluctuate significantly due to the general market and economic conditions and may change for a variety of other reasons, not necessarily related to our
actual operating performance. We have experienced low trading volumes in our common shares, and thus relatively small purchases and sales
can have a significant effect on our stock price. An active trading market for our common shares may not be sustained, which would adversely affect the liquidity and price of our common shares. In addition, the price of our common shares may vary due to general economic conditions and forecasts, our general business condition and the release of our financial reports. If our common shares become delisted from Nasdaq and are quoted on the OTC Bulletin Board (an inter-dealer automated quotation system for equity securities that is not a national securities exchange), the liquidity and price of our common shares may be more limited than if we were quoted or listed on Nasdaq or another national securities exchange. The price of our common shares and its volatility will also be impacted by the number of our common shares that are publicly owned and available for trading. You may be unable to sell your securities unless an active market for our common shares is sustained.
The dual class structure of our ordinary shares and common shares has the effect of concentrating voting control with those shareholders who held our share capital prior to the Merger, including our directors, executive officers and their respective affiliates. This ownership will limit or preclude the ability of holders of our common shares to influence corporate matters, including the election of directors, amendments of our memorandum and articles of association, and any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring shareholder approval.
Our ordinary shares have ten votes per share on matters to be voted on by shareholders, and our common shares have one vote per share. As of December 31, 2021, our directors, executive officers and their affiliates hold in the aggregate 41.5% of the voting power of our issued share capital. Because of the ten-to-one voting ratio between our ordinary shares and our common shares, the holders of our ordinary shares collectively could continue to control a significant percentage of the combined voting power of our share capital and therefore be able to control all matters submitted to our shareholders for their approval. This concentrated control may limit or preclude the ability of the holders of our common shares to influence corporate matters for the foreseeable future, including the election of directors, the removal of Ron Zwanziger, Dave Scott or Jerry McAleer (the “Founder Directors”) from our board of directors, amendments of our memorandum and articles of association, and any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring shareholder approval. This may prevent or discourage unsolicited acquisition proposals or offers for our issued share capital. In addition, each of our Founder Directors are members of our board of directors and cannot be removed from the board of directors without the voting approval of our ordinary shares held by Ron Zwanziger, our Chief Executive Officer and co-founder, and his affiliates. Furthermore, the terms of our arrangements with BMGF, Morningside Venture Investments Limited (“Morningside”), and CVS, grant each of BMGF, Morningside and CVS a right to appoint a director to our board of directors. Under the applicable agreements, the appointment rights shall terminate (i) in the case of BMGF or Morningside, once either party sells or no longer controls more than 25%; or (ii) in the case of CVS, once a sale or combination of sales results in CVS beneficially owning less than 75%, in each case of their respective initial holding of our ordinary shares, as converted from series A preferred shares in connection with the Merger. BMGF’s previous board appointee resigned from our board of directors in April 2021 and BMGF has not exercised its right to appoint a replacement director, but retains its right to do so.
41
Apart from certain limited circumstances described in our Amended and Restated Articles, our ordinary shares must be converted into common shares before they can be sold or transferred. The conversion of ordinary shares to common shares will have the effect, over time, of increasing the relative voting power of those holders of our common shares who retain their shares in the long term. As a result, it is possible that one or more of the persons or entities holding our common shares could gain significant voting control as other holders of our ordinary shares sell or otherwise convert ordinary shares into common shares. We do not expect to issue any additional ordinary shares. Any future issuances of our ordinary shares would be dilutive to holders of common shares.
Future resales of our common shares, warrants or notes, sales of substantial amounts of our common shares in the public market, or the conversion of substantial amounts of our ordinary shares into common shares for sale in the public market, or the perception that these sales and/or conversions may occur, could cause the market price of our securities to decline.
Our amended and restated memorandum of association and articles of association (“Amended and Restated Articles”), which became effective in connection with the consummation of the Merger, imposed a 180-day lock-up period from the date of the closing of the Merger, on certain holders of our common shares. This lock-up period expired on March 28, 2022, following which an aggregate of 40.3 million of our common shares became eligible for sale in the public market, subject to the requirements of Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”), and the restrictions imposed on certain shareholders under our insider trading policy. Additionally, certain holders of our common shares entered into a Sponsor Agreement in connection with the Merger, imposing a one-year lock-up period from the date of the closing of the merger, which will expire on September 29, 2022. Upon the expiration of the applicable lock-up periods, holders of our common shares or warrants may sell large amounts of our common shares and warrants in the open market or in privately negotiated transactions, which could have the effect of increasing the volatility in the trading price of our common shares or warrants.
In addition, sales of substantial amounts of our common shares in the public market, or the conversion of substantial amounts of ordinary shares into common shares for sale in the public market, or the perception that these sales and/or conversions may occur, could cause the market price of our common shares to decline. This could also impair our ability to raise additional capital through the sale of our equity securities. In connection with the consummation of the Merger, we entered into a registration rights agreement (the “Registration Rights Agreement”) providing certain of our shareholders with customary demand registration rights and piggy-back registration rights with respect to registration statements filed by us after the closing of the Merger. In addition, in connection with the Notes offering, we entered into a registration rights agreement providing the holders of Notes with customary demand registration rights (the “Noteholder Registration Rights Agreement” and together with the Registration Rights Agreement, the “Registration Rights Agreements”). Upon the effectiveness of any registration statement we file pursuant to the Registration Rights Agreements, in a registered offering of securities pursuant to the Securities Act, or otherwise in accordance with Rule 144 under the Securities Act, such holders may sell large amounts of common shares, Notes and warrants in the public market or in privately negotiated transactions, which could have the effect of increasing the volatility in the trading price of our common shares or warrants or putting significant downward pressure on the price of our common shares or warrants. Additionally, downward pressure on the market price of our common shares or warrants likely will result from sales of common shares issued in connection with the exercise of warrants. Further, sales of common shares or warrants could encourage short sales by market participants. Generally, short selling means selling a security, contract or commodity not owned by the seller. The seller is committed to eventually purchase the financial instrument previously sold. Short sales are used to capitalize on an expected decline in the security’s price. Short sales of our common shares or warrants could have a tendency to depress the market price of our common shares or warrants, which could increase the potential for short sales.
We cannot predict the size of future issuances of common shares or warrants or the effect, if any, that future issuances and sales of shares of common shares or warrants will have on the market price of our common shares or existing warrants. Sales of substantial amounts of common shares, or the perception that such sales could occur, may adversely affect prevailing market prices of our common shares or warrants.
We rely on key vendors, such as Computershare Trust Company, N.A. for trading and transfer agent roles and other operational needs. These vendors may have strict internal guidelines that we cannot control. For example, shareholders who do not have their accounts set up in line with Computershare's requirements may experience delays in their ability to access and trade their securities. In addition, these key vendors may themselves rely on third party service providers to support their own operations. The failure of any key vendor, or of any service provider to a key vendor, to fulfill its obligations, for any reason, could cause operational issues that could lead to legal liability, regulatory issues, reputational harm and financial losses.
42
There is no guarantee that the public warrants will ever be in the money, and they may expire worthless.
The exercise price for the public warrants is $11.50 per common share. There is no guarantee that the public warrants will ever be in the money prior to their expiration, and as such, such warrants may expire worthless.
We cannot predict the effect our dual class structure may have on the market price of our common shares.
We cannot predict whether our dual class structure will result in a lower or more volatile market price of our common shares, in adverse publicity, or other adverse consequences. For example, certain index providers have announced restrictions on including companies with multiple-class share structures in certain of their indices, including FTSE Russell and S&P Dow Jones which impacted indices include the Russell 2000 and the S&P 500, S&P MidCap 400, and S&P SmallCap 600, which together make up the S&P Composite 1500. Under any such announced policies or future policies, the dual class structure of our share capital could make us ineligible for inclusion in certain indices and, as a result, mutual funds, exchange-traded funds, and other investment vehicles that attempt to passively track those indices would not invest in our common shares. Given the sustained flow of investment funds into passive strategies that seek to track certain indices, exclusion from certain stock indices would likely preclude investment by many of these funds and could make our common shares less attractive to other investors. As a result, the market price of our common shares could be adversely affected. It is unclear what additional effects such policies will have on the valuations of publicly-traded companies excluded from such indices, but it is possible that they may depress valuations, as compared to similar companies that are included or may cause shareholder advisory firms to publish negative commentary about our corporate governance practices or otherwise seek to cause us to change our capital structure.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common shares less attractive to investors or otherwise increase the volatility of the price of our common shares.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we intend to continue to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies.” For example, for as long as we are an “emerging growth company” under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We could be an emerging growth company for up to five years from the closing of the Merger. We cannot predict if investors will find our common shares less attractive because we will rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our share price may be more volatile.
In addition, Section 107 of the JOBS Act provides that an emerging growth company can use the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. Since IFRS makes no distinction between public and private companies for purposes of compliance with new or revised accounting standards, the requirements for our compliance as a private company and as a public company are the same.
We are a foreign private issuer and, as a result, we are not subject to U.S. proxy rules and are subject to Exchange Act reporting obligations that, to some extent, are more lenient and less frequent than those of a U.S. domestic public company. If we lose our foreign private issuer status, which would then require us to comply with the Exchange Act’s domestic reporting regime, we would incur significant legal, accounting and other expenses that we would not incur as a foreign private issuer.
We report under the Exchange Act of 1934, as amended (the “Exchange Act”), as a non-U.S. company with foreign private issuer status. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act, (ii) the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. In addition, foreign private issuers are not required to file their annual report on Form 20-F until four months after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within 75 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers
43
from making selective disclosures of material information. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
In order to maintain our current status as a foreign private issuer, either (a) a majority of our common shares must be directly or indirectly owned of record by non-residents of the U.S. or (b)(i) a majority of our directors and executive officers may not be U.S. citizens or residents, (ii) more than 50% of our assets cannot be located in the U.S. and (iii) our business must be administered principally outside the U.S. If we were to lose this status, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC and stock exchange rules. The regulatory and compliance costs to us if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be significantly higher than the costs we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would increase our legal and financial compliance costs and would make some activities highly time consuming and costly. These rules and regulations could also make it more difficult for us to attract and retain qualified directors.
As a foreign private issuer, and as permitted by the Nasdaq listing requirements, we follow certain home country governance practices rather than the corporate governance requirements of Nasdaq.
The Nasdaq listing rules require listed companies to have, among other things, a majority of its board members be independent. As a foreign private issuer, however, we are permitted to, and we may, follow home country practice in lieu of the above requirements, or we may choose to comply with the Nasdaq listing exchange requirement within one year of listing. The corporate governance practice in our home country, the Cayman Islands, does not require a majority of our board to consist of independent directors. Since a majority of our board of directors may not consist of independent directors, fewer board members may be exercising independent judgment and the level of board oversight on the management of our Company may decrease as a result. In addition, the Nasdaq listing rules also require U.S. domestic issuers to have a compensation committee, a nominating/corporate governance committee composed entirely of independent directors, and an audit committee with a minimum of three members. As a foreign private issuer, we are not subject to these requirements, although we have an audit committee comprising of three independent directors. The Nasdaq listing rules may require shareholder approval for certain corporate matters, such as requiring that shareholders be given the opportunity to vote on all equity compensation plans and material revisions to those plans, as well as certain issuances of share capital. We may consider following home country practice in lieu of the requirements under the Nasdaq listing rules with respect to certain corporate governance standards which may afford less protection to investors.
We have identified material weaknesses in our internal control over financial reporting and if our remediation of such material weaknesses is not effective, or if we fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable laws and regulations could be impaired.
In connection with the audits of our financial statements for the years ended December 31, 2020 and December 31, 2021, we identified certain control deficiencies in the design and operation of our internal control over financial reporting that constituted material weaknesses. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. The material weaknesses specifically resulted from (i) insufficient segregation of duties related to the posting of manual journal entries and (ii) the lack of documented evidence for management review controls related to significant accounting estimates and judgements.
We have insufficient segregation of duty related to the posting of manual journal entries. Additionally, where an independent review does occur, there is insufficient evidence to justify the operation of the control. These control failures are a result of resource constraints which result in inadequate staffing within the finance function to support sufficient segregation of duties and insufficient risk assessment procedures.
We lack documented evidence of review for management review controls related to significant accounting estimates and judgements although reviews were performed by various levels of management. This lack of documented review is a result of controls that are not designed at a sufficient level of detail.
Although we have begun to add appropriate levels of staffing, these material weaknesses have not been remediated as of the time of this Annual Report.
Neither we nor our independent registered public accounting firm has performed an evaluation of our internal control over financial reporting during any period in accordance with the provisions of Sarbanes Oxley. In light of the material weaknesses that were identified in connection with the audits of our financial statements described above, we believe that it is possible that, had we and our independent registered public accounting firm performed an evaluation of our internal control
44
over financial reporting in accordance with the provisions of the Sarbanes Oxley, additional material weaknesses may have been identified.
Our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal control over financial reporting until after we are no longer an “emerging growth company” as defined in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our internal control over financial reporting is documented, designed, or operating. Any failure to implement and maintain effective internal control over financial reporting also could adversely affect the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we will eventually be required to include in our periodic reports that are filed with the SEC. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our common stock. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on the Nasdaq Global Market.
As a result of our debt covenants, our consolidated financial statements contain a statement regarding a material uncertainty that may cast significant doubt about our ability to continue as a going concern.
Based on our current operating plan, we will have sufficient funds to meet our liabilities as they fall due for at least a twelve month period from the approval of this Annual Report, provided we are able to obtain waivers of covenant violations or restructure existing debt obligations in the event we are unable to achieve our covenant obligations. We believe that, if necessary, we will be able to obtain waivers of covenant violations or restructure the existing obligations, although there are no guarantees that these will be achieved. However, these circumstances (obtaining waivers of covenant violations or the restructuring of the existing debt) represent a material uncertainty that may cast significant doubt on our ability to continue as a going concern and therefore, to continue realizing our assets and discharging our liabilities in the normal course of business.
The perception that we might be unable to continue as a going concern may also make it more difficult to obtain financing for the continuation of our operations on terms that are favorable to us, or at all, and could result in the loss of confidence by investors and employees. Our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our consolidated financial statements, and it is likely that our investors will lose all or a part of their investment.
We do not anticipate paying any cash dividends in the foreseeable future.
We currently intend to retain our future earnings, if any, for the foreseeable future, to repay indebtedness and to fund the development and growth of our business. We do not intend to pay any dividends to holders of our common shares. As a result, capital appreciation in the price of our common shares, if any, will be your only potential source of gain on an investment in our common shares. The terms of the 2021 Senior Secured Loan preclude us from paying cash dividends to our shareholders without the consent of Pharmakon.
Shareholders will not be able to exercise preemptive rights and, as a result, may experience substantial dilution upon future issuances of common shares.
Our directors are authorized to issue common shares or grant rights to subscribe for common shares or shares of such undesignated class or classes (however designated) as the directors may determine, up to our authorized share capital from time to time. Our Amended and Restated Articles do not include any preemptive rights to entitle a shareholder to participate in any further issuances of common shares. This could cause existing shareholders to experience substantial dilution of their interest in us.
If equity or industry research analysts publish negative evaluations of LumiraDx, including a downgrade of the price target of our common shares, the price of our common shares could decline.
The trading market for our common shares relies in part on the research and reports that equity and industry research analysts publish about us or our business. We do not control these analysts. If one or more of the analysts covering our business downgrade their evaluations of our common shares, the price of our common shares could decline. If one or more of these analysts cease to cover our common shares, we could lose visibility in the public market for our common shares, which in turn could cause the price of our common shares to decline.
45
Our business and operations could be negatively affected if we become subject to any securities litigation or shareholder activism, which could cause us to incur significant expense, hinder execution of our business and growth strategy and impact the price of our common shares.
Shareholder activism, which could take many forms or arise in a variety of situations, has been increasing recently. Volatility in the price of our common shares or other factors may in the future cause us to become the target of securities litigation or shareholder activism. Securities litigation and shareholder activism could result in substantial costs and divert management’s attention and resources from our business. Additionally, such securities litigation and shareholder activism could give rise to perceived uncertainties as to our future, adversely affect our relationships with service providers or customers and make it more difficult for us to attract and retain qualified personnel. Also, we may be required to incur significant legal fees and other expenses related to any securities litigation and activist shareholder matters. Further, the price of our common shares could be subject to significant fluctuation or otherwise be adversely affected by the events, risks and uncertainties of any securities litigation and shareholder activism.
If we were classified as a “passive foreign investment company” for U.S. federal income tax purposes (“PFIC”), U.S. holders of our common shares would be subject to adverse U.S. federal income tax consequences.
In general, we will be a PFIC for any taxable year in which either: (i) at least 75% of our gross income for such taxable year consists of passive income (e.g., dividends, interest, capital gains, rents, and royalties, other than rents or royalties derived in the active conduct of a trade or business); or (ii) at least 50% of the quarterly average value of the gross assets held by us during such taxable year produce, or are held for the production of, passive income. For purposes of determining whether we are a PFIC, we will be treated as earning and owning our proportionate share of the income and assets, respectively, of any subsidiary corporation in which we own at least 25% of the value of the subsidiary’s stock.
Based on the current and expected composition of our income and assets and the value of our assets, we do not expect to be a PFIC for our taxable year ended December 31, 2021 or in the foreseeable future. However, no assurances regarding our PFIC status can be provided for the taxable year ended December 31, 2021 or any future taxable years. The determination of whether we are a PFIC for any taxable year is a fact-intensive determination that can only be made after the end of each year, and will depend on the composition of our income and assets and the value of our assets from time to time (including the value of our goodwill, which will generally be determined in part by reference to the market price of our common shares, which may fluctuate considerably). The composition of our income and assets will also be affected by the amount of cash that we raise in any future offerings or other financing transactions. Because the value of our goodwill will generally be determined by reference to our market capitalization, we could become a PFIC for any taxable year if the price of our common shares declines significantly while we hold a substantial amount of cash and financial investments. We also could become a PFIC if we do not generate sufficient income from our business in any taxable year relative to the amount of passive income that we generate in such taxable year. In addition, the application of the PFIC rules is subject to some uncertainties and the proper characterization of certain items of our income and assets is not entirely clear. Accordingly, there can be no assurance that we will not be a PFIC for our taxable year ended December 31, 2021 or any future taxable year. We express no belief regarding our PFIC status with respect to any U.S. holder that acquired equity interests (or options or other rights to acquire equity interests) in us prior to the taxable year ended December 31, 2021.
If we were classified as a PFIC, a “U.S. holder” (as defined in the section titled “Certain Material U.S. Federal Income Tax Considerations—Certain Material U.S. Federal Income Tax Considerations”) of our common shares would be subject to adverse U.S. federal income tax consequences, including potential increased tax liability. In addition, for each year during which we were classified as a PFIC, a U.S. holder of our common shares would generally be required to file IRS Form 8621 with such U.S. holder’s U.S. federal income tax return to report certain information concerning its ownership of our common stock. Each U.S. holder of our common shares should consult its own tax advisor regarding the PFIC rules and should read the discussion under “Certain Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Ownership and Disposition of Common Shares and the Ownership and Disposition or Conversion of Public Warrants—U.S. Holders—Passive Foreign Investment Company Rules” in this Annual Report.
U.S. holders that own 10% or more of our equity interests may be subject to adverse U.S. federal income tax consequences under rules applicable to U.S. shareholders of controlled foreign corporations.
A non-U.S. corporation generally will be classified as a controlled foreign corporation for U.S. federal income tax purposes (“CFC”) if “10% U.S. equityholders” (as defined below) own, directly, indirectly or constructively, more than 50% of either the total combined voting power of all classes of stock of such corporation entitled to vote or of the total value of the stock of such corporation. We do not believe that we would be classified as a CFC during the taxable year ended December 31, 2021, although CFC status is determined after taking into account complex constructive ownership rules and, accordingly, there can be no assurance in this regard. However, certain of our subsidiaries are classified as CFCs (as a result of the application of certain constructive ownership rules which treat our U.S. subsidiaries as owning the equity of those subsidiaries), and it is
46
possible that we may be classified as a CFC in the future. The U.S. federal income tax consequences for U.S. holders who at all times are not 10% U.S. equityholders would not be affected by the CFC rules. However, a U.S. holder that owns (or is treated as owning, directly, indirectly or constructively, including by applying certain attribution rules) 10% or more of the combined voting power or value of all of classes of our equity interests (including equity interests attributable to deemed exercise of options and convertible debt instruments) (a “10% U.S. equityholder”) would generally be subject to current U.S. federal income taxation on a portion of our applicable subsidiaries’ earnings and profits (as determined for U.S. federal income tax purposes) and our earnings and profits (if we were classified as a CFC), regardless of whether such 10% U.S. equityholder receives any actual distributions. In addition, if we were classified as a CFC, a portion of any gains realized on the sale of our common shares by a 10% U.S. equityholder may be treated as ordinary income. A 10% U.S. equityholder will also be subject to additional U.S. federal income tax information reporting requirements with respect to our subsidiaries that are classified as CFCs and with respect to us (if we were classified as a CFC) and substantial penalties may be imposed for noncompliance. Each U.S. holder should consult its own tax advisor regarding the CFC rules and whether such U.S. holder may be a 10% U.S. equityholder for purposes of these rules.
Changes in taxation legislation or practice may adversely affect the Company and its group and the tax treatment for holders of our common shares.
Any change in taxation legislation or practice in the U.K. or other jurisdictions to which the Company and its group has exposure could adversely affect the value of the Company and/or affect the post-tax returns to holders of our common shares. Statements in this Annual Report concerning the taxation of the Company and taxation of holders of common shares are based upon current tax law and published practice any aspect of which is, in principle, subject to change that could adversely affect the Company and its group and/or the taxation of holders of common shares, and which may have an adverse effect on the market value of the common shares.
There have been significant recent changes both made and proposed to international tax laws that increase the complexity, burden and cost of tax compliance for all multinational groups. The Organization for Economic Co-operation and Development (“OECD”) is continuously considering recommendations for changes to existing tax laws. We expect to continue to monitor these and other developments in international tax law which may adversely affect the Company and its group and after-tax returns to holders of common shares.
In particular, the tax risks to the Company and its group and to holders of common shares may be affected by the OECD’s Action Plan on Base Erosion and Profit Shifting (the “BEPS Action Plan”). The aim of the BEPS Action Plan is that jurisdictions should change their domestic tax laws and introduce additional or amended provisions in double taxation treaties. Examples of possible outcomes of the BEPS Action Plan could be that the ability of entities such as the Company and members of its group to benefit from reliefs under double taxation treaties, or to obtain tax deductions for finance costs, could be adversely affected, potentially increasing the effective tax rate of the group. Final reports on all action points were published on October 5, 2015, but it remains unclear in many cases whether, when, how and to what extent certain jurisdictions will decide to adopt or further adopt those recommendations and different jurisdictions may implement any such recommendations in different ways. On July 12, 2016, the European Council formally adopted a directive containing a package of measures to combat tax avoidance (“ATAD”). The scope of ATAD was amended and widened by a further directive formally adopted by the European Council on May 29, 2017 (“ATAD 2”). The implementation of ATAD and/or ATAD 2, which (among other initiatives) requires implementation of certain recommendations of the BEPS Action Plan within the E.U., may adversely affect the Company and its group.
In addition, further work is currently being undertaken by the OECD on potential future recommendations related to the challenges arising from the digitalization of the global economy, specifically relating to reform of the international allocation of taxing rights, or Pillar One, and a system ensuring a minimum level of tax for multinational enterprises, or Pillar Two, which may result in additional adverse tax consequences for the Company and its group.
Recently introduced economic substance legislation of the Cayman Islands may adversely impact us or our operations.
The Cayman Islands, together with several other non-E.U. jurisdictions, have recently introduced legislation aimed at addressing concerns raised by the Council of the E.U. as to offshore structures engaged in certain activities which attract profits without real economic activity. With effect from January 1, 2019, the International Tax Cooperation (Economic Substance) Act, (as revised) (the “Substance Act”) came into force in the Cayman Islands introducing certain economic substance requirements for in-scope Cayman Islands entities which are engaged in certain “relevant activities”. As we are a Cayman Islands company, compliance obligations include filing annual notifications for the Company, which need to state whether we are carrying out any relevant activities and whether we are claiming an exemption from the obligations to meet the economic substance tests to the extent required under the Cayman Economic Substance Act (the “substance test”). If we are carrying out such relevant activities, or are claiming such an exemption we are further required to file annually a report as to whether we have satisfied the substance test or the bias on which we are claiming such exemption. As it is a new regime, it is anticipated that the Substance Act will evolve and be subject to further clarification and amendments. We may need to
47
allocate additional resources to keep updated with these developments and may have to make changes to our operations in order to comply with all requirements under the Substance Act. Failure to satisfy these requirements may subject us to penalties under the Substance Act.
We expect the Company and LumiraDx Group to operate so as to be treated solely as a resident of the U.K. for tax purposes, but changes to our management and organizational structure and/or to the tax residency laws of other jurisdictions where we operate may cause the relevant tax authorities to treat the Company as also being a resident of another jurisdiction for tax purposes.
Under current U.K. tax law, if the location of a company’s central management and control is in the U.K., or if a company is incorporated in the U.K., it is regarded as resident for tax purposes in the U.K. unless (i) it is concurrently treated as resident for tax purposes in another jurisdiction (applying the rules of that other jurisdiction for determining tax residency) that has a double tax treaty with the U.K. and (ii) there is a residency tie-breaker provision in that tax treaty which allocates tax residence to that other jurisdiction.
Based upon our anticipated management and organizational structure, we believe that the Company and LumiraDx Group (and the other U.K. incorporated companies in the group) should be regarded as tax resident solely in the U.K. However, because this analysis is highly factual and may depend on future changes in our management and organizational structure, as well as future changes in the tax residency laws of other jurisdictions where we operate, there can be no assurance regarding the determination of the tax residence of such companies in the future.
Should any such company be treated as resident in a jurisdiction other than the U.K. it could be subject to taxation in that jurisdiction and may be required to comply with a number of material and formal tax obligations, including withholding tax and/or reporting obligations provided under the relevant tax law, which could result in additional costs and expenses.
We are a Cayman Islands company. Because judicial precedent regarding the rights of shareholders is more limited under Cayman Islands law than under U.S. law, shareholders may have fewer shareholder rights than they would have under U.S. law.
Our corporate affairs are governed by our then current memorandum and articles of association (as may be amended from time to time), the Companies Act (as revised) of the Cayman Islands and the common law of the Cayman Islands. The rights of shareholders to take action against the directors, actions by minority shareholders and the fiduciary responsibilities of directors are to a large extent governed by the common law of the Cayman Islands. This common law is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, which has persuasive, but not binding, authority on a court in the Cayman Islands. Decisions of courts in other Commonwealth jurisdictions are similarly of persuasive but not binding authority. Decisions of the Privy Council (which is the final Court of Appeal for British overseas territories such as the Cayman Islands) are binding on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of directors under Cayman Islands law are not as clearly defined as they would be under statutes or judicial precedent in some jurisdictions in the U.S. In particular, the Cayman Islands has a less prescriptive body of securities law than the U.S. In addition, some states in the U.S., such as Delaware, have more fulsome and judicially interpreted bodies of corporate law than the Cayman Islands.
In addition, as a Cayman Islands exempted company, our shareholders have no general rights under Cayman Islands law to inspect corporate records and accounts or to obtain copies of lists of shareholders with the exception that the shareholders may request a copy of our then current memorandum and articles of association. Under our Amended and Restated Articles, our directors have discretion to determine whether or not, and under what conditions, our corporate records may be inspected by shareholders, but are not obliged to make them available to shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion. As a result, you may be limited in your ability to protect your interests if you are harmed in a manner that would otherwise enable you to sue in a U.S. federal court. As a result of all of the above, shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of the board or controlling shareholders than they would as shareholders of a U.S. company.
You may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. federal courts may be limited because we are incorporated under Cayman Islands law.
We expect to conduct a significant portion of our operations outside the U.S. through our subsidiaries. The majority of our directors and executive officers reside outside the U.S. and a majority of the group’s assets are located outside of the U.S. As a result, it may be difficult or impossible for you to bring an action against us or against these individuals in the U.S. in the event that you believe that your rights have been infringed under either under U.S. federal or state securities laws or otherwise, or if you have a claim against us. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands may render you unable to enforce a judgment against our assets or the assets of our directors and officers. There is uncertainty as to whether the courts of the Cayman Islands would recognize or enforce judgments of U.S. courts against us or such persons predicated upon the civil liability provisions of the securities laws of the U.S. or any state and it is
48
uncertain whether such Cayman Islands courts would hear original actions brought in the Cayman Islands against us or such persons predicated upon the securities laws of the U.S. or any state. There is no statutory recognition in the Cayman Islands of judgments obtained in the U.S., although the courts of the Cayman Islands will generally recognize and enforce a non-penal judgment of a foreign court of competent jurisdiction without retrial on the merits.
Anti-takeover provisions in our Amended and Restated Articles may discourage, delay or prevent a change in control.
Some provisions in our Amended and Restated Articles, may discourage, delay or prevent a change in control of our Company or management that holders of our common shares may consider unfavorable, including, among other things, the following:
•provisions that permit our board of directors by resolution to issue undesignated classes of shares with such preferred, deferred or other special rights or restrictions as the board of directors may determine in their discretion, without any further vote or action by our shareholders. If issued, the rights, preferences, designations and limitations of any class of undesignated shares could operate to the disadvantage of the outstanding ordinary shares or common shares, the holders of which would not have any pre-emption rights in respect of such an issue of undesignated shares. Such terms could include, among others, preferences as to dividends and distributions on liquidation, or could be used to prevent possible corporate takeovers;
•our shareholders may not take action by written consent, but may only take action at annual or extraordinary meetings of our shareholders. As a result, a holder controlling a majority of our share capital would not be able to amend our Amended and Restated Articles or remove directors without holding a meeting of our shareholders called in accordance with our Amended and Restated Articles. Our Amended and Restated Articles further provide that special meetings of our shareholders may be called only by shareholders holding not less than one-third of the voting rights who are entitled to vote at general meetings. However, shareholders may propose only ordinary resolutions to be put to a vote at such a meeting and shall have no right to propose resolutions with respect to the election, appointment or removal of any person as a director or to amend our Amended and Restated Articles. Our Amended and Restated Articles provide no other right to put any proposals before an annual general meeting or an extraordinary general meeting. These provisions might delay the ability of our shareholders to force consideration of a proposal or for shareholders controlling a majority of our share capital to take any action, including the removal of directors;
•our board of directors is classified into three classes of directors (being the Founder Directors, the Class I directors and the Class II directors). A third party may be discouraged from making a tender offer or otherwise attempting to obtain control of us as it is more difficult and time consuming for shareholders to replace a majority of the directors on a classified board of directors. Shareholders may only remove the Class I directors and Class II directors for cause by way of passing a special resolution; and
•each of the Founder Directors cannot be removed from the board absent the voting approval of the ordinary shares held by Ron Zwanziger, our Chief Executive Officer and co-founder, and his affiliates. This provision would prevent shareholders from removing any of the Founder Directors from their respective positions on the board.
Holders of our common shares may be unable to present proposals before annual general meetings or extraordinary general meetings not called by shareholders.
Cayman Islands law provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. However, these rights may be provided in a company’s articles of association and have been provided for in the Amended and Restated Articles, subject to the restrictions described therein. Advance notice of at least 21 clear days is required for the convening of our annual general shareholders’ meeting and at least 14 clear days’ notice of any other general meeting of our shareholders. A quorum required for a meeting of shareholders consists of at least two shareholders present in person or by proxy, representing not less than one-third in nominal value of the total issued voting shares in the Company. To the extent that shareholders hold in aggregate less than one-third of the outstanding voting shares in the Company, they cannot call general meetings or annual general meetings. To the extent that shareholders hold in the aggregate one third of the outstanding voting shares of the Company, as set out above, an extraordinary general meeting may be convened but shareholders cannot include matters for consideration at such a meeting requiring the approval of a special resolution or are matters relating to the election, appointment, removal of any person as a director or to amend our Amended and Restated Articles.
We may become subject to taxation in the Cayman Islands which would negatively affect our results.
We have received an undertaking from the Governor-in-Cabinet of the Cayman Islands that, in accordance with section 6 of the Tax Concessions Act (as revised) of the Cayman Islands, for a period of 20 years from the date of grant of the
49
undertaking, no law which is enacted in the Cayman Islands imposing any tax to be levied on profits, income, gains or appreciations shall apply to us or our operations and, in addition, that no tax to be levied on profits, income, gains or appreciations or which is in the nature of estate duty or inheritance tax shall be payable (i) on or in respect of the shares, debentures or other obligations of the Company or (ii) by way of the withholding in whole or in part of a payment of dividend or other distribution of income or capital by the Company to its members or a payment of principal or interest or other sums due under a debenture or other obligation of the Company. If we otherwise were to become subject to taxation in the Cayman Islands, our financial condition and results of operations could be materially and adversely affected.
There may be a risk of us being subject to tax in jurisdictions in which we do not currently consider ourselves to have any tax resident subsidiaries or permanent establishments.
Our tax treatment is dependent, among other things, on the jurisdiction of our residence, including the residence of our subsidiaries, for tax purposes. We are a Cayman Islands exempted company with limited liability, resident in U.K. for tax purposes. We attempt to manage our business such that each of our subsidiaries is resident for tax purposes solely in its jurisdiction of incorporation and does not unintentionally create a taxable permanent establishment or other taxable presence in any other jurisdiction.
Risks Related to our Outstanding Convertible Notes
Servicing our debt may require a significant amount of cash. We may not have sufficient cash flow from our business to pay our indebtedness, and we may not have the ability to raise the funds necessary to settle conversions of the Notes or to repurchase the Notes for cash upon a fundamental change, which could adversely affect our business and results of operations.
In March 2022, we issued $56.5 million in aggregate principal amount of 6.00% Convertible Senior Subordinated Notes due 2027 (the “Notes”) in a private offering. The interest rate on the Notes is fixed at 6.00% per annum and is payable semi-annually in arrears on March 1 and September 1 of each year, beginning on September 1, 2022. Our ability to make scheduled payments of the principal of, to pay interest on, or to refinance our indebtedness, including the Notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flows from operations in the future that are sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flows, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional debt financing or equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
Holders of the Notes have the right to require us to repurchase their Notes upon the occurrence of a fundamental change (as defined in the indenture governing the Notes) at a repurchase price equal to 100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest, if any. Upon conversion, unless we elect (or are required) to deliver solely our common shares to settle such conversion (other than paying cash in lieu of delivering any fractional share), we will be required to make cash payments in respect of the Notes being converted. In addition, conversions of Notes in connection with our delivery of a redemption notice (which is only permitted upon the satisfaction of certain requirements set forth in the governing indenture) may require us to pay an interest make-whole payment equal to the remaining scheduled payments of interest that would have been made on the Notes to be converted had such Notes remained outstanding from the conversion date through March 1, 2026, and such interest make-whole payment may be made in cash. We may not have enough available cash or be able to obtain financing at the time we are required to make repurchases or in connection with such conversion and our ability to pay may additionally be limited by law, by regulatory authority or by agreements governing our other indebtedness. Our failure to repurchase the Notes at a time when the repurchase is required by the indenture governing the Notes or to pay any cash payable on future conversions as required by such indenture would constitute a default under such indenture. A default under the indenture or the fundamental change itself could also lead to a default under agreements governing our other indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Notes or make cash payments upon conversions thereof. Additionally, subject to certain exceptions, if we fail to timely file any document or report required under the Exchange Act, in certain circumstances we may be required to pay additional interest of up to 0.50% per annum on our Notes in order to avoid an event of default under the indenture, which may affect our ability to repay the Notes. Furthermore, if we do not remedy such failure within 360 days after receiving notice thereof from the noteholders, there would be an event of default under the indenture.
The Notes are convertible at any time.
The holders of Notes are entitled to convert the Notes at any time prior to 5:00 p.m. (New York City time) on the second scheduled trading day immediately before the maturity date. If one or more holders elect to convert their Notes, unless we elect
50
(or are required) to satisfy our conversion obligation by delivering solely our common shares (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity.
Transactions relating to our Notes may affect the value of our securities.
The conversion of some or all of the Notes would dilute the ownership interests of existing shareholders to the extent we satisfy our conversion obligation by delivering common shares upon any conversion of such Notes. If holders of our Notes elect to convert their Notes, we may elect (or be required to) settle our conversion obligation by delivering to them a significant number of our common shares, which would cause dilution to our existing shareholders. In addition, conversions of Notes in connection with our delivery of a redemption notice (which is only permitted upon the satisfaction of certain requirements set forth in the governing indenture) may require us to pay an interest make-whole payment equal to the remaining scheduled payments of interest that would have been made on the Notes to be converted had such Notes remained outstanding from the conversion date through March 1, 2026, and such interest make-whole payment may be made in our common shares. Furthermore, the conversion rate applicable to the Notes may be increased on March 3, 2023 and March 3, 2024, if the average of the daily volume weighted average prices of our common shares over the 20 consecutive trading days immediately preceding either date is below a specified level, provided that the conversion rate may not be increased to a rate that exceeds 137.9310 common shares per $1,000 principal amount (subject to adjustment in accordance with the terms of the indenture).
The arbitrage or hedging strategy by purchasers of the Notes may affect the value of our common shares.
We expect that many investors in, and potential purchasers of the Notes will employ, or seek to employ, an arbitrage strategy with respect to the Notes. Investors would typically implement such a strategy by selling short our common shares underlying the Notes and dynamically adjusting their short position while continuing to hold the Notes. Investors may also implement this type of strategy by entering into swaps on our common shares in lieu of or in addition to selling short our common shares. This activity could decrease (or reduce the size of any increase in) the market price of our common shares at that time.
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
LumiraDx Limited was incorporated on August 24, 2016 under the laws of the Cayman Islands. On September 29, 2016, we acquired all of the outstanding shares of LumiraDx Holdings Limited in a share for share exchange. On September 28, 2021, we consummated the Merger pursuant to which our wholly owned subsidiary, LumiraDx Merger Sub, Inc., merged with and into CA Healthcare Acquisition Corp., a Delaware corporate (“CAH”), with CAH being the surviving corporation in the Merger.
Our registered office is located at Ocorian Trust (Cayman) Limited, P.O. Box 1350, Windward 3, Regatta Office Park, Grand Cayman KY1-1108, Cayman Islands, and our telephone number is +1 (345) 640-0540.
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Accordingly, we are required to file reports and other information with the Securities and Exchange Commission (the “SEC”), including annual reports on Form 20-F and reports on Form 6-K. The SEC maintains an Internet site at www.sec.gov that contains reports, proxy and information statements and other information we have filed electronically with the SEC. As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We also make available on our investor website, free of charge, the text of our reports on Form 20-F and Form 6-Ks, including any amendments to these reports, as well as certain other SEC filings, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. Our website address is www.lumiradx.com. The reference to our website is an inactive textual reference only, and information contained therein or connected thereto is not incorporated into this Annual Report.
Our capital expenditures for the years ended December 31, 2021 and 2020 amounted to $106.3 million and $64.4 million, respectively. These capital expenditures were primarily related to facilities and equipment for the production of our Platform consumables. If demand for our Platform increases, we anticipate that our capital expenditure requirements will also increase in order to build additional capacity to meet this demand. We anticipate our capital expenditures for the year ending December 31, 2022 to be financed from the cash flows from operating activities and cash reserves, including the net proceeds
51
from our issuance of Notes. For more information on our capital expenditures, see the section of this Annual Report titled “Item 5.B.—Liquidity and Capital Resources.”
B. Business Overview
Summary
We are a next-generation POC diagnostic company addressing the current limitations of legacy POC systems by bringing lab-comparable performance to the POC in minutes on a single instrument with a low cost of ownership. We are focused on transforming community-based healthcare by providing critical diagnostic information to healthcare providers at the point of need, thereby enabling more informed medical decisions to improve health outcomes while lowering costs. We have developed and launched our Platform, which is an integrated system comprised of a small, versatile Instrument, precise, low-cost microfluidic test strips, and seamless, secure digital connectivity. We currently have eight tests commercially available on our Platform and a broad menu of tests in development. Our proprietary Platform is designed to simplify, scale down, and integrate multiple testing methodologies onto a single instrument and offer a broad menu of tests with lab-comparable performance at a low cost and with results generally in 12 minutes or less from sample to result. With our Platform, our goal is to address the key challenges faced by healthcare providers in providing efficient and cost-effective patient care. Our microfluidic technology and Platform have been proven to meet the market needs for fast, high sensitivity, convenient and connected diagnostic test results – for health systems, emergency rooms, retail pharmacy chains and other community settings.
We are initially focused on the development of tests for several of the most common conditions diagnosed or managed in community-based healthcare settings. For many of the tests we commercialize, or plan to commercialize, there are no existing high performance POC alternatives. Our initial authorized and CE Marked tests and those under development are designed to address unmet diagnostic needs in the fields of infectious disease, cardiovascular disease, diabetes, and coagulation disorders. To date, we have developed and launched eight diagnostic tests for use with our Instrument: our SARS-CoV-2 antigen test commercially available (i) under an EUA in the United States which authorizes the emergency use of the test during the period in which an emergency declaration remains in effect, (ii) pursuant to a CE Mark in the European Economic Area and U.K., (iii) pursuant to approvals in Japan and Brazil, and (iv) in Africa and elsewhere based on such approvals, our SARS-CoV-2 antibody test commercially available under an EUA in the United States and a CE Mark in the European Economic Area and U.K., as well as our SARS-CoV-2 antigen pool test, our SARS-CoV-2 & Flu A/B tests, our INR test, CRP test and our D-Dimer test, all of which are CE Marked.
In response to the COVID-19 pandemic and the resulting acute need for timely diagnostic information, we developed our SARS-CoV-2 antigen, SARS-CoV-2 antigen pool, SARS-CoV-2 antibody tests and SARS-CoV-2 & Flu A/B tests for use in community-based healthcare settings. These tests have demonstrated highly accurate results within minutes on our Instrument. We have obtained an EUA and a CE Mark for our SARS-CoV-2 antigen test and antibody test. We have commercialized our SARS-CoV-2 antigen test in Europe, Japan, India, Brazil and the U.S. to customers, including NHS and CVS and have made shipments of Instruments and SARS-CoV-2 antigen test strips to a large number of countries in Africa as part of our collaboration with BMGF. PLOS Medicine recently published a systematic review and meta-analysis of more than 60 SARS-CoV-2 antigen tests and ranked LumiraDx’s SARS-CoV-2 Ag test as most sensitive and accurate.
As of December 31, 2021, we have shipped over 21,000 Instruments, across nearly 100 countries, and have more than 1,600 staff across the globe. Our SARS-CoV-2 antigen test has been authorized by FDA under an EUA only for the qualitative detection of SARS-CoV-2 nucleocapsid protein and has not been authorized for use to detect any other viruses or pathogens. In addition, the CE Mark process is a self-certification process where we self-declare as a manufacturer that we have checked the product meets European Economic Area safety, health and environmental requirements. Our SARS-CoV-2 antigen test has not been cleared or approved by FDA or any other regulatory body, and therefore we cannot, until such time as such clearance or approval has been obtained, market such test in the U.S. following the termination of the EUA.
In laboratory and clinical studies, our SARS-CoV-2 antigen test demonstrated a very low LOD of 32 TCID50 per mL and high sensitivity and specificity within a detection window of 12 days from onset of symptoms and delivered results within 12 minutes or less. Our SARS-CoV-2 antibody test demonstrated 100% sensitivity in fingerstick blood samples collected more than eight days post PCR and delivered results within 11 minutes. We believe that offering our SARS-CoV-2 antigen test and SARS-CoV-2 antibody test on a single Platform with superior performance over a wide detection time has the potential to greatly improve the diagnosis of COVID-19 infection, infectivity and potentially immunity over time; enable large-scale population monitoring and facilitate management of the COVID-19 pandemic. Our SARS-CoV-2 antigen pool test is the first POC test that allows pooling of up to five patient samples in a single test, for higher throughput at lower cost.
52
Our SARS-CoV-2 antigen test is currently being used and implemented in various testing programs across the U.S., U.K., Italy and other European countries, Japan, Brazil, India, Africa and elsewhere, including in accident and emergency departments, care homes, retail pharmacies and other primary care settings. As economies have re-opened with the roll out of COVID-19 vaccines and variants have emerged, we are also supporting testing in schools, workplaces, travel and events where there continues to be a need for diagnostic testing. In addition, in the professional POC settings where our Platform is placed, customers are looking to implement comprehensive POC testing within their institutions leveraging both (i) our broad menu as well as (ii) our quality, compliance and data management infrastructure.
We also see a continued testing opportunity for low complexity mass screening or home COVID-19 testing market. Therefore, based on the same chemistry and test strip design as our SARS-CoV-2 antigen test on our Platform, we are finalizing development of our Amira System, which we are designing as a high-sensitivity mass screening and home testing system for COVID-19. We plan to manufacture and distribute our Amira System at a price and volume that enables both (i) mass testing required to support continued safe re-opening of the economy as well as (ii) broad scale diagnostic testing in high burden countries. Subject to completion of product development, regulatory approval, authorization, certification or clearance for professional and home use, market demand and manufacturing scale-up of the Amira System, we currently expect to launch our Amira System by the summer of 2022, with a manufacturing capacity of building up to 300 million tests per month over time and capability of producing many more for our Amira System, depending on market need for mass screening testing. We anticipate the retail price of our Amira System and Amira COVID-19 test to be between $2.00-$4.00 per test, significantly lower than many existing COVID-19 tests currently on the market as well as the equivalent tests on our Instrument. For very high-volume purchases and shipments to middle and low income countries, or LMICs, we expect the price to be lower. We currently have a prototype Amira System including strips, device and patient application. We expect to move to design freeze at system level shortly. We are simultaneously tooling up high volume manufacturing lines, for the strip and instrument, while we progress through design freeze and the verification and validation (“V&V”) phase. Beyond COVID-19, Amira will be the basis of our home testing platform, potentially bringing fast, accurate, affordable self-testing and monitoring to individuals in their home, empowering them to better manage their health and outcomes.
We have also used our technology to develop two rapid COVID-19 reagent testing kits for use on open molecular systems, LumiraDx SARS-CoV-2 RNA STAR and SARS-CoV-2 RNA STAR Complete. LumiraDx SARS-CoV-2 RNA STAR allows laboratories to utilize their existing molecular lab infrastructure in a high-throughput format by reducing amplification time from approximately one hour down to 12 minutes. LumiraDx SARS-CoV-2 RNA STAR Complete utilizes a direct amplification method that combines lysis and amplification in a single step, detecting SARS-CoV-2 nucleic acid in under 20 minutes, without needing to perform any specimen purification or extraction. We have obtained an EUA for LumiraDx SARS-CoV-2 RNA STAR and both EUA and CE Mark for SARS-CoV-2 RNA STAR Complete and commenced commercial sales. In December of 2021, we achieved several FDA EUA updates for RNA STAR complete molecular reagents including asymptomatic testing, pooling of up to five individuals, and access to 384 well configuration on validated open RT-PCR systems. In addition, through expanded authorization with HealthPulse@Home, we are now able to offer sample collection at home and elsewhere, increasing access to molecular testing. Last month we also achieved MHRA authorization for RNA STAR complete, enabling us to fulfill customer demand in the U.K. for high throughput, high sensitivity and high efficiency testing at events, schools, airports, and other public venues. Looking ahead, The Fast Lab R&D teams continue to innovate this technology with the additional capability of multiplexing. This will allow for simultaneous detection of multiple targets in a single reaction well with the current direct amplification method that is being utilized today for the SARS-CoV-2 RNA STAR Complete. Later in 2022, we expect to file for emergency use authorization with a multiplex respiratory panel while adding more European centric molecular lab offerings mid-year. Beyond the lab, we believe this technology has significant implications for our forthcoming point of care molecular programs.
On the Platform, we currently have a pipeline of more than 30 tests in various stages of development for community-based healthcare settings and plan to launch additional tests, subject to successful development and regulatory approval, authorization, certification or clearance. Our key tests under development include: high sensitivity Troponin I for cardiovascular disease, HbA1c for diabetes and Strep A. Our tests are subject to extensive regulatory requirements and we seek to obtain regulatory approval, authorization, certification or clearance on a test-by-test basis. We are focused on commercializing our tests on pace with receipt of the requisite regulatory approval, authorization, certification or clearance and any delays in commercialization of our tests or decreases in the expected market demand for our tests could adversely impact our operations and financial results. We have also entered into R&D collaborations with well-established diagnostic companies that have market-leading assays and capabilities in specific conditions to further accelerate the expansion of the test menu for our Platform. See the section of this Annual Report titled “Item 4.B. Business Overview—Research and Development” beginning on page 77 for additional details on our R&D collaboration agreements. Additionally, our R&D team is focused on continuous enhancement of our disruptive technologies.
53
The diagnostics industry, including IVD and POC systems, is rapidly evolving, and we face competition from established diagnostics companies as well as new market entrants. We believe the principal competitive factors in our industry include flexibility and ease of use, time to result, accuracy, reputation, price, innovation and compatibility with existing processes. Our eight tests commercially available on the Instrument compare favorably against the current tests available in the market based on sensitivity, precision, time to result and ease of use, and our tests in development are designed and are being validated against their respective lab standard. Many of our competitors have greater brand recognition, resources, sales forces, intellectual property portfolios, larger customer bases and more established and larger scale manufacturing capabilities.
Our proprietary microfluidic test strip is designed to accommodate all of our assays and sample types in a single- design architecture. We can manufacture our test strips at large scale and low cost on our proprietary manufacturing system. We believe our scalable manufacturing process provides us with a sustainable cost position that allows us to provide cost-efficient diagnostic solutions to the POC market. It also enables us to expand into attractive geographies and alternative healthcare settings where high quality POC testing has previously not been feasible.
We believe our Platform and its attractive value proposition will have broad appeal to healthcare providers globally that are seeking innovative POC solutions to improve outcomes and lower costs. As such, we currently have direct sales and marketing operations in 21 countries, including the U.S., most Western European countries, Japan, Colombia, Brazil, India and South Africa and over time plan to further expand to the largest in vitro diagnostic, or IVD, markets, including China and Southeast Asia. We sell mainly to large healthcare systems, government organizations and national pharmacy chains that can deploy comprehensive POC testing across their extensive healthcare provider networks.
The POC Market Opportunity
Overview
IVD tests are used to analyze patient samples to obtain information about a patient’s health status—to screen, diagnose or assess the risk of developing health issues as well as to select the appropriate therapy for a patient or monitor chronic disease patients. IVD testing is one of the most important tools for a healthcare provider to determine the needs of his or her patients and is primarily conducted in one of two locations—either (i) in a central, or “reference,” laboratory, or central lab, or (ii) at the POC, where the healthcare provider first meets with the patient and assesses the patient’s condition. POC locations include hospital emergency departments as well as a range of other community-based healthcare settings, including physician offices, retail pharmacies, urgent care centers, community health clinics and non-traditional health care settings, which we refer to collectively as community-based healthcare settings.
POC tests have numerous advantages over tests performed at central labs. Test results are delivered more quickly than central lab tests since they are performed at or near the site of patient care. This allows for faster and more informed patient care decisions, patient counseling and triaging of patients.
We estimate that the POC market was $12 billion in 2021, growing to $17 billion over the next five years (excluding the market for COVID-19 testing). We believe that the growth rate in the POC market will be larger than the growth rate for the broader IVD testing market for the foreseeable future. Several key trends are contributing to the rapid growth of this market, each of which is driven by healthcare providers’ need for real-time diagnostic information that can be used to improve patient compliance and outcomes while lowering costs relative to hospital-based care.
•Shift towards community-based healthcare. Escalating healthcare costs are driving demand for innovative models and technologies that can lower the cost of care while improving outcomes. POC testing is one of the innovations that is enabling such a shift in care into community-based healthcare settings. These settings provide direct patient access and are more convenient and cost-effective alternatives to hospital emergency departments for nonemergency conditions. Uptake of POC testing in these settings is driven by healthcare providers’ need for real-time diagnostic information that can be applied to improve patient outcomes and compliance, while lowering costs relative to hospital-based care. With reimbursement increasingly based on effectiveness of care, POC testing has been an effective tool for objectively measuring improvements in outcomes particularly for chronic conditions such as diabetes and cardiovascular disease. For example, POC HbA1c testing for diabetes patients at primary care settings enables healthcare providers to guide patient treatment decisions in real time. Similarly, POC flu testing at retail pharmacies allows for actionable results, including immediate access to adequate OTC medicines.
•Improving health outcomes for patients. As general health awareness increases and the cost of healthcare rises, systems, employers and individuals are increasingly focused on prevention and monitoring in order to reduce
54
their healthcare expenses. Thus, employers and systems providing, and individuals purchasing, health benefits are incentivized to better manage health and take steps to reduce the cost of benefits. For all these stakeholders, the greater focus on wellness, prevention and active management of chronic diseases is easier to realize in community-based healthcare settings rather than at the hospital. Furthermore, POC testing has become a useful tool for wellness screening and to support employer-driven diagnostics directly at the workplace. For example, POC COVID-19 testing has been a key tool for employers to safely and effectively re-open their workplaces and keep them open.
•Improvements in POC testing technology. In certain testing areas, technological advancements have closed the quality gap between testing capabilities at central labs and POC locations, leading to higher confidence and greater adoption of POC testing. For example, the introduction of POC molecular tests for Strep A and high precision quantitative tests for HbA1c, have greatly increased clinical utility of these tests and therefore expanded their use at the POC. Additionally, POC testing in hospitals reduces overcrowding and length of stay and accelerates access to care, particularly when used for emergency room triage purposes.
Limitations of Other POC Systems
Despite the trends towards community-based healthcare settings and related need for near patient testing, the promise of better outcomes and lower costs have not been fully realized. We believe that to achieve better health outcomes, healthcare providers require comprehensive diagnostic solutions that can provide fast, accurate test results at the POC, for a broad range of their testing needs all at reasonable cost. The traditional approach to POC test development—initially focusing on a specific medical condition and subsequently designing a test and instrument to deliver that specific application—has limited scalability and has resulted in a proliferation of instruments at the POC with the following major limitations:
•Poor clinical performance in areas of high clinical need. Many of the most common medical conditions diagnosed or managed in community-based healthcare settings require tests that involve complex methodologies to generate the accurate and reliable diagnostic information required for medical decisions. The complexity can range considerably by test depending on the sample type and the concentration and dynamic range of the desired analyte, and thus require many steps in the assay to achieve the desired performance specifications. For example, Troponin assays that are used to rule out a potential heart attack require fast and high sensitivity measurements of very low analyte concentrations seeing the importance of immediate treatment decisions. These complexities have historically been difficult to overcome in benchtop POC systems in a timely manner. Therefore, community-based healthcare providers have sent such assays to central labs.
•Limited test menu. Most currently available POC systems have been designed for a specific application (e.g., molecular, blood-based immunoassay or respiratory immunoassay) and are not readily adapted to other areas. For many conditions, healthcare providers often require multiple parameters to make treatment decisions. For example, proper management of cardiovascular disease patients requires regular monitoring of natriuretic peptides, lipids, ALT/AST, creatinine, blood glucose, electrolytes and other markers. Currently a healthcare provider would require multiple instruments to obtain this information at the POC and instead they are choosing to wait for lab results.
•High cost of total ownership. In order to meet their diagnostic needs, healthcare providers are required to purchase multiple instruments and support the required infrastructure (e.g., refrigeration) to conduct POC testing. In addition, currently available instrument-based POC tests generally have a higher cost per test than their central lab counterparts. The overall cost per reportable result becomes prohibitive to a healthcare provider at the POC in certain areas which we believe leads to suboptimal care.
These limitations have created a POC model for diagnostic testing that has been ineffective, inefficient, costly and inaccessible to a large segment of community-based healthcare settings.
Our Solution
We have developed and launched our Platform with the aim of transforming the delivery of healthcare in community-based healthcare settings. It is designed to deliver accurate results comparable to laboratory reference assays, in an easy-to-use POC solution in minutes. Our Platform comprises (i) a small, light-weight Instrument that is mainly battery operated and capable of going anywhere the patient is located, (ii) precise, low-cost, microfluidic test strips, which share common design features allowing various test strip assay types to be operated, controlled and measured by the Instrument and (iii) seamless, secure digital connectivity.
55
We have spent years developing our Platform and have designed and optimized our Instrument and test strip together to deliver the requisite lab-comparable quality results, where lab references are available, across the full range of assay and sample types, at a low cost and with results generally in 12 minutes or less. Our Instrument has been highly engineered with many innovations which enable precise fluidic control of samples in very low volumes and high sensitivity fluorescent detection of analytes in very low concentration. Our proprietary microfluidic test strip has been designed to be integrated with our Instrument, to perform the specific and precise microfluidic sequence for the assays. Our test strip has been designed with multiple channels, enabling the Instrument to perform either multiple tests or a panel in parallel (e.g., Flu A/B + SARS-CoV-2 antigen), or utilize multiple channels on a single test strip for analytes with the most demanding performance requirements (e.g., SARS-CoV-2 antigen).
While our Instrument has been designed to perform multiple tests, as more tests are added to our Platform, we may encounter design challenges or require updates to our Instrument, which may impact the commercialization of certain diagnostic assays. We have limited experience in marketing and selling our Platform beyond COVID-19 testing and we may not be successful in commercializing our Platform, including gaining market acceptance and competing with other diagnostic test providers. Our Amira System will be subject to similar uncertainties, although they are likely to be more pronounced, given the earlier stage of development of the test strip and instrument and the lack of any clinical testing of the system.
The below illustrations show the various features of our Platform.
|
|
|
|
The LumiraDx Instrument |
Image of the Test Strip (in this example SARS-CoV-2 antigen) |

|

|
Test Strip Inserted into Instrument |
Seamless Connectivity: transferring test results to electronic health record, laboratory information system or patient health record |
56
Our Platform is designed to offer the following benefits:
• Lab-comparable performance at the POC in minutes. Our Platform has been designed to use the same testing methodologies as those used in central lab systems so as to deliver lab-comparable results, where lab references are available, at the POC in minutes, rather than days or weeks. Each test is developed and validated against its respective lab reference standard. We believe that with our Platform, healthcare providers have the benefit of both central lab performance and real-time results.
•Broad menu of tests on a single instrument. Our Platform has been designed to integrate the most commonly used assay technologies (e.g., enzyme, immunoassay, molecular and electrolytes) and sample types (e.g., swab, saliva, blood) into a small, single instrument. As a result, users can replace multiple systems with one instrument. We are building out our menu with further tests, which include tests currently run at the POC, such as CRP and HbA1c, tests not currently available at the POC, such as FDA-defined high sensitivity Troponin I, and diagnostic test panels, such as Flu A/B + SARS-CoV-2.
•Low cost of ownership. Healthcare providers will be able to use a single Instrument with a variety of low-cost test strips as opposed to multiple instruments currently required for POC testing in community-based healthcare settings. We also believe our Platform will provide incremental cost savings, including reduced cost of training, maintenance and test supplies. All of this enables a lower cost per reportable result.
In addition to addressing the fundamental limitations of current POC systems, we have designed our Platform with features that we believe healthcare providers will greatly value:
•Simple workflow and intuitive user interface. Our Instrument provides users with visually easy to follow and step-by-step instructions for entering patient information and performing the test. We strive to standardize the workflow and minimize user steps in each test. We use common sample types (e.g., swab, saliva, blood) with minimal preparation steps. We use automated processes for rolling out additional tests and software upgrades through RFID tags, or “smart labels,” and over-the-cloud updates.
•Seamless connectivity. Our Instrument arrives with out-of-the-box connectivity and self-guided user set up. Our Platform provides data connectivity options for transferring patient data securely via the customer’s existing middleware or via cloud services from our Instrument to the electronic health record, laboratory information system or patient health record.
•Data reporting, analytics and decision support. Our Platform provides options for patient and population data reporting and analytics. For example, we currently market INRstar, a patient reporting and decision support tool, which allows healthcare providers to help manage warfarin patients and to simplify dosing decisions. This is a market leading solution in the U.K., used in over 2,700 primary and secondary care locations across the U.K. It is being expanded in key European markets, including Italy where we have already rolled it out for use with our INR assay.
•System portability and flexibility. Our Instrument is a small, portable device, 2.5 pounds in weight, battery operated and capable of going to wherever the patient is located. Our assays use dry chemistry and as a result can be stored at room temperature eliminating refrigeration requirements and reducing space demands in an already cluttered medical office or lab.
Our Amira System consists of (i) a small, battery operated, disposable device, (ii) the Amira COVID-19 test and (iii) a phone/tablet application for test management and reporting. Our first assay on the Amira System, a high sensitivity COVID-19 Antigen test, is in late stage development and is scheduled to design freeze and enter clinical studies within the next month. We currently have a prototype Amira System including strips, device and patient application and have begun submitting patent applications. We expect to complete POC clinical testing and achieve CE Mark during the first half (“H1”) of 2022. We plan to complete U.S. OTC and POC prospective clinical studies and submit our FDA EUA by mid-year 2022.
Our Strategy
Our goal is to become the market leading provider in POC testing and to establish our Platform as the industry standard. To achieve this objective, we intend to:
•Offer a comprehensive menu of high-performance diagnostic tests for community-based healthcare settings. We believe that delivering a broad menu of diagnostic tests for community-based healthcare on a single Platform is critical to transform the POC market. We are executing a global market-driven menu strategy designed to drive the conversion of our customers’ testing needs onto our Platform. Our tests, both cleared and in
57
development, are initially focused on the most common medical conditions for certain infectious disease, cardiovascular disease, diabetes, and coagulation disorders. Our portfolio includes high-volume tests currently available at the POC (e.g., INR, HbA1c, CRP), tests that currently do not have a viable POC solution (e.g., FDA-defined high-sensitivity Troponin I), and innovative diagnostic test panels, such as Flu A/B + SARS-CoV-2. In addition to developing our own tests, we work with well-established third parties to accelerate menu expansion on our Platform.
•Grow our installed base by executing an institutional sales and channel partnership model. We initially intend to focus our sales efforts on large healthcare systems, government organizations and national pharmacy chains that want to deploy comprehensive POC testing across their networks. We have assembled an experienced commercial team focused on key stakeholder adoption at the senior level of these organizations to deploy our Platform across their extensive healthcare networks. Some examples of this approach include:
•INR testing programs with regional governments in Italy and the U.K.
•BMGF collaboration aimed at implementing POC testing in low and middle income countries, primarily in Africa for the establishment of a primary healthcare model.
•COVID rapid testing program with CVS MinuteClinic nationwide
•D-Dimer testing program just starting in Southern region of Sweden
•Expand into additional healthcare settings and underserved markets. We believe our Platform’s user-friendly setup, competitive cost structure and potential for a broad test menu make it an attractive instrument for roll out in settings where POC testing has traditionally been more challenging, such as low and middle income countries. We intend to leverage our Platform’s adaptable architecture across future Instrument models for additional professional and, over time, home-use settings. We plan further enhancements to our Platform, such as making the Instrument more robust to enable use in more challenging settings such as in areas of extreme heat and dust.
•Continue to innovate to expand into specialty areas. We plan to continue to invest in R&D to expand our test offering into additional specialty areas, such as allergy, toxicology, fertility, veterinary and in-patient hospital, that could benefit from fast, accurate diagnostic test results from our Platform.
•Continue to innovate across our Platform. Our Platform’s connectivity allows information to be managed and shared between patients and healthcare providers to enhance patient experience. Our focus on data driven improvements will also allow us to roll out supply chain improvements and quality control features through direct data communication with our customers. We plan to introduce platform tools that enable customers and users to understand and as well as act on technical issues, test results, test inventory management, etc.
•Continue to expand use of our technology and apply it to non-healthcare settings and applications such as mass population screening and home-testing, initially through the potential launch of our Amira System. We see significant opportunity to make testing more accessible to individuals, in the workplace, at school, and in the home. Based on the same chemistry and strip design as our high performing SARS-CoV-2 antigen test on our Platform, we are finalizing development on our Amira System, which is intended to be a high sensitivity mass screening or home testing system for COVID-19. We plan to distribute the Amira COVID-19 Test at a price and volume that enables high frequency, mass testing at scale - both to potentially control the pandemic in high burden countries as well as to support a continued safe re-opening of economies, subject to regulatory approval, authorization, certification or clearance.
Our Products
Our Instrument
Our Instrument runs a variety of diagnostic testing technologies utilizing our disposable test strips and generates results that are clearly displayed on the Instrument touch-screen generally in under 12 minutes. Our Instrument is designed for use with our approved and future tests, which all share a common design and have been developed for use with very low sample volumes. Our Instrument, in connection with the test strips, is capable of very sensitive measurements at very low levels of concentration. The ability to make measurements at low levels of detection, or LOD, is highly beneficial and directly impacts efforts to identify and detect disease including, for example, COVID-19. We offer flexible placement models including direct purchase or reagent rental.
58
Our Diagnostic Tests on Our Platform
As of the date of this Annual Report, we have eight diagnostic tests available under EUA and/or CE Mark for use with our Instrument.
|
|
|
|
|
|
|
|
|
|
Test |
|
IVD Category |
|
TAM(1) |
|
Current Commercial Markets |
|
Regulatory submissions or certifications in the next 18 months(2) |
SARS-CoV-2 antigen |
|
Immunoassay |
|
$5-15 billion(3) |
|
U.S. (pursuant to EUA), Europe (CE Mark), Japan, Latin America |
|
|
SARS-CoV-2 antigen pool |
|
Immunoassay |
|
$2-6 billion(3) |
|
Europe (CE Mark) |
|
|
SARS-CoV-2 antibody |
|
Immunoassay |
|
$1 billion |
|
U.S. (pursuant to EUA), Europe (CE Mark) |
|
Africa |
INR |
|
Coagulation |
|
$500 million |
|
Europe (CE Mark) |
|
U.S. (510(k)) in H2 2022, Latin America |
D-Dimer |
|
Immunoassay |
|
$700 million |
|
Europe (CE Mark) |
|
U.S. (510(k)) in
H1 2023 |
CRP |
|
Immunoassay |
|
$300 million |
|
Europe (CE Mark) |
|
Africa |
SARS-CoV-2 & Flu A/B |
|
Immunoassay |
|
$1.5-3 billion(3) |
|
Europe (CE Mark), Japan (PDMA) |
|
U.S. (EUA) updated submission in H2 2022 |
(1) |
Global Total Addressable Market: We estimated for each test based on our current assumptions, including the (1) existing market sizes, (2) central lab market that could move to the POC, and (3) expansion of diagnostic testing. |
(2) |
We expect to submit a request for regulatory approval, authorization, certification or clearance or self- certify, as applicable, in the next 18 months in the markets listed for the test. |
(3) |
SARS-CoV-2 antigen TAMs may overlap with each other (e.g., SARS-CoV-2 antigen, SARS-CoV-2 antigen pool, Flu A/B + SARS-CoV-2, RSV + SARS-CoV-2). |
|
|
The Platform is currently the only platform that can run both an INR and D-Dimer test on the same platform (each comprising a different technology – enzyme vs. immunoassay).
We have more than 30 tests in various phases of development. For all our tests in development, we intend to launch them globally over time, subject to successful development and obtaining regulatory approval, authorization, certification or clearance, which we may never obtain. We will focus on the most attractive markets initially. While we have encouraging internal data for many of our diagnostic tests in development, we have not yet performed multi-site, external clinical analyses of most of these tests or otherwise compared our internal results against clinical results, unless stated otherwise in this Annual Report. The chart below summarizes information regarding select tests in development that we believe are important to our strategy of developing a broad menu of tests on a single Platform and are the closest in proximity to regulatory submission or certification. Further information regarding products in development phase to commercialization is provided later in the test section.
59
|
|
|
|
|
|
|
|
|
|
Test |
|
IVD Category |
|
Phase(1) |
|
TAM(2) |
|
Regulatory submissions or certifications in the next 18 months(3) |
SARS-CoV-2 & RSV
|
|
Immunoassay |
|
Verification and Validation |
|
$200-400 million(4) |
|
U.S. (EUA) in H1
2022, Europe (CE
Mark) in H1 2022 |
SARS-CoV-2 Ag Ultra (5 min test) |
|
Immunoassay |
|
Verification and Validation |
|
$5-15 billion(4) |
|
U.S. (EUA) in H1
2022, Europe (CE
Mark) in H1 2022 |
SARS-CoV-2 Ag Ultra pool (5 min test) |
|
Immunoassay |
|
Verification and Validation |
|
$2-6 billion(4) |
|
U.S. (EUA) in H1
2022, Europe (CE
Mark) in H1 2022 |
HbA1c |
|
Immunoassay |
|
Development |
|
$1.3 billion |
|
Europe (CE Mark)
in H1 2022, U.S.
(510(k)) in H2
2022 |
HS Troponin I |
|
Immunoassay |
|
Development |
|
$900 million |
|
Europe (CE Mark) in H1 2022, U.S. (510(k)) in H1 2023 |
NT-proBNP |
|
Immunoassay |
|
Development |
|
$350 million |
|
Europe (CE Mark)
In H1 2022, U.S.
(510(k)) in H1
2023 |
Strep A |
|
Molecular |
|
Feasibility |
|
$300 million |
|
Europe (CE Mark)
in H2 2022, U.S.
(510(k)) in H2
2022 |
TB |
|
Molecular |
|
Feasibility |
|
$250 million |
|
Africa (WHOPQ)
in H1 2023 |
Na,K |
|
Metabolic |
|
Feasibility |
|
$150 million |
|
Europe (CE Mark)
in H2 2022, U.S.
(510(k)) in H1
2023 |
Hemoglobin |
|
Hematology |
|
Feasibility |
|
$400 million |
|
Europe (CE Mark)
in H2 2022, U.S.
(510(k)) in H1
2023 |
BNP |
|
Immunoassay |
|
Feasibility |
|
$350 million |
|
Europe (CE Mark)
in H2 2022, U.S.
(510(k)) in H1
2023 |
(1) |
See the discussion below regarding the different phases of our product development. |
(2) |
Global Total Addressable Market: We estimated for each test based on our assumptions, including the (1) existing market sizes, (2) central lab market that could move to the POC with the right solution, and (3) expansion of diagnostic testing. |
(3) |
We expect to submit a request for regulatory approval, authorization, certification or clearance or self- certify, as applicable, in the next 18 months in the markets listed for the test. |
(4) |
SARS-CoV-2 antigen TAMs may overlap with each other (e.g., SARS-CoV-2 antigen, SARS-CoV-2 antigen pool, Flu A/B + SARS-CoV-2, RSV + SARS-CoV-2). |
|
|
|
|
|
|
60
Our Diagnostic Tests on Our Amira System
In addition to the tests on our Platform, we have started development of our Amira System, which is being developed as a high volume, lower cost mass population screening solution. We are initially focused on COVID-19 testing and we are exploring other applications of our Amira System for mass screening solution testing.
|
|
|
|
|
|
|
|
|
|
Test |
|
IVD Category |
|
Phase (1) |
|
TAM(1) |
|
Expected Regulatory submissions or certifications in the next 18 months |
SARS-CoV2 antigen |
|
Immunoassay |
|
Development |
|
$5-20 billion |
|
U.S. (EUA) in H1 2022, Europe (CE Mark) in H1 2022, Africa |
(1) |
SARS-CoV-2 antigen TAM is estimated to be approximately $5 billion as a low case and $20 billion as a high case. |
|
|
Although we are focused on commercializing our tests, prior to commercialization they must receive regulatory authorization or clearance. We may encounter delays in receiving the necessary regulatory authorization or clearance, which would delay commercialization of our tests and may decrease our expected market demand for our tests and adversely impact our strategy.
We currently generate revenue from sales of the Platform and our SARS-CoV-2 antigen test, SARS-CoV-2 antigen pool test, SARS-CoV-2 & Flu A/B tests, INR test, CRP Test, and D-Dimer test. respectively. For additional information on our revenue, see the section of this Annual Report titled “Item 5. Operating and Financial Review and Prospects” beginning on page 97.
Phases of Development
We identify five phases in our product development process. Each phase of our product development process has well-defined design activities and milestones, timelines, and risk levels at each phase as further described below. In light of our strategy to provide a broad menu of lab-comparable performance diagnostic tests at the POC, with low cost of ownership, our near-term pipeline is comprised of established diagnostic markers with high, existing clinical value. The timelines for our clinical studies vary per assay or target disease diagnosis and depends on the accessibility to clinical samples.
61
Below is a table that summarizes our phases of product development.
|
|
|
|
|
PHASE |
|
MILESTONES |
|
ESTIMATED TIMELINES |
Product concept |
|
• Business case |
|
2 months |
|
|
|
|
|
|
|
• Establishment of target product profile |
|
|
|
|
|
|
|
|
|
• Design control module and core team definition |
|
|
|
|
|
Feasibility |
|
• Demonstrate test strip and assay processing |
|
12 months |
|
|
|
|
(may decrease over time as Platform becomes more stable and uniform) |
|
|
• Define performance and design targets |
|
|
|
|
|
|
|
|
|
• Establish proof-of-concept and verify confidence to meet target product profile |
|
|
|
|
|
|
|
|
|
• Reagent and chemistry developments |
|
|
|
|
|
|
|
|
|
|
Development |
|
• Risk mitigation plans |
|
2 - 6 months |
|
|
|
|
|
|
|
• System integration (hardware, software, test strip, assay script, calibration) |
|
|
|
|
|
|
|
|
|
• Demonstrate performance and manufacturability |
|
|
|
|
|
|
|
|
|
• Pre-clinical studies |
|
|
|
|
|
|
|
|
|
• Establish design freeze and verify readiness for verification and validation |
|
|
|
|
|
Verification and Validation |
|
• Establish process performance qualification (PPQ) – transfer to manufacturing |
|
2 - 4 months |
|
|
|
|
|
|
|
• Complete clinical studies |
|
|
|
|
|
|
|
|
|
• Risk Summary Reports / Residual Risk Assessment reports |
|
|
|
|
|
Regulatory file preparation and submission, followed by launch |
|
• Launch product in self certified markets (CE Mark) |
|
|
|
|
|
|
|
|
|
• Regulatory file submission, such as EUA, and response to regulatory bodies and premarket review by regulatory bodies |
|
|
COVID-19 Tests
Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, is the coronavirus responsible for the COVID-19 pandemic. There are two main types of COVID-19 diagnostic tests:
•Tests that aid in diagnosis of active viral infection: Molecular and antigen tests are used to directly detect the presence of SARS-CoV-2 in respiratory samples such as nasal, nasopharyngeal and oropharyngeal swabs. Molecular tests detect the genetic material, specifically RNA, of the virus, whereas antigen tests detect the
62
proteins expressed on the outside or inside of the virus. These tests allow for accurate identification of a COVID-19 infection from the onset of symptoms, but are not able to detect previous infections.
•Tests that aid in diagnosis of an immune response to COVID-19: An immune response represents the activation of the immune system following exposure to the virus. The response includes activated T cells and B cells (which produce antibodies) that are specific to molecular structures on SARS-CoV-2 and proliferate and attack the invading pathogen. COVID-19 antibody tests are used to directly detect the presence of SARS-CoV-2 IgG, IgM and/or total antibodies in blood samples.
We have obtained an EUA and a CE Mark for our SARS-CoV-2 antigen test and our SARS-CoV-2 antibody test. We have rolled out our SARS-CoV-2 antigen test in a large number of countries in Africa under EUA and applicable local regulations.
SARS-CoV-2 Antigen Test
Our SARS-CoV-2 antigen test has been developed to detect the SARS-CoV-2 virus in respiratory samples such as nasal and nasopharyngeal swabs with results at the POC in 12 minutes or less.
We estimate that the global market for POC COVID-19 testing in 2021 was between $15 billion to $20 billion, including both molecular and lateral flow tests. Though testing rates have been high in the early parts of 2022, continued need for testing globally for the remainder of the year will depend on management of the pandemic in high burden countries, vaccine supply and implementation rates to compared to what is required to achieve herd immunity, and emergence of new variants which are more resistant to vaccines. Regardless of absolute market demand, we do see a continued shift toward fast, high sensitivity POC COVID-19 testing and as such are prioritizing our five minute COVID tests.
Molecular testing is primarily conducted in central labs and therefore requires significant infrastructure, resources and time to deliver patient results. POC molecular tests are commercially available, however they have been limited to the hospital setting due to limited supply.
Antigen lateral flow tests offer fast diagnosis of an active COVID-19 infection, but they are somewhat limited in sensitivity. Unlike lateral flow tests which use old technology developed for at-home pregnancy testing, our SARS-CoV-2 antigen test is a next-generation test system using microfluidic immunofluorescence technology to detect tiny amounts of the virus antigen in just a few microliters of nasal swab extract. We believe our high sensitivity LumiraDx SARS-CoV-2 antigen test has the opportunity to take significant market share of the $15-20 billion POC COVID-19 molecular and lateral flow testing market.
Our SARS-CoV-2 antigen test detects SARS-CoV-2 virus in respiratory samples such as nasal swabs in 12 minutes or less. The performance of our test is attributable to its design as well as the precise microfluidic control of our Instrument. Our test uses SARS-CoV/SARS-CoV-2 specific antibodies in an immunoassay to determine the presence of SARS-CoV-2 Nucleocapsid Protein (“NP”) present in the test sample. Our Instrument generally uses multiple independent assay channels in the test strip to detect the NP antigen in the test sample. It directs fluidic movement and mixing of the reagents and test sample in each test strip channel. A magnetic field is then applied to the measurement zone which retains the magnetic particles and associated SARS-CoV-2 NP immuno-complexes allowing removal of the sample and any unbound label from the measurement zone. Our Instrument measures the fluorescent signal of the immuno-complex fluorescent particles in an essentially dry state which is proportional to the concentration of the SARS-CoV-2 virus NP antigen in the sample. The strip contains on-board control reagents that are used to verify that the test operated correctly. Our SARS-CoV-2 antigen test detects major global SARS-CoV-2 variants including Delta, Gamma, Epsilon, Alpha, Beta and Omicron variants. We are continually testing the new variants of clinical concern as they arise to confirm the performance of our SARS-CoV-2 antigen test.
In a clinical study with 257 patients presenting from zero to 12 days of symptom onset, our SARS-CoV-2 antigen test demonstrated 97.6% positive percent agreement, or PPA, and 96.6% negative percent agreement, or NPA, compared to the reference method, Roche Cobas 6800, and delivered results within 12 minutes or less. The test has high analytical performance with a LOD of 32 TCID50/mL, which is approximately four times lower than currently available POC tests on the market. We believe the superior performance over a wide detection window has the potential to greatly improve the diagnosis of COVID-19 infection and infectivity, enable large-scale population monitoring and facilitate management of the COVID-19 pandemic, however, we only conducted one clinical study with a limited sample size of 257 patients, so this superior performance may not be sustained or replicated as our SARS-CoV-2 antigen tests are used on higher volumes of patients. We obtained an EUA and a CE Mark for our SARS-CoV-2 antigen test. Our SARS-CoV-2 antigen test has been authorized by the FDA under an EUA only for the qualitative detection of SARS-CoV-2 nucleocapsid protein and has not been authorized for use to detect any other viruses or pathogens. In addition, the CE Mark process is a self- certification
63
process where we self-declare as a manufacturer that we have checked the product meets European Economic Area safety, health or environment requirements. Our SARS-CoV-2 antigen test has not been cleared or approved by FDA or any other regulatory body. A summary of the results from the clinical study described above is set out in the following tables.
|
|
|
|
|
|
|
Positive |
Negative |
Total |
SARS-CoV-2 |
Positive |
81 |
6 |
87 |
Ag Test |
Negative |
2 |
168 |
170 |
|
Total |
83 |
174 |
257 |
|
|
|
|
|
|
Value |
95% CI |
SARS-CoV-2 |
PPA |
97.60% |
91.6% - 99.3% |
Ag Test |
NPA |
96.60% |
92.7% -98.4% |
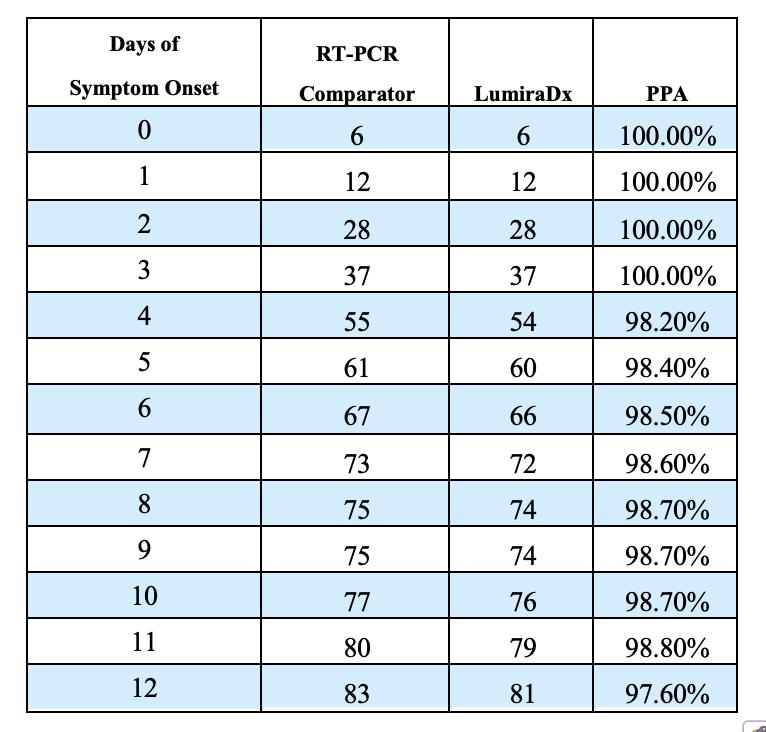
We have also received various external validations of our SARS-CoV-2 antigen test, including from the Swiss and Japanese governments, and the Scandinavian evaluation of laboratory equipment for POC testing (SKUP), which show the performance of our SARS-CoV-2 antigen test.
A recent living systematic review and meta-analysis of SARS-CoV-2 antigen tests by PLOS Medicine, currently including 133 clinical and analytical accuracy studies across 61 antigen tests completed by Lukas Brümmer, Stephan Katzenschlager and their co-workers to inform national and international bodies including WHO, ECDC, and RKI, revealed that LumiraDx’s SARS-CoV-2 antigen test showed the highest overall accuracy.
We have established a proactive system and plan to monitor and evaluate the impact of mutations and viral variants on the performance of our test. The following table shows the currently circulating volatile organic compounds (“VoCs”) and the performance of the LumiraDx SARS-CoV-2 antigen test with the listed VoCs. In- house evaluation has been carried out using in silico analysis and direct testing using either recombinant nucleocapsid protein or independent testing with the viral isolate:
•Alpha Variant, Beta Variant and Gamma Variant – Detection was demonstrated in patient samples by the U.K. Department of Health and Social Care, COVID-19 Technologies Validation Group.
•Beta Variant – Detection was demonstrated by the South African National Health Laboratory Service.
64
•Delta Variant – Detection was demonstrated in patient samples as discussed by the U.K. Department of Health and Social Care, COVID-19 Technologies Validation Group.
•Omicron Variant- Testing with patient live samples was completed by LumiraDx. In addition, a prospective clinical study was carried out by Medical Research Network Diagnostics. Both studies showed that Omicron is detected by the LumiraDx SARS-CoV-2 Ag test with comparable sensitivity to the Delta variant.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LumiraDx SARS-CoV-2 |
|
|
|
|
|
|
|
Antigen Test Result |
WHO Label |
|
Pango Lineage |
Country in which first detected |
|
Nucleocapsid Mutation |
|
(Positive/Negative) |
|
|
|
|
Alpha |
|
B.1.1.7 |
UK, Sept 2020 |
|
D3L, R203K, G204R, S235F |
|
Positive |
|
|
|
|
Beta |
|
B.1351 |
South Africa, May 2020 |
|
T2051 |
|
Positive |
Gal |
|
|
|
Gamma |
|
P.1 |
Brazil, Nov 2020 |
|
P80R, R203K, G204R |
|
Positive |
|
|
|
|
Delta |
|
B.1.617.2 |
India, Oct 2020 |
|
D63G, R203M,G215C, D377Y |
|
Positive |
|
|
|
|
Omicron* |
|
B.1.1.529 |
Multiple Countries |
|
R203K, G2049, P13L, E31-R32-and S33 |
|
Positive |
*Descendent Pango Lineages of Omicron, BA.2 and BA.3 have the additional Nucleocapsid mutation S413R, which was tested in-house using recombinant protein and tested positive at 50 pg/ml on the LumiraDx SARS-CoV-2 Antigen Test.
SARS-CoV-2 Antibody Test
Our SARS-CoV-2 antibody test has been developed to detect presence of SARS-CoV-2 total antibody in a blood sample with performance at the POC and delivers the results in 11 minutes.
Antibody testing is used to understand the virus’s epidemiology in the general population and identify groups at higher risk of infection. In addition, serologic testing can be offered as a method to support diagnosis of acute COVID-19 illness for persons who present late and potentially over time to measure immunity.
Our SARS-CoV-2 antibody test has been developed to detect presence of SARS-CoV-2 total antibody in a blood or plasma sample with high sensitivity and specificity from onset of symptom through disease progression. Our test uses SARS-CoV-2 Spike (S1) and Receptor Binding Domain (RBD) antigens in an immunoassay to determine the presence of SARS-CoV-2 antibodies in the test sample. Our Instrument generally uses multiple independent assay channels in the test strip to detect the antibodies in the test sample. It directs fluidic movement and mixing of the reagents and test sample in each test strip channel. A magnetic field is then applied to the measurement zone which retains the magnetic particles and associated SARS-CoV-2 antibody immuno-complexes allowing removal of the sample and any unbound label from the measurement zone. Our Instrument measures the fluorescent signal of the immuno-complex fluorescent particles in an essentially dry state which is proportional to the concentration of the SARS-CoV-2 antibody in the sample. The strip contains on-board control reagents that are used to verify that the test operated correctly. In clinical studies, our SARS-CoV-2 antibody test has demonstrated high sensitivity and high specificity across the COVID-19 diagnostic window.
Several clinical studies were conducted to determine PPA and NPA of SARS-CoV-2 antibody test in fingerstick blood and plasma samples. In a prospective study of 62 asymptomatic and symptomatic subjects, with fingerstick blood sample collected more than eight days post RT-PCR, the SARS-CoV-2 antibody test demonstrated 100% PPA compared to RT-PCR. In a study of 72 plasma samples collected from symptomatic subjects, the SARS-CoV-2 antibody test demonstrated 97.2% PPA compared to RT-PCR including 90% PPA in the first 14 days post PCR. The test also demonstrated 100% NPA. We believe a fingerstick blood SARS-CoV-2 antibody test with high sensitivity in earlier stages of disease progression may supplement antigen testing to improve COVID-19 diagnosis. A summary of the results from the clinical studies described above is set out in the following tables.
|
|
|
|
|
Positive |
|
Negative |
Sample |
agreement |
|
agreement |
Direct Fingerstick |
100%(62/62) |
|
100%(54/54) |
Fingerstick via Transfer Tube |
100%(62/62) |
|
100%(56/56) |
65
|
|
|
|
|
|
|
Days from RT-PCR to |
|
Number of |
|
|
Sensitivity RT-PCR |
Blood Collection |
|
Samples |
|
|
Comparator |
6 days |
|
|
13 |
|
|
84.60% |
7-13 days |
|
|
7 |
|
|
100% |
14-20 days |
|
|
6 |
|
|
100% |
21 days |
|
|
46 |
|
|
100% |
Total |
|
|
72 |
|
|
97.2% (90.4% – 99.2%) |
We have obtained an EUA and a CE Mark for our SARS-CoV-2 antibody test. The CE Mark process is a self-certification process where we self-certify as a manufacturer that our devices meet the necessary E.U. regulatory requirements. Now that the Transition Period has ended, CE Marks will continue to be recognized in G.B. until June 30, 2023.
The implementation of COVID-19 vaccines may drive an increased demand for SARS-CoV-2 antibody testing as a verification tool around the duration of protective immunity for immunized patients in a real world setting.
SARS-CoV-2 Antigen Pool Test
Pooled testing enables higher test throughput and lower cost per individual which is important for mass testing at schools, workplaces and events.
Pooled testing is generally performed on PCR systems with multiple samples combined into a single reaction to increase the number of samples processed per batch. Follow up testing is done with remnant samples in the lab or by follow up using a rapid test.
The LumiraDx SARS-CoV-2 antigen pool test is intended for qualitative detection of nucleocapsid protein antigen SARS-CoV-2 in one to five individual samples from professionally supervised and self-collected nasal swab samples or professionally collected nasal or nasopharyngeal swab samples which are then pooled for testing. In clinical studies, the LumiraDx SARS-CoV-2 antigen pool test demonstrated 100% positive agreement and 96.6% negative agreement to the individual LumiraDx SARS-CoV-2 antigen test.
|
|
|
|
|
|
|
Positive Pooled Specimens |
Single Swab |
|
|
|
|
|
|
POS |
NEG |
Total |
|
|
|
|
|
|
|
|
|
Pool Test |
POS |
30 |
0 |
30 |
|
|
|
NEG |
0 |
0 |
0 |
|
|
|
Total |
30 |
0 |
30 |
|
|
|
|
|
|
|
Negative Pooled Specimens |
Single Swab |
|
|
|
|
|
|
POS |
NEG |
Total |
|
|
|
|
|
|
|
|
|
Pool Test |
POS |
0 |
1 |
1 |
|
|
|
NEG |
0 |
28 |
28 |
|
|
|
Total |
0 |
29 |
29 |
66
|
|
|
|
|
|
|
Measure |
|
Estimate |
|
95% Wilson Score CI |
PPA |
|
100.00% |
|
88.60% |
|
100.00% |
NPA |
|
N/A |
|
N/A |
|
N/A |
PPV |
|
100.00% |
|
88.60% |
|
100.00% |
NPV |
|
N/A |
|
N/A |
|
N/A |
Prevalence |
|
100.00% |
|
88.60% |
|
100.00% |
OPA |
|
100.00% |
|
88.60% |
|
100.00% |
|
|
|
|
|
|
|
Measure |
|
Estimate |
|
95% Wilson Score CI |
PPA |
|
N/A |
|
N/A |
|
N/A |
NPA |
|
96.60% |
|
82.80% |
|
99.40% |
PPV |
|
0.00% |
|
0.00% |
|
79.30% |
NPV |
|
100.00% |
|
87.90% |
|
100.00% |
Prevalence |
|
0.00% |
|
0.00% |
|
11.70% |
OPA |
|
96.60% |
|
82.80% |
|
99.40% |
We have obtained CE Mark for our LumiraDx SARS-CoV-2 antigen pool test. We believe we are the first and currently only POC antigen pool test commercially available in Europe.
SARS-CoV-2 & Flu A/B Tests
Given that patients with Flu A, Flu B or SARS-CoV-2 antigen present with similar symptoms, having a single test that can provide simultaneous results for multiple conditions will enable healthcare providers to verify infection quicker, begin proper treatment sooner and, if required, initiate isolation precautions, helping to prevent further spread of infection as well as lower costs. Combined testing may also mitigate the problem of testing material shortages, such as swabs or extraction buffers. If SARS-CoV-2 becomes an endemic disease the need for differentiation of the cause of respiratory illness will increase.
We have obtained CE Mark in Europe and PMDA in Japan for our LumiraDx SARS-CoV-2 & Flu A/B tests. We have also submitted an EUA request to the FDA in October 2021. To date we have not yet received authorization for this combo test and currently the FDA has indicated that authorization will be not be provided as further information is required, amongst other things, additional data points related to Flu A/B testing. There can therefore be no guarantee that authorization will be granted by the FDA and timing on updated submissions depends on the prevalence of Flu A/B and our ability to collect further data.
SARS-CoV-2 & RSV Test
The symptoms of RSV or SARS-CoV-2 are very similar and therefore having a single test that can provide simultaneous results will enable healthcare providers to identify the underlying cause, verify infection quicker, begin proper treatment sooner and, if required, initiate isolation precautions, helping to prevent further spread of infection as well as lower costs.
The SARS-CoV-2 & RSV test is currently in verification and validation phase. We plan on obtaining CE Mark and submitting an EUA request to the FDA in H1 2022 for our SARS-CoV-2 & RSV test.
SARS-CoV-2 Ag Ultra
The continued need for testing globally for the remainder of the year will depend on management of the pandemic in high burden countries, vaccine supply and implementation rates and emergence of new variants which are more resistant to vaccines. Regardless of absolute market demand, we do see a continued shift toward fast, high sensitivity POC COVID-19 testing and as such are prioritizing our five-minute COVID tests. High sensitivity results in five minutes will enable routine testing of individuals without compromising performance.
The SARS-CoV-2 Ag Ultra test detects SARS-CoV-2 virus in respiratory samples such as nasal swabs with results at the POC in five minutes and is currently in verification and validation phase. We plan on obtaining CE Mark and submitting an EUA request to the FDA in H1 2022.
67
SARS-CoV-2 Ag Ultra Pool
Pooled testing enables higher test throughput and lower cost per individual which is important for mass testing at schools, workplaces, and events.
The SARS-CoV-2 Ag Ultra Pool test is intended for qualitative detection of nucleocapsid protein antigen of SARS-CoV-2 in one to five individual samples which are pooled for testing to detect the SARS-CoV-2 virus, with results in five minutes. The test is currently in verification and validation phase. We plan on obtaining CE Mark and submitting an EUA request to the FDA in H1 2022.
International Normalized Ratio ("INR") Test
INR is a standardized measurement of the rate at which blood clots. A low INR can indicate an increased risk of blood clots, while an elevated INR can indicate increased risk of excessive bleeding.
Healthcare providers commonly use INR tests to monitor oral anticoagulation therapy with Vitamin-K Antagonist, or VKA, drugs. VKA drugs are often prescribed to patients at risk of forming clots that can lead to strokes. Patients on VKAs require regular INR monitoring to maintain optimal coagulation, but the daily dose of VKA necessary to maintain optimal anticoagulation varies among patients due to factors such as age, body mass index, genetic differences, comorbidities and environmental factors. Therefore, optimal VKA therapy requires regular monitoring of a patient’s INR with an accurate and precise measurement tool.
Our INR test has been validated in various clinical studies against reference lab standard ACL ELITE Pro. One study, the OPTIMAL study, conducted in 11 sites by Glasgow Royal Infirmary, Queen Elizabeth Hospital and Golden Jubilee Hospitals and NHS anti-coagulation services, showed strong correlation between our Platform and laboratory reference method, as well as between the different application methods and test lots (see data chart below). Another study confirmed strong correlation between our INR test results (capillary blood sample) and those obtained from plasma samples using both the ACL Elite and also the Sysmex CS 2100/5100. Feedback from healthcare professionals indicated that overall our Platform was easy to follow and use. Data overall demonstrated that our INR test provided rapid and reliable INR analysis at the POC.
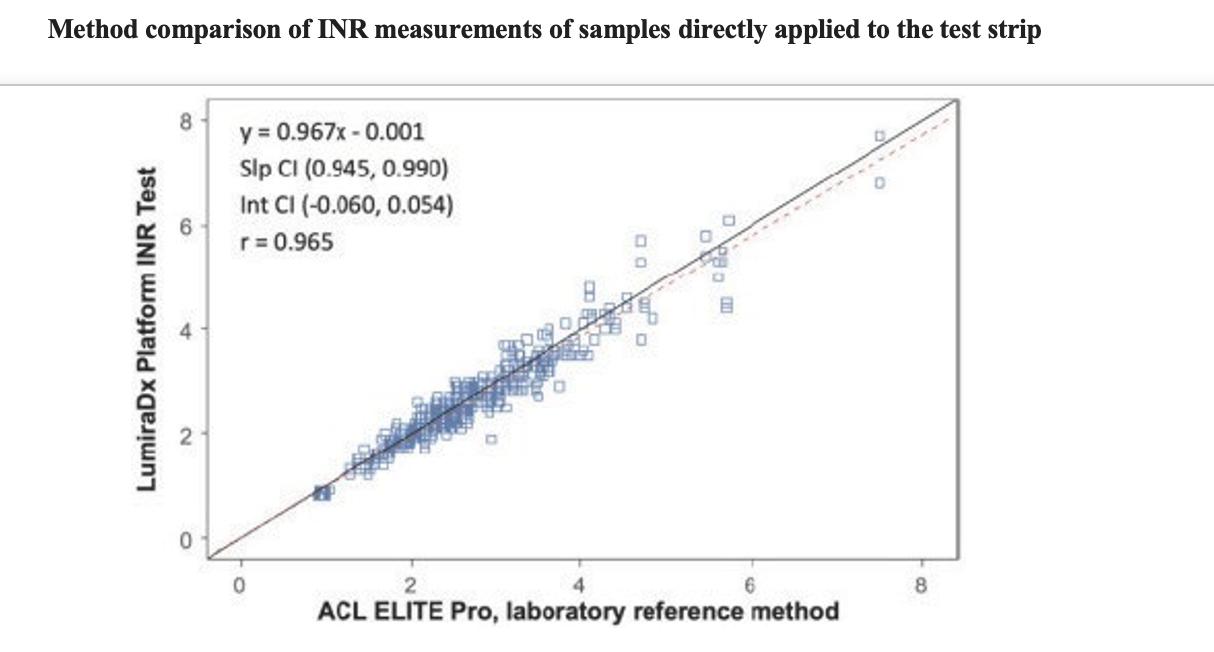
ACL ELITE Pro, IL ACL Elite Pro (Instrumentation Laboratory; Bedford, MA, USA); Int CI, intercept confidence interval; Slp CI, slope confidence interval.
In a clinical study of 596 capillary and venous blood samples collected from 366 patients, our INR test when measured against the Laboratory ACL Elite lab reference method demonstrated strong correlation of 0.965 (95% confidence interval (CI): 0.959, 0.970) when using direct application and 0.958 (95% Cl: 0.950, 0.964) when using a transfer pipette. The established INR range was 0.8-7.5. Precision was measured using samples collected with a transfer pipette (n=291, mean INR 2.525, mean % coefficient of variation (CV) 3.73%) or direct application (n=284 mean INR 2.538, mean % CV 3.46).
We have obtained CE Mark for our INR test and we plan to submit a 510(k) to FDA in the second half (“H2”) of 2022.
68
D-Dimer Test
D-Dimer is a fibrin degradation product, or FDP, a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two D fragments of the fibrin protein joined by a cross-link.
D-Dimer testing is generally used in clinical settings, along with clinical scoring systems and additional testing methods, when there is suspected venous thromboembolism, or VTE, disseminated intravascular coagulation, or DIC, deep vein thrombosis, or DVT, and pulmonary embolism, or PE. Several care guidelines recommend inclusion of a quantitative D-Dimer test for exclusion of VTE in patients presenting with symptoms at primary care. Until the launch of the LumiraDx D-Dimer Test, there were no quantitative POC tests for D-Dimer using a direct fingerstick sample.
A fast and accurate test for D-Dimer is one of the more desired tests by primary care physicians at POC in multiple countries, including the United States and countries in Europe. We believe there is substantial opportunity to bring D-Dimer testing closer to patients with fast accurate test results at POC. Our D-Dimer test delivers quantitative results in six minutes from a fingerstick blood sample. It is currently the only D-Dimer method available on the market today with direct fingerstick sampling capabilities. The test is aimed to be used as an aid in the assessment and diagnosis of patients with suspected VTE, such as DVT and PE. It is designed to be used by healthcare providers or other trained professionals in community-based healthcare or urgent care/hospital ED settings.
We target to expand on our current intended use in H1 2022 to include a clinical cut-off. This would allow us to have an ‘exclusion’ claim, used by healthcare professionals to exclude VTE as a potential diagnosis for patients experiencing symptoms of VTE, in conjunction with additional clinical risk scores. Ruling out VTE at the POC can lead to potentially significant cost savings for healthcare providers as they would no longer require expensive ultrasounds and pulmonary angiograms in secondary care settings to arrive at a rule out diagnosis, also effectively serving to minimize patient stress by making quicker, better-informed decisions at the POC.
In addition, D-Dimer testing is currently also expected to have a utility as part of diagnosis and management of COVID-19 infected patients as D-Dimer has also been shown to be a prognostic indicator for predicting severity of symptoms in patients with COVID-19 infection.
We have obtained CE Mark for our D-Dimer test and we plan to submit a 510(k) to FDA in H1 2023.
The key clinical studies included a (i) method comparison study to evaluate correlation with a laboratory reference method and (ii) precision study. The method comparison was performed using plasma samples from patients (n = 327, range = 60 - 4515 `lg/L FEU). A comparison of 1767 D-Dimer measurements with the LumiraDx D-Dimer Test to the VIDAS Exclusion II D-Dimer assay, a globally recognized laboratory reference method, yielded the following statistics: Slope = 1.02, Intercept = 21, r = 0.92. The LumiraDx D-Dimer Test showed a strong correlation with the laboratory reference method, in particular around the clinically relevant values of <750 `lg/L FEU as highlighted in the below graph.
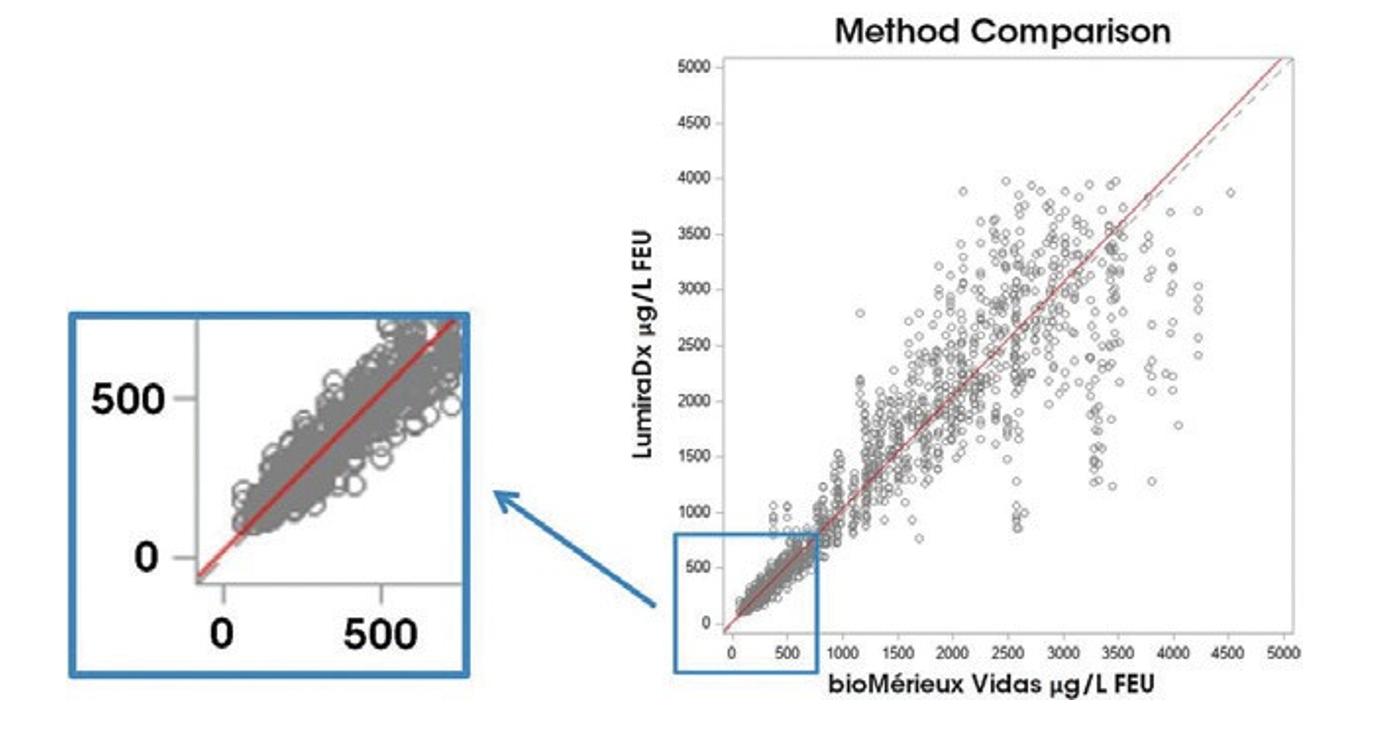
69
A precision study was carried out in citrated venous plasma on a protocol based on CLSI EP5-A3. The study was carried out with three levels of D-Dimer, each was tested in two runs of two replicates per day, for 20 days. The findings of this study are summarized in the table below.
|
|
|
|
|
|
|
|
|
|
|
D-Dimer |
|
Within run |
|
Within day |
|
Between day |
|
Total |
|
n |
concentration |
|
precision |
|
precision |
|
precision |
|
precision |
|
|
(µg/L FEU) |
|
(% CV) |
|
(% CV) |
|
(% CV) |
|
(% CV) |
|
|
291 |
|
9.8 |
|
11.1 |
|
0 |
|
11.1 |
|
80 |
552 |
|
9.4 |
|
9.4 |
|
2.5 |
|
9.7 |
|
80 |
1790 |
|
10.1 |
|
10.1 |
|
0.7 |
|
10.2 |
|
80 |
Feedback from healthcare professionals indicated that overall our Platform was easy to use, results were comparable to their laboratory reference methods, and that having a reliable D-Dimer result at the POC will be able to drive actionable results sooner, as opposed to via secondary care pathways, which can take hours or days to obtain a D-Dimer result from the laboratory before proceeding to next steps in managing patients with suspected VTE.
C-Reactive Protein (CRP) Test
CRP is a circulating protein produced by the liver in response to inflammation caused by tissue damage or infection. CRP has become a universal biomarker of infection and inflammation for a number of diseases and pathophysiological conditions (such as bacterial infections, inflammatory bowel disease and autoimmune disorders) and CRP testing has been shown to reduce the need for antibiotic prescription. CRP testing is used more commonly in European countries, where antibiotic consumption is generally lower than in the U.S.
In addition, CRP testing is currently also expected to have a utility as part of diagnosis and management of COVID-19 infected patients.
The LumiraDx CRP Test achieved CE Mark in January 2022 and has since been introduced into Europe, the Middle East and Africa. We plan to continue to expand our global footprint by completing registrations in other key markets, including South America and Asia.
The key clinical studies included a (i) method comparison study to evaluate correlation with a laboratory reference method and (ii) precision study. The method comparison was performed using three Test Strip lots with plasma samples (Lithium Heparin) sourced from patients presenting with symptoms of respiratory illness, inflammation, or injury, at hospital emergency departments, acute medical units or out-patient clinics. A comparison of 320 CRP measurements with the LumiraDx CRP Test to the RCRP Flex® assay on the Siemens Dimension® Xpand® Plus Integrated Chemistry System analyzed by Passing Bablok regression yielded the following statistics: Slope = 1.054, Intercept = -0.66, r = 0.99. A precision study was carried out in heparinized venous plasma on a protocol based on CLSI EP5-A3. The study was carried out at three concentrations of CRP, each was tested in one run of five replicates per day, for five days across three sites. The results of the precision study are summarized in the below table:
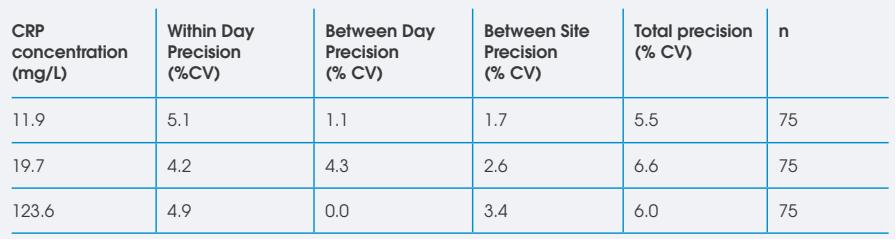
Glycated Hemoglobin (HbA1c) Test
HbA1c is a form of hemoglobin that is chemically linked to a sugar. The formation of the sugar-Hb linkage is due to the presence of excessive sugar in the bloodstream.
70
HbA1c tests show the average level of glucose attached to hemoglobin over the last two to three months (typical life span of a red blood cell). HbA1c is a surrogate biochemical indicator of tissue exposure to elevated glucose. High HbA1c levels may be a sign of diabetes, a chronic condition that can cause serious health problems, including heart disease, kidney disease, and nerve damage. We estimate that 70% of HbA1c tests are conducted in the central lab setting. We believe there is substantial opportunity to bring HbA1c testing closer to patients in an overall effort to improve community-based healthcare and overall patient outcomes.
Our HbA1c test is for use by healthcare professionals in POC settings for the quantitative determination of glycated hemoglobin in human capillary and venous blood samples. This test is to be used as an aid in the monitoring of long-term glycemic control in individuals with diabetes mellitus, and as an aid in identifying patients who may be at risk for developing diabetes. Additionally, HbA1c monitoring at POC enables improved patient physician management of diabetes and comorbidities. Our HbA1c test is in the development phase. We plan to complete the process to obtain a CE Mark in H1 2022 and submit a 510(k) to FDA in H2 2022.
High Sensitivity Troponin I (hs-TnI) Test
Troponin is a complex of three regulatory proteins (Troponin C, Troponin I, and Troponin T) that is integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific Troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocardial infarction, or MI, and acute coronary syndrome.
A Troponin test measures the levels of Troponin T or Troponin I proteins in the blood. These proteins are released when the heart muscle has been damaged and is an indicator of a heart attack. Troponin tests are generally used, together with an electrocardiogram, or ECG, in emergency room patients who present with persistent chest pain, unstable angina or other similar symptoms.
Over time focus has shifted to high sensitivity Troponin I, tests (hs-c Tn) with high precision at very low concentrations allowing accurate quantification of Troponin in the majority of the healthy population. This has enabled cardiologists to develop new algorithms which define a single threshold value (typically 3-6 ng/L c Tn) that identifies patients with suspected acute coronary syndrome at presentation who are at low risk of MI and potentially suitable for immediate discharge therefore reducing hospital crowding and health costs without compromising clinical outcomes. These early rule-out strategies using hs-c Tn assays are now being increasingly employed to safely and effectively manage suspected MI patients.
We estimate 80% of Troponin tests are conducted in the central lab setting because POC alternatives are insufficiently sensitive to measure such low levels at the POC. We believe there is substantial opportunity to bring Troponin testing closer to patients, to reduce lengthy turnaround times generated by sending samples to the lab.
We are developing a highly sensitive Troponin POC test, which is aimed to provide a paradigm shift in suspected MI patients care management and can measure Troponin at very low levels allowing for clinical decisions to be made directly at the POC, in the hospital emergency room or physician office, and ensuring that healthcare providers can rely on the results of this test, and other clinical guidance, to establish if the patient is having a MI or over time can be ruled out and re-directed for further testing in other areas where required.
In addition, it is envisaged that the portability and connectivity of our Platform will, over time, allow suspected MI patient diagnosis, provided for example by paramedics in emergency situations, at the patient’s home or in the ambulance. We have already demonstrated that we can measure at very low concentration and LOD in our SARS-CoV-2 assays. Our high sensitivity Troponin test is in the development phase. We plan to complete the process to obtain a CE Mark H1 2022 and submit a 510(k) to FDA in H1 2023.
Natriuretic Peptides
B-type natriuretic peptides (NT-proBNP & BNP) are proteins naturally produced by the heart and blood vessels. Under normal physiological conditions, they help your body to eliminate fluids, relax blood vessels and move sodium into the renal system. When your heart is damaged, your body secretes higher levels of B-type natriuretic peptides into your bloodstream to try to ease the strain on your heart, of which levels increase as heart failure progresses. Both NT-proBNP & BNP are useful for diagnosing congestive heart failure and for evaluating the risk of a heart attack and other problems in those with existing heart disease. In addition, both markers also have prognostic implications in the settings of sepsis, pulmonary embolism, pulmonary hypertension, and stable coronary artery disease.
71
Our NT-proBNP test is in the development phase and we plan to complete the process to obtain a CE Mark in H1 2022 and submit a 510(k) to FDA in H1 2023. Our BNP test is in the feasibility phase and we plan to submit to IVDR notified body for CE Mark in H2 2022 and submit a 510(k) to FDA in H1 2023.
Strep A
Bacteria called Group A Streptococcus (group A strep) can cause many different infections including Strep Throat, Scarlet fever and Rheumatic fever. Rapid point of care testing for Strep A is commonly utilized to test individuals with a sore throat (Strep throat or pharyngitis) to see if the underlying cause is Group A Strep, rather than a virus which causes the majority of sore throats. A positive Strep A test indicates whether antibiotics are required and helps prevent spread of infection to others. Rapid Strep A tests can help reduce the utilization of unnecessary antibiotics and help individuals return to work or school sooner.
Our molecular Strep A test is in the feasibility phase and we plan to complete the design and development process to submit for CE Mark and 510(k) to FDA in H2 2022.
Tuberculosis
Tuberculosis (TB) is a potentially serious infectious disease that mainly affects the lungs and is caused by Mycobacterium tuberculosis (MTB) bacteria. TB is a large health burden in many countries in Africa, South East Asia and Western Pacific. Once rare in developed countries, TB infections began increasing in 1985, partly because of the emergence of HIV, the virus that causes AIDS. There is large unmet need for rapid TB tests in resource-limited settings that have inadequate or no direct access to laboratories.
Our molecular TB test is in the development phase and we plan to start clinical studies in H1 2022.
Sodium, Potassium (Na, K)
Sodium (Na) and Potassium (K) are electrolytes which are commonly found in the blood. Sodium is a physiological cation that helps to maintain normal distribution of water throughout the body. A sodium test is useful for assessing acid-base balance, water balance, water intoxication, and dehydration. Potassium carries electrical signals throughout the body. It is critical to the proper functioning of nerve and muscles cells – particularly heart muscle cells. A potassium test is useful for evaluation of electrolyte balance, cardiac arrhythmia, muscular weakness, hepatic encephalopathy, and renal failure. Both tests are particularly important across both primary and secondary care settings.
Our Na/K test is currently in the feasibility phase and we plan to submit to IVDR notified body for CE Mark in H2 2022 and submit a 510(k) to FDA in H1 2023.
Hemoglobin
Hemoglobin (Hb) is a protein found in the red blood cells that carries oxygen to the body’s organs and tissues and transports carbon dioxide from your organs and tissues back into your lungs. A low Hb test could reveal that a patient is anemic. Anemia can have many different causes, including vitamin deficiencies, bleeding, or other chronic diseases. An elevated Hb test could reveal that a patient has the blood disorder polycythemia vera, or that a patient is living at a high altitude, a smoker, or is dehydrated.
Our hemoglobin test is in the feasibility phase and we plan to submit to IVDR notified body for CE Mark in H2 2022 and submit a 510(k) to FDA in H1 2023.
Molecular Tests
We have developed over the last few years a proprietary molecular chemistry, qSTAR (Selective Temperature Amplification Reaction), which forms the basis of our molecular assays. This new technology is a non-PCR enzyme-based system with an optimized temperature profile that is suitable to deliver very sensitive, rapid near patient results.
Our molecular tests, which incorporate our proprietary qSTAR technology, are designed to offer many competitive advantages in the market including minimal user steps and reduced sample preparation steps and time. Our molecular tests leverage the same strip design as our other tests, and have the ability to run on our Instrument with results expected in
72
approximately 10-15 minutes. Our molecular test strips are manufactured on the same automated, low-cost manufacturing system as our other test strip designs, using the same base materials.
We have already shown feasibility on a wide range of tests using this molecular technology with our LumiraDx Platform. Currently, we are working on various molecular tests with a focus on infectious diseases, such as HIV and tuberculosis, both of which are being supported by BMGF, and Strep A. Our molecular MTB test is designed to be a true point of care diagnostic test that is safe and easy to use in remote care settings, with high sensitivity results in minutes. Our molecular Strep A test is designed to enable us to offer a comprehensive portfolio of common respiratory conditions, with the clinical benefits offered by high sensitivity Strep A testing. Our molecular pipeline includes additional tests in sexual health and virology. Upon regulatory approval, authorization, certification or clearance and launch of our molecular tests, we would be the only company providing molecular and non-molecular technologies on the same Instrument.
The development of this proprietary technology is being strengthened and enhanced by the research and development efforts of our Fast Lab Solutions team. qSTAR technology is highly applicable to diagnostic testing for COVID-19. Detecting virus via RNA or antigen is the standard of care for COVID-19 diagnosis. But molecular diagnostic testing in the lab is a time consuming and complex process. LumiraDx applied its qSTAR technology to the development of rapid molecular COVID-19 reagent testing kits for use on open molecular systems. The technology allows for the reduction of amplification timing which increases throughput over other molecular platforms, while maintaining the sensitivity of detection.
qSTAR technology can amplify highly conserved regions of the SARS-CoV-2 genome that are smaller in size than other molecular technologies, such as RT-PCR. This reduces or eliminates the risk of mutation occurring in the region selected for detection. Therefore, to date the assay detects all major global SARS-CoV-2 variants including Omicron, Omicron B.2, Delta, Gamma, Epsilon, Alpha, and Beta variants.
We have two COVID-19 reagent kits:
1.LumiraDx SARS-CoV-2 RNA STAR received EUA from FDA on August 11, 2020. The assay allows laboratories to utilize their existing molecular lab infrastructure in a high-throughput format by reducing amplification time from approximately one hour down to 12 minutes. We have commenced sales of our SARS-CoV-2 RNA STAR.
2.LumiraDx SARS-CoV-2 RNA STAR Complete received an EUA from the FDA on October 14, 2020 and CE Mark on June 30, 2021. The assay utilizes a direct amplification method that combines lysis and amplification in a single step, detecting SARS-CoV-2 nucleic acid in under 20 minutes, without needing to perform any specimen purification or extraction. LumiraDx SARS-CoV-2 RNA STAR Complete provides approximately a two- to five-fold increase of testing throughput over common open molecular systems, allowing laboratories to improve turnaround time of patient results and expand their testing capacity with existing instrumentation. In December 2021, we achieved several FDA EUA updates for RNA STAR Complete molecular reagents including asymptomatic testing, pooling of up to five individuals, and access to 384 well configuration on validated open RT-PCR systems. In addition, through expanded authorization with HealthPulse@Home, we are now able to offer sample collection at home and elsewhere, increasing access to molecular testing. In February 2022, we also achieved MHRA authorization for RNA STAR Complete, enabling us to fulfill customer demand in the U.K. for high throughput, high sensitivity and high efficiency testing at events, schools, airports, and other public venues.
As of March 2022, LumiraDx Fast Lab Solutions, which supports high-complexity laboratory testing, has approximately 100 Fast Lab Network Laboratories in the U.S. that are running the RNA STAR Complete test within brick-and-mortar core laboratories, semi-temporary testing pods, mobile laboratories, and remote self-collection services. The Fast Lab Network improves access to diagnostic testing through strategic placement of laboratories across the U.S. that can respond to the ever-changing needs of infectious disease testing with scalable capacity and rapid turnaround times. The goal of this network is to deliver laboratory quality results within 24 hours anywhere and provide rapid response to COVID-19 flare-ups/hotspots. In addition, this network was developed to provide immediate infrastructure when needed to respond to COVID-19 variants, along with potential future outbreaks and pandemics.
Tests for Additional Adjacent Markets / Applications
In addition to our in-house pipeline, we have a number of tests in development through our R&D collaborations with well-established diagnostic companies that have market-leading capabilities in specific disease areas or targets, such as infectious diseases, respiratory assays, enteric diseases and others, and we expect these to lead to multiple test launches in the near term.
73
Furthermore, we expect to investigate other areas of interest in the future such as allergy, toxicology, fertility, veterinary and in-patient hospital where we believe POC testing will improve patient experiences and outcomes.
Our Technology
Our Platform simplifies, scales down, and integrates the techniques used in central lab instruments, to provide a wide range of lab-comparable diagnostic tests on a single POC instrument.
Traditionally, POC companies start with a focus on a particular disease or test, then go on to design the chemistry, assay, and instrument to deliver that application. This approach has demonstrated limited scalability and resulted in a proliferation of instruments at the POC, with inconsistent performance, testing procedure and workflows.
We are taking a fundamentally different approach by developing a unique microfluidic test strip capable of accommodating all our assays in a single design architecture. Our multiple tests can be manufactured at scale and low cost on a single manufacturing system. In addition, we adopted principles from central lab instruments to design an instrument that accurately and reproducibly controls key operational parameters such as fluidic movement, mixing and signal measurement. The technology behind our test strip, Instrument, and connectivity solutions enable a high performance, high quality POC testing Platform at scale.
Our Instrument
Our proprietary Instrument is designed to provide similar methods and features as central lab instruments, such as fluorescent transduction, precise fluidic control and assay precision. It is set up to overcome sample matrix bias through measurement in a liquid free environment and is calibrated against the standard lab reference for applicable samples and tests. Similar to lab instruments, our Instrument has integrated controls of non- specific binding through surface coating or blocking agents. Designing our Instrument from the outset with lab instrument functionality has allowed for improved performance and high sensitivity in a single POC instrument and has enabled innovation across our Platform.
The Instrument performs its analysis when the test strip with applied sample has been inserted in the Instrument and the sample has reacted with the reagents within the test strip. The Instrument then measures the fluorescence in the read area of the test strip by means of spectrophotometer optics or through camera-based particle counts. The Instrument quantifies the amount of analyte present in a sample applied to a test strip using enzyme, immunoassay, molecular or other analytical test principles. The concentration of the analyte in the sample is proportional to the fluorescence detected. The results are displayed on the Instrument touch-screen generally in under 12 minutes. The Instrument provides visual and audible instructions on the Instrument touchscreen to guide the user through the test process. After sample application, the test strip is automatically processed through all stages of the assay including sample movement, sample treatment, reagent interaction, thermal control, assay timing, sample removal and fluorescence measurement reading of the reaction products to provide calibrated qualitative or quantitative assay results. Calibration data for each set of test strips is included in an RFID tag embedded in each box of test strips. For new product launches, the RFID tag also contains the instructions to run new tests.
Given the continuous manufacturing and technological advances, some of our tests in development and future tests that we plan to develop may require an updated version of the Instrument or we may encounter design challenges, which may impact the specifications or commercialization of certain diagnostic assays. We will continue to develop and upgrade our Instrument, including the development of a robust and lower cost version for additional care settings.
Our Test Strip
Our proprietary test strip runs on our Instrument, with a specific and precise microfluidic sequence for each individual assay.
All test strips have certain common design features that allow various assay types to be analyzed, controlled and measured by the Instrument. In addition, the test strips also provide flexibility with regard to internal fluidic/ channel adjustments, enabling adjustments to be made based on the needs of a specific assay, while continuing to be able to interface with the same instrument. In addition, our proprietary manufacturing approach allows all test strips to be produced on a single high speed, high volume, low cost manufacturing system. The simple test strip design uses two main components and easy to source materials common across all our tests. The flexible test strip design further allows for multiple test channels to operate independently within the same test strip, thus allowing one test strip to cover multiple parameters and to enable syndromic panel testing. All assays are designed and tested against current laboratory standards.
74
Our Connectivity Solutions
Our connectivity solutions are designed to ensure easy, secure transfer of data and can be used with mobile, tablet or personal computers to move data from our Instrument.
•Connect Manager is our cloud-based service that provides all capabilities to remotely manage and configure Instruments as well as user access. It has provisions to manage quality control policy, simplify workgroups, produce reports and run data analyses. The ability to manage a large-scale implementation of POC instruments through Connect Manager enables a health care system or large procurer to enforce quality controls policy and effectively manage diagnostic results wherever they are generated in a centralized controlled manner.
•EHR Connect allows for direct integration with existing hospital systems either via cloud or local connectivity. Local connections can be made directly via integration protocols such as HL7, FHIR, GDT/LDT/XDT or through middleware.
Our connectivity solutions enable optimal performance of a POC program. The benefits include:
•management of operators and role-based access, Instrument, quality control policy and training information to meet regulatory and compliance requirements;
•reporting of patient demographics, test results, Instrument function and errors; and
•analytics, including surveillance and hotspot detection.
Quality Controls
Numerous quality controls have been integrated in the design of our Platform from the start to ensure regulatory quality compliance and ease of use. Our Platform is designed with more than 30 automated checks of the correct functioning at power on and during operation, including (i) electrical component operation such as heater operation, battery charge state, mechanical actuators and sensors and optical system performance, (ii) test strip positioning, optics, hematocrit (for blood samples) and checks around test strip expiry and (iii) monitoring of test strip performance and controls during test runtime.
Our Amira System
We have also adapted and simplified our microfluidic technology and next generation point of care testing system for use in non-healthcare settings and applications such as mass screening as well as home testing. The pandemic has highlighted the need for fast, accurate testing in the workplace, in schools, and in the home where it is much more convenient for consumers to access. We believe this trend will continue and that the opportunities are not fully addressed by our Platform. Therefore we view our Amira System as complementary and additional to our Platform strategy, and critical to transforming community-based care. The Amira System uses the same microfluidic strip design and manufacturing process, the same reagents, the same sample management and detection method, and the same workflow as the Platform, but is designed to operate a more targeted menu of tests on a small device and data management system, for self-testing.
The Amira System, initially being developed for COVID-19 testing, consists of (i) a small, battery operated, disposable device, (ii) high sensitivity Amira COVID-19 test and components as well as (iii) a phone/tablet application for test management and reporting. The value proposition of our Amira System is to provide additional testing opportunities for mass screening applications at schools, universities, work screening and testing in the home.
Our first assay on the Amira System, a high sensitivity COVID-19 antigen test, is in late stage development and is scheduled to design freeze and enter clinical studies within the next month. We currently have a prototype Amira System including strips, device and patient application and have begun submitting patent applications. We expect to complete POC clinical testing and achieve CE Mark during H1 2022. We plan to complete US OTC and POC prospective clinical studies and submit our FDA EUA by mid-year 2022.
We currently expect to launch our Amira System by the summer of 2022, with a test strip manufacturing capacity building up to 300 million tests per month over time and capability of producing many more for our Amira System, depending on market need for mass screening testing, subject to successful development and regulatory approval, authorization, certification or clearance. We anticipate the retail price of our Amira System and Amira COVID-19 test to be between $2.00-$4.00 per test, significantly lower than many existing COVID-19 tests currently on the market as well as the equivalent tests on our Instrument.
75
Similar to flu, we believe the sustained COVID-19 testing demand will be met by high sensitivity, device-based point of care tests and that the Amira System will perfectly meet this need. We believe the Amira System, with its high sensitivity, low cost and patient application feature that enables digital interpretation, management and reporting of test results, will be the right testing solution for large scale testing for COVID-19. Its low price point will allow global market access to testing at the scale required for a sustainable pandemic response and recovery.
The Amira System is also the basis of our home testing platform, which we plan to develop a menu of tests to aid in the screening, diagnosis and monitoring of chronic disease such coagulation, COPD, cardiovascular disease and diabetes. In the future, we see opportunities for the Amira System to support mass screening for global and public health priorities such as HIV screening, malaria and dengue.
Market Segments
LumiraDx has a customer focused growth strategy with product roadmaps and partnerships in three key market segments that provide community-based care.
Physician Office/ Retail/ Pharmacy
The physician office, retail, pharmacy market segment is focused on individuals seeking primary care, wellness, urgent care, as well as chronic disease management. It is a rapidly growing segment with individuals looking for faster and more convenient ways to receive their healthcare. The key unmet needs in diagnostic testing are broader menu of tests offered at POC, simplification of POC workflow and instruments, as well as lower cost of ownership. Our three year product roadmap in this segment includes tests for respiratory disease, diabetes, cardiovascular disease, coagulation, and sexual health.
Acute/ Emergency Care
The acute and emergency care market is focused on individuals seeking urgent care for severe and potentially life-threatening conditions. The key unmet needs in diagnostic testing are availability of fast, accurate and easy to use tests at point of care for cardiac disease, respiratory disease, metabolites, blood gases, etc. Our three year product roadmap in this segment includes tests for cardiac disease, metabolites, respiratory disease, and hospital acquired infections.
76
Global Health
The global health market segment encompasses testing for TB, HIV, malaria and maternal and childhood care in developing countries particularly those in sub-Saharan Africa. The key unmet needs in diagnostic testing are the availability of affordable and accurate point of care tests to support primary care as well as public health priorities such as TB and HIV management. LumiraDx has worked with The Bill & Melinda Gates Foundation to provide more than 5,000 platforms across Africa. Our three year product roadmap in this segment includes tests for TB, HIV, malaria, and maternal and childhood care.
|
|
|
|
|
Physician Office / Retail / Pharmacy |
Acute / Emergency Care |
Global Health |
Install Base |
~7500 |
~4500 |
~5000 |
Commercially Available |
INR, D-Dimer, COVID-19 Antigen, COVID-19 Antibody, CRP, COVID-19 + Flu A/B |
D-Dimer, COVID-19 Antigen, COVID-19 Antibody, COVID-19 + Flu A/B |
COVID-19 Antigen, CRP, COVID-19 +Flu A/B |
2022-2023 Launch |
RSV + COVID-19 HbA1c Na, K Hemoglobin, Strep A, NT-proBNP |
RSV + COVID-19 HS Troponin Na, K Hemoglobin, NT-proBNP |
TB , Hemoglobin, HbA1c |
3 year road map |
Sexual Health Diabetes Cardiovascular disease Respiratory |
Cardiac Respiratory Hospital Acquired Infection |
Virology Vector Borne Disease |
Research and Development
We focus our R&D efforts on conceiving and delivering disruptive technologies both at the platform and individual test level. We have invested, and continue to invest, significant time and resources toward improving and expanding our core technologies and tests. We have developed an automated high-speed manufacturing system which can produce a large volume of tests at low cost as well as proprietary technology around our Instrument based on central lab instruments. We are developing a wide variety of new diagnostic tests to be delivered at the POC, with over 30 assays in various stages of development. Our R&D expenses were $107.5 million and $130.2 million for 2020 and 2021, respectively. As of December 31, 2021, we had 451 employees focused on R&D.
In addition to our current pipeline, we plan to dedicate resources to continue our R&D efforts to complete development of our Amira System and populate and accelerate delivery of tests on our Platform. We have a strong internal R&D team focused on Platform-specific matters, including development of the Instrument, manufacturing and specific assays, technologies and conditions. We have also done extensive work over the last few years on Instrument and test strip development which generally benefit additional product development and over time reduce the timelines for feasibility and early stage development, as reflected by the work on our Amira System. Our internal R&D team is highly skilled both technically and scientifically, with many of our employees having advanced degrees in science or engineering.
To allow for additional tests to be developed on our Platform in parallel, we have entered into R&D collaboration agreements with diagnostic companies that have market-leading capabilities in specific conditions or targets and have considerable knowledge of such areas, relationships with key opinion leaders, and access to sample banks and testing. Our R&D collaboration agreements provide for a royalty payment of a certain percentage (typically low- to mid-single digits) of the net sales of any co-developed test products that incorporate the collaborators’ technology or chemistry. We are obligated to make such royalty payments only following the launch of a test product and once revenue has been generated. We have not made any royalty payments under our R&D collaboration agreements to date. Development expenses are typically our responsibility in accordance with an agreed-upon budget, which allows us to monitor and cap such expenses. We reserve the right under our R&D collaboration agreements to continue internal development in parallel on the same test candidates as our collaborators or approach new collaborators; therefore, the agreements are not exclusive.
77
Our collaborations are set up with a uniform approach and standard operating procedures allowing for roll out across multiple collaborators simultaneously. We provide our collaborators with training, an R&D pilot process and equipment. We also provide input on test strip design and printing materials so that our R&D collaborators can leverage our expertise without the need to establish capabilities at their local sites. Once chemistry has been optimized, our collaborators transfer the assays to us for manufacturing. Under these collaborations, we maintain ownership and manufacturing of the tests; collaborators receive R&D funding for development efforts and a royalty for any tests incorporating their proprietary technologies. We currently collaborate with third parties for development of tests covering several disease areas.
In addition, we collaborate closely with scientists, industry experts and key opinion leaders for input and review of our tests. We have established a substantial network of industry leading subject matter experts most often centered around specific conditions and geographies. These collaborators often set the standard for methods and guideline for care providers globally. These relationships are also important for educating the market on the value we offer and for developing innovative testing methodologies enabled by the unique attributes of our Platform.
Manufacturing
We started our product development with a focus on Platform manufacturability and scalability and built upon this with robust instrument architecture, unique chemistry and low-cost test strip designs. Since our inception through December 31, 2021, we have incurred $114 million in costs related to facilities, manufacturing and other equipment.
We believe our automated manufacturing process provides an industry leading cost position that will enable us to provide more cost-efficient diagnostic solutions to the POC market and create new opportunities for the adoption of POC testing in geographies and testing locations where high quality POC testing has not previously been feasible.
We manufacture our test strips on highly automated, manufacturing equipment designed and manufactured specifically for us. All of our test strips are manufactured on a common platform using a high volume, web-based manufacturing process that allows the production of multiple test strip sizes and designs. Utilizing a common platform allows us to leverage volume and have efficient manufacturing costs and provides flexibility to respond more rapidly to changing market demands across our product portfolio.
Our key web-based manufacturing equipment provides an end-to-end solution from input rolls to fully foiled test strips with 100% vision inspection of more than a dozen critical to quality elements. Each test strip is printed with a unique test strip ID (2D bar code) for traceability and quality control.
Our manufacturing facilities are located in Alloa, Scotland, Stirling, Scotland, Glasgow, Scotland, Bathgate, Scotland and San Diego, California. Our current Scotland sites have 246,500 square feet of space dedicated to R&D, manufacturing, packaging and warehousing. Our San Diego sites have 39,800 square feet of space dedicated to R&D, manufacturing and packaging. As of December 31, 2021, we had 604 employees focused on manufacturing and service delivery.
Our Instrument is manufactured by a contract manufacturer, Flextronics, at its facility in Althofen, Austria with components and assemblies supplied by Flextronics and by outside vendors. Our SARS-CoV-2 RNA STAR molecular test kits are manufactured and packaged at our facility in San Diego, California.
78
Sales and Marketing
As of December 31, 2021, we have 201 employees focused on sales and marketing located in 21 countries and plan to open additional sales offices to further expand our presence globally. We have direct sales operations in the U.S., most Western European countries, Japan, India, South Africa, Colombia and Brazil. The organization consists primarily of individuals with multiple years of experience in diagnostics, specifically POC testing, with large diagnostic companies, such as Abbott Laboratories, Roche Holding AG and Danaher Corporation, and many others.
We drive the penetration of our installed base by executing an institutional sales and channel partnership model. We initially intend to focus our sales efforts on large healthcare systems, government organizations and national pharmacy chains that want to deploy comprehensive POC testing across their networks. We have an experienced commercial team focused on leveraging key customers to deploy our Platform across multiple users with our training and support. To drive sales in additional healthcare settings, we will identify key channel partners that are interested in standardizing around a POC testing platform to integrate care across their networks.
Our commercial strategy will vary by health system, but key opinion leaders and certain key accounts will provide important initial clinical evidence of the performance of our Platform and support and validation for the beneficial uses of the technology.
Over time, we plan to operate with a direct sales and marketing presence in each of the largest diagnostic markets and to collaborate with distribution partners and medical wholesalers to ensure broad access to our Platform globally, for example in China and other countries in Southeast Asia. We believe in-country sales and marketing presence enables us to engage in direct sales with large customers, understand local market dynamics and trends, and most effectively deliver localized marketing campaigns. We currently sell our Instruments or place them through reagent rentals.
Competition
The POC market is rapidly evolving, highly competitive for certain product areas and subject to changing technology, shifting client needs and the frequent introduction of new products and services, such as the market demand and introduction of new products for testing of COVID-19. Our competitors range from well-established multinational corporations with multiple product offerings to smaller regional firms and firms with specific disease state offerings. A broad menu of tests available on our Platform will make it more challenging for a single competitor to displace our Platform once installed at a customer location. We believe that our Platform competes on the basis of clinical performance, enhanced turnaround time, test menu breadth on a single instrument, lower cost and return on investment, ease-of-use, seamless connectivity, and multiplex capability.
We primarily face competition in the IVD market from public and private companies such as Abbott Laboratories, Becton, Dickinson and Company, Danaher Corporation, GenMark Diagnostics, Inc., Laboratory Corporation of America Holdings, Quest Diagnostics Incorporated, Quidel Corporation, Roche Diagnostics Corporation, Siemens Healthineers AG, Inc. and many others. For each of our eight available tests, we face competition from other commercially available tests, including:
•For our SARS-CoV-2 antigen test and SARS-CoV-2 antigen pool test: Lateral flow tests such as Quidel Sofia, BD Veritor Plus System, Abbot BinaxNow COVID-19 Ag Card and others, as well as molecular tests such as Cepheid GeneXpert, Abbott ID NOW and lab-based PCR tests.
•For our SARS CoV-2 antibody test: Accelerate Diagnostics BioCheck, Assure Rapid Test, SD Biosensor Q Rapid Test, general lateral flow tests and others.
•For our INR test: Roche Coaguchek and others.
•For our D-Dimer test: Roche Cobas h232, Quidel Triage and others.
•For our SARS-CoV-2 & Flu A/B tests: Quidel Sofia, BD Veritor Plus System, Cepheid GeneXpert and others.
•For our CRP test: Abbott Afinion, Aidian QuickReadGo, and others.
Our tests in development are designed and validated against their respective lab standard.
79
Strategic Partners and Manufacturing and Supply Agreements
Bill & Melinda Gates Foundation
Since 2018, we have collaborated with BMGF with the goal of achieving the following key objectives:
•developing assays with significant potential to improve global health, including a test for HIV viral load;
•accelerating development of LumiraDx’s next-generation instrument that is low-cost, robust and appropriate for all clinical settings, globally; and
•supporting local healthcare delivery partners in foundation-priority markets to incorporate the potential of LumiraDx technology into high-impact, low-cost disruptive diagnostic treatment models.
BMGF has provided funding and support to LumiraDx in support of these objectives through a combination of equity, grants and loans, as further described below.
Cooperation Agreement
We are a party to a cooperation agreement with BMGF, dated July 17, 2018, or the Original Cooperation Agreement, which was amended and restated on October 17, 2019. We entered into the Original Cooperation Agreement in connection with BMGF’s investment of $20.0 million in the Company through its purchase of 3,466,320 LMDX series A preferred shares at a price per share of $5.77. We subsequently entered into an amended and restated Cooperation Agreement with BMGF on October 17, 2019, or the Cooperation Agreement, in connection with BMGF’s loan to the Company in the amount of $18 million, the terms of which are further described in the section of this Annual Report titled “Item 4.B. Business Overview—Strategic Partners and Manufacturing and Supply Agreements—Bill & Melinda Gates Foundation—Note Purchase Agreement” beginning on page 82.
The primary purpose of BMGF’s investments are to further its charitable purpose of, among other things, accelerating the development of lifesaving and low-cost diagnostics to reduce the burden of diseases in low and middle income countries by securing global access commitments in respect of availability and affordability of new, low-cost products and services developed through the use of our capabilities, including in respect of diagnostic tests, integrated POC diagnostic platforms and connected health IT and care solutions.
Under the terms of the Cooperation Agreement, we agreed to the following global access commitments: (i) to develop an assay for HIV viral load and the development of a manufacturing, commercialization and distribution strategy for delivery of POC diagnostics within low and middle income countries, including the HIV viral load assay, or the HIV Viral Load Assay Development Project, (ii) to provide the foundation with an option to continue to provide funding to advance the HIV viral load assay through commercialization and launch, or the HIV Viral Load Assay Launch Project, (iii) to provide the foundation with the option to fund up to five additional assay projects that would use our Platform, or the Additional Assay Projects, (iv) to provide the foundation with the option to fund a project to accelerate commercialization of the Company’s products in certain specified low and middle income countries (with a focus on sub-Saharan Africa), (v) to conduct certain activities in respect of partnering with health systems for low income people in challenging markets and to use good faith efforts to prioritize development of mutually agreed diagnostics and to pursue registration by the World Health Organization as a provider of tests on the WHO Essential Diagnostics Lists or other diagnostic prequalification activities that support commercialization of the Platform in certain low and middle income countries, and (vi) to develop a next-generation diagnostic instrument that is low-cost, robust and appropriate for all clinical settings and intended to improve the access of people in low and middle income countries to low cost POC diagnostics, or the V7 Diagnostic Instrument.
In connection with the HIV Viral Load Assay Development Project, HIV Viral Load Assay Launch Project, and any Additional Assay Projects, we have agreed to certain other global access commitments, including maximum pricing and minimum volume commitments (directed at making such products available to low income people in low and middle income countries at an affordable price) for the assays developed pursuant to such projects. In addition, we have agreed to make the V7 Diagnostic Instrument available to serve low income people in certain low and middle income countries at an affordable price. Our global access commitments under the Cooperation Agreement are ongoing for as long as BMGF exists.
Subject to certain conditions being met, if BMGF reasonably determines that the Company is unlikely to achieve prices and volumes for the HIV viral load assay that are within 20% of the maximum price and minimum volume commitments described in the target product profile agreed by the parties and a third-party would be likely be able to meet such commitments, such third-party may further develop the project and manufacture any resulting products. In such case, to
80
facilitate such development, we would be obligated to either license and transfer our technology to such third-party as needed to enable such third-party’s production, testing, approval and distribution of the HIV viral load assay in the relevant low and middle income countries.
In connection with the projects described above, we granted to BMGF (and/or BMGF supported entities) a worldwide, non-exclusive, non-terminable, perpetual, royalty-free license (with rights to sublicense) to the products, technologies, materials, processes, and other intellectual property and intellectual property rights developed in connection with such projects and our background intellectual property that covers or is used in our Platform’s technology and/or such projects to further develop the projects and manufacture any resulting products in a manner consistent with BMGF’s charitable purpose, or the Global Health License. BMGF has agreed not to exercise its rights under the Global Health License until one of the following “Trigger Events” occurs; (i) a Charitability Default (described below) or (ii) we institute certain bankruptcy, insolvency or similar proceedings or we cease to conduct business in the ordinary course or are no longer a going concern.
In connection with the exercise of the licenses described above, we have agreed to take further actions, including technology transfer as would be commercially reasonable industry practice at the time to accommodate that BMGF, its sublicensees or the relevant BMGF-supported entity can effectively exercise such license and use the related technology and manufacture the relevant products.
A Charitability Default occurs if we (i) materially breach the global access commitments, which includes the failure to conduct the projects as described in the Cooperation Agreement, (ii) misuse the proceeds provided by BMGF, or (iii) fail to comply with certain U.S. legal obligations. Upon written notice that a Charitability Default has occurred, we have a 120-day cure period. If we fail to cure the default, at BMGF’s request we are obligated to (i) redeem (or cause a third-party to purchase) any of our securities that BMGF owns, and (ii) pay to BMGF the entire unpaid principal and accrued and unpaid interest on any outstanding loans from BMGF.
BMGF is entitled to appoint one director to our board and to designate one person to attend all meetings of the board in an observer capacity and receive all documents and materials that are provided to each director. BMGF’s previous board appointee, Amit Thakker, M.D., resigned from our board of directors with effect from April 30, 2021. Dr. Thakker’s resignation was not due to any disagreement with LumiraDx, CAH or any matters relating to the Company’s operations, policies or practices. BMGF has not exercised its right to appoint a replacement director, but retains its right to do so.
Feasibility Grant
We are also a party to a grant agreement with BMGF dated November 5, 2019, or the Grant Agreement, which sets out the terms for us to carry out the Additional Assay Projects described in the Cooperation Agreement. The purpose of the project is to accelerate the development and feasibility assessment of POC diagnostic tests, including a low-cost active tuberculosis test, sickle cell disease test, and three tests for use in maternal and antenatal care. We have agreed to conduct and manage the project in a manner that is consistent with our commitment to disseminate knowledge and information relating to the project and increase availability and accessibility of point of care diagnostic tests. Under the terms of the Grant Agreement, BMGF has granted us $8.0 million. The proceeds of the grant must not be used for any purpose other than the project, and no more than 20% of the proceeds may be used for activities in the U.S. Any funds not used during the course of Grant Agreement must also be returned to BMGF upon expiration or termination of the Grant Agreement. Should any individuals receive compensation paid in whole or in part from the grant proceeds in return for their work on the project, we will track and record the actual time worked by these individuals. We are required to apprise BMGF of our progress against the targets and/or milestones mentioned above.
The parties have agreed to extend the feasibility grant until June 30, 2022, at which time the feasibility activities will have been completed. The parties are currently discussing continuation of work post feasibility. BMGF may terminate the Grant Agreement if (i) BMGF is not reasonably satisfied with our progress on the project, (ii) there are significant changes to our leadership or other factors that BMGF reasonably believes may threaten the success of the project, (iii) there is a change in our control, (iv) there is a change in our tax status, or (v) we fail to comply with the terms of the Grant Agreement.
BMGF-COVID-19 Support Grant
In October 2020, BMGF, with support from Strive Masiyawa, The Rockefeller Foundation and Mastercard Inc., agreed to provide additional grant funding to secure access to a significant number of Instruments and test strips for supply into LMICs. These Instruments and SARS-CoV-2 antigen tests were subsidized by BMGF for deployment in Africa from the third quarter of 2020 to first quarter of 2021. The grant includes an initial commercial implementation plan for roll out in
81
selected countries in Africa. Additional support would be provided by selected procurement partners. The grant includes a similar global access commitment as other earlier grants entered into. In March 2021, BMGF provided an additional grant so secure access to additional SARS-CoV-2 testing so LMICs (which have received instruments) can continue testing at a subsidized rate by the BMGF.
BMGF-Manufacturing Support Grant
In November 2020, BMGF agreed to provide an additional grant to secure access to our strip manufacturing capacity. The grant provides BMGF access to a maximum volume of 50 million strips for 2021 and 150 million strips per year from 2022 for distribution and sale in LMICs/Africa, with flexibility in product mix as forecasted on a quarterly basis. Any test strips and Instruments not covered under the quarterly forecasts or not ordered shall no longer be required to be supplied Instruments to LMICs. Test strips and Instruments would be made available at affordable pricing as agreed between the parties.
Note Purchase Agreement
We have borrowed $18.0 million from BMGF pursuant to a note purchase agreement dated October 17, 2019, or the unsecured loan. The unsecured loan is evidenced by an unsecured subordinated promissory note, accrues interest at the rate of two percent per annum and is subject to default if certain commitments made in the Cooperation Agreement relating to the development of the V7 Diagnostic Instrument are not met, in addition to customary events of default. Unless otherwise extended and subject to any event of default (as detailed in the note purchase agreement), payments are due quarterly and the unsecured loan matures on October 15, 2024.
The indebtedness evidenced by the promissory note ranks (i) pari passu in right of payment to the 5% notes and the 10% notes), and (ii) subordinated to all amounts owed to the holders of senior indebtedness (as described in the note purchase agreement).
While the promissory note is outstanding, we have agreed that we will not pay certain distributions, dividends or undertake returns of capital, without the prior consent of BMGF, among other courses of action detailed in the unsecured loan.
CVS Exclusivity Agreement
On August 3, 2018, we entered into an agreement with CVS, or the CVS Exclusivity Agreement, pursuant to which we granted to CVS the exclusive right to purchase and use our Platform (or any components thereof, including the embedded software) in medical clinics located in retail stores and retail pharmacy businesses, or the Exclusive Field, in the United States and its territories, or the Territory. Such exclusivity continues until one year following the date on which we provide to CVS notice that: (i) we have launched at least one of specified products listed in the CVS Exclusivity Agreement, which does not include COVID-19 tests, for use with our Platform; (ii) all regulatory approvals have been obtained to perform and commercialize the relevant product(s), and (iii) we are in a position to supply commercially reasonable quantities of the Platform under mutually agreed supply and quality agreements, or the Exclusivity Period. We have not yet provided such notice to CVS.
We have agreed to use our commercially reasonable efforts to obtain the regulatory approvals (on a CLlA-waived basis) required for the commercialization of several of the products listed in the CVS Exclusivity Agreement in the Exclusive Field in the Territory within the first 24 months from August 2018. Due to the onset of the COVID-19 pandemic, our primary focus has been on obtaining the approvals required to commercialize our SARS-CoV-2 tests and our other research and development programs have experienced delays. Therefore, obtaining certain of the approvals set out in the CVS Exclusivity Agreement will be delayed.
Prior to the launch of the first commercial supply of products to CVS, we and CVS are obligated to negotiate in good faith the terms of a supply agreement and quality agreement. The supply agreement will grant CVS a license to sell and provide commercial testing services to its customers using the Platform in the Exclusive Field and Non-Exclusive Field in the Territory. Such license will expire at the end of the Term (as defined below). We retain ownership of all intellectual property developed under the CVS Exclusivity Agreement, including rights in any new features and functionality developed for the Platform, as well as all regulatory filings that relate to the Platform.
The CVS Exclusivity Agreement will expire at the end of the Exclusivity Period, or the Term, unless earlier terminated. CVS may terminate the agreement at any time by providing us with written notice. The CVS Exclusivity Agreement may also be terminated by either party: (i) if the other party materially defaults in the performance of its duties, subject to a 90-day cure
82
period; (ii) if there is a material breach of the agreement or the supply agreement that cannot be cured; (iii) if the other party becomes insolvent; or (iv) if there is a force majeure event and the other party cannot perform its duties under the agreement within three months of the event.
CVS Purchase Agreement
On August 14, 2020, we entered into a purchase agreement with CVS, or the CVS Purchase Agreement, pursuant to which we committed to make a minimum monthly quantity of our Instrument and SARS-CoV-2 test strips, as well as ancillary equipment such as collection supplies necessary to administer the SARS-CoV-2 antigen and antibody tests, available to CVS in the United States. If we fail to meet these commitments, it would constitute a breach of the CVS Purchase Agreement. Our initial minimum monthly commitment to CVS was a significant portion of our supply through to the end of 2020. CVS is not obligated to make any minimum purchases under the CVS Purchase Agreement. Any purchases made by CVS are subject to the terms of the CVS Purchase Agreement, as amended. Subject to certain conditions being met, we have also agreed that CVS will receive the best price for products covered as compared to any of our other POC customers in the U.S., other than U.S. Federal or state government agencies. In January 2022, we extended this agreement until December 31, 2022.
All obligations under the CVS Purchase Agreement are subject to us obtaining and maintaining the regulatory approvals required for the use of our Instrument and SARS-CoV-2 test strips in patient care settings outside of the clinical laboratory environment.
Subject to certain termination provisions, the CVS Purchase Agreement, as amended, continues through December 31, 2022 and may be renewed or extended for an additional period of time by written agreement of the parties. CVS may terminate the CVS Purchase Agreement without cause upon 30 days’ notice. In addition, the CVS Purchase Agreement may be terminated (i) by either party if there is a material breach which remains uncured for 30 days, (ii) by either party in the event of the other party’s insolvency or receivership, or (iii) by CVS if we fail to obtain and maintain any required FDA approvals or authorizations. If CVS terminates the CVS Purchase Agreement for cause, CVS may return any unused products to us and receive a full refund of any amounts paid.
Flextronics—Manufacturing Services Agreement
On October 18, 2017, we entered into a manufacturing services agreement, or MSA, with Flextronics, pursuant to which Flextronics has agreed to perform manufacturing, assembling and testing services, and to procure and supply the relevant materials, for the production of our Instrument.
The MSA had an initial two-year term and now automatically renews for successive one-year terms. Either party may terminate the MSA upon six months’ written notice to the other prior to the end of the then-current term that it does not intend to renew the MSA at the end of the term. The MSA may also be terminated by either party if: (i) the other party defaults on any payment, subject to a 14 day cure period, (ii) the other party materially defaults in its performance of its duties under the MSA, subject to a 30-day cure period, (iii) the other party becomes insolvent; or (iv)there is a force majeure event and the other party cannot perform its duties under the MSA within 90 days of the event.
NHS Arrangement
Since September 2020, we have provided our POC SARS-CoV-2 antigen tests to the NHS in the U.K. Under our agreement, the NHS has the right to make reoccurring purchases of our SARS-CoV-2 antigen tests based on their needs and requirements.
83
Intellectual Property
We strive to protect the proprietary technologies that we believe are important to our business, including pursuing and maintaining patent protection intended to cover our Platform technologies, including our Instrument and test strips, and the use of the foregoing in clinical assays, and other inventions that are important to our business. We also protect other valuable aspects of our business as confidential know-how, and if eligible, as trade secrets.
Our commercial success depends in part upon our ability to obtain and maintain patent and other protection for commercially important technologies, inventions, trade secrets and know-how related to our business; defend and enforce our intellectual property rights, particularly our patent rights; preserve the confidentiality of our trade secrets and know-how; and operate without infringing upon the intellectual property rights of others.
The patent positions for diagnostic companies like ours are generally uncertain and can involve complex legal, scientific and factual issues (see, for example, the section of this Annual Report titled “Item 3.D. Risk Factors—Intellectual Property Risks Related to Our Business” beginning on page 33). In addition, the regional or national patent offices worldwide can require the scope of claims pending in a patent application to be significantly reduced or otherwise changed in order to obtain grant of a patent; and the scope, meaning, validity, and/or enforceability of granted claims can be challenged in a variety of proceedings. As a result, we cannot guarantee that any of our Platform technologies, including our Instrument, test strips, and clinical assay products will be protected or remain protectable by enforceable patents. We cannot predict whether any particular patent application that we are currently pursuing in any particular jurisdiction will be granted as a patent or whether the claims of any patents we obtain will sufficiently exclude competitors from making, using, or selling our inventions. Nor can we guarantee that third parties will not circumvent our patent claims by designing around them.
Our patent portfolio consists of patent families assigned to our subsidiaries LumiraDx UK, Ltd. and SureSensors Limited, and includes granted U.S. and ex-U.S. patents, pending provisional or priority applications filed in the U.S. or the U.K., pending U.S. and ex-U.S. patent applications that are undergoing or will undergo substantive examination, and applications filed under the Patent Cooperation Treaty, or PCT, from which we will be able to pursue regional or national phase patent applications that will be subject to substantive examination. We will continue to file additional patent applications as we deem appropriate and of commercial value.
As of March 22, 2022, our patent estate includes at least 10 U.S. patents, at least 100 foreign patents, 13 pending U.S. non-provisional patent applications, four pending PCT patent applications, at least 60 pending foreign patent applications, and two pending U.S. provisional applications relating to our Platform technologies, clinical assays, Amira System, and related technologies, for example, assay formats or protocols that we may implement on our test strips. Patent applications in eight patent families are discussed in more detail below and relate to more current aspects of our Platform technologies, clinical assays, and Amira System. We have granted patents in Argentina, China, Europe, Hong Kong, Japan, and the U.S. in the first patent family and a granted patent in Europe in the fourth patent family, and we will continue to seek patent protection in other jurisdictions for these families, as well as for the other patent families as appropriate. Some of these patent families are still at an early stage of examination or have not yet received substantive examination.
We own at least five families of patent applications, at different stages of filing and prosecution, directed to our Platform technologies, that seek to protect various aspects of our Instrument, test strips, and other technologies generally applicable to our various strip assays. The first patent family has a granted U.S. patent, a granted European patent (which has been validated in the U.K., Germany, France, Ireland, and Italy), a granted European divisional patent and granted Argentinian, Chinese, Russian, South African and Hong Kong patents, and applications pending in the U.S., Canada, Mexico, and a number of countries in Asia; the claims in this family are directed to various aspects of our Instrument and test strips. The term of the validated patents in the U.K., Germany, France, and Italy and the granted Russian and Hong Kong patents will expire, and the term of any patents granted on the noted applications would expire in 2037, in each case subject to the timely payment of the requisite annuities or other renewal fees. A second patent family contains a U.S. application, and regional or national applications in Canada, China, Europe and Japan; the claims in this family are directed to certain aspects of the magnetic capture technologies implemented in our Instrument. The terms of any patents resulting from such regional or national stage applications would expire in 2039, in each case subject to the timely payment of the requisite annuities or other renewal fees. A third patent family contains pending PCT, U.K., and Taiwanese applications directed to additional features of our Instrument and test strip technologies, including specific embodiments of our SARS-CoV-2 tests. We expect to pursue one or more regional or national stage applications based on this PCT application; the terms of any patents resulting from such regional or national stage applications would expire in 2041, in each case subject to the timely payment of the requisite annuities or other renewal fees. A fourth patent family directed to our STAR nucleic acid amplification system has a granted European patent (validated in the U.K., Germany, Ireland, France, Italy, Spain and the Netherlands) which is under
84
opposition, and granted patents in Hong Kong, Japan, Taiwan and Russia and pending applications in the U.S., other North American countries, Argentina, Australia, Brazil, and countries in Asia. The term of the validated patents in the U.K., Germany, Italy, Spain, Ireland, and the Netherlands will expire, and the term of any patents granted from the pending patent applications would expire in 2037, in each case subject to the timely payment of the requisite annuities or other renewal fees. A fifth patent family directed to our qSTAR nucleic acid amplification system has a granted patent in Europe (validated in Germany, France, Ireland, Italy, Spain, Switzerland and the U.K.) and patent applications pending in the U.S., Canada, the European Patent Office, South America, South Africa, Australia, and countries in Asia. The term of any patents granted from these applications would expire in 2039, in each case subject to the timely payment of the requisite annuities or other renewal fees. We plan to seek patent protection for other aspects of our Platform technologies as they are developed.
With regard to our clinical assays, we have applied for and, if available, will continue to apply for, patent protection for the assays that will be implemented on our Platform. We have a sixth patent family containing pending patent applications in Canada, China, Europe, Japan, and the U.S.; the terms of any patents resulting from these applications would expire in 2039, in each case subject to the timely payment of the requisite annuities or other renewal fees. As noted above, the third Platform patent family seeks to protect certain features of our SARS-CoV-2 tests; any regional or national stage applications based on this PCT application that issue as patents would expire in 2041, in each case subject to the timely payment of the requisite annuities or other renewal fees. We have a seventh patent family containing pending patent applications in the U.S. and Europe directed to our HbA1c assay; the terms of any patents resulting from these applications would expire in 2040, in each case subject to the timely payment of the requisite annuities or other renewal fees.
We have an eighth patent family containing an International (PCT) application directed to our Amira System. The term of any resulting patents based on these filings would expire in 2041, in each case subject to the timely payment of the requisite annuities or other renewal fees.
85
The eight patent families, including exemplary jurisdictions where patent applications have been filed, exemplary subject matter being pursed in the applications, and expected expiration dates are summarized in the following table.
|
|
|
|
|
Family
No. |
Exemplary
Jurisdictions |
Patent/Application
and Status |
Exemplary Subject
Matter and Scope(1) |
Expiration(2) |
1 |
China, Europe, Russia and Hong Kong |
Granted European patent validated in France, Germany, Italy, Ireland, & U.K.; granted Chinese, Russian, South African and Hong Kong patents
Pending patent applications: Argentina, Australia, Brazil, Canada, Europe, Japan, Korea, Mexico, Taiwan, U.S. |
Assay system containing improved microfluidic cartridge and reader, and associated assay method – machine and method of use |
2037 |
2 |
Canada, China, Europe, Japan and U.S. |
Pending patent applications: Canada, China, Europe, Japan, U.S. |
Improved magnetic capture assembly for use in reader, associated reader and assay method – machine and method of use |
2039 |
3 |
PCT, U.K. and Taiwan |
Pending patent applications |
Improved assay methods and related cartridges (e.g., assays and cartridges for performing SARS-CoV-2 antigen or antibody tests) – method of use and article of manufacture |
2041 |
4 |
Europe and U.S. |
Granted European patent(3) validated in France, Germany, U.K., Ireland, Italy, Spain Switzerland and the Netherlands
Pending patent applications:
Argentina, Australia, Brazil, Canada, China, Hong Kong, Japan, Korea, Mexico, Russia, Singapore, Taiwan, U.S., South Africa |
STAR amplification – method of use |
2037 |
5 |
Europe and U.S. |
Pending patent applications: Argentina, Brazil, Canada, China, Europe, Hong Kong, Japan, Taiwan, U.S., South Africa |
qSTAR amplification – method of use |
2039 |
6 |
Europe and U.S. |
Pending patent applications: Canada, China, Europe, Japan, U.S. |
Kinetic assay for detecting an analyte such as an enzyme (e.g., PT/INR kinetic assay) and related cartridge – method of use and article of manufacture |
2039 |
7 |
Europe and U.S. |
Pending patent applications: Europe, U.S. |
HbA1c Assays |
2040 |
8 |
PCT |
International PCT patent application pending |
Amira System – method of use and article of manufacture |
2041 |
|
|
|
|
|
(1) |
The general types of subject matter for which patent protection is being pursued includes machines (e.g., a reader), articles of manufacture (e.g., assay strips), and methods of use (e.g., assay methods). |
(2) |
The expiration dates assume that non-provisional patent applications will be filed approximately one year after the earliest priority date and that national stage applications will be filed, as appropriate, and pursued until grant, and that all renewal and annuity fees will be paid. |
(3) |
An Opposition has been filed against our granted European patent covering STAR amplification. |
In most countries worldwide, the term of a utility patent expires 20 years from the earliest effective non-provisional filing date, subject to the timely payment of the requisite annuities or other renewal fees.
We protect other valuable aspects of our business as confidential know-how, and, if eligible, as trade secrets. For example, we protect certain aspects of our manufacturing processes as trade secrets. Although trade secret protection does not expire as long as the protected information is kept secret from the public, it can be challenging to maintain such efforts. We take business-reasonable steps to protect our trade secrets and other confidential proprietary information, including by physically
86
restricting access to our premises and physically and/or electronically securing our confidential information, as well as by requiring our employees, consultants, scientific advisors, contractors and commercial partners to execute non-disclosure agreements. However, third parties may independently develop the subject matter of trade secrets that we hold, in which case we have no remedy if such parties should use such subject matter in furtherance of their own commercial interests. Further, while the law may provide remedies against third-party misappropriation or other unlawful access to our trade secrets and other proprietary information, such remedies may be difficult to obtain in practice and may not make our business whole even if successfully obtained. As a result, we may be unable to meaningfully protect or derive the full benefit of our trade secrets and other valuable proprietary information.
Government Regulation
Our POC diagnostic system and tests are highly regulated IVDs. In addition, we are subject to a variety of regulations and industry standards worldwide governing, among other things, data privacy, distribution of our products and patents and trademark licensing.
The key U.S. and European regulations that are applicable to our business are discussed in more detail below. Whether or not we obtain FDA clearance or approval or a CE Mark for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the use of a diagnostic or other product in those countries. The requirements and processes governing patient consents, product registration and pricing vary from country to country.
U.S. Regulation
Our business is subject to and impacted by extensive and frequently changing laws and regulations in the U.S. at both the federal and state levels. These laws and regulations include those particular to our business and laws and regulations relating to conducting business generally. We also are subject to inspections and audits by governmental agencies. Set forth below are highlights of the key U.S. regulatory schemes applicable to our business.
In the U.S., medical devices, including IVDs, are subject to extensive regulation by FDA, under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations, and other federal and state statutes and regulations. The laws and regulations govern, among other things, medical device development, testing, labeling, storage, premarket clearance or approval, advertising and promotion, reporting, and product sales and distribution. To be commercially distributed in the U.S., medical devices must receive from FDA prior to marketing, unless subject to an exemption, either approval of a PMA (for most Class III devices), clearance of a 510(k) premarket notification or classification pursuant to a de novo submission.
IVDs are types of medical devices that can be used in the diagnosis or detection of diseases, conditions or infections, including, without limitation, the presence of certain chemicals, genetic information or other biomarkers. Predictive, prognostic and screening tests, such as carrier screening tests, can also be IVDs.
Emergency Use Authorizations
The Secretary of Health and Human Services may authorize unapproved medical products, including IVDs, to be marketed in the context of an actual or potential emergency that has been designated by the government. The COVID-19 pandemic has been designated such a national emergency. After an emergency has been announced, the Secretary of Health and Human Services may authorize the issuance of, and the FDA Commissioner may issue EUAs for the use of specific products based on criteria established by statute, including that the product at issue may be effective in diagnosing, treating, or preventing serious or life-threatening diseases when there are no adequate, approved, and available alternatives. An EUA is subject to additional conditions and restrictions and is product specific. For unapproved products authorized under an EUA, including our SARS-CoV-2 antigen test, such conditions on authorization include, to the extent practicable given the applicable circumstances described in an EUA declaration, the obligation to provide facts sheets for healthcare providers administering the product and product recipients, adverse event monitoring and reporting, and recordkeeping and reporting requirements by product manufacturers. FDA may also establish additional discretionary conditions of authorization that FDA deems necessary or appropriate to protect the public health, including conditions and restrictions related to product distribution and product administration and conditions with respect to data collection and analysis concerning the safety and effectiveness of the product. For example, the EUA for our SARS-CoV-2 antigen test contains as conditions of authorization, among other requirements, the requirement that we track adverse events, including any occurrence of false reports and to report such occurrences to FDA, the requirement that we complete a real-time stability study for the test and to notify FDA of the testing results as they become available until completion of the study, and the requirement that we complete flex studies for the product within four months of the date the EUA was granted. An EUA terminates when the emergency determination
87
underlying the EUA terminates. An EUA is not a long-term alternative to obtaining FDA approval, licensure, or clearance for a product. FDA may revoke an EUA where it is determined that the underlying health emergency no longer exists or warrants such authorization, so it is not possible to predict how long an EUA may remain in place.
FDA issued its Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency which described a policy for commercial manufacturers that seek to develop and distribute diagnostic test kits to detect the SARS-CoV-2 virus to clinical laboratories or to healthcare workers for testing. Unless and until an EUA is issued that authorizes additional testing environments for a specific test, under CLIA, use of that test is limited to laboratories certified to perform high complexity testing, including testing at the POC when the site is covered by the laboratory’s CLIA certificate for high-complexity testing. In light of the increasing numbers of COVID-19 cases throughout the country and the urgent need to expand the nation’s capacity for COVID-19 testing during the public health emergency, FDA has stated that it does not intend to object to a commercial manufacturer’s development and distribution of SARS-CoV-2 test kits for specimen testing for a reasonable period of time, where the test has been validated and while the manufacturer is preparing its EUA request, where the manufacturer gives notification of validation to FDA and where the manufacturer provides instructions for use. This same policy also applies for commercial manufacturers that seek to develop and distribute serology tests that identify antibodies to SARS-CoV-2 from clinical specimens. This policy does not apply to at-home testing, including at-home specimen collection.
FDA premarket clearance and approval requirements
The FDCA classifies medical devices into one of three categories based on the risks associated with the device and the level of control necessary to provide reasonable assurance of safety and effectiveness. Class I devices are deemed to be low risk and are subject to the fewest regulatory controls. Many Class I devices are exempt from FDA premarket review requirements. Class II devices, including some software products to the extent that they qualify as a device, are deemed to be moderate risk, and generally require clearance through the premarket notification, or 510(k), process in order to be commercially distributed. Class III devices are generally the highest risk devices and are subject to the highest level of regulatory control to provide reasonable assurance of the device’s safety and effectiveness. Class III devices typically require approval of a PMA by FDA before they are marketed. A clinical study is almost always required to support a PMA application and is sometimes required for 510(k) clearance. All clinical studies of investigational devices must be conducted in compliance with any applicable FDA and Institutional Review Board requirements. Devices that are exempt from FDA premarket review requirements must nonetheless comply with general post-market controls as described below, unless FDA has chosen to exercise enforcement discretion and not regulate them.
510(k) clearance pathway
To obtain 510(k) clearance, a manufacturer must submit a premarket notification demonstrating to FDA’s satisfaction that the proposed device is “substantially equivalent” to a previously 510(k)-cleared device or a device that was in commercial distribution before May 28, 1976 for which FDA has not yet called for submission of PMA applications. The previously cleared device is known as a predicate. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence. FDA’s 510(k) clearance pathway usually takes from three to 12 months, but it can take longer, particularly for a novel type of product.
Before FDA will accept a 510(k) submission for substantive review, FDA will first assess whether the submission satisfies a minimum threshold of acceptability. If FDA determines that the 510(k) submission is incomplete, FDA will issue a “Refuse to Accept” letter which generally outlines the information FDA believes is necessary to permit a substantive review and to reach a determination regarding substantial equivalence. An applicant must submit the requested information within 180 days before FDA will proceed with additional review of the submission. Once the 510(k) submission is accepted for review, by regulation, FDA has 90 calendar days to review and issue a determination. As a practical matter, clearance often takes longer. FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence.
If FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant 510(k) clearance to commercially market the device. If FDA determines that the device is “not substantially equivalent” to a previously cleared device, for example, due to a finding of a lack of a predicate device, that the device has a new intended use or different technological characteristics that raise different questions of safety or effectiveness when the device is compared to the cited predicate device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process. If FDA determines that the information provided in a 510(k) is insufficient to demonstrate substantial
88
equivalence to the predicate device, FDA generally identifies the specific information that needs to be provided so that FDA may complete its evaluation of substantial equivalence, and such information may be provided to the 510(k) within the time allotted by FDA or in a new 510(k) should the original 510(k) have been withdrawn.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) clearance or, depending on the modification, PMA approval. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance. Many minor modifications today are accomplished by a “letter to file” in which the manufacturer documents the rationale for the change and why a new 510(k) is not required. However, FDA may review such letters to file to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
De novo pathway
For novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device, a manufacturer may request a risk-based classification determination for the device in accordance with the “de novo” classification process. This procedure allows a manufacturer whose novel device is automatically classified into Class III to request classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. A medical device may be eligible for de novo classification if the manufacturer first submitted a 510(k) premarket notification and received a determination from FDA that the device was not substantially equivalent or a manufacturer may request de novo classification directly without first submitting a 510(k) premarket notification to FDA and receiving a not substantially equivalent determination. FDA is required to classify the device within 120 days following receipt of the de novo application, although in practice, FDA’s review may take significantly longer. During the pendency of FDA’s review, FDA may issue an additional information letter, which places the de novo request on hold and stops the review clock pending receipt of the additional information requested. In the event the de novo requestor does not provide the requested information within 180 calendar days, FDA will consider the de novo request to be withdrawn. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls, which often include labeling and other restrictions, that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, FDA may reject the de novo request for classification if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed. In the event FDA determines the data and information submitted demonstrate that general controls or general and special controls are adequate to provide reasonable assurance of safety and effectiveness, FDA will grant the de novo request for classification. When FDA grants a de novo request for classification, the device is granted marketing authorization and further can serve as a predicate for future devices of that type, through a 510(k) premarket notification. The de novo route is less burdensome than the PMA process. The de novo route has been used for many IVD products.
PMA pathway
Class III devices require PMA approval before they can be marketed. The PMA pathway requires proof of the safety and effectiveness of the device to FDA’s satisfaction. The PMA pathway is costly, lengthy and uncertain. A PMA application must provide extensive preclinical study and clinical trial data as well as information about the device and its components regarding, among other things, device design, manufacturing and labeling. As part of its PMA review process, FDA will typically inspect the manufacturer’s facilities for compliance with Quality System Regulation, or QSR, requirements, which impose elaborate testing, control, documentation and other quality assurance procedures. The PMA review process typically takes one to three years but can take longer.
Post-market regulation
After a device, including a device exempt from FDA premarket review, is placed on the market, numerous regulatory requirements apply. These include establishment registration and device listing, the QSR, labeling regulations and prohibitions against the promotion of investigational products or “off-label” uses of cleared or approved products, clearance or approval of product modifications to 510(k)-cleared devices, the Medical Device Reporting regulation (which requires that manufacturers report to FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur), the Reports of Corrections and Removals regulation (which requires manufacturers to report product removals and field actions to FDA if initiated to reduce
89
a risk to health posed by the device or to remedy a violation of the FDCA), and post-market surveillance activities and regulations.
Additionally, the manufacturing facilities may be subject to periodic unannounced inspections by government authorities to ensure compliance with QSR and other laws. QSR governs the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of finished devices. Manufacturers may have to provide, on request, electronic or physical records regarding their establishments. Delaying, denying, limiting or refusing inspection by FDA may lead to a product being deemed to be adulterated.
Advertising and promotion of medical devices, in addition to being regulated by FDA, are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Promotional activities for FDA-regulated products have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In general, if FDA determines that our promotional materials or training constitutes promotion of an unapproved or uncleared use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved or uncleared use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
FDA has broad regulatory compliance and enforcement powers. If FDA finds a violation, it can institute a wide variety of compliance or enforcement actions, ranging from an untitled or public warning letter to more severe sanctions such as fines, injunctions and civil penalties; recall or seizure of products; operating restrictions and partial suspension or total shutdown of production; refusing requests for 510(k) clearance or PMA approval of new products; reclassifying devices subject to 510(k) clearance; withdrawing PMAs already granted; and criminal prosecution.
Clinical Laboratory Improvements Amendments
Our IVD devices also are subject to the CLIA and its implementing regulations in the U.S., which establish quality standards for all laboratory testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test is performed. A laboratory is broadly defined to include any facility that performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the impairment of, or assessment of health. The regulations promulgated under CLIA establish three levels of IVD tests: (i) waived; (ii) moderately complex; and (iii) highly complex. When a test is categorized as waived, it may be performed by laboratories that have a Certificate of Waiver.
Tests that are waived by the CLIA regulations are automatically categorized as waived following 510(k) clearance or PMA approval. Otherwise, following clearance or approval, FDA will classify the IVD in accordance with the CLIA regulations. Manufacturers of clinical laboratory test systems, such as IVDs, that are categorized as moderate complexity according to the CLIA categorization criteria may request categorization of the text as waived through a CLIA Waiver by Application submission to FDA. Waived tests are simple laboratory examinations and procedures that have an insignificant risk of an erroneous result, including those that (i) employ methodologies that are so simple and accurate as to render the likelihood of erroneous results negligible or (ii) FDA has determined pose no reasonable risk of harm to patients if the examinations or procedures are performed incorrectly. These tests are waived from regulatory oversight of the user other than the requirement to follow the manufacturer’s labeling and directions for use. Further, when FDA authorizes tests for use at the POC under an EUA, such tests are deemed to be CLIA waived tests. As such, such tests can be performed in a patient care setting that is qualified to have the test performed there as a result of operating under a CLIA Certificate of Waiver for the duration of the emergency declaration.
Federal Communications Commission
Our products contain radio communicating transmitters, which are subject to regulations enforced by the Federal Communications Commission. Such regulations regulate radio transmissions and ensure that radio emitting devices do not degrade or interfere with public transmissions or the public telecommunications network.
HIPAA and the HITECH Act
Under the administrative simplification provisions of HIPAA, as amended by the HITECH Act, the U.S. Department of Health and Human Services issued regulations that establish uniform standards governing the conduct of certain electronic healthcare transactions and protecting the privacy and security of protected health information used or disclosed by
90
healthcare providers and other covered entities and their business associates. The regulations with which we are required to comply have been issued in final form under HIPAA and include: privacy regulations, security regulations and standards for electronic transactions, which establish standards for common healthcare transactions. The privacy and security regulations were extensively amended in 2013 to incorporate requirements from the HITECH Act.
The privacy regulations establish restrictions on the use and disclosure of protected health information by healthcare providers and other covered entities and their business associates. They also set forth certain rights that an individual has with respect to his or her protected health information maintained by a covered entity, including the right to access or amend certain records containing protected health information, or to request restrictions on the use or disclosure of protected health information. The security regulations establish requirements for safeguarding the confidentiality, integrity and availability of protected health information that is electronically transmitted or electronically stored. The HITECH Act, among other things, established certain protected health information security breach notification requirements. A covered entity must notify affected individual(s) and the United States Department of Health and Human Services, and a business associate must notify its respective covered entity, when there is a breach of unsecured protected health information. The HITECH Act also strengthened the civil and criminal penalties that may be imposed against covered entities, business associates, and individuals, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, other federal and state laws may govern the privacy and security of health and other information in certain circumstances, many of which differ from each other in significant ways and may not be preempted by HIPAA, thus complicating compliance efforts. The HIPAA privacy and security regulations establish a uniform federal “floor” that covered entities and their business associates must meet and do not supersede state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing protected health information.
These laws contain significant fines and other penalties for wrongful use or disclosure of protected health information, failure to safeguard protected health information, or failure to notify of a breach of protected health information. Additionally, to the extent that we submit electronic healthcare claims and payment transactions that do not comply with the electronic data transmission standards established under HIPAA and the HITECH Act, payments to us may be delayed or denied.
United States Federal and State Fraud and Abuse Laws
In the United States, there are various fraud and abuse laws with which we must comply and we are potentially subject to regulation by various federal, state and local authorities, including CMS, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the Department of Justice, or DOJ, and individual U.S. Attorney offices within the DOJ, and state and local governments. We also may be subject to foreign fraud and abuse or similar laws.
In the United States, the federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully offering, paying, soliciting or receiving remuneration (including any kickback, bribe, or certain rebates), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual, or purchasing, leasing, ordering, recommending or arranging for the purchase, lease, arrangement, recommendation or order of, any good, facility, item, or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. A person or entity can be found guilty of violating the statute without actual knowledge of the statute or specific intent to violate it. The term remuneration has been interpreted broadly to include anything of value. Courts have stated that a financial arrangement may violate the Anti-Kickback Statute if any one purpose of the arrangement is to encourage patient referrals or other federal healthcare program business, regardless of whether there are other legitimate purposes for the arrangement. Violations are subject to significant civil and criminal fines and penalties for each violation, imprisonment, and exclusion from government healthcare programs. In addition, a claim submitted for payment to any federal healthcare program that includes items or services that were made as a result of a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. There are a number of statutory exceptions and regulatory safe harbors to the federal Anti-Kickback Statute protecting some common activities from prosecution, though the exceptions and safe harbors are drawn narrowly and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Many states also have anti-kickback statutes, some of which may apply to items or services reimbursed by any third-party payor, including commercial insurers.
In addition to the administrative simplification regulations discussed above, HIPAA also created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payors, or obtaining, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by,
91
or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private). A violation of this statute is a felony and may result in fines, imprisonment or exclusion from governmental payor programs such as the Medicare and Medicaid programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact, or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from governmental payor programs. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating the HIPAA fraud provisions without actual knowledge of the statute or specific intent to violate it.
Finally, another development affecting the healthcare industry is the increased enforcement of the FCA and, in particular, actions brought pursuant to the FCA’s “whistleblower” or qui tam provisions. The federal civil and criminal false claims laws, including the FCA and civil monetary penalty laws impose liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to, or approval by Medicare or Medicaid, or other federal governmental payor programs; or knowingly makes, uses, or causes to be made or used, a false record or statement material to a false, fictitious or fraudulent claim or an obligation to pay or transmit money or property to the federal government; or knowingly conceals or knowingly and improperly avoids, decreases or conceals an obligation to pay money to the federal government. The qui tam provisions of the FCA allow a private individual to bring actions on behalf of the federal government alleging that the defendant has defrauded the federal government by submitting a false claim to the federal government and permit such individuals to share in any amounts paid by the entity to the government in fines or settlement. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. When an entity is determined to have violated the FCA, it may be required to pay up to three times the actual damages sustained by the government, plus significant civil penalties for each false claim. These civil penalties are adjusted for inflation periodically.
In addition, various states have enacted false claim laws analogous to the federal FCA, although many of these state laws apply where a claim is submitted to any third-party payor and not merely a governmental payor program.
Other United States Regulatory Requirements
Our laboratories are subject to United States federal, state and local regulations relating to the handling and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemical, biological agents and compounds, blood samples and other human tissue. Typically, we use outside vendors who are contractually obligated to comply with applicable laws and regulations to dispose of such waste. These vendors are licensed or otherwise qualified to handle and dispose of such waste.
The U.S. Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for healthcare employers, including requirements to develop and implement programs to protect workers from exposure to blood-borne pathogens by preventing or minimizing any exposure through needle stick or similar penetrating injuries.
The U.S. federal Physician Payments Sunshine Act created under the ACA, and its implementing regulations, require certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to annually report to CMS information related to direct or indirect payments or other transfers of value made to U.S.-licensed physicians defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Effective January 1, 2022, these reporting obligations include payments and transfers of value made in the previous year to certain non-physician providers, including physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives.
European Regulation
Sales of IVDs in the European Economic Area are subject to the European regulatory framework. The time required to obtain clearance or approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may be different. Set forth below are highlights of the key European regulatory schemes applicable to our business.
92
European Conformity Marking (“CE Mark”) and Certifications
In order to place an IVD, or an accessory to an IVD, on the market in the E.U./European Economic Area, the device must be designed, developed, manufactured, and marketed in compliance with the relevant legal framework. Currently, IVDs must be compliant with Directive 98/79/EEC, or the Directive; however, from May 26, 2022, Regulation (EU) 2017/746, or the Regulation, will replace the Directive. While the new Regulation will have direct effect in all European Economic Area countries, the Directive required national implementing legislation in each country, which had historically led to some variation in the regimes in each country.
Prior to May 26, 2022, IVDs that have been assessed for conformity with the requirements of the Directive, including notably the “essential requirements” set out in Annex I of the Directive, are entitled to bear a CE Mark indicating that the device conforms to the standards required by the Directive. IVDs that have been CE marked may be placed on the market throughout the Member States of the E.U. and the European Economic Area, and other countries that comply with or mirror the Directive.
The method of assessing conformity of IVDs will depend on the type and classification of the IVD. For IVDs that are in the lowest risk classification (meaning that they do not appear in the list set out in Annex II of the Directive nor are they used for the purpose of self-testing by the user/patient), the manufacturer can self-assess that the IVDs comply with the essential requirements in the Directive without any review or intervention by any regulatory body and/or third-party. In doing so, the manufacturer must comply with Common Technical Specifications adopted by the European Commission for certain diagnostic tests, unless they can justify not doing so. The manufacturer may choose to comply with harmonized technical standards adopted by European standards bodies. Although compliance with these standards is not mandatory, compliance raises a presumption of conformity with the essential requirements that each standard addresses.
Once the manufacturer has gathered the technical documentation necessary to demonstrate this in the form of a technical file, it must draw up a declaration of conformity and can then affix a CE Mark to the device and place it on the market. The only additional requirements are (i) that the manufacturer (or its authorized representative if the manufacturer is outside the European Economic Area) must maintain a copy of the relevant technical file, so that it can be inspected by national device regulators; (ii) that the manufacturer and, where relevant, its authorized representative must register themselves and their IVDs, so that these authorities know when the products are to be marketed; and (iii) that the manufacturer must perform device vigilance to monitor the safety and performance of the IVDs on the market, reporting both adverse incidents and any field safety corrective actions, or FSCAs, to the authorities, as appropriate. Challenges by European regulatory authorities may arise subsequently if there is an issue related to the compliance, safety or performance of the device. Such challenges may arise from a routine audit or enquiry by a regulatory authority, or following device vigilance reports by the company or others, or reports of FSCAs by the company, or complaints made by competitors.
Under the Directive, any IVD that is for self-testing or that appears in Annex II (meaning that these devices cannot use the self-certification process) must have their compliance with the Directive reviewed and certified by a European Notified Body. Notified bodies are usually private, non-governmental, independent bodies that are authorized/licensed by governmental authorities to perform conformity assessments. They enter into a contractual arrangement with manufacturers to carry out the conformity assessment of IVDs. The Notified Body will review the technical documentation, including assessing the available clinical evidence, literature data for the product and any available post-market experience. There is some flexibility regarding the conformity assessment procedure the manufacturer uses. If the manufacturer decides to base its conformity assessment on an assessment of its Full Quality Assurance System (rather than a more product-focused “Type Examination”), the Notified Body will also perform an audit of the manufacturer’s quality system against an international standard, EN ISO 13485:2016. If the Notified Body deems the IVD (and where applicable the manufacturer’s quality system) conforms to the Directive it will issue a certificate of conformity for the device and, where applicable, a certificate of conformity for the manufacturer’s quality system, which the manufacturer can use as the basis for its declaration of conformity, then affix a CE Mark and thus place the IVD on the market in the E.U./European Economic Area.
On May 26, 2017 the Regulation entered into force and, from May 26, 2022, the Regulation will apply and will replace the Directive. From that date, IVDs should have been assessed for conformity with the Regulation, and should not be CE marked and placed on the market unless they are in compliance. However, the Regulation provides for a transition period that allows manufacturers or products that benefit from certificates of conformity issued by European Notified Bodies under the Directive prior to May 26, 2022 to continue to place those products on the market through a transitional period, depending on a risk-based product classification scheme. Products deemed lower risk (B) may stay on the market until May 26, 2027, products with medium risk (C) may stay on the market until May 26, 2026 and products with higher risk (D) may stay on the market until May 26, 2025.
93
As with the Directive, the Regulation requires that IVDs must undergo a conformity assessment procedure, have a declaration of conformity drawn up and bear the CE Mark before a manufacturer can place them on the E.U./European Economic Area market. However, the Regulation will up-classify many IVDs that the Directive currently allows manufacturers to self-assess and declare conformity, so that the vast majority of IVDs, including all diagnostic tests, will require a European Notified Body conformity assessment as part of the conformity assessment process. In practice, manufacturers may only be able to self-assess and declare the conformity of consumables and apparatus that are regulated as IVDs, but are not the tests themselves. The Regulation will also provide for greater use of common specifications that are presumed to be binding, unless a manufacturer can justify not doing so.
Following the U.K.’s departure from the E.U. on January 31, 2020, the U.K. continued to follow the same regulations as the E.U. during a Transition Period until the end of 2020. Now that the Transition Period has ended, the U.K. has implemented Directive 98/79/EC into U.K. law (along with other E.U. legislation on medical devices) through the Medical Devices Regulations 2002. Therefore, the two regulatory systems are independent but currently broadly aligned (although under the Northern Irish Protocol, the E.U. regulatory framework will continue to apply in Northern Ireland). The U.K. has implemented certain new regulatory requirements, including that all medical devices and IVDs must be registered with the MHRA before being placed on the G.B. market. There is a grace period to allow time for compliance with the new registration process, with higher risk devices (i.e. List A products) requiring registration by May 1, 2021, and lower risk devices requiring registration later in 2021 (List B products from September 1, 2021 and general IVDs from January 1, 2022). CE marking will continue to be recognized in G.B. for medical devices until June 30, 2023, following which a UKCA mark will be required for a medical device or IVD device to be marketed in G.B. The new E.U. medical device and IVD Regulations will not apply in G.B. and it remains uncertain at present how the U.K. regulatory regime will change in the future and the extent to which it will diverge from E.U. regulations.
General Data Protection Regulation and the U.K. Data Protection Act 2018
The GDPR and the U.K. GDPR and U.K. DPA and other related privacy and data protection legislation in the jurisdictions in which we operate impose strict requirements on controllers and processors of personal data, including special protections for sensitive personal data categories, which include health and genetic information of data subjects. The GDPR and the U.K. GDPR and U.K. DPA impose several requirements on organizations that process such data, including: to observe core data processing principles; to comply with various accountability measures; to provide more detailed information to individuals about data processing activities; to establish a legal basis to process personal data (including enhanced consent requirements); to maintain the integrity, security and confidentiality of personal data; and to report personal data breaches. The GDPR and the U.K. GDPR and U.K. DPA grant individuals a number of data protection rights including, for example, the opportunity to object to the processing of their personal data, allows them to request deletion of personal data in certain circumstances, and provides an individual with an express right to seek legal remedies in the event the individual believes his or her rights have been violated. Further, the GDPR and the U.K. GDPR and U.K. DPA impose strict rules on the transfer of personal data out of the European Economic Area (and the U.K.) (i.e. to “third countries”) to the United States or other regions that have not been deemed to offer “adequate” privacy protections by the European Commission / U.K. Government (as applicable) or a data transfer mechanism has been put in place. Until recently, one such data transfer mechanism was the EU-US Privacy Shield. However, in July 2020 the Court of Justice of the European Union, or CJEU, declared the Privacy Shield to be invalid. The CJEU upheld the validity of the standard contractual clauses, or SCCs, as a legal mechanism to transfer personal data but companies relying on SCCs will need to evaluate and implement supplementary measures that provide privacy protections additional to those provided under SCCs. In turn, the findings of the CJEU will have significant implications for cross-border data flows.
The GDPR and the U.K. GDPR and U.K. DPA may impose additional responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with E.U. and U.K. data protection rules. This may be onerous and adversely affect our business, financial condition, results of operations and prospects. Failure to comply with the requirements of the GDPR and the U.K. GDPR and U.K. DPA and related privacy and data protection legislation may result in a variety of enforcement measures, including significant fines and other administrative measures. The GDPR and the U.K. GDPR and U.K. DPA have introduced substantial fines for breaches of the data protection rules, increased powers for regulators, enhanced rights for individuals, and new rules on judicial remedies and collective redress (the maximum fine is the higher of 20 million Euros (or £17.5 million in the U.K.) or 4% of the total annual worldwide turnover in the preceding financial year). We may be subject to claims by third parties, such as patients or regulatory bodies, that we or our employees or independent contractors inadvertently or otherwise breached GDPR or the U.K. GDPR and U.K. DPA and related data protection rules. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and if we do not prevail, we could be required to pay substantial
94
fines and/or damages and could suffer significant reputational harm. Even if we are successful, litigation could result in substantial cost and be a distraction to management and other employees.
The GDPR and the U.K. GDPR and U.K. DPA are complex laws and the regulatory guidance is still evolving, including with respect to how the GDPR and the U.K. GDPR and U.K. DPA should be applied in the context of transactions from which we may gain access to personal data. Data protection authority activity differs across the E.U. between Member States (and the U.K.), with certain authorities applying their own agenda which shows there is significant uncertainty in the manner in which data protection authorities will seek to enforce compliance with GDPR in the medical and research fields. For example, it is not yet clear if such authorities will conduct random audits of companies subject to the GDPR or the U.K. GDPR and U.K. DPA or will only respond to complaints filed by individuals who claim their rights have been violated. Enforcement actions to date in other industries have resulted in significant fines and other penalties. Failure to comply with the requirements of the GDPR and the related national data protection laws of E.U. Member States, which may deviate slightly from the GDPR, or the U.K. GDPR and U.K. DPA, may result in material fines.
European Fraud and Abuse Laws
In Europe, various countries have adopted anti-bribery laws providing for severe consequences, in the form of criminal penalties and/or significant fines, for individuals and/or companies committing a bribery offense. Violations of these anti-bribery laws, or allegations of such violations, could have a negative impact on our business, results of operations and reputation. For instance, in the U.K., under the Bribery Act 2010, a bribe occurs when a person offers, gives or promises to give a financial or other advantage to induce or reward another individual to improperly perform certain functions or activities, including any function of a public nature.
Bribery of foreign public officials also falls within the scope of the Bribery Act 2010. Infringement of these laws could result in substantial fines and imprisonment.
Coverage, Pricing, and Reimbursement
In the U.S., E.U., and other markets, patients who seek diagnostic services and the providers performing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Accordingly, even if a medical product is authorized for marketing in a given jurisdiction, sales of such products will depend, in significant part, upon the extent to which coverage and reimbursement is provided by third-party payors. In the U.S., this includes government healthcare programs such as Medicare and Medicaid, commercial health insurers, managed care organizations, and other payors.
In the U.S., no uniform policy of coverage and reimbursement for medical products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. Whether a medical product is covered, and at what price, is determined by each payor’s coverage, reimbursement and payment criteria. For example, Medicare provides coverage for items or services that are reasonable and necessary for the diagnosis of an illness, including medically necessary clinical diagnostic laboratory tests. Medicaid provides mandatory coverage for laboratory services and optional coverage (subject to state discretion) for other diagnostic services. A decision by a payor not to cover any of the IVDs we develop could reduce utilization of such products once authorized for marketing and have a material adverse effect on our sales, results of operations, and financial condition. Further, one payor’s determination to provide coverage for a product or services does not assure that such coverage will continue or that other payors will also provide coverage.
The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved, and reimbursement can vary significantly by payor, setting of care, or other factors. For example, government payor reimbursement amounts may be set forth by statute or regulation, whereas private payors may have more flexibility to set reimbursement rates. Additionally, in some settings of care such as in-patient settings, reimbursement for IVD products may be bundled with the cost of other services and not paid for separately. Such bundled payment policies could negatively impact decisions to select other diagnostic testing products over our IVD products, or vice versa.
Further, payors may adopt certain cost-containment measures that affect coverage and reimbursement amounts. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services. Companies may need to conduct expensive pharmacoeconomic studies in order to support coverage and reimbursement determinations, and even with such studies, medical products may not be considered medically necessary or
95
cost effective. Due to these and other factors, reimbursement levels may not be adequate to enable us to maintain prices sufficient to realize an appropriate return on our investment in product development or generate revenue.
The containment of healthcare costs also has become a priority of federal, state and foreign governments as well as other third-party payors. Adoption of cost-containment measures or other policies could further limit a company’s revenue from the sale of any medical products. Coverage policies and reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for a medical product that receives marketing authorization or is CE marked and placed on the market, less favorable coverage policies and reimbursement rates may be implemented or coverage may be ended in the future.
C. Organizational Structure
LumiraDx was incorporated on August 24, 2016. The following table sets forth each of our principal subsidiaries as of December 31, 2021, the countries of organization and the percentage ownership and voting interest held by us (directly or indirectly through our subsidiaries).
|
|
|
|
|
|
|
|
|
|
|
|
|
PROPORTION OF |
|
|
COUNTRY OF
INCORPORATION |
|
|
|
EQUITY SHARES HELD |
NAME |
|
AND RESIDENCE |
|
NATURE OF BUSINESS |
|
BY LUMIRADX |
LumiraDx Brazil Holdings Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
LumiraDx Healthcare Ltda |
|
Brazil |
|
Distributor of medical diagnostics |
|
98% |
LumiraDx Colombia Holdings Limited |
|
United Kingdom |
|
Holding Company |
|
81% |
LumiraDx SAS |
|
Colombia |
|
Distributor of medical diagnostics |
|
100%* |
LumiraDx SAS |
|
France |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx GmbH |
|
Germany |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx AB |
|
Sweden |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx UK Limited |
|
United Kingdom |
|
Manufacture and distribution of medical diagnostics |
|
100% |
LumiraDx Technology Limited |
|
United Kingdom |
|
Research and development |
|
100% |
LumiraDx Ltd. |
|
United Kingdom |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx Group Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
LumiraDx International Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
LumiraDx Investment Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
LumiraDx Care Solutions UK Limited |
|
United Kingdom |
|
Healthcare IT and services |
|
100% |
LumiraDx, Inc |
|
United States |
|
Healthcare IT and services |
|
100% |
ACS Acquisition LLC |
|
United States |
|
Healthcare IT and services |
|
100% |
LumiraDx Healthcare LLC |
|
United States |
|
Healthcare IT and services |
|
100% |
Biomedical Service S.r.l. |
|
Italy |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx AS |
|
Norway |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx GmbH |
|
Austria |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx GmbH |
|
Switzerland |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx Japan Co Ltd |
|
Japan |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx Oy |
|
Finland |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx A/S |
|
Denmark |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx Healthcare S.L. |
|
Spain |
|
Distributor of medical diagnostics |
|
100% |
SureSensors Limited |
|
United Kingdom |
|
Developer and manufacturer of medical diagnostics |
|
100% |
LumiraDx (Pty) Limited |
|
South Africa |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx B.V. |
|
Netherlands |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx Benelux B.V |
|
Netherlands |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx Limited |
|
Ireland |
|
Distributor of medical diagnostics |
|
100% |
LumiraDx Healthcare Private Limited |
|
India |
|
Distributor of medical diagnostics |
|
100% |
CA Healthcare Acquisition Corp. |
|
United States |
|
Holding Company |
|
100% |
*—LumiraDx Colombia Holdings Limited holds 100% of the equity shares of LumiraDx SAS
96
D. Property, Plants and Equipment
We lease all of our facilities. Our main R&D and manufacturing operations are located in Alloa, Scotland, Stirling, Scotland, Glasgow, Scotland, Bathgate, Scotland, San Diego, California and Waltham, Massachusetts. We lease 508,700 square feet across various sites in Scotland and England for R&D, manufacturing, packaging, warehousing and administrative activities. We lease an aggregate of 39,800 square feet in San Diego across two facilities and 30,400 square feet in Waltham for R&D, manufacturing, packaging and administrative activities.
In addition, we lease facilities in Austria, Brazil, Colombia, France, Germany, Italy, Japan, Norway, the Netherlands, South Africa, Spain, Sweden, Switzerland, India and the U.K. for R&D, manufacturing, warehousing, sales and administrative activities.
Lease terms generally vary between one and five years.
The following table outlines our material manufacturing facilities:
|
|
|
|
|
Facility location |
|
Approx. size (sq. ft.) |
|
Lease expiration |
Stirling, Scotland |
|
23,142 |
|
October 24, 2030 |
Motherwell, Scotland |
|
41,568 |
|
September 1, 2040 |
San Diego, California |
|
24,200 |
|
June 3, 2026 |
San Diego, California |
|
15,565 |
|
August 31, 2024 |
Environment, Health and Safety
Our research and development and manufacturing activities take place in our facilities in Alloa, Scotland, Stirling, Scotland, Glasgow, Scotland, Bathgate, Scotland, San Diego, California and Waltham, Massachusetts. For these activities we have obtained the necessary environmental and biohazard permits from the responsible governments. For additional information please see the section of this Annual Report titled “Item 3.D.—Risk Factors—Risks Related to Our Business and Industry.”
None.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
In this section “we,” “us” and “our” refer to LumiraDx. You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and related notes included elsewhere in this Annual Report. The following discussion is based on our financial information prepared in accordance with the IFRS, as issued by the International Accounting Standards Board, or IASB, which may differ in material respects from generally accepted accounting principles in other jurisdictions, including U.S. generally accepted accounting principles, or GAAP. This discussion and other parts of this Annual Report contain forward-looking statements that involve risk and uncertainties, such as statements of our plans, objectives, expectations and intentions. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section of this Annual Report titled “Item 3.D. Risk Factors.” References to the number of shares or options issued by LumiraDx in this section shall be to the number of shares or options issued at December 31, 2021.
We have elected to omit discussion of the earliest of the three years covered by our consolidated financial statements presented in this Annual Report because that disclosure for the fiscal year ended December 31, 2019 was included in our Proxy Statement and Prospectus part of the Registration Statement on Form F-4 (File 333-257745) under the section titled “LumiraDx’s Management’s Discussion and Analysis of Financial Condition and Results of Operations”.
Overview
We are a next-generation POC diagnostic company addressing the current limitations of legacy POC systems by bringing lab-comparable performance to the POC in minutes on a single instrument with a low cost of ownership. We are focused on
97
transforming community-based healthcare by providing critical diagnostic information to healthcare providers at the point of need, thereby enabling more informed medical decisions to improve health outcomes while lowering costs. We have developed and launched our Platform, which is an integrated system comprised of a small, versatile Instrument, precise, low-cost microfluidic test strips, and seamless, secure digital connectivity. We currently have eight tests commercially available on our Platform and a broad menu of tests in development. Our proprietary Platform is designed to simplify, scale down, and integrate multiple testing methodologies onto a single instrument and offer a broad menu of tests with lab-comparable performance at a low cost and with results generally in 12 minutes or less from sample to result. With our Platform, our goal is to address the key challenges faced by healthcare providers in providing efficient and cost-effective patient care. Our microfluidic technology and Platform have been proven to meet the market needs for fast, high sensitivity, convenient and connected diagnostic test results – for health systems, emergency rooms, retail pharmacy chains and other community settings.
We are initially focused on the development of tests for several of the most common conditions diagnosed or managed in community-based healthcare settings. For many of the tests we commercialize, or plan to commercialize, there are no existing high performance POC alternatives. Our initial authorized and CE Marked tests and those under development are designed to address unmet diagnostic needs in the fields of infectious disease, cardiovascular disease, diabetes, and coagulation disorders. To date, we have developed and launched eight diagnostic tests for use with our Instrument: our SARS-CoV-2 antigen test commercially available (i) under an EUA in the United States which authorizes the emergency use of the test during the period in which an emergency declaration remains in effect, (ii) pursuant to a CE Mark in the European Economic Area and U.K., (iii) pursuant to approvals in Japan and Brazil, and (iv) in Africa and elsewhere based on such approvals, our SARS-CoV-2 antibody test commercially available under an EUA in the United States and a CE Mark in the European Economic Area and U.K., as well as our SARS-CoV-2 antigen pool test, our SARS-CoV-2 & Flu A/B tests, our INR test, CRP test and our D-Dimer test, all of which are CE Marked.
In response to the COVID-19 pandemic and the resulting acute need for timely diagnostic information, we developed our SARS-CoV-2 antigen, SARS-CoV-2 antigen pool, SARS-CoV-2 antibody tests and SARS-CoV-2 & Flu A/B tests for use in community-based healthcare settings. These tests have demonstrated highly accurate results within minutes on our Instrument. We have obtained an EUA and a CE Mark for our SARS-CoV-2 antigen test and antibody test. We have commercialized our SARS-CoV-2 antigen test in Europe, Japan, India, Brazil and the U.S. to customers, including NHS and CVS and have made shipments of Instruments and SARS-CoV-2 antigen test strips to a large number of countries in Africa as part of our collaboration with BMGF. PLOS Medicine recently published a systematic review and meta-analysis of more than 60 SARS-CoV-2 antigen tests and ranked LumiraDx’s SARS-CoV-2 Ag test as most sensitive and accurate.
As of December 31, 2021, we have shipped over 21,000 Instruments, across nearly 100 countries, and have more than 1,600 staff across the globe. Our SARS-CoV-2 antigen test has been authorized by FDA under an EUA only for the qualitative detection of SARS-CoV-2 nucleocapsid protein and has not been authorized for use to detect any other viruses or pathogens. In addition, the CE Mark process is a self-certification process where we self-declare as a manufacturer that we have checked the product meets European Economic Area safety, health and environmental requirements. Our SARS-CoV-2 antigen test has not been cleared or approved by FDA or any other regulatory body, and therefore we cannot, until such time as such clearance or approval has been obtained, market such test in the U.S. following the termination of the EUA.
In laboratory and clinical studies, our SARS-CoV-2 antigen test demonstrated a very low LOD of 32 TCID50 per mL and high sensitivity and specificity within a detection window of 12 days from onset of symptoms and delivered results within 12 minutes or less. Our SARS-CoV-2 antibody test demonstrated 100% sensitivity in fingerstick blood samples collected more than eight days post PCR and delivered results within 11 minutes. We believe that offering our SARS-CoV-2 antigen test and SARS-CoV-2 antibody test on a single Platform with superior performance over a wide detection time has the potential to greatly improve the diagnosis of COVID-19 infection, infectivity and potentially immunity over time; enable large-scale population monitoring and facilitate management of the COVID-19 pandemic. Our SARS-CoV-2 antigen pool test is the first POC test that allows pooling of up to five patient samples in a single test, for higher throughput at lower cost.
Our SARS-CoV-2 antigen test is currently being used and implemented in various testing programs across the U.S., U.K., Italy and other European countries, Japan, Brazil, India, Africa and elsewhere, including in accident and emergency departments, care homes, retail pharmacies and other primary care settings. As economies have re-opened with the roll out of COVID-19 vaccines and variants have emerged, we are also supporting testing in schools, workplaces, travel and events where there continues to be a need for diagnostic testing. In addition, in the professional POC settings where our Platform is placed, customers are looking to implement comprehensive POC testing within their institutions leveraging both (i) our broad
98
menu as well as (ii) our quality, compliance and data management infrastructure.
We also see a continued testing opportunity for low complexity mass screening or home COVID-19 testing market. Therefore, based on the same chemistry and test strip design as our SARS-CoV-2 antigen test on our Platform, we are finalizing development of our Amira System, which we are designing as a high-sensitivity mass screening and home testing system for COVID-19. We plan to manufacture and distribute our Amira System at a price and volume that enables both (i) mass testing required to support continued safe re-opening of the economy as well as (ii) broad scale diagnostic testing in high burden countries. Subject to completion of product development, regulatory approval, authorization, certification or clearance for professional and home use, market demand and manufacturing scale-up of the Amira System, we currently expect to launch our Amira System by the summer of 2022, with a manufacturing capacity of building up to 300 million tests per month over time and capability of producing many more for our Amira System, depending on market need for mass screening testing. We anticipate the retail price of our Amira System and Amira COVID-19 test to be between $2.00-$4.00 per test, significantly lower than many existing COVID-19 tests currently on the market as well as the equivalent tests on our Instrument. For very high-volume purchases and shipments to LMICs, we expect the price to be lower. We currently have a prototype Amira System including strips, device and patient application. We expect to move to design freeze at system level shortly. We are simultaneously tooling up high volume manufacturing lines, for the strip and instrument, while we progress through design freeze and the V&V phase.
We have also used our technology to develop two rapid COVID-19 reagent testing kits for use on open molecular systems, LumiraDx SARS-CoV-2 RNA STAR and SARS-CoV-2 RNA STAR Complete. LumiraDx SARS-CoV-2 RNA STAR allows laboratories to utilize their existing molecular lab infrastructure in a high-throughput format by reducing amplification time from approximately one hour down to 12 minutes. LumiraDx SARS-CoV-2 RNA STAR Complete utilizes a direct amplification method that combines lysis and amplification in a single step, detecting SARS-CoV-2 nucleic acid in under 20 minutes, without needing to perform any specimen purification or extraction. We have obtained an EUA for LumiraDx SARS-CoV-2 RNA STAR and both EUA and CE Mark for SARS-CoV-2 RNA STAR Complete and commenced commercial sales. In December of 2021, we achieved several FDA EUA updates for RNA STAR complete molecular reagents including asymptomatic testing, pooling of up to five individuals, and access to 384 well configuration on validated open RT-PCR systems. In addition, through expanded authorization with HealthPulse@Home, we are now able to offer sample collection at home and elsewhere, increasing access to molecular testing. Last month we also achieved MHRA authorization for RNA STAR complete, enabling us to fulfill customer demand in the U.K. for high throughput, high sensitivity and high efficiency testing at events, schools, airports, and other public venues. Looking ahead, The Fast Lab R&D teams continue to innovate this technology with the additional capability of multiplexing. This will allow for simultaneous detection of multiple targets in a single reaction well with the current direct amplification method that is being utilized today for the SARS-CoV-2 RNA STAR Complete. Later in 2022, we expect to file for emergency use authorization with a multiplex respiratory panel while adding more European centric molecular lab offerings mid-year. Beyond the lab, we believe this technology has significant implications for our forthcoming point of care molecular programs.
On the Platform, we currently have a pipeline of more than 30 tests in various stages of development for community-based healthcare settings and plan to launch additional tests, subject to successful development and regulatory approval, authorization, certification or clearance. Our key tests under development include: high sensitivity Troponin I for cardiovascular disease, HbA1c for diabetes and Strep A. Our tests are subject to extensive regulatory requirements and we seek to obtain regulatory approval, authorization, certification or clearance on a test-by-test basis. We are focused on commercializing our tests on pace with receipt of the requisite regulatory approval, authorization, certification or clearance and any delays in commercialization of our tests or decreases in the expected market demand for our tests could adversely impact our operations and financial results. We have also entered into R&D collaborations with well-established diagnostic companies that have market-leading assays and capabilities in specific conditions to further accelerate the expansion of the test menu for our Platform. See the section of this Annual Report titled “Item 4.B. Business Overview—Research and Development” beginning on page 77 for additional details on our R&D collaboration agreements. Additionally, our R&D team is focused on continuous enhancement of our disruptive technologies.
The diagnostics industry, including IVD and POC systems, is rapidly evolving, and we face competition from established diagnostics companies as well as new market entrants. We believe the principal competitive factors in our industry include flexibility and ease of use, time to result, accuracy, reputation, price, innovation and compatibility with existing processes. Our eight tests commercially available on the Instrument compare favorably against the current tests available in the market based on sensitivity, precision, time to result and ease of use, and our tests in development are designed and are being validated against their respective lab standard. Many of our competitors have greater brand recognition, resources, sales
99
forces, intellectual property portfolios, larger customer bases and more established and larger scale manufacturing capabilities.
Our proprietary microfluidic test strip is designed to accommodate all of our assays and sample types in a single-design architecture. We can manufacture our test strips at large scale and low cost on our proprietary manufacturing system. We believe our scalable manufacturing process provides us with a sustainable cost position that allows us to provide cost-efficient diagnostic solutions to the POC market. It also enables us to expand into attractive geographies and alternative healthcare settings where high quality POC testing has previously not been feasible.
We believe our Platform and its attractive value proposition will have broad appeal to healthcare providers globally that are seeking innovative POC solutions to improve outcomes and lower costs. As such, we currently have direct sales and marketing operations in 21 countries, including the U.S., most Western European countries, Japan, Colombia, Brazil, India and South Africa and over time plan to further expand to the largest IVD markets, including China and Southeast Asia. We sell mainly to large healthcare systems, government organizations and national pharmacy chains that can deploy comprehensive POC testing across their extensive healthcare provider networks.
As of December 31, 2021, we have 201 employees focused on sales and marketing located in 21 countries and plan to open additional sales offices to further expand our presence globally. We have direct sales operations in the U.S., most major European countries, Japan, India, South Africa, Colombia and Brazil.
We manufacture our test strips on highly automated, manufacturing equipment designed and manufactured specifically for us. All of our test strips are manufactured on a common platform using a high volume, web-based manufacturing process that allows the production of multiple test strip sizes and designs. Utilizing a common platform allows us to leverage volume and have efficient manufacturing costs and provides flexibility to respond more rapidly to changing market demands across our product portfolio.
As of December 31, 2021, we have raised $1.0 billion through the issuance of debt and equity securities and from our partners since inception. We have primarily deployed this capital to develop and commercialize our Platform and build manufacturing capabilities and a commercial organization that have the potential to deliver on our aspiration to be the global leader in POC diagnostics.
100
Factors Affecting Our Performance
We believe there are several important factors that have impacted and that we expect will impact or will continue to impact our financial performance and results of operations, including:
•COVID-19 test utilization. We believe that our ability to sell POC COVID-19 tests during the pandemic has established strong brand awareness and acceptance of our technology and built an installed base of Instruments with long term point of care customers. The ongoing test utilization of our current installed base will likely be strongly linked to the overall prevalence of COVID-19 infections and the overall need for testing in those settings where Instruments have been installed. Reduced test utilization at these settings would lead to reduced revenue for our COVID-19 test strips.
•Increase the installed base of our Instruments. Our Instrument runs a variety of diagnostic testing technologies utilizing our disposable test strips. We initially intend to focus our sales efforts on large healthcare systems, government organizations and national pharmacy chains that want to deploy comprehensive POC testing across their networks. We believe the successful large-scale deployment of an installed base of Instruments will provide revenue growth in both the near term and the long term through consumption of our current and future assays. We expect our installed base of Instruments to continue to grow as we increase penetration in our existing markets, expand into new markets and add new assays.
•Commercialization of our current and future assays. We believe that delivering a broad menu of diagnostic tests for community-based healthcare on a single Platform is critical to transforming the POC market. We plan to launch 9 tests in the next year, subject to regulatory authorization or clearance. We have a growing pipeline of tests and panels for cardiovascular disease, infectious disease, diabetes, and coagulation disorders, designed to deliver lab-comparable performance. We believe that successful execution of this global market-driven menu strategy will lead to wide adoption of our Platform and high utilization of our diagnostic tests. Any delays in commercialization of our assays or decreases in the expected market demand for our assays could adversely impact our operations and financial results.
•Highly automated, cost efficient manufacturing process. Our proprietary microfluidic test strip is capable of accommodating all of our currently contemplated POC assays within a single design architecture. We manufacture our test strips on highly automated manufacturing equipment designed and manufactured specifically to meet high volume demand at a low cost. We believe the automated manufacturing process of our test strips provides an industry leading cost position. In order to meet the anticipated demand for our Platform, we may need to continue to add manufacturing capacity. This may require continued investments, including the purchase of manufacturing equipment, the lease or purchase of new facilities, leasehold and building improvements to our existing and future facilities, and hiring of new personnel.
•Investment in regulatory approvals, authorizations and clinical trials. We will incur increased costs to conduct clinical trials and to obtain regulatory approvals, authorizations or clearances as we commercialize our products across global markets. Clinical trials demonstrating the acceptable performance of our products may be required in order to obtain regulatory approvals or clearances. Additional regulatory approvals, authorizations or clearances will impact our ability to sell both our Instruments and test strips in various geographies. Any delays in regulatory approvals, authorizations or clearances of our tests or a lack of strong clinical trial evidence for the performance of our tests could adversely impact our operations and financial results.
•Investment in global expansion. We intend to continue to expand the availability of our Platform on a global basis. We intend to establish subsidiaries in additional countries, where appropriate, and hire additional resources in sales, marketing and administration in order to develop the market for our products, engage in sales activities and establish other commercial capabilities to serve the needs our customers. If our investment in our global expansion does not generate expected revenue growth, then our operations and financial results could be adversely impacted.
While each of these areas present significant opportunities for us, they also pose significant risks and challenges that we must address. See the section of this Annual Report titled “Item 3.D. Risk Factors” beginning on page 1 for more information.
101
Components of Results of Operations
Revenue
We expect to continue to derive substantially all our revenue from sales of our Platform, which includes sales of our Instrument, test strips, other related products and services and our Fast Lab Solution. Such sales may have multiple performance obligations under IFRS 15 Revenue from Contracts with Customers, or IFRS 15; therefore, we may recognize revenue associated with a single sale of our Platform both at a point in time and over time. We recognize revenue from the initial sale of the Instrument, test strips and other related products separate from the sale of our connectivity solutions and other services under IFRS 15.
Our Platform will also be made available to customers under operating lease arrangements. Revenue from operating leases are recognized on a straight-line basis over the term or, when lease revenue is entirely variable and subject to subsequent reagent sales, as the performance obligation to deliver reagents is satisfied.
We allocate revenue between products and services based on the relative standalone selling price of each performance obligation.
Products. We derive a significant portion of our product revenue from the sale of our Instrument, test strips, other related products and our Fast Lab Solution. We sell or lease our products directly to users, including healthcare systems, government organizations, national pharmacy chains, diagnostic labs, hospitals and other healthcare providers. In addition, we sell the Instrument, test strips and other related products through wholesalers and distributors. We sell, place free of charge and rent Instruments to customers depending on the needs of the customer and market profile.
During 2019, our instrument and consumable revenue was primarily generated by the resale and distribution of third party medical diagnostic products not related to our Platform. These revenues relate to sales organizations whose operations were acquired by us in anticipation of distributing our proprietary products.
Services. We expect to derive substantially all our service revenue from revenue allocated from the sale of our Platform to our connectivity solutions, such as Connect Manager and EHR Connect. These services allow customers to manage their Instruments and to analyze diagnostic data, provide decision support tools and enforce quality control policies. During 2020 and 2021, less than 1% of our service revenue was derived from sales of our Platform. During this time, the majority of our service revenue related to maintenance on historical software licenses, access to hosted cloud offerings, training, support and other services related to products.
We intend to seek, in the near term, regulatory approval, authorization, certification or clearance for multiple diagnostic assays on our Platform. Assuming we receive regulatory approvals, authorization or clearances, we expect the revenue from sales of our Instrument, test strips and other related products and services to increase significantly.
Costs of Sales and Operating Expenses
Cost of sales. Cost of sales generally consists of the cost of (i) materials and direct labor, including bonus and benefits, (ii) equipment and infrastructure expenses associated with manufacturing and packaging our Platform products, (iii) third party products, (iv) warehousing, handling and shipping costs and (v) the provision of software support and services. Equipment and infrastructure expenses include maintenance and depreciation of manufacturing equipment, facilities costs and amortization of leasehold improvements and of acquired technology. Also included are provisions for excess and obsolete inventory and warranty returns. As we continue to scale our manufacturing operations, improve existing products and introduce new products, it is possible that we will have obsolete parts and materials and our manufacturing output will not match demand, especially in times of volatile demand resulting in write downs for obsolete and short expiry materials and products.
We expect cost of sales to generally increase in line with the increase in the number of Platform products we sell.
Research and development expense. Research and development expense consists of costs incurred to develop our Platform, and includes salaries and benefits, equipment and supplies used in research and development laboratory work, infrastructure expenses, including allocated facility occupancy and information technology costs, contract services, clinical trials and other outside costs, and costs to develop our technology and add additional assays to our Platform. Research and development costs are expensed as incurred.
102
We expect that our research and development expenses will continue to increase as we continue to develop additional assays for our Platform and conduct our ongoing and new clinical trials. These expenses may fluctuate from period to period due to the timing and extent of these expenses incurred within a period.
Selling, marketing and administrative expense. Our selling, marketing and administrative expenses are expensed as incurred and include costs associated with our sales organization, including our direct sales force and sales management, client services, marketing, executive, accounting and finance, legal and human resources functions. These expenses consist primarily of salaries, commissions, bonuses, employee benefits, travel and stock-based compensation, as well as marketing and educational activities and allocated overhead expenses.
We expect our selling, marketing and administrative expenses to increase as we expand our sales force and increase our marketing activities to drive adoption of our Platform. We also expect that our administrative expenses will continue to increase as we increase our headcount and as we incur costs associated with operating as a public company after the Merger, including expenses related to legal, accounting, regulatory, maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums, and investor relations. While we expect these costs to increase in absolute dollars, we expect them to decrease as a percentage of revenue in the long term, though they may fluctuate as a percentage from period to period due to the timing and extent of these expenses.
Listing expenses. Our listing expenses include expenses associated with the Merger with CAH and listing on Nasdaq. These expenses include professional fees incurred with bankers, lawyers, accountants and other advisors as well as an IFRS 2 charge for the difference in the fair value of the shares deemed to have been issued by us in the Merger transaction to CAH shareholders and the net assets of CAH.
Finance Income
Finance income consists of interest earned on our cash and cash equivalents and net foreign currency exchange gains. Our interest income has not been significant to date, but may increase as we invest surplus cash from any future financing transactions in short term, fixed income investments until those proceeds are fully deployed. Net foreign currency exchange gains relate to transactions and asset and liability balances denominated in currencies other than the U.S. dollar, primarily related to our U.K. operations denominated in British pound sterling. We expect our foreign currency gains and losses to continue to fluctuate in the future due to changes in foreign currency exchange rates.
Finance Expense
Finance expense consists primarily of cash and non-cash interest on debt obligations, dividends on our LMDX Series A preferred shares and LMDX Series B preferred shares, changes in fair value of our financial liabilities designated as fair value through profit and loss and net foreign currency exchange losses. Interest expense includes cash interest expense on outstanding debt, as well as non-cash accretion of debt issuance costs and debt proceeds classified as equity under IFRS. Dividends on the LMDX series A preferred shares and LMDX Series B preferred shares accrue cumulatively at an 8% annual rate. All our outstanding LMDX series A preferred shares automatically converted into LMDX ordinary shares immediately prior to the Merger and all our outstanding LMDX Series B preferred shares automatically converted into LMDX common shares immediately prior to the Merger and did not result in cash settlement of the accrued dividends. Our 10% notes and LMDX Series B preferred shares have been designated as financial liabilities at fair value through profit and loss. At each reporting date, these liabilities are re-measured and any increase in liability is recorded as a finance expense. Net foreign currency exchange losses relate to transactions and asset and liability balances denominated in currencies other than the U.S. dollar, primarily related to our U.K. operations denominated in U.K. pound sterling. We expect our finance expense to continue to fluctuate as we manage our debt obligations and due to changes in foreign currency exchange rates.
Provision for Income Taxes
During 2020, benefit from income taxes primarily related to a U.K. tax credit on qualifying research and development expenses. In 2021 we are no longer eligible for the same tax credit. We are now under the Research and Development Expenditure Credit (“RDEC”) scheme in the U.K. and research and development expenditure credits are now recorded as reductions in research and development expenses. We expect to incur tax expense as we recognize income in jurisdictions where no net operating loss carryforwards exist.
103
A. Operating Results
The following table sets forth the significant components of our results of operations for the periods presented.
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED
DECEMBER 31, |
|
|
|
|
2020 |
|
|
2021 |
|
|
|
|
(in thousands) |
|
|
Consolidated Statement of Profit and Loss and Comprehensive Income |
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
Products |
|
$ |
135,656 |
|
|
$ |
415,654 |
|
|
Services |
|
|
3,497 |
|
|
|
5,774 |
|
|
Total revenue |
|
|
139,153 |
|
|
|
421,428 |
|
|
Cost of sales: |
|
|
|
|
|
|
|
Products |
|
|
(84,456 |
) |
|
|
(268,835 |
) |
|
Services |
|
|
(1,750 |
) |
|
|
(1,053 |
) |
|
Total cost of sales |
|
|
(86,206 |
) |
|
|
(269,888 |
) |
|
Gross profit |
|
|
52,947 |
|
|
|
151,540 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
Research and development expenses |
|
|
(107,539 |
) |
|
|
(130,221 |
) |
|
Selling, marketing and administrative expenses |
|
|
(46,129 |
) |
|
|
(130,520 |
) |
|
Listing expenses |
|
|
— |
|
|
|
(36,202 |
) |
|
Total operating expense |
|
|
(153,668 |
) |
|
|
(296,943 |
) |
|
Loss from operations |
|
|
(100,721 |
) |
|
|
(145,403 |
) |
|
Finance income (expense): |
|
|
|
|
|
|
|
Finance income |
|
|
22,500 |
|
|
|
165,426 |
|
|
Finance expense |
|
|
(172,722 |
) |
|
|
(117,934 |
) |
|
Total finance expense, net |
|
|
(150,222 |
) |
|
|
47,492 |
|
|
Loss before provision for income taxes |
|
|
(250,943 |
) |
|
|
(97,911 |
) |
|
Benefit (provision) from income taxes |
|
|
9,946 |
|
|
|
(2,844 |
) |
|
Net loss |
|
$ |
(240,997 |
) |
|
$ |
(100,755 |
) |
|
Comparison of the years ended December 31, 2021 and 2020
Revenue
Products
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Products |
|
$ |
135,656 |
|
|
$ |
415,654 |
|
|
$ |
279,998 |
|
|
|
206.4 |
% |
Product revenue was $415.7 million for the year ended December 31, 2021 compared to $135.7 million for the year ended December 31, 2020, an increase of $280.0 million, or 206.4%. The increase in products revenue was due to a full year of sales of our COVID-19 Platform products. We began sales of our COVID-19 Platform products in September 2020. During the year ended December 31, 2021, revenue from Platform sales and Fast Lab Solutions was $344.8 million and $41.5 million, respectively. During the year ended December 31, 2020, revenue from Platform sales and Fast Lab Solutions was $111.2 million and $1.4 million, respectively. The remainder of product sales was primarily from the resale and distribution of third-party medical diagnostic products. During the years ended December 31, 2021 and 2020, revenue from lease arrangements for diagnostic products was $0.5 and $0.9 million, respectively.
Services
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Services |
|
$ |
3,497 |
|
|
$ |
5,774 |
|
|
$ |
2,277 |
|
|
|
65.1 |
% |
104
Service revenue was $5.8 million for the year ended December 31, 2021 compared to $3.5 million for the year ended December 31, 2020, an increase of $2.3 million, or 65.1%. The increase was a result of an increase in Platform service offerings.
Cost of sales
Products
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Products |
|
$ |
(84,456 |
) |
|
$ |
(268,835 |
) |
|
$ |
(184,379 |
) |
|
|
218.3 |
% |
Cost of sales for products was $268.8 million for the year ended December 31, 2021 compared to $84.5 million for the year ended December 31, 2020, an increase of $184.4 million, or 218.3%. The increase in cost of sales was associated with sales of our COVID-19 Platform products. Product cost of sales as a percentage of product revenue increased slightly from 62% in 2020 to 65% in 2021. This slight increase is a result of the negative impact of inventory scrap and lower initial production yields from our COVID antigen tests early in 2021 as we expanded production to quickly meet market needs.
Services
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Services |
|
$ |
(1,750 |
) |
|
$ |
(1,053 |
) |
|
$ |
697 |
|
|
|
(39.8 |
)% |
Cost of sales for services was $1.1 million for the year ended December 31, 2021 compared to $1.8 million for the year ended December 31, 2020, a decrease of $0.7 million, or 39.8%. The decrease was due to decreases in software hosting costs.
Operating Expenses
R&D Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
R&D expenses |
|
$ |
(107,539 |
) |
|
$ |
(130,221 |
) |
|
$ |
(22,682 |
) |
|
|
21.1 |
% |
R&D expenses were $130.2 million for the year ended December 31, 2021 compared to $107.5 million for the year ended December 31, 2020, an increase of $22.7 million, or 21.1%. The increase in research and development expenses was primarily due to an increase of $12.6 million in personnel-related costs due to increased hiring of R&D personnel, an increase of $4.9 million in facilities and depreciation expense as we expanded our research and development headcount, an increase of $5.4 in costs incurred related to the continued development of the manufacturing process, and an increase of $14.2 million of supplies and laboratory equipment. These increases were partially offset by a decrease of $5.7 million in the use of third-party research development partners, an increase of $7.1 million in grant offsets and a decrease in $2.0 million in development costs related to our Platform instrument.
Selling, Marketing and Administrative Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Selling, marketing and administrative expenses |
|
$ |
(46,129 |
) |
|
$ |
(130,520 |
) |
|
$ |
(84,391 |
) |
|
|
182.9 |
% |
Selling, marketing and administrative expenses were $130.5 million for the year ended December 31, 2021 compared to $46.1 million for the year ended December 31, 2020, an increase of $84.4 million, or 182.9%. The increase was primarily
105
due to an increase of $27.7 million in personnel-related costs as we expanded our sales and marketing headcount to support our growth, an increase of $9.4 million in professional fees including legal and audit fees and an increase of $40.2 million in stock-based compensation expense primarily related to the grant of options to our Founders, for additional information see “Item 6.B Compensation—Founders’ Equity Awards” beginning on page 123.
Listing Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
(in thousands) |
|
|
|
Listing expenses |
|
$ |
— |
|
|
$ |
(36,202 |
) |
|
$ |
(36,202 |
) |
|
100.0% |
Listing expenses were $36.2 million for the year ended December 31, 2021 compared to $nil for the year ended December 31, 2020, an increase of $36.2 million, or 100%. The increase was due to $27.6 million in an IFRS 2 charge for the difference in the fair value of the shares deemed to have been issued by LumiraDx in the Merger transaction to CAH shareholders and the net assets of CAH and due to $8.6 million of transaction costs.
Finance Expense, Net
Finance Income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Finance income |
|
$ |
22,500 |
|
|
$ |
165,426 |
|
|
$ |
142,926 |
|
|
|
635.2 |
% |
Finance income was $165.4 million for the year ended December 31, 2021 compared to $22.5 million for the year ended December 31, 2020, an increase of $142.9 million, or 635.2%. The increase was primarily due to a $101 million gain in 2021 related to fair value adjustments on our 10% notes and LMDX Series B preferred shares which are designated at fair value through profit and loss and $64.1 million of gain related to the conversion of convertible financial instruments as a result of our Merger with CAH. Those increases were partially offset by a decrease of $21.9 million in foreign exchange gains arising from transactions and asset and liability balances denominated in currencies other than the U.S. dollar.
Finance Expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Finance expense |
|
$ |
(172,722 |
) |
|
$ |
(117,934 |
) |
|
$ |
54,788 |
|
|
|
(31.7 |
)% |
Finance expense was $117.9 million for the year ended December 31, 2021 compared to $172.7 million for the year ended December 31, 2020, a decrease of $55 million, or 31.7%%. This decrease was primarily due to a $112 million loss in 2020 related to fair value adjustments on our 10% notes and LMDX Series B preferred shares which are designated at fair value through profit and loss, partially offset by an increase of $43 million in interest costs in connection with the increased borrowings outstanding and an increase of $14.6 million in foreign exchange losses arising from transactions and asset and liability balances denominated in currencies other than the U.S. dollar.
Benefit (provision) for Income Taxes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
CHANGE |
|
|
|
2020 |
|
|
2021 |
|
|
$ |
|
|
% |
|
|
|
(in thousands) |
|
|
|
|
Benefit (provision) for income taxes |
|
$ |
9,946 |
|
|
$ |
(2,844 |
) |
|
$ |
(12,790 |
) |
|
|
(128.6 |
)% |
Provision for income taxes was $2.8 million for the year ended December 31, 2021 compared to a benefit of $9.9 million for the year ended December 31, 2020, a decrease of $12.8 million, or 128.6%. Credit from income taxes in 2020 is primarily
106
related to a U.K. tax credit on qualifying research and development expenses. In 2021 we are no longer eligible for the same tax credit. We are now under the RDEC scheme in the U.K. and research and development expenditure credits are now recorded as reductions in research and development expenses. The tax provision for the year ended December 31, 2021 is primarily attributable to current taxes where net operating loss carryforwards are not available.
B. Liquidity and Capital Resources
Since our inception, we have incurred significant operating losses and negative cash flows from operations. At December 31, 2021, we had an accumulated deficit of $676.2 million. We expect to incur additional operating losses in the near future and our operating expenses will increase as we continue to expand our sales organization, increase our marketing efforts to drive market adoption of our Platform, and invest in the development of new product offerings from our research and development activities. If demand for our Platform increases, we anticipate that our capital expenditure requirements will also increase in order to build additional capacity to meet this demand. Moreover, following the completion of the Merger, we have and will continue to incur additional costs associated with operating as a public company, including expenses related to legal, accounting and financial reporting and regulatory matters, maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums, and investor relations.
The timing and amount of our cost of sales and operating expenditures will depend largely on:
•the cost of purchasing materials to manufacture our products and to maintain sufficient inventory to meet demand;
•the cost of maintaining and expanding our manufacturing capacity;
•the cost of expanding sales, marketing and distribution capabilities in new and existing sales regions in which we may receive marketing approval, authorization, certification or clearance;
•the scope and results of our current and planned research and development activities;
•the outcome, timing and cost of meeting regulatory requirements to commercialize our products in global markets;
•the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights covering our product candidates, including any such patent claims and intellectual property rights that we have licensed under our existing license agreements;
•our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us or our Platform and its components;
•the terms of our existing research and development and commercialization arrangements with third parties, including any minimum commitments in our contractual arrangements with such parties;
•our ability to establish and maintain additional such arrangements on favorable terms and whether and to what extent we retain development or commercialization responsibilities under any new licensing, collaboration, partnership or similar arrangement;
•our need and ability to hire additional management, scientific, medical, accounting and financial reporting and other personnel to scale our Company;
•the costs to operate as a public company, including the need to implement additional financial and reporting systems and other internal systems and infrastructure for our business;
•market acceptance of our product; and
•the effect of competing technological and market developments, including other products that may compete with our Platform.
Through December 31, 2021, we have funded our operations primarily from the issuance of equity securities, convertible preferred stock, convertible notes and debt securities, as well as from revenue from sales of our existing products and services. As of December 31, 2021, we have raised $1.0 billion through the issuance of debt and equity securities and from our partners since inception.
107
In 2020, we secured commitments from investors in our 10% notes totaling $148.9 million and in July 2020, we called and received $74.3 million from investors. The remaining $74.6 million of commitments was available for drawdown until October 31, 2020 but we elected not to call the outstanding amount. A further $1.0 million worth of 10% notes were issued in November 2020 bringing the total amount of 10% notes in issue to $75.3 million.
In November 2020, we raised $164.5 million from investors by issuing 33,008 LMDX Series B preferred shares at an issue price of $5,000, or the second 2020 funding round. The LMDX Series B preferred shares were automatically convertible into LMDX common shares immediately prior to the Merger.
In March 2021, LumiraDx Investment Limited, one of our subsidiaries, entered into a senior secured term loan (as amended from time to time), or the 2021 Senior Secured Loan, with BioPharma Credit Investments V (Master) LP and BPCR Limited Partnership, as lenders and BioPharma Credit PLC, as collateral agent, or collectively, Pharmakon. We have borrowed $300 million under the 2021 Senior Secured Loan, part of which was used to prepay the senior secured term loan originally dated as of October 6, 2020 (as amended on October 16, 2020 and as further amended on January 15, 2021) between LumiraDx Group, one of our subsidiaries, and Silicon Valley Bank, as lender and Jefferies Finance LLC, or Jefferies, as lender and administrative and collateral agent pursuant to which Jefferies originally made available to LumiraDx Group a $100 million senior secured term loan facility and, pursuant to an incremental term loan notice dated as of January 15 2021, Silicon Valley Bank had provided an incremental term loan facility of an additional $40 million.
The 2021 Senior Secured Loan is subject to an interest rate of 8.0% per annum payable in quarterly cash installments. The 2021 Senior Secured Loan matures on March 29, 2024 and contains customary covenants including achieving certain revenue levels for the years ending December 31, 2021, 2022 and 2023. For the 2022 revenue covenant, our short-term revenue prospects will vary with the amount of demand for our SARS-CoV-2 products. While we believe that our SARS-CoV-2 products will remain in high demand as COVID-19 vaccines are rolled out, the availability and efficacy of such vaccines or the mitigation of the COVID-19 pandemic earlier than expected for any other reason could negatively impact demand for our Platform and sales of our Instrument, test strips and other products. In addition, competitors may produce more accurate tests or tests which receive more favorable demand, both of which may impact our revenue streams and ability to meet the revenue covenant.
In September 2021 we completed our Merger with CAH, a special purpose acquisition company. The transaction allowed all holders of preferred shares, Ordinary shares and common shares to receive shares of the continuing publicly listed company and resulted in gross proceeds of $38 million.
We have partnered with BMGF to help them achieve certain key objectives and have received a total of $81.0 million in support from them through a combination of equity, grants and loans. Our $8.0 million grant agreement with BMGF requires us to return any funds not utilized on qualifying expenses by December 31, 2020. Due to the Company’s dedication of resources to respond to the COVID-19 pandemic, the Company and BMGF have reached an agreement for an extension of the grant period to June 30, 2022. As of December 31, 2021, we had available $8.9 million in grant funds that had not been utilized.
On March 2, 2022 we entered into privately negotiated subscription agreements with certain investors wherein we agreed to sell and the investors agreed to purchase $56.5 million of Convertible Senior Subordinated Notes due 2027. The Notes bear annual interest of 6% with interest payable semi-annually in arrears starting September 1, 2022. The Notes will mature on March 1, 2027 and are be convertible at the holder's option at an initial conversion rate of approximately $9.22 per share.
As of December 31, 2021, we had cash and cash equivalents of $132.1 million. Based on our current business plan, we believe that the net proceeds from the Notes, together with our existing cash and cash equivalents, will be sufficient to enable us to fund our operations and capital expenditure requirements for the foreseeable future, provided we are able to obtain waivers of covenant violations or restructure existing debt obligations in the event we are unable to achieve our covenant obligations. To the extent revenue from our Platform grows, we expect our accounts receivable and inventory balances to increase. Any increase in accounts receivable and inventory may not be completely offset by increases in accounts payable and accrued expenses, which could result in greater working capital requirements. The forecast of our capital requirements is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
If our available cash balances and anticipated cash flow from operations, combined with net proceeds of $56.5 million from entering into the Notes are insufficient to satisfy our liquidity requirements, we may seek additional capital.
108
Until such time, if ever, as we can generate sufficient product revenue, we expect to finance our cash needs, as needed, through additional equity and debt financings. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest may be materially diluted, and the terms of such securities could include liquidation or other preferences that adversely affect your rights as a common shareholder. Debt financing and preferred equity financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specific actions, such as incurring additional debt, making capital expenditures, creating liens, redeeming shares or declaring dividends. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we would be required to delay, limit, reduce or terminate our product development or future commercialization efforts, or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Cash Flows
The following table summarizes our cash flows for the periods presented:
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
|
2020 |
|
|
2021 |
|
|
|
(in thousands) |
|
Net cash used in operating activities |
|
$ |
(149,327 |
) |
|
$ |
(134,583 |
) |
Net cash used in investing activities |
|
|
(64,381 |
) |
|
|
(106,346 |
) |
Net cash provided by financing activities |
|
|
236,586 |
|
|
|
219,022 |
|
Net increase (decrease) in cash and cash equivalents |
|
$ |
22,878 |
|
|
$ |
(21,907 |
) |
Operating Activities
During the year ended December 31, 2021, operating activities used $134.6 million of cash, primarily resulting from our net loss of $100.8 million, excluding $35.1 million in non-cash charges and from $68.9 million used by changes in our operating assets and liabilities. Net cash used by changes in our operating assets and liabilities for the year ended December 31, 2021 consisted primarily of a $66.9 million increase in inventories as a result of building stock of raw materials and finished goods to meet customer demand and a $9.5 million decrease in trade payables and other liabilities offset by a $7.5 million increase in trade and other receivables. The changes in trade and other payable and trade and other receivables are largely due to the timing of vendor and customer invoicing and payments.
During the year ended December 31, 2020, operating activities used $149.3 million of cash, primarily resulting from our net loss of $241.0 million, excluding $164.5 million provided by non-cash charges and from $61.5 million used by changes in our operating assets and liabilities. Net cash provided by changes in our operating assets and liabilities for the year ended December 31, 2020 consisted of a $89.2 million increase in trade and other receivables and a $73.3 million increase in inventories, partially offset by a $101.0 million increase in trade payables and other liabilities The increase in trade payables and other liabilities was primarily due to increases in our inventories and operating expenses due to the growth in our business as well as the timing of vendor invoicing and payments. The increase in trade receivables and other receivables is due to an increase in revenue as well as timing of collections from customers.
Investing Activities
During the year ended December 31, 2021, net cash used in investing activities was $106.3 million, consisting solely of $106.3 million in purchases of property, plant and equipment. Purchases of property, plant and equipment were primarily related to facilities and equipment for the production of our Platform consumables.
During the year ended December 31, 2020, net cash used in investing activities was $64.4 million, consisting solely of $64.4 million in purchases of property, plant and equipment. Purchases of property, plant and equipment were primarily related to facilities and equipment for the production of our Platform consumables.
Financing Activities
During the year ended December 31, 2021, net cash provided by financing activities was $219.0 million, primarily consisting of net proceeds of $361.8 million from additional amounts under the 2020 Senior Secured Loan and the 2021 Senior Secured Loan and net proceeds of $38.6 million from the Merger. These increases were offset by $140.6 million in repayments on the 2020 Senior Secured Loan, $29.9 million in net interest payments, $3.6 million in costs related to the early extinguishment of the 2020 Senior Secured Loan and $5.4 million in lease payments.
109
During the year ended December 31, 2020, net cash provided by financing activities was $236.6 million, primarily consisting of net proceeds of $162.4 million from the issuance of our LMDX Series B preferred shares, net proceeds of $62.4 million from entering into the 2020 Senior Secured Loan and $70.9 million in net proceeds from the issuance of the 10% notes. These increases were offset by $40.4 million in repayments on the 2020 Senior Secured Loan, $12.1 million in net interest payments, $3.6 million in costs related to the early extinguishment of the 2020 Senior Secured Loan and $3.1 million in lease payments.
Indebtedness
12% Unsecured Subordinated Loan Notes
In February 2018, we issued a call notice to a group of investors that had accepted our offer to subscribe to up to $76.7 million of our 12% unsecured subordinated loan notes, or the 12% notes. The offer permitted us to call a portion of the 12% notes to be funded during 2018. In February 2018, we issued a call notice for $38.3 million to be funded by the subscribing investors with a maturity date in February 2019. As part of the offer, we agreed to issue 15,461 ordinary shares and to pay $0.8 million in cash as a commitment fee to the investors for their acceptance of the offer.
In August 2018, we received approval from noteholders to prepay the 12% notes. We agreed to pay the full interest due on the 12% notes through to the original maturity date. We converted $35.4 million of principal and $4.3 million of interest into 31,164 LMDX series A preferred shares. Additionally, we paid $2.9 million of principal and $0.4 million of interest in cash to noteholders that elected not to convert the 12% notes into LMDX series A preferred shares.
Unsecured Loan
In October 2019, we issued an unsecured loan in the amount of $18.0 million to BMGF, or the unsecured loan. The terms of the loan include restrictions on the use of the proceeds for specific programs and commitments to provide access to our future products to support the foundation’s charitable purposes. The unsecured loan matures in October 2024 and carries an interest rate of 2% per annum payable in quarterly installments.
11.5% Loan Notes
In September 2019, LumiraDx Investment Limited, our subsidiary, issued senior loan notes in the amount of $40.0 million with an interest rate of 11.5% per annum payable in quarterly installments, or the 11.5% notes. The 11.5% notes were secured generally by all of our assets and were due to mature in September 2023. In conjunction with the 11.5% notes, we also issued the lenders 2,284 warrants to purchase LMDX ordinary shares at an exercise price of $1,459.89 per LMDX ordinary share. The 11.5% notes were prepaid in full in October 2020 and no further amounts can be drawn down from Kennedy Lewis and the other lenders in connection with the 11.5% notes.
5% Convertible Notes
In October and December 2019, we issued an aggregate of $75.2 million 5% unsecured subordinated convertible loan notes, or the 5% notes. The 5% notes have a five-year maturity from their date of issuance and carry an interest rate of 5% per annum, paid semi-annually. The 5% notes automatically converted into LMDX common shares in connection with the Merger.
10% Convertible Notes
In July and November 2020, we issued an aggregate of $75.3 million 10% unsecured subordinated convertible loan notes, or the 10% notes. The 10% notes accrue interest at 10% payable at the same time as repayment of the principal (unless the 10% notes are converted in accordance with their terms). The 10% notes automatically converted into LMDX common shares in connection with the Merger.
2020 Senior Secured Loan
In October 2020, LumiraDx Group, one of our subsidiaries entered into a senior secured term loan, or 2020 Senior Secured Loan, with Jefferies Finance LLC, or Jefferies, as lender and administrative and collateral agent pursuant to which Jefferies originally made available to LumiraDx Group a $100 million senior secured term loan facility. Pursuant to an incremental
110
term loan notice dated as of January 15, 2021, Silicon Valley Bank, or SVB, had provided an incremental term loan facility of an additional $40 million, or the SVB Tranche. The 2020 Senior Secured Loan was secured generally by all of our assets and was originally due to mature in October 2022. In March 2021, the 2020 Senior Secured Loan was repaid and no further amounts can be drawn down from Jefferies or SVB in connection with the 2020 Senior Secured Loan. In connection with the 2020 Senior Secured Loan, on November 6, 2020 we issued to Jefferies the Jefferies warrants to purchase up to 1,000 LMDX common shares at an exercise price equal to $4,644.969 per LMDX common share. In connection with the SVB Tranche, we issued to SVB the SVB warrants to purchase up to 400 LMDX common shares at an exercise price equal to $4,644.969 per LMDX common share.
2021 Senior Secured Loan
In March 2021, LumiraDx Investment Limited, one of our subsidiaries, entered into a senior secured term loan (as amended from time to time), or the 2021 Senior Secured Loan, with BioPharma Credit Investments V (Master) LP and BPCR Limited Partnership, as lenders and BioPharma Credit PLC, as collateral agent, or collectively, Pharmakon. We have borrowed $300 million under the 2021 Senior Secured Loan, part of which was used to prepay the 2020 Senior Secured Loan. The 2021 Senior Secured Loan is subject to an interest rate of 8.0% per annum payable in quarterly cash installments. The 2021 Senior Secured Loan matures on March 29, 2024. The 2021 Senior Secured Loan has been guaranteed and secured by the Company and certain of its subsidiaries. The 2021 Senior Secured Loan contains various covenants that limit our ability to engage in specified types of transactions without the prior consent of Pharmakon, including:
•making certain restricted payments, including paying dividends on, or repurchasing or making distributions with respect to, our shares subject to certain exceptions;
•selling, transferring, leasing or disposing of certain assets;
•encumbering or permitting liens on certain assets;
•incurring certain indebtedness; and
•entering into certain transactions with affiliates.
The 2021 Senior Secured Loan also includes certain financial covenants which require:
•a minimum liquidity level to be maintained which is tested on a monthly basis; and
•a minimum net sales threshold to be met on a trailing twelve-month net sales basis.
Upon the occurrence of a change in control, the 2021 Senior Secured Loan also requires mandatory prepayment of amounts outstanding thereunder. Such change in control may involve one of (i) the persons who are the direct or indirect shareholders of LumiraDx Limited as at March 23, 2021, cease to beneficially own, directly or indirectly, 30% of the then-outstanding shares of LumiraDx, (ii) a sale of all or substantially all of the consolidated assets of LumiraDx Investment Limited and its subsidiaries, (iii) LumiraDx ceasing to own, directly or indirectly, 100% of the equity interests in LumiraDx Investment Limited or (iv) a merger or consolidation of one of LumiraDx, LumiraDx Group or LumiraDx Investment Limited, as applicable, in which such entity is not the surviving entity.
A breach of any of the covenants under the 2021 Senior Secured Loan could result in a default. Upon the occurrence of an event of default under the 2021 Senior Secured Loan, Pharmakon could elect to declare all amounts outstanding to be immediately due and payable and terminate all commitments to extend further credit. Upon the occurrence of insolvency and insolvency proceedings events of default in respect of our U.S. subsidiaries, all amounts outstanding will automatically be immediately due and payable. If we are unable to repay those amounts, Pharmakon could proceed against the collateral granted to secure such indebtedness.
2022 Convertible Notes
On March 2, 2022 we entered into privately negotiated subscription agreements with certain investors wherein the we agreed to sell and the investors agreed to purchase $56.5 million of Convertible Senior Subordinated Notes due 2027. The Notes bear annual interest of 6% with interest payable semi-annually in arrears starting September 1, 2022. The Notes will mature on March 1, 2027 and are be convertible at the holder's option at an initial conversion rate of approximately $9.22 per share.
111
Contractual Obligations and Commitments
Our contractual commitments will have an impact on our future liquidity. The following table summarizes our contractual obligations as of December 31, 2021, which represents contractually committed future obligations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PAYMENTS DUE BY PERIOD |
|
|
|
TOTAL |
|
|
LESS THAN
1 YEAR |
|
|
1-3 YEARS |
|
|
3-5 YEARS |
|
|
MORE THAN
5 YEARS |
|
|
|
(in thousands) |
|
Debt obligations (1) |
|
$ |
369,437 |
|
|
$ |
24,809 |
|
|
$ |
24,690 |
|
|
$ |
319,937 |
|
|
$ |
— |
|
Lease commitments (2) |
|
$ |
46,998 |
|
|
$ |
5,546 |
|
|
$ |
14,646 |
|
|
$ |
10,505 |
|
|
$ |
16,301 |
|
Capital commitments (3) |
|
$ |
5,570 |
|
|
$ |
5,570 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
Total |
|
$ |
422,005 |
|
|
$ |
35,925 |
|
|
$ |
39,336 |
|
|
$ |
330,442 |
|
|
$ |
16,301 |
|
(1)Amounts in the table reflect the contractually required principal and interest payable as of December 31, 2021 pursuant to outstanding borrowings under the unsecured loan with an interest rate of 2.0%, senior secured loans with an interest rate of 8.0%.
(2)Amounts in the table reflect minimum payments due for our leases of office and manufacturing space under operating leases that expire between January 2022 and October 2031.
(3)Amounts in the table reflect amounts due on manufacturing equipment purchases.
C. Research and Development, Patents and Licenses, etc.
For a discussion of our research and development activities, including amounts spent on research and development activities for the last two financial years, see the sections of this Annual Report titled “Item 4.B. Business Overview—Research and Development” beginning on page 77and “Item 5.A. Operating Results” beginning on page 104.
D. Trend Information
Other than as disclosed elsewhere in this Annual Report, we are not aware of any trends, uncertainties, demands, commitments or events that are reasonably likely to have a material adverse effect on our net revenues, income from continuing operations, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future operating results or financial conditions. For more information, see the sections of this Annual Report titled “Item 4.B. Business Overview,” “Item 5.A. Operating Results,” and “Item 5.B. Liquidity and Capital Resources.”
E. Critical Accounting Estimates
Our consolidated financial statements are prepared in accordance with IFRS as issued by the IASB. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our consolidated financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 2 to our consolidated financial statements appearing elsewhere in this Annual Report, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
Our revenue is generated primarily from the sale of diagnostic products, including instruments and consumables. Our services revenue includes the maintenance on historical software licenses, access to hosted cloud offerings and training, support and other services related to our diagnostic products.
Revenue from the sale or lease of goods and services rendered are recognized when a promise in a customer contract (“performance obligation”) has been satisfied by transferring control of the promised goods and services to the customer.
112
Control of a promised good or service refers to the ability to direct the use of, and to obtain substantially all of the remaining benefits from, those goods or services. Control is usually transferred upon shipment or upon receipt of goods by the customer, or as services are rendered, in accordance with the delivery and acceptance terms agreed with the customers. The amount of revenue to be recognized (“transaction price”) is based on the consideration we expect to receive in exchange for our goods and services, excluding amounts collected on behalf of third parties such as value added taxes or other taxes directly linked to sales. If a contract contains more than one performance obligation, the transaction price is allocated to each performance obligation based on their relative standalone selling prices.
The determination of the standalone selling price requires judgment. Our determination of the standalone selling price for each performance obligation varies based on the geography and customer type. Generally, the standalone selling prices are based on observable prices. When observable prices are not available, the standalone selling price for products and services and for determination of amounts allocated for lease consideration in contracts with customers is based on a cost-plus margin approach.
Instruments may be sold together with other goods such as test strips, reagents and other consumables as well as services under a single contract or under several contracts that are combined for revenue recognition purposes. Revenue is recognized upon satisfaction of each of the performance obligations in the contract.
Our sales transactions may consist of various performance obligations that are satisfied at different times. It requires judgment to determine when different obligations are satisfied, including whether enforceable commitments for further obligations exist and when they arise. Depending on the determination of the performance obligations and the point in time or period over which those obligations are fulfilled, this may result in all revenue being calculated at inception, and either being recognized at once or on contract completion, or spread over the term of a longer performance obligation.
In the accounting for contracts that contain promises to deliver more than one good or service, we have to determine how to allocate the total transaction price to the performance obligations of the contract. We allocate the total transaction price of a customer contract to the distinct performance obligations under the contract based on their standalone selling prices. The best evidence of this is an observable price from standalone sales of the good or service to similarly situated customers. However, where standalone selling prices are not observable, it requires judgment to estimate the cost of satisfying a performance obligation and adding an appropriate margin to that good or service and to estimate the standalone selling price for the software using residual method.
Nonrecurring valuations
Our nonrecurring valuations are primarily associated with (i) the application of acquisition accounting and (ii) impairment assessments, both of which require that we make fair value determinations as of the applicable valuation date. In making these determinations, we are required to make estimates and assumptions that affect the recorded amounts, including, but not limited to expected future cash flows, and discount rates, and remaining useful lives of long-lived assets. To assist us in making these fair value determinations, we may engage third party valuation specialists. Our estimates in this area impact, among other items, the amount of depreciation and amortization, impairment charges and income tax expense or credit that we report. Our estimates of fair value are based upon assumptions we believe to be reasonable, but which are inherently uncertain. A significant portion of our long-lived assets were initially recorded through the application of acquisition accounting and all of our long-lived assets are subject to impairment assessments. For additional information, see Notes 11 and 22 to our consolidated financial statements appearing elsewhere in this Annual Report.
We regularly review whether changes to estimated useful lives are required in order to accurately reflect the economic use of our intangible assets with finite lives.
Share-Based Payments
We operate equity-settled, share-based compensation plans under which we receive services or other consideration from employees and other unrelated parties for our equity instruments. The fair value of the services and consideration received in exchange for the grant of options is recognized as an expense and as a component of equity. The total amount to be expensed over the vesting period is determined by reference to the fair value of the options granted. The fair value of the share options was determined using a Black-Scholes valuation model. No performance conditions were included in the fair value calculations.
113
Fair Value of Share Options
We estimate the fair value of each award on the grant date using the Black-Scholes option pricing model. The Black-Scholes model requires the input of highly subjective assumptions, including the expected volatility, the risk-free rate, expected life and the dividend yield. For expected volatility, we have made reference to historical volatility of several comparable companies in the same industry. The expected life is based on the longer of each tranche’s respective weighted-average vesting term or the expected term to a liquidity event. The risk-free rate for periods within the contractual life of the options is based on the market yield of U.S. Treasury Bonds in effect at the time of grant. The dividend yield is based on our expected dividend policy over the contractual life of the options.
The assumptions used to estimate the fair value of the share options granted are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021 |
|
|
|
2020* |
|
|
Employees |
|
|
Founders |
|
Grant date fair value ($) |
|
1,634 to 3,861 |
|
|
1.37 to 4.02 |
|
|
1.26 to 2.85 |
|
Exercise price ($) |
|
1,793 to 3,861 |
|
|
9.89 to 16.39 |
|
|
16.96 to 17.05 |
|
Volatility |
|
35-40% |
|
|
|
40 |
% |
|
|
40 |
% |
Dividend yield |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Expected life of option (years) |
|
2-2.5 |
|
|
4-6.25 |
|
|
4-6.25 |
|
Annual risk free interest rate |
|
0.2 -1.6% |
|
|
0.78-1.22% |
|
|
0.78-1.22% |
|
Total fair value of options granted |
|
$ |
6,716 |
|
|
$ |
8,567 |
|
|
$ |
43,887 |
|
*Amounts not adjusted for 2021 share splits
These assumptions represent our best estimates, but the estimates involve inherent uncertainties and the application of our judgment. As a result, if we use significantly different assumptions or estimates when valuing our options, our share-based compensation expense could be materially different.
Fair Value of LMDX Ordinary Shares
We utilize the fair value of LMDX ordinary shares when determining the fair value of financial instruments, including the 10% notes, and the LMDX Series B preferred shares as well as determining the fair value of our ordinary shares underlying our LMDX options when performing the fair value calculations with the Black-Scholes option pricing model. Therefore, prior to the IPO, our board of directors estimated the fair value of our LMDX ordinary shares at various dates, with input from management, considering the third-party valuations of ordinary shares. The valuations of our LMDX ordinary shares were performed using methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants Audit and Accounting Practice Aid Series: Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the AICPA Practice Guide. In addition, our board of directors considered various objective and subjective factors, along with input from management and the independent third-party valuation firm, to determine the fair value of our LMDX ordinary shares, including: external market conditions affecting the industry, trends within the industry, the results of operations, financial position, status of our research and development efforts, our stage of development and business strategy, and the lack of an active public market for our LMDX ordinary shares, and the likelihood of achieving a liquidity event such as an initial public offering, or IPO.
The valuations of our LMDX ordinary shares were prepared using an option pricing method, or OPM, and a probability-weighted expected return method, or PWERM. The PWERM is a scenario-based methodology that estimates the fair value of ordinary shares based upon an analysis of future values for the company, assuming various outcomes. The ordinary shares’ value is based on the probability-weighted present value of expected future investment returns considering each of the possible outcomes available, as well as the rights of each share class. The future value of the ordinary shares under each outcome is discounted back to the valuation date at an appropriate risk-adjusted discount rate and probability weighted to arrive at an indication of value for the ordinary shares. The OPM treats the LMDX ordinary shares and the LMDX series A preferred shares as call options on the total equity value of a company, with exercise prices based on the value thresholds at which the allocation among the various holders of a company’s securities changes. Under this method, the ordinary shares have value only if the funds available for distribution to shareholders exceeded the value of the preferred share liquidation preferences at the time of the liquidity event, such as a strategic sale or a merger. A discount for lack of marketability of the ordinary shares is then applied to arrive at an estimate of value for the ordinary shares.
114
In addition to considering the results of these third-party valuations, our board of directors considered various objective and subjective factors to determine the fair value of our LMDX ordinary shares as of each grant date, including:
•the prices at which we issued our LMDX ordinary shares and LMDX series A preferred shares and the superior rights and preferences of our LMDX series A preferred shares relative to our LMDX ordinary shares at the time of each grant;
•the progress of our research and development programs;
•our stage of development and our business strategy;
•external market conditions affecting our industry and trends within the industry;
•our financial position, including cash on hand, and our historical and forecasted performance and operating results;
•the lack of an active public market for our LMDX ordinary shares, our LMDX series A preferred shares and LMDX Series B preferred shares;
•the likelihood of achieving a liquidity event, such as an IPO, in light of prevailing market conditions; and
•the analysis of IPOs and the market performance of similar companies in our industry.
The assumptions underlying these valuations represented management’s best estimates, which involved inherent uncertainties and the application of management’s judgment. As a result, if we had used significantly different assumptions or estimates, the fair value of our LMDX ordinary shares and our share-based payment expense could be materially different.
Now that a public trading market for our LMDX common shares, into which our LMDX ordinary shares are convertible, has been established in connection with the completion of the Merger, it is no longer be necessary for our board of directors to estimate the fair value of our LMDX ordinary shares in connection with our accounting for granted stock options and other such awards we may grant, as the fair value of our LMDX ordinary shares will be determined based on the quoted market price of our LMDX ordinary shares.
Product Reserves
We provide standard commercial warranties on our products. Separately, we also periodically perform field service actions related to safety matters and other product campaigns. Pursuant to these warranties and field service actions, we will repair or replace products that are defective in materials or workmanship or that exhibit operational wear and tear. We accrue the estimated cost of both base warranty coverages and field service actions at the time of sale. We are able to service Platform instruments returned to us by customers. We have estimated the number of returned instruments we anticipate being able to service and return to customers and have reserved against those instruments we do not expect to be able to service.
We maintain an allowance for excess or obsolete inventories. The allowance is based on a review of inventory materials on hand, which we compare with estimated future usage. As we continue to scale our manufacturing operations, improve existing products and introduce new products, we expect to procure and produce materials and products that may not be used or sold or may expire. We review our materials and products on hand for their ability to be used in future production or sold to customers. In addition, we review our inventory and compare material costs with current market value and write down any parts with costs in excess of current market value to net realizable value.
These estimates take into consideration historical experience, current contractual and statutory requirements, specific known market events and trends such as competitive pricing and new product introductions, estimated inventory levels, and the shelf life of products. As 2020 was the first year of significant sales of our diagnostic platform, we have limited history to make these estimates. If actual future results vary, these estimates may need to be adjusted, with an effect on sales and earnings in the period of the adjustment. Actual results could differ from these estimates.
Emerging Growth Company and Foreign Private Issuer Status
We qualify as an “emerging growth company” as defined in the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
115
•to the extent that we no longer qualify as a foreign private issuer, (i) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (ii) exemptions from the requirement to hold a non-binding advisory vote on executive compensation, including golden parachute compensation;
•an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; and
•an exemption from compliance with the requirement that the PCAOB has adopted regarding a supplement to the auditor’s report providing additional information about the audit and the financial statements.
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earliest to occur of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; (iii) the date on which we are deemed to be a large accelerated filer under the rules of the SEC; or (iv) the last day of the fiscal year following the fifth anniversary of the closing of the Merger. We may choose to take advantage of some but not all of these exemptions.
In addition, under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. The Company has elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. Further, even after we no longer qualify as an emerging growth company, we may qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements.
We are also a “foreign private issuer.” Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
•the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations with respect to a security registered under the Exchange Act;
•the requirement to comply with Regulation FD, which requires selective disclosure of material information;
•the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and
•the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K upon the occurrence of specified significant events.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents; (ii) more than 50% of our assets are located in the United States; or (iii) our business is administered principally in the United States.
Off-Balance Sheet Arrangements
As of December 31, 2020 and 2021, we have not had any off-balance sheet arrangements as defined in the rules and regulations of the SEC.
Quantitative and qualitative disclosures about market risk
Interest Rate Risk
As of December 31, 2020 and 2021, we had a cash and cash equivalents balance of $161.2 million and $132.1 million, respectively, which comprise cash at bank and in-hand and deposits held at call with banks. We raise debt on a fixed-rate
116
basis for notes in U.S. dollars. We manage risk to protect the net interest result while managing the overall cost of borrowing. A significant change in the market interest rates would not have a material effect on our business, financial condition or results of operations.
Foreign Currency Exchange Risk
We are exposed to foreign exchange risk. The majority of our sales and purchase transactions are denominated in either U.S. dollars or U.K. pound sterling and as such, we are exposed to exchange rate fluctuations between these and other currencies. The exchange risk is managed by maintaining bank accounts denominated in those currencies. During the years ended December 31, 2020 and 2021, we recognized a foreign currency transaction gain and loss of $21.9 million and $14.6 million, respectively. This gain and loss primarily relates to unrealized and realized foreign currency exchange gains or losses as a result of transactions and asset and liability balances denominated in currencies other than the U.S. dollar. All foreign exchange gains and losses are presented within finance income and finance expense in the consolidated statement of profit and loss and comprehensive income for the years ended December 31, 2020 and 2021.
A 10% strengthening of the U.K. pound sterling against the U.S. dollar at December 31, 2021 would have had an impact of increasing the loss before tax for the period by $26.9 million on the basis that all other variables remain constant.
Credit Risk
Credit risk represents the risk of loss that we would incur if operators and counterparties fail to fulfil their credit obligations. The maximum exposure to credit risk is represented by the carrying amount of each financial asset. For banks and financial institutions, we maintain accounts with major international banks with “A” ratings. Credit risk relating to accounts receivable balances are managed on a case-by-case basis. As of December 31, 2020 and 2021, we had trade receivables of $83.9 million and $75.2 million, respectively. New clients are analyzed before standard payment and delivery terms and conditions are offered. The credit quality of the customer is assessed by analyzing its financial position, past experience and other factors. The utilization of credit limits is regularly monitored. Management does not expect any losses, beyond current amounts already included in reserves, from non-performance by these counterparties.
117
Liquidity Risk
Liquidity risk is the risk that we will encounter difficulty in meeting the obligations associated with our financial liabilities that are settled in cash. Cash flow forecasting is performed in our operating entities and aggregated at a consolidated level. We monitor rolling forecasts of our liquidity requirements to ensure we have sufficient cash to meet operational needs. We may be reliant on our ability to raise additional investment capital from the issuance of both debt and equity securities to fund our business operating plans and future obligations.
Recent Accounting Pronouncements
See Note 2 to our consolidated financial statements included elsewhere in this Annual Report for more information.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table lists the names, ages as of March 1, 2022, and positions of the executive officers and directors of LumiraDx.
|
|
|
|
|
|
|
|
|
|
NAME |
|
AGE |
|
POSITION(S) |
Executive Officers: |
|
|
|
|
Ron Zwanziger |
|
67 |
|
Chief Executive Officer, Co-Founder, Chairman and Director |
Dorian LeBlanc, C.P.A. |
|
47 |
|
Chief Financial Officer and Vice President, Global Operations |
Dave Scott, Ph.D. |
|
65 |
|
Chief Technology Officer, Co-Founder and Director |
Jerry McAleer, Ph.D. |
|
66 |
|
Chief Scientist, Co-Founder and Director |
Nigel Lindner, Ph.D. |
|
65 |
|
Chief Innovation Officer |
David Walton, D.M.S. |
|
68 |
|
Chief Commercial Officer |
Peter Scheu |
|
57 |
|
President, North American Commercial Operations |
Veronique Ameye |
|
45 |
|
Executive Vice President and General Counsel |
Pooja Pathak |
|
44 |
|
Chief Product Officer |
Tom Quinlan |
|
60 |
|
Senior Vice President, Instrumentation & Health IT |
|
|
|
Non-Employee Directors: |
|
|
|
|
Donald Berwick, M.D., M.P.P.(1) |
|
75 |
|
Director |
Bruce Keogh, K.B.E., F.R.C.S., F.R.C.P.(2) |
|
67 |
|
Director |
Lurene Joseph(1)(2) |
|
62 |
|
Director |
Lu Huang, M.D.(2) |
|
48 |
|
Director |
Troyen Brennan, M.D., M.P.H. |
|
67 |
|
Director |
George Neble(1) |
|
65 |
|
Director |
Gerald Chan |
|
71 |
|
Director |
(1)Member of the audit committee.
(2)Member of the compensation committee
118
Executive Officers
Ron Zwanziger Mr. Zwanziger is a co-founder of LumiraDx and has served as Chief Executive Officer and Chairman of the LumiraDx group since its inception in November 2014. He has served as a member of LumiraDx’s board of directors since September 2016 when the Company was established as the parent entity of LumiraDx’s group. Mr. Zwanziger brings strategic vision, leadership, extensive business and operating experience and deep knowledge of the industry to the Company. From 2001 to 2014, Mr. Zwanziger served as Chairman, Chief Executive Officer and President of Alere Inc., a diagnostic test manufacturer, or Alere. From 1992 to 2001, he served as Chairman, Chief Executive Officer and President of Alere’s predecessor company, Inverness Medical Technology Inc., or Inverness, until the company was acquired by Johnson & Johnson. From 1981 to 1991, he served as Chairman and Chief Executive Officer of MediSense, Inc., a medical device company. Mr. Zwanziger also previously served on the board of directors of various private and public companies and currently serves on the board of directors of several private companies. Mr. Zwanziger received a B.S. in civil engineering from Imperial College London and an M.B.A from Harvard Business School. We believe that Ron Zwanziger is qualified to serve as a director of LumiraDx based on his experience as the Company’s co-founder and Chief Executive Officer and his experience in the medical diagnostics industry.
Dorian LeBlanc, C.P.A. Mr. LeBlanc has served as LumiraDx’s Chief Financial Officer since November 2016 and has also served as the Vice President, Global Operations since August 2020. Prior to joining LumiraDx, Mr. LeBlanc held several positions with Alere, including as Vice President of Finance, Infectious Disease Global Business Unit from March 2013 to November 2015, and as Vice President Finance and Business Development for Asia Pacific from May 2012 to March 2015. From July 2005 to November 2007, Mr. LeBlanc served as Vice President, Finance at Camden National Corporation. From November 2003 to July 2005, Mr. LeBlanc served as controller for Pierce, a company in the Omnicom group. Mr. LeBlanc received a B.A. in economics from Bowdoin College, and a M.A. in accounting and a M.B.A. from Northeastern University. Mr. LeBlanc is a licensed Certified Public Accountant in the State of Maine.
Dave Scott, Ph.D. Dr. Scott is a co-founder of LumiraDx and has served as the Chief Technology Officer of the LumiraDx group since its inception in November 2014. He has served as a member of LumiraDx’s board of directors since September 2016 when the Company was established as the parent entity of our group. Dr. Scott’s scientific and management background in the diagnostics industry provides the Company with valuable business, research and development, manufacturing and operations expertise. Dr. Scott held several positions with Alere, including as a member of the board of directors, and as a Chief Scientific Officer from 2001 to 2013. From 1999 to 2001, he served as Chairman of Inverness Medical Limited, or Inverness, until the company was acquired by Johnson & Johnson. From 1995 to 1999, Dr. Scott also served as managing director of Inverness. Dr. Scott received a B.S. in microbiology from University of Warwick, and a Ph.D. in biochemistry from University of Kent. We believe that Dr. Scott is qualified to serve as a director based on his experience as LumiraDx’s co-founder and Chief Technology Officer, and his technical and scientific experience in the medical diagnostics industry.
Jerry McAleer, Ph.D. Dr. McAleer is a co-founder of LumiraDx and has served as the Chief Scientist of LumiraDx since its inception in November 2014. He has served as a member of LumiraDx’s board of directors since September 2016. Dr. McAleer brings scientific background in the diagnostics industry, which provides the Company with valuable research and development expertise. Prior to LumiraDx, Dr. McAleer held several positions with Alere, including as a member of the board of directors from 2003 to 2014, as Senior Vice President of Research & Development from 2010 to 2014, and as Vice President Research & Development and Vice President Cardiology from 2001 to 2010. From 1999 to 2001, he served as Vice President of Research & Development of Inverness until the company was acquired by Johnson & Johnson. From 1995 to 1999, Dr. McAleer served as Director of Development of Inverness, Inverness’ primary research and development unit. Dr. McAleer received a B.S. in chemistry, and a M.S. in photochemistry and a Ph.D. in electrochemistry from Southampton University. We believe that Dr. McAleer is qualified to serve as a director of LumiraDx based on his experience as the Company’s co-founder and Chief Scientist and his experience in the medical diagnostics industry.
Nigel Lindner, Ph.D. Dr. Lindner has served as LumiraDx’s Chief Innovation Officer since December 2014. He brings a unique combination of scientific, research and development, management and commercial experience to the Company gained across several industries, including both professional and consumer diagnostics. From December 2011 until July 2014, Dr. Lindner served as the Global Head of Research and Development of Alere. From March 2009 to December 2011, Dr. Lindner served as Chief Executive Officer of Swiss Precision Diagnostics. From June 2007 to March 2009, he served as Vice President of Women’s Health of Inverness. Dr. Lindner received a B.S. in applied biology from University of Hertfordshire and a Ph.D. in biotechnology from Cambridge University.
David Walton, D.M.S. Mr. Walton has served as LumiraDx’s Chief Commercial Officer since August 2014. With over 35 years of experience, Mr. Walton brings significant commercial expertise in the diagnostics industry. Mr. Walton held several positions with Alere, including as President Asia Pacific from August 2009 to September 2014, and as President Europe and
119
Middle East and Asia Pacific from November 2007 to August 2009. Mr. Walton spent 13 years at Unilever PLC, including as Vice President International Business from 1994 to 2007, and Director International Business from 1994 to 2001. Mr. Walton received a B.S. in chemistry from the University of Manchester and a D.M.S. in management from the University of London.
Peter Scheu Mr. Scheu has served as LumiraDx’s President, North American Commercial Operations since August 2016. With over 30 years of healthcare experience, Mr. Scheu brings leadership, strategy development and commercial expertise across numerous provider segments. Mr. Scheu held several positions with Alere, including as President North American Commercial Operations from March 2007 to January 2016, and as Vice President and General Manager, Physician Diagnostics Group from July 2007 to December 2013. From 2004 to 2006, Mr. Scheu served as President Anatomic Pathology at Thermo Fisher Scientific. Mr. Scheu received a B.S. in finance from Miami University.
Veronique Ameye, Esq. Ms. Ameye has served as LumiraDx’s Executive Vice President and General Counsel since February 2015. Previously, Ms. Ameye served as Senior Counsel, Global Mergers and Acquisitions, Commercial Asia Pacific at Alere from May 2007 to January 2015. Ms. Ameye has also worked as a corporate attorney at law firms in Milan, Italy and Brussels, Belgium, including NCTM Studio Legale from 2005 to 2007 and Dal and Veldekens from 2001 to 2005. Ms. Ameye received a law degree from KU Leuven in Belgium, a diplome d’étude specialisés in European law from Université libre de Bruxelles, a degree in economics and competition law from King’s College London and an E.M.B.A. from IE- Brown University.
Pooja Pathak Ms. Pathak joined LumiraDx in February 2020 and currently serves as Chief Product Officer. Ms. Pathak’s experience includes bringing new innovations to market, as well as implementing integrated diagnostic and treatment programs and global public health solutions. From October 2017 to March 2019, Ms. Pathak served as Vice President Global Marketing for Infectious Disease Emerging Markets at Abbott. Ms. Pathak held several positions with Alere, including as Vice President Marketing and Business Development, Africa from September 2015 to September 2017, as Vice President Strategy and Analytics from March 2013 to August 2015 and as Vice President Connected Health from August 2012 to February 2013. From August 2010 to July 2012, Ms. Pathak served as Associate Director, Global Marketing at Novartis. Ms. Pathak received a B.S. in chemical engineering from University of Illinois at Chicago and a M.S. from Massachusetts Institute of Technology.
Thomas Quinlan Mr. Quinlan has served as LumiraDx’s Senior Vice President, Instrumentation and Health IT since October 2017. Mr. Quinlan brings extensive experience delivering large scale service platforms in the financial, telecom and health industries, as well as state-of-the-art diagnostics. From May 2017 to September 2017, Mr. Quinlan served as LumiraDx’s Head of Engineering Product Development, and from March 2016 to April 2017 as a Manager. Previously, Mr. Quinlan served as Executive Vice President of Engineering at Fitlinxx Inc. from December 2007 to March 2016
Non-Employee Directors
Donald Berwick, M.D., M.P.P. Dr. Berwick has served as a member of LumiraDx’s board of directors since January 2018. He is currently President Emeritus and Senior Fellow at the Institute for Healthcare Improvement (“IHI”) a position he has held since December 2014. Previously, Dr. Berwick served as the founding Chief Executive Officer of IHI from 1991 to 2010. In 2015, Dr. Berwick was appointed as an international visiting fellow of the King’s Fund in the U.K. to advise on improvements on health and care in the National Health Service (“NHS”) a position he still holds. In July, 2010, he was appointed by former President Barack Obama as the Administrator of the Centers for Medicare and Medicaid Services, a position he held until December, 2011. Dr. Berwick served two terms on the Governing Council of the Institute of Medicine, (now the National Academy of Medicine), from 2002 to 2007, was a member of International Organization for Migration’s Global Health Board from 2002 to 2009, and served on former President Clinton’s Advisory Commission on Consumer Protection and Quality in the Healthcare Industry from 1996 to 1999. Dr. Berwick was Vice Chair of the U.S. Preventive Services Task Force (1990-1995), independent member of the American Hospital Association Board of Trustees (1996-1999), and as Chair of the National Advisory Council of the Agency for Healthcare Research and Quality (1999-2001). He has also served as Clinical Professor of Pediatrics and Health Care Policy at Harvard Medical School and Professor Health Policy and Management at the Harvard School of Public Health. He currently serves as Lecturer in the Department of Health Care Policy at Harvard Medical School. Since October 2015, Dr. Berwick has served on the board of directors of National Research Corporation, a publicly traded healthcare information and market research services company. Dr. Berwick received an A.B. in social relations from Harvard College, an M.D. from the Harvard Medical School, and an M.P.P. from the Harvard Kennedy School. We believe that Dr. Berwick is qualified to serve as a director based on his knowledge of the healthcare industry, health quality, and improvement requirements, as well as expertise in clinical medical sciences.
120
Bruce Edward Keogh, K.B.E., MD, F.R.C.S., F.R.C.P. Sir Bruce has served as a member of LumiraDx’s board of directors since January 2018. He is currently chair of the Birmingham Women’s and Children’s Hospitals in the U.K., a position he has held since January 2018. Following a distinguished career as a cardiac surgeon and professor at University College London, Sir Bruce was appointed Director General in the Department of Health and Medical Director of the NHS in 2007, a role from which he migrated to the board of a new independent NHS Commissioning Board (NHS England) in 2013. As England’s most senior doctor between 2007-2018, he was responsible for overall clinical policy and strategy and innovation for the NHS. This included medical and dental care, diagnostics and pharmacy. Prior to this, he served as a Commissioner on the boards of the healthcare regulators the Commission for Health Improvement and the Healthcare Commission from 2002 to 2007. He has served as President of the Society for Cardiothoracic Surgery in G.B. and Ireland, Secretary General of the European Association for Cardio-Thoracic Surgery, President of the Cardiothoracic Section of the Royal Society of Medicine and the board of directors of the Society of Thoracic Surgeons in the U.S. and the Council of the Royal College of Surgeons of England. Sir Bruce received a BSc and MB, BS (medical) degree and higher medical research degree from the University of London. He was knighted for services to medicine in 2003 and listed by the Sunday Times in 2014 as one of Britain’s 100 most influential people. We believe that Sir Bruce is qualified to serve as a director based on his knowledge of the healthcare industry, health quality and improvement requirements as well as scientific and medical knowledge.
Lurene Joseph Ms. Joseph has served as a member of LumiraDx’s board of directors since November 2016. Ms. Joseph has been a Director of Maraval Limited since April 2015, providing consulting services to various blue chip organizations on housing, regeneration, socio-economic development. From 2012 to 2015, Ms. Joseph was Chief Executive of Leeds and Partners, a publicly funded inward investment agency. From 2004 to 2012, Ms. Joseph was an Executive Director and latterly Chief Executive of the London Development Agency, a regional body responsible for the economic growth of London including the land assembly and delivery for the London 2012 Olympics. In this position, Ms. Joseph rebuilt and reshaped the organization’s operations, driving improved outcomes with a focus on value for money, and working closely with business leaders, local authorities and with regional, national and international stakeholders. Ms. Joseph has also held various senior roles including Senior Vice President and sat on the Executive Management teams of Shell Oil Europe 1994 to 1999 and Texas Utilities Europe 1999 to 2003. In her early career, Ms. Joseph was a Research Associate at The Financial Research Centre of the University of Manchester Institute of Science and Technology. Over the last 15 years, Ms. Joseph has held various board and committees memberships with private and public sector companies. Ms. Joseph studied the Chartered Institute of Banker’s Examinations and received both a postgraduate Diploma in Business Analysis in 1984 and a M.B.A. from the University of Lancaster. We believe that Ms. Joseph is qualified to serve as a director based on her financial and management experience.
Lu Huang, M.D. Dr. Huang has served as a member of LumiraDx’s board of directors since October 2018. Dr. Huang joined Morningside in 2003 and is currently in charge of Morningside Ventures’ healthcare investment activities in China. With over 19 years’ experience in the venture capital industry, she has led more than 30 healthcare/life science investments in greater China and North America, ranging from bio-pharmaceutical, medical devices and in-vitro diagnostics sectors to healthcare services and IT. Before joining Morningside, Dr. Huang served as a Marketing Associate in the Public Relations & Marketing group at Continuum Health Partners in New York City, which provides integrated healthcare management services throughout the New York metropolitan region. Dr. Huang previously served on the board of directors of Stealth BioTherapeutics Corp., a publicly traded biotechnology company, from June 2018 to December 2020. Dr. Huang obtained her M.D. from Shanghai Jiao Tong University School of Medicine (formerly known as Shanghai Second Medical University) and subsequently worked at the University affiliated hospital. She holds an M.B.A. from St. John’s University. We believe that Lu Huang is qualified to serve as a director based on her knowledge of the healthcare industry, her business and management experience.
Troyen Brennan, M.D., M.P.H. Dr. Brennan has served as a member of LumiraDx’s board of directors since November 2016. Dr. Brennan is Executive Vice President and Chief Medical Officer of CVS Health Corporation, a position he has held since 2008. From 2006 to 2008, Dr. Brennan served as Chief Medical Officer of Aetna Inc., where he was responsible for clinical policies, as well as that company’s full range of clinical operations, disease management programs and patient management services. From 1992 to 2006, Dr. Brennan held several positions with Brigham and Women’s Physician’s Organization, including as President and Chief Executive Officer. Dr. Brennan has also served as a Professor of Medicine at Harvard Medical School and Professor of Law and Public Health at Harvard School of Public Health. He is a member of the Institute of Medicine of the National Academy of Sciences. Dr. Brennan received a B.S. from Southern Methodist University, a M.D. and a M.P.H. from Yale Medical School and a J.D. from Yale Law School. He also received a M.A. from Oxford University, where he was a Rhodes Scholar. We believe that Dr. Brennan is qualified to serve as a director based on his medical and scientific experience and his knowledge of the healthcare industry.
121
George Neble Mr. Neble has served as a member of LumiraDx’s board of directors since July 2020. He currently works as a business advisor for high growth and emerging technology companies. From November 2012 to June 2017, Mr. Neble served as the Northeast Market Leader and Managing Partner of the Boston office of Ernst & Young LLP. From 2002 to 2012, Mr. Neble was a senior assurance partner at Ernst & Young LLP. He has served as a member of the board of directors of publicly traded companies EverQuote, Inc., since May 2018, and Intapp, Inc., since June 2021. He was previously a member of the board of directors of Real Goods Solar, Inc. from June 2019 until July 2021. From 1978 to 2002, Mr. Neble was an Assurance Partner at Arthur Andersen serving primarily emerging and growth-oriented companies. Mr. Neble brings more than 40 years of accounting and auditing experience working with public and private companies. He is a Certified Public Accountant with extensive experience in accounting, SEC and financial reporting matters. Mr. Neble received a B.S. in accounting from Boston College. We believe that Mr. Neble is qualified to serve as a director based on his knowledge of accounting and financial matters as well as audit functions.
Gerald R. Chan, Sc.D. has served as a member of our board of directors since September 2020. Dr. Chan co-founded Morningside Venture Investments, Ltd., a private investment group with venture, private equity and property investments, in 1986. He has served as a member of Scientific Advisory Committee of Brigham and Women’s Hospital since 2018, the Global Advisory Council of Harvard University since 2012 and the Dean’s Board of Advisors of the Harvard T.H. Chan School of Public Health since 2011. and the advisory board of the Johns Hopkins Nanjing Center since 2004. He has served as trustee of Scripps Research Institute since 2016. Dr. Chan has served on the board of directors of Stealth BioTherapeutics Corp., a publicly traded biotechnology company, since 2007, and Apellis Pharmaceuticals, Inc., a publicly traded biopharmaceutical company, since 2013. He has also been a member of the board of directors of Hang Lung Group Limited since 1986 and serves on the boards of several private companies. He previously chaired the Innovation Advisory Committee of Wellcome Trust from 2016 until 2020 and served as a director of Aduro Biotech Inc. from 2014 to 2018. Dr. Chan received his B.S. and M.S. degrees in engineering from the University of California, Los Angeles, and his Master’s degree in medical radiological physics and Doctor of Science degree in radiation biology from Harvard University. He did his post-doctoral training at the Dana-Farber Cancer Institute. We believe that Dr. Chan is qualified to serve on our board of directors because of his extensive experience in life science investments and serving on boards of directors.
Family Relationships
There are no family relationships among any of our executive officers or directors.
Board Diversity
The composition of LumiraDx's board of directors currently includes two individuals who are diverse under the Nasdaq Listing Rule 5605(f) regarding board diversity, representing gender diversity of 20%, as presented in the below Board Diversity Matrix. Under Nasdaq Listing Rule 5605(f), directors who self-identify as (i) female, (ii) an underrepresented minority or (iii) LGBTQ+ are defined as being diverse. The following chart summarizes certain self-identified personal characteristics of our directors, in accordance with Nasdaq Listing Rule 5605(f). Each term used in the table has the meaning given to it in the rule and related instructions.
|
|
|
|
|
Board Diversity Matrix for LumiraDx Limited (As of March 31, 2022) |
Total Number of Directors: |
10 |
|
Female |
Male |
Non-Binary |
Did Not Disclose Gender |
Part I: Gender Identity |
|
|
|
|
Directors |
2 |
8 |
0 |
0 |
Part II: Demographic Background |
|
|
|
|
Underrepresented race, ethnicity, nationality, culture or religious identity |
2 |
0 |
0 |
0 |
LGBTQ+ |
0 |
0 |
0 |
0 |
None of the above |
0 |
5 |
0 |
0 |
Did not disclose |
0 |
3 |
0 |
0 |
122
B. Compensation
Compensation of Management Team and Directors
For the year ended December 31, 2021, the aggregate of cash compensation accrued or paid and benefits in kind provided to the members of our board of directors and our management team for services in all capacities was $4,746,090
(mainly comprised of salary paid to the management team). Other than option grants as described below, the members of our board of directors have not been compensated for their service to the Company for the year ended December 31, 2021. In addition to the amounts described above, the total amount accrued to provide pension, retirement or similar benefits for our officers for the fiscal year ended December 31, 2021 was $233,399.
Effective February 1, 2021, each of our Founder Directors received an increase in annual salary to $515,000. In addition, in January 2021, Dave Scott received a special recognition bonus of $260,000 reflecting his contribution to our COVID-19 testing technology.
Agreements with our Management Team
In part, the compensation amounts described above are paid pursuant to offer letters with each management team member located in the U.S. (Ron Zwanziger, Thomas Quinlan, Peter Scheu, Dorian LeBlanc, and Pooja Pathak) and employment agreements or statement of employment conditions with each management team member located in the U.K. (Nigel Lindner, Jerry McAleer, Dave Scott, David Walton, and Veronique Ameye). The offer letters for our U.S. management team generally provide for at-will employment and eligibility to participate in our U.S.-based employee benefit plans, and do not contain any severance entitlements or non-competition or non-solicitation covenants. The employment agreements set forth for each U.K. management team member generally provide for the term of the employment, eligibility to receive pension benefits according to local law, and eligibility to participate in our U.K.-based employee benefit plans. The following U.K. based management team members are entitled to a payment in lieu of notice for certain non-cause terminations: consisting of four weeks for Dave Scott and Jerry McAleer, one week for Nigel Lindner and David Walton and the greater of: (i) four weeks’ notice; and (ii) one week’s notice for each completed year of service (up to a maximum of twelve weeks) for Veronique Ameye.
Founders’ Equity Awards
Our Founder Directors have contributed to the Company as investors, but unusually had not received any equity compensation since the Company was founded. After receiving independent professional advice on appropriate quantum and conditions, on January 15, 2021, we granted “founder options” over ordinary shares to each of our three Founder Directors. Each Founder Director was granted (i) a fully vested option over 5,235,851 ordinary shares and (ii) following shareholder approval obtained on February 1, 2021, an additional option over 2,819,577 ordinary shares, vesting over a two year period subject to the satisfaction of certain performance conditions. These options have an exercise price of $17.05 per ordinary share and in the normal course expire on the tenth anniversary of the date of grant.
Equity Incentive Plans
Our directors and management team members received the option grants described above pursuant to the LumiraDx Limited Consultants’ and Non-Employees’ Option Scheme, or the Consultant Plan, and the LumiraDx Limited Unapproved Option Scheme with U.S. Appendix, or the Employee Plan, and together with the Consultant Plan, the Prior Equity Plans. In September 2021, our 2021 Stock Option and Incentive Plan became effective upon completion of the Merger, and, following this effectiveness, no options can be granted under the Prior Equity Plans.
Below is a description of the principal terms of the Prior Equity Plans:
|
|
|
|
|
• |
|
Types of Awards. The Prior Equity Plans each permit the award of stock options over ordinary shares. Under the U.S. Appendix to the Employee Plan, incentive stock options may be issued to participants who are subject to tax in the U.S. |
|
|
|
|
|
• |
|
Plan Administration. Our board of directors administers each Prior Equity Plan and has the power to grant option awards under the Prior Equity Plans. |
|
|
|
|
|
• |
|
Award Agreement. Option awards granted under the Prior Equity Plans are evidenced by option certificates that set forth the terms of the option including the number of shares under option, exercise price, vesting schedule and any additional conditions. |
123
|
|
|
|
|
• |
|
Eligibility. Under the Consultant Plan, we may grant awards to consultants, non-employees who provide services to us or our affiliates, or any prospective employee nominated by us. Under the Employee Plan, we may grant awards to any executive director or any employee of us or our affiliates. Awards are made by the board of directors in its discretion, in consultation with our Founder Directors and managers, to further our interests by retaining and incentivizing our management team and board members and, further, by aligning such persons’ interests with our shareholders’ interests. Individual board members do not participate in the decision regarding their own awards. |
|
|
|
|
|
• |
|
Vesting Schedule. In general, the options vest 25% on the 12-month anniversary of the date of grant, 25% on the 24-month anniversary of the date of grant, 25% on the 36-month anniversary of the date of grant, and 25% on the 48-month anniversary of the date of grant. Our board of directors may also specify a different vesting schedule in the relevant option certificate. Accelerated vesting is generally provided upon a sale of the Company and certain reconstructions. There is no accelerated vesting on an initial public offering of our shares. No options accelerated solely as a result of the Merger. |
|
|
|
|
|
• |
|
Exercise of Options. Our board of directors determines the exercise price for each option award, which is stated in the option certificate. The vested portion of each option grant will expire if not exercised prior to the tenth anniversary of the date of grant. |
|
|
|
|
|
• |
|
Leavers. If an optionholder leaves their vested options remain exercisable for a specified length of time, unless the reason for leaving is gross misconduct or a disciplinary reason, in which case the options lapse. Unvested options will lapse on leaving unless the board of directors determines otherwise. |
2021 Stock Option and Incentive Plan
Upon completion of the Merger, our 2021 Stock Option and Incentive Plan, or the 2021 Plan, became effective. The 2021 Plan allows our board of directors or the compensation committee to make equity-based and cash-based incentive awards to eligible individuals, as described in the 2021 Plan and below. The material terms of the 2021 Plan are summarized below.
We initially reserved 30,530,760 common shares, or the Initial Limit, for the issuance of awards under the 2021 Plan. The 2021 Plan provides that the number of shares reserved and available for issuance under the 2021 Plan will automatically increase each January 1, beginning on January 1, 2022, by an amount such that the number of shares reserved and available for issuance under the plan will equal 10% of the issued and outstanding number of ordinary shares and common shares on the immediately preceding December 31, or the Annual Increase. This number is subject to adjustment in the event of a reorganization, recapitalization, reclassification, share dividend, share split, reverse share split or other similar change in our capitalization.
The common shares underlying any awards that are forfeited, cancelled, or otherwise terminated (other than by exercise) under the 2021 Plan or the Prior Equity Plans will be added back to the common shares available for issuance under the 2021 Plan. The maximum aggregate number of shares that may be issued in the form of incentive share options shall not exceed the lesser of (i) the Initial Limit cumulatively increased on January 1, 2022, and on each January 1 thereafter of the Annual Increase for such year, and (ii) 80,000,000 common shares.
The 2021 Plan will be administered by the Administrator, which will be either by our board of directors, the compensation committee or a similar committee performing the functions of the compensation committee and such committee shall be, for any actions taken at or following the effectiveness of this registration statement, comprised of not less than two independent non-employee directors. The Administrator has full power to select, from among the individuals eligible for awards, the individuals to whom awards will be granted, to make any combination of awards to participants, and to determine the specific terms and conditions of each award, subject to the provisions of the 2021 Plan. Persons eligible to participate in the 2021 Plan will be those full- and part-time employees, non-employee directors and consultants as selected from time to time by the Administrator in its discretion.
The 2021 Plan permits the granting of options to purchase common shares intended to qualify as incentive share options under Section 422 of the Code, and options that do not so qualify. The option exercise price of each option will be determined by the Administrator but for options granted to U.S. individuals, subject to certain exceptions, the option exercise price may not be less than 100% of the fair market value of our common shares on the date of grant (and may not be less than 110% of the fair market value of common shares on the date of grant with respect to incentive share options granted to any employee who owns or is deemed to own more than 10% of the combined voting power of all of our classes of such shares as of the date of grant). The term of each option will be fixed by the Administrator and may not exceed 10 years from the date
124
of grant (and may not exceed 5 years from the date of grant with respect to incentive share options granted to any employee who owns or is deemed to own more than 10% of the combined voting power of all of our classes of such as of the date of grant). The Administrator will determine at what time or times each option may be exercised.
The Administrator may award options to purchase common shares to participants subject to time- and/or performance-based vesting conditions and restrictions as it may determine. These conditions and restrictions may include the achievement of certain performance goals and/or continued employment with the Company through a specified vesting period.
The Administrator may award share appreciation rights subject to such time- and/or performance-based vesting and exercisability conditions and restrictions as it may determine. Share appreciation rights entitle the recipient to common shares, or cash, equal to the value of the appreciation in the common share price over the exercise price. The exercise price of each share appreciation right may not be less than 100% of the fair market value of the common shares on the date of grant.
The Administrator may award restricted shares and restricted share units to participants subject to such time- and/or performance-based vesting and exercisability conditions and restrictions as it may determine. These conditions and restrictions may include the achievement of certain performance goals and/or continued employment with us through a specified vesting period. The Administrator may also grant common shares that are free from any restrictions under the 2021 Plan, or unrestricted shares. Unrestricted shares may be granted to 2021 Plan participants in recognition of past services or other valid consideration and may be issued in lieu of cash compensation due to such participant. The Administrator may also grant cash-based awards under the 2021 Plan to participants, subject to the achievement of certain performance goals.
The 2021 Plan provides that in the case of, and subject to, the consummation of a “sale event” as defined in the 2021 Plan, all outstanding awards may be assumed, substituted or otherwise continued by the successor entity.
To the extent that the successor entity does not assume, substitute or otherwise continue such awards, then, except as otherwise provided in the relevant award agreement (i) all time-vesting options and share appreciation rights will automatically become fully vested and exercisable, all other awards with time-based conditions become fully vested and non-forfeitable, and awards with vesting and/or exercisability or settlement conditions and restrictions relating to the attainment of performance goals may become vested and non-forfeitable in connection with a sale event as determined by the Administrator in its sole discretion or to the extent specified in the relevant award agreement, and (ii) upon the effectiveness of the sale event, the 2021 Plan and all awards will automatically terminate. In the event of such termination, (i) individuals holding options and share appreciation rights may be permitted to exercise such options and share appreciation rights (to the extent exercisable) prior to the sale event; or (ii) we may make or provide for a cash payment to participants in respect of their vested and exercisable awards, with the payment to those participants holding options and share appreciation rights equal to the difference between the per share cash consideration payable to shareholders in the sale event and the exercise price of the options or share appreciation rights (to the extent then exercisable), and the payments to the holders of other awards equal to an amount reflecting the per share consideration multiplied by the number of vested shares under such award.
Our board of directors may amend or discontinue the 2021 Plan and the Administrator may amend or cancel outstanding awards for purposes of satisfying changes in law or any other lawful purpose, but no such action may adversely affect rights under an award without the holder’s consent. Except in response to a sale event or a reorganization, recapitalization, reclassification, stock dividend, stock split, reverse stock split or other similar change in our capital stock, the Administrator may not exercise its discretion to reduce the exercise price of outstanding options or share appreciation rights or effect repricing through cancellation for cash or re-grants without prior shareholder approval. Certain amendments to the 2021 Plan require the approval of our shareholders.
The Administrator may modify the terms and conditions of any award granted to individuals outside of the United States to comply with foreign laws and may establish subplans and may modify the exercise procedures and other such procedures to the extent the Administrator determines such actions are necessary or advisable, provided, that no subplan shall increase the number of common shares reserved for issuance under the 2021 Plan.
No awards may be granted under the 2021 Plan after the tenth anniversary of the date upon which our shareholders approve the 2021 Plan and no incentive stock options many be granted after the tenth anniversary of the date the 2021 Plan is approved by our board of directors.
125
2021 Employee Stock Purchase Plan
Upon completion of the Merger, our 2021 Employee Stock Purchase Plan, or the ESPP, became effective. Eligible employees located in the United States may elect to participate in the ESPP, and eligible employees located outside of the United States may elect to participate in a sub-plan to the ESPP intended for participants outside of the United States. The ESPP (but not any sub-plan thereof) is intended to qualify as an “employee stock purchase plan” under Section 423 of the Code. Except where noted, all references to the ESPP in this Annual Report shall be understood to refer to the ESPP and all of its sub-plans, collectively.
The ESPP is intended to provide eligible employees with the opportunity to purchase common shares through accumulated payroll deductions, at a discount from market price, thereby providing eligible employees with the ability to acquire an equity interest in the Company. We believe this incentivizes employees and further aligns their interests with those of our shareholders. The material terms of the ESPP are summarized below.
We initially reserved 15,265,380 common shares for issuance under the ESPP. The ESPP provides that the number of shares reserved and available for issuance under the ESPP will automatically increase on each January 1, beginning on January 1, 2022, by an amount equal to the lesser of (i) 50,000,000 common shares, and (ii) a number of common shares reflecting 5% of our fully diluted share capital as of such date. This number is subject to adjustment in the event of a reorganization, recapitalization, reclassification, share dividend, share split, reverse share split or other similar change in our capitalization.
The ESPP will be administered by the Administrator, which will be either be our board of directors, the compensation committee or a similar committee performing the functions of the compensation committee. The Administrator has the authority to construe, interpret and apply the terms of the ESPP, including the authority to delegate ministerial duties to employees, designate separate offerings under the ESPP, designate subsidiaries as participating in the ESPP, and to determine eligibility under the ESPP.
Any of our employees or those of our designated subsidiaries are eligible to participate in the ESPP, provided that the Administrator will have the authority to designate certain categories of employees as eligible or ineligible to participate in any given Offering Period (as defined below) under the ESPP. However, no employee will be eligible to participate in a given Offering Period if, after receiving a “Purchase Option” (as defined below) for such Offering Period, that employee would hold a number of common shares (or options to purchase common shares under the ESPP) representing 5% or more of the total combined voting power or value of the common shares then outstanding. Eligible employees, or Participants, may participate in the ESPP by completing and submitting to us a subscription agreement authorizing contributions for the ESPP or by following another enrollment procedure determined by the Administrator.
The ESPP operates by offering Participants the right to use accumulated payroll deductions to purchase common shares, or Purchase Options, through a series of successive or overlapping offering periods, or Offering Periods. Offering Periods under the ESPP are typically the consecutive three-month periods running concurrently with each calendar quarter during the year, although the Administrator may change the duration, frequency, start date and end date of Offering Periods in its discretion, provided that Offering Periods may not exceed 27 months. A Participant who enrolls in a given Offering Period under the ESPP will elect to have a set amount, or a Contribution, deducted from each paycheck during the Offering Period, and contributed to an account established for the Participant under the ESPP. A Participant’s Contributions during a single Offering Period may not exceed 15% of his or her compensation during that Offering Period, subject to an overall limit of $25,000 per calendar year. Notwithstanding the foregoing, with respect to Participants in an ESPP sub-plan located outside of the U.S. only, this $25,000 overall limit may be adjusted by the Administrator in its absolute discretion to account for currency exchange rate fluctuations or other reasons.
On the first trading day of each Offering Period, or the Enrollment Date, each Participant in such Offering Period will be granted a Purchase Option. These Purchase Options will be automatically exercised to purchase common shares on the last trading date of the applicable Offering Period, or the Exercise Date, at a price per common share equal to 85% of the lesser of (i) the closing per common share price on the Enrollment Date of the Offering Period, and (ii) the closing per common share price on the Exercise Date of the Offering Period. The maximum possible number of whole common shares will be purchased for each Participant with the accumulated Contributions from his or her account, with any remaining Contribution amounts held over to be applied to the next Offering Period. No Participant may purchase more than 3,500 common shares in a single Offering Period. As soon as reasonably practicable after each Exercise Date on which a Participant’s Contributions are used to purchase common shares, we will arrange for the delivery of such purchased common shares to such Participant in a form determined by the Administrator and pursuant to rules established by the Administrator. No Participant will have any voting, dividend, or other stockholder rights with respect to common shares until those shares have been purchased and delivered to the Participant.
126
Participants are not required to retain common shares purchased under the ESPP for any minimum period of time following delivery of such common shares. However, Participants located in the United States must provide us with prompt notice if they dispose of any common shares acquired under the ESPP (i) within two years after the Enrollment Date of the Offering Period in which such common shares were purchased, or (ii) within one year after the delivery of such common shares to the Participant.
Once a Participant initially enrolls in the ESPP by submitting a subscription agreement (or otherwise enrolls following the applicable enrollment procedure established by the Administrator), the Participant’s enrollment elections will remain in effect for all future Offering Periods, and the Participant will be automatically re-enrolled in each successive Offering Period following the first Offering Period in which he or she elects or participate unless he or she affirmatively withdraws from the ESPP. A Participant may withdraw from the ESPP by submitting a written notice of withdrawal to us or by following a withdrawal procedure determined by the Administrator, in either case, at any time prior to the Enrollment Date for a given Offering Period. If a Participant withdraws from the ESPP prior to the commencement of an Offering Period, no Contributions will be made for that Offering Period or for future Offering Periods unless and until the Participant re-enrolls in the ESPP. A Participant’s withdrawal from an Offering Period will not have any effect on his or her eligibility to participate in future Offering Periods under the ESPP, or in any similar plan that we may adopt in the future. Similarly, a Participant who is already enrolled in an Offering Period in process may withdraw all (but not less than all) of the Contributions credited to his or her account, but not yet used, for that Offering Period pursuant to this same process, and the Participant will similarly be deemed to have withdrawn from the ESPP and all future Offering Periods unless and until he or she re-enrolls. Any withdrawal election must be made at least 15 business days before an Exercise Date in order to be effective before the purchase on that Exercise Date.
If a Participant ceases to be eligible to participate in the ESPP for any reason, he or she will be deemed to have withdrawn from the ESPP. In that event, any outstanding Contributions not yet used will be returned to the Participant or, in the case of a withdrawal due to his or her death, to whoever the Participant has designated as a beneficiary, and such Participant’s Purchase Option will be automatically terminated.
If permitted by the Administrator, a Participant may designate a beneficiary who will receive any common shares and cash, if any, from his or her ESPP account if the Participant dies after the Exercise Date with respect to a given Offering Period, but before the common shares purchased on that Exercise Date are delivered by the Company. In addition, if permitted by the Administrator, a Participant may designate a beneficiary who will receive any cash from the Participant’s ESPP account if the Participant dies prior to the Exercise Date with respect to a given Purchase Option. If a Participant is married and the designated beneficiary is not the Participant’s spouse, the designation will not be effective unless the Participant’s spouse consents to the designation. A designation of beneficiary may be changed by the Participant at any time by notice in a form determined by the Administrator.
Contributions credited to a Participant’s account and any rights with regard to the exercise of an Purchase Option under the ESPP may not be assigned, transferred, pledged or otherwise disposed of in any way, other than by will or the laws of descent and distribution.
If any dividend or other distribution, recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, repurchase, or exchange of common shares or other securities of the Company, or other change in the corporate structure of the Company affecting the common shares, occurs, the Administrator will equitably adjust the number and class of common shares that may be delivered under the ESPP, the purchase price per common share and the number of common shares covered by each Purchase Option under the ESPP that has not yet been exercised in order to prevent dilution or enlargement. In the event of the proposed dissolution or liquidation of the Company, any Offering Period that is in progress will be shortened and will terminate immediately prior to such dissolution or liquidation. In the event of a “sale event,” as defined in the ESPP, each outstanding Purchase Option will be assumed or substituted for an equivalent Purchase Option by the successor corporation; provided, however, that if the successor corporation refuses to assume or substitute the Purchase Option, the Offering Period for that Purchase Option will be shortened and will terminate before the date of the sale event.
The Administrator may amend, suspend or terminate the ESPP at any time and for any reason. If the ESPP is terminated, the Administrator may elect to terminate all outstanding Offering Periods either immediately or upon completion of the purchase of common shares on the next Exercise Date, or the Administrator may permit Offering Periods to expire in accordance with their terms. If the Offering Periods are terminated prior to their expiration, all unused Contributions in the Participants’ accounts will be returned to the Participants.
127
The ESPP became effective upon completion of the Merger, and shall have a term of 10 years, unless terminated earlier by the Administrator.
C. Board Practices
Board of Directors
LumiraDx’s board of directors is currently composed of ten members. As a foreign private issuer, under the Nasdaq listing rules of The Nasdaq Stock Market (“Nasdaq”), LumiraDx is not required to have independent directors on its board of directors, except that the audit committee is required to consist fully of independent directors, subject to certain phase-in schedules. LumiraDx’s board of directors has determined that, of its ten directors, no director other than Ron Zwanziger, Dave Scott, Ph.D., Jerry McAleer, Ph.D. and Troyen Brennan, M.D., M.P.H., has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these other directors is “independent” as that term is defined under Nasdaq listing rules.
The terms of LumiraDx’s arrangements with BMGF, Morningside and CVS grant each of BMGF, Morningside and (under certain circumstances) CVS the right to appoint a director to LumiraDx’s board of directors. Lu Huang is the designated director appointee of Morningside. BMGF has elected not to appoint a director at this time, but retains its right to appoint a director. Under the applicable arrangements, the appointment rights shall terminate, (i) in the case of BMGF or Morningside, once either party sells or no longer controls more than 25%; or (ii) in the case of CVS, once a sale or combination of sales results in it beneficially owning less than 75%, in each case of their respective initial holding of LumiraDx’s ordinary shares. Morningside and BMGF are also entitled to designate one person to attend all meetings of the board of directors in an observer capacity and receive all documents and materials that are provided to each director.
In accordance with the terms of LumiraDx’s Amended and Restated Articles, the board of directors is divided into three groups designated as the Founder Directors and the Class I and Class II directors. Each of the Class I and Class II directors will serve staggered two-year terms. Upon the expiration of the term of either the Class I or the Class II directors, the directors in that class will be eligible to be re-elected for a new two-year term at the annual general meeting of shareholders in the year in which their term expires. Each term of a Class I or Class II director will continue until the election of his or her successor, or his or her earlier death, resignation, or removal (in accordance with the provisions of LumiraDx’s Amended and Restated Articles). Any increase or decrease in the number of the Class I and Class II directors will be distributed among the two classes so as to make the two classes as nearly as equal in number as is reasonably practicable.
The classes are divided as follows:
•The Class I directors are Donald Berwick, George Neble and Lu Huang, and their terms will expire at LumiraDx’s annual general meeting of shareholders to be held in 2022; and
•The Class II directors will be Bruce Keogh, Lurene Joseph, Gerald Chan and Troyen Brennan, and their terms will expire at LumiraDx’s annual general meeting of shareholders to be held in 2023.
The number of Founder Directors is three and is be comprised of LumiraDx’s co-founders, Ron Zwanziger, Dave Scott and Jerry McAleer. The Founder Directors will remain in office until a Founder Director resigns or otherwise ceases to be a director in accordance with the provisions of the Amended and Restated Articles. Any resolution to remove a Founder Director requires the voting approval of the ordinary shares held by Ron Zwanziger, our Chief Executive Officer and co-founder, and his affiliates. In the event a Founder Director retires or otherwise ceases to be a director in accordance with the provisions of the Amended and Restated Articles, the appointment of any replacement Founder Director will require the approval of Ron Zwanziger (for and on behalf of each of the Founder Directors).
The number of directors (other than any alternate directors) is at least three and is subject to any maximum number fixed from time to time by a resolution of the majority of the board of directors and the approval of the Founder Directors. Directors (other than a Founder Director) can be appointed to the board of directors by way of an ordinary resolution or a majority decision of the board of directors, save that any vacancies on the board of directors (other than in the case of the Founder Directors) resulting from death, resignation, disqualification, removal or other cause may be filled by a majority decision of the board of directors only. This classification of the board of directors may have the effect of delaying or preventing changes in control of LumiraDx.
128
Committees of the Board of Directors
The board of directors has two standing committees, the audit committee and the compensation committee. In the future, the board of directors may establish other committees, as it deems appropriate, to assist with its responsibilities.
LumiraDx’s audit committee consists of George Neble, Lurene Joseph and Donald Berwick, and assists the board of directors in overseeing our accounting and financial reporting processes. George Neble serves as chairperson of our audit committee. The audit committee consists exclusively of members of the board who are financially literate, and George Neble is considered an “audit committee financial expert” as defined by applicable SEC rules and has the requisite financial sophistication as defined under the applicable Nasdaq listing rules. The board has determined that all of the members of the audit committee satisfy the “independence” requirements set forth in Rule 10A-3 under the Exchange Act. Members will serve on the audit committee until their resignation or until otherwise determined by the board of directors.
The audit committee meets at least four times per year and oversees and reviews the Company's internal controls, accounting policies and financial reporting, and provides a forum through which the Company’s independent registered public accounting firm reports. The audit committee meets regularly with the Company’s independent registered public accounting firm without management present. The audit committee is governed by a charter compliant with Nasdaq listing rules, outlining the audit committee’s responsibilities, which include:
•recommending the appointment of the independent auditor to the annual general meeting of shareholders;
•the appointment, compensation, retention and oversight of any accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit services;
•pre-approving the audit services and non-audit services to be provided by the Company’s independent auditor before the auditor is engaged to render such services;
•evaluating the independent auditor’s qualifications, performance and independence, and presenting its conclusions to the full board of directors on at least an annual basis;
•reviewing and discussing with management and the Company’s independent registered public accounting firm the Company’s financial statements and financial reporting process; and
•reviewing, approving or ratifying any related party transactions.
LumiraDx’s compensation committee consists of Lurene Joseph, Sir Bruce Keogh and Lu Huang, and assists the board of directors in overseeing our executive compensation decisions and processes. Lurene Joseph serves as chairperson of our compensation committee. The compensation committee meets quarterly and/or as required and is governed by a charter compliant with Nasdaq listing rules, outlining the compensation committee’s responsibilities, which include:
•review of the charter of the compensation committee;
•reviewing and reassessing processes and procedures for considering and determining non-employee director and executive Officer compensation;
•preparing any report on compensation required under the rules and regulations of the SEC, the Nasdaq Stock Market rules, and any other rules and regulations applicable to the Company;
•reviewing and approving incentive-compensation and equity-based plans;
•reviewing and approving matters related to compensation of the Company’s Chief Executive Officer;
•reviewing and approving matters covered under the charter related to compensation of the officers other than the Chief Executive Officer; and
•reviewing and approving matters covered under the charter related to compensation of the Company’s non-employee directors or consulting firms or other outside advisers.
D. Employees
As of December 31, 2021, we had 1,513 employees. Of these employees, 451 were in R&D, 604 were in manufacturing and service delivery, 201 were in sales and marketing and 257 were in general and administrative functions. None of our personnel are covered by a collective bargaining agreement.
129
E. Share Ownership
See “Item 7. Major Shareholders and Related Party Transactions—A. Major Shareholders.”
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
The following table shows the beneficial ownership of LumiraDx’s common shares and ordinary shares, as of December 31, 2021, by:
•each person, or group of affiliated persons, known by us to beneficially own more than 5% of outstanding common shares and ordinary shares;
•each of our directors and executive officers; and
•all of our directors and executive officers as a group.
The number of common shares and ordinary shares beneficially owned by each entity, person, executive officer or director is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any common shares and ordinary shares over which the individual has sole or shared voting power or investment power as well as any common shares and ordinary shares that the individual has the right to acquire within 60 days from December 31, 2021 through the exercise of any option, warrant or other right. Except as otherwise indicated, and subject to applicable community property laws, we believe that the persons named in the table have sole voting and investment power with respect to all ordinary shares held by that person based on information provided to us by such person. This table is based on information supplied by our directors and officers and by Schedules 13D and Schedules 13G, if any, filed with the SEC.
The percentage of outstanding common shares beneficially owned is computed based on 45,241,767 common shares outstanding as of December 31, 2021. The percentage of outstanding ordinary shares beneficially owned is computed based on 207,462,080 ordinary shares outstanding as of December 31, 2021. Common shares and ordinary shares that a person has the right to acquire within 60 days are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person.
130
Unless otherwise noted, the business address of each beneficial owner is c/o Ocorian Trust (Cayman) Limited, PO Box 1350, Windward 3, Regatta Office Park, Grand Cayman KY1-1108.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ordinary Shares |
|
|
Common Shares |
|
|
Total Voting Power |
|
Name of Beneficial Owner |
|
Number of Shares |
|
|
Percentage of Outstanding(1) |
|
|
Number of Shares |
|
|
Percentage of Outstanding(2) |
|
|
|
|
Directors and Executive Officers of LumiraDx: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ron Zwanziger and affiliated entities (3) |
|
|
43,685,394 |
|
|
|
20.5 |
% |
|
|
1,683,414 |
|
|
|
3.7 |
% |
|
|
20.7 |
% |
|
Dorian LeBlanc and affiliated entities (4) |
|
|
2,103,183 |
|
|
|
1.0 |
% |
|
|
19,726 |
|
|
* |
|
|
|
1.0 |
% |
|
Dave Scott (5) |
|
|
15,906,373 |
|
|
|
7.5 |
% |
|
|
- |
|
|
- |
|
|
|
7.5 |
% |
|
Jerry McAleer (6) |
|
|
12,972,528 |
|
|
|
6.1 |
% |
|
|
- |
|
|
- |
|
|
|
6.1 |
% |
|
Nigel Lindner (7) |
|
|
2,670,990 |
|
|
|
1.3 |
% |
|
|
- |
|
|
- |
|
|
|
1.3 |
% |
|
David Walton (8) |
|
|
2,582,546 |
|
|
|
1.2 |
% |
|
|
- |
|
|
- |
|
|
|
1.2 |
% |
|
Peter Scheu (9) |
|
|
1,293,396 |
|
|
* |
|
|
|
- |
|
|
- |
|
|
|
0.6 |
% |
|
Veronique Ameye and affiliated entities (10) |
|
|
2,712,381 |
|
|
|
1.3 |
% |
|
|
19,726 |
|
|
* |
|
|
|
1.3 |
% |
|
Pooja Pathak (11) |
|
|
300,707 |
|
|
* |
|
|
|
- |
|
|
- |
|
|
* |
|
|
Tom Quinlan (12) |
|
|
1,872,875 |
|
|
* |
|
|
|
- |
|
|
- |
|
|
* |
|
|
Donald Berwick (13) |
|
|
353,773 |
|
|
* |
|
|
|
- |
|
|
- |
|
|
* |
|
|
Bruce Keogh (14) |
|
|
353,773 |
|
|
* |
|
|
|
- |
|
|
- |
|
|
* |
|
|
Lurene Joseph (15) |
|
|
353,773 |
|
|
* |
|
|
|
- |
|
|
- |
|
|
* |
|
|
Troyen Brennan (16) |
|
|
353,773 |
|
|
* |
|
|
|
- |
|
|
- |
|
|
* |
|
|
George Neble (17) |
|
|
221,107 |
|
|
* |
|
|
|
29,589 |
|
|
* |
|
|
* |
|
|
Lu Huang |
|
|
- |
|
|
- |
|
|
|
- |
|
|
- |
|
|
|
- |
|
|
Gerald Chan |
|
|
- |
|
|
- |
|
|
|
- |
|
|
- |
|
|
|
- |
|
|
All directors and executive officers as a group (17 individuals) |
|
|
87,736,572 |
|
|
|
38.9 |
% |
|
|
1,752,455 |
|
|
|
3.9 |
% |
|
|
41.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
William Umphrey and Affiliates (18) |
|
|
20,770,405 |
|
|
|
10.0 |
% |
|
|
4,148,026 |
|
|
|
9.1 |
% |
|
|
10.0 |
% |
|
Morningside entities (19) |
|
|
24,296,120 |
|
|
|
11.7 |
% |
|
|
19,495,155 |
|
|
|
40.9 |
% |
|
|
12.4 |
% |
|
Petrichor Healthcare Capital Management LP (20) |
|
|
3,195,284 |
|
|
|
1.5 |
% |
|
|
3,592,622 |
|
|
|
7.6 |
% |
|
|
1.7 |
% |
|
Senvest Management, LLC (21) |
|
|
5,575,474 |
|
|
|
2.7 |
% |
|
|
4,712,096 |
|
|
|
10.2 |
% |
|
|
2.9 |
% |
* Less than one percent.
(1)The percentage of beneficial ownership is based on 207,462,080 ordinary shares outstanding. Under Rule 13d-3 of the Exchange Act, beneficial ownership includes any ordinary shares as to which the holder has sole or shared voting power or investment power and also any ordinary shares which the holder has the right to acquire within 60 days of December 31, 2021 through the exercise of any option, warrant, conversion or any other right, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person.
(2)The percentage of beneficial ownership is based on 45,241,767 common shares outstanding. Under Rule 13d-3 of the Exchange Act, beneficial ownership includes any ordinary shares as to which the holder has sole or shared voting power or investment power and also any ordinary shares which the holder has the right to acquire within 60 days of December 31, 2021 through the exercise of any option, warrant, conversion or any other right, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person.
(3)Consists of (A) 43,685,394 ordinary shares beneficially owned, which includes (i) 37,907,562 ordinary shares outstanding; (ii) 541,981 ordinary shares issuable upon the exercise of the 2016 warrants (such warrants held on Mr. Zwanziger’s behalf by the USB Focus Funds); (iii) 722,759 ordinary shares held on Mr. Zwanziger’s behalf by the USB Focus Funds; and (iv) 5,235,851 ordinary shares issuable upon exercise of options; and (B) 1,683,414 common shares beneficially owned, which includes (i) 1,486,362 common shares; and (iii) 197,052 common shares issuable upon the exercise of the 2020 warrants. In addition, Mr. Zwanziger has unvested options over ordinary shares that are not exercisable within 60 days of December 31, 2021. These securities are owned by Mr. Zwanziger, by Zwanziger
131
Family Ventures LLC and Zwanziger Ventures, entities controlled by Mr. Zwanziger and certain securities held on Mr. Zwanziger’s behalf by the USB Focus Funds.
(4)Consists of (A) 2,103,183 ordinary shares beneficially owned, which includes (i) 215,800 ordinary shares outstanding; and (ii) 1,887,833 ordinary shares issuable upon exercise of options; and (B) 19,726 common shares. These securities are owned by Mr. LeBlanc and by members of his family and by Mohawk Investment Partners and Salem Rentals LLC, entities controlled by Mr. LeBlanc.
(5)Consists of 15,906,373 ordinary shares beneficially owned, which includes (i) 10,670,522 ordinary shares outstanding; and (ii) 5,235,851 ordinary shares issuable upon exercise of options. In addition, Mr. Scott has unvested options over ordinary shares that are not exercisable within 60 days of December 31, 2021.
(6)Consists of 12,972,528 ordinary shares beneficially owned, which includes (i) 7,736,677 ordinary shares outstanding; and (ii) 5,235,851 ordinary shares issuable upon exercise of options. In addition, Mr. McAleer has unvested options over ordinary shares that are not exercisable within 60 days of December 31, 2021.
(7)Consists of 2,670,990 ordinary shares beneficially owned, which includes (i) 902,123 ordinary shares outstanding; and (ii) 1,768,867 ordinary shares issuable upon exercise of options. These securities are owned by Dr. Lindner and Dr. Jayne Ellis, Dr. Lindner’s spouse. In addition, Dr. Lindner has unvested options over ordinary shares that are not exercisable within 60 days of December 31, 2021.
(8)Consists of 2,582,546 ordinary shares beneficially owned, which includes (i) 902,123 ordinary shares outstanding; and (ii) 1,680,424 ordinary shares issuable upon exercise of options. These securities are owned by Mr. Walton and Marianne Walton, Mr. Walton’s spouse.
(9)Consists of 1,293,396 ordinary shares beneficially owned, which includes (i) 55,188 ordinary shares outstanding; and (ii) 1,238,208 ordinary shares issuable upon exercise of options.
(10)Consists of (A) 2,712,381 ordinary shares beneficially owned, which includes (i) 91,272 ordinary shares outstanding; and (ii) 2,621,109 ordinary shares issuable upon exercise of options; and (B) 44,145 common shares beneficially owned, which includes (i) 36,362 common shares outstanding; and (ii) 7,783 common shares issuable upon the exercise of the 2020 warrants. These securities are owed by Ms. Ameye and by Suneet Bakhshi, Ms. Ameye’s spouse and by Jaiventures Limited, an entity controlled by Ms. Ameye.
(11)Consists of 300,707 ordinary shares beneficially owned, which includes 300,707 ordinary shares issuable upon exercise of options. In addition, Ms. Pathak has unvested options over ordinary shares that are not exercisable within 60 days of December 31, 2021.
(12)Consists of 1,872,875 ordinary shares beneficially owned, which includes 1,872,875 ordinary shares issuable upon exercise of options.
(13)Consists of 353,773 ordinary shares beneficially owned, which includes 353,773 ordinary shares issuable upon exercise of options.
(14)Consists of 353,773 ordinary shares beneficially owned, which includes 353,773 ordinary shares issuable upon exercise of options.
(15)Consists of 353,773 ordinary shares beneficially owned, which includes 353,773 ordinary shares issuable upon exercise of options.
(16)Consists of 353,773 ordinary shares beneficially owned, which includes 353,773 ordinary shares issuable upon exercise of options.
(17)Consists of (A) 221,107 ordinary shares beneficially owned, which includes 221,109 ordinary shares issuable upon exercise of options; and (B) 29,589 common shares. In addition, Mr. Neble has unvested options over ordinary shares that are not exercisable within 60 days of December 31, 2021.
(18)Consists of (A) 20,770,405 ordinary shares beneficially owned, which includes (i) 20,114,509 ordinary shares outstanding; (ii) 874,528 ordinary shares held on Mr. and Mrs. Umphrey’s behalf by the USB Focus Funds; and (iii) 655,896 ordinary shares issuable upon the exercise of the 2016 warrants (such warrants held on Mr. and Mrs. Umphrey’s behalf by the USB Focus Funds); and (B) 4,148,026 common shares beneficially owned, which includes (i) 3,748,475 common shares outstanding; and (ii) 399,551 common shares issuable upon the exercise of the 2020 warrants. These securities are owned by (i) Willard L. Umphrey, (ii) Anne M. Umphrey, Mr. Umphrey’s spouse, (iii) Pensco Trust Company, a retirement account of Mr. Umphrey; (iv) and certain securities held on his behalf by USB Focus Fund LumiraDx 1-A, LLC and USB Focus Fund LumiraDx 1-B, LLC (the “USB Focus Funds”), funds established by U.S. Boston Capital Corporation of which Mr. Umphrey is the Principal and Founder.
(19)Consists of (A) 24,296,120 ordinary shares outstanding; and (B) 19,495,984 common shares, which includes (i) 17,128,408 common shares outstanding; and (ii) 2,367,576 common shares issuable upon the exercise of the 2020 warrants. These securities are owed directly by MVIL LLC and Morningside Ventures Investments Limited. The address of MVIL LLC and Morningside Ventures Investments Limited is c/o THC Management Services S.A.M., 2nd Floor, Le Prince De Galles, 3-5 Avenue Des Citronniers, MC 98000, Monaco.
(20)Consists of (A) 3,195,284 ordinary shares beneficially owned, which includes (i) 2,892,100 ordinary shares outstanding and (ii) 303,184 ordinary shares issuable upon exercise of warrants; and (B) 3,592,622 common shares beneficially owned, which includes (i) 2,803,706 common shares outstanding and (ii) 788,916 common shares issuable upon the
132
exercise of warrants. These securities are owed directly by Petrichor Opportunities Fund I Intermediate LP and Petrichor Opportunities Fund LP. The address of Petrichor Healthcare Capital Management LP is 220 East 42nd Street, New York, NY 10017.
(21)Consists of (A) 5,575,474 ordinary shares outstanding; and (B) 4,712,096common shares beneficially owned, which includes (i) 2,783,925 common shares outstanding and (ii) 1,928,171common shares issuable upon the exercise of warrants that are subject to a 9.99% beneficial ownership blocker. These securities are owed directly by Senvest Global (KY) LP, Senvest Master Fund LP and Senvest Technology Partners Master Fund LP. The address of the reporting persons is c/o Senvest Management, LLC, 540 Madison Avenue, 32nd Floor, New York, New York 10022.
Holders
As of December 31, 2021, we had approximately 393 shareholders of record of our ordinary shares and 234 shareholders of record of our common shares. We estimate that as of December 31, 2021, approximately 71% of our outstanding ordinary shares are held by 318 U.S. record holders. We estimate that as of December 31, 2021, approximately 60% of our outstanding common shares are held by 220 U.S. record holders. The actual number of shareholders is greater than this number of record holders and includes shareholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include shareholders whose shares may be held in trust or by other entities.
Significant Changes in Ownership by Major Shareholders
We have experienced significant changes in the percentage ownership held by major shareholders as a result of our Merger. Prior to our Merger, our principal shareholders were William Umphrey and Affiliates and entities affiliated with Morningside Ventures, which held shares representing 10.0% and 12.4% of our outstanding voting power prior to the Merger.
B. Related Party Transactions
The following is a description of certain related party transactions we have entered into since January 1, 2021 with any of our executive officers, directors or their affiliates and holders of more than 10% of any class of our voting securities in the aggregate, which we refer to as related parties, other than compensation arrangements which are described under the Section of this Annual Report titled “Item 6. Directors, Senior Management and Employees.”
Registration Rights
LumiraDx granted each of Zwanziger Family Ventures, CVS and Morningside certain registration rights.
Indemnification Agreements
In connection with the completion of the Merger, LumiraDx entered into indemnification agreements with each of its directors and executive officers. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling LumiraDx pursuant to the foregoing provisions, LumiraDx has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Transactions with Related Companies
From time to time, in the ordinary course of business, LumiraDx may contract for services from companies in which certain of its executive officers or directors may serve as director or advisor. The cost of these services is negotiated on an arm’s length basis and none of these arrangements is material to LumiraDx.
Merger
On September 28, 2021, LumiraDx Limited, an exempted company with limited liability incorporated under the laws of the Cayman Islands (“LumiraDx” or the “Company”), consummated the previously announced merger pursuant to the Agreement and Plan of Merger, dated as of April 6, 2021, as amended pursuant to the Amendment to the Merger Agreement dated August 19, 2021, as further amended pursuant to the Amendment No. 2 to the Merger Agreement dated August 27, 2021 (collectively, the “Merger Agreement”), by and among LumiraDx, LumiraDx Merger Sub, Inc., a newly formed
133
Delaware corporation and wholly owned subsidiary of LumiraDx (“Merger Sub”), and CA Healthcare Acquisition Corp., a Delaware corporation (“CAH”), which, among other things, provides for Merger Sub to be merged with and into CAH with CAH being the surviving corporation in the merger (the “Merger”).
Immediately prior to the effective time of the Merger (the “Effective Time”): (A) each series A 8% cumulative convertible preferred share with a par value of US$0.0000045 each in the capital of LumiraDx that was issued and outstanding converted into one A ordinary share in the capital of LumiraDx (“ordinary shares”) in accordance with the then current memorandum of association and articles of association of LumiraDx; (B) each series B 8% cumulative convertible preferred share with a par value of US$0.0000045 each in the capital of LumiraDx that were issued and outstanding converted into common shares in the capital of LumiraDx (“common shares”) in accordance with the then current memorandum of association and articles of association of LumiraDx; (C) the 5% unsecured subordinated convertible loan notes of LumiraDx converted into 9,195,340 common shares; and (D) the 10% unsecured subordinated convertible loan notes of LumiraDx converted into 7,802,080 common shares. Immediately thereafter (but prior to the Effective Time), LumiraDx effected a subdivision of each ordinary share and each common share into such number of ordinary shares and common shares (as applicable) calculated in accordance with the terms of the Merger Agreement at a conversion factor of 1.60806264:1 to achieve an exchange ratio in the Merger of one common share for each share of common stock of CAH (the “Merger Subdivision”) which subdivided the par value of each ordinary share and common share to US$0.0000028 per share. We refer to these steps collectively as the “Capital Restructuring.”
Pursuant to the Merger Agreement and following the Capital Restructuring, each outstanding share of CAH Class B common stock converted into shares of CAH common stock immediately prior to the Effective Time, and at the Effective Time each outstanding share of CAH common stock was automatically canceled and extinguished and reissued to LumiraDx as one share of common stock of CAH, in consideration for the right to receive one common share. The outstanding CAH public warrants, by their terms, automatically entitled the holders to purchase common shares upon the completion of the Merger (the “public warrants”). In addition, pursuant to the amended and restated sponsor agreement, dated April 6, 2021, as amended pursuant to the Amendment to the Sponsor Agreement dated August 19, 2021, by and among CAH, sponsor and the CAH initial stockholders (the “Sponsor Agreement”), CA Healthcare Sponsor LLC, a Delaware limited liability company, exchanged all 4,050,000 CAH private placement warrants for 405,000 common shares.
The Merger Agreement also contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions.
Convertible Notes Offering
On March 2, 2022 we entered into privately negotiated subscription agreements with certain investors wherein we agreed to sell and the investors agreed to purchase $56.5 million of Convertible Senior Subordinated Notes due 2027. An affiliate of Ron Zwanziger, the Chief Executive Officer, co-founder and a director of the Company, purchased $2 million of Notes in the offering.
In connection with the issuance of the Notes, the Company and certain of the investors, including an affiliate of Mr. Zwanziger, entered into the Registration Rights Agreement, dated March 3, 2022. Pursuant to the terms of the Registration Rights Agreement, the Company has agreed to file a resale registration statement covering the resale of the Notes and the common shares issued or potentially issuable upon conversion of the Notes with the SEC within 60 calendar days after the closing of the Notes offering.
Related Party Transactions Policy
In connection with the completion of the Merger, LumiraDx adopted a written related party transactions policy requiring that such transactions be approved by LumiraDx’s audit committee. Pursuant to the related party transactions policy, the audit committee has the primary responsibility for reviewing and approving “related person transactions,” which are transactions between LumiraDx and related parties in which the related party has a direct or indirect material interest. For purposes of the policy, a related party is defined as a director, executive officer, nominee for director, or greater than 5% beneficial owner of any class of LumiraDx voting securities, and their immediate family members.
C. Interests of Experts and Counsel
134
Not applicable.
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
Financial Statements
See the section of this Annual Report titled “Item 18. Financial Statements,” which contains our financial statements prepared in accordance with IFRS.
Legal Proceedings
From time to time, we may be a party to litigation or subject to claims incident to the ordinary course of business. There are currently no claims or actions pending against us that, in the opinion of our management, are likely to have a material adverse effect on our business.
Dividends and Dividend Policy
LumiraDx has never declared or paid any cash dividend on the ordinary shares or the common shares, and does not anticipate declaring or paying any cash dividends on the ordinary shares or common shares in the foreseeable future. LumiraDx intends to retain all available funds and any future earnings to fund the commercialization of its products and expansion of its business.
LumiraDx is a holding company that does not conduct any business operations of its own. As a result, LumiraDx is dependent upon cash dividends, distributions and other transfers from its subsidiaries to make dividend payments, and such subsidiaries may be restricted in their ability to pay dividends or distributions, or make other transfers to us. In addition, the terms of the Senior Secured Loan preclude LumiraDx from paying cash dividends to its shareholders without the consent of Pharmakon.
However, if LumiraDx does pay a cash dividend on the common shares or ordinary shares in the future, it may only pay such dividend out of its profits available for distribution or (subject to applicable solvency requirements) share premium or contributed surplus under Cayman Islands law. LumiraDx’s board of directors will have complete discretion regarding the declaration and payment of dividends, and the Founder Directors will be able to influence its dividend policy. The amount of any future dividend payments we may make will depend on, among other factors, our strategy, future earnings, financial condition, cash flow, working capital requirements, capital expenditures, contractual restrictions and applicable provisions of the Amended and Restated Articles.
B. Significant Changes
A discussion of the significant changes in our business can be found under “Item 4. Information on the Company—A. History and Development of the Company” and “Item 4. Information on the Company—B. Business Overview.”
ITEM 9. THE OFFER AND LISTING
A. Offer and Listing Details
See “Item 9. The Offer and Listing—C. Markets.”
B. Plan of Distribution
Not applicable.
C. Markets
LumiraDx’s common shares and public warrants are listed on the Nasdaq Global Market under the symbols “LMDX” and “LMDXW”, respectively.
D. Selling Shareholders
135
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
LumiraDx is an exempted company incorporated in the Cayman Islands with limited liability. The affairs of LumiraDx are governed by its Amended and Restated Articles, the Companies Act (as revised) of the Cayman Islands, or the Cayman Companies Act, and the common law of the Cayman Islands.
As of December 31, 2021, LumiraDx’s authorized share capital is $10,290 divided into (i) 1,769,292,966 A ordinary shares (“ordinary shares”) with a par value (to seven decimal places) of US$0.0000028 per ordinary share, (ii) 1,769,292,966 common shares with a par value (to seven decimal places) of US$0.0000028 per common share and (iii) 136,414,068 undesignated shares of such class or classes (however designated) and having such rights as the board of directors may determine in accordance with the provisions of the Amended and Restated Articles.
Ordinary Shares and Common Shares
General
In accordance with the Amended and Restated Articles, the following summarizes the rights of the holders of the ordinary shares and the common shares:
•all resolutions to be voted on by shareholders at an extraordinary general meeting or at an annual general meeting will be held by way of a poll;
•each holder of ordinary shares is entitled to ten votes per ordinary share on matters to be voted on by shareholders;
•each holder of common shares is entitled to one vote per common share on matters to be voted on by shareholders;
•the holders of ordinary shares and common shares shall be entitled to receive notice of, attend, speak and vote by way of a poll at an extraordinary general meeting or at an annual general meeting (as if they were one class of shares);
•the holders of ordinary shares and common shares shall be entitled to receive such dividends as may be declared by the board of directors, which shall be distributed pro rata (as if they were one class of shares) according to the number of ordinary shares and common shares held by the relevant holder and as recommended by the directors and declared by the shareholders of LumiraDx;
•all of the issued and outstanding ordinary shares and common shares are fully paid and non-assessable;
•the ordinary shares and common shares are issued in registered form, and are issued when registered in the register of members of LumiraDx;
•LumiraDx’s board of directors may issue undesignated shares in one or more series and designate the price, rights, preferences, privileges and restrictions of such shares without any further vote or action by LumiraDx’s shareholders;
•LumiraDx’s shareholders who are non-residents of the Cayman Islands may freely hold and vote their ordinary shares and common shares; and
136
•LumiraDx may not issue shares to bearer.
Dividends
The holders of the ordinary shares and common shares are entitled to such dividends as may be declared by the board of directors of LumiraDx pro rata (equally as if they were one class of shares) according to the number of ordinary shares and common shares held. In addition, LumiraDx’s shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by the directors. Under the Cayman Companies Act, a Cayman Islands company may pay a dividend out of either: (i) profits available for distribution; or (ii) share premium or contributed surplus, provided that in no circumstances may a dividend be paid if this would result in the company being unable to pay its debts as they fall due in the ordinary course of business.
Subject to any special rights attaching to or the terms of issue of any share, all dividends shall be declared and paid according to the amounts paid up on the shares and shall be apportioned and paid pro rata according to the amounts paid up on the shares during any part or parts of the period in respect of which the dividend is paid.
No dividend or other moneys payable by LumiraDx on or in respect of any share shall bear interest against LumiraDx, unless otherwise provided by the terms on which such shares were issued or the provisions of a separate agreement between the holder of that share and LumiraDx. Any dividend unclaimed after a period of six years from the date that such dividend became due for payment shall be forfeited and shall revert to LumiraDx.
Any general meeting declaring a dividend may by ordinary resolution of the shareholders, upon the recommendation of the board of directors, direct payment or satisfaction of such dividend wholly or in part by the distribution of specific assets other than cash, and in particular of paid up shares or debentures of any other company. The directors may, if authorized by ordinary resolution of shareholders, offer any holders of shares the right to elect to receive in lieu of a dividend an allotment of shares credited as fully paid up, subject to such exclusions as the board of directors may deem necessary or desirable.
No shareholder shall be entitled to receive any dividend or other distribution in respect of any share held by him, her or it unless all calls or other sums payable by him, her or it in respect of that share have been paid.
Voting Rights
The voting rights attaching to the ordinary shares and the common shares are as follows:
•at any general meeting (being extraordinary general meetings and annual general meetings) all resolutions put to the vote of the meeting shall be decided by way of a poll;
•every holder of an ordinary share who is present in person or by proxy shall have ten votes for each ordinary share of which he/she/it is the holder; and
•every holder of a common share who is present in person or by proxy shall have one vote for each common share of which he/she/it is the holder.
An ordinary resolution to be passed by the shareholders requires the affirmative vote of a simple majority of the votes cast by the shareholders entitled to vote who are present in person or by proxy at a general meeting, while a special resolution requires the affirmative vote of no less than two thirds of the votes cast by the shareholders entitled to vote who are present in person or by proxy at a general meeting. A special resolution will be required for important matters such as approving a winding up of LumiraDx, a reduction in LumiraDx’s share capital or removing a director (other than a Founder Director) for cause. LumiraDx’s shareholders may effect certain changes by ordinary resolution, including increasing the amount of LumiraDx’s authorized share capital, consolidating and dividing all or any of the LumiraDx’s share capital into shares of larger amounts than existing shares and cancelling any authorized but unissued shares. Any resolution to remove a Founder Director requires the voting approval of the ordinary shares held by Ron Zwanziger and his affiliates.
Restrictions on Voting
No shareholder shall be entitled to vote at any general meeting or at any separate class meeting in respect of any shares held by him, her or it unless all calls or other sums payable by him, her or it in respect of that share have been paid.
137
The board of directors may from time to time make calls upon the shareholders in respect of any money unpaid on their shares and each shareholder shall (subject to at least 14 clear days’ notice specifying the time or times and place of payment) pay at the time or times so specified the amount called on his, her or its shares.
Voluntary Conversion of Ordinary Shares
At the option of the relevant holder, each ordinary share is convertible into one common share at any time.
Transfer of Ordinary Shares
Except as provided herein, no ordinary share may be transferred unless such ordinary share is first converted into a common share in accordance with the terms of the Amended and Restated Articles. Ordinary shares may be transferred (without first being converted into common shares), subject to certain conditions in the following circumstances:
(i)through a Permitted Transfer or a Mandatory Transfer (each, as described below);
(ii)pursuant to a bona fide third party offer to acquire control of LumiraDx;
(iii)pursuant to a court order as a result of divorce;
(v)by a corporate shareholder to an affiliate of such corporate shareholder.
A transfer of ordinary shares through a Permitted Transfer or in accordance with (iv) and (v) above is permitted, provided that no public disclosure or filing under the Exchange Act is required as a result of the relevant transfer other than certain permitted filings.
These restrictions on transfers of ordinary shares do not prevent holders of ordinary shares from establishing a 10b5-1 plan, although sales made under that plan are subject to the restrictions stated above. Such establishment, together with (i)-(v) above, are referred to as the “Limited Circumstances.”
For purposes of the Amended and Restated Articles:
•the following transfers of ordinary shares are “Permitted Transfers”:
otransfers by one holder of ordinary shares to another holder of ordinary shares;
otransfers to and from employee trusts;
otransfers to Privileged Relations (which, in general, includes shareholders and certain of their family members) (if being transferred by an employee) or to trusts established for the benefit of Privileged Relations or charities and/or their nominees (“Family Trust”); and
otransfers by a corporate shareholder to another member of its wholly owned group; and
•the following transfers of ordinary shares are “Mandatory Transfers”:
oif any trust ceases to be a Family Trust or there ceases to be any beneficiaries of the Family Trust, a transfer back to the settlor of that Family Trust or to a Privileged Relation of the settlor or to another Family Trust of the settlor;
oif any Privileged Relation ceases to be a Privileged Relation of the original shareholder, a transfer back to the original shareholder or to another Privileged Relation of the original shareholder or to another Family Trust of the original shareholder; and
oif a corporate shareholder ceases to be member of the same wholly owned group as the original corporate shareholder, a transfer back to the original corporate shareholder or to another member of the original corporate shareholder’s wholly owned group.
Restriction on further issuance of Ordinary Shares
138
The Amended and Restated Articles provide that except for the issuance of ordinary shares issuable upon the exercise of rights outstanding at the date of adoption of the Amended and Restated Articles (such as under existing equity award plans or existing warrants over ordinary shares), no further ordinary shares can be issued.
Transfer of Common Shares
Subject to the restrictions contained in the Amended and Restated Articles, the Nasdaq listing rules or any relevant securities laws and the restrictions imposed on certain of the common shares issued to the sponsor pursuant to the terms of the Sponsor Agreement, the common shares are freely tradeable (without restriction) and any holders may transfer all or any of his, her or its common shares by an instrument of transfer in any usual or common form or any other form approved by the board of directors.
Liquidation
On a winding up of LumiraDx, if the assets available for distribution among the holders of the ordinary shares and common shares shall be more than sufficient to repay the whole of the share capital at the commencement of the winding up, the surplus will be distributed among the holders of the ordinary shares and common shares (equally as if they were one class of shares) on a pro rata basis in proportion to the number of ordinary shares and common shares held by them. If LumiraDx’s assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by the holders of the ordinary shares and common shares in proportion to the number of the ordinary shares and common shares held by them.
The liquidator may, with the sanction of a special resolution of LumiraDx’s shareholders and any other sanction required by the Cayman Companies Act, divide among the shareholders in species or in kind the whole or any part of the assets of LumiraDx, and may for that purpose value any assets and determine how the division shall be carried out as between LumiraDx’s shareholders or different classes of shareholders.
Because LumiraDx is an exempted company with “limited liability” incorporated under the Cayman Companies Act, the liability of LumiraDx’s shareholders is limited to the amount, if any, unpaid on the shares respectively held by them. The Amended and Restated Articles contain a declaration that the liability of LumiraDx’s shareholders is so limited.
Repurchase Rights
Any repurchase of shares by LumiraDx as may be agreed with the relevant shareholders shall be approved by the board of directors in accordance with the Cayman Companies Act and the Amended and Restated Articles, and LumiraDx may make a payment in respect of such repurchase in any manner authorized by the Companies Act and the Amended and Restated Articles, including out of capital. A payment out of capital by a Cayman Islands company is not lawful unless immediately following the date on which the payment out of capital is proposed to be made, the company shall be able to pay its debts as they fall due in the ordinary course of business. Only shares that are fully paid may be repurchased, and there must be at least one share remaining in issue following the repurchase.
Variations of Rights of Shares
If at any time LumiraDx’s share capital is divided into different classes of shares, all or any of the rights attached to any class of shares may be varied with the consent in writing of the holders of not less than three-fourths of the shares of that class or with the sanction of a special resolution passed at a general meeting of the holders of the shares of that class. The rights conferred upon the holders of the shares of any class issued with preferred or other rights will not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such existing class of shares.
Notwithstanding the foregoing, the board of directors may issue undesignated shares without further action by the shareholders.
General Meetings of Shareholders
139
Shareholder meetings may be convened by a majority of the board of directors of LumiraDx. As a Cayman Islands exempted company, LumiraDx is not required by the Cayman Companies Act to convene annual general meetings of its shareholders. However, the Amended and Restated Articles and LumiraDx’s corporate governance guidelines provide that in accordance with the Nasdaq Stock Market (“Nasdaq”) listing rules in each year LumiraDx may hold an annual general meeting of shareholders. The annual general meeting shall be held at such time and place as may be determined by the board of directors in accordance with the provisions of the Amended and Restated Articles. All annual general meetings and extraordinary general meetings will be chaired by the Chairman (as defined below) or a member of the board of directors.
The Cayman Companies Act provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. However, these rights may be provided in a company’s articles of association. The Amended and Restated Articles provide that upon the requisition of shareholders representing not less than one-third of the voting rights entitled to vote at general meetings, the board will convene an extraordinary general meeting and put the resolutions so requisitioned to a vote at such meeting. However, shareholders may propose only ordinary resolutions (and not a special resolution) to be put to a vote at such meeting and shall have no right to propose resolutions with respect to the election, appointment or removal of directors or to amend the Amended and Restated Articles. The Amended and Restated Articles provide no other right to put any proposals before annual general meetings or extraordinary general meetings.
Advance notice of at least 21 clear days is required for the convening of the annual general meeting and 14 clear days’ notice for the convening of any extraordinary general meeting of shareholders. All general meetings of shareholders shall occur at such time and place as determined by the directors and set forth in the notice for such meeting.
No business shall be transacted at any general meeting unless a quorum is present. At least two shareholders present in person or by proxy and entitled to vote shall be a quorum for all purposes.
Class Meetings
The provisions in the Amended and Restated Articles relating to extraordinary general meetings shall apply, mutatis mutandis, to every separate extraordinary general meeting of the holders of a class of shares.
Nomination, Election and Removal of Directors
Number
The number of directors (other than any alternate directors) is subject to a minimum of three and is subject to any maximum number fixed from time to time by a resolution of the majority of the board of directors and the approval of the Founder Directors.
Appointment
The Amended and Restated Articles provide that persons standing for election as directors at a duly constituted general meeting with requisite quorum will be elected by an ordinary resolution of shareholders, which requires the affirmative vote of a simple majority of the votes cast on the resolution by the shareholders entitled to vote who are present in person or by proxy at the meeting. Without prejudice to the power to appoint any person to be a director by shareholder resolution, the board of directors have the power to appoint any person to be a director (other than a Founder Director), either to fill a casual vacancy or as an addition to the existing board of directors.
The Amended and Restated Articles further provide that the board of directors are divided into three groups designated as the Founder Directors and the Class I and Class II directors. Each of the Class I and Class II directors shall serve staggered two-year terms. Upon the expiration of the term of either the Class I or the Class II directors, the directors in that class will be eligible to be re-elected for a new two-year-term at the annual general meeting of shareholders in the year in which their term expires. Directors assigned to Class I shall initially serve until the first annual general meeting of shareholders following the effectiveness date of the Amended and Restated Articles and directors assigned to Class II shall initially serve until the second annual general meeting of shareholders following the effectiveness date of the Amended and Restated Articles. Each term of a Class I or Class II director will continue until the election of his or her successor, or his or her earlier death, resignation, or removal in accordance with the provisions of the Amended and Restated Articles. Any increase or decrease in the number of the Class I and Class II directors will be distributed among the two classes so as to make the two classes as nearly as equal in number as is reasonably practicable.
For so long as Ron Zwanziger, Dave Scott and Jerry McAleer (the “Founders”) and each of their respective affiliates control, directly or indirectly, any of the ordinary shares then outstanding, Ron Zwanziger (for and on behalf of each of the Founders)
140
shall be entitled to nominate and have appointed (and remove and replace) by written notice to LumiraDx three directors (the “Founder Directors”). The number of Founder Directors is three. The Founder Directors will remain in office until a Founder Director resigns or otherwise ceases to be a director in accordance with the provisions of the Amended and Restated Articles. Any resolution to remove a Founder Director requires the voting approval of the ordinary shares held by Ron Zwanziger and his affiliates (for and on behalf of the Founder Directors).
Re-election/Removal
A director will be removed from office automatically if, among other things, the director (i) is prohibited from being a director by any applicable law; (ii) dies or becomes bankrupt or makes any arrangement or composition with his or her creditors generally; (iii) is found of unsound mind; (iv) resigns his or her office by notice in writing to LumiraDx; or (v) save in the case of a Founder Director, that person has, for more than six consecutive months, been absent without permission of the directors from meetings of the board and the directors make a decision that that person’s office be vacated. In addition, any director, other than a Founder Director, may be removed by special resolution for cause. The notice of any meeting at which a resolution to remove a director shall be proposed or voted upon must contain a statement of the intention to remove that director and such notice must be served on that director not less than 10 business days before the meeting. Such director is entitled to attend the meeting and be heard on the motion for his or her removal.
Proceedings of Board of Directors
The Amended and Restated Articles provide that LumiraDx’s business is to be managed and conducted by the board of directors. The quorum necessary for the transaction of business of the directors is two eligible directors, provided that at least one director is a Founder Director.
In addition, the Amended and Restated Articles provide that the board of directors may from time to time at its discretion exercise all powers of LumiraDx to raise capital or borrow money, to mortgage or charge all or any part of the undertaking, property and assets (present and future) and uncalled capital and, subject to the Cayman Companies Act, issue debentures, bonds and other securities of LumiraDx, whether outright or as collateral security for any debt, liability or obligation of LumiraDx or of any third party.
Chairman
Unless otherwise agreed by the holder(s) of the majority of the ordinary shares at the relevant time with the approval of the ordinary shares held by Ron Zwanziger and his affiliates, the chairman of the board of directors will be Ron Zwanziger, or the Chairman. The chairman of the board of directors has a casting vote if the numbers of votes for and against any board resolution are equal.
Directors’ Interests
The directors of LumiraDx may authorize, to the fullest extent permitted by Cayman Companies Act and the Nasdaq listing rules, any matter proposed to them which would otherwise result in a director infringing his or her duty to avoid a situation in which he or she has, or can have, a direct or indirect interest that conflicts, or possibly may conflict, with the interests of LumiraDx. A director shall not, save as otherwise agreed by him or her, be accountable to LumiraDx for any benefit which he or she derives from any matter authorized by the directors and any contract, transaction or arrangement relating thereto shall not be liable to be avoided on the grounds of any such benefit.
A director who is in any way, whether directly or indirectly, interested in a proposed or existing transaction or arrangement with LumiraDx shall declare the nature of his or her interest at a meeting of the directors. Provided it is permitted by Cayman Companies Act and the Nasdaq listing rules, and provided he or she has disclosed to the other directors the nature and extent of his or her interest, a director may be a party to, or otherwise directly or indirectly interested in any contract, arrangement or proposal with LumiraDx and may participate in the meeting on which the relevant resolution is being voted upon.
If a question arises at a meeting of the board of directors or of a board committee as to the right of a director to vote or be counted in the quorum, and such question is not resolved by such director voluntarily agreeing to abstain from voting or not to be counted in the quorum, the question shall be determined by a majority of votes of the remaining directors present at the meeting or if there is an equality of votes, the chairman of the board of directors shall have a second or casting vote and his or her ruling in relation to any director other than himself shall be final and conclusive except in a case where the nature or extent of the interest of the director concerned has not been fairly disclosed.
Directors’ Fees and Remuneration
141
Each of the directors shall be paid a fee at such rate as may from time to time be determined by the board of directors (or for the avoidance of doubt any duly authorized board committee). Each director may be paid for reasonable expenses properly incurred in connection with their attendance at and returning from meetings of the board or board committees or general meetings or separate meetings of the holders of classes of shares or of debentures and shall be paid all expenses properly incurred by him or her in the conduct of the business of LumiraDx or in the discharge of his or her duties as a director.
Inspection of Books and Records
Holders of the ordinary shares and common shares have no general right under Cayman Companies Act to inspect or obtain copies of the list of shareholders of LumiraDx or any corporate records, provided that they are entitled to a copy of the Amended and Restated Articles. Copies of annual financial statements will also be provided to LumiraDx’s registered office service provider in the Cayman Islands to allow for compliance with certain obligations under Cayman law.
Changes in Capital
The shareholders of LumiraDx may from time to time by ordinary resolution:
•increase the share capital by such sum, to be divided into shares of such classes and amount, as the resolution shall prescribe;
•consolidate and divide all or any of the share capital into shares of a larger amount than the existing shares of LumiraDx;
•sub-divide LumiraDx’s existing shares, or any of them into shares of a smaller amount, provided that in the subdivision the proportion between the amount paid and the amount, if any, unpaid on each reduced share shall be the same as it was in case of the share from which the reduced share is derived; or
•cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person and diminish the amount of LumiraDx’s share capital by the amount of the shares so cancelled.
LumiraDx’s shareholders may by special resolution, subject to any confirmation or consent required by the Cayman Companies Act, reduce the share capital of LumiraDx or any capital redemption reserve in any manner permitted by law.
Restrictive Provisions
Under the Amended and Restated Articles, in connection with any change of control, merger or sale of LumiraDx, the holders of ordinary shares and common shares shall receive the same consideration with respect to their ordinary shares and common shares in connection with any such transaction.
Exempted Company
LumiraDx is an exempted company with limited liability incorporated under the Cayman Companies Act. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. As an exempted company, LumiraDx has received an undertaking from the Governor-in-Cabinet of the Cayman Islands that, for a period of 20 years from the date of the grant of the undertaking, no law which is thereafter enacted in the Cayman Islands imposing any tax to be levied on profits or income or gains or appreciations will apply to LumiraDx and its operations; and, in addition, that no tax to be levied on profits, income, gains or appreciations or which is in the nature of estate duty or inheritance tax will be payable on or in respect of LumiraDx’s shares, debentures or other obligations, or by way of the withholding in whole or in part of any relevant payment. “Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on the shares of LumiraDx.
Register of Members
Under the Cayman Companies Act, LumiraDx must keep a register of members and there should be entered therein:
•the names and addresses of the shareholders of LumiraDx, a statement of the shares held by each member (such statement will distinguish each share by its number (where applicable), confirm the amount paid or agreed to be considered as paid on the shares of each member, confirm the number and category of the shares held by each member and confirm whether each relevant category of shares held by a member carries voting rights under the articles of association of LumiraDx, and if so, whether such voting rights are conditional);
•the date on which the name of any person was entered on the register as a member; and
•the date on which any person ceased to be a member.
142
Under the Cayman Companies Act, the register of members of a company is prima facie evidence of the matters set out in the register (that is, the register of members will raise a presumption of fact on the matters referred to above unless rebutted) and a member registered in the register of members is deemed as a matter of Cayman Companies Act to have legal title to the shares as set against its name in the register of members. The shareholders recorded in the register of members are be deemed to have legal title to the shares set against their names.
If the name of any person is incorrectly entered in or omitted from the register of members, or if there is any default or unnecessary delay in entering on the register the fact of any person having ceased to be a member of the company, the person or member aggrieved (or any member of the company or the company itself) may apply to the Grand Court of the Cayman Islands for an order that the register be rectified, and the Court may either refuse such application (with or without cost to be paid the applicant) or it may, if satisfied of the justice of the case, make an order for the rectification of the register.
LumiraDx’s register of members is maintained by Ocorian Trust (Cayman) Limited.
Differences in Corporate Law
The Cayman Companies Act is derived, to a large extent, from the older Companies Acts of England and Wales (being the Companies Act 1985) but does not enact all of the provisions of the more recent Companies Act of England and Wales (being the Companies Act 2006). In addition, the Cayman Companies Act differs from laws applicable to United States corporations and their shareholders. Set forth below is a summary of certain significant differences between the provisions of the Cayman Companies Act applicable to LumiraDx and the comparable laws applicable to companies incorporated in the State of Delaware in the United States.
Mergers and Similar Arrangements
The Cayman Companies Act permits mergers and consolidations between Cayman Islands companies and between Cayman Islands companies and non-Cayman Islands companies. For these purposes, (i) “merger” means the merging of two or more constituent companies and the vesting of their undertaking, property and liabilities in one of such companies as the surviving company, and (ii) a “consolidation” means the combination of two or more constituent companies into a consolidated company and the vesting of the undertaking, property and liabilities of such companies to the consolidated company. In order to effect such a merger or consolidation, the directors of each constituent company must approve a written plan of merger or consolidation, which must then be authorized by (a) a special resolution of the shareholders of each constituent company, and (b) such other authorization, if any, as may be specified in such constituent company’s articles of association. The plan of merger or consolidation must be filed with the Registrar of Companies of the Cayman Islands together with a declaration as to the solvency of the consolidated or surviving company, a list of the assets and liabilities of each constituent company and an undertaking that a copy of the certificate of merger or consolidation will be given to the members and creditors of each constituent company and that notification of the merger or consolidation will be published in the Cayman Islands Gazette. Dissenting shareholders have the right to be paid the fair value of their shares if they follow the required procedures under the Cayman Companies Act subject to certain exemptions. The fair value of the shares will be determined by the Cayman Islands court if it cannot be agreed among the parties. Court approval is not required for a merger or consolidation effected in compliance with these statutory procedures.
A merger between a Cayman parent company and its Cayman subsidiary or subsidiaries does not require authorization by a resolution of shareholders provided that a copy of the plan of merger is given to every shareholder of each subsidiary company to be merged (unless that shareholder agrees otherwise). For this purpose a subsidiary is a company of which at least 90% of the issued shares entitled to vote are owned by the parent company.
The consent of each holder of a fixed or floating security interest of a constituent company is required unless this requirement is waived by a court in the Cayman Islands.
Except in certain limited circumstances, a dissenting shareholder of a Cayman Islands constituent company is entitled to payment of the fair value of his, her or its shares upon dissenting from a merger or consolidation. The exercise of such dissenter rights will preclude the exercise by the dissenting shareholder of any other rights to which he, she or it might otherwise be entitled by virtue of holding shares, except for the right to seek relief on the grounds that the merger or consolidation is void or unlawful. Dissent rights do not extend to shares for which an open market exists on a recognized stock exchange or share quotation system.
In addition, there are statutory provisions that facilitate the reconstruction and amalgamation of companies, provided that the arrangement is approved by a majority in number of each class of shareholders and creditors with whom the arrangement is to be made, and who must, in addition, represent three-fourths in value of each such class of shareholders or creditors, as the case
143
may be, that are present and voting either in person or by proxy at a meeting, or meetings, convened for that purpose. The convening of the meetings and subsequently the arrangement must be sanctioned by the Grand Court of the Cayman Islands. While a dissenting shareholder has the right to express to the court the view that the transaction ought not to be approved, the court can be expected to approve the arrangement if it determines that:
•the statutory provisions as to the required majority vote have been met;
•the shareholders have been fairly represented at the meeting in question and the statutory majority are acting bona fide without coercion of the minority to promote interests adverse to those of the class;
•the arrangement is such that may be reasonably approved by an intelligent and honest man of that class acting in respect of his or her interest; and
•the arrangement is not one that would more properly be sanctioned under some other provision of the Cayman Companies Act.
When a takeover offer is made and accepted by holders of 90% of the shares affected within four months the offeror may, within a two-month period commencing on the expiration of such four month period, require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection can be made to the Grand Court of the Cayman Islands but this is unlikely to succeed in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or collusion.
If an arrangement and reconstruction is thus approved, or if a takeover offer is made and accepted, a dissenting shareholder would have no rights comparable to appraisal rights, which would otherwise ordinarily be available to dissenting shareholders of Delaware corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
Shareholders’ Suits
In principle, LumiraDx will normally be the proper plaintiff to sue for a wrong done to it, and as a general rule, a derivative action may not be brought by a minority shareholder. However, based on English law authorities, which would in all likelihood be of persuasive authority in the Cayman Islands, the Cayman Islands courts can be expected to follow and apply the common law principles (namely the rule in Foss v. Harbottle and the exceptions thereto) so that a non-controlling shareholder may be permitted to commence a class action against or derivative actions in the name of LumiraDx to challenge:
•an act that is illegal or ultra vires with respect to LumiraDx and is therefore incapable of ratification by the shareholders;
•an act that, although not ultra vires, requires authorization by a qualified (or special) majority (that is, more than a simple majority) that has not been obtained; and
•an act that constitutes a “fraud on the minority” where the wrongdoers are themselves in control of LumiraDx.
Indemnification of Directors and Executive Officers and Limitation of Liability
The Cayman Companies Act does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. The Amended and Restated Articles provide that LumiraDx shall indemnify its officers and directors against all actions, proceedings, costs, charges, expenses, losses, damages or liabilities incurred or sustained by such directors or officers, other than by reason of such person’s dishonesty, willful default or fraud, in or about the conduct of LumiraDx’s business or affairs (including as a result of any mistake of judgment) or in the execution or discharge of his or her duties, powers, authorities or discretions, including without prejudice to the generality of the foregoing, any costs, expenses, losses or liabilities incurred by such director or officer in defending (whether successfully or otherwise) any civil proceedings concerning LumiraDx or its affairs in any court whether in the Cayman Islands or elsewhere. This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation. In addition, LumiraDx has entered into indemnification agreements with its directors and executive officers that provide such persons with additional indemnification beyond that provided in the Amended and Restated Articles.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling LumiraDx under the foregoing provisions, LumiraDx has been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
144
Anti-Takeover Provisions in the Amended and Restated Articles
The Amended and Restated Articles include a number of provisions that could deter hostile takeovers or delay or prevent changes in control of the board of directors or management team. They are also designed, in part, to encourage persons seeking to acquire control of LumiraDx to negotiate first with the board of directors of LumiraDx. LumiraDx believes that the benefits of the increased protection of an ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire LumiraDx because negotiation of these proposals could result in an improvement of their terms. Such provisions include:
•Dual Class Stock. The Amended and Restated Articles provide for a dual class structure, which provides the current holders of the ordinary shares and the Founder Directors with significant influence over all matters requiring shareholder approval, including the election of directors and significant corporate transactions, such as a merger or other sale of LumiraDx of its assets.
•Board of Directors Vacancies. The Amended and Restated Articles authorize only the board of directors to fill vacant directorships, including newly created seats. In addition, the number of directors constituting the board of directors (other than alternative directors) shall be at least three and is subject to any maximum number fixed from time to time by a resolution of a majority of the board of directors and the approval of the Founder Directors. These provisions would prevent a shareholder from increasing the size of the board of directors and then gaining control of the board of directors by filling the resulting vacancies with its own nominees. This will make it more difficult to change the composition of the board of directors and promote continuity of management.
•Classified Board. The Amended and Restated Articles provide that the board of directors is classified into three classes of directors (being the Founder Directors, the Class I directors and the Class II Directors). A third-party may be discouraged from making a tender offer or otherwise attempting to obtain control of LumiraDx as it is more difficult and time consuming for shareholders to replace a majority of the directors on a classified board of directors.
•Shareholder Action; Special Meeting of Shareholders. The Amended and Restated Articles provide that the LumiraDx shareholders may not take action by written consent, but may only take action at an annual general meeting or extraordinary general meeting of the shareholders. As a result, a holder controlling a majority of LumiraDx’s share capital would not be able to amend the Amended and Restated Articles or remove directors without holding a meeting of the shareholders called in accordance with the Amended and Restated Articles. The Amended and Restated Articles further provide that special meetings of the shareholders may be called only by shareholders holding not less than one-third of the voting rights who are entitled to vote at general meetings. However, shareholders may propose only ordinary resolutions to be put to a vote at such meetings and shall have no right to propose resolutions with respect to the election, appointment or removal of directors or to amend the Amended and Restated Articles. The Amended and Restated Articles provide no other right to put any proposals before annual general meetings or extraordinary general meeting. These provisions might delay the ability of the shareholders to force consideration of a proposal or for shareholders controlling a majority of LumiraDx’s share capital to take any action, including the removal of directors.
•Founder Directors. The Amended and Restated Articles provide that any resolution to remove a Founder Director requires the voting approval of the ordinary shares held by Ron Zwanziger, LumiraDx’s Chief Executive Officer and co-founder, and his affiliates. This provision would prevent shareholders from removing any of the Founder Directors from their respective positions on the board who LumiraDx considers as being fundamental to the running and continued development LumiraDx’s business.
•Class I and Class II Directors Removed Only for Cause. The Amended and Restated Articles provide that shareholders may only remove the Class I and Class II directors for cause by way of passing a special resolution.
•Issuance of Undesignated Shares. The board of directors has the authority, without further action by the shareholders, to issue undesignated shares of par value with rights and preferences, including voting rights, designated from time to time by the board of directors. The existence of authorized but unissued undesignated shares would enable the board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest, or other means.
Under the Cayman Companies Act, the directors may only exercise the rights and powers granted to them under the Amended and Restated Articles, as amended and restated from time to time, for what they believe in good faith to be in the best interests of LumiraDx and for a proper purpose.
145
Directors’ Fiduciary Duties
Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself or herself of, and disclose to shareholders, all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he or she reasonably believes to be in the best interests of the corporation. He or she must not use his or her corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interests of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally.
In general, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Should such evidence be presented concerning a transaction by a director, a director must prove the transaction was procedurally fair and provided fair value to the corporation. As a matter of Cayman law, a director of a Cayman Islands company is in the position of a fiduciary with respect to the company and therefore owes the following duties to the company—a duty to act bona fide in the best interests of the company, a duty not to make a profit based on his or her position as director (unless the company permits him or her to do so), a duty not to put himself or herself in a position where the interests of the company conflict with his or her personal interest or his or her duty to a third-party, and a duty to exercise powers for the purpose for which such powers were intended. A director of a Cayman Islands company owes to the company a duty to act with skill and care. It was previously considered that a director need not exhibit in the performance of his or her duties a greater degree of skill than may reasonably be expected from a person of his or her knowledge and experience. However, English and Commonwealth courts have moved towards an objective standard with regard to the required skill and care, and these authorities are likely to be followed in the Cayman Islands. In fulfilling their duty of care to LumiraDx, the directors must ensure compliance with the Amended and Restated Articles, as amended and restated from time to time. The Company has the right to seek damages if a duty owed by any of the directors is breached.
Shareholder Proposals
While the Delaware General Corporation Law does not provide shareholders with an express right to put any proposal before the annual meeting of shareholders, under applicable common law, Delaware corporations generally afford shareholders an opportunity to make proposals and nominations provided that they comply with the notice provisions in the certificate of incorporation or bylaws. A special meeting may be called by the board of directors or any other person authorized to do so in the organizational documents, and shareholders may be precluded from calling special meetings.
The Cayman Companies Act provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. However, these rights may be provided in a company’s articles of association. The Amended and Restated Articles allow the shareholders of LumiraDx holding not less than one-third of the voting rights entitled to vote at general meetings to requisition an extraordinary general meeting of the shareholders, in which case the board is obliged to convene an extraordinary general meeting and to put the resolutions so requisitioned to a vote at such meeting. However, the shareholders of LumiraDx may propose only ordinary resolutions to be put to a vote at such meetings and shall have no right to propose resolutions with respect to the election, appointment or removal of directors or to amend the Amended and Restated Articles. The Amended and Restated Articles provide no other right to put any proposals before annual general meetings or extraordinary general meetings. As a Cayman Islands exempted company, LumiraDx is not obligated by law to call shareholders’ annual general meetings. However, the Amended and Restated Articles and LumiraDx’s corporate governance guidelines provide that LumiraDx may call an annual general meeting each year.
Cumulative Voting
Under the Delaware General Corporation Law, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director. As permitted under the Cayman Companies Act, the Amended and Restated Articles do not provide for cumulative voting. As a result, LumiraDx’s shareholders are not afforded any less protections or rights on this issue than shareholders of a Delaware corporation.
Removal of Directors
Under the Delaware General Corporation Law, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides
146
otherwise. Under the Amended and Restated Articles, a director will be removed from office automatically if, among other things, the director (i) is prohibited from being a director by any applicable law; (ii) dies or becomes bankrupt or makes any arrangement or composition with his or her creditors generally; (iii) is found of unsound mind; (iv) resigns his or her office by notice in writing to LumiraDx; or (v) save in the case of a Founder Director, that person has, for more than six consecutive months, been absent without permission of the directors from meetings of the board and the directors make a decision that that person’s office be vacated. The Class I and Class II directors can also be removed for cause by way of the shareholders passing a special resolution. A Founder Director can only be removed from office on a resolution being proposed which is approved by the ordinary shares held by Ron Zwanziger and his affiliates.
Transactions with Interested Shareholders
The Delaware General Corporation Law contains a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not to be governed by such statute by amendment to its certificate of incorporation or bylaws that is approved by its shareholders, it is prohibited from engaging in certain business combinations or mergers with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or a group who or which owns or owned 15% or more of the target’s outstanding voting stock or who or which is an affiliate or associate of the corporation and owned 15% or more of the corporation’s outstanding voting stock within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The statute does not apply if, among other things, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the merger, business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware corporation to negotiate the terms of any acquisition transaction with the target’s board of directors.
The Cayman Companies Act has no comparable statute. As a result, LumiraDx cannot avail itself of the types of protections afforded by the Delaware General Corporation Law. However, although the Cayman Companies Act does not regulate transactions between a company and its significant shareholders, it does provide that such transactions must be entered into bona fide in the best interests of the company and for a proper corporate purpose and not with the effect of constituting a fraud on the minority shareholders.
Dissolution; Winding Up
Under the Delaware General Corporation Law, unless the board of directors approves the proposal to dissolve, dissolution must be approved by shareholders holding 100% of the total voting power of the corporation. Only if the dissolution is initiated by the board of directors may it be approved by a simple majority of the corporation’s outstanding shares. Delaware law allows a Delaware corporation to include in its certificate of incorporation a supermajority voting requirement in connection with dissolutions initiated by the board of directors.
Under the Cayman Companies Act, a company may be voluntarily wound up upon the shareholders passing a special resolution (being two-thirds of the total voting rights). In addition, a company may be wound up by an order of the courts of the Cayman Islands. The court has authority to order winding up in a number of specified circumstances including where it is, in the opinion of the court, just and equitable to do so.
Variation of Rights of Shares
Under the Delaware General Corporation Law, a corporation may vary the rights of a class of shares with the approval of a majority of the outstanding shares of such class, unless the certificate of incorporation provides otherwise.
Under the Cayman Companies Act and the Amended and Restated Articles, if LumiraDx’s share capital is divided into more than one class of shares, LumiraDx may materially and adversely vary the rights attached to any class only with the consent in writing of the holders of not less than three-fourths of the shares of that class or with the sanction of a special resolution passed at a general meeting of the holders of the shares of that class.
Amendment of Governing Documents
Under the Delaware General Corporation Law, a corporation’s certificate of incorporation may be amended only if adopted and declared advisable by the board of directors and approved by a majority of the outstanding shares entitled to vote, and the bylaws may be amended with the approval of a majority of the outstanding shares entitled to vote and may, if so provided in the certificate of incorporation, also be amended by the board of directors. Under the Cayman Companies Act and the Amended and Restated Articles, the Amended and Restated Articles may only be amended by special resolution of our shareholders.
147
Rights of Non-Resident or Foreign Shareholders
There are no limitations imposed by the Amended and Restated Articles on the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. In addition, there are no provisions in the Amended and Restated Articles governing the ownership threshold above which shareholder ownership must be disclosed.
Directors’ Power to Issue Shares
Under the Amended and Restated Articles, the board of directors is empowered to issue or allot shares or grant options or warrants and analogous equity-based rights with or without preferred, deferred, qualified or other special rights or restrictions. In particular, pursuant to the Amended and Restated Articles, the board of directors has the authority, without further action by the shareholders, to issue all or any part of LumiraDx’s authorized but unissued share capital and, subject to the provisions of the Amended and Restated Articles, to fix the designations, powers, preferences, privileges, and relative participating, optional or special rights and the qualifications, limitations or restrictions therefrom, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights of the common shares.
The board of directors, without shareholder approval, may issue undesignated shares with voting, conversion or other rights that could adversely affect the voting power and other rights of holders of the common shares. Subject to the directors’ duty of acting in the best interest of the company, such undesignated shares can be issued quickly with terms calculated to delay or prevent a change in control of us or make removal of management more difficult. Additionally, the issuance of undesignated shares may have the effect of decreasing the market price of the common shares, and may adversely affect the voting and other rights of the holders of the common shares.
Inspection of Books and Records
Holders of the ordinary shares and common shares have no general right under the Cayman Companies Act to inspect or obtain copies of the list of shareholders or the corporate records. However, LumiraDx will provide its shareholders with annual audited financial statements. Copies of annual financial statements will also be provided to LumiraDx’s registered office service provider in the Cayman Islands to allow for compliance with certain obligations under Cayman law. Stockholders of a Delaware corporation have the right to inspect the books and records of the corporation and the stock ledger for any proper purpose under the Delaware General Corporation Law, and a stockholder list must be available for inspection at annual and special meetings of the stockholders.
C. Material Contracts
For information on our material contracts, please see the section of this Annual Report titled "Item 4.B. Business Overview—Strategic Partners and Manufacturing and Supply Agreements."
D. Exchange Controls
There is no exchange control legislation or regulation in the Cayman Islands, except by way of such as freezing of funds of, and/or prohibition of new investments in, certain jurisdictions subject to international sanction.
E. Taxation
The following summary contains a description of certain material U.S. federal income tax consequences of the ownership and disposition of common shares and public warrants of LumiraDx. This summary should not be considered a comprehensive description of all the tax considerations that may be relevant to the decision to own or dispose of common shares or public warrants.
Certain Material U.S. Federal Income Tax Considerations
This discussion does not address all U.S. federal income tax consequences that may be relevant to your particular circumstances. In addition, it does not address consequences relevant to holders subject to special rules, including, without limitation:
|
|
|
|
|
• |
|
U.S. expatriates and former citizens or long-term residents of the United States; |
|
|
|
|
|
• |
|
persons subject to the alternative minimum tax; |
148
|
|
|
|
|
• |
|
persons that hold common shares or public warrants, as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction, constructive sale, wash sale or other integrated transaction; |
|
|
|
|
|
• |
|
banks, insurance companies and other financial institutions; |
|
|
|
|
|
• |
|
brokers, dealers or traders in securities; |
|
|
|
|
|
• |
|
“controlled foreign corporations,” “passive foreign investment companies” and corporations that accumulate earnings to avoid U.S. federal income tax; |
|
|
|
|
|
• |
|
S corporations, partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
|
|
|
|
|
• |
|
tax-exempt organizations or governmental organizations; |
|
|
|
|
|
• |
|
persons subject to special tax accounting rules as a result of any item of gross income with respect to their common shares or public warrants being taken into account in an applicable financial statement; |
|
|
|
|
|
• |
|
U.S. holders (as defined below) whose functional currency is not the U.S. dollar; |
|
|
|
|
|
• |
|
persons who own 5% or more of the total outstanding common shares or public warrants, or the total outstanding equity of LumiraDx, in each case, directly, indirectly or constructively, by vote or value; |
|
|
|
|
|
• |
|
persons that acquired or will hold common shares or public warrants pursuant to an exercise of employee options, in connection with employee incentive plans or otherwise as compensation; |
|
|
|
|
|
• |
|
regulated investment companies or real estate investment trusts; |
|
|
|
|
|
• |
|
tax-qualified retirement plans; and |
|
|
|
|
|
• |
|
“qualified foreign pension funds” as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds. |
If you are a partnership (or other pass-through entity) for U.S. federal income tax purposes, the tax treatment of your partners (or other owners) will generally depend on the status of the partners, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships (or other pass-through entities) and the partners (or other owners) in such partnerships (or such other pass-through entities) should consult their own tax advisors regarding the U.S. federal income tax consequences to them relating to the matters discussed below.
For purposes of this discussion, a “U.S. holder” is a beneficial owner of shares of common shares or public warrants, as the case may be, who or that is, for U.S. federal income tax purposes:
|
|
|
|
|
• |
|
an individual who is a citizen or resident of the United States; |
|
|
|
|
|
• |
|
a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) organized in or under the laws of the United States, any state thereof or the District of Columbia; |
|
|
|
|
|
• |
|
an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
|
|
|
|
|
• |
|
an entity treated as a trust that (i) is subject to the primary supervision of a court within the United States and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code) or (ii) was in existence on August 20, 1996 and has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
Also, for purposes of this discussion, a “Non-U.S. holder” is any beneficial owner of common shares or public warrants, as the case may be, who or that is neither a U.S. holder nor an entity or arrangement classified as a partnership for U.S. federal income tax purposes.
The following does not purport to be a complete analysis of all potential tax effects stemming from the ownership of common shares or public warrants. The effects of other U.S. federal tax laws, such as estate and gift tax laws or the Medicare contribution tax on net investment income and any applicable state, local or non-U.S. tax laws are not discussed. This discussion is based on the Code, Treasury regulations promulgated thereunder, judicial decisions and published rulings and administrative pronouncements of the IRS, in each case in effect as of the date of this Annual Report. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect holders to which this discussion applies and could affect the accuracy of the statements
149
herein. LumiraDx has not sought, and will not seek, any rulings from the IRS regarding the matters discussed below. There can be no assurance that the IRS or a court will not take a contrary position to that regarding tax consequences discussed below.
THIS DISCUSSION IS FOR INFORMATIONAL PURPOSES ONLY AND IS NOT TAX ADVICE. INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
U.S. Federal Income Tax Consequences of the Ownership and Disposition of Common Shares and the Ownership and Disposition or Conversion of Public Warrants
U.S. Holders
Distributions on Common Shares.
As discussed above under “Item 8. Financial Statements—Dividends and Dividend Policy”, LumiraDx does not anticipate declaring or paying any cash distributions on the common shares in the foreseeable future. However, if LumiraDx were to make distributions of cash or property on the common shares (other than certain pro rata distributions of its common shares), subject to the discussion of the PFIC rules below, any such distributions will generally constitute dividends to the extent of LumiraDx’s current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of LumiraDx’s current and accumulated earnings and profits will be treated first as a nontaxable return of capital to the extent of the U.S. holder’s tax basis in its common shares and thereafter as capital gain, which will be either long-term or short-term capital gain depending upon whether the U.S. holder held its common shares for more than one year. LumiraDx does not maintain calculations of its earnings and profits under U.S. federal income tax principles and, accordingly, a U.S. holder should expect that distributions on its common shares will be treated entirely as dividends for U.S. federal income tax purposes. Because LumiraDx is not a U.S. corporation, U.S. holders that are corporations will not be entitled to claim a dividend received deduction with respect to any distributions they receive from LumiraDx. Dividends paid with respect to common shares will be treated as foreign source income and will generally be “passive category income” for purposes of computing allowable foreign tax credits for U.S. foreign tax credit purposes.
Dividends received by a U.S. holder on its common shares will generally be taxed as ordinary income for U.S. federal income tax purposes. However, a non-corporate U.S. holder may be eligible for taxation at the lower rates applicable to long-term capital gain, provided that such dividends constitute qualified dividend income with respect to such U.S. holder. Qualified dividend income generally includes a dividend paid by a foreign corporation if (i) the stock with respect to which the dividend is paid is readily tradable on an established securities market in the United States (such as the Nasdaq Stock Market, on which the common shares are listed), (ii) the foreign corporation is not a PFIC for the taxable year during which the dividend is paid and the immediately preceding taxable year (as discussed below), and (iii) the U.S. holder has owned the stock for more than 60 days during the 121-day period beginning 60 days before the date on which the stock become ex-dividend (and has not entered into certain risk limiting transactions with respect to such stock).
Sale or Other Taxable Disposition of Common Shares or Public Warrants.
Subject to the discussion of the PFIC rules below, a U.S. holder will generally recognize capital gain or loss upon a sale, exchange or other taxable disposition of its common shares or public warrants in an amount equal to the difference between the amount realized by the U.S. holder from such sale, exchange or other taxable disposition and the U.S. holder’s tax basis in such common shares or public warrants. Such gain or loss will be long-term capital gain or loss if the U.S. holder’s holding period is greater than one year at the time of the sale, exchange or other taxable disposition. Such capital gain or loss will generally be treated as U.S.-source income or loss, as applicable, for U.S. foreign tax credit purposes.
Exercise, Lapse or Redemption of a Public Warrant.
Except as discussed below with respect to the cashless exercise of a public warrant, a U.S. holder generally will not recognize gain or loss upon the acquisition of a common share on the exercise of a public warrant for cash. A U.S. holder’s initial tax basis in a common share received upon exercise of the public warrant generally will equal the sum of the U.S. holder’s tax basis in the public warrant immediately prior to exercise and the exercise price of such public warrant. It is unclear whether a U.S. holder’s holding period for the common share received upon exercise of the public warrant will commence on the date
150
of exercise of the public warrant or the day following the date of exercise of the public warrant; in either case, the holding period will not include the period during which the U.S. holder held the public warrant. If a public warrant is allowed to lapse unexercised, a U.S. holder generally will recognize a capital loss equal to such holder’s tax basis in the public warrant.
The tax consequences of a cashless exercise of a public warrant are not clear under current law. A cashless exercise may not be taxable, either because the exercise is not a realization event or because the exercise is treated as a “recapitalization” for U.S. federal income tax purposes. In either situation, a U.S. holder’s tax basis in the common shares received generally would equal the U.S. holder’s tax basis in the public warrants exercised therefor. If the cashless exercise were not a realization event, it is unclear whether a U.S. holder’s holding period for the common shares will commence on the date of exercise of the public warrant or the day following the date of exercise of the public warrant. If the cashless exercise were treated as a recapitalization, the holding period of the common shares would include the holding period of the public warrants exercised therefor.
It is also possible that a cashless exercise could be treated in whole or in part as a taxable exchange in which gain or loss would be recognized with respect to the portion of the exercised public warrants treated as surrendered to pay the exercise price of the public warrants. In such event, a U.S. holder could be deemed to have surrendered a number of warrants having an aggregate value (as measured by the excess of the fair market value of the common shares over the exercise price of the public warrants) equal to the exercise price for the total number of public warrants to be exercised (i.e., the public warrants underlying the number of common shares actually received by the U.S. holder pursuant to the cashless exercise). In this case, the U.S. holder would recognize capital gain or loss in an amount equal to the difference between the value of the public warrants deemed surrendered and the U.S. holder’s tax basis in such public warrants. Such gain or loss would be long-term or short-term, depending on the U.S. holder’s holding period in the public warrants deemed surrendered. In this case, a U.S. holder’s tax basis in the common shares received would equal the sum of the U.S. holder’s tax basis in the public warrants exercised and the exercise price of such public warrants. It is unclear whether a U.S. holder’s holding period for the common shares would commence on the date following the date of exercise or on the date of exercise of the public warrant; in either case, the holding period would not include the period during which the U.S. holder held the public warrant. Alternative characterizations are also possible (including as a taxable exchange of all of the public warrants surrendered by the U.S. holder for common shares received upon exercise). Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, including when a U.S. holder’s holding period would commence with respect to the common shares received, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. holders should consult their tax advisors regarding the tax consequences of a cashless exercise.
If LumiraDx redeems the public warrants for cash pursuant to the terms thereof or if LumiraDx purchases public warrants in an open market transaction, such redemption or purchase generally will be treated as a taxable disposition to the U.S. Holder, taxed as described in the section of this Annual Report titled “Item 10.E. Certain Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Ownership and Disposition of Common Shares and the Ownership and Disposition or Conversion of Public Warrants—U.S. Holders—Sale or Other Taxable Disposition of Common Shares or Public Warrants” beginning on page 150.
Possible Constructive Distributions.
The terms of each public warrant provide for an adjustment to the number of common shares for which the public warrant may be exercised or to the exercise price of the public warrant in certain events. Depending on the circumstances, such adjustments may be treated as constructive distributions. An adjustment which has the effect of preventing dilution pursuant to a bona fide reasonable adjustment formula generally is not taxable. The U.S. holders of the warrants would, however, be treated as receiving a constructive distribution from LumiraDx if, for example, the adjustment increases the warrant holders’ proportionate interest in LumiraDx’s assets or earnings and profits (e.g., through an increase in the number of common shares that would be obtained upon exercise or through a decrease to the exercise price) as a result of a taxable distribution of cash or other property to the holders of common shares. Any such constructive distribution would generally be subject to tax as described in the section of this Annual Report titled “Item 10.E. Certain Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Ownership and Disposition of Common Shares and the Ownership and Disposition or Conversion of Public Warrants—U.S. Holders—Distributions on Common Shares” beginning on page 150 in the same manner as if the U.S. holders of the warrants received a cash distribution from LumiraDx equal to the fair market value of such increased interest resulting from the adjustment, and such U.S. holder would increase its basis in its public warrants in an amount equal to the amount of such constructive distribution that is treated as a dividend.
151
Passive Foreign Investment Company Rules.
In general, LumiraDx will be a PFIC with respect to a U.S. holder if, for any taxable year in which such holder held common shares or public warrants, either: (i) at least 75% of LumiraDx’s gross income for such taxable year consists of passive income (e.g., dividends, interest, capital gains, rents and royalties, other than any rents or royalties derived in the active conduct of a trade or business); or (ii) at least 50% of the quarterly average value of the gross assets held by LumiraDx during such taxable year produce, or are held for the production of, passive income. For purposes of determining whether LumiraDx is a PFIC, LumiraDx will be treated as earning and owning its proportionate share of the income and assets, respectively, of any subsidiary corporation in which it owns at least 25% of the value of the subsidiary’s stock.
Based on the current and expected composition of LumiraDx’s income and assets and the value of LumiraDx’s assets, LumiraDx does not expect to be a PFIC for its taxable year ended December 31, 2021 or in the foreseeable future. However, no assurances regarding LumiraDx’s PFIC status can be provided for the taxable year ended December 31, 2021 or any future taxable years. The determination of whether LumiraDx is a PFIC for any taxable year is a fact-intensive determination that can only be made after the end of each year, and will depend on the composition of its income and assets and the value of its assets from time to time (including the value of its goodwill, which will generally be determined in part by reference to the market price of the common shares, which may fluctuate considerably). The composition of LumiraDx’s income and assets will also be affected by the amount of cash that it raises in any future offerings or other financing transactions. Because the value of LumiraDx’s goodwill will generally be determined by reference to its market capitalization, LumiraDx could become a PFIC for any taxable year if the price of its common shares declines significantly while LumiraDx holds a substantial amount of cash and financial investments. LumiraDx also could become a PFIC if it does not generate sufficient income from its business in any taxable year relative to the amount of passive income that it generates in such taxable year. In addition, the application of the PFIC rules is subject to some uncertainties and the proper characterization of certain items of its income and assets is not entirely clear. Accordingly, there can be no assurance that LumiraDx will not be a PFIC for its taxable year ended December 31, 2021 or any future taxable year. No belief is expressed regarding LumiraDx’s PFIC status with respect to any U.S. holder that acquired equity interests (or options or other rights to acquire equity interests) in LumiraDx prior to the taxable year ended December 31, 2021.
If LumiraDx were a PFIC for any taxable year in which a U.S. holder owned common shares or public warrants, the U.S. holder would be subject to special tax rules with respect to any “excess distribution” such U.S. holder receives and any gain such U.S. holder recognizes from a sale or other disposition (including, under certain circumstances, a pledge) of common shares or public warrants, unless the common shares or public warrants constitute “marketable securities,” and such U.S. holder makes a mark-to-market election as discussed below. An “excess distribution” is the portion of any distribution received by the U.S. holder on common shares or public warrants in a taxable year in excess of 125% of the average annual distributions received by such U.S. holder in the three preceding taxable years, or, if shorter, the U.S. holder’s holding period for such common shares or public warrants. Under these special tax rules:
|
|
|
|
|
• |
|
the excess distribution or the gain will be allocated ratably over the U.S. holder’s holding period for its common shares or public warrants; |
|
• |
|
the amount of such excess distribution or the gain allocated to the taxable year of disposition, and any taxable year prior to the first taxable year in which LumiraDx became a PFIC, will be treated as ordinary income; and |
|
|
|
|
|
• |
|
the amount of such excess distribution or the gain allocated to each other year will be subject to the highest U.S. federal income tax rate in effect for that year for individuals or corporations, as appropriate, and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
If LumiraDx were a PFIC for any taxable year and its common shares were treated as “marketable stock,” a U.S. holder would be allowed to make a “mark-to-market” election with respect to its common shares. Common shares or public warrants will be treated as “marketable stock” if they are “regularly traded” on certain U.S. stock exchanges or on a foreign stock exchange that meets certain conditions. For these purposes, common shares or public warrants will be considered regularly traded during any calendar year during which they are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. The common shares and public warrants are listed on the Nasdaq Stock Market, which is a qualified exchange for these purposes. Consequently, if the common shares and public warrants remain listed on the Nasdaq Stock Market and are regularly traded, it is expected that the mark-to-market election would be available to U.S. holders if LumiraDx is a PFIC.
A U.S. holder that makes a mark-to-market election must include in ordinary income for each year an amount equal to the excess, if any, of the fair market value of common shares or public warrants at the close of the taxable year over the U.S.
152
holder’s adjusted tax basis in common shares or public warrants. An electing holder may also claim an ordinary loss deduction for the excess, if any, of the U.S. holder’s adjusted basis in common shares or public warrants over the fair market value of the common shares or public warrants at the close of the taxable year, only to the extent of any net mark-to-market gains for prior years. Gains from an actual sale or other disposition of common shares or public warrants will be treated as ordinary income, and any losses incurred on a sale or other disposition of such common shares or public warrants will be treated as an ordinary loss to the extent of any net mark-to-market gains for prior years. Once made, the election cannot be revoked without the consent of the IRS, unless the common shares or public warrants cease to be marketable.
LumiraDx does not intend to provide the information necessary for U.S. holders to make qualified electing fund elections which, if available, would result in tax treatment different from the general tax treatment for PFICs described above. In addition, a U.S. holder of warrants would not be permitted to make a qualified electing fund election with respect to such warrants.
If LumiraDx were a PFIC in any year with respect to which a U.S. holder owns common shares or public warrants, LumiraDx will continue to be treated as a PFIC with respect to such U.S. holder in all succeeding years during which the U.S. holder owns common shares or public warrants, regardless of whether LumiraDx continues to meet the tests described above, unless LumiraDx ceases to be a PFIC and the U.S. holder has made a “deemed sale” election under the PFIC rules.
If LumiraDx were a PFIC for any taxable year and any entity in which LumiraDx owns equity interests in were also a PFIC, U.S. holders would be deemed to own a proportionate amount (by value) of the shares of each lower-tier PFIC and would be subject to U.S. federal income tax according to the rules described below on (i) certain distributions by the lower-tier PFIC and (ii) dispositions of shares of the lower-tier PFIC, in each case as if the U.S. holders held such shares directly, even though the U.S. holder would not receive any proceeds of those distributions or dispositions. It is unclear whether a mark-to-market election can be made with respect to any lower-tier PFIC, even if the shares of such lower-tier PFIC are themselves “marketable stock.”
If LumiraDx were a PFIC for any taxable year in which a U.S. holder owned common shares or public warrants, the U.S. holder generally would be required to file IRS Form 8621 with the U.S. holder’s U.S. federal income tax return for each year to report the U.S. holder’s ownership of such common shares or public warrants and, in the event a U.S. holder that is required to file IRS Form 8621 does not file such form, the statute of limitations on the assessment and collection of U.S. federal income taxes of such U.S. holder for the related tax year may not close until three years after the date that the required information is filed.
Information Reporting and Backup Withholding.
Payments of dividends on, and sales proceeds from the disposition of, common shares and public warrants made to U.S. holders may be subject to information reporting requirements unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a credit against a U.S. holder’s U.S. federal income tax liability, or refunded to such U.S. holder, provided that the required information is timely furnished to the IRS.
Certain Additional Reporting Requirements.
Individual U.S. holders (and to the extent specified in applicable Treasury regulations, certain U.S. holders that are entities) that hold “specified foreign financial assets” whose aggregate value exceeds $75,000 at any time during the taxable year or $50,000 on the last day of the taxable year (or such higher amounts as prescribed by applicable Treasury regulations) are required to file a report on IRS Form 8938 with information relating to such assets for each such taxable year. Specified foreign financial assets would include, among other things, common shares and public warrants, unless the common shares or public warrants are held in an account maintained by a U.S. financial institution. Substantial penalties apply to any failure to timely file IRS Form 8938. Additionally, in the event a U.S. holder that is required to file IRS Form 8938 does not file such form, the statute of limitations on the assessment and collection of U.S. federal income taxes of such holder for the related tax year may not close until three years after the date that the required information is filed. U.S. holders should consult their own tax advisors regarding their reporting obligations with respect to specified foreign financial assets.
153
Non-U.S. Holders
Tax Consequences of Distributions
Distributions paid to a non-U.S. holder in respect of common shares (and constructive distributions paid to a non-U.S. holder in respect of public warrants, as described in the section of this Annual Report titled “Item 10.E. Certain Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Ownership and Disposition of Common Shares and the Ownership and Disposition or Conversion of Public Warrants—U.S. Holders—Possible Constructive Distributions” beginning on page 151) generally will not be subject to U.S. federal income tax, unless the distributions are treated as dividends and are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that such holder maintains in the United States). Dividends that are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base in the United States) generally will be subject to U.S. federal income tax at the same regular U.S. federal income tax rates applicable to a comparable U.S. holder and, in the case of a non-U.S. holder that is a corporation for U.S. federal income tax purposes, also may be subject to an additional branch profits tax at a 30% rate or a lower applicable tax treaty rate, but will not be subject to the gross basis U.S. federal income tax described above, as long as the non-U.S. holder provides proper certification (generally on an IRS Form W-8ECI).
Tax Consequences of Sale or Other Taxable Disposition
A non-U.S. holder generally will not be subject to U.S. federal income tax on any gain attributable to a sale or other taxable disposition of common shares or public warrants unless (i) such gain is effectively connected with its conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base that such holder maintains in the United States) or (ii) the non-U.S. holder is an individual who was present in the United States for 183 days or more in the taxable year of such disposition and certain other requirements are met.
Exercise, Lapse or Redemption of a Public Warrant
The U.S. federal income tax treatment of a non-U.S. holder’s exercise of a public warrant, or the lapse of a public warrant held by a non-U.S. holder, generally will correspond to the U.S. federal income tax treatment of the exercise or lapse of a public warrant by a U.S. holder, as described under the section of this Annual Report titled “Item 10.E. Certain Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Ownership and Disposition of Common Shares and the Ownership and Disposition or Conversion of Public Warrants—U.S. Holders—Exercise, Lapse or Redemption of a Public Warrant” beginning on page 150, although, the U.S. federal income tax treatment for a non-U.S. holder of a redemption of public warrants for cash (or if LumiraDx purchases public warrants in an open market transaction), or to the extent that a cashless exercise results in a taxable exchange, would be similar to those described in the section of this Annual Report titled “Item 10.E. Certain Material U.S. Federal Income Tax Considerations—U.S. Federal Income Tax Consequences of the Ownership and Disposition of Common Shares and the Ownership and Disposition or Conversion of Public Warrants—Non-U.S. Holders—Tax Consequences of Sale or Other Taxable Disposition” beginning on page 154.
Information Reporting and Backup Withholding.
If you are a non-U.S. holder, you may be required to establish your exemption from information reporting and backup withholding by certifying your non-U.S. status on IRS Form W-8BEN, W-8BEN-E, W-8ECI or W-8IMY, as applicable.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a credit against a non-U.S. holder’s U.S. federal income tax liability, or refunded to such U.S. holder, provided that the required information is timely furnished to the IRS.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
154
H. Documents on Display
We are subject to the informational requirements of the Exchange Act. Accordingly, we are required to file reports and other information with the SEC, including annual reports on Form 20-F and reports on Form 6-K. The SEC maintains an Internet site at www.sec.gov that contains reports, proxy and information statements and other information we have filed electronically with the SEC. As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We also make available on our website, free of charge, our Annual Report and the text of our reports on Form 6-K, including any amendments to these reports, as well as certain other SEC filings, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. Our website address is www.lumiradx.com. The reference to our website is an inactive textual reference only, and information contained therein or connected thereto is not incorporated into this Annual Report.
I. Subsidiary Information
Not applicable.
155
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
As of December 31, 2020 and 2021, we had a cash and cash equivalents balance of $161.2 million and $132.1 million, respectively, which comprise cash at bank and in-hand, and deposits held at call with banks. We raise debt on a fixed-rate basis for notes in U.S. dollars. We manage risk to protect the net interest result while managing the overall cost of borrowing. A significant change in the market interest rates would not have a material effect on our business, financial condition or results of operations.
Foreign Currency Exchange Risk
We are exposed to foreign exchange risk. The majority of our sales and purchase transactions are denominated in either U.S. dollars or U.K. pound sterling and as such, we are exposed to exchange rate fluctuations between these and other currencies. The exchange risk is managed by maintaining bank accounts denominated in those currencies. During the years ended December 31, 2020 and 2021, we recognized a foreign currency transaction gain of $21.9 million and loss of 14.6 million, respectively. This gain primarily relates to unrealized and realized foreign currency exchange gains or losses as a result of transactions and asset and liability balances denominated in currencies other than the U.S. dollar. All foreign exchange gains and losses are presented within finance income and finance expense in the consolidated statement of profit and loss and comprehensive income for the years ended December 31, 2020 and 2021.
A 10% strengthening of the U.K. pound sterling against the U.S. dollar at December 31, 2021 would have had an impact of increasing the loss before tax for the period by $26.9 million on the basis that all other variables remain constant.
Credit Risk
Credit risk represents the risk of loss that we would incur if operators and counterparties fail to fulfil their credit obligations. The maximum exposure to credit risk is represented by the carrying amount of each financial asset. For banks and financial institutions, we maintain accounts with major international banks with “A” ratings. Credit risk relating to accounts receivable balances are managed on a case-by-case basis. As of December 31, 2020 and 2021, we had trade receivables of $83.9 million and $79.2 million, respectively. New clients are analyzed before standard payment and delivery terms and conditions are offered. The credit quality of the customer is assessed by analyzing its financial position, past experience and other factors. The utilization of credit limits is regularly monitored. Management does not expect any losses, beyond current amounts already included in reserves, from non-performance by these counterparties.
Liquidity Risk
Liquidity risk is the risk that we will encounter difficulty in meeting the obligations associated with our financial liabilities that are settled in cash. Cash flow forecasting is performed in our operating entities and aggregated at a consolidated level. We monitor rolling forecasts of our liquidity requirements to ensure we have sufficient cash to meet operational needs. We may be reliant on our ability to raise additional investment capital from the issuance of both debt and equity securities to fund our business operating plans and future obligations.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
156
D. American Depositary Shares
Not applicable.
157
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
A. Defaults
None.
B. Arrears and Delinquencies
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
None.
ITEM 15. CONTROLS AND PROCEDURES
A. Disclosure Controls and Procedures
As required by Rule 13a-15 under the Exchange Act, management, including our chief executive officer and our chief financial officer, has evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. Disclosure controls and procedures include, without limitations, controls and procedures designed to ensure that information required to be disclosed by us in our reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding our required disclosures.
Based on the foregoing, our chief executive officer and our chief financial officer have concluded that, as of December 31, 2021, our disclosure controls and procedures were not effective due to the material weaknesses in our internal control over financial reporting primarily related to (i) insufficient segregation of duties related to the posting of manual journal entries, and (ii) the lack of documented evidence for management review controls related to significant accounting estimates and judgements.
We have developed a remediation plan designed to address these material weaknesses and other existing deficiencies. We have re-designed the key processes and included significant measures to ensure an effective internal control over financial reporting. We are currently implementing these processes to ensure operating effectiveness. In doing so, we rely on the assistance of external advisors with expertise in these matters. Additionally, we have and continue to train our accounting and finance staff and hired financial reporting personnel, to develop and implement appropriate internal controls and reporting procedures.
B. Management’s Annual Report on Internal Control over Financial Reporting
This Annual Report does not include a report of management’s assessment regarding internal control over financial reporting due to a transition period established by rules of the SEC for newly public companies.
C. Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include an attestation report of the Company’s registered public accounting firm due to a transition period established by rules of the SEC for newly public companies and because we are an emerging growth Company under the JOBS Act.
158
D. Changes in Internal Control Over Financial Reporting
None.
ITEM 16. RESERVED
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
The audit committee of our board of directors include Donald Berwick, Lurene Joseph, and George Neble. Each member of the Audit Committee satisfies the “independence” requirements set forth in Rule 10A-3 under the Exchange Act and is financially literate. George Neble qualifies as an “audit committee financial expert” as defined in applicable SEC rules.
ITEM 16B. CODE OF ETHICS
We have adopted a Code of Business Conduct and Ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Our Code of Business Conduct and Ethics is available on our website at www.lumiradx.com. We intend to disclose any amendment to the code, or any waivers of its requirements, in our Annual Report on Form 20-F. For the year ended December 31, 2021, we did not grant any waivers of the Code of Business Conduct and Ethics.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended
December 31, |
|
|
|
2020 |
|
|
2021 |
|
|
|
(in thousands) |
|
Audit Fees |
|
$ |
1,225 |
|
|
$ |
1,441 |
|
Audit-Related Fees |
|
|
— |
|
|
|
— |
|
Tax Fees |
|
|
— |
|
|
|
— |
|
All Other Fees |
|
|
— |
|
|
|
— |
|
Total Fees |
|
$ |
1,225 |
|
|
$ |
1,441 |
|
For the years ended December 31, 2020 and 2021, KPMG LLP, London, United Kingdom
, was the Company’s auditor.
Audit fees include the standard audit work performed each fiscal year necessary to allow the auditor to issue an opinion on our financial statements and to issue an opinion on the local statutory financial statements. Audit fees also include services such as reviews of quarterly financial results and review of securities offering documents.
Audit-related fees consisted of fees billed for assurance and related services that were reasonably related to the performance of the audit or review of our financial statements or for services that were traditionally performed by the external auditor.
Tax fees are fees billed for professional services for tax compliance, tax advice and tax planning.
The Audit Committee evaluates the qualifications, independence and performance of the independent auditor as well as pre-approves and reviews the audit and non-audit services to be performed by the independent auditor. In accordance with this policy, all services performed by and fees paid to KPMG LLP were approved by the Audit Committee.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
During the year ended December 31, 2021, no purchases of our equity securities were made by or on behalf of us or any affiliated purchaser.
159
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
ITEM 16G. CORPORATE GOVERNANCE
As a “foreign private issuer,” as defined by the SEC, we are permitted to follow home country corporate governance practices, instead of certain corporate governance standards required by Nasdaq for U.S. companies. Accordingly, we follow Cayman Islands corporate governance rules in lieu of certain of Nasdaq’s corporate governance requirements. The significant differences between our Cayman Islands corporate governance rules and Nasdaq’s corporate governance requirements are set forth below:
•Executive Sessions. IM5605-2 of the Nasdaq Rules requires independent directors of a Nasdaq listed company to meet regularly in executive session (without members of management present), and such executive sessions should occur at least twice a year. In this regard we have elected to adopt the practices of our home country, the Cayman Islands, which practices which do not require independent directors to meet regularly in executive sessions separate from the full board of directors.
•Nomination of Directors. Rule 5605(e)(1) of the Nasdaq Rules requires an issuer to have independent director oversight of director nominations. We follow Cayman Islands practice which does not require director nominations be made or recommended solely by independent directors. Rule 5605(e)(2) of the Nasdaq Rules requires the director nominations process be addressed by a formal written charter or board resolution. We follow Cayman Islands practice which does not require us to have a formal written charter addressing the director nominations process.
•Composition of Board. Rule 5605(b)(1) of the Nasdaq Rules requires a Nasdaq listed company to have a majority of the board of directors be independent. In this regard we have elected to adopt the practices of the Cayman Islands, which practices do not require a majority independent board of directors.
•Compensation Committee. Rule 5605(d)(2) of the Nasdaq Rules requires a Nasdaq listed company to have a compensation committee of its board of directors consisting of independent directors. We follow Cayman Islands practice which does not require us to have an independent compensation committee of our board of directors. Rule 5605(d)(1) of Nasdaq Rules requires a Nasdaq listed company to have a formal written charter addressing the responsibilities and authorities of its compensation committee. We follow Cayman Islands practice which does not require us to have a formal written charter for such compensation committee.
•Shareholder Meeting Quorum. Rule 5620(c) requires a Nasdaq listed company to provide for a quorum for meetings of its holders of common stock to be no less than 33 1/3% of the then outstanding shares of its common voting shares. In this regard we have elected to adopt the practices of the Cayman Islands, which practices do not require such minimum quorum for meetings of shareholders.
•Shareholder Approval. Rule 5635(c) requires shareholder approval for certain issuances of securities. In this regard we have elected to adopt the practices of our home country. In accordance with the provisions of our Amended and Restated Memorandum and Articles of Association, our board of directors is authorized to issue securities, including ordinary shares, preferred shares, warrants and convertible notes. Rule 5635(d) requires shareholder approval in order to enter into any transaction, other than a public offering, involving the sale, issuance or potential issuance by the Company of common shares (or securities convertible into or exercisable for common shares) equal to 20% or more of the outstanding share capital of the company or 20% or more of the voting power outstanding before the issuance for less than the greater of book or market value of the ordinary shares. We follow Cayman Islands law with respect to any requirement to obtain shareholder approval in connection with any private placements of equity securities.
•Director Compensation. As permitted by the listing requirements of Nasdaq, we have also opted out of the requirements of Nasdaq Listing Rule 5250(b)(3), which requires an issuer to disclose information regarding third party compensation of its directors or director nominees.
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
160
PART III
ITEM 17. FINANCIAL STATEMENTS
See “Item 18. Financial Statements” of this Report.
ITEM 18. FINANCIAL STATEMENTS
Financial statements are filed as part of this Annual Report beginning on page F-1.
ITEM 19. EXHIBITS
The following documents are filed as part of this Annual Report or incorporated by reference herein:
|
|
|
Exhibit
Number |
|
Description |
|
|
1.1 |
Amended and Restated Memorandum and Articles of Association of LumiraDx Limited (incorporated by reference to Exhibit 1.1 to the Company’s Report on Form 20-F (File No. 001-40852) filed with the SEC on September 29, 2021). |
|
|
2.1 |
Specimen Common Share Certificate of LumiraDx Limited (incorporated by reference to Exhibit 4.5 to Amendment No. 3 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on September 1, 2021). |
|
|
2.2 |
Specimen Ordinary Share Certificate of LumiraDx Limited (incorporated by reference to Exhibit 4.6 to Amendment No. 3 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on September 1, 2021). |
|
|
2.3 |
Amended and Restated Warrant Agreement, dated as of September 28, 2021, by and among Continental Stock Transfer & Trust Company, LumiraDx Limited, Computershare Inc., Computershare Trust Company, N.A. and CA Healthcare Acquisition Corp., including Specimen Warrant Certificate of LumiraDx Limited(incorporated by reference to Exhibit 2.3 to the Company’s Report on Form 20-F (File No. 001-40852) filed with the SEC on September 29, 2021). |
|
|
2.4 |
Warrant Instrument in Respect of Warrants to Subscribe for Ordinary Shares in LumiraDx Limited, dated as of October 3, 2016, issued by the Company to certain warrant holders (incorporated by reference to Exhibit 4.10 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
2.5 |
Form of Warrant Instrument in Respect of Warrants to Subscribe for Ordinary Shares in LumiraDx Limited, dated as of September 20, 2019, issued by the Company to certain warrant holders (incorporated by reference to Exhibit 4.11 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
2.6 |
Warrant Instrument in Respect of Warrants to Subscribe for Common Shares in LumiraDx Limited, dated as of July 1, 2020, issued by the Company to certain warrant holders (incorporated by reference to Exhibit 4.16 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
2.7 |
Warrant Instrument in Respect of Warrants to Subscribe for Common Shares in LumiraDx Limited, dated as of November 6, 2020, issued by the Company to Jefferies Finance LLC (incorporated by reference to Exhibit 4.17 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
2.8 |
Warrant Instrument in Respect of Warrants to Subscribe for Common Shares in LumiraDx Limited, dated as of January 20, 2021, issued by the Company to Silicon Valley Bank (incorporated by reference to Exhibit 4.18 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
2.9 |
Form of Warrant Instrument in Respect of Warrants to Subscribe for Common Shares in LumiraDx Limited, to be entered into between LumiraDx Limited and BPCR Limited Partnership and Biopharma Credit Investments V (Master) LP (incorporated by reference to Exhibit 4.19 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
161
|
|
|
2.10 |
Loan Agreement, dated as of March 23, 2021 by and among LumiraDx Investment Limited, as borrower, LumiraDx Limited, as a credit party and issuer of the warrants thereunder, LumiraDx Group Limited, as a credit party and parent, BPCR Limited Partnership and BioPharma Credit Investments V (Master) LP, as lenders and BioPharma Credit PLC, as collateral agent (incorporated by reference t[o Exhibit 10.14 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
2.11 |
Intercreditor Agreement, dated as of March 29, 2021, by and among, inter alia, LumiraDx Limited, BioPharma Credit PLC, Wilmington Trust SP Services (London) Limited and certain subsidiaries of LumiraDx Limited (incorporated by reference to Exhibit 10.16 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
|
2.12* |
Description of Securities. |
|
|
4.1 |
Agreement and Plan of Merger, dated as of April 6, 2021, by and among LumiraDx Limited, LumiraDx Merger Sub, Inc., and CA Healthcare Acquisition Corp. (incorporated by reference to Exhibit 2.1 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.2 |
Amendment No. 1 to the Agreement and Plan of Merger, dated as of August 19, 2021, by and among LumiraDx Limited, LumiraDx Merger Sub, Inc., and CA Healthcare Acquisition Corp. (incorporated by reference to Exhibit 2.2 to Amendment No. 1 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on August 20, 2021). |
|
|
4.3 |
Amendment No. 2 to the Agreement and Plan of Merger, dated as of August 27, 2021, by and among LumiraDx Limited, LumiraDx Merger Sub, Inc., and CA Healthcare Acquisition Corp. (incorporated by reference to Exhibit 3.2 to Amendment No. 2 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on August 27, 2021). |
|
|
4.4 |
Amended and Restated Registration Rights Agreement by and among LumiraDx Limited, CAH, Sponsor and the other parties named therein (incorporated by reference to Exhibit 4.4 to the Company’s Report on Form 20-F (File No. 001-40852) filed with the SEC on September 29, 2021). |
|
|
4.5# |
LumiraDx Limited 2021 Stock Option and Incentive Plan (incorporated by reference to Exhibit 4.5 to the Company’s Report on Form 20-F (File No. 001-40852) filed with the SEC on September 29, 2021). |
|
|
4.6# |
LumiraDx Limited 2021 Employee Stock Purchase Plan (incorporated by reference to Exhibit 4.6 to the Company’s Report on Form 20-F (File No. 001-40852) filed with the SEC on September 29, 2021). |
|
|
4.7# |
Aegle Care (Holdings) Limited EMI Option Scheme (incorporated by reference to Exhibit 10.20 to Post-effective Amendment No. 1 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on September 27, 2021). |
|
|
4.8# |
LumiraDx Limited Consultants’ and Non-Employees’ Option Scheme (incorporated by reference to Exhibit 10.1 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.9# |
LumiraDx Limited Unapproved Option Scheme with U.S. Appendix (incorporated by reference to Exhibit 10.2 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.10# |
Form of Indemnification Agreement by and between LumiraDx Limited and each of its directors and executive officers (incorporated by reference to Exhibit 10.4 to Amendment No. 2 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on August 27, 2021). |
|
|
4.11 |
Standard Industrial/Commercial Multi-Tenant Lease, dated as of July 29, 2016, by and between LumiraDx Limited and South Cedros Associates, LLC, as amended by Lease Modification and Extension, dated as of June 2, 2020, by and between the Registrant and South Cedros Associates, LLC (incorporated by reference to Exhibit 10.13 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.12† |
Amended and Restated Cooperation Agreement, dated as of October 17, 2019, by and between LumiraDx Limited and the Bill & Melinda Gates Foundation (incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
162
|
|
|
|
4.13† |
Note Purchase Agreement, dated as of October 17, 2019, by and between LumiraDx Limited and the Bill & Melinda Gates Foundation, and the Unsecured Subordinated Promissory Note issued thereunder (incorporated by reference to Exhibit 10.6 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.14† |
Grant Agreement, dated as of November 5, 2019, by and between LumiraDx Limited and the Bill & Melinda Gates Foundation, as amended (incorporated by reference to Exhibit 10.7 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.15† |
Grant Agreement, dated as of October 6, 2020, by and between LumiraDx Limited and the Bill & Melinda Gates Foundation (incorporated by reference to Exhibit 10.8 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.16† |
Exclusivity Agreement, dated as of August 3, 2018, by and between LumiraDx Limited and CVS Pharmacy, Inc. (incorporated by reference to Exhibit 10.9 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.17† |
Purchase Agreement, dated August 14, 2020, by and between LumiraDx Inc. and CVS Pharmacy, Inc. (incorporated by reference to Exhibit 10.10 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.18†* |
First Amendment, dated January 1, 2022, to the Purchase Agreement, dated August 14, 2020, by and between LumiraDx Inc. and CVS Pharmacy, Inc. |
|
|
4.19† |
Manufacturing Services Agreement, dated as of October 18, 2017, by and between the LumiraDx UK Limited and Flextronics Medical Sales and Marketing, Ltd., as amended by the Affiliate Adoption Agreement No. 2, dated as of January 17, 2020 (incorporated by reference to Exhibit 10.11 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.20 |
Assignation and Variation, dated as of October 28, 2015, by and among LumiraDx Limited, Alere Technologies Limited and The Ashtenne Industrial Fund LP, as amended by Minute of Variation and Extension of Lease, dated as of October 16, 2019, by and between LumiraDx Limited and The Ashtenne Industrial Fund LP (incorporated by reference to Exhibit 10.12 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.21 |
Loan Agreement, dated as of March 23, 2021 by and among LumiraDx Investment Limited, as borrower, LumiraDx Limited, as a credit party and issuer of the warrants thereunder, LumiraDx Group Limited, as a credit party and parent, BPCR Limited Partnership and BioPharma Credit Investments V (Master) LP, as lenders and BioPharma Credit PLC, as collateral agent (incorporated by reference to Exhibit 10.14 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.22† |
Grant Agreement, dated as of November 7, 2020, by and between LumiraDx Limited and the Bill & Melinda Gates Foundation (incorporated by reference to Exhibit 10.15 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.23 |
Intercreditor Agreement, dated as of March 29, 2021, by and among, inter alia, LumiraDx Limited, BioPharma Credit PLC, Wilmington Trust SP Services (London) Limited and certain subsidiaries of LumiraDx Limited (incorporated by reference to Exhibit 10.16 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.24 |
Registration Rights Agreement, dated as of August 8, 2018, by and between the Registrant and the investors named therein (incorporated by reference to Exhibit 10.17 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
4.25 |
Registration Rights Agreement, dated as of November 30, 2020, by and between the Registrant and the investors named therein (incorporated by reference to Exhibit 10.18 to the Company’s Registration Statement on Form F-4 (File No. 333-257745) filed with the SEC on July 7, 2021). |
|
|
163
|
|
4.26 |
Indenture (including form of Note as Exhibit A), dated as of March 3, 2022, by and between LumiraDx Limited and U.S. Bank Trust Company, National Association, as trustee (incorporated by reference to Exhibit 4.1 to the Company's Form 6-K, filed with the SEC on March 3, 2022). |
|
|
4.27 |
Form of 6.00% Convertible Senior Subordinated Notes due 2027 (included within Exhibit 4.26). |
|
|
4.28 |
Registration Rights Agreement, dated as of March 3, 2022, by and among LumiraDx Limited and the Investors party thereto (incorporated by reference to Exhibit 4.3 to the Company's Form 6-K, filed with the SEC on March 3, 2022). |
|
|
4.29 |
Form of Subscription Agreement (incorporated by reference to Exhibit 10.1 to the Company's Form 6-K, filed with the SEC on March 1, 2022). |
|
|
4.30†* |
Lease, dated as of September 20, 2020, by and among BNP Paribas Depositary Services (Jersey) Limited and BNP Paribas Depositary Services Limited, as Trustees of the Mayfair Capital Commercial Property Trust, and LumiraDX UK LTD. |
|
|
4.31†* |
Lease, dated as of December 7, 2020, by and among Drawbridge Nancy Ridge, LLC, and LumiraDX, Inc., as amended on August 17, 2021. |
|
|
8.1* |
List of subsidiaries of LumiraDx Limited. |
|
|
12.1* |
Certification of Principal Executive Officer pursuant to Rule 13a-14(a)/15d-14(a). |
|
|
12.2* |
Certification of Principal Financial Officer pursuant to Rule 13a-14(a)/15d-14(a). |
|
|
13.1** |
Certification of Principal Executive Officer pursuant to 18 U.S.C. 1B350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
13.2** |
Certification of Principal Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
15.1* |
Consent of KPMG LLP, independent public registered accounting firm for LumiraDx Limited. |
|
|
|
|
|
101.INS* |
Inline XBRL Instance Document. |
|
|
101.SCH* |
Inline XBRL Taxonomy Extension Schema Document. |
|
|
101.DEF* |
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
101.CAL* |
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
101.LAB* |
Inline XBRL Taxonomy Extension Label Linkbase Document. |
|
|
101.PRE* |
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
104* |
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
* Filed herewith.
** Furnished herewith.
# Indicates a management contract or compensatory plan.
† Portions of this exhibit (indicated by brackets and asterisks) have been omitted in accordance with the rules of the SEC.
164
165
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this report on its behalf.
Date: April 13, 2022
|
|
|
|
|
|
|
|
|
|
LumiraDx Limited |
|
|
By: |
|
/s/ Ron Zwanziger |
|
|
Name: |
|
Ron Zwanziger |
|
|
Title: |
|
Chief Executive Officer, Chairman, and Director |
166
INDEX TO FINANCIAL STATEMENTS
|
|
|
|
|
Page |
Consolidated Financial Statements of LumiraDx Limited |
|
|
|
Report of Independent Registered Public Accounting Firm (KPMG LLP, London, United Kingdom, Auditor Firm ID: 1118) |
F-1 |
|
|
Consolidated Statement of Profit and Loss and Comprehensive Loss for the years ended December 31, 2021, 2020, and 2019 |
F-2 |
|
|
Consolidated Statement of Financial Position as of December 31, 2021 and 2020 |
F-3 |
|
|
Consolidated Statement of Changes in Equity for the years ended December 31, 2021, 2020, and 2019 |
F-4 |
|
|
Consolidated Statement of Cash Flows for the years ended December 31, 2021, 2020, and 2019. |
F-6 |
|
|
Notes to Consolidated Financial Statements |
F-7 |
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
of LumiraDx Limited:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of LumiraDx Limited and subsidiaries (the Company) as of December 31, 2021 and 2020, the related consolidated statements of profit and loss and comprehensive loss, changes in equity, and cash flows for each of the years in the three-year period ended December 31, 2021, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2021, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company could be dependent on its ability to obtain waivers of covenant violations or restructure its existing debt obligations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ KPMG LLP
We have served as the Company’s auditor since 2015.
London, United Kingdom
April 13, 2022
F-1
LUMIRADX LIMITED
Consolidated Statement of Profit and Loss and Comprehensive Loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED
DECEMBER 31,
2019 |
|
|
YEAR ENDED
DECEMBER 31,
2020 |
|
|
YEAR ENDED
DECEMBER 31,
2021 |
|
|
|
Note |
|
(in thousands, except share data and EPS) |
|
Revenue |
|
|
|
|
|
|
|
|
|
|
|
Products |
|
4 |
|
$ |
19,802 |
|
|
$ |
135,656 |
|
|
$ |
415,654 |
|
Services |
|
4 |
|
|
3,340 |
|
|
|
3,497 |
|
|
|
5,774 |
|
Total Revenue |
|
|
|
|
23,142 |
|
|
|
139,153 |
|
|
|
421,428 |
|
Cost of sales |
|
|
|
|
|
|
|
|
|
|
|
Products |
|
|
|
|
(12,469 |
) |
|
|
(84,456 |
) |
|
|
(268,835 |
) |
Services |
|
|
|
|
(1,853 |
) |
|
|
(1,750 |
) |
|
|
(1,053 |
) |
Total Cost of Sales |
|
|
|
|
(14,322 |
) |
|
|
(86,206 |
) |
|
|
(269,888 |
) |
Gross Profit |
|
|
|
|
8,820 |
|
|
|
52,947 |
|
|
|
151,540 |
|
Research and development expenses |
|
|
|
|
(86,546 |
) |
|
|
(107,539 |
) |
|
|
(130,221 |
) |
Selling, marketing and administrative expenses |
|
|
|
|
(37,294 |
) |
|
|
(46,129 |
) |
|
|
(130,520 |
) |
Listing expenses |
|
27 |
|
|
- |
|
|
|
- |
|
|
|
(36,202 |
) |
Operating Loss |
|
|
|
|
(115,020 |
) |
|
|
(100,721 |
) |
|
|
(145,403 |
) |
Finance income |
|
6 |
|
|
11,705 |
|
|
|
22,500 |
|
|
|
165,426 |
|
Finance expense |
|
6 |
|
|
(39,335 |
) |
|
|
(172,722 |
) |
|
|
(117,934 |
) |
Net finance income/(expense) |
|
|
|
|
(27,630 |
) |
|
|
(150,222 |
) |
|
|
47,492 |
|
Loss before Tax |
|
|
|
|
(142,650 |
) |
|
|
(250,943 |
) |
|
|
(97,911 |
) |
Tax credit/(expense) for the period |
|
7 |
|
|
9,541 |
|
|
|
9,946 |
|
|
|
(2,844 |
) |
Loss for the period |
|
|
|
$ |
(133,109 |
) |
|
$ |
(240,997 |
) |
|
$ |
(100,755 |
) |
Loss/(gain) attributable to non-controlling interest |
|
9 |
|
|
(302 |
) |
|
|
(17 |
) |
|
|
174 |
|
Net loss attributable to equity holders of parent—basic and diluted |
|
|
|
$ |
(132,807 |
) |
|
$ |
(240,980 |
) |
|
$ |
(100,929 |
) |
Net loss per share attributable to equity holders of parent—basic and diluted |
|
8 |
|
$ |
(1.01 |
) |
|
$ |
(1.82 |
) |
|
$ |
(0.62 |
) |
Weighted-average number of Ordinary Shares used in loss per share—basic and diluted |
|
8 |
|
|
131,757,738 |
|
|
|
132,192,880 |
|
|
|
163,255,784 |
|
Other Comprehensive Loss: |
|
|
|
|
|
|
|
|
|
|
|
Items that may be reclassified subsequently to profit or loss |
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation differences - foreign operations |
|
|
|
|
(7,580 |
) |
|
|
(17,560 |
) |
|
|
199 |
|
Total Comprehensive loss for the year |
|
|
|
|
(140,689 |
) |
|
|
(258,557 |
) |
|
|
(100,556 |
) |
Total comprehensive loss attributable to: |
|
|
|
|
|
|
|
|
|
|
|
Equity holders of the parent |
|
|
|
|
(140,389 |
) |
|
|
(258,544 |
) |
|
|
(100,730 |
) |
Non-controlling interest |
|
9 |
|
|
(300 |
) |
|
|
(13 |
) |
|
|
174 |
|
Total |
|
|
|
$ |
(140,689 |
) |
|
$ |
(258,557 |
) |
|
$ |
(100,556 |
) |
The accompanying notes are an integral part of these financial statements.
F-2
LUMIRADX LIMITED
Consolidated Statement of Financial Position
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AS OF DECEMBER 31, 2020 |
|
|
AS OF DECEMBER 31, 2021 |
|
|
|
Note |
|
(in thousands, except share data) |
|
ASSETS |
|
|
|
|
|
|
|
|
Non–Current Assets |
|
|
|
|
|
|
|
|
Other non-current assets |
|
|
|
$ |
241 |
|
|
$ |
569 |
|
Intangibles and goodwill |
|
10 |
|
|
40,723 |
|
|
|
37,048 |
|
Right-of-use assets |
|
24 |
|
|
10,386 |
|
|
|
27,746 |
|
Property, plant and equipment |
|
11 |
|
|
87,082 |
|
|
|
173,397 |
|
Total Non-Current Assets |
|
|
|
|
138,432 |
|
|
|
238,760 |
|
Current Assets |
|
|
|
|
|
|
|
|
Inventories |
|
12 |
|
|
85,516 |
|
|
|
149,055 |
|
Tax receivable |
|
7 |
|
|
20,680 |
|
|
|
15,022 |
|
Trade and other receivables |
|
13 |
|
|
109,295 |
|
|
|
109,798 |
|
Restricted cash |
|
2 |
|
|
2,455 |
|
|
|
- |
|
Cash and cash equivalents |
|
|
|
|
158,717 |
|
|
|
132,145 |
|
Total Current Assets |
|
|
|
|
376,663 |
|
|
|
406,020 |
|
TOTAL ASSETS |
|
|
|
$ |
515,095 |
|
|
$ |
644,780 |
|
LIABILITIES AND EQUITY |
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
Non-Current Liabilities |
|
|
|
|
|
|
|
|
Debt due after more than one year |
|
17 |
|
$ |
(139,734 |
) |
|
$ |
(301,129 |
) |
Preferred shares |
|
16 |
|
|
(451,721 |
) |
|
|
— |
|
Lease liabilities |
|
|
|
|
(8,991 |
) |
|
|
(25,514 |
) |
Stock warrants |
|
27 |
|
|
- |
|
|
|
(10,407 |
) |
Deferred tax liabilities |
|
19 |
|
|
(1,230 |
) |
|
|
(779 |
) |
Total Non-Current Liabilities |
|
|
|
|
(601,676 |
) |
|
|
(337,829 |
) |
Current Liabilities |
|
|
|
|
|
|
|
|
Debt due within one year |
|
17 |
|
|
(147,238 |
) |
|
|
(191 |
) |
Government and other grants |
|
21 |
|
|
(44,037 |
) |
|
|
(38,941 |
) |
Trade and other payables |
|
20 |
|
|
(95,246 |
) |
|
|
(99,641 |
) |
Lease liabilities due within one year |
|
|
|
|
(2,114 |
) |
|
|
(5,582 |
) |
Total Current Liabilities |
|
|
|
|
(288,635 |
) |
|
|
(144,355 |
) |
Equity |
|
|
|
|
|
|
|
|
Share capital and share premium |
|
14 |
|
|
(152,732 |
) |
|
|
(754,023 |
) |
Foreign currency translation reserve |
|
|
|
|
19,905 |
|
|
|
19,706 |
|
Other reserves |
|
14 |
|
|
(99,821 |
) |
|
|
(104,957 |
) |
Accumulated deficit |
|
|
|
|
607,657 |
|
|
|
676,223 |
|
Total equity attributable to equity holders of the parent |
|
|
|
|
375,009 |
|
|
|
(163,051 |
) |
Non-controlling interests |
|
9 |
|
|
207 |
|
|
|
455 |
|
Total Equity |
|
|
|
|
375,216 |
|
|
|
(162,596 |
) |
TOTAL EQUITY AND LIABILITIES |
|
|
|
$ |
(515,095 |
) |
|
$ |
(644,780 |
) |
The accompanying notes are an integral part of these financial statements.
F-3
LUMIRADX LIMITED
Consolidated Statement of Changes in Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SHARE
CAPITAL |
|
|
SHARE
PREMIUM |
|
|
TRANSLATION
RESERVES |
|
|
OTHER
RESERVES |
|
|
ACCUMULATED
DEFICIT |
|
|
TOTAL |
|
|
NON-
CONTROLLING
INTEREST |
|
|
TOTAL
EQUITY |
|
|
|
(in thousands) |
|
Balance at January 1, 2019 |
|
$ |
0 |
|
|
$ |
152,125 |
|
|
$ |
5,241 |
|
|
$ |
49,582 |
|
|
$ |
(241,031 |
) |
|
$ |
(34,083 |
) |
|
$ |
106 |
|
|
$ |
(33,977 |
) |
Loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(132,807 |
) |
|
|
(132,807 |
) |
|
|
(302 |
) |
|
|
(133,109 |
) |
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currency translation differences |
|
|
— |
|
|
|
— |
|
|
|
(7,582 |
) |
|
|
— |
|
|
|
— |
|
|
|
(7,582 |
) |
|
|
2 |
|
|
|
(7,580 |
) |
Total comprehensive loss for the
period |
|
|
— |
|
|
|
— |
|
|
|
(7,582 |
) |
|
|
— |
|
|
|
(132,807 |
) |
|
|
(140,389 |
) |
|
|
(300 |
) |
|
|
(140,689 |
) |
Shares issued |
|
|
— |
|
|
|
2,601 |
|
|
|
|
|
|
(255 |
) |
|
|
— |
|
|
|
2,346 |
|
|
|
— |
|
|
|
2,346 |
|
Equity compensation plans |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,970 |
|
|
|
3,970 |
|
|
|
— |
|
|
|
3,970 |
|
Equity conversion feature of
convertible notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
17,065 |
|
|
|
- |
|
|
|
17,065 |
|
|
|
— |
|
|
|
17,065 |
|
Issue of other equity instruments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
491 |
|
|
|
— |
|
|
|
491 |
|
|
|
— |
|
|
|
491 |
|
Shares repurchased |
|
|
— |
|
|
|
(2,035 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,035 |
) |
|
|
— |
|
|
|
(2,035 |
) |
Transaction with owners, recognized
directly in equity |
|
|
— |
|
|
|
566 |
|
|
|
— |
|
|
|
17,301 |
|
|
|
3,970 |
|
|
|
21,837 |
|
|
|
— |
|
|
|
21,837 |
|
Changes in non-controlling interests |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Balance at December 31, 2019 |
|
$ |
0 |
|
|
$ |
152,691 |
|
|
$ |
(2,341 |
) |
|
$ |
66,883 |
|
|
$ |
(369,868 |
) |
|
$ |
(152,635 |
) |
|
$ |
(194 |
) |
|
$ |
(152,829 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2020 |
|
$ |
0 |
|
|
$ |
152,691 |
|
|
$ |
(2,341 |
) |
|
$ |
66,883 |
|
|
$ |
(369,868 |
) |
|
$ |
(152,635 |
) |
|
$ |
(194 |
) |
|
$ |
(152,829 |
) |
Loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(240,980 |
) |
|
|
(240,980 |
) |
|
|
(17 |
) |
|
|
(240,997 |
) |
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currency translation differences |
|
|
— |
|
|
|
— |
|
|
|
(17,564 |
) |
|
|
— |
|
|
|
— |
|
|
|
(17,564 |
) |
|
|
4 |
|
|
|
(17,560 |
) |
Total comprehensive loss for the
period |
|
|
— |
|
|
|
— |
|
|
|
(17,564 |
) |
|
|
— |
|
|
|
(240,980 |
) |
|
|
(258,544 |
) |
|
|
(13 |
) |
|
|
(258,557 |
) |
Equity compensation plans |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3,191 |
|
|
|
3,191 |
|
|
|
— |
|
|
|
3,191 |
|
Issue of other equity instruments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
32,938 |
|
|
|
— |
|
|
|
32,938 |
|
|
|
— |
|
|
|
32,938 |
|
Shares issued on exercise of share options |
|
|
— |
|
|
|
41 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
41 |
|
|
|
— |
|
|
|
41 |
|
Transaction with owners, recognized
directly in equity |
|
|
— |
|
|
|
41 |
|
|
|
— |
|
|
|
32,938 |
|
|
|
3,191 |
|
|
|
36,170 |
|
|
|
— |
|
|
|
36,170 |
|
Changes in non-controlling interests |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Balance at December 31, 2020 |
|
$ |
0 |
|
|
$ |
152,732 |
|
|
$ |
(19,905 |
) |
|
$ |
99,821 |
|
|
$ |
(607,657 |
) |
|
$ |
(375,009 |
) |
|
$ |
(207 |
) |
|
$ |
(375,216 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2021 |
|
$ |
0 |
|
|
$ |
152,732 |
|
|
$ |
(19,905 |
) |
|
$ |
99,821 |
|
|
$ |
(607,657 |
) |
|
$ |
(375,009 |
) |
|
$ |
(207 |
) |
|
$ |
(375,216 |
) |
Loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(100,929 |
) |
|
|
(100,929 |
) |
|
|
174 |
|
|
|
(100,755 |
) |
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currency translation differences |
|
|
— |
|
|
|
— |
|
|
|
199 |
|
|
|
— |
|
|
|
— |
|
|
|
199 |
|
|
|
— |
|
|
|
199 |
|
Total comprehensive loss for the
period |
|
|
— |
|
|
|
— |
|
|
|
199 |
|
|
|
— |
|
|
|
(100,929 |
) |
|
|
(100,730 |
) |
|
|
174 |
|
|
|
(100,556 |
) |
Equity compensation plans |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
33,909 |
|
|
|
33,909 |
|
|
|
— |
|
|
|
33,909 |
|
F-4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issue of other equity instruments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,136 |
|
|
|
— |
|
|
|
5,136 |
|
|
|
— |
|
|
|
5,136 |
|
Shares issued on exercise of share options |
|
|
— |
|
|
|
104 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
104 |
|
|
|
— |
|
|
|
104 |
|
Conversion of debt and preferred shares in merger |
|
|
— |
|
|
|
576,210 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
576,210 |
|
|
|
— |
|
|
|
576,210 |
|
Shares issued in merger |
|
|
— |
|
|
|
24,977 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
24,977 |
|
|
|
— |
|
|
|
24,977 |
|
Transaction with owners, recognized
directly in equity |
|
|
— |
|
|
|
601,291 |
|
|
|
— |
|
|
|
5,136 |
|
|
|
33,909 |
|
|
|
640,336 |
|
|
|
— |
|
|
|
640,336 |
|
Changes in non-controlling interests |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,546 |
) |
|
|
(1,546 |
) |
|
|
(422 |
) |
|
|
(1,968 |
) |
Balance at December 31, 2021 |
|
$ |
0 |
|
|
$ |
754,023 |
|
|
$ |
(19,706 |
) |
|
$ |
104,957 |
|
|
$ |
(676,223 |
) |
|
$ |
163,051 |
|
|
$ |
(455 |
) |
|
$ |
162,596 |
|
The accompanying notes are an integral part of these financial statements.
F-5
LUMIRADX LIMITED
Consolidated Statement of Cash Flows
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED
DECEMBER 31,
2019 |
|
|
YEAR ENDED
DECEMBER 31,
2020 |
|
|
YEAR ENDED
DECEMBER 31,
2021 |
|
|
|
(in thousands, except share data) |
|
Cash Flows from Operating Activities |
|
|
|
|
|
|
|
|
|
Loss for the period |
|
$ |
(133,109 |
) |
|
$ |
(240,997 |
) |
|
$ |
(100,755 |
) |
Adjustments to reconcile loss for the year to net cash used
in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
5,502 |
|
|
|
8,527 |
|
|
|
22,868 |
|
Amortization |
|
|
2,494 |
|
|
|
2,387 |
|
|
|
2,827 |
|
Net finance expenses |
|
|
6,001 |
|
|
|
126,774 |
|
|
|
(63,625 |
) |
Equity based share based payment transactions |
|
|
3,970 |
|
|
|
3,191 |
|
|
|
33,909 |
|
Increase in tax receivable |
|
|
(9,549 |
) |
|
|
(11,269 |
) |
|
|
(4,663 |
) |
Accrued preferred shares dividends |
|
|
21,600 |
|
|
|
23,578 |
|
|
|
16,156 |
|
Listing charge |
|
|
- |
|
|
|
- |
|
|
|
27,607 |
|
Changes to working capital: |
|
|
|
|
|
|
|
|
|
Inventories |
|
|
(8,389 |
) |
|
|
(73,302 |
) |
|
|
(66,874 |
) |
Trade and other receivables |
|
|
6,388 |
|
|
|
(89,213 |
) |
|
|
7,511 |
|
Trade payables and other liabilities |
|
|
13,337 |
|
|
|
100,997 |
|
|
|
(9,544 |
) |
Net Cash used in Operating Activities |
|
|
(91,755 |
) |
|
|
(149,327 |
) |
|
|
(134,583 |
) |
Cash Flows from Investing Activities |
|
|
|
|
|
|
|
|
|
Purchases of property, plant, equipment |
|
|
(10,625 |
) |
|
|
(64,381 |
) |
|
|
(106,346 |
) |
Purchases of intangible assets |
|
|
(102 |
) |
|
|
- |
|
|
|
- |
|
Cash paid for business acquisitions, net of cash received |
|
|
(581 |
) |
|
|
- |
|
|
|
- |
|
Net Cash used in Investing Activities |
|
|
(11,308 |
) |
|
|
(64,381 |
) |
|
|
(106,346 |
) |
Cash Flows from Financing Activities |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of preferred shares |
|
|
- |
|
|
|
162,401 |
|
|
|
- |
|
Proceeds from debt issuance, net of issuance costs |
|
|
55,769 |
|
|
|
62,391 |
|
|
|
361,830 |
|
Proceeds from issuance of convertible notes, net of issuance costs |
|
|
71,932 |
|
|
|
70,917 |
|
|
|
- |
|
Proceeds from issuance of share capital |
|
|
- |
|
|
|
- |
|
|
|
38,568 |
|
Shares issued on the exercise of share options |
|
|
- |
|
|
|
41 |
|
|
|
104 |
|
Repayment of principal portion of lease liabilities |
|
|
(1,866 |
) |
|
|
(3,054 |
) |
|
|
(5,429 |
) |
Cash interest paid, net of interest received |
|
|
(3,771 |
) |
|
|
(12,114 |
) |
|
|
(29,894 |
) |
Early extinguishment of debt |
|
|
- |
|
|
|
(3,600 |
) |
|
|
(3,637 |
) |
Repurchase of shares |
|
|
(2,035 |
) |
|
|
|
|
|
|
Cash issued for non-controlling interest |
|
|
|
|
|
- |
|
|
|
(1,968 |
) |
Repayments of debt |
|
|
(49,328 |
) |
|
|
(40,396 |
) |
|
|
(140,552 |
) |
Net Cash generated from Financing Activities |
|
|
70,701 |
|
|
|
236,586 |
|
|
|
219,022 |
|
Net (Decrease) / Increase in Cash and Cash Equivalents |
|
$ |
(32,362 |
) |
|
$ |
22,878 |
|
|
$ |
(21,907 |
) |
Movement in Cash and Cash Equivalents |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at the beginning of the year |
|
$ |
171,273 |
|
|
$ |
139,387 |
|
|
$ |
161,172 |
|
Exchange loss on cash and cash equivalents |
|
|
476 |
|
|
|
(1,093 |
) |
|
|
(7,120 |
) |
Net Increase / (decrease) in cash and cash equivalents |
|
|
(32,362 |
) |
|
|
22,878 |
|
|
|
(21,907 |
) |
Cash and Cash Equivalents at the end of the year |
|
$ |
139,387 |
|
|
$ |
161,172 |
|
|
$ |
132,145 |
|
The accompanying notes are an integral part of these financial statements.
F-6
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except share and per share data)
1. GENERAL INFORMATION
These consolidated financial statements are the annual financial statements of LumiraDx Limited (“the Company”) and its subsidiaries (“the Group”) (“the Financial Statements”).
The Company is an exempted company limited by shares incorporated in the Cayman Islands (registered number 314391) with registered offices situated at the offices of Ocorian Trust (Cayman) Limited, PO Box 1350, Windward 3, Regatta Office Park,, Grand Cayman KY1-1108. The subsidiaries of the Company are listed in Note 9.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The principal accounting policies applied in the preparation of these Financial Statements are set out below. These policies have been consistently applied, unless otherwise stated.
2.1 Basis of preparation of Financial Statements
The Financial Statements of LumiraDx Limited have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”). These Financial Statements were authorized for issue by the Board on April 12, 2022.
The Financial Statements have been prepared under the historical cost convention.
The preparation of Financial Statements in conformity with IFRS requires the use of certain critical accounting estimates. It also requires management to exercise its judgement in the process of applying the Group’s accounting policies. The areas involving a higher degree of judgement or complexity, or areas where assumptions and estimates are significant to the consolidated Financial Statements, are disclosed in Note 3.
LumiraDx Limited was incorporated on August 24, 2016. On September 29, 2016, the Company acquired all of the outstanding shares of LumiraDx Holdings Limited in a share for share exchange. LumiraDx Holdings Limited was incorporated on September 1, 2014. The consolidated Financial Statements of LumiraDx Limited have been prepared as if the share exchange had occurred on September 1, 2014 to reflect the continuous operations of the Company.
Going concern
During the year ended December 31, 2021 the Group incurred a loss for the year of $100,755 (2020: $240,997), and operating cash outflows of $134,583 (2020: $149,327). As of December 31, 2021 the Group had net assets of $162,596 (2020: $(375,216)). The Group has financed its operations principally through issuances of debt and equity securities, and the Group requires ongoing additional funding to continue to develop its commercial operations and research and development projects for future products.
The financial statements have been prepared on a going concern basis which the directors consider to be appropriate for the following reasons.
The directors have prepared cash flow forecasts for a period of at least 12 months from the date of approval of these financial statements which indicate that the Group will have sufficient funds to meet their liabilities as they fall due for that period (the going concern period).
The Group has minimum committed expenses including payroll for current employees, lease and other contractual commitments and interest payments on debt obligations of approximately $13,000 per month; however, the Group will be required to spend considerably more in order to continue to execute on its entire strategic business plan.
As discussed in Note 17, in January 2021 the Group drew an additional $35,000 on its 2020 Senior Secured Loan and issued an additional $40,000 in senior notes on the same terms as the Senior Secured Loan. In March 2021 the Group refinanced the $100,000 in outstanding amounts under the 2020 Senior Secured Loan and the $40,000 borrowed in January 2021 such that
F-7
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
at the reporting date debt outstanding consisted primarily of a $300,000 loan (the 2021 Senior Secured Loan). In September 2021 the Group completed a merger with CA Healthcare Acquisition Corp., a special purpose acquisition company. The transaction allowed all holders of preferred shares, A Ordinary shares and common shares to receive shares of the publicly listed company and resulted in gross proceeds of $38,000 to the Group.
The 2021 Senior Secured Loan matures in March 2024 and contains customary covenants including achieving certain revenue levels for the years ending December 31, 2021, 2022 and 2023. The Group met the 2021 revenue covenant. For the 2022 revenue covenant, the Group’s short-term revenue prospects will vary with the amount of demand for its SARS-CoV-2 products. While the directors believe that the Group’s SARS-CoV-2 products will continue to remain in high demand as COVID-19 vaccines are available, the continued efficacy of such vaccines or the mitigation of the COVID-19 pandemic earlier than expected for any other reason could negatively impact demand for the Group’s Platform and sales of its Instrument, test strips and other products. In addition, competitors may produce more accurate tests or tests which receive more favorable demand, both of which may impact the Group’s revenue streams and ability to meet the revenue covenant. In a severe but plausible scenario, cashflows indicate that the revenue covenant will not be met and therefore the Group would be required to obtain waivers of covenant violations or restructure existing debt obligations.
In March 2022 the Group entered into privately negotiated subscription agreements with certain investors wherein the Group sold and investors purchased $56,500 of Convertible Senior Subordinated Notes due 2027. The notes bear annual interest of 6%, payable semi-annually in arrears starting September 1, 2022. The notes mature on March 1, 2027 and are convertible at the holder’s option at an initial conversion rate of approximately $9.22 per share.
The directors believe that, if necessary, they will be able to obtain waivers of covenant violations or restructure the existing obligations, although there are no guarantees that these will be achieved. The directors believe the Group and company will be able to meet their liabilities as they fall due for the going concern period and have therefore prepared the financial statements on a going concern basis.
However, these circumstances represent a material uncertainty that may cast significant doubt on the Group's and the company’s ability to continue as a going concern and therefore, to continue realizing their assets and discharging their liabilities in the normal course of business. The financial statements do not include any adjustments that would result from the basis of preparation being inappropriate.
The Merger
On April 6, 2021, the Company entered into an initial business combination agreement (“the Merger”) with CA Healthcare Acquisition Corp. (“CAH”), a publicly-held special purpose acquisition company. The shareholders of CAH agreed to exchange their interests for new common shares in the share capital of the Company. Prior to the Merger, CAH was a newly-formed shell with no active trade or business, and all relevant assets, liabilities, income and expenses. Because CAH is not considered a business, the merger is not considered a business combination, and instead is accounted for as a reverse recapitalization, whereby the Company issues shares in exchange for the net assets of CAH represented by cash, which had a value of approximately $38,000 upon closing of the transaction, and its listed status. The excess of the fair value of the equity instruments issued by the Company over the identifiable net assets of CAH represents payment for the listing status and is recorded as a listing expense in the income statement under IFRS 2 Share-based Payment. The Merger completed on September 28, 2021 (the “acquisition date”). At the acquisition date, the Company became the ultimate legal parent of CAH. The Company’s common shares are traded on the NASDAQ Global Market under the ticker symbol LMDX and its warrants are traded under LMDXW. The Company’s A Ordinary shares are not publicly traded.
2.2 Basis of consolidation
The consolidated Financial Statements consolidate the Financial Statements of LumiraDx Limited and its subsidiary undertakings made up to December 31, 2021, 2020, and 2019.
Subsidiaries are all entities over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the investee and has the ability to affect those returns through its power over the investee. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. They are deconsolidated from the date that control ceases.
F-8
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
The Group applies the acquisition method to account for business combinations. The consideration transferred for the acquisition of a subsidiary is the fair values of the assets transferred, the liabilities incurred to the former owners of the acquiree and the equity interests issued by the Group. The consideration transferred includes the fair value of any asset or liability resulting from a contingent consideration arrangement. Identifiable assets acquired and liabilities and contingent liabilities assumed in a business combination are measured initially at their fair values at the acquisition date. The Group recognizes any non-controlling interest in the acquiree on an acquisition-by-acquisition basis, either at fair value or at the non-controlling interest’s proportionate share of the recognized amounts of acquiree’s identifiable net assets.
Acquisition-related costs are expensed as incurred.
If the business combination is achieved in stages, the acquisition date carrying value of the acquirer’s previously held equity interest in the acquiree is re-measured to fair value at the acquisition date; any gains or losses arising from such re-measurement are recognized in the consolidated statement of comprehensive income.
Any contingent consideration to be transferred by the Group is recognized at fair value at the acquisition date. Subsequent changes to the fair value of the contingent consideration that is deemed to be an asset or liability is recognized in accordance with IFRS 9 in the consolidated statement of comprehensive income. Contingent consideration that is classified as equity is not re-measured, and its subsequent settlement is accounted for within equity.
Inter-company transactions, balances and unrealized gains on transactions between Group companies are eliminated. Unrealized losses are also eliminated. When necessary, amounts reported by subsidiaries have been adjusted to conform with the Group’s accounting policies.
Investments in subsidiaries are accounted for at cost less impairment. Where necessary, adjustments are made to the financial statements of subsidiaries to bring the accounting policies used into line with those used by other members of the Group.
2.3 Investments
The major investments of the Group are listed in Note 9. Ownership interests equal voting rights.
The Group assesses investments for impairment whenever events or changes in circumstances indicate that the carrying value of an investment may not be recoverable. If any such indication of impairment exists, the Group makes an estimate of the recoverable amount. If the recoverable amount of the cash-generating unit is less than the value of the investment, the investment is considered to be impaired and is written down to its recoverable amount. Any impairment loss is recognized immediately in profit or loss.
Investments in equity instruments are required to be measured at fair value through profit or loss with the irrevocable option at inception to present changes in fair value in other comprehensive income.
2.4 Changes in accounting policy and disclosure
In 2021 the Group did not implement, nor were they aware of, any new accounting pronouncements that had a material impact on the Group’s financial statements.
2.5 Revenue recognition
The Group’s revenue is generated primarily from the sale of diagnostic products, including instruments and consumables. The Group’s services revenue includes the maintenance on software licenses, access to hosted cloud offerings and training, support and other services related to the Group’s diagnostic products.
Revenue from the sale or lease of goods and services rendered are recognized when a promise in a customer contract (“performance obligation”) has been satisfied by transferring control of the promised goods and services to the customer. Control of a promised good or service refers to the ability to direct the use of, and to obtain substantially all of the remaining benefits from, those goods or services. Control is usually transferred upon shipment or upon receipt of goods by the customer, or as services are rendered, in accordance with the delivery and acceptance terms agreed with the customers. The amount of revenue to be recognized (“transaction price”) is based on the consideration the Group expects to receive in
F-9
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
exchange for its goods and services, excluding amounts collected on behalf of third parties such as value added taxes or other taxes directly linked to sales. If a contract contains more than one performance obligation, the transaction price is allocated to each performance obligation based on their relative standalone selling prices.
The determination of the standalone selling price requires judgment. The Group’s determination of the standalone selling price for each performance obligation varies based on the geography and customer type. Generally, the standalone selling prices are based on observable prices. When observable prices are not available, the standalone selling price for products and services and for determination of amounts allocated for lease consideration in contracts with customers is based on a cost-plus margin approach.
Instruments may be sold together with other goods such as test strips, reagents and other consumables as well as services under a single contract or under several contracts that are combined for revenue recognition purposes. Revenue is recognized upon satisfaction of each of the performance obligations in the contract.
2.6 Research and development
Expenditure on research and development activities is recognized in profit or loss as incurred. The Group will capitalize development expenditures once the Group incurs expenditures related to technologies or products under development with proven technical feasibility. The development projects undertaken by the Group are subject to technical, regulatory and other uncertainties, such that, technical feasibility is deemed not to have been met prior to obtaining marketing approval by the regulatory authorities in major markets.
2.7 Foreign Currency Translation
(a) Functional and presentation currency
Items included in each of the Financial Statements of the Group’s entities are measured using the currency of the primary economic environment in which the entity operates (“functional currency”). The Group Financial Statements are presented in U.S. Dollars which is the Group’s presentation currency.
(b) Transactions and balances
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of the transactions or valuation where such items are re-measured. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in the statement of profit and loss and comprehensive income. All foreign exchange gains and losses are presented in the income statement within Finance income and Finance expense.
(c) Group companies
The results and financial position of all the Group entities (none of which has the currency of a hyperinflationary economy) that have a functional currency different from the presentation currency are translated into the presentation currency as follows:
•assets and liabilities for each Statement of Financial Position presented are translated at the closing rate at the date of that Statement of Financial Position;
•income and expenses for each statement of comprehensive income are translated at average exchange rates (unless this average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case income and expenses are translated at the dates of the transactions); and
•all resulting exchange differences are recognized in other comprehensive income.
Goodwill and fair value adjustments arising on the acquisition of a foreign entity are treated as assets and liabilities of the foreign entity and translated at the closing rate. Exchange differences arising are recognized in other comprehensive income.
F-10
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
2.8 Property, Plant and Equipment
All property, plant and equipment is stated at historical cost less depreciation. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Group and the cost of the item can be measured reliably. All other repairs and maintenance are charged to the Statement of Profit and Loss and Comprehensive Income during the financial period in which they are incurred. No depreciation is charged on assets in the course of construction ahead of their productive use.
Depreciation on assets is calculated using the straight-line method to allocate their cost or revalued amounts to their residual values over their estimated useful lives, as follows:
•Land and buildings—length of the lease up to 15 years
•Plant and equipment—3-15 years
•Fixtures and fittings—3-7 years
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at the end of each reporting period. An asset’s carrying amount is written down immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount.
Gains and losses on disposal are determined by comparing proceeds with carrying amount. These are included in the Statement of Profit and Loss and Comprehensive Income.
2.9 Right-of-Use Assets
The Group assesses whether a contract is or contains a lease at inception of a contract. The Group recognizes a right-of-use asset and a corresponding lease liability with respect to all lease agreements in which it is the lessee, except for short-term leases (defined as leases with a lease term of 12 months or less) and leases of low value assets. For these leases, the Group recognizes the lease payments as an operating expense on a straight-line basis over the term of the lease unless another systematic basis is more representative of the time pattern in which economic benefits from the leased asset are consumed.
The lease liability is initially measured at the present value of the lease payments that are not paid at the commencement date, discounted by using the rate implicit in the lease. If this rate cannot be readily determined, the Group uses its incremental borrowing rate which is based on the Group’s recent borrowings.
Lease payments included in the measurement of the lease liability comprise:
•fixed lease payments (including in-substance fixed payments), less any lease incentives;
•variable lease payments that depend on an index or rate, initially measured using the index or rate at the commencement date;
•the amount expected to be payable by the lessee under residual value guarantees;
•the exercise price of purchase options, if the lessee is reasonably certain to exercise the options; and
•payments of penalties for terminating the lease, if the lease term reflects the exercise of an option to terminate the lease.
The lease liability is presented as a separate line in the consolidated statement of financial position.
The lease liability is subsequently measured by increasing the carrying amount to reflect interest on the lease liability (using the effective interest method) and by reducing the carrying amount to reflect the lease payments made.
F-11
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
The Group remeasures the lease liability, making a corresponding adjustment to the related right-of-use asset) whenever:
•the lease term has changed or there is a change in the assessment of exercise of a purchase option, in which case the lease liability is remeasured by discounting the revised lease payments using a revised discount rate.
•the lease payments change due to changes in an index or rate or a change in expected payment under a guaranteed residual value, in which cases the lease liability is measured by discounting the revised lease payments using the initial discount rate (unless the lease payments change is due to a change in a floating interest rate, in which case a revised discount rate is used).
•a lease contract is modified and the lease modification is not accounted for as a separate lease, in which case the lease liability is remeasured by discounting the revised lease payments using a revised discount rate.
The Group did not make any such adjustments during the periods presented.
The right-of-use assets comprise the initial measurement of the corresponding lease liability, lease payments made at or before the commencement day and any initial direct costs. They are subsequently measured at cost less accumulated depreciation and impairment losses.
Whenever the Group incurs an obligation for costs to dismantle and remove a leased asset, restore the site on which it is located or restore the underlying asset to the condition required by the terms and conditions of the lease, a provision is recognized and measured under IAS 37 Provisions, Contingent Liabilities and Contingent Assets. The costs are included in the related right-of-use asset, unless those costs are incurred to produce inventories.
Right-of-use assets are depreciated over the shorter period of lease term and useful life of the underlying asset. If a lease transfers ownership of the underlying asset or the cost of the right-of-use asset reflects that the Group expects to exercise a purchase option, the related right-of-use asset is depreciated over the useful life of the underlying asset. The depreciation starts at the commencement date of the lease.
The right-of-use assets are presented as a separate line in the consolidated statement of financial position.
The Group applies IAS 36 Impairment of Assets to determine whether a right-of-use asset is impaired and accounts for any identified impairment loss as described in Note 2.10 (d).
Variable rents that do not depend on an index or rate are not included in the measurement the lease liability and the right-of-use asset. The related payments are recognized as an expense in the period in which the event or condition that triggers those payments occurs and are recorded as an operating expense in the Consolidated Statement of Profit and Loss and Comprehensive Income.
As a practical expedient, IFRS 16 permits a lessee not to separate non-lease components, and instead account for any lease and associated non-lease components as a single arrangement. The Group has not used this practical expedient.
2.10 Intangible assets
(a) Goodwill
Goodwill arises on the acquisition of businesses and represents the excess of the consideration transferred over the fair value of the identifiable net assets acquired. If the total of consideration transferred, non-controlling interest recognized and previously held interest measured at fair value is less than the fair value of the net assets of the subsidiary acquired, in the case of a bargain purchase, the difference is recognized directly in the income statement.
For the purpose of impairment testing, goodwill acquired in a business combination is allocated to each of the cash generating units (“CGUs”), or groups of CGUs, that is expected to benefit from the synergies of the combination. Each unit or group of units to which the goodwill is allocated represents the lowest level within the entity at which the goodwill is
F-12
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
monitored for internal management purposes. Goodwill is monitored at the operating segment level. Currently the Group operates in a single segment and the goodwill is assessed at a single CGU.
Goodwill impairment reviews are undertaken annually or more frequently if events or changes in circumstances indicate a potential impairment. The carrying value of the CGU containing the goodwill is compared to the recoverable amount, which is the higher of value in use and the fair value less costs of disposal. Any impairment is recognized immediately as an expense and is not subsequently reversed.
(b) Patents
Acquired patents and patent applications are shown at acquired cost less accumulated amortization. Amortization will be calculated using the straight line method to allocate the cost of patents over their estimated useful economic lives, calculated as the lower of management’s estimated useful life or the time remaining on the granted patent, once brought into use.
(c) Intangible assets acquired in a Business Combination
Intangible assets acquired in a business combination and recognized separately from goodwill are initially recognized at their fair value at the acquisition date (which is regarded as their cost). Subsequent to initial recognition, intangible assets acquired in a business combination are reported at cost less accumulated amortization and accumulated impairment losses, on the same basis as intangible assets that are acquired separately. Separately recognized intangible assets comprise customer relationships and contracts, supplier relationships, technology and software. Amortization is calculated either using the straight line method or over the asset’s economic useful life based on cash flow projections. Customer related intangibles and supplier relationships are amortized over 7 to 10 years. Technology and software are amortized over 8 to 10 years.
(d) Impairment of Non-Financial Assets
Assets not ready for use are not subject to amortization and are tested annually for impairment. Assets that are subject to amortization or depreciation are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs to sell and value in use. For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash-generating units). Non-financial assets other than goodwill that suffered impairment are reviewed for possible reversal of the impairment at each reporting date.
2.11 Financial instruments
(a) Classification
The Group classifies its financial instruments in the following categories (as disclosed in Note 22): amortized cost or fair value through profit or loss (equity investments).
Financial assets and liabilities are recognized when the Group becomes a party to the contractual provisions of the instrument.
Financial liabilities at amortized cost comprise trade and other payables, loans and other financial liabilities.
(b) Recognition and Measurement
At initial recognition, the Group measures a financial asset at its fair value plus, in the case of a financial asset not at fair value through profit or loss, transaction costs that are directly attributable to the acquisition of the financial asset. Subsequently, loans and receivables are measured at amortized cost (with the exception of equity investments which are measured at fair value through profit or loss) using the effective interest method less a provision for impairment.
The Group’s financial liabilities consist of trade and other payables, notes payable and preferred shares. These financial instruments are assessed under IFRS 9, to determine if the instrument qualifies to be accounted for under the fair value through profit or loss (“FVTPL”) method or at amortized cost.
F-13
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Financial liabilities held at amortized cost are initially recognized at the amount to be required to be paid, less, when material, a discount to reduce the payables to fair value. Financing costs are recorded as a reduction of the proceeds from the financing. If the costs relate to more than one element of a financing transactions, the financing costs are recorded as a proportional reduction of the proceeds of the separate elements. Financial liabilities are subsequently measured at amortized cost using the effective interest method.
Financial liabilities held at FVTPL are initially recognised at fair value. After initial recognition, these financial liabilities are re-measured at FVTPL using an appropriate valuation technique.
Financial liabilities are classified as current liabilities if payment is due within twelve months. Otherwise, they are presented as non-current liabilities.
(c) Derecognition
The Group derecognizes a financial asset when the contractual rights to the cash flows from the asset expire, or it transfers the rights to receive the contractual cash flows on the financial asset in a transaction in which substantially all the risks and rewards of the ownership of the financial asset are transferred. Any interest in transferred financial assets that is created or retained by the Group is recognized as a separate asset or liability.
Derecognition also takes place for certain assets when the Group write-off balances pertaining to the assets deemed to be uncollectible.
The Group derecognizes a financial liability when its contractual obligations are discharged, cancelled or expire. Where there has been a significant modification of a financial liability the Group derecognizes the original financial liability and recognizes the modified liability at fair value with any difference between the amortized cost of the derecognized liability and the fair value of the modified liability being recognized in comprehensive income.
(d) Impairment of financial assets
An impairment assessment is carried out annually and when there is evidence that an asset may be impaired. When the recoverable amount of an asset, being the higher of its fair value less costs of disposal and its value in use, is less than its carrying value, then the carrying value is reduced to its recoverable amount.
For the loans and receivables category, the amount of the loss is measured as the difference between the asset’s carrying amount and the present value of estimated future cash flows (excluding future credit losses that have not been incurred), discounted at the financial asset’s original effective interest rate. The asset’s carrying amount is reduced, and the loss is recognized in the income statement. If a loan or held-to-maturity investment has a variable interest rate, the discount rate for measuring any impairment loss is the current effective interest rate determined under the contract. As a practical expedient, the Group may measure impairment on the basis of an instrument’s fair value using an observable market price.
When a subsequent event causes the amount of impairment loss to decrease, the impairment loss is reversed through the Consolidated Statement of Profit and Loss and Comprehensive Income. Evidence of impairment may include indications that the debtors or a Group of debtors is experiencing significant financial difficulty, default or delinquency in interest or principal repayments, the probability that they will enter bankruptcy or other financial reorganization, and where observable data indicate that there is a measurable decrease in the estimated future cash flows, such as changes in arrears or economic conditions that correlate with defaults.
The Group recognizes loss allowances for expected credit losses (“ECL”) for financial assets measured at amortized cost.
For trade and other receivables, the Group measures the allowance for doubtful accounts at an amount equal to lifetime ECL.
Financial assets are written off (either partially or in full) when there is no realistic prospect of recovery. This is generally the case when the Group determines that the customer does not have assets or sources of income that could generate sufficient cash flows to repay the amounts subject to the write-off.
F-14
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Inventories are stated at the lower of cost and net realizable value. The cost of finished goods, work in process includes raw materials, direct labor and other directly attributable costs and overheads based upon the normal capacity of production facilities. Cost is determined using the weighted average method. Net realizable value is the estimated selling price less cost to completion and selling expenses.
2.13 Trade and other receivables
Trade receivables are amounts due from customers for merchandise sold or services performed in the ordinary course of business. If collection is expected in one year or less (or in the normal operating cycle of the business if longer), they are classified as current assets. If not, they are presented as non-current assets.
Trade and other receivables are carried at the original invoiced amount less allowances made for doubtful accounts, trade discounts, cash discounts and similar allowances. An allowance for doubtful accounts is recorded for expected credit losses over the term of the receivables. These are based on specific indicators, such as the ageing of customer balances and other specific credit circumstances. Trade and other receivables are written off when there is no reasonable expectation of recovery. The Group applies the simplified approach prescribed by IFRS 9, which requires / permits the use of the lifetime expected loss provision from initial recognition of the receivables.
2.14 Cash and cash equivalents
In the Consolidated Statement of Cash Flows, cash and cash equivalents comprise cash at bank and in hand, deposits held at call with banks and bank overdrafts. In the Consolidated Statement of Financial Position, bank overdrafts, if any, are shown within borrowings in current liabilities.
2.15 Restricted cash
Restricted cash consist of deposits that are required as collateral for letters of credit for vendor deposits.
2.16 Trade and other payables
Trade payables are obligations to pay for goods or services that have been acquired in the ordinary course of business from suppliers. Trade payables are classified as current liabilities if payment is due within one year or less (or in the normal operating cycle of the business if longer). If not, they are presented as non-current liabilities.
Trade and other payables are initially measured at fair value and are subsequently measured at amortized cost using the effective interest method.
2.17 Provisions and charges
Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax rate that reflects current market assessments of the time value of money and the risks specific to the obligation. The present value of the liability is remeasured at the reporting date.
Borrowing costs are recognized in the Consolidated Statement of Profit and Loss and Comprehensive Income in the period in which they are incurred.
2.19 Share capital
Ordinary Shares are classified as equity when there is no obligation to transfer cash or other assets. Incremental costs directly attributable to the issue of equity instruments are shown in equity as a deduction from the proceeds, net of tax. Incremental costs directly attributable to the issue of equity instruments as consideration for the acquisition of a business are included in
F-15
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
the cost of acquisition. The Company’s Series A Preferred Shares have been classified as a compound financial instrument as described in Note 16. The Company’s Series B Preferred Shares have been classified as financial liability held at FVTPL.
On February 1, 2021 the Board of Directors of the Company approved a stock split of the issued and outstanding A Ordinary and common shares of the Company on a 220 for 1 basis. In accordance with IAS 33, the earnings per share calculations have been presented for the stock split retrospectively. In connection with the merger with CAH, in order to achieve an exchange ratio of one LMDX common share for each CAH share, the Company effected a subdivision, immediately prior to the merger, of all issued, and authorized but unissued, LMDX ordinary shares and LMDX common shares at a ratio of 1.60806264:1.
The Company operates equity-settled, share-based compensation plans under which the entity receives services or other consideration from employees and other unrelated parties for equity instruments of the Company. The fair value of the services and consideration received in exchange for the grant of options is recognized as an expense and as a component of equity. The total amount to be expensed over the vesting period is determined by reference to the fair value of the options granted.
2.21 Taxation
The tax expense or credit comprises current and deferred tax. It is calculated using tax rates that have been enacted or substantively enacted by the Statement of Financial Position date. Subsidiaries within the Group may be eligible for tax credits related to qualifying research and development expenditures. The Group records an asset as a reduction in tax expense when it determines the receipt of a tax credit is probable.
Deferred tax is accounted for using the balance sheet liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the financial statements and the corresponding tax basis used in the computation of taxable profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences and deferred tax assets are recognized to the extent that it is probable that taxable profits will be available against which deductible temporary differences can be utilized. Such assets and liabilities are not recognized if the temporary difference arises from goodwill (or negative goodwill) or from the initial recognition (other than in a business combination) of other assets and liabilities in a transaction, which affects neither the tax profit nor the accounting profit.
Deferred tax assets are recognized only to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilized.
Deferred tax liabilities are recognized for taxable temporary differences arising on investments in subsidiaries and associates, and interests in joint ventures, except where the Group is able to control the reversal of the temporary difference and it is probable that the temporary difference will not reverse in the foreseeable future. Deferred tax is calculated at the tax rates that are expected to apply to the period when the asset is realized or the liability is settled. Deferred tax is charged or credited in the Consolidated Statement of Profit and Loss and Comprehensive Income, except when it relates to items credited or charged directly to equity, in which case the deferred tax is also dealt with in equity. Deferred tax assets and liabilities are offset when they relate to income taxes levied by the same taxation authority and the Group intends to settle its current tax assets and liabilities on a net basis.
2.22 Pension Obligations
The Group makes contributions to defined contribution pension plans for employees. The Group has no legal or constructive obligations to pay further contributions. The contributions are recognized as employee benefit expense when they are paid. In 2021 expenses for the Group’s defined contribution plans were $3,111 (2020: $1,569, 2019: $1,061).
F-16
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
3. CRITICAL ACCOUNTING ESTIMATES AND JUDGEMENTS
Use of estimates and judgements
The preparation of financial statements in conformity with IFRSs requires management to make judgements, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, income and expenses. The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis of making the judgements about carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods if the revision affects both current and future periods. In particular, information about significant areas of estimation uncertainty and critical judgements in applying accounting policies that have the most significant effect on the amount recognized in the financial statements are described below.
Revenue Recognition
The Group’s sales transactions may consist of various performance obligations that are satisfied at different times. It requires judgment to determine when different obligations are satisfied, including whether enforceable commitments for further obligations exist and when they arise. Depending on the determination of the performance obligations and the point in time or period over which those obligations are fulfilled, this may result in all revenue being calculated at inception, and either being recognized at once or on contract completion or spread over the term of a longer performance obligation.
In the accounting for contracts that contain promises to deliver more than one good or service, the Group has to determine how to allocate the total transaction price to the performance obligations of the contract. The Group allocates the total transaction price of a customer contract to the distinct performance obligations under the contract based on their standalone selling prices. The best evidence of this is an observable price from the standalone sales of the good or service to similarly situated customers. However, where standalone selling prices are not observable, it requires judgment to estimate the cost of satisfying a performance obligation and adding an appropriate margin to that good or service.
Nonrecurring valuations
The Group’s nonrecurring valuations are primarily associated with (i) the application of acquisition accounting and (ii) impairment assessments, both of which require value in use determinations as of the applicable valuation date. In making these determinations, the Group is required to make estimates and assumptions that affect the recorded amounts, including, but not limited to expected future cash flows, and discount rates, and remaining useful lives of long-lived assets. To assist in making these fair value determinations, the Group may engage third party valuation specialists. Estimates in this area impact, among other items, the amount of depreciation and amortization, impairment charges and income tax expense or credit. Estimates of fair value are based upon assumptions management believes to be reasonable, but which are inherently uncertain. A significant portion of the Group's long-lived assets were initially recorded through the application of acquisition accounting and all of the Group's long-lived assets are subject to impairment assessments.
The Group regularly review whether changes to estimated useful lives are required in order to accurately reflect the economic use of our intangible assets with finite lives.
The Group had net intangible assets of $24,732 and $21,442 as of December 31, 2020 and 2021, respectively.
Share-Based Payments
The Group operates equity-settled, share-based compensation plans under which the Group receives services or other consideration from employees and other unrelated parties for our equity instruments. The fair value of the services and consideration received in exchange for the grant of options is recognized as an expense and as a component of equity. The total amount to be expensed over the vesting period is determined by reference to the fair value of the options granted. The fair value of the share options was determined using a Black-Scholes valuation model. No performance conditions were included in the fair value calculations.
F-17
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Fair Value of Share Options
The fair value of each award on the grant date is estimated using the Black-Scholes option pricing model. The Black-Scholes model requires the input of highly subjective assumptions, including the expected volatility, the risk-free rate, expected life and the dividend yield. The expected volatility is based on the historical volatility of several comparable companies in the same industry. The expected life is based on the longer of each tranche’s respective weighted-average vesting term or the expected term to a liquidity event. The risk-free rate for periods within the contractual life of the options is based on the market yield of U.S. Treasury Bonds in effect at the time of grant. The dividend yield is based on the Company’s expected dividend policy over the contractual life of the options.
The assumptions used to estimate the fair value of the share options granted are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021 |
|
|
|
2020* |
|
|
Employees |
|
|
Founders |
|
Grant date fair value ($) |
|
1,634 to 3,861 |
|
|
1.37 to 4.02 |
|
|
1.26 to 2.85 |
|
Exercise price ($) |
|
1,793 to 3,861 |
|
|
9.89 to 16.39 |
|
|
16.96 to 17.05 |
|
Volatility |
|
35-40% |
|
|
|
40.0 |
% |
|
|
40.0 |
% |
Dividend yield |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Expected life of option (years) |
|
2-2.5 |
|
|
4-6.25 |
|
|
4-6.25 |
|
Annual risk free interest rate |
|
0.2-1.6% |
|
|
0.78-1.22% |
|
|
0.78-1.22% |
|
Total fair value of options granted |
|
$ |
6,716 |
|
|
$ |
8,567 |
|
|
$ |
43,887 |
|
*Amounts not adjusted for 2021 share splits
Fair Value of Ordinary Shares
The Group utilized the fair value of ordinary shares when determining the fair value of financial instruments including the 2020 Convertible Notes and Series B preferred shares as well as determining the fair value of the ordinary shares underlying its options when performing the fair value calculations with the Black-Scholes option pricing model. Therefore, prior to the merger, the directors have estimated the fair value of the Group’s ordinary shares at various dates, with input from management, considering the third-party valuations of ordinary shares. The valuations of ordinary shares were performed using methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants Audit and Accounting Practice Aid Series: Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the AICPA Practice Guide. In addition, the directors considered various objective and subjective factors, along with input from management and the independent third-party valuation firm, to determine the fair value of ordinary shares, including: external market conditions affecting the industry, trends within the industry, the results of operations, financial position, status of our research and development efforts, our stage of development and business strategy, and the lack of an active public market for the Group’s ordinary shares, and the likelihood of achieving a liquidity event such as an initial public offering, or IPO.
The valuations of the Group’s ordinary shares, prior to the merger, were prepared using an option pricing method, or OPM, and a probability-weighted expected return method, or PWERM. The PWERM is a scenario-based methodology that estimates the fair value of ordinary shares based upon an analysis of future values for the Group, assuming various outcomes. The ordinary shares’ value is based on the probability-weighted present value of expected future investment returns considering each of the possible outcomes available, as well as the rights of each share class. The future value of the ordinary shares under each outcome is discounted back to the valuation date at an appropriate risk-adjusted discount rate and probability weighted to arrive at an indication of value for the ordinary shares. The OPM treats ordinary shares and preferred shares as call options on the total equity value of a company, with exercise prices based on the value thresholds at which the allocation among the various holders of a company’s securities changes. Under this method, the ordinary shares have value only if the funds available for distribution to shareholders exceeded the value of the preferred share liquidation preferences at the time of the liquidity event, such as a strategic sale or a merger. A discount for lack of marketability of the ordinary shares is then applied to arrive at an estimate of value for the ordinary shares.
F-18
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
In addition to considering the results of these third-party valuations, the directors considered various objective and subjective factors to determine the fair value of ordinary shares as of each grant date, including:
•the prices at which the Group issued ordinary and preferred shares and the superior rights and preferences of the preferred shares relative to the ordinary shares at the time of each grant;
•the progress of the Group’s research and development programs;
•the stage of development and the Group’s business strategy;
•external market conditions affecting the Group’s industry and trends within the industry;
•the Group’s financial position, including cash on hand, and historical and forecasted performance and operating results;
•the lack of an active public market for the Group’s ordinary shares and preferred shares;
•the likelihood of achieving a liquidity event, such as an IPO, in light of prevailing market conditions; and
•the analysis of IPOs and the market performance of similar companies in the Group’s industry.
The Group utilized the fair value of its Ordinary shares when determining the fair value of financial instruments including the 2020 Convertible Notes (Note 17) and Series B Preferred Shares (Note 16), as well as determining the Group estimates.
The assumptions underlying these valuations represented management’s best estimates, which involved inherent uncertainties and the application of management’s judgment. As a result, if the assumptions or estimates used had been significantly different, the fair value of ordinary shares and share-based payment expense could be materially different.
Product Reserves
The Group provides standard commercial warranties on its products. Separately, the Group also periodically performs field service actions related to safety matters and other product campaigns. Pursuant to these warranties and field service actions, the Group will repair or replace products that are defective in materials or workmanship. The Group accrues the estimated cost of both base warranty coverages and field service actions at the time of sale. Provisions for warranties of $5,801 (2020: $6,557) are recorded in the balance sheet within trade and other payables (Note 20). The Group is able to service Platform instruments returned by customers. The Group has estimated the number of returned instruments that are anticipated being able to be serviced and returned to customers and have reserved against those instruments not expected to be serviced.
The Group maintains an allowance for excess or obsolete inventories. The allowance is based on a review of inventory materials on hand, which the Group compares with estimated future usage. In addition, the Group reviews the inventories and compares parts costs with current market value and writes down any parts with costs in excess of current market value to net realizable value. Provisions for inventories of $22,829 (2020: $13,186) are recorded in the balance sheet within inventory.
These estimates take into consideration historical experience, current contractual and statutory requirements, specific known market events and trends such as competitive pricing and new product introductions, estimated inventory levels, and the shelf life of products. As 2020 was the first year of significant sales of its diagnostic platform, the Group has limited history to make these estimates. If actual future results vary, these estimates may need to be adjusted, with an effect on sales and earnings in the period of the adjustment. Actual results could differ from these estimates.
4. Revenue
Disaggregation of Revenue
F-19
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
REVENUE STREAM |
|
REVENUE
FROM
CONTRACTS
WITH
CUSTOMERS |
|
|
REVENUE
FROM
OTHER
SOURCES |
|
|
TOTAL |
|
|
REVENUE
FROM
CONTRACTS
WITH
CUSTOMERS |
|
|
REVENUE
FROM
OTHER
SOURCES |
|
|
TOTAL |
|
|
REVENUE
FROM
CONTRACTS
WITH
CUSTOMERS |
|
|
REVENUE
FROM
OTHER
SOURCES |
|
|
TOTAL |
|
Products |
|
$ |
18,817 |
|
|
$ |
985 |
|
|
$ |
19,802 |
|
|
$ |
133,794 |
|
|
$ |
862 |
|
|
$ |
135,656 |
|
|
$ |
414,114 |
|
|
$ |
1,540 |
|
|
$ |
415,654 |
|
Services |
|
|
3,340 |
|
|
|
— |
|
|
|
3,340 |
|
|
|
3,497 |
|
|
|
— |
|
|
|
3,497 |
|
|
|
5,774 |
|
|
|
— |
|
|
|
5,774 |
|
Total Revenue |
|
$ |
22,157 |
|
|
$ |
985 |
|
|
$ |
23,142 |
|
|
$ |
137,291 |
|
|
$ |
862 |
|
|
$ |
139,153 |
|
|
$ |
419,888 |
|
|
$ |
1,540 |
|
|
$ |
421,428 |
|
Revenue from diagnostic products is recognized at the time the performance obligations are met. Service revenue is recognized over the contractual term. Revenue from other sources represents lease revenue on instruments.
Contract Balances
Service revenue is typically billed in advance giving rise to a contract liability balance. The deferred balance as of December 31, 2021 and 2020 is $1,517 and $1,760, respectively. As the Company generally recognizes revenue as goods are sold for product revenue, the Company does not have other material contract asset or liability balances as of December 31, 2021.
Remaining performance obligations in (partially) unsatisfied long-term contracts:
|
|
|
|
|
|
|
|
|
|
|
DEFERRED
REVENUE |
|
|
|
2020 |
|
|
2021 |
|
Balance at start of the period |
|
|
2,639 |
|
|
|
1,760 |
|
Recognized revenue from prior years' invoicing |
|
|
(2,348 |
) |
|
|
(1,760 |
) |
Amounts invoiced to be recognized over time |
|
|
2,509 |
|
|
|
4,149 |
|
Recognized revenue from current year invoicing |
|
|
(1,131 |
) |
|
|
(2,703 |
) |
Foreign exchange impact |
|
|
91 |
|
|
|
71 |
|
Balance at end of the period |
|
|
1,760 |
|
|
|
1,517 |
|
Remaining performance obligations in (partially) unsatisfied long-term contracts are included in deferred revenue. For contracts that have an original duration of one year or less, the Group has elected the practical expedient to not disclose the transaction price for remaining performance obligations at the end of each reporting period and at which point in time the Company expects to recognize these sales.
5. SEGMENTS
Basis for segmentation:
The CEO is the Group’s chief operating decision maker (“CODM”). The regular internal reporting to the CEO, which fulfils the criteria to constitute a segment, is done for the Group as a whole, and therefore the total Group is the company’s only segment.
F-20
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Revenue from external customers by country, based on the location of the customer is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
ANALYSIS OF REVENUE BY COUNTRY: |
|
2019 |
|
|
2020 |
|
|
2021 |
|
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
20 |
|
|
$ |
54,655 |
|
|
$ |
250,755 |
|
Italy |
|
|
5,993 |
|
|
|
24,098 |
|
|
|
65,659 |
|
United Kingdom |
|
|
5,373 |
|
|
|
39,936 |
|
|
|
56,282 |
|
Germany |
|
|
282 |
|
|
|
1,462 |
|
|
|
16,261 |
|
Colombia |
|
|
8,177 |
|
|
|
8,789 |
|
|
|
12,101 |
|
Sweden |
|
|
1,097 |
|
|
|
3,128 |
|
|
|
6,954 |
|
Netherlands |
|
|
— |
|
|
|
101 |
|
|
|
2,610 |
|
Switzerland |
|
|
63 |
|
|
|
581 |
|
|
|
2,491 |
|
Brazil |
|
|
1,758 |
|
|
|
3,209 |
|
|
|
2,320 |
|
Japan |
|
|
— |
|
|
|
— |
|
|
|
2,251 |
|
Austria |
|
|
104 |
|
|
|
1,622 |
|
|
|
2,002 |
|
Spain |
|
|
— |
|
|
|
— |
|
|
|
1,014 |
|
Denmark |
|
|
— |
|
|
|
1,354 |
|
|
|
98 |
|
Other |
|
|
275 |
|
|
|
218 |
|
|
|
630 |
|
Total revenue |
|
$ |
23,142 |
|
|
$ |
139,153 |
|
|
$ |
421,428 |
|
In 2021 the Group had 1 significant customer which accounted for 45% of the Group's revenue.
In 2020 the Group had 2 significant customers which accounted for 29% and 17% of the Group’s revenue, respectively.
Non-current assets by country are as follows:
|
|
|
|
|
|
|
|
|
|
|
ANALYSIS OF NON-CURRENT ASSETS BY COUNTRY: |
|
|
|
2020 |
|
|
2021 |
|
United Kingdom |
|
|
|
$ |
115,135 |
|
|
$ |
199,312 |
|
United States |
|
|
|
|
7,985 |
|
|
|
22,537 |
|
Italy |
|
|
|
|
9,280 |
|
|
|
10,600 |
|
Colombia |
|
|
|
|
4,306 |
|
|
|
3,780 |
|
Sweden |
|
|
|
|
593 |
|
|
|
630 |
|
Other |
|
|
|
|
1,133 |
|
|
|
1,901 |
|
Total |
|
|
|
$ |
138,432 |
|
|
$ |
238,760 |
|
F-21
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
6. FINANCE INCOME AND FINANCE EXPENSE
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
Net gain on conversion of convertible financial instruments |
|
$ |
- |
|
|
$ |
- |
|
|
$ |
64,143 |
|
Change in fair value of 2020 convertible notes (Note 17) |
|
|
- |
|
|
|
- |
|
|
|
52,267 |
|
Change in fair value of Series B preferred shares (Note 16) |
|
|
- |
|
|
|
- |
|
|
|
48,956 |
|
Interest Income |
|
|
1,978 |
|
|
|
581 |
|
|
|
60 |
|
Foreign exchange gain |
|
|
9,727 |
|
|
|
21,908 |
|
|
|
- |
|
Other |
|
|
|
|
|
11 |
|
|
|
- |
|
Finance income |
|
$ |
11,705 |
|
|
$ |
22,500 |
|
|
$ |
165,426 |
|
|
|
|
|
|
|
|
|
|
|
Interest expense (cash) |
|
$ |
(5,749 |
) |
|
$ |
(12,695 |
) |
|
$ |
(29,954 |
) |
Interest expense (non-cash) |
|
|
(11,044 |
) |
|
|
(18,152 |
) |
|
|
(43,939 |
) |
Lease liability interest expense (Note 24) |
|
|
(396 |
) |
|
|
(751 |
) |
|
|
(2,501 |
) |
Foreign exchange loss |
|
|
- |
|
|
|
- |
|
|
|
(14,594 |
) |
Dividend on preferred shares (Note 16) |
|
|
(21,600 |
) |
|
|
(23,578 |
) |
|
|
(16,156 |
) |
Debt extinguishment fee (cash) |
|
|
- |
|
|
|
- |
|
|
|
(3,636 |
) |
Debt extinguishment fee (non-cash) |
|
|
(520 |
) |
|
|
(5,647 |
) |
|
|
(4,170 |
) |
Change in fair value of 2020 convertible notes (Note 17) |
|
|
- |
|
|
|
(102,548 |
) |
|
|
- |
|
Change in fair value of Series B preferred shares (Note 16) |
|
|
- |
|
|
|
(9,351 |
) |
|
|
- |
|
Change in fair value of Stock Warrants (Note 27) |
|
|
- |
|
|
|
- |
|
|
|
(2,875 |
) |
Other |
|
|
(26 |
) |
|
|
- |
|
|
|
(109 |
) |
Finance expense |
|
$ |
(39,335 |
) |
|
$ |
(172,722 |
) |
|
$ |
(117,934 |
) |
7. INCOME TAXES
|
|
|
|
|
|
|
|
|
|
|
|
|
TAX CREDIT FOR THE PERIOD |
|
2019 |
|
|
2020 |
|
|
2021 |
|
Current income credit / (tax) |
|
|
|
|
|
|
|
|
|
- Current year |
|
$ |
8,228 |
|
|
$ |
10,320 |
|
|
$ |
(4,087 |
) |
- Prior years |
|
|
1,030 |
|
|
|
(767 |
) |
|
|
— |
|
Total current income credit / (tax) |
|
|
9,258 |
|
|
|
9,553 |
|
|
|
(4,087 |
) |
Deferred income tax credit |
|
|
|
|
|
|
|
|
|
- Current year |
|
|
283 |
|
|
|
393 |
|
|
|
1,243 |
|
- Prior years |
|
|
0 |
|
|
|
0 |
|
|
|
0 |
|
Total deferred income credit |
|
|
283 |
|
|
|
393 |
|
|
|
1,243 |
|
Total income tax credit/(expense) |
|
$ |
9,541 |
|
|
$ |
9,946 |
|
|
$ |
(2,844 |
) |
Included in the current year income credit are amounts related to research and development tax credits of $NaN (2020: $10,479, 2019: $8,976) in respect of the current year and $NaN (2020: $772, 2019: $804) in respect of prior years.
The prior year adjustment, which is primarily related to the research and development tax credit, has arisen following an increase in the eligible expenditure included within the claim filing made with the tax authorities.
In 2021, the group transitioned from the small company scheme to the research and development expenditure credit scheme ("RDEC"), see Note 21.
F-22
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Reconciliation of effective tax rate:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
Loss for the period before taxation |
|
$ |
142,650 |
|
|
$ |
250,943 |
|
|
$ |
97,911 |
|
Tax benefit at standard U.K. rate at 19% |
|
|
27,104 |
|
|
|
47,679 |
|
|
|
18,603 |
|
Difference in overseas tax rates |
|
|
409 |
|
|
|
145 |
|
|
|
(700 |
) |
Expenses not deductible for tax purposes |
|
|
(5,345 |
) |
|
|
(5,389 |
) |
|
|
7,359 |
|
Tax losses for which no deferred tax asset was recognized |
|
|
(14,683 |
) |
|
|
(37,694 |
) |
|
|
(21,887 |
) |
Share-based payment (not deductible for tax purposes) |
|
|
(693 |
) |
|
|
(572 |
) |
|
|
(6,443 |
) |
Research and development credit |
|
|
3,943 |
|
|
|
4,804 |
|
|
|
— |
|
Adjustments for prior year |
|
|
(1,030 |
) |
|
|
767 |
|
|
|
— |
|
Other timing differences and adjustments |
|
|
(164 |
) |
|
|
206 |
|
|
|
224 |
|
Income tax credit/(expense) |
|
$ |
9,541 |
|
|
$ |
9,946 |
|
|
$ |
(2,844 |
) |
Effective tax rate |
|
|
7 |
% |
|
|
4 |
% |
|
|
-3 |
% |
In the March 3, 2021 U.K. budget, it was announced that the U.K. tax rate will increase to 25% from April 1, 2023. This will not have a consequential effect on the Group’s recognized deferred taxes, however the Group has substantial unrecognized U.K. net operating losses (Note 19).
8. EARNINGS PER SHARE
The calculation of basic and diluted earnings per share has been calculated by dividing the loss for the period attributable to ordinary shareholders of $100,929 (2020: $240,980, 2019: $132,807), by the weighted average number of A Ordinary shares outstanding of 163,255,784 (2020: 132,192,880, 2019: 131,757,738) during the year ended December 31, 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss attributable to ordinary shareholders: |
|
2019 |
|
|
2020 |
|
|
2021 |
|
|
|
BASIC |
|
|
DILUTED |
|
|
BASIC |
|
|
DILUTED |
|
|
BASIC |
|
|
DILUTED |
|
Loss for the year, attributable to equity holders of the parent |
|
$ |
(132,807 |
) |
|
$ |
(132,807 |
) |
|
$ |
(240,980 |
) |
|
$ |
(240,980 |
) |
|
$ |
(100,929 |
) |
|
$ |
(100,929 |
) |
Loss attributable to ordinary shareholders |
|
|
(132,807 |
) |
|
|
(132,807 |
) |
|
|
(240,980 |
) |
|
|
(240,980 |
) |
|
|
(100,929 |
) |
|
|
(100,929 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average number of ordinary shares: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BASIC |
|
|
DILUTED |
|
|
BASIC |
|
|
DILUTED |
|
|
BASIC |
|
|
DILUTED |
|
Issued ordinary shares at January 1 |
|
|
133,597,362 |
|
|
|
133,597,362 |
|
|
|
132,188,281 |
|
|
|
132,188,281 |
|
|
|
132,204,201 |
|
|
|
132,204,201 |
|
Effect of shares issued |
|
|
(1,839,624 |
) |
|
|
(1,839,624 |
) |
|
|
4,599 |
|
|
|
4,599 |
|
|
|
31,051,583 |
|
|
|
31,051,583 |
|
Weighted-average number of ordinary shares |
|
|
131,757,738 |
|
|
|
131,757,738 |
|
|
|
132,192,880 |
|
|
|
132,192,880 |
|
|
|
163,255,784 |
|
|
|
163,255,784 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BASIC |
|
|
DILUTED |
|
|
BASIC |
|
|
DILUTED |
|
|
BASIC |
|
|
DILUTED |
|
Loss per share |
|
$ |
(1.01 |
) |
|
$ |
(1.01 |
) |
|
$ |
(1.82 |
) |
|
$ |
(1.82 |
) |
|
$ |
(0.62 |
) |
|
$ |
(0.62 |
) |
On February 1, 2021 the Board of Directors of the Company approved a stock split of the issued and outstanding A Ordinary and common shares of the Company on a 220 for 1 basis. In accordance with IAS 33, the earnings per share calculations have been presented for the stock split retrospectively. In connection with the merger, in order to achieve an exchange ratio of one LMDX common share for each CAH share, the Company effected a subdivision, immediately prior to the merger, of all issued, and authorized but unissued, LMDX ordinary shares and LMDX common shares at a ratio of 1.60806264:1. The denominator has been calculated to reflect the share splits.
The Company’s potentially dilutive securities, which include stock options, convertible preferred shares, convertible notes and warrants, have been excluded from the computation of diluted net loss per share as the effect would be to reduce the net loss per share. Therefore, the weighted-average number of A Ordinary and common shares outstanding used to calculate both basic and diluted net loss per share attributable to A Ordinary and common shareholders is the same. The Company excluded the following potential A Ordinary shares and common shares, presented based on amounts outstanding at each period end,
F-23
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
from the computation of diluted net loss per share attributable to ordinary shareholders and common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
|
|
|
|
|
|
|
|
|
YEAR ENDED DECEMBER 31, |
|
|
|
2020 |
|
|
2021 |
|
Convertible preferred shares (as converted to A Ordinary shares) |
|
|
87,711,133 |
|
|
|
— |
|
Options to purchase A Ordinary shares |
|
|
57,212,650 |
|
|
|
83,573,631 |
|
Convertible Debt (as converted to common shares) |
|
|
25,944,000 |
|
|
|
— |
|
Warrants to purchase A Ordinary shares |
|
|
5,430,781 |
|
|
|
5,430,781 |
|
Warrants to purchase common shares |
|
|
6,200,947 |
|
|
|
13,578,294 |
|
|
|
|
182,499,511 |
|
|
|
102,582,706 |
|
9. INVESTMENTS
The following table summarizes the information relating to each of the Group’s subsidiaries with Non-controlling interests.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2021 |
|
|
|
LUMIRADX
COLOMBIA
HOLDINGS
LIMITED* |
|
|
LUMIRADX
HEALTHCARE
LTDA. |
|
|
LUMIRADX
COLOMBIA
HOLDINGS
LIMITED* |
|
|
LUMIRADX
HEALTHCARE
LTDA. |
|
Non-current assets |
|
$ |
430 |
|
|
$ |
93 |
|
|
$ |
189 |
|
|
$ |
237 |
|
Current assets |
|
|
6,986 |
|
|
|
2,001 |
|
|
|
8,627 |
|
|
|
1,517 |
|
Non-current liabilities |
|
|
(6,119 |
) |
|
|
(3,016 |
) |
|
|
(5,643 |
) |
|
|
(3,594 |
) |
Current liabilities |
|
|
(1,730 |
) |
|
|
(504 |
) |
|
|
(3,213 |
) |
|
|
(543 |
) |
Net assets/(liabilities) (100%) |
|
|
(433 |
) |
|
|
(1,426 |
) |
|
|
(40 |
) |
|
|
(2,383 |
) |
Carrying amount of Non-controlling interest |
|
|
921 |
|
|
|
(1,128 |
) |
|
|
805 |
|
|
|
(1,260 |
) |
Revenue |
|
|
8,789 |
|
|
|
3,208 |
|
|
|
12,101 |
|
|
|
2,320 |
|
Profit/(loss) |
|
|
817 |
|
|
|
(2,023 |
) |
|
|
1,609 |
|
|
|
(879 |
) |
Other comprehensive gain |
|
|
(5 |
) |
|
|
40 |
|
|
|
- |
|
|
|
- |
|
Total comprehensive profit/(loss) (100%) |
|
|
812 |
|
|
|
(1,983 |
) |
|
|
1,609 |
|
|
|
(879 |
) |
Profit/(loss) allocated to non-controlling interest |
|
|
286 |
|
|
|
(303 |
) |
|
|
306 |
|
|
|
(132 |
) |
Other comprehensive loss allocated to non-
controlling interest |
|
|
(2 |
) |
|
|
6 |
|
|
|
— |
|
|
|
— |
|
Cash flows from operating activities |
|
|
731 |
|
|
|
(352 |
) |
|
|
1,810 |
|
|
|
(701 |
) |
Cash flows from investment activities |
|
|
(184 |
) |
|
|
(18 |
) |
|
|
(98 |
) |
|
|
(38 |
) |
Cash flows from financing activities |
|
|
— |
|
|
|
700 |
|
|
|
- |
|
|
|
500 |
|
Net increase/(decrease) in cash and cash equivalents |
|
$ |
547 |
|
|
$ |
330 |
|
|
$ |
1,712 |
|
|
$ |
(239 |
) |
*—Represents the consolidation of LumiraDx Colombia Holdings Limited and LumiraDx SAS, a wholly owned subsidiary of LumiraDx Colombia Holdings Limited
External parties hold 19% (2020: 35%) of the share capital of LumiraDx Colombia Holdings Limited. In 2021 the Group acquired an additional 16% of LumiraDx Colombia Holdings Limited for $1,968.
External parties hold 15% (2020: 15%) of the share capital of LumiraDx Healthcare, Ltda.
F-24
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
All subsidiary undertakings are included in the consolidation. LumiraDx Group Limited is held directly by the Company; all other subsidiaries are held indirectly. The proportion of the voting rights in the subsidiary undertaking held directly by the Company does not differ from the proportion of equity shares held.
|
|
|
|
|
|
|
|
|
Principal Subsidiaries |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PROPORTION OF
EQUITY SHARES HELD
BY COMPANY |
NAME |
|
COUNTRY OF
INCORPORATION AND
RESIDENCE |
|
NATURE OF
BUSINESS |
|
2020 |
|
2021 |
LumiraDx Brazil Holdings Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
|
100% |
LumiraDx Healthcare Ltda |
|
Brazil |
|
Distributor of medical diagnostics |
|
98% |
|
98% |
LumiraDx Colombia Holdings Limited |
|
United Kingdom |
|
Holding Company |
|
65% |
|
81% |
Lumira SAS |
|
Colombia |
|
Distributor of medical diagnostics |
|
100% * |
|
100% * |
Lumira SAS |
|
France |
|
Distributor of medical diagnostics |
|
n/a |
|
100% |
LumiraDx GmbH |
|
Germany |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx AB |
|
Sweden |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx UK Limited |
|
United Kingdom |
|
Manufacture and distribution of medical diagnostics |
|
100% |
|
100% |
LumiraDx Technology Limited |
|
United Kingdom |
|
Research and development |
|
100% |
|
100% |
LumiraDx Ltd. |
|
United Kingdom |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx Group Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
|
100% |
LumiraDx International Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
|
100% |
LumiraDx Investment Limited |
|
United Kingdom |
|
Holding Company |
|
100% |
|
100% |
LumiraDx Care Solutions UK Limited |
|
United Kingdom |
|
Healthcare IT and services |
|
100% |
|
100% |
LumiraDx, Inc |
|
United States |
|
Healthcare IT and services |
|
100% |
|
100% |
ACS Acquisition LLC |
|
United States |
|
Healthcare IT and services |
|
100% |
|
100% |
LumiraDx Healthcare LLC |
|
United States |
|
Healthcare IT and services |
|
100% |
|
100% |
Biomedical Service S.r.l. |
|
Italy |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx AS |
|
Norway |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx GmbH |
|
Austria |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx GmbH |
|
Switzerland |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx Japan KK |
|
Japan |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx Oy |
|
Finland |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx A/S |
|
Denmark |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx Healthcare S.L. |
|
Spain |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
SureSensors Ltd. |
|
United Kingdom |
|
Developer and manufacturer of medical diagnostics |
|
100% |
|
100% |
LumiraDx (Pty) Limited |
|
South Africa |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx B.V. |
|
Netherlands |
|
Distributor of medical diagnostics |
|
100% |
|
100% |
LumiraDx Benelux B.V. |
|
Netherlands |
|
Distributor of medical diagnostics |
|
n/a |
|
100% |
LumiraDx Limited |
|
Ireland |
|
Distributor of medical diagnostics |
|
n/a |
|
100% |
LumiraDx Healthcare Private Limited |
|
India |
|
Distributor of medical diagnostics |
|
n/a |
|
100% |
CA Healthcare Acquisition Corp. |
|
United States |
|
Holding Company |
|
0% |
|
100% |
*—LumiraDx Colombia Holdings Limited holds 100% of the equity shares of LumiraDx SAS
10. GOODWILL AND INTANGIBLE ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GOODWILL |
|
|
PATENTS |
|
|
CUSTOMER
INTANGIBLES |
|
|
SUPPLIER
RELATIONSHIPS |
|
|
TECHNOLOGY
AND
SOFTWARE |
|
|
TOTAL |
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At 1 January 2019 |
|
$ |
13,635 |
|
|
$ |
18,020 |
|
|
$ |
8,731 |
|
|
$ |
2,856 |
|
|
$ |
11,177 |
|
|
$ |
54,419 |
|
Additions |
|
|
— |
|
|
|
102 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
102 |
|
Acquisition of subsidiaries |
|
|
1,506 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,506 |
|
Exchange differences |
|
|
250 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
250 |
|
At 31 December 2019 |
|
|
15,391 |
|
|
|
18,122 |
|
|
|
8,731 |
|
|
|
2,856 |
|
|
|
11,177 |
|
|
|
56,277 |
|
Amortisation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At 1 January 2019 |
|
|
— |
|
|
|
2,312 |
|
|
|
2,977 |
|
|
|
757 |
|
|
|
6,711 |
|
|
|
12,757 |
|
Charge for the period |
|
|
— |
|
|
|
930 |
|
|
|
890 |
|
|
|
286 |
|
|
|
388 |
|
|
|
2,494 |
|
Impairments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Exchange differences |
|
|
— |
|
|
|
(532 |
) |
|
|
167 |
|
|
|
— |
|
|
|
(142 |
) |
|
|
(507 |
) |
At 31 December 2019 |
|
|
— |
|
|
|
2,710 |
|
|
|
4,034 |
|
|
|
1,043 |
|
|
|
6,957 |
|
|
|
14,744 |
|
Net Book Value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At 31 December 2019 |
|
$ |
15,391 |
|
|
$ |
15,412 |
|
|
$ |
4,697 |
|
|
$ |
1,813 |
|
|
$ |
4,220 |
|
|
$ |
41,533 |
|
F-25
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2020 |
|
$ |
15,391 |
|
|
$ |
18,122 |
|
|
$ |
8,731 |
|
|
$ |
2,856 |
|
|
$ |
11,177 |
|
|
$ |
56,277 |
|
Additions |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Acquisition of subsidiaries |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Exchange differences |
|
|
600 |
|
|
|
549 |
|
|
|
408 |
|
|
|
— |
|
|
|
156 |
|
|
|
1,713 |
|
At December 31, 2020 |
|
|
15,991 |
|
|
|
18,671 |
|
|
|
9,139 |
|
|
|
2,856 |
|
|
|
11,333 |
|
|
|
57,990 |
|
Amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2020 |
|
|
— |
|
|
|
2,710 |
|
|
|
4,034 |
|
|
|
1,043 |
|
|
|
6,957 |
|
|
|
14,744 |
|
Charge for the period |
|
|
— |
|
|
|
831 |
|
|
|
951 |
|
|
|
286 |
|
|
|
319 |
|
|
|
2,387 |
|
Impairments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Exchange differences |
|
|
— |
|
|
|
54 |
|
|
|
62 |
|
|
|
— |
|
|
|
20 |
|
|
|
136 |
|
At December 31, 2020 |
|
|
— |
|
|
|
3,595 |
|
|
|
5,047 |
|
|
|
1,329 |
|
|
|
7,296 |
|
|
|
17,267 |
|
Net Book Value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2020 |
|
$ |
15,991 |
|
|
$ |
15,076 |
|
|
$ |
4,092 |
|
|
$ |
1,527 |
|
|
$ |
4,037 |
|
|
$ |
40,723 |
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2021 |
|
$ |
15,991 |
|
|
$ |
18,671 |
|
|
$ |
9,139 |
|
|
$ |
2,856 |
|
|
$ |
11,333 |
|
|
$ |
57,990 |
|
Additions |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Exchange differences |
|
|
(385 |
) |
|
|
(178 |
) |
|
|
(278 |
) |
|
|
— |
|
|
|
(48 |
) |
|
|
(889 |
) |
At December 31, 2021 |
|
|
15,606 |
|
|
|
18,493 |
|
|
|
8,861 |
|
|
|
2,856 |
|
|
|
11,285 |
|
|
|
57,101 |
|
Amortization |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2021 |
|
|
— |
|
|
|
3,595 |
|
|
|
5,047 |
|
|
|
1,329 |
|
|
|
7,296 |
|
|
|
17,267 |
|
Charge for the period |
|
|
— |
|
|
|
890 |
|
|
|
1,317 |
|
|
|
286 |
|
|
|
334 |
|
|
|
2,827 |
|
Exchange differences |
|
|
— |
|
|
|
(16 |
) |
|
|
(21 |
) |
|
|
— |
|
|
|
(4 |
) |
|
|
(41 |
) |
At December 31, 2021 |
|
|
— |
|
|
|
4,469 |
|
|
|
6,343 |
|
|
|
1,615 |
|
|
|
7,626 |
|
|
|
20,053 |
|
Net Book Value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2021 |
|
$ |
15,606 |
|
|
$ |
14,024 |
|
|
$ |
2,518 |
|
|
$ |
1,241 |
|
|
$ |
3,659 |
|
|
$ |
37,048 |
|
Amortization of $2,652 (2020: $2,224, 2019: $1,911) has been charged to Selling, marketing, and administrative expenses and $175 (2020: $163, 2019: $583) Research and development expenses.
|
|
|
|
|
|
|
|
|
Intangible assets in use |
|
Type of intangible
asset |
|
Net book value |
|
|
Remaining
amortization period |
Acquired Patents |
|
Patents |
|
|
8,033 |
|
|
9 years |
Acquired Technology |
|
Technology |
|
|
356 |
|
|
2 years |
Acquired Supplier relationships |
|
Supplier relationships |
|
|
1,243 |
|
|
4 years |
Acquired Customer-related intangible |
|
Customer-related |
|
|
2,517 |
|
|
5-6 years |
Acquired Technology |
|
Technology |
|
|
1,180 |
|
|
7 years |
|
|
|
|
|
|
|
|
Intangible assets under development |
|
Type of intangible asset |
|
Net book value |
|
|
Remaining amortization period |
Technology License |
|
Technology |
|
|
2,120 |
|
|
n/a |
Patent License |
|
Patents |
|
|
5,301 |
|
|
n/a |
Patents |
|
Patents |
|
|
692 |
|
|
n/a |
F-26
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Impairment Review—Goodwill
Goodwill is tested for impairment at the operating segment level, this being the level at which goodwill is monitored for internal management purposes. As detailed in Note 5, the Group does not have multiple operating segments and is engaged in a single business activity of the manufacture and sale of point of care medical diagnostics. The recoverable amount of the goodwill has been calculated with reference to the present value of the future cash flows expected to be derived from the cash generating unit (value in use). In calculating this value, management have used the following assumptions:
•Five years of cash flow projections are based on the Group’s long term financial projections, including the launch and commercialization of its new diagnostic products and services. These projects are based on the Group's existing commercial experience and its overall understanding of new product launches.
•A terminal value based on a perpetual growth rate of 5% (2020 3%) for free cash flow
•A discount rate of 25% (2020: 25%) calculated using a risk-free interest rate of 1.5% (2020: 1.5%) and appropriate market risk and small company specific risk premiums
Reasonable changes in (i) the Group's ability to achieve the commercial milestones included in the projections, (ii) the discount rate, or (iii) the perpetual growth rate would not lead to an impairment.
Impairment Review—Intangible Assets
Whilst the Group has no intangible assets with indefinite useful lives, there are intangible assets under development which are not yet available for use. These represent elements of the underlying technology which will ultimately support the Group’s future product launches.
The recoverable amount of the assets have been calculated with reference to the present value of the future cash flows expected to be derived from the assets (value in use). In calculating this value, management have used the following assumptions:
•Five years of cash flow projections are based on the Group’s long term financial projections, including the launch and commercialization of products and services related to the underlying technology. These projects are based on the Group's existing commercial experience and its overall understanding of new product launches.
•A discount rate of 25% (2020: 25%) calculated using a risk-free interest rate of 1.5% (2020: 1.5%) and appropriate market risk and small company specific risk premiums
Reasonable changes in (i) the Group's ability to achieve the commercial milestones included in the projections, (ii) the discount rate, or (iii) the perpetual growth rate would not lead to an impairment.
F-27
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
11. PROPERTY, PLANT AND EQUIPMENT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LAND AND
BUILDINGS |
|
|
FIXTURES
AND
FITTINGS |
|
|
PLANT AND
EQUIPMENT |
|
|
UNDER
CONSTRUCTION |
|
|
TOTAL |
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At 1 January 2019 |
|
$ |
2,180 |
|
|
$ |
2,314 |
|
|
$ |
14,140 |
|
|
$ |
3,312 |
|
|
$ |
21,946 |
|
Additions |
|
|
188 |
|
|
|
581 |
|
|
|
2,409 |
|
|
|
7,542 |
|
|
|
10,720 |
|
Transfers |
|
|
602 |
|
|
|
216 |
|
|
|
(88 |
) |
|
|
(730 |
) |
|
|
— |
|
Acquisition of subsidiaries |
|
|
— |
|
|
|
— |
|
|
|
633 |
|
|
|
— |
|
|
|
633 |
|
Disposals |
|
|
— |
|
|
|
(29 |
) |
|
|
(209 |
) |
|
|
— |
|
|
|
(238 |
) |
Exchange differences |
|
|
84 |
|
|
|
33 |
|
|
|
402 |
|
|
|
308 |
|
|
|
827 |
|
At 31 December 2019 |
|
|
3,054 |
|
|
|
3,115 |
|
|
|
17,287 |
|
|
|
10,432 |
|
|
|
33,888 |
|
Accumulated Depreciation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At 1 January 2019 |
|
|
442 |
|
|
|
1,266 |
|
|
|
3,178 |
|
|
|
— |
|
|
|
4,886 |
|
Charge for the period |
|
|
478 |
|
|
|
622 |
|
|
|
2,681 |
|
|
|
— |
|
|
|
3,781 |
|
Transfers |
|
|
110 |
|
|
|
108 |
|
|
|
(218 |
) |
|
|
— |
|
|
|
— |
|
Disposals |
|
|
— |
|
|
|
(17 |
) |
|
|
(126 |
) |
|
|
— |
|
|
|
(143 |
) |
Exchange differences |
|
|
37 |
|
|
|
35 |
|
|
|
151 |
|
|
|
— |
|
|
|
223 |
|
At 31 December 2019 |
|
|
1,067 |
|
|
|
2,014 |
|
|
|
5,666 |
|
|
|
— |
|
|
|
8,747 |
|
Carrying Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At 31 December 2019 |
|
$ |
1,987 |
|
|
$ |
1,101 |
|
|
$ |
11,621 |
|
|
$ |
10,432 |
|
|
$ |
25,141 |
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2020 |
|
$ |
3,054 |
|
|
$ |
3,115 |
|
|
$ |
17,287 |
|
|
$ |
10,432 |
|
|
$ |
33,888 |
|
Additions |
|
|
3,686 |
|
|
|
1,115 |
|
|
|
25,831 |
|
|
|
33,749 |
|
|
|
64,381 |
|
Transfers |
|
|
— |
|
|
|
(22 |
) |
|
|
22 |
|
|
|
— |
|
|
|
— |
|
Disposals |
|
|
— |
|
|
|
(126 |
) |
|
|
(137 |
) |
|
|
(406 |
) |
|
|
(669 |
) |
Exchange differences |
|
|
366 |
|
|
|
64 |
|
|
|
1,799 |
|
|
|
2,090 |
|
|
|
4,319 |
|
At December 31, 2020 |
|
|
7,106 |
|
|
|
4,146 |
|
|
|
44,802 |
|
|
|
45,865 |
|
|
|
101,919 |
|
Accumulated Depreciation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2020 |
|
|
1,067 |
|
|
|
2,014 |
|
|
|
5,666 |
|
|
|
— |
|
|
|
8,747 |
|
Charge for the period |
|
|
841 |
|
|
|
618 |
|
|
|
4,258 |
|
|
|
— |
|
|
|
5,717 |
|
Transfers |
|
|
— |
|
|
|
(1 |
) |
|
|
1 |
|
|
|
— |
|
|
|
— |
|
Disposals |
|
|
— |
|
|
|
(47 |
) |
|
|
(135 |
) |
|
|
— |
|
|
|
(182 |
) |
Exchange differences |
|
|
151 |
|
|
|
52 |
|
|
|
352 |
|
|
|
— |
|
|
|
555 |
|
At December 31, 2020 |
|
|
2,059 |
|
|
|
2,636 |
|
|
|
10,142 |
|
|
|
— |
|
|
|
14,837 |
|
Carrying Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2020 |
|
$ |
5,047 |
|
|
$ |
1,510 |
|
|
$ |
34,660 |
|
|
$ |
45,865 |
|
|
$ |
87,082 |
|
Cost |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2021 |
|
$ |
7,106 |
|
|
$ |
4,146 |
|
|
$ |
44,802 |
|
|
$ |
45,865 |
|
|
$ |
101,919 |
|
Additions |
|
|
28,047 |
|
|
|
4,144 |
|
|
|
72,186 |
|
|
|
1,969 |
|
|
|
106,346 |
|
Transfers |
|
|
— |
|
|
|
2,137 |
|
|
|
(2,137 |
) |
|
|
— |
|
|
|
— |
|
Disposals |
|
|
(67 |
) |
|
|
(452 |
) |
|
|
(91 |
) |
|
|
— |
|
|
|
(610 |
) |
Exchange differences |
|
|
(562 |
) |
|
|
(322 |
) |
|
|
(2,084 |
) |
|
|
(574 |
) |
|
|
(3,542 |
) |
At December 31, 2021 |
|
|
34,524 |
|
|
|
9,653 |
|
|
|
112,676 |
|
|
|
47,260 |
|
|
|
204,113 |
|
Accumulated Depreciation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At January 1, 2021 |
|
|
2,059 |
|
|
|
2,636 |
|
|
|
10,142 |
|
|
|
— |
|
|
|
14,837 |
|
Charge for the period |
|
|
2,773 |
|
|
|
2,204 |
|
|
|
12,298 |
|
|
|
— |
|
|
|
17,275 |
|
Transfers |
|
|
— |
|
|
|
1,686 |
|
|
|
(1,686 |
) |
|
|
— |
|
|
|
— |
|
Disposals |
|
|
(21 |
) |
|
|
(366 |
) |
|
|
(91 |
) |
|
|
— |
|
|
|
(478 |
) |
Exchange differences |
|
|
(106 |
) |
|
|
(223 |
) |
|
|
(589 |
) |
|
|
— |
|
|
|
(918 |
) |
At December 31, 2021 |
|
|
4,705 |
|
|
|
5,937 |
|
|
|
20,074 |
|
|
|
— |
|
|
|
30,716 |
|
Carrying Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2021 |
|
$ |
29,819 |
|
|
$ |
3,716 |
|
|
$ |
92,602 |
|
|
$ |
47,260 |
|
|
$ |
173,397 |
|
Depreciation expense of $5,355 (2020: $1,676, 2019: $1,333) has been charged to Research and development expenses and $11,920 (2020: $4,041, 2019: $2,448) to Selling, marketing and administrative expenses.
F-28
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Assets under construction are comprised of manufacturing equipment to be placed in service in 2022. Commitments related to property, plant and equipment are referenced in Note 23.
12. INVENTORY
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2021 |
|
Finished goods |
|
$ |
50,054 |
|
|
$ |
78,245 |
|
Raw Materials |
|
|
37,789 |
|
|
|
86,613 |
|
WIP |
|
|
10,859 |
|
|
|
7,026 |
|
Total Inventory |
|
$ |
85,516 |
|
|
$ |
149,055 |
|
The increase in inventory for the year ended December 31, 2021 is a result of the building of inventory to meet the continued growth in product demand that has occurred since commercialization of COVID-19 products.
During 2021, the amount of inventories recognized as an expense within cost of sales was $114,195 (2020: $70,261, 2019: $11,914). The amount of inventory write-downs recognized as an expense was $6,830 (2020: $16,493, 2019: $120).
Included in reserves is $12,891 (2020: $8,148) related to returned instruments that management expects to be unable to refurbish.
13. TRADE AND OTHER RECEIVABLES
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2021 |
|
Trade receivables |
|
$ |
83,941 |
|
|
$ |
75,207 |
|
Reserves on trade receivables |
|
|
(661 |
) |
|
|
(1,681 |
) |
Prepaids |
|
|
10,970 |
|
|
|
20,349 |
|
Other receivables |
|
|
4,011 |
|
|
|
9,408 |
|
VAT receivable |
|
|
11,034 |
|
|
|
6,515 |
|
Total trade and other receivables |
|
$ |
109,295 |
|
|
$ |
109,798 |
|
Trade receivables comprise customer receivables and include an allowance for doubtful accounts of $1,681 (2020: $661). Trade receivables relate to existing customers with no significant defaults in the past. The Group has 0 material reserve for expected credit losses in respect of Other receivables as of December 31, 2021 and 2020. The Group retains all risks associated with these receivables until fully recovered.
The fair value of all receivables is the same as their carrying values stated above.
The maximum exposure to credit risk at the reporting date is the carrying value of each class of receivable mentioned above. The Group does 0t hold any collateral as security.
14. SHARE CAPITAL, PREMIUM AND OTHER RESERVES
Share capital and share premium
LumiraDx Limited was incorporated on August 24, 2016 with an authorized share capital of 5,000,000 A Ordinary Shares of par value $0.001 each and 5,000,000 Common Shares of par value $0.001 each. On September 29, 2016, the Company acquired 100% of the issued share capital of LumiraDx Holdings Limited following the agreement of an Exchange Offer, which was effective from September 28, 2016. LumiraDx Limited acquired all shares in LumiraDx Holdings Limited, and in
F-29
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
exchange LumiraDx Limited issued to the shareholders of LumiraDx Holdings Limited a corresponding number of shares on a share-for-share basis.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SHARES AUTHORIZED, FULLY PAID AND ALLOCATED |
|
A ORDINARY
SHARES |
|
|
A ORDINARY
SHARES |
|
|
COMMON SHARES |
|
|
COMMON SHARES |
|
|
|
2020 |
|
|
2021 |
|
|
2020 |
|
|
2021 |
|
In issue at start of period |
|
|
373,652 |
|
|
|
373,697 |
|
|
|
— |
|
|
|
— |
|
February Subdivision (220:1) |
|
|
— |
|
|
|
81,839,643 |
|
|
|
— |
|
|
|
— |
|
Issued for cash |
|
|
45 |
|
|
|
104,200 |
|
|
|
— |
|
|
|
— |
|
Issued in other transactions |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,307,607 |
|
Merger Subdivision at the LMDX Conversion Factor (1.60806264:1) |
|
|
— |
|
|
|
78,446,580 |
|
|
|
— |
|
|
|
4,796,852 |
|
Shares issued upon conversion of financial instruments |
|
|
— |
|
|
|
46,797,960 |
|
|
|
— |
|
|
|
35,137,307 |
|
In issue at December - fully paid and allocated |
|
|
373,697 |
|
|
|
207,562,080 |
|
|
|
— |
|
|
|
45,241,766 |
|
On February 1, 2021 the Board of Directors of the Company approved a stock split of the issued and outstanding A Ordinary and common shares of the Company on a 220 for 1 basis. In accordance with IAS 33, the earnings per share calculations have been presented for the stock split retrospectively. In connection with the merger, in order to achieve an exchange ratio of one LMDX common share for each CAH share, the Company effected a subdivision, immediately prior to the merger, of all issued, and authorized but unissued, LMDX ordinary shares and LMDX common shares at a ratio of 1.60806264:1.
During September 2021, the Company completed its merger (Note 27) and all outstanding convertible instruments at the time of the merger converted into A ordinary and Common shares.
Translation reserve
The translation reserve comprises all foreign exchange differences arising since the date of incorporation from the translation of the financial statements of operations with functional currencies different from the Company.
Other reserves
Other reserves are comprised of warrants and debt conversion rights. On September 28, 2016, the Company amended its Secured Fixed Rate Loan Notes and granted the Acquisition Note Holder (Note 17) the right to convert 50% of the principal amount of the Acquisition Notes into A Ordinary Shares of the Company at a conversion prices of $611.63 per share. The issue date fair value of the loan conversion rights is included in Other reserves. In 2018, the Acquisition Note Holder converted 25% of the principal amount and the Company issued 1,586 A Ordinary shares. In 2019, the Acquisition Note Holder converted the remaining 25% of the principal amount and the Company issues 1,587 A Ordinary Shares.
During 2018, the Company issued 212,718 Preferred Shares (Note 16), which have been treated as a compound instrument in accordance with IFRS 9. The conversion feature of the Preferred Shares has been included in Other reserves at an issue date fair value of $47,264.
During 2019, as part of its senior debt offering, as described in Note 17, the Company issues 2,284 warrants to purchase it’s A Ordinary shares at a fixed price of $1,459.89 per share.
During 2019, the Company issued convertible notes (Note 17), which have been treated as a compound instrument in accordance with IFRS 9. The conversion feature of the convertible notes has been included in Other reserves at an issue date fair value of $17,065.
During 2020, as part of its 2020 Convertible Notes (Note 17) the Company issued 16,528 warrants to acquire Common Shares at a strike price of $1,793.38.
During October 2020, as part of its 2020 Senior Secured Loan (Note 17) the Company issued 1,000 warrants to acquire Common shares at a strike price of $4,644.96.
F-30
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
During March 2021, as part of its 2021 Senior Secured Loan (Note 17) the Company issued the warrants to purchase up to 1,485,848 common shares at an exercise price equal to $10.00 per common share.
15. SHARE BASED PAYMENTS
Share options are granted to directors, employees and certain service providers. The share options have a vesting period of 1-4 years with shares being exercisable pro rata per year from the date of issue. All share options granted have a contractual life of 10 years from the date of grant. Share options are settled in equity.
For the employee based share options, if the owner of the share option ceases to be employed by the Company, in most cases the option lapses within a short period of departure of such employee. 6,773,837 share options have been forfeited to date. Management has not anticipated any stock options to be forfeited due to termination of employment prior to the assumed exercise date.
Movements on number of share options and their related exercise price are as follows:
|
|
|
|
|
|
|
|
|
|
|
NUMBER OF
OPTIONS |
|
|
WEIGHTED
AVERAGE
EXERCISE
PRICE |
|
Outstanding at January 1, 2020 |
|
|
54,198,851 |
|
|
$ |
1.67 |
|
Granted |
|
|
3,073,940 |
|
|
|
9.39 |
|
Exercised |
|
|
(15,920 |
) |
|
|
(2.56 |
) |
Forfeited |
|
|
(44,221 |
) |
|
|
(3.81 |
) |
Outstanding at December 31, 2020 |
|
|
57,212,650 |
|
|
|
2.09 |
|
Granted |
|
|
26,557,293 |
|
|
|
16.45 |
|
Exercised |
|
|
(104,200 |
) |
|
|
(1.00 |
) |
Forfeited |
|
|
(92,112 |
) |
|
|
(9.72 |
) |
Outstanding at December 31, 2021 |
|
|
83,573,631 |
|
|
|
6.72 |
|
Exercisable at December 31, 2020 |
|
|
45,770,544 |
|
|
|
1.44 |
|
Exercisable at December 31, 2021 |
|
|
66,322,324 |
|
|
$ |
5.28 |
|
On February 1, 2021 the Board of Directors of the Company approved a stock split of the issued and outstanding A Ordinary and common shares of the Company on a 220 for 1 basis. In accordance with IAS 33, the earnings per share calculations have been presented for the stock split retrospectively. In connection with the merger, in order to achieve an exchange ratio of one LMDX common share for each CAH share, the Company effected a subdivision, immediately prior to the merger, of all issued, and authorized but unissued, LMDX ordinary shares and LMDX common shares at a ratio of 1.60806264:1.
On January 15, 2021, the Company granted “founder options” over ordinary shares to each of the 3 Founder Directors. Each Founder Director was granted a fully vested option over 5,235,851
ordinary shares. On April 15, 2021, the Company granted each Founder Director a further option over 2,819,577 ordinary shares. These options will vest over a two year period subject to the satisfaction of performance conditions. In each instance, the exercise price of these options is equal an exercise price per ordinary share of $17.05.
In 2021, 104,200 options (2020: 15,920) were exercised at a weighted average exercise price of $1.00 (2020: $2.56). The options outstanding at December 31, 2021 have an exercise price in the range of $0.2 to 17.05 (2020: $0.2to10.91) and a weighted average contractual life of 6.63 years (2020: 6.47).
Share based compensation expense of $2,406 (2020: $1,890, 2019: $2,523) has been charged to Research and development expenses and $31,503 (2020: $1,301, 2019: $1,447) to Selling, marketing and administrative expenses.
F-31
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
16. PREFERRED SHARES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PREFERRED
SHARES |
|
|
DIVIDENDS |
|
|
TOTAL |
|
Balance at January 1, 2020 |
|
$ |
221,927 |
|
|
$ |
26,713 |
|
|
$ |
248,640 |
|
Issuance, net of related costs |
|
|
162,401 |
|
|
|
— |
|
|
|
162,401 |
|
Accretion of issuance costs |
|
|
7,751 |
|
|
|
— |
|
|
|
7,751 |
|
Dividends accrued |
|
|
— |
|
|
|
23,578 |
|
|
|
23,578 |
|
Fair value adjustment of convertible feature |
|
|
9,351 |
|
|
|
— |
|
|
|
9,351 |
|
Balance at December 31, 2020 |
|
|
401,430 |
|
|
|
50,291 |
|
|
|
451,721 |
|
Accretion of issuance costs |
|
|
8,498 |
|
|
|
— |
|
|
|
8,498 |
|
Dividends accrued |
|
|
0 |
|
|
|
16,156 |
|
|
|
16,156 |
|
Converted to Share Premium from Merger |
|
|
(409,928 |
) |
|
|
(66,447 |
) |
|
|
(476,375 |
) |
Balance at December 31, 2021 |
|
$ |
0 |
|
|
$ |
0 |
|
|
$ |
0 |
|
Series A Preferred Shares
In July 2018, the Company’s Board of Directors authorized the Company to raise up to $300 million through the issue of up to 236,353 new Series A 8% Cumulative Convertible Preferred Shares (“Series A Preferred Shares”).
The outstanding Series A Preferred Shares have been treated as a compound instrument in accordance with IFRS 9 as the Company has a contractual obligation to deliver: i) cash upon maturity; and/or ii) a requirement to deliver A Ordinary shares upon conversion. The Series A Preferred Shares are convertible into A Ordinary shares at the option of the holder and mandatorily convertible into A Ordinary shares upon listing on a public market if at a price above the liquidation preference and accrued and unpaid dividends. Each Series A Preferred Share, including any accrued dividends, is convertible into one A Ordinary share.
In accordance with IFRS 9, the redemption feature qualifies as a liability at fair value with the residual proceeds allocated to conversion feature recorded within equity as Other reserves.
The Series A Preferred Shares accrue an 8% cumulative annual dividend until the earlier of (i) the date seven years from their issue (ii) the date the Preferred Shares are converted in accordance with their terms or (iii) the date the Company is liquidated. NaN dividends will be paid on the A Ordinary Shares for so long as the Preferred Shares are in issue.
The Series A Preferred Shares carry a preferential right to share in the proceeds of a liquidation of the Company, and will rank senior to the A Ordinary Shares and the Common Shares of the Company on liquidation.
Each of the Series A Preferred Shares shall automatically convert to A Ordinary Shares in connection with an IPO or sale of the Company, provided that the value of an A Ordinary Share at that time is not less than the aggregate of the issue price of such Preferred Share and the dividend accrued on each such Preferred Share. Each Preferred Shareholder may convert their Preferred Shares to A Ordinary Shares at any time.
During 2018, the Company issued a total of 212,718 Series A Preferred Shares. Loan Noteholders of the Company’s 12% Loan Notes were given the opportunity to convert the proceeds of the prepayment of their Loan Notes to subscribe for Series A Preferred Shares. An amount of $39,672, representing the principal and accrued interest, was converted from Loan Notes into Preferred Shares. The issue date fair value of the conversion feature of the Series A Preferred Shares has been recorded as $47,264 in Other reserves (Note 14).
One of the investors in the Series A Preferred Shares had the right to subscribe for an additional $30,000 of Series A Preferred Shares on or before June 18, 2019 at the fair market value on the date of subscription. This option expired in 2019 without being exercised.
In connection with the September 2021 merger (Note 27), all outstanding Series A Preferred Shares converted into A Ordinary Shares on a one to one basis.
F-32
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Series B Preferred Shares
In October 2020, the Company’s Board of Directors authorized the Company to raise up to $200 million through the issue of up to 40,000 new Series B 8% Cumulative Convertible Preferred Shares (“Series B Preferred Shares”).
The Series B Preferred Shares accrue an 8% cumulative annual dividend until the earlier of (i) the date seven years from their issue (ii) the date the Preferred Shares are converted in accordance with their terms or (iii) the date the Company is liquidated. NaN dividends will be paid on the A Ordinary Shares for so long as the Preferred Shares are in issue.
The Series B Preferred Shares carry a preferential right to share in the proceeds of a liquidation of the Company, and will rank senior to the A Ordinary Shares and the Common Shares of the Company and pari passu with the Series A Preferred Shares on liquidation.
Each of the Series B Preferred Shares shall automatically convert to Common Shares in connection with an IPO or sale of the Company at a share price not more than the fully diluted share capital divided by $4 billion and not less than the fully diluted share capital divided by $6.4 billion. Each Preferred Shareholder may convert their Preferred Shares to Common Shares at any time.
The variable conversion feature constitutes an embedded derivative as the conversion feature is a component of the host instrument that would allow for the cash flows of the combined instrument to be changed according to the value of a financial variable. In accordance with IFRS 9, the Company has elected to record the entire instrument at fair value through profit or loss. The change in fair value of $48,956 (2020: $9,351, 2019: $NaN) has been charged to finance expenses.
During 2020, the Company issued a total of 33,008 Series B Preferred Shares for gross proceeds of $164.5 million
In connection with the September 2021 merger (Note 27), Series B Preferred Shares converted into 12,543,492 Common Shares.
17. DEBT
This note provides information about the contractual terms of the Group’s interest-bearing loans and borrowings, which are measured at amortized cost.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENCY |
|
NOMINAL
INTEREST
RATE |
|
YEAR OF
MATURITY |
|
2020
FACE
VALUE |
|
|
2020
CARRYING
AMOUNT |
|
|
2020
FAIR
VALUE |
|
|
2021
FACE
VALUE |
|
|
2021
CARRYING
AMOUNT |
|
|
2021
FAIR
VALUE |
|
Unsecured Loan |
|
USD |
|
2.00% |
|
2024 |
|
$ |
18,000 |
|
|
$ |
18,000 |
|
|
$ |
18,849 |
|
|
$ |
14,242 |
|
|
$ |
14,242 |
|
|
$ |
14,557 |
|
Convertible Notes |
|
USD |
|
5.00% |
|
2024 |
|
|
75,156 |
|
|
|
59,113 |
|
|
|
62,530 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
2020 Convertible Notes |
|
USD |
|
10.00% |
|
2021 |
|
|
75,370 |
|
|
|
146,844 |
|
|
|
146,844 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
2020 Senior Secured Loans |
|
USD |
|
8.00% |
|
2022 |
|
|
65,000 |
|
|
|
62,339 |
|
|
|
62,351 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
2021 Senior Secured Loans |
|
USD |
|
8.00% |
|
2024 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
300,000 |
|
|
|
286,815 |
|
|
|
283,893 |
|
Instrument Financing Loans |
|
EUR |
|
1.70-2.60% |
|
2022-2023 |
|
|
676 |
|
|
|
676 |
|
|
|
676 |
|
|
|
263 |
|
|
|
263 |
|
|
|
263 |
|
2020 Convertible Notes
In April 2020, the Company opened a 2020 Funding Round, pursuant to which it had received commitments from investors to lend funds to the Company up to the amount of $148,900 in aggregate. The committed amounts were available for drawdown by the Company for the period (the “Call Option Period”) starting on July 1, 2020 and ending on October 31, 2020. Following a call of the funds and receipt of the committed amounts, the Company agreed to issue relevant Loan Note certificates (“2020 Convertible Notes”) to the Investors in respect of their relevant actual committed amounts. The relevant 2020 Convertible Notes shall be for a term of 360 days from the date of issue and shall entitle its holders to 10% interest from the date of issue of such 2020 Convertible Notes. Such interest will be paid on the date the relevant 2020 Convertible Notes are repaid.
In consideration for committing to lend their respective committed amounts to the Company, the Company agreed to pay to the relevant Investors agreeing to lend the Committed Amounts: (i) a commitment fee to be satisfied by the issue to such Investors of a total of up to a maximum of 16,528 warrants over common shares, to be allocated between such Investors based on their to their Actual Committed Amounts (the “Consideration Shares”) and (ii) cash fee, in aggregate, equivalent to
F-33
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
1 (1) per cent of the total aggregate amount raised by the Company pursuant to the Offer, to be allocated between the Investors pro rata to their Actual Committed Amounts (the “Cash Fee”).
On July 1, 2020, the Company called and received $74,300 of the committed amount. In November 2020 warrants for 16,528 shares were issued to the Investors and recorded in Other reserves in equity. These 2020 Convertible Notes automatically convert into common shares upon an initial public offering at a share price not more than the fully diluted share capital divided by $1,800,000 and not less than the fully diluted share capital divided by $3,600,000 depending on the number of instruments sold during 2020.
The variable conversion feature constitutes an embedded derivative as the conversion feature is a component of the host instrument that would allow for the cash flows of the combined instrument to be changed according to the value of a financial variable. In accordance with IFRS 9, the Company has elected to record the entire instrument at fair value through profit or loss.
In connection with the September 2021 merger (Note 27), the 2020 Convertible Notes converted into Common Shares.
2020 Senior Secured Loan
In October 2020, the Company entered into a senior secured term loan and security agreement (“2020 Senior Secured Loan”) to borrow up to $100,000. The Company borrowed $65,000 in October 2020 which was used, in part, to repay the Senior Secured Loan. Under the terms of the 2020 Senior Secured Loan an additional $35,000 was available upon the achievement of certain commercial milestones. On January 13, 2021, the Company achieved the commercial milestones and drew an additional $35,000 on the 2020 Senior Secured Loan.
The 2020 Senior Secured Loan is subject to an interest rate of 8.0% per annum payable in quarterly installments, which, under the terms of the 2020 Senior Secured Loan, was due to increase after January 31, 2021 if an “IPO” or “Qualifying Investment” was not completed before January 31, 2021. The issuance of the Series B Preferred Shares in November 2020 constitutes a “Qualifying Investment” for the purposes of the 2020 Senior Secured Loan, and therefore, the interest rate will remain at 8% per annum for the term of the 2020 Senior Secured Loan. The 2020 Senior Secured Loan also provides an ability to incur additional incremental term loans, or incremental term loans, in an aggregate amount of up to $150,000 during the initial 12 month period on an uncommitted basis. The Senior Secured Loan matures October 5, 2022. Debt issuance costs were recorded as a reduction of the proceeds. The discount on the issuance will be recognized using the effective interest method until the maturity date of October 5, 2022.
In connection with the 2020 Senior Secured Loan, on November 6, 2020 the Company issued warrants to purchase up to 1,000 common shares at an exercise price equal to $4,644.96 per common share, recorded in Other Reserves (Note 14).
On January 15, 2021 the Group agreed to borrow $40,000 in incremental term loans under and on substantially the same terms as the 2020 Senior Secured Loan.
2021 Senior Secured Loan
On March 23, 2021, the Group refinanced the $100,000 in outstanding amounts under the 2020 Senior Secured Loan and the $40,000 borrowed in January 2021 with a $300,000 loan (“2021 Senior Secured Loan”). The loan matures in three years and bears interest at 8% annually, paid quarterly. Debt issuance costs were recorded as a reduction of the proceeds. The discount on the issuance will be recognized using the effective interest method until the maturity date.
In connection with the 2021 Senior Secured Loan, the Company issued warrants to purchase up to 1,485,848 common shares at an exercise price equal to $10.00 per common share, recorded in Other Reserves (Note 14).
Convertible Notes
In October and December 2019, the Company issued convertible loan notes (“Convertible Notes”) in an aggregate value of $75,156. The Convertible Notes have a five year maturity from their date of issue and carry an interest rate of 5%, paid semi-annually. Holders may convert the Convertible Notes into Common Shares of the Company at their election at a fixed price. Convertible Notes automatically convert into Common Shares upon a qualifying IPO. The Company may force conversion of
F-34
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
the Convertible Notes on a qualifying equity funding round. In the event the Company is prevented by a senior creditor from repaying the Convertible Notes upon maturity, the Convertible Note holder may choose to extend the maturity of the Convertible Notes. Of the Convertible Notes issued, $1,353 of notes were issued in settlement of debt issuance costs on January 2, 2020 and have been included in the balance at December 31, 2019.
The outstanding Convertible Notes have been treated as a compound instrument in accordance with IFRS 9 as the Company has a contractual obligation to deliver: i) cash upon maturity; and/or ii) a requirement to deliver A Ordinary shares upon conversion. In 2020, the issue date fair value of the conversion feature of the Convertible Notes was recorded as $17,065 in Other reserves (Note 14).
In connection with the September 2021 merger (Note 27), these Convertible Notes converted into A Ordinary Shares.
Senior Secured Loans
On September 20, 2019, LumiraDx Investment Ltd, a subsidiary of the Company, issued Senior Secured Loans in the amount of $40,000. The Senior Secured Loans are secured generally by all the Group’s assets and mature on September 20, 2023. The Senior Secured Loans carry an interest rate of 11.5% paid quarterly. The Group can draw two additional loans in the amount of $25,000 each upon the achievement of certain commercial milestones. The Company also issued the lenders 2,284 10 year warrants to purchase A Ordinary Shares at a fixed price of $1,459.89 per share.
The fair value of the warrants was recorded in Other reserves in equity. Senior Secured Loans were recorded at a fair value of $37,265. Debt issuance costs were recorded as a reduction of the proceeds. The discount on the issuance will be recognized using the effective interest method until the maturity date of September 20, 2023.
In October 2020, in connection with the issuance of the 2020 Senior Secured Loan, LumiraDx Investment Ltd settled the balance of the Senior Secured Loans with a payment of $43,600, including $3,600 incurred as a prepayment penalty (Note 6).
Unsecured Loans
On October 17, 2019, the Group issued an Unsecured Loan in the amount of $18,000 to a tax-exempt private foundation. The terms of the loan include restrictions on the use of the proceeds for specific programs and commitments to provide access to the Group’s future products to support the foundation’s charitable purposes. The Unsecured Loan matures on October 17, 2024. The Unsecured Loan carries an interest rate of 2% paid quarterly.
Instrument Financing Loans
F-35
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Instrument financing loans are used to finance the cost of installing instruments at customer locations where the Group retains title of the instruments.
|
|
|
|
|
|
Balance at January 1, 2020 |
|
$ |
111,923 |
|
Changes from financing cash flows |
|
|
|
Proceeds from borrowings, net of issuance costs |
|
|
|
2020 Convertible Notes |
|
|
70,917 |
|
2020 Senior Secured Loan |
|
|
62,391 |
|
Repayments of borrowings |
|
|
|
Senior Secured Loans |
|
|
(40,000 |
) |
Instrument Financing Loans |
|
|
(396 |
) |
Total changes from financing cash flows |
|
|
92,912 |
|
Other changes |
|
|
|
Warrants |
|
|
|
2020 Convertible Notes |
|
|
(32,531 |
) |
2020 Senior Secured Loan |
|
|
(407 |
) |
Loss on extinguishment of debt |
|
|
|
Senior Secured Loans |
|
|
2,047 |
|
Change in fair value |
|
|
|
2020 Convertible Notes |
|
|
102,548 |
|
Amortization of debt issuance costs |
|
|
|
Convertible Notes |
|
|
3,636 |
|
Senior Secured Loans |
|
|
500 |
|
2020 Convertible Notes |
|
|
5,909 |
|
2020 Senior Secured Loan |
|
|
355 |
|
Foreign exchange impact |
|
|
|
Instrument Financing Loans |
|
|
80 |
|
Total other changes |
|
|
82,137 |
|
Balance at December 31, 2020 |
|
|
286,972 |
|
Less: Debt due within one year |
|
|
(147,238 |
) |
|
|
$ |
139,734 |
|
Changes from financing cash flows |
|
|
|
Proceeds from borrowings, net of issuance costs |
|
|
|
2020 Senior Secured Loan |
|
$ |
34,125 |
|
Incremental term loan |
|
|
39,000 |
|
2021 Senior Secured Loan |
|
|
288,513 |
|
Instrument Financing Loans |
|
|
192 |
|
Repayments of borrowings |
|
|
|
2020 Senior Secured Loan |
|
|
(100,000 |
) |
Incremental term loan |
|
|
(40,000 |
) |
Instrument Financing Loans |
|
|
(552 |
) |
Total changes from financing cash flows |
|
|
221,278 |
|
Other changes |
|
|
|
Reclassification of Unsecured Loan amounts to grant liability in accordance with IAS 20 |
|
|
(3,758 |
) |
Warrants |
|
|
|
2021 Senior Secured Loan |
|
|
(5,136 |
) |
Conversion to equity |
|
|
|
Convertible Notes |
|
|
(61,980 |
) |
2021 Convertible Notes |
|
|
(125,652 |
) |
Loss on extinguishment of debt |
|
|
|
2020 Senior Secured Loan |
|
|
3,170 |
|
Incremental term loan |
|
|
1,000 |
|
Change in fair value |
|
|
|
2021 Convertible Notes |
|
|
(52,267 |
) |
Amortization of debt issuance costs |
|
|
|
2020 Senior Secured Loan |
|
|
366 |
|
Convertible Notes |
|
|
2,866 |
|
2021 Convertible Notes |
|
|
31,075 |
|
2021 Senior Secured Loan |
|
|
3,438 |
|
Foreign exchange impact |
|
|
|
Instrument Financing Loans |
|
|
(52 |
) |
F-36
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
|
|
|
|
|
Total other changes |
|
|
(206,930 |
) |
Balance at December 31, 2021 |
|
|
301,320 |
|
Less: Debt due within one year |
|
|
(191 |
) |
|
|
$ |
301,129 |
|
18. LEASE LIABILITY
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2021 |
|
Due in less than one year |
|
$ |
3,149 |
|
|
$ |
5,546 |
|
Due between one and five years |
|
|
9,018 |
|
|
|
25,151 |
|
Due in more than five years |
|
|
2,269 |
|
|
|
16,301 |
|
Total |
|
$ |
14,436 |
|
|
$ |
46,998 |
|
19. DEFERRED TAX ASSET AND LIABILITY
The analysis of deferred tax assets and deferred tax liabilities is as follows:
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2021 |
|
Deferred taxes: |
|
|
|
|
|
|
- Liabilities |
|
$ |
1,230 |
|
|
$ |
779 |
|
Total net deferred tax liabilities |
|
$ |
1,230 |
|
|
$ |
779 |
|
The analysis and movement of deferred tax assets and liabilities is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
JANUARY 1,
2021 |
|
|
RECOGNIZED
IN INCOME |
|
|
RECOGNIZED
IN EQUITY |
|
|
DECEMBER 31,
2021 |
|
Deferred tax liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets |
|
$ |
1,727 |
|
|
$ |
(383 |
) |
|
$ |
(106 |
) |
|
$ |
1,238 |
|
Deferred tax assets |
|
|
|
|
|
|
|
|
|
|
|
|
Net operating losses and other
timing differences |
|
|
(497 |
) |
|
|
197 |
|
|
|
(159 |
) |
|
|
(459 |
) |
Net deferred tax liability |
|
$ |
1,230 |
|
|
$ |
(186 |
) |
|
$ |
(265 |
) |
|
$ |
779 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
JANUARY 1,
2020 |
|
|
RECOGNIZED
IN INCOME |
|
|
RECOGNIZED
IN EQUITY |
|
|
DECEMBER 31,
2020 |
|
Deferred tax liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets |
|
$ |
2,036 |
|
|
$ |
(393 |
) |
|
$ |
84 |
|
|
$ |
1,727 |
|
Deferred tax assets |
|
|
|
|
|
|
|
|
|
|
|
|
Net operating losses and other
timing differences |
|
|
(477 |
) |
|
|
- |
|
|
|
(20 |
) |
|
|
(497 |
) |
Net deferred tax liability |
|
$ |
1,559 |
|
|
$ |
(393 |
) |
|
$ |
64 |
|
|
$ |
1,230 |
|
Deferred tax assets are recognized for tax losses carried forward to the extent that the realization of the related tax benefit through future taxable profits is probable. The realization of the tax benefit related to losses in certain jurisdictions were determined to not be probable. As such, the Group did not recognize deferred tax assets of $78,602 for U.K. tax losses and $10,615 for U.S. tax losses and other temporary timing differences. The Group has material carried forward tax losses in the U.K. and U.S. Losses in the U.K. do not expire whereas losses in the U.S. expire on various dates up to 2037 if not utilized.
The utilization of the U.S. net operating loss carry-forwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to ownership changes that have occurred previously or that could occur in the future. These ownership changes may limit the amount of U.S. net operating loss carry-forwards that can be utilized annually to offset future taxable income. The Company has not yet completed an evaluation of ownership changes through December 31, 2019. To the extent an ownership change has occurred or does occurs in the future, the U.S. net operating loss carry-forwards may be subject to limitation.
F-37
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
20. TRADE AND OTHER PAYABLES
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2021 |
|
Trade payables |
|
$ |
57,898 |
|
|
$ |
59,718 |
|
Accrued expenses and other liabilities |
|
|
23,301 |
|
|
|
26,366 |
|
Accrued interest |
|
|
5,730 |
|
|
|
6,239 |
|
Warranty provision |
|
|
6,557 |
|
|
|
5,801 |
|
Deferred revenue |
|
|
1,760 |
|
|
|
1,517 |
|
Total trade and other payables |
|
$ |
95,246 |
|
|
$ |
99,641 |
|
21. GOVERNMENT AND OTHER GRANTS
The Group has received grants from government and private entities. These include grants in respect of research and development activities, expansion of manufacturing capabilities and deployment of the Group’s products in certain geographical markets.
The Group has recorded $8,602, $1,473 and $75 as a reduction in research and development expenses in 2021, 2020, and 2019 respectively, to reflect the usage of grant funds and research and development tax expenditures. The Group had liabilities of $38,941 and $44,037 as of December 31, 2021 and 2020, respectively, for these unspent grant funds.
As of December 31, 2021 the Group had $26,211 (2020: $10,000) related to a grant for manufacturing equipment. The Group will recognize the grant over the useful life of the equipment. In 2021, the Group reduced manufacturing expenses by $3,784 (2020: $NaN, 2019: $NaN).
22. FINANCIAL RISK MANAGEMENT
Financial liabilities not measured at fair value are calculated based on the present value of future principal and interest cash flows discounted at the market rate of interest at the balance sheet date.
The Group’s activities expose it to a variety of financial risks: market risk (including currency risk and cash flow and interest rate risk), credit risk and liquidity risk.
Market risk
(a) Currency risk
The majority of the Group’s sales and purchase transactions are denominated in either U.S. dollars or U.K. pound sterling. The exchange risk is managed by maintaining bank accounts denominated in those currencies.
F-38
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
A 10% strengthening of the U.K. pound sterling against the U.S. dollar at December 31, 2021 would have had an impact of increasing the loss before tax for the period by $26,910 on the basis that all other variables remain constant.
The Group considers the impact of foreign currency risk to be not significant given the Group’s net balance sheet exposure.
The carrying amounts of the Group’s trade and other receivables are denominated in the following currencies:
|
|
|
|
|
|
|
|
|
|
|
2020 |
|
|
2021 |
|
US Dollars |
|
|
45,863 |
|
|
|
51,568 |
|
U.K. Pounds |
|
|
46,093 |
|
|
|
27,089 |
|
Euros |
|
|
10,825 |
|
|
|
24,057 |
|
Colombian Pesos |
|
|
3,473 |
|
|
|
3,398 |
|
Swiss Francs |
|
|
8 |
|
|
|
992 |
|
Swedish Krona |
|
|
1,903 |
|
|
|
954 |
|
Brazilian Reals |
|
|
900 |
|
|
|
783 |
|
Other |
|
|
230 |
|
|
|
957 |
|
|
|
|
109,295 |
|
|
|
109,798 |
|
(b) Cash flow and interest rate risk
The Group mainly raises debt on a fixed rate basis for bonds and notes in U.S. dollars. The primary objective of the Group’s interest rate management is to protect the net interest result while managing the overall cost of borrowing.
The Group’s debt, including Preferred Shares, is carried at fixed interest rates.
Credit risk
Credit risk represents the risk of loss the Group would incur if operators and counterparties fail to fulfil their credit obligations. The maximum exposure to credit risk is represented by the carrying amount of each financial asset. For banks and financial institutions, the Group maintains it accounts with major international banks with “A” ratings. Credit risk relating to accounts receivable balances are managed on a case-by-case basis.
At December 31, 2021 the Group has trade receivables of $75,207 (2020: $83,941). New clients are analyzed before standard payment and delivery terms and conditions are offered. The credit quality of the customer is assessed taking into account its financial position, past experience and other factors. The utilization of credit limits is regularly monitored. Management does not expect any losses, beyond current amounts already included in reserves, from non-performance by these counterparties. Movement in the loss allowances against trade receivables is as follows:
|
|
|
|
|
Loss allowance as of January 1, 2020: |
|
$ |
674 |
|
Loss allowance recognized during the year |
|
|
119 |
|
Balances written off during the year |
|
|
(132 |
) |
Balances recovered during the year |
|
|
0 |
|
Loss allowance at December 31, 2020: |
|
|
661 |
|
Loss allowance recognized during the year |
|
|
1,253 |
|
Balances written off during the year |
|
|
(222 |
) |
Balances recovered during the year |
|
|
(12 |
) |
Loss allowance at December 31, 2021: |
|
$ |
1,680 |
|
At December 31, 2021 trade receivables of $1,811 (2020: $3,581) were past due but not impaired. These relate to a number of independent customers for whom there is no recent history of default. The ageing analysis of these receivables is 3 months and above.
At December 31, 2021 trade receivables included 1 significant customer that accounted for 29% of the balance.
F-39
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
Liquidity risk
Liquidity risk is the risk that the Group will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled in cash. Cash flow forecasting is performed in the operating entities of the Group and aggregated by Group Finance. Group Finance monitors rolling forecasts of the Group’s liquidity requirements to ensure it has sufficient cash to meet operational needs. The Group may be reliant on its ability to raise additional investment capital from the issuance of both debt and equity securities to fund its business operating plans and future obligations. The Group believes it will continue to be successful in raising additional investment capital to meet its obligations.
The following are the undiscounted contracted maturities of financial liabilities, including interest payments for the period ending December 31, 2021:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-DERIVATIVE FINANCIAL
LIABILITY |
|
EFFECTIVE
INTEREST
RATE* |
|
|
YEAR OF
MATURITY |
|
|
CARRYING
AMOUNT |
|
|
CONTRACTUAL
CASH FLOWS |
|
|
LESS
THAN 1
YEAR |
|
|
1—2
YEARS |
|
|
2—5
YEARS |
|
Unsecured Loan |
|
|
10.55 |
% |
|
2024 |
|
|
|
14,242 |
|
|
|
15,040 |
|
|
|
285 |
|
|
|
285 |
|
|
|
14,471 |
|
2021 Senior Secured Loan |
|
|
10.02 |
% |
|
|
2024 |
|
|
|
286,815 |
|
|
|
354,133 |
|
|
|
24,333 |
|
|
|
24,333 |
|
|
|
305,467 |
|
Instrument Financing Loans |
|
1.7-2.6% |
|
|
2022-2023 |
|
|
|
263 |
|
|
|
263 |
|
|
|
191 |
|
|
|
72 |
|
|
|
— |
|
Trade and other payables |
|
|
|
|
|
|
|
|
99,641 |
|
|
|
99,641 |
|
|
|
99,641 |
|
|
|
— |
|
|
|
— |
|
Total |
|
|
|
|
|
|
|
|
400,961 |
|
|
|
469,078 |
|
|
|
124,450 |
|
|
|
24,690 |
|
|
|
319,937 |
|
*—the effective interest rate for both the Convertible Notes and Preferred Shares include the accretion of the portion of proceeds allocated to equity (Notes 16 and 17)
The following are the undiscounted contracted maturities of financial liabilities, including interest payments for the period ending December 31, 2020:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NON-DERIVATIVE FINANCIAL
LIABILITY |
|
EFFECTIVE
INTEREST
RATE* |
|
|
YEAR OF
MATURITY |
|
|
CARRYING
AMOUNT |
|
|
CONTRACTUAL
CASH FLOWS |
|
|
LESS
THAN 1
YEAR |
|
|
1—2
YEARS |
|
|
2—5
YEARS |
|
Unsecured Loan |
|
|
10.55 |
% |
|
2024 |
|
|
|
18,000 |
|
|
|
19,369 |
|
|
|
360 |
|
|
|
360 |
|
|
|
18,649 |
|
Convertible Notes |
|
|
11.38 |
% |
|
|
2024 |
|
|
|
59,113 |
|
|
|
71,616 |
|
|
|
3,758 |
|
|
|
3,758 |
|
|
|
64,100 |
|
2020 Convertible Notes |
|
|
21.67 |
% |
|
|
2021 |
|
|
|
146,844 |
|
|
|
79,025 |
|
|
|
79,025 |
|
|
|
— |
|
|
|
— |
|
2020 Senior Secured Loans |
|
|
10.32 |
% |
|
|
2022 |
|
|
|
62,401 |
|
|
|
74,161 |
|
|
|
5,200 |
|
|
|
68,961 |
|
|
|
— |
|
Instrument Financing Loans |
|
1.7-2.6% |
|
|
2021-2023 |
|
|
|
676 |
|
|
|
705 |
|
|
|
409 |
|
|
|
212 |
|
|
|
84 |
|
Series A Preferred Shares |
|
|
11.45 |
% |
|
2025 |
|
|
|
277,995 |
|
|
|
421,199 |
|
|
|
— |
|
|
|
— |
|
|
|
421,199 |
|
Series B Preferred Shares |
|
|
8.23 |
% |
|
2027 |
|
|
|
173,726 |
|
|
|
257,462 |
|
|
|
— |
|
|
|
— |
|
|
|
257,462 |
|
Trade and other payables |
|
|
|
|
|
|
|
|
139,283 |
|
|
|
139,283 |
|
|
|
139,283 |
|
|
|
— |
|
|
|
- |
|
Total |
|
|
|
|
|
|
|
|
878,038 |
|
|
|
1,062,820 |
|
|
|
228,035 |
|
|
|
73,291 |
|
|
|
761,494 |
|
23. COMMITMENTS
Capital Commitments
Capital expenditure contracted for at the end of the reporting period but not yet incurred is as follows:
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2020 |
|
|
As of December 31, 2021 |
|
Capital |
|
$ |
51,264 |
|
|
$ |
15,641 |
|
Inventory |
|
|
35,631 |
|
|
|
43,573 |
|
Total |
|
$ |
86,895 |
|
|
$ |
59,214 |
|
The capital commitments relate to contracts to purchase property, plant and equipment.
F-40
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
24. LEASES—GROUP AS LESSEE
The Group leases various offices and facilities. The lease terms are between 1-10 years.
|
|
|
|
|
Right-of-use assets |
|
|
|
Net Carrying Amount |
|
|
|
December 31, 2020 |
|
$ |
10,386 |
|
December 31, 2021 |
|
|
27,746 |
|
Depreciation expense for the year ended |
|
|
|
December 31, 2020 |
|
$ |
2,810 |
|
December 31, 2021 |
|
|
5,593 |
|
During 2021, additions to right-of-use assets amounted to $23,038 (2020: $10,233).
|
|
|
|
|
|
|
|
|
|
|
|
|
AMOUNTS RECOGNIZED IN PROFIT AND LOSS |
|
2019 |
|
|
2020 |
|
|
2021 |
|
Depreciation expense of right-of-use-assets |
|
$ |
1,643 |
|
|
$ |
2,810 |
|
|
$ |
5,593 |
|
Interest expense on lease liabilities |
|
|
396 |
|
|
|
751 |
|
|
|
2,501 |
|
|
|
$ |
2,039 |
|
|
$ |
3,561 |
|
|
$ |
8,094 |
|
At December 31, 2021 the Group is 0t committed to any material short-term leases.
Variable lease payment terms are deemed an insignificant portion of the overall liability at December 31, 2021.
The total cash outflow for leases in 2021 amounted to $5,429 (2020: $3,054, 2019: $1,866).
25. RELATED PARTY TRANSACTIONS
During 2019, Zwanziger Family Ventures subscribed to the Convertible Notes issued by the Company. At December 31, 2021, the Company had accrued interest on the Zwanziger Family Ventures note of $NaN (2020: $30).
During 2020, Zwanziger Family Ventures subscribed to the Convertible Notes issued by the Company. At December 31, 2021, the Company had accrued interest on the Zwanziger Family Ventures note of $NaN (2020: $252).
The Company’s Directors are the Key Management Personnel for the Group. The total Director’s emoluments for 2021 were $30,197 (2020: $661, 2019: $783). Included in the Director’s emoluments for 2021 is $28,376 of stock compensation expense (2020: $62, 2019: $133).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
Salaries and wages |
|
$ |
534 |
|
|
$ |
537 |
|
|
$ |
1,743 |
|
Stock compensation expense |
|
|
133 |
|
|
|
62 |
|
|
$ |
28,376 |
|
Pension and other post-employment benefits |
|
|
32 |
|
|
|
33 |
|
|
$ |
60 |
|
Other employee benefits |
|
|
84 |
|
|
|
29 |
|
|
$ |
18 |
|
Total |
|
$ |
783 |
|
|
$ |
661 |
|
|
$ |
30,197 |
|
For the purposes of these remuneration disclosures the values for equity compensation plans are calculated based on the fair value used in Note 15.
26. ULTIMATE CONTROLLING PARTY
NaN one party or Company of shareholders has a controlling interest in the Company.
27. LISTING EXPENSES
As described in Note 2, the Merger led to a share listing expense. The Company issued shares with a fair value of $52.5 million to CAH shareholders, comprised of the fair value of the Company’s shares that were issued to CAH shareholders of $9.89 per share. In exchange, the Company received the identifiable net assets held by CAH, which had a fair value upon
F-41
LUMIRADX LIMITED
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(In thousands, except share and per share data)
closing of $24.9 million. The excess of the fair value of the equity instruments issued over the fair value of the identified net assets received, represents a non-cash expense in accordance with IFRS 2. This one-time expense as a result of the transaction, in the amount of $27.6 million, is recognized as a share listing expense presented as part of the financial result within the Consolidated Statement of Profit or Loss. In addition, the Company incurred other transaction related expenses of $8.6 million.
|
|
|
|
|
|
|
|
|
|
|
Amount |
|
|
Number of Shares |
|
(a) Shares issued to CAH shareholders |
|
|
|
|
|
5,307,607 |
|
(b) Opening price of LMDX shares on NASDAQ as of September 29, 2021 |
|
$ |
9.89 |
|
|
|
|
(c) Fair value of LMDX shares issued to CAH shareholders (a * b) |
|
|
52,492 |
|
|
|
|
(d) CAH cash in trust |
|
|
38,244 |
|
|
|
|
(e) CAH other assets |
|
|
325 |
|
|
|
|
(f) CAH liabilities |
|
|
(13,683 |
) |
|
|
|
(g) Net assets of CAH (d + e + f) |
|
|
24,886 |
|
|
|
|
IFRS 2 listing expense (c - g) |
|
$ |
27,606 |
|
|
|
|
28. EVENT AFTER THE REPORTING PERIOD
2022 Convertible Notes
On March 2, 2022 the Company entered into privately negotiated subscription agreements with certain investors wherein the Company agreed to sell and the investors agreed to purchase $56.5 million of Convertible Senior Subordinated Notes due 2027. The notes bear annual interest of 6% with interest payable semi-annually in arrears starting September 1, 2022. The notes will mature on March 1, 2027 and are be convertible at the holder's option at an initial conversion rate of approximately $9.22 per share.
F-42







