CONFIDENTIAL TREATMENT REQUESTED BY BERKELEY LIGHTS, INC.
PURSUANT TO 17 C.F.R. Section 200.83
and could result in the award of substantial damages against us, including treble damages, attorney’s fees, costs and expenses if we are found to have willfully infringed. In the event of a successful claim of infringement against us, we may be required to pay damages and ongoing royalties, and obtain one or more licenses from third parties, or be prohibited from selling certain products or services. We may not be able to obtain these licenses on acceptable or commercially reasonable terms, if at all, or these licenses may be non-exclusive, which could result in our competitors gaining access to the same intellectual property. In addition, we could encounter delays and incur significant costs, in product or service introductions while we attempt to develop alternative products or services, or redesign our products or services, to avoid infringing third party patents or proprietary rights. Defense of any lawsuit or failure to obtain any of these licenses or to develop a workaround could prevent us from commercializing products or services, and the prohibition of sale or the threat of the prohibition of sale of any of our products or services could materially affect our business and our ability to gain market acceptance for our products or services.
In addition, our agreements with some of our customers, suppliers or other entities with whom we do business require us to defend or indemnify these parties to the extent they become involved in infringement claims, including the types of claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, financial condition, results of operations and prospects.
Claims by AbCellera and the University of British Columbia that we infringe their intellectual property rights may adversely affect our business, financial condition, results of operations and prospects.
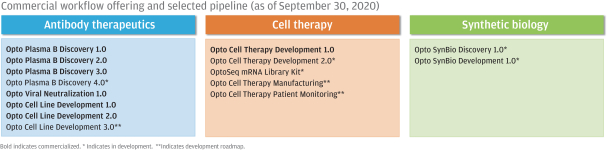
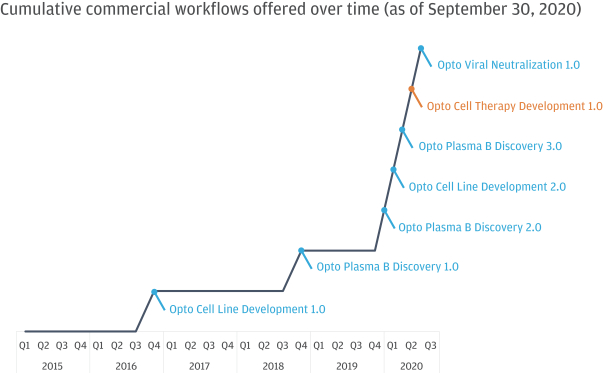
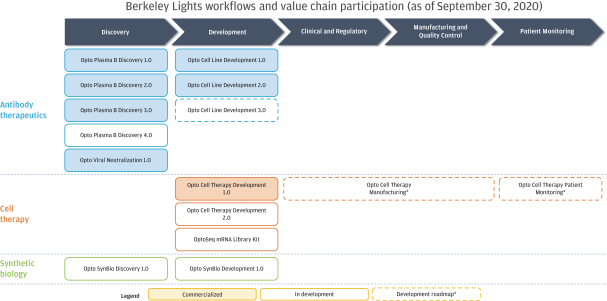
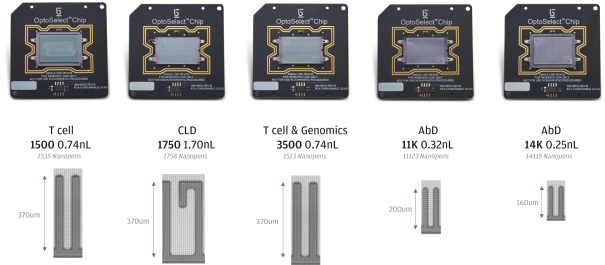
In July 2020, AbCellera Biologics Inc. (“AbCellera”) filed a complaint in the United States District Court for the District of Delaware, alleging that we infringed and continue to infringe, directly and indirectly, the following patents exclusively licensed by AbCellera by making, using, offering for sale, selling and/or importing our Beacon and Culture Station instruments and the OptoSelect chips, and sale of the Opto Plasma B Discovery Workflow: U.S. Patent Nos. 10,107,812, 10,274,494, 10,466,241, 10,578,618, 10,697,962, 10,087,408, 10,421,936 and 10,704,018 (“AbCellera I”). In August 2020, AbCellera filed a second complaint in the United States District Court for the District of Delaware, making the same allegations with regard to U.S. Patent Nos. 10,718,768, 10,738,270, 10,746,737, and 10,753,933 (“AbCellera II”). In September 2020, AbCellera filed amended complaints in each of AbCellera I and AbCellera II adding The University of British Columbia as a named plaintiff. Also in September 2020, AbCellera and The University of British Columbia filed a third complaint in the United States District Court for the District of Delaware, making the same allegations with regard to U.S. Patent Nos. 10,775,376, 10,775,377, and 10,775,378 (“AbCellera III”). AbCellera and The University of British Columbia are seeking, among other things, judgment of infringement, a permanent injunction and damages (including lost profits, a reasonable royalty, reasonable costs and attorney’s fees, and treble damages for willful infringement). In addition to procedural motions, we have filed an answer and counterclaims in response to each of the AbCellera I, AbCellera II and AbCellera III lawsuits. Our counterclaims in each lawsuit include counts for declaratory judgment of non-infringement of the asserted patents, for declaratory judgment of invalidity of the asserted patents, and for declaratory judgment of unenforceabity of the asserted patents due to inequitable conduct. The AbCellera I, AbCellera II and AbCellera III lawsuits are pending.
In August 2020, we filed a complaint in in the United States District Court for the Northern District of California against AbCellera and Lineage BioSciences, Inc., an entity previously acquired by AbCellera. The complaint includes two counts of unfair competition and one count of a declaratory judgment of non-infringement of U.S. Patent No. 10,058,839. We are seeking, among other things, damages and a judgment of non-infringement. In October 2020, we filed an amended complaint asserting the same three counts and AbCellera and Lineage filed a motion to dismiss the amended complaint. This lawsuit remains pending.
49