Exhibit 99.1

NEURO INNOVATION BROAD PIPELINE OF LATE STAGE PRODUCT CANDIDATES NYSE: BHVN © 2019 Biohaven Pharmaceuticals Inc. All rights reserved. Biohaven Investor Presentation October 3, 2019

Disclaimer SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 2 This presentation contains forward - looking statements within the meaning of “safe harbor” provisions of The Private Securities Litigation Reform Act of 1995, including: statements about our plans to develop and commercialize our product candidates, our planned clinical trials for our rimegepant , vazegepant (BHV - 3500), troriluzole , BHV - 0223, BHV - 5000 and verdiperstat development programs, the timing of the availability of data from our clinical trials, the timing of our planned regulatory filings, the tim ing of and our ability to obtain and maintain regulatory approvals for our product candidates and the clinical potential utility of our pro duct candidates, alone and as compared to other existing or potential treatment options. These statements involve substantial know n and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward - looking statements and from the Company's current expectations. We may not actually achieve the plans, intentions or expectations disclosed in our forwar d - looking statements, and you should not place undue reliance on our forward - looking statements. The forward - looking statements in this presentation represent our views as of the date of this presentation. Subsequent events and developments may cause ou r views to change. However, while we may elect to update these forward - looking statements at some point in the future, we have no obligation to do so except to the extent required by applicable law. You should, therefore, not rely on these forward - looking statements as representing our views as of any date subsequent to the date of this presentation. For further information rega rdi ng these risks, uncertainties and other factors, you should read the “Risk Factors” section of the Annual Report on Form 10 - K for the year ended December 31, 2018, the Company’s Quarterly Report on Form 10 - Q filed with the Securities and Exchange Commission (the ”SEC”) on August 9, 2019 and the Company’s other reports filed with the SEC. References to www.biohavenpharma.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on such website into this presentation by reference. This presentation also contains market data and other statistical information that are based on independent industry publicat ion s, reports by market research firms or published independent sources. Some market data and statistical information are also base d on the Company's good faith estimates, which are derived from management's knowledge of its industry and such independent sources referred to above. While the Company is not aware of any misstatements regarding the market and industry data presented herein, such data involve risks and uncertainties and are subject to change based on various factors.
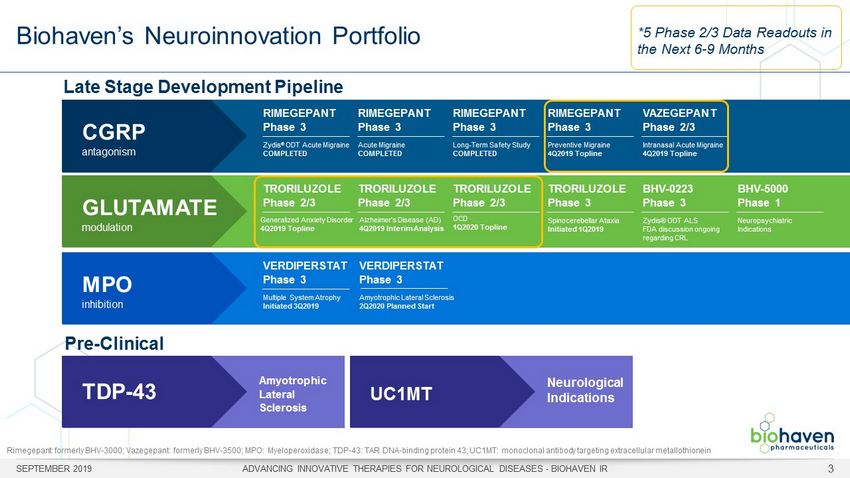
Biohaven’s Neuroinnovation Portfolio SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 3 Late Stage Development Pipeline Pre - Clinical CGRP antagonism RIMEGEPANT Phase 3 Zydis ® ODT Acute Migraine COMPLETED RIMEGEPANT Phase 3 Acute Migraine COMPLETED RIMEGEPANT Phase 3 Long - Term Safety Study COMPLETED RIMEGEPANT Phase 3 Preventive Migraine 4Q2019 Topline VAZEGEPANT Phase 2/3 Intranasal Acute Migraine 4Q2019 Topline GLUTAMATE modulation TRORILUZOLE Phase 2/3 Generalized Anxiety Disorder 4Q2019 Topline TRORILUZOLE Phase 2/3 Alzheimer’s Disease (AD) 4Q2019 Interim Analysis TRORILUZOLE Phase 2/3 OCD 1Q2020 Topline TRORILUZOLE Phase 3 Spinocerebellar Ataxia Initiated 1Q2019 BHV - 0223 Phase 3 Zydis ® ODT ALS FDA discussion ongoing regarding CRL BHV - 5000 Phase 1 Neuropsychiatric Indications MPO inhibition VERDIPERSTAT Phase 3 Multiple System Atrophy Initiated 3Q2019 *5 Phase 2/3 Data Readouts in the Next 6 - 9 Months TDP - 43 Amyotrophic Lateral Sclerosis UC1MT Neurological Indications VERDIPERSTAT Phase 3 Amyotrophic Lateral Sclerosis 2Q2020 Planned Start Rimegepant : formerly BHV - 3000; Vazegepant : formerly BHV - 3500; MPO: Myeloperoxidase; TDP - 43: TAR DNA - binding protein 43; UC1MT: monoclonal antibody targeting extracellula r metallothionein

Aspire to Achieve • 3 Marketed drugs by 2021 • Grow while maintaining lean, efficient and high performing culture meeting patient needs growing value SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 4 Goal:

CGRP PLATFORM Therapies for Migraine
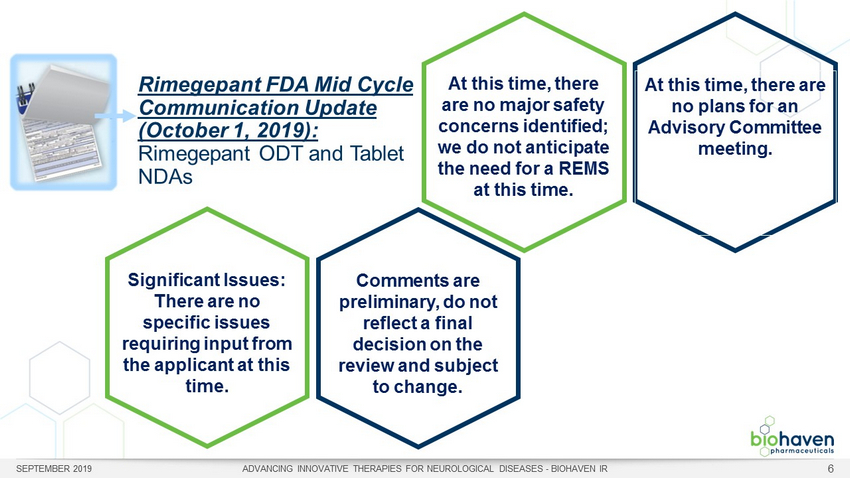
SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 6 Rimegepant FDA Mid Cycle Communication Update (October 1, 2019): Rimegepant ODT and Tablet NDAs At this time, there are no major safety concerns identified; we do not anticipate the need for a REMS at this time. At this time, there are no plans for an Advisory Committee meeting. Significant Issues: There are no specific issues requiring input from the applicant at this time. Comments are preliminary, do not reflect a final decision on the review and subject to change.

Multiple Formulations With the Potential to Meet Patient Needs from Acute to Preventive Treatment of Migraine SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 7 NO JECTION™ CGRP Drug Delivery Platform 1. Exclusive World - Wide License with Catalent for use of Zydis ® Fast Dissolve Technology in our migraine product candidates 2. Aptar Pharma Unidose System (UDS) single shot nasal technology Oral Rapid Dissolving 1 Intranasal 2 Vazegepant Rimegepant

Biohaven Strategy: Advance Oral Agents with Potential Dual - Therapy Action (Acute and Preventive Tx) ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR PREVENTIVE TREATMENT • Indicated based on headache frequency & headache related impairment, including: • Chronic Migraine (CM) patients (~3.6M) • Patients appropriate for prevention without CM diagnosis (~10.4M) • These patients also need acute treatment • CGRP agents targeting indication: • Antibodies: Alder, Amgen, Lilly, Teva • Small molecules: Biohaven, Allergan ~36M People with Migraine in the U.S. 1 1. American Migraine Foundation 2. ICHD - 3b International Classification of Headache Disorders 3. Lipton RB, Bigal ME, Diamond M, et al. Neurology . 2007;68(5):343 - 349. ~14M ( 39 % 3 ) ~36M ( 100 %) ACUTE TREATMENT • 100% of patients need acute therapy • Diagnosis: Migraine with/without aura 2 • Take as needed to abort attack • CGRP agents targeting indication: • Small molecules: Biohaven, Allergan Stylized pie - chart: NOT brain anatomy SEPTEMBER 2019 8

Rimegepant Value Proposition: “One and Done” SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 9 Rapid Pain Relief Zydis ® ODT Durable Benefits through 2 days Placebo - like Tolerability

Rimegepant Clinical Trials Published in NEJM and The Lancet Articles SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 10 Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised , phase 3, double - blind, placebo - controlled trial. Rimegepant, an Oral Calcitonin Gene – Related Peptide Receptor Antagonist, for Migraine Study 302 Study 303
Rimegepant 75 mg Zydis ® ODT Phase 3 Results: Study 303 in the Acute Treatment of Migraine

Rimegepant ODT Broad Spectrum of Efficacy (Study 303) Superiority Over Placebo on 21 Consecutive, Hierarchically - Tested Endpoints SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 12 Favors Placebo Favors Rimegepant 1º Pain freedom, 2 hrs 21.2 10.9 10.4 (6.5, 14.2) Freedom from MBS, 2 hrs 35.1 26.8 8.3 (3.4, 13.2) 2º Pain relief, 2 hrs 59.3 43.3 16.1 (10.8, 21.3) Ability to function normally, 2 hrs 38.1 25.8 12.3 (7.4, 17.2) Sustained pain relief, 2 - 24 hrs 47.8 27.7 20.1 (15.1, 25.2) Sustained freedom from MBS, 2 - 24 hrs 27.1 17.7 9.3 (4.9, 13.7) No rescue medication within 24 hrs b 85.8 70.8 15.0 (10.7, 19.3) Sustained ability to function normally, 2 - 24 hrs 29.6 16.9 12.7 (8.3, 17.2) Sustained pain relief, 2 - 48 hrs 42.2 25.2 16.9 (12.0, 21.9) Sustained freedom with MBS, 2 - 48 hrs 23.2 16.4 6.7 (2.5, 11.0) Sustained ability to function normally, 2 - 48 hrs 26.0 15.4 10.6 (6.3, 14.9) Freedom from photophobia, 2 hrs 33.4 24.5 8.8 (3.7, 13.9) Ability to function normally, 90 mins 30.2 21.3 8.9 (4.3, 13.6) Pain relief, 90 mins 49.6 37.2 12.4 (7.1, 17.6) Sustained pain freedom, 2 - 24 hrs 15.7 5.6 10.1 (6.9, 13.4) Freedom from MBS, 90 mins 27.4 21.5 5.8 (1.2, 10.4) Pain freedom, 90 mins 15.1 7.3 7.8 (4.4, 11.1) Freedom from photophobia, 2 hrs 41.7 30.2 11.5 (5.3, 17.7) Sustained pain freedom, 2 - 48 hrs 13.5 5.4 8.0 (4.9, 11.1) Pain relief, 60 mins 36.8 31.2 5.5 (0.5, 10.6) Ability to function normally, 60 mins 22.3 15.8 6.4 (2.3, 10.6) Freedom from nausea, 2 hrs 51.0 45.2 5.9 ( - 0.9, 12.7) No pain relapse, 2 - 48 hrs 63.4 50.0 13.3 ( - 0.4, 27.1) 3 Categories: 2 hr effects , Early Action (60 - 90 min) , Durable Effect (24 - 48 hr ) Rimegepant % Placebo % Difference (95% CI) -30 -25 -20 -15 -10 -5 0 5 10 15 20 25 30

RIMEGEPANT PHASE 3 – STUDY 303, 75 MG ZYDIS ® ODT SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 13 Efficacy Highlights: Rimegepant Zydis ® ODT Shows Early and Sustained Effects with a Single Dose Pain Relief *60 min *90 min *2 hr *24 hr *48 hr Sustained Sustained Return to Normal Function Sustained Sustained Pain Freedom MBS Freedom Sustained Sustained *p < 0.05 vs. placebo *60 min *90 min *2 hr *24 hr *48 hr *90 min *2 hr *24 hr *48 hr

RIMEGEPANT PHASE 3 – STUDY 303, 75 MG ZYDIS ® ODT Pain Relief: Rimegepant Zydis ® ODT Shows Early Separation From Placebo Beginning within 15 Minutes and is Statistically Significant by 60 Minutes SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 1. Pain Relief is defined as patients who have either mild - pain or no - pain during the specified interval. Estimates computed usi ng the mITT population and CMH methods. Subjects using rescue medications at or before the assessment, and subjects not providing data, are classified as failures. 14 0 10 20 30 40 50 60 15 min 30 min 45 min 60 min 90 min 120 min Single Dose of Rimegepant, No Rescue Meds % of Patients with Pain Relief Pain Relief 0 - 2 Hours 1 Post - Single Dosing with Rimegepant 75 mg Zydis ® ODT Time Rimegepant 75 mg (n=669) Placebo (n=682) 19 й 59 й37 й50 й8 й 28 й** * p < 0.05, ** p < 0.0001 ** *
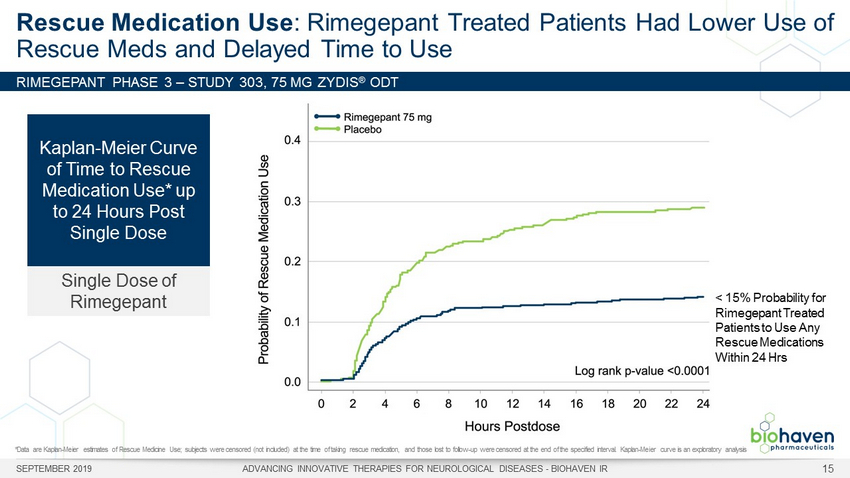
RIMEGEPANT PHASE 3 – STUDY 303, 75 MG ZYDIS ® ODT Rescue Medication Use : Rimegepant Treated Patients Had Lower Use of Rescue Meds and Delayed Time to Use *Data are Kaplan - Meier estimates of Rescue Medicine Use; subjects were censored (not included) at the time of taking rescue medi cation, and those lost to follow - up were censored at the end of the specified interval. Kaplan - Meier curve is an exploratory ana lysis Probability of Rescue Medicine Use 0.5 0.4 0.3 0.2 0.1 0.0 0 2 4 6 8 10 12 14 16 18 20 24 Time (hours) Rimegepant (n=537) Placebo (n=535) 22 Kaplan - Meier Curve of Time to Rescue Medication Use* up to 24 Hours Post Single Dose Single Dose of Rimegepant SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 15 Log rank p - value: < 0.0001 Rimegepant (n=669) Placebo (n=682) < 15% Probability for Rimegepant Treated Patients to Use Any Rescue Medications Within 24 Hrs

RIMEGEPANT PHASE 3 – STUDY 303, 75 MG ZYDIS ® ODT Safety Profile: Rimegepant 75 mg ODT was Well Tolerated and Low Rates of AEs SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 16 • The most common AEs were nausea and urinary tract infection (≤1.6%) • There were no treatment - related increases in transaminase level > 3x ULN • One rimegepant - treated and one placebo - treated subject had a transaminase level >3x ULN, but neither was assessed by the principal investigator as related to study medication • No subjects experienced elevations in bilirubin >2x the ULN Rimegepant 75 mg ODT n=682, n (%) Placebo N=693, n (%) Participants with AEs 90 (13.2) 73 (10.5) AEs reported by ≥ 1% of subjects Nausea 11 (1.6) 3 (.4) Urinary tract infection 10 (1.5) 4 (.6) AEs related to treatment 47 (6.9) 36 (5.2) Serious AEs 0 (.0) 0 (.0) ULN: Upper limit of normal

RIMEGEPANT (BHV - 3000) PHASE 3 – 301 , 302 (75 MG TABLET) & 303 (75 MG ZYDIS ® ODT) Pooled Liver Function Test (LFT) Profile: Rimegepant Liver Safety was Similar to Placebo Across Studies ALT or AST Rimegepant N=1771 Placebo N=1785 > ULN 2 48 (2.7%) 52 (2.9%) > 3x ULN 2 (0.1%) 2 (0.1%) > 5x ULN 1 (0.06%) 3 0 > 10x ULN 0 0 > 20x ULN 0 0 Pooled LFT Results from Studies 301, 302, and 303 1 1. AST/ALT categories are not mutually exclusive; No bilirubin elevations > 2x ULN across Studies 301, 302 and 303 2. Upper limit of normal; ALT: alanine aminotransferase; AST: aspartate aminotransferase. 3. AST elevation, Not Drug - Related as deemed by the investigator: subject newly initiated weight - lifting with laboratory results consistent with muscle injury. SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 17

RIMEGEPANT PHASE 3 – STUDY 303, 75 MG ZYDIS ® ODT Summary : Rimegepant 75 mg ODT – Profile of Rapid and Sustained Effect in the Acute Treatment of Migraine SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 18 • Rapid onset of pain relief with single dose • Numerical separation from placebo as early as 15 minutes and statistically significant by 60 minutes • Significantly greater percentage of patients returned to normal functioning by 60 minutes (p = 0.0025) • Achieved statistical significance on regulatory co - primary endpoints of pain freedom (p < 0.0001) and freedom from most bothersome symptom (p = 0.0009) at 2 hours • Superiority over placebo in 21 consecutive, prespecified, hierarchically - tested efficacy outcome measures, including: • Clinical effect observed from 2 through 48 hours on freedom from pain (p < 0.0001), pain relief (p < 0.0001), freedom from most bothersome symptom (p = 0.0018), and freedom from functional disability (p < 0.0001) • Safety profile comparable to placebo, including liver function tests
RIMEGEPANT LONG TERM SAFETY – STUDY 201, INTERIM ANALYSIS 13JUN2 019 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 19 • 111,573 doses of rimegepant 75 mg administered across 1,798 patients with migraine • Median number of days in study 278 • <3% of subjects discontinued due to AE • Interim hepatic data were reviewed by an external independent panel of liver experts, who: • Provided a consensus opinion based upon Drug - Induced Liver Injury Network (DILIN) causality assessment • Did not assess any liver cases as “probably related” to study drug; and no Hy’s Law cases identified • Concluded there was no liver safety signal detected through the data analysis cut - off date, including a subset of patients with near - daily dosing (≥14 doses/month) • In aggregate, the panel noted that, compared to placebo arms of other migraine treatments, there was a very low incidence of overall elevations of liver laboratory abnormalities (1.2% incidence of serum ALT or AST > 3x ULN) Overall LFT Results: Open Label Long - Term Safety Study Completed

RIMEGEPANT (BHV - 3000) LONG TERM SAFETY STUDY – STUDY 201, INTER IM ANALYSIS 13JUN2019 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 20 • Subjects in the 12 - week scheduled every other day plus as needed dosing on alternate days (QOD+PRN) cohort (n=286) were exposed to 11,312 rimegepant 75 mg tablets • Median number of tablets per 4 week period 14.2 • Median number of days in study 85 • Rimegepant was well tolerated; only 2.8% of subjects were reported to have discontinued due to an adverse event • During the treatment period in the QOD+PRN cohort, no rimegepant - treated subjects experienced ALT or AST levels >3x the upper limit of normal (n= 279 subjects with liver function test data) • No bilirubin elevations >2x the upper limit of normal Fixed - Dosing Cohort LFT Results: Open Label Long - Term Safety Study

RIMEGEPANT LONG TERM SAFETY – STUDY 201, INTERIM ANALYSIS 13JUN2 019, ON - TREATMENT CENTRAL LABS Fixed - Dosing (QOD+PRN) Study 201 Cohort LFT Results ALT or AST Rimegepant N=286 > 3x ULN 0 > 5x ULN 0 > 10x ULN 0 > 20x ULN 0 Total Bilirubin > 2x ULN 0 Alkaline Phosphatase > 2x ULN 0 LFT Results from Studies 201 (QOD+PRN Cohort) SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 21

• 48.4% of subjects in the fixed - dosing QOD+PRN cohort experienced ≥50% reduction from baseline during month 3 in the frequency of monthly migraine days with moderate - to - severe pain intensity RIMEGEPANT LONG TERM SAFETY STUDY – STUDY 201 Rimegepant Fixed - Dosing Cohort Preliminarily Shows Reduction in Moderate - Severe Migraine Days* Irrespective of Baseline Migraine Frequency SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 22 48.4% 48.6% 46.2% 0% 10% 20% 30% 40% 50% 60% All <14 >14 Patients Showing 50% Reduction in Moderate - Severe Migraine Days Migraine Frequency in Observation N = 244 N = 218 N = 26 ALL < 14 ≥ 14 *Reduction in migraine days is an exploratory analysis

33.0 20.7 19.1 17.8 17.2 15 20 25 30 35 baseline 12 24 36 52 Mean MIDAS Total Score N=1631 N=1211 N=1085 N=917 N=1789 P<.0001 versus baseline – all time points Time (weeks) *Lower score reflects improvement Improvement in Migraine - Specific Disability after Dosing with Rimegepant 75 mg up to Once Daily for the Acute Treatment of Migraine Severe Moderate Level of Disability* MIDAS: Migraine Disability Assessment Test SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 23

TIME (weeks) Absenteeism Days of missed work, school, household, social or leisure activities due to migraine P <.0001 vs baseline for all timepoints 17.8 10.7 9.6 8.9 8.9 16.2 10 9.4 8.7 8.4 5 8 11 14 17 20 baseline 12 24 36 52 ABSENTEEISM PRESENTEEISM Presenteeism Days when migraine interfered with work, school, household, social or leisure activities Mean Item Score Treatment with Rimegepant 75 mg up to Once Daily Reduces Lost Productivity Time by Approximately 50% *Lower score reflects improvement 24 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR

Treatment with Rimegepant 75 mg up to Once Daily Improves Outcomes on Health Related Quality of Life ( HRQoL ) 14.5 15.4 17.4 17.6 11.5 12.8 13.7 14.4 15.4 15.8 17 .0 17.2 0 2 4 6 8 10 12 14 16 18 20 0 Weeks 12 Weeks 24 Weeks 36 Weeks 52 Weeks Restrictive Role Preventative Role Emotional Function P<.0001 – each domain - all time points Improvement 0 Migraine Specific Quality of Life ( MSQoL v2.1) Mean Change from Baseline SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 25

Prevention Strategy: Redefine the Treatment Paradigm as a Continuum SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 26 • Rimegepant acute efficacy and durability of action demonstrated in three Phase 3 Trials • Early signals from 201 data and 303 study suggest that Rimegepant has the potential for “dual - therapy action” in both acute and preventive treatment of migraine: • Acute efficacy over placebo • Rapid onset of Pain Relief: separating at 15 min, significant at 60 min 1 • Return to Normal Function at 60 min 1 • Sustained benefit seen through 48 hours 1 • Long half - life provides potential for “protection” from recurrence 2 • Low number of patients taking “day 2” dosing 2 • Frequency of migraines appeared to decrease over time 2 • Unique approach to migraine treatment differentiates from competitors • Phase 3 randomized, placebo - controlled trial is ongoing to evaluate rimegepant prevention 1. 303 Study : (BHV3000 - 303) | Acute Treatment of Migraine. 2. 201 Study: (BHV3000 - 201) | Open Label Long - Term Safety Study. Goal of Dual - Therapy Action: Acute effect + preventive effect = consistent and comprehensive benefit to patients

FIRST INTRANASAL FORMULATION OF A SMALL MOLECULE CGRP RECEPTOR A NTAGONIST SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 27 • Superior chemical attributes • Potent antagonist at the human CGRP receptor • Highly soluble and high free fraction • US composition of matter protection to March 2031 a • Multiple potential routes of delivery • nasal, inhalation and oral • Favorable safety profile in preclinical studies even at high doses • Intranasal initial development for acute treatment • Potential for rapid onset • Phase 2/3 initiated March 2019, Topline Phase 2/3 data anticipated 4Q2019 Vazegepant (BHV - 3500) : Third Generation CGRP Receptor Antagonist hCGRP K i = 23 pM Vazegepant hCGRP K i = 32 pM Rimegepant vazegapant structural diversity vs. rimegepant a patent expiration, not including patent term adjustment or any potential patent term extensions
CGRP Platform Development Milestones & Next Steps SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 28 2Q19 Initiate a Phase 2 proof of concept trial to evaluate the safety and efficacy of rimegepant in patients with treatment refractory trigeminal neuralgia 2Q19 Two NDAs for rimegepant Zydis ® ODT and tablet formulations for acute treatment of migraine 2Q19 Reported results from regularly scheduled dosing cohort in rimegepant long - term safety study 4Q19 Phase 2/3 topline results expected for intranasal vazegepant in acute treatment of migraine 4Q19 Phase 3 topline data expected for rimegepant in preventive treatment of migraine

Rimegepant Commercial & Marketing Update

SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 30 Biohaven’s Commercial and Launch Readiness Remain on Timelines Region Business Director (RBDs): COMPLETED hires District Business Manager (DBMs): Hires: 50% hire completed, on timelines Neuroscience and Primary Care Sales Specialists: Hires: Recruitment & preparation activities on timelines for 4Q2019 hires 2Q2019 3 Q2019 4 Q2019 • Hiring for Region Business Directors (RBD), District Business Managers (DBM) and National Account Directors have remained on schedule to support 1Q2020 rimegepant commercial launch • Highly experienced Commercial Leadership and Managed Markets team in place • Payor discussions remain on track • Commercial operations facility leased in healthcare corridor between Philadelphia and Central NJ

Biohaven’s Experienced Commercial Leaders Mark Glackin • >30 yrs • Allergan/GSK Adrienne Ross • >30 yrs • BMS Jimi Ayodele • >25 yrs • PWC/Otsuka Chris Barrett • >25 yrs • Schering/BI/DBV Cassi O’Neil • >15 yrs • BMS/AZ/ITV HCP Marketing Chris Deluzio • >20 yrs • BMS/BI/AZ/Takeda Graham Goodrich • >20 yrs • Merck/BI Sanjay Keshav • >20 yrs • ZS/BI//Takeda Brian Fitzpatrick • >25 yrs • JJ/Endo/AZ Courtney Cupples • >20 yrs • Genzyme/Alexion Brand Marketing Brian Phillips • >30 yrs • GSK/Mallinckrodt Launch Project Management Portfolio Strategy Managed Markets & Gov’t Affairs Finance - Commercial Sales/Commercial Operations Marketing Strategic Communications Comm Learning/ Development Commercial Operations Sales & Marketing Operations Matt Hubbard • >20 yrs • Alexion BJ Jones • >20 years • BMS/BI/AZ/Takeda CCO, Migraine & Common Disease Migraine Strategy SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 31

SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 32 Biohaven Managed Markets and National Accounts Team K. Floeder, RPh • 25 years industry experience • 16 years account management Key Accounts • CVS • Aetna G. Tadlock, RPh • 25+ years industry experience • 13 years account management Key Accounts • Anthem • Humana S. Hagenbrock, RN • 25+ years industry experience • 20 years account management Key Accounts • HCSC • Centene J. Daddio • 22 years industry experience • 16 years account management Key Accounts • ESI • Cigna Chris Barrett SVP, Managed Markets and Government Affairs • > 25 years industry experience • Formerly Schering Plough, Boehringer Ingelheim, DBV Paul Sbrilli , BPharm VP, Access, Reimbursement & Payor Relations • > 20 years industry experience • Formerly Biogen, Bausch and Lomb, AstraZeneca, Pfizer, Wyeth, Lederle Laboratories

33 Bernadette Raymond Industry: 30 yrs RBD: 15 yrs Biohaven’s Highly Experienced Region Business Directors (RBD) Lead National Commercial Sales Efforts Chris Deluzio SVP Sales/ Comm Ops BJ Jones CCO, Migraine & Common Diseases Chad McMahon Industry: 23 yrs RBD: 3 yrs Brian Keenan Industry: 20 yrs RBD: 5 yrs Ann Marie Hilson Industry: 29 y rs RBD: 19 yrs Stan Metter Industry: 23 yrs RBD: 3 yrs Tim Lee Industry: 20 yrs RBD: 4 yrs Todd Snook Industry: 31 y rs RBD: 15 yrs SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR Bernadette Sgrizzi Industry: 19 yrs RBD: 2 yrs

GLUTAMATE PLATFORM Therapies for Neurologic and Neuropsychiatric Indications
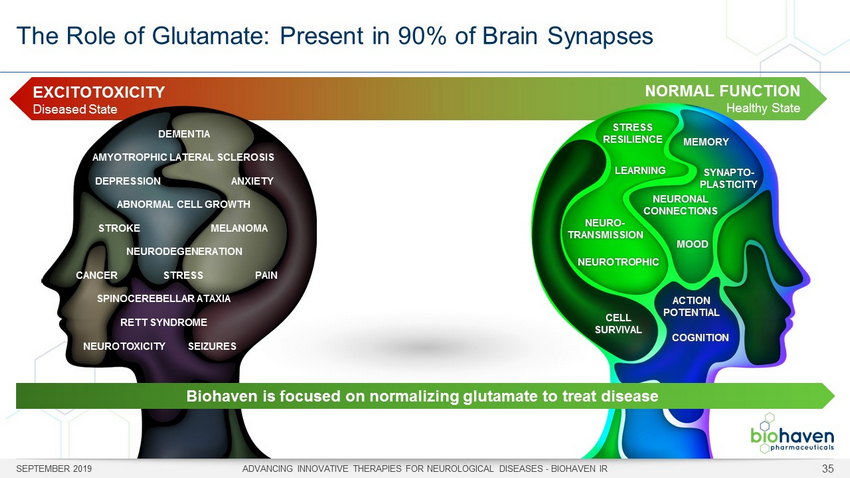
NORMAL FUNCTION Healthy State The Role of Glutamate: Present in 90% of Brain Synapses SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 35 EXCITOTOXICITY Diseased State AMYOTROPHIC LATERAL SCLEROSIS SPINOCEREBELLAR ATAXIA DEMENTIA NEURODEGENERATION NEUROTOXICITY SEIZURES DEPRESSION ANXIETY STRESS CANCER PAIN STROKE MELANOMA ABNORMAL CELL GROWTH RETT SYNDROME NEURO - TRANSMISSION MEMORY CELL SURVIVAL SYNAPTO - PLASTICITY LEARNING NEUROTROPHIC STRESS RESILIENCE ACTION POTENTIAL COGNITION MOOD NEURONAL CONNECTIONS Biohaven is focused on normalizing glutamate to treat disease
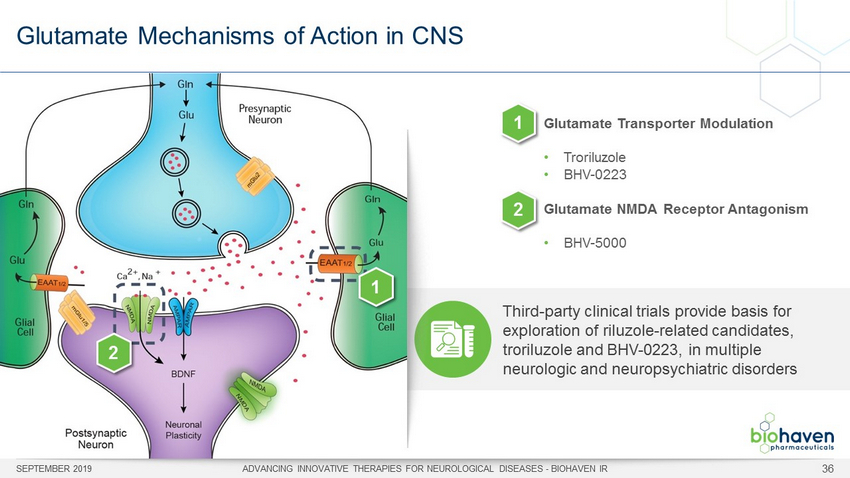
Glutamate Mechanisms of Action in CNS SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 36 Glutamate Transporter Modulation • Troriluzole • BHV - 0223 Glutamate NMDA Receptor Antagonism • BHV - 5000 Third - party clinical trials provide basis for exploration of riluzole - related candidates, troriluzole and BHV - 0223, in multiple neurologic and neuropsychiatric disorders 1 2 1 2

UNMET MEDICAL NEED IN ALS SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 37 Riluzole ( Rilutek ® ) is approved for the treatment of patients with amyotrophic lateral sclerosis (ALS) and proven to extend survival • Originally marketed by Sanofi, received FDA approval in 1995 • In 2013, the FDA approved the first generic versions of riluzole • Doses above 100 mg for efficacy not approved due to dose - dependent liver effects BENEFITS x Mechanism of action well understood x Neuroprotective, survival benefit in ALS x Well tolerated, safe in clinical settings at approved dose LIMITATIONS ✘ Twice daily dosing, low bioavailability ✘ Fasting required for 6 hours/day, can’t be taken with meals ✘ Dose dependent LFT liability (1) ✘ Marked PK variability ✘ High drug burden relative to efficacy (2) ✘ Only one approved indication (ALS) Riluzole ( Rilutek ® ): Use and Limitations 1. LFT = liver function test 2. Poor oral bioavailability results in a high liver burden relative to efficacy as ~40% is either not absorbed or is metabol ize d in the liver
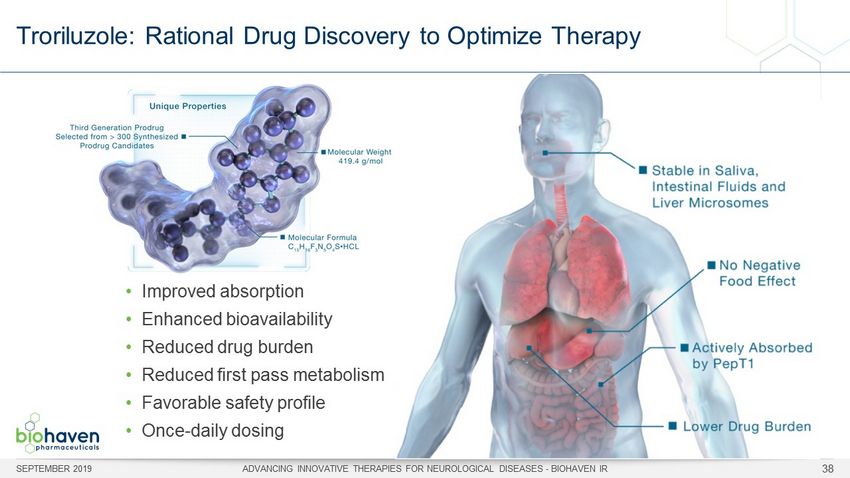
Troriluzole: Rational Drug Discovery to Optimize Therapy SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 38 • Improved absorption • Enhanced bioavailability • Reduced drug burden • Reduced first pass metabolism • Favorable safety profile • Once - daily dosing

Peptide Transporter 1 Enhances Absorption of Troriluzole PepT1 PepT1 = Peptide Transporter 1 ACTIVE ABSORPTION IN INTESTINAL TRACT BY PEPT1 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 39

Social Anxiety Disorder Generalized Anxiety Disorder Bipolar Depression Troriluzole: Targeted Lead - Indication Development Strategy SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 40 * = Third - party study / collaboration ongoing or planned (ADCS Collaboration for AD; ET Study Group Collaboration for ET) Lead indications across an array of potential neurologic and neuropsychiatric indications Obsessive - Compulsive & Related Disorders Neurodegenerative Disorders Mild - to - Moderate Alzheimer’s Disease* Prodromal Alzheimer’s Disease ALS (High Dose) Affective Disorders Cerebellar Disorders Spinocerebellar Ataxia (SCA) Friedreich’s Ataxia Sporadic Ataxia Other Ataxias Essential Tremor* Obsessive - Compulsive Disorder Trichotillomania Hoarding Disorder
TRORILUZOLE (BHV - 4157) PHASE 2/3 TRIAL DESIGN GENERALIZED ANXIETY DISORDER • Chronic or excessive worry, restlessness, fatigue, difficulty concentrating, insomnia • Impairs ability to function socially or at work • Irritable bowel - like gastrointestinal issues • Significant unmet need • GAD has a 12 - month prevalence in the United States of 3% • 3,500,000 (est. treatment resistant in U.S.) TRORILUZOLE PHASE 2/3 TRIAL DESIGN • Multicenter (US only), randomized, double - blind, placebo - controlled trial in outpatients with GAD • Troriluzole 100 mg vs Placebo BID*, N=372 • Primary Outcome: Change on HAM - A from baseline to Week 8 Expanding Biohaven’s Glutamate Modulating Platform into Generalized Anxiety Disorder (GAD) NBC New, 28 - JUL - 18 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 41 * BID: twice daily

BHV - 0223 DESIGN • Double - blind, placebo controlled crossover trial with two Impromptu Speech Task sessions • Subjects diagnosed with Social Anxiety Disorder • N=22 randomized (21 completed) • BHV - 0223 vs PBO, dosed 1 hour prior to public speaking stress task; separated by two to ten days to allow for medication washout Yale POC Study: Anti - Anxiety Effects of BHV - 0223 Primary outcome: trial powered at 80%, to detect an effect size of 0.58, at an alpha of p=0.10; prespecified analysis showed p=0.056 and likelihood - based analysis showed p=0.0259 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 42
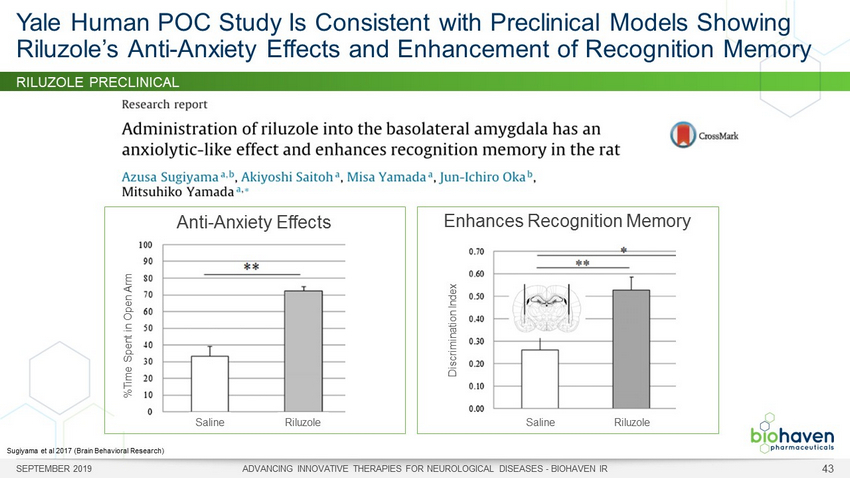
RILUZOLE PRECLINICAL Yale Human POC Study Is Consistent with Preclinical Models Showing Riluzole’s Anti - Anxiety Effects and Enhancement of Recognition Memory Sugiyama et al 2017 (Brain Behavioral Research) SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 43 Anti - Anxiety Effects %Time Spent in Open Arm Saline Riluzole Enhances Recognition Memory Discrimination Index Saline Riluzole
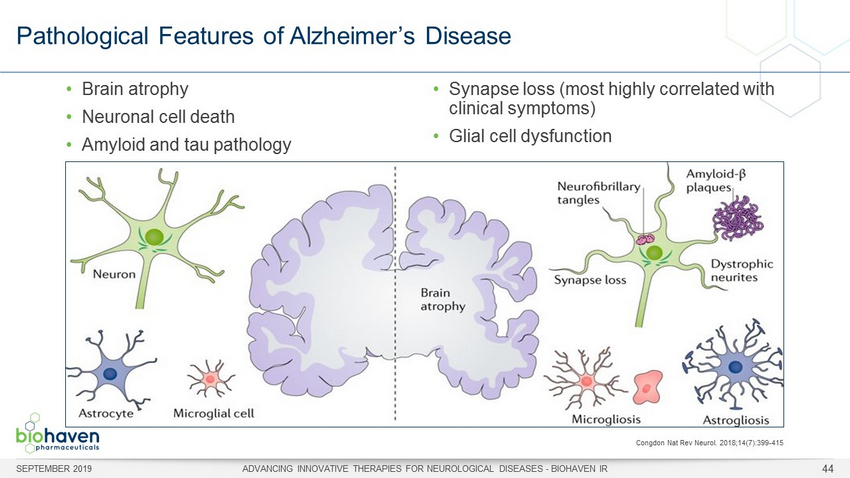
Pathological Features of Alzheimer’s Disease • Brain atrophy • Neuronal cell death • Amyloid and tau pathology • Synapse loss (most highly correlated with clinical symptoms) • Glial cell dysfunction Congdon Nat Rev Neurol. 2018;14(7):399 - 415 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 44
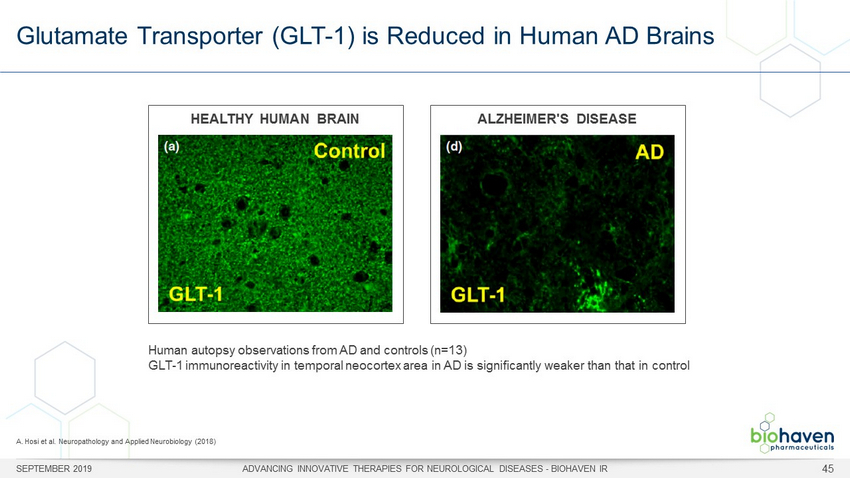
Glutamate Transporter (GLT - 1) is Reduced in Human AD Brains SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 45 Human autopsy observations from AD and controls (n=13) GLT - 1 immunoreactivity in temporal neocortex area in AD is significantly weaker than that in control A. Hosi et al. Neuropathology and Applied Neurobiology (2018) HEALTHY HUMAN BRAIN ALZHEIMER'S DISEASE

Increases GLT - 1 Glutamate Transporter 4 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 46 Riluzole Rescues Symptoms, Function and Pathology in AD Animal Models 1 Pereira Proc Natl Acad Sci 2014; 2 Hunsberger J Neurochem 2015; 3 Hunsberger, Metab Brain Dis 2016; 4 Pereira Molecular Psychiatry 2017; 5 Okamoto Transl Psychiatry 2018 Reduces Amyloid and Tau Pathology Reduces amyloid plaque 5 Reduces p - tau 2 Control TauP301L Riluzole + TauP301L CP - 13/Actin (Relative Ratio) Rescues Cognitive Symptoms (Learning and Memory) Water Maze Errors Control TauP301L Riluzole + TauP301L Morris water maze 2,3 Time (min) Exploration Time (sec) Aged - control Young - control Aged - treated Y - maze Riluzole - treated aged animals perform like young - controls 1 Riluzole - treated tau - overexpressing animals perform like controls Vehicle control RLZ treated

TRORILUZOLE (BHV - 4157) PHASE 2/3 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 47 • Key entry criteria • Diagnosis of mild to moderate Alzheimer’s disease with Mini - Mental State Exam (MMSE) score of 14 – 24 • Co - primary efficacy endpoints • Alzheimer's Disease Assessment Scale (ADAS) - Cog11 • Clinical Dementia Rating - Sum of Boxes (CDR - SB) • Secondary efficacy endpoints • Brain volumes: MRI imaging • Activities of daily living: ADCS - ADL • Neuropsychiatric: NPI • Neuropsychological: NTB • Cognitive: MMSE/ MoCA • Sample size: 292 subjects • Randomization: 1:1 • Collaborator: Alzheimer’s Disease Cooperative Study Troriluzole Phase 2/3 Clinical Trial Design in AD Troriluzole 280 mg QD Placebo QD Screening Phase 42 days Randomized Phase 48 weeks R

Cerebellar Ataxias SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 48 • Characterized by: • Poor balance with falls • Dysarthria / dysphagia • Incoordination of limbs • Cognitive impairment • Postural or kinetic tremor • Oculomotor dysfunction • Spinocerebellar ataxia (SCA; >40 subtypes) • Affects 1 – 5.6 per 100,000 (estimated 3,200 – 18,000 in US) • Neurodegeneration of cerebellum and input/output tracts • Relentlessly progressive, often fatal (aspiration) • Increasing disability over time (requiring wheelchair, assistance with activities of daily living) • The 6 most common genotypes caused by triplet repeat expansion mutations, sharing many phenotypic features • Genetic anticipation in some: subsequent generations affected at earlier ages and with greater severity • Other ataxias have similar core features • Can be recessive, dominant, immune, mitochondrial, post - stroke, e.g., Friedreich’s ataxia, ataxia telangiectasia, multiple system atrophy — cerebellar type Atrophy of Cerebellum and Linked Structures Spinocerebellar Ataxia type 2 Cerebellar and brainstem volume loss No FDA - approved medications for SCA

Troriluzole Treated SCA Patients Treated for 1 Year Compared to Matched Ashizawa Natural History Cohort SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 49 • Post - hoc analysis of patients enrolled in long - term extension of Phase 2b/3 troriluzole SCA trial • Primary efficacy endpoint: change from baseline in the Total SARA Score after 48 weeks • Patients from BHV4157 - 201 trial versus eligibility criteria matched Ashizawa Natural History cohort: • SCA Genotype • SCA1, SCA2, SCA3, SCA6 • Age at baseline • 18 to 75 years of age • Gender • SARA Score at baseline • ≥ 8 and ≤ 30, and • Initial SARA gait item score ≥ 2 Troriluzole Study (BHV4157 - 206) in Spinocerebellar Ataxia (SCA) Achieved Phase 2/3 start in 1Q19 SCA Patients on Troriluzole vs. Natural History Cohort 2 ANCOVA model with fixed effects for cohort, sex, & SCA genotype with age and baseline SARA scores as covariates 1 Matched on eligibility criteria Least Squares Mean 2 Change in Total SARA Score (from baseline ± SE) Difference: - 1.41 ± 0.411 (95% confidence interval of - 2.22 to - 0.60) suggesting therapeutic benefits of troriluzole (p=0.0007) SARA: Scale for the Assessment and Rating of Ataxia

Study BHV4157 - 206: Randomized Controlled Trial of Troriluzole in SCA Key Entry Criteria • SCA genotypes ( SCA1, SCA2, SCA3, SCA6, SCA7, SCA8, SCA10) Design • Sample size: 230 subjects • Randomization: 1:1 • Stratification: by SCA genotype • Dose: Troriluzole 200 mg QD vs. Placebo QD • Primary Outcome: Modified SARA Scale • FDA aligned outcome measure Status • Phase 3 (initiated 1Q19) SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 50 Troriluzole 200 mg QD Placebo QD Screening Phase 6 weeks Randomization Phase 48 weeks R Extension Phase 48 weeks Troriluzole 200 mg QD SARA: Scale for the Assessment and Rating of Ataxia
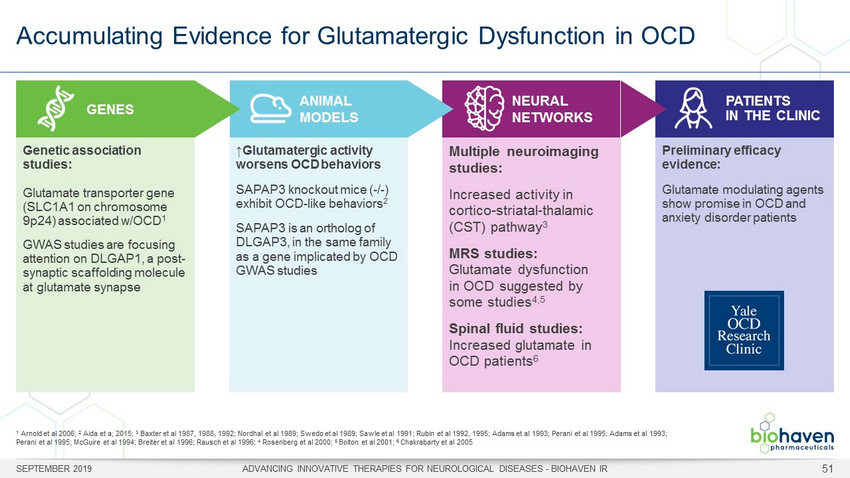
Accumulating Evidence for Glutamatergic Dysfunction in OCD SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 51 GENES ANIMAL MODELS NEURAL NETWORKS PATIENTS IN THE CLINIC Genetic association studies: Glutamate transporter gene (SLC1A1 on chromosome 9p24) associated w/OCD 1 GWAS studies are focusing attention on DLGAP1, a post - synaptic scaffolding molecule at glutamate synapse ↑Glutamatergic activity worsens OCD behaviors SAPAP3 knockout mice ( - / - ) exhibit OCD - like behaviors 2 SAPAP3 is an ortholog of DLGAP3, in the same family as a gene implicated by OCD GWAS studies Multiple neuroimaging studies: Increased activity in cortico - striatal - thalamic (CST) pathway 3 MRS studies: Glutamate dysfunction in OCD suggested by some studies 4,5 Spinal fluid studies: Increased glutamate in OCD patients 6 Preliminary efficacy evidence: Glutamate modulating agents show promise in OCD and anxiety disorder patients 1 Arnold et al 2006; 2 Aida et a; 2015; 3 Baxter et al 1987, 1988, 1992; Nordhal et al 1989; Swedo et al 1989; Sawle et al 1991; Rubin et al 1992, 1995; Adams et al 1993; Perani et al 1995; Adams et al 1993; Perani et al 1995; McGuire et al 1994; Breiter et al 1996; Rausch et al 1996; 4 Rosenberg et al 2000; 5 Bolton et al 2001; 6 Chakrabarty et al 2005
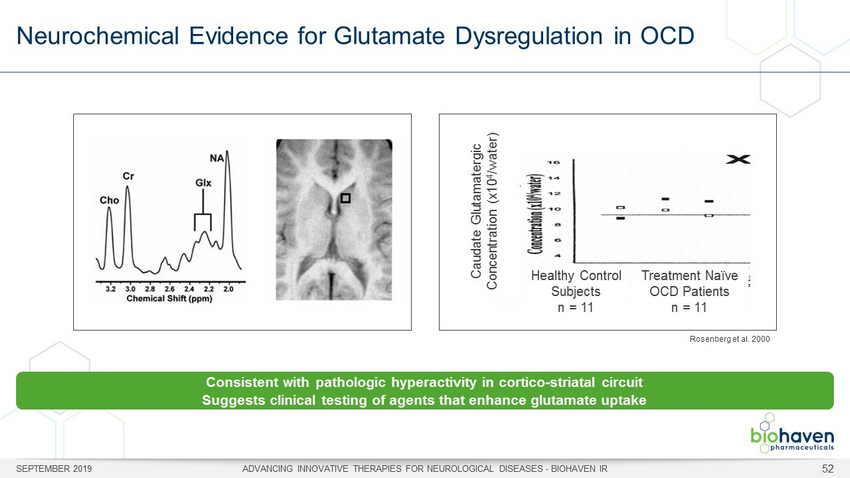
Neurochemical Evidence for Glutamate Dysregulation in OCD SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 52 Rosenberg et al. 2000 Consistent with pathologic hyperactivity in cortico - striatal circuit Suggests clinical testing of agents that enhance glutamate uptake Healthy Control Subjects n = 11 Treatment Naïve OCD Patients n = 11 Caudate Glutamatergic Concentration ( x10 4 /water)
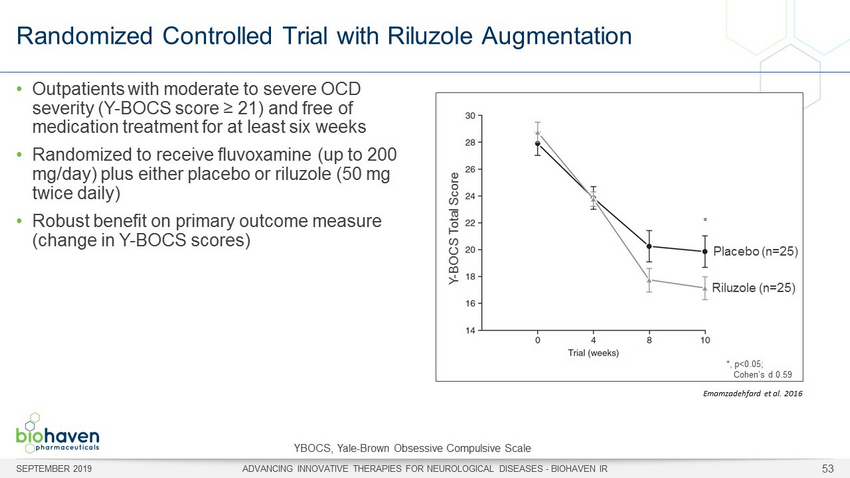
Randomized Controlled Trial with Riluzole Augmentation SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 53 • Outpatients with moderate to severe OCD severity (Y - BOCS score ≥ 21) and free of medication treatment for at least six weeks • Randomized to receive fluvoxamine (up to 200 mg/day) plus either placebo or riluzole (50 mg twice daily) • Robust benefit on primary outcome measure (change in Y - BOCS scores) *, p<0.05; Cohen’s d 0.59 * Placebo (n=25) Riluzole (n=25) Y - BOCS Total Score YBOCS, Yale - Brown Obsessive Compulsive Scale Emamzadehfard et al. 2016

Study BHV4157 - 202: Randomized Controlled Trial of Troriluzole in OCD KEY ENTRY CRITERIA • Moderate to severe OCD and inadequate response to standard of care DESIGN • Sample size: 226 subjects • Randomization: 1:1 • Dose: Troriluzole 200 mg QD vs. Placebo QD (in patients on standard of care) • Primary Outcome: Y - BOCS, a precedented outcome measure accepted by FDA STATUS • Enrollment active • Expect to complete enrollment in 2019 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 54 SOC + Troriluzole 200 mg QD SOC + Placebo QD Screening Phase 42 days Randomization Phase 12 weeks R Extension Phase 48 weeks SOC + Troriluzole 200 mg QD Subjects (n=226) with Moderate to severe OCD and inadequate response to standard of care SOC, standard of care YBOCS, Yale - Brown Obsessive Compulsive Scale

BHV - 0223 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 55 • Amyotrophic Lateral Sclerosis (ALS) is a progressive neurodegenerative disease that causes muscle weakness, difficulties with breathing and swallowing, and death • Generic riluzole has been proven to extends survival in ALS • Yet, swallowing is a challenge for patients with ALS • About 1/3 of patients have dysphagia at diagnosis; over 80% during advanced disease • Many crush riluzole tablet and mix with food, yet label cautions results in lower drug levels • Fasting required 3 hours per dose (fast 2 hours before and one hour after meals) • BHV - 0223 is a proprietary, novel formulation of riluzole optimized for sublingual administration • BHV - 0223 rapidly dissolves when placed under tongue • Active ingredient efficiently absorbed by sublingual mucosa • No need to swallow tablet with liquid BHV - 0223 is Optimized to Address Riluzole Delivery Limitations for ALS Caution: New Drug - Limited by United States law to investigational use BHV - 0223 is an investigational drug not currently approved for the treatment of ALS

BHV - 0223 BHV - 0223 Phase 1 Single Dose PK Results SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 56 Observations • (A) Sublingual (SL) 35 mg dose of BHV - 0223 vs. 50 mg dose of oral Rilutek ( riluzole ) • (B) SL 40 mg BHV - 0223 vs. 50 mg Rilutek • BHV - 0223 associated with less PK variability • Oral Rilutek associated with large PK variability • Lower exposures in some patients • Attributed to poor bioavailability and first - pass metabolism of oral dosing C 0 50000 100000 150000 200000 250000 300000 350000 400000 0.0 0.5 0.1 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 0 50000 100000 150000 200000 250000 300000 350000 400000 0.0 0.5 0.1 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 Rilutek (50 mg tablet) BHV - 0223 (35 mg SL) Actual Time (h) C Mean Plasma Riluzole Concentration (pg/mL) Mean Individual Results Mean Individual Results Time Post - Dose (h) BHV - 0223 is an investigational drug not currently approved for the treatment of ALS A. 35 mg BHV - 0223 vs 50 mg Rilutek B. 40 mg BHV - 0223 vs 50 mg Rilutek
BHV - 5000 SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 57 • Potential first - in - class, next - generation NMDA receptor antagonist • Exclusive license from AstraZeneca • Low - trapping agent • Orally bioavailable prodrug of the IV drug lanicemine • NMDA modulation has the potential for applicability across a number of CNS disorders • BHV - 5000 demonstrate markedly mitigated risk of dissociative effects in the clinic • Attributed to its distinct ability to uncouple from the NMDA receptor more freely than other agents • Well tolerated in a Phase 1 single and multiple ascending dose trial • BHV - 5000 doses up to 95 mg studied to date in Phase 1 • Active metabolite, lanicemine , has been administered to ~770 subjects in single or multiple doses in 18 clinical trials; generally well tolerated BHV - 5000: A Novel Low - Trapping NMDA Antagonist

Glutamate Platform Development Milestones & Next Steps SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 58 3Q2019 Complete Response Received, ongoing discussions with FDA for BHV - 0223 NDA for treatment of ALS via 505(b)(2) 4Q2019 Futility analysis expected in Phase 2/3 trial of troriluzole in Alzheimer’s Disease 4Q2019 Expected to complete enrollment in Phase 2/3 trial of troriluzole in OCD 4Q2019 Expected to complete enrollment in Phase 2/3 trial of troriluzole in GAD 1Q2019 Achieved Phase 3 start in trial of troriluzole in Spinocerebellar Ataxia (SCA) ALS: amyotrophic lateral sclerosis, AD: Alzheimer’s Disease, OCD: Obsessive - Compulsive Disorder, GAD: Generalized Anxiety Disorder, SCA: Spinocerebellar Ataxia

MPO PLATFORM Therapies for Neuroinflammation
BHV - 3241 FOR NEUROINFLAMMATION SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 60 • Rare, rapidly progressive and fatal neurodegenerative disease • Prevalence: 2 – 5 per 100,000 • Clinical symptoms • Parkinsonism: characteristic tremor (not responsive to L - DOPA), rigidity, dysarthria, falls • Cerebellar ataxia • Autonomic failure: orthostatic hypotension, urinary dysfunction, erectile dysfunction • Prognosis: more rapidly progressive than Parkinson’s disease • Time to loss of ambulation: 3.5 – 5 years • Mean survival from symptom onset: 6 – 10 years • Pathology: glial cytoplasmic inclusions (GCIs) containing alpha - synuclein • Disease mechanisms: oxidative stress and neuroinflammation • No disease modifying treatments • Symptomatic and palliative management Multiple System Atrophy (MSA)

BHV - 3241 FOR NEUROINFLAMMATION SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 61 BHV - 3241 Background • Myeloperoxidase (MPO) enzyme • Key mediator of oxidative and inflammatory processes that lead to neurodegeneration • Promotes alpha - synuclein aggregation • Increased in human MSA brains in areas of neurodegeneration 1 • BHV - 3241 ( verdiperstat ) • Potent , first - in - class, brain - penetrant MPO inhibitor Developed by AstraZeneca (formerly AZ3241) 1. Neurotox Res 2012;21(4):393 - 404
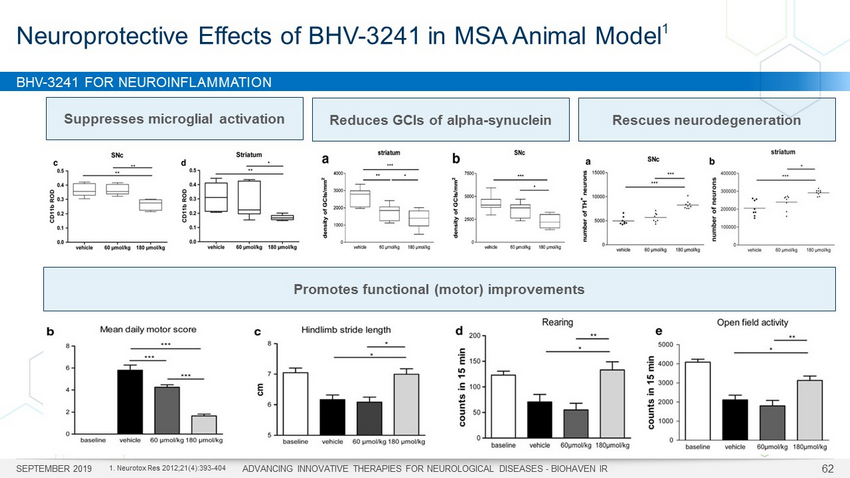
BHV - 3241 FOR NEUROINFLAMMATION SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 62 Neuroprotective Effects of BHV - 3241 in MSA Animal Model 1 Reduces GCIs of alpha - synuclein Promotes functional (motor) improvements Suppresses microglial activation RLZ treated Rescues neurodegeneration 1. Neurotox Res 2012;21(4):393 - 404
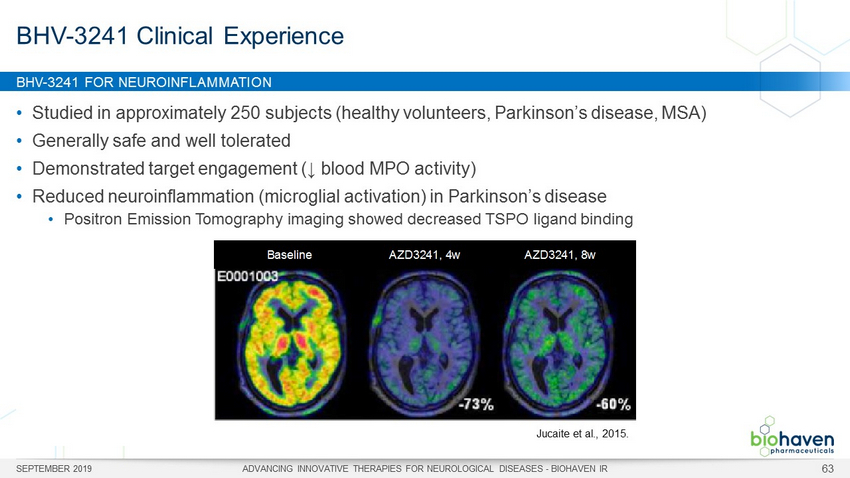
BHV - 3241 FOR NEUROINFLAMMATION SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 63 • Studied in approximately 250 subjects (healthy volunteers, Parkinson’s disease, MSA) • Generally safe and well tolerated • Demonstrated target engagement ( ↓ blood MPO activity) • Reduced neuroinflammation (microglial activation) in Parkinson’s disease • Positron Emission Tomography imaging showed decreased TSPO ligand binding BHV - 3241 Clinical Experience Jucaite et al., 2015. Baseline AZD3241, 4w AZD3241, 8w
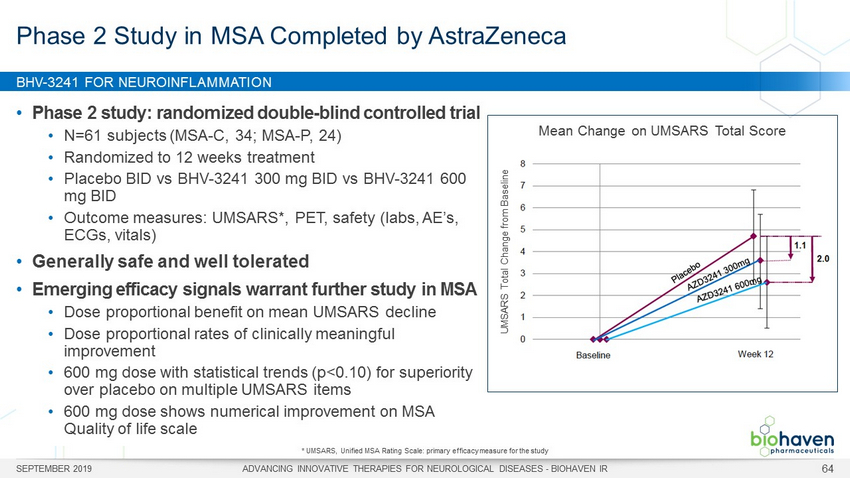
BHV - 3241 FOR NEUROINFLAMMATION SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 64 • Phase 2 study: randomized double - blind controlled trial • N=61 subjects (MSA - C, 34; MSA - P, 24) • Randomized to 12 weeks treatment • Placebo BID vs BHV - 3241 300 mg BID vs BHV - 3241 600 mg BID • Outcome measures: UMSARS*, PET, safety (labs, AE’s, ECGs, vitals) • Generally safe and well tolerated • Emerging efficacy signals warrant further study in MSA • Dose proportional benefit on mean UMSARS decline • Dose proportional rates of clinically meaningful improvement • 600 mg dose with statistical trends (p<0.10) for superiority over placebo on multiple UMSARS items • 600 mg dose shows numerical improvement on MSA Quality of life scale Phase 2 Study in MSA Completed by AstraZeneca * UMSARS, Unified MSA Rating Scale: primary efficacy measure for the study Mean Change on UMSARS Total Score UMSARS Total Change from Baseline
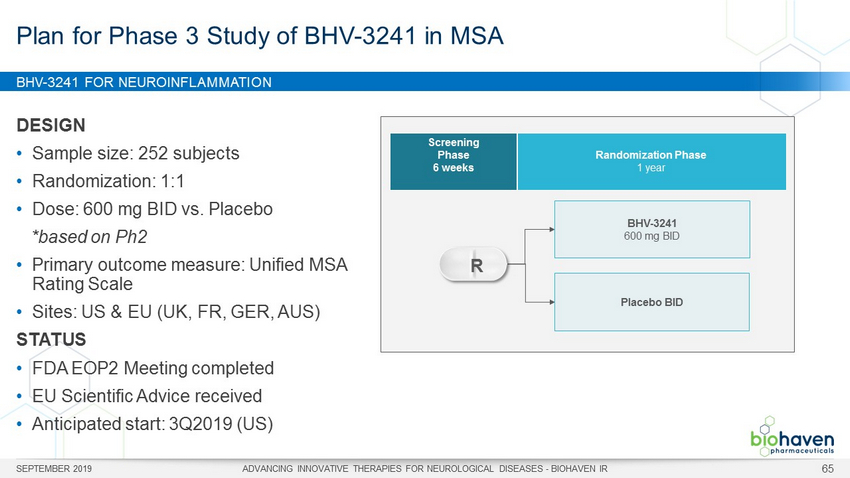
BHV - 3241 FOR NEUROINFLAMMATION SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 65 DESIGN • Sample size: 252 subjects • Randomization: 1:1 • Dose: 600 mg BID vs. Placebo *based on Ph2 • Primary outcome measure: Unified MSA Rating Scale • Sites: US & EU (UK, FR, GER, AUS) STATUS • FDA EOP2 Meeting completed • EU Scientific Advice received • Anticipated start: 3Q2019 (US) Plan for Phase 3 Study of BHV - 3241 in MSA BHV - 3241 600 mg BID Placebo BID Screening Phase 6 weeks Randomization Phase 1 year R

SEPTEMBER 2019 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES - BIOHAVEN IR 66 MPO Platform Development Milestones & Next Steps Received Orphan Disease designation from FDA On track to Initiate a Phase 3 clinical trial for the treatment of MSA, a rare, rapidly progressive and fatal neuroinflammatory disease with no cure or effective treatments 1Q2019 3Q2019 BHV - 3241 FOR NEUROINFLAMMATION

Thank You! www.biohavenpharma.com Zydis ® is a registered trademark of Catalent. Rilutek ® is a registered trademark of Covis Pharma B.V. NYSE: BHVN © 2019 Biohaven Pharmaceuticals Inc. All rights reserved.


































































