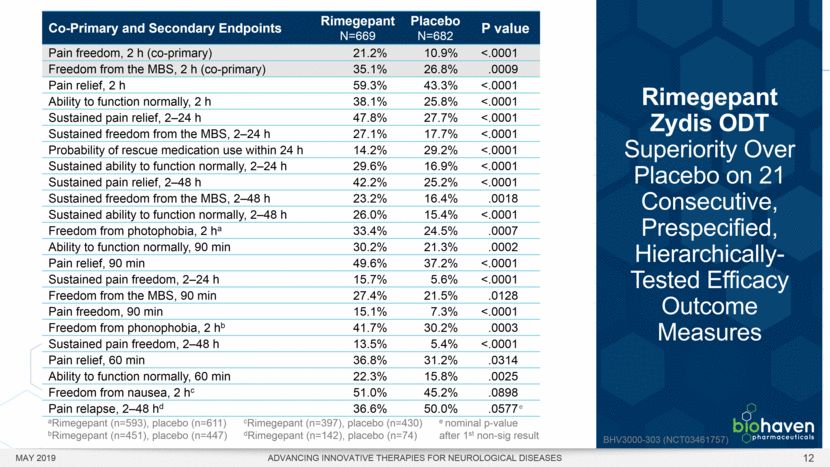
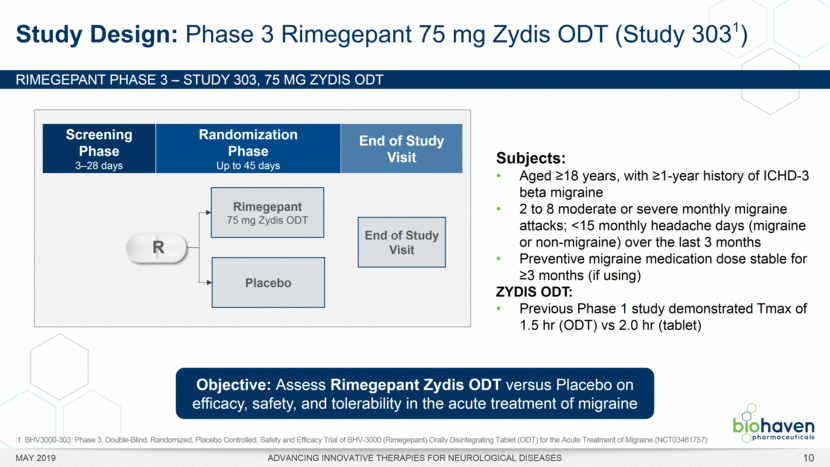
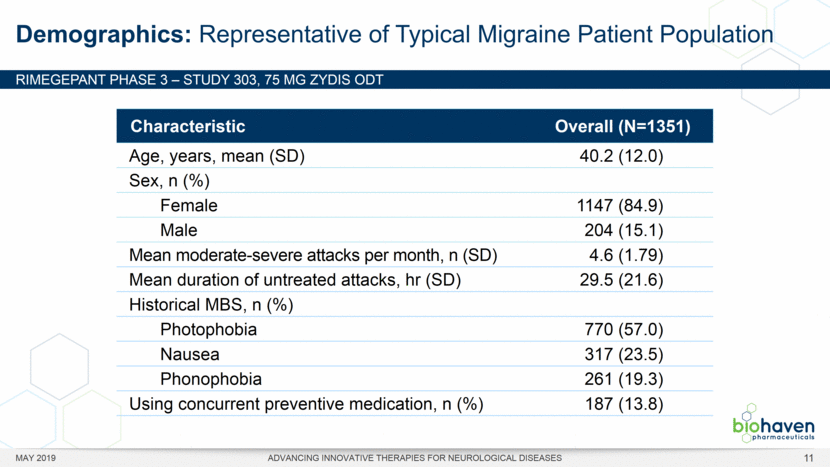
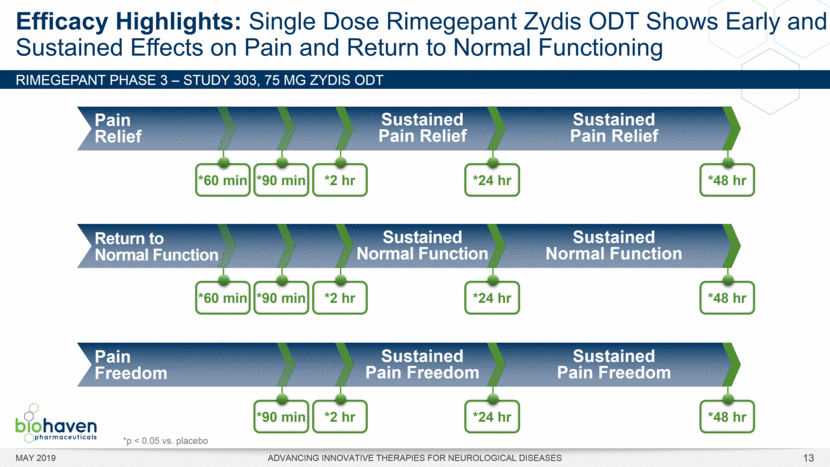
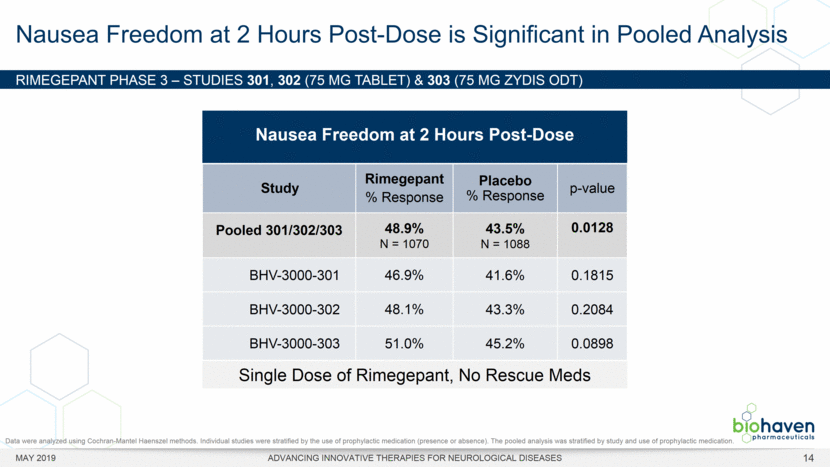
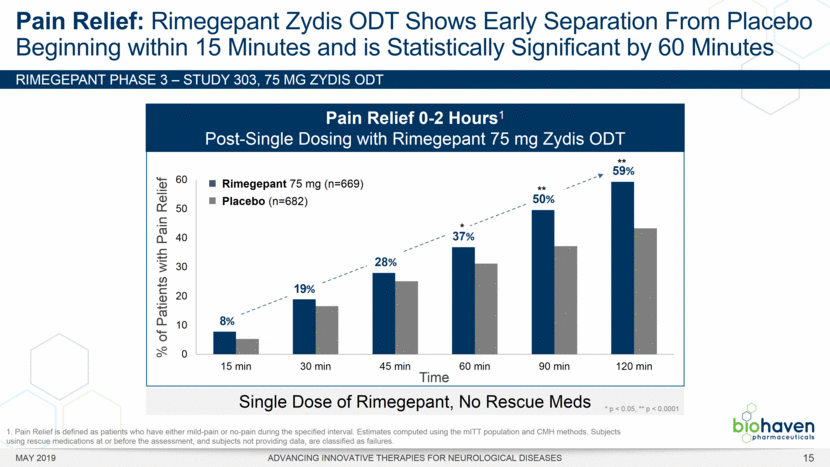
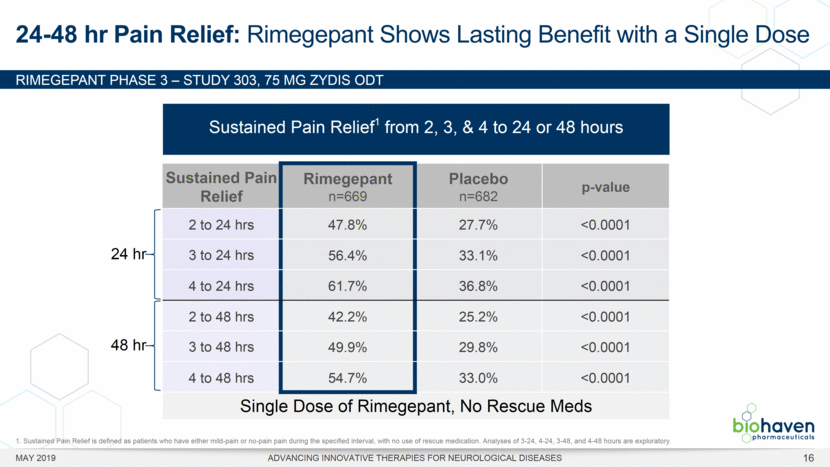
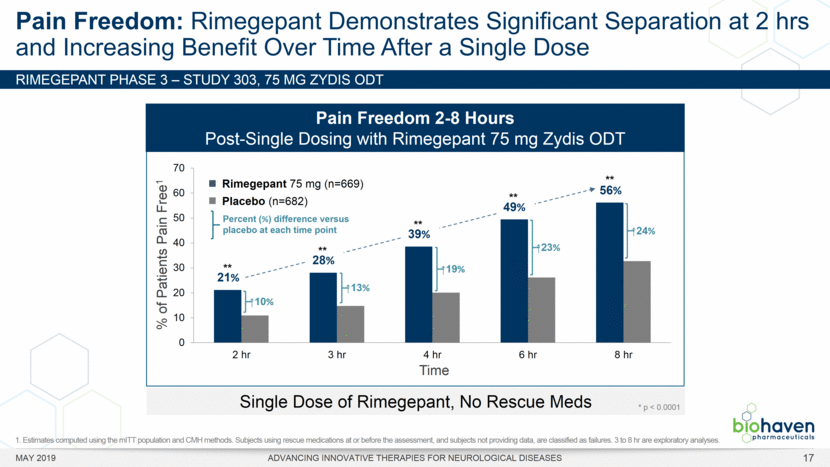
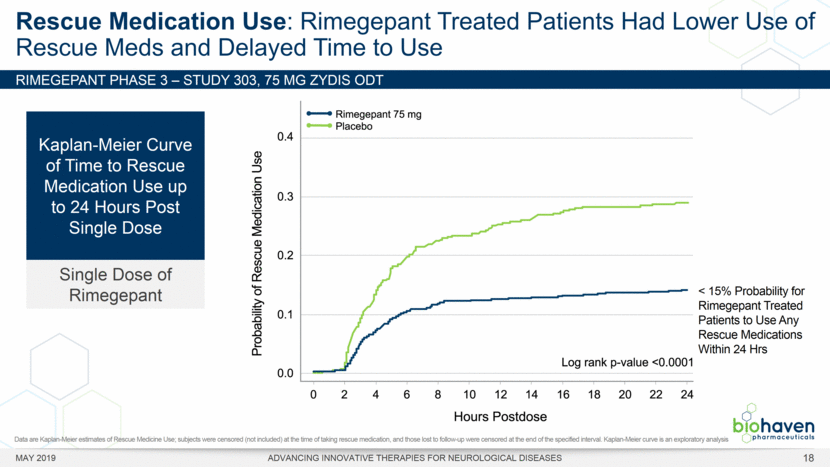
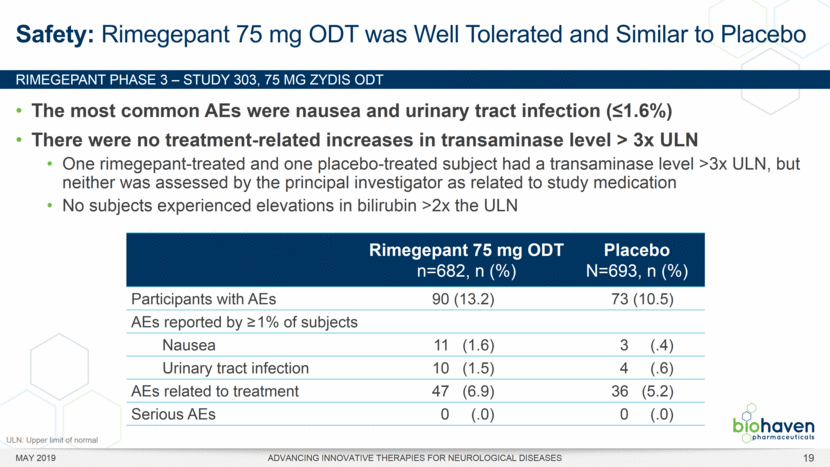
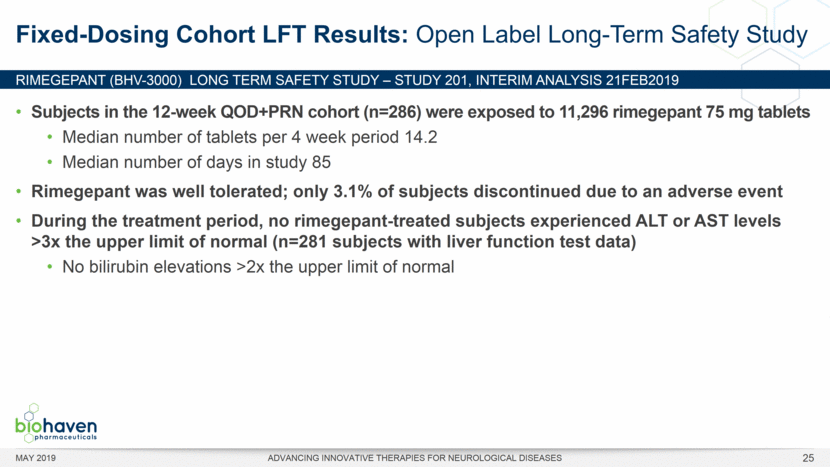
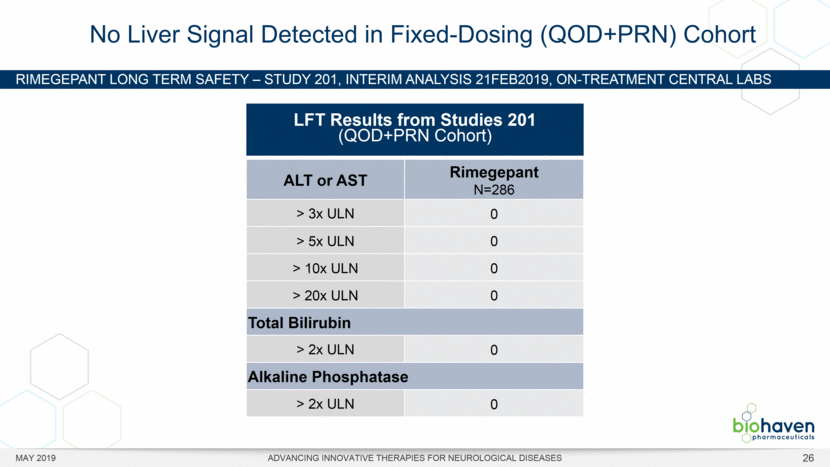
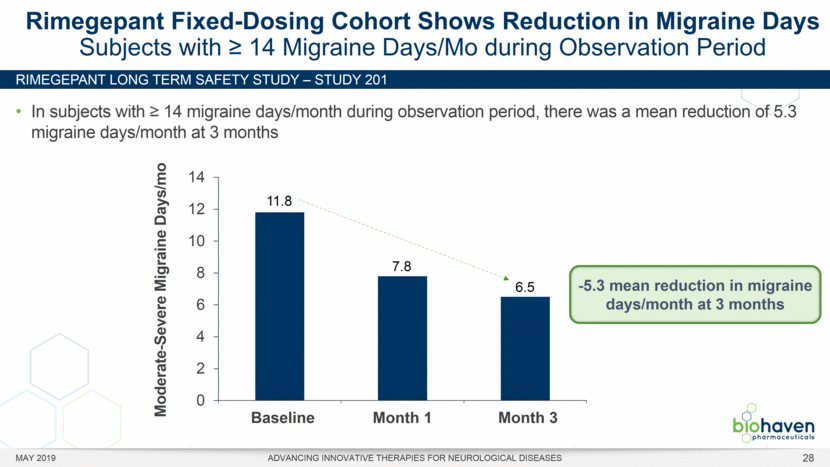
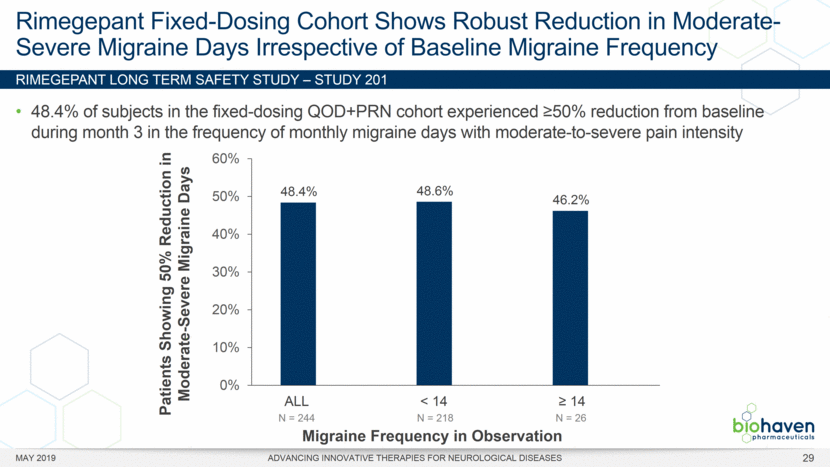
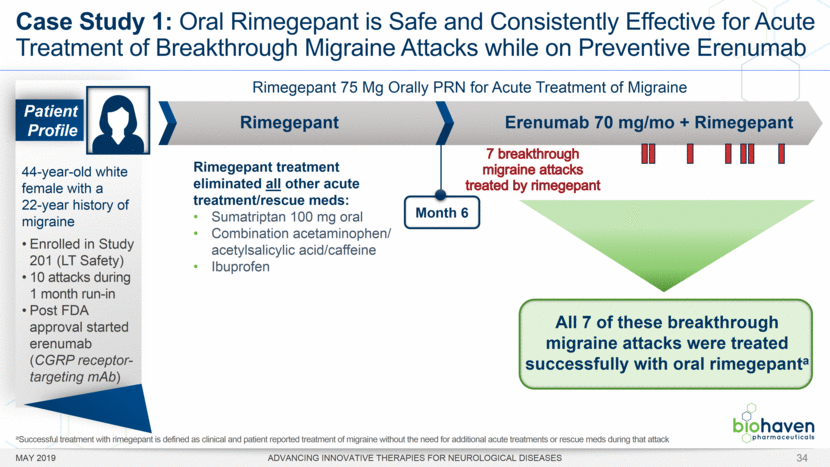
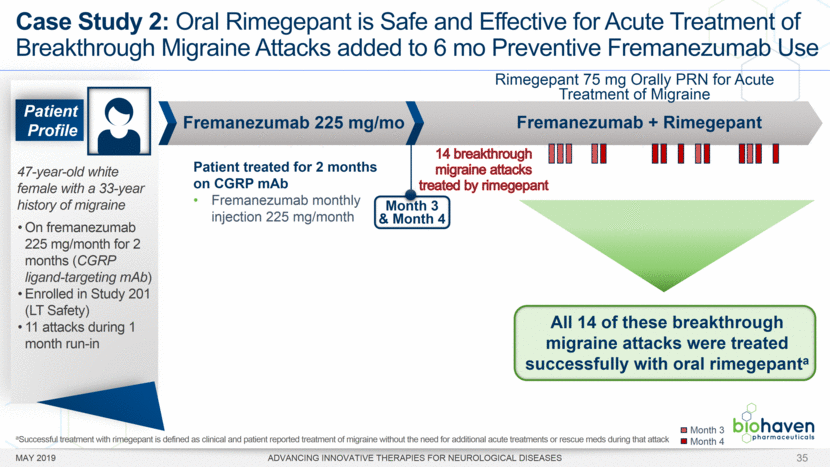
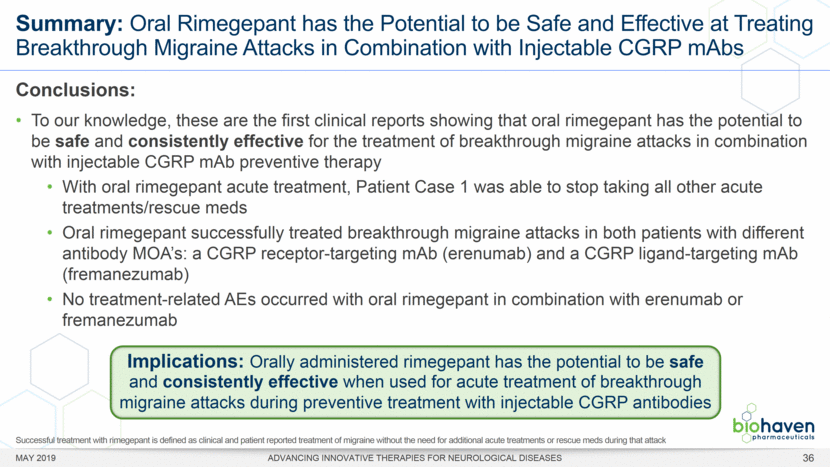
MAY 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES 12 Rimegepant Zydis ODT Superiority Over Placebo on 21 Consecutive, Prespecified, Hierarchically-Tested Efficacy Outcome Measures Co-Primary and Secondary Endpoints Rimegepant N=669 Placebo N=682 P value Pain freedom, 2 h (co-primary) 21.2% 10.9% <.0001 Freedom from the MBS, 2 h (co-primary) 35.1% 26.8% .0009 Pain relief, 2 h 59.3% 43.3% <.0001 Ability to function normally, 2 h 38.1% 25.8% <.0001 Sustained pain relief, 2–24 h 47.8% 27.7% <.0001 Sustained freedom from the MBS, 2–24 h 27.1% 17.7% <.0001 Probability of rescue medication use within 24 h 14.2% 29.2% <.0001 Sustained ability to function normally, 2–24 h 29.6% 16.9% <.0001 Sustained pain relief, 2–48 h 42.2% 25.2% <.0001 Sustained freedom from the MBS, 2–48 h 23.2% 16.4% .0018 Sustained ability to function normally, 2–48 h 26.0% 15.4% <.0001 Freedom from photophobia, 2 ha 33.4% 24.5% .0007 Ability to function normally, 90 min 30.2% 21.3% .0002 Pain relief, 90 min 49.6% 37.2% <.0001 Sustained pain freedom, 2–24 h 15.7% 5.6% <.0001 Freedom from the MBS, 90 min 27.4% 21.5% .0128 Pain freedom, 90 min 15.1% 7.3% <.0001 Freedom from phonophobia, 2 hb 41.7% 30.2% .0003 Sustained pain freedom, 2–48 h 13.5% 5.4% <.0001 Pain relief, 60 min 36.8% 31.2% .0314 Ability to function normally, 60 min 22.3% 15.8% .0025 Freedom from nausea, 2 hc 51.0% 45.2% .0898 Pain relapse, 2–48 hd 36.6% 50.0% .0577 aRimegepant (n=593), placebo (n=611) bRimegepant (n=451), placebo (n=447) cRimegepant (n=397), placebo (n=430) dRimegepant (n=142), placebo (n=74) BHV3000-303 (NCT03461757) e nominal p-value after 1st non-sig result e